Implantable and lumen-supporting stents and related methods of manufacture and use
a technology of stents and lumens, applied in the field of implantable medical devices, can solve the problems of reducing the service life of the stent, unable to meet the exactness of the actual stent, and the specific material properties assigned to the modeled stents based on these commercial stents, so as to improve the lateral bending characteristics, improve the service life, and enhance the deflection characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
I. INTRODUCTION
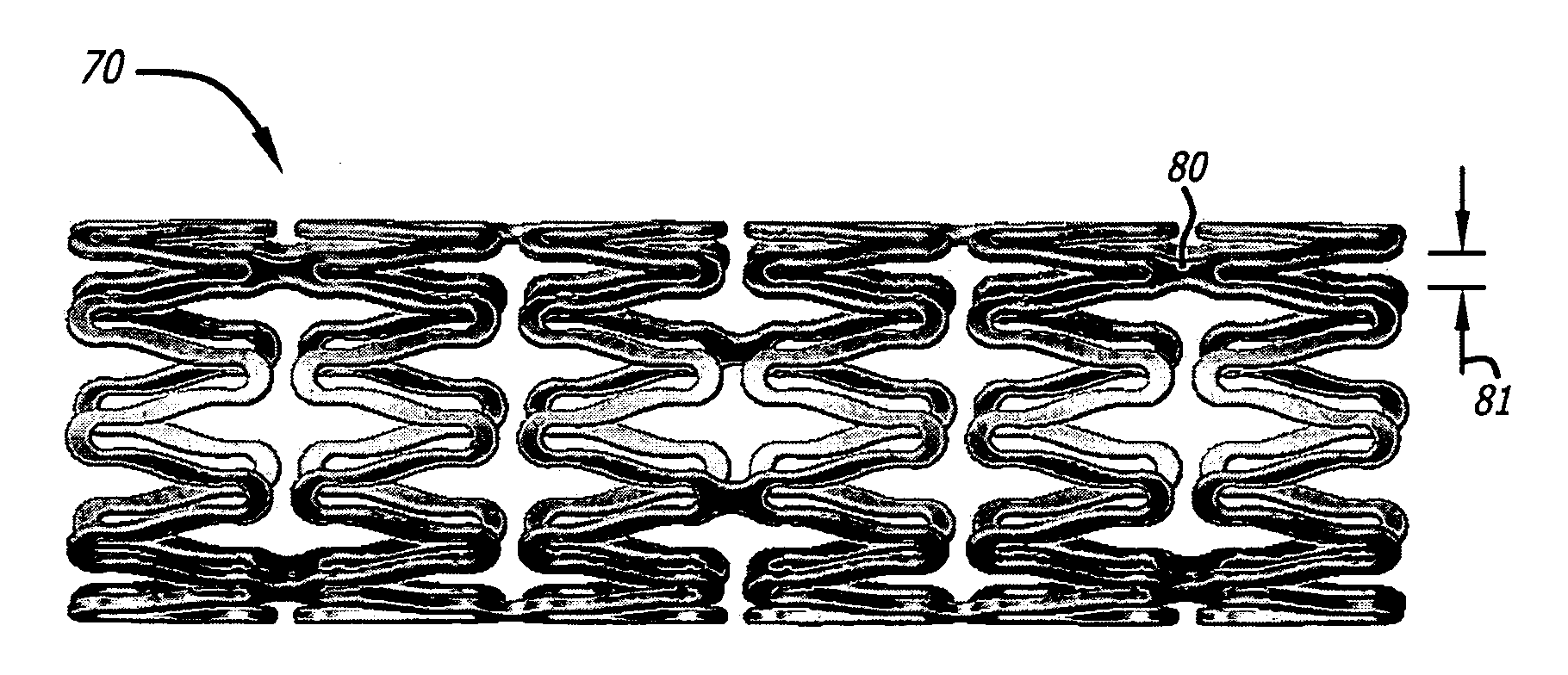
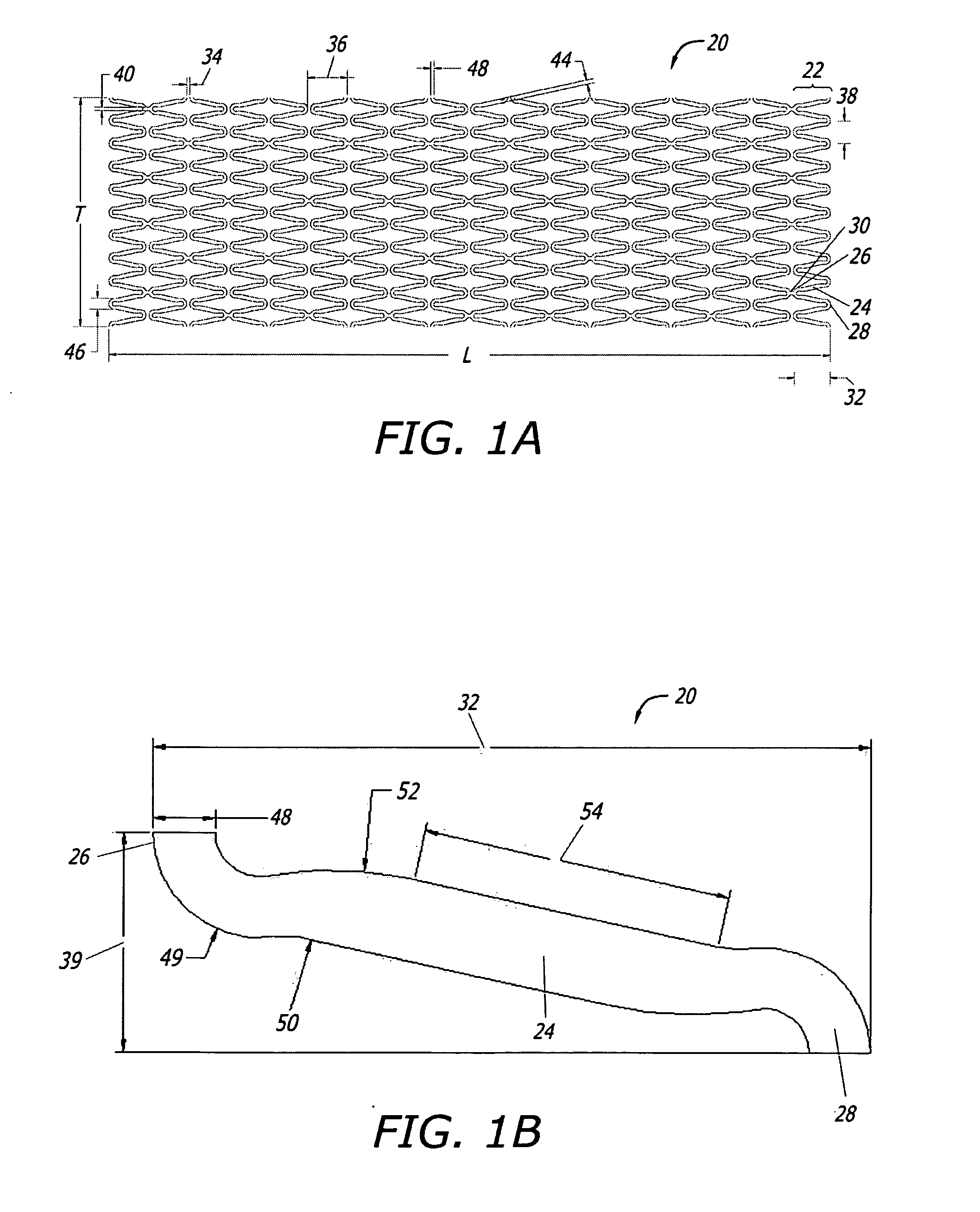
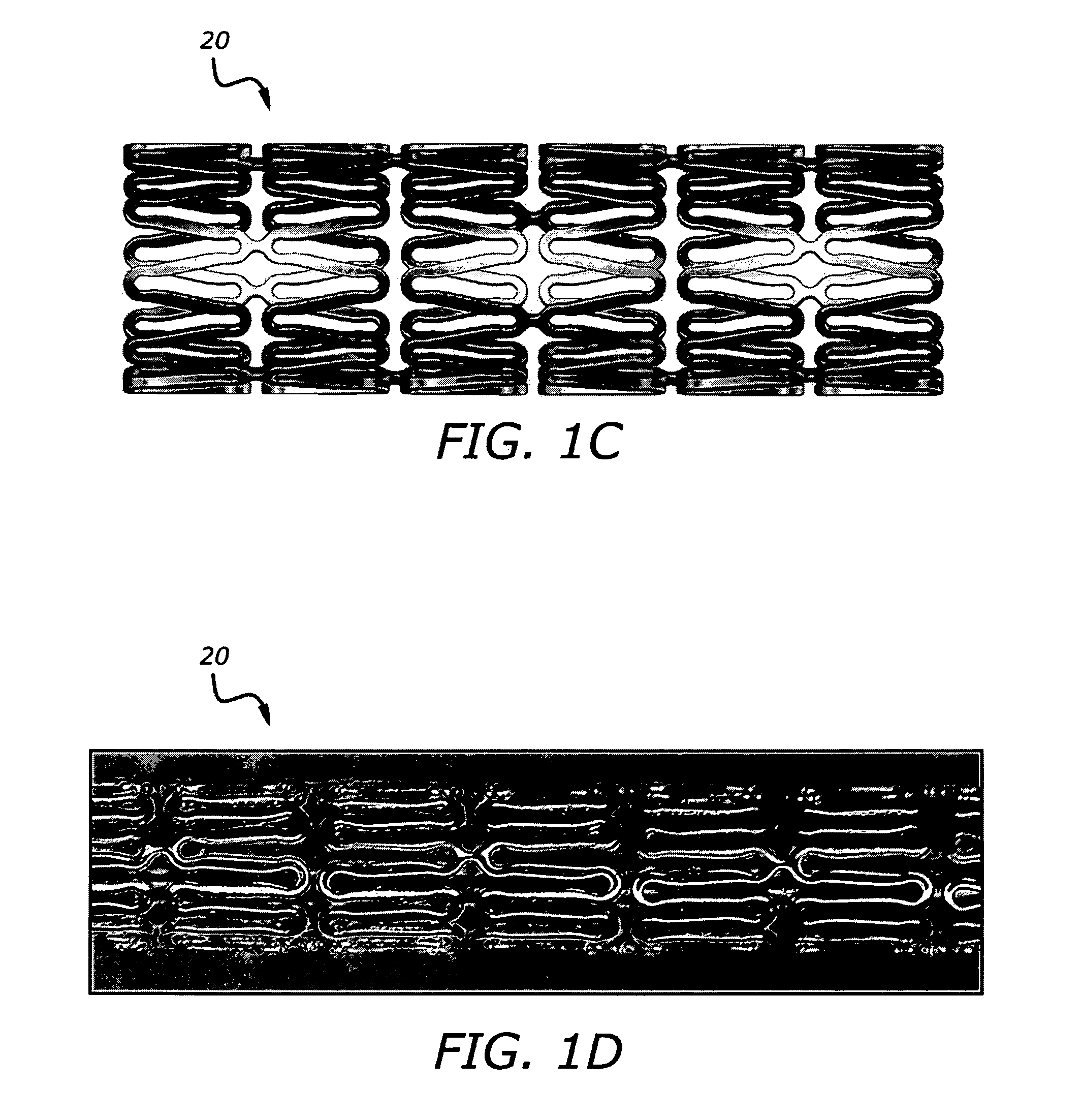
[0193]Certain aspects of exemplary stent constructions consistent with the present embodiments were modeled using 3-Dimensional Finite Element Analysis (3-D FEA). These 3-D FEA models were subjected to certain simulated deflection forces in order to calculate, represent, and evaluate material strain distributions variously within and between representative stent segments of the models under conditions related to radial expansion and lateral bending, respectively.
[0194]Similar 3-D FEA modeling and simulation was performed for two additional stent designs intended to represent certain commercially available stents. More specifically, these two additional stent models were intended to represent certain aspects of two cobalt-chromium stents of different designs, and from two different manufacturers, and that represent the top selling bare metal stents in the world: the “DRIVER®” stent commercially sold by Medtronic, and the “VISION®” stent commercially sold formerly by G...
example 2
[0239]Twenty pigs underwent coronary artery placement of 22 stents of two types as follows: commercially available Driver® stents (n=10); and a physical embodiment similar to that described above with respect to FIGS. 2A-D, referred to here as “Model #2” (n=12) as incorporating similar features as presented for Model #2 in Example #1 simulations presented above. Stents used from each group were provided for intended use in 3.0 mm or 3.5 mm diameter sizes, and were all 18 mm in length. Average vessel diameter at implant was 2.89 mm for the Model #1 group, and 3.04 mm for the Driver® group.
[0240]At day 28, animals were re-studied for angiographic endpoints variously represented in Tables 2A-4B. Certain criteria were measured or calculated and included in these Tables as follows. “Balloon-to-Artery” ratio is a measure of balloon oversizing as related to reference vessel diameter at the time of implant. “Acute gain” is the increase in vessel diameter due to stenting, measured immediatel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com