Patents
Literature
102 results about "Culture viruses" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method for preparing II-type pig's ring-virus nucleic vaccine and the use thereof
InactiveCN1579553AShorten the production cycleAvoid pollutionViral antigen ingredientsGenetic material ingredientsPeripheral blood mononuclear cellBiology
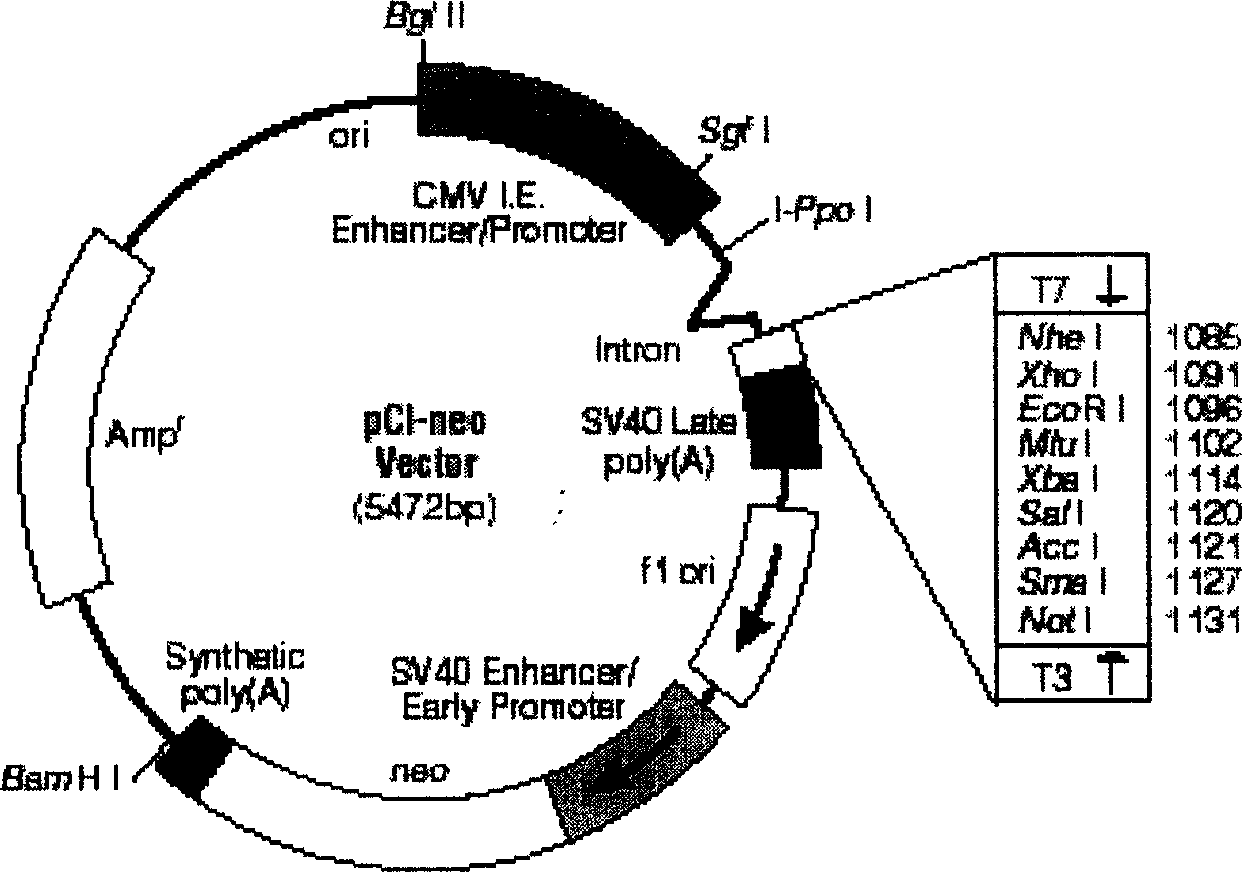
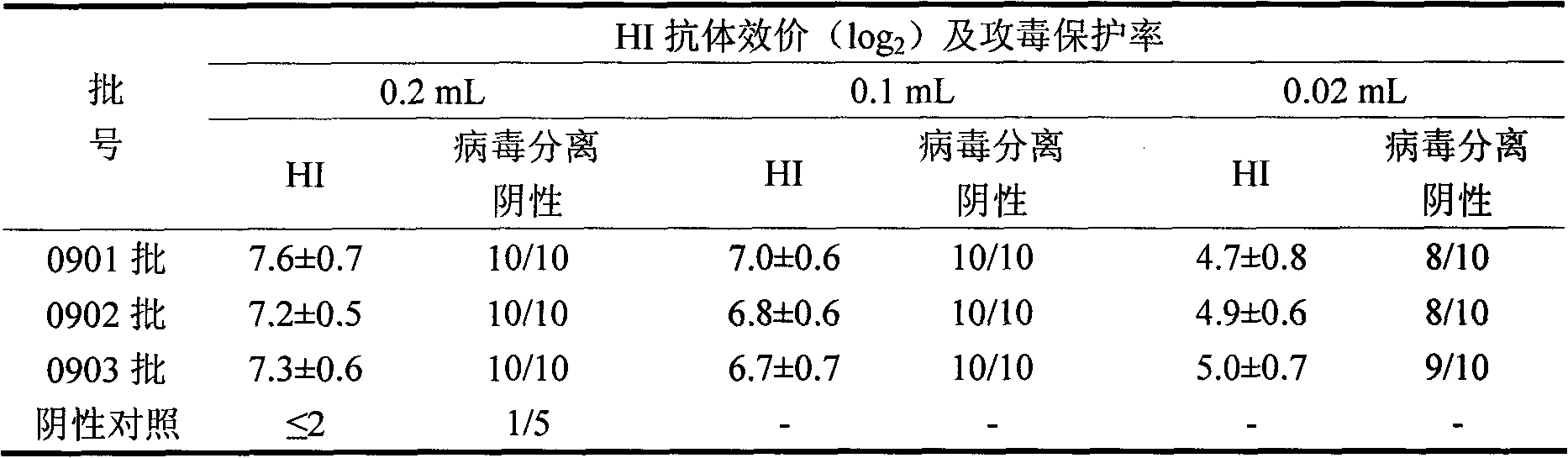
The invention discloses a manufacturing method and application of II type pig ring virus nucleic acid vaccine. The method is: 1) designs the specific primer, uses PCV2 Hangzhou (HZ201) gene group as template, closes ORF1, ORF2, ORF3 and ORF4 genes of PCV-2 with PCR method, and they are constructed into eucaryon expressing carrier with pCI-neo; 2) separates the pig external blood single nucleus cell, clones the genes IFN, IL-2 and IL-4 with RT-PCR method, and they are constructed into eucaryon expressing carrier with pCI-neo; 3) based on above mentioned reconstructed carrier, constructs the fused expressing carrier of the other genes of PCV2, ORF2 and PCV2 or pig cell factor gene; the merits of the invention lie in: (1) it needs not to culture virus, the producing period is short; (2) it needs not cell culture, thus can prevent the contamination from other pig source virus; (3) it does not express the pathogenesis protein which is harmful to body, the safety is high. (4) it can activates body secretion and cell immunity replay at the same time.
Owner:ZHEJIANG UNIV
Preparation method and product of H9N2 subtype avian influenza inactivated vaccine
ActiveCN101816785AHigh titerSimple production methodAntiviralsAntibody medical ingredientsVirus multiplicationVaccine Production
The invention relates to a preparation method and a product of an H9N2 subtype avian influenza inactivated vaccine. The technical points of the invention mainly relate to the screening, the determination and the domestication of a virus-adapted cell line, the primary amplification cultivation and the continuous cultivation of a virus-adapted cell, the preparation of virus fluid by virus-inoculated culture and the preparation of final inactivated vaccine products. Firstly, the invention avoids the virus propagating method using a large amount of chick embryos in the avian influenza production at present, thereby avoiding the problem of biological potential safety hazards, and overcoming the problem that the mass production of vaccines is enslaved to the supply of the chick embryos; secondly, the invention provides a safe, continuous and closed cell culture virus production method, is used for the preparation of the H9N2 subtype avian influenza inactivated vaccine, enables the use of the cell culture method, and can simultaneously produce high-titer viruses to meet the requirements for the immunological production; and finally, the vaccine production method of the invention is simple and fast, thereby realizing the fast vaccine supply at the epidemic situation.
Owner:扬州优邦生物药品有限公司
Porcine pseudorabies virus strain as well as inactivated vaccine and applications thereof
ActiveCN103305474APromote rapid proliferationHigh titerMicroorganism based processesAntiviralsLaboratory cultureVirus strain
The invention discloses a porcine pseudorabies virus strain as well as an inactivated vaccine and applications thereof, belonging to the field of separation and application of the porcine pseudorabies virus strain. The invention firstly provides a porcine pseudorabies virus BJ strain separated from diseased pig tissues, and the microbial preservation number of the porcine pseudorabies virus BJ strain is CGMCC (China General Microbiological Culture Collection Center) No.7351. The invention discloses a method for preparing the inactivated vaccine by applying the porcine pseudorabies virus BJ strain. The method comprises the steps of culturing a virus strain to obtain a virus solution; adding an inactivator, and inactivating and concentrating the virus solution; and evenly mixing an adjuvant and the virus solution, and emulsifying to obtain the inactivated vaccine. The technological parameters of the inactivated vaccine preparation method are further optimized, and the immune protection efficacy and safety of the inactivated vaccine can be improved. Shown by the immune protection efficacy and safety tests, the porcine pseudorabies inactivated vaccine prepared has good immune protection efficacy and safety, and can be clinically used for preventing or treating porcine pseudorabies.
Owner:泰州博莱得利生物科技有限公司 +2
Decreasing potential iatrogenic risks associated with influenza vaccines
InactiveUS20090081252A1Improve securitySsRNA viruses negative-senseViral antigen ingredientsC6 cellsMammal
Influenza viruses for use in preparing human vaccines have traditionally been grown on embryonated hen eggs, although more modern techniques grow the virus in mammalian cell culture e.g. on Vero, MDCK or PER.C6 cell lines. The inventor has realised that the conditions used for influenza virus 5 culture can increase the risk that pathogens other than influenza virus may grow in the cell lines and have identified specific contamination risks. Suitable tests can thus be performed during manufacture in order to ensure safety and avoid iatrogenic infections.
Owner:NOVARTIS AG
Decreasing potential iatrogenic risks associated with influenza vaccines
InactiveUS20120034600A1Increased risk of contaminationSsRNA viruses negative-senseViral antigen ingredientsFlu immunizationInfluenza virus culture
Influenza viruses for use in preparing human vaccines have traditionally been grown on embryonated hen eggs, although more modern techniques grow the virus in mammalian cell culture e.g. on Vero, MDCK or PER.C6 cell lines. The inventor has realised that the conditions used for influenza virus culture can increase the risk that pathogens other than influenza virus may grow in the cell lines and have identified specific contamination risks. Suitable tests can thus be performed during manufacture in order to ensure safety and avoid iatrogenic infections.
Owner:NOVARTIS AG
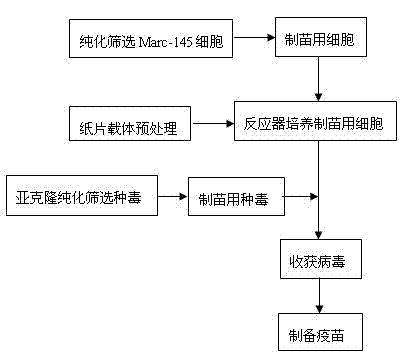
Preparation method of porcine reproductive and respiratory syndrome vaccines by utilizing bioreactor
ActiveCN102552896AUniform stateSmall batch-to-batch varianceViral antigen ingredientsAntiviralsImmune effectsTGE VACCINE
A preparation method of porcine reproductive and respiratory syndrome vaccines by utilizing a bioreactor utilizes a stream perfusion type bioreactor as a cultivation tool. The preparation method comprises the steps of a cultivating cells for preparing the vaccines; b vaccinating and cultivating viruses; c harvesting virus liquor; d preparing the vaccines and the like. The preparation method has the advantages that the preparation method is simple and convenient in operation, low in contamination probability and capable of remarkably reducing preparation cost, the vaccines are large in yield, uniform and stable in quality and good in immune effect, and the like. The whole preparation process does not involve other problems of biological safety and public hygiene so that the preparation method is suitable for large-scale preparation of the porcine reproductive and respiratory syndrome vaccines.
Owner:RINGPU (BAODING) BIOLOGICAL PHARMACEUTICAL CO LTD
Method for Propagating Infectious Bursal Virus with Chicken Embryo Origin Cell Line to Prepare Inactivated Vaccine and Combined Vaccine
InactiveCN102258777AGuaranteed to be pureAvoid Biosafety HazardsViral antigen ingredientsAntiviralsVaccine ProductionEmbryo
The invention relates to a method for preparing a vaccine by breeding infectious bursal disease virus (IBDV) by a chicken embryo source cell line. The method mainly comprises the following steps of: 1) subculturing cells DF-1 for preparing vaccines; 2) breeding IBDV HQ cell seeds; 3) breeding virus liquid for preparing the vaccines; 4) concentrating and inactivating the virus liquid for preparingthe vaccine; 5) preparing other virus liquid of newcastle disease method, newcastle disease-infectious bronchitis virus method, and newcastle disease-infectious bronchitis virus-egg drop syndrome method combined vaccine and concentrating; and 6) proportioning inactivated combined vaccine, emulsifying and sub-packaging. The production process is simple, and stable and is easy to operate, eliminates biological potential safety hazard existing in the conventional vaccine production, and overcomes the defects that large-scale production of the vaccines is limited by supply of chicken embryos; cost and batch-to-batch variation are reduced; the virus titer and the quality of vaccine are improved; basis is laid for culturing virus liquid on large scale by a suspension culture technology in vaccine industry; and the produced IBDV inactivated vaccine and combined vaccine have high safety and immune efficacy, and have the complete immune protection effect on IBDV attack.
Owner:POULTRY DISEASE RES INST OF HENAN AGRI UNIV
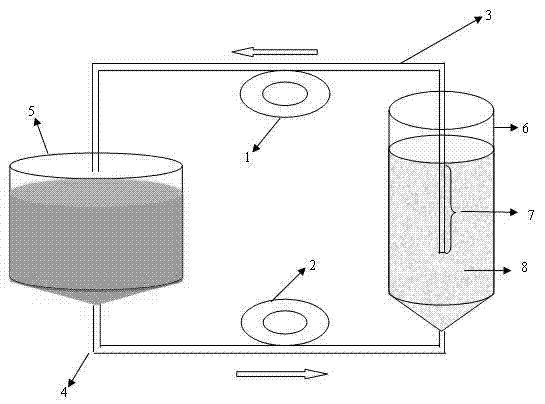
Safety high-efficient continuous enclosed type cell culture and virus production-inactivation system
ActiveCN1840651AIncrease productionReduce the effect of proliferationTissue/virus culture apparatusProcess engineeringProduct gas
The safe high-efficiency continual closed cell culture virus generation / deactivation system comprises: a cell amplification unit connected to a gas supplying unit, a steam generator, a supplying device for cell growth medium, a primary fill and collection device, a heat source supply device, and a acid / alkali liquid supply device; a virus amplification unit connected to a gas supplying unit, a basic medium supply device, a cell / carrier separator, a heat source supply device, and a acid / alkali liquid supply device; and a old carrier collector. This invention integrates cell and virus amplification processes for automation control and standard production with little pollution.
Owner:上海丽坤生物科技股份有限公司
Method for breeding Pseudostellaria heterophylla by virus-free tissue culture
InactiveCN102524067AImprove stress resistanceIncrease productionPlant tissue cultureHorticulture methodsBiotechnologyPseudostellaria
The invention relates to a method for breeding Pseudostellaria heterophylla by virus-free tissue culture. The method comprises the following steps of: selecting explants, performing induction culture, detecting viruses, performing enrichment culture, performing rooting culture, propagating virus-free tissue-culture seedlings, acclimating, transplanting, and producing virus-free Pseudostellaria heterophylla stock seeds, first generation seeds and second-generation propagation seeds (production seeds). The method is easy to operate and has a low inoculation contamination rate and a high survival rate. The virus elimination operation is simple, rapid and complete, the propagation coefficient is increased by 10-15 times, and the propagation speed is high. The virus-free tissue-culture seedlings are suitable for industrialized production. The root tubers produced from the virus-free tissue-culture seedlings have resistance, are better in yield and quality than virus-infected seedlings, and can be produced commercially.
Owner:贵州昌昊中药发展有限公司 +1
Oriental lily seed ball virus removing method
InactiveCN102577965AReduce cross infectionImprove survival ratePlant tissue cultureHorticulture methodsViral testCallus
The invention provides an oriental lily seed ball virus removing method, which specifically comprises the following steps of: A) stripping of explant growth points, B) heat treatment virus removal, C) cleaning and sterilization of explants, D) acquisition, induction and culture of growth points, E) virus-free culture and F) virus detection. The oriental lily seed ball virus removing method has the advantages that the defects existing in the prior art are overcome, the defects caused by a reason that a single method is used are overcome by comprehensively using a heat treatment virus removing method, a shoot tip culture virus removing method and a callus virus removing method, the probability of virus cross infection can be effectively reduced, the virus removal rate is high, the survival rate is high and the culture period of seed balls can be effectively shortened.
Owner:YUXI MINGZHU FLOWER
Production method of transmissible gastroenteritis virus vaccine
ActiveCN103550771AHigh potencyReduce manufacturing costMicroorganism based processesAntiviralsViral VaccineBottle
The invention discloses a production method of a transmissible gastroenteritis virus vaccine. The production method comprises the steps of carrying out subculture on cells for production so as to form monolayer cells; multiplying virus seeds for production, namely inoculating the basic virus seeds to the monolayer cells for culturing; dissociating the monolayer cells into a monolayer cell suspension liquid, and inoculating the cell suspension liquid into a bioreactor for culturing; multiplying vaccine culturing virus liquid, namely inoculating the virus seeds for production to the cells to be cultured after the quantity of the cells reaches 5*106-5*107 unit / ml; and harvesting the virus liquid. The production method has the advantages that the production cost can be greatly lowered, the production cycles are short, each production cycle only lasts for 5-7 days, and compared with that of transmissible gastroenteritis virus generated by an existing spinner bottle culture method, the titer of the transmissible gastroenteritis virus produced by the method is higher; the automation degree is high, few workers are required, the production process is simple and stable, the operation is easy, the yield is high, the occupied area is small, the production scale can be easily and rapidly expanded, and the quality is balanced and stable basically; the environmental pollution is slight and is easy to avoid.
Owner:成都史纪生物制药有限公司
Tissue culture rapid propagation method for jonquil
InactiveCN103651133AHigh reproductive coefficientShort cyclePlant tissue cultureHorticulture methodsAlcoholSugar
The invention belongs to the technical field of jonquil tissue culture, and especially relates to a tissue culture rapid propagation method for jonquil. The method comprises the following steps: preparing mediums, wherein the mediums comprise a basic medium and mediums in all tissue culture stages, the compositions of the basic medium comprise MS, cane sugar with a concentration of 8% and 0.7% of agar, the pH value of the basic medium is 5.6-5.8, the compositions of an induction callus medium comprise MS + 1.0 mg / L of 6-BA + 0.2 mg / L of NAA or MS + 1.0 mg / L of 6-BA + 0.1 mg / L of NAA, the compositions of a propagation medium comprise MS + 1.0 mg / L of 6-BA + 0.1 mg / L of NAA, and the compositions of a rooting medium comprise MS + 0.2 mg / L of NAA; culturing virus-free tissue culture seedlings; selecting explants and disinfecting, specifically choosing jonquil leaves as explants, washing with alcohol with a concentration of 75% for half a minute, and then performing disinfection processing for 10 min by using HgCl with a concentration of 0.1%; and transplanting the tissue culture seedlings. The method is high in propagation coefficient, short in period and low in cost.
Owner:DASHUN INT FLOWER
Method for proliferating avian influenza viruses in bioreactor with cell carrier
InactiveCN102127524AUniform product qualityStable virulenceMicroorganism based processesViruses/bacteriophagesAutomatic controlVaccine Production
The invention discloses a method for replicating and proliferating avian influenza viruses in the passage cells which are absorbed and grow in the microcarrier of a bioreactor. The invention is based on the method which uses cells as carrier to proliferate viruses in the bioreactor. The obtained avian influenza viruses and the avian influenza virus vaccine product have uniform quality and stable toxicity. The automatic control of vaccine production can be realized, the problem of large-scale production and application can be solved, the production matrix of the influenza vaccine can be completely changed, the technology that chick embryo is adopted to culture viruses can be changed, the problems caused by chick embryo such as adventitious agent pollution and viral pollution and the interferences caused by heterologous proteins such as chick embryo can be reduced, the purity, safety and immune effect of vaccine can be increased and the method can play an important role in the prevention of avian influenza.
Owner:深圳市南海潮生物技术有限公司
Process for preparing human diploid cell rabies vaccine through Celligen310 bioreactor
InactiveCN103083654ASimple purification processSimple preparation processPowder deliveryAntiviralsRabies virus strainTGE VACCINE
The invention belongs to the field of biological products, in particular to a process for preparing rabies vaccine by culturing human diploid cells WI-38 and MRC-5 (called as human diploid cells as follows) as toxigenic cells through a Celligen310 bioreactor and taking a rabies virus PM strain as a virus seed, wherein the finished vaccine is prepared by comprising the steps of resuscitating human diploid cells, culturing and proliferating the human diploid cells, inoculating the virus seed of the rabies virus PM strain onto the human diploid cells, domesticating, inoculating and culturing virus seeds, collecting virus filtrate, inactivating, purifying, concentrating, adding a protective solution and the like. Verification indexes of the vaccine meet standards of Chinese Pharmacopoeia of 2010 version. The process is characterized in that the human diploid cells are cultured and prepared as matrix cells by using the Celligen310 bioreactor; therefore, exogenous pollution factors and tumorigenicity of animal passage cells can be avoided. As the inoculated virus strain is the rabies virus PM strain in the preparation process of the vaccine, the immune effect is better than that of the traditional vaccine. The vaccine prepared by using the process has the advantages of being high in purity, good in immune effect and high in safety.
Owner:万里明
MDBK acclimation suspension method and two-stage virus production process
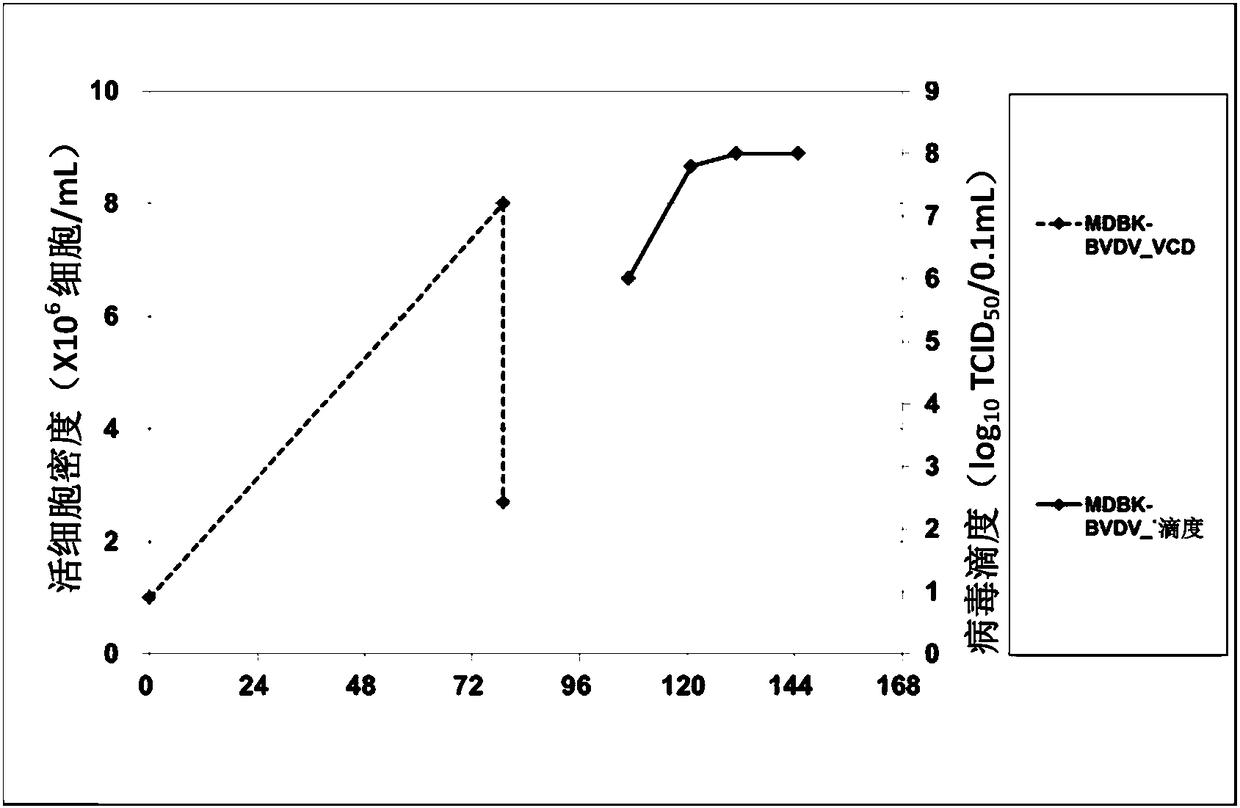
PendingCN108570454AHigh densityTo achieve growthSsRNA viruses positive-senseViral antigen ingredientsCytopathic effectSerum free
The invention relates to an MDBK cell acclimation suspension method and a two-stage virus production process. According to the MDBK cell acclimation suspension method, adherent MDBK cells are acclimated to adapt to serum-free suspension culture and used as host cells for producing viruses which can have cytopathic effect or sensitivity on suspension MDBK cells by two-stage culture virus inoculation; when the cells grow to 2.0-20.0*10<6> cells / mL, diluting with a production culture medium by 1-5 times is performed to reach a density range of 1.0-10.0*10<6> cells / mL, and then virus inoculation is carried out or virus inoculation is performed before dilution. The suspension MDBK cells are adaptive to suspension growth in the serum-free chemical-defined culture medium, and the two-stage virusproduction process is simple and easy in amplification; liquid changing is avoided, and high culture medium utilization efficiency is achieved; production and growth culture media can be identical ornot and can be mixed proportionally to realize cell secondary growth and viral expression promotion.
Owner:上海健士拜生物科技有限公司 +2
H9N2 subtype avian influenza virus strain as well as inactivated vaccine and application thereof
The invention discloses an H9N2 subtype avian influenza virus strain as well as an inactivated vaccine and application thereof, and belongs to the field of separation and application of avian influenza virus strains. The H9N2 subtype avian influenza virus strain J / ZY strain is separated from a suspected H9N2 avian influenza pathogenetic chicken flock, and the microbial collection number of the strain is CGMCC No. 8853. The invention further discloses a preparation method for the H9N2 subtype avian influenza inactivated vaccine, and the preparation method comprises the following steps: culturing viruses to obtain a virus supernate; adding an inactivating agent to inactivate the virus supernate; mixing adjuvants and the virus supernate uniformly, and emulsifying to obtain the inactivated vaccine. Immune protection experiments prove that the separated H9N2 subtype avian influenza virus strain J / ZY strain serving as a vaccine strain has good immunogenicity and a relatively high antibody level can be produced after an animal is immunized by the strain.
Owner:JILIN ZHENGYE BIOLOGICAL PROD
Novel process for preparing influenza virus split vaccine
ActiveCN102133399AHigh yieldIncrease the effective antigen contentAntiviralsAntibody medical ingredientsHemagglutininPurification methods
The invention relates to a novel process for preparing influenza virus split vaccine, comprising the following steps of: (1) inoculating and culturing viruses: inoculating working seed lot viruses diluted to virus amount of 2.0-0.6LgEID50 / ml in chick embryo allantois, and culturing at 33-35 DEG C for 48-72h; (2), harvesting and inactivating viruses and concentrating a virus harvest liquid; (3) purifying and splitting viruses: adding TritonX-100 with volume ratio of final concentration of 0.3-0.5% and deoxysodium cholate in a purified virus liquid, mixing evenly and placing at 20-30 DEG C for 90-120min; (4) adding a PB split agent; and (5) sterilizing, filtering and then preparing a semi-finished product: diluting each stock solution to hemagglutinin content of 30micrograms / strain / ml by 0.01mol / L of PBS (Phosphate Buffered Saline) with a pH value of 7.2, and filtering by a filter membrane with aperture of 0.22micron to obtain a semi-finished product. The novel process for preparing influenza virus split vaccine has the beneficial effects that: due to the increase of a novel purification method, high yield can be achieved, effective antigen content can be improved and the contents of residual ovalbumin and split agent causing inoculation reaction can be greatly reduced.
Owner:ZHEJIANG TIANYUAN BIO PHARM CO LTD
Virus and vaccine of porcine reproductive and respiratory syndrome and preparation method of same
ActiveCN101979514AHigh viral titerHigh poison priceViral antigen ingredientsAntiviralsFreeze-dryingCells/microL
The invention discloses a method for preparing virus of porcine reproductive and respiratory syndrome on a large scale. In the method, the virus of the porcine reproductive and respiratory syndrome is prepared in a cell microcarrier suspension culture system by a bioreactor. The method comprises the following steps of: inoculating host cells for preparing the virus to a carrier tank containing culture solution and a microcarrier, and mixing the cells and the microcarrier uniformly to ensure that the cells are attached to the microcarrier; providing sufficient nutrients and appropriate gas environment for the cells under the appropriate culture environment to ensure that the cells are grown until the cells are in an amount which are 10 to 20 times of the inoculation concentration on the microcarrier; preparing virus suspension from the virus of the porcine reproductive and respiratory syndrome by using cell maintenance culture solution to ensure that the suspension is adsorbed to the cells; culturing the virus under the appropriate culture environment; culturing continuously for 2 to 3 days to obtain virus solution; and after the virus solution passes inspection, performing freeze thawing on the virus solution twice at the temperature of -20 DEG C, and inactivating and purifying to prepare an inactivated vaccine of the porcine reproductive and respiratory syndrome or adding a freeze-drying protective agent for freeze drying to prepare a live vaccine of the porcine reproductive and respiratory syndrome. The method has large production scale, high yield of single batch and low production cost.
Owner:PU LIKE BIO ENG
PK15 cell acclimation suspension method and two-stage virus production process
PendingCN108570445ATo achieve growthTo achieve the role of replication and proliferationArtificial cell constructsViruses/bacteriophagesCytopathic effectChemical composition
The invention relates to a PK15 cell acclimation suspension method and a two-stage virus production process. According to the PK15 cell acclimation suspension method, PK15 cells are acclimated to adapt to serum-free suspension culture and used as host cells for producing viruses which can have cytopathic effect or sensitivity on suspension PK15 cells by two-stage culture virus inoculation; when the cells grow to 2.0-20.0*10<6> cells / mL, diluting with a production culture medium by 1-5 times is performed to reach a density range of 1.0-10.0*10<6> cells / mL, and then virus inoculation is carriedout or virus inoculation is performed before dilution. The suspension PK15 cells are adaptive to suspension growth in the serum-free chemical-defined culture medium, and the two-stage virus productionprocess is simple and easy in amplification; liquid changing is avoided, and high culture medium utilization efficiency is achieved; production and growth culture media can be identical or not and can be mixed proportionally to realize cell secondary growth and viral expression promotion.
Owner:上海健士拜生物科技有限公司 +2
Tissue culture bulb preserving method for thunberg fritillary virus
InactiveCN101215550AEasy to carry and exchangeConvenient for virus researchMicrobiological testing/measurementViruses/bacteriophagesPlant virusViral test
The invention relates to a method for preserving tissue culture bulbs of fritillary viruses, which belongs to the technical field of plant virus preservation and comprises following steps: identifying pathogeny species of the viruses, preparing culture medium, culturing virus-free tissue culture bulbs, testing virus ELISA, vaccinating virus by friction, and preserving virus bulbs. The invention has the advantages that the tissue culture bulbs of the fritillary viruses are used to infect the purified viruses, and then the viruses of fritillary tissue culture bulbs are preserved, which overcomes the defects in traditional preservation, can prevent viruses from confounding and losing, is easy to extract and prepare infectious titer, provides virus with high purity for preparing antiviral breeding and antiviral serum, lowers preserving cost, increases preserving effects, and is easy to carry and communicate.
Owner:ZHEJIANG ACADEMY OF AGRICULTURE SCIENCES
Preparation method for avian influenza virus split vaccine
InactiveCN103520716AImproving immunogenicityBroad antigen spectrumAntiviralsAntibody medical ingredientsAvian influenza virusFlu immunization
The invention discloses a preparation method for an avian influenza virus split vaccine. The preparation method comprises the following steps of constructing H7N9 avian influenza working seed lot virus seeds, inoculating and cultivating viruses, inactivating the viruses, clarifying and concentrating virus harvest liquid, purifying the viruses, splitting the viruses, inactivating the viruses again, removing sugar and a splitting agent, filtering bacteria, performing filtration and preparing semi-finished products. The H7N9 influenza vaccine prepared by the method is high in immunogenicity, wide in antigen spectrum, stable in quality, high in purity, high in safety, low in cost and suitable for large-scale production and has a large application value.
Owner:CHENGDU KANGHUA BIOLOGICAL PROD
Cell strain for producing goatpox vaccine
InactiveCN104694457AImprove adaptabilityLow risk of recoveryMicrobiological testing/measurementMicroorganism based processesGreen monkeyEngineering
The invention relates to the field of vaccine preparation, and particularly relates to a cell strain for producing a goatpox vaccine. The cell strain is an African green monkey kidney cell Vero B10 and is collected at China General Microbiological Culture Collection Center with the collection number of CGMCC No. 9705. Compared with a primary cell production method, a preparation method of the cell strain has the advantages that a goatpox virus strain AV41 is cultivated by using a VeroB10 cell, good in virus adaptability and low in strong recurrence risk; a passage cell vaccine is superior to a primary cell vaccine in terms of virus yield, uniformity, pureness and the like; the cost is low, and animals are not needed; and the safety of the passage cell vaccine is superior to that of the primary cell vaccine, and the immunity efficiency of the passage cell vaccine is not lower than that of the primary cell vaccine.
Owner:SHANDONG LVDU BIO SICIENCE & TECH
Slow virus purification method
ActiveCN107384877AKeep aliveImprove recycling efficiencyRecovery/purificationReverse transcribing RNA virusesMolecular sievePurification methods
The invention provides a slow virus purification method. The slow virus purification method comprises the following steps: S1, culturing viruses; S2, carrying out purification pretreatment on the viruses; carrying out centrifugal separation on the slow viruses obtained through amplification, and collecting supernatant as a virus harvesting liquid; firstly carrying out primary filtration treatment on the virus harvesting liquid, carrying out secondary filtration treatment on the virus liquid obtained through filtration, collecting a virus filtrate, carrying out ultrafiltration concentration, then centrifuging, collecting the supernatant, then carrying out tertiary filtration treatment, and collecting a filtrate; and S3, carrying out membrane chromatography treatment on the collected virus purification pretreatment sample, then carrying out ultrafiltration treatment, and purifying by adopting molecular sieve chromatography, wherein matrix used by a chromatography membrane is a regenerated cellulose skeleton during the membrane chromatography. By adopting the technical scheme of the invention, virus recycling efficiency is improved on the basis of guaranteeing slow virus activity; and operation is simple, amplification is easy, the virus harvest liquid can be treated in a large scale, and the slow virus purification method provided by the invention has good repeatability and stability.
Owner:SHEN ZHEN TSINGHUA YUANXING BIO PHARM SCI & TECHNOL
Method for large scale cultivation in bioreactor
ActiveCN102732487AEasy to operateReduce workloadMicroorganism based processesViruses/bacteriophagesVaccine ProductionFixed bed
The invention discloses a method for large scale cultivation in a bioreactor, comprising the following steps: (1) making a recombinant H5N1 avian influenza virus adapt to MDCK cells; (2) preparing a seed lot by using the adapted virus strain as a reactor large-scale culture virus, wherein the seed lot virus generation is no less than F6, the hemagglutination titer is no less than 28, and EID50 is no less than 107.5; (3) culturing the MDCK cells by using polyester sheets as a carrier and the reactor as a basket fixed bed, and pouring the cells on every gram of the carrier, wherein the culture medium is newborn calf serum DMEM; (4) inoculating the virus on the cultured MDCK cells, washing the cells by using a virus diluent, conducting virus inoculation, after absorption, and replacing a cell maintenance media; (5) maintaining the MDCK cells and conducting virus propagation, stirring, and replacing the cell maintenance media; and (6) monitoring the hemagglutination titer and harvesting the virus, and harvesting the reactor to culture a volume virus liquid. According to the invention, the method is easy to operate and the operation is simple, the HA titer of the virus after adaption is higher than 2<7>, and the problem that the culture virus titer cannot satisfy the demand of produce vaccine in the existing culture of the recombinant H5 subtype avian influenza virus on the MDCK cells is solved.
Owner:WUHAN KEQIAN BIOLOGY CO LTD
Inactivated vaccine of porcine epidemic diarrhea virus and preparation method of inactivated vaccine
ActiveCN107412763AEasy to prepareImprove securityOrganic active ingredientsViral antigen ingredientsTGE VACCINEIntestinal mucosa
The invention belongs to the field of biological products, and in particular relates to an inactivated vaccine for porcine epidemic diarrhea virus which is produced by ST cells and a preparation method of the inactivated vaccine. The method comprises four steps, namely amplification culture of the ST cells, virus inoculation, multiplying of vaccine producing virus liquid, and product preparation. The vaccine, which is produced by the method, is high in immunization level and short in immunological window phase; an immunity period is obviously prolonged; and total secretory antibody (total SIgA) in intestinal mucosa is remarkably improved.
Owner:广州渔跃生物技术有限公司
Comprehensive detoxification method of lily bulbs
The invention provides a comprehensive detoxification method of lily bulbs. The method concretely comprises the following steps: 1, picking off explant growing points; 2, carrying out heat treatment detoxification; 3, cleaning and disinfecting explants; 4, and carrying out collection and induction culture on the growing pints; 5, carrying out detoxifying culture; and 6, detecting viruses. The method solves disadvantages in the prior art, solves the disadvantages of individual methods through comprehensively utilizing heat treatment detoxification, meristem culture detoxification and callus detoxification methods, and also has the advantages of effective reduction of the virus cross infection change, high detoxification rate, high survival rate, and effective shortening of the bulb culture period.
Owner:SONGTAO HONGFA MEAT FOOD CO LTD
Preparation method of rainbow trout infectious haematopoietic necrosis virus (IHNV) inactivated vaccine and application thereof
InactiveCN108114275AGood inactivation effectImprove securitySsRNA viruses negative-senseViral antigen ingredientsInfectivityInfectious haematopoietic necrosis virus
The invention discloses a preparation method of a rainbow trout infectious haematopoietic necrosis virus (IHNV) inactivated vaccine. The method comprises the following steps of S1, recovering and purifying an FHM cell; S2, basically culturing the FHM cell; S3, amplifying and culturing the FHM cell; S4, culturing an IHNV virus; S5, purifying the IHNV virus; S6, inactivating the IHNV virus. The IHNVinactivated vaccine prepared through the method is applied to preventing infectious haematopotietic necrosis; the IHNV inactivated vaccine successfully prepared through the method is good in inactivation effect, high in safety and high in relative immune protective rate; meanwhile, the preparation method is simple in process, beneficial to reducing a large-scale production cycle and cost of the virus, and capable of being used for industrially preparing the IHNV inactivated vaccine for preventing infectious haematopotietic necrosis.
Owner:CHENGDU ACAD OF AGRI & FORESTRY SCI +1
Method for carrying out virus removal on prunus salicina during tissue culture and rapid propagation
InactiveCN104115750AVigorousGood lookingHorticulture methodsPlant tissue culturePrunus salicinaVirus detection
The invention discloses a method for carrying out virus removal on prunus salicina during tissue culture and rapid propagation. The method for carrying out virus removal on prunus salicina during tissue culture and rapid propagation comprises the following steps: 1) selecting an implant and carrying out pre-treatment; 2) peeling stem tips; 3) carrying out induction culture on the stem tips; 4) carrying out multiplication culture; 5) carrying out rooting culture; 6) culturing virus-free seedlings; and 7) detecting viruses and bacteria. The method for carrying out virus removal on prunus salicina during tissue culture and rapid propagation has the advantages that a stem tip virus removal method, a high-temperature treatment virus removal method and a chemical reagent virus removal method are combined, a good virus removal effect in tissue culture and rapid propagation of prunus salicina seedling culture is realized, the obtained virus-free seedlings are subjected to virus detection, and results show that viruses of all the seedlings are successfully removed, and no prune dwarf virus (PDV) or prunus necrotic ringspot virus (PNRSV) infection is found. By utilizing the method disclosed by the invention for carrying out virus removal on prunus salicina, new virus-free prunus salicina seedlings are cultured through tissue culture, and then domestication and transplantation are carried out, so that the aims of increasing the yield and improve the fruit flavour are realized.
Owner:GUIZHOU YANHE WUJIANG BIOTECH DEV
MDCK (Madin-Darby Canine Kidney) clone cell strains and application thereof
ActiveCN104694458AHigh potencyImprove performanceMicroorganism based processesVertebrate cellsVaccine ProductionTGE VACCINE
The invention discloses MDCK (Madin-Darby Canine Kidney) clone cell strains and application thereof, belonging to the field of MDCK clone cell strains. A limiting dilution process is adopted to perform MDCK unicell cloning, and subcloning is further performed to finally obtain 2 monoclonal cell strains with excellent performance. The two monoclonal cell strains have higher growth rate than the female parent, can well reproduce avian influenza virus, obviously enhances the duplication level of the virus in cells, and obtains the virus solution with higher potency. Compared with the virus by female parent cell culture, the HA potency titer is obviously enhanced. The microbe collection numbers of the two cell strains are respectively CGMCC No.10008 and CGMCC No.10009. The MDCK clone cell strains disclosed by the invention can efficiently culture viruses, obviously enhances the virus titer, and has high application value in influenza virus vaccine production.
Owner:哈药集团生物疫苗有限公司
Large-scale culture method of H9N2 subtype avian influenza virus
InactiveCN104560888AStable proliferationEasy to operateMicroorganism based processesViruses/bacteriophagesVaccine ProductionAvian influenza virus
The invention discloses a large-scale culture method of an H9N2 subtype avian influenza virus. According to the large-scale culture method, the H9N2 virus is rapidly adapted to MDCK, the HA titer of the virus is 28, MDCK cells are cultured as seed cells by taking a 7.5L reactor as a seed cell tank and cytodex1 as a carrier, the seed cells are amplified to large-scale culture in a 40L fixed bed basket type stirring system reactor, and 80L of H9N2 viruses of which the hemagglutination titer (HA) is up to 210 can be obtained. The virus produced by using the method is high in titer and large in yield, the method is easy in standardization and is applicable to all H9 subtype influenza viruses, and the difficulty that the culture virus titer cannot meet vaccine production when the H9N2 avian influenza virus is cultured on the MDCK cells is solved.
Owner:HUAZHONG AGRI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com