Anti-Cyclin D1 human single-chain antibody
A single source, antibody technology, applied in the direction of antibodies, anti-animal/human immunoglobulins, anti-tumor drugs, etc., can solve the problems of strong immunogenicity and short half-life, and achieve the effect of avoiding toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
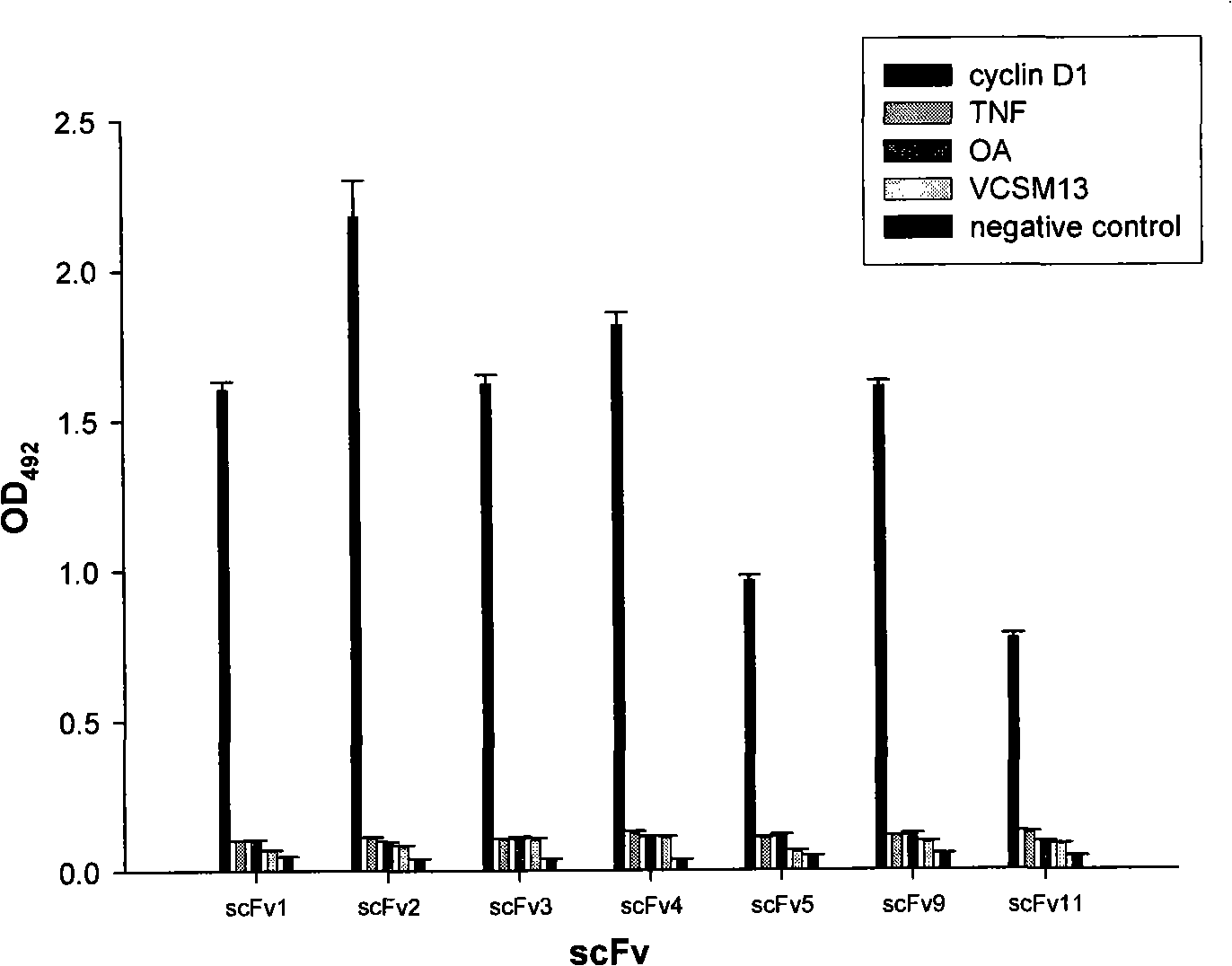
[0038] Example 1 Screening of specific anti-human cyclin D1 human single-chain antibody
[0039] In order to deeply study the mechanism of action of cyclin D1 in the occurrence and development of tumors, and to better target cyclin D1 to carry out research on tumor treatment and diagnosis, the present invention uses recombinant human cyclin D1 protein expressed in prokaryotic and purified by Ni-NTA Agarose as an antigen , through the "adsorption-elution-amplification" panning process to screen the specific anti-human cyclinD1 single-chain antibody from the human phage antibody library, and analyze and identify the specificity and binding activity of the obtained antibody by ELISA method. details as follows:
[0040] 1. Experimental materials
[0041] Human phage single-chain antibody library, E.coli HB2151, XL1-Blue and helper phage VCSM13 were provided by Professor Wang Yan of Beijing Naval General Hospital, prokaryotic expression and recombinant human cyclinD1 protein purif...
Embodiment 2
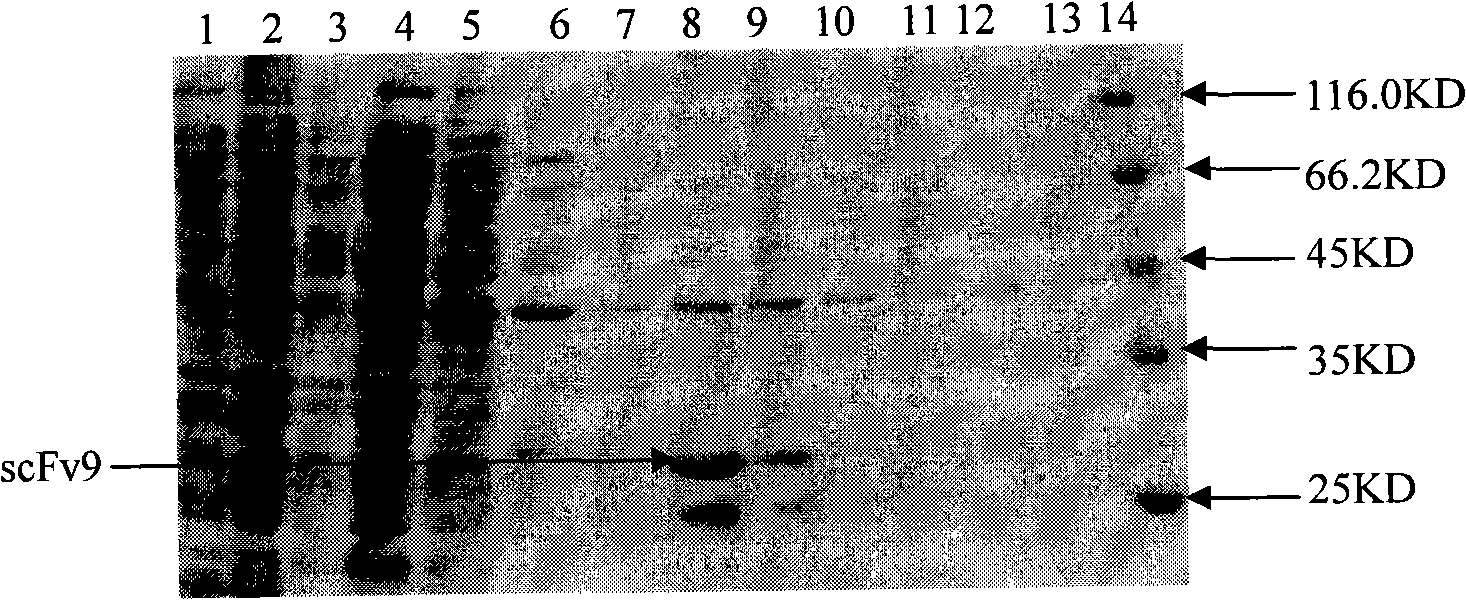
[0054] Example 2 Soluble expression, purification and identification of anti-Cyclin D1 human single-chain antibody
[0055] One experimental material
[0056] Escherichia coli XL1-Blue, HB2151 as above, Coomassie Brilliant Blue G-250, PMSF, anti-v5 antibody were purchased from Sigma Company, anti-cyclin D1 antibody was purchased from NeoMARKERS HRP-labeled goat anti-mouse IGg was purchased from Dingguo Bioengineering Company , His Trap HP Kit, PD-10 desalting column were purchased from Amersham Bioscience.
[0057] Two experimental methods
[0058] The plasmid containing the anti-CylinD1 single-chain antibody (ADκ) gene was transformed into the expression host strain HB2151. Pick a single clone in 5ml 2YT (100μg / ml Amp + , 10ug / ml ter + ) at 30°C at 180-200rpm overnight, transfer the overnight culture at a ratio of 1:100, and continue culturing at 30°C until OD 600 When it is 0.5-1.0, add IPTG to a final concentration of 1 mM to induce overnight. The bacterial liquid sup...
Embodiment 3
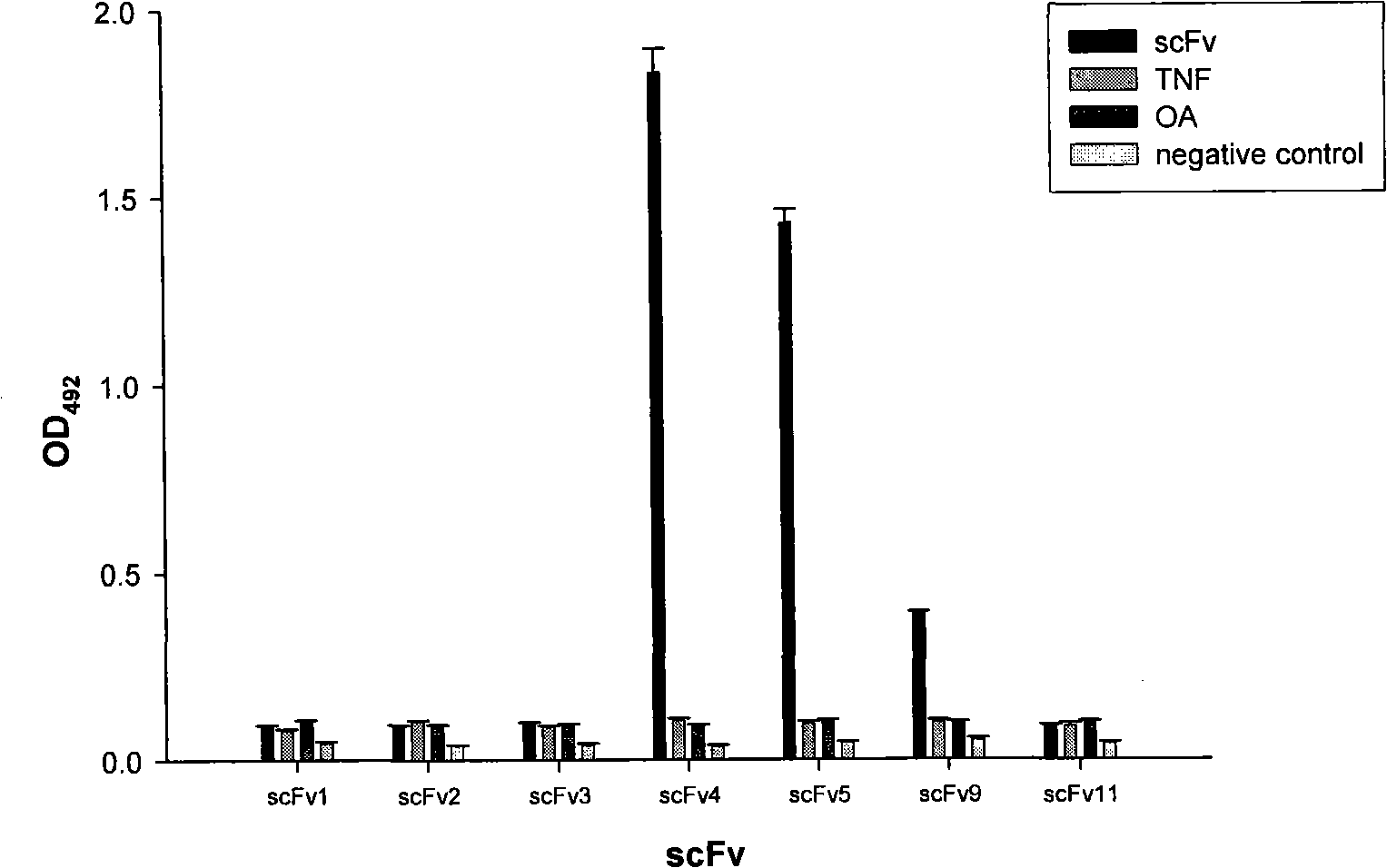
[0061] Example 3 Activity characterization of anti-Cyclin D1 human single-chain antibody (ADκ)
[0062] 1. Experimental material Same as above
[0063] Two experimental methods
[0064] 1. Detection of ADκ activity by competitive ELISA
[0065] Coat the ELISA plate with 1-10 μg / ml cyclin D1 protein and overnight at 4°C, block with 0.1% BSA-PBST for 2 hours the next day, then add 50 μl ADκ, and then add 50 μl anti-cyclin D1 polyclonal antibody (with 0.1% BSA-PBST PBST diluted 1:200) was used as a competitive inhibitor, anti-cyclin D1 polyclonal antibody was not added as a positive control, and 50 μl of Ami-V5 mouse monoclonal antibody (in 0.1% BSA-PBST) was added after incubation for 2 h. 1:5000 dilution) and incubate for 2 hours, then add 50 μl of HRP-goat anti-mouse IgG (diluted 1:1000 with 0.1% BSA-PBST), use OPD as the substrate to develop color in the dark, and read the value of OD492. The inhibition rate was calculated using the following formula:
[0066] Inhibition ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com