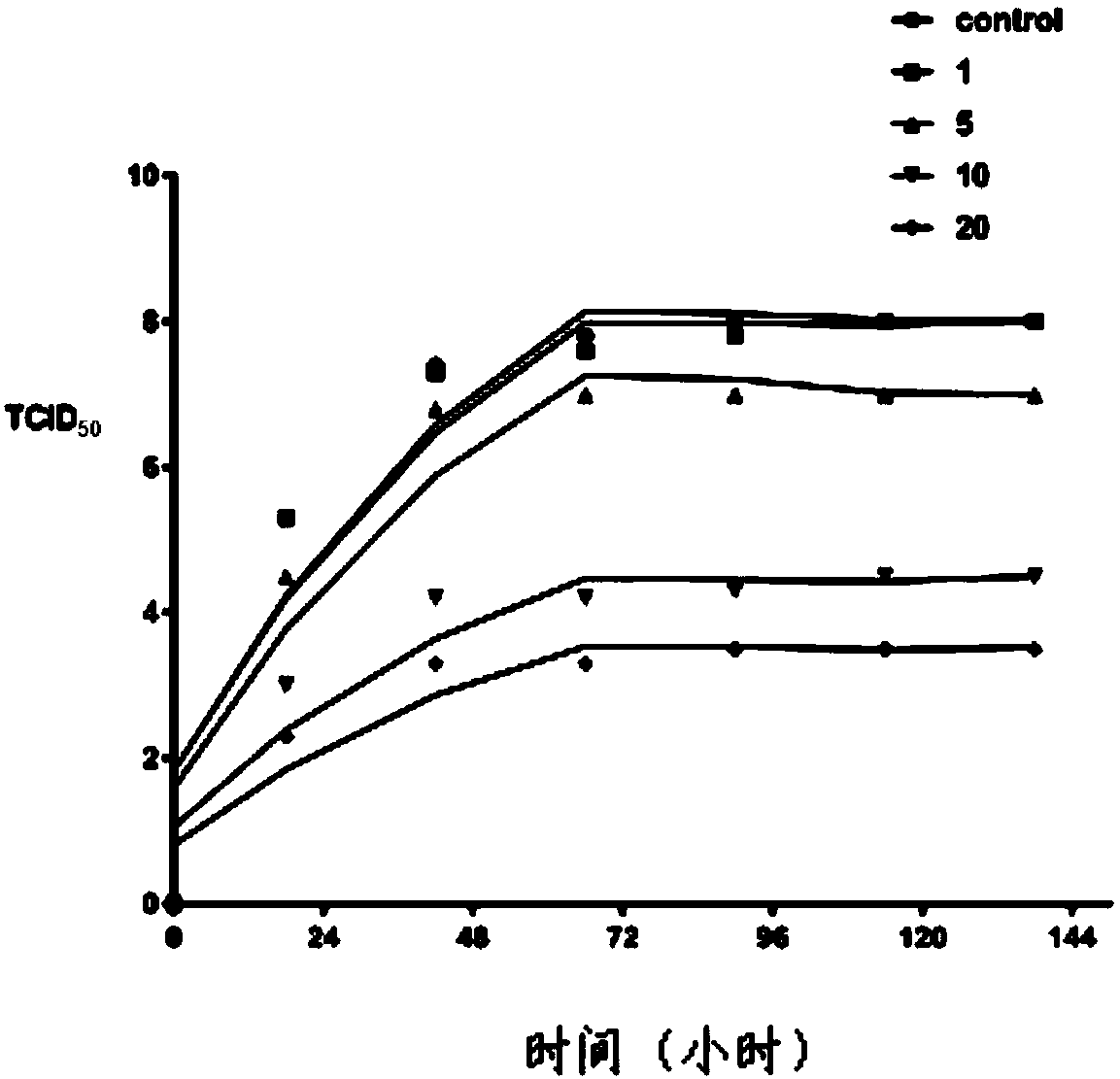
Infectious bovine rhinotracheitis virus gD protein antigen epitope polypeptide, and inhibitor, monoclonal antibody and application of polypeptide
A technology of rhinotracheitis virus and protein antigen, applied in the fields of molecular biology and medicine, can solve the problems of economic loss, secondary bacterial infection, disease control and other problems in the cattle industry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The preparation of embodiment 1 monoclonal antibody
[0033] 1. Immunization of mice
[0034] Immunization of mice Three 4-6-week-old female BALB / c mice were immunized with prokaryotically expressed recombinant gD-pET-28a protein purified by cutting gel, and immunized three times in total, with two weeks between each immunization, and the immunization dose was 60 μg / Only, for the first time, an equal amount of Freund's complete adjuvant was used to emulsify with protein, and for the second and third times, an equal amount of Freund's incomplete adjuvant was used for emulsification, and the immunization route was subcutaneous immunization. Immunization was boosted by intraperitoneal injection of 30 μg purified recombinant protein (without any adjuvant) before fusion.
[0035] 2. Cell Fusion
[0036] The feeder layer cells were prepared 1 day before fusion, and BALB / c mouse peritoneal macrophages were plated in 96-well cell culture plates according to conventional meth...
Embodiment 2
[0039] Identification of embodiment 2 monoclonal antibody
[0040] 1. Subclass identification of monoclonal antibodies
[0041] The subclass of the anti-gD protein monoclonal antibody 2B6 was identified as IgG2a / κ by the antibody subclass identification kit.
[0042] 2. Western blot test
[0043] The reactivity of monoclonal antibody and recombinant protein was verified by Western blot. The purified recombinant protein was transferred to PVDF membrane after SDS-PAGE, the positive hybridoma cell line 2B6 culture supernatant was used as the primary antibody, HRP-labeled goat anti-mouse IgG was used as the secondary antibody, and DAB was used for color development ( figure 1 ).
[0044] The reactivity of monoclonal antibody with IBRV was verified by Western blot. The virus purified by sucrose gradient centrifugation was transferred to PVDF membrane, the positive hybridoma cell line 2B6 culture supernatant was used as the primary antibody, HRP-labeled goat anti-mouse IgG was u...
Embodiment 3
[0048] Example 3 Screening of antigenic epitopes
[0049] 1. Preliminary identification of antigenic epitopes
[0050] Referring to the amino acid sequence of BHV-1gD, the experiment designed 3 pairs of primers to divide the gD protein into 3 segments, each segment has partial amino acid overlap, and the upstream of each pair of primers was inserted into the BamH Ⅰ site, and the downstream of each pair was inserted into the EcoRI site. After amplification and purification by PCR, enzyme digestion and connection with pGEX-6P-1 vector, (PCR system, enzyme digestion system and connection system are shown in Table 2-5) the recombinant protein containing GST tag was expressed, and each fusion short was determined by Western blot. Reactivity of peptides with MAb 2B6. After thrice truncated expression ( Figure 4 ), the size of the epitope was preliminarily determined to be 15 amino acids, namely 323 GEPKPGPSDADRPE 337 .
[0051] Table 1 Design of primers for preliminary epitope...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com