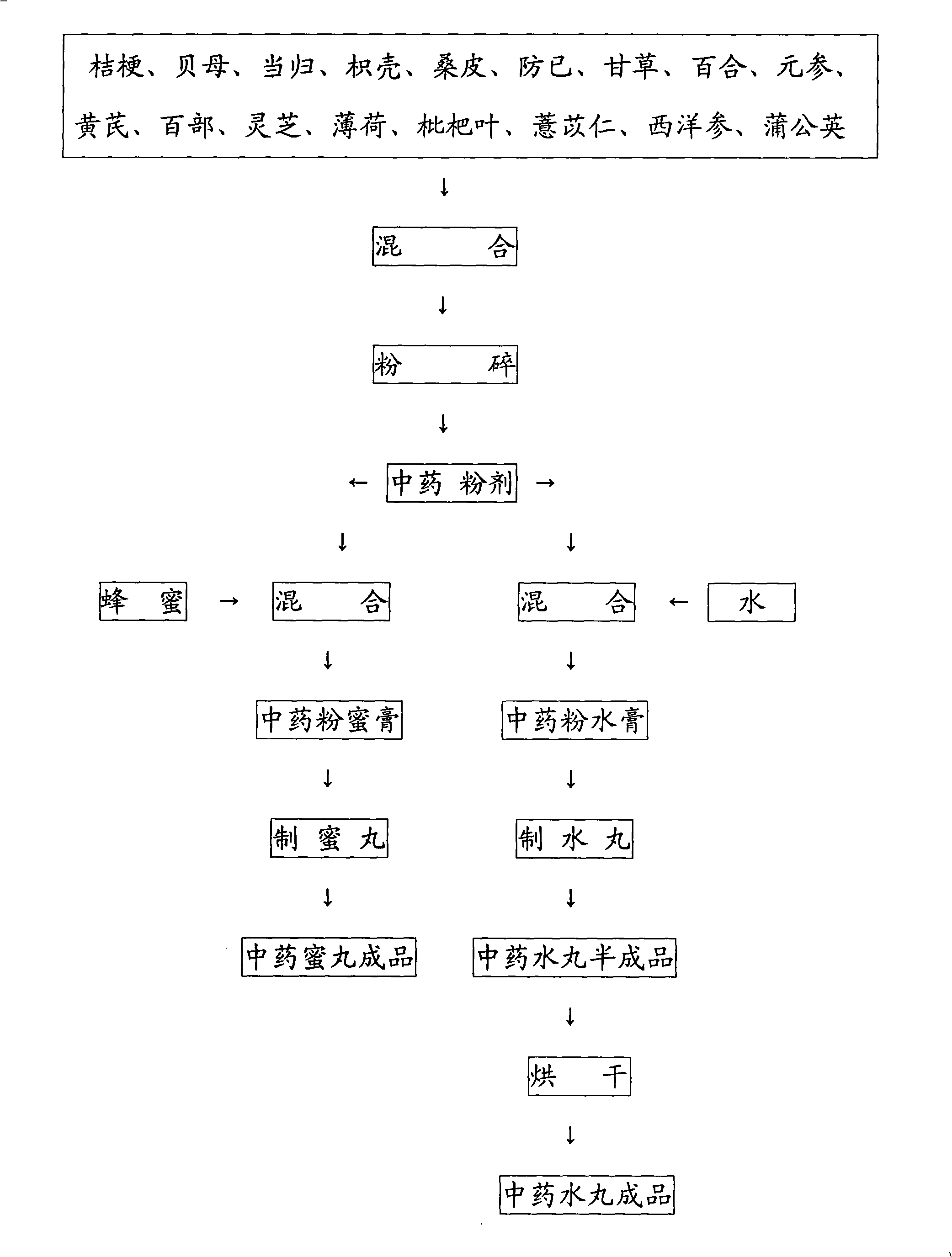Patents
Literature
362 results about "Lung fibrosis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
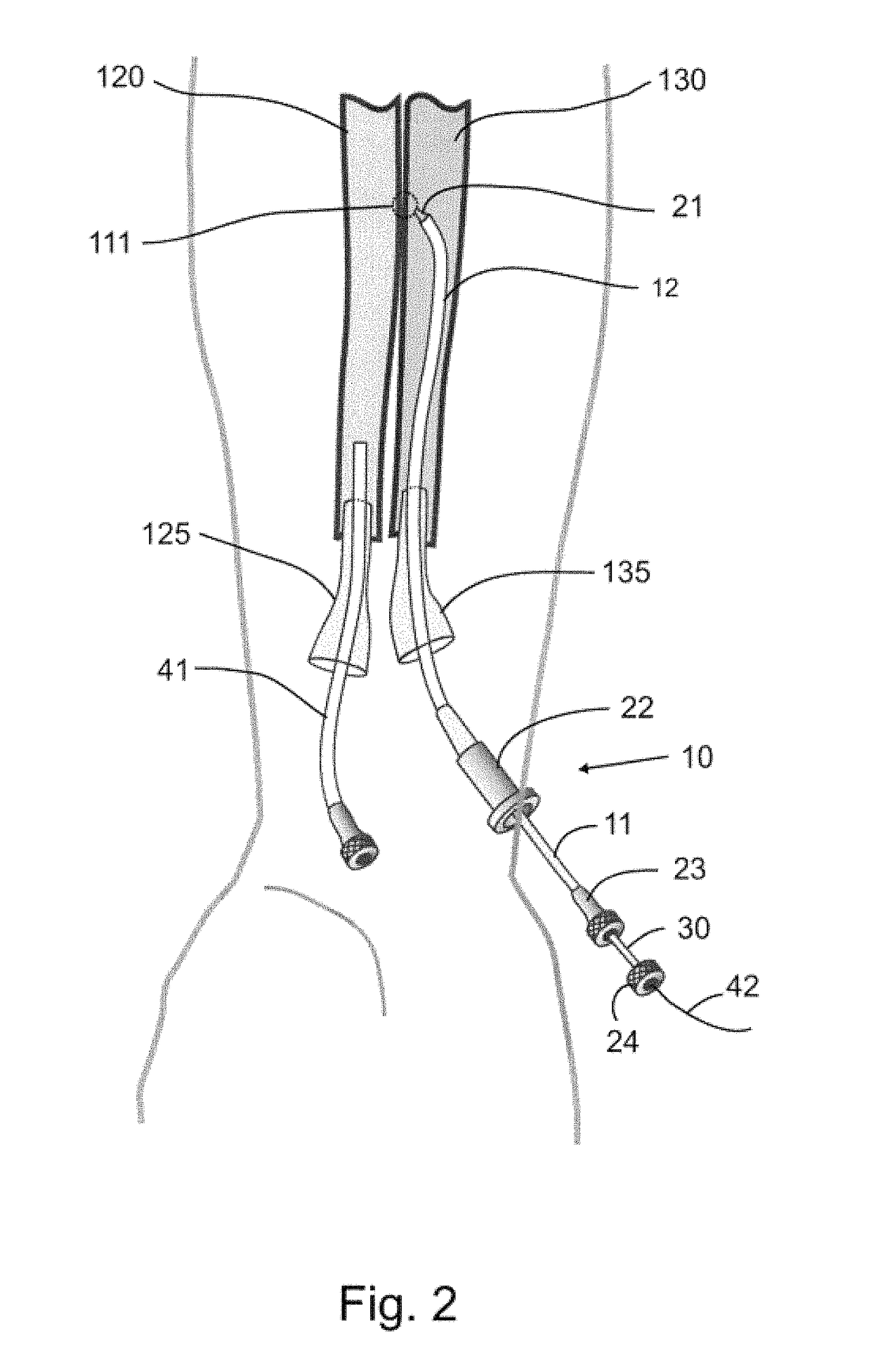
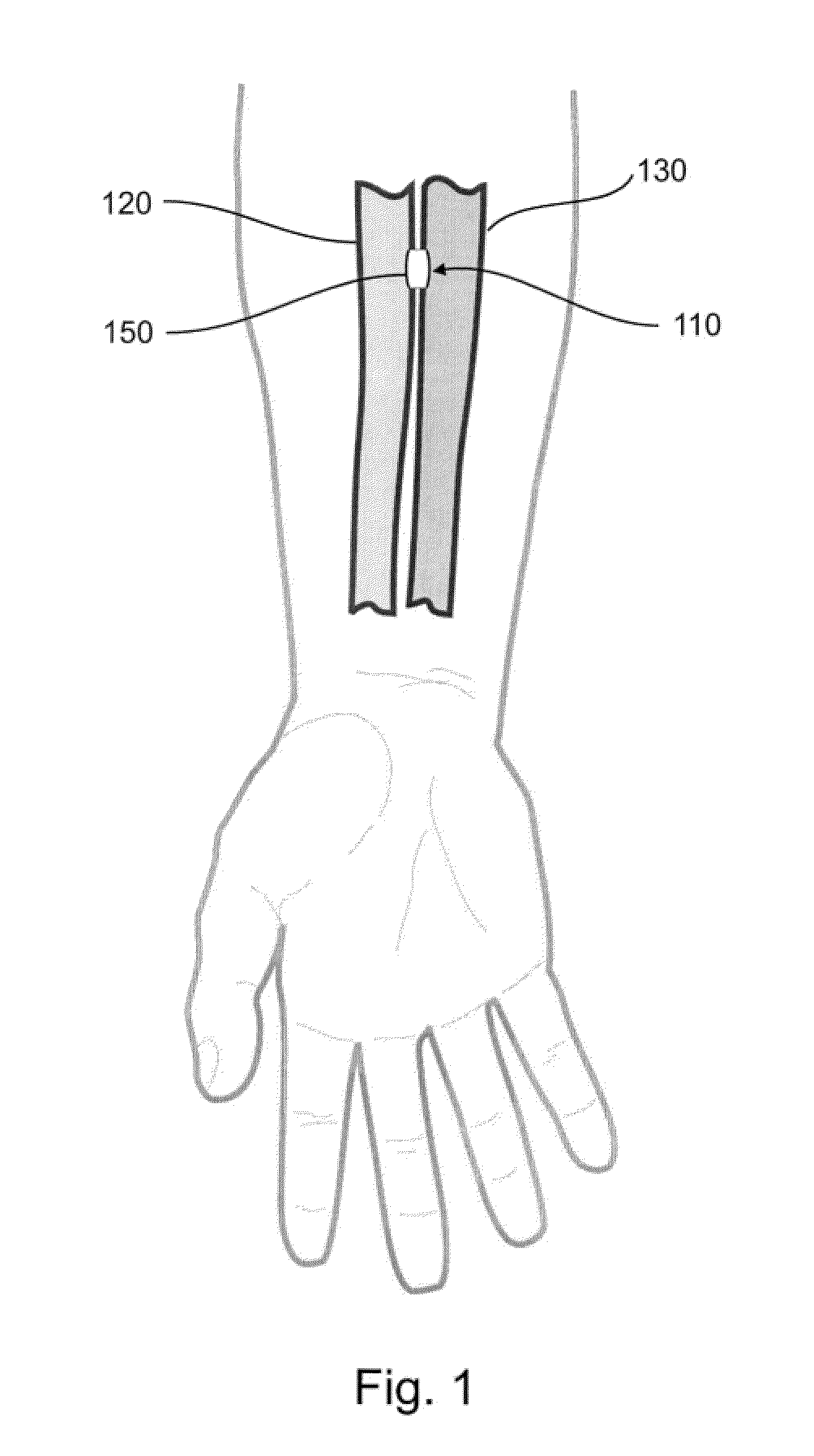
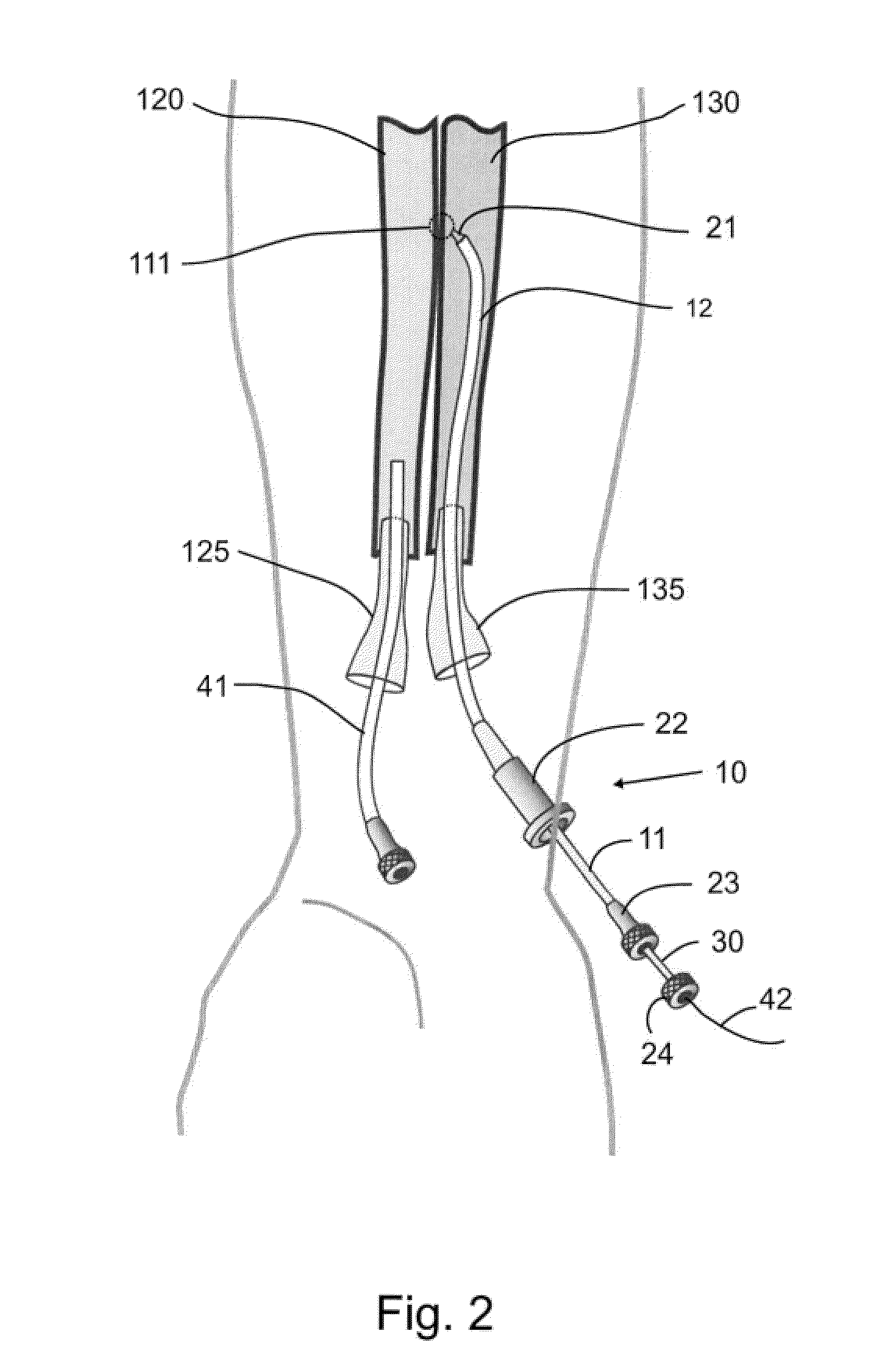
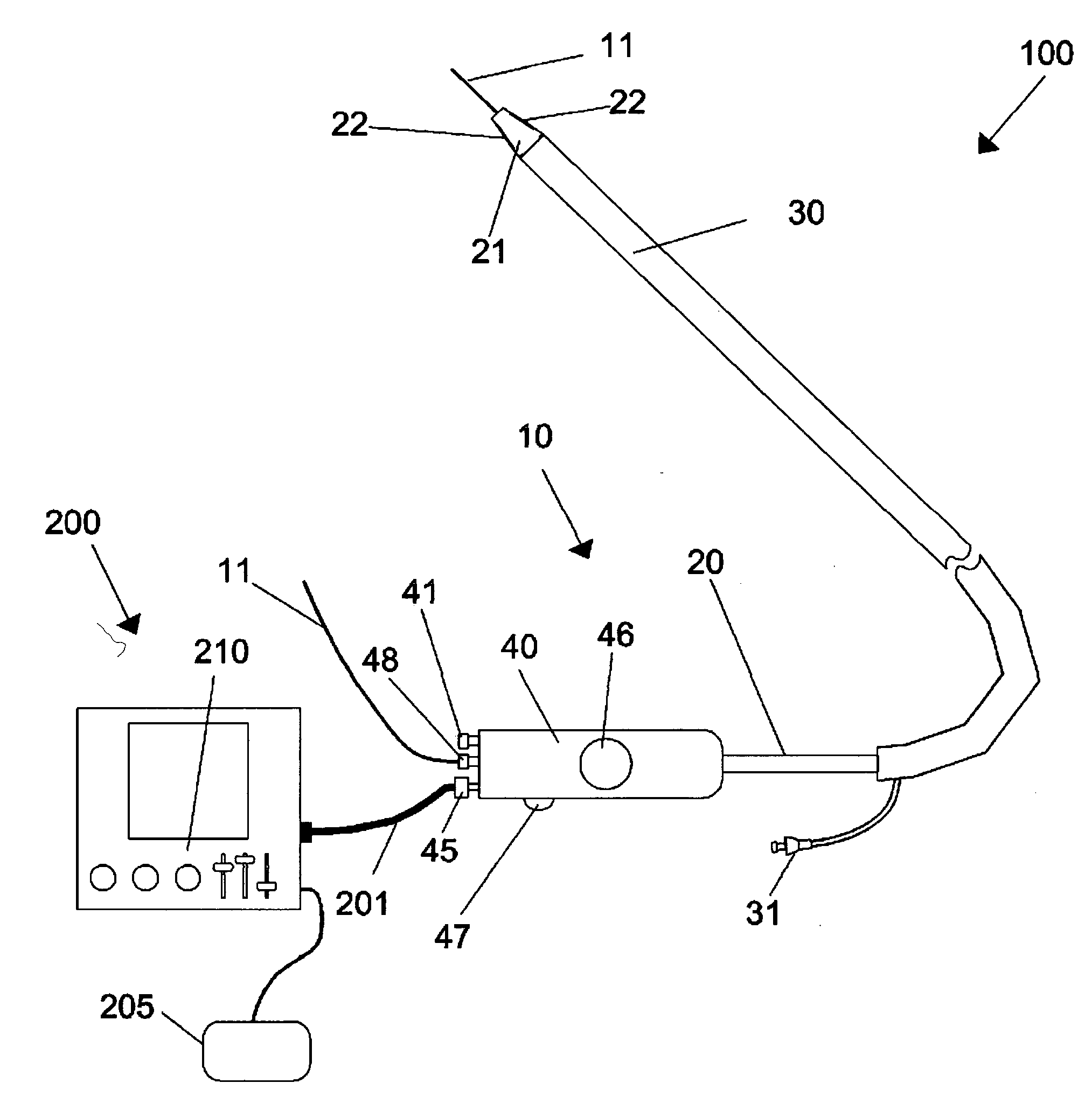
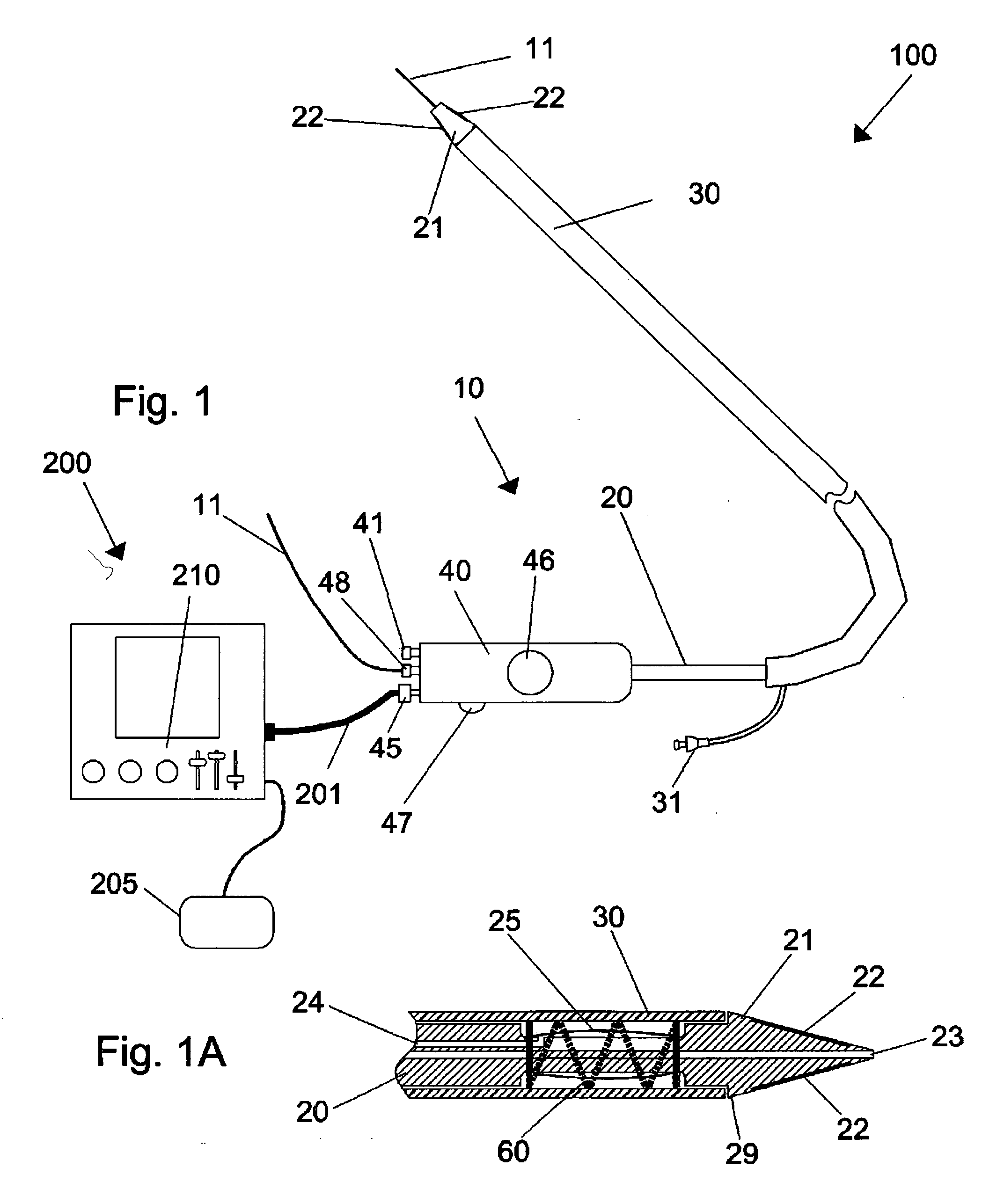
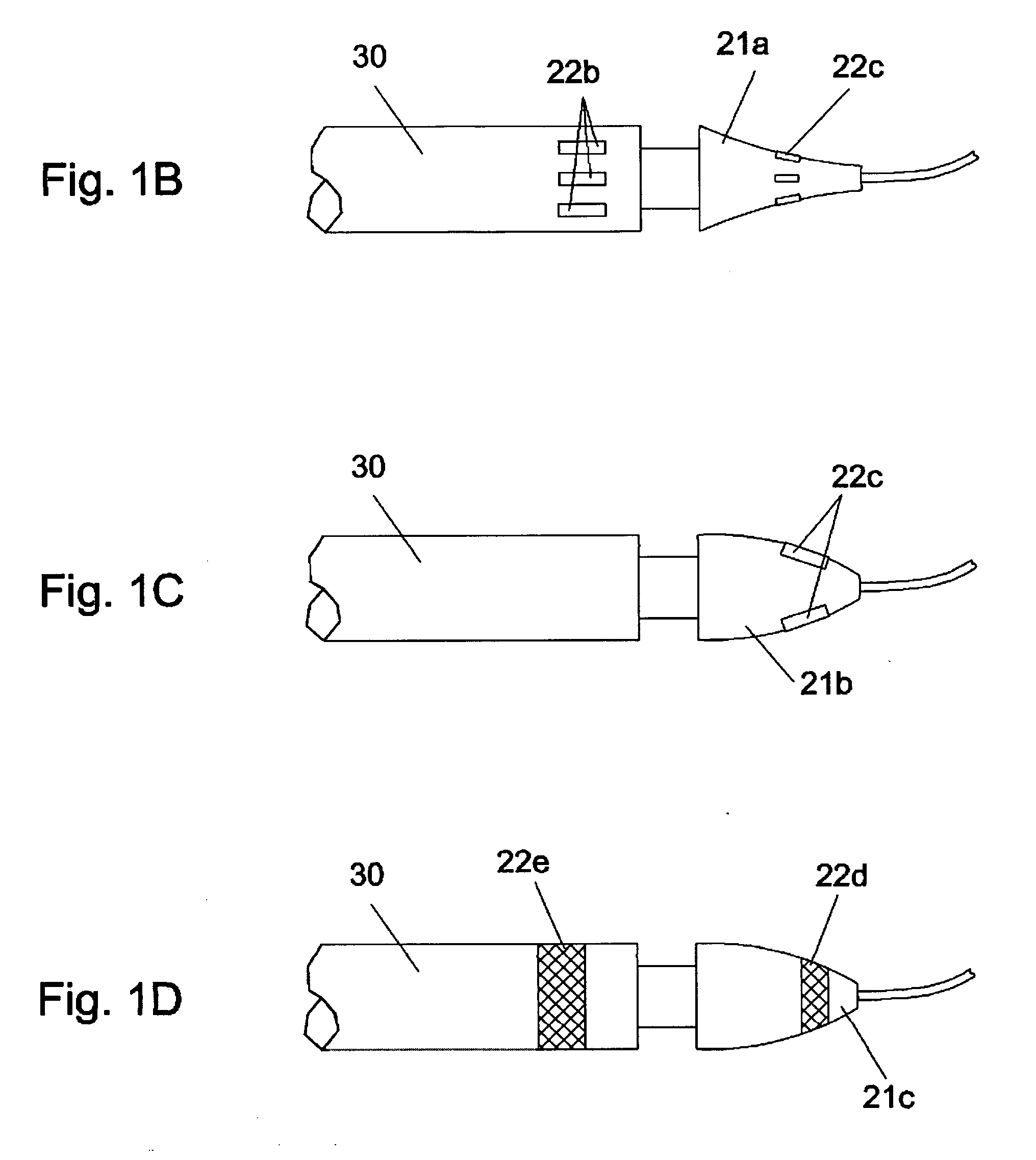
Devices, systems, and methods for energy assisted arterio-venous fistula creation
InactiveUS20060111704A1Avoid bleedingProviding tensionStentsSurgical instruments for heatingRESPIRATORY DISTRESS SYNDROME ADULTDisease
Devices, systems and methods are disclosed for the formation of an arteriovenous fistula. Embodiments include catheter apparatus including an ablation element for creating and / or modifying the fistula. The devices, systems and methods can be used to treat patients with one or more numerous ailments including chronic obstructive pulmonary disease, congestive heart failure, hypertension, hypotension, respiratory failure, pulmonary arterial hypertension, lung fibrosis and adult respiratory distress syndrome.
Owner:EDWARDS LIFESCIENCES CORP
Compositions, formulations and kit with anti-sense oligonucleotide and anti-inflammatory steroid and/or obiquinone for treatment of respiratory and lung disesase
InactiveUS20070021360A1Decreased airwayOrganic active ingredientsBiocideDiseaseAntiendomysial antibodies
A pharmaceutical composition and formulations comprise preventative, prophylactic or therapeutic amounts of an oligo(s) anti-sense to a specific gene(s) or its corresponding mRNA(s), and a glucocorticoid and / or non-glucocorticoid steroid or a ubiquinone or their salts. The agents, composition and formulations are used for treatment of ailments associated with impaired respiration, bronchoconstriction, lung allergy(ies) or inflammation, and abnormal levels of adenosine, adenosine receptors, sensitivity to adenosine, lung surfactant and ubiquinone, such as pulmonary fibrosis, vasoconstriction, inflammation, allergies, allergic rhinitis, asthma, impeded respiration, lung pain, cystic fibrosis, bronchoconstriction, COPD, RDS, ARDS, cancer, and others. The present treatment is effectively administered by itself for conditions without known therapies, as a substitute for therapies exhibiting undesirable side effects, or in combination with other treatments, e.g. before, during and after other respiratory system therapies, radiation, chemotherapy, antibody therapy and surgery, among others. Each of the agents of this invention may be administered directly into the respiratory system so that they gain direct access to the lungs, or by other effective routes of administration. A kit comprises a delivery device, the agents and instructions for its use.
Owner:EPIGENESIS PHARMA LLC
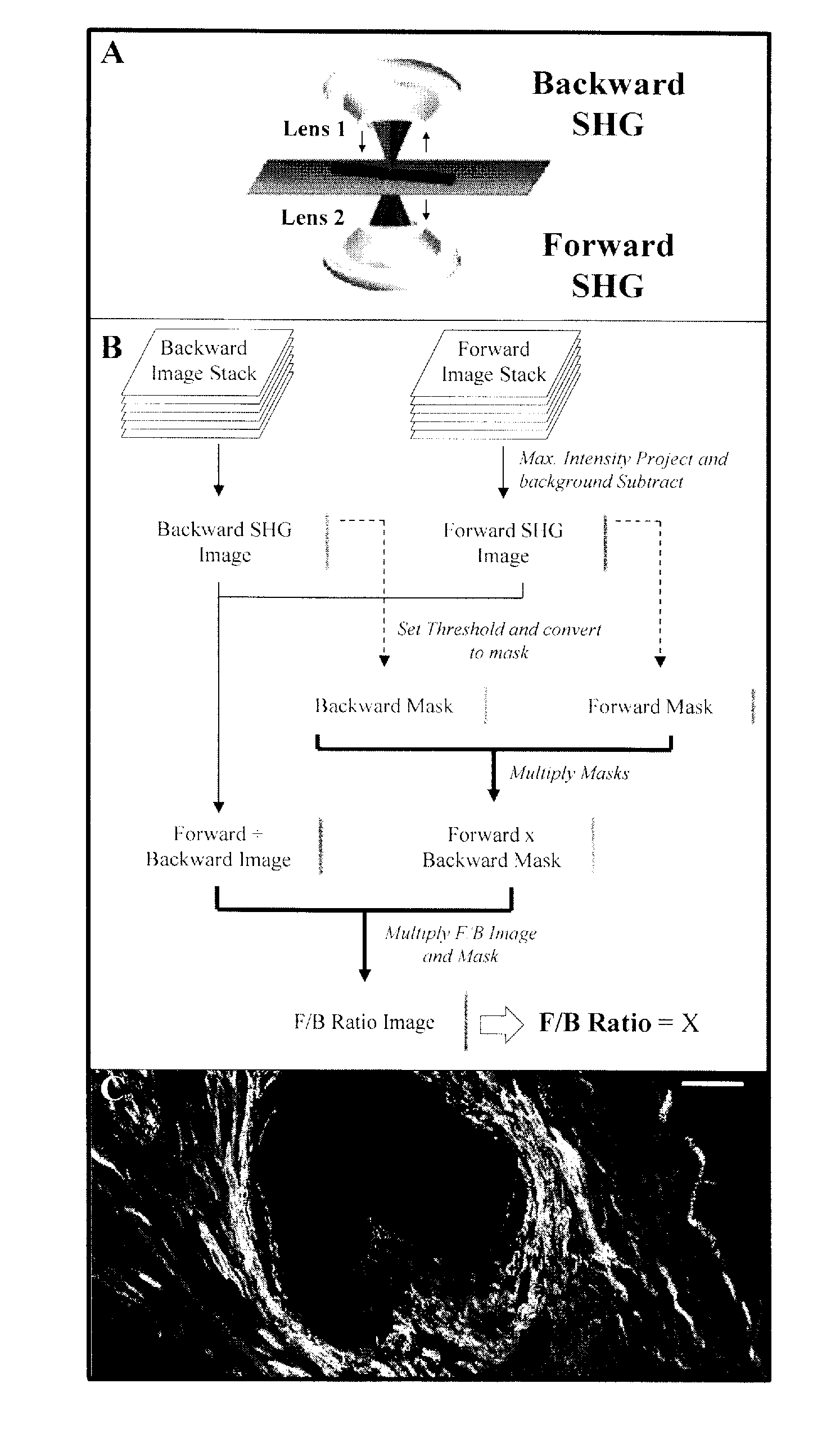
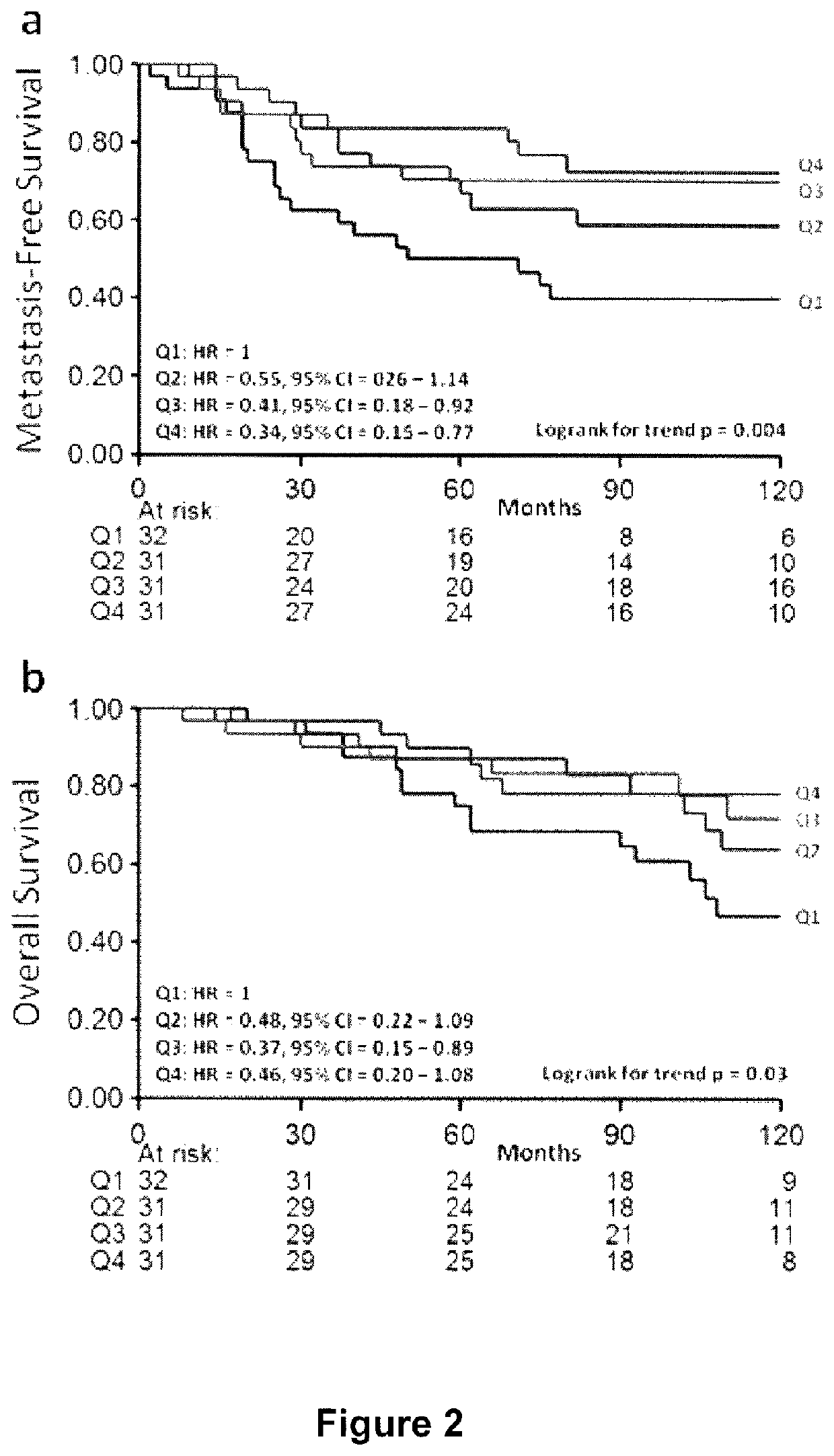
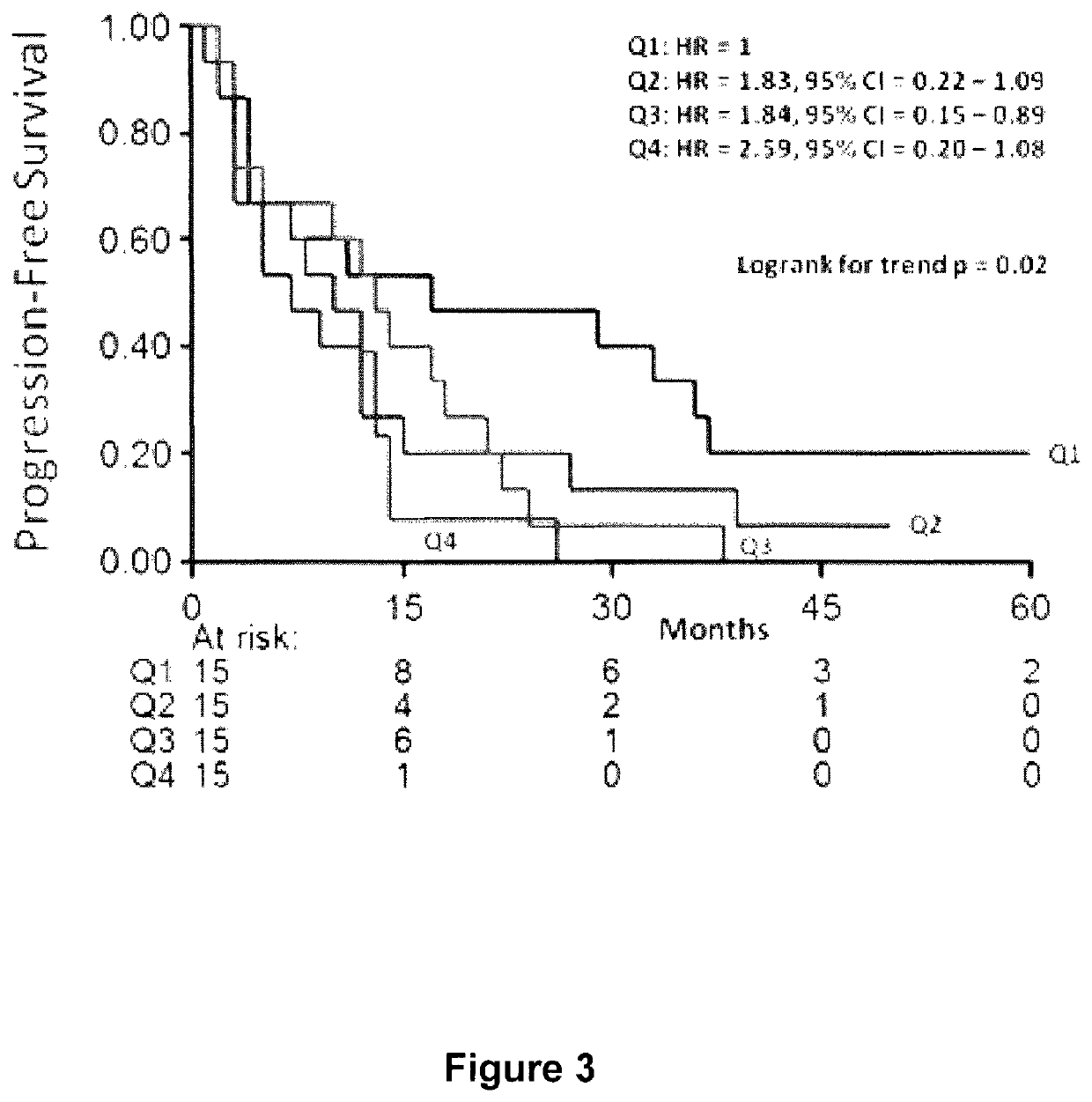
Method and apparatus to diagnose the metastatic or progressive potential of cancer, fibrosis and other diseases
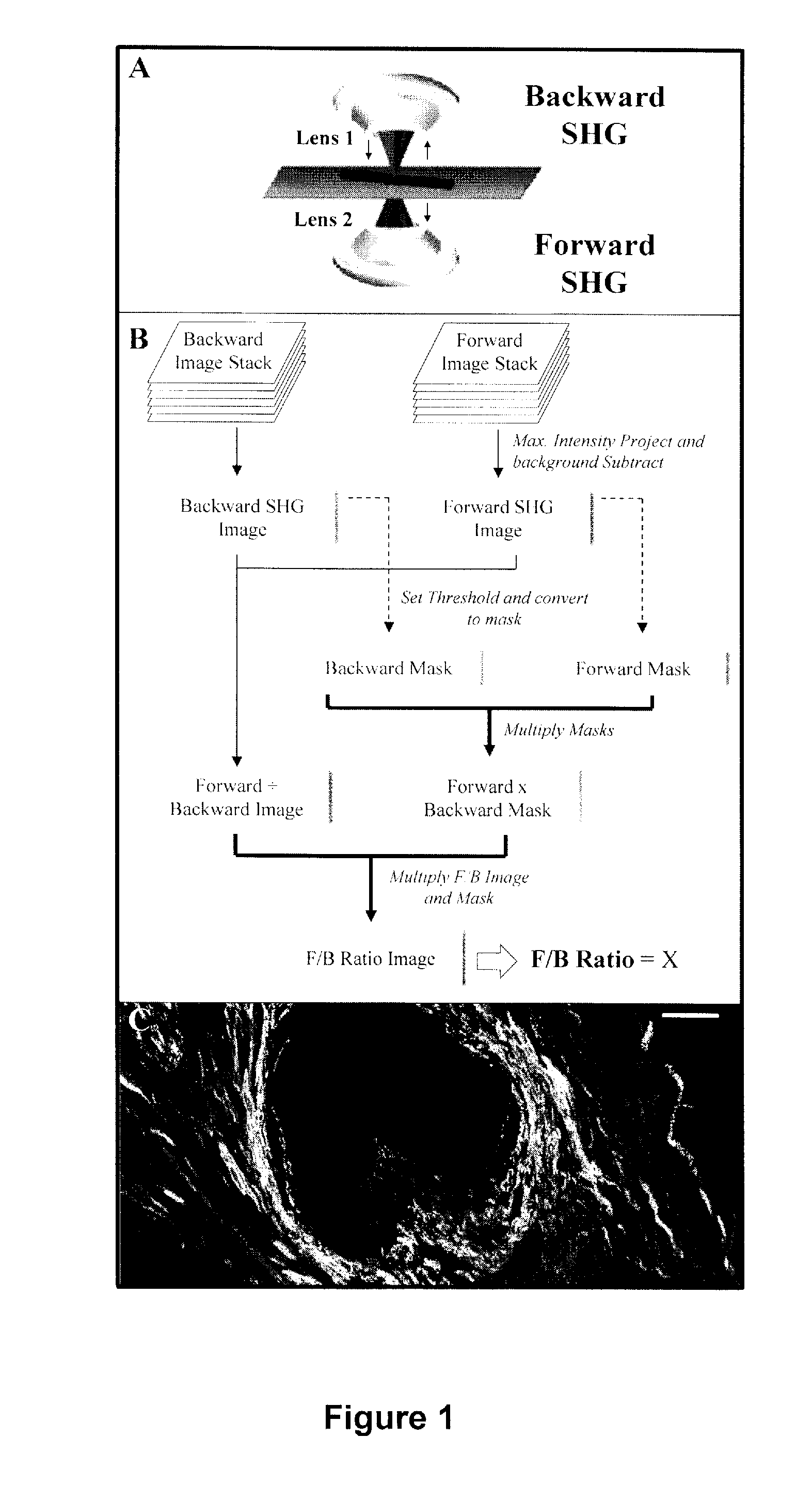
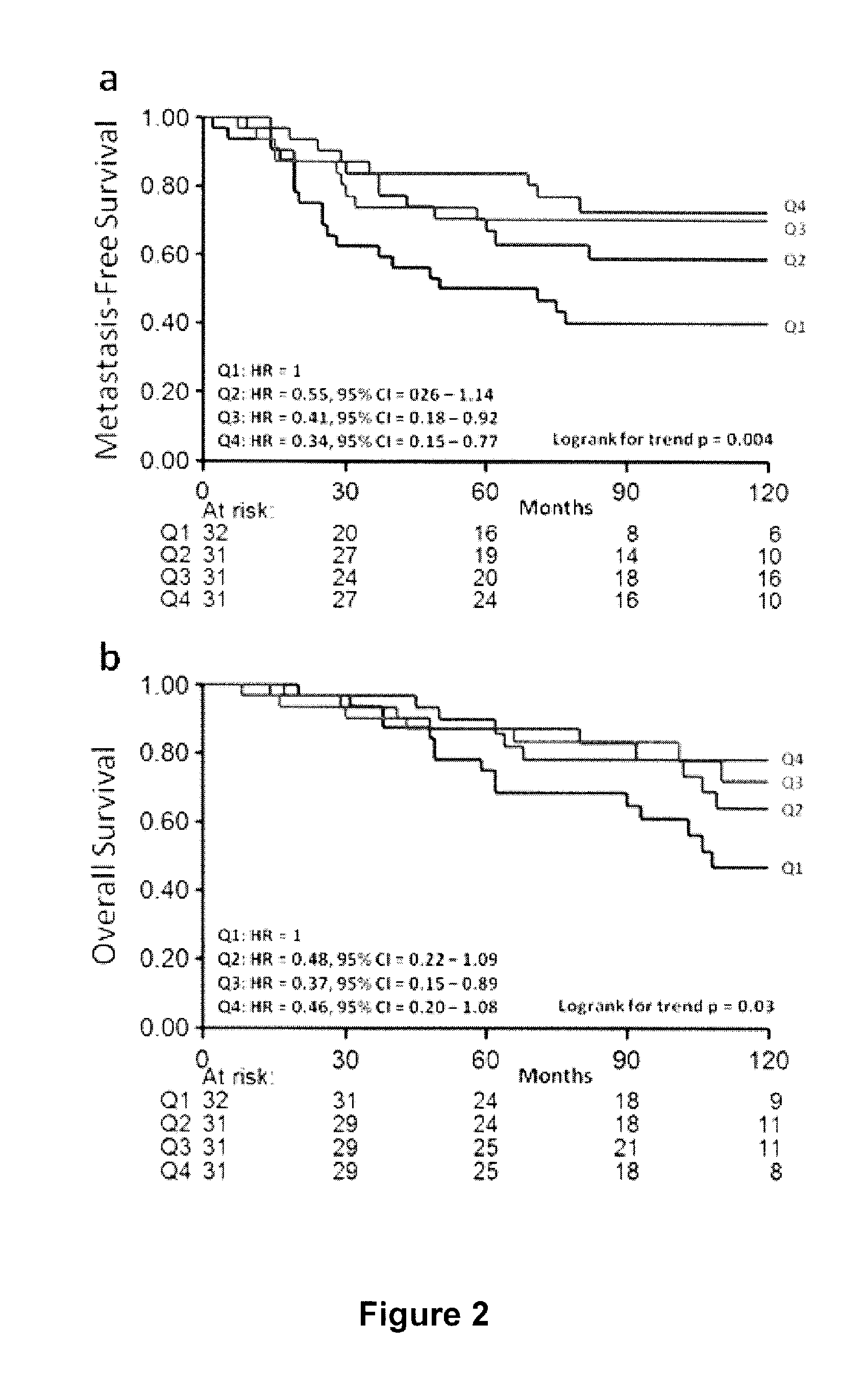
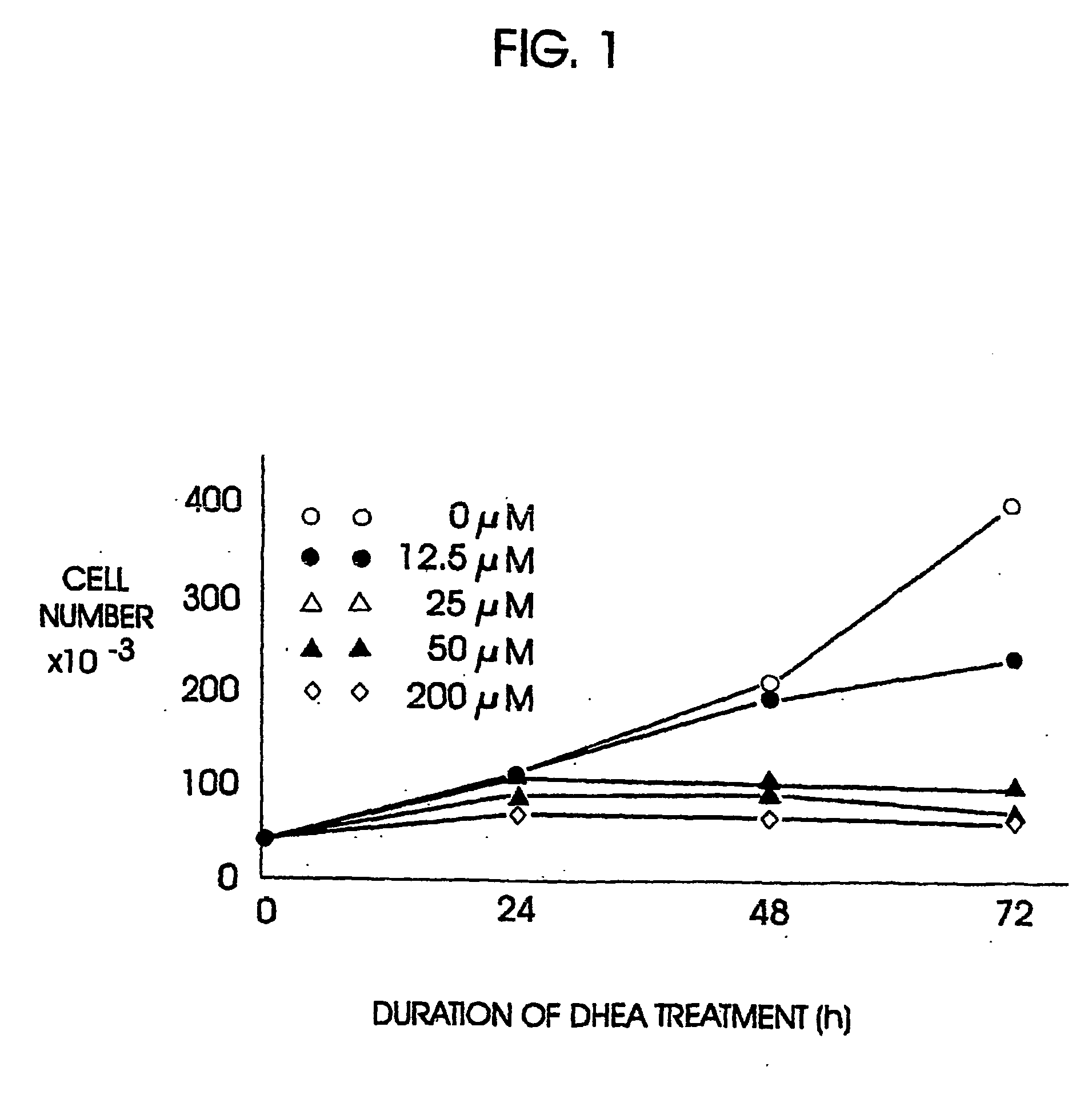
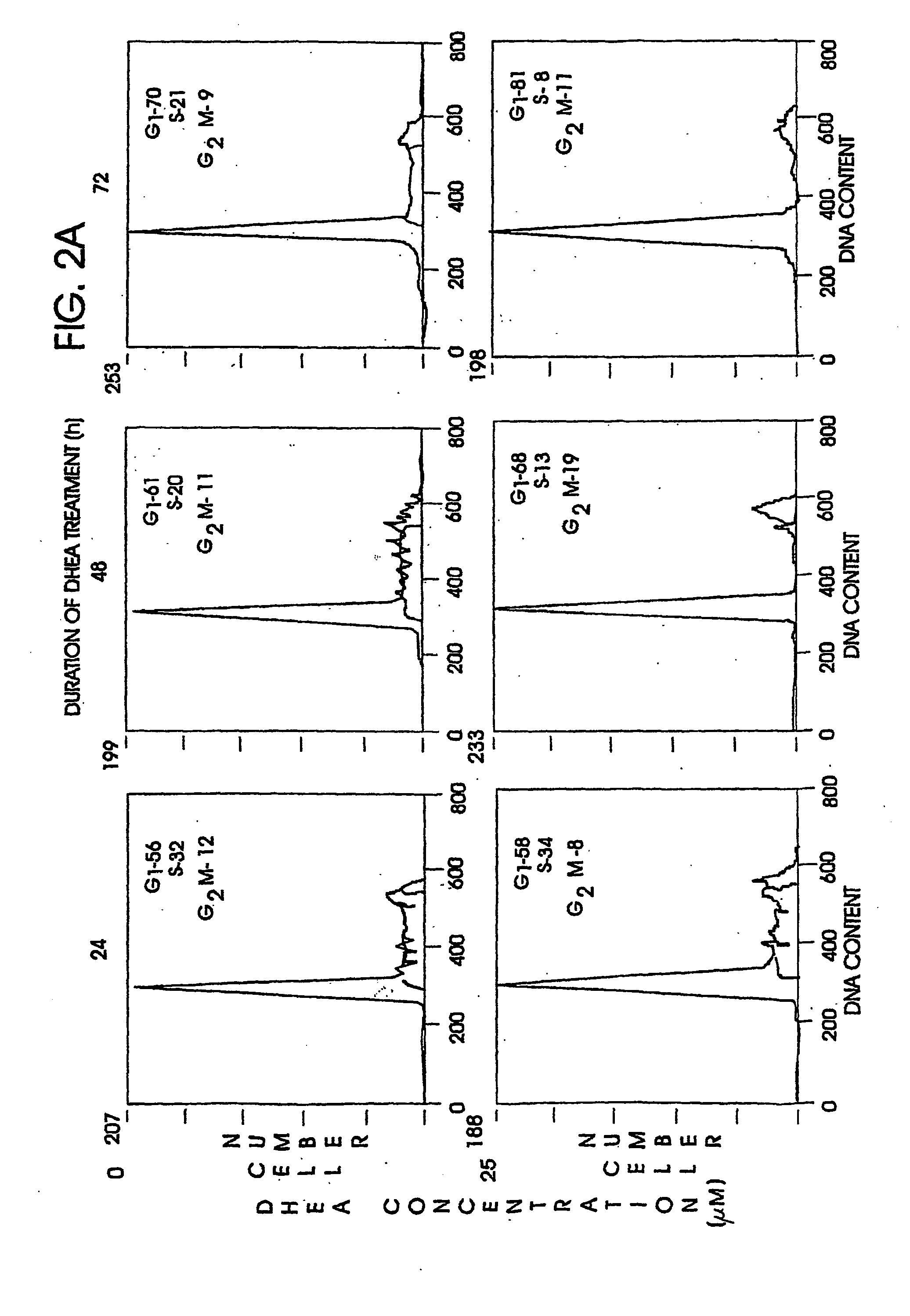
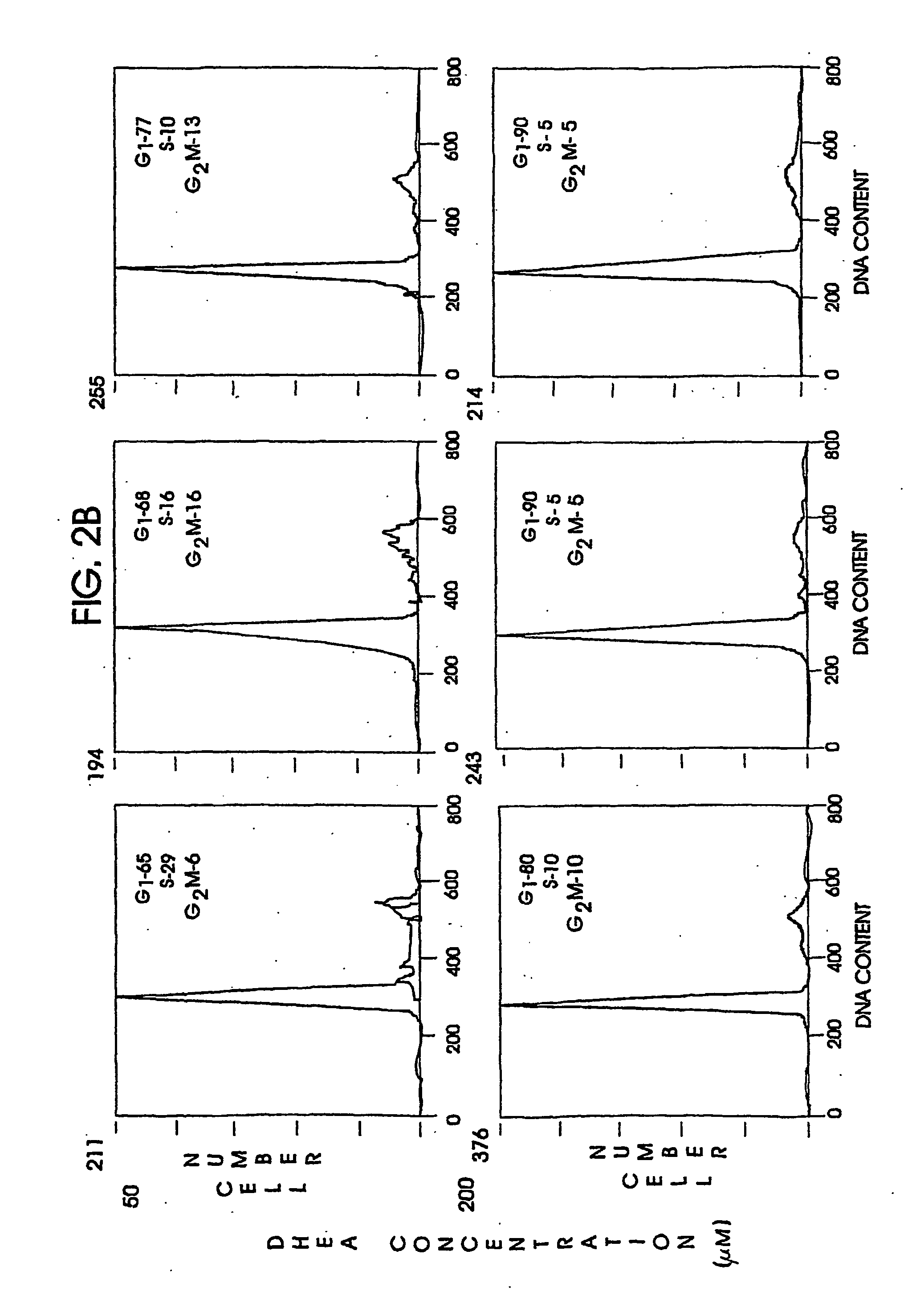
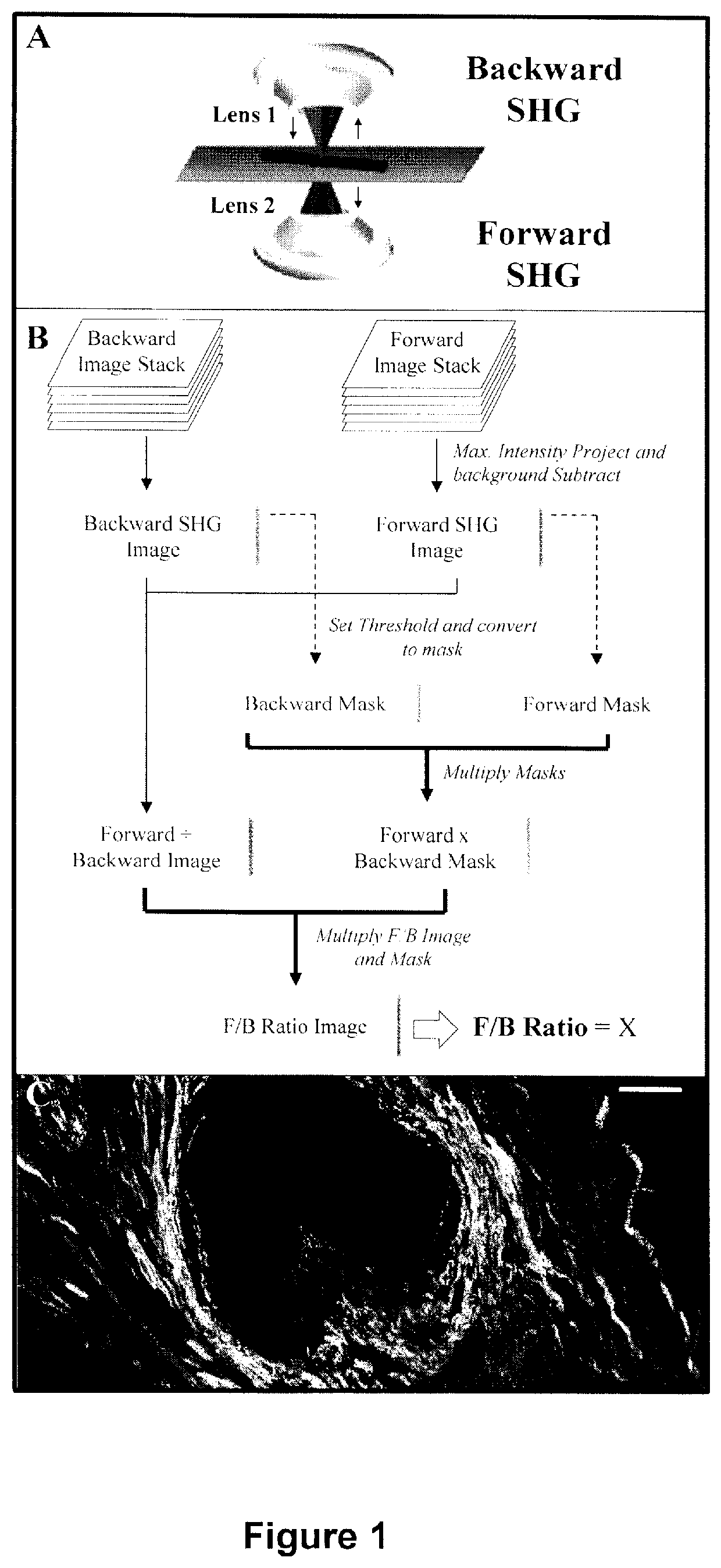
A method and apparatus for determining the progressive potential of a disease is disclosed. The forward to backward propagating second harmonic generation signal derived from a second harmonic generation instrument is used to assess the collagen microstructure of imaged body tissue by way of numerical values that are in turn used to determine the progressive or metastatic potential of the disease. The disease may, for example, be a cancer such as breast cancer, lung fibrosis, colorectal adenocarcinoma, or the like. The apparatus may include in vivo instruments or laboratory diagnostic instruments with methods disclosed herein.
Owner:UNIVERSITY OF ROCHESTER
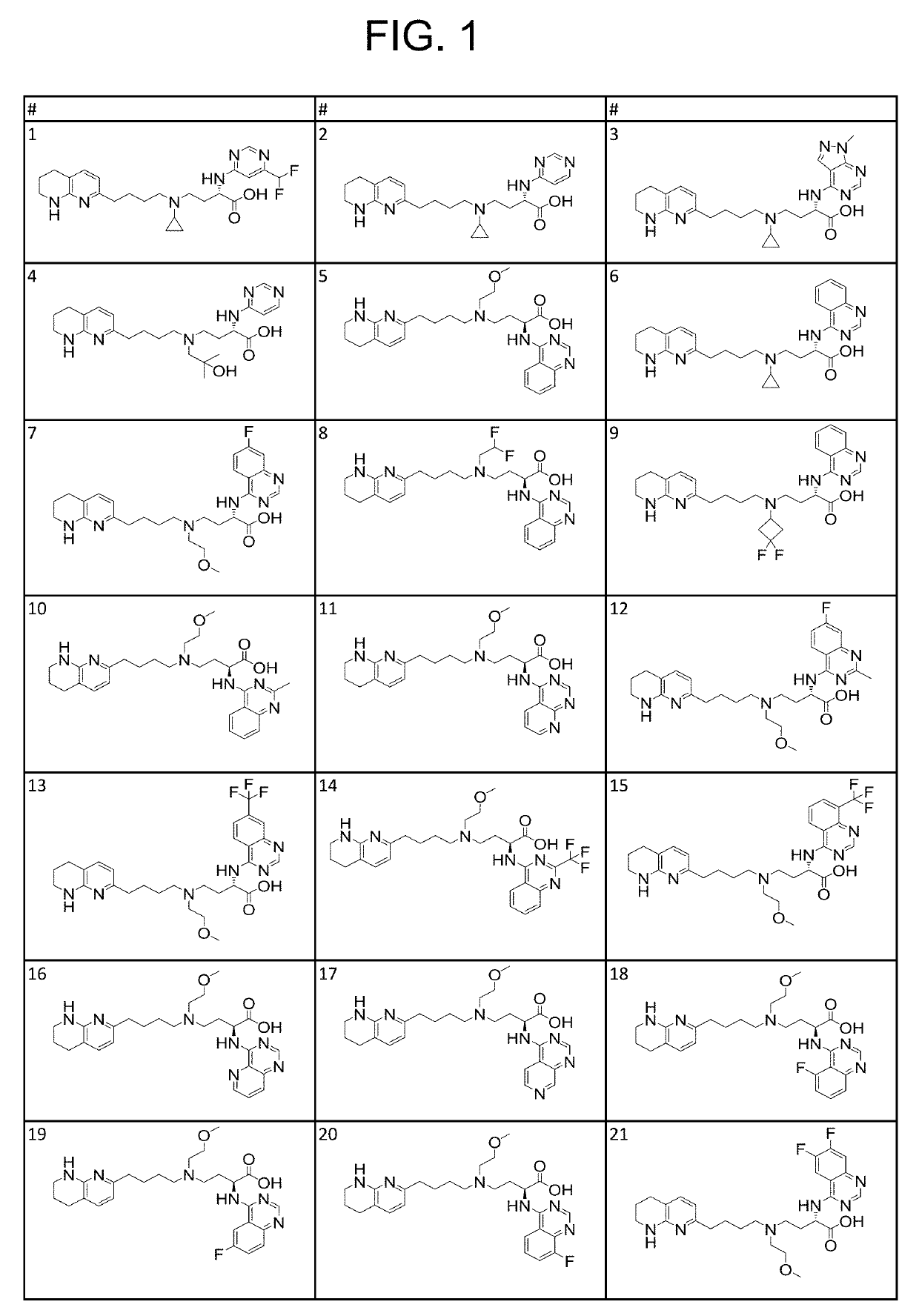
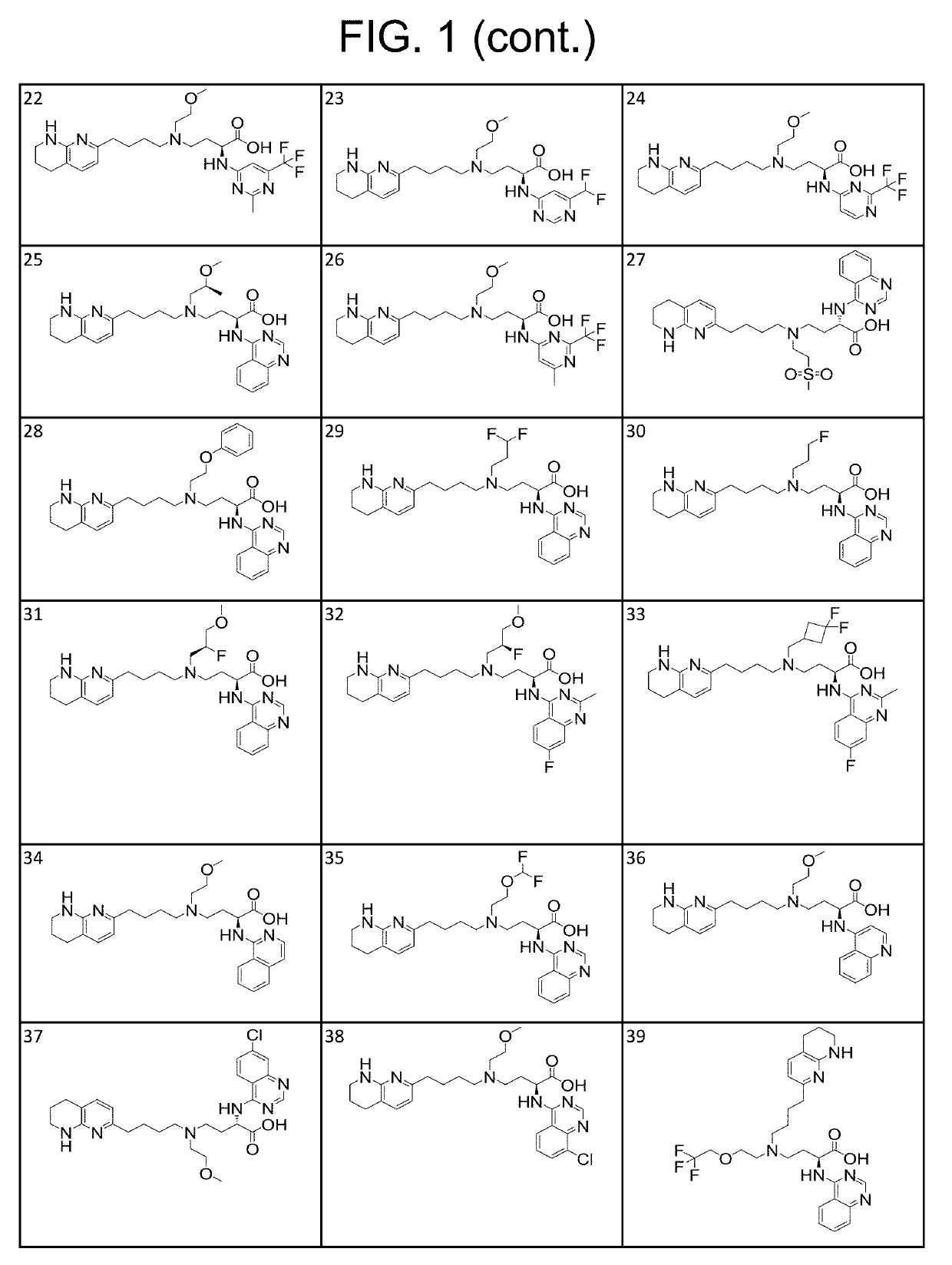
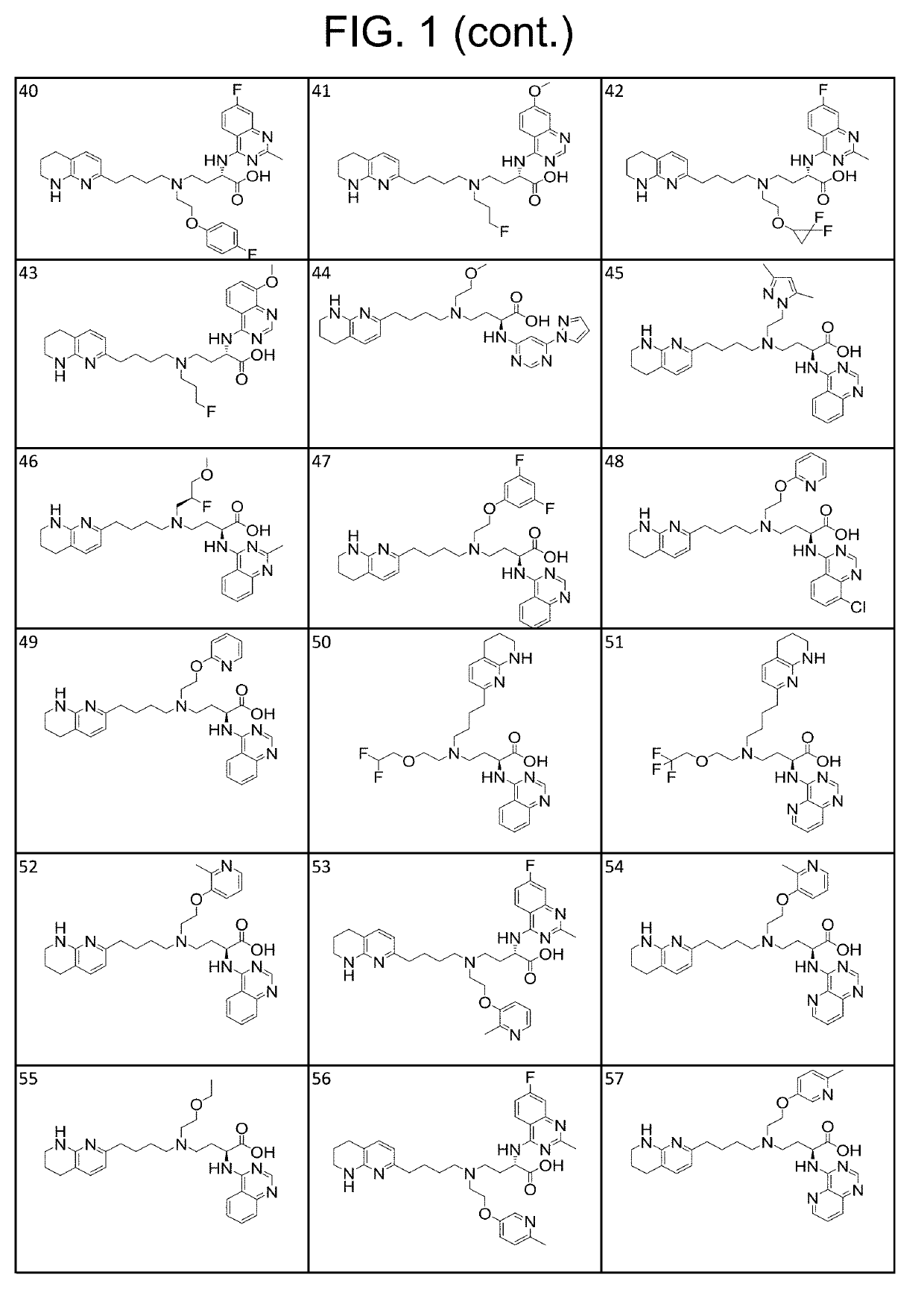
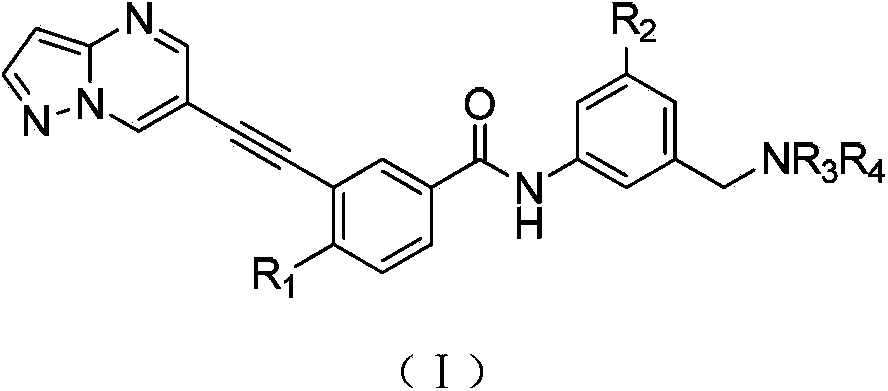
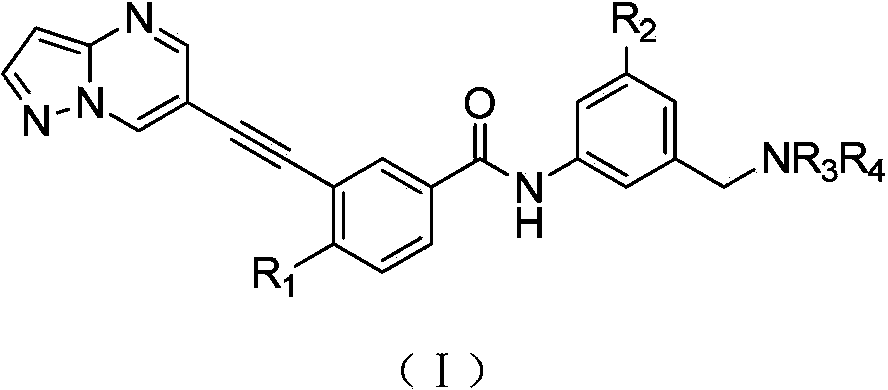
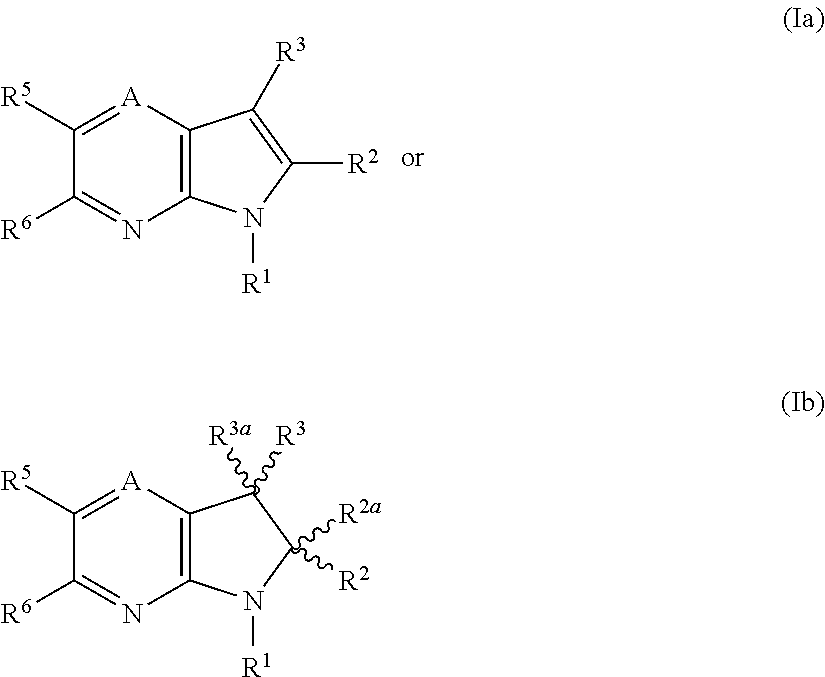
1H-pyrazolo[3,4-b]pyridines and therapeutic uses thereof
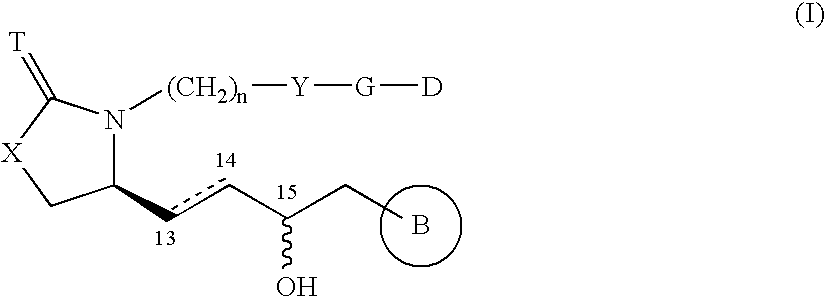
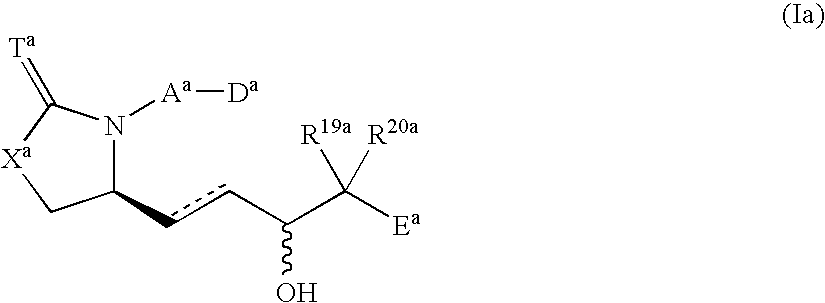
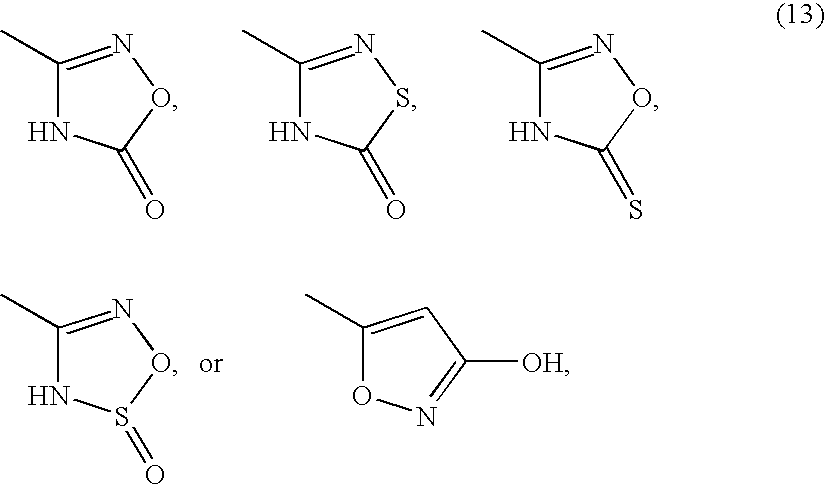
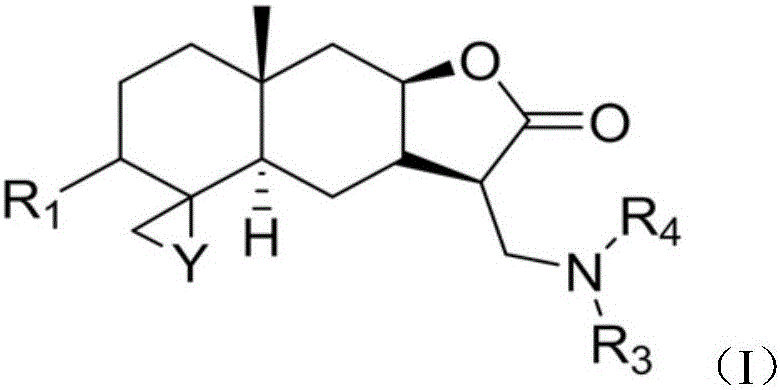
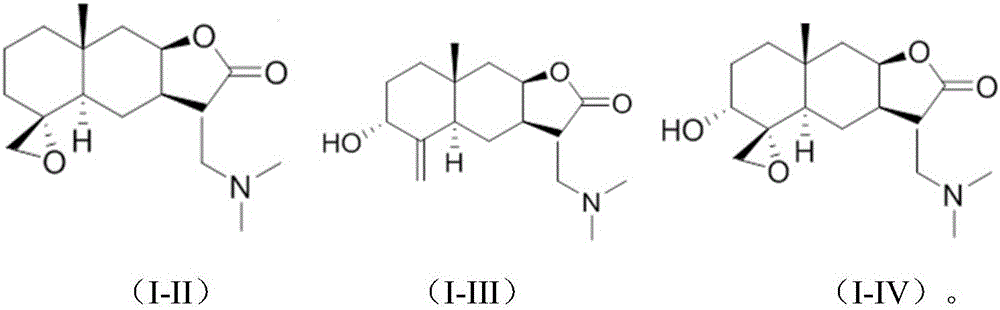
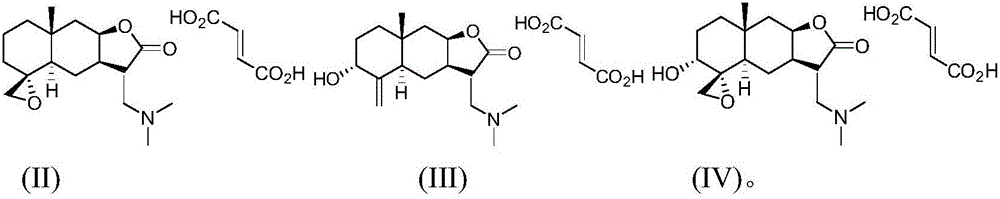
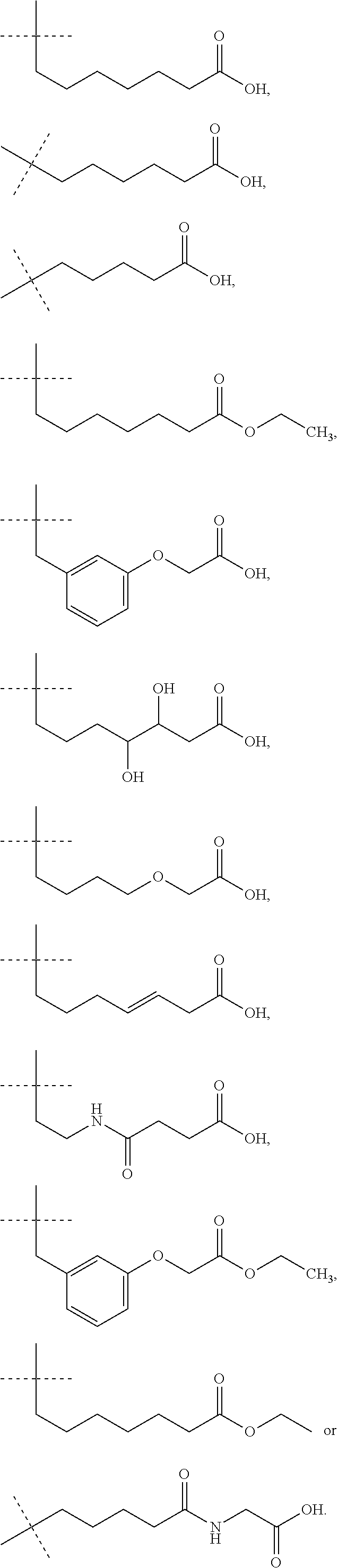
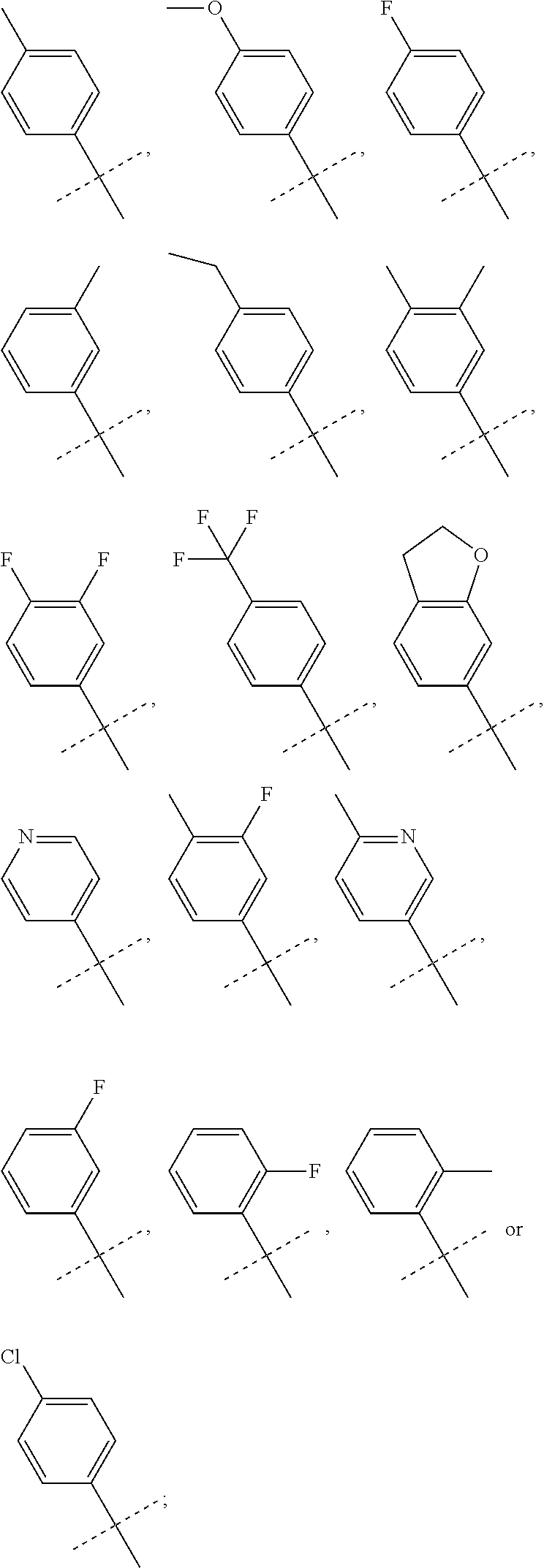
Provided herein are compounds according to Formulas (I) or (II) and pharmaceutically acceptable salts thereof, and compositions comprising the same, for use in various methods, including treating cancer, abnormal cellular proliferation, angiogenesis, Alzheimer's disease, lung disease, osteoarthritis, idiopathic pulmonary fibrosis and neurological conditions / disorders / diseases.
Owner:BIOSPLICE THERAPEUTICS INC
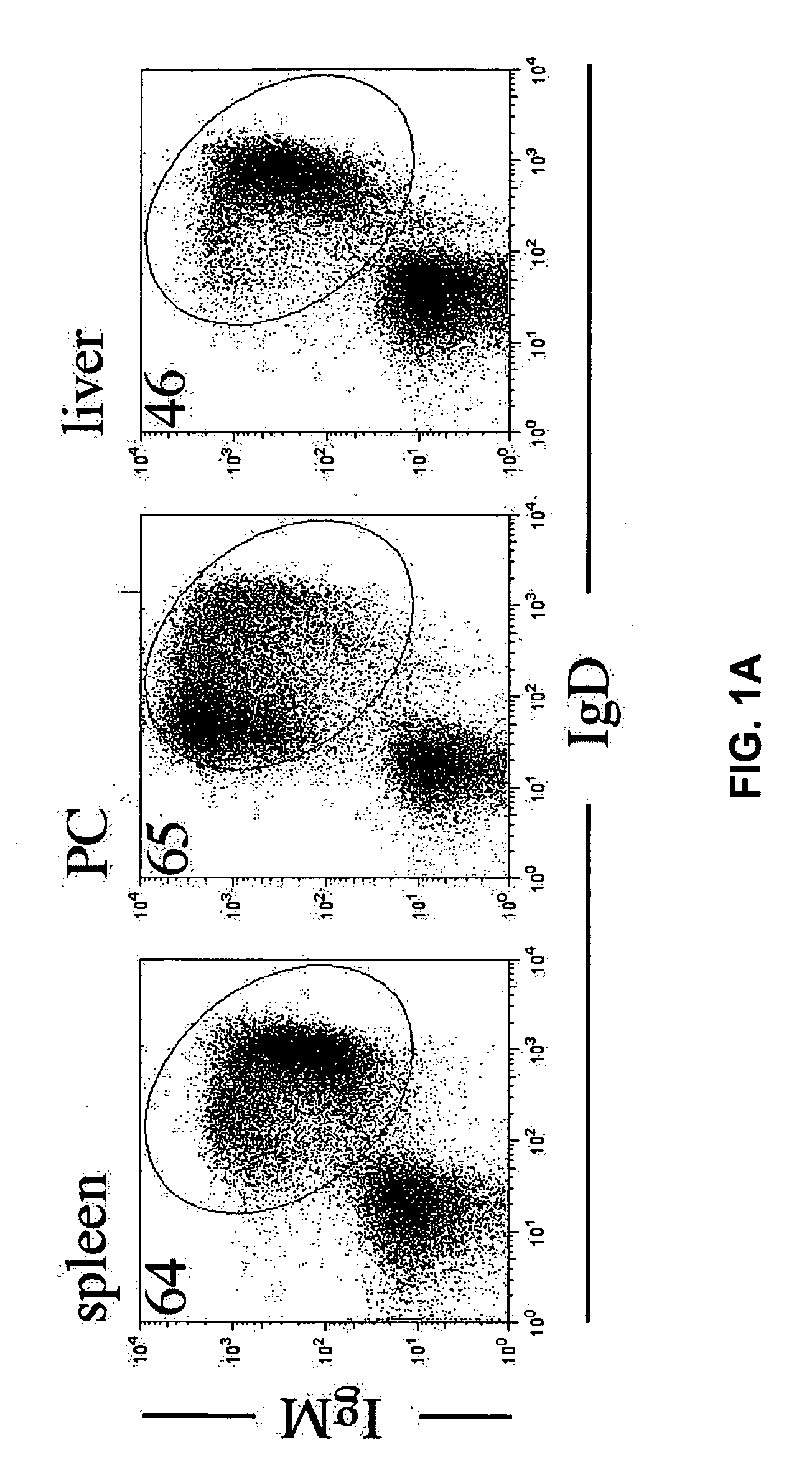
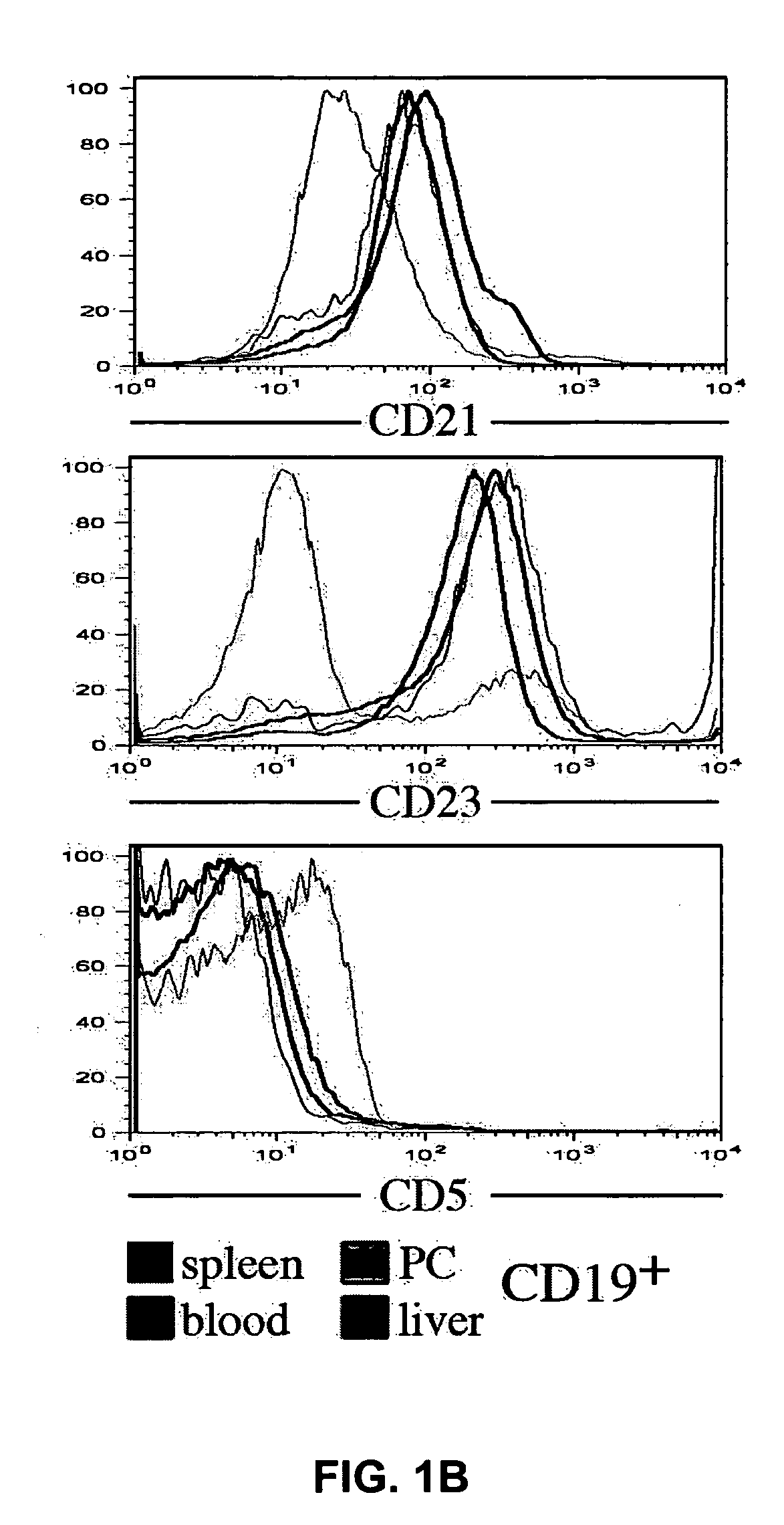
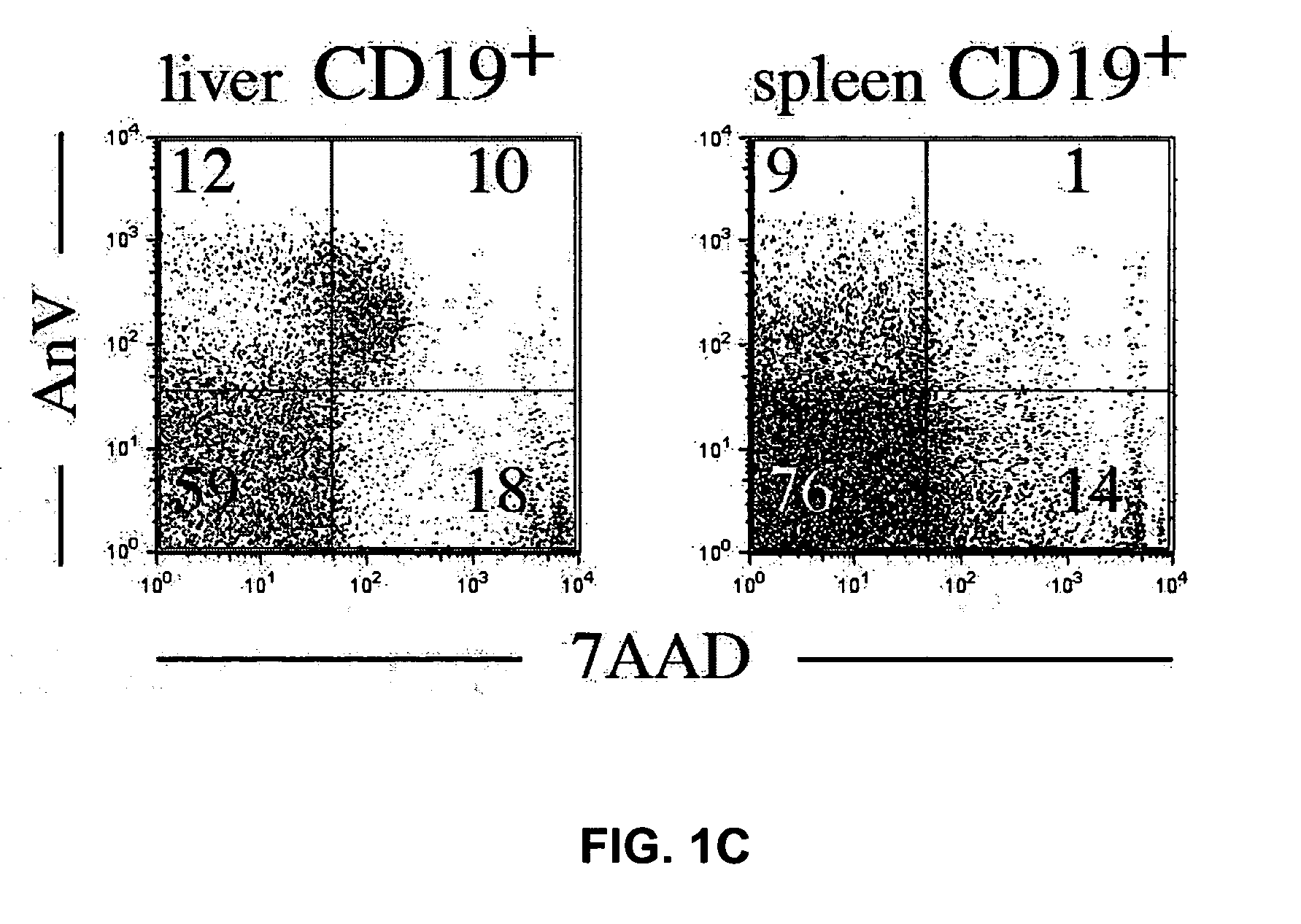
Methods for treating fibrotic conditions
The present invention is directed to methods for treating fibrosis conditions, such as liver, kidney and lung fibrosis, as well as fibrosis conditions of other tissues of the body. The methods of the invention comprise administering to a patient in need of such treatment a therapeutically effective amount of a B-cell antagonist. Exemplary B-cell antagonists that can be used in the practice of the methods of the invention include antibodies against B-cell surface antigens (e.g., antibodies against CD20), and BAFF antagonists.
Owner:BIOGEN INC
Method And Apparatus To Diagnose The Metastatic Or Progressive Potential Of Cancer, Fibrosis And Other Diseases
A method and apparatus for determining the progressive potential of a disease is disclosed. The forward to backward propagating second harmonic generation signal derived from a second harmonic generation instrument is used to assess the collagen microstructure of imaged body tissue by way of numerical values that are in turn used to determine the progressive or metastatic potential of the disease. The disease may, for example, be a cancer such as breast cancer, lung fibrosis, colorectal adenocarcinoma, or the like. The apparatus may include in vivo instruments or laboratory diagnostic instruments with methods disclosed herein.
Owner:UNIVERSITY OF ROCHESTER
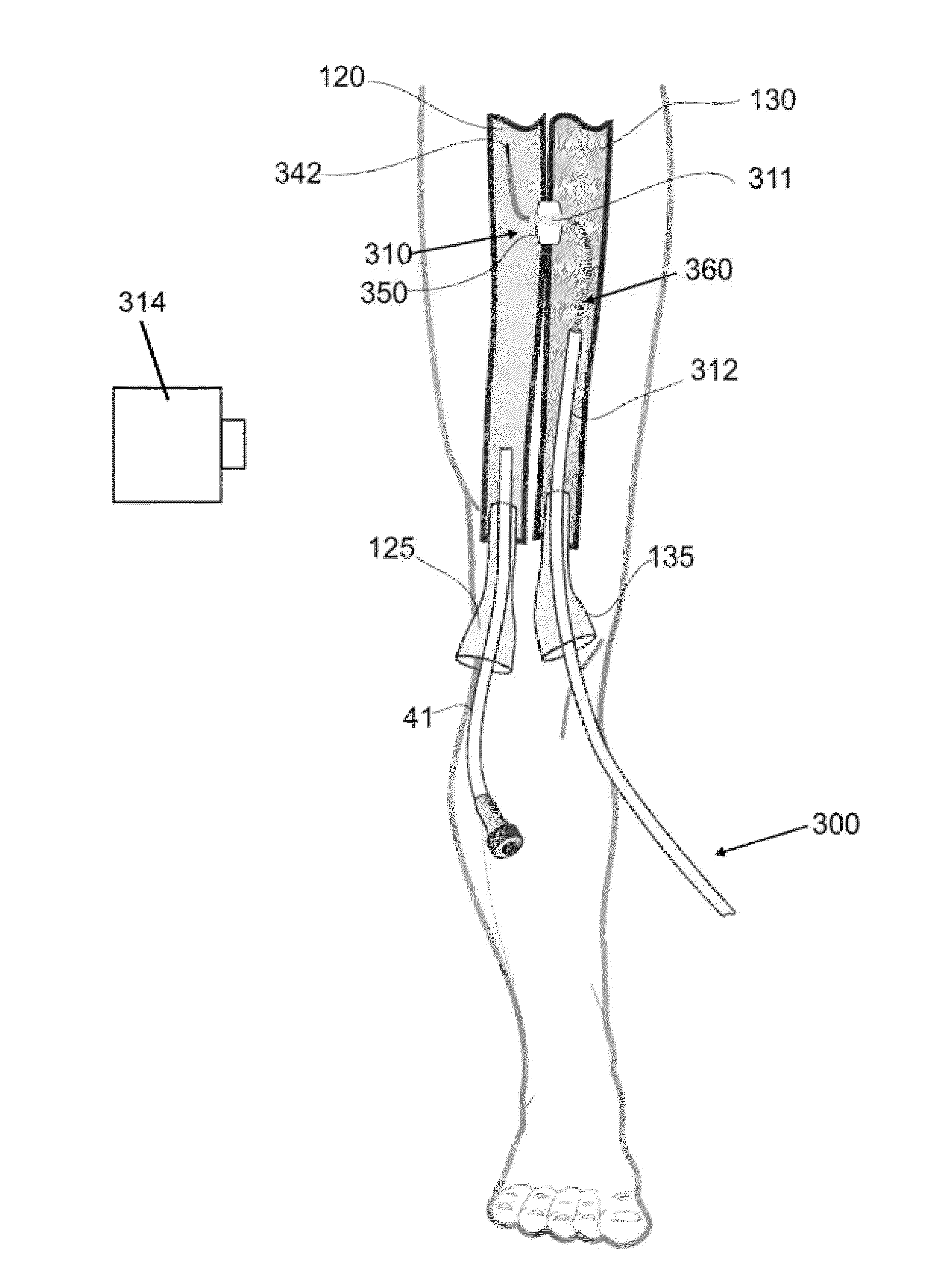
Devices, systems, and methods for peripheral arteriovenous fistula creation
ActiveUS9782533B2Good treatment effectReduce risk and adverse eventOther blood circulation devicesMedical devicesRESPIRATORY DISTRESS SYNDROME ADULTDisease
Owner:EDWARDS LIFESCIENCES CORP
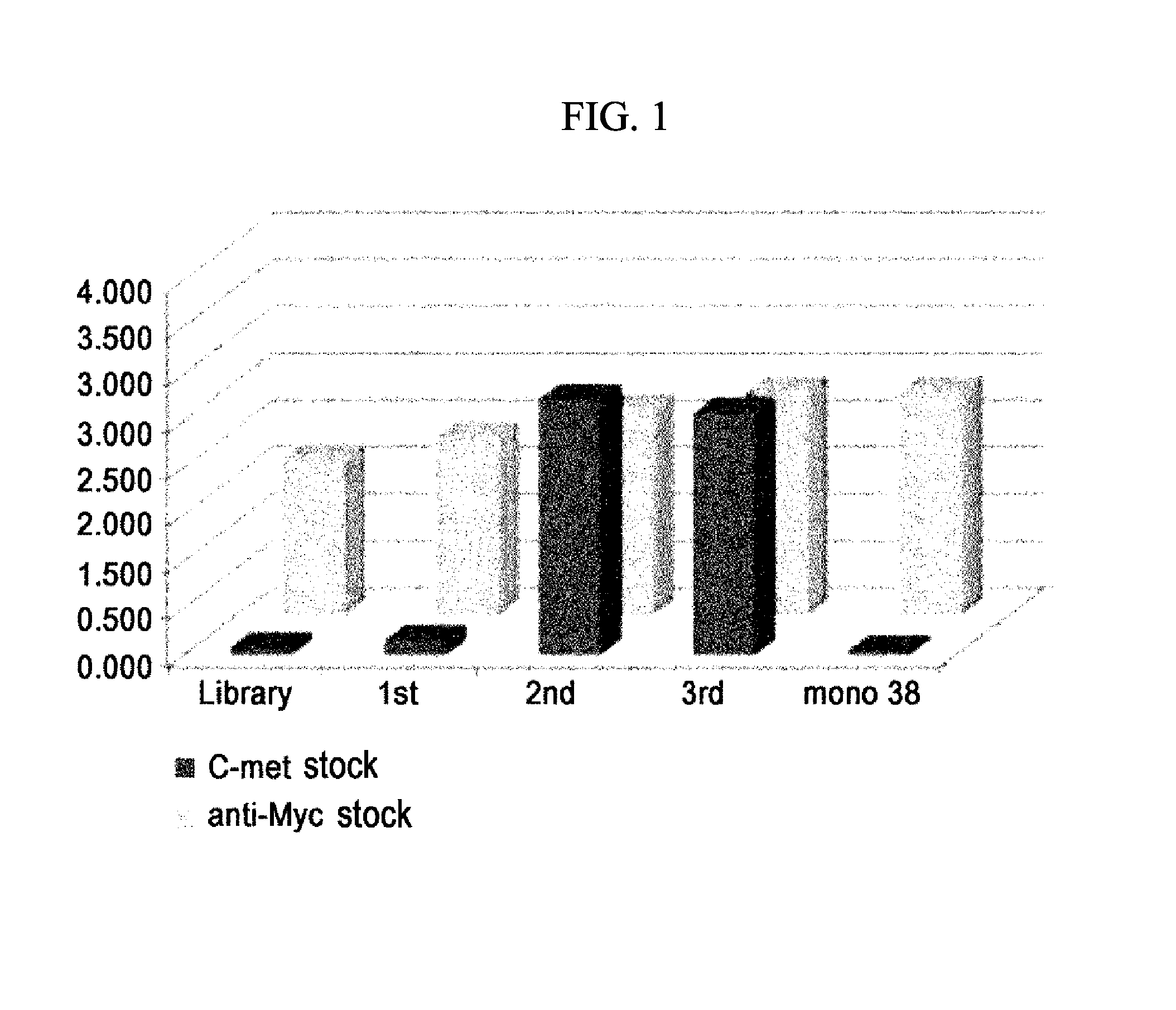
Anti-c-met antibody having hgf activity and use thereof
InactiveUS20140193431A1Animal cellsNervous disorderComplementarity determining regionBiological activation
Disclosed are a human antibody comprising a human complementarity-determining region (CDR), which binds specifically to c-Met, and a framework region (FR), a polynucleotide encoding the human antibody, an expression vector comprising the polynucleotide, a transformant transformed with the expression vector, a method of producing the human antibody B7 by culturing the transformant, a wound healing composition comprising the human antibody as an active ingredient, a cell regeneration composition comprising the antibody as an active ingredient, and a drug conjugate comprising a drug linked to the human antibody. The c-Met-specific human antibody can function as an HGF mimic that can be used as a wound healing composition. The antibody can be widely used to determine the treatment and prognosis of various diseases, including neuronal infarction, progressive nephropathy, liver cirrhosis, lung fibrosis, kidney injury, liver injury, lung injury, and ulcerative wounds, which are treated by activation of HGF or c-Met.
Owner:Y BIOLOGICS INC +1
Amino acid compounds and methods of use
The invention relates to compounds of formula (A) and formula (I):or a salt thereof, wherein R1, R2, R10, R11, R12, R13, R14, R15, R16, q and p are as described herein. Compounds of formula (A), formula (I), and pharmaceutical compositions thereof are αvβ6 integrin inhibitors that are useful for treating fibrosis such as idiopathic pulmonary fibrosis (IPF) and nonspecific interstitial pneumonia (NSIP).
Owner:PLIANT THERAPEUTICS INC
Devices, systems, and methods for peripheral arteriovenous fistula creation
ActiveUS20140188028A1Reduce spreadPromote healingOther blood circulation devicesMedical devicesRESPIRATORY DISTRESS SYNDROME ADULTFibrosis
Devices, systems and methods are disclosed for the formation of an arteriovenous fistula in the limb of the patient. Embodiments include an apparatus for the creation, modification and maintenance of a fistula, including the modification of an existing dialysis fistula; and a method of supplying oxygenated blood to the venous circulation of a patient. A kit of anastomotic implants is described which supports a broad base of patient anatomies and fistula locations. The devices, systems and methods can be used to treat patients with one or more numerous ailments including chronic obstructive pulmonary disease, congestive heart failure, hypertension, hypotension, respiratory failure, pulmonary arterial hypertension, lung fibrosis and adult respiratory distress syndrome.
Owner:EDWARDS LIFESCIENCES CORP
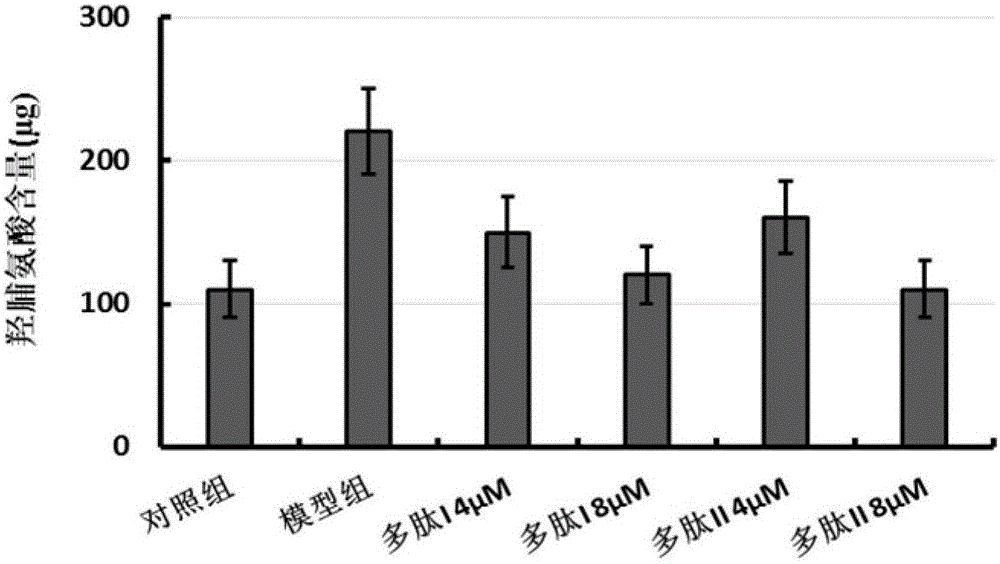
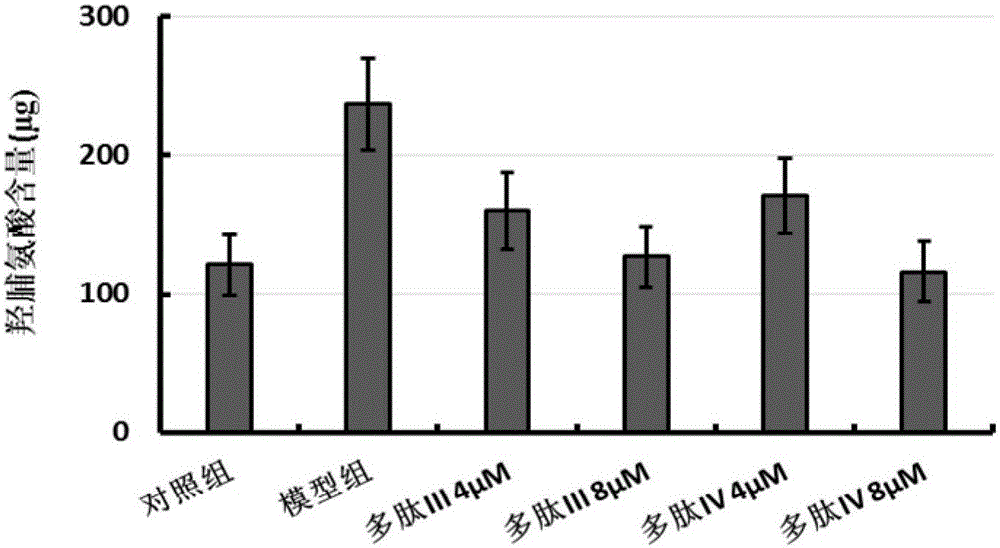
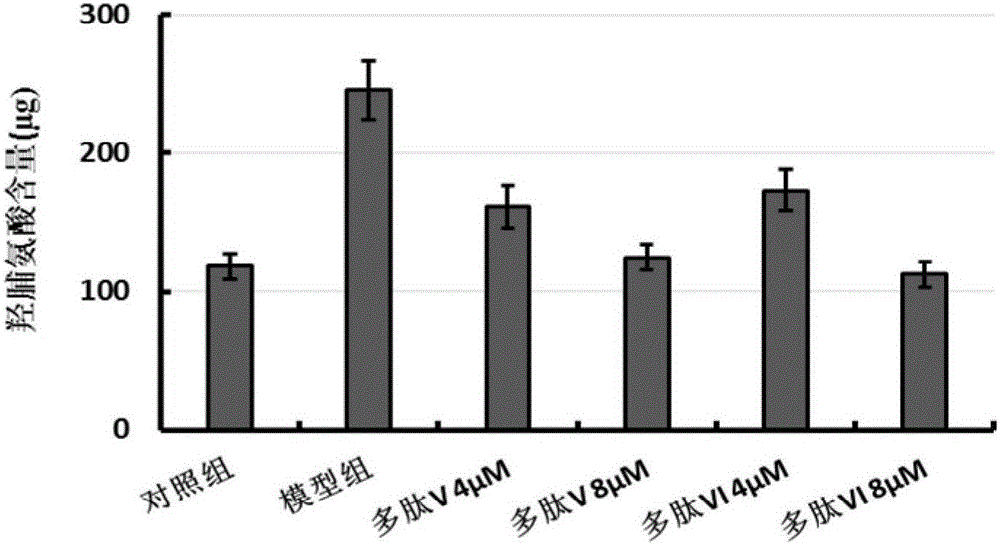
Multifunctional fusion polypeptide and preparation method and application thereof
ActiveCN105713095AReduced hydroxyproline contentEasy to synthesizePolypeptide with localisation/targeting motifPeptide/protein ingredientsHydroxyprolineMedicine
The invention discloses a multifunctional fusion polypeptide and a preparation method and application thereof, and belongs to the field of biological pharmacy. The fusion polypeptide has the structural domains of Pro-(D-Pyr)-(D-Cys)-Bip-Arg-Gly-Glu, Ile-Val-Arg-Arg-Ala-Asp-Arg-Ala-Ala-Val-Pro, Arg-Gly-Asp and Gly-Gly-Gly-Gly; the fusion polypeptide can treat human lung fibrosis, pathological changes of lung tissue, lung cancers and other tumors, and in a lung fibrosis cell model, the fusion polypeptide can significantly reduce the hydroxyproline content in model group cells and inhibit the process of lung fibrosis. It is shown through MTT tests that the fusion polypeptide can inhibit proliferation of multiple kinds of anthropogenic tumor cells; the fusion polypeptide is prepared through an artificial synthesizing method, the preparation method is simple, and a good application prospect is achieved.
Owner:NANJING ANJI BIOLOGICAL TECH CO LTD
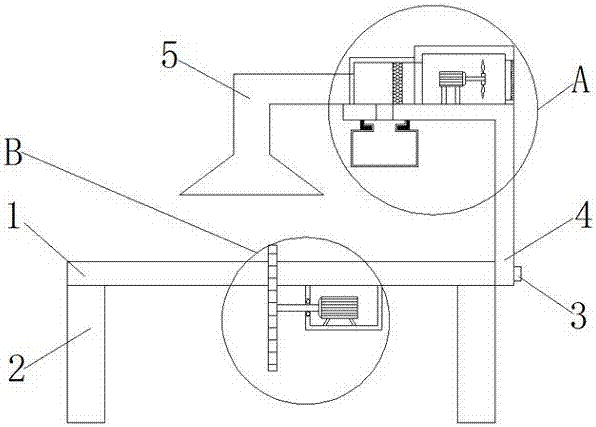
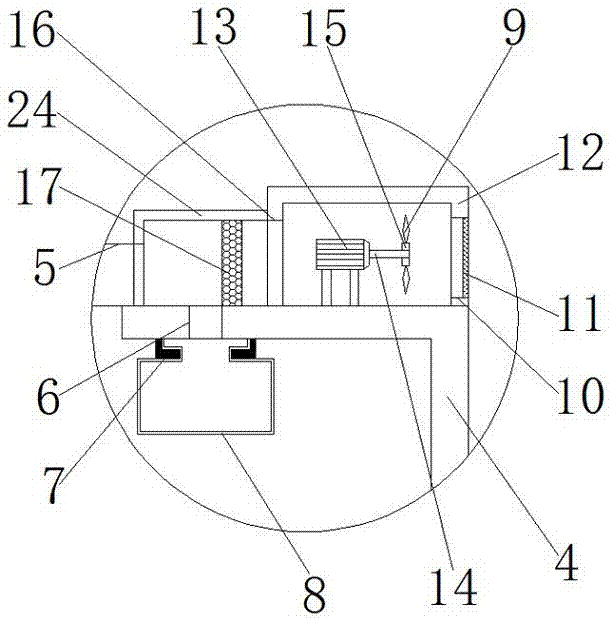
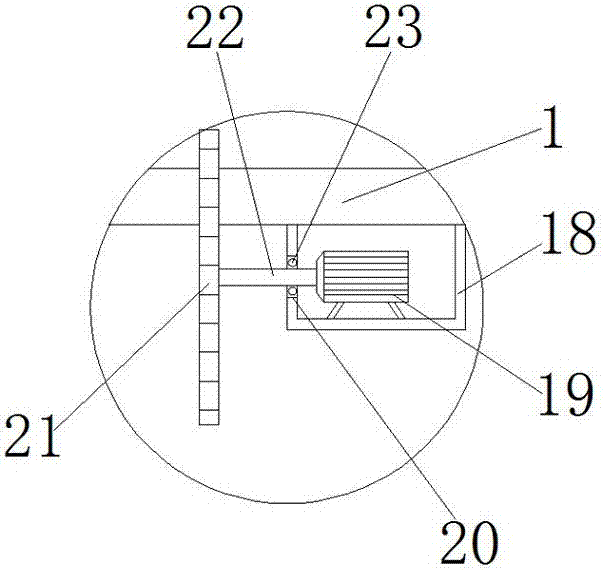
Cutting machine with dust collecting function
InactiveCN107042229AWith dust removal functionDust removal function hasMetal sawing accessoriesDispersed particle filtrationDiseaseArchitectural engineering
The invention provides a cutting machine with a dust collecting function and relates to the technical field of railways. The cutting machine with the dust collecting function comprises a cutting machine worktable. An L-shaped supporting frame is fixedly connected to the right side of the cutting machine worktable through a screw. A first motor bin is fixedly connected to the top of the L-shaped supporting frame. The top of the L-shaped supporting frame is located in a first motor bin and is fixedly connected with a first motor, and the output end of the first motor is fixedly connected with a first rotating shaft. The cutting machine with the dust collecting function is provided with an air outlet, the first motor, fan blades, a filtering net, a dust collecting box and a dust collecting pipe so that the cutting machine can have a dust collecting function, and the problems that most of current cutting machines need to be used for cutting through manual work, multiple metal particles can be mixed in air in the cutting process, the body is likely to be injured when workers inhale metal particles for a long time, and even irreversible diseases such as lung fibrosis and lung cancer are caused are effectively solved.
Owner:于法周
Application of gene modified mesenchymal stem cell in pulmonary fibrosis treatment
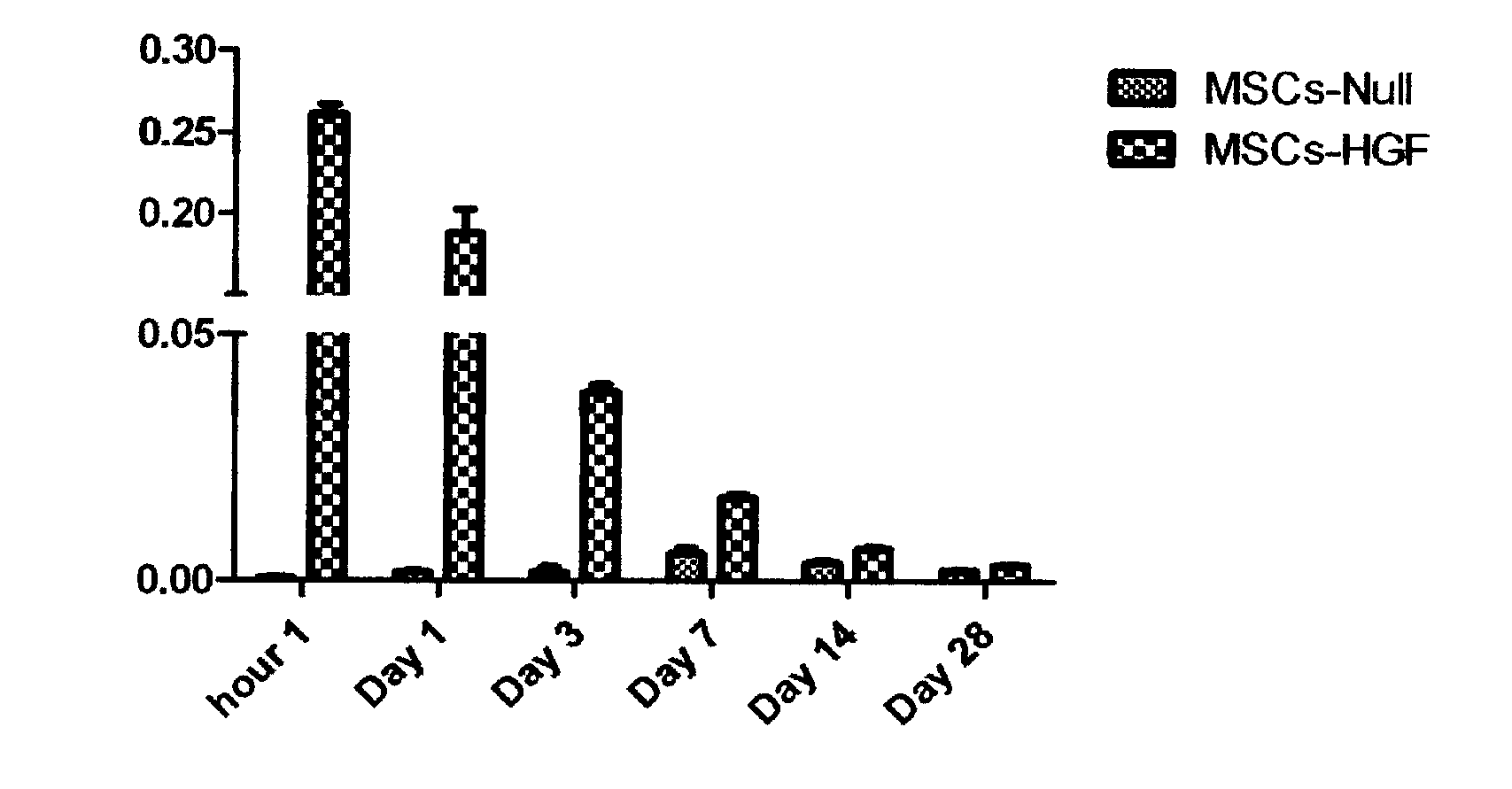
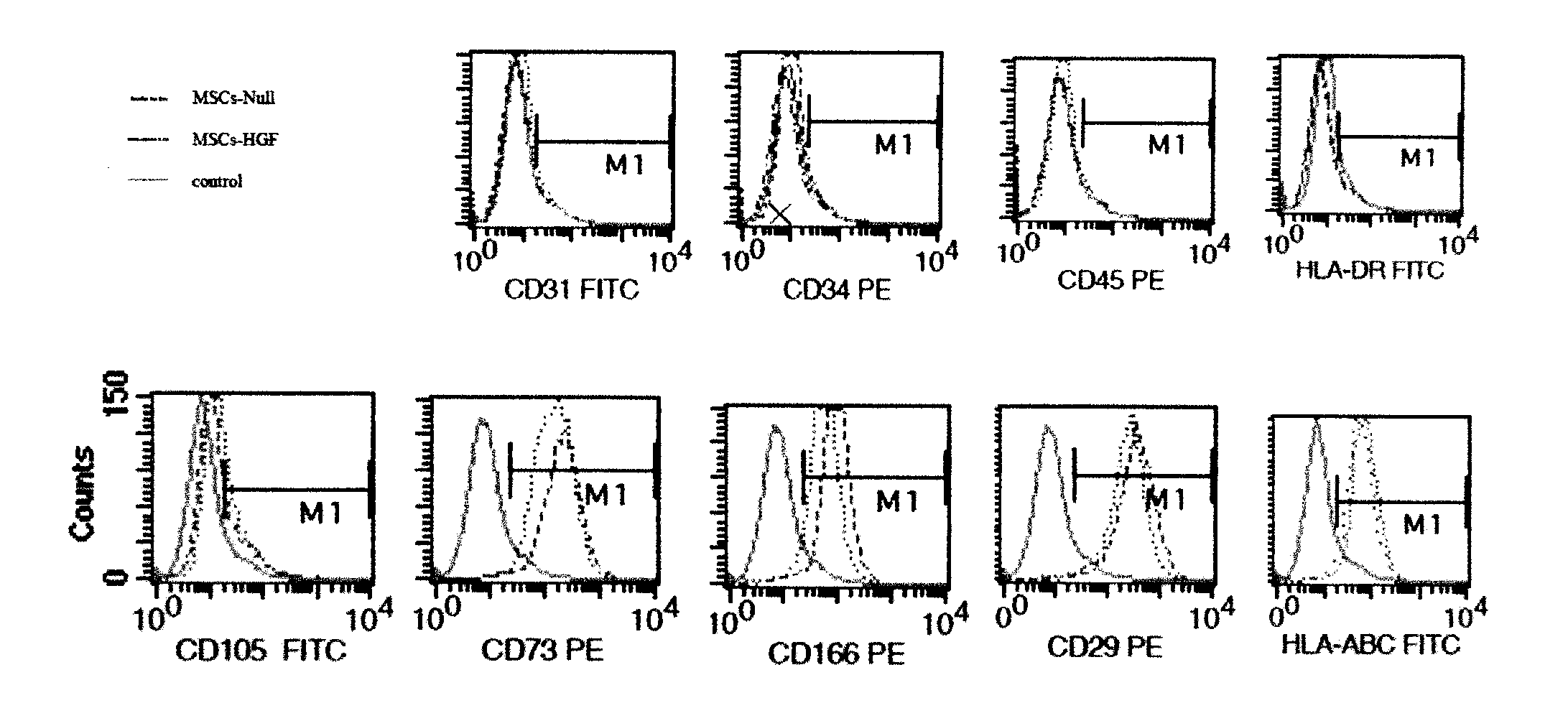
InactiveCN103203025AHigh activityPromote proliferationPeptide/protein ingredientsGenetic material ingredientsBone marrowFactor ii
Relating to the fields of biotechnologies and gene therapy, the invention provides application of a gene modified mesenchymal stem cell in pulmonary fibrosis treatment. The gene modified mesenchymal stem cells are obtained through: in-vitro isolated culture and amplification of a mesenchymal stem cell (MSC) deriving from bone marrow and an umbilical cord, and recombinant adenovirus Ad-HGF mediated in-vitro modification of the MSC by a hepatocyte growth factor (HGF). By transplanting the gene modified MSC to a C57 mouse to intervene in radiation induced lung injury and fibrosis, exudation of a plurality proteins including albumin, IgM and the like from an alveolar space can be reduced, local inflammatory responses of the lung can be alleviated, and expression of TNF-alpha, soluble ICAM-1 and multiple factors is inhibited, expression of the profibrotic factor TGF-beta, the collagen gene col1 alpha 1 and col 3 alpha 1 can be inhibited, and pulmonary tissue collagen fiber deposition is reduced. The expression results of endogenous HGF and its receptor cmet show that endogenous HGF expression can be induced and endogenous MSC can home to injured parts. Therefore, the employment of HGF modified MSC in treatment of lung injury and fibrosis brought by various pathogenic causes is of great significance.
Owner:INST OF RADIATION MEDICINE ACAD OF MILITARY MEDICAL SCI OF THE PLA
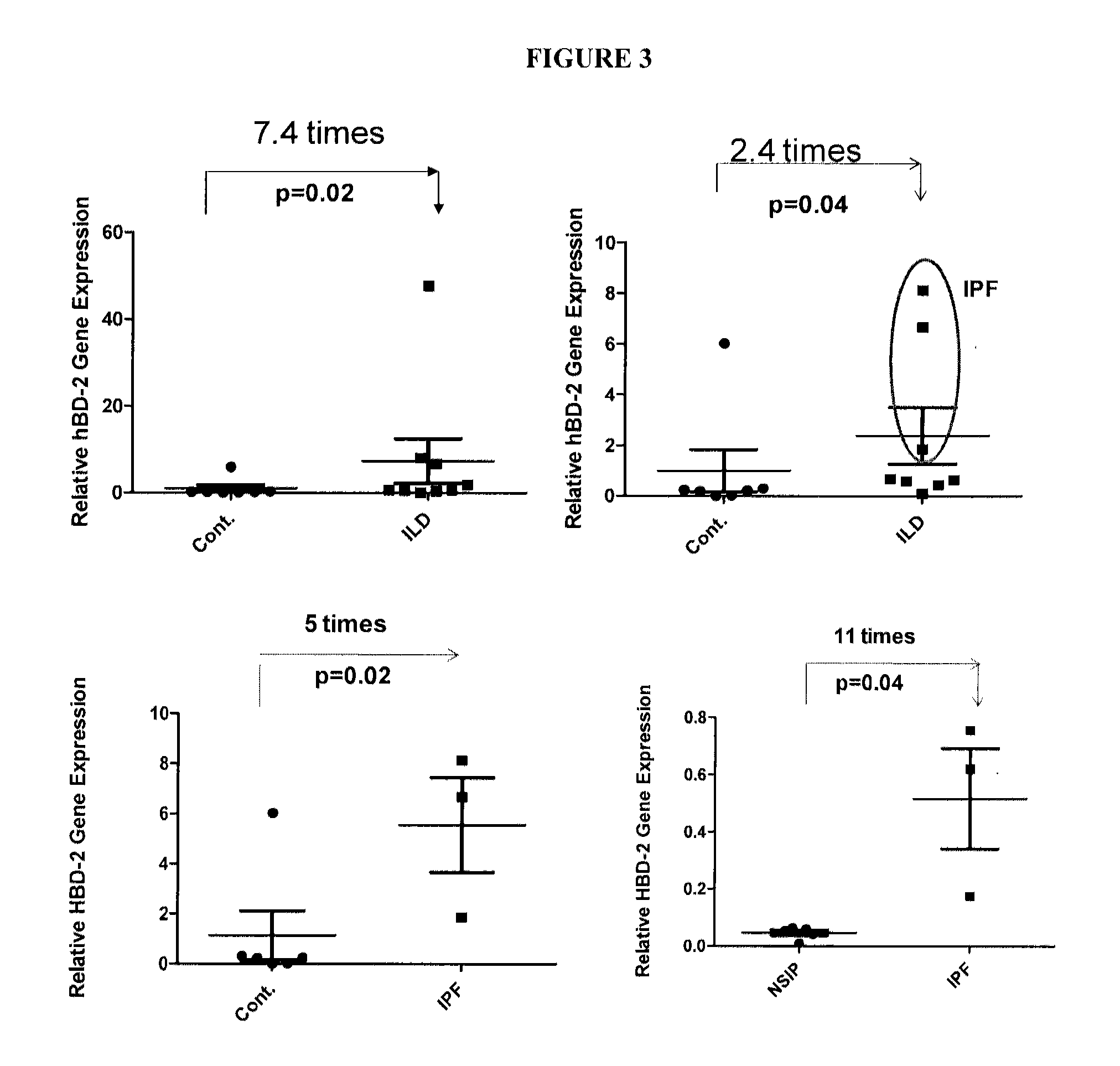
Differential gene expression for detecting and/or differentiating lung disease
Disclosed herein are methods, constructs, kits, and the like, which can be used for detecting and / or differentiating interstitial lung disease. For example, idiopathic pulmonary fibrosis (IPF) and nonspecific interstitial pneumonia (NSIP) can be detected and / or differentiated using at least one biomarker.
Owner:GEORGE MASON UNIVERSITY
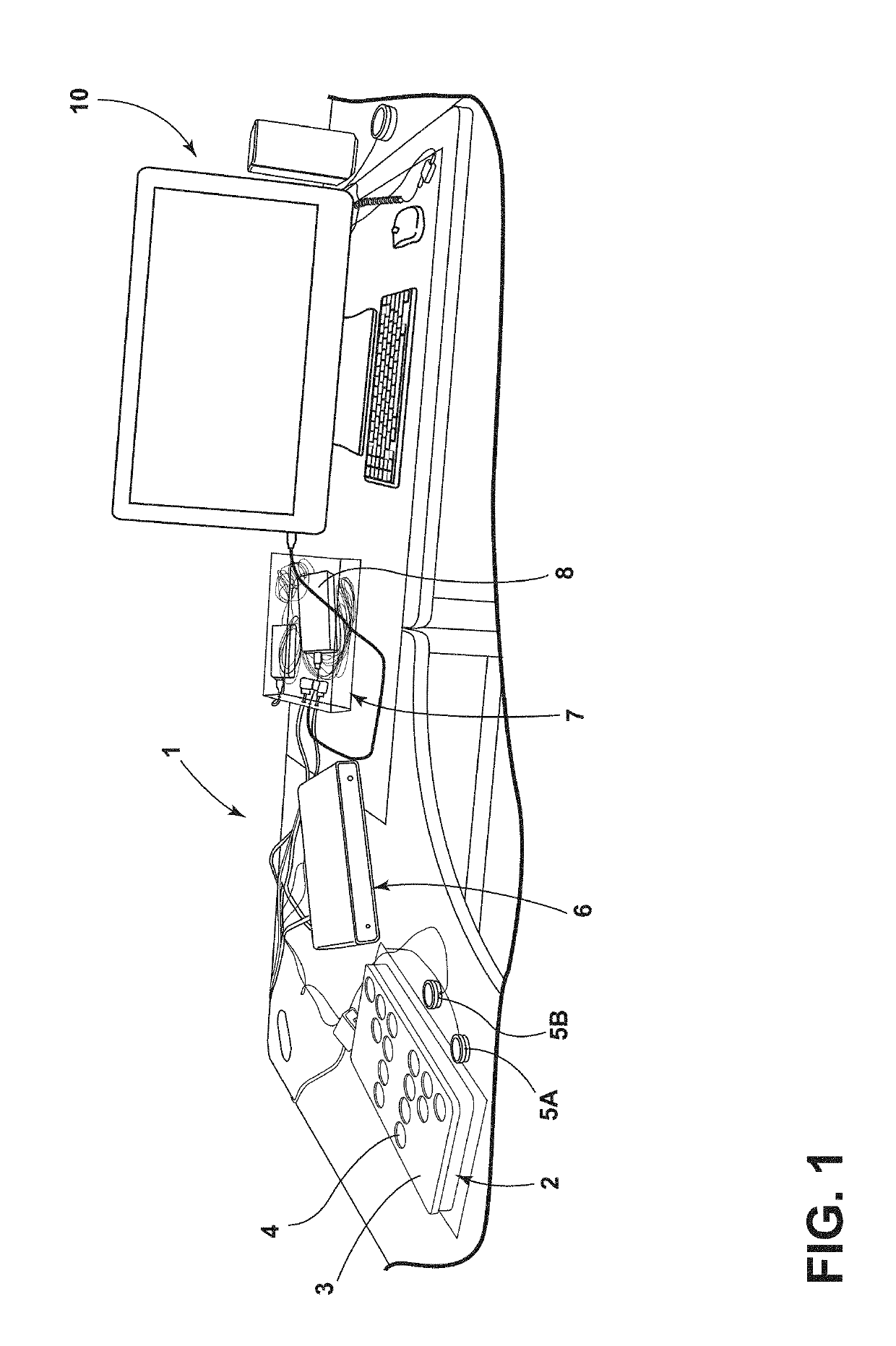
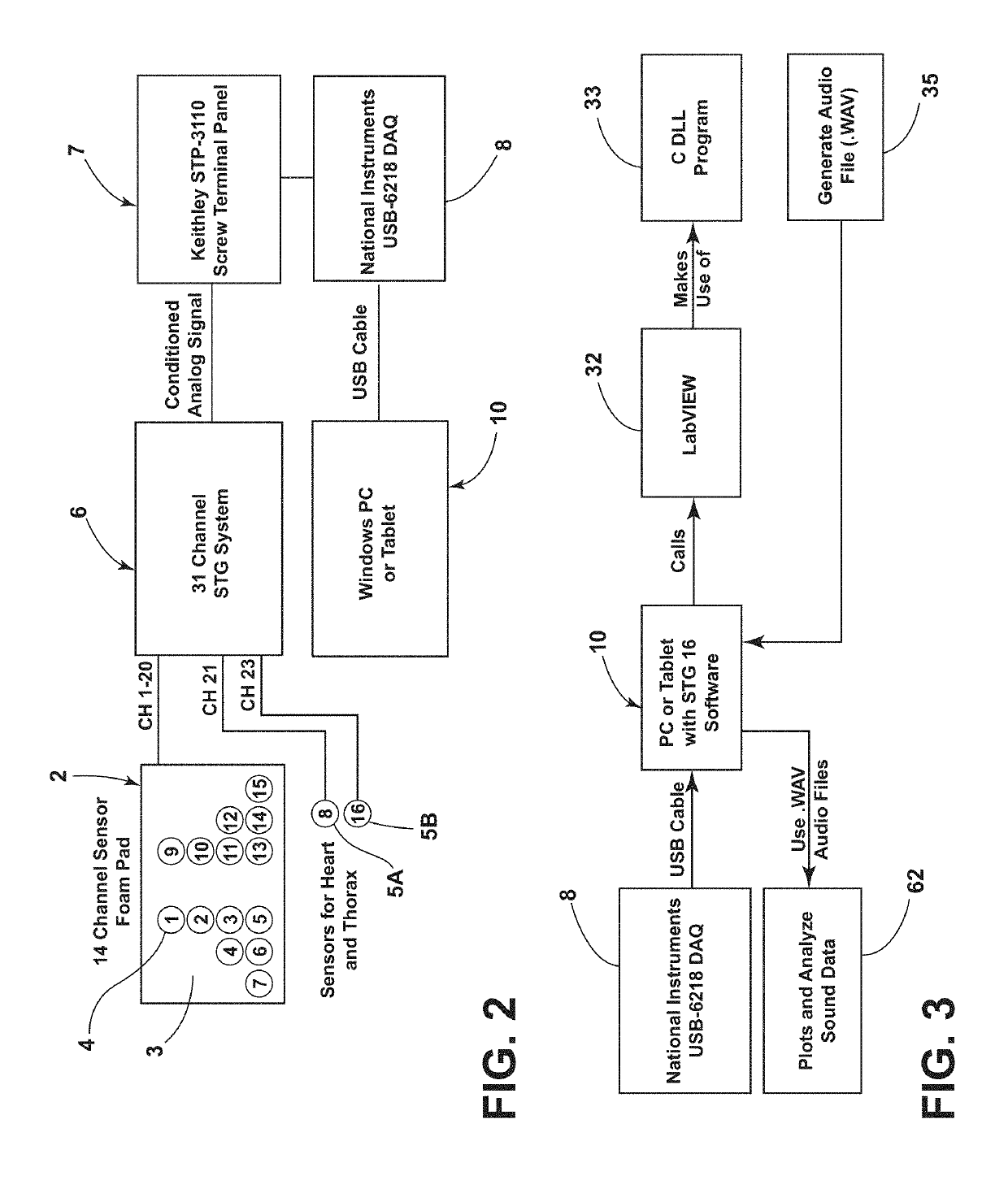
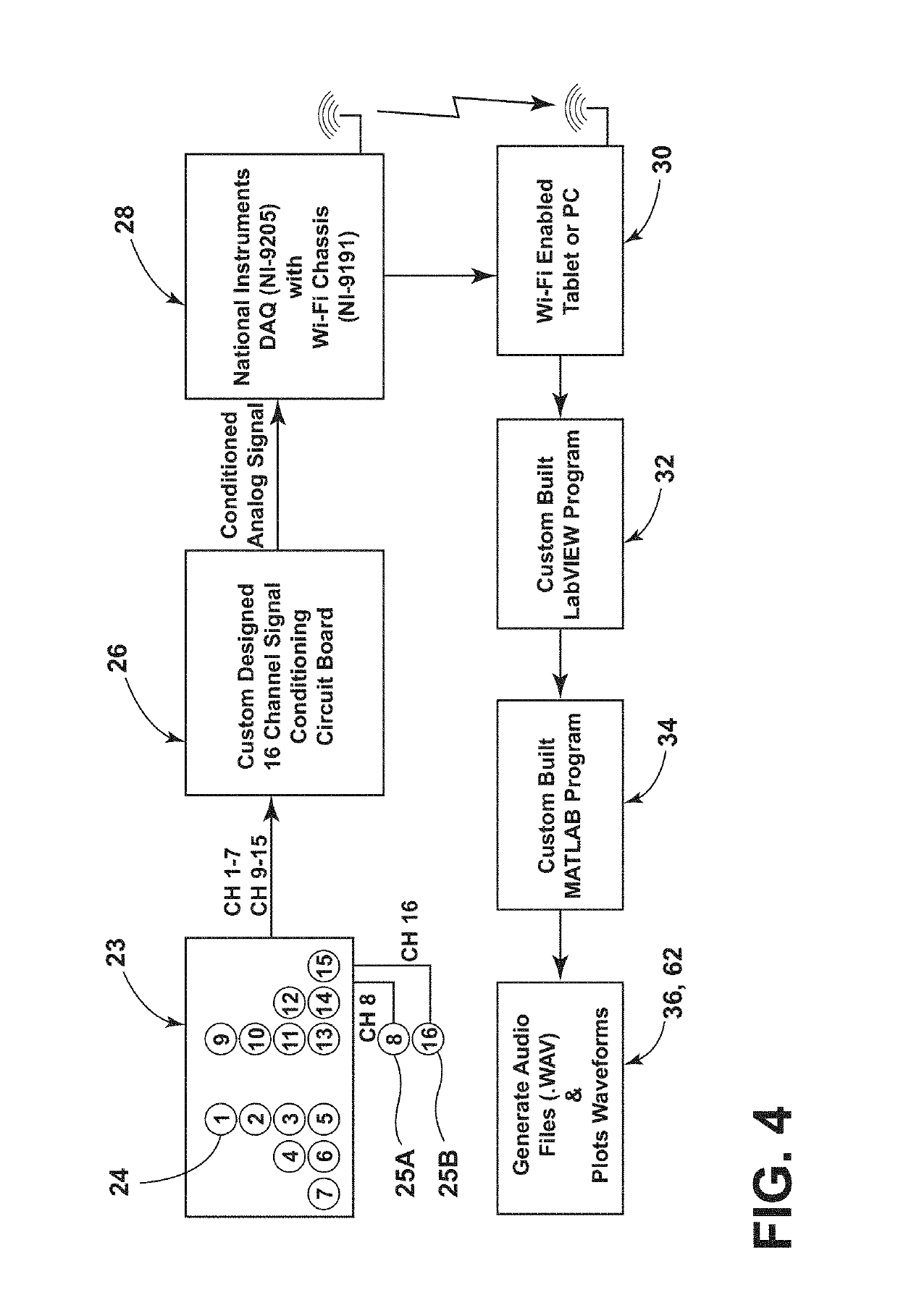
Stethographic device
ActiveUS20190298269A1Improve accuracyImprove abilitiesStethoscopeWireless architecture usageCongestive heart failure chfStethoscope
A multichannel stethographic device includes a plurality of individual stethoscopes that may be embedded in a foam pad or surface mounted on a thin flexible substrate. Additional stethoscopes for the heart and thorax may also be utilized. The system may include a signal conditioning circuit, wireless DAQ module, and software (algorithms). The systems may be configured to identify and diagnose various disease conditions such as pneumonia, chronic obstructive pulmonary disease (COPD), asthma, congestive heart failure (CHF), interstitial pulmonary fibrosis (IPF), and vocal cord dysfunction (VCD).
Owner:WESTERN MICHIGAN UNIVERSITY
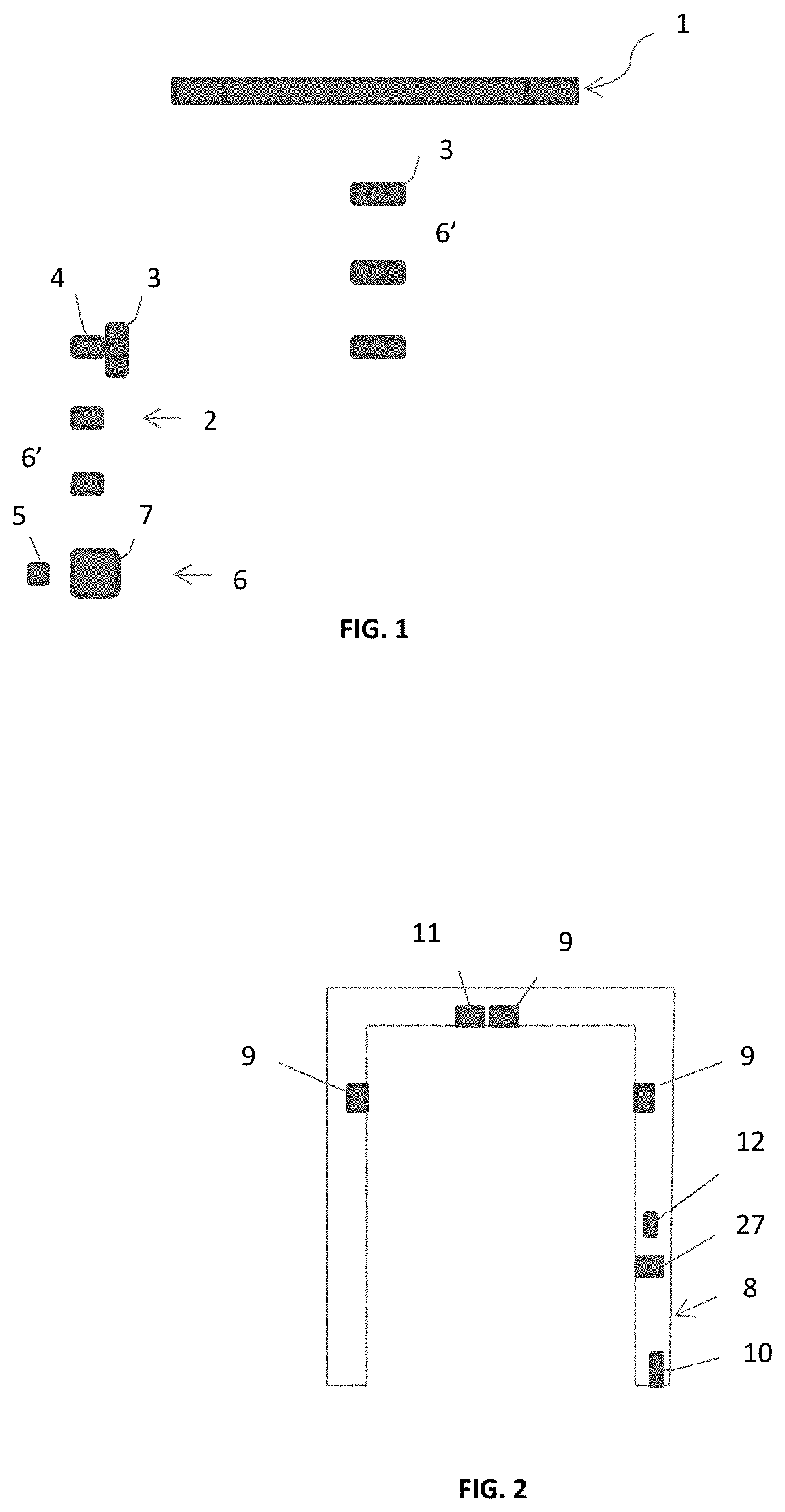
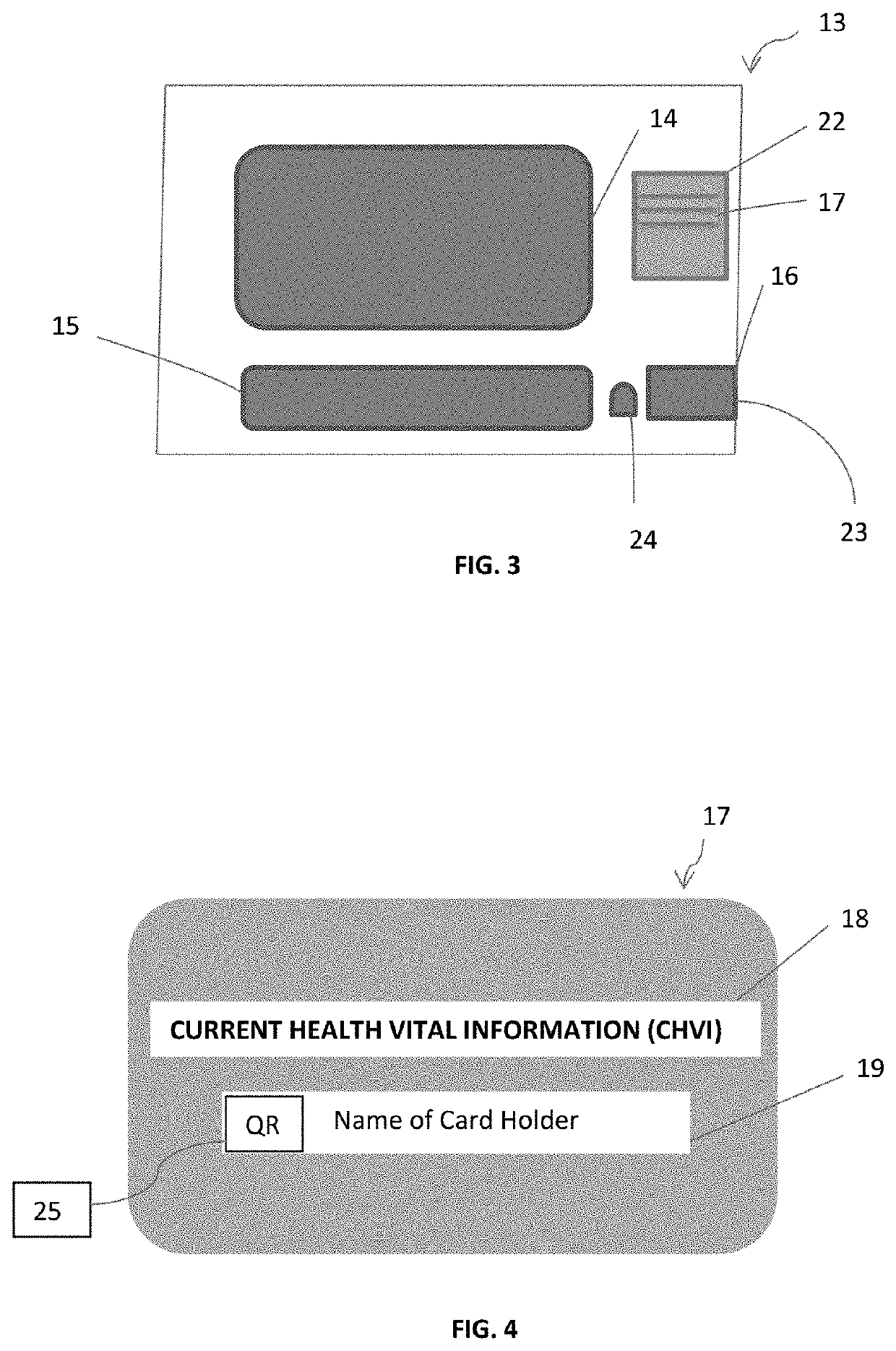
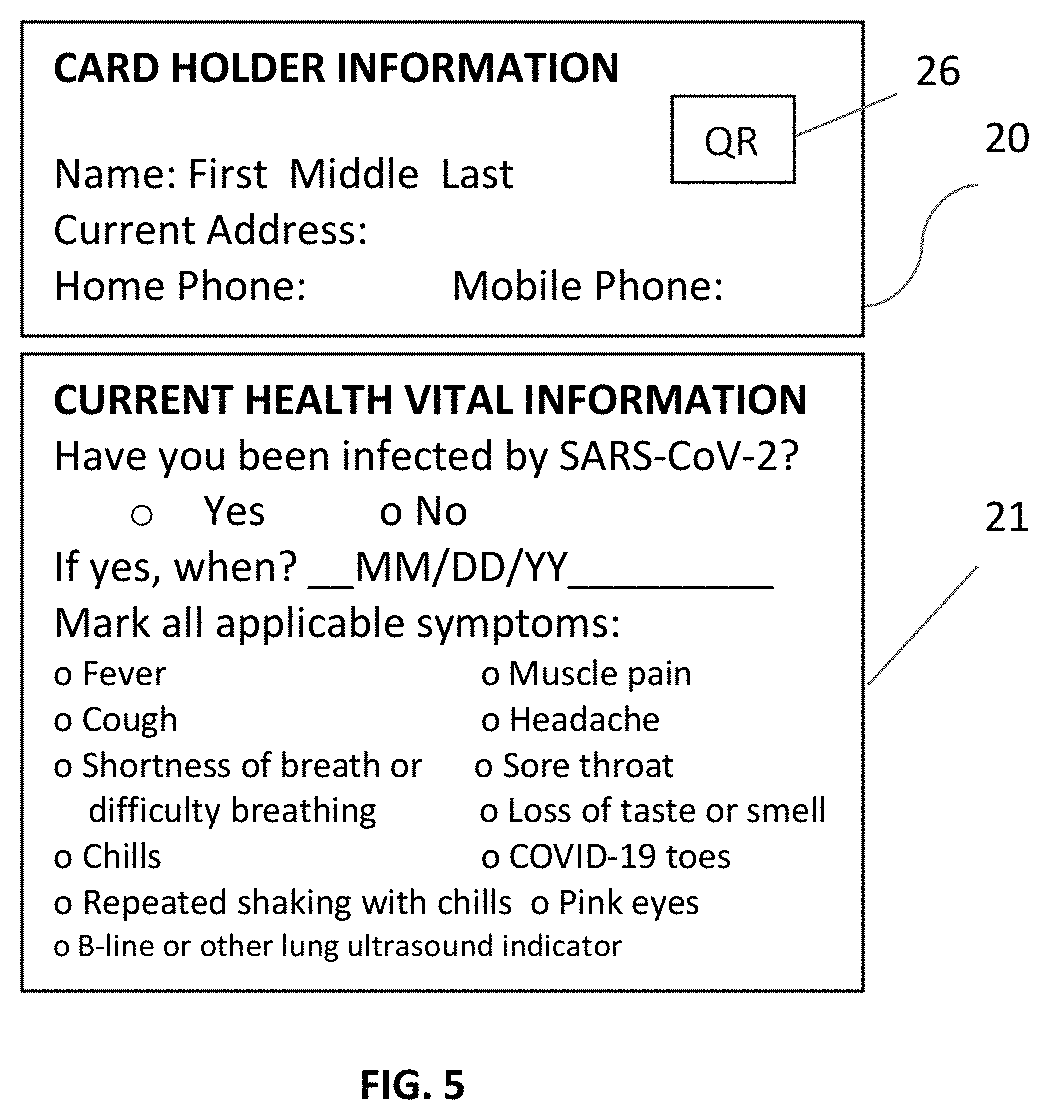
COVID-19 symptoms alert machine (CSAM) scanners
ActiveUS10888283B1Easy to useEconomical in cost to manufactureComputerised tomographsRespiratory organ evaluationDiseaseThroat soreness
A COVID-19 Symptoms Alert Machine (CSAM) scanner, or apparatus, is described herein. This apparatus employs Artificial Intelligent (AI) technology in combination with the latest mobile device technology (viz. smart phone / smart watch) to quickly help track down people who have COVID-19 symptoms anywhere and anytime, isolate them, and professionally handle them, not allowing SARS-CoV-2 virus to spread. CSAM automatically measures body temperature and assesses lung conditions such as pulmonary fibrosis and B-lines (for asymptomatic people), and other current health vital information (CHVI), furnished by the participant, such as fever, sore throat, headache, and body ache to generate an alert signal when COVID-19 symptoms are found significant and to send it out to a COVID-19 control center. The alerted participant is then immediately required to go to the COVID-19 control center or be picked up by a special COVID-19 emergency vehicle for isolation and further evaluation and testing. If the testing turns out to be COVID-19 positive, the participant will be quarantined and treated appropriately according to COVID-19 protocol until he / she is tested COVID-19 negative. In the meantime, people who have been in close physical contact with this participant will be alerted and requested to be immediately checked for COVID-19 symptoms. If anyone is found to have COVID-19 symptoms, then he / she must go through the same protocol. The process is repeated until all people in the cluster are tested COVID-19 negative. This will ensure that SARS-CoV-2 virus for this cluster has been completely eliminated. A rapid deployment of this type of apparatus throughout communities where people tend to congregate such as superstores, supermarkets, and any other establishments, small or large, can help to contain the rapid spread of the disease, as well as to give more confidence to the general public. People, who pass through this apparatus without an alert signal, should feel more confident in carrying out their activities, though social distancing and other COVID-19 precautionary requirements should still be maintained. The concept can be further expanded to cover shopping malls, concert halls, sports arenas, and any other large events including highways and freeways with the help of mobile phone technologies, transponders, and other mobile devices. By working on the 0.6% (around 2 million infected people in the US as of June 2020) quickly and effectively, instead of on the 99.4% (330 million, the remaining population) by locking people at home and closing down all businesses and activities; we can save a significant amount of money and hassles. (A long lockdown can also lead to a collapse of our economy and can consequently lead to a worldwide calamity.) In this way the 99.4% will not be burdened with the virus problem and can live normally without having to take any test. It is probably the only effective approach in solving the COVID-19 problem at the moment because vaccines and known COVID-19 cures are not yet available. Even if SARS-CoV-2 vaccines are available presently, they may not be practical to implement economically and operationally in time to contain the virus worldwide due to the massive amount of people (viz. over 7 billion).
Owner:BENJAUTHRIT BOONSIENG +3
Human source Fab antibody for anti recombined alkalescent fibroblast growth factor and application
InactiveCN101092457AHigh affinityImprove featuresImmunoglobulins against growth factorsAntibody ingredientsSurface displayFibrosis
This invention discloses anti-recombinant alkaline fibroblast growth factor human-derived Fab antibody and its application. The antibody segment genome comprises: heavy chain Fd chain and light chain kappa chain. This invention utilizes phage surface display technique to successfully construct kappa chain and Fd chain genes of human-derived Fab antibody that can neutralize recombinant human bFGF or FGF-2. Functional neutralizing antibody that can specifically bind bFGF or FGF-2 is obtained in prokaryotic cells, yeast cells, insect cells and eukaryotic cells, which can be used to prepare antibody drugs that can diagnose and treat tumors, and inhibit kidney, liver and lung fibrosis. The bFGF or FGF-2 human-derived Fab antibody has high affinity and specificity, and can be directly used to develop antibody drugs.
Owner:JINAN UNIVERSITY
Stem cell derived exosome preparation for preventing and treating lung jury
InactiveCN109078020AGood treatment effectPharmaceutical delivery mechanismUnknown materialsTissue repairTherapeutic effect
The invention provides a stem cell derived exosome preparation for preventing and treating lung jury. Lung injury includes acute lung injury, radiation lung injury, pulmonary fibrosis, silicosis and the like. The preparation is prepared from stem cell derived exosomes, and after a trachea cannula or intravenous injection reaches injured lungs, recovery of injured tissue structure and function is promoted; the preparation is an acellular preparation, so that the risk of cell mutation is lowered; the stem cell derived exosomes repair and maintain the function in lung tissue; treatment effect oflung injury and fibrosis is enhanced.
Owner:NANKAI UNIV
Compound used for discoidin domain receptor micro-molecule inhibitor, and its application
ActiveCN103965195AInhibitory activityAvoid side effectsOrganic active ingredientsOrganic chemistryMedicineHepatic fibrosis
The invention discloses a compound having a structure represented by formula (I) and used for a discoidin domain receptor micro-molecule inhibitor, or its pharmaceutically acceptable salt or stereo isomer or prodrug molecule. The above compounds can effectively inhibit the activity of the discoidin domain receptor, and can be used for preparing drugs for preventing and treating inflammations, liver fibration, kidney fibration, lung fibration, skin scars or atherosclerosis.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
Chinese medicine for treating lung cyst, pulmonary fibrosis and suppressing cough and preparation method thereof
The invention provides a traditional Chinese medicine for treating pulmonary cyst, pulmonary fibrosis and relieving cough and the preparation method thereof, which relates to the technical field of traditional Chinese medicines. The active ingredients of the traditional Chinese medicine consist of the following materials comprising platycodon root, fritillaria, Chinese angelica, bitter orange, mulberry root bark, fourstamen stephania root, liquoric root, bulbus lilii, membranous milkvetch root, radix stemonae, lucid ganoderma, peppermint, loquat leaf, semen coicis, root of American ginseng and dandelion respectively, the weights of which are equal to each other and range from 25 to 35g. In the method, the traditional Chinese medicines with active ingredients are taken as the raw materials, which are mixed and crushed to 100 to 300 meshes to obtain traditional Chinese medicine powder; wherein, the powder is added with bee honey to be made into traditional Chinese medicine honey pills with a specification of 6 to 10g / pill or added with water to be made into traditional Chinese medicine water pills with a specification of 3 to 5g / pill. The medicine is suitable for patients with the pulmonary cyst, the pulmonary fibrosis and the cough. Either one honey pill or one water pill can be taken orally before breakfast, lunch and supper everyday, with good curative effect, short treatment course, low dose, and low cost.
Owner:荣玉明
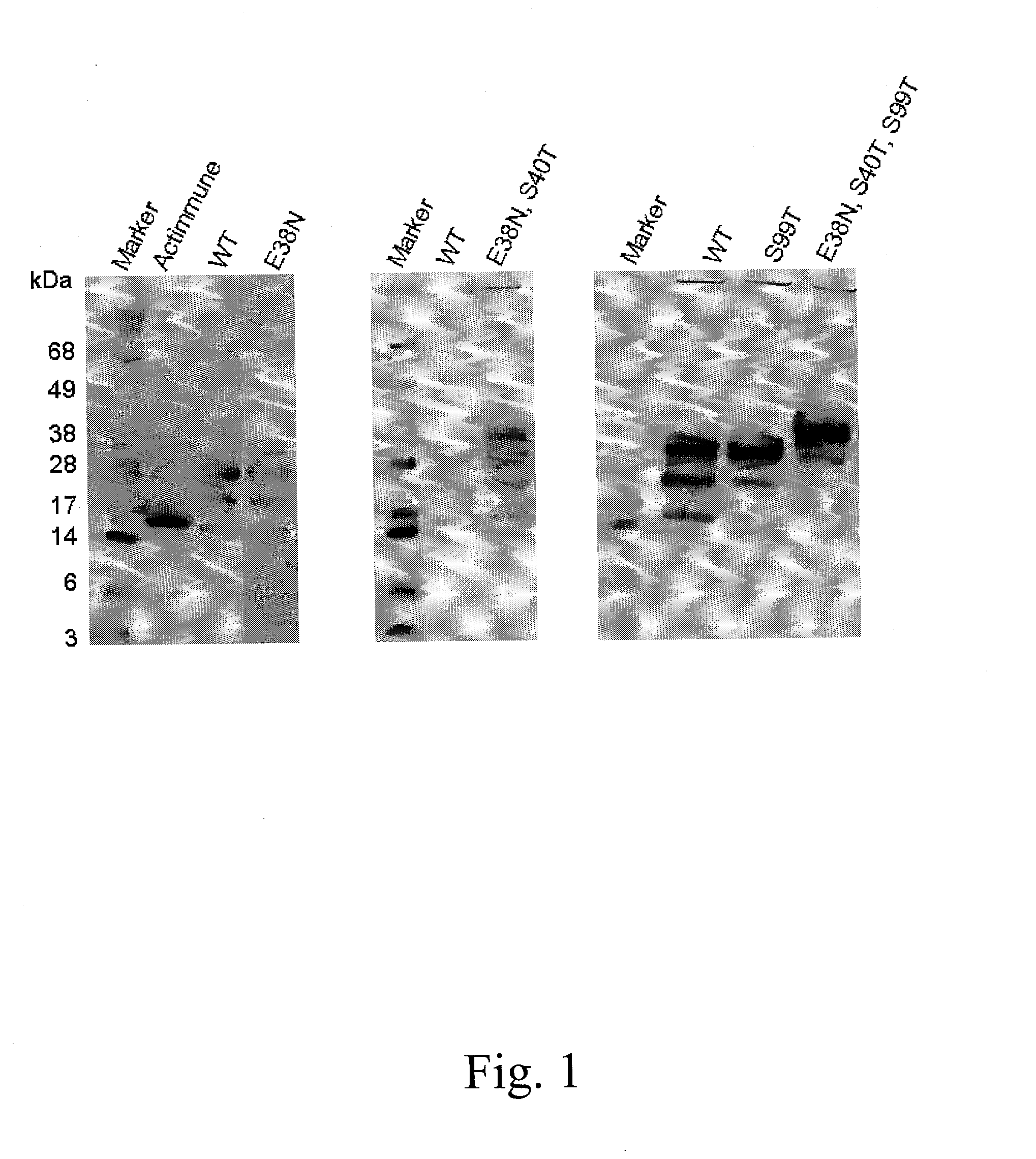
Interferon gamma polypeptide variants
InactiveUS20020192183A1Renal clearance is reducedHigh molecular weightSugar derivativesPeptide/protein ingredientsInterstitial lung diseaseDisease
The present invention relates to novel interferon gamma polypeptide variants having interferon gamma (IFNG) activity, methods for their preparation, pharmaceutical compositions comprising the polypeptide variants and their use in the treatment of diseases, in particular for the treatment of interstitial pulmonary diseases, such as idiopathic pulmonary fibrosis. These novel polypeptide variants all comprise the substitution S99T as compared to the amino acid sequence of huIFNG or fragments thereof. By performing this mutation the naturally occurring N-glycosylation site present at position 97 is significantly better utilized. Preferably, the variants comprise further modifications, e.g. in order to increase the AUC of such variants when administered subcutaneously.
Owner:PERSEID THERAPEUTICS
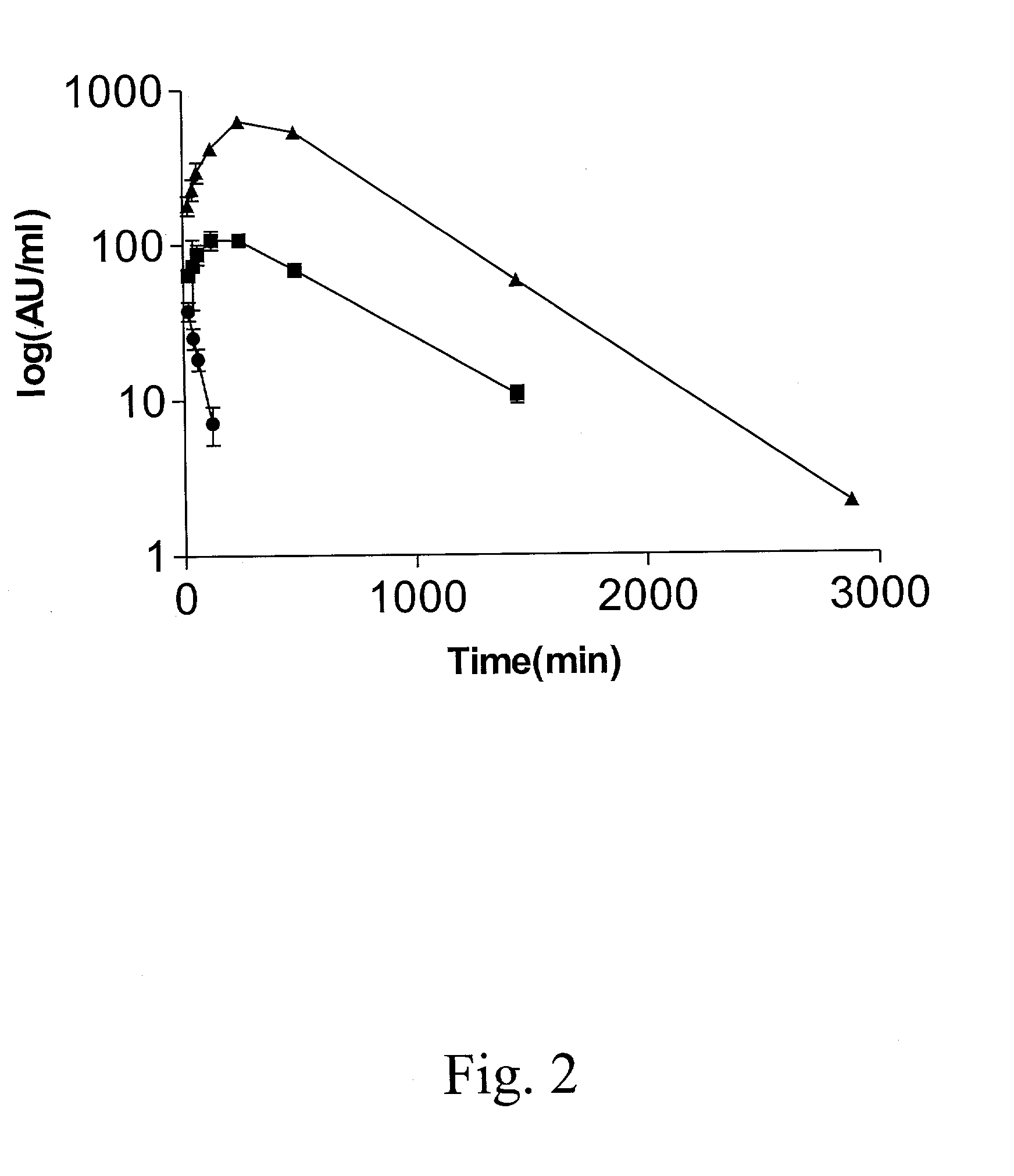
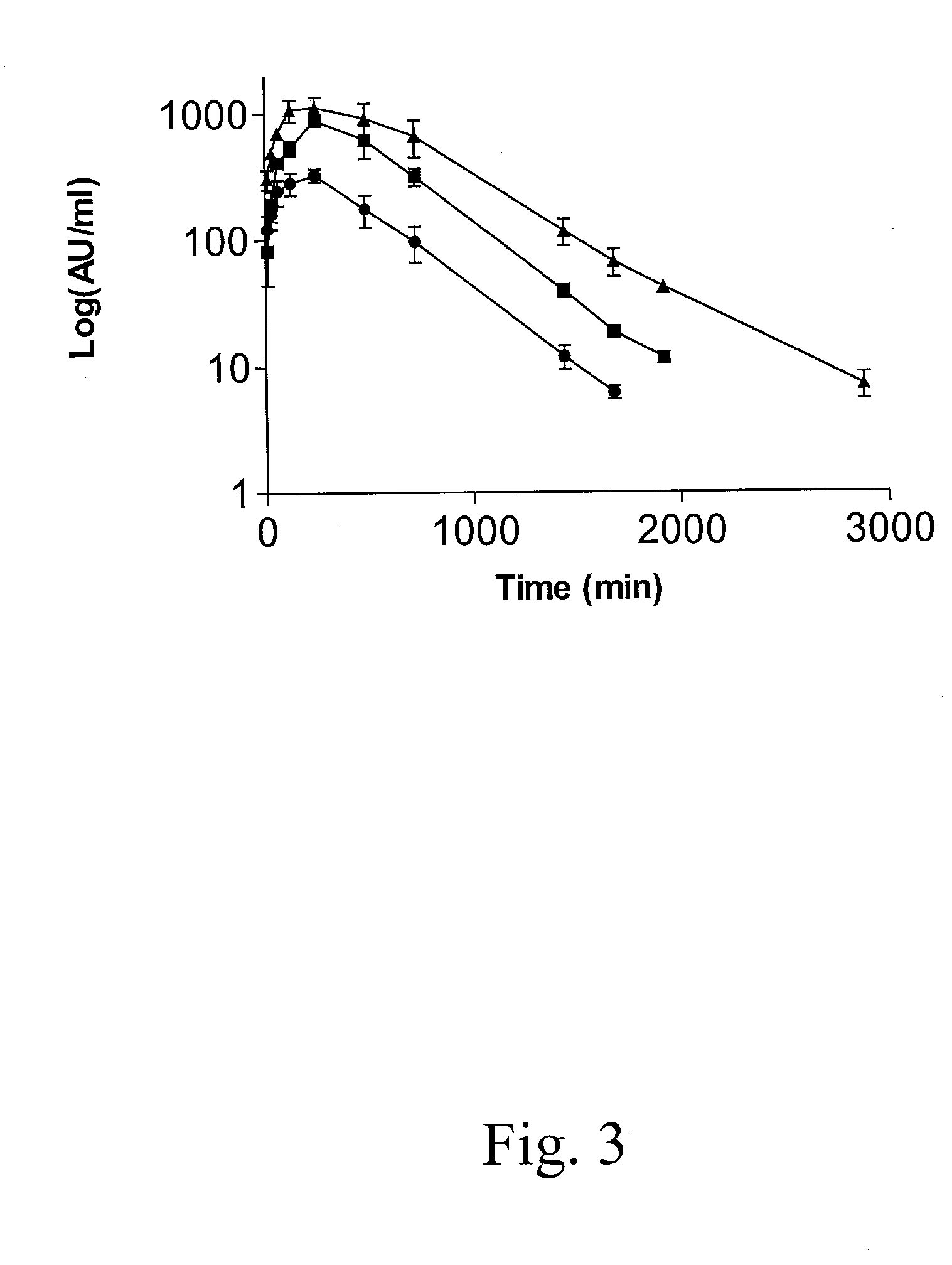
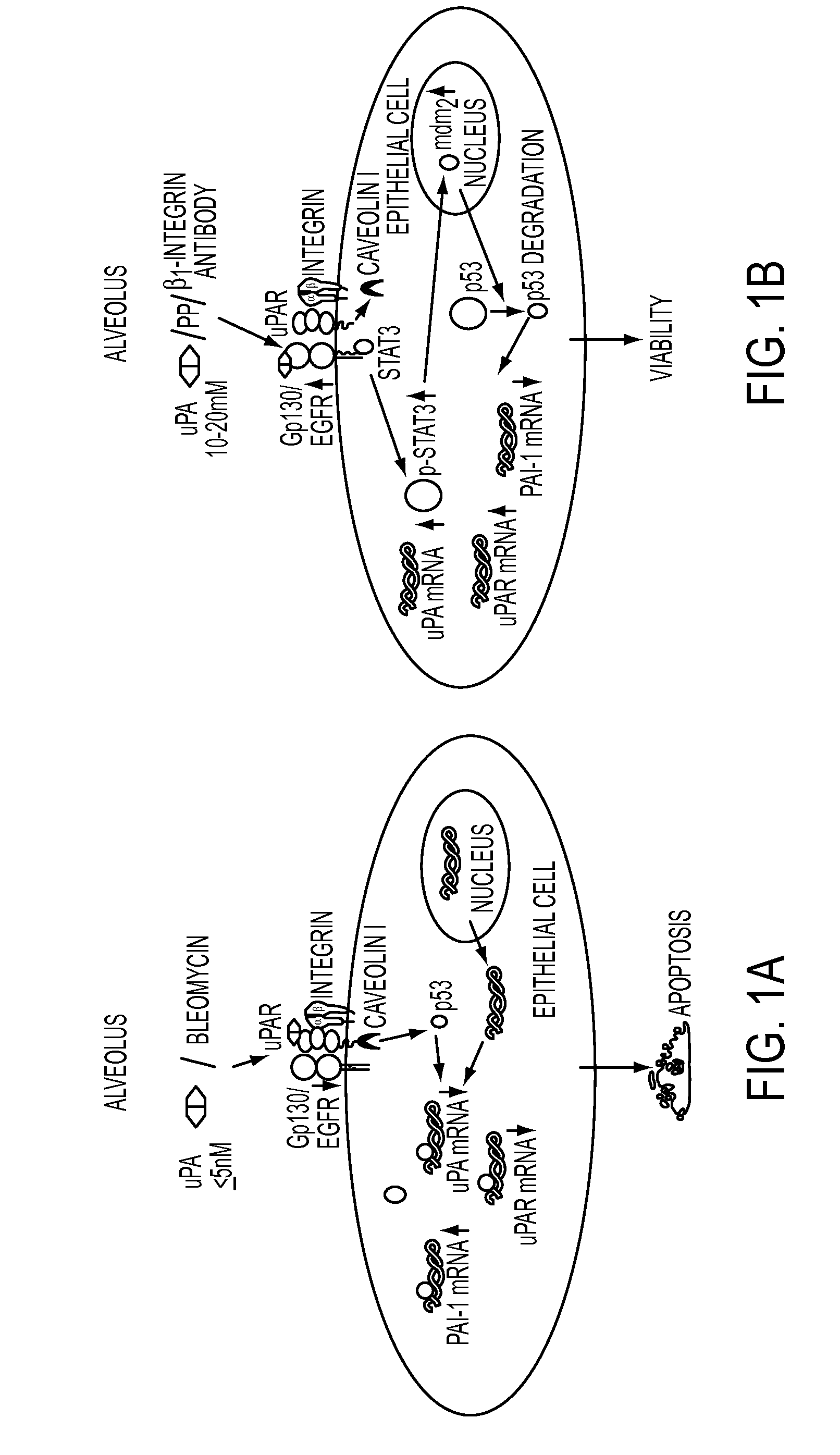
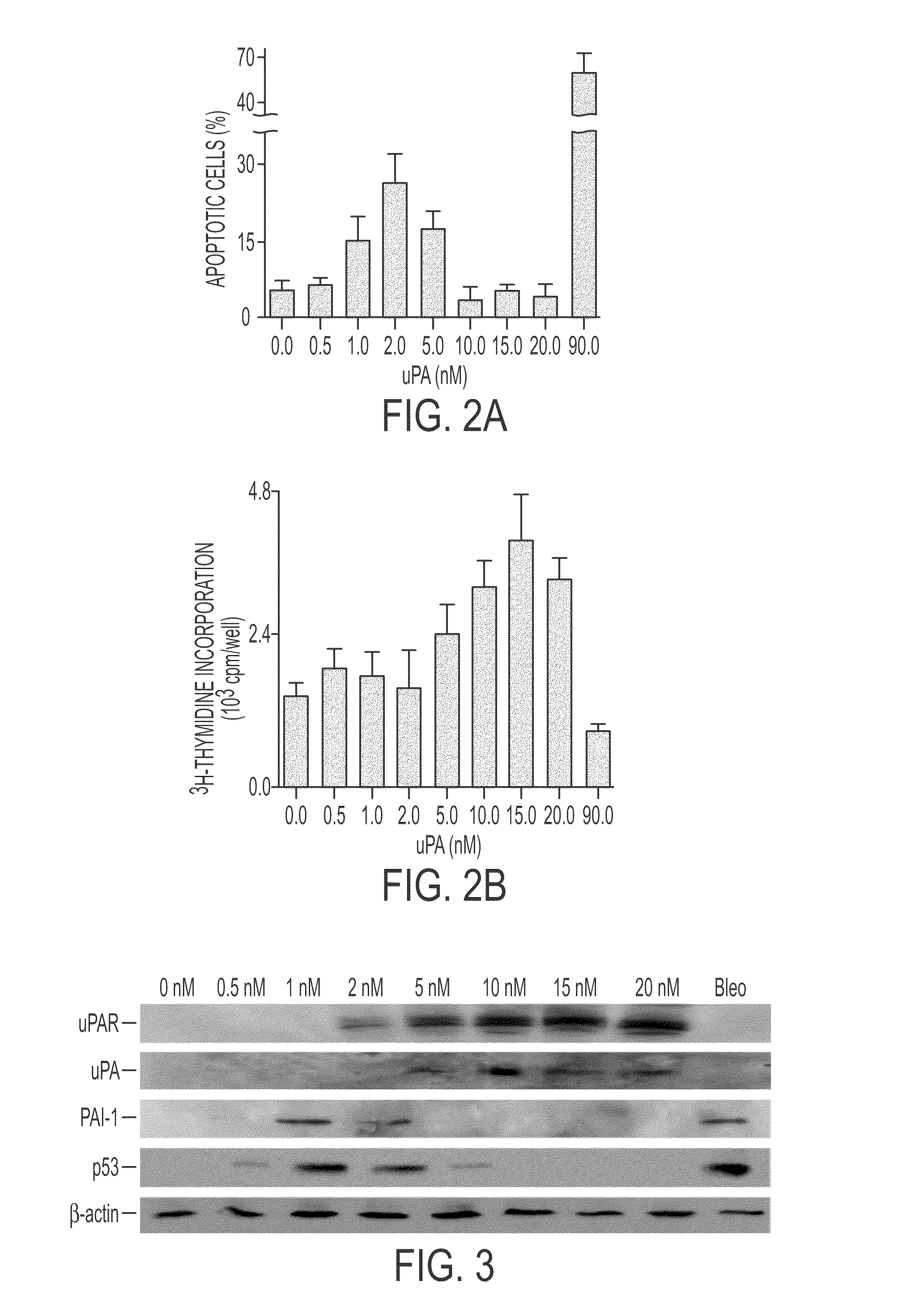
Peptide inhibition of lung epithelial apoptosis and pulmonary fibrosis
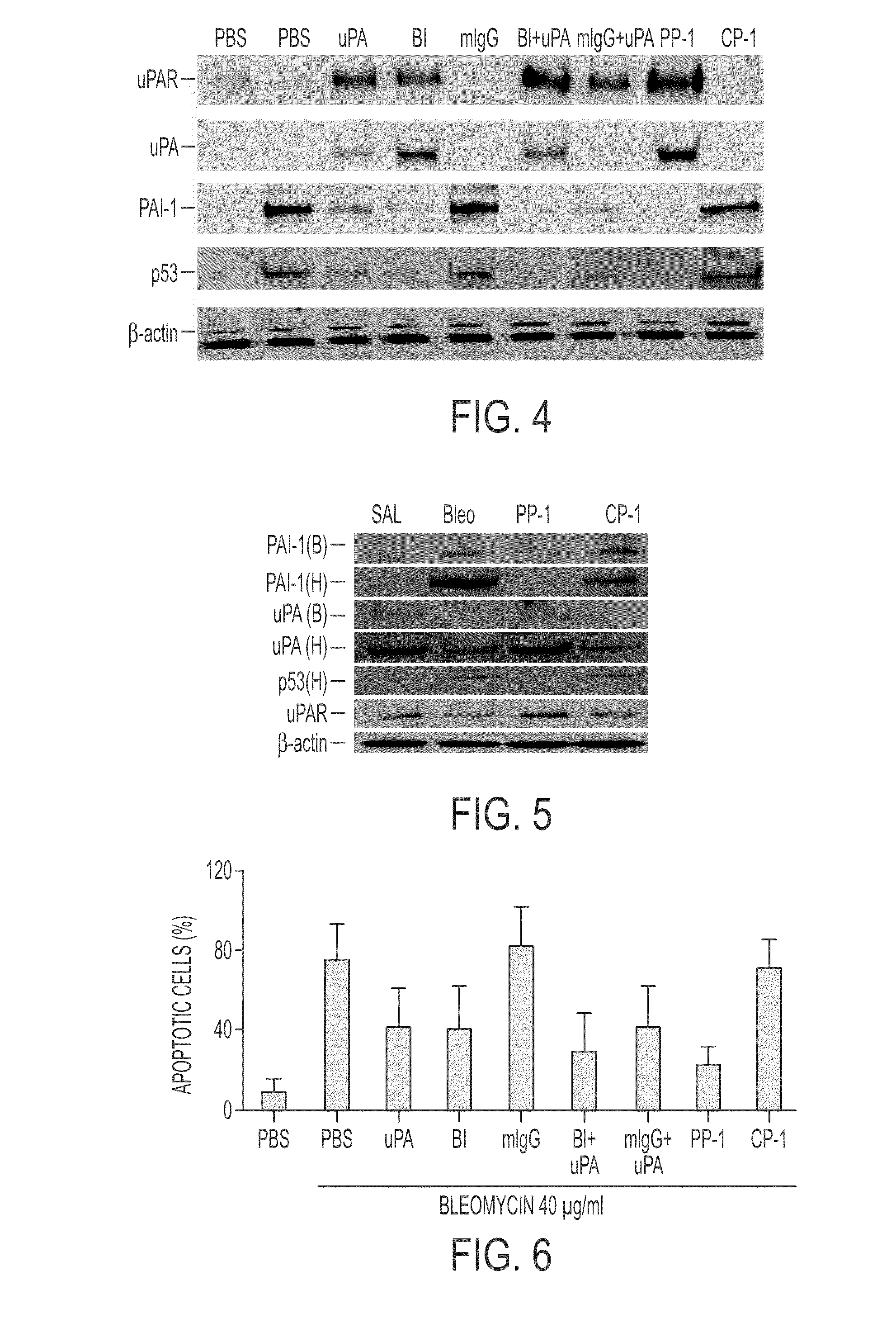
ActiveUS8697840B2High expressionPolypeptide with localisation/targeting motifCell receptors/surface-antigens/surface-determinantsZymogenReceptor
During lung injury, p53 expression increases, inducing plasminogen activator inhibitor-1 (PAI-1) while inhibiting expression of urokinase-type plasminogen activator (uPA) and its receptor (uPAR), resulting in apoptosis of lung epithelial cells (LECs). In the bleomycin lung injury model, p53 and PAI-1 are induced while uPA and uPAR are inhibited. A 20 residue peptide DGIWKASFTTFTVTKYWFYR termed PP-1 (the Cav-1 scaffolding domain) or peptide NYHYLESSMTALYTLGH, termed PP-2, protected LECs from bleomycin-induced apoptosis in vitro and in vivo and prevented subsequent pulmonary fibrosis by attenuating lung epitheilial damage. Pharmaceutical compositions, peptide multimers and deliverable polypeptides comprising the above peptides are dislcosed. The peptides and functional variants, peptide multimers, cell-targeted polyepeptides and pharmaceutical compositions are used in methods for inhibiting apoptosis of injured or damaged lung epithelial cells and for treating acute lung injury and consequent pulmonary fibrosis.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
8-azaprostaglandin derivatives and medical use thereof
The pharmaceutical composition comprising the compound of the invention having 8-azaprostaglandin skeleton represented by formula (I) (wherein, all the symbols have the same meanings as that of the specification.) a salt thereof, a solvate thereof or a cyclodextrin clathrate thereof, or a prodrug thereof and them as active ingredient have EP4 agonistic action and thus are considered useful for the prevention and / or treatment of immunological diseases, asthma, neuronal cell death, arthritis, lung failure, pulmonary fibrosis, pulmonary emphysema, bronchitis, chronic obstructive pulmonary disease, liver damage, acute hepatitis, nephritis, renal insufficiency, hypertension, myocardial ischemia, systemic inflammatory response syndrome, sepsis, hemophagous syndrome, macrophage activation syndrome, Still's disease, Kawasaki disease, burn, systemic granulomatosis, ulcerative colitis, Crohn's disease, hypercytokinemia at dialysis, multiple organ failure, shock and glaucoma, etc. Furthermore, the compounds also have an action of accelerating bone formation, so it is expected to be useful for the prevention and / or treatment of diseases associated with loss in bone mass, for example, primary osteoporosis, secondary osteoporosis, bone metastasis of cancer, hypercalcemia, Paget's disease, bone loss, osteonecrosis, bone formation after bone operation, alternative treatment for bone grafting.
Owner:ONO PHARMA CO LTD
Application of mesenchymal stem cell exosome to preparation of medicament for preventing and treating radiation-induced lung injury (RILI)
InactiveCN109432130AImprove securityChemically stablePharmaceutical delivery mechanismUnknown materialsInflammatory factorsLesion
The invention discloses application of a mesenchymal stem cell exosome to preparation of a medicament for preventing and treating radiation-induced lung injury (RILI). After a radiated mouse with lunginjury is treated by adopting tail vein injection administration of the mesenchymal stem cell exosome, inflammatory factor level is effectively lowered (IL-1beta, IL-6, TNF-alpha, TGF-beta); the survival rate of the mouse is improved effectively; and pneumonia and lung fibrosis lesion caused by the RILI are reduced. The mesenchymal stem cell exosome has the advantages of high security, stable chemical property and easiness in storage, and a new way is provided for safely and effectively treating the RILI.
Owner:中科广聚(北京)生物医学技术中心有限公司 +1
Complex vitamin and amino acid oral liquid as well as preparation method and application thereof
ActiveCN105708829AReasonable compositionMaintain nutritional balanceDispersion deliveryMetabolism disorderThreonineTryptophan
The invention belongs to the field of a health care product, and in particular relates to a complex vitamin and amino acid oral liquid as well as a preparation method and application thereof. The complex vitamin and amino acid oral liquid provided by the invention mainly consists of isoleucine, threonine, valine, aspartic acid, arginine hydrochloride, lysine hydrochloride, phenylalanine, methionine, leucine, tryptophan, nicotinamide, vitamin B1, vitamin B2, vitamin B6 and water. The complex vitamin and amino acid oral liquid provided by the invention is reasonable in compatibility of various components, and is capable of effectively keeping nutrient balance in human bodies; and meanwhile, the oral liquid also has functions of boosting immunity of organism and relieving fatigue of the human bodies. At the same time, upon tests, the complex vitamin and amino acid oral liquid has a certain effect on improving idiopathic pulmonary fibrosis.
Owner:GUANGDONG JIABO PHARM CO LTD
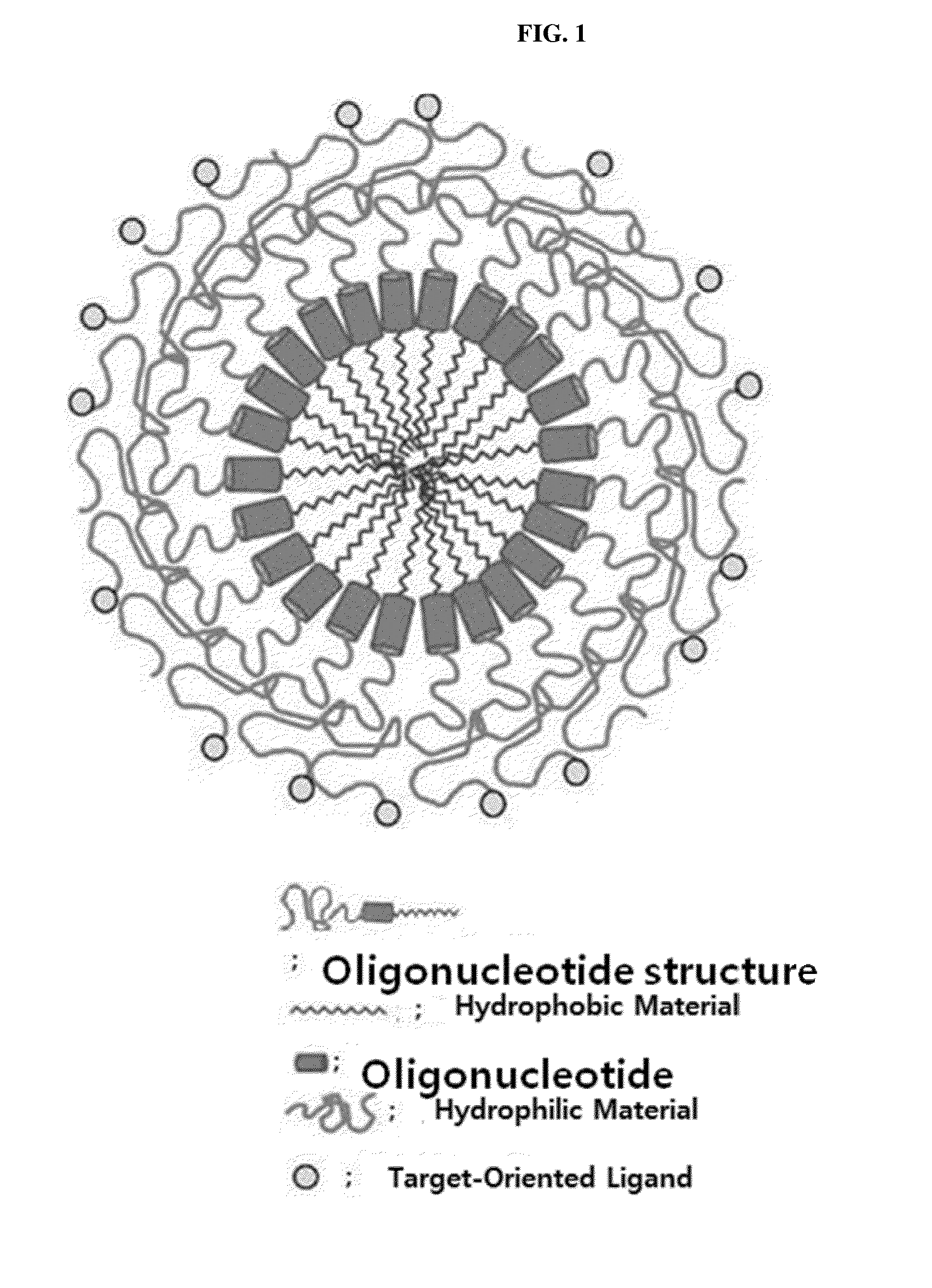
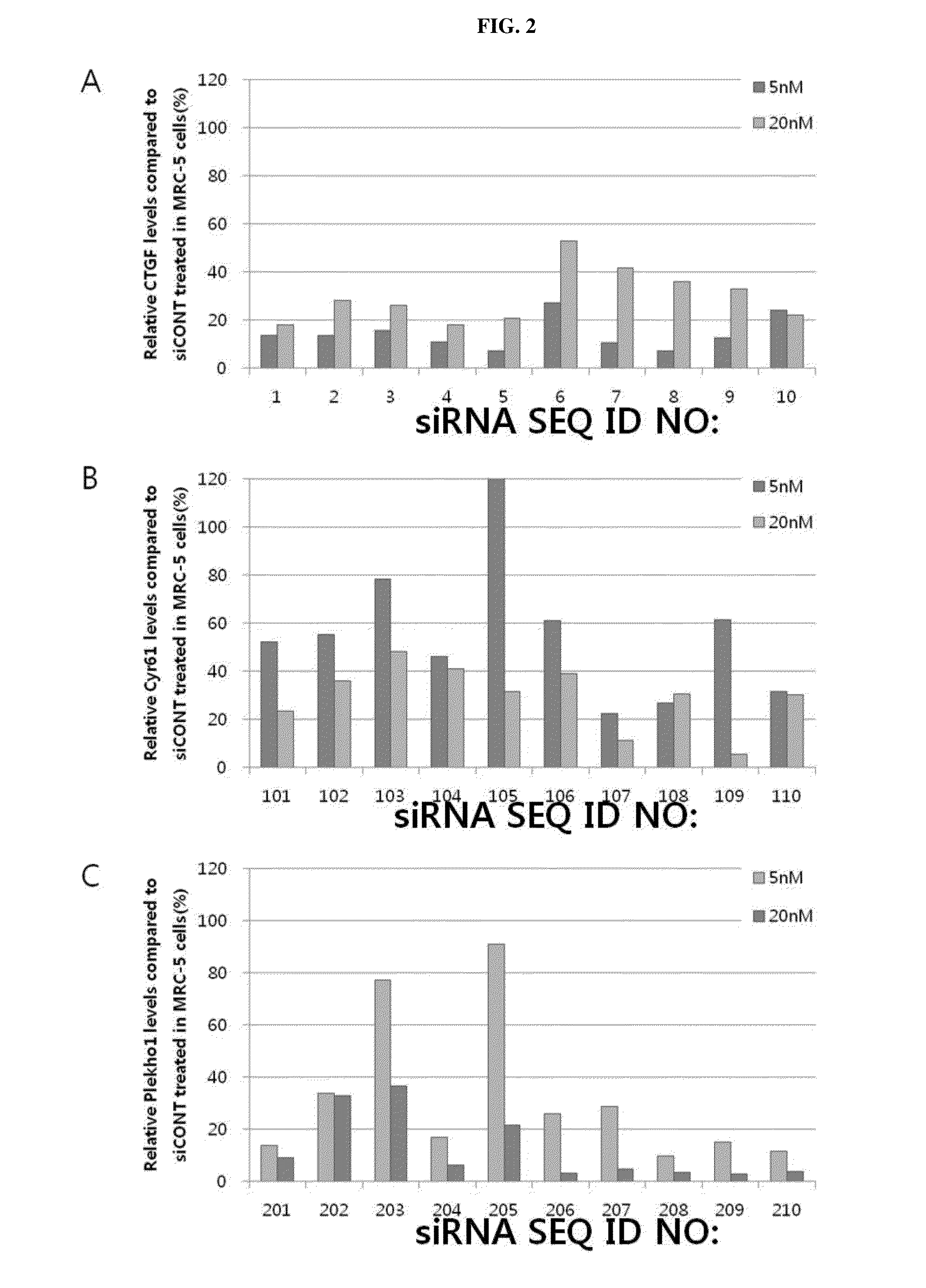
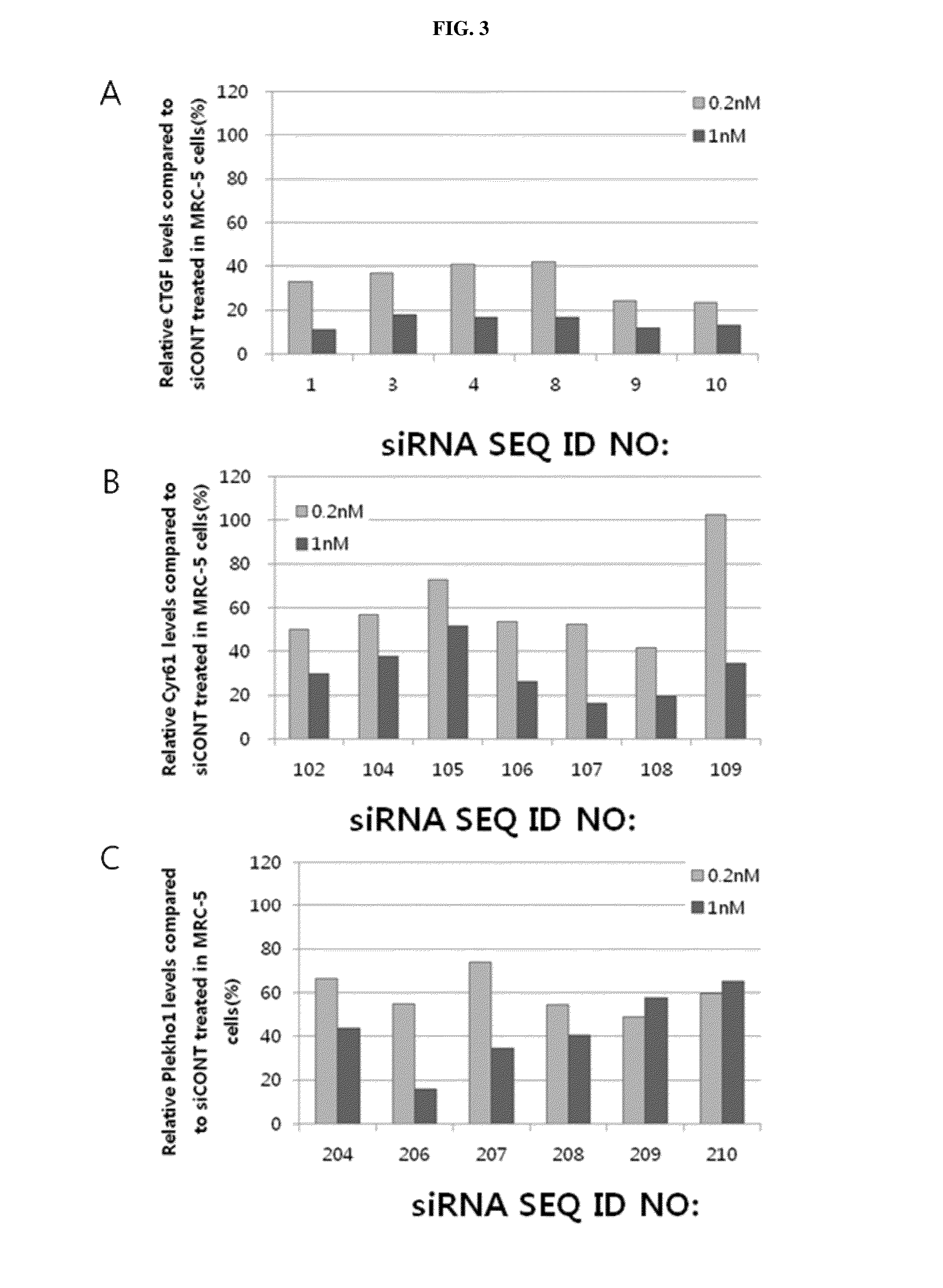
Respiratory disease-related gene specific sirna, double-helical oligo RNA structure containing sirna, compositon containing same for preventing or treating respiratory disease
InactiveUS20160122764A1Improve efficiencyInhibit expressionAntibacterial agentsSugar derivativesDiseaseCYR61
The present invention relates to a gene specific siRNA related with respiratory diseases, particularly, to a gene specific siRNA related with idiopathic pulmonary fibrosis and chronic obstructive pulmonary disease (COPD), and a highly efficient double-helical oligo RNA structure containing the same, wherein the double-helical oligo RNA structure has a structure in which hydrophilic and hydrophobic materials are bonded at the both ends of the double-helical RNA (siRNA) using a simple covalent bond or a linker-mediated covalent bond to be effectively transferred into a cell, and may be converted into nanoparticles by the hydrophobic interaction of the double-helical oligo RNA structure in a solution. It is desirable that the siRNA contained in the double-helical oligo RNA structure is a siRNA specific to a CTGF, Cyr61, or Plekho1, which are genes related with respiratory diseases, particularly idiopathic pulmonary fibrosis and COPD. In addition, the present invention relates to a method for producing the double-helical oligo RNA structure and a pharmaceutical composition containing the double-helical oligo RNA structure for preventing or treating respiratory diseases, particularly idiopathic pulmonary fibrosis and COPD.
Owner:BIONEER
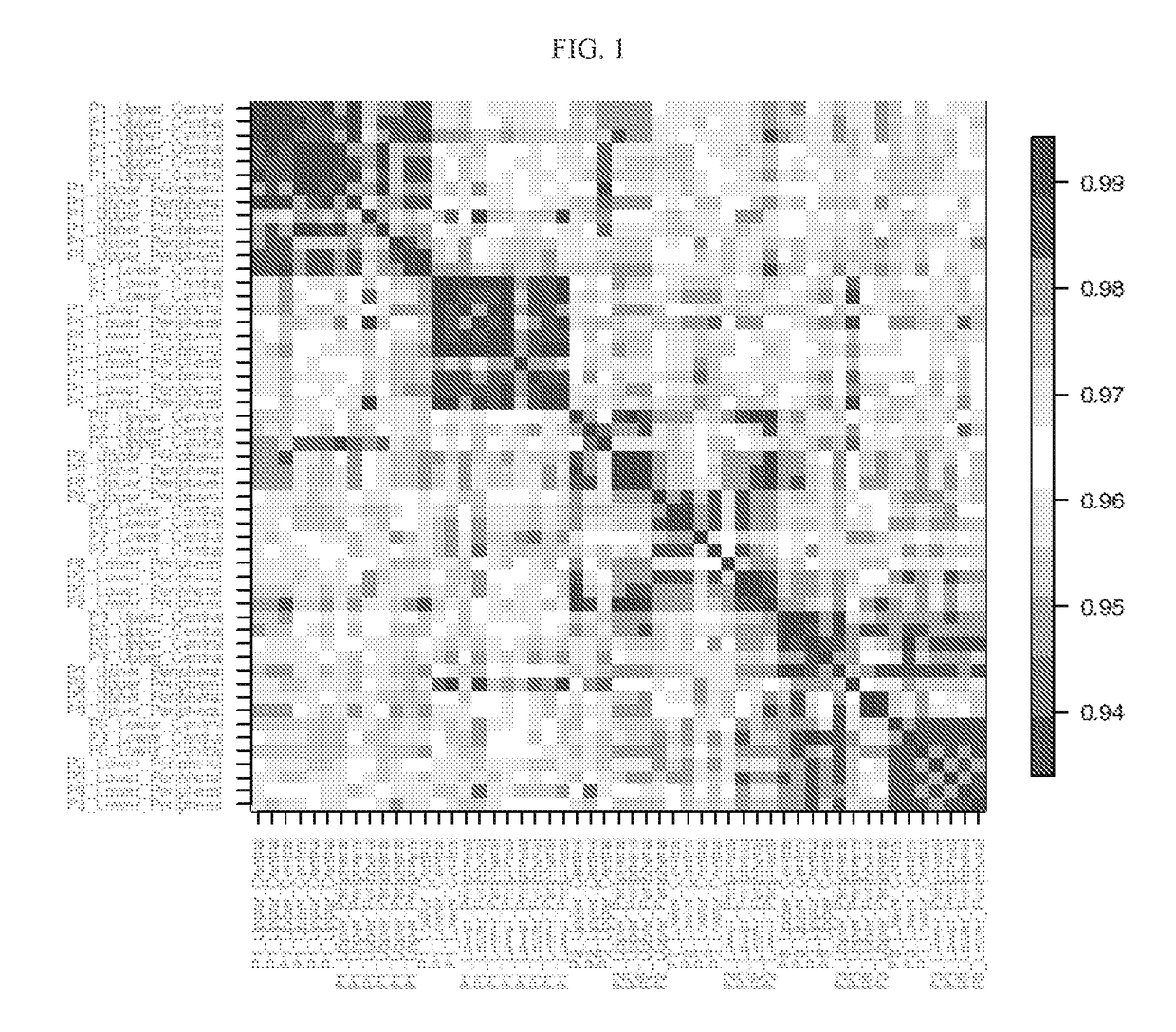
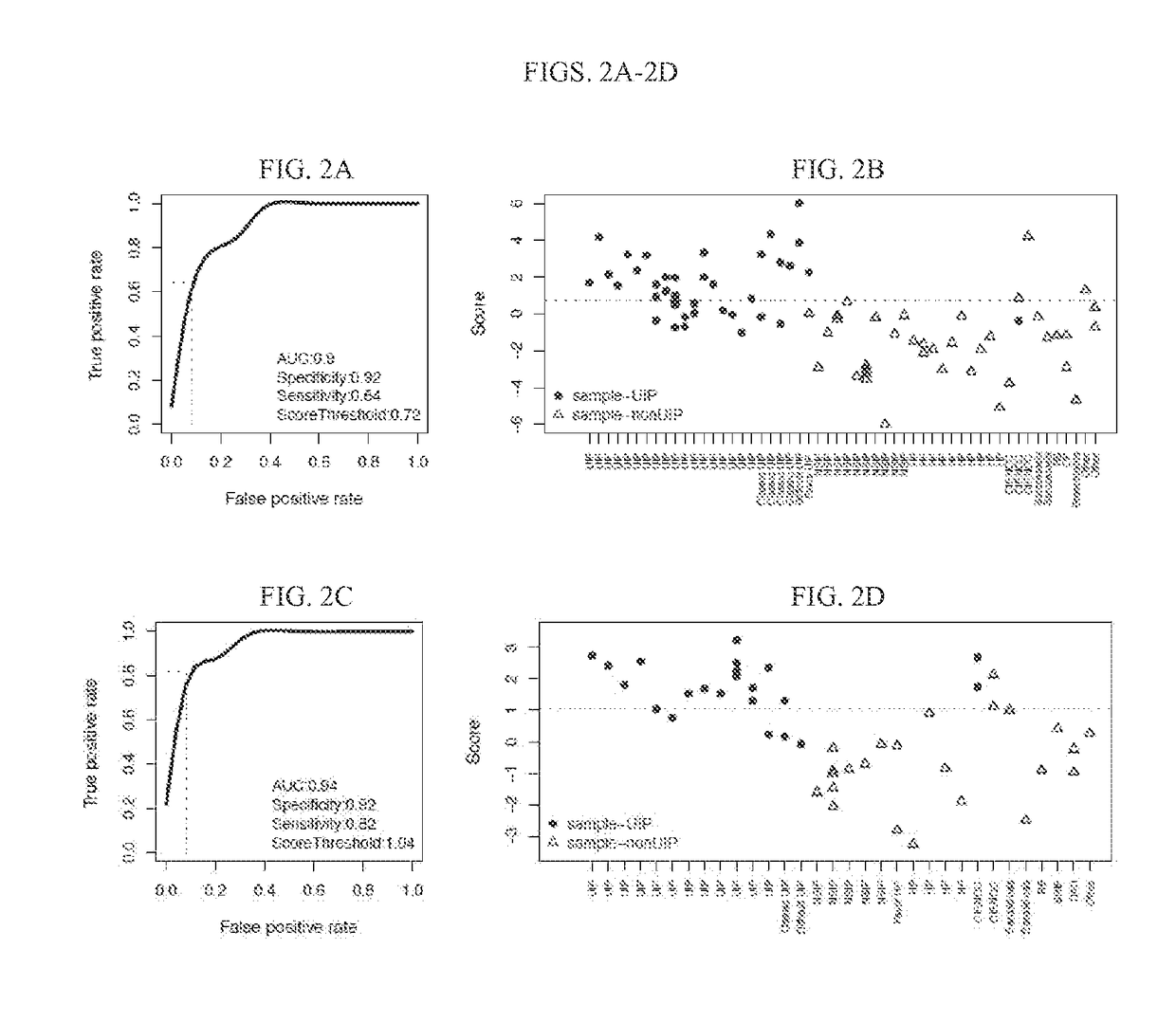
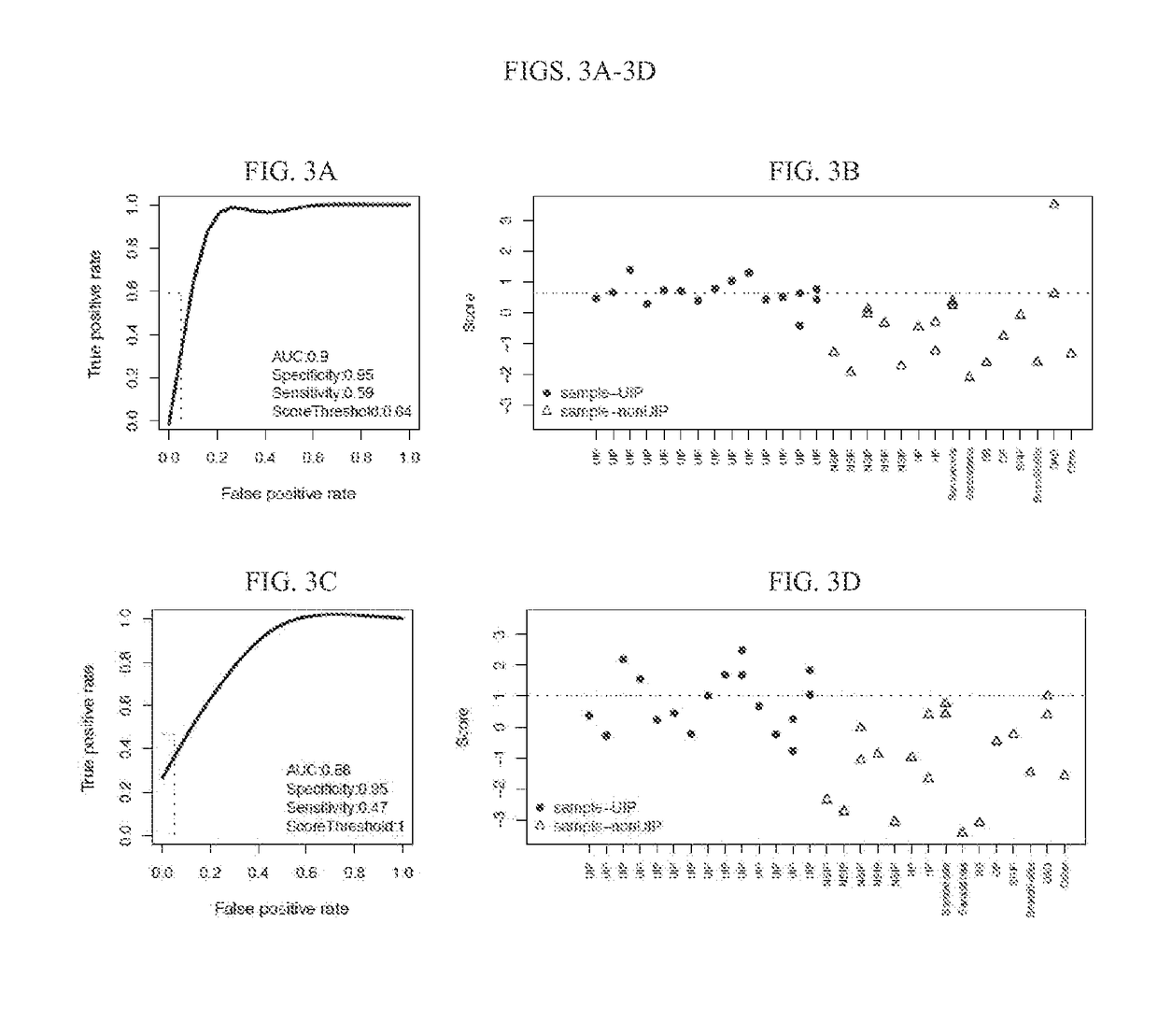
Systems and methods of diagnosing idiopathic pulmonary fibrosis on transbronchial biopsies using machine learning and high dimensional transcriptional data
InactiveUS20170335396A1Microbiological testing/measurementRespiratory disorderRadiologyBronchial epithelium
The present invention provides systems, methods, and classifiers for differentiating between samples as usual interstitial pneumonia (UIP) or non-UIP.
Owner:VERACYTE INC
Application of isoalantolactone derivative and salt thereof in preparation of medicines for treating lung fibration
ActiveCN106496243AGood treatment effectOrganic active ingredientsOrganic chemistryBenzoic acidPhosphomolybdic acid
The invention relates to an application of an isoalantolactone derivative and a salt thereof in the preparation of medicines for treating lung fibration, and provides an isoalantolactone derivative represented by formula (I). An acid for forming the salt is an inorganic acid or an organic acid, the inorganic acid is selected from hydrofluoric acid, hydrochloric acid, hydrobromic acid, hydroiodic acid, sulfuric acid, nitric acid, phosphoric acid, carbonic acid, boric acid, seleninic acid, phosphomolybdic acid, phosphorous acid and sulfurous acid, and the organic acid is selected from citric acid, maleic acid, D-malic acid, L-malic acid, DL-malic acid, L-lactic acid, D-lactic acid, DL-lactic acid, oxalic acid, methanesulfonic acid, pentanoic acid, oleic acid, lauric acid, p-methyl benzenesulfonic acid, 1-naphthalenesulfonic acid, 2-naphthalenesulfonic acid, phthalic acid, tartaric acid, propane diacid, succinic acid, fumaric acid, glycollic acid, thioglycollic acid, glycine, sarcosine, sulfonic acid, nicotinic acid, methylpyridine acid, isonicotinic acid, benzoic acid and substituted benzoic acid.
Owner:NANKAI UNIV
IP receptor agonist heterocyclic compounds
The present invention provides heterocyclic derivatives which activate the IP receptor, processes for preparing them, pharmaceutical compositions comprising said derivatives and uses thereof. Activating the IP receptor signaling pathway is useful to treat many forms of PAH, pulmonary fibrosis and exert beneficial effects in fibrotic conditions of various organs in animal models and in patients.
Owner:NOVARTIS AG
Application of effective parts of astragalus mongholicus and hedysarum polybotrys
InactiveCN102885878AIncreased lung coefficientImproved lung coefficientOrganic active ingredientsRespiratory disorderHedysarumAstragalus mongholicus
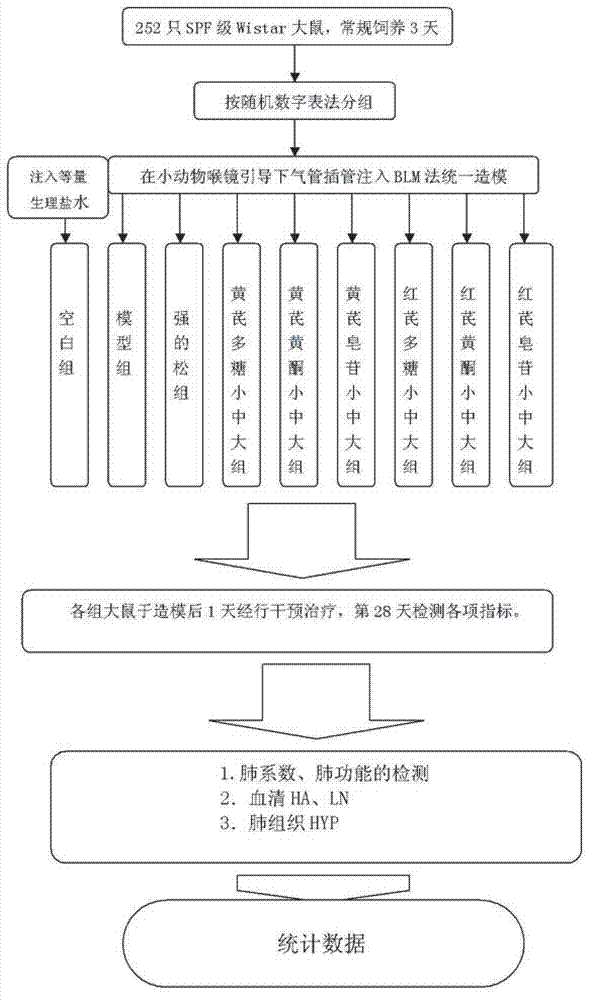
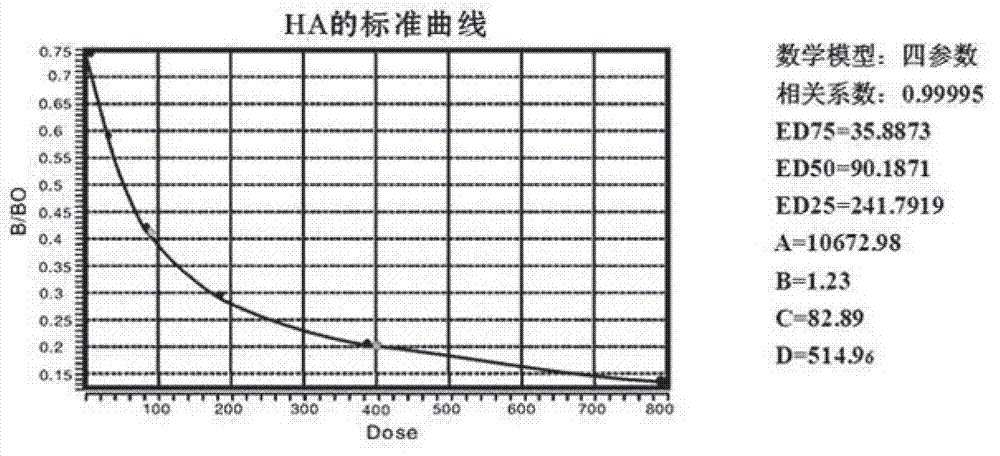
The invention belongs to the traditional Chinese medicine field and relates to the preparation of effective parts of astragalus mongholicus and hedysarum polybotrys and selection thereof for pulmonary fibrosis model intervention so as to obtain further study on the effective parts having the optimal curative effect. An interstitial pulmonary fibrosis model of rats is established through modeling; and compared with the normal rats, the rats having interstitial pulmonary fibrosis have obvious difference and pathologic change typical cases in various related detection indexes and pathologic changes. The various effective parts of astragalus mongholicus and hedysarum polybotrys are capable of suppressing various related indexes of the interstitial pulmonary fibrosis model of rats induced by bleomycin and preventing the development of interstitial pulmonary fibrosis, wherein low and medium dose groups of hedysarum polybotrys flavone have the optimal effect. The degree of the effect of the effective parts of astragalus mongholicus and hedysarum polybotrys on stopping the progress of interstitial pulmonary fibrosis is related to appropriate concentrations of equivalent dugs. Through comprehensive assessment, the hedysarum polybotrys flavone is better in influence on the lung function, HA, LN, HYP and the like of the rats having interstitial pulmonary fibrosis than other effective parts.
Owner:GANSU UNIV OF CHINESE MEDICINE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![1H-pyrazolo[3,4-<i>b</i>]pyridines and therapeutic uses thereof 1H-pyrazolo[3,4-<i>b</i>]pyridines and therapeutic uses thereof](https://images-eureka.patsnap.com/patent_img/ab7b9af2-18c0-4461-a0e2-7121e6fc6aba/US08618128-20131231-C00001.png)
![1H-pyrazolo[3,4-<i>b</i>]pyridines and therapeutic uses thereof 1H-pyrazolo[3,4-<i>b</i>]pyridines and therapeutic uses thereof](https://images-eureka.patsnap.com/patent_img/ab7b9af2-18c0-4461-a0e2-7121e6fc6aba/US08618128-20131231-C00002.png)
![1H-pyrazolo[3,4-<i>b</i>]pyridines and therapeutic uses thereof 1H-pyrazolo[3,4-<i>b</i>]pyridines and therapeutic uses thereof](https://images-eureka.patsnap.com/patent_img/ab7b9af2-18c0-4461-a0e2-7121e6fc6aba/US08618128-20131231-C00003.png)