Novel fusion protein, pharmaceutical composition and preparation method therefor and use thereof
A technology of fusion protein and composition, which is applied in the field of fusion protein to achieve the effects of alleviating the pain of drug administration, significant tumor growth, and reducing the number of times
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1 protein preparation
[0028] A novel fusion protein, the general structural formula of which is as follows:
[0029] X-linked peptide-Y-linked peptide-IgGFc fragment; wherein, X is VEGF-Trap and its derivatives; Y is a PD-1 antibody fragment and its derivatives or a PD-1 antagonist fragment and its derivatives.
[0030] In this embodiment, X is VEGF-Trap, and its nucleotide sequence is shown in SEQ ID NO: 4; Y is a PD-1 antibody fragment, and its nucleotide sequence is shown in SEQ ID NO: 6; the connecting peptide is the formula ( The amino acid sequence shown by Gly-Gly-Gly-Gly-Ser) n, n in the connecting peptide is 3; the nucleotide sequence of the IgGFc fragment is shown in SEQ ID NO: 16; that is, the gene for synthesizing the fusion protein in this embodiment The nucleotide sequence of the fragment is shown in SEQ ID NO:18.
[0031] The specific synthetic route of the above-mentioned novel fusion protein in this embodiment is as follows:
[0032] 1. Con...
Embodiment 2
[0049] Example 2. VNI Binding Kinetic Analysis
[0050] (1) Binding kinetic analysis of VNI and VEGF-A
[0051] Bind 25 μg / ml Biotin-hVEGF-A to SAsensor through Loading; set the concentration range according to the pre-experiment: 10000, 5000, 1000, 500, 100 nM respectively, and use SampleDiluentbuffer as blank control. Kinetic analysis on the OctetQK detection platform was used to detect the kinetic parameters of hVEGF-A, VNI, VEGF-Trap and PD1 antibodies. The test steps are set as shown in Table 1:
[0052] Detection steps the solution step type detection time (s) 1 Sample Diluent buffer Baseline 120 2 Biotin-hVEGF-A Loading 360 3 Sample Diluent buffer Baseline 120 4 sample Association 720 5 Sample Diluent buffer Dissociation 1800
[0053] The experimental results obtained through the above test steps are shown in Table 2:
[0054] KD (M) kon(1 / Ms) kon Error kdis(1 / s) kdis Error Full R...
Embodiment 3
[0063] Example 3. In vitro biological activity detection of VNI.
[0064] (1) Comparative study on the effect on HUVEC cell proliferation
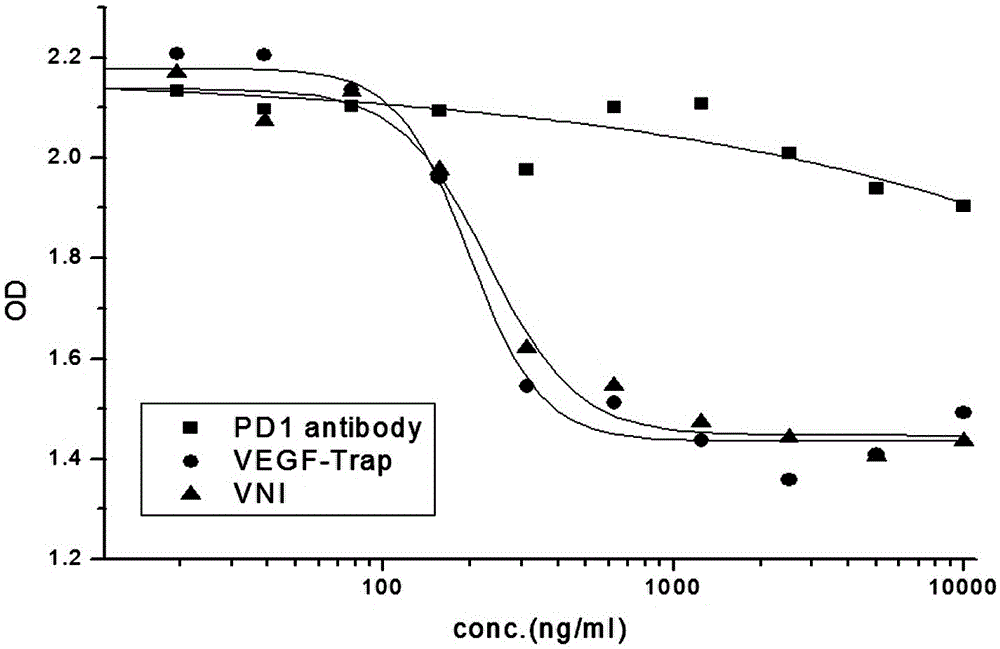
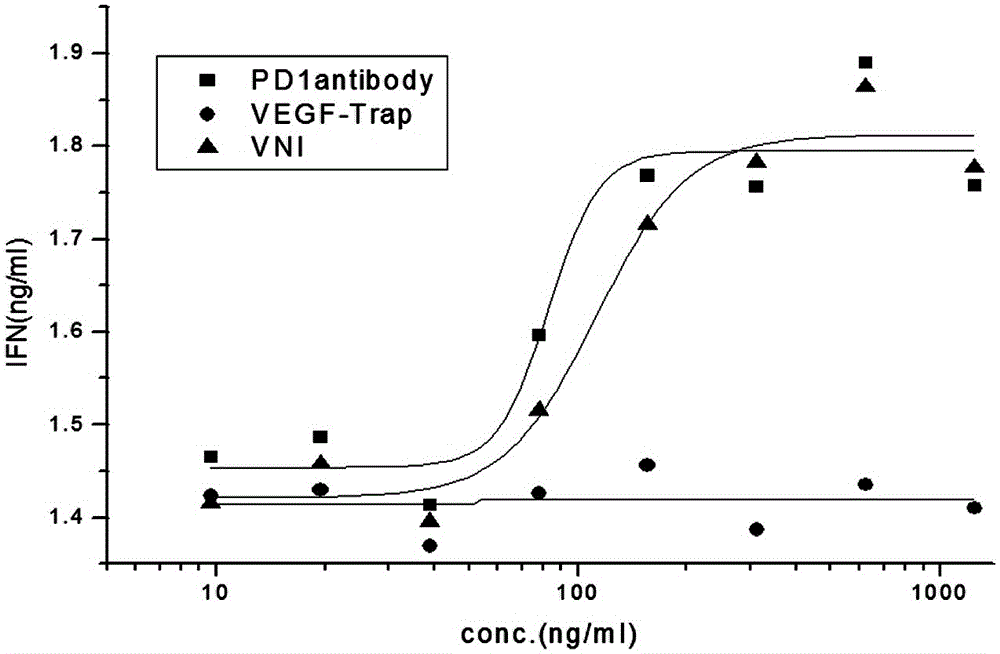
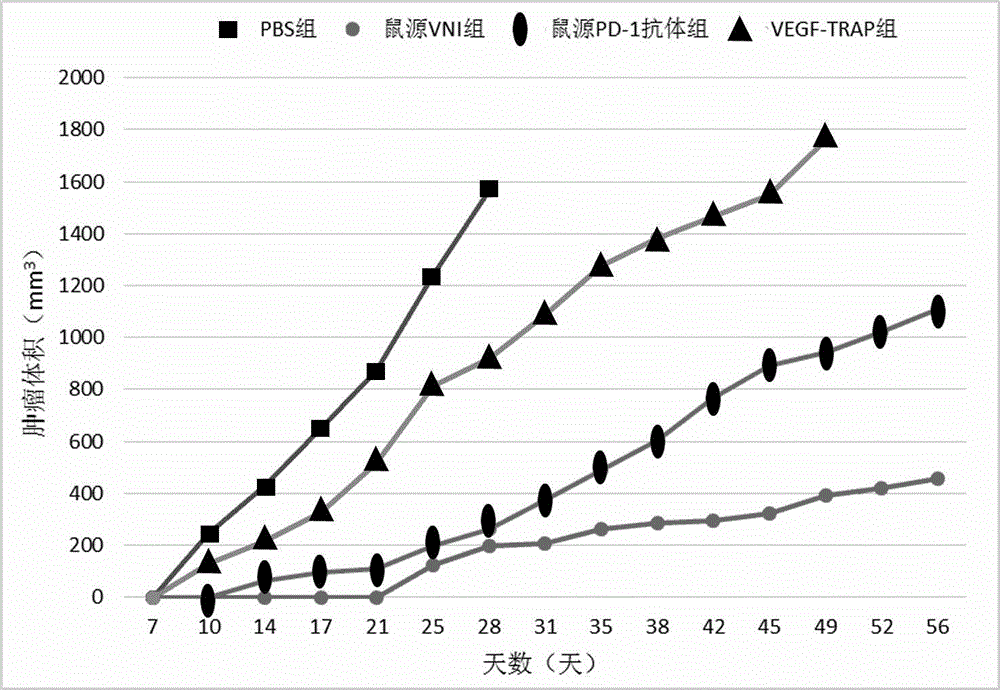
[0065] HUVEC cells in the logarithmic growth phase were seeded in 96-well culture plates, 3×10 3 cells / well, 100μl / well, 37℃, 5%CO 2 Cultivate for 20 hours; prepare different molar concentrations of VNI, PD-1 antibody and VEGF-Trap (9.7-10000ng / ml) in Base medium, mix with 40ng / ml VEGF respectively, and incubate at 37°C for 2 hours; then inoculate with HUVEC cells 96-well plate, 100 μl / well, 3 replicate wells for each group, 37°C, 5% CO 2 Continue to incubate for 96 hours, add Base medium and complete medium respectively to the negative control group; add CCK-8 reagent, 20 μl / well, incubate at 37°C in the dark for 2 hours, detect the absorbance at 450nm, and the EC50 value curve of the effect of VNI on HUVEC cell proliferation Schematic such as figure 1 As shown, the experimental results are shown in Table 5.
[0066] sampl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com