Helicobacter pylori epitope vaccine, design method thereof, preparation method thereof and application thereof
A Helicobacter pylori epitope vaccine technology, applied in the field of biomedicine, can solve problems such as inability to effectively inhibit urease activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0061] Example 1: Molecular structure design of Helicobacter pylori epitope vaccine CTB-UA
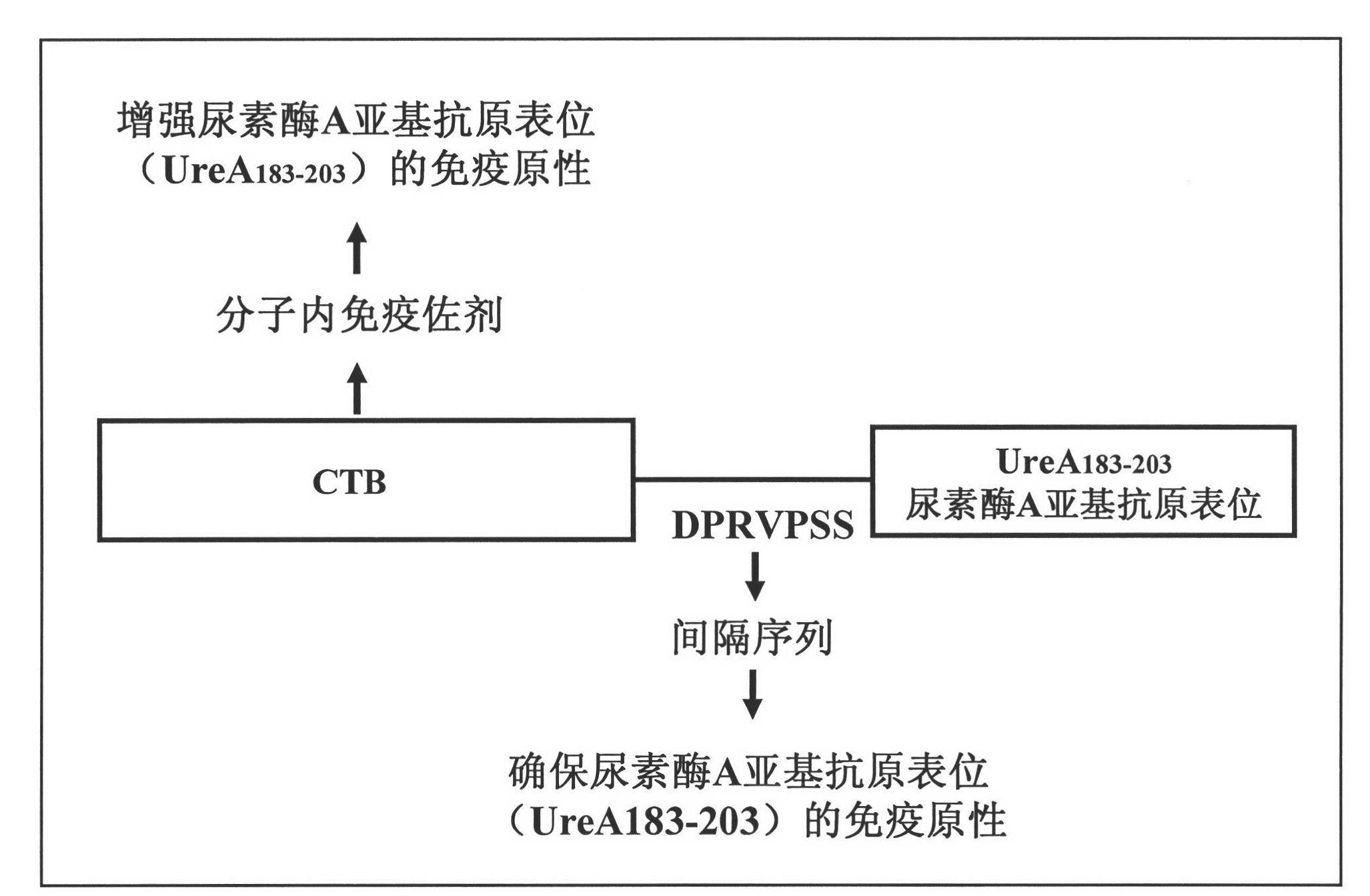
[0062] CTB forms a pentameric structure and combines with ganglioside GM1, which is very important for CTB to function as a mucosal immune adjuvant. Experimental studies have shown that when exogenous proteins are coupled to the C-terminus of CTB, the fusion protein can form a pentameric structure to the greatest extent, and has the strongest binding activity with ganglioside GM1. Therefore, in the design of the new Hp multi-epitope vaccine, the urease multi-epitope peptide (UE) was fused to the C-terminus of CTB to maintain the mucosal immune adjuvant activity of CTB to the greatest extent. Therefore, in the molecular structure design of Hp epitope vaccine CTB-UA, the urease A subunit epitope (UreA 183-203 ) is fused to the C-terminus of CTB.
[0063] In the design and research of enterotoxigenic Escherichia coli (ETEC) vaccine LTB-ST, it was shown that when 7 amino acids (DPRVPSS) ...
example 2
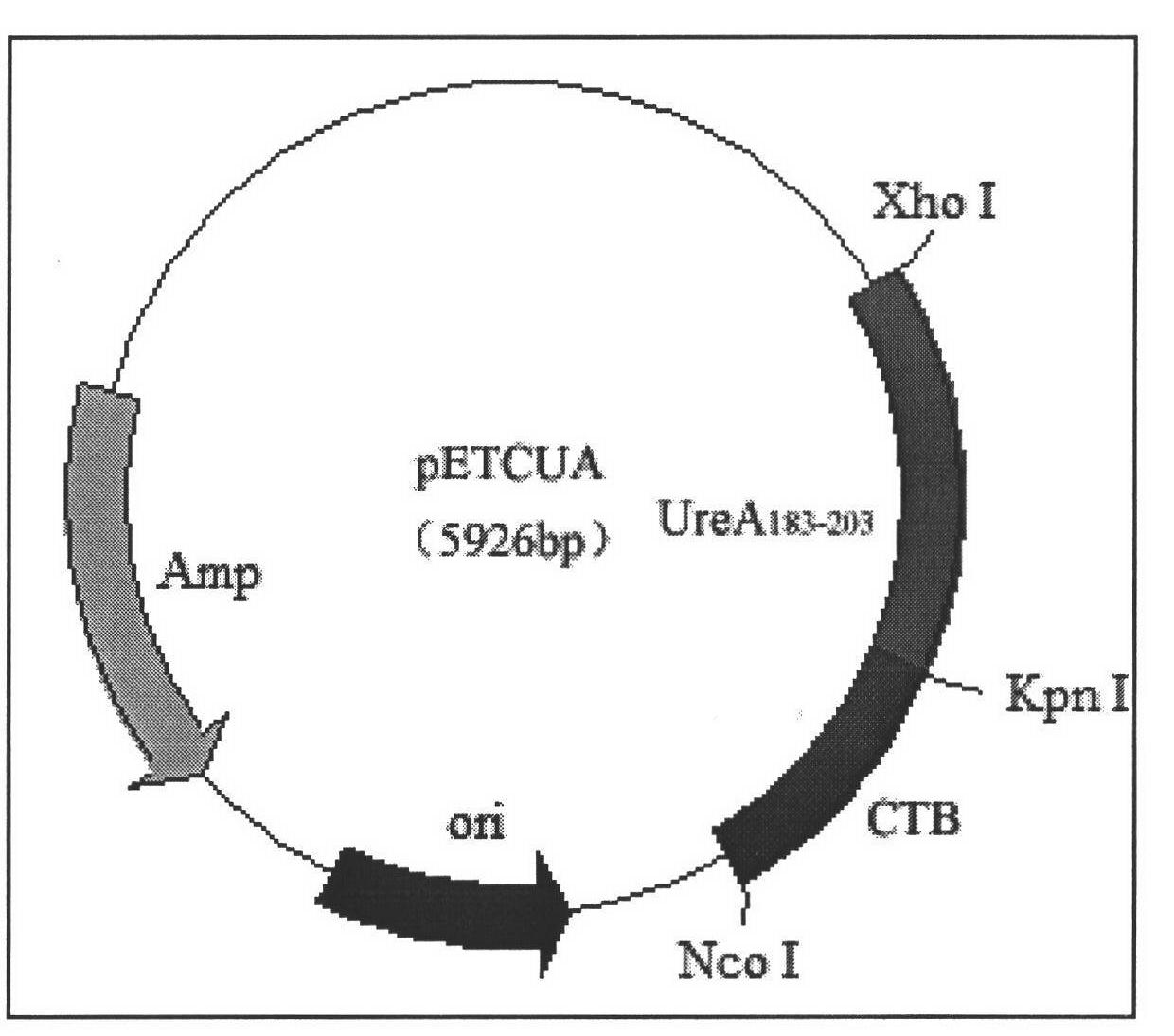
[0065] Example 2: Construction of recombinant expression vector pETCUA (containing fusion gene CTB-UA)
[0066] (1) Urease A subunit epitope UreAB 183-203B PCR synthesis of gene sequence
[0067] Primer P1-UreAB was designed using Primer Premier V5.0 183-203B and P2-UreAB 183-203B . Primer P1-UreAB 183-203B and P2-UreAB 183-203B The sequence and characteristics are as follows:
[0068] Primer P1-UreAB 183-203B :C C;
[0069] Primer P2-UreAB 183-203B :
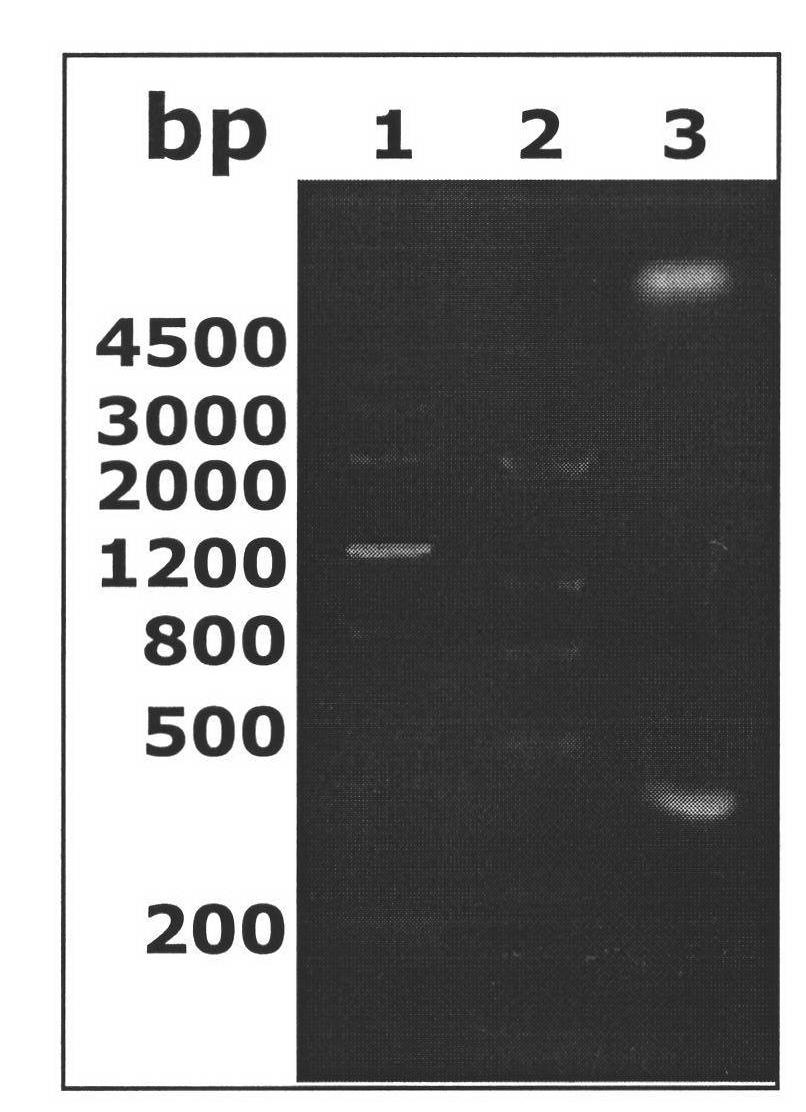
[0070] Primer P1-UreAB 183-203The sticky end (C) with Kpn I restriction site at the front end and the sticky end (C) with Xho I restriction site at the back end are marked in black bold font; primer P2-UreAB 183-203 The sticky end with Xho I restriction site at the front end (TCGAG), and the sticky end with Kpn I restriction site at the back end (GGTAC), are marked in bold black font; primer P1-UreAB 183-203 and P2-UreAB 183-203 The middle part is the complementary urease A subunit epitope UreAB 183-203 an...
example 3
[0081] Example 3: Prokaryotic expression of Hp epitope peptide fusion protein CTB-UA
[0082] Transform the correct recombinant expression plasmid pETCUA into E.coli BL21(DE3) strain. On the pre-prepared LB plate containing 50 μg / mL Amp, inoculate the loop-streaked genetically engineered strain BL21(DE3) / pETCUA, place it upside down in a 37°C incubator, and after cultivating overnight for 12-16 hours, pick a single colony and inoculate it on a plate containing In LB medium with 50 μg / mL Amp, culture at 37°C, 180 rpm, for 12-16 hours. Inoculate the recombinant bacteria with 2% inoculum in LB medium containing 50 μg / mL Amp, shake overnight at 37°C and 180 rpm, and inoculate the bacterial solution at 1% inoculum in LB liquid culture containing 50 μg / mL Amp the next morning After shaking the flask at 37°C and 180rpm for 3 hours in the medium, IPTG was added to make the final concentration reach 1mmol / L, and the expression was induced at 37°C and 180rpm. The empty vector strain BL...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com