Cancer therapy based on tumor associated antigens derived from cyclin d1
a technology of cyclin d1 and tumors, applied in the field of cyclin-derived peptides, can solve the problem of not providing precise information as to the use of the antigens transcribed from these genes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
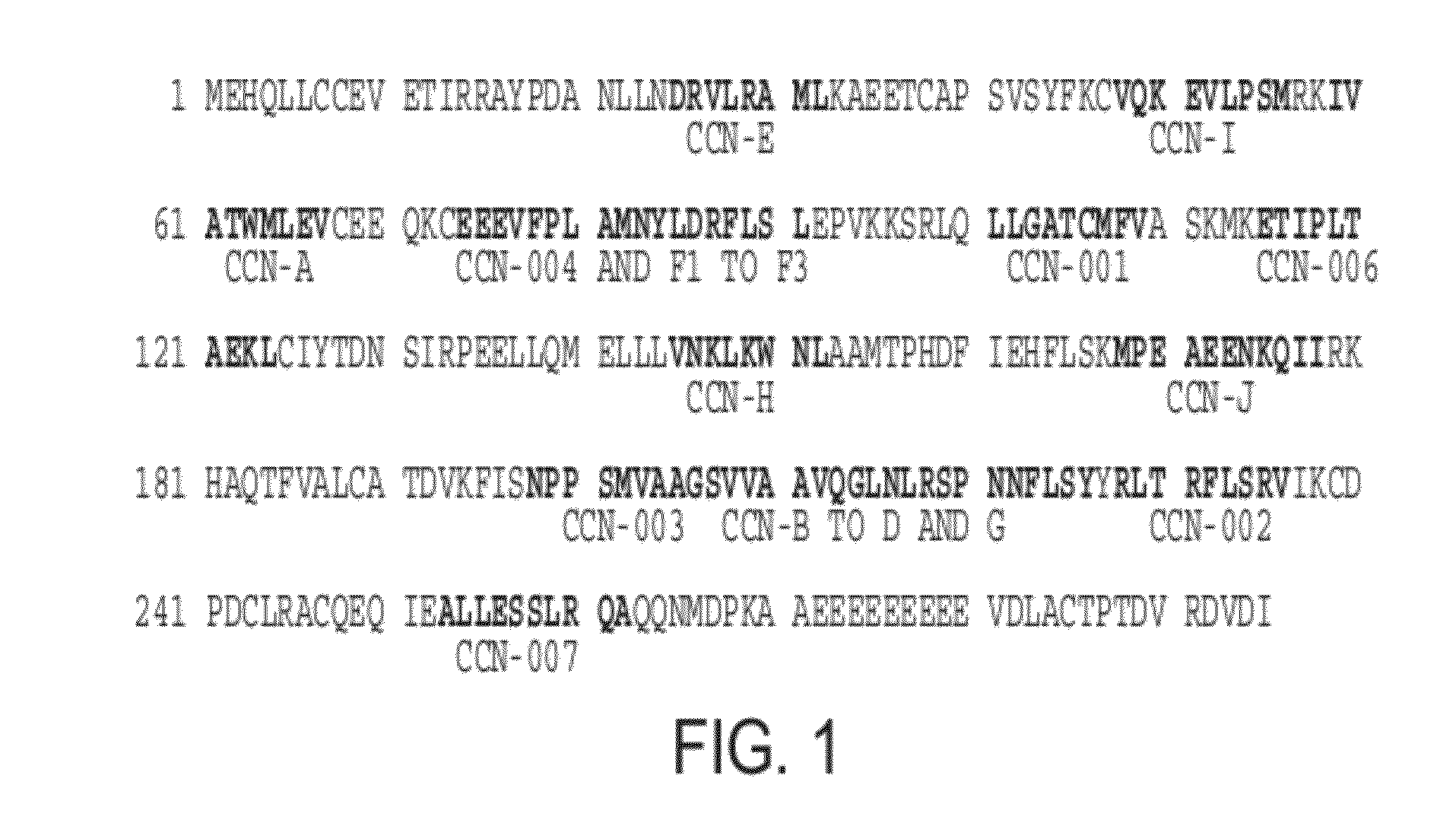
1. Identification and Characterization of Peptides as Used According to the Present Invention
[0093]In general, the peptides as used according to the present invention were identified using the XPRESIDENT® technology as described (see, for example, Weinschenk et al. Integrated Functional Genomics Approach for the Design of Patient-individual Antitumor Vaccines, CANCER RESEARCH 62, 5818-5827, Oct. 15, 2002) on the basis of renal cancer cells. The average overexpression of cyclin D1 (CCND1) in ccRCC against the average expression in normal tissues was 3.0-fold, and 5.7-fold in primary tumors and 5.4-fold in metastases. 55% of primary tumors showed an overexpression against normal kidney.
2. HLA-Restriction of the Peptides as Identified
[0094]For CCN-001 and CCN-002, a good binding to HLA A*02 was predicted using the SYFPEITHI routine (Rammensee et al., 1997; Rammensee et al., 1999). Good binding of CCN-001 to HLA A*0201 was confirmed by an ELISA-based method (Sylvester-Hvid et al., 2002)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pharmaceutical composition | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com