Anti-SARS-CoV-2 S1-RBD monoclonal antibody and application thereof
A monoclonal antibody, sars-cov-2s1-rbd technology, applied in antiviral immunoglobulins, instruments, peptides, etc., can solve the problems of false negative test results, cumbersome operation, large human error, etc., and achieve subjective factor error. The effect of small size, low color rendering background, and short detection period
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The preparation of embodiment 1 mouse anti-SARS-CoV-2 S1 protein monoclonal antibody
[0043] The monoclonal antibody of the present invention uses SARS-CoV-2 S1 recombinant protein as the immunogen and is secreted from the hybridoma cell line obtained from immunized mice, and is hereinafter referred to as 28D9 and 6B11. Wherein, the amino acid sequence of the heavy chain variable region of monoclonal antibody 28D9 is shown in SEQ ID NO.1, and the amino acid sequence of the light chain variable region is shown in SEQ ID NO.2; the amino acid sequence of the heavy chain variable region of monoclonal antibody 6B11 is shown in SEQ ID NO.2. The amino acid sequence is shown in SEQ ID NO.3, and the amino acid sequence of the light chain variable region is shown in SEQ ID NO.4. Specific steps are as follows:
[0044] (1) Five 8-week-old and healthy female purebred Balb / c mice were selected for antigen immunization with SARS-CoV-2 S1 recombinant protein (purchased from Beijing ...
Embodiment 2
[0056] Example 2 Double Antibody Sandwich ELISA Detection
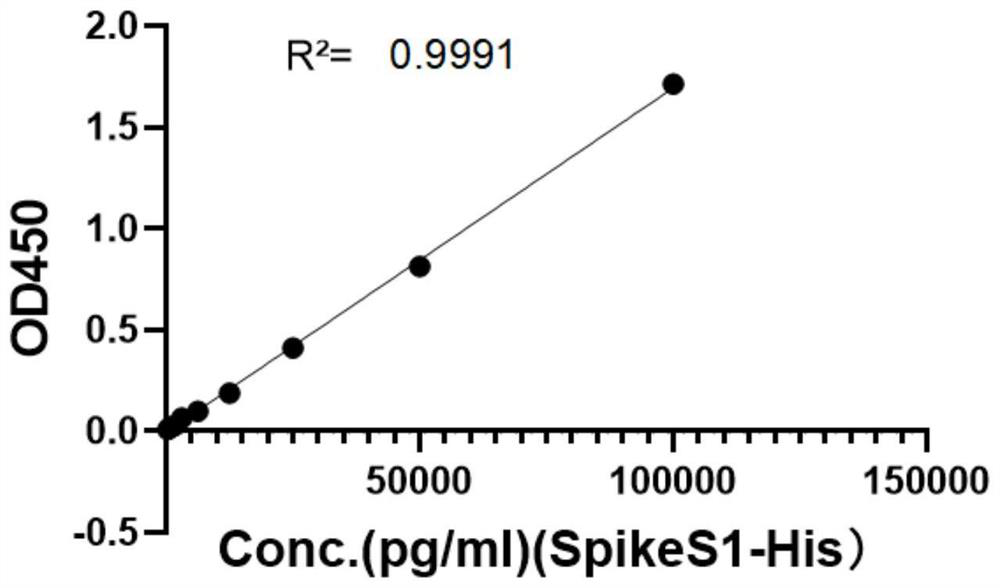
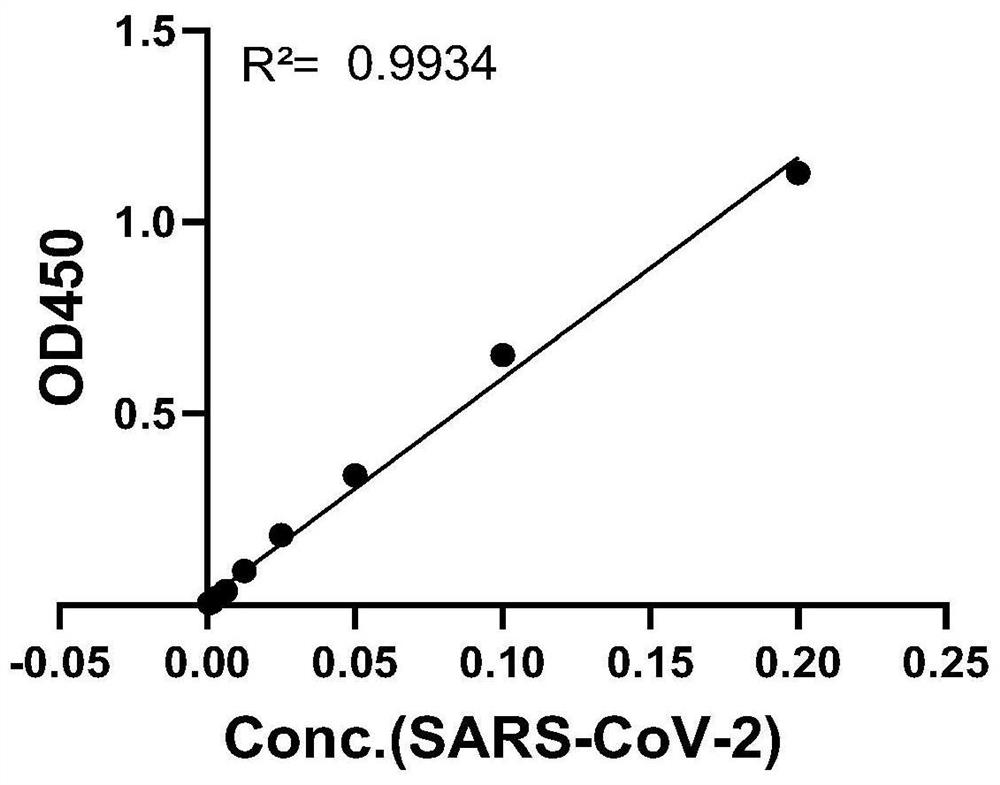
[0057] In order to obtain antibody pairs suitable for quantitative detection of SARS-CoV-2 virus or its S1 protein, the following double-antibody sandwich ELISA crossover experiment was performed to obtain purified monoclonal antibodies 26D8, 28D9, 30G10, and 6B11, and these 4 antibodies were labeled separately HRP (Baiying Biotechnology Co., Ltd. provides labeling HRP services), and obtained 4 kinds of enzyme-labeled monoclonal antibodies HRP-26D8, HRP-28D9, HRP-30G10, and HRP-6B11. Monoclonal antibodies 26D8, 28D9, 30G10, and 6B11 were coated on 96-well ELISA plates, and incubated overnight at 4°C for 20h; after washing, the blocking solution was blocked at 37°C for 2h. After washing, add SARS-CoV-2 S1 recombinant protein to be detected (diluted to 20000pg / ml in blocking solution) or SARS-CoV-2 inactivated virus vaccine (gifted by Institute of Medical Biology, Chinese Academy of Medical Sciences, diluted 20 times in...
Embodiment 3
[0064] One of the purposes of the present invention is to apply the best antibody obtained from the antibody provided by the present invention to double-antibody sandwich ELISA, which can quantitatively detect SARS-CoV-2 virus or its S1 protein. In order to achieve the high sensitivity of the present invention, A series of experiments were optimized, and the technical scheme is as follows:
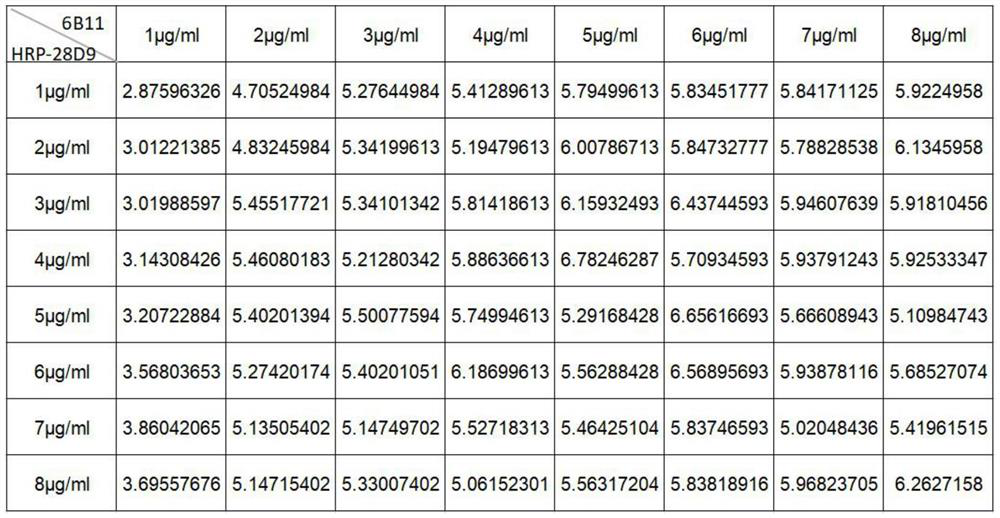
[0065] (1) Determination of optimal antibody dilution concentration
[0066] Fix the concentration of the test substance (when the test sample is S1 protein, the concentration is 25000pg / ml; when the test sample is an inactivated SARS-CoV-2 virus vaccine, the dilution factor is 30 times), the coated antibody (when When the sample to be tested is S1 protein, the coating antibody is 6B11; when the sample to be tested is an inactivated SARS-CoV-2 virus vaccine, the coating antibody is 28D9) and the enzyme-labeled antibody (HRP-28D9) are set at different concentrations ( 1, 2, 3, 4, 5, 6, 7, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com