Bovine viral diarrhea, bovine infectious rhinotracheitis, bovine parainfluenza triple inactivated vaccine and preparation method thereof
A technology for bovine viral diarrhea and inactivated vaccine, applied in the field of veterinary biological products, can solve the problems of slow growth of beef cattle and recovered cattle, decline of milk production, loss of cattle industry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] ——Preparation of bovine viral diarrhea, bovine infectious rhinotracheitis, bovine parainfluenza triple inactivated vaccine (L strain + J1 strain + S strain)
[0066] 1. Antigen Preparation
[0067] In order to solve a series of problems that BVDV, IBRV, BPIV3 adapt to spinner bottle adherent culture, suspension culture, microcarrier suspension culture cell bovine kidney cell line MDBK (from China Veterinary Microorganism Culture Preservation Management Center) process, the invention provides The invention discloses a method for preparing BVDV-L strain, IBRV-J1 strain and BPIV3-S strain by utilizing spinner bottle adherent culture, suspension culture technology and microcarrier suspension culture technology.
[0068] (1) Prepare BVDV-L strain, IBRV-J1 strain, and BPIV3-S strain antigens by using spinner bottle adherent culture cells
[0069] 1) Cultivate cells: First, resuscitate the bovine kidney cell line MDBK into a cell flask, grow for about 48 hours, digest with ED...
Embodiment 2
[0091] ——The finished product inspection of bovine viral diarrhea, bovine infectious rhinotracheitis, and bovine parainfluenza triple inactivated vaccine (L strain + J1 strain + S strain)
[0092] 1. Sterility test: according to the appendix of the current "Chinese Veterinary Pharmacopoeia", it should meet the requirements. The above test results are shown in Table 2.
[0093] Table 2 Physical properties, sterility test results
[0094]
[0095] 2. Safety inspection:
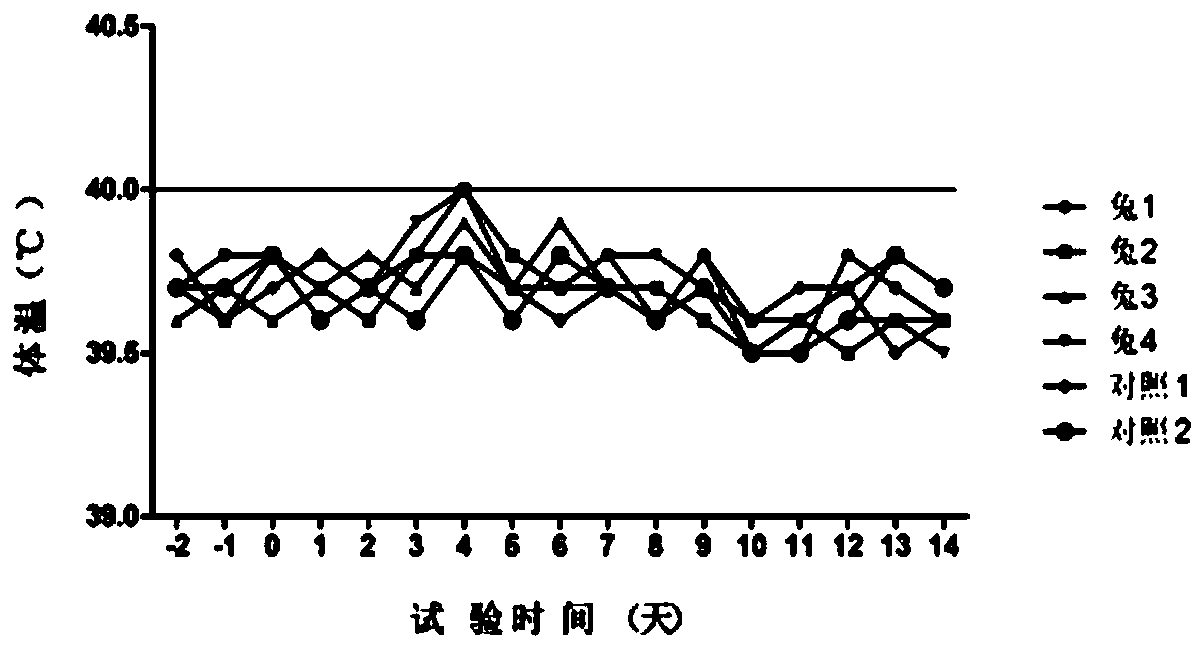
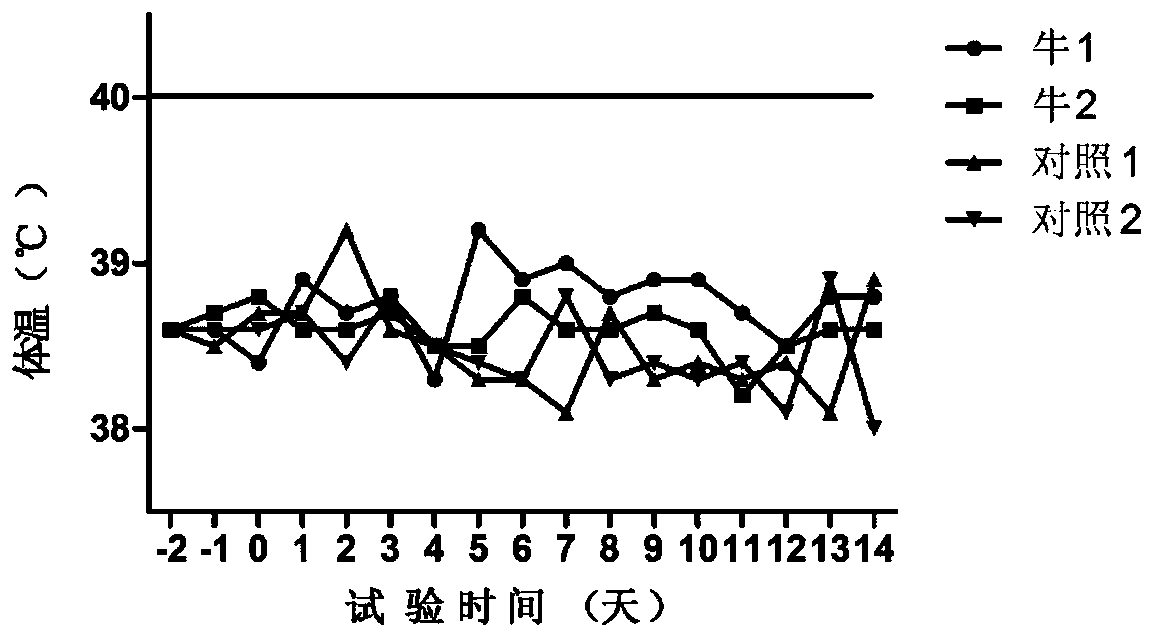
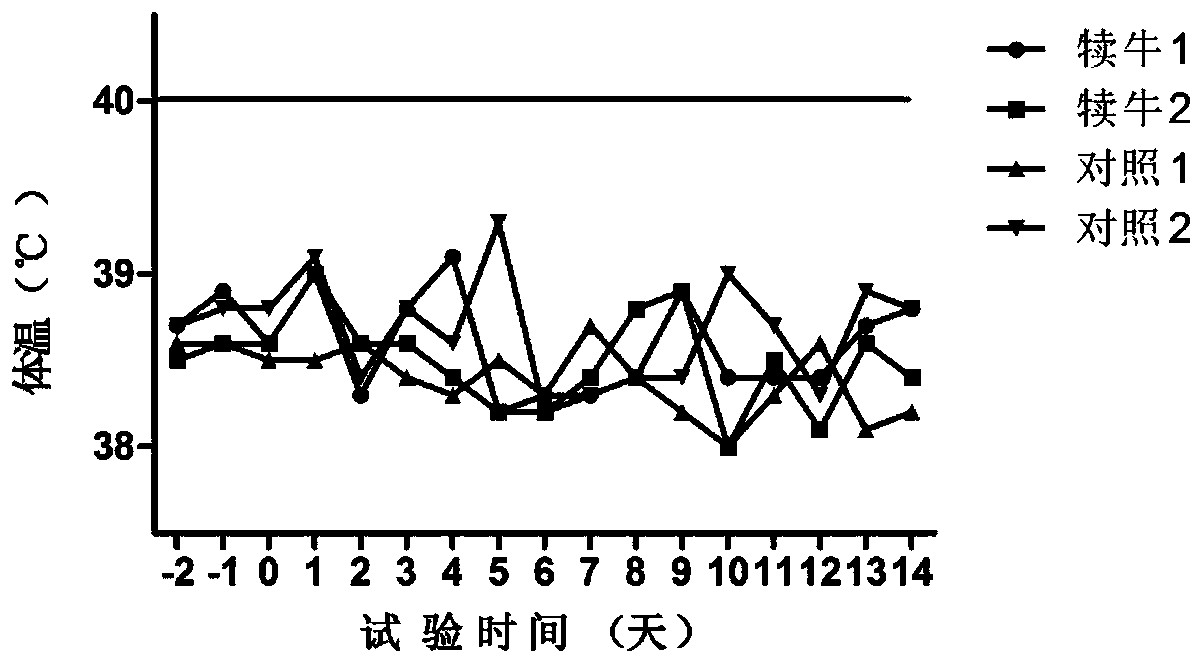
[0096] (1) Substitute animal test: use 6 healthy rabbits weighing 1.5 to 2.0 kg, including 4 in the immunization group and 2 in the control group, inject 1.0ml of vaccine into each leg muscle of the rabbits in the immunization group (two-point injection, 0.5ml / point), each leg of rabbits in the control group was injected intramuscularly with 1.0ml of cell culture (two-point injection, 0.5ml / point), observed continuously for 7 days and measured body temperature at fixed points every afternoon. There is no ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com