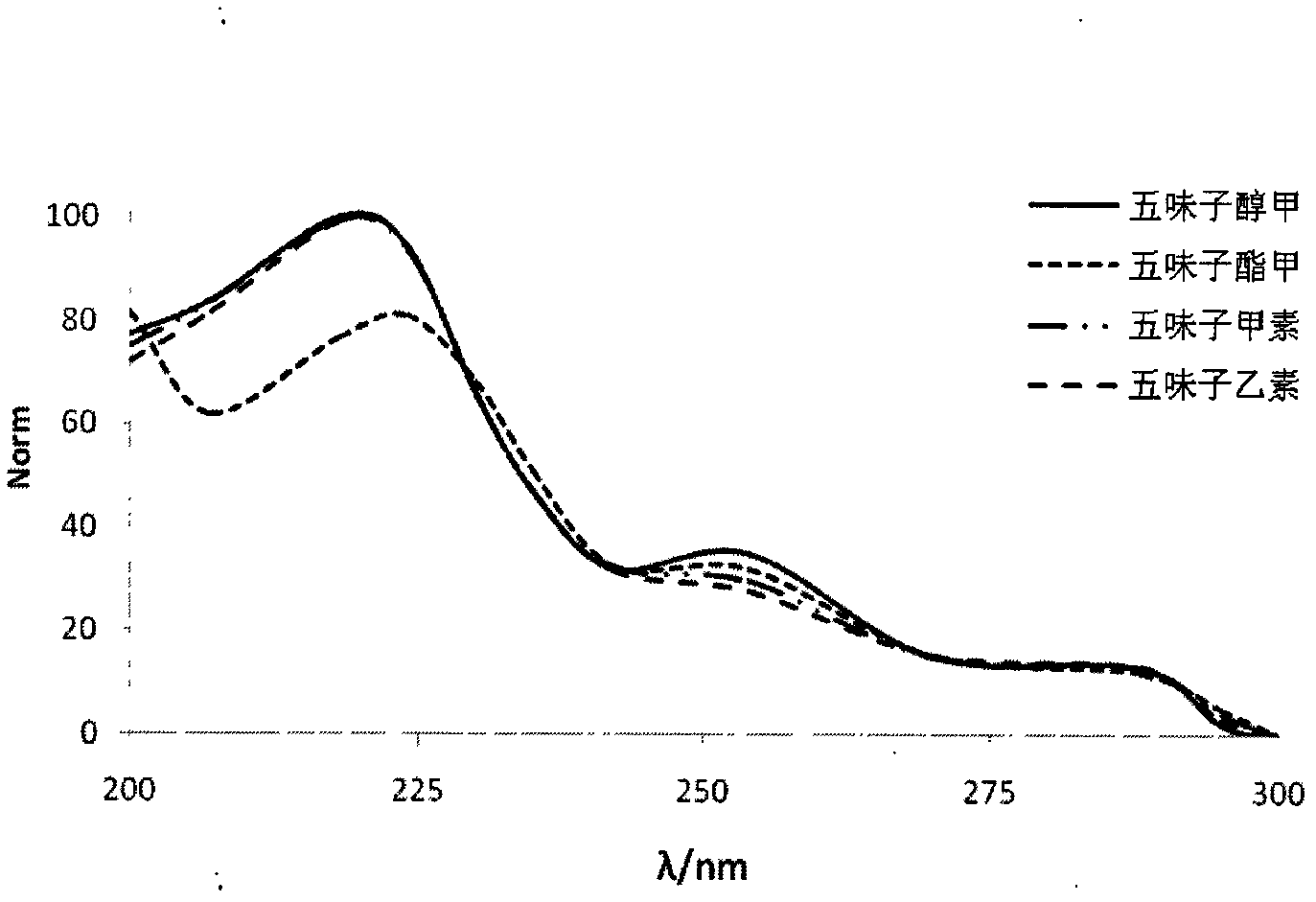
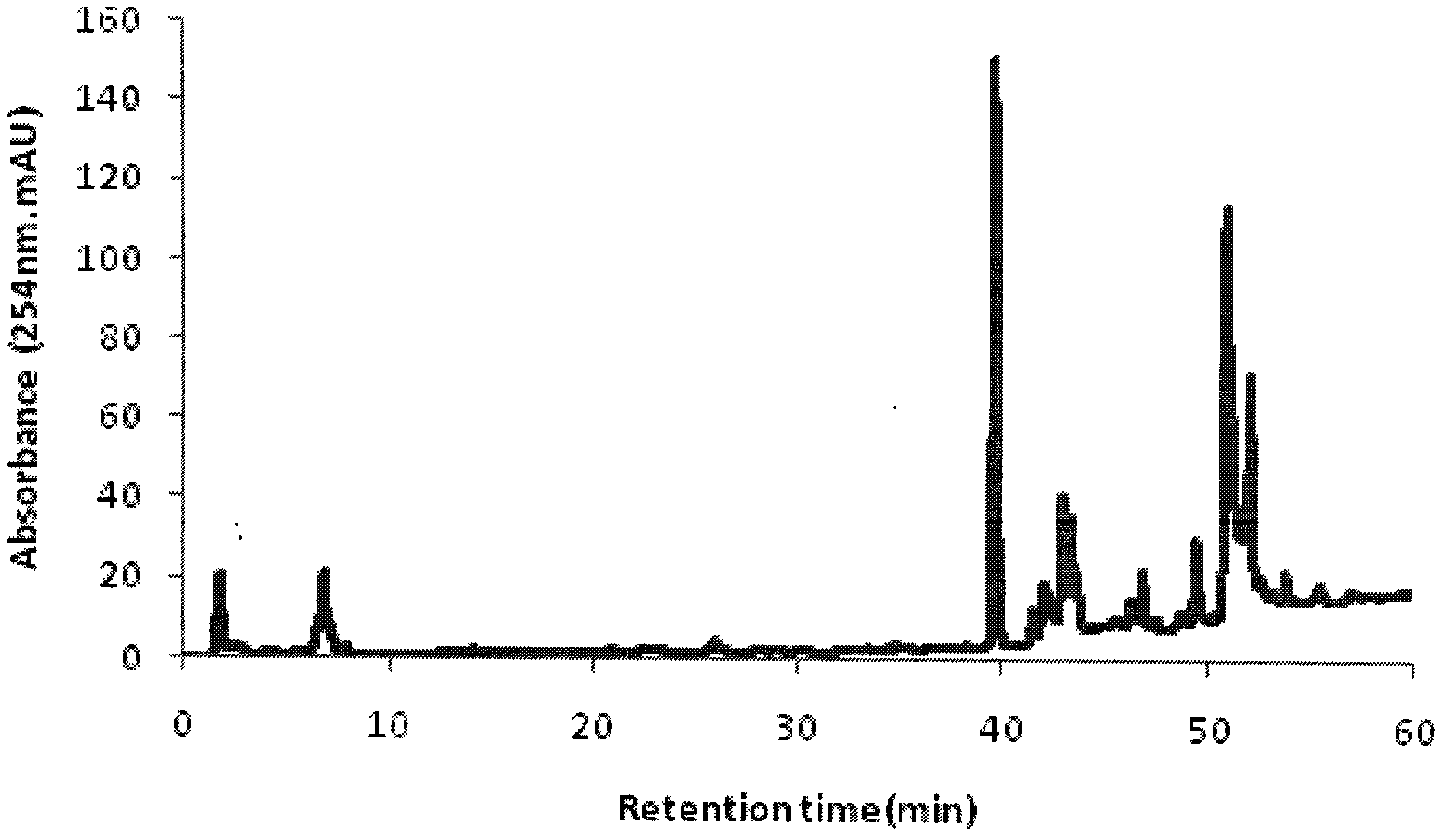
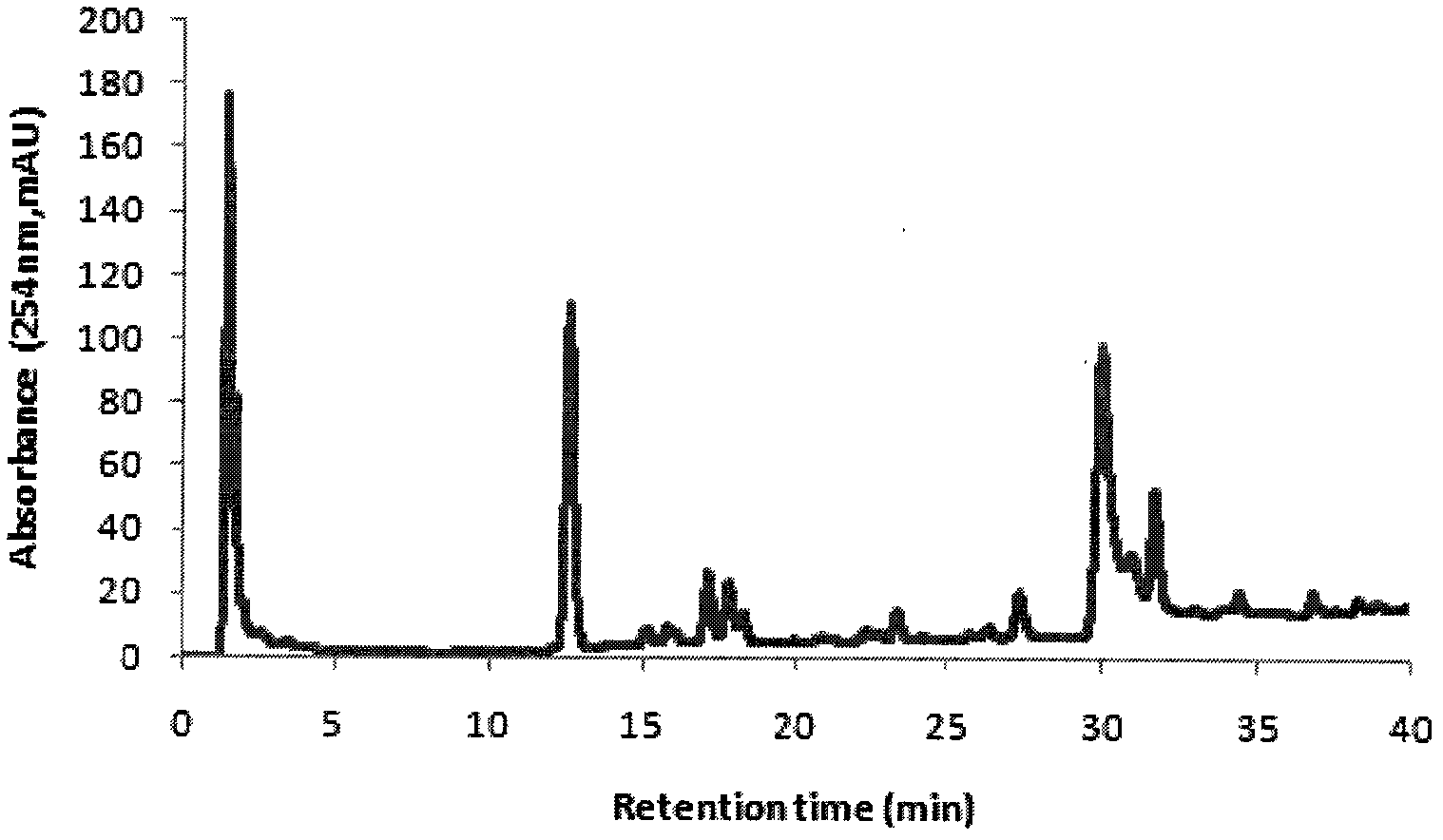
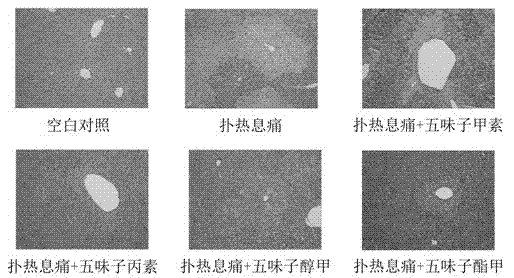
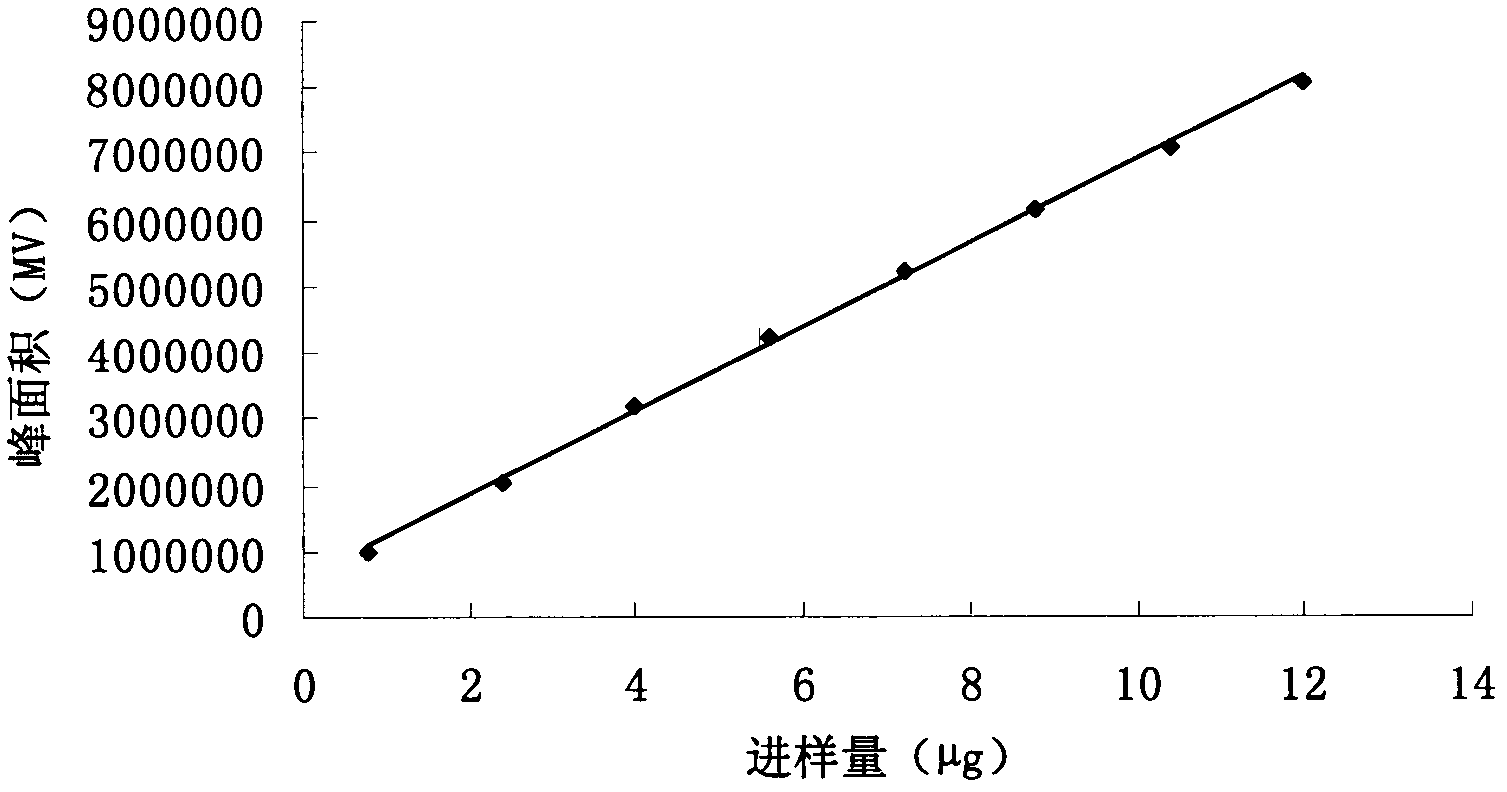
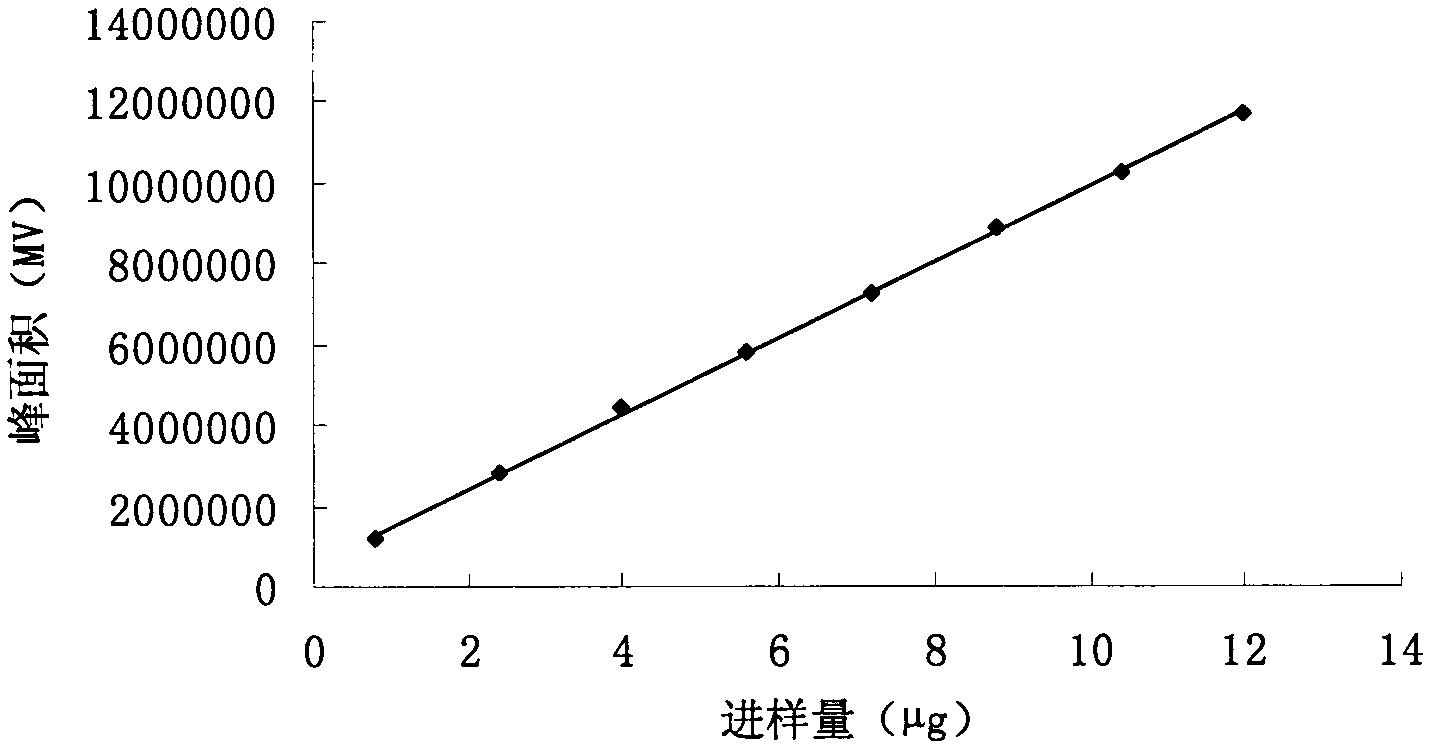
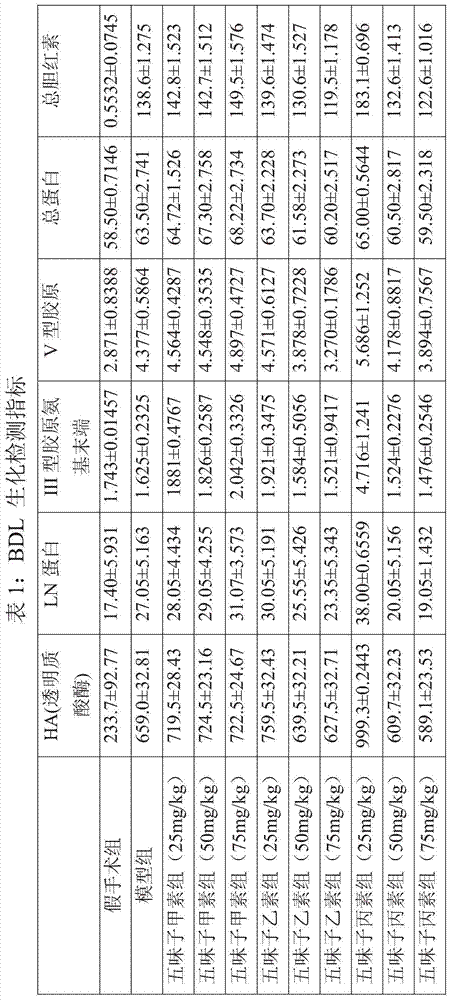
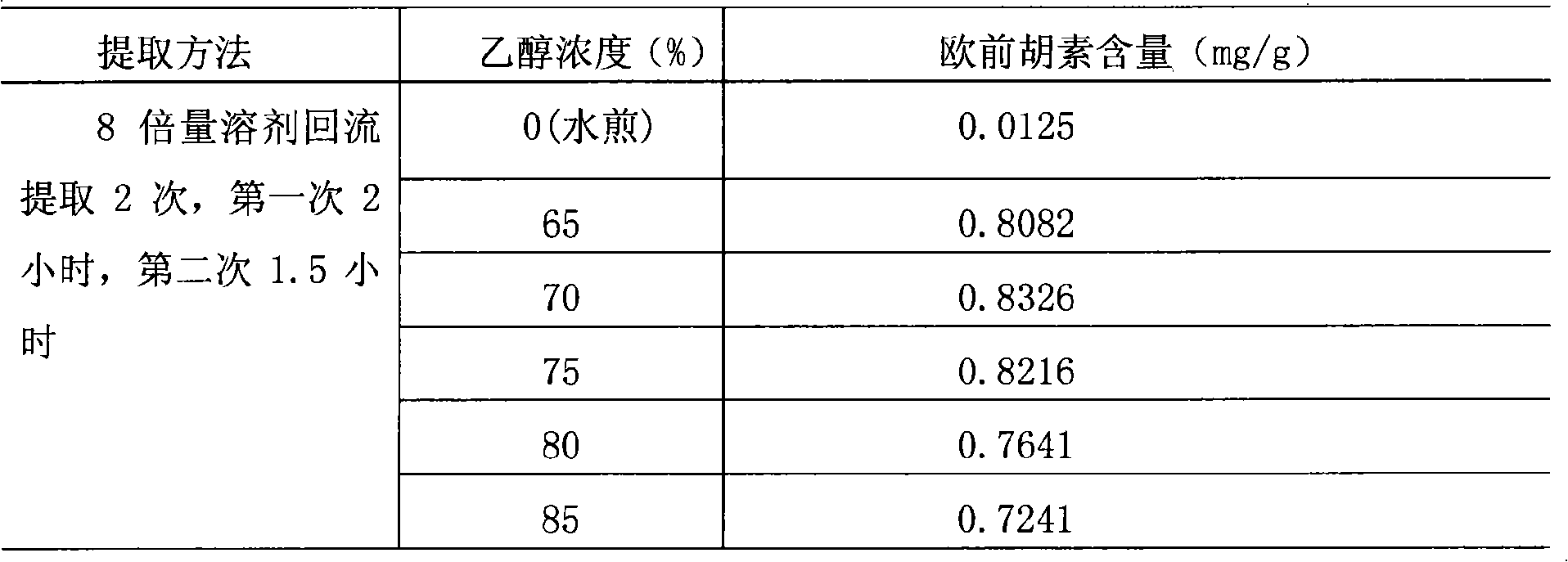
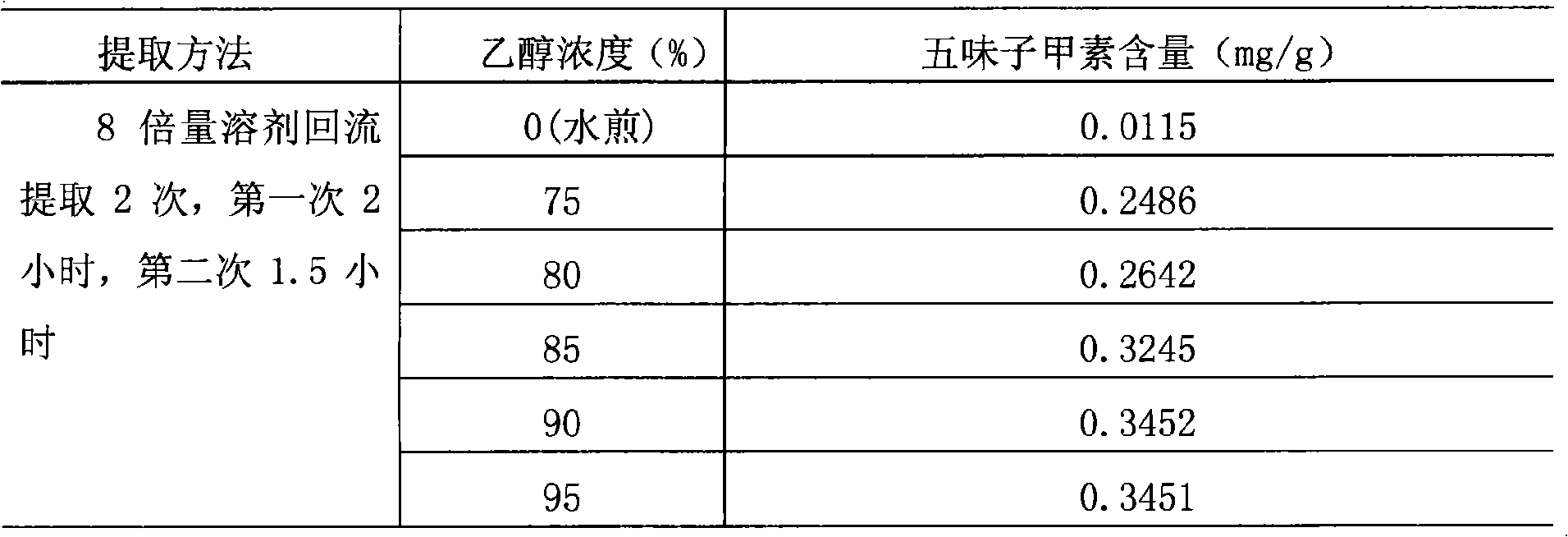
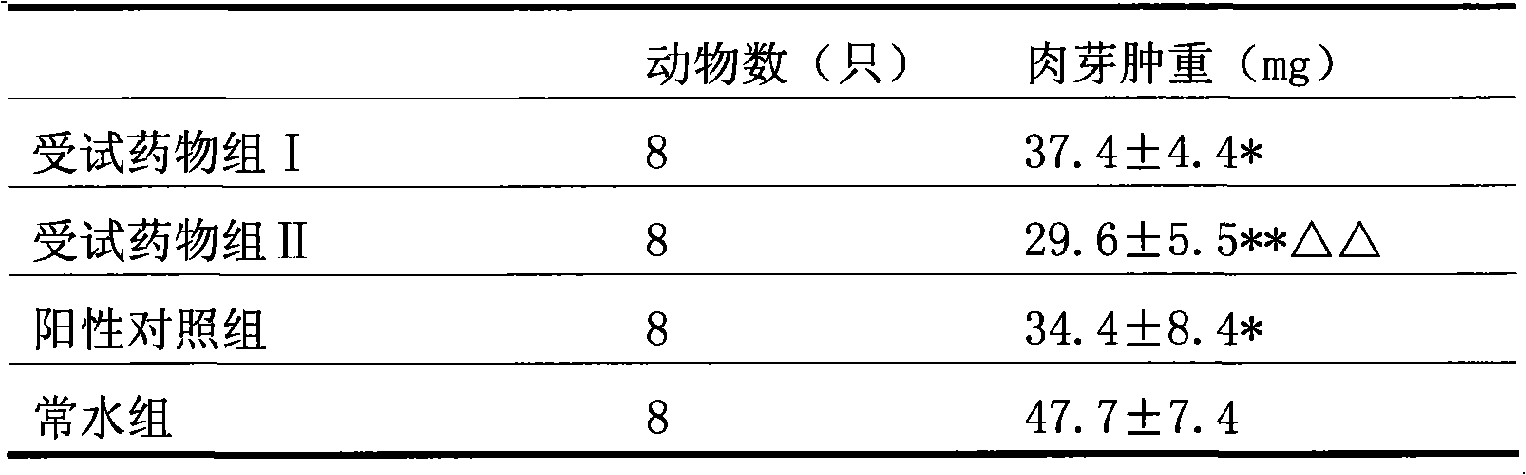
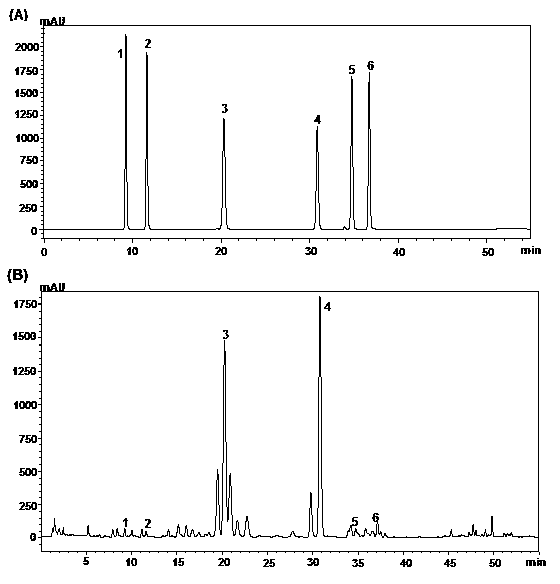
Patents
Literature
44 results about "Deoxyschizandrin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Deoxyschizandrin is a bio-active isolate of Schisandra chinensis. Deoxyschizandrin has been found to act as an agonist of the adiponectin receptor 2 (AdipoR2).
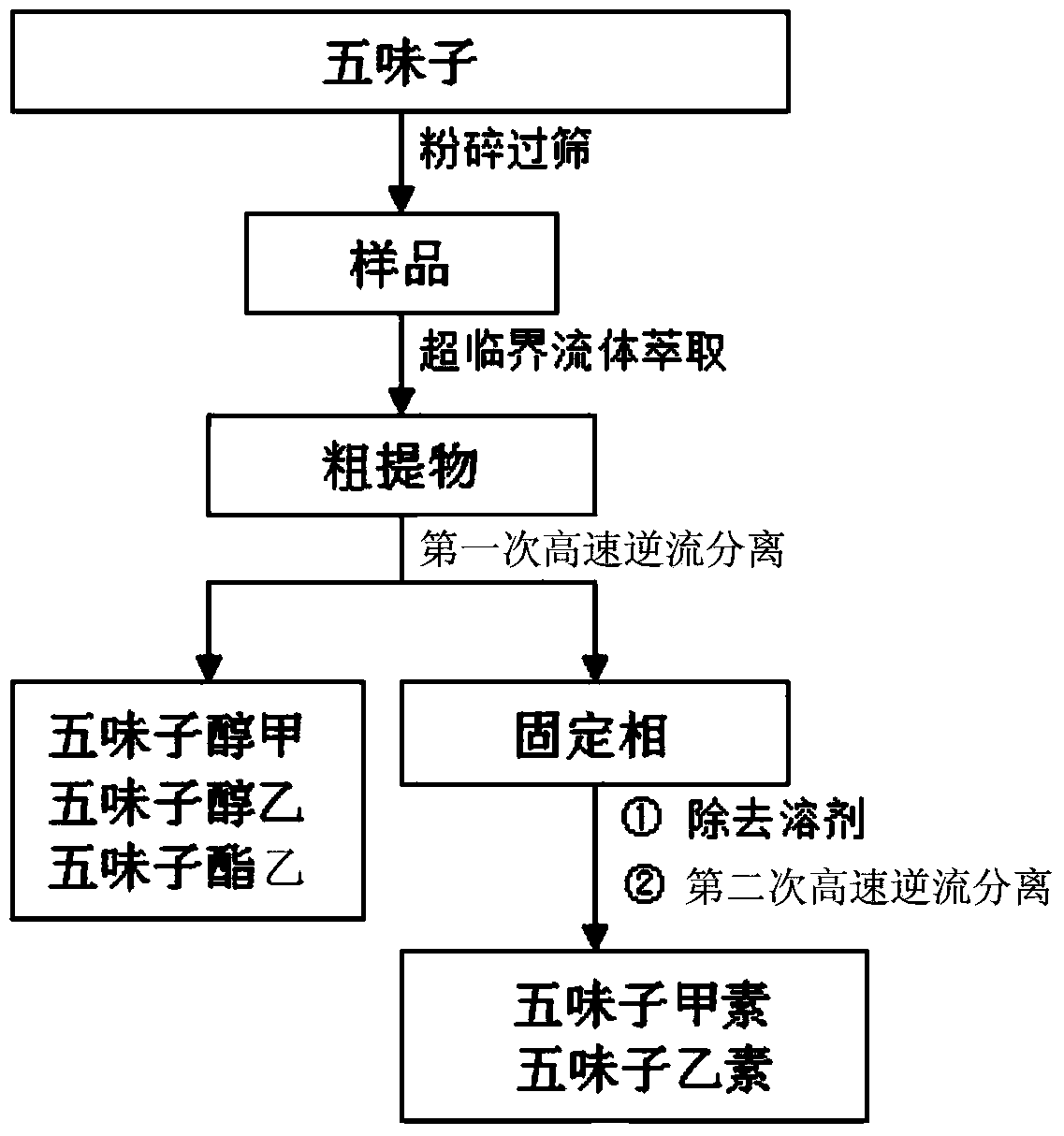
Chinese magnoliavine fruit monomer composition separation preparation method
InactiveCN101709059ANo lossHigh recovery rateOrganic chemistryOrganic compound preparationMonomer compositionEthyl acetate
The invention relates to a Chinese magnoliavine fruit monomer composition separation preparation method; ethanol extracts from Chinese magnoliavine fruit are extracted by petroleum ether, chromatography is carried out to the petroleum ether extracts by a silicagel column, and then the petroleum ether is eluted by petroleum ether-ethylacetate, and HPLC is used for monitoring, and then crude extract A and crude extract B are fraction-collected, and eluted; the volume ratio of eluant petroleum ether and ethylacetate is 3-5:1, the crude extract A and crude extract B are respectively applied to a high-speed counter-current chromatography for separation, so as to obtain SCHisanhenol, deoxyschizandrin, schizandrin B, schisandrin C, schizandrol A, schizandrol B and schisantherrin B monomers, and the purity is higher than 98 percent.
Owner:华美恒盛(北京)科技有限公司
Method for extracting, separating and preparing lignin monomers from schisandra chinensis
ActiveCN103467438AEasy to separate and purifySimple extraction stepsOrganic compounds purification/separation/stabilisationEther separation/purificationChromatographic separationEthyl acetate
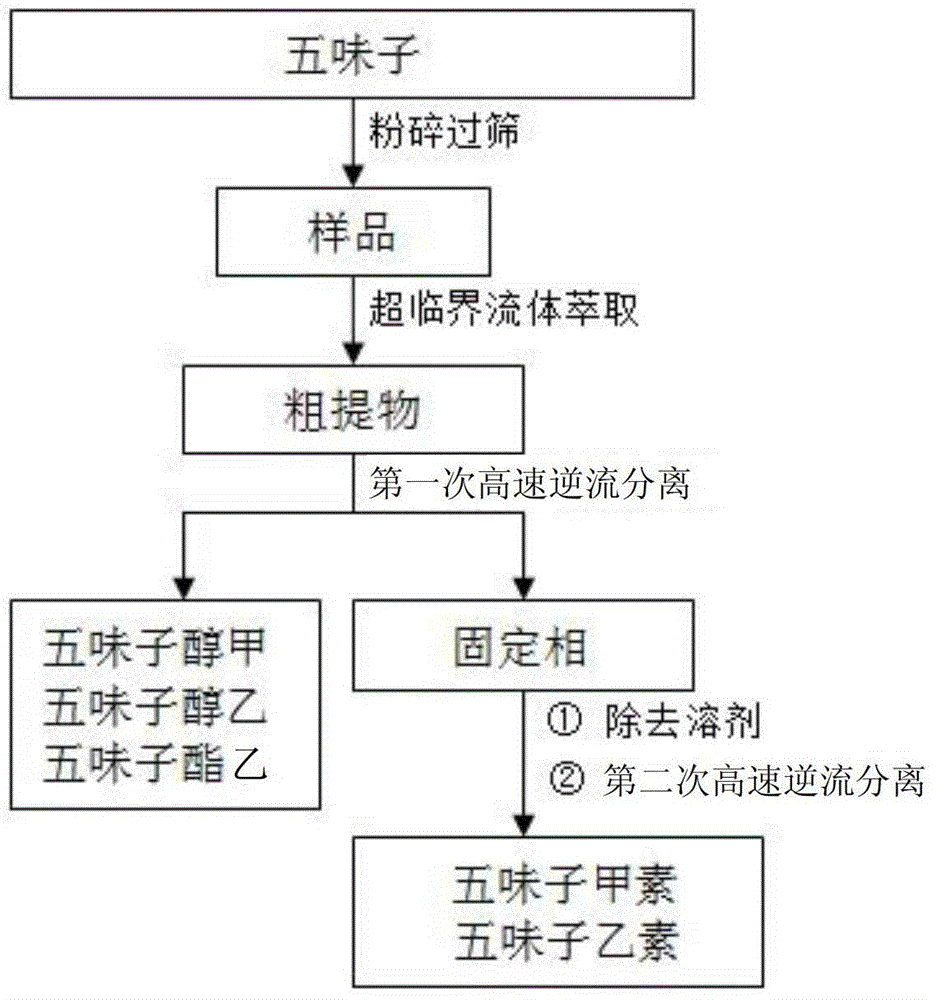
The invention discloses a method for extracting, separating and preparing lignin monomers from schisandra chinensis. The method comprises the following steps: performing supercritical extraction on schisandra chinensis medicinal powder by using CO2 to obtain a crude extract; performing primary high-speed counter-current chromatographic separation on the crude extract; forming a solvent system A by using hexane, ethyl acetate, methyl alcohol and water in the volume ratio of (2-10):(0-10):(0-10):5, wherein the upper phase A is a stationary phase A and the lower phase A is a mobile phase A; preparing a first schizandrol, a second schizandrol and a third schizandrol; recovering the separated stationary A to serve as a sample to be tested; performing secondary high-speed counter-current chromatographic separation; forming a solvent system B by using hexane, ethyl acetate, methyl alcohol and water in the volume ratio of (1-10):(0-4):(0-9):1, wherein the upper phase B is a stationary phase B, and the lower phase B is a mobile phase B; preparing deoxyschizandrin and schisandrin b. By adopting the method which integrates supercritical extraction and high-speed counter-current chromatographic separation, the deoxyschizandrin and the schisandrin b can be directly used for separating and purifying subsequent monomers without complex sample treatment after the extraction; the steps of extracting and separating are simple and efficient.
Owner:ZHEJIANG UNIV OF TECH
Compositions and methods for prostate and kidney health and disorders, an herbal preparation
A composition including an aliquot of the herb Herba Epimedii; and an aliquot of at least three supplemental herbs selected from the group consisting of Fructus Rosae Laevigatae; Fructus Rubi; Fructus Psoralea; Radix Morindae Officinalis; Fructus Schisandrac Chinensis; Fructus Ligustri Lucidi; Semen Cuscutae; and Radix Astragali. A composition including icariin; ursolic acid; ellagic acid; psoralen; deoxyschizandrin; oleanolic acid; quercetin; aslvagaloside; and an extract of the herb Radix Morindae Officinalis. Methods including administering a composition directed at treatment of various kidney disorders or the promotion of kidney health and to the overall health of the kidney, including the use of a composition in the treatment of prostate cancer, prophylatic prostate health, reduction of polyuria, incontinence, proteinuria, as well as for sexual satisfaction.
Owner:SIBONI GRP
Compositions and methods for prostate and kidney health and disorders, an herbal preparation
A composition including an aliquot of the herb Herba Epimedii; and an aliquot of at least three supplemental herbs selected from the group consisting of Fructus Rosae Laevigatae; Fructus Rubi; Fructus Psoralea; Radix Morindae Officinalis; Fructus Schisandrac Chinensis; Fructus Ligustri Lucidi; Semen Cuscutae; and Radix Astragali. A composition including icariin; ursolic acid; ellagic acid; psoralen; deoxyschizandrin; oleanolic acid; quercetin; aslvagaloside; and an extract of the herb Radix Morindae Officinalis. Methods including administering a composition directed at treatment of various kidney disorders or the promotion of kidney health and to the overall health of the kidney, including the use of a composition in the treatment of prostate cancer, prophylatic prostate health, reduction of polyuria, incontinence, proteinuria, as well as for sexual satisfaction.
Owner:SIBONI GRP
Traditional Chinese medicine preparation lumbus-strengthening kidney-tonifying pill detection method
InactiveCN104483315AStrong process controllabilityImprove quality controlComponent separationMaterial analysis by optical meansControllabilityHigh-performance liquid chromatography
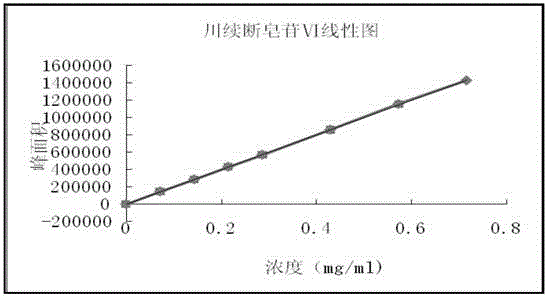
The invention discloses a traditional Chinese medicine preparation lumbus-strengthening kidney-tonifying pill detection method, according to the method, rehmannia glutinosa, yam, tuckahoe, schisandra, chrysanthemum and astragalus in a lumbus-strengthening kidney-tonifying pill can be microscopically identified, a radix angelicae sinensis referenee crude herb, ginsenoside Rg1, ginsenoside Re, ginsenoside Rb1, dipsacus asper saponin VI, astragaloside and deoxyschizandrin are used as reference substances, whether the lumbus-strengthening kidney-tonifying pill contains the radix angelicae sinensis ,ginseng, dipsacus asper, astragalus and schisandra can be identified by thin-layer chromatography; the dipsacus asper saponin VI content in the lumbus-strengthening kidney-tonifying pill preparation can be detected by HPLC, according to the detection method, the quantitative index is that the dipsacus asper saponin VI (C47H76O18) content in every 1g of the traditional Chinese medicine preparation lumbus-strengthening kidney-tonifying pill may not be less than 0.60mg, the controllability of quality standards of the traditional Chinese medicine preparation lumbus-strengthening kidney-tonifying pill can be improved to further ensure the product intrinsic quality and effect, so that the quality standard is more perfect, and the drug quality control level is improved.
Owner:JINGFUKANG PHARMA GRP CHIFENG DANLONG PHARMA CO LTD
Method for measuring content of Shenqi blood sugar reducing preparation and application thereof in overall quality control
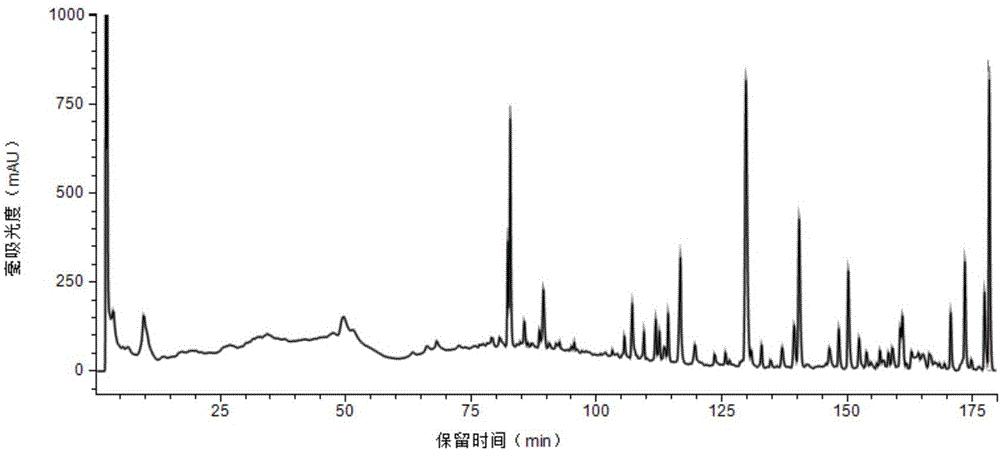
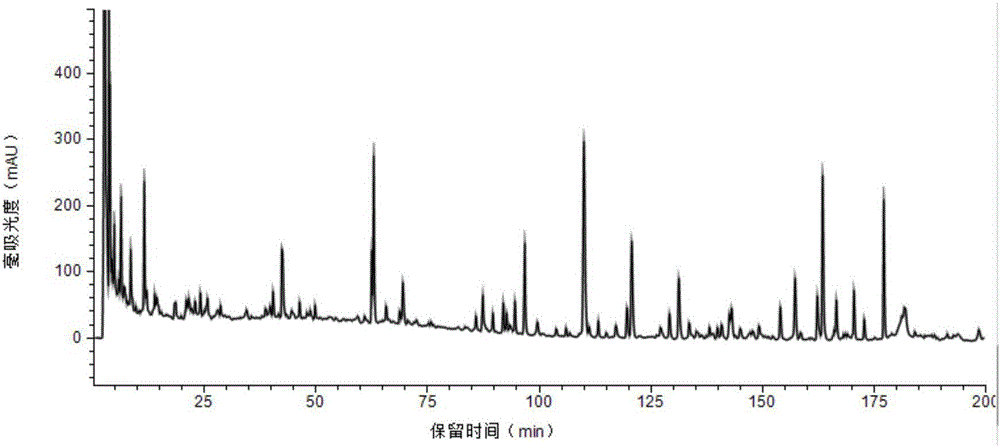
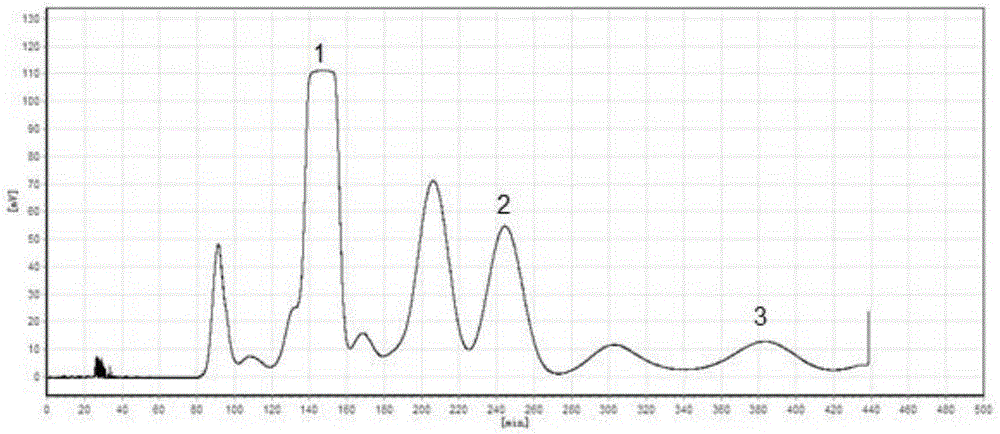
The invention provides a method for measuring the content of a Shenqi blood sugar reducing preparation. The method is used for measuring the content of 10 active ingredients in the Shenqi blood sugar reducing preparation: ginsenoside Rb1, ginsenoside Rc, ginsenoside Rd, ginsenoside Rg1, ginsenoside Re, astragaloside, deoxyschizandrin, schisandrol A, schisandrol B. In the invention, with the guidance of blood sugar reducing activity of a compound, multiple active substances in the compound are detected, and the quality level of the whole compound preparation can be reflected better; meanwhile, a Q-MS ion monitoring mode is selected for content measurement, and the mass spectrum quantification has high accuracy, specificity and sensitivity and is superior to traditional HPLC analysis method; and moreover, fingerprint is established according to the liquid phase diagrams of different batches of Shenqi blood sugar reducing preparations obtained by the method, the similarity is evaluated, the inherent quality of the Shenqi blood sugar reducing preparation product is evaluated more scientifically and comprehensively, and a basis is provided for establishing a quality standard of higher level.
Owner:ZHEJIANG UNIV OF TECH
Process for preparing high-purity deoxyschizandrin
InactiveCN101070314ANo lossNo pollution in the processOrganic compounds purification/separation/stabilisationDeoxyschizandrinAcetonitrile
This invention relates to a method of preparing high-purity Schisandrin B. The invention adopts analysis mode high speed counter-current chromatography to proceed small scale preparation, adopt semipreparative type high speed counter-current chromatography to proceed large-scale preparation. The feature is that in solvent system the volume ratio of normal hexane, methanol, water and acetonitrile is 5 - 11 : 3 - 6 : 2 - 5 : 1, and the best volume ratio is 10:5:4:3. The invention does not need solid padding, has no irreversible adsorption and no pollution.The purity of gained Schisandrin B can reach 98%upwards.
Owner:赵景辉
Quality control method of south schizandrol extract oral solid formulated product
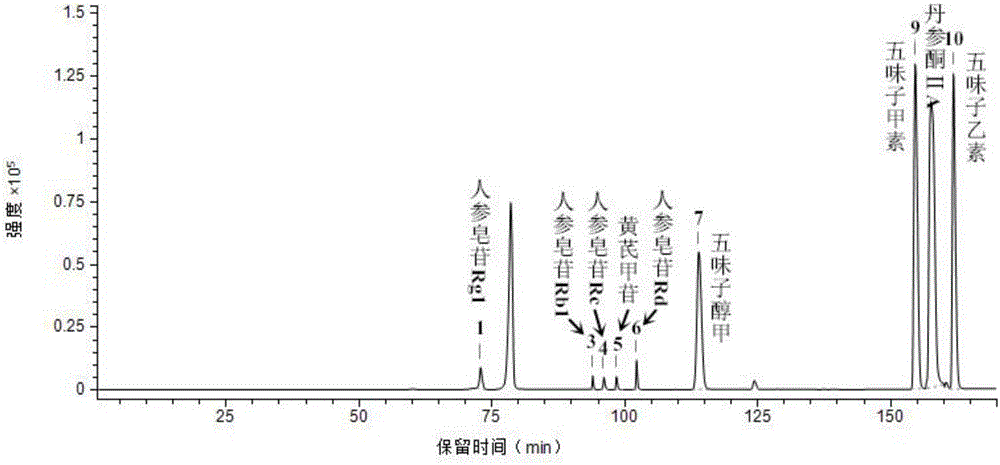
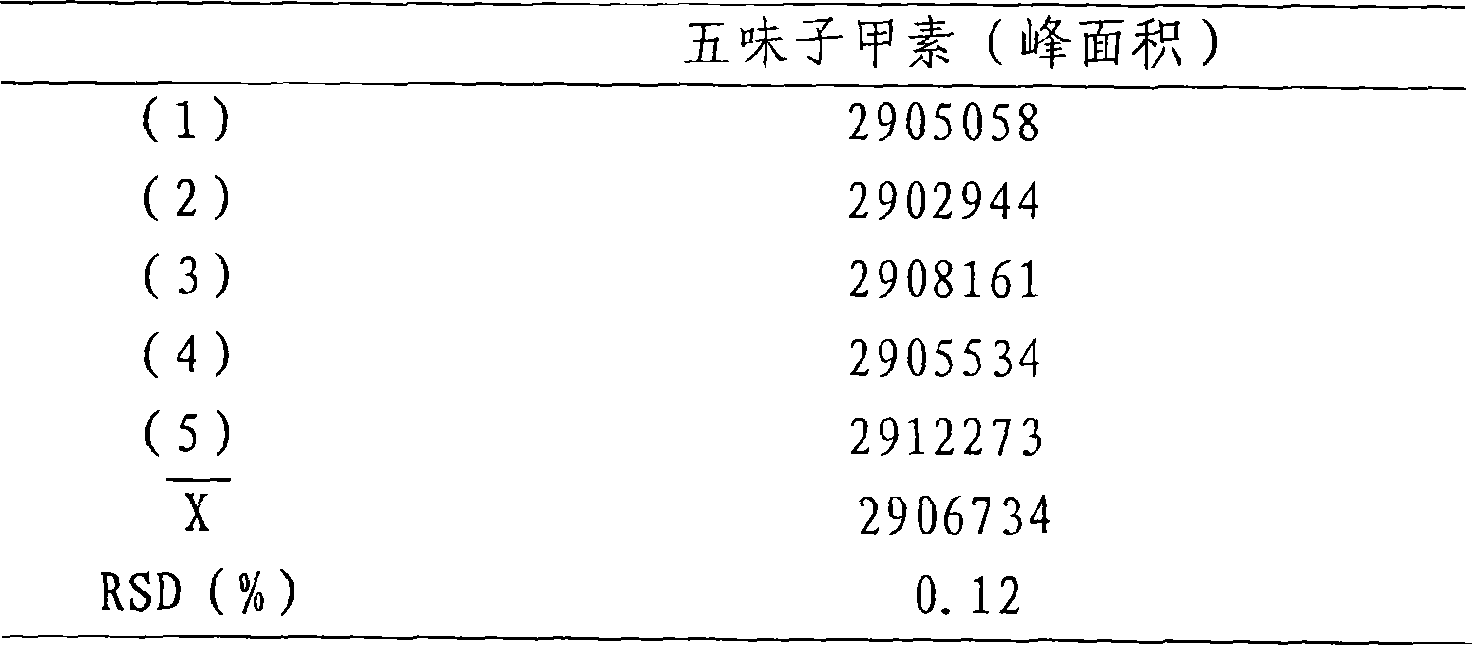
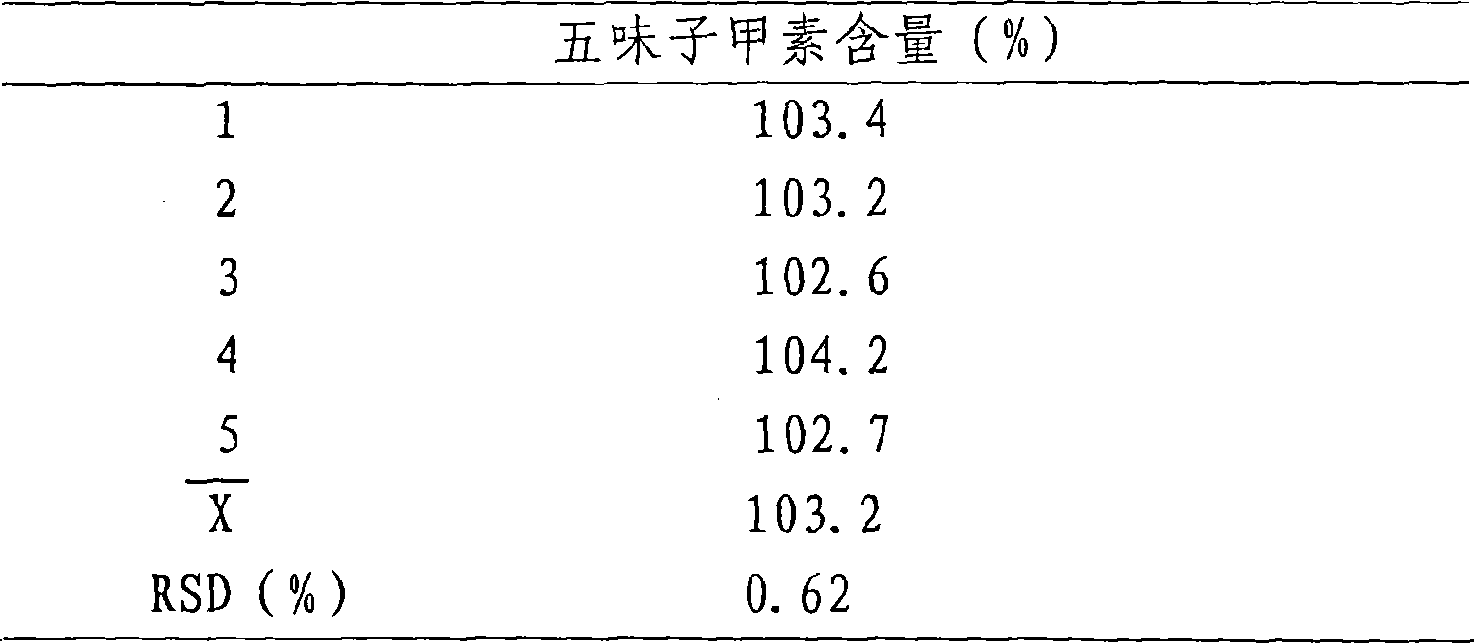
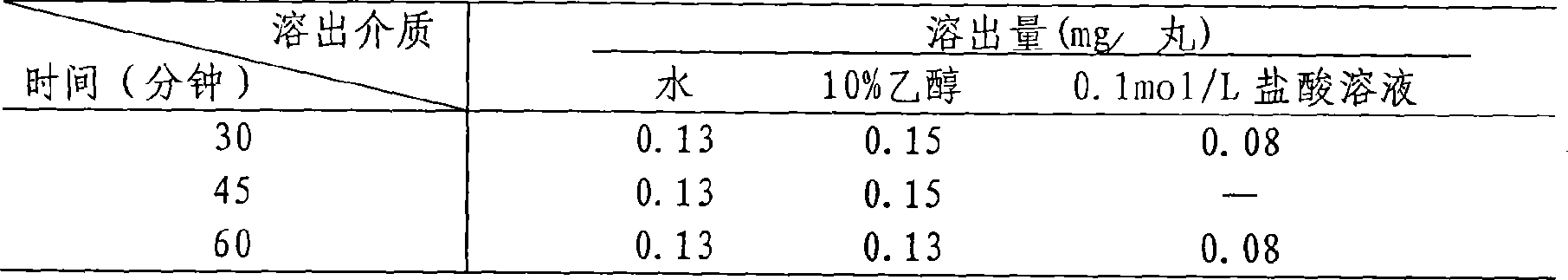
The invention relates to a method for detecting dissolving rate of oral administration solid preparation which is prepared by using schisandra fruit extract as the active constituent, wherein ethanol solution, or thin hydrochloric acid or water is used as the dissolving out medium, and high performance liquid phase chromatographic method is employed to detect the dissolving quantity of Deoxyschizandrin in the oral administration solid preparation of schizandrol extract.
Owner:HONGYI SCI & TECH CO LTD NANCHANG
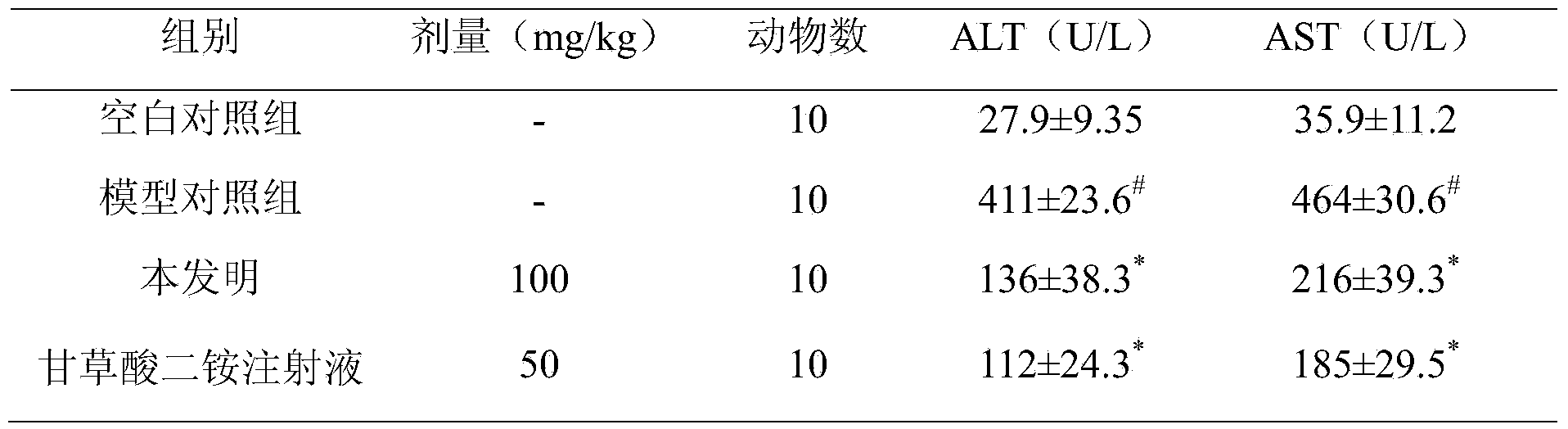
Application of deoxyschizandrin in preparation of drug for treating cholestasis liver injury
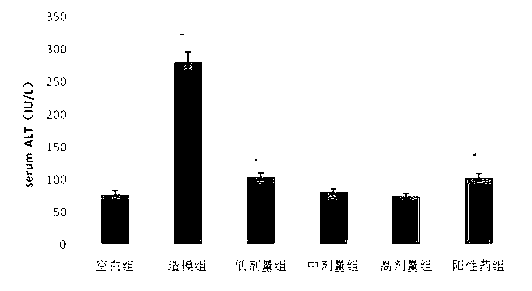
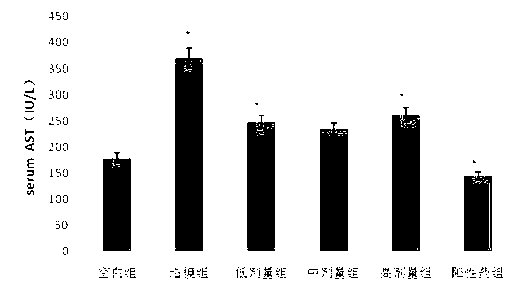
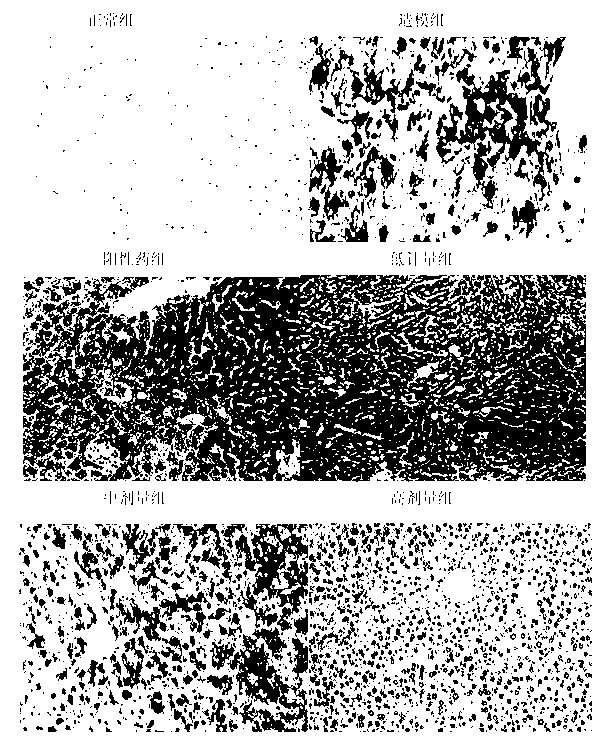
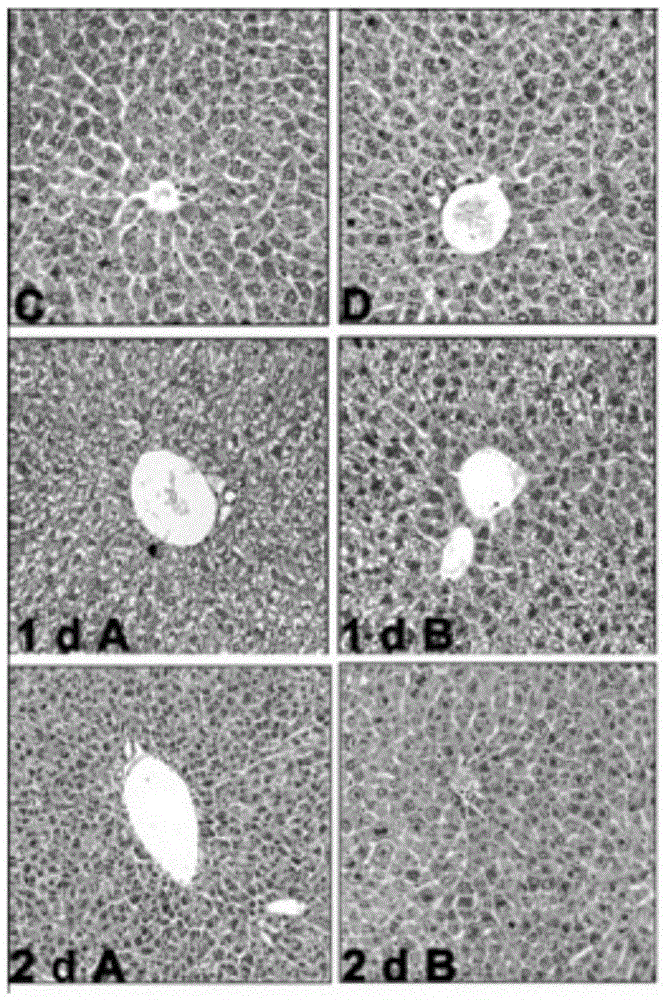
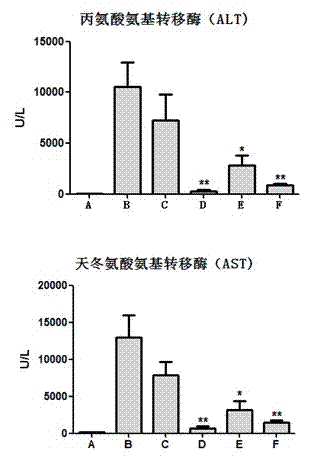
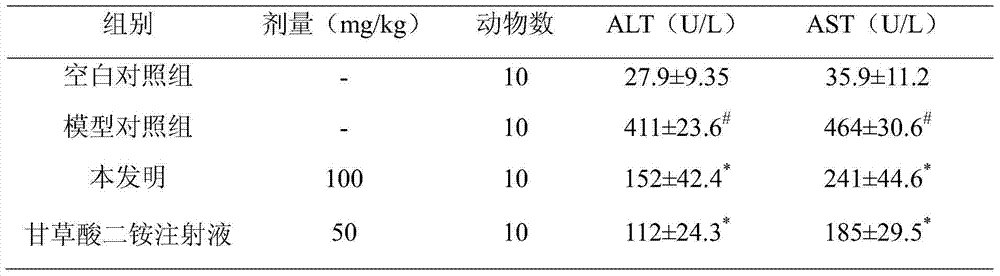
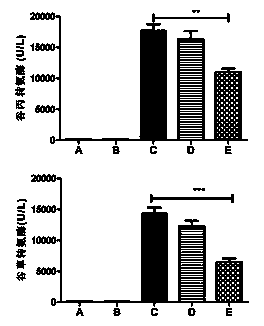
The invention discloses an application of deoxyschizandrin in preparation of drug for treating cholestasis liver injury. Deoxyschizandrin has molecular formula of C24H32O6 and molecular weight of 416.51, and has the structural formula shown in the description. Mouse liver injury induced by application of excessive lithocholic acid (LCA) is taken as a model. After application of deoxyschizandrin, alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels in serum are significantly lowered, while necrosis degree of liver cells is significantly improved. The results indicate that deoxyschizandrin has treatment effect for liver injury induced by LCA, and can be used for treating cholestasis liver injury clinically.
Owner:INST OF RADIATION MEDICINE ACAD OF MILITARY MEDICAL SCI OF THE PLA
Application of fructus schisandrae sphenantherae extract in preparation of liver regeneration medicine
ActiveCN105456481AGood conditionRecovery functionDigestive systemPlant ingredientsCyclin D1Additive ingredient
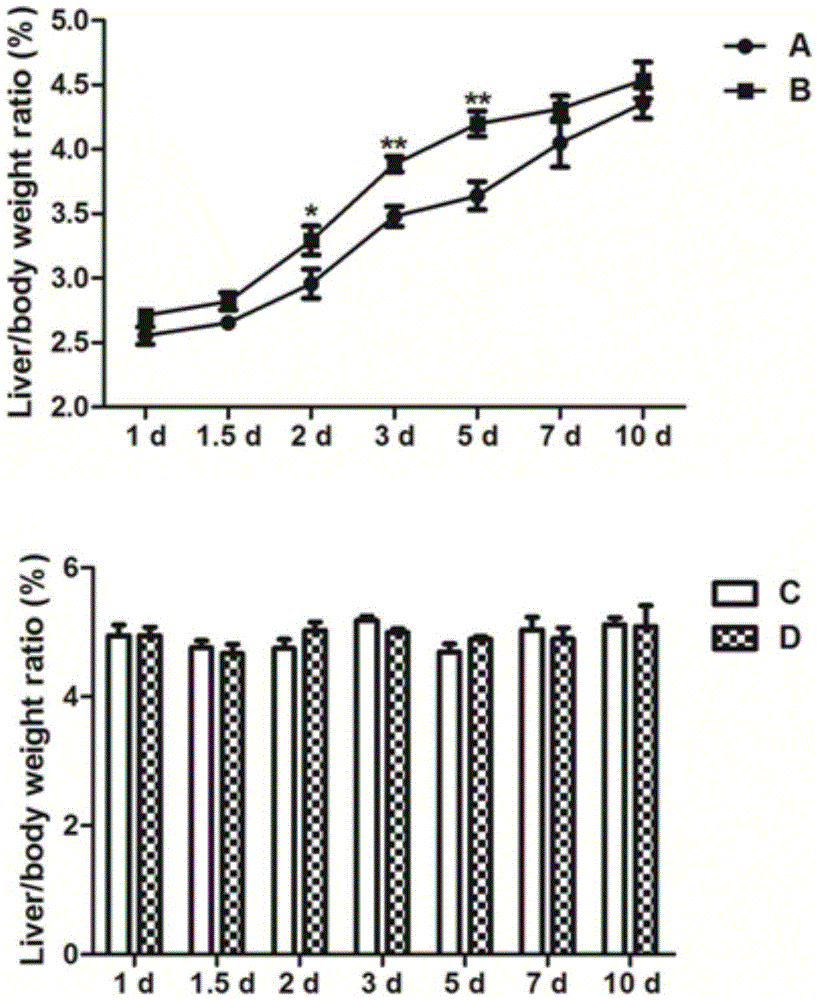
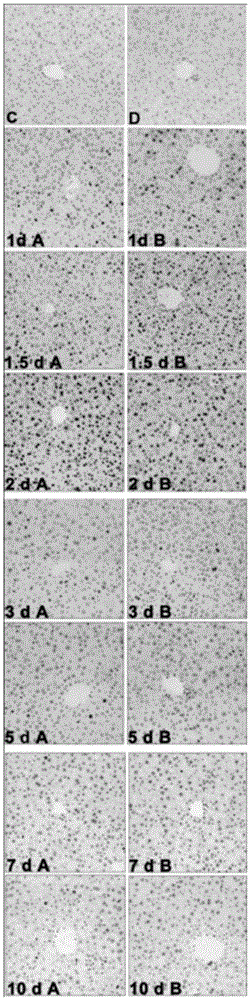
The invention discloses application of a fructus schisandrae sphenantherae extract in preparation of a liver regeneration medicine. The fructus schisandrae sphenantherae extract is an alcohol extract of dried fruits of schisandrae sphenantherae, and the main effective ingredients of the fructus schisandrae sphenantherae extract comprise schisantherin and deoxyschizandrin. A partial hepatectomy mouse model is utilized, the fructus schisandrae sphenantherae extract is cooperatively applied, a result shows that the fructus schisandrae sphenantherae extract has the characteristics of being capable of significantly improving the quick recovering capacity of the liver-weight ratio and improving the quick proliferation capacity of liver cells, the mechanism relates to significant activation of protein expression of a related cell cycle, the manifestation is that cyclin D1 and PCNA proteins are significantly up-regulated, and meanwhile obvious improvement on liver tissue inflammatory infiltration and liver cell swelling is achieved. Experimental results show that the fructus schisandrae sphenantherae extract supplies a novel method and treatment means to liver regeneration after hepatectomy.
Owner:SUN YAT SEN UNIV
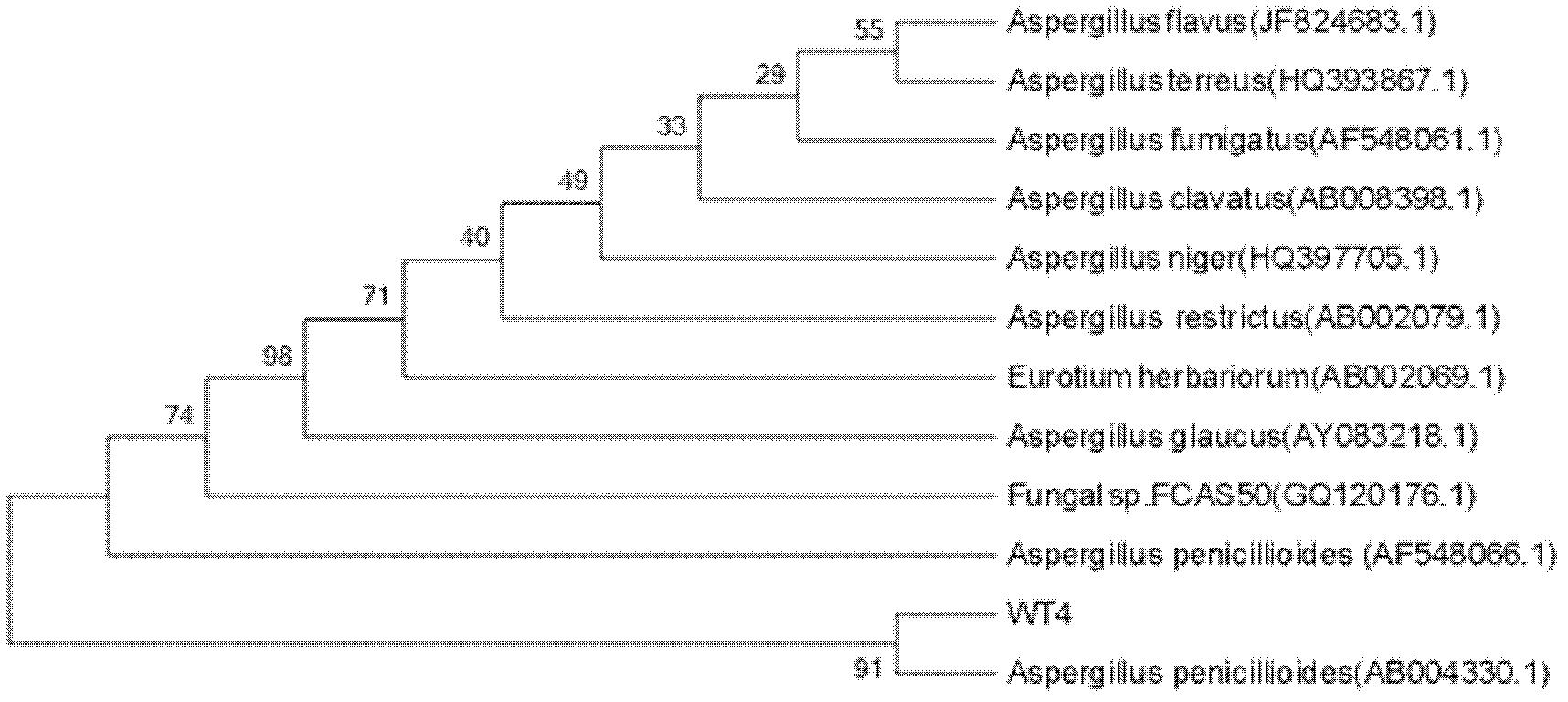
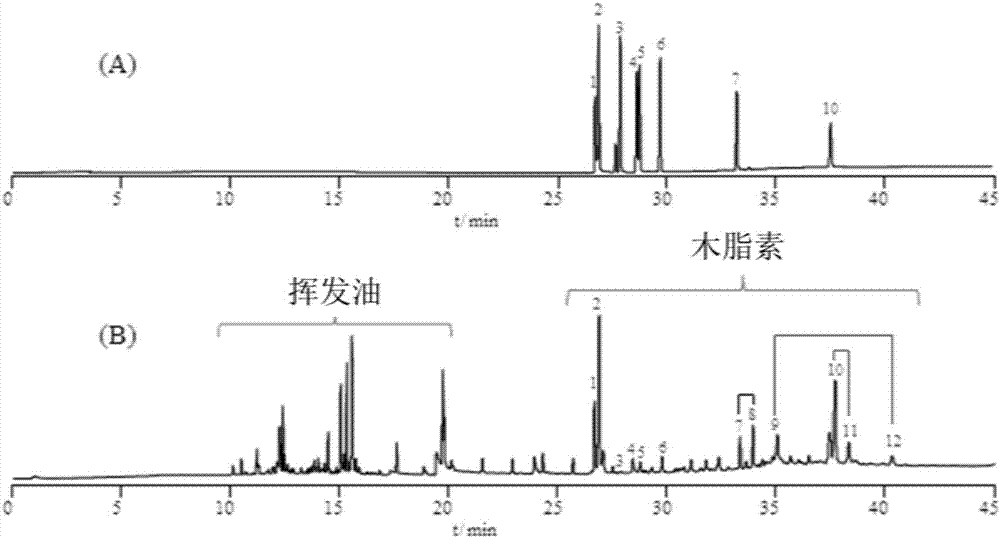
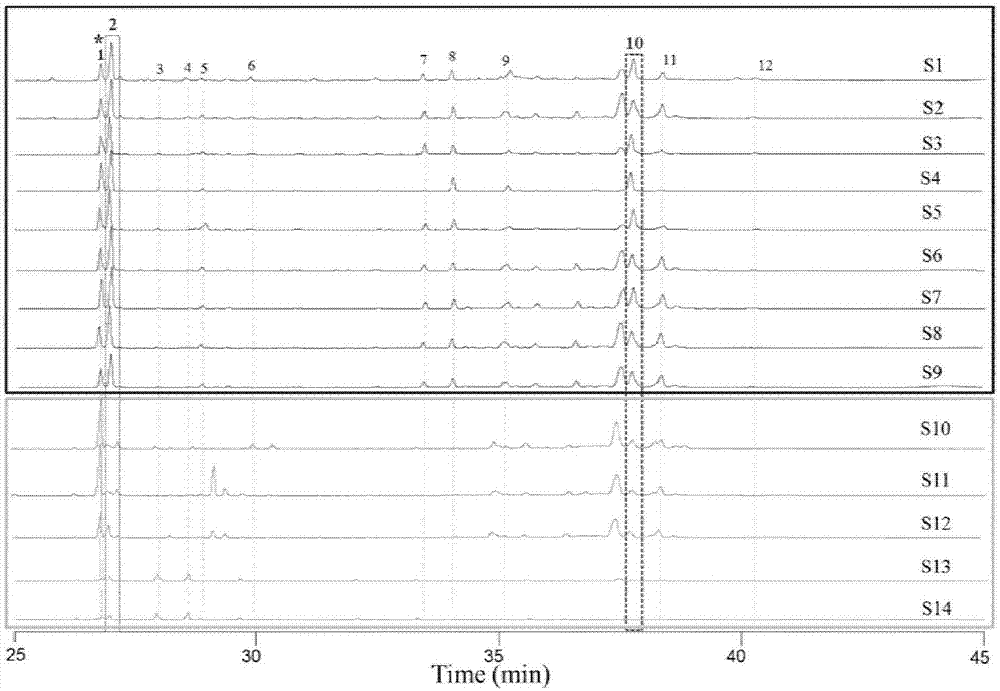
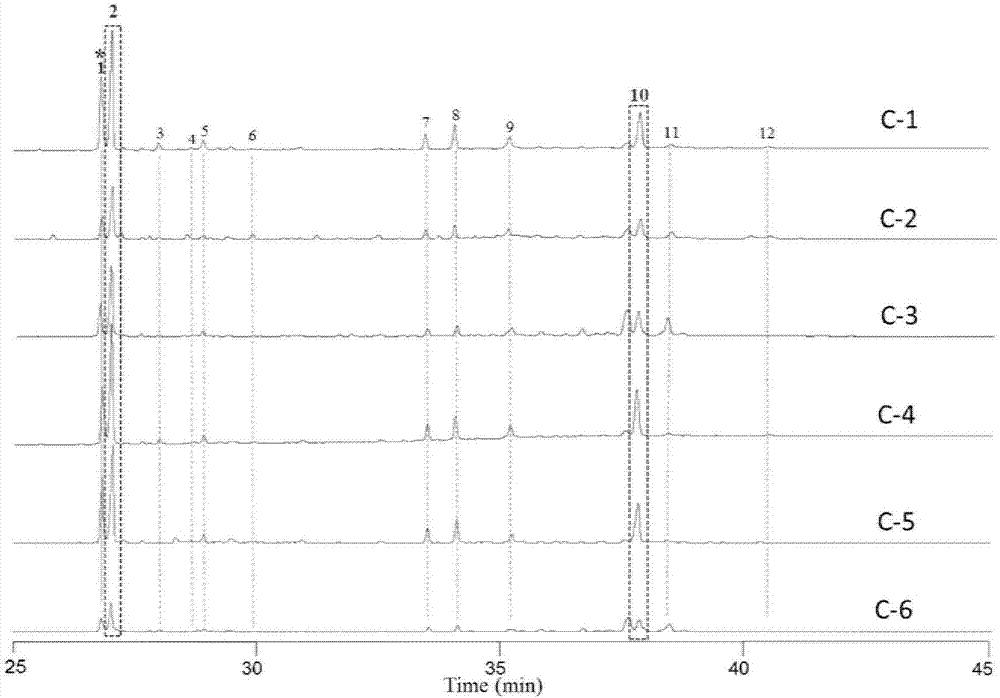
Endophytic fungi for improving content of main active ingredients of schisandra chinensis through fermentation method
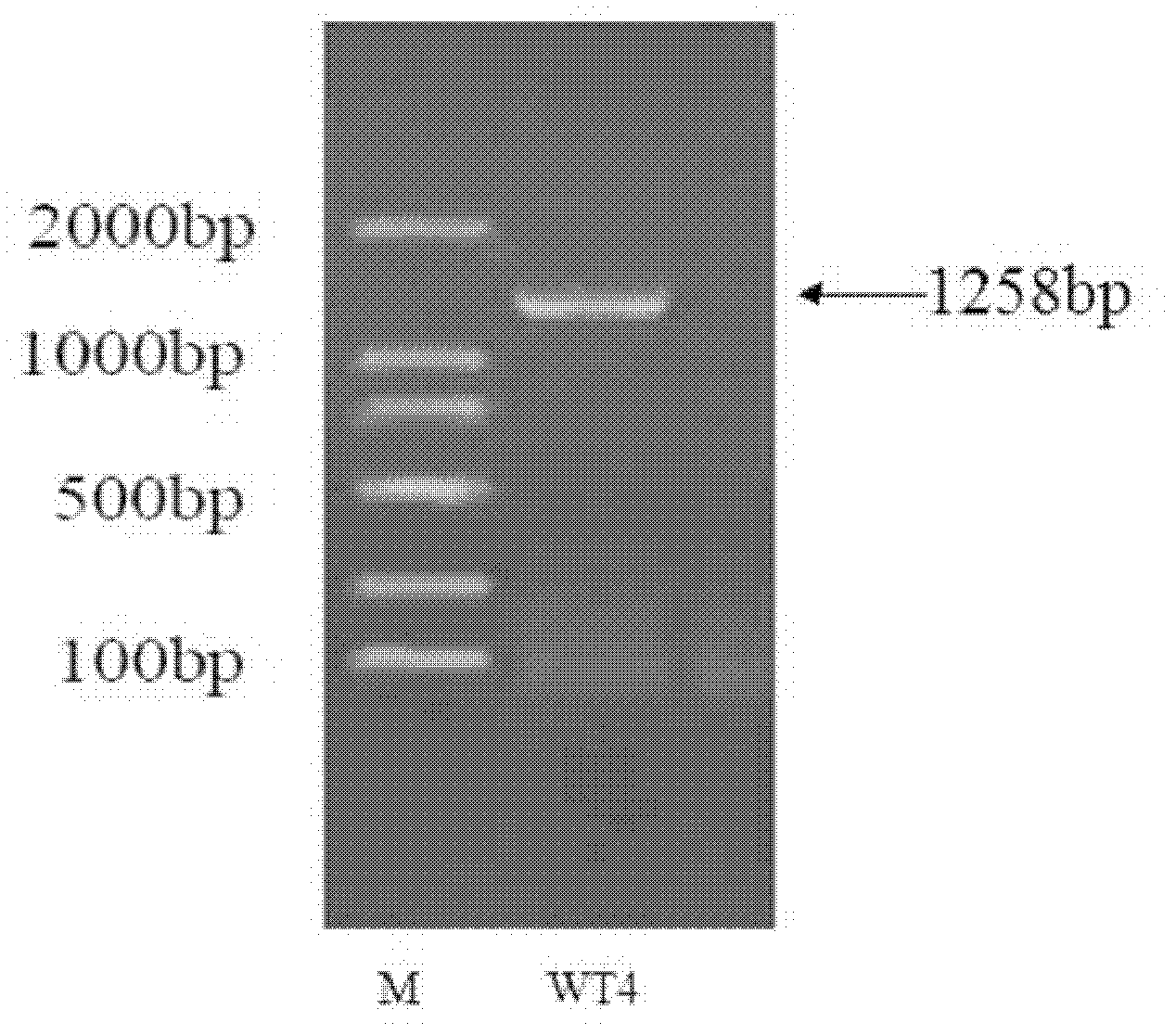
InactiveCN102660466AHigh content of ingredientsSave drug resourcesFungiMicroorganism based processesBiotechnologyDeoxyschizandrin
The invention relates to an endophytic fungi for improving content of main active ingredients of schisandra chinensis through a fermentation method. The endophytic fungi is Aspergilluspenicillioides WT4 which is preserved in the China Center for Type Culture Collection (CCTCC) with preservation numbers of CCTCC No: M2012043 in Wuhan University in Wuhan city, and the preservation date is February 29, 2012. The WT4 in the endophytic fungi can remarkably improve the main active ingredients of the schisandra chinensis, such as schizandrin, schisantherin, deoxyschizandrin and schisandrin b. Therefore, in the raw material production with the purpose of obtaining the schizandrin, the schisantherin, the deoxyschizandrin and the schisandrin b, people can select schisandra chinensis endophytic fungi WT4 to ferment the schisandra chinensis to improve the yield and save medicinal resources.
Owner:HEILONGJIANG UNIV
Method for evaluating quality of FRUCTUSSCHISANDRAE SPHENANTHERAE in commercially available Chinese patent medicines by using flash-gas chromatography
ActiveCN107064363ASimple and fast operationEnvironmental assessment quality methodComponent separationRetention timeLignan
The invention relates to a method for evaluating quality of FRUCTUSSCHISANDRAE SPHENANTHERAE in commercially available Chinese patent medicines by using a flash-gas chromatography. The method comprises the steps of putting a powdered or liquid Chinese patent medicine sample, which contains the FRUCTUSSCHISANDRAE SPHENANTHERAE, into a pyrolyzer, and placing the pyrolyzer at a sample inlet of a gas chromatograph; when the temperature of the pyrolyzer reaches a certain value, carrying out sample introduction; detecting the sample by using the gas chromatograph to obtain a gas chromatogram; carrying out qualitative analysis on a lignan component in the Chinese patent medicine sample by using standard material retention time, and determining the corresponding peak positions of anwuligan, deoxyschizandrin and schisantherin in the gas chromatogram; (2) then, using the flash-gas chromatography to carry out quantitative analysis on the deoxyschizandrin and the schisantherin in the Chinese patent medicine sample by means of an external standard method; if the quantitative result shows that the relative contents of the deoxyschizandrin and the schisantherin in the Chinese patent medicine sample are within a range of 1.50-2.0, proving that the quality of the FRUCTUSSCHISANDRAE SPHENANTHERAE in the Chinese patent medicine sample is better, and otherwise, proving that the quality of the fructus schisandrae in the Chinese patent medicine sample is poorer. The method has the characteristics of being simple, environmentally friendly and efficient.
Owner:ZHEJIANG UNIV OF TECH
Method for carrying out simultaneous quantitative analysis on four lignan components in Chinese magnoliavine raw material and Chinese magnoliavine extract
InactiveCN102419350ARealize simultaneous measurementSolve the problem that the raw materials of Schisandra chinensis cannot be measured at the same timeComponent separationLignanColumn temperature
The invention discloses a method for carrying out simultaneous determination on contents of schisandrin, schisantherin, deoxyschizandrin and schisandrin B in a Chinese magnoliavine raw material or a Chinese magnoliavine extract. The method employs means of crushing, sieving, methanol ultrasonic extraction, calibrating volume and filtering to treat schisandra chinensis or a Chinese magnoliavine extract to obtain a sample solution for testing; a high performance liquid chromatography is utilized for simultaneous determination on contents of four lignan components. The method utilizes a reverse direction distribution chromatographic theory to carry out analysis on the sample by gradient elution, under conditions of a column temperature of 25-45 DEG C, mobile phases of methanol and 0.05-0.2% trifluoroacetic acid aqueous solution (methanol and trifluoroacetic acid aqueous solution in a volume ratio of 50:50-100:0), an elution time of 30-60 min, a flow velocity of 0.7-1.5 ml / min and a detection wavelength of 210-280 nm. The quality detection method is simple, rapid, sensitive and accurate.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI +1
Application of schisandra chinensis monomer compound in preparation of drugs for prevention and treatment of hepatotoxicity caused by acetaminophen
ActiveCN103751174ACurb consumptionImprove necrosisDigestive systemEther/acetal active ingredientsLiver necrosisAlanine aminotransferase
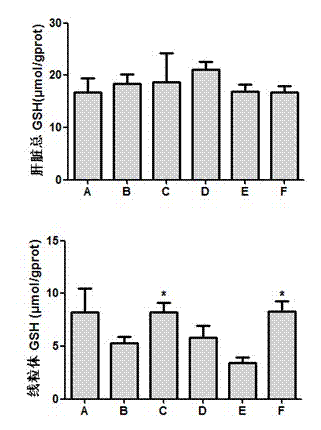
The invention discloses an application of a schisandra chinensis monomer compound in preparation of drugs for prevention and treatment of hepatotoxicity caused by acetaminophen (APAP), wherein the schisandra chinensis monomer compound is deoxyschizandrin, schisandrin C, schisandrin or schisantherin a. Experiment results show that: with the schisandra chinensis monomer compound, the ALT content and the AST content in the APAP-induced liver injury animal model can be significantly reduced, consumption of glutathione in liver cells can be inhibited, the liver cell necrosis degree can be effectively improved, and the schisandra chinensis monomer compound provides significant treatment and protection effects for hepatotoxicity symptoms caused by APAP.
Owner:SUN YAT SEN UNIV
Chinese magnoliavine active extract
InactiveCN107513008AHigh purityHigh adsorption rateEther separation/purificationReflux extractionDeoxyschizandrin
The invention discloses a Chinese magnoliavine active extract, which comprises schisandrin, deoxyschizandrin, and schisandrin B. The preparation method comprises reflux extraction, concentration and water precipitation, adsorption, elution, and drying. Ethanol (85-95%) is used to extract the Chinese magnoliavine active substances through reflux extraction; then the active substances are purified by water precipitation, adsorption, macroporous resin elution, and reversed-phase high-performance liquid chromatography to obtain schisandrin, deoxyschizandrin, and schisandrin B, and the product quality is high. The production technology is simple, each operation has accurate data, the repeatability is good, the product quality is stable, ethanol is low in toxicity, and the extracted Chinese magnoliavine active substances are very safe and green.
Owner:浦江县美泽生物科技有限公司
Application of deoxyschizandrin in preparation of cosmetics or externally-applied medicines with functions of resisting light aging or removing acnes and diminishing inflammation
InactiveCN104546611AIncrease secretionReduce decompositionCosmetic preparationsAntipyreticDeoxyschizandrinMedicine
The invention relates to an application of deoxyschizandrin in preparation of cosmetics or externally-applied medicines with functions of resisting light aging or removing acnes and diminishing inflammation. The mass content of Chinese magnoliavine fruit lignan in deoxyschizandrin is not less than 5%, and the use amount of deoxyschizandrin in the cosmetics or the externally-applied medicines is 0.01-10% in percentage by mass. The application disclosed by the invention has the advantages that researches show that deoxyschizandrin has relatively good anti-light-aging effects, also has a relatively good anti-inflammatory effect on inflammation caused by propionibacterium acne, and can be used for preparing the cosmetics or the externally-applied medicines with the effects of resisting light aging or removing the acnes and diminishing inflammation.
Owner:SHANGHAI INOHERB COSMETIC +1
Process for preparing high-purity deoxyschizandrin
InactiveCN101070313ANo lossNo pollution in the processOrganic compounds purification/separation/stabilisationDeoxyschizandrinProcess conditions
This invention relates to a preparation method of high-purity deoxyschizandrin. The process as follows: the solvent system includes normal hexane, methanol and water; the volume ratio of normal hexane, methanol and water is 2 - 6: 3 - 7: 4 - 8; firstly dissolve deoxyschizandrin crude extract perpared by traditional craft in 2ml - 10ml bottom phase; adopt analysis mode high speed counter-current chromatography to proceed small scale preparation, adopt semipreparative type high speed counter-current chromatography to proceed large-scale preparation. The invention does not need solid padding, has no irreversible adsorption and no pollution. The purity of gained deoxyschizandrin can reach 94%upwards.
Owner:赵景辉
Strain of endophytic fungus of fermenting schisandra chinensis to improve main active components of schisandra chinensis
InactiveCN102628018AHigh in active ingredientsHigh content of active ingredientsFungiMicroorganism based processesDeoxyschizandrinActive component
The invention relates to a strain of endophytic fungus, in particular to a strain of endophytic fungus of fermenting schisandra chinensis to improve main active components of schisandra chinensis. The strain of endophytic fungus of fermenting schisandra chinensis to improve main active components of schisandra chinensis is aspergillus niger WJ1, which is preserved in China Center for Type CultureCollection on Feb. 29th, 2012, the preservation number is CCTCC No. M2012044, and the preservation address is Wuhan University in Wuhan. The aspergillus niger WJ1 provided in the invention can substantially improve main active components including schisadrol A, schisantherin A, deoxyschizandrin, schisandrin B, and the like of schisandra chinensis. Thus, in raw material productions aiming at obtaining schisadrol A, schisantherin A, deoxyschizandrin, and schisandrin B, the schisandra chinensis endophytic fungus WJ1 can be selected to ferment schisandra chinensis, thereby improving yield and saving medicine resources.
Owner:HEILONGJIANG UNIV
Preparation for auxiliary protection against chemical liver injury
The invention discloses a preparation for auxiliary protection against chemical liver injury. The preparation is mainly prepared by deoxyschizandrin and procyanidine, and has a precise curative effect on liver injuries of various types.
Owner:苏州天南星生物科技有限公司
Deoxyschizandrin-containing drug composition and application thereof
PendingCN108685885AAvoid formingEther/acetal active ingredientsBlood disorderDeoxyschizandrinFundamental study
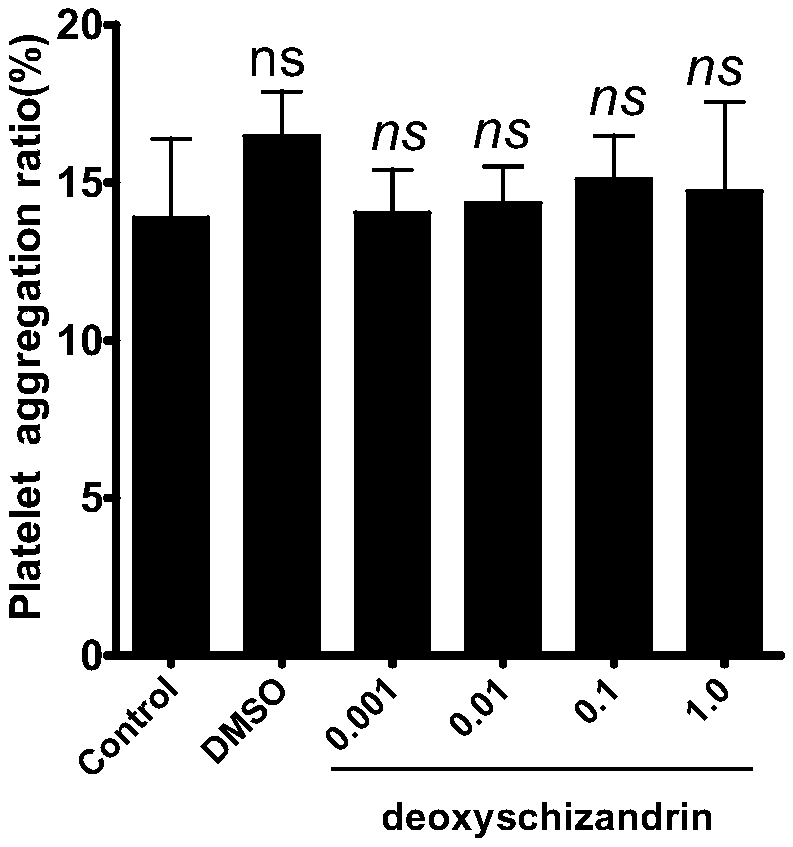
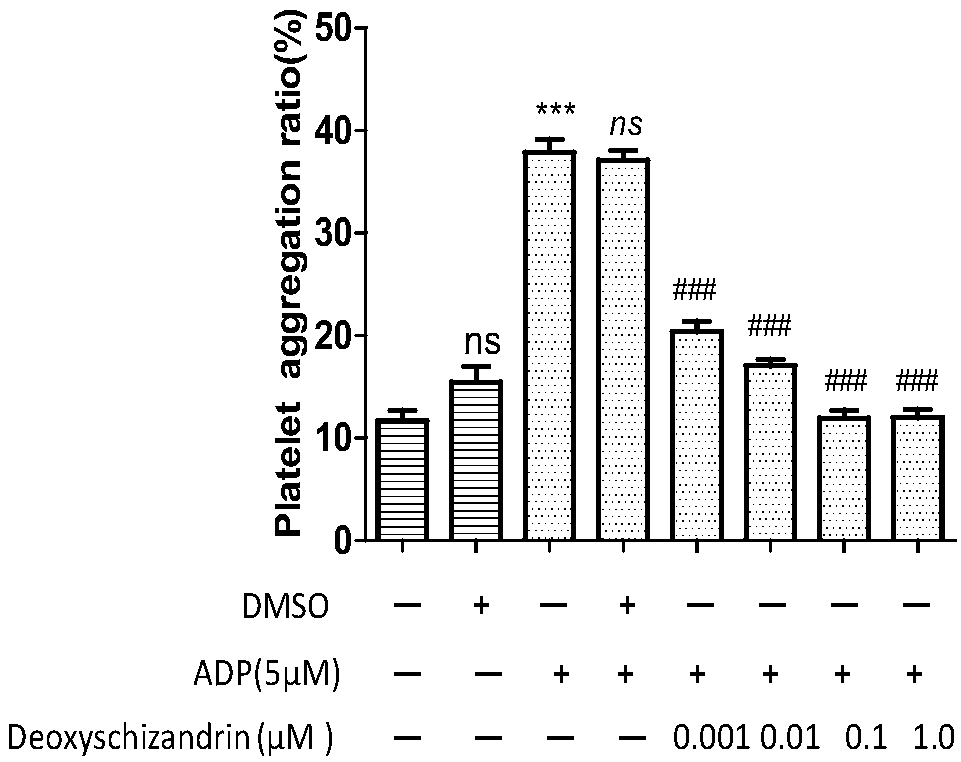
The invention relates to a traditional Chinese medicine monomer, i.e. deoxyschizandrin. The traditional Chinese medicine monomer can inhibit the ADP-induced platelet aggregation promotion effect. Theinvention further discloses the fundamental research of deoxyschizandrin as a novel anti-platelet aggregation drug in thrombosis.
Owner:NANHUA UNIV
Extraction method of lignans in schisandra chinensis rattan stems
InactiveCN102552408BGrowth age requirements are not highImprove extraction efficiencyPlant ingredientsDeoxyschizandrinLignan
Owner:SHAANXI NORMAL UNIV
Application of schisandrin B and schisandrin C in the preparation of anti-hepatic fibrosis drugs
ActiveCN104873491BHas anti-hepatic fibrosis effectDigestive systemEther/acetal active ingredientsLignanSchizandrin B
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
A method for determining the fingerprint of Qibai Pingfei granules and its fingerprint
ActiveCN107782833BMonitor qualityAvoid one-sidednessComponent separationDeoxyschizandrinSchisandrin B
The invention discloses a method for determining the fingerprint of a Qibai Pingfei granule, and the fingerprint of the granule. The method comprises the following steps: respectively carrying out extraction on a Qibai Pingfei granule sample and schizandrin by using a methanol solution to obtain a sample solution and a reference substance solution, and carrying out high performance liquid chromatography on the sample solution and the reference substance solution by using the schizandrin, caffeic acid, ferulic acid, deoxyschizandrin, schisandrin B and schisantherin A as reference substances toobtain the fingerprint. The fingerprint of the Qibai Pingfei granule, established by using the method, can effectively characterize the quality of the Qibai Pingfei granule, and is in favor of comprehensively monitoring the quality of medicinal materials. The method has the advantages of simplicity, stability, high precision and good reappearance, and can be used to rapidly and accurately identifythe authenticity and the quality.
Owner:JIANGSU KANION PHARMA CO LTD
Compound preparation
The invention discloses a compound preparation, which is mainly prepared from deoxyschizandrin, puerarin and procyanidine as main raw materials. The preparation has an accurate curative effect on various of liver injuries, and has the characteristics of being quick to take effect, short in treatment course, low in cost, small in side effect and the like.
Owner:苏州天南星生物科技有限公司
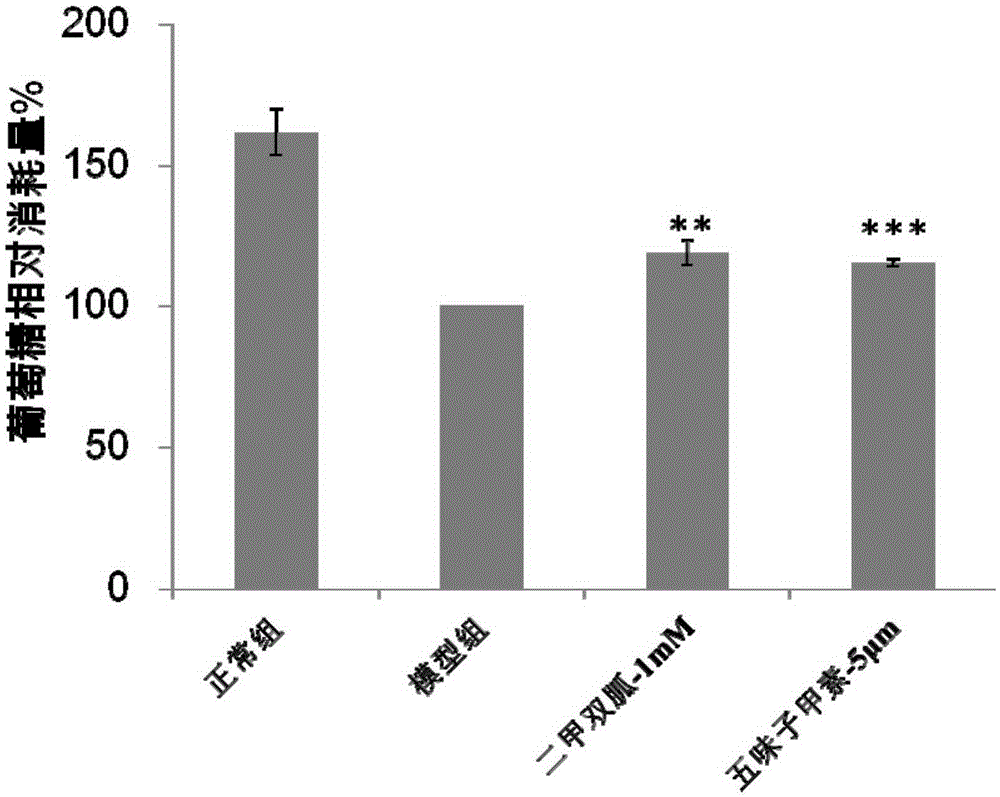
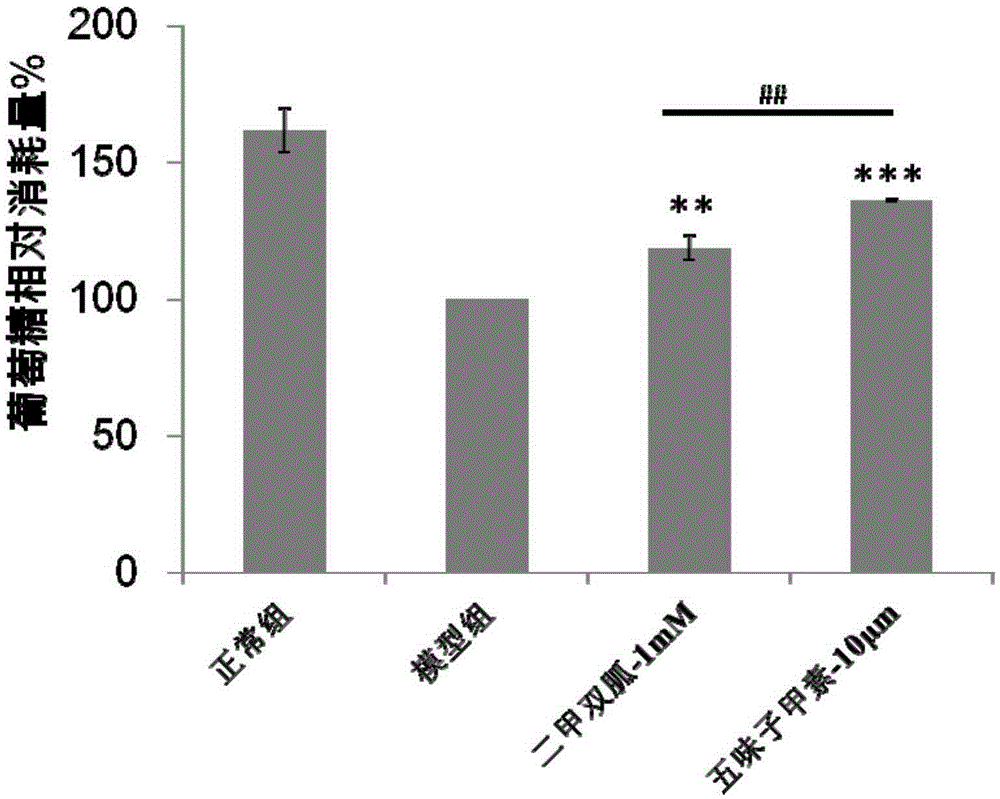
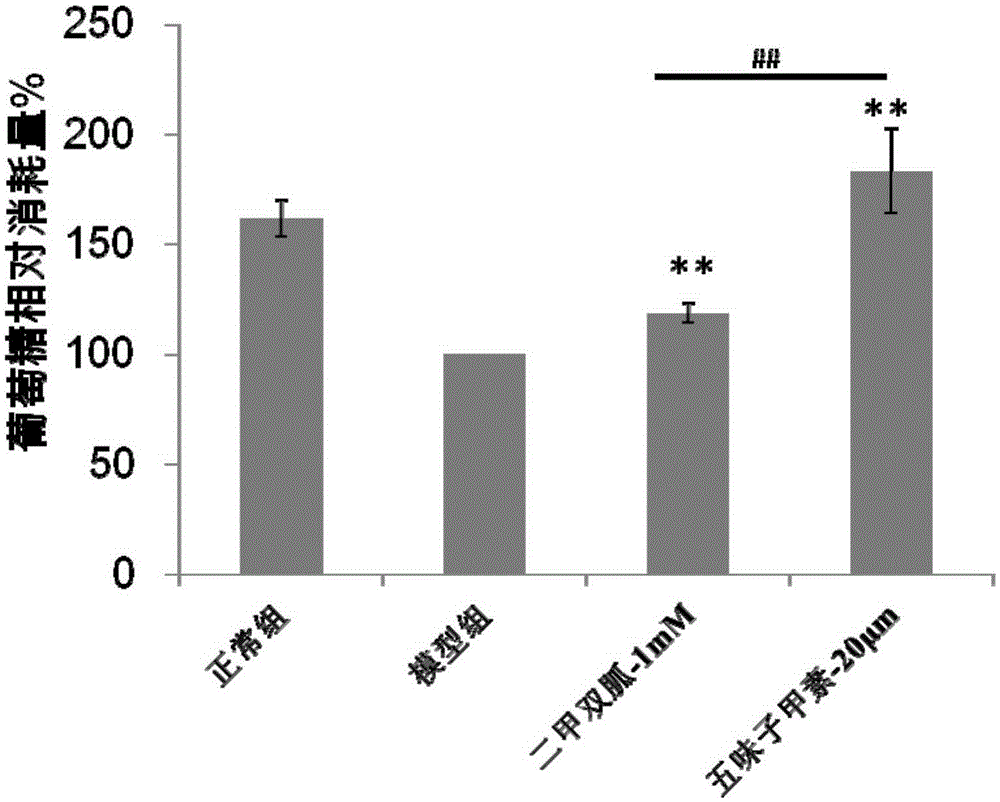
Application of deoxyschizandrin to preparation of medicine for treating diabetes
InactiveCN105232498ALower blood sugar levelsHigh sensitivityMetabolism disorderEther/acetal active ingredientsDeoxyschizandrinBULK ACTIVE INGREDIENT
The invention discloses application of deoxyschizandrin to preparation of a medicine for treating diabetes and provides a pharmaceutical composition taking the deoxyschizandrin as an active ingredient. The pharmaceutical composition is capable of decreasing blood sugar remarkably and is far higher than first-line medicine metformin in the market in acting sensitivity.
Owner:ZHEJIANG UNIV
Method for preparing Chinese medicinal preparation for treating urinary system infection
InactiveCN101564452AHigh extraction rateGood curative effectUrinary disorderGranular deliveryDeoxyschizandrinMedicine
The invention relates to a method for preparing a Chinese medicinal preparation for treating urinary system infection. The preparation method takes effective components represented by imperatorin, deoxyschizandrin and berberine as indexes, and determines that angelica dahurica is extracted twice with 70 percent ethanol, 2 hours for the first time and 1.5 hours for the second time; shizandra is extracted twice with 90 percent ethanol, 2 hours for the first time and 1.5 hours for the second time; phellodendron is separately added with water and decocted twice, 2 hours for the first time and 1.5 hours for the second time; and decoction solutions are mixed and filtered when the merged solutions are still hot. The preparation method has the advantage of giving better play to the effect of the prescription in clearing heat, treating stranguria, promoting urination and relieving pain.
Owner:BEIJING ASIA EAST BIO PHARMA CO LTD
Application of Schisandra sphenanthera Rehd.et Wils. extract in preparation of medicament for treating liver injury caused by acetaminophen
InactiveCN103638109ALessen liver damageCurb consumptionPowder deliveryDigestive systemAlanine aminotransferaseTherapeutic effect
The invention discloses an application of a Schisandra sphenanthera Rehd.et Wils. extract in preparation of a medicament for treating a liver injury caused by acetaminophen (APAP). The main ingredients of the Schisandra sphenanthera Rehd.et Wils extract are schisantherin and deoxyschizandrin. Experiments find that the Schisandra sphenanthera Rehd.et Wils. extract can significantly resist serum ALT (alanine aminotransferase)and AST (aspartate amino transferase)level rise and necrosis of liver cells caused by the liver injury caused by APAP, and inhibits consumption of reductive glutathione in the liver cells, so as to relieve the liver injury degree caused by the APAP. The mechanism relates to activation of an Nrf2-ARE (NF-E2-related factor 2-antioxidative responsive element) signal channel, is manifested by raising mRNA (messenger Ribonucleic Acid) expression of Nrf2, facilitating Nrf2 nuclear translocation, starting a downstream medicament-metabolizing enzyme and transcribing an antioxidant gene. The experiment result shows that the Schisandra sphenanthera Rehd.et Wils. extract has a treatment effect on the liver injury caused by the APAP, and novel method and means are provided for clinical prevention and treatment of the liver injury caused by the APAP.
Owner:SUN YAT SEN UNIV
Method for extracting, separating and preparing lignin monomers from schisandra chinensis
ActiveCN103467438BEasy to separate and purifySimplified extraction stepsOrganic compounds purification/separation/stabilisationEther separation/purificationChromatographic separationEthyl acetate
Owner:ZHEJIANG UNIV OF TECH
Application of schisandrin in inhibiting the growth of multi-drug resistant Escherichia coli
ActiveCN108113980BMitigate or resolve drug-resistant infectionsReduce fatality rateAntibacterial agentsEther/acetal active ingredientsEscherichia coliResistance infection
Owner:NINGBO MUNICIPAL CENT FOR DISEASE CONTROL & PREVENTION
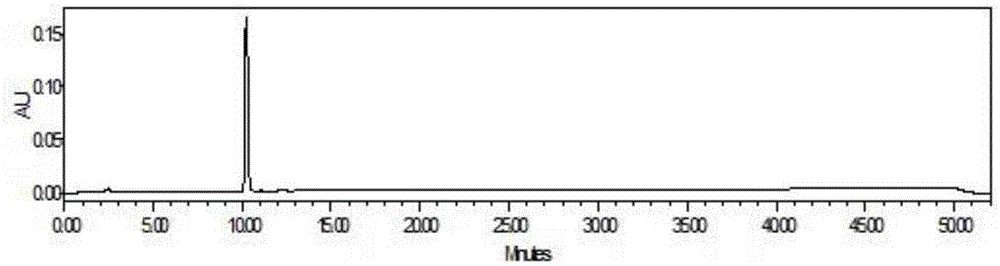
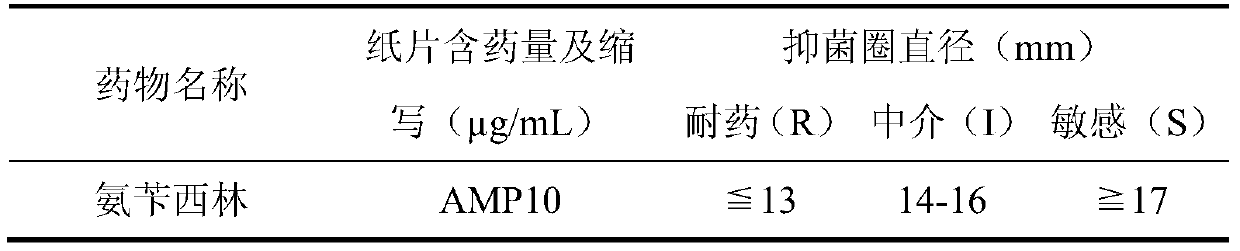
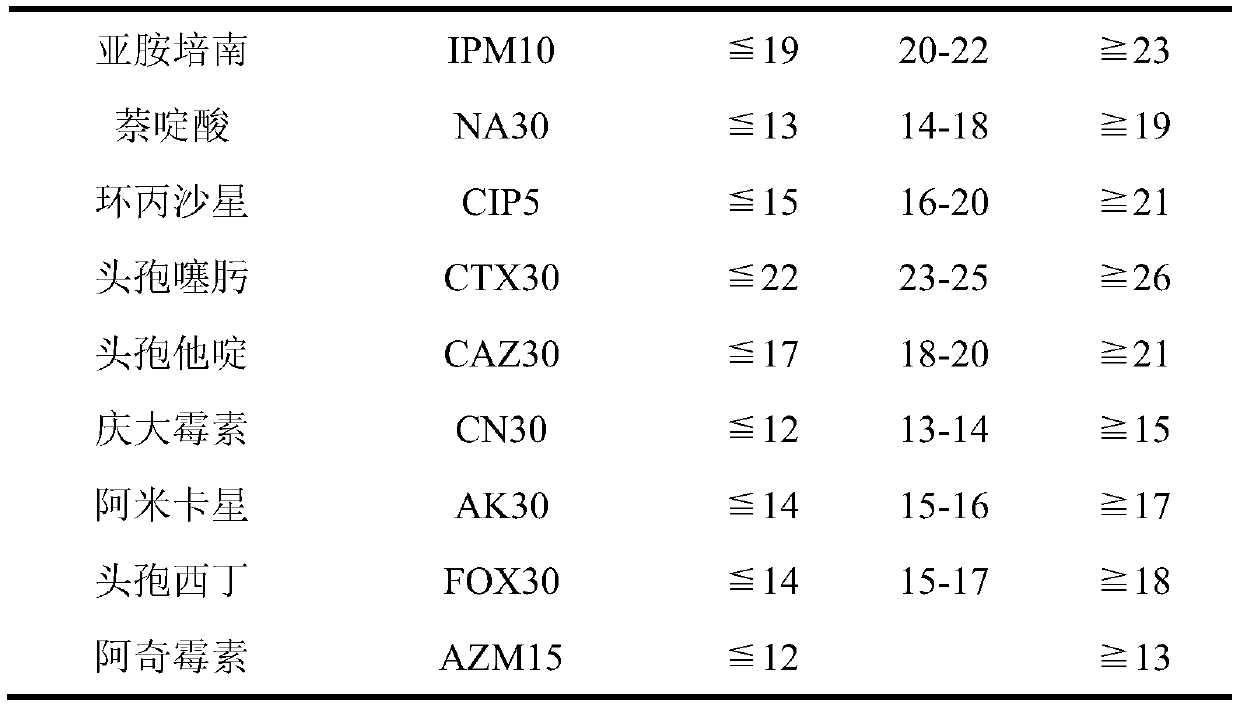
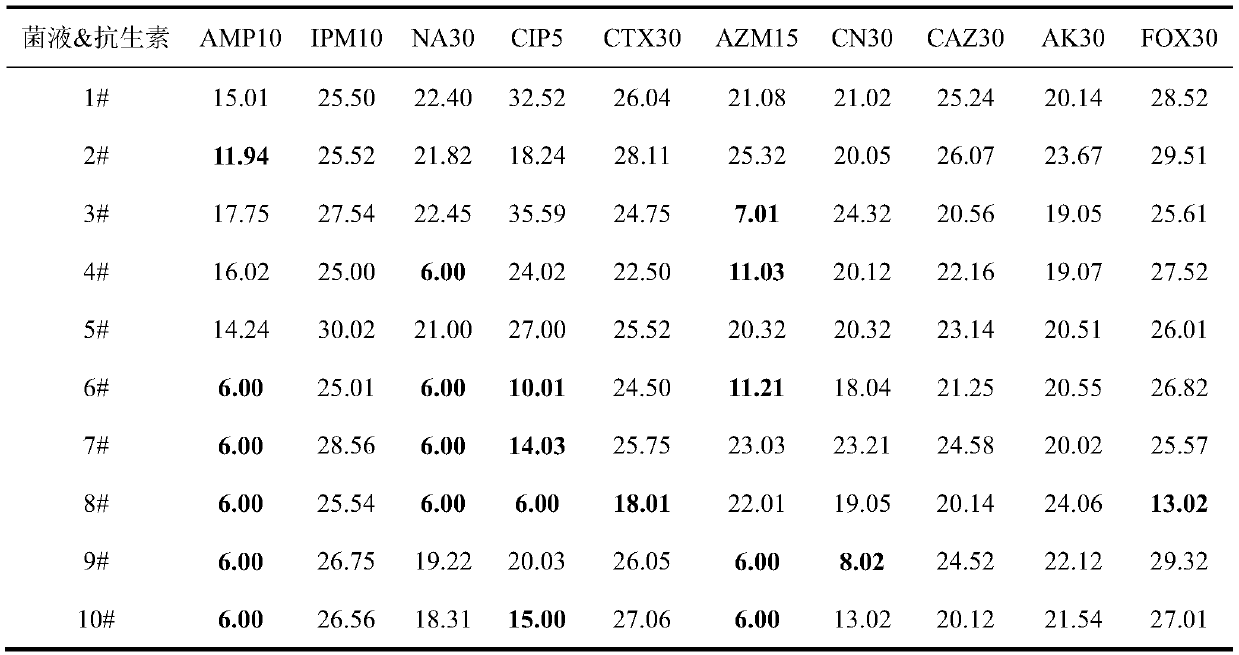
Applications of deoxyschizandrin in inhibiting growth of multi-drug-resistance escherichia coli
ActiveCN108113980AMitigate or resolve drug-resistant infectionsReduce fatality rateAntibacterial agentsEther/acetal active ingredientsEscherichia coliInfections problems
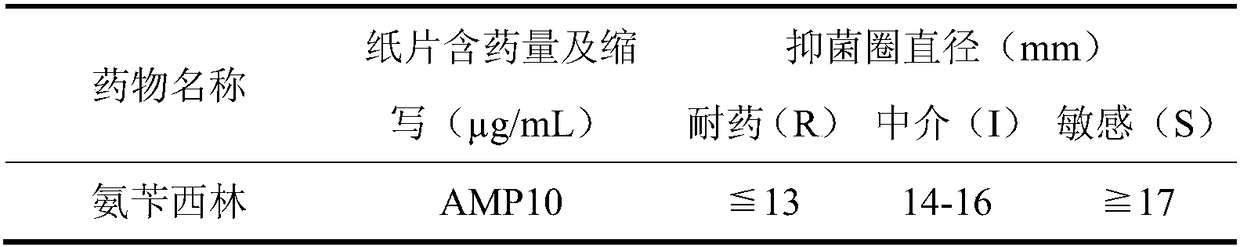
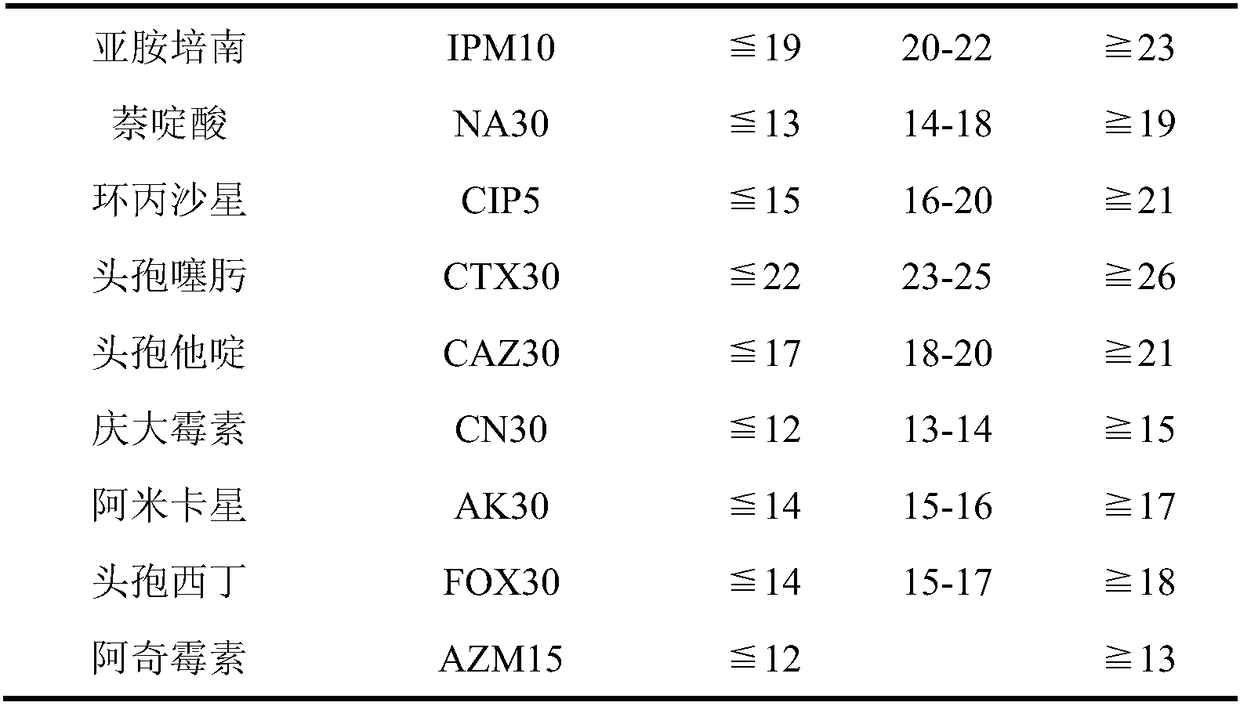
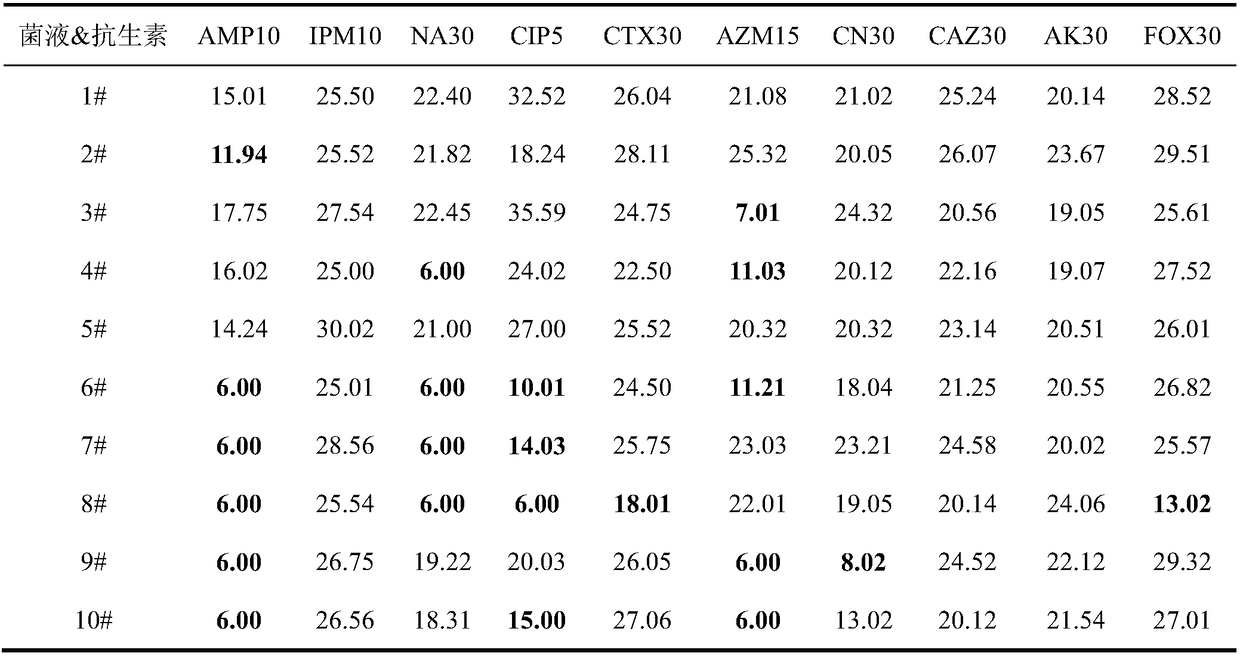
The invention discloses applications of deoxyschizandrin in inhibiting growth of multi-drug-resistance escherichia coli. Deoxyschizandrin has good in-vitro killing action on human multi-drug-resistance Escherichia coli resisting ampicillin, nalidixic acid, cefotaxime, ciprofloxacin, cefoxitin and the like, the deoxyschizandrin can inhibit the growth of multi-drug-resistance escherichia coli at theminimum bactericidal concentration of 25.57 microg / mL and minimum inhibitory concentration of 3.13 microg / mL. The invention provides the inhibition of deoxyschizandrin on human multi-drug-resistanceescherichia coli. The deoxyschizandrin can effectively relieve or solve the drug-resistance infection problem of the multi-drug-resistance escherichia coli, reduce the case fatality rate, thus havingimportant practical significance on providing a new concept for inhibiting the human multi-drug-resistance Escherichia coli.
Owner:NINGBO MUNICIPAL CENT FOR DISEASE CONTROL & PREVENTION
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com