Quality control method of south schizandrol extract oral solid formulated product
A quality control method and the technology of Schisandra chinensis, which are applied in the field of detecting the dissolution of Schisandrin A in oral solid preparations of Schisandra chinensis extract, and can solve problems such as no research reports, patent documents, and no dissolution inspection items.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0008] Determination according to high performance liquid chromatography (Appendix VID of Chinese Pharmacopoeia 2005 edition).
[0009] Chromatographic conditions and system suitability test: Octadecylsilane bonded silica gel was used as filler; methanol-water (70:30) was used as mobile phase; detection wavelength was 250nm. The number of theoretical plates should not be less than 2000 based on the peak of schisandrin A.
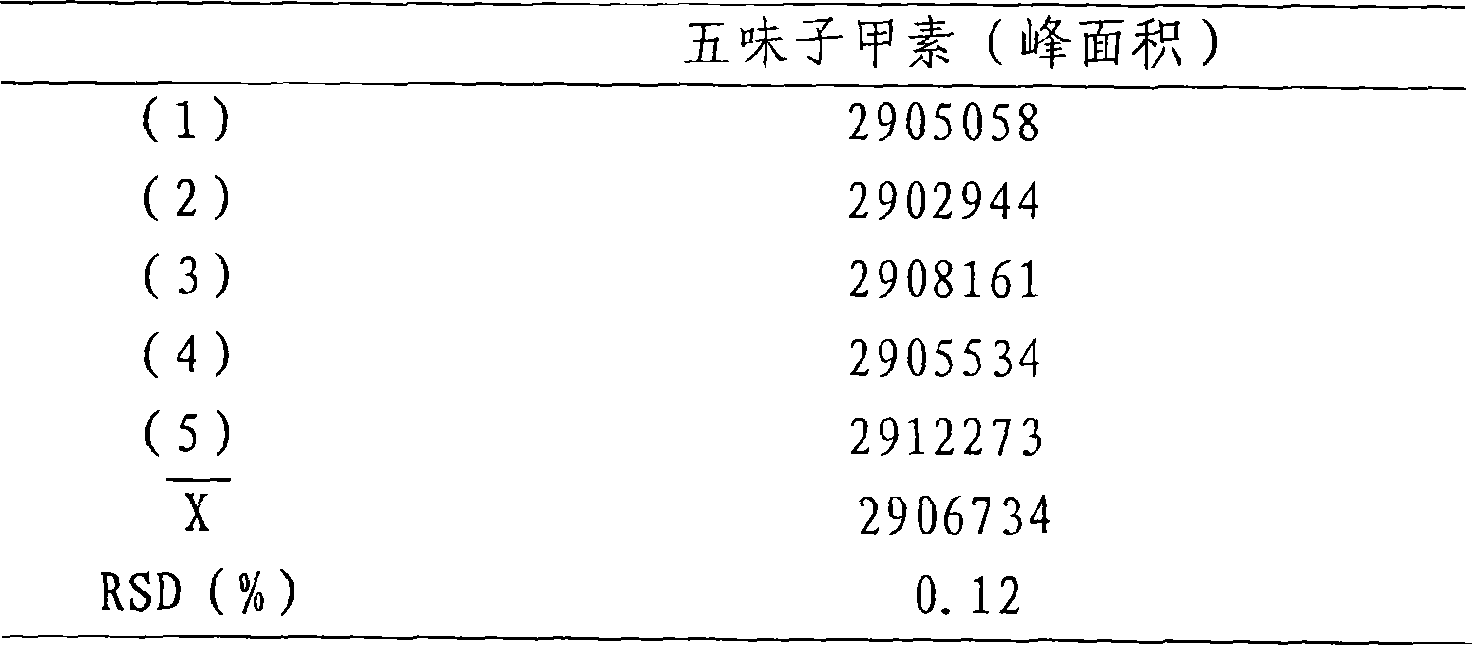
[0010] Take about 10 mg of Schizandrin A reference substance, accurately weigh it, put it in a 50ml measuring bottle, add an appropriate amount of methanol, dissolve it by ultrasonic (40KHZ power 120W), and dilute to the mark with methanol. Precisely draw 3.0, 4.0, 5.0, 6.0, 7.0ml of the above solutions into 10ml measuring bottles respectively, dilute to the mark with methanol, shake well, and make standard solutions of serial concentrations. Inject 20 μl of samples respectively and record the chromatograms. The results are shown in Table 1.
[0011] Table...
Embodiment 2
[0017] Determination according to high performance liquid chromatography (Appendix VID of Chinese Pharmacopoeia 2005 edition).
[0018] Chromatographic conditions and system suitability test: Octadecylsilane bonded silica gel was used as filler; methanol-water (70:30) was used as mobile phase; detection wavelength was 250nm. The number of theoretical plates should not be less than 2000 based on the peak of schisandrin A.
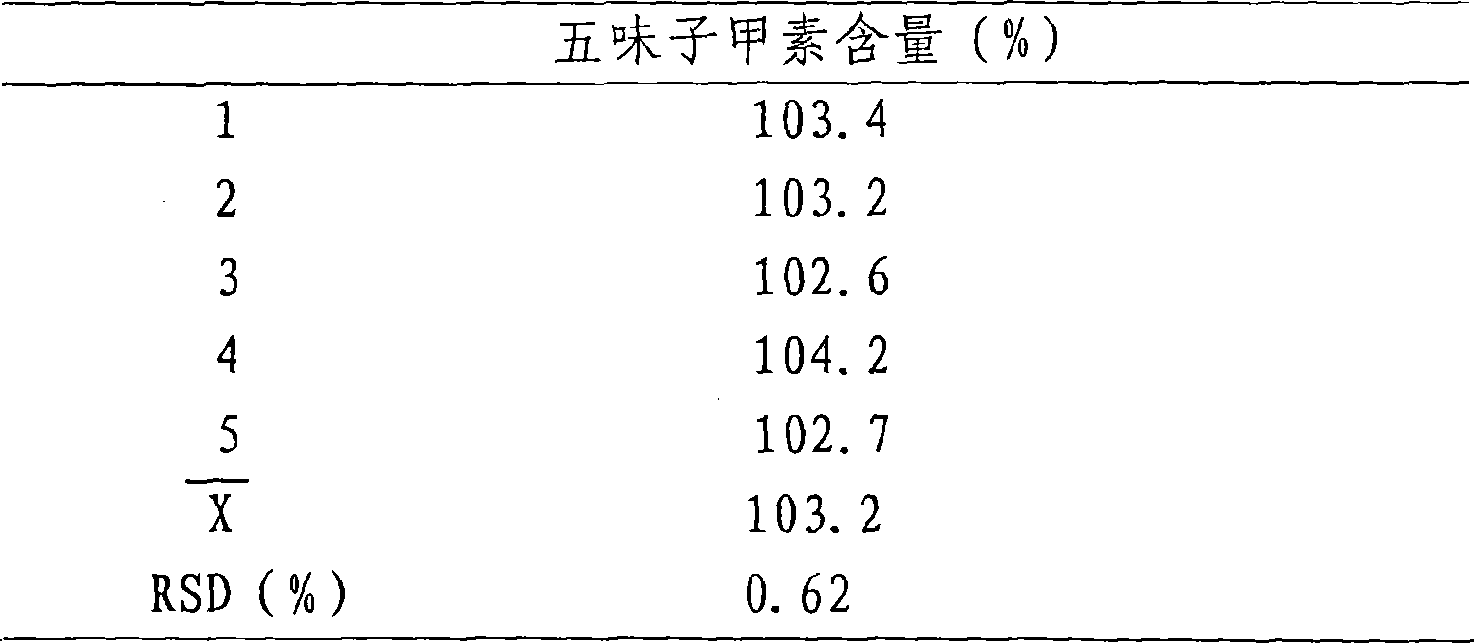
[0019] Take the Schizandrin A reference substance solution with a concentration of 100 μg / ml, and measure it at 0, 2, 4, 6, and 8 hours under the above-mentioned chromatographic conditions, and the results are shown in Table 2.
[0020] Table 2. Schizandrin A content determination solution stability test results
[0021] Placement time (h)
[0022] The results showed that the test solution was stable within 8 hours.
Embodiment 3
[0024] Determination according to high performance liquid chromatography (Appendix VID of Chinese Pharmacopoeia 2005 edition).
[0025] Chromatographic conditions and system suitability test: Octadecylsilane bonded silica gel was used as filler; methanol-water (70:30) was used as mobile phase; detection wavelength was 250nm. The number of theoretical plates should not be less than 2000 based on the peak of schisandrin A.
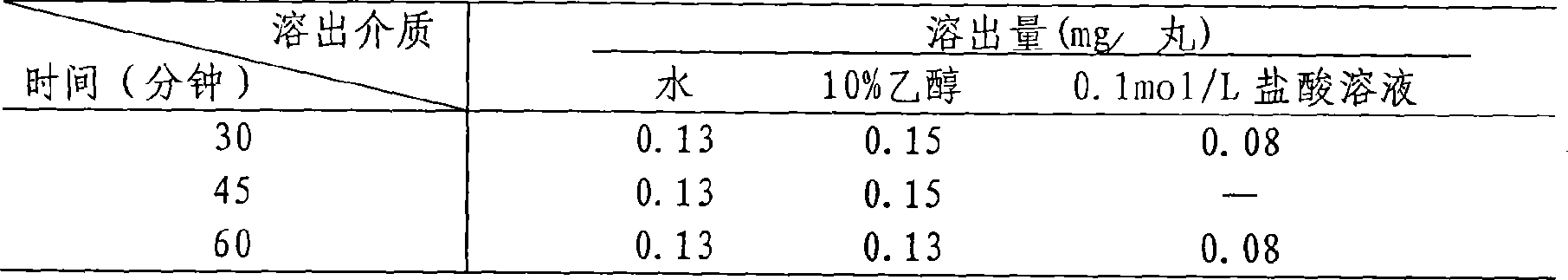
[0026] Preparation of reference substance solution: Take an appropriate amount of Schisandrin A reference substance, accurately weigh it, add methanol to make a solution containing 100 μg per 1 ml, and obtain it.
[0027] The preparation of need testing solution: get the Schisandrin A reference substance that takes by prescription ratio and accurately weigh a certain amount and mix with adjuvant, take appropriate amount, accurately weigh, put in 10ml measuring bottle, add methanol appropriate amount, ultrasonic treatment (frequency 40KHZ, power 120W) for 20...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com