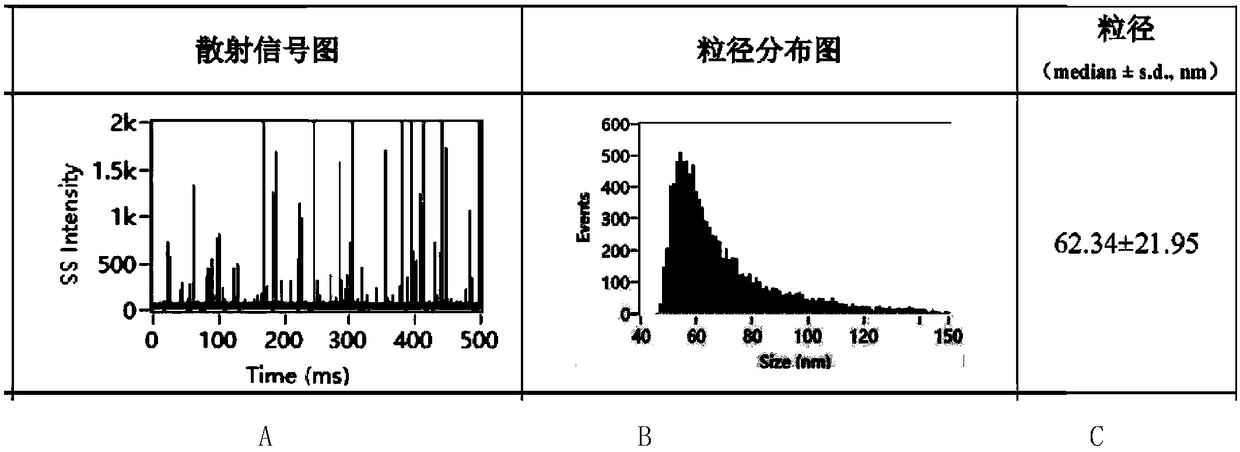
Patents
Literature
46 results about "Early hcc" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Methods and compositions for the diagnosis for early hepatocellular carcinoma
ActiveUS20080038736A1Microbiological testing/measurementBiological testingCyclin D1Early Hepatocellular Carcinoma
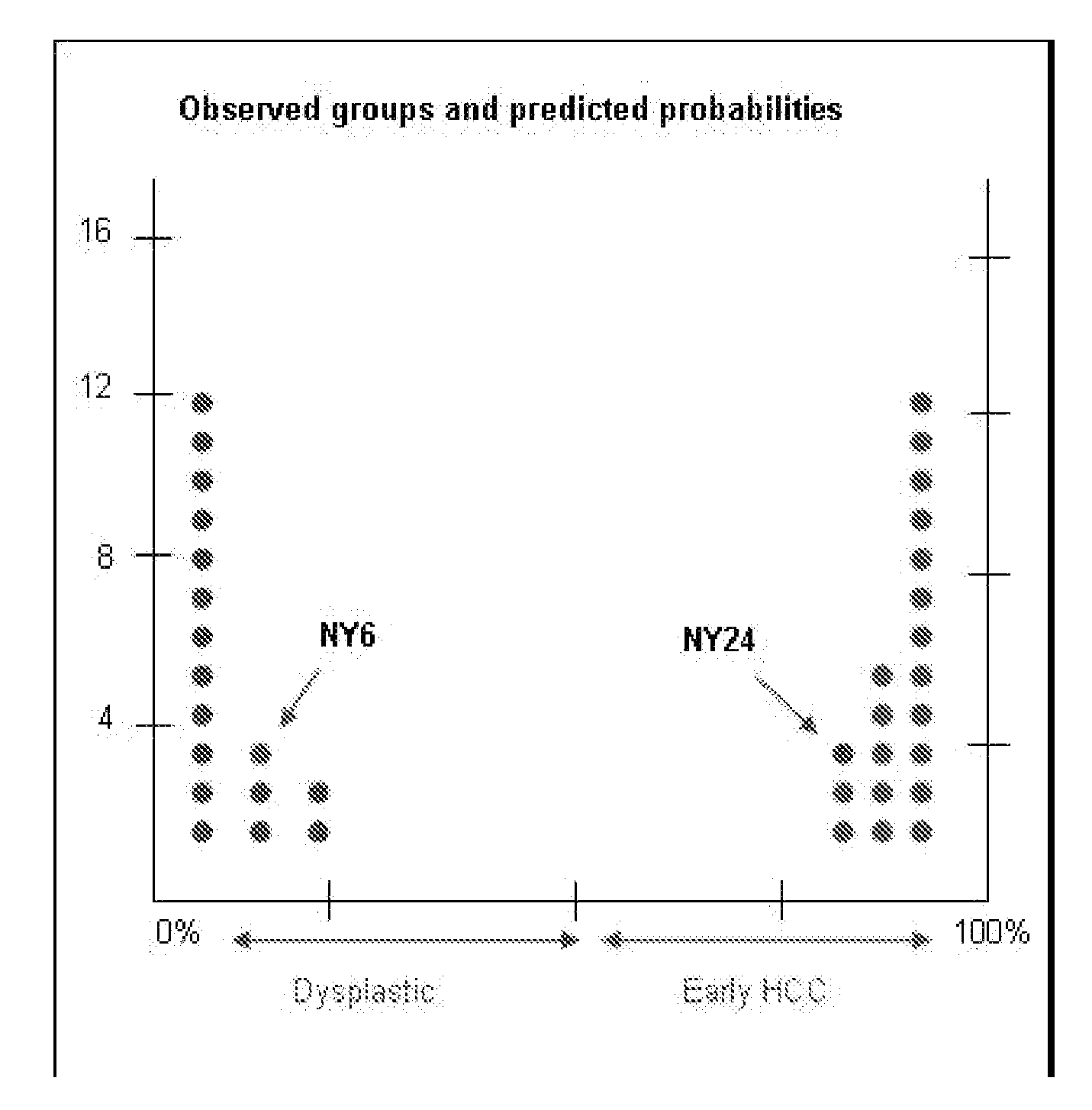
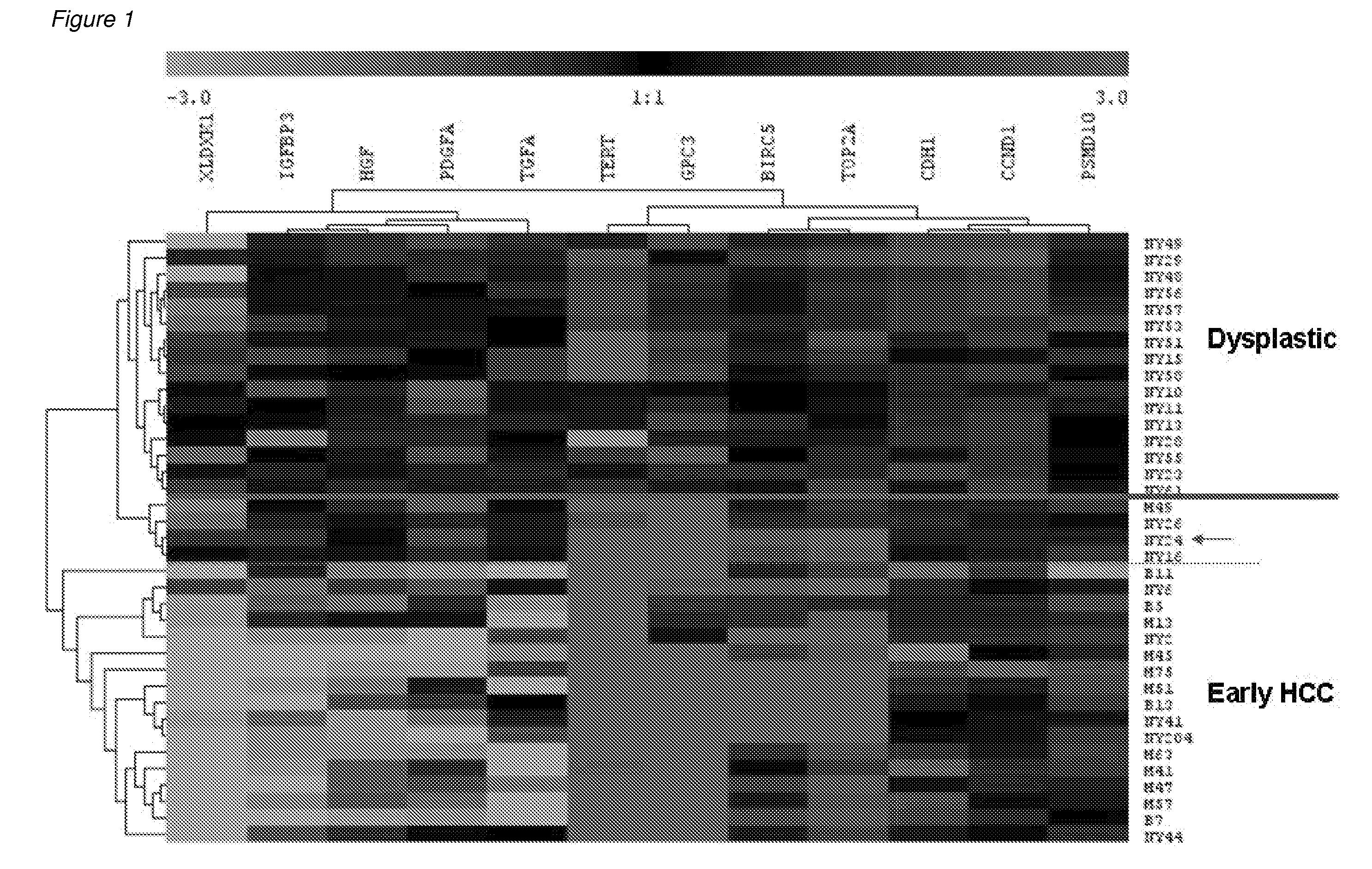
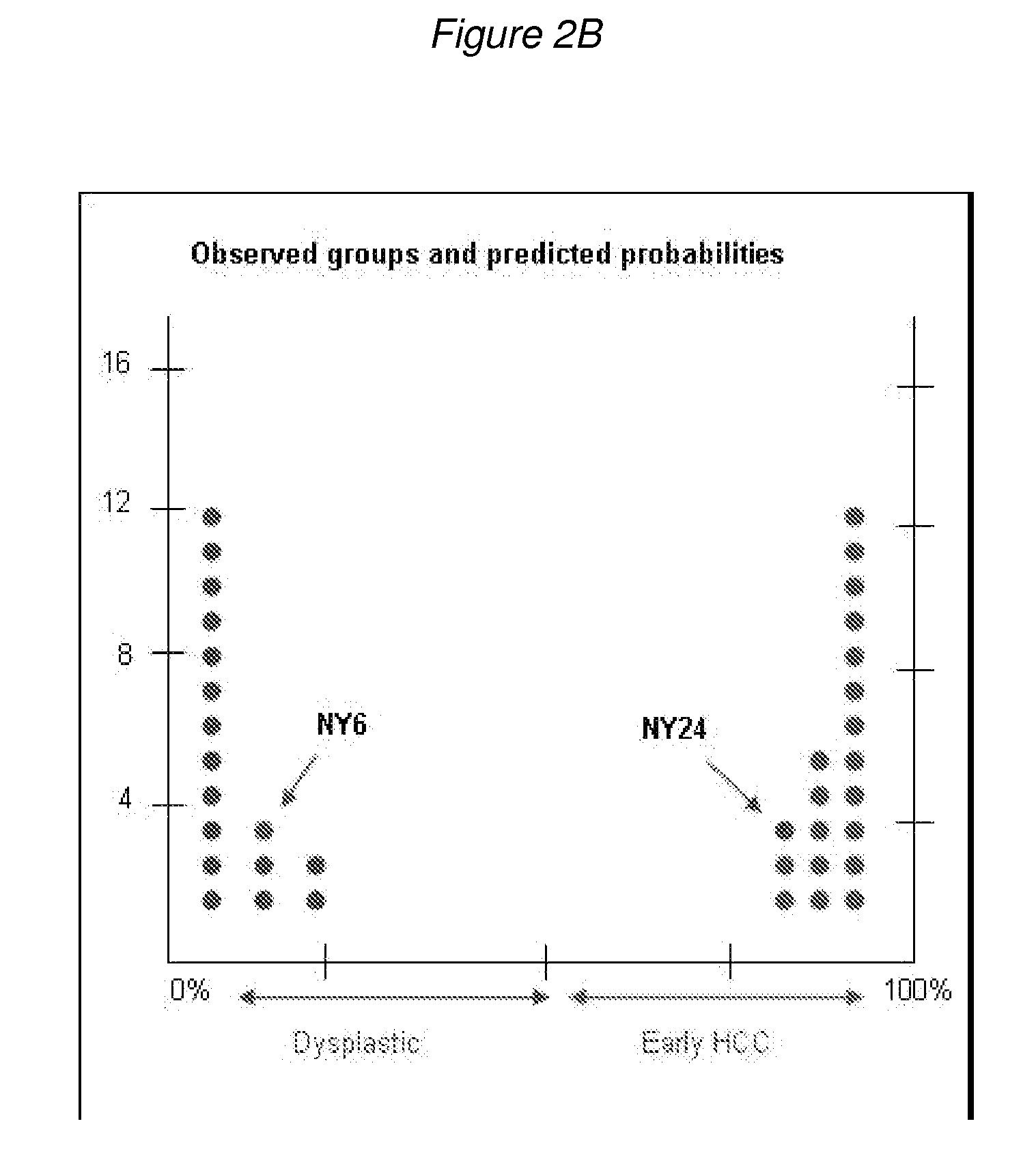
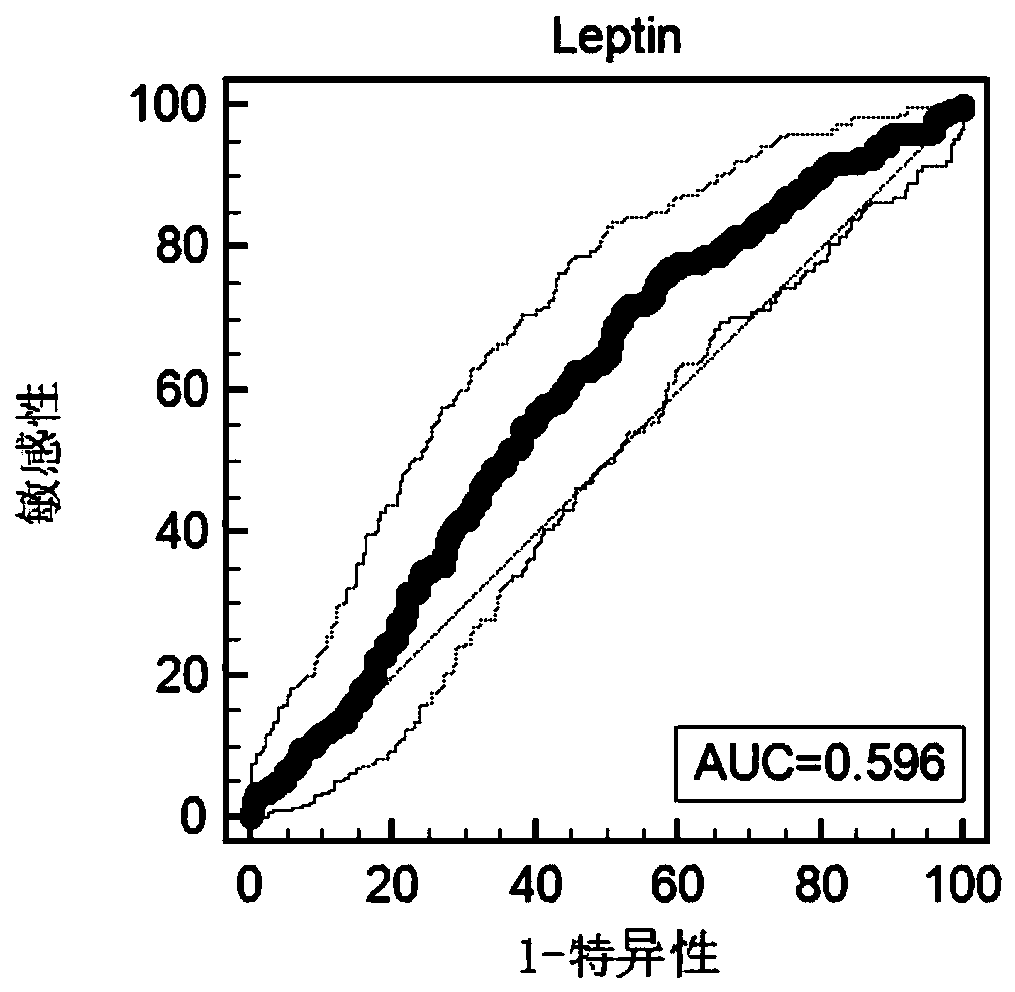
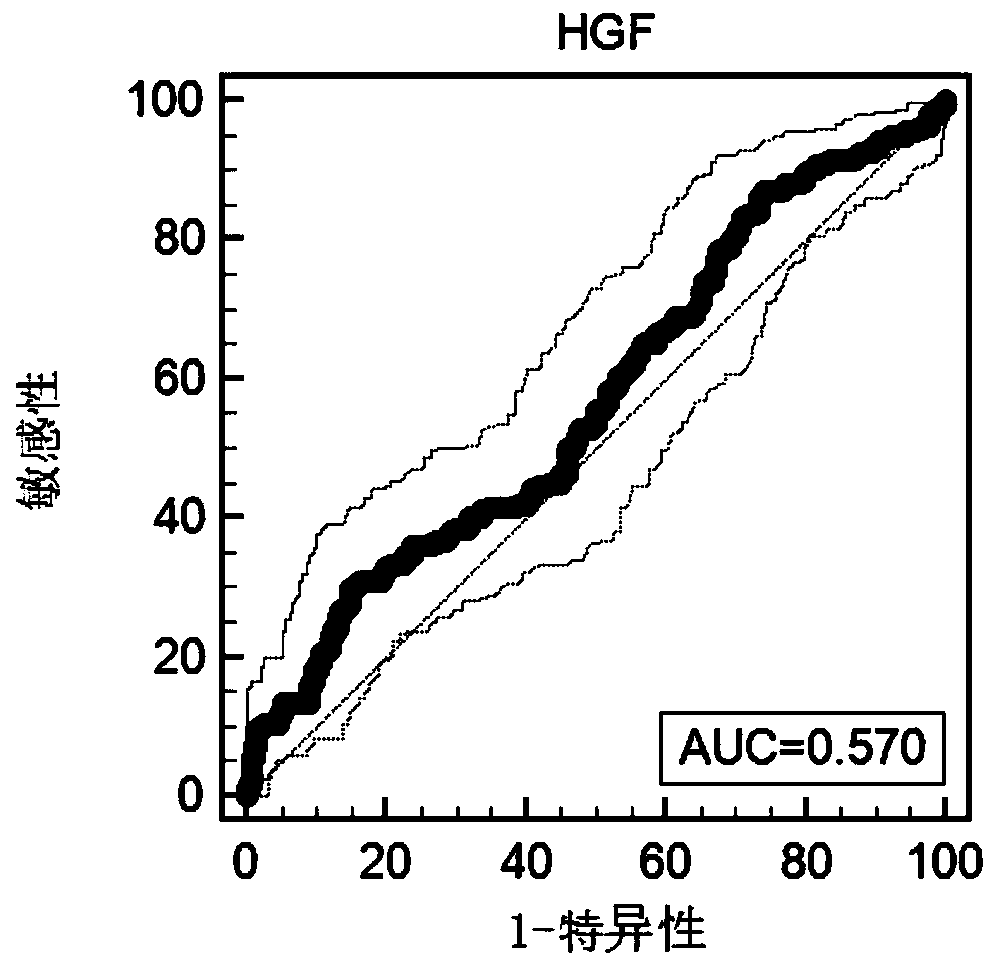
Methods and compositions are provide to allow discrimination of dysplastic nodules from early HCC nodules. More specifically, it has been determined that TERT, GPC3, gankyrin, survivin, TOP2A, LYVE1, Ecadherin, IGFBP3, PDGFRA, TGFA, cyclin D1 and HGF are differentially expressed in HCC as compared to normal liver cells and liver cells that have dysplastic, non-cancerous nodules.
Owner:MT SINAI SCHOOL OF MEDICINE
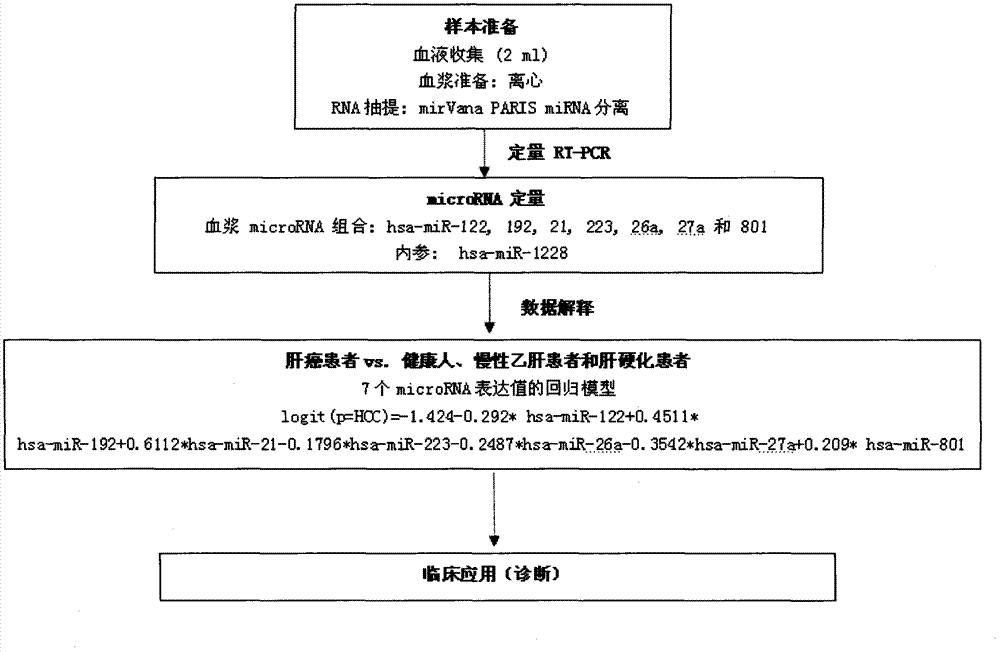
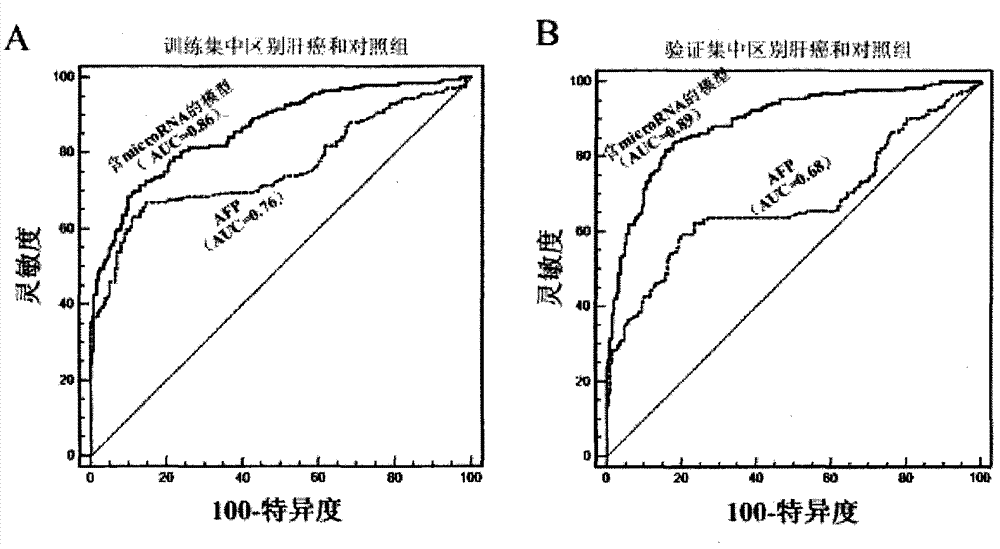
Liver cancer diagnostic marker composed of blood plasma microRNA (micro ribonucleic acid) and new method for diagnosing liver cancer
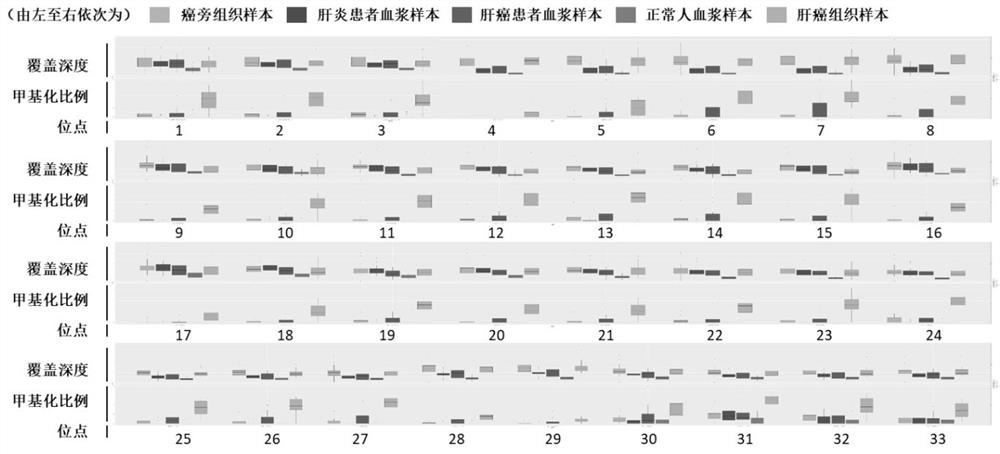
ActiveCN102776185AMicrobiological testing/measurementDNA/RNA fragmentationChronic hepatitisEarly Hepatocellular Carcinoma
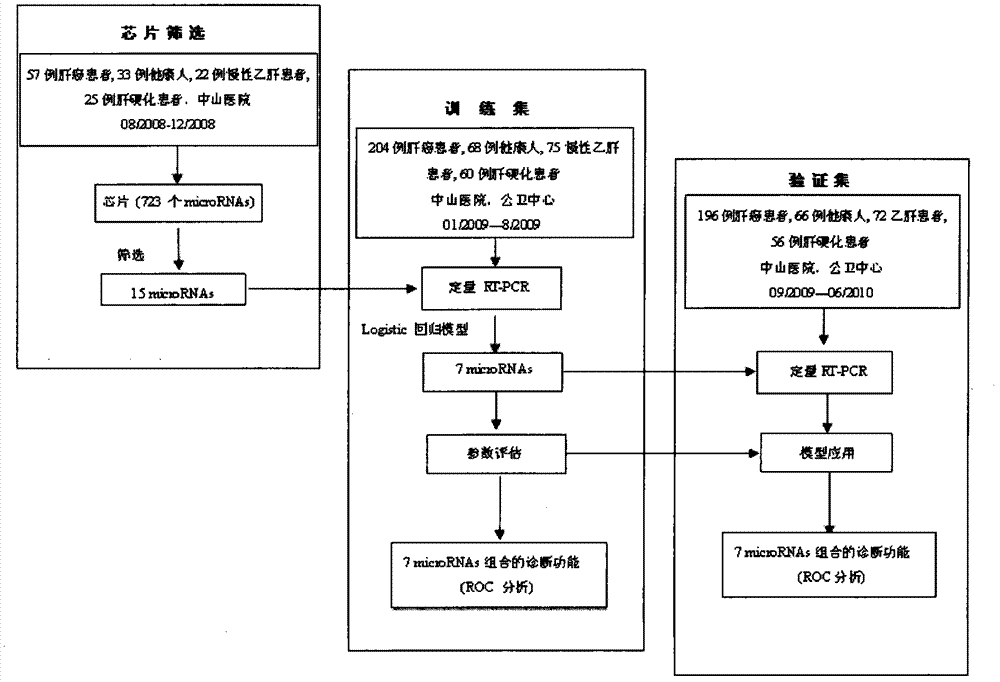
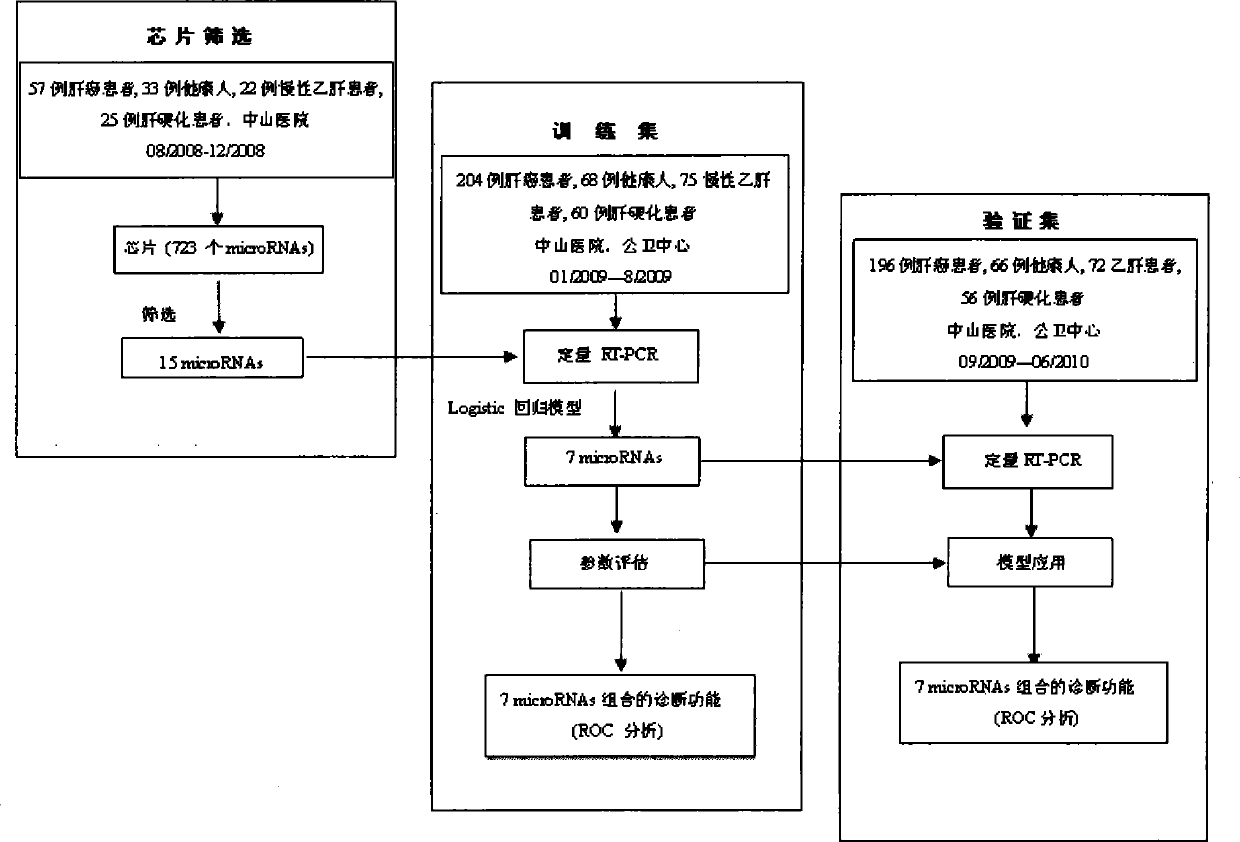
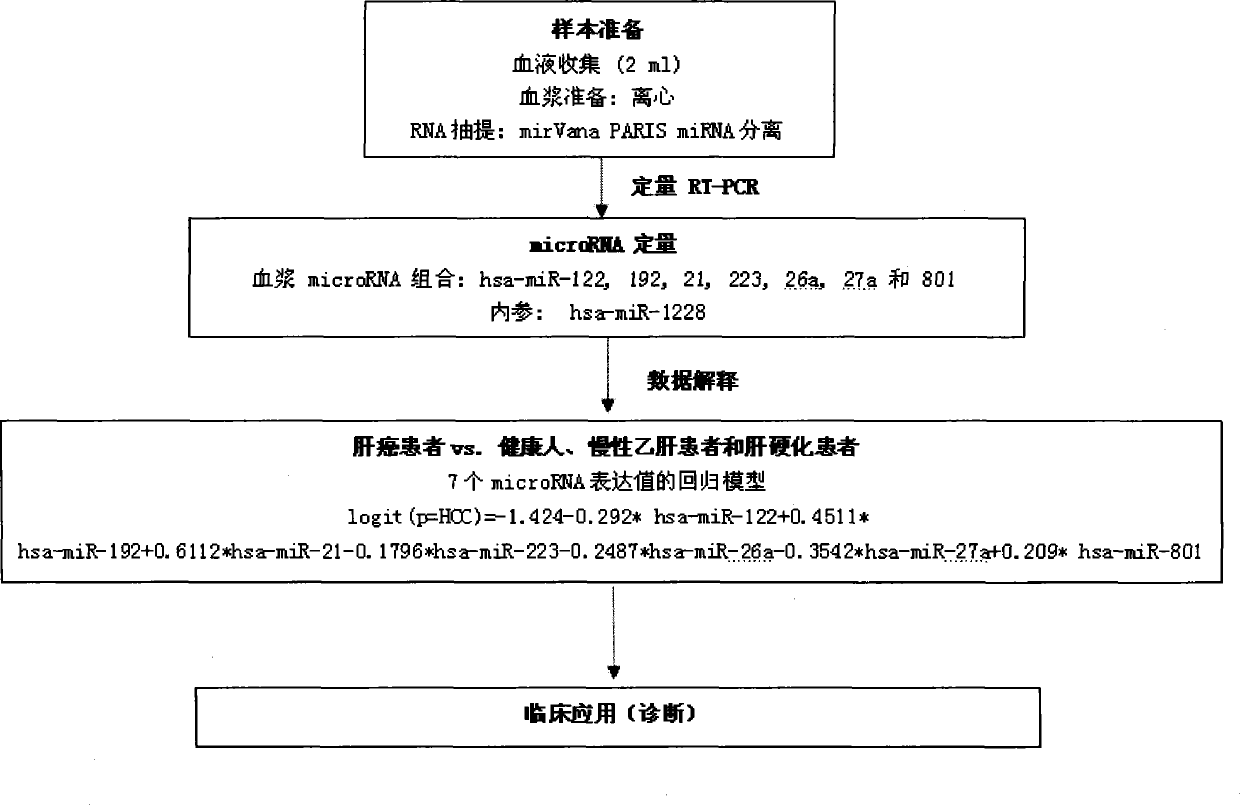
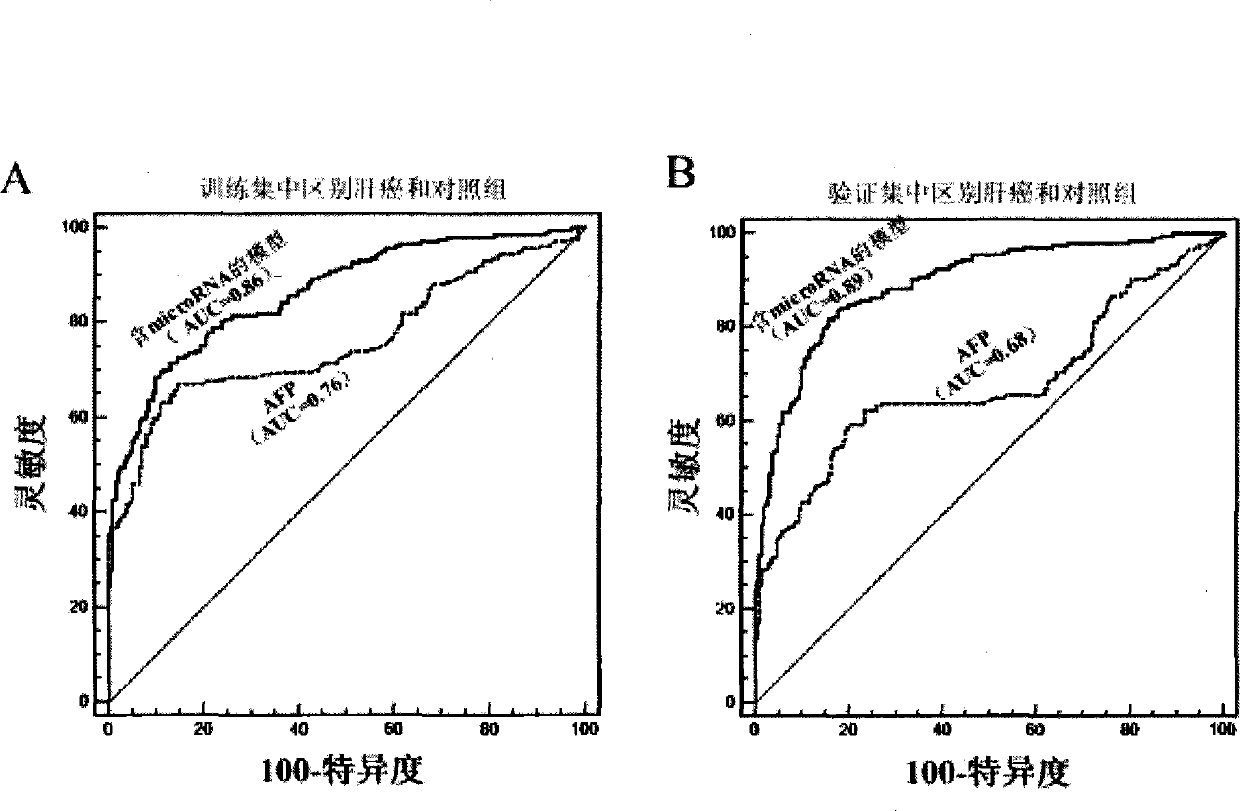
The invention relates to a liver cancer diagnostic marker composed of blood plasma microRNA (micro ribonucleic acid), a kit containing the marker and a new method for diagnosing liver cancer (in particular early liver cancer). The liver cancer diagnostic marker is composed of a plurality of nucleic acid molecules, wherein each nucleic acid molecule is used for coding at least one microRNA sequence; and preferably, the liver cancer diagnostic marker is composed of nucleic acid molecules for coding hsa-miR-122, hsa-miR-192, hsa-miR-21, hsa-miR-223, hsa-miR-26a, hsa-miR-27a, hsa-miR-801 and hsa-miR-1228. The kit can be applied to diagnosing hepatocellular carcinoma, in particular early hepatocellular carcinoma, or to differentiating the blood plasma of at least one hepatocellular carcinoma sufferer from the blood plasma of at least one healthy individual, and the blood plasma of at least one chronic hepatitis b sufferer or the blood plasma of at least one cirrhosis sufferer.
Owner:JUSBIO SCI SHANGHAI CO LTD
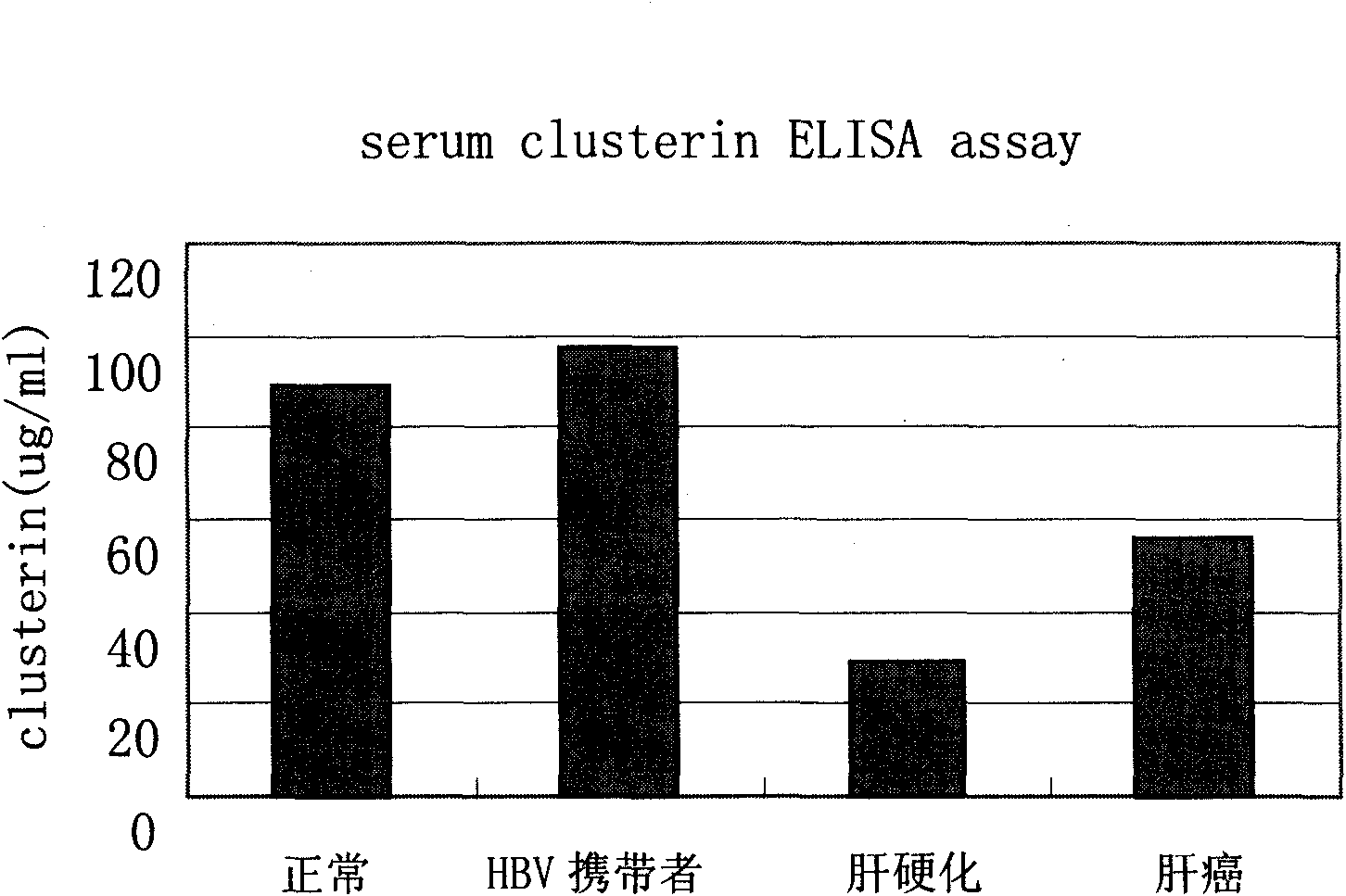
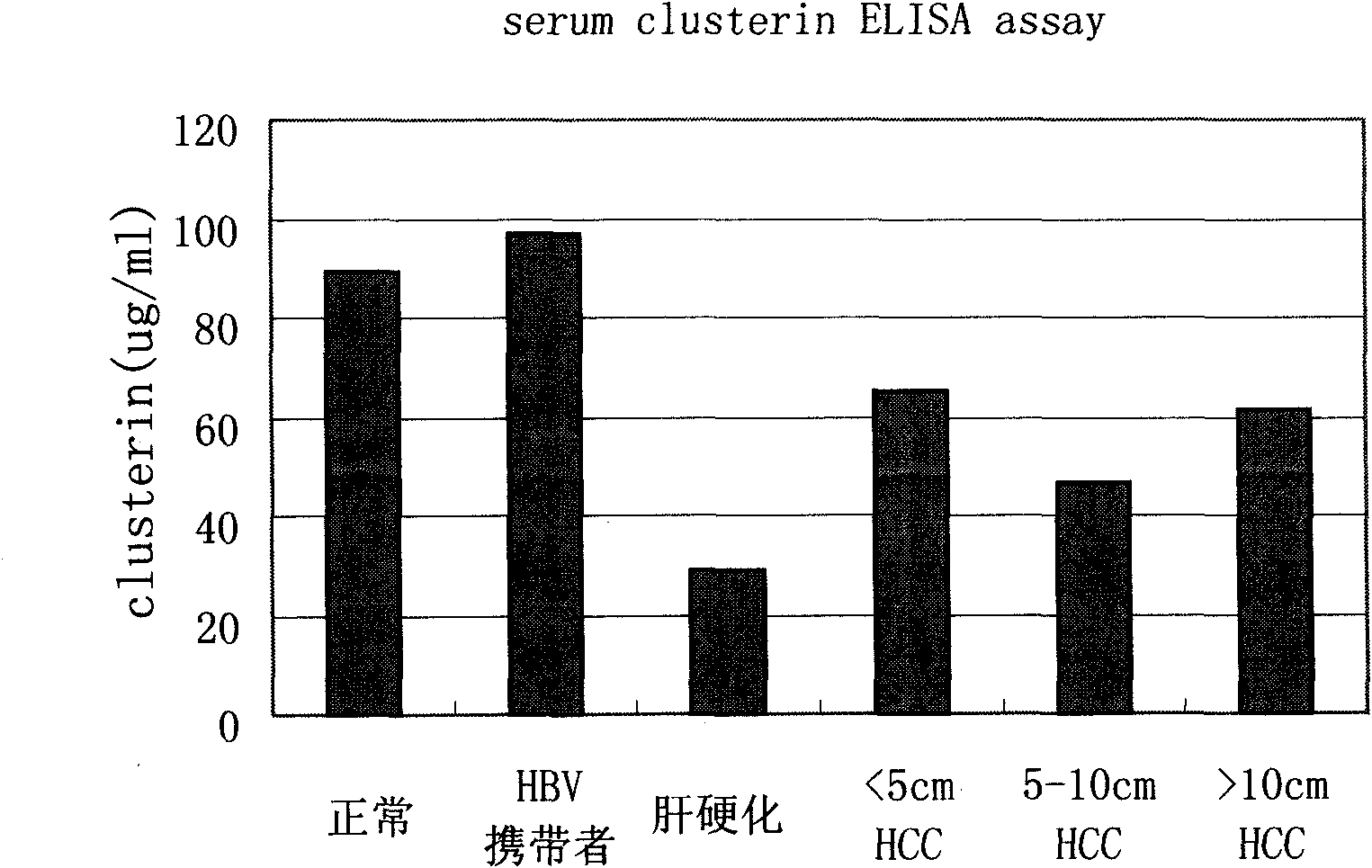
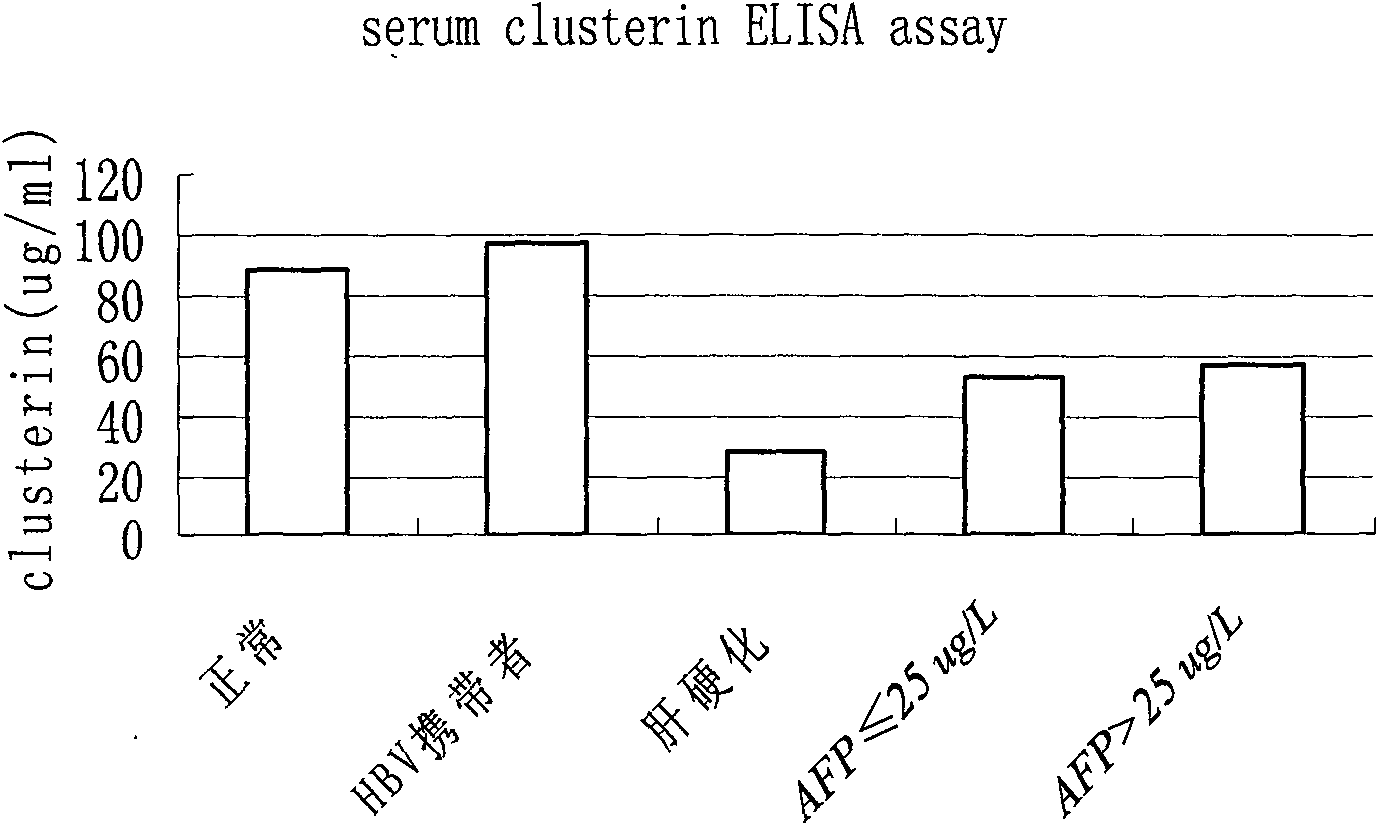
Kit for distinguishing and diagnosing hepatocirrhosis and early liver cancer and application of serum clusterin in preparing same
The invention discloses a kit for diagnosing primary hepatoma, preparation method and application thereof. The kit can quickly and accurately carry out the clusterin detection so as to carry out quickauxiliary diagnosis on the primary hepatoma of each period; and the kit especially has an important clinical application value for distinguishing hepatocirrhosis and early liver cancer. The kit comprises an enzyme-linked immunological diagnosis system established on the basis of a double-antibody sandwich principle. The kit has high sensitivity, strong specificity, simple operation, stable reagent, good repeatability, easy popularization and application and extensive market prospect.
Owner:SUN YAT SEN UNIV
Gene chip for predicting liver cancer metastasis and recurrence risk and manufacturing method and using method thereof
InactiveCN101812507AImprove survivalAccurate predictionMicrobiological testing/measurementLymphatic SpreadCvd risk
The technical scheme of the invention provides a gene chip for predicting liver cancer metastasis and recurrence risk, which comprises a substrate and a probe arranged on the substrate, wherein the probe consists of 149 genes. The gene chip for predicting the liver cancer metastasis and the recurrence risk has the advantages that: due to the adoption of genome comparison research, a large sample independently proves that a prediction model can accurately predict and evaluate the metastasis potential of liver cancer patients. Therefore, postoperative survival and metastasis, and recurrence of the patient can be accurately predicted (even early liver cancer patients can be accurately predicted). The gene chip contributes to early identifying or predicting high-risk patients so as to perform intensive monitoring and effective intervention on the high-risk patients to further prolong the life of the tumor patients.
Owner:ZHONGSHAN HOSPITAL FUDAN UNIV
Kit for early screening of liver cancer and using method of kit
ActiveCN108753979AHigh incidence of methylationDiagnosis applicableMicrobiological testing/measurementDNA/RNA fragmentationForward primerLiver cancer
The invention belongs to the field of molecular biology and particularly relates to a kit for early screening of liver cancer and a using method of the kit. The invention firstly discovers that SCAND3can serve as a methylation marker for detecting the liver cancer. Sequences of primers for detecting SCAND3 methylation are as follows: the sequence of a forward primer is shown as SEQ ID NO. 1; thesequence of a reverse primer is shown as SEQ ID NO. 2; the sequence of a fluorescence probe is SEQ ID NO. 3; the 5' terminal of the probe is labeled with FAM, and the 3' terminal of the probe is labeled with MGB. The SCAND3 is the methylation marker for early screening of the liver cancer, is used for liver cancer diagnosis, and has the advantages of high sensitivity and strong specificity, and particularly in a patient having the AFP (Alpha-Fetoprotein) of less than 400 ug / L, the SCAND3 methylation incidence reaches 60%. Therefore, the kit disclosed by the invention takes the SCAND3 as the methylation marker, is applicable to diagnosis of the liver cancer, and is particularly applicable to diagnosis of early liver cancer.
Owner:ANHUI DAJIAN MEDICAL TECH CO LTD
Biomarker used for early diagnoses of liver cancer and application thereof
InactiveCN108504739AAchieve early screeningAchieve early diagnosisMicrobiological testing/measurementDNA/RNA fragmentationDiseaseFeces
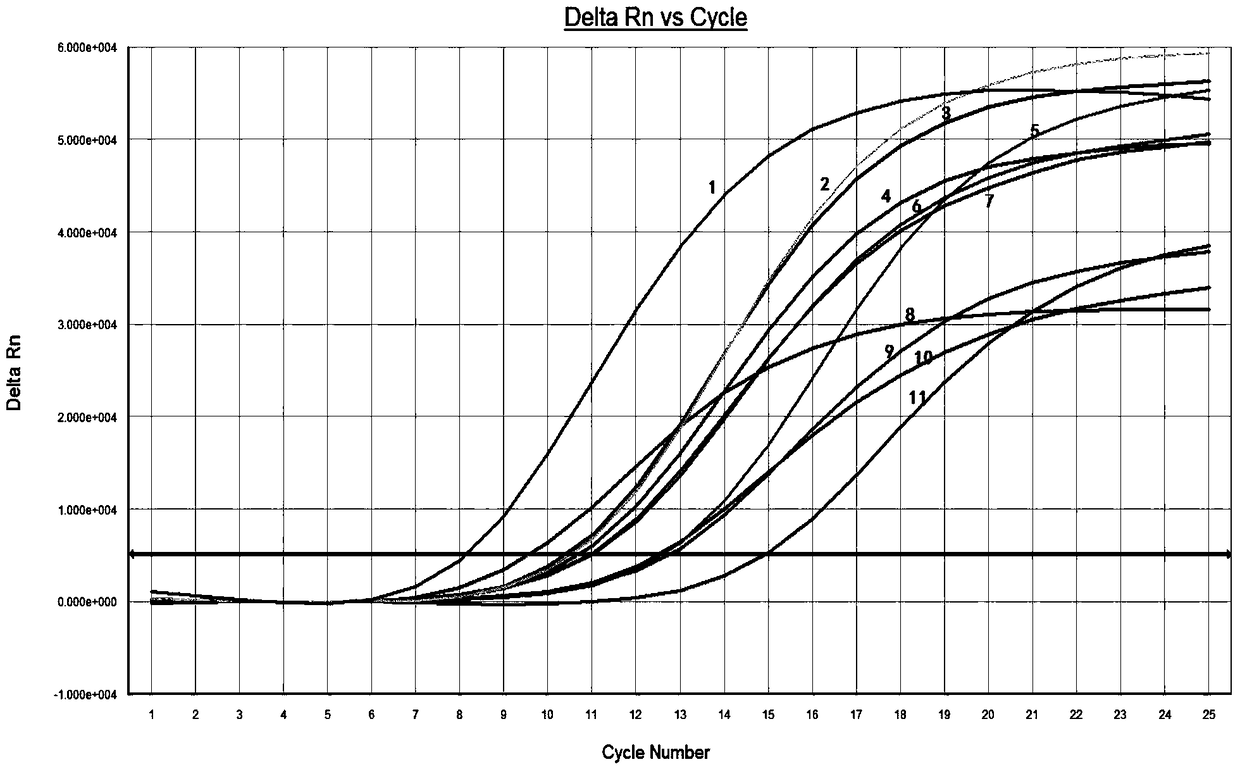
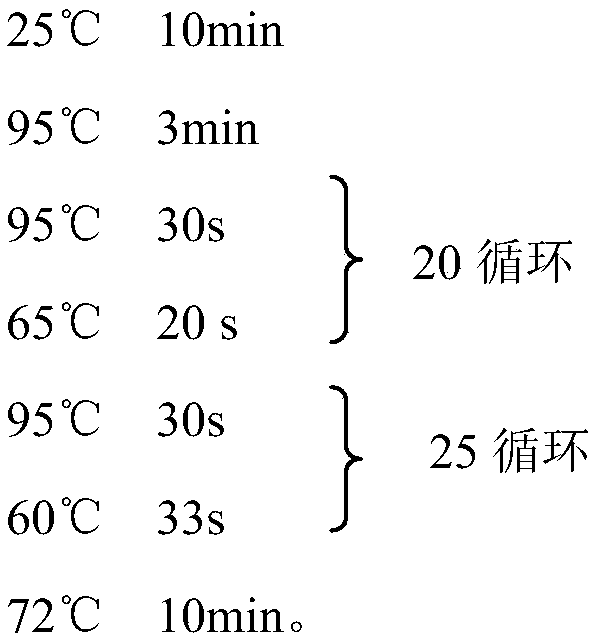
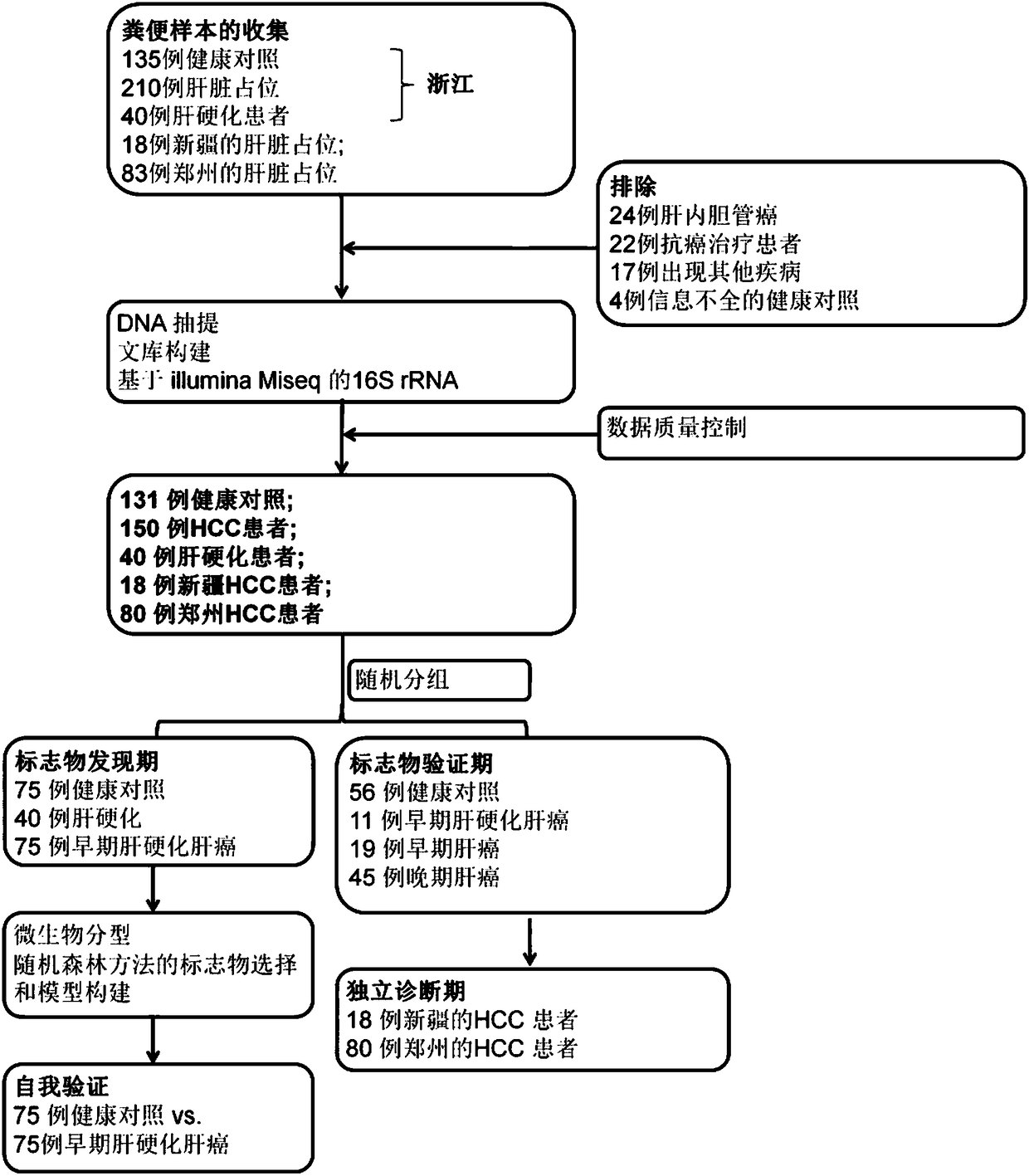
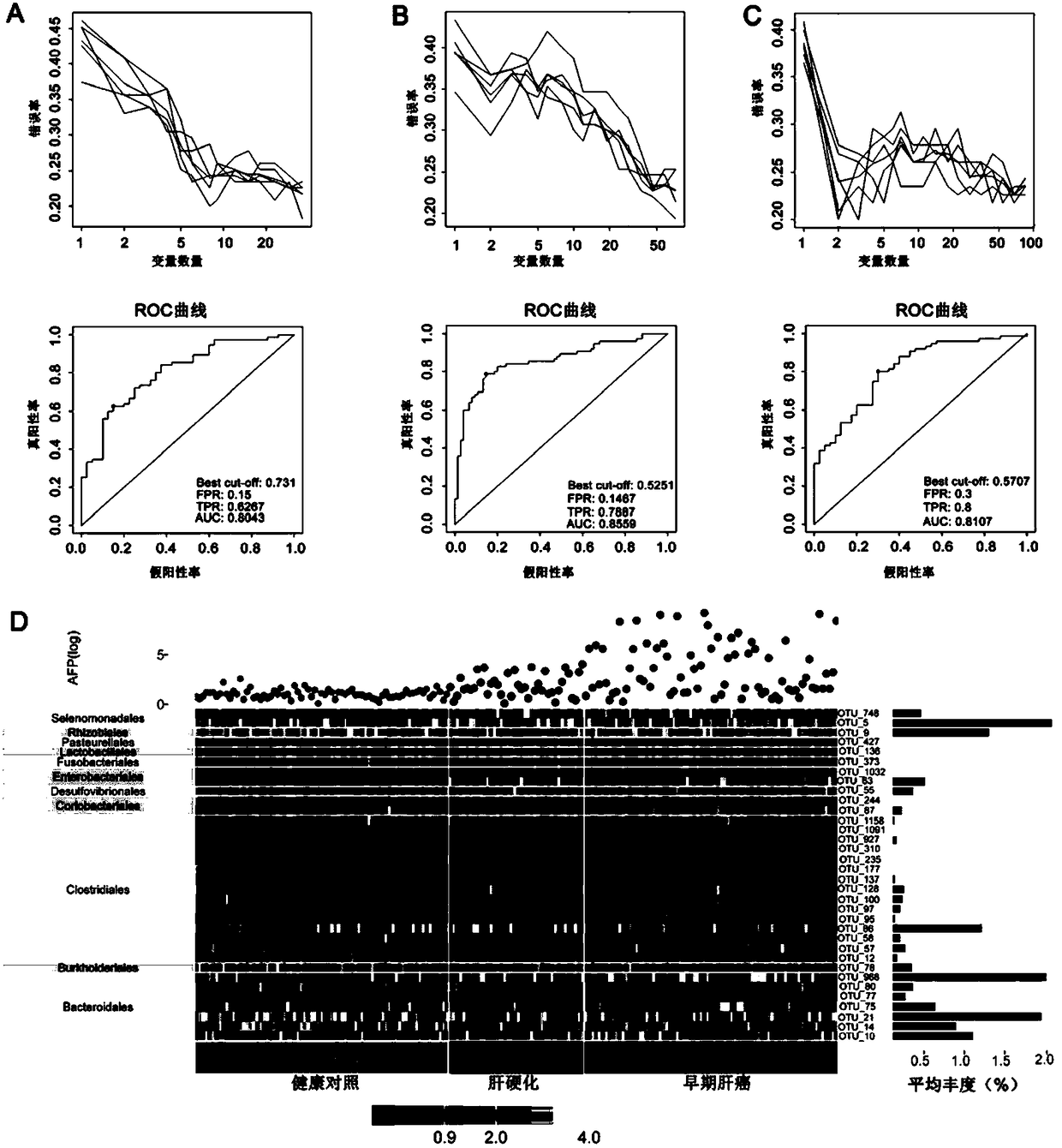
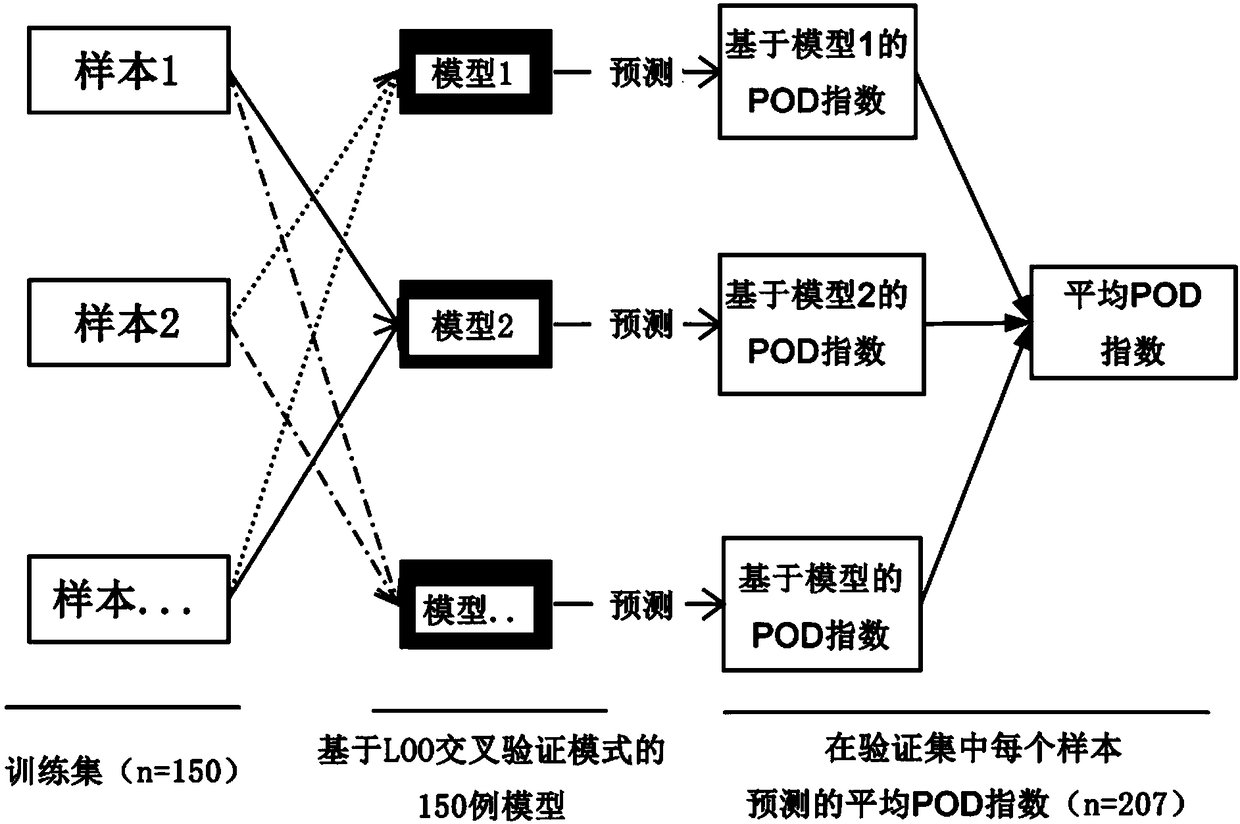
The invention relates to a marker for intestinal microecology and application thereof, in particular to a biomarker used for early diagnoses of liver cancer. The biomarker used for early diagnoses ofliver cancer is composed of 34 kinds of genes shown as SEQ ID NO:1-34, and the genes are enriched in intestinal tracts. The invention also provides a detection reagent, and the reagent includes a primer used for detecting the 34 kinds of genes shown as SEQ ID NO:1-34. Feces of patients in groups are collected through a noninvasive method, and 16S rRNA Miseq sequencing of intestinal florae is conducted. During a discovery period of the biomarker, through a random forest law, microorganism gene markers with specificity of early liver cancer are identified among patients suffering from early cirrhosis and liver cancer, patients suffering from cirrhosis and healthy comparisons, an index of a probability of disease (POD) of liver cancer is created, and verification of the markers during the discovery period is achieved. During the verification period of the markers, the POD index of the patients during the verification period is calculated, and verification of the diagnosis value of the patients suffering from liver cancer during the verification period according to the POD index is achieved.
Owner:ZHEJIANG UNIV
Screening method of early-stage liver cancer diagnosis markers for people with liver cirrhosis and hepatitis
The invention belongs to the technical field of clinical medical diagnosis and relates to a screening method of early-stage liver cancer diagnosis markers for people with liver cirrhosis and hepatitis. The screening method of the early-stage liver cancer diagnosis markers for people with liver cirrhosis and hepatitis liver cirrhosis and hepatitis comprises the following steps of: S1, acquiring data, specifically, collecting a target plasma sample and detecting metabolites in the plasma sample; S2, constructing a discrimination model used for distinguishing people with liver cirrhosis from patients with the primary liver cancer and distinguishing people with the hepatitis from patients with the primary liver cancer; S3, screening markers; and S4, verifying diagnosis capability. According to the screening method of early-stage liver cancer diagnosis markers for people with liver cirrhosis and hepatitis, a high performance liquid chromatography-mass spectrometry system is utilized to obtain the metabolic profiles of blood plasma of patients with liver cirrhosis, hepatitis and hepatocellular carcinoma, a potential tumor metabolic marker group is found, and a diagnosis model is constructed and is used for assisting early diagnosis of tumors of groups with high risk of liver cancer.
Owner:MEI HOSPITAL UNIV OF CHINESE ACAD OF SCI
Kit for carrying out early warning and screening on liver cancer
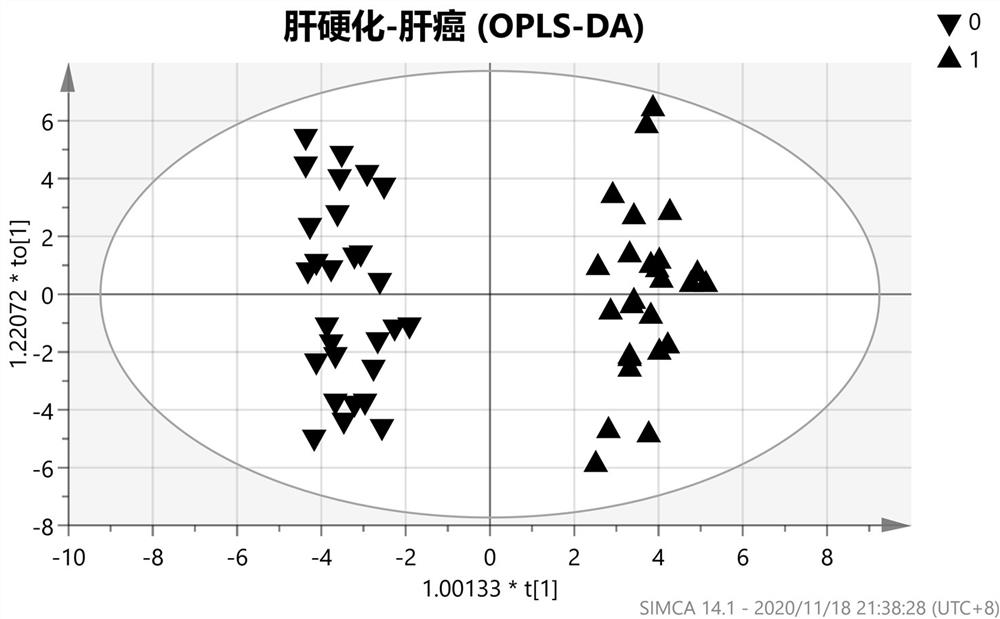
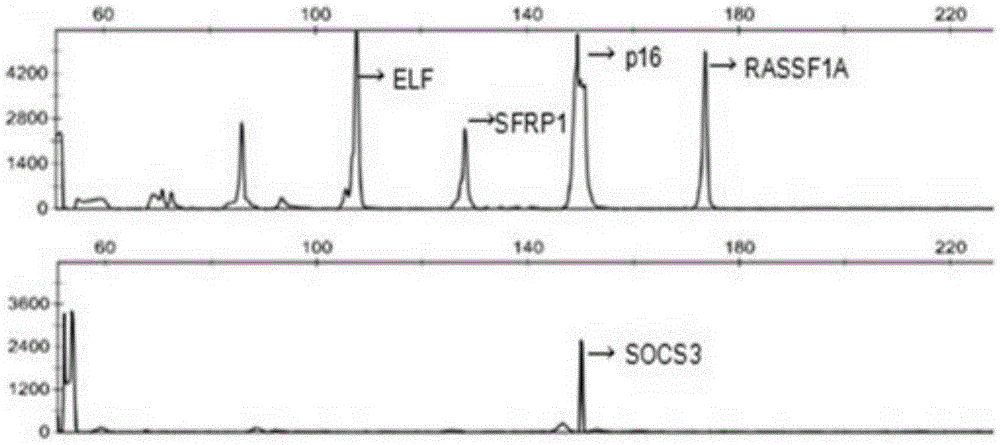
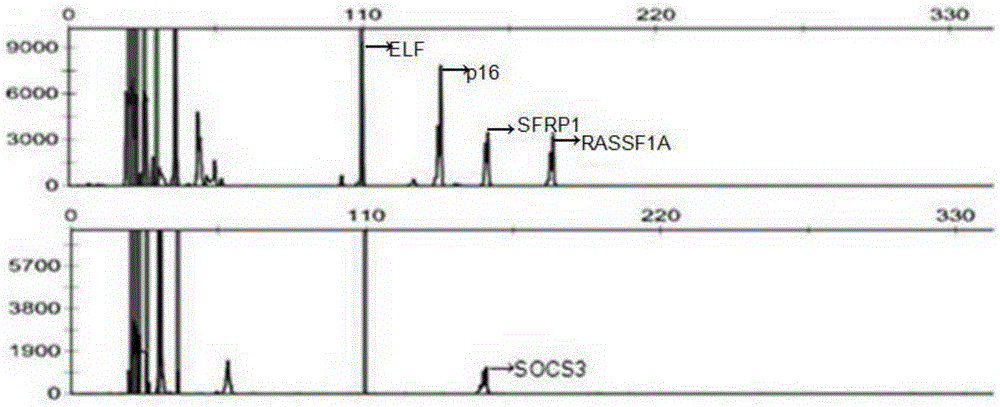
The invention belongs to the field of molecular biology (tumor screening), particularly relates to a technology for carrying out early warning and screening on liver tumor, and aims to solve the technical problem to provide a new choice for early prediction of the liver tumor. The technical scheme of the invention is that a kit for carrying out early warning and screening on liver tumor comprises a primer which is used for amplifying the following genes of an RASSF1A (Ras-association Domain Family 1A) gene of which a promoter is in a CpG island methylation state, a p16 gene, ELF (Embryonic Liver Fodrin), an SOCS (Suppressors of Cytokine Signaling)-3 gene, an SFRP1 (Secreted Frizzled-Related Protein 1) gene and a genome repetitive sequence LINE1 (Long Interspersed Nuclear Elements 1). According to the kit disclosed by the invention, a new effective inspection method is provided for carrying out early warning and screening on early liver tumor and liver cirrhosis; the cost is low, the use is very convenient, the flexibility is high, the specificity is good, and the application prospect is very good.
Owner:SICHUAN UNIV
Application of plasma exosome miR-455-3p as early liver cancer diagnostic marker
PendingCN109439757AEasy to carry outQuantitatively accurateMicrobiological testing/measurementDNA/RNA fragmentationExosomeBlood plasma
The invention belongs to the fields of biotechnology and medicine, and particularly relates to application of plasma exosome miR-455-3p as an early liver cancer diagnostic marker.
Owner:BEIJING YOUAN HOSPITAL CAPITAL MEDICAL UNIV +1
Transcriptome-sequencing-based gene group for early diagnosis of liver cancer and prognostic evaluation and application thereof
ActiveCN107058550AEasy diagnosisReduce misdiagnosis rateMicrobiological testing/measurementDNA/RNA fragmentationCvd riskTranscriptome Sequencing
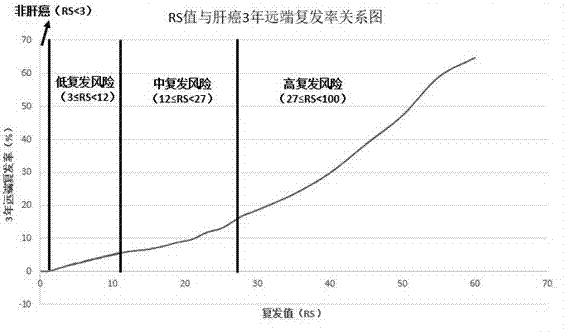
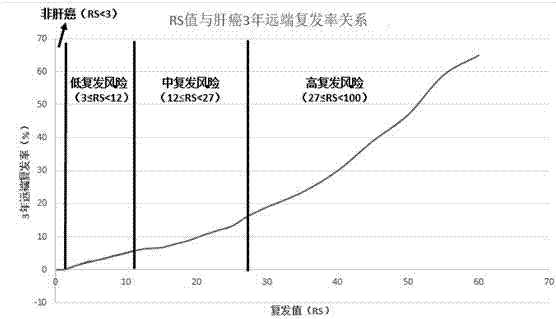
The invention discloses a transcriptome-sequencing-based gene group for early diagnosis of liver cancer and prognostic evaluation and application thereof; the gene group comprises four genes, GPC3, HSP70, GS and PIVKA-II. Recurrence risk value is calculated according to expression amounts of the four genes, whether a patient under test carries early liver cancer or not and prognostic recurrence rate of a patient with liver cancer are judged according to the recurrence risk value. An algorithm to calculate the recurrence risk value via the expression amounts is RS=0.080*GP3+0.098*HSP70+0.047*GS+0.13*PIVKA-II. Preclinical verification results are precise, diagnostic sensitivity for early primary liver cancer reaches 100%, and diagnostic sensitivity for non-liver cancers reaches 100%, prediction sensitivity to a low-recurring risk patient reaches 100%, prediction sensitivity to a medium-recurring risk patient reaches 96.774%, prediction sensitivity to a high-recurring risk patient reaches 99.375%, and detection capacity and efficiency are improved with detection cost greatly reduced; clear tips can be provided upon a patient's prognosis.
Owner:上海东方肝胆外科医院
A kit for auxiliary diagnosis of early early liver cancer and a detection method thereof
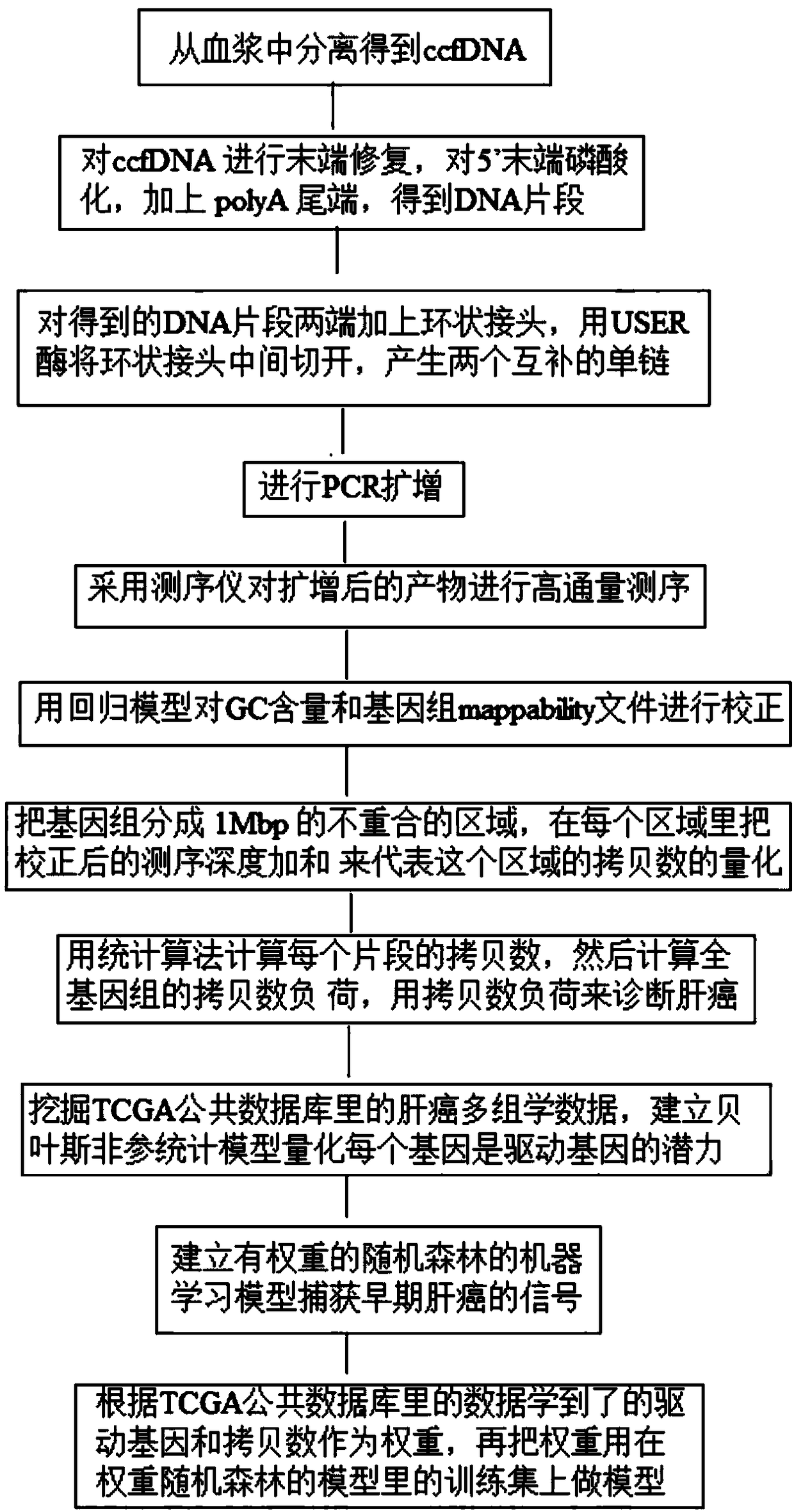
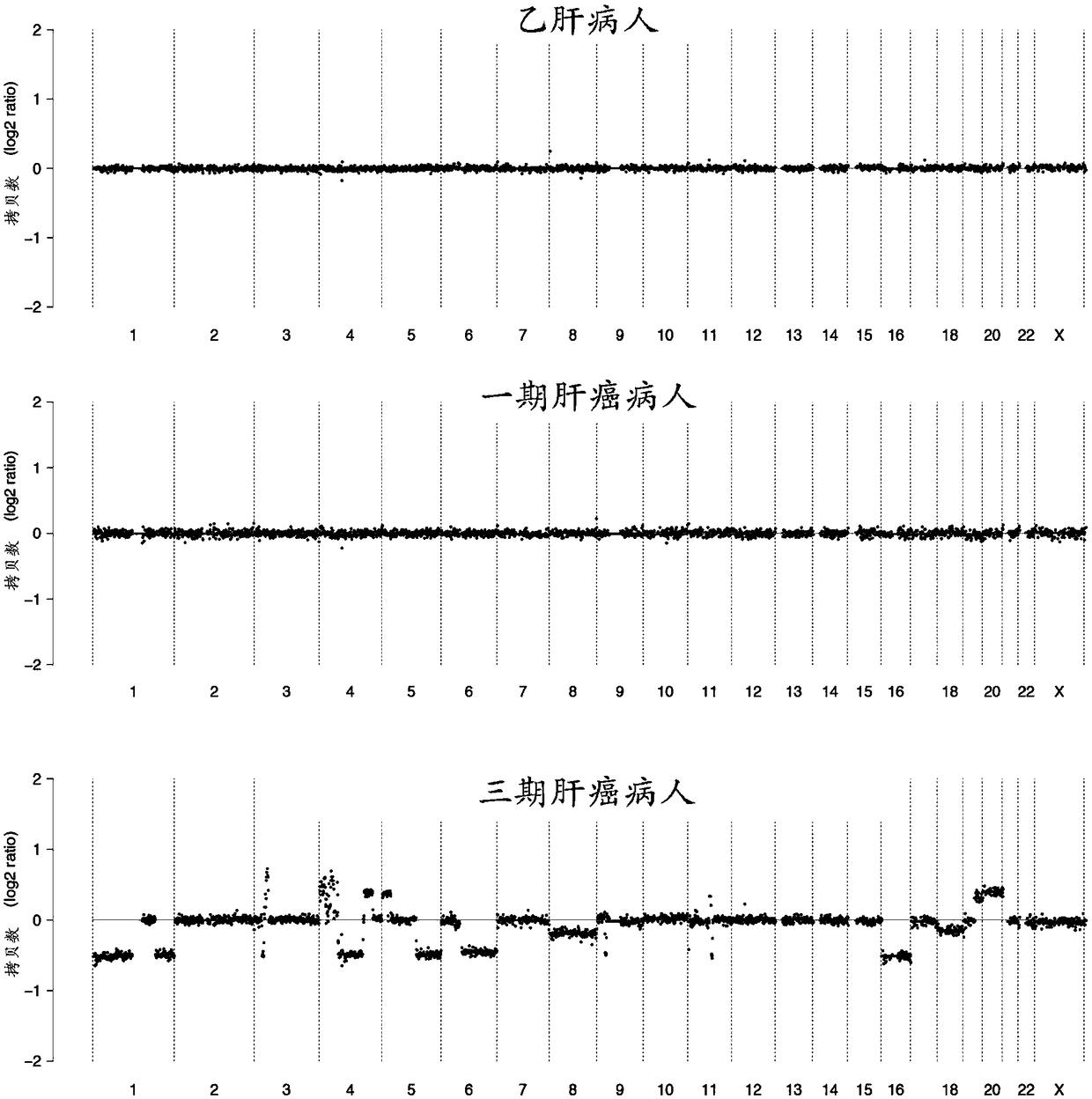
InactiveCN109182526AAchieve early diagnosisImprove accuracyMicrobiological testing/measurementBiostatisticsMagnetic beadGenomic data
The invention discloses a kit for auxiliary diagnosis of early liver cancer and a detection method thereof, which comprises the following reagents: a ccfDNA terminal treatment system, a ring linker reaction system, 0.06-0.15 U / ul USER enzyme, PCR amplification system, magnetic beads; The whole ccfDNA genome is sequenced by sequencing library. After genome-wide data processing, statistics and machine learning model were established to detect the abnormal copy number of ccfDNA in patients so as to achieve the early diagnosis of liver cancer. Such a detection method maximizes the accuracy of fluid biopsy for the diagnosis of early liver cancer, especially for the detection of primary liver cancer.
Owner:杭州翱锐生物科技有限公司
Application of taking EPS8L2 as biomarker for indicating intrahepatic nodule and pre-warning liver cancer at early stage
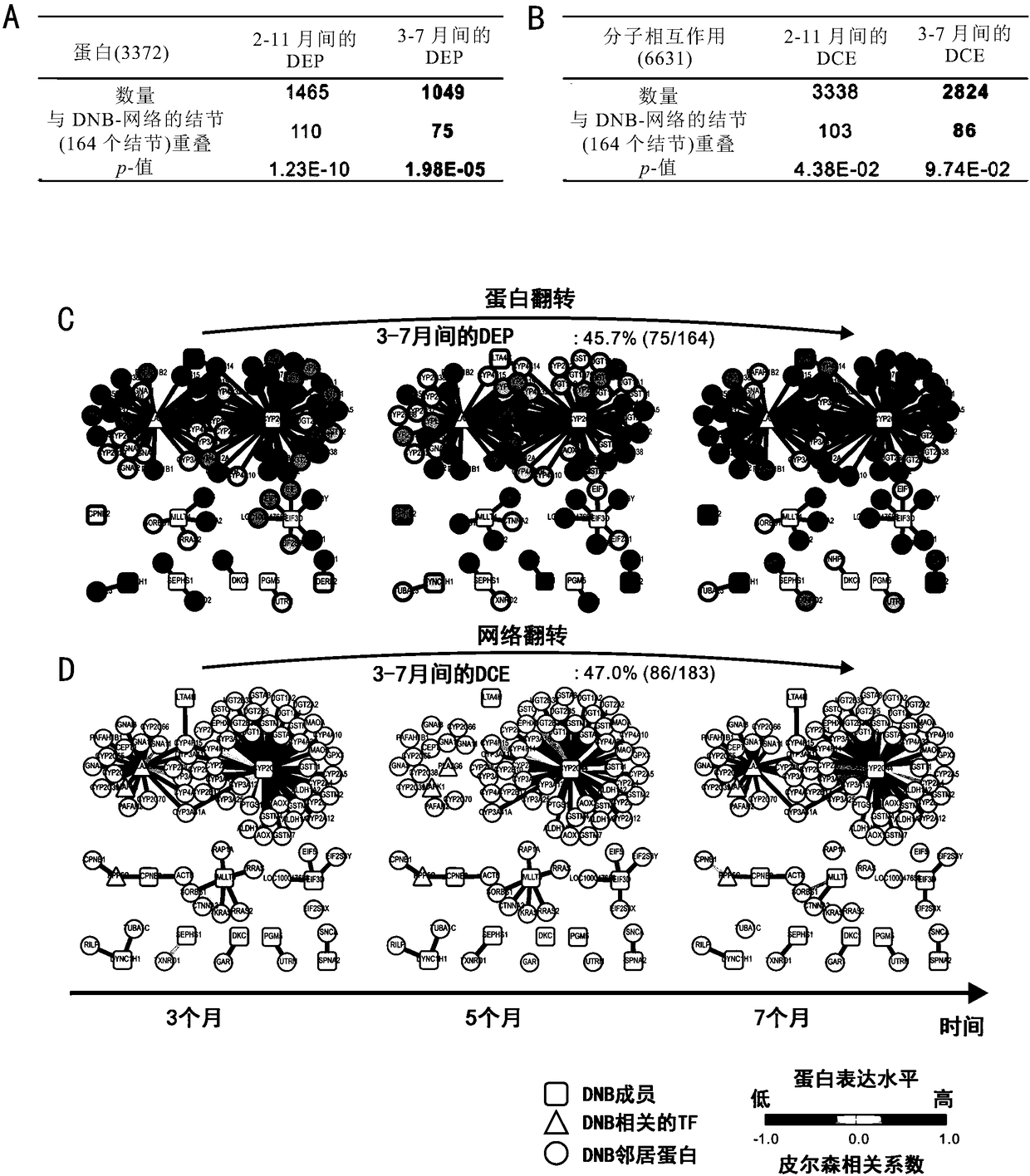
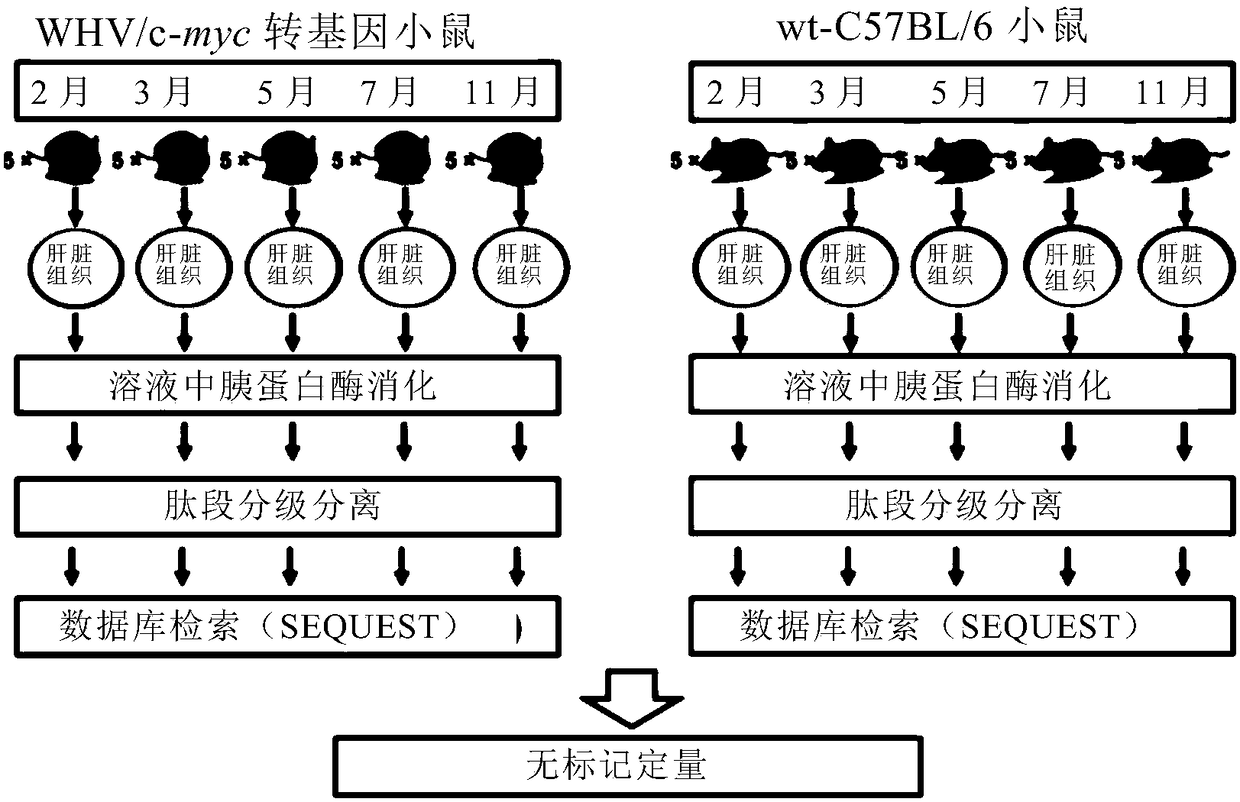
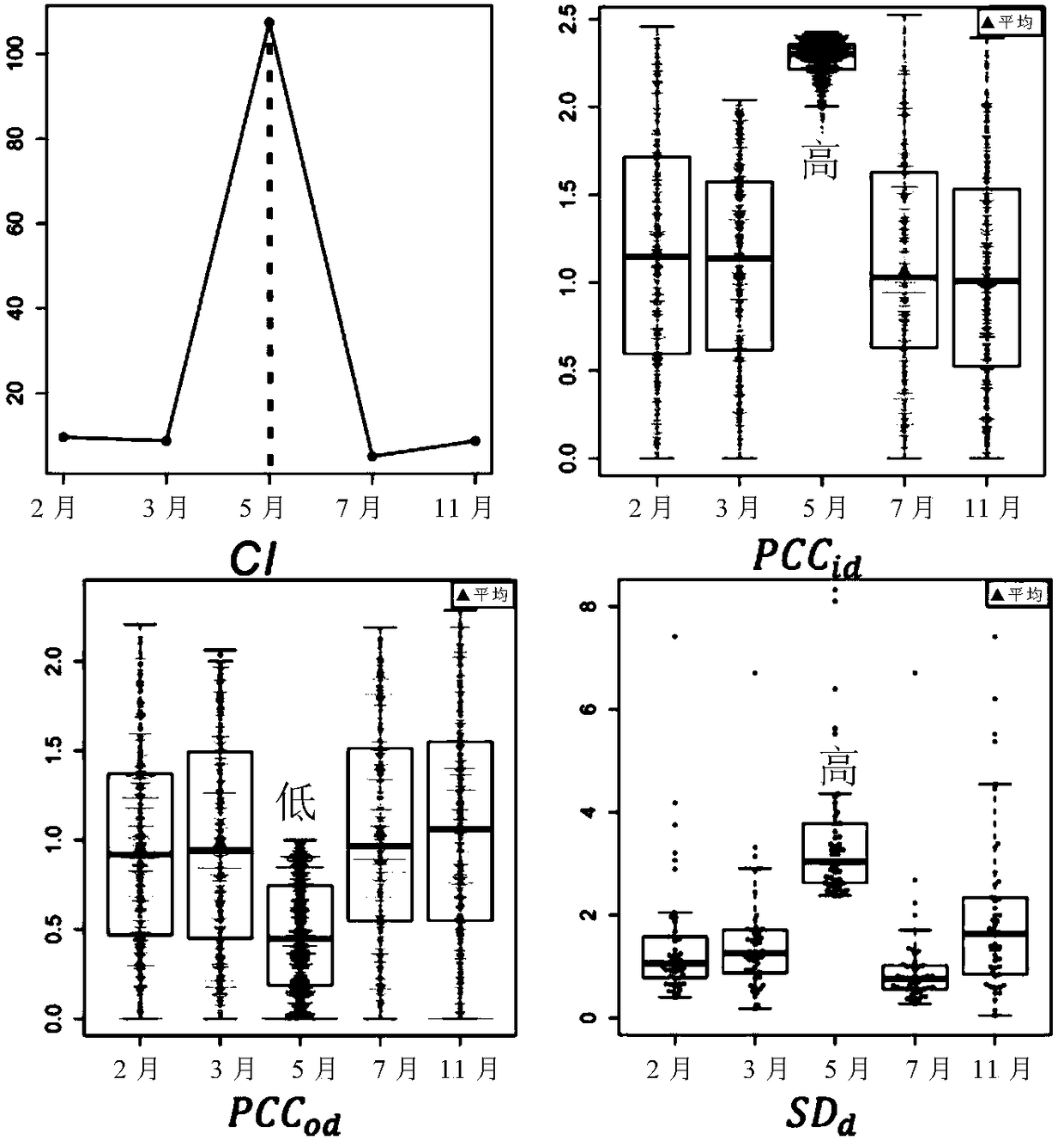
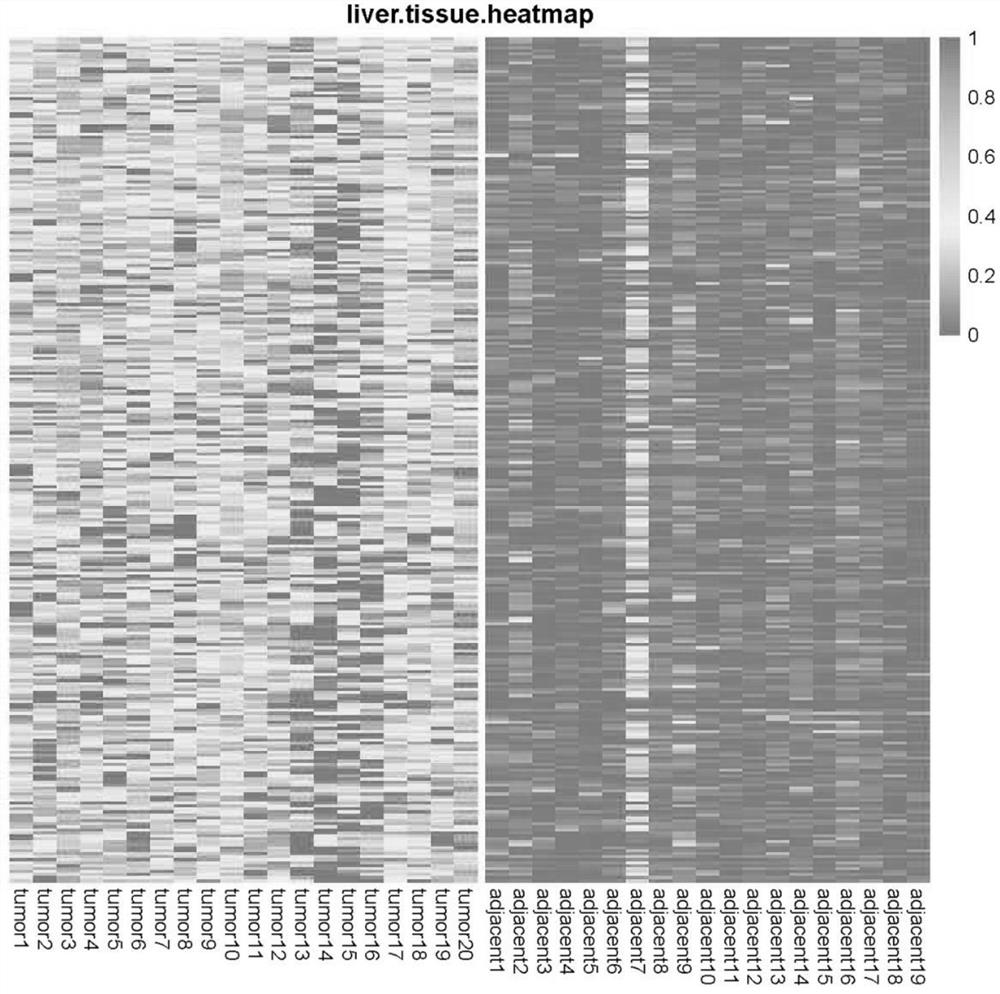
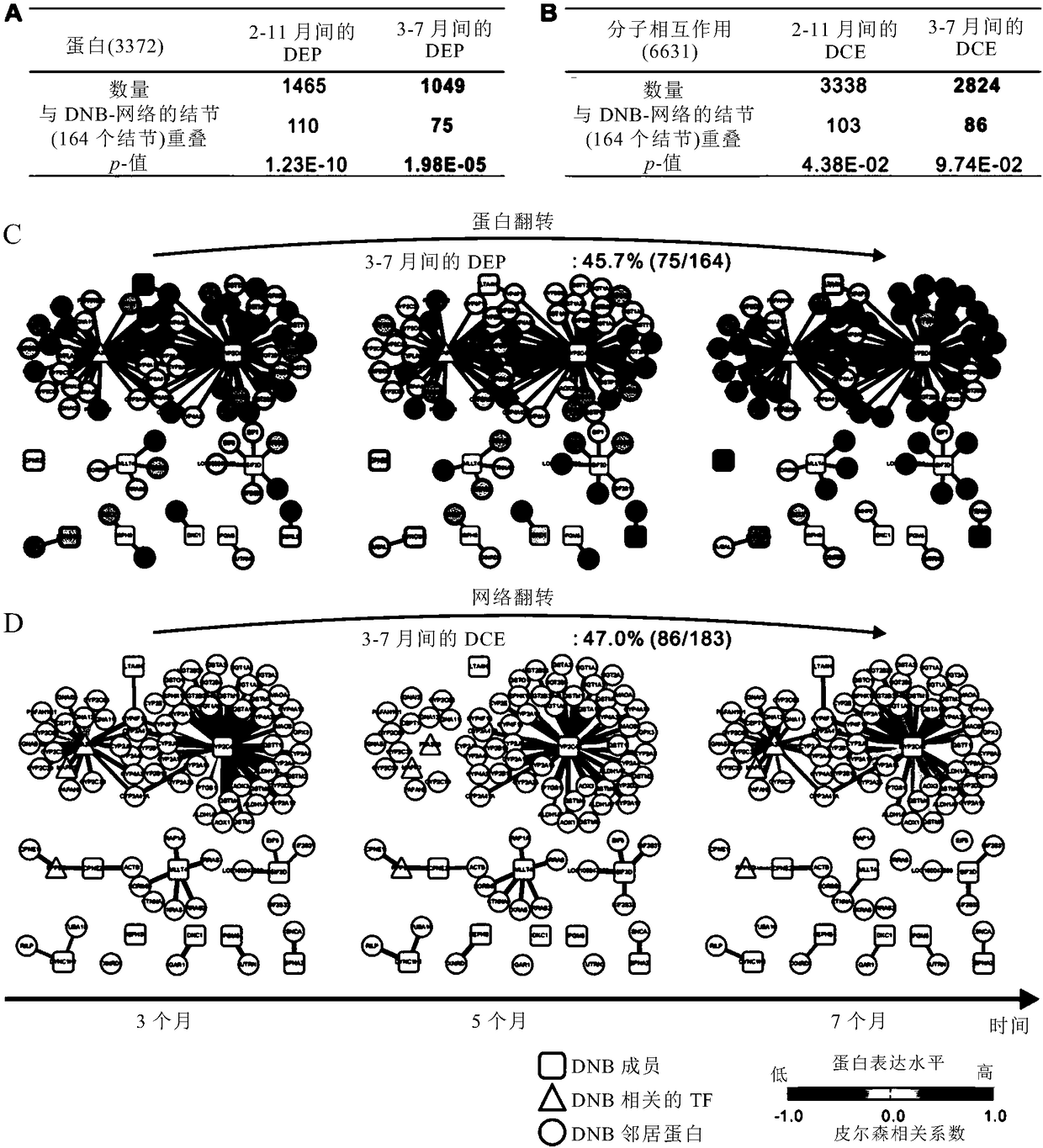
The invention relates to application of taking EPS8L2 as a biomarker for indicating an intrahepatic nodule and pre-warning liver cancer at an early stage, in particular to application of the followingreagents in preparing a kit for diagnosing the intrahepatic nodule of an object or diagnosing whether the object is in a vicious transformation critical phase from hepatitis to liver cancer, and thereagents comprise: (1) a reagent which is specifically combined with EPS8L2; (2) a reagent which is randomly selected and specifically combined with LTA4H; and (3) a reagent which is randomly selected and specifically combined with PLA2G6. The key critical stage of transformation from hepatitis to liver cancer is found based on an analysis on time sequence dynamics and network correlation, so that a novel direction is provided for clinically being applied to early-stage liver cancer treatment of a patient with hepatitis, judging whether the patient is in the critical stage of vicious transformation from hepatitis to liver cancer, and preventing deterioration of hepatitis and liver cancer.
Owner:SHANGHAI INST OF BIOLOGICAL SCI CHINESE ACAD OF SCI +1
Novel multi-target combined marker for early detection of liver cancer and application thereof
ActiveCN114592066AHigh sensitivityStrong specificityMicrobiological testing/measurementMaterial analysisHepatitisNovel gene
Owner:杭州翱锐基因科技有限公司
Application of microRNA in serum serving as diagnostic marker of liver cancer
ActiveCN103924002AHigh sensitivityImprove featuresMicrobiological testing/measurementDNA/RNA fragmentationSerum igeSerum samples
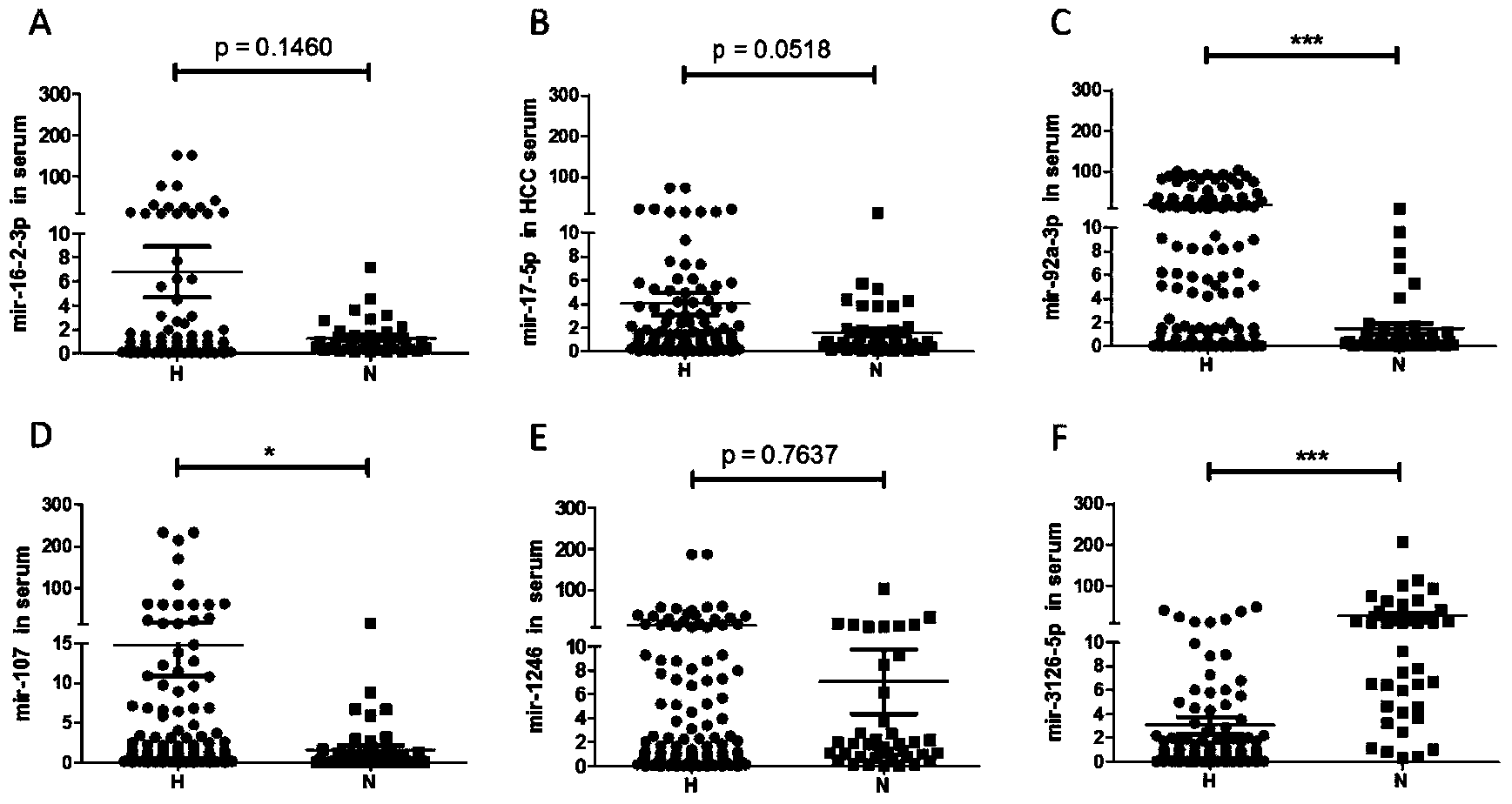
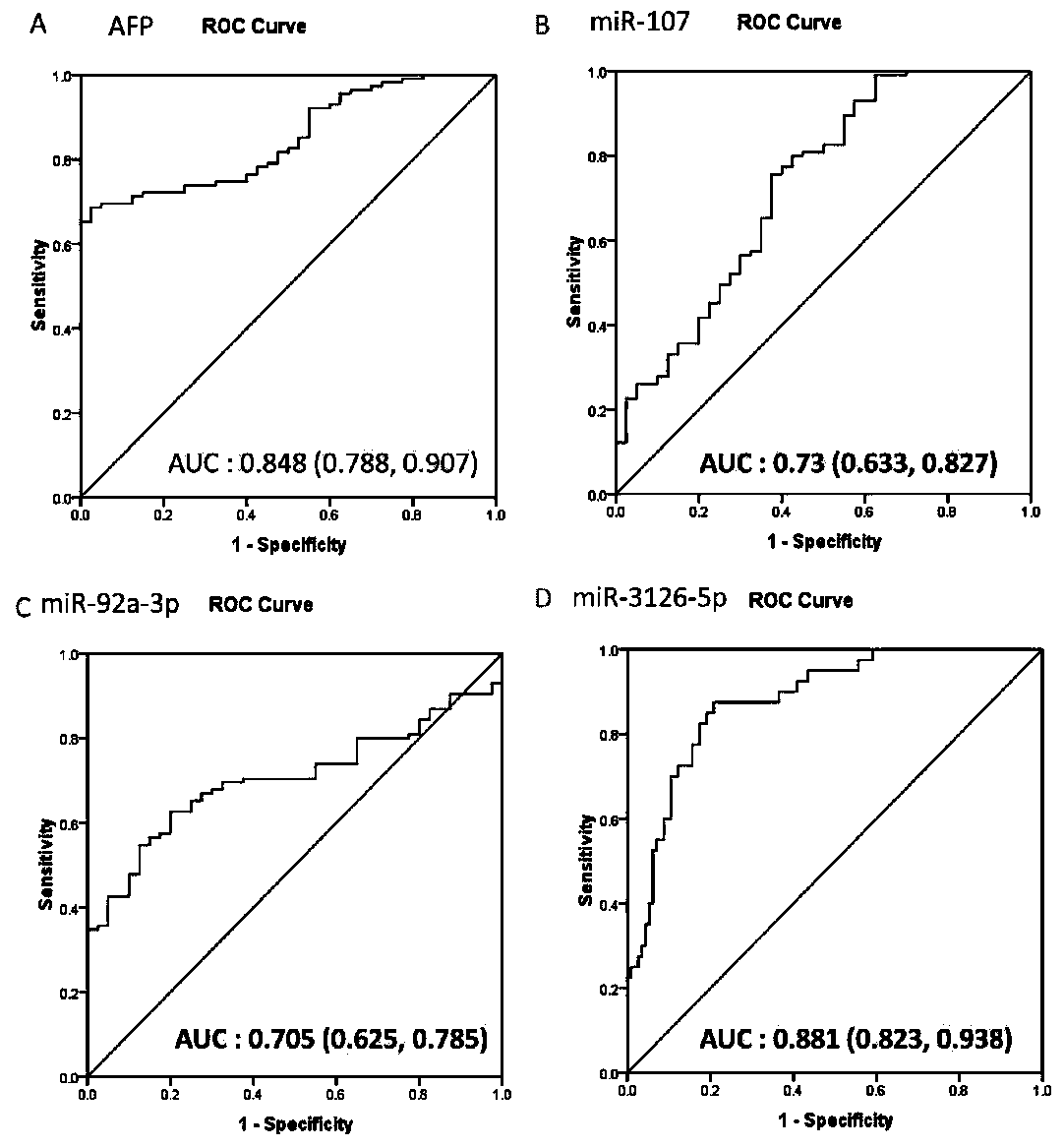
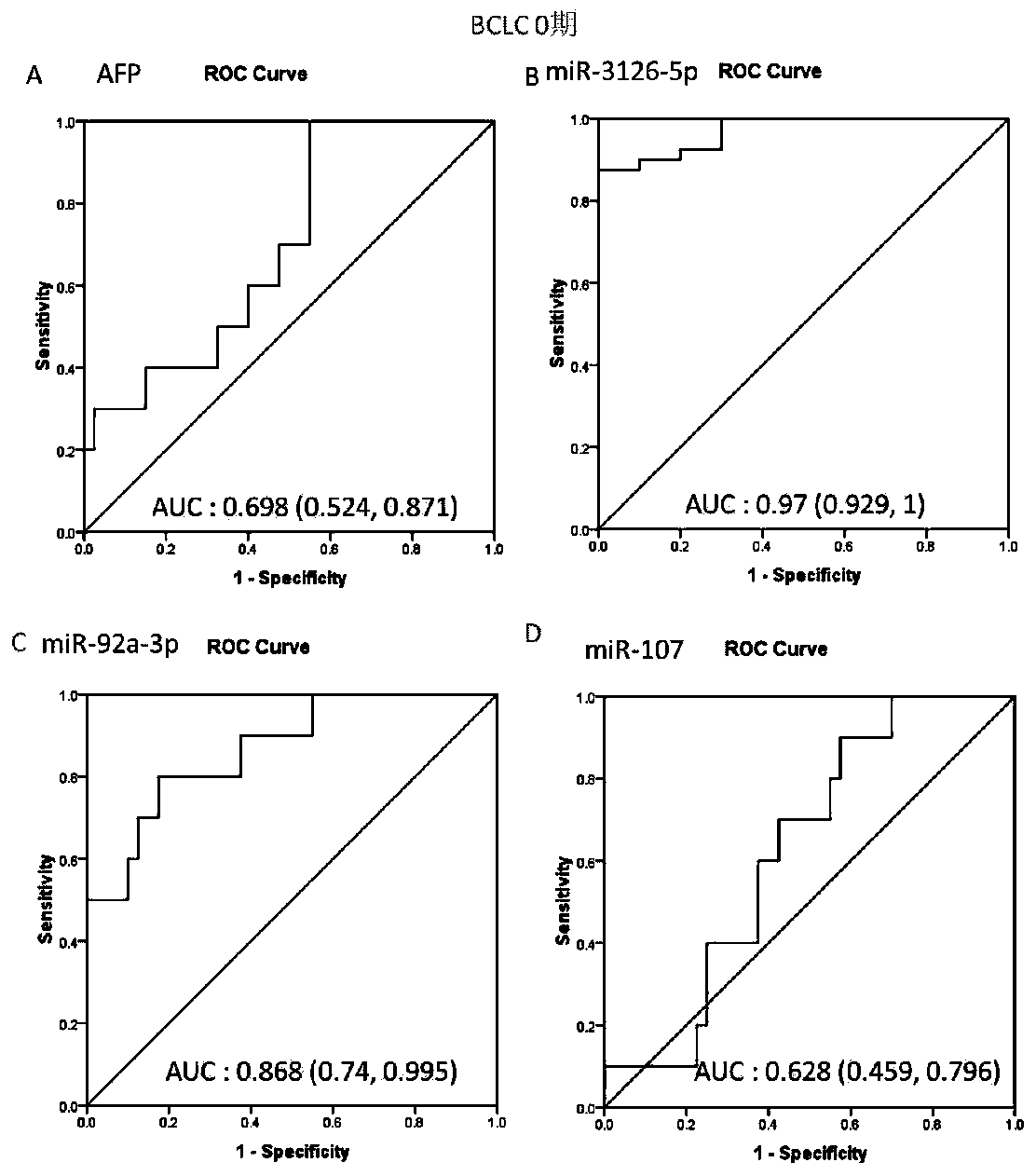
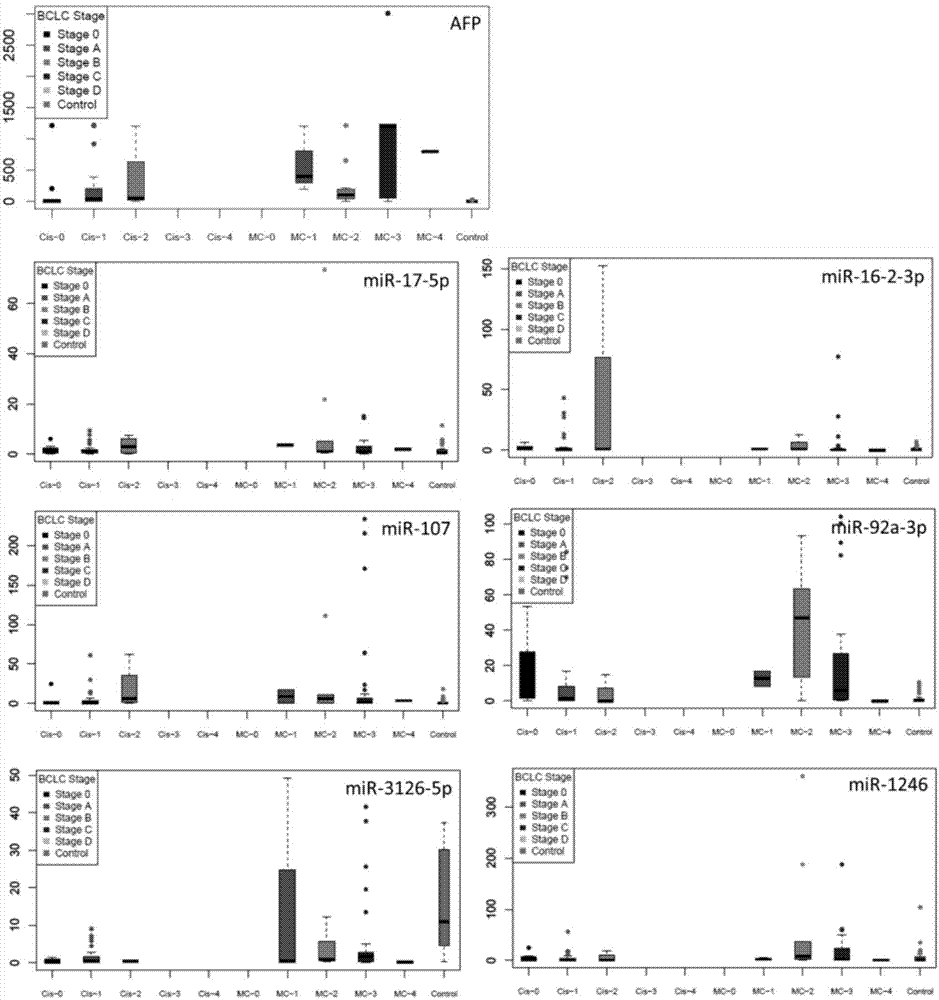
The invention discloses an application of microRNA-miR-3126-5p in serum serving as a diagnostic marker of liver cancer. In the invention, a high-throughput chip is used for detecting expression of microRNA in liver cancer cells at different development stages and in clinical liver cancer serum samples, candidate microRNA is selected, the expression of the candidate microRNA in the serum samples is detected to find that the miR-3126-5p has good sensitivity and specificity on diagnosis of liver cancer and especially has a good diagnostic value in diagnosis of early liver cancer and liver cancer with low AFP expression. On the basis, the invention develops a primer, a kit and a detection method for detecting the miR-3126-5p, which have good development prospects and clinical application values. The sequence of the primer is as shown in SEQ ID NO.2, and the kit is composed of a microRNA extraction reagent, a reverse transcription reagent, a specific amplification primer and a PCR amplification reagent.
Owner:青岛瑞思德医学检验实验室有限公司
MiRNA composition and kit therefore for diagnosis of early hepatocellular carcinoma
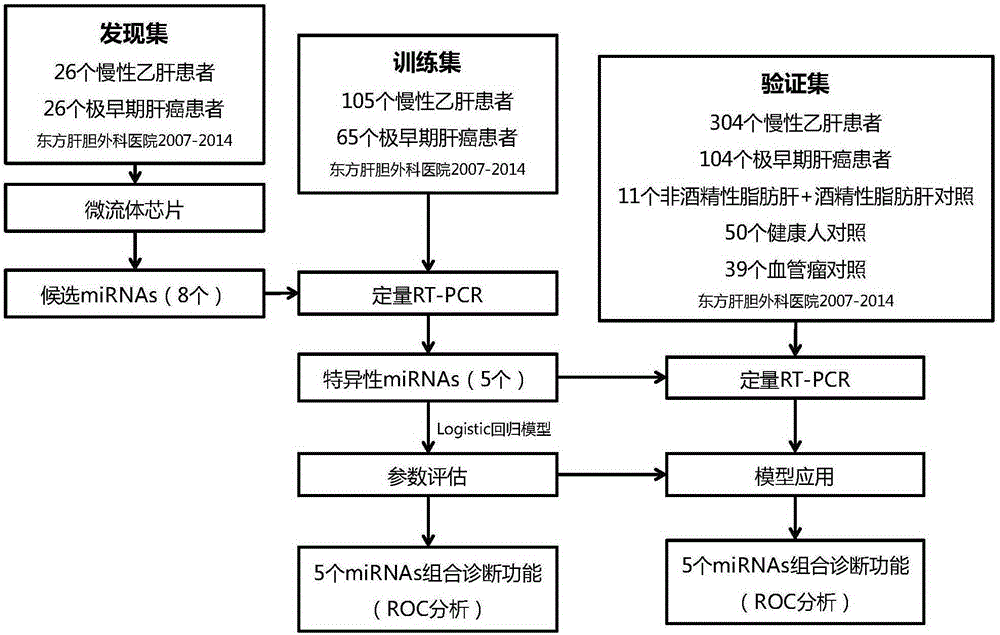
The invention relates to a miRNA composition and a kit thereof for diagnosis of early hepatocellular carcinoma. The method discloses a method for the diagnosis of hepatocellular carcinoma, and in particular early hepatocellular carcinoma, through cooperative detection of expression of five miRNAs for the first time; and the invention also provides a reagent for implementing the diagnosis.
Owner:HEPATOBILIARY SURGERY HOSPITAL SECOND MILITARY MEDICAL UNIV
Application of LTA4H as biomarker for indicating intrahepatic nodule and pre-warning liver cancer at early stage
The invention relates to application of LTA4H as a biomarker for indicating an intrahepatic nodule and pre-warning liver cancer at an early stage, in particular to application of the following reagents in preparing a kit for diagnosing the intrahepatic nodule of an object or diagnosing whether the object is in a vicious transformation critical phase from hepatitis to liver cancer, and the reagents comprise: (1) a reagent which is specifically combined with LAT4H; (2) a reagent which is specifically combined with PLA2G6; and (3) a reagent which is randomly selected and specifically combined with EPS8L2. The key critical stage of transformation from hepatitis to liver cancer is found based on an analysis on time sequence dynamics and network correlation, so that a novel direction is provided for being clinically applied to early-stage liver cancer treatment of a patient with hepatitis, judging whether the patient is in the critical stage of vicious transformation from hepatitis to liver cancer, and preventing deterioration of hepatitis and liver cancer.
Owner:SHANGHAI INST OF BIOLOGICAL SCI CHINESE ACAD OF SCI +1
Application of serum microRNA as early diagnostic marker for hepatocellular carcinoma metastasis
The invention discloses an application of miR-107 combined with miR-1246 as an early diagnostic marker for hepatocellular carcinoma metastasis. Researches find that miR-107 combined with miR-1246 for diagnosis of early liver cancer metastasis is superior to alpha fetal protein (AFP), and has good sensitivity and specificity, and the miR-107 and miR-1246 combined with AFP diagnosis is more excellent. As to metastasis diagnosis of low alpha fetal protein secretion liver cancer (AFP is smaller than 200), the miR-1246 is superior to the APF, the miR-107 combined with miR-1246 diagnosis is much superior to the APF, and the miR-107 and miR-1246 combined with AFP are more excellent. On the basis, a primer, a kit and a detection method for detecting the miR-107 and the miR-1246 are developed, and the serum microRNA has a good development prospect and clinical application value. A sequence of the primer is shown in SEQ ID NO.3 and 4, and the kit is formed by a specific amplification primer and a required reagent for PCR amplification.
Owner:青岛瑞思德医学检验实验室有限公司
Early warning method for liver cancer, detection kit for early warning and detection method
PendingCN112280867AIncreased sensitivityImprove featuresMicrobiological testing/measurementDNA methylationA-DNA
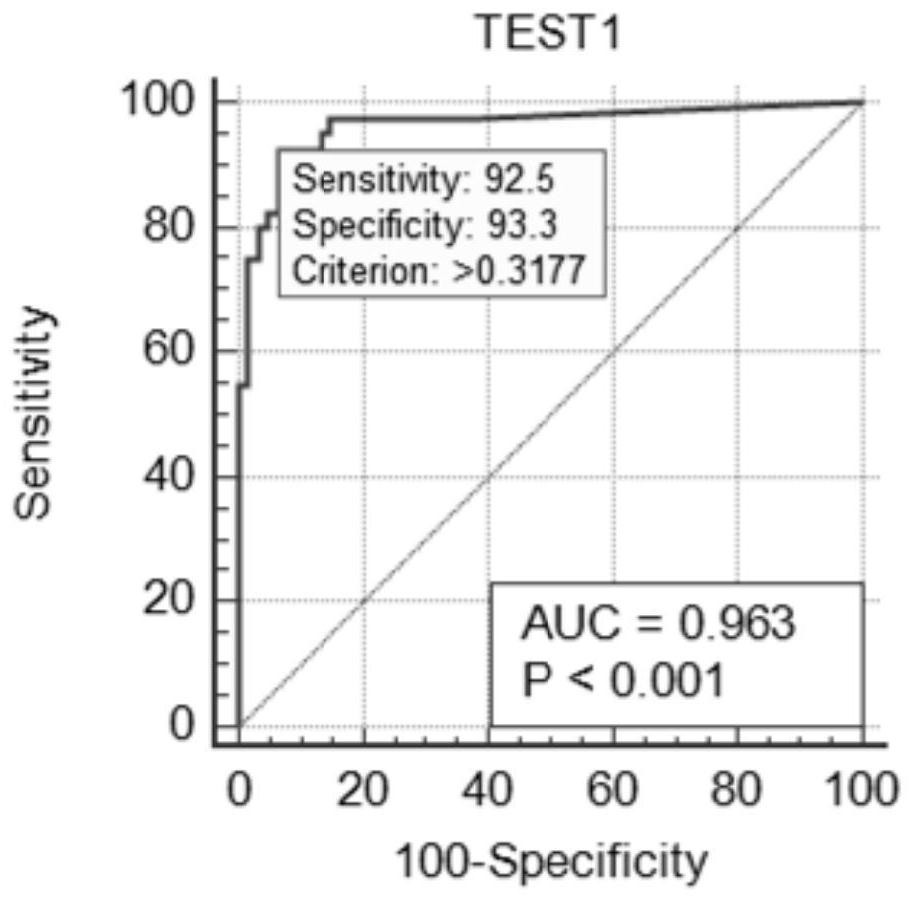
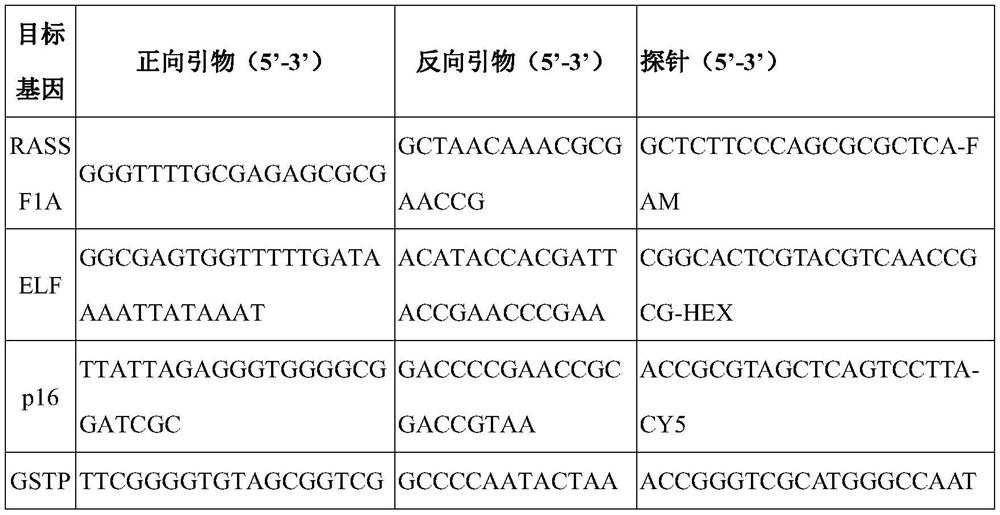
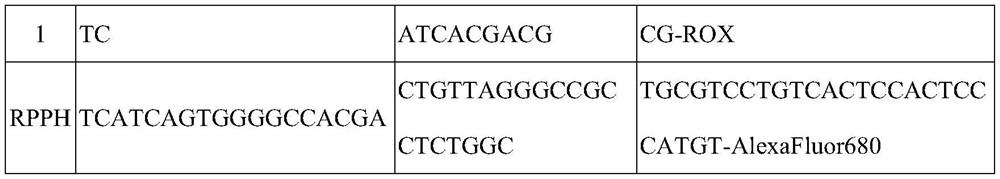
The invention discloses an early warning method for liver cancer, a detection kit for early warning and a detection method, and belongs to the technical field of biology. The early warning method comprises the following steps: detecting DNA methylation levels of RASSF1A, ELF, p16 and GSTP1 genes in a peripheral blood sample through a PCR method, calculating a risk value of suffering from liver cancer, and carrying out early warning according to a calculation result. The calculation formula is as follows: Y is equal to 1.208163-0.00653*Ct(RASSF1A)-0.00751*Ct(ELF)-0.00555tCT(p16)-0.00457*Ct(GSTP1), wherein when Y is larger than or equal to 0.3177, the risk is judged to be high, and when Y is smaller than 0.3177, and the risk is judged to be low. The multi-gene methylation joint detection kitfor early warning of liver cancer comprises a DNA methylation detection reagent for specifically detecting the RASSF1A, ELF, p16 and GSTP1 genes in the peripheral blood sample, an endogenous gene andan endogenous gene detection reagent. The high-risk early warning result and the clinical diagnosis result are high in conformity, good application prospects are achieved for diagnosis of early livercancer, the survival rate of liver cancer patients is increased, and the cure rate of liver cancer patients is increased.
Owner:苏州天隆生物科技有限公司
Ultra-early liver cancer screening and evaluating product based on saliva specific glycoprotein sugar chain structure and application
PendingCN111239396ARapid identificationAccurate identificationMedical automated diagnosisMaterial analysisSaliva samplePharmaceutical drug
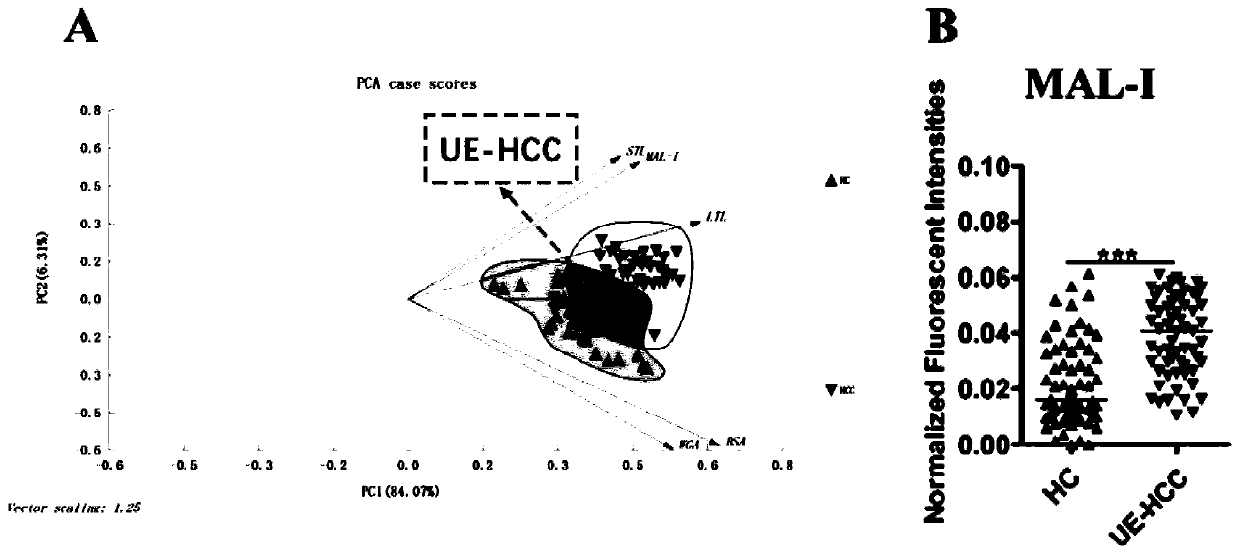
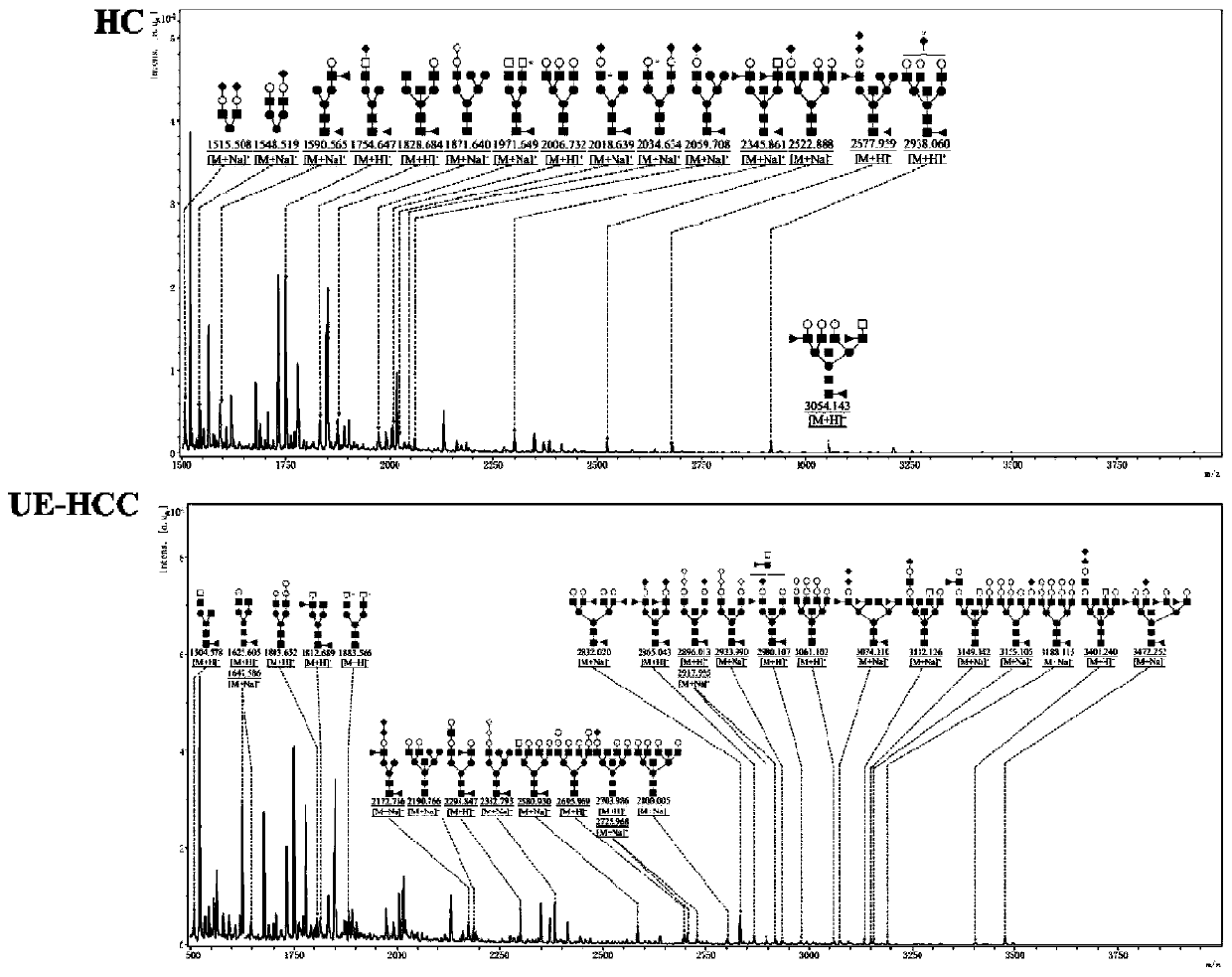
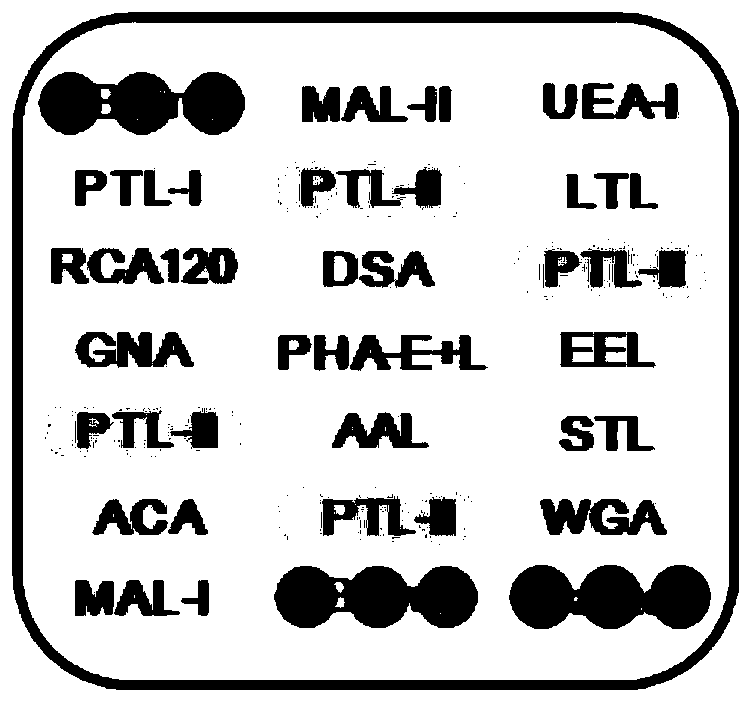
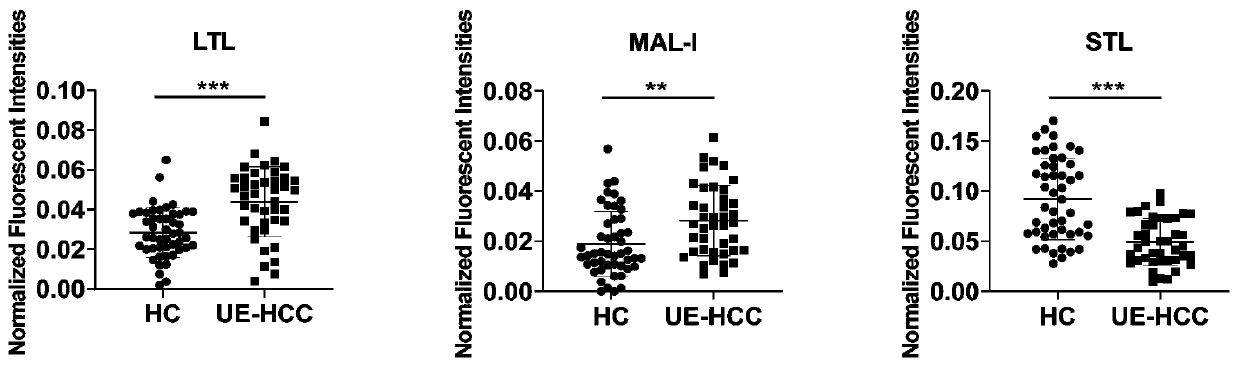
The invention discloses an ultra-early liver cancer related screening and evaluating product based on a saliva specific glycoprotein sugar chain structure and application. Aiming at a saliva sample, aproduct for screening, early diagnosis, risk assessment, drug screening and / or curative effect assessment of the ultra-early liver cancer is provided, whether the saliva sample contains any one or any combination of specific 26 N-sugar chains or not is detected, and whether a saliva sample main body is an ultra-early liver cancer patient (not a liver cirrhosis patient) or not can be judged. Correspondingly, lectin playing a role in specific binding recognition is MAL-I and can be independently used as a reagent for recognizing a saliva specific glycoprotein sugar chain structure to be used for preparing related medical products.
Owner:深圳格道糖生物技术有限公司
Construction method of liver cancer early screening model, detection device and computer readable medium
PendingCN113421608AIncrease contentHigh clinical application valueMedical simulationBiostatisticsOncologyBiology
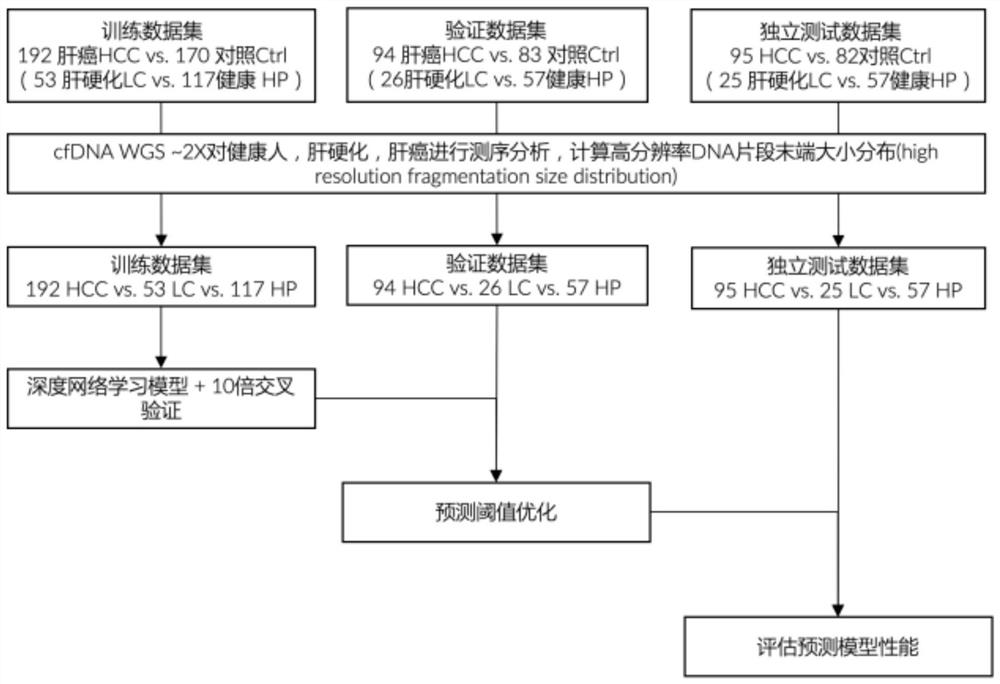
The invention relates to a construction method of a liver cancer early screening model, a detection device and a computer readable medium. The lengths of WGS cfDNA read segments of 170 contrast groups and 192 liver cancer patients are counted, and the number of total fragments (40-300 bp), the number of short fragments (40-80 bp) and the number of super-long fragments (200-300 bp) are different between the number of total fragments (40-300 bp) and the number of short fragments (40-80 bp). Meanwhile, the number of fragments with different lengths is counted through long and short arms of chromosomes, and the two groups also have significant differences. According to the invention, a diagnosis model of the relationship between DNA fragment size sheet distribution and terminal sequence proportion and liver cancer is provided for the first time based on plasma cfDNA high-throughput low-depth sequencing, and the model not only can diagnose early liver cancer, but also can distinguish liver cirrhosis, and has the advantages of noninvasive detection, low throughput, and high detection specificity and sensitivity.
Owner:GENESEEQ TECH INC +1
Biomarker, kit and detection method for liver cancer diagnosis
ActiveCN114441760AHigh sensitivityImprove featuresDisease diagnosisBiological testingBlood plasmaCancer research
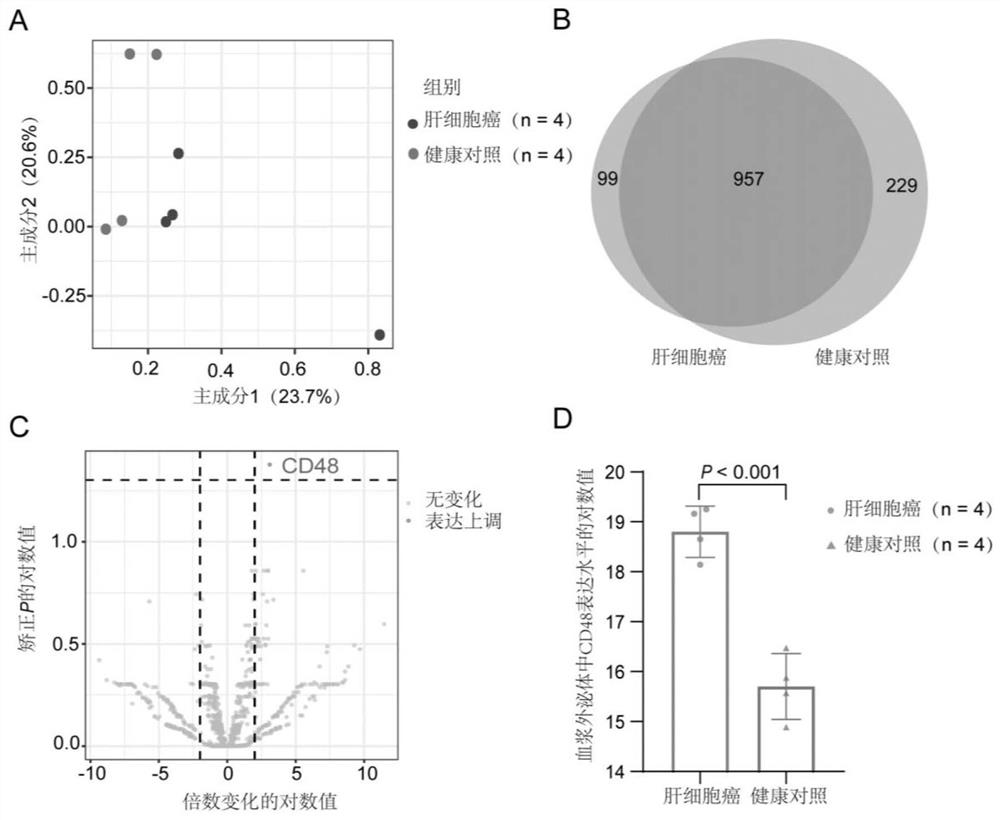
The invention discloses a biomarker, a kit and a detection method for liver cancer diagnosis, belongs to the technical field of molecular markers, and aims to solve the technical problem of how to improve the sensitivity and / or specificity of hepatocellular carcinoma diagnosis. The invention provides an application of a biomarker and / or a substance for detecting the biomarker in hepatocellular carcinoma diagnosis or preparation of a product for hepatocellular carcinoma diagnosis. The biomarker is a plasma exosome CD48 protein or a combination of the plasma exosome CD48 protein and a plasma AFP (Alpha Fetoprotein). The biomarker CD48 protein and the detection method have the characteristics of strong sensitivity and high specificity, and the sensitivity can reach 0.928, so that the missed diagnosis rate (false negative rate) is low, the diagnosis rate of early liver cancer can be improved when the biomarker CD48 protein is applied to clinical diagnosis of hepatocellular carcinoma patients, sub-clinical liver cancer or latent liver cancer can be screened out from people, and the clinical application prospect is wide. The cure rate of liver cancer can be increased and the death rate can be reduced.
Owner:ACADEMY OF MILITARY MEDICAL SCI
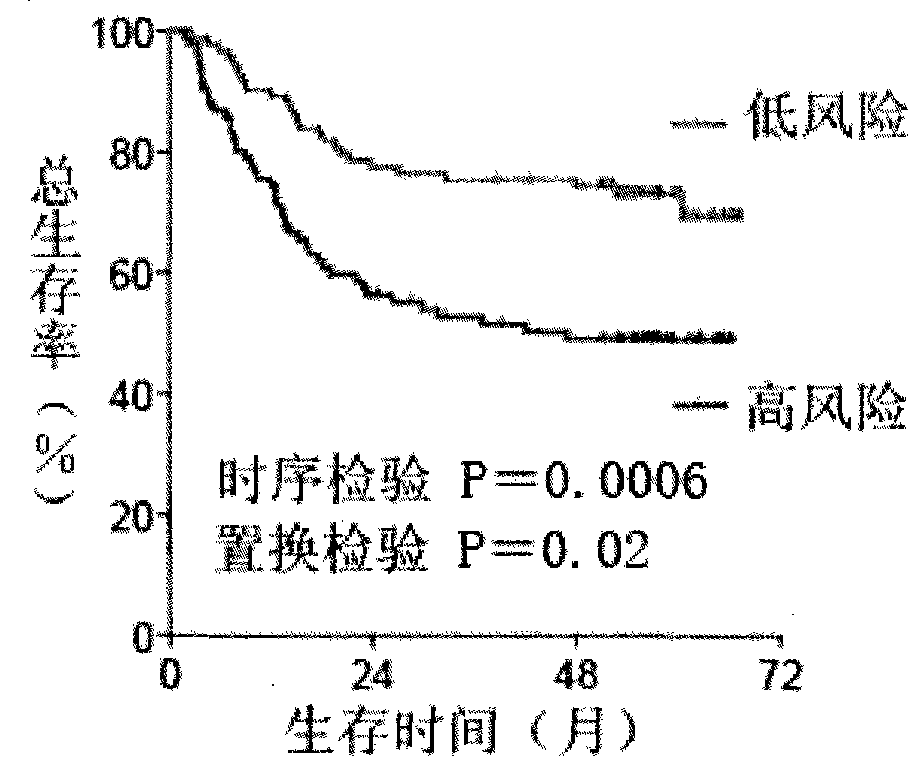
Combined marker for predicting liver cancer risk of male hepatitis B virus infected person and application thereof
PendingCN114487426AReduce monitoringReduce frequencyBiological material analysisMedical automated diagnosisHBsAgWhite blood cell
The invention discloses a combined marker for predicting the liver cancer risk of male hepatitis B virus infected persons and application of the combined marker. The combined marker comprises at least one of gamma-glutamyl transpeptidase, platelets, leukocytes, albumin and liver cirrhosis indexes, alpha fetoprotein and abnormal prothrombin. According to the invention, early warning can be realized 1-2 years before HCC nodules appear in iconography in liver cancer high-risk patients, and the early liver cancer (lt; and meanwhile, the worry of many low-risk asymptomatic HBsAg positive persons is relieved, and the HCC monitoring and screening frequency is reduced. And 4) economic and effective layered screening can be realized from the aspect of public health.
Owner:CANCER INST & HOSPITAL CHINESE ACADEMY OF MEDICAL SCI +1
Liver cancer diagnostic marker composed of blood plasma microRNA (micro ribonucleic acid) and new method for diagnosing liver cancer
ActiveCN102776185BMicrobiological testing/measurementDNA/RNA fragmentationChronic hepatitisEarly Hepatocellular Carcinoma
Owner:JUSBIO SCI SHANGHAI CO LTD
A combined diagnostic kit for opn, gdf15, nse, trap5 and opg
ActiveCN107462720BStrong specificityHigh sensitivityDisease diagnosisBiological testingDiagnosis earlyAfter treatment
The invention relates to the field of diagnosis kits, particularly to application of OPN, GDF15, NSE, TRAP5 and OPG to preparation of early liver cancer diagnosis detecting reagents or kits. The invention also discloses a kit which is used for diagnosing early liver cancer in a combined mode by detecting content of OPN, GDF15, NSE, TRAP and OPG in plasma. The kit has the advantages of being high in specificity and sensitivity, can be applied to early diagnosis of liver cancer, therapeutic effect evaluation during treatment and post-treatment metastasis and recurrence monitoring and can provide detecting results earlier than clinical symptoms. The kit and detecting technology is mature, short in cycle and good in specificity.
Owner:SHANGHAI CHANGHAI HOSPITAL
Application of specific lectin combination in construction of test tool for identifying ultra-early liver cancer based on saliva glycoprotein sugar chain
ActiveCN111381033ARapid identificationAccurate identificationMedical automated diagnosisMaterial analysisSaliva sampleSpecific lectin
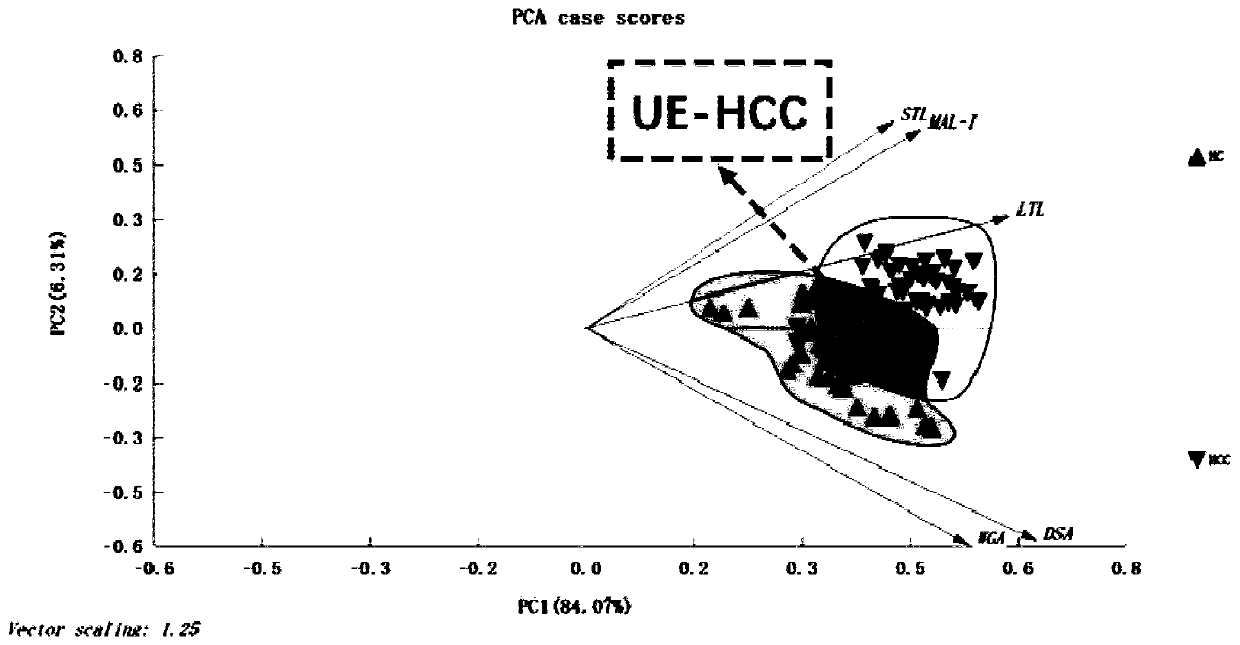
The invention discloses an application of a specific lectin combination in construction of a test tool for identifying ultra-early liver cancer based on a saliva glycoprotein sugar chain, wherein thespecific lectin combination is selected from three kinds of lectin of LTL, STL and MAL-I; compared with a liver cirrhosis sample, an ultra-early liver cancer sample has the advantages that LTL and MAL-I are significantly up-regulated, and STL is significantly down-regulated. Besides, an accurate test model is constructed, and a user can calculate the following detection values according to a lectin test result; if the detection value is greater than or equal to 0.4931, it is indicated that the main body to which the saliva sample belongs is an ultra-early liver cancer patient (UE-HCC), otherwise, it is indicated that the main body to which the saliva sample belongs is a liver cirrhosis patient (HC).
Owner:深圳格道糖生物技术有限公司
Gene chip of prediction of recurrence after hepatocellular carcinoma operation
InactiveCN101560554AImproved prognosisReduce postoperative recurrence rateNucleotide librariesMicrobiological testing/measurementAdjuvant therapyBiology
The invention provides a gene chip of prediction of recurrence after hepatocellular carcinoma operation. The invention is characterized in that the chip takes EPAS1, JMJD1C, NEDD9, EPC2 and USP36 genes as detecting sites, takes beta-actin as the actin antibody and takes upstream primers and downstream primers on the EPAS1, JMJD1C, NEDD9, EPC2 and USP36 genes as detecting probes. The gene chip has the characteristics of accurate and fast diagnosis and prediction, simple method and easy promotion and application and the like. The gene can be used for clinically guiding adjuvant therapy after the operation of liver cancer, and reduce recurrence rate of high-risk patients with liver cancer after operation, thus improving the prognosis of patients with liver cancer. The group of gene labels has important clinical significance to the judgment of prognosis of patients with feminine alpha-fetoprotein and carcinoma in the early stage.
Owner:ZHONGSHAN HOSPITAL FUDAN UNIV
DNA methylation biomarker or composition for early liver cancer detection and application of DNA methylation biomarker or composition
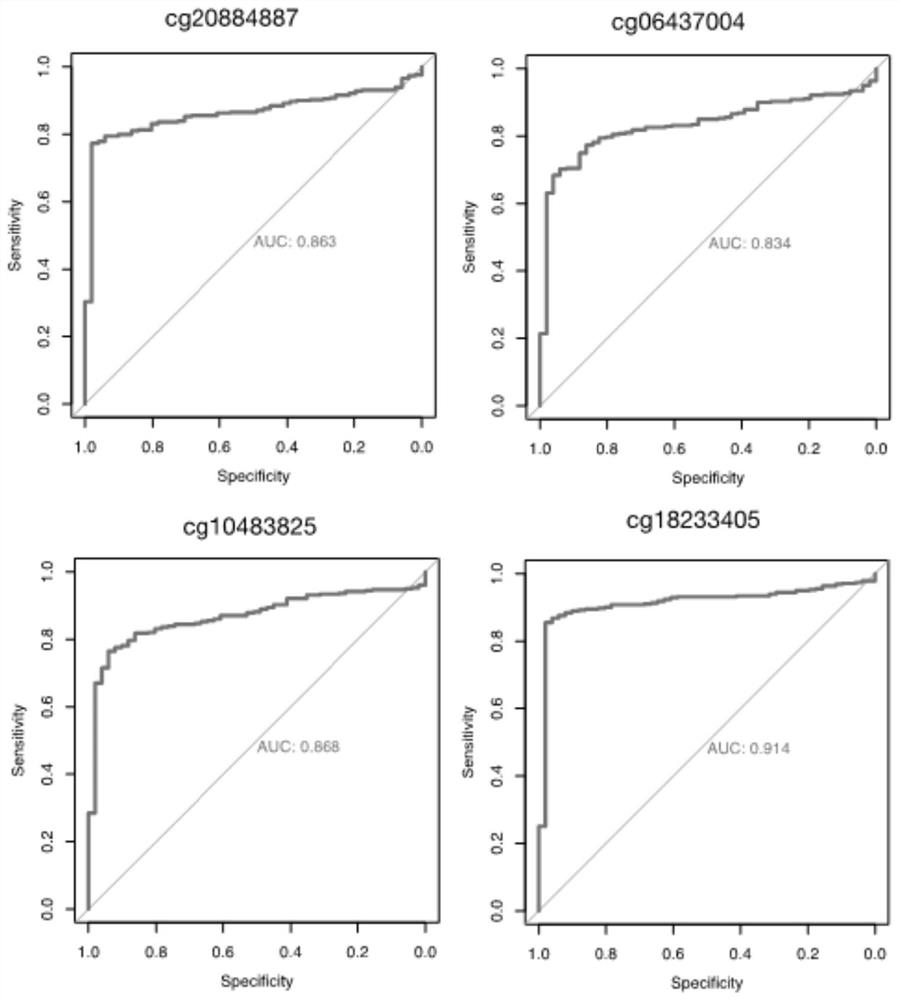
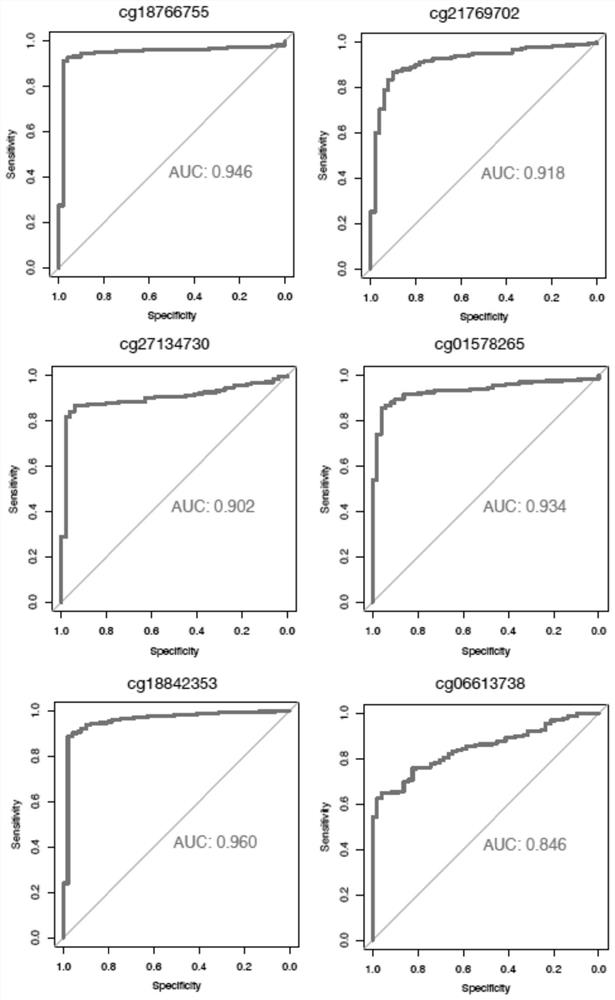
ActiveCN114657247AImprove the detection rateImprove forecast accuracyMicrobiological testing/measurementLibrary creationDNA methylationA-DNA
The invention discloses a DNA (deoxyribonucleic acid) methylation biomarker or a DNA methylation biomarker combination for early liver cancer detection, which comprises at least one of the following methylation markers: cg2088488887, cg06437004, cg10483825, cg18233405, cg18766755, cg21769702, cg27134730, cg01578265, cg18842353 and cg06613738. The invention discloses ten specific methylation markers related to occurrence of liver cancer, and the specific methylation markers can be used for remarkably improving the detection rate of early liver cancer. After the methylation marker is further combined with age and gender information, the predicted AUC value of a trained model can reach 0.917 when the model is used for distinguishing benign liver diseases (hepatitis and cirrhosis) and early liver cancer, and the model is suitable for large-scale popularization and application due to noninvasiveness of plasma detection.
Owner:北京莱盟君泰国际医疗技术开发有限公司
Probe pair, primer and kit used for prognosis evaluation of primary liver cancer
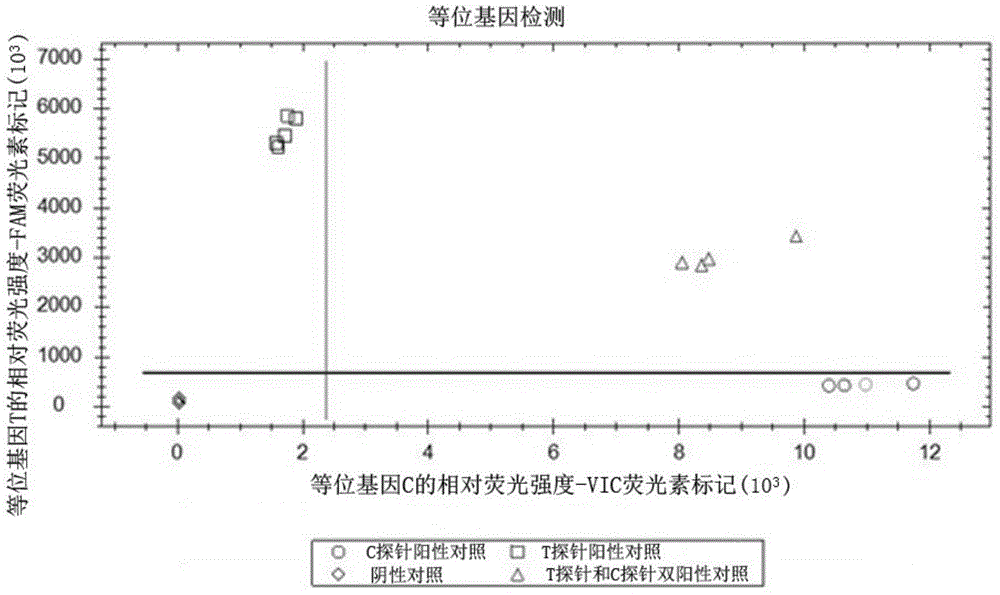
ActiveCN104962619AReduce false positive rateIncrease the probability of specific pairingMicrobiological testing/measurementDNA/RNA fragmentationWilms' tumorCoding region
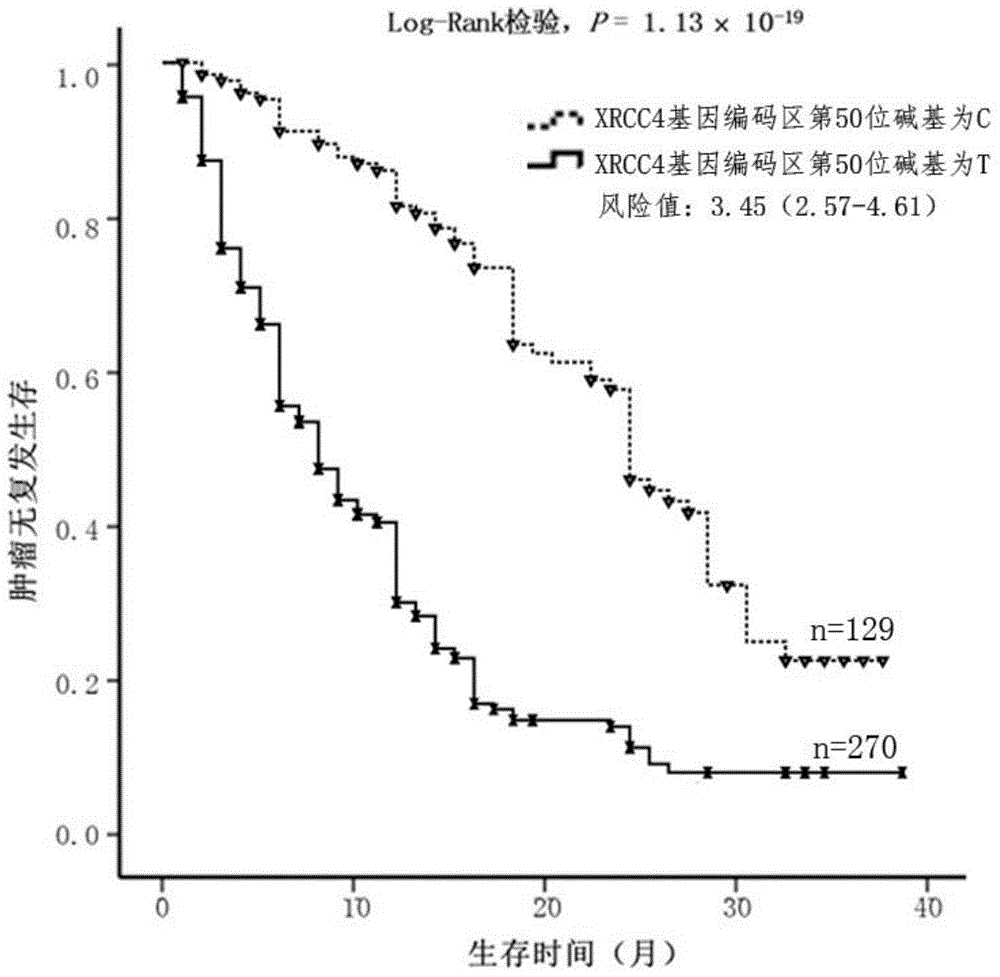
The invention discloses a probe pair and primer used for detecting C-to-T mutation of a 50 base of a coding region of the human XRCC4 gene. The probe pair is composed of a probe T as shown in SEQ ID No. 4 and a probe C as shown in SEQ ID No. 5. The primer is composed of an upstream primer as shown in SEQ ID No. 2 and a downstream primer as shown in SEQ ID No. 3. The invention further discloses a kit including the probe pair and the primer. The kit can be used for detecting whether C-to-T mutation of the 50 base of the coding region of the XRCC4 gene exists in a tumor tissue sample of a patient with early-stage primary liver cancer, which is favorable for prognosis evaluation of early-stage primary liver cancer.
Owner:武汉双旋生物科技有限责任公司
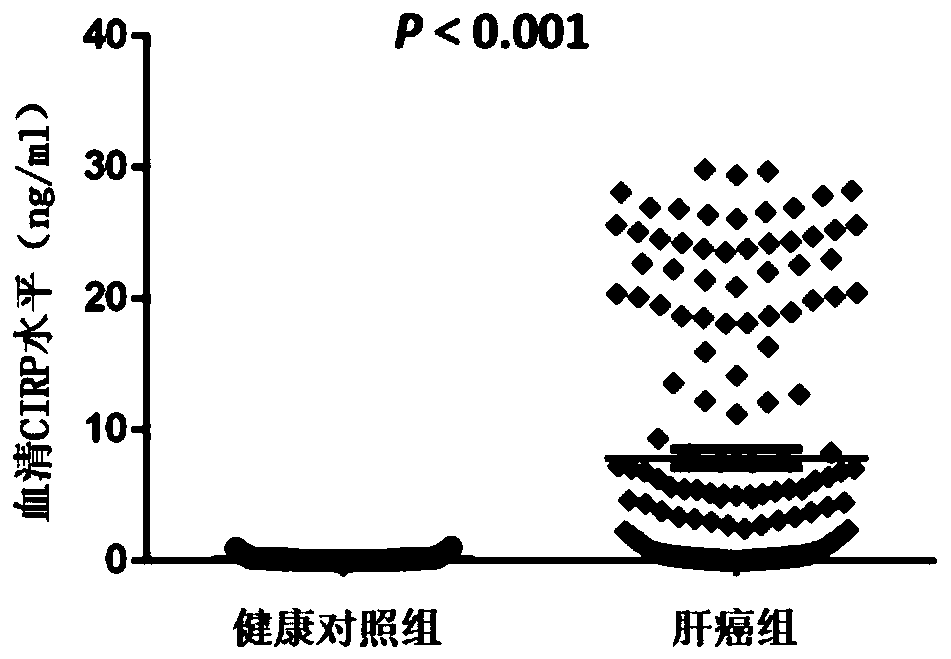
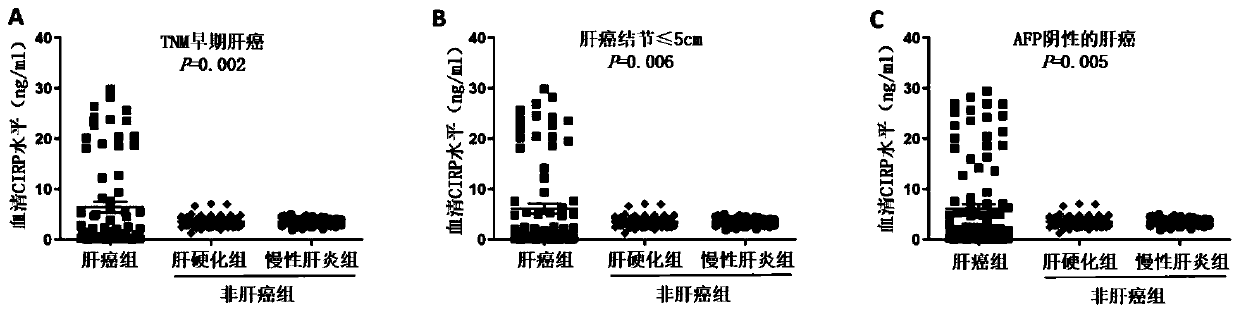
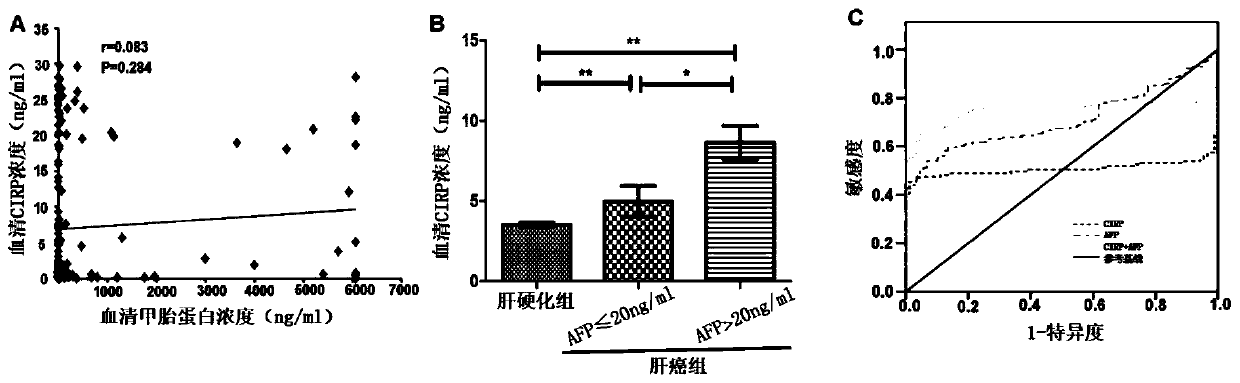
Tumor marker serum cold-inducible RNA-binding protein for liver cancer and application thereof
The invention relates to the field of early diagnosis of tumors and biomedicine, in particular to application of a serum cold-inducible RNA-binding protein (CIRP) as a tumor marker for liver cancer. The invention describes the application of a concentration detection reagent for the serum CIRP in preparing an early tumor diagnosis or prognosis detection reagent for liver cancer. According to the invention, the increased CIRP secretion in the serum identified by the standard ELISA method can be used as a diagnostic marker for early liver cancer, can be used for evaluation of prognosis, and hasa significant clinical application prospect.
Owner:THE FIRST AFFILIATED HOSPITAL OF MEDICAL COLLEGE OF XIAN JIAOTONG UNIV
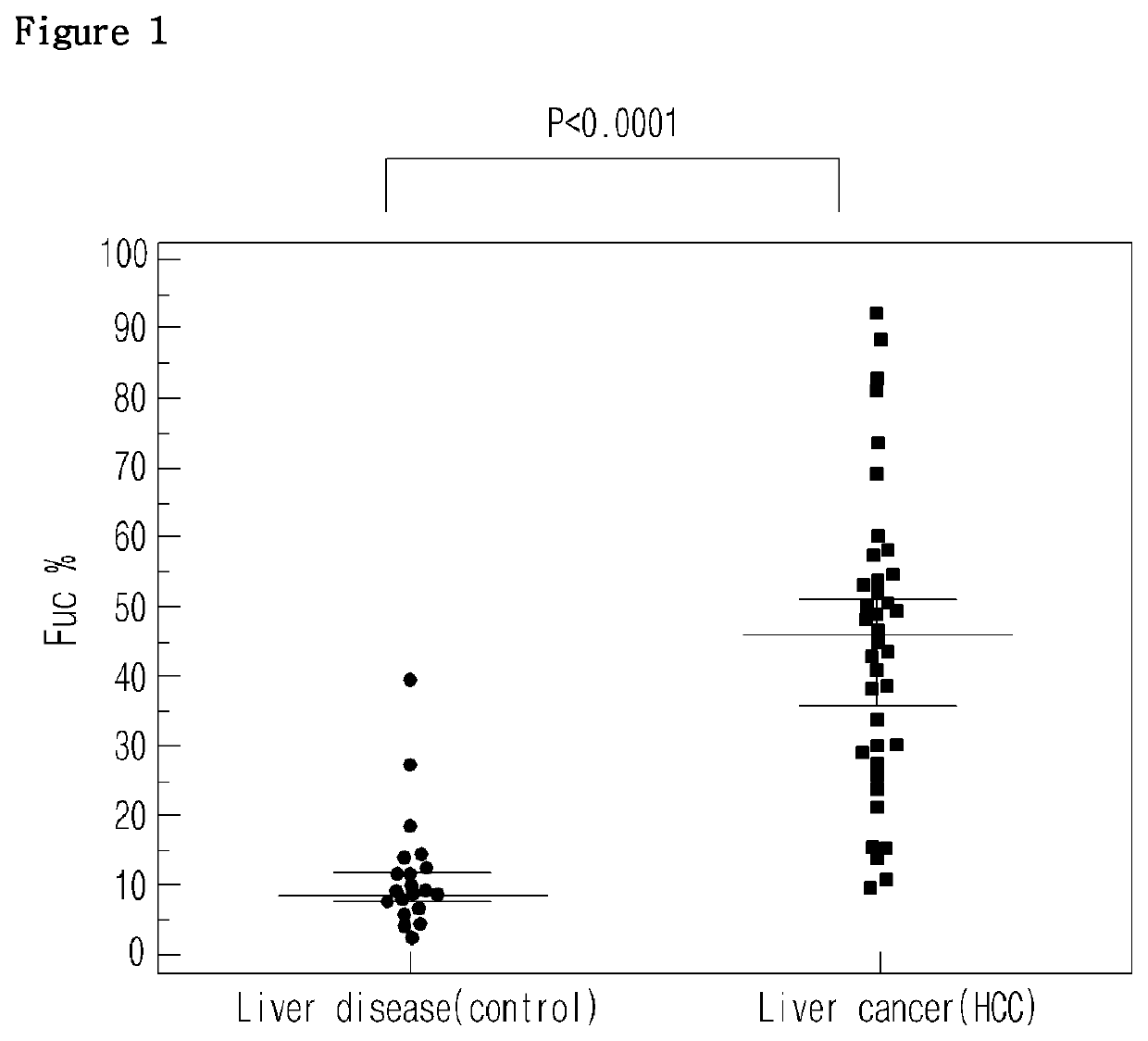
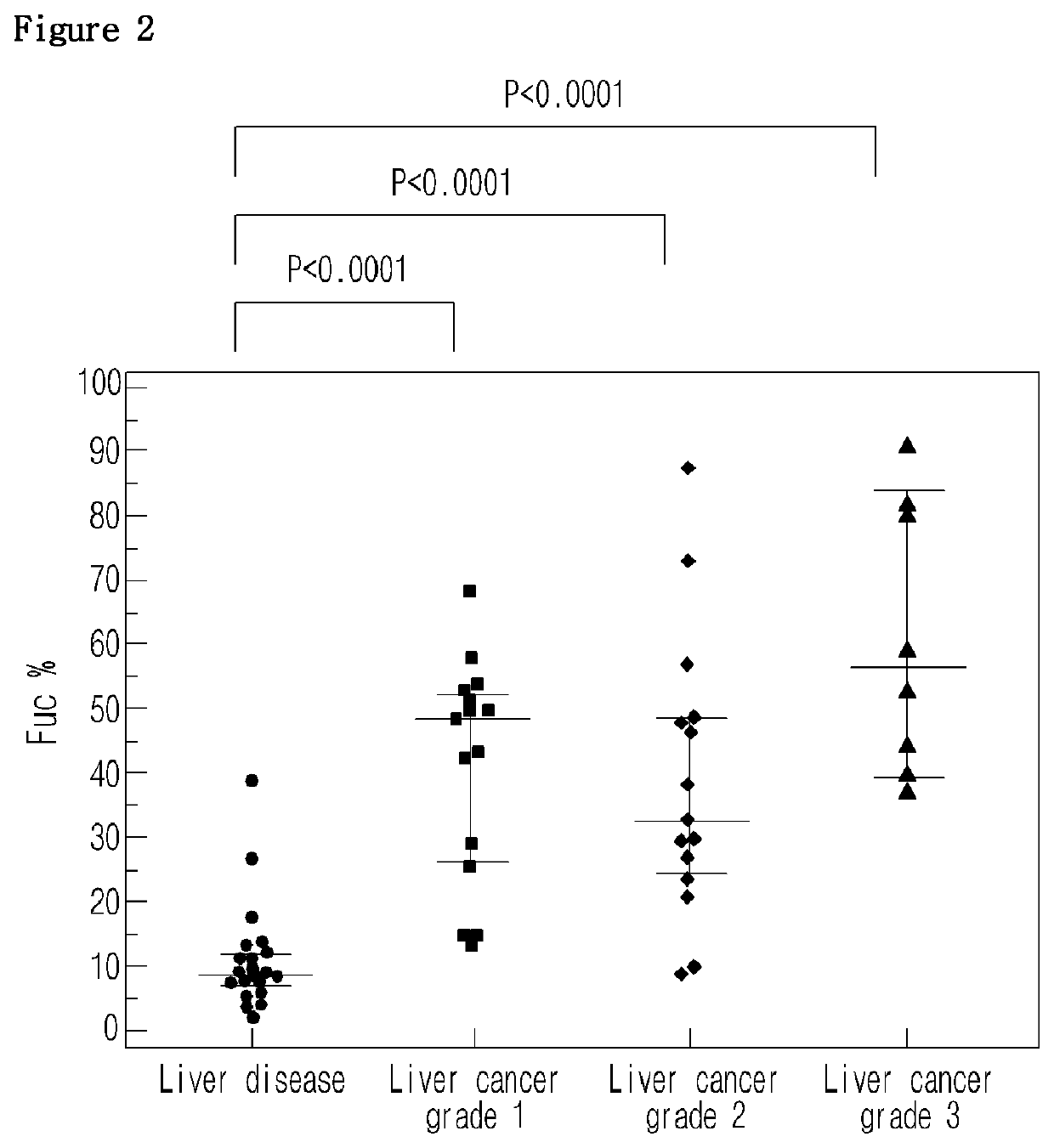
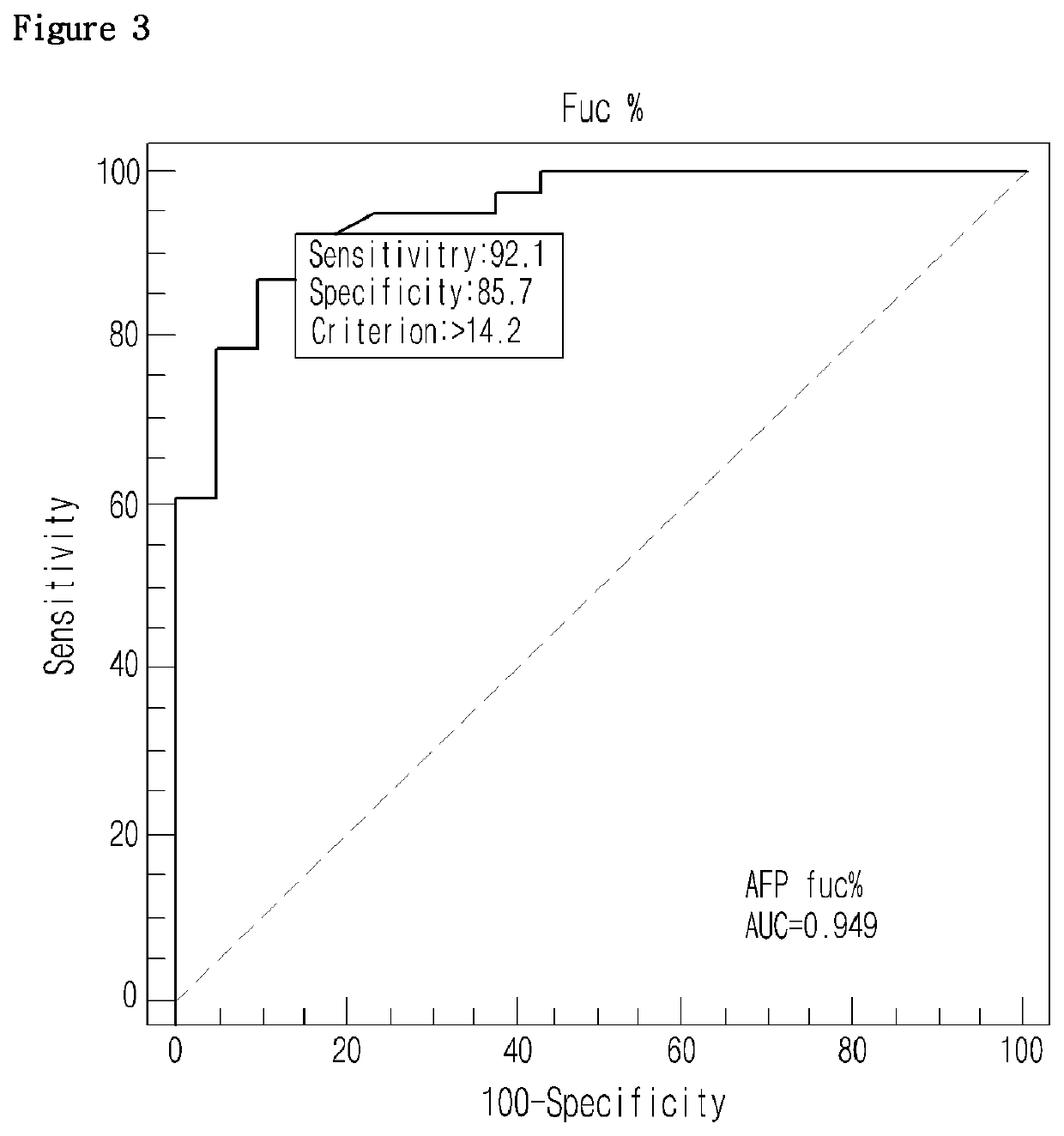
Diagnostic method of liver cancer using α-fetoprotein derived glycopeptides by mass spectrometry
ActiveUS10697971B2High fucosylation rateEasy to useMass spectrometric analysisDisease diagnosisFucosylationMass Spectrometry-Mass Spectrometry
The present invention relates to a diagnosis method of liver cancer using mass spectrometry of α-fetoprotein derived glycopeptide. Particularly, according to the AFP glycopeptide analysis method of the invention, fucosylation rate of the glycopeptide having the sequence composed of Val-Asn-Phe-Thr-Glu-Ile-Gln-Lys is analyzed to diagnose liver cancer in the early developmental grade. In particular, fucosylation rate is higher in the liver cancer patients than in other liver disease patients, so that the comparison of the fucosylation rate can be useful to diagnose or distinguish liver cancer from other liver diseases in the early HCC patients.
Owner:KOREA BASIC SCI INST
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com