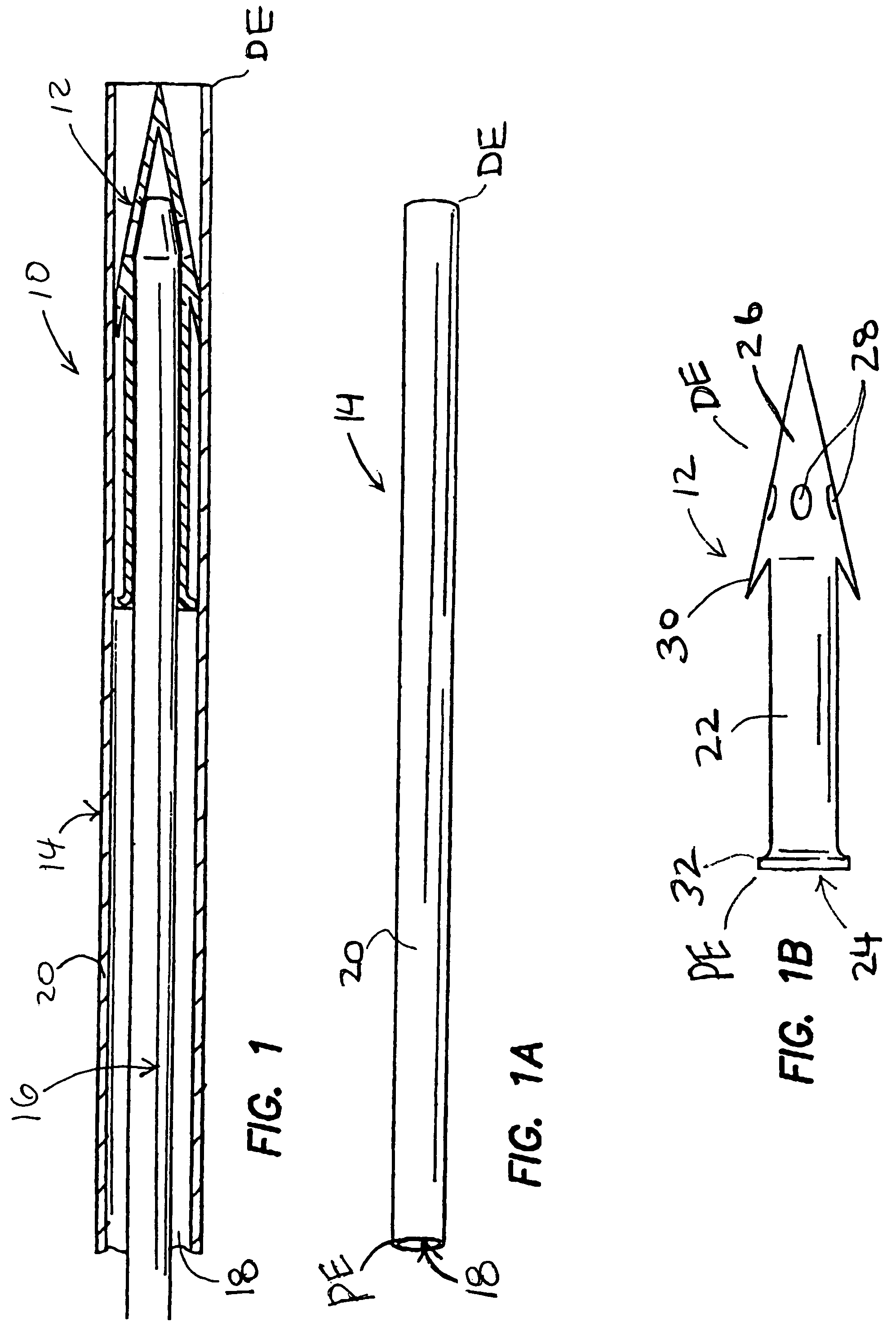
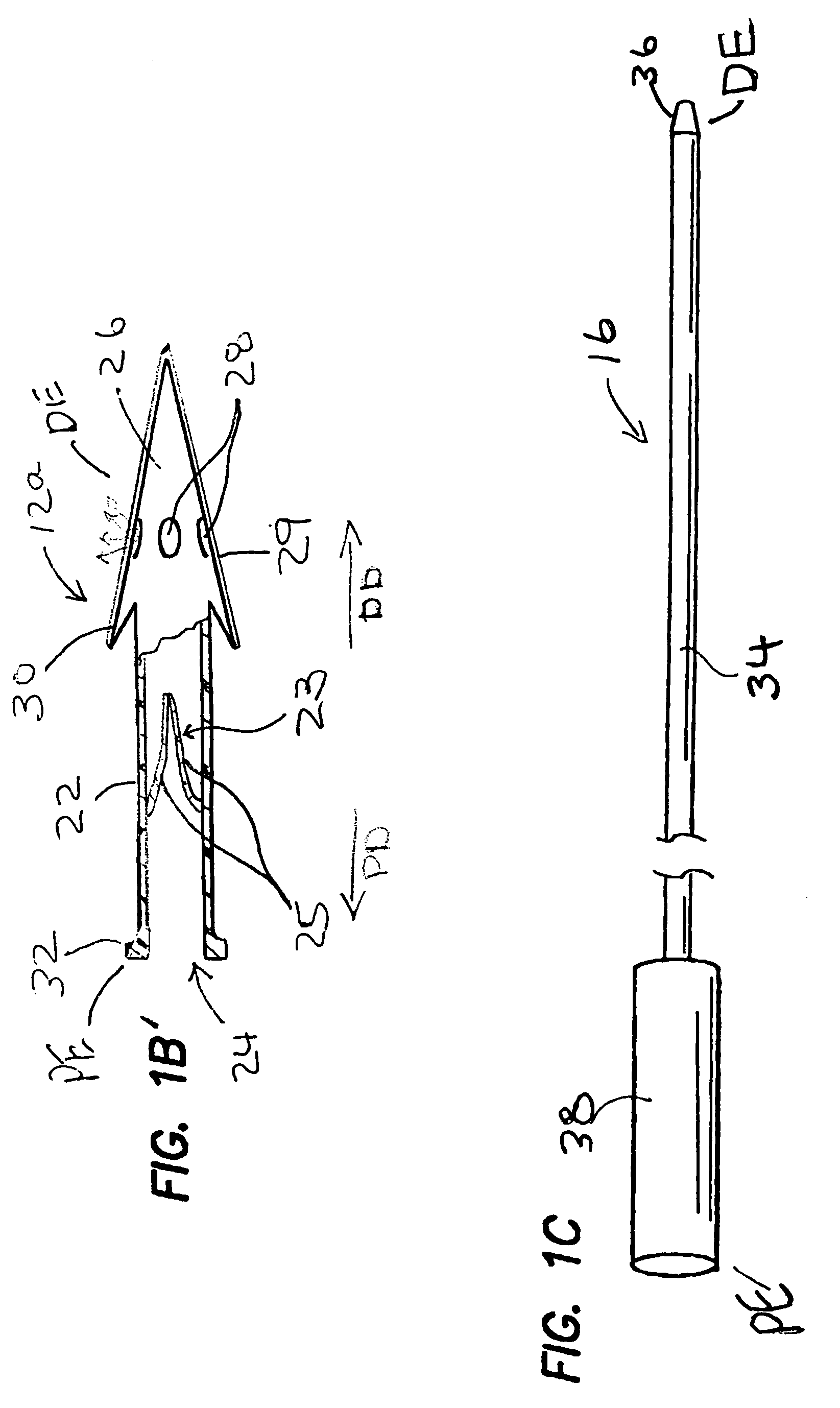
Patents
Literature
95 results about "Stent insertion" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
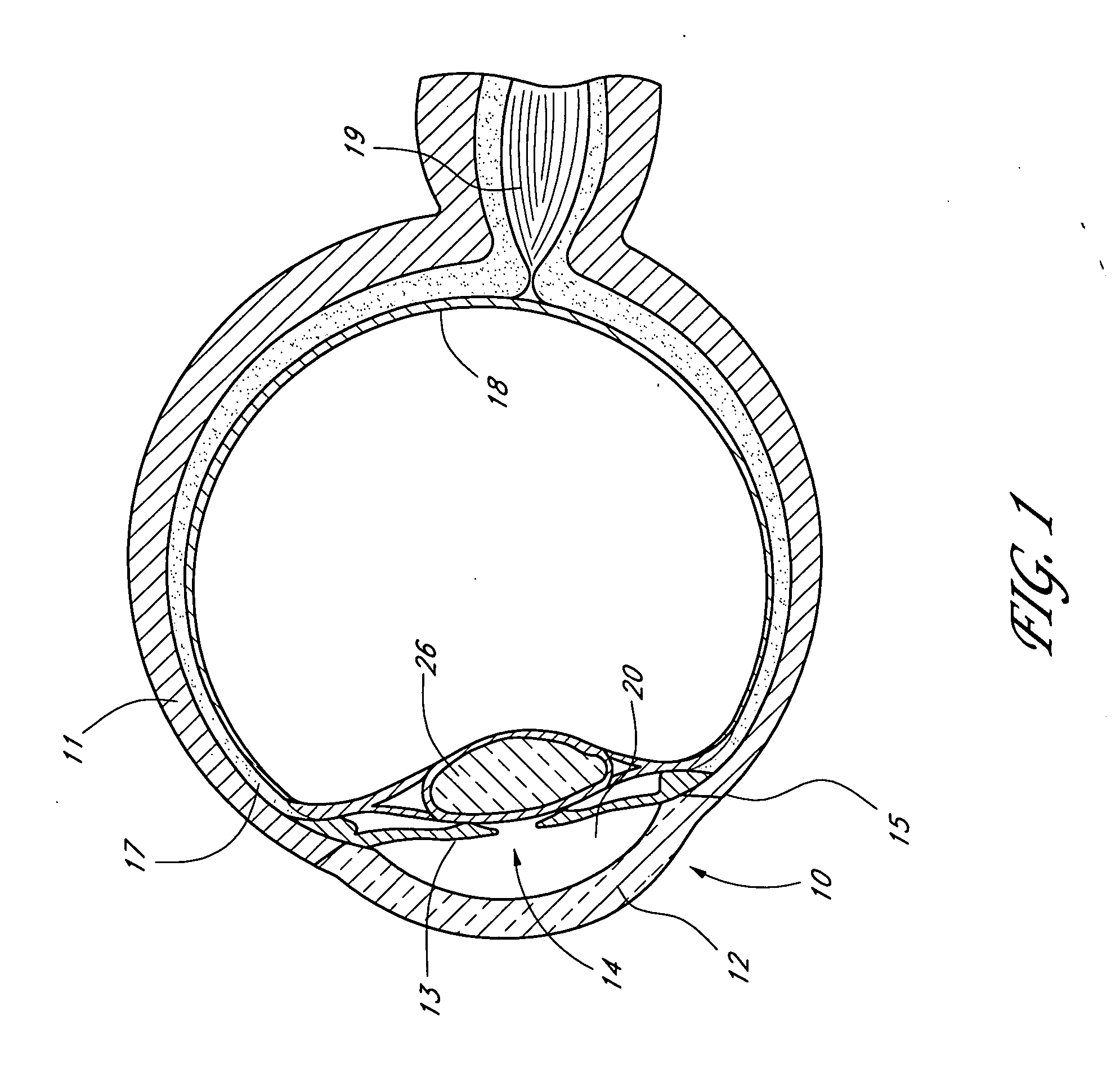
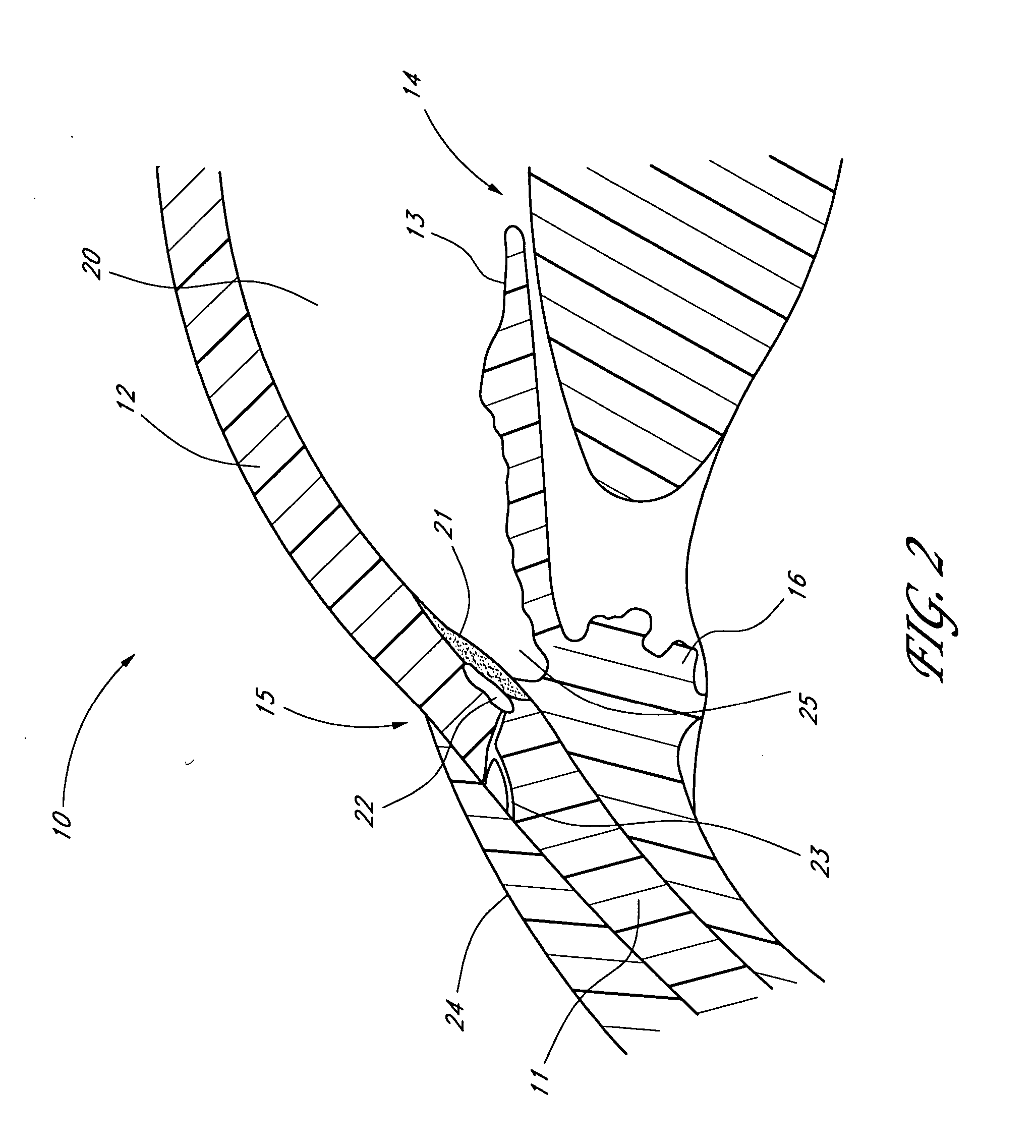
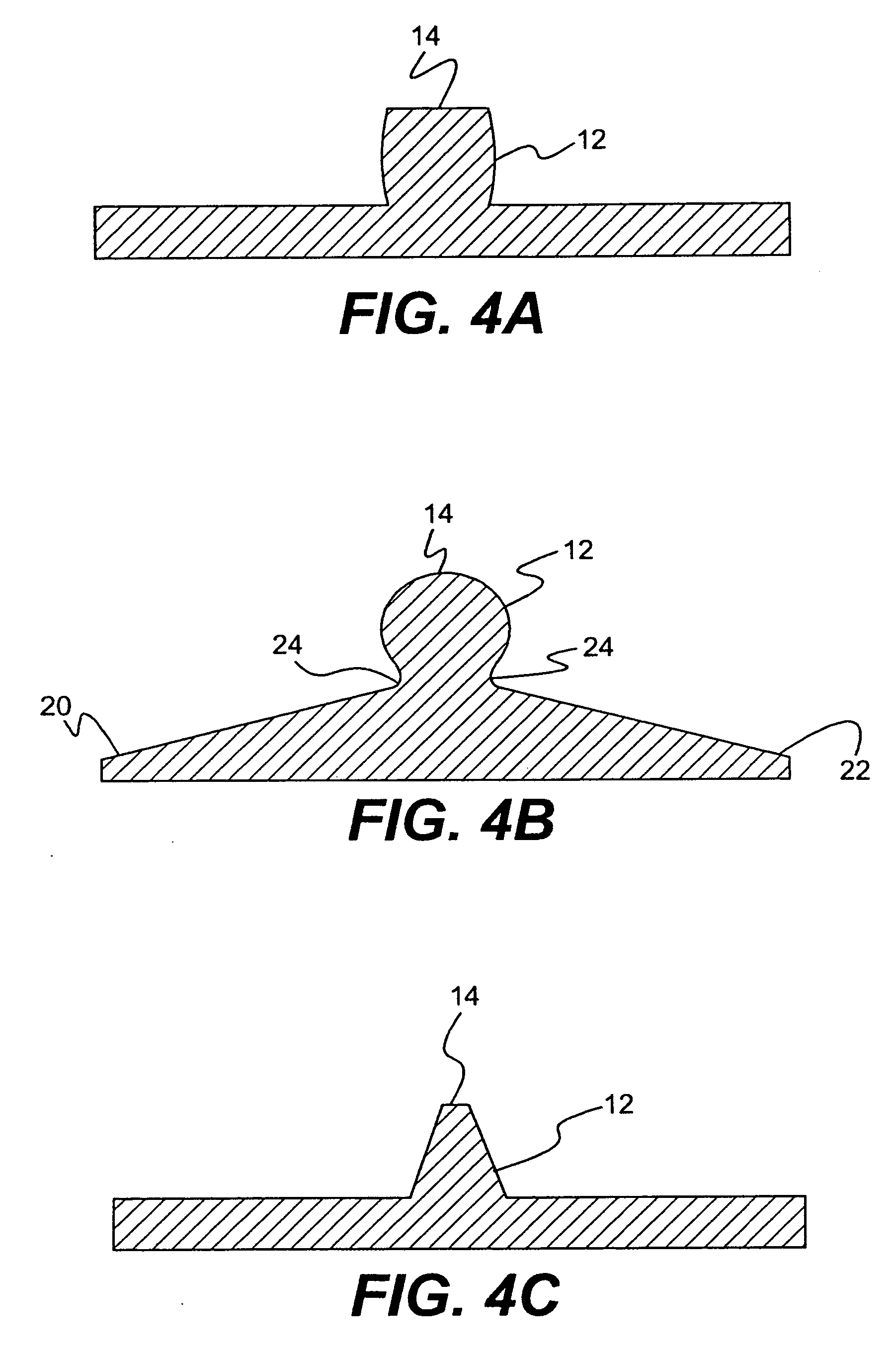
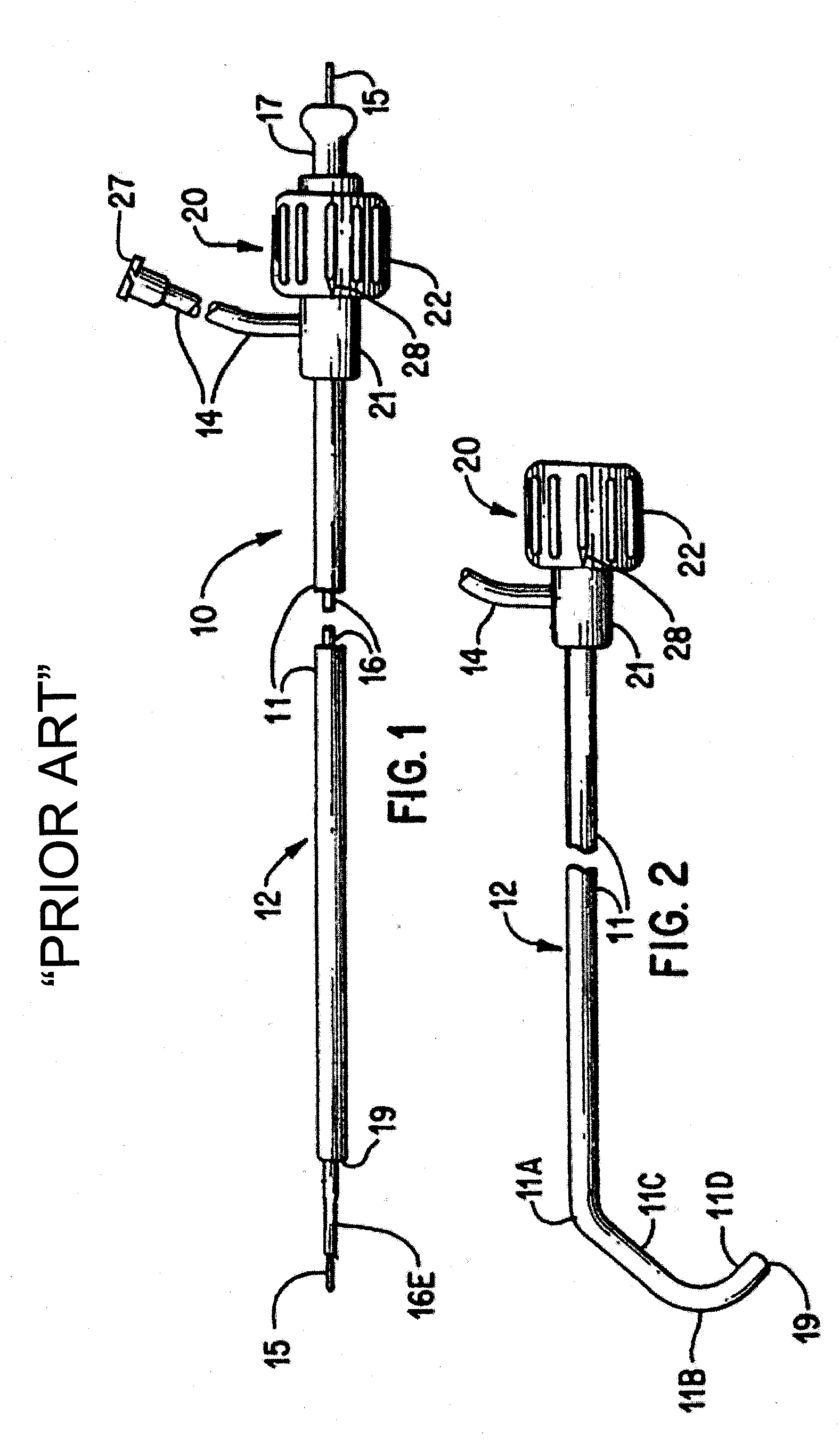
Angioplasty and stent insertion is used to treat narrowing in an artery. Angioplasty uses a small, sausage-shaped balloon to stretch the artery open and improve blood flow. The stent is a small, metal cylinder that acts like a small scaffold to hold the artery permanently open.
System and methods for performing endovascular procedures
InactiveUS20060058775A1Procedure is complicatedEasy to controlStentsGuide needlesExtracorporeal circulationAtherectomy
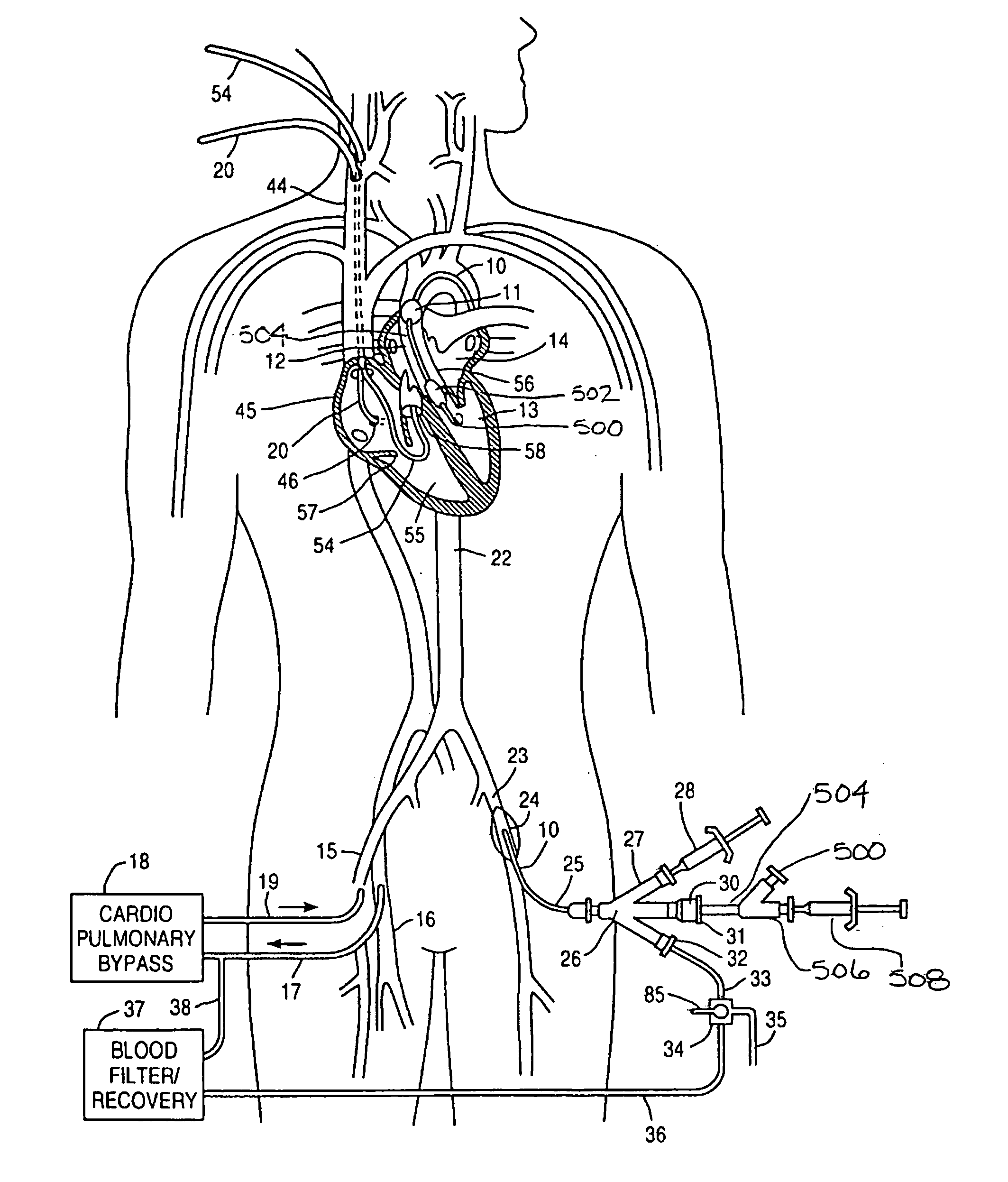
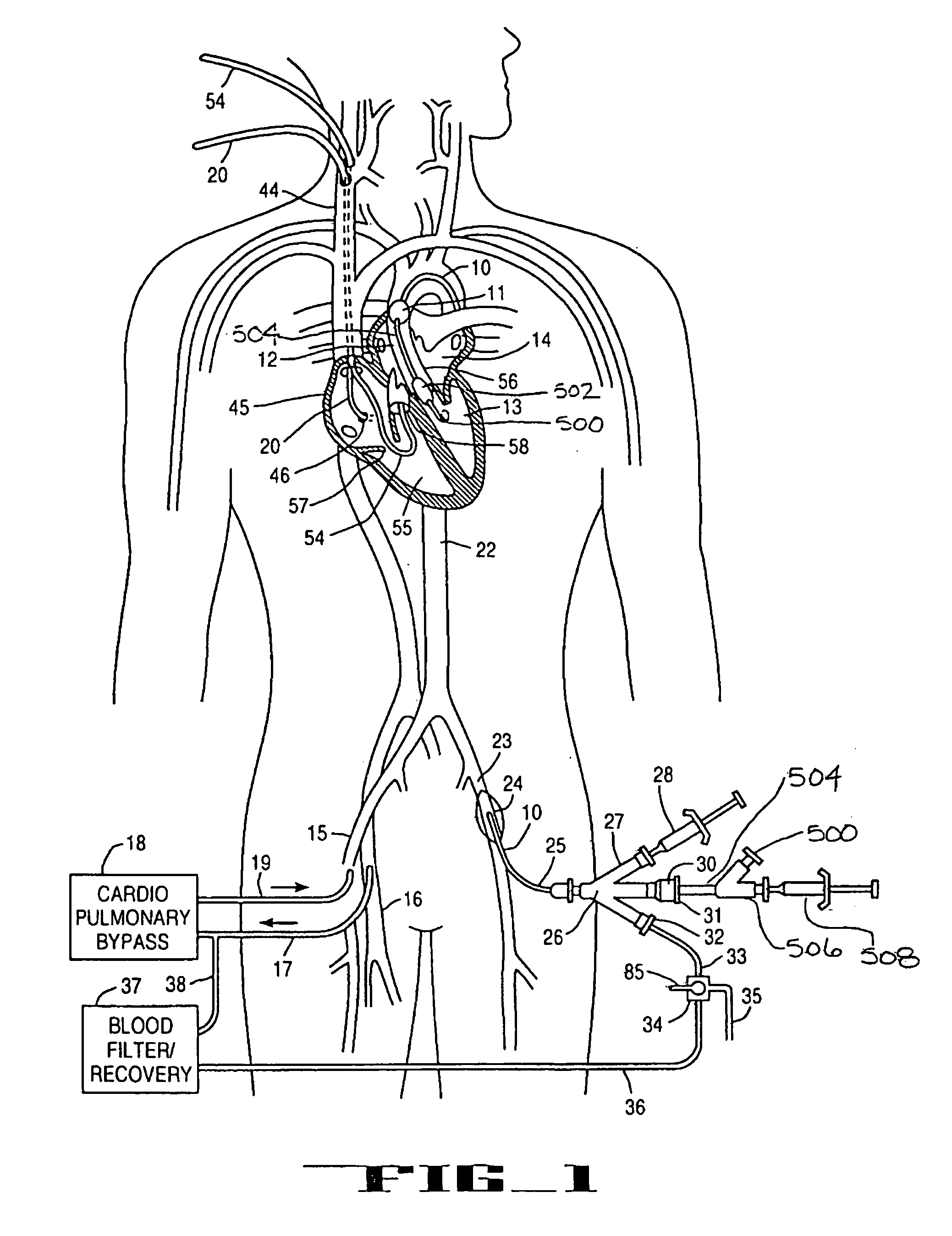
A system for inducing cardioplegic arrest and performing an endovascular procedure within the heart or blood vessels of a patient. An endoaortic partitioning catheter has an inflatable balloon which occludes the ascending aorta when inflated. Cardioplegic fluid may be infused through a lumen of the endoaortic partitioning catheter to stop the heart while the patient's circulatory system is supported on cardiopulmonary bypass. One or more endovascular devices are introduced through an internal lumen of the endoaortic partitioning catheter to perform a diagnostic or therapeutic endovascular procedure within the heart or blood vessels of the patient. Surgical procedures such as coronary artery bypass surgery or heart valve replacement may be performed in conjunction with the endovascular procedure while the heart is stopped. Embodiments of the system are described for performing: fiberoptic angioscopy of structures within the heart and its blood vessels, valvuloplasty for correction of valvular stenosis in the aortic or mitral valve of the heart, angioplasty for therapeutic dilatation of coronary artery stenoses, coronary stenting for dilatation and stenting of coronary artery stenoses, atherectomy or endarterectomy for removal of atheromatous material from within coronary artery stenoses, intravascular ultrasonic imaging for observation of structures and diagnosis of disease conditions within the heart and its associated blood vessels, fiberoptic laser angioplasty for removal of atheromatous material from within coronary artery stenoses, transmyocardial revascularization using a side-firing fiberoptic laser catheter from within the chambers of the heart, and electrophysiological mapping and ablation for diagnosing and treating electrophysiological conditions of the heart.
Owner:EDWARDS LIFESCIENCES LLC
Deformable scaffolding multicellular stent
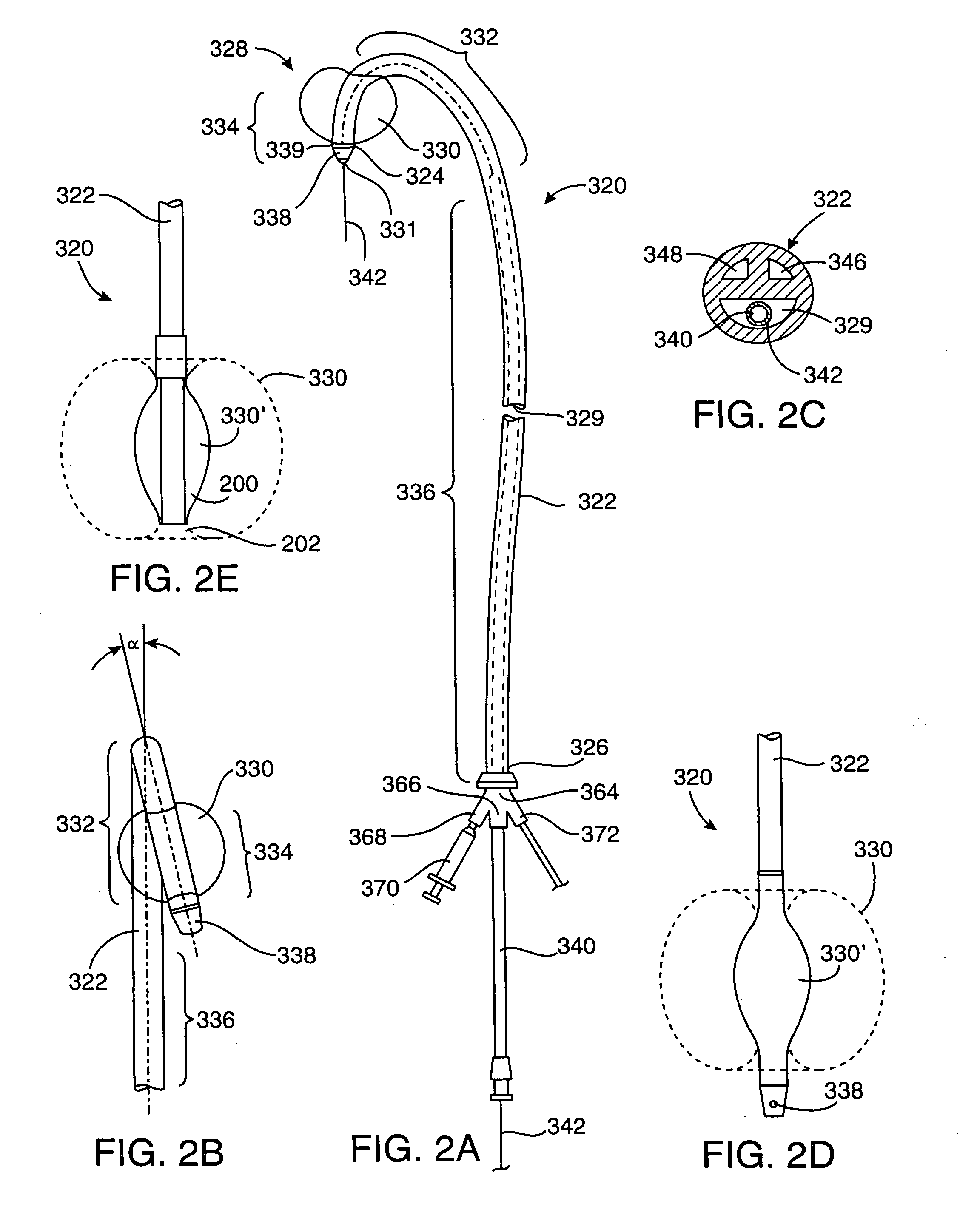
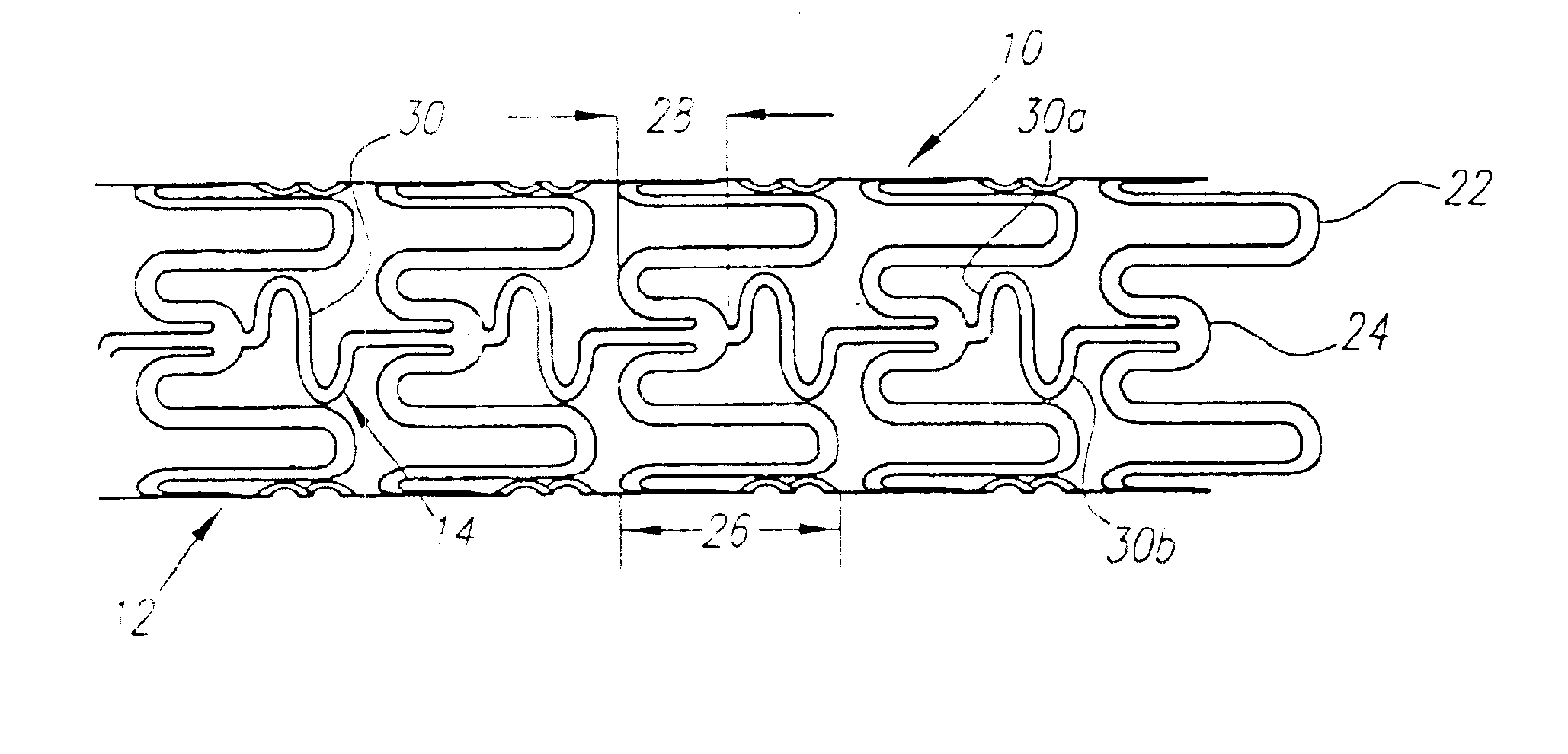
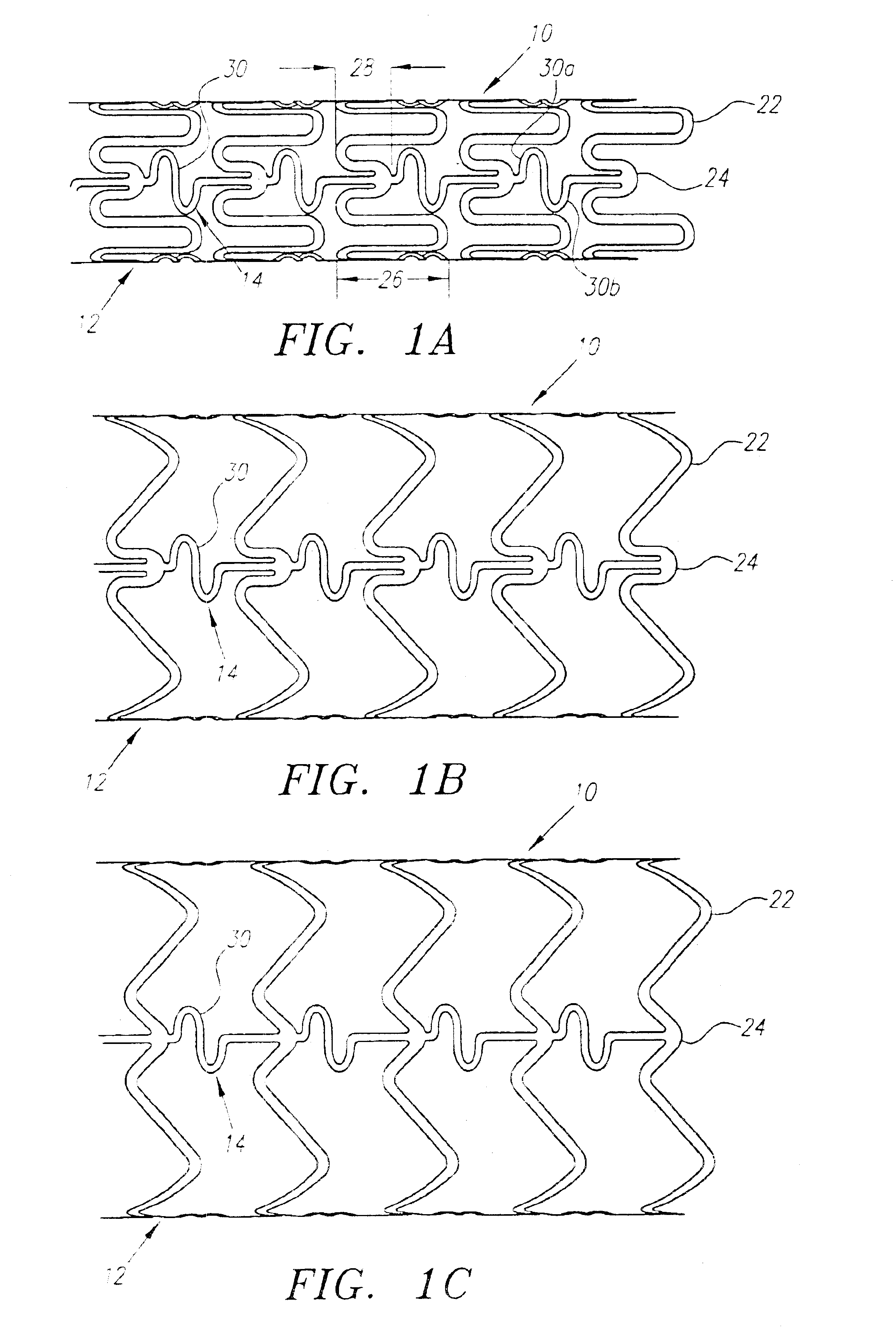
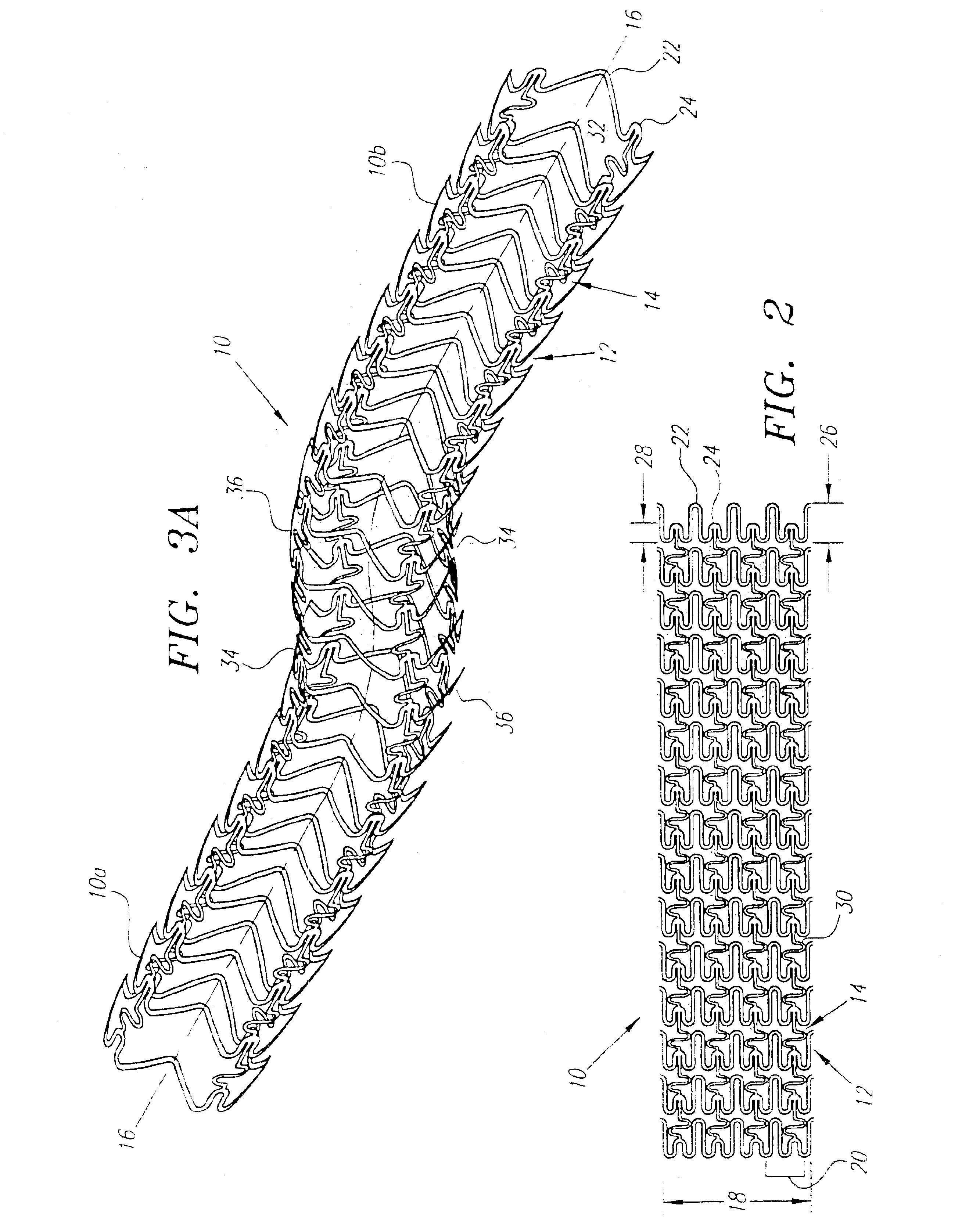
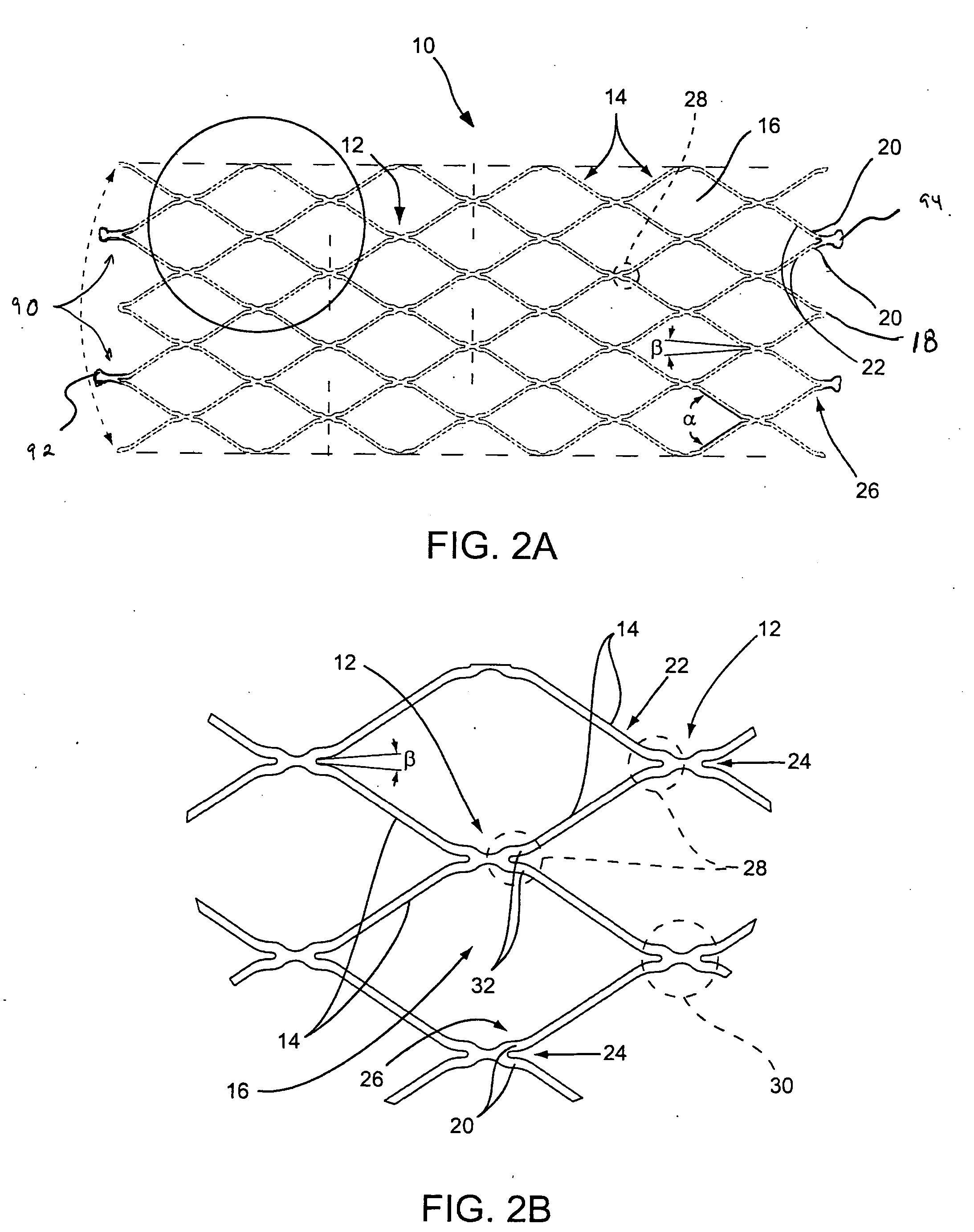
A plastically deformable stent for implantation within a body passage includes a plurality of cylindrical segments, and a plurality of connectors extending between adjacent segments. Each segment has an alternating pattern of curvilinear elements extending about its circumference, including first and second sets of curvilinear elements having different resistances to expansion, and preferably defining “U” shapes with alternating lengths that are connected to one another to define a substantially sinusoidal pattern. The connectors define a sinusoidal shape adapted to extend and compress axially substantially evenly when the adjacent segments are subjected to bending. The stent may be delivered on a device including an elongate member with a nose cone, an expandable member, and a proximal shoulder thereon, and an outer sheath for slidably receiving the elongate member therein. The outer sheath and / or nose cone may have perfusion holes for allowing continued perfusion of fluid during stent delivery. The device may be used in a method for implanting a stent within a curved region of a body passage, particularly for creating and / or maintaining a channel connecting a vein to an adjacent artery, preferably in the coronary system.
Owner:MEDTRONIC VASCULAR INC
Spinal disc annulus reconstruction method and deformable spinal disc annulus stent
InactiveUS20060173545A1Reduce riskImprove integritySuture equipmentsTelevision system detailsInsertion stentNormal cell
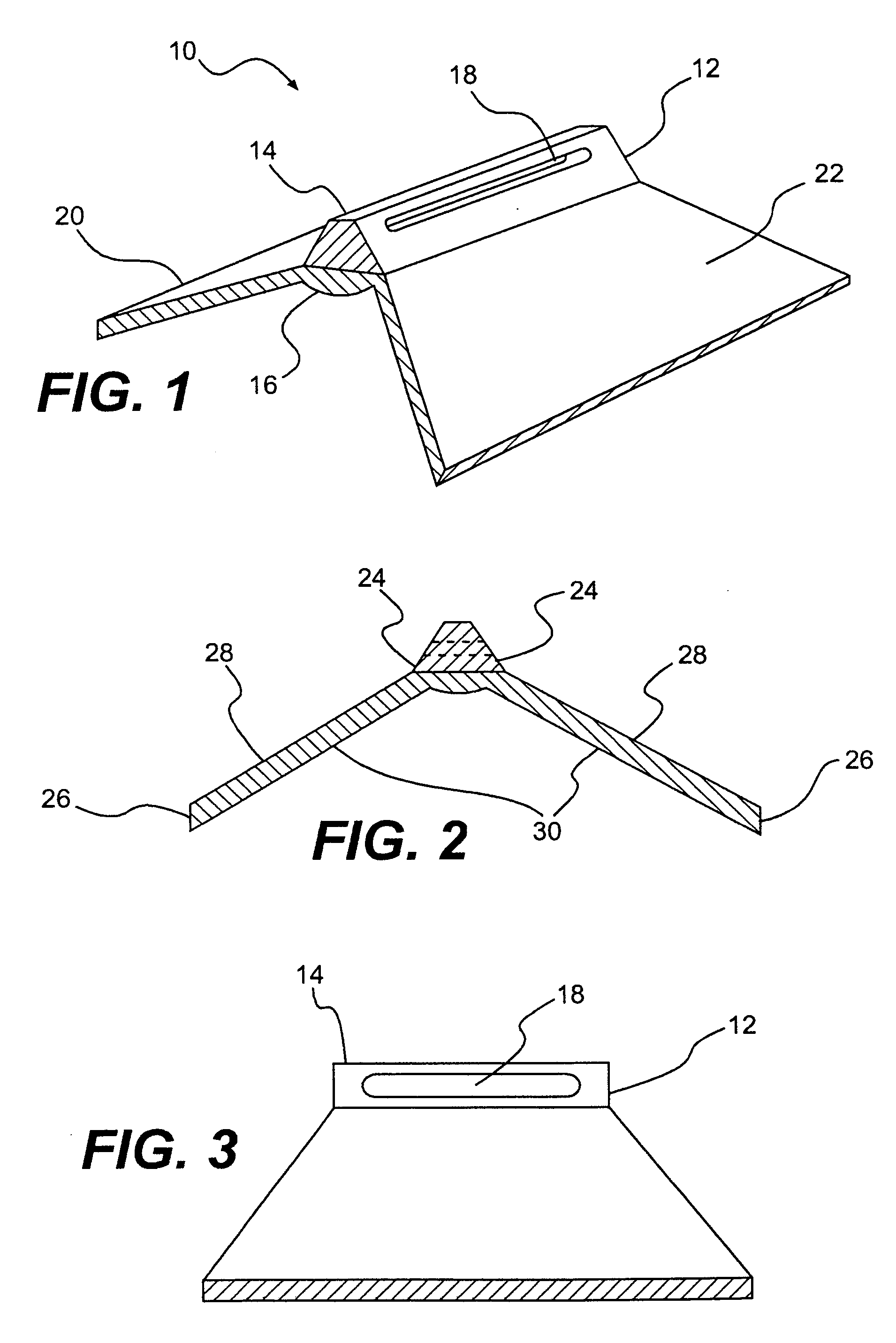
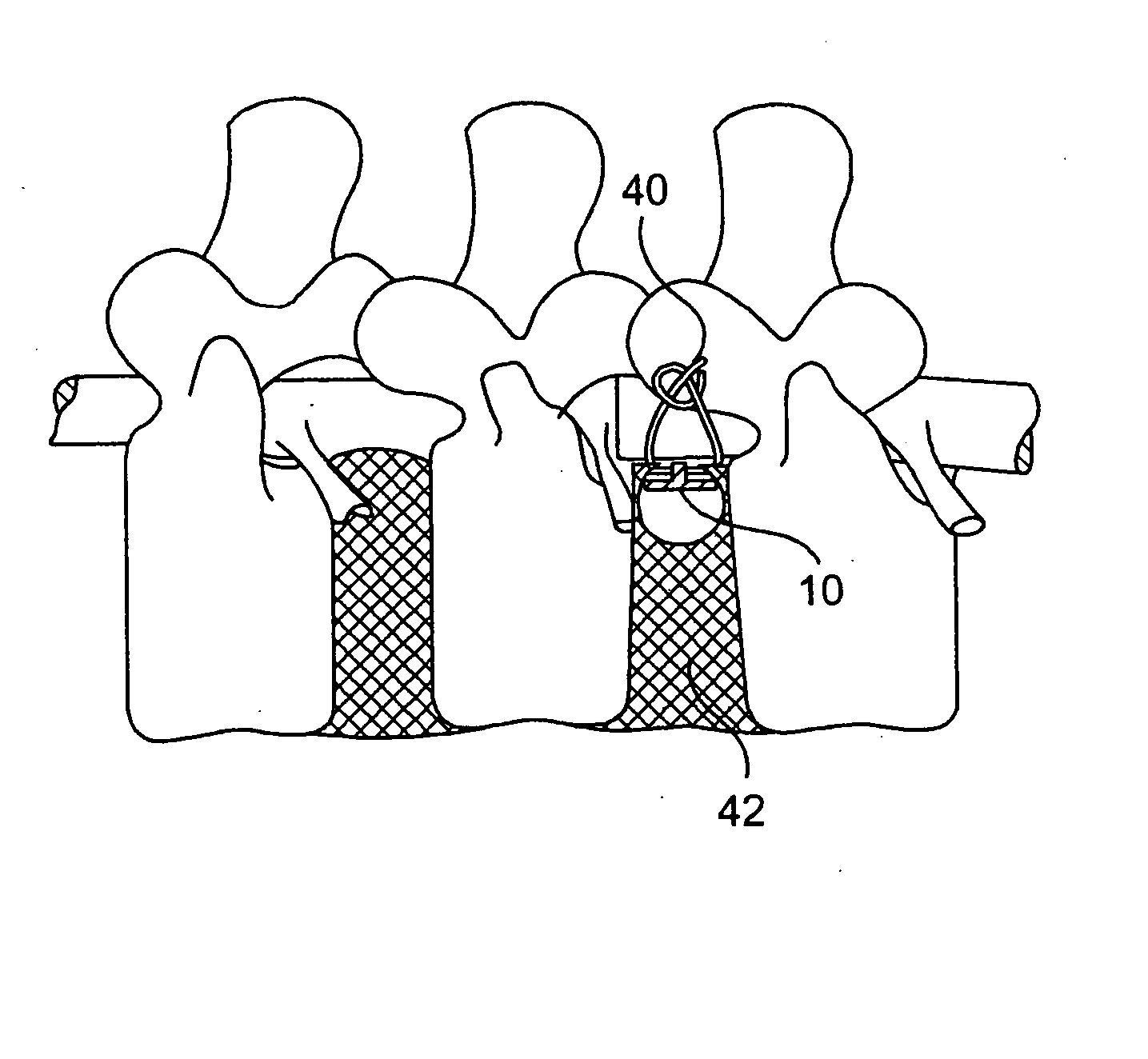
A spinal disc annulus repair stent for repair and reconstruction of the spinal disc wall (annulus) after surgical invasion or pathologic rupture, which may incorporate suture closure or other means of stent insertion and fixation, designed to reduce the failure rate of conventional surgical procedures on the spinal discs. In an illustrative embodiment, the design of the spinal disc annulus stent advantageously allows ingrowth of normal cells of healing in an enhanced fashion strengthening the normal reparative process.
Owner:ANULEX TECH
Glaucoma stent system
Surgical methods and related medical devices for treating glaucoma are disclosed. The method comprises trabecular bypass surgery, which involves bypassing diseased trabecular meshwork with the use of a stent implant. The stent implant is inserted into an opening created in the trabecular meshwork by a piercing member that is slidably advanceable through the lumen of the stent implant for supporting the implant insertion. The stent implant is positioned through the trabecular meshwork so that an inlet end of the stent implant is exposed to the anterior chamber of the eye and an outlet end is positioned into fluid collection channels at about an exterior surface of the trabecular meshwork or up to the level of aqueous veins.
Owner:GLAUKOS CORP
Spinal disc annulus reconstruction method and deformable spinal disc annulus stent
InactiveUS7052516B2Reduce riskImprove integritySuture equipmentsTelevision system detailsSurgical departmentStent insertion
A spinal disc annulus repair stent for repair and reconstruction of the spinal disc wall (annulus) after surgical invasion or pathologic rupture, which may incorporate suture closure or other means of stent insertion and fixation, designed to reduce the failure rate of conventional surgical procedures on the spinal discs. In an illustrative embodiment, the design of the spinal disc annulus stent advantageously allows ingrowth of normal cells of healing in an enhanced fashion strengthening the normal reparative process.
Owner:KRT INVESTORS
Glaucoma stent system
Owner:GLAUKOS CORP
Spinal disc annulus reconstruction method and deformable spinal disc annulus stent
InactiveUS20060167553A1Reduce riskImprove integritySuture equipmentsTelevision system detailsIntervertebral discInsertion stent
A spinal disc annulus repair stent for repair and reconstruction of the spinal disc wall (annulus) after surgical invasion or pathologic rupture, which may incorporate suture closure or other means of stent insertion and fixation, designed to reduce the failure rate of conventional surgical procedures on the spinal discs. In an illustrative embodiment, the design of the spinal disc annulus stent advantageously allows ingrowth of normal cells of healing in an enhanced fashion strengthening the normal reparative process.
Owner:KRT INVESTORS
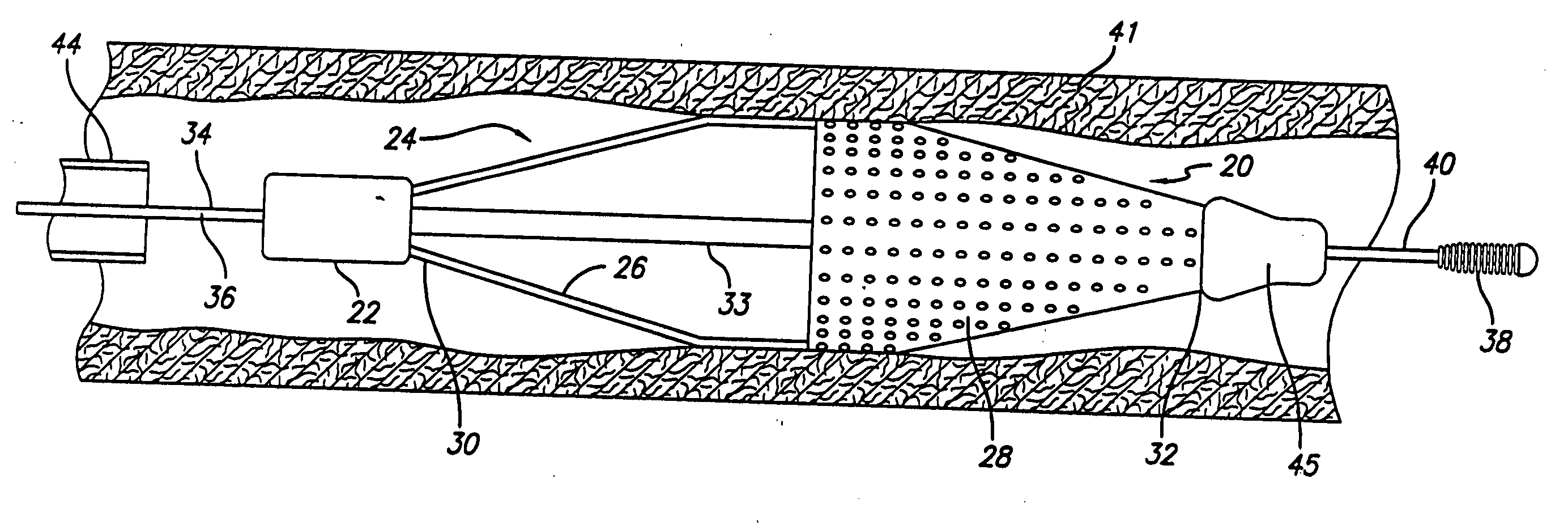
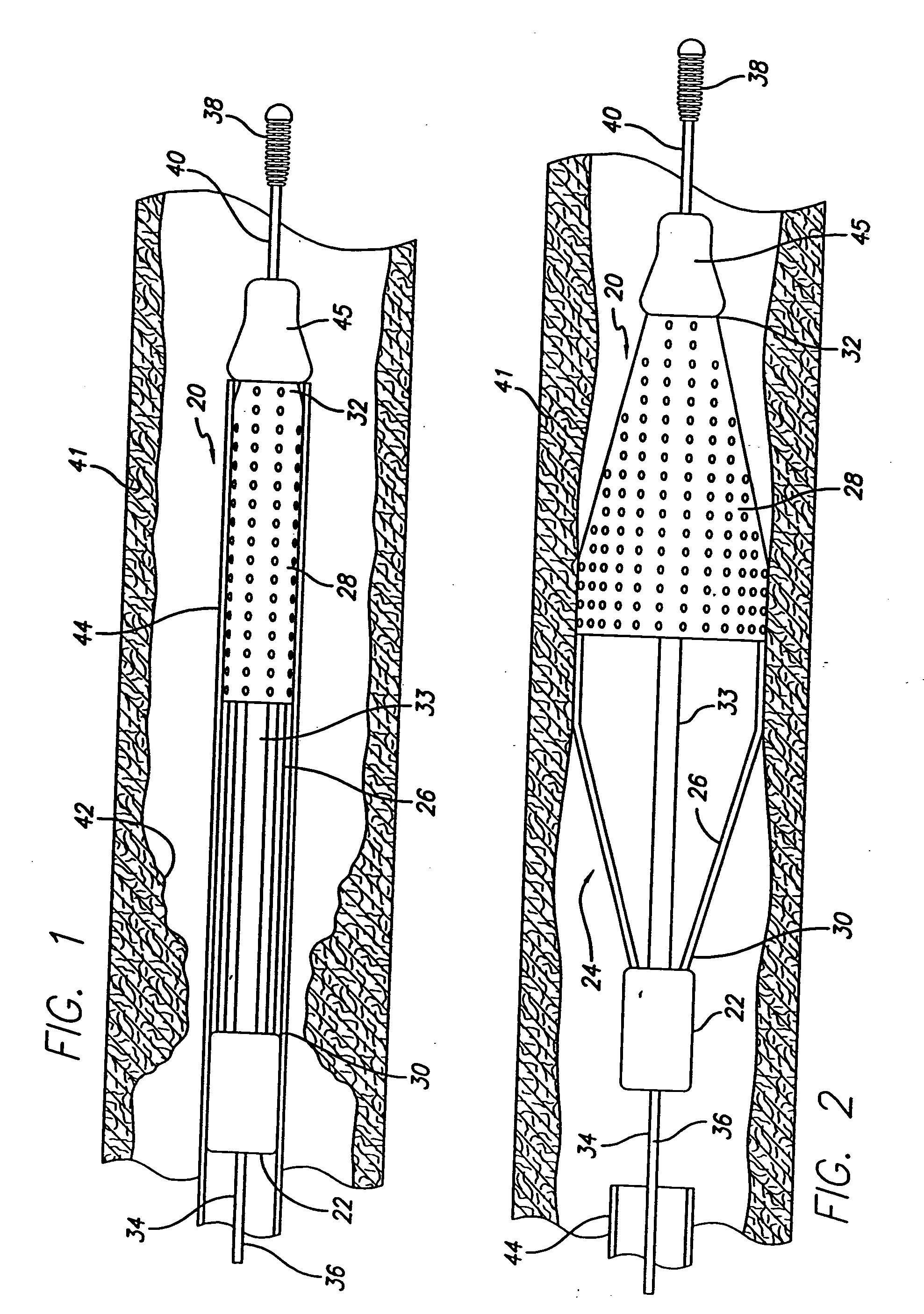
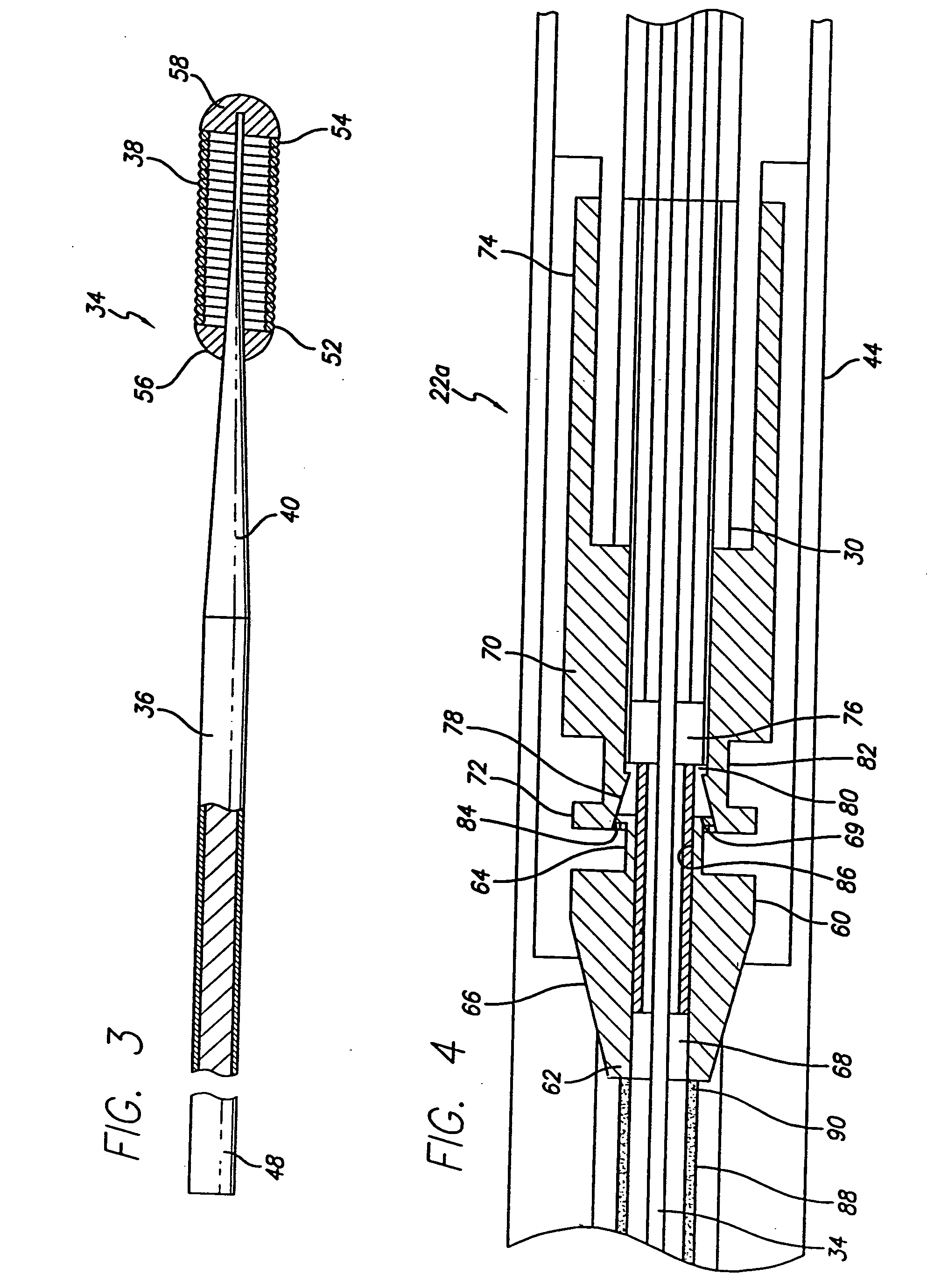
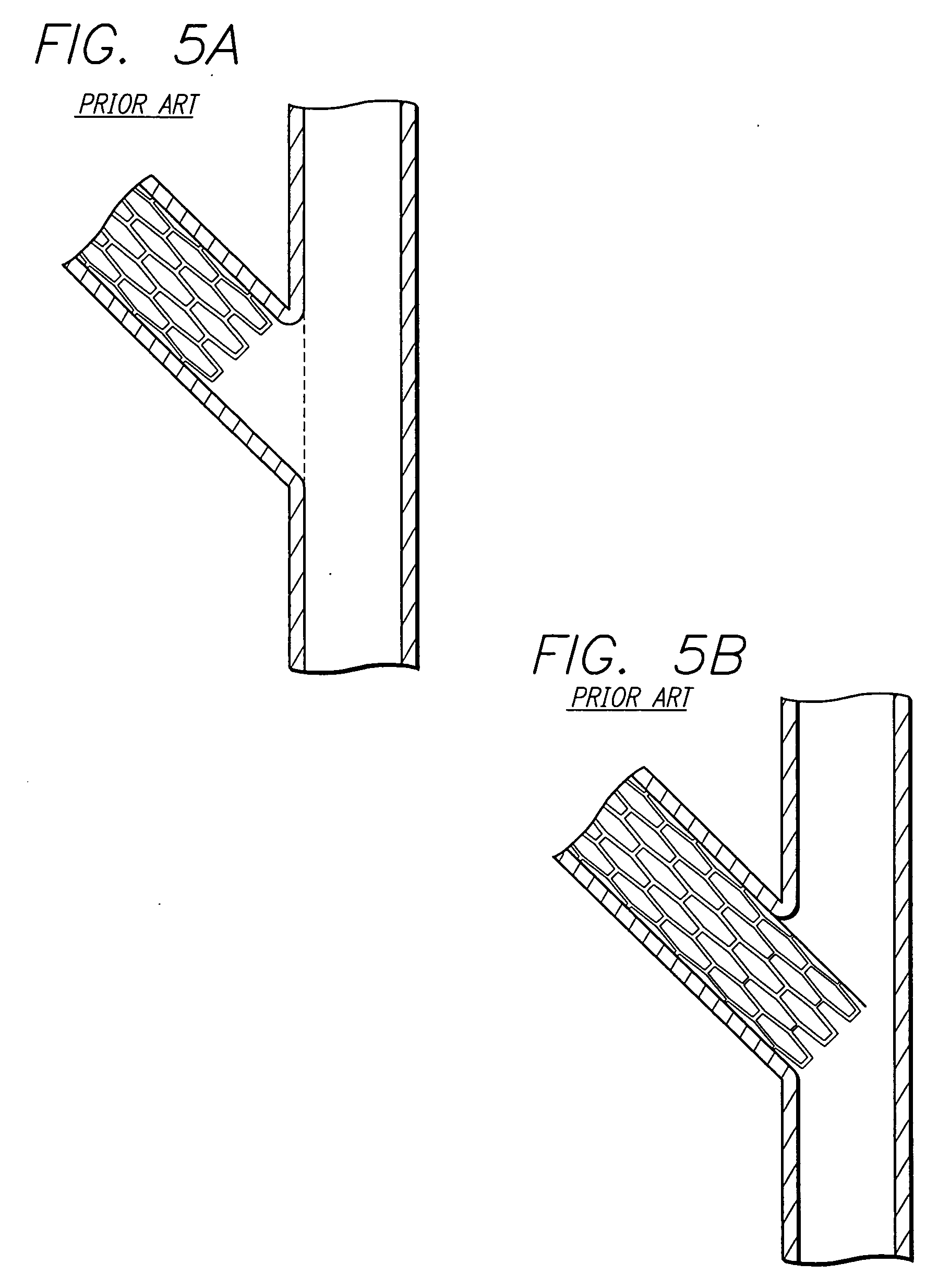
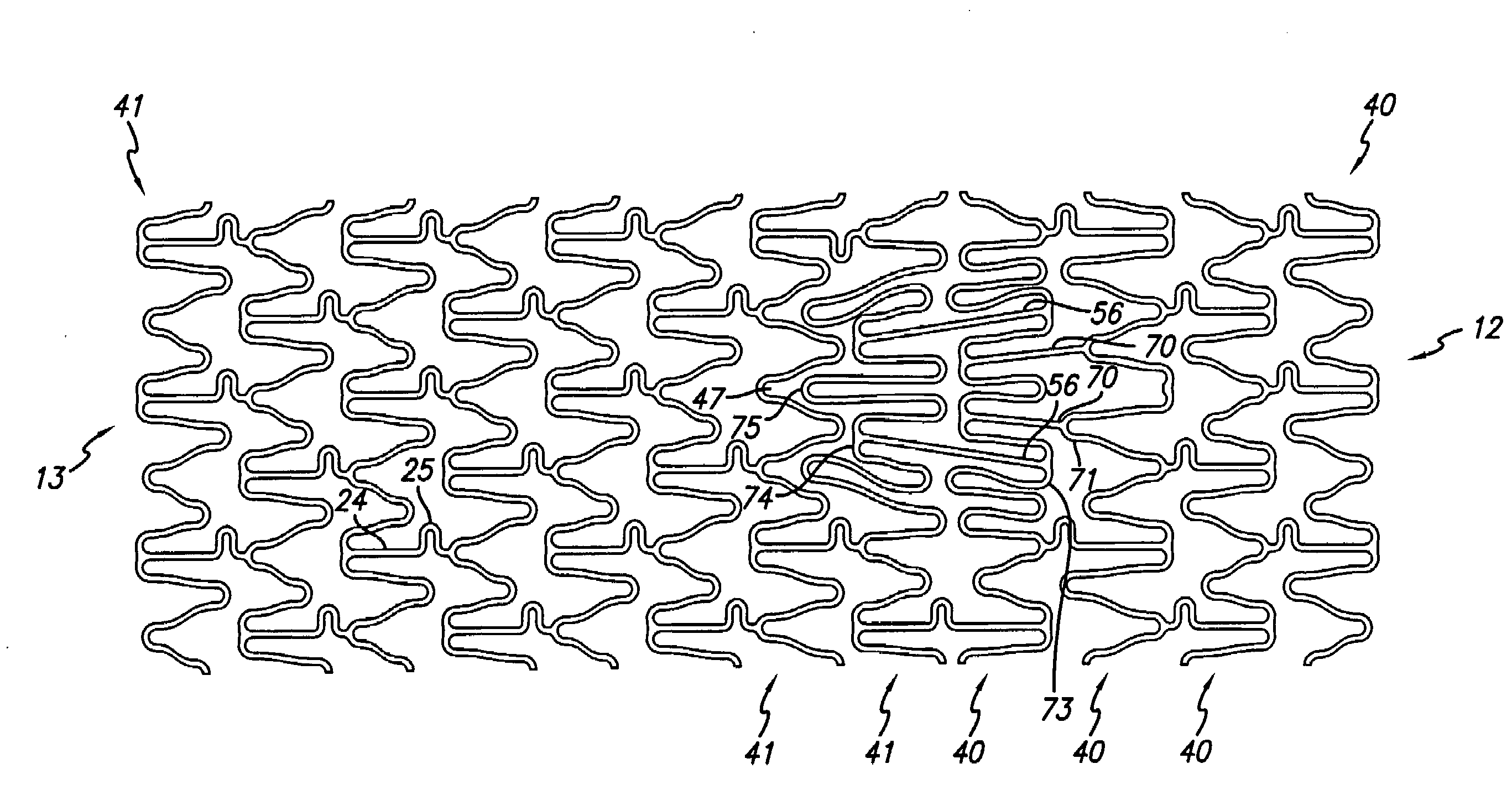
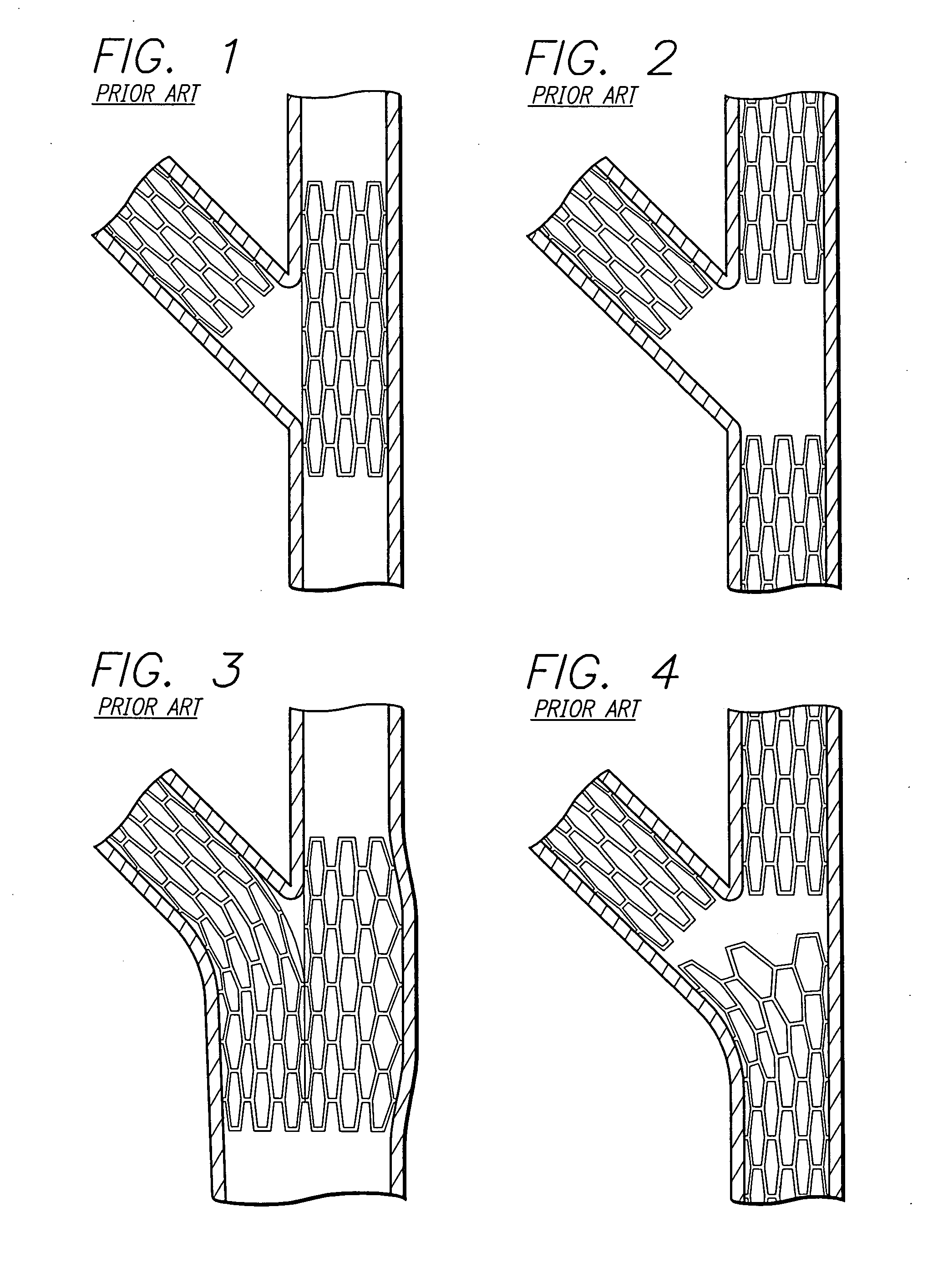
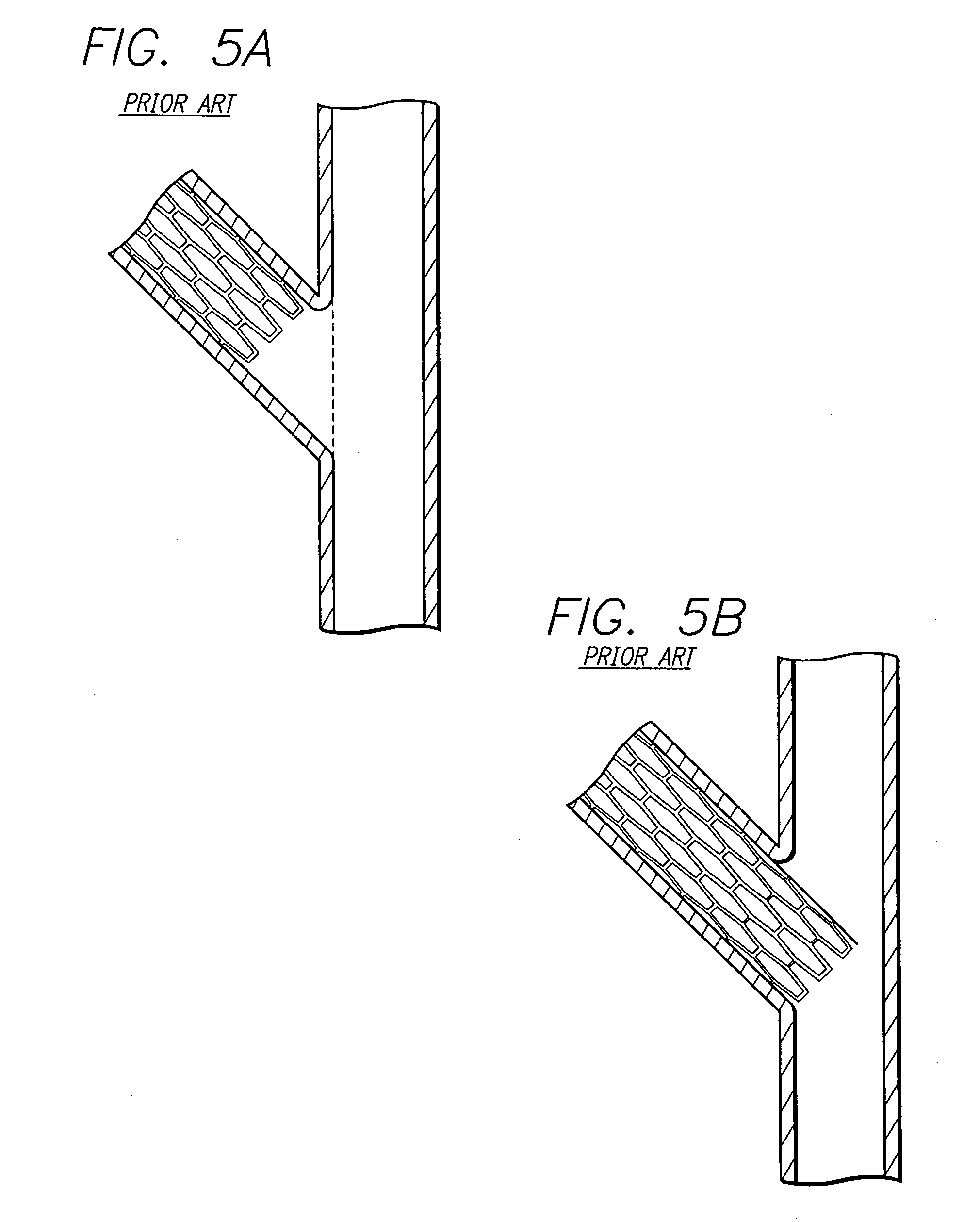
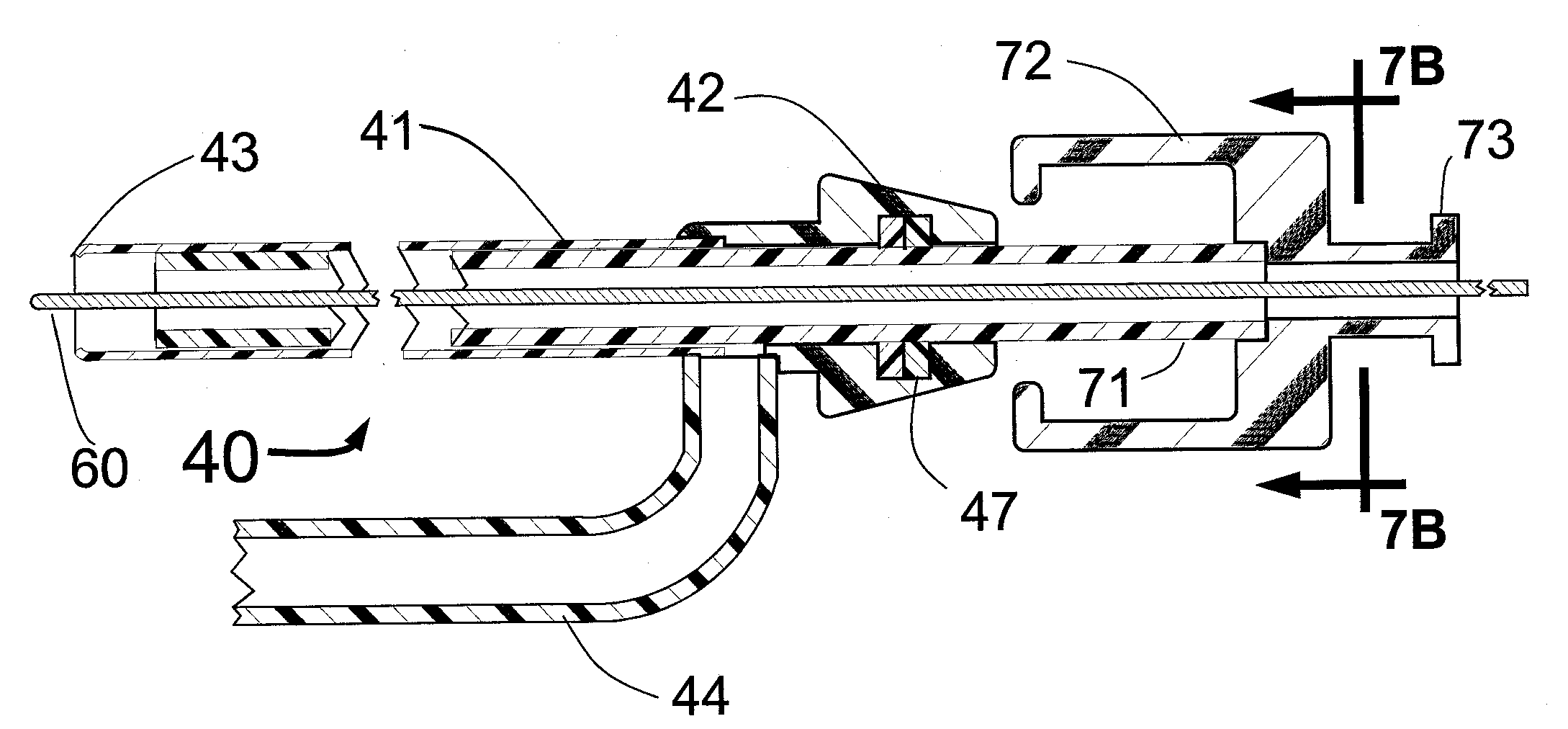
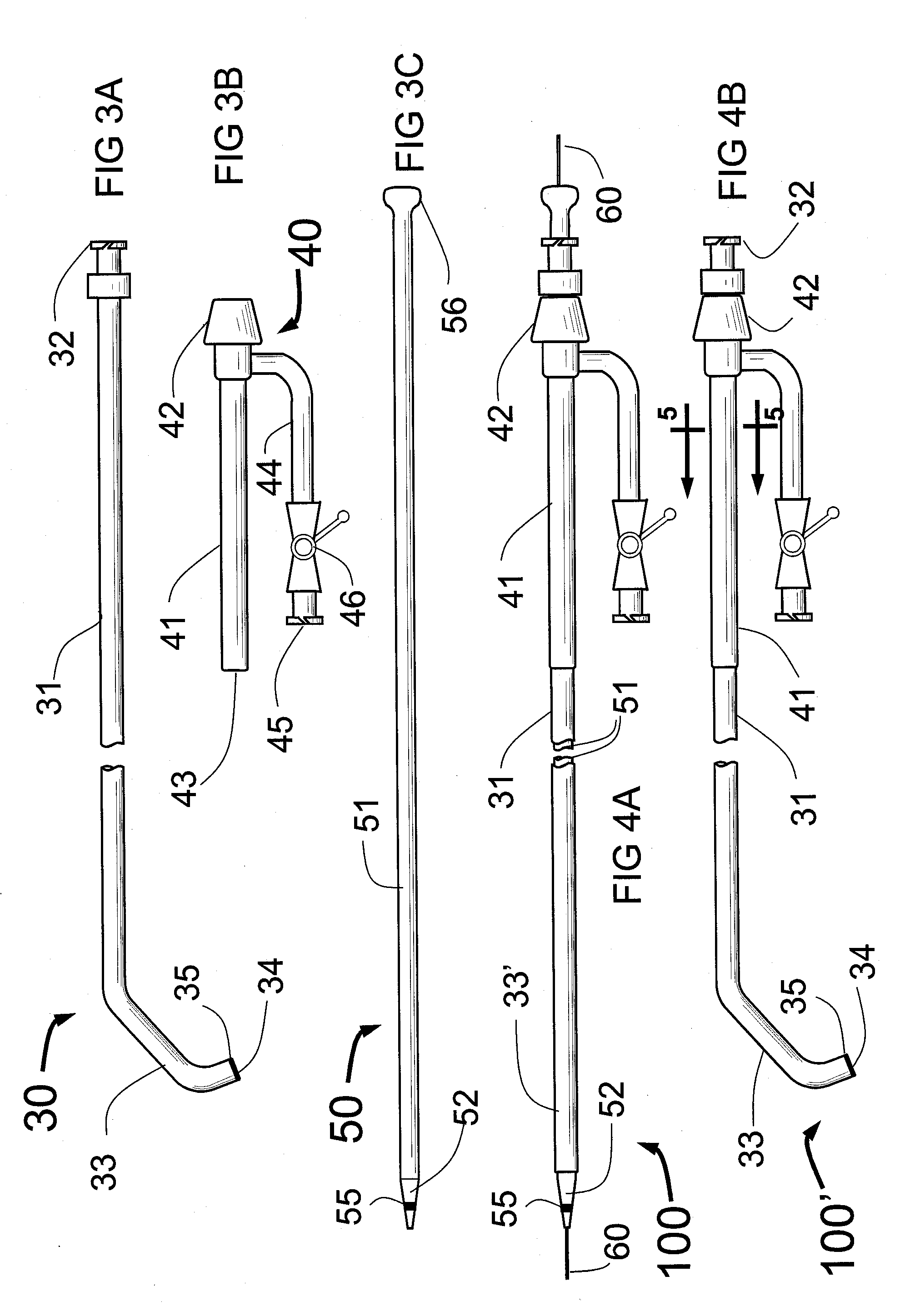
Method and apparatus for stenting comprising enhanced embolic protection coupled with improved protections against restenosis and thrombus formation
Apparatus and methods for stenting are provided comprising a stent attached to a porous biocompatible material that is permeable to endothelial cell ingrowth, but impermeable to release of emboli of predetermined size. Preferred stent designs are provided, as well as preferred manufacturing techniques. Apparatus and methods are also provided for use at a vessel branching. Moreover, embodiments of the present invention may comprise a coating configured for localized delivery of therapeutic agents. Embodiments of the present invention are expected to provide enhanced embolic protection, improved force distribution, and improved recrossability, while reducing a risk of restenosis and thrombus formation.
Owner:ABBOTT LAB VASCULAR ENTERPRISE
Stent delivery system
InactiveUS20070073379A1Maximize often conflicting objectiveClose toleranceStentsBlood vesselsBody organsAnatomical structures
Medical device and methods for delivery or implantation of prostheses within hollow body organs and vessels or other luminal anatomy are disclosed. The subject technologies may be used in the treatment of atherosclerosis in stenting procedures.
Owner:BIOSENSORS INT GROUP
Methods and apparatus for stenting comprising enhanced embolic protection coupled with improved protecions against restenosis and thrombus formation
InactiveUS20070179593A1Shorten the lengthImprove radial stiffnessStentsSurgeryPercent Diameter StenosisThrombus
Apparatus and methods for stenting are provided comprising a stent attached to a porous biocompatible material that is permeable to endothelial cell ingrowth, but impermeable to release of emboli of predetermined size. Preferred stent designs are provided, as well as preferred manufacturing techniques. Apparatus and methods are also provided for use at a vessel branching. Moreover, embodiments of the present invention may comprise a coating configured for localized delivery of therapeutic agents. Embodiments of the present invention are expected to provide enhanced embolic protection, improved force distribution, and improved recrossability, while reducing a risk of restenosis and thrombus formation.
Owner:ABBOTT LAB VASCULAR ENTERPRISE
Methods and apparatus for stenting comprising enhanced embolic protections coupled with improved protections against restenosis and trombus formation
InactiveUS20070179601A1Shorten the lengthImprove radial stiffnessStentsSurgeryInsertion stentPercent Diameter Stenosis
Apparatus and methods for stenting are provided comprising a stent attached to a porous biocompatible material that is permeable to endothelial cell ingrowth, but impermeable to release of emboli of predetermined size. Preferred stent designs are provided, as well as preferred manufacturing techniques. Apparatus and methods are also provided for use at a vessel branching. Moreover, embodiments of the present invention may comprise a coating configured for localized delivery of therapeutic agents. Embodiments of the present invention are expected to provide enhanced embolic protection, improved force distribution, and improved recrossability, while reducing a risk of restenosis and thrombus formation.
Owner:ABBOTT LAB VASCULAR ENTERPRISE
Locking component for an embolic filter assembly
A locking component for locking a medical device onto a guide wire. Such medical devices include, for example, an embolic filter assembly used to capture embolic material that may be created and released into a patient's vasculature during a stenting or angioplasty procedure. The embolic filter assembly tracks along the guide wire, and is delivered to a treatment site where it is locked in place and deployed. The locking component enables the filter assembly to lock onto any standard guide wire, and does not require a modified guide wire that has a specially-designed fitting or stop to accomplish the locking function.
Owner:ABBOTT CARDIOVASCULAR
Devices and methods for percutaneous endarterectomy
Devices and methods are provided for percutaneously treating atherosclerotic plaques within blood vessels. Atherosclerotic plaques cause significant morbidity and mortality by narrowing the arteries, which adversely affects blood flow, and by acting as a source for thrombi and emboli thus causing acute organ ischemia. Current treatments include open surgery with its inherent drawbacks, and stenting, which is less invasive but leaves the plaque material in the artery, which promotes restenosis. The present invention combines the advantages of both approaches. In general, the invention provides tools that enable percutaneously dissecting the plaque from the arterial wall and removing it from the body.
Owner:ANGIOWORKS MEDICAL
Stent covered by a layer having a layer opening
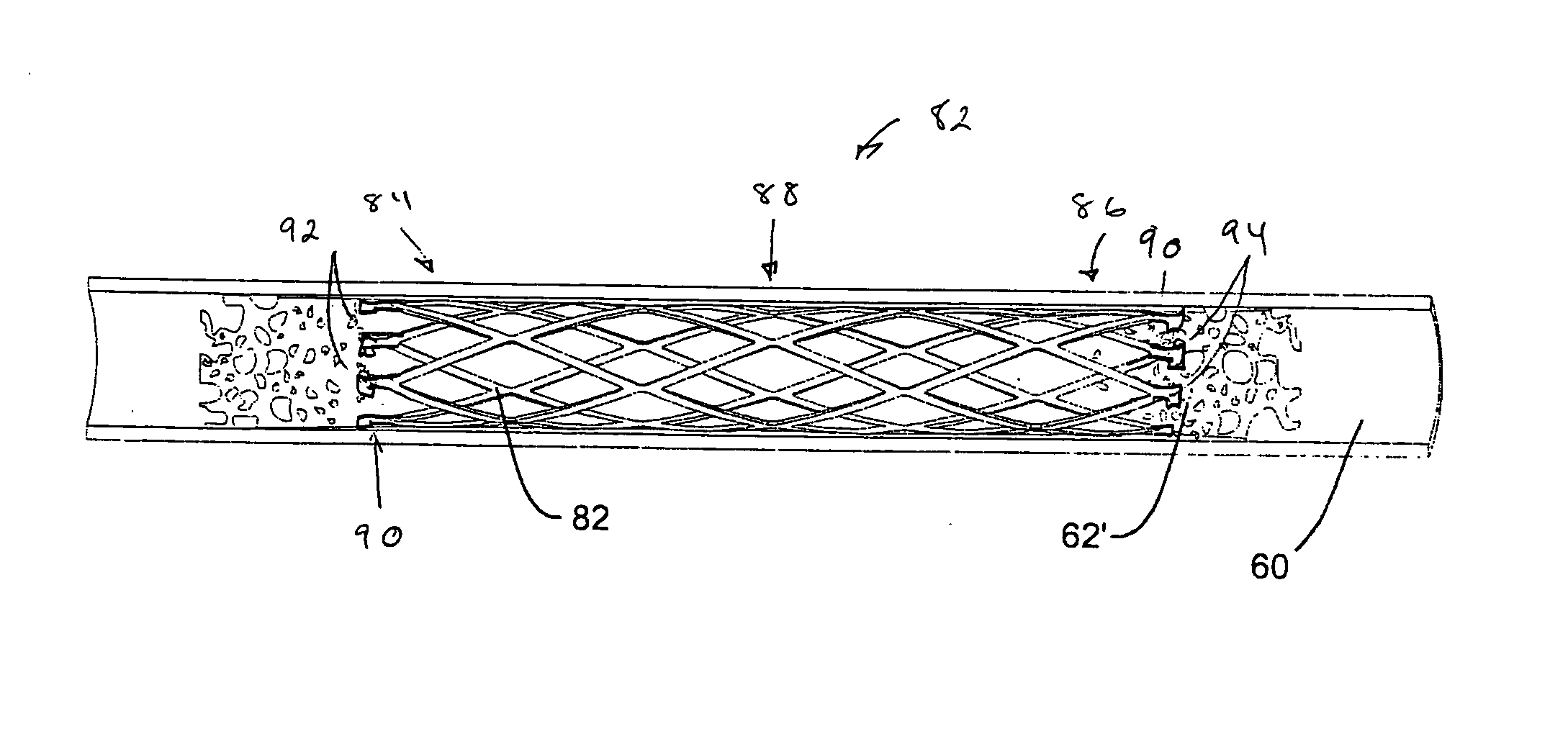
InactiveUS20050228482A1Modulate vascular toneEnhance macrophage-mediated microbial killingStentsBlood vesselsThree vesselsBiomedical engineering
A drug eluting stent has a frame with at least one frame opening, and the frame is covered with an outer continuous layer and / or an inner continuous layer. The outer and / or inner layers further include a layer opening that at least partially overlaps with the frame opening to form a continuous opening having a size that allows endothelialization when the stent is implanted into a blood vessel.
Owner:HERZOG WILLIAM +2
Catheter assembly and method for treating bifurcations
InactiveUS20080009933A1Improve delivery capabilitiesSave effortStentsBlood vesselsInsertion stentBlood vessel
An improved stent design and stent delivery catheter assembly for repairing a main vessel and a side branch vessel forming a bifurcation. The stent includes rings aligned along a common longitudinal axis and connected by links, where the stent has one or more portals for aligning with and partially expanding into the opening to the side branch vessel. The stent is implanted at a bifurcation so that the main stent section is in the main vessel, and the portal section covers at least a portion of the opening to the side branch vessel. A second stent can be implanted in the side branch vessel and abut the expanded central section to provide full coverage of the bifurcated area in the main vessel and the side branch vessel. Radiopaque markers on the stent and on the tip of the delivery catheter assist in aligning the portal section with the opening to the side branch vessel.
Owner:ABBOTT CARDIOVASCULAR
Stent and catheter assembly and method for treating bifurcations
ActiveUS20080009932A1Improve delivery capabilitiesSave effortStentsBlood vesselsInsertion stentGuide tube
An improved stent design and stent delivery catheter assembly for repairing a main vessel and a side branch vessel forming a bifurcation. The stent includes rings aligned along a common longitudinal axis and connected by links, where the stent has one or more portals for aligning with and partially expanding into the opening to the side branch vessel. The stent is implanted at a bifurcation so that the main stent section is in the main vessel, and the portal section covers at least a portion of the opening to the side branch vessel. A second stent can be implanted in the side branch vessel and abut the expanded central section to provide full coverage of the bifurcated area in the main vessel and the side branch vessel. Radiopaque markers on the stent and on the tip of the delivery catheter assist in aligning the portal section with the opening to the side branch vessel.
Owner:ABBOTT CARDIOVASCULAR
System and method for performing angiography and stenting
InactiveUS20110301684A1Reduce riskThin componentGuide needlesStentsInsertion stentAngiographic catheters
An integrated catheter system for performing angiography on a human patient, the integrated catheter system consisting of an angiographic catheter onto which a thin-walled sheath is co-axially mounted. The angiographic catheter having an essentially straight and elongated proximal section in the form of a cylindrical shaft that is surrounded for less than one-half of its length by the thin-walled sheath that has an outer diameter that is less than 0.25 mm greater than the outside diameter of the angiography catheter. The strength to prevent buckling of the thin-walled sheath being provided by the shaft of the angiographic catheter. The integrated catheter system also being ideal for the placement of stents using small diameter stent delivery systems such as the stent-on-a-wire system.
Owner:SVELTE MEDICAL SYST
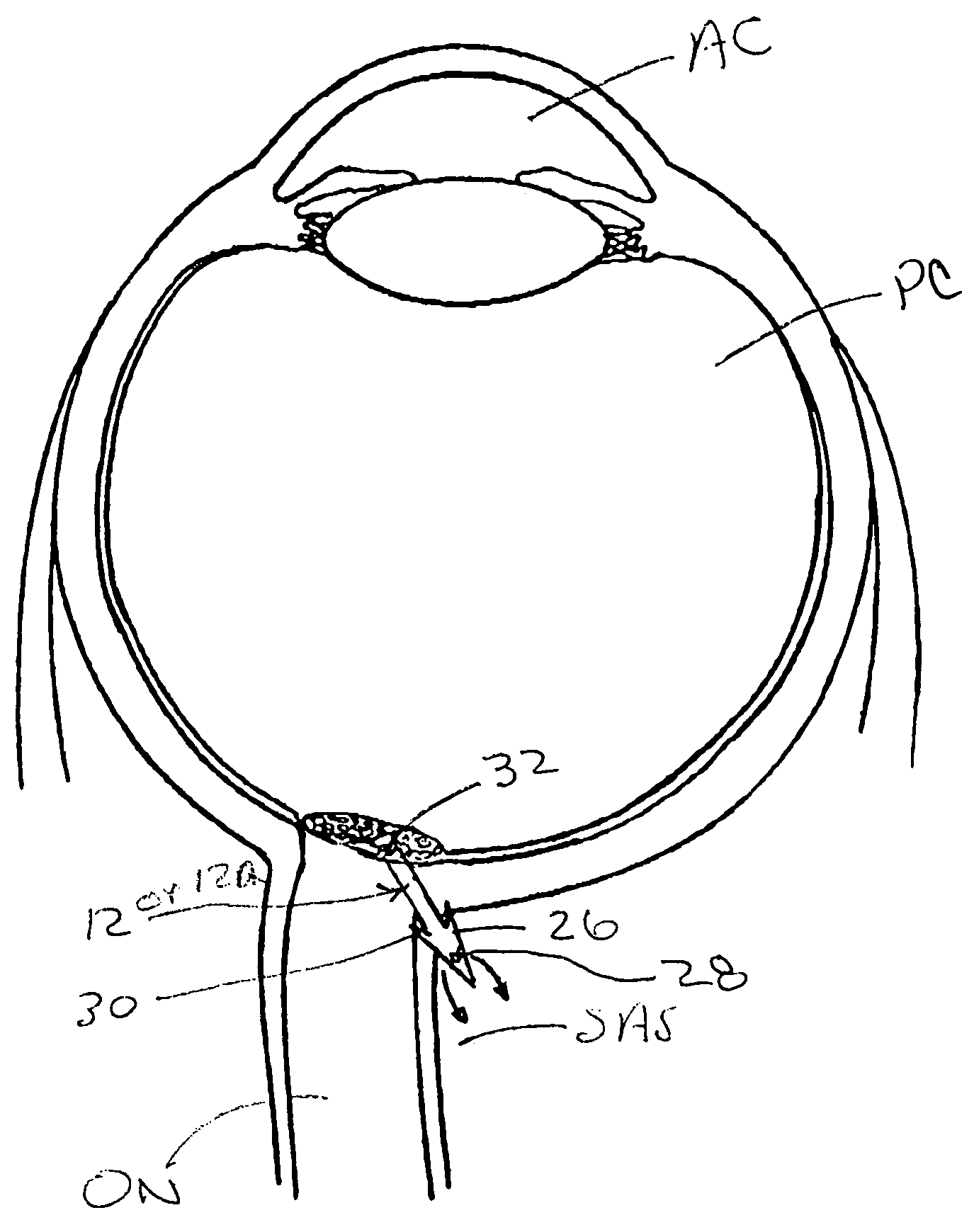
Methods and devices for draining fluids and lowering intraocular pressure
ActiveUS7354416B2Prevent and deter cloggingPrevent unwanted backflow of fluidEye implantsEye surgerySubarachnoid spaceOptic nerve
Methods, devices and systems for draining fluid from the eye and / or for reducing intraocular pressure. A passageway (e.g., an opening, puncture or incision) is formed in the lamina cribosa or elsewhere to facilitate flow of fluid from the posterior chamber of the eye to either a) a subdural location within the optic nerve or b) a location within the subarachnoid space adjacent to the optic nerve. Fluid from the posterior chamber then drains into the optic nerve or directly into the subarachnoid space, where it becomes mixed with cerebrospinal fluid. In some cases, a tubular member (e.g., a shunt or stent) may be implanted in the passageway. A particular shunt device and shunt-introducer system is provided for such purpose. A vitrectomy or vitreous liquefaction procedure may be performed to remove some or all of the vitreous body, thereby facilitating creation of the passageway and / or placement of the tubular member as well as establishing a route for subsequent drainage of aqueous humor from the anterior chamber, though the posterior chamber and outwardly though the passageway where it becomes mixed with cerebrospinal fluid.
Owner:QUIROZ MERCADO HUGO +1
Tubular restraint release approaches for electrolytic implant delivery system
InactiveUS20070100419A1Reduce forceParticularly space-efficientStentsBlood vesselsBody organsProsthesis
Medical devices and methods for delivery or implantation of prostheses within hollow body organs and vessels or other luminal anatomy are disclosed. The subject technologies may be used in the treatment of atherosclerosis in stenting procedures or be used in variety of other procedures. The systems may employ a self expanding stent restrained by one or more members released by an electrolytically erodable latch. Such release means do not connect directly to the implant, though one or more portions may contact it.
Owner:BIOSENSORS INT GROUP
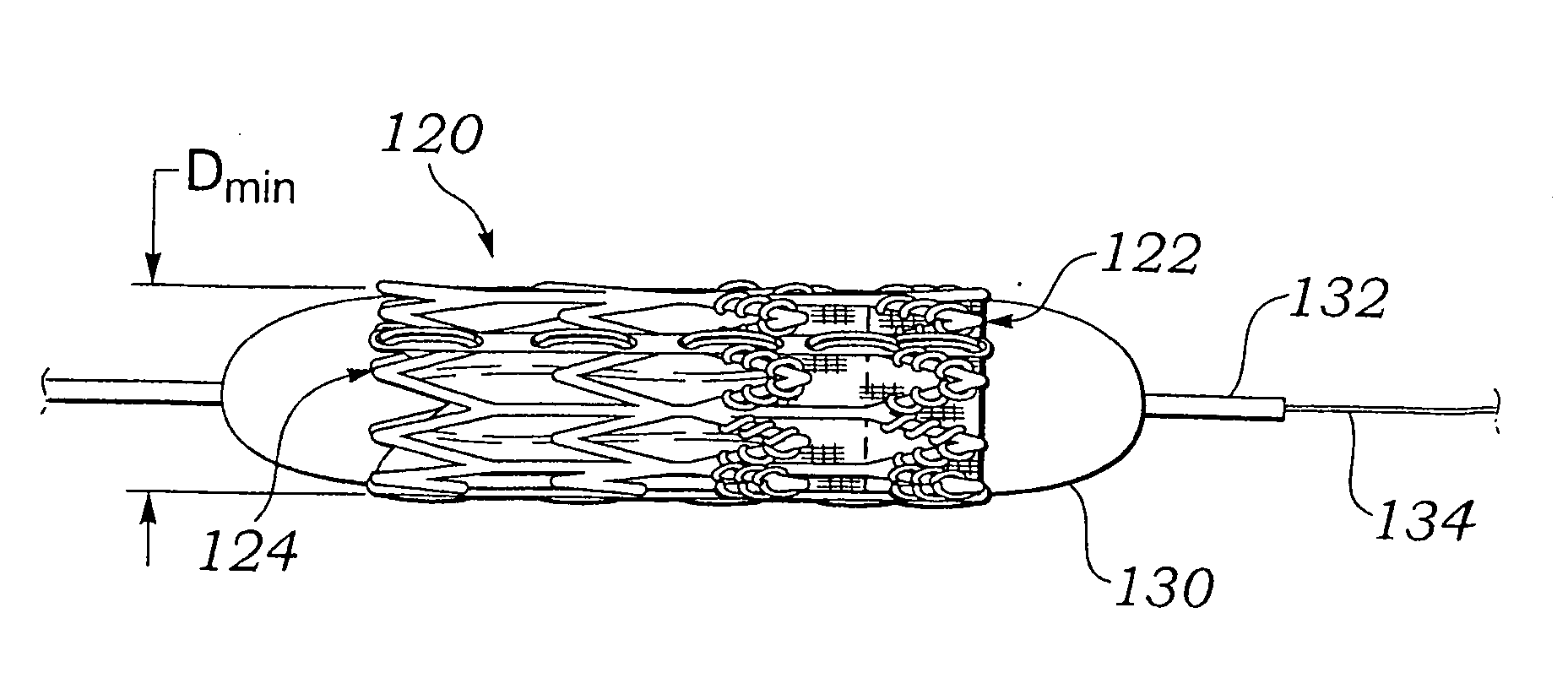
Method of crimping a prosthetic valve
ActiveUS20070061009A1Prevent rotationMost efficientStentsBalloon catheterProsthetic valveProsthetic heart
An improved crimping mechanism and method well-suited for use with stented prosthetic heart valves. The crimping mechanism includes a plurality of jaws configured for linear non-rotational movement toward a central axis. A rotational plate is formed with a plurality of spiral grooves or tracks for engaging the jaws. Rotational movement of the spiral tracks produces linear movement of the jaws. Nesting of the inner ends of the jaws permits each to be acted on along different radial lines while their inner faces move together evenly to reduce the crimping aperture in a smooth fashion. The crimping mechanism is particularly well-suited for use with stented prosthetic heart valves, such as a prosthetic aortic valve, though it can also be applied to other stented heart valves, venous valves, and even stent grafts which tend to be fairly large.
Owner:EDWARDS LIFESCIENCES CORP
Spinal disc annulus reconstruction method and deformable spinal disc annulus stent
InactiveUS20060287731A1Reduce riskImprove integritySuture equipmentsTelevision system detailsInsertion stentNormal cell
A spinal disc annulus repair stent for repair and reconstruction of the spinal disc wall (annulus) after surgical invasion or pathologic rupture, which may incorporate suture closure or other means of stent insertion and fixation, designed to reduce the failure rate of conventional surgical procedures on the spinal discs. In an illustrative embodiment, the design of the spinal disc annulus stent advantageously allows ingrowth of normal cells of healing in an enhanced fashion strengthening the normal reparative process.
Owner:KRT INVESTORS
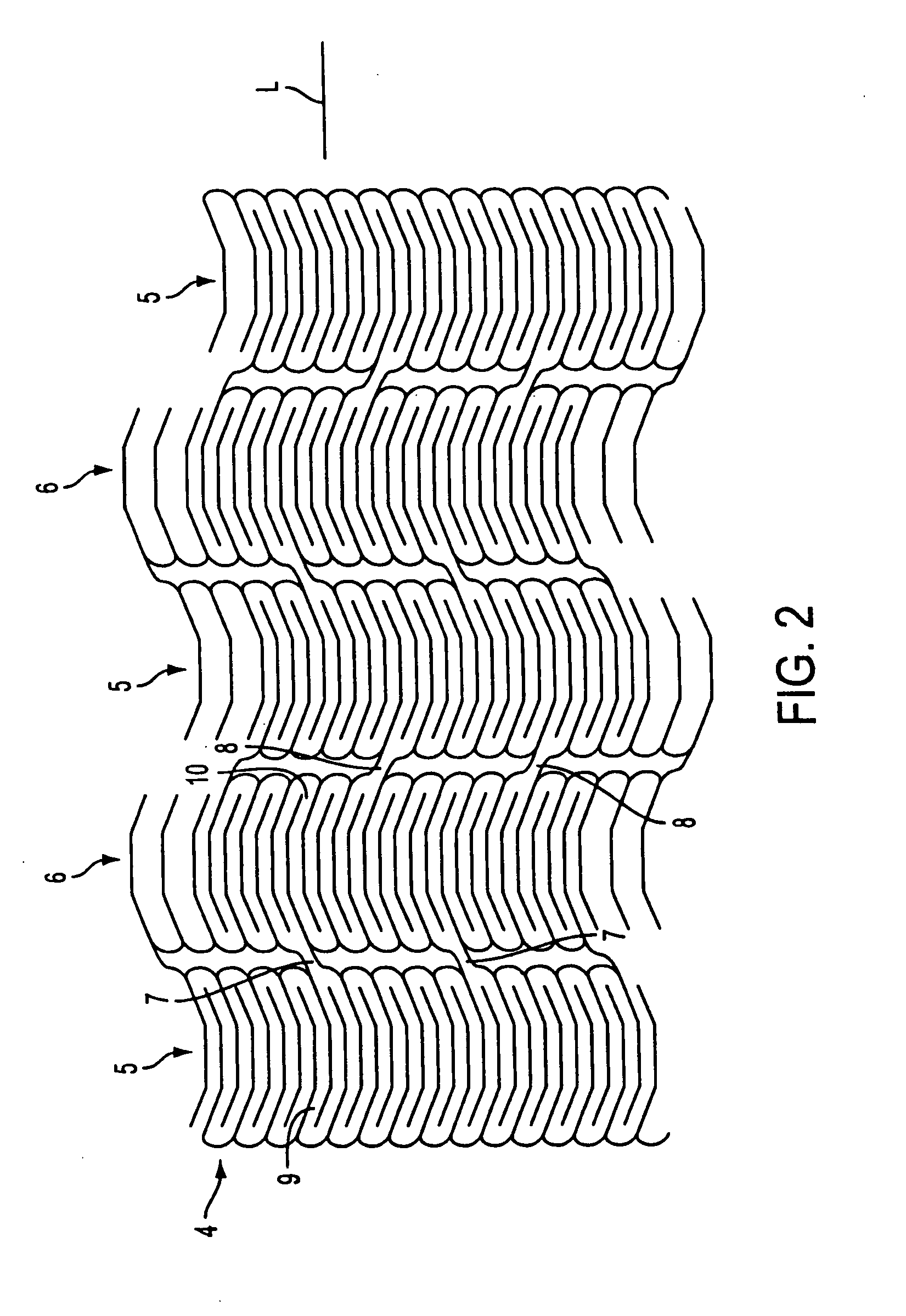
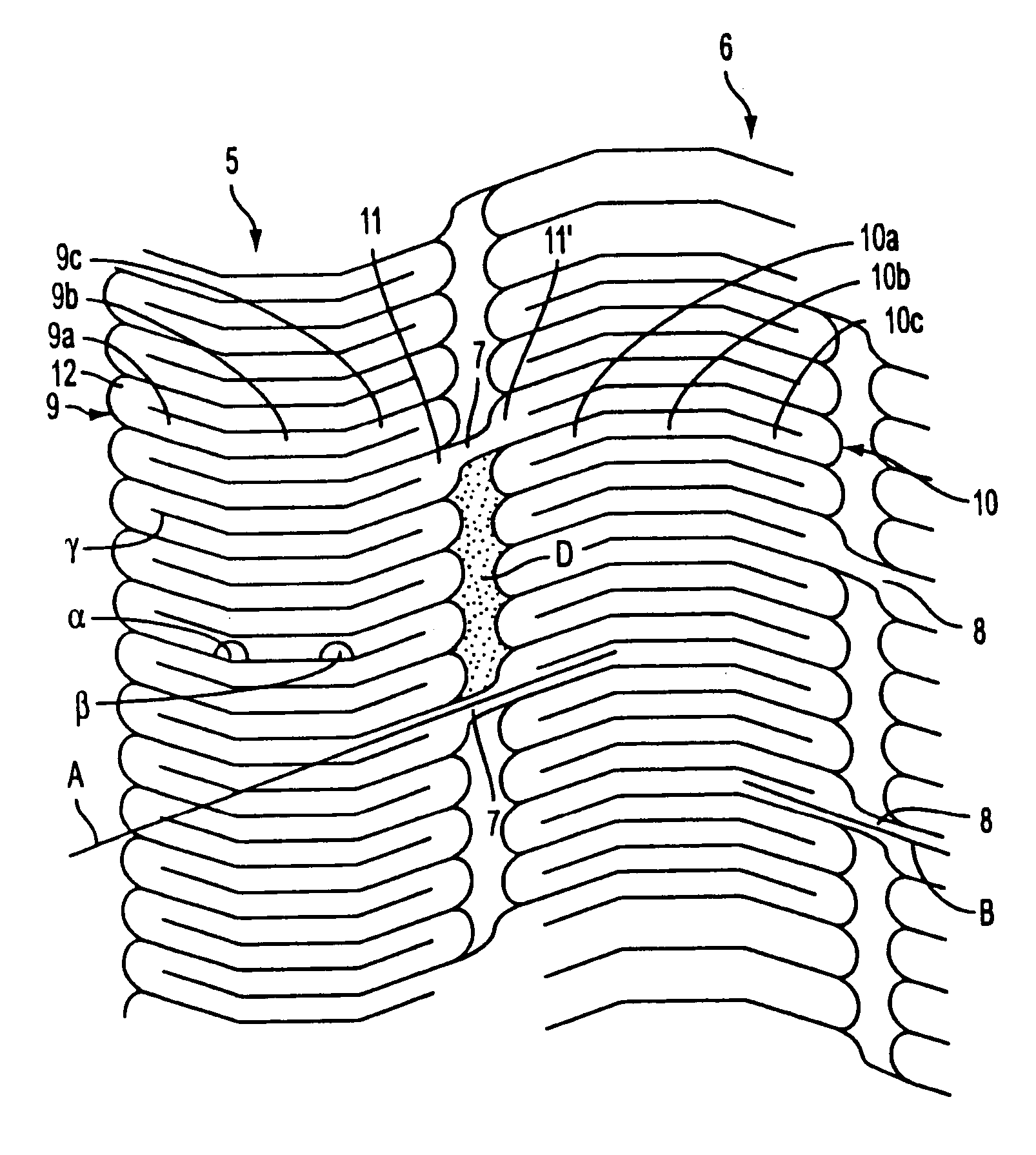
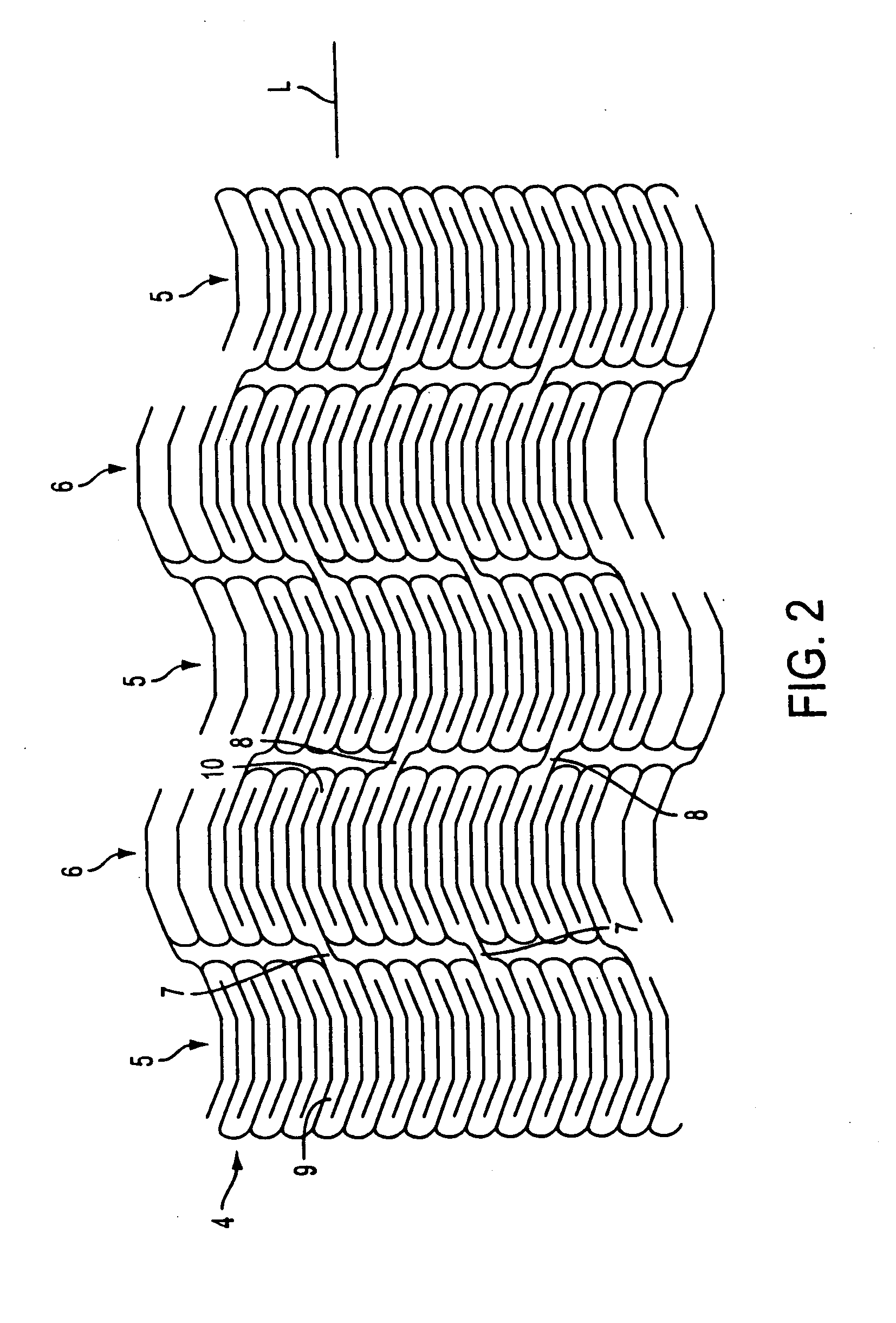
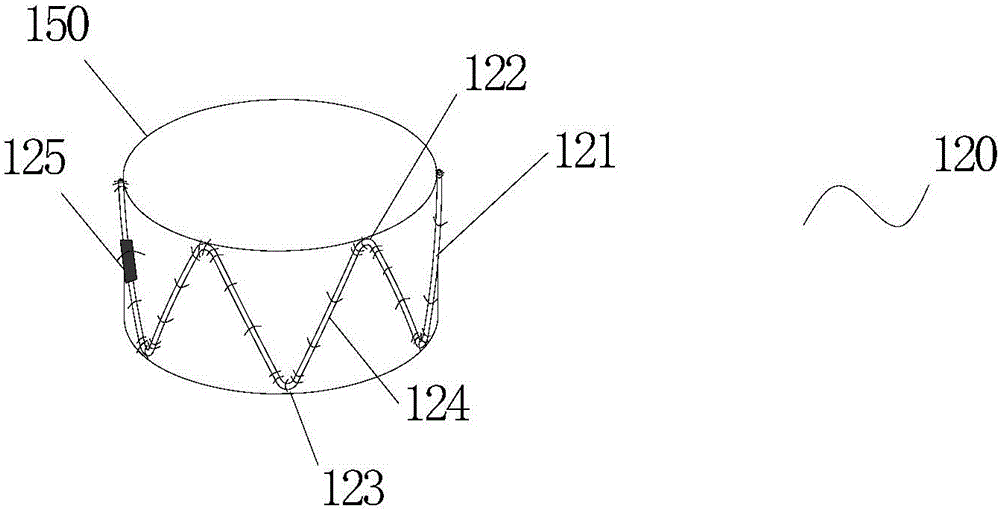
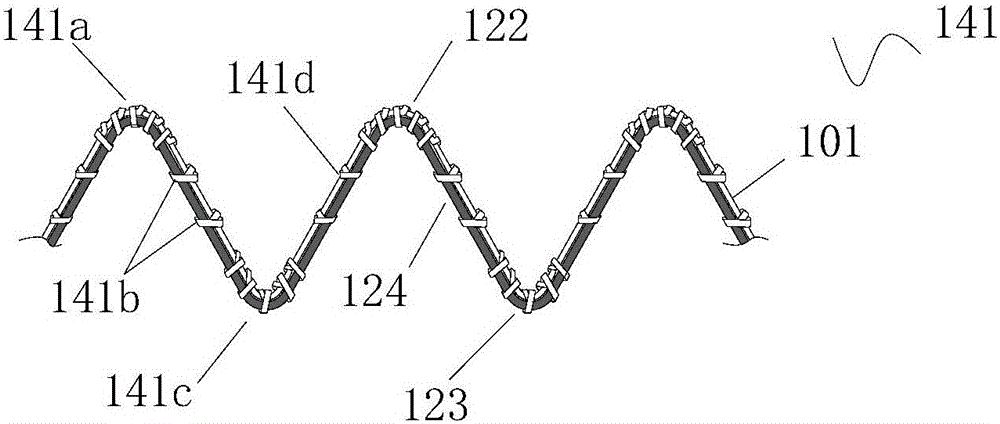
Stent for Vessel
ActiveUS20070282428A1Improve mass productionEasy to manufactureStentsBlood vesselsInsertion stentLiving body
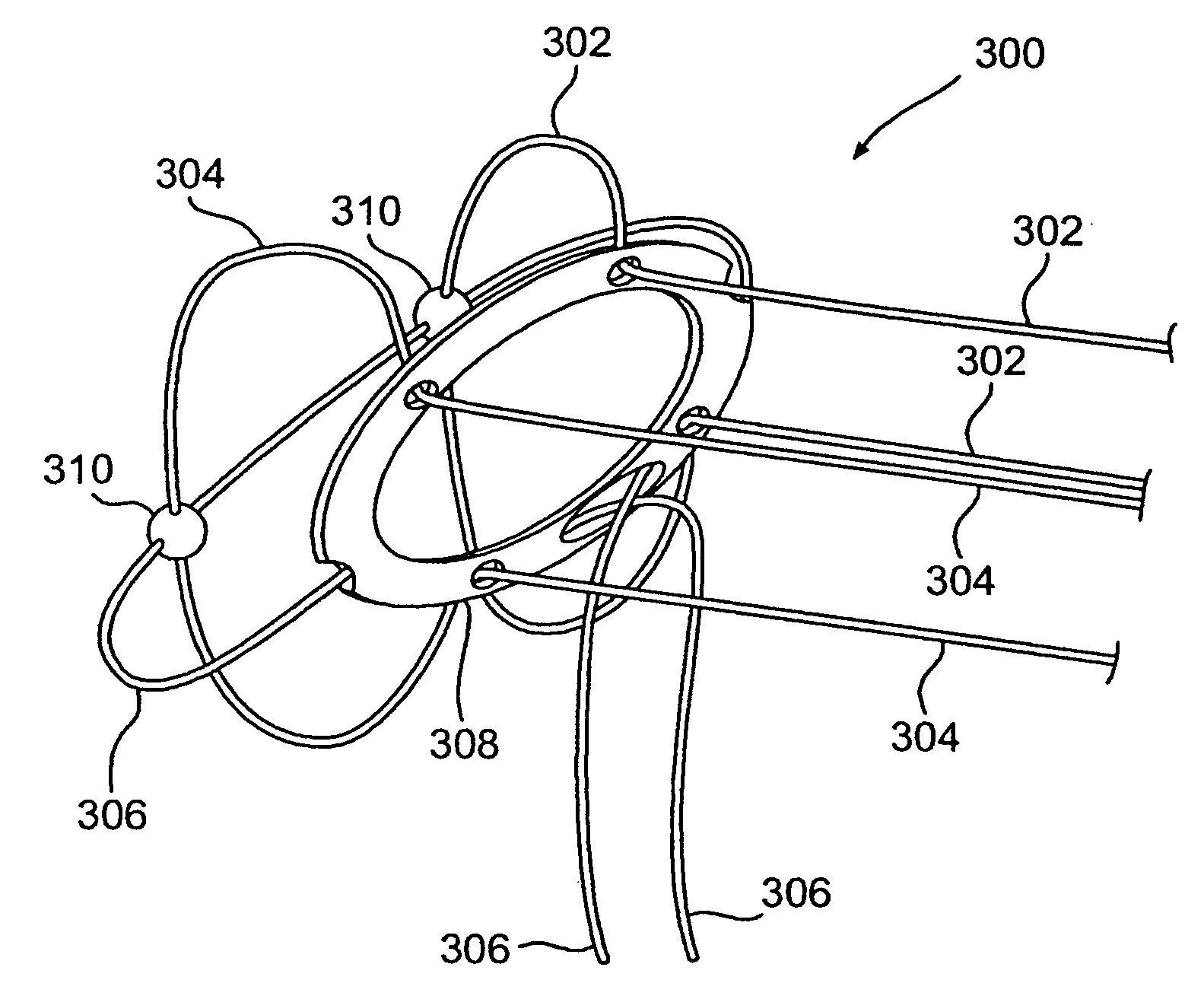
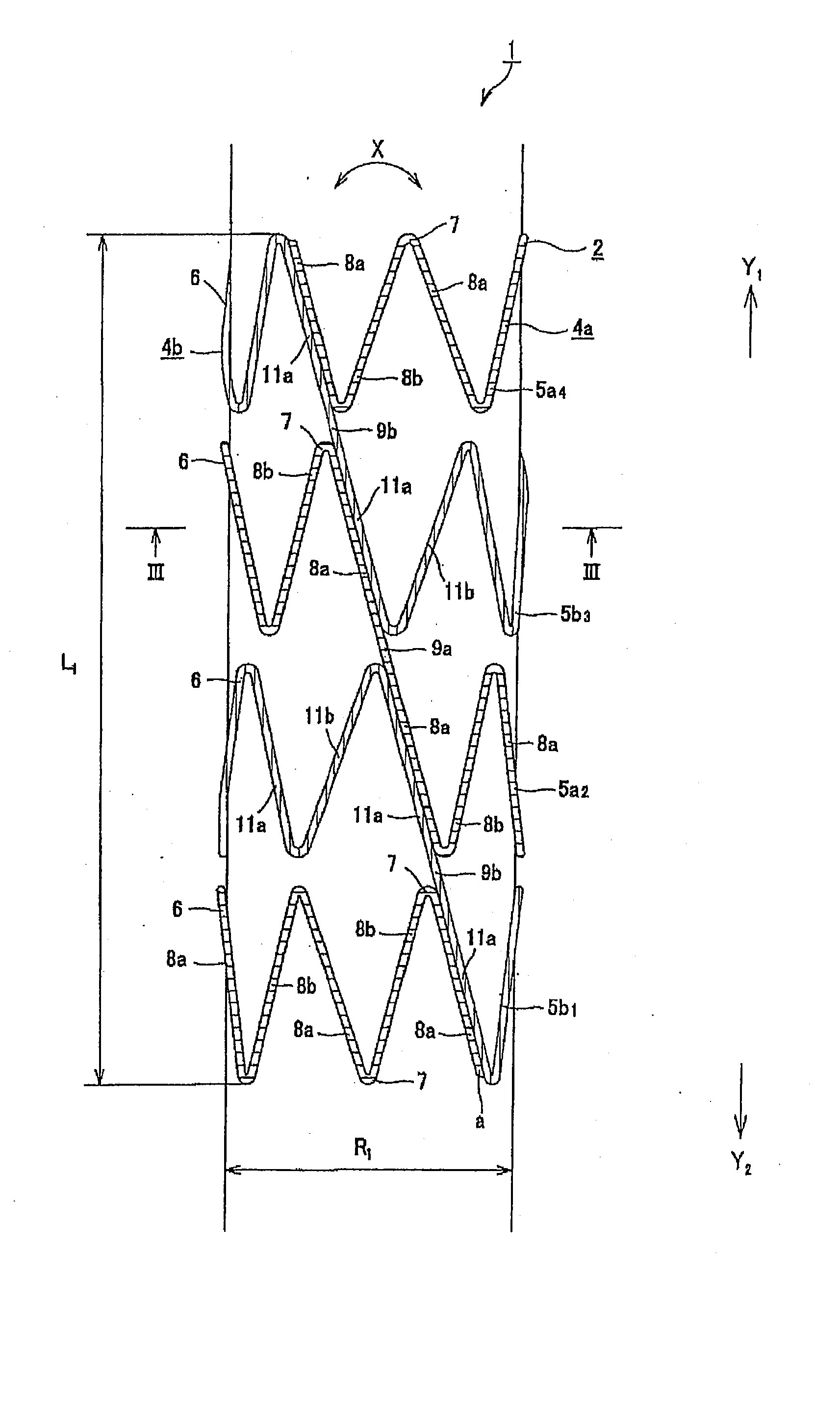
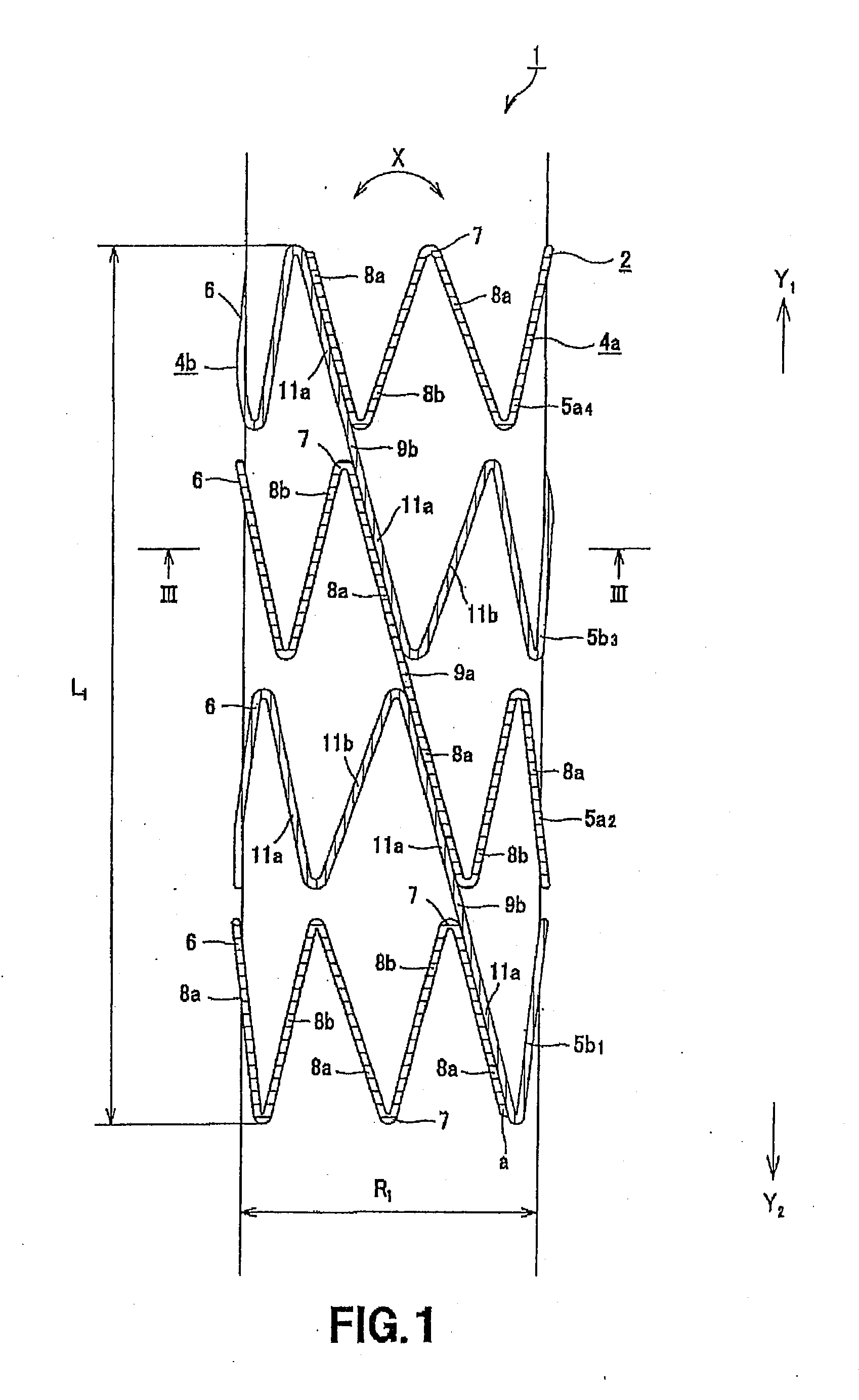
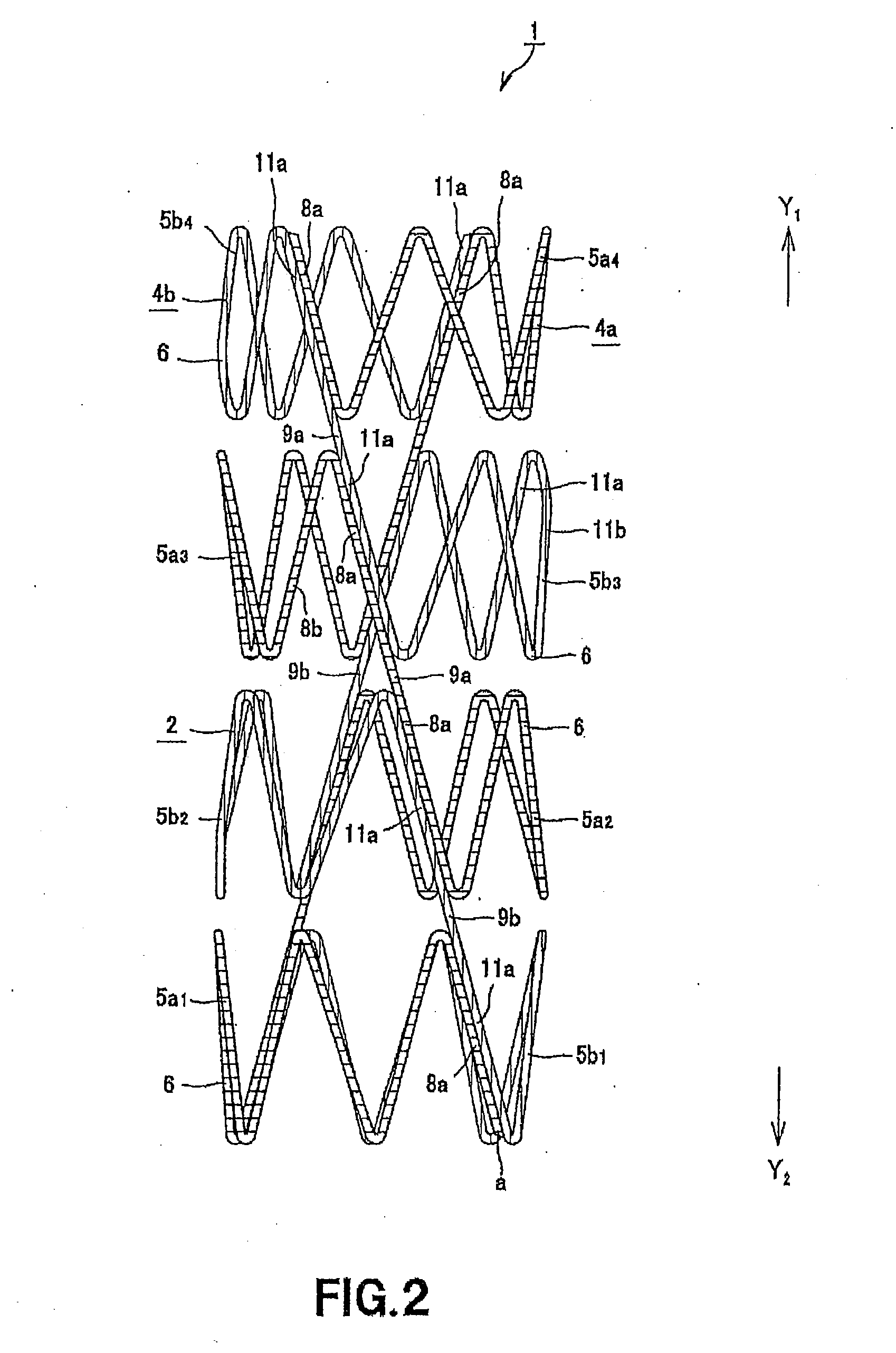
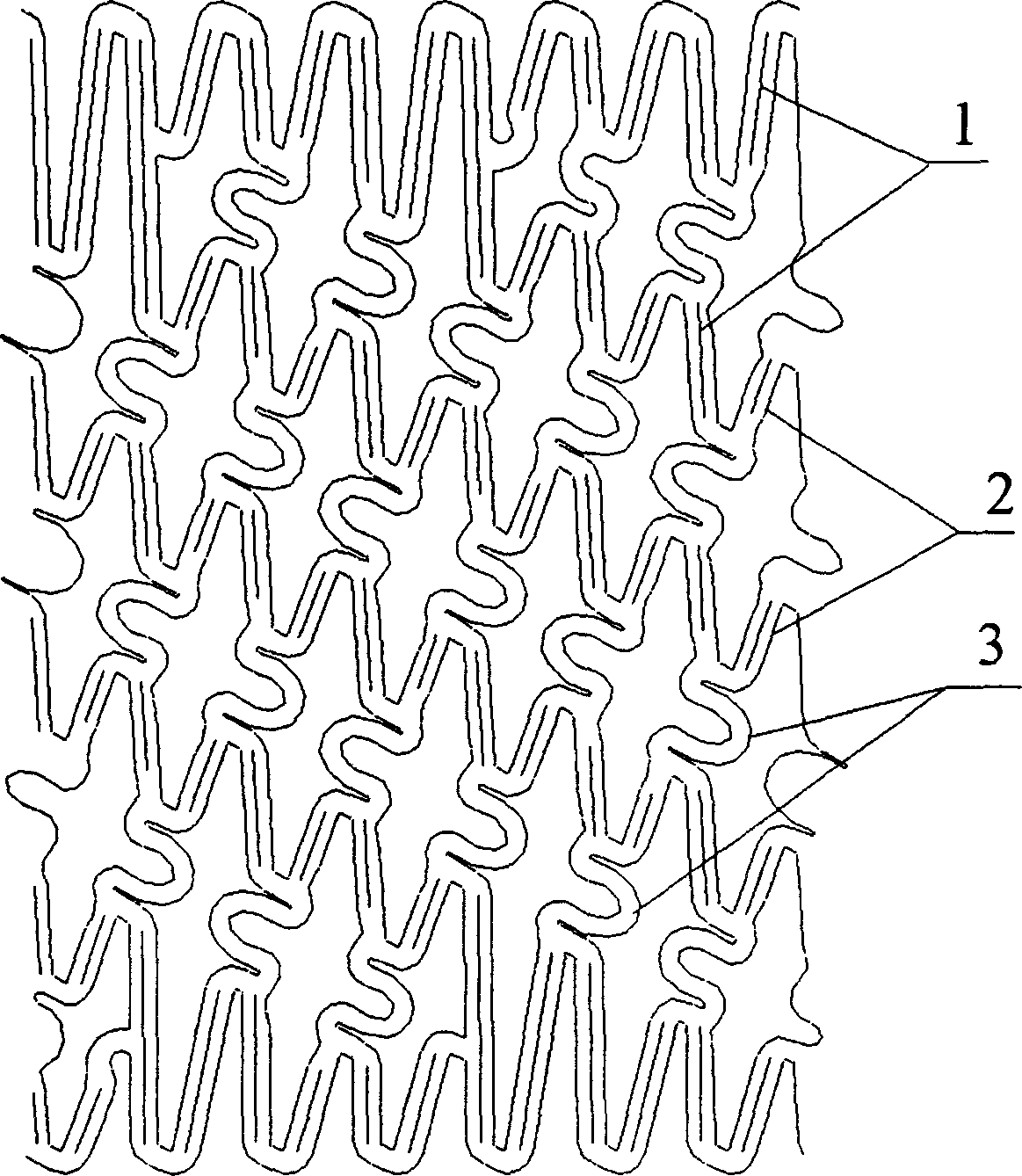
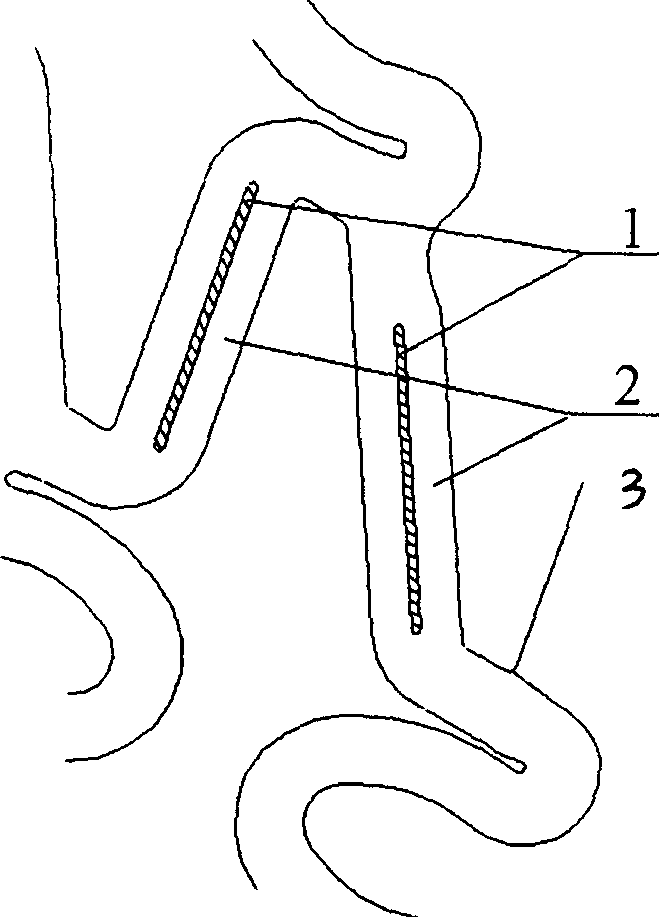
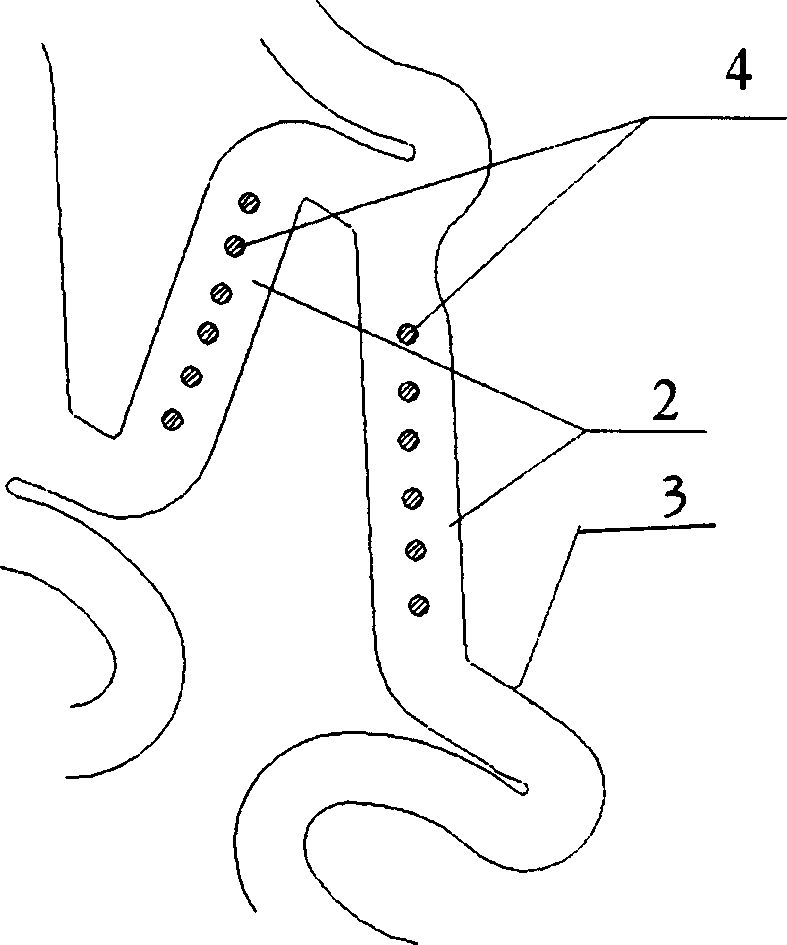
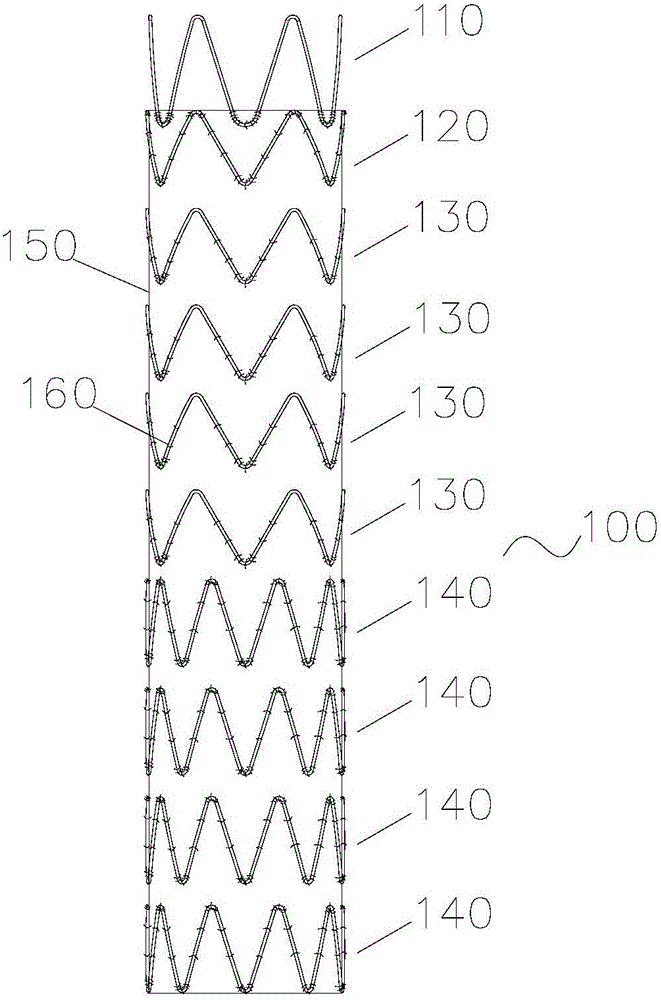
Disclosed is a stent for a vessel implanted and left in the vessel of a living body to support the lumen of the vessel from inside. The stent for a vessel consists of a plurality of stent constituting members (4a and 4b), formed by combining a plurality of the tubular-body constituting elements (5a and 5b) in multistage, each of the tubular-body forming elements (5) comprising a part of a tubular member (2) is formed by bending a yarn (3) of a biodegradable polymer in a zigzag design. Each of stent constituting members (4a and 4b) is comprised with tubular segments (6) formed by combining the tubular-body forming elements (5a and 5b) facing each other, and by continuously joining at least neighboring parts of each tubular-body forming element (5a and 5b) together, to comprise a continuously joined tubular body (2).
Owner:IGAKI IRYO SEKKEI
Balloon Catheter
A balloon catheter is provided for the implantation of a stent in a mammalian duct or cavity, comprising an elongate distal section and an expandable first balloon accommodating the section, further comprising means for the supply of a pressure medium for the expansion of the balloon, and means for heating the pressure medium, the catheter being provided with an elongate stent mounted onto the balloon. The catheter contains second means for establishing outwardly directed expansion of the stent at a sight or location selected from the two ends of the stent so that the stent will remain in position as implanted after removal of the catheter from the duct or cavity; and a method for the implantation of a stent in a human prostatic urethra.
Owner:WALLSTEN MEDICAL
Apparatus for aiding the flow of blood through patient's circulatory system
InactiveUS20060178731A1Sustained beneficialAvoid the needControl devicesBlood pumpsVeinInsertion stent
A percutaneous implanted device for temporarily sustaining a patient awaiting a heart transplant. The device includes a radially expandable stent mounted upon the balloon section of a balloon catheter. A venous biological valve is mounted at each end of the stent and an inflatable annular-shaped diaphragm is secured to the stent between the valves, which permit the flow of blood in one direction and prevent the flow of blood in the opposite direction. The diaphragm is attached to a pump which inflates the diaphragm after the stent is implanted in a blood vessel in relation to the heart's rhythm to assist the heart in moving blood through the patient's circulatory system.
Owner:NUMED INC
Lumen stent
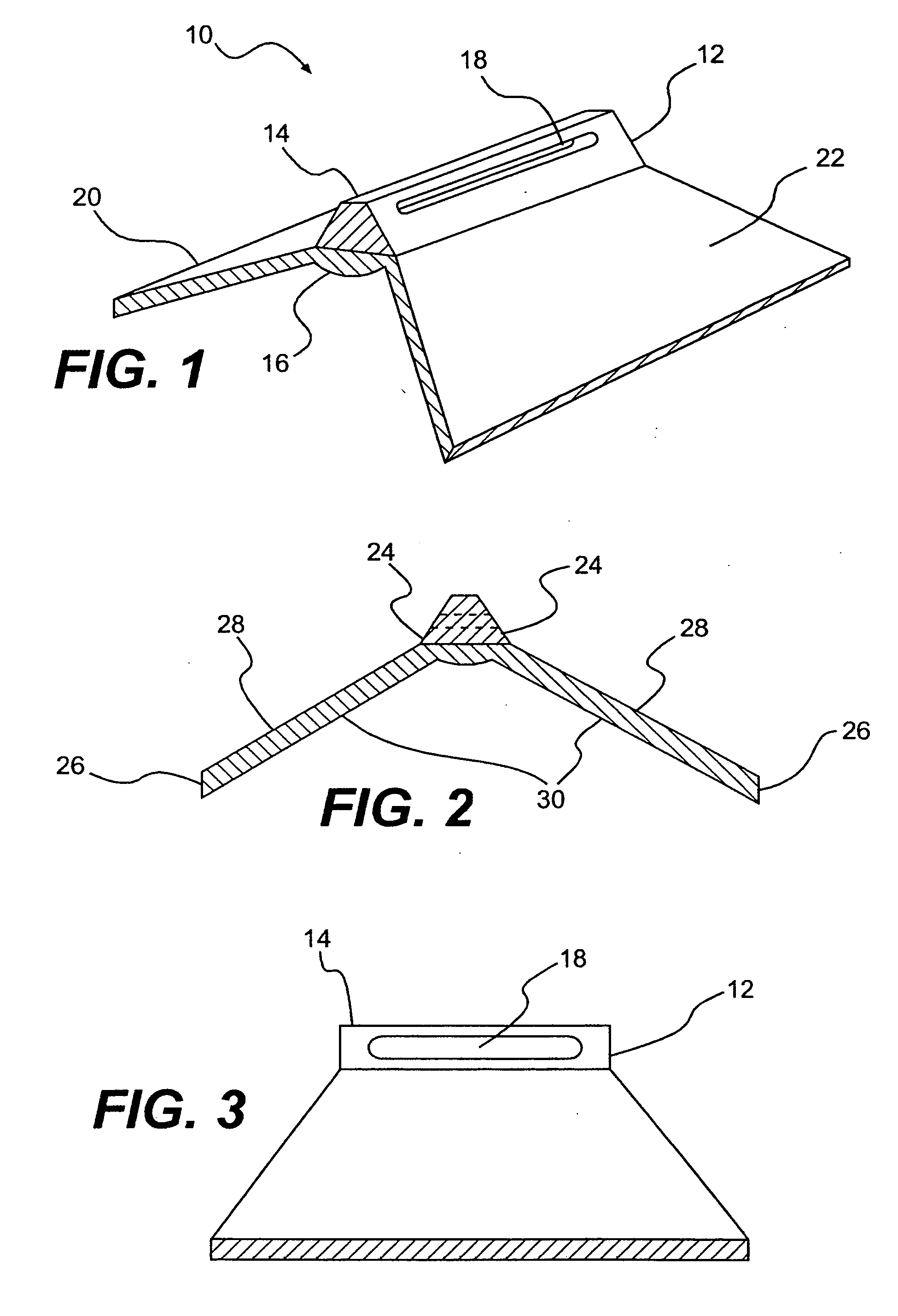
The invention discloses a lumen stent which includes a first tube body and a second tube body. The second tube body sleeves outside the first tube body and has at least one end in hermetic connection with the outer surface of the first tube body. The second tube body includes a thin film body which covers at least part of the first tube body and a second radial supporting structure which is disposed in the maximum radius-length region of the thin film body and surrounds the maximum radius-length region. The second radial supporting structure has radius-length supporting property. According to the embodiments of the invention, the implantation of the lumen stent can form a semi-enclosed gap between the first tube body and the second tube body, or form a semi-enclosed gap between the second tube body and a tube wall, so that blood that flow into the aforementioned gap can serve as a filling material and block off I type endoleak channel and prevent blood from flowing into a tumor body or an interlayer position.
Owner:LIFETECH SCIENTIFIC (SHENZHEN) CO LTD
Ten-thousandths scale metal reinforced stent delivery guide sheath or restraint
InactiveUS20060276886A1Produced cost-effectivelyReduce frictionStentsCatheterProsthesisHigh intensity
A high-strength thin-walled tubular material having a lubricious polymer inner layer is disclosed. The tubing may be used in constructing delivery systems for radially expanding prostheses for use in the treatment of atherosclerosis in stenting procedures or a variety of other procedures.
Owner:BIOSENSORS INT GROUP
Biodegradable stent with laminated coatings
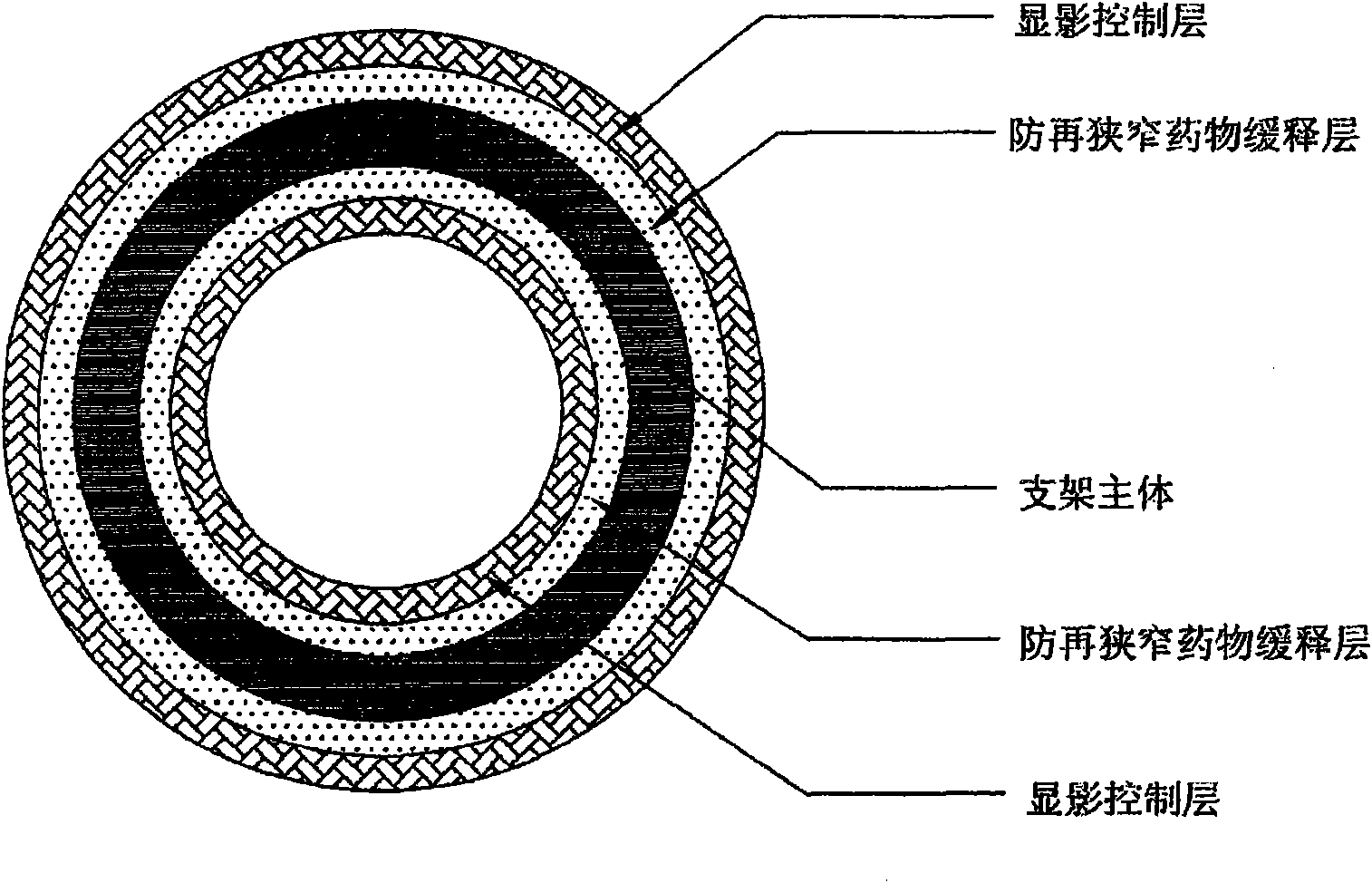
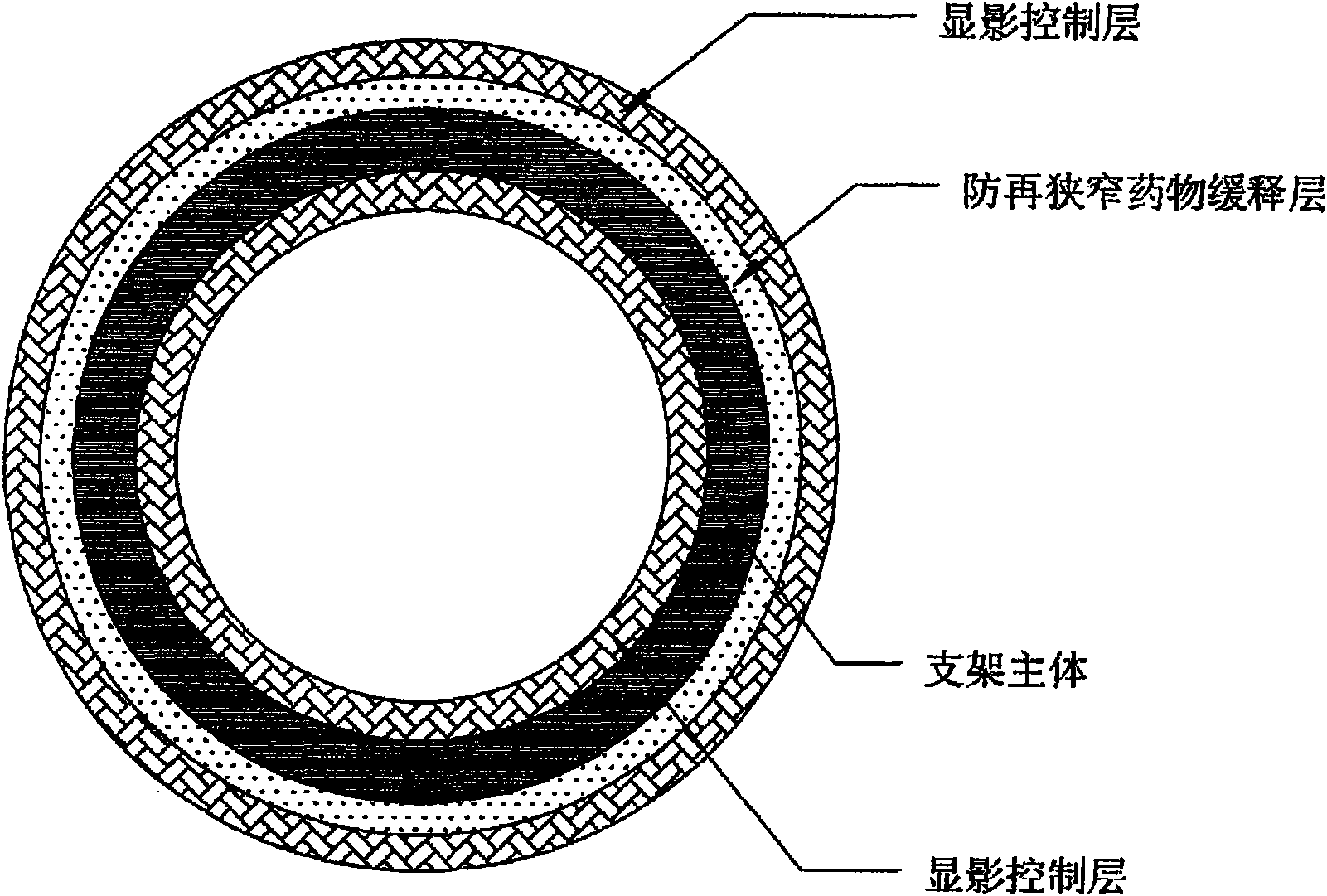
InactiveCN102151185AStable supportImprove the development effectStentsSurgeryControl layerVascular endothelium
The invention belongs to the field of medical apparatus and relates to a biodegradable stent with laminated coatings. The stent comprises a stent main body with a supporting role and at least two layers selected from a medicine coating, an endothelial cell growth promotion layer and a developing control layer; the main body material of the stent is a biodegradable polymer with higher mechanical strength; after implanted into a pathological position, the stent has strong supporting force and can play a role of supporting a pathological blood vessel in a short time; the developing control layer can help a doctor accurately observe the position and the expansion consistency of the stent in the operation so as to prevent adverse events, such as displacement, instant collapse, and the like from happening; medicines for restricting neointimal hyperplasia in the medicine coating can prevent restenosis of the blood vessel after the stent is implanted for a short time; and the endothelial cell growth promotion layer can promote endothelial cells to grow on the surface of the stent to wrap the stent in the vascular endothelium so as to prevent degradable fragments of the stent from falling into the blood to cause embolism.
Owner:SHANGHAI MICROPORT MEDICAL (GROUP) CO LTD
Catheter assembly and method for treating bifurcations
An improved stent design and stent delivery catheter assembly for repairing a main vessel and a side branch vessel forming a bifurcation. The stent includes rings aligned along a common longitudinal axis and connected by links, where the stent has one or more portals for aligning with and partially expanding into the opening to the side branch vessel. The stent is implanted at a bifurcation so that the main stent section is in the main vessel, and the portal section covers at least a portion of the opening to the side branch vessel. A second stent can be implanted in the side branch vessel and abut the expanded central section to provide full coverage of the bifurcated area in the main vessel and the side branch vessel. Radiopaque markers on the stent and on the tip of the delivery catheter assist in aligning the portal section with the opening to the side branch vessel.
Owner:ABBOTT CARDIOVASCULAR
Vascular stent mounted with non penetrable slot or blind hole and its preparing method
The present invention proposes one kind of blood vessel rack with blind slot or blind hole, and belongs to medical equipment technology. The blood vessel rack consists of straight main bearing bars and connecting bars, and the main bearing bars have blind slot or blind hole in the outer surface for embedding medicine coating. The blind slots or blind holes in the main bearing bars have depth not affecting the bearing capacity of the bars, the slots may be in different shapes, and the holes may be distributed regularly or irregularly. The blind slots or blind holes are made through laser machining, electric beam machining or chemical etching. The blood vessel rack of the present invention has improved medicine coating attaching capacity, prolonged medicine releasing period and less re-stenosis.
Owner:BEIJING AMSINO MEDICAL
Plastic covered aortic dissection stent and aortic dissection stent
ActiveCN105726164AImprove flexibilityHigh strengthStentsBlood vesselsAortic dissectionInsertion stent
The invention discloses a plastic covered aortic dissection stent and an aortic dissection stent.The plastic covered aortic dissection stent comprises a tubular covering membrane and multiple annular supports sequentially sewn on the covering membrane in the axial direction and is characterized in that the annular supports on one part of the covering membrane are semi-sewn supports, the semi-sewn supports are provided with non-sewn areas which can be separated from the covering membrane, and the covering membrane corresponding to the internally-bent side of the non-sewn areas breaks away from the semi-sewn supports to be folded internally in a bent state.The semi-sewn supports are distributed at the bent positions after the plastic covered aortic dissection stent is implanted.The number of the semi-sewn supports is at least two sequentially-adjacent circles.When the plastic covered aortic dissection stent is bent, non-sewn areas of every two adjacent annular supports located on the internally-bent side can be overlapped, and the covering membrane in the non-sewn areas is folded towards the inside of the stent to maintain the bent state of the covered stent.
Owner:HANGZHOU WEIQIANG MEDICAL TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com