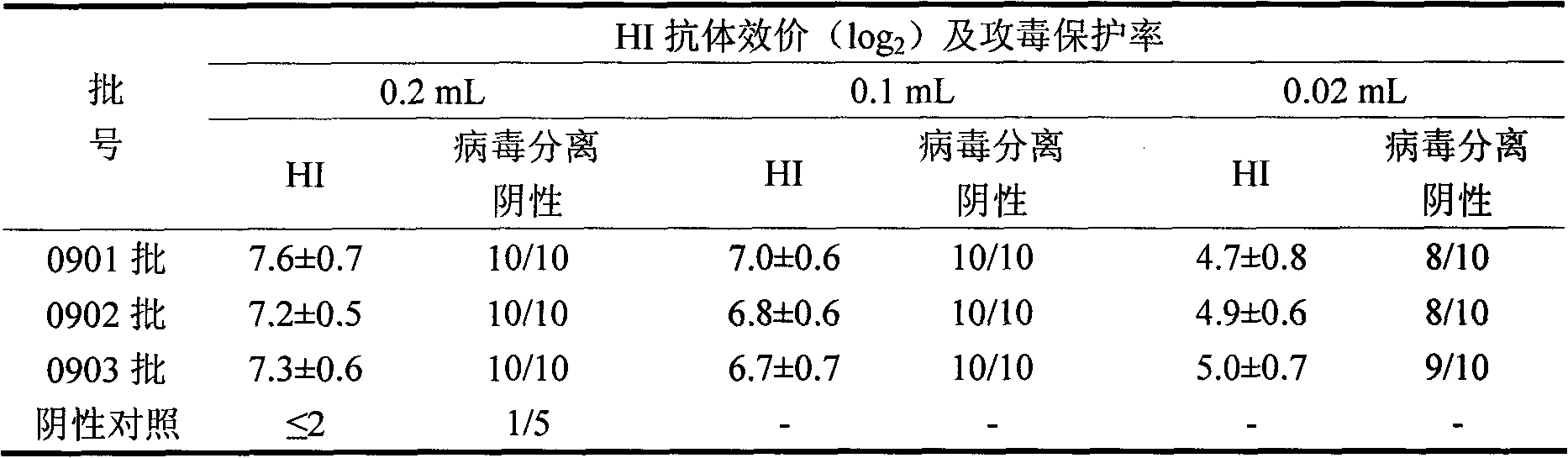
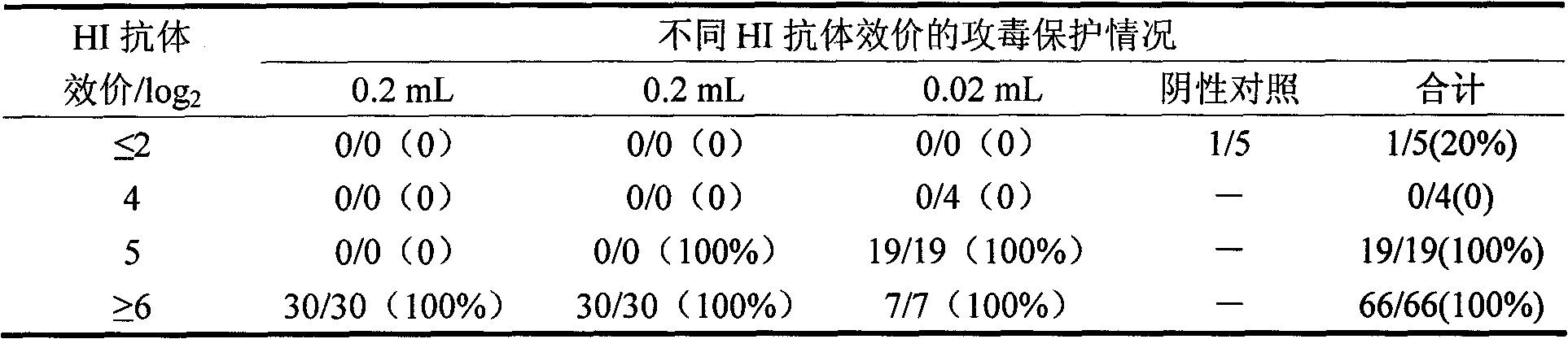
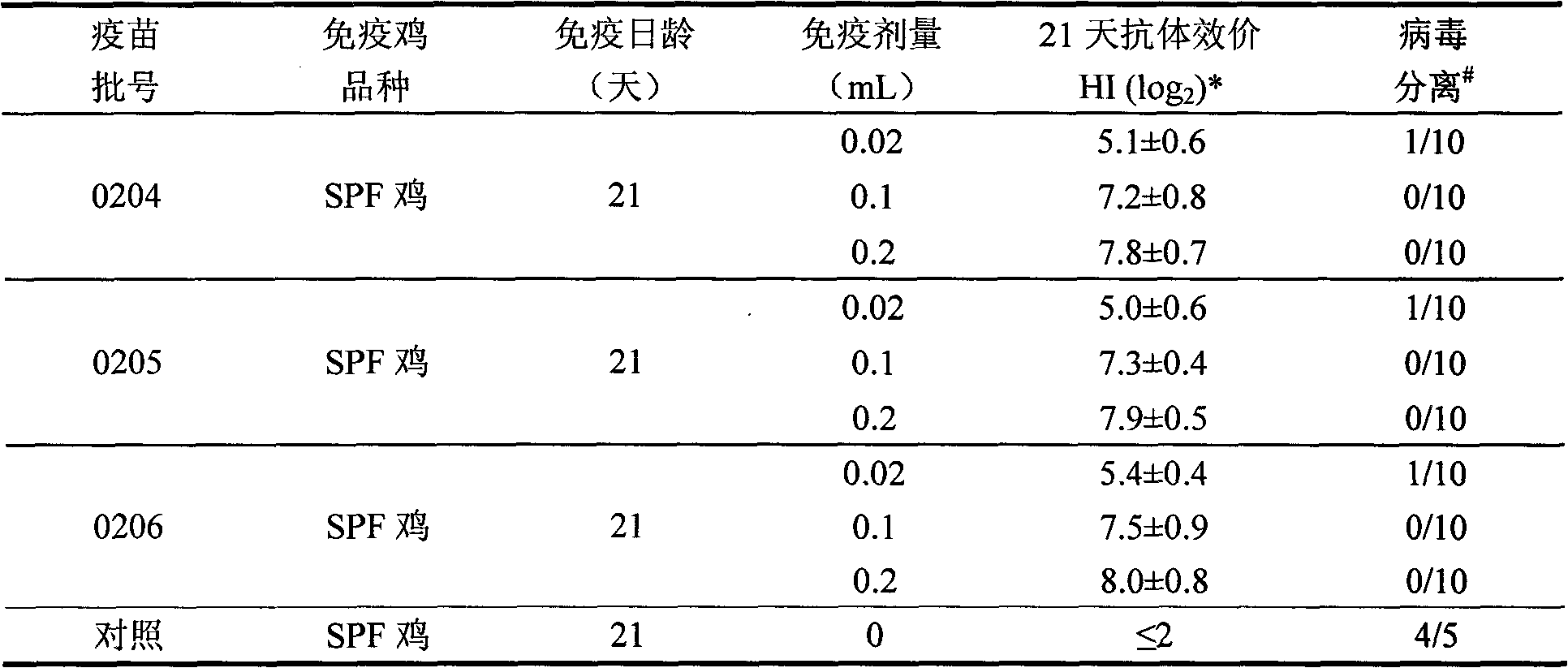
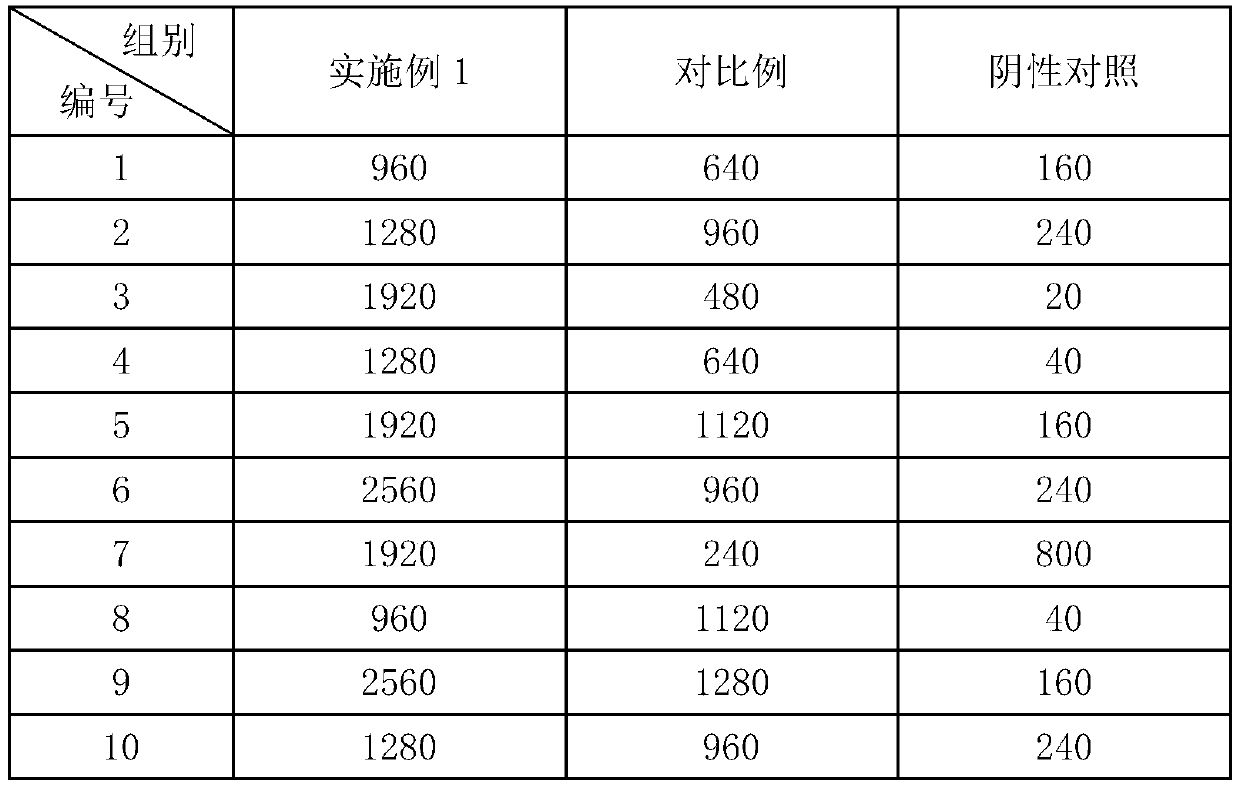
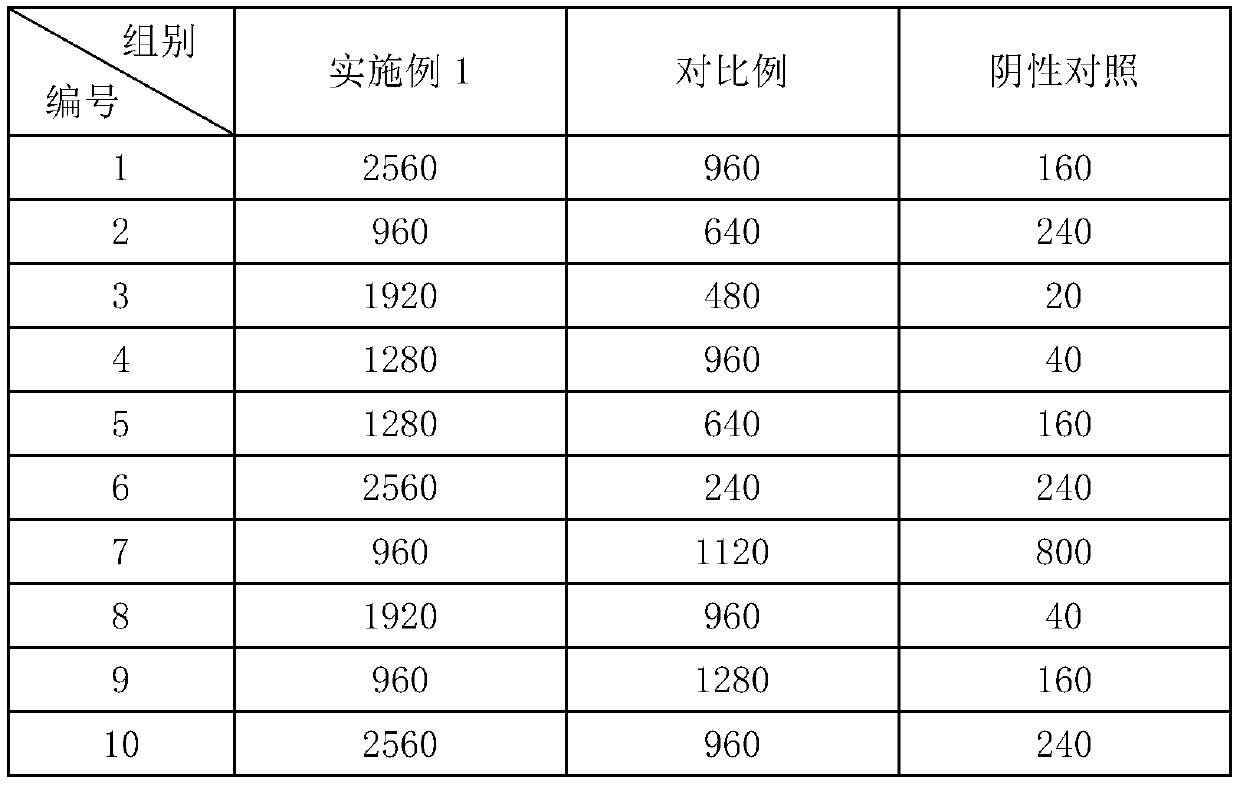
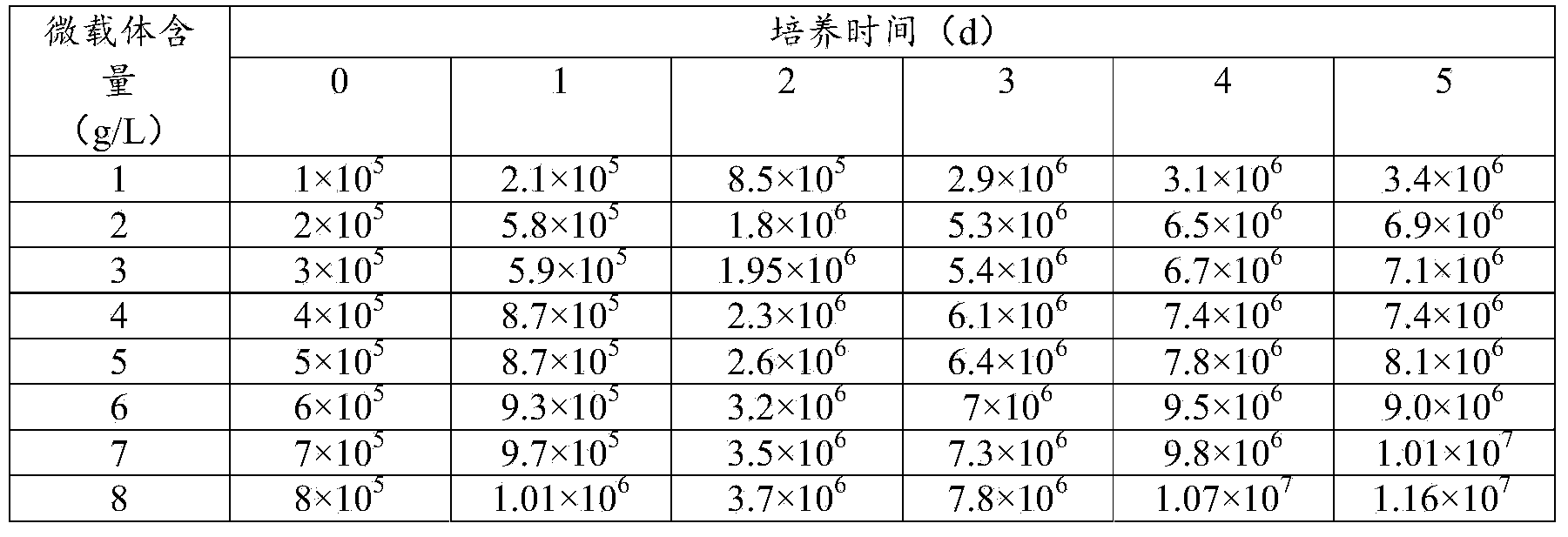
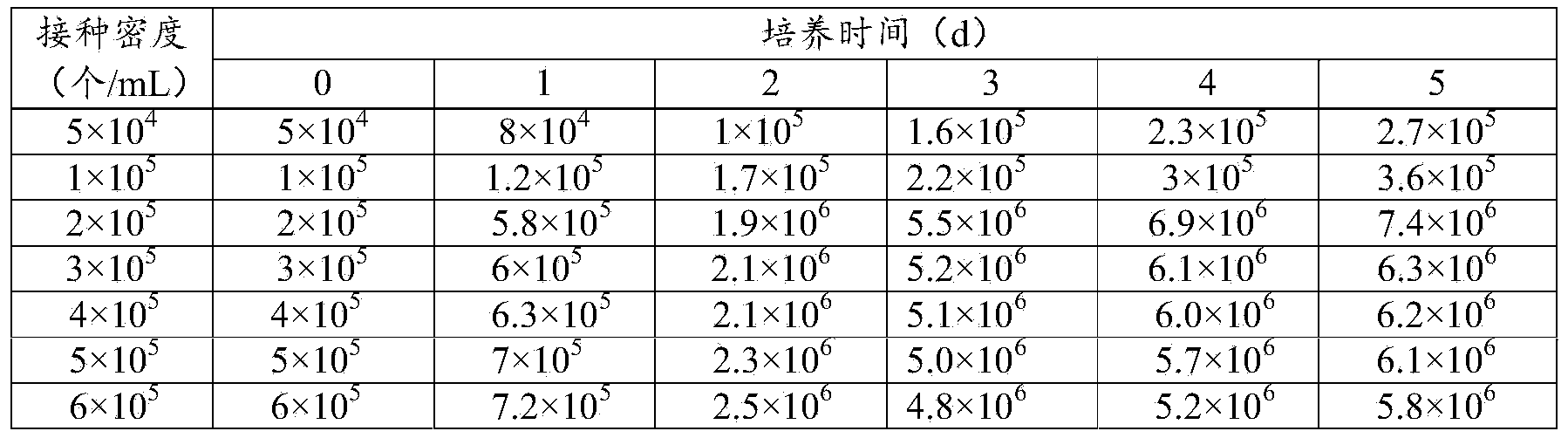
Patents
Literature
115 results about "Virus multiplication" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Virus Multiplication. Abstract. The multiplication of a plant virus covers all the events from the entry of the virus particles into the initially infected cells, via the spread of the virus through the plant, to the formation of complete virus particles ready for transmission to other plants.
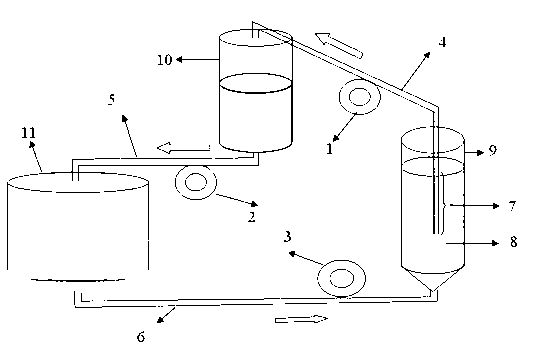
Preparation method and product of H9N2 subtype avian influenza inactivated vaccine
ActiveCN101816785AHigh titerSimple production methodAntiviralsAntibody medical ingredientsVirus multiplicationVaccine Production
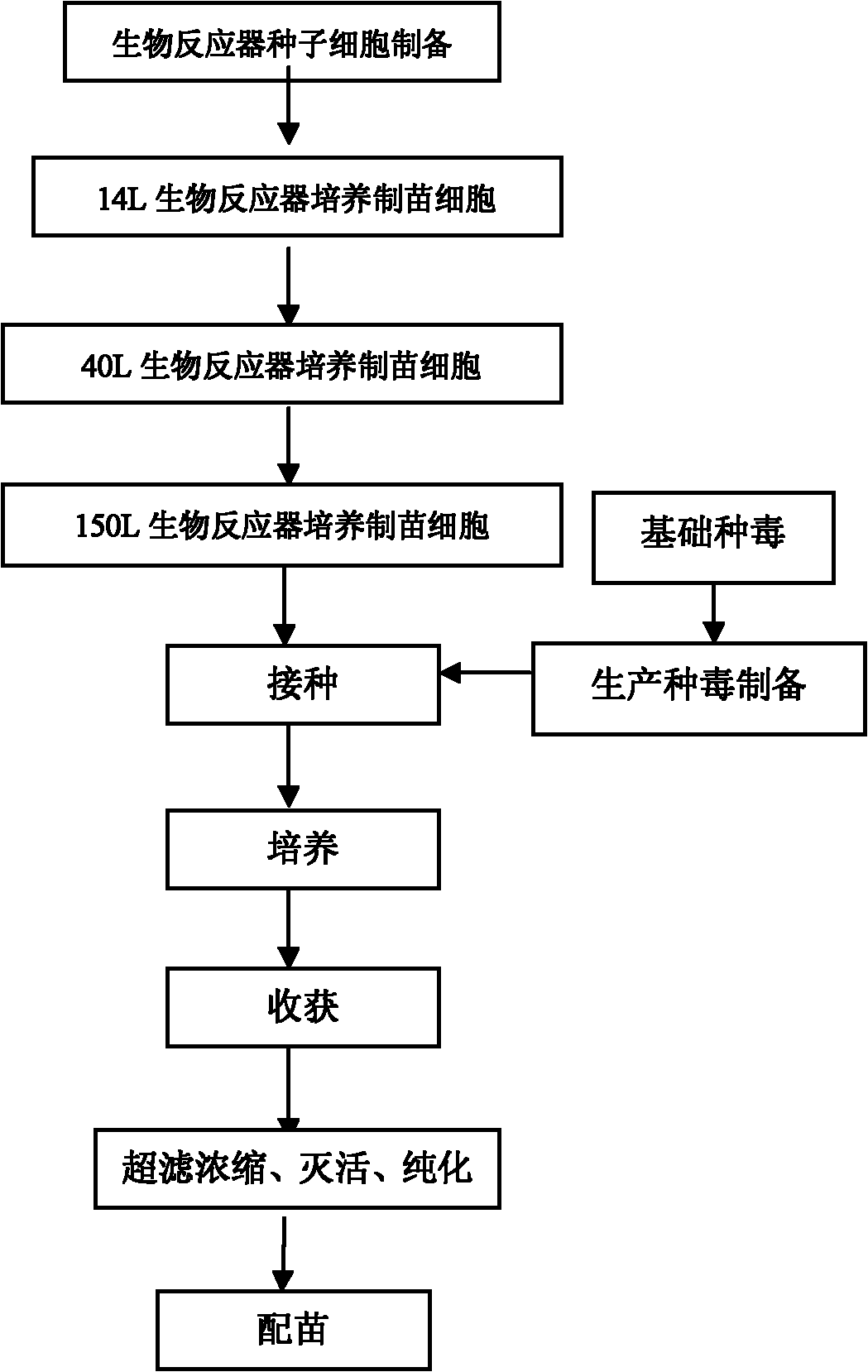
The invention relates to a preparation method and a product of an H9N2 subtype avian influenza inactivated vaccine. The technical points of the invention mainly relate to the screening, the determination and the domestication of a virus-adapted cell line, the primary amplification cultivation and the continuous cultivation of a virus-adapted cell, the preparation of virus fluid by virus-inoculated culture and the preparation of final inactivated vaccine products. Firstly, the invention avoids the virus propagating method using a large amount of chick embryos in the avian influenza production at present, thereby avoiding the problem of biological potential safety hazards, and overcoming the problem that the mass production of vaccines is enslaved to the supply of the chick embryos; secondly, the invention provides a safe, continuous and closed cell culture virus production method, is used for the preparation of the H9N2 subtype avian influenza inactivated vaccine, enables the use of the cell culture method, and can simultaneously produce high-titer viruses to meet the requirements for the immunological production; and finally, the vaccine production method of the invention is simple and fast, thereby realizing the fast vaccine supply at the epidemic situation.
Owner:扬州优邦生物药品有限公司
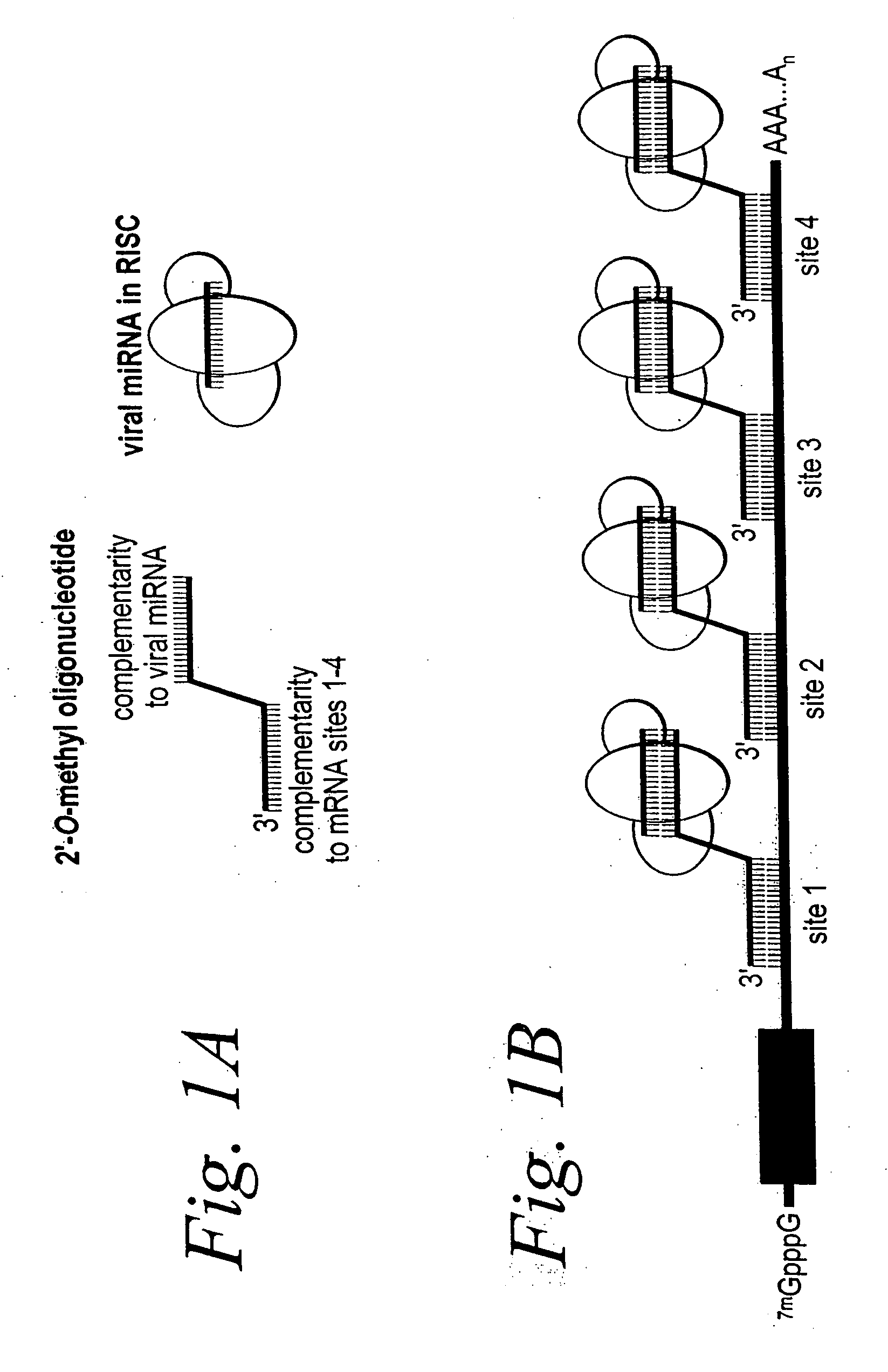
Dual functional oligonucleotides for use as anti-viral agents
InactiveUS20060293267A1Improve in vivo stabilitySugar derivativesActivity regulationTarget mrnaViral infection
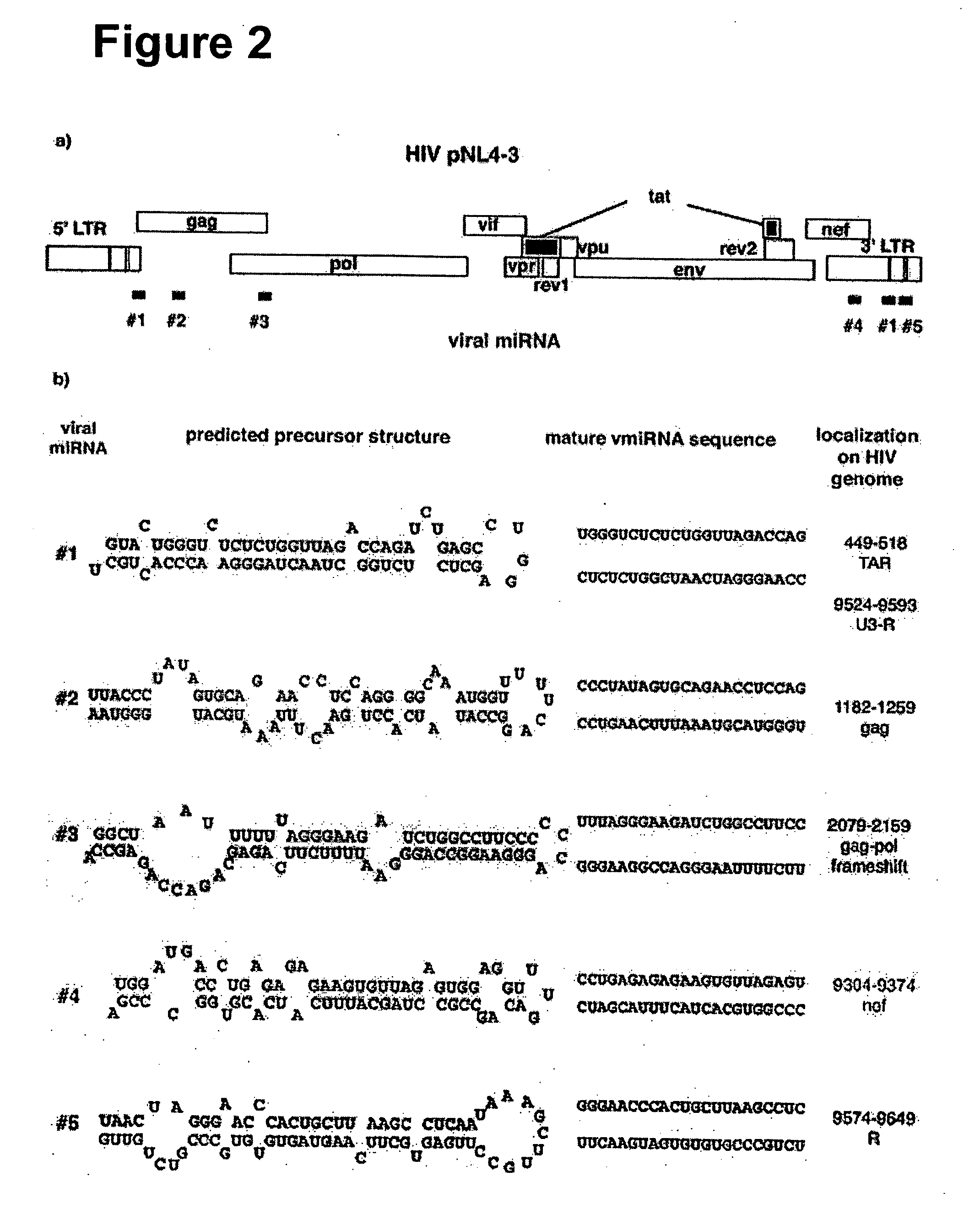
The present invention is based, in part, on the discovery that endogenous mRNAs, such as viral miRNAs, can be recruited for translational repression of target mRNAs, such as viral target mRNAs. The RNA-silencing agents and the methods described herein, thereby provide a means of treating viral infections, of treating diseases or disorders caused by viral infections, or for preventing viral propagation. The RNA-silencing agents of the present invention have an mRNA targeting moiety, a linking moiety, and a viral miRNA recruiting moiety.
Owner:MASSACHUSETTS UNIV OF
Torque teno virus (TTV) isolates and compositions
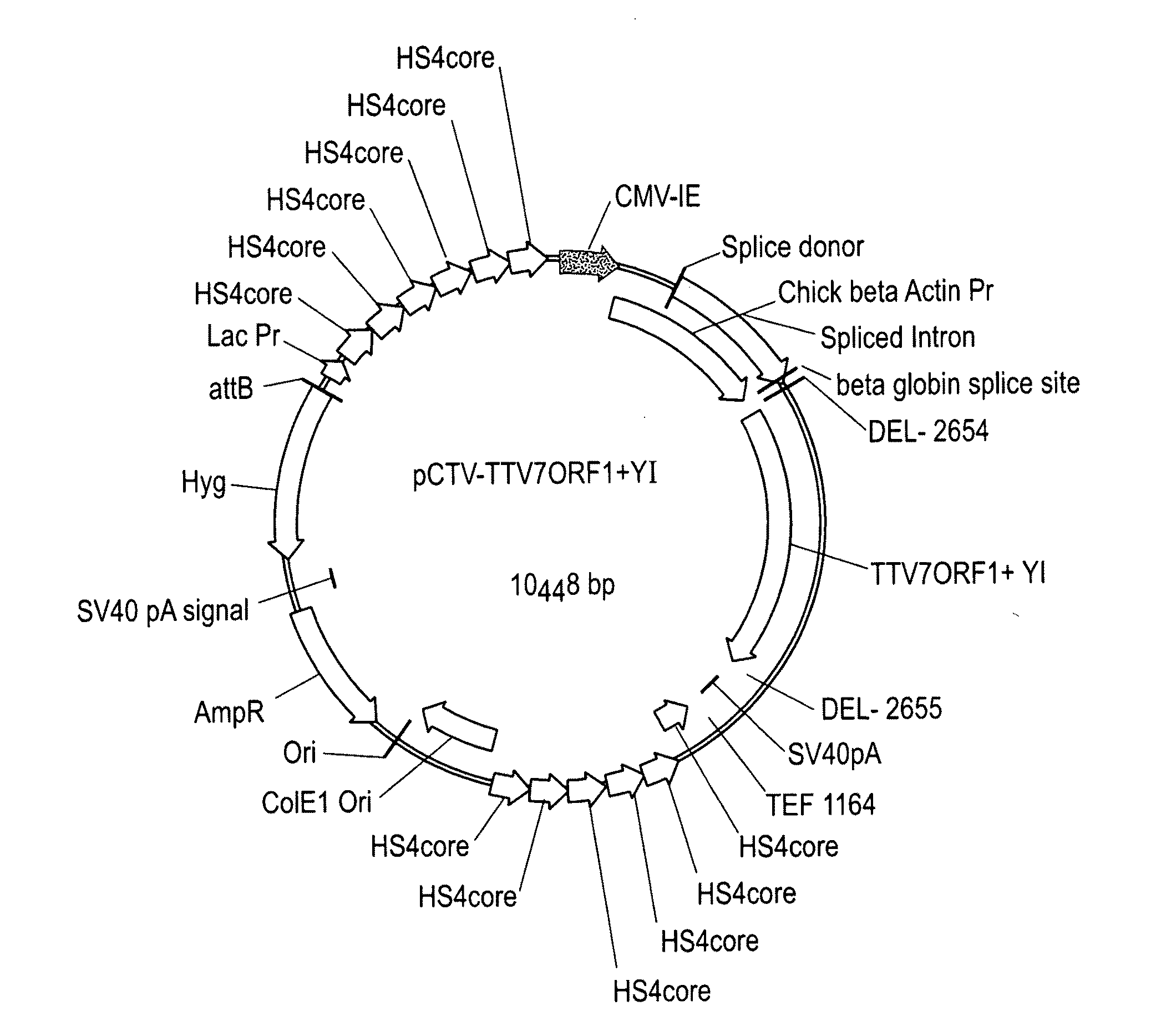
The present invention is directed to novel nucleotide and amino acid sequences of Torque teno virus (“TTV”), including novel genotypes thereof, all of which are useful in the preparation of vaccines for treating and preventing diseases in swine and other animals. Vaccines provided according to the practice of the invention are effective against multiple swine TTV genotypes and isolates. Diagnostic and therapeutic polyclonal and monoclonal antibodies are also a feature of the present invention, as are infectious clones useful in the propagation of the virus and in the preparation of vaccines. Particularly important aspects of the invention include vaccines that provide TTV ORF1 protein, or peptide fragments thereof, as antigen.
Owner:ZOETIS SERVICE LLC
Compounds that modulate negative-sense, single-stranded RNA virus replication and uses thereof
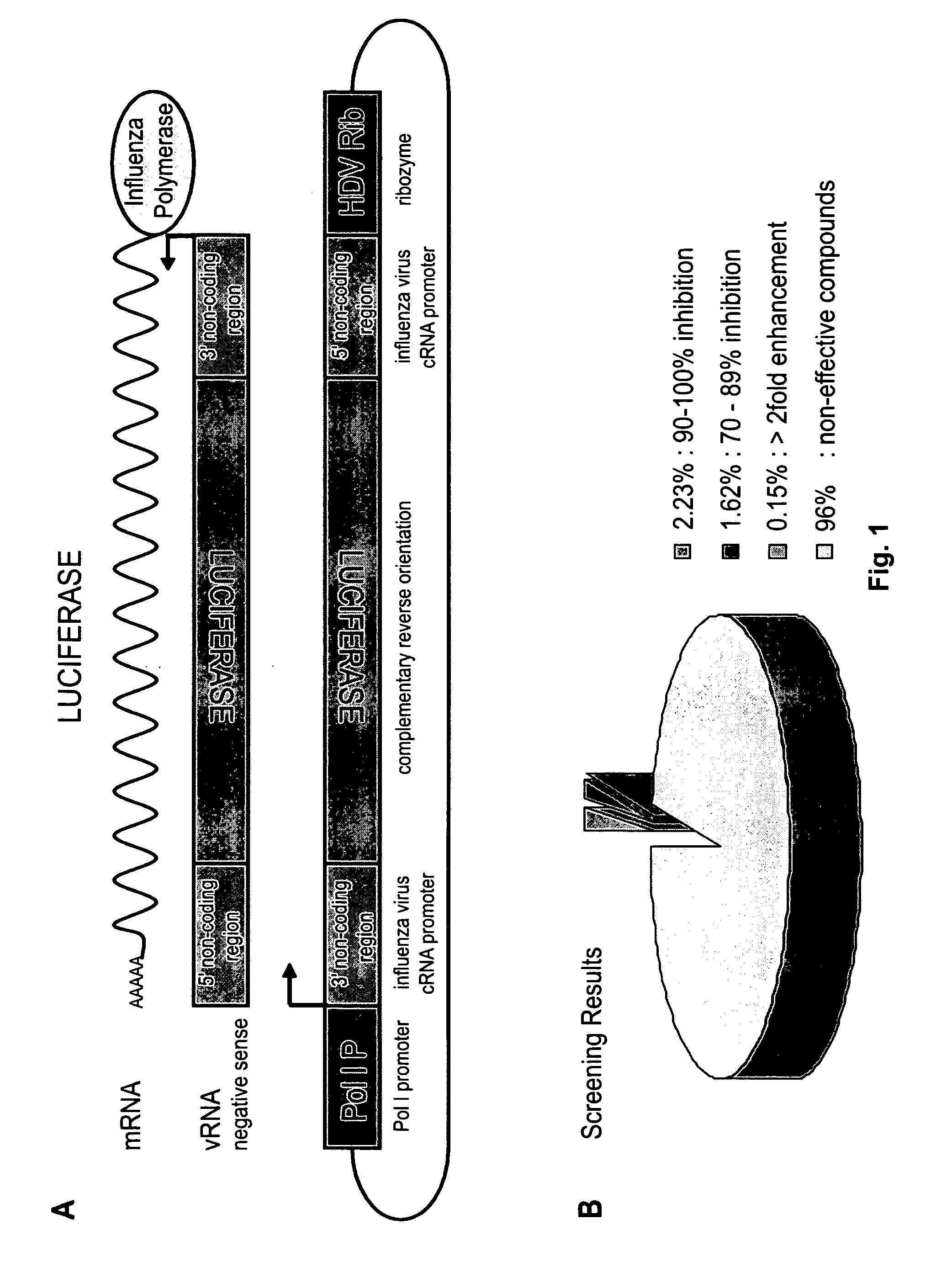
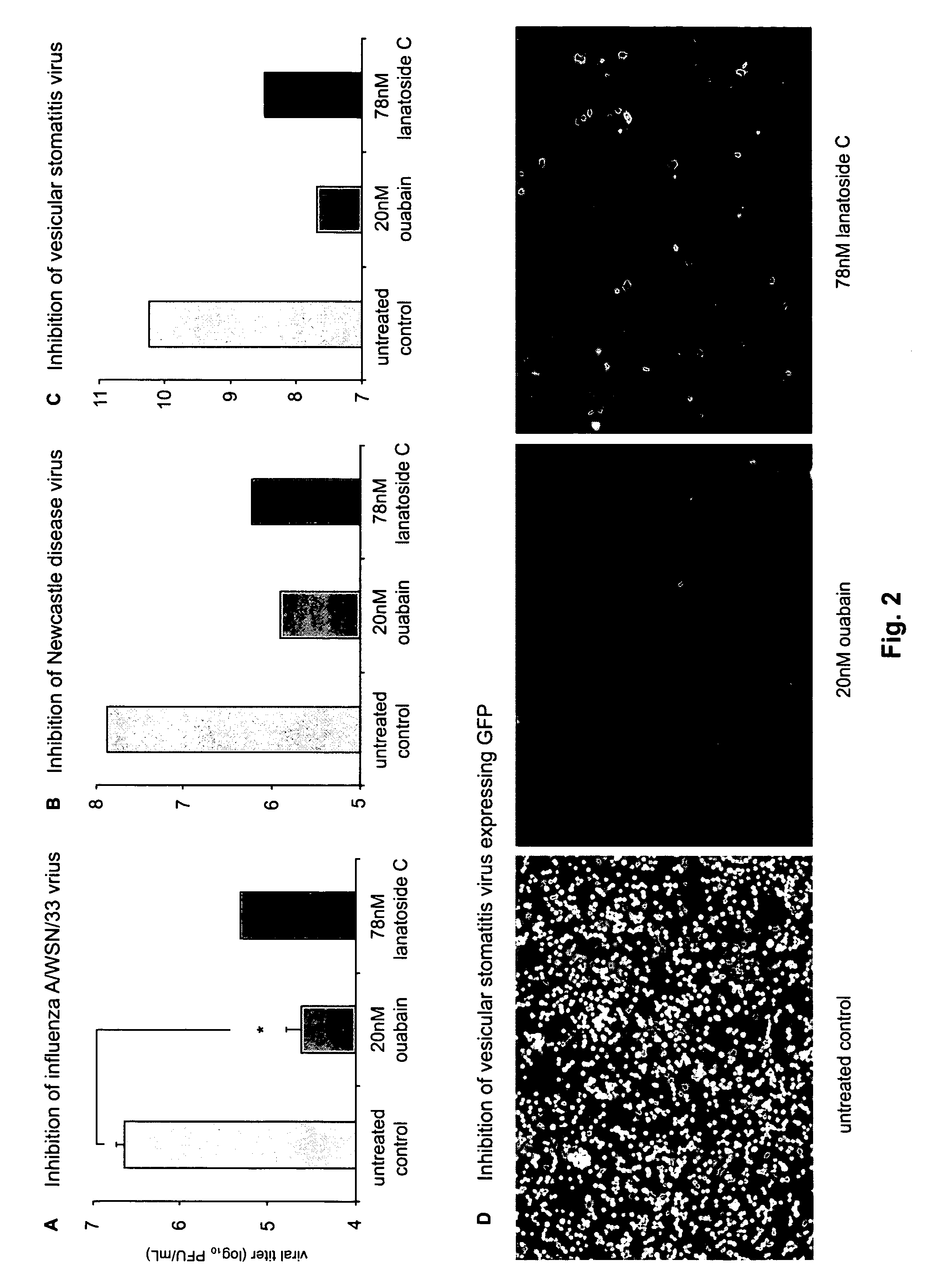
ActiveUS20110105423A1Easy to copyUtility in propagationBiocideOrganic chemistrySingle-Stranded RNAVirus multiplication
The present invention relates to compounds that modulate the replication of negative-sense, single-stranded RNA viruses, such as influenza virus, and the use of such compounds. The invention relates to methods for increasing the titer of negative-sense, single-stranded RNA viruses, such as influenza virus, in substrates for virus propagation (e.g., tissue culture). The invention also relates to the use of compounds that decrease virus replication as antiviral agents. The invention further relates to methods for identifying compounds that modulate the replication of negative-sense, single-stranded RNA viruses, in particular, influenza virus.
Owner:MT SINAI SCHOOL OF MEDICINE
Method for preparing live vaccines of hog cholera and product thereof
InactiveCN101879311ASmall batch-to-batch quality varianceStable production processInactivation/attenuationAntiviralsVaccine ProductionFreeze-drying
The invention discloses a method for preparing live vaccines of hog cholera and a product thereof. The preparation method comprises the following steps of: (1) culturing porcine passage cell lines; (2) inoculating the porcine passage cell lines with live vaccine production seed viruses of the hog cholera to obtain attenuated vaccine strains of the hog cholera; (3) performing virus multiplication on the attenuated vaccine strains of the hog cholera; (4) measuring the virus titer of multiplication virus suspension by adopting an immunofluorescence method; and (5) adding a freeze-drying protective agent and antibiotics into the virus suspension which is detected to be qualified for vaccine matching and freeze-drying. The preparation method has the advantages of producing the live vaccines of the hog cholera by using the cell lines so as to achieve small quality differences among batches and the characteristics of simple and stable process, easy operation, high yield, low cost, the feasibility and extendibility of industrial production and the like, and measuring the virus titer of the multiplication virus suspension by adopting the immunofluorescence method so as to achieve sensitive, fast, specific and accurate detection, high repeatability and reliable results. The live vaccines of the hog cholera prepared by the method can completely protect pigs from the attacks of violent hog cholera viruses.
Owner:武华
Method for producing rabies viruses by suspension culture of BHK21 cells
InactiveCN101851608AHigh titerQuality improvementMicroorganism based processesViruses/bacteriophagesAutomatic controlCell culture media
The invention provides a method for producing rabies viruses by the suspension culture of BHK21 cells, which comprises a step of performing suspension culture of BHK21 cells in bioreactor containing a BHK21 cell culture medium, wherein the conditions for the suspension culture in the step include a temperature of 32 to 36 DEG C, a pH value of 7.0 to 8.0 and a dissolved oxygen concentration of 30 to 50 percent; and the BHK21 cell culture medium comprises the components shown in a table 1. The method overcomes the biases of the prior art and realizes the production of the rabies viruses by the suspension culture of BHK21 cells in the bioreactor. The obtained rabies viruses can be used for producing rabies vaccine. Due to the automatic culture environment parameter control of the bioreactor, the cells grow and the viruses propagate in more favorable environments, the virus titer is improved and the large-scale automatic continuous production can be realized.
Owner:BEIJING SKYWING TECH CO LTD
Method for diagnosing and alleviating the symptoms of chronic fatigue syndrome
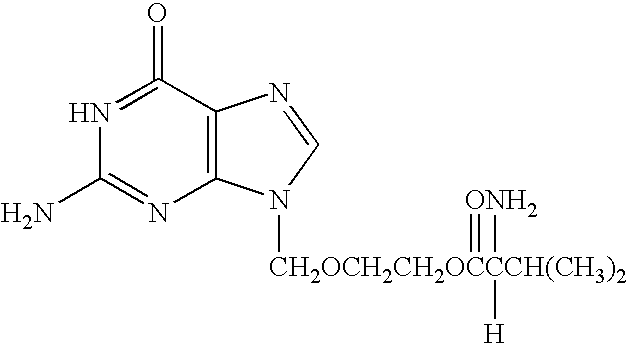
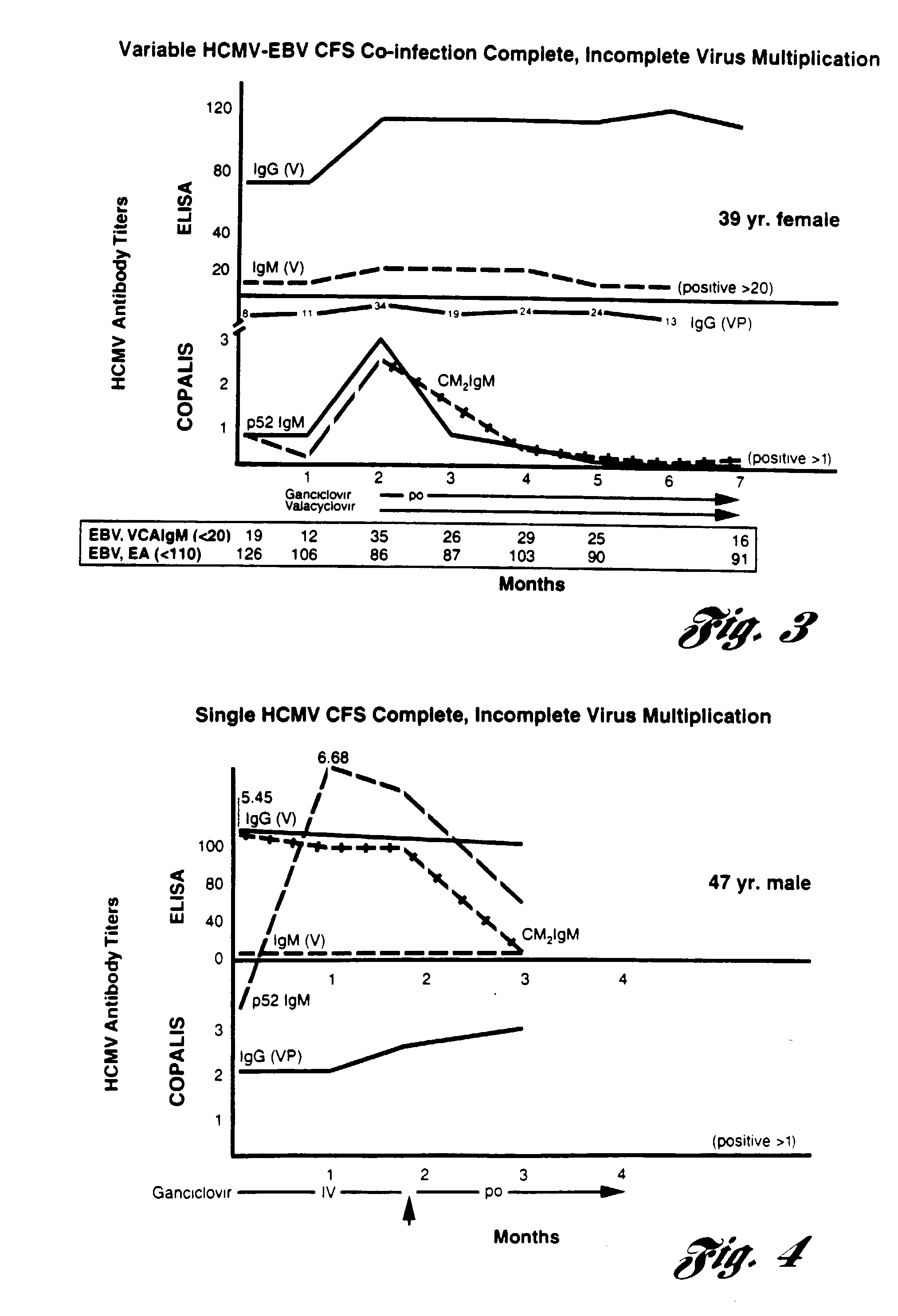
InactiveUS20040072144A1Relieve symptomsEfficient multiplicationBiocideMicrobiological testing/measurementVirus multiplicationClinical trial
A method for alleviating chronic fatigue syndrome with the administration of antiviral agents. Based on clinical tests, chronic fatigue syndrome is a persistent herpes virus infection including incomplete virus multiplication and thus administration of antiviral agents are shown to alleviate the symptoms associated with the disorder. Based on therapeutic trials, patients receiving the recommended antiviral treatment, have experienced significant reduction or elimination of the symptoms associated with chronic fatigue syndrome. A method of diagnosis of the chronic fatigue syndrome is further disclosed.
Owner:LERNER A MARTIN
Avian influenza virus inactivated vaccine and preparation method thereof
InactiveCN102600464AAchieve mass productionIncrease volumeAnimal cellsAntiviralsAdjuvantVaccine Production
The invention discloses an avian influenza virus inactivated vaccine and a preparation method thereof. Adaptive avian influenza viruses are adaptive to screening and domestication of a continuous cell line, and after the viruses are adaptive to cells, the number of the viruses is continuously increased until the viruses are inoculated into a cell culture bottle or a bioreactor to culture. After the obtained culture is inactivated, the obtained culture and mineral oil adjuvant are mixed and emulsified so as to prepare the vaccine. A virus reproduction method for producing an avian vaccine by means of utilizing a large quantity of chicken embryo is omitted, resource consumption is reduced, environmental pollution is decreased, biosafety is guaranteed, cost is lowered, and production efficiency is improved. By the aid of the preparation method, high-potency viruses can be produced to prepare corresponding vaccines, and requirements of fast and high-efficient vaccine production are met.
Owner:SINOPHARM YANGZHOU VAC BIOLOGICAL ENG CO LTD +1
Production process of fusion expression recombinant chicken interferon alpha
InactiveCN106399321AStrong antiviral activityEfficient expressionFermentationInterferonsEscherichia coliInclusion bodies
The invention discloses a production process of fusion expression recombinant chicken interferon alpha. The process includes the steps of: S1, according to the preference of Escherichia coli codon, conducting codon optimization on a chicken interferon alpha gene sequence published in Genebank, and artificially synthesizing the chicken interferon alpha gene; S2, according to the codon optimized chicken interferon alpha gene, designing three specific primers; S3, constructing recombinant chicken interferon alpha plasmid containing ProS2 dissolution promoting label; S4, transforming and identifying the recombinant expression plasmid; S5, conducting inducible expression of recombinant chicken interferon alpha; S6, extracting an expression product and conducting protein renaturation purification: S61, inclusion body extraction and treatment; S62, inclusion body denaturation; S63, denaturation solution renaturation; and S64, nickel column affinity purification. By means of cell cytopathic inhibition, the invention detects that the interferon has the activity of inhibiting vesicular stomatitis virus proliferation, and the activity unit reaches 7.32*10<7>UI / mg.
Owner:SOUTH CHINA AGRI UNIV +1
Use of active ingredients for the prophylaxis and/or therapy of viral diseases
The invention relates to the use preferably of at least one active ingredient for the prophylaxis and / or therapy of a viral disease, wherein this active ingredient inhibits at least one component of the cellular signal transduction pathway for the activation of the transcription factor NF-kB such that the virus multiplication is inhibited. The present invention relates furthermore to the local, preferably aerogenic administration of the active ingredient according to the invention for inhibiting a virus multiplication. The active ingredient according to the invention may be combined with at least one further antivirally effective substance for the prophylaxis and / or therapy of a viral disease.
Owner:INAMED GMBH INST FUR AEROSOLMEDIZIN
Nasal spray preparation for resisting respiratory virus infection
PendingCN111529685AExpand the scope ofImprove adaptabilityOrganic active ingredientsPeptide/protein ingredientsLiposomeSaline
The invention relates to a nasal spray preparation for resisting respiratory virus infection. The preparation comprises liposome-loaded angiotensin converting enzyme 2 (ACE2) recombinant protein, sialic acid, natural polysaccharide and normal saline. The nasal spray preparation is mainly prepared from the liposome-loaded ACE2 extracellular domain recombinant protein, can competitively inhibit thecombination of viruses and host cells, prevents and treats respiratory tract infection caused by various viruses, and enhances the antiviral range and clinical indications of the nasal spray preparation for resisting respiratory tract virus infection. In addition, the nasal spray preparation can be used for directly spraying medicines to virus propagation parts, and is convenient and simple to use.
Owner:XIAMEN NUOKANGDE BIOLOGICAL TECH CO LTD +1
Virus-like particles for treatment of viral infections
The invention provides virus-like particles for treatment of viral infections based on the virus causing the infection. The virus-like particles comprise the virus recombinant proteins that form a capsid, recombinant virus membrane proteins attached to the capsid and vRNA packaged within said capsid. The vRNA is generated from a DNA sequence encoding a polypeptide capable of specifically binding to a constant region of a nonstructural protein of the virus that is essential for propagation of the virus.
Owner:BEN GURION UNIVERSITY OF THE NEGEV
Production method of canine parvovirus inactivated vaccine by utilizing bioreactor
InactiveCN102861329AAvoid process problemsAvoid timeAntiviralsAntibody medical ingredientsAntigenAdjuvant
The invention discloses a production method of a canine parvovirus inactivated vaccine by utilizing a bioreactor. The production method comprises the following steps: (1) culturing a vaccine producing cell by applying a microcarrier and chip carrier system of the bioreactor; (2) vaccinating a canine parvovirus and performing virus multiplication culture; (3) harvesting virus culture fluid; and (4) inactivating the virus fluid with BEI (binary ethyleneimine), and adding adjuvant to prepare the vaccine. The canine parvovirus inactivated vaccine has the advantages of high density of cultured cell, high virus titer, uniform and stable quality, controllable process and high production efficiency, and the defects of large difference among product batches, low antigen content and low production efficiency in a conventional spinner bottle or chick embryo production process can be overcome.
Owner:WUHAN CHOPPER BIOLOGY
Influenza virus subunit vaccine and preparation method thereof
InactiveCN104888212AHigh purityQuality improvementAntiviralsAntibody medical ingredientsHemagglutininAdjuvant
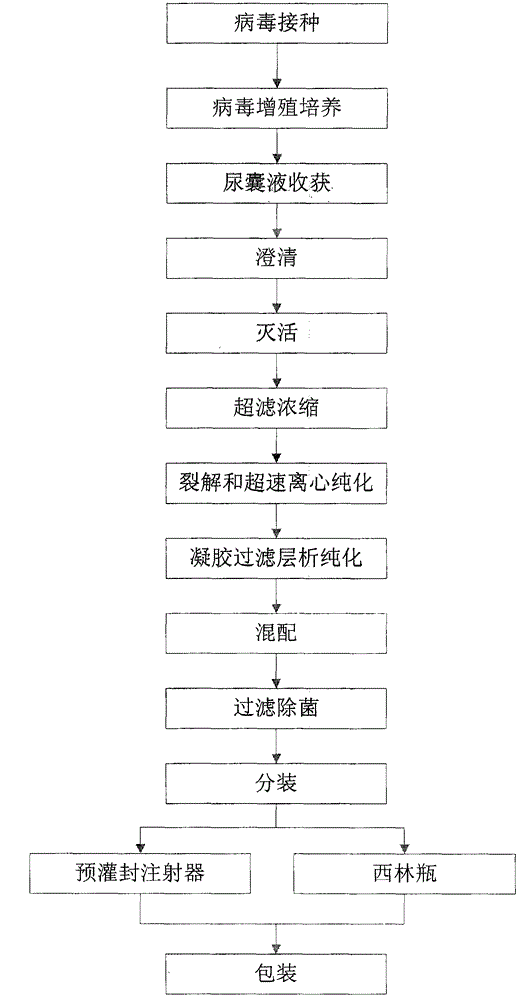
The invention discloses an influenza virus subunit vaccine and a preparation method thereof. The influenza virus subunit vaccine is formed by further purifying cracked viral proteins through a cracking agent and a new purification method; each agent of subunit vaccine comprises more than 80% of H1N1, H3N2 and B type influenza hemagglutinin, and does not comprise adjuvant, thiomersal or other corrosion removers. The invention further provides the preparation method of the influenza virus subunit vaccine. The preparation method of the influenza virus subunit vaccine comprises the following steps of virus inoculation, virus multiplication culture, allantonic fluid harvesting, clarification, inactivation, ultrafiltration and concentration, cracking and overspeed centrifugal purification, gel filtration chromatography purification, mixture, filtration sterilization, subpackage, packaging and the like. The influenza virus subunit vaccine can improve safety, eliminate untoward effects caused by adjuvant and eliminate the toxic and side effects caused by thiomersalate.
Owner:ZHONGYI ANKE BIOTECH CO LTD
Method for producing blue-ear disease vaccine by culturing sensitive cell
InactiveCN101612395AQuality improvementIncrease cell densityViral antigen ingredientsAntiviralsHigh cellDisease
The invention provides a method for producing a blue-ear disease vaccine by culturing a sensitive cell. The method comprises the following steps: 1) culturing the sensitive cell on a microcarrier in a bioreactor; 2) inoculating a porcine reproductive and respiratory syndrome virus to the sensitive cell cultured on the microcarrier; 3) continuously culturing the sensitive cell; and 4) obtaining virus solution to produce the blue-ear disease vaccine. The method produces the blue-ear disease vaccine by culturing the sensitive cell in the bioreactor by applying microcarrier culture technology. The microcarrier provides larger surface area, the cell can reproduce to generate higher cell density, and automatically controlled culture environment parameters of the bioreactor ensure that the cell growth and virus propagation can be in an excellent environment, so the method can improve the virus titer, and ensures more stable vaccine quality.
Owner:扬州优邦生物药品有限公司
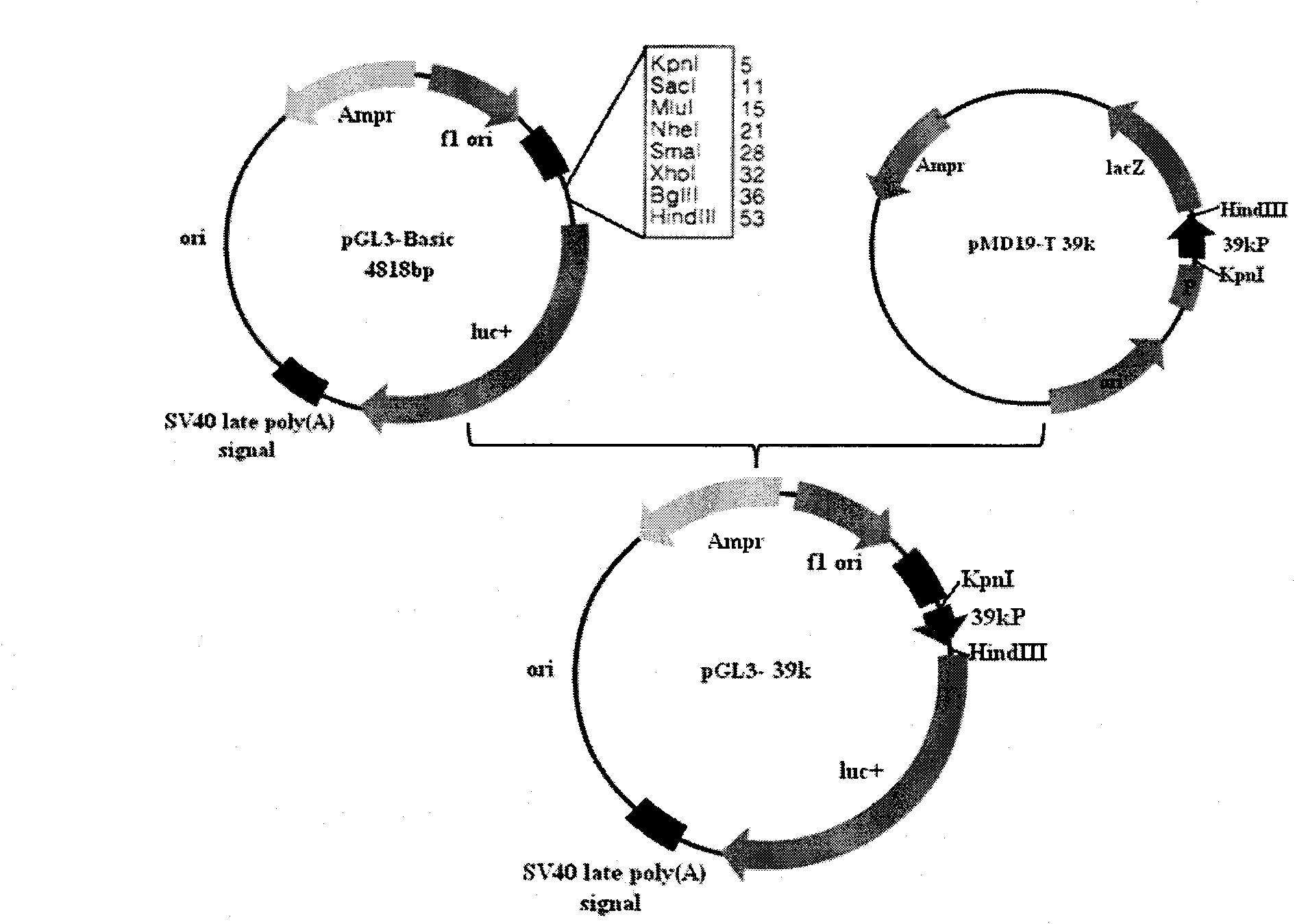
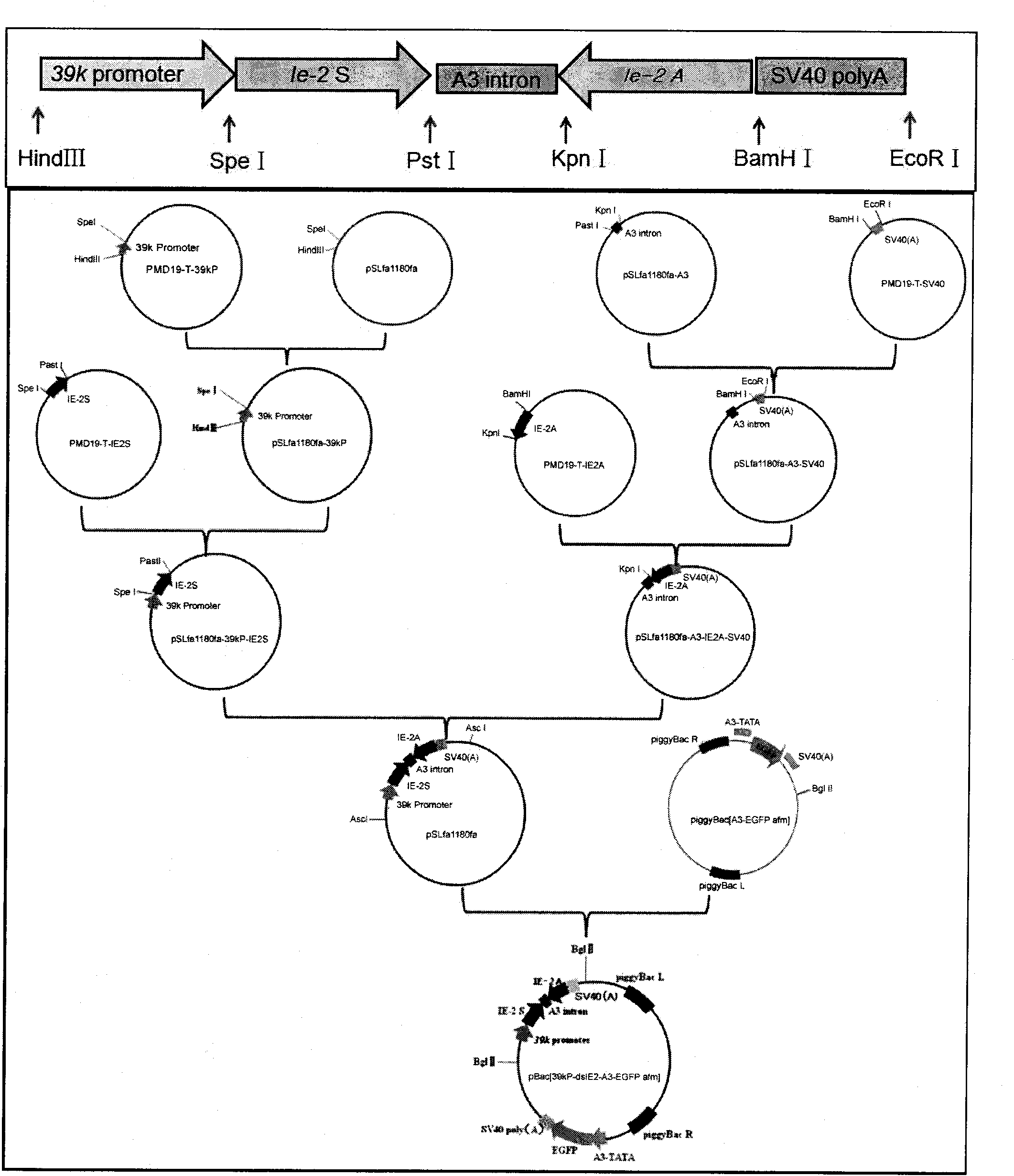
Bombyx mori nuclear polyhydrosis virus (BmNPV) 39k inducible promoter and application thereof
InactiveCN101914537APriming activityPrevent proliferationFungiBacteriaVirus multiplicationBombyx mori
The invention discloses a bombyx mori nuclear polyhydrosis virus (BmNPV) 39k inducible promoter and application thereof. A nucleotide sequence of the promoter is shown as SEQ ID No.1; the promoter has obvious BmNPV inducible promotion activity and can make cells start foreign gene expression when the cells are subjected to the BmNPV infection; an RNAi vector using a BmNPV multiplication essential gene as a target can be built by utilizing the promoter; the abundant expression of shRNA can be performed when the bombyx mori is subjected to the BmNPV infection; the shRNA is cut into siRNA in a cell by an enzyme; specific degradation is performed on the BmNPV multiplication essential gene mRNA to initiate the BmNPV multiplication essential gene to be transcribed and then silenced, so the aim of inhibiting the virus multiplication is fulfilled, and the promoter can be used for preventing and controlling the BmNPV infection of the bombyx mori, performing genetic engineering breeding of bombyx mori anti-BmNPV strains and providing a good reference mode for transgenic therapy of other biologic virus diseases.
Owner:SOUTHWEST UNIVERSITY
Method for industrially producing swine parvovirus vaccine by utilizing bioreactor
ActiveCN102038945AHigh titerHigh degree of automation controlMicroorganism based processesAntiviralsHigh cellUltrafiltration
The invention provides a method for industrially producing a swine parvovirus vaccine by utilizing a bioreactor, comprising the following steps of: (1) sterilizing a micro-carrier and the bioreactor, adding a cell growth solution, inoculating, preparing the vaccine and culturing with cells, inoculating a swine parvovirus after the cell on the micro-carrier forms a compact single layer, and continuously culturing to propagate the virus; (2) stopping culturing until the cytopathy reaches more than 80%, and harvesting a virus solution; (3) carrying out ultrafiltration concentration and virus inactivation on the harvested virus solution; and (4) purifying and inactivating the virus through a column chromatography method to prepare the vaccine. The invention has the advantages of favorable controllability of processing parameters, high cell density and virus titer, favorable vaccine safety, stable and reliable quality, high production efficiency, and the like.
Owner:WUHAN CHOPPER BIOLOGY
Preparation method of avian infectious brunchitis virus HA antigen
InactiveCN101455838AExtended shelf lifeEasy to storeAntiviralsAntibody medical ingredientsAntigenSerum ige
The invention discloses a method for preparing a chicken infectious bronchitis virus HA antigen. The chicken infectious bronchitis virus HA antigen is prepared by the steps of virus multiplication, virus solution concentration and phospholipase C treatment, and antigen inactivation and stabilization. The stability of the prepared HA antigen at a temperature of 4 DEG C is prolonged to more than 6 months from the original 24 hours; besides, the valence of antigen is stable so as to solve the difficult problems which are not solved for a long time that the antigen is temporarily prepared for the test of each time and needs to be detected before use, and reduce the workload of the HI test. The prepared IBV HA antigen can be successfully applied to detecting the valence of antibody of serum HI after the chicken IBV vaccine immunization, and solves the problem that the detection of the IBV vaccine immunization effect is realized through neutralization tests in China for a long time, thereby wasting time and energy and having the danger of poison diffusion.
Owner:HENAN AGRICULTURAL UNIVERSITY
Application of H3N2 canine influenza virus CGD1
ActiveCN103223162AReduce severityShorten the detox periodAntiviralsAntibody medical ingredientsTGE VACCINEMicrobiological culture
The invention belongs to the technical field of preparation of virus vaccine, and concretely discloses an application of an H3N2 canine influenza virus CGD1. According to the invention, three self-isolated, identified and preserved strains of H3N2 canine influenza virus CGD1, CGD2 and CGD3 are used to inoculate chick embryo for subcultring, and the CGD1 is found to be good in genetic stability. Therefore, the CGD1 is selected for plaque purification to breed an H3N2 canine influenza virus vaccine strain CIVGDYM1, and the vaccine strain has been preserved in China general microbiological culture collection center, with an accession number being CGMCCNO: 7218. By using the H3N2 canine influenza virus vaccine strain CIVGDYM1 to inoculate the chick embryo for virus breeding, and through gaining allantoic fluid of the chick embryo, inactivating, preparing vaccine and other steps, the safe and effective H3N2 canine influenza virus inactivated vaccine can be prepared.
Owner:SOUTH CHINA AGRI UNIV
Tetravalent influenza virus subunit vaccine and preparation method thereof
ActiveCN111420044AReduce contentExtended shelf lifeSsRNA viruses negative-senseViral antigen ingredientsVirus multiplicationUltrafiltration
The invention belongs to the technical field of biology, and particularly relates to a tetravalent influenza virus subunit vaccine and a preparation method thereof. Each dose of the tetravalent influenza virus subunit vaccine contains H1N1, H3N2 and two types of B; and the tetravalent influenza virus subunit vaccine is prepared by virus inoculation, virus multiplication culture, allantoic fluid harvesting, clarification, inactivation, ultrafiltration concentration, cracking and ultracentrifugal purification, mixing, filtration sterilization, split charging and packaging, wherein the inactivation process comprises the following steps: firstly, adding a carboxymethyl glucan solution into monovalent virus harvesting liquid, and then adding formaldehyde for inactivation. According to the method disclosed by the invention, the content of free formaldehyde in the prepared tetravalent influenza virus subunit vaccine is reduced, the storage life of the vaccine is prolonged, and the antibody level after vaccine immunization is increased.
Owner:ZHONGYI ANKE BIOTECH CO LTD
Method for producing avian influenza vaccine by using WAVE bioreactor
ActiveCN103394081AImprove cultivation efficiencyIncrease production capacityAntiviralsAntibody medical ingredientsVirus multiplicationVaccine Production
The invention discloses a method for producing an avian influenza vaccine by using a WAVE bioreactor, which comprises the following steps: (1) cell culture: inoculating cells onto a micro supporter of a cell culture bag, adding a cell culture medium, and shaking the cell culture bag to perform cell culture; (2) virus solution multiplication: settling the micro supporter, washing the cultured cells, inoculating with an avian influenza virus seed, adding a virus multiplication culture medium, and further performing cell culture, thus obtaining a virus solution; and (3) inactivating the virus solution, and emulsifying to obtain the avian influenza vaccine. The method disclosed by the invention optimizes the technological parameters in the process of culturing MDCK cells by using a WAVE bioreactor, effectively improves the MDCK cell culture efficiency, and finally increases the avian influenza vaccine production efficiency remarkably. The immunoprotection efficacy experiment proves that the avian influenza vaccine prepared by the method disclosed by the invention has exact immunoprotection efficacy for poultry.
Owner:黑龙江省百洲生物工程有限公司
Reovirus vaccine and its preparing method
ActiveCN1884497AFill in the gapsImprove immunityViral antigen ingredientsAntiviralsMicroorganismFowl
A reovirus vaccine and its method for preparation belong to the technical sphere of druggist sundries and are used to solve the indigenous fowl reovirus infection vaccine problem. The separated reovirus is of CGMCC No.1713 as its preserved number and has been preserved in China microbial bacterial preservation management committee common microorganism center on May 16th, 2006. The said vaccine is prepared by steps including virus isolation from organ of diseased chicken, serum check, virus breeding and generation and cryodesiccation. The invention separates the virus from the diseased chicken with ARV infection of indigenous fowls, applies them in immune chicken, and produces immune response within 1 week and produces strong immunity within 2 weeks. The immune time is 2-2.5 months, and the detoxicating protecting rate during immune period is 94.8%-98.8%, which is explicitly higher than import vaccine protecting rate and can make up for the domestic defect of vaccine vacancy in reovirus affection.
Owner:RINGPU (BAODING) BIOLOGICAL PHARMACEUTICAL CO LTD
Full-suspension culture method for avian influenza virus
ActiveCN107904215ANon-tumorigenicEasy to useSsRNA viruses negative-senseRecovery/purificationVirus multiplicationAvian influenza virus
The invention discloses a full-suspension culture method for avian influenza virus. The full-suspension culture method comprises the following steps: step 1, taking EB66 cells for growth culture; step2, when the density of the EB66 cells grows to be suitable for virus inoculation, utilizing a fed-batch fluid-replacement virus inoculation technology to inoculate avian influenza virus into the EB66cells and performing virus multiplication culture; step 3, 24h after virus inoculation, sampling every 12h to determine HV titer of the virus and obtaining and storing the virus when the virus HA titer reaches to the maximum to obtain cultured avian influenza virus. According to the full-suspension culture method disclosed by the invention, the EB66 cells are utilized to perform avian influenza virus culture, so that the defect that a lot of miscellaneous protein is prone to being introduced into when a traditional chick embryo culture technology is utilized for production is overcome, and occurrence of chicken immunity side reaction is effectively reduced; meanwhile, titer and purity of the cultured avian influenza virus are improved; furthermore, production quality of the avian influenza vaccine is improved.
Owner:ZHAOQING INST OF BIOTECHNOLOGY CO LTD +2
Preparation method and product of swine fever live vaccine
ActiveCN102294029ASmall batch-to-batch quality varianceStable production processInactivation/attenuationAntiviralsImmunofluorescenceVirus multiplication
The invention discloses a preparation method and product of a swine fever live vaccine. The preparation method comprises the following steps: (1) culturing a swine-derived continuous cell line; (2) inoculating the swine-derived continuous cell line into a seed virus for producing the swine fever live vaccine, thereby obtaining a swine fever weak-virus vaccine strain; (3) carrying out virus multiplication on the swine fever weak-virus vaccine strain; (4) determining the virus value of the multiplied virus suspension by an immunofluorescence method; and (5) adding a freeze-drying protective agent and antibiotics into the qualified virus suspension to carry out vaccine preparation and freeze-drying. Since the swine fever live vaccine is prepared from the cell line, the difference of quality among different batches is small, and the invention has the characteristics of simple and stable technique, high yield, low cost and the like, is easy to operate, and has feasibility and amplification of industrialized mass production. In addition, the immunofluorescence method, which has the advantages of high detection sensitivity, high speed, high specificity, high accuracy, high repetitiveness and reliable result, is used for determining the virus value of the multiplied virus suspension. The swine fever live vaccine disclosed by the invention can 100% protect the attack of swine fever strong virus.
Owner:华威特(江苏)生物制药有限公司
Collecting method for insect virus breed
InactiveCN1584021AIncrease productionWays to Simplify CollectionBiocideViruses/bacteriophagesVirus multiplicationCollection methods
A collecting method in insect viruses breed includes: 1) feeding three-year old larva by 500,000 polyhedrons / ml concentration for 3 days, 2) primary collecting larva after experimenting 7-8 days under 24deg.C environment,3) extracting virus and processing viral imagocide after reserving 20-30 days under 15deg.C homeothermal condition. It achieves high insect virus output, simple viral collecting process, electrical save.
Owner:TEA RES INST CHINESE ACAD OF AGRI SCI
Method for producing human diploid cell encephalitis B inactivated vaccine
InactiveCN102406927AImprove quality and safetyImprove quality stabilityViral antigen ingredientsAntiviralsUltrafiltrationDiploid cells
The invention relates to the technical field of biology, in particular to a method for producing a human diploid cell encephalitis B inactivated vaccine. The method sequentially comprises the following steps of: resuscitating and subculturing a human diploid cell strain; culturing the human diploid cell strain by using a multi-stage bioreactor; inoculating an encephalitis B virus strain, and performing virus multiplication culture; harvesting a virus culture solution; and inactivating the virus culture solution, performing ultrafiltration, purifying, adding a glycoprotein protective agent, and preparing the vaccine. Human diploid cells are used as a cell matrix, so that the vaccine is safe and is free from exogenous factor pollution; the human diploid cells are cultured by the bioreactor through stage-by-stage linear amplification, so that the large-scale production of the vaccine with low cost, high quality, safety and stability is easy to implement; and the titer of the virus harvested solution is subjected to sampling inspection every day, so that the high expression of the harvested virus culture is ensured, and production quality is ensured.
Owner:CHENGDU KANGHUA BIOLOGICAL PROD
Pharmaceutical composition for the prophylaxis and/or treatment of virus diseases
The invention relates to the use of at least one active substance for producing a pharmaceutical composition for the prophylaxis and / or treatment of at least one viral disease. It is characterized by active substance(s) which inhibit(s) either at least two kinases or at least one SEK kinase of a cellular signal transmission path such that virus multiplication is inhibited.
Owner:LUDWIG STEPHAN +3
Method for preparing rabies vaccine
ActiveCN103285390AIncrease in sizeImprove the growing environmentAntiviralsTissue/virus culture apparatusVaccinationOrganism
The invention discloses a method for preparing a rabies vaccine, and belongs to the technical field of organisms. The method comprises the following steps of: a, cell inoculation; b, cultivation of cells for seeding; c, virus vaccination, d, virus cultivating and harvesting; and e, vaccine preparation. By adopting the method, the virus is rejuvenated; the reproductive capacity of the virus is enhanced; the multiplication titer and the harvesting yield of the virus are improved by adopting a torrent perfusion-type bioreactor culture system; the entire production process is not involved with other problems of biosafety and public health; and the method is suitable for mass production.
Owner:RINGPU (BAODING) BIOLOGICAL PHARMACEUTICAL CO LTD
Method for preparing avian influenza virus and inactivated vaccine thereof with Vero passage cells
ActiveCN102586195AReduce manufacturing costAntiviralsViruses/bacteriophagesHemagglutininVaccine Production
The invention discloses a method for preparing an avian influenza virus and an inactivated vaccine thereof with Vero passage cells. According to the method disclosed by the invention, the Vero passage cells are adopted to replace chicken embryo to culture influenza virus, so that the problems of chicken embryo remnant and exogenous virus inflection are solved and the immunogenicity of the cultured virus is more stable. On the other hand, for avoiding the phenomenon that the cracking of HA is influenced because protease is acted by an inhibitor in a maintaining liquid to inactivate, pancreatinis coated with chitosan and then is added in a cell maintaining liquid, so that the pancreatin is slowly released in the maintaining liquid and the defect that the cracking of hemagglutinin is influenced because the pancreatin is inactivated in the reproduction process of viruses is overcome. In addition, the probability that the cells are polluted since the pancreatin is added for multiple timesis avoided. The virus cultured by the method disclosed by the invention is high in titer and favorable in stability and is suitable for large-scale vaccine production.
Owner:哈药集团生物疫苗有限公司
New hepatitis A inactivated vaccine virus strain and method for culturing same
ActiveCN101525597AIncrease productionQuality improvementInactivation/attenuationAntiviralsEarly generationAntigen
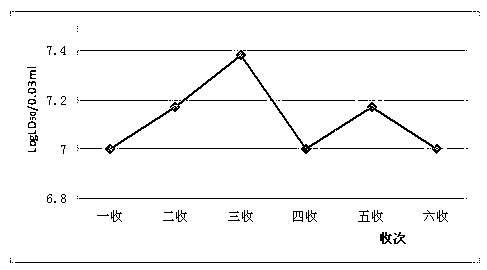
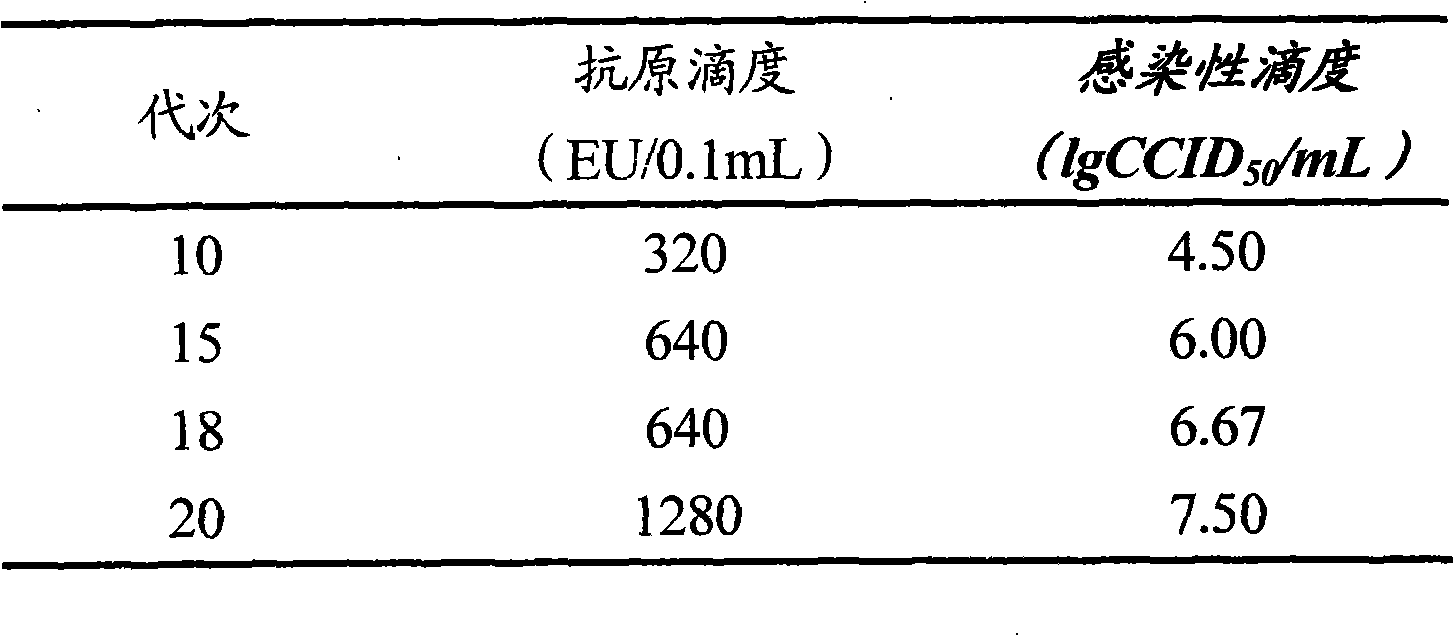
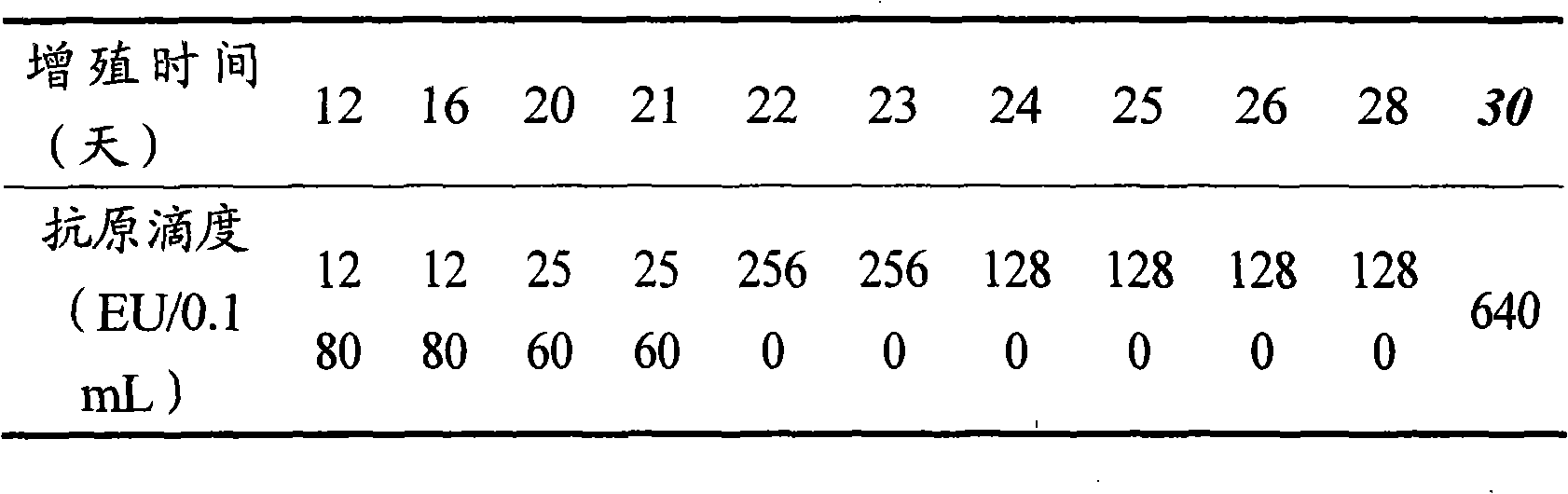
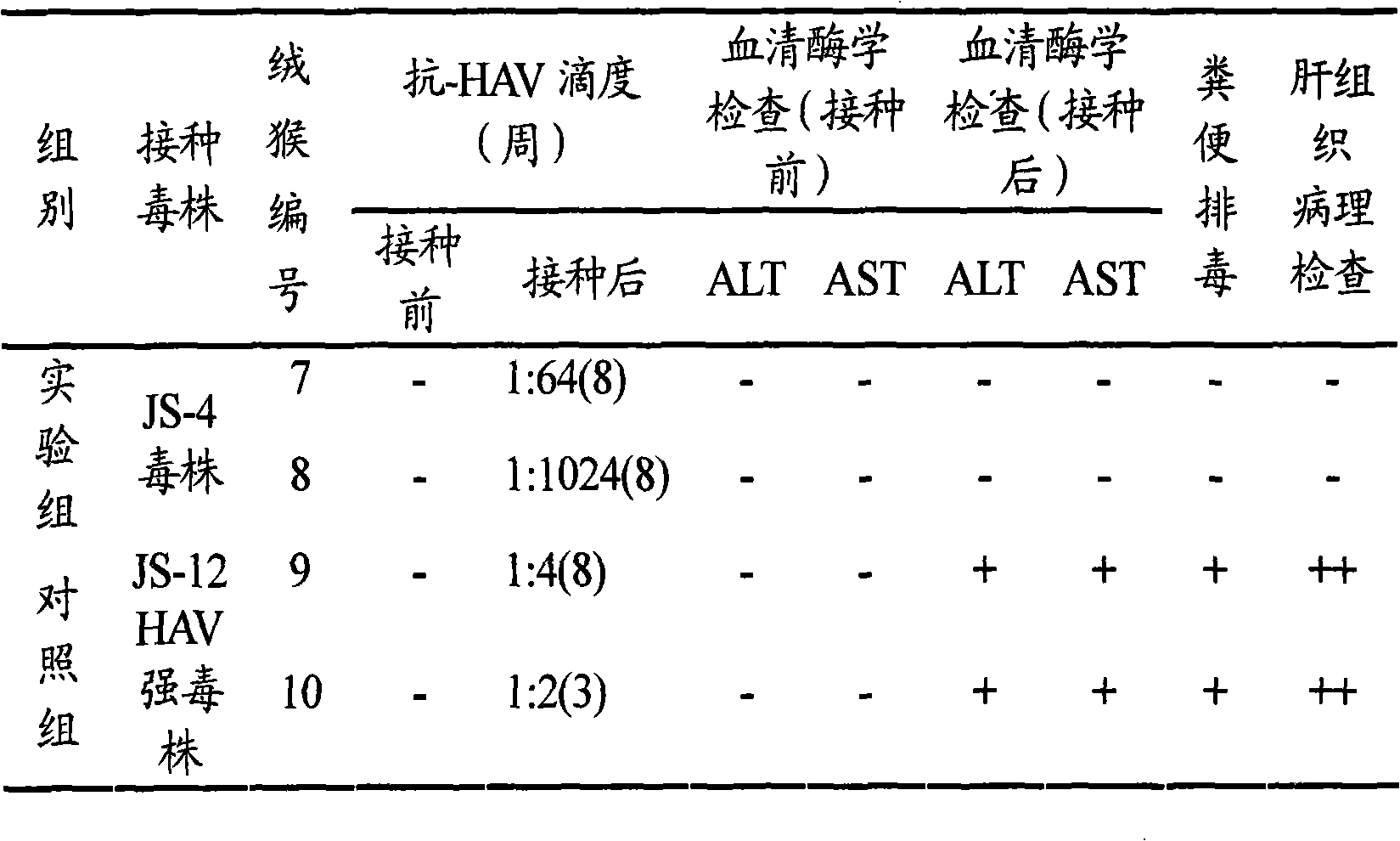
The invention provides a new hepatitis A virus JS-4 strain for preparing hepatitis A inactivated vaccine and a method for culturing the same. The strain is separated from dejecta of a patient infected with acute hepatitis A and is transmitted to a Vero cell for adapted culture; through neutralization tests and other methods, the strain is proved to be hepatitis A virus; in the adaptation process, the subculture cycle of early generation is 28 days; when the strain is transmitted to the tenth generation, the cycle is shortened to 21 days; the strain is continuously cultured for ten generations; the culture temperature is between 35 and 36 DEG C; the cycle for virus propagation is shortened from 28 to 21 days; the antigen titer can reach 1:640-1:1,280; and the infectious titer is between 106.67 and 107.50 CCID50 / ml. The marmoset virulence test proves that the strain has weak virulence, is reliable in safety and has good immunogenicity and protective effect when the strain is used for producing the hepatitis A inactivated vaccine and is an ideal strain for producing the hepatitis A inactivated vaccine.
Owner:JIANGSU SIMCERE VAXTEC BIO PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com