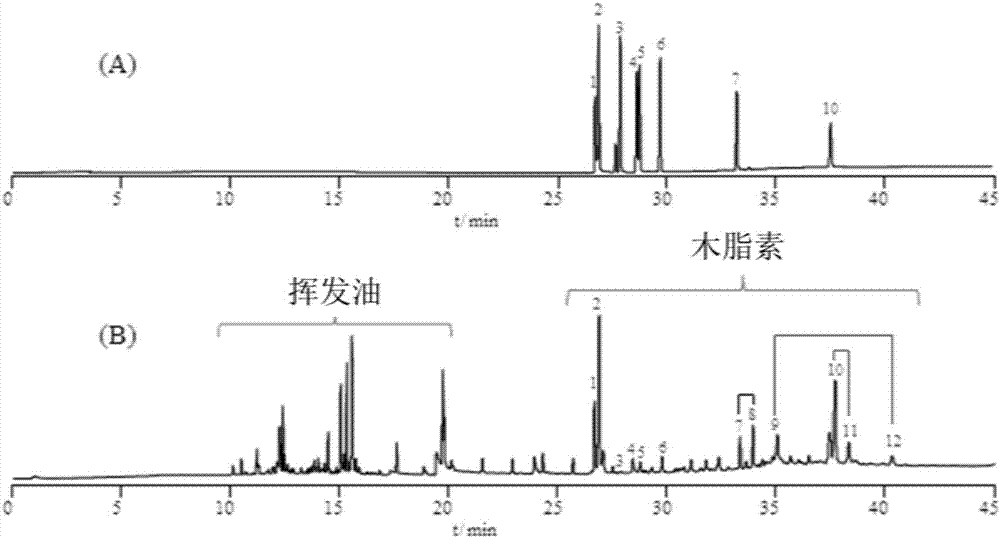
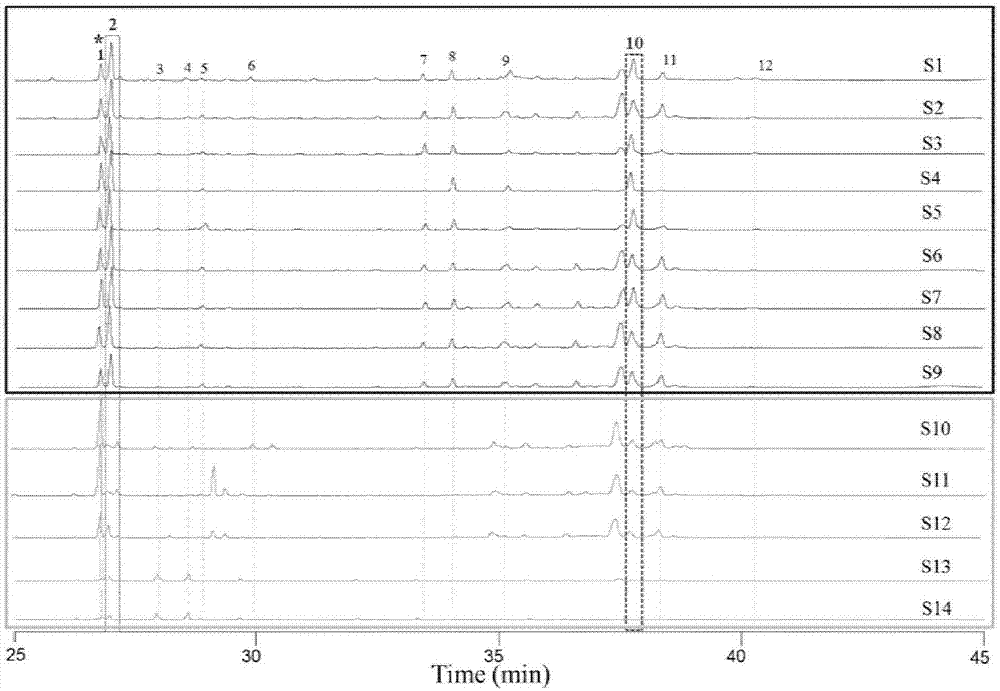
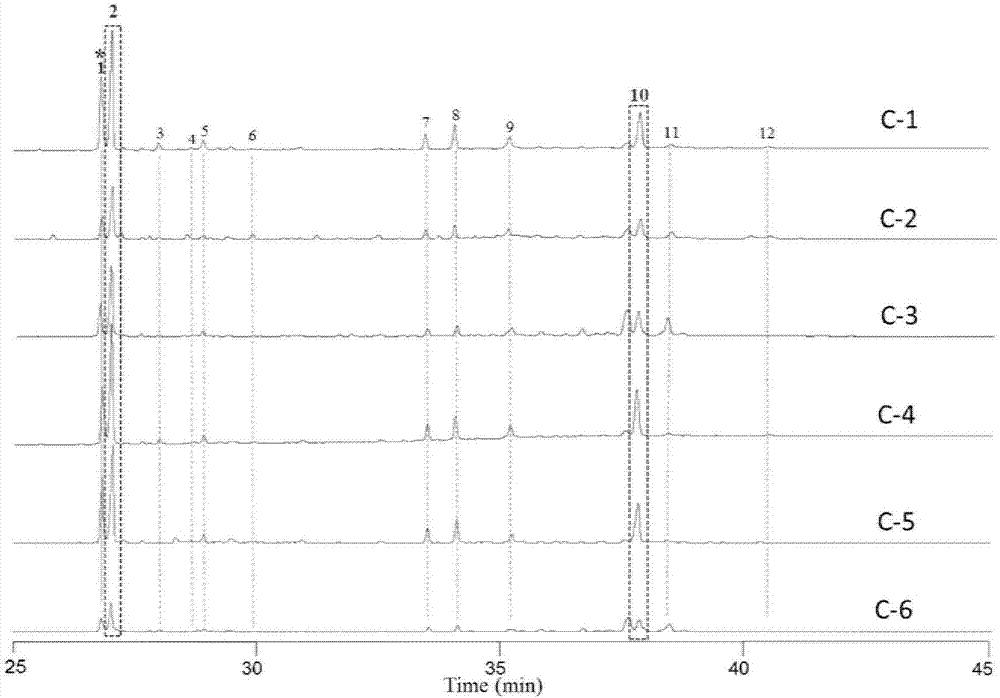
Method for evaluating quality of FRUCTUSSCHISANDRAE SPHENANTHERAE in commercially available Chinese patent medicines by using flash-gas chromatography
A technology of Schisandra chinensis and gas chromatography, which is applied in the field of evaluating the quality of Schisandra chinensis, a commercially available Chinese patent medicine, can solve the problems that there are no literature reports on the quality evaluation method of Schisandra chinensis, and achieve the effects of saving experimental time, simplifying experimental steps, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 Qualitative analysis of lignans in the raw medicinal materials of Schisandra chinensis
[0029] (1) Instruments and reagents
[0030] American ThermoFinnigan Trace DSQ gas chromatography / mass spectrometer (GC-MS); American ThermoFinnigan Trace GC Ultra gas chromatograph with hydrogen flame detector (FID); Japanese Frontier PY-3030D double-click vertical micro-furnace cracker.
[0031] Purchase locally produced schisandra from Shaanxi, Sichuan, Shanxi, and pharmacies, remove visible branches, sawdust and other sundries, bake at 60°C for 5 hours, take out and quickly grind through a 120-mesh sieve to make schisandra powder for later use. See Table 1 for the list of samples.
[0032] Table 1: List of raw medicinal materials of Schisandra chinensis
[0033]
[0034] (2) Experimental method
[0035] Accurately weigh 0.3 mg of Schisandra chinensis (S1) powder produced in Shaanxi, put it into a sample cup, fix it on the injection rod, and put it into the cracke...
Embodiment 2
[0043] The investigation of embodiment 2 reproducibility
[0044] American Thermo Trace GC ultra gas chromatograph (UA-5 metal capillary column, 30m×0.25mmi.d.×0.25μm, film thickness 5% methylpolysiloxane, Japan), Japan Frontier PY-3030D double-click vertical type Micro Furnace Cracker.
[0045] Gas phase conditions: heating program: initial temperature 50°C, rise to 200°C at a rate of 10°C / min, then rise to 300°C at a rate of 10°C / min, and keep for 10 minutes; the temperature of the injection port is 300°C, and the temperature of the detector is 300°C ℃, split ratio 30:1; carrier gas nitrogen or helium, flow rate 1.0mL / min.
[0046] Cracker conditions: cracking furnace temperature: 300°C; interface temperature between cracker and gas chromatograph: 300°C.
[0047] Accurately weigh 3 parts of 0.3 mg of Southern Schisandra powder prepared by the method in Example 1 and put it into a sample cup, fix it on the injection rod, and put it into the cracker installed above the GC in...
Embodiment 3
[0049] Example 3 Quantitative analysis of lignans in the raw medicinal materials of Schisandra chinensis
[0050] The gas chromatograph of ThermoFinnigan Trace in the United States and the Japan Frontier PY-2020iD double-click vertical micro-furnace cracker.
[0051] The gas phase conditions are: the chromatographic column is a UA-5 metal capillary column (30m×0.25mmi.d.×0.25μm, film thickness 5% methyl polysiloxane, Japan). Raise to 200°C at a rate of ℃ / min, then rise to 300°C at a rate of 10°C / min, and hold for 10 minutes; the temperature of the injection port is 300°C, the temperature of the detector is 300°C, and the split ratio is 30:1; the carrier gas is nitrogen or Helium, flow rate 1.0mL / min.
[0052] Cracker conditions: cracking furnace temperature: 300°C; interface temperature between cracker and gas chromatograph: 300°C.
[0053] Make 8 lignan standard products into 20-10000 mg / L methanol solution (mixed standard), draw 1 microliter of the solution and inject it i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com