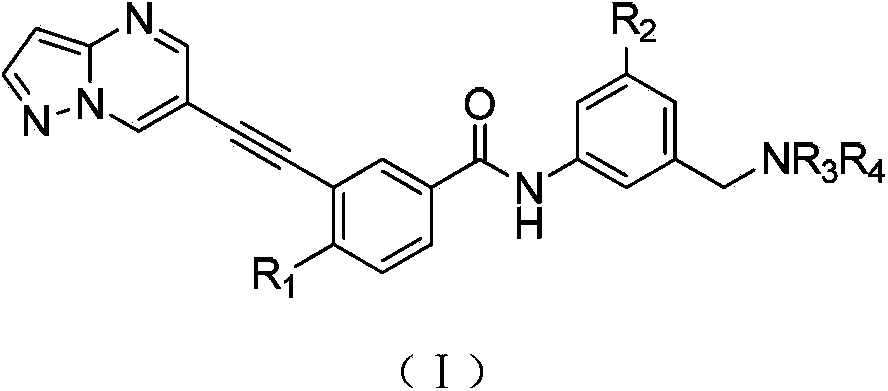
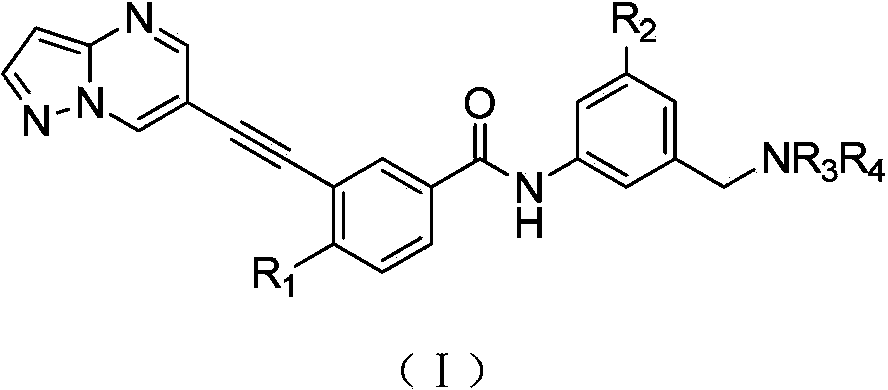
Compound used for discoidin domain receptor micro-molecule inhibitor, and its application
A small molecule inhibitor, domain technology, applied in the field of chemical medicine to avoid toxic side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] 4-Methyl-N-(3-((4-methylpiperazine-1-substituted)methyl)-5-(trifluoromethyl)phenyl)-3-(pyrazol[1,5-a ]pyrimidine-6-substituted ethynyl)benzamide (7-4090)
[0084] (4-Methyl-N-(3-((4-methylpiperazin-1-yl)methyl)-5-(trifluoromethyl)phenyl)-3-(pyrazolo[1,5-a]pyrimidin-6-ylethynyl)benzamide)
[0085]
[0086] Step 1. Methyl3-ethynyl-4-methylbenzoate (Methyl3-ethynyl-4-methylbenzoate)
[0087]
[0088] Dissolve methyl 3-iodo-4-methylbenzoate (27.61 g, 100 mmol) in ethyl acetate (300 mL) and add Pd(PPh 3 ) 2 Cl 2 (0.70g, 1mmol), CuI (0.19g, 1mmol), and triethylamine (30.4g, 300mmol). Argon was replaced 3 times and stirred overnight at room temperature. The reaction solution was filtered with celite, and the filtrate was concentrated and subjected to column chromatography. A white solid (20.9 g, 85.0%) was obtained. The above product (19.7g, 80mmol) was dissolved in methanol (300mL), potassium carbonate (16.6g, 120mmol) was added with stirring, and stirred at room...
Embodiment 2
[0109] 4-Methyl-N-(3-(morpholinylmethyl)-5-(trifluoromethyl)phenyl)-3-(pyrazol[1,5-a]pyrimidine-6-substituted ethynyl)benzene Formamide (7-4086)
[0110] (4-Methyl-N-(3-(morpholinomethyl)-5-(trifluoromethyl)phenyl)-3-(pyrazolo[1,5-a]pyrimidin-6-ylethynyl)benzamide)
[0111]
[0112] The synthesis method is as in Example 1.
[0113] H NMR (400MHz, DMSO-d 6 ( d,J=2.0Hz,1H),8.20(s,1H),8.04(s,1H),7.98(dd,J=8.0,1.6Hz,1H),7.56(d,J=8.0Hz,1H), 7.38(s,1H),6.85(dd,J=2.0,0.4Hz,1H),3.60(t,J=4.4Hz,4H),3.56(s,2H),2.60(s,3H),2.40(t ,J=4.4Hz,4H).
[0114] LC-MS (ESI): m / z520[M+H] + ;518[M-H] - .
Embodiment 3
[0116] 4-Methyl-N-(3-(piperidine-1-substituted methyl)-5-(trifluoromethyl)phenyl)-3-(pyrazol[1,5-a]pyrimidine-6-substituted Ethynyl)benzamide (7-4144)
[0117] (4-Methyl-N-(3-(piperidin-1-ylmethyl)-5-(trifluoromethyl)phenyl)-3-(pyrazolo[1,5-a]pyrimidin-6-ylmethyl)benzamide)
[0118]
[0119] The synthesis method is as in Example 1.
[0120] 1 H NMR (400MHz, DMSO-d 6 ) δ10.58 (s, 1H), 9.58 (d, J=1.2Hz, 1H), 8.72 (d, J=2.0Hz, 1H), 8.34 (d, J=2.4Hz, 1H), 8.22 (d, J=1.2Hz,1H),8.19(s,1H),8.01(s,1H),7.97(dd,J=8.0,1.6Hz,1H),7.55(d,J=8.0Hz,1H),7.35( s,1H),6.85(d,J=2.0Hz,1H),3.51(s,2H),2.59(s,3H),2.35(brs,4H),1.52(quint,J=4.2Hz,4H), 1.42-1.38 (m, 2H).
[0121] LC-MS (ESI): m / z518[M+H] + ;516[M-H] - .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com