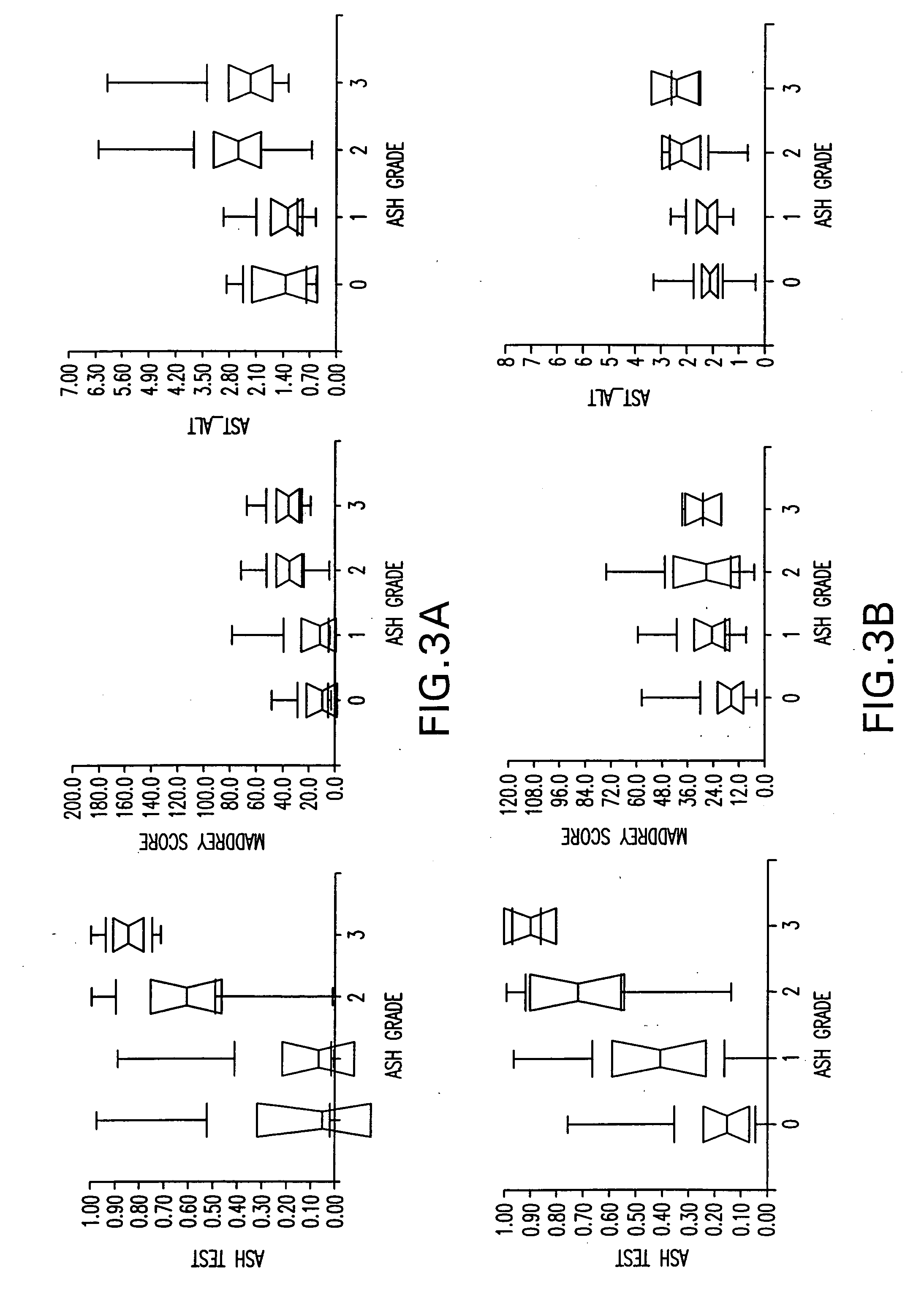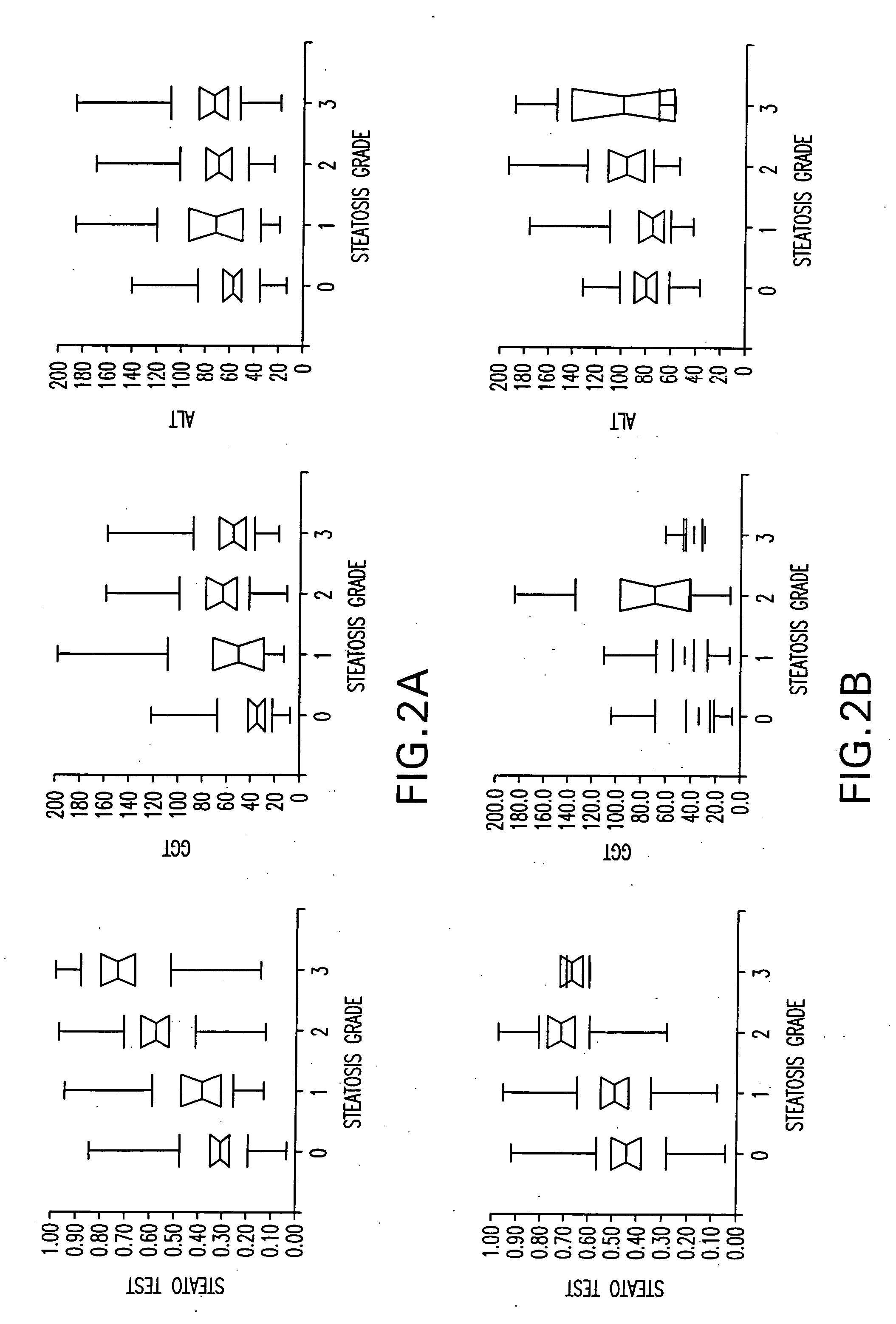Patents
Literature
188 results about "Serum concentration" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Serum concentration is the measure of a compound found in the blood's liquid. Human blood is made up of two components: blood cells and plasma. Blood cells contain white and red blood cells.
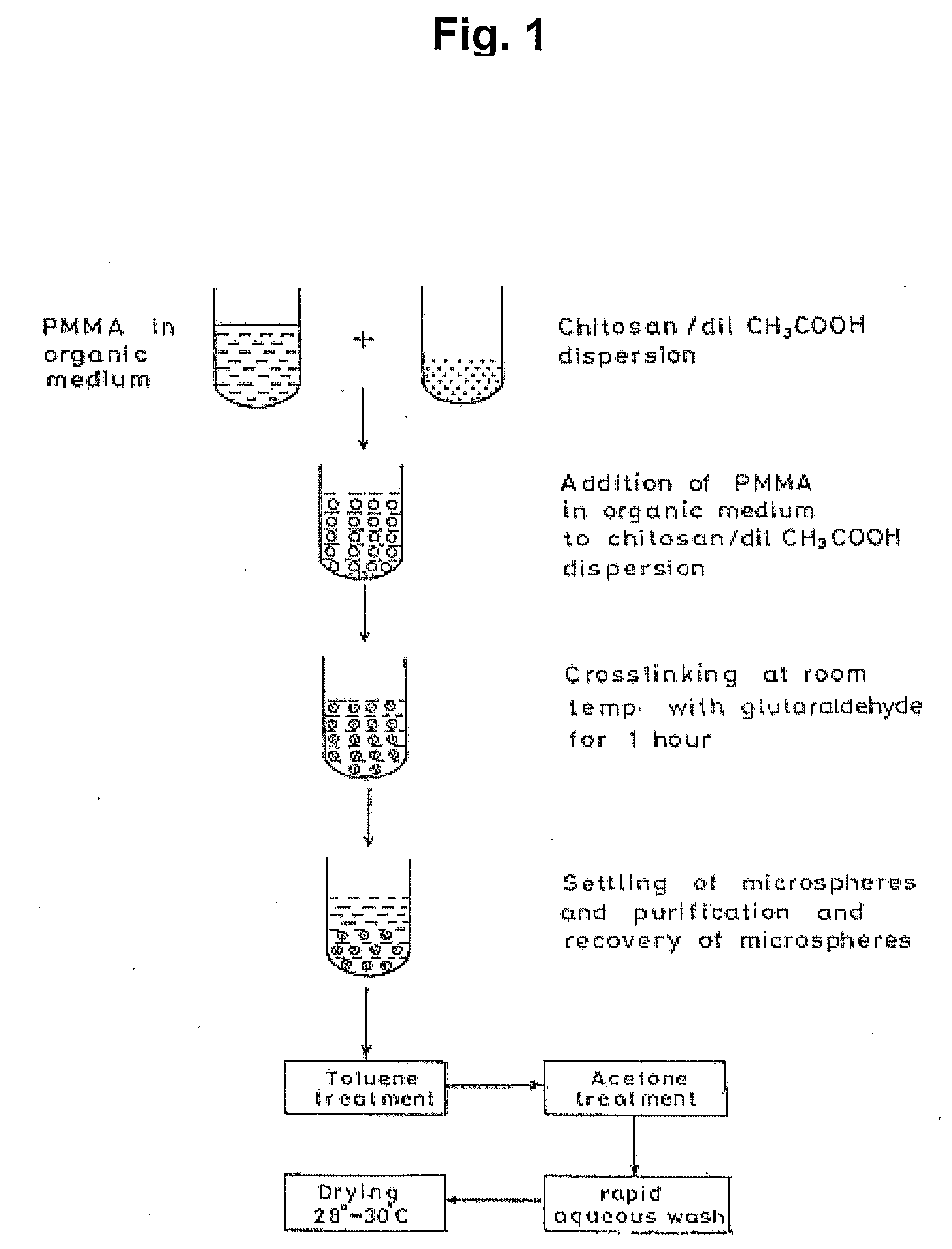
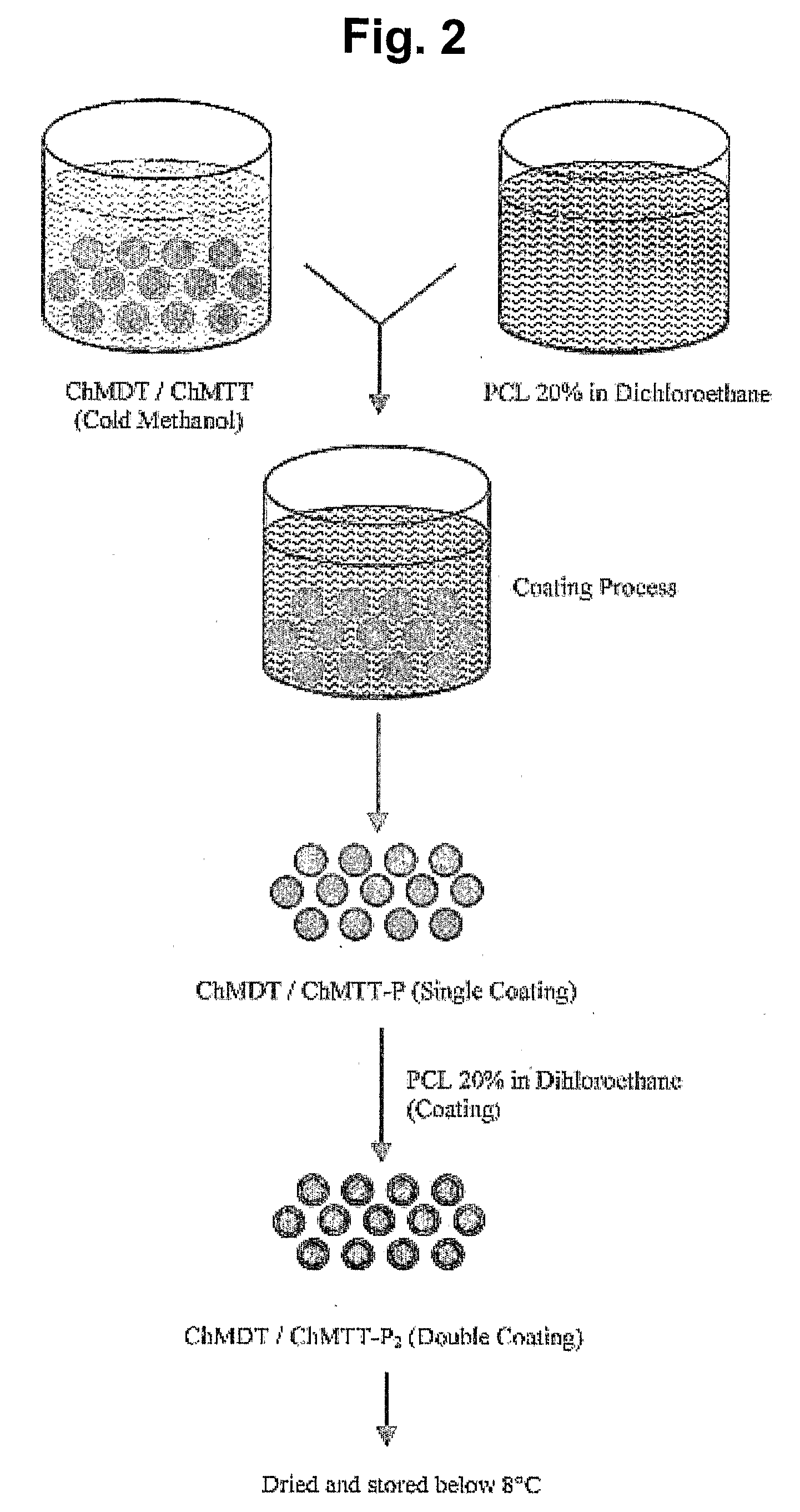
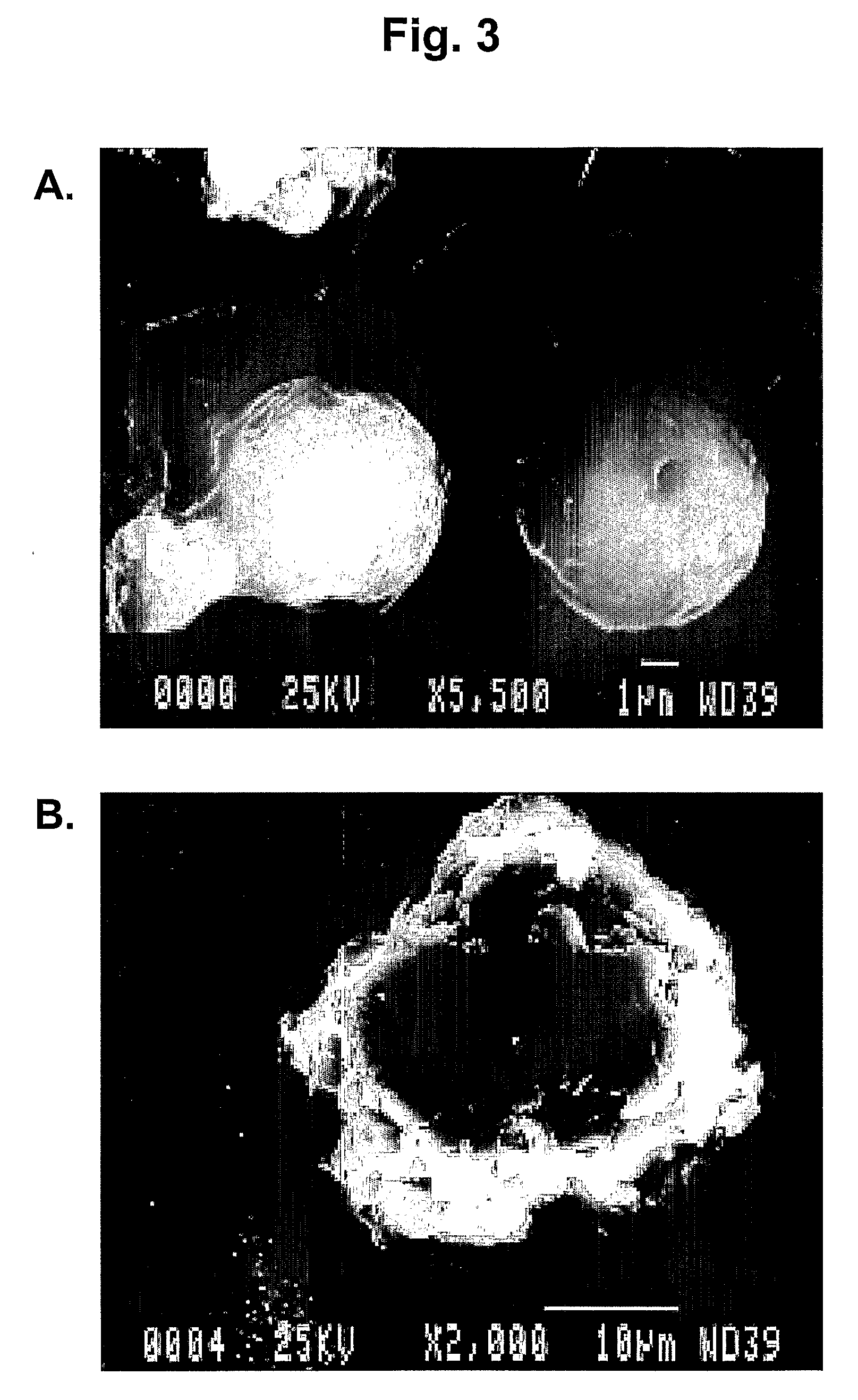
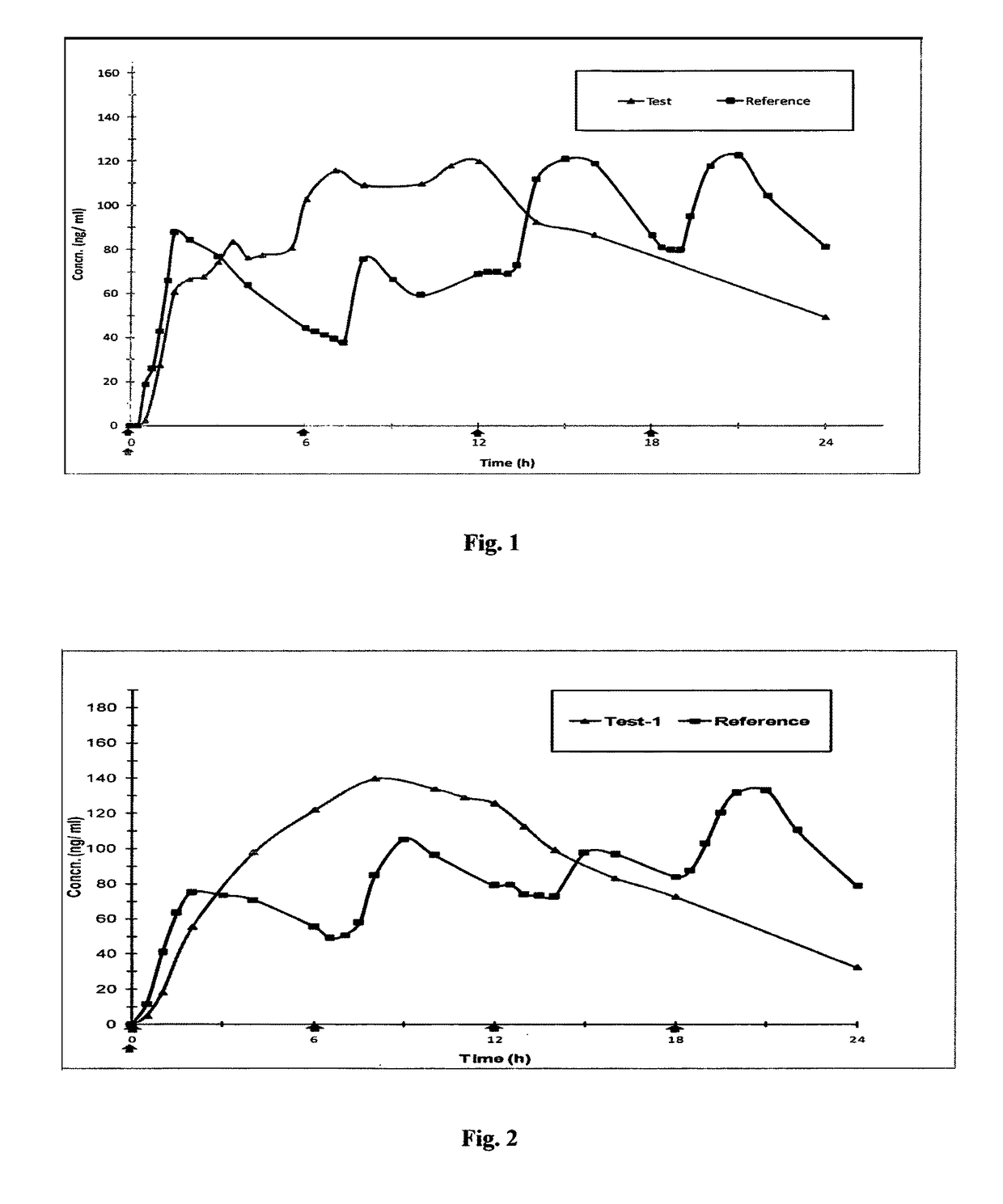
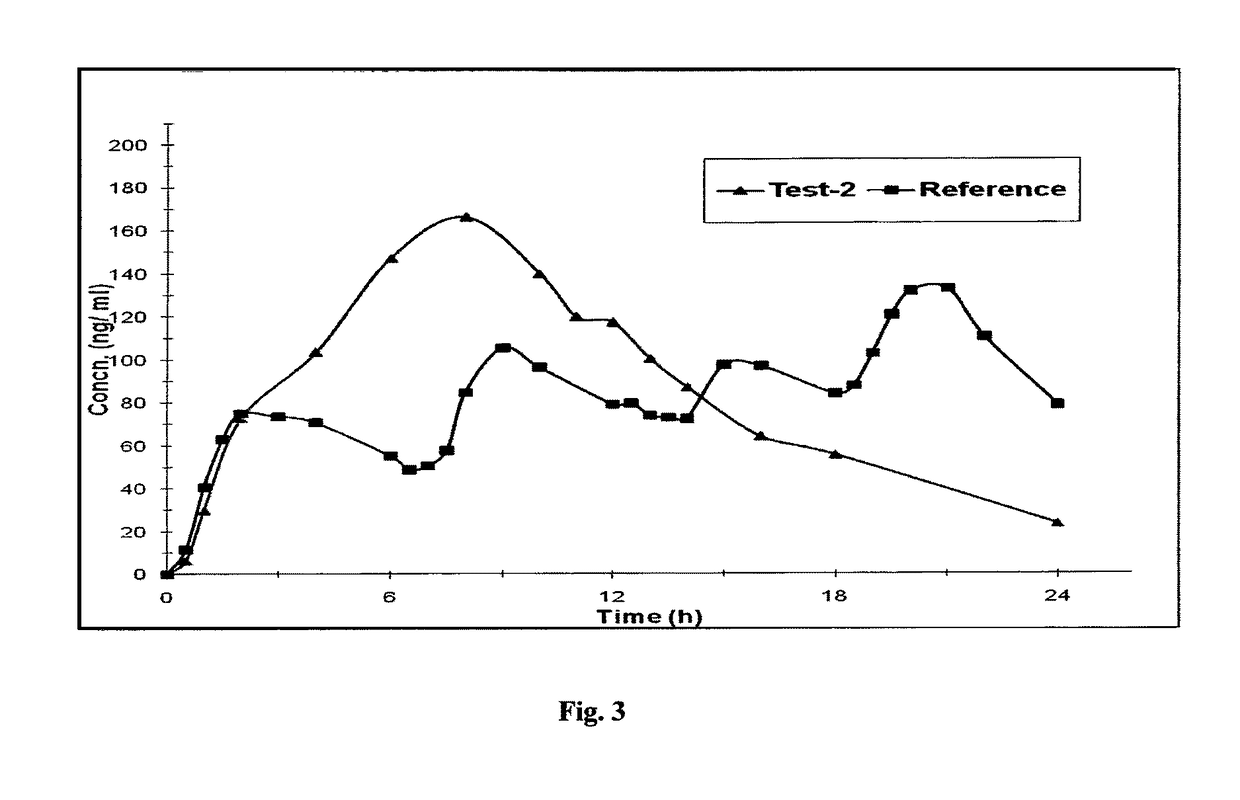
Microparticles and Nanoparticles for the Transmucosal Delivery of Therapeutic and Diagnostic Agents
InactiveUS20080102114A1Easy to transportEasy to demonstrateOrganic active ingredientsPeptide/protein ingredientsMicrosphereMicroparticle
The invention relates to compositions and methods for the administration of therapeutic and / or diagnostic agents such as polypeptides to a mammal, and in particular, compositions suitable for oral administration. The invention provides polymeric particles, and in particular, nano / microparticles such as, but not limited to, microspheres and nanospheres, as well as methods of synthesizing them. The invention also provides methods of increasing the serum concentration of a therapeutic agent such as a polypeptide by orally administering polymeric particles comprising the therapeutic agent. The compositions of the invention allow the absorption of polypeptides through intestinal mucosa and intestinal cells and into the bloodstream of a mammal. The invention further provides a method of treating type II diabetes through the oral administration of compositions comprising insulin and also provides a related glucose-responsive insulin delivery system.
Owner:KORITALA PANDURANGA RAO +1
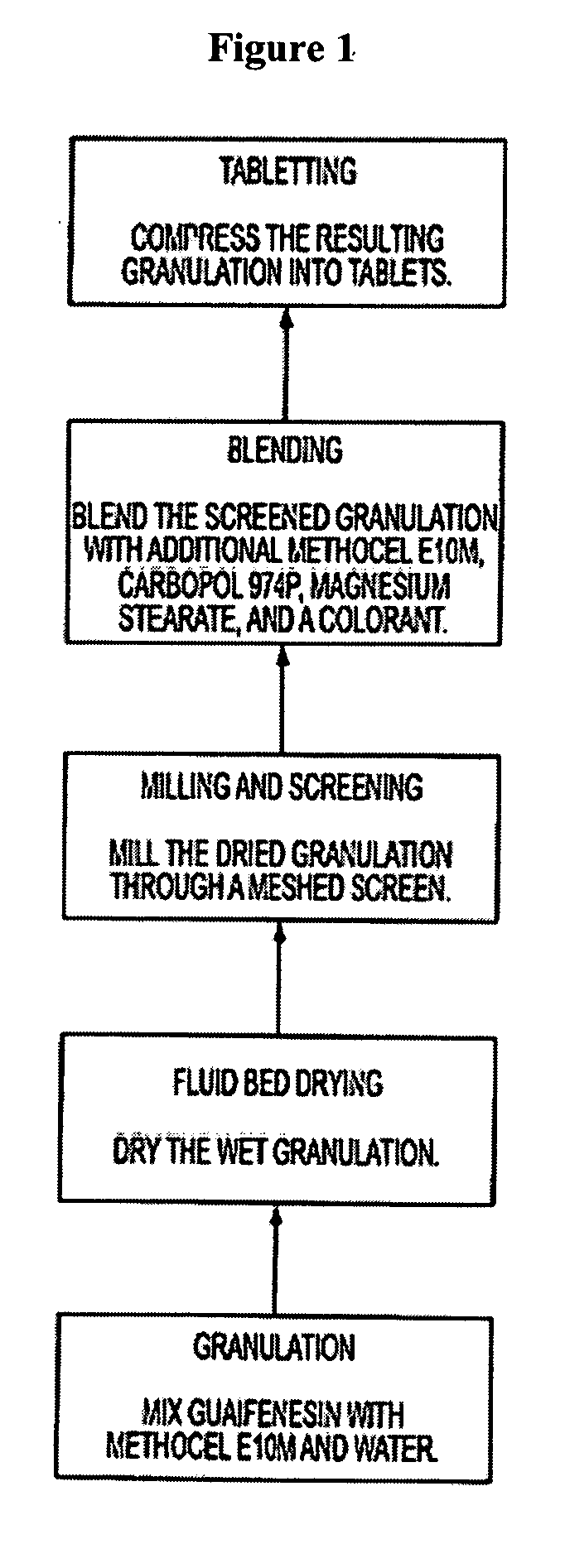
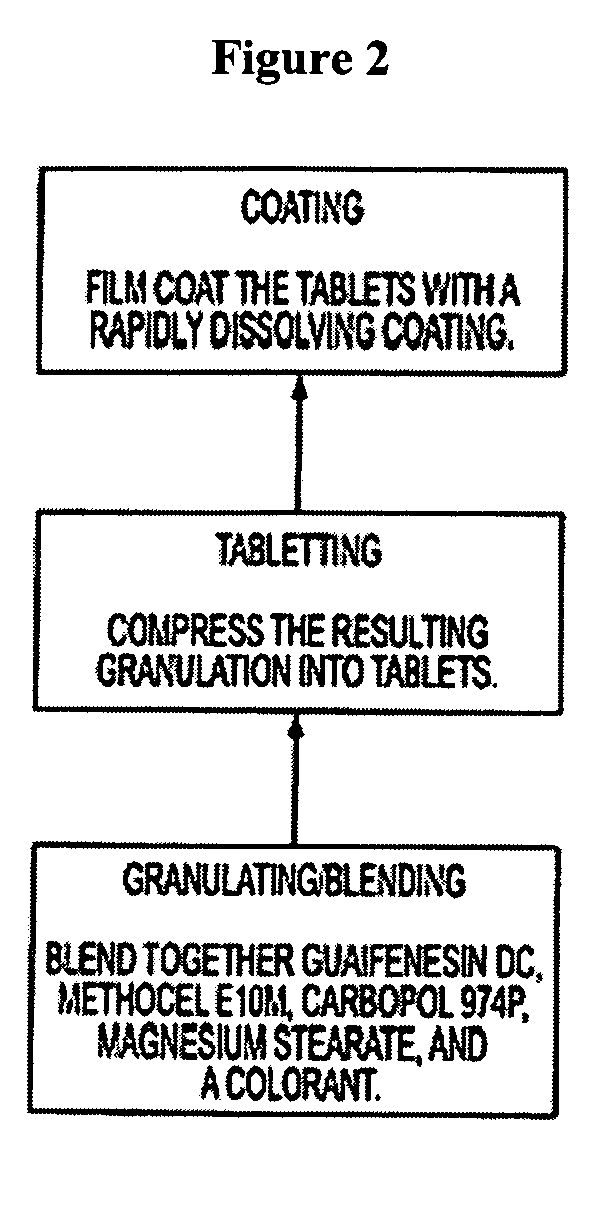
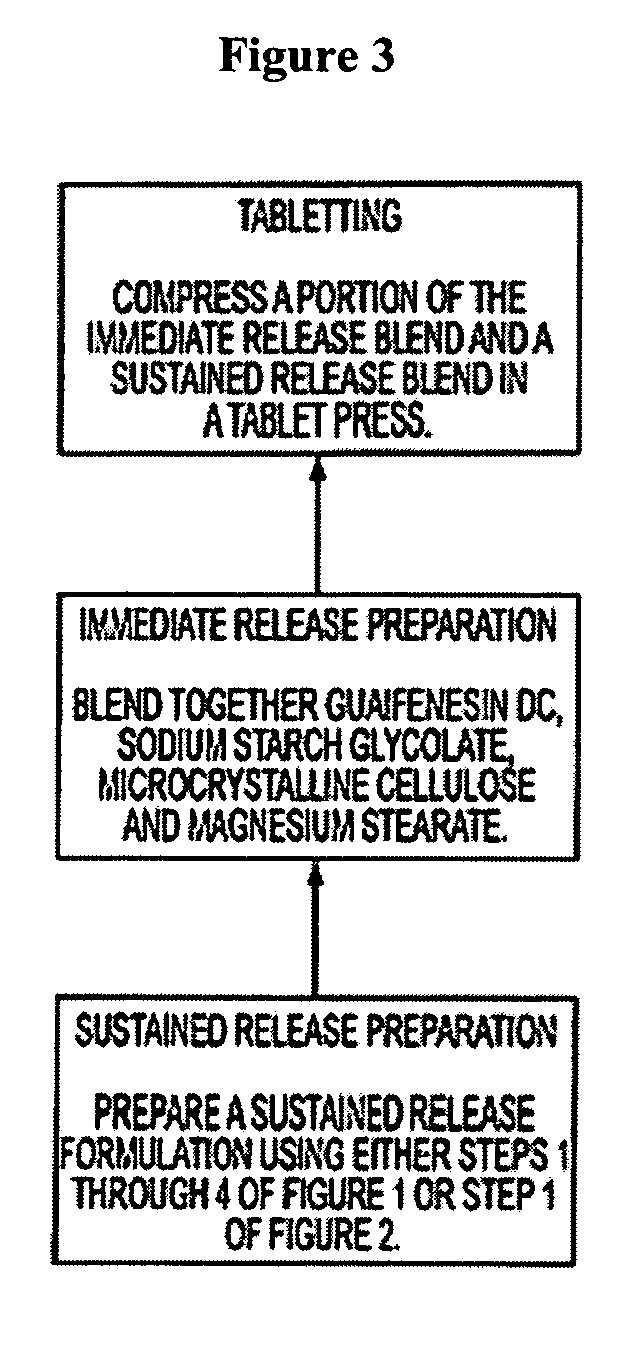
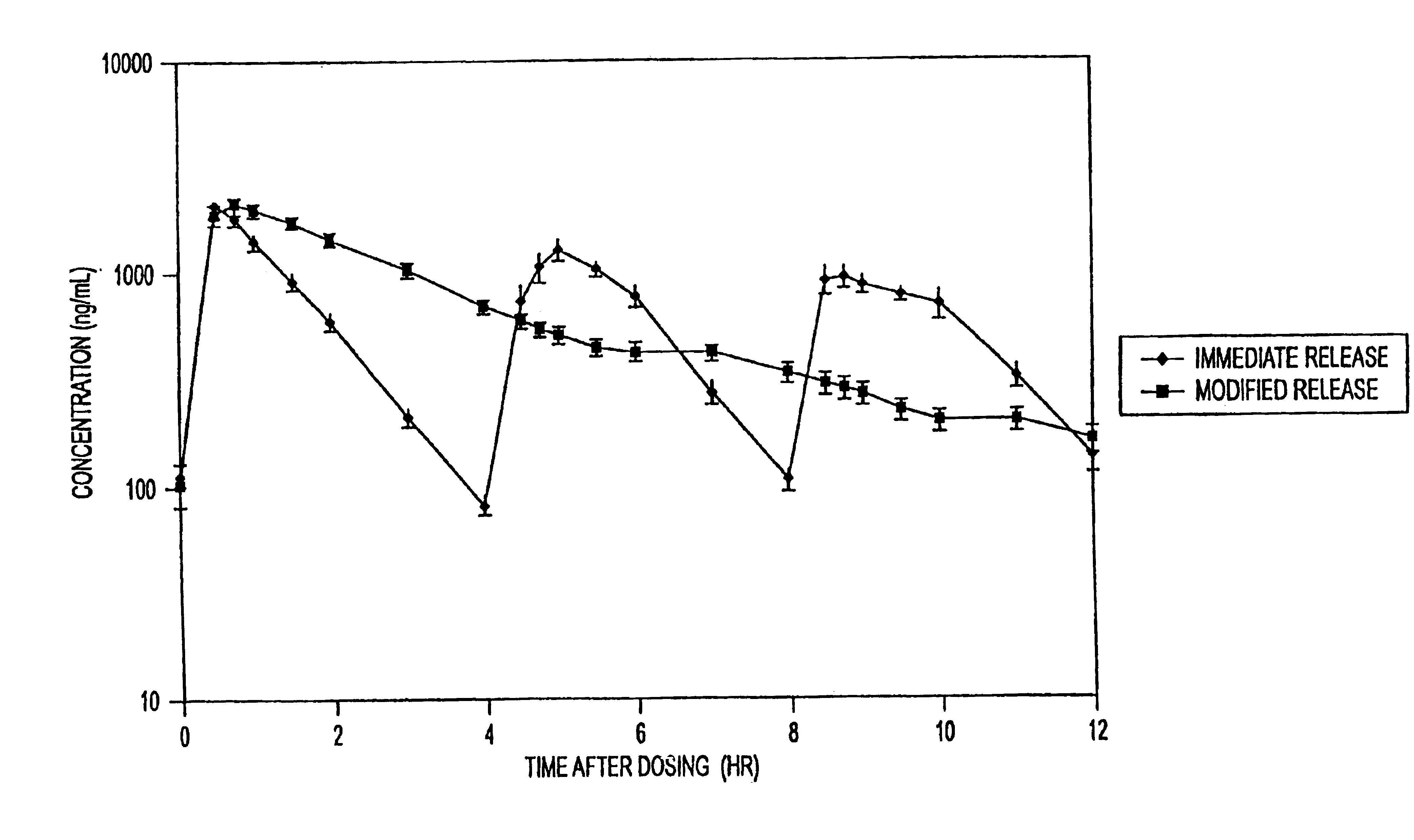
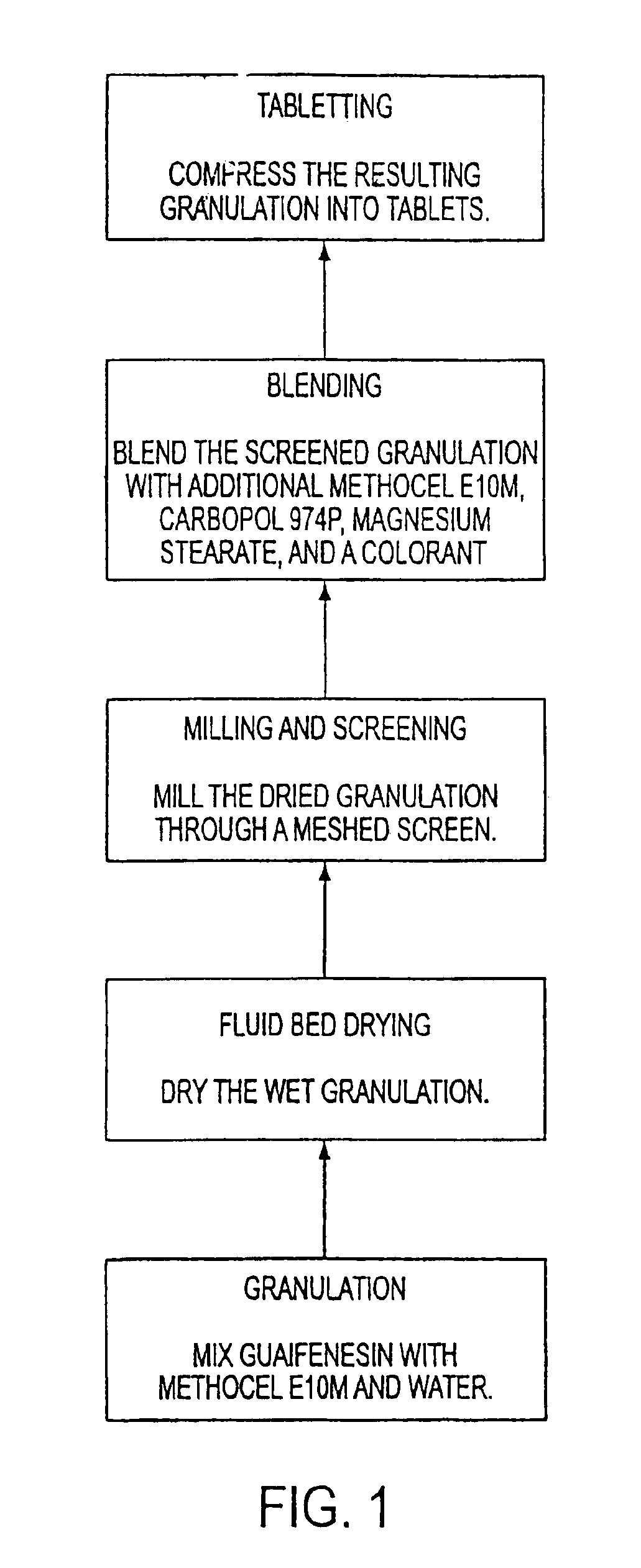
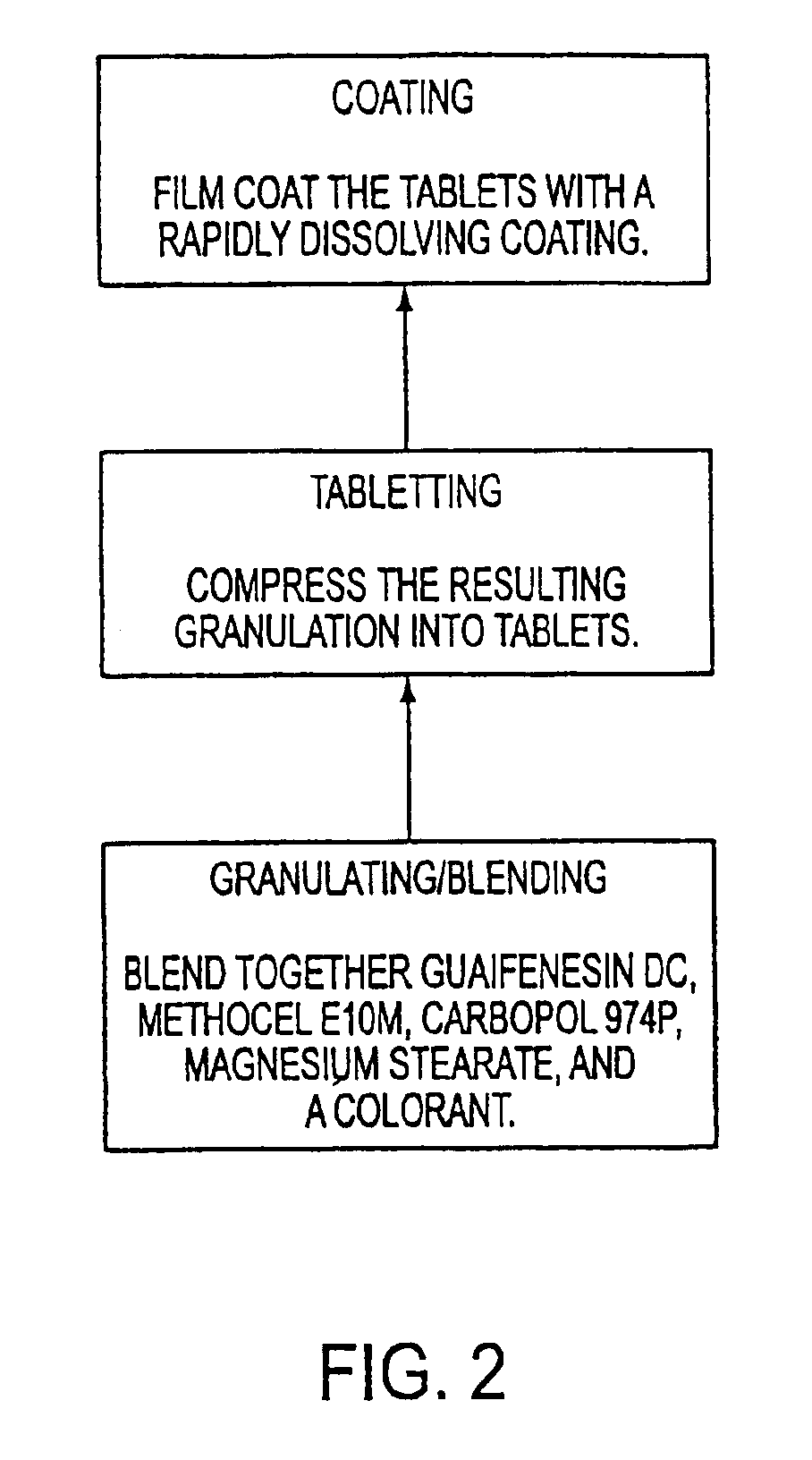
Sustained release formulations of guaifenesin and additional drug ingredients
InactiveUS20050276852A1Increase in drug strengthSustained releaseOrganic non-active ingredientsEther/acetal active ingredientsSerum concentrationImmediate release
The invention relates to a novel pharmaceutical sustained release formulation of guaifenesin and at least one additional drug ingredient. The formulation may comprise a hydrophilic polymer, preferably a hydroxypropyl methylcellulose, and a water-insoluble polymer, preferably an acrylic resin, in a ratio range of about one-to-one (1:1) to about nine-to-one (9:1), more preferably a range of about three-to-two (3:2) to about six-to-one (6:1), and most preferably in a range of about two-to-one (2:1) to about four-to-one (4:1) by weight. This formulation capable of providing therapeutically effective bioavailability of guaifenesin for at least twelve hours after dosing in a human subject. The invention also relates to a modified release product which has two portions: a first portion having an immediate release formulation of guaifenesin and a second portion having a sustained release formulation of guaifenesin, wherein one or both portions has at least one additional drug ingredient. The modified release product has a maximum guaifenesin serum concentration equivalent to that of an immediate release guaifenesin tablet, and is capable of providing therapeutically effective bioavailability of guaifenesin for at least twelve hours after dosing in a human subject.
Owner:RB HEALTH US LLC
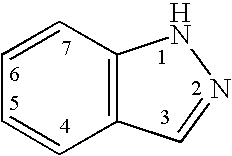
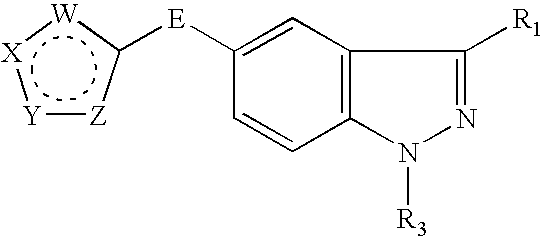
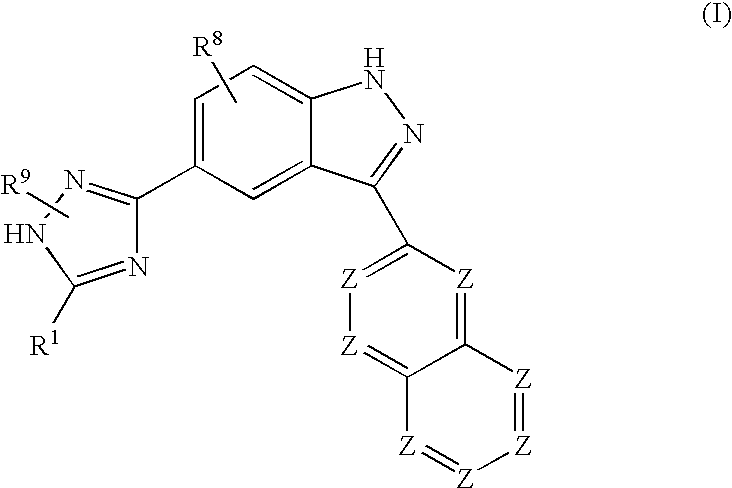
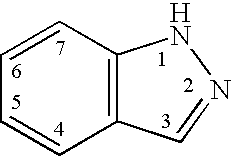
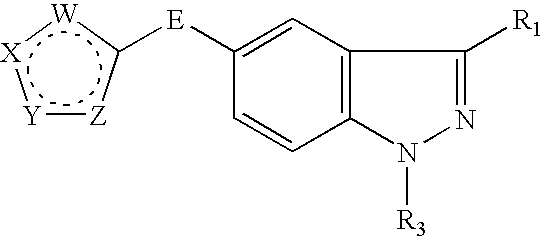
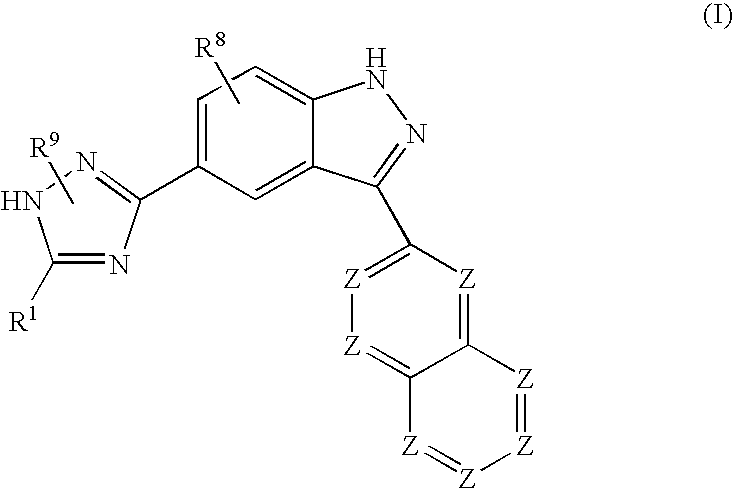
Indazole compounds and methods of use thereof
InactiveUS20060004043A1Modulating levelUseful in treatmentAntibacterial agentsBiocideAutoimmune conditionProtein kinase A signaling
This invention is directed to Indazole Compounds or pharmaceutically acceptable salts, solvates and hydrates thereof. The Indazole Compounds have utility in the treatment or prevention of a wide range of diseases and disorders that are responsive to the inhibition, modulation or regulation of kinases, such as inflammatory diseases, abnormal angiogenesis and diseases related thereto, cancer, atherosclerosis, a cardiovascular disease, a renal disease, an autoimmune condition, macular degeneration, disease-related wasting, an asbestos-related condition, pulmonary hypertension, diabetes, obesity, pain and others. Thus, methods of treating or preventing such diseases and disorders are also disclosed, as are pharmaceutical compositions comprising one or more of the Indazole Compounds. This invention is based, in part, upon the discovery of a novel class of 5-triazolyl substituted indazole molecules that have potent activity with respect to the modulation of protein kinases. Thus, the invention encompasses orally active molecules as well as parenterally active molecules which can be used at lower doses or serum concentrations for treating diseses or disorders associated with protein kinase signal transduction.
Owner:BHAGWAT SHRIPAD S +10
Sustained release formulations of guaifenesin and additional drug ingredients
InactiveUS6955821B2Increase in drug strengthSustained releaseEther/acetal active ingredientsOrganic non-active ingredientsSerum concentrationImmediate release
The invention relates to a novel pharmaceutical sustained release formulation of guaifenesin and at least one additional drug ingredient. The formulation may comprise a hydrophilic polymer, preferably a hydroxypropyl methylcellulose, and a water-insoluble polymer, preferably an acrylic resin, in a ratio range of about one-to-one (1:1) to about nine-to-one (9:1), more preferably a range of about three-to-two (3:2) to about six-to-one (6:1), and most preferably in a range of about two-to-one (2:1) to about four-to-one (4:1) by weight. This formulation capable of providing therapeutically effective bioavailability of guaifenesin for at least twelve hours after dosing in a human subject. The invention also relates to a modified release product which has two portions: a first portion having an immediate release formulation of guaifenesin and a second portion having a sustained release formulation of guaifenesin, wherein one or both portions has at least one additional drug ingredient. The modified release product has a maximum guaifenesin serum concentration equivalent to that of an immediate release guaifenesin tablet, and is capable of providing therapeutically effective bioavailability of guaifenesin for at least twelve hours after dosing in a human subject.
Owner:RB HEALTH US LLC
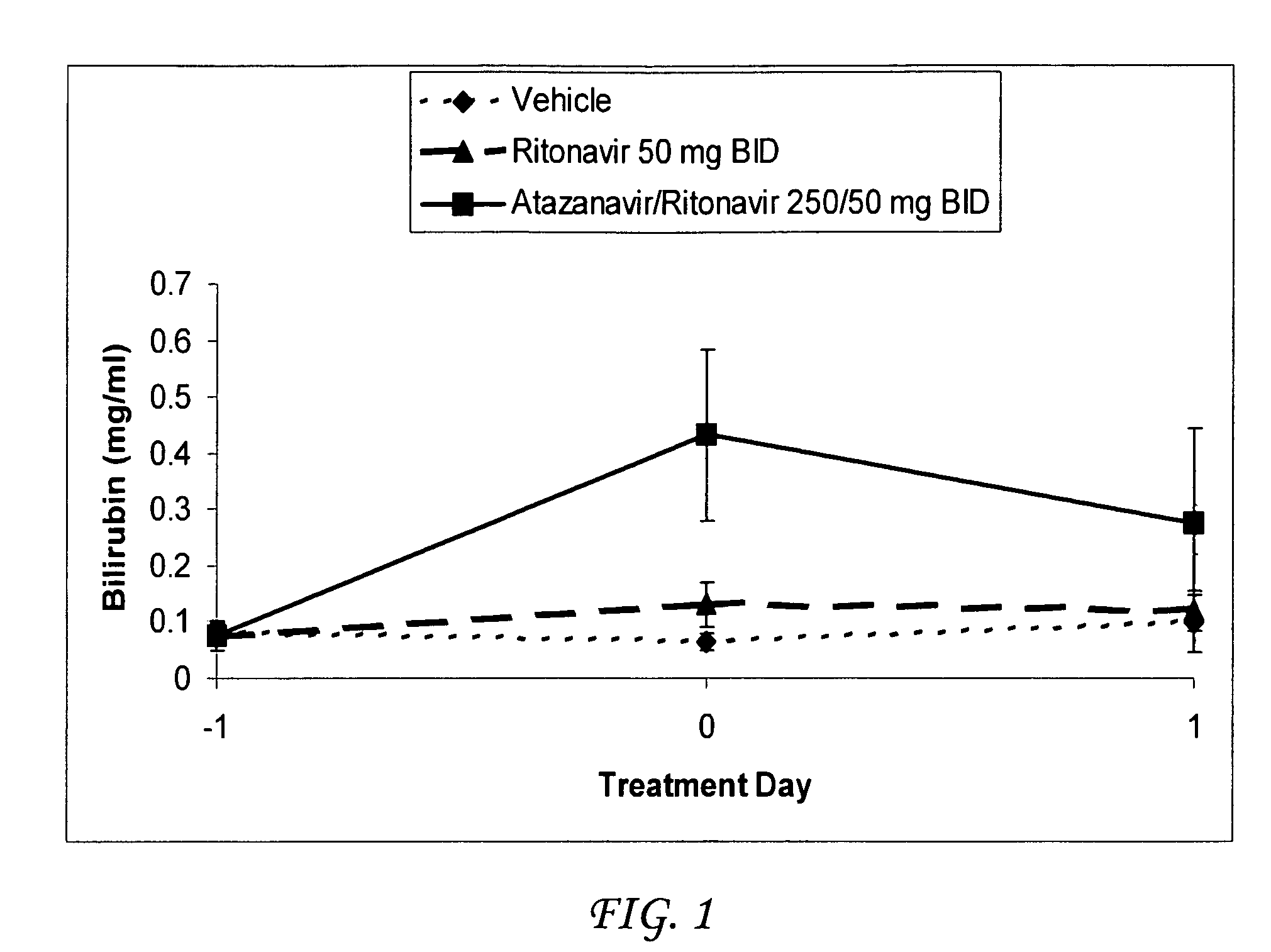
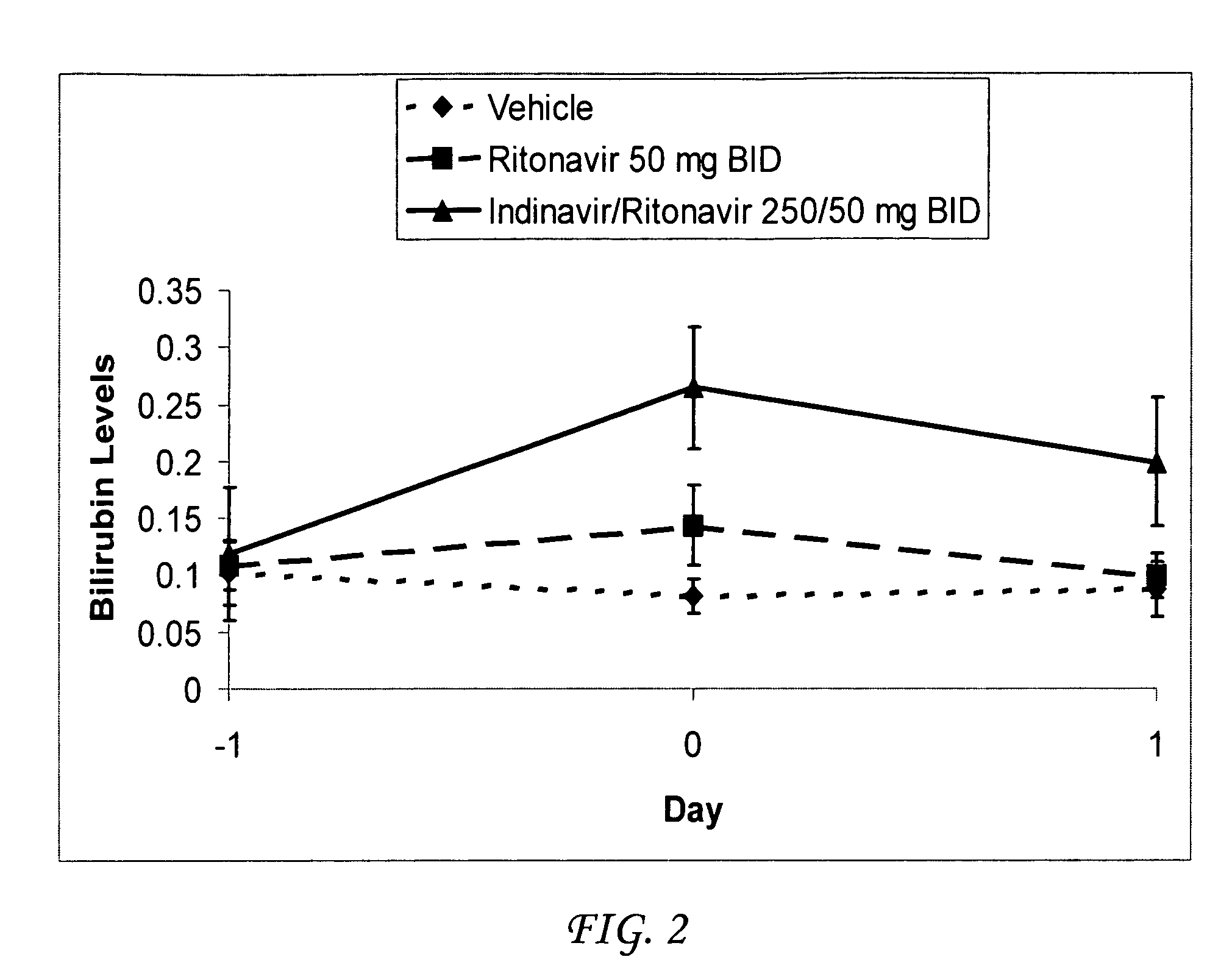
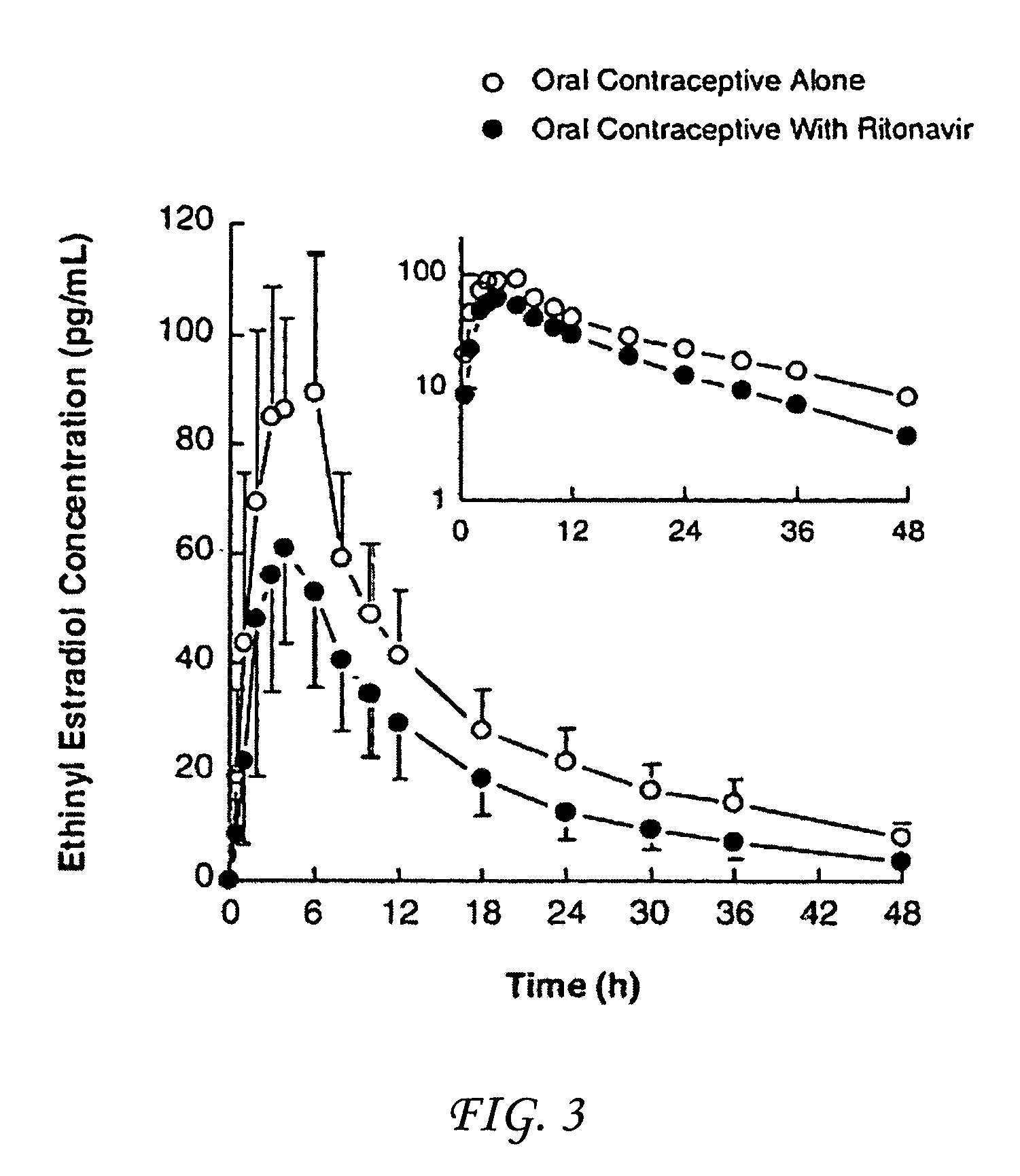
Method for treating a disease, disorder or adverse effect caused by an elevated serum concentration of an UGT1A1 substrate
The present invention is directed to a method for inducing UGT1A1 isoform expression for treatment of a disease, disorder or adverse effect caused by an elevated serum concentration of an UGT1A1 substrate comprising the step of administering to a subject an effective amount of ritonavir. In particular, the present invention is directed to a method of treating unconjugated hyperbilirubinemia by UGT1A1 induction comprising the step of administering to a subject an effective amount of ritonavir.
Owner:ABBVIE INC
Complete medium with low serum concentration for cultivating mesenchymal stem cells and method for cultivating mesenchymal stem cells using same
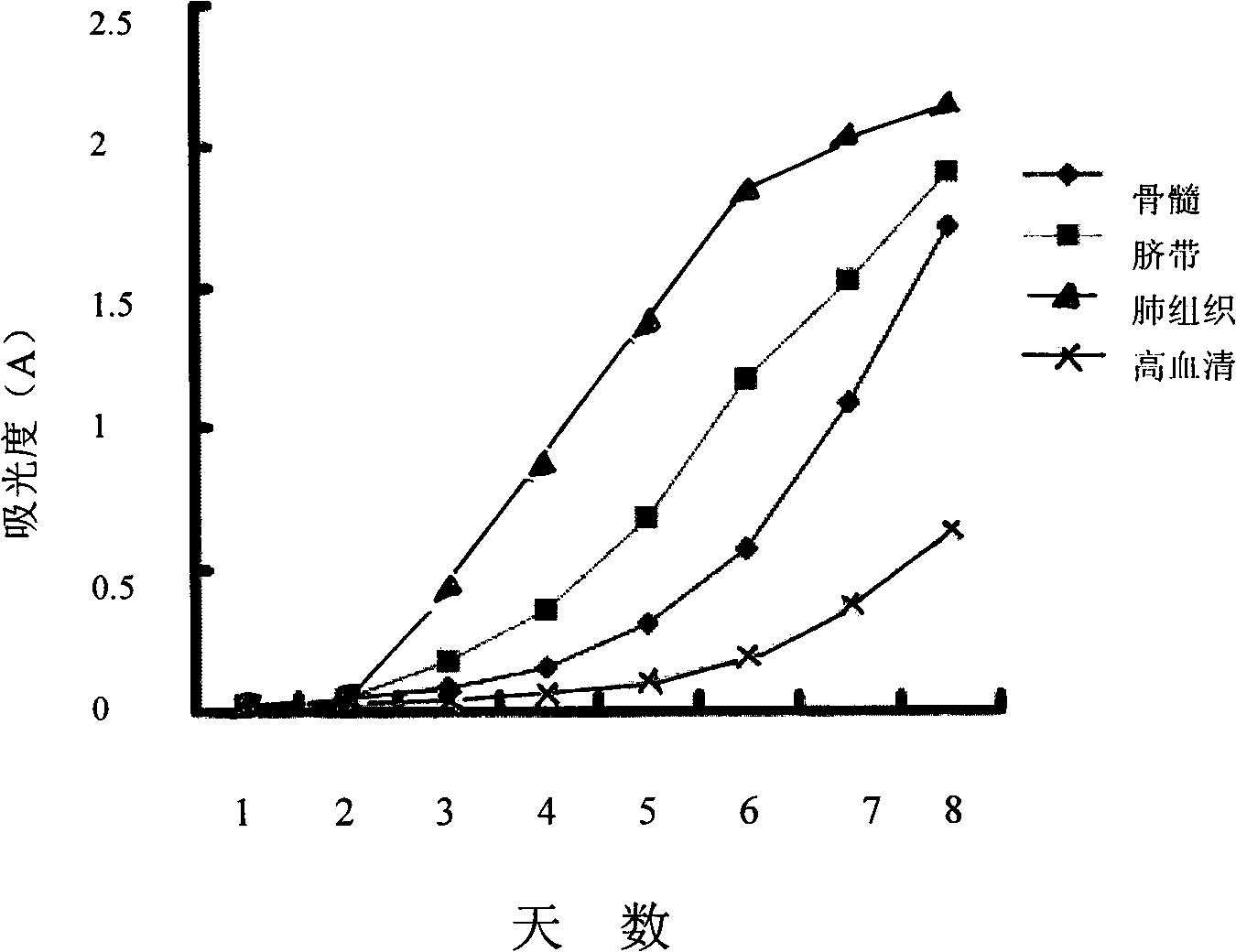
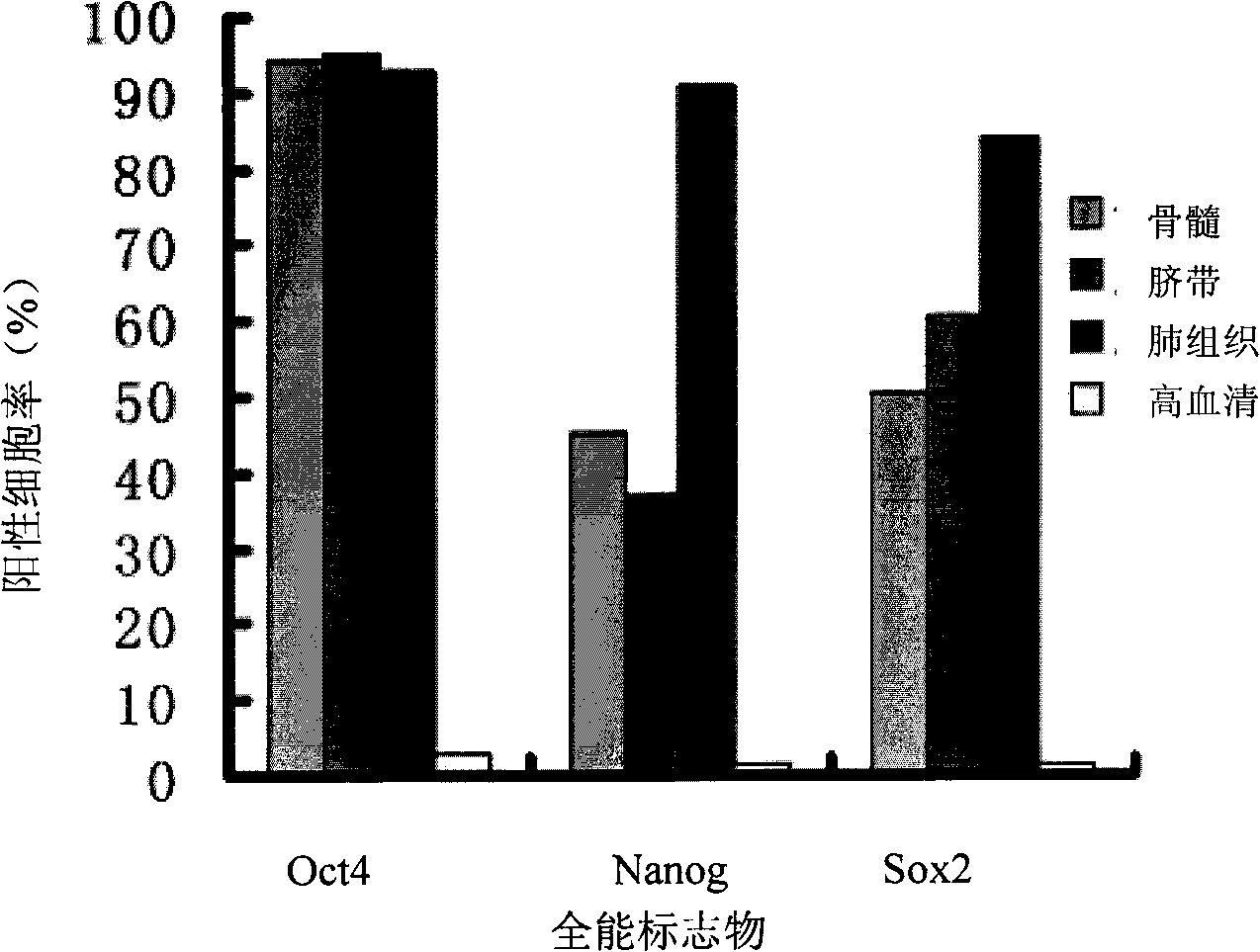
The invention discloses a complete medium with low serum concentration for cultivating mesenchymal stem cells and a method for cultivating the mesenchymal stem cells using same. The complete medium comprises a cell basic medium, fetal calf serum with final concentration of 1-100 mul / ml, an epidermal growth factor with final concentration of 1-100 ng / ml, and a basic fibroblast growth factor with final concentration of 1-100 ng / ml. The complete medium with low serum concentration successfully reaches equal or even better function of promoting cell proliferation than a culture reagent with high serum concentration. The cultured cells have the typical biological characteristics of mesenchymal stem cells, and can also express an omnipotent mark of the embryonic stem cell and high express the idiosyncratic mark of the neuron under the condition of in vitro inducement. And the difference between the cell batches is little, the cost is low and the security is good. Compared with the prior cultivating method, the method has advantages of simple operation, low probability of pollution and high success ratio of cultivating cells.
Owner:INST OF HEMATOLOGY & BLOOD HOSPITAL CHINESE ACAD OF MEDICAL SCI +1
Recombinantly expressed insulin polypeptides and uses thereof
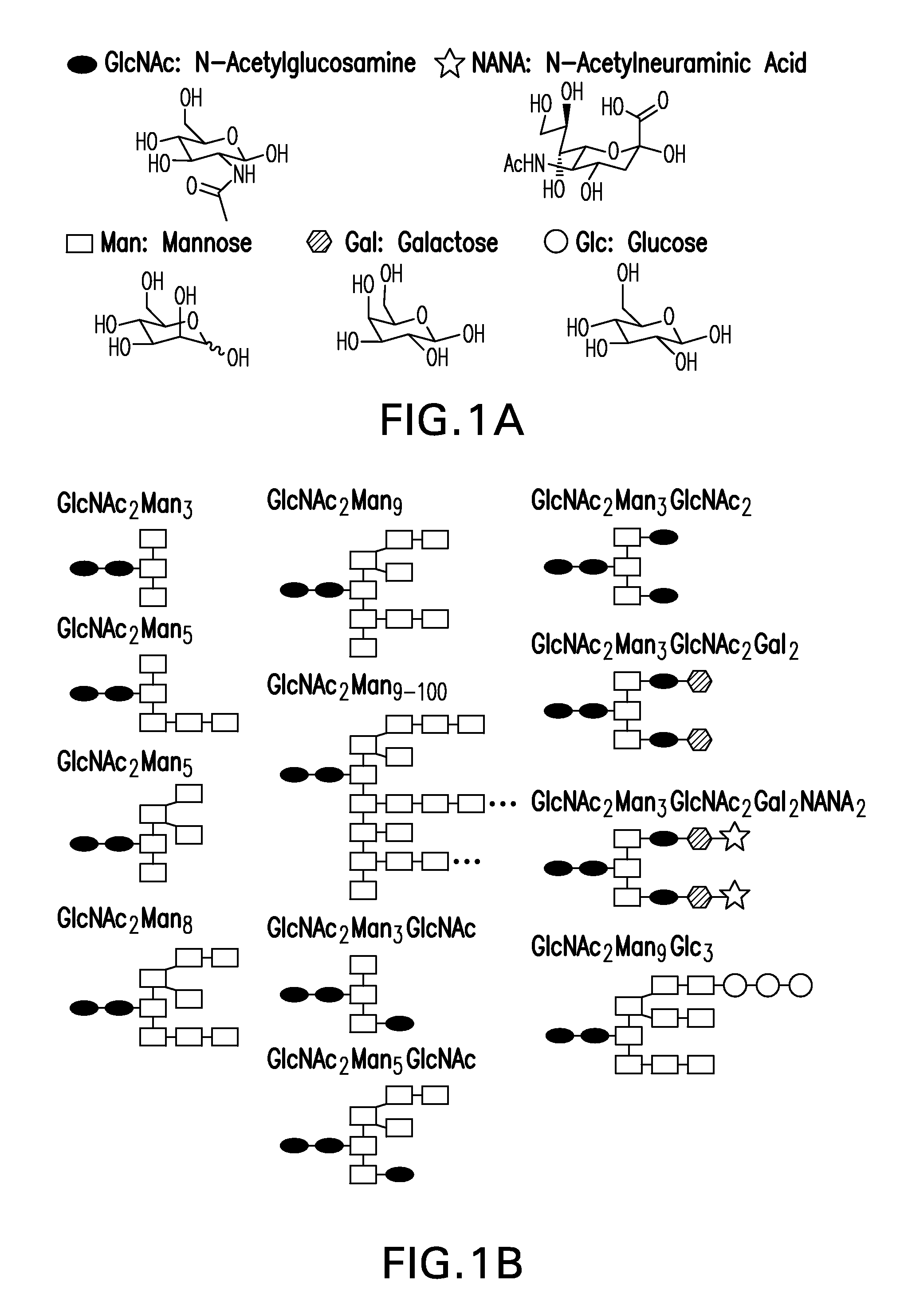
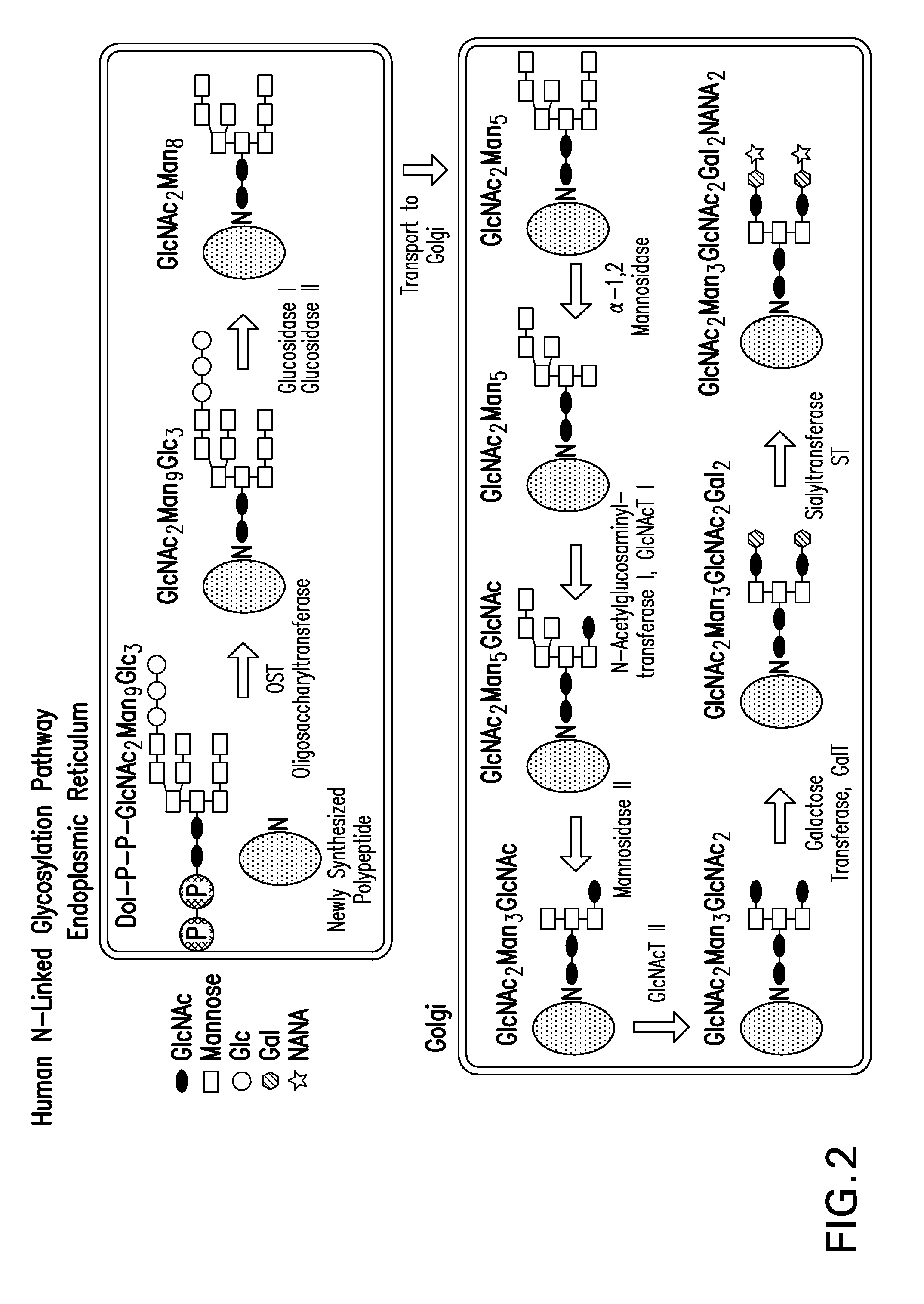
The present disclosure provides recombinantly expressed insulin polypeptides that comprise an N-linked glycan motif. The N-linked glycan motif is not present in wild-type insulins and enables the recombinant expression of glycosylated insulin polypeptides (e.g., in yeast cells). Based on results obtained with synthetic glycosylated insulin conjugates we predict that when these recombinant glycosylated insulin polypeptides are administered to a mammal, at least one pharmacokinetic or pharmacodynamic property of the glycosylated insulin polypeptide will be sensitive to serum concentrations of glucose (or an exogenous saccharide such as alpha-methyl mannose). Exemplary insulin polypeptides, polynucleotides encoding these insulin polypeptides, glycosylated insulin polypeptides, pharmaceutical formulations and sustained release formulations are provided in addition to methods of use and preparation.
Owner:SMARTCELLS
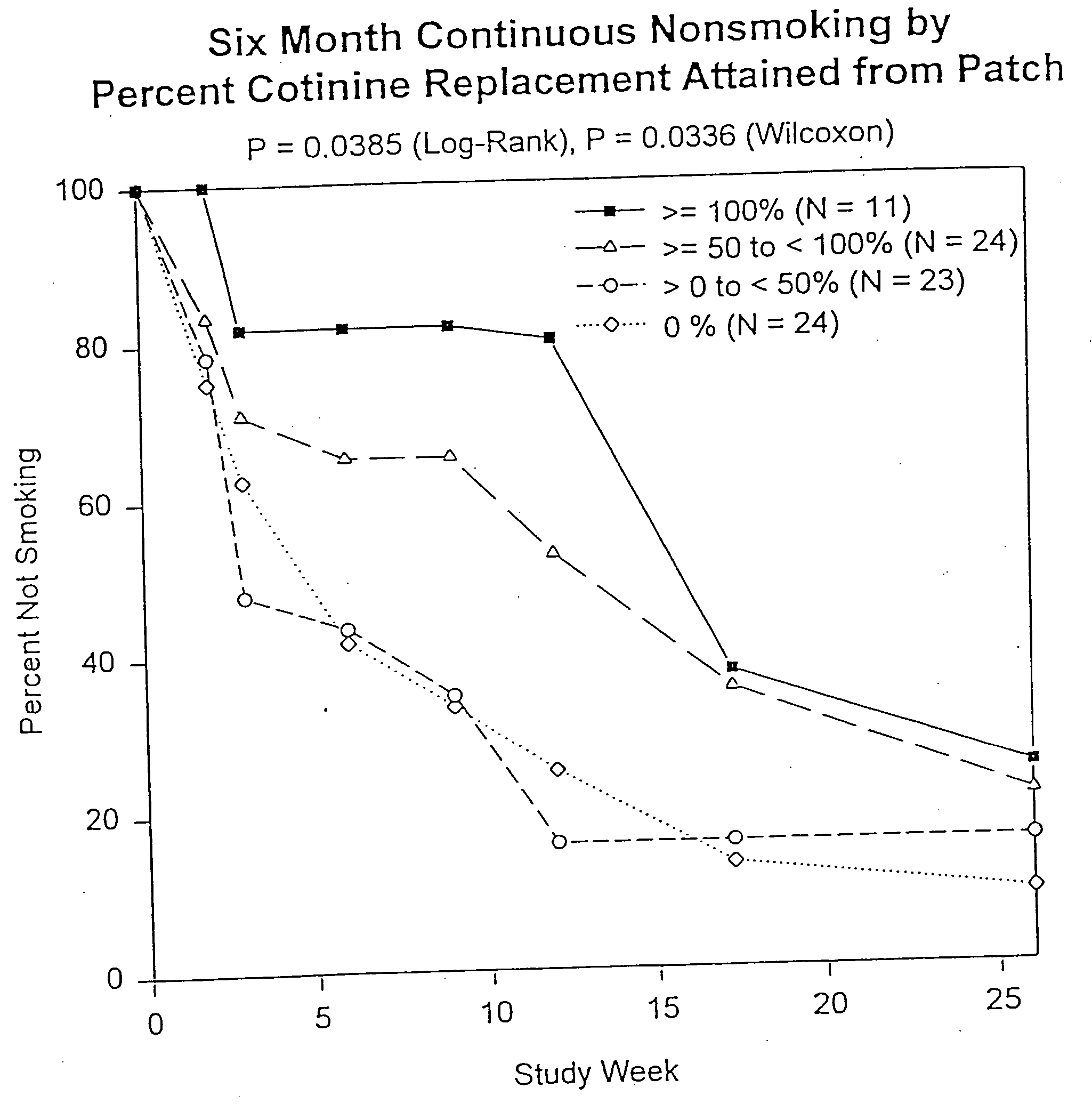
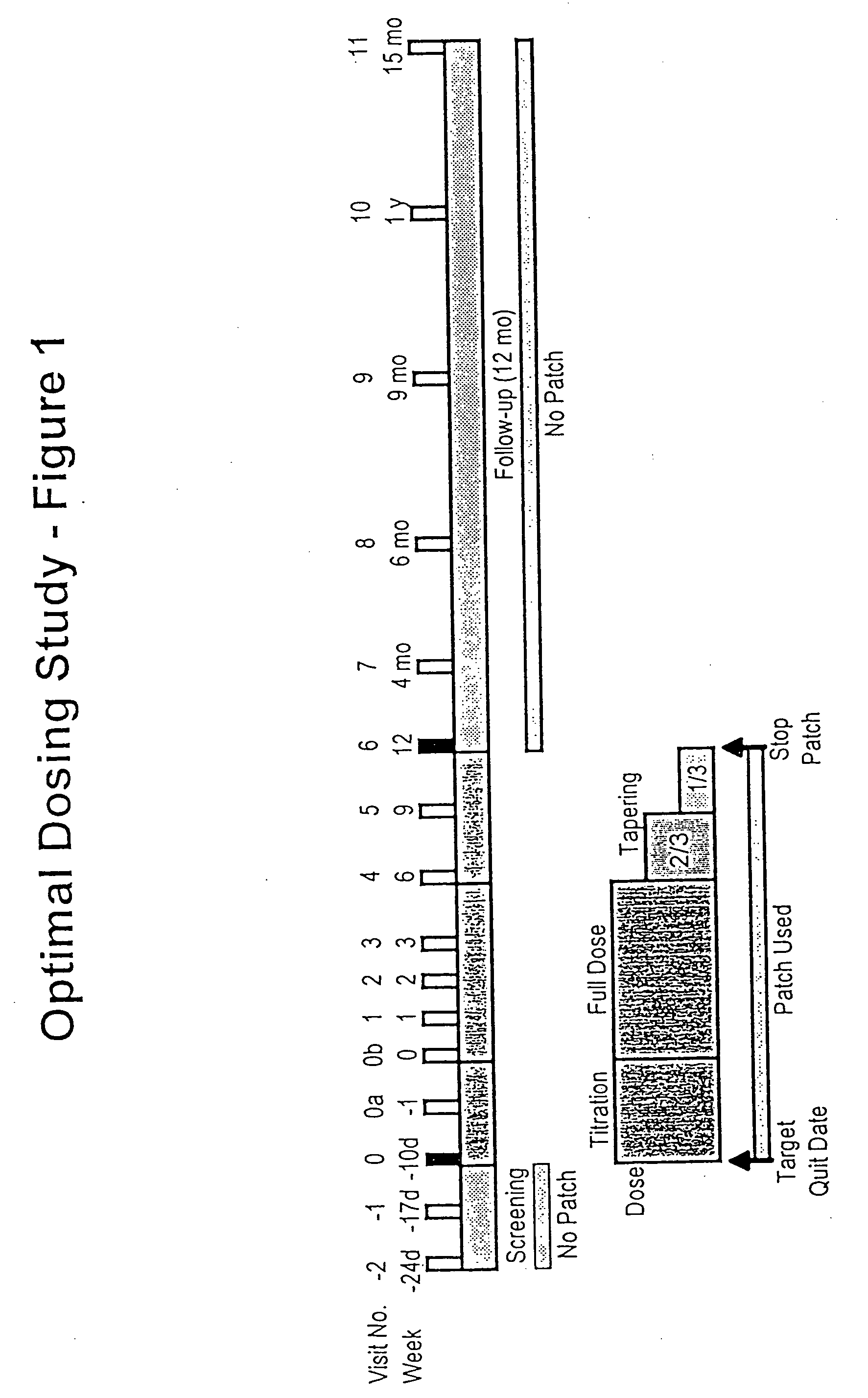
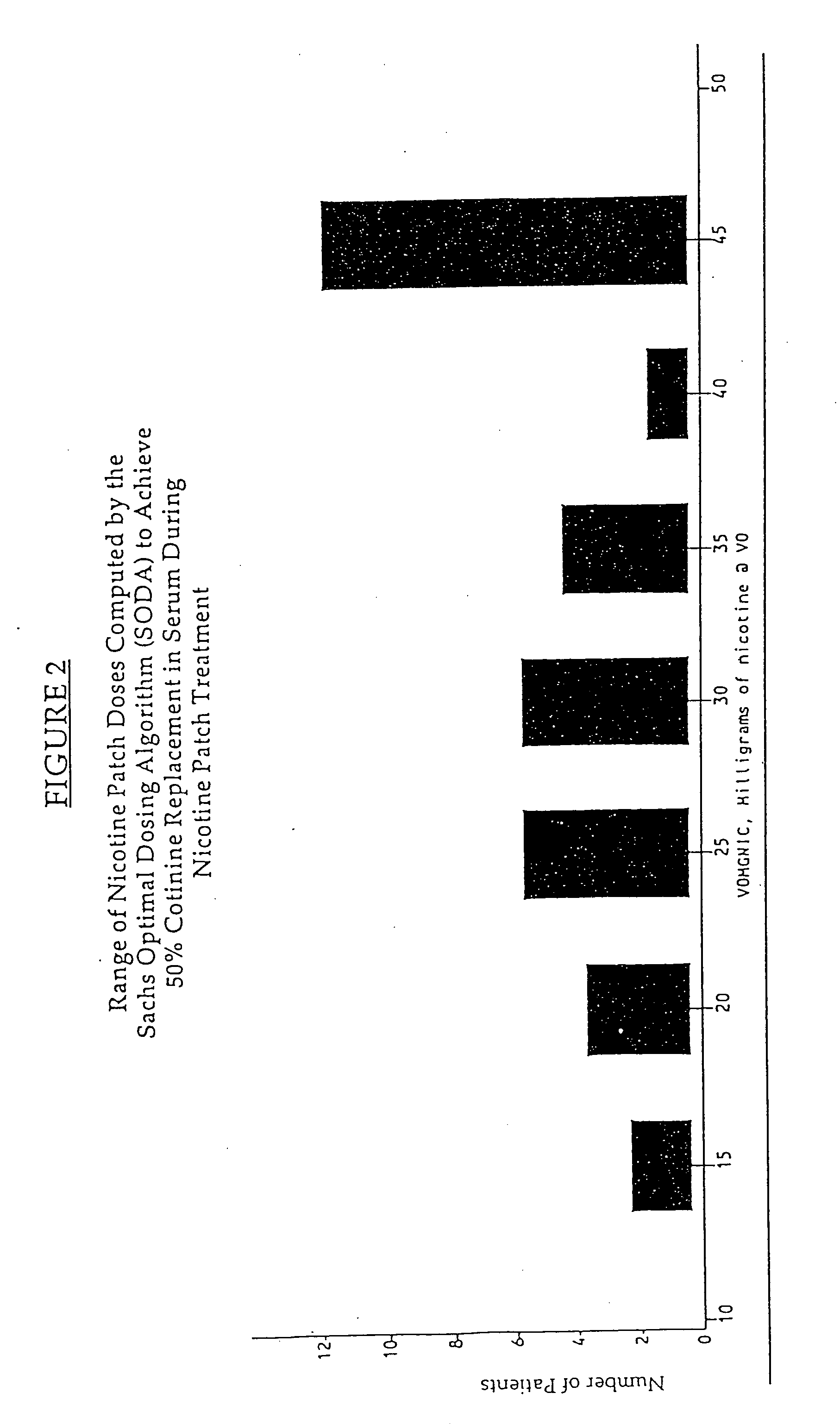
Methods for nicotine replacement dosage determination
InactiveUS20040006113A1Increased riskQuickly reachCompounds screening/testingBiocideNicotine replacementsSerum concentration
Owner:SACHS DAVID P L
High-strength testosterone undecanoate compositions
ActiveUS9034858B2Improve performanceReduce loadOrganic active ingredientsCapsule deliveryOral medicationSerum concentration
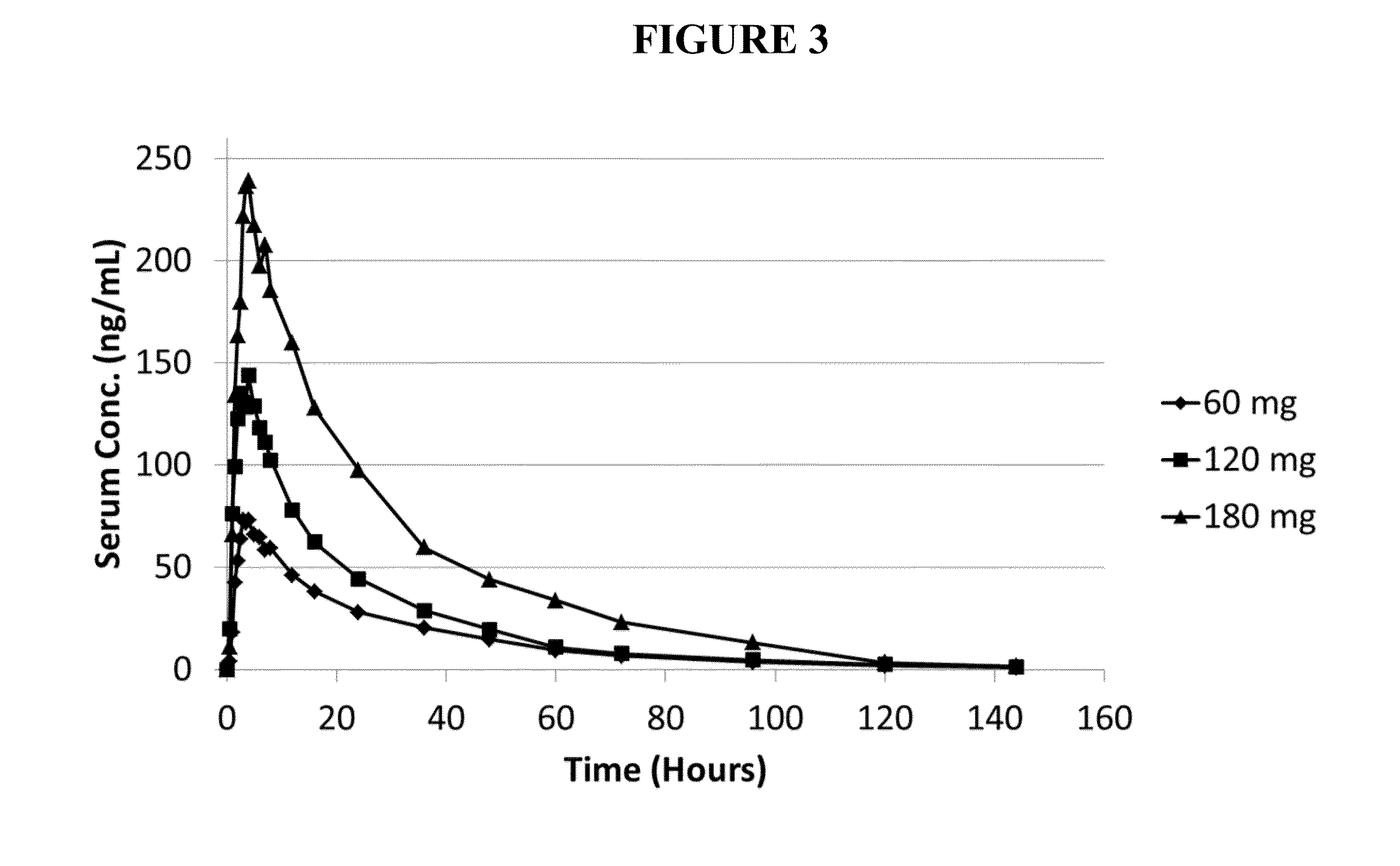
The present disclosure is drawn to pharmaceutical compositions and oral dosage capsules containing testosterone undecanoate, as well as related methods. The capsule includes a capsule shell and a capsule fill. The capsule fill can include a solubilizer and about 14 wt % to about 35 wt % testosterone undecanoate based on the total capsule fill. The oral dosage capsule is such that when a single oral administration to a male subject of one or more capsules with a total testosterone undecanoate daily dose of about 350 mg to about 650 mg it provides a ratio of serum testosterone Cmax to serum testosterone Cave of about 2.7 or less. In yet another embodiment, a method for providing a serum concentration of testosterone within a target serum testosterone concentration Cave range for a male subject is provided.
Owner:LIPOCINE
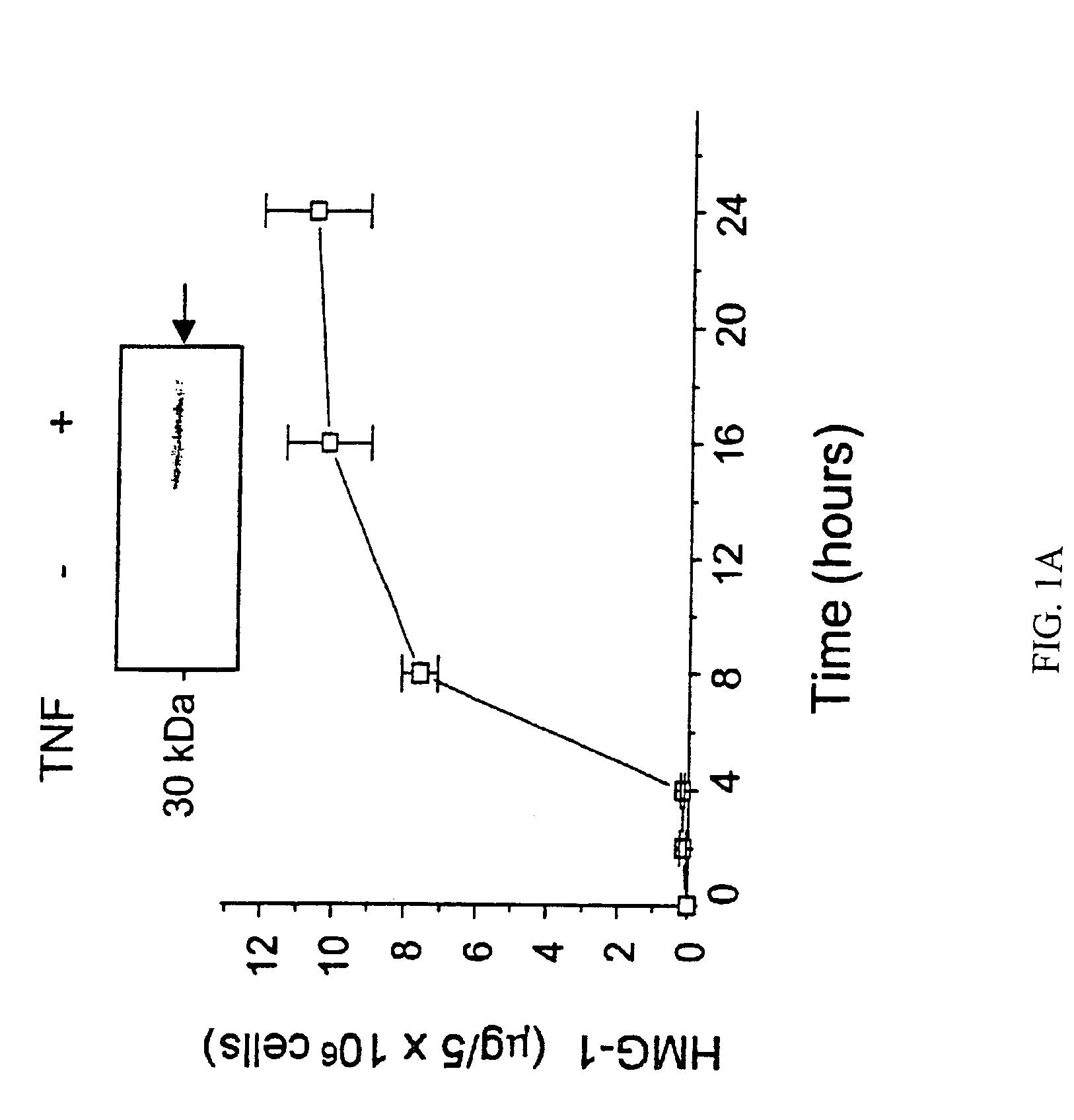
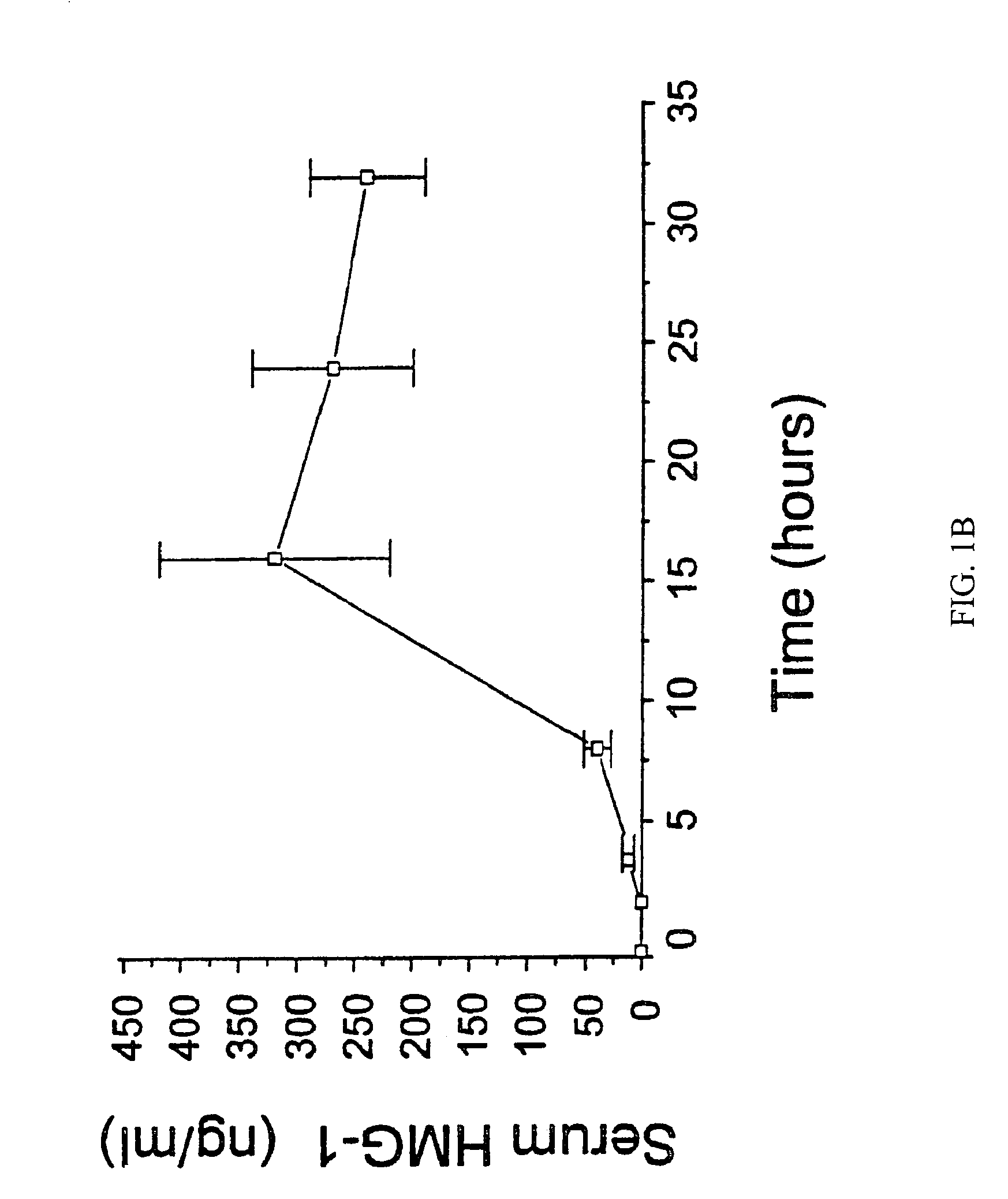
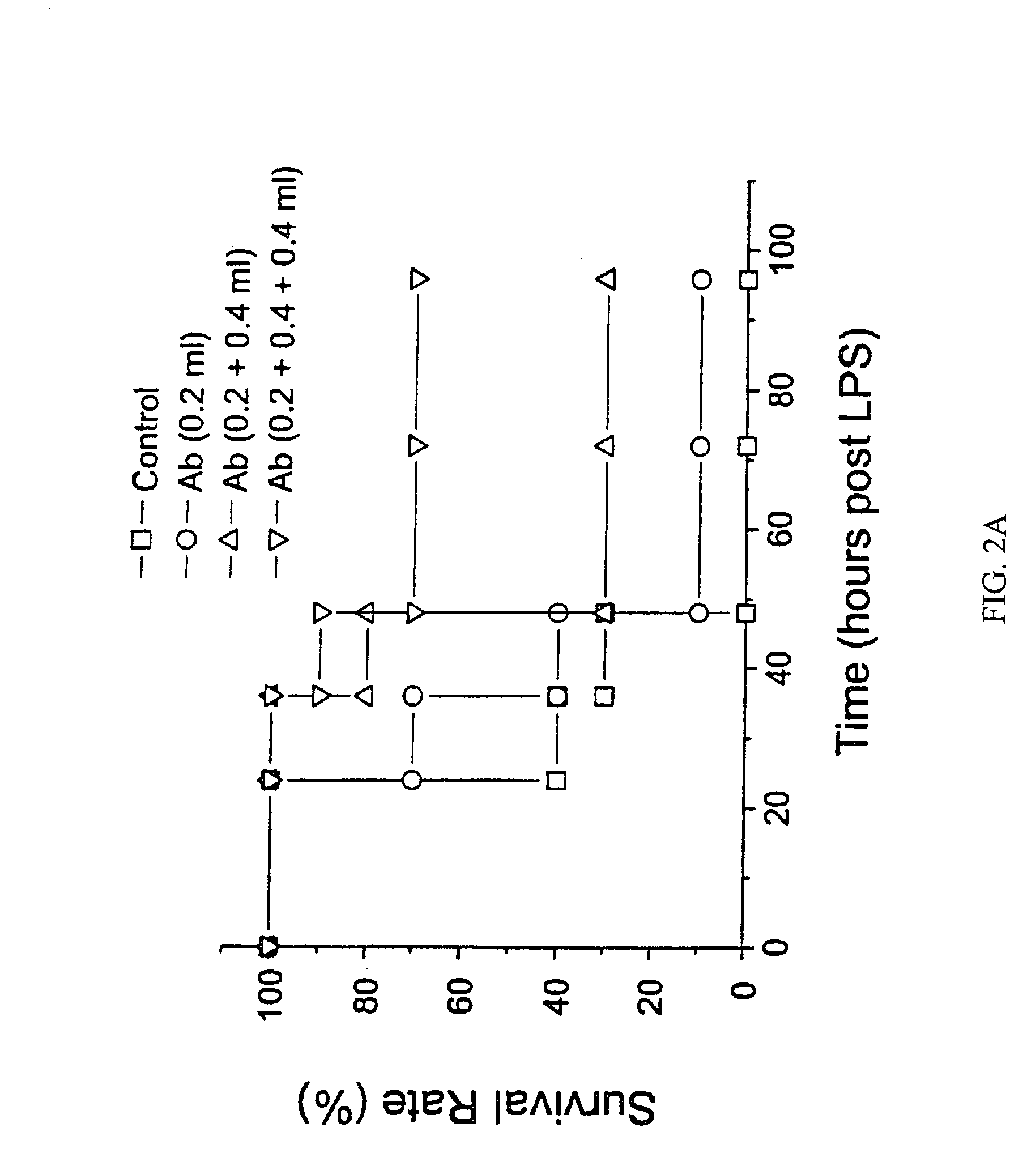
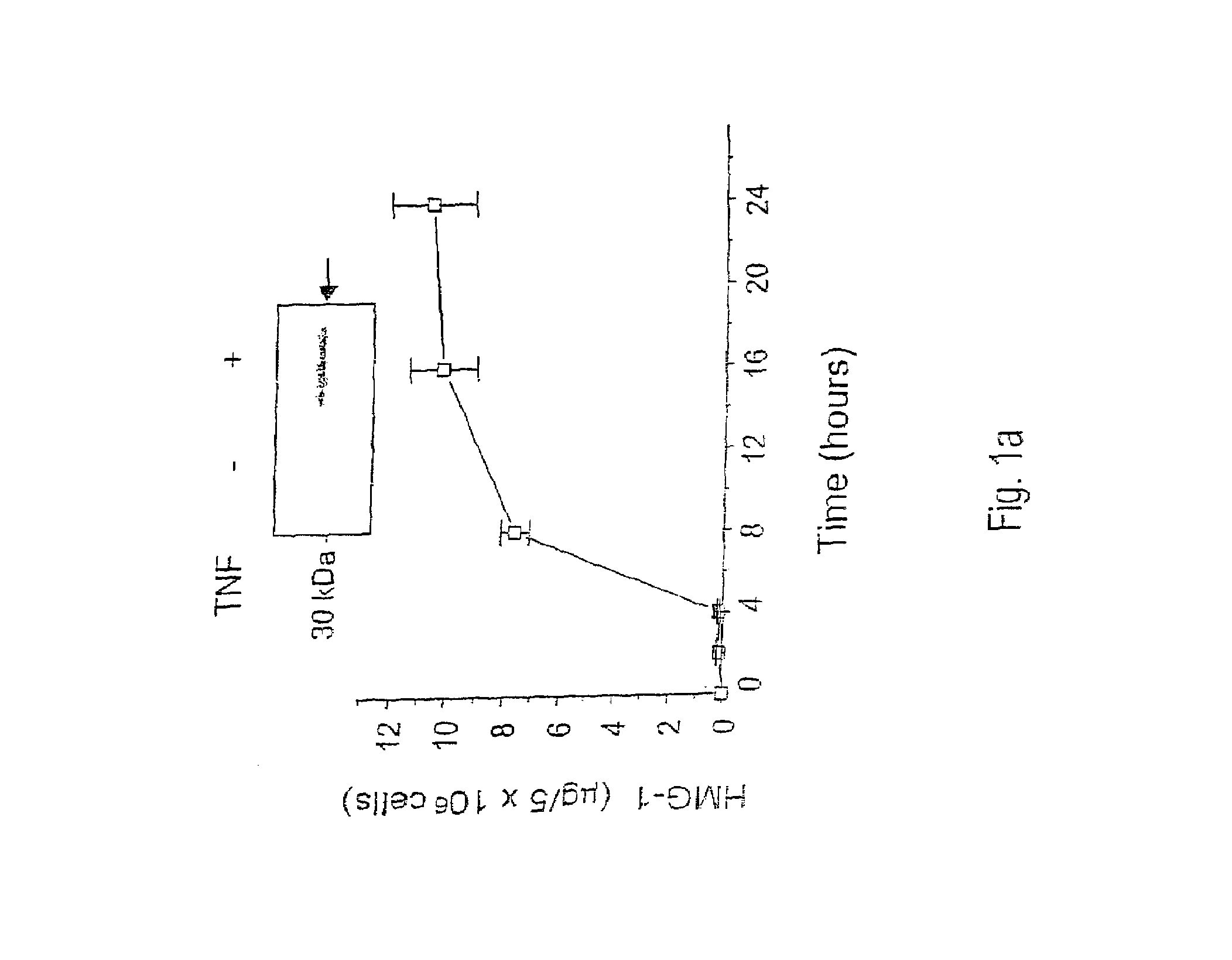
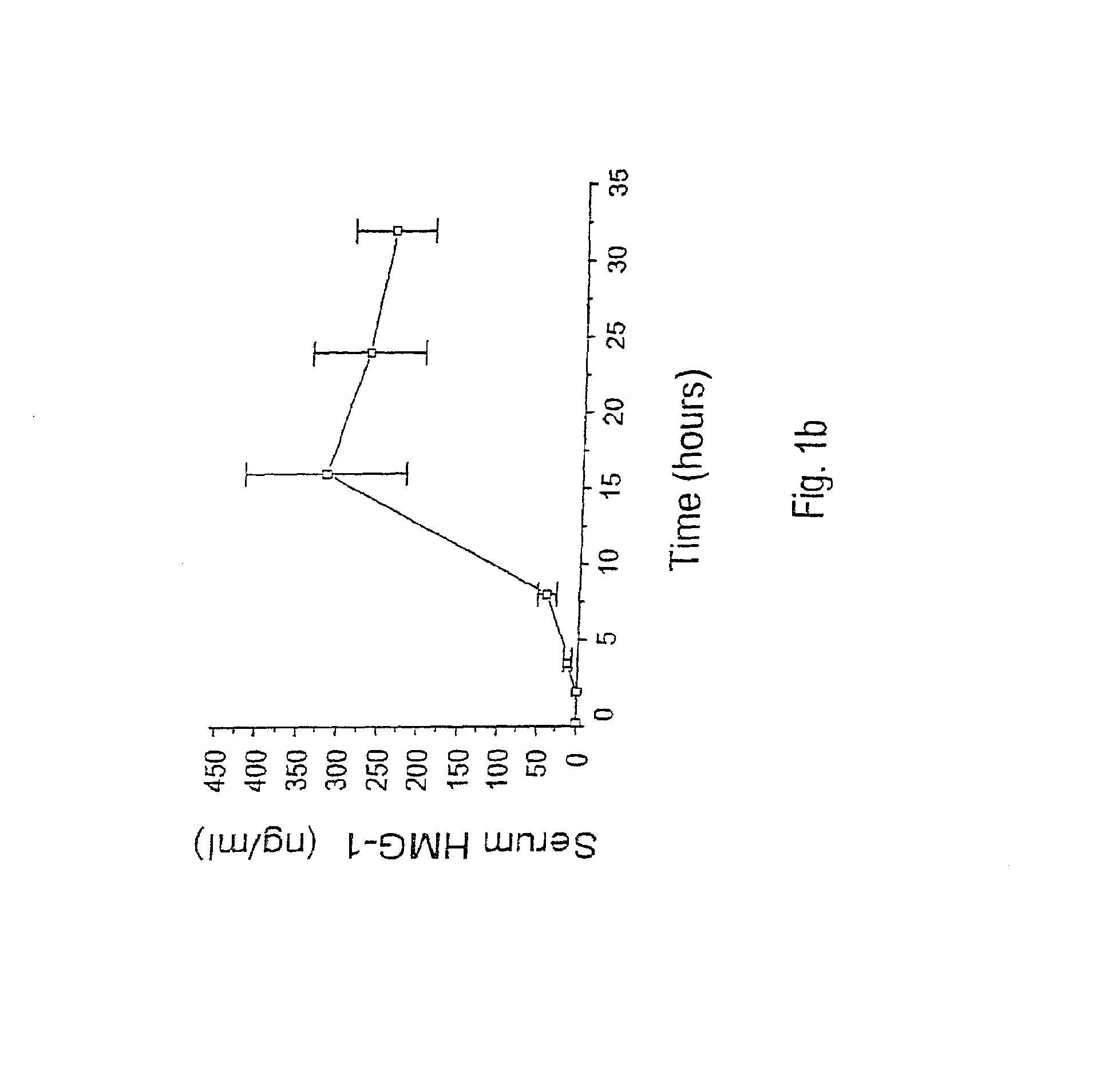
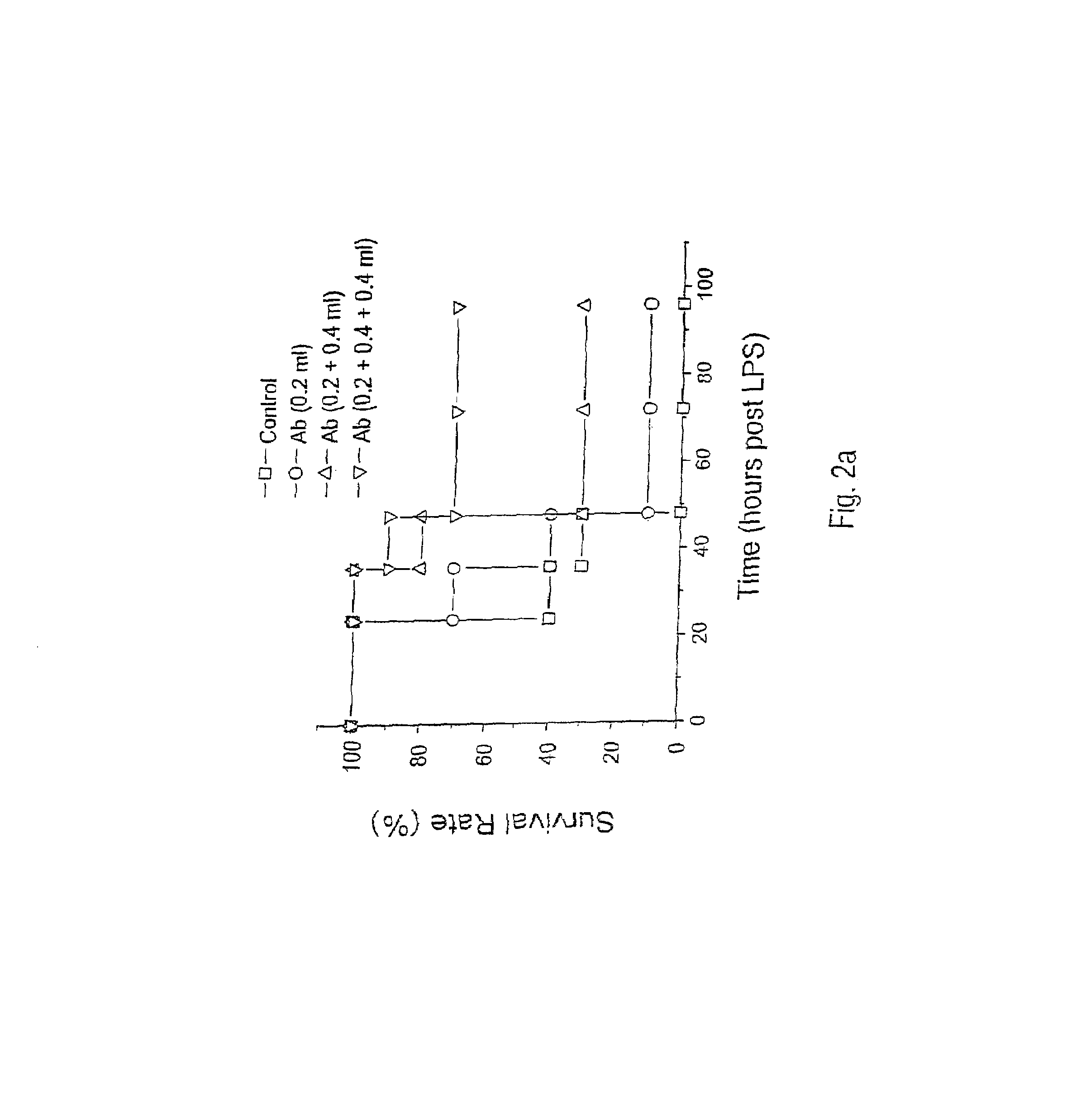
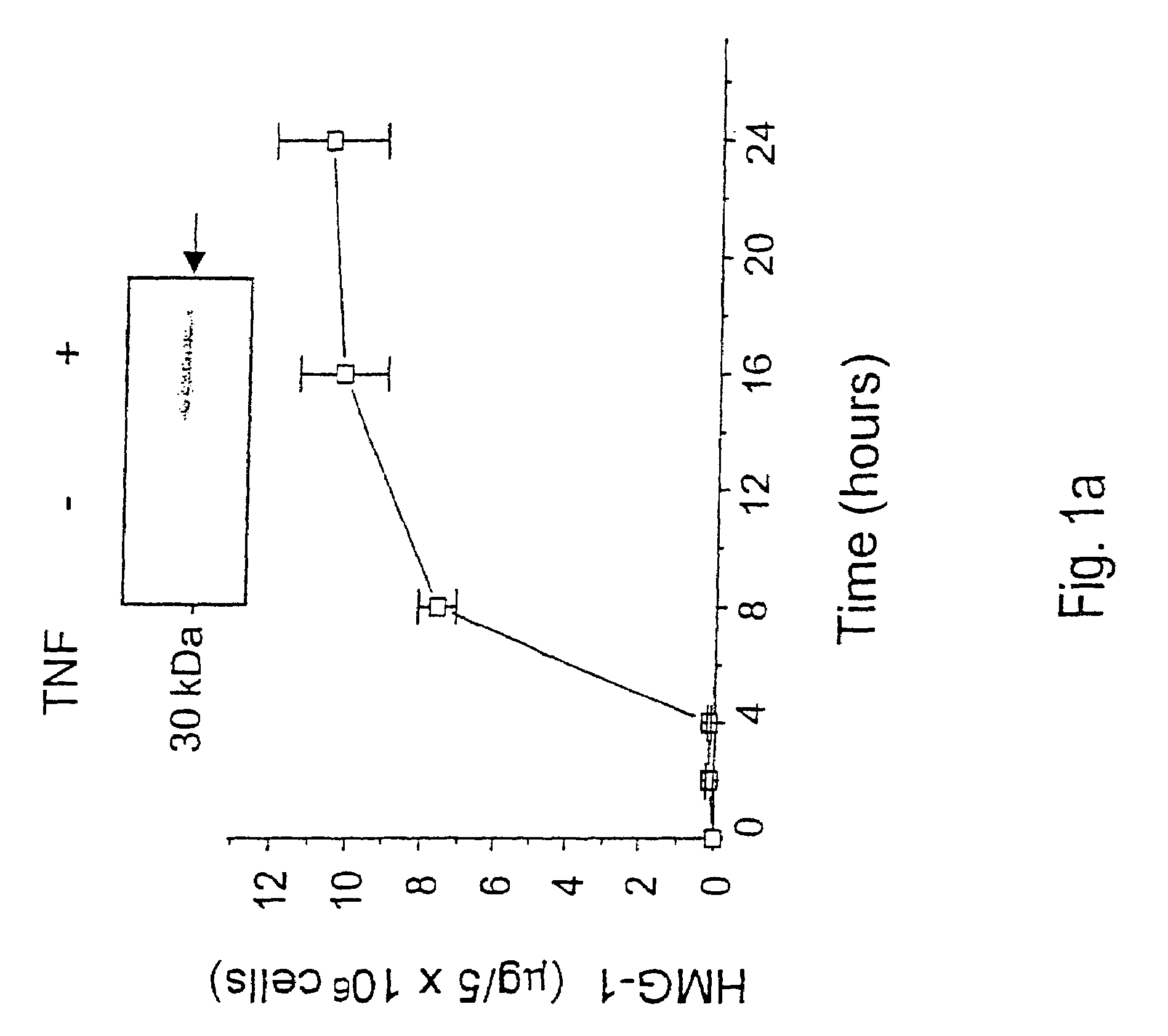
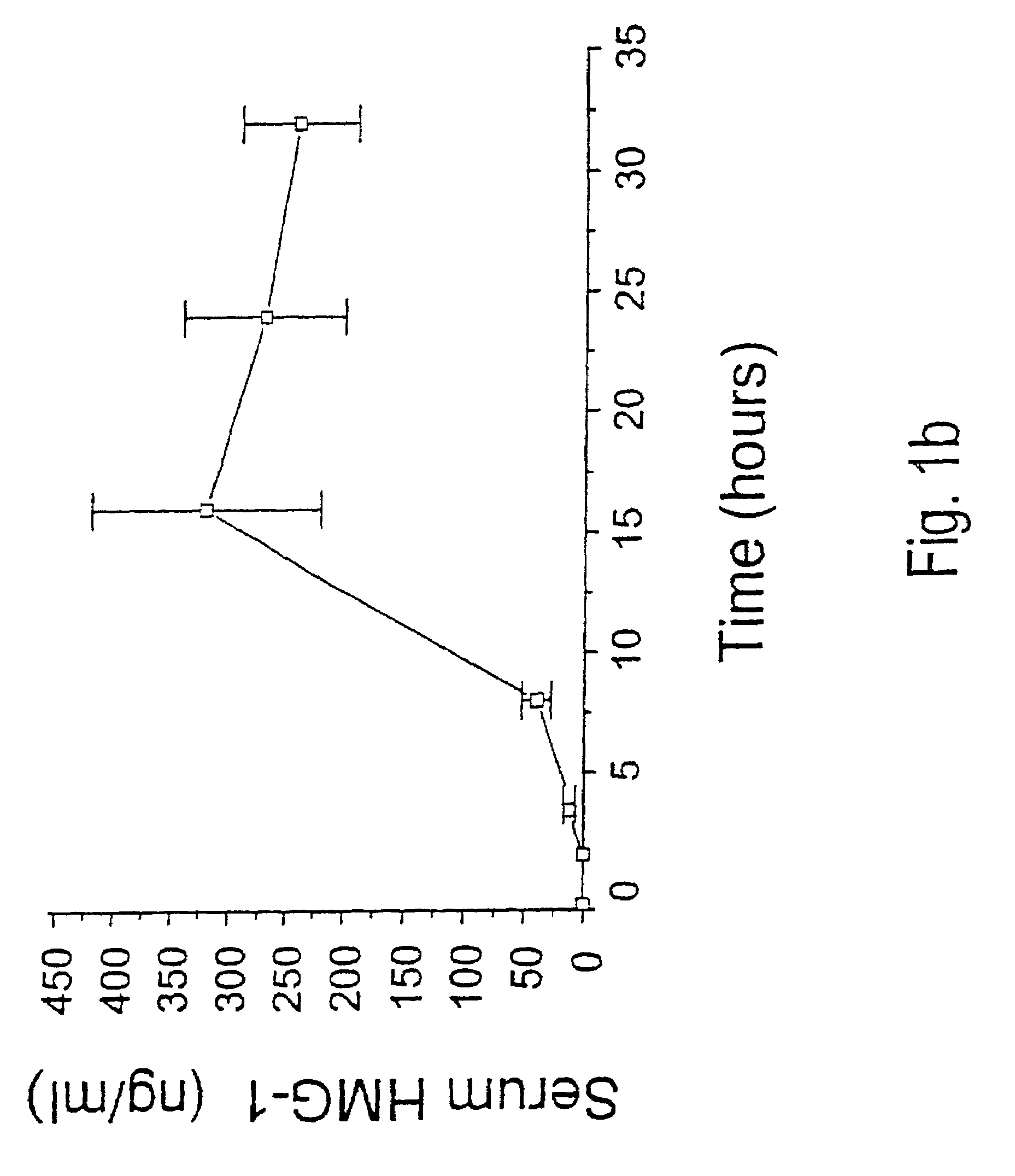
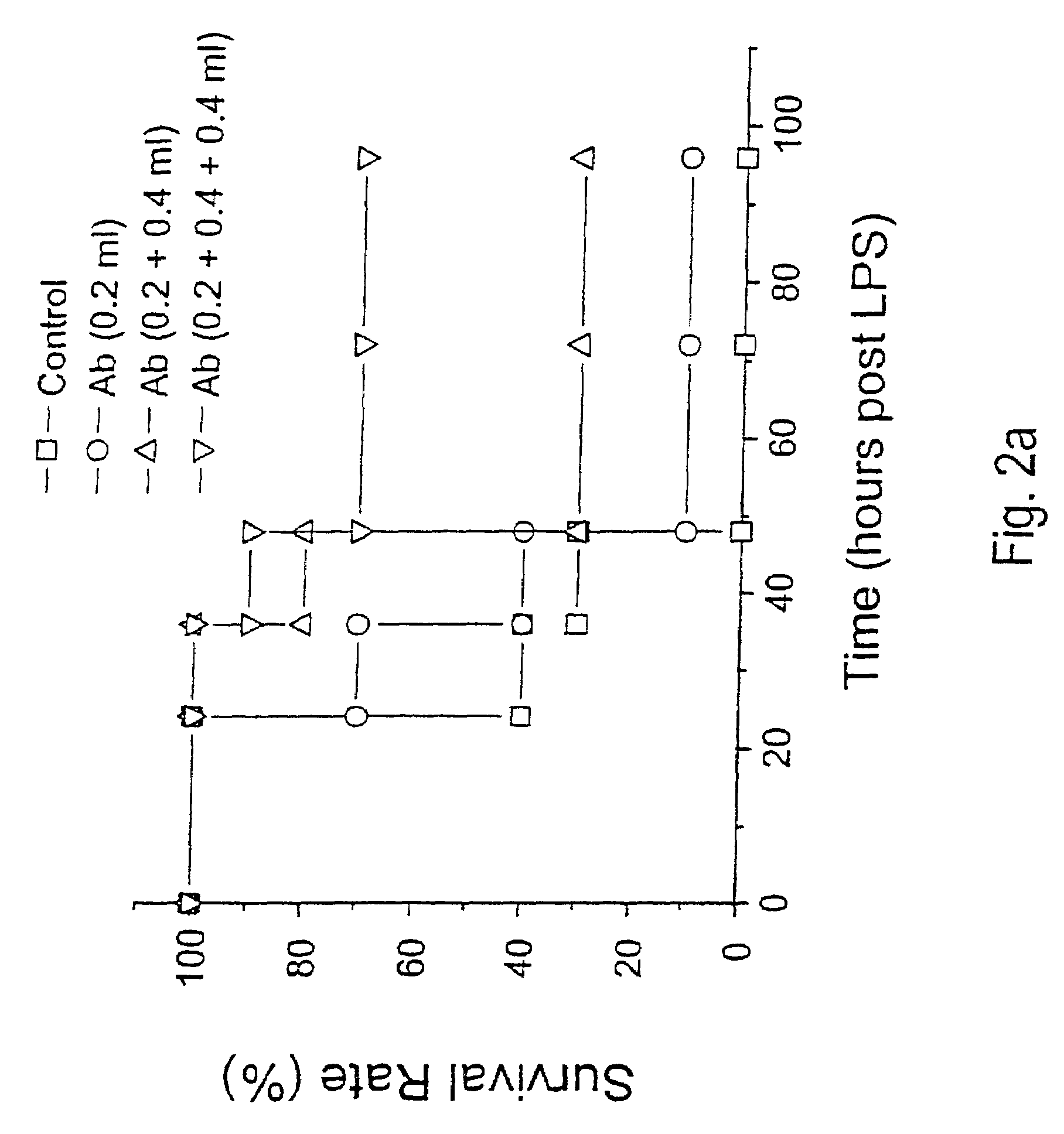
Antagonists of HMG1 for treating inflammatory conditions
Owner:THE FEINSTEIN INST FOR MEDICAL RES
Antagonists of HMG1 for treating inflammatory conditions
There is disclosed a pharmaceutical composition and method for treating sepsis, including septic shock and ARDS (acute respiratory distress syndrome), comprising administering an effective amount of a HMG1 antagonist. There is further disclosed a diagnostic method for monitoring the severity or potential lethality of sepsis or septic shock, comprising measuring the serum concentration of HMG1 in a patient exhibiting or at risk or exhibit sepsis or septic shock symptoms. Lastly, there is disclosed a pharmaceutical composition and method for effecting weight loss or treating obesity, comprising administering an effective amount of HMG1 or a therapeutically active HMG1 fragment.
Owner:THE FEINSTEIN INST FOR MEDICAL RES
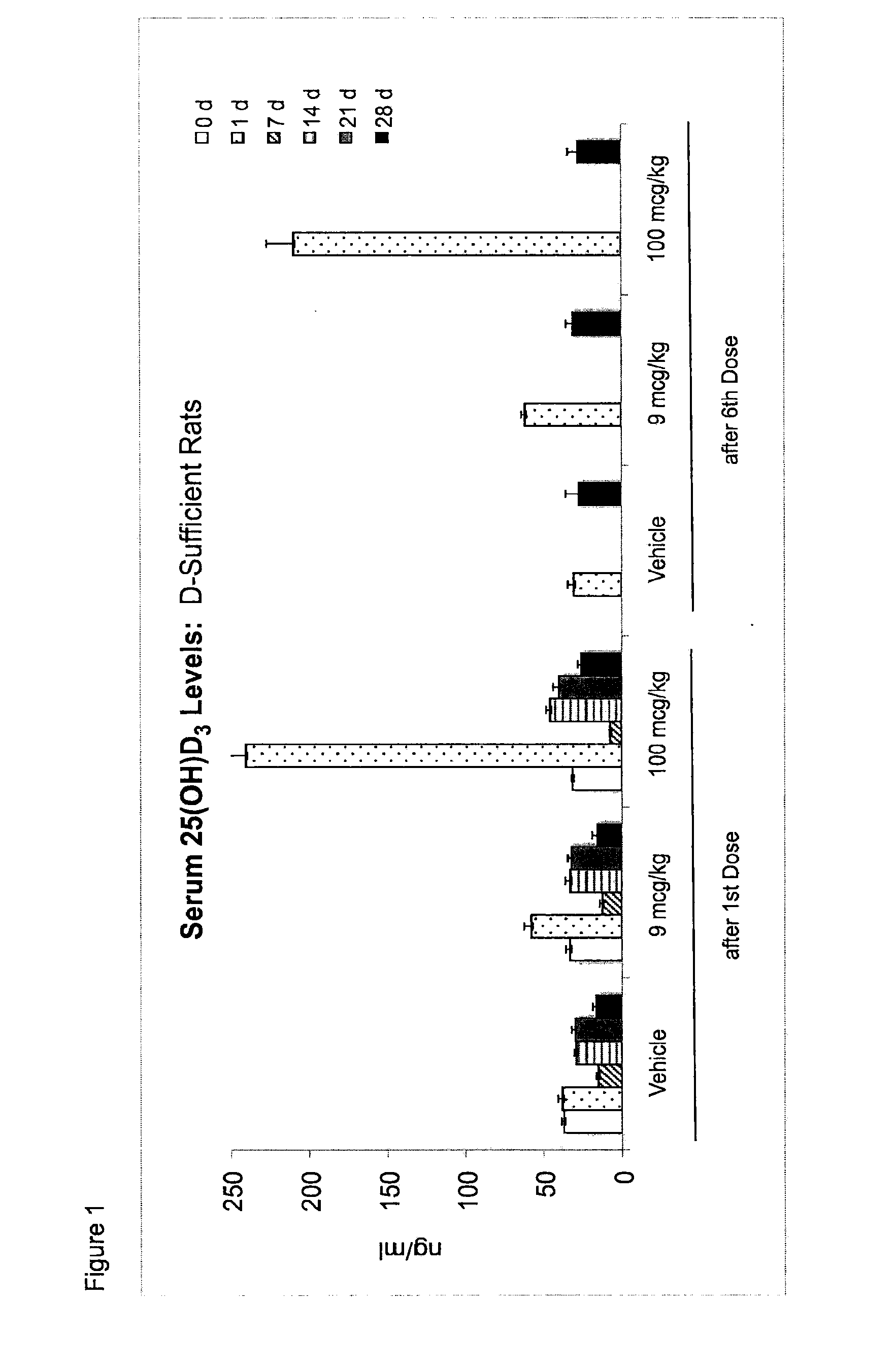
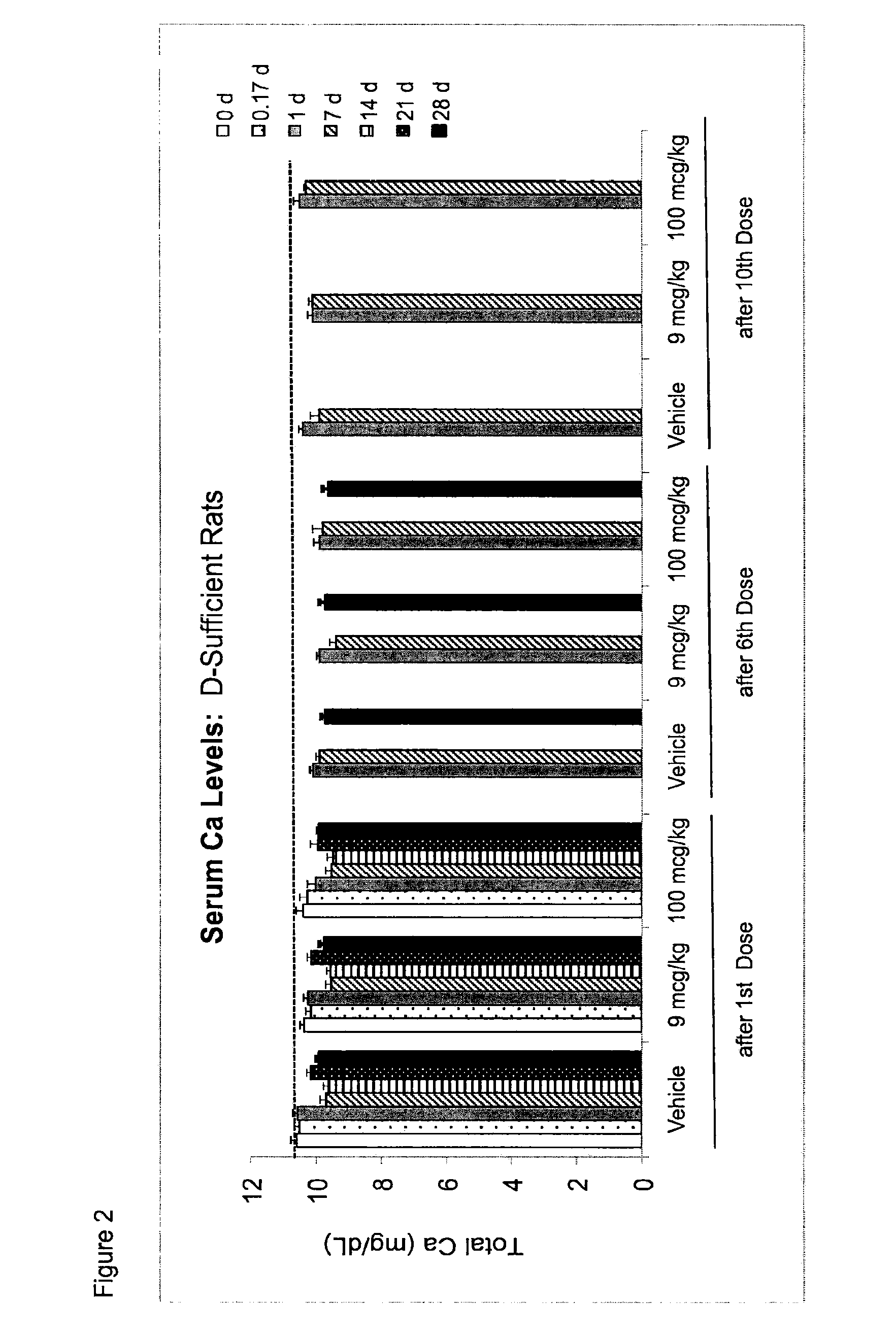
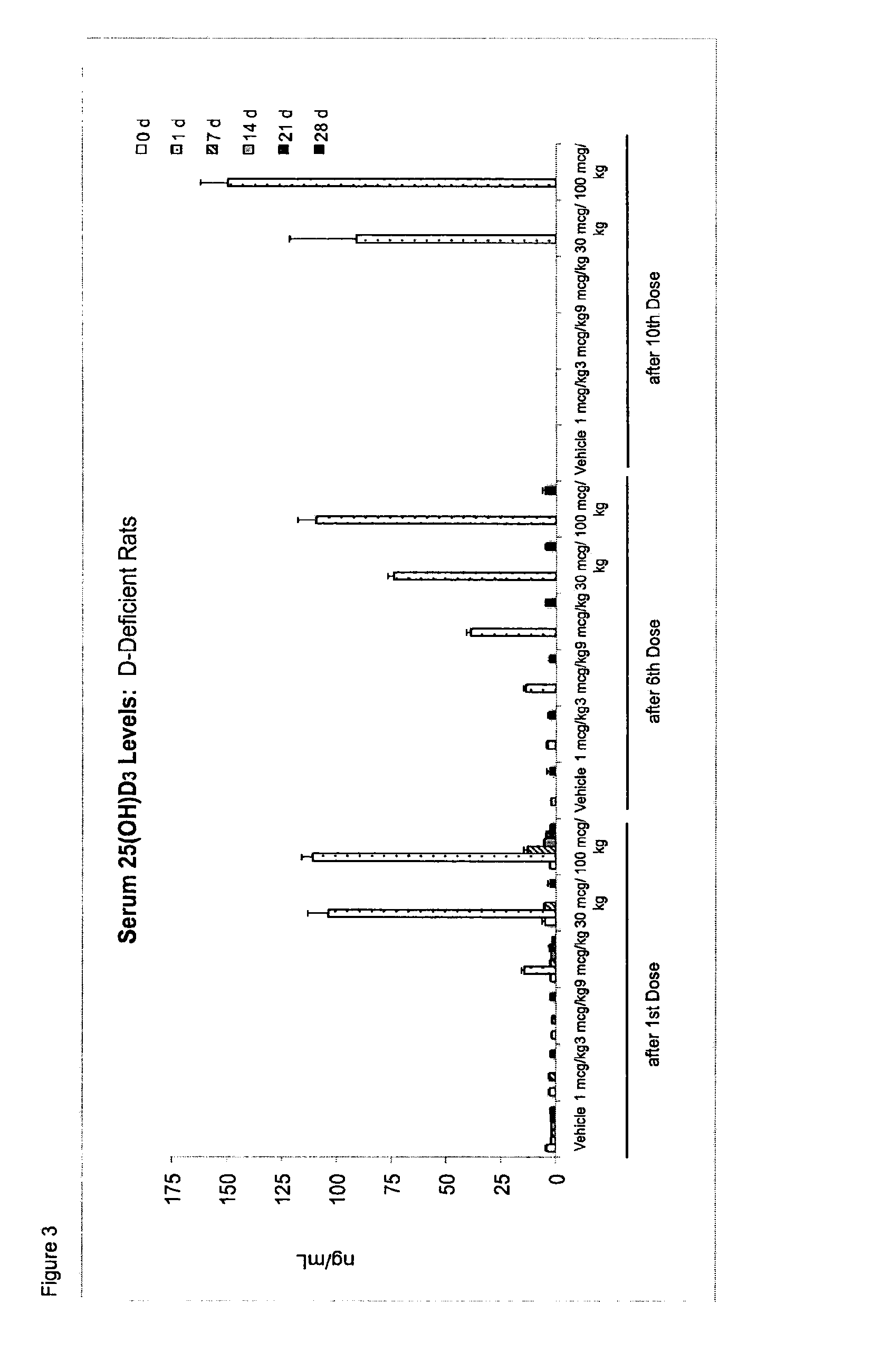
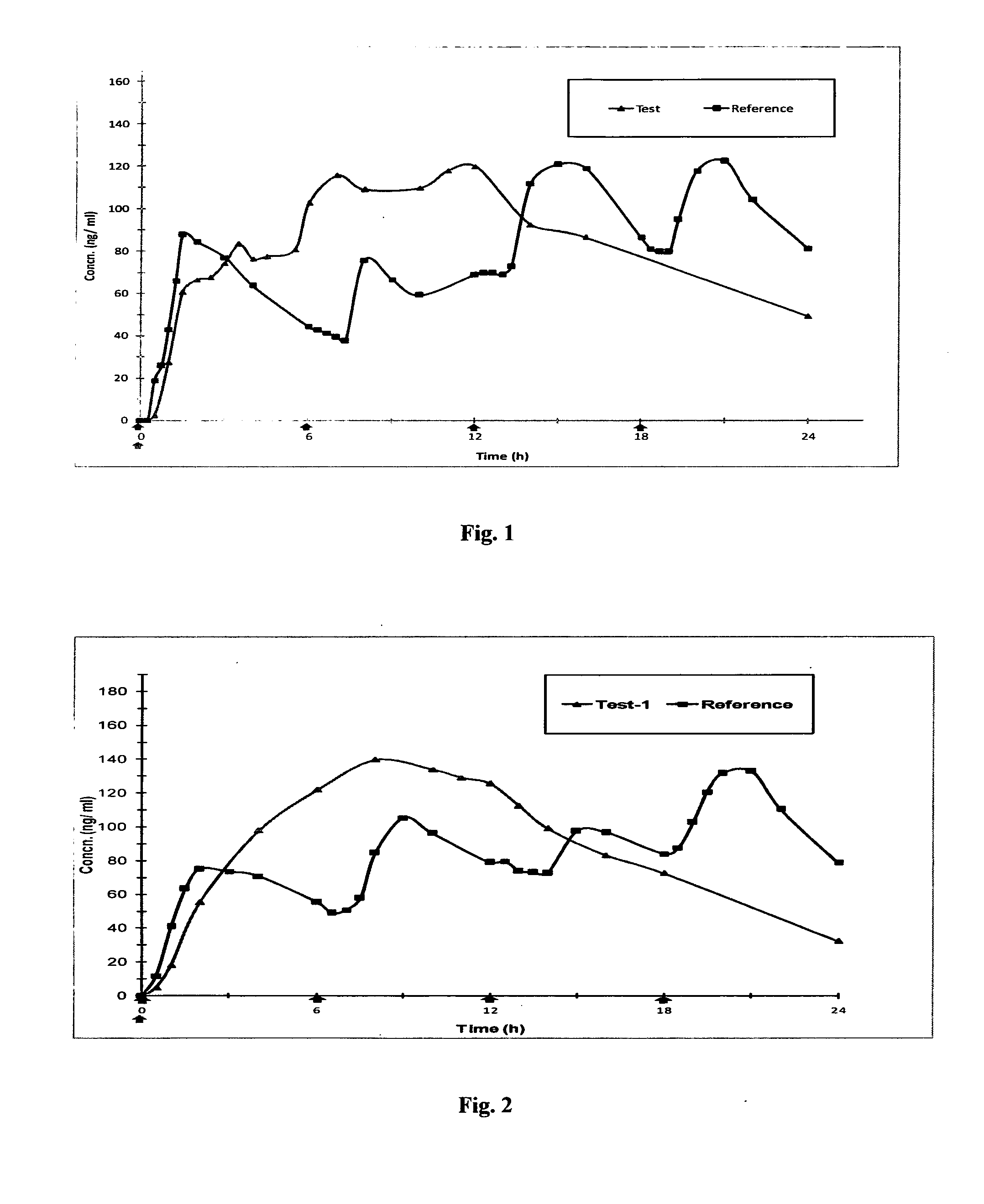
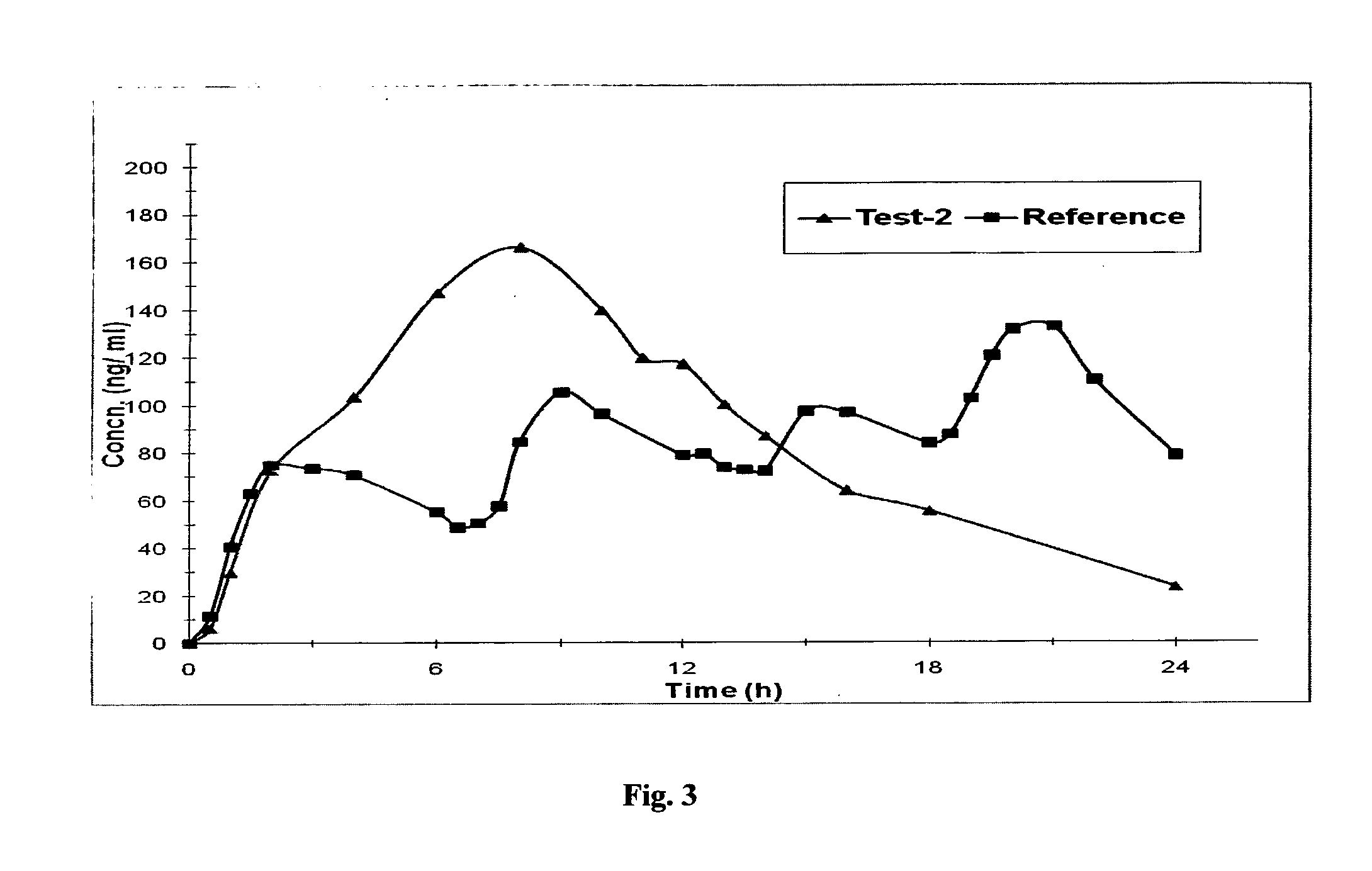
Once-a-week administration of 25-hydroxy vitamin d3 to sustain elevated steady-state pharmacokinetic blood concentration
An oral dosage form comprising a single dose of 25-hydroxy-vitamin D3 sufficient to elevate the serum level in a human to a concentration in the range of 30 ng / ml to 200 ng / ml for at least 7 days and a pharmaceutically suitable oral carrier system, wherein subsequent single doses at least every 7 days are sufficient to sustain the serum level in a human to a concentration in the range of 30 ng / ml to 200 ng / ml at steady-state pharmacokinetics is disclosed. A method of elevating and sustaining the blood level concentration of 25-hydroxy-vitamin D3 in a human in need thereof comprising orally administering or parenterally administering by injection or infusion, at least once every 7 days, a single dose of 25-hydroxy-vitamin D3 sufficient to elevate the serum level in a human to a concentration in the range of 30 ng / ml to 200 ng / ml for at least 7 days, wherein the single doses orally administered at least every 7 days are sufficient to sustain the serum level in a human to a concentration in the range of 30 ng / ml to 200 ng / ml at steady-state pharmacokinetics is disclosed. The human in need thereof may be a human deficient in vitamin D having a serum level concentration of 25-hydroxy-vitamin D3 less than 30 ng / ml.
Owner:WISCONSIN ALUMNI RES FOUND
Controlled release pharmaceutical compositions of tapentadol
A once daily controlled release pharmaceutical compositions comprising tapentadol, wherein preferably the mean Tmax of tapentadol is reached after 10 hours of administration of the composition. The composition comprises tapentadol, such that it maintains serum concentration of tapentadol of at least about 20 ng / ml for at least about 17 hours after oral administration of the composition. According to one embodiment the controlled release pharmaceutical composition comprises tapentadol, which is gastroretentive.
Owner:LUPIN LTD
High-strength testosterone undecanoate compositions
ActiveUS9358241B2Improve performanceReduce loadOrganic active ingredientsGranular deliveryOral medicationSerum concentration
The present disclosure is drawn to pharmaceutical compositions and oral dosage capsules containing testosterone undecanoate, as well as related methods. The capsule includes a capsule shell and a capsule fill. The capsule fill can include a solubilizer and about 14 wt % to about 35 wt % testosterone undecanoate based on the total capsule fill. The oral dosage capsule is such that when a single oral administration to a male subject of one or more capsules with a total testosterone undecanoate daily dose of about 350 mg to about 650 mg it provides a ratio of serum testosterone Cmax to serum testosterone Cave of about 2.7 or less. In yet another embodiment, a method for providing a serum concentration of testosterone within a target serum testosterone concentration Cave range for a male subject is provided.
Owner:LIPOCINE
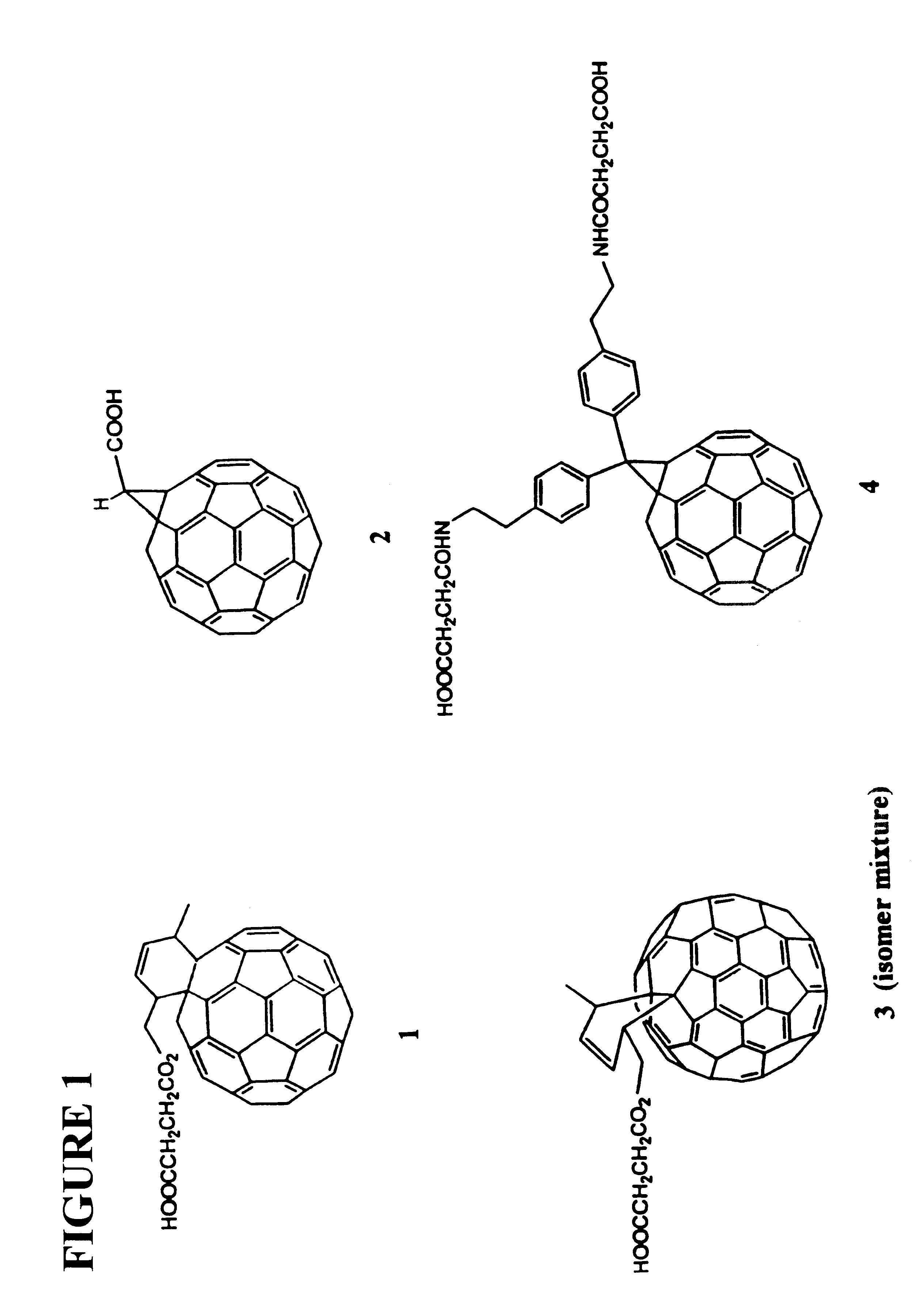
Antibodies specific for fullerenes
This invention provides a hybridoma produced by the fusion of a mouse antibody-producing cell and a mouse myeloma which is designated 1-10F-8A and deposited with the ATCC under Accession Number PTA-279, said hybridoma producing a monoclonal antibody which binds to fullerene C60. This invention provides a mouse monoclonal antibody specific for a fullerene-C60 and produced by the mouse monoclonal antibody-producing hybridoma designated 1-10F-8A. The invention provides the amino acid and encoding nucleic acid sequences of the heavy and light chains of the 1-10F-8A monoclonal antibody. This invention also provides methods of determining a serum concentration of a fullerene in a subject and of purifying a fullerene from a sample.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
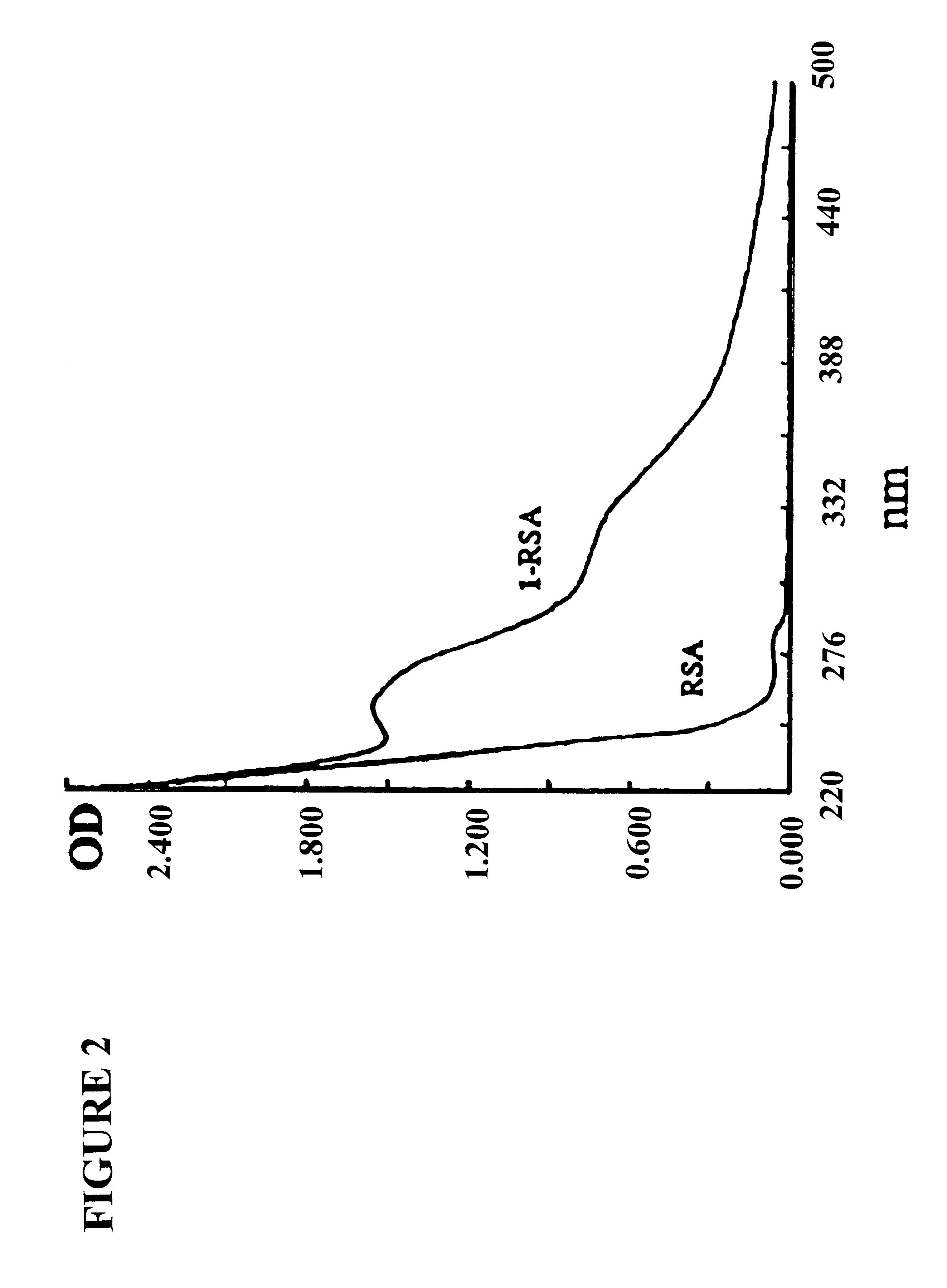
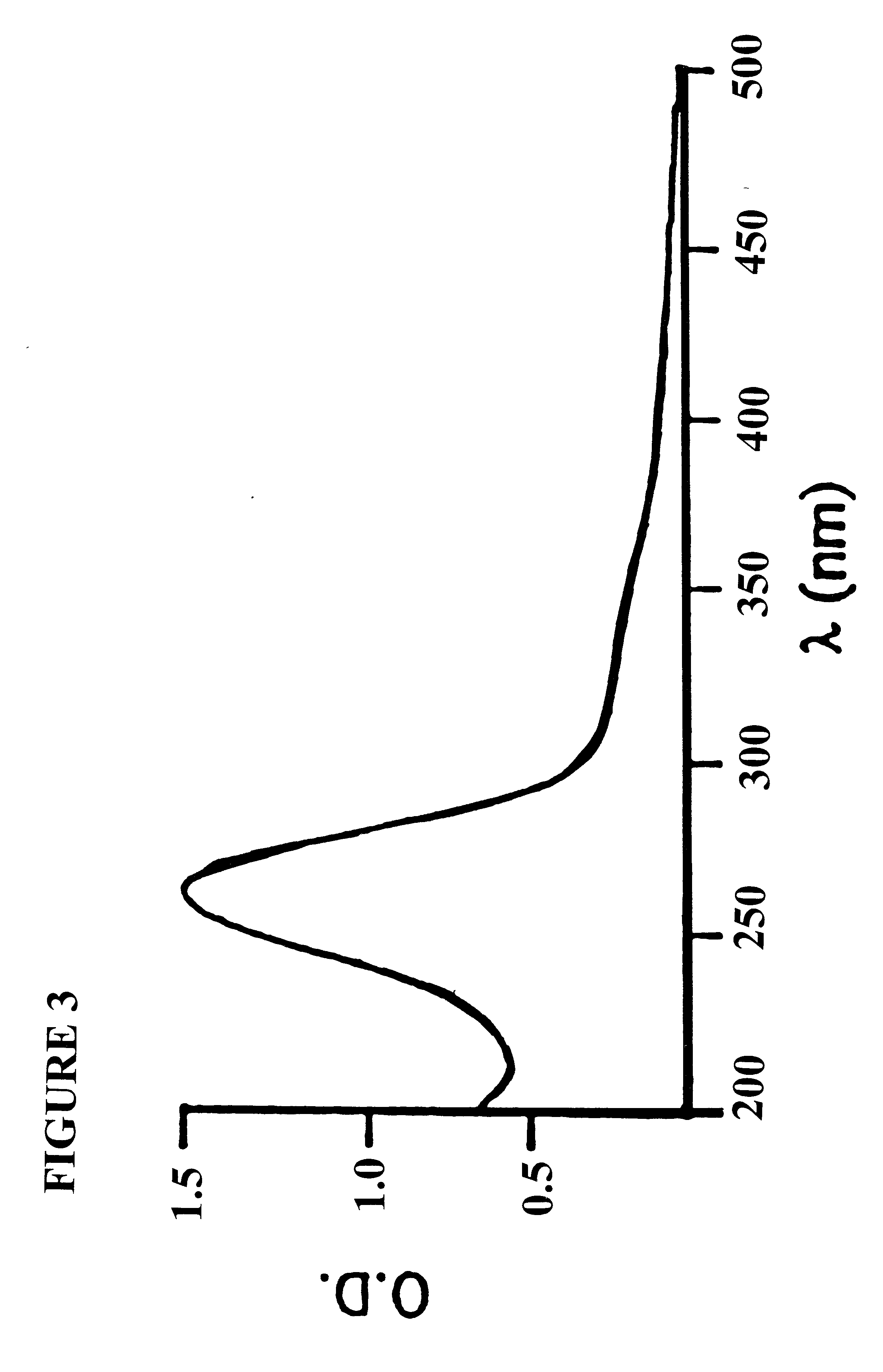
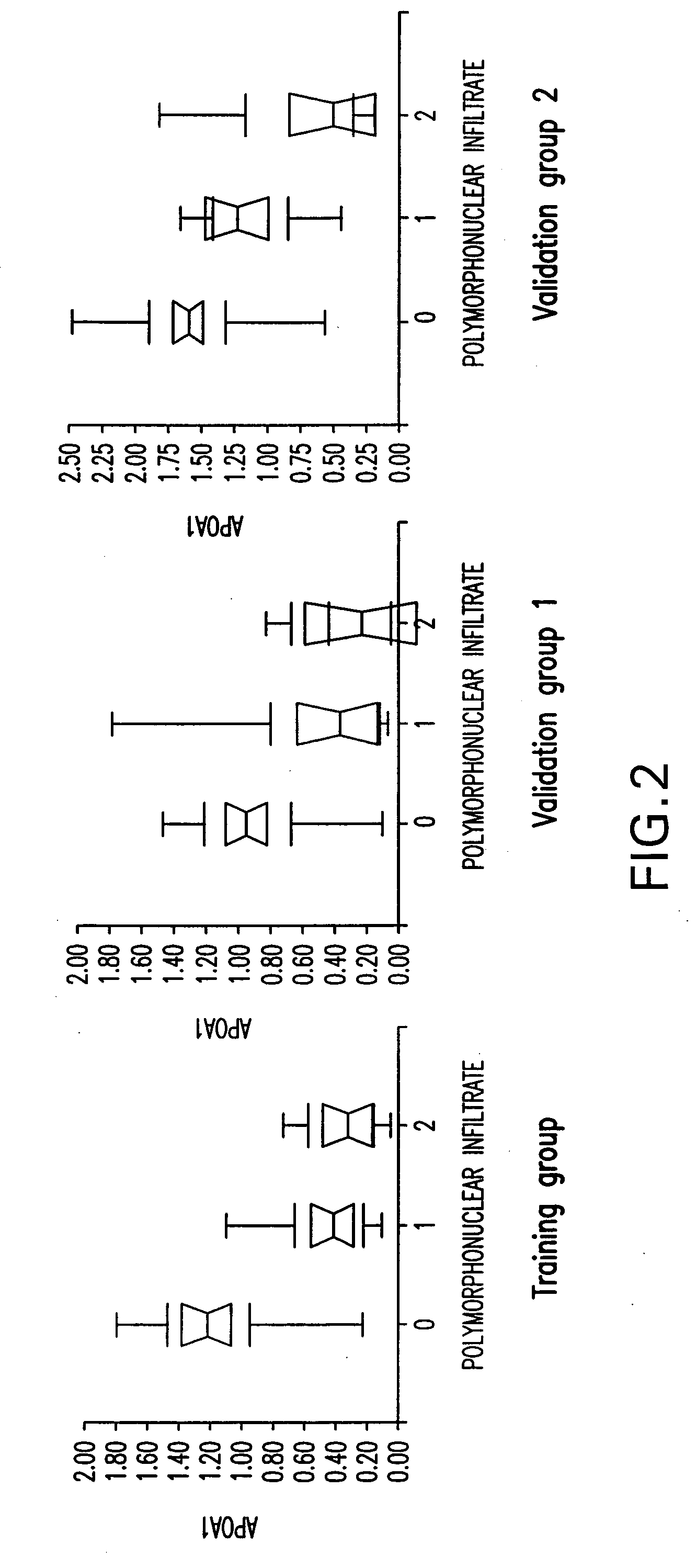
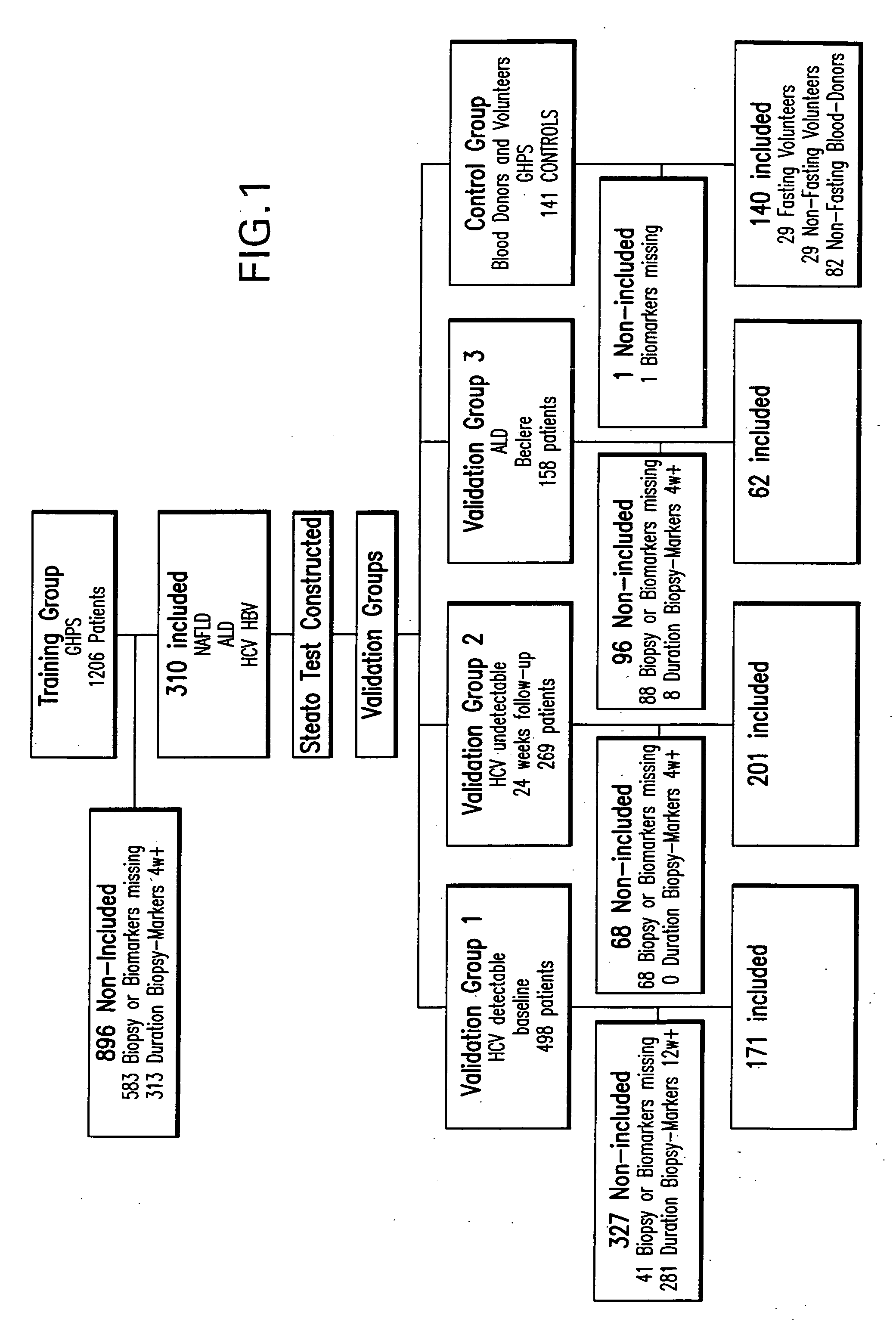
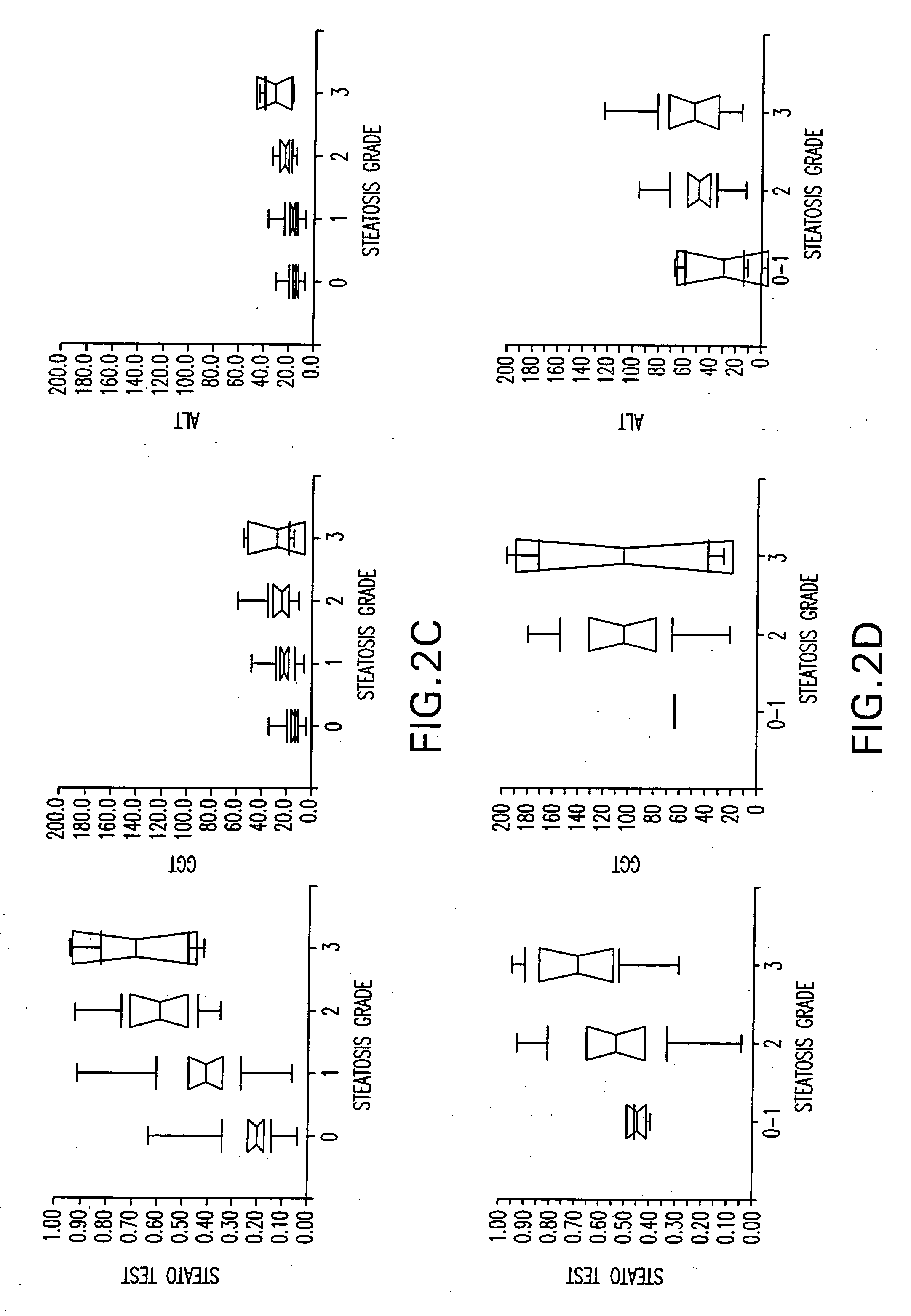
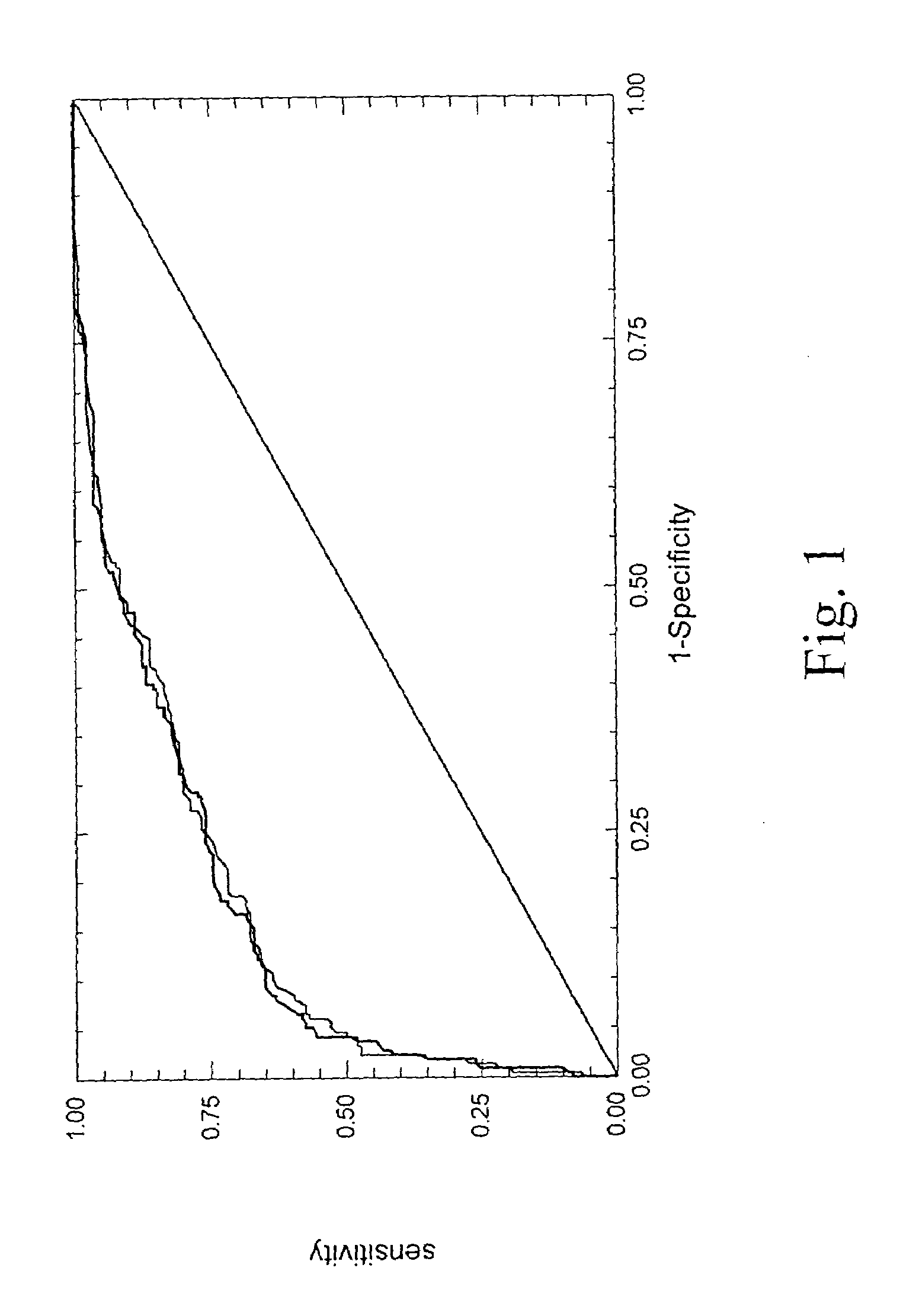
Diagnosis method of alcholic or non-alcoholic steato-hepatitis using biochemical markers
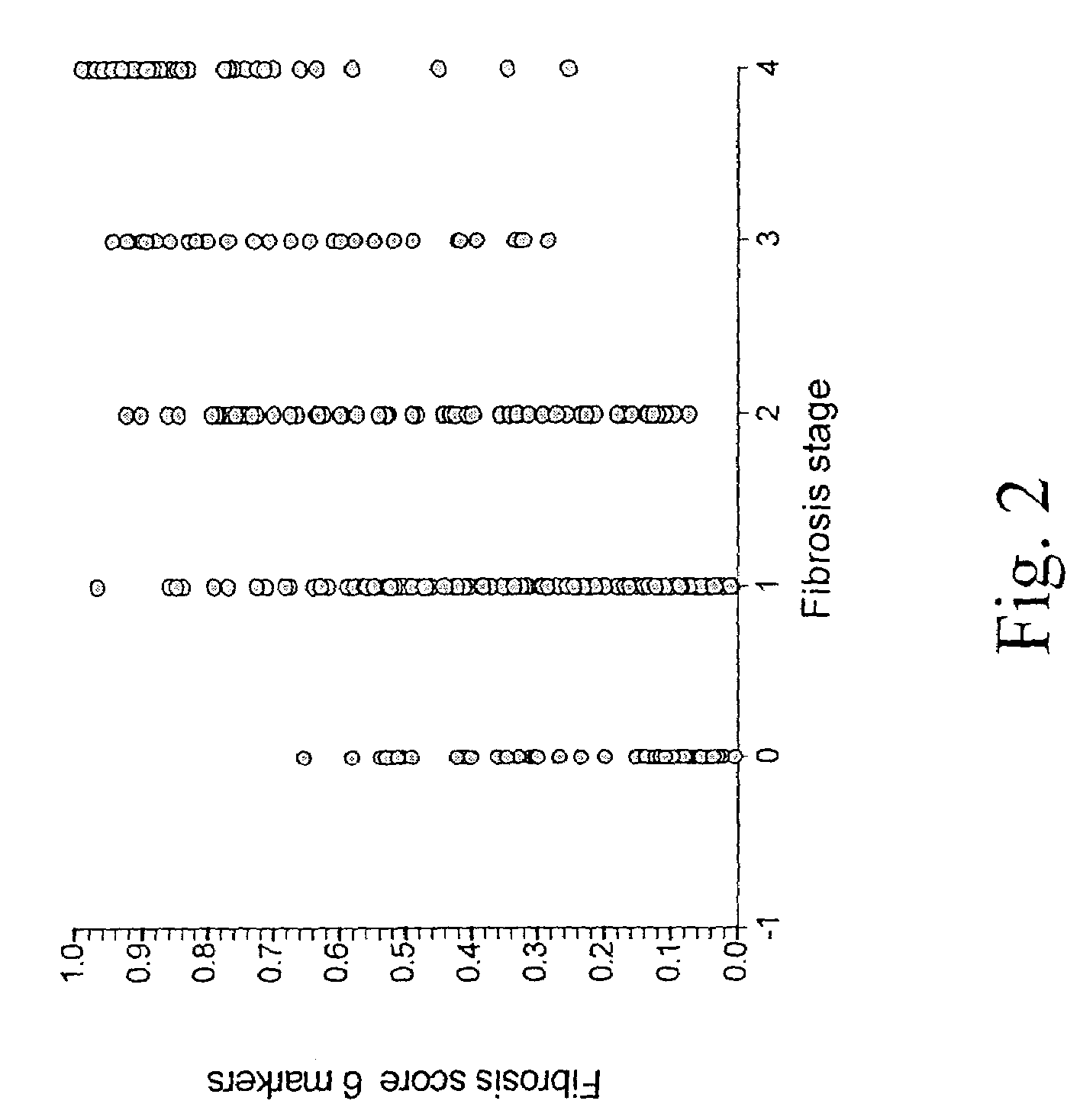
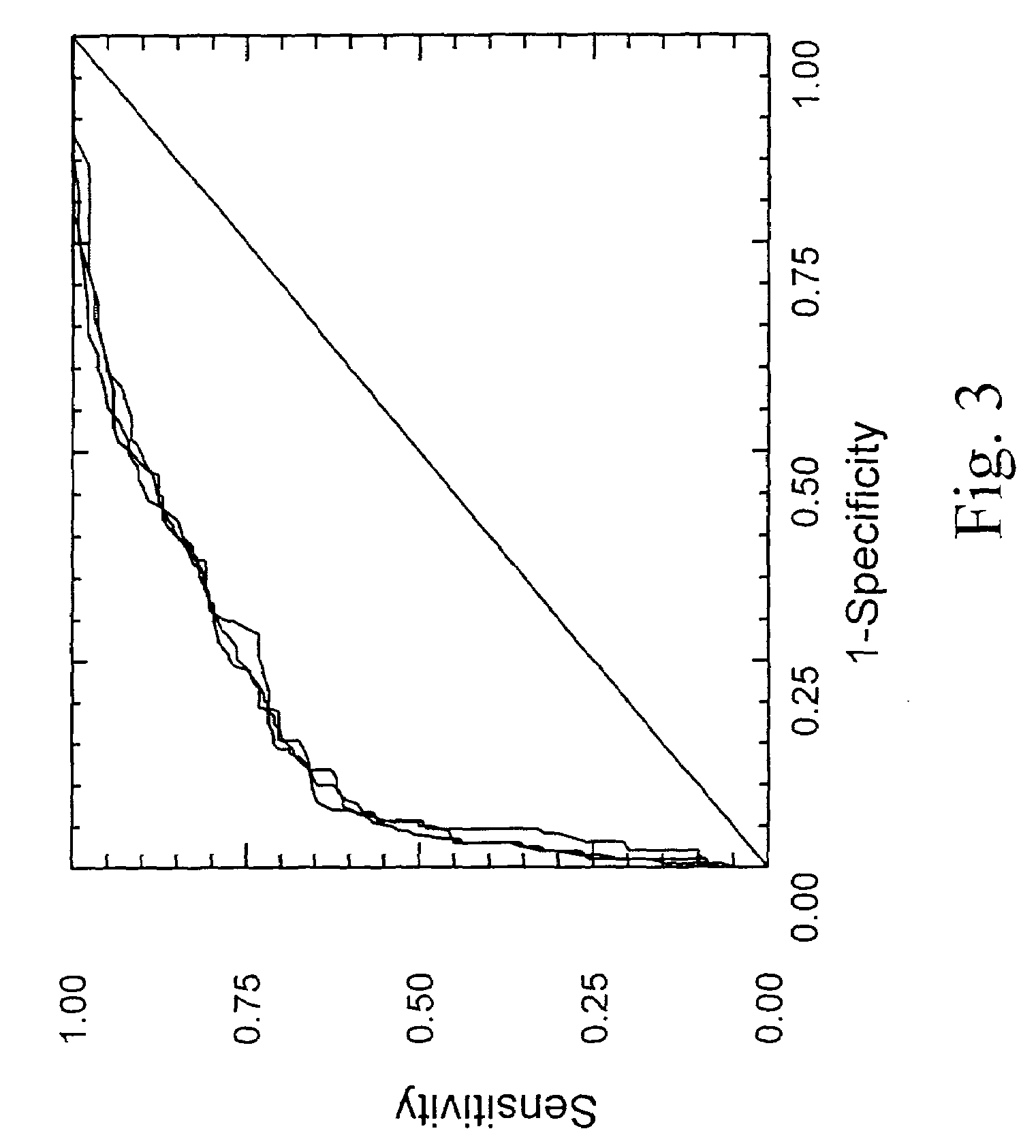
ActiveUS20060172286A1Reduce in quantityLow costMicrobiological testing/measurementDiagnostic recording/measuringLiver fibrosisBiochemical markers
The present invention is drawn to a new diagnosis method for detecting the extent of alcoholic or non-alcoholic steato-hepatitis in a patient, in particular in a patient suffering from a disease involving alcoholic or non-alcoholic steato-hepatitis or who already had a positive diagnosis test of liver fibrosis and / or presence of liver necroinflammatory lesions, by using the serum concentration of easily detectable biological markers. The invention is also drawn to diagnosis kits for the implementation of the method.
Owner:ASSISTANCE PUBLIQUE HOPITAUX DE PARIS
Controlled release pharmaceutical compositions of tapentadol
A once daily controlled release pharmaceutical compositions comprising tapentadol, wherein preferably the mean Tmax of tapentadol is reached after 10 hours of administration of the composition. The composition comprises tapentadol, such that it maintains serum concentration of tapentadol of at least about 20 ng / ml for at least about 17 hours after oral administration of the composition. According to one embodiment the controlled release pharmaceutical composition comprises tapentadol, which is gastroretentive.
Owner:LUPIN LTD
High-strength testosterone undecanoate compositions
InactiveUS20140309202A1Improve sexual symptomImproves and enhances physicalOrganic active ingredientsPowder deliverySerum igeSerum concentration
The present disclosure is drawn to pharmaceutical compositions and oral dosage capsules containing testosterone undecanoate, as well as related methods. The capsule includes a capsule shell and a capsule fill. The capsule fill can include a solubilizer and about 14 wt % to about 35 wt % testosterone undecanoate based on the total capsule fill. The oral dosage capsule is such that when a single oral administration to a male subject of one or more capsules with a total testosterone undecanoate daily dose of about 350 mg to about 650 mg it provides a ratio of serum testosterone Cmax to serum testosterone Cave of about 2.7 or less. In yet another embodiment, a method for providing a serum concentration of testosterone within a target serum testosterone concentration Cave range for a male subject is provided.
Owner:LIPOCINE
Antagonists of HMG1 for treating inflammatory conditions
There is disclosed a pharmaceutical composition and method for treating sepsis, including septic shock and ARDS (acute respiratory distress syndrome), comprising administering an effective amount of a HMG1 antagonist. There is further disclosed a diagnostic method for monitoring the severity or potential lethality of sepsis or septic shock, comprising measuring the serum concentration of HMG1 in a patient exhibiting or at risk or exhibit sepsis or septic shock symptoms. Lastly, there is disclosed a pharmaceutical composition and method for effecting weight loss or treating obesity, comprising administering an effective amount of HMG1 or a therapeutically active HMG1 fragment.
Owner:THE FEINSTEIN INST FOR MEDICAL RES
Indazole compounds and methods of use thereof
This invention is directed to Indazole Compounds or pharmaceutically acceptable salts, solvates and hydrates thereof. The Indazole Compounds have utility in the treatment or prevention of a wide range of diseases and disorders that are responsive to the inhibition, modulation or regulation of kinases, such as inflammatory diseases, abnormal angiogenesis and diseases related thereto, cancer, atherosclerosis, a cardiovascular disease, a renal disease, an autoimmune condition, macular degeneration, disease-related wasting, an asbestos-related condition, pulmonary hypertension, diabetes, obesity, pain and others. Thus, methods of treating or preventing such diseases and disorders are also disclosed, as are pharmaceutical compositions comprising one or more of the Indazole Compounds. This invention is based, in part, upon the discovery of a novel class of 5-triazolyl substituted indazole molecules that have potent activity with respect to the modulation of protein kinases. Thus, the invention encompasses orally active molecules as well as parenterally active molecules which can be used at lower doses or serum concentrations for treating diseases or disorders associated with protein kinase signal transduction.
Owner:BHAGWAT SHRIPAD S +10
High-strength testosterone undecanoate compositions
ActiveUS20140303130A1Improve performanceIncrease loadPowder deliveryOrganic active ingredientsSerum igeOral medication
The present disclosure is drawn to pharmaceutical compositions and oral dosage capsules containing testosterone undecanoate, as well as related methods. The capsule includes a capsule shell and a capsule fill. The capsule fill can include a solubilizer and about 14 wt % to about 35 wt % testosterone undecanoate based on the total capsule fill. The oral dosage capsule is such that when a single oral administration to a male subject of one or more capsules with a total testosterone undecanoate daily dose of about 350 mg to about 650 mg it provides a ratio of serum testosterone Cmax to serum testosterone Cave of about 2.7 or less. In yet another embodiment, a method for providing a serum concentration of testosterone within a target serum testosterone concentration Cave range for a male subject is provided.
Owner:LIPOCINE
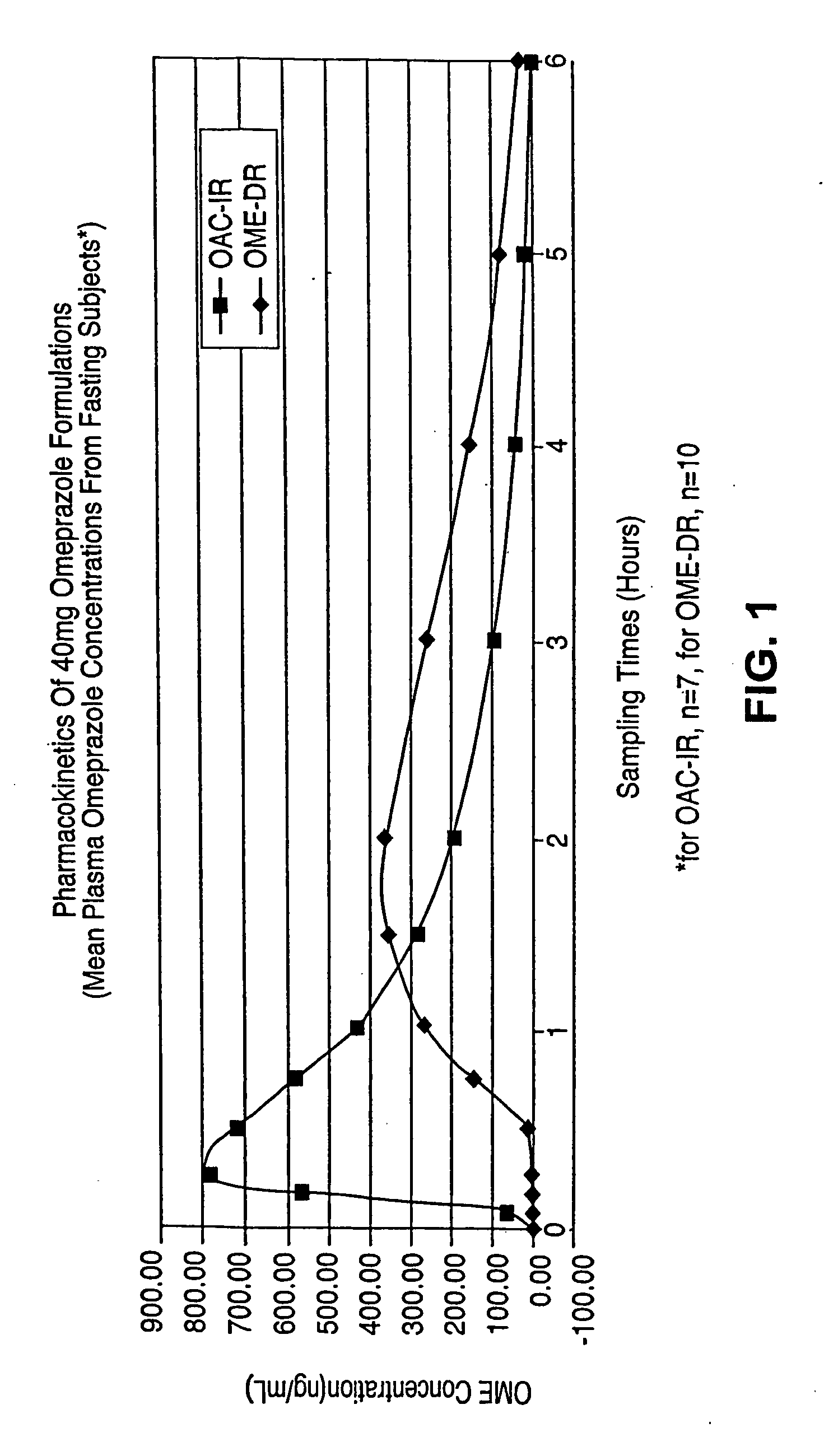
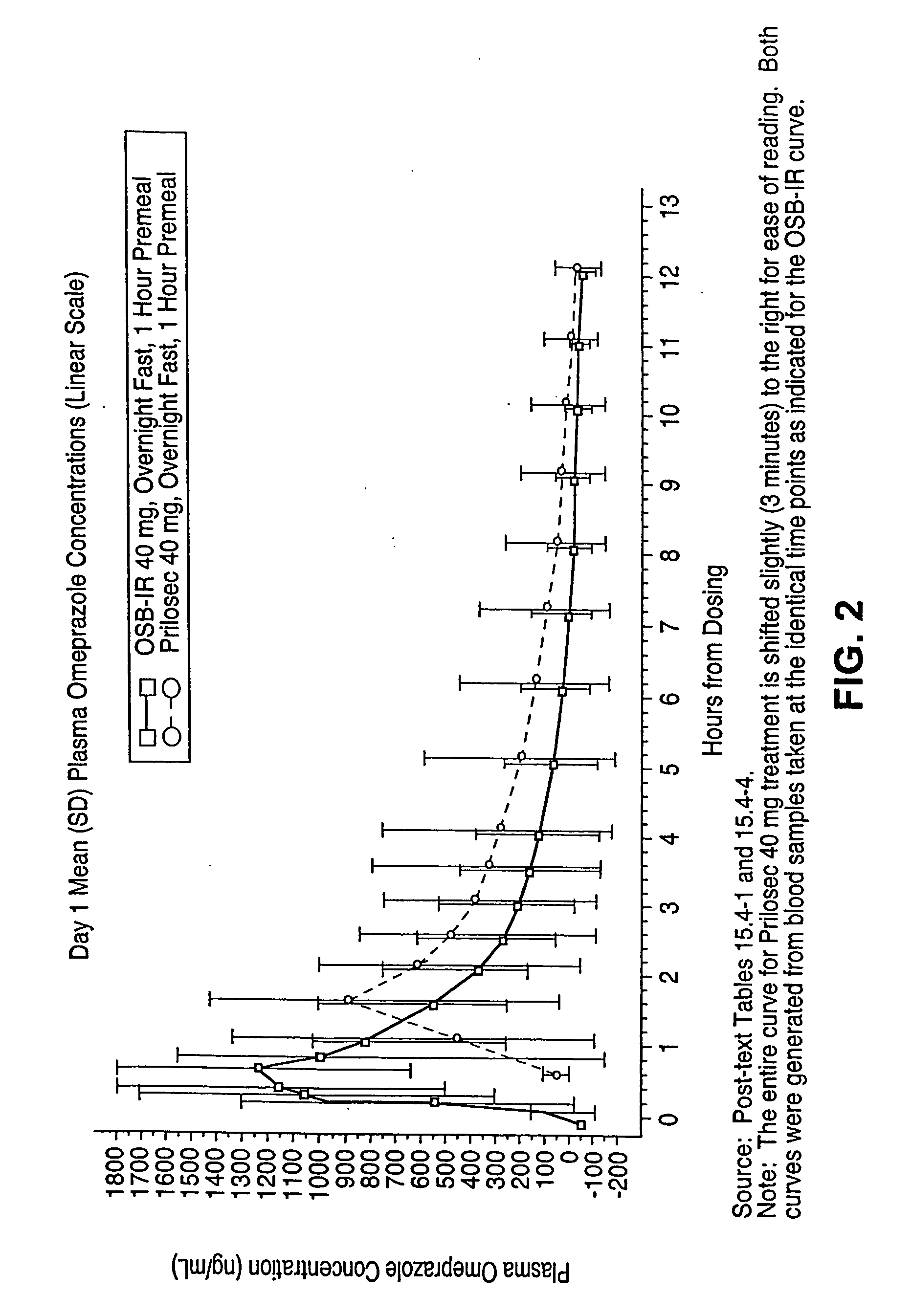
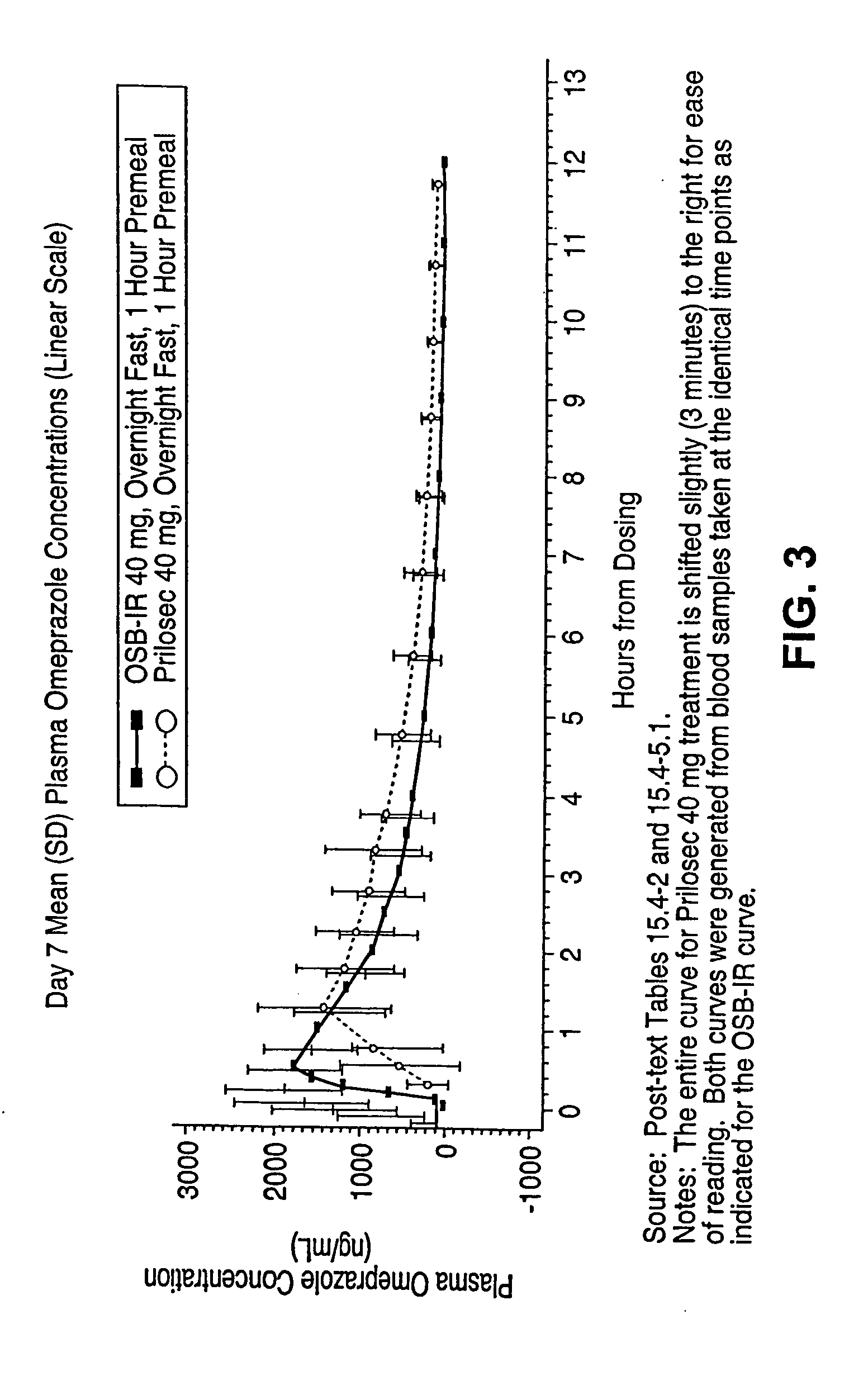
Novel formulation, omeprazole antacid complex-immediate release for rapid and sustained suppression of gastric acid
InactiveUS20050220870A1Reduce productionInhibit and reduce degradationBiocideDispersion deliveryImmediate releaseGastric fluid
The present invention is directed to methods, kits, combinations, and compositions for treating, preventing or reducing the risk of developing a gastrointestinal disorder or disease, or the symptoms associated with, or related to a gastrointestinal disorder or disease in a subject in need thereof. In one aspect, the present invention provides a pharmaceutical composition comprising a proton pump inhibiting agent and a buffering agent for oral administration and ingestion by a subject. Upon administration, the composition contacts the gastric fluid of the stomach and increases the gastric fluid pH of the stomach to a pH that substantially prevents or inhibits acid degradation of the proton pump inhibiting agent in the gastric fluid and allows a measurable serum concentration of the proton pump inhibiting agent to be absorbed into the blood serum of the subject.
Owner:SANTARUS
Diagnosis method of hepatic steatosis using biochemical markers
ActiveUS20060173629A1Low costReduce riskData processing applicationsMicrobiological testing/measurementLiver fibrosisBiochemical markers
The present invention is drawn to a new diagnosis method for detecting the extent of hepatic steatosis in a patient, in particular in a patient who suffers from a disease involving hepatic steatosis, or who already had a positive diagnosis test of liver fibrosis and / or presence of liver necroinflammatory lesions, by using the serum concentration of easily detectable biological markers. The invention is also drawn to diagnosis kits for the implementation of the method.
Owner:ASSISTANCE PUBLIQUE HOPITAUX DE PARIS
Diagnosis method of inflammatory, fibrotic or cancerous disease using biochemical markers
InactiveUS7225080B2Reduce in quantityLow costAmplifier modifications to reduce noise influenceDigital computer detailsLiver fibrosisBiochemical markers
The present invention is drawn to a new diagnosis method for detecting the extend of a inflammatory, fibrotic or cancerous disease in a patient, in particular liver fibrosis, in particular in a patient infected with hepatitis C virus, by using the serum concentration of easily detectable biological markers. The invention is also drawn to diagnosis kits for the implementation of the method.
Owner:ASSISTANCE PUBLIQUE HOPITAUX DE PARIS
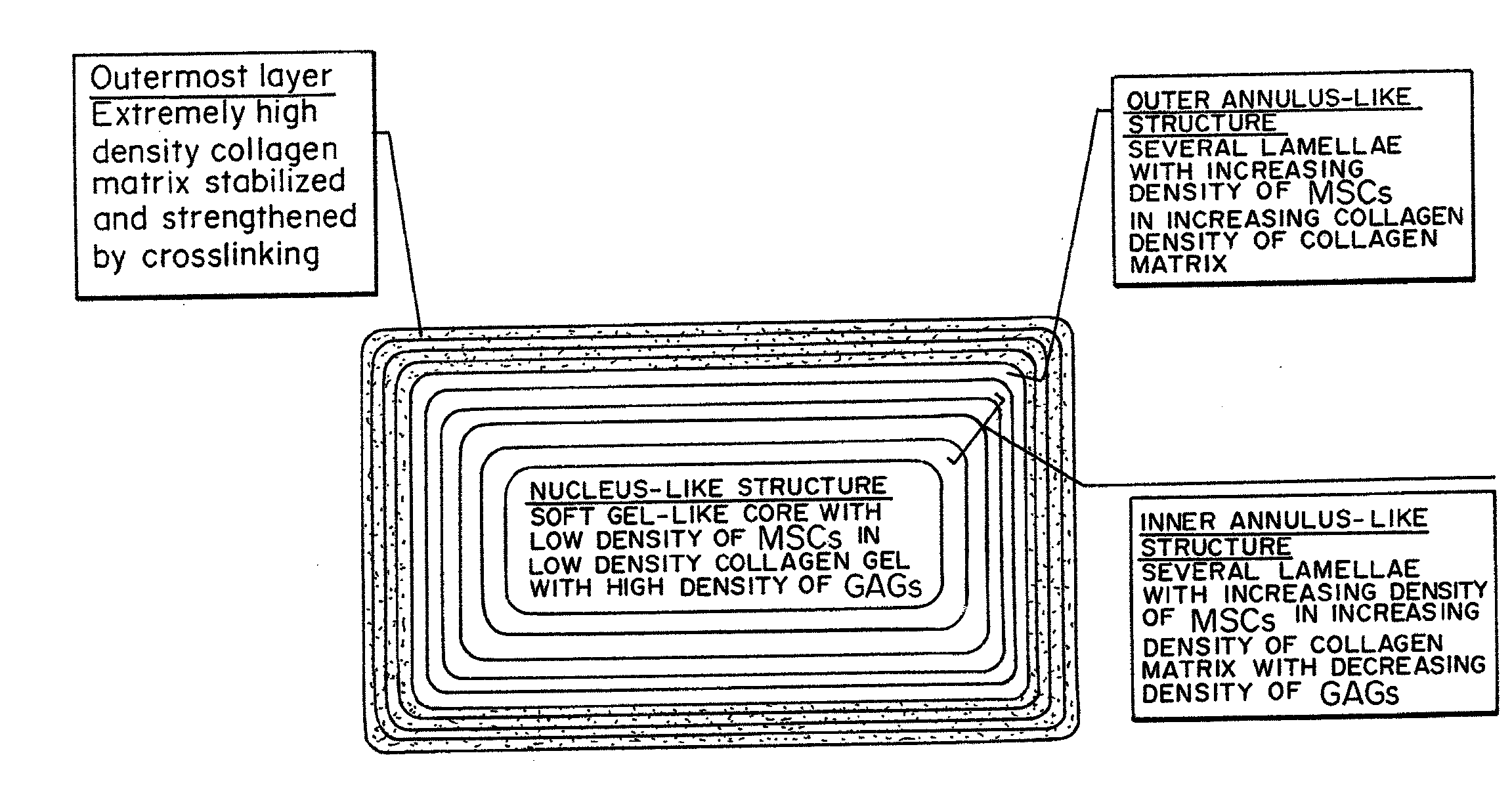
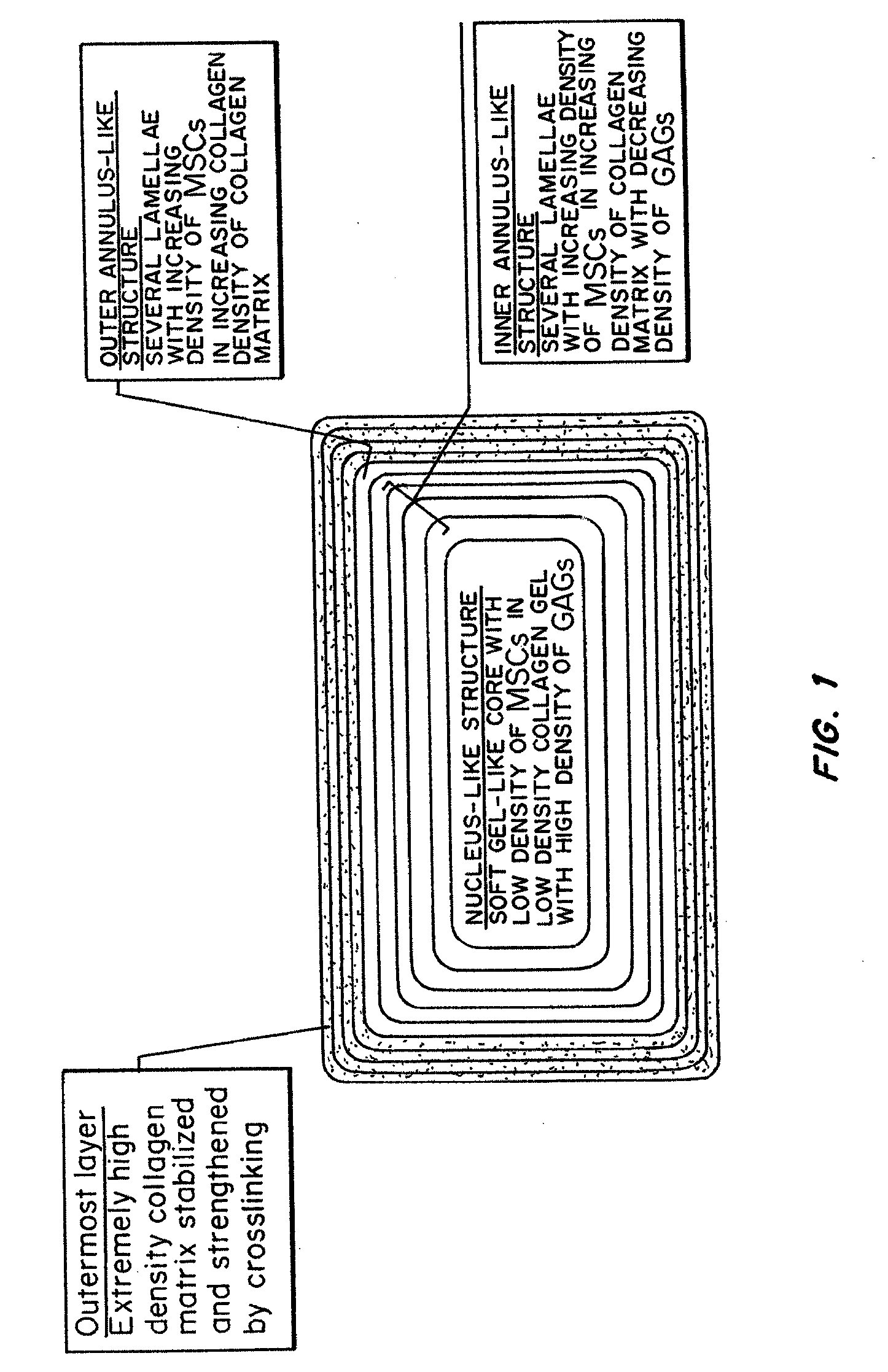
Bioengineered Intervertebral Discs and Methods for Their Preparation
ActiveUS20070292514A1Equally distributedMinimize timePowder deliveryPeptide/protein ingredientsCellular componentCell-Extracellular Matrix
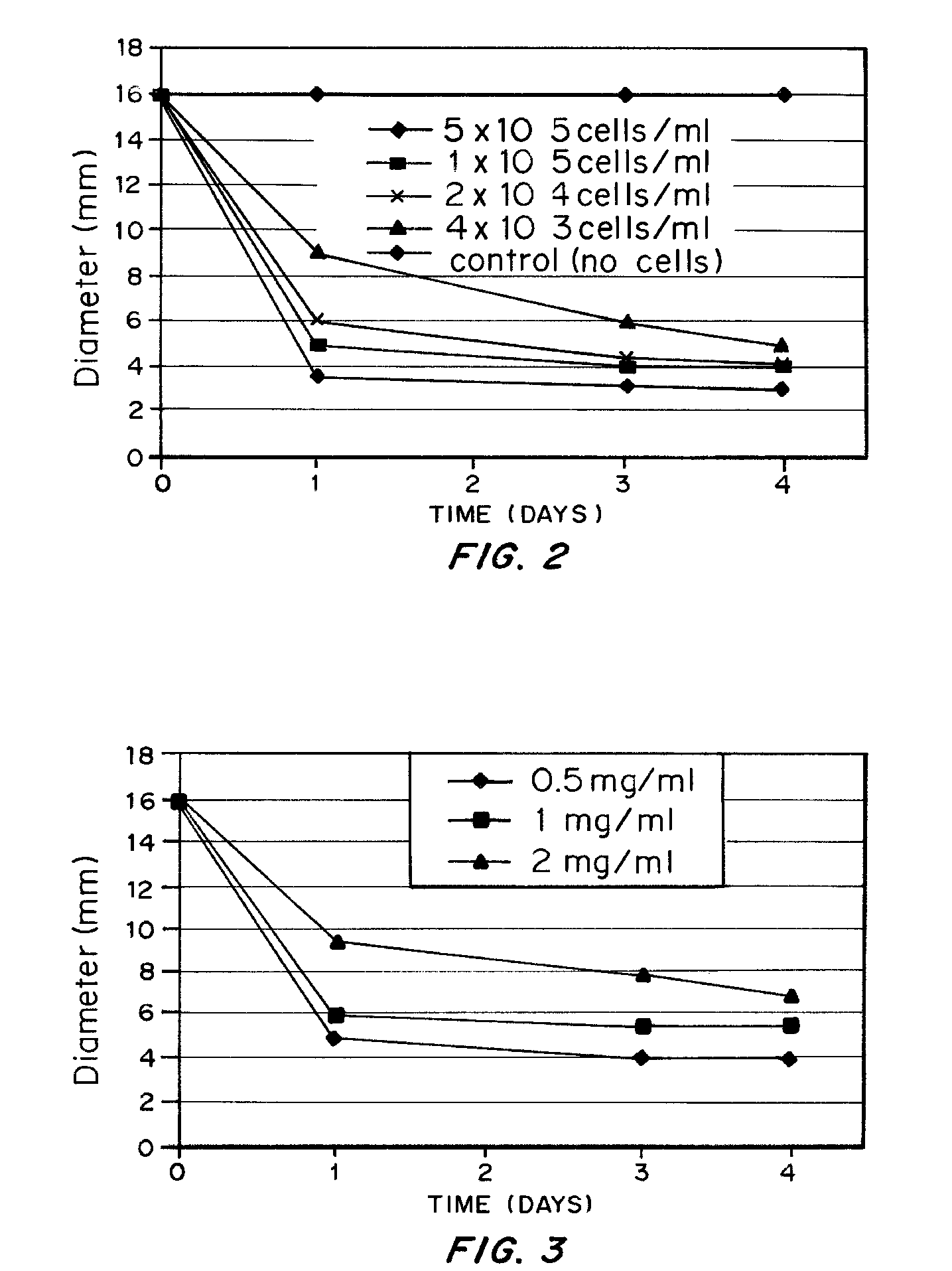
A bioengineered IVD for disc replacement has been developed that has mechanical and structural support characteristics similar to those of native IVD. Extracellular matrix (ECM) provides support to living cell components and interacts with the living cellular components during the fabrication process without introducing toxicity. The composition can be produced from both natural or synthetic source but preferably natural and induced to self-assemble or reconstitute into its solid form under conditions that are mild enough to support cellular survival and growth. The cells induce a volume change of the structures, leading to changes in dimension, ECM density, cell density mechanical property and stability, etc. The extent of the change i volume of the composition can be precisely controlled by factors such as the density of the ECM, the density of the living cells, the timing for interaction and the serum concentration. Increased structural support is provided by crosslinking.
Owner:THE UNIVERSITY OF HONG KONG
Methods for acute and long-term treatment of substance abuse
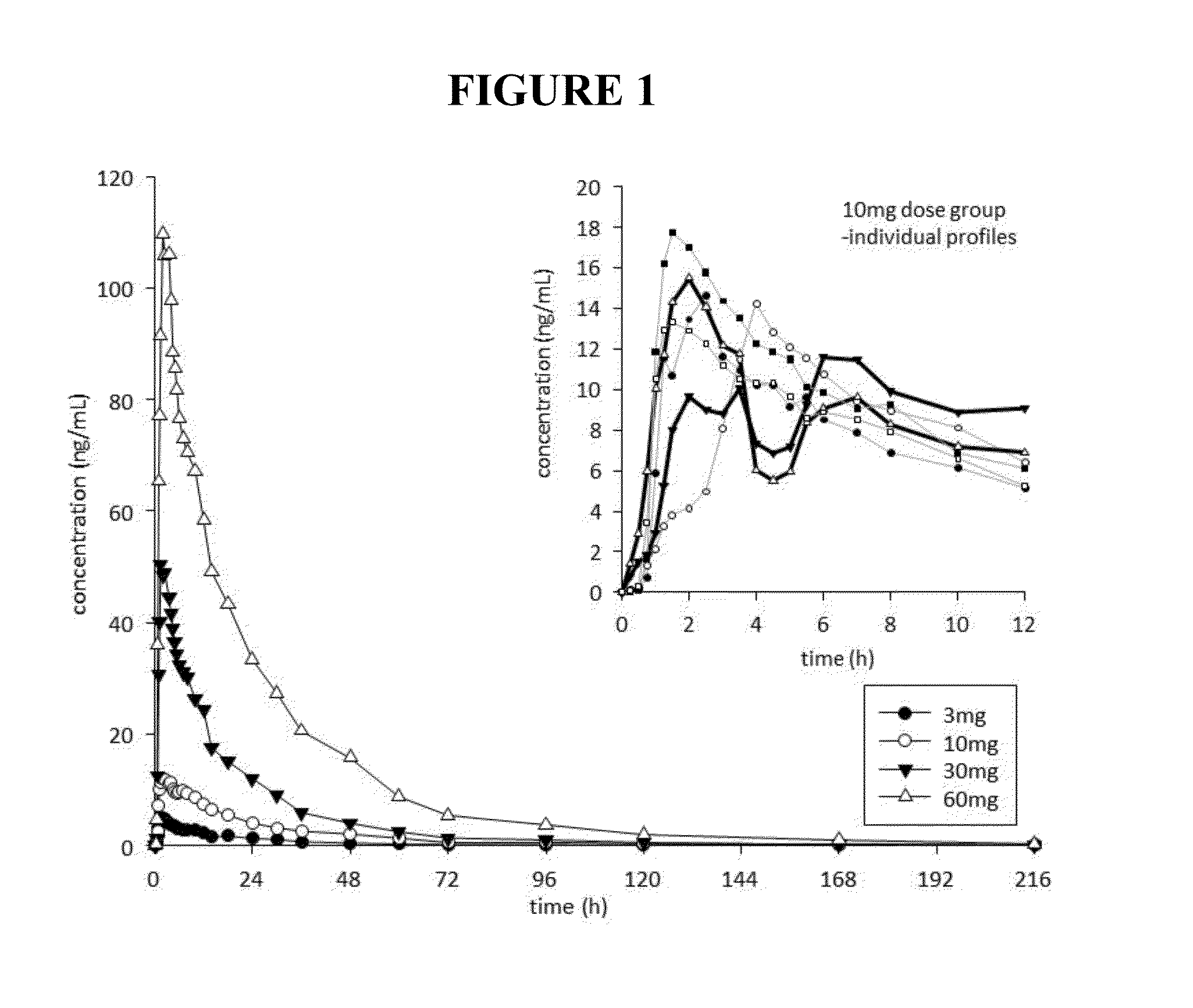
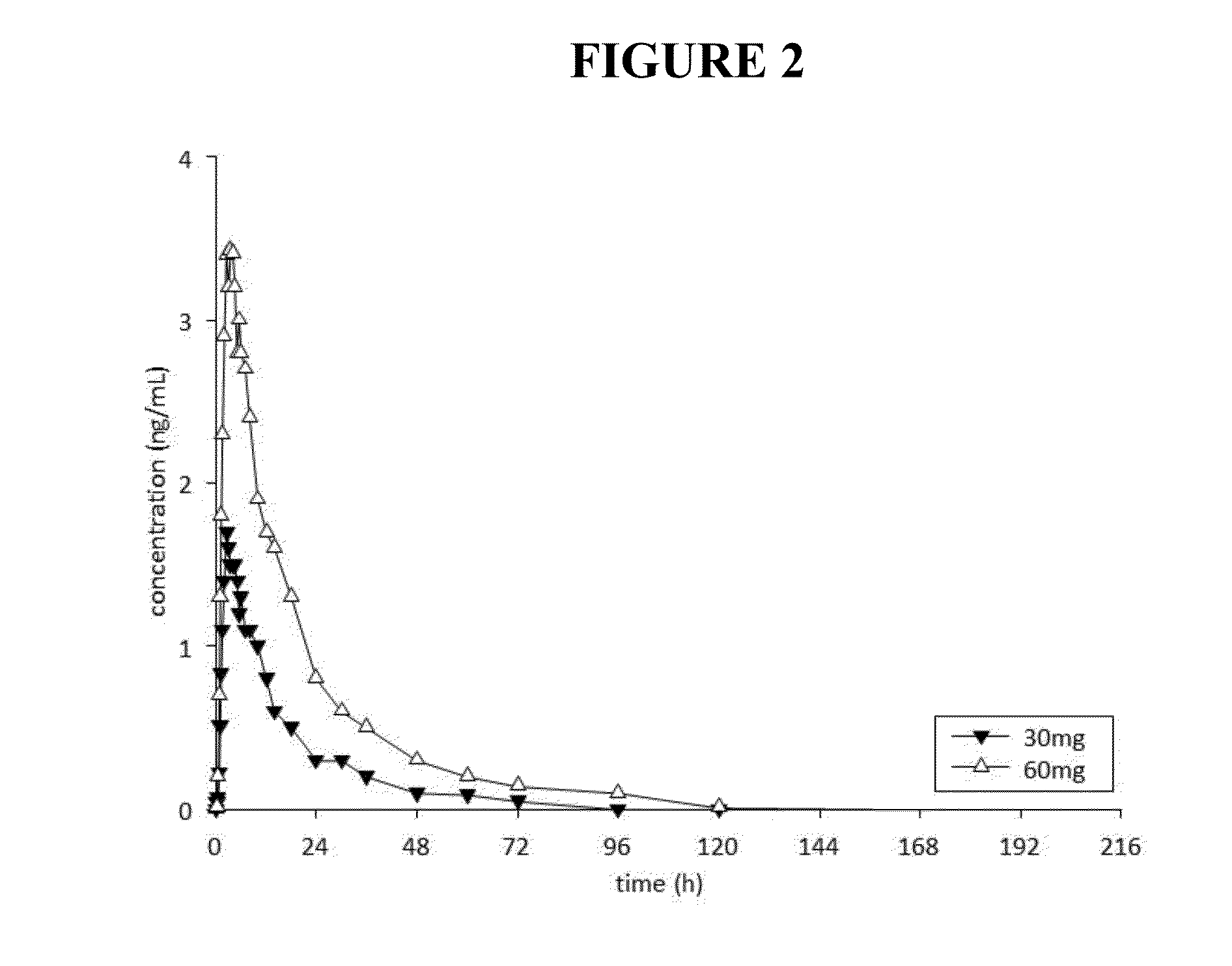
This invention is directed to a method of treating drug addiction, including acute and post-acute withdrawal symptoms, comprising treating an addicted patient with noribogaine or a pharmaceutically acceptable salt and / or solvate thereof at a dosage that provides a therapeutic serum concentration. In one embodiment, the average serum concentration is 50 ng / mL to 180 ng / mL under conditions where the QT interval prolongation does not exceed about 50 milliseconds, and preferably about 30 milliseconds.
Owner:DEMERX
Method and compositions for transdermal administration of antimicrobial medications
InactiveUS20060222692A1Avoid gastrointestinal side effectsReduce the possibilityAntibacterial agentsBiocideSerum concentrationMedicine
The present invention relates to methods and compositions for improved efficacy and delivery of time-dependent antimicrobial drug compositions to a patient. Transdermal dosage forms and methods for steady-state delivery of drug to produce and maintain a serum concentration of drug above the minimum inhibitory concentration or minimum microbicidal concentration are provided.
Owner:FAIRFIELD CLINICAL TRIALS
Anti-cd22 Anti-idiotypic antibodies and uses thereof
ActiveUS20150175711A1Not applyBiological material analysisArtificial cell constructsComplement-dependent cytotoxicityAntibody fragments
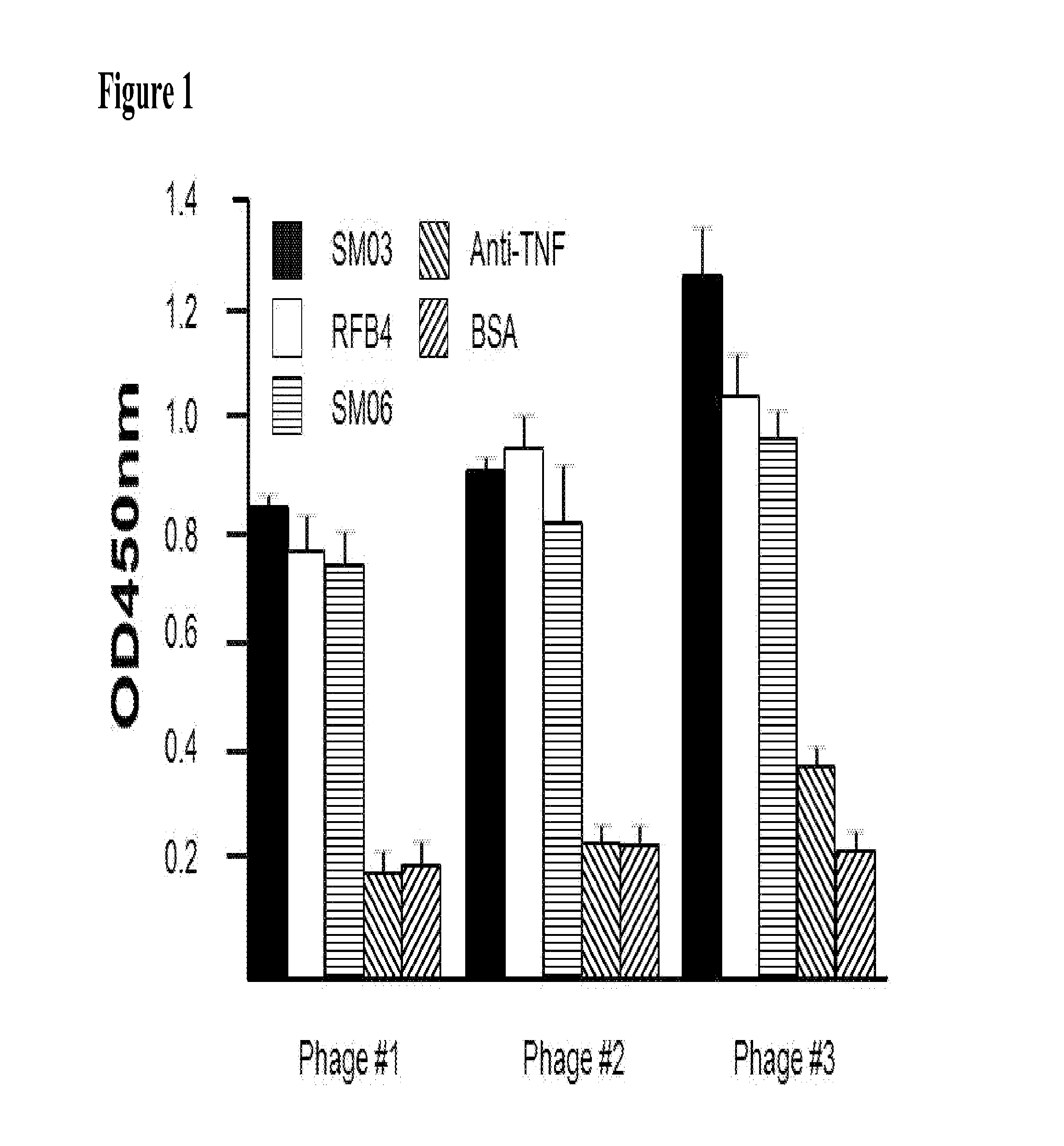
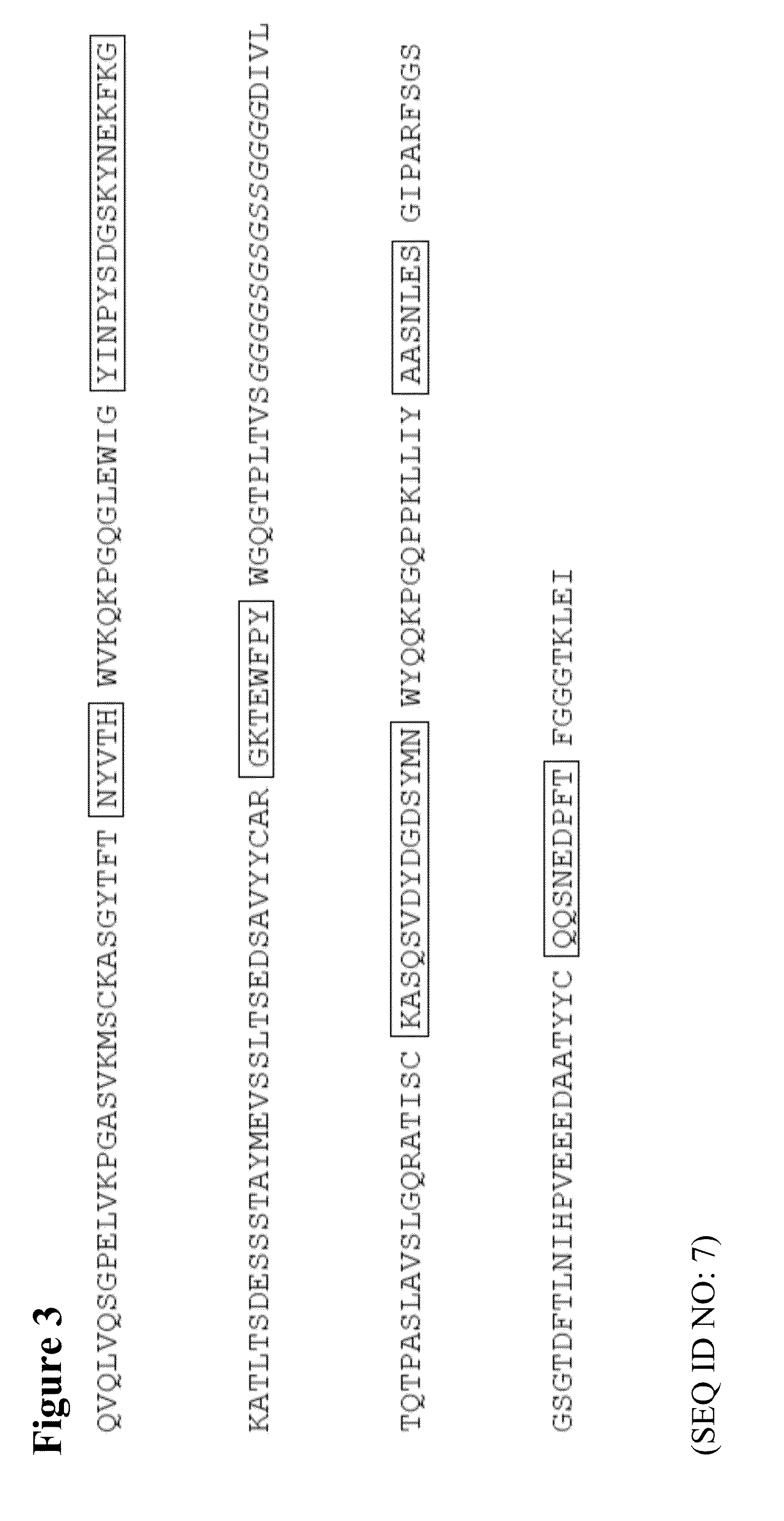
The present invention describes the generation of an anti-idiotype single-chain Fv (scFv) antibody specific for the murine (RFB4), chimeric (SM03) and humanized (SM06) versions of an anti-CD22 antibody (the anti-CD22 antibodies). The present invention further describes the construction of a murine IgG2a / kappa immunoglobulin carrying the variable region sequences of the anti-idiotype scFv sequences. Additionally, the present invention provides a cell line capable of producing an anti-idiotype murine antibody specific for the anti-CD22 antibodies. The present invention is directed against a method for identifying and evaluating the activities and concentration of the anti-CD22 antibodies. Additionally, the present invention provides a method for evaluating serum concentration of the anti-CD22 antibodies that are being used clinically. The present invention is also directed against a method to detect HAMA, HACA and HAHA responses in patients treated with the anti-CD22 antibodies. Specifically, the present invention is directed against the establishment of a cell line expressing surface concentration of the antibody of the invention; the said cell line expressing surface anti-idiotype antibodies or antibody fragments will be used as the target cell line for evaluating the functional activities of the anti-CD22 antibodies via complement dependent cytotoxicity (CDC) and / or antibody dependent cell cytotoxicity (ADCC) activities.
Owner:SINOMAB BIOSCI
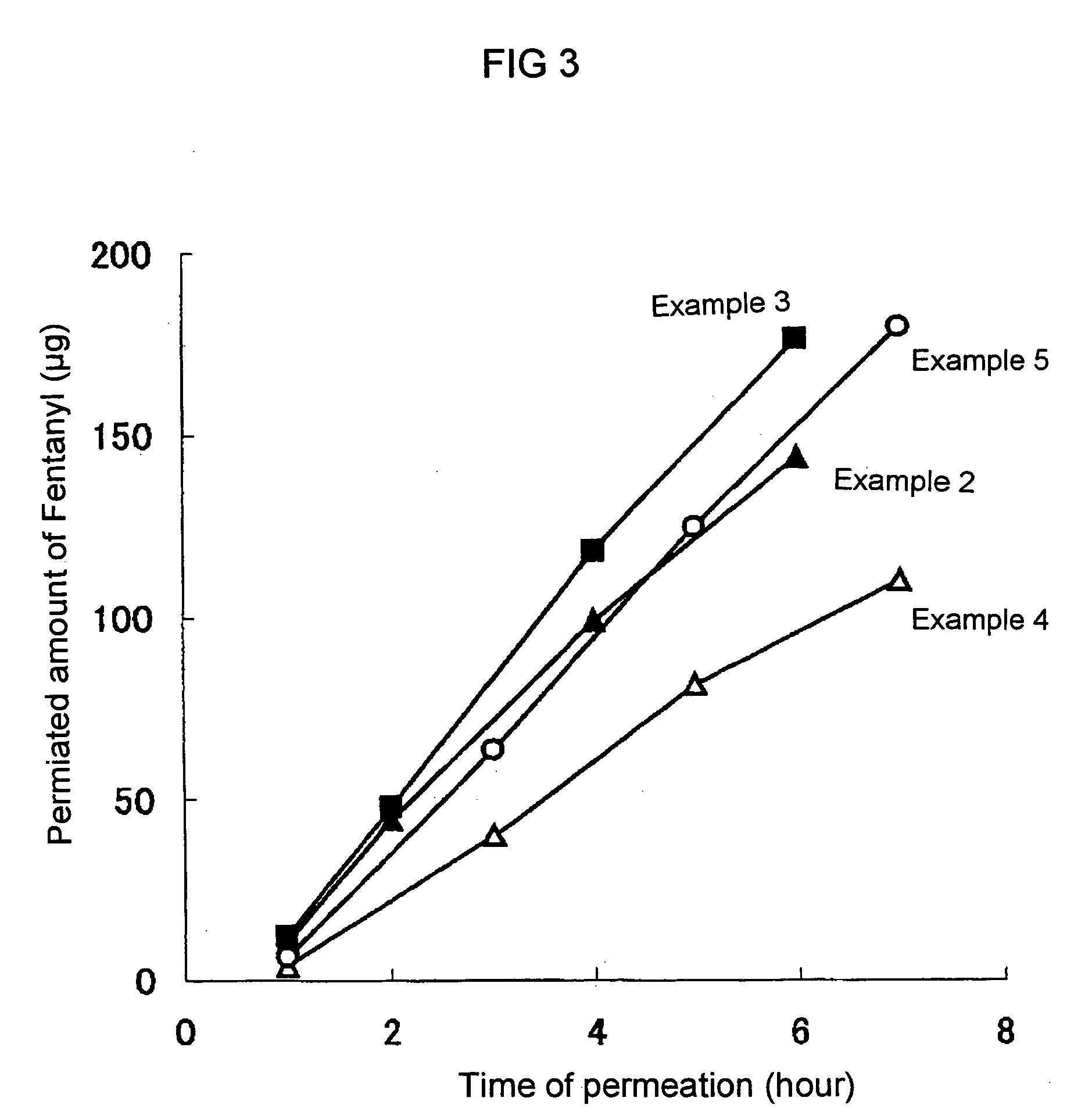
Phentanyl-containing adhesive patch for application to oral-cavity mucosa
ActiveUS20050232982A1Sufficient adhesivityEasy to handleBiocideNervous disorderAdhesiveBULK ACTIVE INGREDIENT
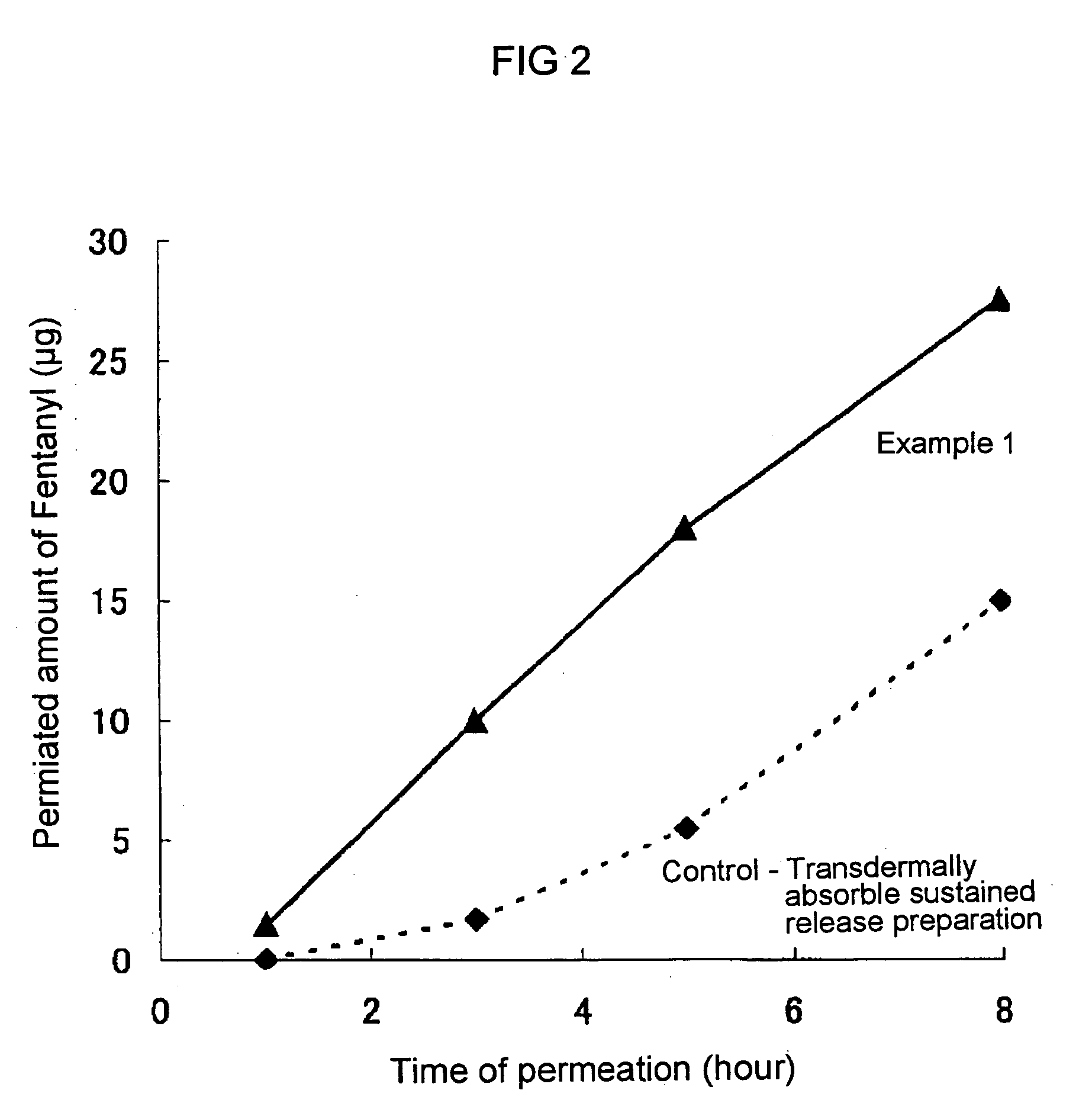
To provide a patch containing fentanyl for mucous membrane of the oral cavity (oral transmucosal fentanyl), which rapidly increases the serum concentration of the drug, is easy in handling and is superior in safety. A patch containing fentanyl for mucous membrane of the oral cavity, which can be prepared by laminating on one side of a drug layer which contains fentanyl or its salt as an active ingredient, methyl vinyl ether-maleic anhydride copolymer as an adhesive, and at least one substance selected from the group consisting of hydroxypropylcellulose, hydroxypropylmethylcellulose and hydroxyethylcellulose as a thickener, a support layer hardly soluble or insoluble in water, and a backing in their order.
Owner:TEIKOKU SEIYAKU KK TEIKOKU SEIYAKU CO LTD
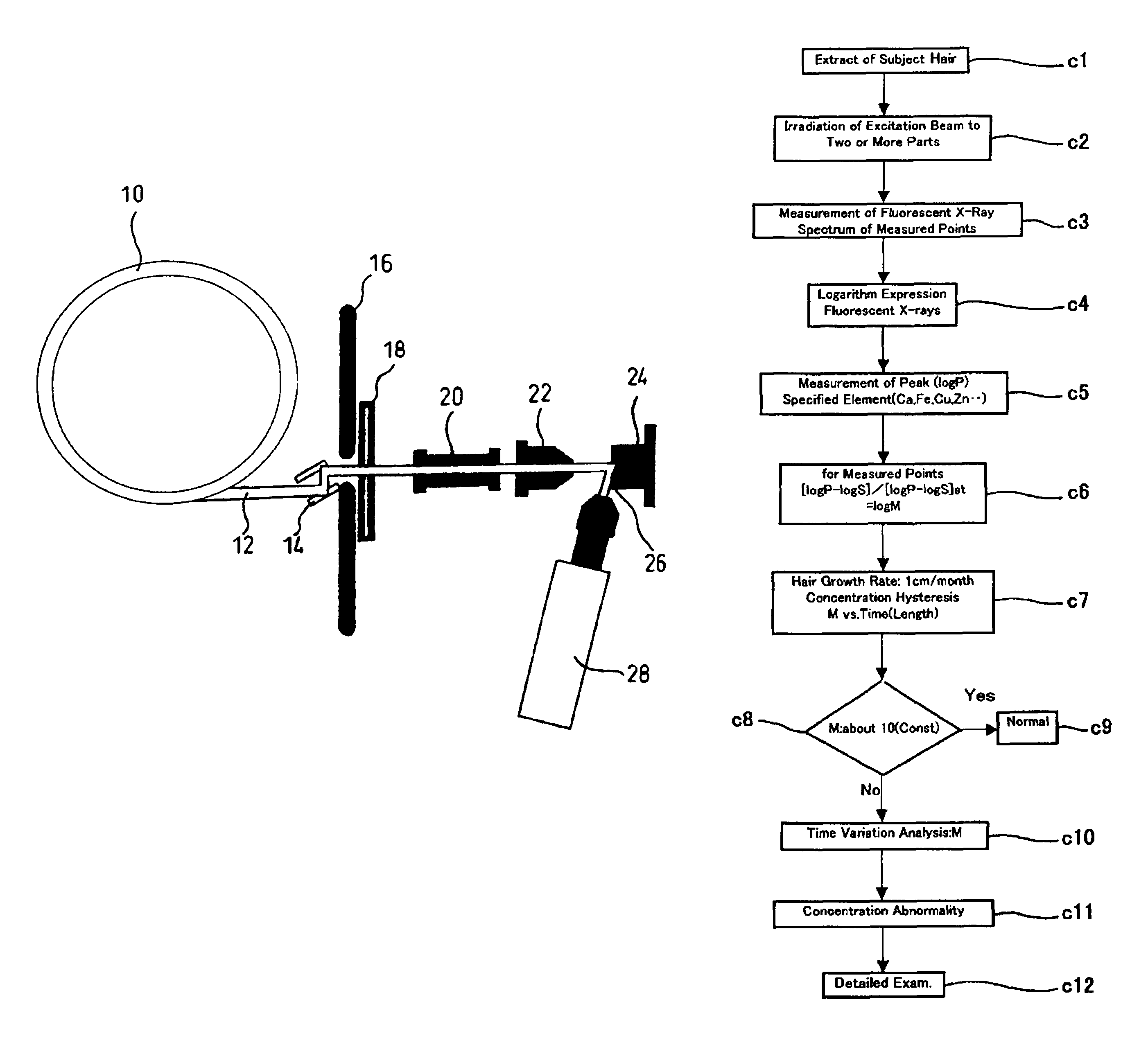
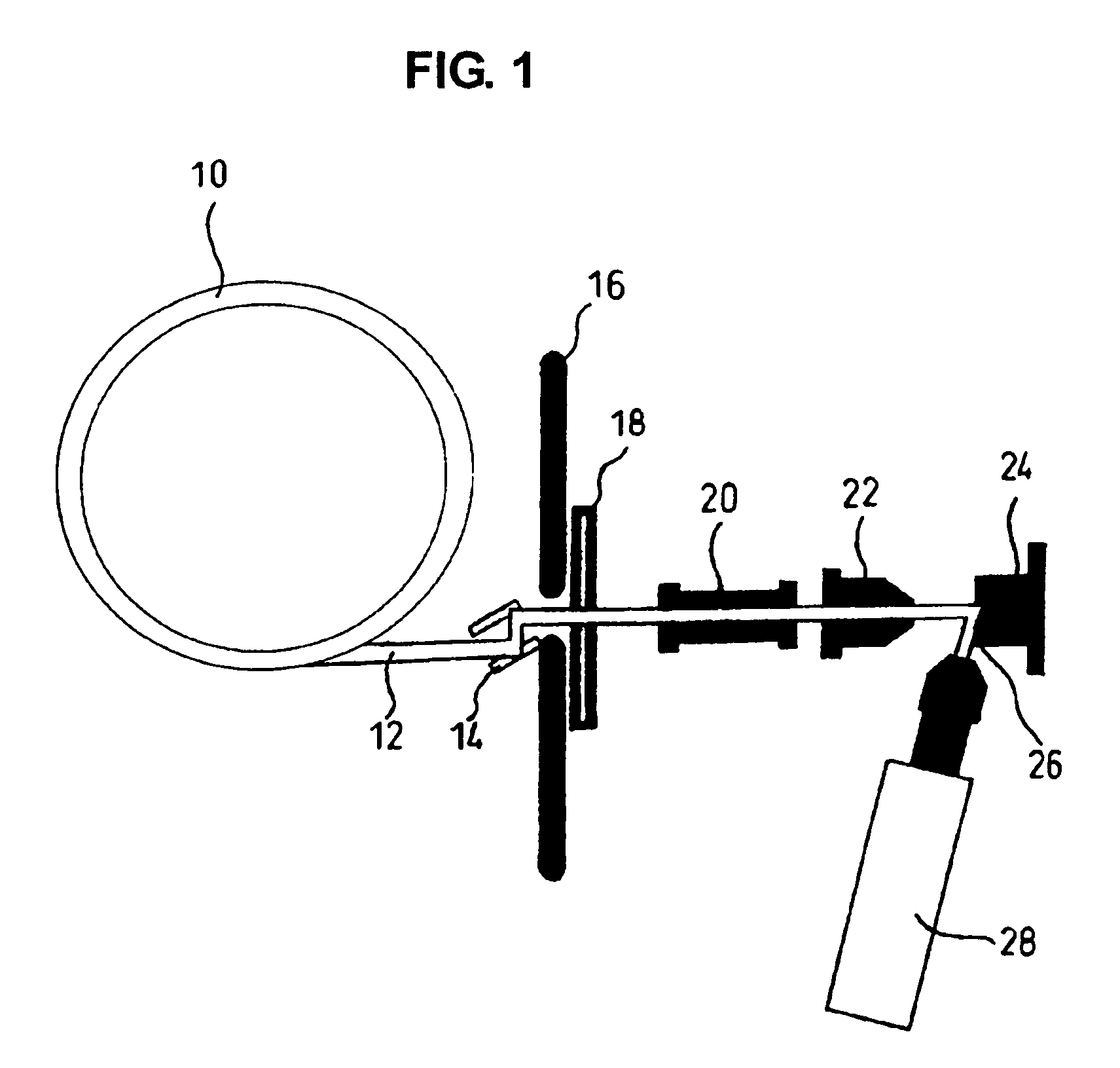
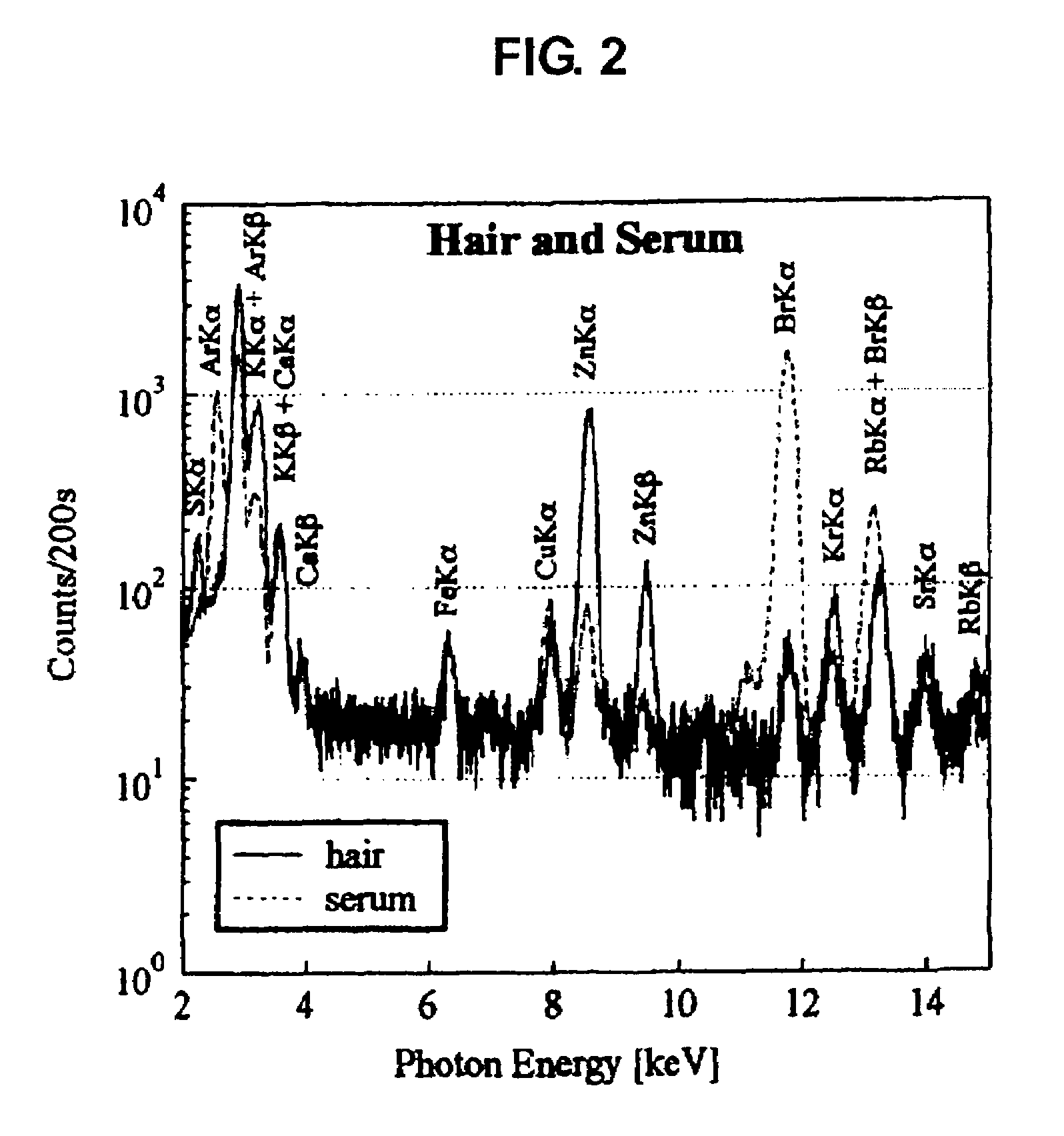
Method for evaluating physical conditions using head hair or body hair
InactiveUS7688943B2Easy to handleEasy to implementMaterial analysis using wave/particle radiationX-ray spectral distribution measurementFluorescent spectraSerum protein
Elemental concentrations in hair (head and body hair) and dried serum have been measured by x-ray fluorescence analysis using synchrotron radiation. The relative concentration defined by log P−log S are obtained from the fluorescent spectra, where P is the peak height for the element and S is the background height. The observation shows that hair has two separate [Ca] concentration levels, the upper level and lower level. Since the content in hair growing at a steady state must be equal to the supply from serum, the upper and the lower level of hair [Ca] are attributed to open and close Ca ion channels of the hair matrix cells and can be derived from the serum concentrations of Ca ion and Ca atoms included in serum protein, respectively. The hair analysis is useful for cancer detection and protection as well as for diagnosing the Ca metabolism.
Owner:CHIKAWA JUN ICHI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com