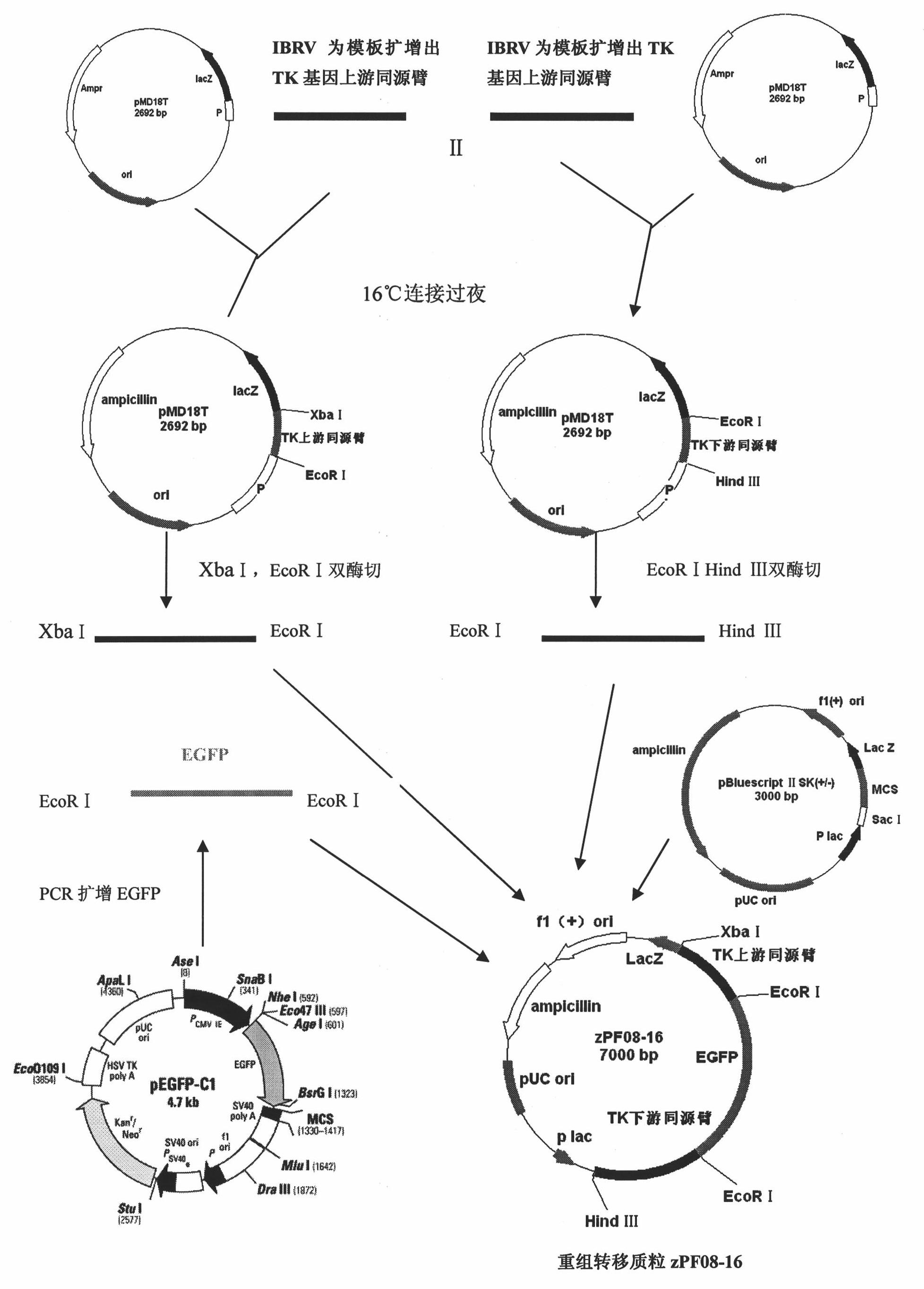
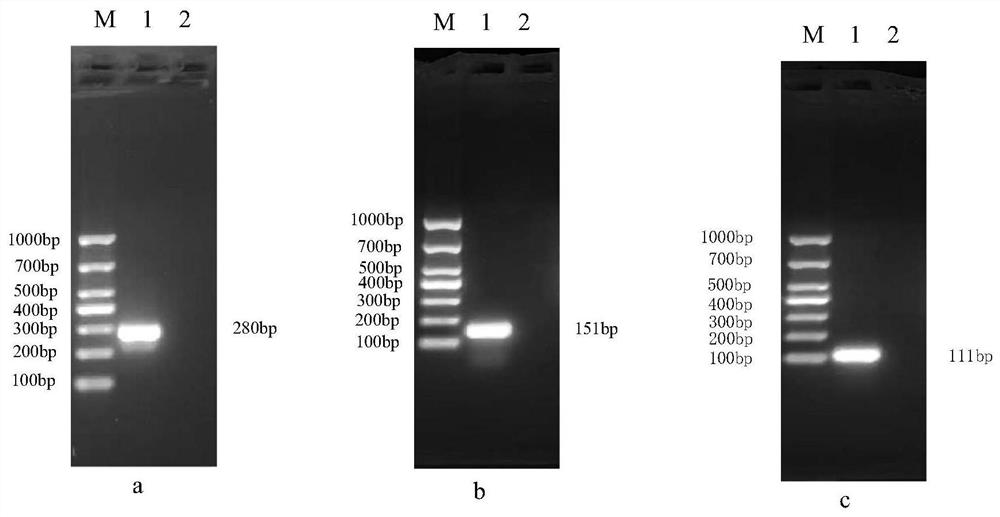
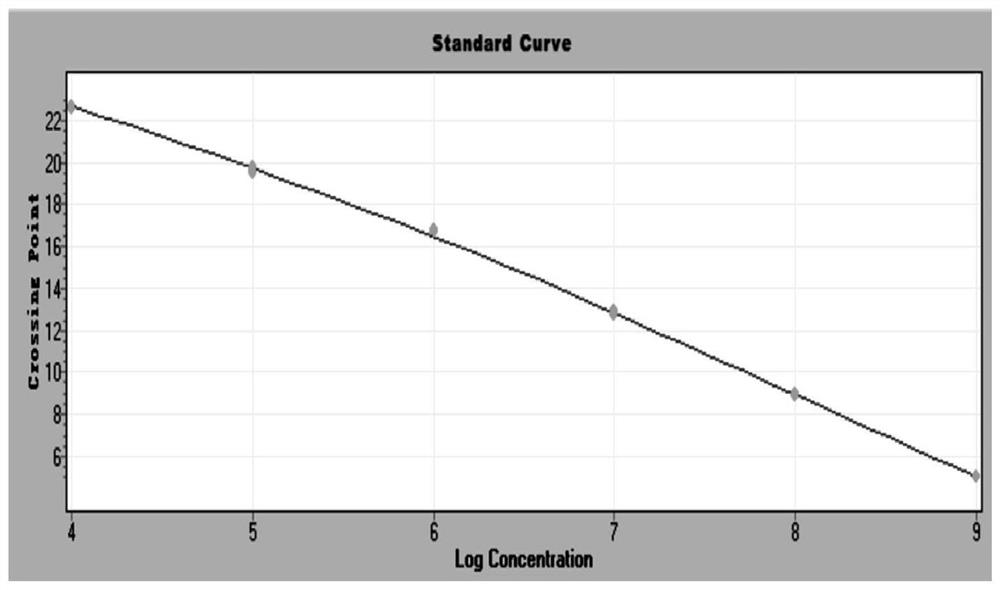
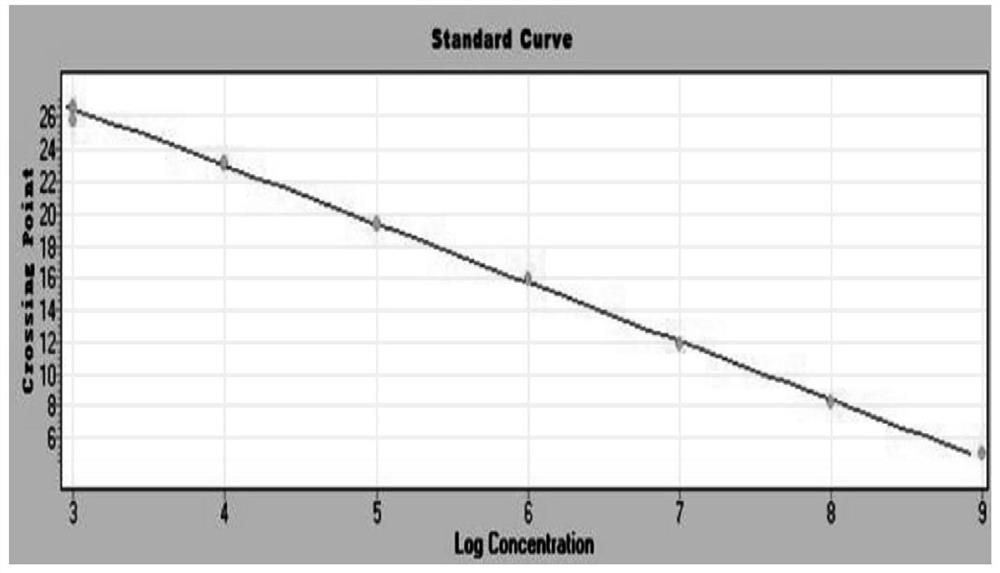
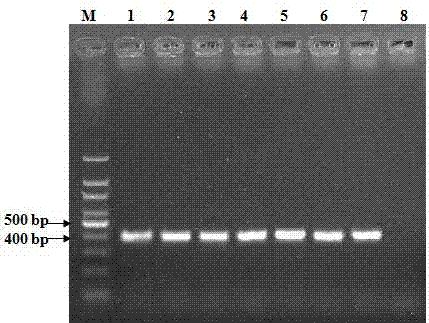
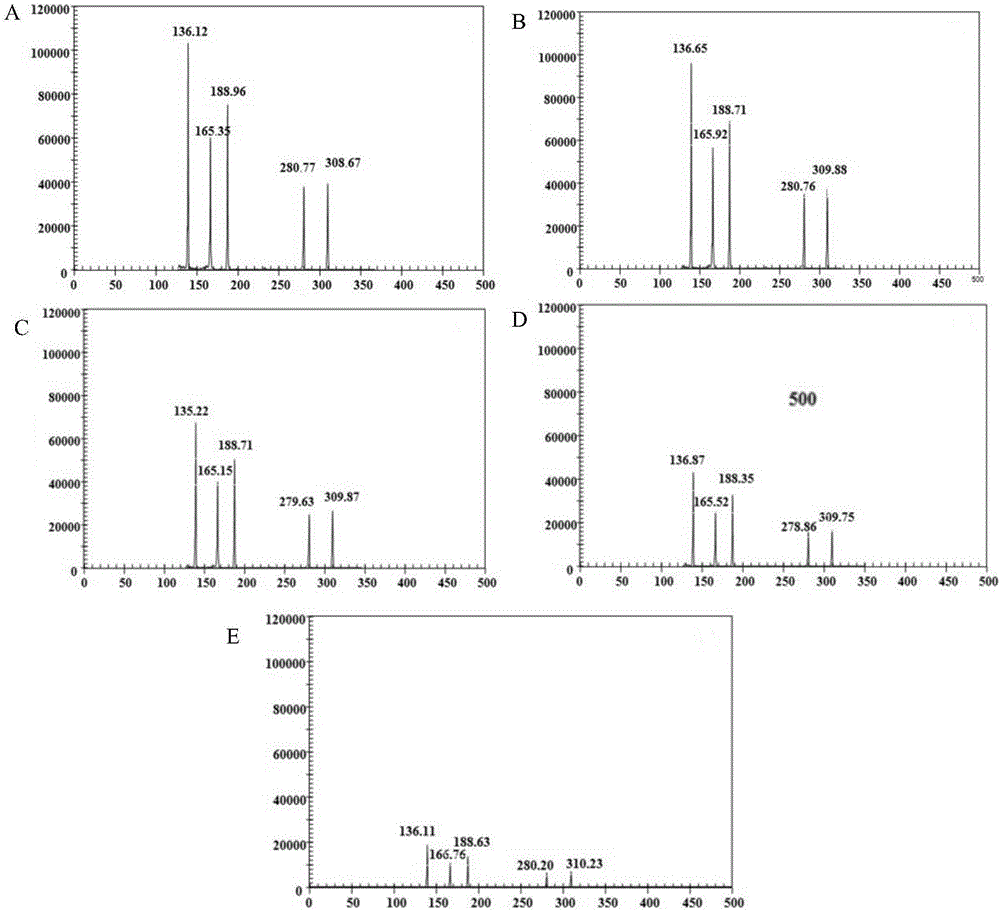
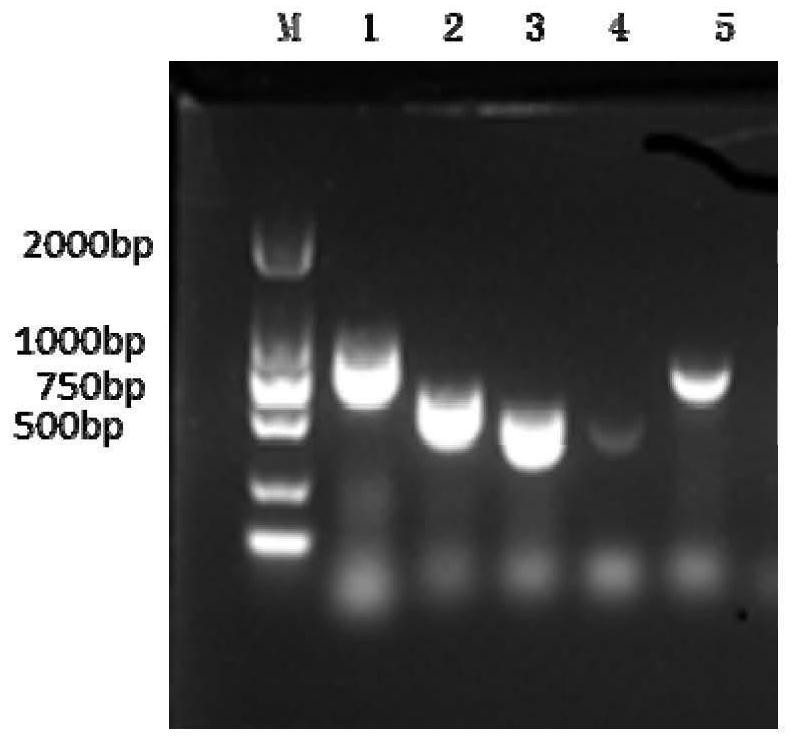
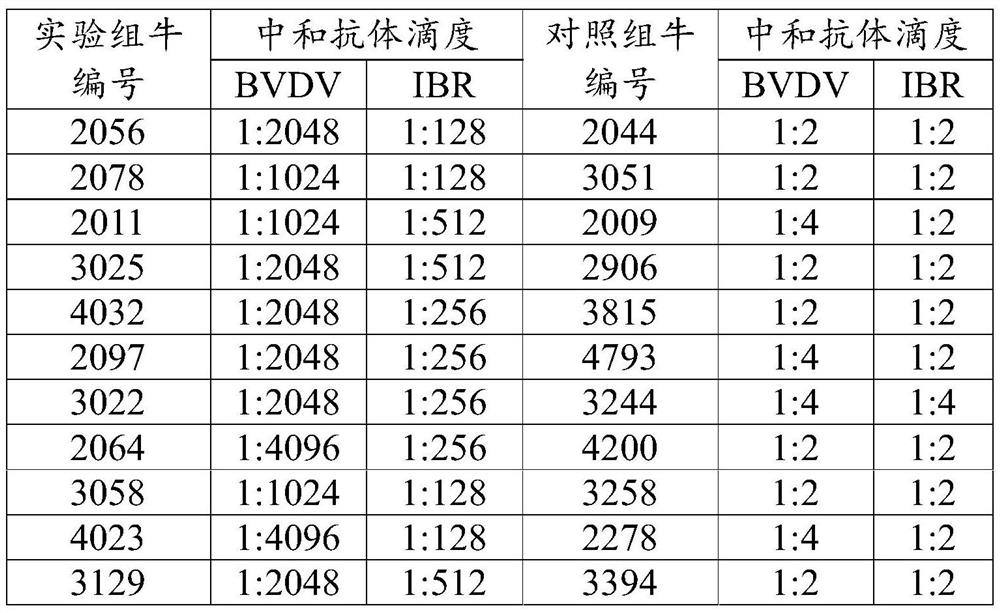
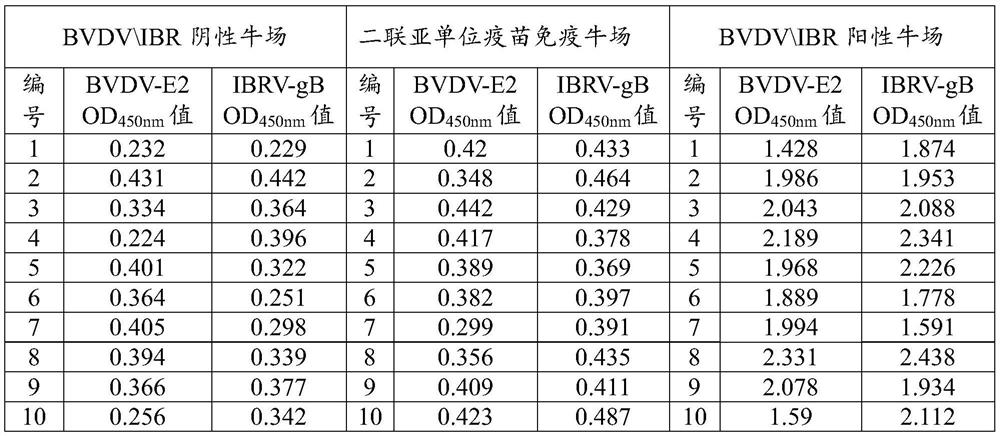
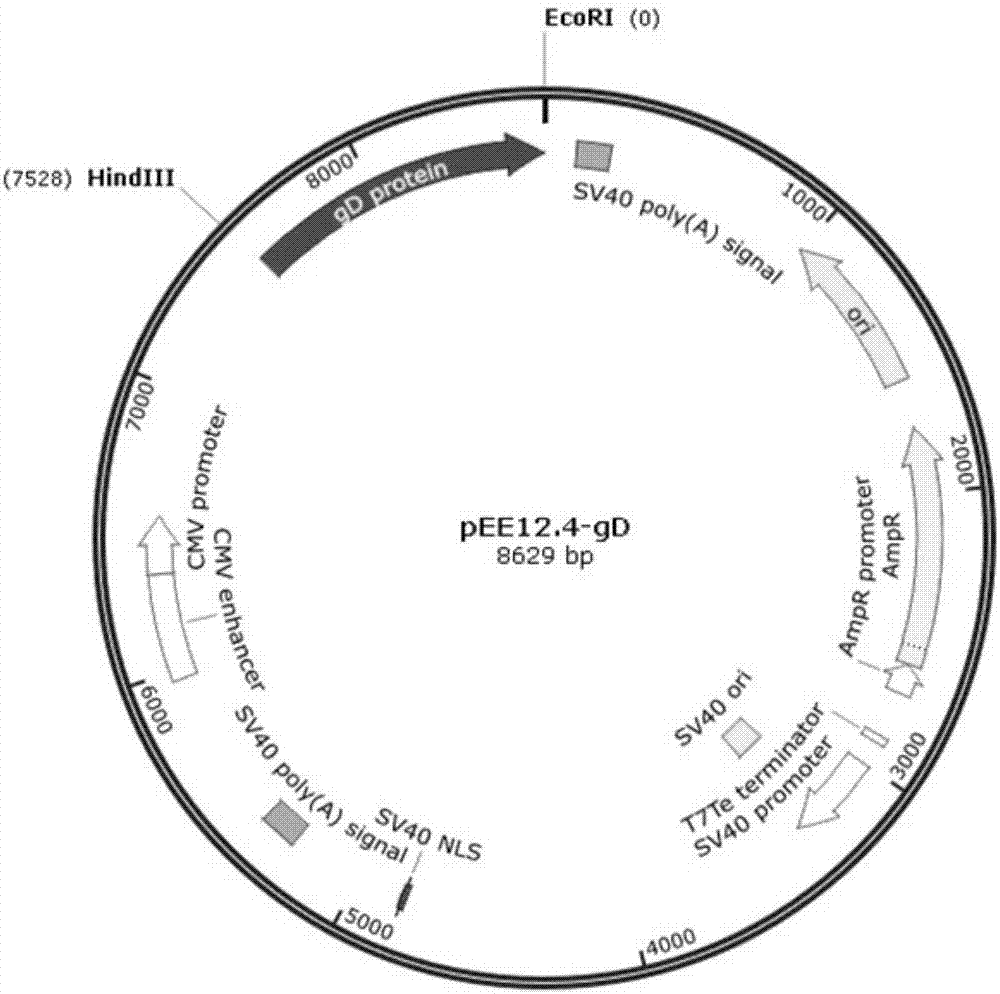
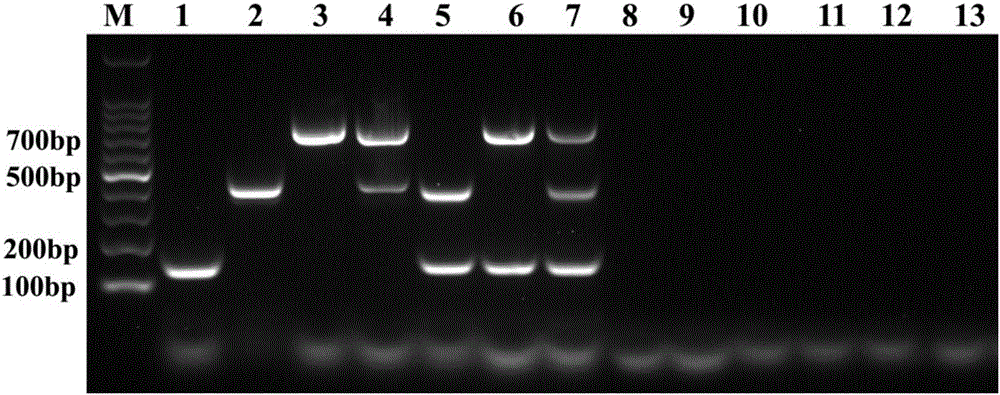
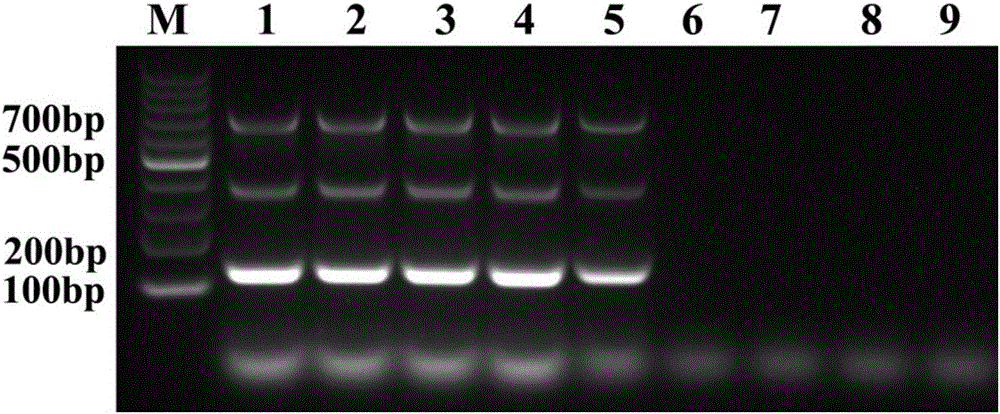
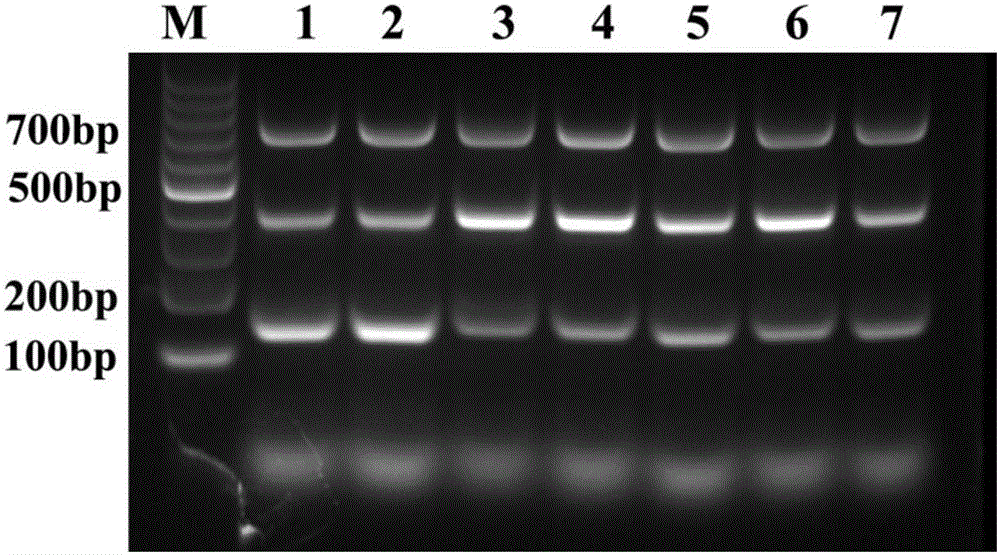
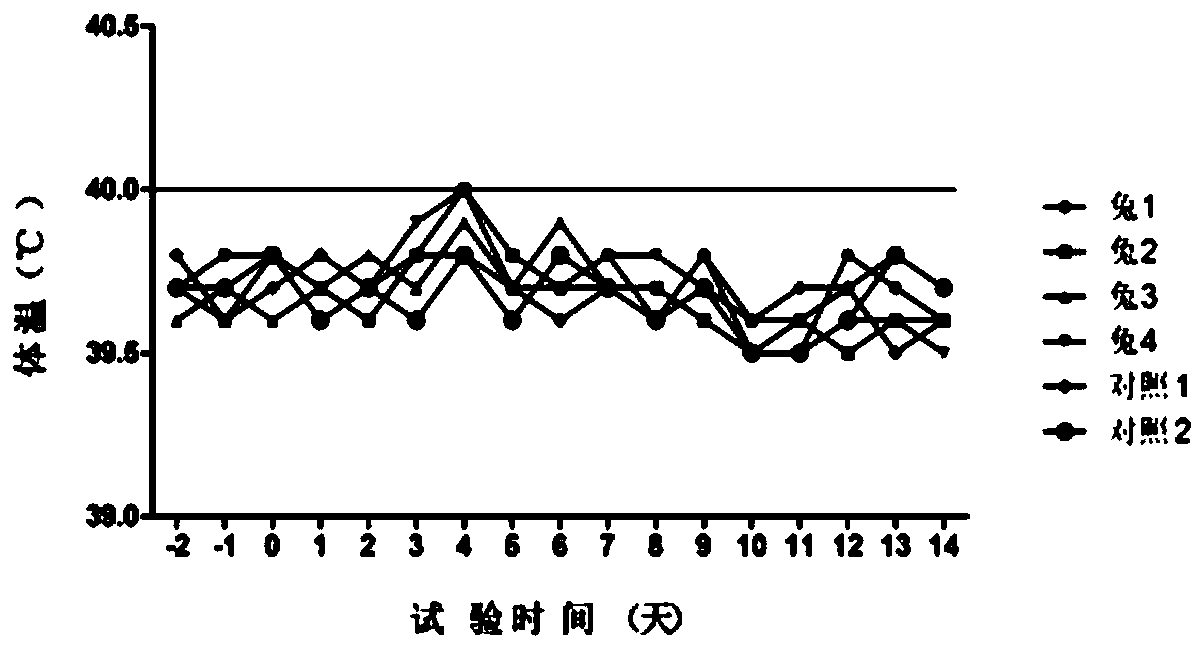
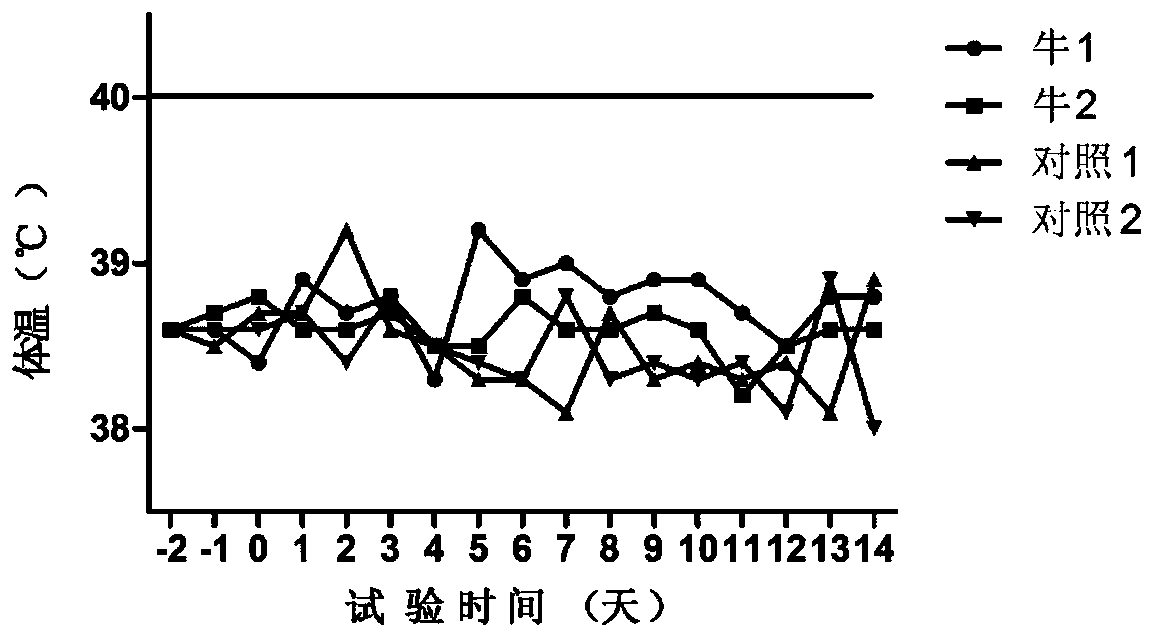
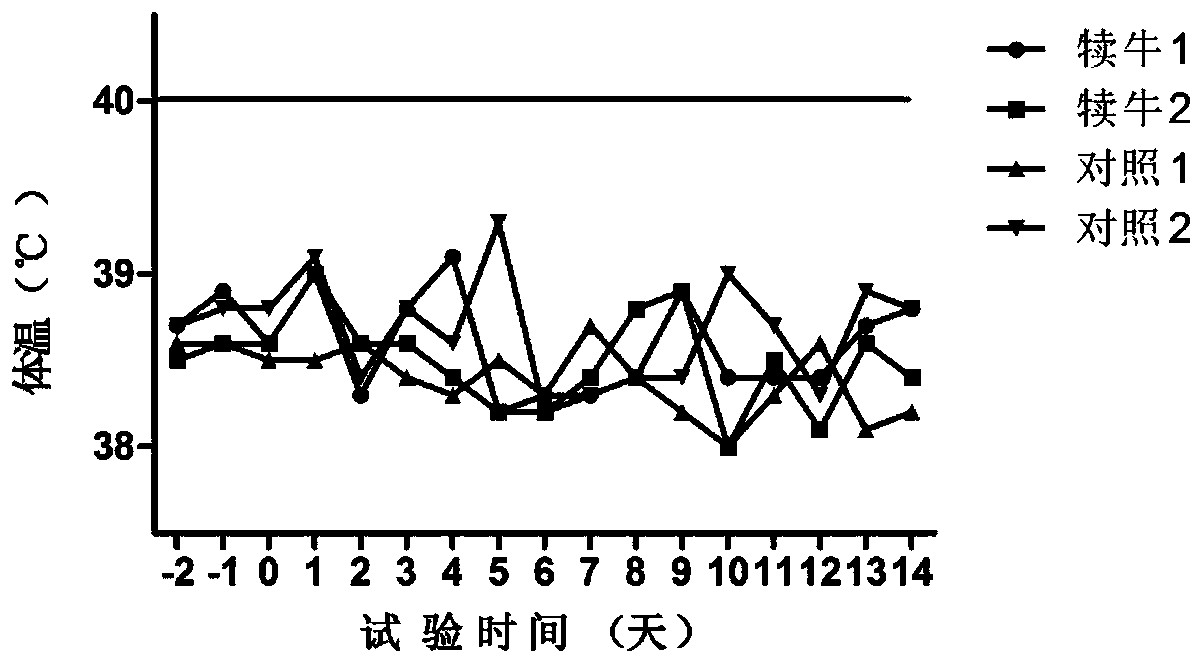
Patents
Literature
75 results about "Infectious bovine rhinotracheitis virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Infectious Bovine Rhinotracheitis (IBR) is a highly contagious, infectious disease that is caused by Bovine Herpesvirus-1 (BHV-1). In addition to causing respiratory disease, this virus can cause conjunctivitis, abortions, encephalitis, and generalized systemic infections.
Multiplex-PCR (polymerase chain reaction) detection kit for bovine respiratory disease complex and preparation method thereof
InactiveCN103498009AAccurate detectionImprove featuresMicrobiological testing/measurementPositive controlBovine Viral Diarrhea Viruses
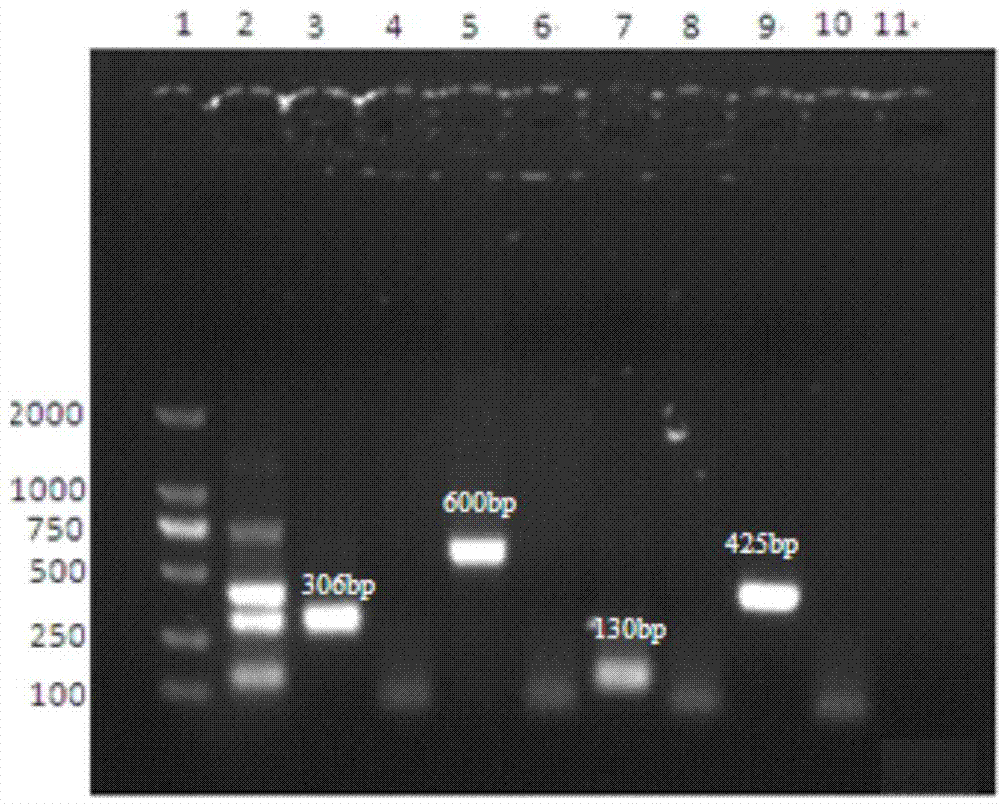
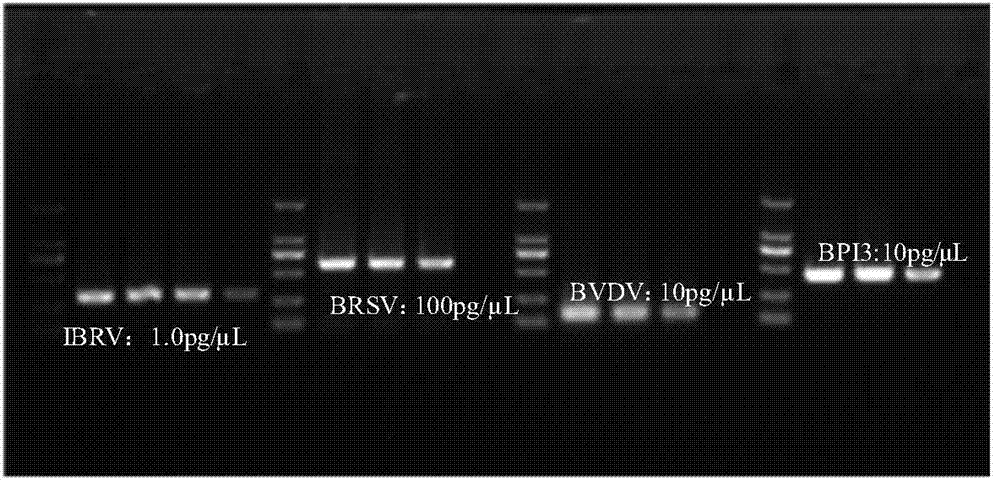
The invention discloses a multiplex-PCR (polymerase chain reaction) detection kit for bovine respiratory disease complex and a preparation method of the multiplex-PCR detection kit and relates to the field of detection of main viruses of bovine respiratory diseases. The problem that four pathogens of the bovine respiratory disease complex cannot be simultaneously and effectively detected in a multiplex-PCR method for detecting the bovine respiratory disease complex is solved. The kit comprises MightyAmp DNA polymerase, a 2xBuffe Mix buffer solution, sterile double distilled water, and four pairs of specific primers for identifying infectious bovine rhinotracheitis virus, bovine respiratory syncytial virus, bovine viral diarrhea virus and bovine parainfluenza 3 virus respectively, and also comprises positive control plasmids of the four viruses. The kit can simultaneously detect nucleic acids containing the four viruses in the same reaction system, is high in specificity and sensitivity and can accurately detect hosts and pathogenetic animals which suffer from invisible infection or continuously take viruses in the group of cattle, infectivity is avoided, the safety is high, the result can be detected in a short time, and time and labor are saved.
Owner:INST OF SPECIAL ANIMAL & PLANT SCI OF CAAS +1
Method for detecting infectious bovine rhinotracheitis virus and application thereof
InactiveCN101701265ASensitive detectionHigh detection sensitivityMicrobiological testing/measurementMicroorganism based processesInfectious bovine rhinotracheitis virusPcr method
The invention discloses a method for detecting infectious bovine rhinotracheitis virus. Internal amplification control (IAC) markers indicating the false negative are added to a polymerase chain reaction (PCR) system; an IAC fragment is built through the rearrangement of the base, the design of the primers and the PCR gene amplification with the bypass method; the added concentration of an internal maker template is 10<2> MuL in each reaction process, the added concentration of the Mg2+ is 2.0 mmol / L, the added concentration of the each dNTP is 0.3 mmol / L, the added concentration of each primer is 0.5 mmol / L, and the added concentration of a probe is 0.2 mmol / L; the primers are amplified at 50 DEG C for 2min in 1 cycle, 95 DEG C for 5min in 1 cycle, 95 DEG C for 15s in 45 cycles and 60 DEG C for 60s in 45 cycles. Therefore, the existence of inhibitory components or the occurrence of false negative indication can be accurately showed. The method is 10-100 times more sensitive than the conventional PCR method and the virus isolation test method and can be widely used for detecting the infectious bovine rhinotracheitis virus.
Owner:新疆出入境检验检疫局检验检疫技术中心
Infectious bovine rhinotracheitis virus gD protein and application thereof
InactiveCN105753947AHigh sensitivityStrong specificityVirus peptidesBiological material analysisCelluloseSerum samples
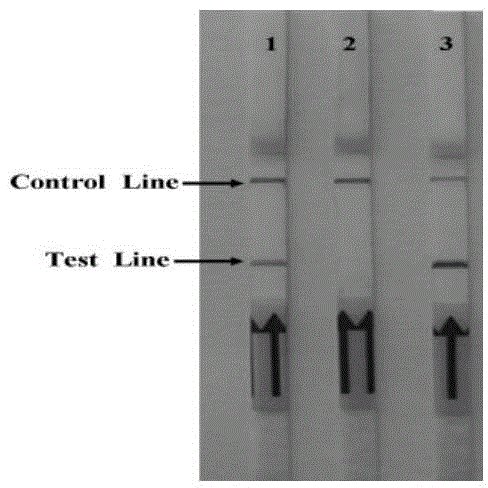
The invention discloses a test strip for detecting infectious bovine rhinotracheitis, which comprises a PVC backboard, a sample pad, a gold mark pad, a cellulose membrane and a water absorbing pad. The test strip is characterized in that infectious bovine rhinotracheitis virus gD protein according to the claim 1, marked by colloidal gold, is coated on the gold mark pad; and the infectious bovine rhinotracheitis virus gD protein and a rabbit anti-infectious bovine rhinotracheitis virus gD protein antibody are separately coated on a detection line and a quality control line of the cellulose membrane. The test strip for detecting the infectious bovine rhinotracheitis virus antibody in a bovine serum sample is high in sensitivity, is good in specificity, is quick and convenient to detect, is easy to use, and is free of special apparatus equipment, can detect results within 10 minutes, and is very convenient for being popularized and used in the basic-level breeding unit.
Owner:NBGEN
Detection primer, kit and detection method of infectious bovine rhinotracheitis virus
InactiveCN104726614ARapid IBRv pathogen detection guaranteeSimple IBRv pathogen detection guaranteeMicrobiological testing/measurementMicroorganism based processesInfectious bovine rhinotracheitis virusInfectivity
The invention relates to a detection primer, a kit and a detection method of an infectious bovine rhinotracheitis virus. The detection primer comprises a pair of external primers and a pair of internal primers, wherein the external primers are F3:5'-GTGCGTCTGCAGTCTGAG-3', and B3:5'-GCTGTACACACGGTCTCGGAG-3'; and the internal primers are FIP:5'-TGCGGATGAGCGCGCAGTCTTTTTCGACGAGGCTCCCT-3', and BIP:5'-ACGAGACGTGCATCTTCCACCGTACGGCGACGCGAAG-3'. The invention further designs a kit and a loop-mediated isothermal amplification detection method aiming at the detection primer. The detection primer is good in rapid primer detection specificity; and the detection method is fast, simple, convenient, accurate and cheap, and is especially suitable for basic clinical veterinarians.
Owner:HENAN UNIV OF ANIMAL HUSBANDRY & ECONOMY
Primer, probe and kit for detecting infectious bovine rhinotracheitis viruses
ActiveCN104862419AHigh sensitivityImprove featuresMicrobiological testing/measurementMicroorganism based processesForward primerInfectious bovine rhinotracheitis virus
The invention relates to a primer, a probe and a kit for detecting infectious bovine rhinotracheitis viruses, and discloses a primer and probe combination used for detecting the infectious bovine rhinotracheitis viruses according to an RPA technology, the forward primer sequence of the primer and probe combination is shown as SEQ ID No.1; the reverse primer sequence is shown as SEQ ID No.2; the probe sequence is shown as SEQ ID No.3. The invention further discloses the kit for detecting the infectious bovine rhinotracheitis viruses. The primer and the probe are adopted for detection, a clinical sample is only subjected to virus DNA crude extraction and RPA isothermal amplification, the result can be displayed on a lateral flow chromatography test strip, a heat circular reaction is not needed, and amplification needs not to be performed in a PCR instrument; the primer, the probe and the kit have the advantages of being high in sensitivity, strong in specificity, simple in reaction procedure, short in detection time, and suitable for clinical field detection in a non-lab environment, and have a wide application prospect.
Owner:DAIRY CATTLE RES CENT SHANDONG ACADEMY OF AGRI SCI
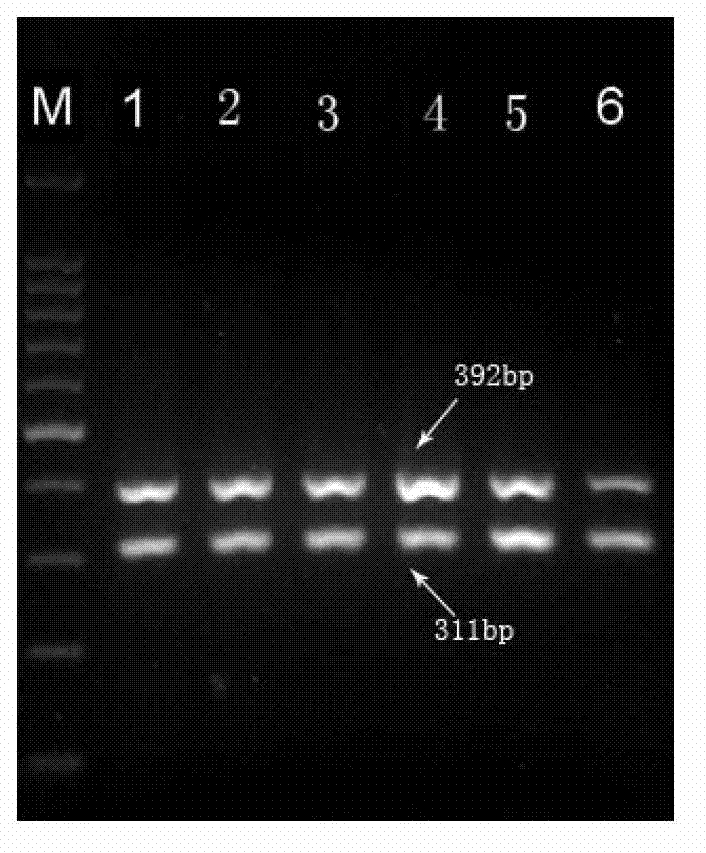
Multiple PCR primer used for simultaneously detecting infectious Bovine Rhinotracheitis virus and akabane virus as well as its design method
InactiveCN103160615ARapid detectionSimple and fast operationMicrobiological testing/measurementMicroorganism based processesAgricultural scienceInfectious bovine rhinotracheitis virus
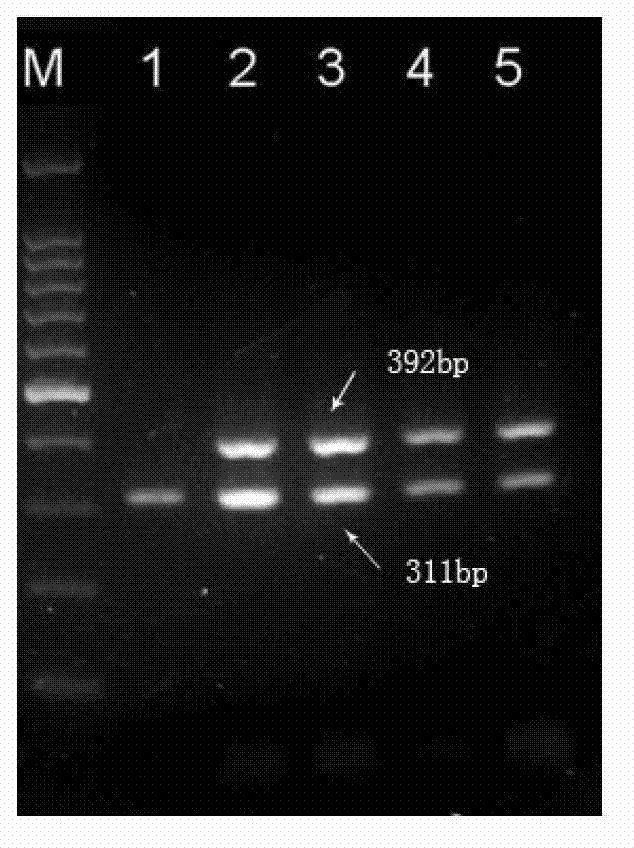
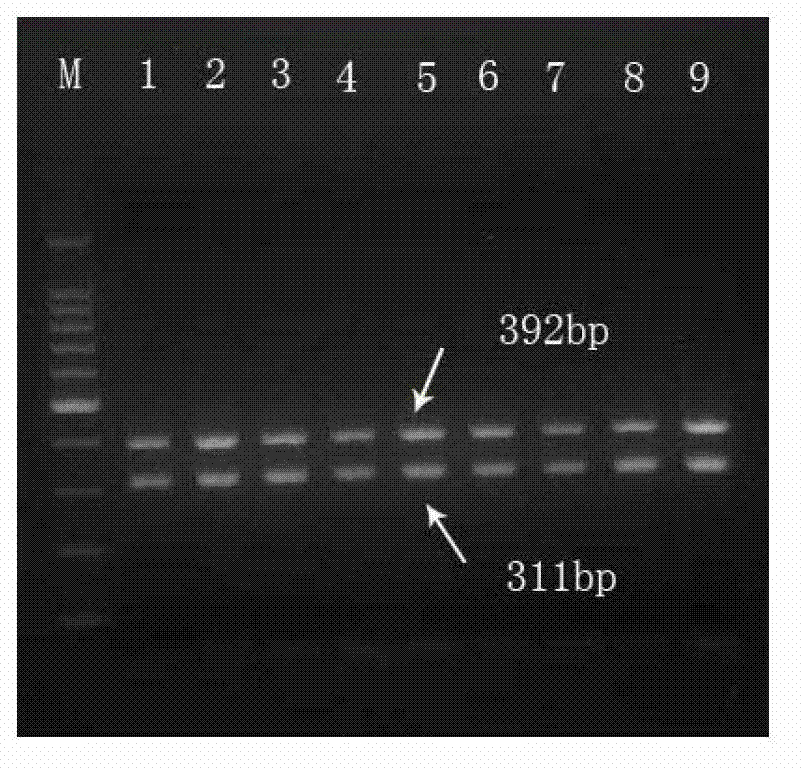
The invention relates to a multiple PCR primer used for simultaneously detecting infectious Bovine Rhinotracheitis virus and akabane virus as well as its design method, and relates to detection of infectious Bovine Rhinotracheitis virus and akabane virus. The size of a PCR amplification fragment corresponded to an infectious Bovine Rhinotracheitis virus primer in the multiple PCR primer is 311bp, the size of the PCR amplification fragment corresponded to akabane virus id 392bp. The method comprises the following steps: 1) designing multiple PCR primer combination; 2) screening the primer in the primer combination, keeping the primer which can not synthesize a primer dimer; 3) determining the competition advantage and disadvantage states of the kept primer, comparing GC% and base number in the kept primer, selecting the kept primer with high GC content and determining as the primer with excellent competition state, performing a step 5); otherwise, performing a step 4); 4) selecting the primer with poor competition state again, wherein the concrete step repeats the step 2) and determining the kept primer according to the step 3) again; and 5) performing amplification on the primer with the excellent competition state.
Owner:厦门佰能检验技术服务有限公司
Multiple-connection probe amplification detection kit, primer and probe for simultaneously detecting five cow disease viruses
ActiveCN105002301AGuaranteed sensitivityEnable multiple detectionMicrobiological testing/measurementMicroorganism based processesLeucosisBovine Viral Diarrhea Viruses
The invention discloses a multiple-connection probe amplification detection kit, primer and probe for simultaneously detecting the bluetongue viruses, the infectious bovine rhinotracheitis viruses, the bovine viral diarrhea viruses, the enzootic bovine leucosis viruses and the foot and mouth disease viruses. The multiple-connection probe is shown in sequence tables from SEQ ID NO:1 to SEQ ID NO:10. The primer is shown in sequence tables from SEQ ID NO:11 to SEQ ID NO:12. The primer, the probe and / or the multiple-connection probe amplification detection kit including the primer and the probe can detect the five vital cow disease pathogenies including the bluetongue viruses, the infectious bovine rhinotracheitis viruses, the bovine viral diarrhea viruses, the enzootic bovine leucosis viruses and the foot and mouth disease viruses at the same time, the detection time and cost are saved, and epidemic diseases can be diagnosed in time.
Owner:PEOPLES REPUBLIC OF CHINA BEIJING ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Dot immunogold filter kit for detecting IBR (infectious bovine rhinotracheitis) virus antibody and detection method thereof
The invention discloses a dot immunogold filter kit for detecting an IBR (infectious bovine rhinotracheitis) virus antibody and a detection method thereof. The kit comprises a) an infectious bovine rhinotracheitis virus antigen, b) a gold marked goat anti-bovine antibody, c) a cleaning solution, and d) a confining liquid. The method comprises the following steps: dotting the infectious bovine rhinotracheitis virus antigen on a nitrocellulose film; closing, and adding a serum sample to be detected; cleaning, and detecting the infectious bovine rhinotracheitis virus antibody by using the gold marked goat anti-bovine antibody as colloidal gold marked protein. Detection of the infectious bovine rhinotracheitis virus antibody by adopting the kit disclosed by the invention has the advantages of specificity, sensitivity, quickness, reliability, intuitive effect, easily determined result and the like, special equipment is not required, and the detection result can be preserved for inspection.
Owner:INSPECTION & QUARANTINE TECH CENT OF FUJIAN ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Infectious bovine rhinotracheitis vaccine and preparation method thereof
InactiveCN102049043AAntiviralsAntibody medical ingredientsInfectious bovine rhinotracheitis virusTiter
The invention relates to an infectious bovine rhinotracheitis vaccine and a preparation method thereof, belongs to the technical field of veterinary biological products, and is used for solving the problem to control the endemic infectious bovine rhinotracheitis by a vaccine. The vaccine is prepared by the following steps of: separating virus from a tissue of aborted fetus of cow infected with the infectious bovine rhinotracheitis; breeding; detecting titer; inactivating and emulsifying and the like. The vaccine can be used for controlling the endemic infectious bovine rhinotracheitis.
Owner:TIANJIN RINGPU BIO TECH
Detection method for infectious bovine rihinotracheitis virus in aerosol
ActiveCN103981283ASimple and fast operationEasy to operateMicrobiological testing/measurementMicroorganism based processesInfectious laryngotracheitis virusInfectious bovine rhinotracheitis virus
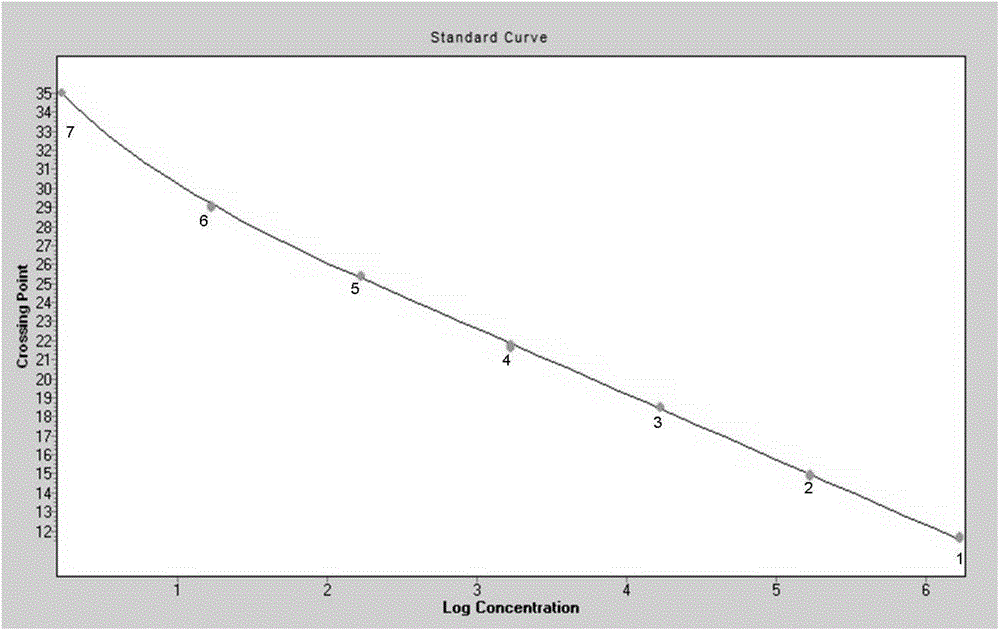
The invention discloses a detection method for the infectious bovine rihinotracheitis virus in an aerosol. The method comprises the following steps: (1) acquisition of an aerosol sample; (2) extraction of genome total DNA of the aerosol sample; (3) detection: a step of carrying out PCR amplification; (4) establishment of a standard curve and a melting curve: a step of establishing the standard curve of positive standard plasmid and the melting curve of an amplification system; and (5) judgment: a step of judging whether the aerosol sample contains the infectious bovine rihinotracheitis virus. The invention further discloses specific primers (as shown in SEQ ID No. 3 and 4) and a kit (composed of the specific primers, the infectious bovine rihinotracheitis virus positive standard plasmid pEASY-T3-D, a SYBRGreenI real-time fluorescent quantitative PCR reagent and ddH2O). The method provided by the invention is applicable to detection of an infectious bovine rihinotracheitis virus aerosol sample and to detection of samples like clinical blood, milk and tissue and has wide application prospects.
Owner:DAIRY CATTLE RES CENT SHANDONG ACADEMY OF AGRI SCI
Detection kit for detecting infectious bovine rhinotracheitis virus antibody
InactiveCN106405093ALow costEasy to produceBiological material analysisInfectious bovine rhinotracheitis virusMonoclonal antibody
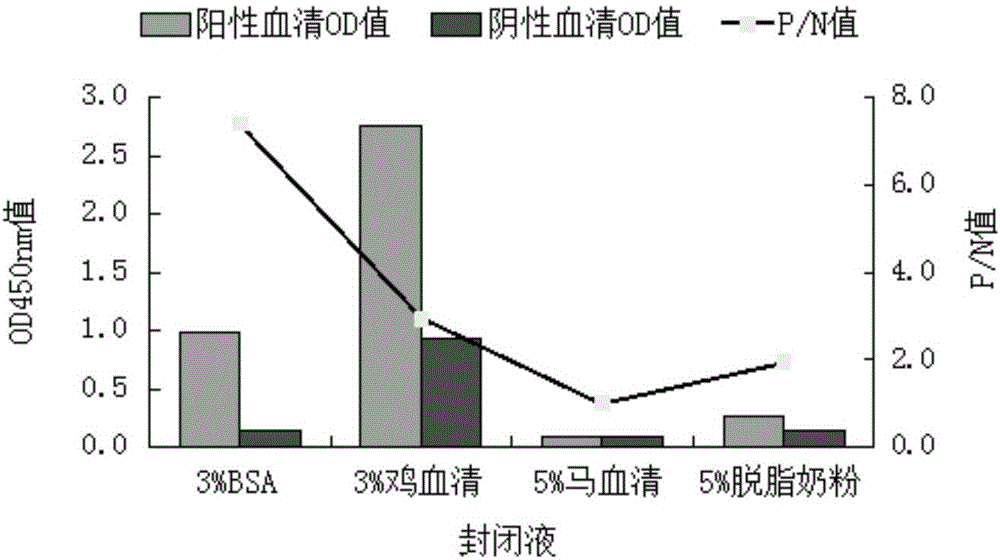
The invention provides a detection kit for detecting an infectious bovine rhinotracheitis virus antibody. The detection kit comprises an elisa plate for enveloping a monoclonal antibody and an IBRV antigen of infectious bovine rhinotracheitis virus gD protein, an IBRV positive standard serum, a negative serum, an enzyme labeling secondary antibody, a substrate developing solution and a stop solution. Infectious bovine rhinotracheitis viruses in infected MDBK cell lysates are captured in the elisa plate by using the monoclonal antibody of the infectious bovine rhinotracheitis virus gD protein, and the optimal enveloping concentrations of the monoclonal antibody and the virus antigen are determined through a square titration method. The detection kit provided by the invention has good specificity and sensitivity, is high in neutralization coincidence rate, and has a good application prospect.
Owner:BEIJING ACADEMY OF AGRICULTURE & FORESTRY SCIENCES
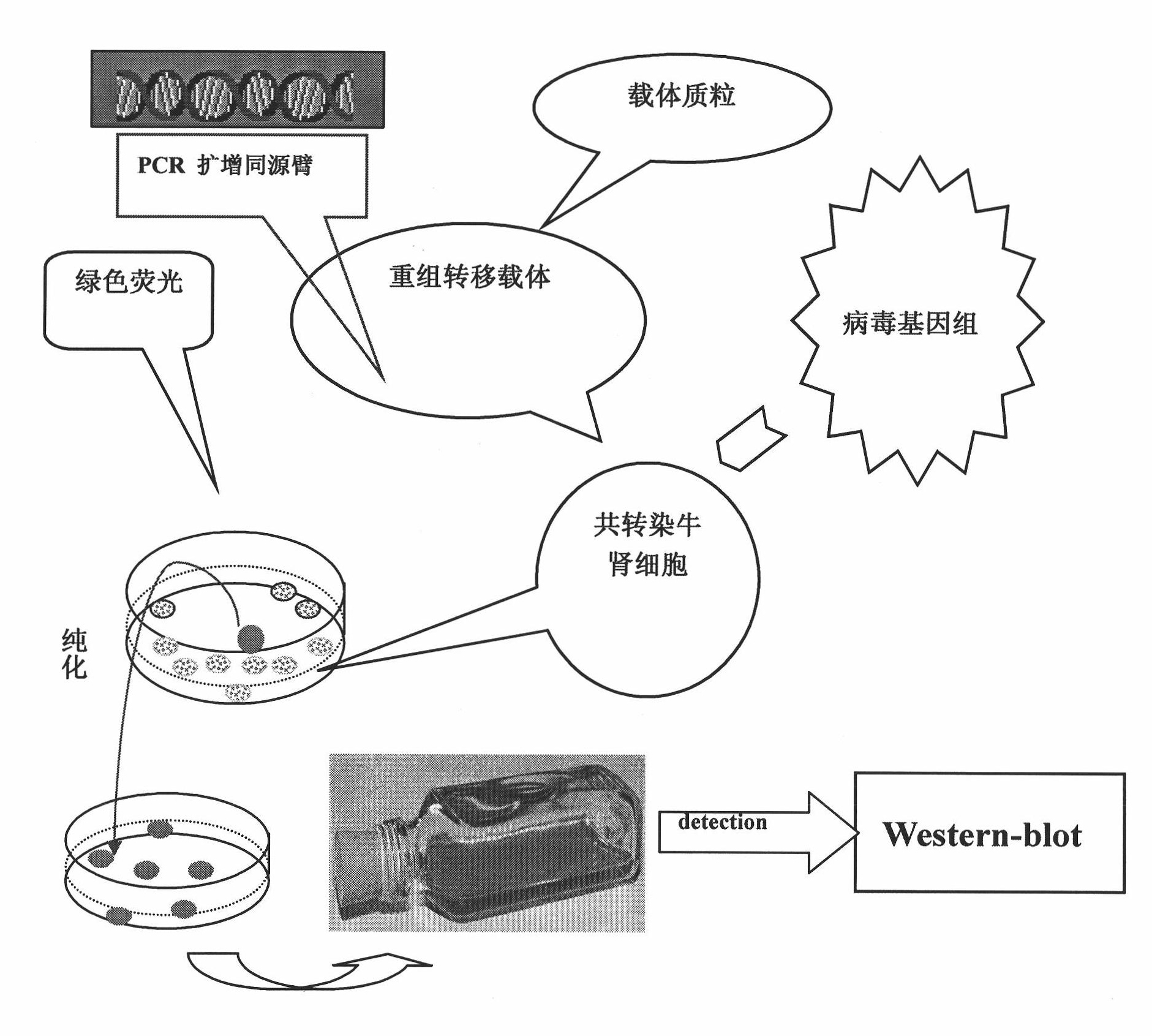
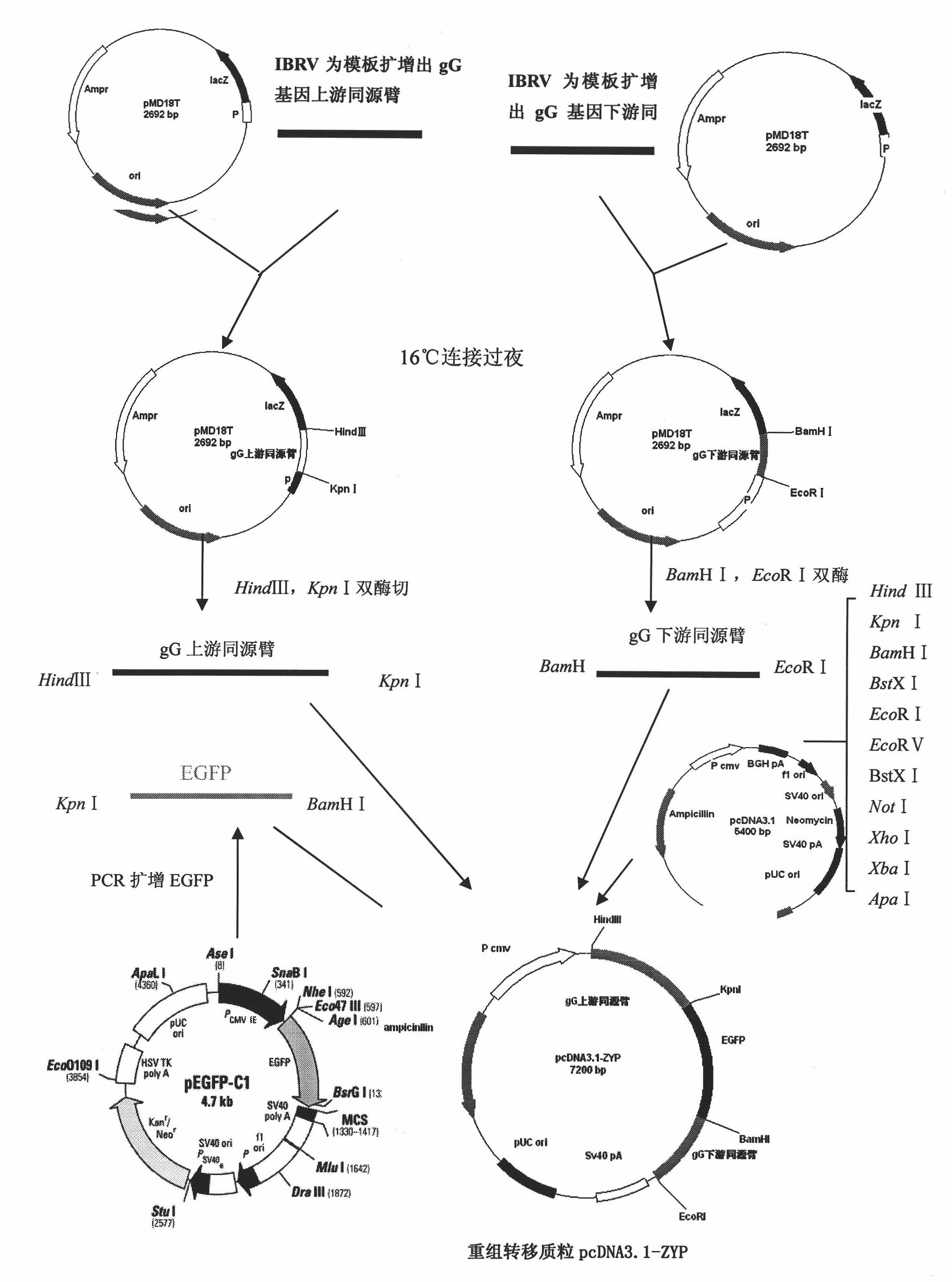
Infectious bovine rhinotracheitis virus recombinant strain, construction method and use thereof
ActiveCN101353670AViruses/bacteriophagesAntibody medical ingredientsInfectious bovine rhinotracheitis virusTransfer vector
The invention discloses an IBRV recombinant transfer vector and a constructing method thereof, and also discloses a recombinant strain IBRV46 obtained by the recombinant transfer vector and an IBRV genome after being co-transfect. A gE gene in the recombinant transfer vector, including the 1134bp comprising an initiation codon ATG is deleted, the position where the gE gene is deleted is inserted with a LacZ expression cassette. The recombinant strain of the invention can be used for preparing a vaccine for preventing and curing IBRV and can also be used for preparing diagnostic reagent. According to the conventional molecular biological method or serological method, the strain-deleted infection of the gE gene is distinguished from the wild strain infection. Additionally, the strain can be further remodeled or the nonessential genes of the strain are deleted, therefore, a mutated strain is obtained, and the vaccine, etc., can be further prepared.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
gg and TK gene-deleted recombinant infectious bovine rhinotracheitis virus and application
ActiveCN101818130ALow toxicityImmunity has no effectMicroorganism based processesAntiviralsDiseaseAnimal virus
The invention belongs to the fields of animal virology and animal genetically engineered technology, and in particular relates to a gG and TK gene-deleted recombinant infectious bovine rhinotracheitis virus, a genetic engineering vaccine and application. The recombinant infectious bovine rhinotracheitis virus strain IBRV delta gG / delta TK prepared by the invention is preserved in China Center for Type Culture Collection, with the preservation number of CCTCC No: V200915; and gG and TK genes are deleted in the strain, and the strain does not comprise foreign genes. An intermediate strain, namely recombinant infectious bovine rhinotracheitis virus GAZH-2009-01 is preserved in China Center for Type Culture Collection, with the preservation number of CCTCC No: V200910; and gG and TK genes are deleted in the strain and the strain comprises reporter gene EGFP. The invention also discloses application of the recombinant infectious bovine rhinotracheitis virus IBRV delta gG / delta TK in preparing the genetic engineering vaccine for the infectious bovine rhinotracheitis and preventing and controlling infectious bovine rhinotracheitis virus diseases.
Owner:HUAZHONG AGRI UNIV
RAA primer, probe and method for detecting knopvelsiekte virus
ActiveCN110592286AAccurate detectionNo cross reactionMicrobiological testing/measurementDNA/RNA fragmentationBovine parainfluenza virusBovine Viral Diarrhea Viruses
The invention discloses a primer, a probe and a method for detecting knopvelsiekte virus by a RAA fluorescence method. The primer and the probe are suitable for the detection by the RAA fluorescence method, can accurately detect knopvelsiekte virus plasmids without cross reaction with mycoplasma, bovine infectious rhinotracheitis virus, bovine viral diarrhea virus, bovine parainfluenza virus, bovine respiratory syncytial virus, goat pox virus and sheep pox virus, and have a specificity of 100%. The method is fast and easy to achieve high throughput, and can reduce time and cost for detection.The method for rapid detection of DNA of the knopvelsiekte virus based on the RAA fluorescence method has high sensitivity reaching 10 copies / reaction.
Owner:CHINA ANIMAL HEALTH & EPIDEMIOLOGY CENT +1
Special primer for one-tube multiplex fluorescence PCR detection of viruses BVDV, BRV and BCV and application of special primer
ActiveCN112176105AHigh sensitivityNo cross reactionMicrobiological testing/measurementMicroorganism based processesEscherichia coliMultiplex
The invention discloses a special primer for one-tube multiplex fluorescence PCR detection of viruses BVDV, BRV and BCV and application of the special primer, and belongs to the technical field of molecular biological detection. The optimal multiplex fluorescence PCR reaction conditions are designed for the special primer; the lowest detection limits of the primer and the reaction conditions on plasmid standard substances of BVDV, BRV and BCV are 1.19*10<2> copies / mu L, 3.89*10<1> copies / mu L and 3.74*10<1> copies / mu L respectively, the lowest sensitivity is 100 times higher than that of conventional PCR, and the sensitivity is high. The method only specifically amplifies BVDV, BRV and BCV, has no cross reaction with Escherichia coli, salmonella and infectious bovine rhinotracheitis virus,and has strong specificity. The intra-group variable coefficient is less than 1%, the inter-group variable coefficient is less than 1%, and the repeatability is good.
Owner:HEBEI AGRICULTURAL UNIV. +1
PCR amplification primer for rapid detection of bovine infectious rhinotracheitis virus and applications thereof
InactiveCN107881259AHigh sensitivityShort timeMicrobiological testing/measurementDNA/RNA fragmentationForward primerInfectious bovine rhinotracheitis virus
The invention discloses a PCR amplification primer for the rapid detection of a bovine infectious rhinotracheitis virus and applications thereof, and belongs to the fields of animal bacteriology and molecular biology. The PCR amplification primer is composed of a forward primer having a nucleotide sequence represented by SEQ ID No.1 and a reverse primer having a nucleotide sequence represented bySEQ ID No.2. The PCR amplification primer is used to prepare a PCR amplification kit for detecting the bovine infectious rhinotracheitis virus. The provided PCR detection method using the kit has theadvantages of high specificity, high sensitivity, good repeatability, and high credibility. The bovine infectious rhinotracheitis virus can be detected specifically. The detection results can be obtained rapidly and accurately. At the same time, the cost is lower, the operation is simple, and the kit is suitable for being used in grassroots units; and a rapid, accurate and simple detection tool isprovided for laboratory rapid identification and large scale epidemiological investigation of bovine infectious rhinotracheitis virus.
Owner:GUANGXI VETERINARY RES INST
Blocking ELISA kit for detecting neutralizing antibody of infectious bovine rhinotracheitis virus and application of blocking ELISA kit
ActiveCN112979789AObvious negative reactionStrong specificityBiological material analysisImmunoglobulins against virusesElisa kitInfectious bovine rhinotracheitis virus
The invention relates to detection of a neutralizing antibody of an infectious bovine rhinotracheitis virus, in particular to a blocking ELISA kit for detecting the neutralizing antibody of the infectious bovine rhinotracheitis virus and application of the blocking ELISA kit. The invention provides a monoclonal antibody with neutralizing activity for resisting infectious bovine rhinotracheitis virus. The monoclonal antibody is secreted by hybridoma cells with the preservation number of CGMCC No.21015. The invention further provides a kit for detecting the neutralizing antibody of the infectious bovine rhinotracheitis virus. The kit comprises the monoclonal antibody. Based on the monoclonal antibody and truncated gD protein designed in the invention, a blocking ELISA method for detecting a neutralizing antibody of the infectious bovine rhinotracheitis virus is established, and the blocking ELISA method has the advantages of strong specificity, high sensitivity and good repeatability.
Owner:BEIJING ACADEMY OF AGRICULTURE & FORESTRY SCIENCES
Infectious bovine rhinotracheitis virus gD protein antigen epitope polypeptide, and inhibitor, monoclonal antibody and application of polypeptide
InactiveCN107586322AVirus peptidesImmunoglobulins against virusesAdjuvantInfectious bovine rhinotracheitis virus
The invention belongs to the field of molecular biology and medicine, and specifically discloses an infectious bovine rhinotracheitis virus gD protein antigen epitope polypeptide and an application ofthe polypeptide in preparation of a reagent or a medicament for detecting or treating infectious bovine rhinotracheitis. The invention provides a monoclonal antibody for resisting the infectious bovine rhinotracheitis virus gD protein, and simultaneously the antigen epitope of the infectious bovine rhinotracheitis virus gD protein is screened out as <323>GEPKPGPSPDADRPE<337> (the shortest epitopesequence is 7 amino acid peptide fragments: <323>GEPKPGP<329>). The recombinant protein based on the antigen epitope can specifically be used to detect infectious bovine rhinotracheitis serum; in addition, a small-molecule inhibition drug designed based on the antigen epitope can block virus infection; and meanwhile, the multi-copy repeated epitope vaccine constructed on the basis of the antigenepitope can induce a high-titer gD protein antibody under the assistance of an appropriate adjuvant, and has a relatively high neutralizing antibody titer. The invention lays a foundation for establishing a detection method and researching and developing vaccines for the infectious bovine rhinotracheitis.
Owner:HAINAN UNIVERSITY
Competitive ELISA antibody detection kit for infectious bovine rhinotracheitis virus and application thereof
ActiveCN113461808AStrong specificityIncreased sensitivityImmunoglobulins against virusesTissue cultureInfectious bovine rhinotracheitis virusHybridoma cell
The invention relates to antibody detection of infectious bovine rhinotracheitis virus, in particular to a competitive ELISA antibody detection kit for the infectious bovine rhinotracheitis virus and the application of the competitive ELISA antibody detection kit. The invention provides the monoclonal antibody for resisting infectious bovine rhinotracheitis virus. The monoclonal antibody is secreted by hybridoma cells with the preservation number of CGMCC (China General Microbiological Culture Collection Center) No.23005. The invention further provides an antibody detection kit for the infectious bovine rhinotracheitis virus, and the antibody detection kit comprises the monoclonal antibody. Based on the monoclonal antibody and the truncated infectious bovine rhinotracheitis virus gB protein designed and expressed in the invention, an antibody detection method of the infectious bovine rhinotracheitis virus is established. The method has the advantages of high specificity, high sensitivity, simplicity and convenience in operation and rapidness.
Owner:BEIJING ACADEMY OF AGRICULTURE & FORESTRY SCIENCES
Primer combination and GeXP detection method for simultaneously identifying 5 bovine viral dermatitis viruses
InactiveCN105969913APromote healthy developmentImprove throughputMicrobiological testing/measurementMicroorganism based processesDisease epidemiologyInfectious laryngotracheitis virus
The invention discloses a primer combination and GeXP detection method for simultaneously identifying 5 bovine viral dermatitis viruses. The primer combination is composed of a primer pair I, a primer pair II, a primer pair III, a primer pair IV and a primer pair V. The invention also discloses a GeXP detection method for simultaneously identifying foot-and-mouth disease virus, bluetongue virus, vesicular stomatitis virus, bovine viral diarrhoea virus and infectious bovine rhinotracheitis virus. The GeXP detection method can simultaneously identify the 5 bovine viral dermatitis viruses. The method has the characteristics of higher flux, higher specificity and higher sensitivity, can be used for monitoring of bovine disease epidemiology and differential diagnosis of unexpected epidemic situations, and ensures the healthy development of cattle raising industry.
Owner:GUANGXI VETERINARY RES INST
Bovine viral diarrhea-bovine infectious rhinotracheitis bivalent subunit vaccine and preparation method and application thereof
PendingCN107174660AImproving immunogenicityImprove securitySsRNA viruses positive-senseViral antigen ingredientsAntigenAdjuvant
The invention discloses a bovine viral diarrhea-bovine infectious rhinotracheitis bivalent subunit vaccine and a preparation method and application thereof, and belongs to the technical field of animal vaccines and animal biological products. The vaccine comprises bovine viral diarrhea virus E2 protein, bovine infectious rhinotracheitis virus gD protein and pharmaceutically acceptable adjuvant. The preparation method for the vaccine comprises the following steps that: 1) preparing the bovine viral diarrhea virus E2 protein and the bovine infectious rhinotracheitis virus gD protein; 2) mixing the bovine viral diarrhea virus E2 protein and the bovine infectious rhinotracheitis virus gD protein prepared in the 1) to prepare antigen liquid; 3) carrying out mixing emulsion on the anti-agent liquid with ISA 201VG at an volume ratio of 46:54. The vaccine has the advantages of high immunogenicity, high safety and no immunity interference; in addition, cows can be effectively prevented and protected from the infection of the bovine viral diarrhea virus and the bovine infectious rhinotracheitis virus, an effect of two prevention functions by one injection can be achieved, time and labor are saved, and cost is saved.
Owner:NOVO BIOTECH CORP
Inactivation inspection method suitable for BVDV, IBRV, PIV3 and BRSV inactivated vaccines
InactiveCN108152498AGuaranteed infection timeImprove the detection rateMaterial analysisFreeze thawingAntigen
The invention discloses an inactivation inspection method suitable for BVDV, IBRV, PIV3 and BRSV inactivated vaccines, and relates to the field of biological medicines, in particular to an inactivation inspection method for BVDV, IBRV, PIV3 and BRSV inactivated vaccines. The inactivation inspection method comprises the following steps: (1) adsorbing an inactivated virus liquor on an appropriate cell for 40-60 min, and shaking for 2-3 times in the period; (2) after adsorption, adding a certain volume of a cell maintenance fluid in a cell spinner bottle, continuously performing culture, observing cytopathy, as for the virus fluid free from cytopathy, harvesting a cell culture fluid, and performing freeze thawing for 2-3 times; and (3) taking 10-15 ml of the frozen and thawed cell culture fluid, inoculating the taken cell culture fluid on the appropriate cell for adsorption, and continuously performing culture. Observation and fluorescence detection are performed, and if no specific fluorescence appears, complete inactivation is manifested. According to the technical scheme disclosed by the prevention, the anti-gen concentration and the virus infection time are guaranteed. The virus detection rate of the inactivation inspection method is high, and the bio-safety of the inactivated vaccines can be effectively ensured.
Owner:华威特(江苏)生物制药有限公司
Bovine viral diarrhea-infectious bovine rhinotracheitis bivalent subunit fusion vaccine and identification method thereof
ActiveCN112125961AImprove securityNo immune interferenceSsRNA viruses positive-senseViral antigen ingredientsAntigen epitopeAntigen
The invention relates to the technical field of biology, and particularly provides a bovine viral diarrhea-infectious bovine rhinotracheitis bivalent subunit fusion vaccine and an identification method thereof. The inventor optimizes genes of BVDV-NS3 and IBRV-VP8, and performs fusion expression on antigen epitope proteins of the BVDV-NS3 and the IBRV-VP8 to obtain polypeptide fragment compositions coded by SEQ ID NO.1 and SEQ ID NO.2. The polypeptide fragment compositions can be used for preparing bivalent subunit fusion vaccines, good protective efficacy can be generated after animals are immunized, the purpose of preventing bovine viral diarrhea and infectious bovine rhinotracheitis at the same time is achieved, and the effect of preventing two diseases by one injection is achieved. Meanwhile, a detection kit provided by the invention can be used for rapidly identifying and diagnosing whether cattle are immunized with vaccine strains or infected wild strains, so that the purification of bovine viral diarrhea virus and infectious bovine rhinotracheitis virus is realized.
Owner:天康制药股份有限公司
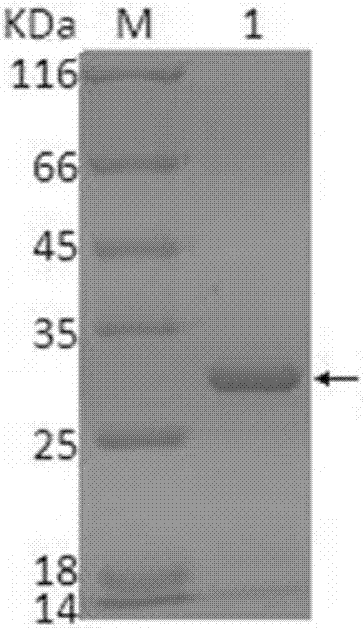
Preparation of CHO cell expressed infectious bovine rhinotracheitis virus protein gD and subunit vaccine thereof and application
PendingCN107973840AIncrease productionImprove securityViral antigen ingredientsVirus peptidesProtein targetVaccine Production
The invention discloses preparation of CHO cell expressed recombinant infectious bovine rhinotracheitis virus protein gD and a subunit vaccine thereof and an application and belongs to the technical fields of animal vaccines and veterinary biologicals. The condition that the vaccine can generate relatively high humoral immunity in bovine bodies is proven. The object of the invention is to providea preparation method capable of industrially producing the infectious bovine rhinotracheitis virus recombinant subunit vaccine on a large scale. The reparation method for the recombinant subunit vaccine comprises the following steps: 1) cloning an eukaryotic expression vector containing a protein gD coding gene; 2) transfecting CHO cells, and obtaining suspending CHO cell strains, which stably andefficiently express the protein gD, in a selecting, screening and acclimatizing manner; 3) subjecting the cell strains obtained in the step 2) to fermented culture, and carrying out purification, soas to obtain recombinant protein gD; and 4) uniformly mixing the recombinant protein gD and ISA 201 VG thoroughly, thereby obtaining the recombinant subunit vaccine. According to the method provided by the invention, target protein can be obtained from cell culture supernatant, the yield reaches up to 2g / L to 3g / L, the protein purification time is shortened, the vaccine production steps are simplified, and the vaccine production cost is greatly reduced.
Owner:NOVO BIOTECH CORP
Anti-IBRV single-chain antibody and preparation method and application thereof
InactiveCN107312087ASmall molecular weightEasy constructionImmunoglobulins against virusesAntiviralsEscherichia coliWAS PROTEIN
The invention provides an anti-IBRV single-chain antibody which is protein consisting of a heavy chain variable region shown as SEQ ID NO.1, a light chain variable region shown as SEQ ID NO.2 and a connecting peptide for connecting the two regions and having the characteristic of resisting an infectious bovine rhinotracheitis peplos gD protein antibody. The invention also provides a preparation method of the single-chain antibody. The single-chain antibody can be specifically combined with IBRV and can be also specifically combined with IBV gD protein in an escherichia coli expression product. An indirect immunofluorescence assay shows that the single-chain antibody can identify the IBRV infected in the bovine kidney cell MDBK, and the single-chain antibody has the good application value when applied to infectious bovine rhinotracheitis detection and development of treatment preparations.
Owner:BEIJING ACADEMY OF AGRICULTURE & FORESTRY SCIENCES
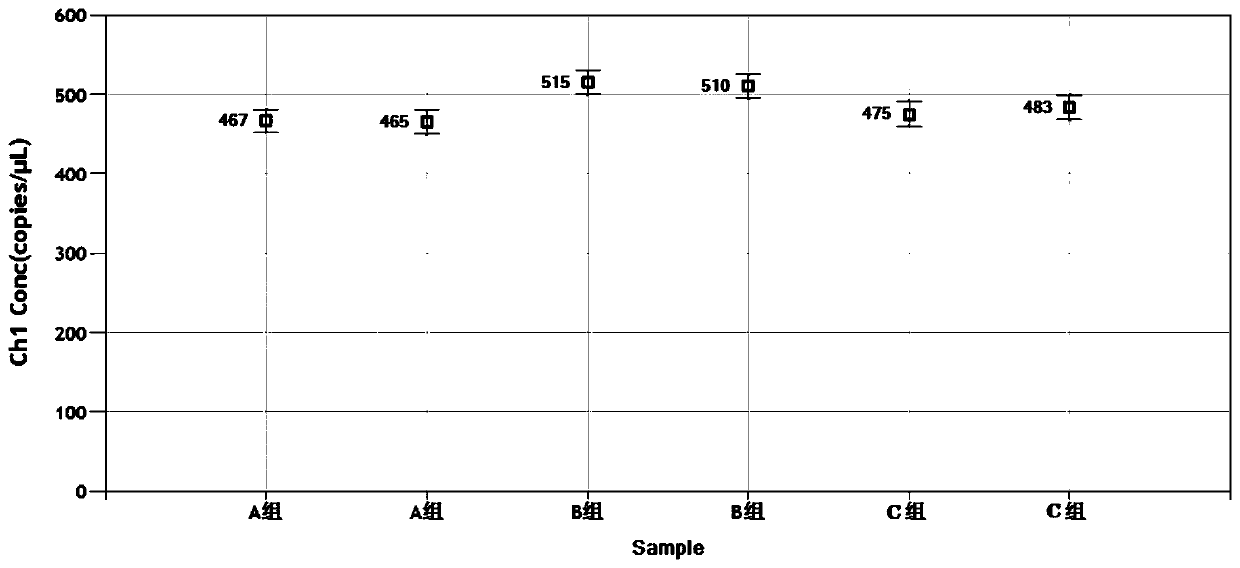
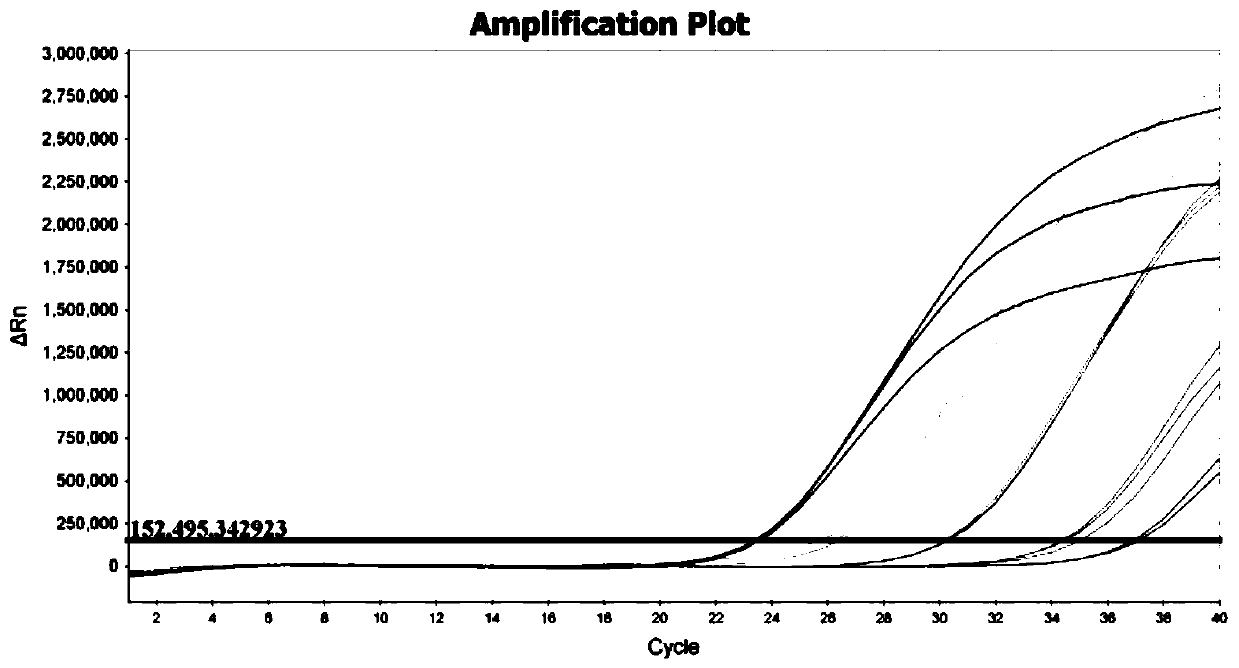
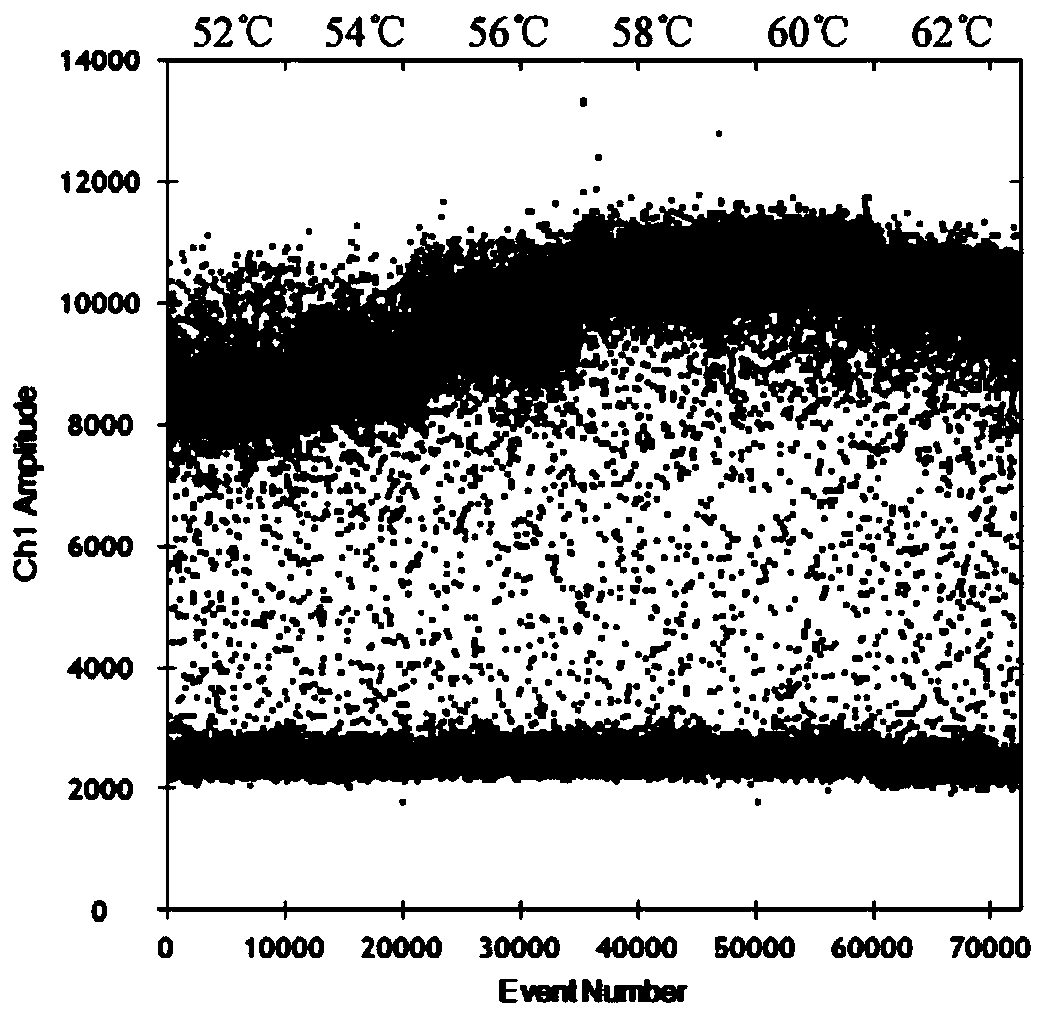
Nucleic acid, kit and droplet type digital PCR method for detecting infectious bovine rhinotracheitis virus
InactiveCN111560471ARealize precise quarantineHigh detection sensitivityMicrobiological testing/measurementMicroorganism based processesForward primerInfectious Disorder
The invention provides a nucleic acid for detecting infectious bovine rhinotracheitis virus, a kit and a droplet type digital PCR method. The nucleic acid comprises a forward primer, a reverse primerand a probe, the sequence of the forward primer is shown as SEQ ID NO. 1, the sequence of the reverse primer is shown as SEQ ID NO. 2, and the sequence of the probe is shown as SEQ ID NO. 3. The sensitivity of the droplet type digital PCR detection kit for detecting the infectious bovine rhinotracheitis virus is 1copies / mu L, and only the infectious bovine rhinotracheitis virus can be specificallydetected. When the kit is used for detecting suspected samples of infectious bovine rhinotracheitis viruses, results show that the accuracy of the kit is higher than that of existing qPCR, detectionof trace viruses can be achieved, and absolute quantification can be conducted on nucleic acid without a standard curve. The detection method provided by the invention can realize high-specificity, high-sensitivity and absolute quantitative detection of the infectious bovine rhinotracheitis virus.
Owner:北京市动物疫病预防控制中心
Primer group for identifying mycoplasma bovis, bovine viral diarrhea virus and infectious bovine rhinotracheitis virus and application thereof
ActiveCN106754911AHigh sensitivityHigh clinical application valueMicrobiological testing/measurementDNA/RNA fragmentationAgricultural scienceBovine Viral Diarrhea Viruses
The invention discloses a primer group for identifying a mycoplasma bovis, a bovine viral diarrhea virus and an infectious bovine rhinotracheitis virus and an application thereof. According to the invention, a primer composition formed by a primer pair A, a primer pair B and a primer pair C is protected. The primer pair A is formed by a primer F1 as shown in a sequence 1 and a primer R1 as shown in a sequence 2; the primer pair B is formed by a primer F2 as shown in a sequence 3 and a primer R2 as shown in a sequence 4; and the primer pair C is formed by a primer F3 as shown in a sequence 5 and a primer R3 as shown in a sequence 6. By adopting the primer pair A, the primer pair B and the primer pair C, the mycoplasma bovis, the bovine viral diarrhea virus and the infectious bovine rhinotracheitis virus are detected through triple two-temperature PCR, and the primer group has the advantages of being good in specificity, high in sensitivity, good in universality, convenient and fast, and can be used for clinical differential diagnosis and epidemiological investigation. A novel technique is provided for prevention and control of cattle diseases, and the primer group has very high clinical application value.
Owner:GUANGXI VETERINARY RES INST
Bovine viral diarrhea, bovine infectious rhinotracheitis, bovine parainfluenza triple inactivated vaccine and preparation method thereof
ActiveCN106729692BSsRNA viruses negative-senseSsRNA viruses positive-senseAntigenBovine Viral Diarrhea Viruses
The invention relates to a triple inactivated vaccine for preventing bovine viral diarrhea, infectious bovine rhinotracheitis and bovine parainfluenza and preparation method thereof. The active ingredients of the vaccine include the inactivated antigens of bovine viral diarrhea virus, infectious bovine rhinotracheitis virus and bovine parainfluenza type-3 virus; and in the triple inactivated vaccine, virus multiplication is performed by use of a passage cell line of cell spinner bottle, full suspension and micro-carrier culture, and the vaccine preparation technology and the like are optimized. The safety and efficacy test results of the vaccine indicate that after bovine is immunized with the triple inactivated vaccine provided by the invention, local and whole-body adverse reaction is avoided, and all bovines gain immune protection, thus the vaccine is safe and reliable, an aim of 'one injection and three preventions' is achieved, and economic loss is reduced.
Owner:QILU ANIMAL HEALTH PROD
Antibody detection kit for infectious bovine rihinotracheitis virus and application thereof
ActiveCN109374886AEasy to operateReduce the difficulty of operationMaterial analysisPositive controlInfectious bovine rhinotracheitis virus
The invention provides an antibody detection kit for an infectious bovine rihinotracheitis virus. The detection kit is composed of a recombinant gD antigen coated elisa plate, negative control, positive control, a horse radish peroxidase marked IBR specific monoclonal antibody, a sample diluting solution, a washing solution, a primer solution and a terminating solution. By means of the kit, whether a cattle is infected with infectious bovine rihinotracheitis or not can be detected within a relatively short time, and a corresponding management policy is made. For a complex situation of currentanimal disease prevention and control, the kit has a very wide market prospect and can play a great role in basic level detection and government regulation.
Owner:北京纳百生物科技有限公司
Multiplex Ligation Probe Amplification Detection Kit, Primers and Probes for Simultaneous Detection of Five Bovine Disease Viruses
ActiveCN105002301BGuaranteed sensitivityEnable multiple detectionMicrobiological testing/measurementMicroorganism based processesMultiplex ligation-dependent probe amplificationBovine Viral Diarrhea Viruses
The invention discloses a multiple-connection probe amplification detection kit, primer and probe for simultaneously detecting the bluetongue viruses, the infectious bovine rhinotracheitis viruses, the bovine viral diarrhea viruses, the enzootic bovine leucosis viruses and the foot and mouth disease viruses. The multiple-connection probe is shown in sequence tables from SEQ ID NO:1 to SEQ ID NO:10. The primer is shown in sequence tables from SEQ ID NO:11 to SEQ ID NO:12. The primer, the probe and / or the multiple-connection probe amplification detection kit including the primer and the probe can detect the five vital cow disease pathogenies including the bluetongue viruses, the infectious bovine rhinotracheitis viruses, the bovine viral diarrhea viruses, the enzootic bovine leucosis viruses and the foot and mouth disease viruses at the same time, the detection time and cost are saved, and epidemic diseases can be diagnosed in time.
Owner:PEOPLES REPUBLIC OF CHINA BEIJING ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com