Traditional Chinese medicine composition capable of ventilating the lung and relieving asthma and preparation and quality control method thereof
A quality control method and composition technology, applied in the direction of drug combinations, pharmaceutical formulas, medical preparations containing active ingredients, etc., can solve the problems of ineffective control of recurrence, toxic and side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0051] Experimental example 1. the optimal experiment of Chinese medicine group and thing capsule preparation technology of the present invention
[0052] 1. Screening of the amount of water added for water extraction
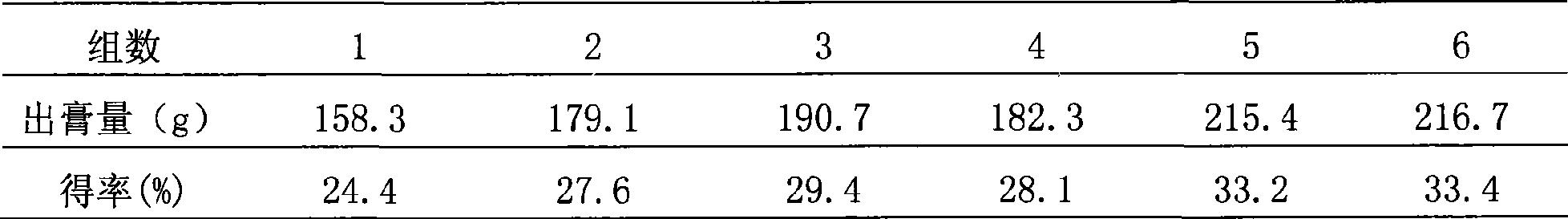
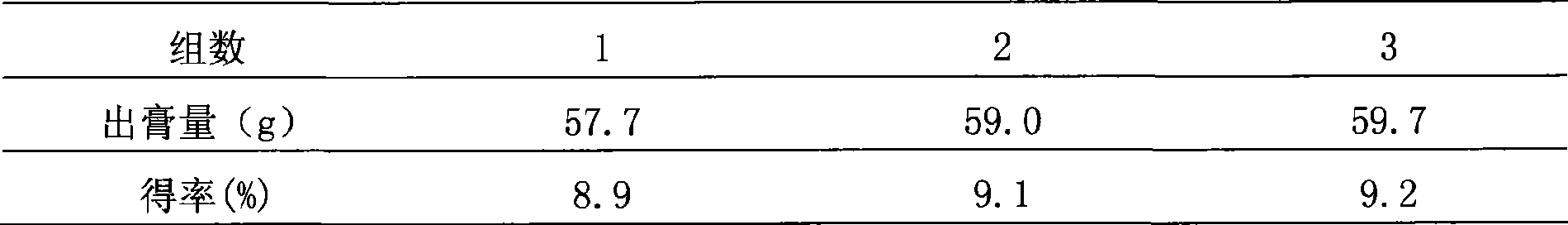
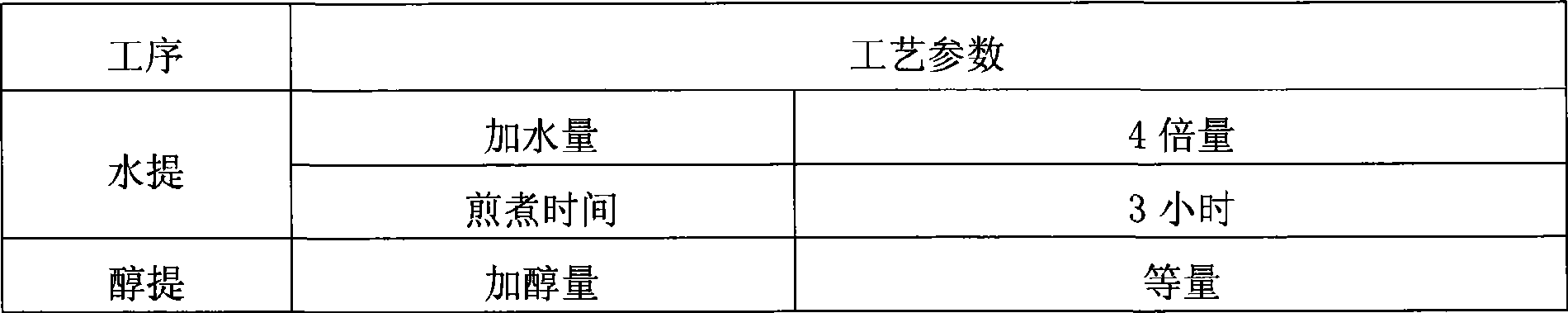
[0053] Configure 5 parts of medicinal materials, 648.8g each, and divide them into three groups for testing. The first group added 6 times and 4 times the amount of water, the second group added 8 times and 6 times the amount of water, and the third group added 10 times the amount of water. 8 times the amount of water, add 2 times the amount of water to the fourth group of countercurrent circulation extraction process, add 4 times the amount of water to the fifth group of countercurrent circulation extraction process, add 6 times the amount of water to the sixth group of countercurrent circulation extraction process, and use the amount of clear paste after water extraction as an indicator , to determine the amount of water added, the results are shown in Table ...
experiment example 2
[0067] Experimental example 2. The preferred experiment of the processing technology of bitter almond in the Chinese medicine group and thing of the present invention
[0068] The traditional Chinese medicine bitter almond contains the cough-relieving ingredient amygdalin. Under appropriate conditions, such as being exposed to moisture, decocting and soaking in water, the amygdalinase contained in it can easily decompose the amygdalin, thereby reducing or even losing the efficacy of the medicine. Therefore, bitter almonds need to be processed to achieve the purpose of destroying enzymes and protecting glycosides. Due to the current traditional processing methods: water decoction method and stir-frying method are not effective in inactivating enzymes and protecting glycosides, and the quality is difficult to control, so this paper introduces steam hot pressing method and traditional method for comparative experiments to explore better processing methods .
[0069] 1 Instrument...
experiment example 3
[0092] Experimental example 3. Pharmacodynamics experiment
[0093] In order to prove the curative effect of the present invention, we have carried out following pharmacodynamic experiment.
[0094] The drug group of the present invention used in the following pharmacodynamic tests is the capsule prepared according to Example 4;
[0095] Positive control drug: granules prepared by the following method:
[0096] Ephedra 1200g, ginger 1200g, schisandra (made) 150g, roasted licorice 45g
[0097] 1000g made from 627g sucrose and 260g dextrin
[0098] The above four herbs are decocted twice with water, the first time is 3 hours, the second time is 2 hours, the decoction is combined, filtered, the filtrate is concentrated under reduced pressure to a clear paste with a relative density of 1.08 (60°C), and let it cool to At room temperature, add an equal amount of ethanol, stir well, let stand for 24 hours, take the supernatant, recover the ethanol under reduced pressure and concen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com