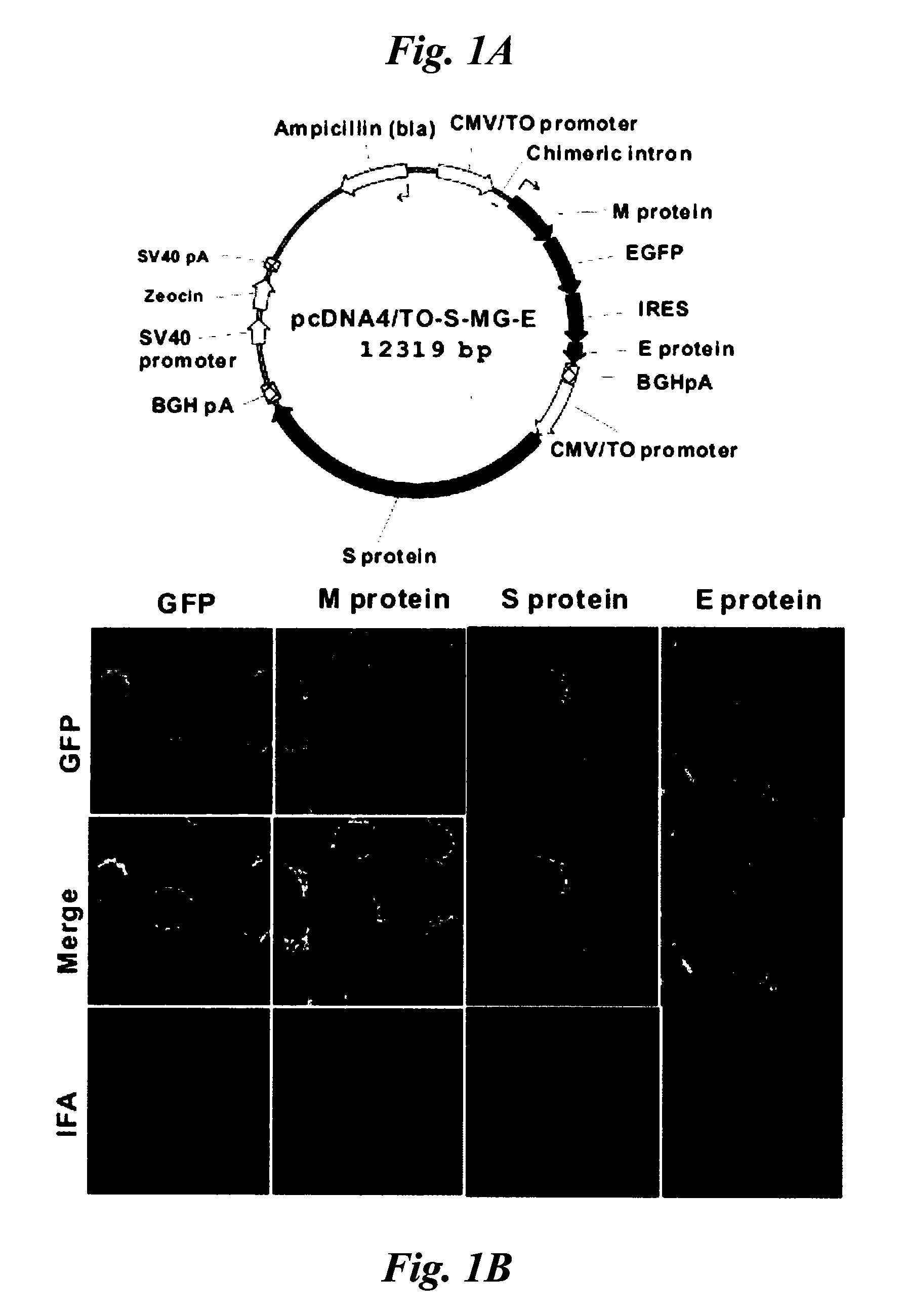
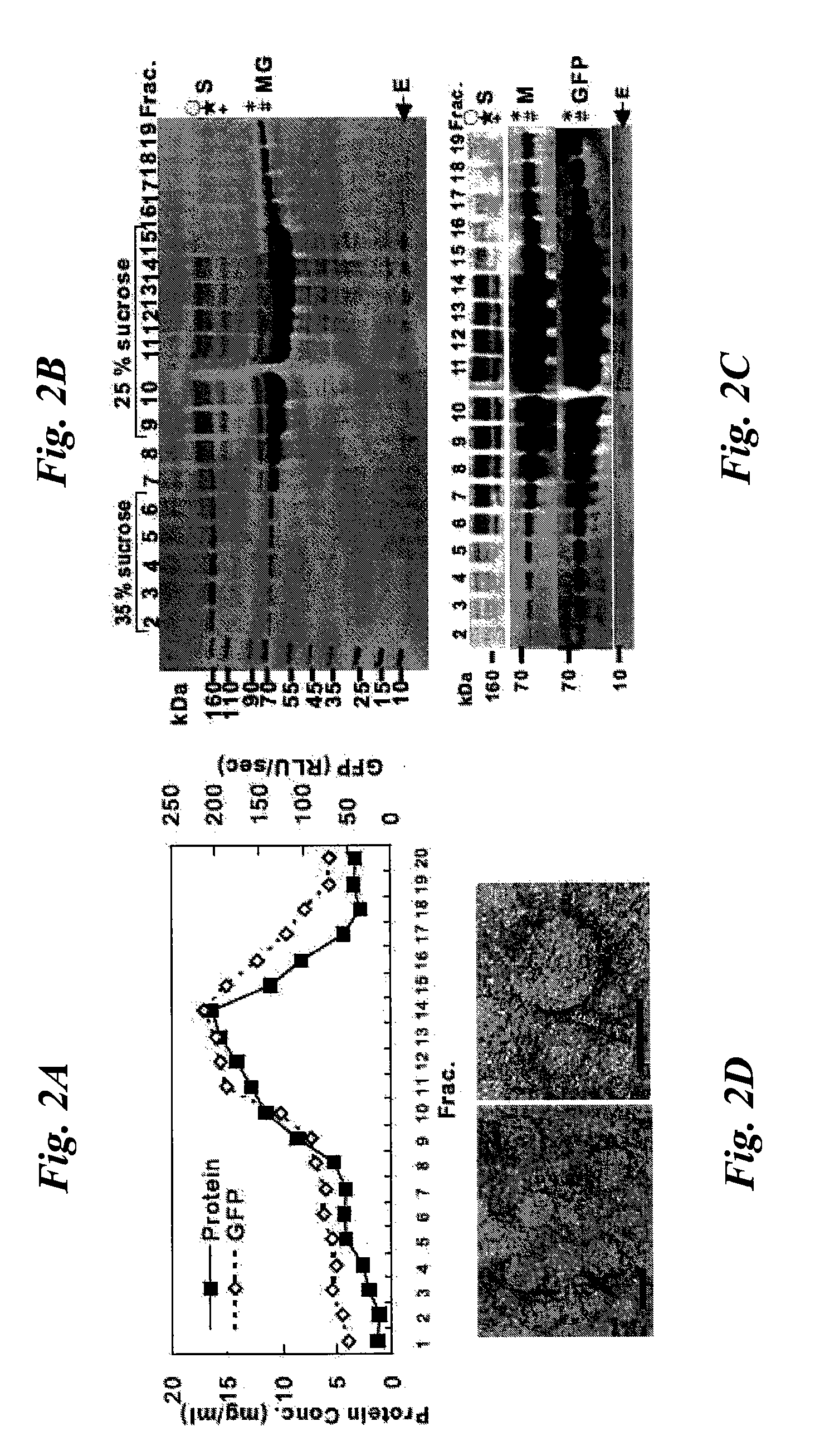
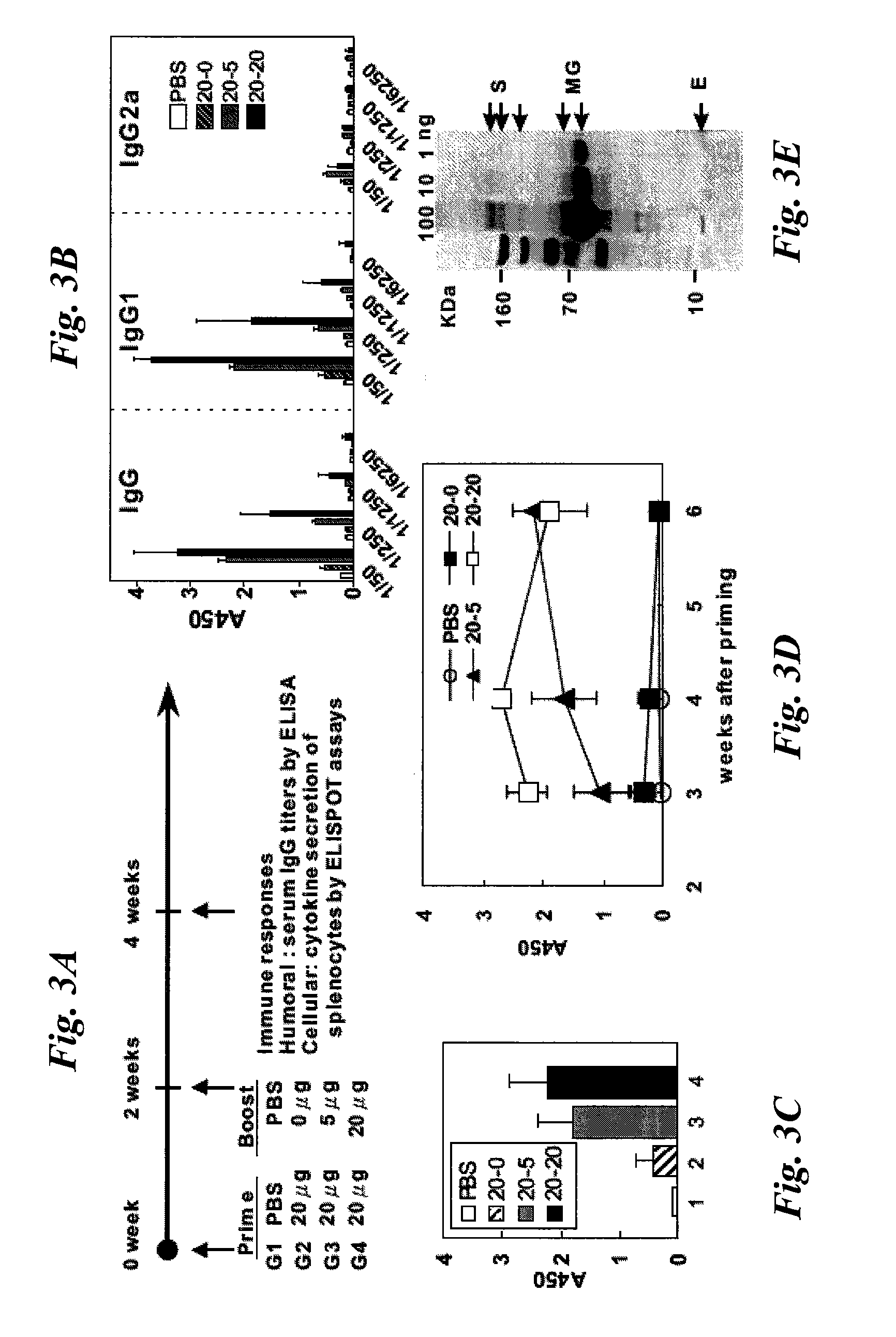
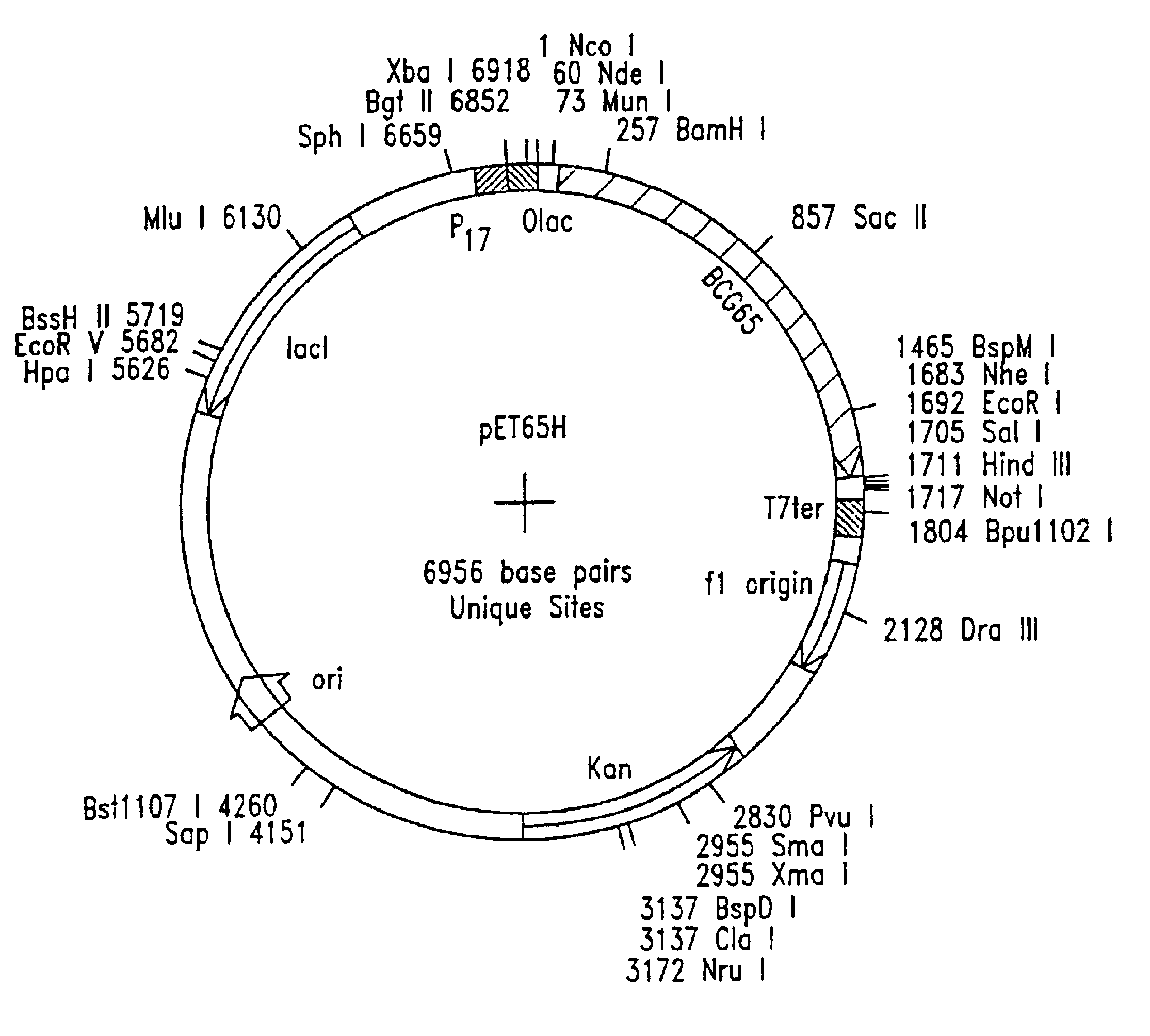
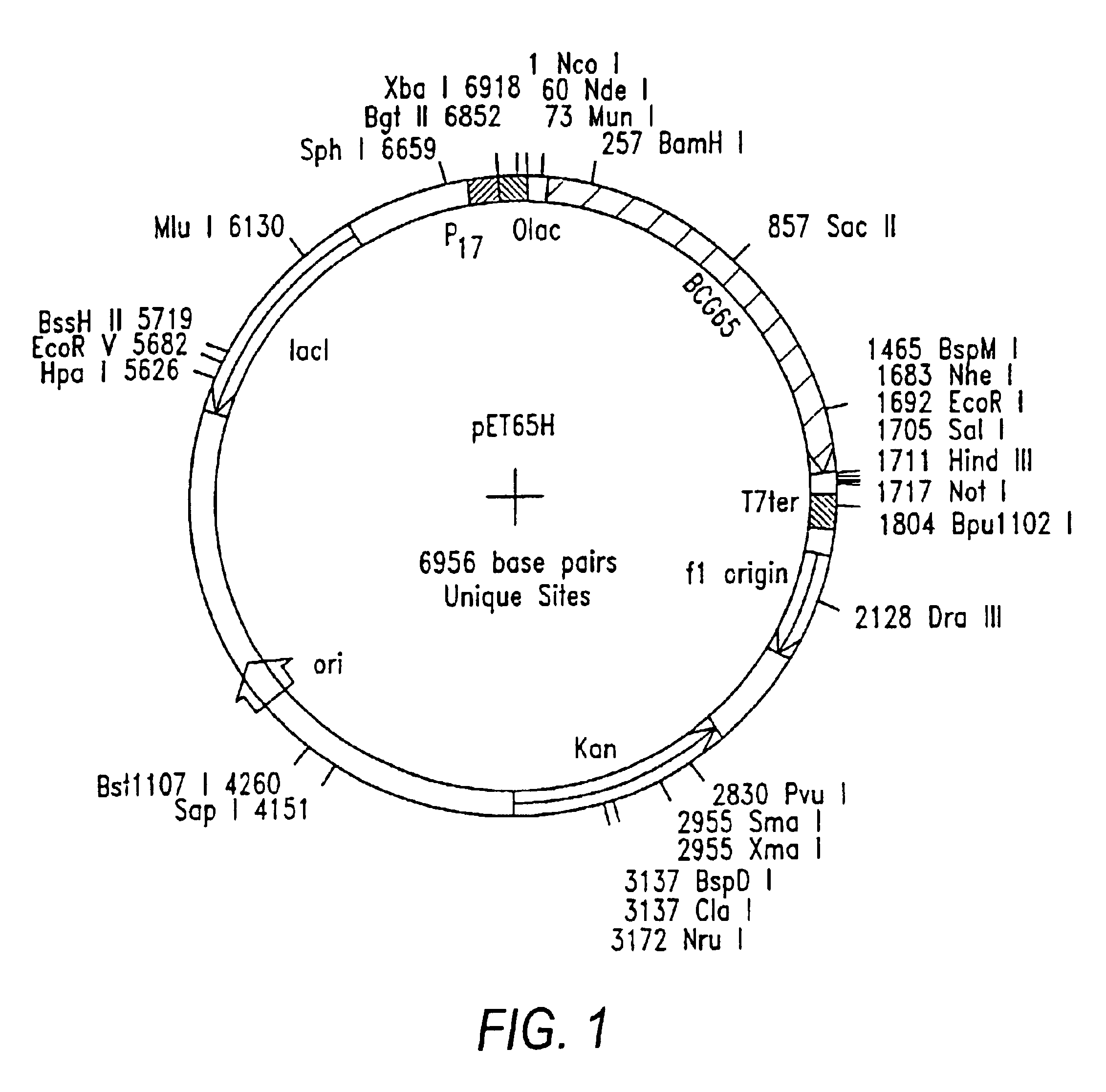
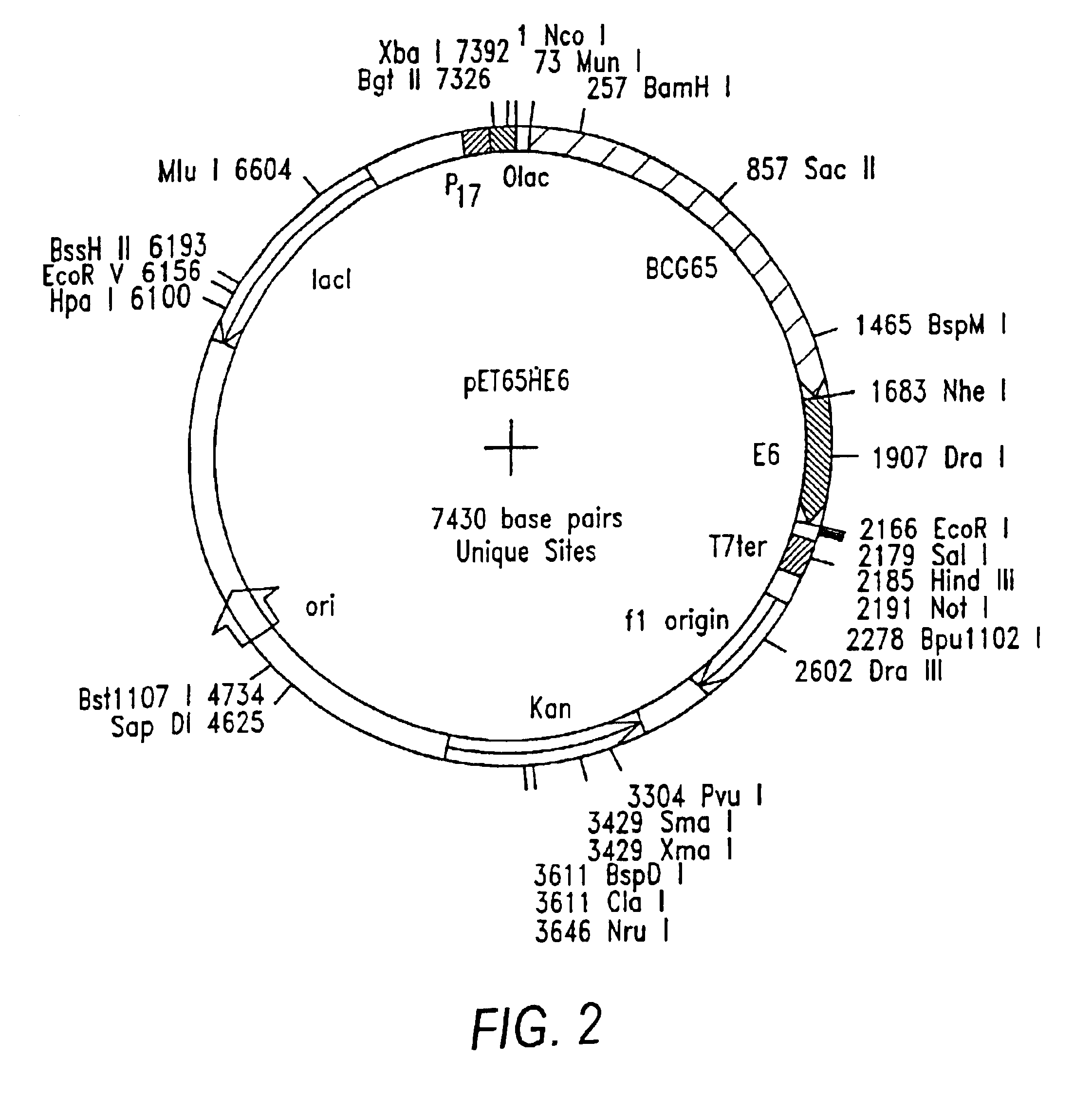
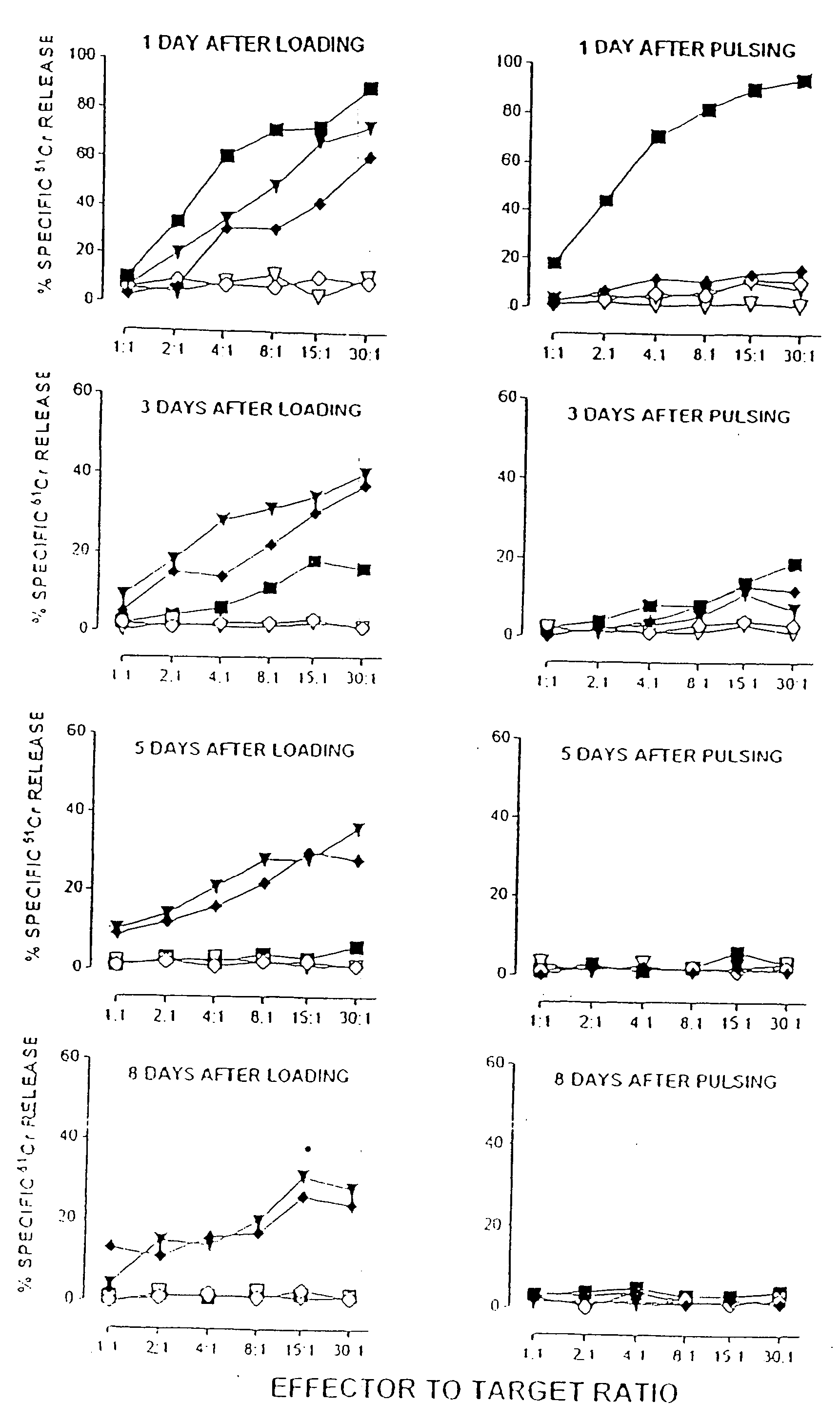
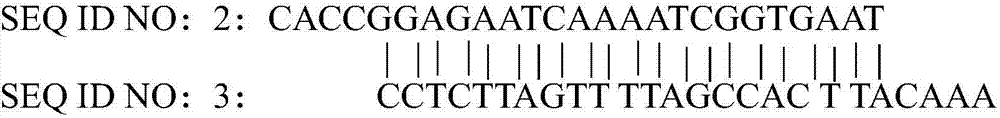Patents
Literature
739 results about "Cell immunity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cellular immunity, also known as cell-mediated immunity, is an important aspect of the immune system that allows the body to attack invading organisms on a cellular level.
Novel methods for therapeutic vaccination
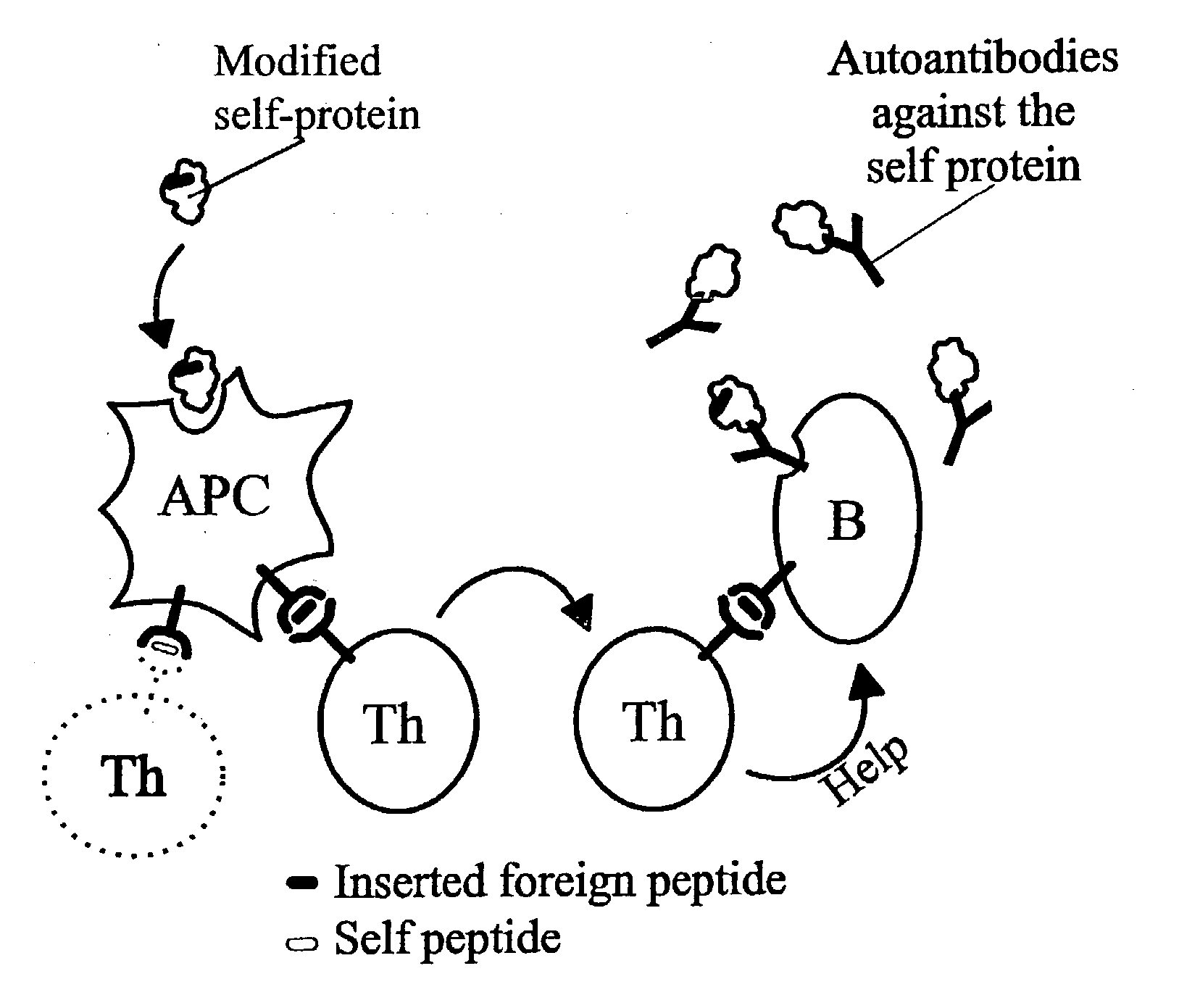
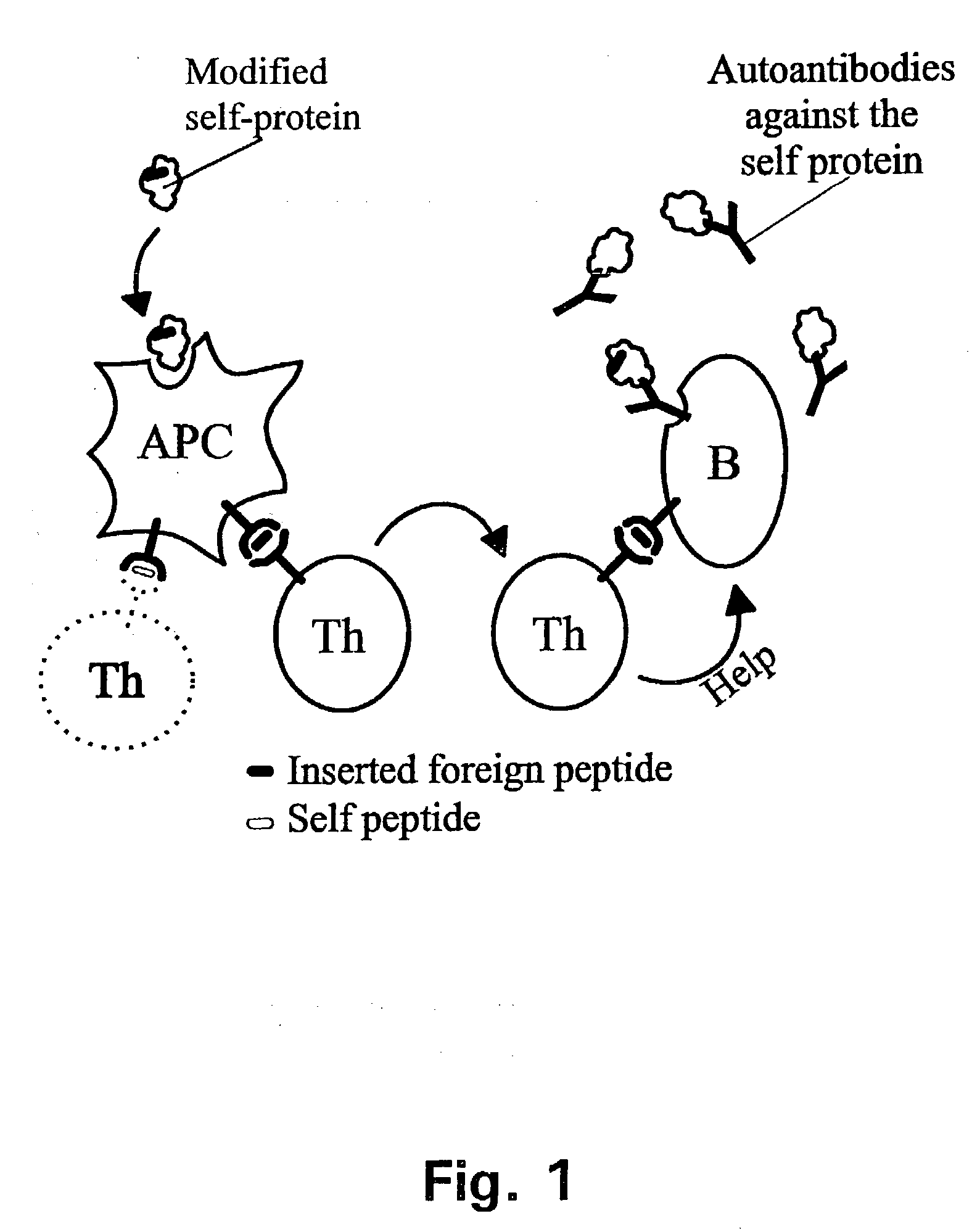
A method is disclosed for inducing cell-mediated immunity against cellular antigens. More specifically, the invention provides for a method for inducing cytotoxic T-lymphocyte immunity against weak antigens, notably self-proteins. The method entails that antigen presenting cells are induced to present at least one CTL epitope of the weak antigen and at the same time presenting at least one foreign T-helper lymphocyte epitope. In a preferred embodiment, the antigen is a cancer specific antigen, e.g. PSM, Her2, or FGF8b. The method can be exercised by using traditional polypeptide vaccination, but also by using live attenuated vaccines or nucleic acid vaccination. The invention furthermore provides immunogenic analogues of PSM, Her2 and FGF8b, as well as nucleic acid molecules encoding these analogues. Also vectors and transformed cells are disclosed. The invention also provides for a method for identification of immunogenic analogues of weak or non-immunogenic antigens.
Owner:BAVARIAN NORDIC AS
Redirection of cellular immunity by receptor chimeras
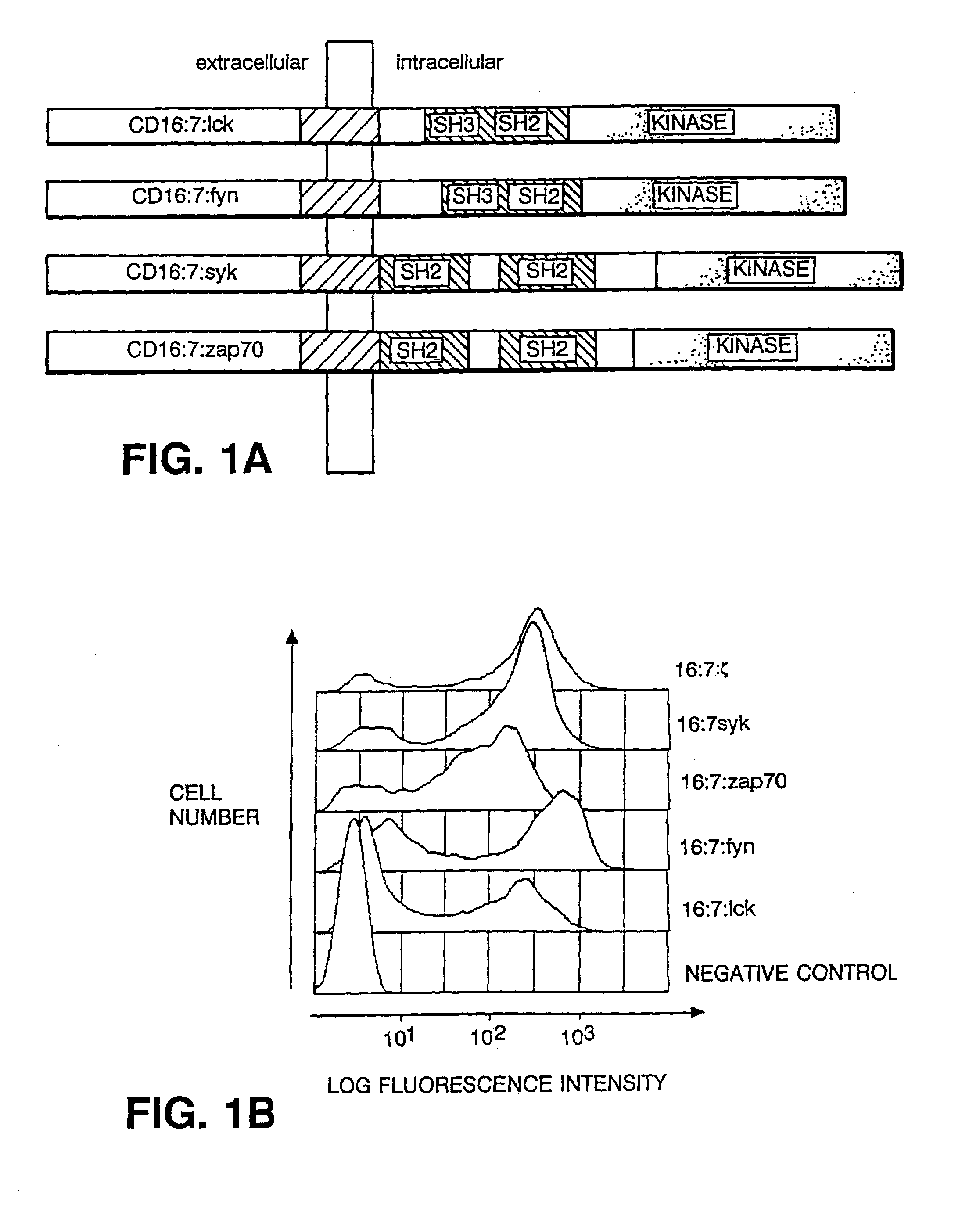
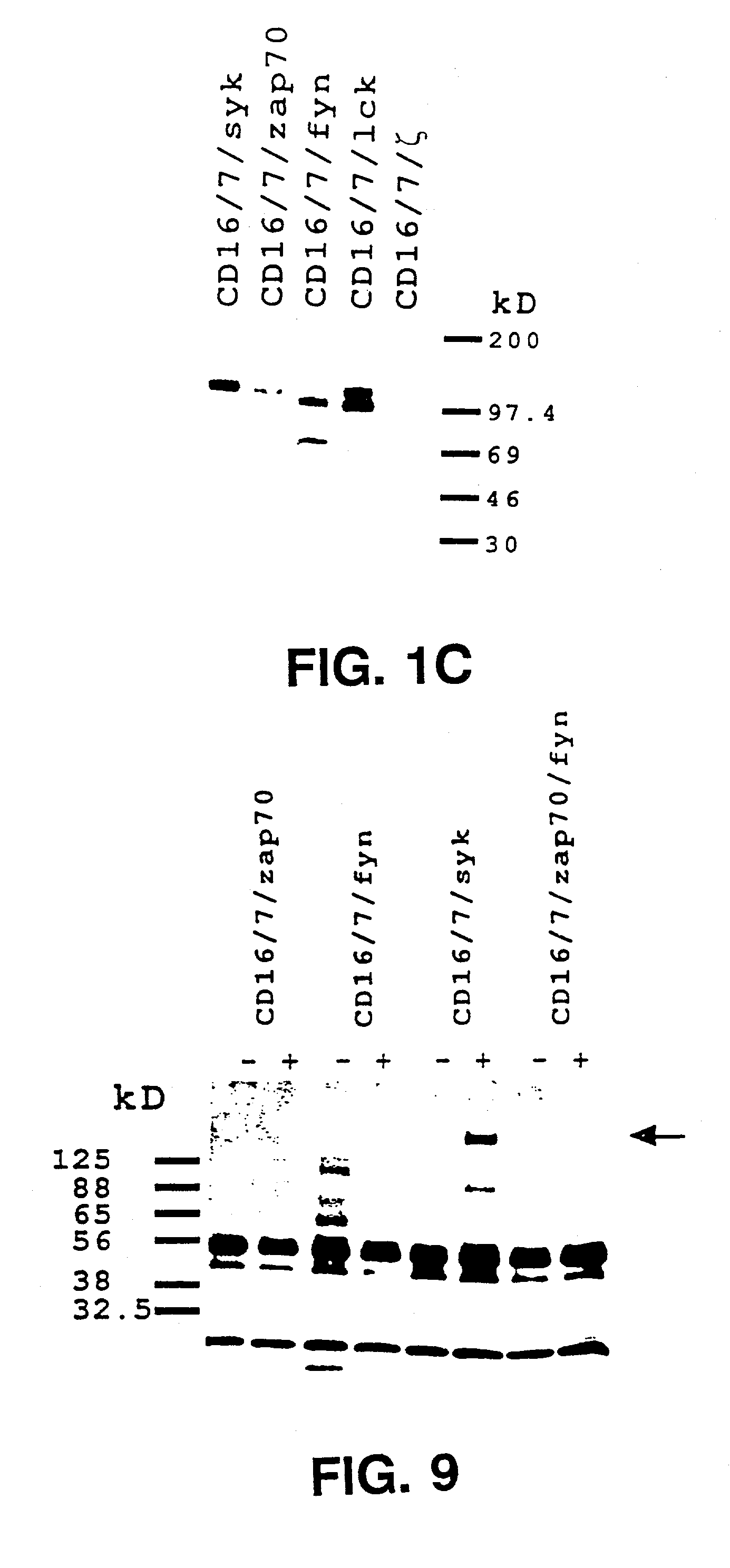
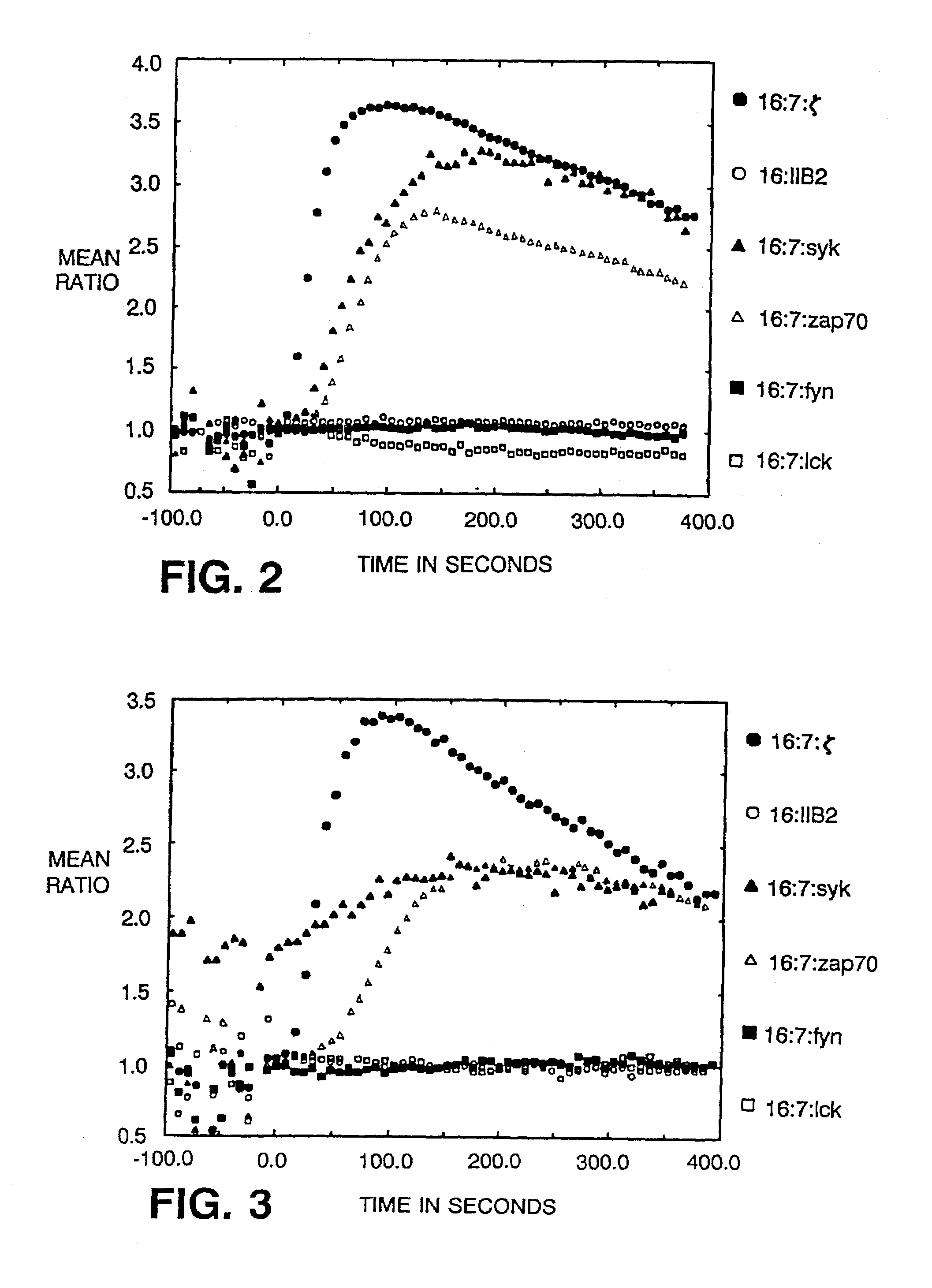
InactiveUS7049136B2Enhances immune system responseImprove responseVirusesPeptide/protein ingredientsAbnormal tissue growthCancer cell
Disclosed is a method of directing a cellular response in a mammal by expressing in a cell of the mammal a chimeric receptor which causes the cells to specifically recognize and destroy an infective agent, a cell infected with an infective agent, a tumor or cancerous cell, or an autoimmune-generated cell. Also disclosed are cells which express the chimeric receptors and DNA encoding the chimeric receptors.
Owner:THE GENERAL HOSPITAL CORP
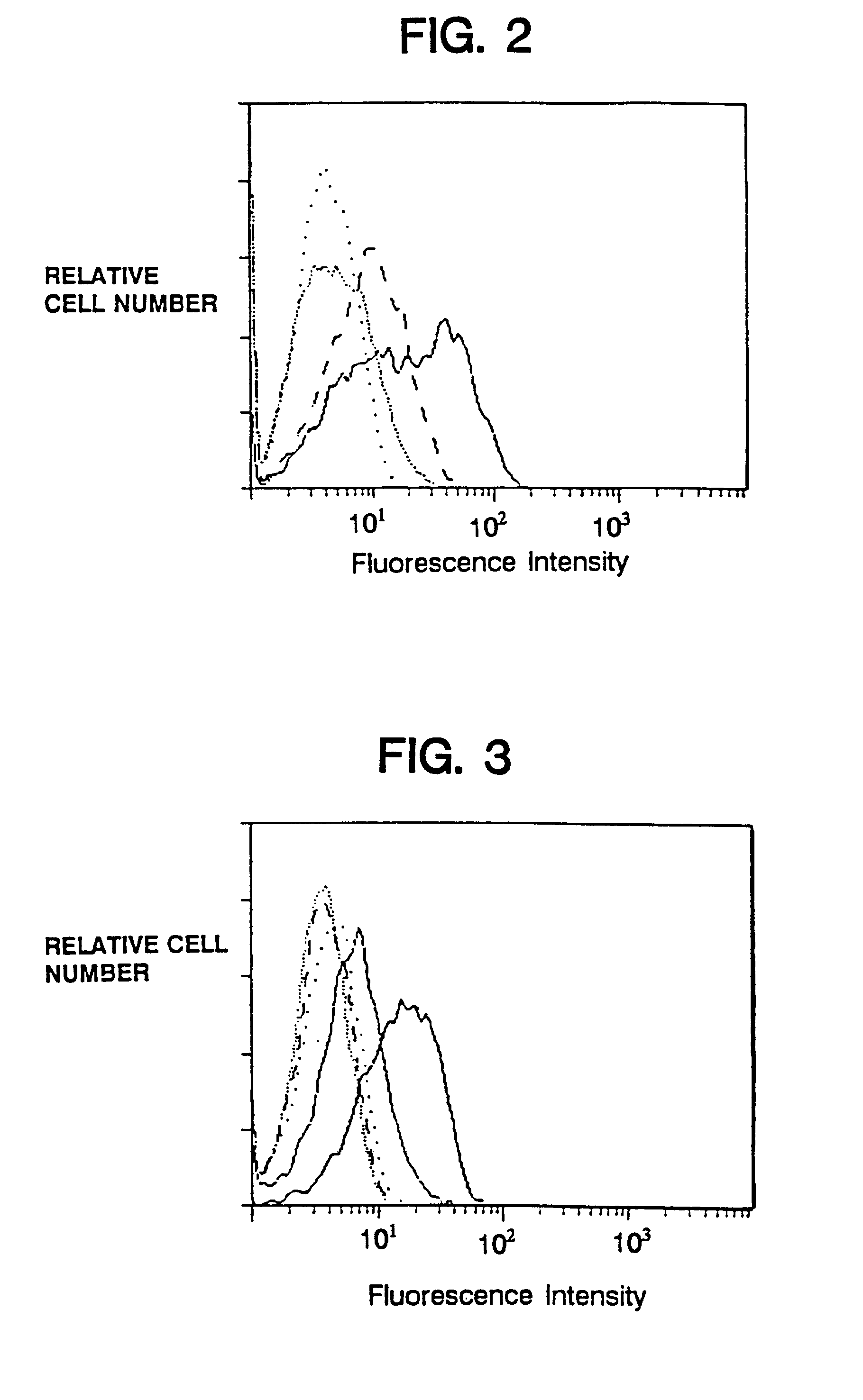
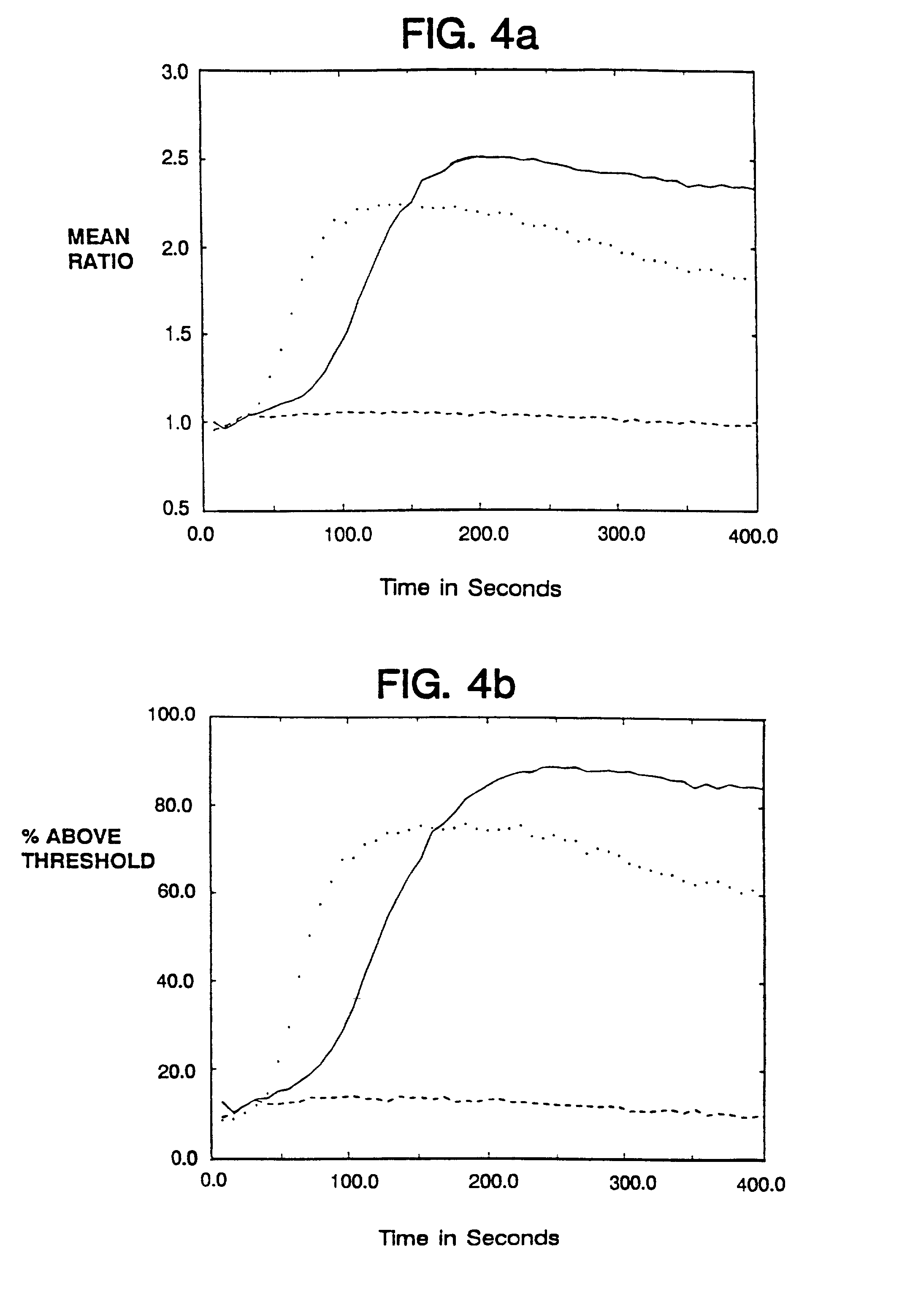
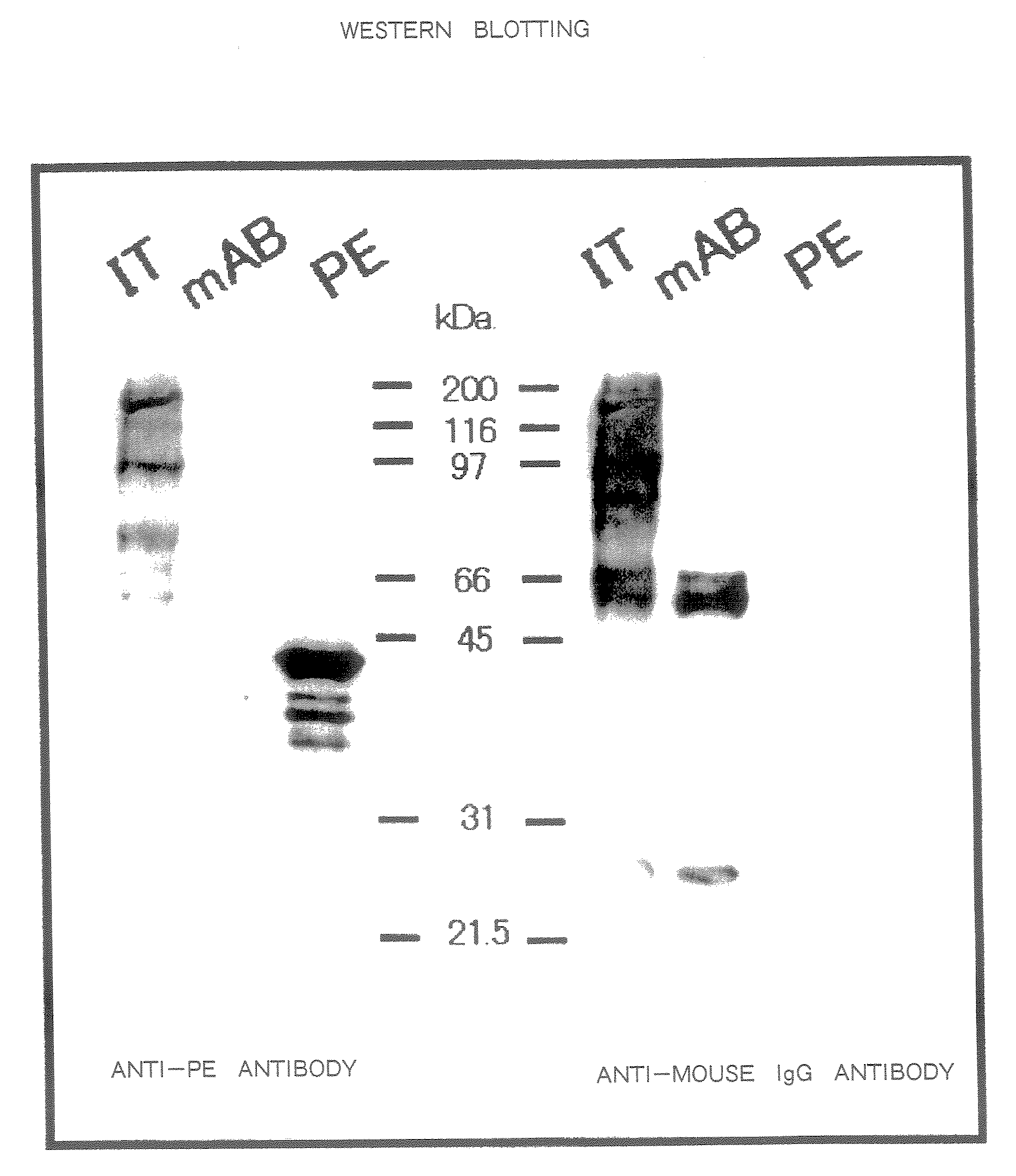
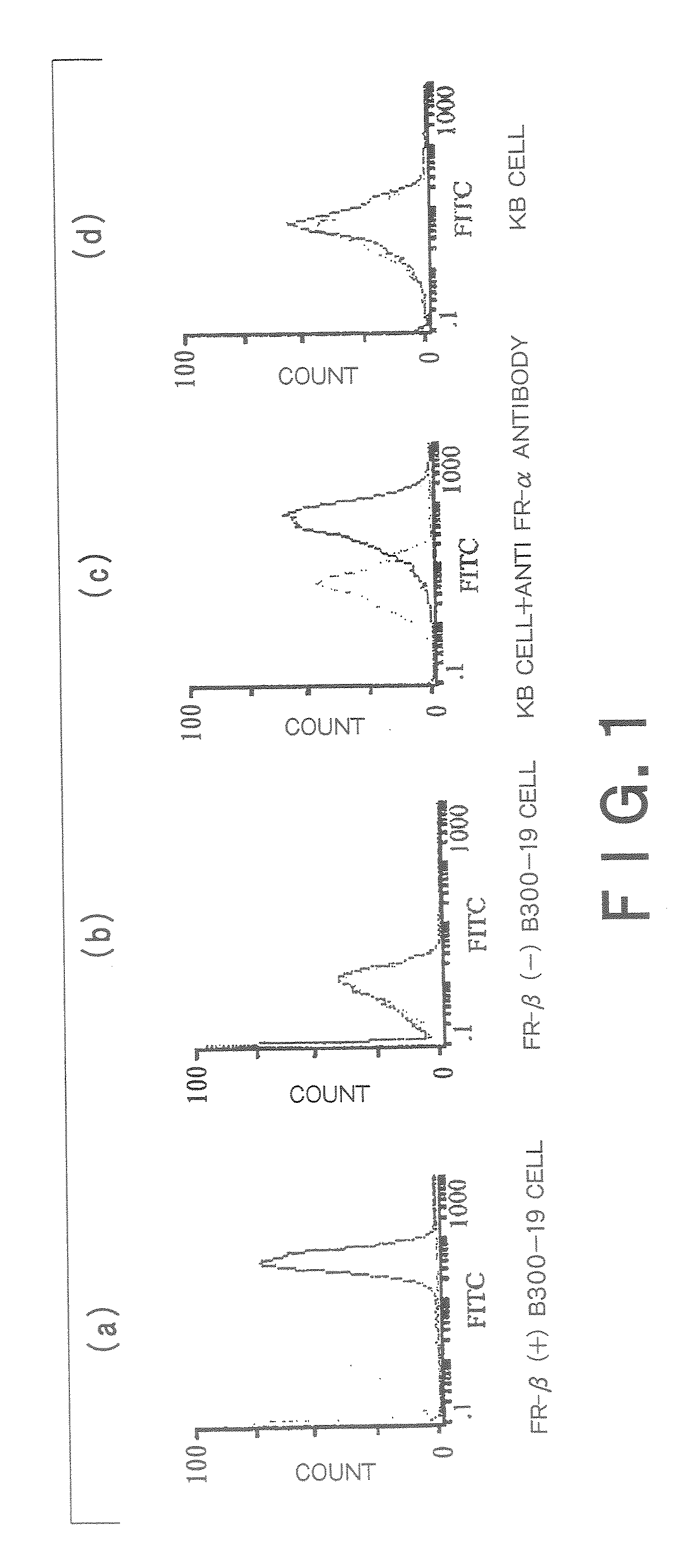
Therapeutic Medicine Containing Monoclonal Antibody Against Folate Receptor Beta (Fr-Beta)
InactiveUS20080260812A1Good chemical stabilitySufficient amountPeptide/protein ingredientsAntipyreticMacrophage activation syndromeArthritis therapy
An objective of the present invention is to provide a therapeutic agent for treating rheumatoid arthritis, juvenile rheumatoid arthritis, macrophage activation syndrome, septicemia, and FR-β expressing leukemia, which induces apoptosis in activated macrophages and folate receptor beta (FR-β) expressing leukemia cells to specifically destroy these cells. An FR-β monoclonal antibody of the present invention is preferably an IgG-type monoclonal antibody which specifically reacts with a human-type FR-β antigen and is produced from a clone resulting from immunization with an FR-β expressing B300-19 cell. The FR-β monoclonal antibody of the present invention specifically reacts with the FR-β antigen of activated macrophages and FR-β expressing leukemia cells and a therapeutic agent of the present invention contains an FR-β antibody immunotoxin which causes apoptosis in activated macrophages and FR-β expressing leukemia cells, as an active ingredient. Further, suitable administration form for the therapeutic agent of the present invention includes intravenous injection as well as joint injection in the case of therapeutic agents for rheumatoid arthritis and juvenile arthritis.
Owner:TAKAMI MATSUYAMA KAGOSHIMA UNIV +1
Compositions and methods for eliciting an immune response to escape mutants of targeted therapies
InactiveUS20100034840A1Reduce resistanceSymptoms improvedAntibacterial agentsOrganic active ingredientsMolecular Targeted TherapiesMutant
Provided herein are cells, vectors and viruses in association with a mutant polypeptide that has emerged in response to a therapeutic or prophylactic agent; compositions comprising such cells, vectors and viruses and methods for their use in eliciting an immune response to the mutant polypeptide. In some examples, the immune response is a cellular immune response.
Owner:GLOBE IMMUNE INC
Redirection of cellular immunity by protein tyrosine kinase chimeras
InactiveUS7320787B2Facilitates specific recognitionFacilitates destructionBiocidePeptide/protein ingredientsDna encodingProtein-Tyrosine Kinases
Disclosed is a method of directing a cellular response in a mammal by expressing in a cell of the mammal a chimeric receptor which causes the cells to specifically recognize and destroy an infective agent, a cell infected with an infective agent, a tumor or cancerous cell, or an autoimmune-generated cell. The chimeric receptor includes an extracellular portion which is capable of specifically recognizing and binding the target cell or target infective agent, and (b) an intracellular portion of a protein-tyrosine kinase which is capable of signalling the therapeutic cell to destroy a receptor-bound target cell or a receptor-bound target infective agent. Also disclosed are cells which express the chimeric receptors and DNA encoding the chimeric receptors.
Owner:THE GENERAL HOSPITAL CORP
Molecular vaccines employing nucleic acid encoding anti-apoptotic proteins
InactiveUS20070026076A1Increase the number ofEasy to demonstratePowder deliveryVirusesDendritic cellVaccine Potency
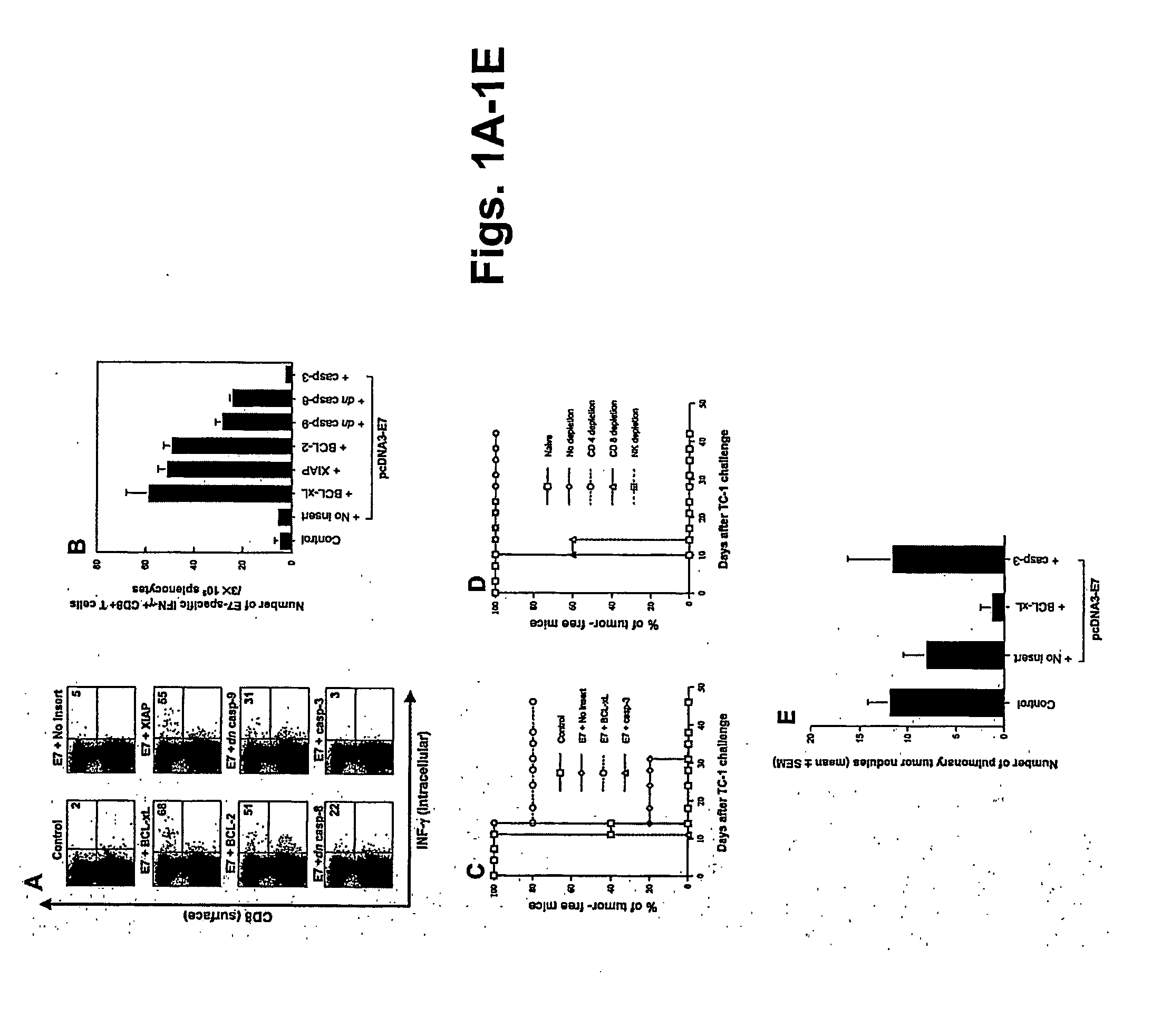
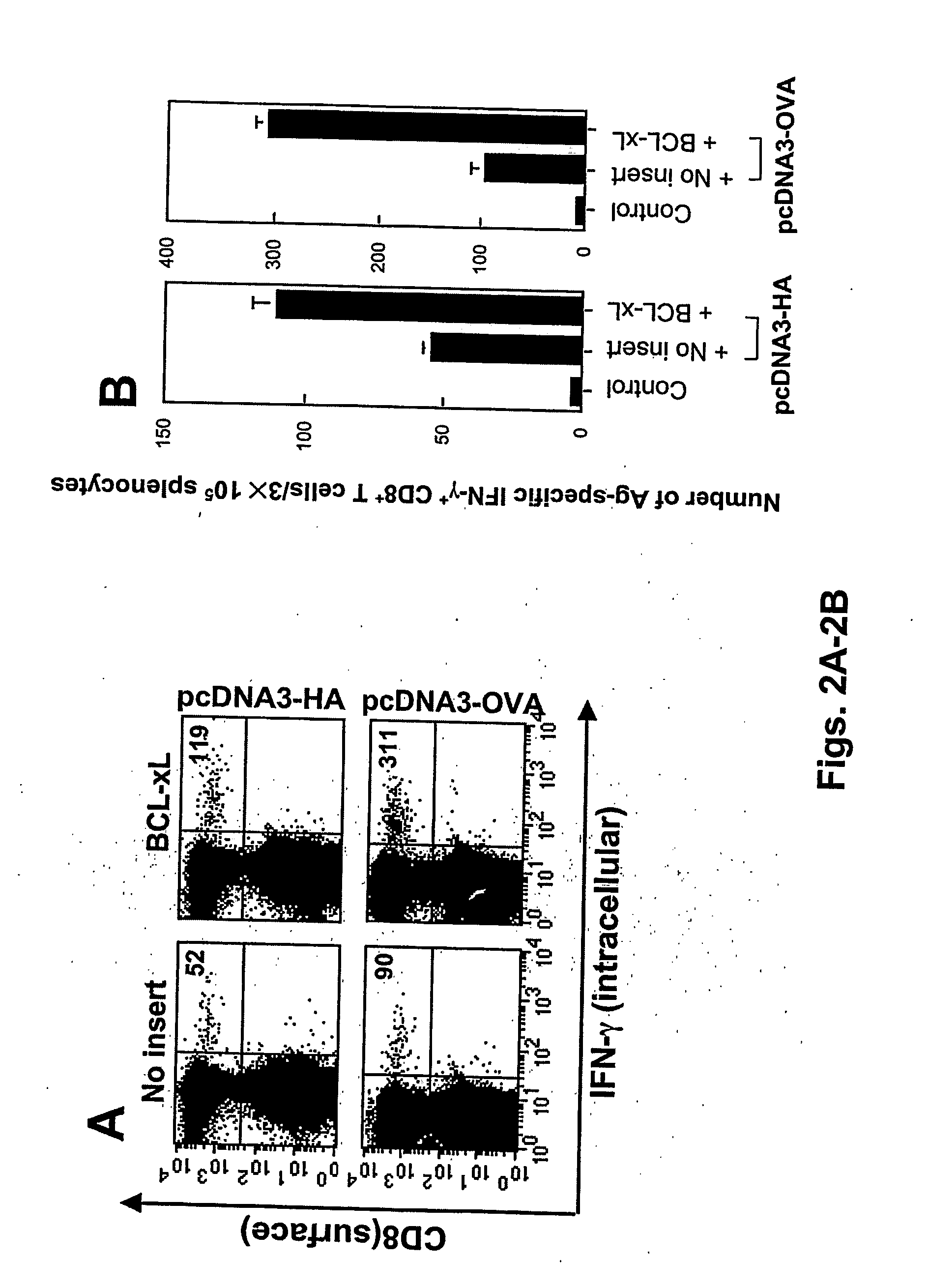
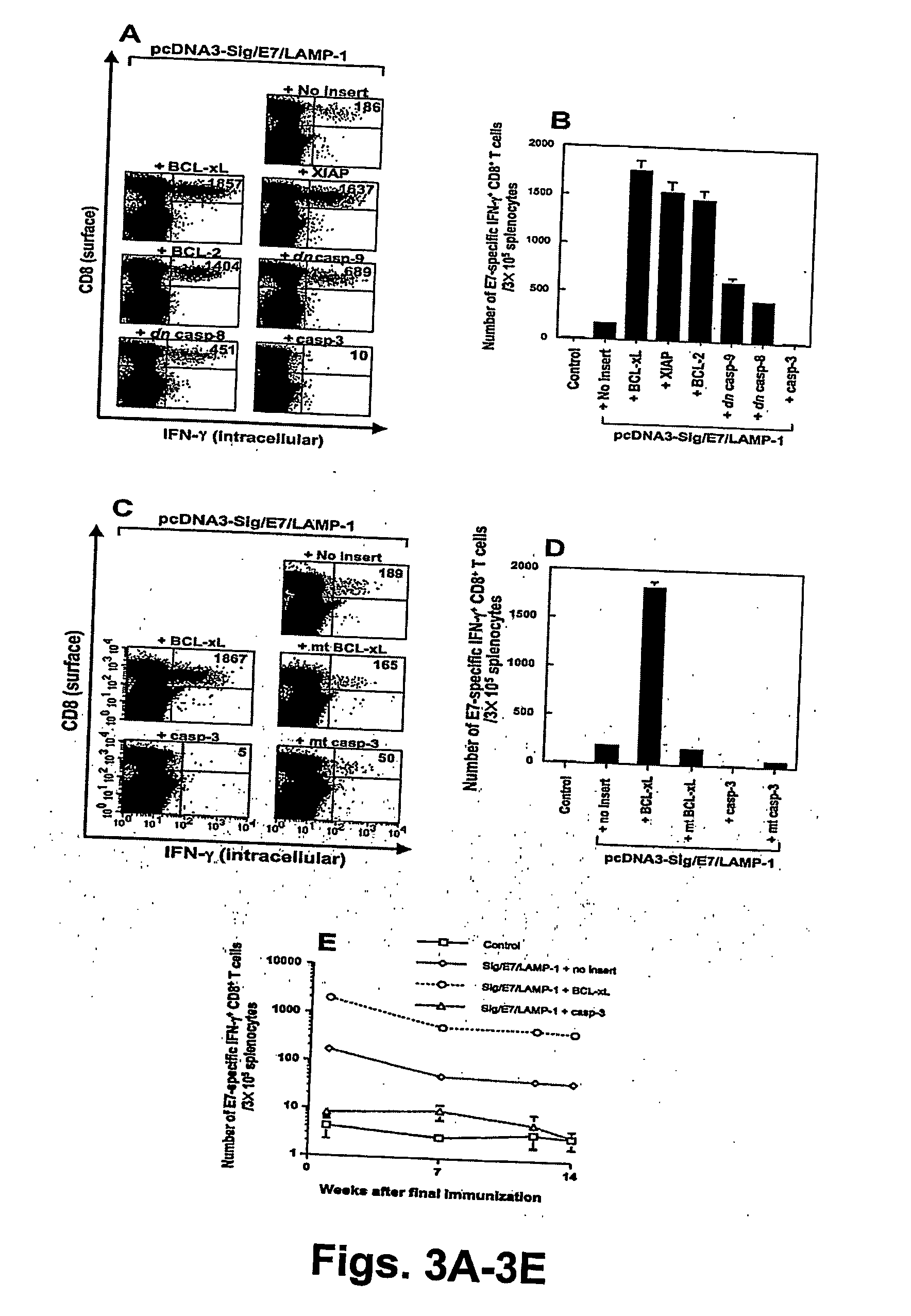
T cell immune responses are enhanced by presentation of antigen to CD8+ T cells using a chimeric nucleic acid immunogen or vaccine that links DNA encoding an antigen with DNA encoding a polypeptide that targets or translocates the antigenic polypeptide to which it is fused (immunogenicity-potentiating polypeptides or “IPP”). By inhibiting apoptosis in the vicinity of a T cell responses to such a nucleic acid immunogen, even more potent immune responses are attained. The present strategy prolongs the survival of DNA-transduced cells, including dendritic cells (DCs), thereby enhancing the priming of antigen-specific T cells and increase potency. Co-delivery of DNA encoding an inhibitor of apoptosis, including (a) BCL-xL, (b) BCL-2, (c) XIAP, (d) dominant negative caspase-9, or (e) dominant negative caspase-8, or (f) serine protease inhibitor 6 (SPI-6) which inhibits granzyme B, with DNA encoding an antigen, prolongs the survival of transduced DCs and results in significant enhancement of antigenspecific T cell immune responses that provide potent antitumor effects. Thus, co-administration of a DNA vaccine encoding antigen linked to an IPP along with one or more DNA constructs encoding an anti-apoptotic protein provides a novel way to enhance vaccine potency.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
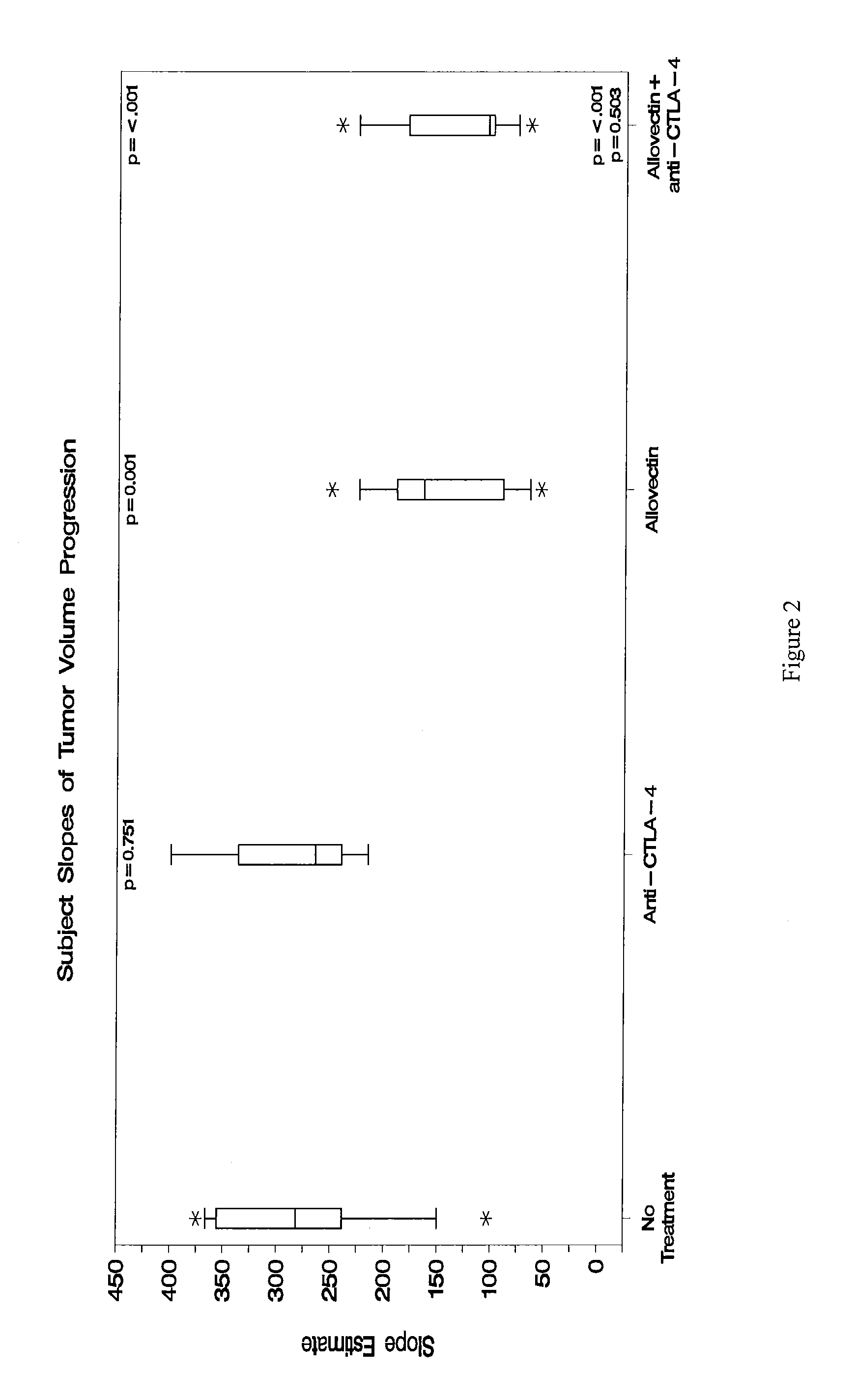
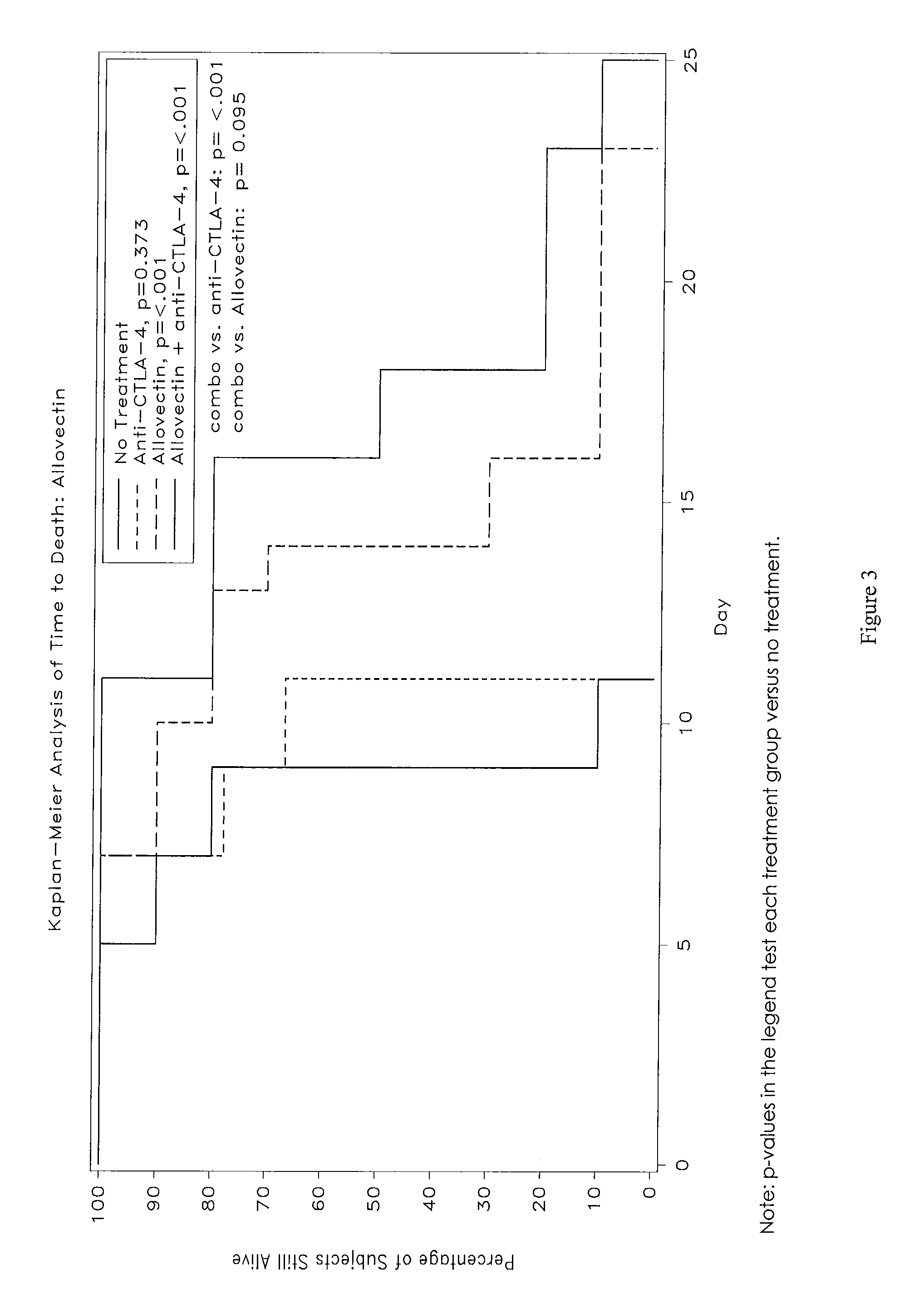
Synergistic Anti-tumor efficacy using alloantigen combination immunotherapy
InactiveUS20130280265A1Increased activationOrganic active ingredientsAntibody ingredientsImmunotherapeutic agentEfficacy
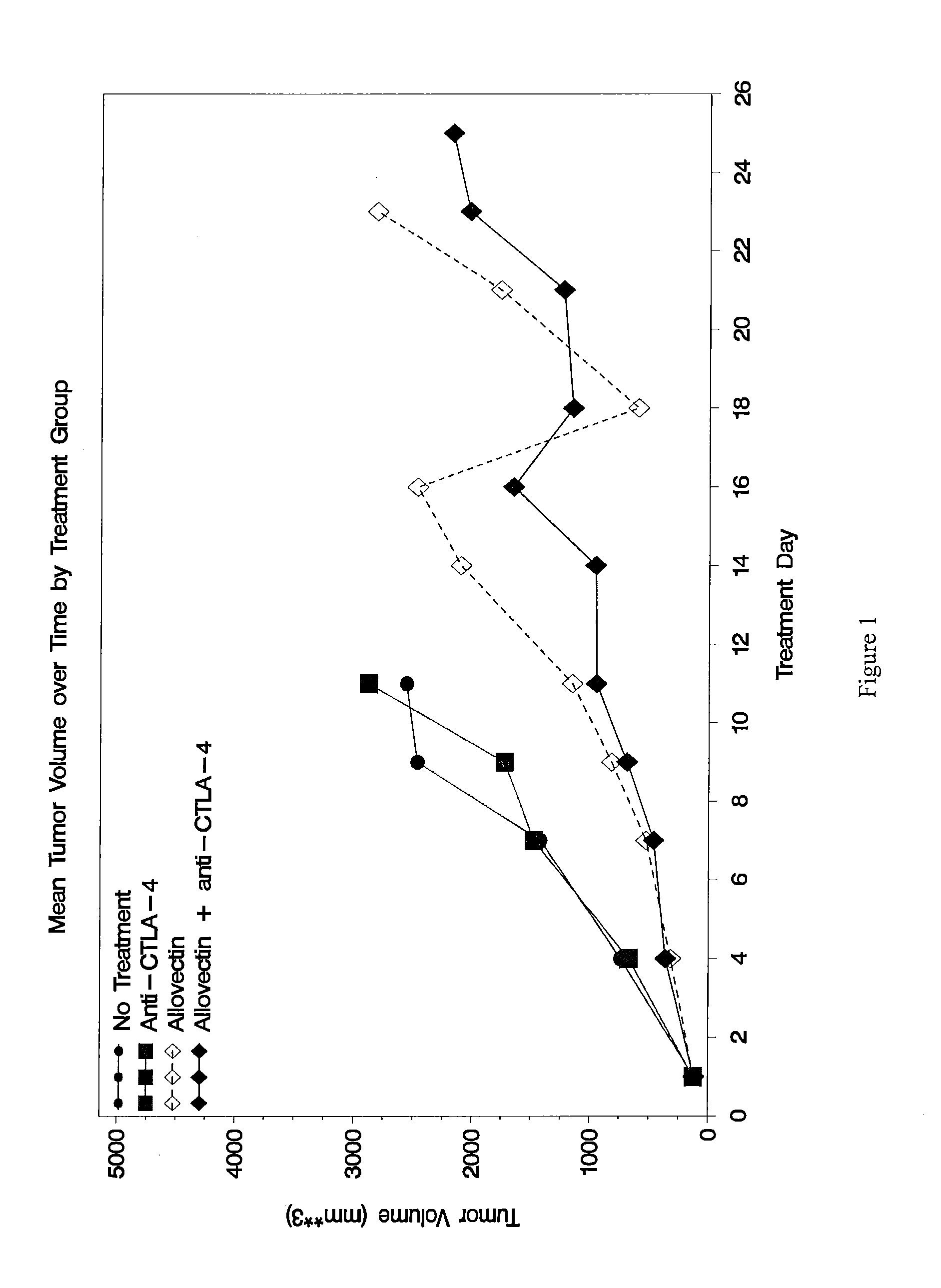
The present disclosure provides combinations of immunotherapeutics and methods for treating medical conditions that are characterized by the lack of an effective immune response, for example as would result following a down-regulation of MHC class I, such as in cancer. The immunotherapeutic compositions of the invention, which can be used to treat the medical conditions, include one or more immunostimulatory antibodies or molecules having specificity for CTLA-4, PD-1, PD-L1, PD-L2, CD40, OX40, CD137, GITR, ILT2, or ILT3, or ligands for these molecules (e.g., an isolated fully-human monoclonal antibody) in association with one or more alloantigens, such as, vector(s) capable of expressing protein(s) or peptide(s) that stimulate T-cell immunity against tissues or cells, formulated in a pharmaceutically acceptable carrier. The proteins or peptides may comprise class I major histocompatibility complex (MHC) antigens, β2-microglobulins, or cytokines. The MHC antigen may be foreign to the subject. The MHC antigen may be HLA-B7.
Owner:VICAL INC
Method for preparing II-type pig's ring-virus nucleic vaccine and the use thereof
InactiveCN1579553AShorten the production cycleAvoid pollutionViral antigen ingredientsGenetic material ingredientsPeripheral blood mononuclear cellBiology
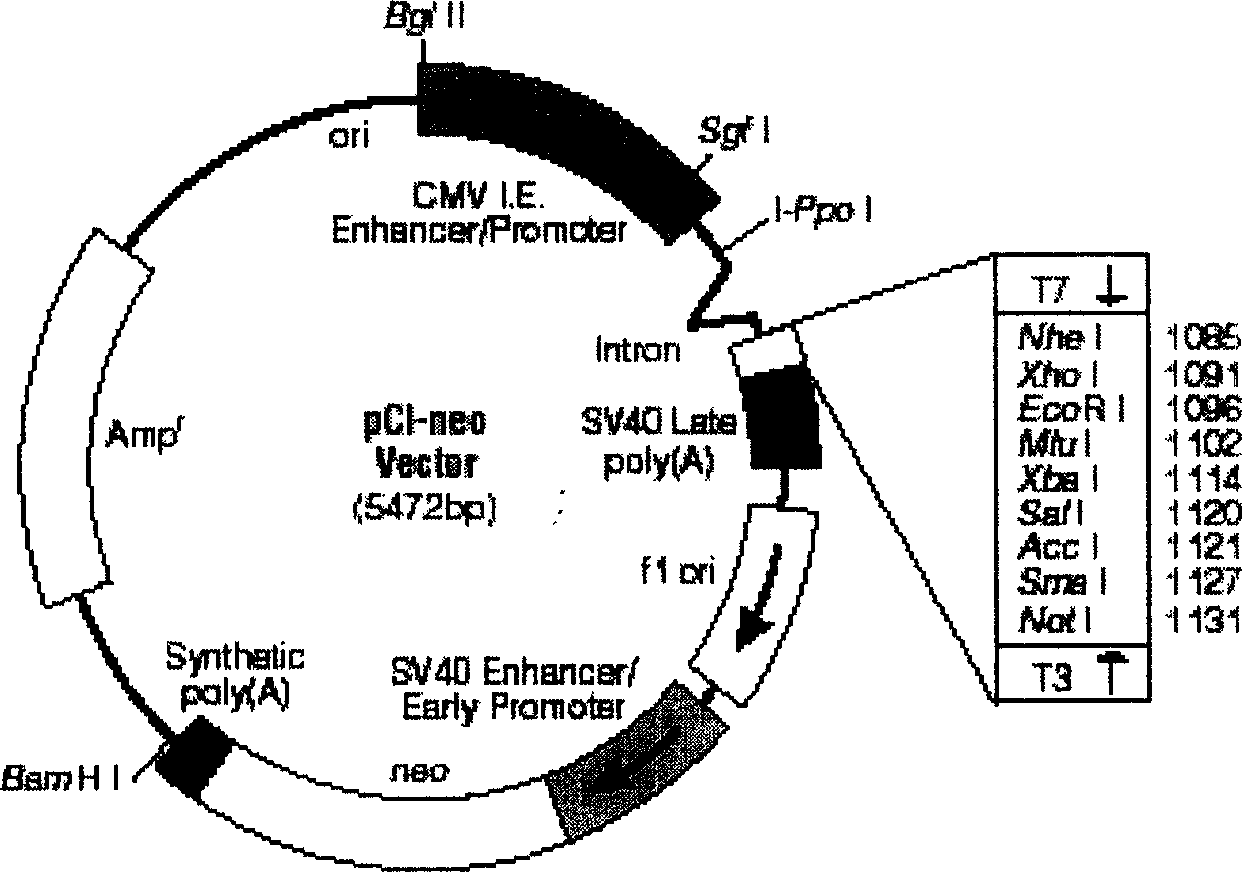
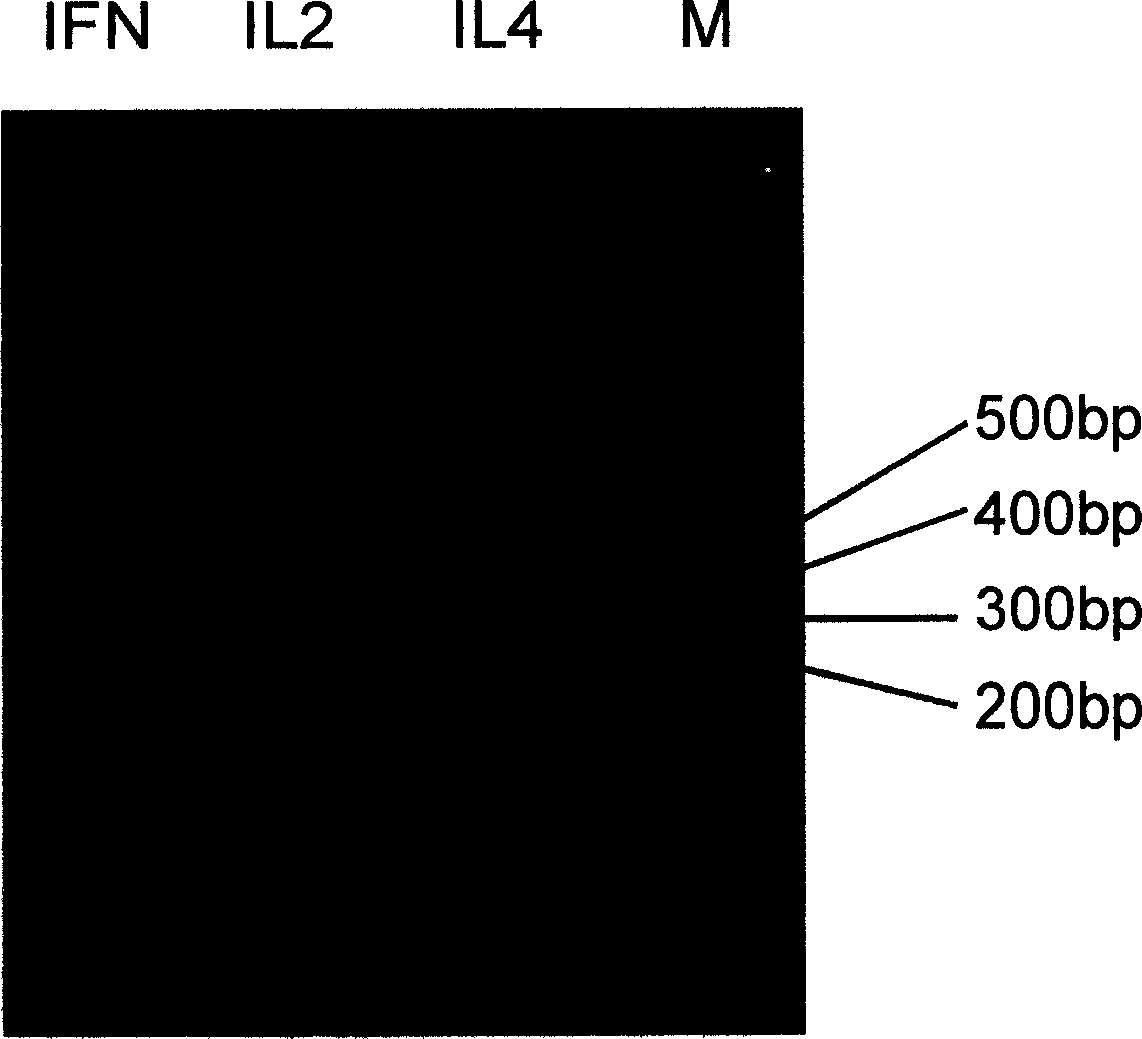
The invention discloses a manufacturing method and application of II type pig ring virus nucleic acid vaccine. The method is: 1) designs the specific primer, uses PCV2 Hangzhou (HZ201) gene group as template, closes ORF1, ORF2, ORF3 and ORF4 genes of PCV-2 with PCR method, and they are constructed into eucaryon expressing carrier with pCI-neo; 2) separates the pig external blood single nucleus cell, clones the genes IFN, IL-2 and IL-4 with RT-PCR method, and they are constructed into eucaryon expressing carrier with pCI-neo; 3) based on above mentioned reconstructed carrier, constructs the fused expressing carrier of the other genes of PCV2, ORF2 and PCV2 or pig cell factor gene; the merits of the invention lie in: (1) it needs not to culture virus, the producing period is short; (2) it needs not cell culture, thus can prevent the contamination from other pig source virus; (3) it does not express the pathogenesis protein which is harmful to body, the safety is high. (4) it can activates body secretion and cell immunity replay at the same time.
Owner:ZHEJIANG UNIV
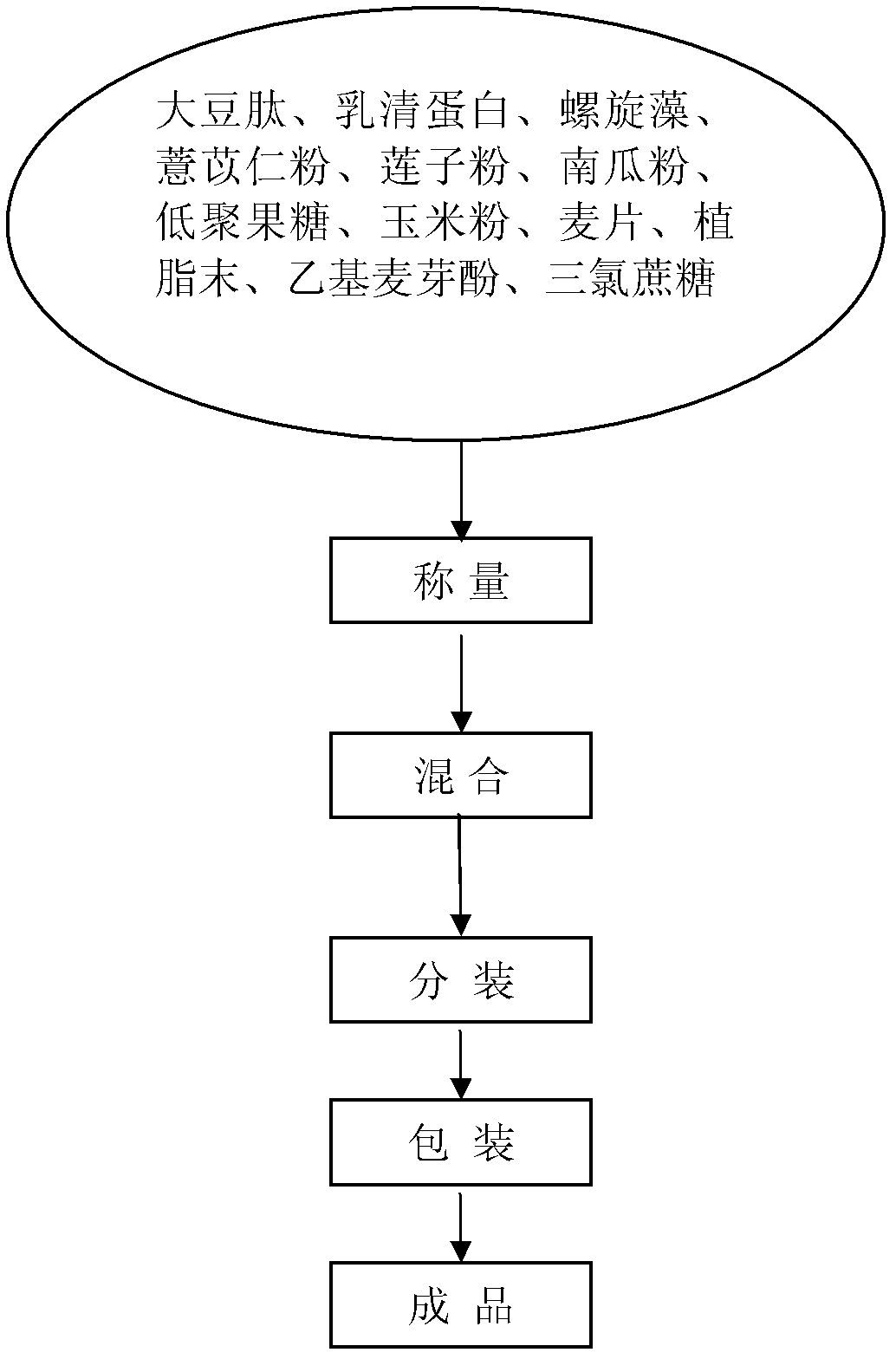
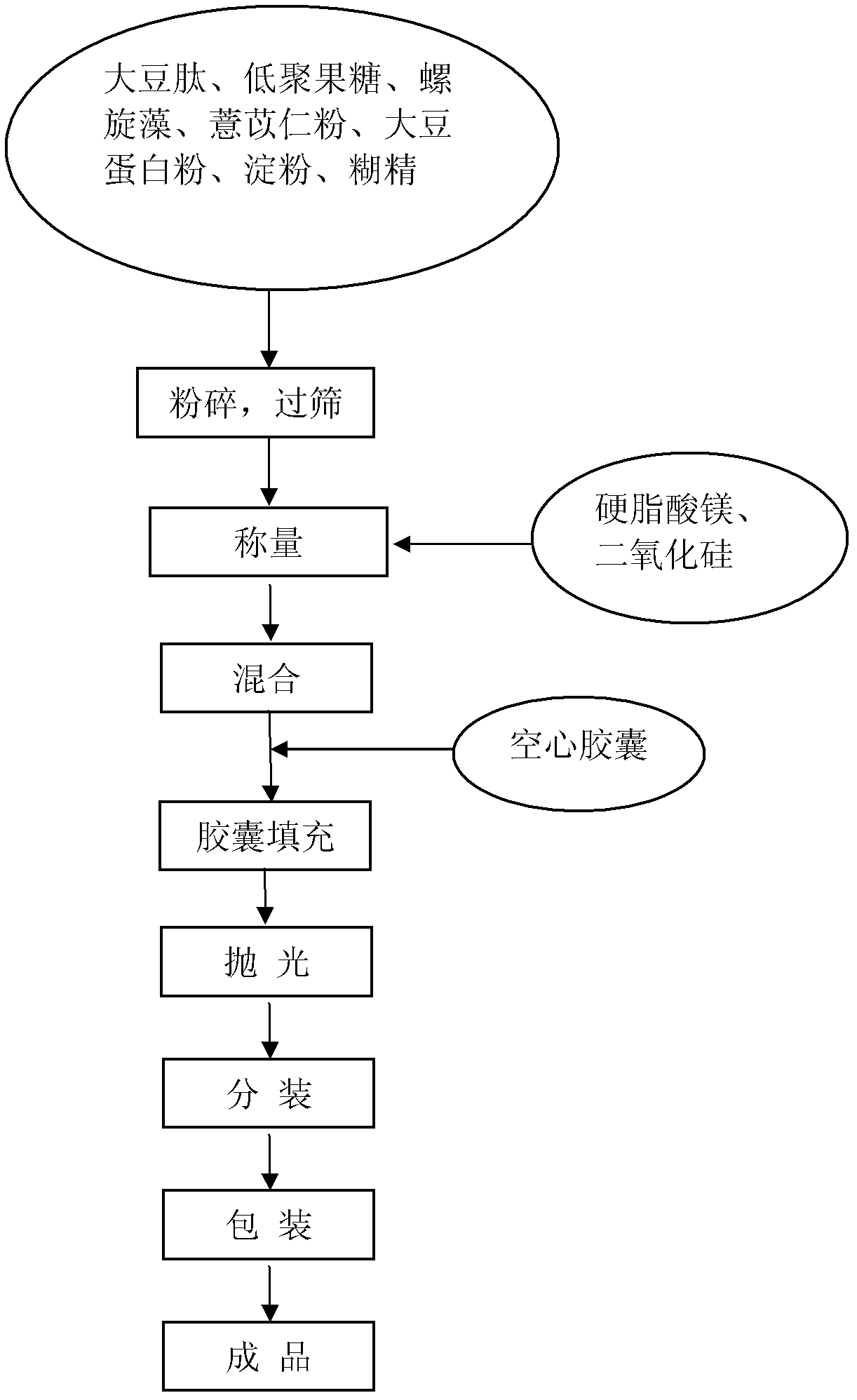
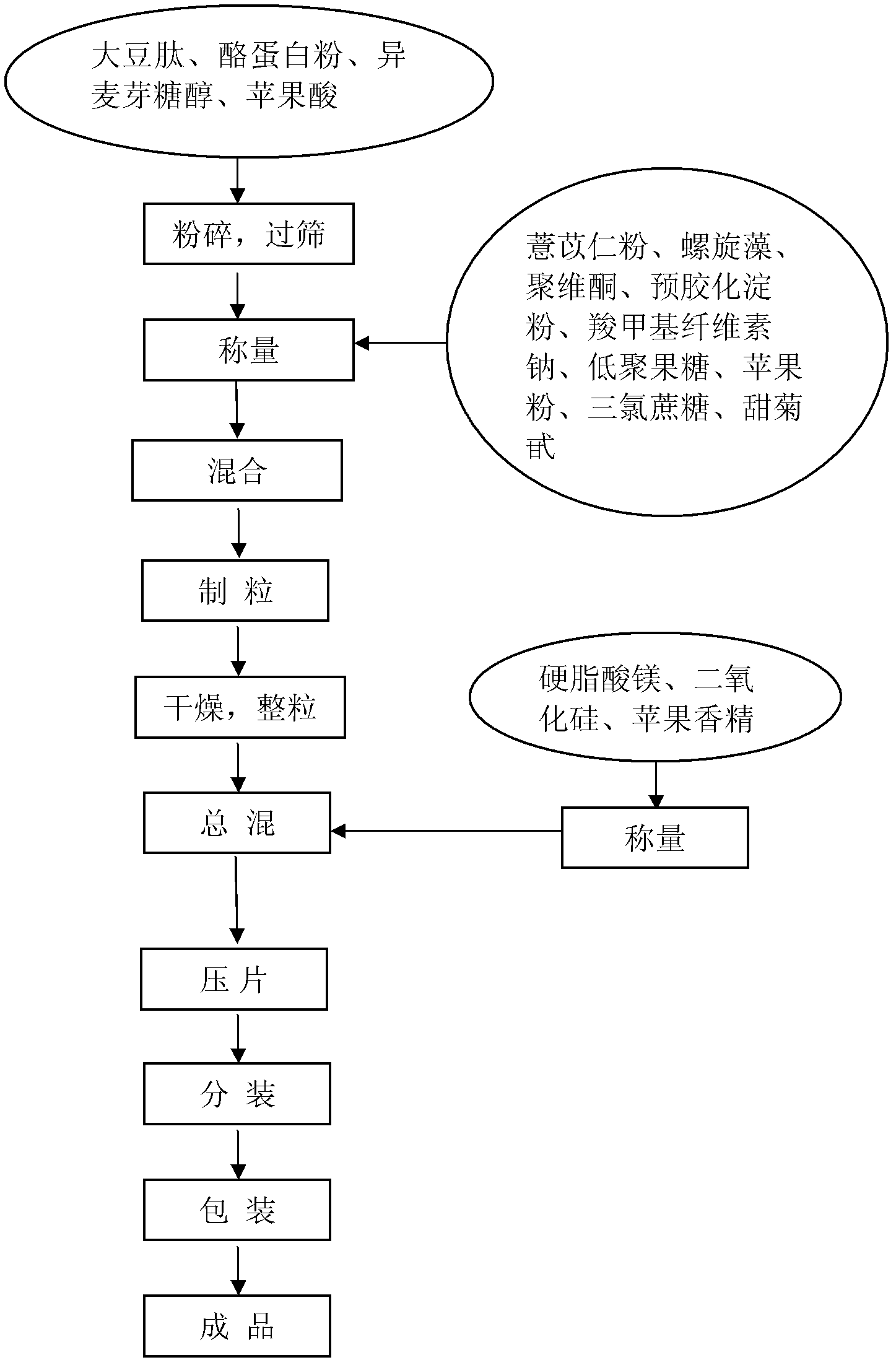
Healthcare food capable of strengthening immunity and preparing method thereof
The invention discloses a healthcare food capable of strengthening immunity. The healthcare food comprises, by weight percentage, 1% to 50% of soybean peptide, 0.5% to 30% of protein powder, 1% to 30% of fructo-oligosaccharide, 0.1% to 30% of coix seed powder, 0.1% to 10% of spirulina and 15% to 85% of auxiliary materials. According to the healthcare food, materials which are nutritious and effective are selected to be subjected to scientific formulating prescription, nutrition, safety, palatability and efficacy are taken as principles, and effects of strengthening cell immune function and humoral immune function are achieved, thereby immunity strengthening is achieved.
Owner:PERFECT CHINA
High-yield Transgenic Mammalian Expression System for Generating Virus-like Particles
ActiveUS20100166769A1Improving immunogenicityStimulate immune responseSsRNA viruses negative-senseSsRNA viruses positive-senseMammalVirus-like particle
Virus-like particles (VLPs) of mammalian-hosted viruses, such as SARS-CoV and influenza viruses, have been recombinantly produced from Vero cells. The VLPs closely emulate the exterior of authentic virus particles and are highly immunogenic. They can elicit not only humoral but also cellular immune responses in a mammal. Compositions and methods related to the VLPs are also described.
Owner:ACAD SINIC
Immune responses against HPV antigens elicited by compositions comprising an HPV antigen and a stress protein or an expression vector capable of expression of these proteins
InactiveUS6900035B2Enhance immune responseVirusesAntibody mimetics/scaffoldsHPV AntigenInfected cell
This document describes compositions and methods for inducing an immune response (e.g., a cellular response such as a cell-mediated cytolytic immune response) to a human papillomavirus (HPV) antigen, which can be displayed by HPV or exhibited by infected cells (e.g., cells from cervical and other tumors). The HPV protein can be joined to a stress protein by chemical conjugation or noncovalently using linking moieties, or by fusion (e.g., a recombinant fusion protein). Also described are expression vectors containing sequences encoding HPV antigens and stress proteins, which can be introduced into cells of a subject or cells ex vivo. Also described are compositions that include a stress protein linked to an HPV antigen and another pharmacologically acceptable component and stress protein—HPV antigen fusions and conjugates. These compositions can be used to induce or enhance an immune response against HPV and cells that exhibit HPV antigens, including HPV-associated tumors.
Owner:NVENTA BIOPHARMACEUTICALS CORP
Use of polymeric nanoparticles for vaccine delivery
InactiveUS20080044484A1Enhance antigen presentationImprove efficiencyBiocidePowder deliveryCancer cellT lymphocyte
The invention relates generally to the treatment and prevention of human cancer and viral diseases. More specifically, this invention relates to development of a new generation of vaccines that rely on eliciting cellular immune responses, specifically induction of cytotoxic T lymphocytes (CTL), against cancer cells and virus-infected cells via administration of a polymeric nanoparticle containing a vaccine comprising a fusion peptide or a modified peptide. Such a fusion peptide is composed of an insertion signal sequence and a peptide derived from a tumor antigen or a viral antigen, which improves antigen presentation and induces CTL with higher efficiency against cancer cells and virus-infected cells. An exemplary peptide utilized in the invention is Mart-1:27-35 peptide.
Owner:RGT UNIV OF CALIFORNIA
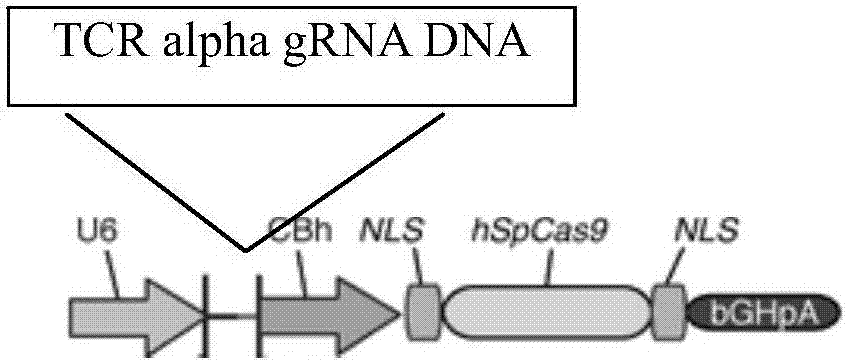
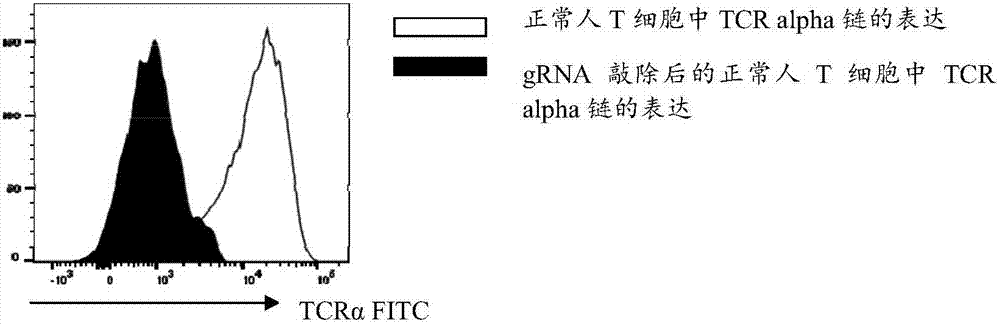
gRNA subjected to wild type T cell TCR alpha chain knockout and method
InactiveCN107236741AHigh gene knockout efficiencyEasy to prepareImmunoglobulin superfamilyStable introduction of DNAMolecular biologyT cell immunity
The invention discloses a gRNA subjected to wild type T cell TCR alpha chain knockout and a method. The sequence of the gRNA is shown as SEQ ID NO:1. By using a CRISPR / Cas 9 technology, the gRNA and the CRISPR / Cas9 perform co-infection on T cells; the wild type T cell TCR alpha chain knockout is performed; the T cells lack of wild type T cell TCR alpha chains are built; the gRNA can be used for CAR-T or TCR-T cellular immunity treatment. The gRNA has high knockout rate; the preparation method is relatively simple and easy; the T cells lack of wild type T cell TCR alpha chains can be fast and efficiently provided for clinics.
Owner:THE FIFTH AFFILIATED HOSPITAL OF GUANGZHOU MEDICAL UNIV
Methods and reagents for vaccination which generate a CD8 T cell immune response
New methods and reagents for vaccination are described which generate a CD8 T cell immune response against malarial and other antigens such as viral and tumour antigens. Novel vaccination regimes are described which employ a priming composition and a boosting composition, the boosting composition comprising a non-replicating or replication-impaired pox virus vector carrying at least one CD8 T cell epitope which is also present in the priming composition.
Owner:OXXON THERAPEUTICS LTD
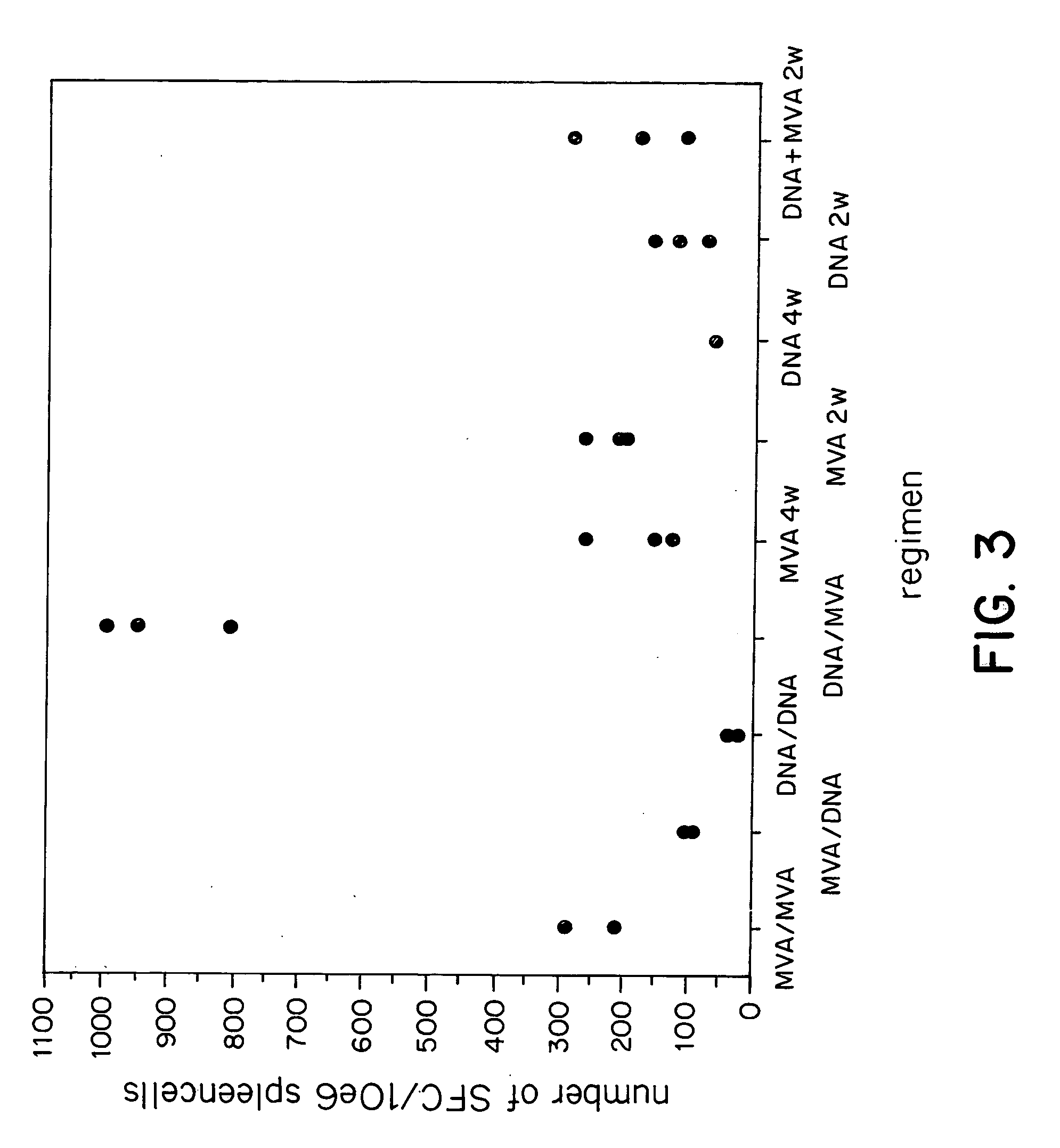
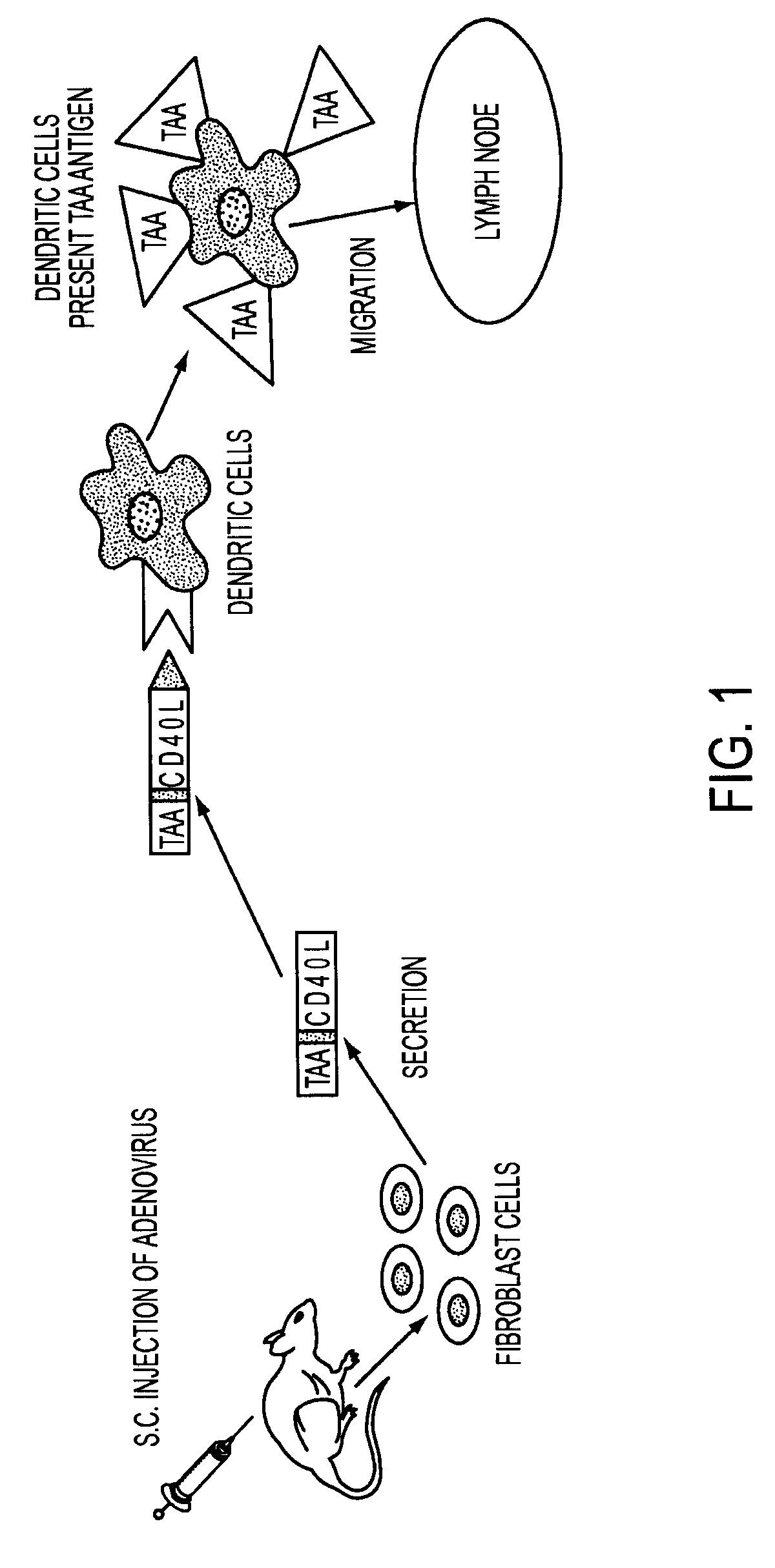
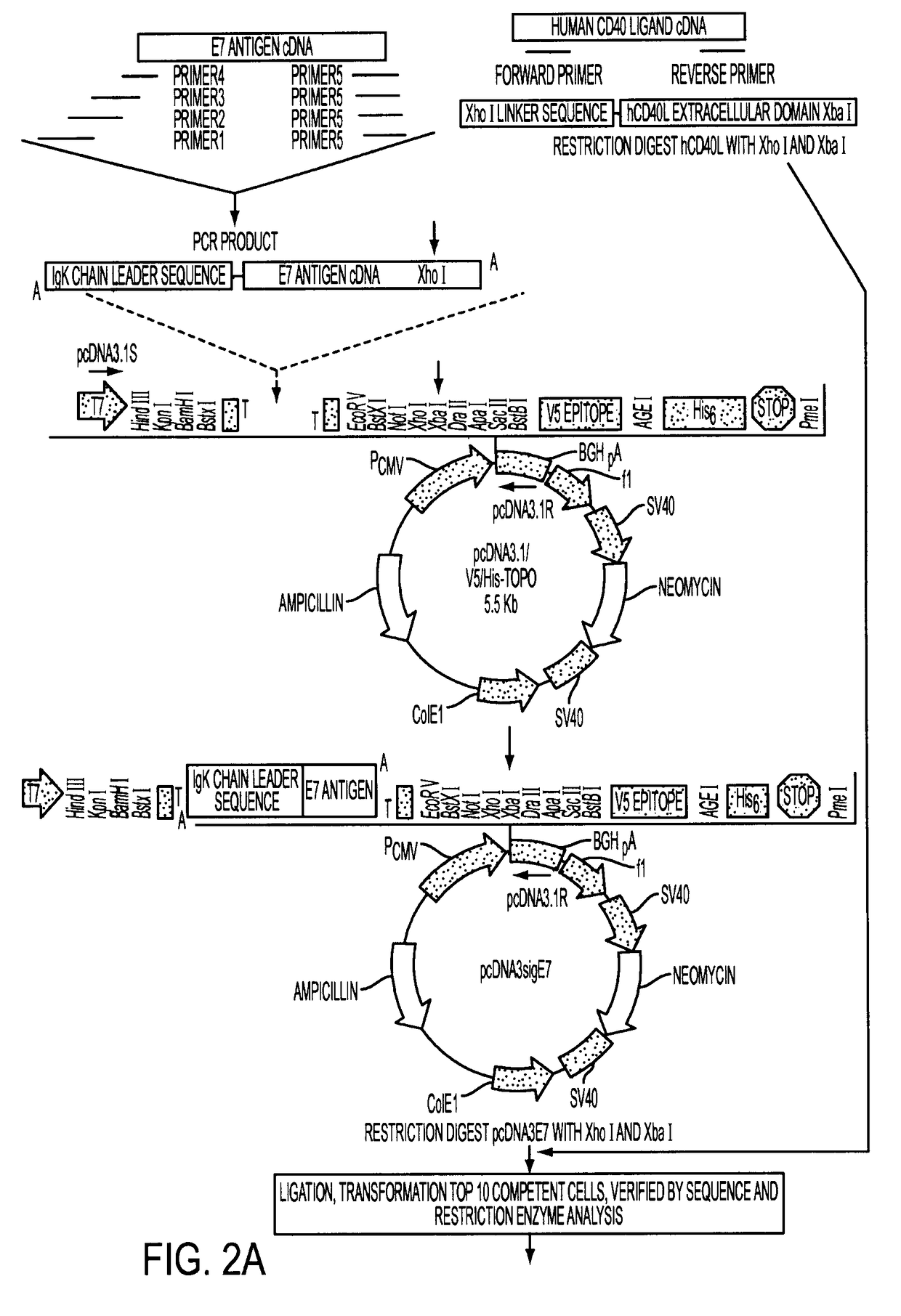
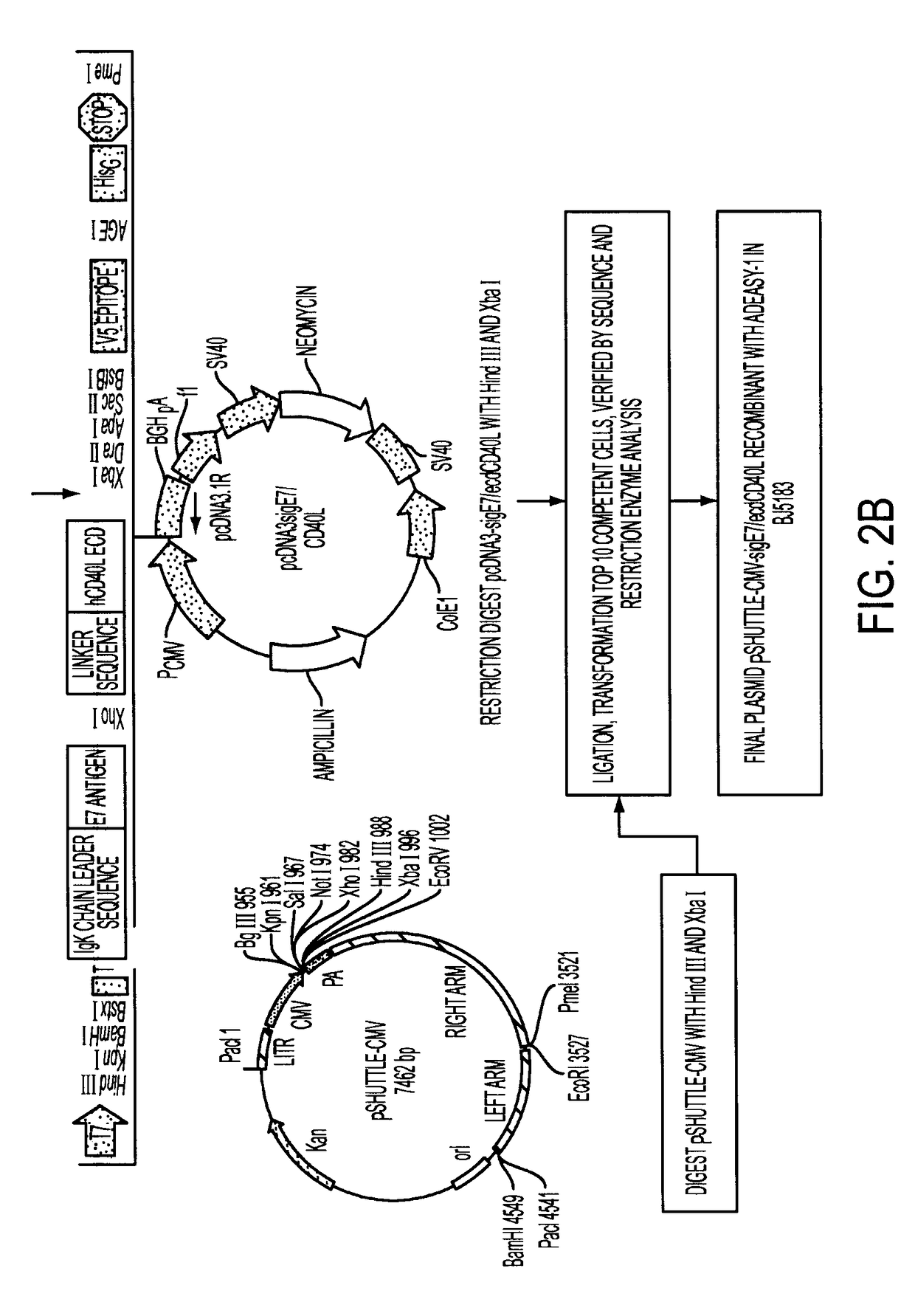
Adenoviral expression vector comprising a CD40L fusion protein adapted to elicit cellular immunity
ActiveUS8119117B2Enhance immune responseLong-lasting immunityVirusesAntibody mimetics/scaffoldsTransmembrane domainTumor antigen
Provided are adenoviral vectors for generating an immune response to antigen. The vectors comprise a transcription unit encoding a secretable polypeptide, the polypeptide comprising a secretory signal sequence upstream of a tumor antigen upstream of CD40 ligand, which is missing all or substantially all of the transmembrane domain rendering CD40L secretable. Also provided are methods of generating an immune response against cells expressing a tumor antigen by administering an effective amount of the invention vector. Further provided are methods of generating an immune response against cancer expressing a tumor antigen in an individual by administering an effective amount of the invention vector. Still further provided are methods of generating immunity to infection by human papilloma virus (HPV) by administering an effective amount of the invention vector which enocodes the E6 or E7 protein of HPV. The immunity generated is long term.
Owner:VAXUM
Immunogenic compositions and methods of using the compositions for inducing humoral and cellular immune responses
ActiveUS20120328655A1Reduce the possibilityAntibacterial agentsAntimycoticsInfectious DisorderLymphocyte
Compositions and methods are provided herein for improved dual immunization strategies that induce in a subject an immune response that includes a humoral immune response and cellular immune response, both CD4 and CD8 T lymphocyte immune responses, thereby providing a complete adaptive immune response to one or more antigens. The methods described are therefore useful for treating and / or preventing (i.e., reducing the likelihood or risk of occurrence) different diseases, disorders, and conditions such as cancers and infectious diseases for which induction of both a humoral immune response and cellular immune response is desired and beneficial.
Owner:IMMUNE DESIGN CORP
Reprogramming immortalized b cells
ActiveUS20120058562A1High reprogramming efficiencySave stepsGenetically modified cellsArtificial cell constructsLymphocyteB cell
Methods and composition for providing induced pluripotent stem (iPS) cells are provided. For example, in certain aspects methods including reprogramming B lymphocytes transformed by episomal vectors such as Epstein-Barr virus-based vectors are described. Furthermore, the invention provides induced pluripotent stem cells essentially free of exogenous elements and having B cell immunoglobin variable region rearrangement.
Owner:FUJIFILM CELLULAR DYNAMICS INC
HCV fusion proteins with modified NS3 domains
InactiveUS20050074465A1Improve protectionImprove responseSsRNA viruses positive-senseAntibody mimetics/scaffoldsEpitopeMutated protein
The invention provides HCV fusion proteins that include a mutated NS3 protease domain, fused to at least one other HCV epitope derived from another region of the HCV polyprotein. The fusions can be used in methods of stimulating a cellular immune response to HCV, such as activating hepatitis C virus (HCV)-specific T cells, including CD4+ and CD8+ T cells. The method can be used in model systems to develop HCV-specific immunogenic compositions, as well as to immunize a mammal against HCV.
Owner:CHIRON CORP
Novel vaccine for preventing COVID-19 and preparation method thereof
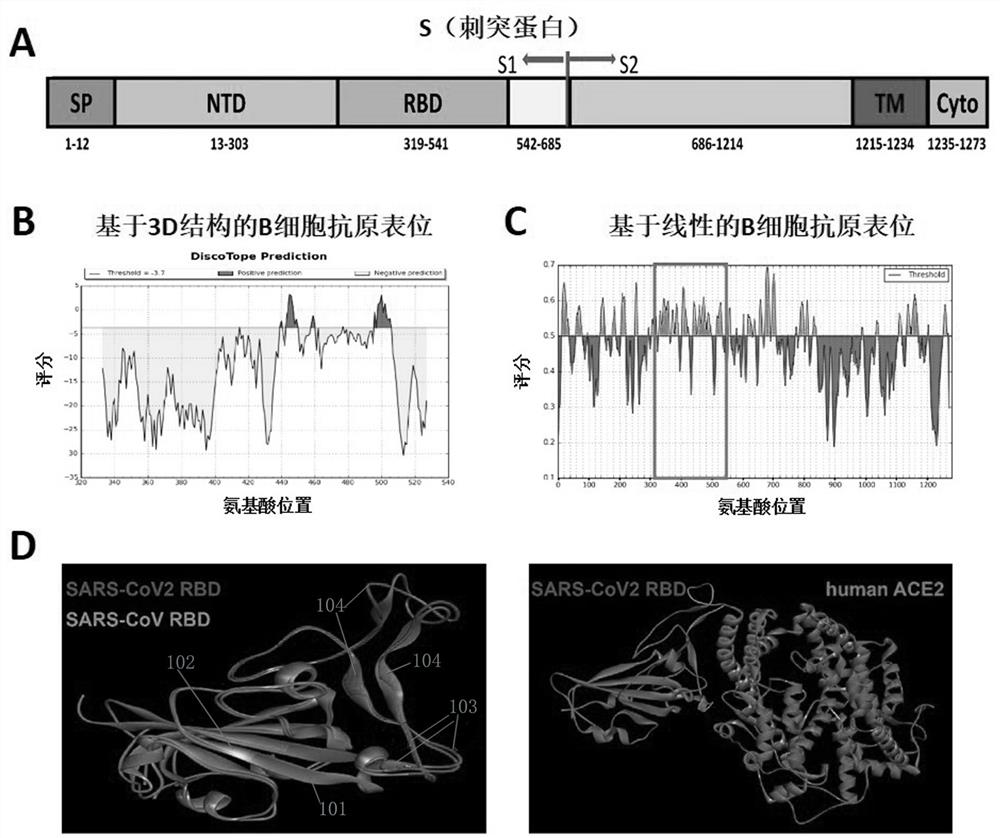
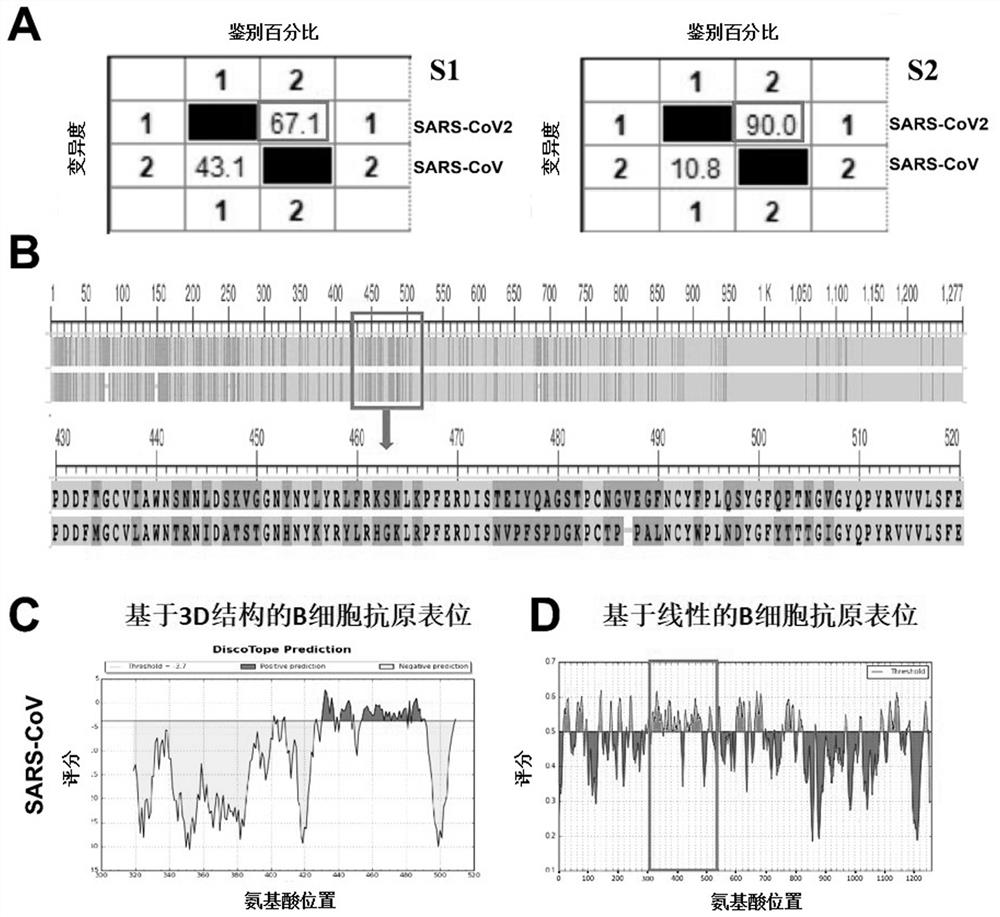
ActiveCN111939250AHighly conservativeAntibody induction ability is weakSsRNA viruses positive-senseAntibody mimetics/scaffoldsNucleotideReceptor
Provided is a novel vaccine for preventing COVID-19, the nucleotide sequence of an antigen of the novel vaccine is SEQ NO: 1, the amino acid sequence of the antigen of the novel vaccine is SEQ NO: 2,and the antigen of the vaccine comprises two functional parts: an S protein receptor binding structural domain capable of inducing a specific neutralizing antibody and a T cell related N protein truncated peptide fragment capable of inducing and activating effector T cells; The vaccine disclosed by the invention has the characteristics that the T cell related N protein truncated peptide fragment has weak capability of inducing the generation of the N protein antibody, so that a vaccine inoculator and a COVID-19 infected patient can be identified by using the N protein antibody, and the vaccineantigen does not induce the generation of the N protein antibody, so that lung injuries can be reduced, and the vaccine is safer. The cell vaccine disclosed by the invention is low in manufacturing cost, and can induce generation of virus-specific neutralizing antibodies and T cell immune response.
Owner:ZHENGZHOU UNIV
Antiviral agents and vaccines against influenza
InactiveUS20090208531A1SsRNA viruses negative-senseOrganic active ingredientsHemagglutininPrime boost
These vaccines target H5N1, H1, H3 and other subtypes of influenza and are designed to elicit neutralizing antibodies, as well as cellular immunity. The DNA vaccines express hemagglutinin (HA) or nucleoprotein (NP) proteins from influenza which are codon optimized and / or contain modifications to protease cleavage sites of HA which affect the normal function of the protein. Adenoviral constructs expressing the same inserts have been engineered for prime boost strategies. Protein-based vaccines based on protein production from insect or mammalian cells using foldon trimerization stabilization domains with or without cleavage sites to assist in purification of such proteins have been developed. Another embodiment of this invention is the work with HA pseudotyped lentiviral vectors which would be used to screen for neutralizing antibodies in patients and to screen for diagnostic and therapeutic antivirals such as monoclonal antibodies.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
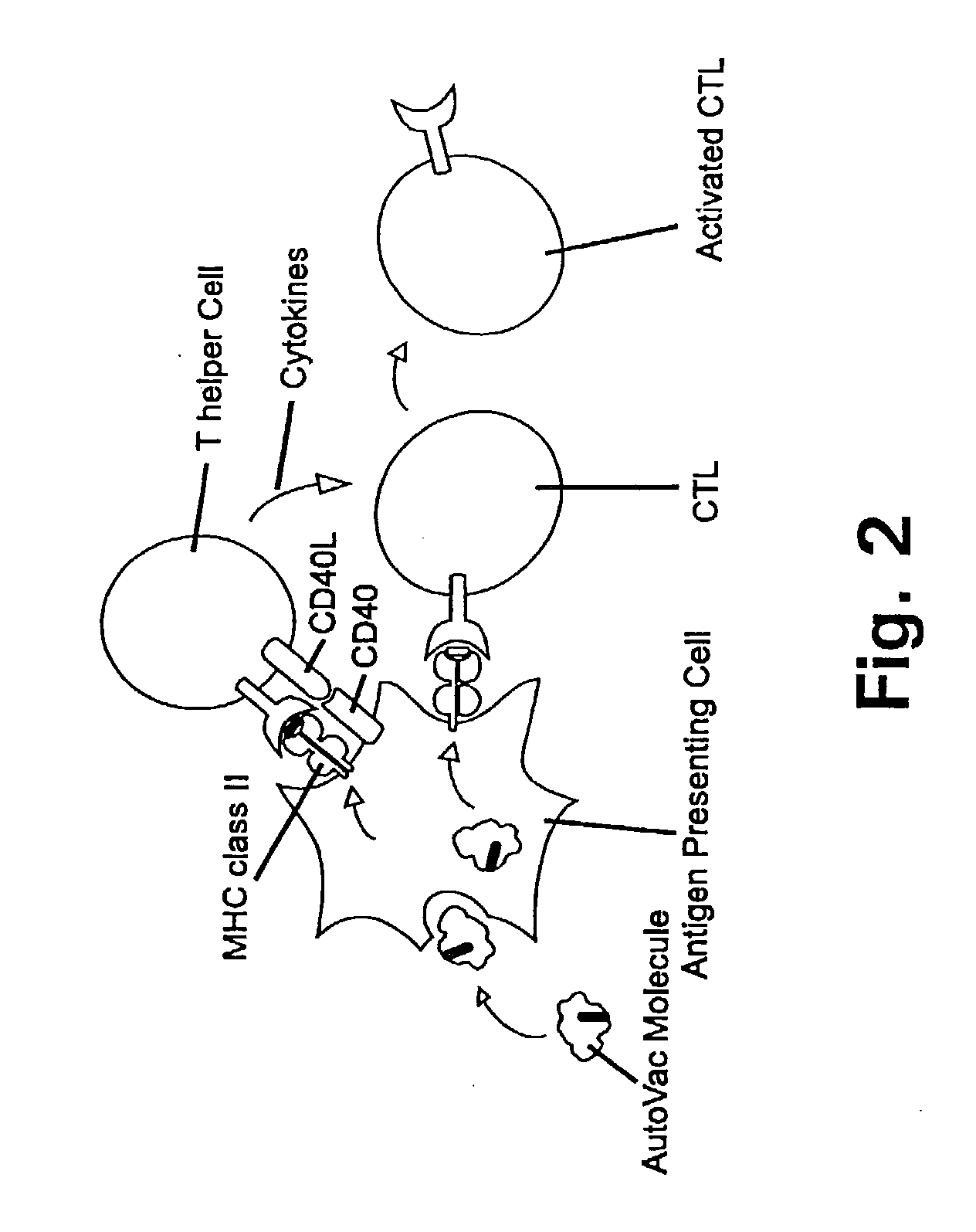
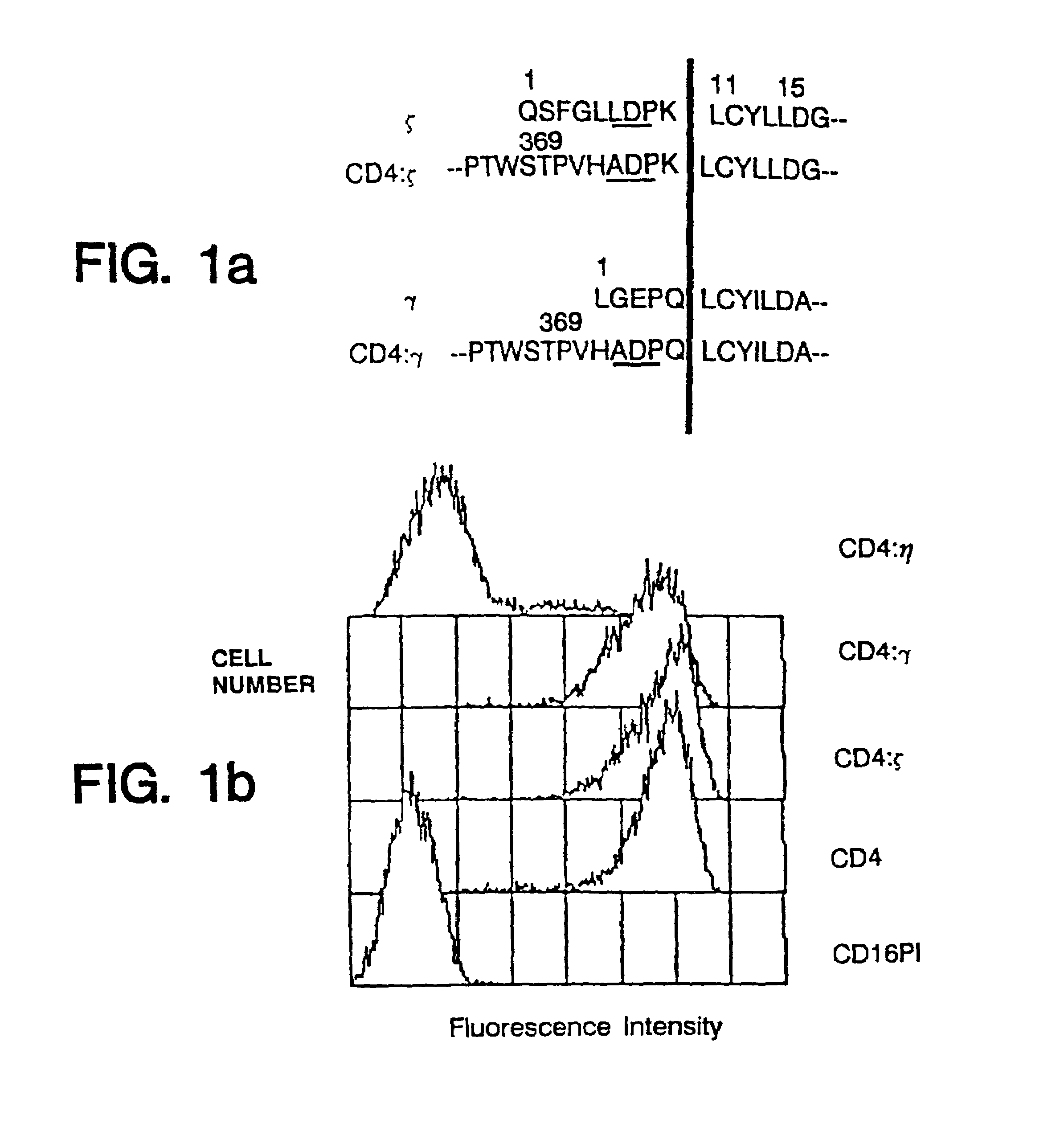
Targeted cytolysis of HIV-infected cells by chimeric CD4 receptor-bearing cells
InactiveUS7094599B2Promote absorptionProlong lifeVirusesPeptide/protein ingredientsMammalEphA Receptors
Disclosed is a method of directing a cellular immune response against an HIV-infected cell in a mammal involving administering to the mammal an effective amount of therapeutic cells which express a membrane-bound, proteinaceous chimeric receptor comprising (a) an extracellular portion which includes a fragment of CD4 which is capable of specifically recognizing and binding the HIV-infected cell but which does not mediate HIV infection and (b) an intracellular portion which is capable of signalling the therapeutic cell to destroy the receptor-bound HIV-infected cell. Also disclosed are cells which express the chimeric receptors and DNA and vectors encoding the chimeric receptors.
Owner:THE GENERAL HOSPITAL CORP
Nucleic acids and corresponding proteins useful in the detection and treatment of various cancers
ActiveUS20070031335A1Organic active ingredientsPeptide/protein ingredientsNovel genePassive Immunizations
Novel genes designated and set forth in FIG. 2 and their respective encoded proteins, and variants thereof, are described wherein a gene of the invention exhibits tissue specific expression in normal adult tissue, and is aberrantly expressed in the cancers such as those listed in Table I. Consequently, of gene products of a gene of FIG. 2 provide diagnostic, prognostic, prophylactic and / or therapeutic targets for cancer. A gene of FIG. 2 or fragment thereof, its encoded protein, or variants thereof, or a fragment thereof, can be used to elicit a humoral or cellular immune response; antibodies or T cells reactive with a gene product of FIG. 2 can be used in active or passive immunization.
Owner:AGENSYS
Device for inducing an immune response in cancer therapy
A device for inducing an immune response in cancer therapy in which isolated living malignant tissue is enclosed in a space formed by a microporous membrane and a holder which is introduced into a body cavity so that macromolecular and cellular immune system components can pass freely in both directions through the membrane while malignant cells of the tumor tissue remain trapped inside.
Owner:KALINA VLADIMIR +2
Bursa of Fabricius heptapeptide with immune regulation effect
InactiveCN101830968ASynthetic technology is matureImprove efficiencyPeptide preparation methodsAntibody medical ingredientsSide effectImmunologic Competence
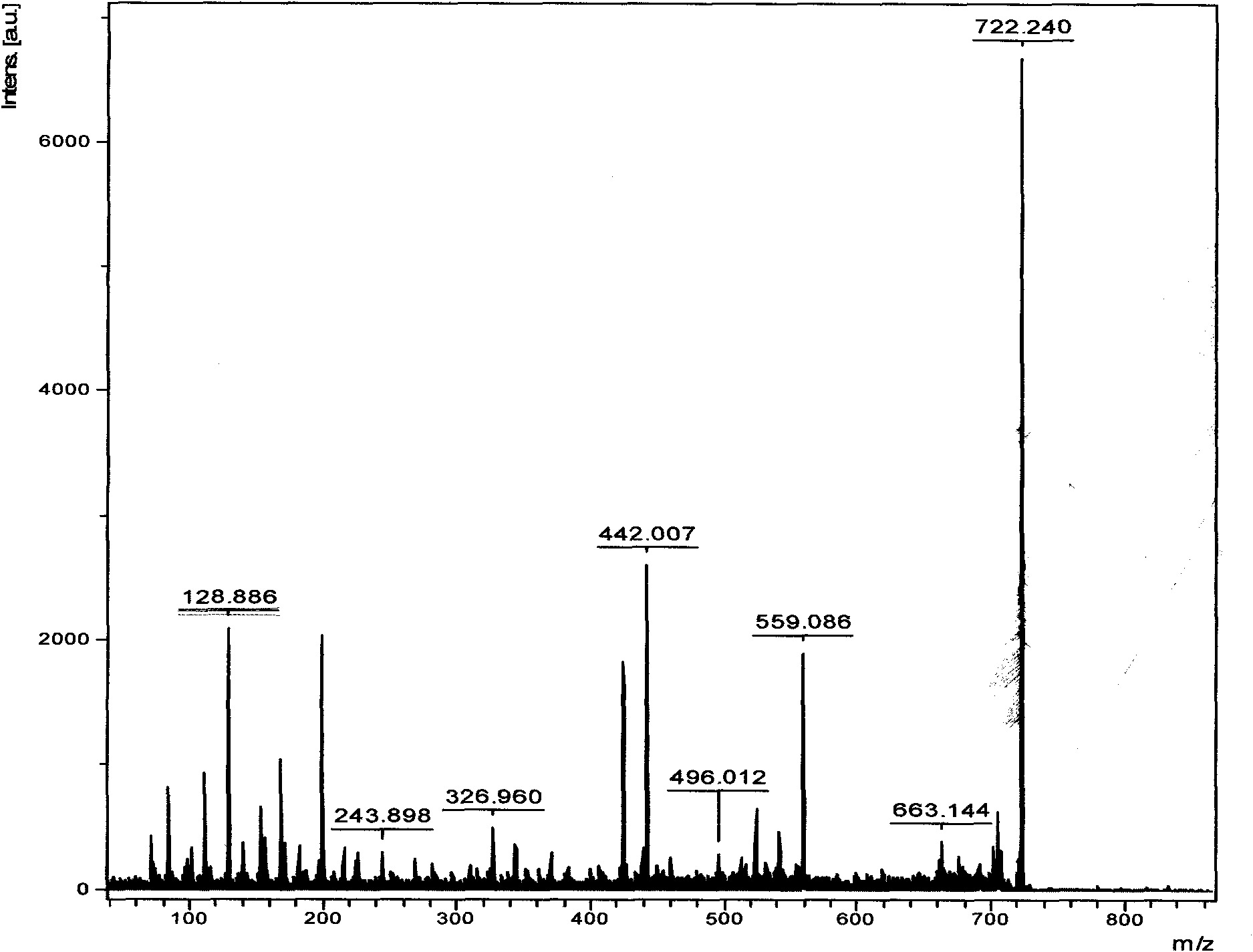
The invention relates to bursa of Fabricius heptapeptide with immune regulation effect and application thereof in immunity (in improving the immune capability of animals, improving the immune effect of vaccines and affecting the activity of tumor cells), and belongs to the field of immunology. The molecular weight of the separated heptapeptide is 722.240, the amino acid sequence is EPASGMM, and the heptapeptide has a simple structure, no toxic or side effect and extremely weak immunogenic property. The heptapeptide can be separated and extracted from bursa of Fabricius, also can be chemically synthesized, has low cost, and can be massively produced. The bursa of Fabricius heptapeptide has induction effect on the production of antibody and subtype thereof, and meanwhile can regulate the production of cell factors, partition of T lymphocytes and proliferation of spleen cells, and promote the immune reaction of the cells. The bursa of Fabricius heptapeptide is an immune regulation factor on functions, has wide application prospect on the aspects of immune regulation, immune therapy and the like, and can be applied in the fields of basic immune research, clinical application research and the like.
Owner:NANJING AGRICULTURAL UNIVERSITY
Method to improve the immune function of t cells
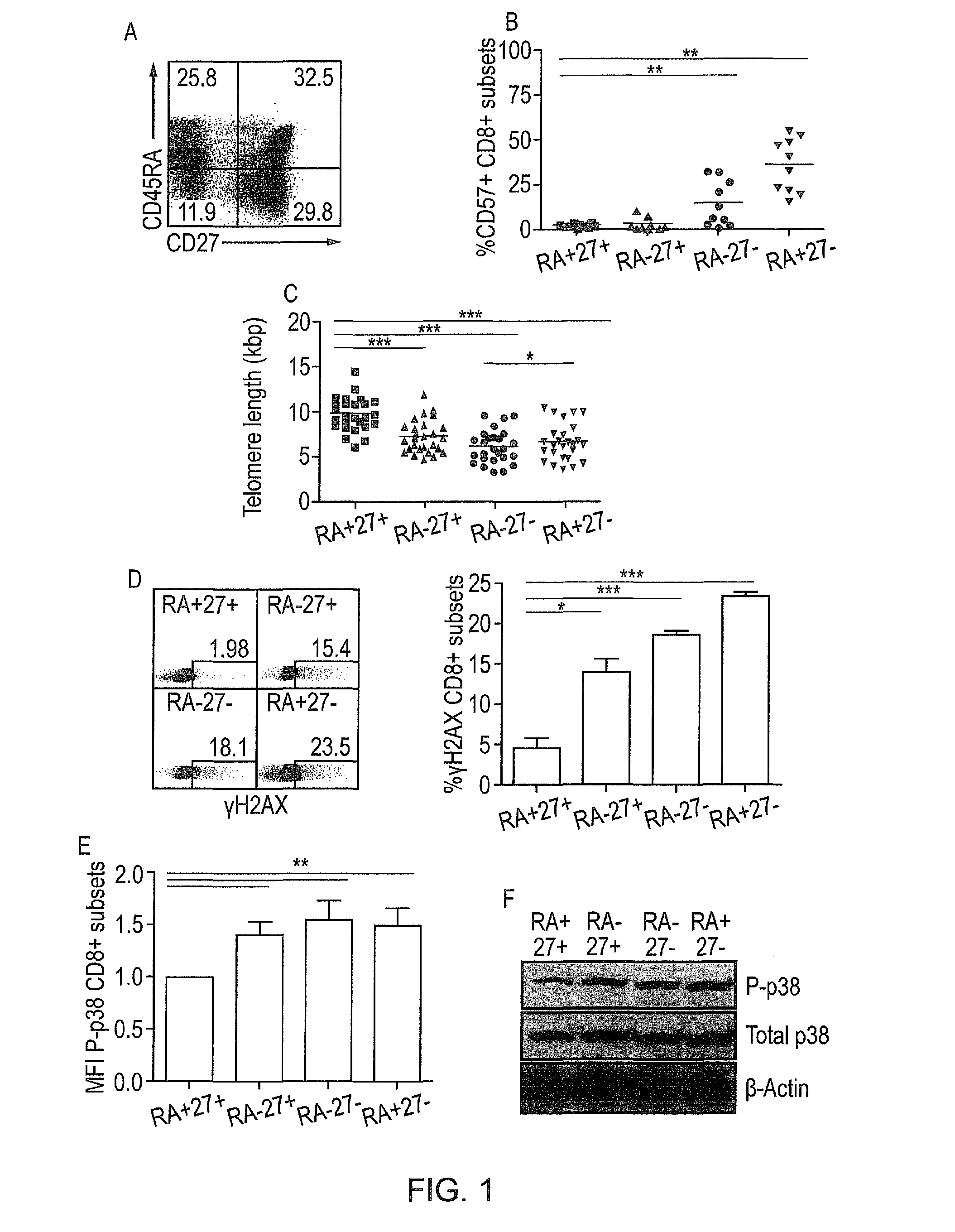
InactiveUS20150017185A1Enhance immune responseEnhancing function of T cellOrganic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsCentral Memory T-CellP38map kinase
The present invention provides a method for enhancing the immune function of a memory T cell which comprises the step of coinhibting signalling via an inhibitory receptor which regulates T cell exhaustion and via the p38 MAP kinase signalling pathway in the T cell, and a method for treating and / or preventing an immune condition in a subject, which comprises the step of enhancing the immune function of a memory T cell in the subject by such a method. There is also provided a pharmaceutical composition or kit comprising an agent capable of inhibiting signalling via an inhibitory receptor which regulates T cell exhaustion, such as PD-1, and an agent capable of inhibiting the p38 MAP kinase signalling pathway.
Owner:UCL BUSINESS PLC
Novel fusion protein, pharmaceutical composition and preparation method therefor and use thereof
ActiveCN105111314AGrowth inhibitionSynergistic effectAntibody ingredientsPharmaceutical non-active ingredientsAntiendomysial antibodiesAntibody fragments
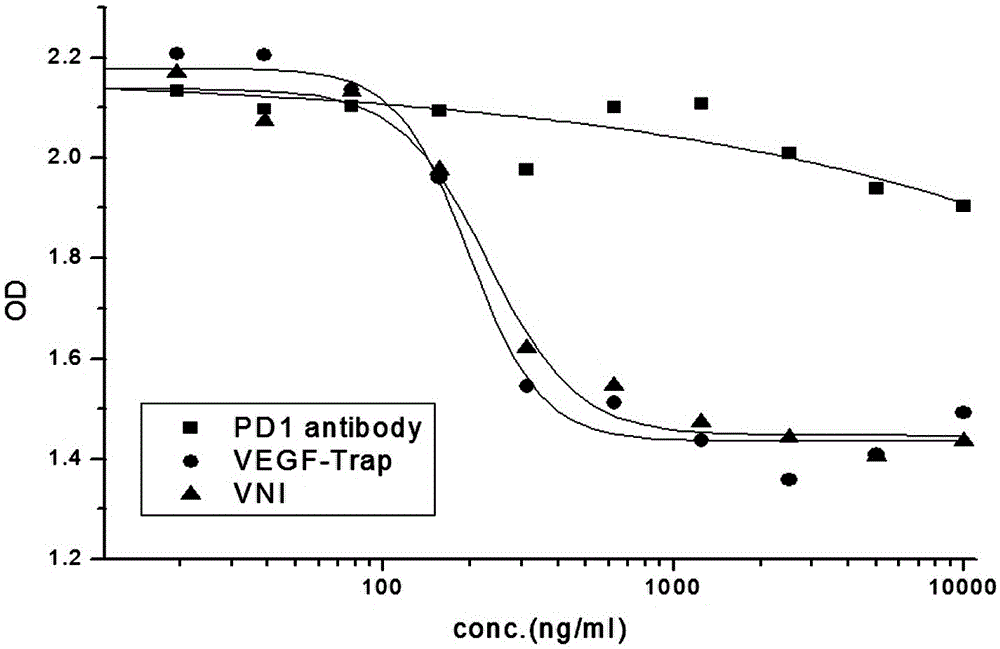
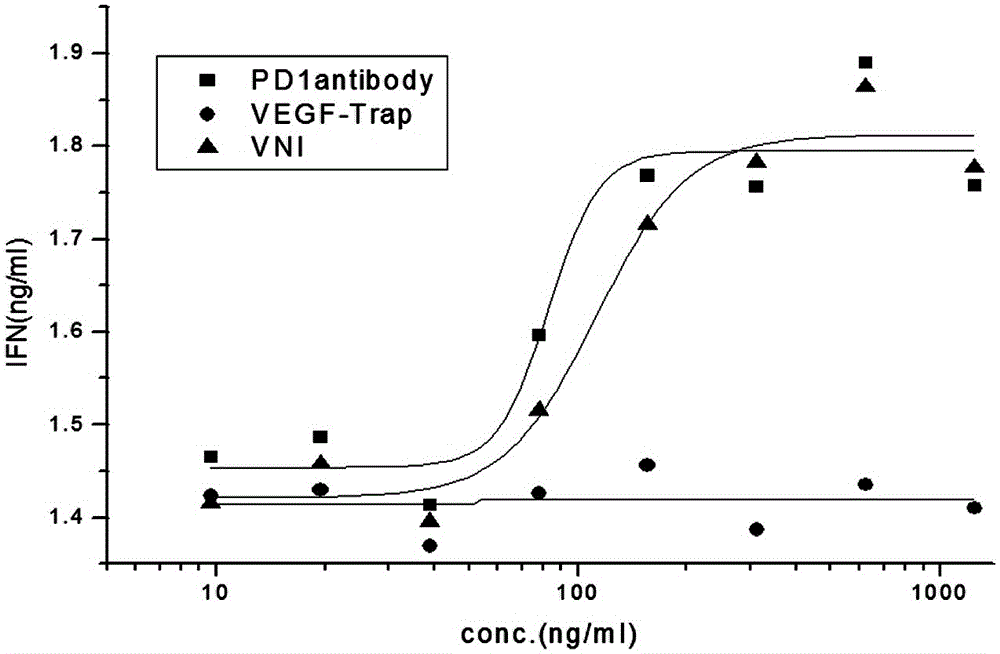
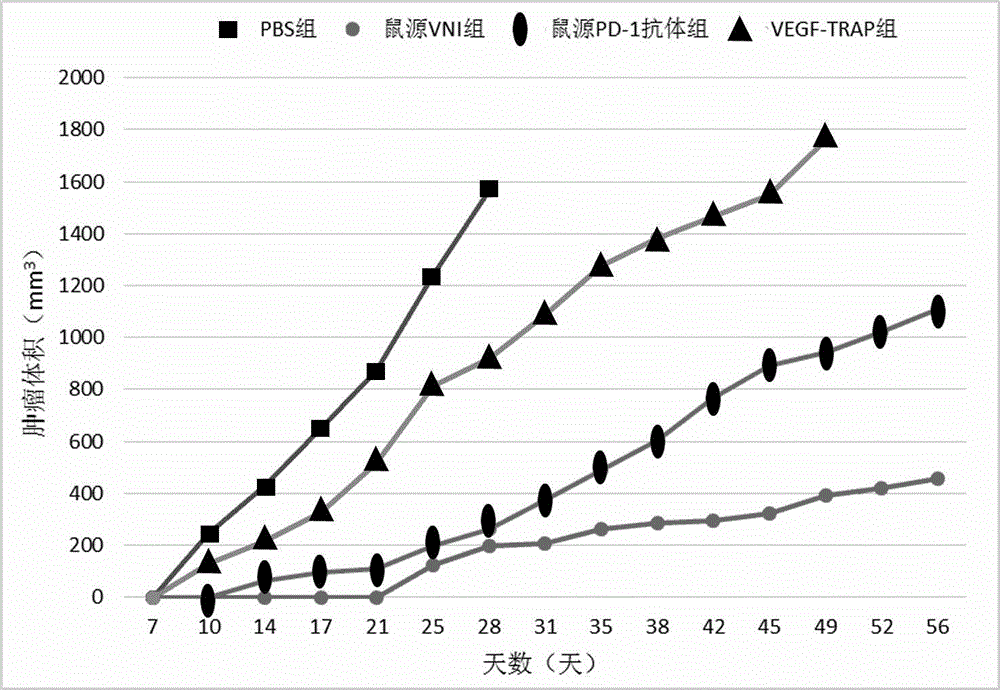
The invention discloses a novel fusion protein, a pharmaceutical composition formed by the novel fusion protein, a preparation method for the novel fusion protein and use of the novel fusion protein. According to novel fusion protein, the structural general formula is X-connecting peptide-Y-connecting peptide-IgG Fc fragment or Y-connecting peptide-X-connecting peptide-IgG Fc fragment, wherein X is VEGF-Trap and derivatives thereof, and Y is a PD-1 antibody fragment and derivatives thereof or a PD-1 antagonist fragment and derivatives thereof. The fusion protein plays roles in suppressing regenerated blood vessels and activating T cellular immunity and plays a role in coordinating and promoting two functions, so that the function of inhibiting tumor growth is more remarkable.
Owner:DONGGUAN YUNJING BIOTECHNOLOGY CO LTD
Preparation of specific tumor killing cell
ActiveCN102526716ABreak immune tolerancePowerful killing functionMammal material medical ingredientsBlood/immune system cellsCell immunityT lymphocyte
The invention relates to an antitumor cell immunotherapy technology, in particular to preparation of a specific tumor killing cell. The preparation method disclosed by the invention comprises the following steps of: 1, sampling a single prokaryotic cell from peripheral blood; 2, separating a DC (Dendritic Cell) from a T cell; 3, maturing the DC and preparing a DC vaccine; 4, preparing a CIK (Cytokine Induced Killer); 5, preparing a CTL (cytotoxic T lymphocyte); and 6, preparing a specific DC-CIK-CTL cell preparation.
Owner:玥特农生物科技河北有限责任公司
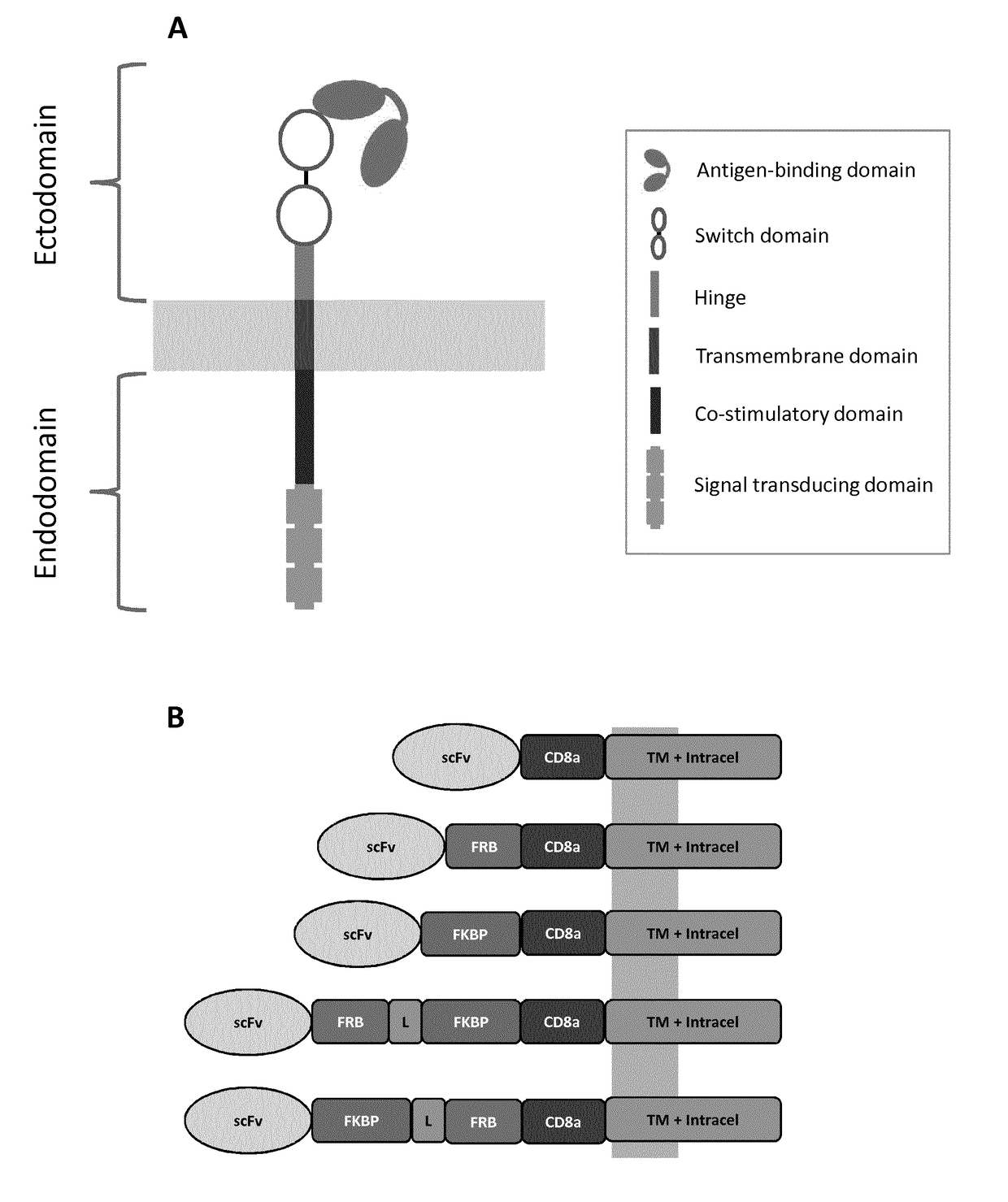
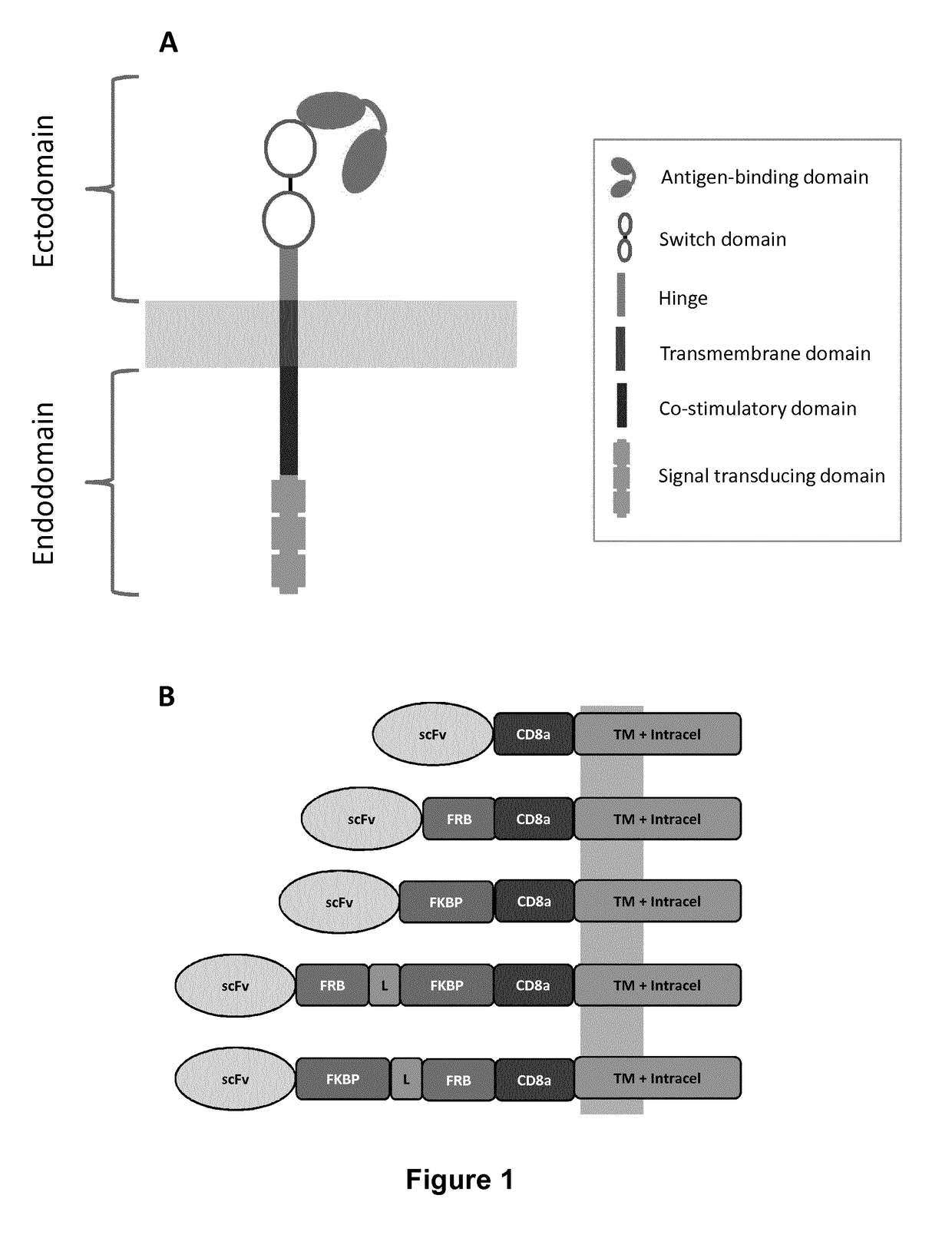
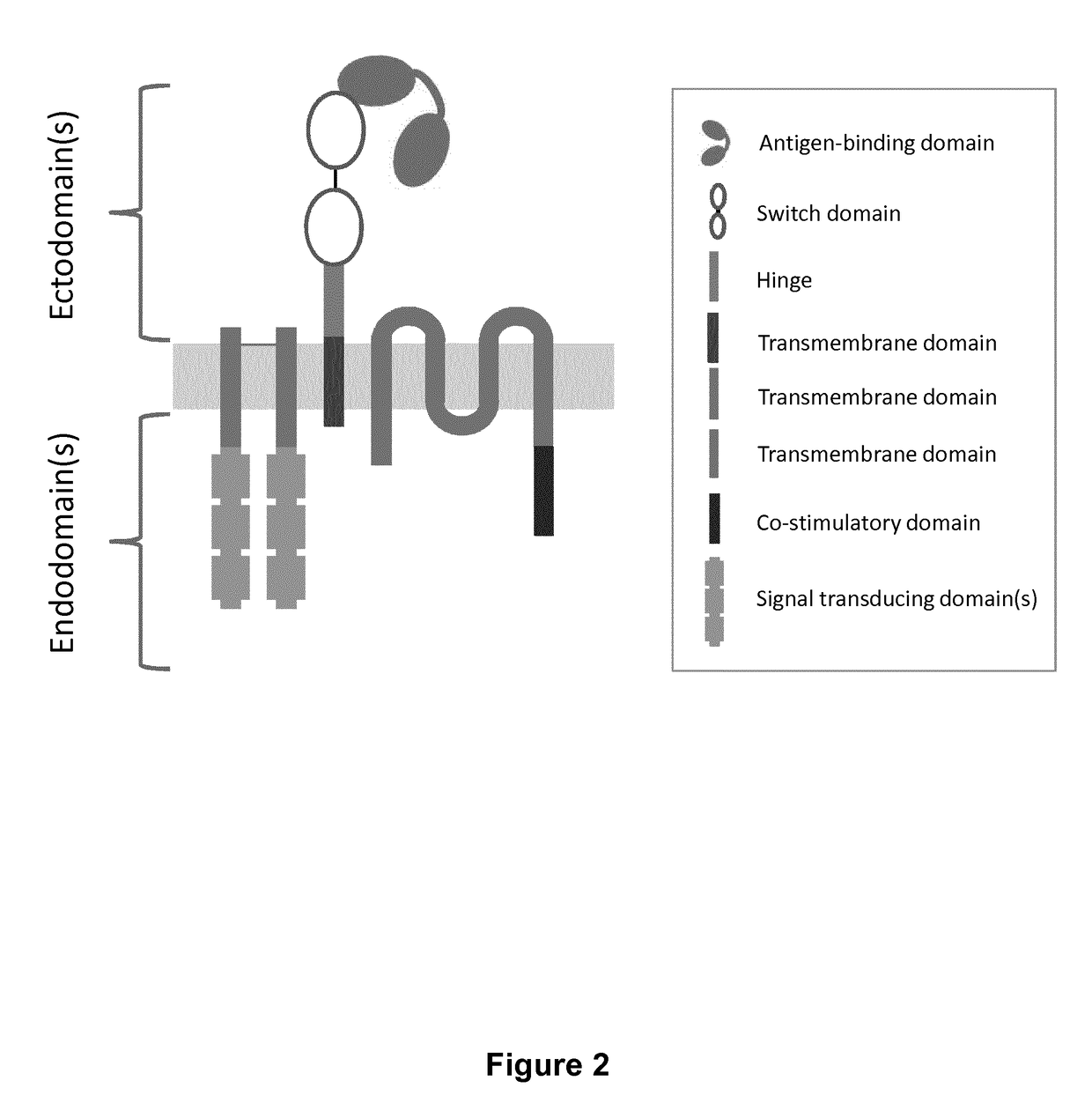
Chimeric antigen receptors with integrated controllable functions
ActiveUS20180237533A1Suppress mutationReliable functionPolypeptide with localisation/targeting motifOrganic active ingredientsAntigen receptorsT lymphocyte
The present invention relates to the field of cell immunotherapy and more particularly to a new generation of chimeric antigen receptors (CAR), allowing the control of immune cells endowed with such CARs through the interaction with small molecules. More particularly, the present invention relates to chimeric antigen receptor which comprise in at least one ectodomain a molecular switch turning the antigen binding function of the receptor from an off to on state, and vice versa. The present invention thus provides more controlled and potentially safer engineered CAR endowed immune cells, such as T-lymphocytes.
Owner:CELLECTIS SA
Vaccine for inducing specific immunity of tumor and application thereof
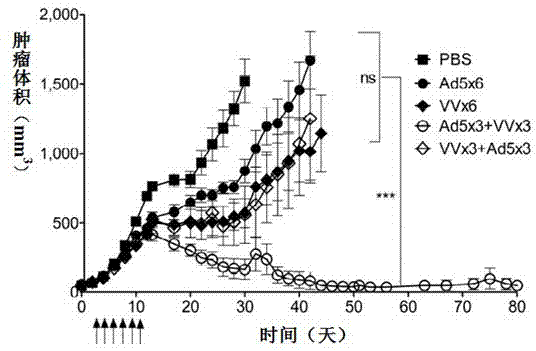
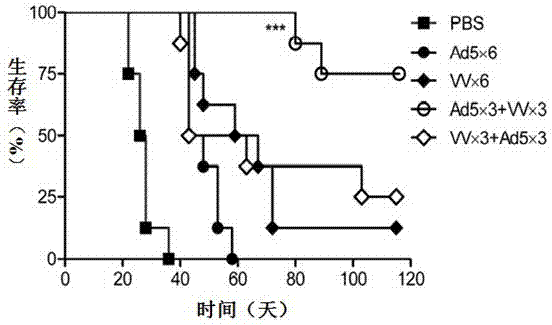
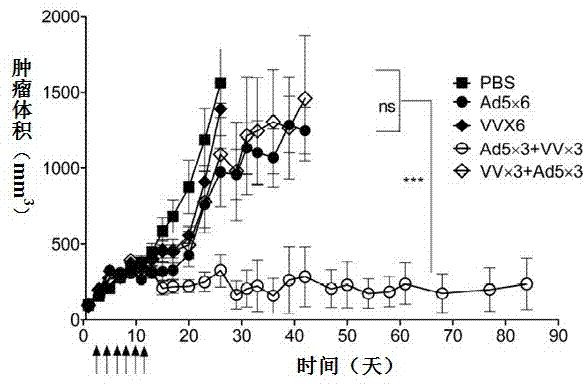
InactiveCN103110939AEnhance tumor immunityStrong immune responseViral antigen ingredientsUnknown materialsOncolytic adenovirusSpecific immunity
The invention relates to a vaccine comprising an adenovirus vector and a vaccinia virus vector and an application of the vaccine in treating tumor. The vaccine comprises oncolytic adenovirus and vaccinia virus; the two viruses are used in sequence, wherein the oncolytic adenovirus is the reproductive human adenovirus type 5 and the vaccinia virus is the vaccinia virus for smallpox vaccine. According to the vaccine, the oncolytic adenovirus and the vaccinia virus are prepared into the vaccine by sequential combination, so the antineoplastic activity can be improved, the stronger cellular immune response can be generated compared with the effect of reversely associated virus or separate virus, the using effect is better and the long-term specific immunity of tumor is shown. The tumor vaccine can eradicate the tumor and generate the long-term specific immunity of tumor and can be used in the treatment of various tumors of the human or animals; and meanwhile, the vaccine can effectively prevent the tumor from relapsing and has a wide application prospect.
Owner:ZHENGZHOU UNIV +1
Immune cell microfluid array
InactiveCN1403817AImprove the efficiency of differentiation antigen detectionMicrobiological testing/measurementBiological testingAntigenWhite blood cell
The present invention relates to one kind of immune cell microfluid array belonging to the field of cell immunity test technology. On the polymer substrate of the immune cell microfluid array, there are orderly arranged pores communicated via inclined micro tubes. By using the present invention, once operation can detect cell with corresponding leucocyte differentiating antigen, and thus judges the kind of leucocyte differentiating antigen and the growth and decline rule of the leucocyte differentiating antigen. The present invention results in high working efficiency.
Owner:中国人民解放军南京军区联勤部军事医学研究所
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com