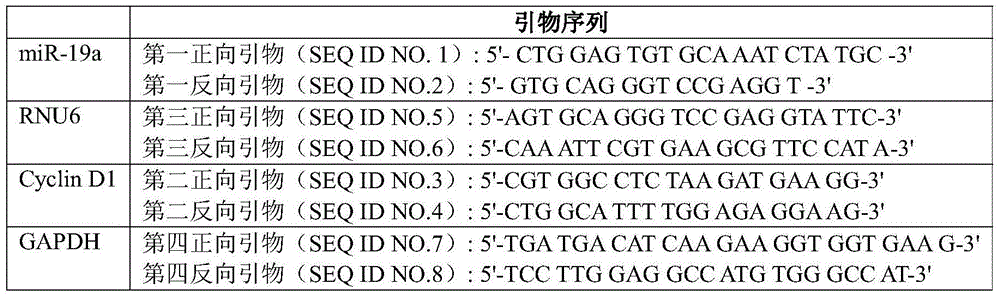
Patents
Literature
58 results about "Cyclin D1" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cyclin D1 is a protein that in humans is encoded by the CCND1 gene.
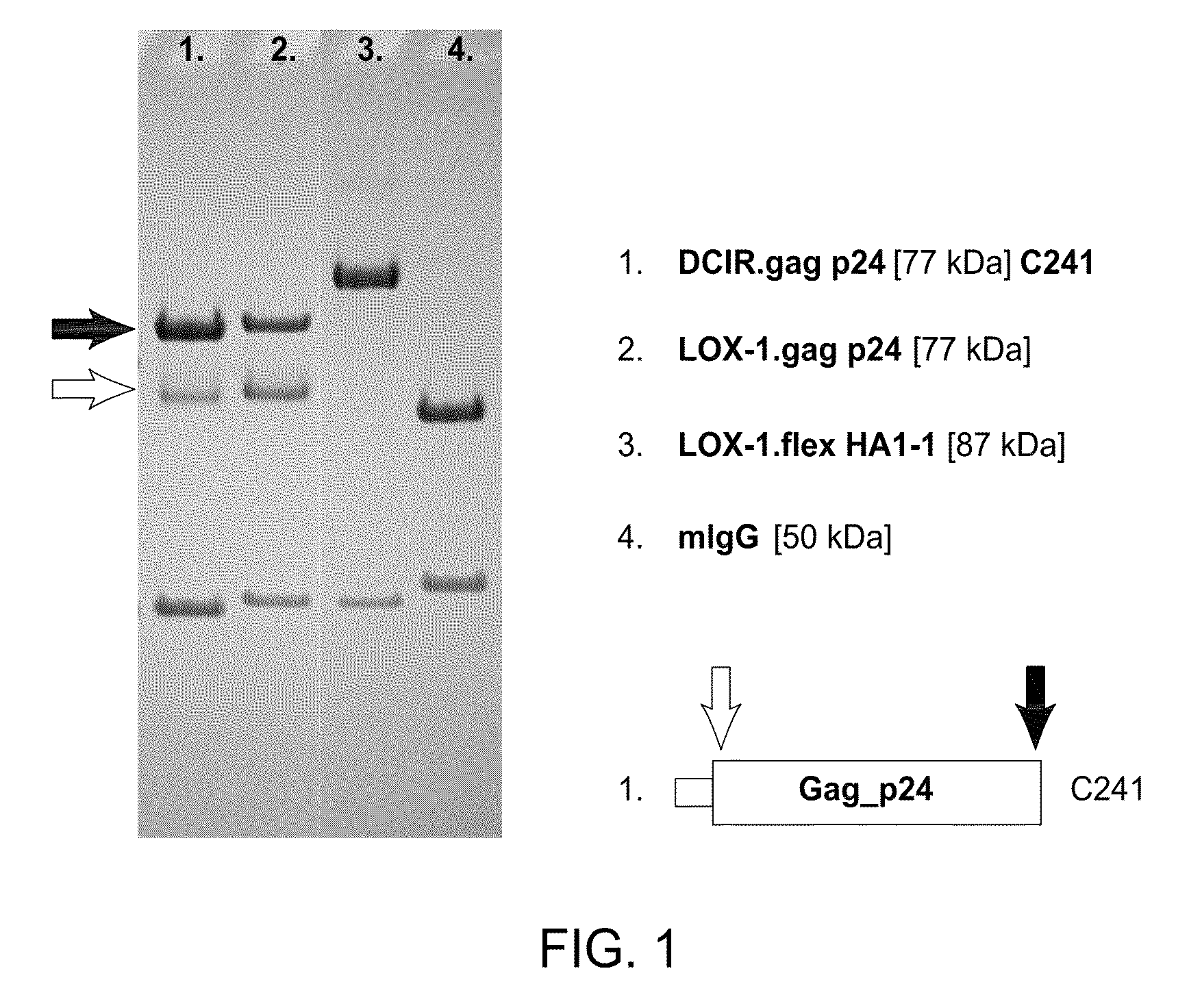
HIV vaccine based on targeting maximized gag and nef to dendritic cells
ActiveUS20100135994A1Improve efficiencyIncrease flexibilityBiocidePeptide/protein ingredientsCyclin D1Dendritic cell
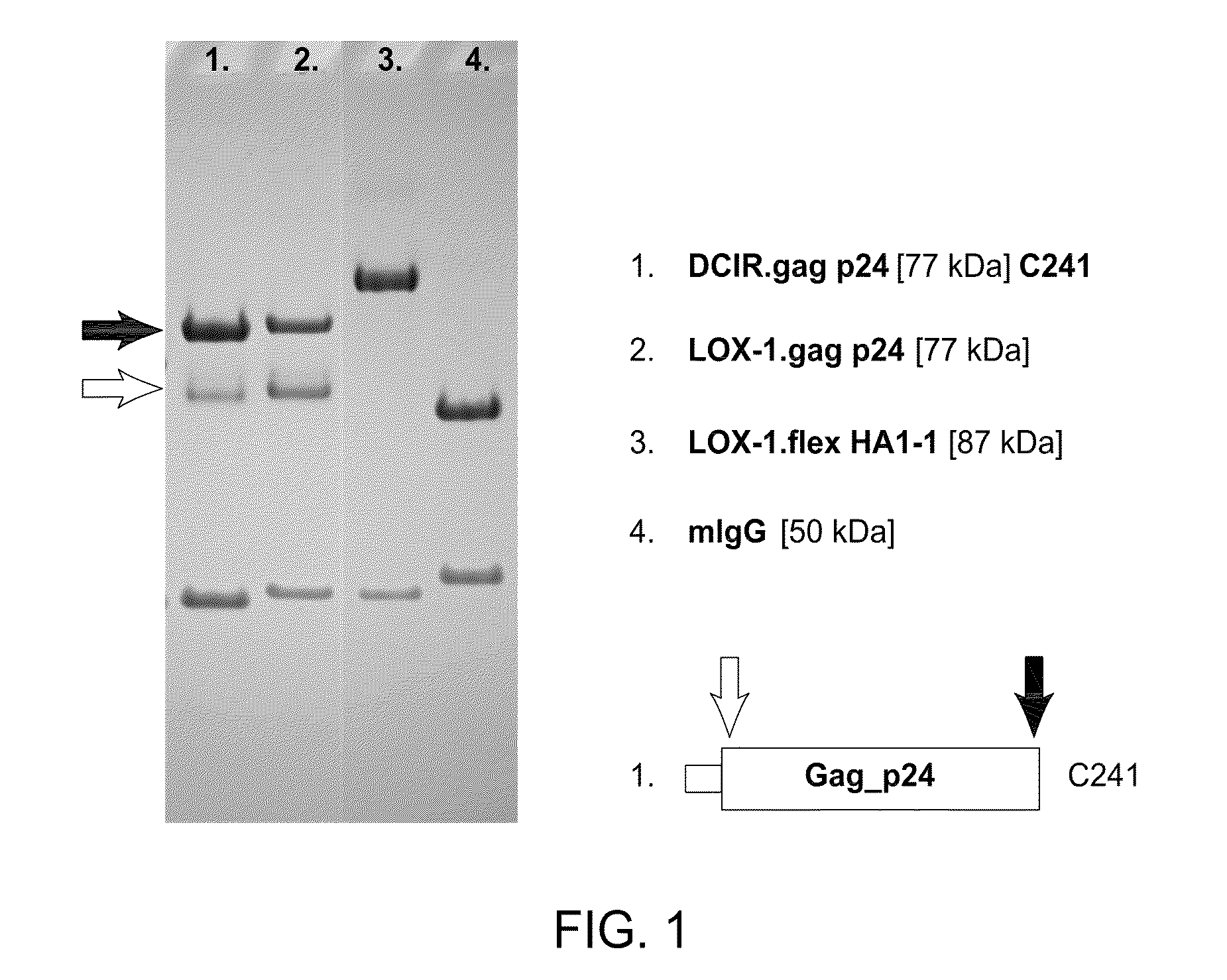
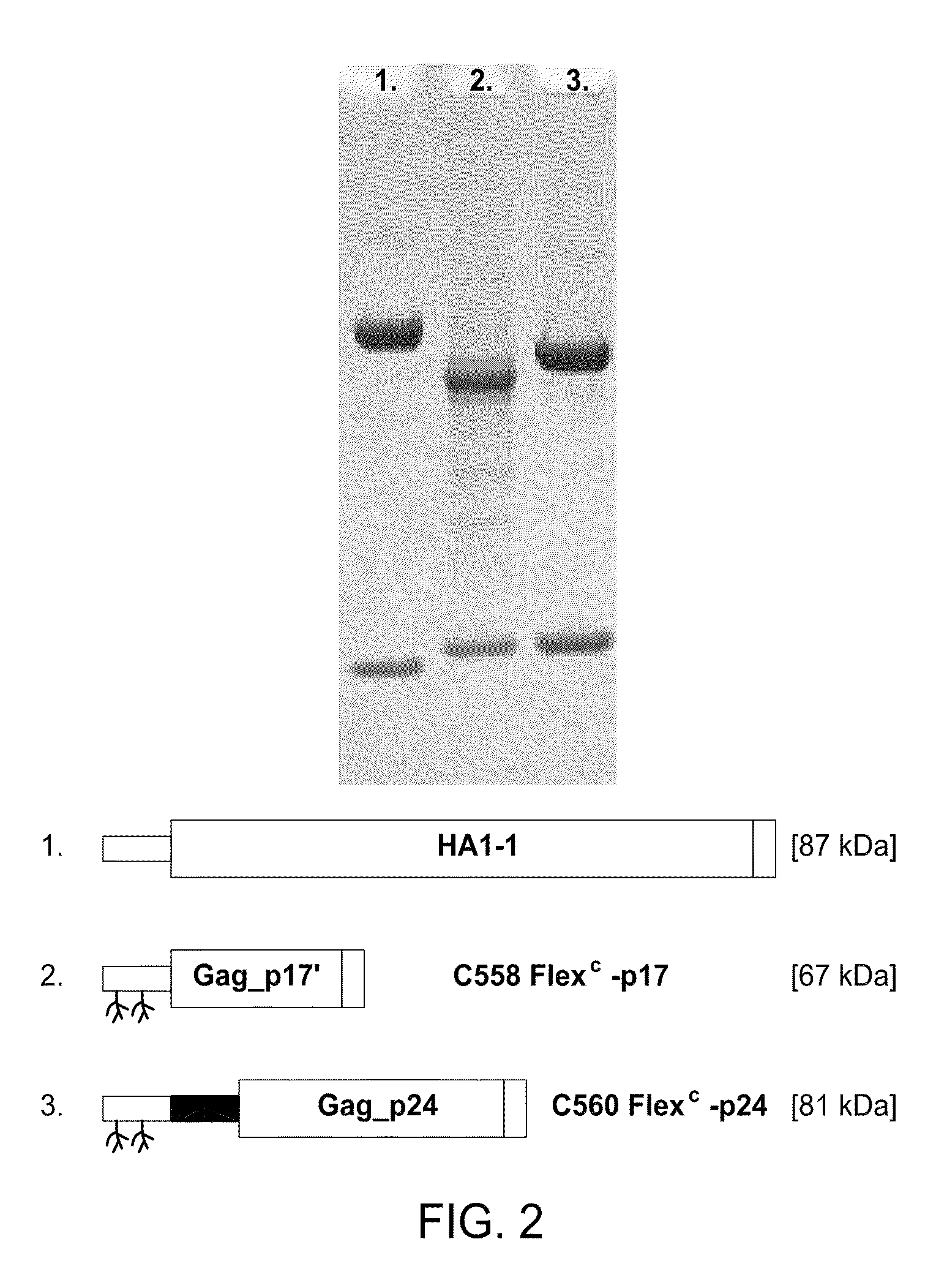
The present invention includes compositions and methods for making and using a vaccine that includes a DC-specific antibody or fragment thereof to which an engineered Gag antigen is attached to form an antibody-antigen complex, wherein the Gag antigen is less susceptible to proteolytic degradation by eliminating one or more proteolytic sites or a DC-specific antibody or fragment thereof to which an engineered Nef antigen is attached to form an antibody-antigen complex, wherein the Nef antigen comprises one or more codon usage optimization that increase antibody-antigen complex secretion, or both, wherein the vaccine is able to elicit an HIV-specific T cell immune response to Gag p17, Gag p24, Nef and / or Cyclin D1.
Owner:BAYLOR RES INST
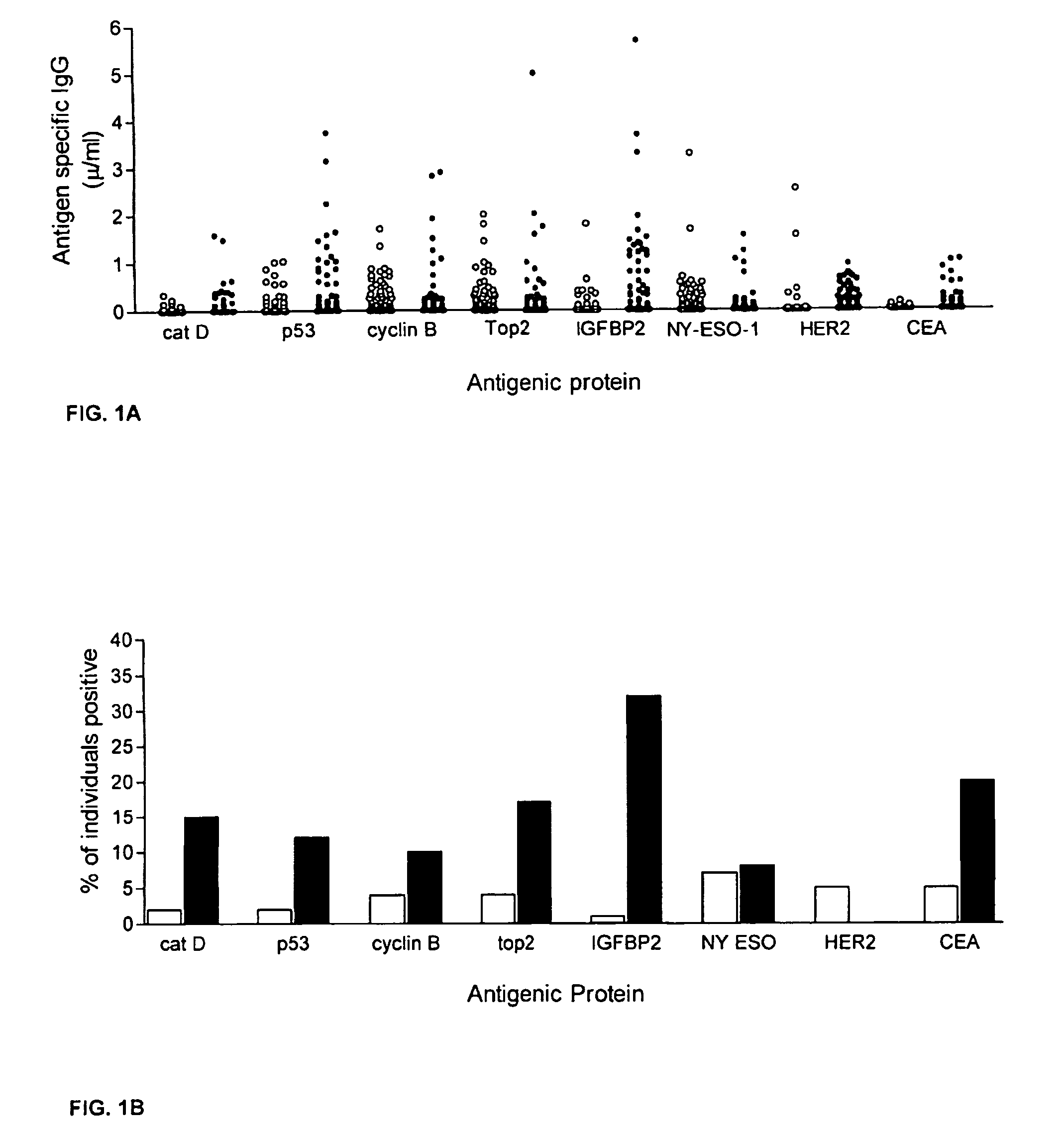
Diagnostic panel of cancer antibodies and methods for use
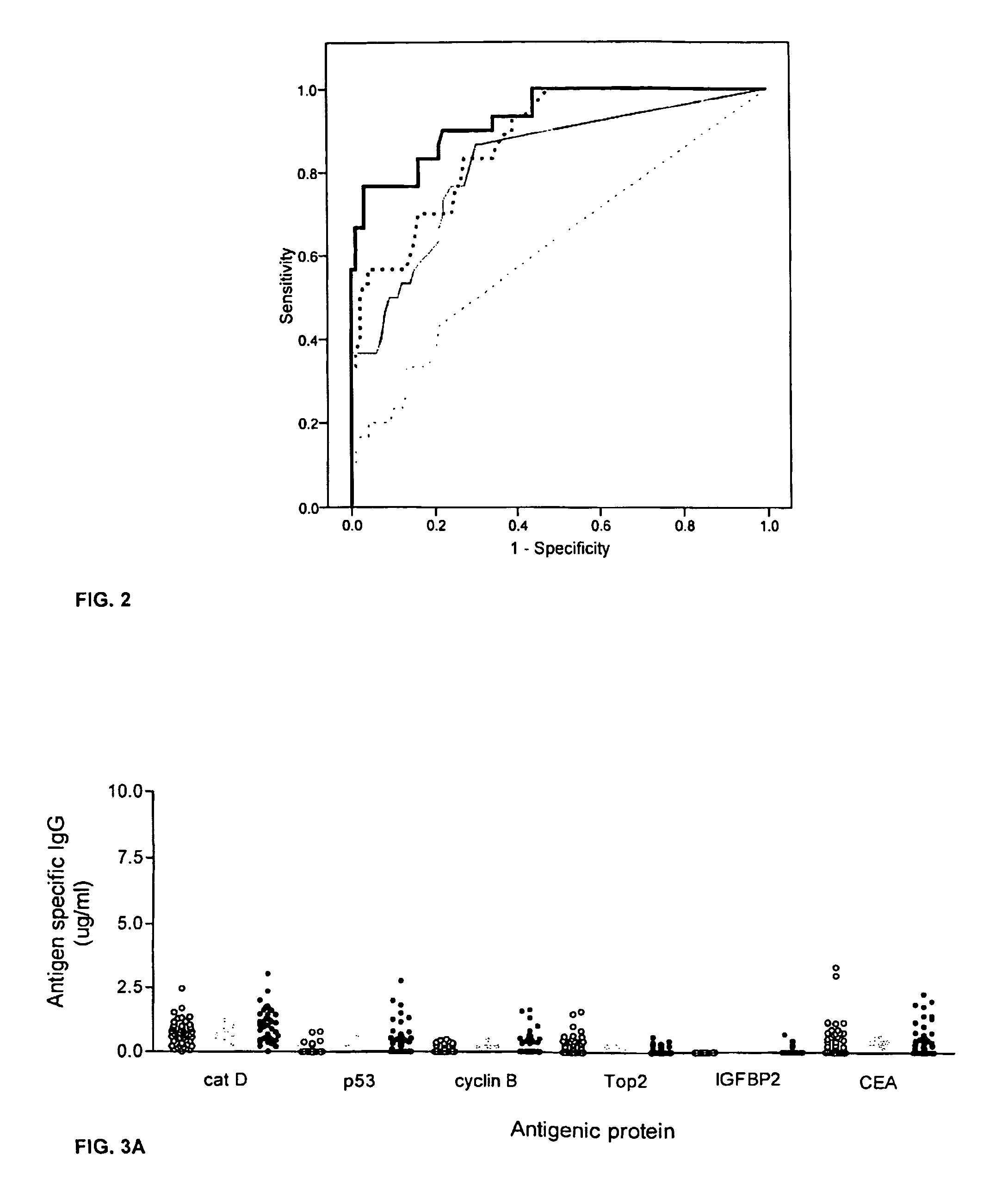
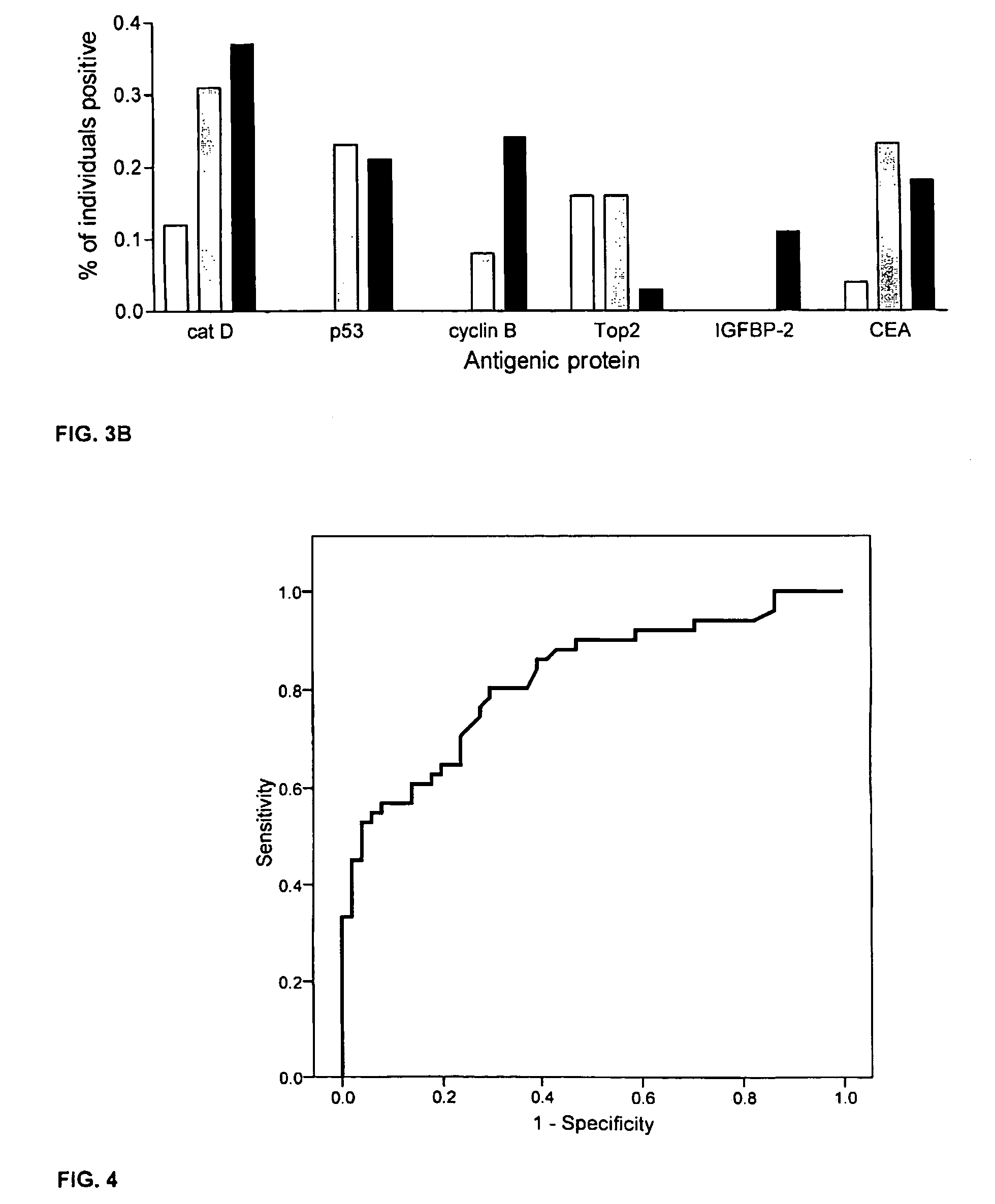
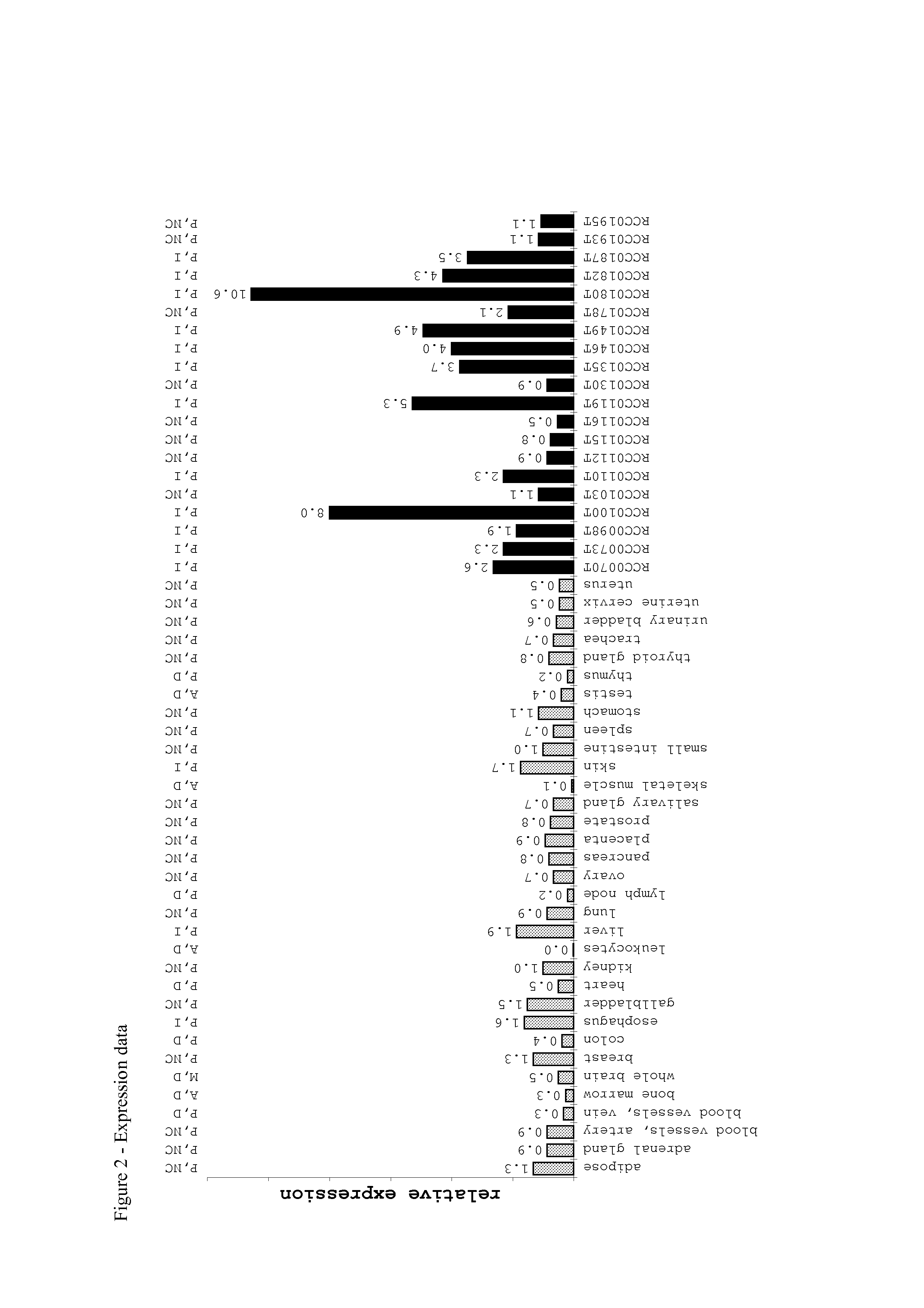
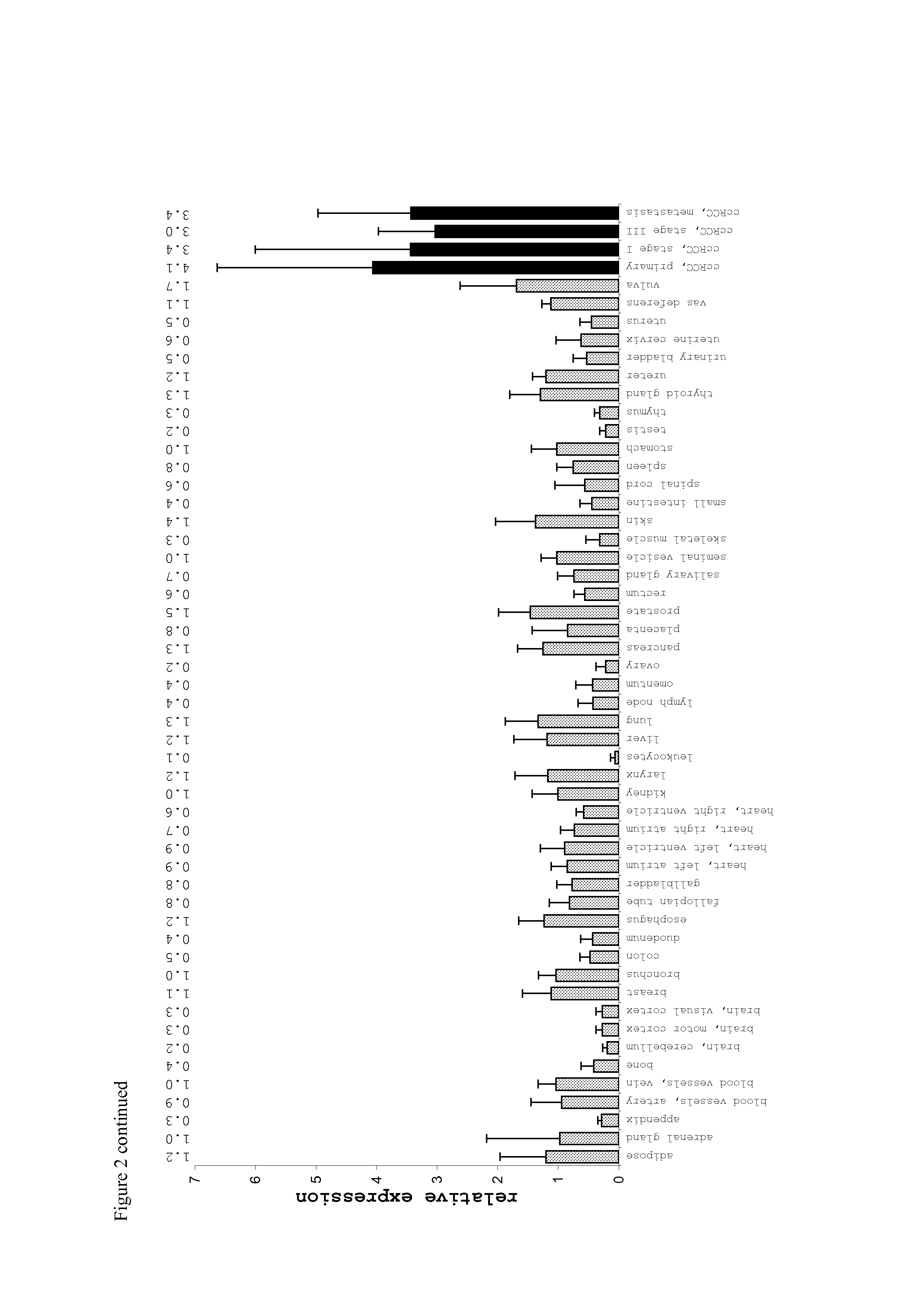
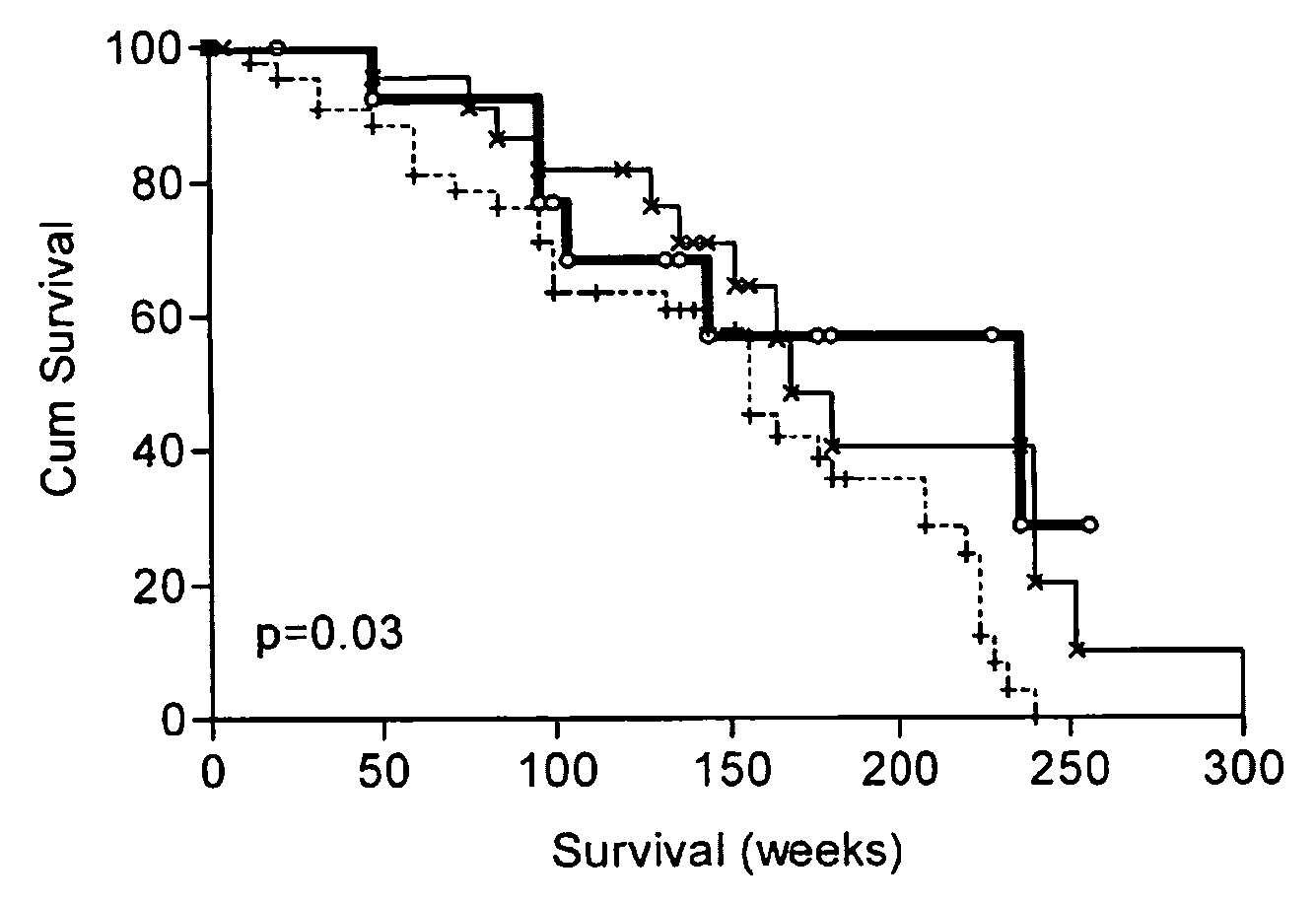
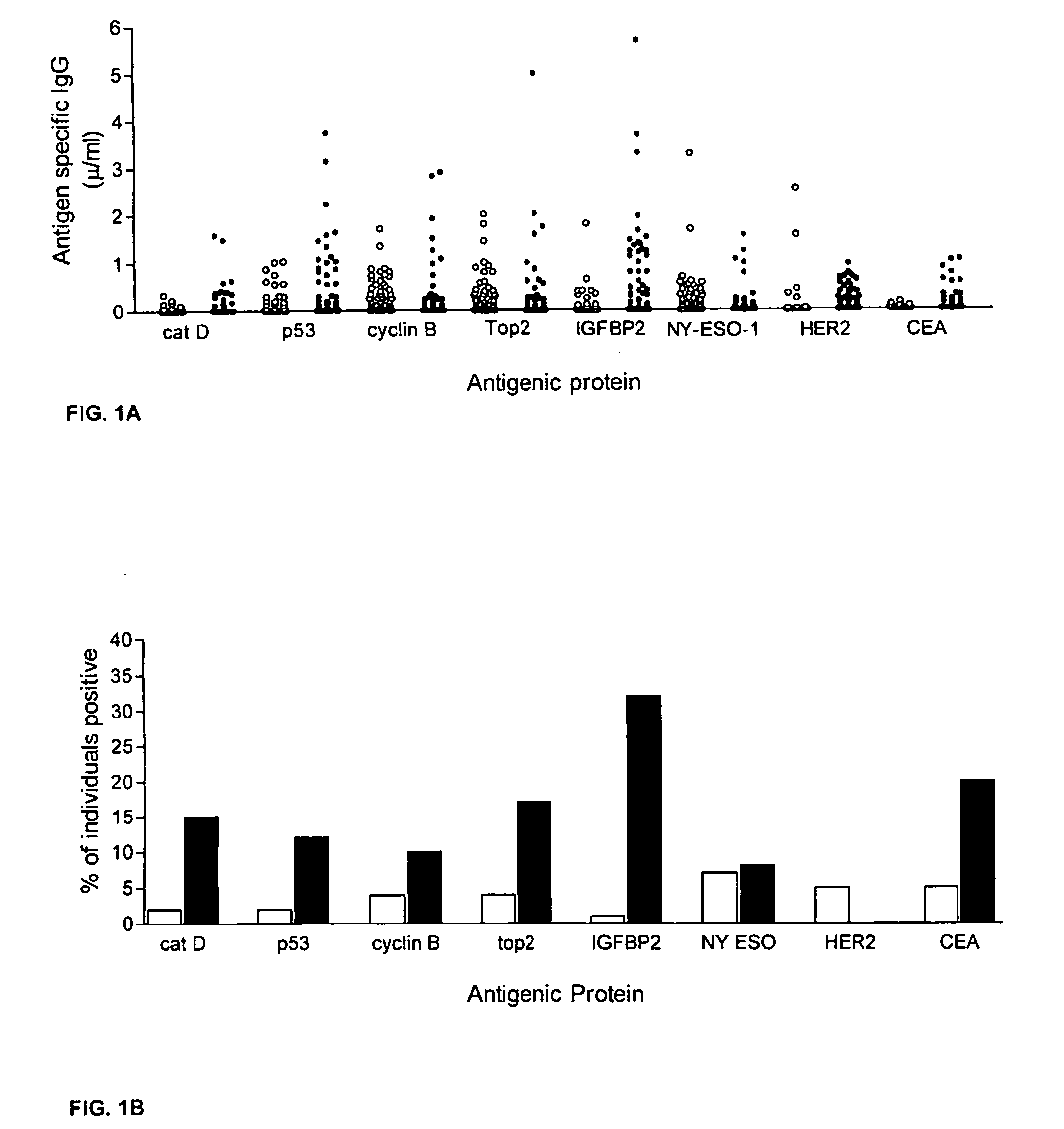
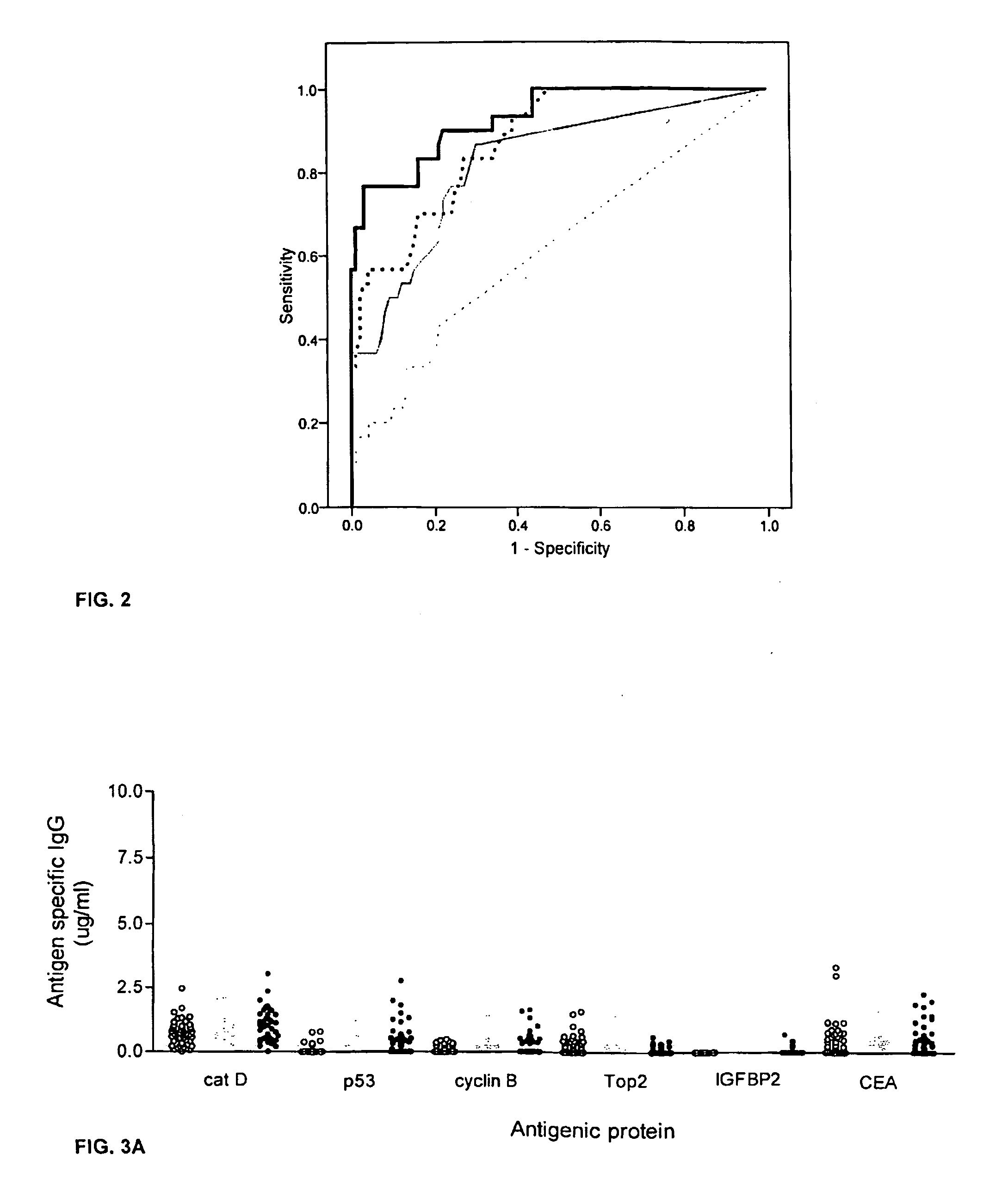
The invention provides a method for detection of a malignancy in a specimen of bodily fluid. The method comprises contacting the specimen with at least two antigens selected from the group consisting of p53, IGFBP2, Topo2α, cathepsin D, cyclin B, cyclin D1, MUC1, HER-2 / neu and CEA. The method further comprises incubating the specimen and the antigen for a duration and under conditions that are sufficient for the formation of immunocomplexes; and detecting the presence or absence of immunocomplex formation between the antigens and antibodies specific for the antigens in the specimen, thereby determining the presence or absence of the malignancy. Also provided is a method for monitoring the effectiveness of cancer therapy related to a malignancy in a warm-blooded animal, a method for distinguishing between Stage I and Stage II colorectal cancer in a specimen of bodily fluid.
Owner:UNIV OF WASHINGTON
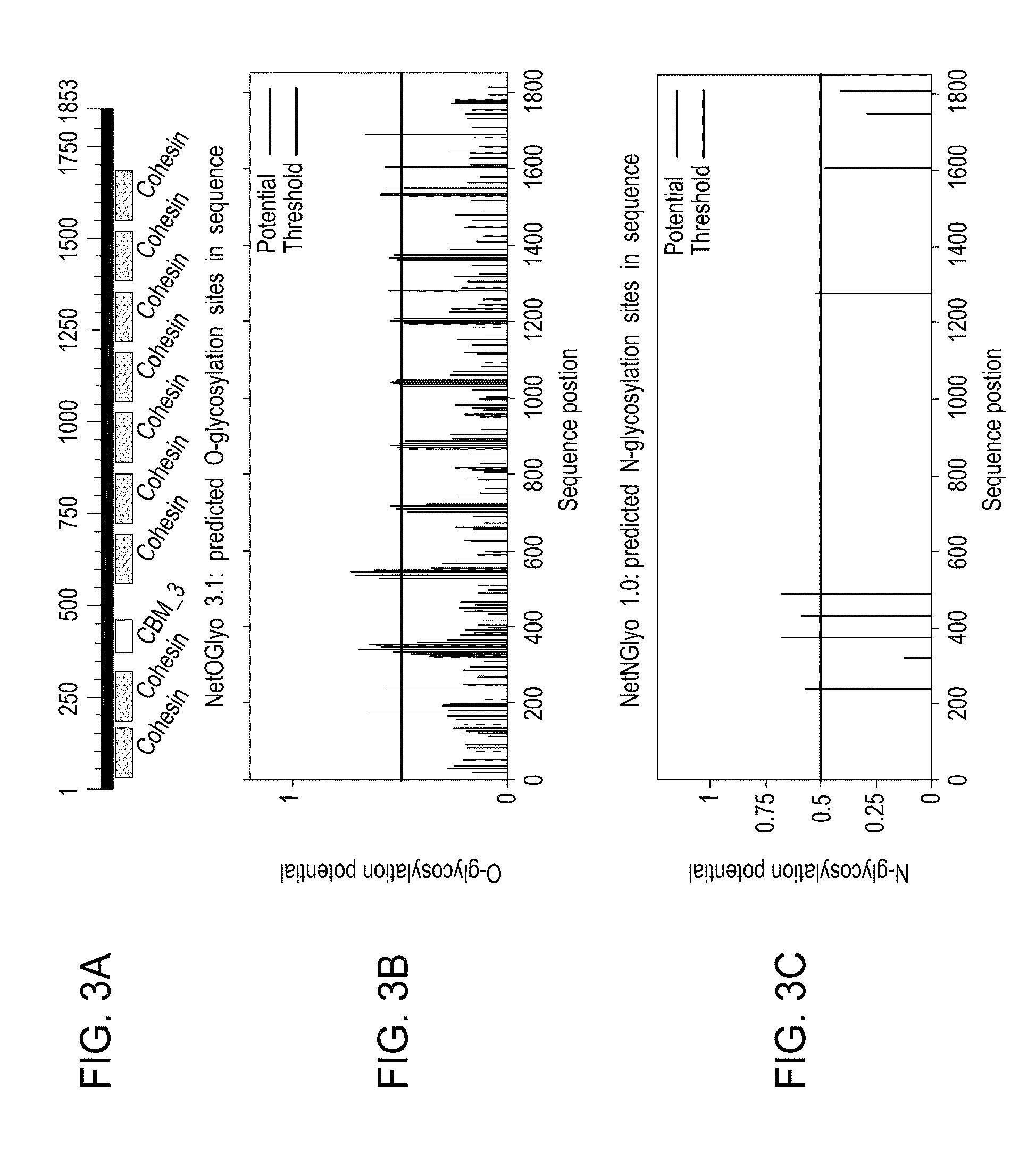
Cancer therapy based on tumor associated antigens derived from cyclin d1
The present invention relates to cyclin D1-derived peptides for use in the improved treatment of cancer in a patient, particularly in the form of a combination therapy using a vaccine. Other aspects relate to the use of the peptides or a combination thereof as a diagnostic tool.
Owner:IMMATICS BIOTECHNOLOGIES GMBH
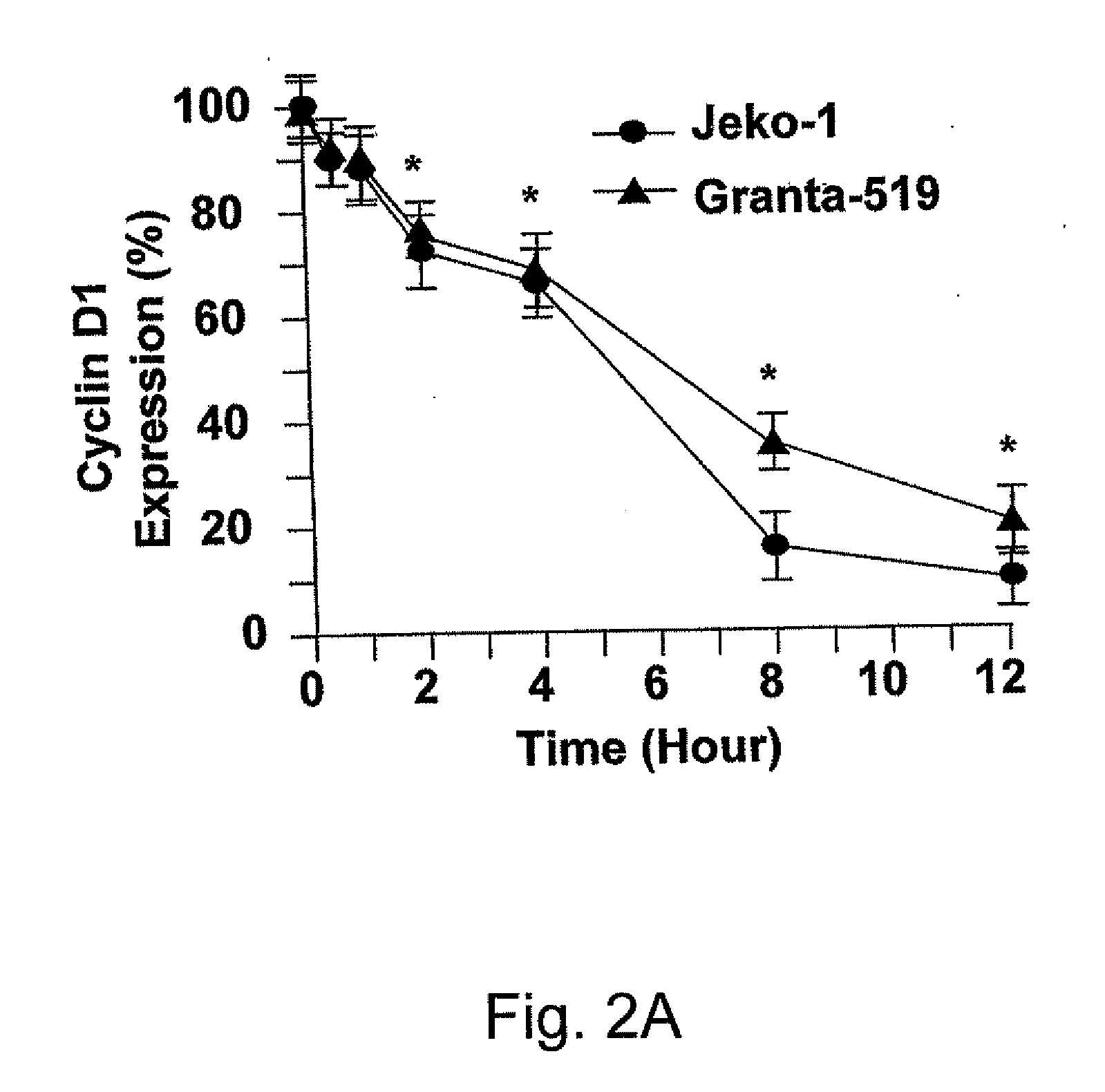
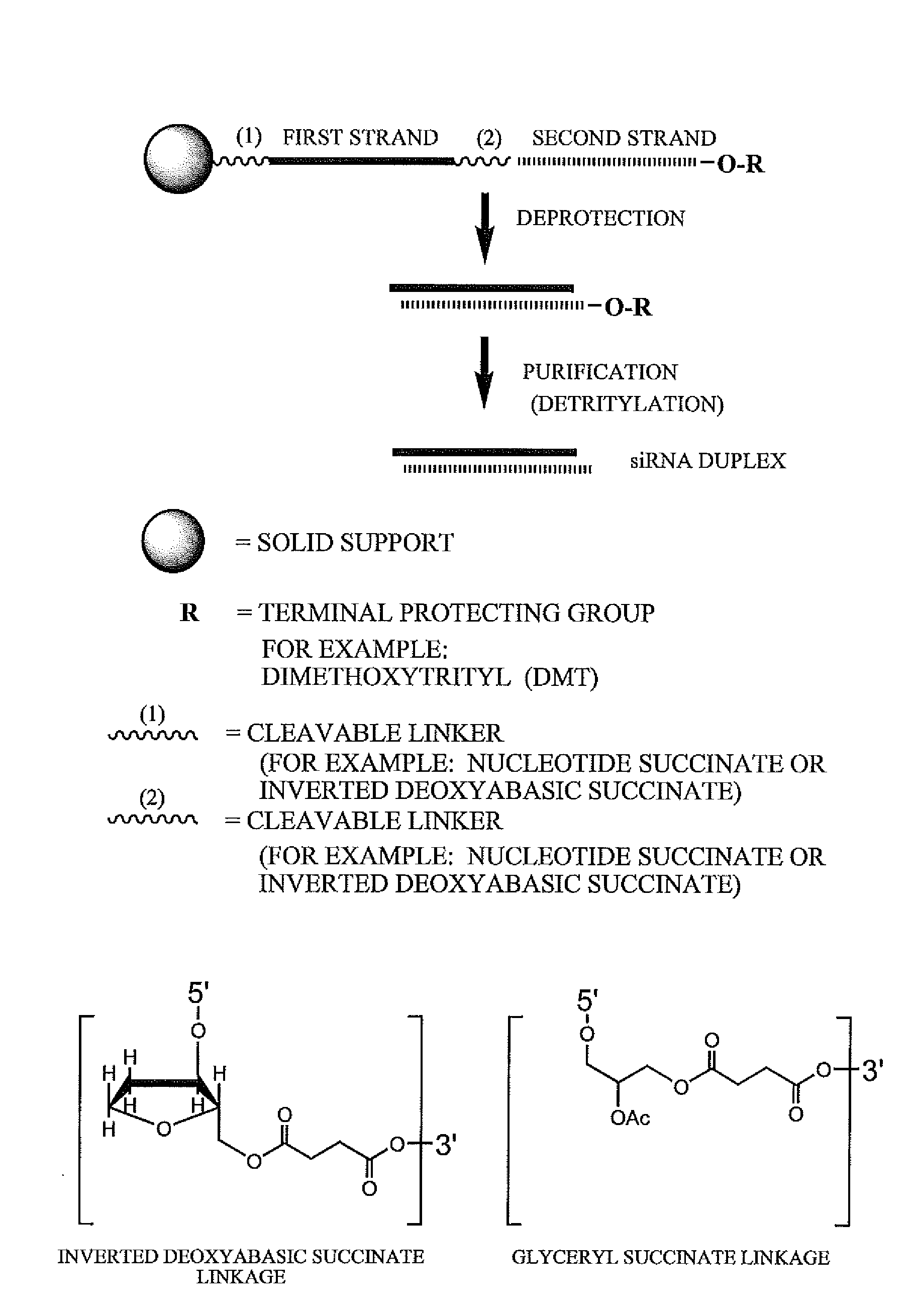
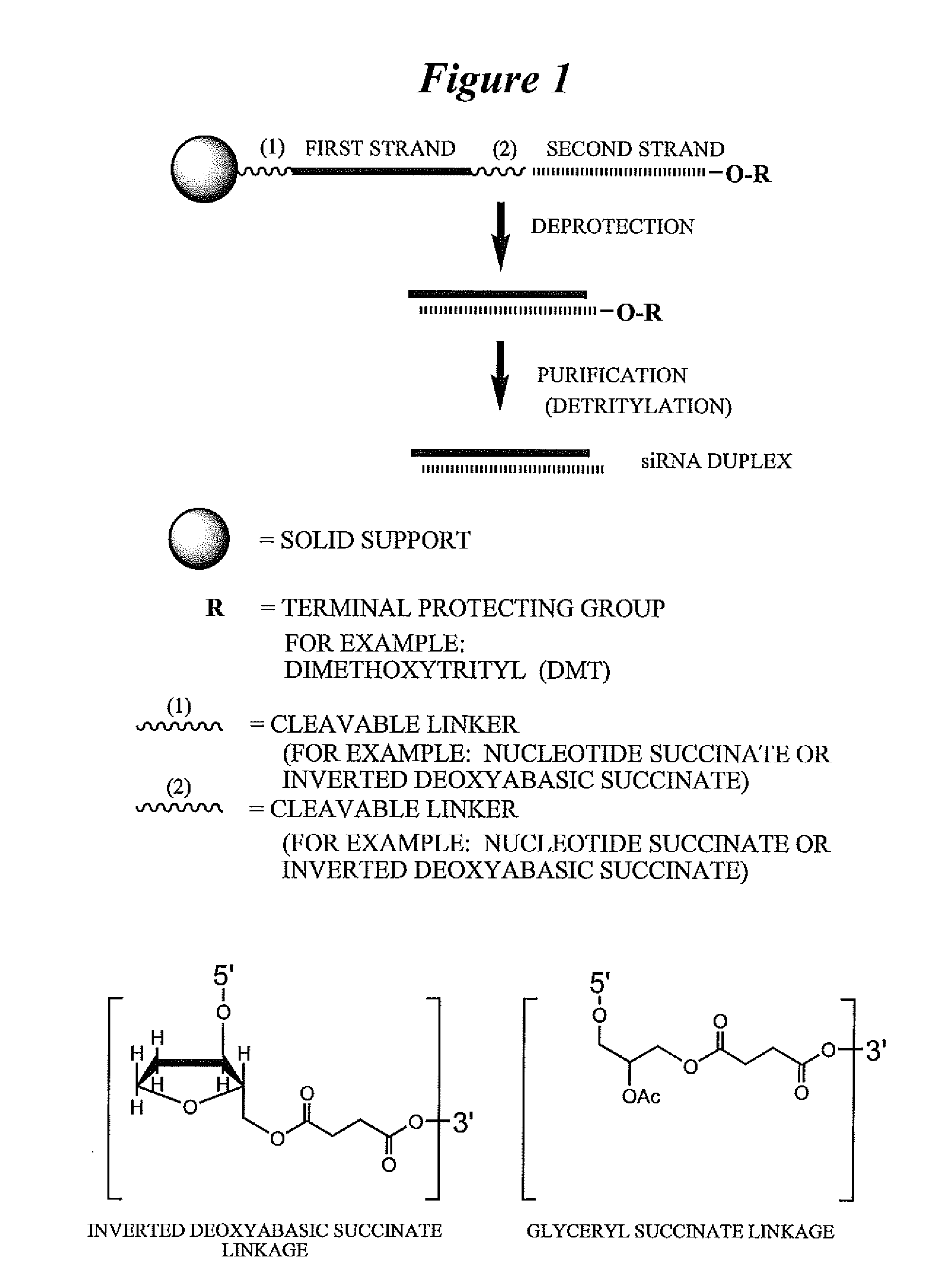
RNA interference mediated inhibition of cyclin D1 gene expression using short interfering nucleic acid (siNA)
InactiveUS20050164224A1Improve bioavailabilityMinimize the possibilityCompounds screening/testingPeptide/protein ingredientsCyclin D1Cyclin
This invention relates to compounds, compositions, and methods useful for modulating cyclin (e.g., cyclin D1) and / or cyclin dependent kinase (CDK) gene expression using short interfering nucleic acid (siNA) molecules. This invention also relates to compounds, compositions, and methods useful for modulating the expression and activity of other genes involved in pathways of cyclin (e.g., cyclin D1) and / or cyclin dependent kinase (CDK) gene expression and / or activity by RNA interference (RNAi) using small nucleic acid molecules. In particular, the instant invention features small nucleic acid molecules, such as short interfering nucleic acid (siNA), short interfering RNA (siRNA), double-stranded RNA (dsRNA), micro-RNA (miRNA), and short hairpin RNA (shRNA) molecules and methods used to modulate the expression of cyclin (e.g., cyclin D1) and / or cyclin dependent kinase (CDK) genes.
Owner:SIRNA THERAPEUTICS INC
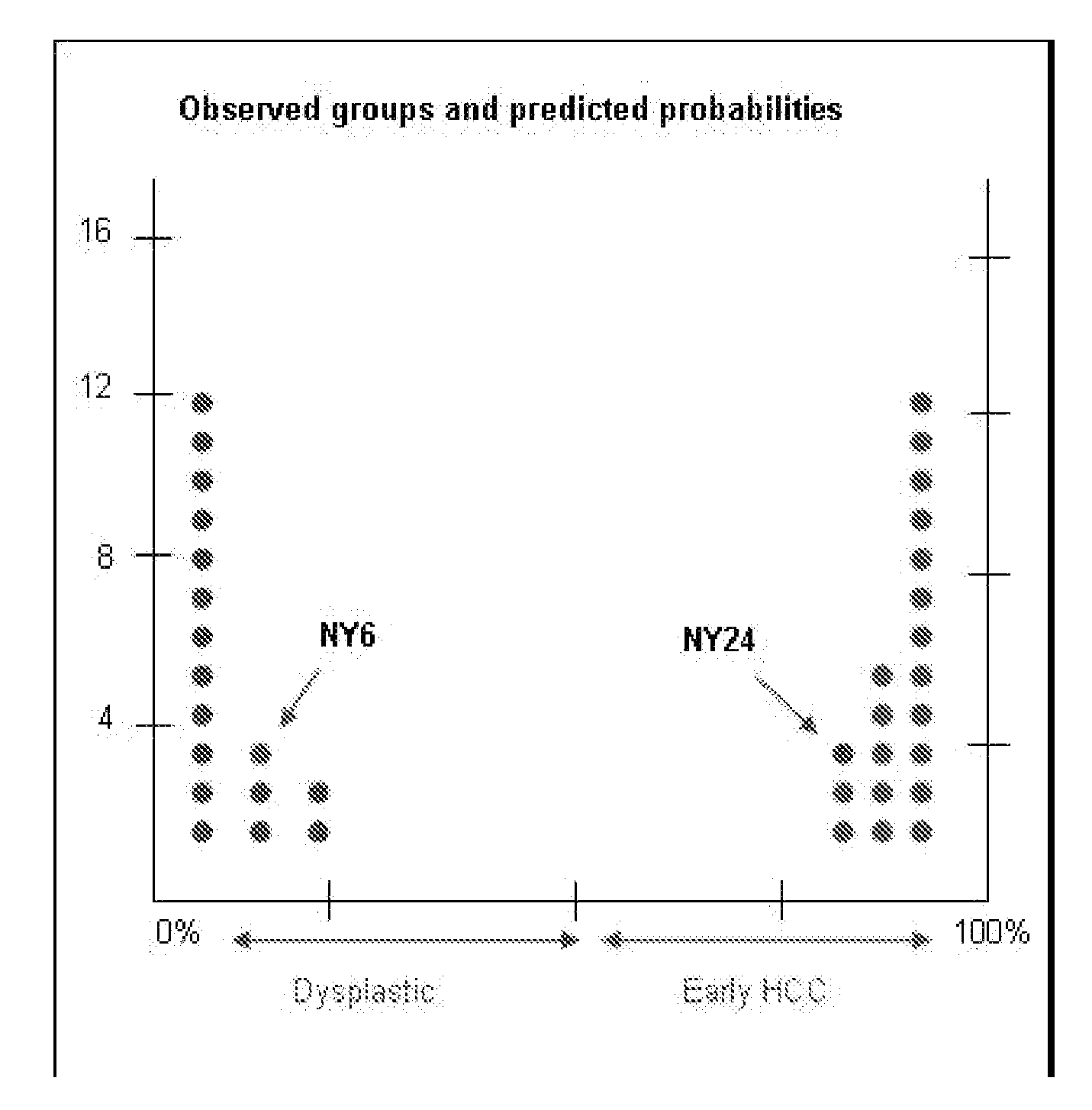
Methods and compositions for the diagnosis for early hepatocellular carcinoma
ActiveUS20080038736A1Microbiological testing/measurementBiological testingCyclin D1Early Hepatocellular Carcinoma
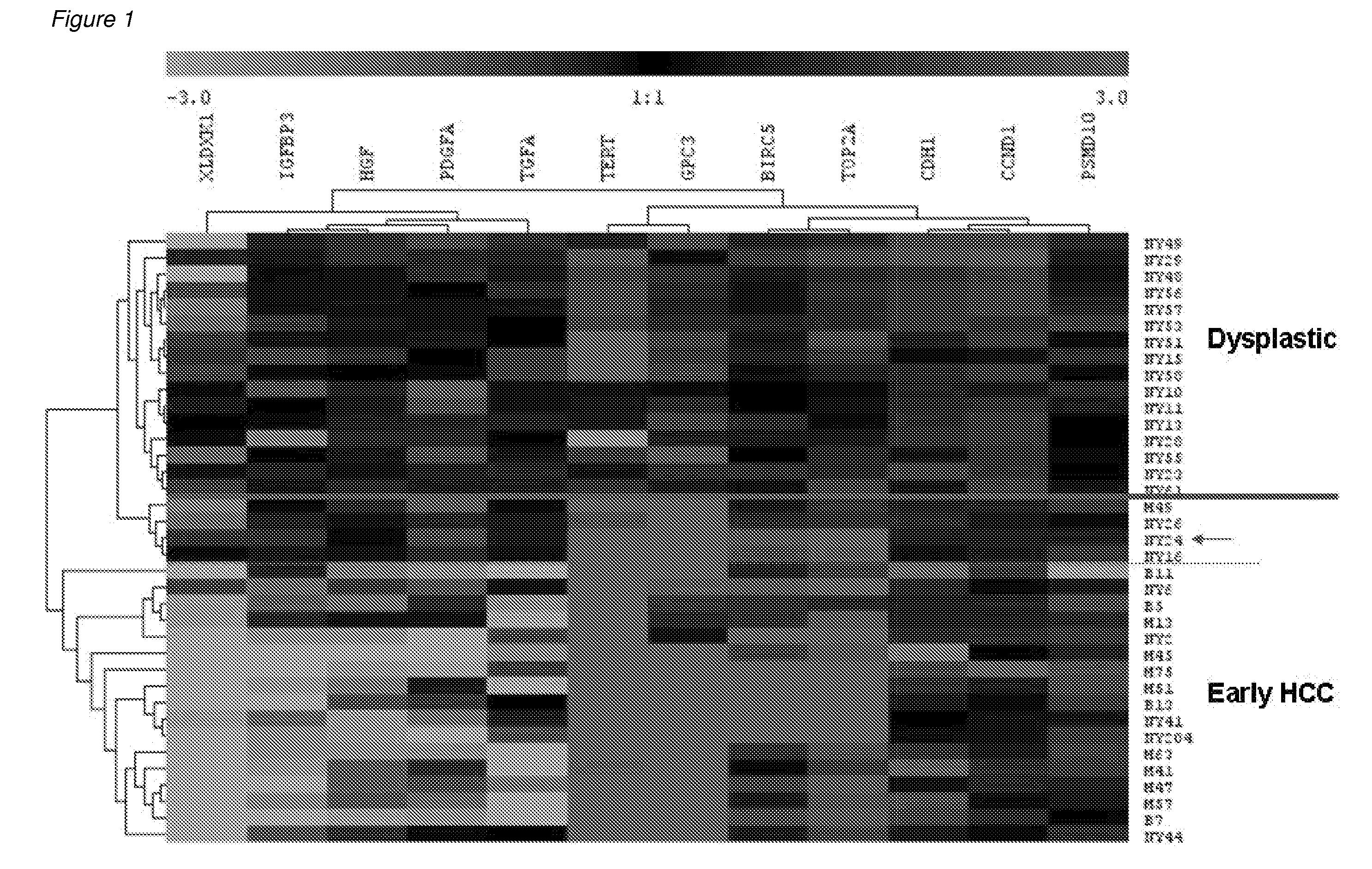
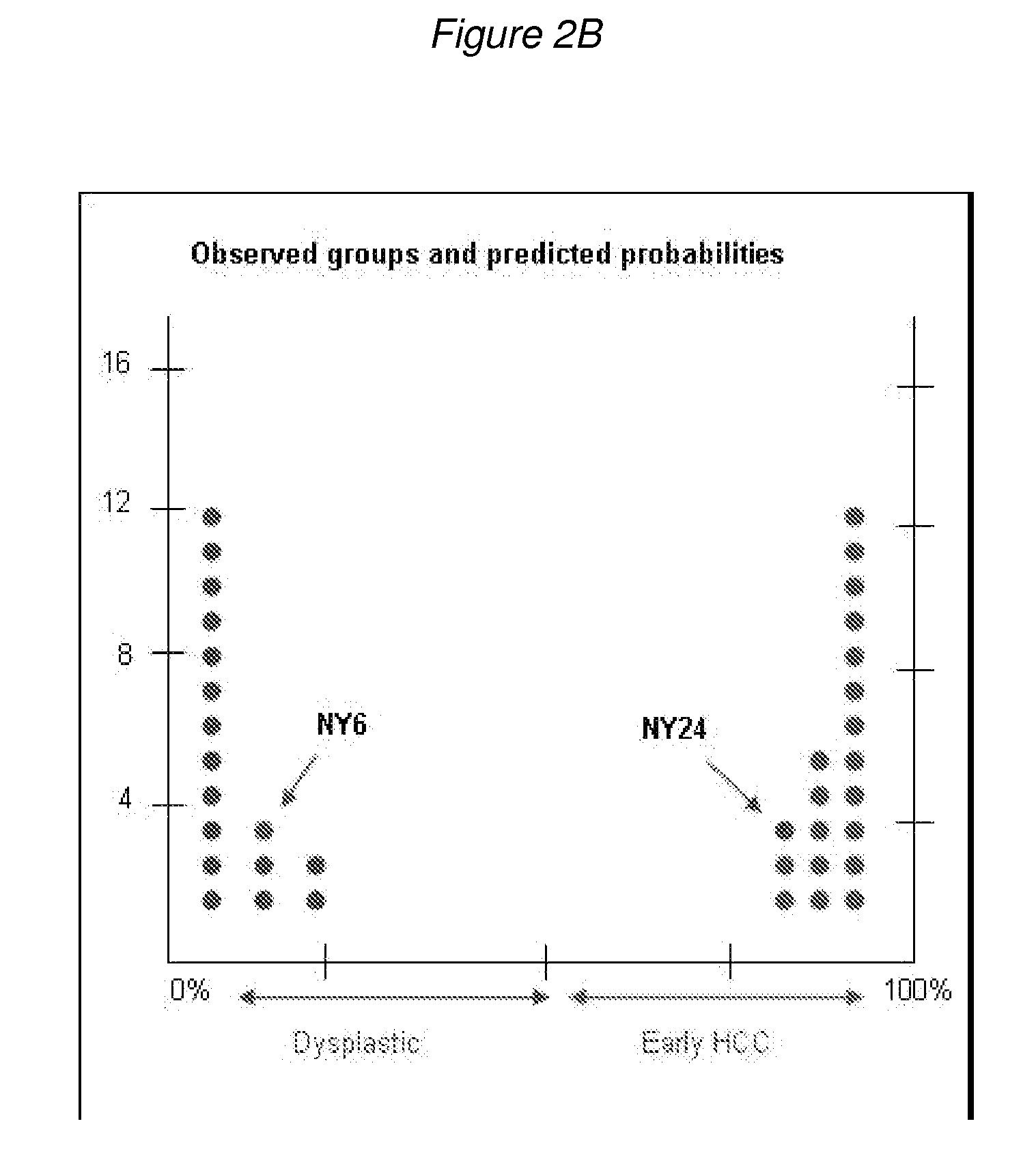
Methods and compositions are provide to allow discrimination of dysplastic nodules from early HCC nodules. More specifically, it has been determined that TERT, GPC3, gankyrin, survivin, TOP2A, LYVE1, Ecadherin, IGFBP3, PDGFRA, TGFA, cyclin D1 and HGF are differentially expressed in HCC as compared to normal liver cells and liver cells that have dysplastic, non-cancerous nodules.
Owner:MT SINAI SCHOOL OF MEDICINE
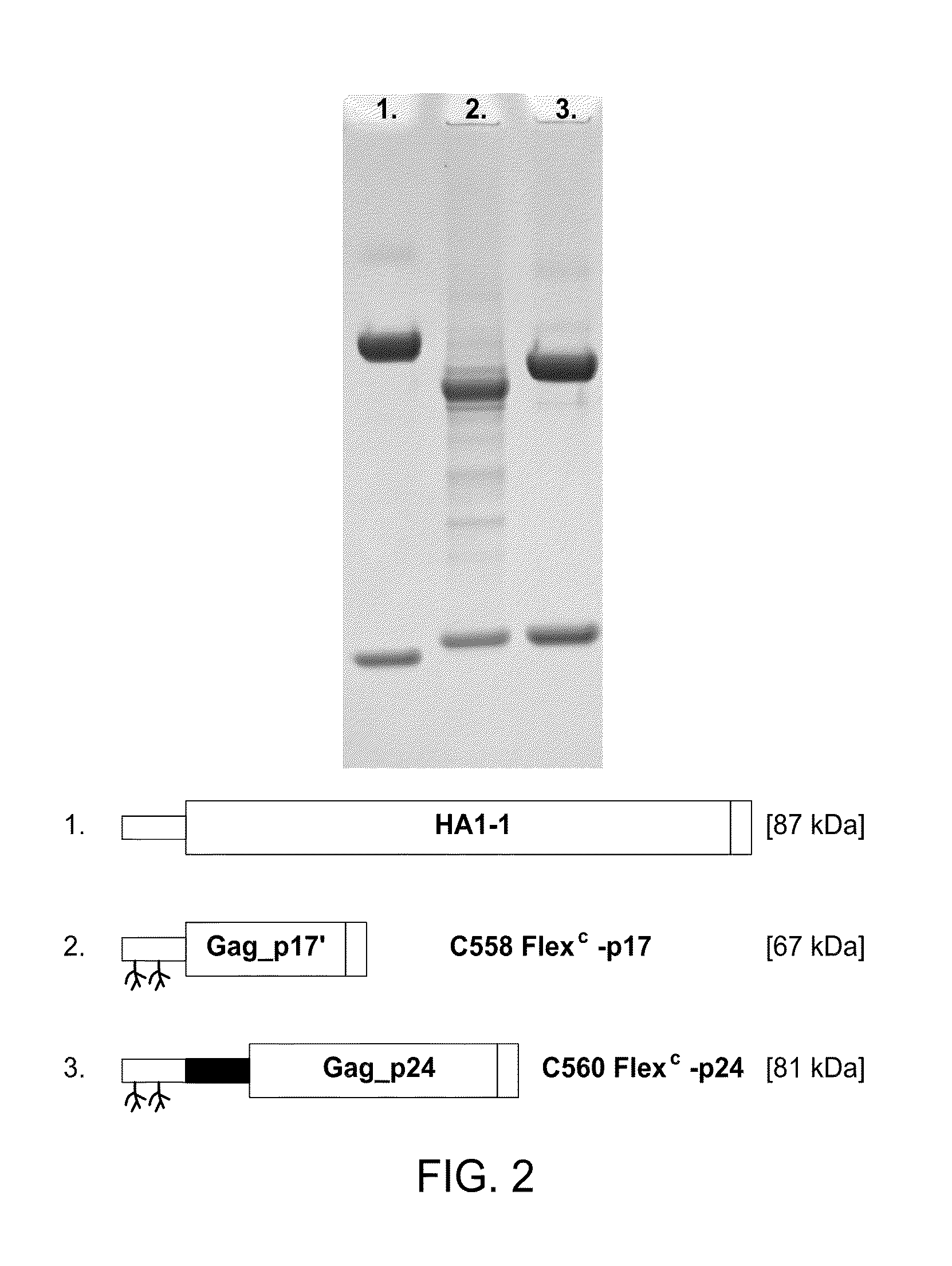
Dendritic cell-specific antibody conjugate comprising anti-CD40 monoclonal antibodies conjugated to HIV-1 Gag/Nef
ActiveUS9109011B2Improve efficiencyIncrease flexibilityBiocidePeptide/protein ingredientsCyclin D1Dendritic cell
The present invention includes compositions and methods for making and using a vaccine that includes a DC-specific antibody or fragment thereof to which an engineered Gag antigen is attached to form an antibody-antigen complex, wherein the Gag antigen is less susceptible to proteolytic degradation by eliminating one or more proteolytic sites or a DC-specific antibody or fragment thereof to which an engineered Nef antigen is attached to form an antibody-antigen complex, wherein the Nef antigen comprises one or more codon usage optimization that increase antibody-antigen complex secretion, or both, wherein the vaccine is able to elicit an HIV-specific T cell immune response to Gag p17, Gag p24, Nef and / or Cyclin D1.
Owner:BAYLOR RES INST
Cancer therapy based on tumor associated antigens derived from cyclin d1
The present invention relates to cyclin D1-derived peptides for use in the improved treatment of cancer in a patient, particularly in the form of a combination therapy using a vaccine. Other aspects relate to the use of the peptides or a combination thereof as a diagnostic tool.
Owner:IMMATICS BIOTECHNOLOGIES GMBH
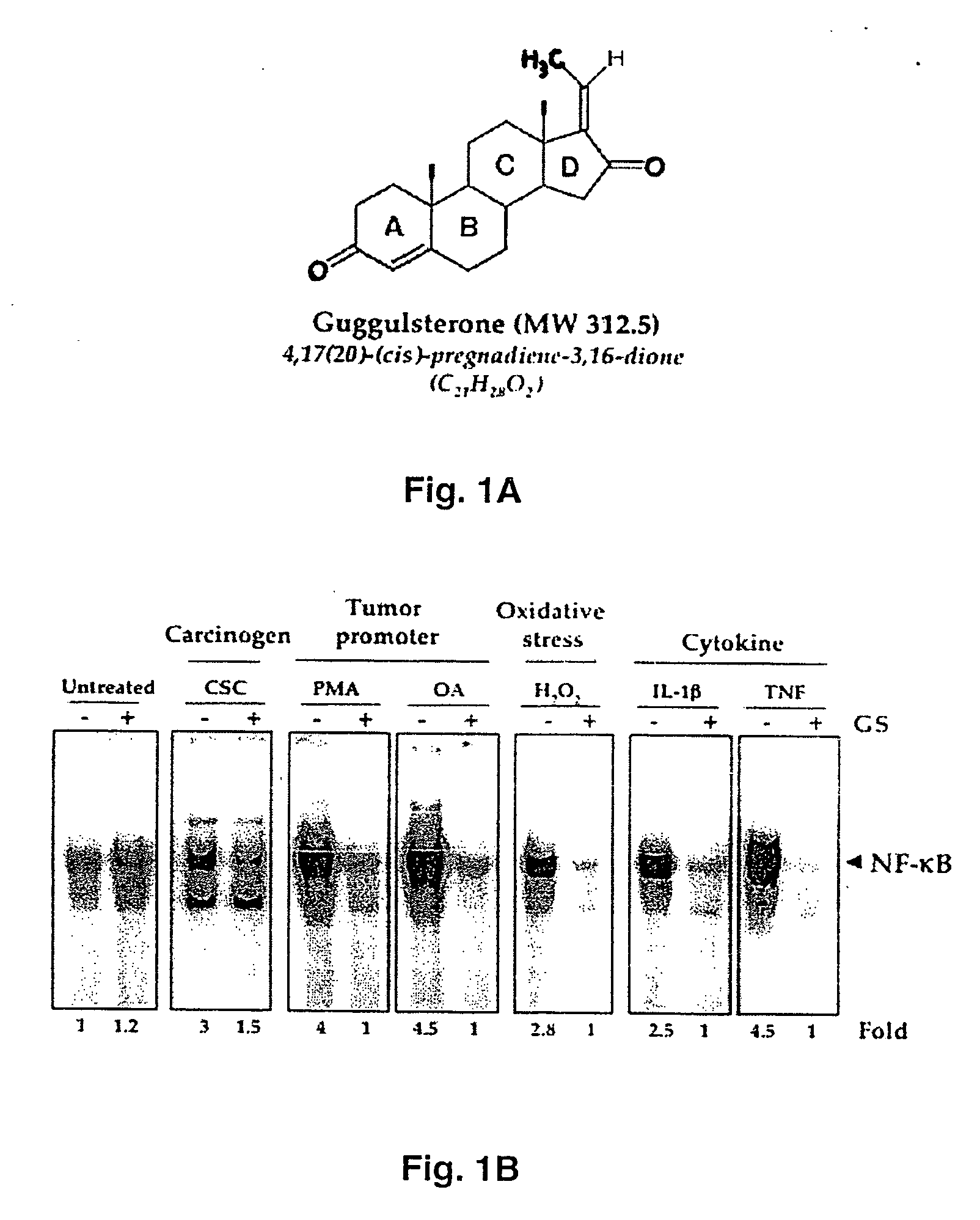
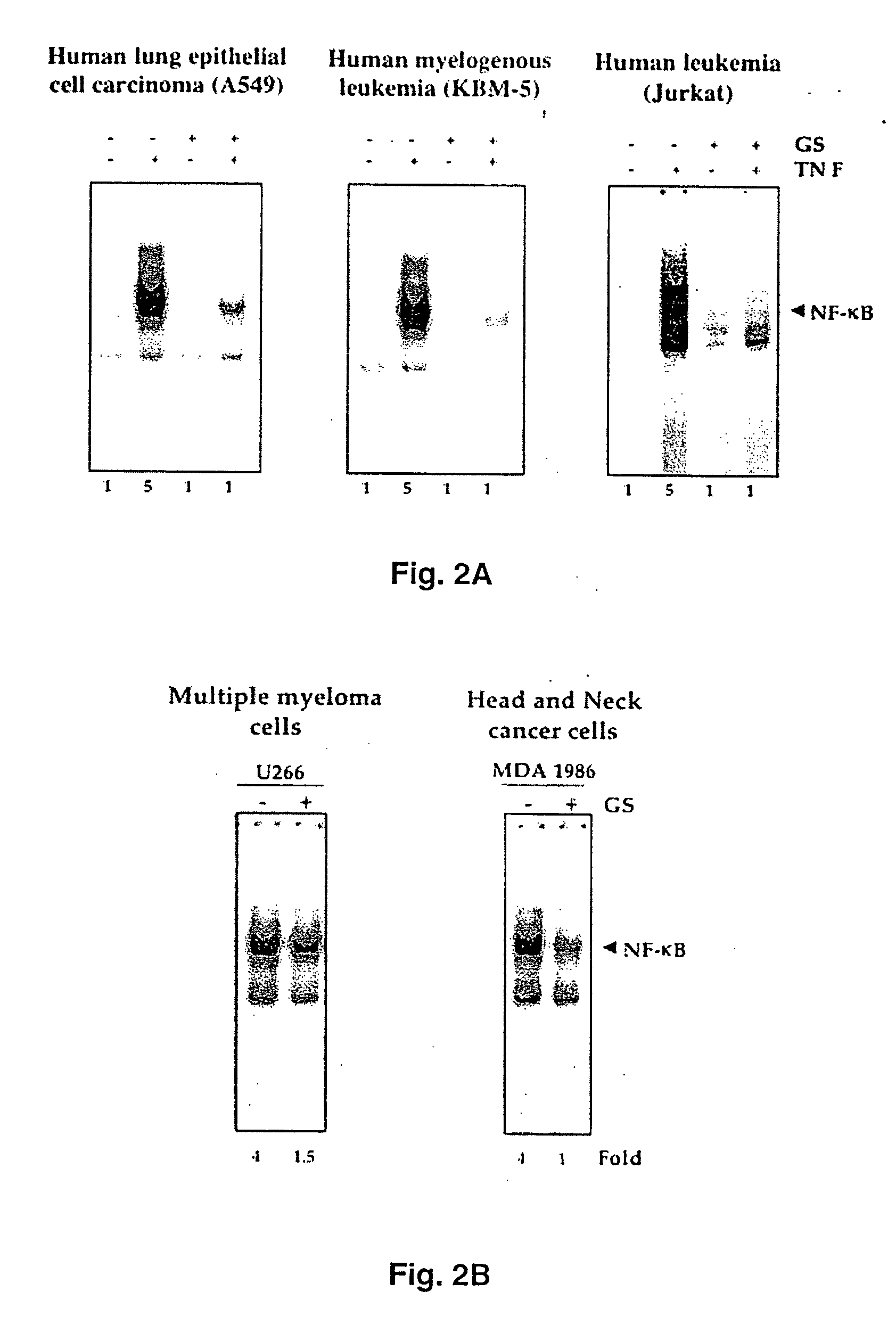
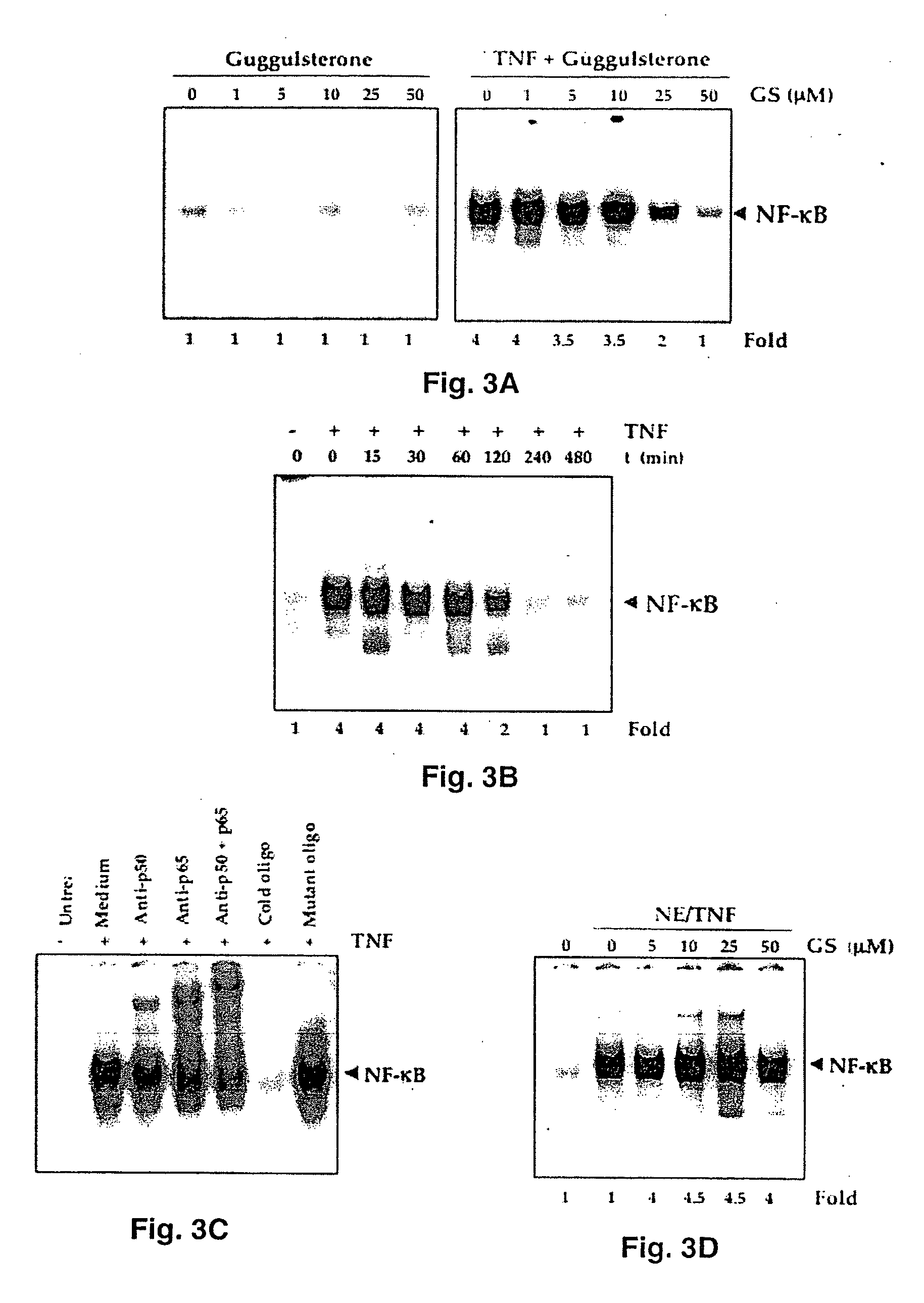
Guggulsterone: an inhibitor of nuclear factor - kappaB and IkappaBalpha kinase activation and uses thereof
InactiveUS20060019907A1Good effectHigh activityBiocideOrganic active ingredientsLymphatic SpreadCyclin D1
The present invention provides an inhibitor of NF-κB, guggulsterone and its analogs. Guggulsterone suppresses NF-κB activation induced by TNF, phorbol ester, okadaic acid, cigarette smoke, H2O2 and IL-1β, as well as constitutive NF-κB activation expressed in most tumor cells. One mechanism by which guggulsterone inhibits activation of NF-κB is through suppression of IκBα phosphorylation and IκBα degradation. NF-κB-dependent gene transcription is modulated by guggulsterone and its analogs. In particular, induction by TNF, TNFR1, TRADD, TRAF2, NIK and IKK, is modulated by guggulsterone and its analogs. In addition, guggulsterone decreased the expression of genes involved in anti-apoptosis (IAP1, XIAP, Bfl-1 / A1, bcl-2, cFLIP, survivin), proliferation (cyclin D1, c-myc) and metastasis (MMP-9, COX2 and VEGF).
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
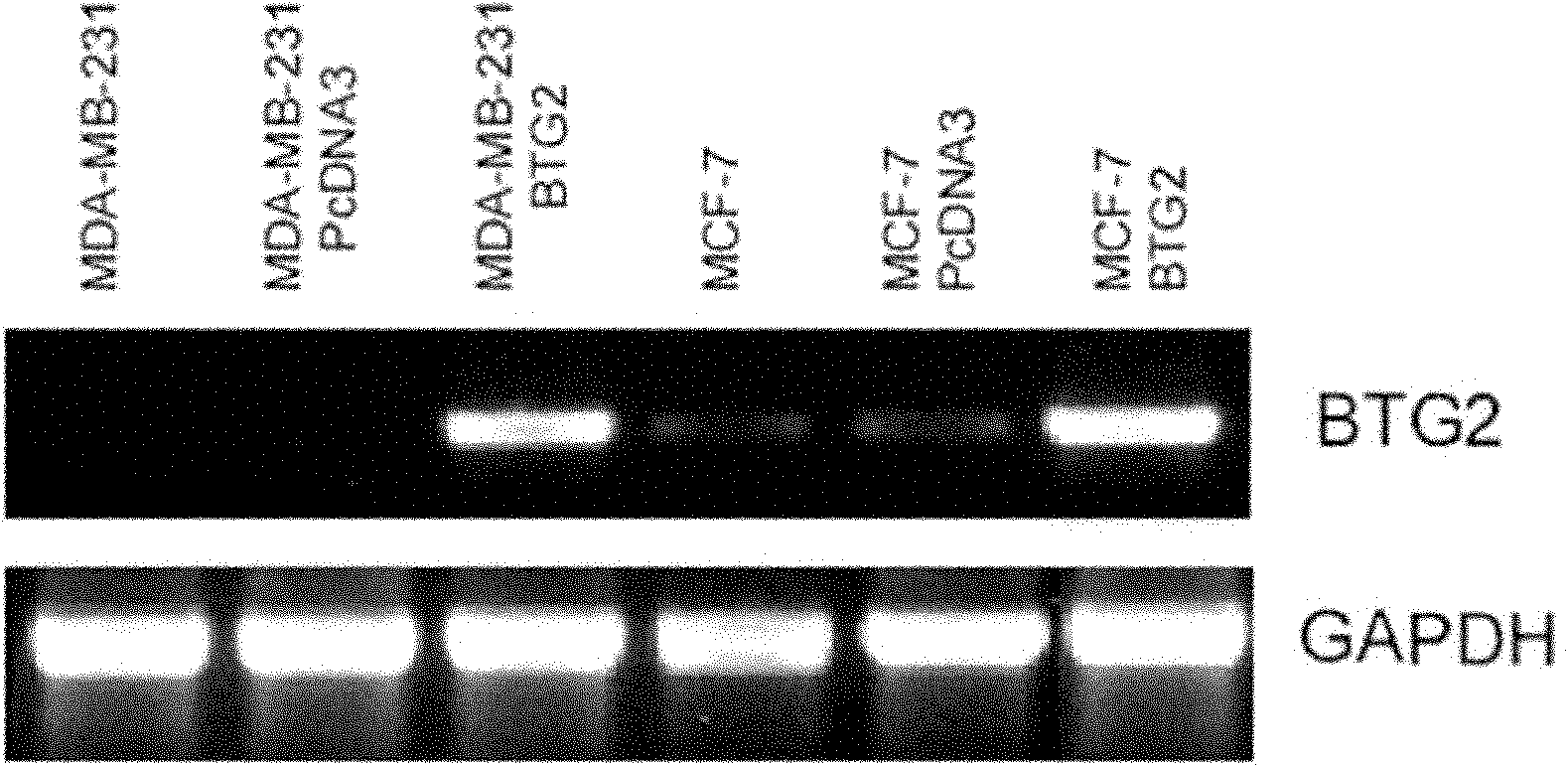
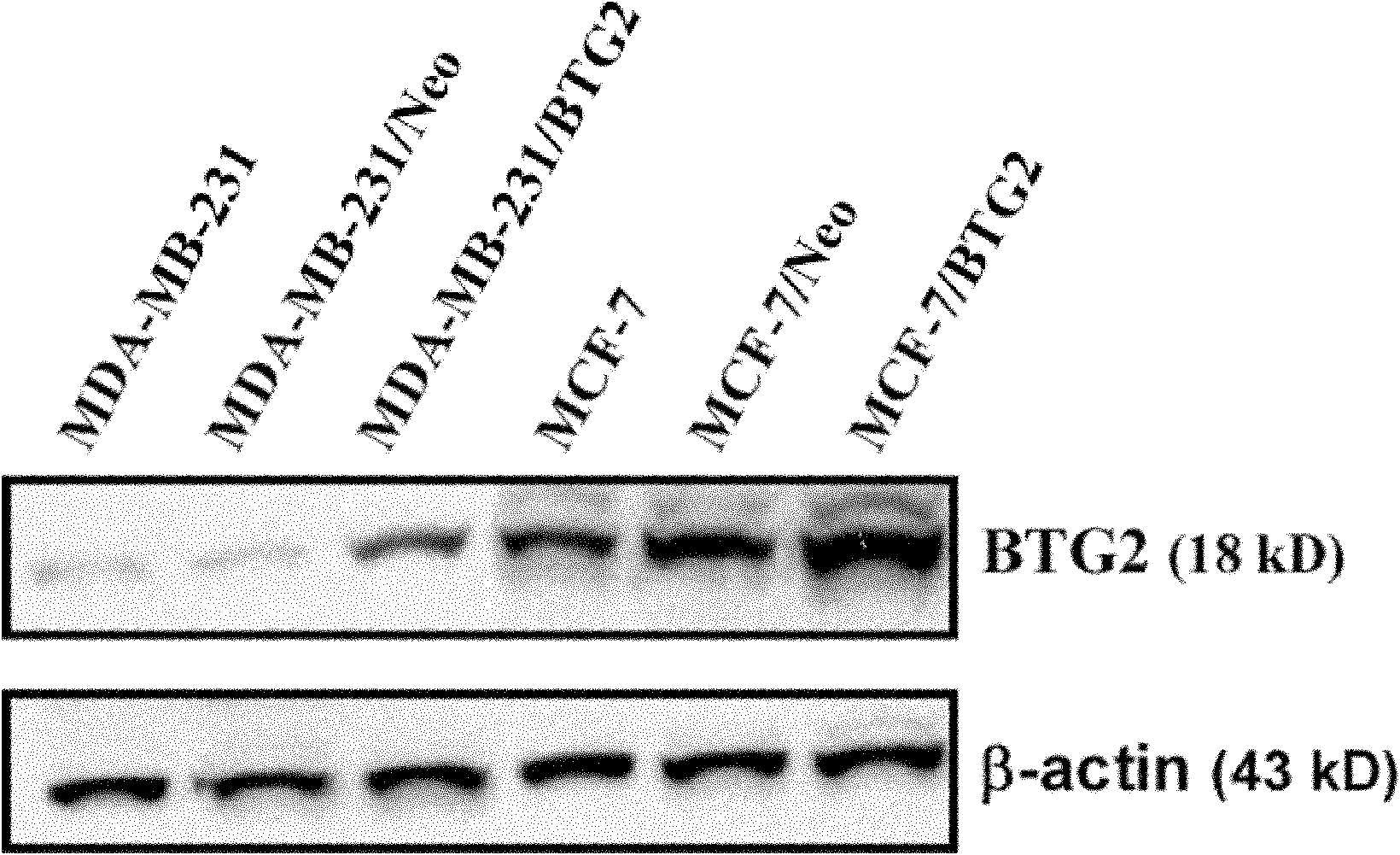
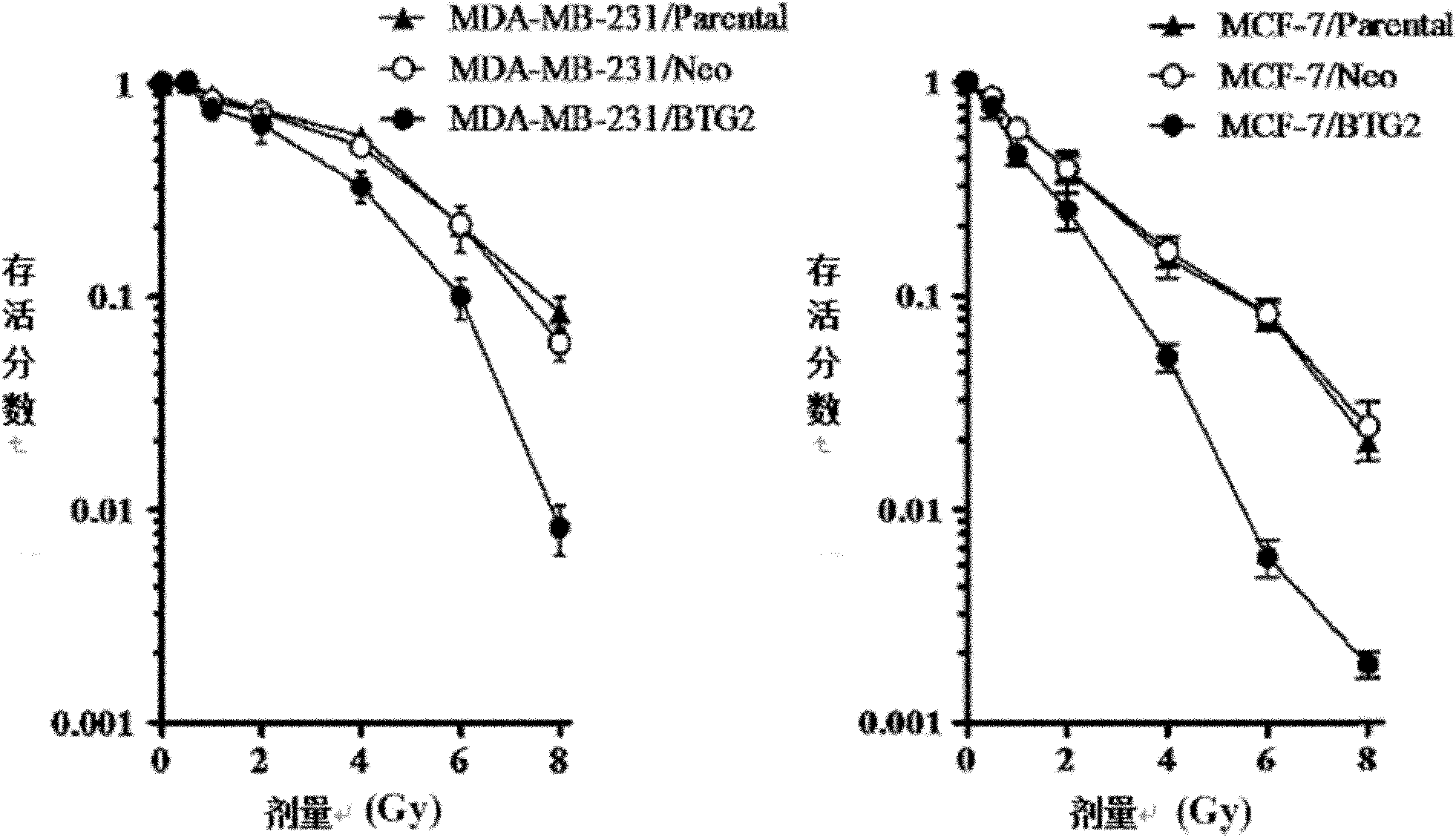
Application of BTG2 (B cell translocation gene 2) in preparing radiation sensitizing agent
InactiveCN102028957APromote apoptosisIncreased sensitivityGenetic material ingredientsAntineoplastic agentsCyclin D1Mda mb 231
The invention belongs to the field of radiation sensitizing agents, in particular to an application of BTG2 (B cell translocation gene 2) in preparing a tumor radiation sensitizing agent for ionizing radiation including X-rays. The invention discloses an application of liposome mediated eukaryotic expression plasmid pcDNA3-BTG2 in preparing the radiation sensitizing agent, wherein the liposome mediated eukaryotic expression plasmid pcDNA3-BTG2 is transfected to MCF-7 (macrophage chemotactic factor-7) breast cancer cells and MDA-MB-231 breast cancer cells and expressed successfully, and the BTG2 protein can up-regulate the proapoptosis Bax protein and down-regulate the cell cycle regulatory protein Cyclin D1, and by promoting the cell apoptosis, G0 / G1 phase arrest of the cells is promoted, thereby increasing the sensitivity of the breast cancer cells to ionizing radiation including the X-rays.
Owner:SUZHOU UNIV
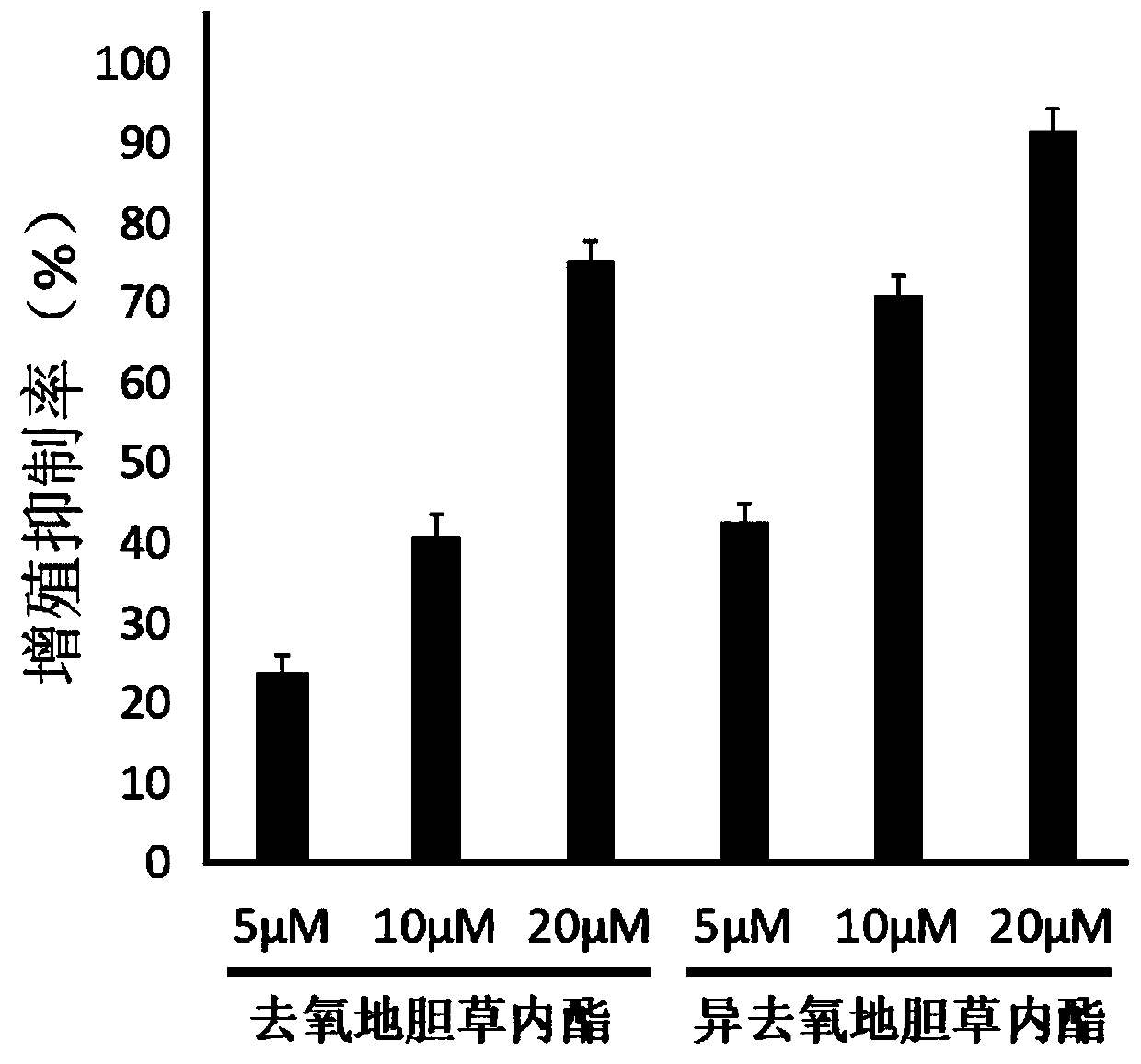
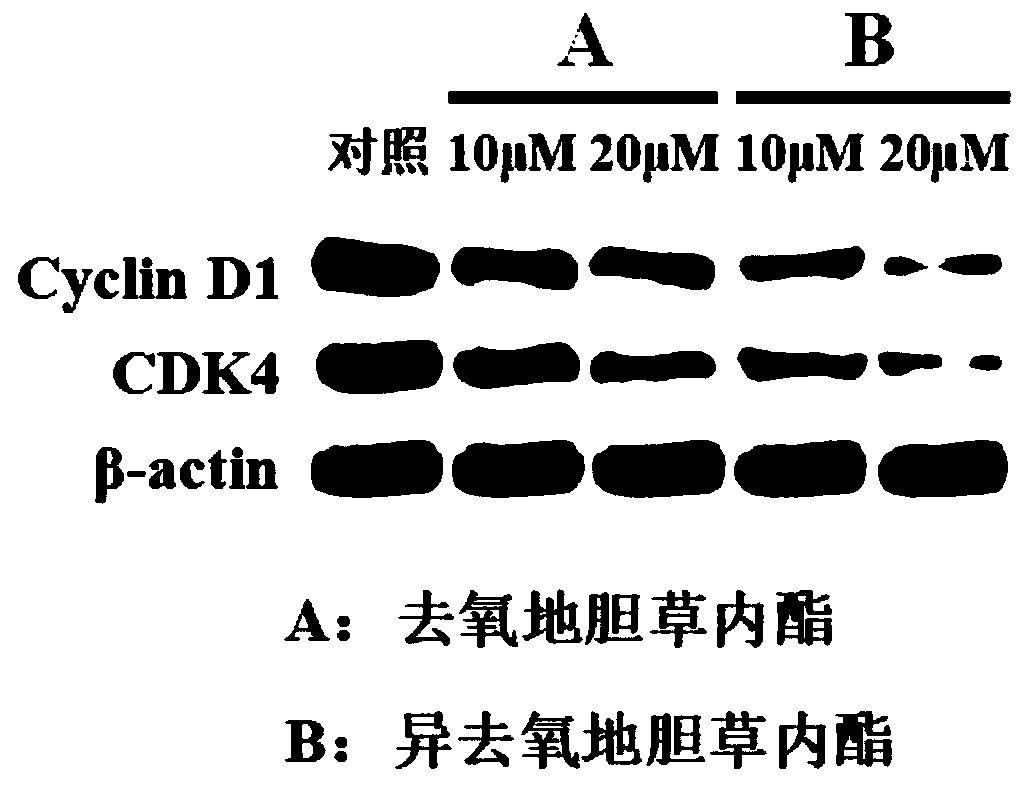
Application of deoxyelephantolide or isodeoxyelephantolide in preparation of a medicine used for resisting lung fibrosis
ActiveCN111084773ADivide and proliferateProliferation inhibitionOrganic active ingredientsRespiratory disorderCell divisionCyclin D1
The invention discloses an application of deoxyelephantolide or isodeoxyelephantolide in preparation of a medicine used for resisting lung fibrosis. Researches find that the deoxyelephantolide or theisodeoxyelephantolide can be used for effectively inhibiting the proliferation of human embryonic lung fibroblasts HFL1. Western Blot results show that the proliferation inhibition effect of the deoxyelephantolide or the isodeoxyelephantolide on human embryonic lung fibroblasts HFL1 is likely to be related with the situation that the human embryonic lung fibroblasts is blocked in a G1 phase and can not realize cell division and proliferation through down regulation of the expression of cyclin D1 and CDK4. Therefore, the deoxyelephantolide or the isodeoxyelephantolide has the prospect of beingdeveloped into the medicine used for resisting the lung fibrosis.
Owner:仁合熙德隆药业有限公司
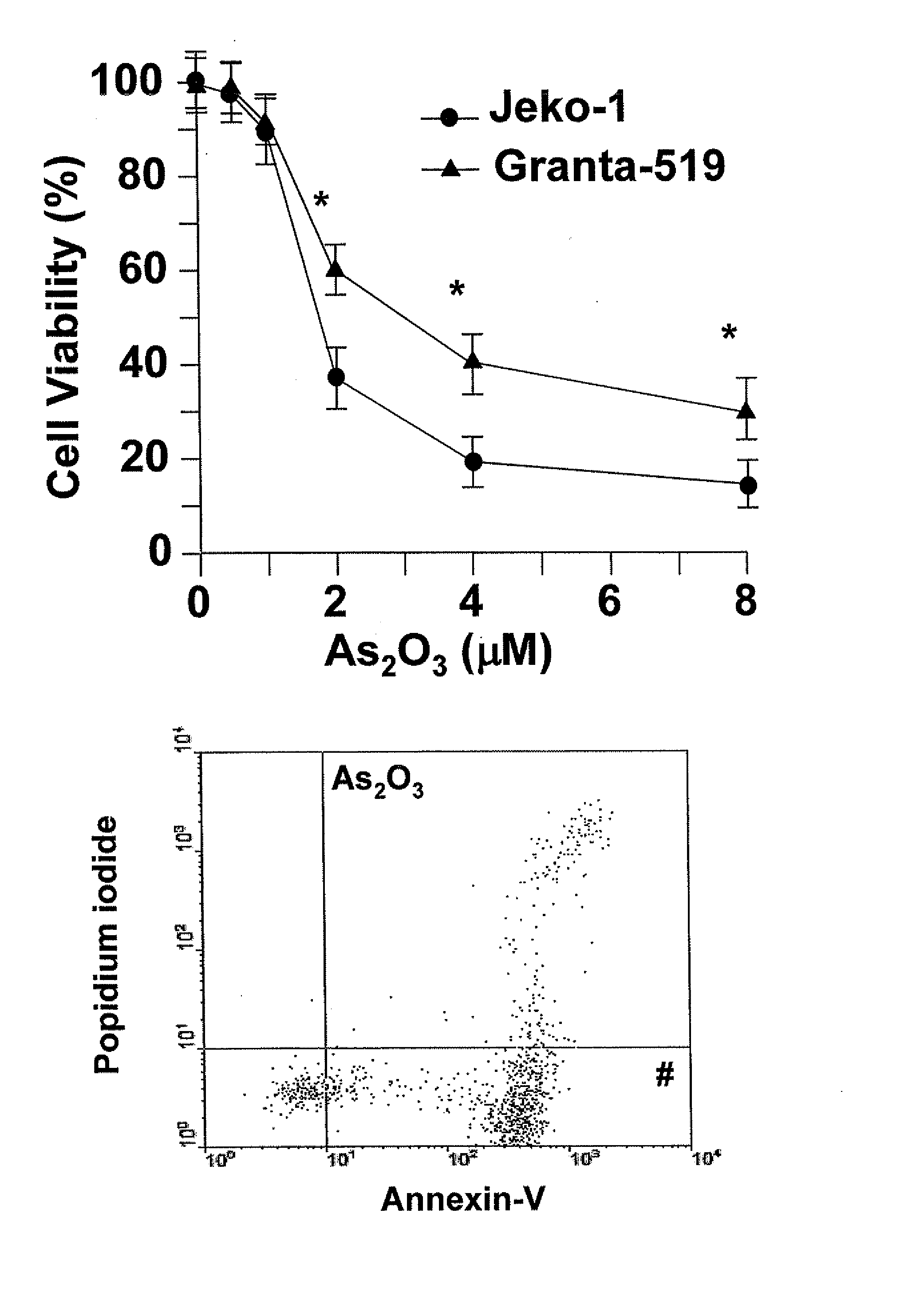
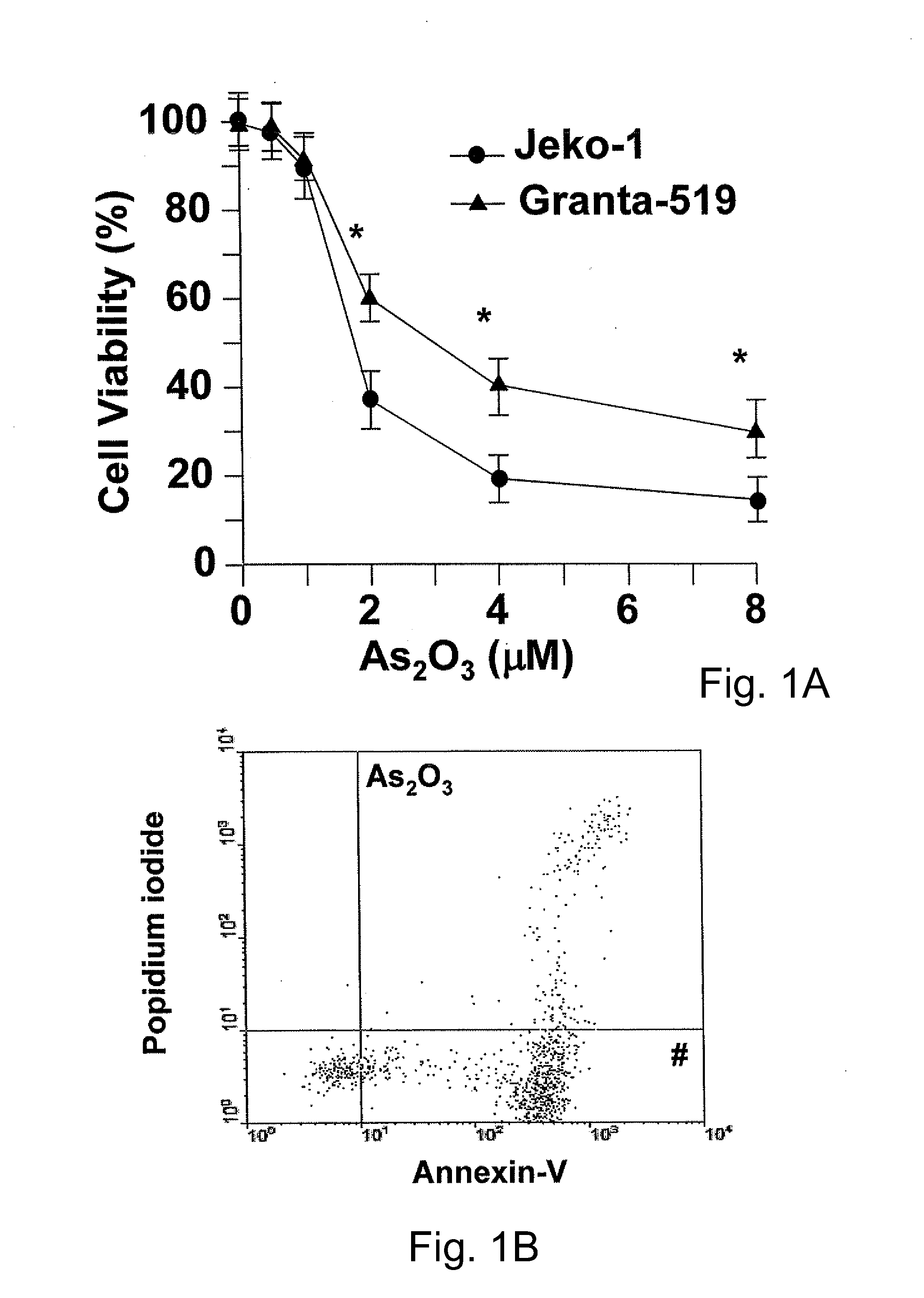
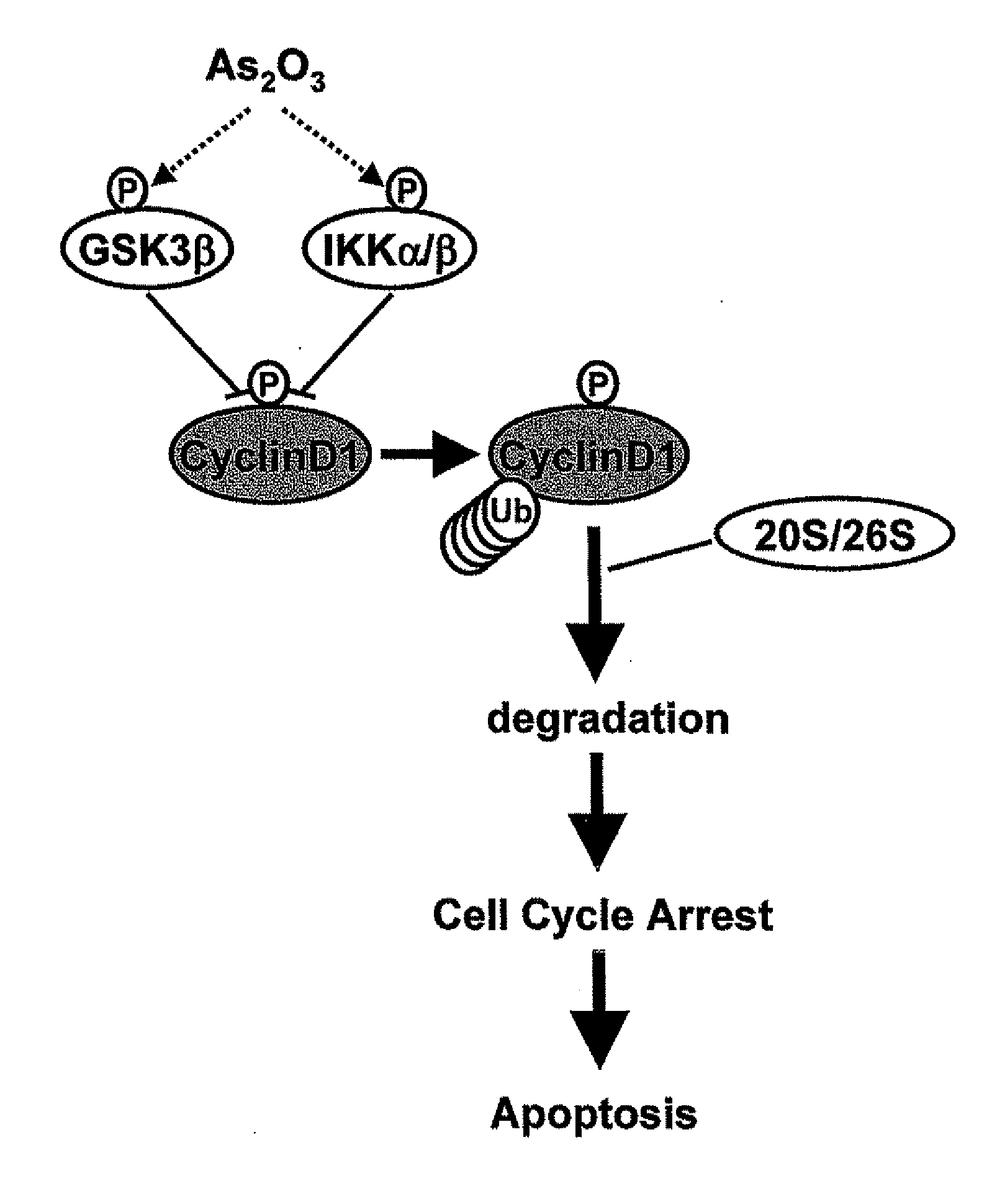
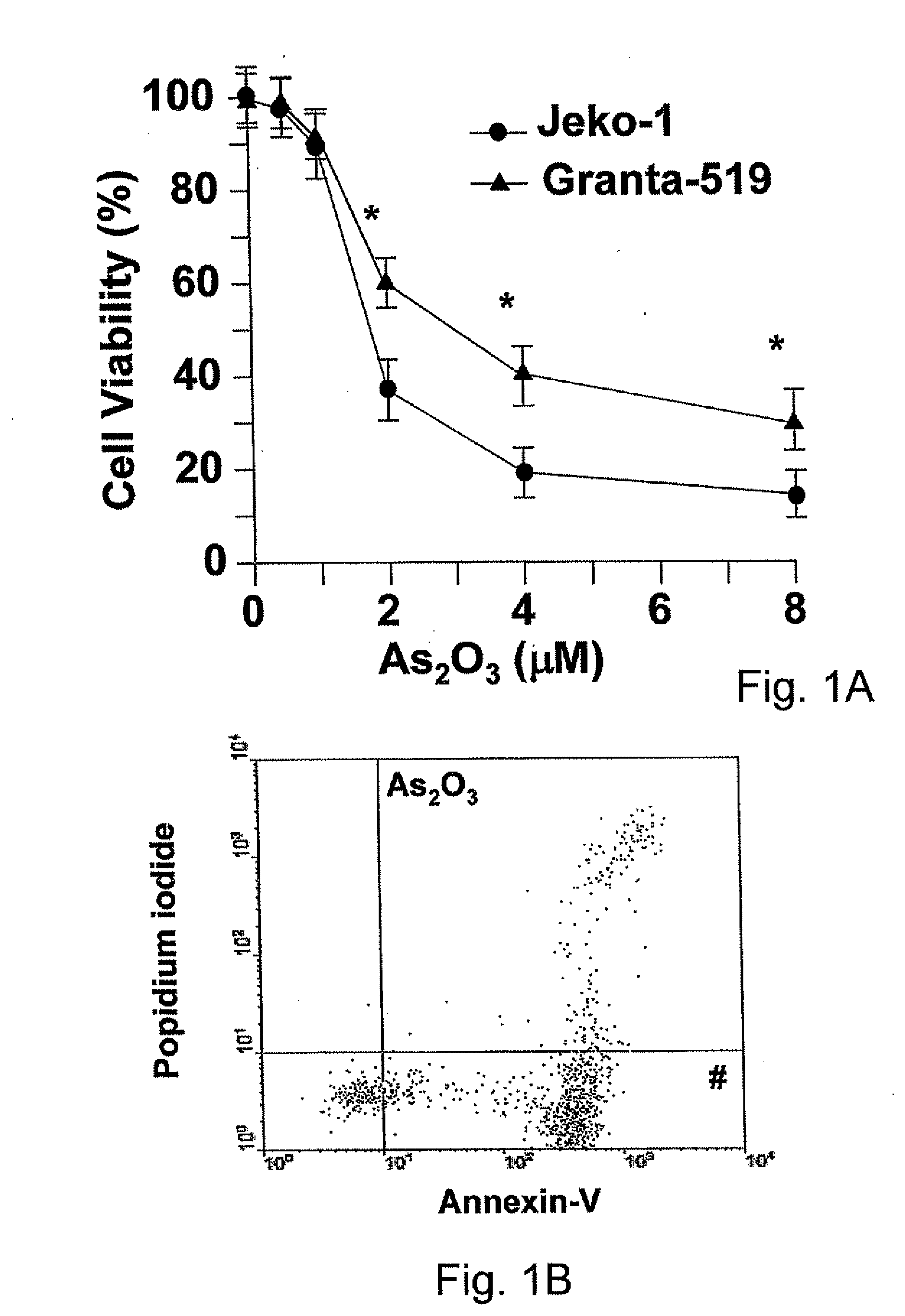
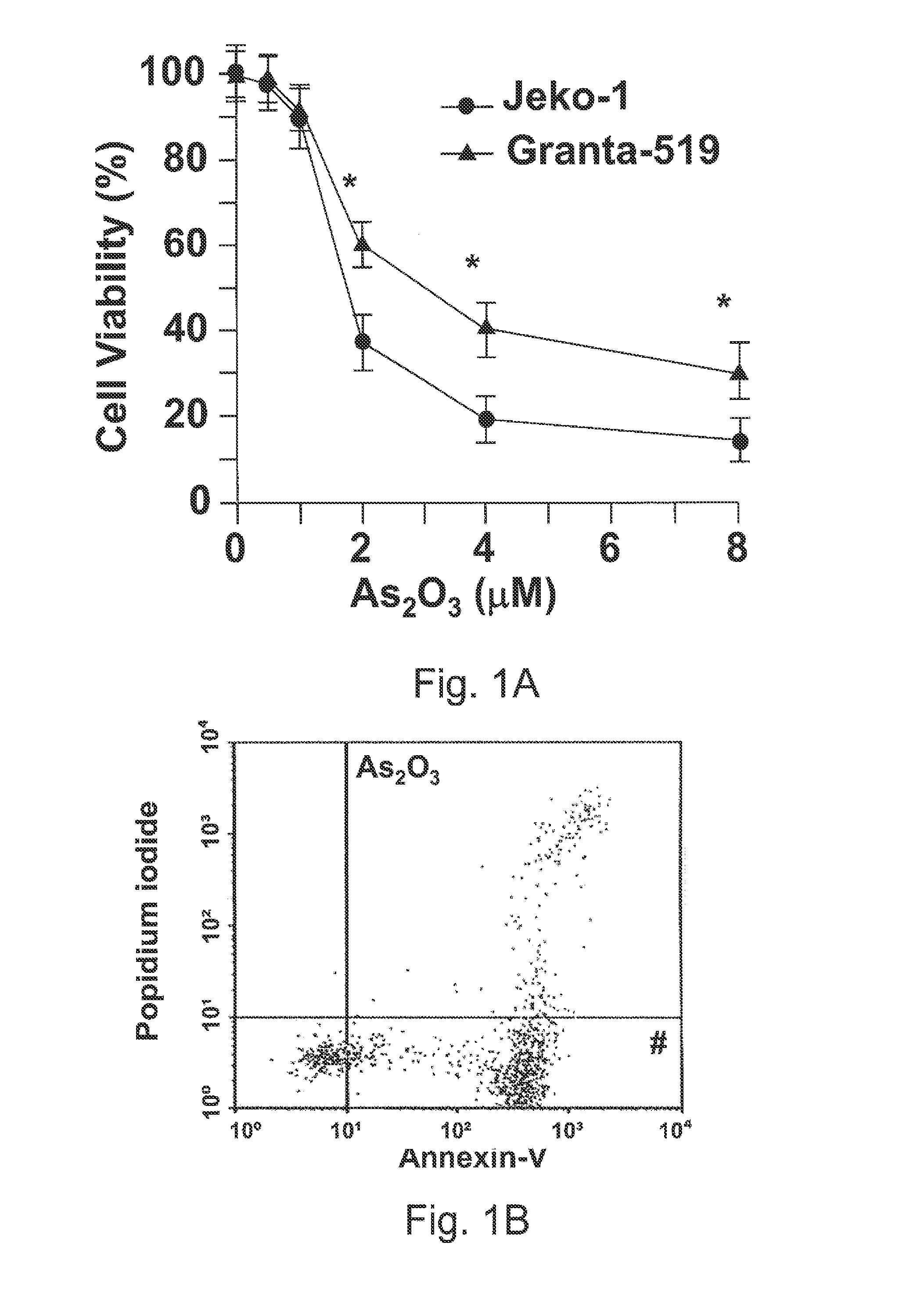
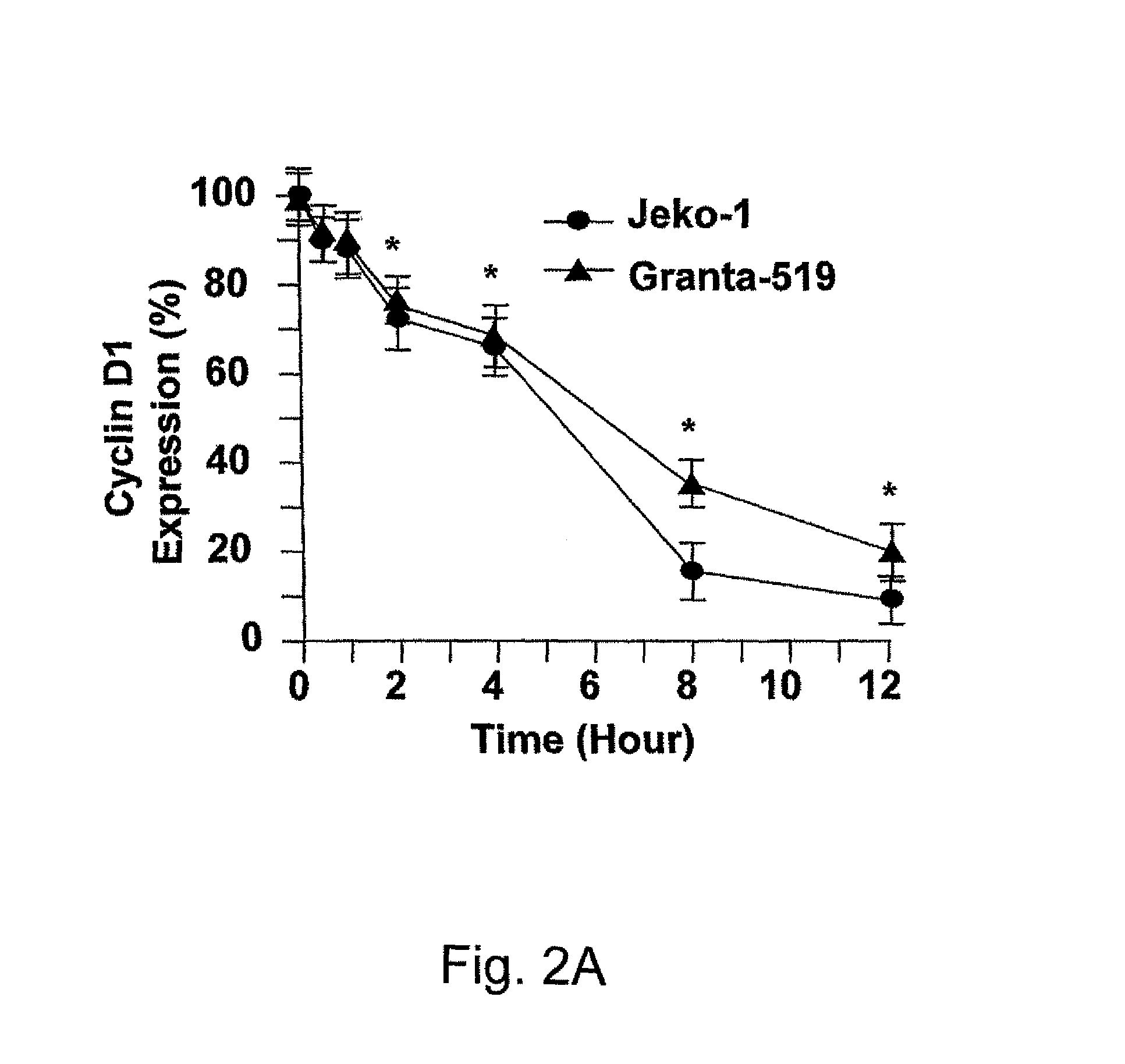
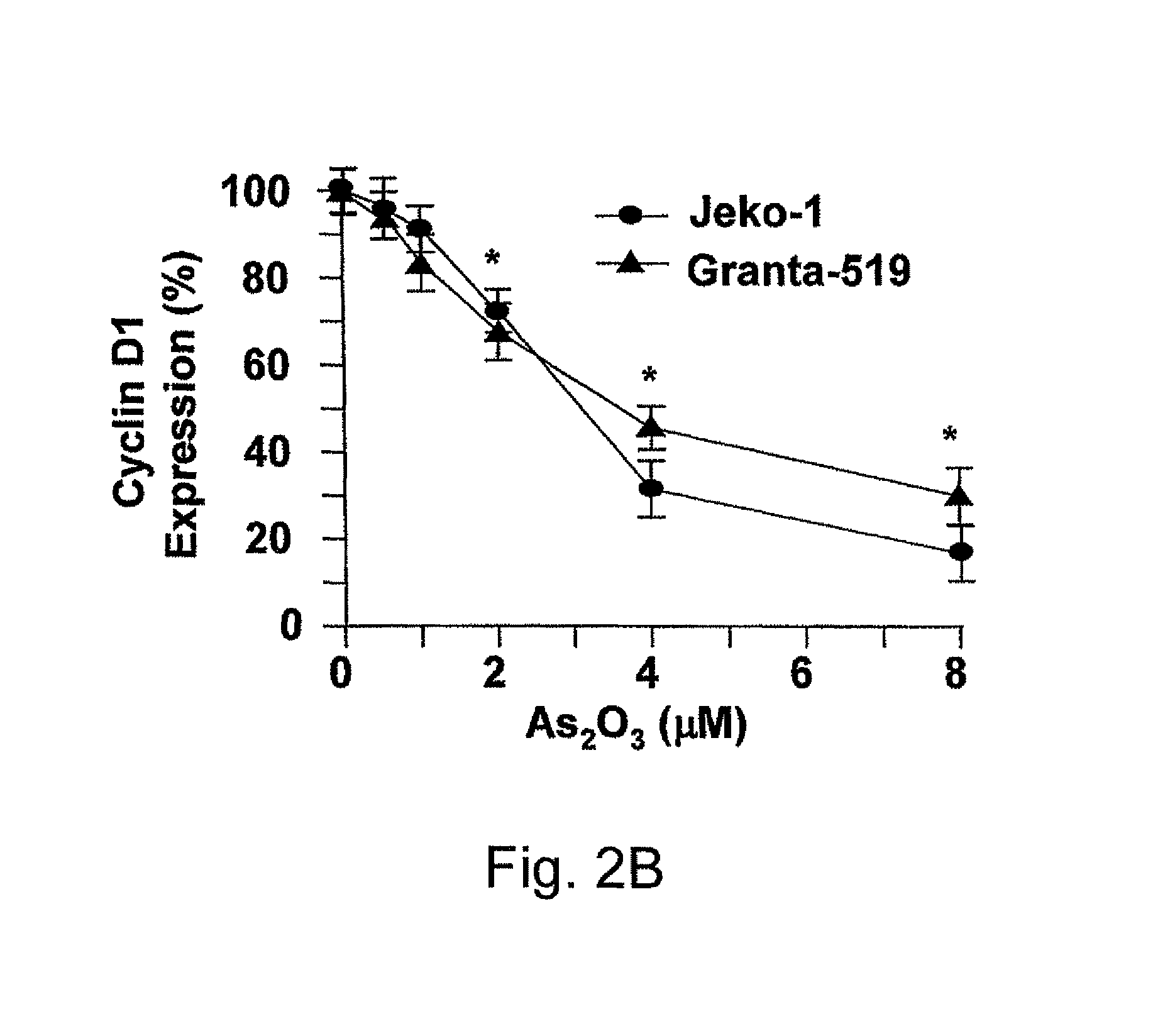
Method for inhibiting cancer using arsenic trioxide
The invention provides a method for treating cancers that are dependent on cyclin D1 for proliferation, survival, metastasis and differentiation, involving administering a composition containing an effective amount of arsenic trioxide to an affected patient. The arsenic trioxide can be administered orally, for example, as a solution, suspension, syrup, emulsion, tablet, or capsule. The composition can also contain one or more pharmaceutically acceptable carriers and / or excipients.
Owner:THE UNIVERSITY OF HONG KONG
Antibodies for treating cancer
InactiveUS20110027296A1Immunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsCyclin D1Therapeutic treatment
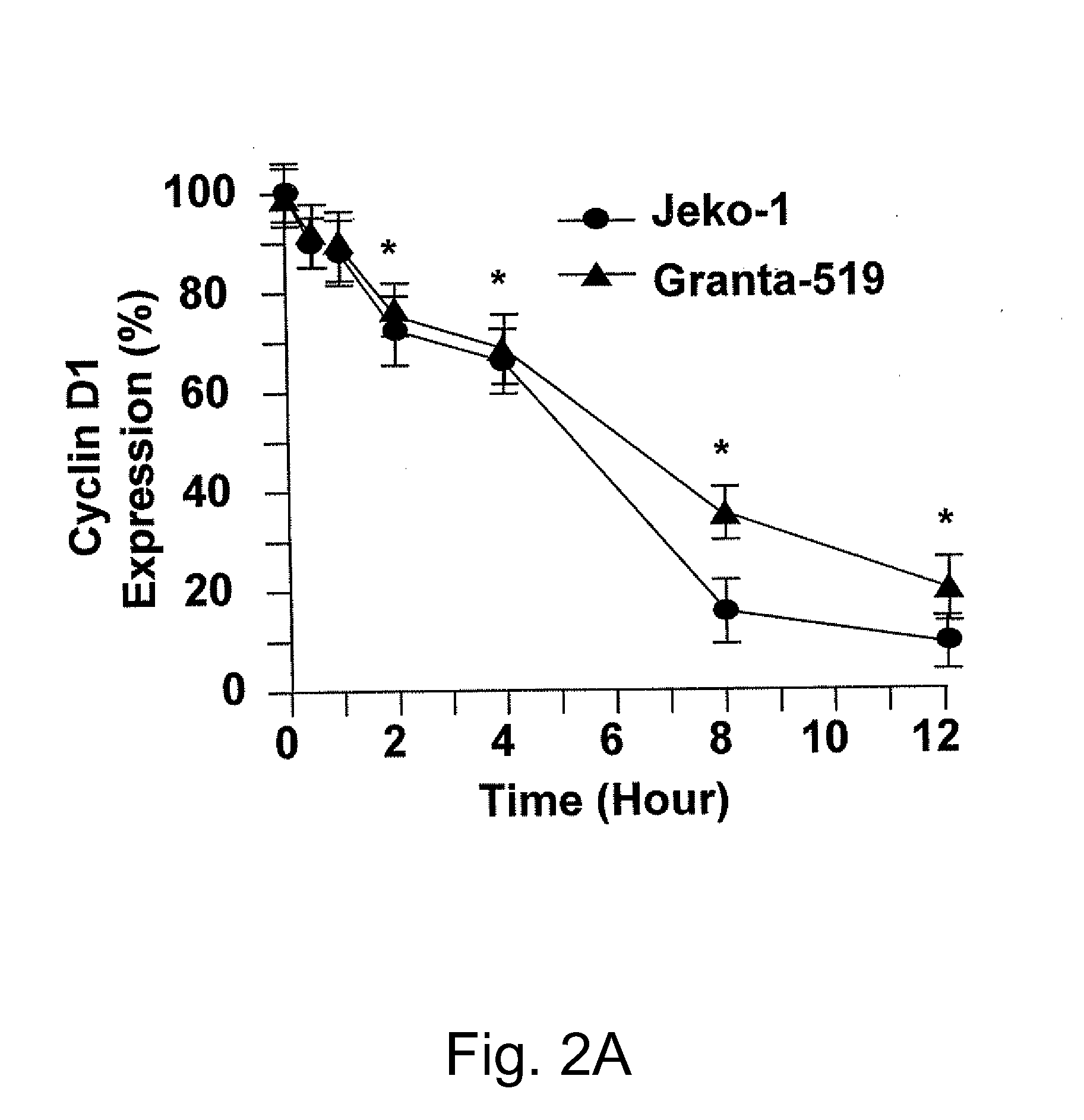
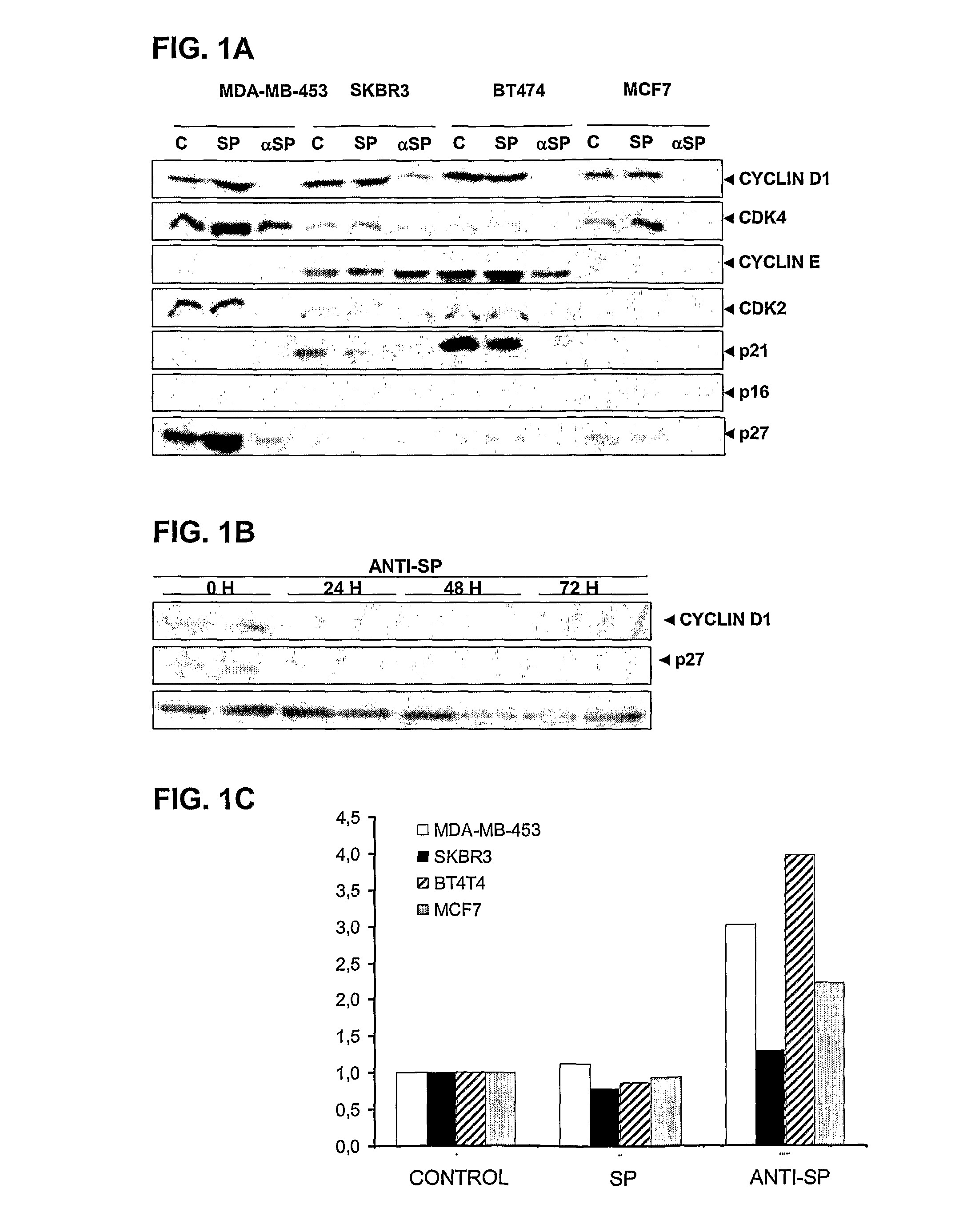
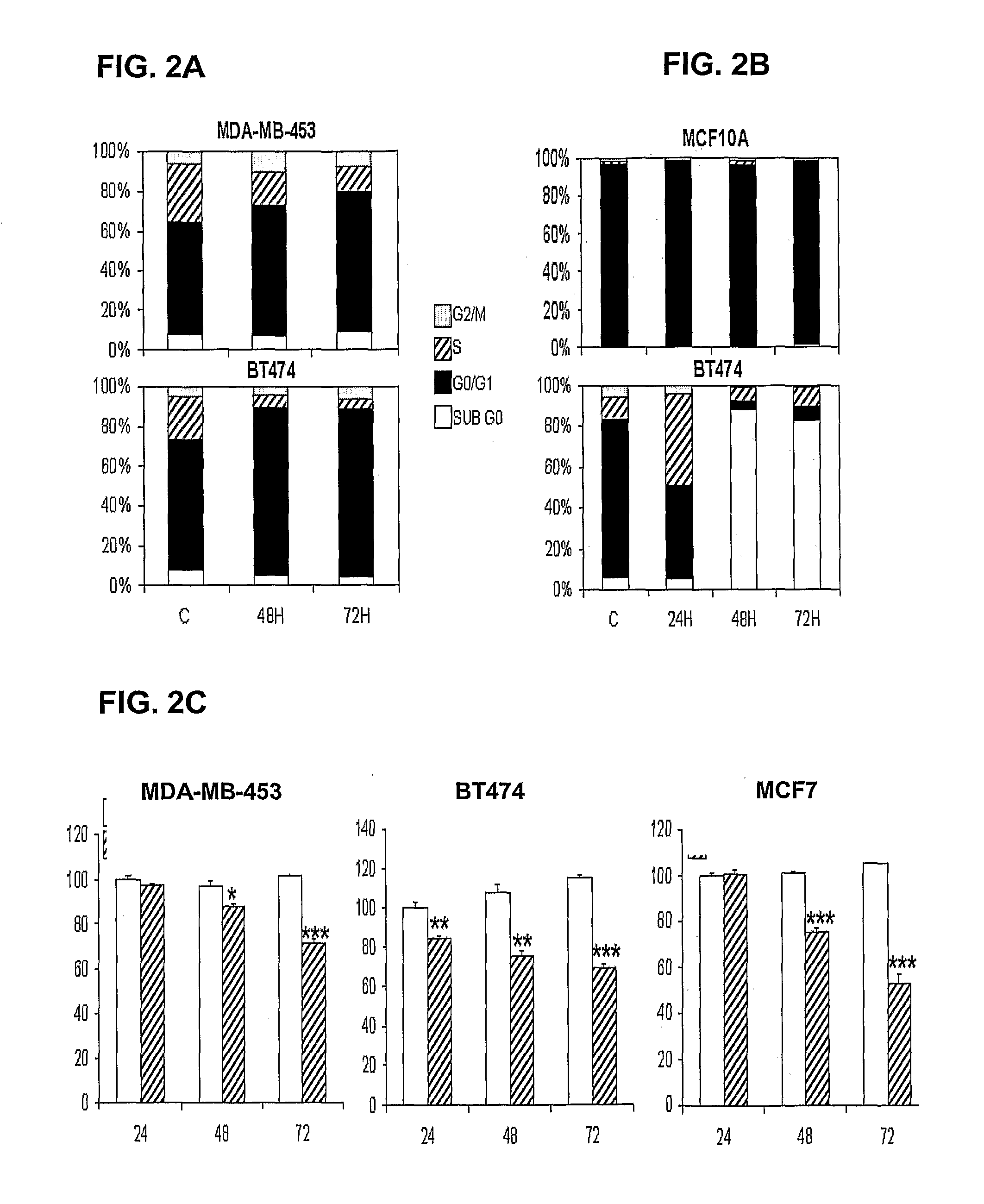
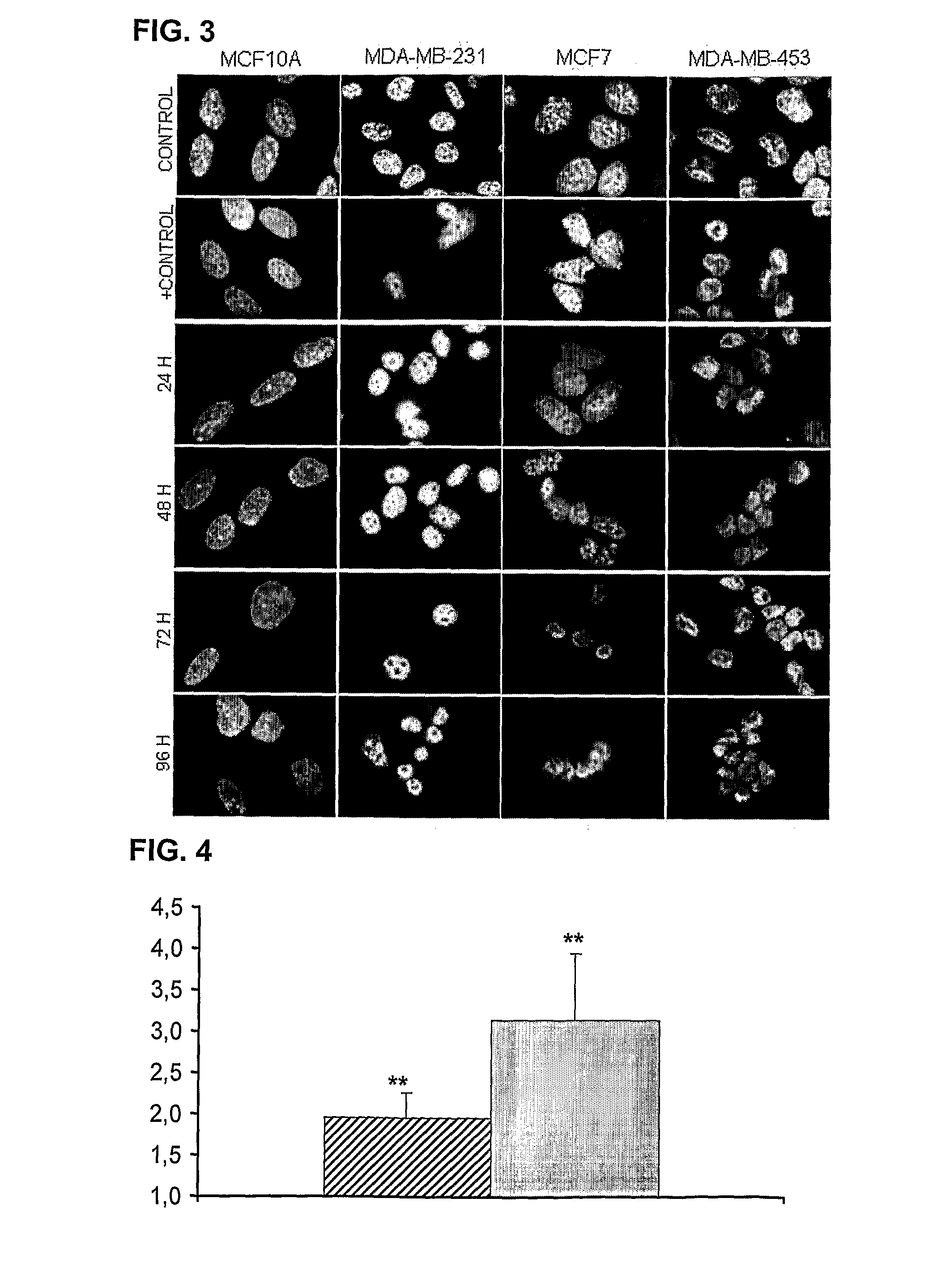
The invention relates to the use of an antibody or a fragment thereof, specific against substance P, for the manufacture of a medicament for therapeutic treatment of a cancer expressing NK1 receptor and / or a ErbB receptor family member (i.e. the epidermal growth factor receptors family), by direct administration to a mammal, including a human. The administration of an antibody against substance P to a panel of breast cancer cell lines, inhibits HER2 activation and expression, causing a downregulation of different genes involved in cell cycle progression, like cyclin D1, cyclin E, cdk4 and cdk2. A decrease in proliferation and an arrest in G1 phase of cell cycle are observed. On the other hand, the antibody causes an induction of apoptosis.
Owner:UNIV DE BARCELONA +2
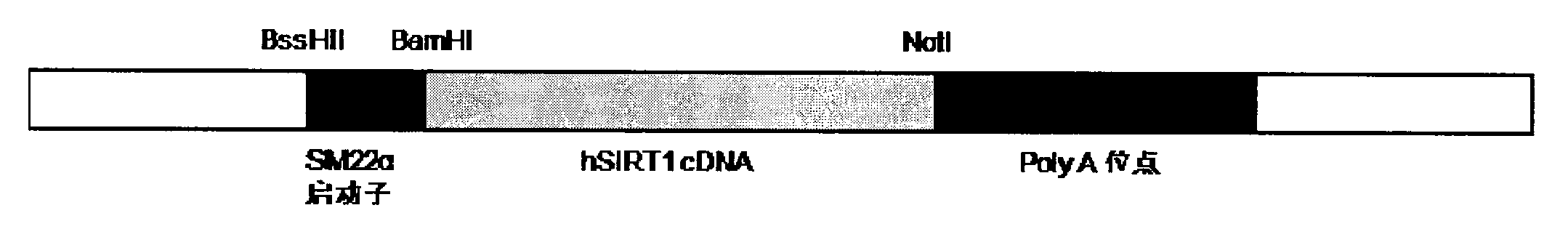
Application of SIRT (Silent Mating Type Information Regulation 2 Homolog 1) to prepare medicine used for regulating down the expression of cyclin D1
InactiveCN102008718AInhibition formationPeptide/protein ingredientsGenetic material ingredientsCyclin D1Viral vector
The invention relates to the application of SIRT1 (Silence Information Regulator Tuin1) to prepare medicine used for regulating down the expression of cyclin D1. Particularly, the medicine is of a delegiert protein type, and the form of the medicine can be any form suitable for the application of protein medicine. The medicine is a reorganization nucleic acid construction body capable of expressing SIRT1 protein. The expression carrier of the medicine can be any expression carrier which is suitable for gene therapy and comprises a virus carrier, such as a reorganization retrovirus carrier, an adenovirus carrier, an adeno-associated virus carrier, a herpes simplex virus carrier, a vaccinia virus carrier, and the like and a nonviral carrier, such as a bacteriophage carrier, a replicon carrier, and the like.
Owner:THE INST OF BASIC MEDICAL SCI OF CHINESE ACAD OF MEDICAL SCI
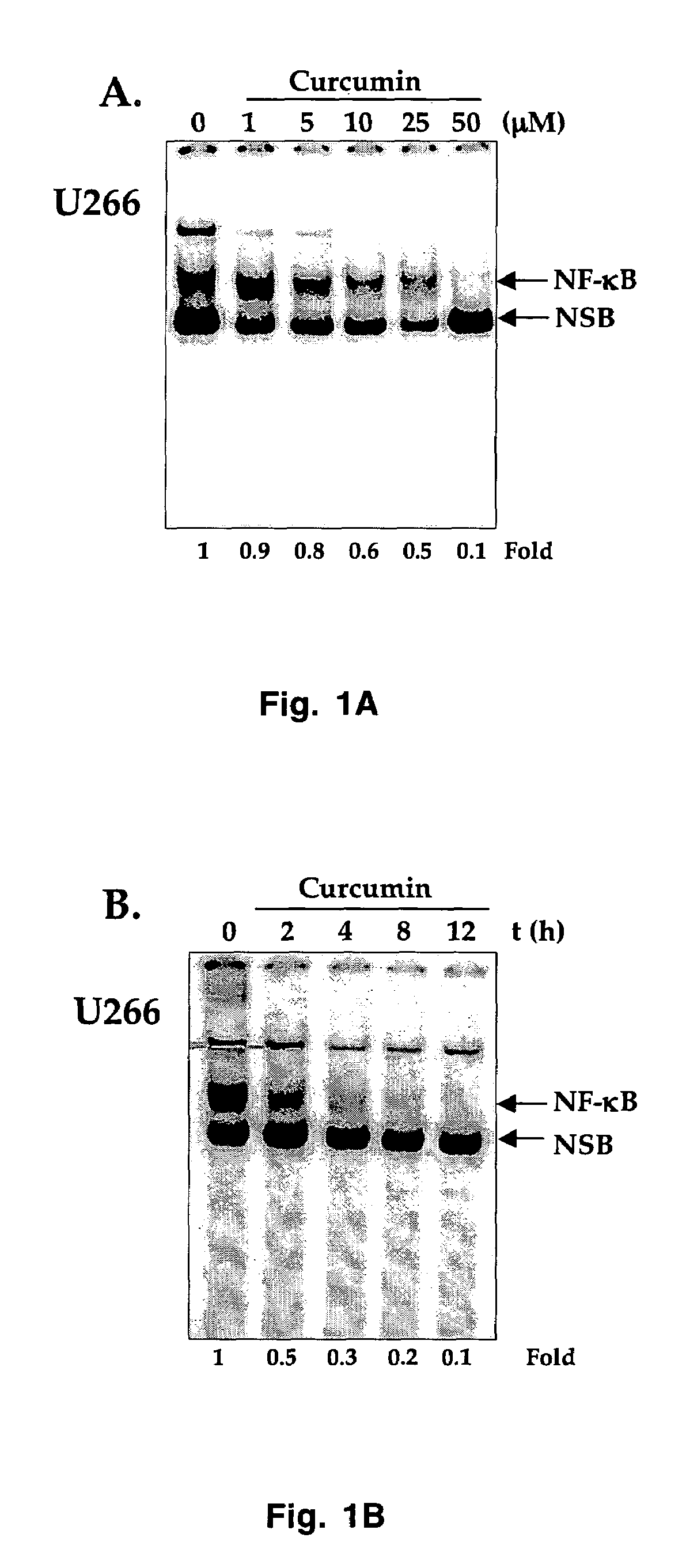
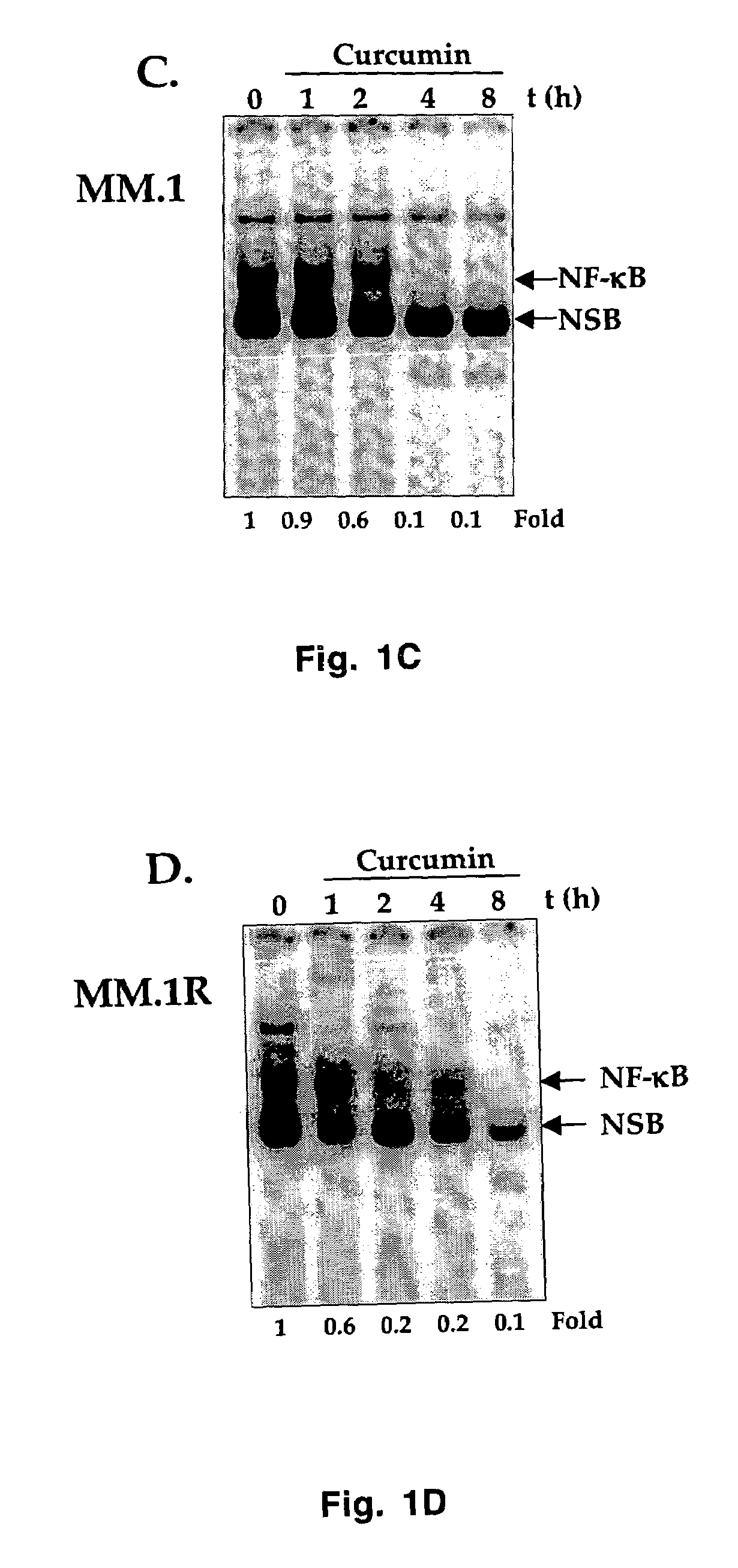
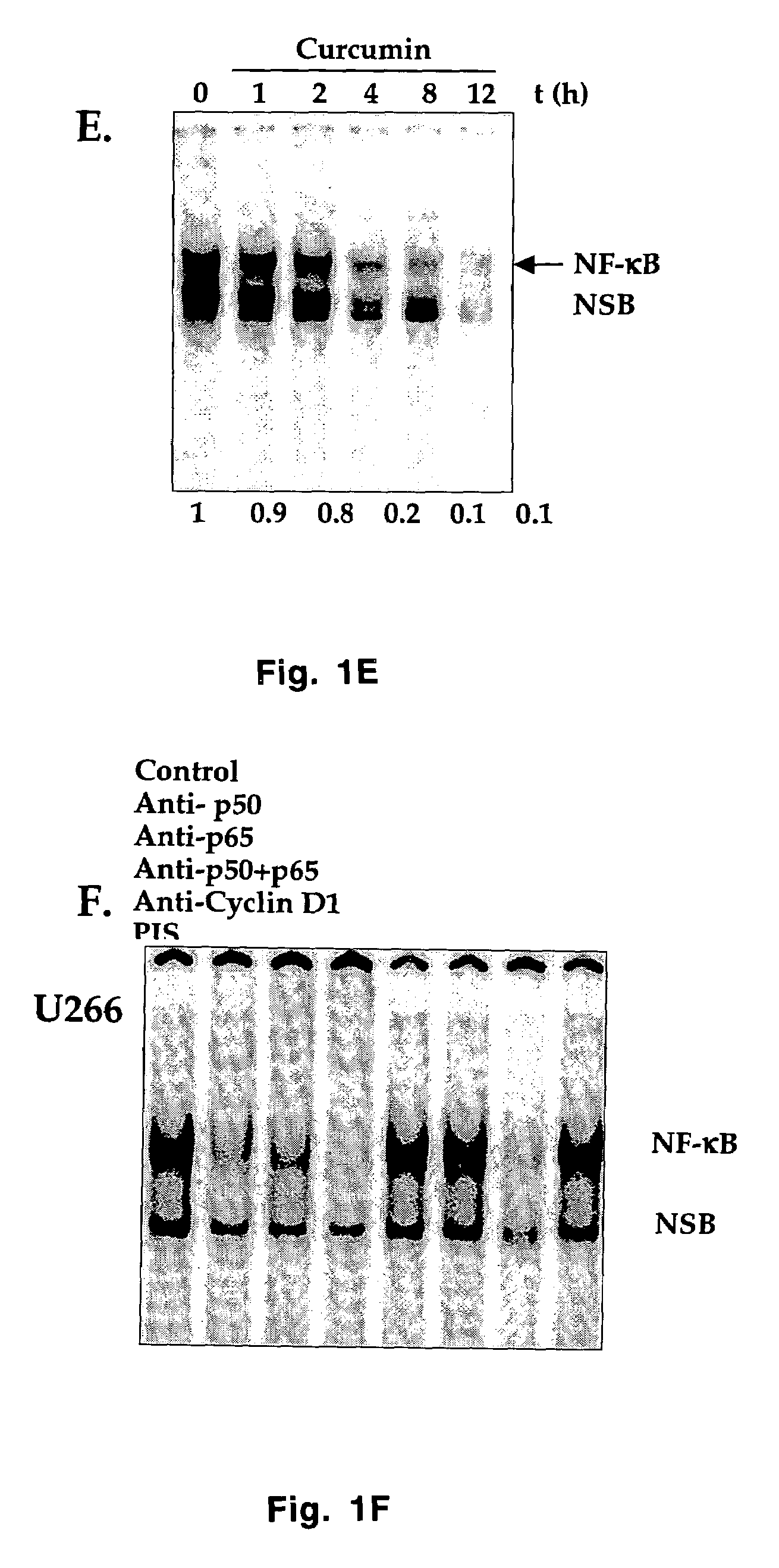
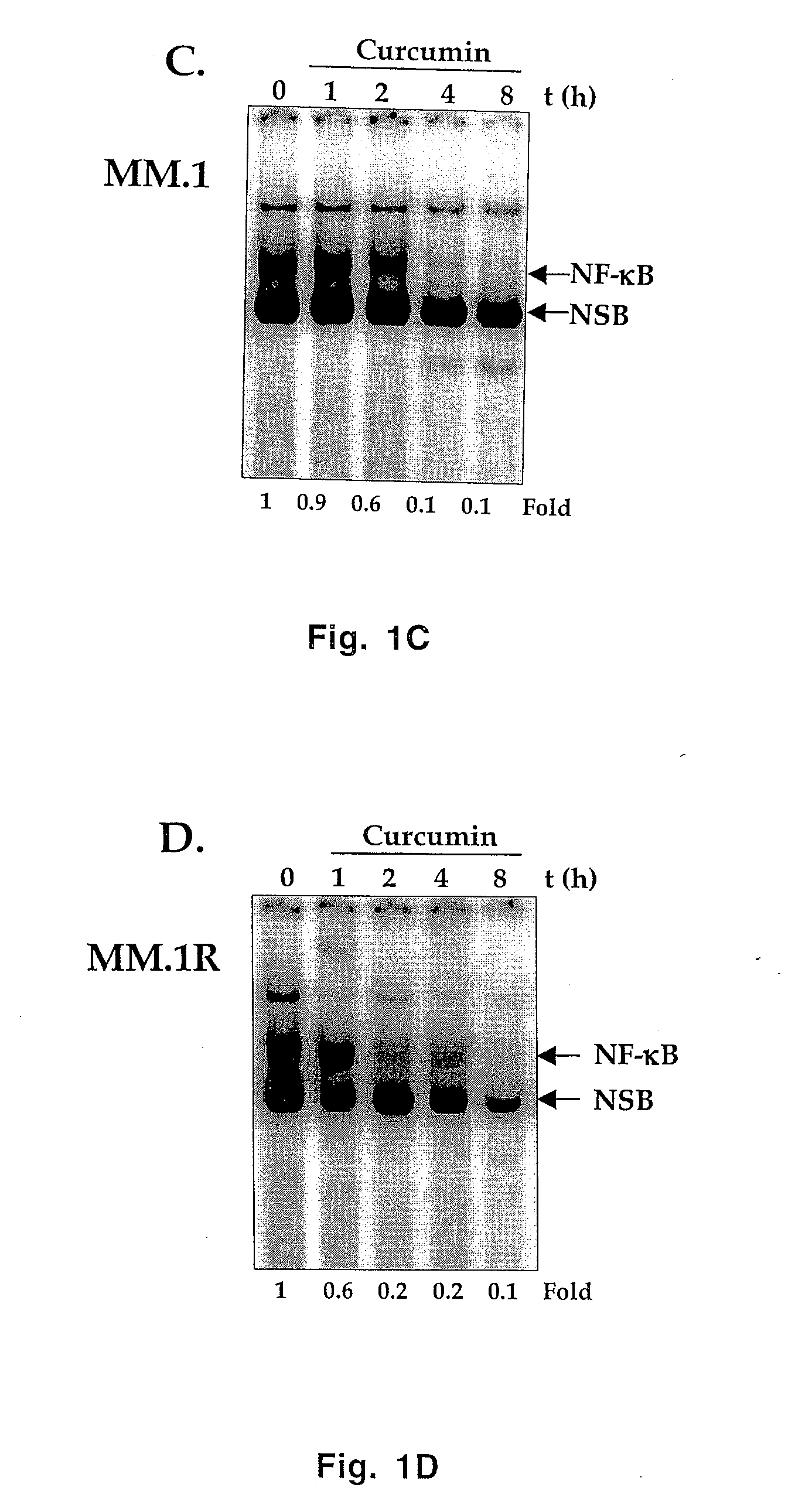
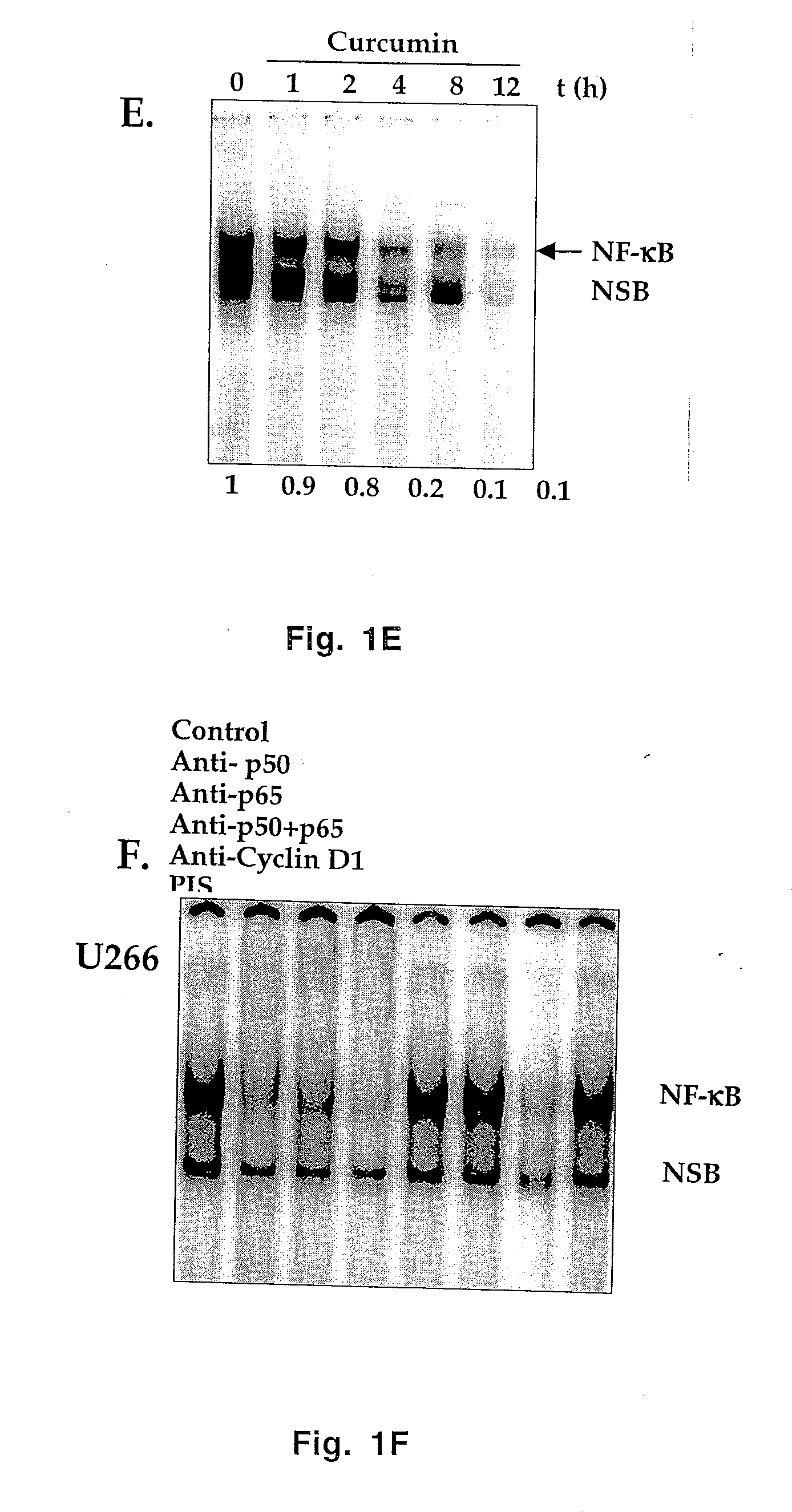
Treatment of human multiple myeloma by curcumin
InactiveUS7196105B2Suppression of and arrestLittle and toxicityBiocidePeptide/protein ingredientsInterleukin 6Cyclin D1
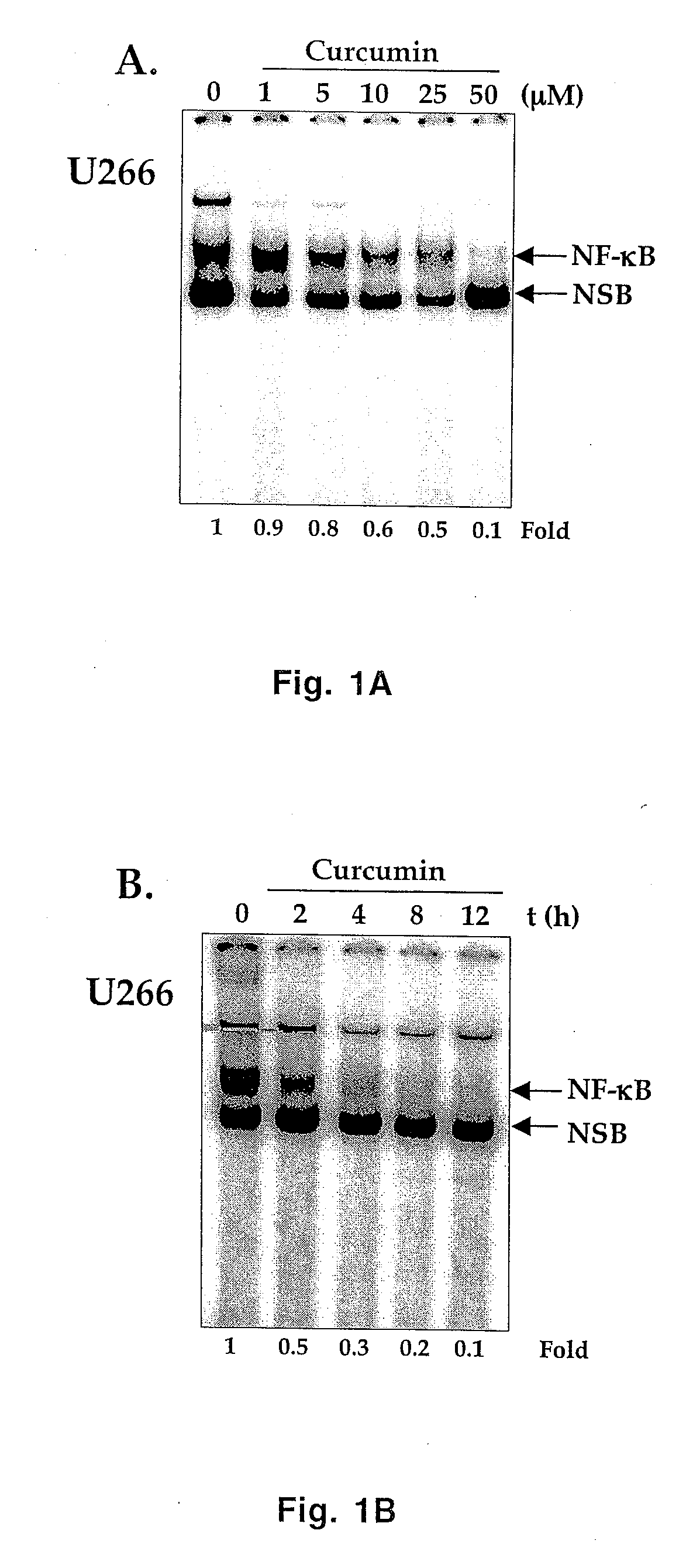
All multiple myeloma cell lines examined showed constitutively active IκB kinase (IKK), IκBα phosphorylation and constitutively active NF-κB. Curcumin, a chemopreventive agent, suppressed constitutive IκBα phosphorylation through inhibition of IKK activity and downregulated NF-κB. Curcumin also downregulated expression of NF-κB-regulated gene products such as IκBα, Bcl-2, Bcl-xL, cyclin D1 and interleukin-6. Consequently, curcumin suppressed multiple myeloma cell proliferation and arrested cells at the G1 / S phase of the cell cycle. Curcumin also induced apoptosis and chemosensitivity to vincristine. Overall, results presented herein provide a molecular basis for the treatment of multiple myeloma patients with this pharmacologically safe agent.
Owner:RES DEVMENT FOUND
Method for Inhibiting Cancer Using Arsenic Trioxide
The invention provides a method for treating cancers that are dependent on cyclin D1 for proliferation, survival, metastasis and differentiation, involving administering a composition containing an effective amount of arsenic trioxide to an affected patient. The arsenic trioxide can be administered orally, for example, as a solution, suspension, syrup, emulsion, tablet, or capsule. The composition can also contain one or more pharmaceutically acceptable carriers and / or excipients.
Owner:THE UNIVERSITY OF HONG KONG
RNA Interference Mediated Inhibition of Cyclin D1 Gene Expression Using Short Interfering Nucleic Acid (siNA)
InactiveUS20090137512A1Improve bioavailabilityMinimize the possibilitySenses disorderNervous disorderCyclin D1Cyclin
This invention relates to compounds, compositions, and methods useful for modulating cyclin (e.g., cyclin D1) and / or cyclin dependent kinase (CDK) gene expression using short interfering nucleic acid (siNA) molecules. This invention also relates to compounds, compositions, and methods useful for modulating the expression and activity of other genes involved in pathways of cyclin (e.g., cyclin D1) and / or cyclin dependent kinase (CDK) gene expression and / or activity by RNA interference (RNAi) using small nucleic acid molecules. In particular, the instant invention features small nucleic acid molecules, such as short interfering nucleic acid (siNA), short interfering RNA (siRNA), double-stranded RNA (dsRNA), micro-RNA (miRNA), and short hairpin RNA (shRNA) molecules and methods used to modulate the expression of cyclin (e.g., cyclin D1) and / or cyclin dependent kinase (CDK) genes.
Owner:SIRNA THERAPEUTICS INC
Diagnostic panel of cancer antibodies and methods for use
InactiveUS20090215091A1Determine effectivenessBiological material analysisBiological testingImmune complex formationCyclin D1
The invention provides a method for detection of a malignancy in a specimen of bodily fluid. The method comprises contacting the specimen with at least two antigens selected from the group consisting of p53, IGFBP2, Topo2α, cathepsin D, cyclin B, cyclin D1, MUC1, HER-2 / neu and CEA. The method further comprises incubating the specimen and the antigen for a duration and under conditions that are sufficient for the formation of immunocomplexes; and detecting the presence or absence of immunocomplex formation between the antigens and antibodies specific for the antigens in the specimen, thereby determining the presence or absence of the malignancy. Also provided is a method for monitoring the effectiveness of cancer therapy related to a malignancy in a warm-blooded animal, a method for distinguishing between Stage I and Stage II colorectal cancer in a specimen of bodily fluid.
Owner:UNIV OF WASHINGTON
Treatment of Human Multiple Myeloma by Curcumin
InactiveUS20060233899A1Suppression of and arrestLittle and toxicityBiocidePeptide/protein ingredientsInterleukin 6Phosphorylation
All multiple myeloma cell lines examined showed constitutively active IκB kinase (IKK), IκBα phosphorylation and constitutively active NF-κB. Curcumin, a chemopreventive agent, suppressed constitutive IκBα phosphorylation through inhibition of IKK activity and downregulated NF-κB. Curcumin also downregulated expression of NF-κB-regulated gene products such as IκBα, Bcl-2, Bcl-xL, cyclin D1 and interleukin-6. Consequently, curcumin suppressed multiple myeloma cell proliferation and arrested cells at the G1 / S phase of the cell cycle. Curcumin also induced apoptosis and chemosensitivity to vincristine. Overall, results presented herein provide a molecular basis for the treatment of multiple myeloma patients with this pharmacologically safe agent.
Owner:AGGARWAL BHARAT
Use of molecular marker detection object in preparation of kit for detecting liver cancer
InactiveCN104673894AOvercome the defect that the accuracy rate is still relatively lowImprove accuracyMicrobiological testing/measurementCyclin D1Liver cancer
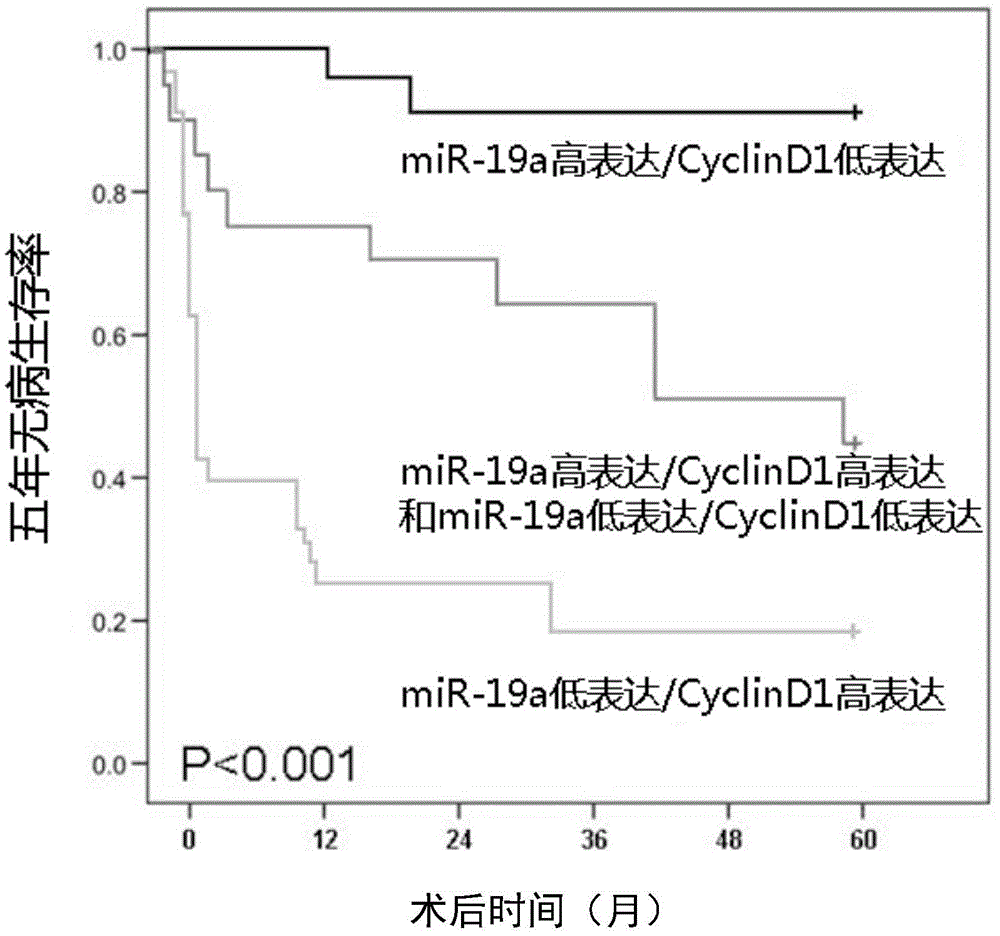
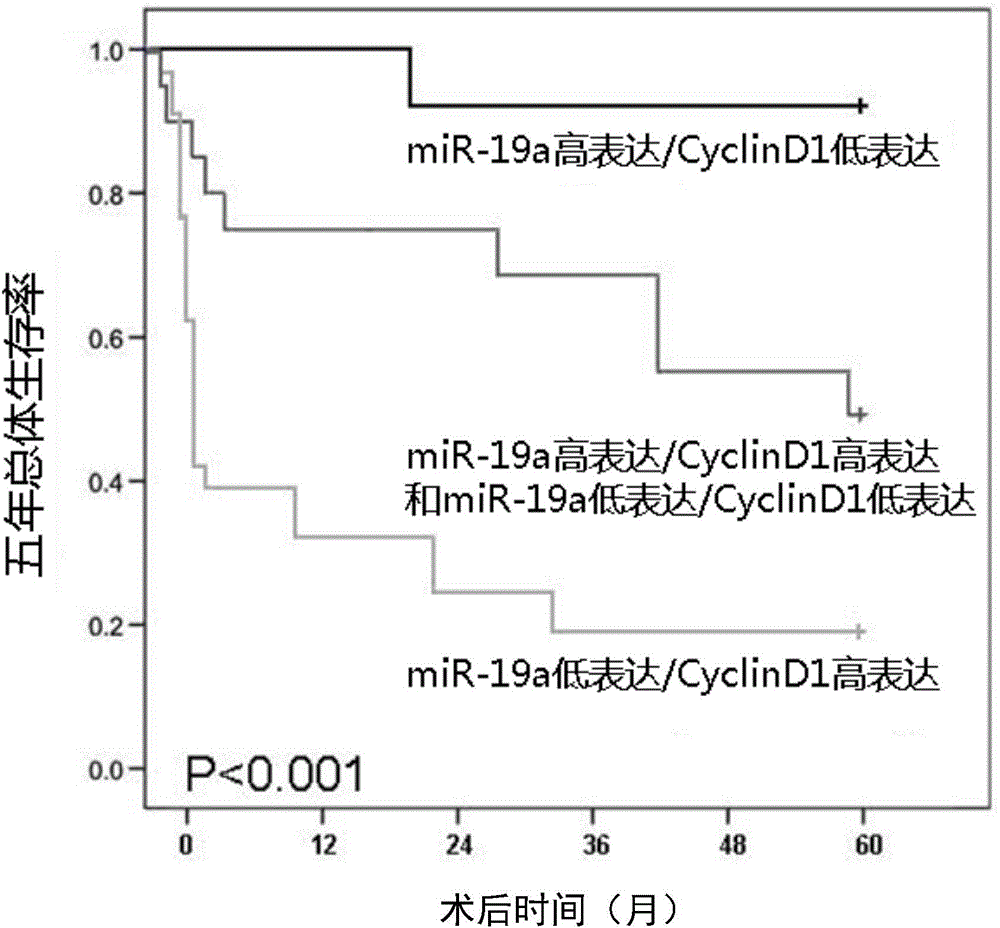
The invention provides use of a molecular marker detection object in preparation of a kit for detecting liver cancer. The molecular marker detection object comprises a detection object which can specifically detect miR-19a and a detection object which can specifically detect cell cycle proteins D1(Cyclin D1). According to the use of the molecular marker detection object, the accuracy rate of detection of histopathologic stage and poor prognosis of the liver cancer can be improved.
Owner:INST OF CHINESE MATERIA MEDICA CHINA ACAD OF CHINESE MEDICAL SCI +1
Method for inhibiting cancer using arsenic trioxide
The invention provides a method for treating cancers that are dependent on cyclin D1 for proliferation, survival, metastasis and differentiation, involving administering a composition containing an effective amount of arsenic trioxide to an affected patient. The arsenic trioxide can be administered orally, for example, as a solution, suspension, syrup, emulsion, tablet, or capsule. The composition can also contain one or more pharmaceutically acceptable carriers and / or excipients.
Owner:THE UNIVERSITY OF HONG KONG
Method of screening for melanoma by detecting an increase in cyclin D1
The present invention provides methods of screening for the presence of premalignant melanocytes in a sample from a patient. The methods comprise contacting a nucleic acid sample from a biological sample from the patient with a probe which binds selectively to a target polynucleotide sequence on a chromosomal region which is amplified in melanoma cells.
Owner:RGT UNIV OF CALIFORNIA
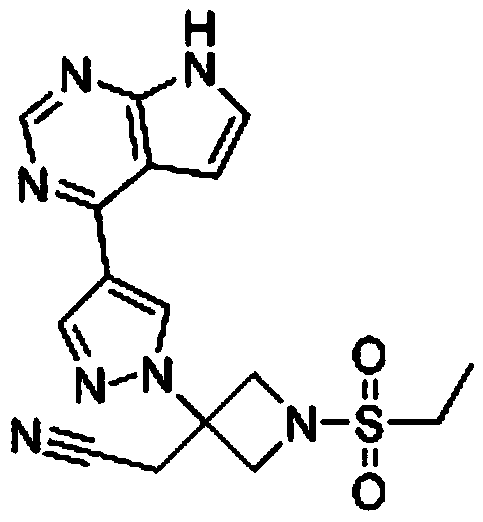
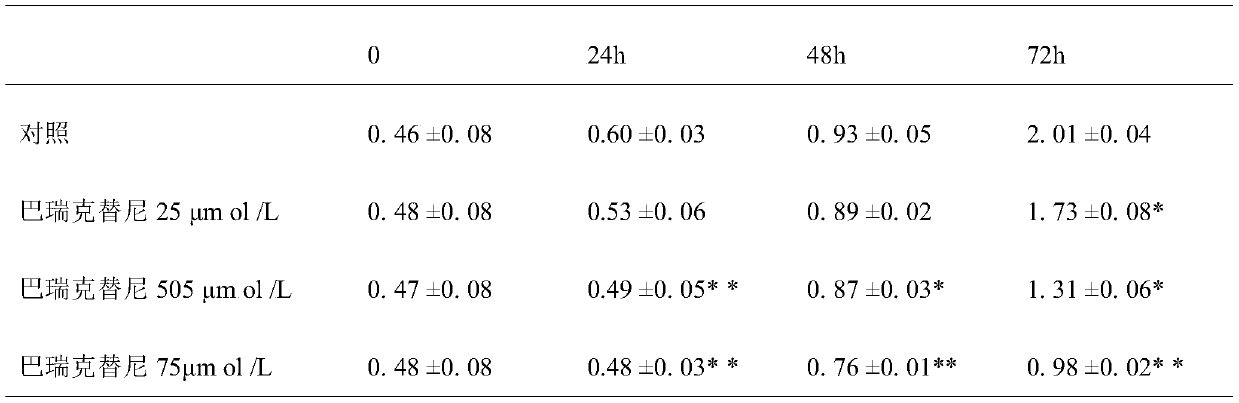
Application of Baricitinib in preparation of medicament for treating senile calcific heart valve disease
InactiveCN110327346ALower the targetGood conditionOrganic active ingredientsCardiovascular disorderDiseaseHeart valvular disease
The invention relates to new application of a medicament, in particular to application of Baricitinib in preparation of a medicament for treating senile calcific heart valve disease. The research of the invention shows that: by inhibiting a JAK pathway and selectively activating a substrate-STAT of the Baricitinib, the substrate-STAT is translocated to the nucleus so as to act on downstream targetgenes such as cyclin D1, BCL-xL, C-MYC, P21 WAF1 / CIP1 and the like, and the cell proliferation, differentiation and apoptosis is regulated, so that degeneration, fibrosis, thickening and calcification of heart valve connective tissues of a patient are obviously improved, and cardiomyocytes are protected. Compared with the prior art, the medicament can reduce various indexes of patients with thesenile calcific heart valve disease, improve the disease condition and improve the life quality of the patients with the senile calcific heart valve disease.
Owner:HUISHENG MEDICAL TECH XUZHOU CO LTD
Kit for predicting recurrence and transference risk of patient suffering from early-stage operable esophageal squamous carcinoma
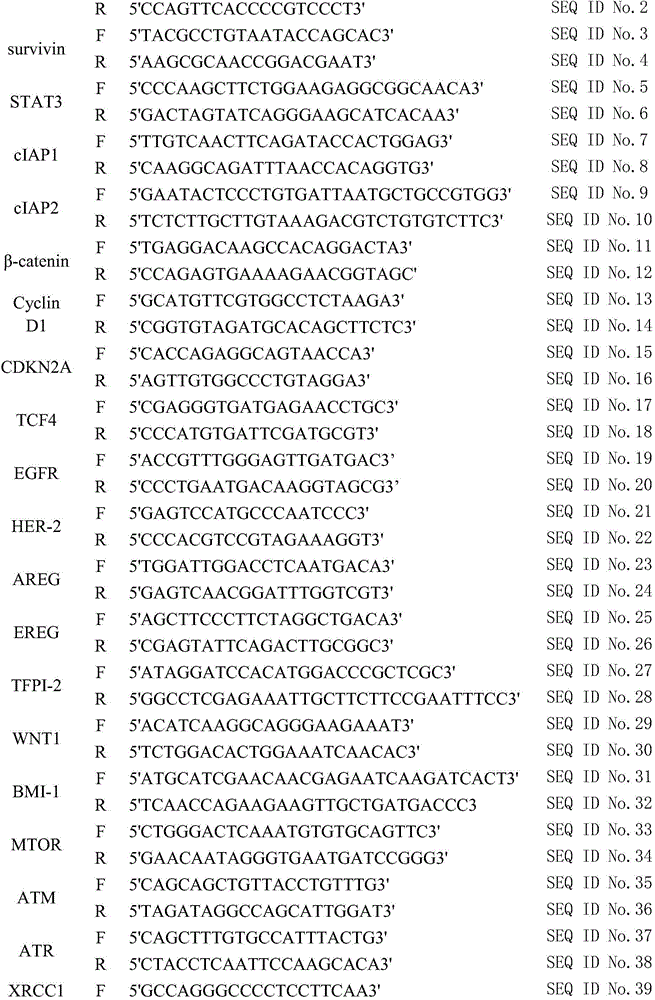
InactiveCN106498085AAccurate transferReduce transferMicrobiological testing/measurementWNT1Cyclin D1
The invention provides a kit for predicting recurrence and transference risk of a patient suffering from early-stage operable esophageal squamous carcinoma. The kit contains amplification primers of 32 genes of BCL2, Survivin, STAT3, cIAP1, cIAP2, beta-catenin, Cyclin D1, CDKN2A, TCF4, EGFR, HER-2, AREG, EREG, TFPI-2, WNT1, BMI-1, MTOR, ATM, ATR, XRCC1, XRCC2, XRCC3, XRCC4, XRCC5, HIF-1alpha, HIF-2alpha, VEGF, RAD51, beta-actin, GAPDH, GUS and TFRC in all. The kit is capable of accurately predicting transference potential of early-stage operable esophageal squamous carcinoma, so that the transference and recurrence risk of the patient suffering from the early-stage operable esophageal squamous carcinoma after radical operation can be accurately predicted and evaluated, patients with high risk can be particularly monitored and effectively interfered, recurrence and transference of esophageal squamous carcinoma can be reduced, and prognosis of the patients can be further improved.
Owner:JIANGSU PROVINCE HOSPITAL
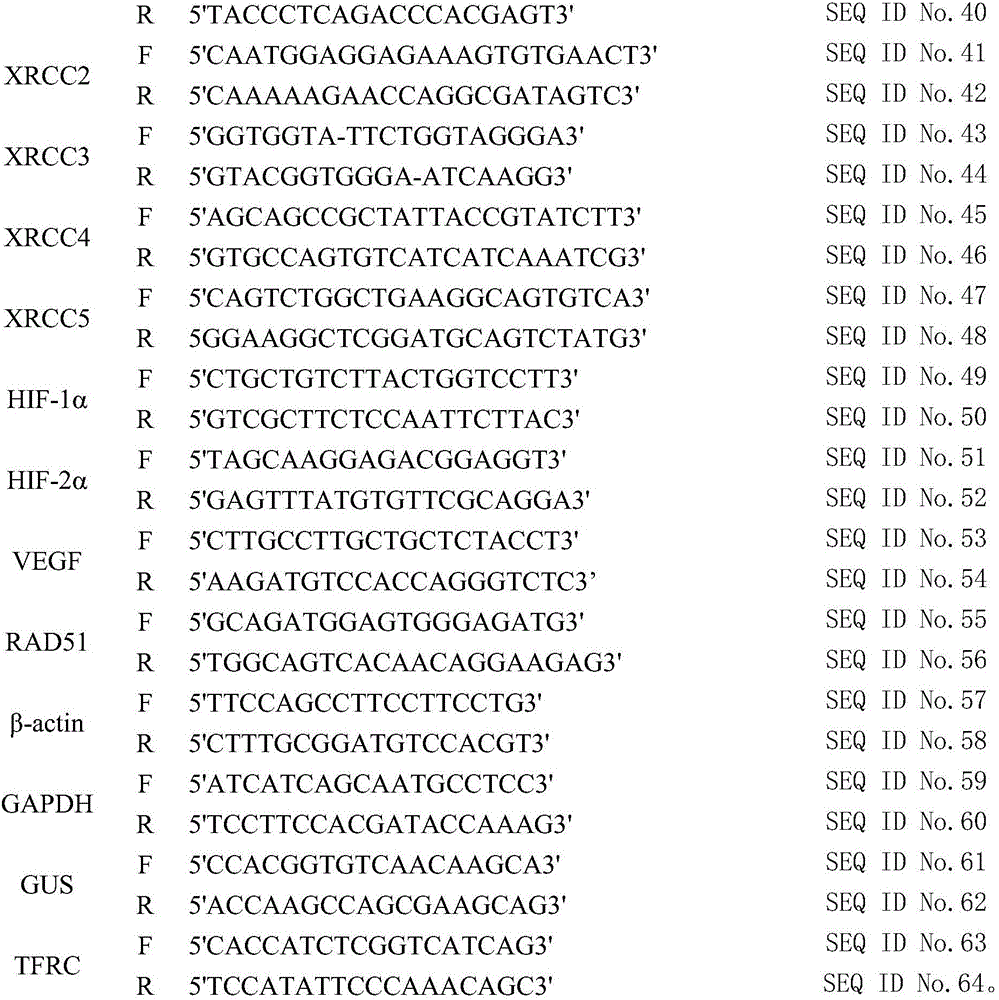
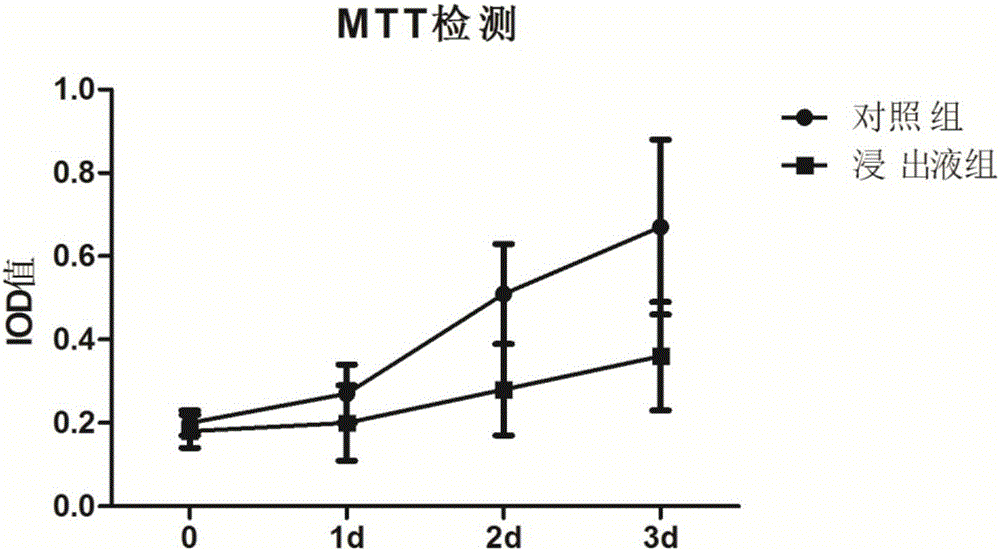
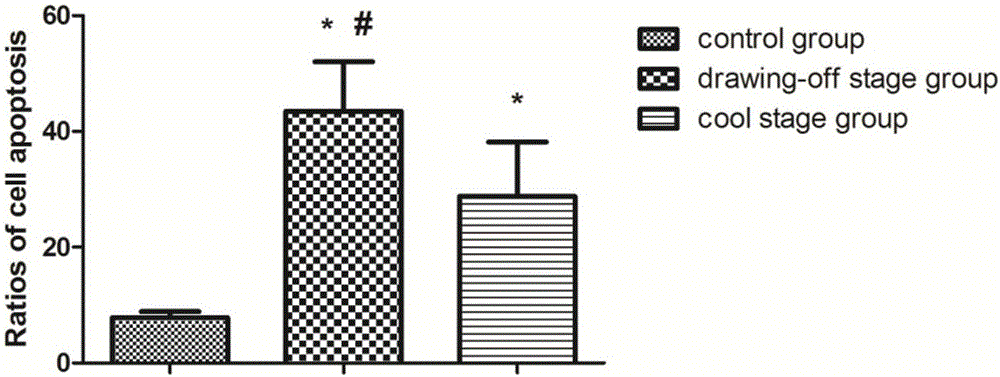
Testing method of influence of polymethyl methacrylate on spinal metastasis tumor cell
InactiveCN106480182APromote growthIncrease apoptosis rateMicrobiological testing/measurementBiological material analysisCyclin D1Polymethyl methacrylate
The invention discloses a testing method of influence of polymethyl methacrylate on spinal metastasis tumor cell. The testing method includes steps of treating a primary culture spinal metastasis tumor for 24 h by PMMA bone cement at different stages; measuring temperatures of inner part and surface of the bone cement, and respectively observing three different polymerization stages which are polymerization period, wire-drawing period, cooling and condensing period; particularly observing the influence of heat released by the bone cement in the wire drawing period on the tumor cell proliferation and apoptosis; measuring the cell apoptosis and cell cycle by a flow cytometry; measuring relationship among PMMA and Bcl-2, Bax, Caspase 3, Caspase 8, Fas and PARP in cell apoptosis; cyclin D1. The invention learns about the molecular mechanism of the in-vitro PMMA for the primary culture spinal metastasis tumor, and provides a solid theoretical basis for treating spine metastatic tumors by percutaneous centrum injected bone cement.
Owner:THE FIRST AFFILIATED HOSPITAL OF CHONGQING MEDICAL UNIVERSITY
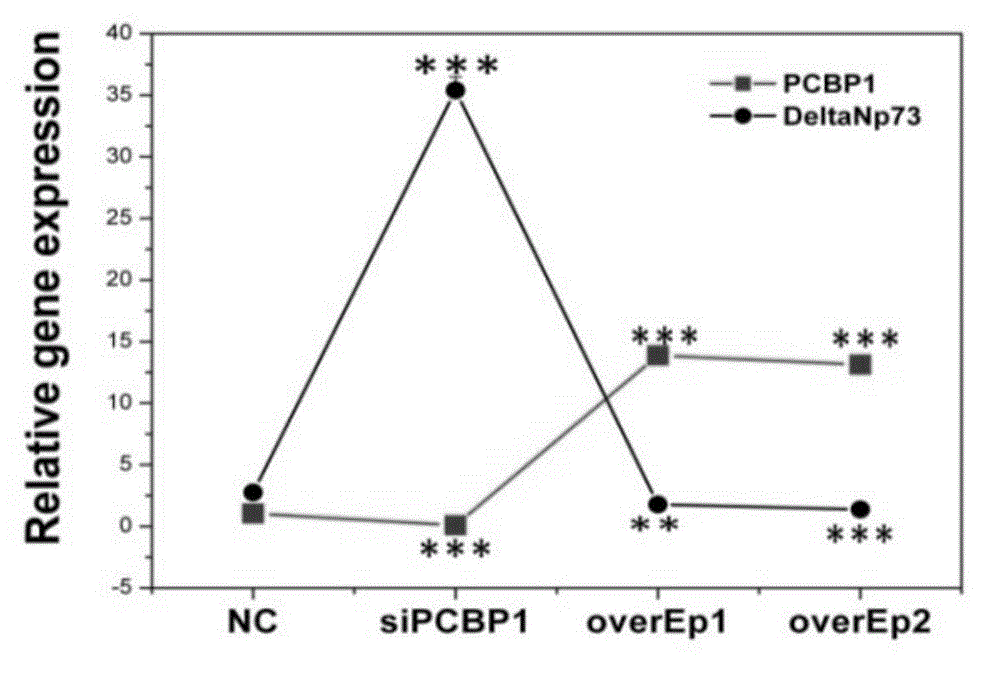
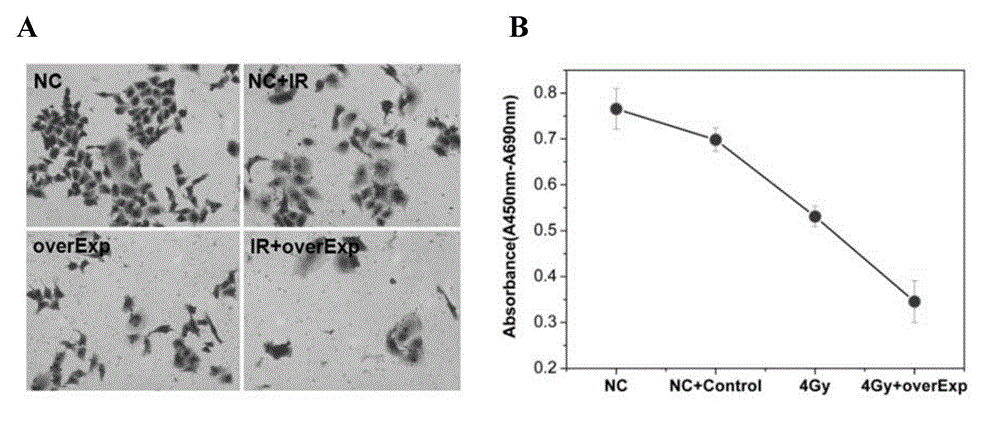
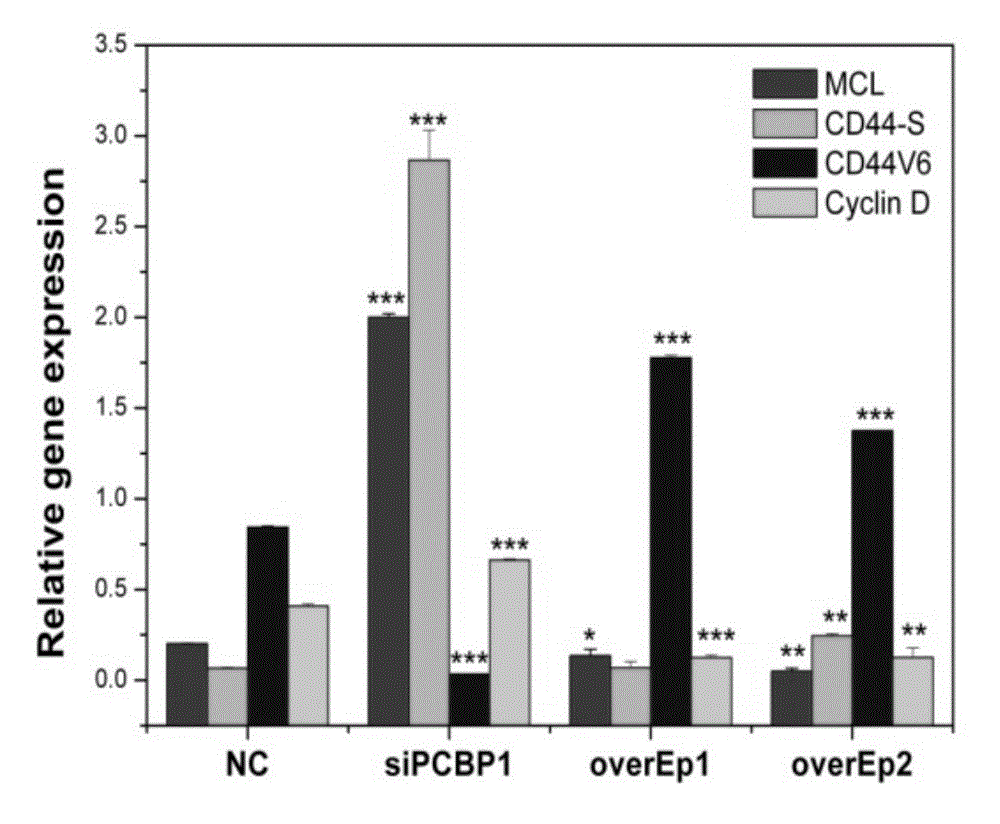
Application of PCBP1 gene in preparing radiotherapy sensitivity enhancing kit
InactiveCN104338150ASignificant radiosensitization synergistic effectPrevent proliferationGenetic material ingredientsAntineoplastic agentsCyclin D1Lymphatic Spread
The invention discloses application of a PCBP1 gene in preparing a radiotherapy sensitivity enhancing kit, and in particular relates to application of the PCBP1 gene in preparing a sensitivity enhancing kit for tumor radiotherapy utilizing ionizing radiation including heavy ion rays. The nucleotide sequence of the PCBP1 gene is as shown in SEQ ID NO.1 and the PCBP1 gene is established in eukaryotic expression plasmid. Together with heavy ion beams, the PCBP1 gene regulates and controls expression of the CD44v6 gene related to tumor metastasis and invasion, inhibits proliferation, migration and invasion of HeLa tumor cells, remarkably down-regulates the cell cycle regulatory protein Cyclin D1, prompts cells to generate G0 / G1 retardation, enhances the radiotherapy sensitivity of HeLa cells, up-regulates pro-apoptotic protein Tap73 and expression of the mitochondria apoptosis pathway MCL, down-regulates the apoptosis inhibition DelNp73 protein, and induces apoptosis of many HeLa cells.
Owner:INST OF MODERN PHYSICS CHINESE ACADEMY OF SCI
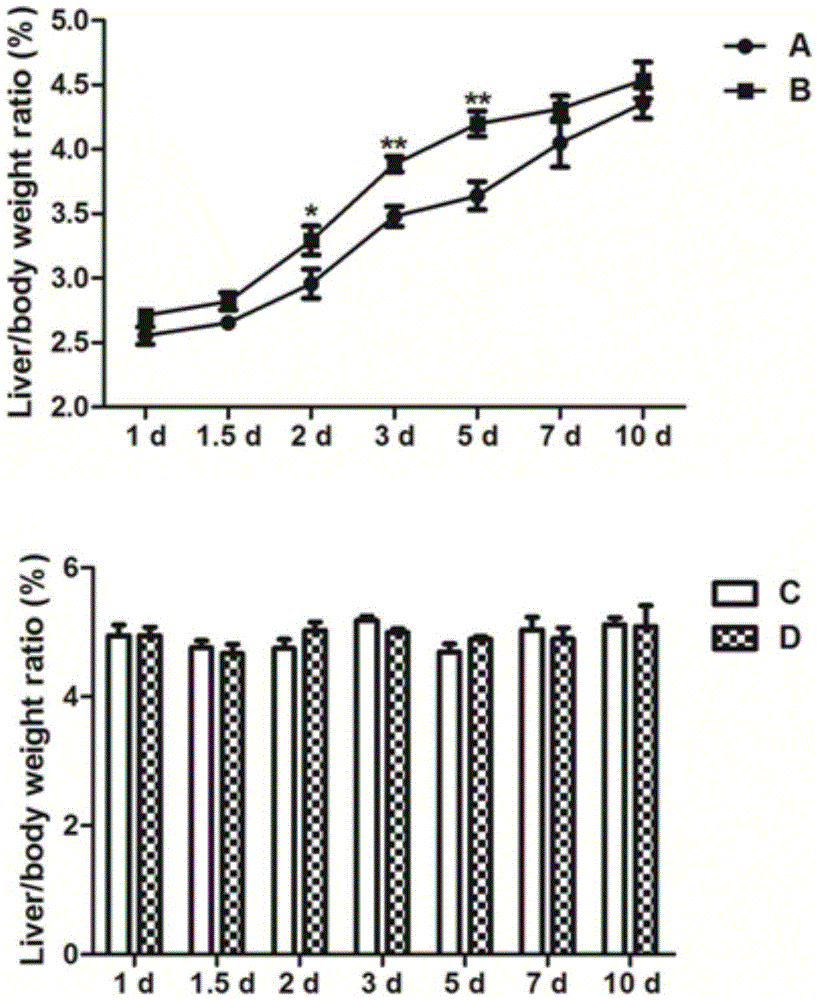
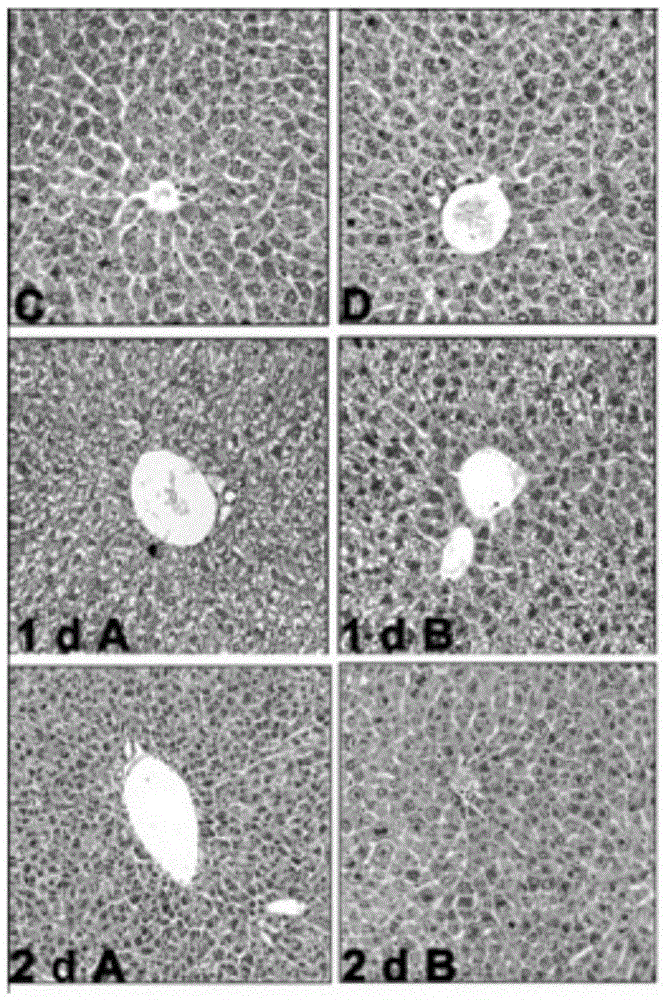
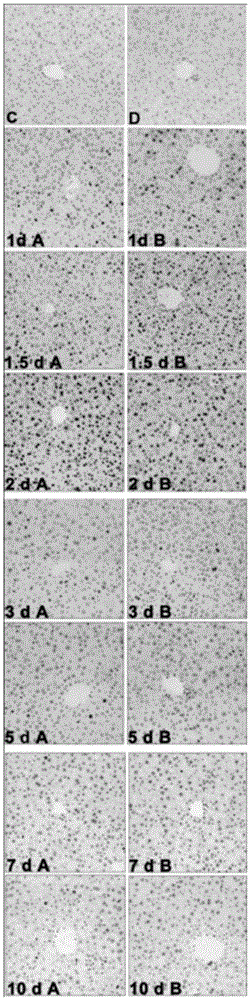
Application of fructus schisandrae sphenantherae extract in preparation of liver regeneration medicine
ActiveCN105456481AGood conditionRecovery functionDigestive systemPlant ingredientsCyclin D1Additive ingredient
The invention discloses application of a fructus schisandrae sphenantherae extract in preparation of a liver regeneration medicine. The fructus schisandrae sphenantherae extract is an alcohol extract of dried fruits of schisandrae sphenantherae, and the main effective ingredients of the fructus schisandrae sphenantherae extract comprise schisantherin and deoxyschizandrin. A partial hepatectomy mouse model is utilized, the fructus schisandrae sphenantherae extract is cooperatively applied, a result shows that the fructus schisandrae sphenantherae extract has the characteristics of being capable of significantly improving the quick recovering capacity of the liver-weight ratio and improving the quick proliferation capacity of liver cells, the mechanism relates to significant activation of protein expression of a related cell cycle, the manifestation is that cyclin D1 and PCNA proteins are significantly up-regulated, and meanwhile obvious improvement on liver tissue inflammatory infiltration and liver cell swelling is achieved. Experimental results show that the fructus schisandrae sphenantherae extract supplies a novel method and treatment means to liver regeneration after hepatectomy.
Owner:SUN YAT SEN UNIV
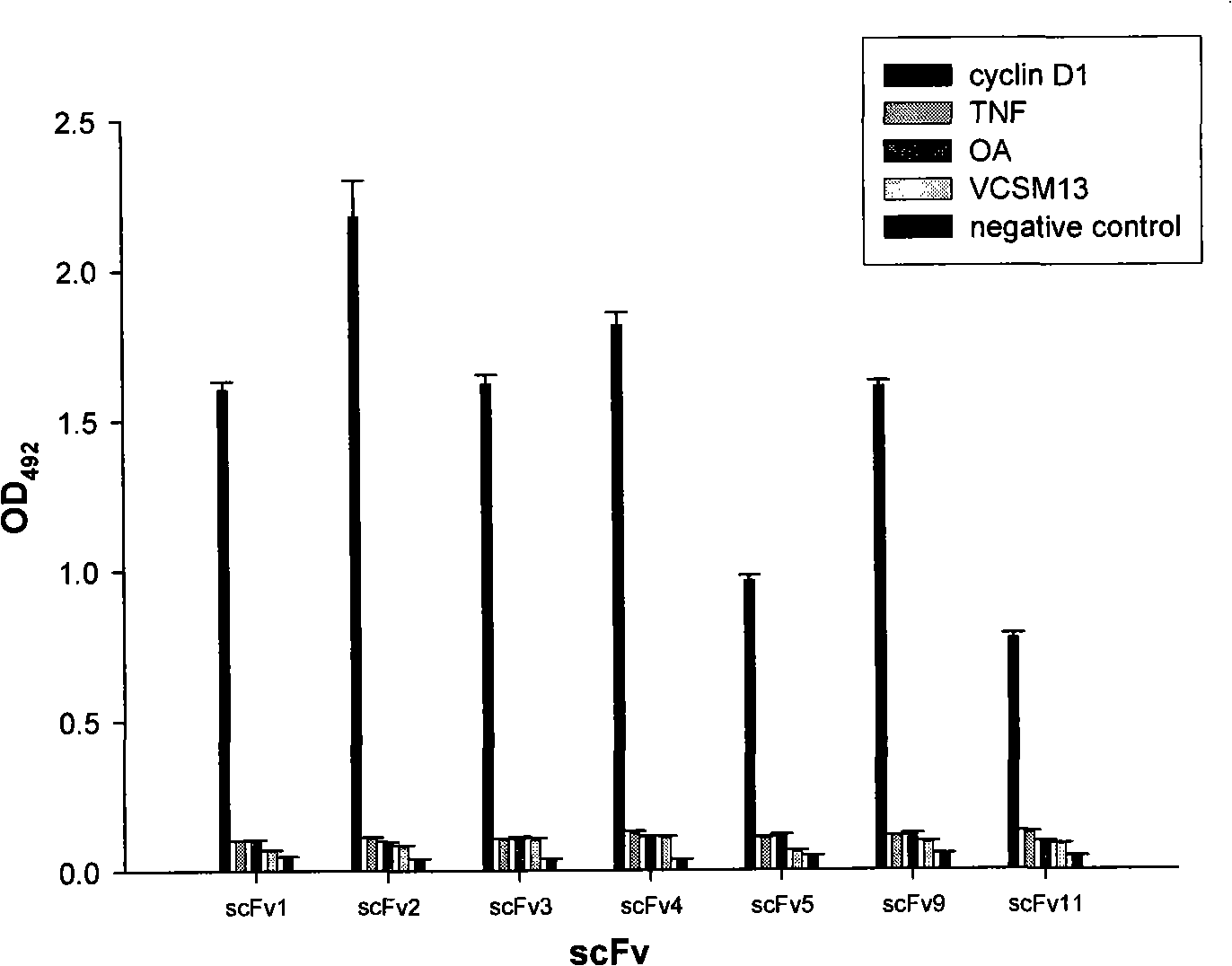
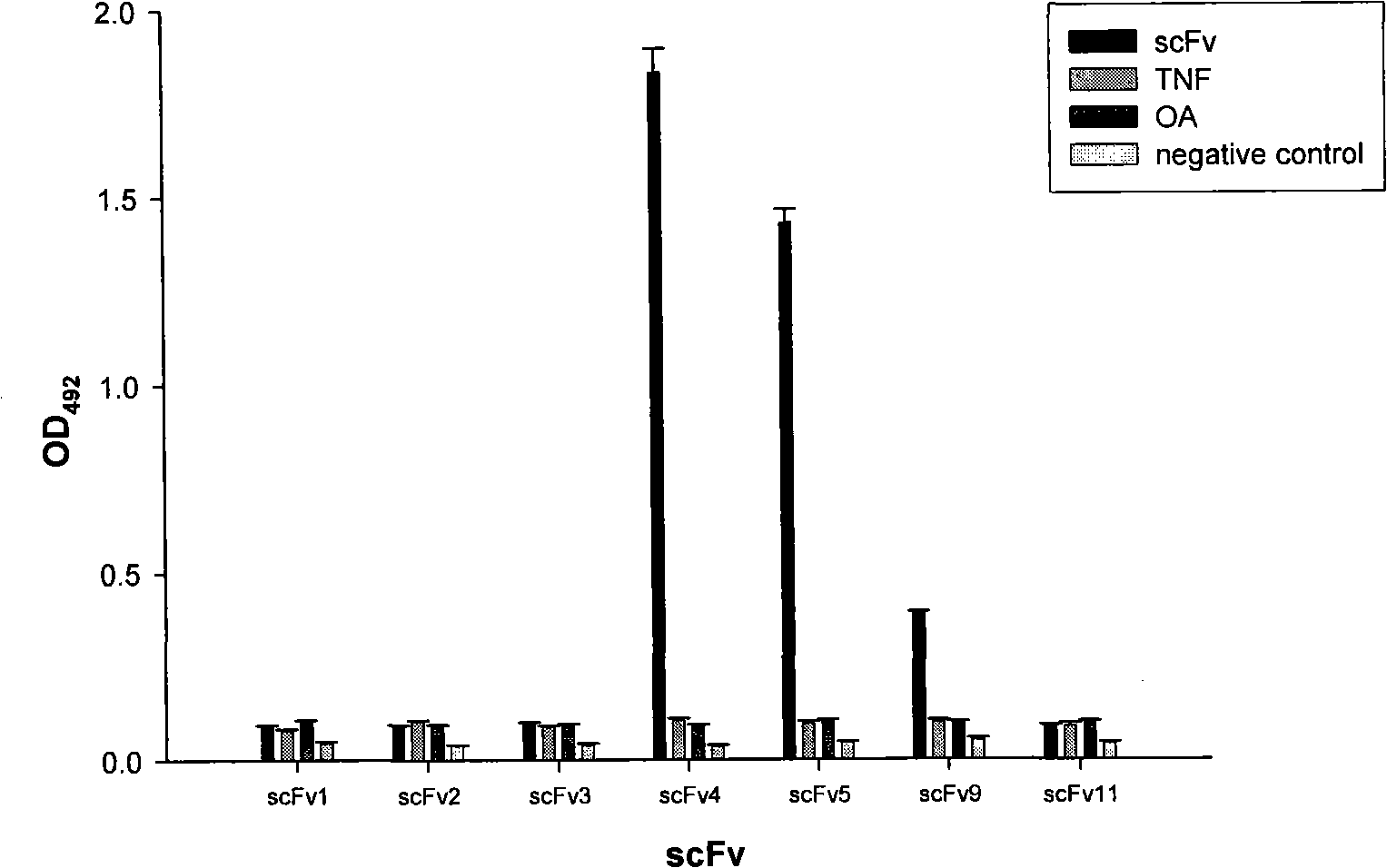
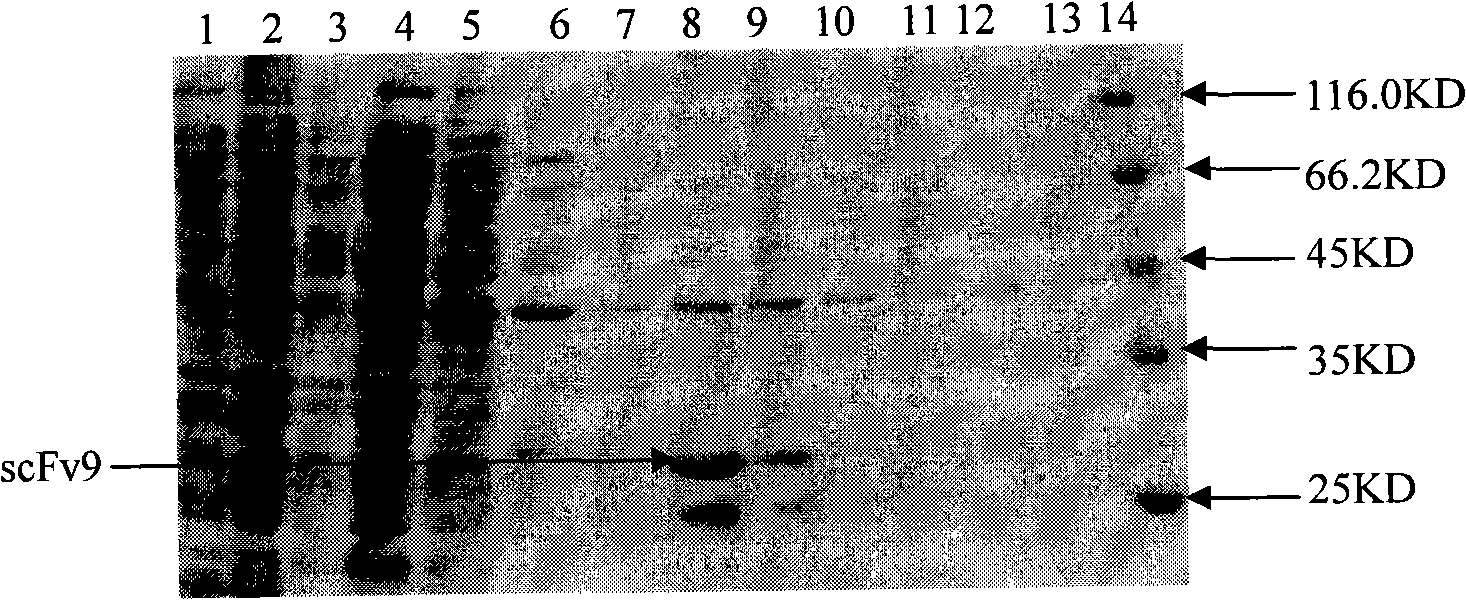
Anti-Cyclin D1 human single-chain antibody
InactiveCN101280014AAvoid side effectsImmunoglobulins against animals/humansAntibody ingredientsCyclin D1Single-Chain Antibodies
The invention relates to an anti-CyclinD1 human single-chain antibody which is composed of IgK leader peptide, anti-CyclinD1 human single-chain antibody, E-tag peptide and KDEL gene coding sequence left on endoplasmic reticulum. The anti-CyclinD1 human single-chain antibody can be specifically combined with CyclinD1 protein after dissoluble expression, purification and being activated. If being recombined in a eucaryon expression vector after the addition of the localization signals in the cell left on the endoplasmic reticulum, the anti-CyclinD1 human single-chain antibody can restrain the proliferation of tumor cells after the transfection of the tumor cells.
Owner:JILIN UNIV
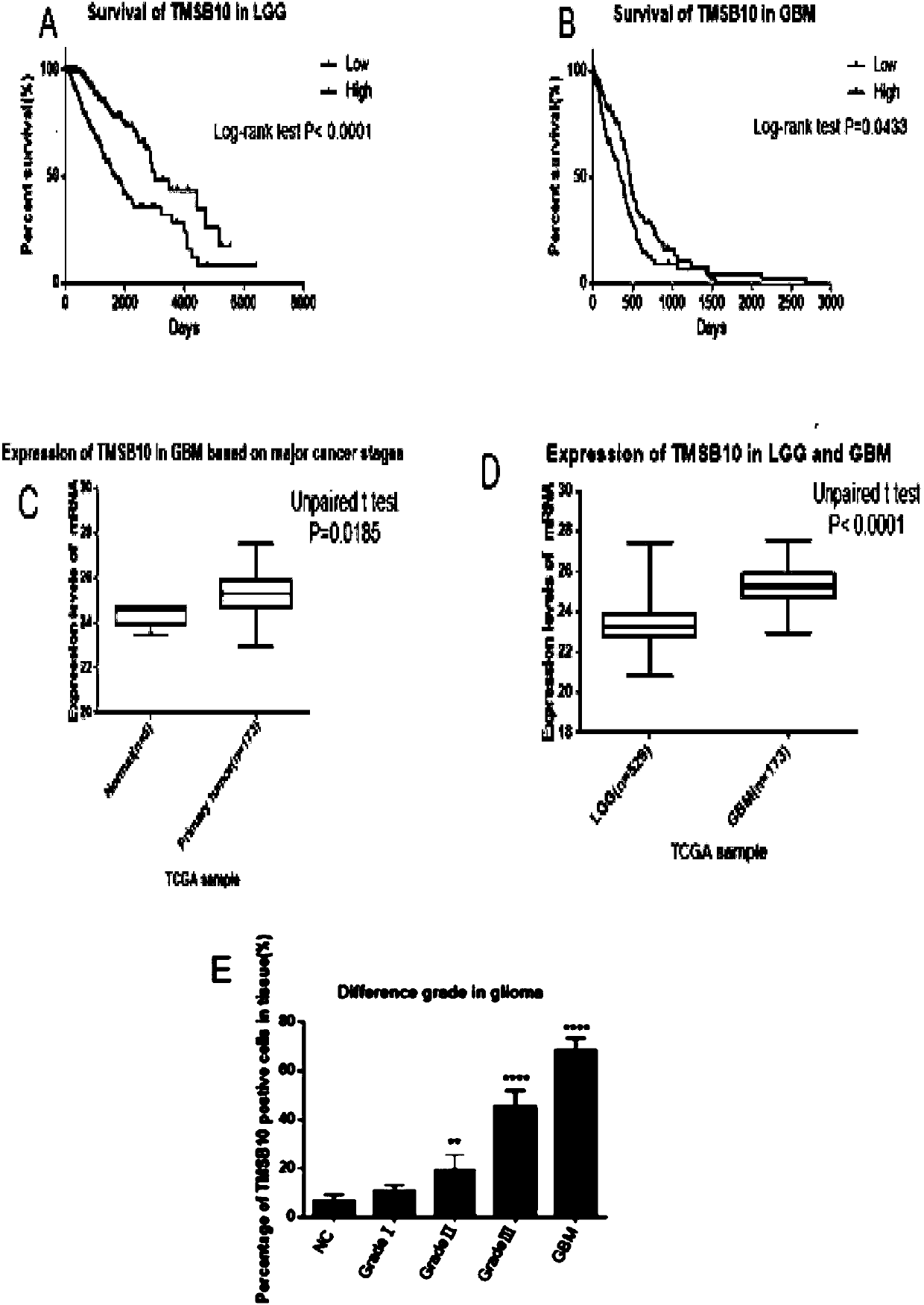
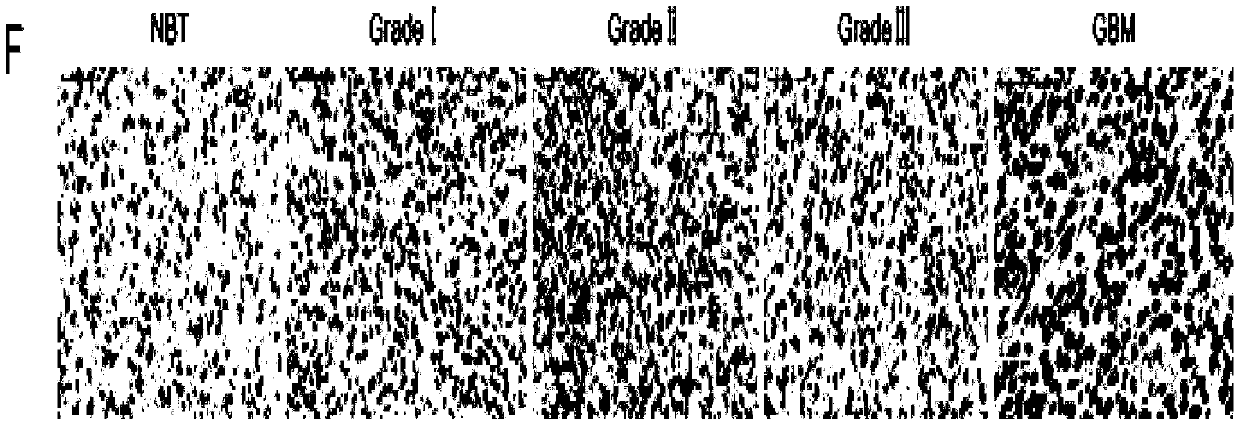
Application of TMSB10 in diagnosis and treatment of glioma
InactiveCN109593860AReduce tumorigenicity in vivoThe value of good practical applicationMicrobiological testing/measurementBiological material analysisCyclin D1Treatment targets
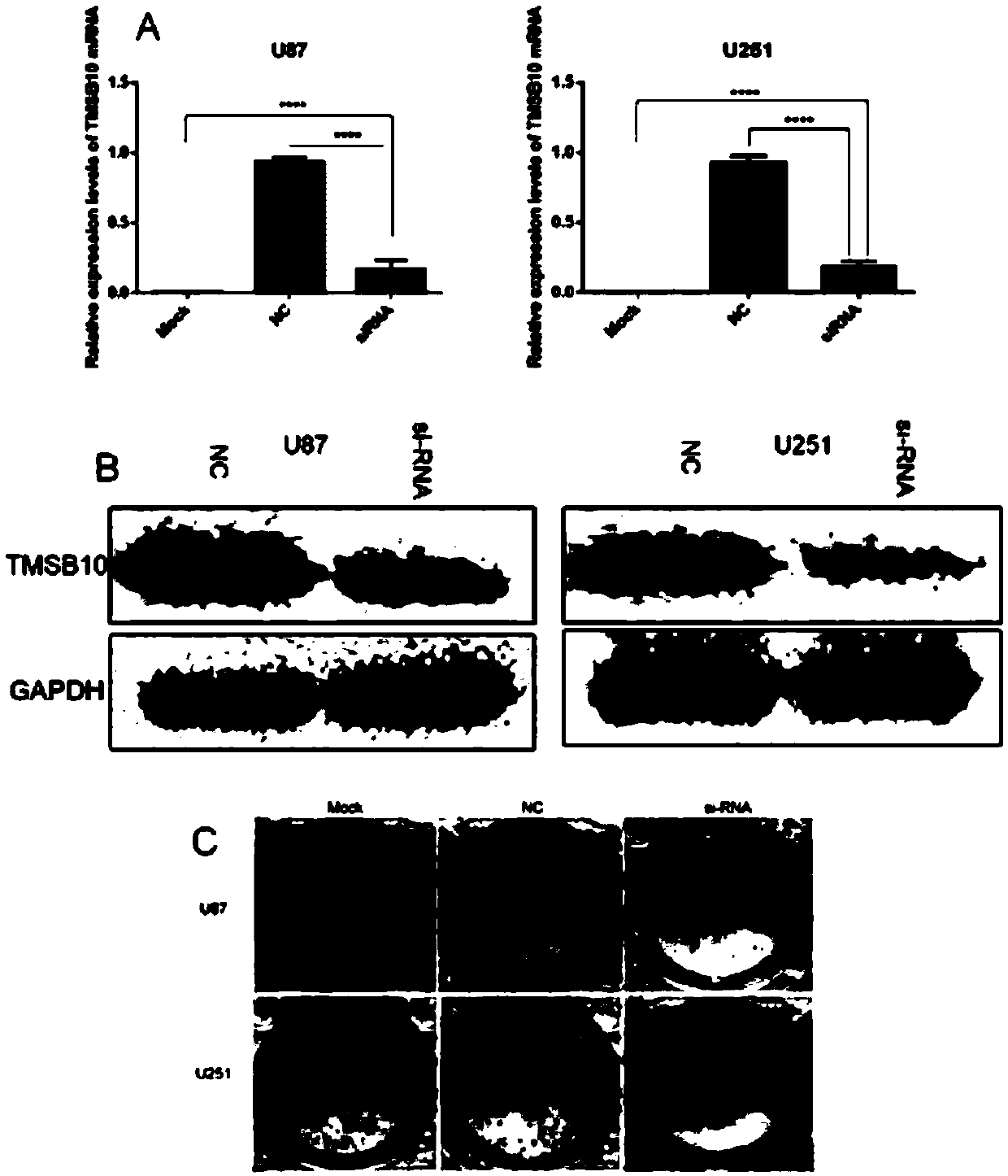
The invention provides an application of TMSB10 in diagnosis and treatment of glioma. Specifically, research finds that expression of TMSB10 increases with malignancy degree of glioma and is negativecorrelation with the survival rate. The TMSB10 silences and inhibits proliferation, attack and migration (MMP-9 and N-Cadherin) in U87MG and U251 cell lines, induces cell apoptosis, inhibits in-vitrocell cycles (p21, CDK4 and cyclin D1) through PI3K-AKT / ERK signal pathways (PI3K, P-AKT and p-ERK) and reduces in-vivo tumorigenicity. It indicates that the TMSB10 is an oncogene participating in occurrence and development of glioma and can be used as a biomarker and treatment target of the glioma.
Owner:SHANDONG UNIV QILU HOSPITAL
Application of caffeic acid 3,4-dyhydroxy phenethyl ester to preparing medicine for preventing colorectal cancer
InactiveCN108992434APrevent intrusionPrevent infiltrationOrganic active ingredientsAntineoplastic agentsDimethylhydrazineCyclin D1
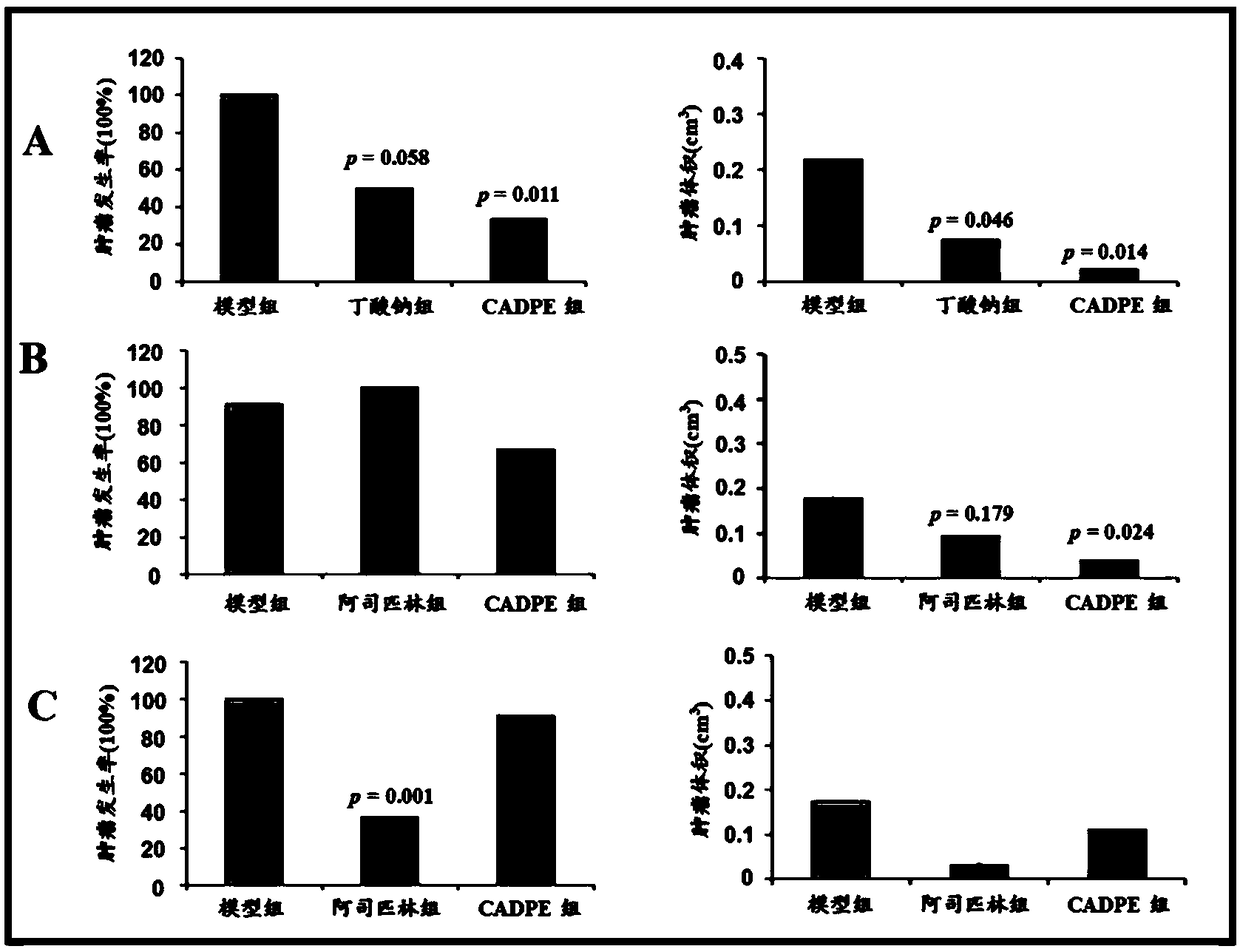
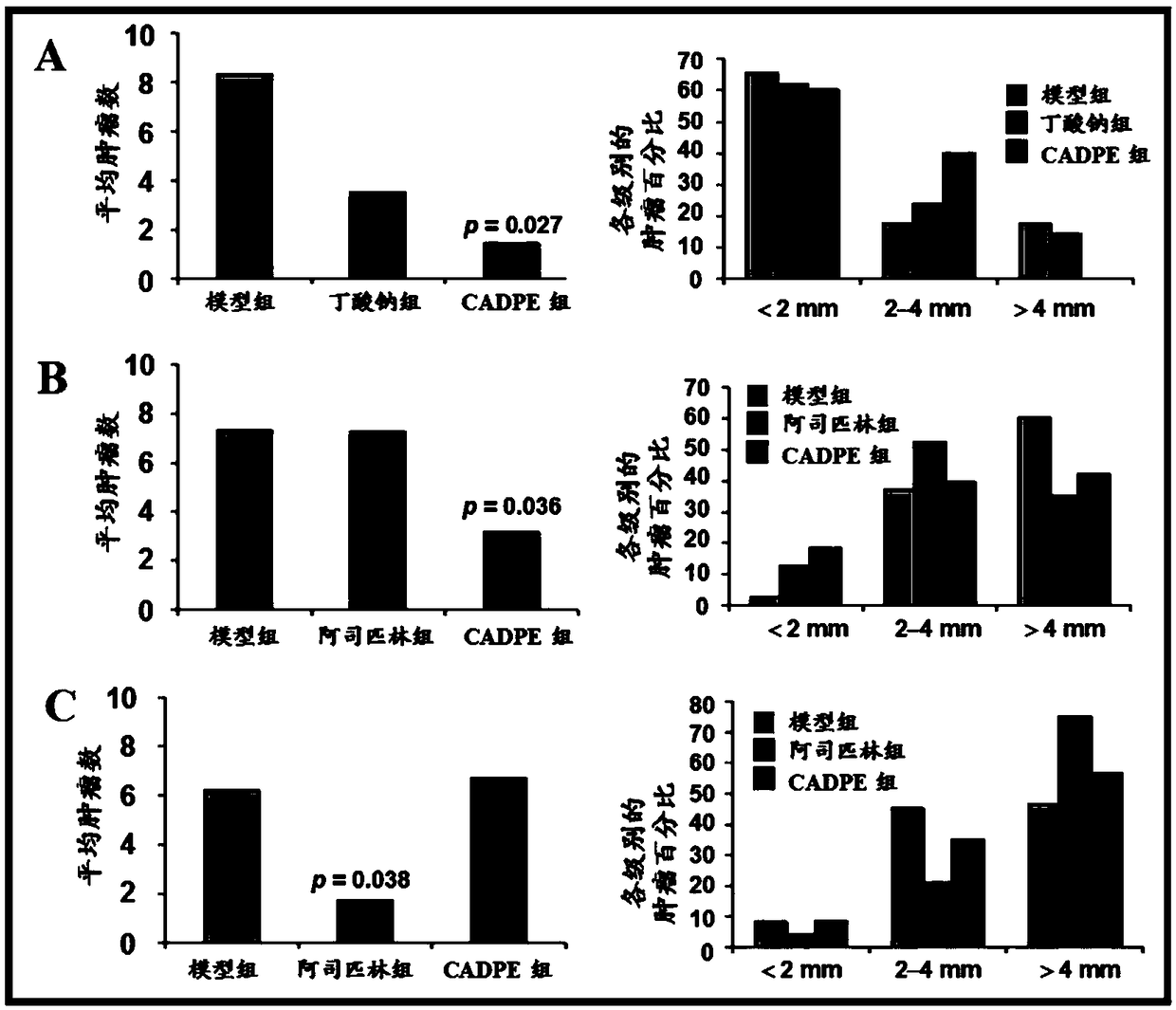
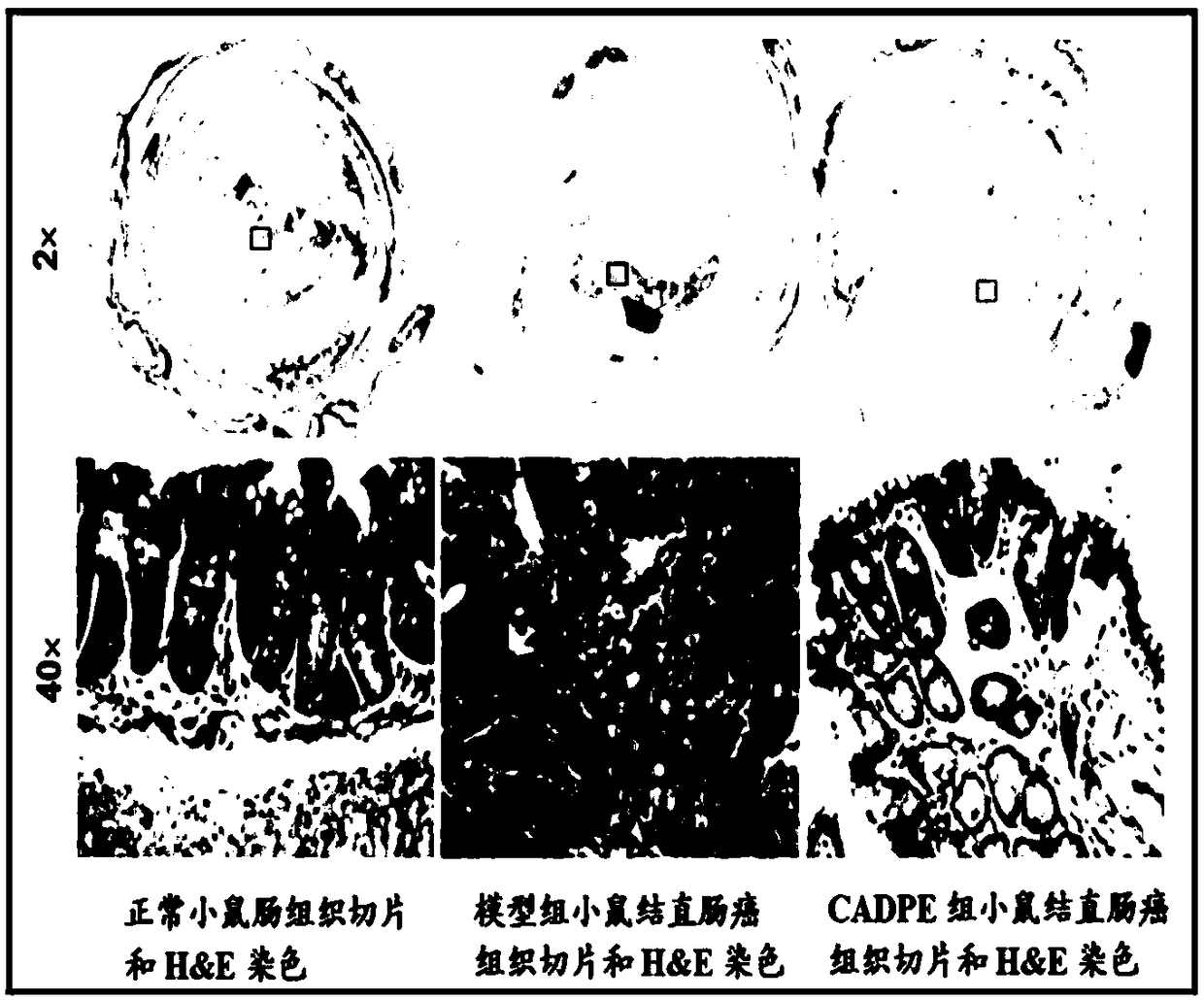
The invention provides application of caffeic acid 3,4-dyhydroxy phenethyl ester (CADPE) to preparing a medicine for preventing colorectal cancer. The medicine is prepared from CADPE as an active component and a pharmaceutically acceptable carrier. The CAPDE is capable of effectively preventing early-stage occurrence of ICR (Institute for Cancer Research) mouse colorectal cancer induced by 1,2-dimethylhydrazine (DMH) and dextran sulphate sodium salt, has no conspicuous toxicity, and has the function mechanisms of inhibiting inflammatory cell invasion and infiltration and inhibiting NF-KappaB expression in monocyte, inhibiting expression of beta-catenin in aberrant crypt, reducing expression of cell proliferin PCNA and Cyclin D1, an anti-apoptoasis protein surviving and a cancer gene c-Myc,and inhibiting tumor vessel proliferation. Novel treatment means are provided for clinical prevention and treatment on colorectal cancer.
Owner:ZHEJIANG UNIV
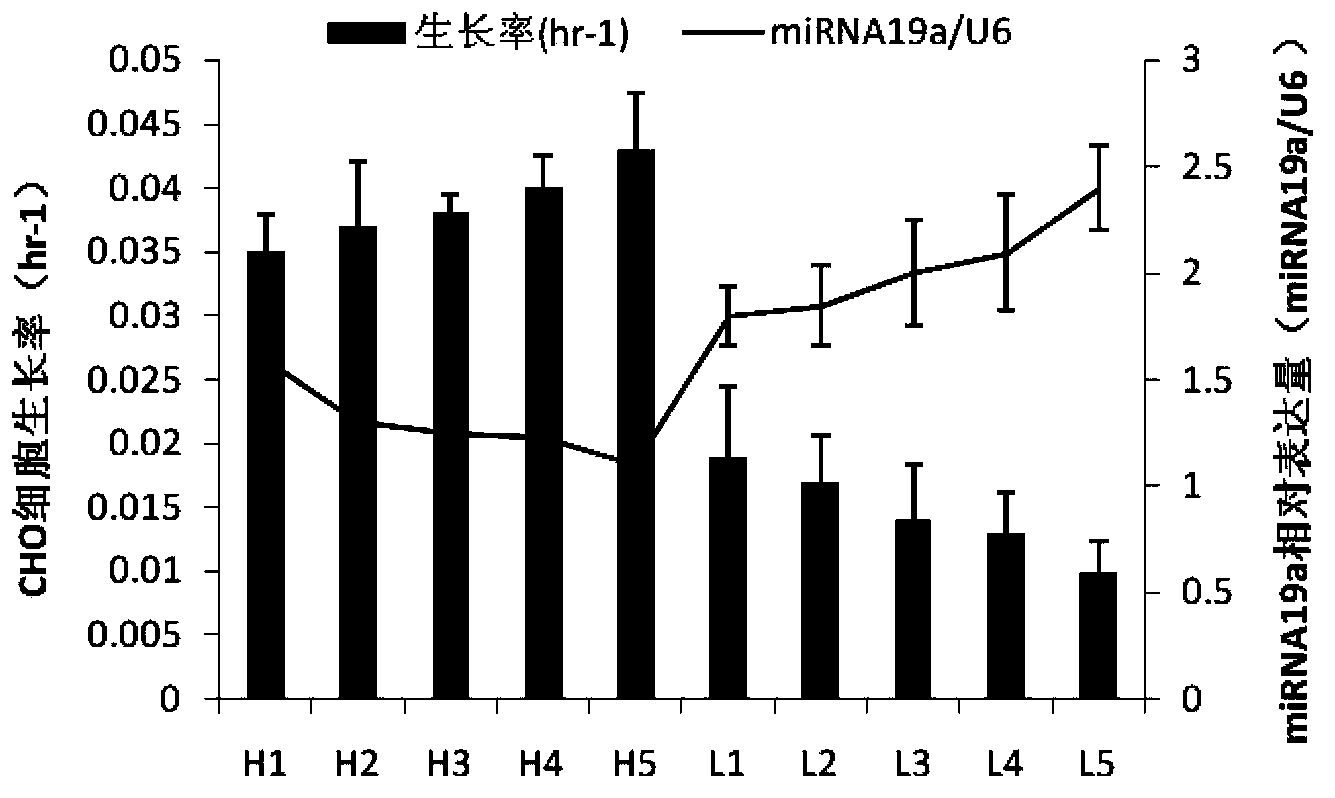
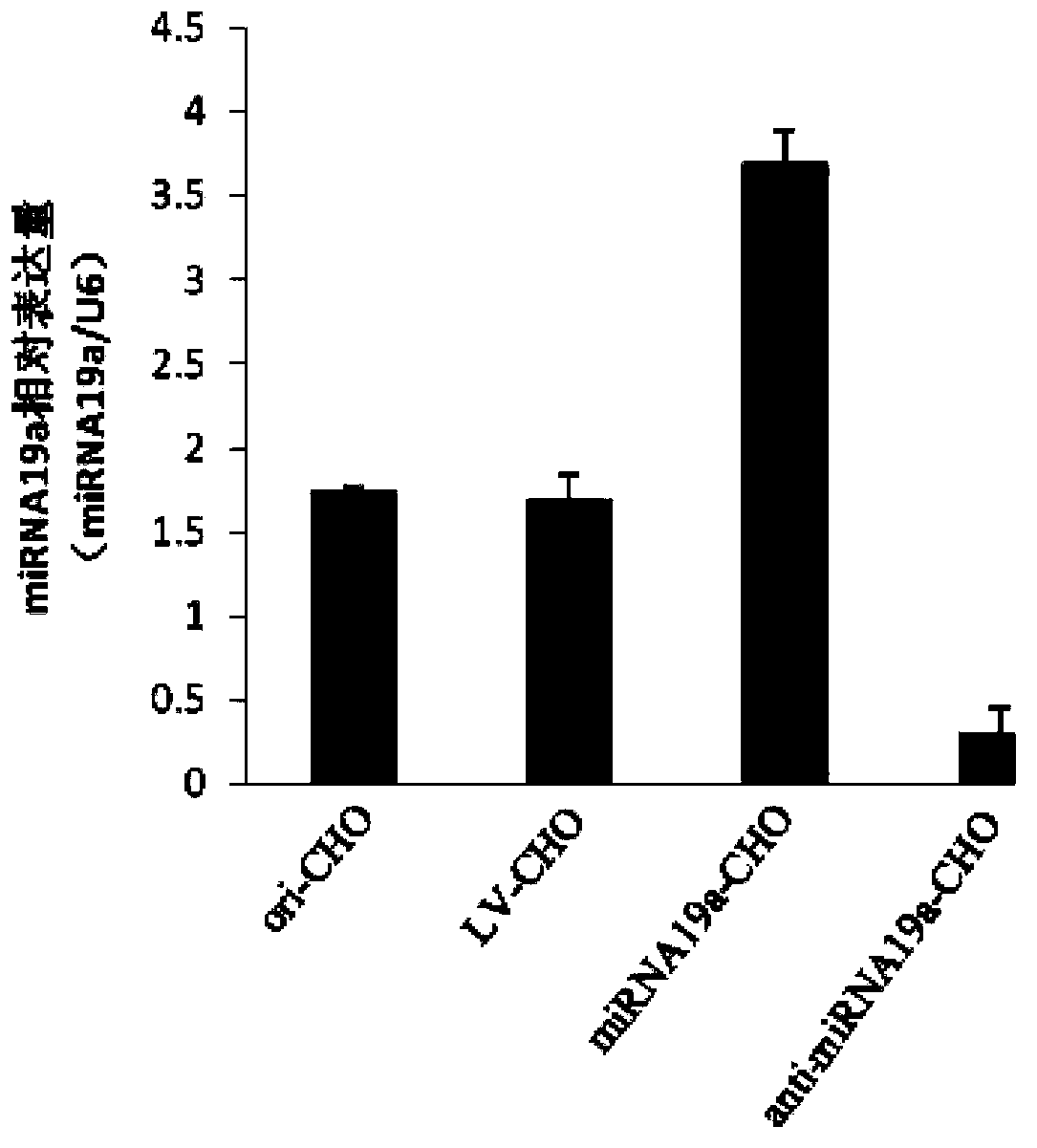
Application of miRNA-19a (micro ribonucleic acid-19a) serving as target in regulation of mammal host cell proliferation
InactiveCN103397034AVector-based foreign material introductionDNA/RNA fragmentationCyclin D1Genetic engineering
The invention discloses an application of miRNA-19a (micro ribonucleic acid-19a) serving as a target in regulation of mammal host cell proliferation. A genetic engineering technology is used for inhibiting or promoting the expression quantity of the miRNA-19a in a mammal host cell, so that the expression quantity of cell cycle protein Cyclin D1 closely related to cell proliferation can be indirectly promoted or inhibited, and further the cell proliferation is promoted or inhibited, thus the purpose of regulating the mammal host cell proliferation is achieved.
Owner:CHONGQING UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com