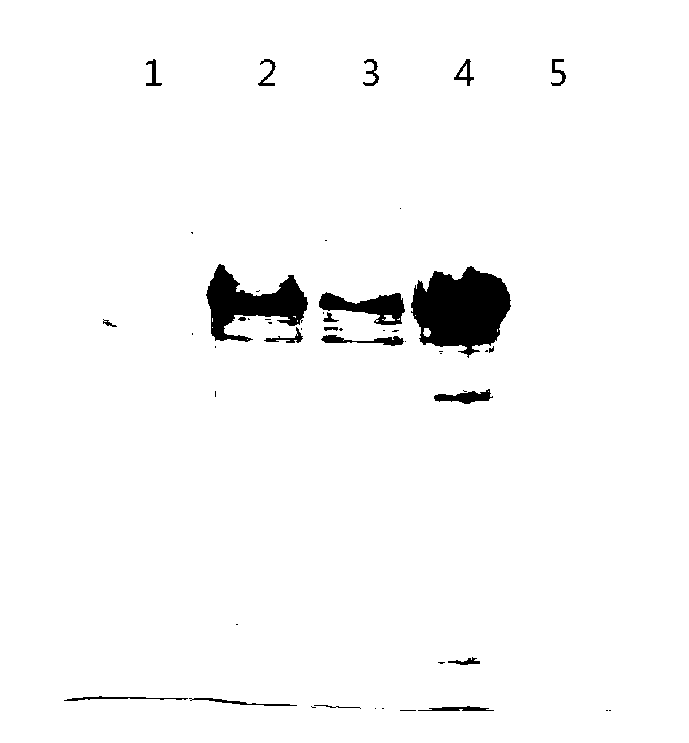
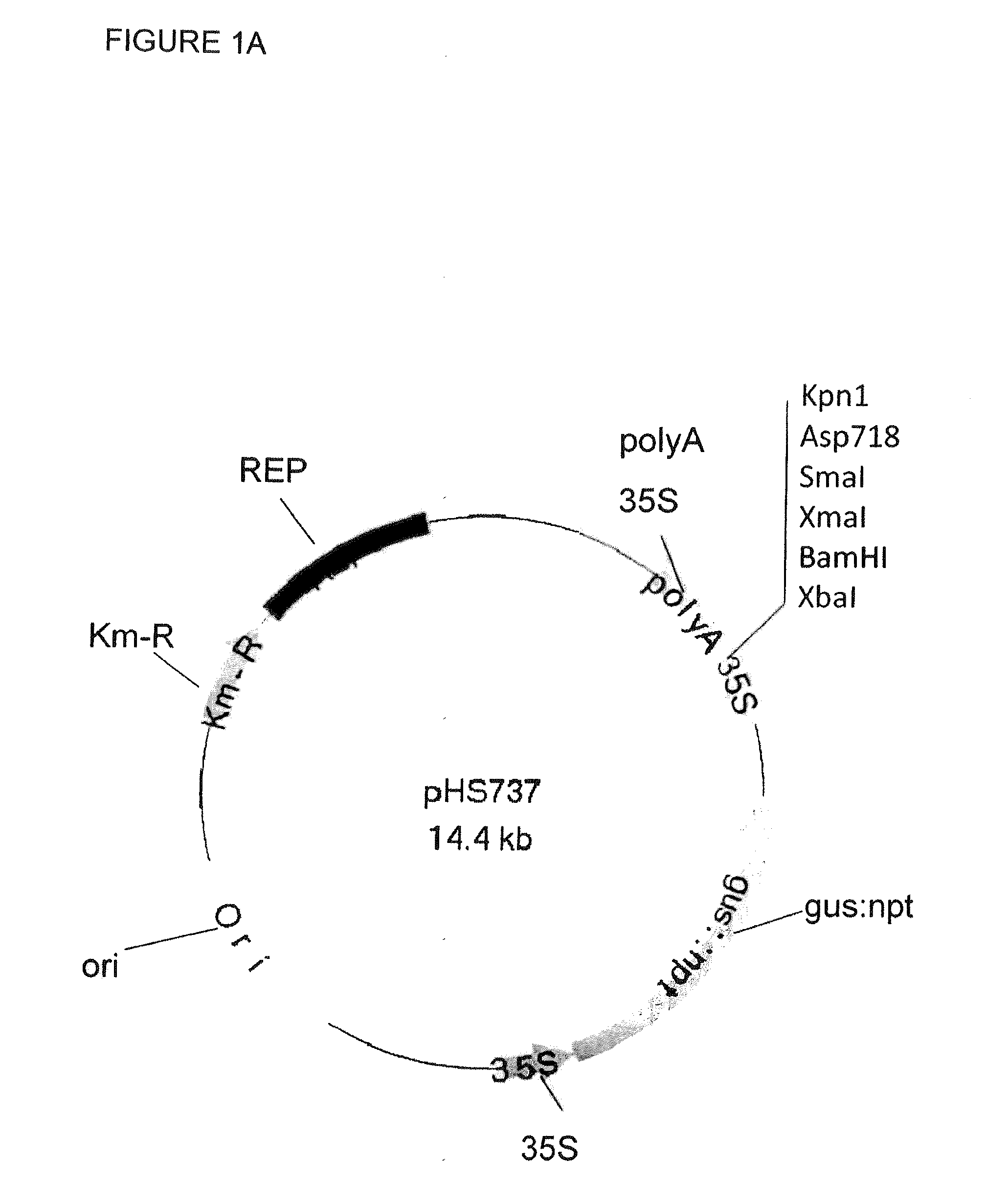
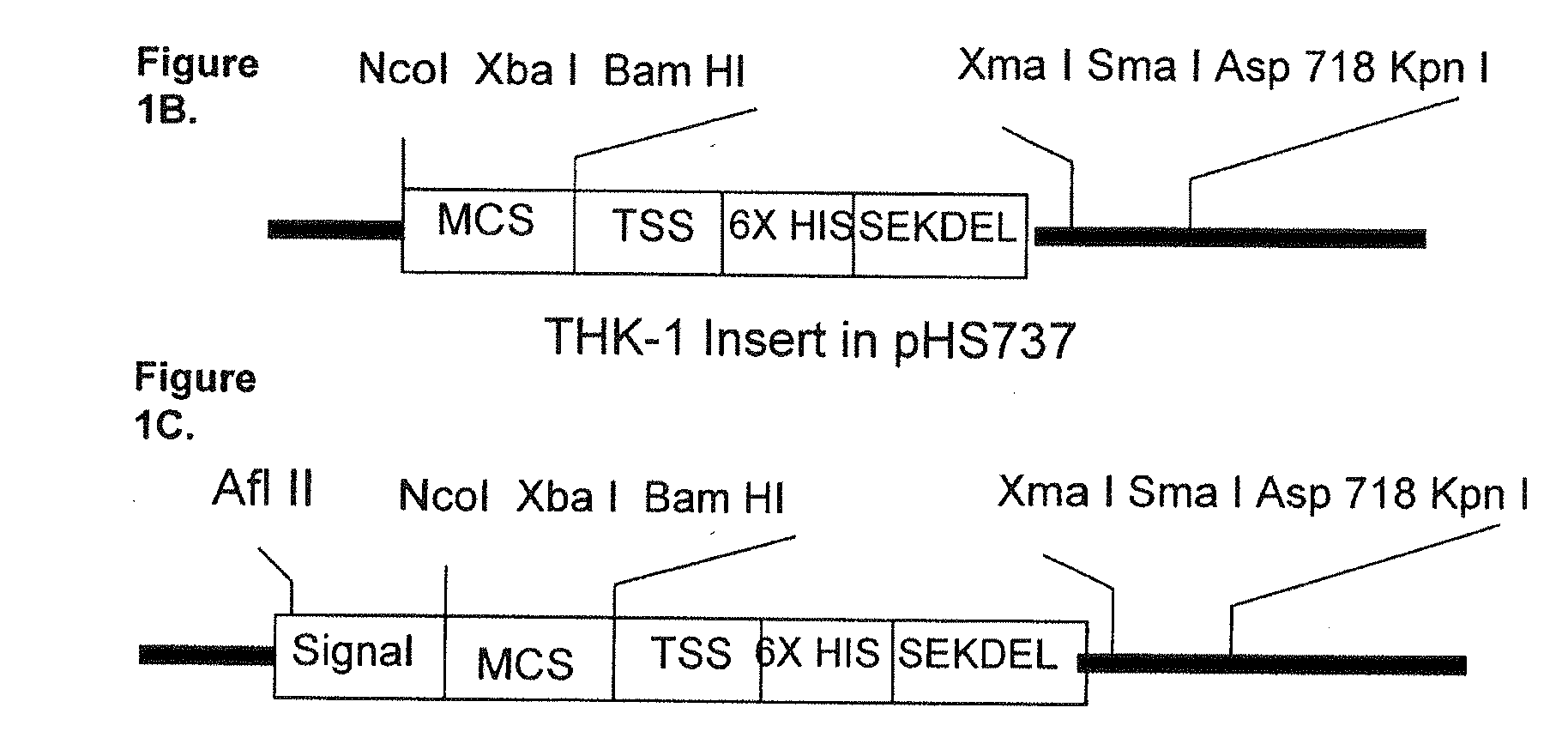
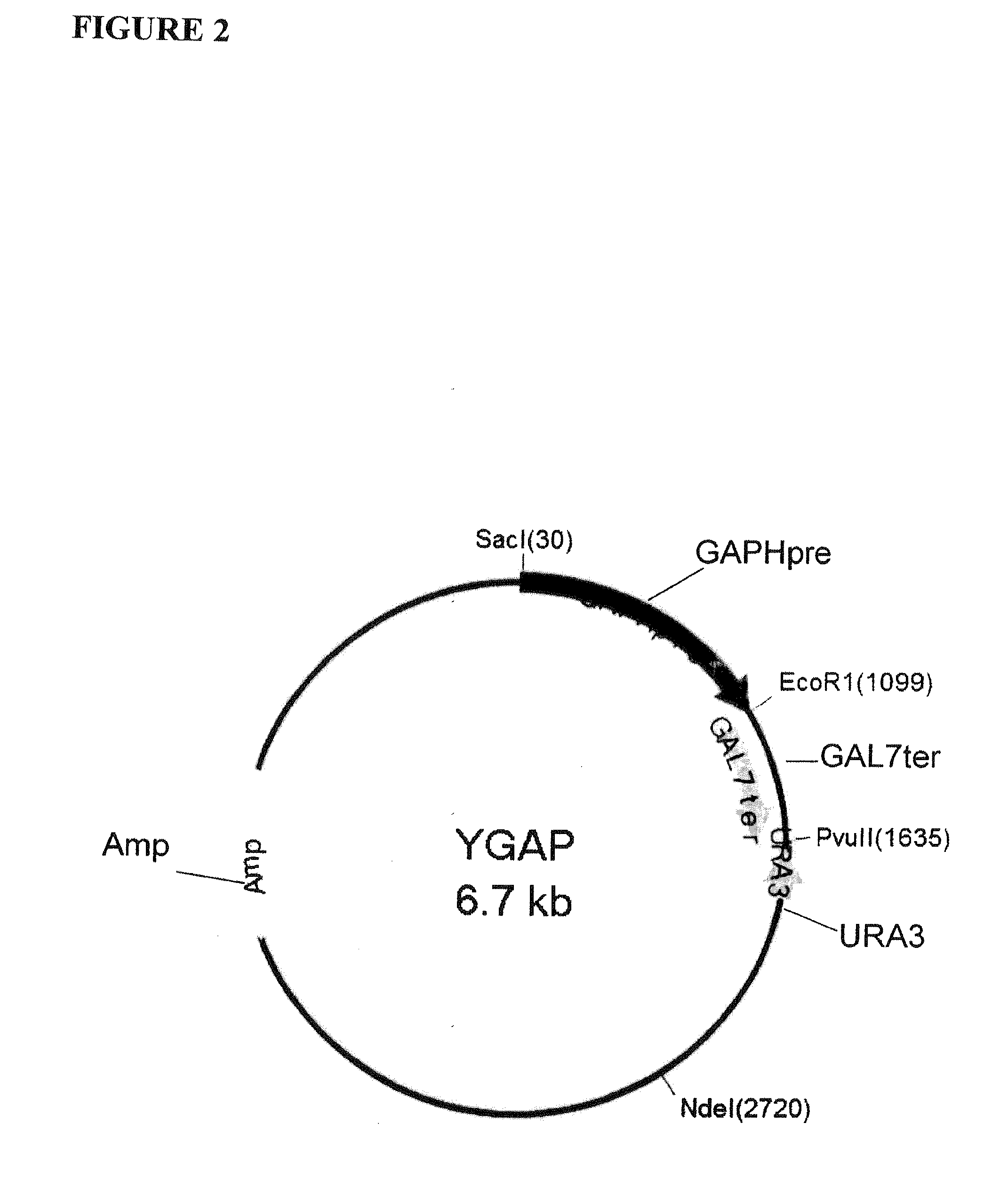
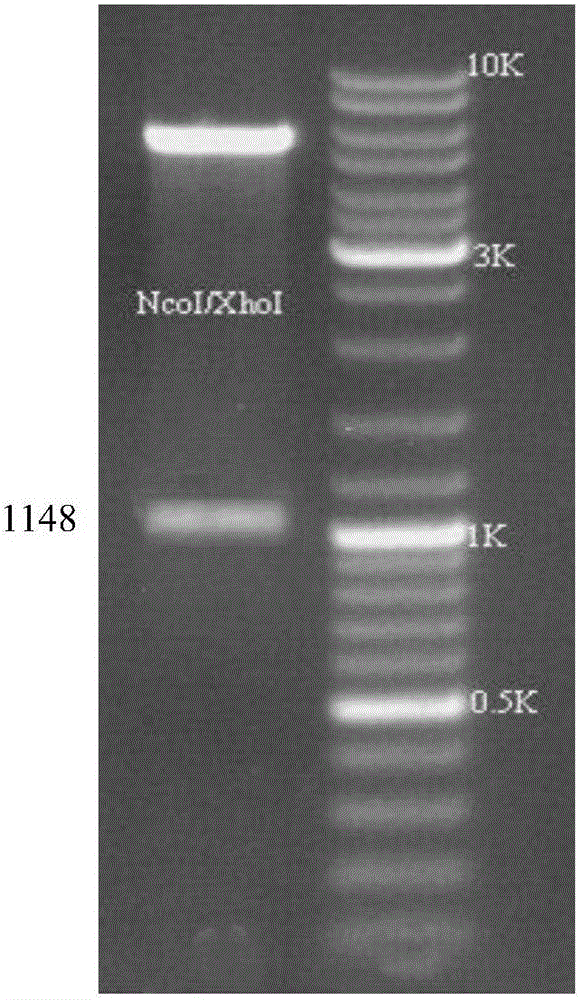
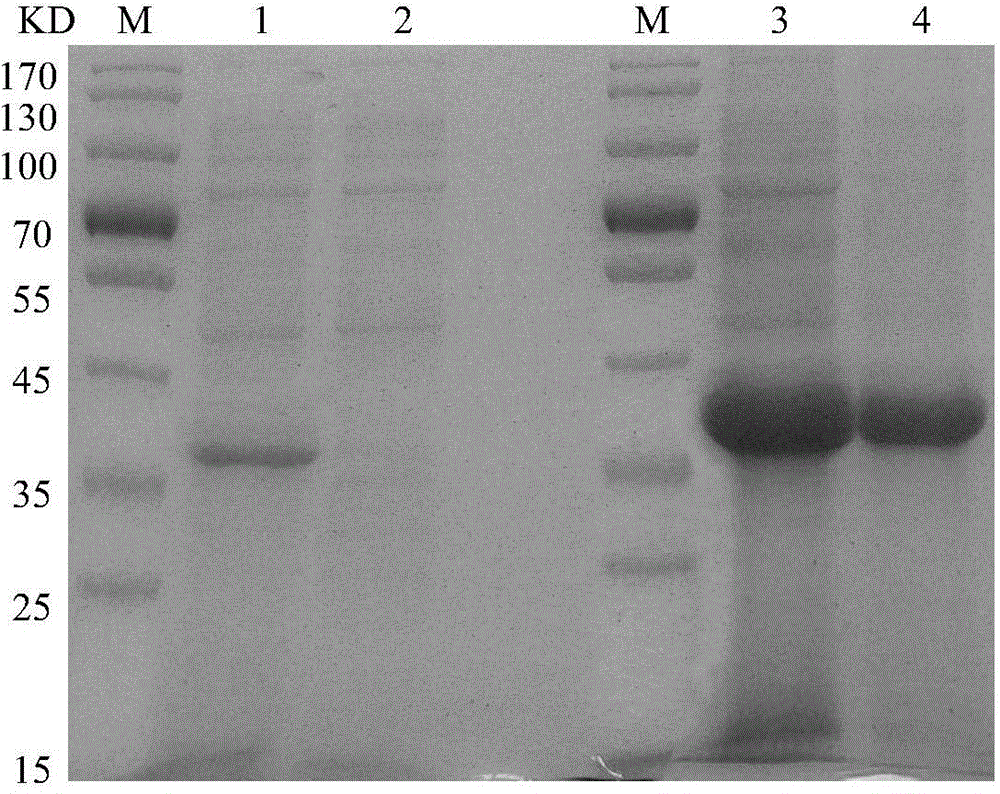
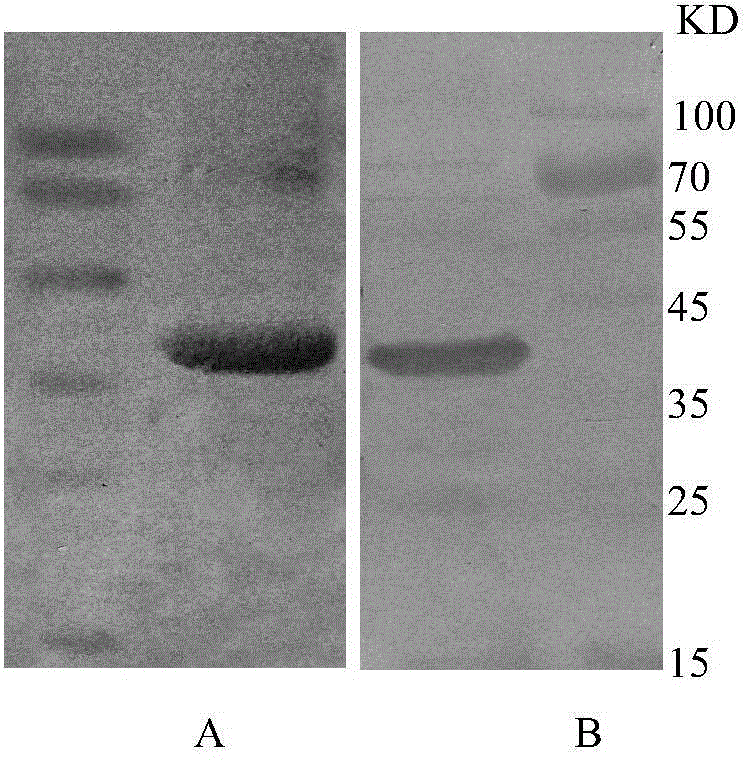
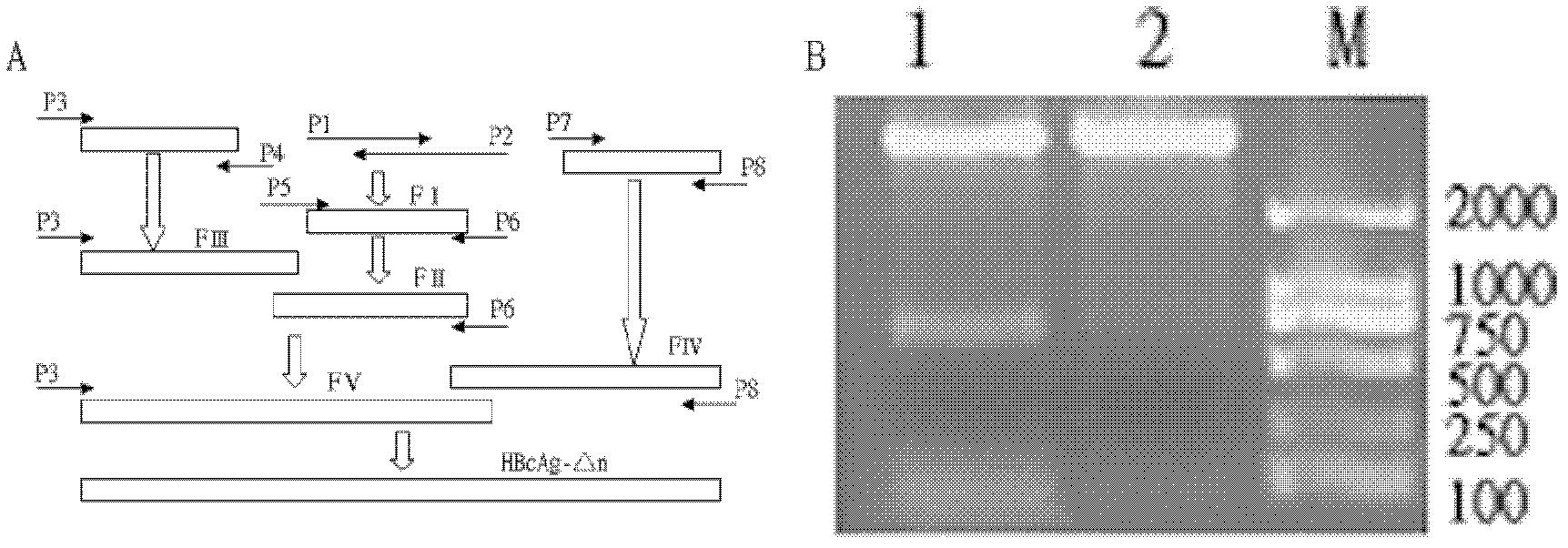
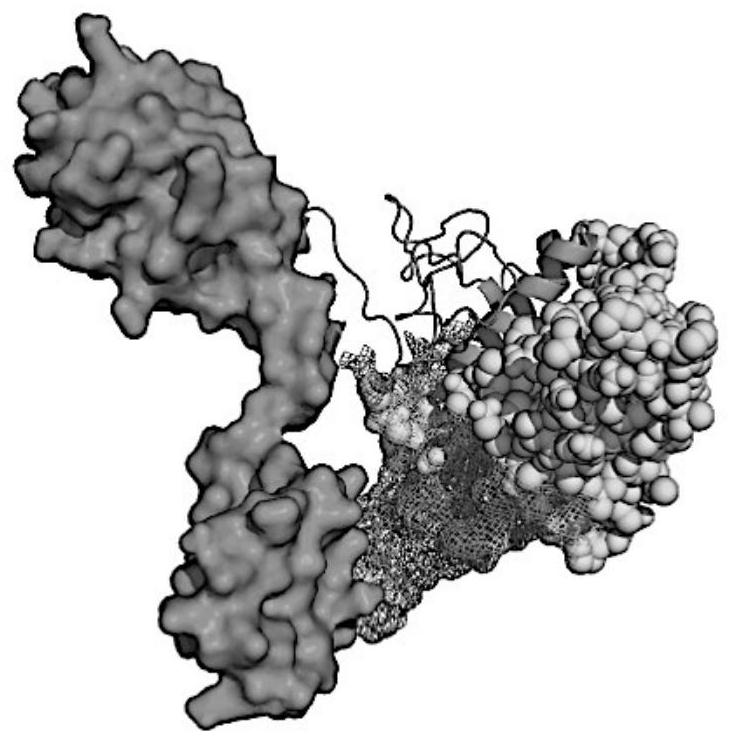
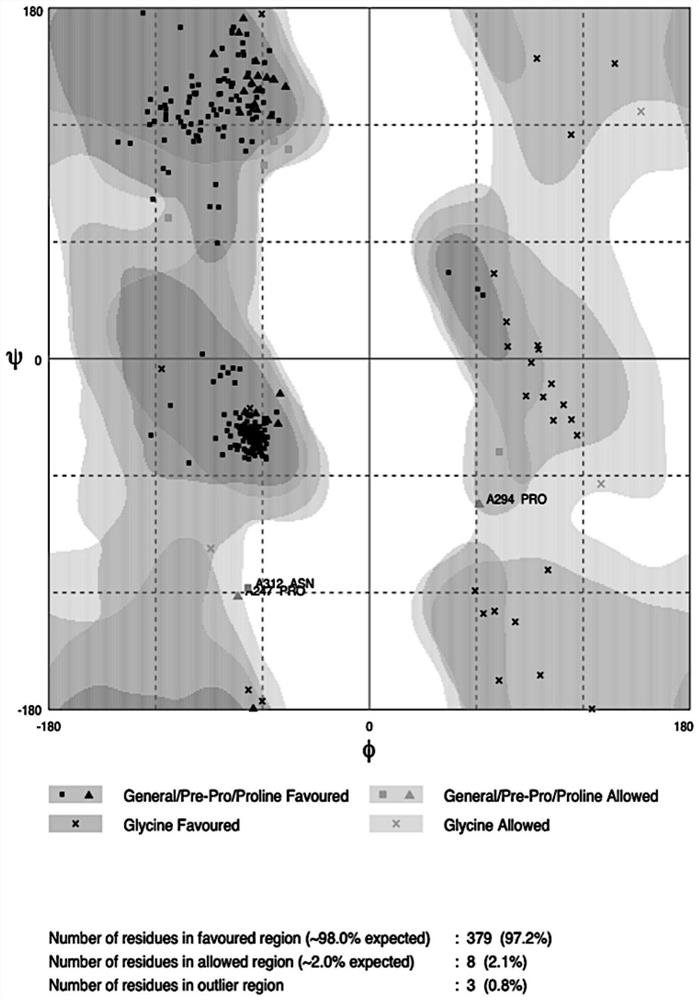
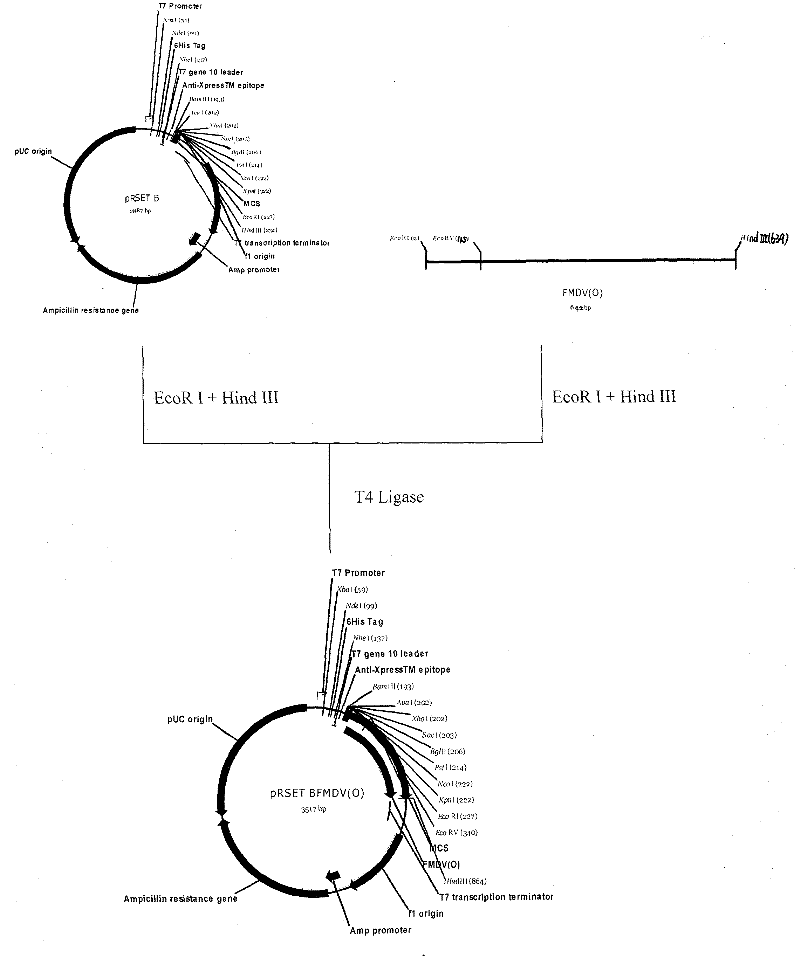
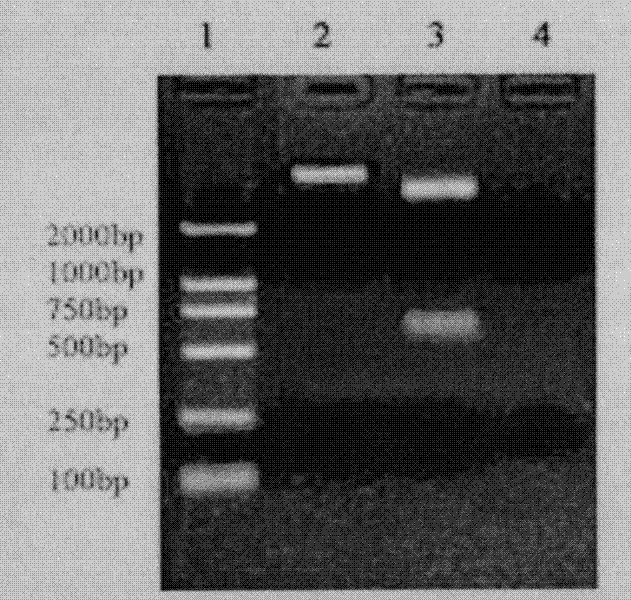
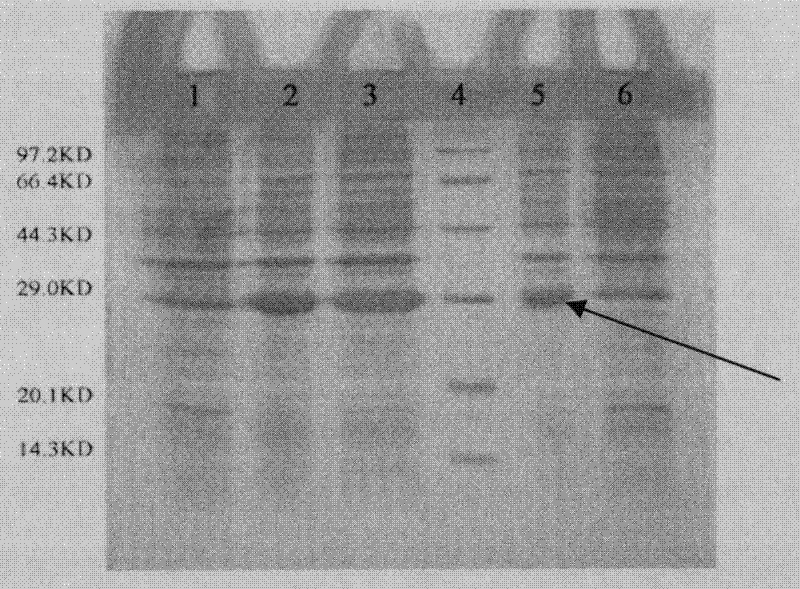
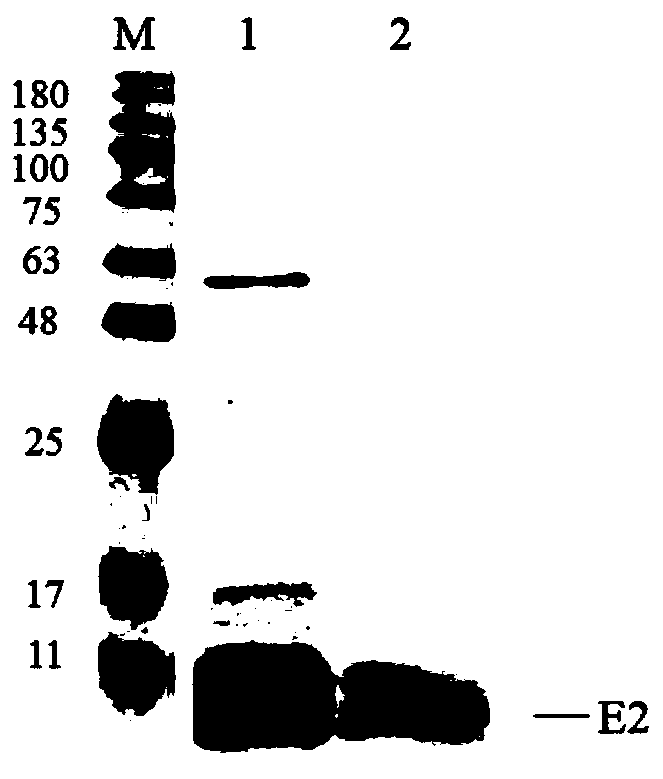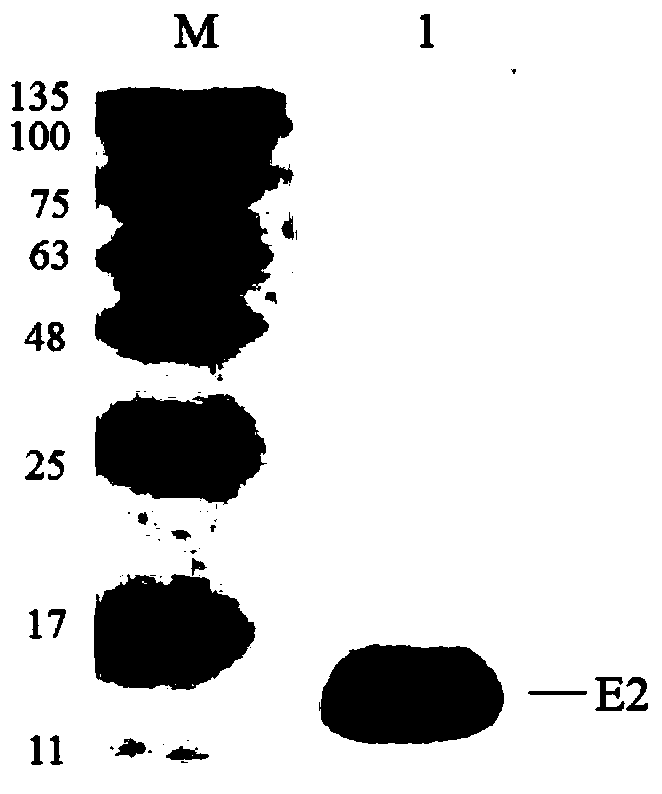Patents
Literature
126 results about "Epitope vaccine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Foot-and-mouth disease genetic engineering mixed epitope vaccine and preparation method thereof
ActiveCN103007273AGood immune protectionUniform response levelAntiviralsAntibody medical ingredientsGenetic engineeringPolyinosinic Acids
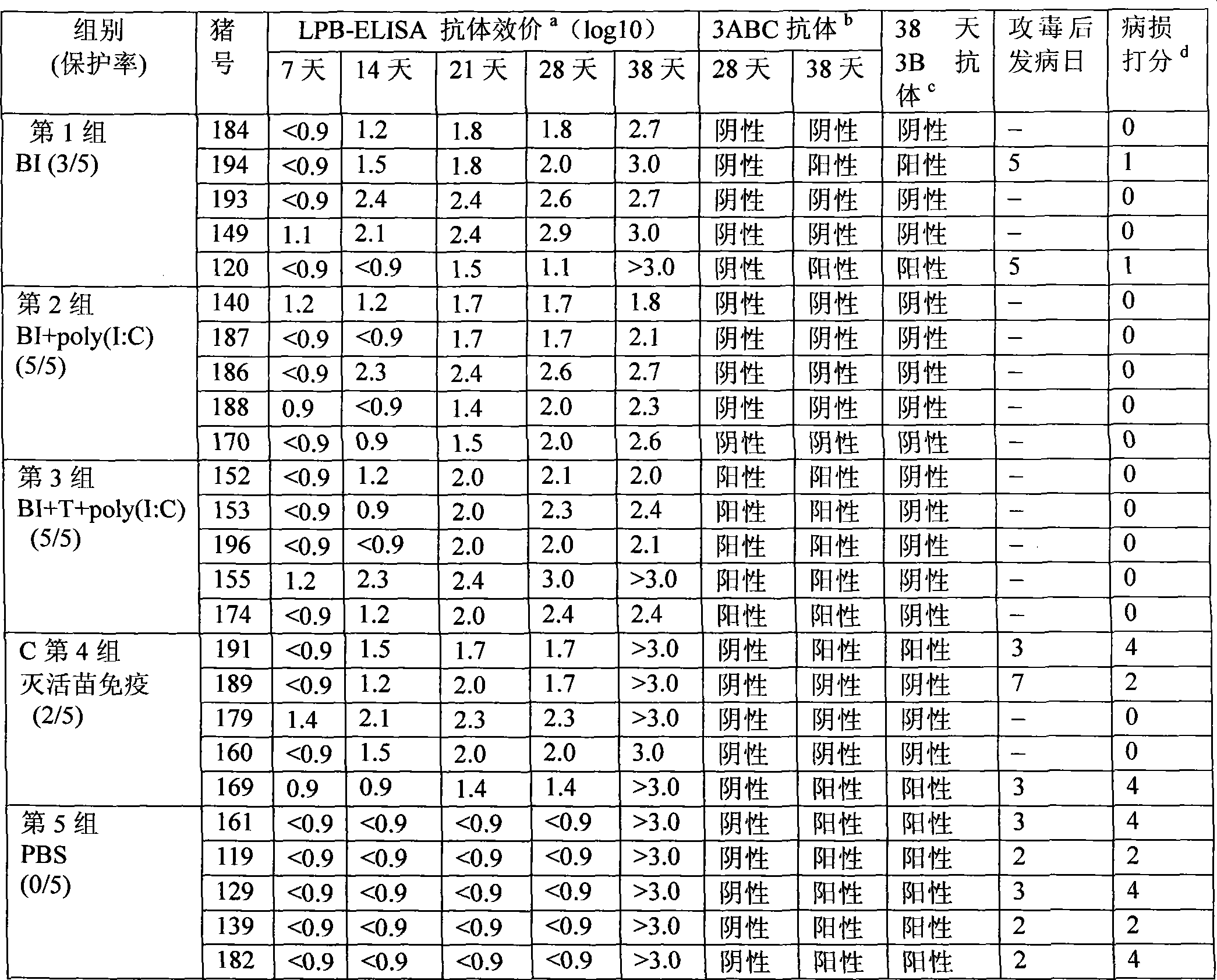
The invention discloses a foot-and-mouth disease genetic engineering mixed epitope vaccine and a preparation method thereof. The vaccine consists of the following four parts: a serial B cell epitope recombinant protein BI consisting of main neutralizing epitops of O-type foot-and-mouth disease viruses in Cathay, Transasia and Mya 98 pedigrees with a gene sequence of SEQ ID NO:1 and an amino acid sequence of SEQ ID NO:2, a T-cell epitope recombinant protein TI consisting of serial connection of universal T-cell epitope and a plurality of foot-and-mouth disease virus specific T-cell epitopes with a gene sequence of SEQ ID NO:3 and an amino acid sequence of SEQ ID NO:4, Toll-like receptor 3 agonist-polyinosinic acid-polycytidysic acid and / or Toll-like receptor7 / 8 agonist-R848 serving as immunopotentiator, and 201 oil adjuvant. When being used for immunizing a pig, the BI and TI mixed epitope vaccine prepared by utilizing the method can produce a protective immunization effect the same as or better than that of an inactivated influenza virus Vaccines, and has a cross protection effect to viruses of the three pedigrees, so that the vaccine is a novel immune-enhanced O-type foot-and-mouth genetic engineering mixed epitope vaccine.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Epitope screening method capable of exciting anti-mycobacterium tuberculosis protective immunological reaction of body and uses
ActiveCN101289496AHelp predictHelp determineAntibacterial agentsPeptide preparation methodsScreening methodPeptide vaccine
The invention relates to a selection method for epitope which can stimulate the protective immune response of the body anti-mycobacterium tuberculosis and the function thereof, in particular to the molecule mimic peptide of the epitope with vaccine development prospect from the mycobacterium tuberculosis and the coding DNA thereof. The selection method of epitope which can stimulate the protective immune response of the body anti-mycobacterium tuberculosis and the function thereof provides T lymphocyte epitope contained in one important gene Ag85B in the research of mycobacterium tuberculosis and the method of deducting or selecting the epitope, which is beneficial to further developing novel multivalent and poly epitope tuberculosis vaccine and prevent and control the happening and development of tuberculosis; the method makes a foundation of the future development of synthesizing peptide vaccine epitope vaccine and dna vaccine by using epitope and provides molecule mimic peptide of epitope which can stimulate the protective immune response of the body anti-mycobacterium tuberculosis and the peptide has the amino acid sequence of FVRSSNLKFQDAYNA(SEQ ID NO:1).
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
Epitope peptide H362 of HN protein in peste des petits ruminants virus (PPRV), and determination, preparation method and application thereof
ActiveCN107216372AStrong green fluorescenceGood reactogenicitySsRNA viruses negative-senseViral antigen ingredientsF proteinHN Protein
The invention relates to an epitope peptide H362 of an HN protein in PPRV, and determination, a preparation method and application thereof. The amino acid sequence of the epitope peptide is H362: <362>EANWVVPSTDVRDL<375>. The invention detects reactogenicity of a monoclonal antibody and PPRV and specificity of the monoclonal antibody; according to detection results, the monoclonal antibody has good reactogenicity to rPPRV-HN-F protein; immunoinformatic technology is cooperatively used for predicating the B cell epitope of the HN protein; an aminated ELISA plate is employed for detecting candidate epitopes and the monoclonal antibody 10E3, and the epitope peptide H362 corresponding to 10E3 is determined; and determination of the epitope peptide lays a theoretical foundation for preparation of epitope vaccine antigens and diagnostic reagent antigens for PPRV.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Helicobacter pylori multivalent epitope vaccine and preparation method thereof
ActiveCN105169381ASmall molecular weightBiological toxicity avoidanceBacteriaDigestive systemEscherichia coliBiology
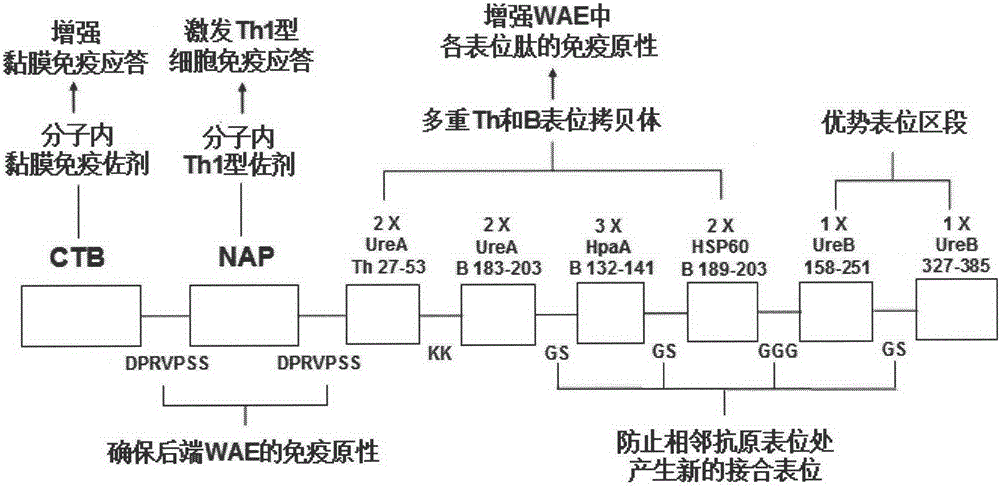
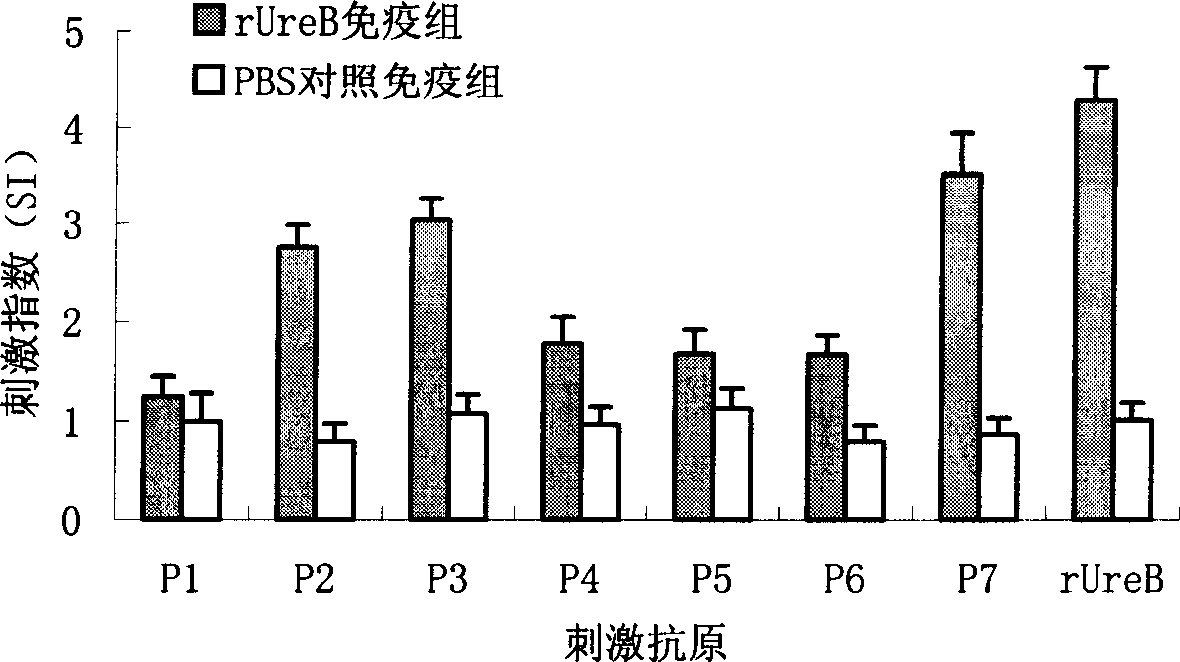
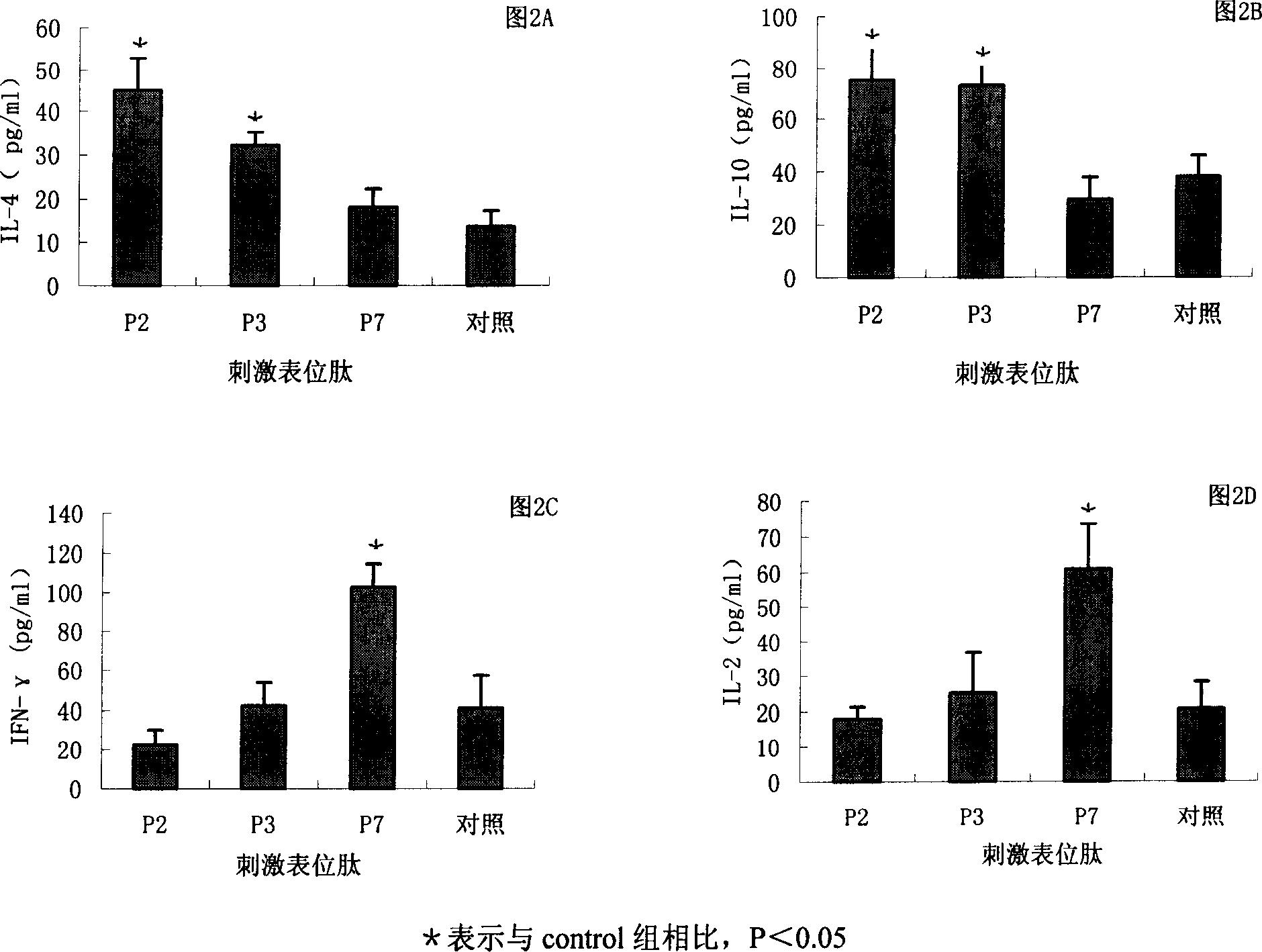
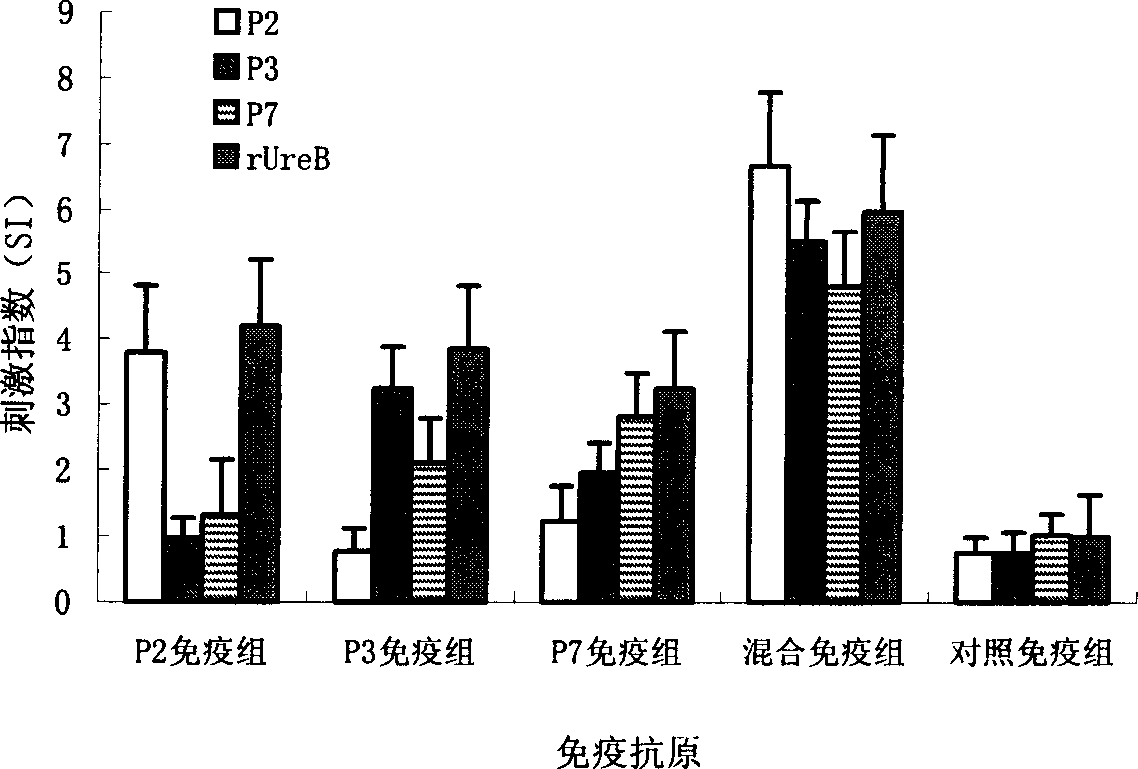
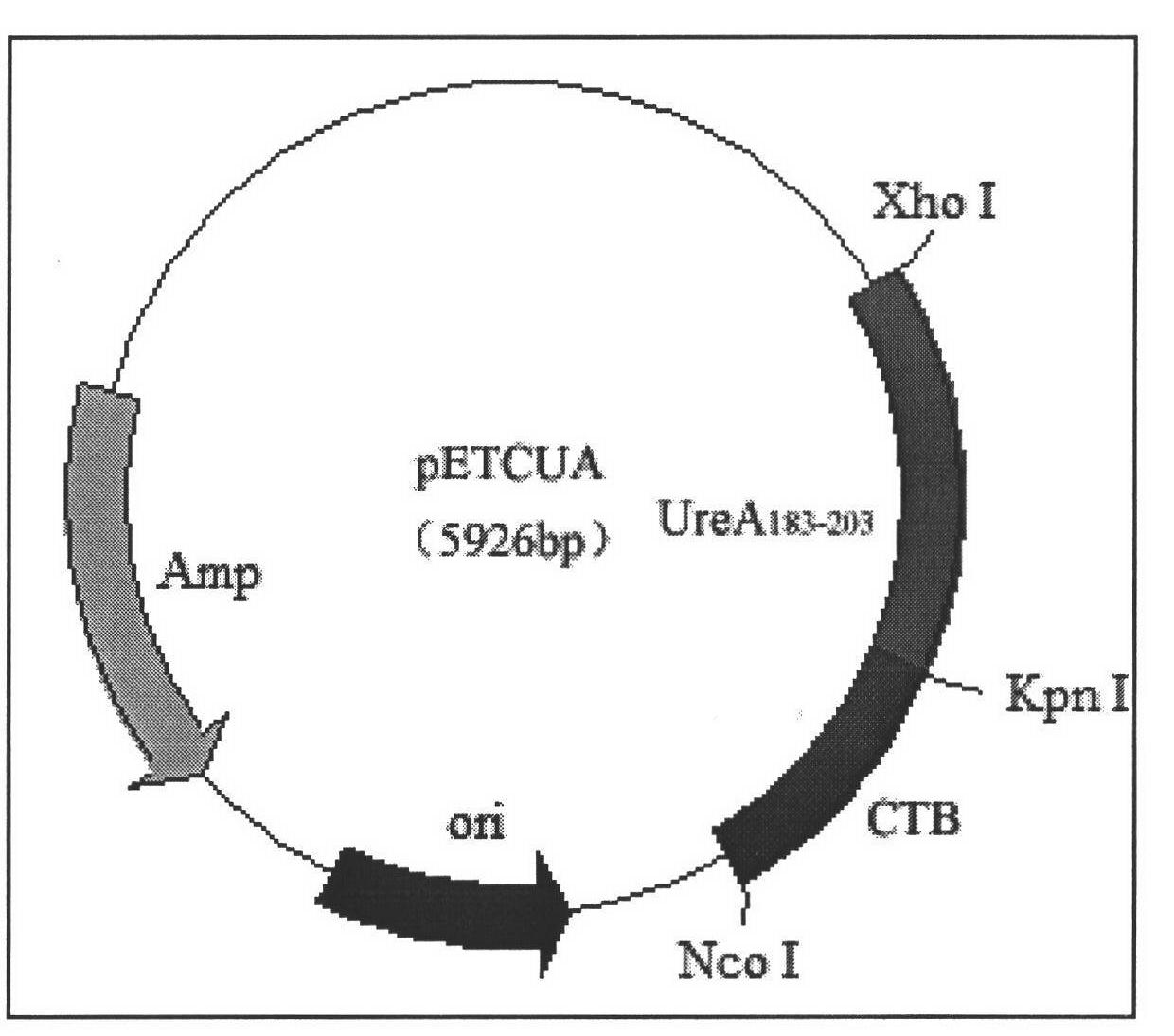
The present invention provides a Helicobacter pylori multivalent epitope vaccine, wherein the activity is a polypeptide, and the polypeptide comprises urease subunit A, urease subunit B, adhesin HpaA, heat shock protein HSP60 advantage Th, B cell epitope or segment, neutrophil activating protein NAP and cholera toxin subunit B. According to the present invention, the artificial gene is synthesized through the gene synthesis technology, and comprises the gene sequences of urease subunit A, urease subunit B, adhesin HpaA, heat shock protein HSP60 advantage Th, B cell epitope or segment and neutrophil activating protein NAP, the artificial gene is coupled to cholera toxin subunit B gene to form a fusion gene, the fusion gene is expressed through escherichia coli, and protein purification is performed to obtain the multivalent epitope vaccine; and the multivalent epitope vaccine can stimulate the body to produce the T cell immune response and the antibody humoral immunoresponse against urease, adhesin HpaA, heat shock protein HSP60 and neutrophil activating protein NAP, and can be used for prevention and treatment of Helicobacter pylori infection-related diseases.
Owner:NINGXIA MEDICAL UNIV
Helicobacter Pylori urease B subunit Th epitope peptide, its coding DNA, vaccine and uses
InactiveCN1807452AHas the effect of clearing HpImproving immunogenicityAntibacterial agentsPeptide/protein ingredientsPylorusHelicobacter pylori gastritis
The invention provides three Th cell epitope peptide and code DNA of pylorus spirillum urease B subunit, also providing a bacterin of B cell epitope which contains the triepitope peptide and an additional urease B subunit. Among them, the epitope peptide is the polypeptide which has one of the amino acid residue sequences as follows: 1) hasing amino acid sequence of sequence 2, sequence3 and sequence 7 in sequence table; 2) replacing, deleting or appending the amino acid sequence of sequence 2, sequence3 and sequence 7 in sequence table by one or several amino acid residue to form derivanting polypeptide. The coded DNA is one of the following sequences: 1) hasing the ribonucleotide sequence of sequence 8, sequence 9 and sequence sequence 10 table. 2) Hasing the ribonucleotide sequence of same coded product with sequence 8, sequence 9 and sequence10 in sequence table. The epitope vaccine which contains the polypeptide of this invention has the protein vaccine of amino acid sequence in sequence 12 of sequence table. The vaccine or medication which is made by using the polypeptide of the invention as active component can clean out the infection caused by pylorus spirillum and has wide application prospect in medical realm.
Owner:ARMY MEDICAL UNIV
CTL (Cytotoxic T Lymphocyte) epitope peptide of foot-and-mouth disease virus type O and screening method of CTL epitope peptide
ActiveCN103864905AImprove bindingConvenient researchSsRNA viruses positive-senseVirus peptidesCtl epitopeDisease
The invention discloses a CTL (Cytotoxic T Lymphocyte) epitope peptide of a foot-and-mouth disease virus type O as well as a screening method and application of the CTL epitope peptide. The CTL epitope peptide is composed of nine amino acid residues, and the amino acid sequence of the CTL epitope peptide is as follows: Ala-Thr-Arg-Val-Thr-Glu-Leu-Leu-Tyr. The epitope peptide has relatively strong combining capacity with SLA (Swineleukocyteantigen)-I proteins from various strains of swine and can induce cytotoxic immune response so as to be suitable for preparing vaccines for preventing and controlling foot-and-mouth disease viruses of various strains of swine and wide in application range. According to the invention, a CTL simulated epitope peptide of a foot-and-mouth disease virus is combined with a single-chain molecule of SLA-I of six strains of constructed swine in vitro, thus a polypeptide which can be combined with a complex can be screened through mass spectrum measurement; in addition, a simulated epitope peptide which can be induced to generate the immune response capacity of T cells is determined through ELISPOT (Enzyme-Linked Immunospot Assay) detection. The invention provides a method for screening and authenticating the CTL epitope of the foot-and-mouth disease virus in a large scale, and lays the foundation for researching and preparing a multi-epitope vaccine of a foot-and-mouth disease.
Owner:DALIAN UNIV
Helicobacter pylori epitope vaccine, design method thereof, preparation method thereof and application thereof
InactiveCN102151332AAntibacterial agentsAntibody medical ingredientsEscherichia coliSpecific antibody
The invention provides a helicobacter pylori epitope vaccine. The active constituent of the helicobacter pylori epitope vaccine is a polypeptide and mainly consists of a cholera toxin B subunit and a B cell antigen epitope from a urease A subunit. A preparation method of the helicobacter pylori epitope vaccine comprises the following steps of: synthesizing the nucleotide sequence of the B cell antigen epitope from the urease A subunit by a PCR (polymerase chain reaction) technology, coupling the nucleotide sequence with the gene sequence of the cholera toxin B subunit to form into a fusion gene, and expressing the fusion gene in the escherichia coli by an expression vector to obtain the fusion protein of the epitope vaccine by means of protein purification. The helicobacter pylori epitope vaccine can be used for inducing the human body to generate an epitope specific antibody which is higher in titer to the urease. In the biologic medical field, the epitope vaccine can be used for preventing and curing the relevant diseases caused by the helicobacter pylori infection, thereby being great in economic benefit and social benefit.
Owner:CHINA PHARM UNIV
Bovine A-type foot-and-mouth disease multi-epitope vaccine, and preparation method and application thereof
The invention discloses a bovine A-type foot-and-mouth disease multi-epitope recombinant vaccine, and a preparation method and an application thereof, and belongs to the field of veterinary vaccine research. The preparation method comprises the following steps: carrying out reasonable serial connection on the dominant antigen epitopes of bovine A-type foot-and-mouth disease virus representative strains once pandemic in China by adopting an antigenized antibody strategy and a brand new reverse vaccinology technology and strategy, coupling with a bovine IgG immunostimulatory fragment (IgG heavy chain constant region), cloning into a prokaryotic expression vector to construct a recombinant expression vector, transforming Escherichia coli cells to express a recombinant antigen, purifying by adopting Ni-NAT column chromatography, quantifying by a Bio-Rad protein quantification kit, and preparing the vaccine individually or combining with a recombinant foot-and-mouth disease virus 3D protein. A result of animal immune experiments shows that the recombinant protein or combined vaccine can stimulate the body to produce a protective antibody with high titer and also can protect immune animals against a virus attack, so the bovine A-type foot-and-mouth disease multi-epitope recombinant vaccine has a good application prospect.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
B cell antigen epitope polypeptide of infectious bronchitis virus isolate S1 protein, and vaccine thereof
ActiveCN105949321AInhibits IBV detoxificationEffective protectionSsRNA viruses positive-senseViral antigen ingredientsB-Cell EpitopesFhit gene
The invention provides a B cell antigen epitope polypeptide of a chicken infectious bronchitis virus Shanghai 1208 isolate S1 protein, and belongs to the field of gene and protein engineering. The B cell antigen epitope polypeptide can be combined to prepare an IBV multi-epitope vaccine. The IBV B cell antigen epitope can cause high-level immunoresponse.
Owner:SHANGHAI VETERINARY RES INST CHINESE ACAD OF AGRI SCI
Rabies virus glycoprotein and nucleoprotein antigen epitope polypeptides, and screening and identification method and application thereof
The invention belongs to the technical field of biology, and particularly relates to screening and identification of antigen epitope polypeptides. The invention discloses screening, identification and application of a series of rabies virus glycoprotein and nucleoprotein antigen epitope polypeptides. The rabies virus glycoprotein and nucleoprotein are predicted by biological information means to obtain the candidate epitope polypeptides; and a lymphopoiesis experiment, ELISPOT experiment and a stream-type cell method are utilized to carry out in-vitro experimental verification on the subsequent epitope polypeptides to obtain the four rabies virus protein antigen epitope polypeptides. The invention is characterized in that the antigen epitope polypeptides respectively comprise a Th epitope and a CTL epitope, can stimulate the lymphopoiesis of the vaccine-immunized mouse in vitro and induce the cells to secrete related cell factors, and have the functions of killing virus-infected cells and stimulating the generation of the antibody. The invention can be used for developing rabies virus epitope vaccines and detecting the vaccine effect, and has important value for developing and producing immunologic function detection kits for rabies virus vaccines.
Owner:FUDAN UNIV
Epitope delivery system with Escherichia coli heat labile enterotoxin B subunits serving as carriers
The invention relates to design techniques of an antigenic epitope delivery system and preparation purification techniques, and relates to application of the antigenic epitope delivery system to epitope vaccine development and preparation. An epitope delivery system with Escherichia coli heat labile enterotoxin B subunits serving as carriers is characterized in that Escherichia coli heat labile enterotoxin B (LTB) subunits are connected with specific peptides by means of recombinant DNA (deoxyribose nucleic acid) technology, and then the corresponding B lymphocyte epitope or T lymphocyte epitope is connected to the LTB subunits. According to experiments, the B lymphocyte epitope carried by the antigenic epitope delivery system can effectively impel animals to generate antibodies aiming at the epitope; and the T lymphocyte epitope carried by the antigenic epitope delivery system can effectively impel animals to generate CD8 lymphocyte cells aiming at the epitope. Therefore, the antigenic epitope delivery system can play a significant role in development of epitope vaccines.
Owner:冯强
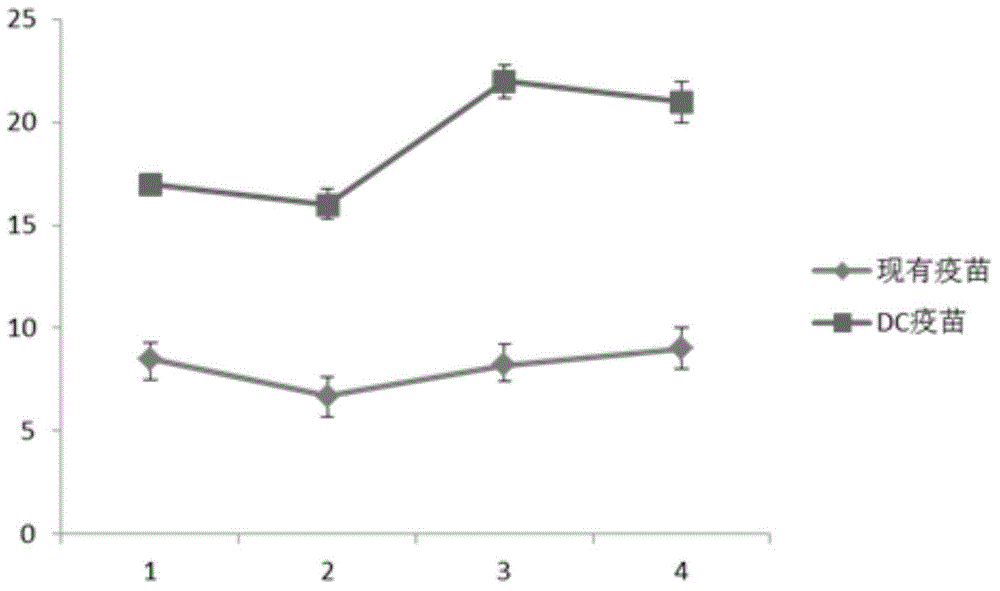
DC-based HCV epitope vaccine and preparation method thereof
InactiveCN105031641ARegulate the body's immune mechanismStable traitsAntiviralsBlood/immune system cellsCD34Epitope vaccine
The invention relates to a DC-based HCV epitope vaccine and a preparation method thereof. The preparation method comprises 1, carrying out CD34<+> and / or CD14<+> cell induced culture to obtain immature DC, 2, adding linear polypeptide comprising HCVNS3 and HCVNS5 polypeptide chains into the immature DC and carrying out co-culture, and 3, adding a tumor necrosis factor-alpha and interleukin or lipopolysaccharide into the culture and carrying out culture for 7-13 days until the DC is mature. The DC-based HCV epitope vaccine has stable properties and strong immunogenicity and can well adjust a HCV virus-resistant body immunization mechanism.
Owner:SHEN ZHEN ISTEM REGENERATIVE MEDICINE SCI TECH CO LTD
Helicobacter pylori multiple-epitope fusion protein and multiple-epitope vaccine prepared by helicobacter pylori multiple-epitope fusion protein
InactiveCN102838680AAntibacterial agentsBacterial antigen ingredientsSpecific iggHelicobacter pylori
The invention relates to a helicobacter pylori multiple-epitope fusion protein and a multiple-epitope vaccine prepared by the helicobacter pylori multiple-epitope fusion protein. An amino acid sequence of the helicobacter pylori multiple-epitope fusion protein is shown as SEQ ID NO:1. Fusion proteins in the multiple-epitope vaccine can induce generation of specificity sIgA in stomach tissues and formation of phigh-potency specificity IgG in blood serum, can effectively reduce constant value quantity of helicobacter pylori in a stomach of a mouse and has obvious protection effects.
Owner:ARMY MEDICAL UNIV
Multi epitope vaccine for poultry
InactiveUS20110078828A1Low costMany timesPeptide/protein ingredientsProtozoaPoultry diseaseCoccidiosis
Antigenic polypeptides, capable of inducing an immune response against multiple parasites, and methods of designing such polypeptides, are provided. Also provided by the invention are polynucleotides encoding such polypeptides, as well as recombinant vectors and transformed host cells containing the said polynucleotides. Oral administration and intramuscular injection of the polypeptides provides vaccination protection against infection from Eimeria parasites that result in the poultry disease coccidiosis.
Owner:GUARDIAN BIOTECHNOLOGIES
Epitope vaccine for resisting A/B subgroup avian leucosis virus infection and preparation method and application of epitope vaccine
ActiveCN104548087ALow costEase of mass productionAntiviralsAntibody medical ingredientsLeucosisNucleotide
The invention relates to the field of animal virology and immunology, and provides an epitope vaccine for resisting A / B subgroup avian leucosis virus infection. The epitope vaccine is prepared by highly active recombinant protein His-cENV which is obtained through screening and purification after prokaryotic expression and a freund's adjuvant in a united manner, wherein the nucleotide sequence for encoding the recombinant protein His-cENV is shown as SEQ ID NO.1. Through the epitope vaccine, 7-day-old breeding poultry chicks are immune and can produce 1:128000 neutralizing antibodies. Vitro virus neutralization experiments and animal experiments show that the epitope vaccine can neutralize different ALV-A / B isolated strains, so that chicken flocks are effectively protected to resist the infection of ALV-A / B strains. Because the epitope vaccine is based on a multi-epitope antigen gene sequence which is originated form env, the defect of ALV-A / B virus variation is overcome, a new era of the ALV-A / B vaccine is opened, a new way of resisting the ALV-A / B infection is provided, and a technical support for preventing and controlling the ALV-A / B is provided.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
EGFR and HER2 combined polypeptide epitope vaccine
InactiveCN102357246AHigh antibody titerLow antibody titerAntibody medical ingredientsHybrid peptidesB-Cell EpitopesEpitope vaccine
The invention discloses an EGFR and HER2 combined polypeptide epitope vaccine; two B cell epitope mimic polypeptide I and II of HER2 of the HER family, and a B cell epitope mimic polypeptide of EGFR of the HER family are inserted in a serial form between the 78th amino acid and the 79th amino acid of a hepatitis B core antigen HBcAg so as to form fusion protein which is the combined polypeptide epitope vaccine. The EGFR and HER2 combined polypeptide epitope vaccine of the invention breaks through the limitations of existing single epitope vaccines, and the combined epitope vaccine is providedwith more extensive antineoplastic activity.
Owner:JIANGSU PROVINCE INST OF TRADITIONAL CHINESE MEDICINE
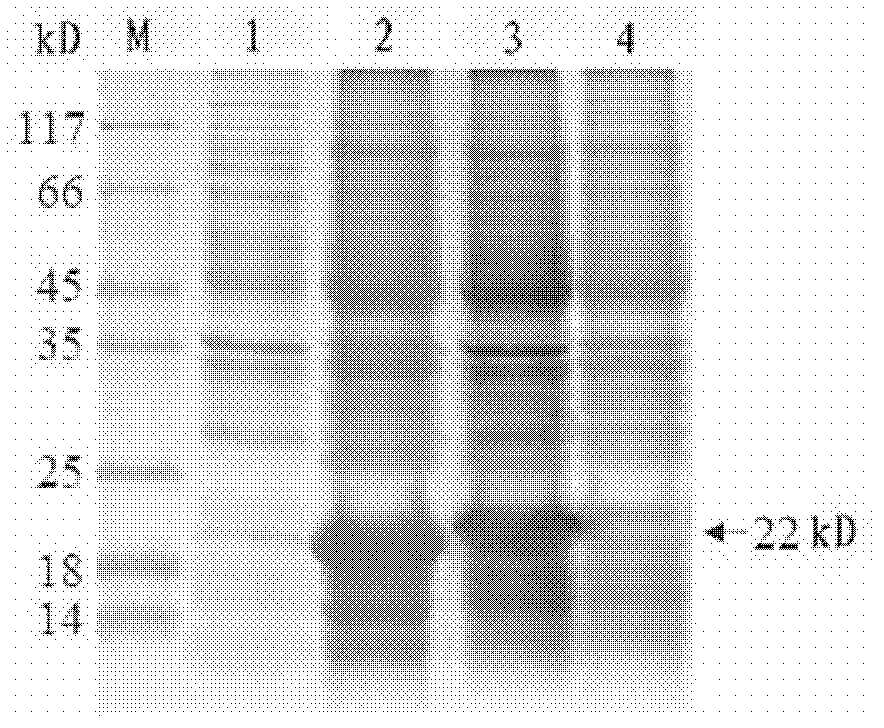
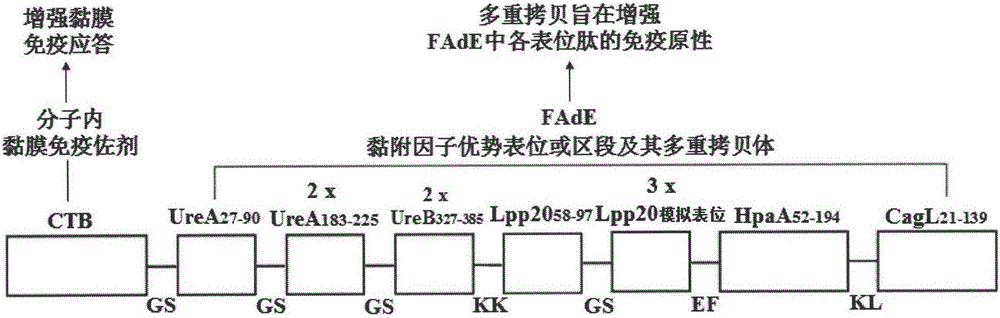
Helicobacter pylori tetravalent adhesion multi-epitope vaccine and preparation method thereof
ActiveCN105126093ABiological toxicity avoidanceProtect against immunopathological damageAntibacterial agentsPeptide preparation methodsEscherichia coliDisease
The invention provides a multi-epitope vaccine for a helicobacter pylori tetravalent adhesion, wherein the activity of the multi-epitope vaccine is presented as a polypeptide which mainly consists of urease A and B subunits, superior Th and B cell epitopes or fragments of three outer membrane proteins (Lpp20, HpaA and CagL) as well as cholera toxin B subunit. According to the invention, an artificial gene is synthesized by virtue of gene synthesis technology, wherein the synthesized artificial gene consists of urease A and B subunits, and superior Th and B cell epitopes or fragments of three outer membrane proteins (Lpp20, HpaA and CagL), and the artificial gene is coupled with gene sequence of the cholera toxin B subunit, so as to form a fusion gene. The fusion gene is expressed by escherichia coli, and upon protein purification, the tetravalent adhesion multi-epitope vaccine is obtained. The vaccine can be used for inducing a body to generate T cellullar immunologic response and specific antibody humoral immune response in accordance with the urease A and B subunits and the three outer membrane proteins (Lpp20, HpaA and CagL); and the vaccine is suitable for preventing and controlling helicobacter pylori infection related diseases.
Owner:NINGXIA MEDICAL UNIV
Infectious bovine rhinotracheitis virus gD protein antigen epitope polypeptide, and inhibitor, monoclonal antibody and application of polypeptide
InactiveCN107586322AVirus peptidesImmunoglobulins against virusesAdjuvantInfectious bovine rhinotracheitis virus
The invention belongs to the field of molecular biology and medicine, and specifically discloses an infectious bovine rhinotracheitis virus gD protein antigen epitope polypeptide and an application ofthe polypeptide in preparation of a reagent or a medicament for detecting or treating infectious bovine rhinotracheitis. The invention provides a monoclonal antibody for resisting the infectious bovine rhinotracheitis virus gD protein, and simultaneously the antigen epitope of the infectious bovine rhinotracheitis virus gD protein is screened out as <323>GEPKPGPSPDADRPE<337> (the shortest epitopesequence is 7 amino acid peptide fragments: <323>GEPKPGP<329>). The recombinant protein based on the antigen epitope can specifically be used to detect infectious bovine rhinotracheitis serum; in addition, a small-molecule inhibition drug designed based on the antigen epitope can block virus infection; and meanwhile, the multi-copy repeated epitope vaccine constructed on the basis of the antigenepitope can induce a high-titer gD protein antibody under the assistance of an appropriate adjuvant, and has a relatively high neutralizing antibody titer. The invention lays a foundation for establishing a detection method and researching and developing vaccines for the infectious bovine rhinotracheitis.
Owner:HAINAN UNIVERSITY
Epitope polypeptide combination capable of inducing immunity and application thereof
PendingCN113372417AVerify immune response propertiesHighly conservativeSsRNA viruses positive-senseViral antigen ingredientsAntigen epitopeCtl epitope
The invention discloses an epitope polypeptide combination capable of inducing immunity and application thereof, belongs to the technical field of biology, and aims to carry out molecular design of related vaccines by utilizing immunoinformatics on the basis of epitope analysis optimization. Based on a structural antigen epitope vaccine design strategy, a B cell epitope, a Th epitope and a CTL epitope on a new coronavirus S protein are determined through immunoinformatics to induce a main neutralizing antibody, activate cellular immune response and induce body fluid and cellular immune balance. The epitope vaccine is designed through connection of a molecular adjuvant and candidate antigen epitopes, antigenicity, physicochemical properties, protein secondary structure and tertiary structure modeling of the epitope vaccine are analyzed, vaccine conformation B cell epitopes are analyzed by means of a structural biological tool, and immune response characteristics of the vaccine are verified through molecular docking with TLR4 and immune response simulation stimulation. Information analysis results show that the designed candidate epitope combination has well balanced humoral immune and cellular immune response capabilities.
Owner:SHANTOU UNIV MEDICAL COLLEGE
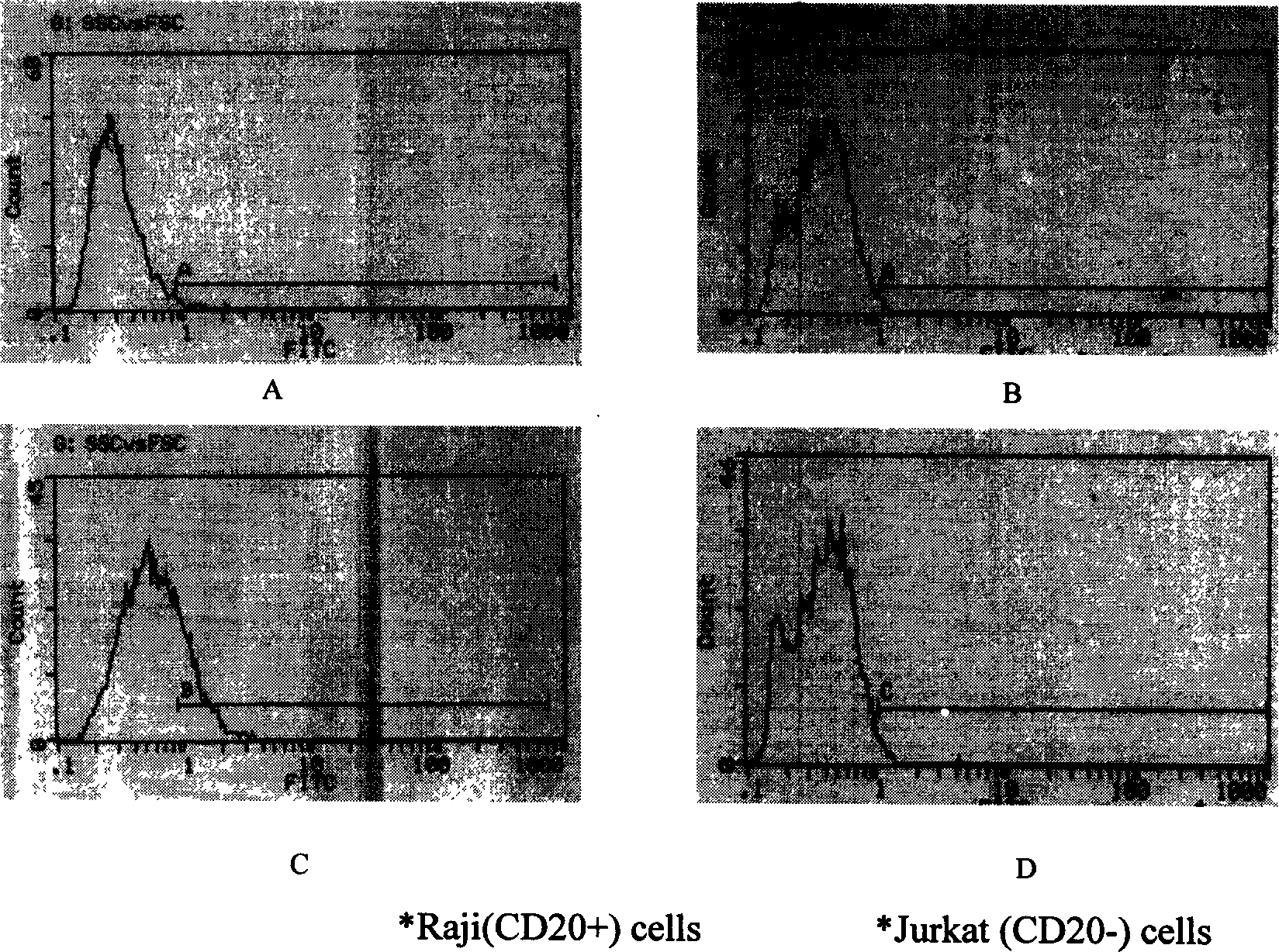
12 amino acid analog epi-position of human b cell specificity membrane molecule CD20 and polypeptide epi-position vaccine configurated by said analog epi-position
InactiveCN1786021AEfficient killingReduce the burden onPeptidesAntibody ingredientsChemical synthesisCD20
The present invention discloses a simulation epitope of 12 amino acids of human cell B specificity expression membrane molecule CD 20 and its polypeptide epitope vaccine constructed by using said simulation spitope. Said invention also provides a method for screening simulation epitope of CD 20 molecule by using Rituximab as ligand, and provides its amino acid sequence, Gln-Asp-Lys-Leu-Th-Gln-Try-Pro-Lys-Try-Leu-Glu. Said invention also provides a method for chemically-synthesizing simulation epitope of 12 amino acids and making it be chemically-coupled with keyhole limpet hemo cyanin (KLH) to obtain the successfully-constructed vaccine. Besides, said invention also provides the concrete application of said vaccine.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
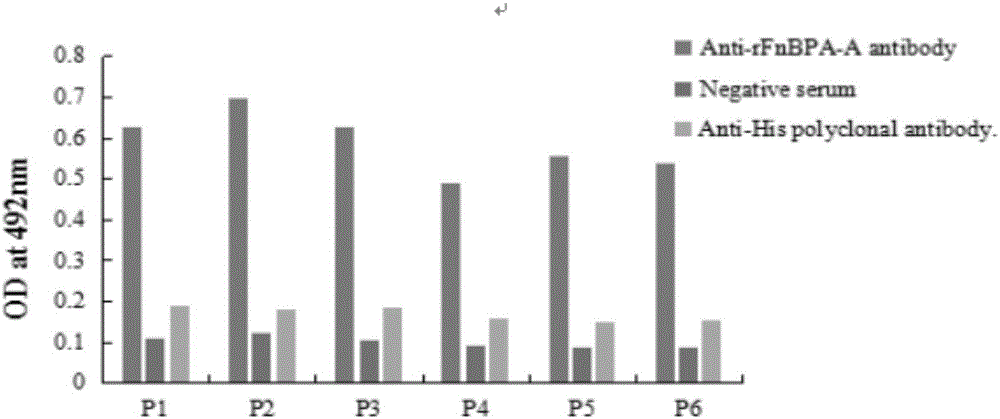
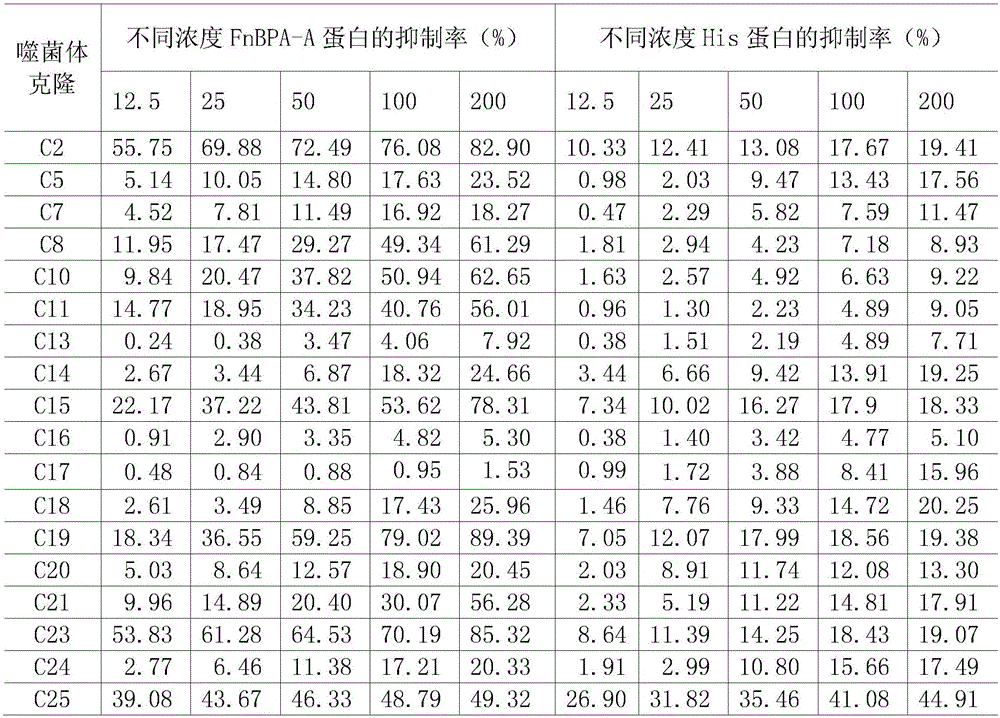
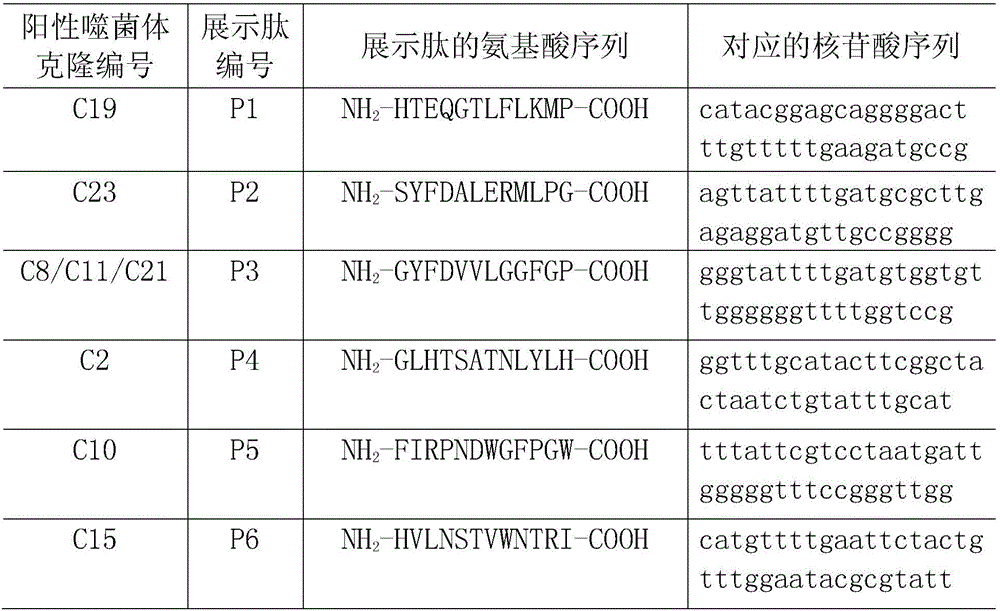
Staphylococcus aureus FnBPA-A protein mimic epitope peptides having immunizing protection, mimic epitope peptide composition, and applications of mimic epitope peptides and mimic epitope peptide composition
ActiveCN106478777AEffective combinationEffective adhesionAntibacterial agentsAntibody mimetics/scaffoldsStaphylococcus cohniiMass ratio
The invention relates to staphylococcus aureus FnBPA-A protein mimic epitope peptides having immunizing protection, a mimic epitope peptide composition, and applications of the mimic epitope peptides and the mimic epitope peptide composition. Two immunoprotective mimic epitope peptides provided by the invention have the amino acid sequences respectively shown in SEQ ID NO:1 and SEQ ID NO:2, the mimic epitope peptide composition consists of two polypeptides shown in SEQ ID NO:1 and SEQ ID NO:2, and the mass ratio of the polypeptide shown in SEQ ID NO:1 to the polypeptide shown in SEQ ID NO:2 is 2 to 1. Experiment animal immunoprotective tests show that the two mimic epitope peptides can stimulate a body to produce high-level specific antibodies and have a certain degree of immunizing protection; moreover, the mimic epitope peptide composition has the immunoprotective effect on staphylococcus aureus infection better than that of an FnBPA-A holoprotein. Therefore, the mimic epitope peptides and the composition thereof can be used as effective components for development of multi-epitope vaccines of staphylococcus aureus and prevention of cow mastitis caused by staphylococcus aureus.
Owner:ANHUI AGRICULTURAL UNIVERSITY
O-type foot-and-mouth disease multi-epitope vaccine with cross immunity protective efficiency
The invention relates to preparation and application of a multi-epitope vaccine with cross immunity protective efficiency to O-type foot-and-mouth disease viruses such as OZK / 93, OR / 80MF8, OS / 99MF8, OHK93 and the like. The vaccine contains two sections of T cell auxiliary antigenic epitope polypeptides, 4-7 sections of antigenic epitope polypeptides related to main outer membrane proteins VP1, VP2 and VP3 of different foot-and-mount disease strains. The invention also relates to a preparation method of the vaccine and a clinical immunology application method. The vaccine has stable production preparation process and is applicable to mass production, experiments show that the multi-epitope vaccine is safe to use, infection of different O-type foot-and-mouth circulating strains can be effectively prevented and effective antibody titer can last for at least seven months.
Owner:QINGDAO MINGQIN BIOLOGICAL TECH CO LTD
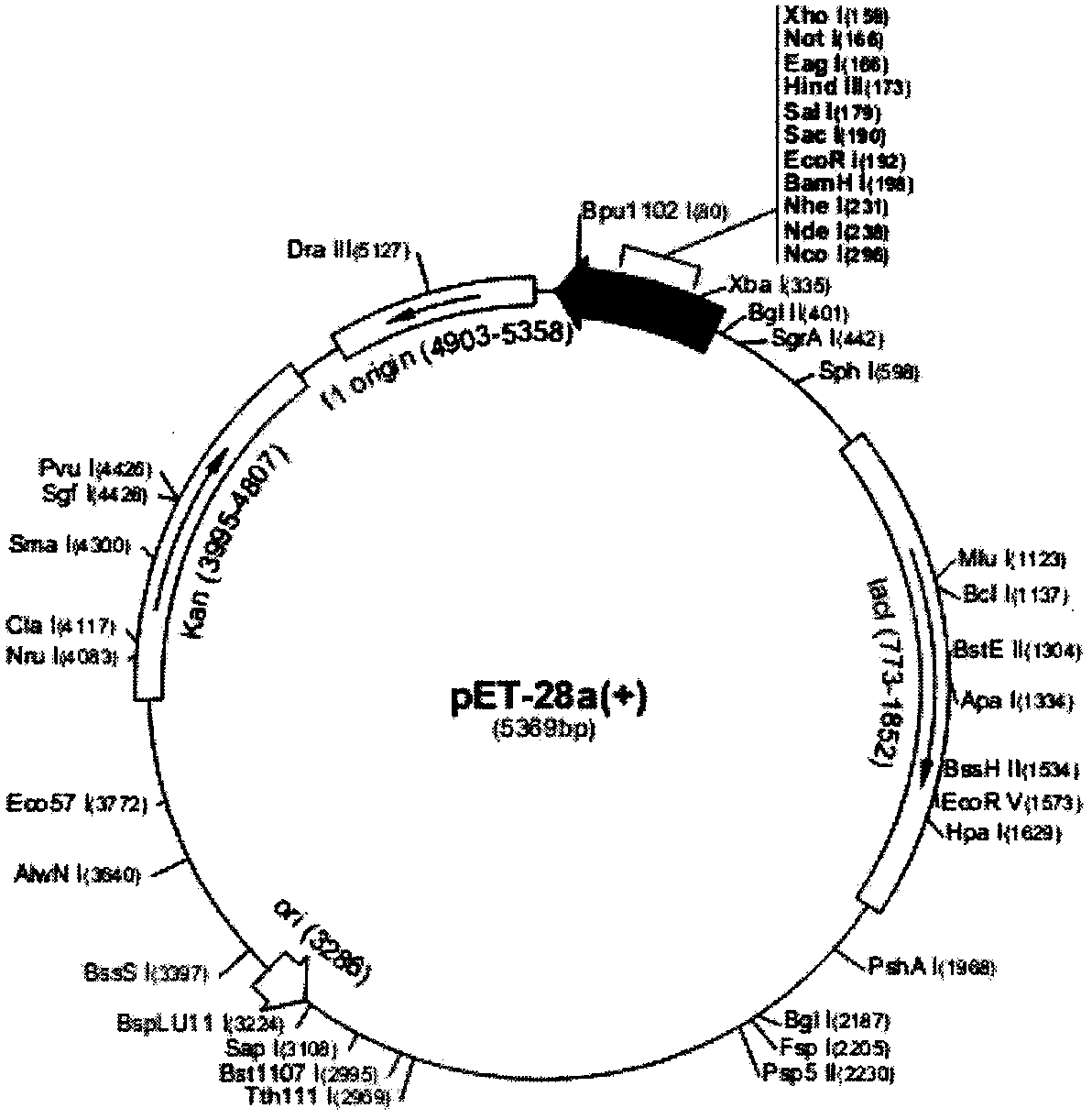
Bovine viral diarrhea virus (BVDV) E2 protein antigen multi-epitope fusion peptide as well as preparation and application thereof
ActiveCN108614121AAccurate diagnosisAccurate immune evaluationSsRNA viruses positive-senseAntibody mimetics/scaffoldsEscherichia coliBovine Viral Diarrhea Viruses
The invention discloses a bovine viral diarrhea virus (BVDV) E2 multi-epitope fusion peptide and a preparation method thereof. The preparation method comprises the following steps: performing gene synthesis on two respective epitope tandem sequences of optimized E2 proteins such as BVDV-1 and BVDV-2, cloning the sequences to a pET-28a (+) vector, transforming Escherichia coli DH-5alpha, transforming Escherichia coli BL21 from an accurate recombinant vector, performing IPTG inducible expression, and performing Ni-NTA purification, so as to obtain the BVDV E2 multi-epitope fusion peptide. Afterbeing subjected to animal immunization, the E2 multi-epitope fusion peptide is capable of producing a neutralizing antibody of the BVDV, and can be used for research and development of BVDV epitope vaccines; an ELISA (Enzyme-linked Immuno Sorbent Assay) detection kit prepared by utilizing the E2 multi-epitope fusion peptide can be used for BVDV antibody detection of cattle, and has the advantagesof being high in specificity, high in sensitivity, excellent in repeatability and easy to operate.
Owner:SHANDONG NORMAL UNIV
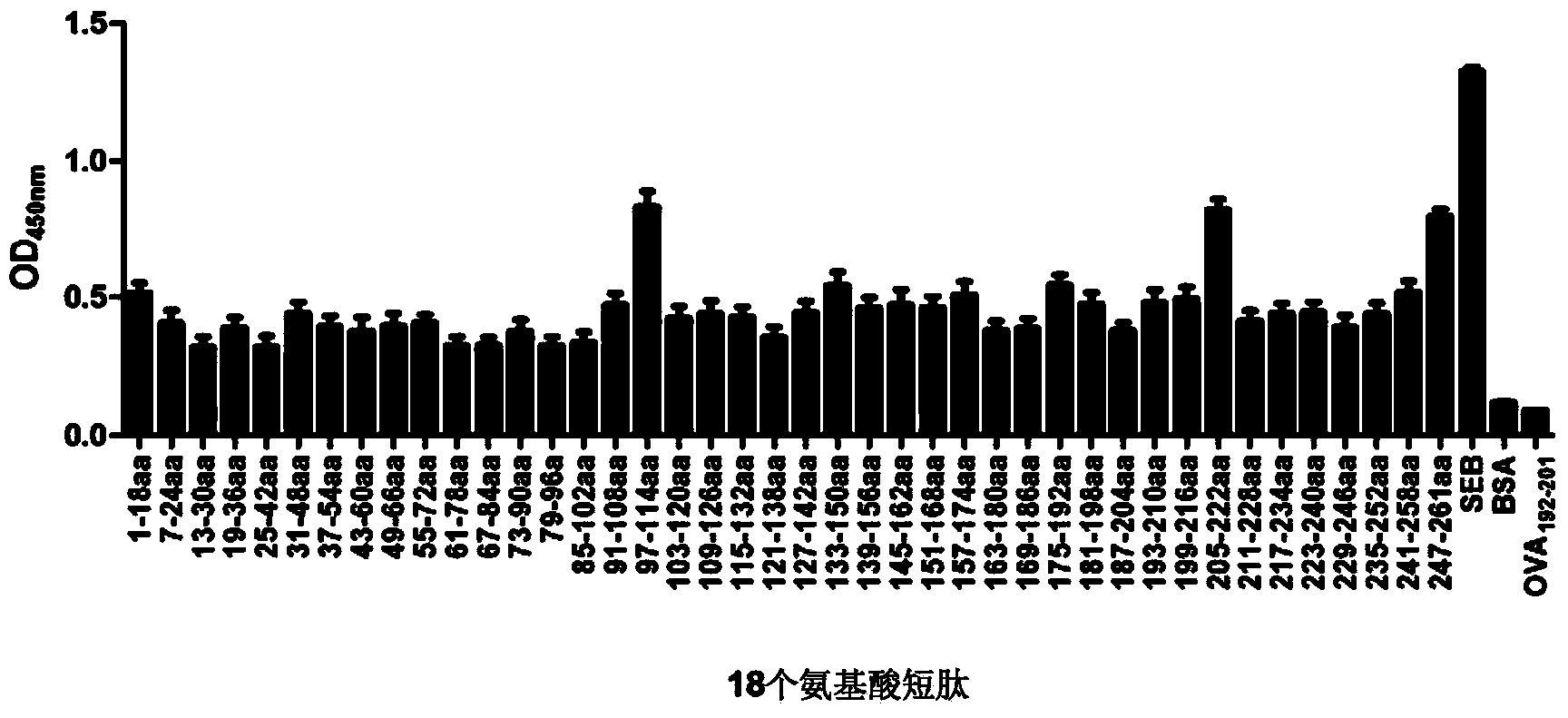
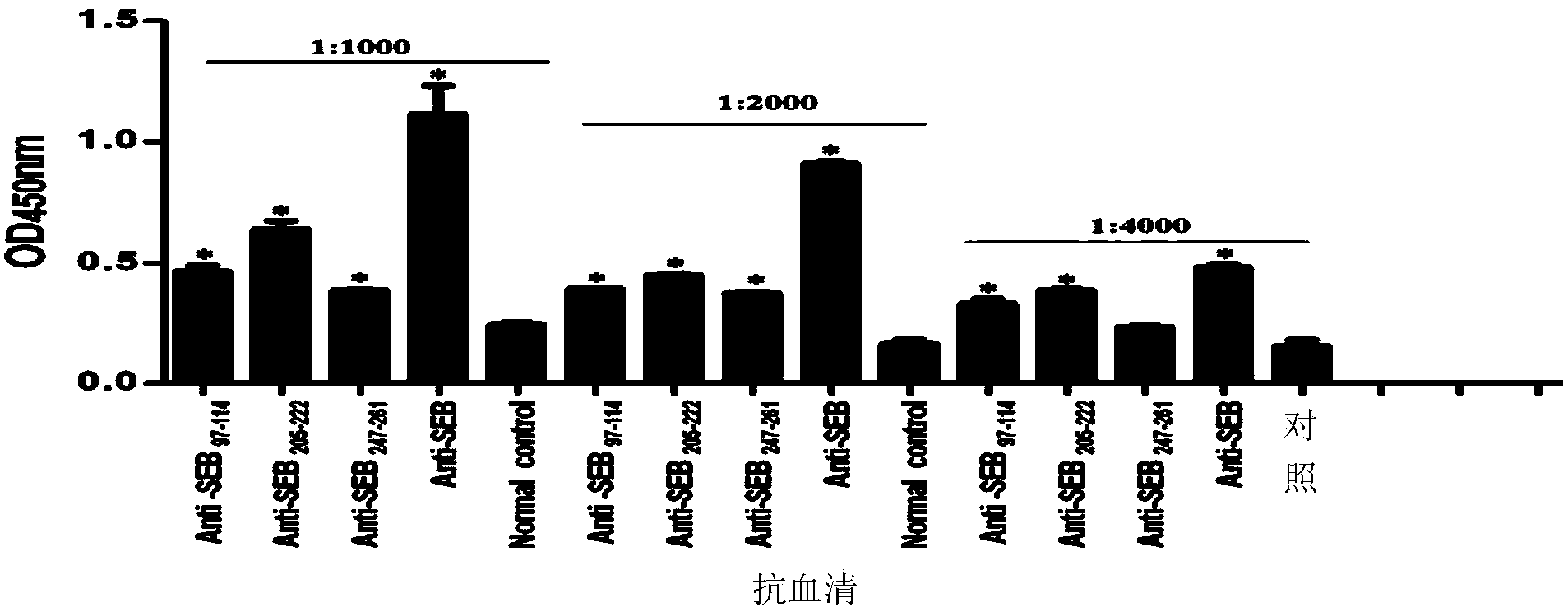
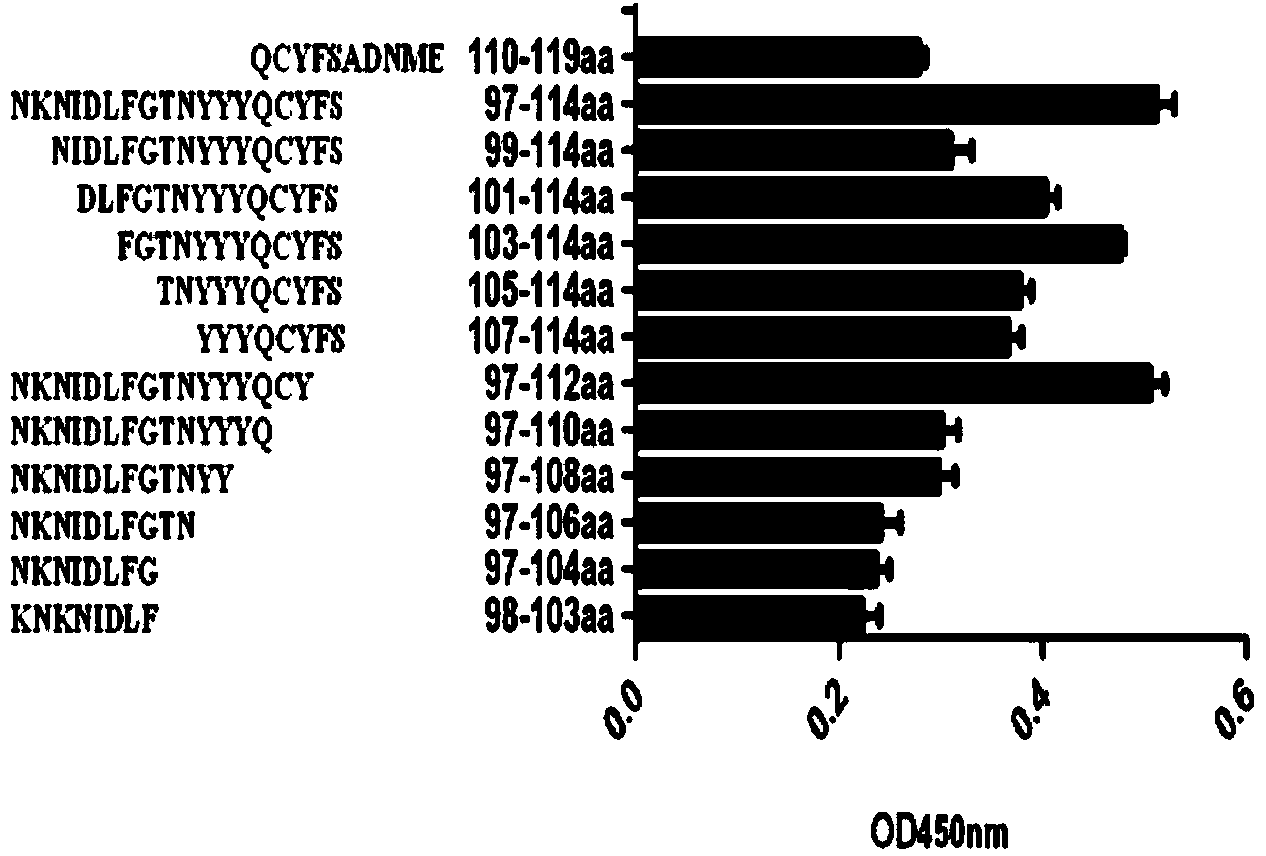
B cell immunodominant epitope peptide of staphylococcus aureus enterotoxin B and preparation method and application thereof
InactiveCN103772493AImproving immunogenicitySimple methodAntibacterial agentsBiological material analysisStaphylococcus aureus enterotoxin BImmunodominance
The invention relates to a B cell immunodominant epitope peptide of staphylococcus aureus enterotoxin B and a preparation method and application thereof, and concretely provides a B cell immunodominant epitope peptide of staphylococcus aureus enterotoxin B. Amino acid sequences of the B cell immunodominant epitope peptide are shown in SEQ ID NO: 17, 35 and 42. Corresponding amino acid sequences of core epitopes of the B cell immunodominant epitope peptide are respectively shown in SEQ ID NO: 48, 54 and 69. According to the preparation method, the B cell immunodominant epitope peptide of the staphylococcus aureus enterotoxin B is assayed by an overlapping peptide binding titration method, so that the screening method is simple, convenient, quick and accurate and is free from omission. The immunodominant epitope peptide does not contain unnecessary or even harmful parts, so that the risk of vaccines prepared from the immunodominant epitope peptide during use is lowered; the immunodominant epitope peptide has a relatively high vaccine application value and can be applied to the preparation of epitope vaccines and / or prevention vaccines of the staphylococcus aureus enterotoxin B.
Owner:ARMY MEDICAL UNIV
Anti-influenza A virus and novel universal epitope vaccine and preparing method thereof
The invention discloses a method for preparing anti-influenza A virus and novel universal epitope vaccine. The method comprises the following steps: combining a network server and related software to predict functional epitopes of NP, M1 and HA that are related with influenza protection antigen via a bioinformatics method, obtaining a total of 8 epitopes from H1N1, H3N1, H3N2, H5N1, H5N2 subtype CTL epitope and a B-cell epitope, and adding a flexible segment GPGPG between every two of the epitopes to act as linker and naming the linked product as HMN; linking a nucleotide sequence that corresponds to the epitope polypeptide to a plasmid carrier, performing prokaryotic expression, and adding an adjuvant to the purified expressed protein to prepare the vaccine, wherein the vaccine is used to perform immunization in mice. Western blot test proves that the recombinant polypeptide is effective in gene expression and has antigenicity. The T-lymphocyte subset index of the test group is higher than that of the control group in the mice immunization test, and the result indicates that the polypeptide can induce BALC / c mice to generate specific humoral and cellular immune responses specific to the selected epitopes and proves that the polypeptide has strong immunogenicity.
Owner:HENAN AGRICULTURAL UNIVERSITY
Human metapneumovirus multi-epitope antigen and application thereof
The invention relates to a human metapneumovirus multi-epitope antigen and an application thereof. The human metapneumovirus multi-epitope antigen is formed by combining B cell epitope sequences, Th cell epitope sequences and CTL cell epitope sequences. The epitope sequences in various types are connected in series through connexon. The invention further provides preparing, series connection and expression of human metapneumovirus B cell epitope gene segments, CTL cell epitope gene segments and Th cell epitope gene segments, the human metapneumovirus multi-epitope antigen is prepared, and the cellular-immunity and humoral-immunity activating response level of the human metapneumovirus multi-epitope antigen is evaluated. An evaluation experiment indicates that the human metapneumovirus multi-epitope antigen can activate CTL immunity and Th immunity in cell immunity and can also activate humoral immunity, the certain neutralization effect on hMPV is achieved during an in-vitro experiment, and the human metapneumovirus multi-epitope antigen can be used for developing epitope vaccine, establishing an immunology diagnosis method, researching disease epidemiology, carrying out hMPV infection immunity treatment and the like.
Owner:TIANJIN CENT FOR DISEASE CONTROL & PREVENTION
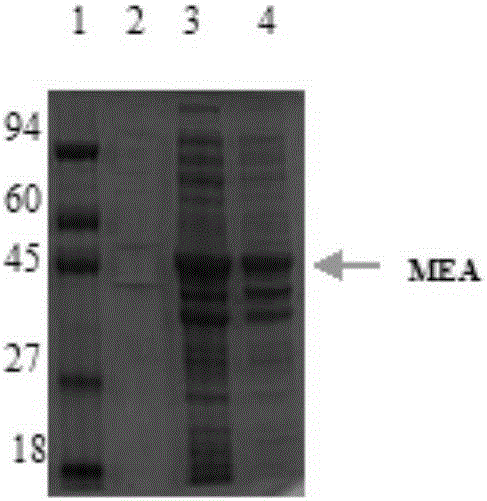
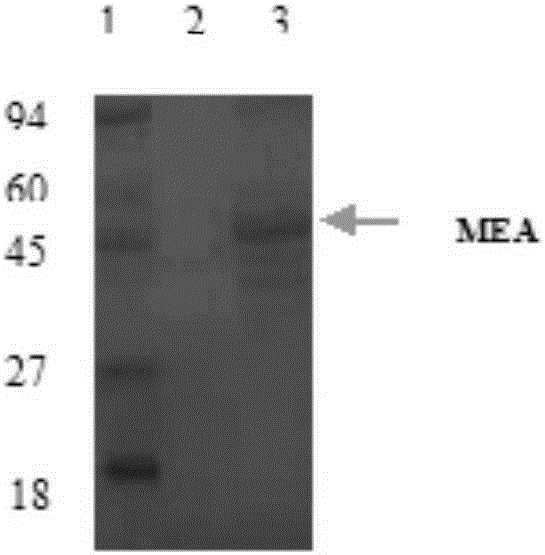
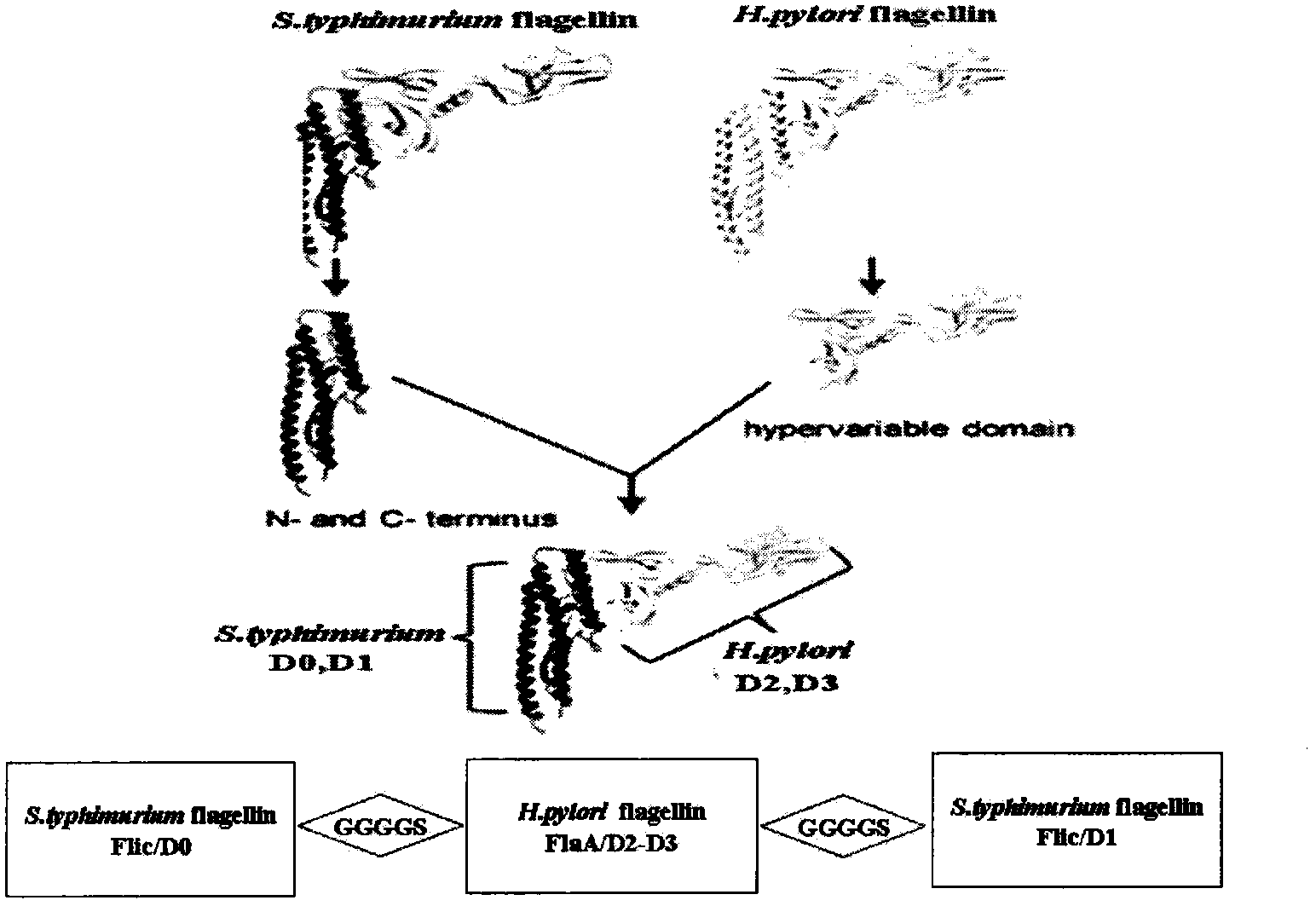
Novel chimeric flagellin adjuvant of helicobacter pylori multi-epitope vaccine
The invention provides a novel chimeric flagellin adjuvant of a helicobacter pylori multi-epitope vaccine. An active ingredient of the novel chimeric flagellin adjuvant of the helicobacter pylori multi-epitope vaccine is a polypeptide chain which mainly consists of regions D0 and D1 of salmonella typhimurium flagellin Flic, and regions D2 and D3 of helicobacter pylori flagellin F1aA. A nucleotide sequence of the protein adjuvant is synthesized through a PCR (polymerase chain reaction) technology, expressed in escherichia coli by utilizing an expression vector, and the adjuvant protein is obtained after protein purification.
Owner:CHINA PHARM UNIV
Epitope vaccine for resisting subgroup J avian leukosis virus infection as well as preparation method and application of epitope vaccine
ActiveCN104306995ALow costEase of mass productionGenetic material ingredientsAntiviralsNucleotideAvian leukosis viruses
The invention relates to the fields of animal virology and immunology, and provides an epitope vaccine for resisting subgroup J avian leukosis virus infection. The vaccine is prepared from screened and purified high-activity recombinant protein His-cENV after prokaryotic expression with the combination of a freund's adjuvant, wherein the nucleotide sequence of the encoding recombinant protein His-cENV is as shown in SEQ ID NO.1. 7-day breeding poultry chicks by utilizing epitope vaccine immunity can produce neutralizing antibodies of 1 to 128000; in-vitro virus neutralization experiments and animal experiments show that the epitope vaccine can neutralize different ALV-J separation strains, and effectively protect the chicks so as to resist ALV-J strain infection. Env source based multi-epitope antigen gene sequence of the epitope vaccine overcomes the virus variation of ALV-J, develops the ALV-J vaccine new era, provides a new means for resisting ALV-J infection, and provides technical support for ALV-J prevention and control.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
Eukaryotic expression method of fish viral hemorrhagic septicemia virus G gene
InactiveCN105132438AIncrease productionImmunogenicDepsipeptidesGenetic engineeringMolecular diagnostic techniquesViral hemorrhagic septicemia
The invention discloses a eukaryotic expression method of a fish viral hemorrhagic septicemia virus G gene. The method includes the following steps of (1) gene synthesis of a main antigenic domain optimized by codons, (2) construction of a reconstructed baculovirus transfer vector, (3) construction of shuttle plasmids and (4) analysis and identification of reconstructed Bacmid transfection and expression products. By means of the method, efficient expression of the G protein antigenic domain in a Bac-to-Bac baculovirus system; the activity of the expression protein is primarily identified and analyzed through Western blotting, it is proved that the expression protein has immunogenicity, and a foundation is provided for further epitope vaccine development and VHSV molecular diagnosis technology establishment.
Owner:INSPECTION & QUARANTINE TECH CENT SHANDONG ENTRY EXIT INSPECTION & QUARANTINE BUREAU
American-type PRRSV N protein antigenic epitope and applications thereof
InactiveCN109554375APerfect epitope mapHighly conservativeSsRNA viruses positive-senseViral antigen ingredientsEpitope vaccineBioinformatics
The invention discloses an American-type PRRSV N protein antigenic epitope and applications thereof, and aims to solve the technical problem of insufficient research on PRRSV N protein cell epitopes.The invention designs a short peptide, and the amino acid sequence of the short peptide is as shown in SEQ NO.1 or is an amino acid sequence which is derived from the amino acid sequence as shown in the SEQ ID NO.1 and has the same function. The American-type PRRSV N protein antigenic epitope is screened, and comprises the amino acid sequence of the short peptide. The short peptide or the American-type PRRSV N protein antigenic epitope are applied in preparing an antibody for preventing and / or treating American-type PRRSV. The invention provides a novel N-protein antigenic epitope, completes the identification of the epitope, improves the epitope map of the N protein, lays a foundation for the research of American-type PRRSV cross-protective epitope vaccines, and has great significance fordifferentiated detection on American-type PRRSV and European-type PRRSV.
Owner:ZHENGZHOU UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com