Competitive ELISA antibody detection kit for infectious bovine rhinotracheitis virus and application thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
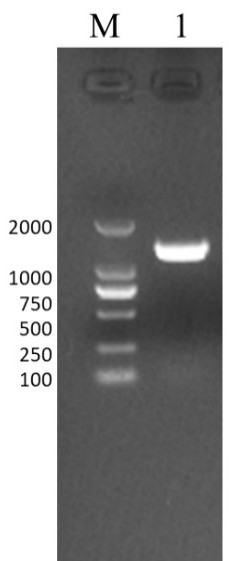
[0057] Embodiment 1. Design and expression of IBRV recombinant gB protein
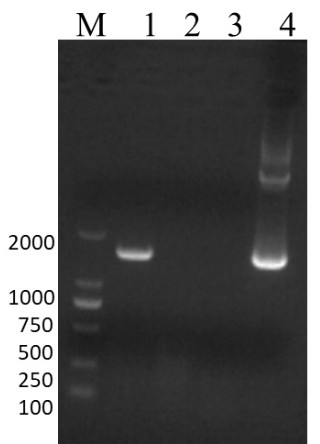
[0058] 1. Sequence optimization and primer synthesis
[0059] Download the IBRV gB gene sequence (GeneID: 4783419) from the GenBank database, analyze the sequence using the online analysis website (http: / / www.cbs.dtu.dk / services / TMHMM / ), and design a truncated gB gene sequence ( SEQ ID NO: 1), which encodes a truncated gB protein (SEQ ID NO: 2). In order to secrete and express the truncated gB protein in insect cells, the codon optimization of the truncated gB gene sequence was performed according to insect cell preference to obtain the target gene sequence (SEQ ID NO: 3). Entrust Sangon Bioengineering (Shanghai) Co., Ltd. to synthesize the target gene. According to the base sequence of the baculovirus expression vector and the target gene sequence, a pair of homology arm amplification primers were designed, and restriction sites NdeI (CATATG) and XhoI (CTCGAG) were added to the upstream and downstre...
Embodiment 2
[0079] Example 2. Preparation and screening of anti-IBRV monoclonal antibodies
[0080] 1. Animal Immunization
[0081] First immunization: After mixing the purified IBRV virus liquid (Bartha Nu / 67 standard strain) with an equal volume of Freund's complete adjuvant (Sigma-Aldrich, product number F5881) and emulsifying completely, immunize 6-8 week-old SPF grade female BALB / c mice, the immunization dose was 50 μg / mouse. Two weeks after the first immunization, an equal volume of IBRV virus fluid (Bartha Nu / 67 standard strain) was fully emulsified with Freund's incomplete adjuvant (Sigma-Aldrich, product number F5506), and the second, third and third times were carried out. 4 times of immunization, the immunization dose was 50μg / monkey, and the interval between each immunization was 2 weeks.
[0082] 2. Serum titer determination
[0083] The indirect ELISA method for hybridoma screening was established by using the positive serum of the mice after three immunizations, and the...
Embodiment 3
[0107] Example 3. Establishment of IBRV competition ELISA antibody detection method
[0108] 1. Horseradish peroxidase-labeled monoclonal antibody
[0109] The 1F24 monoclonal antibody was labeled with horseradish peroxidase (HRP) by the sodium periodate method, and the steps were as follows:
[0110] (1) Weigh 5 mg of HRP (Sigma-Aldrich, SRE0082) and dissolve it in 1000 μL of double-distilled water, and continue to add 500 μL of newly prepared 0.1M NaIO 4 , mix well, and incubate at 4°C in the dark for 30min.
[0111] (2) Add 500 μL of freshly prepared 0.2M ethylene glycol (Sigma-Aldrich, 324558) solution, mix well, and incubate at 4°C in the dark for 30 minutes.
[0112] (3) Add 5 mg of purified 1F24 monoclonal antibody to the above solution, mix well and put it into a pre-treated dialysis bag (Solarbio, MW14000), dialyze overnight in 0.1M carbonate buffer solution with pH 9.6, and dialyze The medium was changed three times during this period.
[0113] (4) Pour the dialy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com