Patents
Literature
250 results about "T cell immunity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
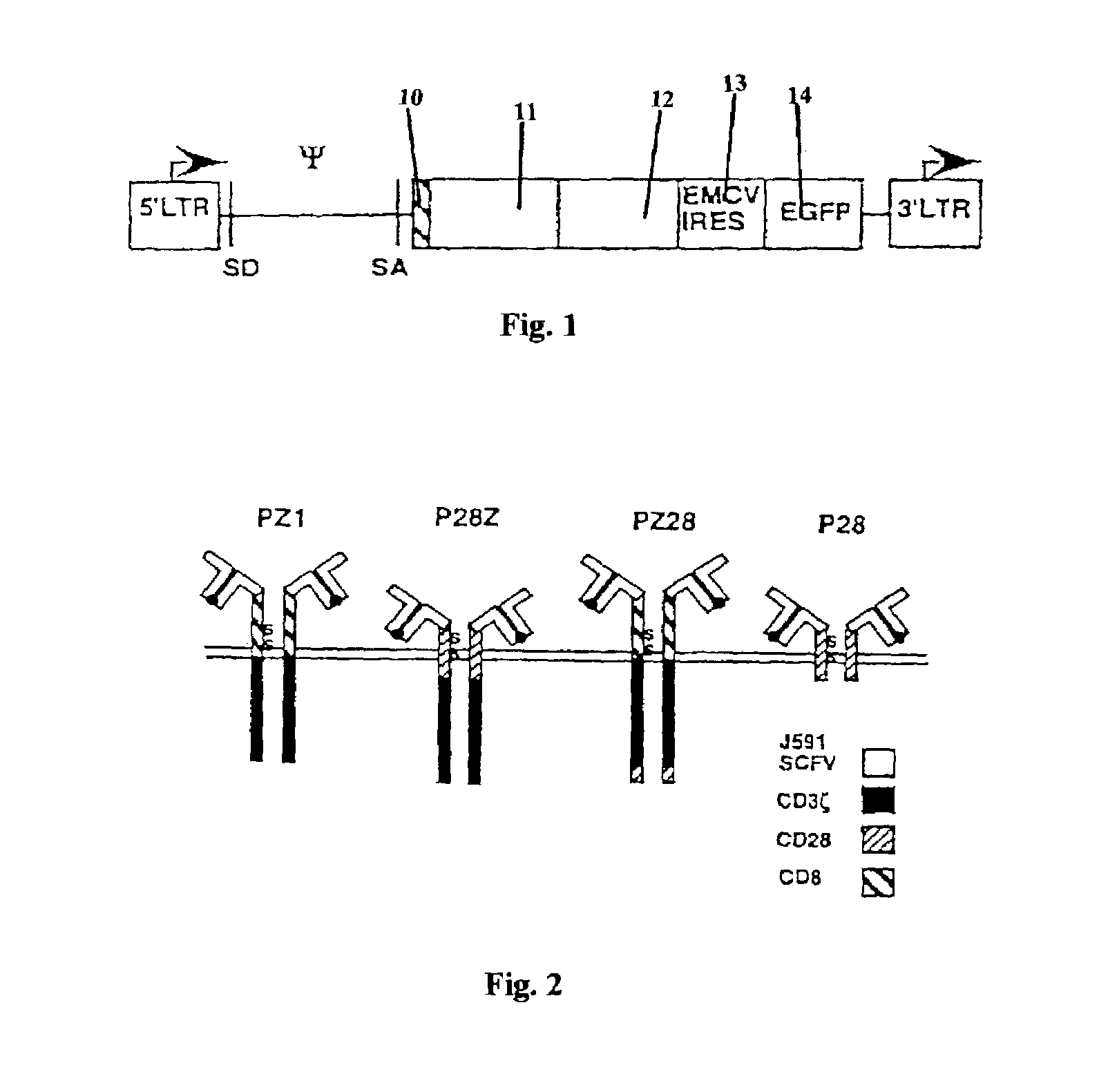
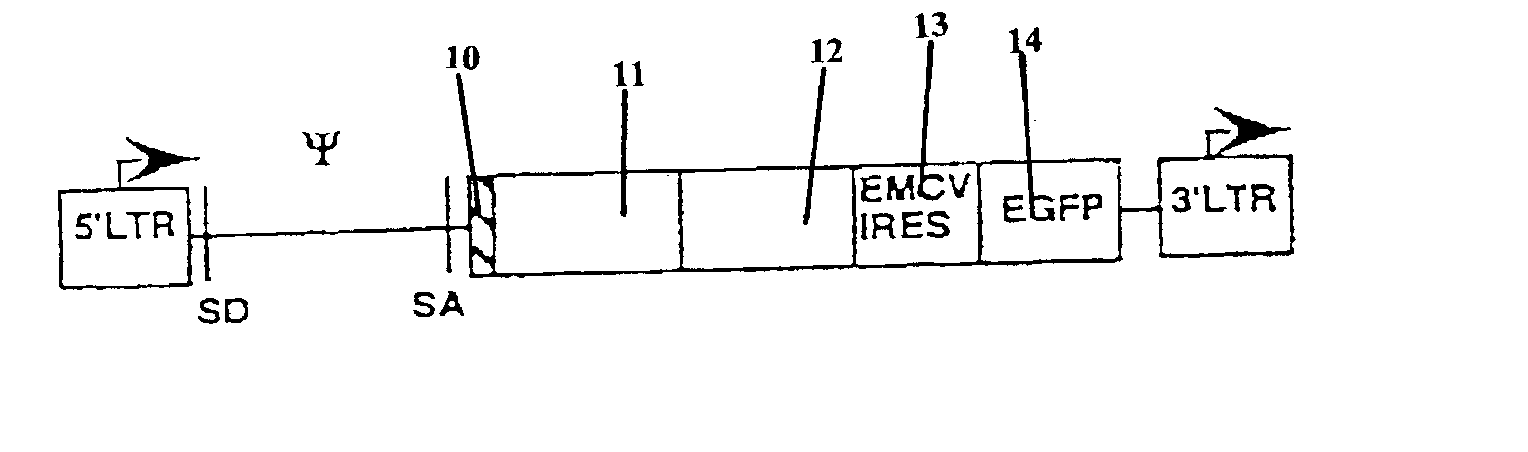
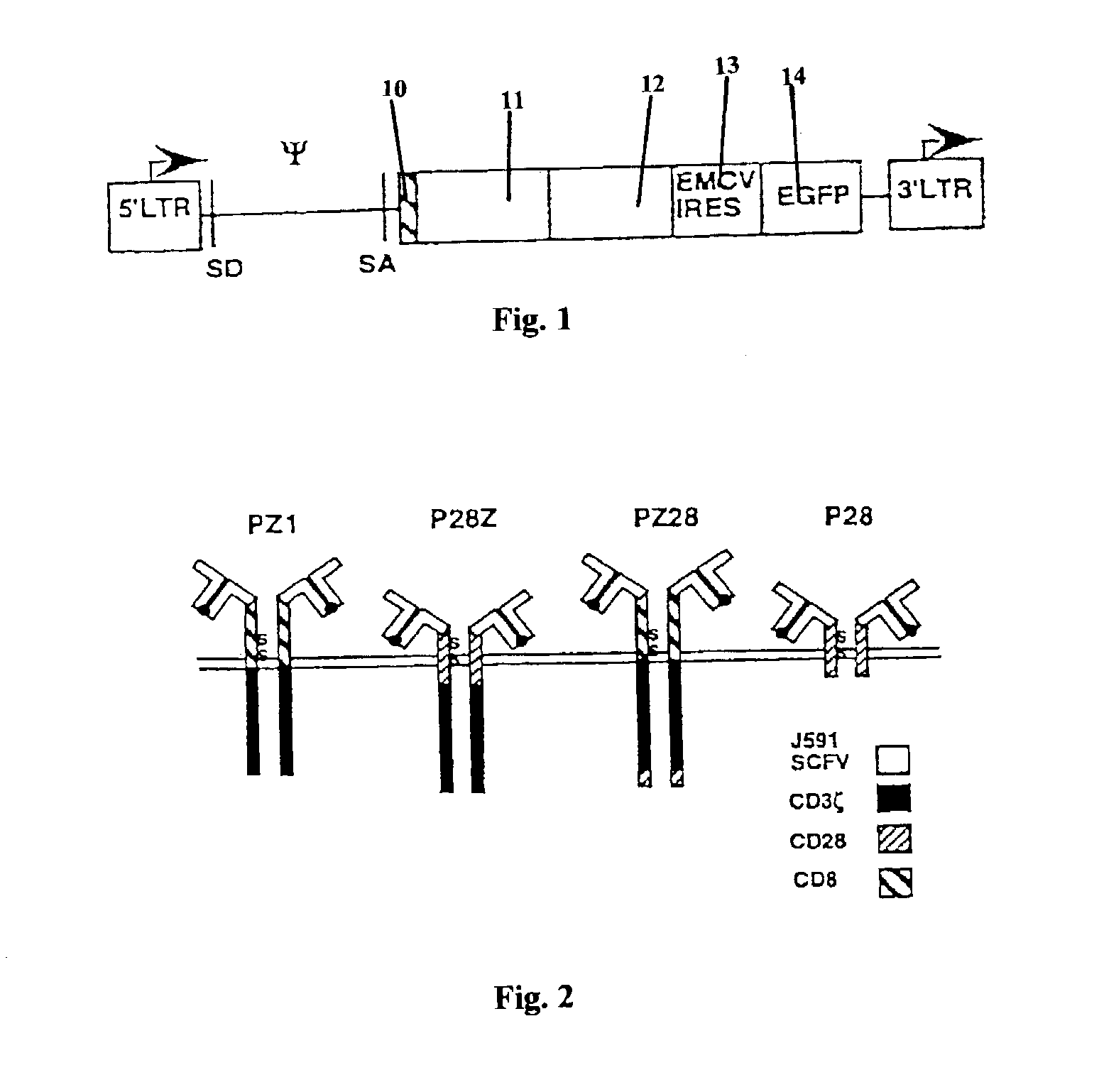
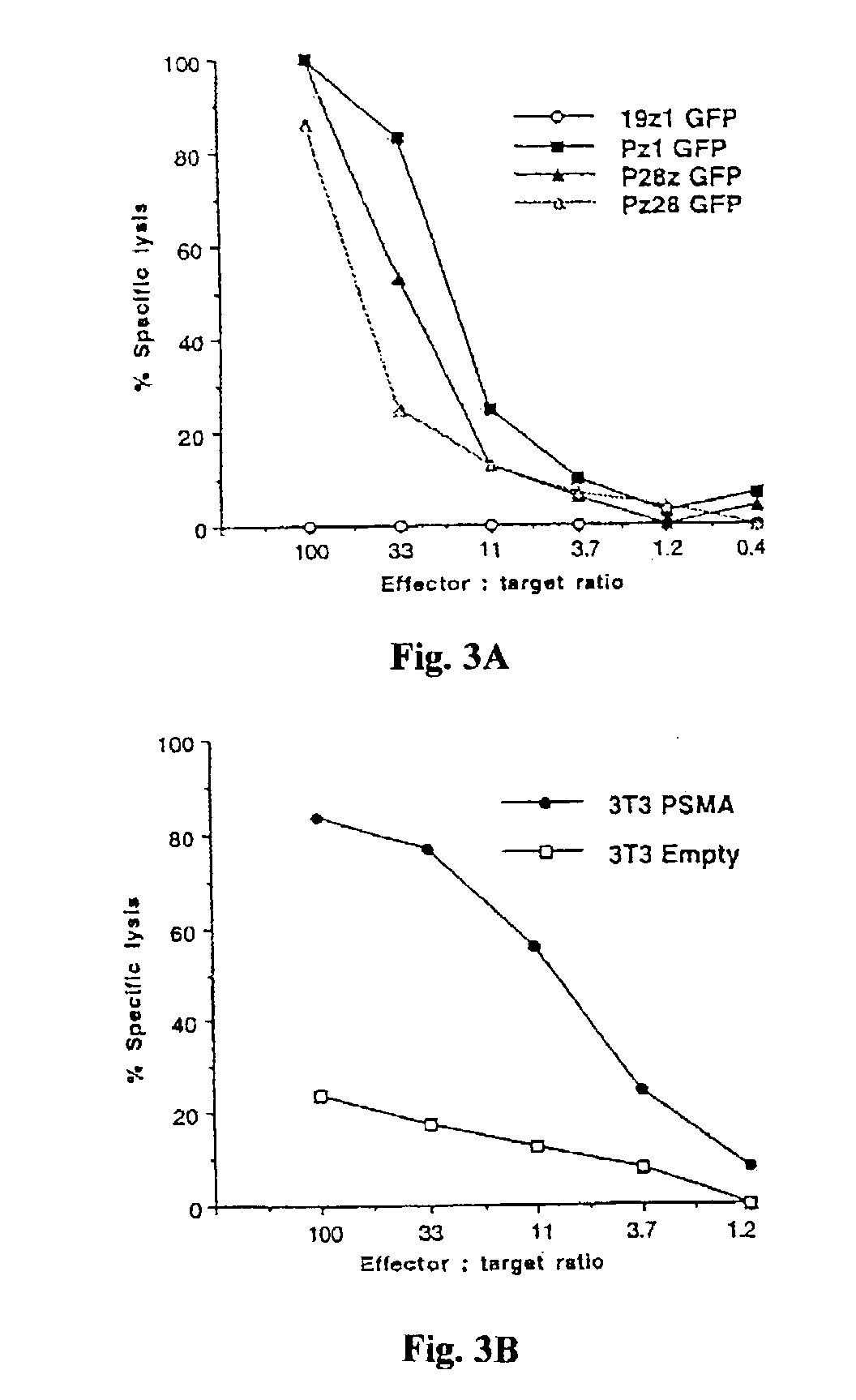
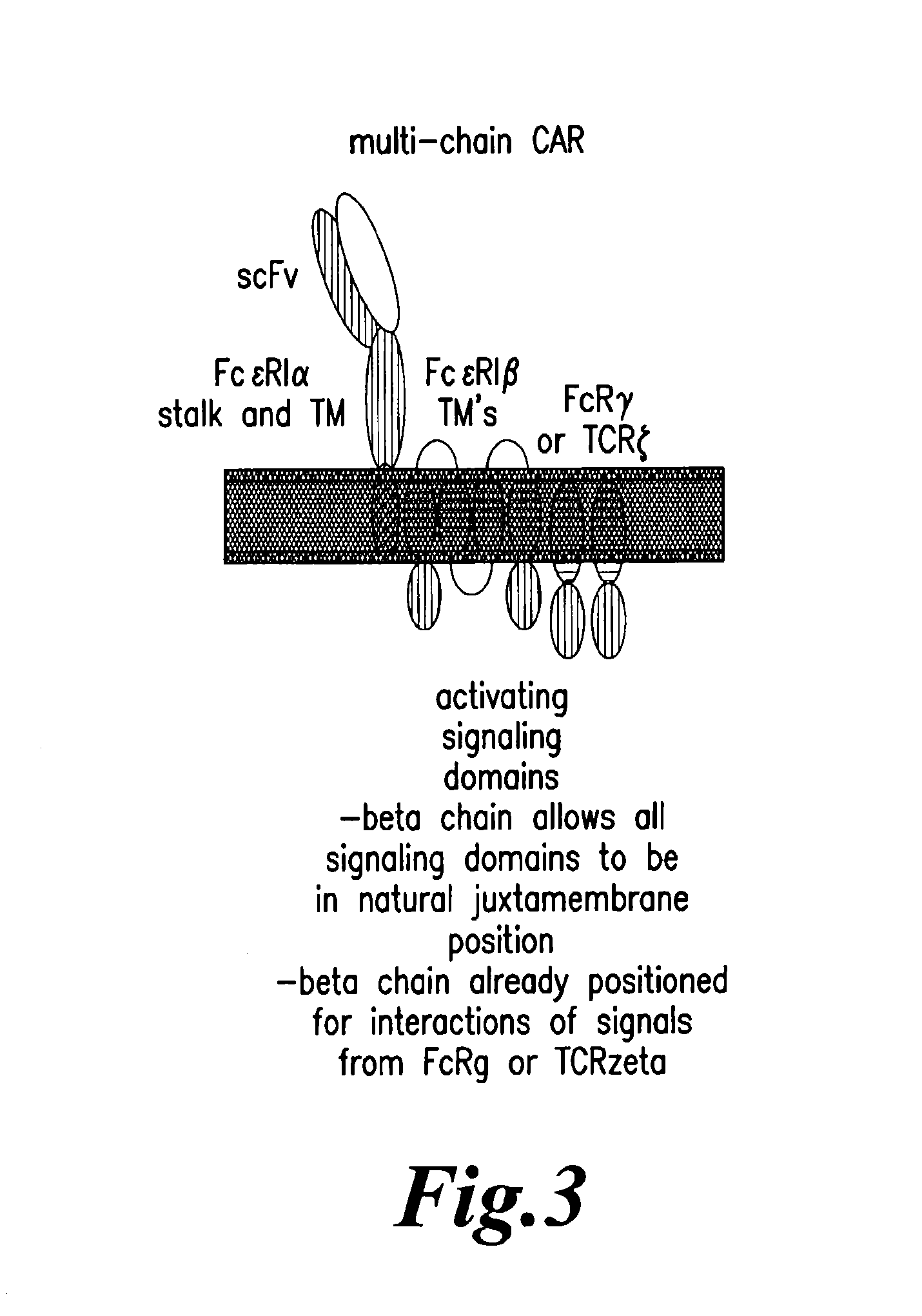
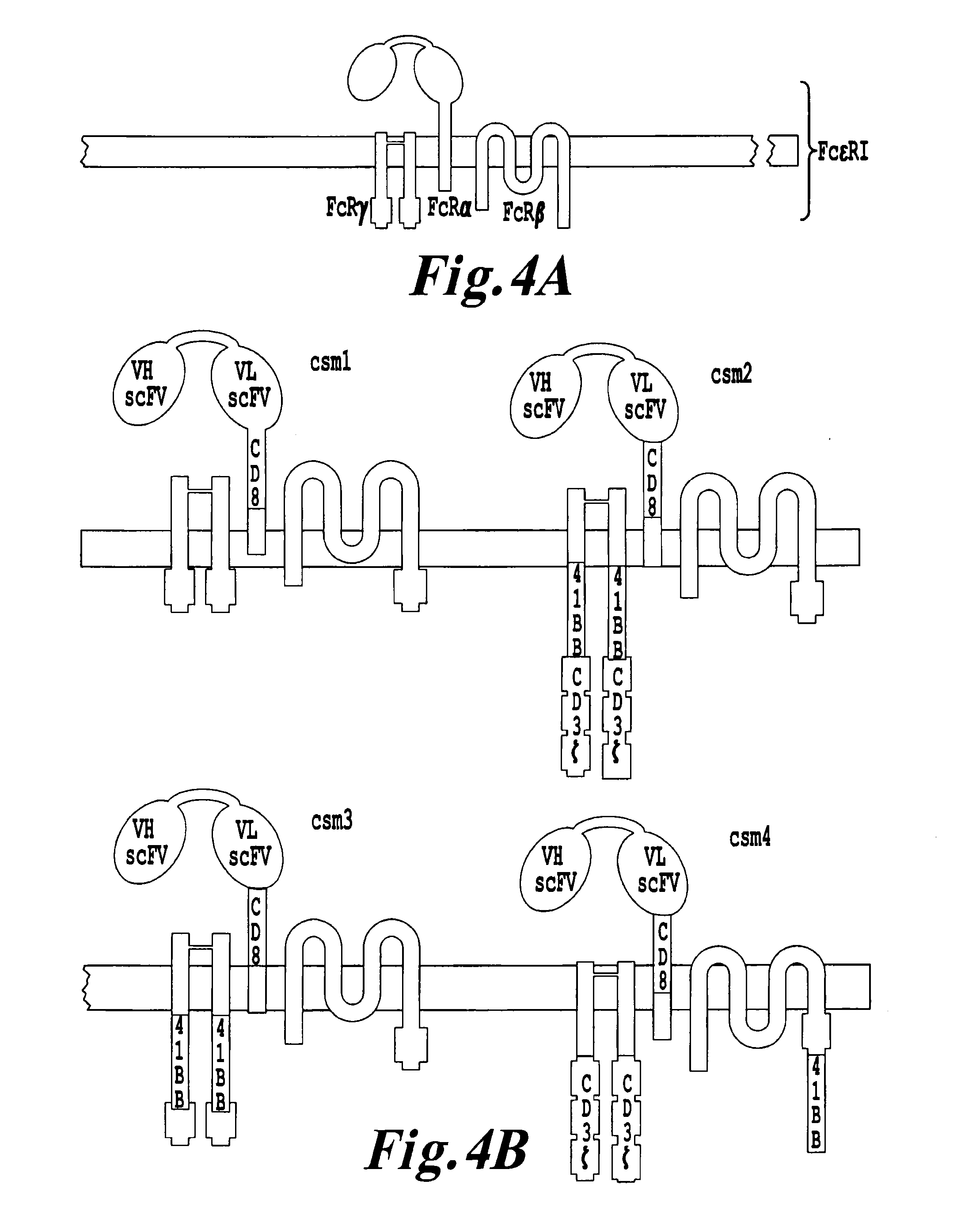
Nucleic acids encoding chimeric T cell receptors
ActiveUS7446190B2Antibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsCytotoxicityBiological activation
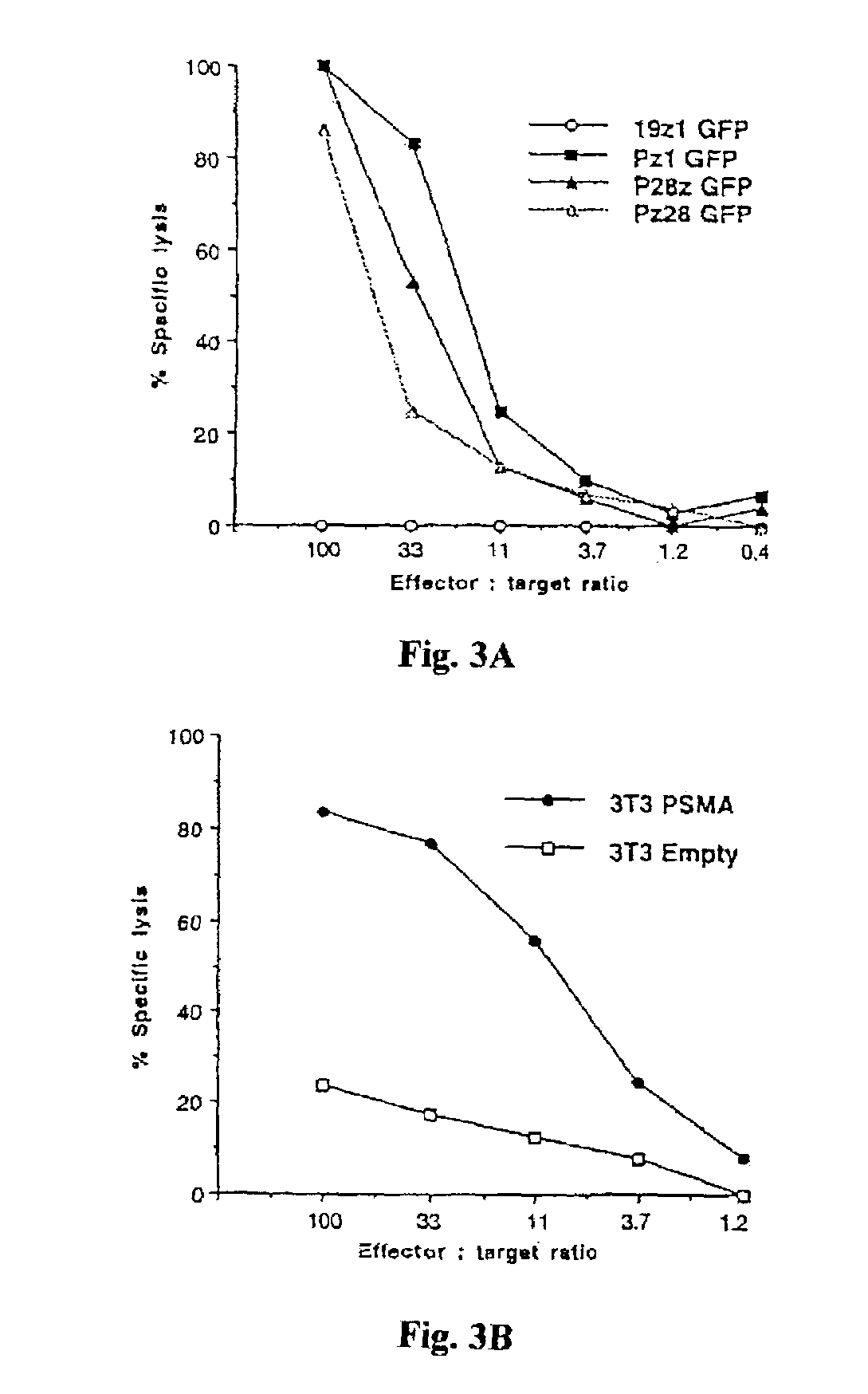
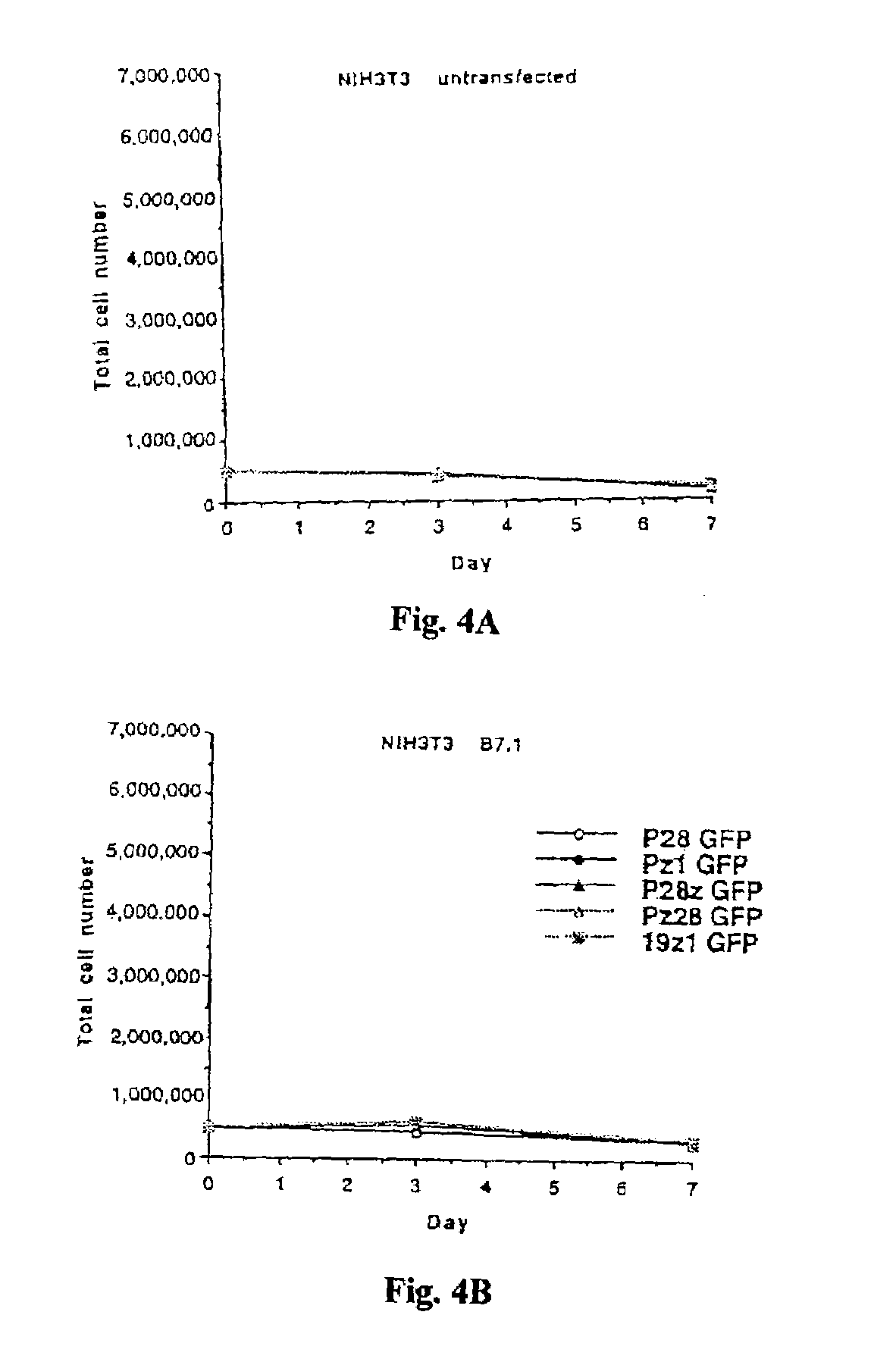
Chimeric T cell receptors (TCR) are provided that combine, in a single chimeric species, the intracellular domain of CD3 ζ-chain, a signaling region from a costimulatory protein such as CD28, and a binding element that specifically interacts with a selected target. When expressed, for example in T-lymphocytes from the individual to be treated for a condition associated with the selected target, a T cell immune response is stimulated in the individual to the target cells. The chimeric TCR's are able to provide both the activation and the co-stimulation signals from a single molecule to more effectively direct T-lymphocyte cytotoxicity against the selected target and T-lymphocyte proliferation.
Owner:SLOAN KETTERING INST FOR CANCER RES
Chimeric T cell receotors
ActiveUS20040043401A1Minimize the numberSmall doseAntibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsLymphocyte proliferationCytotoxicity
Chimeric T cell receptors (TCR) are provided that combine, in a single chimeric species, the intracellular domain of CD3 zeta-chain, a signaling region from a costimulatory protein such as CD28, and a binding element that specifically interacts with a selected target. When expressed, for example in T-lymphocytes from the individual to be treated for a condition associated with the selected target, a T cell immune response is stimulated in the individual to the target cells. The chimeric TCR's are able to provide both the activation and the co-stimulation signals from a single molecule to more effectively direct T-lymphocyte cytotoxicity against the selected target and T-lymphocyte proliferation.
Owner:SLOAN KETTERING INST FOR CANCER RES
Synergistic Anti-tumor efficacy using alloantigen combination immunotherapy
InactiveUS20130071403A1Increased activationOrganic active ingredientsAntibody ingredientsImmunotherapeutic agentIrritation
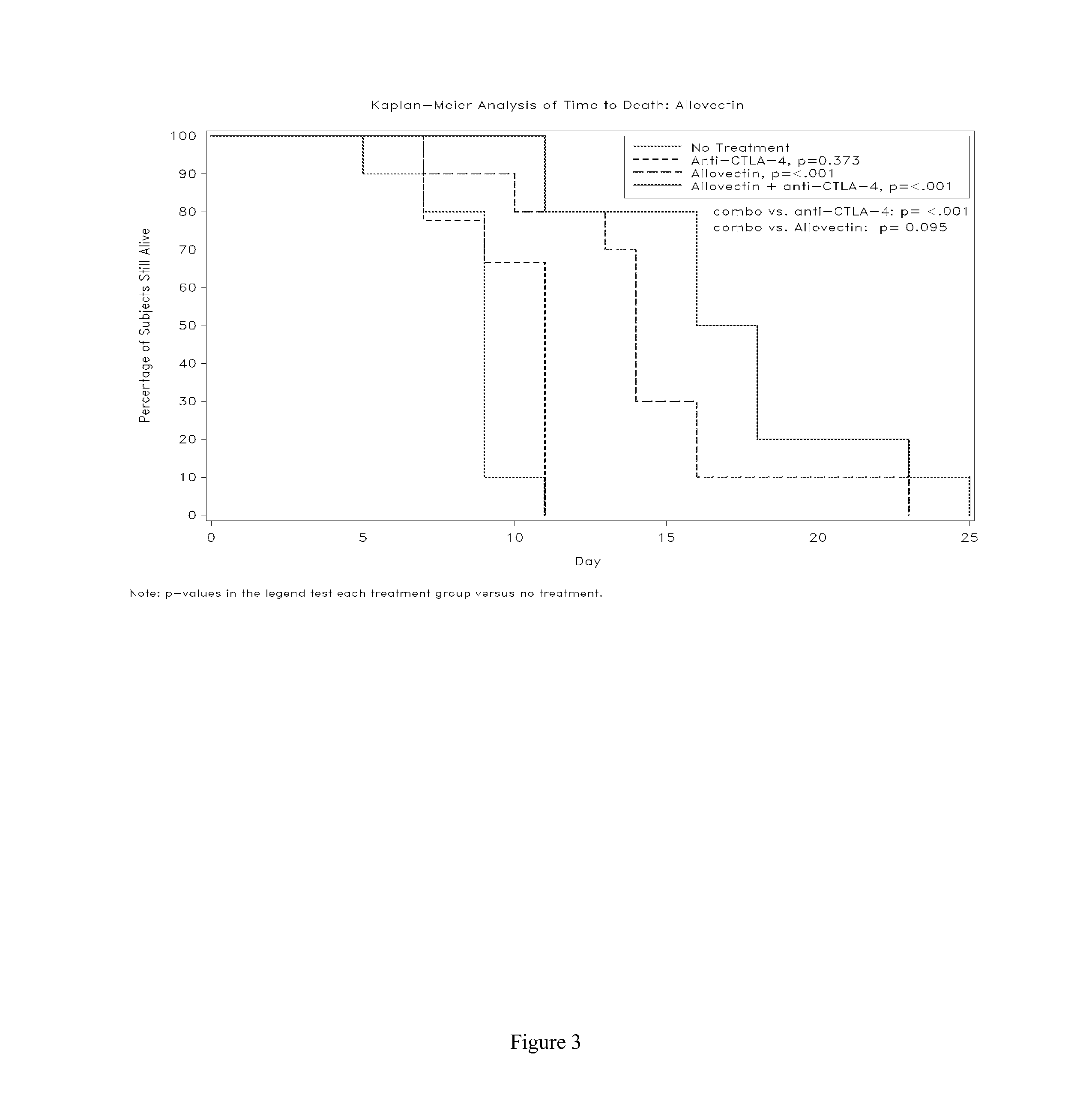
The present disclosure provides combinations of immunotherapeutics and methods for treating medical conditions that are characterized by the lack of an effective immune response, for example as would result following a down-regulation of MHC class I, such as in cancer. The immunotherapeutic compositions of the invention, which can be used to treat the medical conditions, include one or more immunostimulatory antibodies or molecules having specificity for CTLA-4, PD-1, PD-L1, PD-L2, CD40, OX40, CD137, GITR, ILT2, or ILT3, or ligands for these molecules (e.g., an isolated fully-human monoclonal antibody) in association with one or more alloantigens, such as, vector(s) capable of expressing protein(s) or peptide(s) that stimulate T-cell immunity against tissues or cells, formulated in a pharmaceutically acceptable carrier. The proteins or peptides may comprise class I major histocompatibility complex (MHC) antigens, β2-microglobulins, or cytokines. The MHC antigen may be foreign to the subject. The MHC antigen may be HLA-B7.
Owner:VICAL INC
Methods for engineering highly active t cell for immunotheraphy
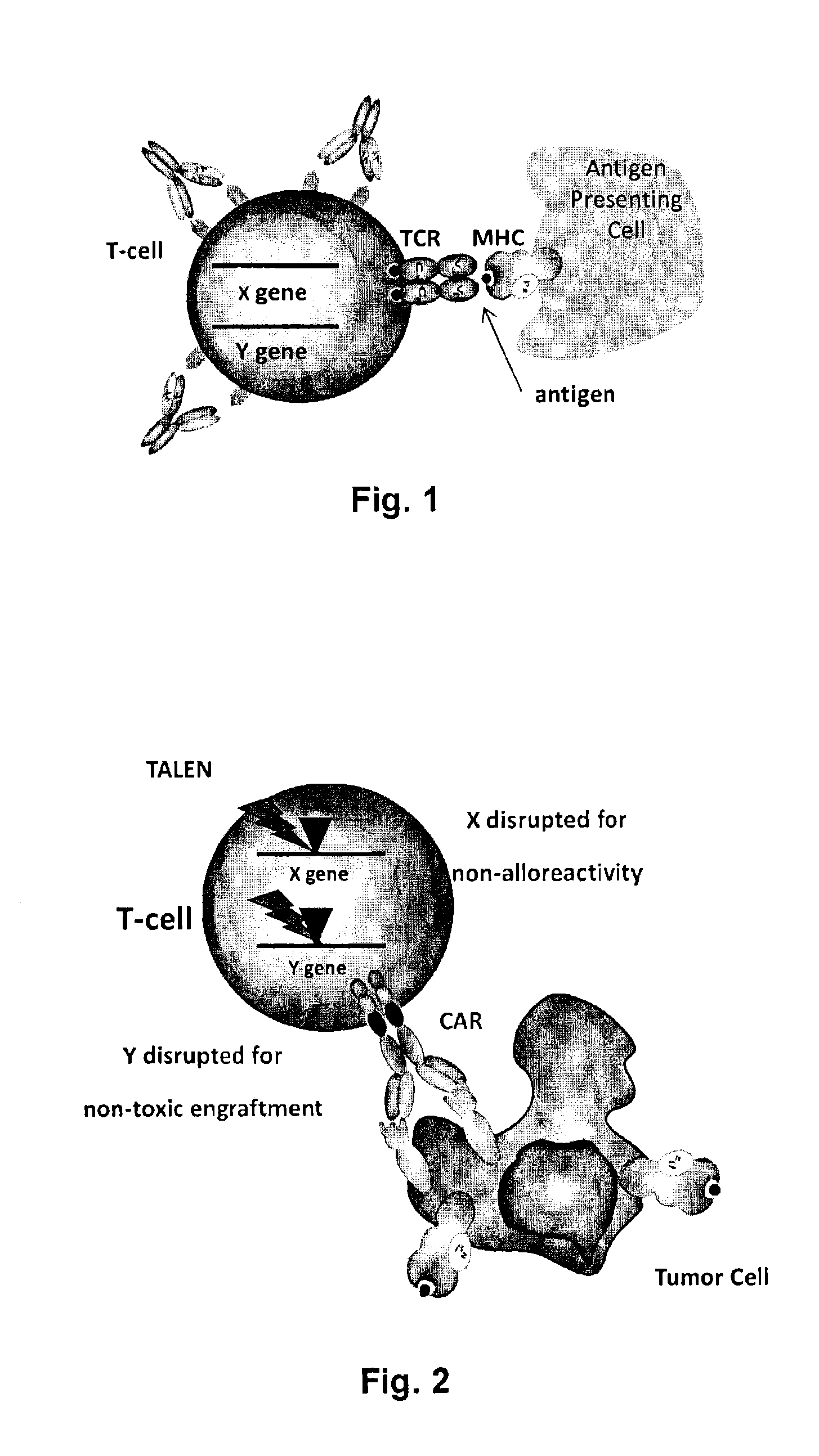
The present invention relates to methods for developing engineered T-cells for immunotherapy and more specifically to methods for modifying T-cells by inactivating at immune checkpoint genes, preferably at least two selected from different pathways, to increase T-cell immune activity. This method involves the use of specific rare cutting endonucleases, in particular TALE-nucleases (TAL effector endonuclease) and polynucleotides encoding such polypeptides, to precisely target a selection of key genes in T-cells, which are available from donors or from culture of primary cells. The invention opens the way to highly efficient adoptive immunotherapy strategies for treating cancer and viral infections.
Owner:CELLECTIS SA
Immunotoxin fusion proteins and means for expression thereof
InactiveUS7696338B2Facilitating proteolytic processingInduce immune toleranceSugar derivativesAntibody mimetics/scaffoldsYeastT cell immunity
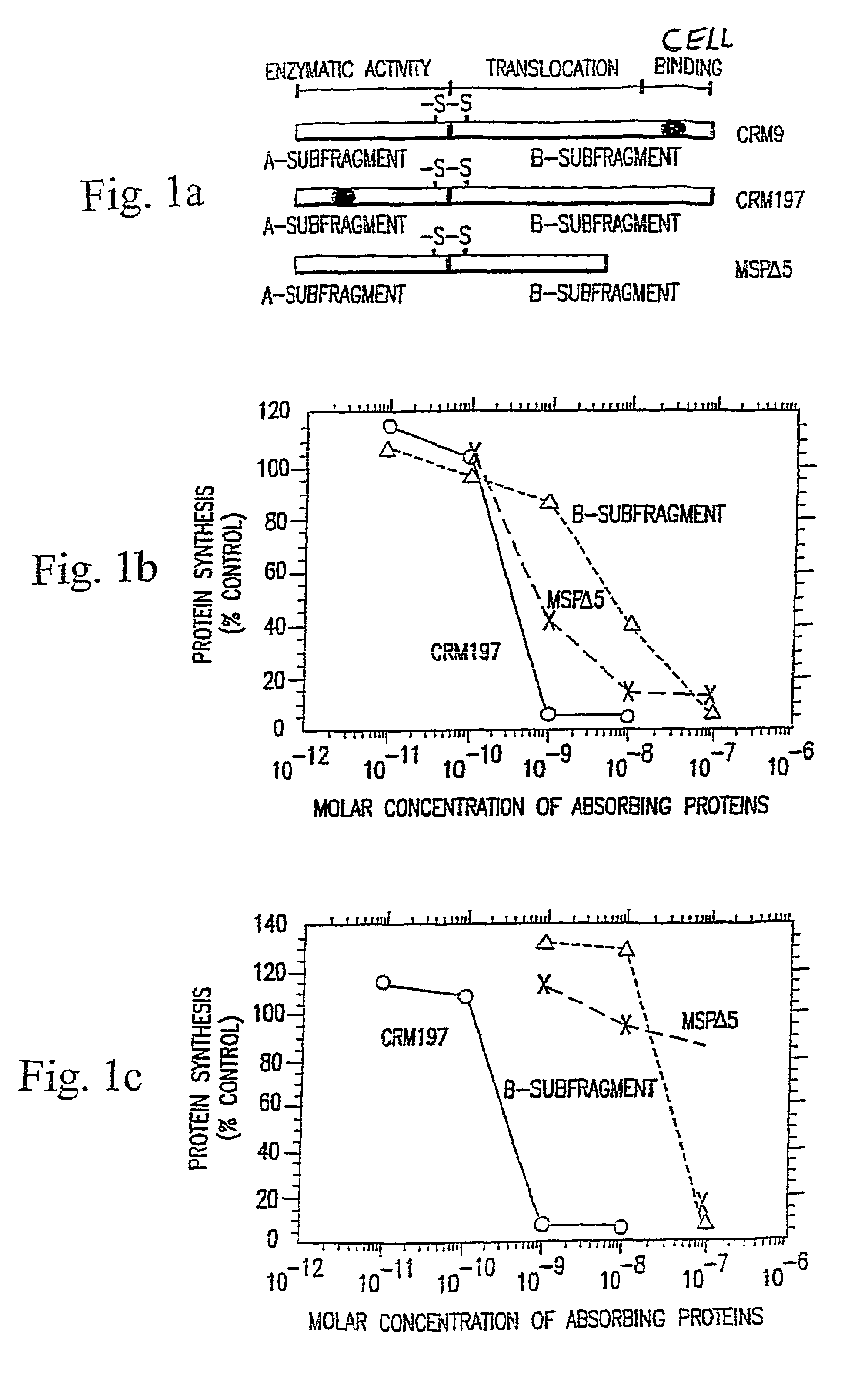
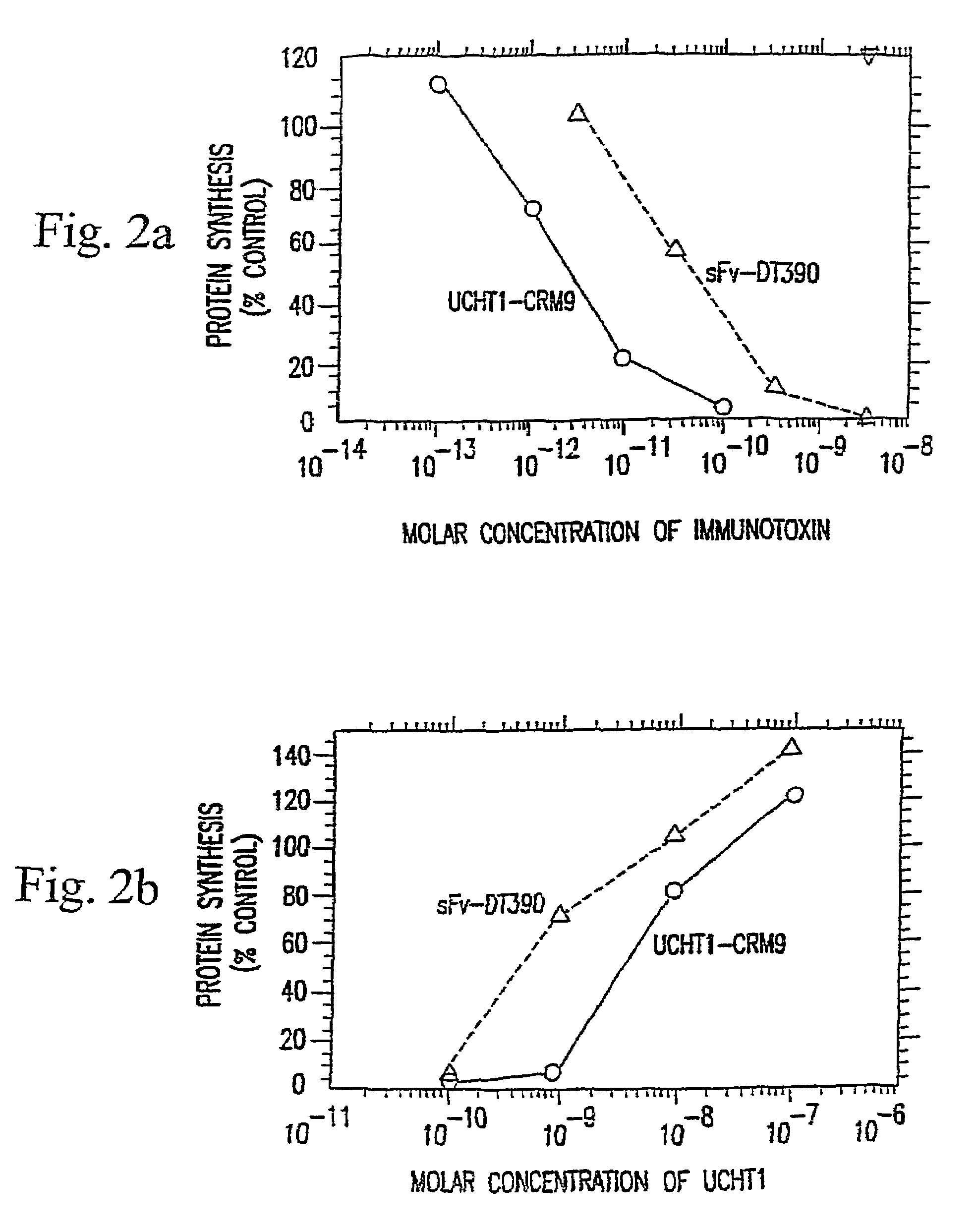
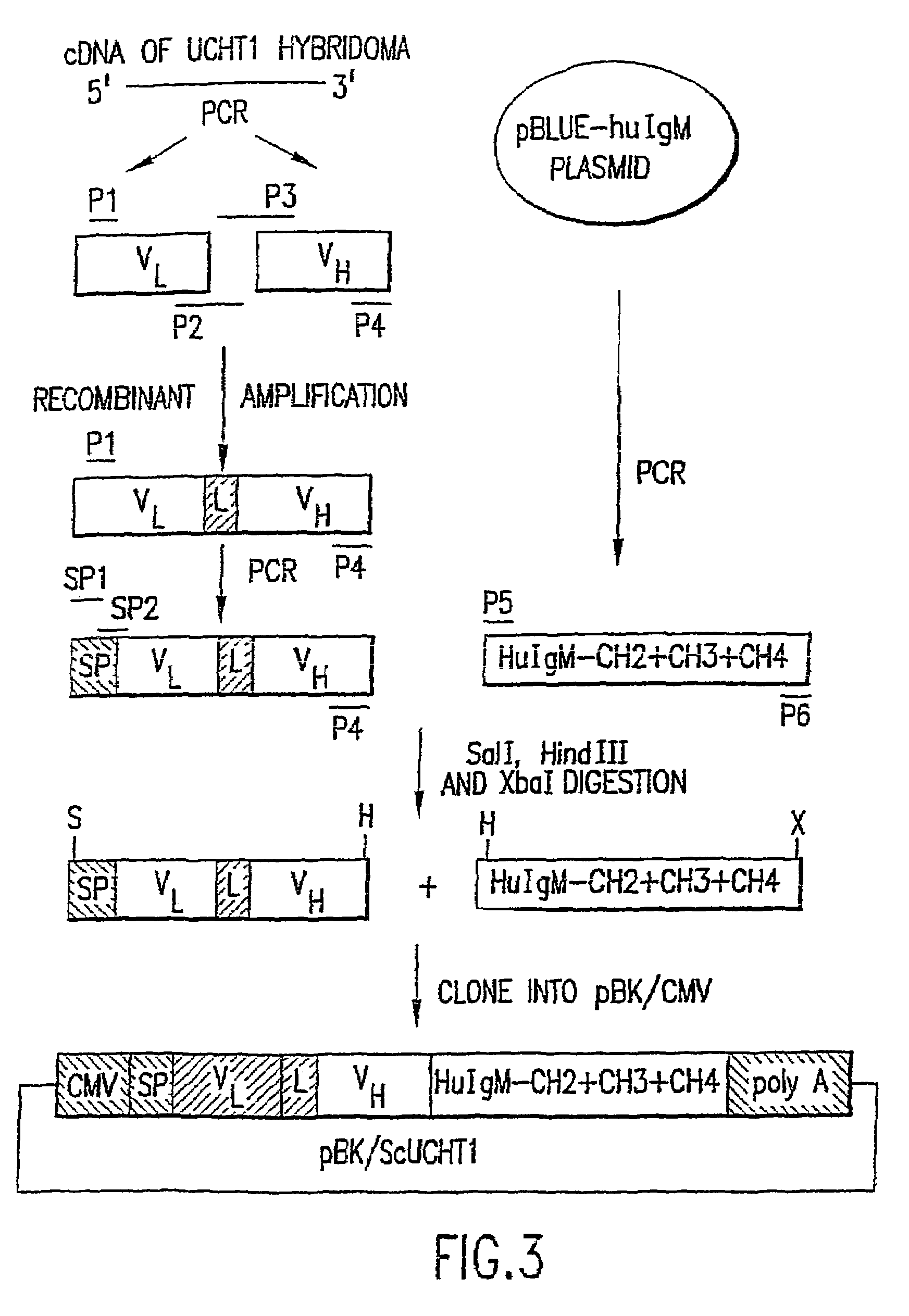
The present invention described and shown in the specification and drawings provides novel recombinant DT-based immunotoxins, and, more specifically anti-T cell immunotoxin fusion proteins. Also provided are immunotoxins that can be expressed in bacterial, yeast, or mammalian cells. The invention also provides means for expression of the immunotoxin fusion protein.
Owner:THE GOVERNMENT OF THE UNITED STATES OF AMERICA REPRESENTED BY THE DEPT OF HEALTH & HUMAN SERVICES NAT INST OF HEALTH OFFICE OF TECH TRANSFER +1
Use of PD-L3 proteins and PD-L3 specific antibodies or antibody fragments to regulate CD4+ and CD8+ T cell immunity
The present invention relates to novel regulatory T cell proteins. One protein, designated PD-L3, resembles members of the PD-L1 family, and co-stimulates αCD3 proliferation of T cells in vitro. A second, TNF-like, protein has also been identified as being upregulated upon αCD3 / αGITR stimulation. This protein has been designated Treg-sTNF. Proteins, antibodies, activated T cells and methods for using the same are disclosed.
Owner:TRUSTEES OF DARTMOUTH COLLEGE THE
HIV vaccine based on targeting maximized gag and nef to dendritic cells
ActiveUS20100135994A1Improve efficiencyIncrease flexibilityBiocidePeptide/protein ingredientsCyclin D1Dendritic cell
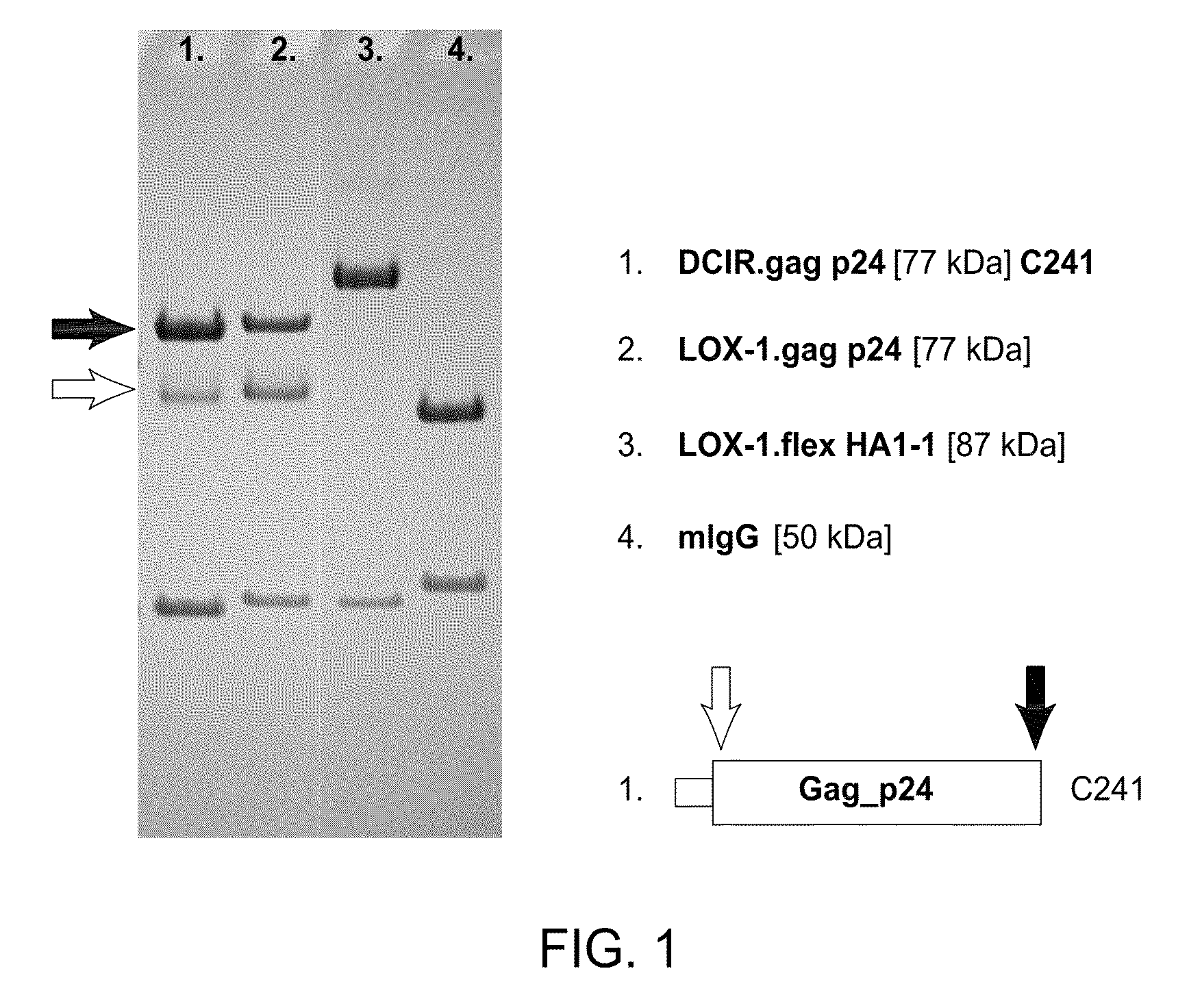
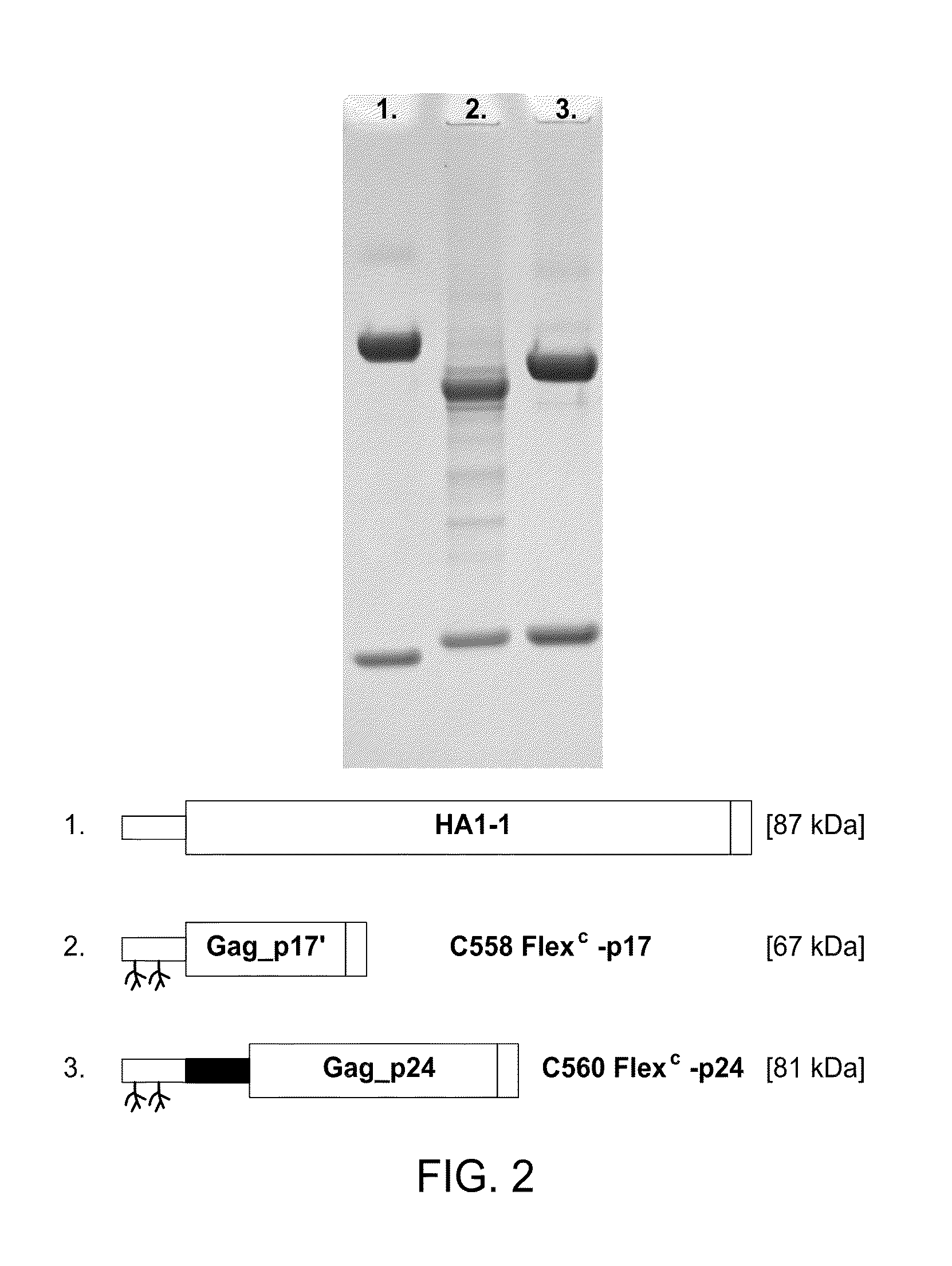
The present invention includes compositions and methods for making and using a vaccine that includes a DC-specific antibody or fragment thereof to which an engineered Gag antigen is attached to form an antibody-antigen complex, wherein the Gag antigen is less susceptible to proteolytic degradation by eliminating one or more proteolytic sites or a DC-specific antibody or fragment thereof to which an engineered Nef antigen is attached to form an antibody-antigen complex, wherein the Nef antigen comprises one or more codon usage optimization that increase antibody-antigen complex secretion, or both, wherein the vaccine is able to elicit an HIV-specific T cell immune response to Gag p17, Gag p24, Nef and / or Cyclin D1.
Owner:BAYLOR RES INST
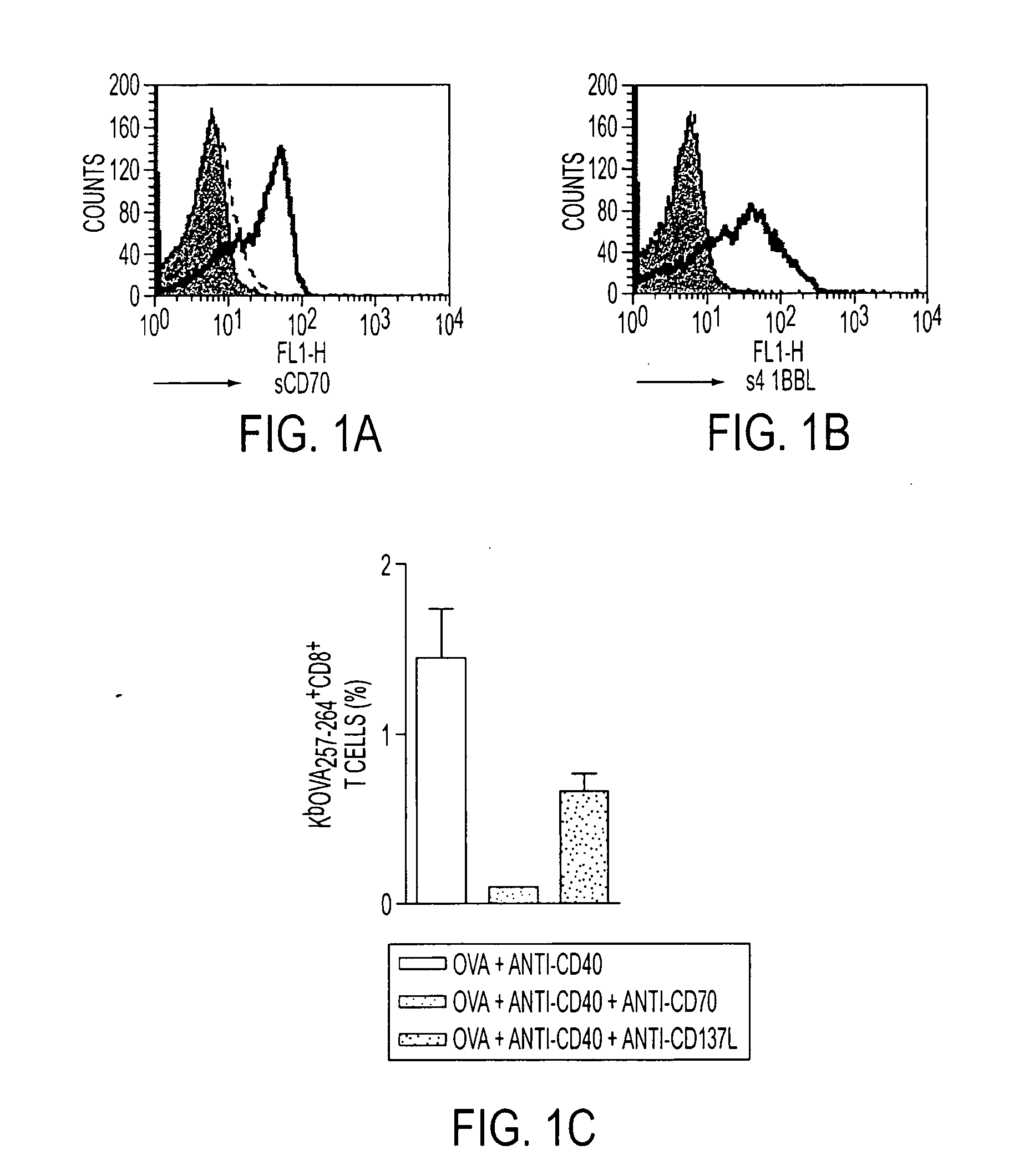
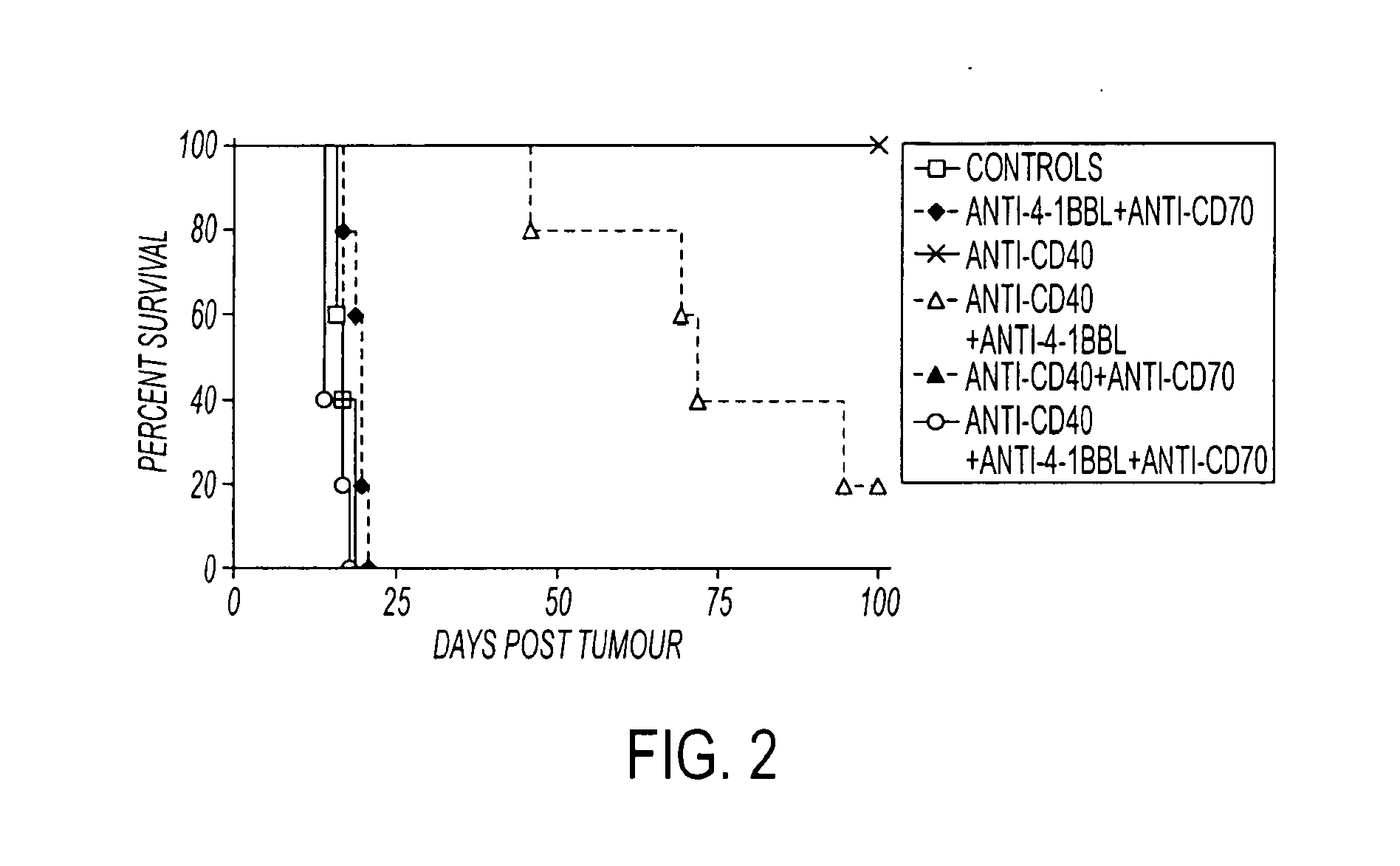
Human immune therapies using a CD27 agonist alone or in combination with other immune modulators
ActiveUS8481029B2Promotes strong expression of 4-1BBImprove responseAntibacterial agentsNervous disorderAutoimmune responsesImmune modulator
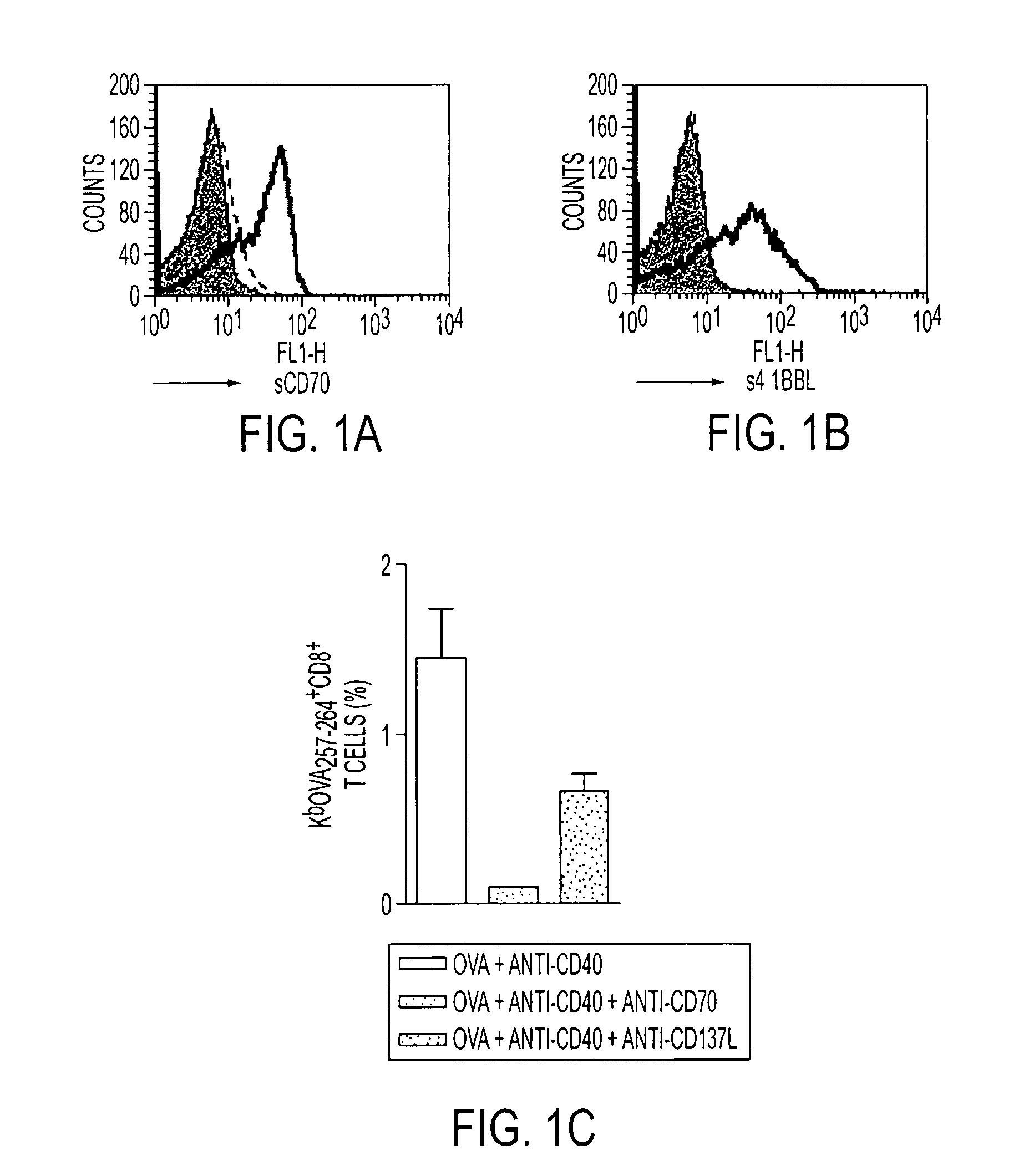
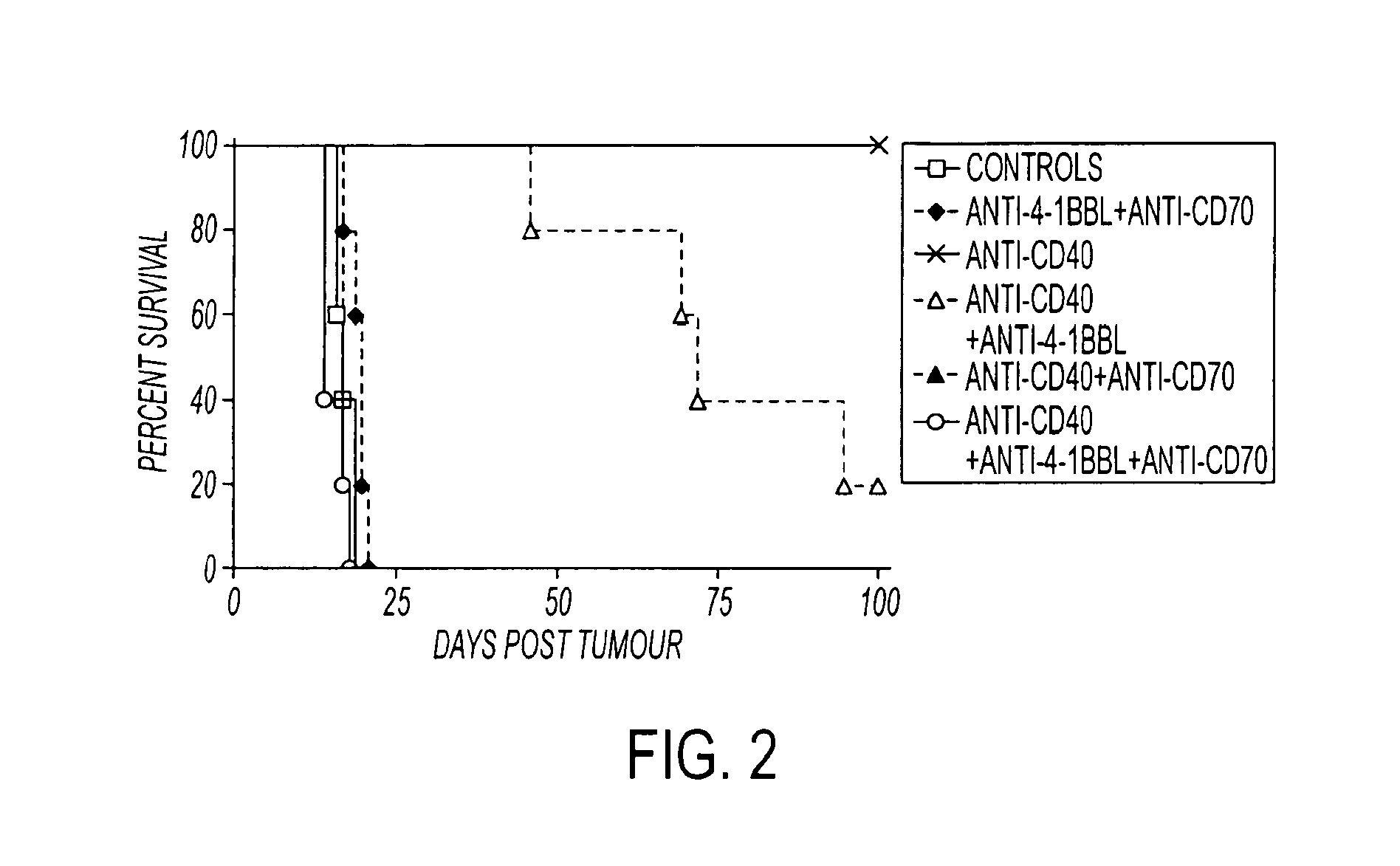
Methods of inducing T cell proliferation and expansion in vivo for treating conditions wherein antigen-specific T cell immune response are therapeutically desirable such as cancer, infection, inflammation, allergy and autoimmunity and for enhancing the efficacy of vaccines are provided. These methods comprise the administration of at least one CD27 agonist, preferably an agonistic CD27 antibody, alone or in association with another moiety such as immune stimulant or immune modulator such as an anti-CD40, OX-40, 4-IBB, or CTLA-4 antibody or an agent that depletes regulatory cells, or a cytokine. These mono and combination therapies may also optionally include the administration of a desired antigen such as a tumor antigen, an allergen, an autoantigen, or an antigen specific to an infectious agent or pathogen against which a T cell response (often CD8+) is desirably elicited.
Owner:UNIV OF SOUTHAMPTON
Antigen specific T cell therapy
Provided are methods for generating immune cells of the desired type and specificity in a host. The methods may be used to treat a disease or disorder, such as a tumor in a patient. Target cells, preferably hematopoietic stem cells such as primary bone marrow cells are transfected with a polynucleotide encoding a T cell receptor with the desired specificity. The transfected cells are then transferred to the host where they develop into mature, functional immune cells. The source of the T cell receptor can determine the stem cell's fate. Thus transfecting stem cells with TCRs from cytotoxic cells will lead to the generation of cytotoxic T cells in the host, while TCRs from helper cells will produce helper cells. Both arms of T cell immunity can be generated simultaneously in a host. Additionally, the immune response to the desired antigen can be further stimulated by immunizing the host with the antigen.
Owner:CALIFORNIA INST OF TECH
Novel pd1 isoforms, and uses thereof for potentiating immune responses
ActiveUS20140302070A1Prevention treatment ameliorationPeptide/protein ingredientsGenetic material ingredientsAutoimmune conditionAdjuvant
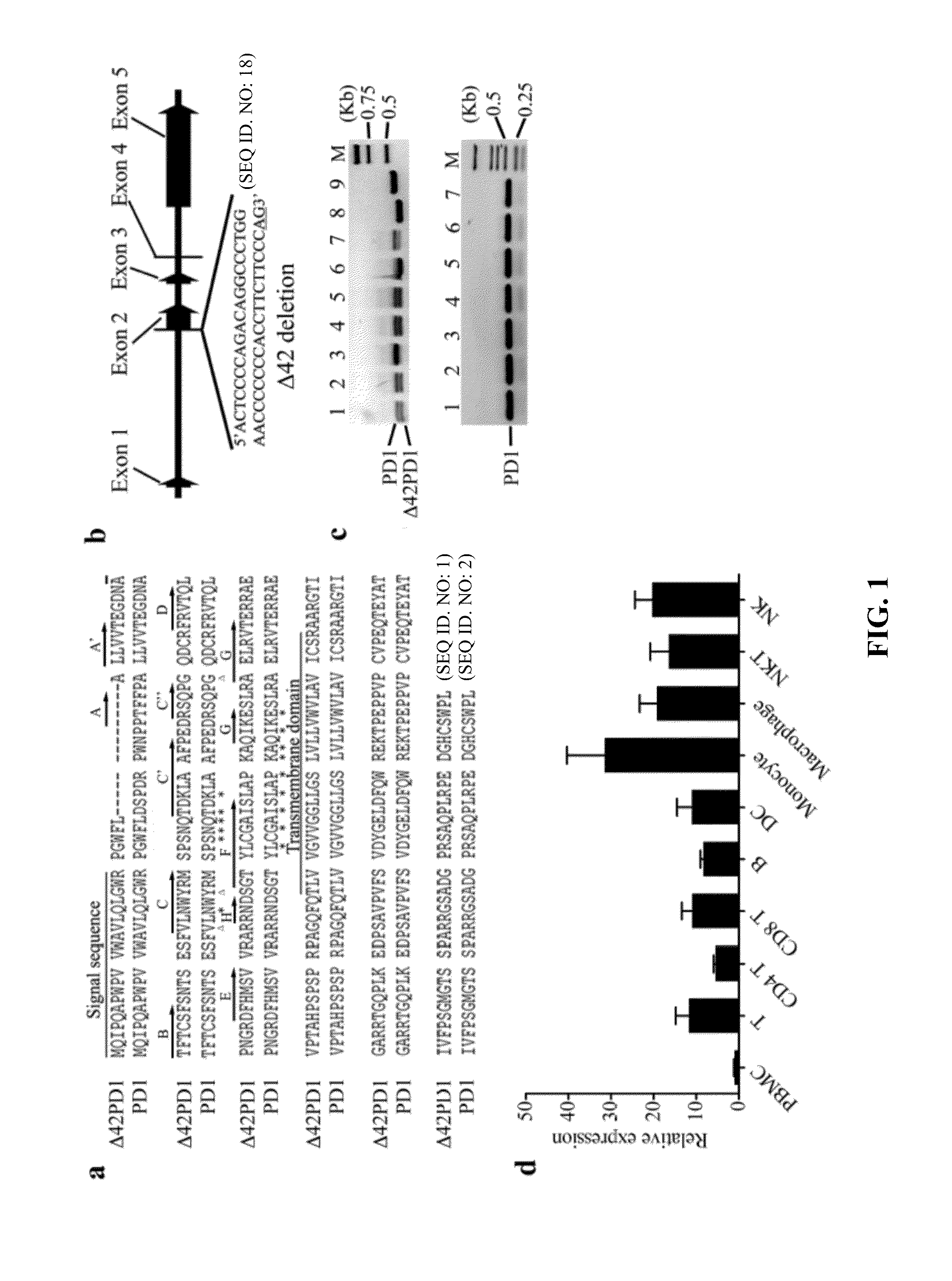
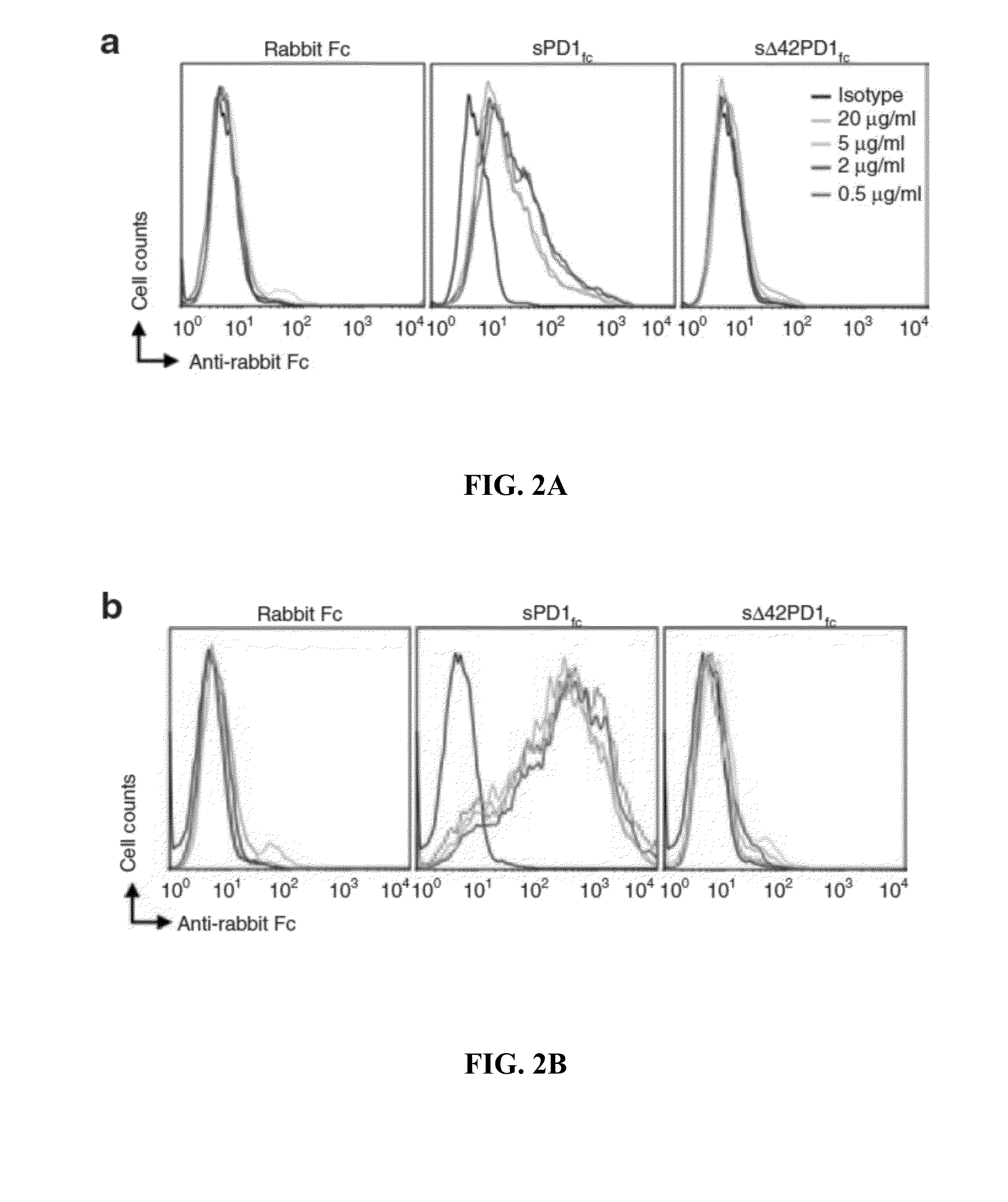
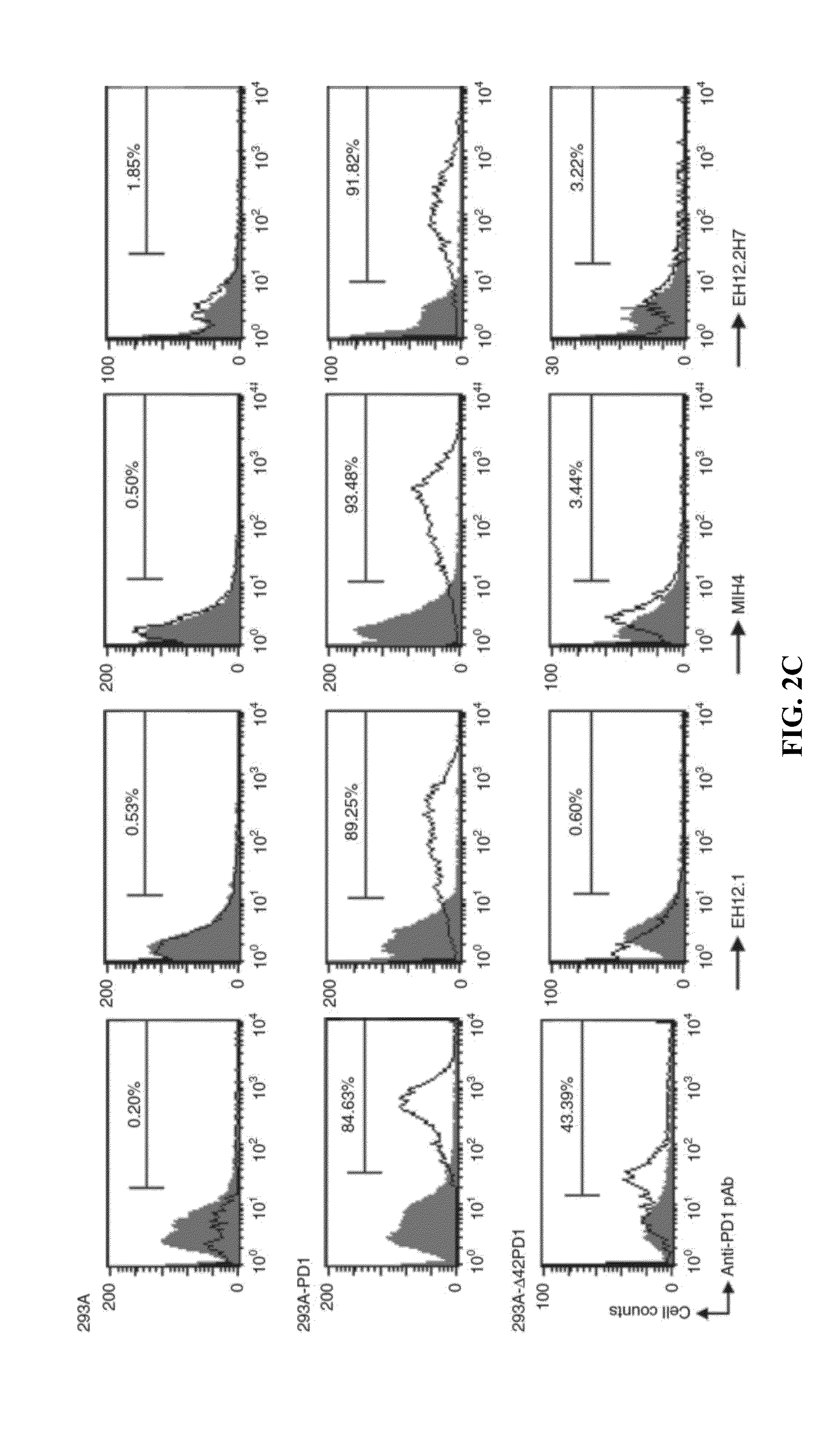
In one embodiment, the present invention provides a new isoform of human PD1 (Δ42PD1) that contains a 42-nucleotide in-frame deletion located at exon 2 domain. Δ42PD1 does not engage PD-L1 / PD-L2, and can induce the production of pro-inflammatory cytokines In one embodiment, Δ42PD1 can be used as an intramolecular adjuvant to develop a fusion DNA vaccine for enhancing antigen-specific CD8+ T cell immunity and for prevention of pathogenic infection and / or cancer. In one embodiment, soluble Δ42PD1 protein could be a therapeutic target for autoimmune diseases. In other embodiments, proteins or peptides or nucleic acids encoding proteins or peptides containing Δ42PD1 could be used as immunogens for developing antibodies binding specifically to Δ42PD1. In yet another embodiment, neutralizing antibodies could block sΔ42PD1 function and accordingly could be used as treatment for autoimmune disorders.
Owner:VERSITECH LTD
Compositions and methods for treating inflammation and auto-immune diseases
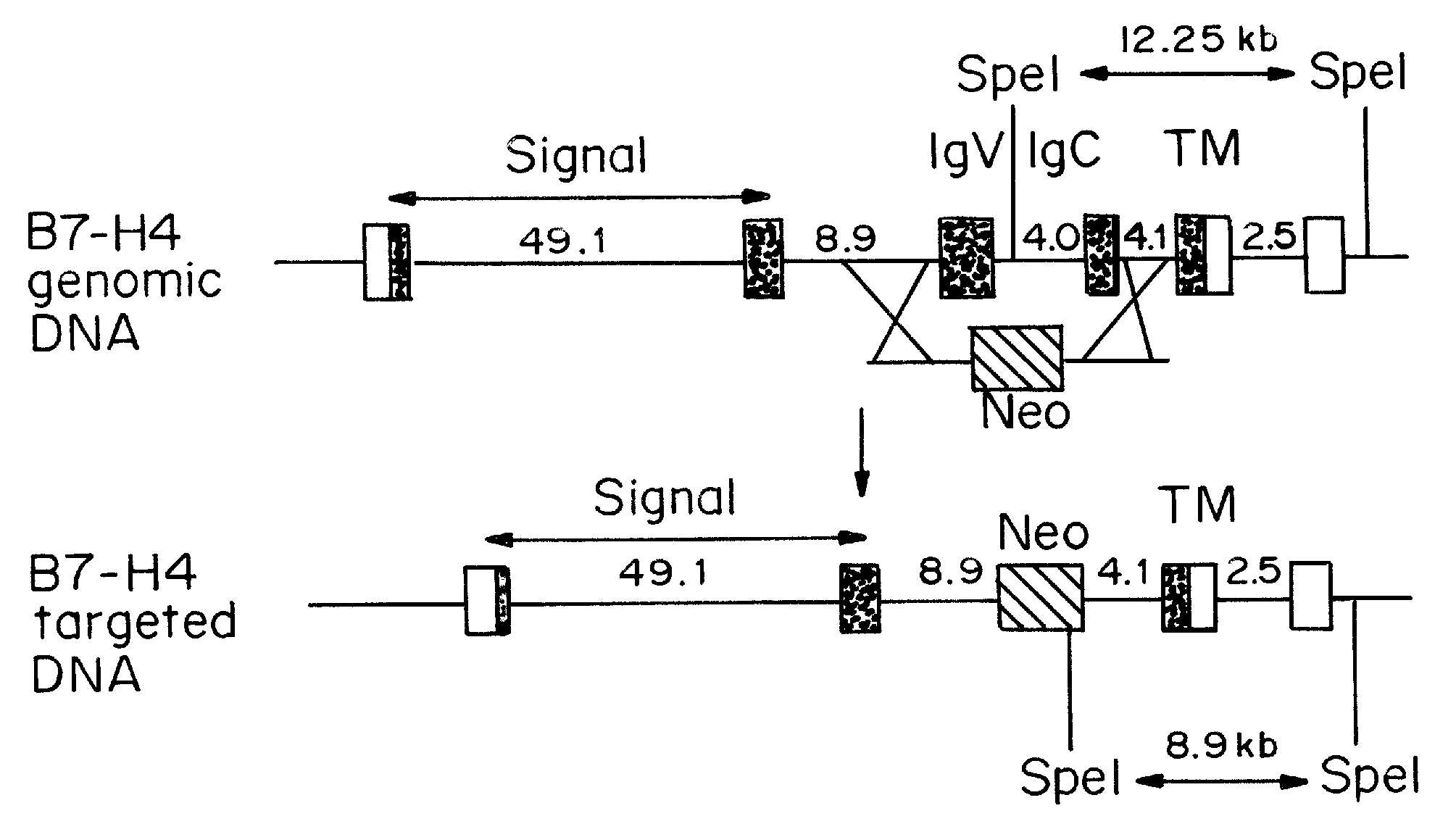
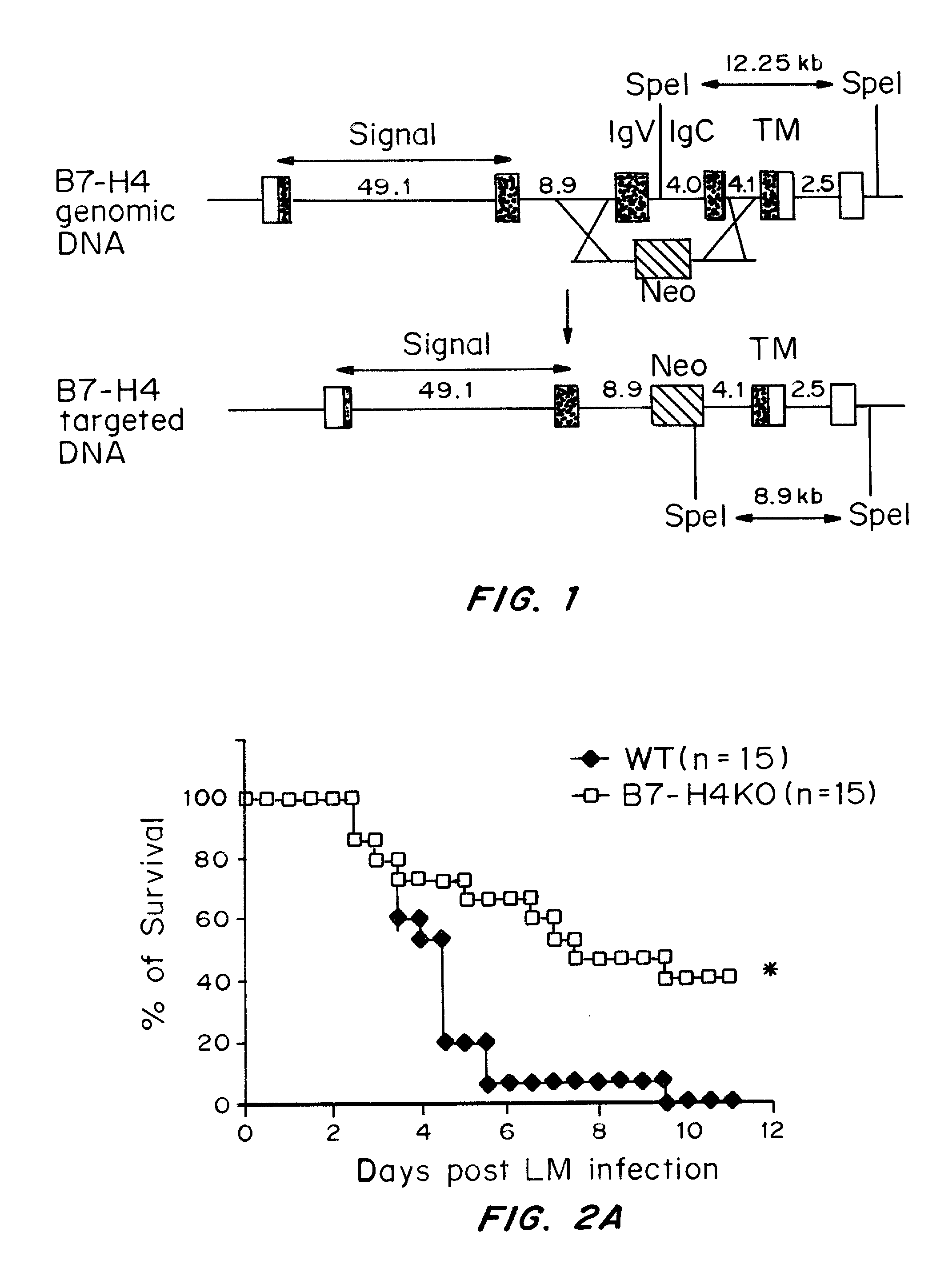
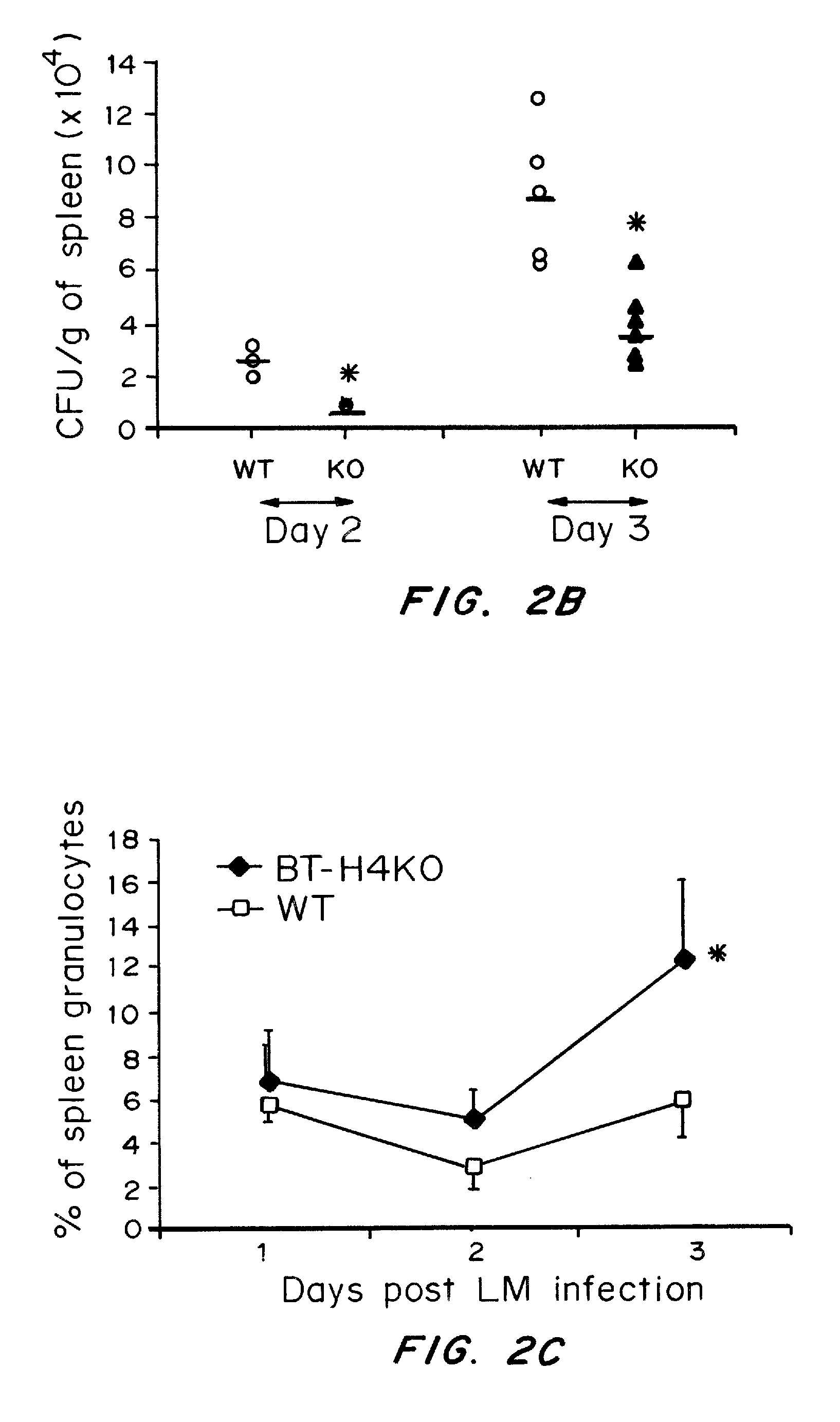
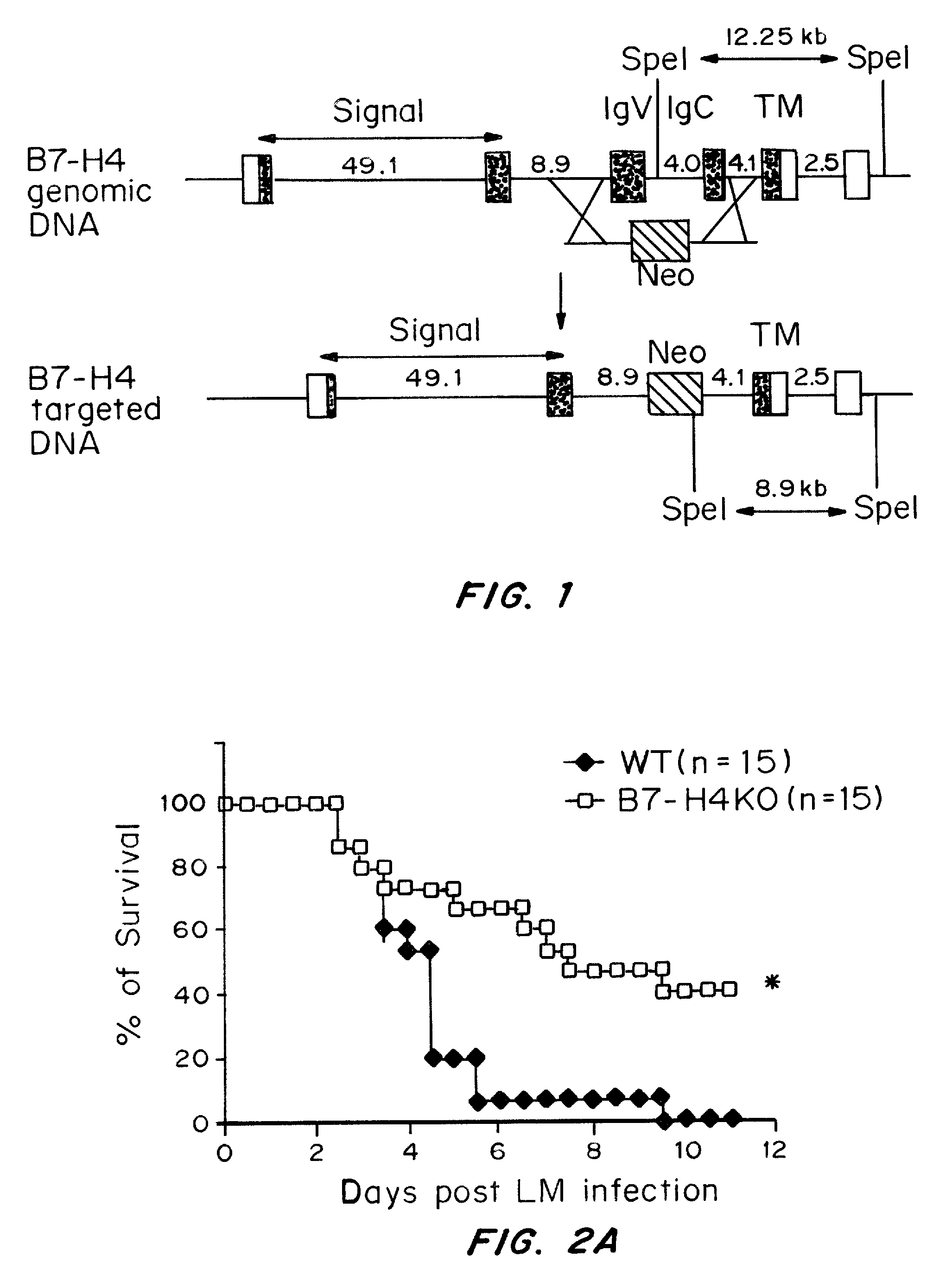
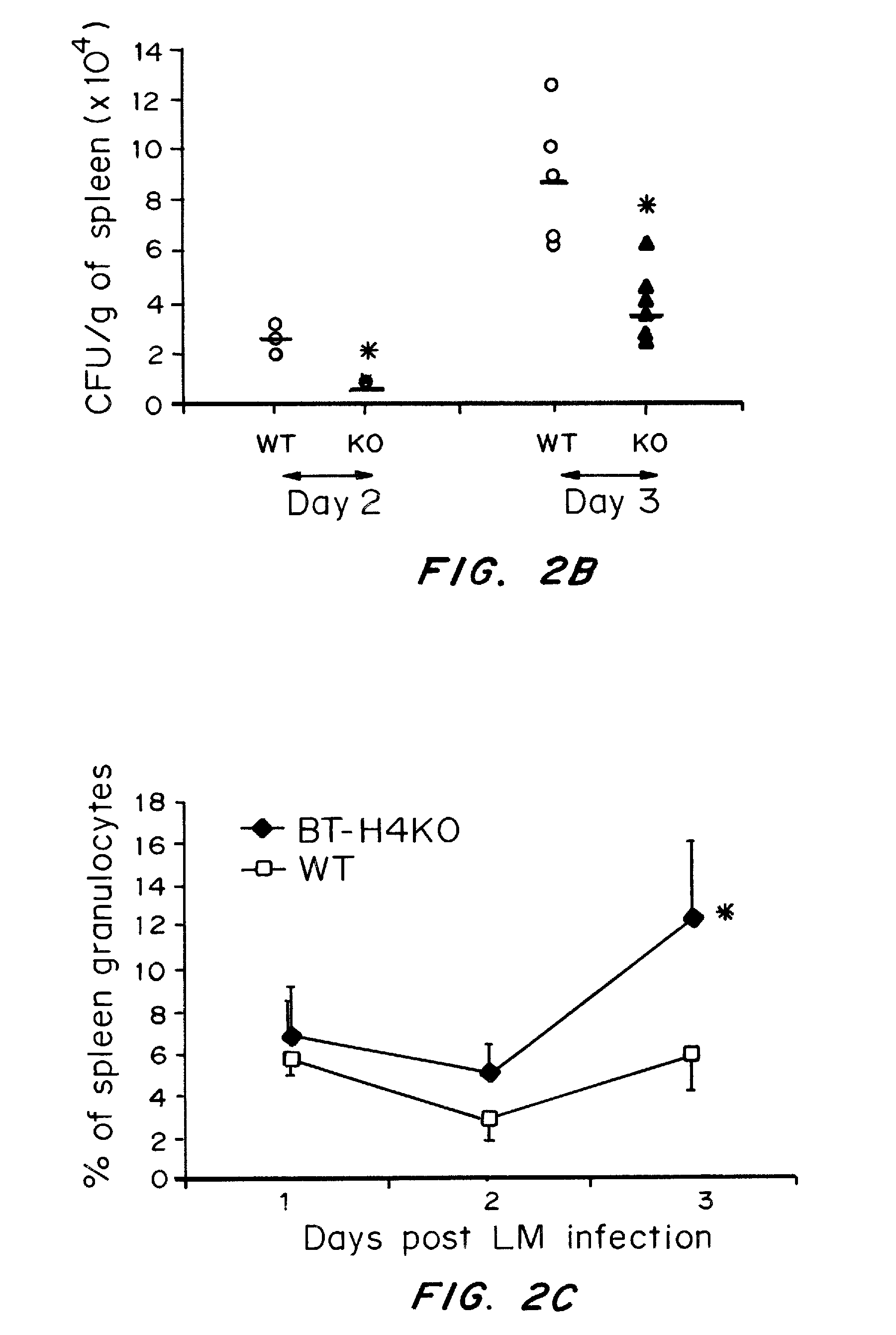
InactiveUS20080160036A1Reduce and inhibit and mitigate inflammatory responseEfficient methodAntibacterial agentsSenses disorderAutoimmune diseaseT cell immunity
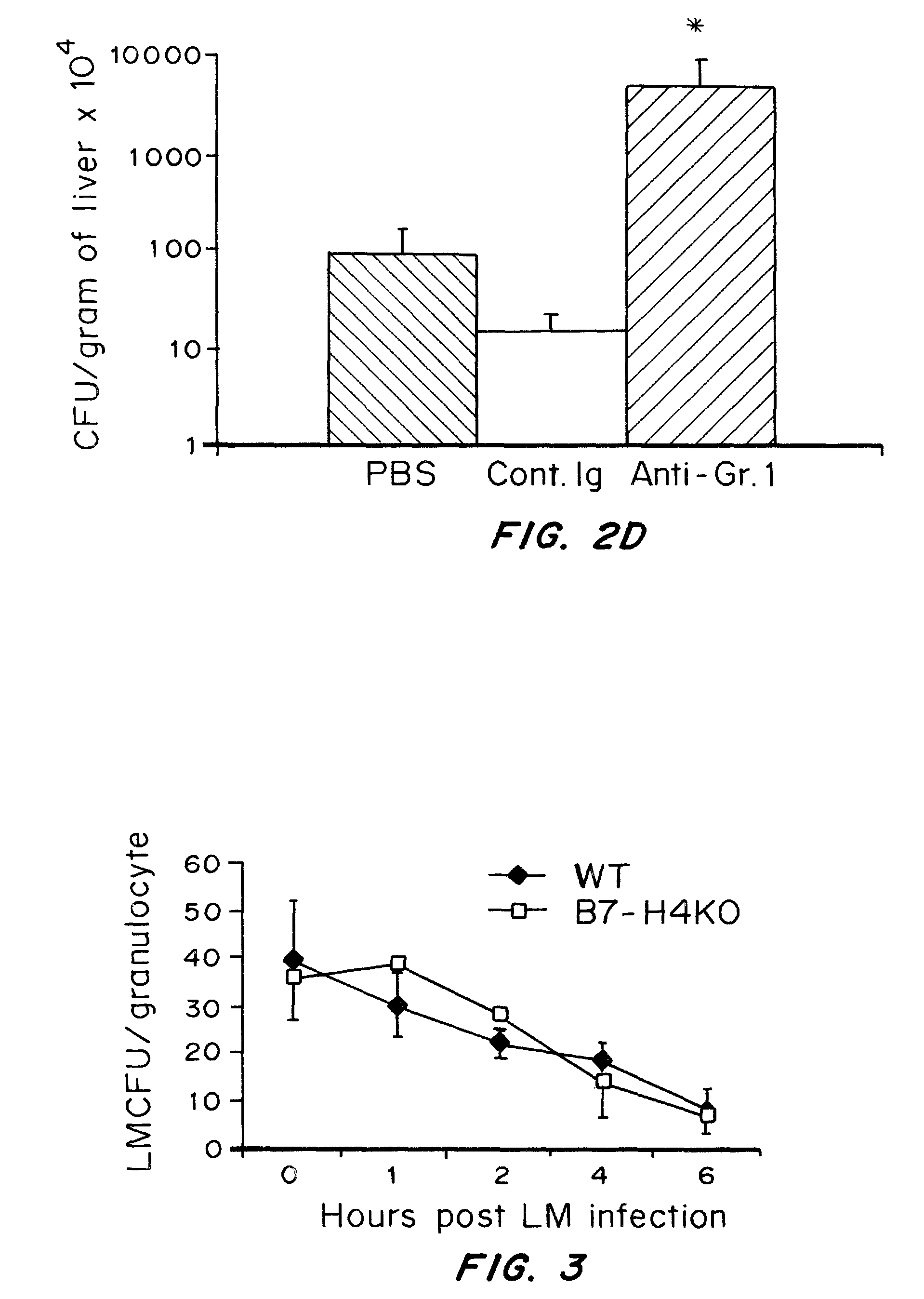
Compositions containing soluble B7-H4 (sH4) antagonists in an amount effective to reduce, inhibit, or mitigate an inflammatory response in an individual and methods for the treatment or prophylaxis of inflammatory disorders and autoimmune diseases or disorders are provided. Soluble H4 has been discovered to interfere with B7-H4 activity including B7-H4's activity as an inhibitor of T cell immunity. Thus, interference of sH4 biological activity is an effective method to restore B7-H4 activity and thereby provide an effective method for treating inflammatory diseases or disorders including autoimmune diseases or disorders.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Compositions and methods for treating inflammation and auto-immune diseases
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
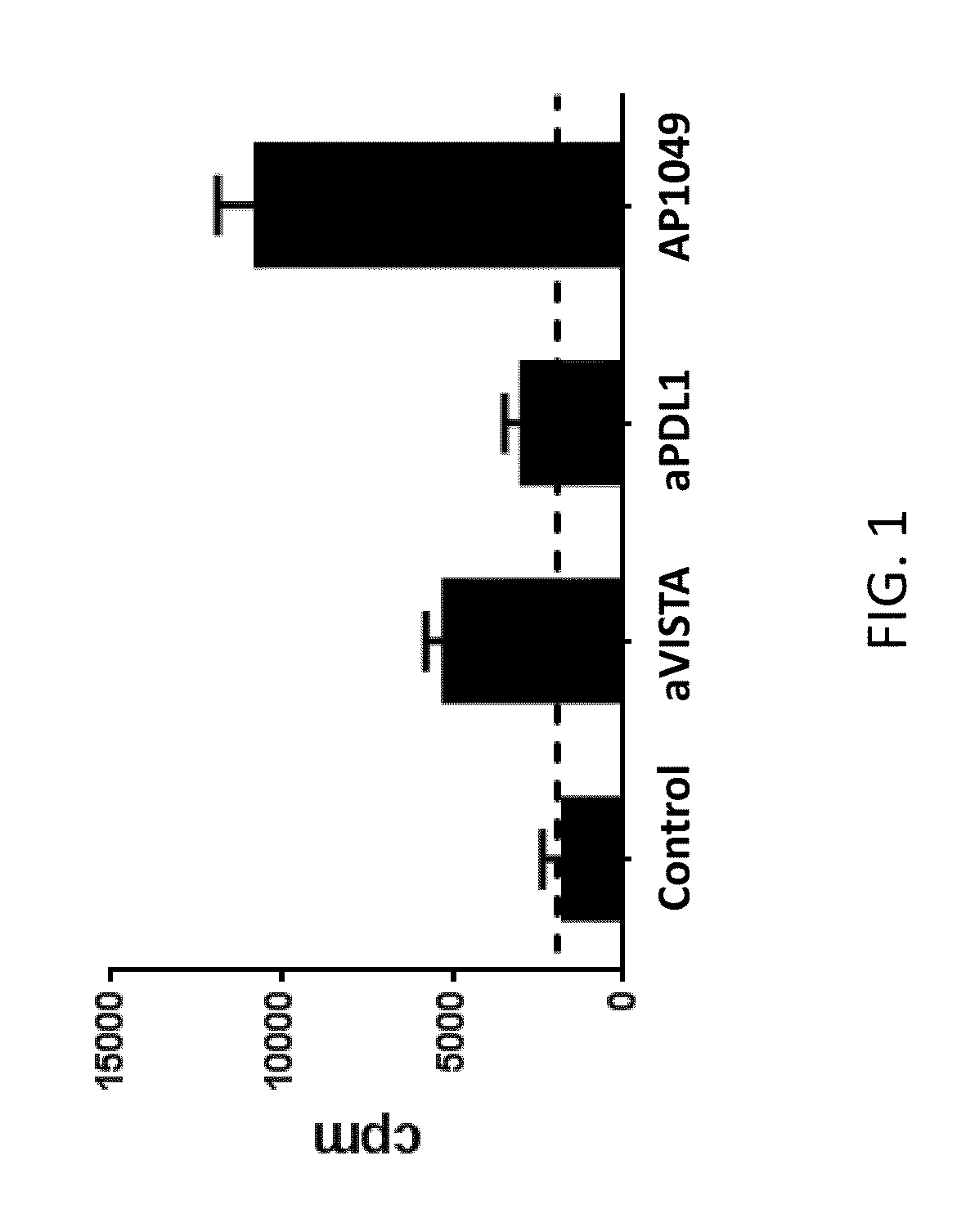
VISTA Antagonist and Methods of Use
The present invention is directed to synergic or additive therapies comprising the administration of a VISTA antagonist and a PD-1, PD-L1 or POD-L3 antagonist; or the combination of a VISTA agonist and a -1, PD-L1 or POD-L3 agonist which combinations respectively elicit an additive or synergistic effect at promoting T cell immunity or inhibiting T cell immunity, i.e., CD4, CD8 or Th1 immunity. The agonists and antagonists may be in the same or separate compositions and may be administered together or separately administered in either order.
Owner:NOELLE RANDOLPH J +4
Innate immune system-directed vaccines
InactiveUS20080226667A1Improve responseEnhancing adaptive immune responseBacterial antigen ingredientsViral antigen ingredientsAntigenInnate immune system
The present invention provides novel vaccines, methods for the production of such vaccines and methods of using such vaccines. The novel vaccines of the present invention combine both of the signals necessary to activate native T-cells—a specific antigen and the co-stimulatory signal—leading to a robust and specific T-cell immune response.
Owner:YALE UNIV
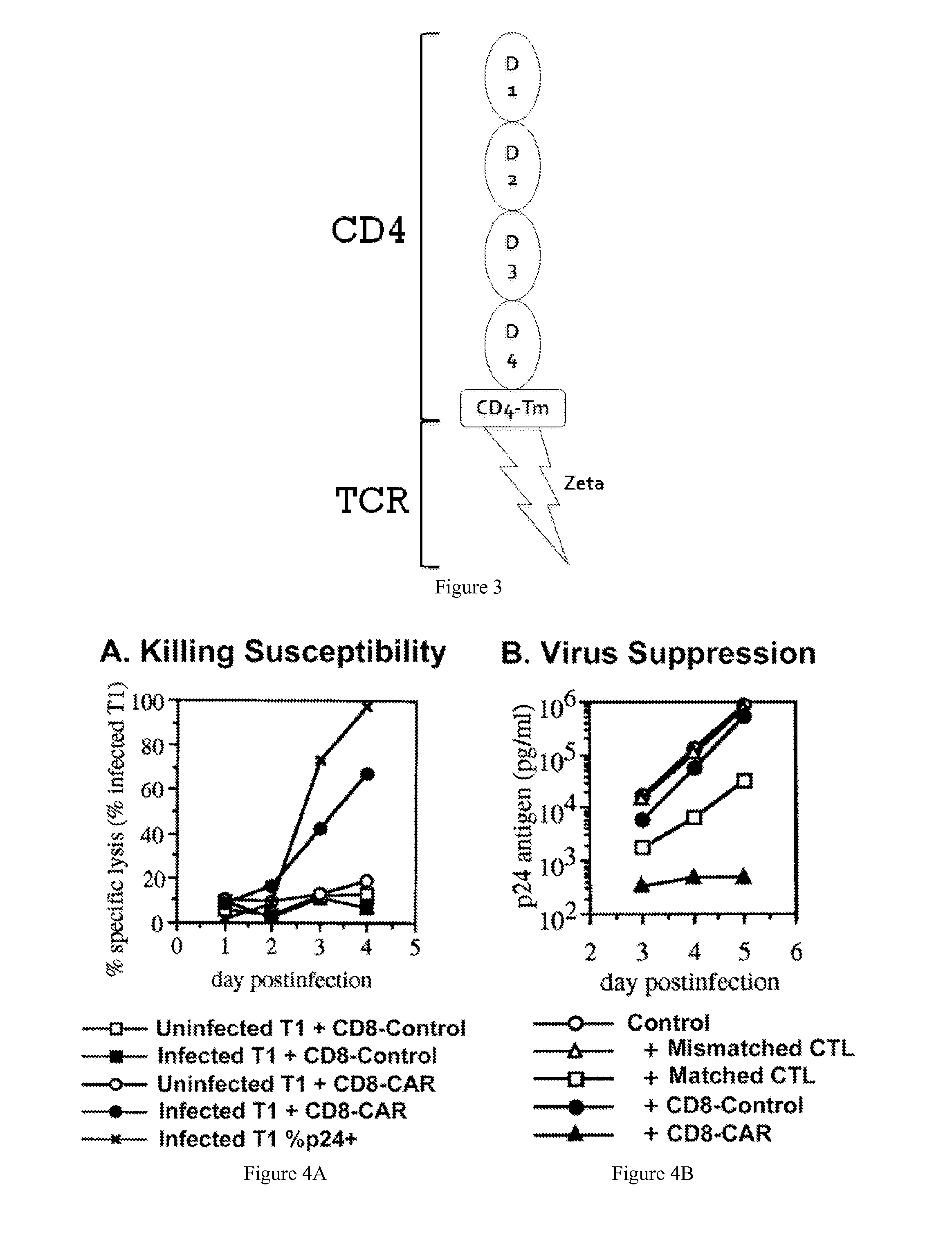
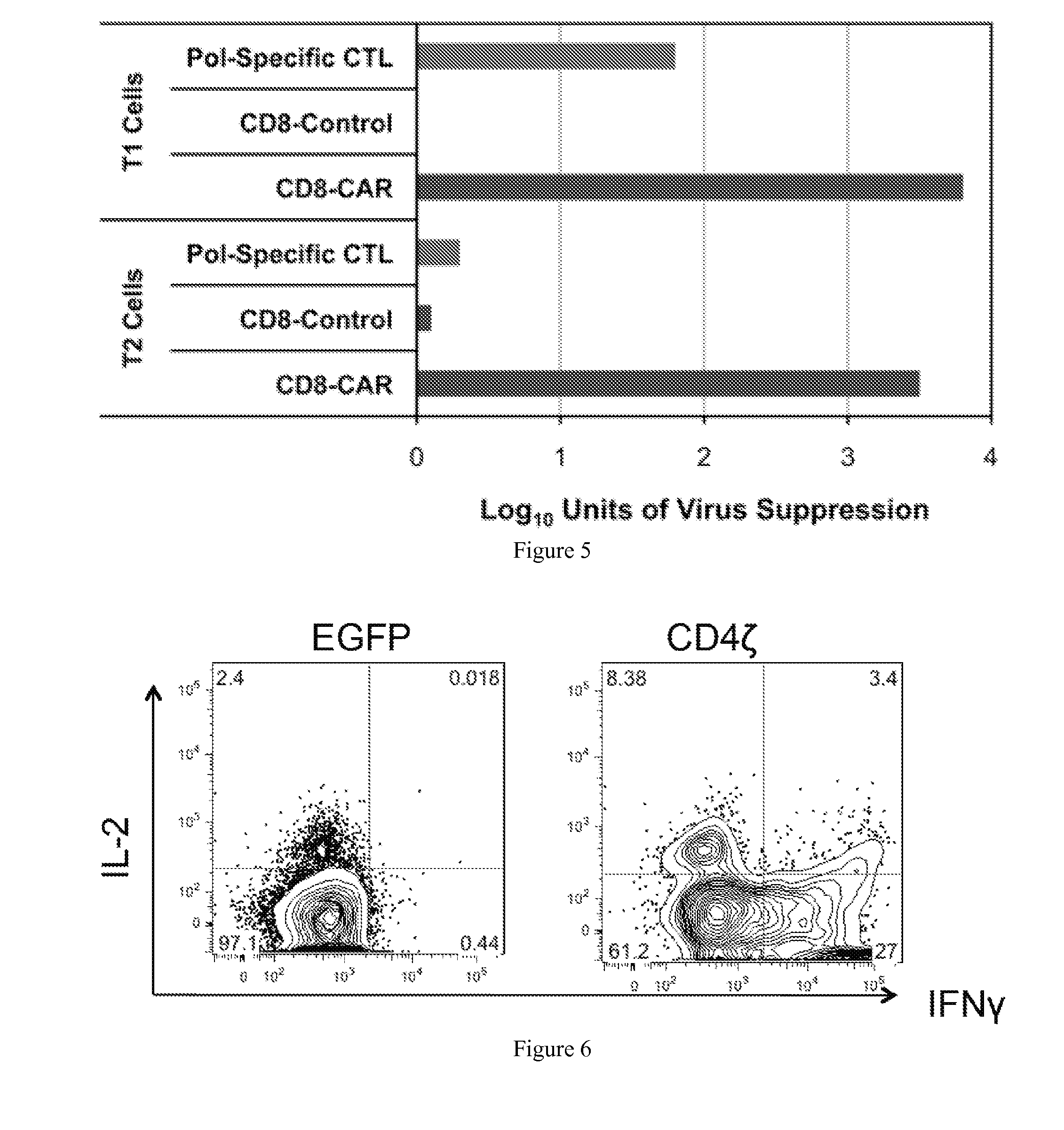
Engineering Antiviral T Cell Immunity through Stem Cells and Chimeric Antigen Receptors
The HIV-specific cytotoxic T lymphocyte (CTL) response is a critical component in controlling HIV replication and is an important part of the ultimate failure to eradicate the virus. Disclosed herein are methods for genetically enhancing the HIV-specific CTL response to allow long-term viral suppression or viral clearance. Human hematopoietic stem cells (HSCs) were genetically modified such that they differentiate into mature CTLs that will kill HIV infected cells. As disclosed herein, the functional effector cells are not human leukocyte antigen (HLA)-restricted. As disclosed herein, stem cells are transduced with non HLA-restricted chimeric antigen receptors (CARs) that allow the recognition of HIV or HIV infected cells when expressed by a CTL.
Owner:RGT UNIV OF CALIFORNIA
Dendritic cell-specific antibody conjugate comprising anti-CD40 monoclonal antibodies conjugated to HIV-1 Gag/Nef
ActiveUS9109011B2Improve efficiencyIncrease flexibilityBiocidePeptide/protein ingredientsCyclin D1Dendritic cell
The present invention includes compositions and methods for making and using a vaccine that includes a DC-specific antibody or fragment thereof to which an engineered Gag antigen is attached to form an antibody-antigen complex, wherein the Gag antigen is less susceptible to proteolytic degradation by eliminating one or more proteolytic sites or a DC-specific antibody or fragment thereof to which an engineered Nef antigen is attached to form an antibody-antigen complex, wherein the Nef antigen comprises one or more codon usage optimization that increase antibody-antigen complex secretion, or both, wherein the vaccine is able to elicit an HIV-specific T cell immune response to Gag p17, Gag p24, Nef and / or Cyclin D1.
Owner:BAYLOR RES INST
Innate immune system-directed vaccines
InactiveUS20070160623A1Stimulating innate immune responseEnhancing adaptive immune responseAntibody mimetics/scaffoldsTissue cultureAntigenInnate immune system
The present invention provides novel vaccines, methods for the production of such vaccines and methods of using such vaccines. The novel vaccines of the present invention combine both of the signals necessary to activate native T-cells—a specific antigen and the co-stimulatory signal—leading to a robust and specific T-cell immune response.
Owner:MEDZHITOV RUSLAN M +1
Immuno-Oncolytic Therapies
ActiveUS20160235793A1Reduced responseImprove immunityViral antigen ingredientsUnknown materialsAbnormal tissue growthInterleukin-18 binding protein
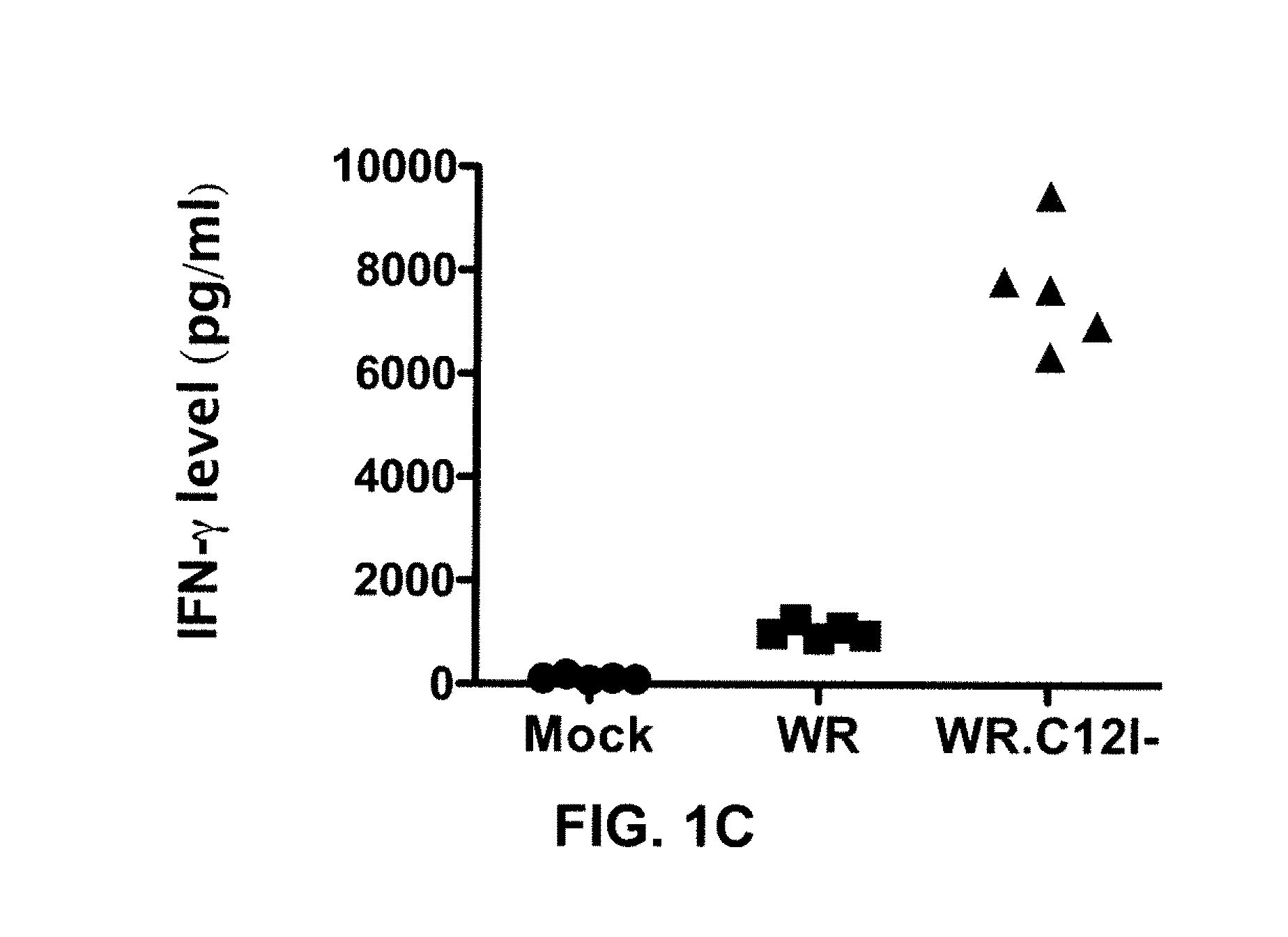
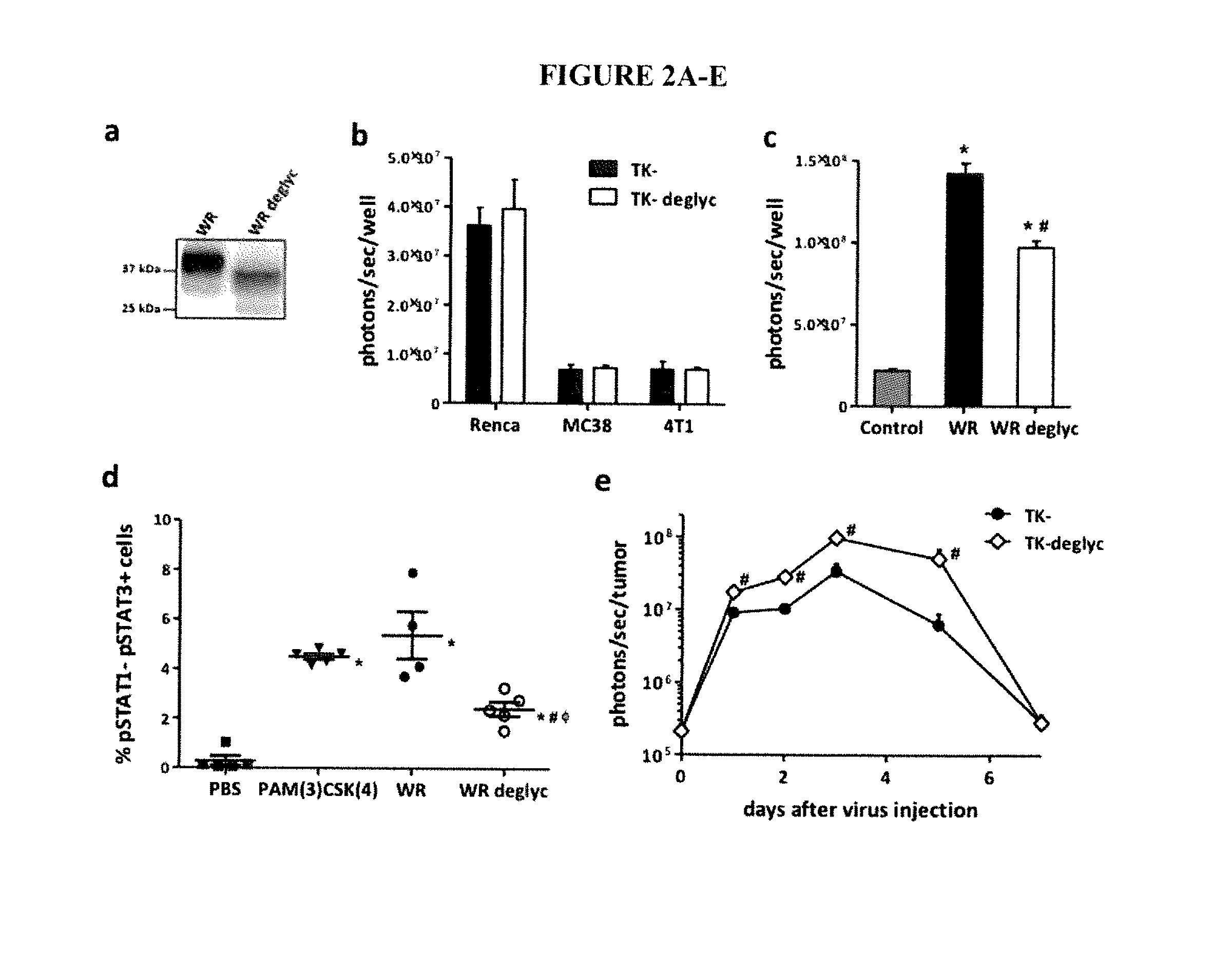
The present invention relates to oncolytic vaccinia viruses which have been modified to promote anti-tumor immunity and / or reduce host immunity and / or antibody response against the virus. It is based, at least in part, on the discovery that oncolytic vaccinia virus (i) bearing a genome deletion of a gene that reduces T cell immunity (interleukin-18 binding protein); (ii) treated with a sialidase enzyme which is believed to reduce TLR2 activation and therefore the antibody response; (iii) carrying a gene that enhances cytotoxic T lymphocyte induction (e.g., TRIF) and / or (iv) reduces tumor myeloid-derived suppressor cells by reducing prostaglandin E2 reduces tumor growth. Accordingly, the present invention provides for immunooncolytic vaccinia viruses and methods of using them in the treatment of cancers.
Owner:UNIVERSITY OF PITTSBURGH
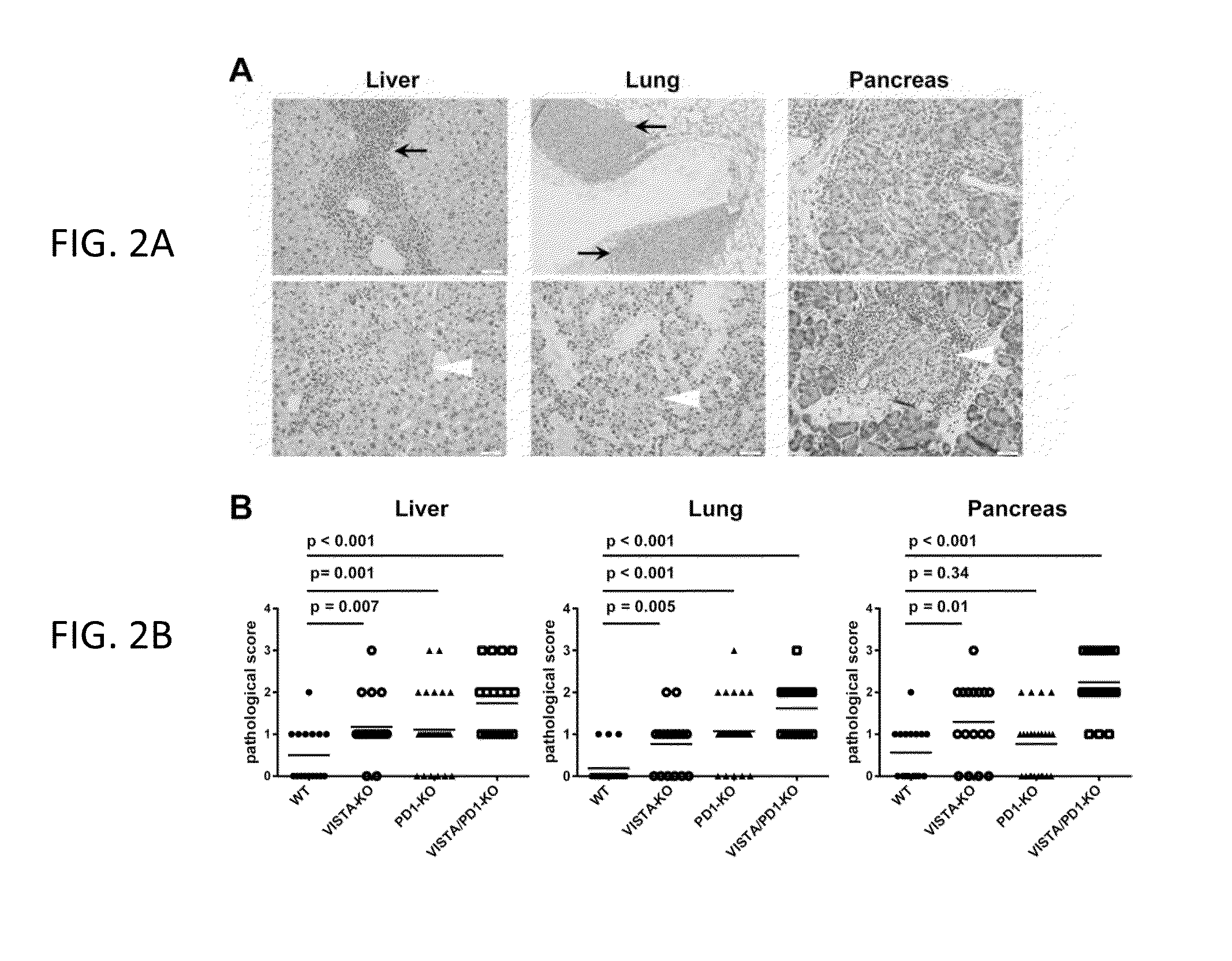
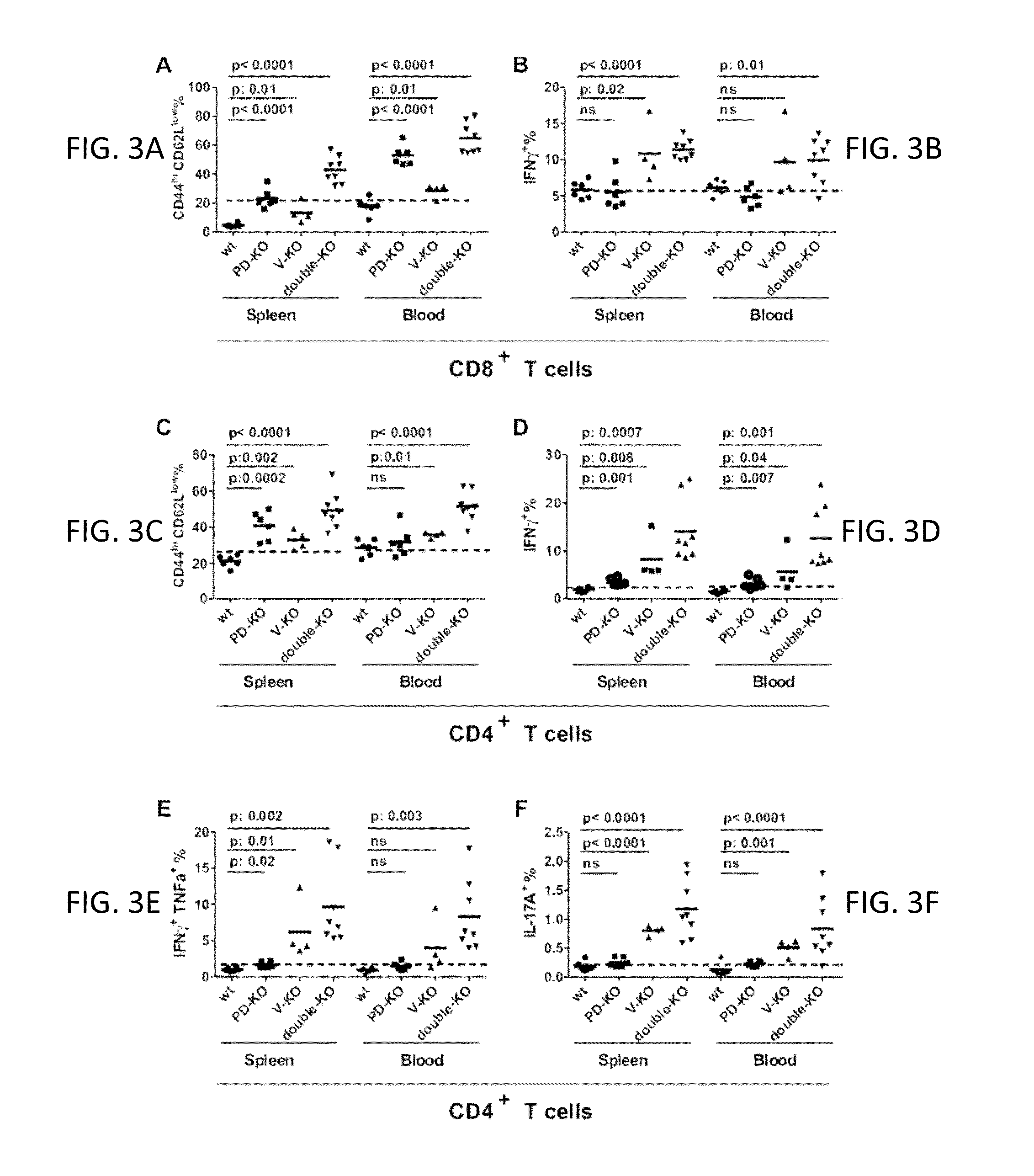
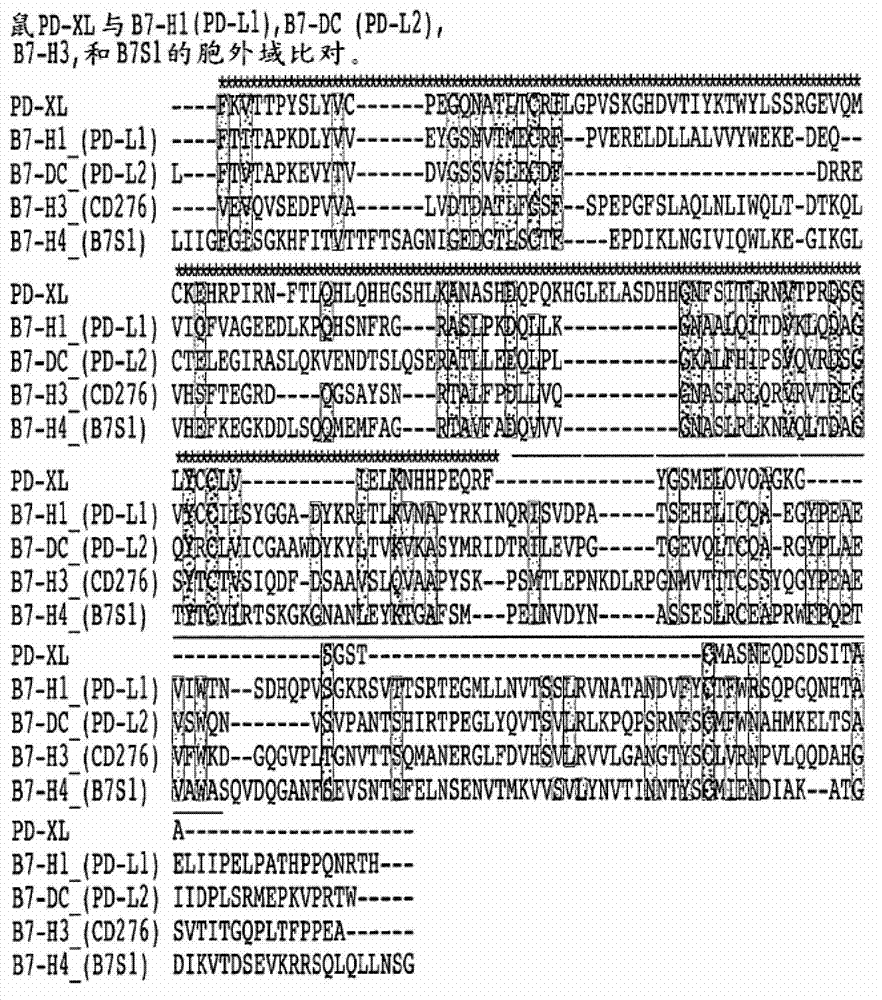
VISTA regulatory T cell mediator protein, VISTA binding agents and use thereof
The present invention relates to a novel regulatory T cell protein. This protein, designated PD-L3 OR VISTA resembles members of the PD-L1 family, identified a novel and structurally-distinct, Ig-superfamily inhibitory ligand, whose extracellular domain bears homology to the B7 family ligand PD-L1. This molecule is designated as PD-L3 OR VISTA or V-domain Immunoglobulin Suppressor of T cell Activation (VISTA). Expression of VISTA is primarily within the hematopoietic compartment and is highly regulated on myeloid APCs and T cells. Therapeutic intervention of the VISTA inhibitory pathway represents a novel approach to modulate T cell-mediated immunity for the treatment of a wide variety of cancers, e.g., ovarian, bladder cancer and melanomas. Also, VISTA proteins, especially multimeric VISTA proteins and antibodies may be used to suppress T cell immunity in autoimmune disease, allergy, infection and inflammatory conditions, e.g. multiple sclerosis and artritic conditions such as RA.
Owner:TRUSTEES OF DARTMOUTH COLLEGE THE
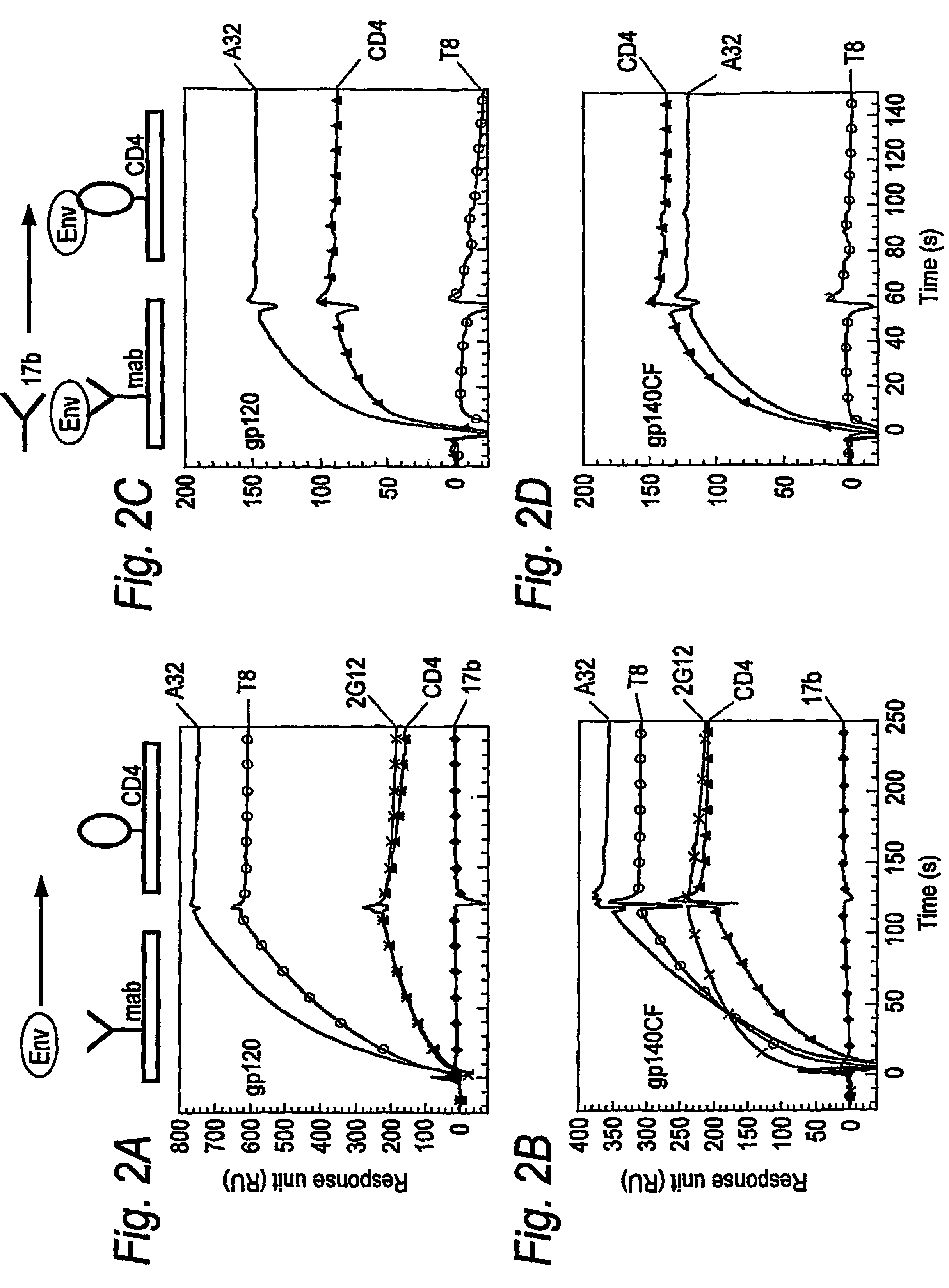
Nucleic acids encoding modified human immunodeficiency virus type 1 (HIV-1) group M consensus envelope glycoproteins
The present invention relates, in general, to an immunogen and, in particular, to an immunogen for inducing antibodies that neutralizes a wide spectrum of HIV primary isolates and / or to an immunogen that induces a T cell immune response. The invention also relates to a method of inducing anti-HIV antibodies, and / or to a method of inducing a T cell immune response, using such an immunogen. The invention further relates to nucleic acid sequences encoding the present immunogens.
Owner:UNIVERSITY OF ALABAMA +2
Vaccine immunotherapy for immune suppressed patients
A method for overcoming mild to moderate immune suppression includes the steps of inducing production of naïve T-cells and restoring T-cell immunity. A method of vaccine immunotherapy includes the steps of inducing production of naïve T-cells and exposing the naïve T-cells to endogenous or exogenous antigens at an appropriate site. Additionally, a method for unblocking immunization at a regional lymph node includes the steps of promoting differentiation and maturation of immature dendritic cells at a regional lymph node and allowing presentation of processed peptides by resulting mature dendritic cells, thus, for example, exposing tumor peptides to T-cells to gain immunization of the T-cells. Further, a method of treating cancer and other persistent lesions includes the steps of administering an effective amount of a natural cytokine mixture as an adjuvant to endogenous or exogenous administered antigen to the cancer or other persistent lesions.
Owner:BROOKLYN IMMUNOTHERAPEUTICS LLC
Immunotherapy for reversing immune suppression
InactiveUS7731945B2Restoring T cell immunityPromoting differentiationAntibacterial agentsOrganic active ingredientsRegion lymph nodeDendritic cell
A method for overcoming immune suppression includes the steps of inducing production of naïve T cells and restoring T cell immunity. A method of vaccine immunotherapy includes the steps of inducing production of naïve T cells and exposing the naïve T cells to endogenous or exogenous antigens at an appropriate site. Additionally, a method for unblocking immunization at a regional lymph node includes the steps of promoting differentiation and maturation of immature dendritic cells at a regional lymph node and allowing presentation of processed peptides by resulting mature dendritic cells, thus, for example, exposing tumor peptides to T cells to gain immunization of the T cells. Further, a method of treating cancer and other persistent lesions includes the steps of administering an effective amount of a natural cytokine mixture as an adjuvant to endogenous or exogenous administered antigen to the cancer or other persistent lesions; preferably the natural cytokine mixture is administered in combination with thymosin α1.
Owner:BROOKLYN IMMUNOTHERAPEUTICS LLC
Modified HIV-1 clade C envelope glycoprotein immunogens comprising deletions in the gp120/gp41 cleavage site and gp41 fusion domain
The present invention relates, in general, to an immunogen and, in particular, to an immunogen for inducing antibodies that neutralize a wide spectrum of HIV primary isolates and / or to an immunogen that induces a T cell immune response. The invention also relates to a method of inducing anti-HIV antibodies, and / or to a method of inducing a T cell immune response, using such an immunogen. The invention further relates to nucleic acid sequences encoding the present immunogens.
Owner:DUKE UNIV
Antigen composition used for immunodetection of tuberculosis infected cell and application thereof
ActiveCN104628833AIncrease reaction strengthHigh strengthAntibacterial agentsBacterial antigen ingredientsInfected cellEpitope
The invention provides an antigen composition used for immunodetection of a tuberculosis infected cell. The antigen composition comprises mycobacterium tuberculosis CFP-10, ESAT-6 and Rv3873 antigens and / or antigen epitope peptides derived from the antigens. In an optimized embodiment, the antigen composition comprises all the antigen epitope peptides contained in the mycobacterium tuberculosis CFP-10, ESAT-6 and Rv3873 antigens. The invention also provides an application of the antigen composition to preparation of detection reagents used for detecting specific T cell immune response caused by tuberculosis infection in vitro. After lymphocytes of human or animals are stimulated by the antigen composition, cell factors secreted by a tuberculosis specific T cell are detected.
Owner:ICDC CHINA CDC +1
Enhancing the t-cells stimulatory capacity of human antigen presenting cells and their use in vaccination
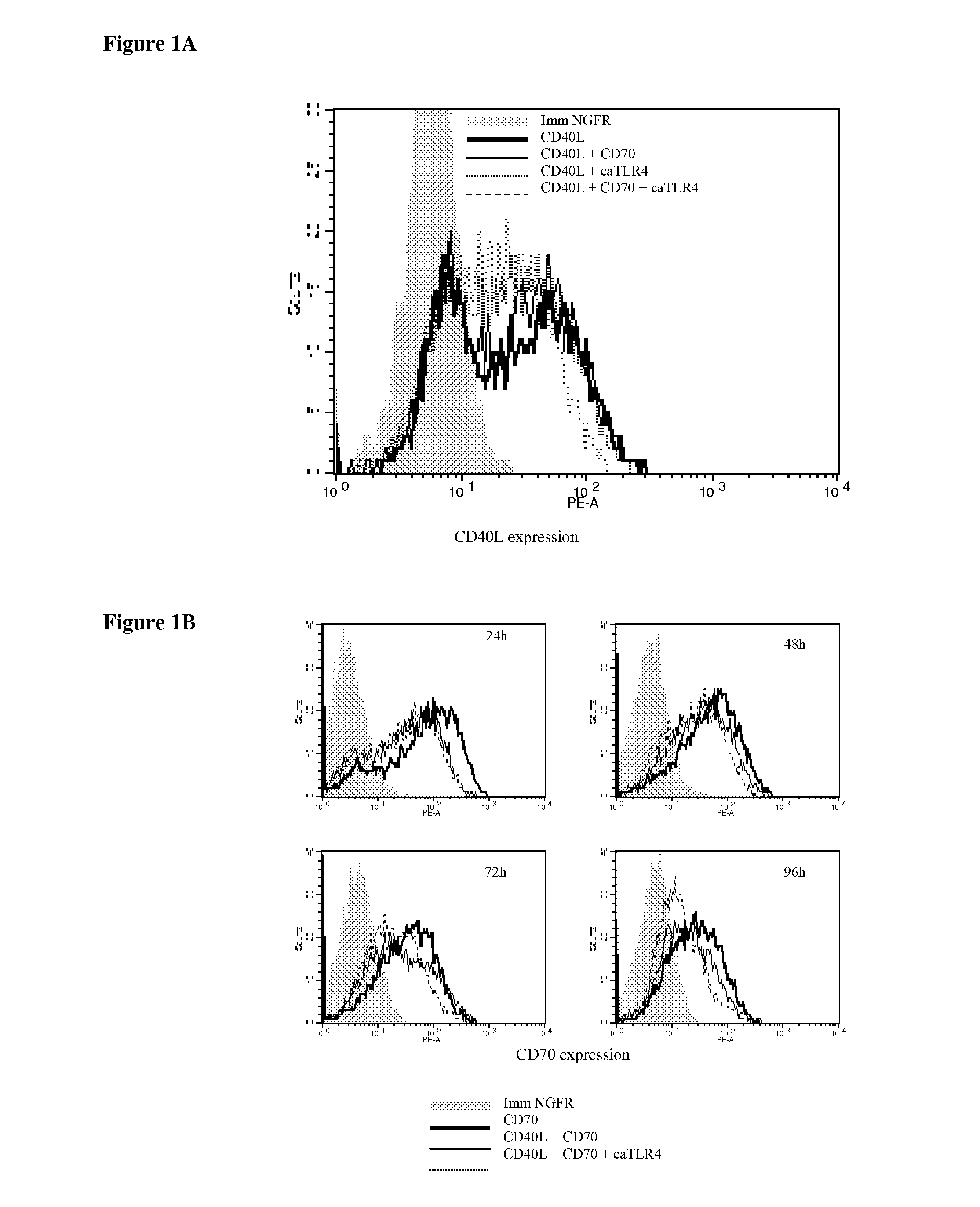
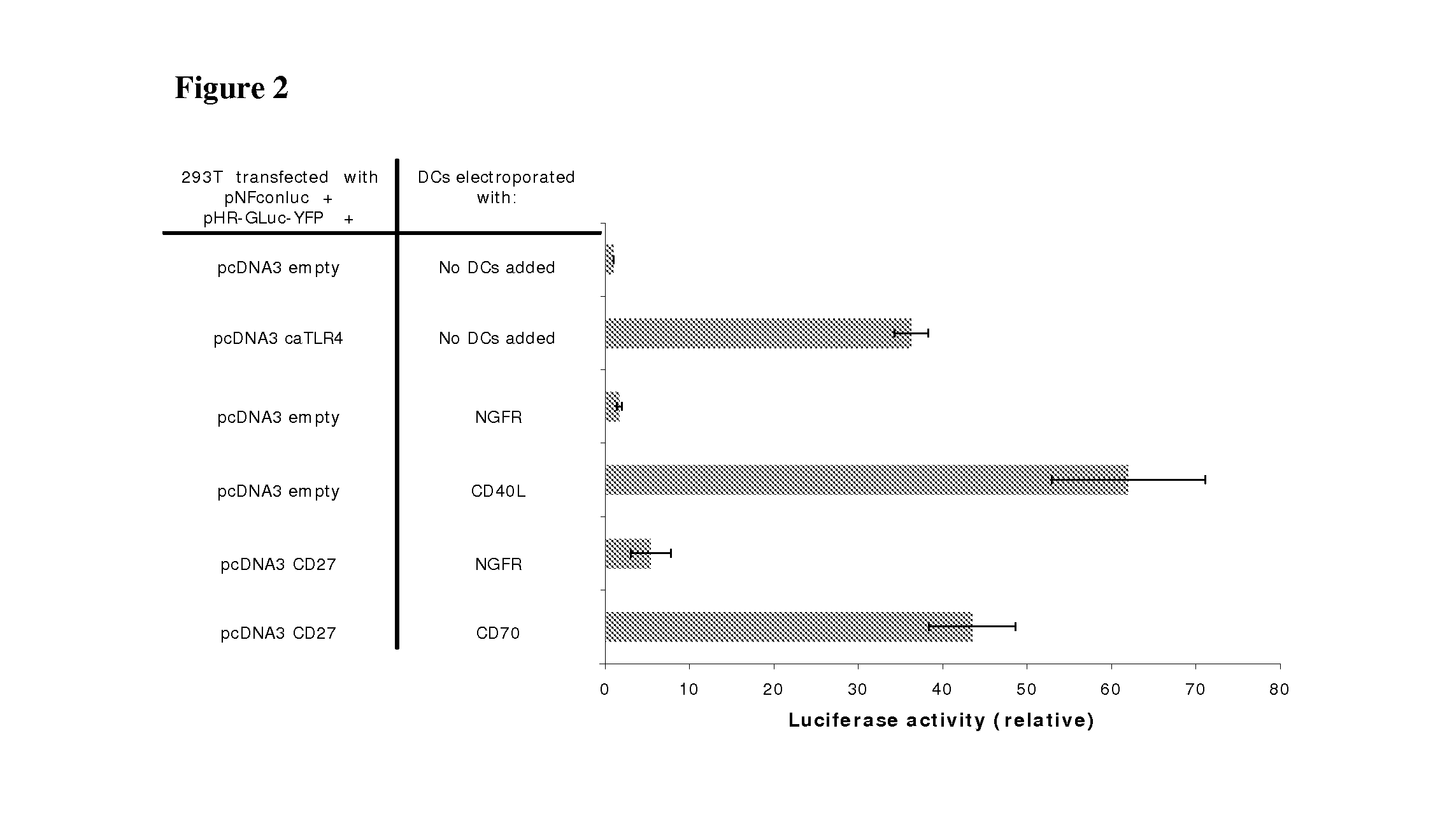
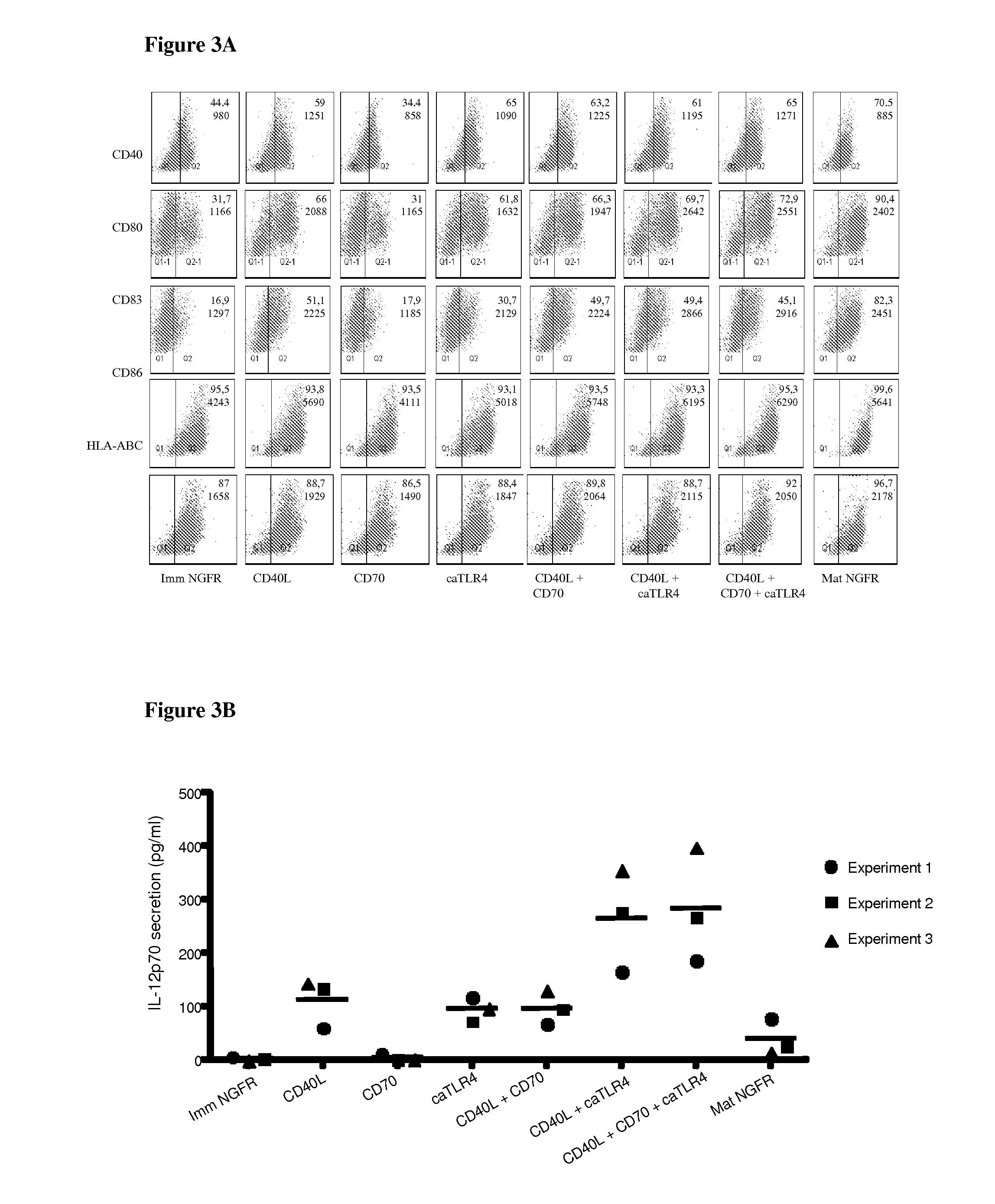
With the current invention, we provide new methods of enhancing the T-cell stimulatory capacity of human dendritic cells (DCs) and their use in cancer vaccination. The method comprises the introduction of different molecular adjuvants to human DCs through transfection with at least two mRNA or DNA molecules encoding markers selected from the group of: CD40L, CD70, constitutively active TLR4 (caTLR4), IL-12p70, EL-selectin, CCR7 and / or 4-1 BBL; or in combination with inhibition of SOCS, A20, PD-L1 and / or STAT3 expression, for example through siRNA transfection. We could show a clear increase in the immunostimulatory capacity of DCs obtained in this way, enabling them to elicit an unexpectedly high T-cell immune response in vitro. Introduction of at least two of the above molecules, in combination with a tumor-specific antigen enables the DCs to elicit a significant host-mediated T-cell immune response in vivo against the tumor antigen and thus makes them very attractive in the manufacturing of anti-cancer vaccines.
Owner:VRIJE UNIV BRUSSEL
Methods of inducing immune responses through the administration of auxtrophic attenuated dal/dat double mutant Listeria strains
The present invention includes a method of eliciting a T-cell immune response to an antigen in mammal. The method of eliciting a T-cell immune response includes administering mammal an auxotrophic attenuated strain of listeria which expresses the antigen. The auxotrophic attenuated strain of listeria includes a mutation in at least one gene whose protein product is essential for growth of bacteria.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Car expression vector and car-expressing T cells
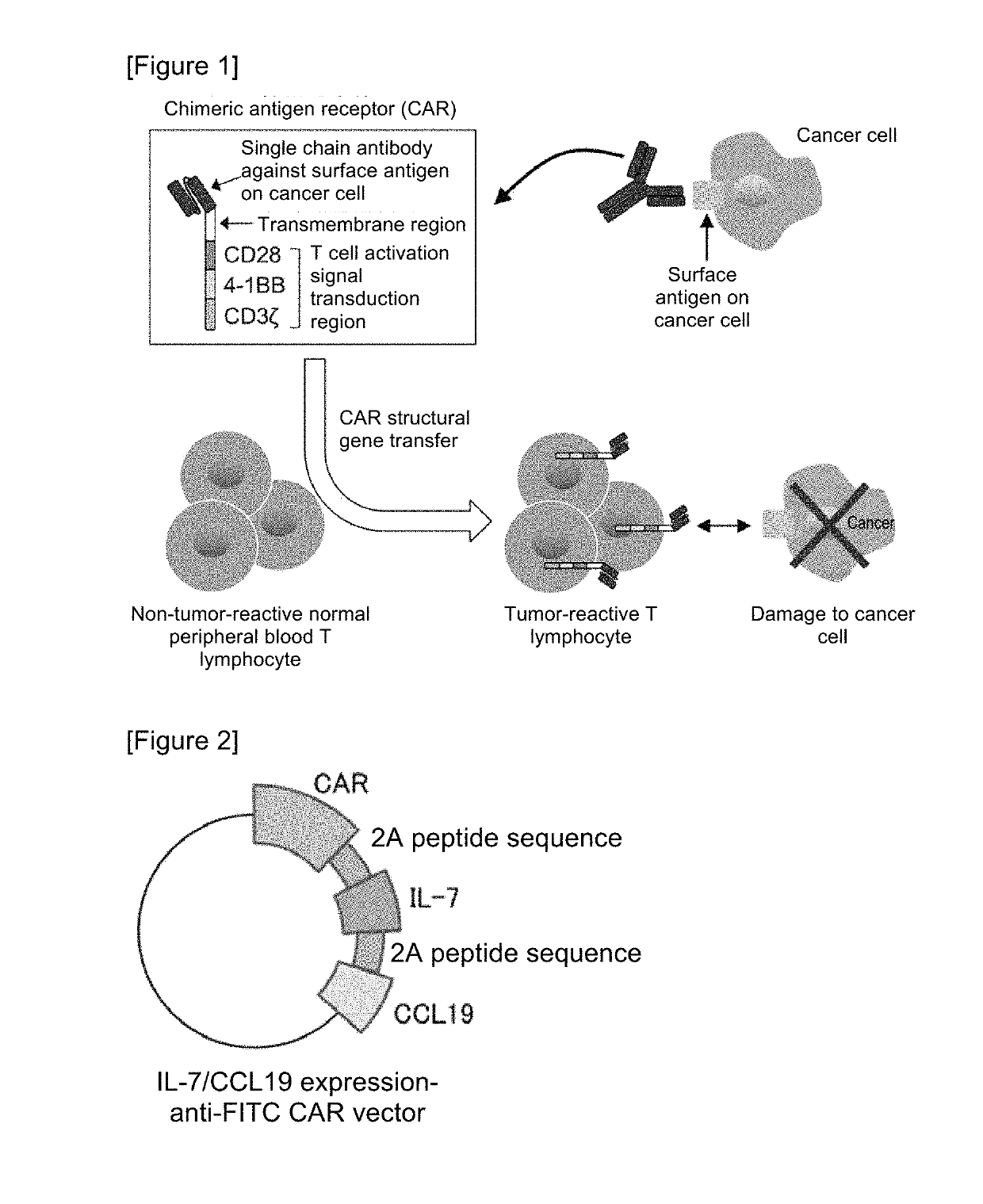
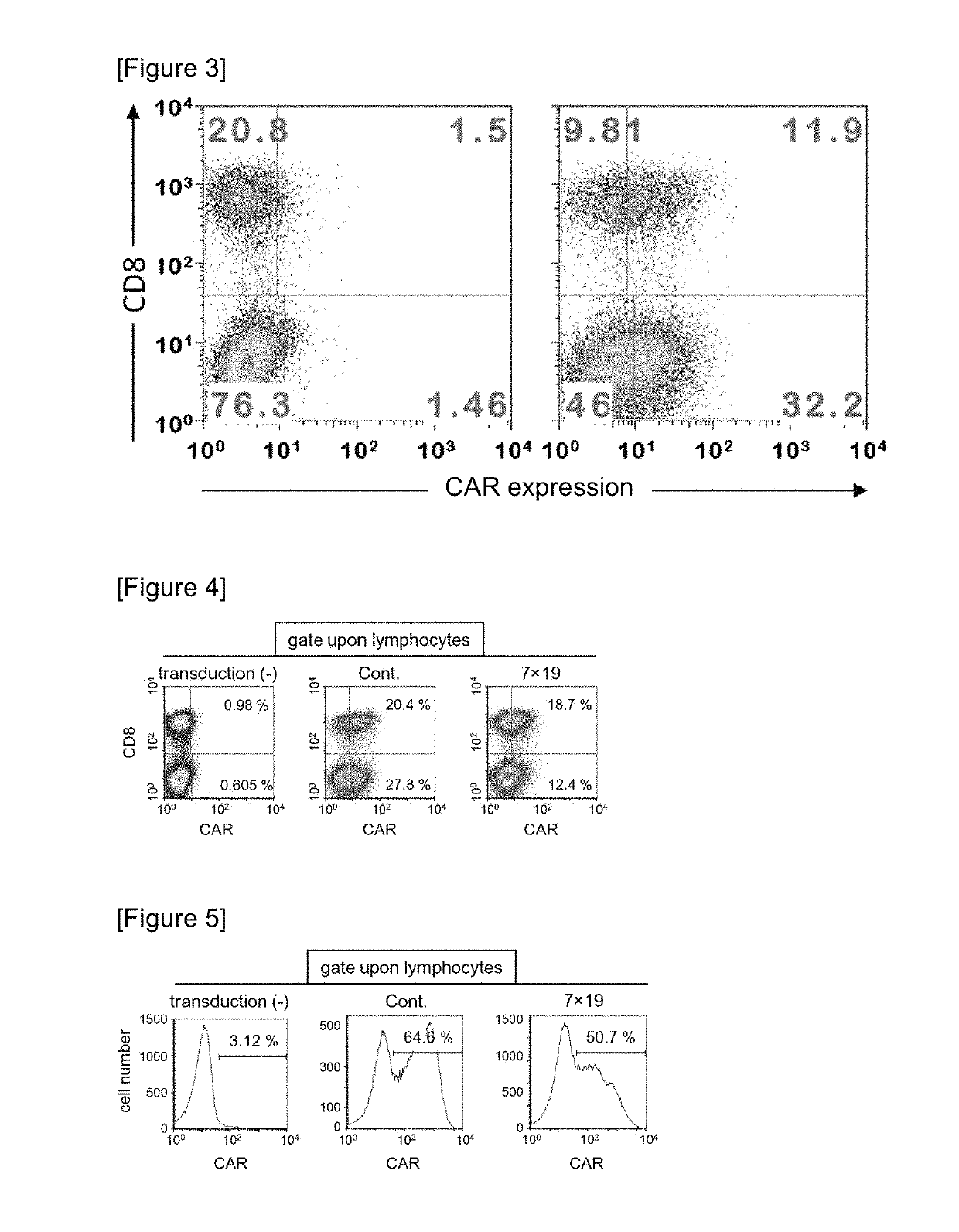
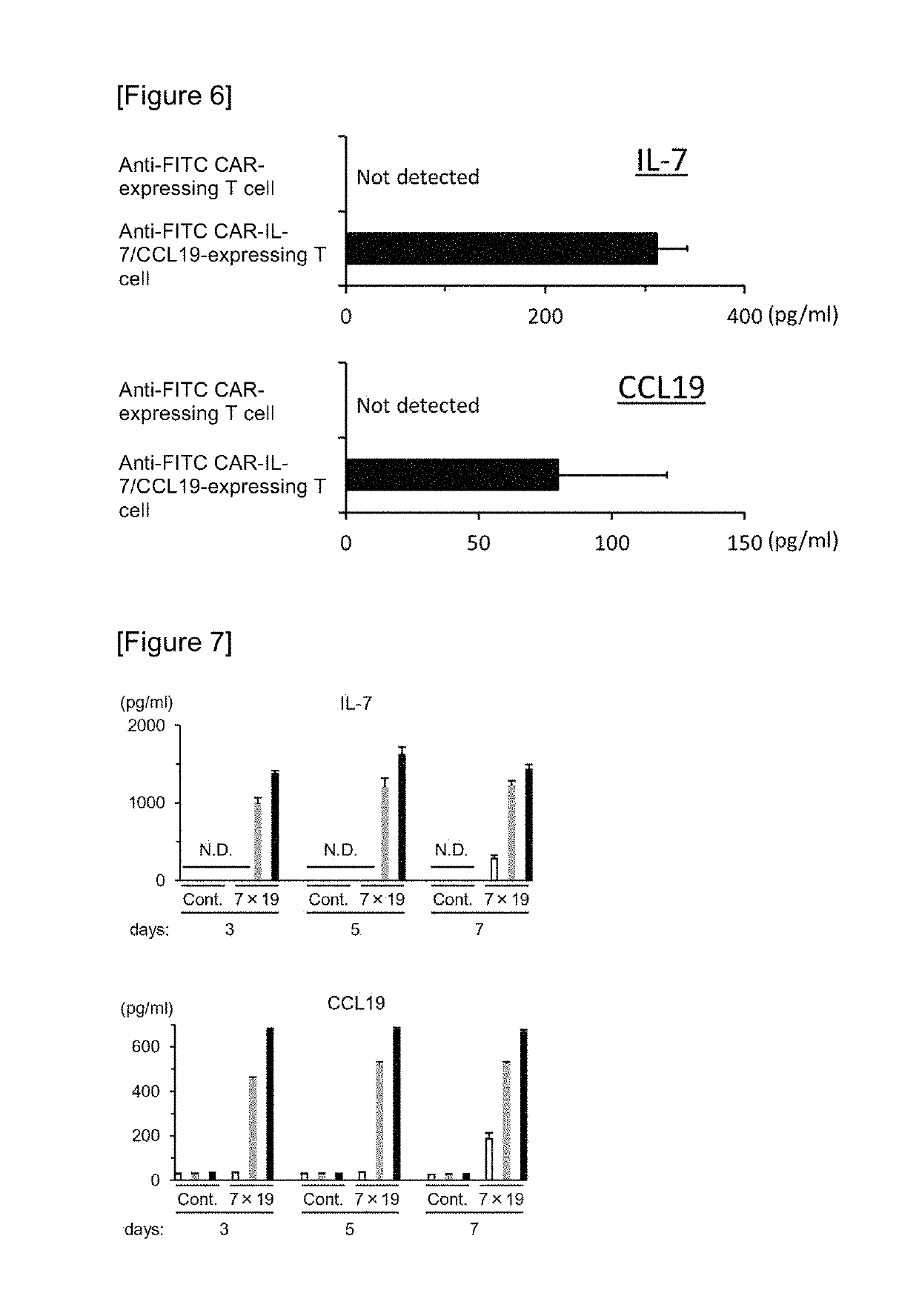
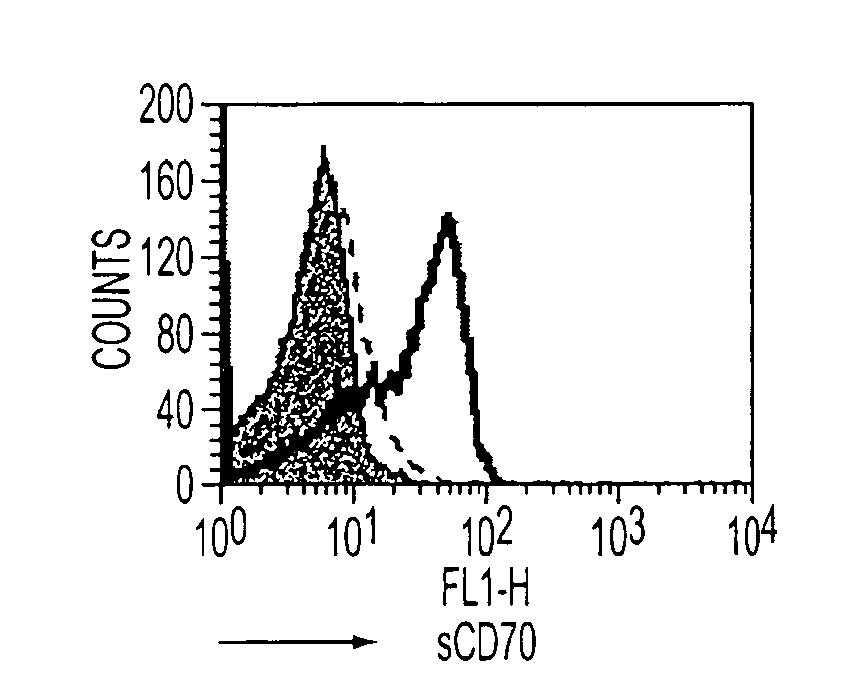
ActiveUS10316102B2Good treatment effectVirusesAntibody mimetics/scaffoldsAntigen receptorsChimeric antigen receptor
Owner:YAMAGUCHI UNIV
Human immune therapies using a CD27 agonist alone or in combination with other immune modulators
ActiveUS20110033449A1Profound effect on anti-tumor immunityImprove scalabilityAntibacterial agentsAntimycoticsAutoimmune responsesImmune modulator
Owner:UNIV OF SOUTHAMPTON
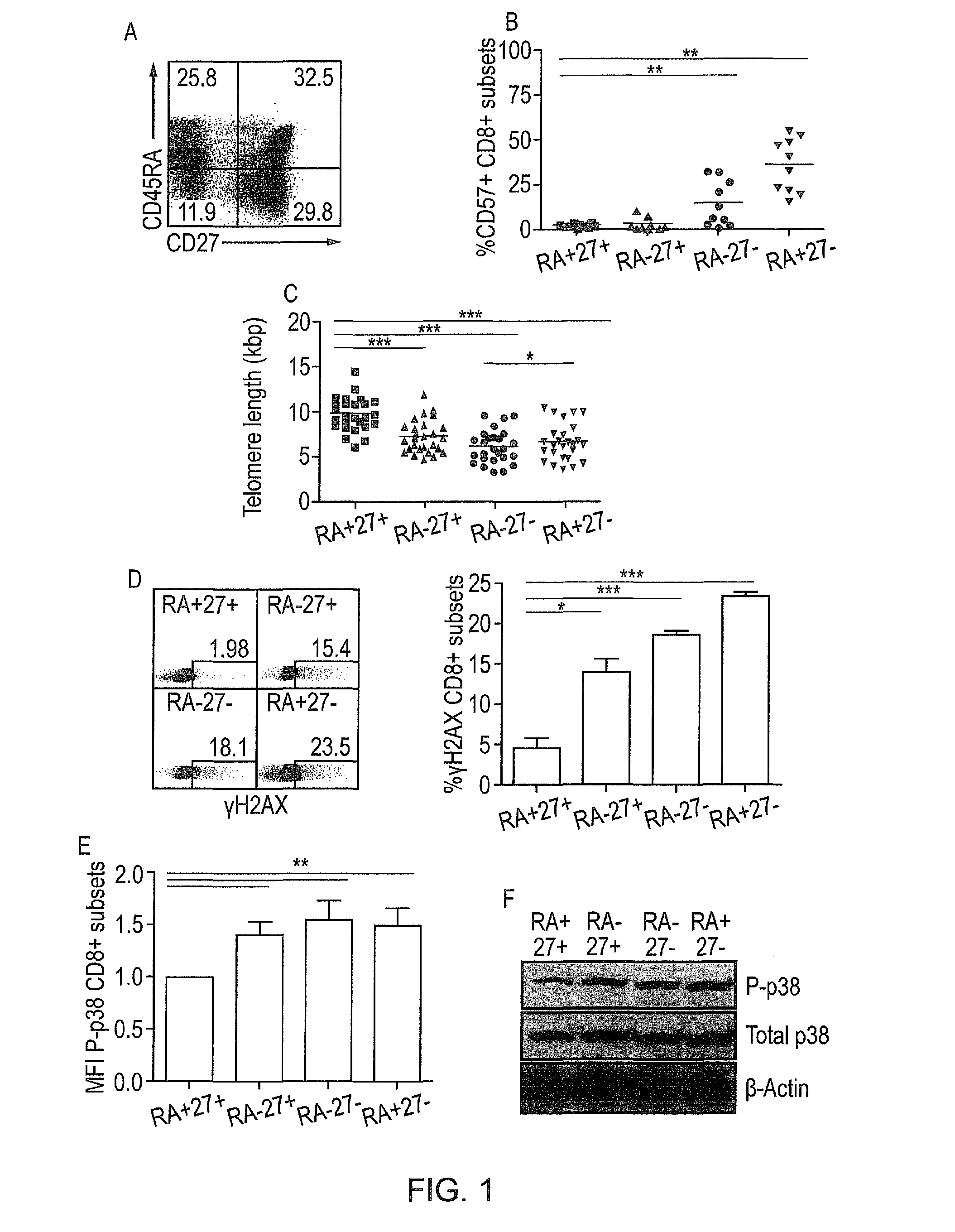
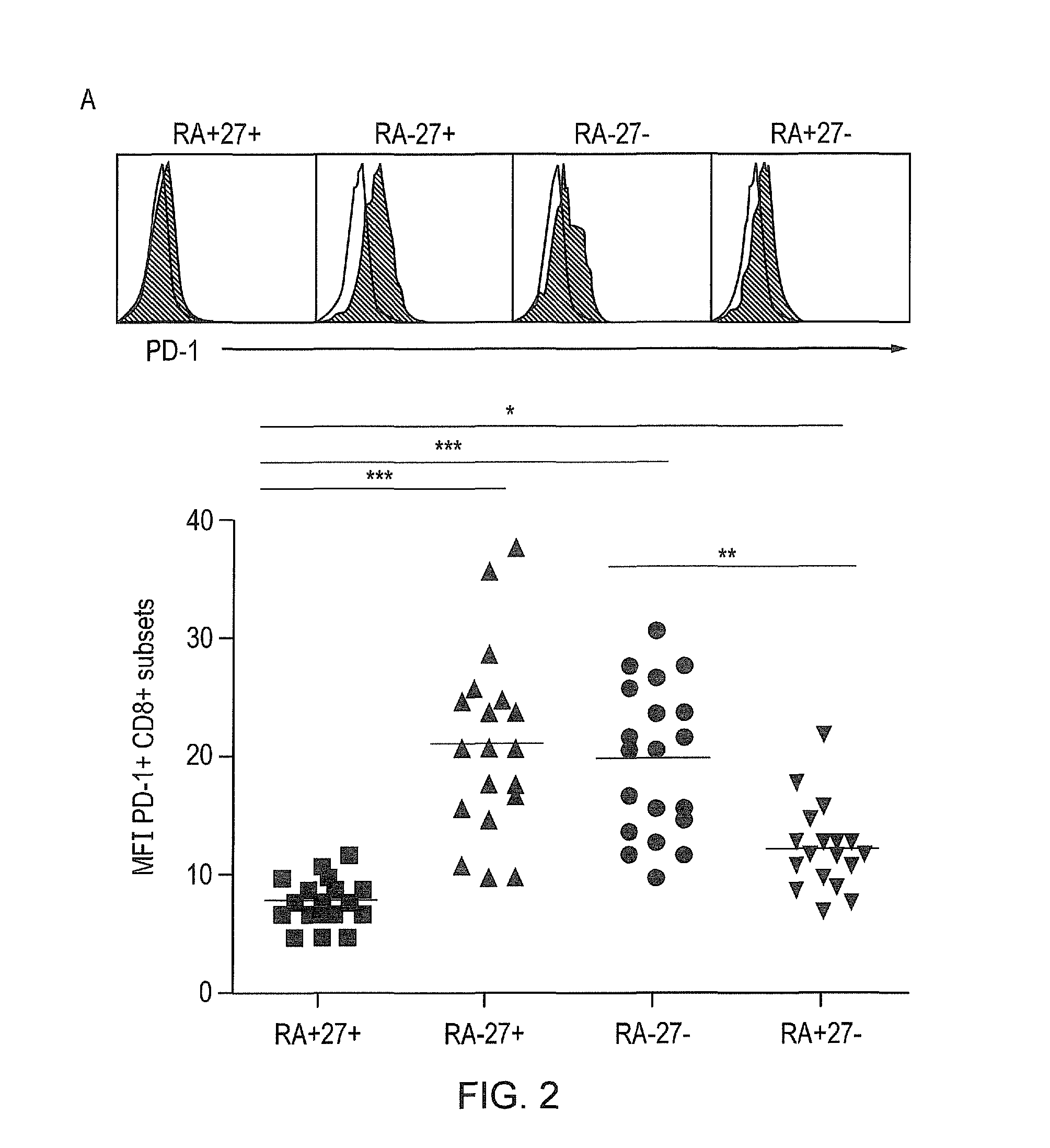
Method to improve the immune function of t cells
InactiveUS20150017185A1Enhance immune responseEnhancing function of T cellOrganic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsCentral Memory T-CellP38map kinase
The present invention provides a method for enhancing the immune function of a memory T cell which comprises the step of coinhibting signalling via an inhibitory receptor which regulates T cell exhaustion and via the p38 MAP kinase signalling pathway in the T cell, and a method for treating and / or preventing an immune condition in a subject, which comprises the step of enhancing the immune function of a memory T cell in the subject by such a method. There is also provided a pharmaceutical composition or kit comprising an agent capable of inhibiting signalling via an inhibitory receptor which regulates T cell exhaustion, such as PD-1, and an agent capable of inhibiting the p38 MAP kinase signalling pathway.
Owner:UCL BUSINESS PLC
Immunotherapy for reversing immune suppression
InactiveUS20110044941A1Promoting differentiation and maturationRestore immunityAntibacterial agentsOrganic active ingredientsRegion lymph nodeDendritic cell
A method for overcoming immune suppression includes the steps of inducing production of naïve T cells and restoring T cell immunity. A method of vaccine immunotherapy includes the steps of inducing production of naïve T cells and exposing the naïve T cells to endogenous or exogenous antigens at an appropriate site. Additionally, a method for unblocking immunization at a regional lymph node includes the steps of promoting differentiation and maturation of immature dendritic cells at a regional lymph node and allowing presentation of processed peptides by resulting mature dendritic cells, thus, for example, exposing tumor peptides to T cells to gain immunization of the T cells. Further, a method of treating cancer and other persistent lesions includes the steps of administering an effective amount of a natural cytokine mixture as an adjuvant to endogenous or exogenous administered antigen to the cancer or other persistent lesions; preferably the natural cytokine mixture is administered in combination with thymosin α1.
Owner:IMMUNO RX
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com