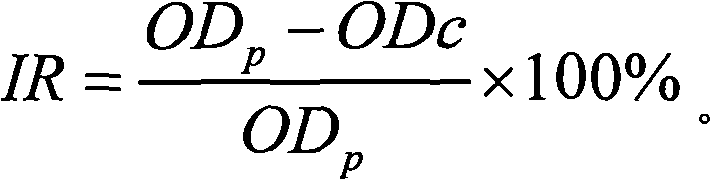Patents
Literature
1019 results about "Coating antigen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method of detecting residue of small-molecule substance harmful to human body and a special kit
InactiveCN101762706AExcitation spectrum widthNarrow emission spectrumFluorescence/phosphorescenceHuman bodyBiology
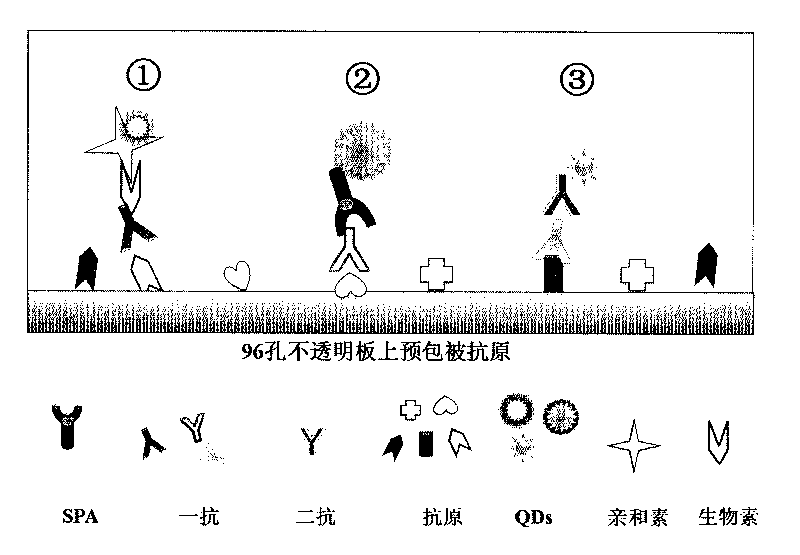
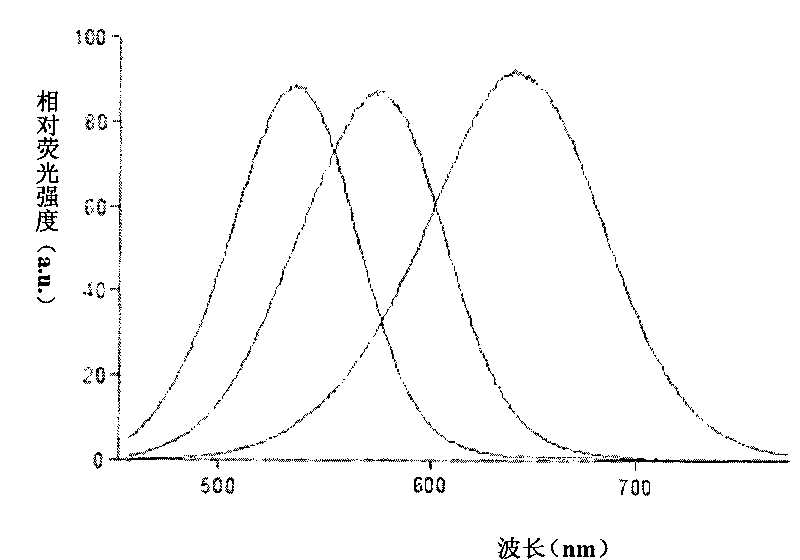
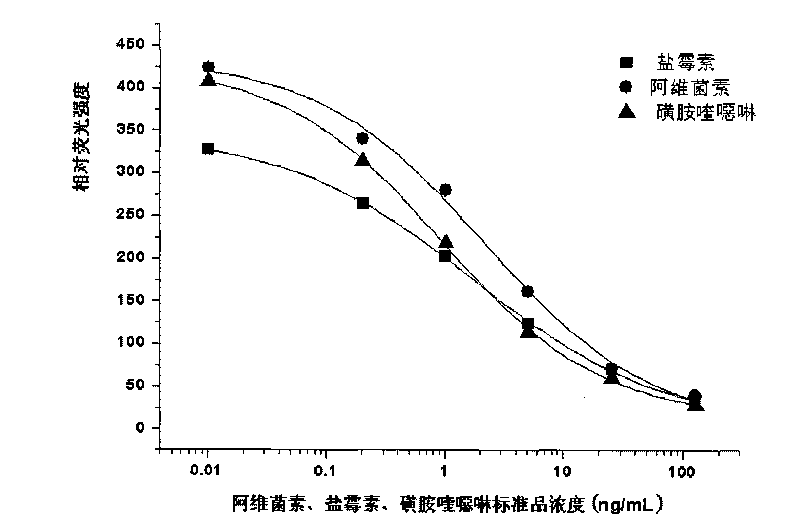
The invention discloses a method of detecting the residue of small-molecule substances harmful to the human body and a special kit. The special kit comprises a non-transparent micro-porous plate and a light-emitting compound, wherein each hole of the non-transparent micro-porous plate is filled with a coat antigen which is simultaneously coated with three kinds of small-molecule substances. The invention makes full use of the multi-color marking function of QDs, establishes a novel kit for simple and rapid detection of the residue and a method thereof, and realizes the multi-color marking through indirect marking of polyclonal antibodies and monoclonal antibodies in the veterinary drug by coupling the QDs with different particle sizes and targets with functional groups (such as an amino) with specific surfaces. The method comprises: obtaining quantum dots with different fluorescent characteristics through separation and purification, namely, multi-color antibody markers, using the multi-color antibody markers as fluorescent probes, and establishing a reaction system for synchronous analysis of various antigen components of different kinds, thereby realizing the synchronous detection of multiple kinds of residues of the veterinary drug in animal food. Moreover, the method has the advantages of simple operation, high fluorescence intensity and long stabilization time.
Owner:CHINA AGRI UNIV
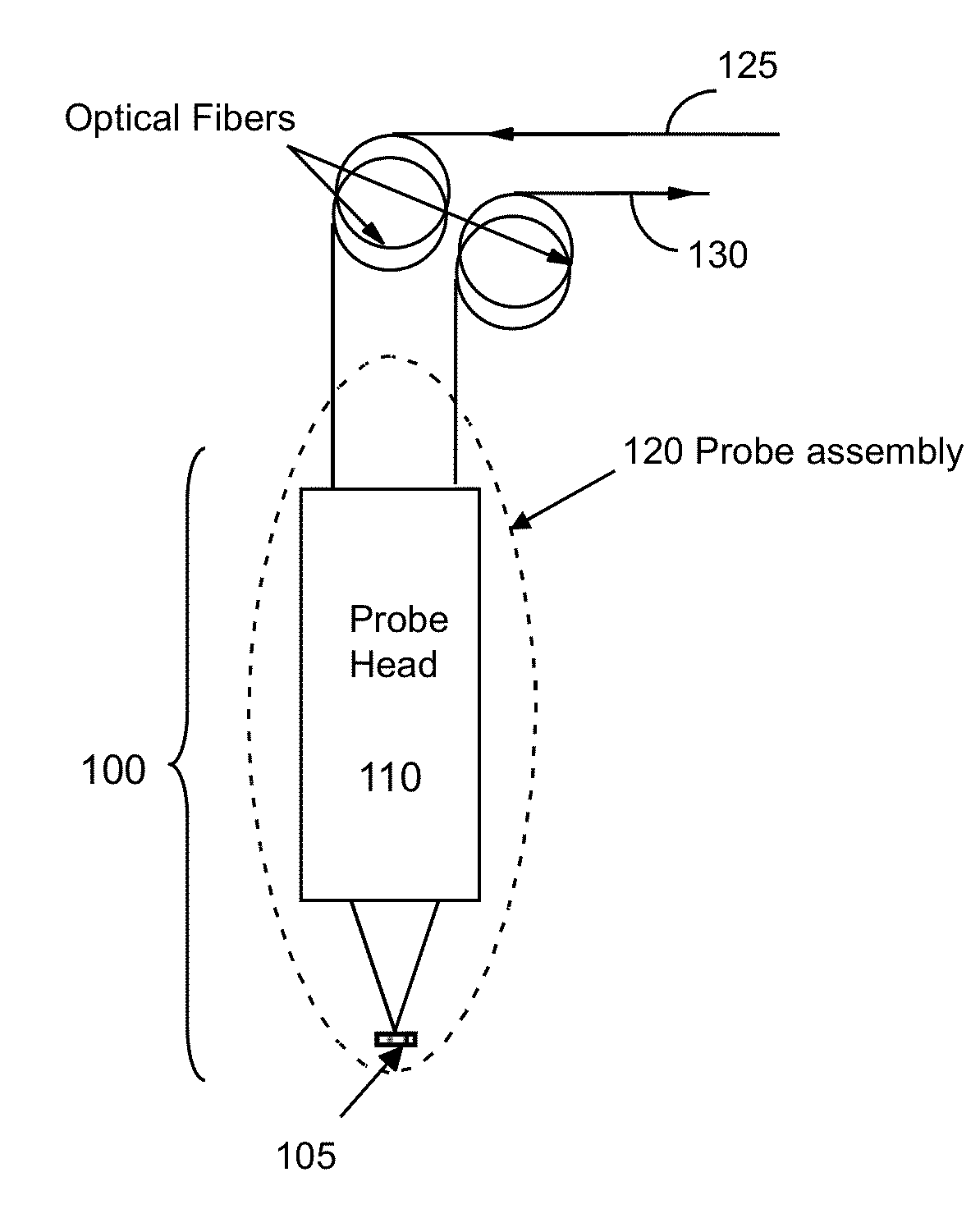
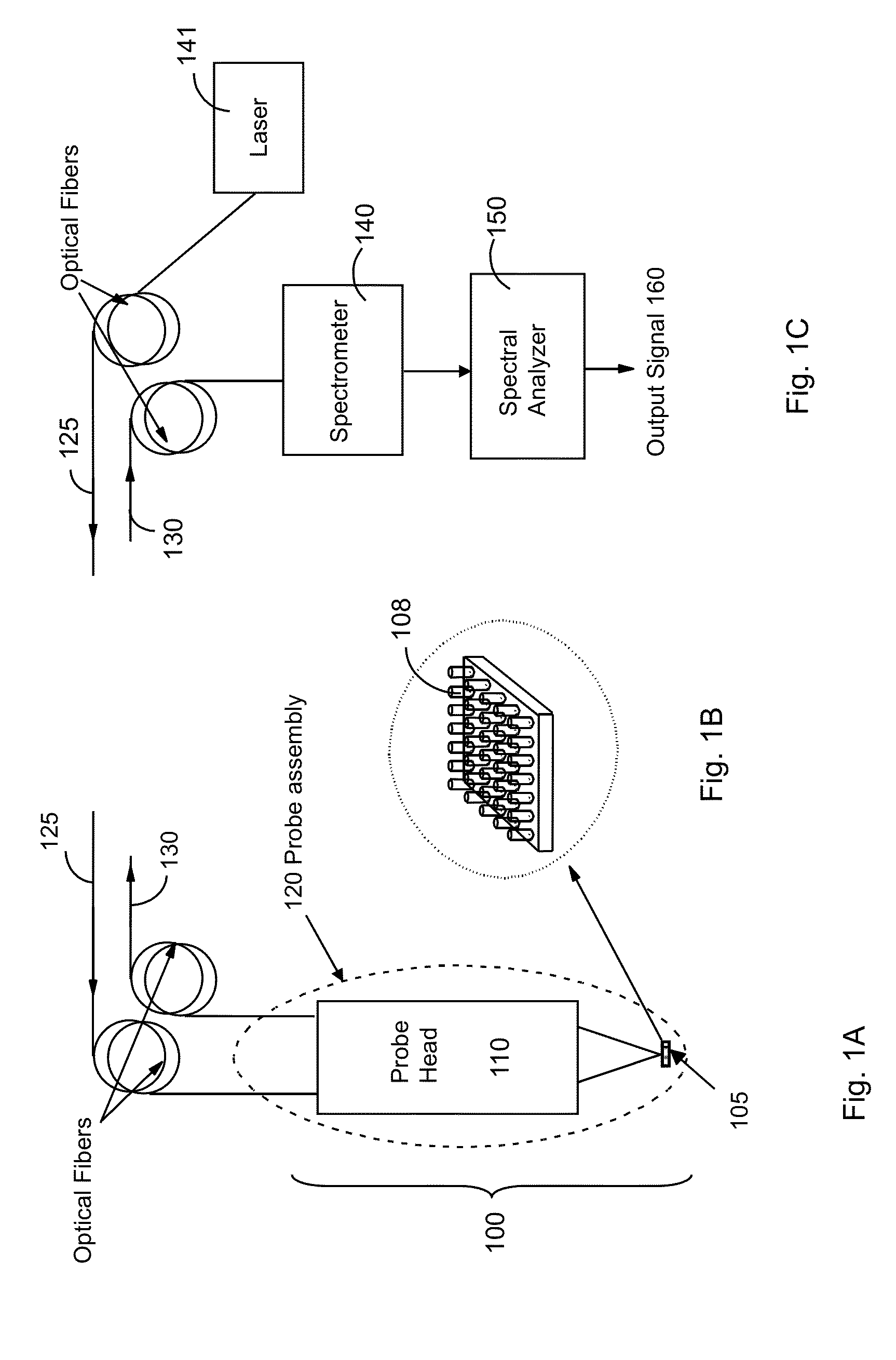
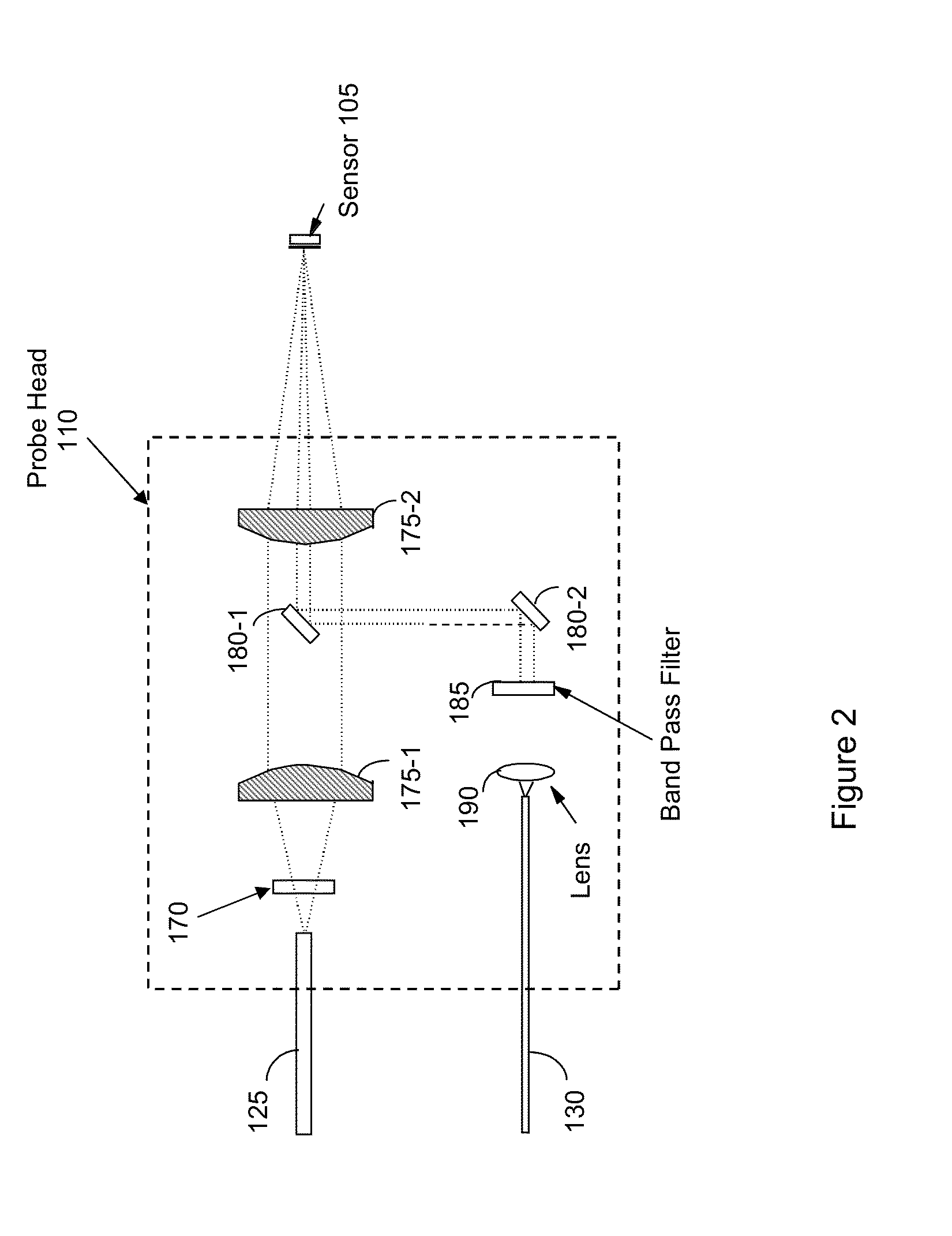
Analyzing chemical and biological substances using nano-structure based spectral sensing
ActiveUS20130115717A1High sensitivityStrong specificityMaterial nanotechnologyComponent separationLaser lightNanostructure
An integrated chromatography-immunoassay system for integrated chromatography-immunoassay system includes a chromatographic unit that receives labeled nano-structured probes comprising nano particles and antibodies attached to the nano particles, and a test membrane comprising coating antigens. The chromatographic unit allows the labeled nano-structured probes to diffuse there through and into the test membrane, wherein the antibodies on the nano particles are bound to the coating antigens. A laser device emits a laser light to illuminate the labeled nano-structured probes having the antibodies bound to the coating antigens on the test membrane. A spectral analyzer obtains a Raman spectrum from light scattered from the labeled nano-structured probes having the antibodies bound to the coating antigens on the test membrane, and to identify a spectral signature in the Raman spectrum associated with the antibody-antigen pair, which enables detection and identification of the antibody.
Owner:EXCELLENT CAPACITY LTD
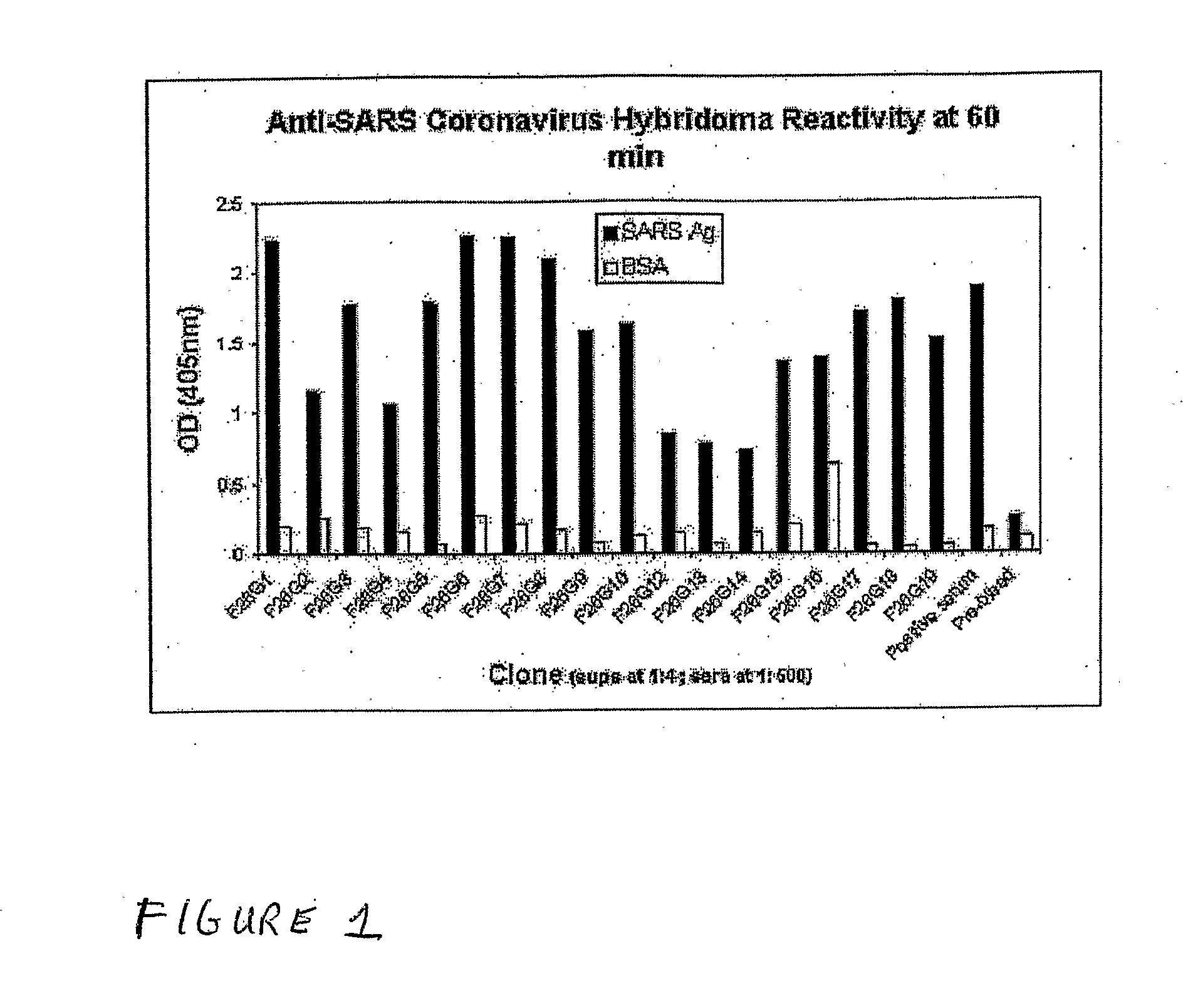
Anti-Sars Monoclonal Antibodies
ActiveUS20080081047A1Sugar derivativesViral antigen ingredientsDrug biological activityWestern immunoblot
Monoclonal antibody reagents that recognize the SARS-coronavirus (SARS-HCoV) are needed urgently. In this report we describe the development and immunochemical characterisation of mAbs against the SARS-HCoV based upon their specificity, binding requirements, and biological activity. Initial screening by ELISA, using highly purified virus as the coating antigen, resulted in the selection of seventeen mAbs. Five mAbs exhibited Western immunoblot reactivity with the denatured spike protein, of which two demonstrated the ability to neutralize SARS-HCoV in vitro. Another four Western immunoblot-negative mAbs also neutralize the virus. These antibodies will be useful for the development of diagnostic tests, pathogenicity and vaccine studies.
Owner:HER MAJESTY THE QUEEN & RIGHT OF CANADA REPRESENTED BY THE MIN OF HEALTH
Detection kit for diagnosing RA, preparating kit, and method for completing standard of quality detection
InactiveCN1796997AIncreased sensitivityQuick checkImmunoglobulinsBiological testingAnti ccp antibodiesArthritis
The invention discloses a diagnosis reagent box for arthritis pauperum and the preparing method thereof as well as a method of completing a quality detection standard. The reagent box is a reagent box for detecting cyclic citrullinated peptide antibodies. The preparing method comprises coating antigens, preparing a contrast and preparing a liquid reagent. The quality detection standard comprises a finished product batched detection standard and a finished product periodic detection standard. And the reagent box has very high positive forecasting value, simple to operate and high- sensitivity and able to be used in fast detection of large numbers of samples and qualitative and quantitative detection of anti-CCP antibodies.
Owner:SHANGHAI KEXIN BIOTECH
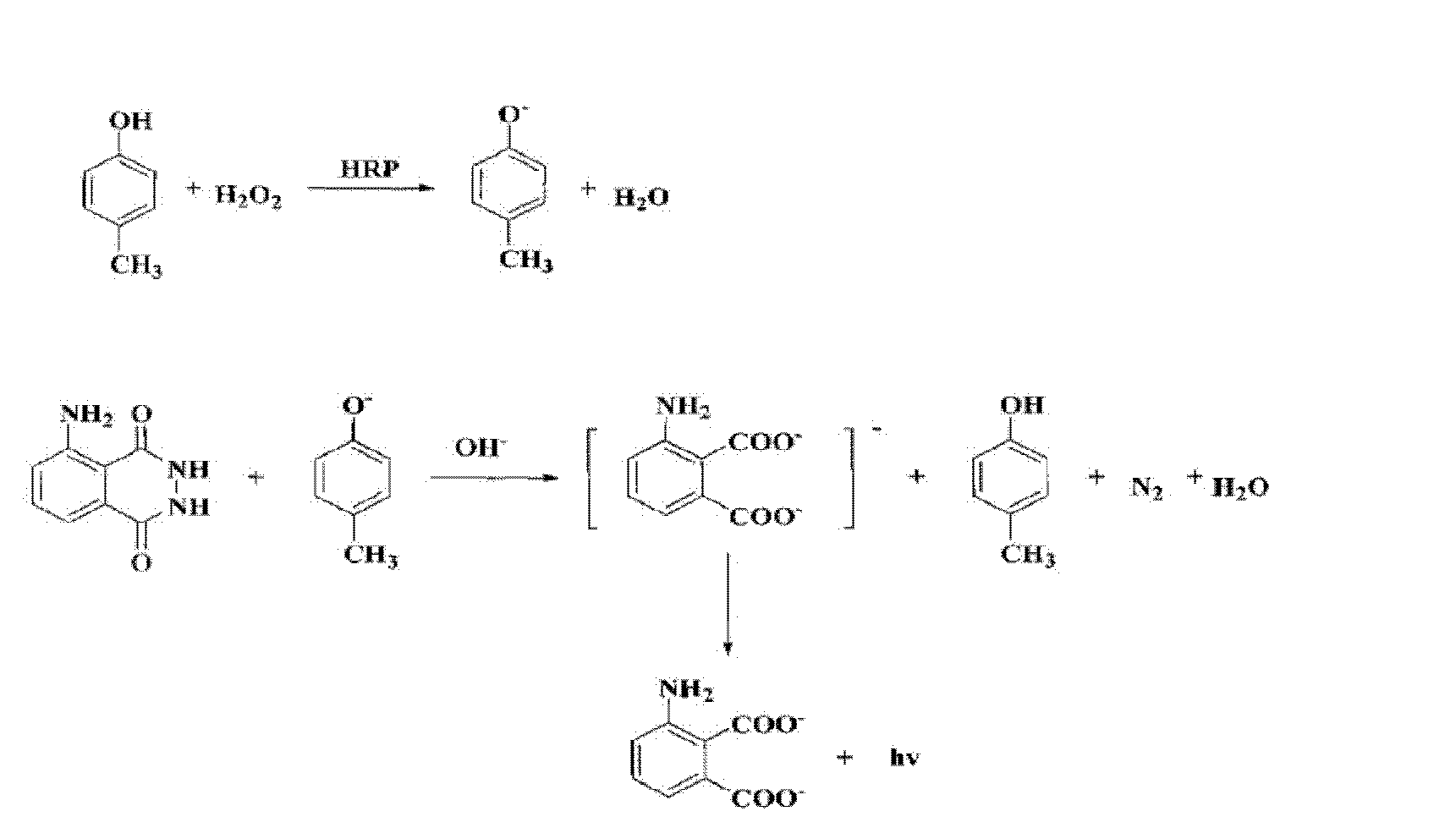
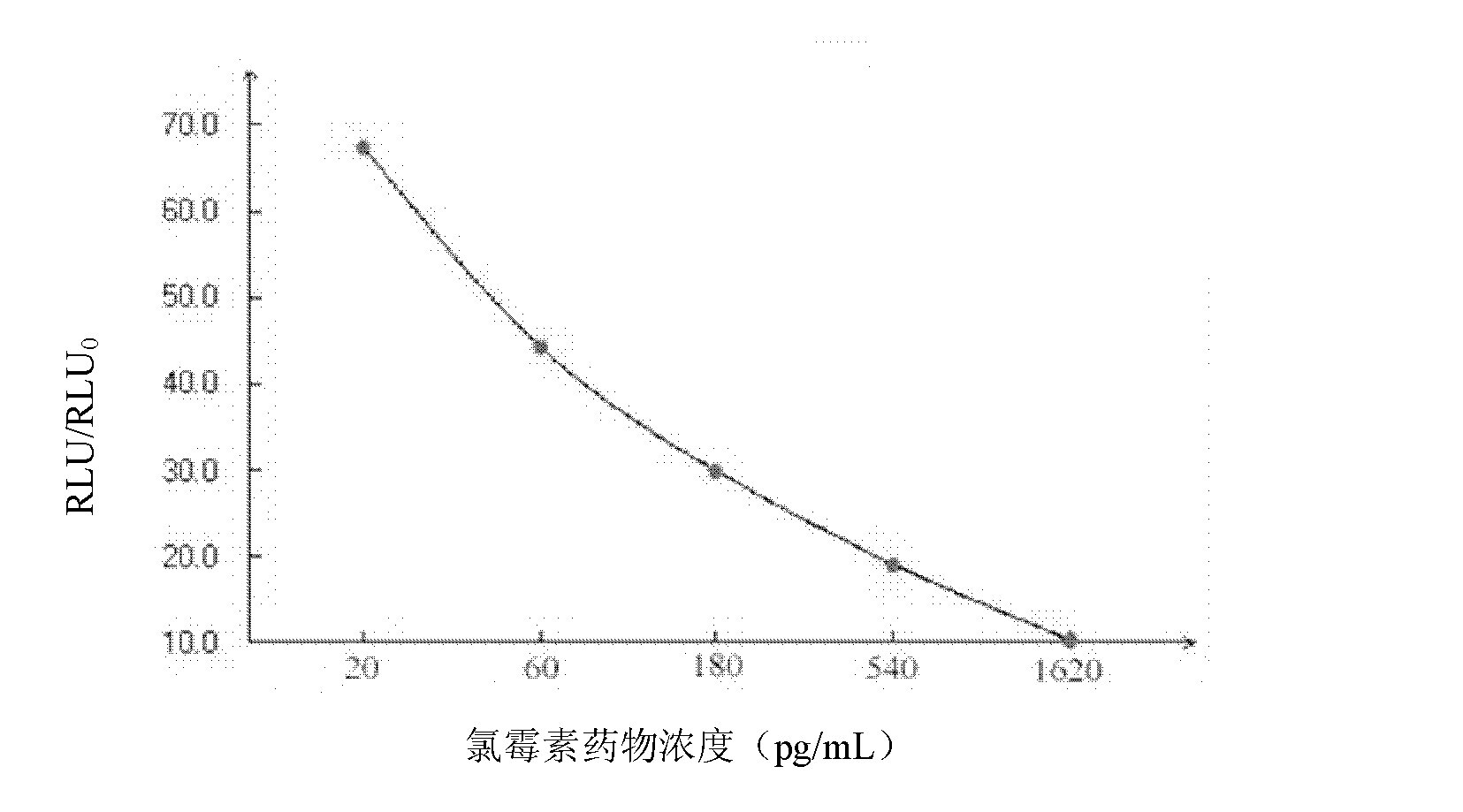
Chloramphenicol chemiluminescence enzyme-linked immunodetection kit
The present invention discloses a chloramphenicol chemiluminescence enzyme-linked immunodetection kit, which comprises a kit body, an enzyme label plate placed inside the kit body, and reagents placed inside the kit body, and is characterized in that every hole of the enzyme label plate is coated with coating antigen, the coating antigen is a chloramphenicol and carrier protein conjugate, and the reagents comprise horseradish peroxidase-labeled chloramphenicol monoclonal antibody, a series of chloramphenicol standard solutions, a concentrated phosphate buffer, a concentrated washing solution and a chemiluminescence solution. The chloramphenicol chemiluminescence enzyme-linked immunodetection kit has characteristics of high sensitivity, simple and rapid detection, and high accuracy, provides a substantially reduced operation time compared to the conventional colorimetric ELISA method, and can be used for detection of chloramphenicol residues in animal tissues (pork, chicken, pork liver and chicken liver), aquatic products (fish and shrimp) and milk.
Owner:BEIJING KWINBON BIOTECH
Analyzing chemical and biological substances using nano-structure based spectral sensing
InactiveCN103698510AHigh detection sensitivityImprove featuresRaman scatteringLaser lightNanostructure
An integrated chromatography-immunoassay system for integrated chromatography-immunoassay system includes a chromatographic unit that receives labeled nano-structured probes comprising nano particles and antibodies attached to the nano particles, and a test membrane comprising coating antigens. The chromatographic unit allows the labeled nano-structured probes to diffuse there through and into the test membrane, wherein the antibodies on the nano particles are bound to the coating antigens. A laser device emits a laser light to illuminate the labeled nano-structured probes having the antibodies bound to the coating antigens on the test membrane. A spectral analyzer obtains a Raman spectrum from light scattered from the labeled nano-structured probes having the antibodies bound to the coating antigens on the test membrane, and to identify a spectral signature in the Raman spectrum associated with the antibody-antigen pair, which enables detection and identification of the antibody.
Owner:OPTO TRACE SUZHOU TECH
Porcine epidemic diarrhea recombinant baculovirus gene engineering subunit vaccine, preparation method and application thereof
InactiveCN103585625AImprove abilitiesTargetedMicroorganism based processesAntiviralsGenetic engineeringTGE VACCINE
The invention belongs to the technical field of biological vaccine preparation, and particularly relates to a porcine epidemic diarrhea (PED) recombinant baculovirus gene engineering subunit vaccine, a preparation method and an application thereof. According to the present invention, S1 gene and M gene of the current new PEDV epidemic strain are selected as reference sequences, a baculovirus expression system is adopted to express S1 protein or partial S1 protein and M protein, and the obtained recombinant protein is prepared into a subunit vaccine for effectively controlling PED occurrence; with the PED recombinant baculovirus gene engineering subunit vaccine produced by using the method, the defect of the current PEDV traditional vaccine is solved; and the PED recombinant baculovirus gene engineering subunit vaccine can be used for prevention and treatment of PEDV infections and related diseases caused by PEDV, and can further be used for preparation of coating antigen of PEDV detection antibody ELISA kits.
Owner:SOUTH CHINA AGRI UNIV
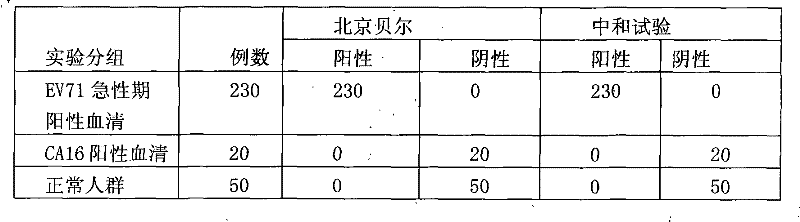
Diagnostic reagent kit (enzyme-linked immunosorbent assay (ELISA)) for enterovirus (EV) 71-type antibody (immune globulin M (IgM))
InactiveCN102243232AEasy to operateAvoid distractionsColor/spectral properties measurementsEnterovirusAbzyme
The invention relates to the field of biomedicine, in particular to an enzyme-linked immunization diagnostic reagent kit for detecting an enterovirus (EV) 71-type antibody (immune globulin M (IgM)), and a preparation method and application of the diagnostic reagent kit. The probability of hand-foot-and-mouth disease and severe infection (viral encephalitis, viral cerebrospinal meningitis and pulmonary edema) caused by EV71 type is relatively higher, and case fatality rate is relatively higher and can be 10 to 25 percent. The enzyme-linked immunization diagnostic reagent kit of the EV71-IgM antibody can be used for diagnosing the infection of the EV71 type. According to related documents about the detection of the EV71-IgM, EV71 virus cultures serving as indirect enzyme-linked immuno sorbent assay (ELISA) of envelope antigens has defects in such aspects as specificity, sensitivity and stability, and due to high cultivation cost and low efficiency, a large amount of virus cannot be supplied to the market. In order to overcome the defects, the invention provides the reagent kit which is used for detecting the EV71-IgM in human blood serum, required by clinical examination, simple and convenient to operate and applicable to all medical disease control departments, and the preparation method and the application of the reagent kit. The invention has the technical scheme that: firstly, the human blood serum is added into a micro-pore plate, wherein the IgM antibody is obtained by an anti-mu chain which is pre-enveloped on the micro-pore plate, and other uncombined components are washed and removed; secondly, an enzyme labeling object is added, the EV71-IgM in the obtained IgM can be combined with the specificity of an EV71 recombinant antigen which is labeled by horse radish peroxidase (HRP), and after washing, the HRP can react with substrates which are added subsequently; and finally, the aim of detecting the EV71-IgM antibody is fulfilled.
Owner:BEIJING BEIER BIOENG
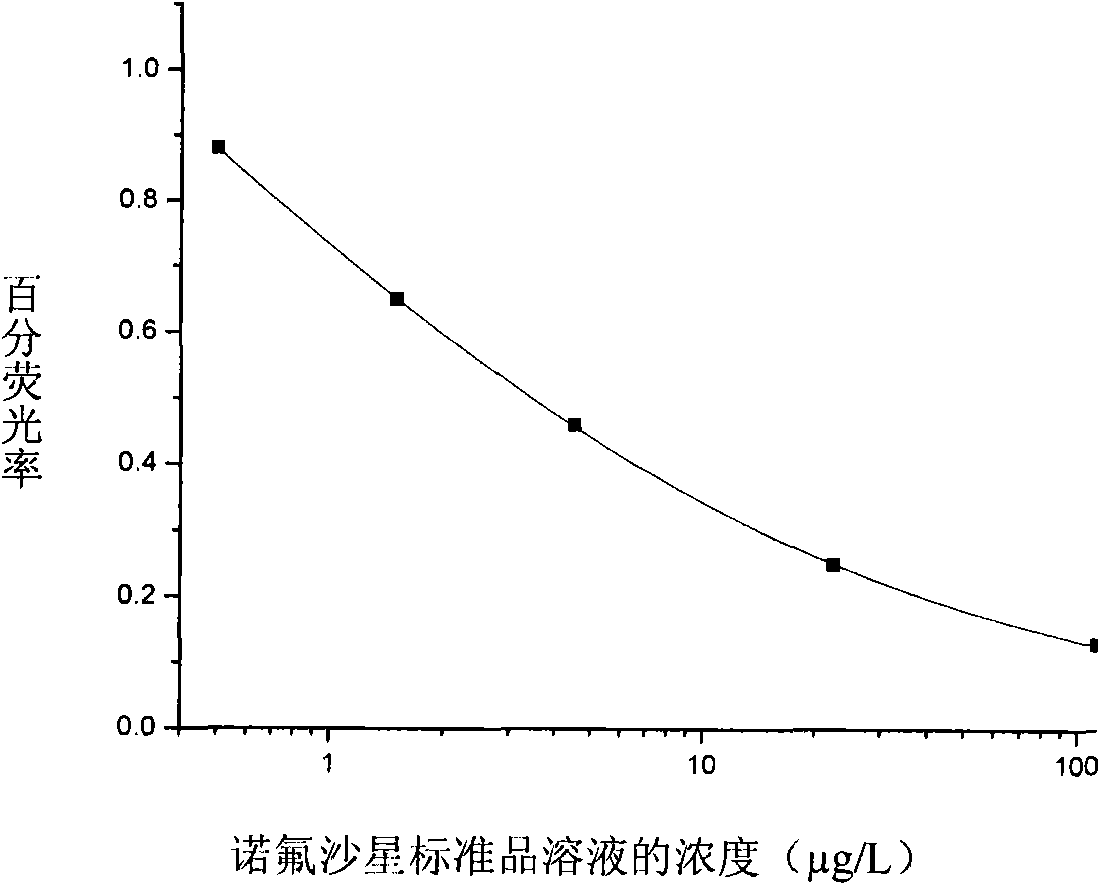
Method and special quantum dot fluorescent immunoassay kit for detecting quinolone compounds
ActiveCN101915844AConducive to preservationImprove stabilityMicroorganism based processesTissue cultureCompound specificFluorescence
The invention discloses a method and a special quantum dot fluorescent immunoassay kit for detecting quinolone compounds. The quantum dot fluorescent immunoassay kit for detecting the quinolone compounds provided by the invention comprises specific antibodies, coating antigens and standard solution of the quinolone compounds, wherein the coating antigens are conjugates of hapten and carrier proteins of the quinolone compounds. Experiments prove that the kit of the invention has the characteristics of simple sample pretreatment process, simple and convenient operation, low cost, high specificity, high sensitivity, high accuracy, on-site monitoring, suitability for screening of a large number of samples, and the like.
Owner:CHINA AGRI UNIV
Indirect ELISA (Enzyme-Linked Immunosorbent Assay) method and kit for detecting haemophilus parasuis antibodies
InactiveCN101968490AGood specificityHigh sensitivityBacteria peptidesBiological testingEpidemiologic surveyEnzyme
The invention discloses indirect ELISA (Enzyme-Linked Immunosorbent Assay) method and kit for detecting haemophilus parasuis antibodies. The indirect ELISA method comprises the steps of: taking a haemophilus parasuis OMPP5 protein as an envelope antigen; wherein the judgment standard is as followed: OD450 value of to-be-detected serum is greater than 0.375, OD450 value of the to-be-detected serum / OD450 value of standard negative serum is greater than or equal to 2.1. The kit for realizing the method comprises an envelope buffer solution, confining liquid, a washing buffer solution, an antibody diluent, a chromogenic substrate solution and a stop solution, wherein the envelop antigen used in the method is the haemophilus parasuis OMPP5 protein. Proved by a specificity test, a blocking-up experiment, a repeatability test and clinic application detection, the invention has the characteristics of excellent specificity, high sensitiveness, good repeatability, rapidness, simpleness and accuracy, and can be used in clinic large-scale detection of haemophilus parasuis antibodies and epidemiological survey.
Owner:广东省农业科学院兽医研究所
Nano antibody of anti-deoxynivalenol antibody
InactiveCN104592389AHave immune response propertiesGood effectImmunoglobulinsGenetic engineeringEpitopeEndurance capacity
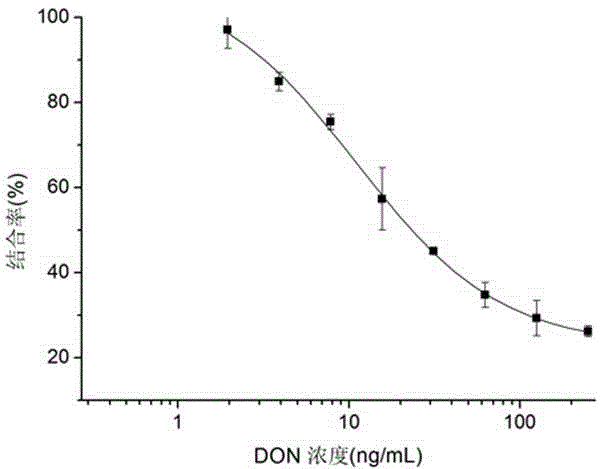
The invention belongs to the technical field of biology and specifically relates to a preparation method and an application of a nano antibody capable of specially binding with an anti-deoxynivalenol antibody. The amino acid sequence of the nano antibody is SEQ ID No. 1. The invention also relates to a nucleotide for encoding amino acid. The nano antibody is capable of taking the place of the expensive high-toxicity DON standard substances, and can be applied to the immunological detection of the DON as a competitive antigen or a solid-phase envelope antigen; the nano antibody has immunoreaction characteristic similar to that of natural DON molecule, and is excellent in effect. Compared with the traditional antigen mimic epitopes based on polypeptides and the traditional anti-idiotype antibodies based on IgG, the nano antibody has the characteristics of more stable structure, acid-alkali resistance, high temperature resistance, high detection sensitivity and the like, and therefore, the immunodetection stability of the nano antibody is greatly improved, and meanwhile, the endurance capacity of the nano antibody to the environment is also improved.
Owner:NANCHANG UNIV
Competitive ELISA kit for peste-des-petits-ruminants antibody detection and preparation method thereof
ActiveCN102967710ANo cross reactionStrong specificityImmunoglobulins against virusesBiological testingSerum igeElisa kit
Belonging to the field of biotechnologies, the invention discloses a competitive ELISA kit for detection of a peste-des-petits-ruminants virus antibody. The kit comprises a detection system composed of a coating antigen reaction solution and a monoclonal antibody reaction solution. The kit adopts prokaryotically expressed peste-des-petits-ruminants Nigeria 75 / 1 strain N protein as the coating antigen and employs a monoclonal antibody against N protein as the competitive antibody. The antibody against a peste-des-petits-ruminants virus in sheep serum is detected according to a competitive ELISA principle. The kit provided in the invention can rapidly and specifically detect the peste-des-petits-ruminants virus antibody in serum, and simultaneously has the advantages of large-scale production of monoclonal antibodies, good reaction specificity, high sensitivity, simple operation, low cost, stable, reliable and easily observable reaction results, thus being very suitable for import and export quarantine of sheep, food hygiene and screening of large batches of samples in livestock breeding farms, and being easy for large-scale popularization and application.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
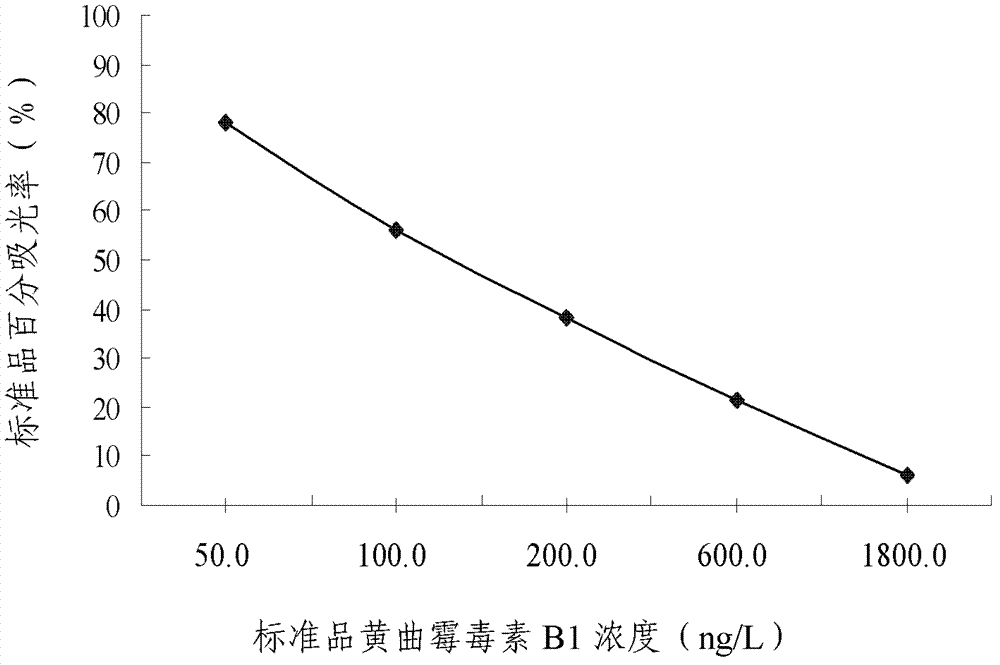
Enzyme-linked immunosorbent assay kit for detecting aflatoxin B1-containing medicine and application for same
The invention provides an enzyme-linked immunosorbent assay kit for detecting an aflatoxin B1-containing medicine and an application for the same. The enzyme-linked immunosorbent assay (ELISA) kit comprises an ELISA plate coated with a coating antigen, an enzyme label, aflatoxin B1 specific antibody working solution (contained in the case that the coating antigen on the ELISA plate and the enzyme label are enzyme-labelled antibodies or enzyme-labelled antigens), aflatoxin B1 standard substance solution, substrate developing solution, stopping solution, concentrated washing solution and concentrated compound solution. The method for detecting aflatoxin B1 by virtue of the kit provided by the invention comprises the following steps of: performing sample pre-treatment at first, and then detecting by virtue of the kit, and finally analysing the detected result. The enzyme-linked immunosorbent assay kit provided by the invention can be used for detecting the residual amount of aflatoxin B1 in samples such as oil, peanuts and grains, as well as is simple and convenient to operate, low in expense, high in sensitivity, capable of being monitored in the field, and suitable for screening lots of samples.
Owner:BEIJING KWINBON BIOTECH
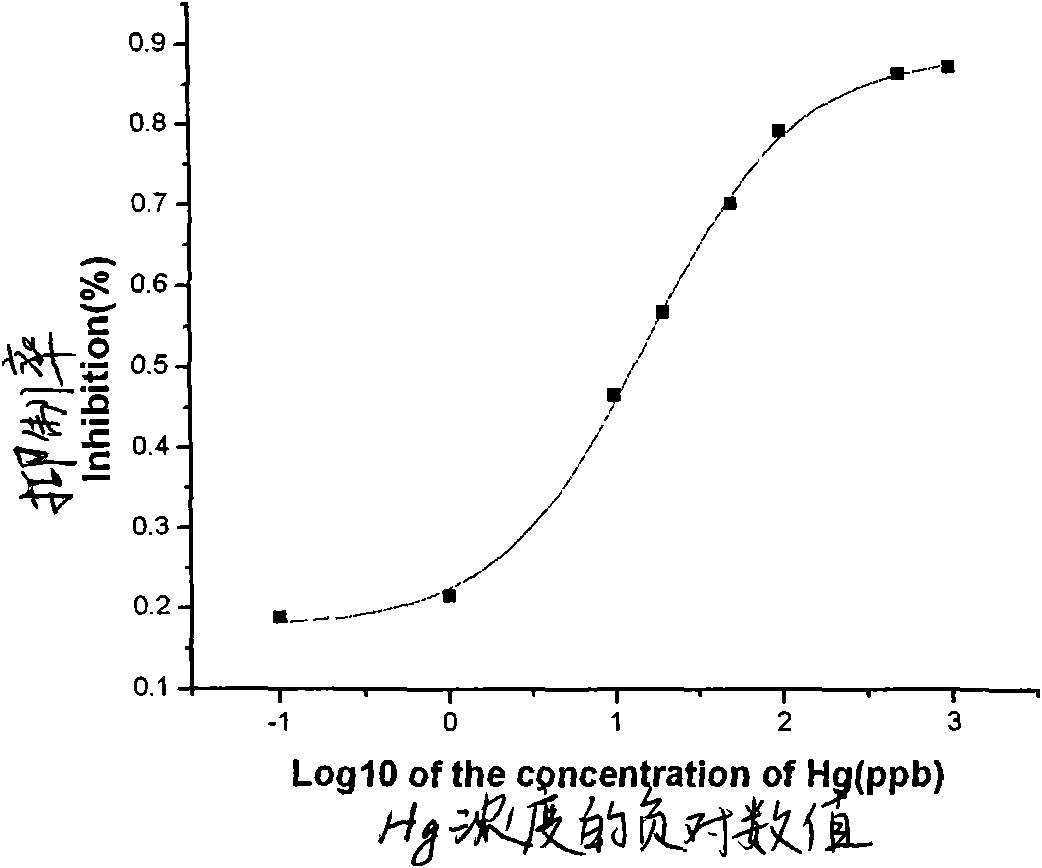
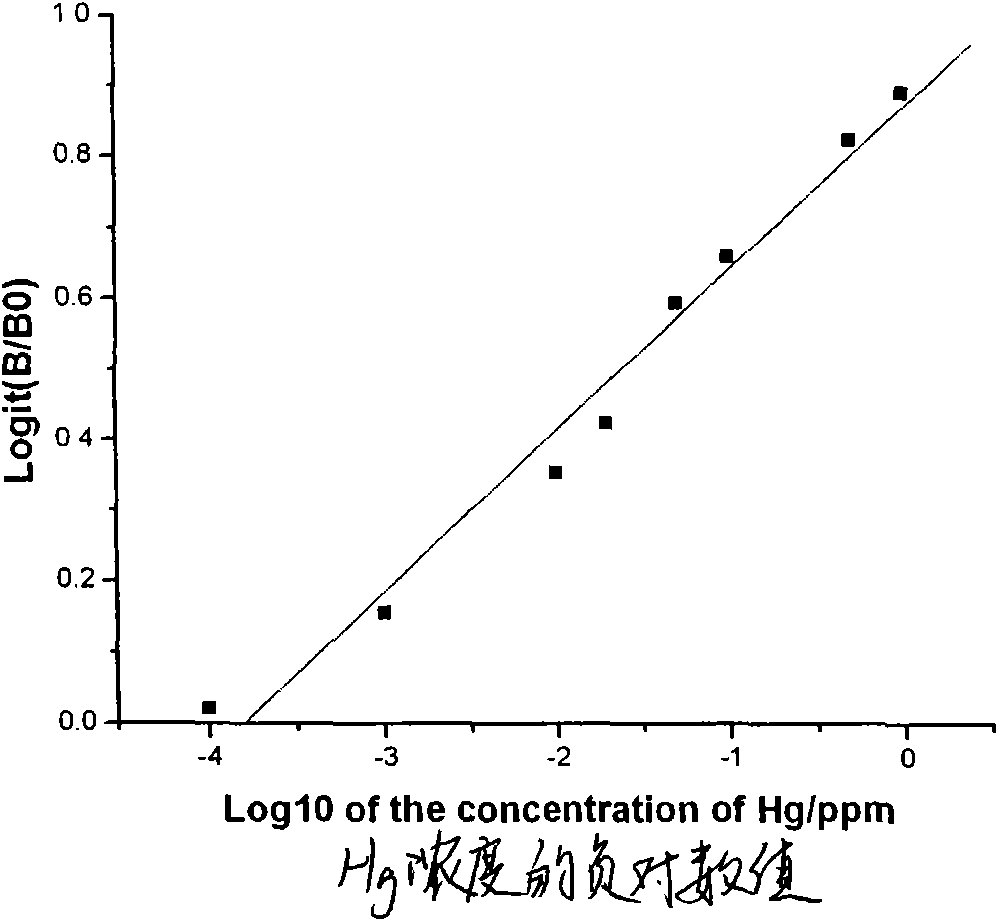
Indirect competitive enzyme-linked immunosorbent assay for measuring heavy metal mercury
The invention discloses an indirect competitive enzyme-linked immunosorbent assay (ELISA) for measuring heavy metal mercury, belonging to a method for measuring heavy metal mercury in an environment water sample. The method comprises the following steps: taking a monoclonal antibody for specially identifying mercury-chelating agent EDTA compound Hg-EDTA as the base, coating the coated antigen mercury-chelating agent-albumen on a 96-hole ELISA plate; incubating at 4 DEG C over night and then sealing by phosphate buffer solution PBS including 1% of gelatin, after washing the ELISA plate, addinga mixing solution of the specificity mercury monoclonal antibody and samples to be measured, incubating at 37 DEG C and then washing the plate, adding an ELISA secondary antibody, incubating at 37 DEGC, adding an enzyme reaction substrate after washing the plate, and adding a reaction stop solution for stopping the reaction after the incubation, and then measuring the absorbance value of each hole by an ELISA reader; obtaining a standard competitive inhibit curve, then executing Logit transition on the curve, and drawing the standard curve of the mercury in the sample to carry out the quantitative analysis. The method is suitable for the measurement of trace mercury in an environment water sample, simultaneously provides the technical support for the fast scene measurement of heavy metalpollution emergency in order to remedy the pollution accident and go back to work in time.
Owner:NANJING UNIV
CSFV antibody detection system and preparation method thereof
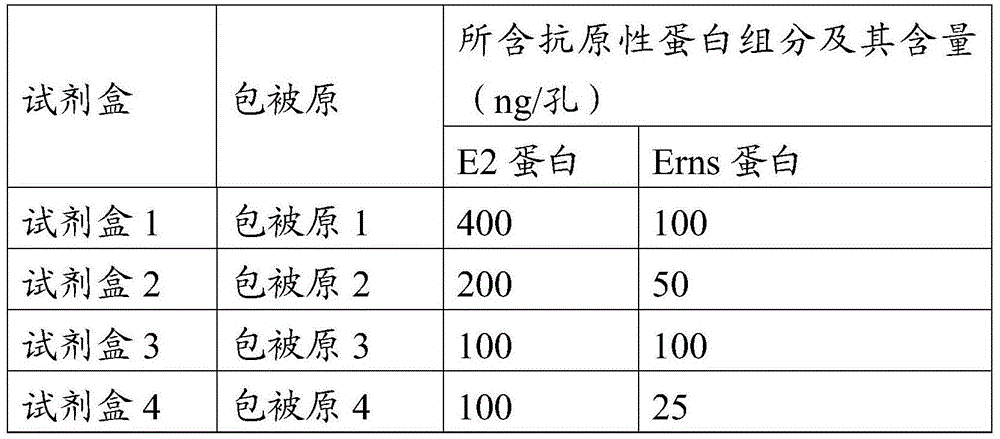
ActiveCN105527442AHigh detection rate sensitivityImprove capture efficiencyBiological testingSerum igeE2 protein
The invention provides a CSFV antibody detection system and a preparation method thereof. A coating antigen of the detection system contains CSFV E2 protein and Erns protein. The CSFV E2 protein and Erns protein are recombinant proteins expressed by eucaryon, correct spatial conformation and posttranslational modification process can be guaranteed, antigen is capable of effectively combining with the antibody in serum, and specificity, sensitivity and repeatability of detection can be increased. The system and the method can be sued for diagnosis of CSFV antibody in prevention and control of CSFV as well as immunization evaluation of a CSFV vaccine.
Owner:LUOYANG PULIKE WANTAI BIOTECH
Indirect ELISA (enzyme linked immunosorbent assay) kit for detecting porcine epidemic diarrhea virus antibody
ActiveCN103675274AStrong specificityIncreased sensitivityBiological material analysisSerum igeEpidemic diarrhea
The invention discloses an indirect ELISA (enzyme linked immunosorbent assay) kit for detecting a porcine epidemic diarrhea virus antibody. The indirect ELISA kit comprises a coated ELISA plate, negative control serum, positive control serum, ELISA secondary antibody, a concentrated cleaning solution, a sample diluent, a developing liquid and a stop buffer, wherein the coated ELISA plate utilizes recombinant protein Nh as a coating antigen. Research and practice show that the indirect ELISA kit has the characteristics of high specificity and sensitiveness, simplicity in operation and the like, is easy to popularize and use in a large range, has a broad market prospect, and can be used for the fields of serological investigation, antibody monitoring and vaccine immunity effect evaluation and the like of epidemic diarrhea infection condition.
Owner:GUANGXI UNIV
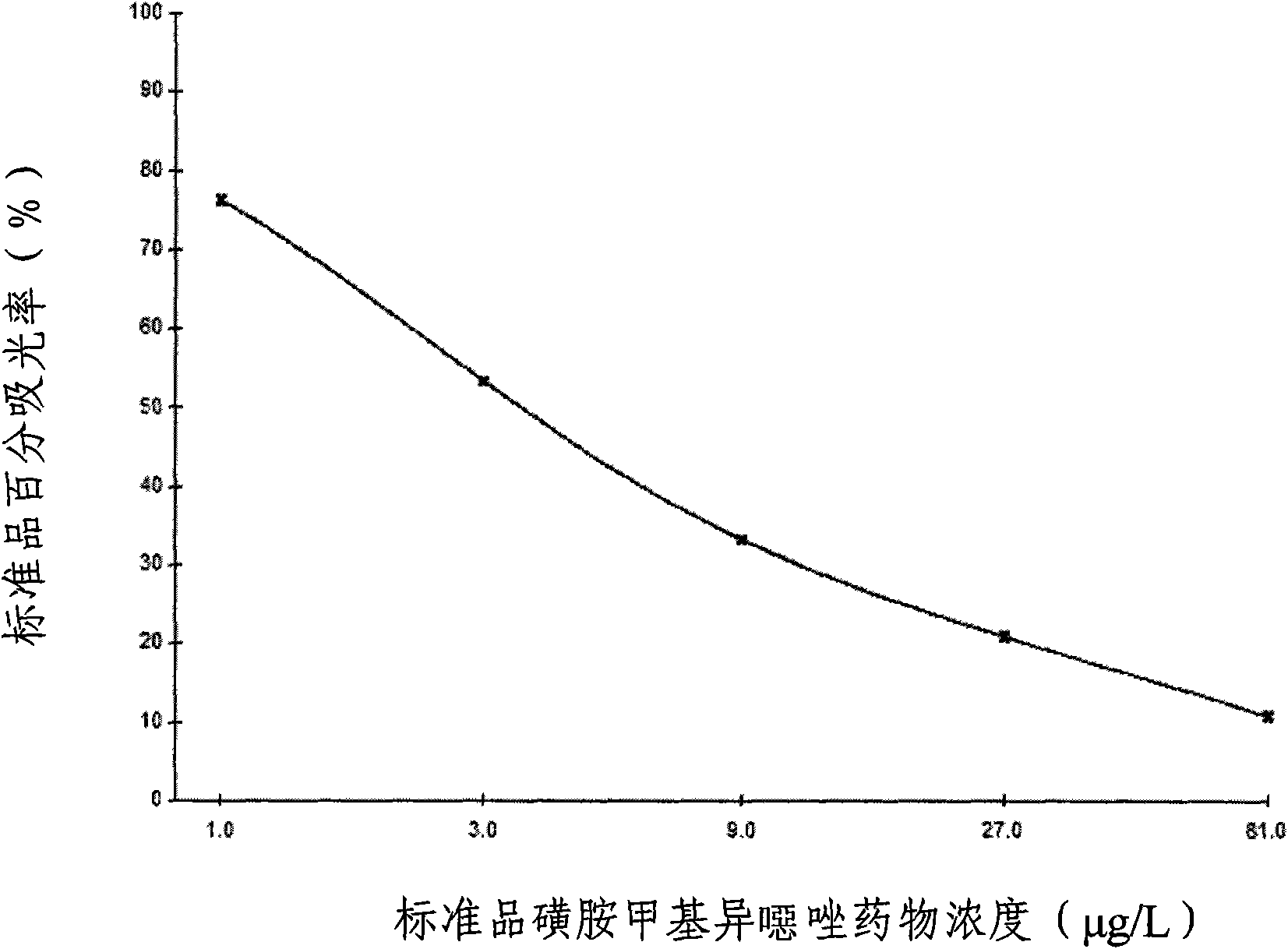
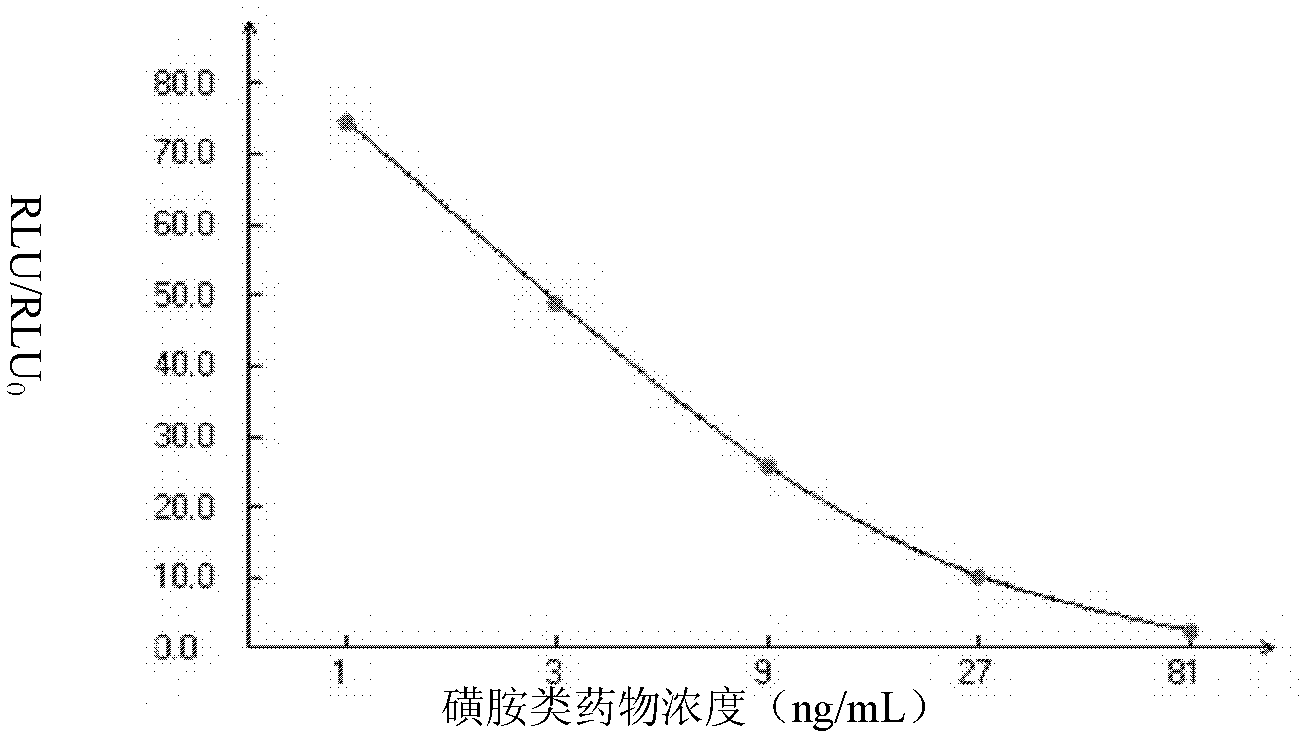
Enzyme-linked immunosorbent inspect kit for inspecting sulfa drugs and method thereof
The invention provides an enzyme-linked immunosorbent kit for inspecting sulfa drugs, comprising an ELISA plate which is coated with coating antigen, an enzyme label, sulfa drug specific antibody working liquid (being contained when the antigen is coated on the ELISA plate and the enzyme label is enzyme labeling antibody or antibody is coated on the ELISA plate and the enzyme label is enzyme labeling antigen), sulfamethoxy-isoxazole standard product solution, substrate color development solution, stop solution, concentrated washing liquid and concentrated complex solution. The invention further discloses a method which applies the enzyme-linked immunosorbent kit for inspecting the sulfa drugs, and the method comprises the steps of firstly carrying out the pre-treatment on a sample, then using the kit for inspecting and finally analyzing the inspection result. The provided enzyme-linked immunosorbent kit can be used for inspecting the residual amount of the sulfa drugs in animal tissues(chicken, pork, fish and shrimp), honey, eggs, milk, feeds and other samples, the operation is simple, the cost is low, the sensitivity is high and the enzyme-linked immunosorbent kit can be monitore d on-site and is applicable in screening mass samples.
Owner:BEIJING WANGER BIOTECH
Kit for detecting peste des petits ruminants virus hemagglutinin protein antibody and application method of kit
InactiveCN105158480AGood response specificityHigh sensitivitySsRNA viruses negative-senseVirus peptidesAntigenCrop livestock
The invention provides a kit for detecting a peste des petits ruminants virus hemagglutinin protein (H protein) antibody. The kit takes recombinant protein of extracellular region sequence code of peste des petits ruminants virus hemagglutinin protein gene as an envelope antigen. The invention further provides an application method of the kit. The kit can quickly specifically detect the peste des petits ruminants virus H protein antibody in serum, and is good in reaction specificity and high in sensitivity. The operation is simple; the cost is low; and the reaction result is stable and reliable, and is easy to observe. The kit is suitable for monitoring the vaccine immunity effect of import and export immunity, food sanitation, and a livestock farm of peste des petits ruminants.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Mycoplasma bovis diagnosis reagent and its application
InactiveCN103172752AReduce manufacturing costSimple and fast operationBiological testingHybrid peptidesMycoplasma antibodySpecific antibody
The invention relates to the diagnostic medicine of animals, especially relates to a mycoplasma bovis diagnosis technology, and concretely relates to a multi-epitope fusion antigen having an amino acid sequence represented by SEQ ID NO:1 or SEQ ID NO:2, and its application in the preparation of a mycoplasma bovis diagnosis reagent. The diagnosis reagent can be used as a solid phase vector coating antigen of an indirect ELISA kit and is combined with its specific antibody, a horseradish peroxidase coupled anti-cattle IgG antibody is added and incubated, and a color development reaction is carried out, and the color development degree is proportional to the amount of the anti-mycoplasma bovis antibody in a sample to be measured. The technology has the advantages of simple operation, no need of complex equipment, low technical requirements on the laboratorial conditions and experiment personals, low detection cost, and suitableness for the large-scale development in the basic level and the culture farm; the multi-epitope fusion antigen has a low making cost and is suitable for large-scale application; and has the advantages of high sensitivity and specificity, small batch difference, and high detection result consistence because of the adoption of multi-epitope as a target.
Owner:重庆市动物疫病预防控制中心 +1
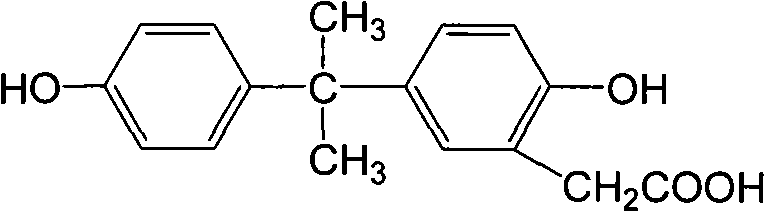
Preparation method of anti-bisphenol A monoclonal antibody
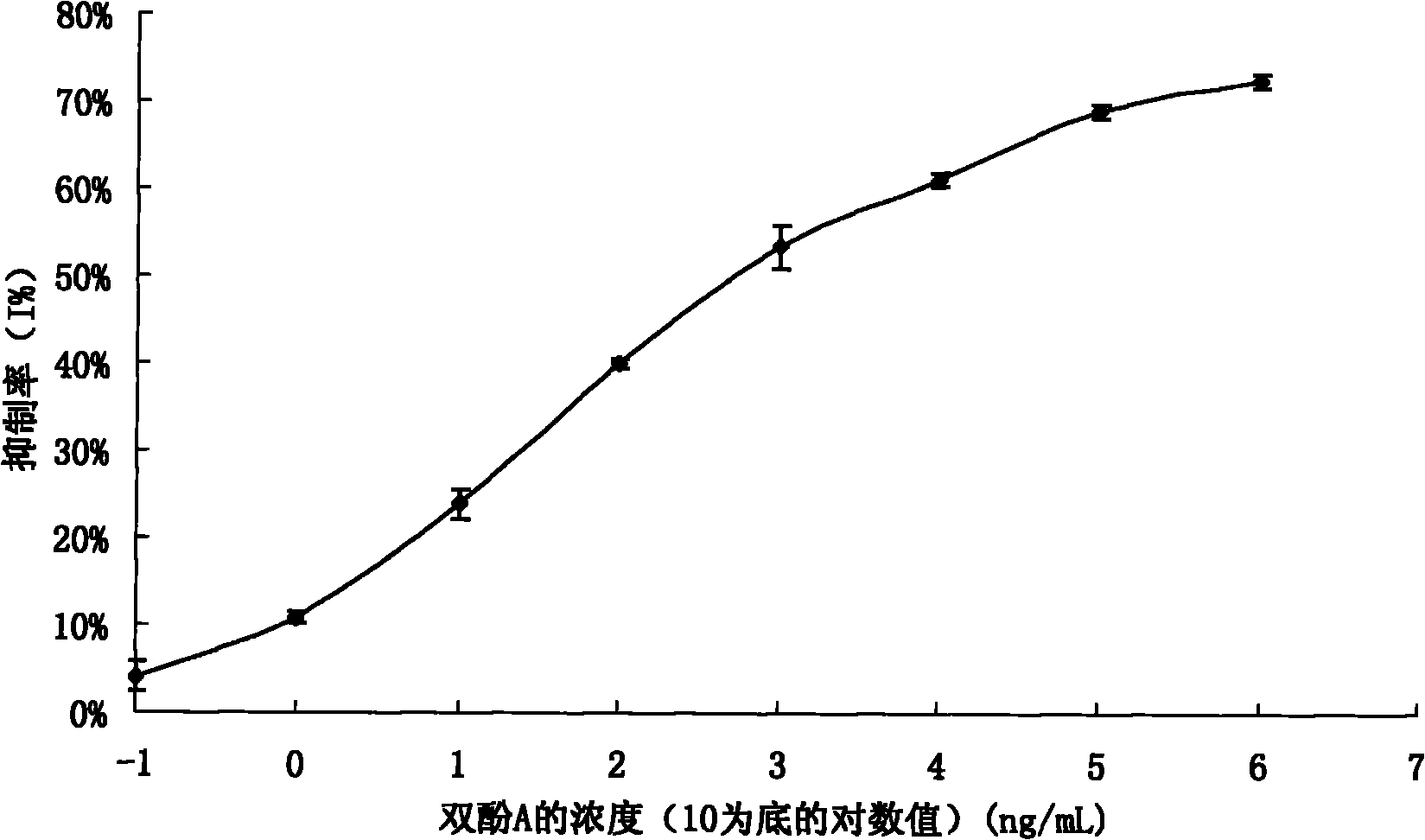
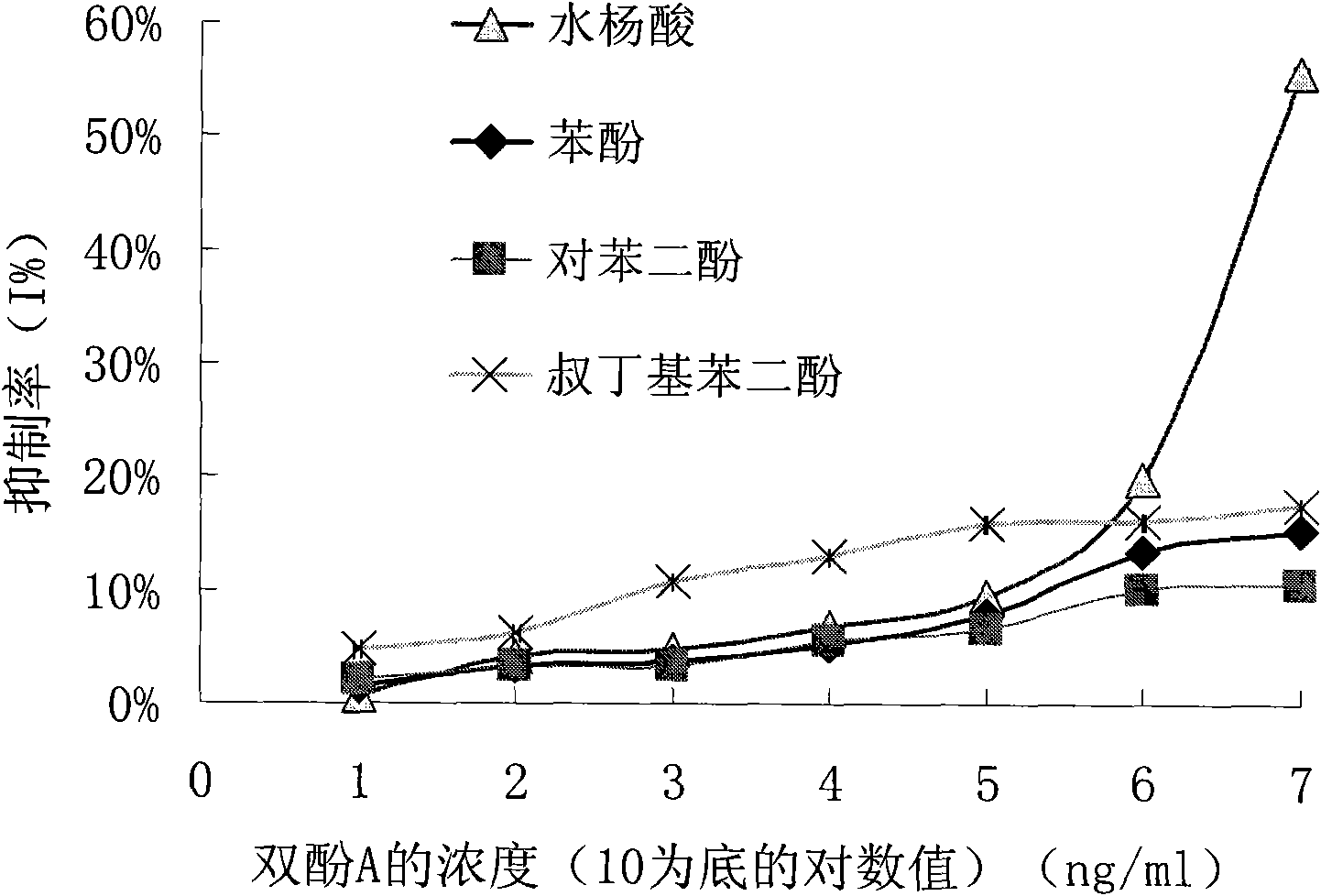
InactiveCN101863981AEffective monitoringRapid Field MonitoringSerum albuminTissue culturePolyethylene glycolCarrier protein
The invention discloses a preparation method of an anti-bisphenol A monoclonal antibody. The preparation method comprises the following steps of: combining bisphenol A and a macromolecule carrier protein to prepare an artificial immunity antigen and a coating antigen; preparing splenocyte suspension from a bisphenol A artificial immunity antigen immunity mouse; fusing the mouse splenocyte and mouse myeloma cells SP2 / 0 in the presence of polyethylene glycol; detecting by hybridoma technology and subcloning for many times to obtain positive hybridoma cells which can stably secrete bisphenol A antibody, wherein the secreted antibody belongs to the IgG1 subclass; and establishing a bisphenol A indirect competition ELISA (Enzyme-Linked Immunosorbent Assay) test by utilizing the monoclonal antibody secreted by cell lines with high antibodytiter, wherein the minimum limit of detection can reach 0.324ng / mL. The antibody has no cross reaction with compounds with benzene ring structures, such as benzene, phenol, tert-butylphenol, p-hydroxylphengl, o-hydroxybenzoic acid and the like, has strong specificity and can be used for immunity detection of bisphenol A.
Owner:GUANGDONG UNIV OF TECH
Fluorescence immunoassay chromatography test paper for cefalexin residue and preparation of test paper
ActiveCN103901201AEasy to handleThe determination method is simpleMaterial analysisGlass fiberCefalexin
The invention discloses a piece of fluorescence immunoassay chromatography test paper which is rapid, sensitive, simple and convenient to operate to test the residual quantity of cefalexin, and preparation of the test paper. The test paper comprises a sample pad, a binding pad, a nitrocellulose membrane and a water absorbing pad, which are adhered to a substrate in a mutual lap joint manner in sequence, wherein the nitrocellulose membrane is coated with a detection line and a quality control line; the binding pad is coated with a cefalexin monoclonal antibody with a fluorescent mark. The preparation method of the fluorescence immunoassay chromatography test paper for cefalexin residue comprises the following steps: synthesizing cefalexin-ovalbumin coating antigen, preparing a goat anti-mouse immune globulin antibody, coating the cefalexin-ovalbumin coating antigen and the goat anti-mouse immune globulin antibody onthe nitrocellulose membrane to serve as the detection line and the quality control line, preparing a fluorescence nano-particle mark cefalexin monoclonal antibody, then coating glass fiber to serve as the binding pad, sequentially adhering the sample pad, the binding pad, the nitrocellulose membrane and the water absorbing pad to a back plate in the lap joint manner in sequence, cutting the obtained test paper into the width of 4mm, and preserving at normal temperature.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
ELISA reagent box for detecting pig reproduction and respiratory syndrome antibody and usage thereof
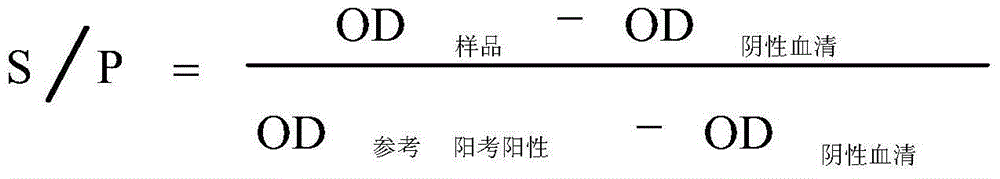
The invention is an ELISA reagent box to detect pig progenitive and respiratory syndrome (PRRS) antibody as well as detecting method. The reagent box including: ELISA slat with reformed N protein as coating antigen, PBS solution, blood serum diluted solution, rabbit anti-pig enzyme-labeled bi-antibody, color-developing substrate, terminating liquor, PRRS positive blood serum, PRRS negative blood serum, etc. After tested by the indexes of peculiarity, sensitivity, repetition and so on, the detecting method is used to detect the blood serum sample. Its advantages: it has good peculiarity, able to be largely produced and has low detecting cost. The detecting coincidence with ELISA reagent box of IDEXX Company is 91%, largely reducing the detecting cost.
Owner:NANJING AGRICULTURAL UNIVERSITY
Kit for quantitatively testing free triiodothyronine and preparation method thereof
ActiveCN101949942AIncrease the effective coating amountReduce the impactBiological testingAntigenMagnetic bead
The invention discloses a kit for quantitatively testing free triiodothyronine, which comprises 3,3'-L-Diiodothyronine-gelatin enveloped magnetic bead suspension, a free triiodothyronine series calibrator, a horse radish peroxidase labeled free triiodothyronine antibody, chemiluminescent substrate A solution, chemiluminescent substrate B solution, and PBS buffer solution. The invention also discloses a method for preparing the kit. The kit has the advantages that: the chemiluminescence technology is combined with the immunomagnetic bead technology, the immunomagnetic beads are taken as a reaction carrier, the effective envelop amount of antigen is increased, raw materials are saved and the detection sensitivity and detection speed are obviously improved. A method for enveloping antigen analogs by the labeled antibody is adopted, so the analogs can be specifically combined with the antibody of hormone, but the combination capacity with thyroxine-binding protein is greatly reduced, and the influence of the binding protein on a measurement system is reduced to a large extent.
Owner:AUTOBIO DIAGNOSTICS CO LTD
Competitive ELISA (Enzyme-Linked Immuno Sorbent Assay) kit for detecting antibody of African swine fever virus and application thereof
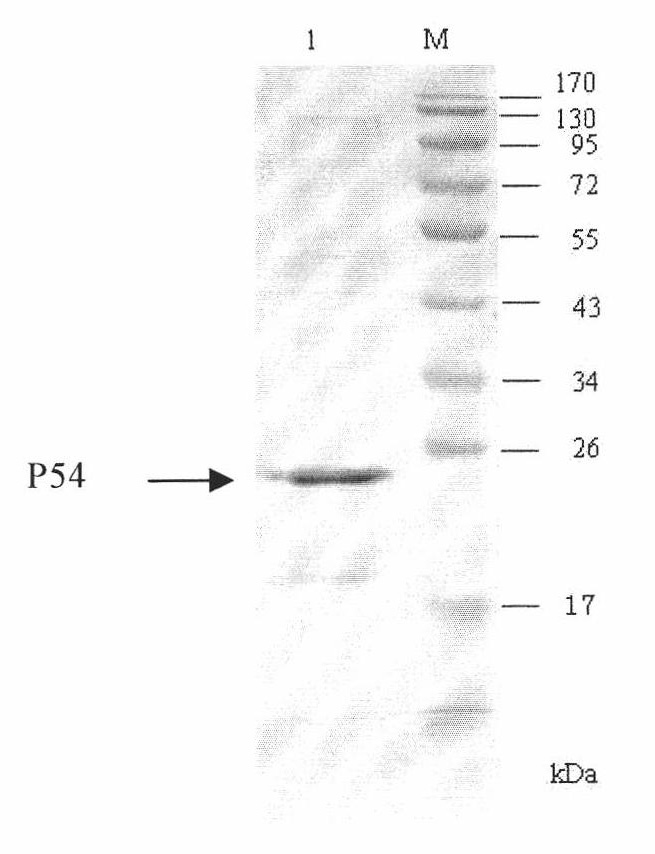
The invention discloses a competitive ELISA (Enzyme-Linked Immuno Sorbent Assay)kit for detecting an antibody of an African swine fever virus and application thereof, belonging to the technical field of organisms. The kit is used for detecting an antibody of the African swine fever virus in pig serum by adopting prokaryotically expressed recombinant P54 protein as an envelope antigen according toa competitive ELISA principle. The envelope antigen in a 96 pore plate in the kit is prokaryotically expressed recombinant P54 protein and has favorable antigenicity. The enzyme-linked immuno kit provided by the invention comprises the P54 protein enveloped 96 pore plate, positive control, negative control, a horseradish peroxidase marked monoclonal antibody, a concentrated cleaning solution, serum diluent, a TMB substrate and a stopping solution. The kit can be used for screening samples in bulk, main reagents in the kit are provided in a working solution way, and the use is convenient.
Owner:ANIMAL & PLANT & FOOD INSPECTION CENT OF TIANJIN ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Kit for distinguishing and diagnosing capripox field virus infection, preparation and detection method thereof
InactiveCN102183643AEasy to purifyEasy to manufactureDepsipeptidesFermentationCapripox virusAttenuated vaccine
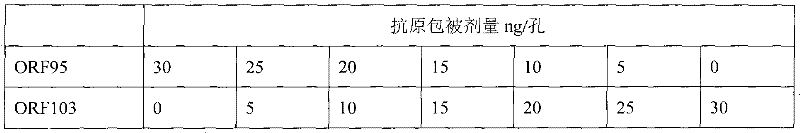
The invention discloses an ELISA (Enzyme-Linked Immunosorbent Assay) antibody detection kit for distinguishing and diagnosing capripox field virus infection. The indirect ELISA antibody detection kit for distinguishing and diagnosing capripox field virus infection, disclosed by the invention, is internally provided with a coated antigen ELISA antibody detection board and an ELIAS secondary antibody, wherein the coated antigen is a mixture of a recombination capripox virus ORF95 protein and an ORF103 protein. The recombination proteins of ORF95 and ORF103, adopted by the invention, are convenient to prepare and purify in large quantities. The kit disclosed by the invention has a strong specificity, can effectively exclude the interference of the capripox attenuated-vaccine immunity, and specifically detects the capripox field virus infection.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
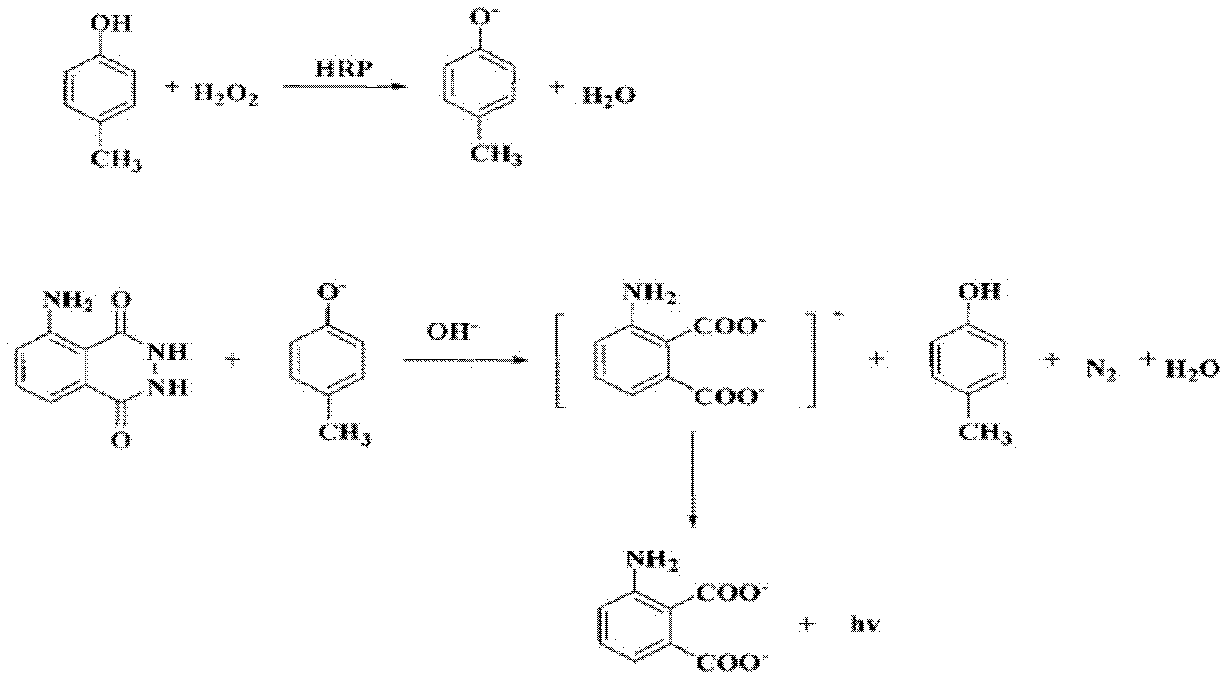
Sulfanilamide drug chemiluminescence enzyme-linked immunodetection kit
The present invention discloses a sulfanilamide drug chemiluminescence enzyme-linked immunodetection kit, which comprises a kit body, an enzyme label plate placed inside the kit body, and reagents placed inside the kit body, and is characterized in that every hole of the enzyme label plate is coated with coating antigen, the coating antigen is a sulfanilamide mother nucleus and carrier protein conjugate, and the reagents comprise sulfanilamide monoclonal antibody, horseradish peroxidase-labeled goat anti-mouse antibody, a series of sulfanilamide standard solutions, a concentrated phosphate buffer, a concentrated washing solution and a chemiluminescence solution. The sulfanilamide drug chemiluminescence enzyme-linked immunodetection kit has characteristics of high sensitivity, simple and rapid detection, high accuracy, and more drug detection types, provides a substantially reduced operation time compared to the conventional colorimetric ELISA method, and can be used for detection of residues of the 17 sulfanilamide drugs in animal tissues (pork, chicken, pork liver and chicken liver), aquatic products (fish and shrimp), eggs, milk and milk powder.
Owner:BEIJING KWINBON BIOTECH
ELISA (enzyme linked immunosorbent assay) kit for detecting PPRV (peste des petits ruminants virus) antibody
ActiveCN107238702AEasy to monitorStrong specificitySsRNA viruses negative-senseAntibody mimetics/scaffoldsAntigenElisa kit
The invention discloses an ELISA (enzyme linked immunosorbent assay) kit for detecting a PPRV (peste des petits ruminants virus) antibody. The ELISA kit for detecting the PPRV antibody comprises an envelope antigen, wherein the envelope antigen is a recombinant PPRV H-F fusion protein which is a protein of a) or b) as follows: a) protein formed by an amino acid sequence shown in SEQ ID No.2; b) soluble protein obtained from the amino acid sequence shown in SEQ ID No.2 through substitution and / or deletion and / or addition of one or several amino acid residues. The ELISA kit for detecting the PPRV antibody not only can be used for diagnosing PPR (peste des petits ruminants), but also can be used for evaluating a vaccine immunity effect, further can detect PPR rapidly and accurately and is beneficial to clinical monitoring of PPR, thereby playing a positive role in better control of spread of PPR in China.
Owner:CHINA ANIMAL DISEASE CONTROL CENT
Multi-antigen ELISA (Enzyme Linked Immunosorbent Assay) kit for detecting African swine fever virus antibody
The invention provides a multi-antigen enzyme linked immunosorbent assay (ELISA) kit for detecting an African swine fever virus (ASFV) antibody, belonging to the field of a biotechnology and diagnosis and research of animal-borne diseases. The multi-antigen enzyme linked immunosorbent assay kit comprises expression and purification of three types of ASFV recombined antigens, preparation of positive and negative control blood serum of an ASFV antibody, optimal envelope antigen combination and concentration determination, optimization of multi-antigen ELISA (MA-ELISA (Microalbumin-Enzyme Linked Immunosorbent Assay)) reaction parameters, determination of an ASFV antibody negative blood serum critical value, MA-ELISA detection artificial infection and determination of sensitivity, specificity and repeatability of field blood serum samples. By detecting and testifying a large quantity of known blood serum samples, the sensitivity, the specificity and the repeatability of detecting the ASFV antibody by the MA-ELISA are obviously higher than those of an ELISA method recommended by World Organization for Animal Health and oversea similar kits; and the multi-antigen enzyme linked immunosorbent assay kit can be used for ASFV serological diagnosis, epidemiological investigation and live pig import and export quarantine inspection.
Owner:YANGZHOU UNIV
Indirect ELISA kit for detecting African swine fever virus antibody and application thereof
InactiveCN102236017AImmunoglobulinsMaterial analysisAfrican swine fever virus AntibodyPositive control
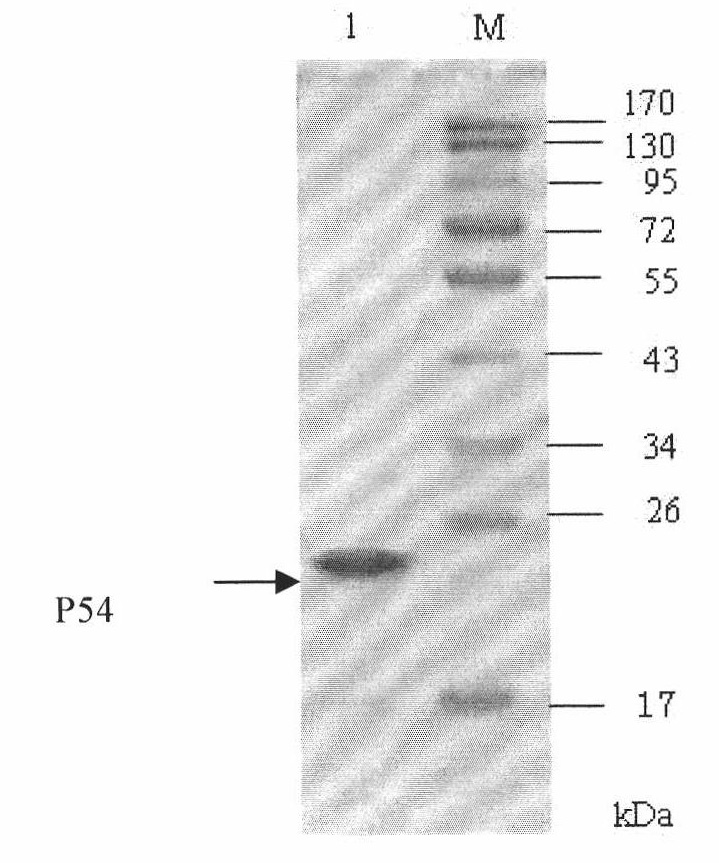
The invention discloses an indirect ELISA kit for detecting an African swine fever virus antibody and an application thereof, and belongs to the technical field of biology. The kit adopts prokaryotic expression recombinant P30 protein as a coating antigen, and detects the antibody of African swine fever virus in porcine serum based on the indirect ELISA principle. The coating antigen in a 96-well plate of the kit is prokaryotic expression recombinant P30 protein which has good antigenicity. The enzyme-linked immunoassay kit provided by the invention comprises a 96-well plate coated with P30 protein, a positive control, a negative control, a horseradish peroxidase-labeled rabbit anti-porcine IgG polyclonal antibody, a concentrated washing liquid, a serum diluent, a TMB substrate, and a terminating liquid. The kit of the invention is applicable to the screening of large quantities of samples, and main reagents in the kit are provided in a form of operating fluid which is convenient for use.
Owner:陈文刚
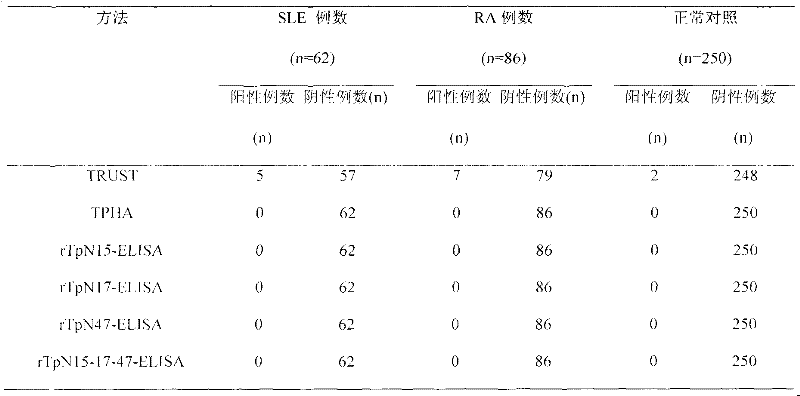
Preparation method of rTpN15-17-47-ELISA for detecting syphilis serum antibody
InactiveCN102183646AOvercome the disadvantage of high costGood antigenicityBacteriaFermentationSyphilisNucleotide
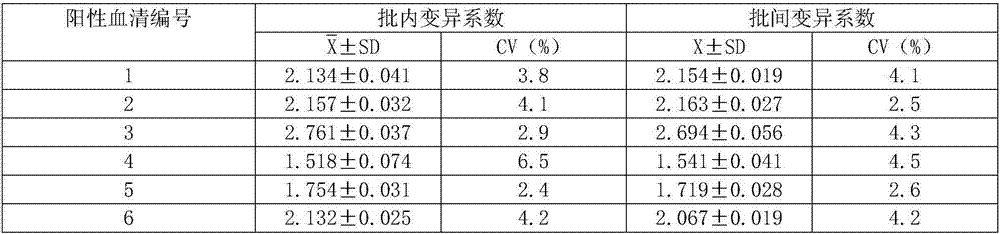
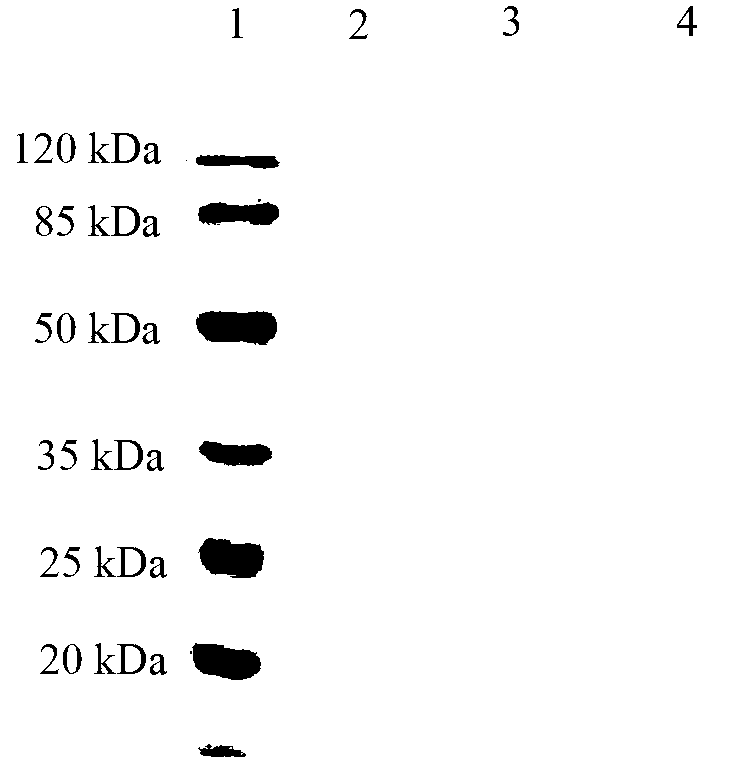
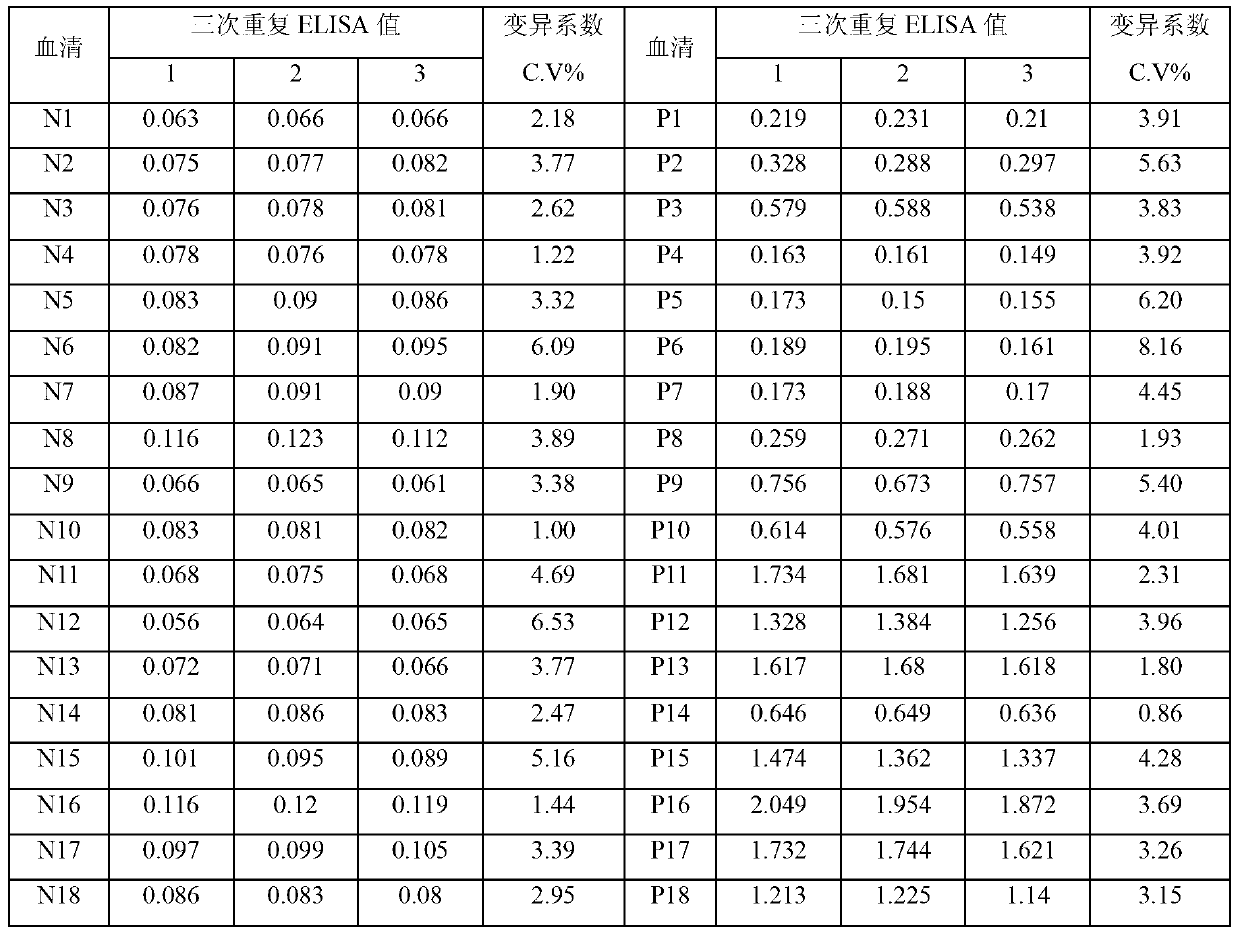
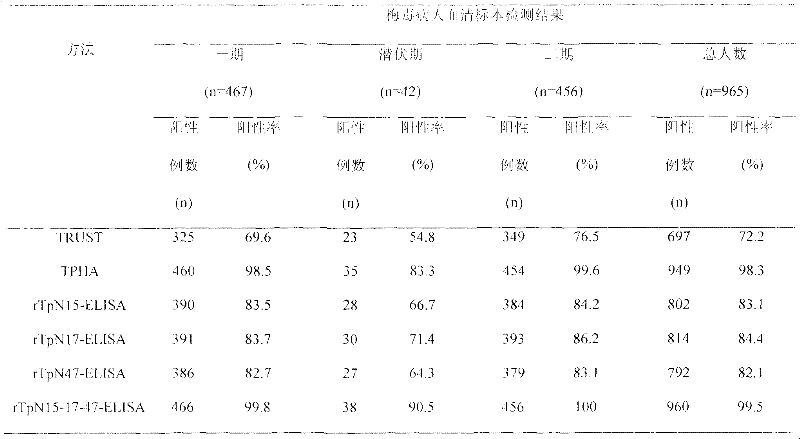
The invention relates to a syphilis serological screening or diagnosis method. The purpose is that the method has the characteristics of low price, high speed, sensitivity and specificity. The technical scheme provided by the invention is that: a preparation method of rTpN15-17-47-ELISA for detecting syphilis serum antibodies comprises the following steps in turn: constructing an artificial fusion gene tpN15-17-47 of tpN15, tpN17 and tpN47 and a prokaryotic expression system E.coliBL21DE3Pet42a-tpN15-17-47 by gene technology, purifying the recombinant expression product rTpN15-17-47 as a coating antigen, establishing rTpN15-17-47-ELISA for detecting syphilis serum antibodies. The fusion gene tpN15-17-47 has a nucleotide sequence and an amino acid sequence as shown in sequence table 5.
Owner:孙爱华 +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com