Novel technique for preparing schizandrol A and schizandrol B
A technology of schisandrin B and schisandrin A, applied in the field of medicine, can solve problems such as quality decline, low purity, rising price, etc., and achieve the effects of alleviating shortage, simple process and convenient operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
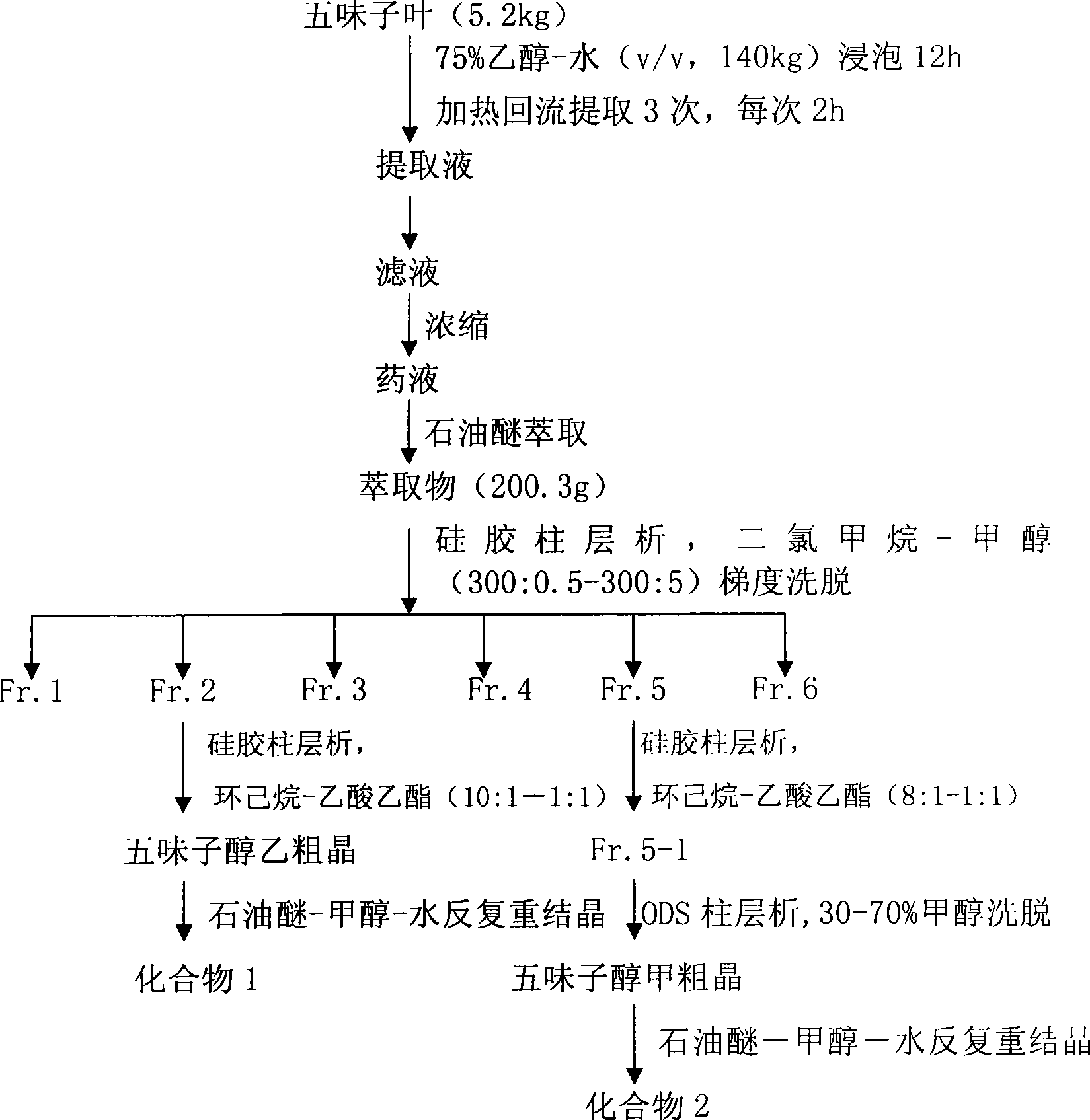
[0018] 5.2 kg of schisandra leaves were crushed, added with 80% acetone and extracted under reflux for 3 times, each time for 2 hours, filtered, and the combined filtrates were concentrated under reduced pressure to 3.5 L of medicinal solution with a density of 0.982. Add an equal volume of petroleum ether for extraction 6 times, and concentrate the petroleum ether layer to dryness under reduced pressure to obtain 150 g of the extract. The extract is subjected to silica gel column chromatography, and the volume ratio of dichloromethane-methanol (300:0.5—300:5) is Gradient elution of the mixed solution, roughly divided into 6 sections, TLC detection, collecting the second section rich in schisandra alcohol B, and then performing a second silica gel column chromatography, using cyclohexane-ethyl acetate (10:1- 1:1) mixed solution with a volume ratio of 1:1) was eluted, TLC was detected, and the part rich in schisandra alcohol B was collected, and recrystallized repeatedly with me...
Embodiment 2
[0020] 5.2 kg of schisandra leaves were crushed, added with 75% ethanol, heated and refluxed for 3 times, 2 hours each time, filtered, and the combined filtrate was concentrated under reduced pressure to 4.5 L of medicinal solution with a density of 0.982. An equal volume of petroleum ether was added for extraction 6 times, and the petroleum ether layer was concentrated to dryness under reduced pressure to obtain 203 g of the extract. The extract was subjected to silica gel column chromatography, and the volume ratio of dichloromethane-methanol (300:0.5—300:5) was Gradient elution of the mixed solution, roughly divided into 6 sections, TLC detection, collecting the second section rich in schisandra alcohol B, and then performing a second silica gel column chromatography, using cyclohexane-ethyl acetate (10:1- 1:1) mixed liquid with a volume ratio of 1:1) was eluted, TLC was detected, and the part rich in Schisandrin B was collected, petroleum ether-methanol-water was recrystall...
Embodiment 3
[0022] 5.2 kg of schisandra leaves were crushed, added with 75% ethanol, heated and refluxed for 3 times, 2 hours each time, filtered, and the combined filtrate was concentrated under reduced pressure to 4.5 L of medicinal solution with a density of 0.982. Adsorbed on the HPD100 macroporous resin on the liquid medicine, and eluted with water, 30%, and 40-90% ethanol successively, collected 40-90% ethanol eluate, recovered ethanol and evaporated to dryness in a water bath to obtain 160.8 g of eluate, and washed Carry out silica gel column chromatography, and use dichloromethane-methanol (300:0.5—300:5) volume ratio mixed solution gradient elution, roughly divide into 6 sections, TLC detection, collect the second phase rich in schisandra alcohol B Section part, then perform secondary silica gel column chromatography, elute with the mixed solution of cyclohexane-ethyl acetate (10:1-1:1) volume ratio, TLC detection, collect the part rich in schisandra alcohol B, Repeated recrystal...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com