Mycoplasma pneumoniae antibody detection kit and application thereof
A detection technology for mycoplasma pneumoniae and antibodies, applied in the biological field, can solve the problems of difficult storage and poor thermal stability, and achieve the effects of wide application, improved stability and high precision
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0067] 3. Preparation of streptavidin-coated magnetic microspheres:
[0068] (1) Preparation of acetic acid buffer with pH 3.6: Weigh 2.55 g of sodium acetate trihydrate and dissolve it in 4500 mL of purified water, then add 14 mL of acetic acid to mix, and make up to 5000 mL to obtain acetate buffer with pH of 3.6.
[0069] (2) Magnetic microsphere connection (magnetic microsphere connection CMC method):
[0070]In the magnetic microsphere, add the above-mentioned pH 3.6 acetate buffer suspension of the coating volume equivalent, wherein the magnetic microsphere concentration is 20mg / mL, and then add the 1-cyclohexyl-2-morpholinoethyl that the concentration is 10mg / mL Carbodiimide p-toluenesulfonate (CMC) was added with 12 μg streptavidin (SA) according to 1 mg of magnetic microspheres to form a reaction system. The above reaction system was put into a constant temperature shaking water bath box at 37°C for reaction for 24 hours.
[0071] (3) Cleaning of magnetic microspher...
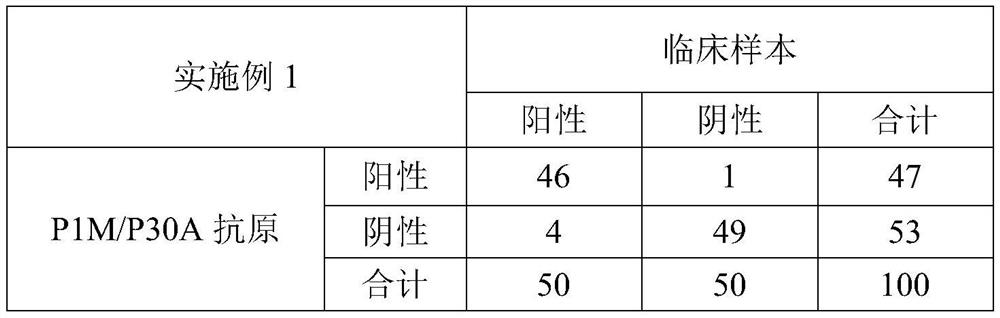
Embodiment 1
[0096] In this example, a reaction system of streptavidin-coated magnetic microspheres and biotin-labeled P1M / P30A antigen was used.
[0097] Biotin-labeled P1M / P30A antigen: The working concentration of biotin is 100ng / mL, and the working concentration of P1M / P30A antigen is 500ng / mL. During the process of labeling the antigen, take 1.5mg of P1M / P30A antigen and adjust it to 1.5mL with dialysate, and stir at room temperature After dialysis for 3 hours, the molar ratio of biotin to P1M / P30A antigen was 10:1, and the biotin-labeled P1M / P30A antigen was labeled according to the aforementioned preparation process of biotin-labeled P1M / P30A antigen.
[0098] Streptavidin-coated magnetic microspheres: the diameter of the magnetic microspheres is 1 μm, the working concentration is 0.5 mg / mL, and the working concentration of streptavidin is 10 μg / mL. The process of making the balls is coated.
[0099] In this example, alkaline phosphatase (ALP)-labeled anti-human IgM antibody, NaOH ...
Embodiment 2
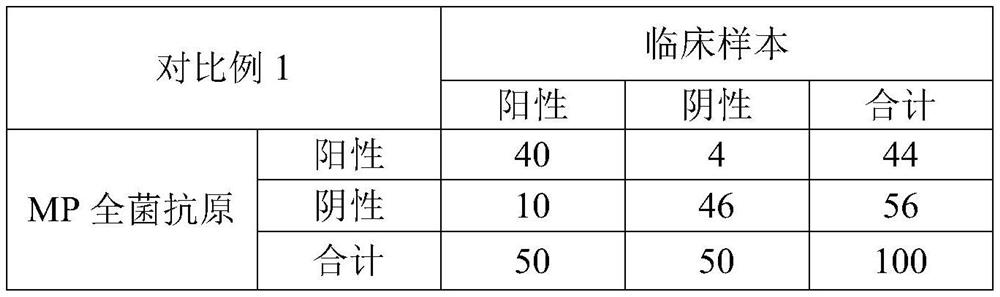
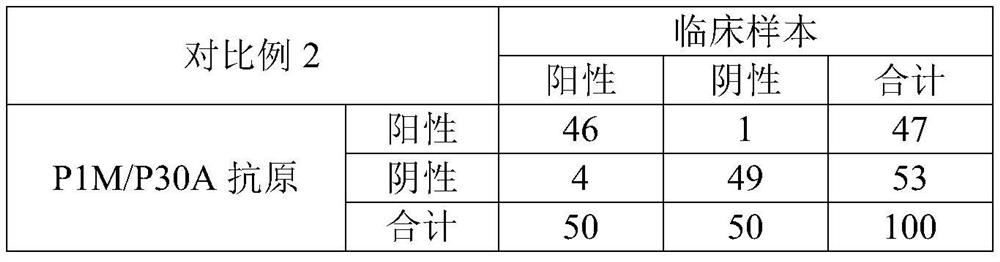
[0108] The AES concentration in the working solution of antigen and antibody in Example 1 was changed to 0.02 wt %, and other conditions were the same as those in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com