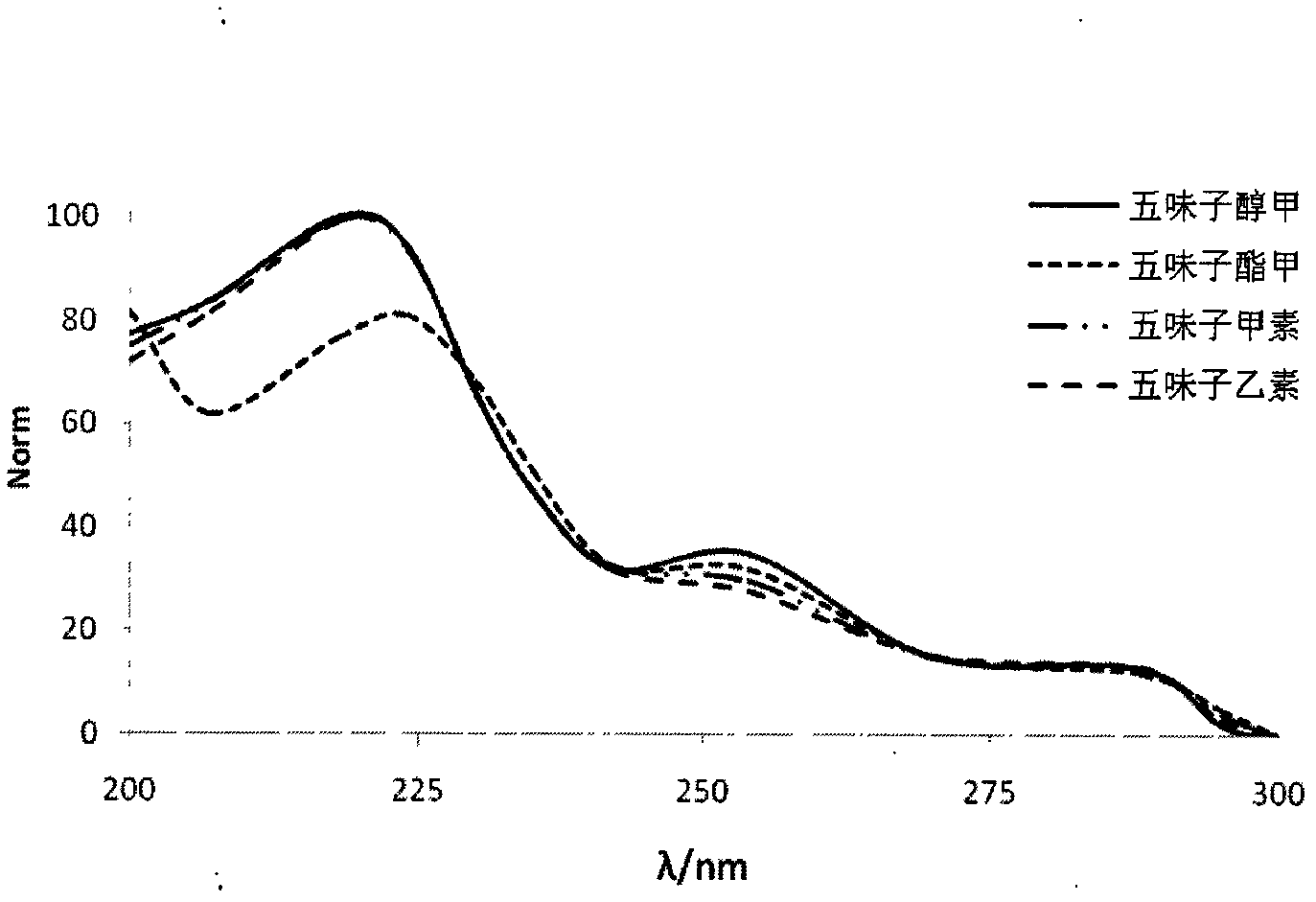
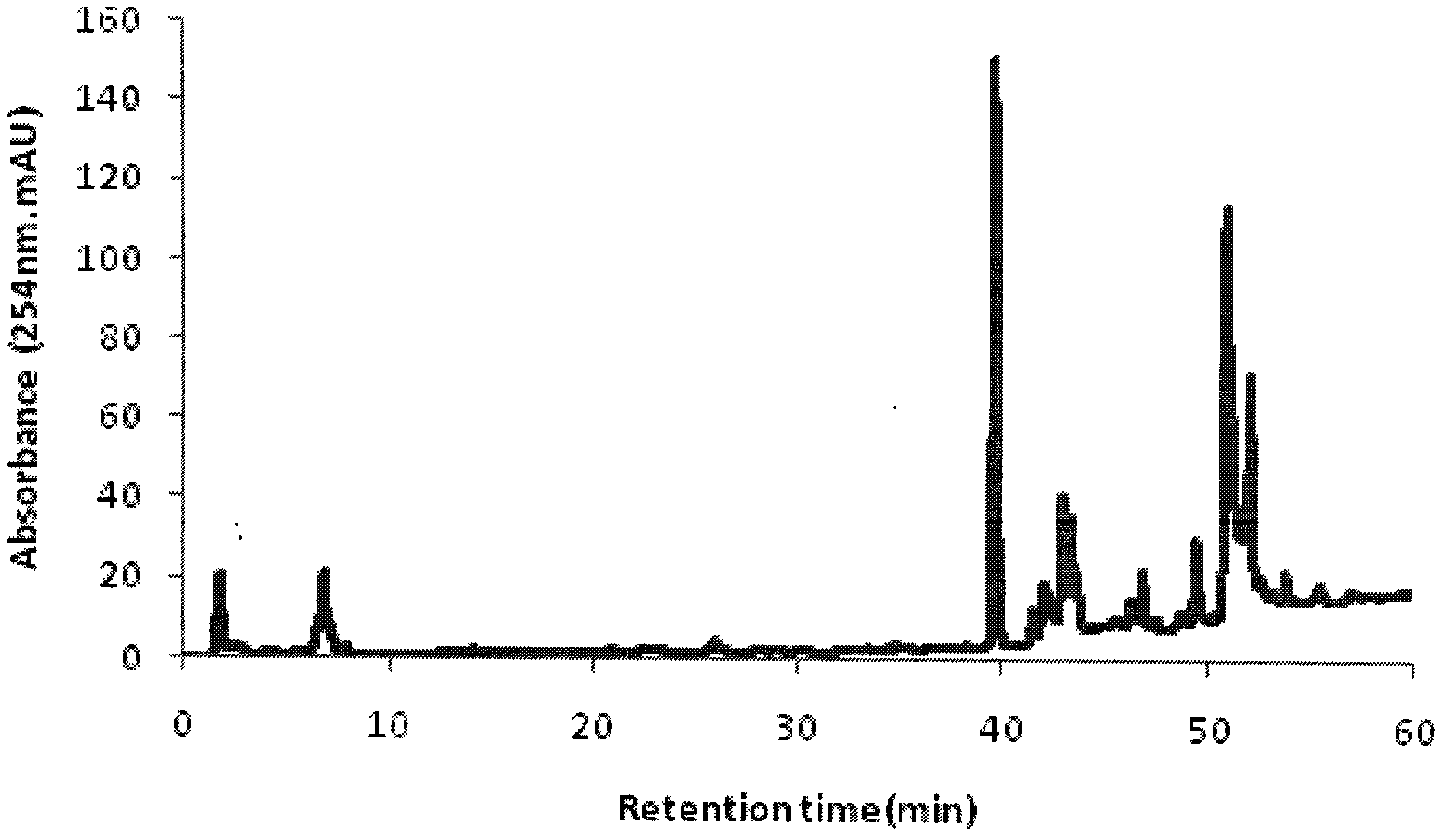
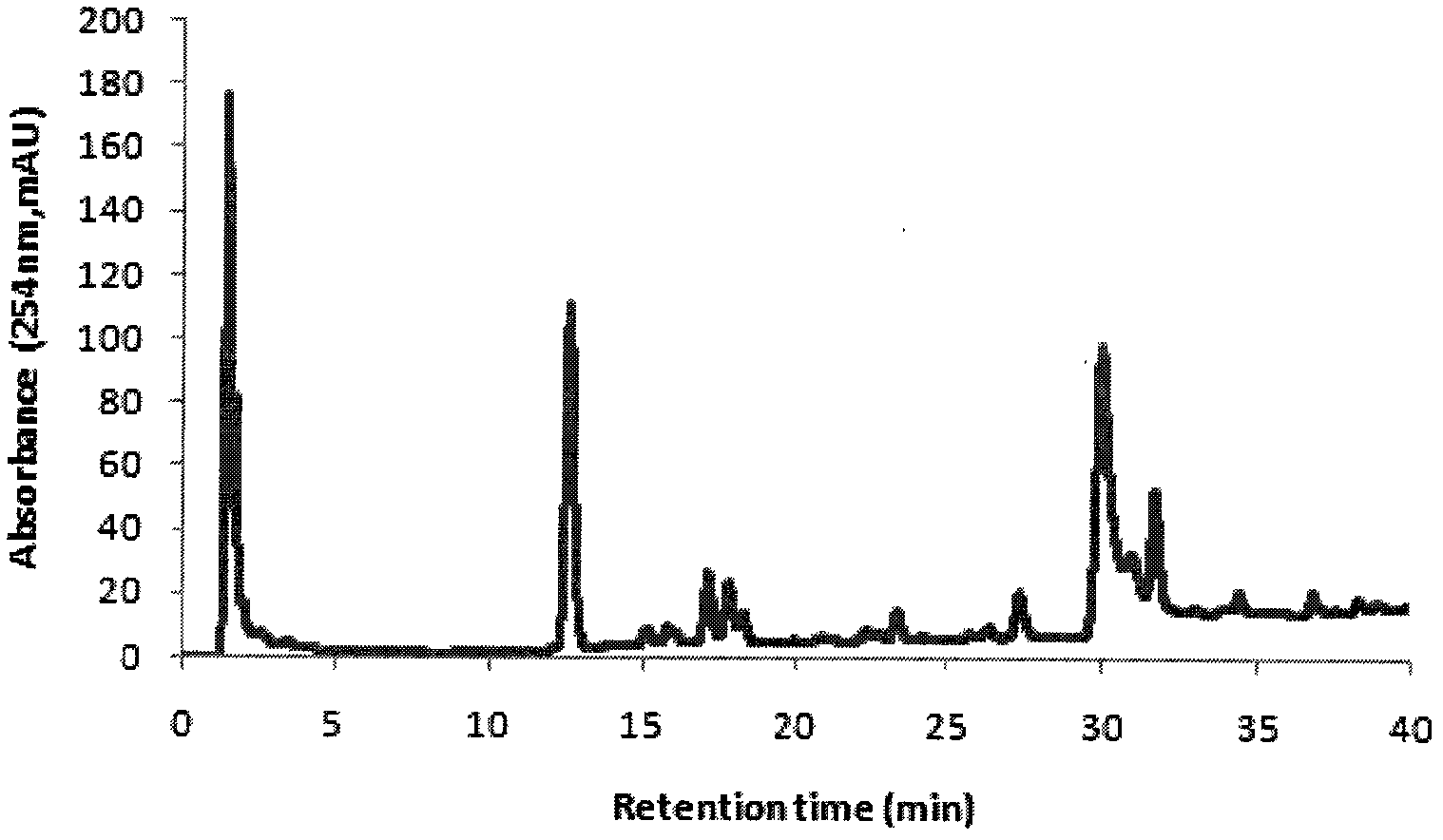
Method for carrying out simultaneous quantitative analysis on four lignan components in Chinese magnoliavine raw material and Chinese magnoliavine extract
A technology of schisandrin A and schisandrin B, which is applied to the analysis of materials, measuring devices, and material separation, can solve the problems of incomplete application and imperfection of analysis conditions, and achieve the effect of simple pretreatment process and high extraction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Embodiment 1: Determination of HPLC analysis conditions
[0068] In this embodiment, the Agilent high performance liquid chromatography system: quaternary pump G1311A, online degasser G1379A, autosampler G1329A, variable wavelength detection 1314A; reagents: methanol and acetonitrile are dedicated to HPLC, and water is deionized water.
[0069] 1. Selection of chromatographic column and mobile phase
[0070] Due to the complexity and diversity of traditional Chinese medicine ingredients, the determination of the analysis method must first be based on its characteristics, to find the chromatographic mode and the best column system. For the analysis of general medicinal materials or the total extract of the compound, choose the reversed-phase liquid chromatography mode, and establish a pure water reversed-phase To the analysis method of the full concentration range of traditional Chinese medicine high performance liquid chromatography non-aqueous reversed-phase, ionic and...
Embodiment 2
[0085] Embodiment 2: quantitative correlation test
[0086] The standard solution of preparation is changed sample size by above-mentioned analysis condition and carries out chromatographic analysis, the chromatographic peak area obtained and solution concentration are done linear regression analysis, and the regression curve obtained has good linear relation ( Figure 9 ).
Embodiment 3
[0087] Embodiment 3: instrument precision experiment
[0088] Under the optimal chromatographic conditions, 25 μl of the reference solution was precisely drawn and injected in parallel for 10 times. It can be seen from the measurement results that the relative deviations of the four substances are all less than 3.0%, and the reliability of the measurement results can meet the analysis requirements.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com