Stem cell derived exosome preparation for preventing and treating lung jury
An exosome and stem cell technology, applied in the field of stem cell therapy and regenerative medicine, can solve the problem of lack of a good treatment method, and achieve the effect of promoting recovery and enhancing the therapeutic effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Example 1, the present invention provides a method for preparing an exosome preparation derived from stem cells.
[0016] Reagents such as cell culture medium, penicillin / streptomycin, trypsin, sodium pyruvate, and glutamine were all purchased from Gibco Company; culture flasks and well plates required for cell culture were purchased from Corning Company. The serum used for cell culture was Hyclone, and the serum concentration was 10%.
[0017] Refer to "Animal Cell Culture (Sixth Edition)" for common cytological procedures such as cell recovery, culture, subculture, and cryopreservation.
[0018] Preparation of exosome-free serum: Fetal bovine serum was centrifuged at 120,000 g at 4°C for 2 h, the supernatant was extracted (exosomes were precipitated), filtered and sterilized with a 0.22 μm filter, and prepared to a final concentration of 10% for culture Base spare.
[0019] Collection of exosome conditioned medium: Stem cell culture, when the growth reaches 80%, rem...
Embodiment 2
[0028] Example 2, the present invention provides a method for identifying stem cell-derived exosome preparations.
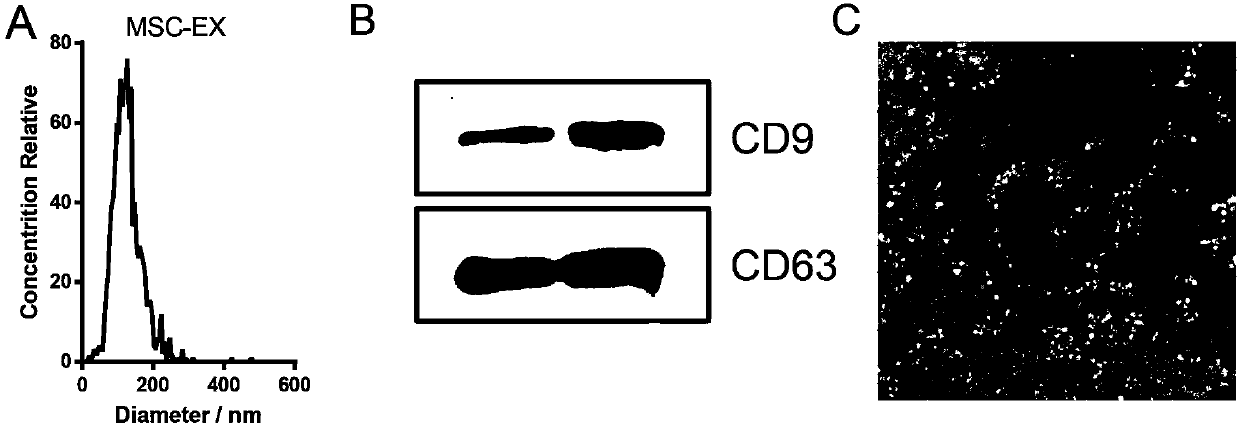
[0029] Size analysis of exosomes: Dilute the exosomes adjusted to the above concentration 400 times with pure water, and then use the nanoparticle tracking analyzer to analyze and image the suspended exosomes one by one. The analysis results are as follows: figure 1 a. The particle size of exosomes derived from stem cells is 0-200nm, with an average particle size of 120nm.
[0030] Analysis of exosome surface protein expression: detection of exosome surface protein markers by Western blot, the experimental steps are as follows:
[0031] (1) Protein sample preparation: Add 5× loading buffer (Kangwei reagent) to the above quantified exosomes, bathe in boiling water for 10 minutes, and store at -80°C.
[0032] (2) Preparation of polyacrylamide electrophoresis separation gel
[0033] Clean the long and short glass plates and the combs required for film preparation...
Embodiment 3
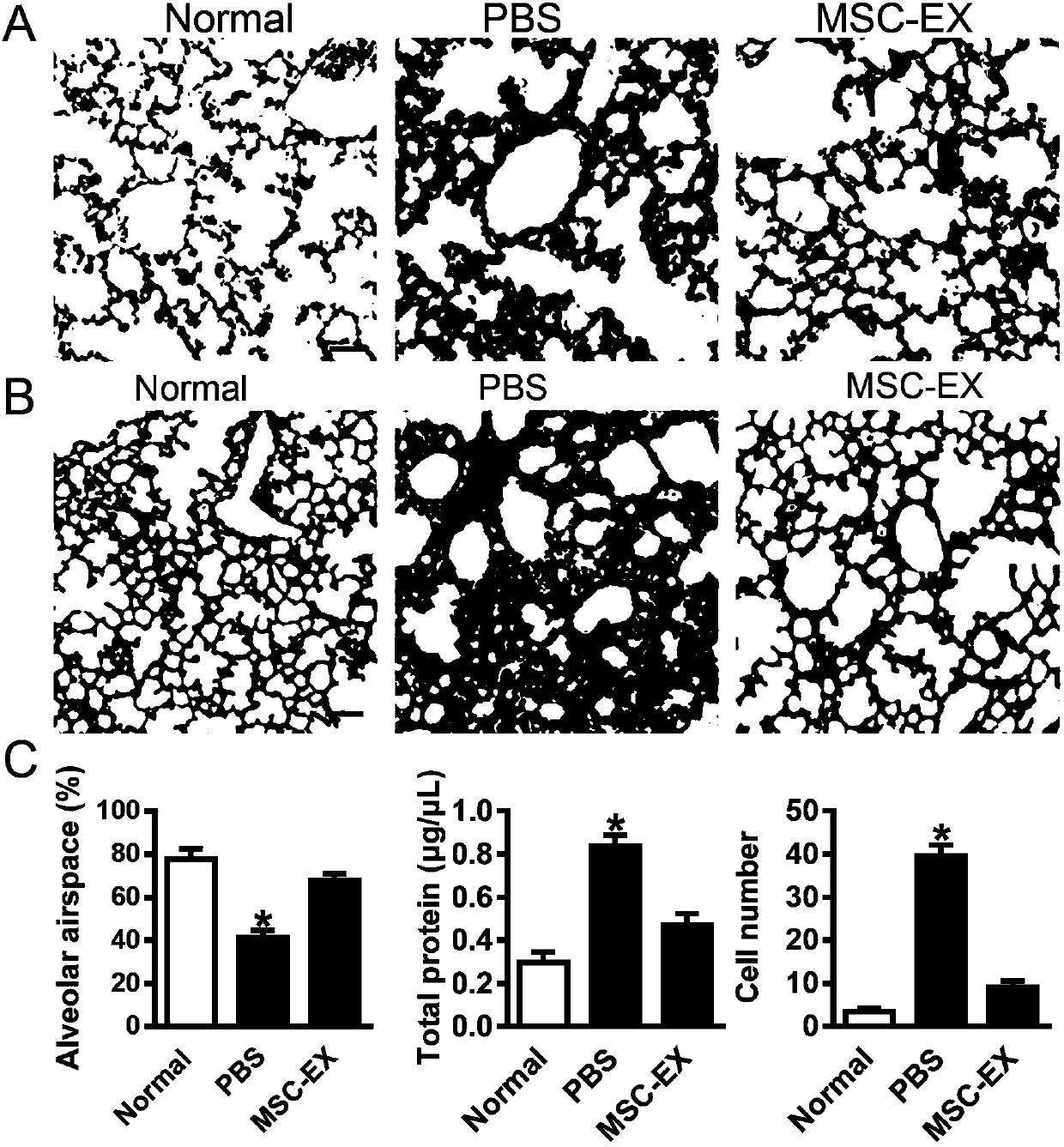
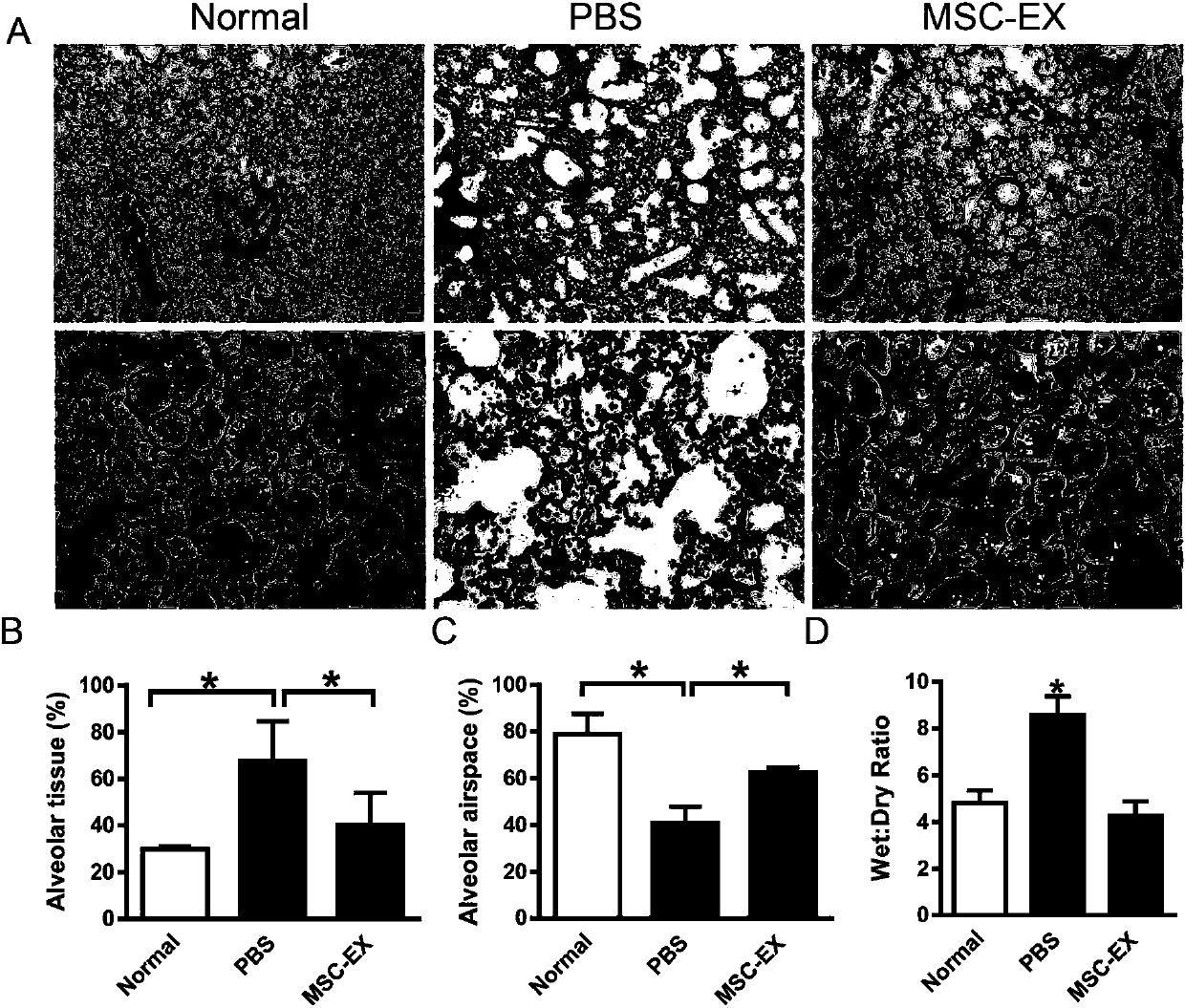
[0052] Example 3, the present invention provides methods for constructing mouse models of acute lung injury, radiation lung injury, pulmonary fibrosis, and silicosis.
[0053] Establishment of acute lung injury in mice: weigh the mice, anesthetize the mice with intraperitoneal injection of 4% chloral hydrate (330 mg / kg); fix the anesthetized mice in supine position, and fix the limbs and head teeth; cold light source lamp Irradiate from between the chest and neck; pull out the tongue with tweezers to expose the oral cavity; wipe off the saliva in the oral cavity with a cotton swab, and adjust the position of the light source to see the opening and closing movement of the pharynx, and the bright dot in the middle is the entrance of the trachea; Cannulation was performed with a 22G cannula; after intubation, 10 8 CFU E. coli (dissolved in 100 μL PBS). The untreated group was used as the normal control group, and the animals were randomly divided into two groups after surgery: t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com