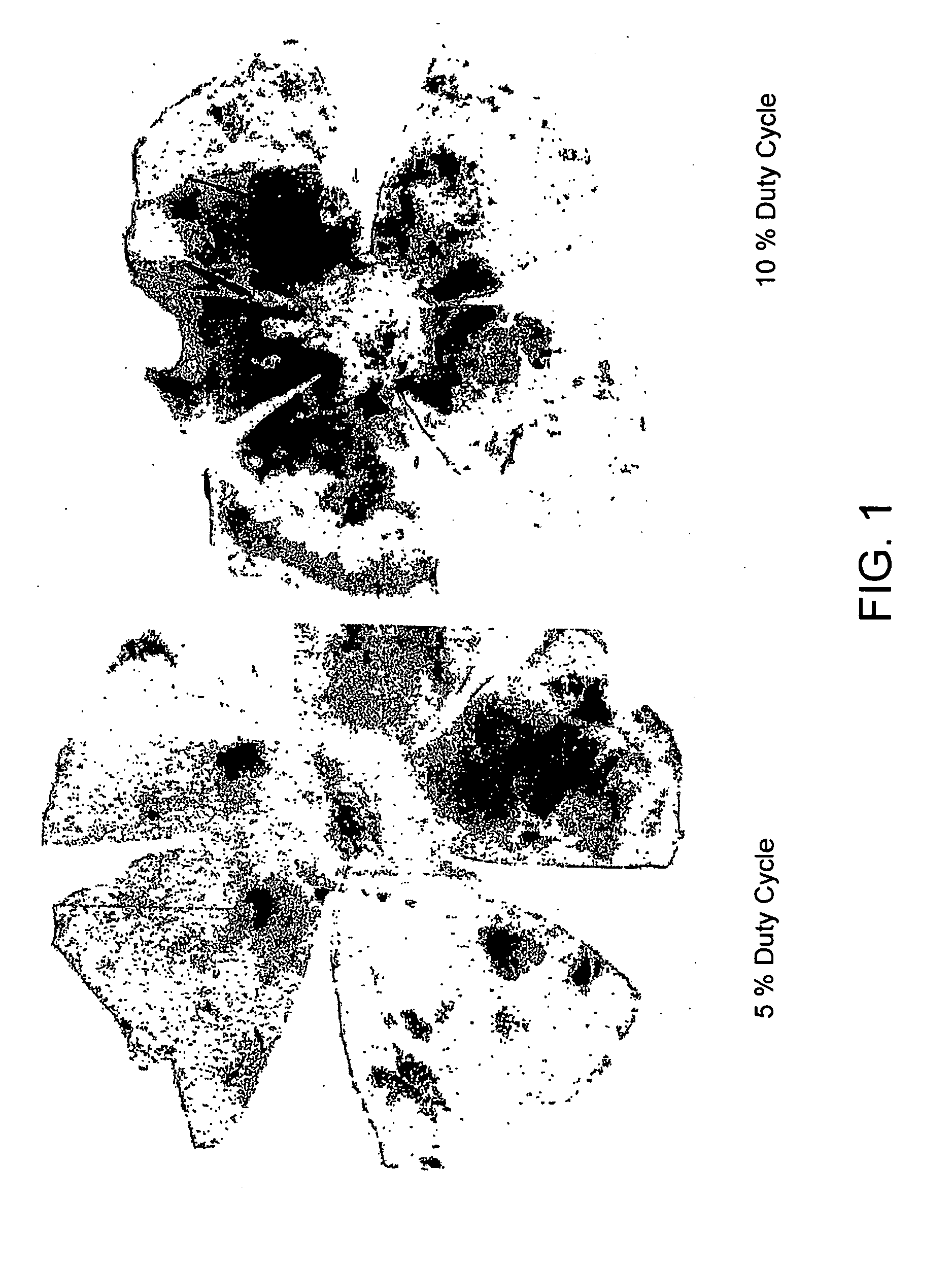
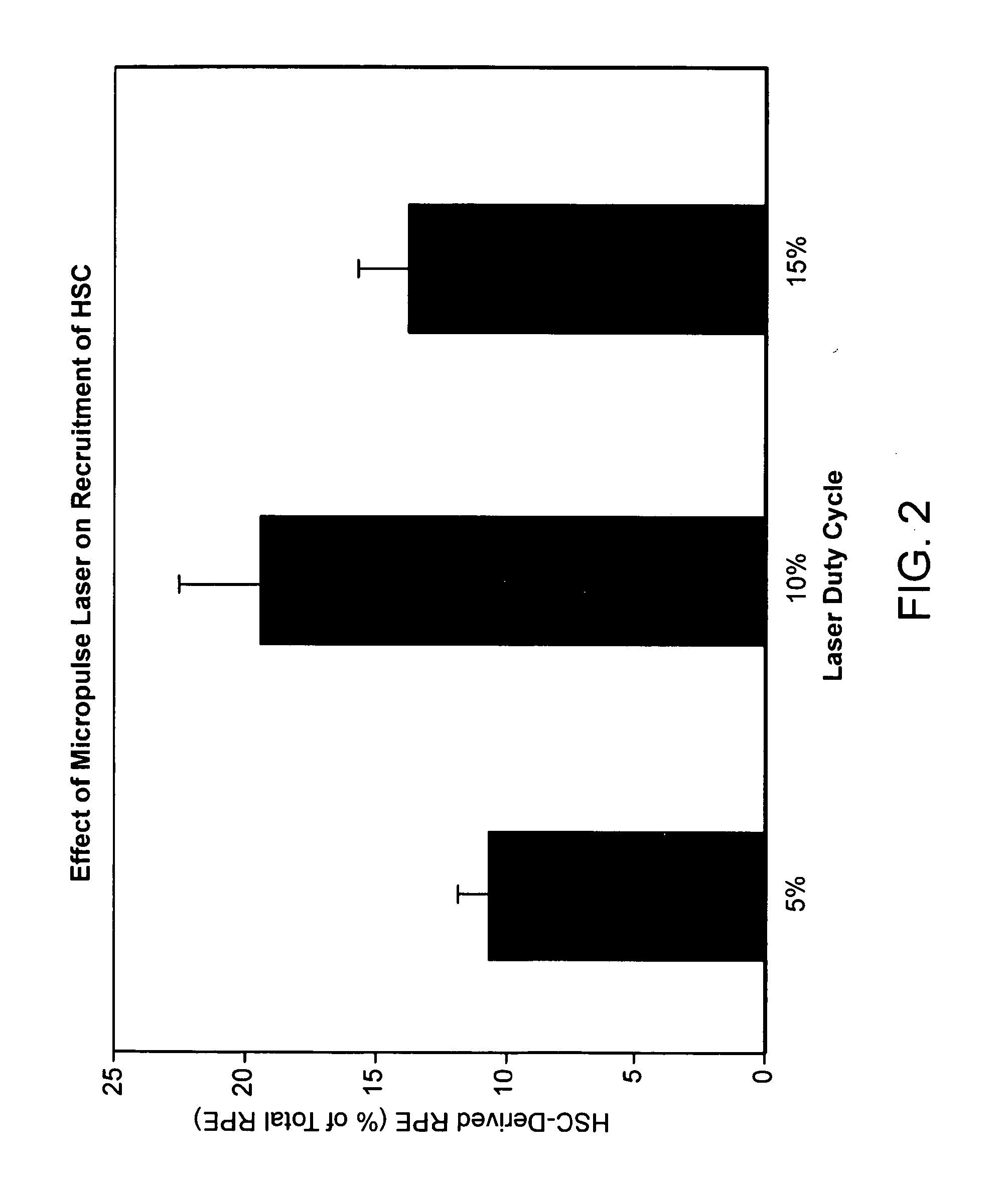
Patents
Literature
425 results about "Liver regeneration" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Liver regeneration is the process by which the liver is able to replace lost liver tissue from growth from the remaining tissue. The liver is the only visceral organ that possesses the capacity to regenerate. The liver can regenerate after either surgical removal or after chemical injury. It is known that as little as 25% of the original liver mass can regenerate back to its full size. The process of regeneration in mammals is mainly compensatory growth because only the mass of the liver is replaced, not the shape. However, in lower species such as fish, both liver size and shape can be replaced.
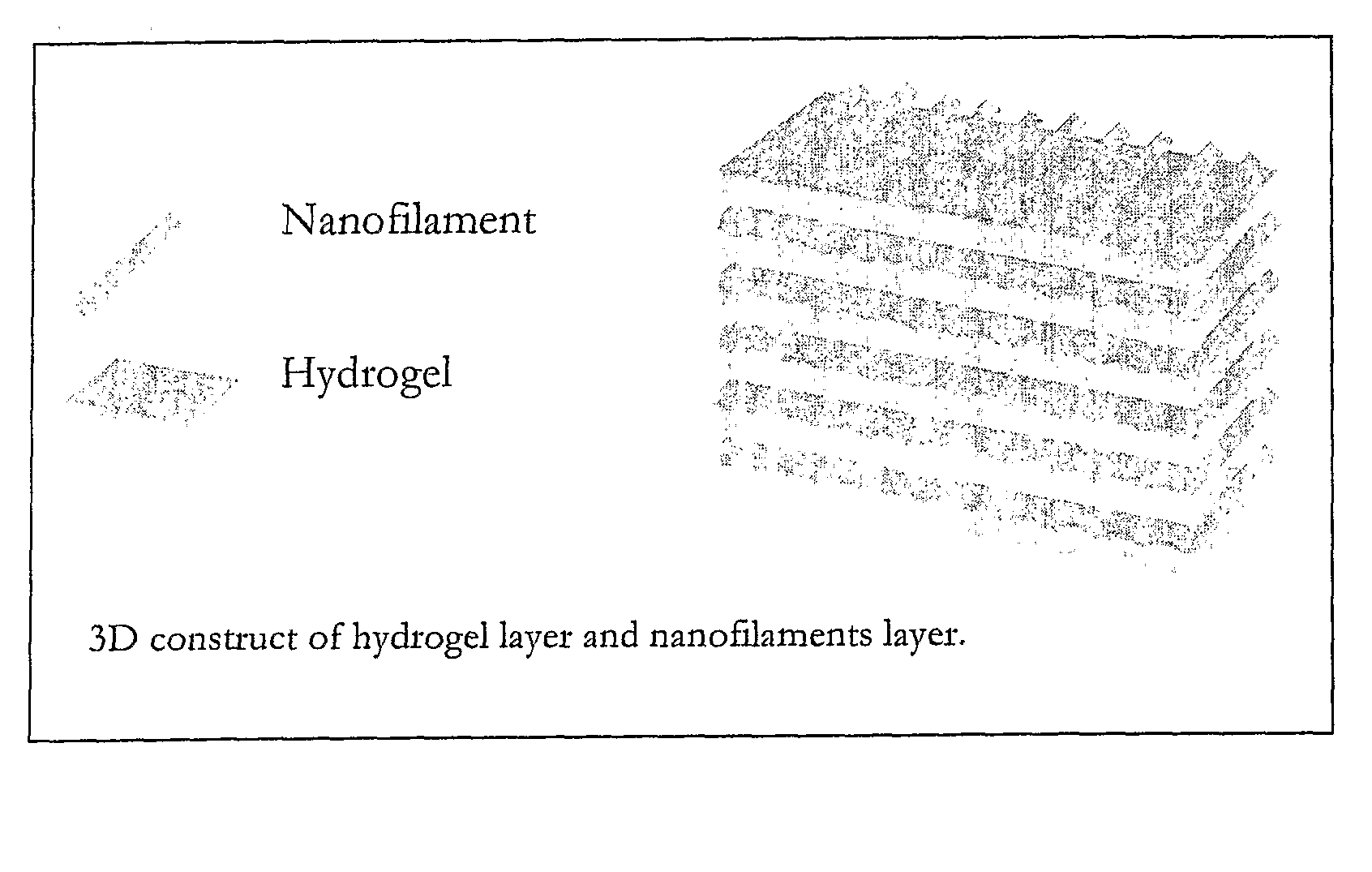
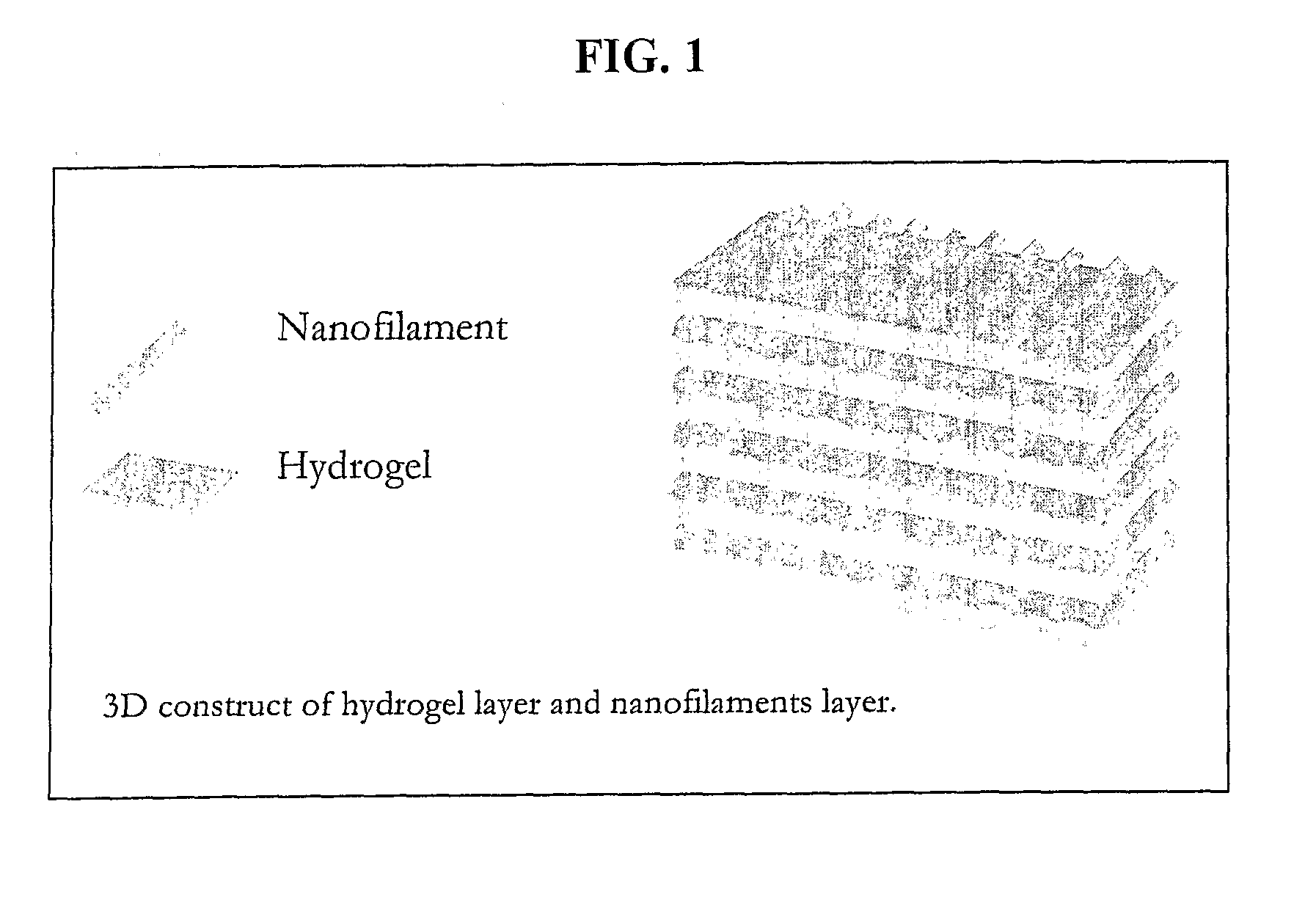
Nanofilament Scaffold for Tissue Regeneration
A scaffold for tissue regeneration is provided. In a preferred embodiment, the scaffold is implantable in a patient in need of nerve or other tissue regeneration and includes a structure which has a plurality of uniaxially oriented nanofibers made of at least one synthetic polymer. Preferably, at least 75% of the nanofibers are oriented within 20 degrees of the uniaxial orientation. The scaffold beneficially provides directional cues for cell and tissue regeneration, presumably by mimicking the natural strategy using filamentous structures during development and regeneration.
Owner:GEORGIA TECH RES CORP
Muscle regeneration promoter
ActiveUS20100008907A1Promote muscle regenerationIncrease the number ofPeptide/protein ingredientsMuscular disorderCell growthSignal Pathways
Owner:OSAKA UNIV
Biomaterials for guided tissue regeneration and drug delivery
The present invention is directed to compositions and methods of using compositions comprising a scaffold with growth factors chemically immobilized thereto for inducing chondrogenesis and / or osteogenesis when implanted in vivo or osteogenesis or chondrogenesis in cultures in vitro. The compositions and methods enhance bone and cartilage growth. Also described are compositions and methods for targeted drug delivery.
Owner:GELWELL BIOTECH CORP
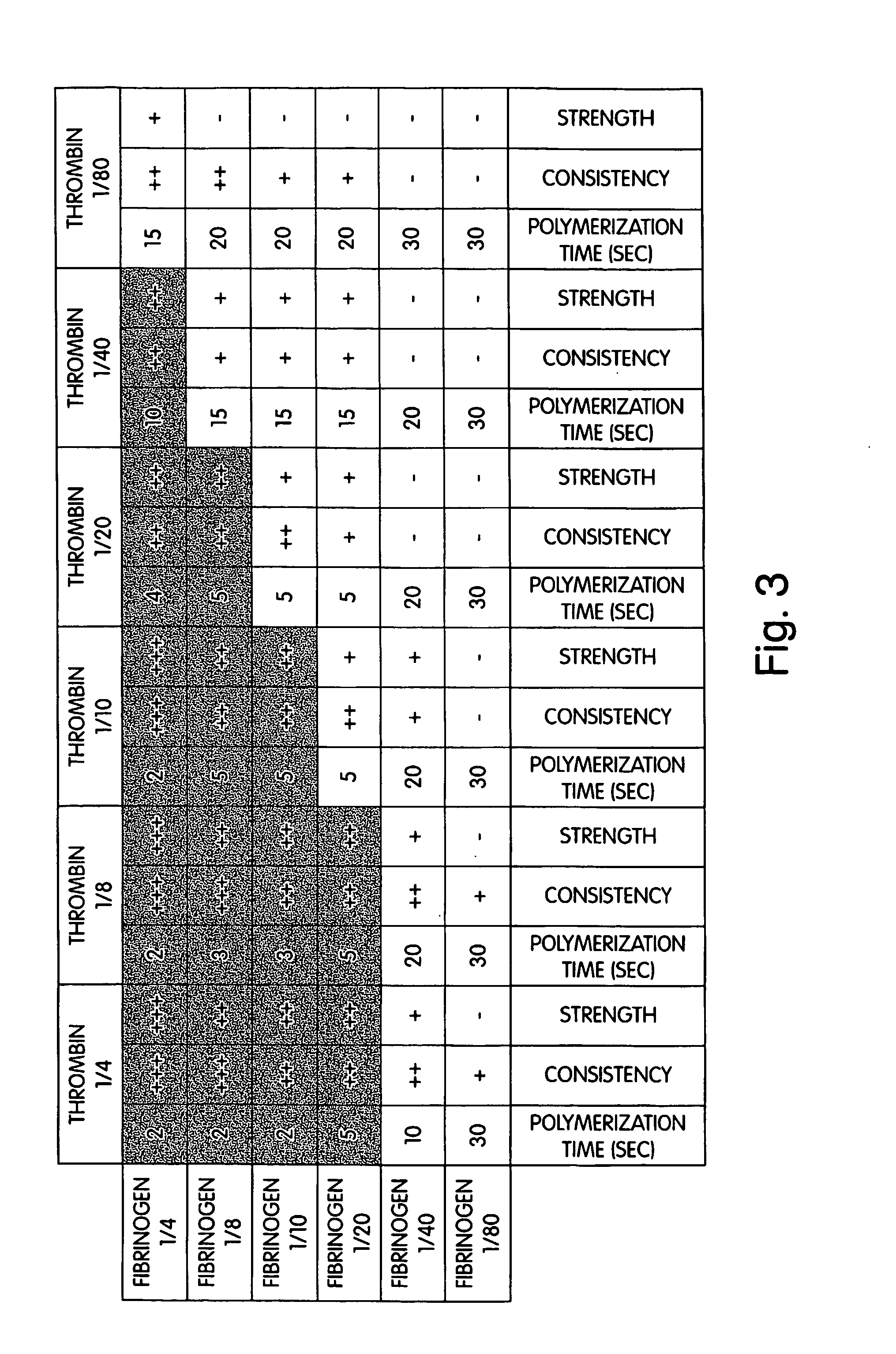
Scaffolds with oxygen carriers, and their use in tissue regeneration
Provided are fibrin or silk matrices comprising an oxygen carrier, and matrices, which comprise an oxygen carrier and mesenchymal stem cells. Also provided are methods of generating and using same for ex vivo or in vivo tissue regeneration and / or repair such as for treating a non-union bone fracture and a condition requiring spinal fusion.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD
Method for directed cell in-growth and controlled tissue regeneration in spinal surgery
ActiveUS20100028309A1Prevention and minimization of uncontrolled adhesionPrevention and minimization of and scar formationBiocideSurgerySpinal columnMammal
The present invention relates to a method for directed cell in-growth and controlled tissue regeneration to prevent post-surgical or post-traumatic adhesion and fibrosis formation on the injured surface of a tissue selected from the group consisting of spinal column tissue, dura mater, and spinal nerves in a mammal, comprising the step of providing, covering and separating the tissue with a bioactive biofunctional, non-porous, microscopically multilayered collagen foil biomatrix, and to a method for treating a defect in a mammal comprising the step of providing, covering and separating said tissue with a bioactive biofunctional, non-porous, microscopically multilayered collagen foil biomatrix.
Owner:BAXTER INT INC +1
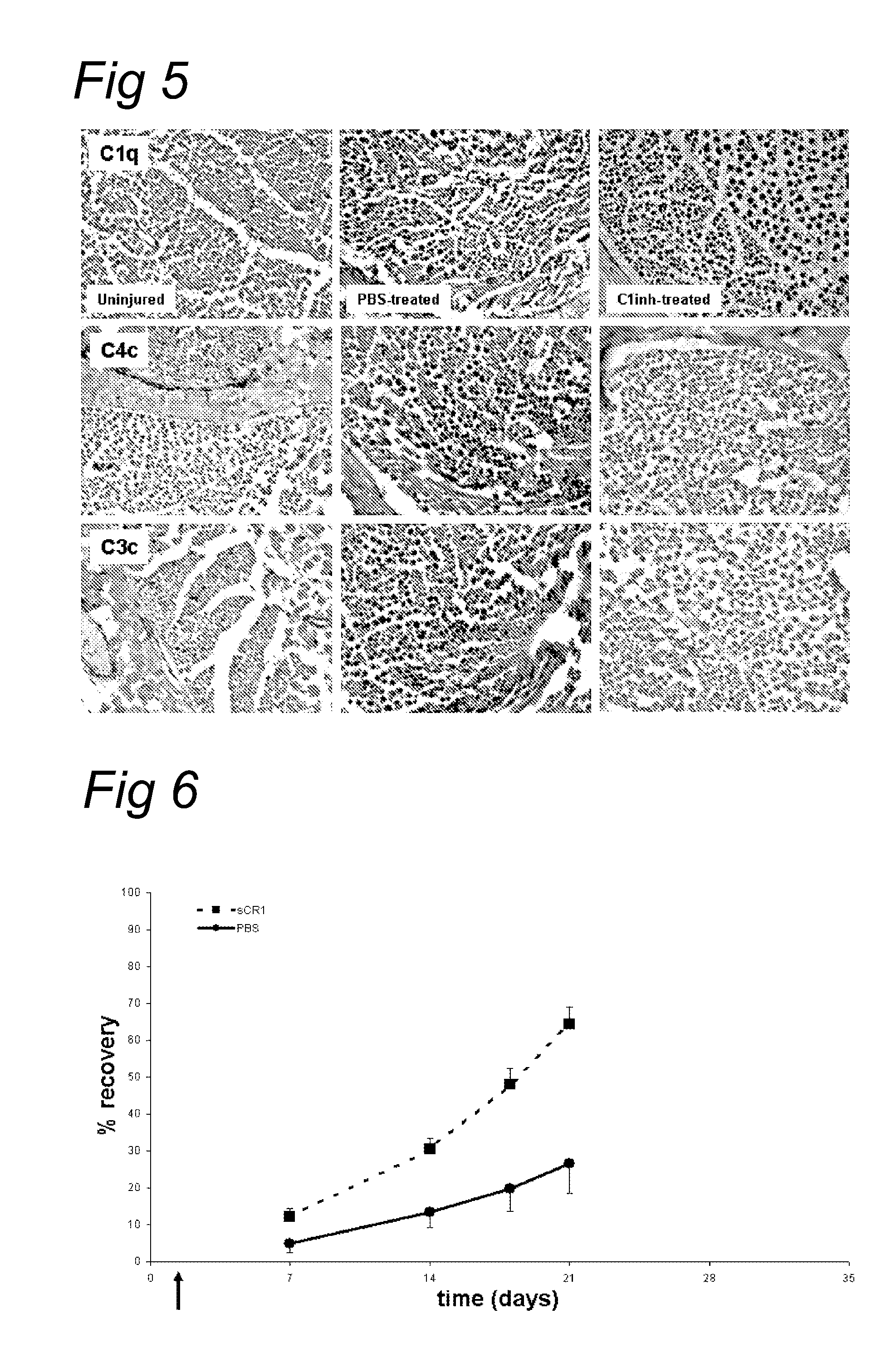
Complement inhibition for improved nerve regeneration
ActiveUS8703136B2Improve regenerative abilityPromote regenerationNervous disorderPeptide/protein ingredientsDiseaseNervous system
The present invention relates to methods and medicaments used for treating conditions that require axonal regeneration, e.g. in mammals affected by injury or disease of the central or peripheral nervous system. The medicaments used in these methods facilitate axonal regeneration by inhibition of the complement system. Conditions requiring axonal regeneration that may be treated in accordance with the invention include physical injuries as well as neurodegenerative disorders of the peripheral or central nervous system.
Owner:REGENESANCE
Medical guide tubes
Polymeric fibers were microbraided around a mandrel to make a tubular guide tube for nerve regeneration. The polymer used for the fibers was one of poly(L-lactide-co-glycolide) (10:90 PLGA) and chitosan. These polymers are biodegradable and biocompatible. The tubes were studied for their surface morphology and swelling behavior. Biological performance of the tubes was examined in the rat sciatic nerve model with a 12 mm gap. One month after implantation nine out of ten rats showed successful nerve regeneration. Morphometric analysis of regenerated nerves confirmed the quality of the regeneration compatible with those offered by other types of biodegradable nerve guide tubes. The tubes were flexible, permeable and showed no swelling.
Owner:NAT UNIV OF SINGAPORE +1
Pluripotent therapeutic compositions and uses thereof
InactiveUS20090274660A1Reduce riskReduce significant riskBiocideOrganic active ingredientsSelf-healingAnticarcinogen
Synthetic Stem Cell-like Tissue Healing and Regeneration Medication with Anti-inflammatory, Protein Synthesis, Enzyme Deficiency Activation and Genetic Therapy, and Anti-cancer Agent derived from a series of inventions that include these products of Biomolecular Engineering, Drug Discovery from a Biologic Periodic Table of Applied Biochemistry and Biophysics. Tissue has a self healing effect promoting tissue healing and tissue regeneration. Not only does it maintain good health but also it has been observed that the patient's blood is withdrawn from the patient and applied to the ulcer has healing qualities. Cartilage placed in a wound promotes and accelerates wound healing. The anabolic biochemical and biophysical equivalent of tissue has been found in these embodiments to have the same pharmacologic qualities, when devoid of genetic DNA mismatch and other catabolic factors including the catabolic effects of microorganism overgrowth that lacks pro-biotic qualities. The healing efficacy of these tissue components gives us further appreciation of the protective action of human tissue over and above and other than the immune protective system or perhaps an integral component part of the immune system.
Owner:IMMUNOPATH PROFILE INC A CORP OF PA
Growth factor slow-release type double-layered artificial skin
ActiveCN101716376AImprove waterproof performanceImprove breathabilitySkin implantsMicrospherePolyethylene glycol
The invention relates to a growth factor slow-release type double-layered artificial skin which is used for repairing injured skin with related growth factor slow-release functions and a preparation method thereof. The artificial skin is divided into two layers of an epidermal layer and an enderonic layer, wherein the epidermal layer is constituted by a micropore double-layered thin film with the functions of water proofing, ventilation and enderonic protection; the materials of the upper layer which contacts with air is polyurethane, or silicon rubber, or polyethylene glycol, or ethylene terephthalate and other medical materials; the lower layer which contacts with the dermis is a natural biological macromolecule thin film which is constructed by silk fibroin and chitosan; the enderonic layer of the artificial skin is constituted by collagen, polysaccharide and microspheres carrying growth factors related to skin regeneration and repairing; the microspheres have good slow-release function, and the slow-release period is equal to or slightly longer than the degradation period of the collagen and polysaccharide and can be matched with the skin regeneration and repairing period; and the thickness of the artificial skin is in a range of 0.5-1.5mm and can be regulated according to requirements.
Owner:SHENZHEN QIKANG MEDICAL DEVICES
Support for tissue regeneration and process for producing the same
ActiveUS7445793B2Excellent in retention and grafting abilityPrevent intrusionBiocidePowder deliveryFreeze-dryingBiology
Owner:GC CORP +2
Injectable composite material capable of promoting bone regeneration and repair and preparation method thereof
The invention discloses an injectable composite material capable of promoting bone regeneration and repair. The injectable composite material is prepared by mixing sodium alginate, chitosan, multiple trace element, calcium phosphate porous microsphere and bioactive glass nanometer granules, preparing the mixture by using deionized water and cell culture fluid, and compounding the prepared mixture. The injectable composite material comprises the following components in percentage by mass: 0.10 to 0.50 percent of sodium alginate, 0.01 to 0.20 percent of chitosan, 5 to 30 percent of multiple trace element codoped calcium phosphate porous microsphere, 0.05 to 0.50 percent of bioactive glass, 25 to 55 percent of cell culture fluid and 30 to 45 percent of deionized water. The preparation process is simple; the prepared injectable composite material has the characteristics of excellent injectability and quick degradation; and a hydrogel network can concentrate calcium, phosphorous ion and trace elements degraded and released by inorganic particles and can promote the migration, growth, multiplication and differentiation of bone cell, thereby having effects of quickly inducing bone regeneration and promoting bone repair on endosteal microdamage, fracture or bone defect.
Owner:ZHEJIANG UNIV
Method and apparatus for stimulating nerve regeneration
A method is provided for stimulating nerve growth, especially for nerve regeneration after damage to the nerve. The method comprises: applying thermal energy to one or more nerve segments adjacent a damaged region of the nerve, such that nerve fibers from the treated adjacent segment are stimulated to grow and extend toward the damaged region. The method may be used to enhance mechanical stimulative effect at a terminus of a severed nerve or a region of nerve injured by crush or other physical forces. The method may also be used to treat nerve segments retrograde up the nerve fiber and to increase the response of the injured nerve to regrowth and extension rapidly. An apparatus for delivery of thermal energy to the distal terminus of the severed nerve or region is disclosed. Thermal conductive or electromagnetic energy is delivered through a probe having a handle and a shaft. An energy delivery portion of the probe is configured to apply thermal energy to the distal segments of the severed nerve to promote rapid and more extensive growth of the nerve cells.
Owner:HUGH R SHARKEY
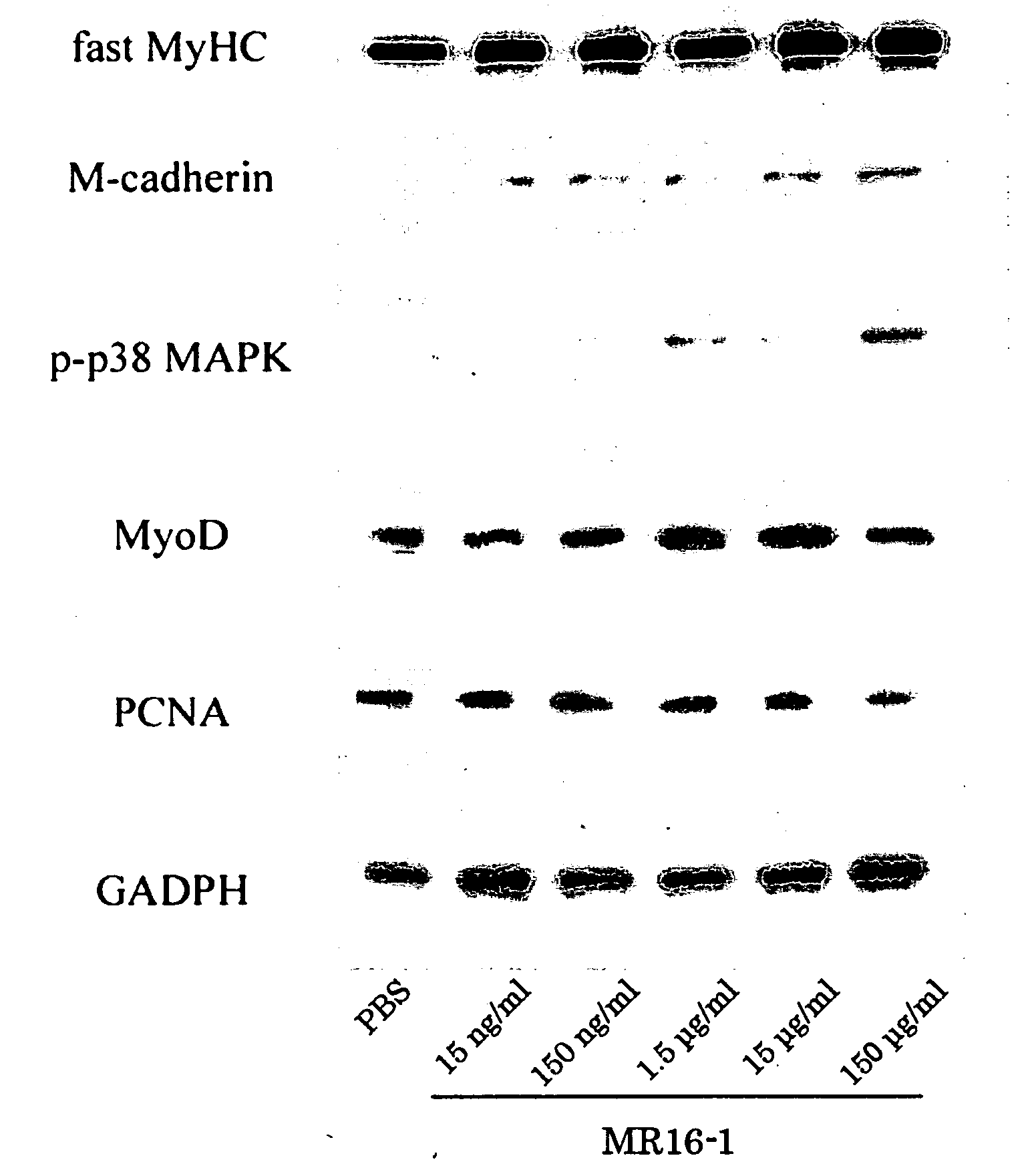
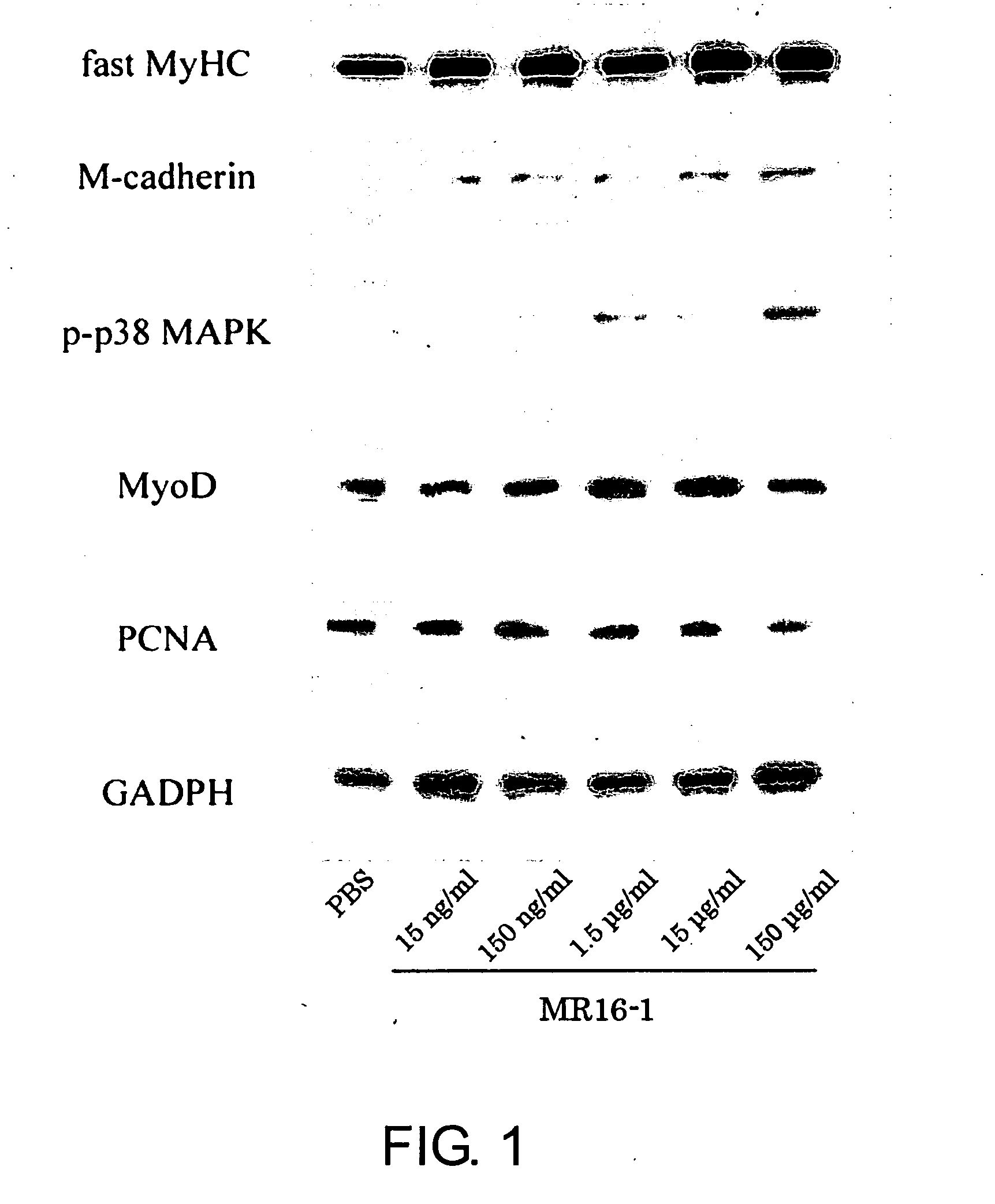
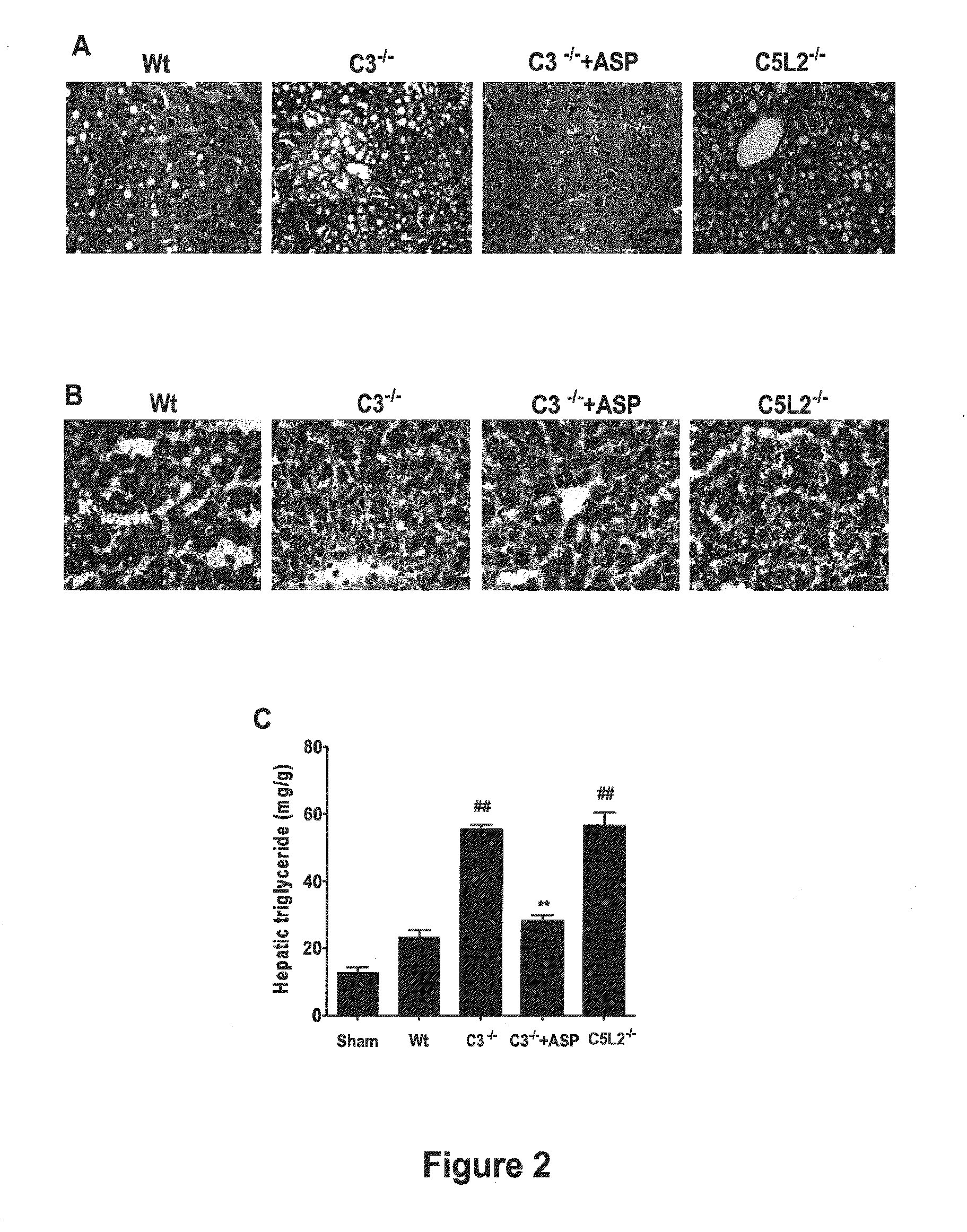
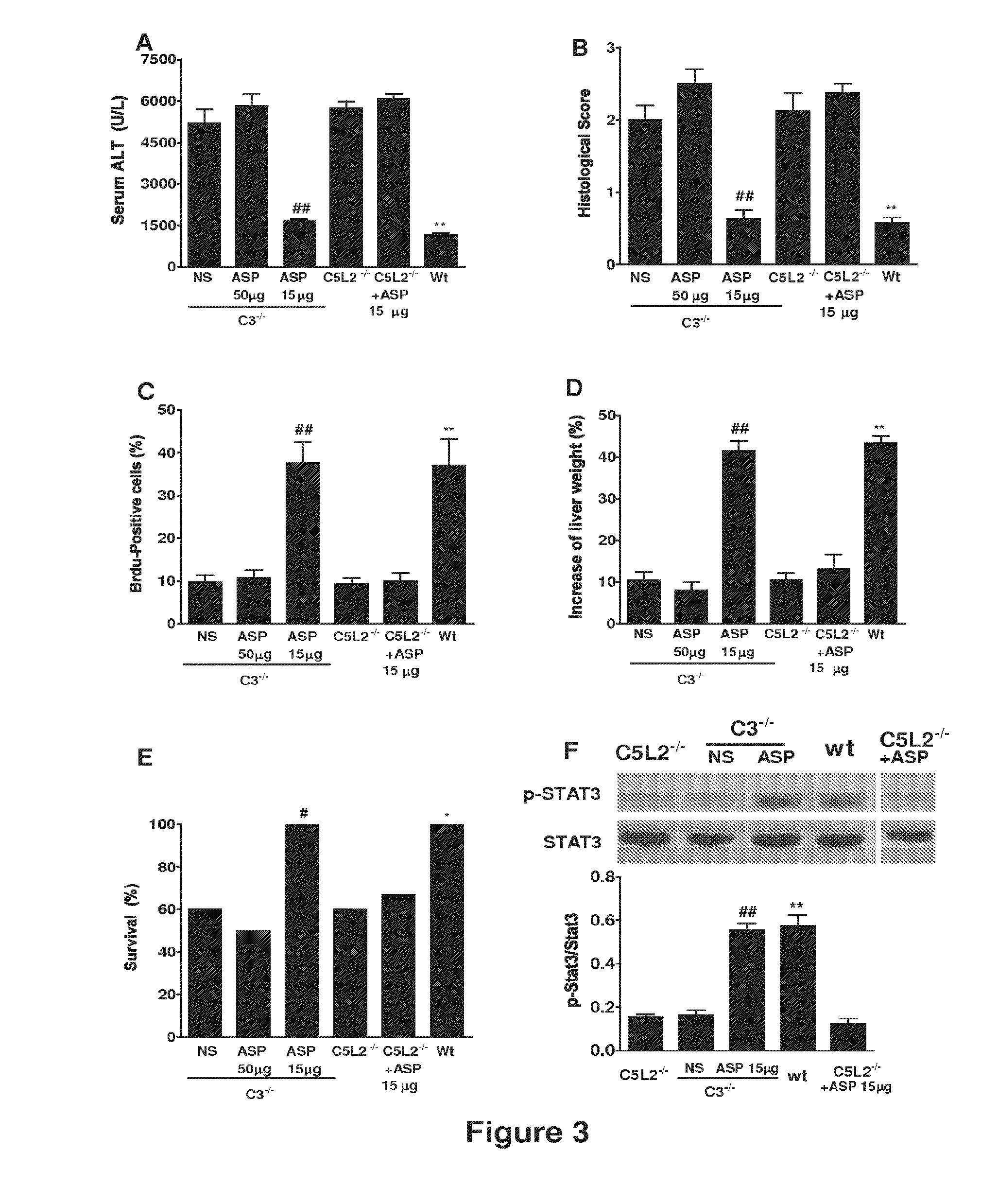
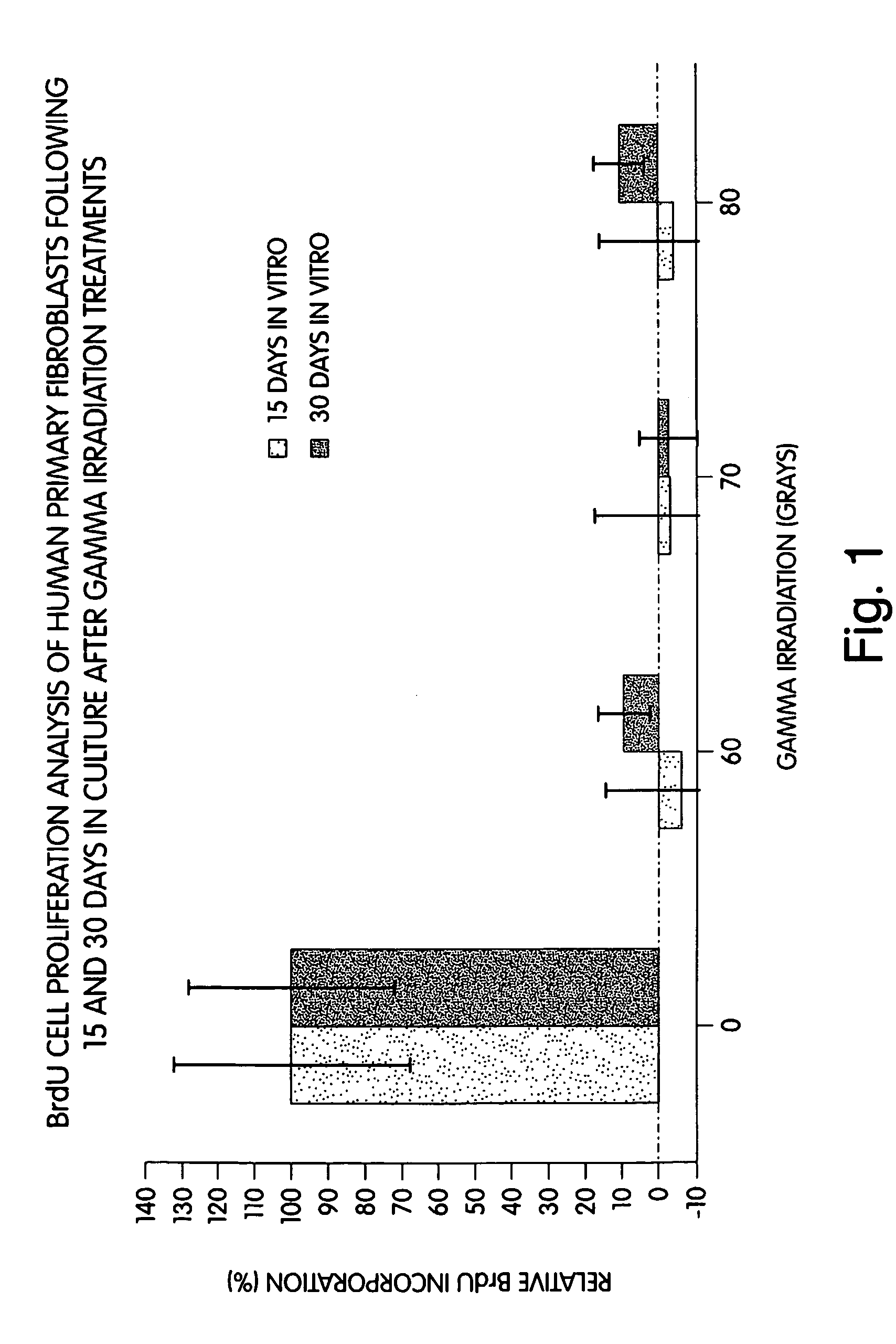
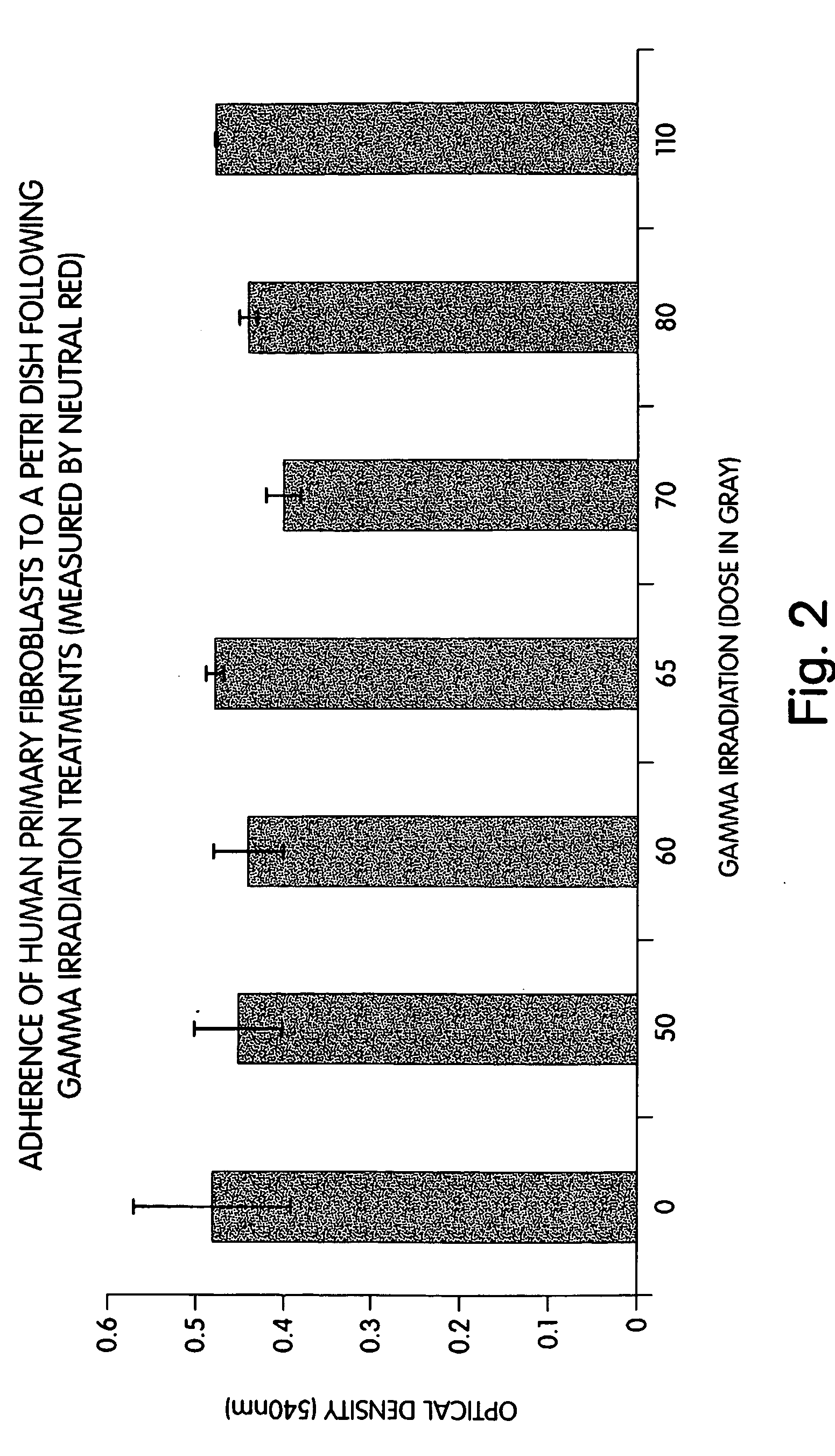
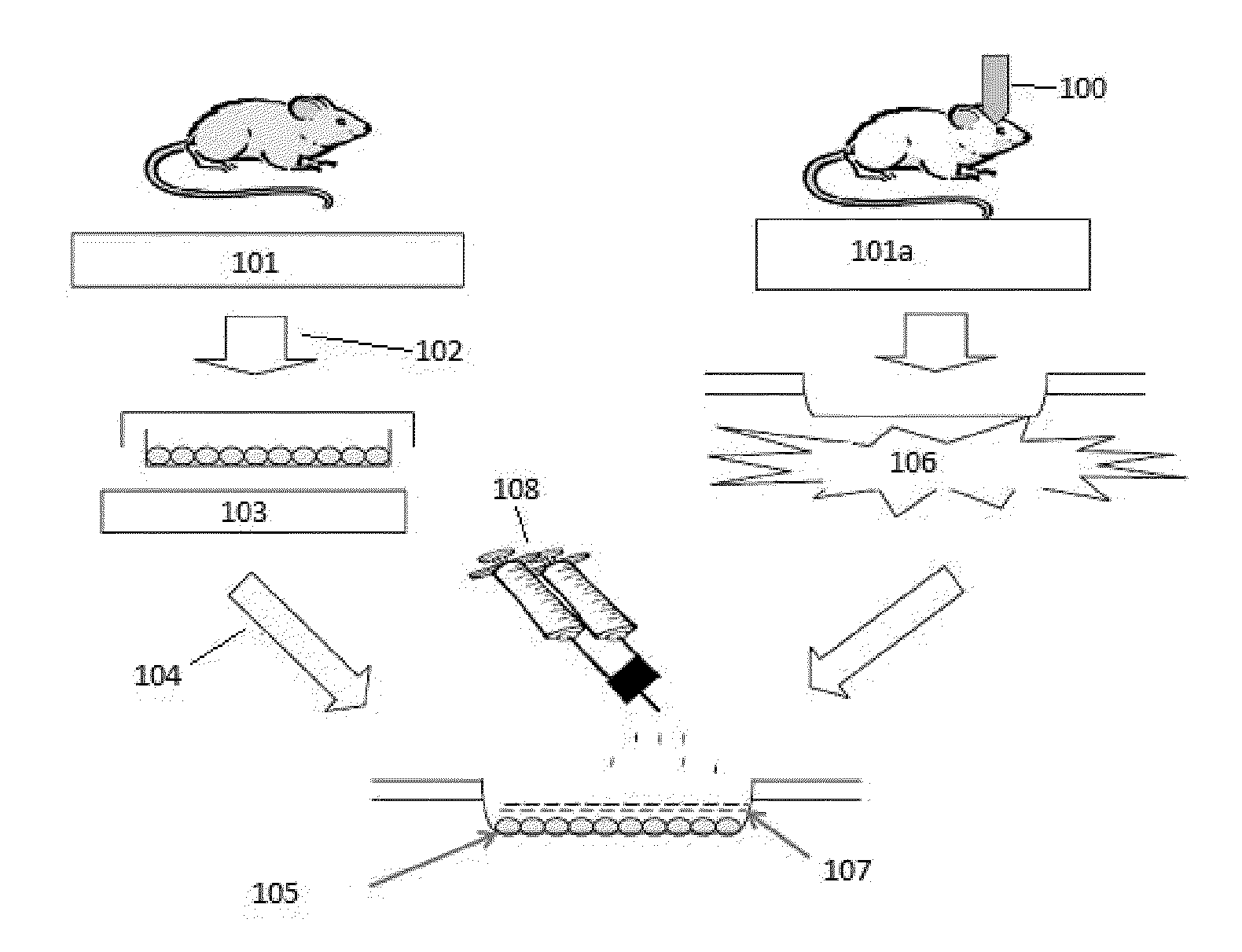
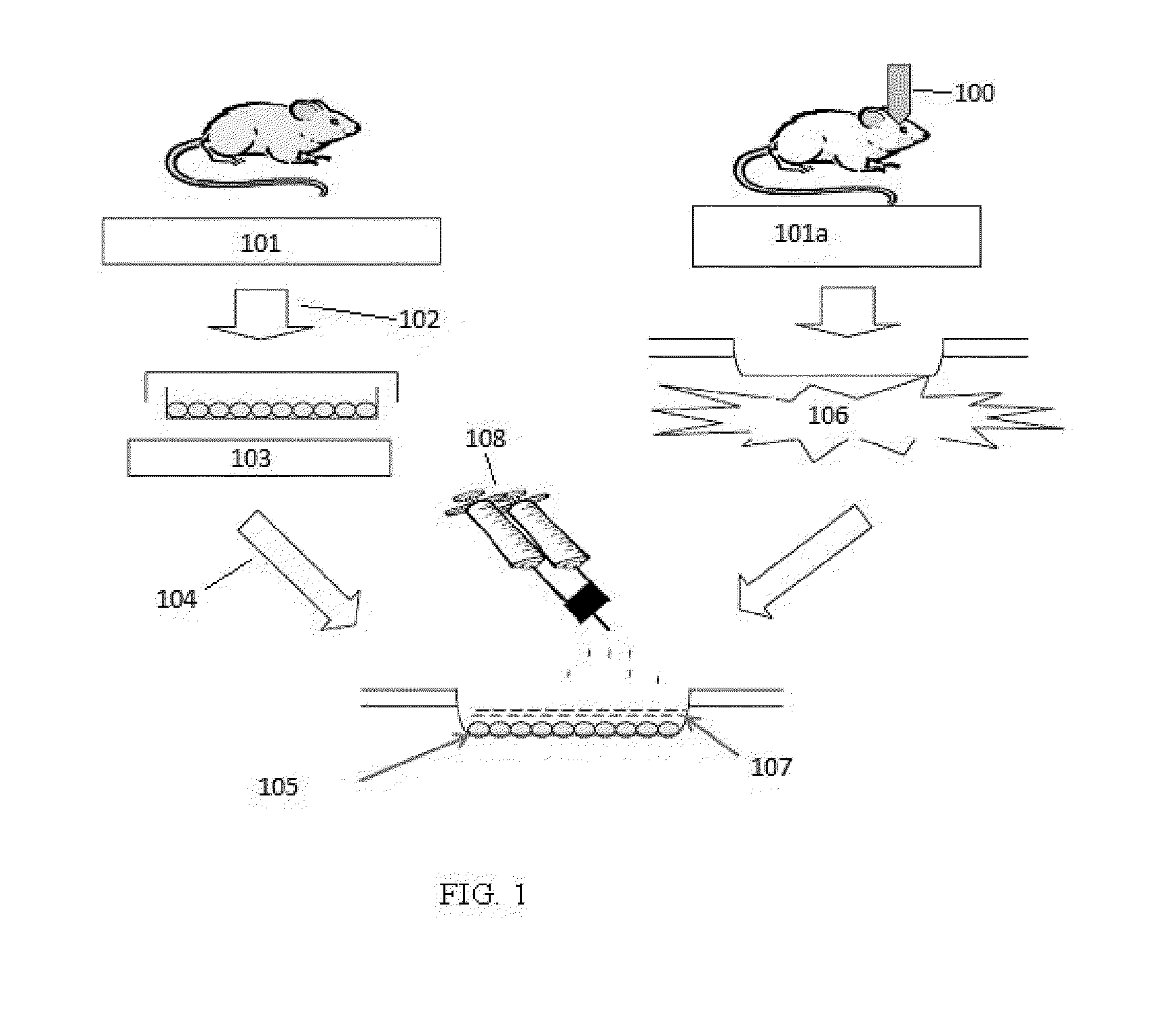
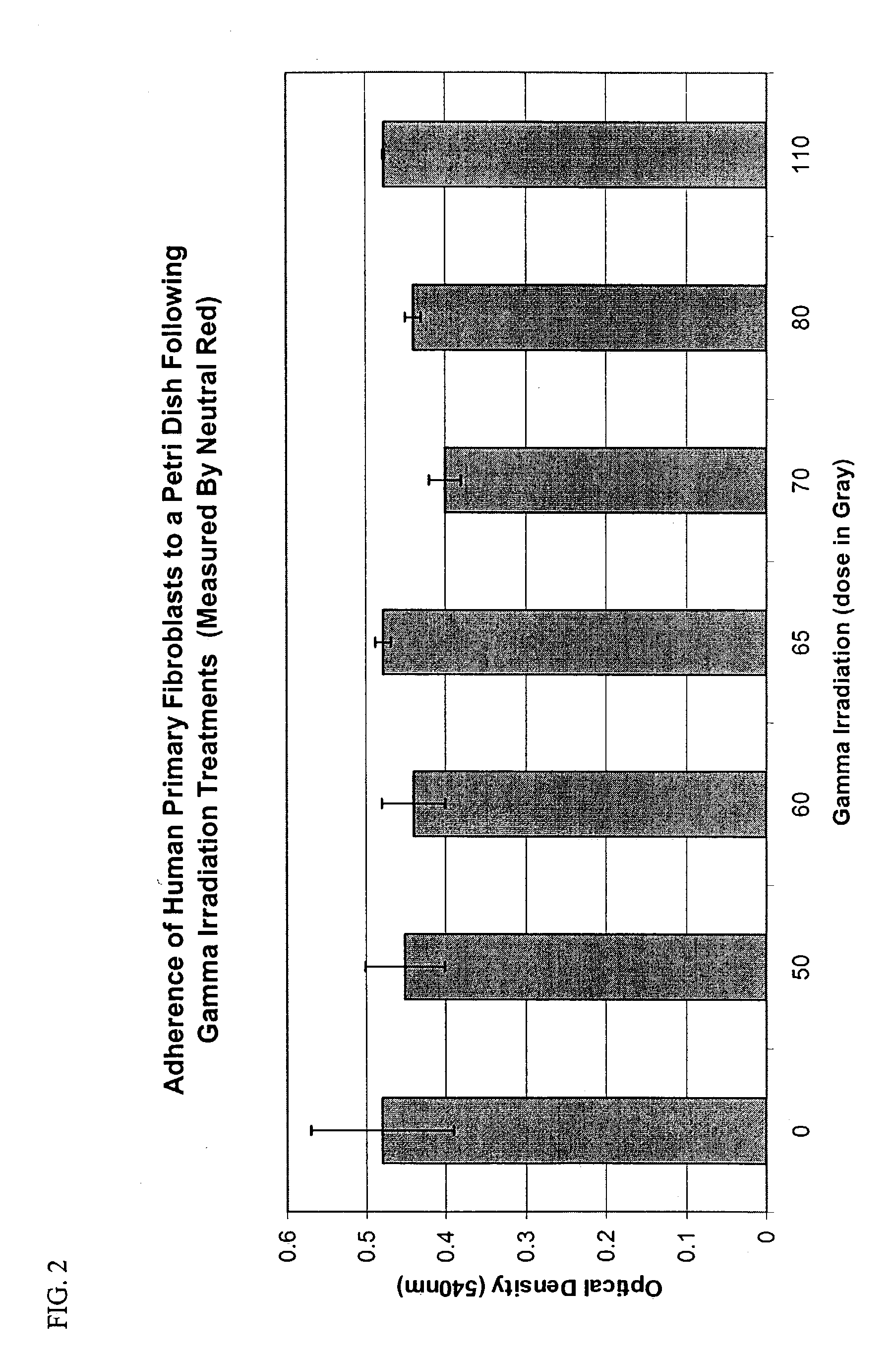
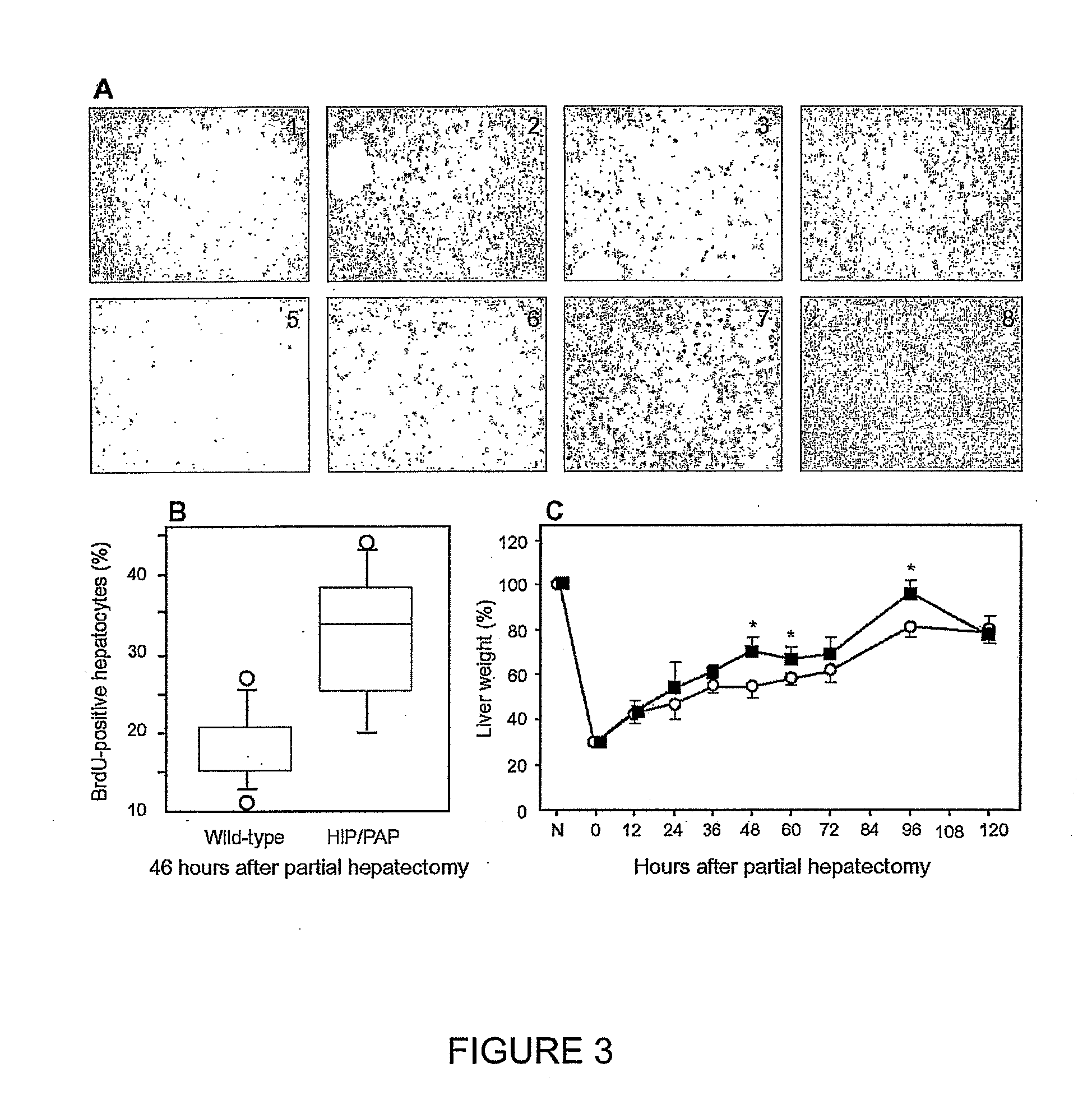
Methods of stimulating liver regeneration
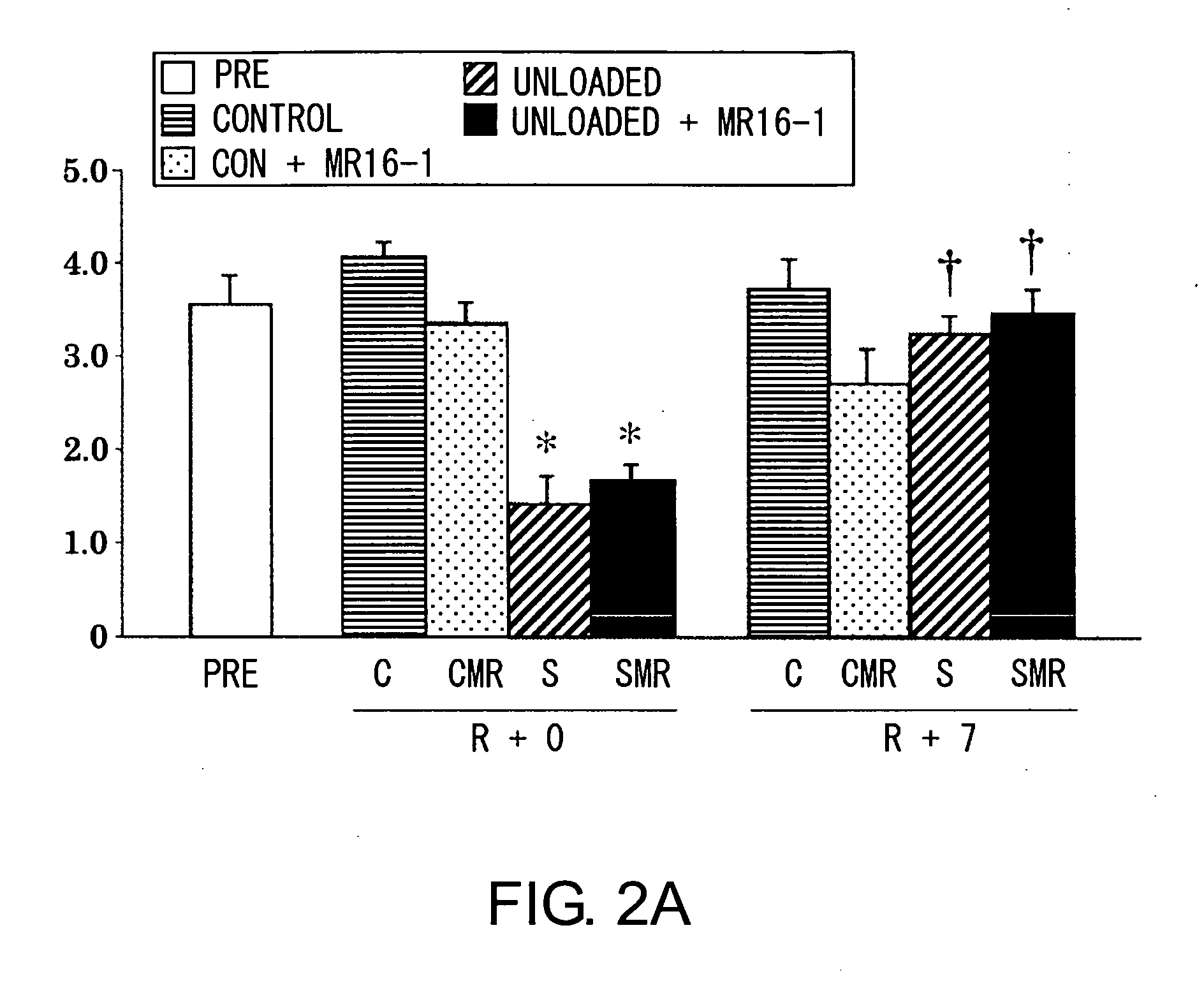
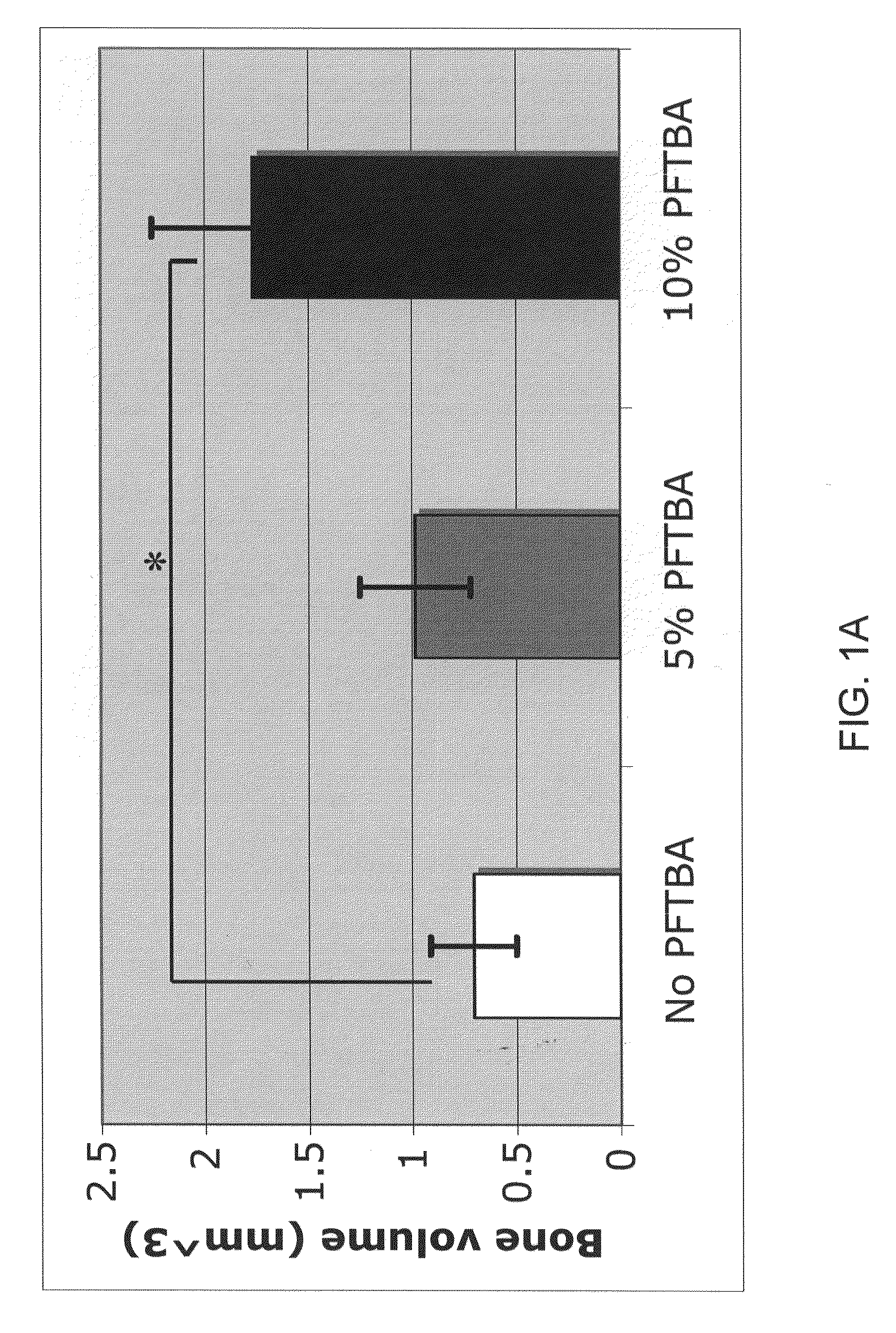
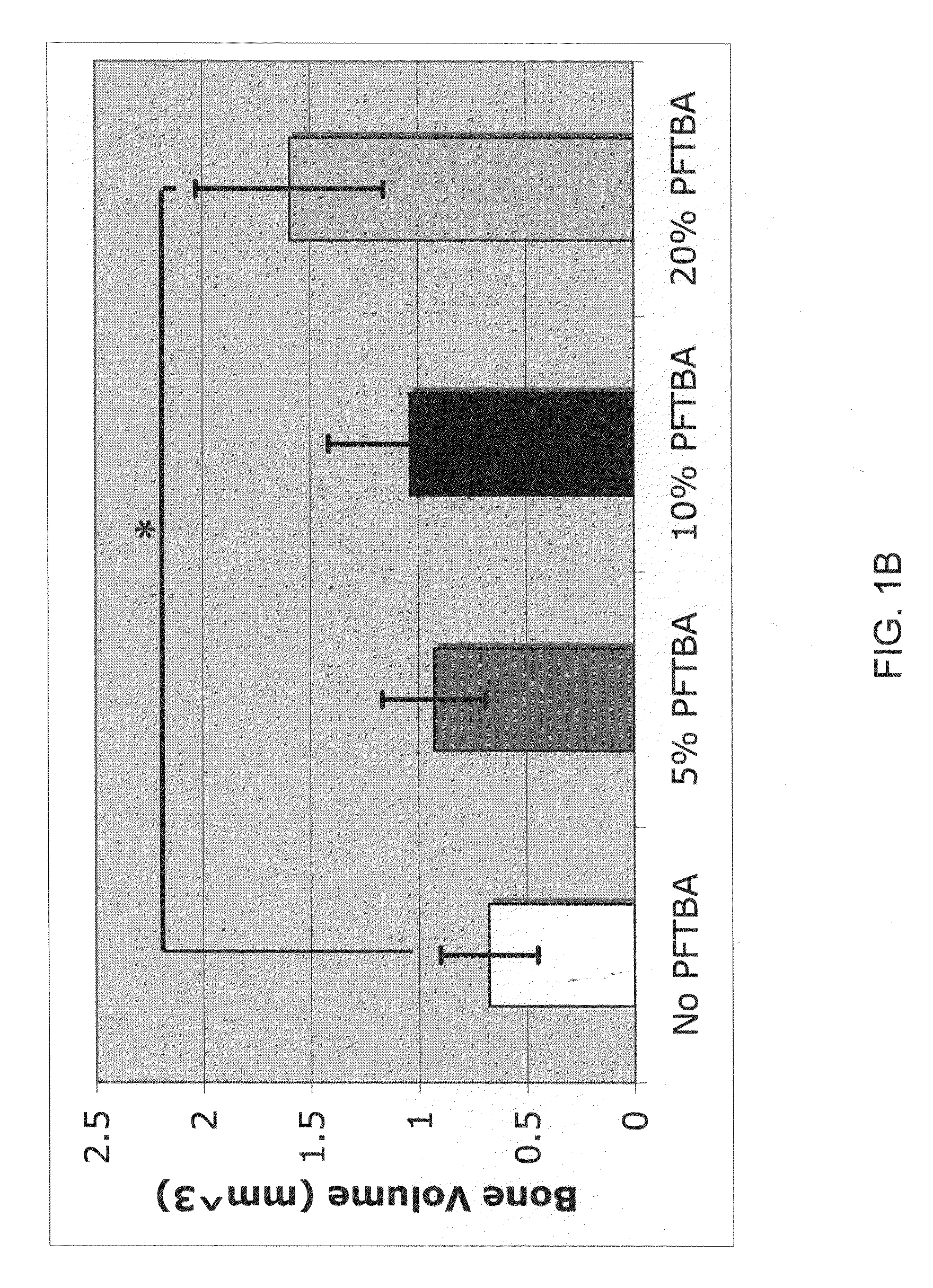
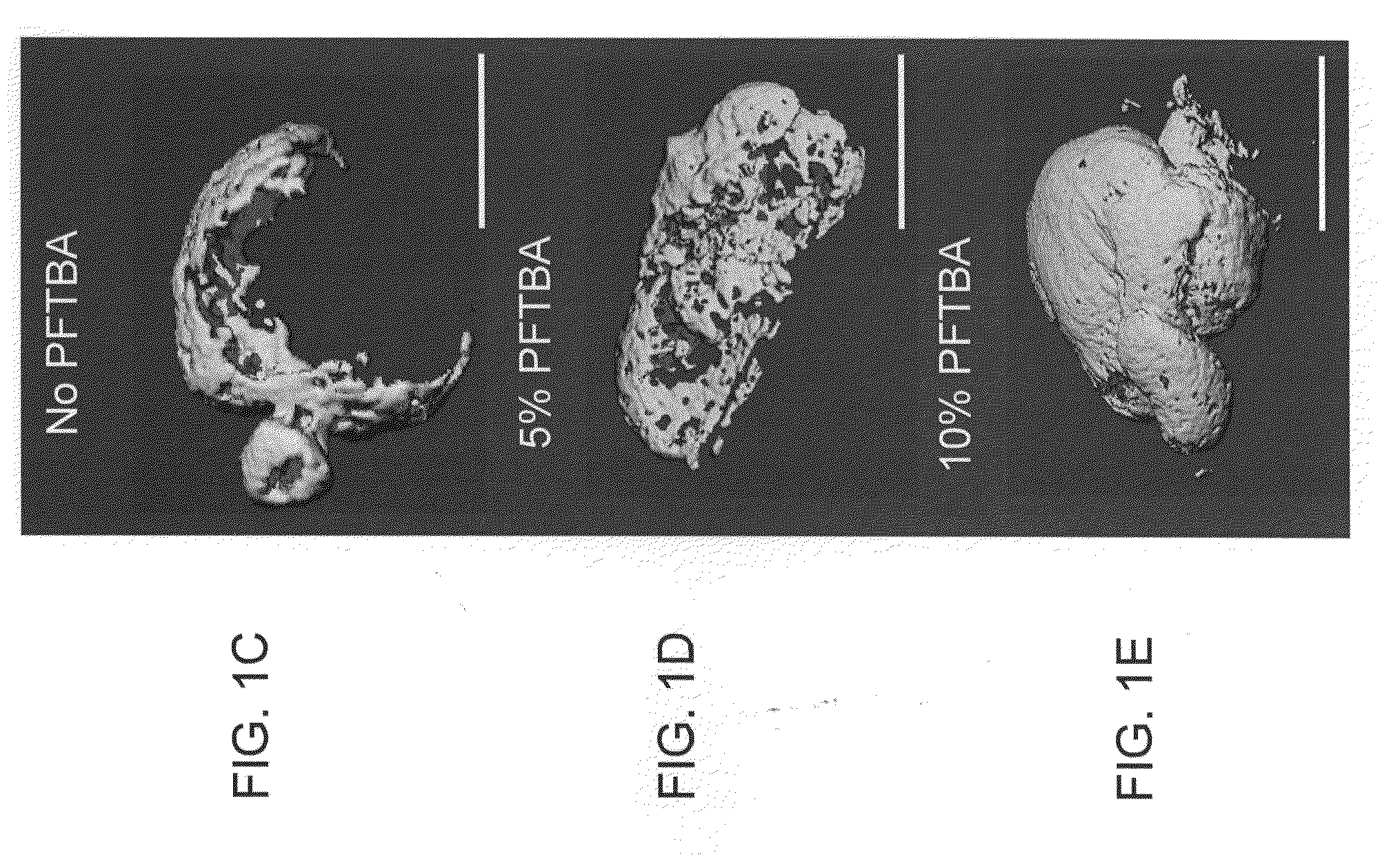
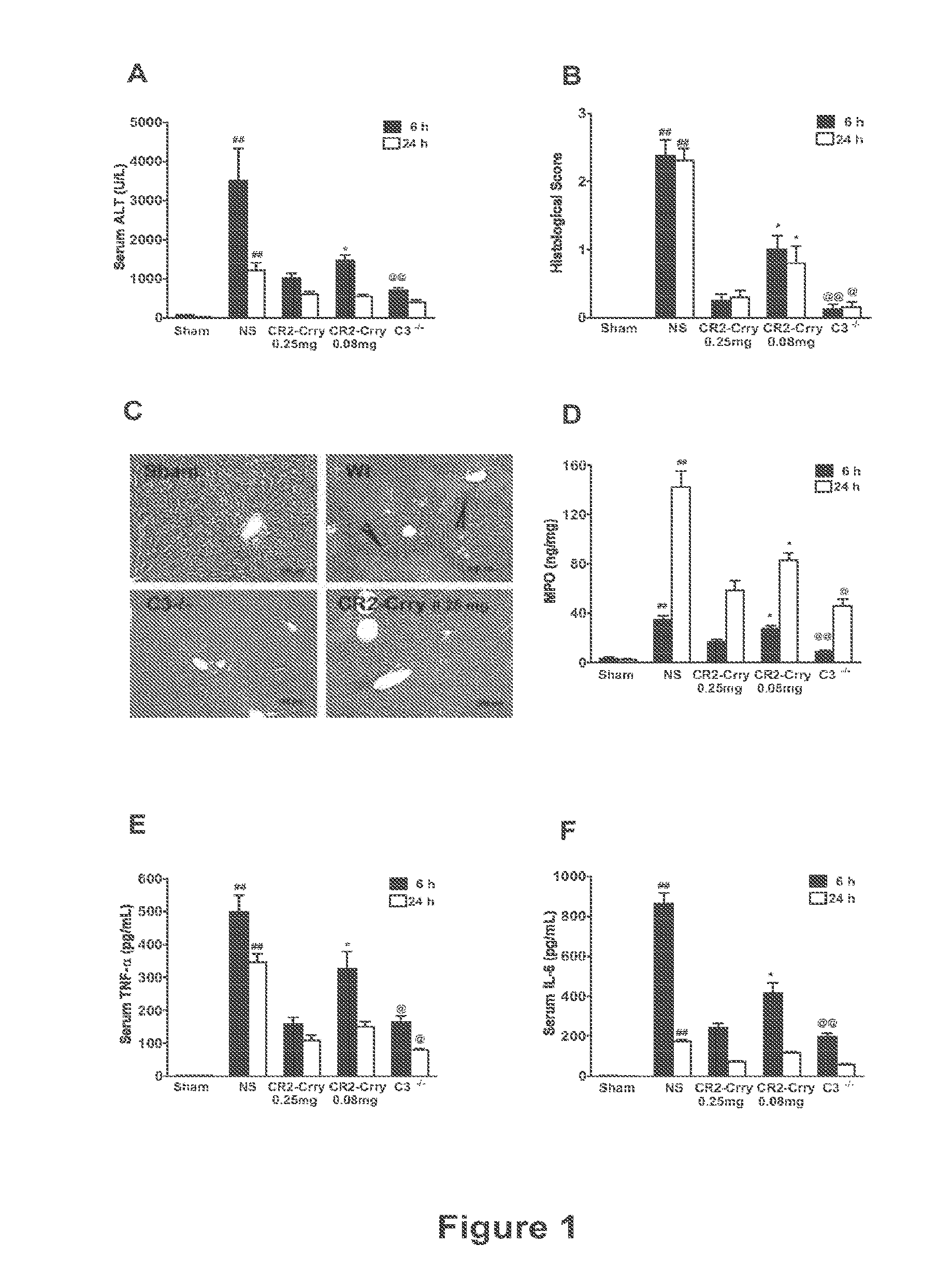
InactiveUS20120171206A1Reduce activationPromoting liver regenerationDigestive systemSaccharide peptide ingredientsSize liverPartial hepatectomy
Provided herein are methods and compositions, including pharmaceutical compositions, for stimulating liver regeneration after partial hepatectomy, massive liver resection and toxic injury, or following liver transplantation, including small-for-size liver transplantation, by inhibiting activation of complement.
Owner:MUSC FOUND FOR RES DEV
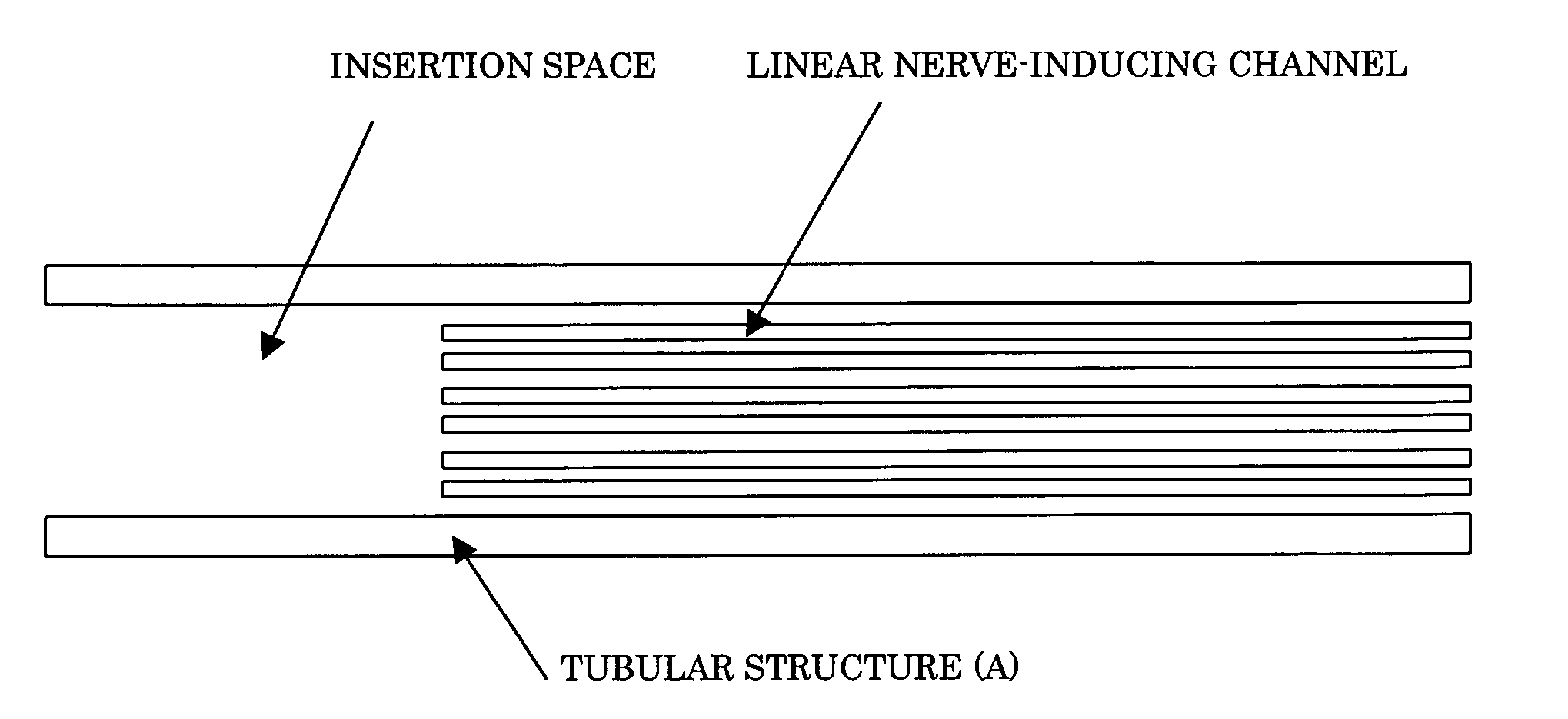
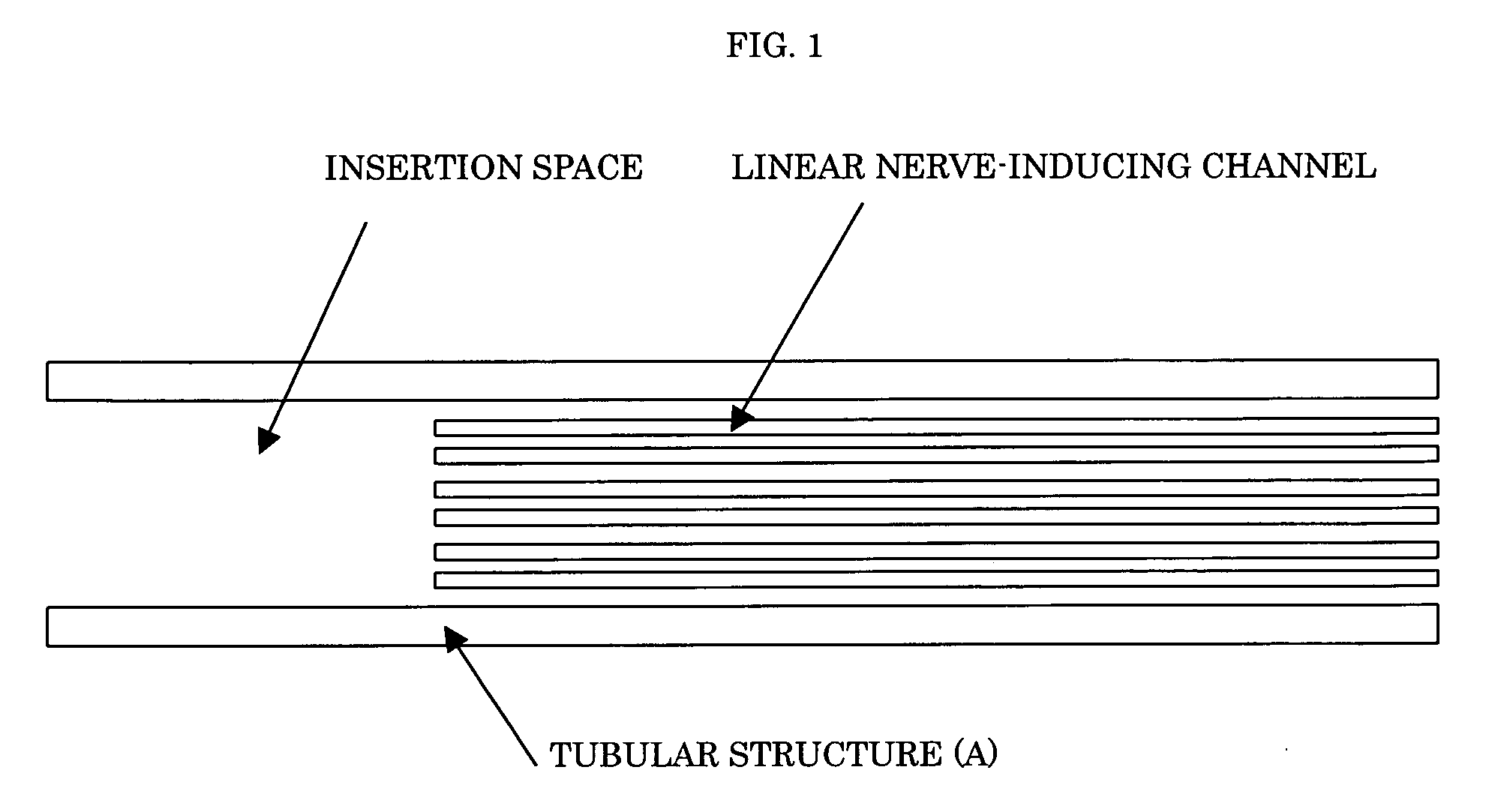
Nerve regeneration-inducing tube comprising
ActiveUS20060100647A1Guaranteed efficient growthPromote efficient proliferationTubular organ implantsTissue regenerationAnatomyAbsorbent material
The present invention provides a nerve regeneration-inducing tube in which nerve is inserted into a tubular structure and can be easily sutured and fixed without resort to any special instruments or operations, thereby allowing nerve cells to efficiently proliferate and grow in the correct direction. The nerve regeneration-inducing tube of the present invention includes: a tubular structure (A) made of a biodegradable material or a bioabsorbable material and provided inside with a matrix (B) having linear nerve-inducing channels and being made of a biodegradable material or a bioabsorbable material; and a definite space part provided at one end of the tubular structure (A).
Owner:NIPRO CORP +1
Methods and compositions for tissue regeneration
InactiveUS20060121002A1Reestablish integrityInhibit excessive scar formationPeptide/protein ingredientsGenetic material ingredientsCell-Extracellular MatrixInjury mouth
The present invention provides the use and composition of matter of angiogenic or other growth / cytokine factors expressed by mixtures of allogeneic human cell strains or lines of various types and stages of differentiation. Also provided are unencapsulated preparations (mixed with or applied to extracellular matrix material or synthetic biocompatible substances) for the purpose of temporary application to wounds or defects in the skin or other tissues for the restoration of blood supplying connective tissue to enable organ-specific cells to reestablish organ integrity as well as to inhibit excessive scar formation.
Owner:SMITH & NEPHEW INC
Adipose-derived stem cells for tissue regeneration and wound healing
Compositions and methods for promoting tissue regeneration, particularly skin regeneration, with adipose-derived stem cells are provided. Additionally methods and compositions for promoting tissue regeneration with adipose-derived stem cell side population cells are provided. The adipose-derived cells are administered in a tissue regenerating effect amount optionally with a bioactive agent. Additionally the adipose derived cells can be autologous or syngeneic.
Owner:PRIMEGEN BIOTECH LLC
System and method for tissue generation and bone regeneration
ActiveUS8518123B2Promotes bone formationIncrease surface areaImpression capsBone implantDamages tissueSurgical site
A system and method for the repair of damaged tissue and bones, congenitally missing tissue / cosmetic reconstruction of tissue is described. The system has a layered porous structure with a sufficiently large area of exposed pores to promote neo-vascularization as well as bone and tissue formation. The disclosed porous implant system can contain bioactive agents necessary for rapid tissue formation and keep ingrowth of unwanted tissue out of the implant surgical site. The implant can be reinforced with an additional, stronger polymer layer and / or may include an endoskeleton or exoskeleton for dimensional stability.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ARKANSAS
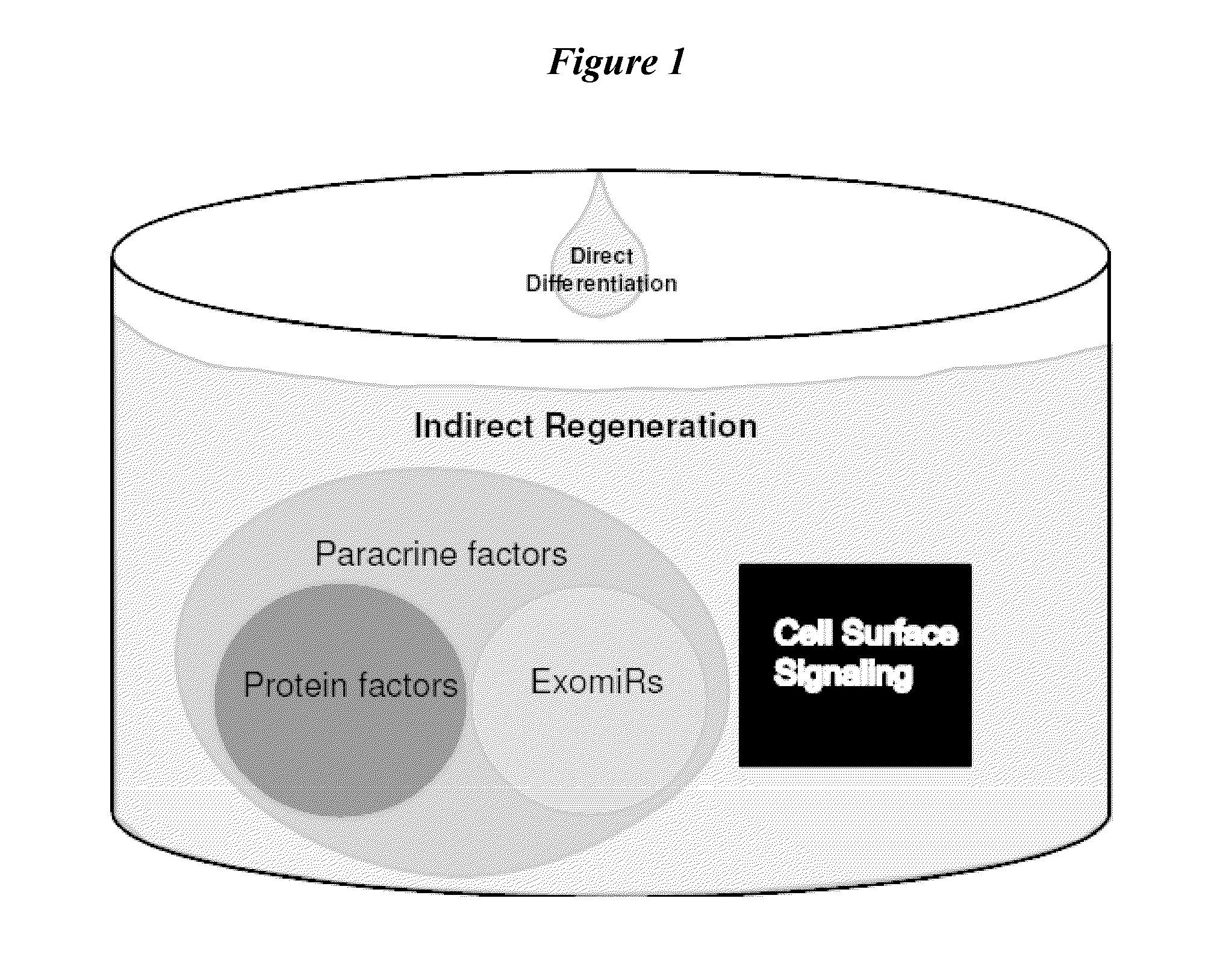
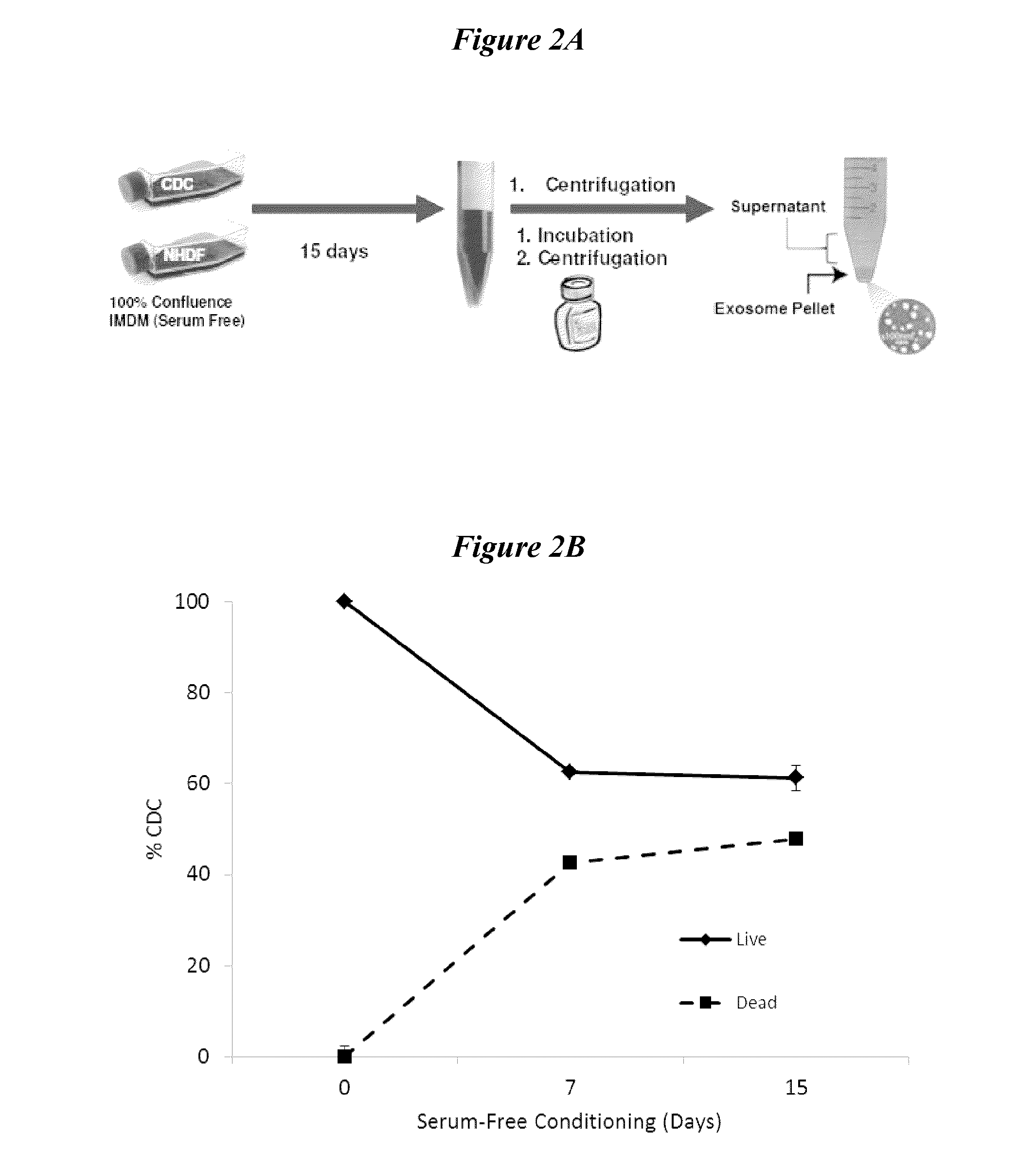
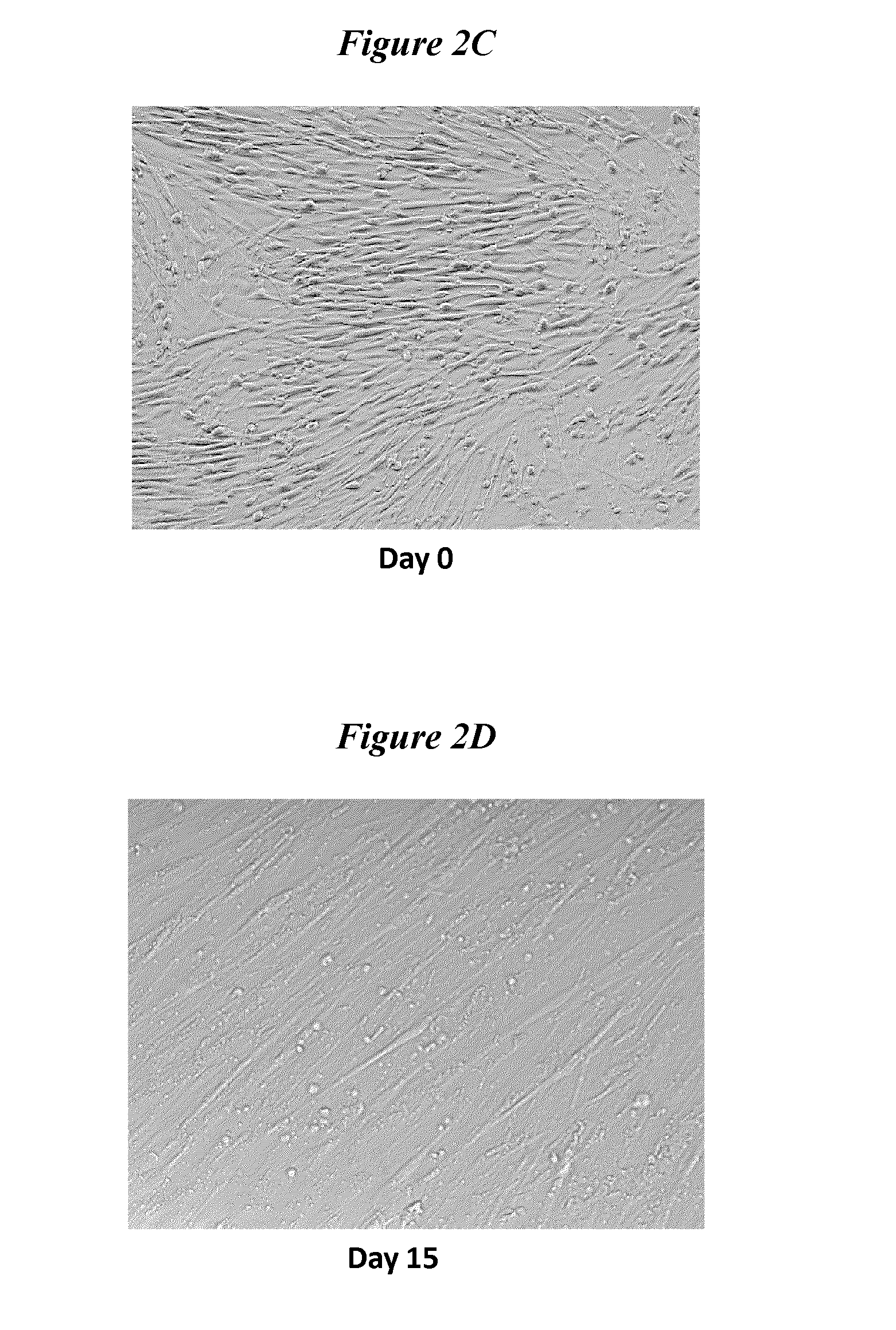
Exosomes and micro-ribonucleic acids for tissue regeneration
ActiveUS20150203844A1Improve survivabilityFunction increaseOrganic active ingredientsAntipyreticMicroRNATissue viability
Several embodiments relate to methods of repairing and / or regenerating damaged or diseased tissue comprising administering to the damaged or diseased tissues compositions comprising exosomes. In several embodiments, the exosomes comprise one or more microRNA that result in alterations in gene or protein expression, which in turn result in improved cell or tissue viability and / or function.
Owner:CEDARS SINAI MEDICAL CENT
Exogenous matrix-supported topical application of stem cells to organ surface
A process of tissue regeneration is provided that includes the administration of a stem cell topically to a surface of a tissue. The tissue being in vivo in a subject or in vitro. The tissue has a biocompatible matrix applied around the stem cell and in contact with the surface. After sufficient time, the stem cell infiltrates and regenerates the tissue. A composition is also provided that includes an injured or diseased tissue with a plurality of stem cells proximal to the tissue surface in a biocompatible. A process of increasing a level of cytokines, chemokines, growth factors, anti-apoptotic or anti-necrotic factors in a tissue is provided that includes the administration of a stem cell topically to a surface of a tissue in a biocompatible matrix. After sufficient time, the stem cell releases soluble pro-regenerative factors to increase the levels in the tissue.
Owner:BANYAN BIOMARKERS INC
Use of heat shock activators for tissue regeneration
InactiveUS20100204093A1Reduce probabilityHigh expressionBiocidePeptide/protein ingredientsHeat shockMedicine
The present invention generally provides therapeutic compositions and methods for treating a disease, disorder, or injury characterized by a deficiency in cell number. The method involves inducing a heat shock response in tissue or organ effected by disease and recruiting stem cells to repair or regenerate the disease-effected tissue.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
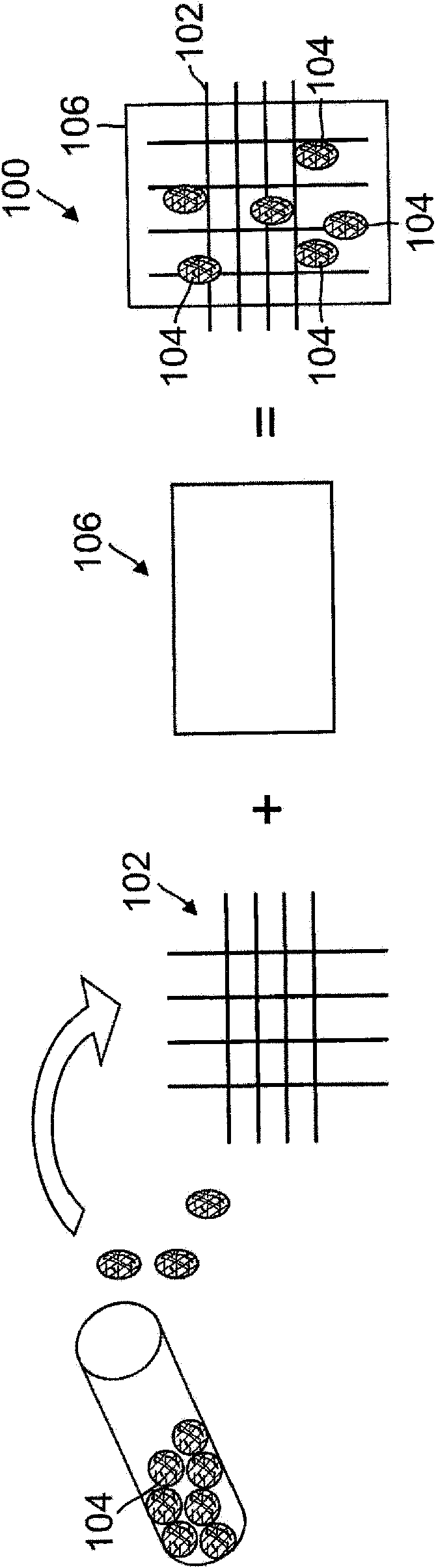
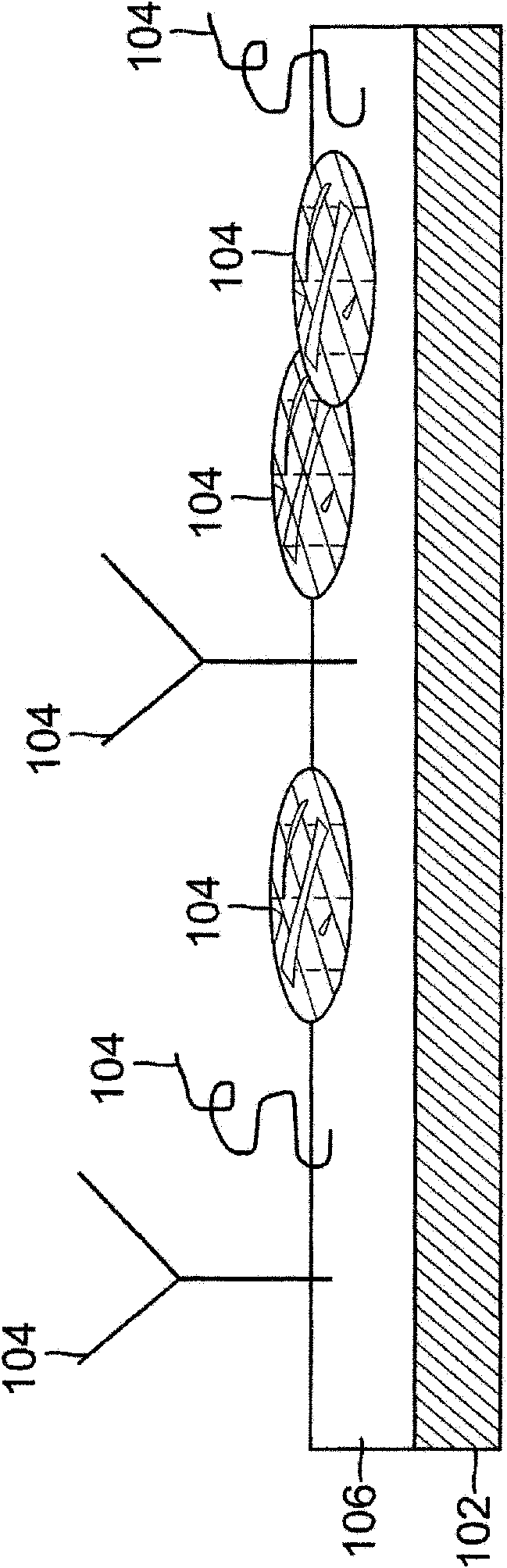
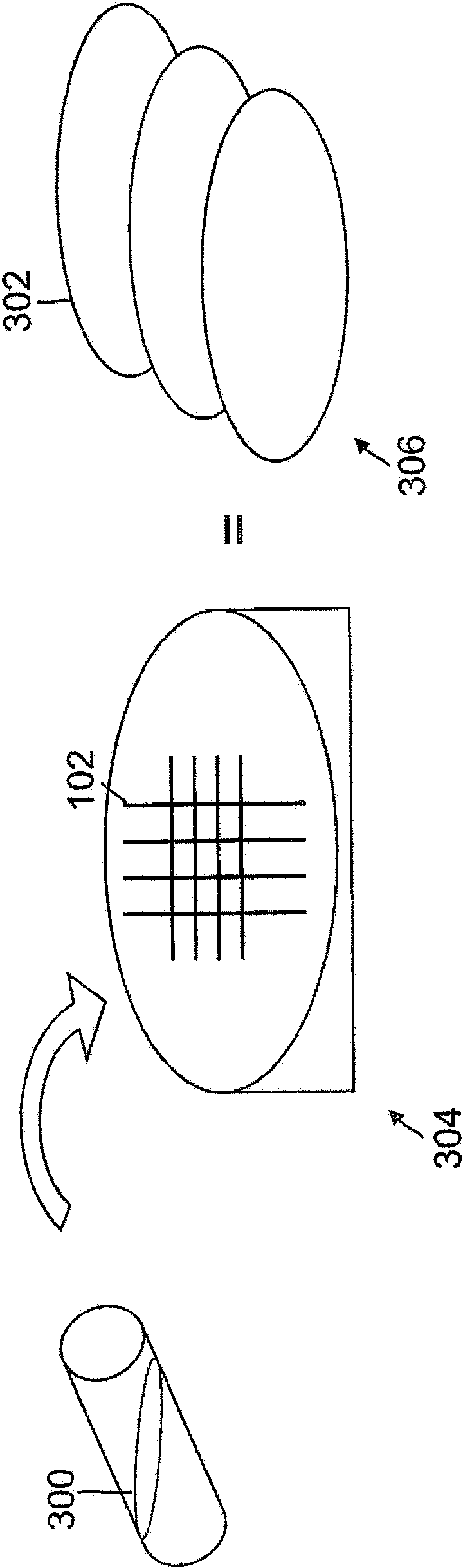
Programmed-release, nanostructured biological construct for stimulating cellular engraftment for tissue regeneration
The present invention relates to a biologically engineered construct, comprising a polymeric biomatrix (100) designed with a nanophase texture (106) and a therapeutic agent (104 or 300) for the purpose of tissue regeneration and / or controlled delivery of regenerative factors and therapeutic substances after it is implanted into tissues, vessels, or luminal structures within the body. The therapeutic agent (104 or 300) may be a therapeutic substance (104) or a biological agent (300), such as antibodies, ligands, or living cells. The nanophase construct is designed to maximize lumen size, promote tissue remodeling, and ultimately make the implant more biologically compatible. The nano-textured polymeric biomatrix (100) may comprise one or more layers containing therapeutic substances (104) and / or beneficial biological agents (300) for the purpose of controlled, physiological, differential substance / drug delivery into the luminal and abluminal surfaces of the vessel or lumen, and the attraction of target molecules / cells that will regenerate functional tissue. The topographic and biocompatible features of this layered biological construct provides an optimal environment for tissue regeneration along with a pro grammed -release, drug delivery system to improve physiological tolerance of the implant, and to maximize the cellular survival, migration, and integration within the implanted tissues.
Owner:杰伊·N·沙皮拉 +1
Methods of treatment using an EP2 selective receptor agonist
InactiveUS20050203086A1Reduce generationEasy to integrateBiocideAmide active ingredientsMetastatic bone tumorCartilage repair
The present invention relates to methods of treating pulmonary hypertension, facilitating joint fusion, facilitating tendon and ligament repair, reducing the occurrence of secondary fracture, treating avascular necrosis, facilitating cartilage repair, facilitating bone healing after limb transplantation, facilitating liver regeneration, facilitating wound healing, reducing the occurrence of gastric ulceration, treating hypertension, facilitating the growth of tooth enamel or finger or toe nails, treating glaucoma, treating ocular hypertension, and repairing damage caused by metastatic bone disease using an EP2 selective receptor agonist.
Owner:PFIZER INC
Methods and compositions for tissue regeneration
The present invention provides the use and composition of matter of angiogenic or other growth / cytokine factors expressed by mixtures of allogeneic human cell strains or lines of various types and stages of differentiation. Also provided are unencapsulated preparations (mixed with or applied to extracellular matrix material or synthetic biocompatible substances) for the purpose of temporary application to wounds or defects in the skin or other tissues for the restoration of blood supplying connective tissue to enable organ-specific cells to reestablish organ integrity as well as to inhibit excessive scar formation.
Owner:SMITH & NEPHEW INC
Method to improve hydroxyapatite implantation and stimulate bone regeneration
InactiveUS20020127261A1Favorable to tissue growth of tissueReduced affinityBone implantJoint implantsApatiteTissue Compatibility
Hydroxyapatite is treated by a combination of nitridation and the application of bone morphogenetic protein to improve the tissue compatibility and affinity of the hydroxyapatite, rendering the hydroxyapatite more useful as a material for biomedical implants.
Owner:RGT UNIV OF CALIFORNIA
Composition and method for dermal regeneration
InactiveUS20100255076A1Augmentation and regeneration and healingPromotes collagen productionBiocideCosmetic preparationsPharmaceutical formulationExcipient
The present invention relates to glucommannan oligosaccharides and polysaccharides that possess one or more properties selected from skin regeneration, wound healing and skin augmentation. More particularly, the invention relates to the use of glucomannan oligosaccharides and polysaccharides for one or more of skin regeneration, wound healing and skin augmentation agents in a mammal. The invention also relates to a pharmaceutical formulation for treating skin of a subject comprising an active component comprising glucomannan in a suitable diluent, excipient or physical form such as dermal scaffold or sponge, the active component being capable of promoting an accumulation of fibroblasts in the skin and stimulating production of collagen in the skin.
Owner:ULTRACEUTICALS R&D
Cosmetic dermabrasion treatment system
The present invention provides a complementary three-step dermabrasion system that provides treatment for skin conditions such as acne scars, wound scars, and other visually unappealing skin disfigurements, which constitutes the following, (1) Dermal Resurfacing with an adhesive composition, (2) Dermal Polishing with a polishing composition, and (3) Dermal Regeneration with a collagen-building and cell-proliferative composition.
Owner:GUPTA SHYAM K
Nervous duct and membrane with fixed nerve regeneration promoting agent with surface modified by plasma and their preparations
InactiveCN1439432AMeet the requirements of subsequent immobilization reactionsMeet the requirements of immobilization reactionCatheterCoatingsFiberAnatomy
A nerve catheter or membrane for regenerating or repairing nerve features that a plasma surface modification method is used to immobilize the nerve regeneration promoter on its surface and the fibres, non-woven fabric, or sponge filler which are coaxially inserted in said catheter to induce the nerve cells to grow along surface, resulting in higher effect.
Owner:DONGHUA UNIV
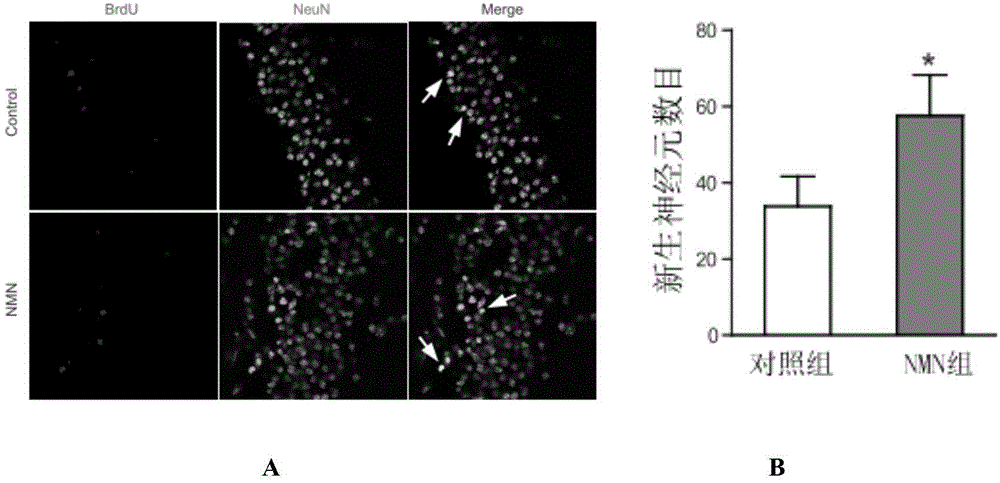
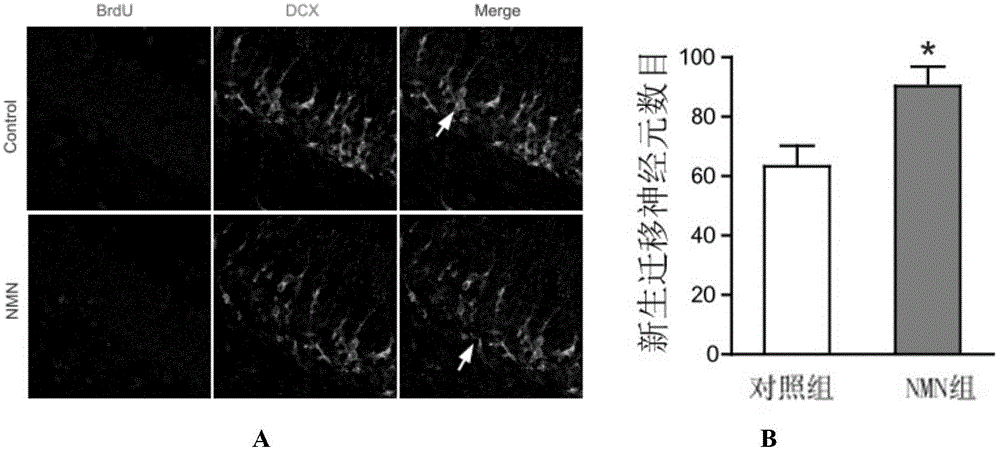
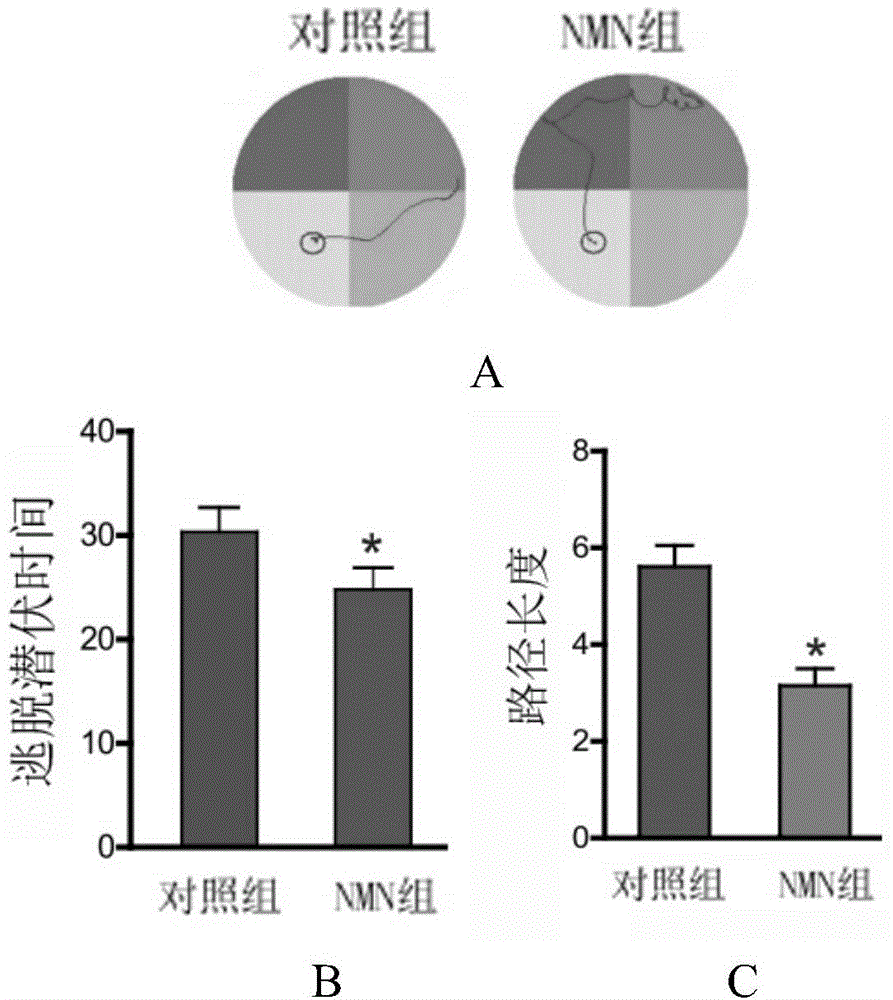
Application of nicotinamide mononucleotide in preparation of medicines for promotion of nerve regeneration after cerebral ischemia
ActiveCN104367587APromote differentiationImprove neurological impairmentOrganic active ingredientsNervous disorderNerves regenerationNicotinamide mononucleotide
The invention relates to the technical field of medicines. The present invention provides application of nicotinamide mononucleotide in preparation of medicines for promotion of nerve regeneration after cerebral ischemia, and the nicotinamide mononucleotide can be used as an active pharmaceutical ingredient and a pharmaceutically acceptable supplementary material for preparation of medicine compositions. The nicotinamide mononucleotide compound itself is an endogenous protective substance, and the available data do not report the adverse reaction, so that the nicotinamide mononucleotide as a medicine or a health product is high in safety and wide in therapeutic window.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
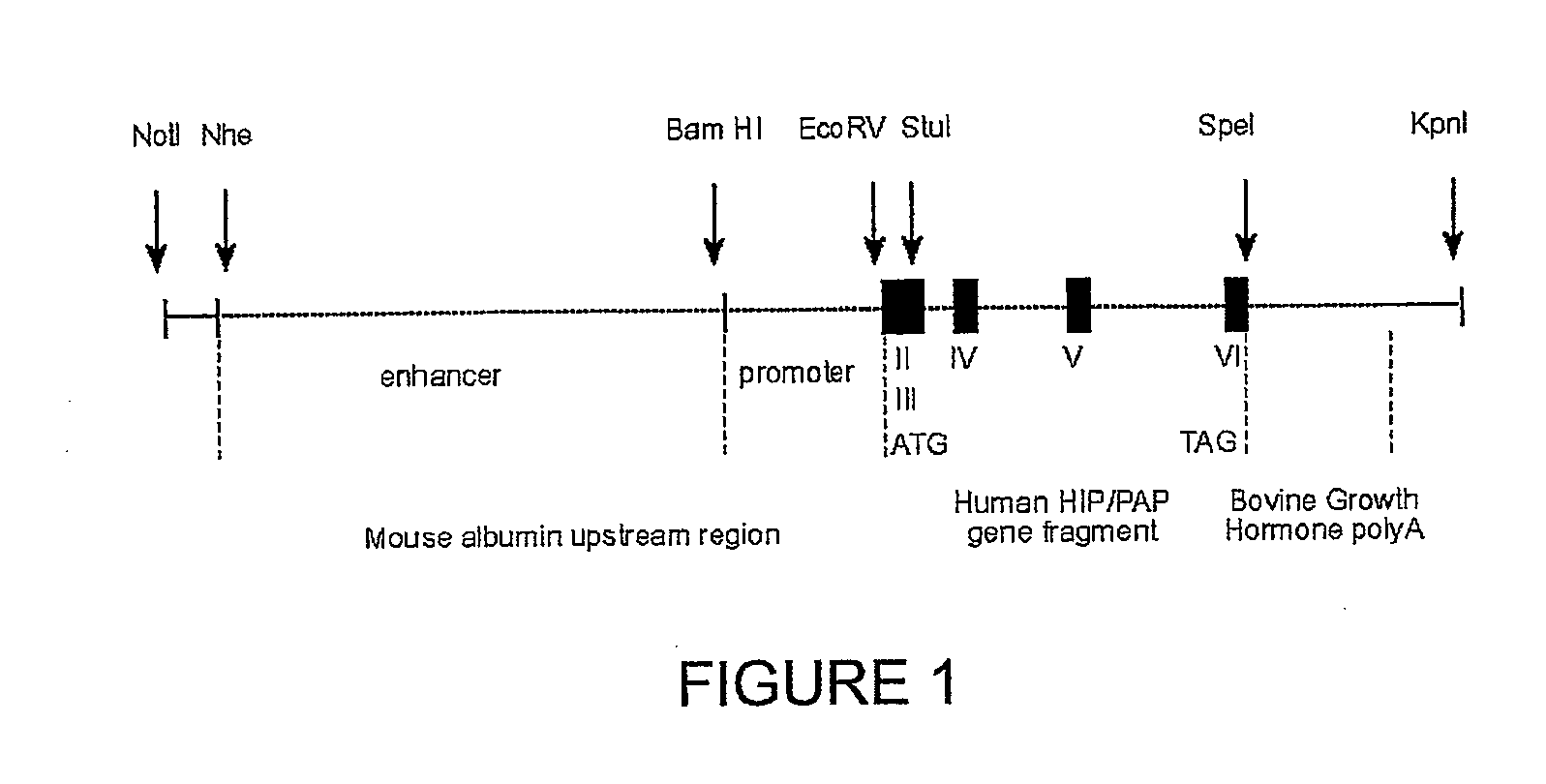
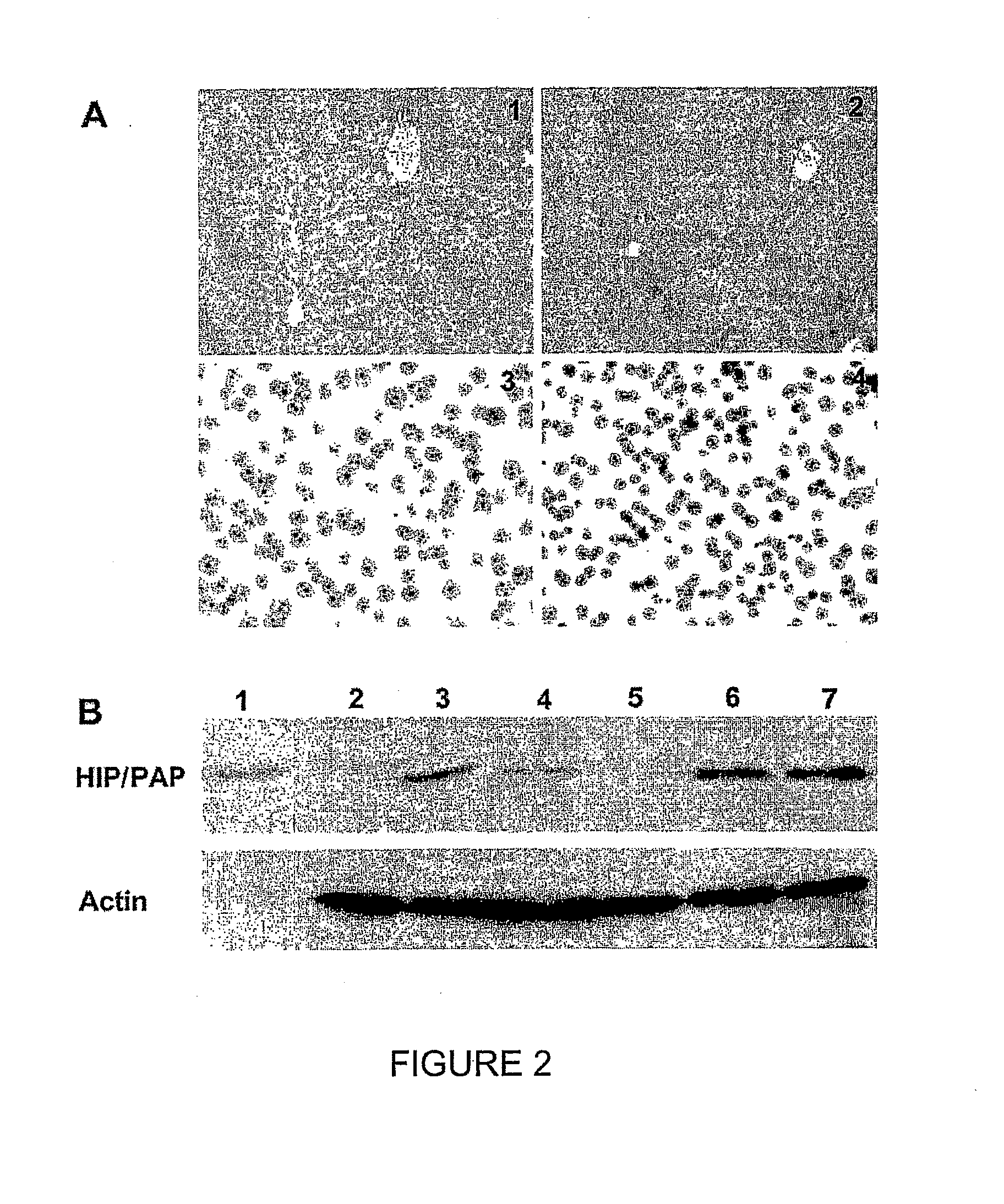
HIP/PAP Polypeptide Composition for Use in Liver Regeneration and for the Prevention of Liver Failure
Owner:VIVO BIOSCI CO LTD
Neural restoration material combined with acellular nerve application
ActiveCN105169486AGood biocompatibilityNo toxicityProsthesisBiocompatibility TestingNeurotrophic factors
The invention aims to provide a neural restoration material combined with the acellular nerve application. The neural restoration material is prepared from nerves of an acellular allogene or acellular heterogeneous living organism, a restoration material, a microtubulin inhibitor, neurotrophic factors and stem cells. The neural restoration material disclosed by the invention has good biocompatibility, can provide nutrition supplies needed by nerve regeneration and restoration, is capable of maintaining an optimal physicochemical and biology microenvironment for nerve regeneration for a long time and has dual functions of preventing nerve tumor formation and promoting defect nerve restoration.
Owner:WENZHOU MEDICAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com