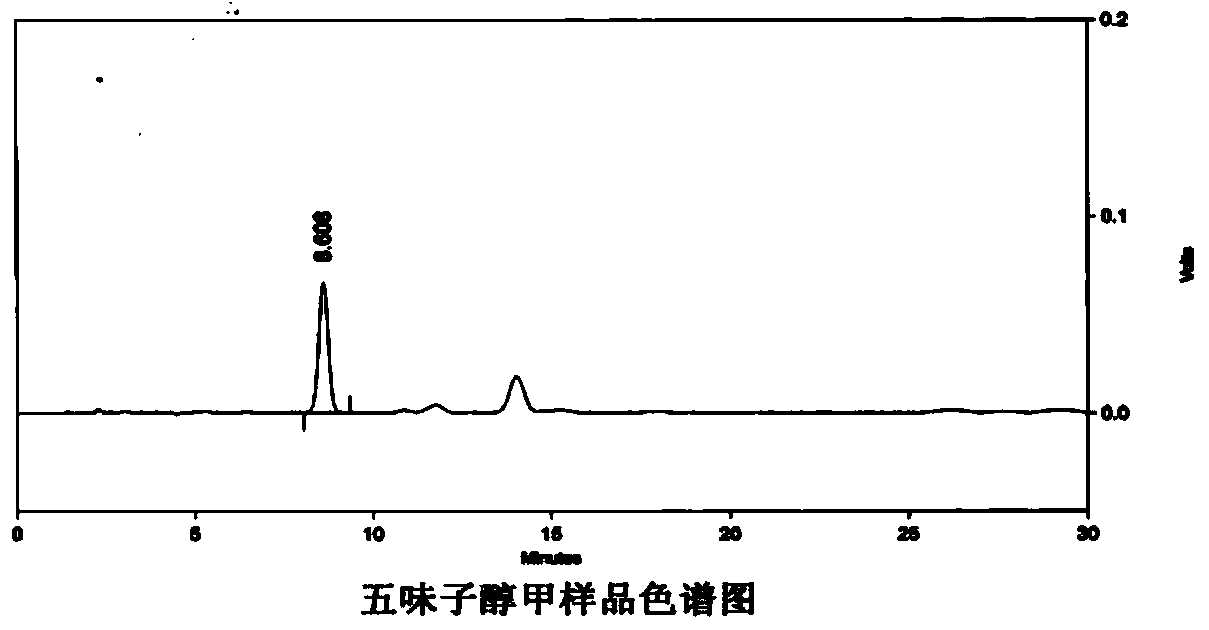
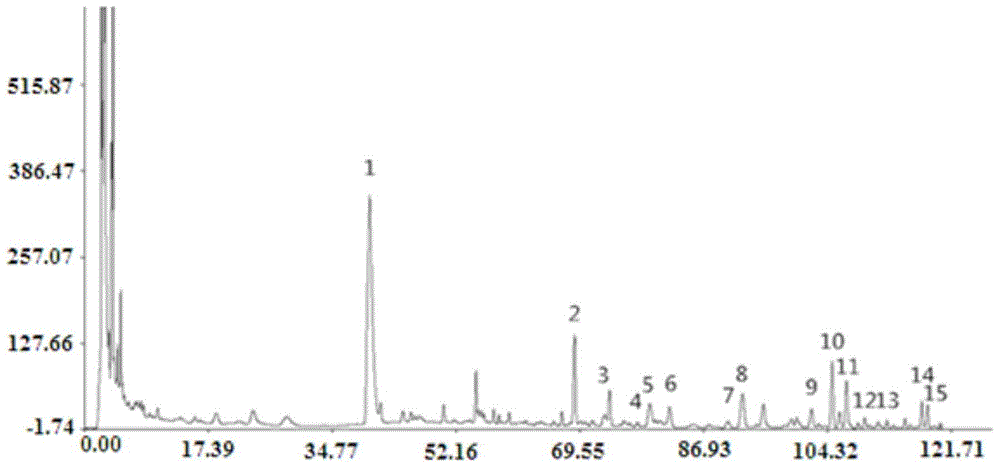
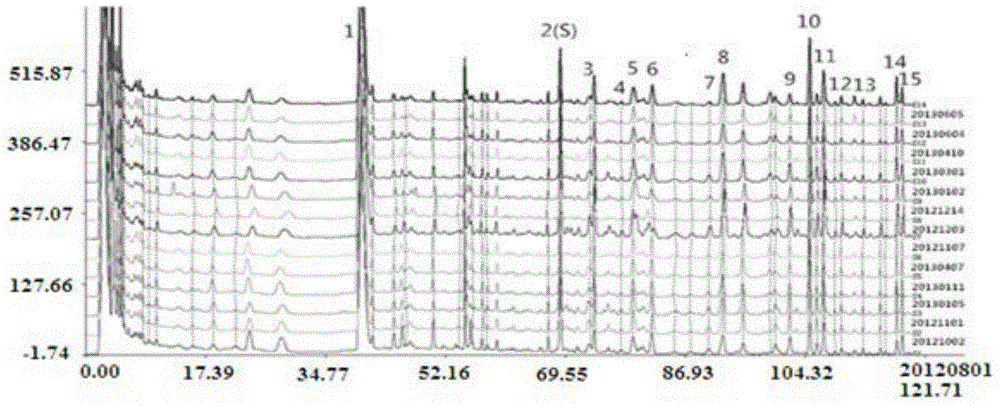
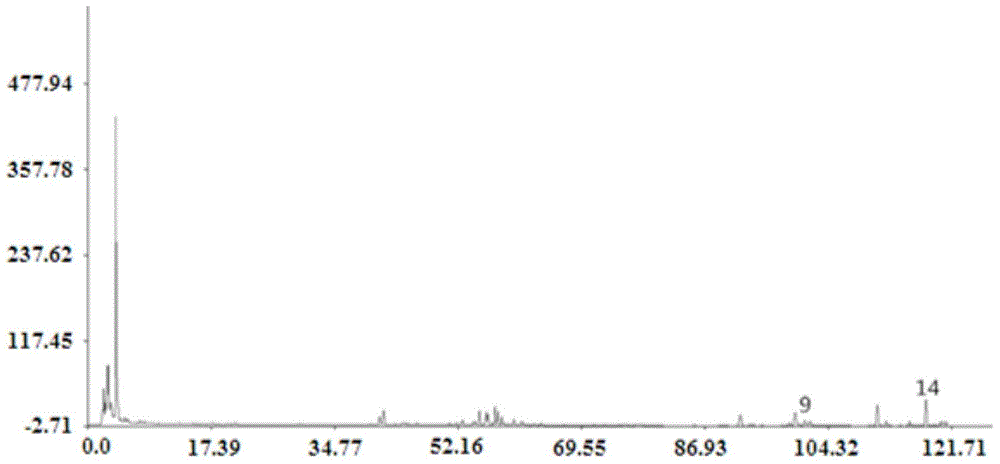
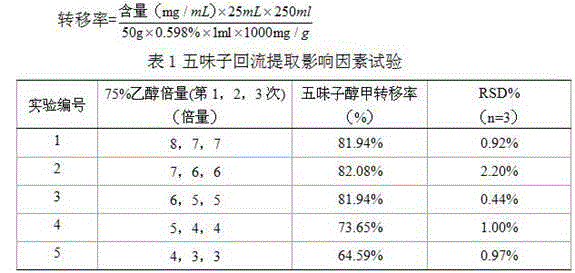
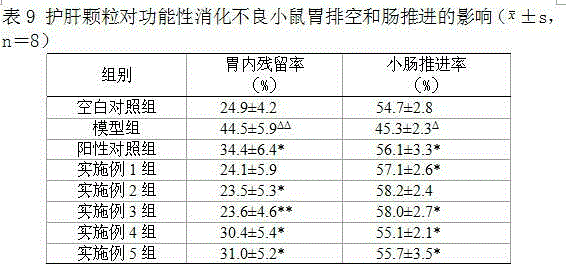
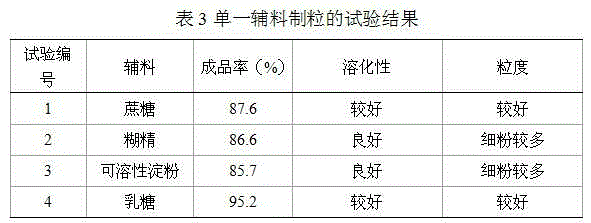
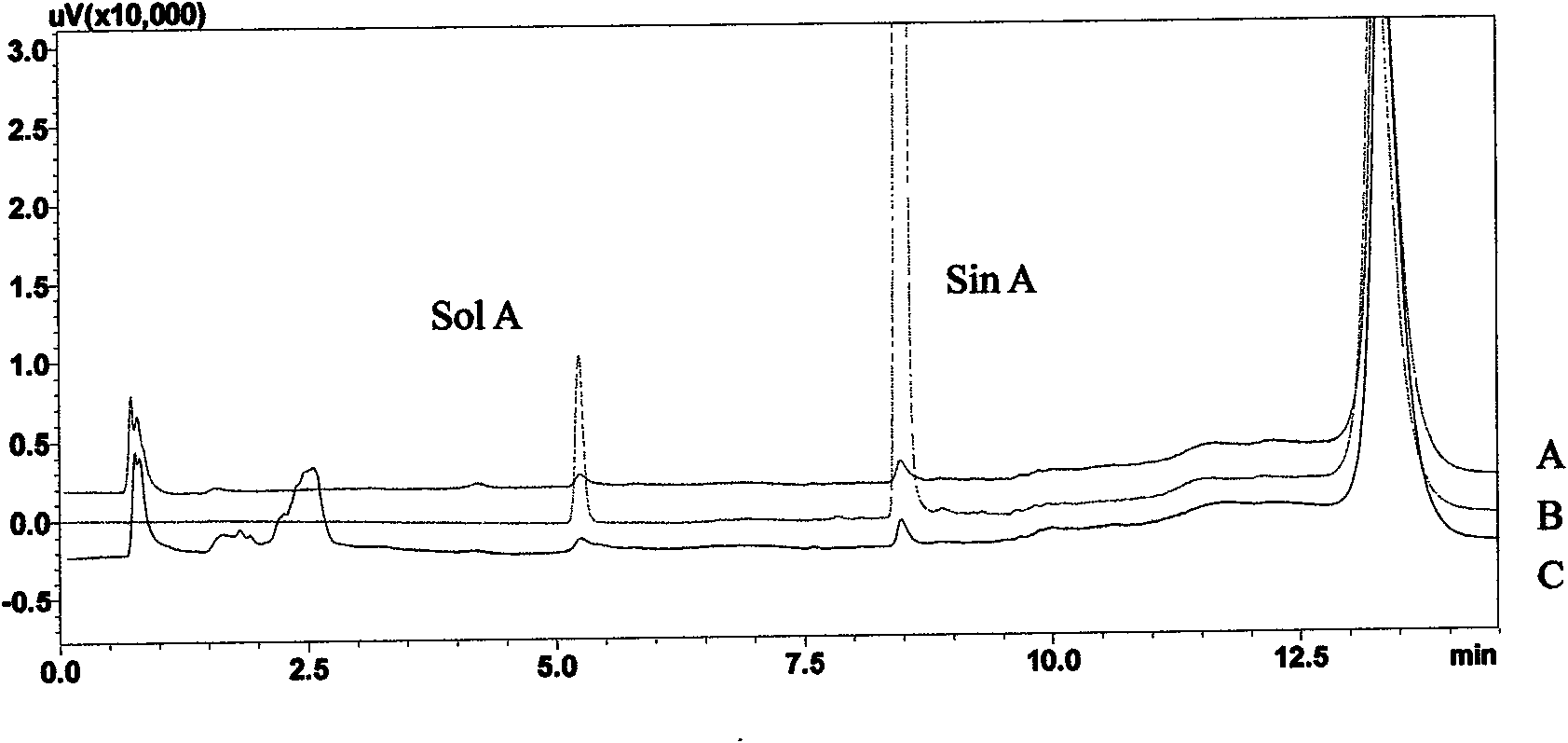
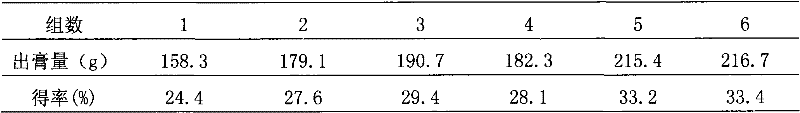
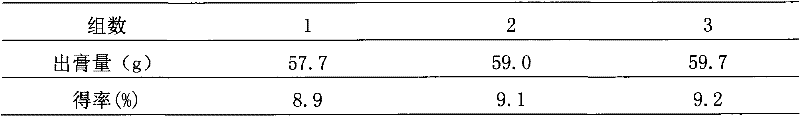
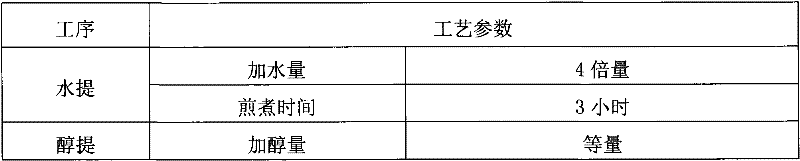
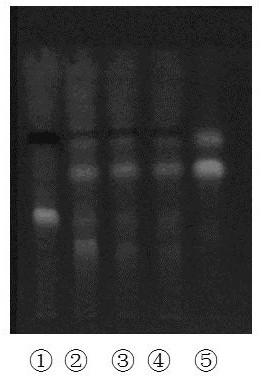
Patents
Literature
40 results about "Schizandrol A" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Chinese magnoliavine fruit monomer composition separation preparation method
InactiveCN101709059ANo lossHigh recovery rateOrganic chemistryOrganic compound preparationMonomer compositionEthyl acetate
The invention relates to a Chinese magnoliavine fruit monomer composition separation preparation method; ethanol extracts from Chinese magnoliavine fruit are extracted by petroleum ether, chromatography is carried out to the petroleum ether extracts by a silicagel column, and then the petroleum ether is eluted by petroleum ether-ethylacetate, and HPLC is used for monitoring, and then crude extract A and crude extract B are fraction-collected, and eluted; the volume ratio of eluant petroleum ether and ethylacetate is 3-5:1, the crude extract A and crude extract B are respectively applied to a high-speed counter-current chromatography for separation, so as to obtain SCHisanhenol, deoxyschizandrin, schizandrin B, schisandrin C, schizandrol A, schizandrol B and schisantherrin B monomers, and the purity is higher than 98 percent.
Owner:华美恒盛(北京)科技有限公司
Novel technique for preparing schizandrol A and schizandrol B
ActiveCN101503341ASimple processEasy to operateNervous disorderEther separation/purificationBiotechnologyChromatographic separation
The invention belongs to the field of medical technology and discloses a new preparation technique of schizandrol B and schizandrol A in schisandra chinensis baill leaves. The technique comprises the steps: schisandra chinensis baill leaves are taken as raw material, ground and then processed by one or more technique(s) in solvent extraction method, organic solvent extraction method, resin adsorption method, normal phase column chromatography, reversed-phase column chromatography and the like, namely, the schisandra chinensis baill leaves are taken as raw material, extracting solution containing the schizandrol A and the schizandrol B is extracted by organic solvent; after extract is processed by repeated column chromatographic separation, the flowing parts containing the schizandrol A and the schizandrol B are collected, and the schizandrol A and the schizandrol B are obtained after repeated recrystallization of the condensate; the purities of the schizandrol A and the schizandrol B can be more than 98% by HPLC detection. The preparation method has simple technique, stable purity and good reproduction quality, can be used for replacing shizandra fruit to extract and prepare standard product, fully utilizes the resource of shizandra and relieve the situation that the supply of the shizandra fruit is not adequate to the demand.
Owner:SHENYANG PHARMA UNIVERSITY
Method for controlling the quality of schisandra raw material fingerprint in the plant medicine for improving hemorheology
ActiveCN101040909AGuarantee normal implementationMeet the identificationComponent separationPlant ingredientsColumn temperaturePhase gradient
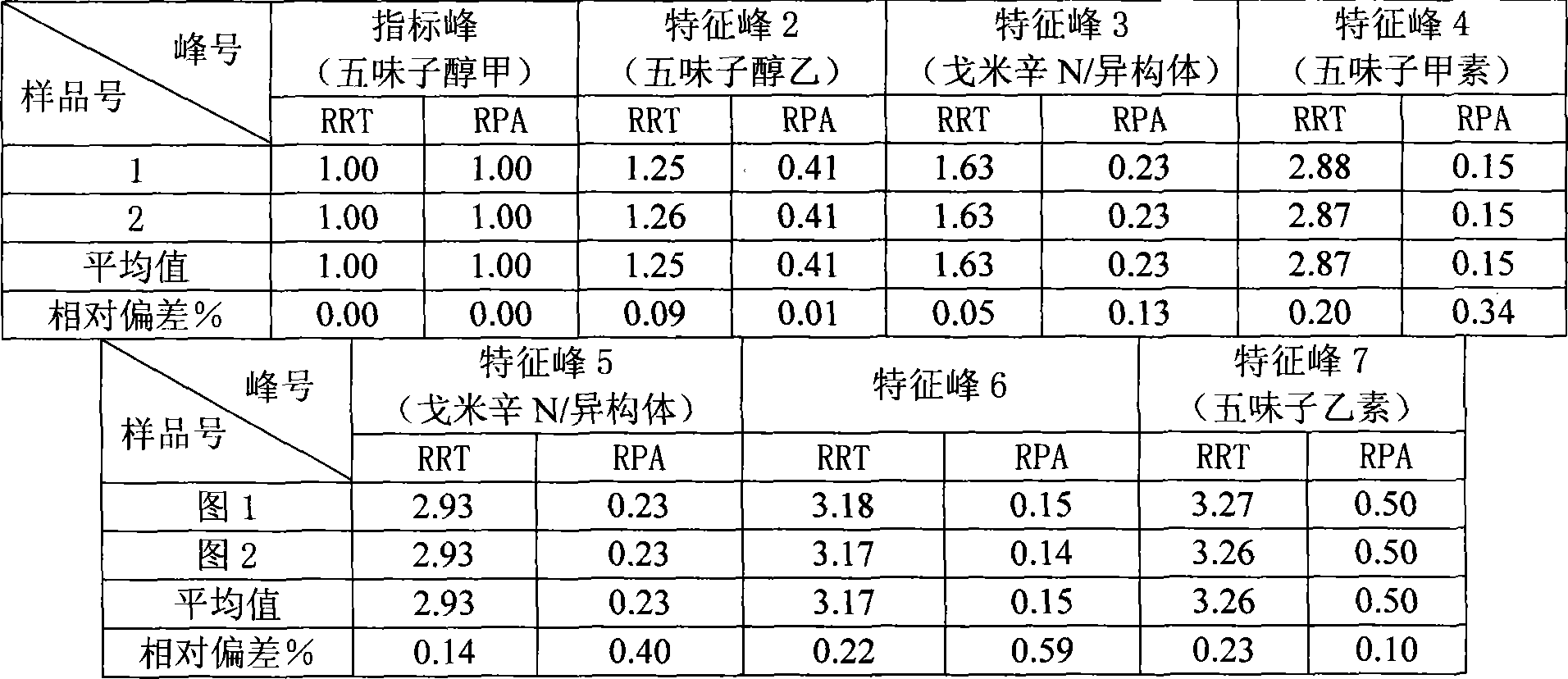
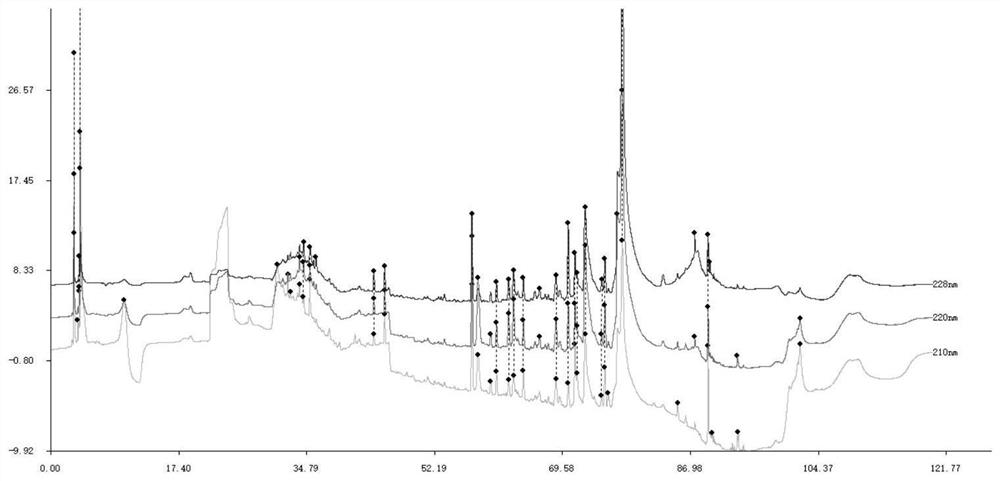
The invention relates to a schisandra fruit fingerprint spectrum quality control method, comprising that (1), adding 0.5g schisandra fruit to extract via microwave for 50min, filtering, (2), washing flow phase gradient that the spectrum column is Eclipse, as 0-40min, 60-20% A, 40-80% B, flow speed is 0.8-1.2ml / min, the wavelength is 210-280nm, the column temperature is 20-40Deg. C, and the sample amount is 10-20ul, (3), building standard fingerprint spectrum that the first peak is schisandra fruit alcohol 1, the third peak is schisandra fruit alcohol 2, the fourth peak is gemixin N / isomer, the sixth peak is schisandra fruit I element, the seventh and eighth peaks are gemixin N / isomer, the ninth peak is schisandra fruit II element, (4), controlling the quality of fingerprint spectrum that the check peak relative holding times are 1.00, 1.25, 1.63, 1.88, 3.18 and 3.27, (5), the schisandra fruit planting collecting method. The invention can control the quality of materials to assure the stable quality of product.
Owner:SHANGHAI MODERN CHINESE TRADITIONAL MEDICINE TECH DEV
Traditional Chinese medicine composition capable of ventilating the lung and relieving asthma and preparation and quality control method thereof
The invention discloses a pharmaceutical composition for ventilating lung and relieving asthma, a preparation method and a quality control method. The pharmaceutical composition comprises the following pharmaceutical raw materials: ephedra herb, raw ginger, cassia twig, bitter apricot seed, Chinese magnoliavine fruit (prepared) and prepared liquoric root, the preparation method is as follows: the five herbal medicines except the bitter apricot seed are decocted by adding water through a countercurrent circulatory extraction process and concentrated to obtain a clear paste after reducing pressure, ethanol is added, then the mixture is stirred evenly and placed staticly, supernatant liquid is taken, the ethanol is recovered, and the concentration is carried out to obtain a thick paste; the bitter apricot seed is arranged in a hot pressure sterilization pot for hot pressure steaming and boiling and then is arranged in a small amount of cold water for soaking instantly after being taken out, an apricot processed product can be obtained by removing seed coats and being dried; the quality control method is as follows: the thin layer identification is carried out on the ephedra herb, the raw ginger and the liquoric root, the content measurement is carried out on ephedrine in the ephedra herb and schizandrin in the Chinese magnoliavine fruit. The pharmaceutical composition has good effects on ventilating lung and relieving asthma.
Owner:BEIJING ASIA EAST BIO PHARMA CO LTD
Chinese medicinal injectable powder and quality control method thereof
The invention relates to Chinese medicinal injectable powder and a quality control method thereof. The Chinese medicinal injectable powder consists of red ginseng, dwarf lilyturf tuber and Chinese magnoliavine fruit in part by weight, wherein the sum of the main active ingredient content of the injectable powder cannot be lower than 4.0mg / g; and the active ingredients comprise ginsenosides Rf, Rb1, Rb2, Rb3, Rd, Rg3 and F2, and schizandrol A. The quality control method comprises the steps of making quality standards, measuring the content, comparing the content of a sample to be measured with the quality standards, and judging whether the content reaches the preset standards or not so as to control the product quality. The method has the advantages of quickness, accuracy, sensitivity, contribution to industrial production inspection, and capability of effectively controlling the quality of a finished product.
Owner:TIANJIN TASLY ZHIJIAO PHARMA
Quality detection method for liver protection dropping pill of traditional Chinese medicine preparation
ActiveCN103808842AQuality improvementEfficient detectionComponent separationChlorogenic acidMedicine
The invention relates to a quality detection method for a liver protection dropping pill of traditional Chinese medicine preparation. The quality detection method comprises the following steps: measuring the content of effective components of the liver protection dropping pill, including saikoside a and schizandrin, and identifying schisandra chinensis, pulvis fellis suis and artemisia capillaris in the liver protection dropping pill. The quality detection method also comprises the following detection steps: (1) with the saikoside a as a reference substance, measuring whether a radix bupleuri component is contained in a liver protection dropping pill recipe by adopting a high efficiency liquid chromatography method; (2) with the schizandrin as the reference substance, measuring whether a schizandrin component is contained in the liver protection dropping pill by adopting the high efficiency liquid chromatography method; (3) with the schisandrin b as the reference substance, identifying whether a schisandra chinensis component is contained in the liver protection dropping pill by adopting a thin-layer chromatography method; (4) with hyodeoxycholic acid as the reference substance, identifying whether a pulvis fellis suis component is contained in the liver protection dropping pill by adopting the thin-layer chromatography method; and (5) with chlorogenic acid as the reference substance, identifying whether an artemisia capillaris component is contained in the liver protection dropping pill by adopting the thin-layer chromatography method. The quality detection method disclosed by the invention can be used for effectively and reliably controlling the quality of the liver protection dropping pill, and the method is scientific, feasible and reliable.
Owner:HEILONGJIANG KUIHUA PHARMA
Method for enriching and purifying schizandrol A in schisandra
ActiveCN101786945AHigh adsorption selectivityParsing fastIon-exchange process apparatusEther separation/purificationThermal stabilityAdsorption selectivity
The invention belongs to the field of natural organic chemistry, relating to a method for enriching and purifying schizandrol A in schisandra by utilizing macroporous adsorbent resin. The invention is characterized in that the macroporous adsorption technology, which is used for separating and purfying the schizandrol A, has good adsorption selectivity on the schizandrol A, rapid adsorption, rapid resolution and large adsorption capacity; and the extraction is convenient and fast, the source of raw materials is abundant, the production cost is low, the separating effect is obvious, the extraction purity is high, and the semi-finished product of the schizandrol A of which the content is more than 35% and the final product of the schizandrol A of which the content is more than 98% can be obtained, so that the defects that relatively low extraction rate and low extraction purity of routine extraction are overcomed. The macroporous absorbent resin selected by the invention has stable physicochemical property, larger surface area, fast exchange speed, high mechanical strength, strong anti-pollution ability and good thermostability, thereby being capable of selectively adsorbing the schizandrol A from solution by physical adsorption with the characteristics of fast adsorption, fast resolution and larger adsorption capacity.
Owner:HEILONGJIANG DINGHENGSHENG PHARM CO LTD
Fingerprint spectrum detection method and fingerprint spectrum of Yixinshu preparation
The invention discloses a fingerprint spectrum detection method and a fingerprint spectrum of a Yixinshu preparation. The fingerprint spectrum detection method comprises the following steps: by taking a schizandrin reference substance as reference, measuring the fingerprint spectrum by adopting high performance liquid chromatography (HPLC). The similarity of the HPLC fingerprint spectrum of the Yixinshu preparation established in the method and a reference spectrum is more than 0.9, the quality of the fingerprint spectrum can be effectively characterized, and the product quality is comprehensively monitored. Moreover, a common mode of the HPLC characteristic fingerprint spectrum of the Yixinshu preparation is established, 15 common peaks are calibrated, the established fingerprint spectrum is high in technical content, the simplicity and one-sidedness of the quality control of the Yixinshu preparation are avoided, and the possibility that the quality of the artificially handled product reaches the standard is reduced.
Owner:GUIZHOU XINBANG PHARMACEUTICAL CO LTD
Detection method of traditional Chinese medicine composition for relieving lung and relieving asthma
The invention discloses a method for detecting a Chinese medicinal composition for freeing lung and relieving asthma. The Chinese medicinal composition consists of the following raw material medicines: ephedra, ginger, cassia twig, bitter apricot seed, Chinese magnoliavine fruit (prepared) and roasted liquorice. The detection method comprises the steps of detecting the ephedrine content of the ephedra and detecting the Schizandrol content of the Chinese magnoliavine fruit.
Owner:BEIJING ASIA EAST BIO PHARMA CO LTD
New application of schizandrol A, schizandrin and schizandrol extract to prevention and treatment of senile dementia
The invention belongs to the technical field of medicaments and discloses application of schizandrol A, schizandrin and a schizandrol extract to prevention and treatment of senile dementia. The invention particularly relates to the effect of the schizandrol extract, the schizandrol A and the schizandrin of improving learning memory disorder of dementia mice induced by injecting Abeta1-42 into lateral ventricle and discusses the action mechanism. The result shows that the schizandrol extract, the schizandrol A and the schizandrin can obviously improve working memory disorder and spatial discrimination learning memory disorder of the mice, can improve SOD and GSH-Px activity, reduce MDA content, can increase GSH content and can reduce GGSG content, and shows that the schizandrol extract, the schizandrol A and the schizandrin can eliminate free radicals generated by in vivo oxidation stress through various routes, can inhibit lipid peroxidation and can improve oxidation resistance of an organism so as to improve oxidative stress damage caused by Abeta1-42. The schizandrol extract, the schizandrol A and the schizandrin can be applied to preparing medicaments or health-care food for preventing and treating senile dementia.
Owner:SHENYANG PHARMA UNIVERSITY
Extractive of fructus schisandrae and preparation method and application thereof
The invention discloses an extractive of fructus schisandrae and particularly relates to an ethanol extractive extracted from roots of fructus schisandrae and containing 15% of schizandrin or an ethanol extractive extracted from xylem of rhizome of fructus schisandrae and containing 18% of schizandrin. By means of the extractive, the problem of shortage in the fructus schisandrae raw material is solved, resources of the roots of the fructus schisandrae and the abandoned xylem of the rhizome of the fructus schisandrae in processing are made full use of, and waste is reduced. The extractive of fructus schisandrae is high in content of lignans such as schizandrin and different in content and composition of lignans compared with fruits and rattan of the fructus schisandrae and has unique activity in pharmacological activity. The invention further discloses the preparation method of the extractive of fructus schisandrae and the application of the extractive of fructus schisandrae in the aspect of hangover cure and liver protection.
Owner:吉林省华惠生物科技有限公司
Extraction of schizandrol A
Extraction of Schisandra chinensis alcohol-A is carried out by extracting Schisandra chinensis by solvent, extracting extract containing Schisandra chinensis alcohol-A, column chromatography separating, recovering elutropic solvent in eluent containing Schisandra chinensis alcohol-A and obtaining the final product concentrate. It is cheap and simple, has higher purity, recovery rate and quality.
Owner:朱萧俊
Method for determining fingerprint of Qibai Pingfei granule, and fingerprint of granule
ActiveCN107782833AReduce the possibilityThe method is simpleComponent separationChemistryFingerprint
The invention discloses a method for determining the fingerprint of a Qibai Pingfei granule, and the fingerprint of the granule. The method comprises the following steps: respectively carrying out extraction on a Qibai Pingfei granule sample and schizandrin by using a methanol solution to obtain a sample solution and a reference substance solution, and carrying out high performance liquid chromatography on the sample solution and the reference substance solution by using the schizandrin, caffeic acid, ferulic acid, deoxyschizandrin, schisandrin B and schisantherin A as reference substances toobtain the fingerprint. The fingerprint of the Qibai Pingfei granule, established by using the method, can effectively characterize the quality of the Qibai Pingfei granule, and is in favor of comprehensively monitoring the quality of medicinal materials. The method has the advantages of simplicity, stability, high precision and good reappearance, and can be used to rapidly and accurately identifythe authenticity and the quality.
Owner:JIANGSU KANION PHARMA CO LTD
Liver-protecting granule for poultry and preparation method thereof
ActiveCN104083510AIncrease contentImprove transfer rateDigestive systemUnknown materialsBiotechnologyFatty liver
The invention discloses a liver-protecting granule for poultry and a preparation method thereof. The granule is prepared from radix bupleuri, oriental wormwood, radix isatidis, schisandra chinensis, pulvis fellis suis and mung bean, and is modified from the liver-protecting tablet. The liver-protecting granule has the effects of soothing liver and regulating qi, strengthening spleen and promoting digestion, and are used for fatty liver syndrome of chicken; furthermore, the extraction process is improved, and the yield of schizandrin extracted from schisandra chinensis is relatively high; specific auxiliary dosage is adopted, so that the granule has high yield, excellent solubility and excellent granularity.
Owner:江西中成中药原料有限公司
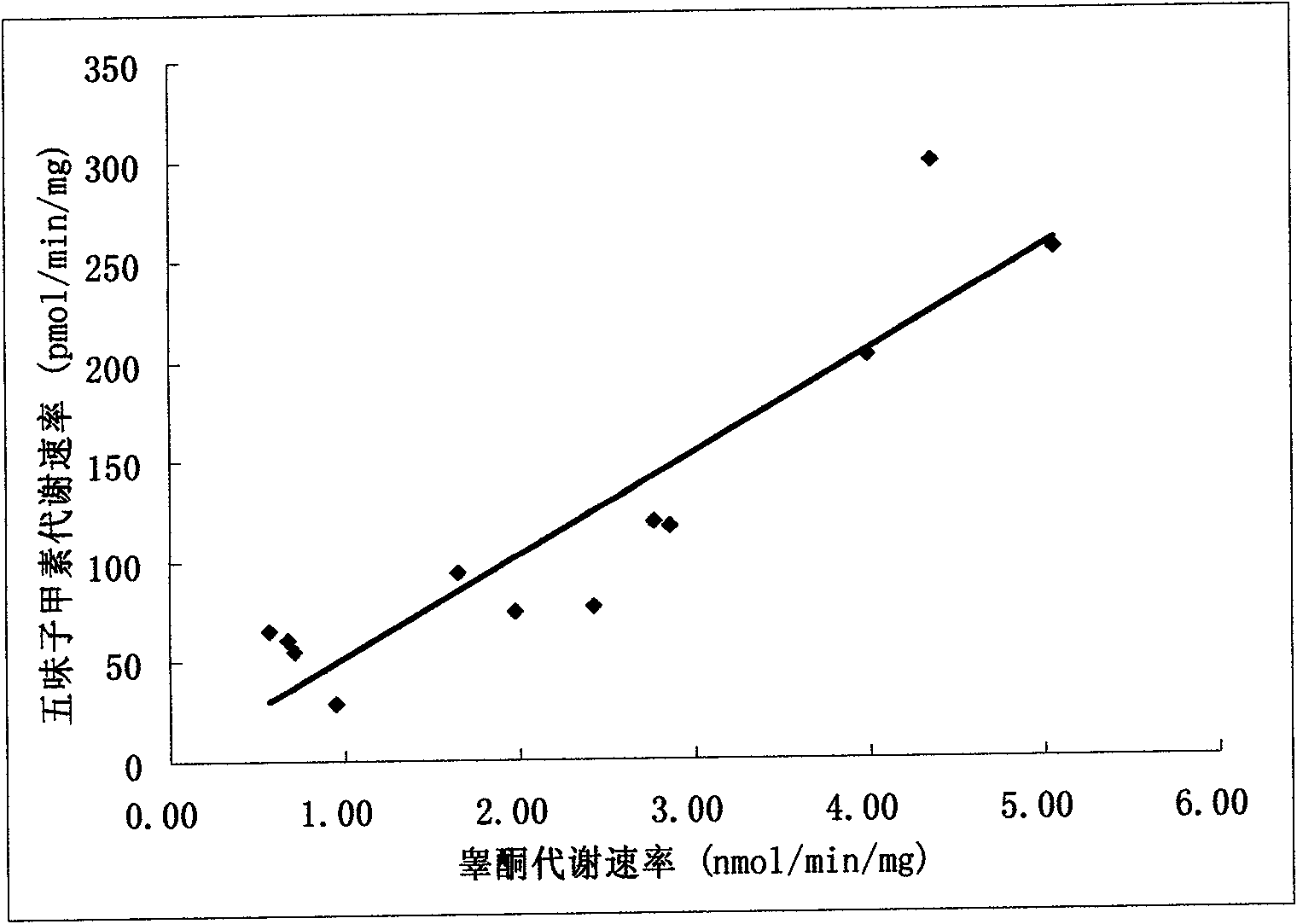
Application of schizandrin A in detection of cytochrome P4503A enzymatic activity and detection method thereof
ActiveCN101608212ALow toxicityHigh substrate conversion rateMicrobiological testing/measurementIn-vivo testing preparationsMetaboliteFeces
The invention provides application of schizandrin A in detection of cytochrome P4503A enzymatic activity and a detection method thereof. The schizandrin A can be used as probe substrate to detect the cytochrome P4503A enzymatic activity, and the schizandrin A or other medicines are used as specific probe substrate to obtain schizandrin to be used as metabolite at the reaction temperature between 10 DEG C and 60 DEG C. The decrement of the schizandrin A substrate or the metabolite generation amount of the schizandrin A is used as an evaluation index of cytochrome P4503A subfamily enzymatic activity. Or a mammal to be detected is enabled to take the schizandrin A or other medicines with 0.01-1000mg / kg of weight of the mammal through an intravenous injection, muscle injection or oral way; a time point is selected within 0-72h, and then a biological sample of blood plasma, urine, excrement or bile of the mammal to be detected is collected; and the decrement of the schizandrin A substrate or the metabolite generation amount of the schizandrin A is measured to be used as the evaluation index of the cytochrome P4503A subfamily enzymatic activity.
Owner:ZHANGJIAGANG IND TECH RES INST CO LTD DALIAN INST OF CHEM PHYSICS CHINESE ACADEMY OF SCI
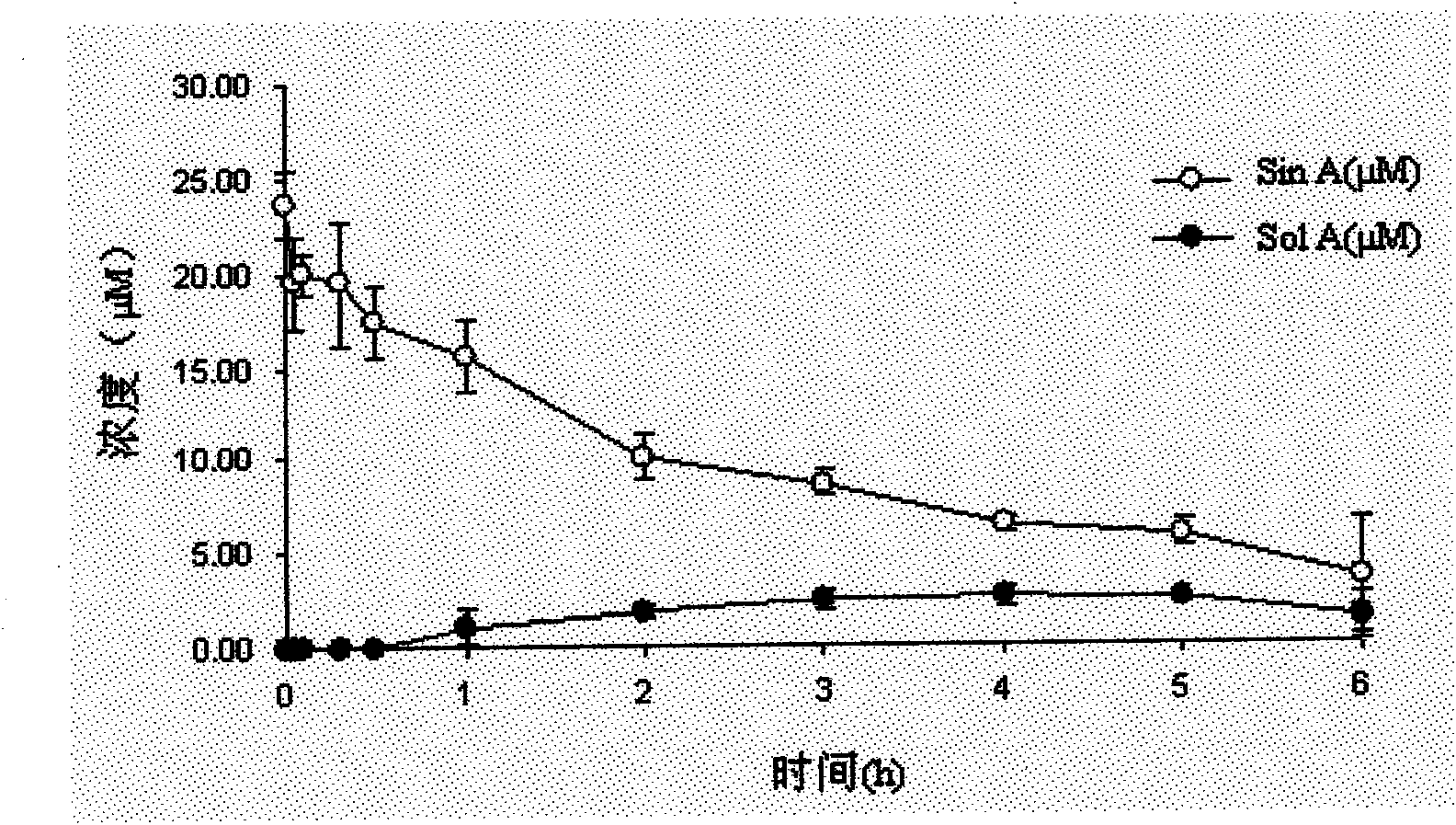
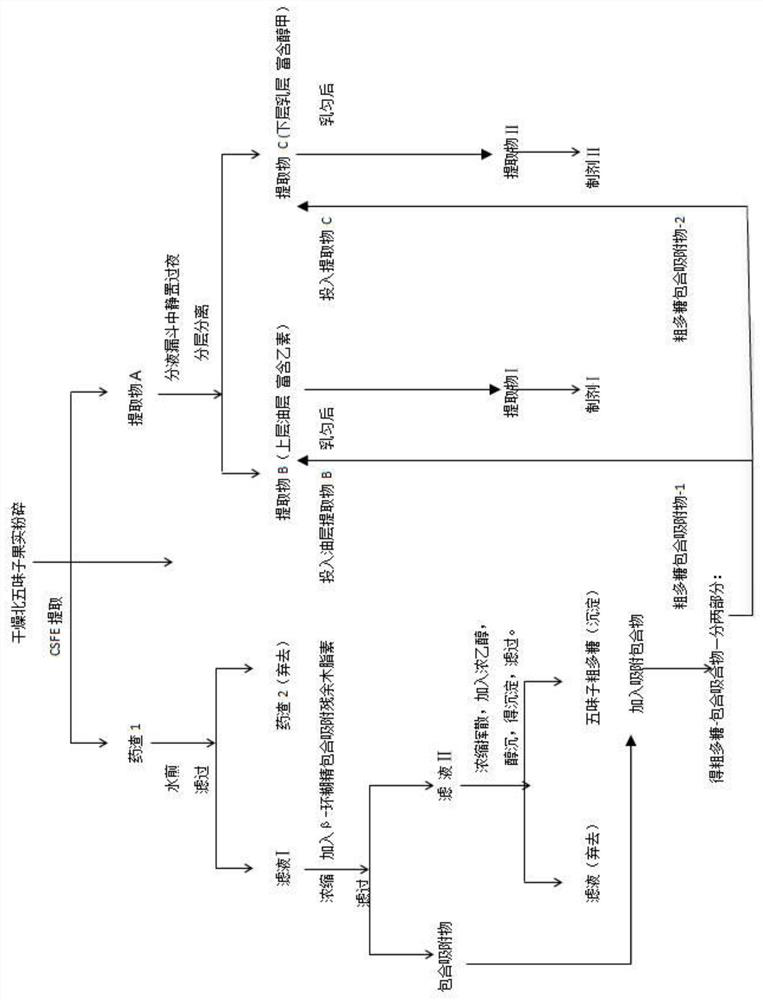
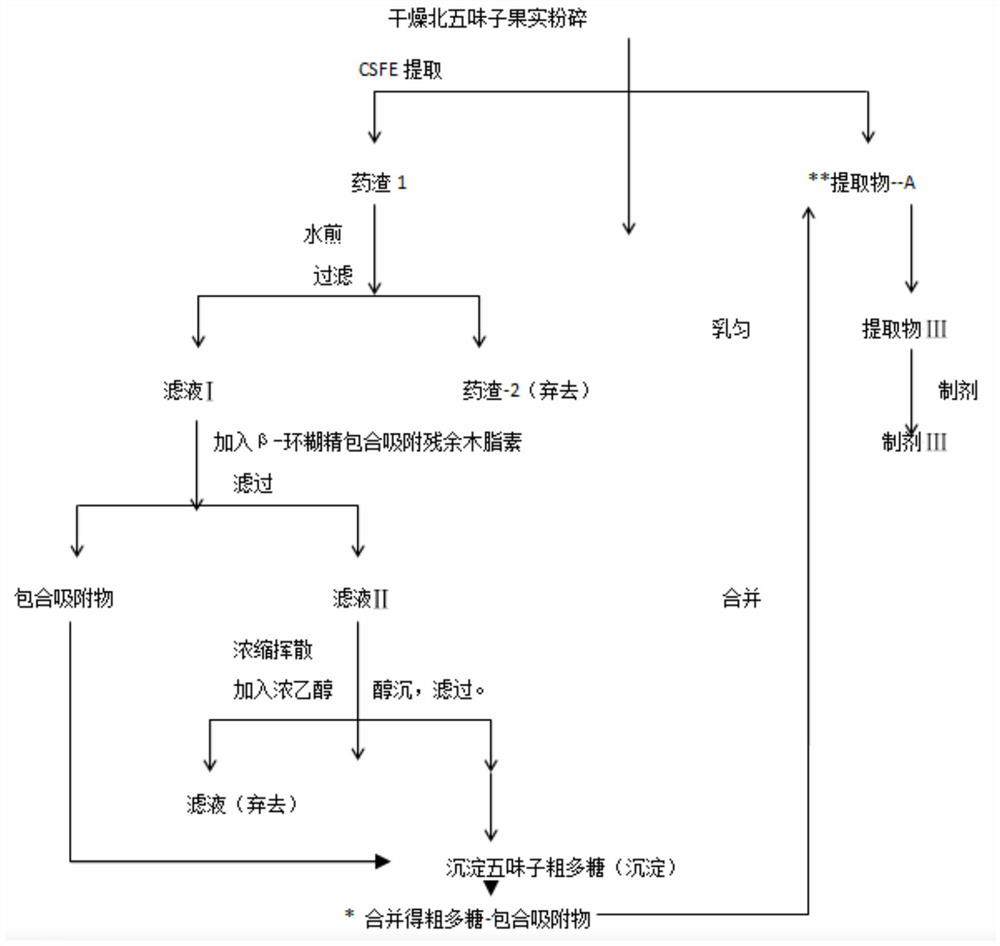
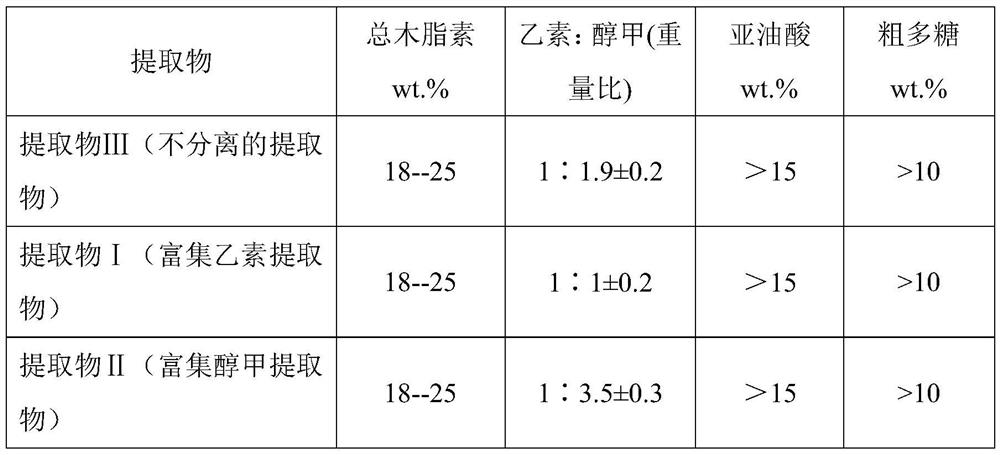
Three Chinese magnoliavine fruit extracts as well as preparation process and application thereof
ActiveCN112791137AIncreased total lignan contentEasy to separateNervous disorderBulk chemical productionBiotechnologyCyclodextrin
The invention discloses three Chinese magnoliavine fruit extracts as well as a preparation process and application thereof, and belongs to the technical field of medicines. The preparation method of the Chinese magnoliavine fruit extract comprises the following steps: treating Chinese magnoliavine fruit by using a CSFE method to obtain an extract A, and placing the extract at room temperature for layering to obtain an extract B (rich in schisandrin B) and an extract C (rich in schizandrin); boiling the decoction dregs, adding beta-cyclodextrin into filtrate to carry out inclusion adsorption on residual fat-soluble active ingredients in the decoction dregs, and filtering to obtain the inclusion adsorption lignan; performing concentration and alcohol precipitation on the filtrate to obtain the Chinese magnoliavine fruit polysaccharide; respectively combining the inclusion adsorption lignan and polysaccharide with the extracts A, B and C to obtain an extract I, an extract II and an extract III. The content of effective parts of the three extracts accounts for more than 50% of the extracts, the three extracts are composed of three active components including total lignan, linoleic acid and polysaccharide, and the total lignan is 18-25%. Different new drugs can be developed from the three extracts according to different proportions of schisandrin B and schizandrin for resisting depression, promoting sleep, promoting intelligence and other indications.
Owner:沈嘉
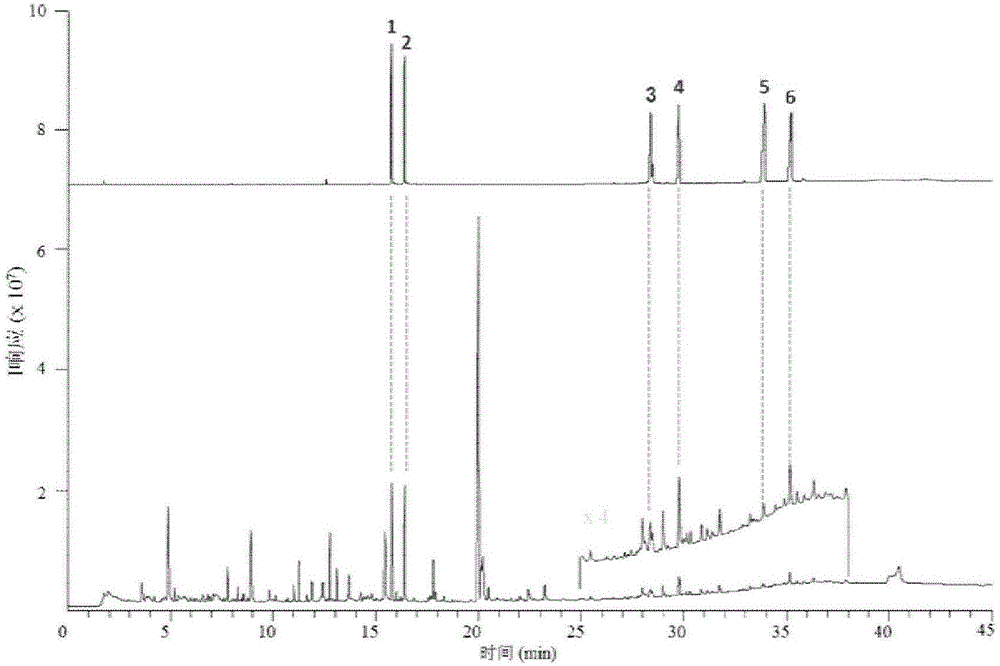
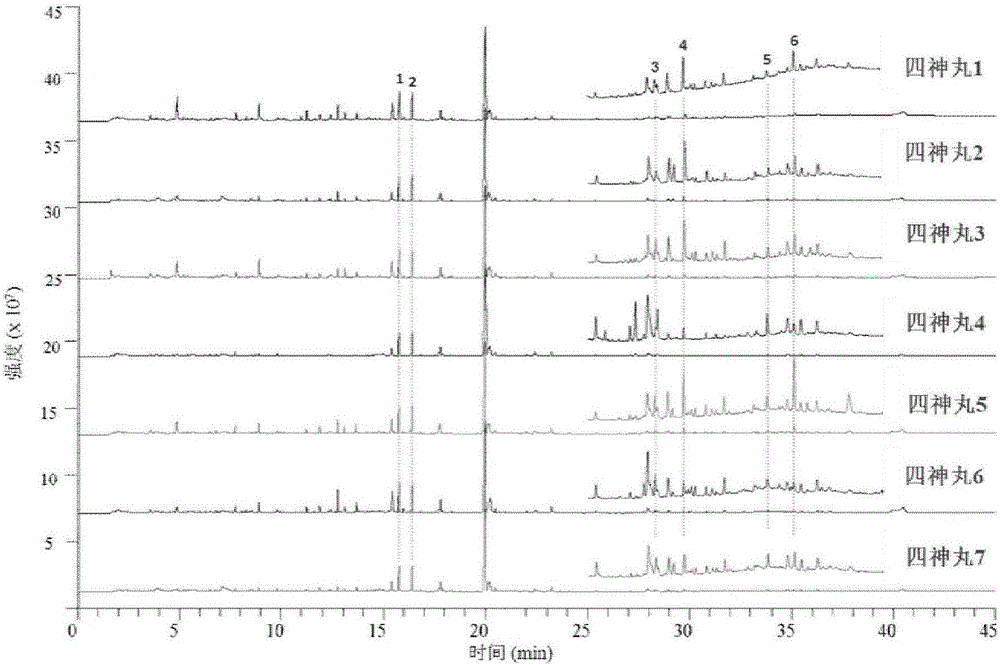
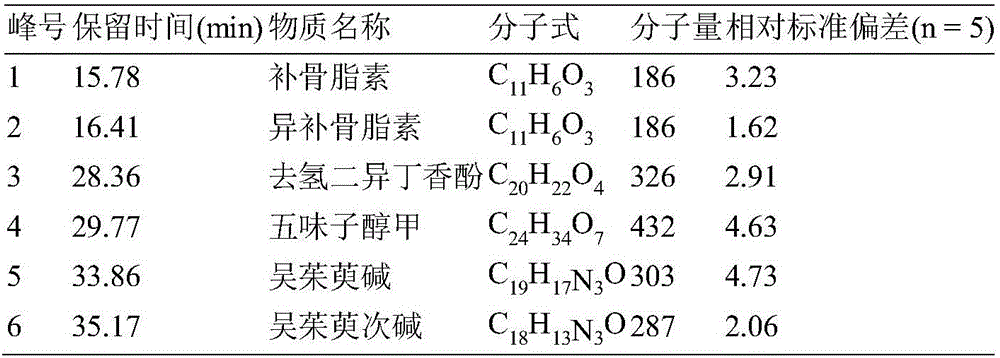
Method for determining index components in Sishen pill through flash evaporation-gas chromatography technology
ActiveCN107436331ASimple and fast operationEnvironmental Quality Evaluation MethodComponent separationEvodiamineGas phase
The invention provides a method for simultaneously determining psoralen, isopsoralen, dehydrodiisoeugenol, schizandrol A, evodiamine and rutaecarpine in a Sishen pill through a flash evaporation-gas chromatography technology. The method comprises the following steps: carrying out flash evaporation-gas chromatography detection on a Sishen pill sample to obtain a qualitative analysis result, carrying out an external standard technology to establish a standard working curve, and acquiring quantitative analysis results of the index components in the Sishen pill sample. The method adopting the flash evaporation-gas chromatography to realize the direct introduction of a solid sample without a complex extraction process or a solvent, so the method is simple to operate, and is environmentally-friendly; and the method adopting the flash evaporation-gas chromatography to quantitatively detect the index components in the Sishen pill can effectively save the experiment time and simplify experiment steps, so the above quality evaluation method has the characteristics of simplicity, environmental friendliness and high efficiency, and is of very far-reaching significance to evaluate the quality of the Sishen pill.
Owner:ZHEJIANG UNIV OF TECH
Magnolia vine fruit oil with human body heath care function
InactiveCN101904366AThere was a dose-dependent reduction between the groupsEdible oils/fatsBiotechnologyPharmaceutical Aids
The invention relates to magnolia vine fruit oil with a human body heath care function, which is prepared by directly extracting plant magnolia vine fruits. The magnolia vine fruit oil mainly comprises the following effective components by weight percent: 0.5-0.75% of schizandrol A, 0.08-0.12% of tablet, capsule or oral liquid can be prepared bymagnolia vine fruit oil as the main active component and coordinating with pharmaceutically acceptable carriers or auxiliary materials, which has the health care functions of protecting liver, relaxing vessels, reducing blood fat and the like.
Owner:张守勤
Weight reducing tea
InactiveCN105053372APromote decompositionInhibit synthesisPre-extraction tea treatmentAnimal sciencePreservative
The invention discloses weight reducing tea which is prepared from the following raw materials in parts by weight: 20-30 parts of semen cassiae, 20-30 parts of lotus leaves, 20-30 parts of ginsengs, 20-30 parts of golden camellia and 0.05-0.10 part of a preservative, wherein the biological preservative is schisantherin A and / or schizandrin A. The weight reducing tea can promote fat decomposition, suppress synthesis of fatty acid, adjust material metabolism and relax bowel and is effective, healthy and weight-reducing.
Owner:SHANGHAI QIUCHENG NEW MATERIAL TECH
Quality detection method of kidney-nourishing and nerve-calming pills
ActiveCN108037200AStrong specificityIncreased durabilityComponent separationThin layer chromatographicSemen
The invention discloses a quality detection method of kidney-nourishing and nerve-calming pills. The quality detection method comprises thin-layer chromatographic qualitative identification and content detection of schizandrin. According to the quality detection method of the kidney-nourishing and nerve-calming pills, the thin-layer chromatographic qualitative identification for prepared polygonummultiflorum and semen cuscutae in the kidney-nourishing and nerve-calming pills is firstly built, and the content measurement is performed on the schizandrin. The detection method is relatively highin specificity and durability, and the accuracy, the repetitiveness and the stability of the detection method all can meet scientific research and production requirements, so that the stability and the controllability of product quality can be effectively guaranteed.
Owner:GUANGDONG YIHETANG PHARMA CO LTD
Fructus schisandrae extract as well as preparation method and application thereof
The invention discloses a fructus schisandrae extract which refers to a fructus schisandrae rhizome xylem ethanol extract which contains 18% of schizandrin, and is extracted from fructus schisandrae rhizome xylem. According to the fructus schisandrae extract, the problem of shortage of fructus schisandrae can be solved, fructus schisandrae roots and the fructus schisandrae rhizome xylem thrown away in the processing process are fully utilized, and the waste is reduced; the extract is high in lignans such as schizandrin with different compositions and contents in comparison with those in fructus schisandrae fruits and rattans, and is unique in pharmacological activity. The invention further discloses a preparation method for the fructus schisandrae extract and an application of the fructus schisandrae extract to dispelling an alcohol effect and protecting the liver.
Owner:吉林省华惠生物科技有限公司
Traditional Chinese medicine composition capable of ventilating the lung and relieving asthma and preparation method thereof
The invention discloses a pharmaceutical composition for ventilating lung and relieving asthma, a preparation method and a quality control method. The pharmaceutical composition comprises the following pharmaceutical raw materials: ephedra herb, raw ginger, cassia twig, bitter apricot seed, Chinese magnoliavine fruit (prepared) and prepared liquoric root, the preparation method is as follows: thefive herbal medicines except the bitter apricot seed are decocted by adding water through a countercurrent circulatory extraction process and concentrated to obtain a clear paste after reducing pressure, ethanol is added, then the mixture is stirred evenly and placed staticly, supernatant liquid is taken, the ethanol is recovered, and the concentration is carried out to obtain a thick paste; the bitter apricot seed is arranged in a hot pressure sterilization pot for hot pressure steaming and boiling and then is arranged in a small amount of cold water for soaking instantly after being taken out, an apricot processed product can be obtained by removing seed coats and being dried; the quality control method is as follows: the thin layer identification is carried out on the ephedra herb, theraw ginger and the liquoric root, the content measurement is carried out on ephedrine in the ephedra herb and schizandrin in the Chinese magnoliavine fruit. The pharmaceutical composition has good effects on ventilating lung and relieving asthma.
Owner:BEIJING ASIA EAST BIO PHARMA CO LTD
A quality detection method for Zishen Ningshen Pills
ActiveCN108037200BStrong specificityIncreased durabilityComponent separationThin layer chromatographicSchizandrol A
The invention discloses a quality detection method of kidney-nourishing and nerve-calming pills. The quality detection method comprises thin-layer chromatographic qualitative identification and content detection of schizandrin. According to the quality detection method of the kidney-nourishing and nerve-calming pills, the thin-layer chromatographic qualitative identification for prepared polygonummultiflorum and semen cuscutae in the kidney-nourishing and nerve-calming pills is firstly built, and the content measurement is performed on the schizandrin. The detection method is relatively highin specificity and durability, and the accuracy, the repetitiveness and the stability of the detection method all can meet scientific research and production requirements, so that the stability and the controllability of product quality can be effectively guaranteed.
Owner:GUANGDONG YIHETANG PHARMA CO LTD
Extraction of schizandrol A
Owner:朱萧俊
Determination method for lignan content
InactiveCN103808813AImprove extraction efficiencyEasy to separateComponent separationLignanVolumetric flask
The invention especially relates to a determination method for lignan content, belonging to the technical field of content determination. The method provided by the invention has the advantages of high efficiency and simple and flexible operation. The method comprises the following steps: weighing and putting schisandrol A, schisandrol B and a schisandrol A reference substance in a same volumetric flask, adding methanol to a scale, carrying out shaking up and filtration and taking a subsequent filtrate as a mixed standard reference substance solution A; weighing and putting deoxyschizandrin, schisandrin B, a schisandrin C control product in a same volumetric flask, adding methanol to a scale, carrying out shaking up and filtration and taking a subsequent filtrate as a mixed standard reference substance solution B; weighing Chinese magnoliavine fruit, smashing and grinding the Chinese magnoliavine fruit, then carrying out sieving, weighing the obtained powder, adding 75% alcohol, weighing the weight, carrying out ultrasonic treatment for 10 to 60 min, taking an obtained substance out, cooling the substance to room temperature, adding 75% ethanol to compensate for lost weight, carrying out shaking up and filtration and taking a subsequent filtrate so as to obtain a to-be-determined sample solution; and respectively weighing the mixed standard reference substance solutions A and B, respectively diluting the solutions 1 time and respectively injecting the solutions into a liquid chromatograph.
Owner:陶建臣
A kind of fingerprint detection method of traditional Chinese medicine composition
The invention provides a fingerprint detection method for a traditional Chinese medicine composition. The method comprises the following steps: preparation of a test solution: taking a traditional Chinese medicine composition to prepare a test solution; preparation of a reference solution: taking schisandrin, Prepare reference solution; Chromatographic conditions: take acetonitrile-phosphoric acid aqueous solution as mobile phase, gradient elution, the concentration of phosphoric acid aqueous solution is 0.05%~0.2%; Determination method: draw reference solution and test solution respectively, inject into liquid phase Chromatograph, measurement; wherein, the traditional Chinese medicine composition is made from the following raw materials by weight: 5-20 parts by weight of Taizishen, 5-20 parts by weight of Shuidi, 4-10 parts by weight of Lycium barbarum, 4-10 parts by weight of Schisandra chinensis parts, 4-10 parts by weight of Polygala radix, 4-10 parts by weight of Shichangpu, and 5-20 parts by weight of Poria.
Owner:张环宇
Identification and adulteration inspection methods for component of fruit of Chinese magnoliavine in eyesight-improving pills with stem of noble dendrobium
PendingCN112858564AEnsure medication safetyThe test result is accurateComponent separationAgainst vector-borne diseasesBiotechnologyThin layer chromatographic
The invention discloses identification and adulteration inspection methods for the component of the fruit of Chinese magnoliavine in eyesight-improving pills with the stem of noble dendrobium. According to the identification method, the eyesight-improving pill with the stem of noble dendrobium is identified by taking schizandrin as a reference substance through thin-layer chromatography. The adulteration inspection method comprises the following steps: detecting the contents of schizandrin and anwuligan in the eyesight-improving pill with the stem of noble dendrobium by adopting a high performance liquid chromatography-mass spectrometry, and then judging the authenticity of the stem of noble dendrobium in the eyesight-improving pill according to the ratio of the content of the anwuligan to the content of the schizandrin. By establishing a thin-layer chromatography identification method and a high performance liquid chromatography-mass spectrometry detection method for the stem of noble dendrobium in the eyesight-improving pill, the quality condition of the stem of noble dendrobium in the eyesight-improving pill is investigated, whether a production enterprise has the behavior of no feeding or adulterated feeding or not is checked, the method is simple, the cost is low, and the result is accurate. The medication safety of the eyesight-improving pill can be effectively ensured.
Owner:广西壮族自治区食品药品检验所
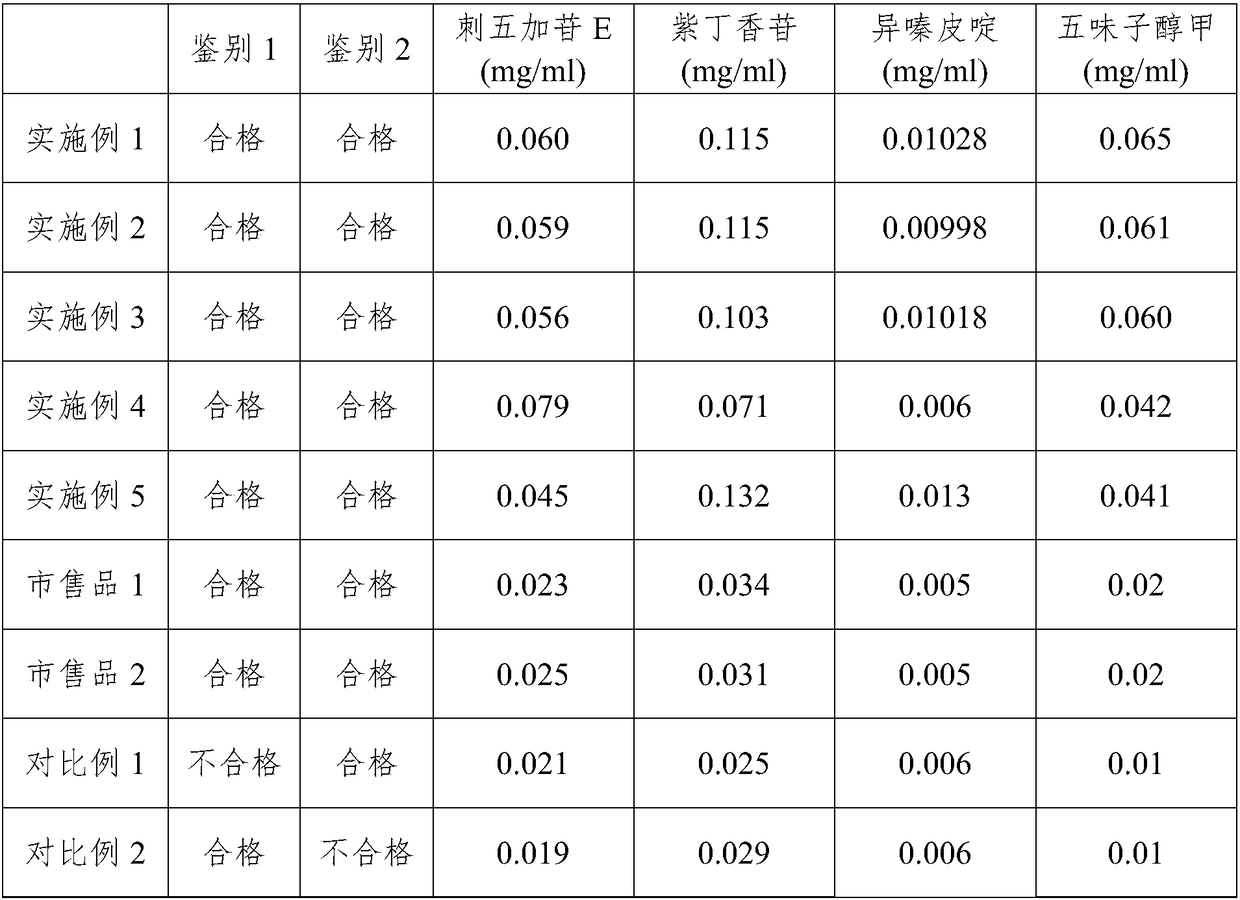
A pharmaceutical composition for treating neurasthenia and its preparation method
The invention relates to a pharmaceutical composition for treating neurasthenia and a preparation method thereof. The pharmaceutical composition comprises the following active ingredients: 0.03-0.5mg / ml eleutheroside E, 0.05-0.5mg / ml syringin, 0.005-0.05mg / ml isofraxidin and 0.03-0.5mg / ml schizandrin; preferably, 0.05-0.2mg / ml eleutheroside E, 0.1-0.3mg / ml syringin, 0.01-0.03mg / ml isofraxidin and 0.05-0.2mg / ml schizandrin. The pharmaceutical composition provided by the invention has the advantages that the preparation process is simple and feasible, the properties of the traditional Chinese medicine components are fully considered in the preparation process, excessive impurities are removed by macroporous adsorption resin to improve the concentration of active ingredients in the preparation and play the effects of the traditional Chinese medicine components; meanwhile, the used resin can be repeatedly used, so that resource consumption can be reduced. The traditional Chinese medicine composition prepared in the invention has a remarkable curative effect.
Owner:安徽九洲方圆制药有限公司
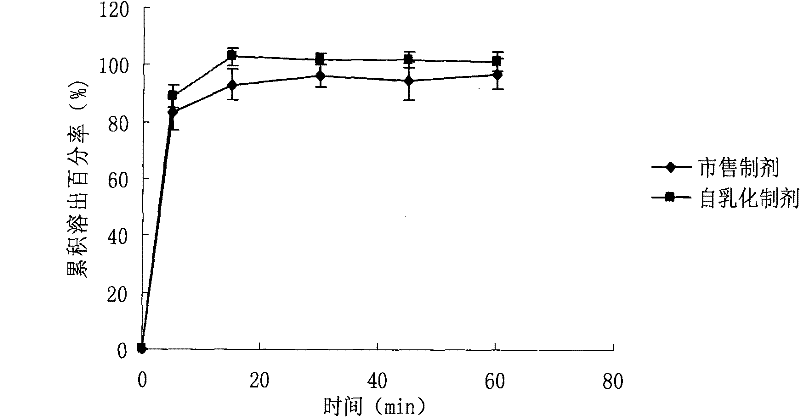
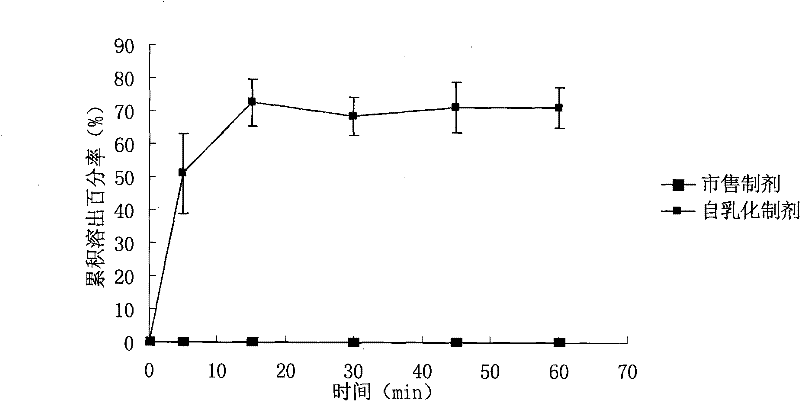
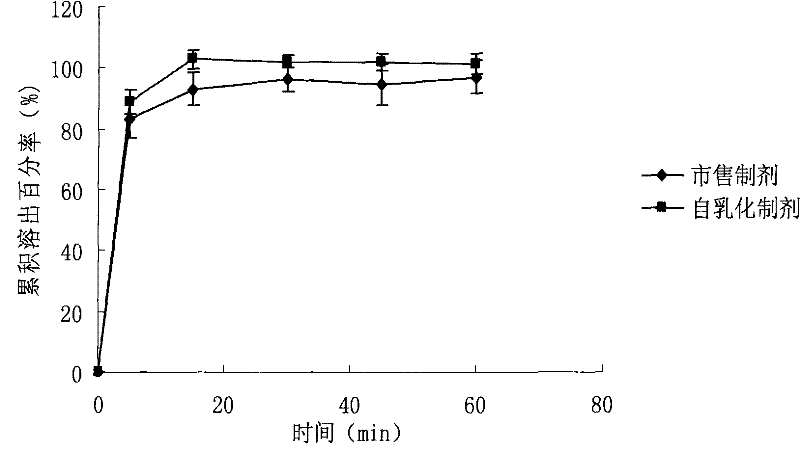
WuRenChun self-emulsifying soft capsule preparation and preparation method
ActiveCN101574397BGood water solubilityImprove bioavailabilityDigestive systemCapsule deliveryOil phaseDissolution
The invention relates to a WuRenChun self-emulsifying soft capsule preparation and a preparation method; WuRenChun (Shizandra ethanol extract containing 2-5% (W / W) of Schizandrol A and 1-3% (W / W) of Schizandrin B) is used for preparing the self-emulsifying soft capsule for curing acute, chronic and metastatic hepatitis, and reducing serum glutamic pyruvic transminase. The WuRenChun self-emulsifying soft capsule preparation comprises: WuRenChun, oil phase, surfactant and auxiliary surfactant, and the component proportion is 1:0.25-3.25:1.5-3.5:2.5-1.25. The preparation method is : the oil phase, the surfactant and the auxiliary surfactant are mixed evenly, the WuRenChun bulk drug is added to be mixed fully with intravascular ultrasound at 40 DEG C until the drug is fully dissolved, the obtained content is pressed into soft capsules according to the conventional method. The invention can enhance the dissolution degree of the WuRenChun, improve the bioavailability and then improve the healing effect.
Owner:HARBIN MEDICAL UNIVERSITY
Chinese magnoliavine fruit monomer composition separation preparation method
InactiveCN101709059BNo lossHigh recovery rateOrganic chemistryOrganic compound preparationMonomer compositionEthyl acetate
The invention relates to a Chinese magnoliavine fruit monomer composition separation preparation method; ethanol extracts from Chinese magnoliavine fruit are extracted by petroleum ether, chromatography is carried out to the petroleum ether extracts by a silicagel column, and then the petroleum ether is eluted by petroleum ether-ethylacetate, and HPLC is used for monitoring, and then crude extract A and crude extract B are fraction-collected, and eluted; the volume ratio of eluant petroleum ether and ethylacetate is 3-5:1, the crude extract A and crude extract B are respectively applied to a high-speed counter-current chromatography for separation, so as to obtain SCHisanhenol, deoxyschizandrin, schizandrin B, schisandrin C, schizandrol A, schizandrol B and schisantherrin B monomers, and the purity is higher than 98 percent.
Owner:华美恒盛(北京)科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com