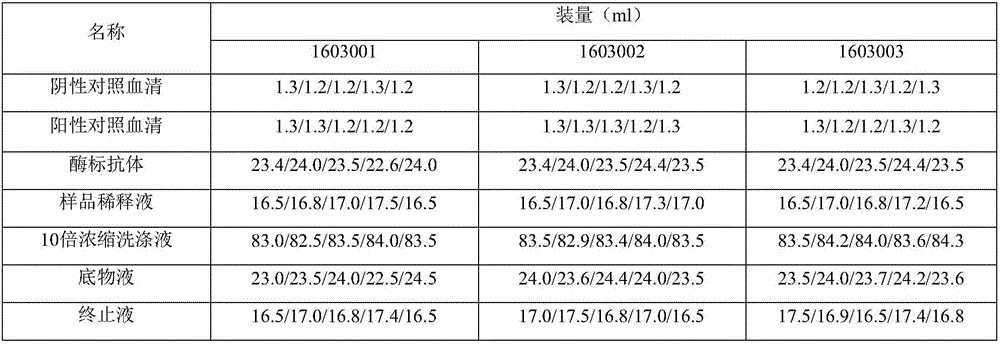
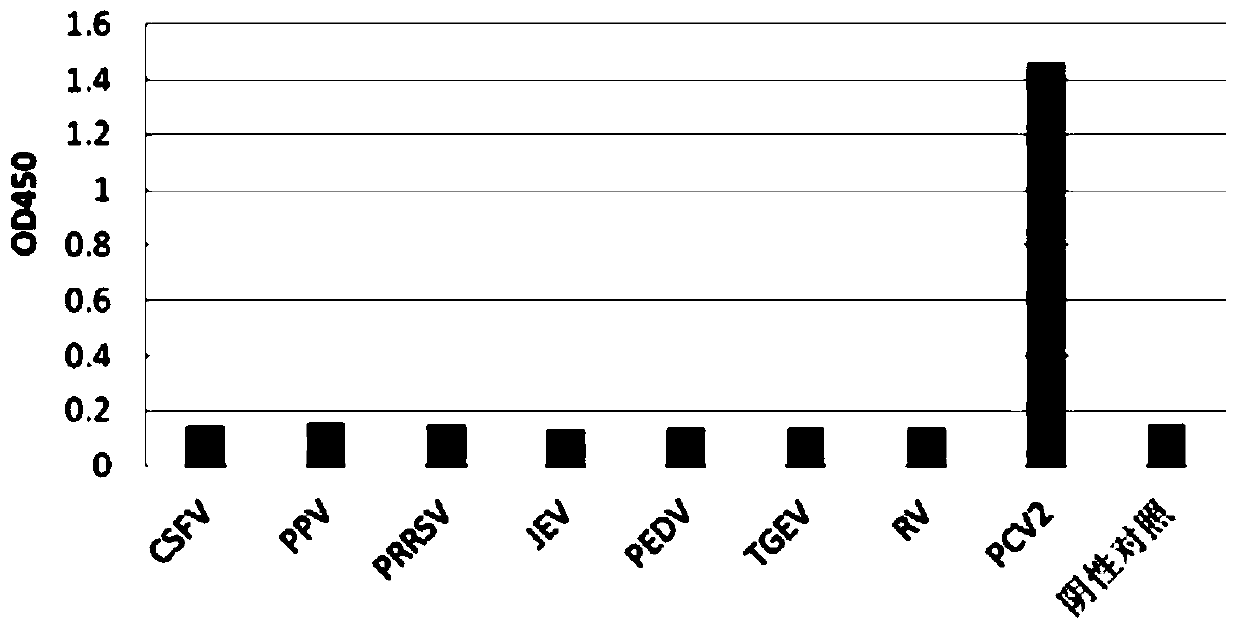
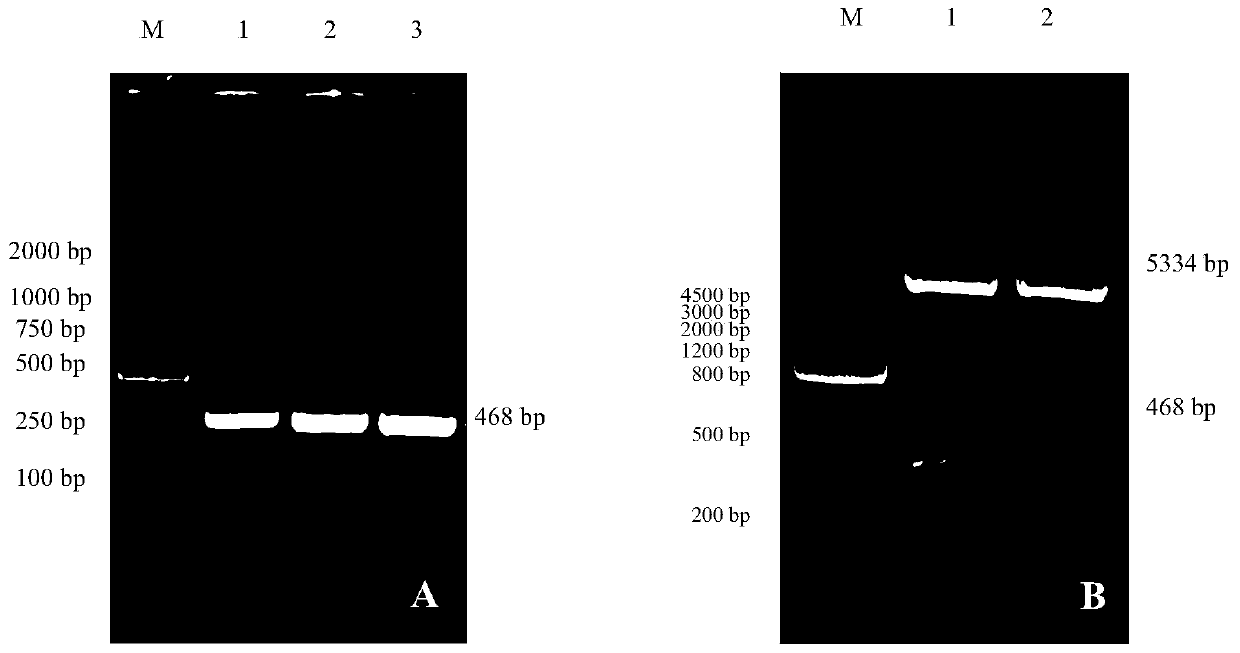
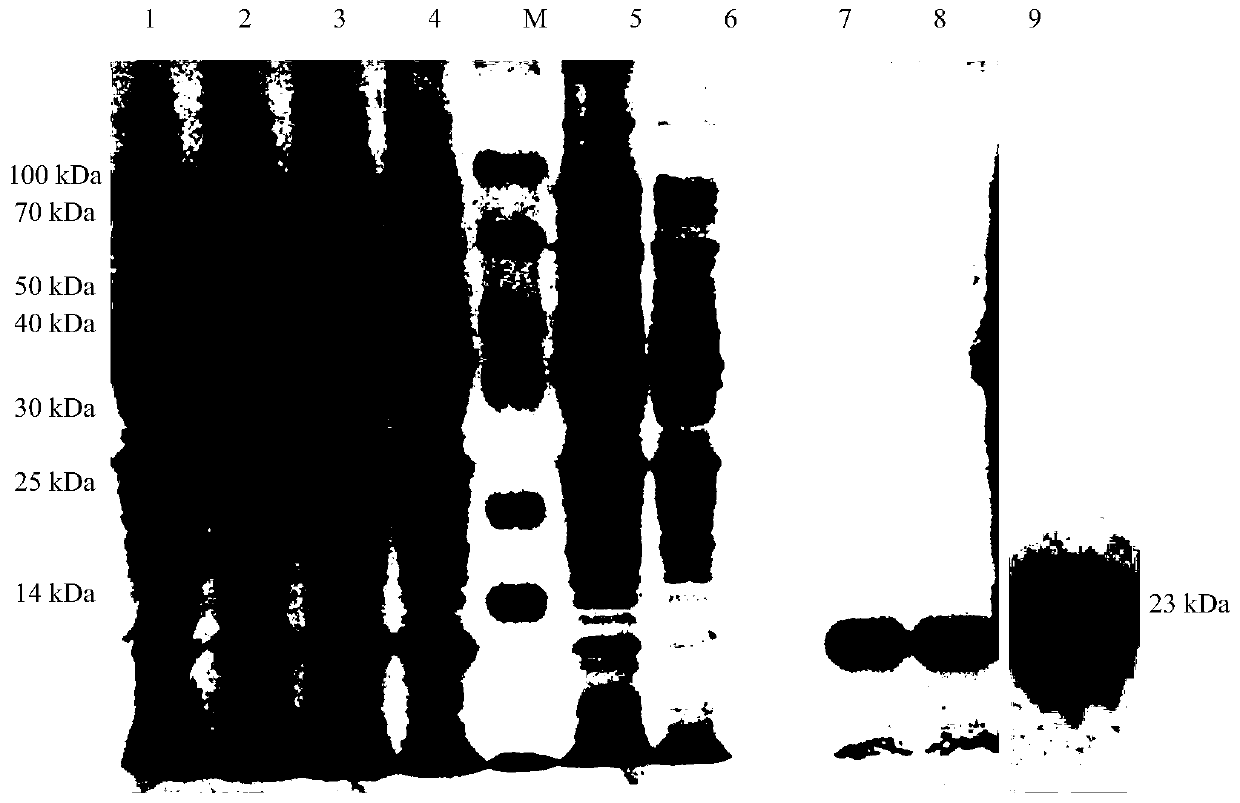
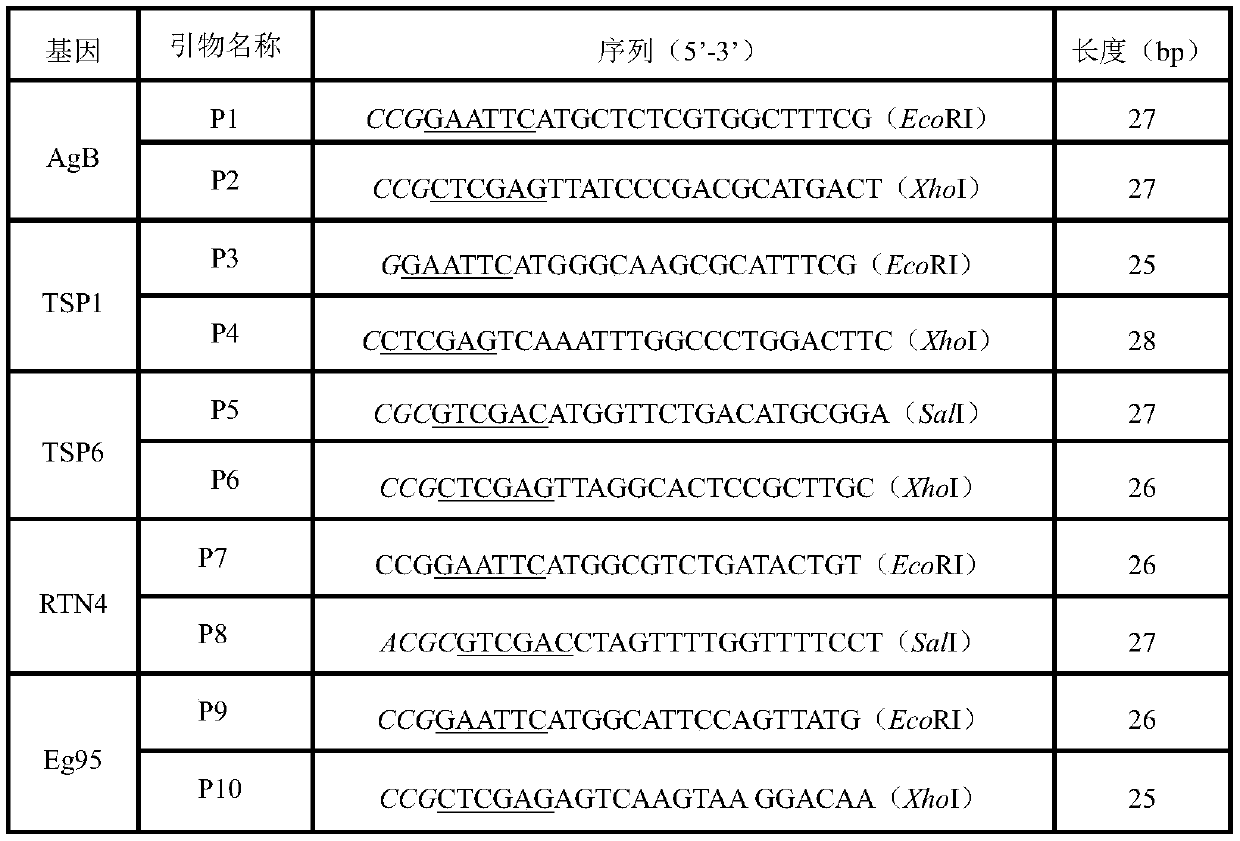
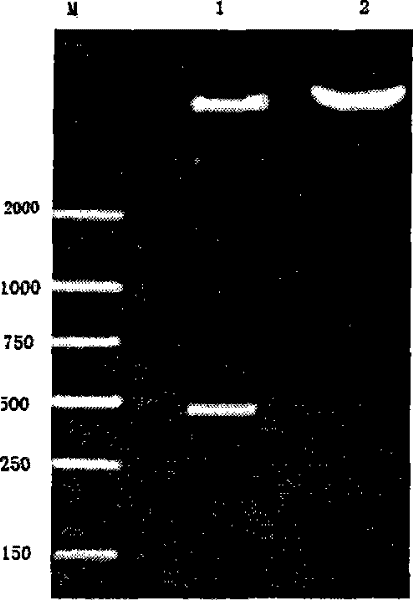
Patents
Literature
56results about How to "Good reactogenicity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Acellular pertussis vaccines and methods of preparation thereof
InactiveUS6696065B1Increase contentEnhance immune responseBiocideSsRNA viruses positive-sensePoliomyelitisTetanus toxoids
A multi-component vaccine composition is described comprising acellular pertussis vaccine components, diphtheria toxoid, tetanus toxoid and inactivated poliovirus. The composition also may contain a conjugate of a capsular polysaccharide on Haemophilus influenzae type b and tetanus toxoid or diphtheria toxoid, which may be reconstituted from a lyophilized state by the other component. The administration of the multiple component vaccine resulted in no diminution of the immunogenicity of any component as a result of interference by other components of the vaccine.
Owner:SANOFI PASTEUR LTD
Indirect ELISA reagent kit for detecting antibodies against rabbit hemorrhagic disease virus
InactiveCN101533016AGood reactogenicityGuaranteed high sensitivityColor/spectral properties measurementsHemorrhagic diseasesAntigenicity
The invention relates to an ELISA reagent kit for detecting antibodies against rabbit hemorrhagic disease virus (RHDV), and belongs to the field of biotechnology. The reagent kit adopts recombined VP60 capsid protein expressed by a baculovirus expression system as envelope antigen, detects the antibodies against rabbit hemorrhagic disease virus in serum of a rabbit according to an indirect ELISA principle, and is used for rabbit hemorrhagic disease vaccine serological detection in a rabbit group. The core substance envelope antigen of the reagent kit is the recombined VP60 capsid protein expressed by the baculovirus expression system, which has good antigenicity like natural RHDV but no potential poison spreading danger like the RHDV. The reagent kit used to detect serum samples has the advantages of low background reaction and obvious contrast of OD values of negative and positive serum development. The reagent kit has low production cost, strong specificity and high sensitivity, can detect samples in a large quantity, and has important application value in aspects such as rabbit hemorrhagic disease immunity monitoring and the like.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
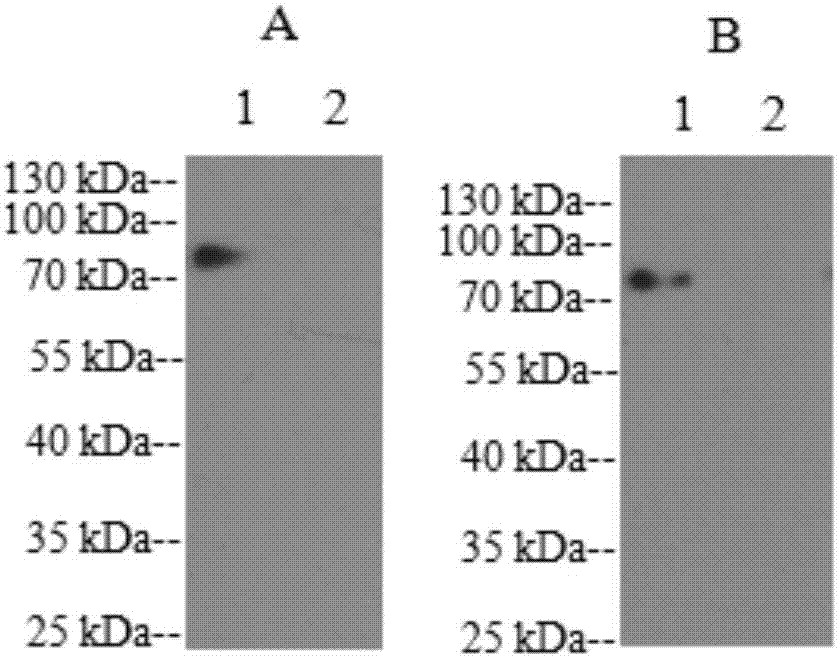
Epitope peptide H362 of HN protein in peste des petits ruminants virus (PPRV), and determination, preparation method and application thereof
ActiveCN107216372AStrong green fluorescenceGood reactogenicitySsRNA viruses negative-senseViral antigen ingredientsF proteinHN Protein
The invention relates to an epitope peptide H362 of an HN protein in PPRV, and determination, a preparation method and application thereof. The amino acid sequence of the epitope peptide is H362: <362>EANWVVPSTDVRDL<375>. The invention detects reactogenicity of a monoclonal antibody and PPRV and specificity of the monoclonal antibody; according to detection results, the monoclonal antibody has good reactogenicity to rPPRV-HN-F protein; immunoinformatic technology is cooperatively used for predicating the B cell epitope of the HN protein; an aminated ELISA plate is employed for detecting candidate epitopes and the monoclonal antibody 10E3, and the epitope peptide H362 corresponding to 10E3 is determined; and determination of the epitope peptide lays a theoretical foundation for preparation of epitope vaccine antigens and diagnostic reagent antigens for PPRV.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Preparation and use of giant panda Ascaris schroederi antigen
InactiveCN101348787AGood reactogenicityImprove diagnostic toolsGenetic material ingredientsAntiparasitic agentsBacteroidesAntigen
The invention discloses preparation and application of a giant panda Ascaris schroederi Mcintosh antigen, which belong to the giant panda Ascaris schroederi Mcintosh prevention, treatment and detection field. The method comprises the following steps: a Ascaris schroederi Mcintosh antigen gene primer is designed; total RNA of ascarids undergoes reverse transcription by the RT-PCR method to synthesize cDNA, and then the cDNA is taken as a template for PCR amplification of a target product; the purified target product is connected with a pMD18-T vector and then converted into DH5 alpha competent bacteria; positive recombinant clone is selected through flat screening and culture of the bacteria, and the antigen genes have a Bs-Ag1gene, a Bs-Ag2 gene and a Bs-Ag3 gene after culture and sequencing; and then recombinant plasmids which are accurately sequenced are converted into Escherichia coli BL21 competent cells for mass expression of proteins after construction of the recombinant plasmids, induction expression and purification of the recombinant proteins. After detection and immunologic tests of the product and ELISA detection and analysis of a test animal antibody IgG, the Bs-Ag1gene, the Bs-Ag2 gene and the Bs-Ag3 gene of the giant panda Ascaris schroederi Mcintosh antigen can be taken as candidate genes for preparing genetic engineering vaccines through the sascarids; and the recombinant proteins Bs-Ag1, Bs-Ag2 and Bs-Ag3 have good reactionogenicity and can be used for detecting infection of giant panda Ascaris by the ELISA method.
Owner:SICHUAN AGRI UNIV
Preparation method of anti-canine parvovirus protein VP2 specific IgY
InactiveCN103570830AGood reactogenicityImprove bindingEgg immunoglobulinsImmunoglobulins against virusesEscherichia coliEnzyme digestion
The invention discloses a preparation method of an anti-canine parvovirus protein VP2 specific IgY, which comprises the following steps: (1) designing a pair of primers according to CPV-VP2 gene sequence, carrying out PCR (polymerase chain reaction) amplification on the CPV-VP2 gene, connecting to a pMD18-T vector, transforming DH5alpha competent cell, carrying out blue-white screening, extracting the plasmid, carrying out digest enzyme digestion ion analysis, carrying out positive plasmid sequencing, and carrying out comparative analysis on the sequencing result; (2) expression and purification of VP2 protein in Escherichia coli: carrying out BamH I and Xho I double-enzyme digestion on the pMD18-T-VP2 and pET-32a,connecting to the target segment, constructing the pET-32a-VP2 expression vector, transforming Bal21(DE3)pLysS competent bacteria, carrying out enzyme digestion and PCR identification, optimizing the IPTG (isopropyl-beta-D-thiogalactopyranoside) induction concentration and time, carrying out mass induction expression, and purifying the recombinant protein by using a Ni<+> affinity column; and (3) preparation of anti-VP2-IgY antibody: immunizing a laying hen by using the purified VP2 protein, extracting the specific IgY antibody by using PEG 6000, and carrying out SDS-PAGE (sodium dodecyl sulfate polyacrylamide gel electrophoresis) analysis. The anti-VP2-IgY antibody extracted by the method can be well combined with the VP2 protein to carry out non-cross reaction on the degradation segment.
Owner:NORTHWEST A & F UNIV
1-type duck hepatitis A virus VP2 recombinant protein, ELISA kit and preparation method thereof
ActiveCN105273065AGood reactogenicitySsRNA viruses positive-senseVirus peptidesDuck hepatitis A virusInclusion bodies
The invention belongs to the technical field of bioengineering and particularly relates to a 1-type duck hepatitis A virus VP2 recombinant protein, an ELISA kit and a preparation method thereof. The amino acid sequence of the 1-type duck hepatitis A virus VP2 recombinant protein is shown as SEQ ID NO:1. The preparation method comprises the following steps of: acquiring a VP2 target segment; constructing recombinant expression plasmid pProEx-HTb-VP2; preparing VP2 recombinant protein. The successfully-obtained VP2 recombinant protein is expressed in an insoluble inclusion body, the VP2 recombinant protein has good reactogenicity with rabbit-anti-DHAV-1 serum, and proved by the above, prokaryotic expression is successfully obtained by VP2 protein of DHAV-1; the ELISA kit for detecting 1-type duck hepatitis A virus antibody is established by the expressed VP2 recombinant protein, and provides test data and basic materials for detecting the DHAV-1 antibody and further performing relevant study of the DHAV-1.
Owner:SICHUAN AGRI UNIV
Blocking ELISA antibody detection kit for porcinecircovirus type 2 and preparation method of blocking ELISA antibody detection kit
InactiveCN106841609ANo immune cross-reactivityReduce non-specific reactionsBiological material analysisProkaryotic expressionMonoclonal antibody
The invention relates to a blocking ELISA antibody detection kit for porcinecircovirus type 2 and a preparation method of the blocking ELISA antibody detection kit. The blocking ELISA antibody detection kit for the porcinecircovirus type 2 comprises a coated plate, a PVC2 enzyme labelled antibody, sample diluent, a concentrated washing solution (10*), a positive serum contrast, a negative serum contrast, TMB substrate diluent and a stop solution, wherein the coated plate is prepared from a purified porcine circovirus type 2 nucleocapsid protein CapC protein serving as a non-antigen, and the PVC2 enzyme labelled antibody is prepared from monoclonal antibody hybridoma which is labeled with horse radish peroxidase and secretes PCV2. According to the preparation method, the CapC protein is expressed by virtue of a prokaryotic expression system, and the blocking ELISA is established by taking a monoclonal antibody of PCV2 as the enzyme labelled antibody; and the blocking ELISA antibody detection kit has the characteristics of high specificity and sensitivity and can be applied to the detection of PCV2 antibodies of a porcine serum sample.
Owner:JIANGSU NANNONG HI TECH +1
Porcine circovirus type 2 double antibody sandwich ELISA reagent kit and application thereof
InactiveCN109799351ANo cross reactivityGood reactogenicityBiological testingAntigenPorcine circovirus
The invention relates to a porcine circovirus type 2 double antibody sandwich ELISA reagent kit and a detection method. The reagent kit includes an enzyme label plate coated with a monoclonal antibodyagainst a porcine circovirus Cap protein, a blocking buffer, a primary antibody, an enzyme-labeled secondary antibody, concentrated washing liquid, a colored solution, a stopping solution, a standard, a positive contrast, and a negative contrast. The porcine circovirus type 2 double antibody sandwich ELISA has the advantages of short detection cycle, simple operation, high sensitivity, strong specificity and simple equipment requirements and can perform qualitative, semi qualitative and quantitative analysis. Moreover, the reagent kit can achieve rapid detection of large quantities of samples. The invention also provides an application of the reagent kit in detecting an antigen of a porcine circovirus type 2 inactivated vaccine.
Owner:TIANJIN RINGPU BIO TECH
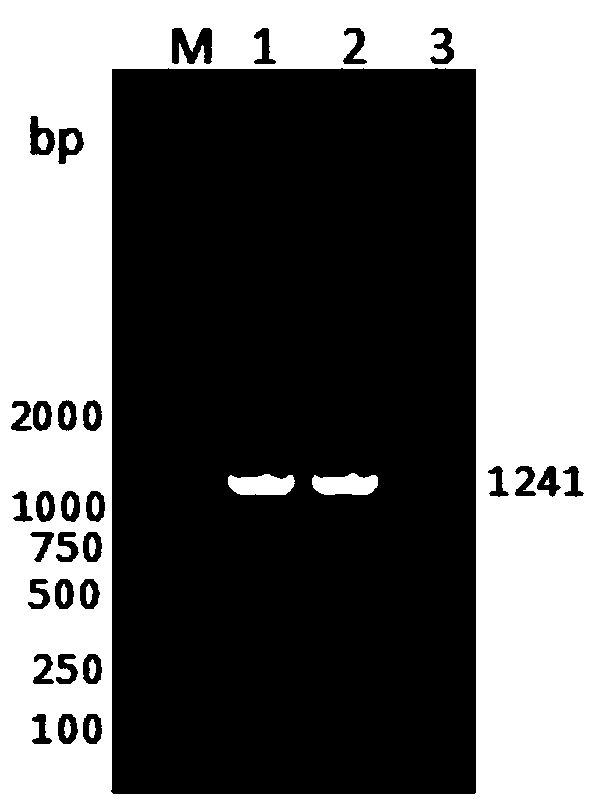
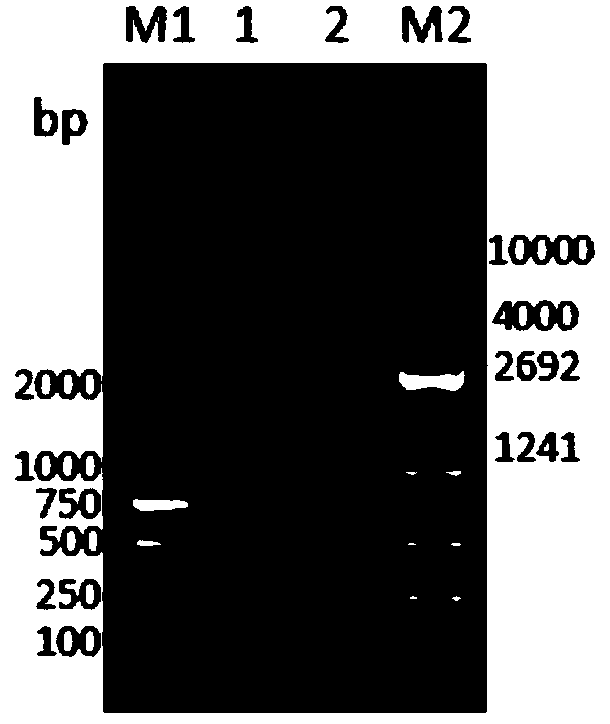
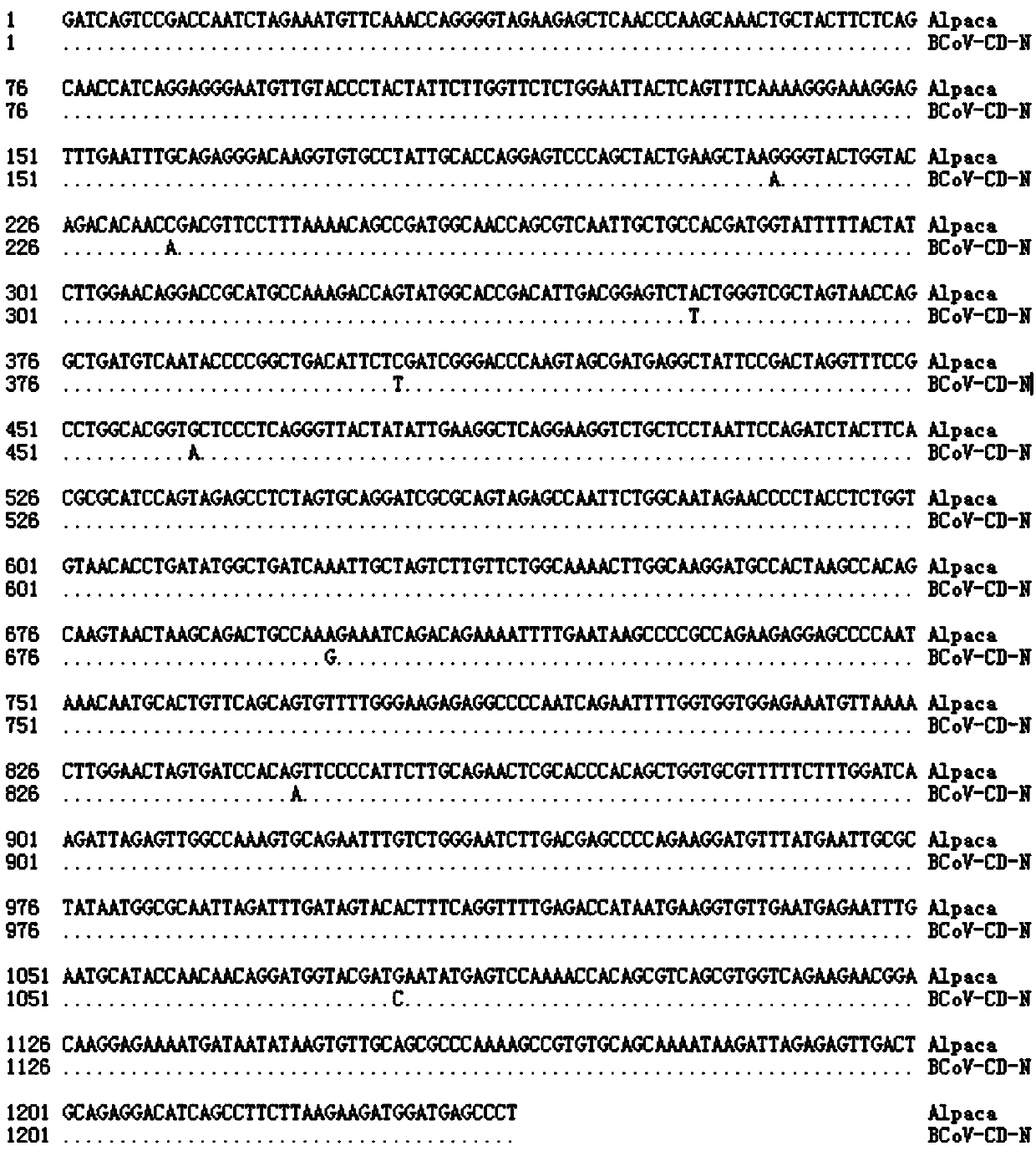
ELISA (Enzyme-Linked Immuno Sorbent Assay) detection kit for bovine coronavirus
ActiveCN108318686AImproving immunogenicityStrong conservativeSsRNA viruses positive-senseVirus peptidesEscherichia coliCompanion animal
The invention discloses an ELISA (Enzyme-Linked Immuno Sorbent Assay) detection kit for bovine coronavirus. The ELISA detection kit is characterized in that purified BCoV-CD isolate recombinant pET-32a-N protein is used as coating antigen, reaction conditions are optimized, and an indirect ELISA diagnosis method for detecting bovine-serum characteristic N protein antibodies. The ELISA detection kit disclosed by the invention has the beneficial effects that escherichia coli is adopted for prokaryotic expression, the raw-material sources are wide, the preparation is easy, the standardization iseasy, and the production and detection processes are safer and more reliable, so that the ELISA detection kit is suitable for being promoted and used in basic units.
Owner:HEILONGJIANG BAYI AGRICULTURAL UNIVERSITY
Recombinant S1 protein of novel mutant strain of porcine epidemic diarrhea virus and subunit vaccine of recombinant S1 protein
ActiveCN104046637AGood reactogenicityAntiviralsAntibody medical ingredientsPseudomonas aeruginosa exotoxin ASeroconversion
The invention discloses a recombinant S1 protein of a novel mutant strain of a porcine epidemic diarrhea virus and a subunit vaccine of the recombinant S1 protein. The protein is obtained by the steps of cloning a fusion gene fragment PE(delta III)-S1(m)-KDEL3 containing a KDEL3 gene sequence, a gene sequence of III-region-deleted pseudomonas aeruginosa exotoxin A and an S1(m) gene sequence to a baculovirus vector to obtain a recombinant vector; carrying out recombinant vector site specificity transposition on a seroconversion DH10Bac competent cell of the recombinant vector to obtain a recombinant bacmid; transfecting the recombinant bacmid to an Sf9 cell under the mediation of a liposome to obtain a recombinant Sf9 cell; expressing by using the recombinant Sf9 cell. According to the invention, a PE(delta III)-S1(m)-KDEL3 fusion protein is firstly expressed by using a baculovirus expression system on the basis of optimizing the predilection of a codon of mammalian with S1 genes, so that the recombinant baculovirus for efficiently and accurately expressing the fusion protein is obtained. Proved by IFA and Western blot, the recombinant baculovirus can be used for accurately expressing the fusion protein, and the fusion protein has favorable reactionogenicity.
Owner:NANJING AGRICULTURAL UNIVERSITY
Recombinant porcine circovirus type 2 Cap protein with tandem dominant epitope, and application thereof
ActiveCN110423269AImprove the level ofImproving immunogenicityViral antigen ingredientsVirus peptidesEscherichia coliNucleotide
The present invention belongs to the field of biotechnology and particularly relates to a recombinant porcine circovirus type 2 Cap protein with a tandem dominant epitope, and an application thereof.An amino acid sequence of the recombinant porcine circovirus type 2 Cap protein with the tandem dominant epitope is shown as SEQ ID No.1 and a nucleotide sequence encoding the above protein is shown in SEQ ID No.2. A double promoter transfer vector containing the above nucleotide sequence is further constructed to transform escherichia coli to obtain recombinant baculovirus plasmids; and then therecombinant baculovirus plasmids transfect insect cells to obtain recombinant baculoviruses, a recombinant protein expression amount is 914 [mu]g / ml, and the recombinant porcine circovirus type 2 Capprotein can specifically bind to positive serum and has good immunological reactivity. The provided subunit vaccine can stimulate mice to produce a high level of humoral immune responses after immunizing the mice, can induce a specific immune response of body after immunizing piglets, and can provide a good immune protection effect for the challenged piglets.
Owner:SOUTH CHINA AGRI UNIV
Trichina recombinant protein and application thereof
The invention discloses a preparation method of a recombinant protein and in particular relates to a TsSerpin recombinant protein and application thereof. The preparation method of the TsSerpin recombinant protein comprises the following steps: carrying out reverse transcription, with total RNA (Ribonucleic Acid) extracted from pig trichina muscle larva as a template and Oligo(dT)18Primer as a primer; with the obtained reverse transcription Cdna (Deoxyribose Nucleic Acid) as a template and SEQ ID No.1 and SEQ ID No.2 as primers, carrying out PCR (Polymerase Chain Reaction) amplification, to obtain pig trichina TsSerpin gene; and carrying out the treatment including recombinant plasmid construction, escherichia coli conversion and the like to obtain target protein. The recombinant protein disclosed by the invention can be used for preparing an antigen for detecting trichinosis.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
1-type duck hepatitis A virus VP4 recombinant protein, ELISA kit and preparing method of 1-type duck hepatitis A virus VP4 recombinant protein
ActiveCN105330727AGood repeatabilityHigh sensitivitySsRNA viruses positive-senseVirus peptidesDuck hepatitis A virusElisa kit
The invention belongs to the technical field of bioengineering, and particularly relates to 1-type duck hepatitis A virus VP4 recombinant protein, an ELISA kit and a preparing method of the 1-type duck hepatitis A virus VP4 recombinant protein. The amino acid sequence of the 1-type duck hepatitis A virus VP4 recombinant protein is shown as SEQ ID NO:1. The preparing method of the 1-type duck hepatitis A virus VP4 recombinant protein includes the following steps of obtaining VP4 target segments; constructing recombinant expression plasmids pET-32c-VP4; preparing the VP4 recombinant protein. The recombinant prokaryotic expression plasmids are successfully constructed, and soluble expression of the VP4 recombinant protein is successfully obtained and has good reactogenicity with rabbit anti-DHAV-1 serums, which shows that the VP4 protein of DHAV-1 successfully obtains prokaryotic expression; the ELISA kit for detecting the 1-type duck hepatitis A virus antibodies is built and provides test data and basic materials for detecting the DHAV-1 antibodies and further carrying out DHAV-1 related research.
Owner:SICHUAN AGRI UNIV
Babesia orientilas thrombin gene 1 and protein coded by same
InactiveCN105524935ABiologically activeGood reactogenicityMicrobiological testing/measurementFermentationAntigenDisease
The invention discloses a babesia orientilas thrombin gene 1 which has the nucleotide sequence shown in SEQ ID NO:1. The invention further discloses recombinant protein obtained by coding the babesia orientilas thrombin gene 1, wherein the recombinant protein has the amino acid sequence shown in SEQ ID NO:2. The recombinant protein can effectively detect the existence of antigens of a water buffalo infected with the babesia orientilas, has good reactogenicity, belongs to a candidate factor for developing a serological diagnosis method for detecting the babesia orientilas disease, and is beneficial for controlling the prevalence and spread of the babesiosis disease of the water buffalo.
Owner:HUAZHONG AGRI UNIV
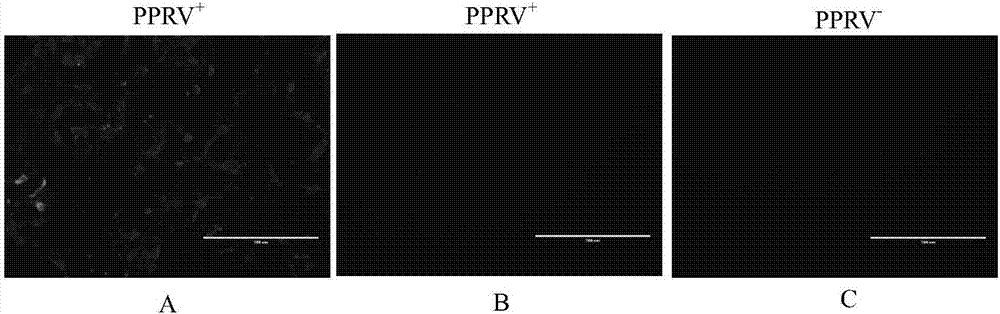
Peste des petits ruminant virus HN protein epitope peptide as well as determination and preparation method and application thereof
PendingCN107033225AStrong green fluorescenceImprove responseSsRNA viruses negative-senseViral antigen ingredientsProtein targetHN Protein
The invention relates to peste des petits ruminant virus HN protein epitope peptide. The amino acid sequences of the epitope peptide are as follows: H123: <123>KFLNPDREYDFRDLR<137>, or / and H185: <185>GTGCLGRTVTRA<196>, or / and H487: <487>IRGPRGRCH<495>, or / and H569: <569>ECFPWYHKVWCYHDCLI<585>. B cell epitopes of a target protein are predicted by virtue of multiple immunoinformatic software, the different predicted epitopes are respectively artificially synthesized, the reactogenicity of the predicted epitopes is verified by virtue of an indirect ELISA method, an aminated ELISA Plate is coated with different polypeptides, and the reactogenicity with an antibody of HN protein is detected, and therefore, the B cell epitopes of the PPRV HN protein are authenticated.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Recombinant E protein ELISA kit for detecting antibody against Japanese encephalitis in pig
InactiveCN1766620AStrong specificityGood reactogenicityColor/spectral properties measurementsJapanese encephalitisAntigen
The invention relates to a recombinant E protein agent box for detecting Japanese cerebritis antibody in the field of biology technology. The agent comprises: ELISA batten which uses the recombinant E protein as the coated antibody, PBS solution, blood dilution solution, color developing base material, ending solution, JEV anodic serum and JEV cathodic serum and so on. The method can be used in other serum sample detection after peculiar, sensitive and repeating detection.
Owner:NANJING AGRICULTURAL UNIVERSITY
Peste des petits ruminant virus F protein epitope peptide as well as determination and preparation method and application thereof
ActiveCN107033226AStrong green fluorescenceImprove responseSsRNA viruses negative-senseViral antigen ingredientsProtein targetF protein
The invention relates to peste des petits ruminant virus F protein epitope peptide. The amino acid sequences of the epitope peptide are respectively as follows: F176: <176>DYINNELVPSVHRMSCEL<193>, or / and F347: <347>MSPLLQECFRGSTKS<361>, or / and F391: <391>CKCYTTETVINQDPDKLL<408>, or / and F417: <417>TVINQDPDKGPVGSREYPD<435>, or / and F430: <430>SREYPDSVYLH<440>, or / and F502: <502>VSLGLVTLICCCKGRCRNK<520>. B cell epitopes of a target protein are predicted, the different predicted epitopes are respectively artificially synthesized, the reactogenicity with an antibody of F protein is detected, and therefore, the B cell epitopes of the PPRV F protein are authenticated.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
O type foot-and-mouth disease virus artificial recombinant antigen and preparation and application thereof
ActiveCN106397546AEasy to operateEasy to identifySsRNA viruses positive-senseBacteriaPolyclonal antibodiesGene
The invention discloses O type foot-and-mouth disease virus artificial recombinant antigen and preparation and application thereof and belongs to the field of biological preparations; an amino acid sequence of the artificial recombinant antigen comprises peptide segments shown as in Seq ID No. 4; the amino acid sequence of the artificial recombinant antigen is shown specifically as in Seq ID No. 5. The invention also claims a gene fragment to encode the artificial recombinant antigen, as well as a recombinant expression vector to express the artificial recombinant antigen and related transformants. The O type foot-and-mouth disease virus artificial recombinant antigen has the advantages such as low production cost, good safety, good antigenicity and good convenience of large-scale production, and polyclonal antibodies produced by foot-and-mouth disease virus natural infections and artificial immunity can be efficiently distinguished.
Owner:北京世纪元亨动物防疫技术有限公司
Canine adenovirus type-1 antibody ELISA detection kit and application thereof
ActiveCN110568189ASensitiveIncreased sensitivityMaterial analysisAgainst vector-borne diseasesAntigenProkaryotic expression
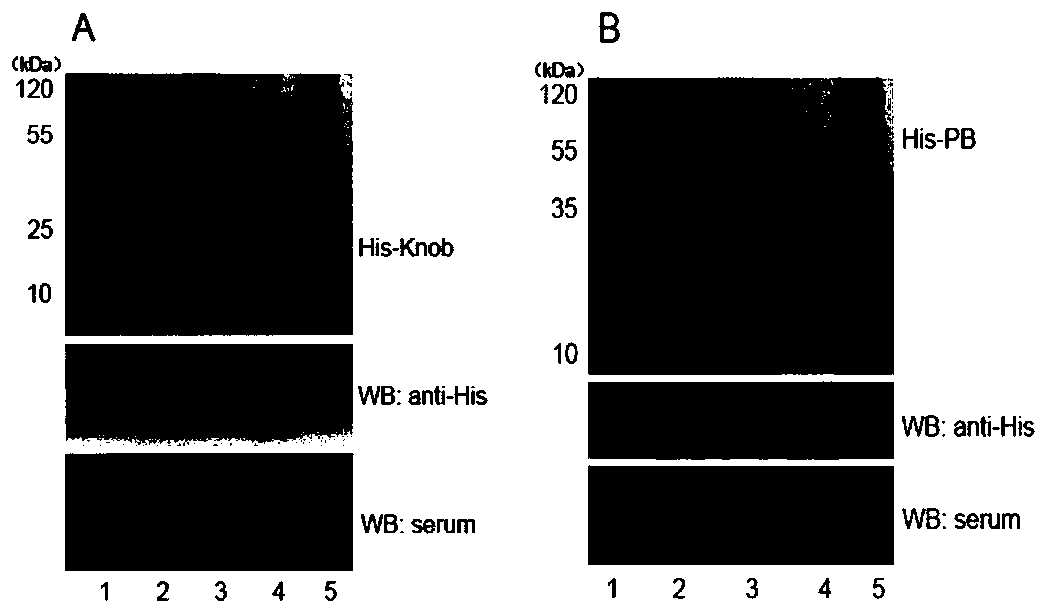
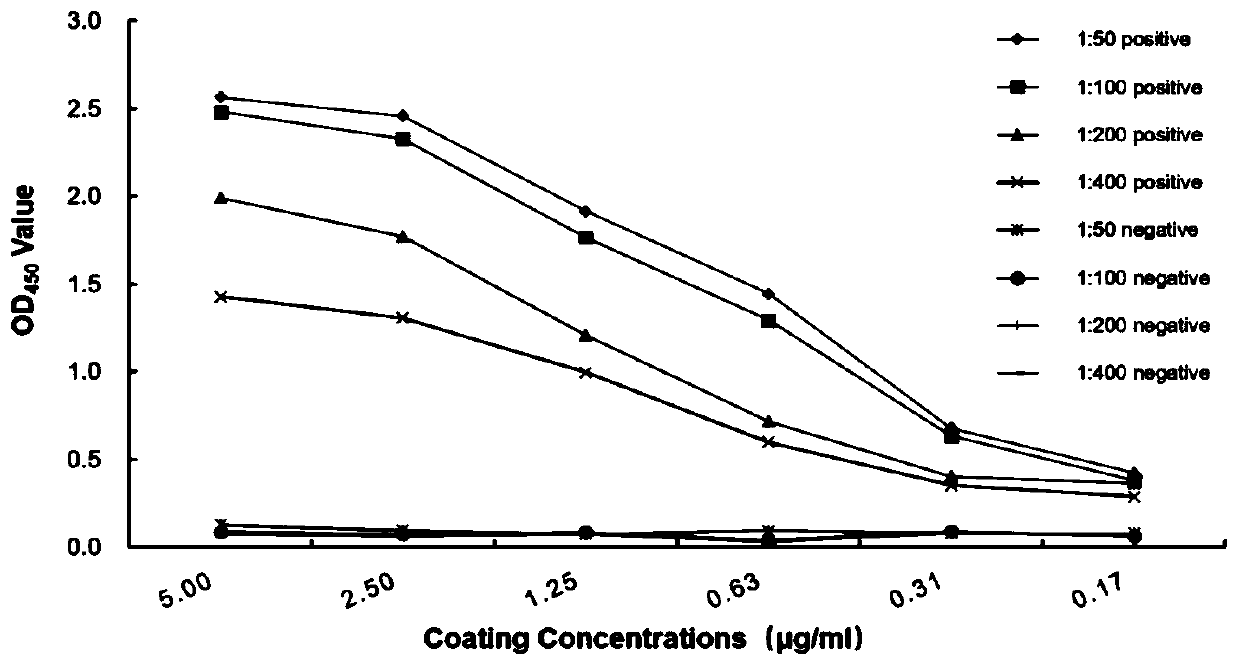
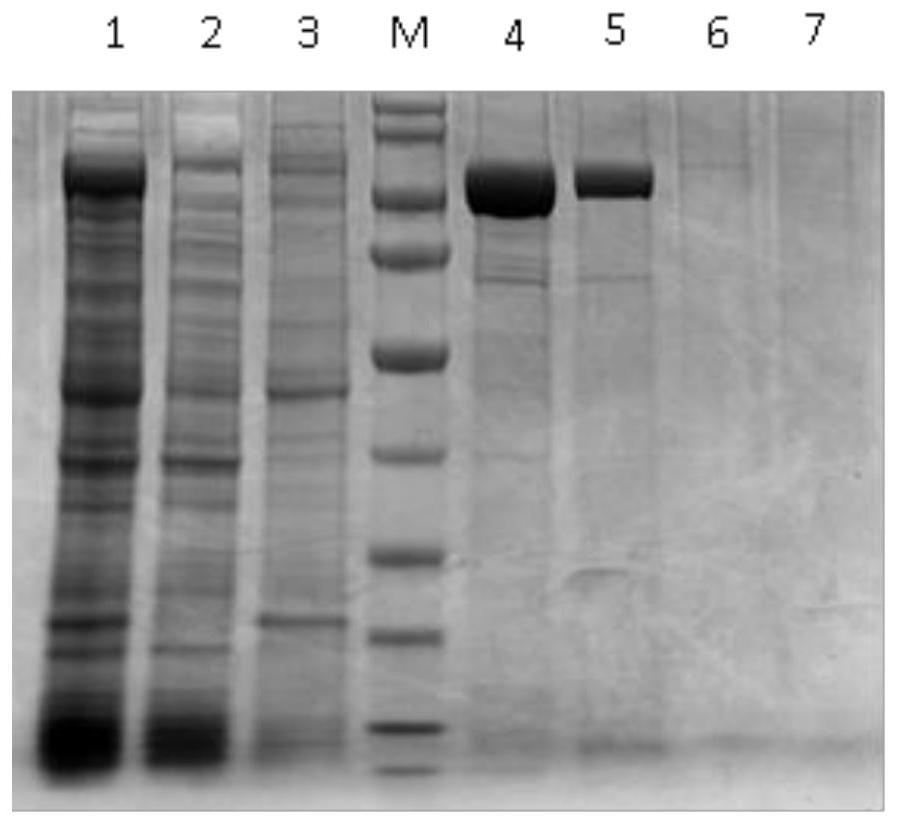
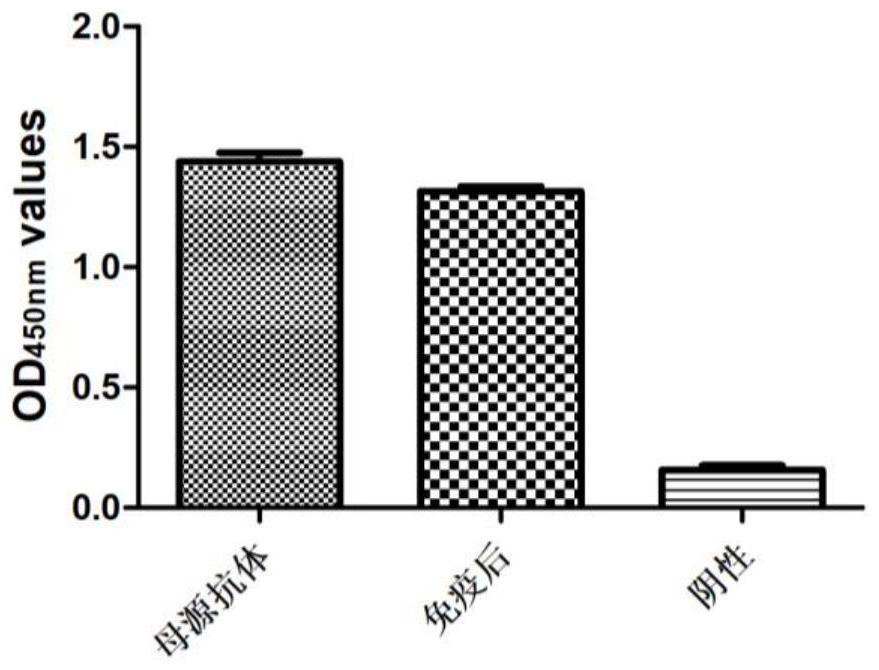
The invention discloses a canine adenovirus type-1 (CAdV-1) antibody ELISA detection kit and application thereof. According to the detection kit, a pCold II prokaryotic expression system is utilized to prepare a PB (penton base) and Knob protein with good reactogenicity, the PB and the Knob protein are subjected to a large quantity of induced expressions, two types of protein after renaturation are used as envelop antigens respectively and compared with a purified CAdV-1 totivirus, and finally the Knob protein is determined as the optimal ELISA envelop antigen. On this basis, the ELISA detection kit with high efficiency, good sensitivity and good specificity for a CAdV-1 serum antibody is provided; results of simultaneous sample detection through the detection kit and an SN method show that the sensitivity of the ELISA detection kit is 97.14%, the specificity is 90.00%, and the SN coincidence rate is 93.57%; and the detection kit has the advantages of high sensitivity, good specificity, strong repeatability and the like.
Owner:INST OF SPECIAL ANIMAL & PLANT SCI OF CAAS
Coating antigen for detecting mycoplasma synoviae antibody, kit and detection method thereof
PendingCN114324859AEfficient detectionInfection controlMaterial analysisMycoplasma synoviaeNucleotide
The invention relates to the technical field of biology, in particular to a coating antigen for detecting a mycoplasma synoviae antibody, a kit and a detection method thereof. Detecting that a coating antigen of the mycoplasma synoviae antibody is a recombinant EFG protein; the amino acid sequence of the recombinant EFG protein comprises a sequence as shown in SEQ ID NO: 1; the nucleotide sequence of the recombinant EFG protein comprises a sequence as shown in SEQ ID NO: 2. The recombinant EFG protein is used as the coating antigen of the ELISA method for detecting the mycoplasma synoviae antibody, can be used for clinical detection of the mycoplasma synoviae serum antibody, and efficiently detects the mycoplasma synoviae antibody in a farm; according to the present invention, the recombinant EFG protein is adopted as the coating antigen of the ELISA method for detecting the mycoplasma synoviae antibody, such that the detection method has good reactogenicity, specificity, sensitivity and repeatability, can be used for clinical mycoplasma synoviae serum antibody detection, provides certain guidance for the formulation of the mycoplasma synoviae vaccine immune program, and provides the important significance for the clinical detection of the mycoplasma synoviae serum antibody. Therefore, the aim of preventing and controlling mycoplasma synoviae infection is achieved.
Owner:WENS FOODSTUFF GRP CO LTD +1
ELISA kit for detecting echinococcus granulosus infection of livestock such as dogs and sheep
PendingCN113341160AHigh sensitivityGood specificityBiological material analysisBiological testingEpidemiologyWestern blot
The invention discloses an ELISA (enzyme-linked immuno sorbent assay) kit for detecting echinococcus granulosus infection of livestock such as dogs and sheep. A coating antigen in the kit is a recombinant echinococcus granulosus 14-3-3 zeta protein. According to the invention, the echinococcus granulosus recombinant protein rEg14-3-3 zeta is cloned and expressed for the first time; western blot shows that the recombinant protein has good reactogenicity; it is found that after rEg14-3-3 zeta immunizes mice, good body fluid and cellular immune response is induced and generated, and Th1 type immune response is mainly induced and generated. Furthermore, an indirect ELISA method for detecting the echinococcus granulosus antibody is established on the basis of the rEg14-3-3 zeta protein, an ELISA kit is prepared and has good sensitivity, specificity and repeatability, and an effective method is provided for epidemiological investigation and diagnosis of echinococcus granulosus infection and cystic echinococcosis of livestock.
Owner:SHANGHAI VETERINARY RES INST CHINESE ACAD OF AGRI SCI
Duck hepatitis virus polyprotein antigen sense region DHAV-Mag genetic recombination protein and preparation method and application thereof
InactiveCN105330728AHigh purityReduce manufacturing costSsRNA viruses positive-senseVirus peptidesDuck hepatitis A virusDuck viral hepatitis
The invention discloses a duck hepatitis virus polyprotein antigen sense region DHAV-Mag genetic recombination protein and a preparation method and application thereof, and belongs to the field of biological medicine. The preparation method of the DHAV-Mag genetic recombination protein comprises the following steps of a, DHAV-Mag DNA fragment cloning and expression vector construction; b, DHAV-Mag genetic recombination protein expression; c, DHAV-Mag genetic recombination protein purification. The DHAV-Mag genetic recombination protein is used for successfully obtaining a polyclonal antibody for an antigen immune animal, preliminary study is performed on detection and immune protection effect of the DHAV-Mag genetic recombination protein and the polyclone antibody, and a new approach is provided for detection and prevention of the duck hepatitis virus.
Owner:SICHUAN AGRI UNIV
Caprine arthritis encephalitis virus-encephalitis virus serum antibody diagnosis labelled protein gp135 and preparation method thereof
InactiveCN107488219AGood reactogenicityEase of industrial productionVirus peptidesMicroorganism based processesAntigenNucleotide
The invention belongs to the technical field of biology, and concretely relates to caprine arthritis encephalitis virus-encephalitis virus serum antibody diagnosis labelled protein gp135. The caprine arthritis encephalitis virus-encephalitis virus serum antibody diagnosis labeled protein gp135 contains an optimized gp135 protein nucleotide sequence shown as SEQ ID NO.1 and the other sequences with consistency being not less than 95%; and contains the optimized gp135 protein nucleotide sequence shown as SEQ ID NO.2 and the other sequences with consistency being not less than 95%. The gp135 diagnosis labelled protein expressed through pichia yeast GS115 has good reactogenicity and is convenient for industrial production, and provides antigen for developing a CAEV high flux serological diagnosis method.
Owner:新疆畜牧科学院
Seneca recombinant virus of recombinant O-type foot-and-mouth disease virus epitope genes, recombinant vaccine as well as preparation method and application of recombinant vaccine
ActiveCN111996203AHigh titerReduce pathogenicitySsRNA viruses positive-senseViral antigen ingredientsGenetic engineeringSenecavirus
The invention provides a Seneca recombinant virus of recombinant O-type foot-and-mouth disease virus epitope genes, a recombinant vaccine and a preparation method and application of the recombinant vaccine, and relates to the technical field of genetic engineering. According to the invention, the full-length cDNA of SVV / FJ / 001 strain is obtained, deletion and mutation transformation are carried out on the 5'UTR, meanwhile, the tandem O-type FMDV recombinant epitope genes are fused into SVA cDNA, and the Seneca recombinant virus of recombinant foot-and-mouth disease antigen epitope is constructed. The recombinant virus can express the foot-and-mouth disease B cell epitope and T cell epitope fused into the SVA cDNA, and an expression product has good reactogenicity. The pathogenicity of therecombinant virus is remarkably reduced, even no pathogenicity is caused to pigs, and the biological safety of the virus strain is remarkably improved; and the prepared inactivated vaccine has good immunogenicity, can generate a specific antibody for FMDV while effectively stimulating an SVA neutralizing antibody, and can be used for preventing and controlling Seneca virus and one or more non-Seneca viruses.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
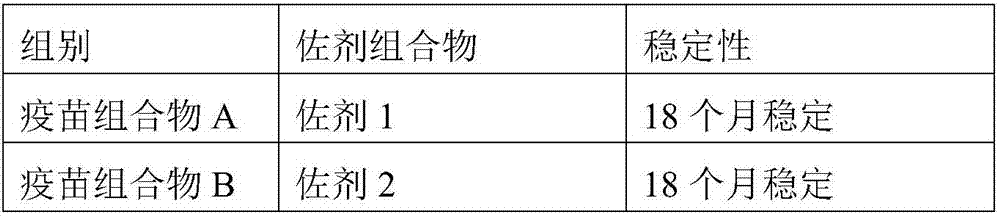
Adjuvant composition and preparation method and application thereof
ActiveCN107496914AStrong reactogenicityReduced reactogenicityAntibacterial agentsBacterial antigen ingredientsAntigenCross-link
The invention relates to the technical field of animal biological products, in particular to an adjuvant composition and a preparation method and application thereof. The adjuvant composition comprises acrylic acid polymer-epsilon-polylysine nano-particles; and specifically, 100 parts by weight of the adjuvant composition comprises 0.001 to 5 parts by weight of acrylic acid polymer-epsilon-polylysine nano-particles. The acrylic acid polymer-epsilon-polylysine nano-particles contained in the adjuvant composition are prepared through a simple electrostatic interaction, and the adjuvant composition is free of cross-linking among proteins harmful for bodies, so that the adjuvant composition has extremely high safety and effectiveness, is applicable to be used as an adjuvant, and overcomes problems existing when polyacrylic acid is used as the adjuvant. Moreover, a vaccine composition formed by the adjuvant composition and an antigen has extremely high stability for long-term storage, and can be safely and effectively applied to various types of subjects.
Owner:LUOYANG SEIWEI BIOTECHNOLOGIES CO LTD
Anti-SARS-CoV-2 S1-RBD monoclonal antibody and application thereof
ActiveCN113929773AHigh potencyHigh sensitivityBiological material analysisImmunoglobulins against virusesHeavy chainProtein s antigen
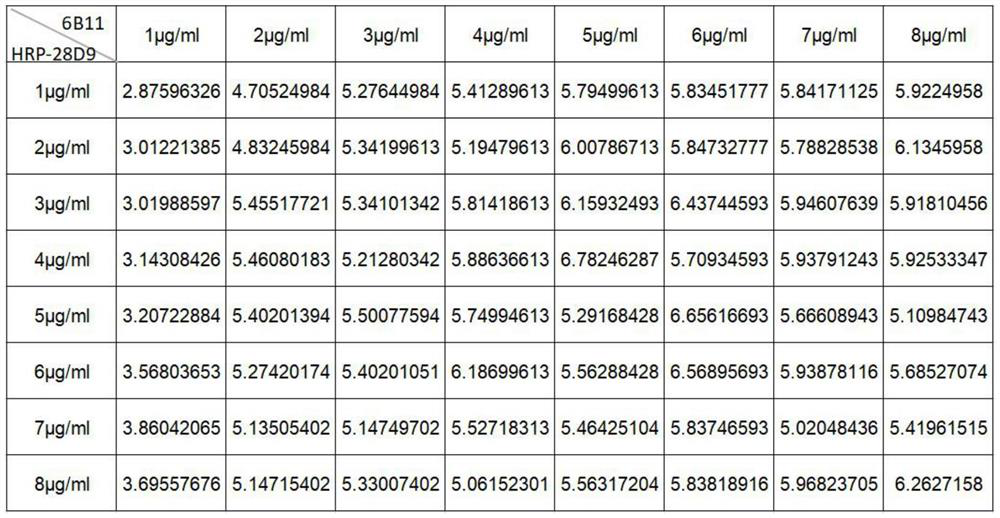
The invention relates to an anti-SARS-CoV-2S1-RBD monoclonal antibody 28D9. A heavy chain of the monoclonal antibody 28D9 is IgG1 type, a light chain of the monoclonal antibody 28D9 is Kappa type, an amino acid sequence of a heavy chain variable region is as shown in SEQ ID NO.1, and an amino acid sequence of a light chain variable region is as shown in SEQ ID NO.2. The antibody has native conformation epitopes, has good reactogenicity with SARS-CoV-2S1 protein and SARS-CoV-2S1-RBD, and has the characteristics of high specificity, high sensitivity, high titer and the like. A double-antibody sandwich ELISA detection method is established on the basis of the SARS-CoV-2S1 protein epitope, can be used for quantitatively detecting the SARS-CoV-2 inactivated virus and the SARS-CoV-2S1 protein, and is good in repeatability, high in precision, high in sensitivity, low in color development background and good in linearity.
Owner:国际遗传工程和生物技术中心泰州区域研究中心 +3
A kind of swine fever virus blocking ELISA antibody detection kit and its application
ActiveCN104483490BIncrease culture densityEasy extractionSsRNA viruses positive-senseVirus peptidesPositive controlViral antibody
Owner:WUHAN KEQIAN BIOLOGY CO LTD
A kind of recombinant porcine circovirus type 2 cap protein with dominant epitope in series and its application
ActiveCN110423269BImprove the level ofImproving immunogenicityViral antigen ingredientsVirus peptidesDual promoterNucleotide
The invention belongs to the field of biotechnology, and in particular relates to a recombinant porcine circovirus type 2 Cap protein in series with dominant epitopes and an application thereof. The amino acid sequence of the recombinant porcine circovirus type 2 Cap protein of the tandem dominant epitope is shown in SEQ ID No.1, and the nucleotide sequence encoding the protein is shown in SEQ ID No.2. The present invention further constructs a dual-promoter transfer vector containing the above-mentioned nucleotide sequence, and transforms Escherichia coli to obtain a recombinant baculovirus plasmid; then transfects insect cells to obtain a recombinant baculovirus, and its recombinant protein expression reaches 914 μg / ml, can specifically combine with positive serum, and has good immunological reactivity. The subunit vaccine provided by the invention can stimulate mice to produce a high level of humoral immune response after immunization of mice; can induce a specific immune response in the body after immunization of piglets, and can provide good immune protection for challenged piglets.
Owner:SOUTH CHINA AGRI UNIV
Preparation method and application of multi-epitope fusion diagnostic antigen protein of Echinococcus granulosus
InactiveCN106397610BGood reactogenicityImprove featuresAntibody mimetics/scaffoldsBiological material analysisEscherichia coliCyst
The invention relates to a multi-epitope fusion antigen protein for echinococcosis granulosis cyst diagnosis and a preparation method and application of the multi-epitope fusion antigen protein. The method comprises the steps that protein genes with echinococcosis granulosis cyst immunogenicity are screened out, dominant linear epitopes of the antigen gene coding protein is analyzed by means of the bioinformatics means, the epitopes are connected in series according to a certain sequence, the multi-epitope fusion antigen gene Eg-meAgl is constructed and expressed in escherichia coli, and the recombinant antigen protein Eg-meAgl with the diagnostic value and high immuneoreactivity is obtained. The prepared echinococcosis granulosis cyst recombinant multi-epitope fusion antigen Eg-meAgl has the high specificity and sensitivity, and is the valuable Eg infection diagnosis antigen, and a novel method for antigen protein preparation is provided for echinococcosis granulosis cyst (echinococcosis) diagnosis.
Owner:SHIHEZI UNIVERSITY
Preparation and use of giant panda Ascaris schroederi antigen
InactiveCN101348787BGood reactogenicityImprove diagnostic toolsGenetic material ingredientsAntiparasitic agentsBacteroidesAntigen
Owner:SICHUAN AGRI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com