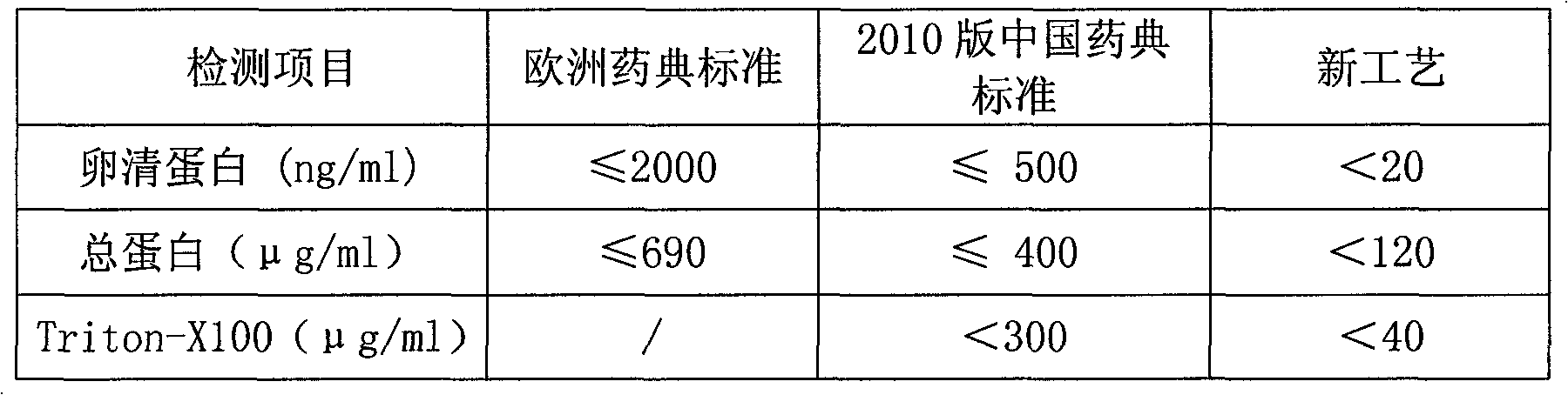
Novel process for preparing influenza virus split vaccine
An influenza virus and new process technology, applied in the direction of antiviral agents, medical preparations containing active ingredients, and antibody medical ingredients, etc., can solve the problems of small side effects, large side effects, and children cannot be vaccinated, so as to improve the yield and reduce the content, the effect of increasing the effective antigen content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0019] The present invention will be further described below in conjunction with embodiment:
[0020] The new process for preparing the influenza virus split vaccine has the following steps:
[0021] (1), virus inoculation and cultivation: inoculate and dilute to the virus amount 2.0-6.0LgEID in the allantoic cavity of chicken embryo 50 / ml working seed batch virus species (the working seed batch virus strain is recommended by the World Health Organization (WHO) and approved by the national drug management department of the year's epidemic strains or similar strains of influenza A and B viruses in conventional methods. Prepare the main generation virus seed bank, and then prepare the working virus seed according to the same method), and culture at 33-35°C for 48-72 hours;
[0022] (2), virus harvesting: screen live chicken embryos, put 2~8 ℃ after certain time cold embryo, harvest allantoic fluid in the container, after clarification and filtration, each container is sampled ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com