Novel chimeric flagellin adjuvant of helicobacter pylori multi-epitope vaccine
A technology of Helicobacter pylori and flagellin, which is applied in the field of biomedicine, can solve the problems of stimulating mucosal immune response and recognition, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
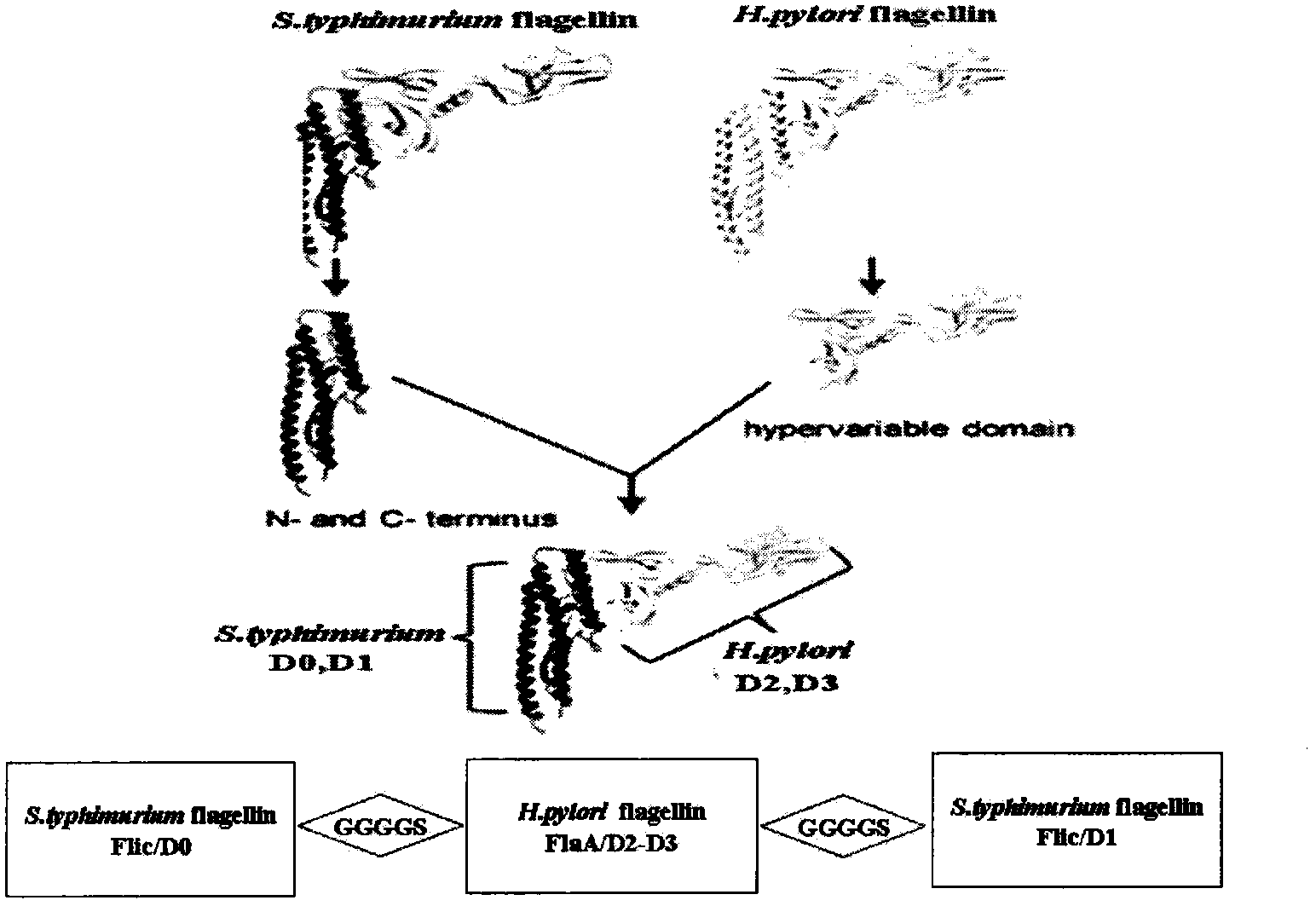
[0030] Example 1: Molecular structure design of novel chimeric flagellin CF
[0031] Search the nucleotide sequence of D0 and D1 regions of Salmonella typhimurium flagellin Flic (GenBank: AEO27871.1) and the nucleotide sequence of D2 and D3 regions of Helicobacter pylori 26695 flagellin FlaA in the GenBank database, wherein Flic The nucleotide sequence corresponding to the D0 region is 1-426, the nucleotide sequence corresponding to the D1 region of Flic is 1255-1515, and the nucleotide sequence corresponding to the D2-D3 region of FlaA is 559-909.
[0032] According to the analysis of the bioinformatics software DNAstar, the spacer sequence (GGGGS) is easy to form turns and β-sheets, and is not easy to form α-helices. It is a flexible structure and can effectively space Flic / D0, FlaA / D2-D3 and Flic / D1. It is beneficial to the effective presentation of the Helicobacter pylori flagellin FlaA epitope and shows a high renaturation efficiency.
[0033] Results: The molecular struct...
example 2
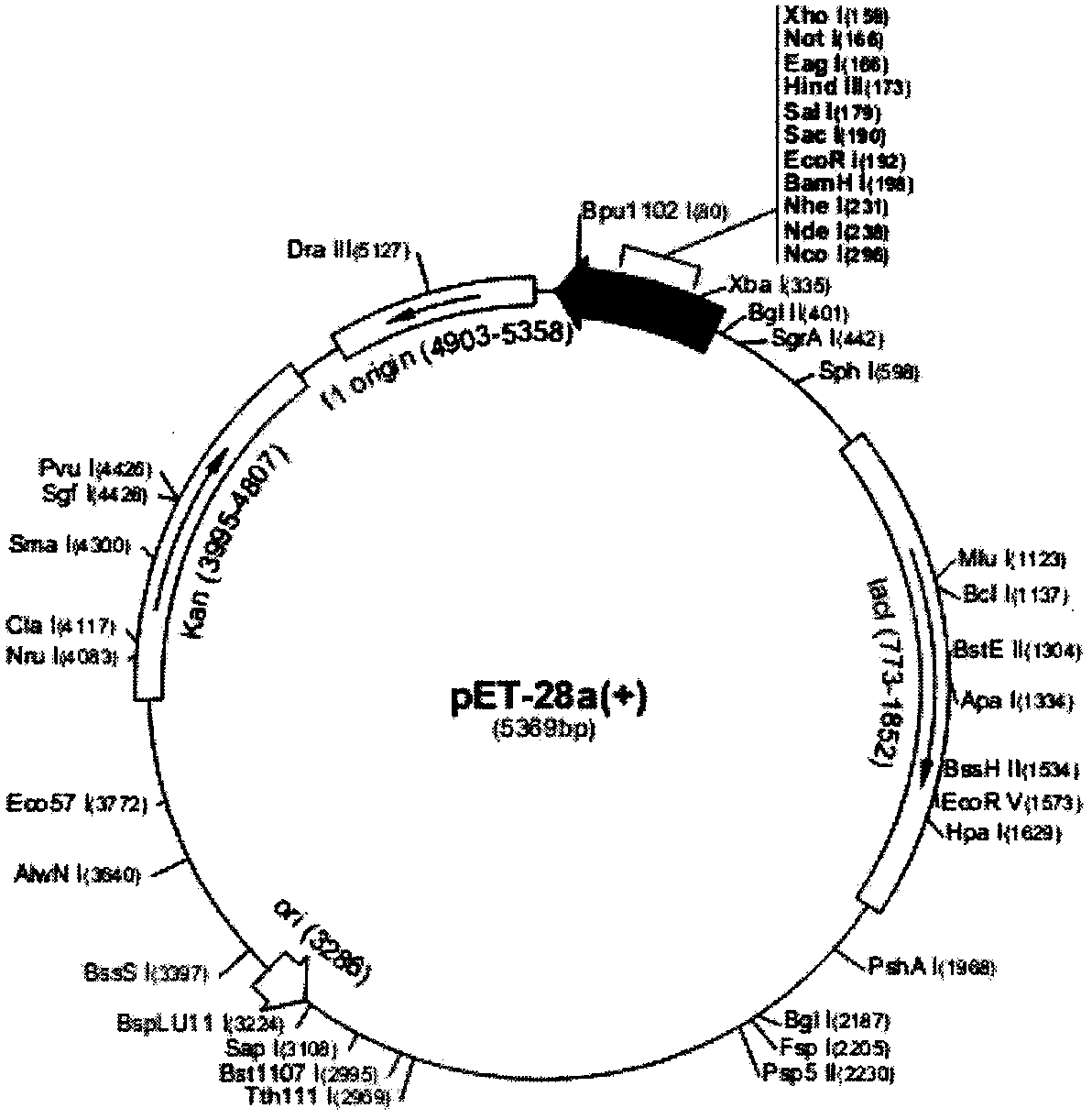
[0034] Example 2: Construction of recombinant expression vector pETCF (containing chimeric gene CF)
[0035] The gene sequence of the designed chimeric flagellin CF was sent to Nanjing Sipujin Biological Co., Ltd. for codon optimization and whole gene synthesis, and cloned into pET28a.
[0036] Results: Supkin Biological Co., Ltd. used T7 promoter and terminator universal primers for gene sequencing, and proved that the nucleotide sequence of the chimeric gene CF in pETCF synthesized by PCR was completely correct, and there was no frameshift mutation. The plasmid map of the recombinant expression vector pETCF such as ( figure 2 ) shown.
example 3
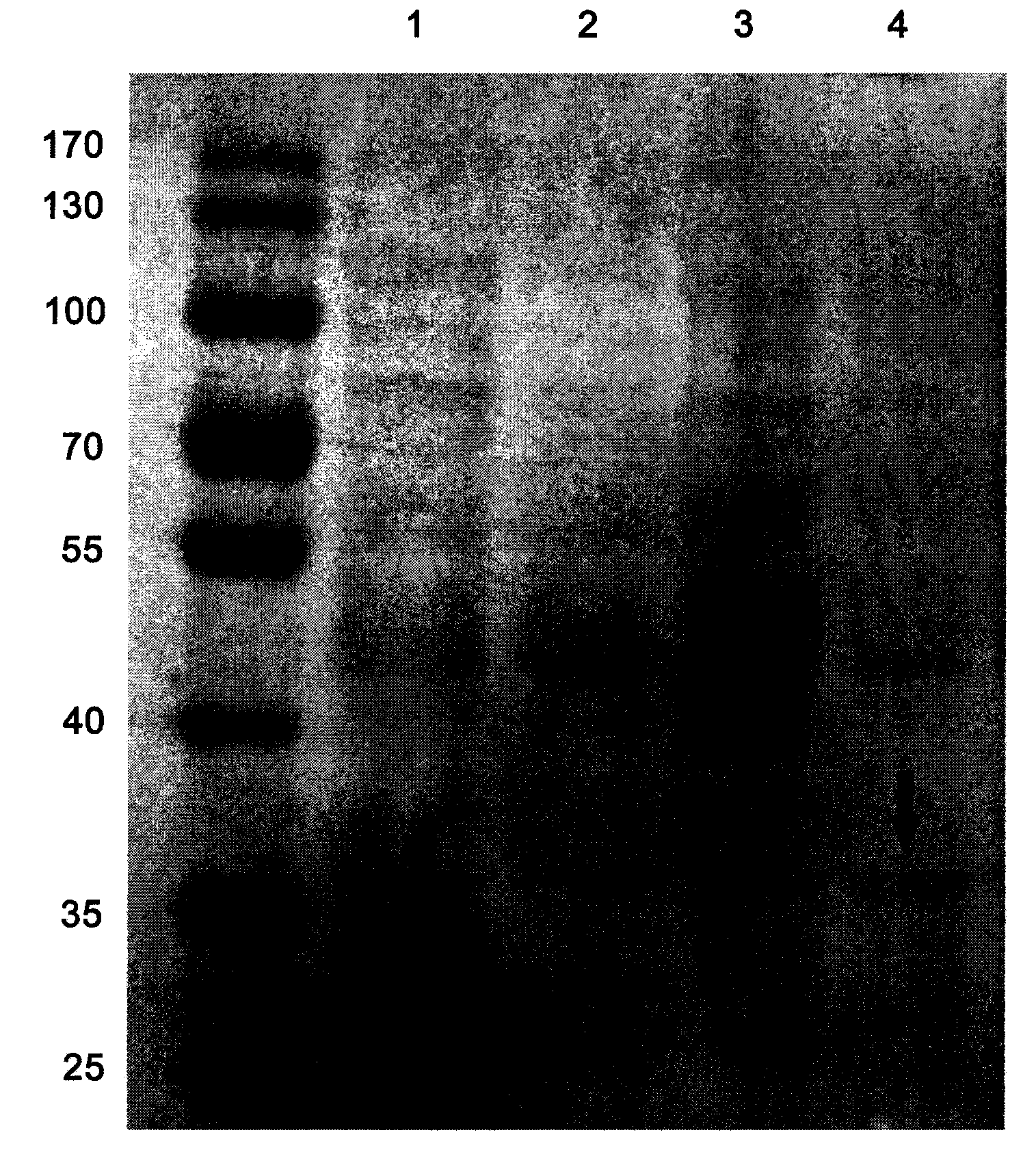
[0037] Example 3: Prokaryotic Expression of Chimeric Flagellin
[0038] The recombinant plasmid pETCF synthesized by Sipujin Biological Co., Ltd. was transformed into E.coli BL21(DE3) strain. On the pre-prepared LB plate containing 50 μg / mL Kan, inoculate the loop-streaked genetically engineered strain BL21(DE3) / pETCF, place it upside down in a 37°C incubator, and after cultivating overnight for 12-16 hours, pick a single colony and inoculate it in In LB medium containing 50 μg / mL Kan, culture at 37°C, 180 rpm, for 12-16 hours. Sent to Sipujin Biological Co., Ltd. for sequence determination.
[0039] Inoculate the correctly sequenced bacterial species with 2% inoculum in LB medium containing 50 μg / mL Kan, shake overnight at 37°C and 180 rpm, and inoculate the bacteria solution with 1% inoculum in LB medium containing 50 μg / mL Kan the next morning. In the LB liquid medium, shake the flask at 37°C and 180rpm for 3h (OD value is 0.6-0.8), add IPTG to make the final concentratio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com