Bovine viral diarrhea-bovine infectious rhinotracheitis bivalent subunit vaccine and preparation method and application thereof
A technology for bovine viral diarrhea and rhinotracheitis, applied in biochemical equipment and methods, vaccines, viruses, etc., can solve the problems of poor immune effect of vaccines, hidden dangers of biological safety, virus mutation, etc., and achieve no immune interference, immune Strong originality and cost-saving effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: the preparation of bovine viral diarrhea virus E2 protein and bovine infectious rhinotracheitis virus gD protein
[0029] 1.1 Refer to the patent application document of the Chinese invention patent application publication number CN 105924506 A or the invention patent application document of the company with the application number 201611208261.8 or other protein expression methods (such as prokaryotic expression system expression) or bovine virus in other patents or literature The preparation method of bovine viral diarrhea virus E2 protein prepares the bovine viral diarrhea virus E2 protein.
[0030] 1.2 Refer to the application number 201610225968.3 for invention patent application documents or other protein expression methods (such as prokaryotic expression system expression, insect baculovirus expression, CHO cell expression system, etc.) or bovine infectious rhinotracheitis virus gD protein in other patents or literature The preparation method prepare...
Embodiment 2
[0031] Embodiment 2: the preparation of bovine viral diarrhea-bovine infectious rhinotracheitis dual subunit vaccine (illustrate with the preparation of 2ml / head part, 200ml altogether)
[0032] The consumables and materials used for the preparation of vaccines need to undergo aseptic treatment in advance, and the preparation process is completed in a biological safety cabinet or other instruments or environments that can ensure that the entire preparation process is sterile.
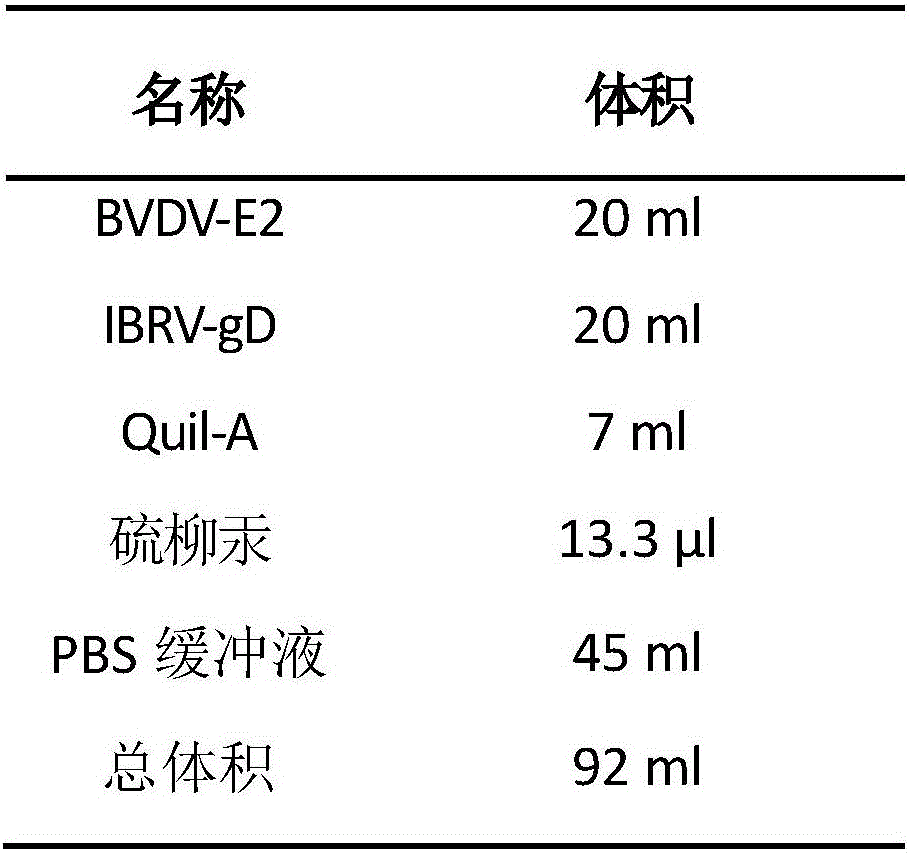
[0033] 1. Preparation of ISA 201 VG: According to the volume ratio of antigen solution and adjuvant of 46:54, measure 108ml of adjuvant into a pre-prepared reagent bottle, seal it, and preheat it in a 33°C water bath for about 30 minutes.
[0034] 2. According to the volume ratio of antigen solution and adjuvant is 46:54, the total volume of aqueous phase is 92ml. According to the protein concentration of bovine viral diarrhea virus E2 (BVDV-E2) and the protein concentration of bovine infectious rhinotr...
Embodiment 3
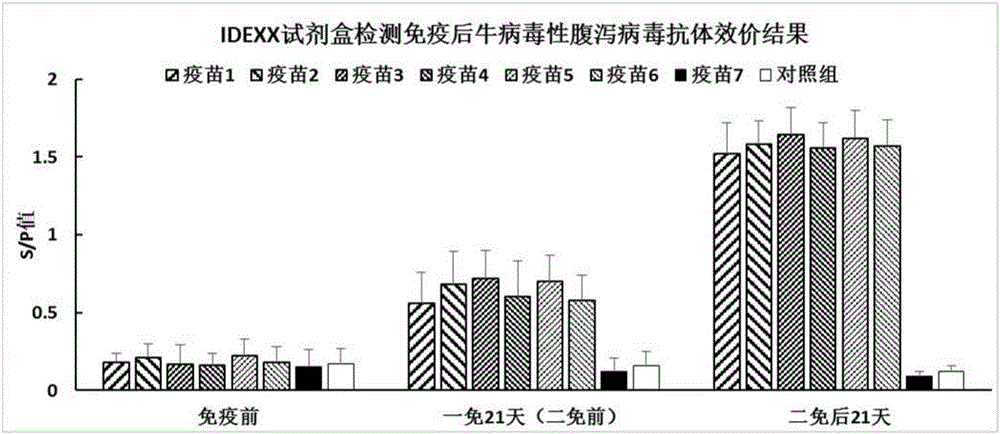
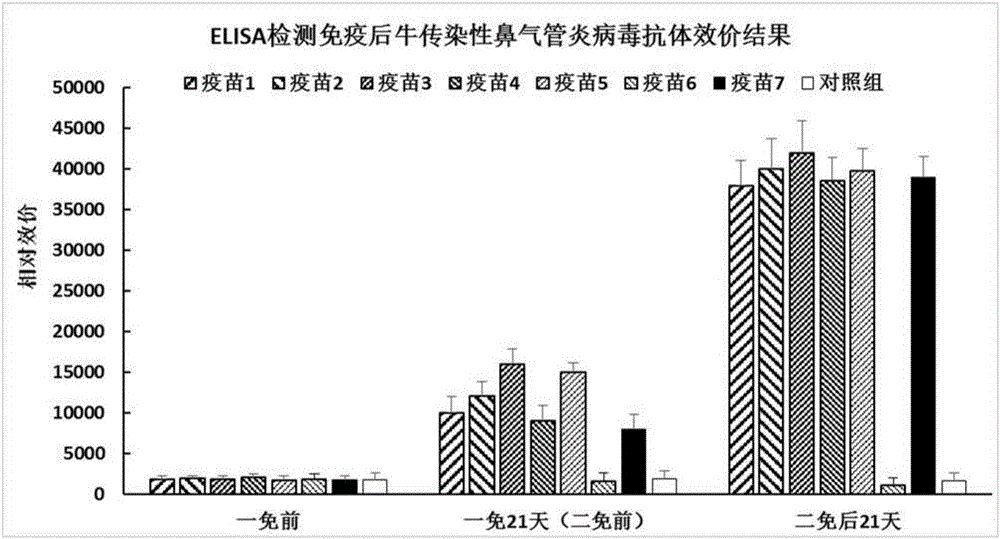
[0040] Embodiment 3: The immunization experiment of bovine viral diarrhea-bovine infectious rhinotracheitis dual subunit vaccine
[0041] 3.1 Vaccine preparation
[0042] Proteins and vaccines were prepared according to the methods of Examples 1 and 2, and the specific vaccine information is shown in Table 2 below:
[0043] Table 2
[0044]
[0045] 3.2 Immunization experiment
[0046] Screen 40 calves aged 4-5 months (negative for BVDV and IBRV antigen antibodies), and randomly divide them into 8 groups, 5 calves in each group. One group is used as the blank control group, and the other 7 groups are used as the immunization group, and are immunized with vaccines 1 to 7 respectively. . The blank control group was injected intramuscularly with 2ml of normal saline each time, and the other 6 groups of immunized groups were injected intramuscularly with 2ml of the corresponding vaccine each time. Three weeks after the initial immunization, a booster immunization was given o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com