Patents
Literature
591 results about "Adjacent segment" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Adjacent segment disease is a progressed form of adjacent segment degeneration, a condition that often occurs after a spinal fusion or other back surgery.
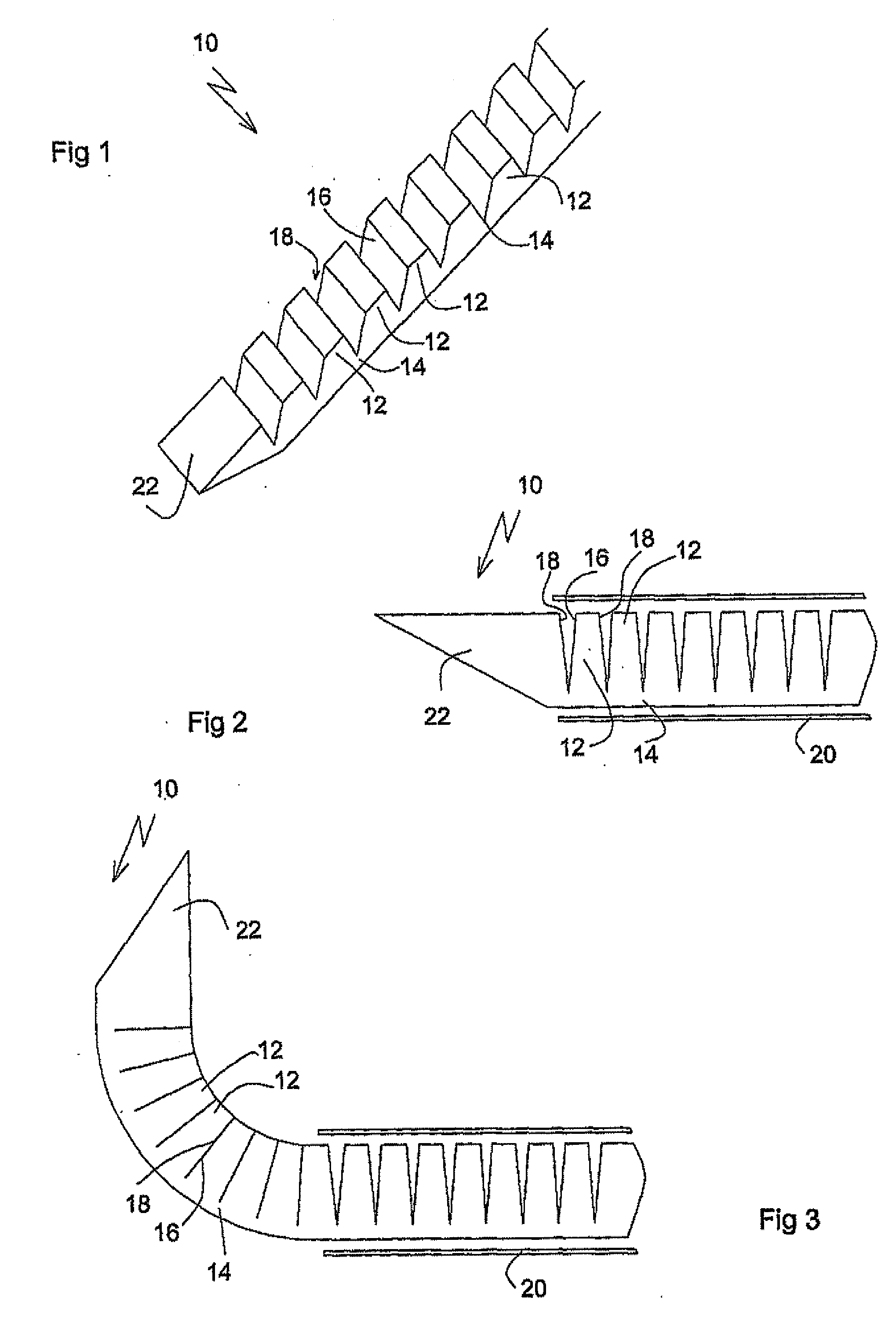
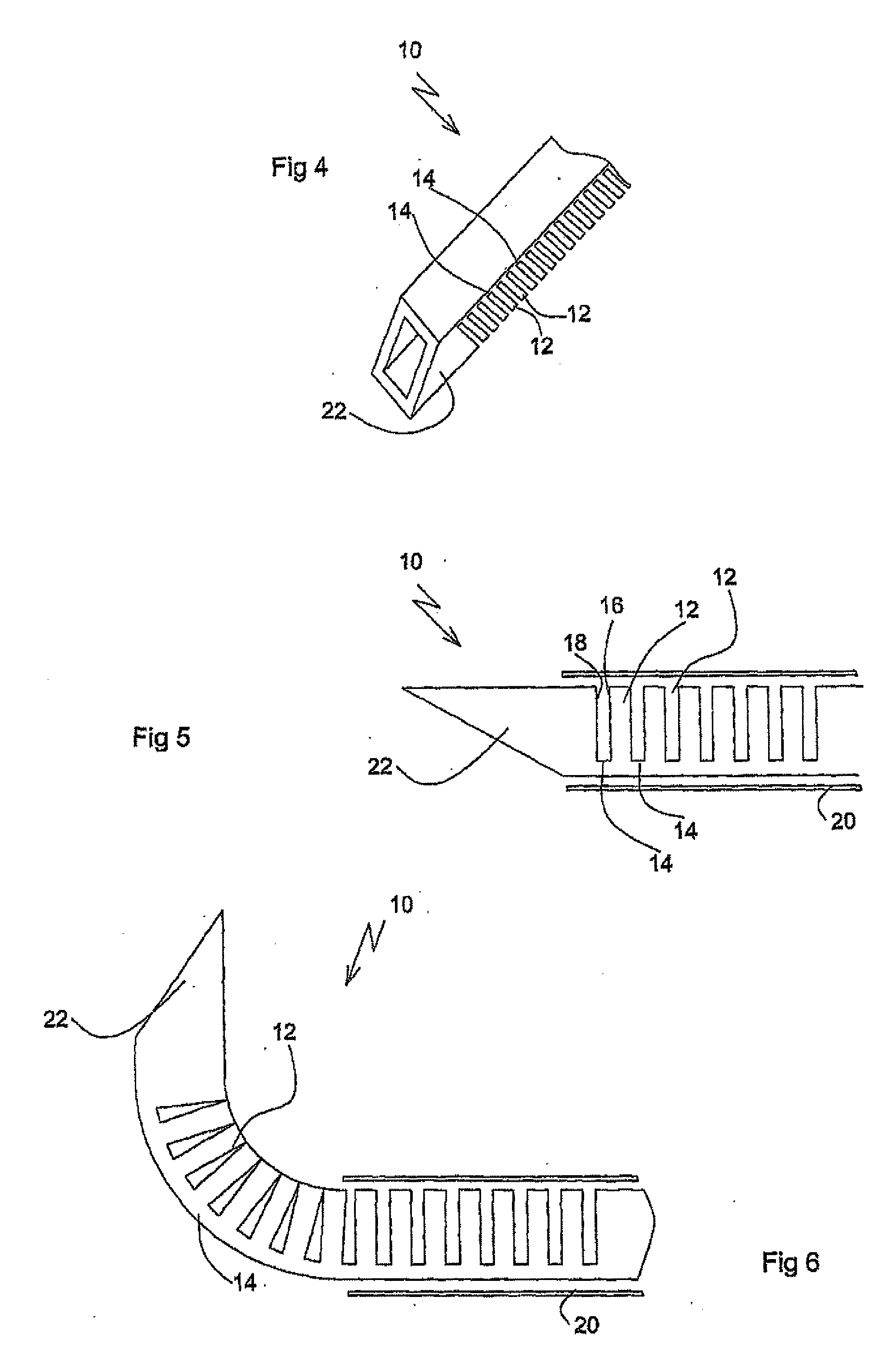
Activated polymer articulated instruments and methods of insertion
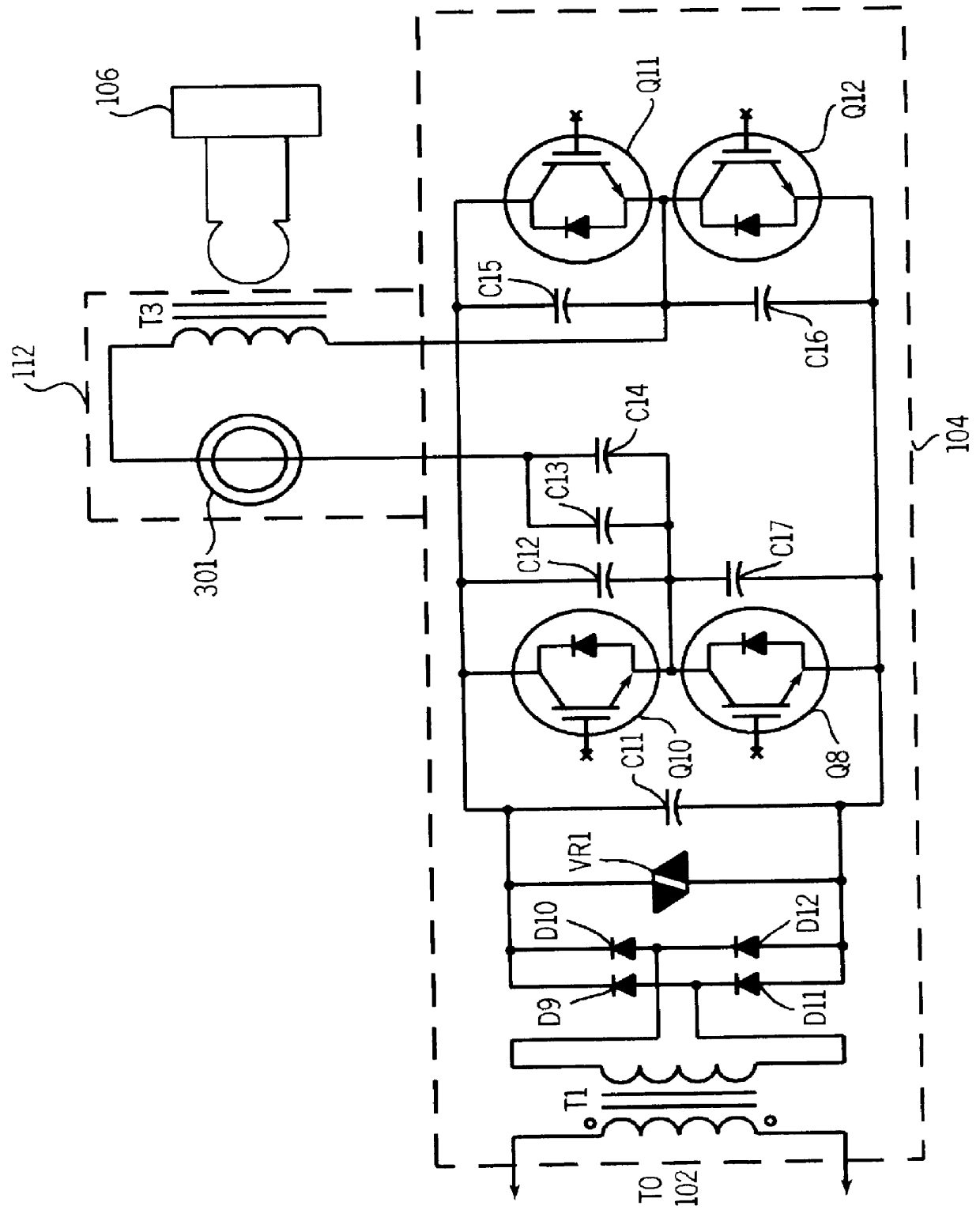
An electro-polymeric articulated endoscope and method of insertion are described herein. A steerable endoscope having a segmented, elongated body with a manually or selectively steerable distal portion and an automatically controlled proximal portion can be articulated by electro-polymeric materials. These materials are configured to mechanically contract or expand in the presence of a stimulus, such as an electrical field. Adjacent segments of the endoscope can be articulated using the electro-polymeric material by inducing relative differences in size or length of the material when placed near or around the outer periphery along a portion of the endoscope.
Owner:INTUITIVE SURGICAL
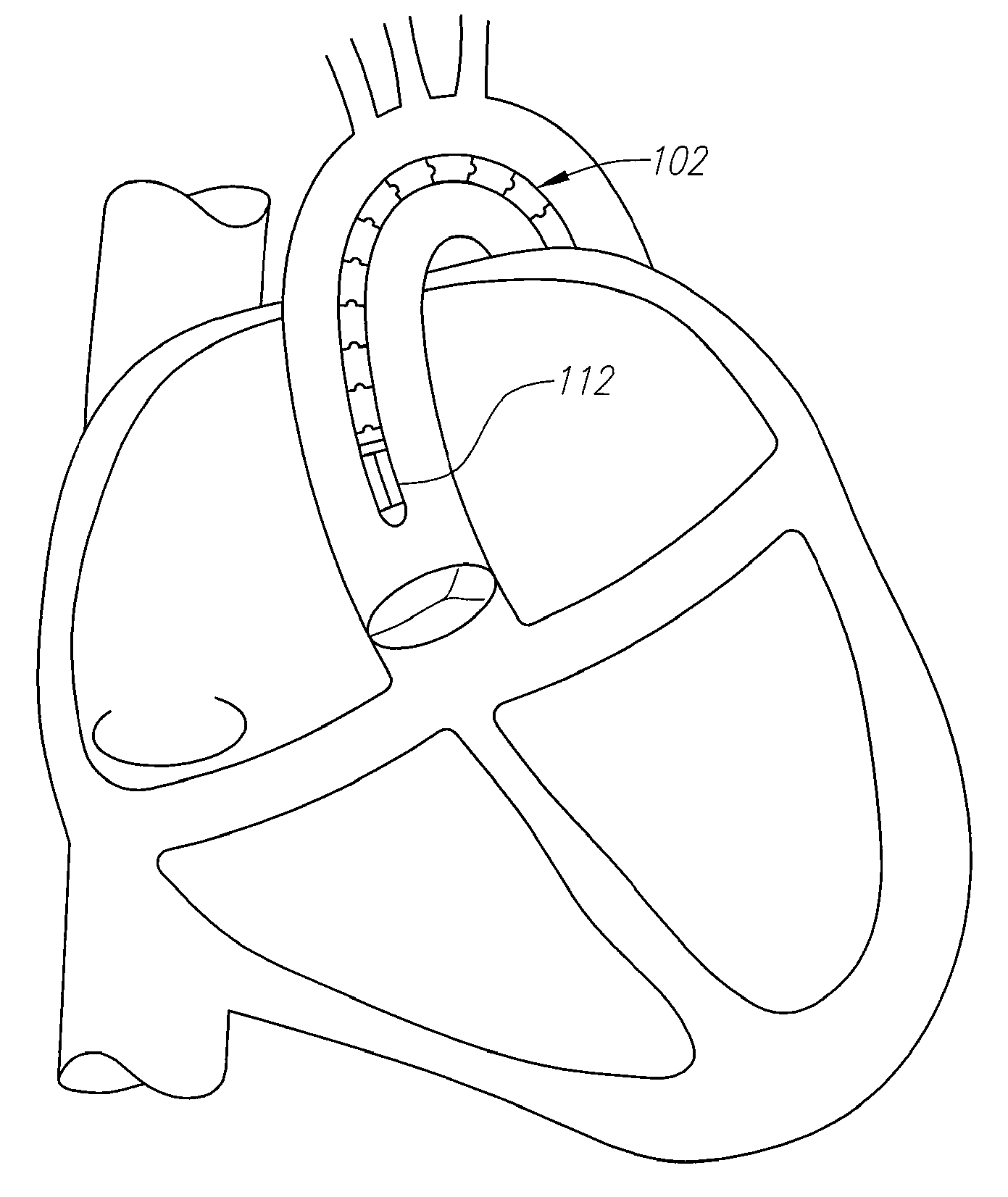
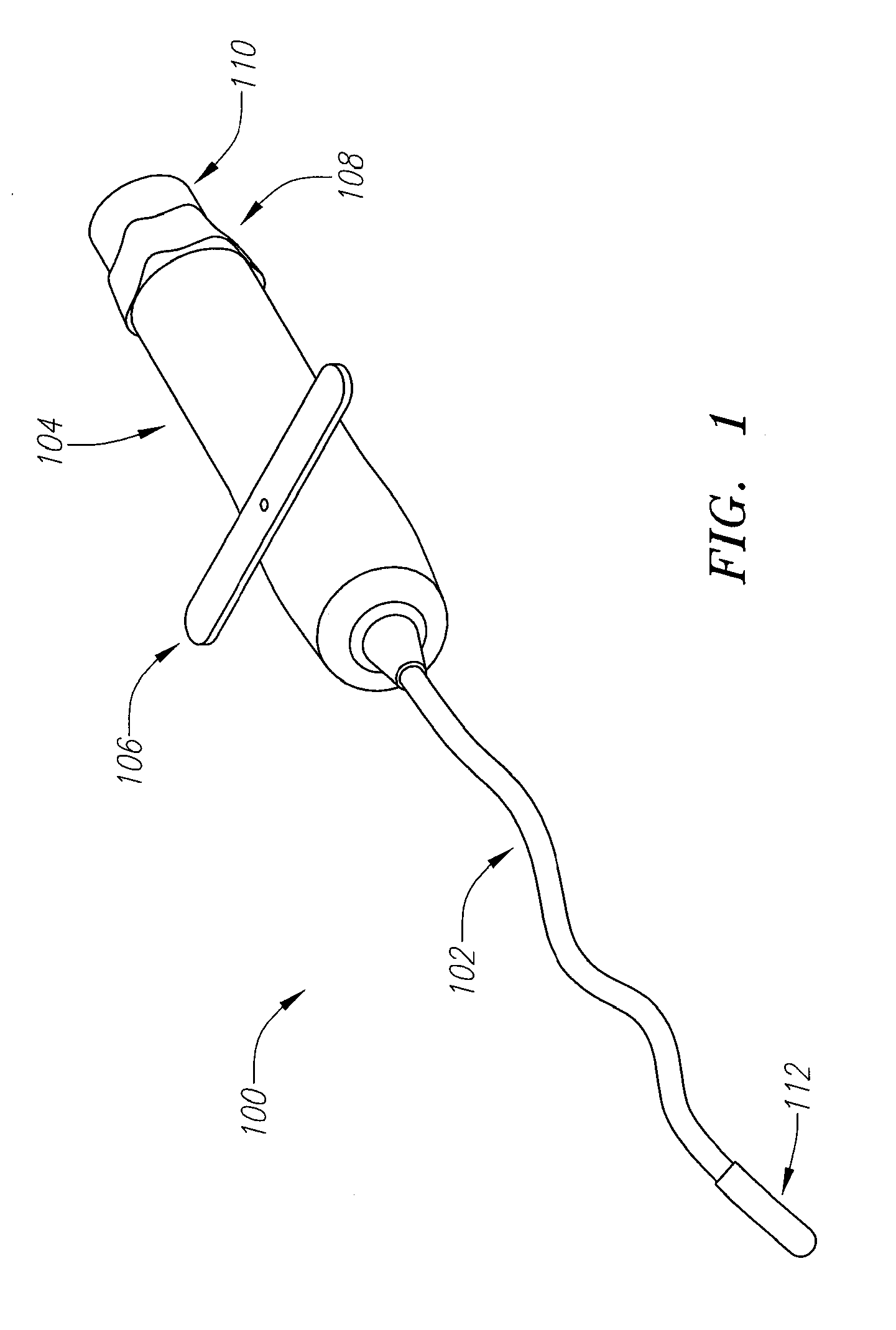
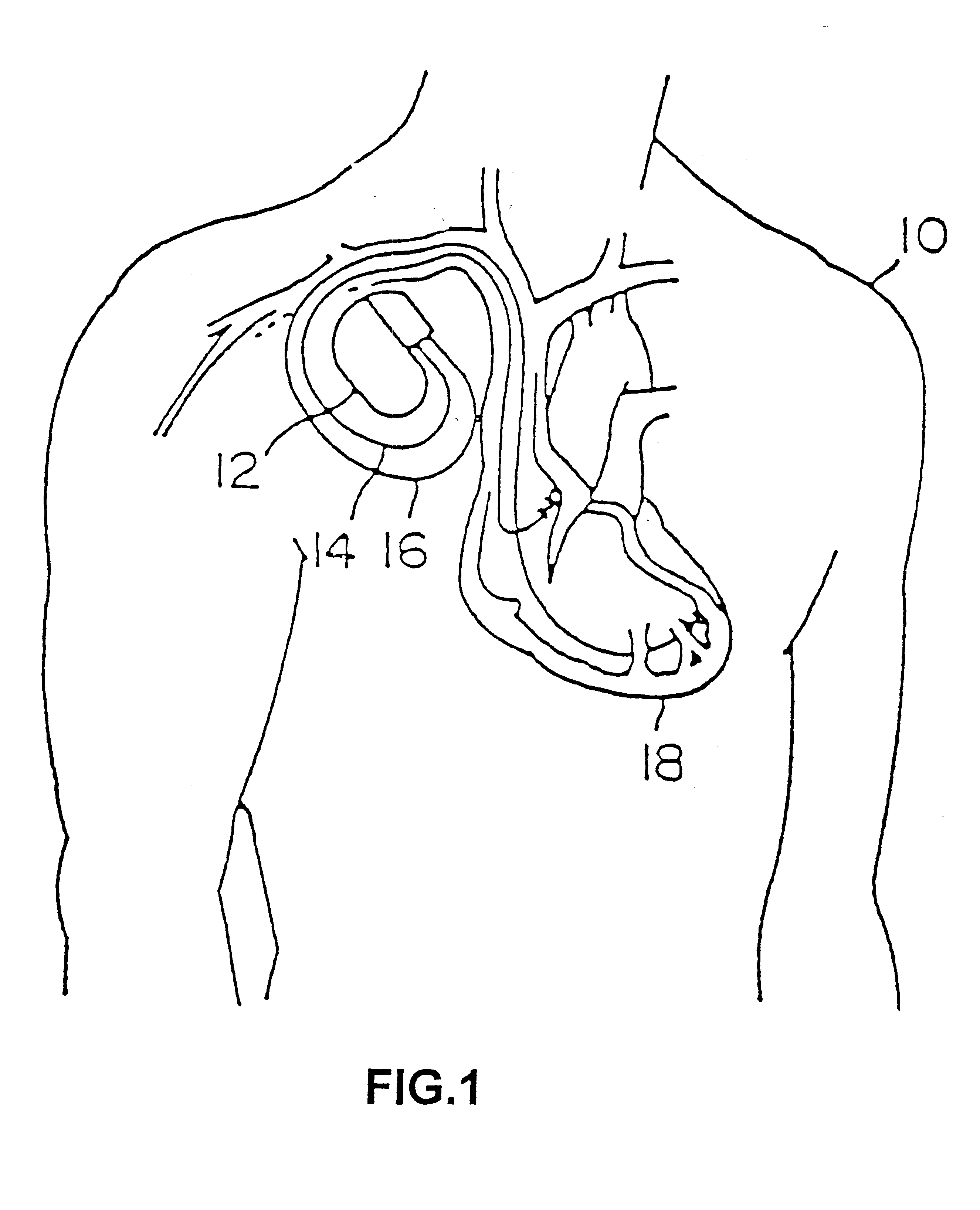
Percutaneous transcatheter repair of heart valves
Apparatus, systems, and methods are provided for repairing heart valves through percutaneous transcatheter delivery and fixation of annuloplasty rings to heart valves. An annuloplasty ring includes an outer hollow member including a plurality of segments. Adjacent segments cooperate with one another to change the outer hollow member from an elongate insertion geometry to an annular operable geometry. The annuloplasty ring also includes an internal anchor member located at least partially within the outer hollow member. The internal anchor member includes a plurality of anchors configured to attach the annuloplasty ring to tissue of a heart valve annulus. The internal anchor member is configured to move the plurality of anchors with respect to a plurality of windows in the outer hollow member to selectively deploy the plurality of anchors through the respective windows.
Owner:VALCARE INC
Deformable scaffolding multicellular stent
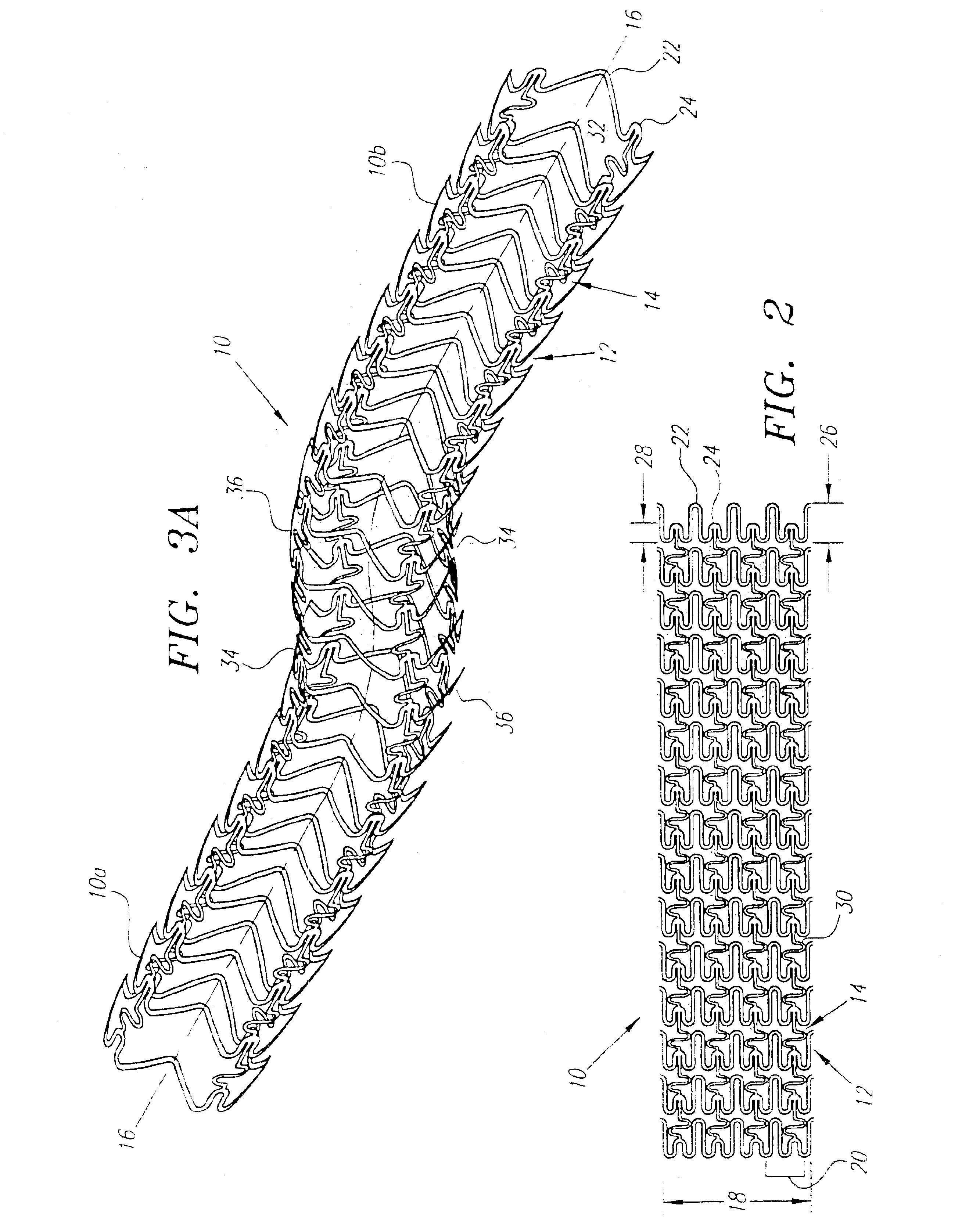
A plastically deformable stent for implantation within a body passage includes a plurality of cylindrical segments, and a plurality of connectors extending between adjacent segments. Each segment has an alternating pattern of curvilinear elements extending about its circumference, including first and second sets of curvilinear elements having different resistances to expansion, and preferably defining “U” shapes with alternating lengths that are connected to one another to define a substantially sinusoidal pattern. The connectors define a sinusoidal shape adapted to extend and compress axially substantially evenly when the adjacent segments are subjected to bending. The stent may be delivered on a device including an elongate member with a nose cone, an expandable member, and a proximal shoulder thereon, and an outer sheath for slidably receiving the elongate member therein. The outer sheath and / or nose cone may have perfusion holes for allowing continued perfusion of fluid during stent delivery. The device may be used in a method for implanting a stent within a curved region of a body passage, particularly for creating and / or maintaining a channel connecting a vein to an adjacent artery, preferably in the coronary system.
Owner:MEDTRONIC VASCULAR INC
Percutaneous transcatheter repair of heart valves
Apparatus, systems, and methods are provided for repairing heart valves through percutaneous transcatheter delivery and fixation of annuloplasty rings to heart valves. An annuloplasty ring includes an outer hollow member including a plurality of segments. Adjacent segments cooperate with one another to change the outer hollow member from an elongate insertion geometry to an annular operable geometry. The annuloplasty ring also includes an internal anchor member located at least partially within the outer hollow member. The internal anchor member includes a plurality of anchors configured to attach the annuloplasty ring to tissue of a heart valve annulus. The internal anchor member is configured to move the plurality of anchors with respect to a plurality of windows in the outer hollow member to selectively deploy the plurality of anchors through the respective windows.
Owner:VALCARE INC
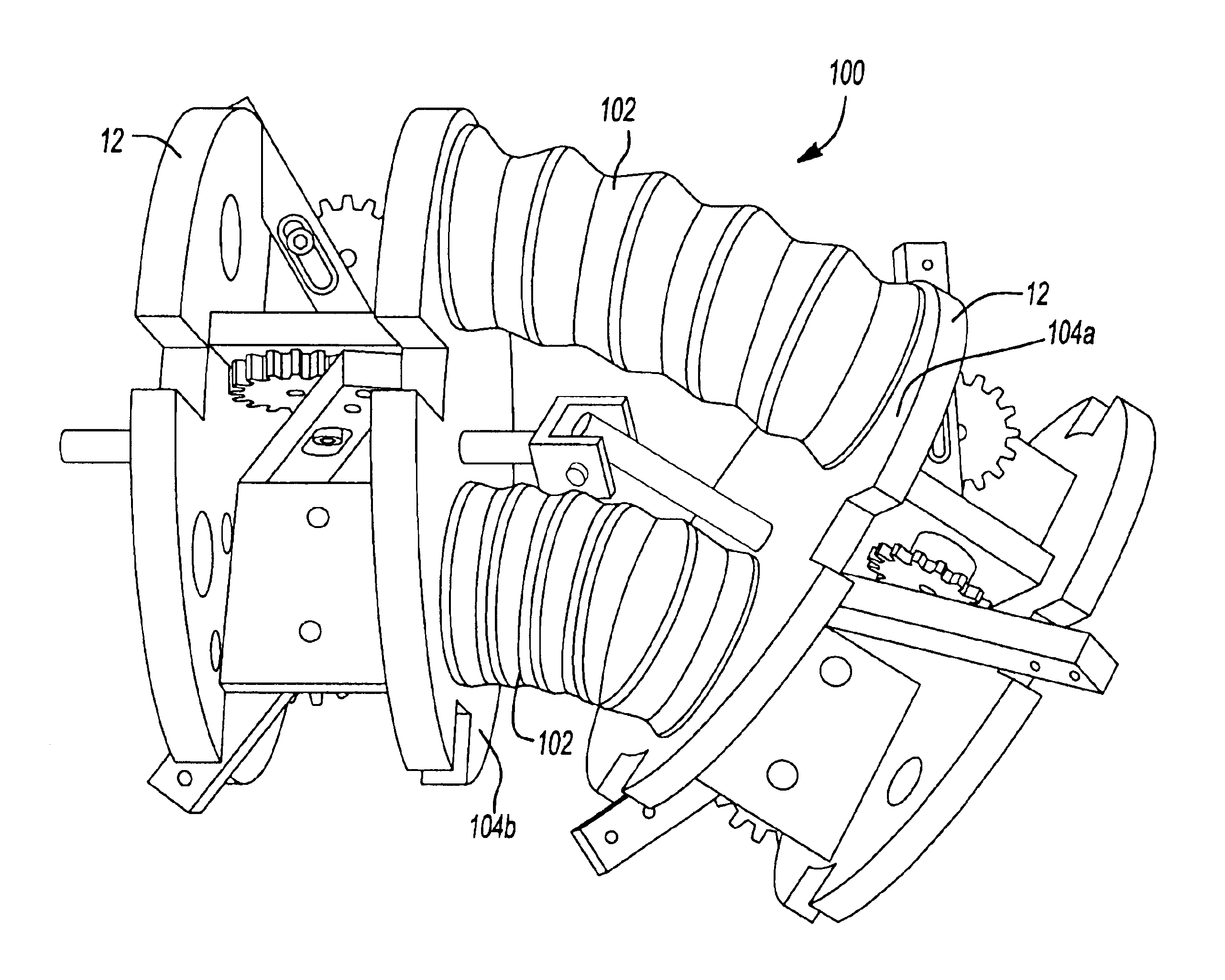
Modular femoral component for a total knee joint replacement for minimally invasive implantation
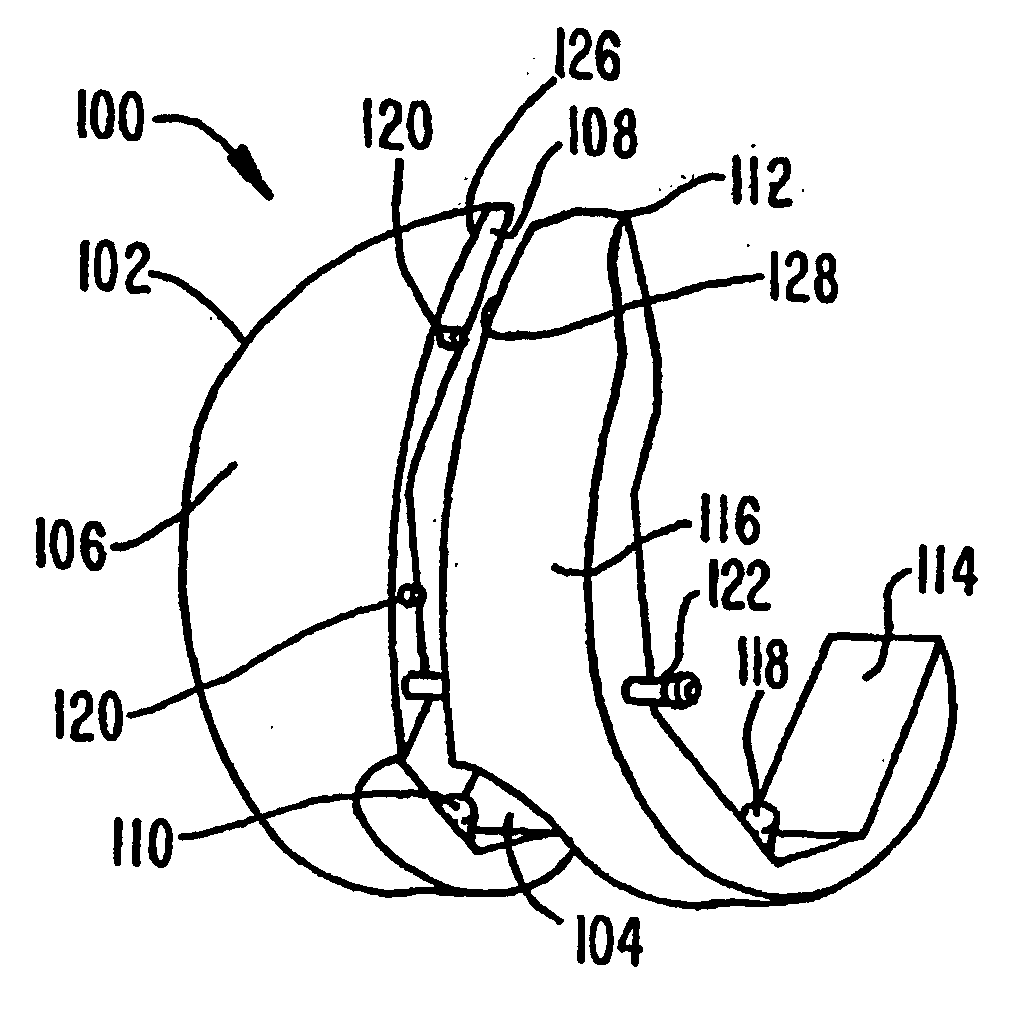
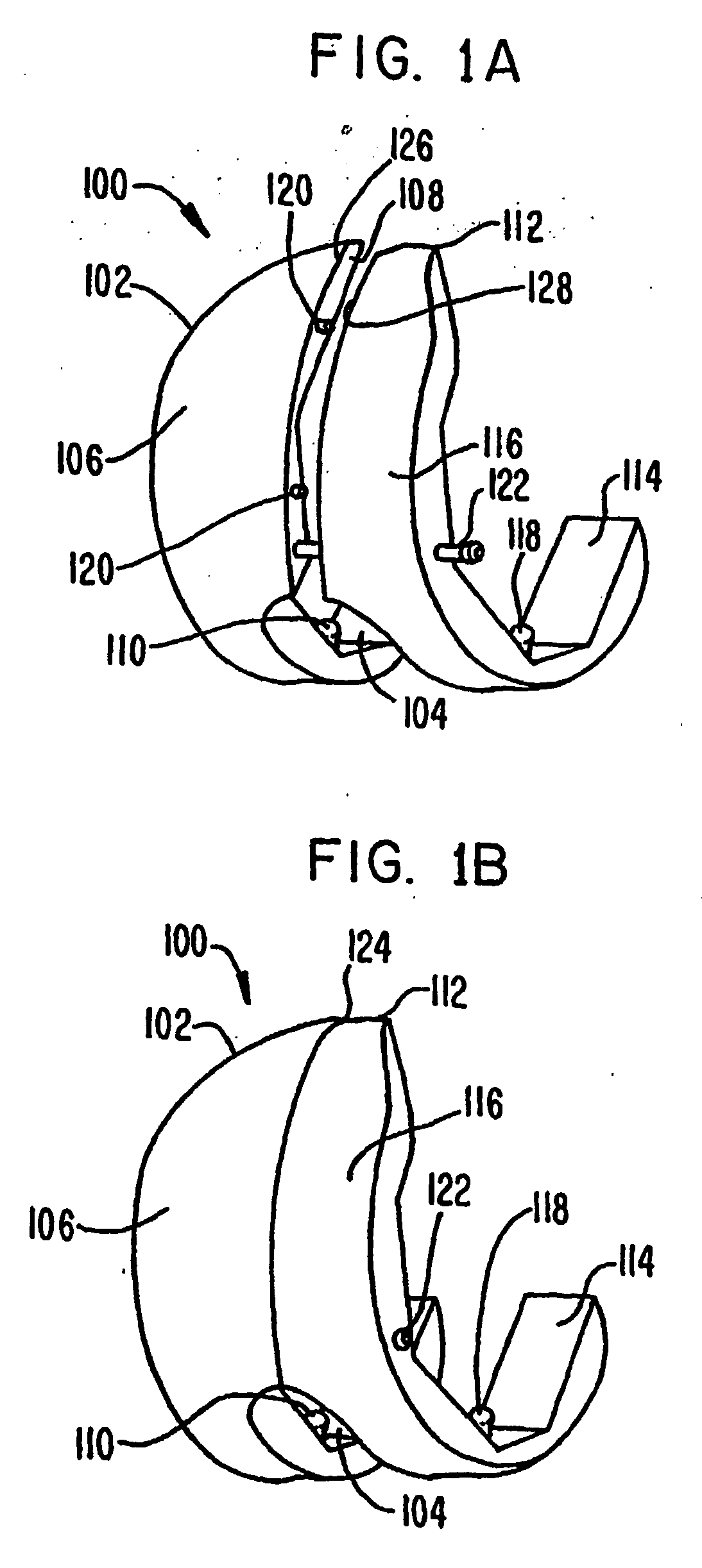
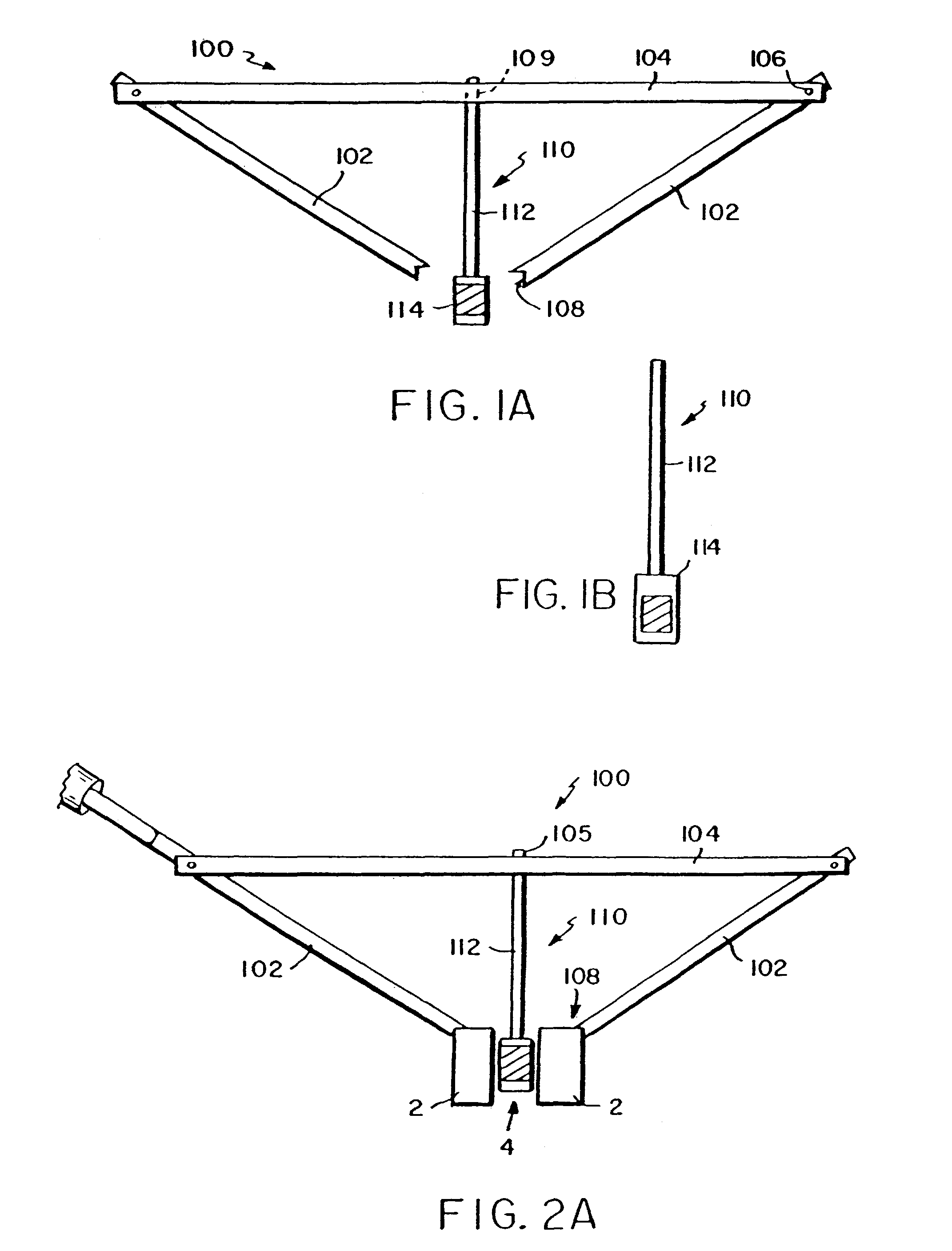
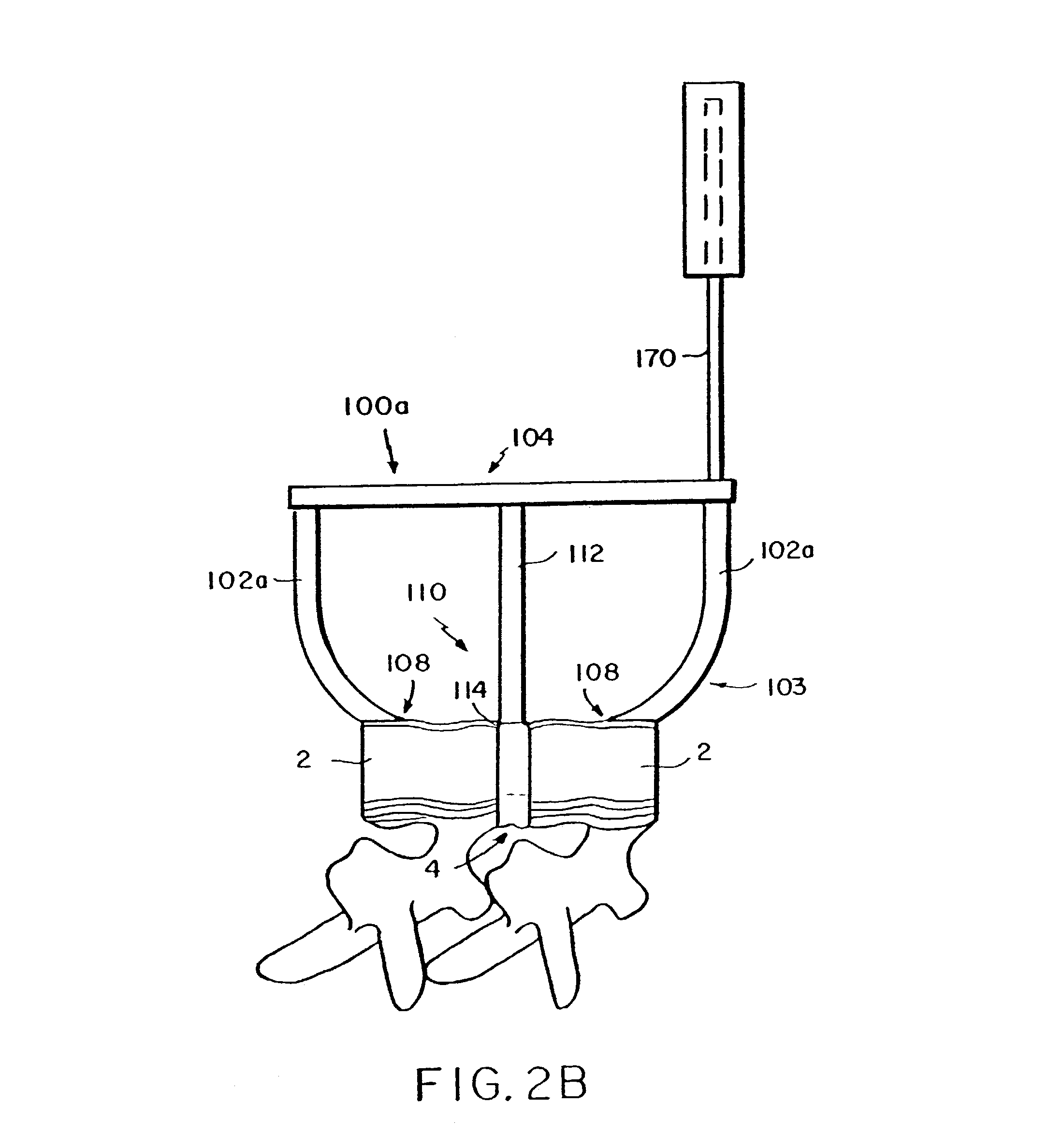
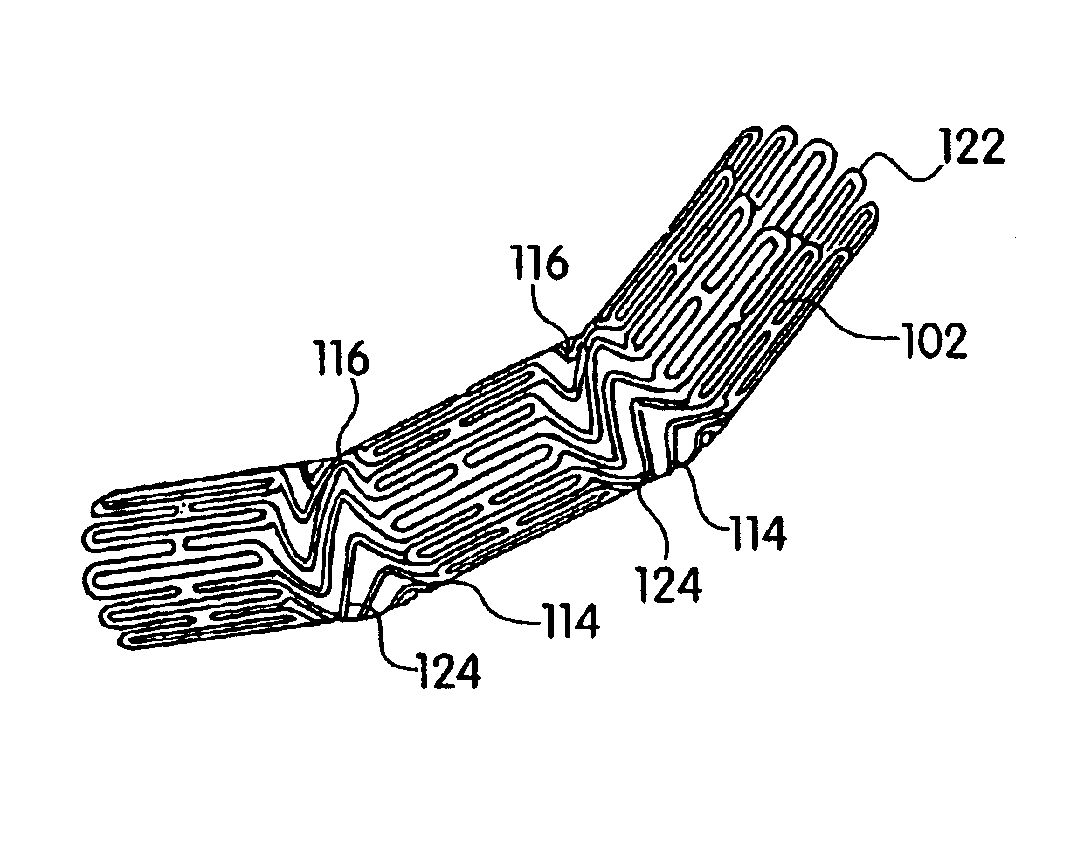
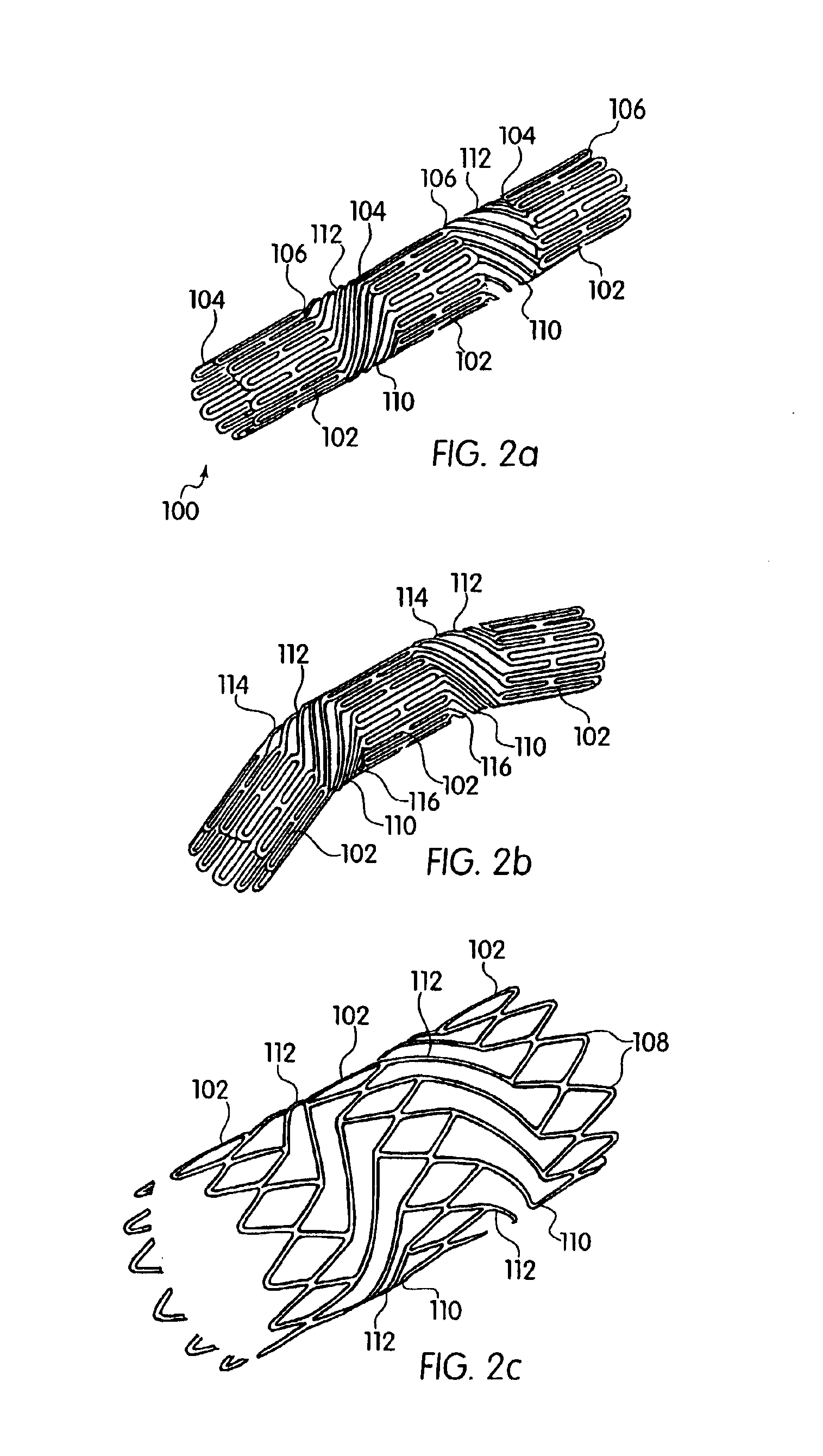
A femoral component (100) for a total knee joint replacement has a modular structure including a number of segments (102, 112), each of the segments (102, 112) having a femoral fixation surface (104, 114) for attachment to the distal end of a femur and at least one assembly surface (108) for joining with an adjacent segment (102, 112) of the modular femoral component (100). The assembly surfaces (108) are generally planar and arranged to be oriented generally in a plane extending in a proximal-distal direction and in an anterior-posterior direction when the femoral fixation surface (104, 114) is positioned on the distal end of the femur. Although the assembly surfaces (108) are generally planar, they may be shaped or provided with complementary structures (120) to assure self-alignment when the segments (102, 112) are assembled.
Owner:MAKO SURGICAL CORP
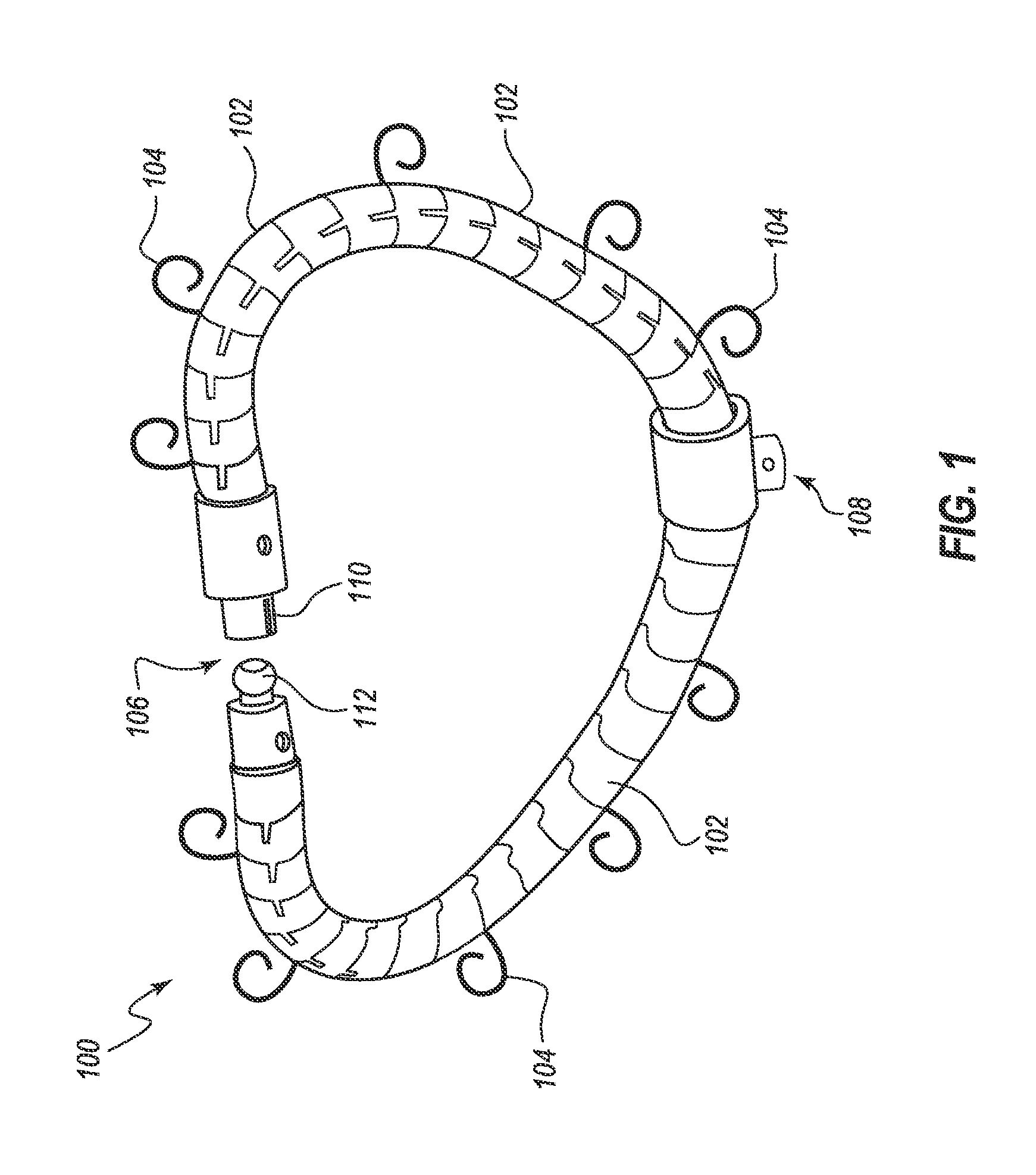
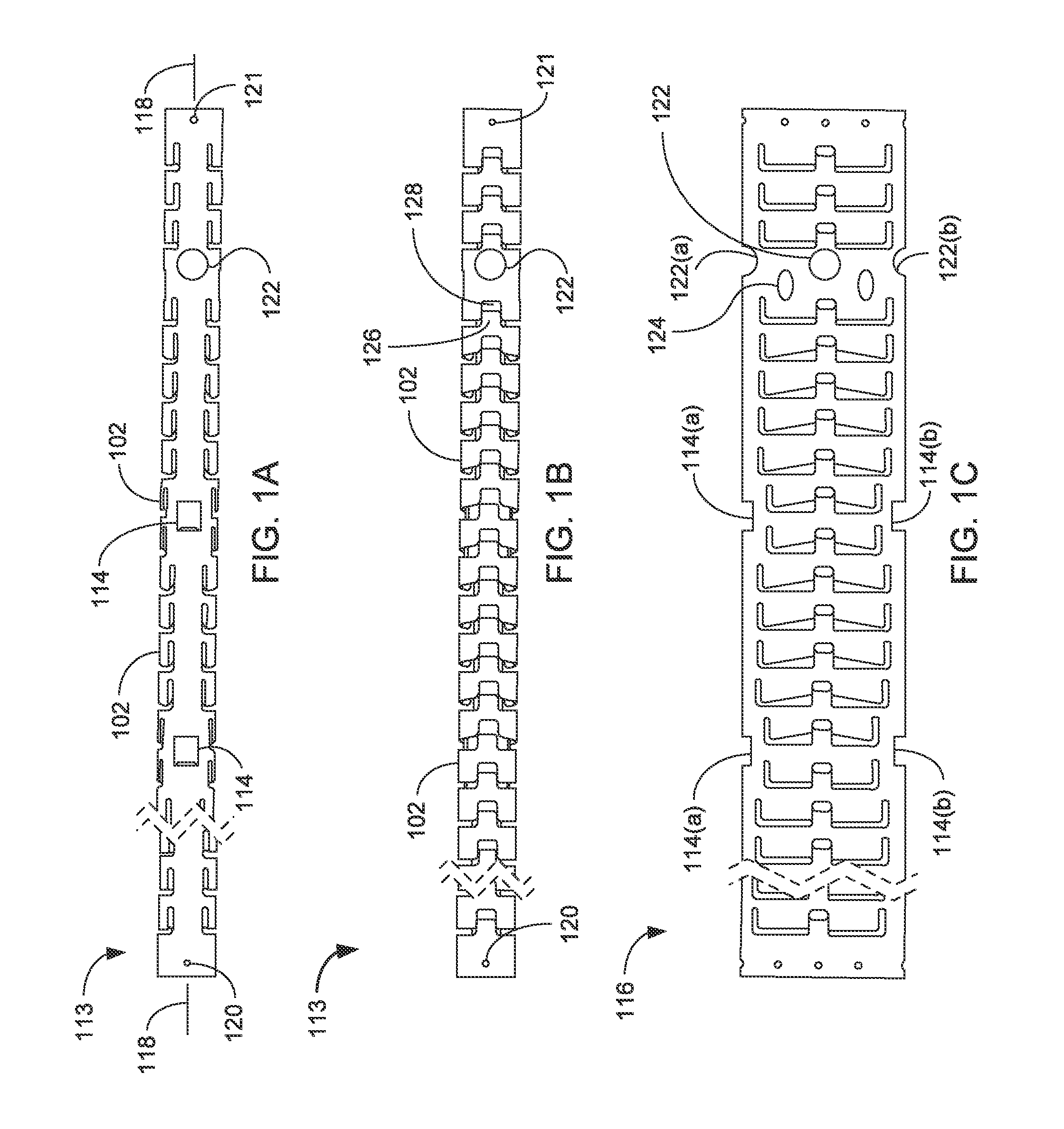
Devices For Introduction Into A Body Via A Substantially Straight Conduit To Form A Predefined Curved Configuration, And Methods Employing Same
InactiveUS20080208255A1Avoid lateral displacementIncrease the effective heightInternal osteosythesisJoint implantsCatheterAbutment
A device for introduction into a body in a straight configuration and assuming within the body a predefined curved configuration, includes an elongated element formed from a number of segments interconnected so as to form effective hinges therebetween. When the elongated element is confined to a straight state, the effective hinges transfer compressive forces from each segment to the next so that the elongated element can be pushed to advance it through a conduit. When the elongated element is not confined to a straight state, the effective hinges allow deflection of each segment relative to adjacent segments until abutment surfaces of the segments come into abutment, thereby defining a fully flexed state of the elongated element with a predefined curved configuration. The device can be produced with a wide range of two-dimensional and three-dimensional curved forms, and has both medical and non-medical applications.
Owner:SEASPINE
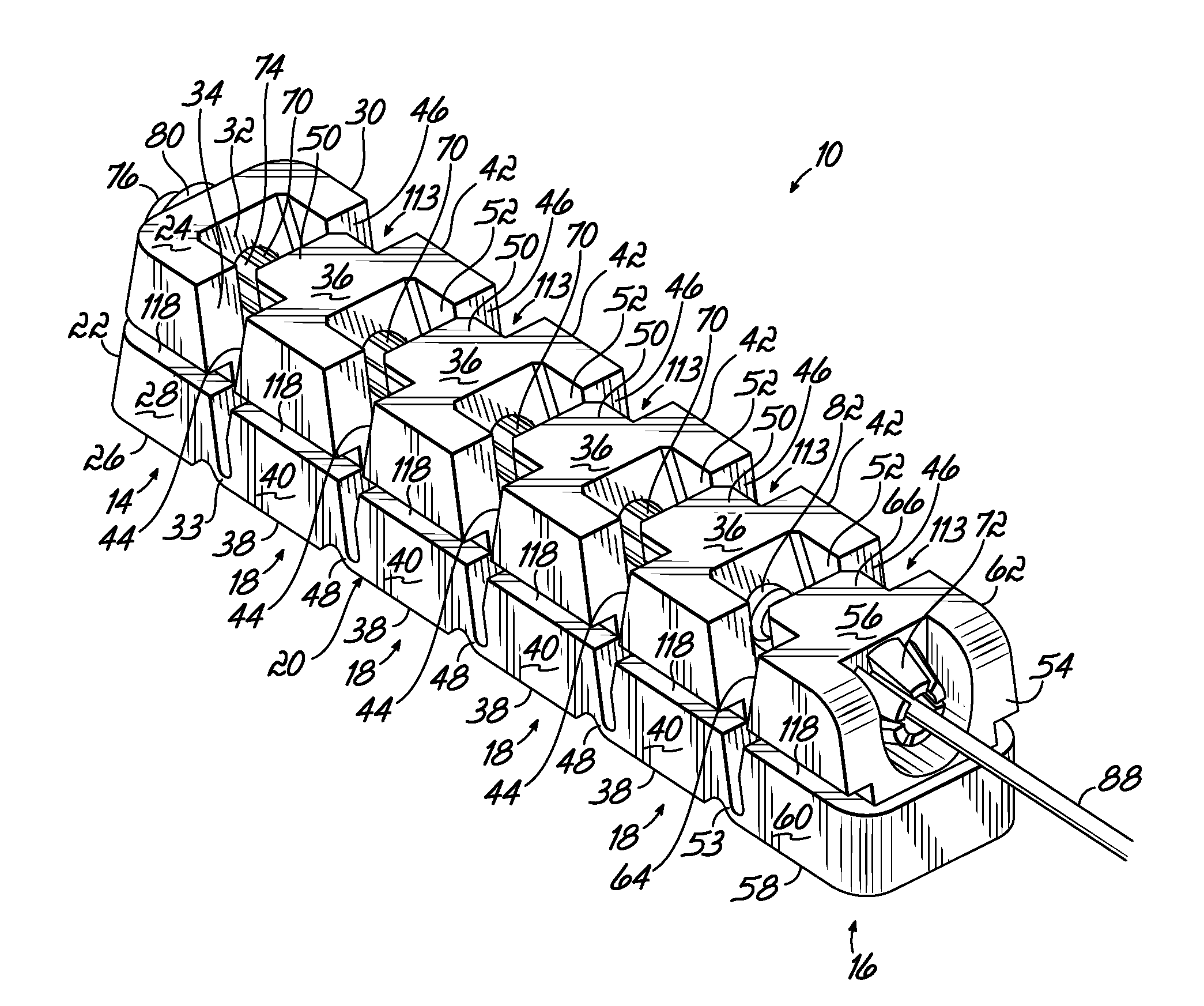
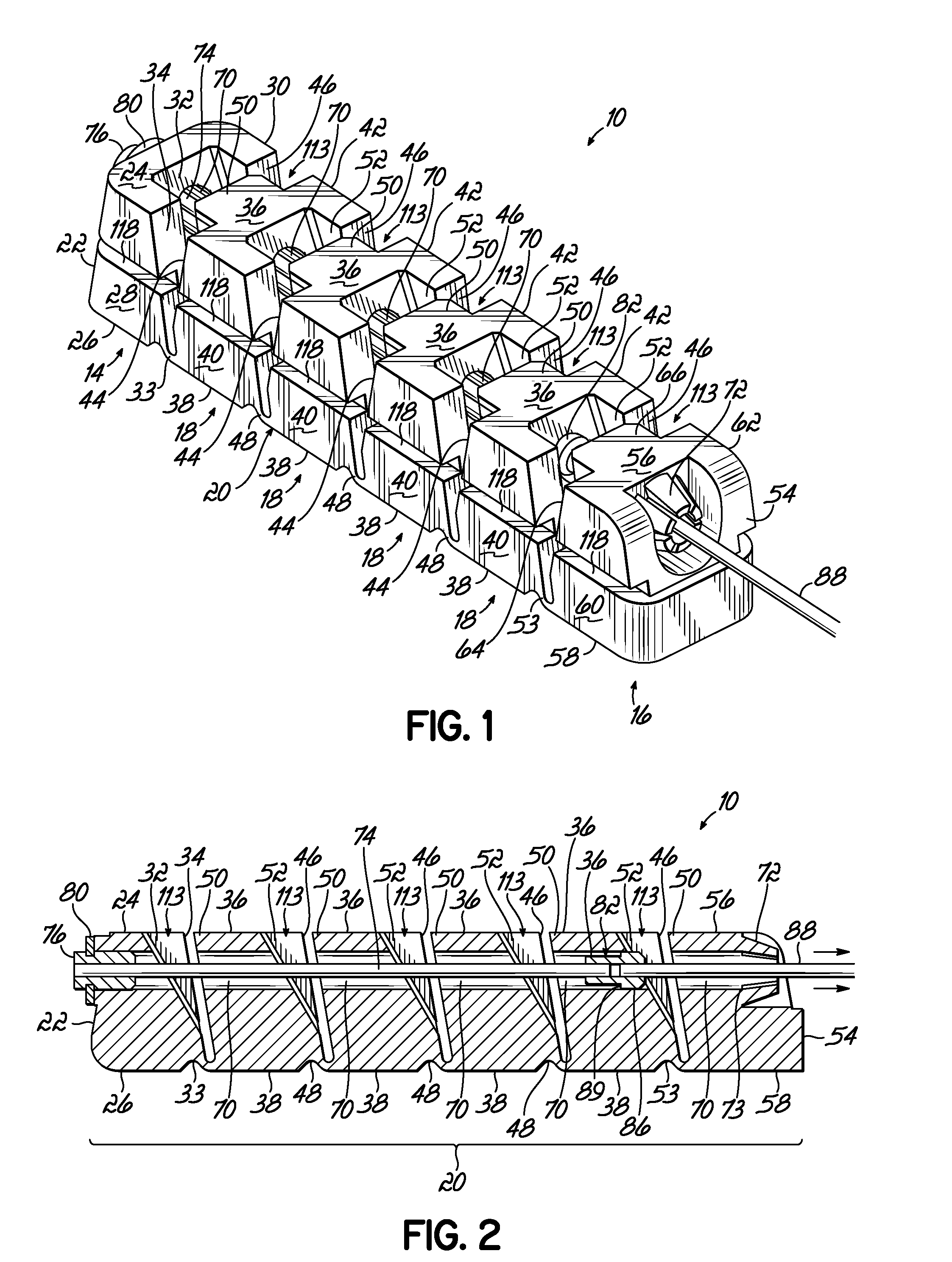
Deployable segmented tlif device
A segmented intervertebral body fusion support includes a plurality of segments, the segments including an initial segment, a final segment and at least one intermediate segment. The intermediate segment has a generally trapezoidal configuration and the initial and final segments include tapered side walls providing triangular gaps between adjacent segments. A draw wire is fixed to the first segment and passes through the remaining segments. By pulling the draw wire relative to the segments, the segments are drawn together in a generally arcuate configuration. The draw wire includes an enlargement that passes through the final segment and engages a plurality of fingers on the final segment, which prevents the draw wire from retracting, maintaining the arcuate configuration. The segmented device can be inserted through a laparoscopic device into the intervertebral space and can be subsequently drawn into the arcuate configuration to establish the desired intervertebral spacing.
Owner:ZIMMER SPINE INC
Turbine blades made from multiple single crystal cast superalloy segments
InactiveUS6331217B1Improve bindingQuality improvementPropellersFrom frozen solutionsTurbine bladeSingle crystal
Large gas turbine blades made from separate cast segments of superalloys are disclosed. The turbine blade is designed such that bond lines between adjacent segments are placed in low stress regions of the blade. The cast superalloy segments of the blades are aligned and fitted together with specified tolerances. The turbine blade segments are then joined by transient liquid phase bonding, followed by a controlled heat treatment which produces the desired microstructure in the bond region. The method allows for the production of large, high quality turbine blades by joining small, high quality cast superalloy sections, in comparison with prior attempts to cast large turbine blades as single pieces which have produced very low yields and high individual component costs.
Owner:SIEMENS ENERGY INC
Elongate Flexible Torque Instruments And Methods Of Use
Torque shafts and other related systems and methods are described herein. In one embodiment, the torque shafts are both flexible and capable of transmitting torque. An apparatus for transmission of torque includes an elongate body, comprising a plurality of joint segments, each joint segment configured to pivot with respect to an adjacent segment and being further configured to have at least two link elements.
Owner:AORTX
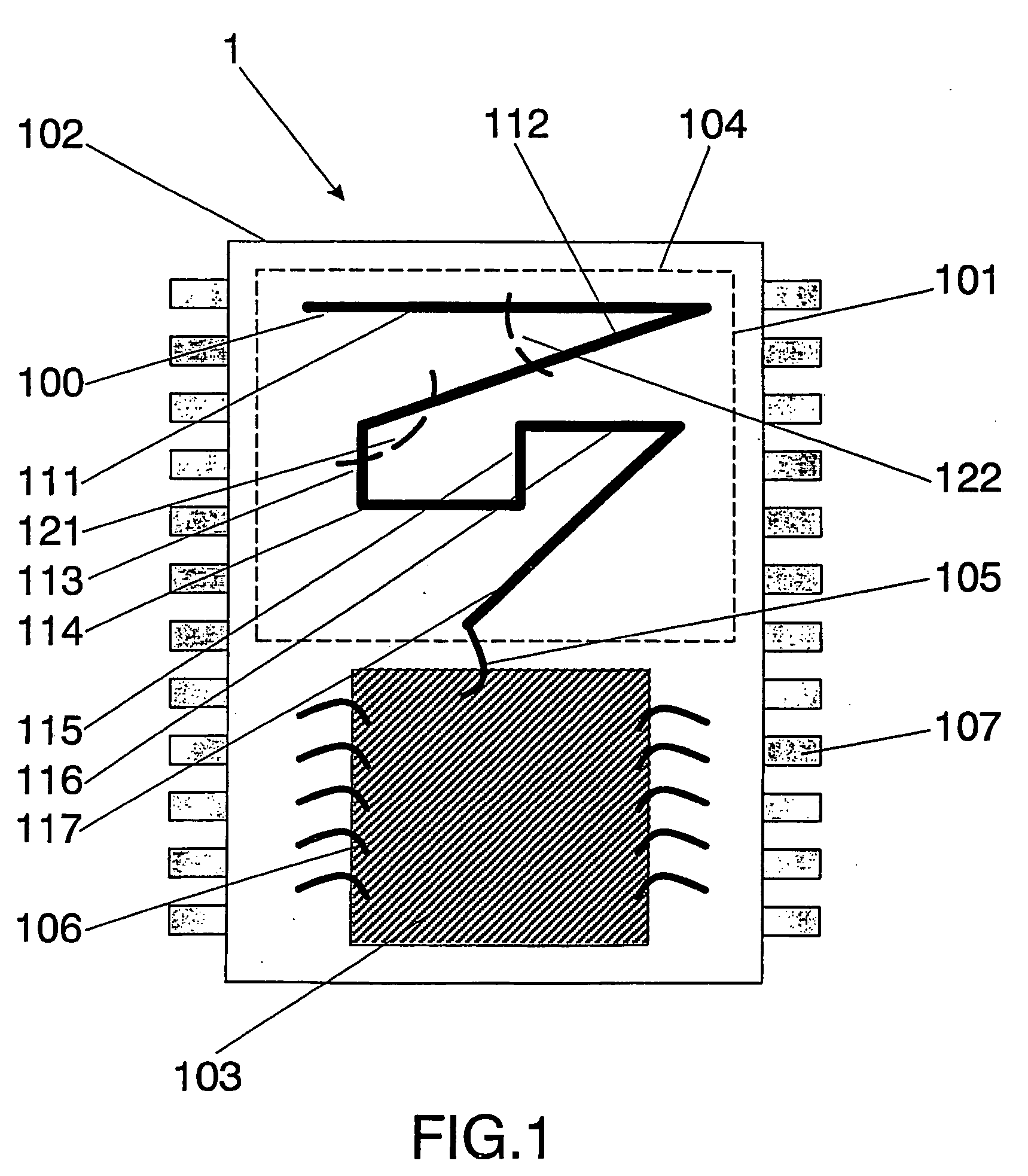
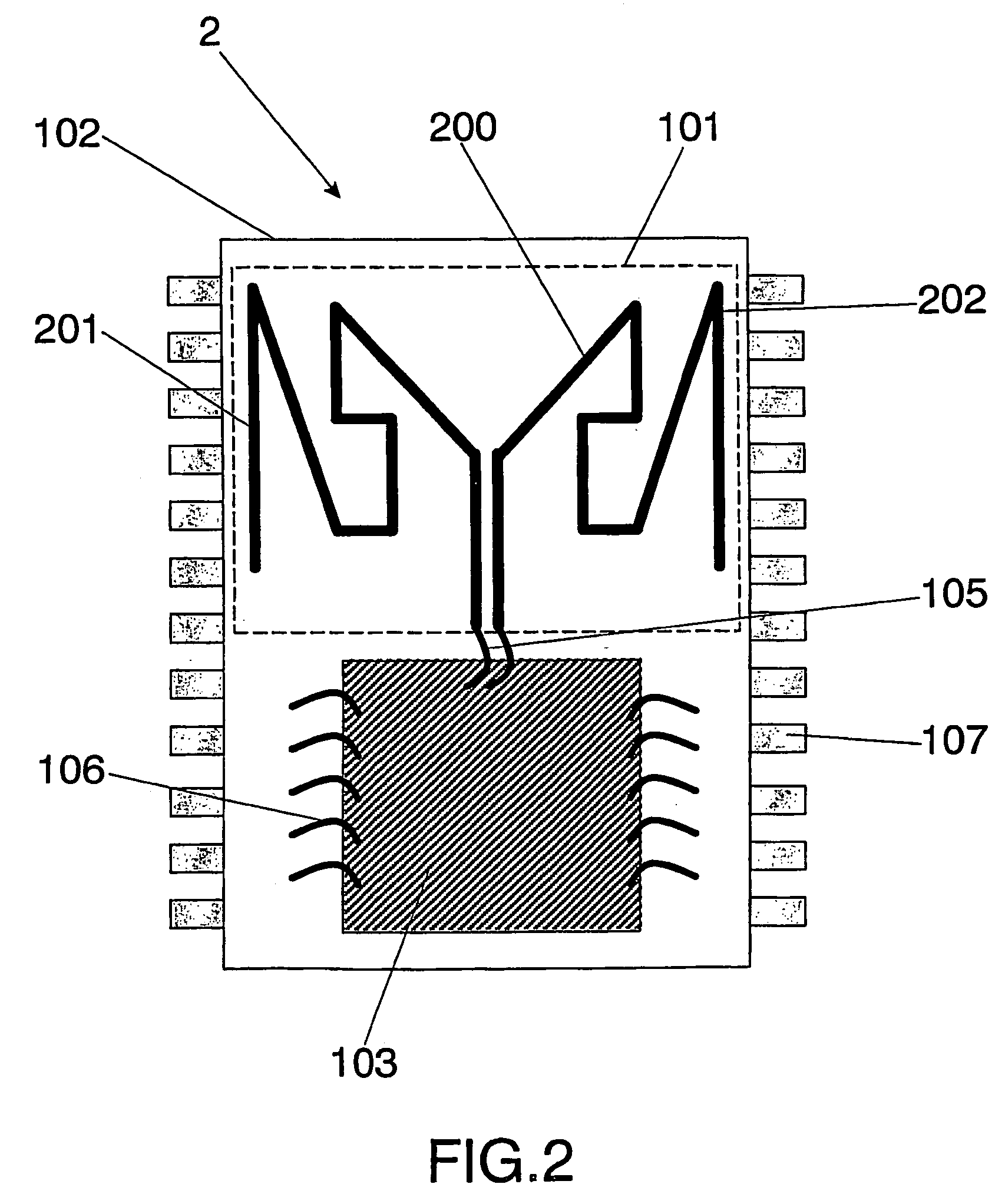
Integrated circuit package including miniature antenna
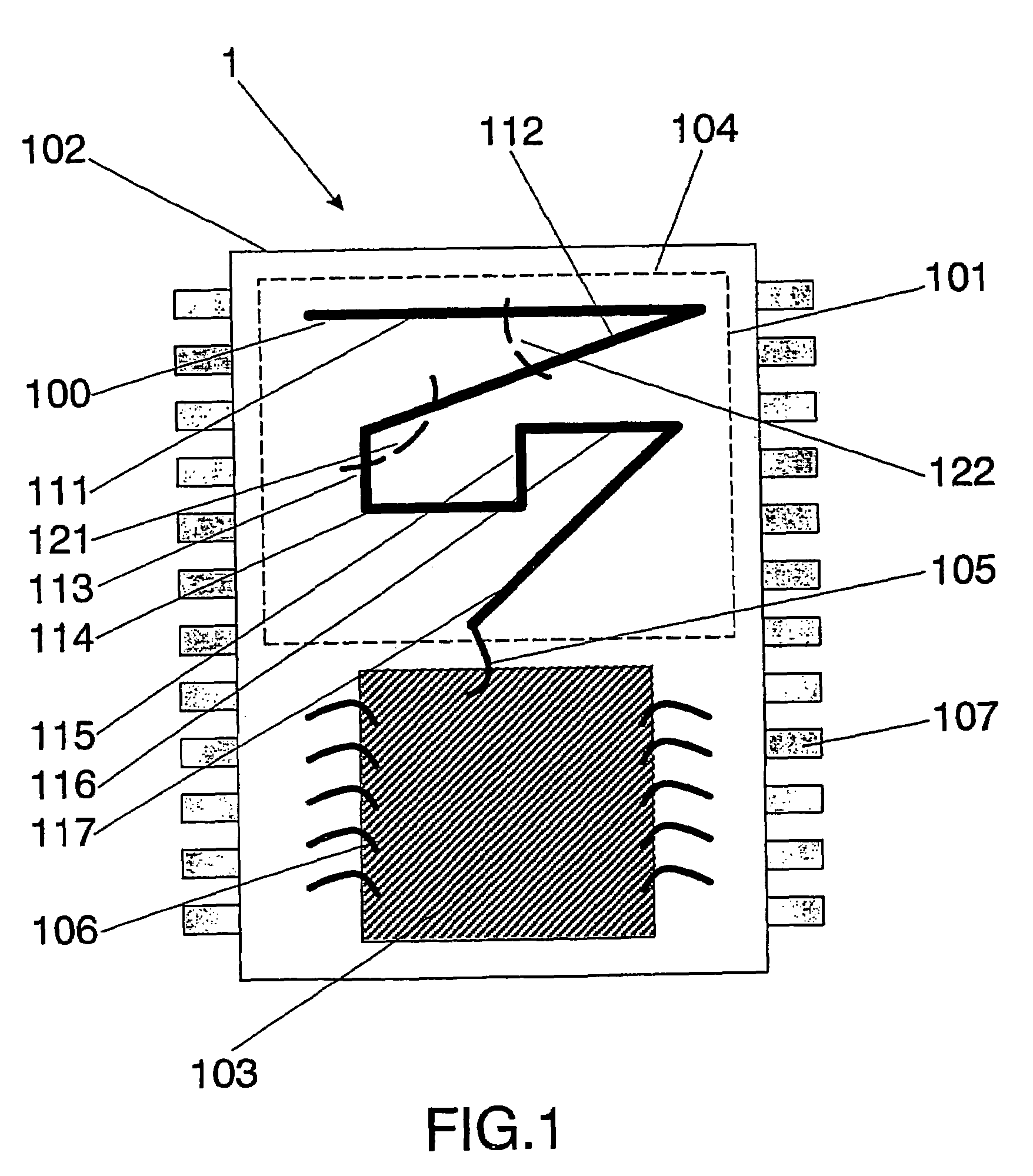
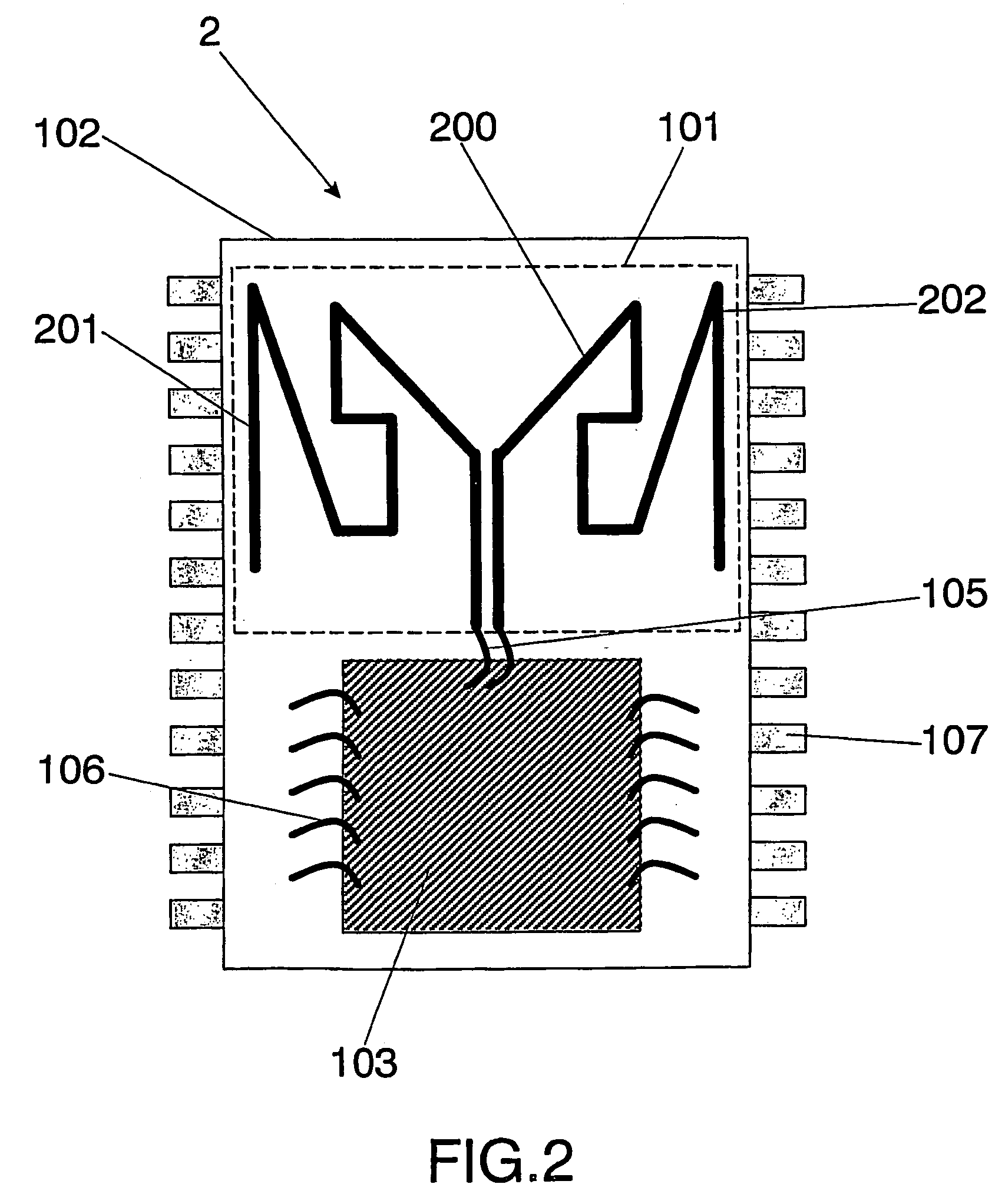
InactiveUS7095372B2Minimize coiling effectEfficient integrationResonant long antennasSimultaneous aerial operationsSemiconductor chipEngineering
The present invention relates to an integrated circuit package comprising at least one substrate, each substrate including at least one layer, at least one semiconductor die, at least one terminal, and an antenna located in the integrated circuit package, but not on said at least one semiconductor die. The conducting pattern comprises a curve having at least five sections or segments, at least three of the sections or segments being shorter than one-tenth of the longest free-space operating wavelength of the antenna, each of the five sections or segments forming a pair of angles with each adjacent segment or section, wherein the smaller angle of each of the four pairs of angles between sections or segments is less than 180° (i.e., no pair of sections or segments define a longer straight segment), wherein at least two of the angles are less than 115°, wherein at least two of the angles are not equal, and wherein the curve fits inside a rectangular area the longest edge of which is shorter than one-fifth of the longest free-space operating wavelength of the antenna.
Owner:FRACTUS
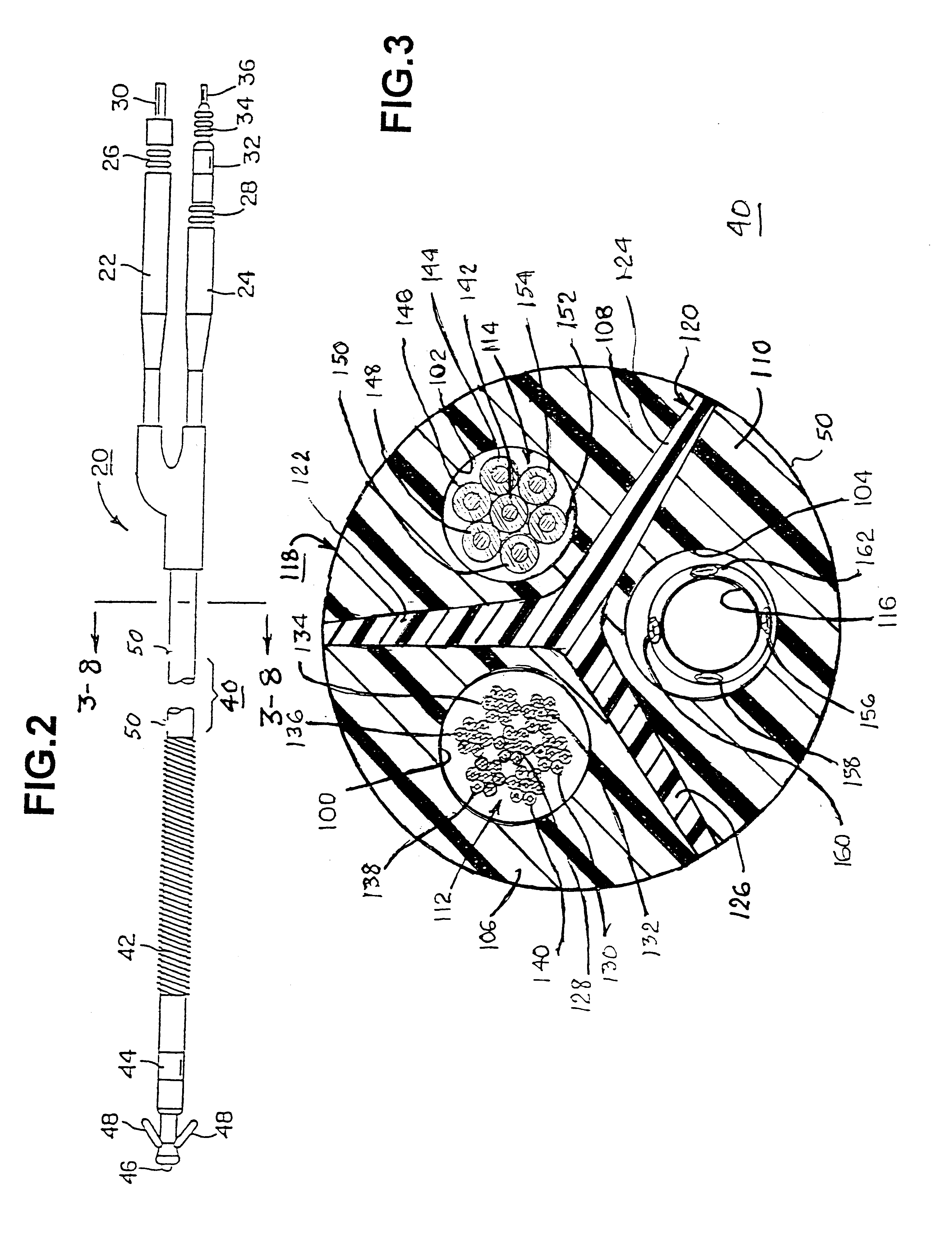
Triaxial fiber optic force sensing catheter
ActiveUS20090177095A1High sensitivityLow profileElectrocardiographyStrain gaugeGratingFiber Bragg grating
A fiber optic force sensing assembly for detecting forces imparted at a distal end of a catheter assembly. The structural member may include segments adjacent each other in a serial arrangement, with gaps located between adjacent segments that are bridged by flexures. Fiber optics are coupled to the structural member. In one embodiment, each fiber optic has a distal end disposed adjacent one of the gaps and oriented for emission of light onto and for collection of light reflected from a segment adjacent the gap. The optical fibers cooperate with the deformable structure to provide a change in the intensity of the reflected light, or alternatively to provide a variable gap interferometer for sensing deformation of the structural member. In another embodiment, the gaps are bridged by fiber Bragg gratings that reflect light back through the fiber optic at central wavelengths that vary with the strain imposed on the grating.
Owner:ST JUDE MEDICAL INT HLDG SARL
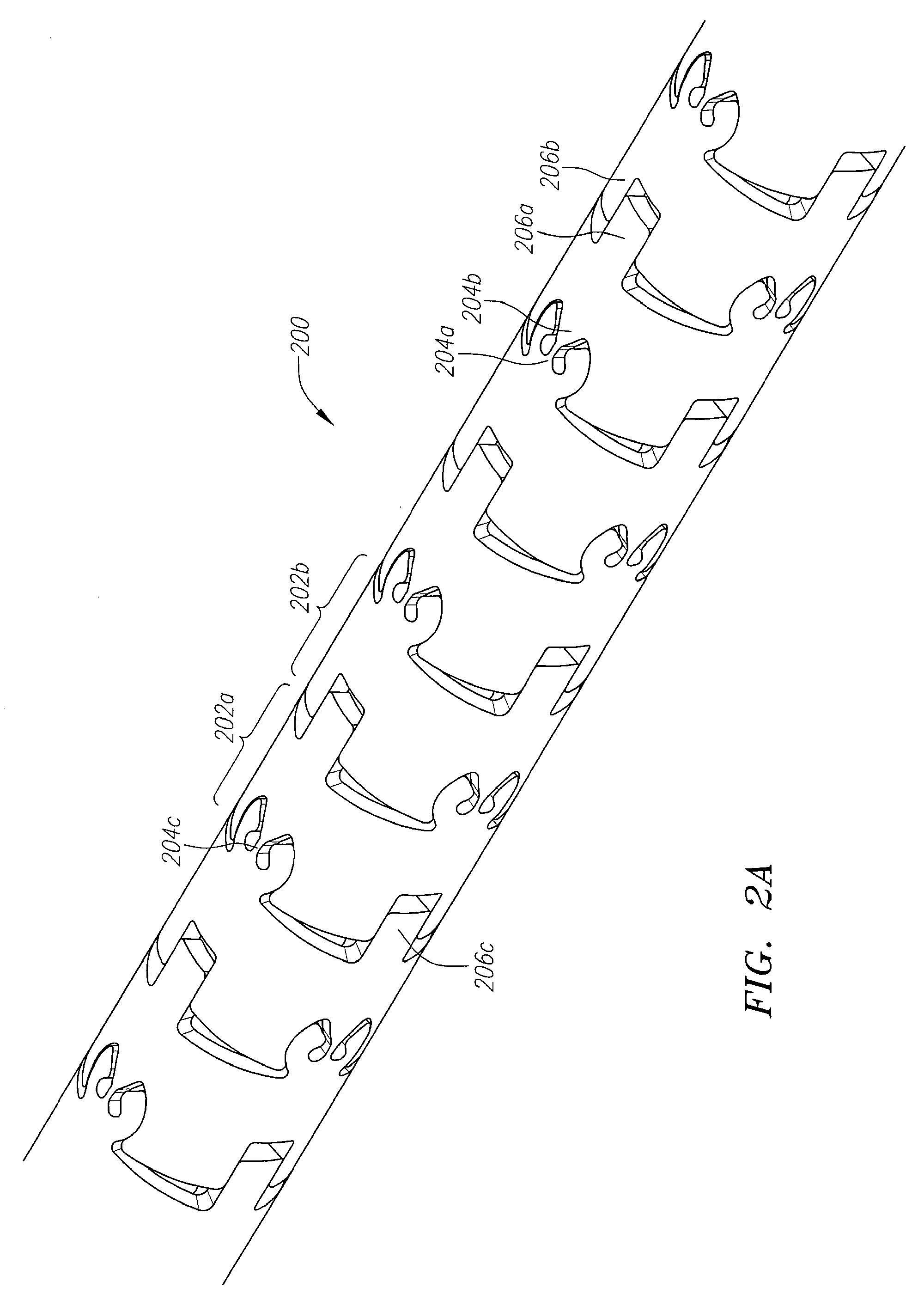
Implantable and lumen-supporting stents and related methods of manufacture and use
InactiveUS20090105809A1Improve expansion propertiesImprove featuresStentsBlood vesselsVisibilityLateral bending
An implantable stent includes multiple circumferential segments that surround a bore and are connected in series along a length to form a tubular wall. Multiple adjacent alternating opposite facing crowns arranged along each segment's circumference are bridged by struts. The struts include a series of staggered arcuate edges with limited flats to provide a limited region of maximum width between significantly extended reducing diameter tapers at either end where they transition into the crowns. Connections between adjacent segments are wider and stiffer than the struts and strut-crown transitions in the segments. The crowns include inner and outer radii with off-set centers along a common axis to provide medial crown peaks along the axis that are wider than the narrowed crown shoulders on either side of the axis and from which the tapered struts extend. Material strain and flexure along the stent during lateral bending is distributed mainly within the segments, e.g. along the struts or crowns, versus at the connections between segments. Material strain and deformation during radial expansion is principally concentrated at the crown shoulders and tapered transition region with the struts. Particular closed-open-closed arrangements along the stent length are disclosed, though with fewer stent connections in the relatively “closed” end-portions along the stent than are provided by other typically “open” cell stents in prior use. Enhanced combinations of performance characteristics are provided regarding visibility, trackability, expansion characteristics, fatigue failures, coating integrity, and local drug delivery from the stent.
Owner:MEDLOGICS DEVICE CORP
Activated polymer articulated instruments and methods of insertion
An electro-polymeric articulated endoscope and method of insertion are described herein. A steerable endoscope having a segmented, elongated body with a manually or selectively steerable distal portion and an automatically controlled proximal portion can be articulated by electro-polymeric materials. These materials are configured to mechanically contract or expand in the presence of a stimulus, such as an electrical field. Adjacent segments of the endoscope can be articulated using the electro-polymeric material by inducing relative differences in size or length of the material when placed near or around the outer periphery along a portion of the endoscope.
Owner:INTUITIVE SURGICAL OPERATIONS INC
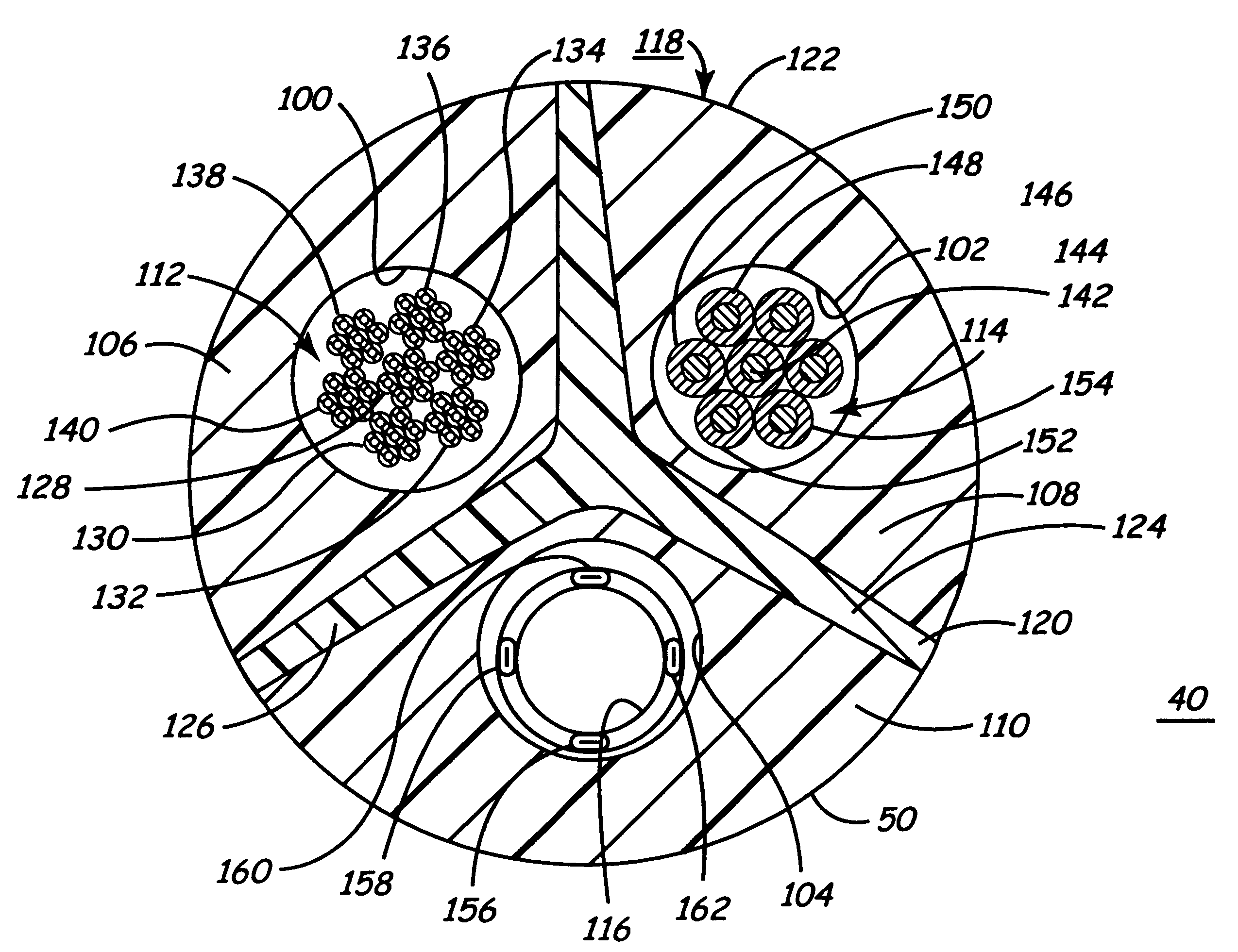
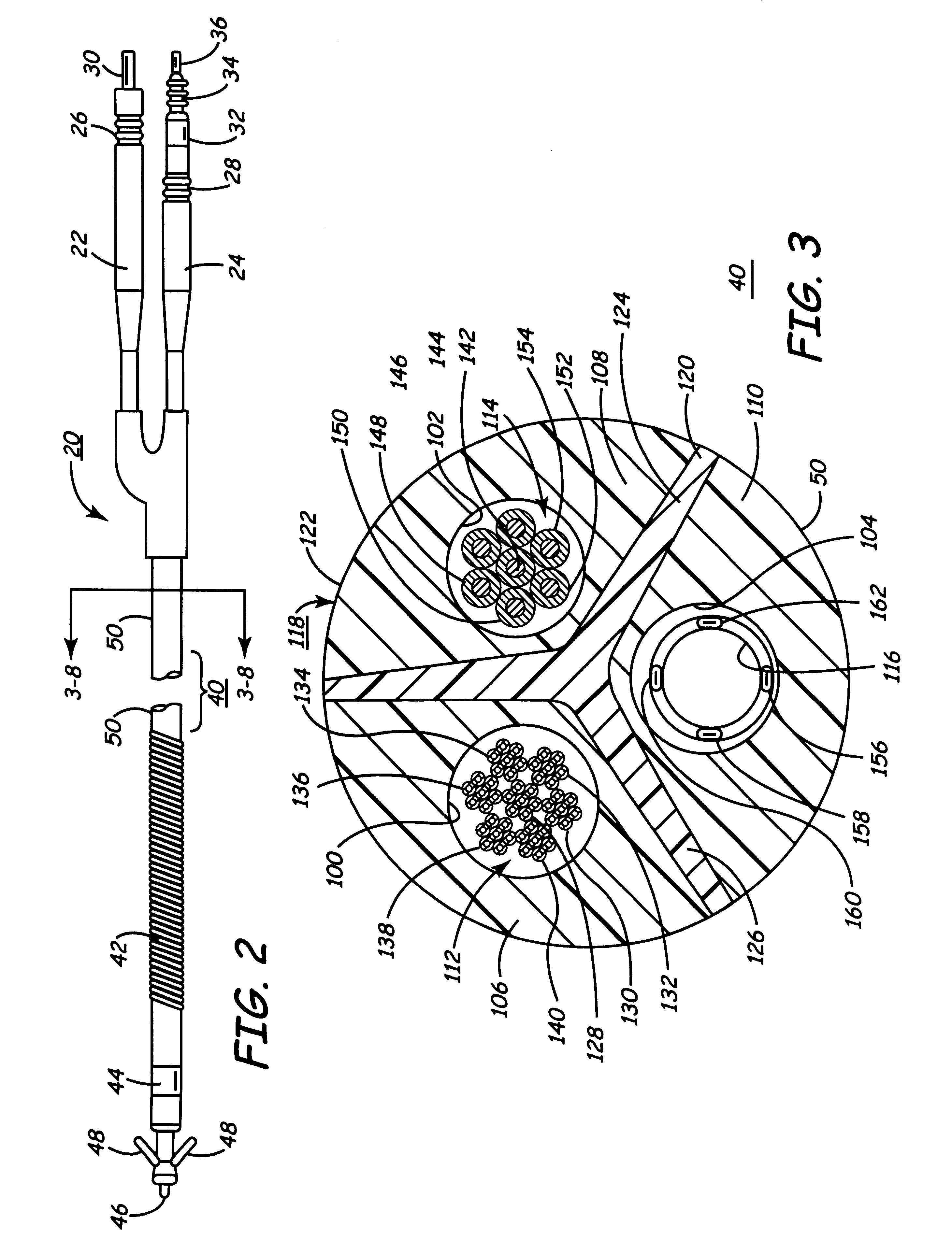
Co-extruded, multi-lumen medical lead
InactiveUS6434430B2Transvascular endocardial electrodesDiagnostic recording/measuringElectrical stimulationsBending stiffness
Owner:MEDTRONIC INC
Integrated, proportionally controlled, and naturally compliant universal joint actuator with controllable stiffness
InactiveUS6870343B2Limited stiffnessMinimize airflowProgramme-controlled manipulatorComputer controlProportional controlUniversal joint
An apparatus for traversing obstacles having an elongated, round, flexible body that includes a plurality of segments interconnected by an integrated joint actuator assembly. The integrated joint actuator assembly includes a plurality of bellows-type actuators individually coupling adjacent segments to permit pivotal actuation of the apparatus therebetween. A controller is employed to maintain proper positional control and stiffness control while minimize air flow.
Owner:RGT UNIV OF MICHIGAN
Support frame for an embolic protection device
InactiveUS6964672B2Reduce the overall diameterEasy to deploySurgeryDilatorsEmbolic Protection DevicesEngineering
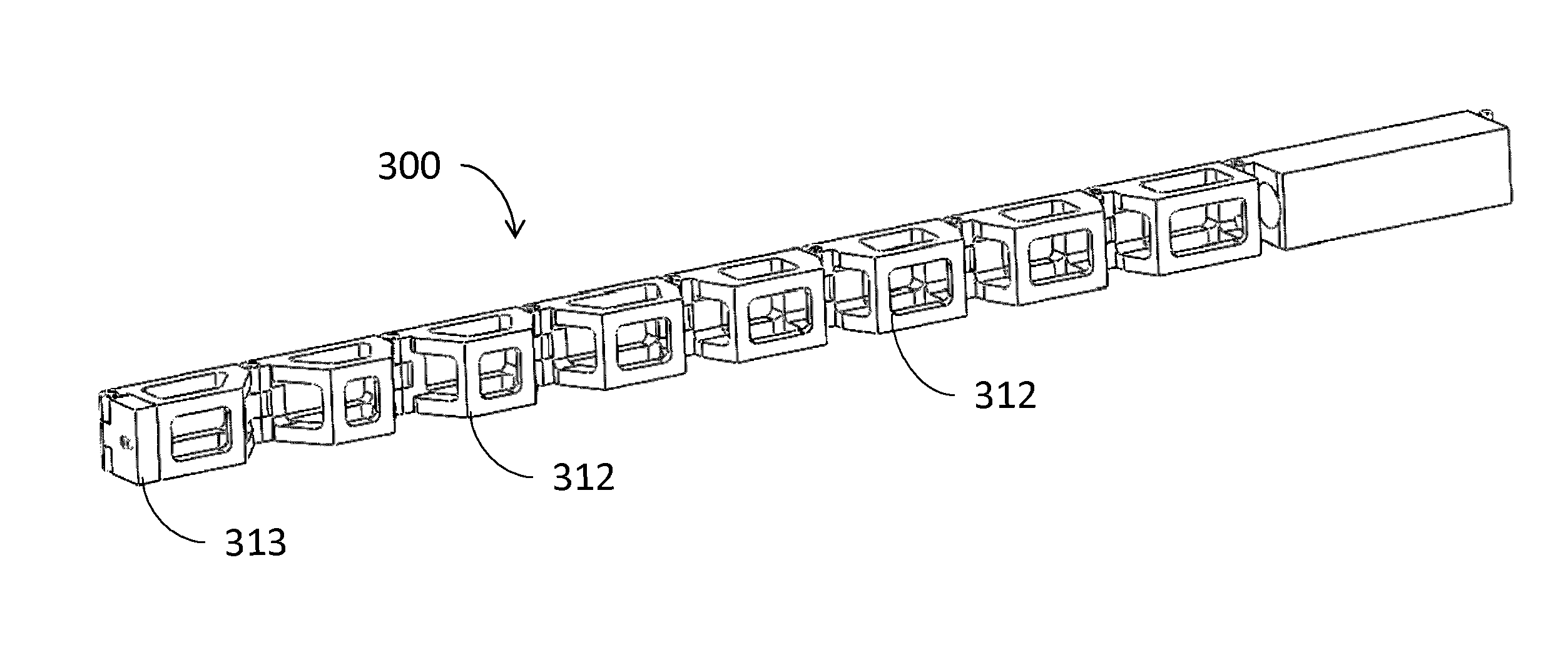
An embolic protection device comprises a collapsible filter body and a filter support for the filter body. The filter support comprises a support frame defined by at least two wire segments having terminations. The terminations of adjacent segments are fixed relative to one another and extend generally parallel in a bifilar manner for enhanced trackability and flexibility.
Owner:SALVIAC
Co-extruded, multi-lumen medical lead
InactiveUS6400992B1Transvascular endocardial electrodesExternal electrodesElectrical stimulationsBending stiffness
Medical electrical leads for sensing or electrical stimulation of body organs or tissues, particularly implantable cardiac leads for delivering pacing pulses and cardioversion / defibrillation shocks, and / or sensing the cardiac electrogram (EGM) or other physiologic data and their methods of fabrication are disclosed. A lead body sheath is co-extruded in a co-extrusion process using bio-compatible, electrically insulating, materials of differing durometers in differing axial sections thereof, resulting in a unitary lead body sheath having differing stiffness sections including axial segments or webs or lumen encircling rings or other structures in its cross-section. The lead body sheath is co-extruded to have an outer surface adapted to be exposed to the environment or to be enclosed within an outer sheath and to have a plurality of lead conductor lumens for receiving and enclosing a like plurality of lead conductors of the same or differing types. The lead body sheath can be co-extruded of a plurality of sheath segments containing a lead conductor lumen and formed of a first durometer material or of differing durometer materials. A web of a further durometer material can be co-extruded extending between the adjoining boundaries of the axial sheath segments and bonding the adjacent segments together. The lead body sheath can be tailored to exhibit differing bending stiffnesses away from the lead body sheath axis in selected polar directions around 360° circumference of the sheath body.
Owner:MEDTRONIC INC
Tool and corresponding method for removal of material from within a body
A device for removing material from within the body, includes an elongated element formed from hollow segments sequentially interconnected at effective hinges. The device assumes an insertion configuration for insertion of the segments sequentially through an opening of a first dimension into the body. A portion of the elongated element inserted into the body progressively assumes a material removing configuration in which a relative position of each segment relative to an adjacent segment is delineated by the effective hinge together with additional abutment surfaces defining a fully deflected state of the effective hinge. The material removing configuration has at least two dimensions exceeding the first dimension. At least two of the segments are formed with at least one cutting configuration deployed so as to collect material into a hollow volume of the segment during progressive formation of the material removing configuration as the elongated element is advanced.
Owner:NLT SPINE
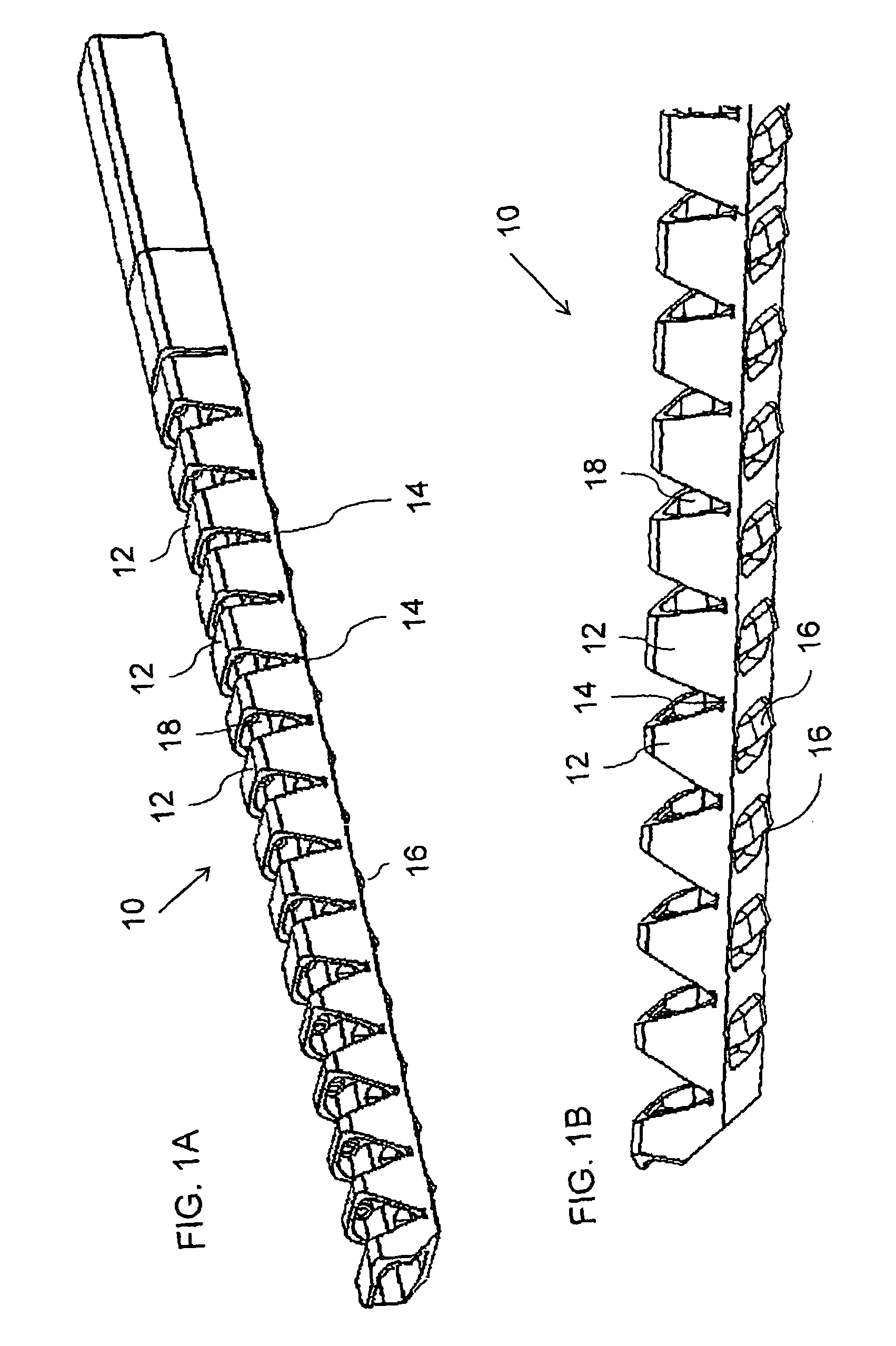
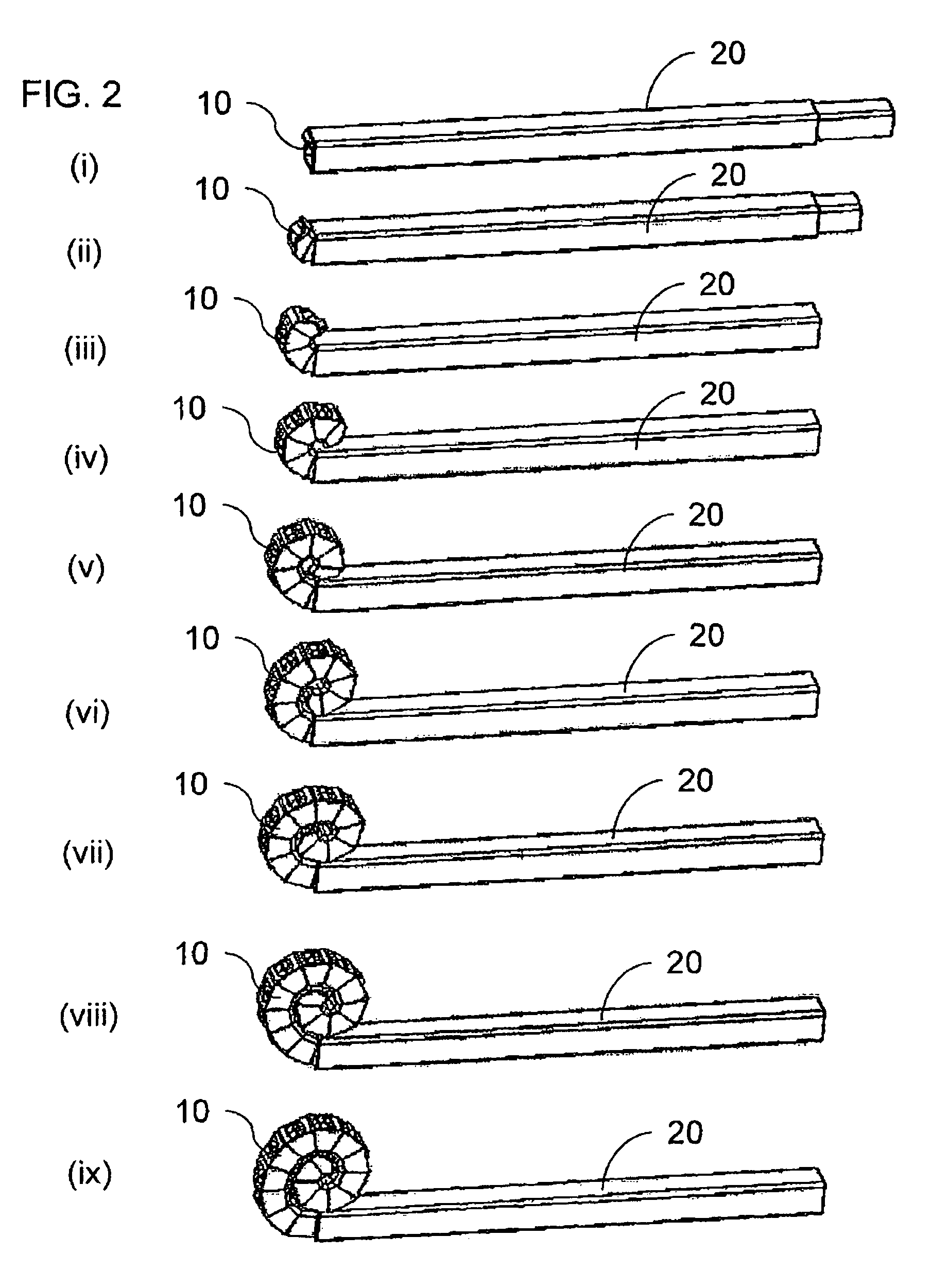
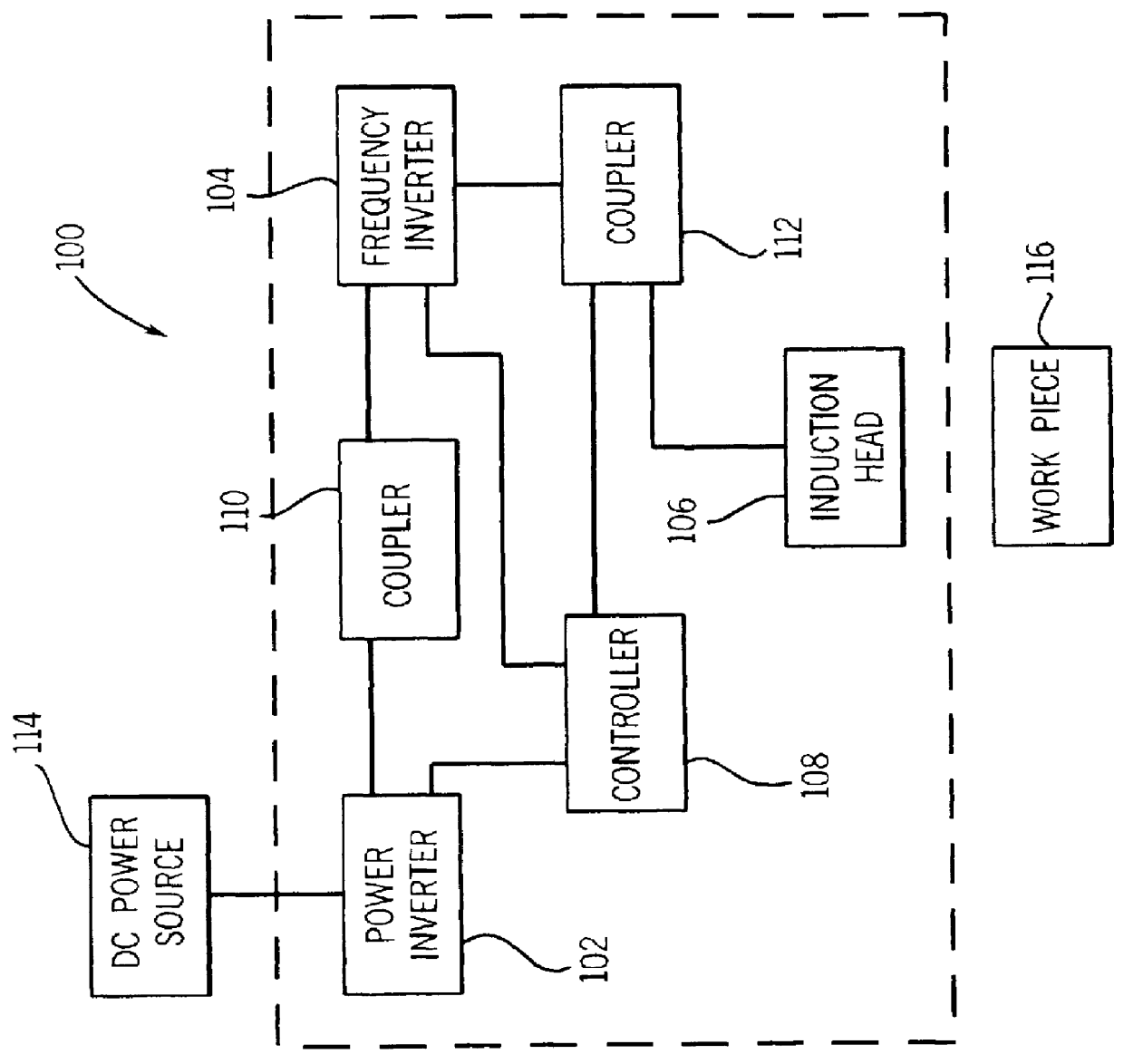
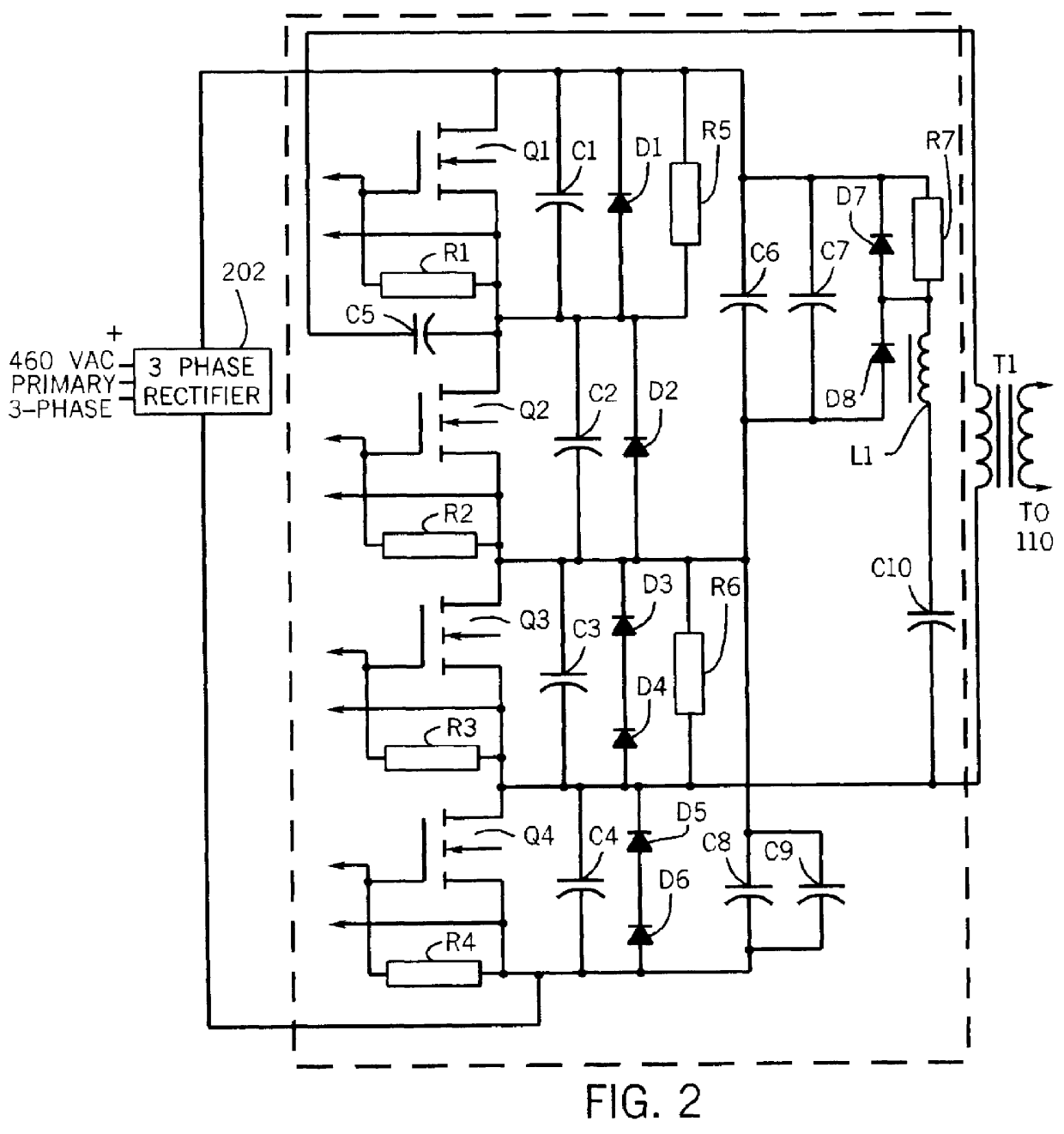
Multiple head inductive heating system
InactiveUS6043471ACoil arrangementsInduction current sourcesHeating systemElectrical and Electronics engineering
A system and method for inductively heating a workpiece includes a controller and a plurality of power supplies that receive and send signals to the controller. Induction heads receive power from the power supplies. The induction heads may be aligned with adjacent segments of the workpiece, and can span the perimeter of the workpiece. The gap between adjacent induction heads is less than one half the size of the adjacent induction heads, and preferably the induction heads abut or substantially abut. Each of the power supplies include feedback for controlling the power delivered to the segments of the workpiece. In alternative embodiments the feedback may be based on the current or power provided to the induction heads, or the power provided to the workpiece.
Owner:ILLINOIS TOOL WORKS INC
Tool and corresponding method for removal of material from within a body
A device for removing material from within the body, includes an elongated element formed from hollow segments sequentially interconnected at effective hinges. The device assumes an insertion configuration for insertion of the segments sequentially through an opening of a first dimension into the body. A portion of the elongated element inserted into the body progressively assumes a material removing configuration in which a relative position of each segment relative to an adjacent segment is delineated by the effective hinge together with additional abutment surfaces defining a fully deflected state of the effective hinge. The material removing configuration has at least two dimensions exceeding the first dimension. At least two of the segments are formed with at least one opening, typically provided with a cutting configuration, deployed so as to receive material into a hollow volume of the segment during progressive formation of the material removing configuration as the elongated element is advanced.
Owner:NLT SPINE
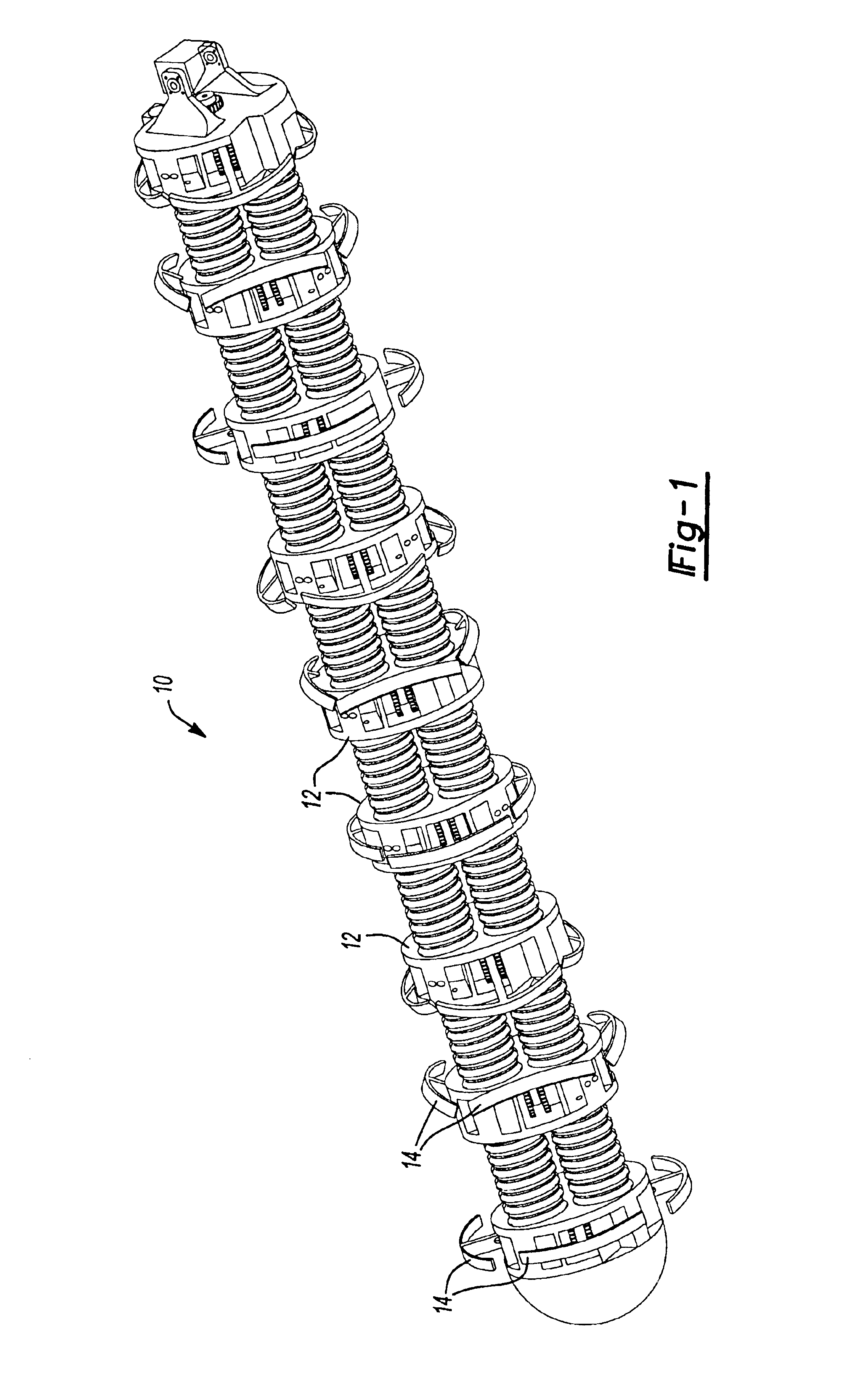
Systems, methods, devices and device kits for fixation of bones and spinal vertebrae
InactiveUS8021401B2Avoid problemsMaintain alignmentInternal osteosythesisDiagnosticsIntervertebral spaceAlternative methods
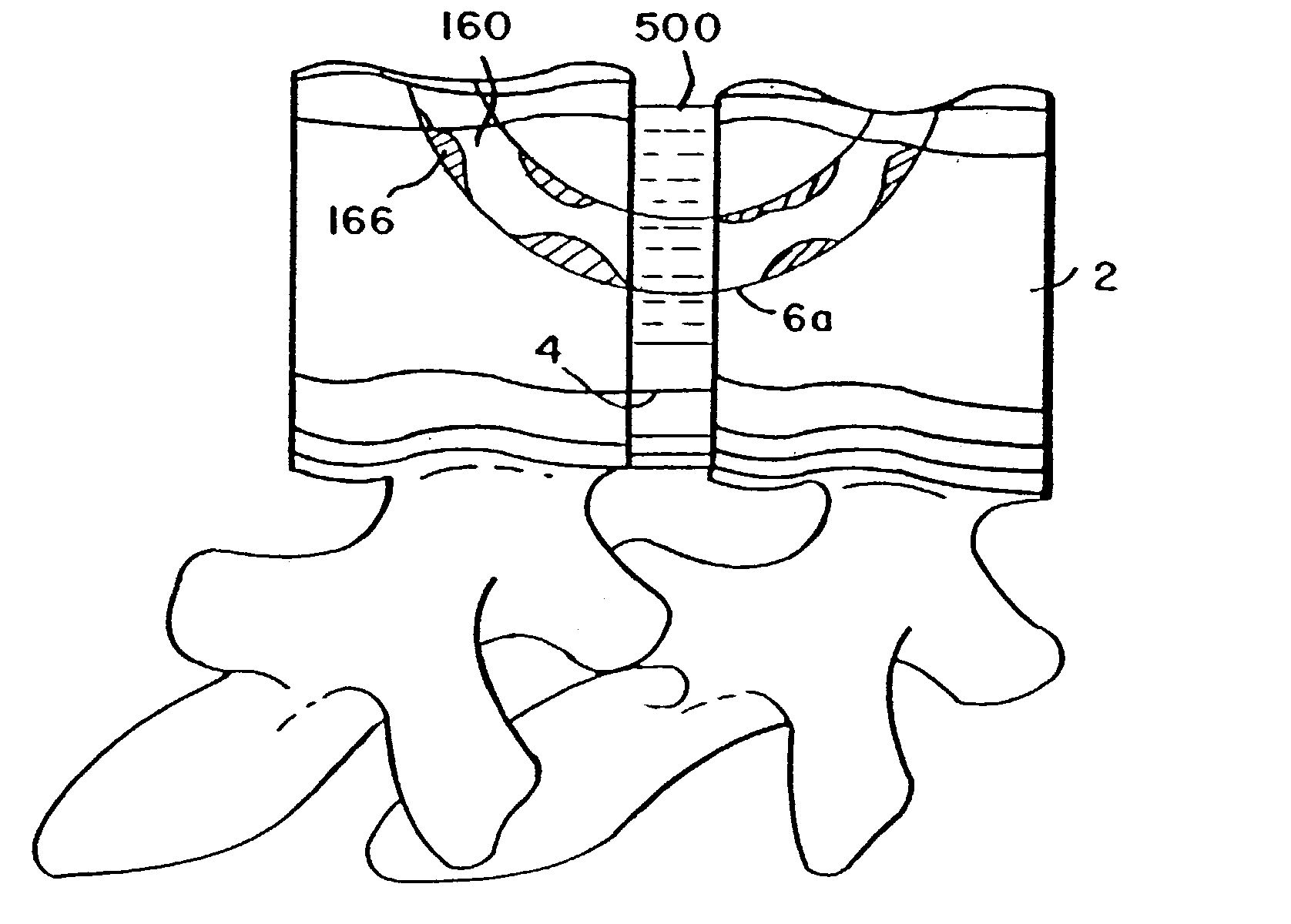
Featured are methods for fixing adjacent vertebrate of a spine that utilize an implant member, which preferably is arcuate. Preferred methods of the invention for stabilizing adjacent vertebrae of the spine, include forming an aperture in each of the adjacent vertebrae and inserting an implant into the apertures formed in each of the adjacent vertebrae so that the implant extends between the adjacent vertebrae and through the intervertebral space. An alternative method for fixing adjacent vertebrae of a spine includes the step of forming a common channel in and between the adjacent vertebrae and inserting an implant in the channel so as to bridge between the adjacent vertebrae. Also featured are methods for stabilizing adjacent segments of a bone, fixation systems and fixation kits.
Owner:K2M
Integrated circuit package including miniature antenna
InactiveUS20060033664A1Small sizeMinimize coiling effectResonant long antennasSimultaneous aerial operationsSemiconductor chipEngineering
The present invention relates to an integrated circuit package comprising at least one substrate, each substrate including at least one layer, at least one semiconductor die, at least one terminal, and an antenna located in the integrated circuit package, but not on said at least one semiconductor die. The conducting pattern comprises a curve having at least five sections or segments, at least three of the sections or segments being shorter than one-tenth of the longest free-space operating wavelength of the antenna, each of the five sections or segments forming a pair of angles with each adjacent segment or section, wherein the smaller angle of each of the four pairs of angles between sections or segments is less than 180° (i.e., no pair of sections or segments define a longer straight segment), wherein at least two of the angles are less than 115°, wherein at least two of the angles are not equal, and wherein the curve fits inside a rectangular area the longest edge of which is shorter than one-fifth of the longest free-space operating wavelength of the antenna.
Owner:FRACTUS
Electrical lead for an electronic device such as an implantable device
Owner:SURGIVISION +1
LED collimator having spline surfaces and related methods
InactiveUS20090128921A1Sacrificing performanceGood collimationPoint-like light sourceCondensersLight sourceAdjacent segment
A TIR collimator for an LED light source includes a body portion having a reflective surface, wherein the reflective surface includes a plurality of segments. Respective segments of the reflective surface have corresponding cross-sectional profiles defined by different low-order polynomial functions, such that the overall cross-sectional profile of the reflective surface constitutes a spline, i.e., a piecewise polynomial function. The respective segments are configured to achieve substantial collimation of the output light. In one example, the cross-sectional profiles of adjacent segments of the reflective surface are defined by different low-order polynomials. Additionally, two or more adjacent segments may have respective cross-sectional profiles which together are defined by a Bezier curve, so as to provide smooth transitions between adjacent segments of the spline reflective surface.
Owner:PHILIPS SOLID STATE LIGHTING SOLUTIONS
Articulated stent
An articulated stent for delivering through a bodily conduit, for example, a peripheral or coronary artery, which has one or more curved portions and for implantation therein. The articulated stent includes at least two substantially rigid segments and a flexible connector for connecting adjacent segments. The connector assumes a cylindrical configuration when relaxed and a differentially stretched and compressed curved configuration when flexed.
Owner:MEDINOL LTD
Stacked die BGA or LGA component assembly
InactiveUS7215018B2Semiconductor/solid-state device detailsSolid-state devicesEpoxyConductive polymer
The present invention provides an apparatus for vertically interconnecting semiconductor die, integrated circuit die, or multiple die segments. Metal rerouting interconnects which extend to one or more sides of the die or segment can be optionally added to the die or multi die segment to provide edge bonding pads upon the surface of the die for external electrical connection points. After the metal rerouting interconnect has been added to the die on the wafer, the wafer is optionally thinned and each die or multiple die segment is singulated from the wafer by cutting or other appropriate singulation method. After the die or multiple die segments are singulated or cut from the wafer, insulation is applied to all surfaces of the die or multiple die segments, openings are made in the insulation above the desired electrical connection pads, and the die or multiple die segments are placed on top of one another to form a stack. Vertically adjacent segments in the stack are electrically interconnected by attaching a short flexible bond wire or bond ribbon to the exposed electrical connection pad at the peripheral edges of the die which protrudes horizontally from the die and applying electrically conductive polymer, or epoxy, filaments or lines to one or more sides of the stack.
Owner:INVENSAS CORP
Nanowhiskers with PN junctions, doped nanowhiskers, and methods for preparing them
InactiveUS20050006673A1Good electrical conductivityPermitted diffusionPolycrystalline material growthNanoinformaticsHeterojunctionP–n junction
Nano-engineered structures are disclosed, incorporating nanowhiskers of high mobility conductivity and incorporating pn junctions. In one embodiment, a nanowhisker of a first semiconducting material has a first band gap, and an enclosure comprising at least one second material with a second band gap encloses said nanoelement along at least part of its length, the second material being doped to provide opposite conductivity type charge carriers in respective first and second regions along the length of the of the nanowhisker, whereby to create in the nanowhisker by transfer of charge carriers into the nanowhisker, corresponding first and second regions of opposite conductivity type charge carriers with a region depleted of free carriers therebetween. The doping of the enclosure material may be degenerate so as to create within the nanowhisker adjacent segments having very heavy modulation doping of opposite conductivity type analogous to the heavily doped regions of an Esaki diode. In another embodiment, a nanowhisker is surrounded by polymer material containing dopant material. A step of rapid thermal annealing causes the dopant material to diffuse into the nanowhisker. In a further embodiment, a nanowhisker has a heterojunction between two different intrinsic materials, and Fermi level pinning creates a pn junction at the interface without doping.
Owner:QUNANO
Shroud segment and assembly with surface recessed seal bridging adjacent members
A turbine engine shroud segment is provided with a radially outer surface including a pair of spaced apart, opposed first and second edge portion surface depressions, for example spaced circumferentially, having a seal surface shaped to receive, in a surface depression formed between assembled adjacent segments in a shroud assembly, or axially assembled adjacent segments and engine members, a radially outer fluid seal member. The depression portions of a shroud segment are joined with the radially outer surface of the shroud segment through an arcuate transition surface. Stresses generated during engine operation in the shroud material are reduced, enabling practical use of a low ductility material, for example a ceramic matrix composite. The edge portion surface depressions are provided with a first shape and the fluid seal member, disposed at the depression, is provided with a surface of a second shape matched in shape with the first shape.
Owner:GENERAL ELECTRIC CO
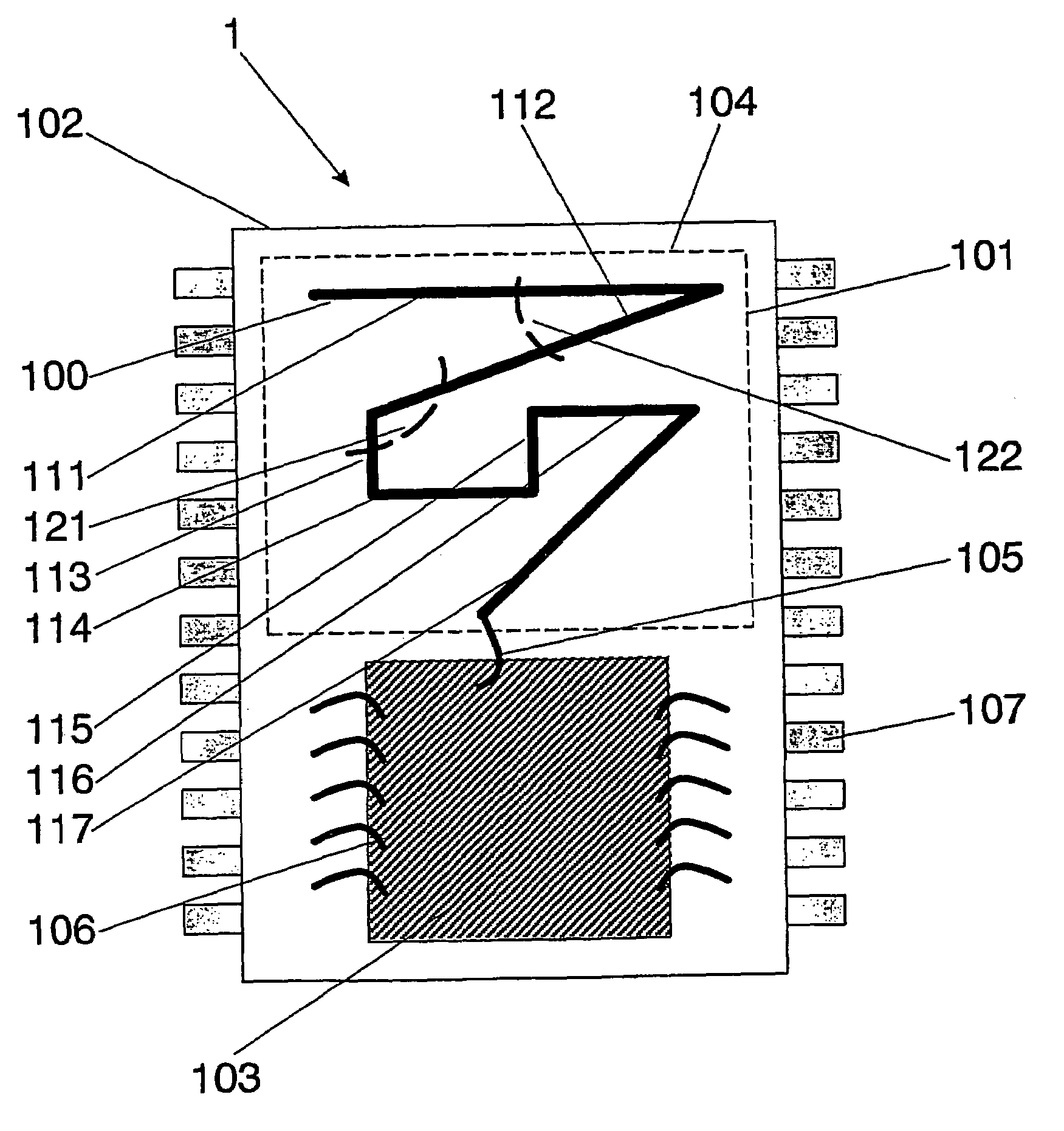
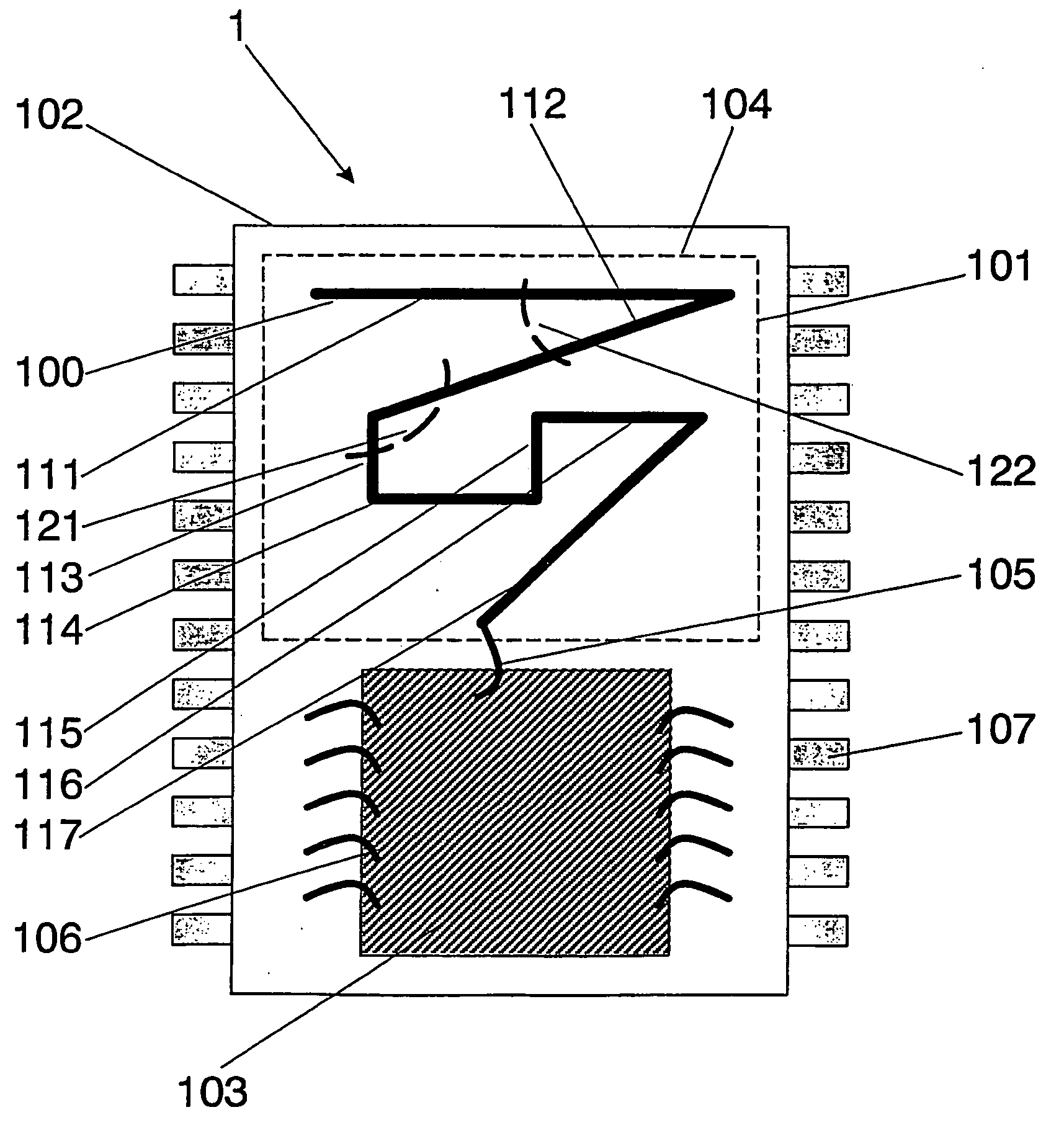
Wireless Implantable Medical Device
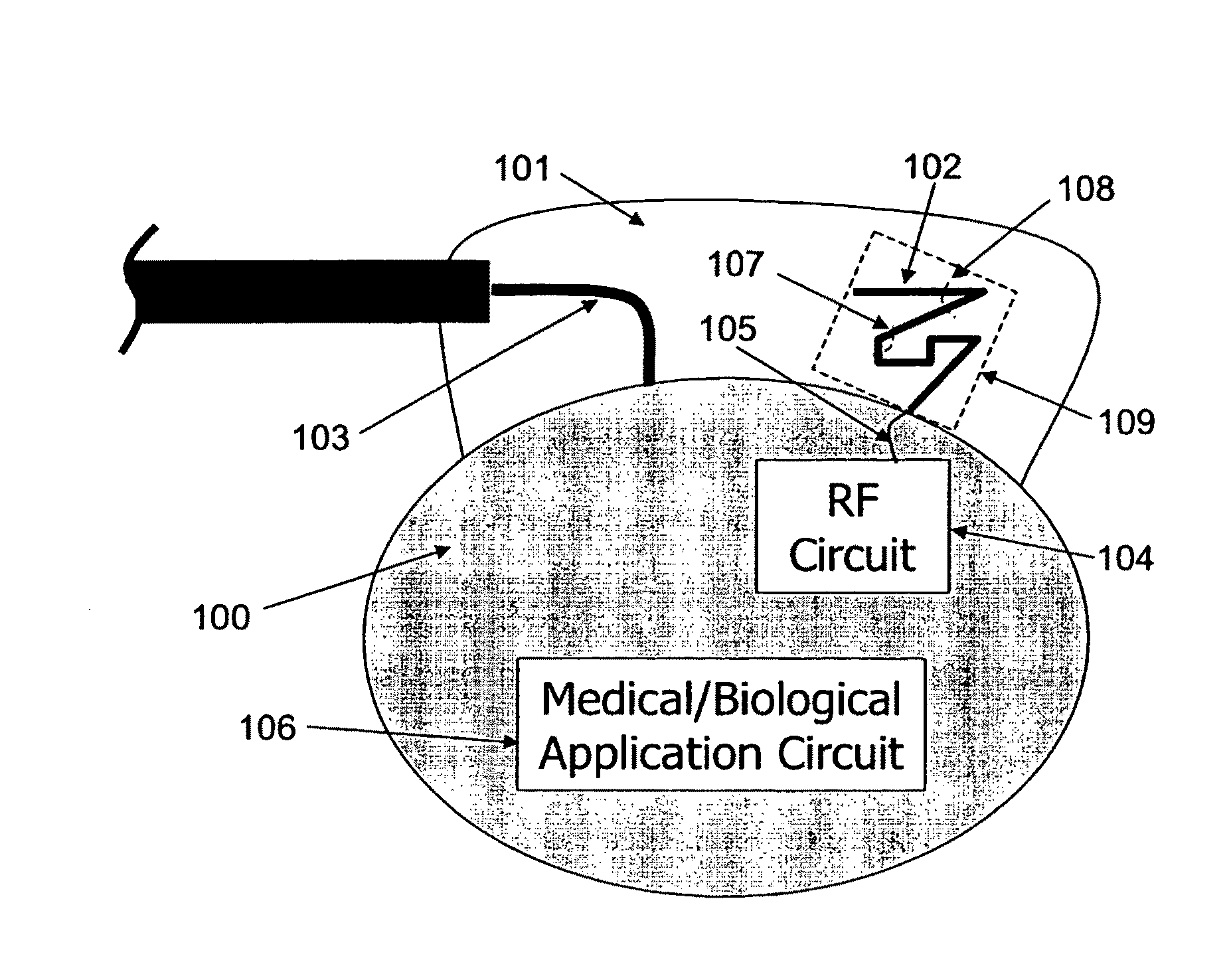
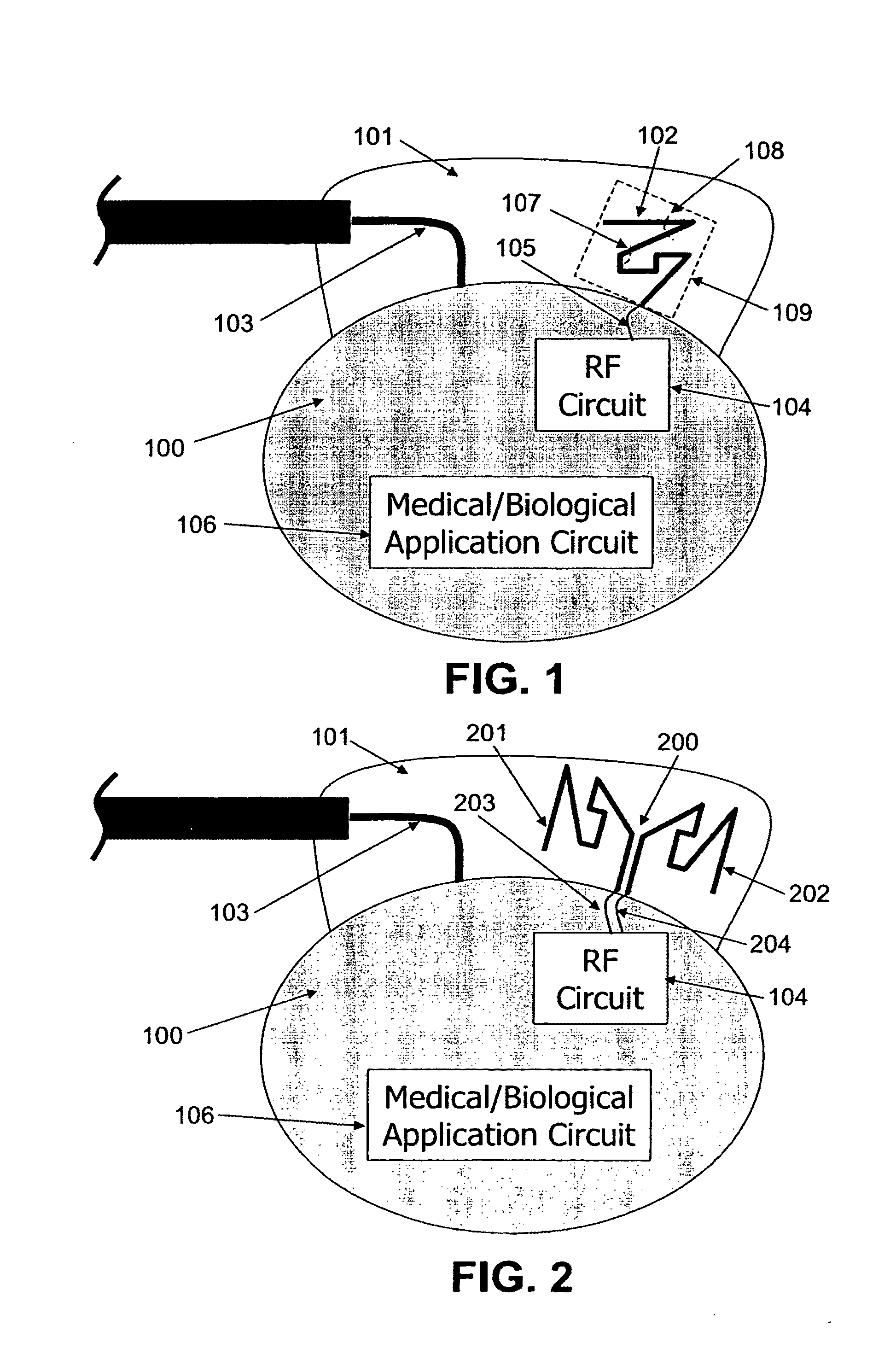
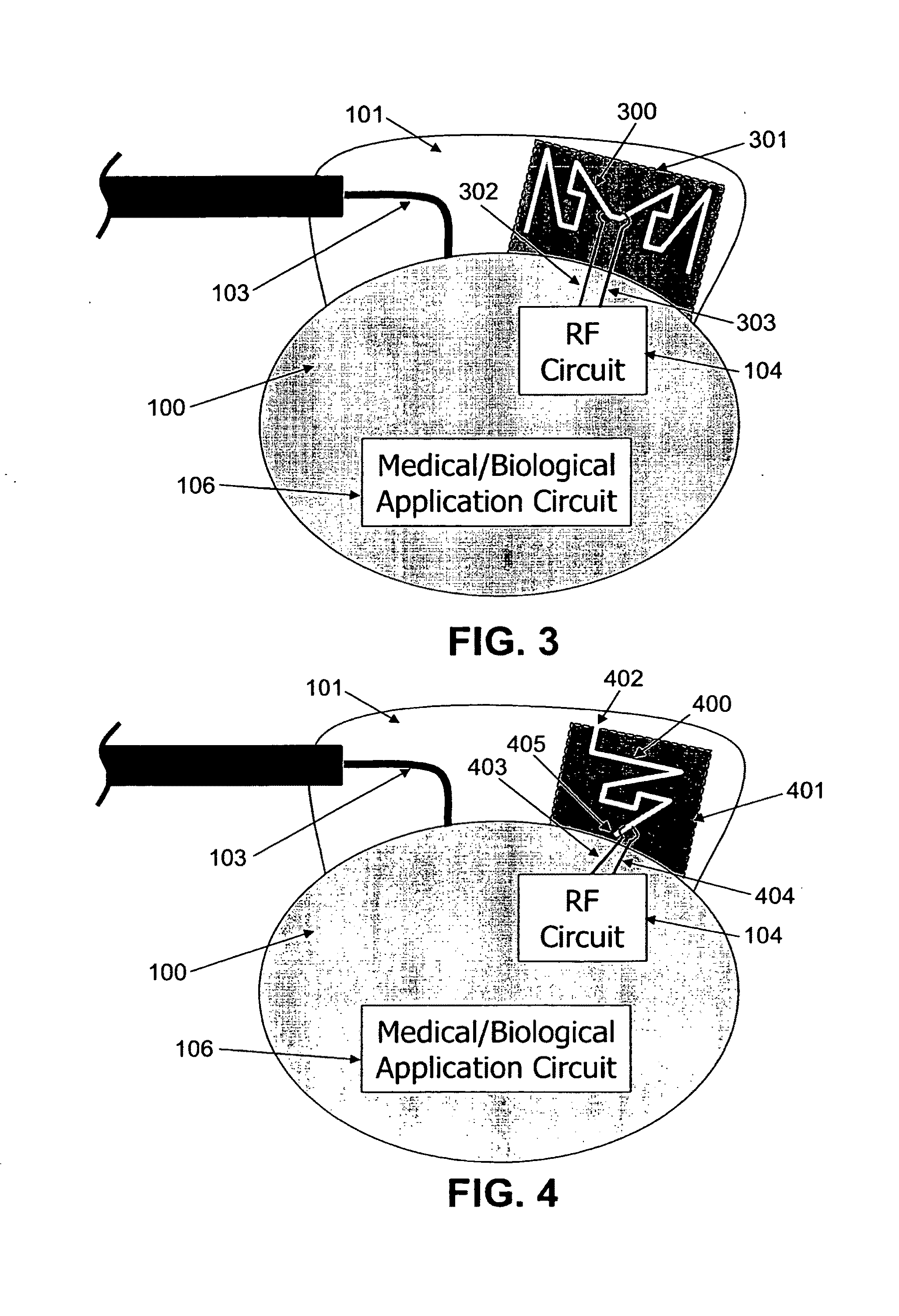
ActiveUS20090248112A1Provide goodLow dielectric constantElectrotherapyRadiating elements structural formsElectromagnetic couplingElectricity
One aspect of the invention relates to an implantable medical device comprising a device housing (100), at least one radio frequency circuit (104) for radio frequency communication, at least one antenna, at least one terminal to electromagnetically couple said at least one antenna to said at least one radio frequency circuit, and a dielectric compartment (101, 1661) that encompasses at least a portion of said at least one antenna. The antenna comprises a conducting pattern, at least a portion of which is shaped as a curve, wherein said curve comprises at least five segments, wherein each of said at least five segments forms an angle with each adjacent segment in said curve, wherein at least three of the at least five segments of said curve are shorter than one-fifth of the longest free-space operating wavelength of the antenna, wherein each angle between adjacent segments is less than 180°, and at least two of the angles between adjacent sections are less than approximately 115°.
Owner:FRACTUS
Device for precision positioning of instruments at a MRI scanner
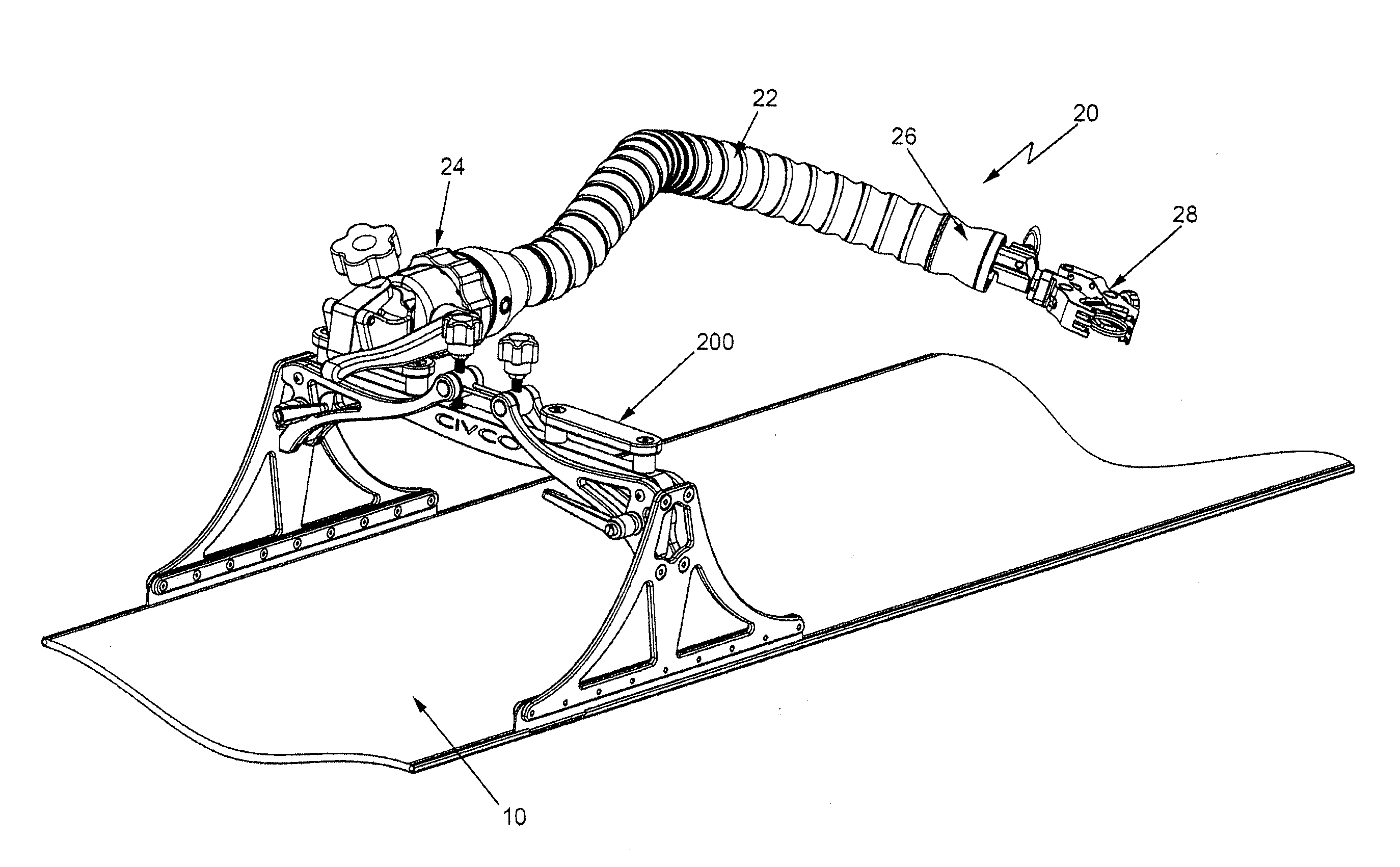
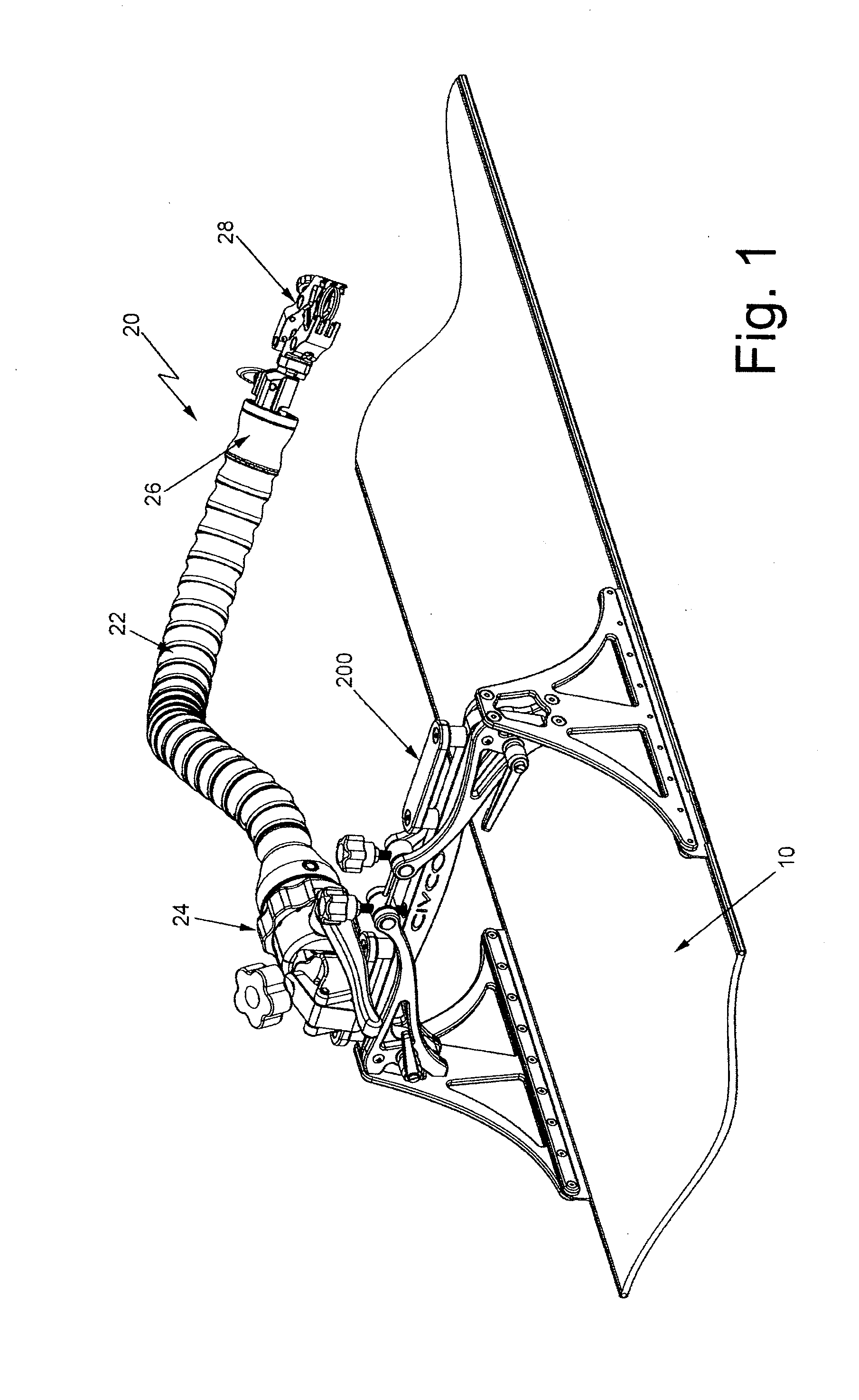
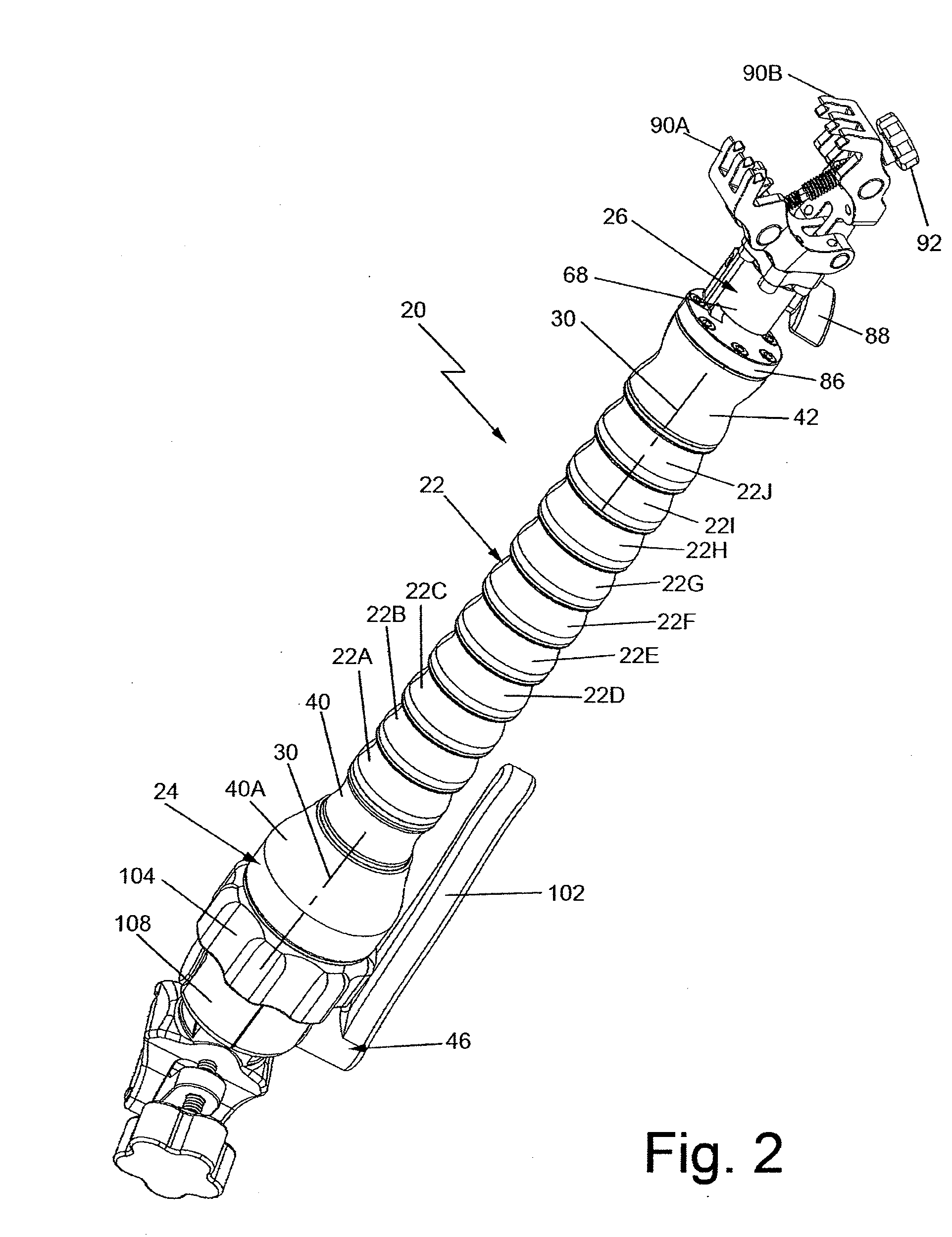
A device in the form of an elongated articulating arm having a base for mounting it on or at the MRI apparatus. The free end of the arm is arranged to releasably mount any type of device, e.g., a clamp, a bracket, a biopsy needle guide, etc. The arm includes a plurality of interconnected segments each of which is arranged to pivot with respect to adjacent segments. A flexible elongated adjustable tensioning member extends through the arm between the base and the distal end portion to enable the arm to be moved or bent into a desired shape when tension is released and then held in that shape when tension is applied.
Owner:CIVCO MEDICAL INSTR CO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com