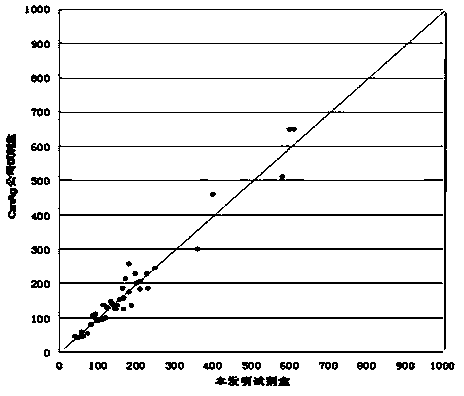
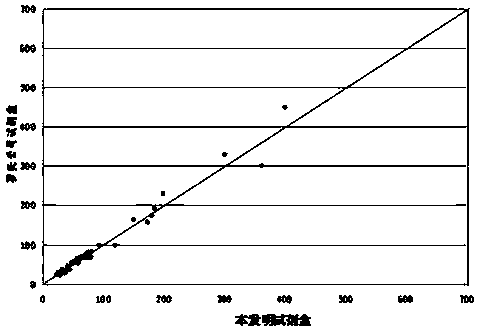
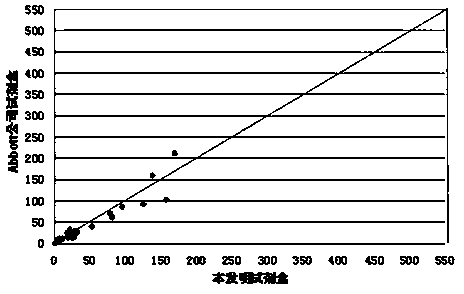
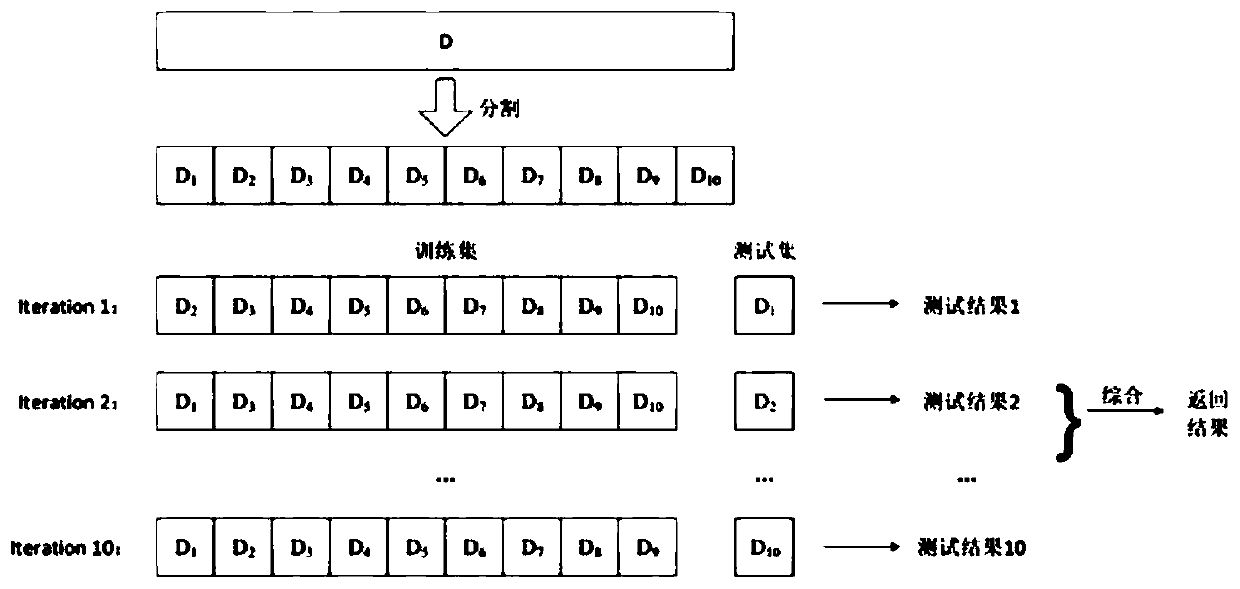
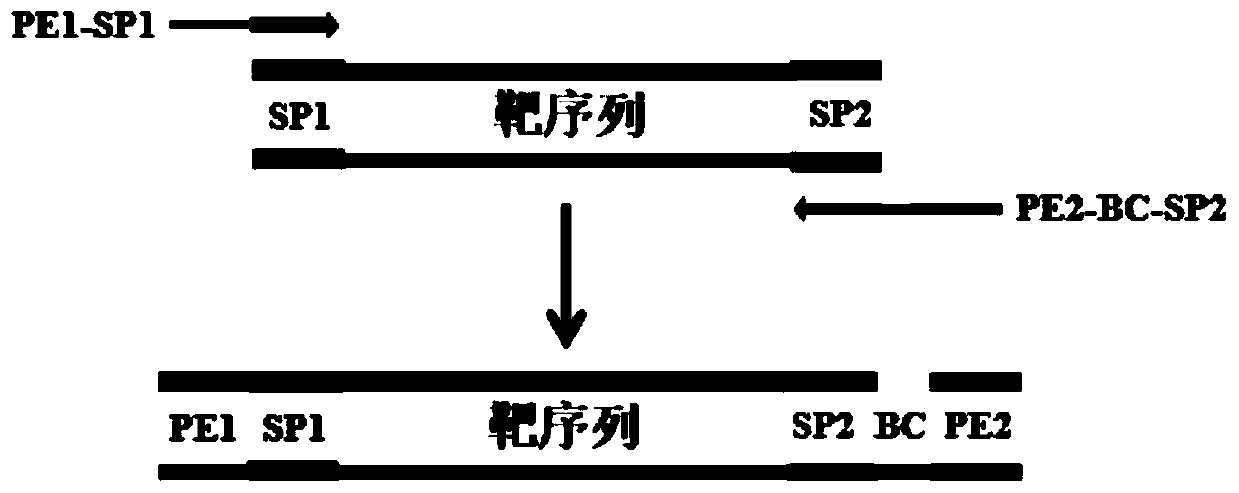
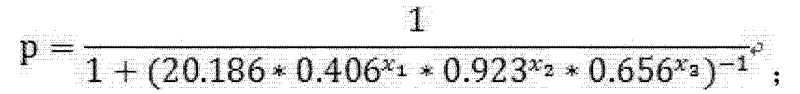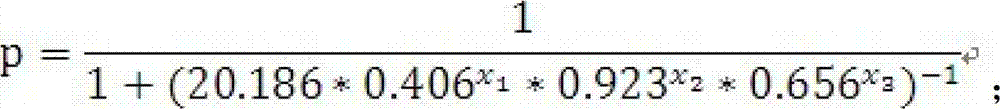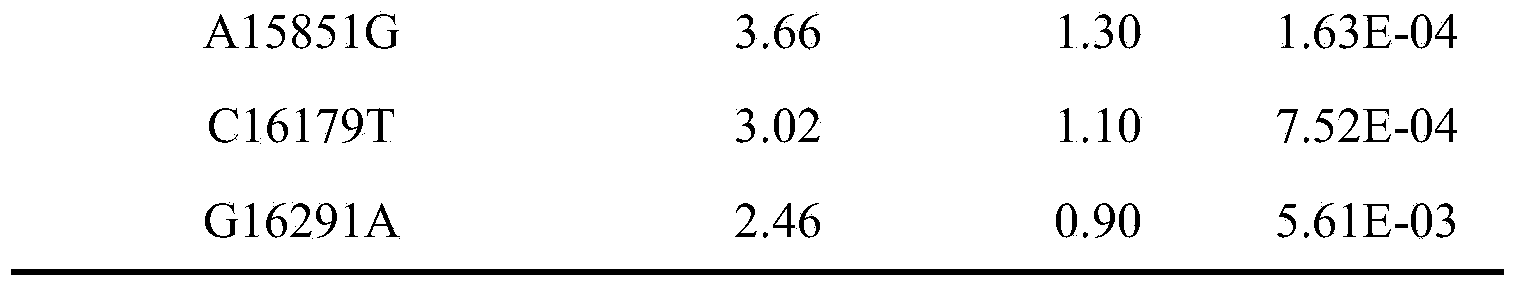Patents
Literature
468 results about "AIDS diagnosis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Multichannel micro-fluidic chip specially used for AIDS diagnosis and comprising quasi-one-dimensional sensitive electrodes
InactiveCN101581725AFast diagnosisReduce diagnostic costsMaterial electrochemical variablesMagnetic beadCapillary channel
The invention relates to a multichannel micro-fluidic chip specially used for AIDS diagnosis and comprising quasi-one-dimensional sensitive electrodes, belonging to the field of test. One of the hopes in the progress of medical techniques is to quickly diagnose AIDS at low cost. The invention provides a micro-fluidic chip that uses a plurality of AIDS characteristic antibodies to simultaneously detect and quickly diagnose AIDS. The chip is provided with three micro liquid storage tanks. The key points of the proposal lie in that the inner of the chip contains capillary channels of parallel structure, the parallel structure contains four micro-channels mutually connected in parallel, four working electrodes of bead string structure in total are respectively arranged in the four micro-channels, the substances on the superficial coats of the four working electrodes of bead string structure are respectively four antibody substances, namely, AIDS antibodies p24, gp41, gp120 and gp36. The application of the chip is conducive to increasing the diagnosis efficiency of AIDS as shown by the structural characteristics.
Owner:NINGBO UNIV
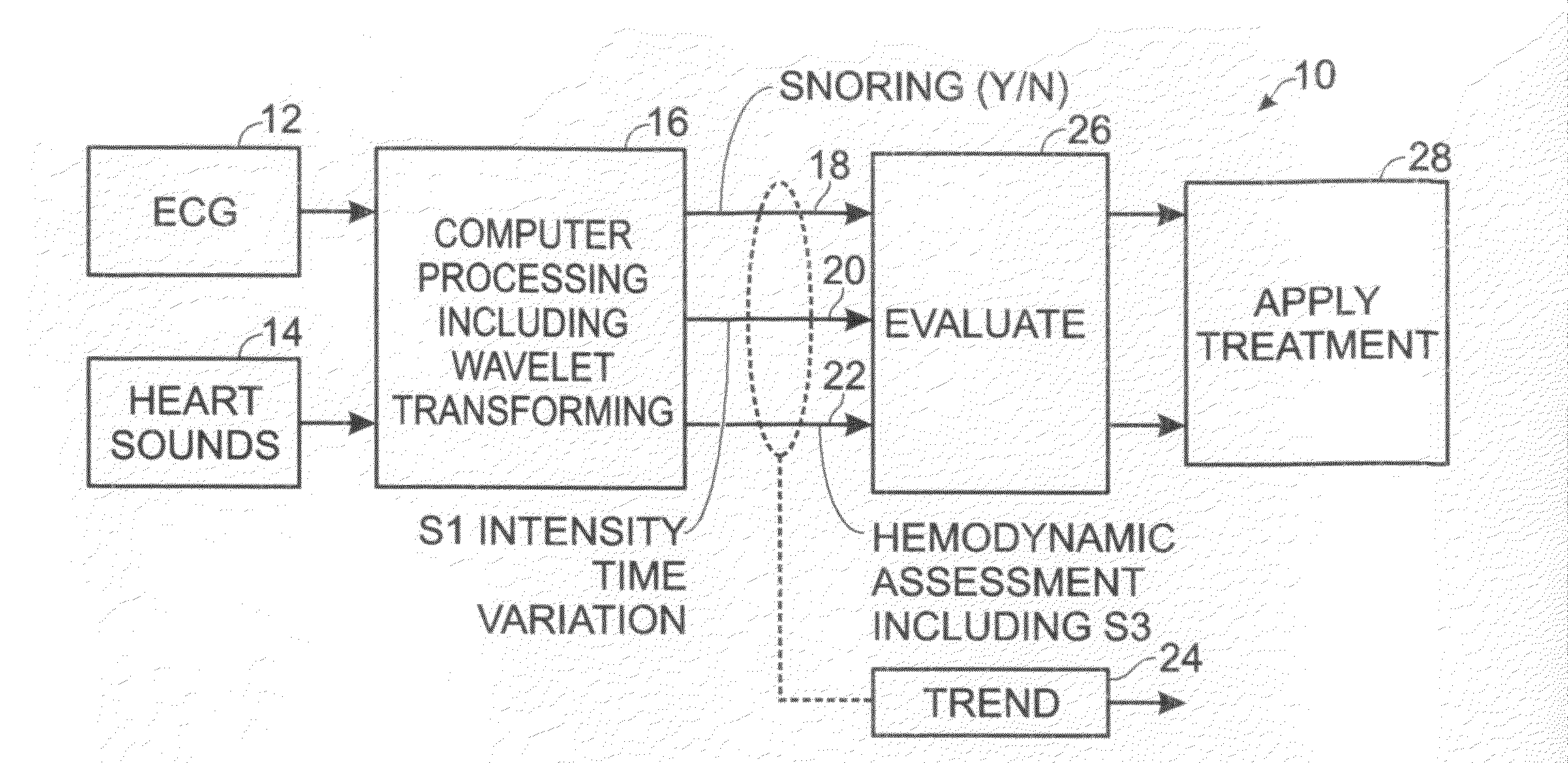
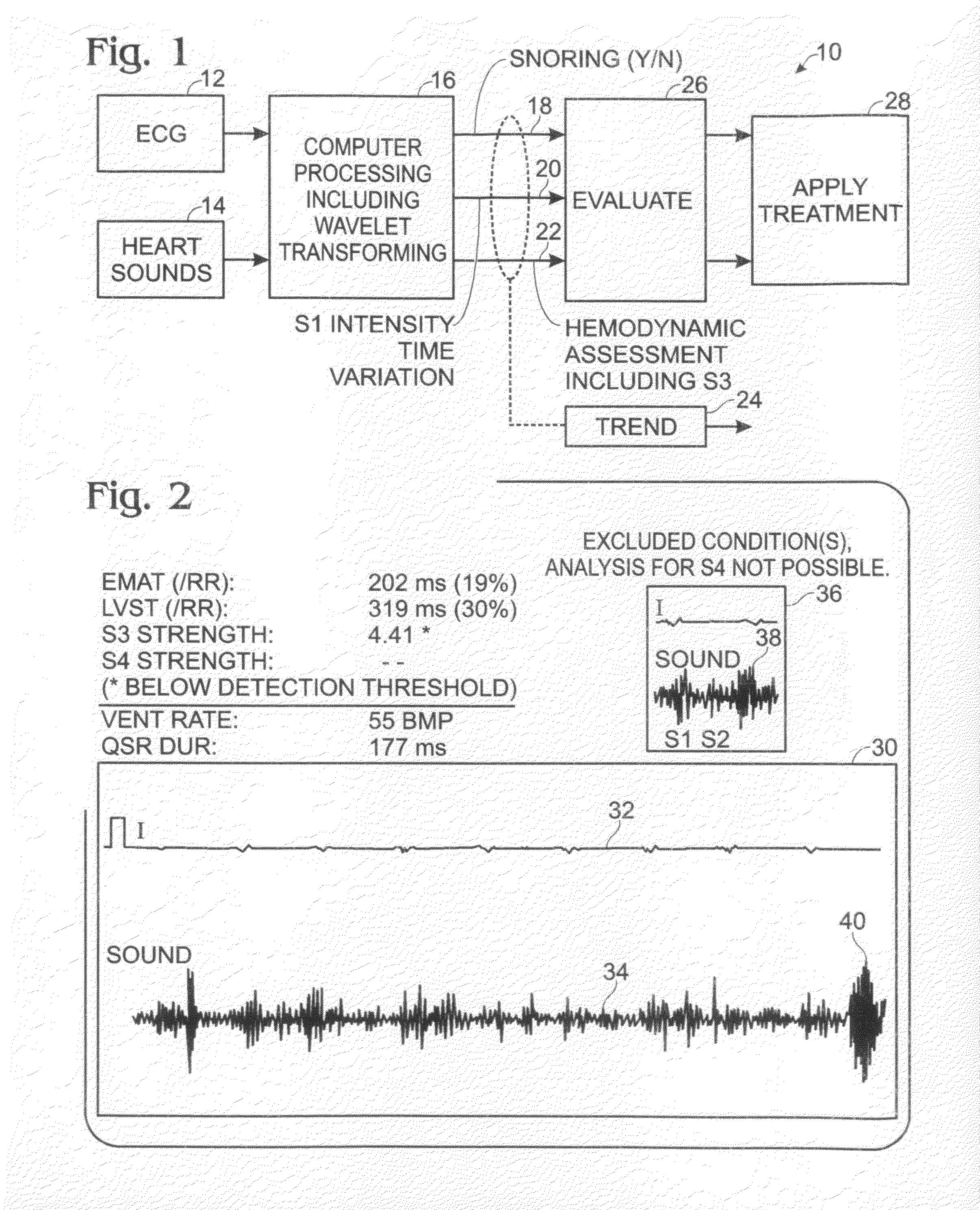
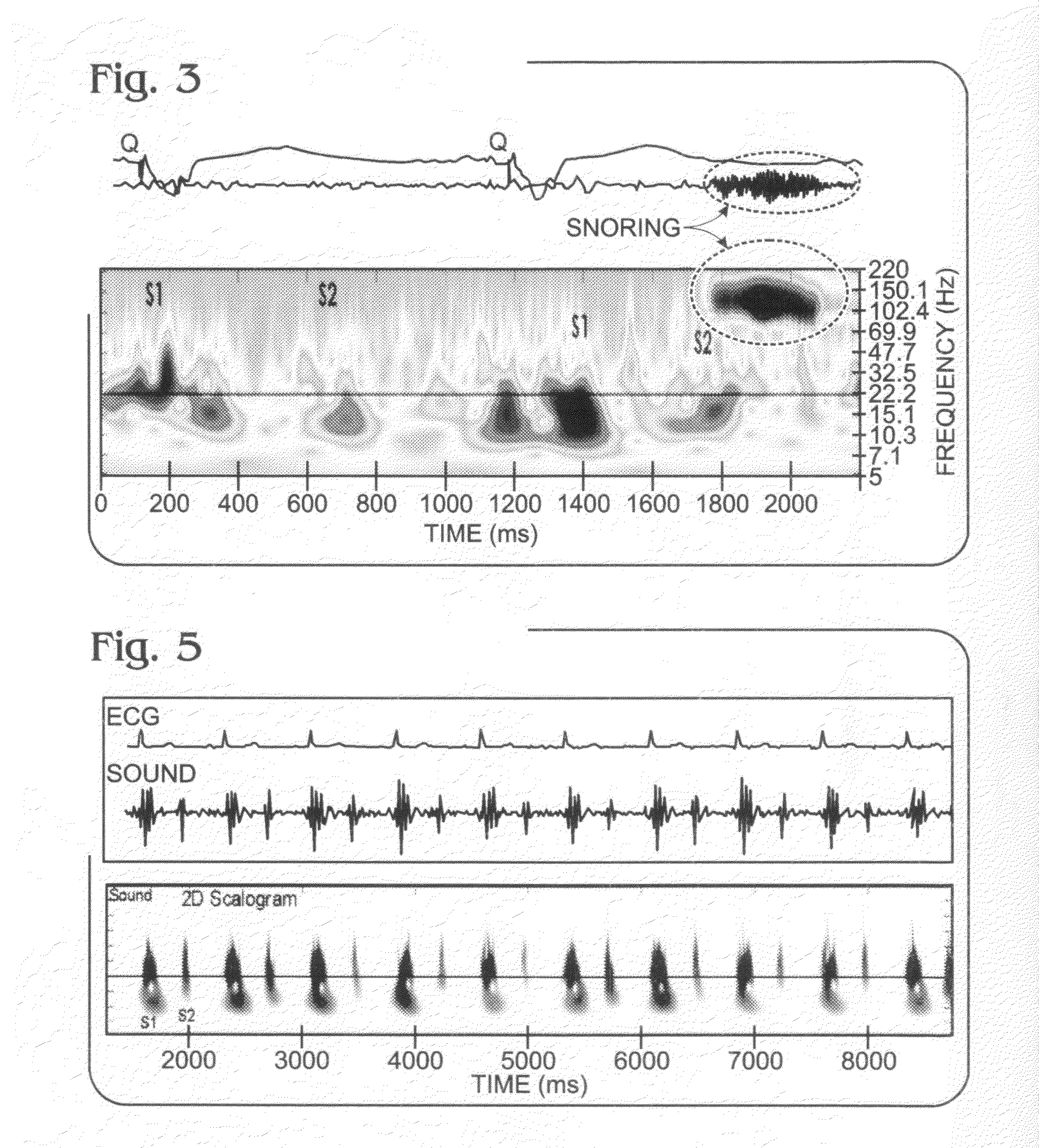
Differential apneic detection in aid of diagnosis and treatment
Owner:INOVISE MEDICAL
Tools for aiding in the diagnosis of neurodegenerative diseases
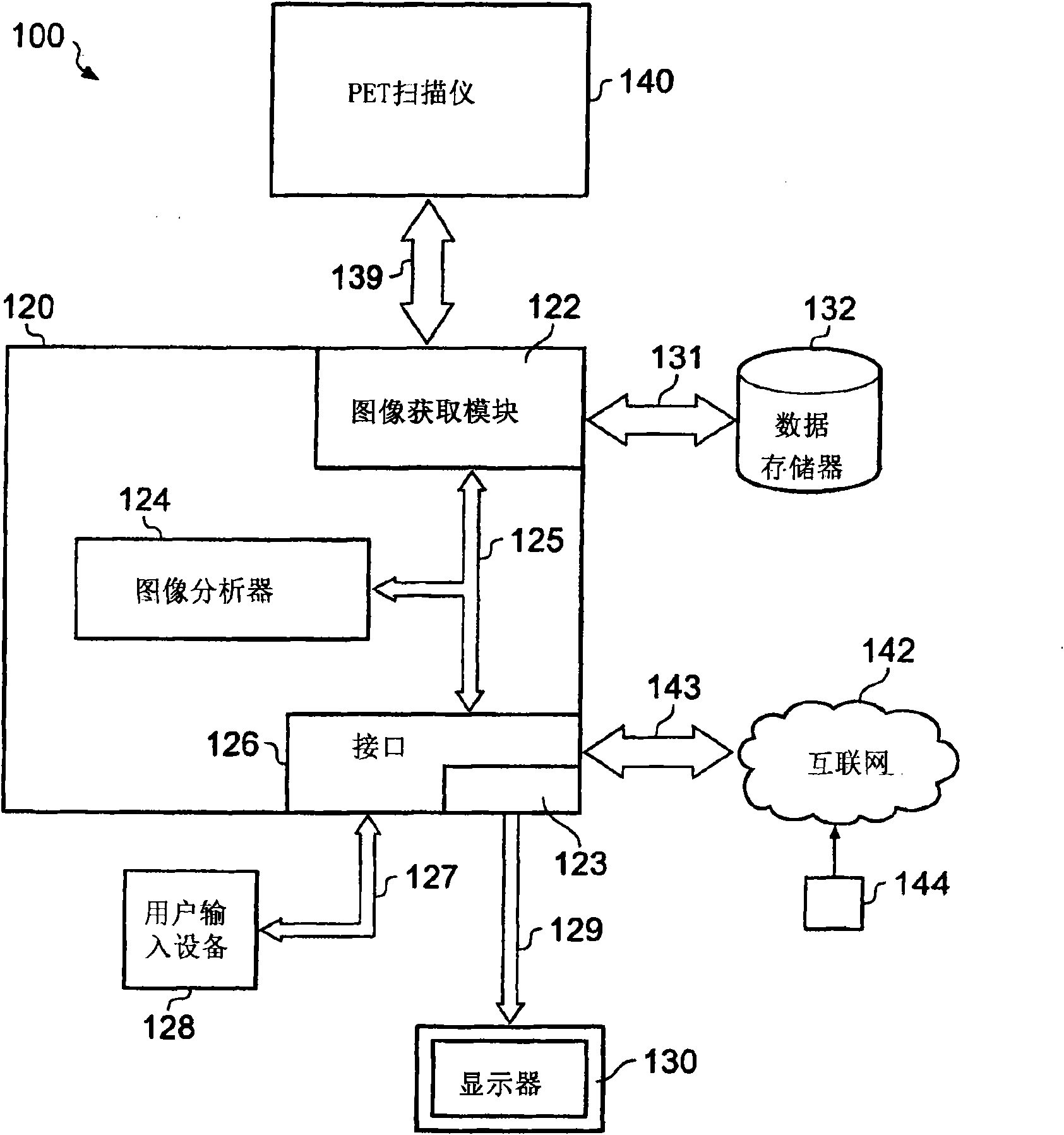
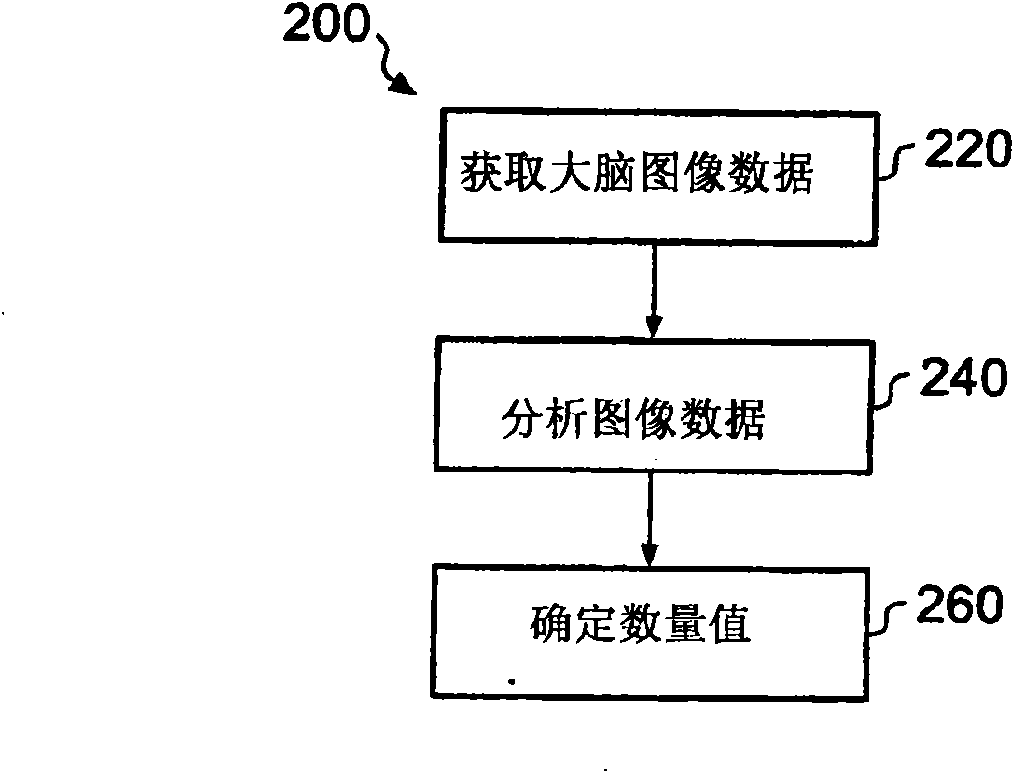
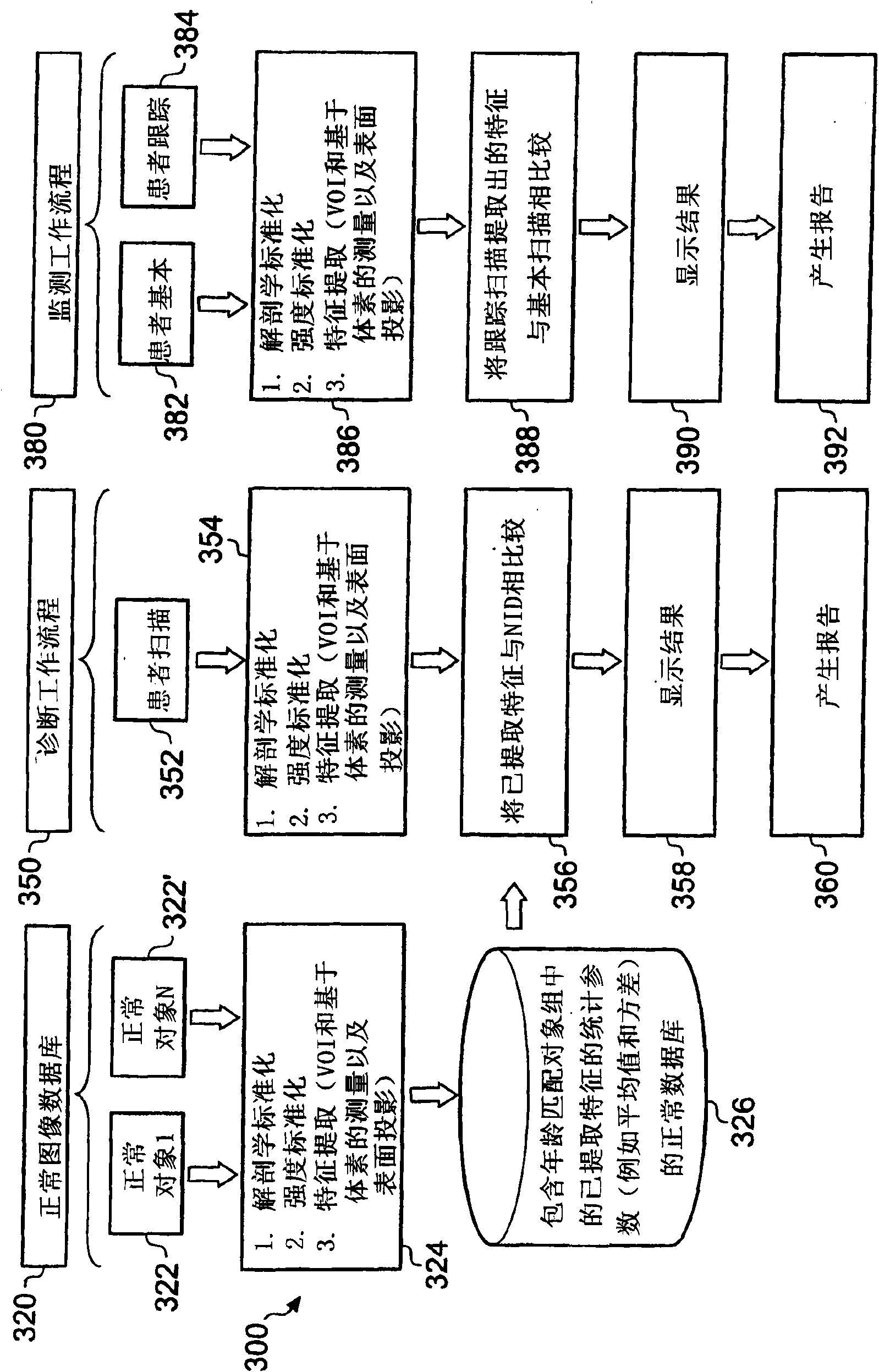
A system (100) and method for clinical evaluation of neurodegenerative disease present in a subject is described. The system (100) comprises an image acquisition module (122) operable to acquire image data representative of a brain of a subject, and an image analyser (124). The image analyser (124) is operable to determine a quantitative value from the image data that is indicative of the level of neurodegenerative disease present in the brain of the subject. Various embodiments of the invention provide a tool that aids in improved early diagnosis and monitoring of neurodegenerative diseases, such as, for example, Alzheimer's disease (AD).
Owner:GE HEALTHCARE LTD
Kits for Auxiliary Diagnosis of Tuberculosis
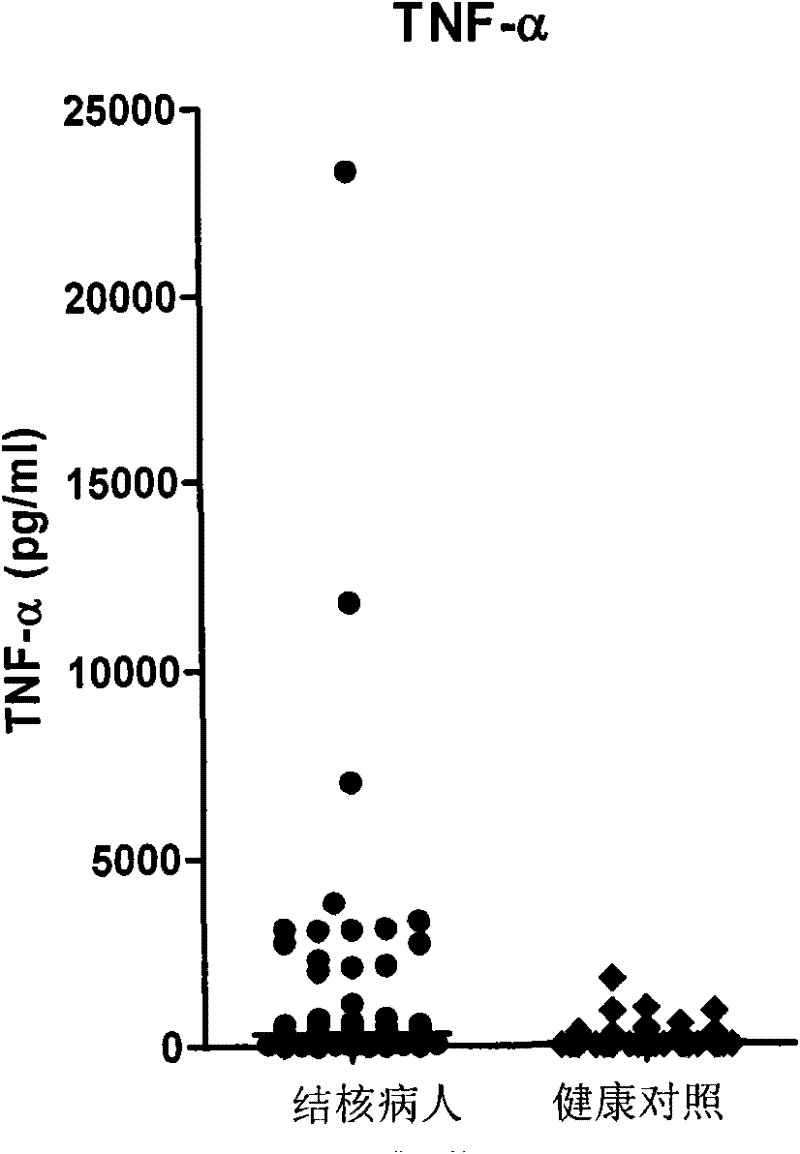
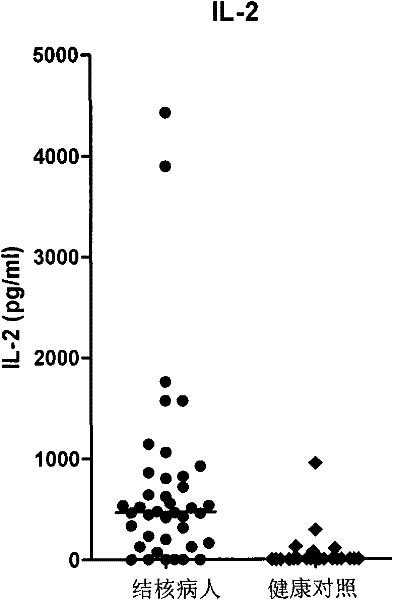
InactiveCN102297968AIncreased sensitivityReduce positive rateImmunoglobulins against cytokines/lymphokines/interferonsDepsipeptidesAIDS diagnosisIfn gamma
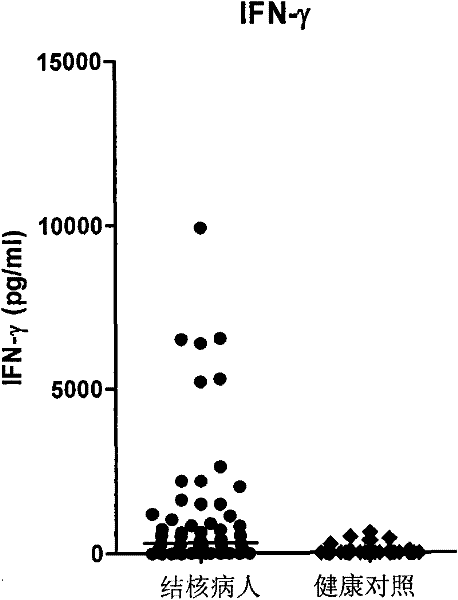
The invention discloses a kit for assisted diagnosis of tuberculosis, which comprises a specific antibody composition, wherein the specific antibody composition comprises the following five antibodies: (1) IFN-gamma antibody, (2) TNF-alpha antibody, (3) IL-2 antibody, (4) MIG antibody and (5) IP-10 antibody. The kit disclosed by the invention can distinguish a Mycobacterium tuberculosis infected person from a BCG vaccinee. Compared with the ELISPOT tuberculosis diagnosis method, the tuberculosis diagnosis kit based on multi-molecular marker detection has obviously enhanced sensitivity to active tuberculosis (from 72% to 89%), and the positive rate for normal healthy persons is obviously reduced (from 27% to 16%).
Owner:程小星 +3
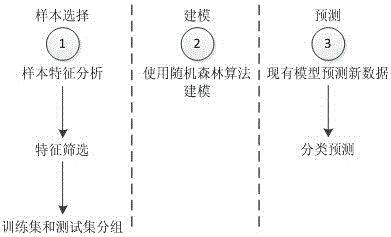
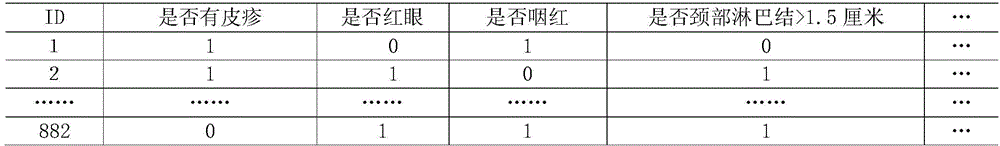
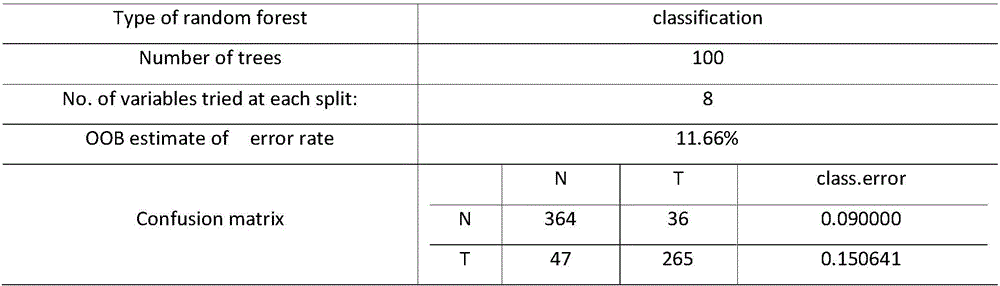
Kawasaki disease classification and prediction method based on medical data modeling
ActiveCN106339593AReduce misdiagnosis rateImprove follow-up treatment processSpecial data processing applicationsICT adaptationData setFeature set
The invention provides a Kawasaki disease classification and prediction method based on medical data modeling. The method comprises the following steps: S1, data sample selection: extracting a valid sample which can be modeled from a sample data set; S2, feature sieving: sieving 19 features conforming to field medical auxiliary diagnosis application from a feature set for constructing sample data for modeling; S3, Kawasaki disease classifying model construction and evaluation: fitting an Xtrain data set on a training set by using a random forest classifying method, recording optimal modeling parameters and weights of all selected features, and classifying and predicting a test set sample according to the classifying model. In the Kawasaki disease classification and prediction method, Kawasaki disease relevant data are analyzed and modelled systemically, and an evaluation method for model prediction is provided, so that the model can be used for performing effective auxiliary diagnosis on a Kawasaki disease of a patient based on Kawasaki disease data, effective prevention and intervention and treatment are performed at the early stage of attacking, and a basis is laid for a best treatment effect.
Owner:北京万灵盘古科技有限公司
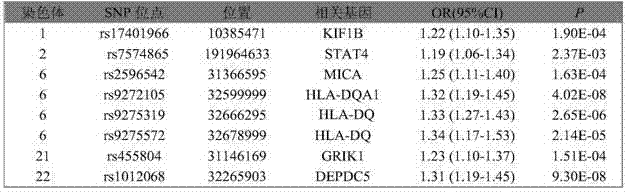
SNP marker related to primary hepatocellular carcinoma auxiliary diagnosis and application of SNP marker
InactiveCN104745710AHelps reflect disease stateQuickly and accurately grasp the conditionMicrobiological testing/measurementDNA/RNA fragmentationHepatic carcinomaWilms' tumor
The invention belongs to the genetic engineering and tumour medicine fields and discloses an SNP marker related to primary hepatocellular carcinoma auxiliary diagnosis and an application of the SNP marker. The marker is a combination of rs7574865, rs1012068, rs17401966, rs2596542, rs455804, rs9272105, rs9275319 and rs9275572. The marker can be used for preparing a hepatic carcinoma auxiliary diagnosis kit.
Owner:SHANGHAI ROSETTA BIOTECH CO LTD
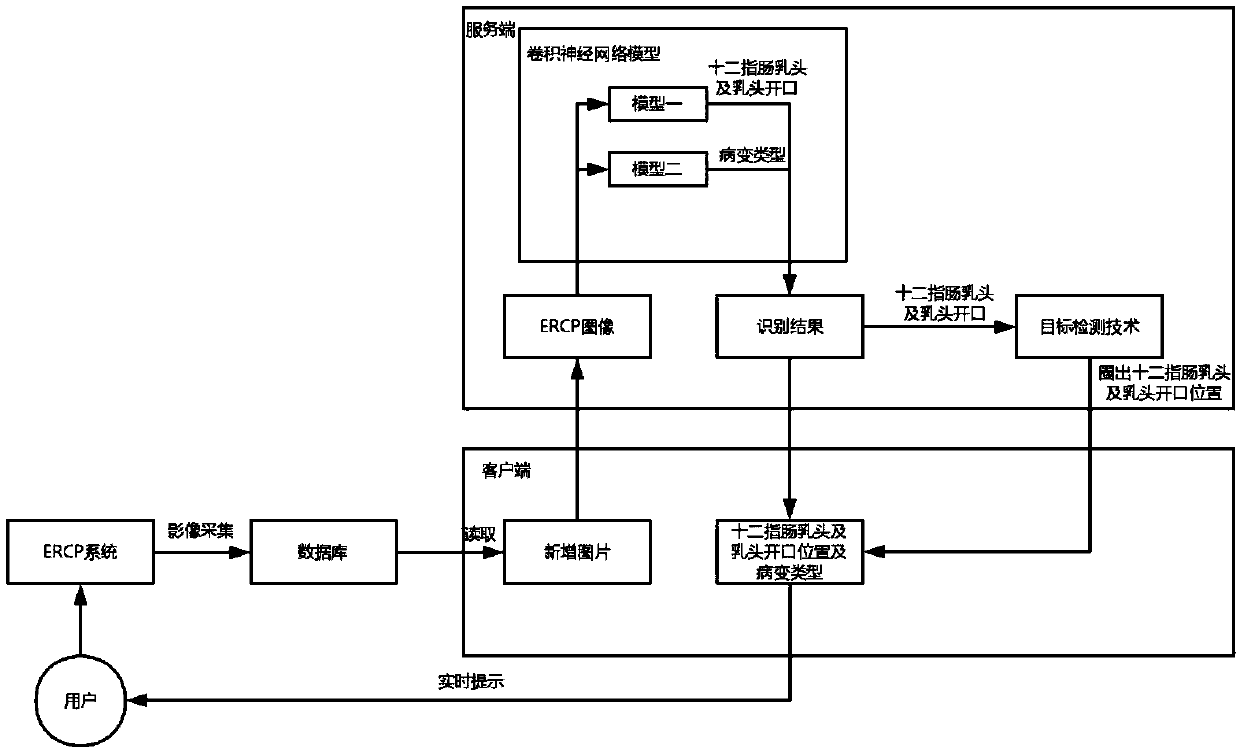
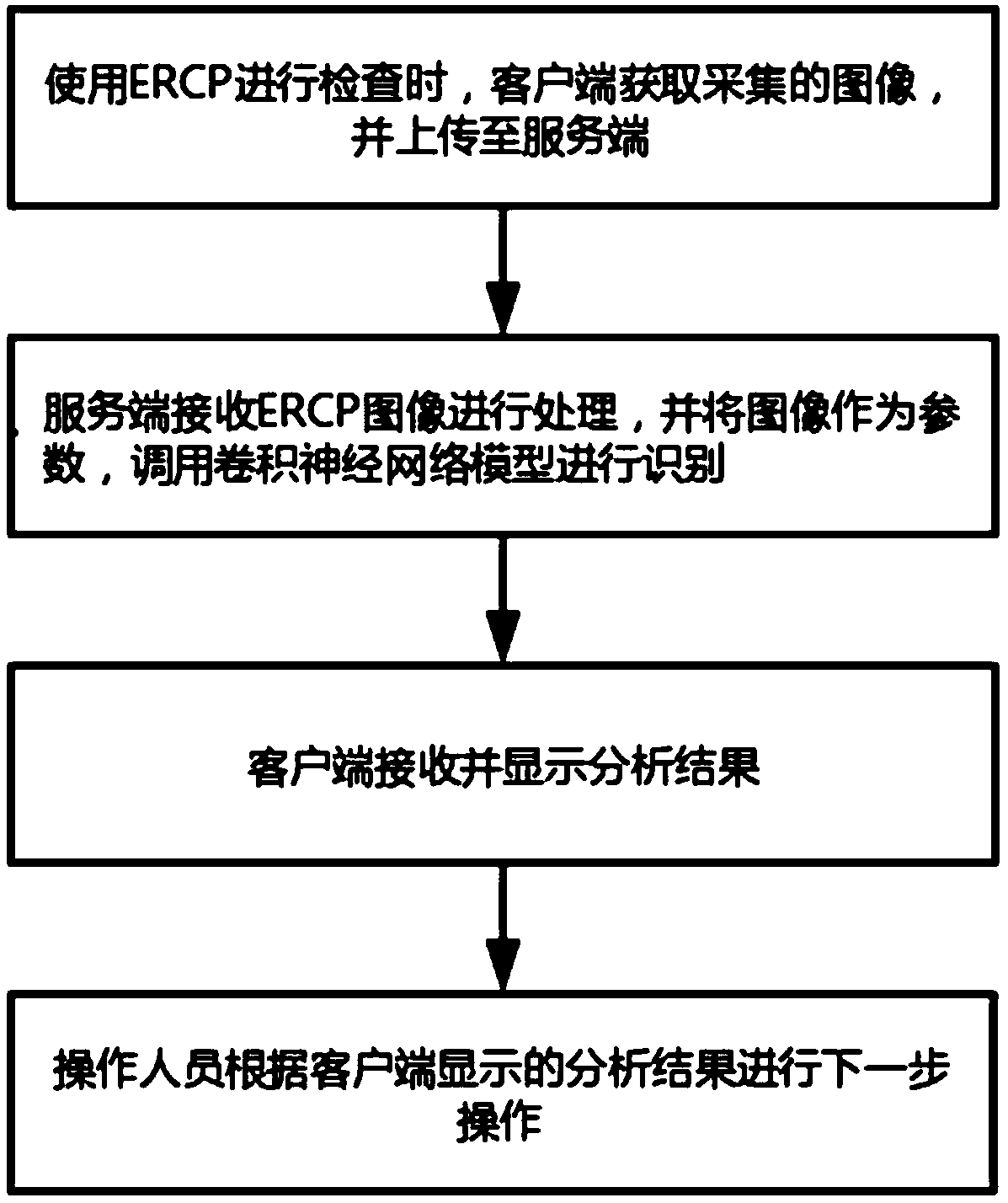
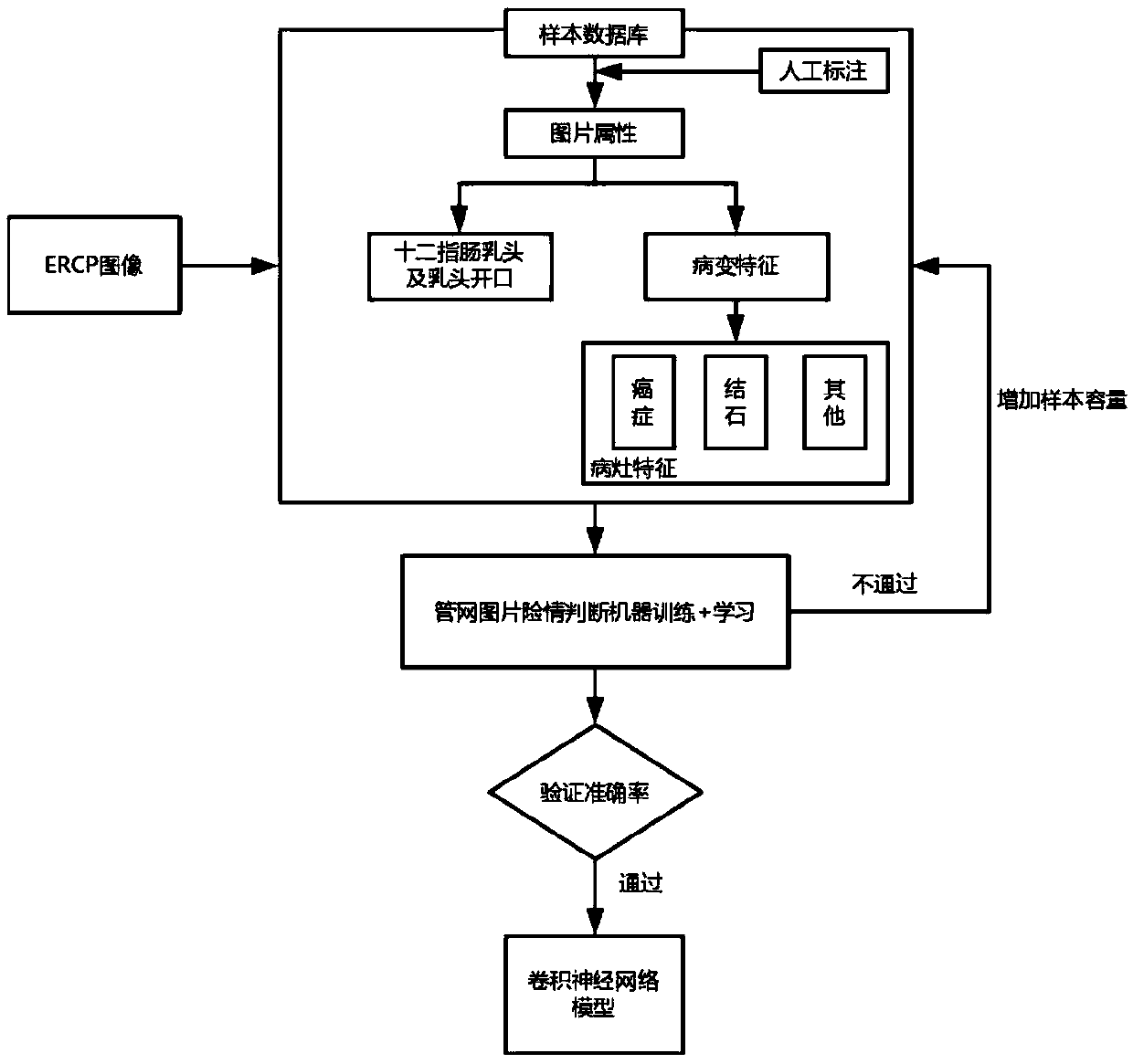
A real-time auxiliary diagnosis system and method for endoscopic retrograde pancreatic duct radiography
InactiveCN109584229AImprove intubation success rateImprove accuracy and effectivenessImage enhancementImage analysisMissed diagnosisComputer science
The invention discloses a real-time auxiliary diagnosis system and method for endoscopic retrograde pancreatic duct radiography. The system comprises a client, a server and a database, An ERCP image is collected through a client side, the obtained ERCP image is input into a trained deep learning model to be recognized and classified, and corresponding image information (including duodenal papillaand papilla opening recognition and disease diagnosis) is obtained and fed back to the client side in time. A doctor can be helped to determine the duodenal papilla and the papilla opening position, and the intubation success rate is increased; In addition, doctors can be assisted to diagnose diseases, misdiagnosis and missed diagnosis are prevented, and the diagnosis accuracy and the working efficiency are improved.
Owner:武汉大学人民医院
Human immunodeficiency virus (HIV) antibody detection kit and preparation method thereof
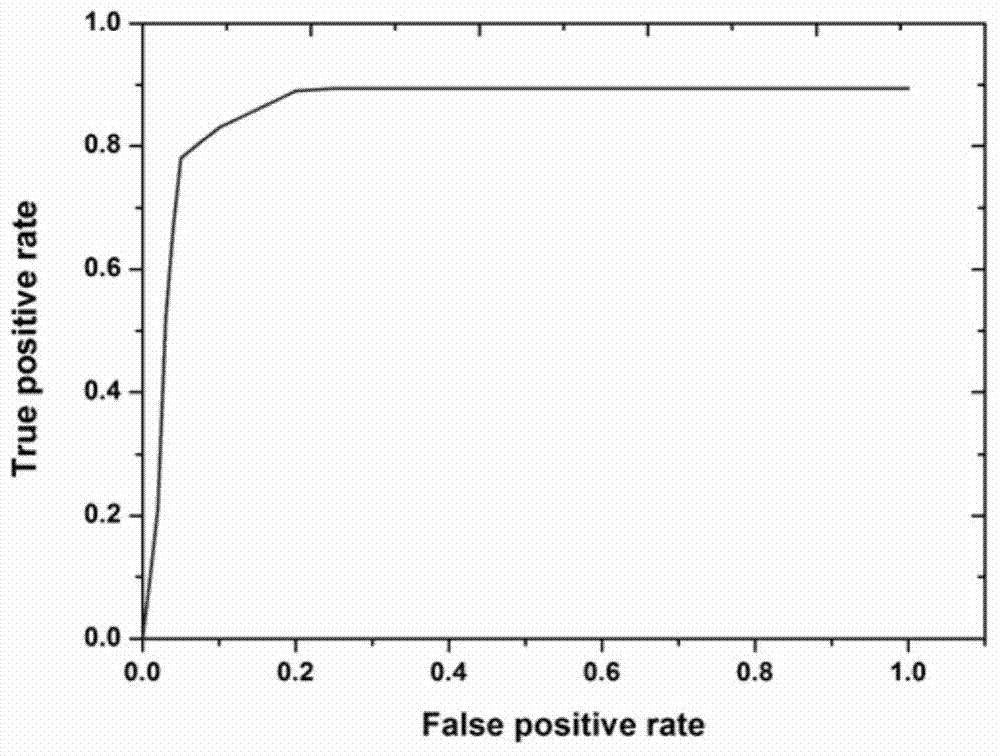
InactiveCN104090101AHigh sensitivityStrong specificityChemiluminescene/bioluminescencePositive controlTrue positive rate
The invention belongs to the technical field of immunologic diagnosis and particularly relates to a human immunodeficiency virus (HIV) antibody detection kit using a microparticle chemiluminiscence method and a preparation method of the kit. The kit is composed of magnetic microparticles for detecting an HIV antibody, a tracing conjugate for detecting the HIV antibody, a negative control, a I type positive control, a II type positive control and an analyzing buffering solution. The invention further discloses the preparation method of the detection kit, which adopts a particle chemiluminiscence immunoassay technology; compared with ELISA (Enzyme-Linked Immunosorbent Assay), the technology has higher sensitivity and specificity and is suitable for the clinical auxiliary diagnosis of HIV.
Owner:威海威高生物科技有限公司
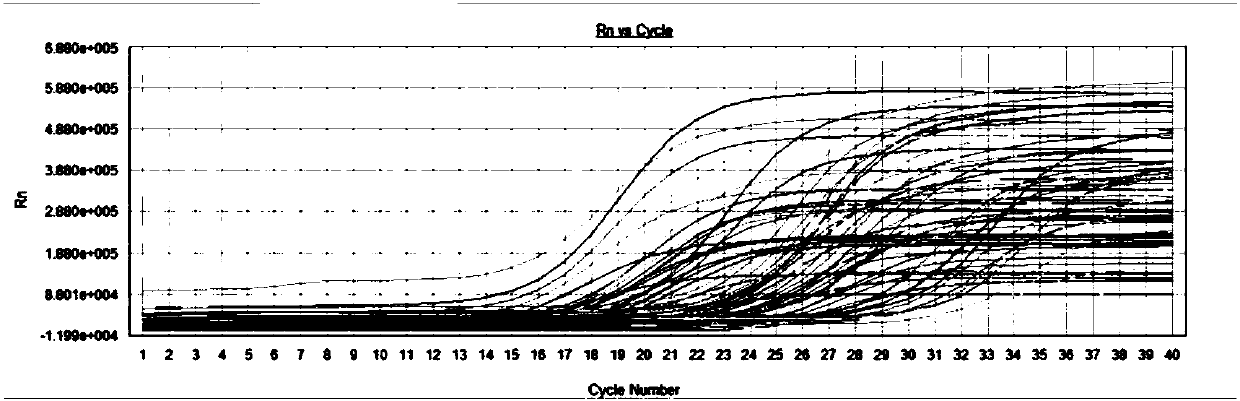
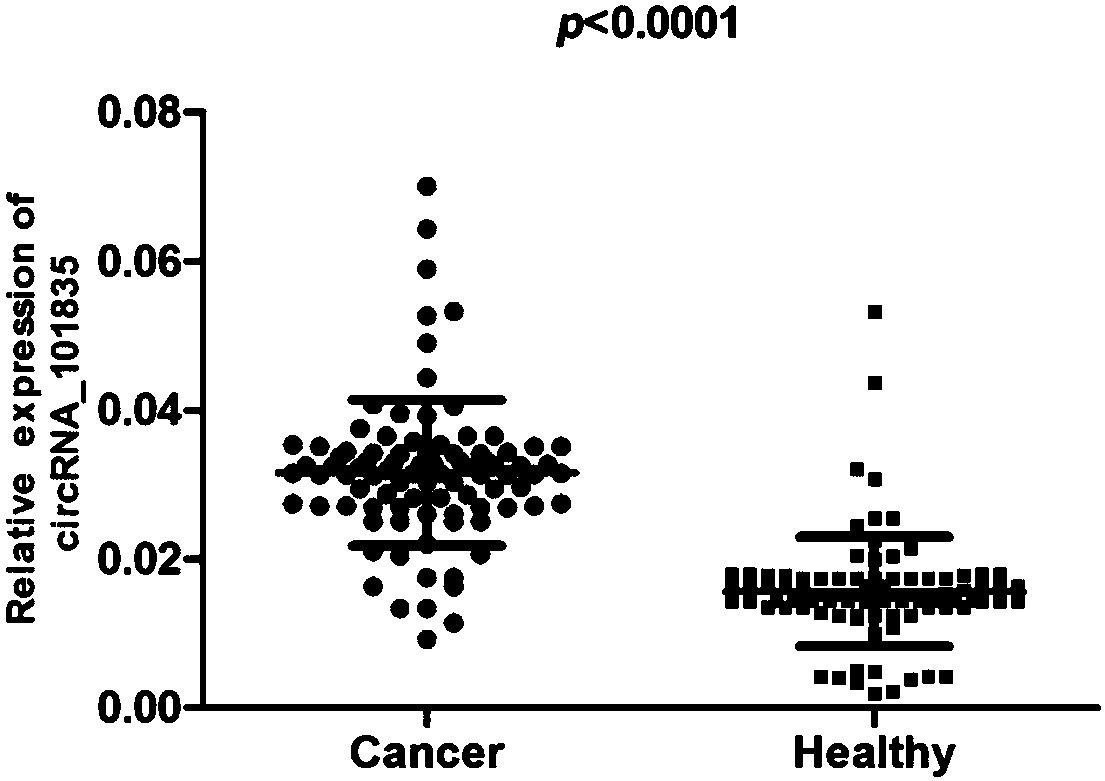
Primers, kit and method to detect circular DNA circRNA_101835 and their application
InactiveCN107858435AMicrobiological testing/measurementDNA/RNA fragmentationFluorescencePolymerase chain reaction
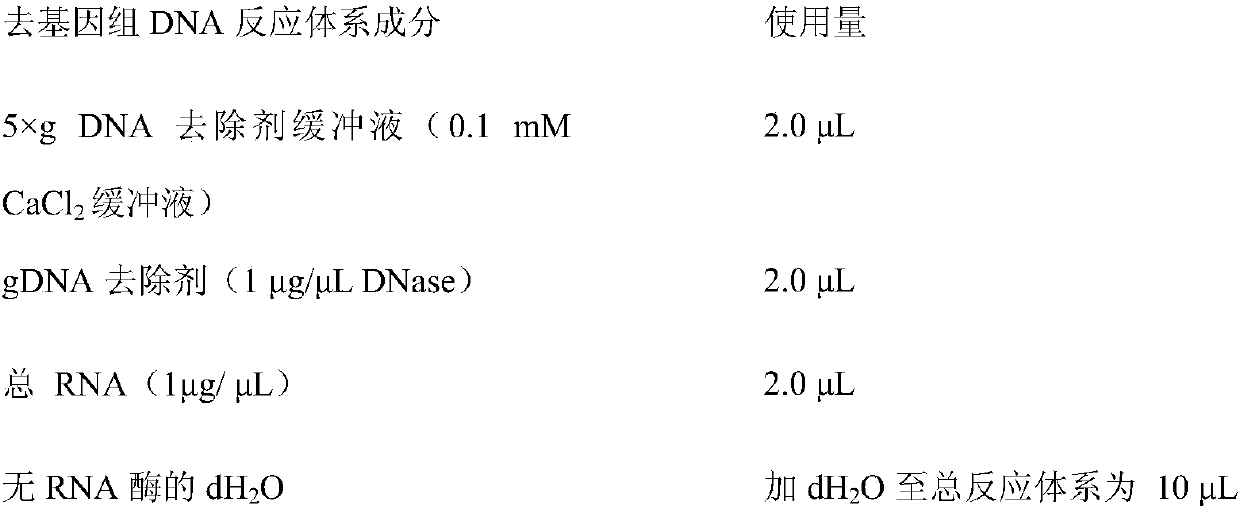
The invention relates to primers, kit and method to detect circular DNA circRNA_101835 and their application. The primers to detect circular DNA circRNA_101835 are involved. The kit includes a blood sample RNA extracting reagent, the primers, a genome-removed DNA reaction system, a reverse transcription reaction system, and a qPCR (quantitative polymerase chain reaction) operation reaction system.The invention discloses expression of the circular DNA circRNA_101835 in people with gastric cancer and normal people and the use of fluorescence quantitative PCR to detect the circular DNA circRNA_101835. The circular DNA circRNA_101835 is suitable for assisted diagnosis of gastric cancer and evaluation on cancer treatment effect and prognosis condition.
Owner:ZHENJIANG NO 1 PEOPLES HOSPITAL
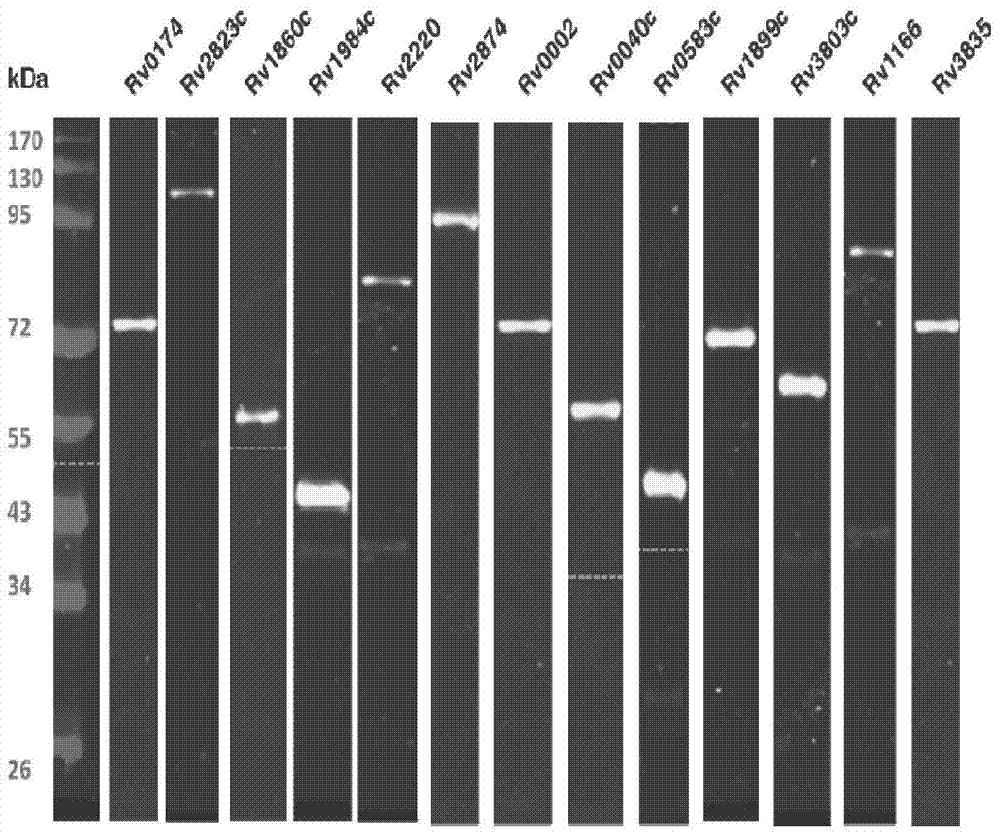
Application of mycobacterium tuberculosis protein in preparation of products used for diagnosis of latent tuberculosis infection
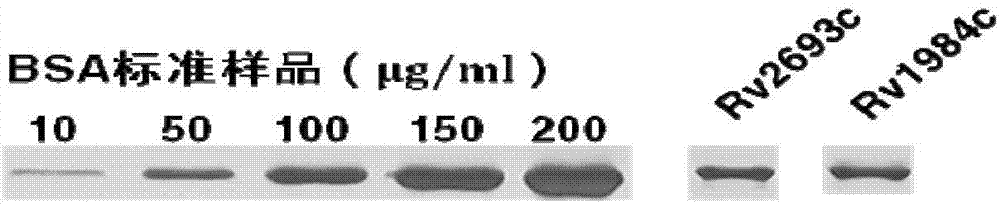
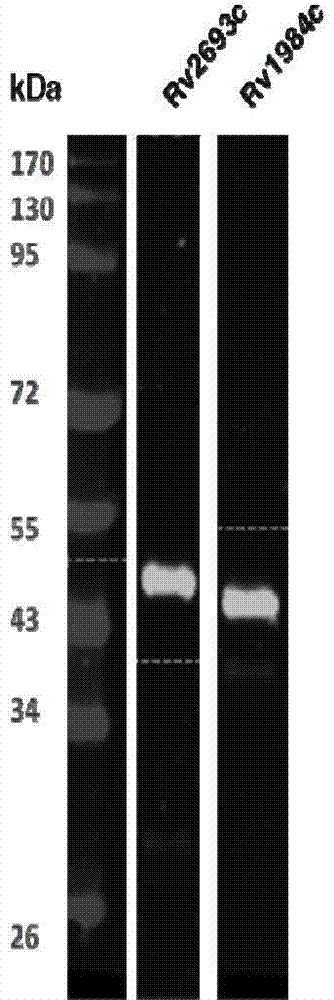
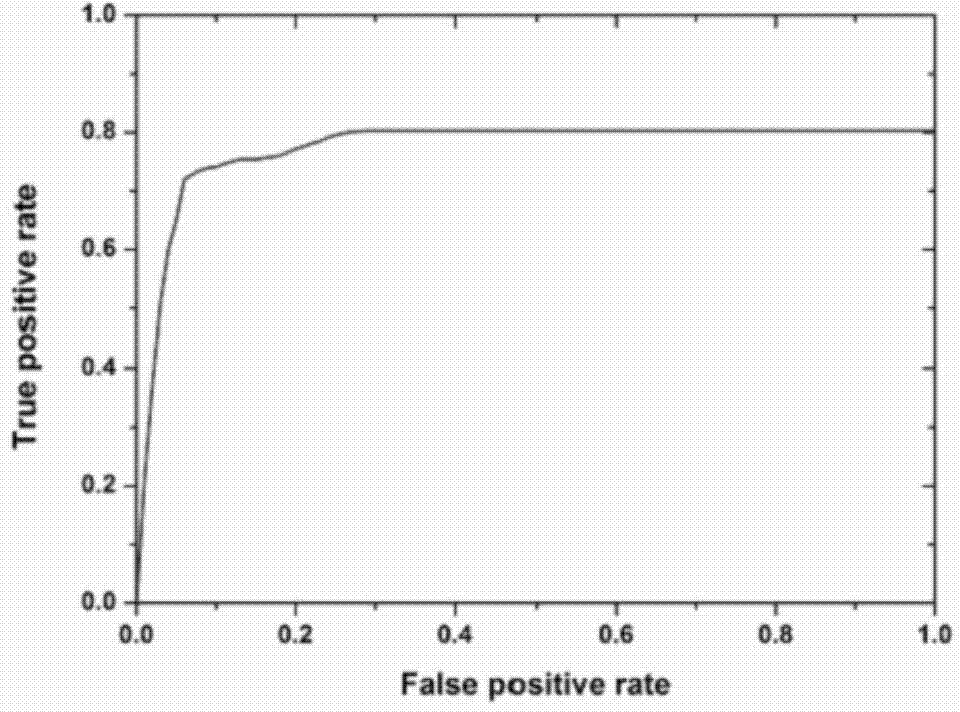
The invention provides mycobacterium tuberculosis protein Rv2693c and / or Rv1984c in development and / or designing of products capable of discrimination, diagnosis, auxiliary diagnosis, screening and / or auxiliary screening of latent tuberculosis infection. The invention further provides protein chips prepared from the two mycobacterium tuberculosis proteins. The protein chips prepared in the invention are used for detecting the levels of IgM antibodies respectively corresponding to the twp proteins in serum of a patient with latent tuberculosis infection and of a normal person, and detection results of the antibodies respectively corresponding to the three protein are analyzed together so as to determine whether an examined person suffers from latent tuberculosis infection; detection results show that optimal operating points of the protein chips provided by the invention in auxiliary diagnosis of latent tuberculosis infection have specificity of 80.3% and sensitivity of 75.6%, both higher than indexes of diagnosis of latent tuberculosis infection in the prior art.
Owner:TB HEALTHCARE BIOTECHNOLOGY (GUANGDONG) CO LTD
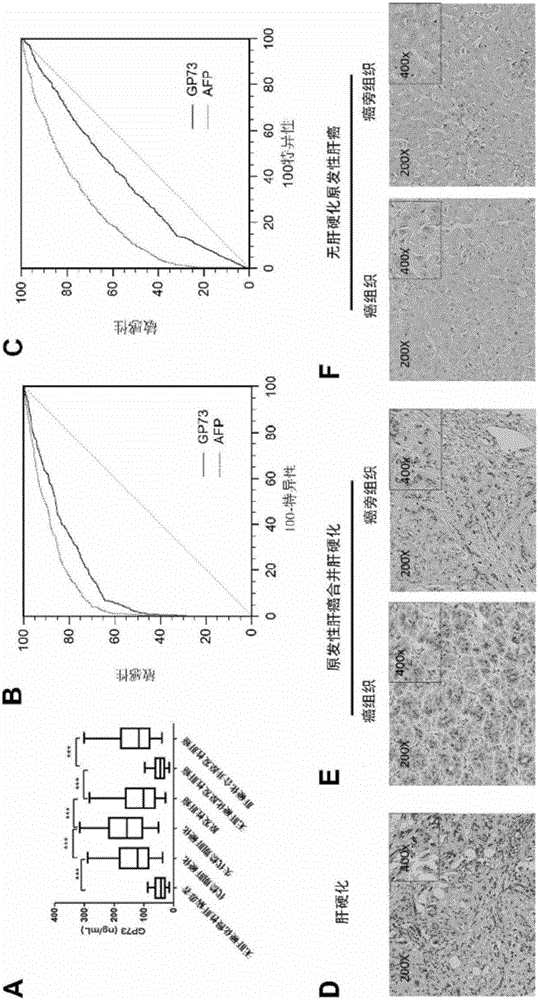
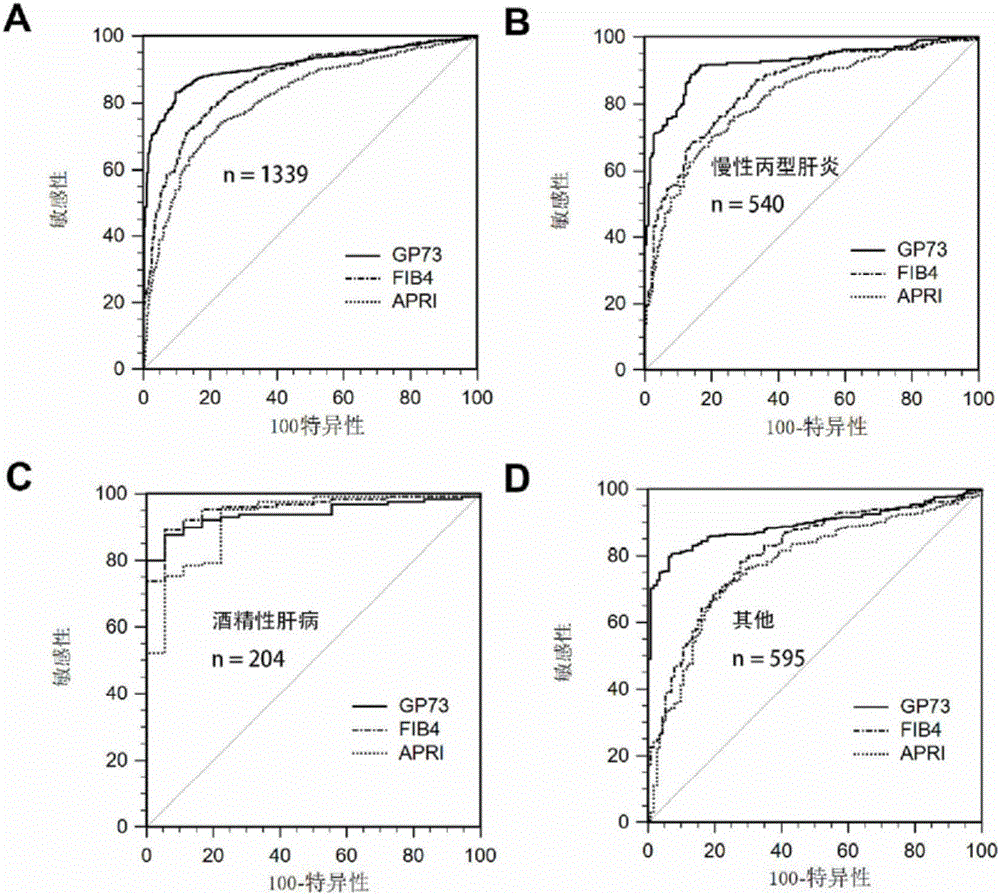
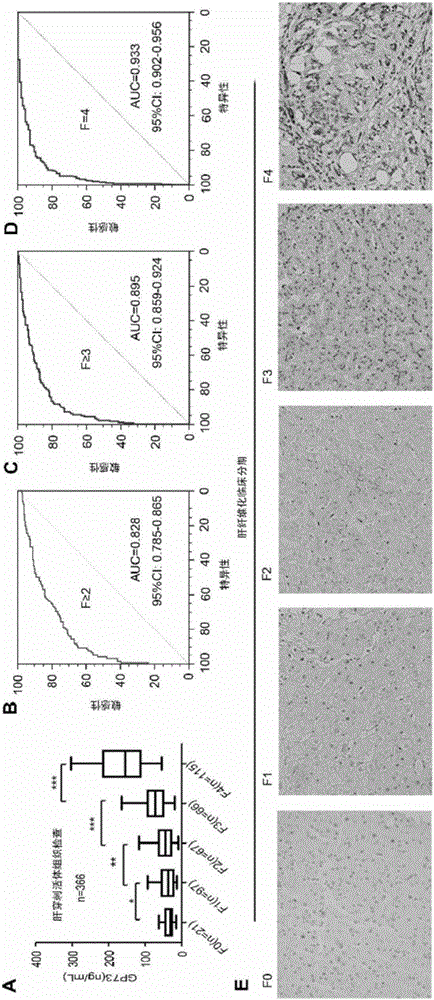
Novel liver cirrhosis or liver fibrosis marker
ActiveCN106405104AHigh sensitivityImprove the ability to distinguishDisease diagnosisBiological testingDiseaseAIDS diagnosis
The invention relates to the field of diagnosis and treatment of liver cirrhosis and liver fibrosis, in particular to application of Golgi protein 73(GP73) and matter for detecting the concentration of the Golgi protein 73(GP73) in preparation of products for screening or auxiliarily diagnosing liver cirrhosis or liver fibrosis related diseases or products for auxiliarily judging prognosis of the diseases. It is proved that the Golgi protein 73(GP73) can serve as a novel liver cirrhosis marker, also has diagnosis value on liver cirrhosis caused by non-b hepatitis virus infection, and has no diagnosis value on primary liver cancer.
Owner:PEKING UNIV
Application of mycobacterium tuberculosis proteins in preparation of products used for diagnosis of latent tuberculosis infection
The invention provides 13 mycobacterium tuberculosis proteins in development and / or designing of products capable of discrimination, diagnosis, auxiliary diagnosis, screening and / or auxiliary screening of latent tuberculosis infection. The invention further provides protein chips prepared from 13 mycobacterium tuberculosis protein antigens. The protein chips prepared in the invention are used for detecting the levels of IgG antibodies respectively corresponding to the 13 protein antigens in serum of a patient with latent tuberculosis infection and of a normal person, and detection results of the antibodies respectively corresponding to the three protein are analyzed together so as to determine whether an examined person suffers from latent tuberculosis infection; detection results show that optimal operating points of the protein chips provided by the invention in auxiliary diagnosis of latent tuberculosis infection have specificity of 89.4% and sensitivity of 80.6%, both higher than indexes of diagnosis of latent tuberculosis infection in the prior art.
Owner:广东希格生物科技有限公司
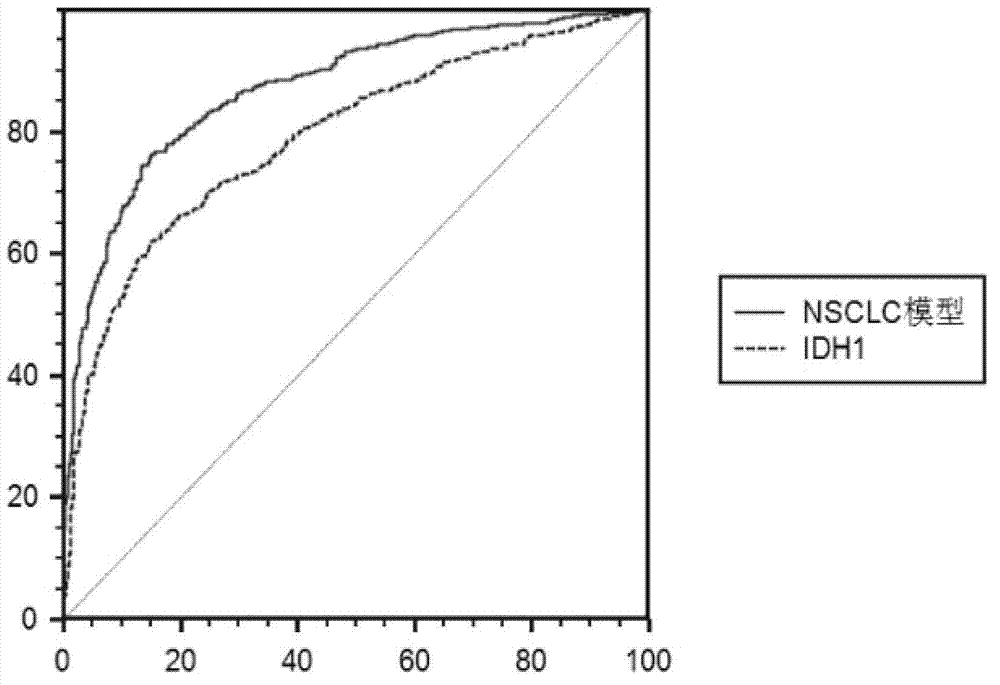
Test kit of auxiliary diagnosis of non-small cell lung cancer patients
ActiveCN103163293AIncreased sensitivityIncrease credibilityBiological testingIDH1Diagnosis standards
The invention discloses a test kit of auxiliary diagnosis of non-small cell lung cancer patients. The test kit comprises a product used for detecting protein landmark isocitrate dehydrogenase 1(IDH1), a product used for detecting protein landmark carbonic anhydrase 125 (CA125), a product used for detecting protein landmark CYFRA21-1 and a carrier on which a functional expression p=1 / (1+(20.186*0.406<x1>*0.923<x2>*0.656<x3>)<-1>) is recorded, wherein x1 represents the concentration of IDH1, x2 represents the concentration of CA125, and x3 represents the concentration of CYFRA21-1. The test kit is adopted and the non-small cell lung cancer patients are diagnosed in an auxiliary mode according to corresponding diagnosis standards, the test kit has the advantages of being high in sensitivity and strong in specificity, reliability of the diagnosis is far higher than that of diagnosis which is carried out with each single protein landmark, and the test kit has great value and application prospects for diagnosis and treatment of non-small cell lung cancer.
Owner:CANCER INST & HOSPITAL CHINESE ACADEMY OF MEDICAL SCI
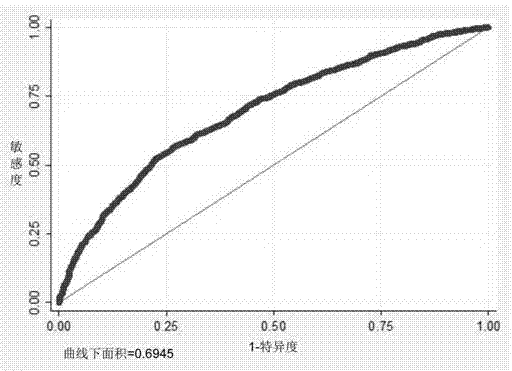
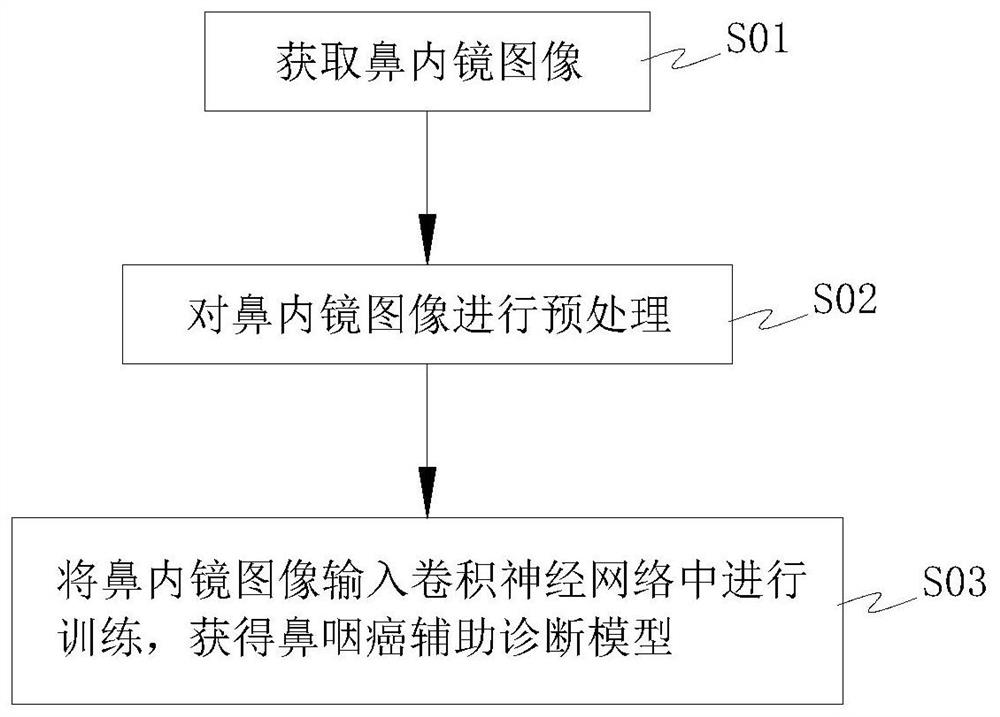
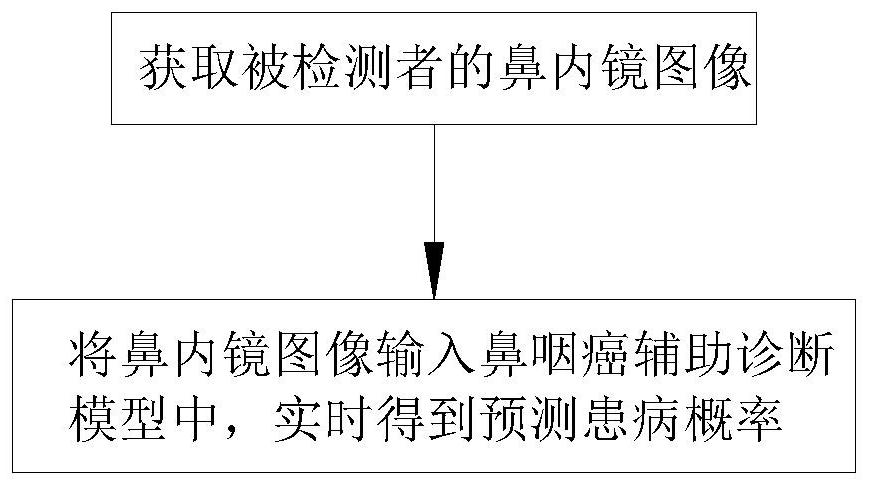
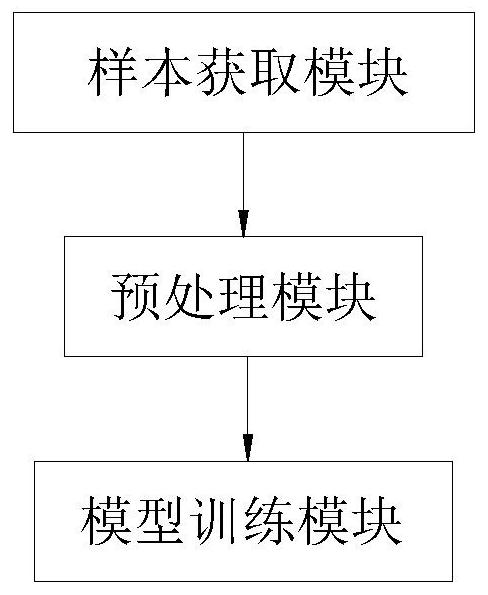
Nasopharyngeal carcinoma auxiliary diagnosis model construction and auxiliary diagnosis method and system
Owner:THE FIRST AFFILIATED HOSPITAL OF SUN YAT SEN UNIV +1
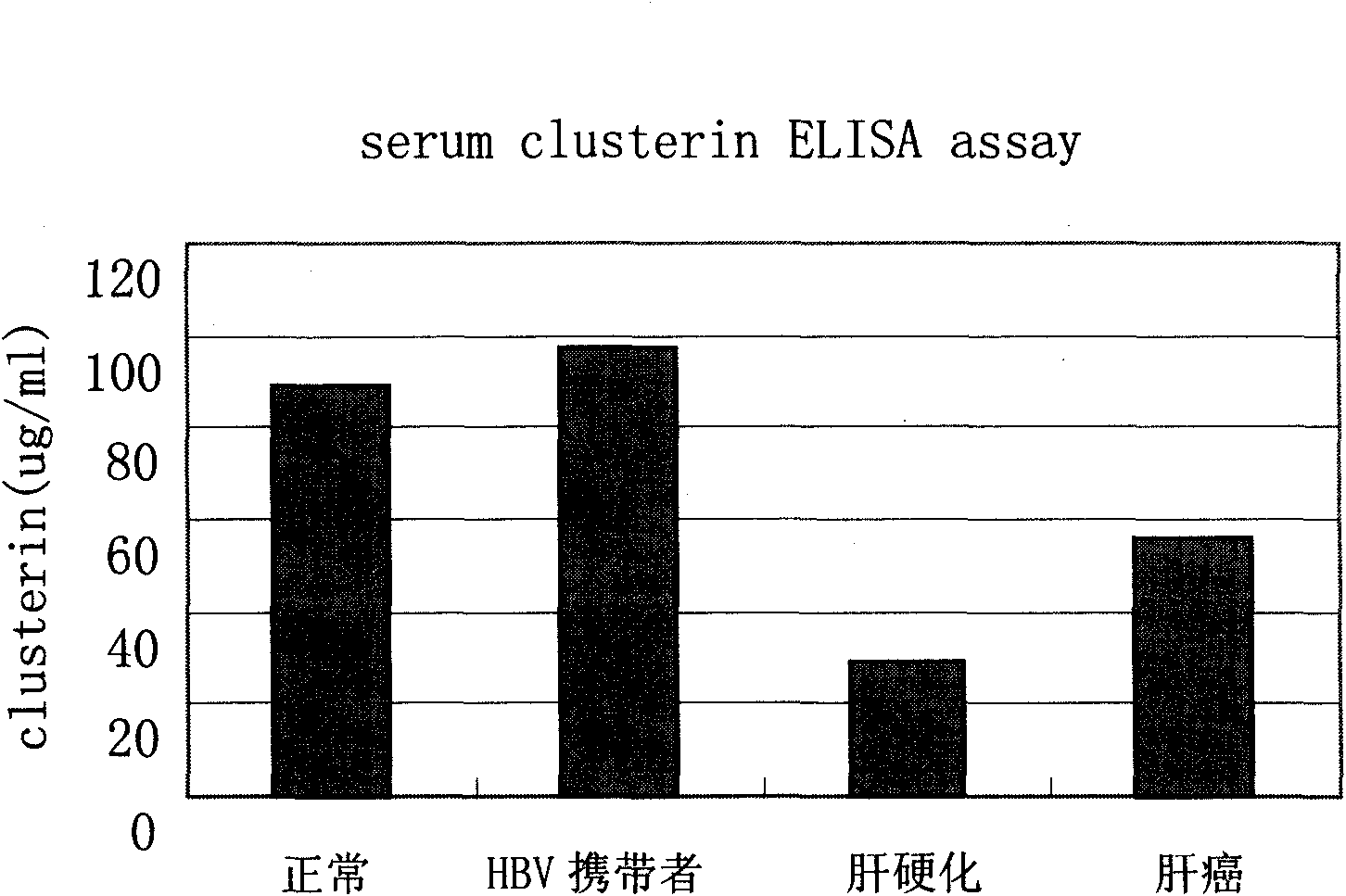
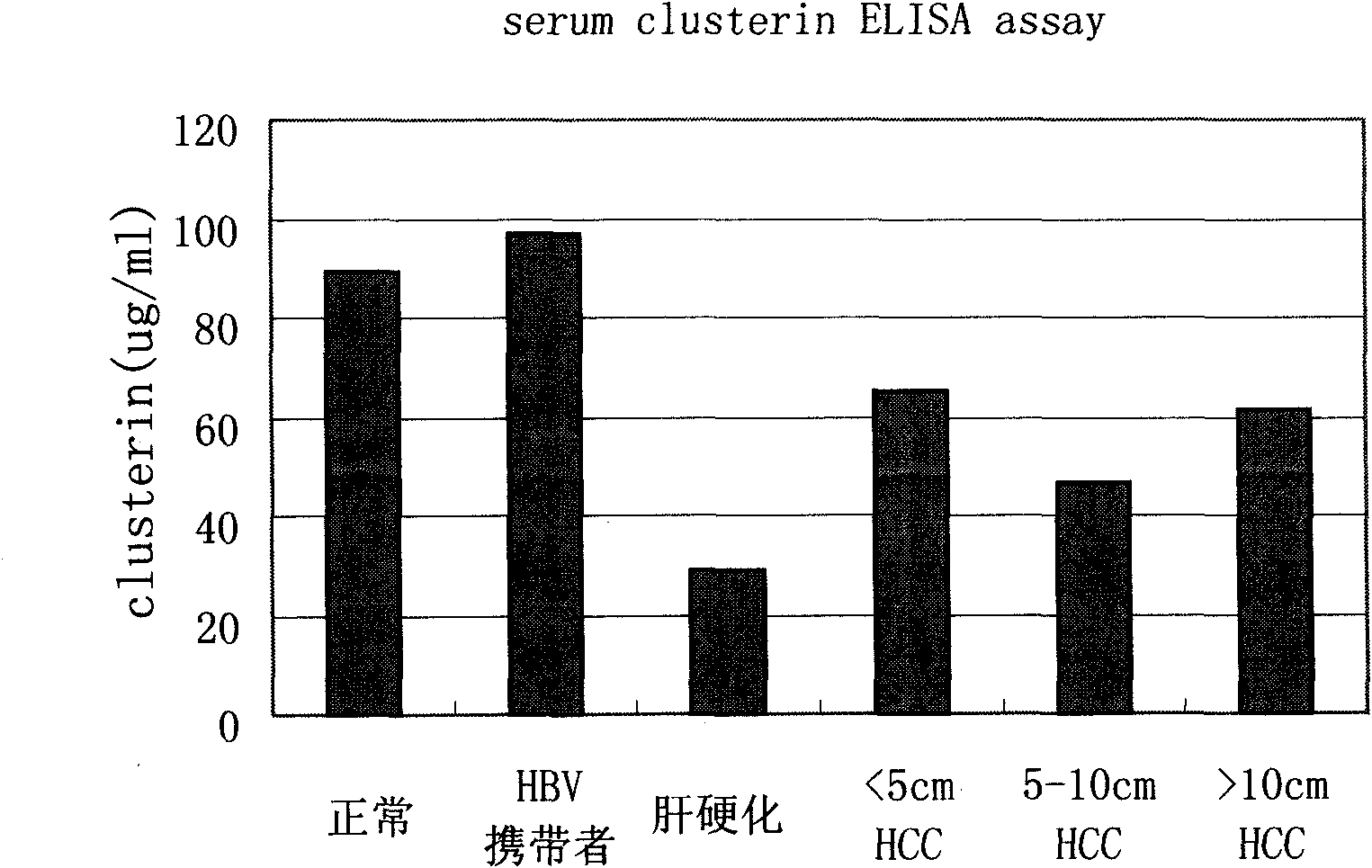
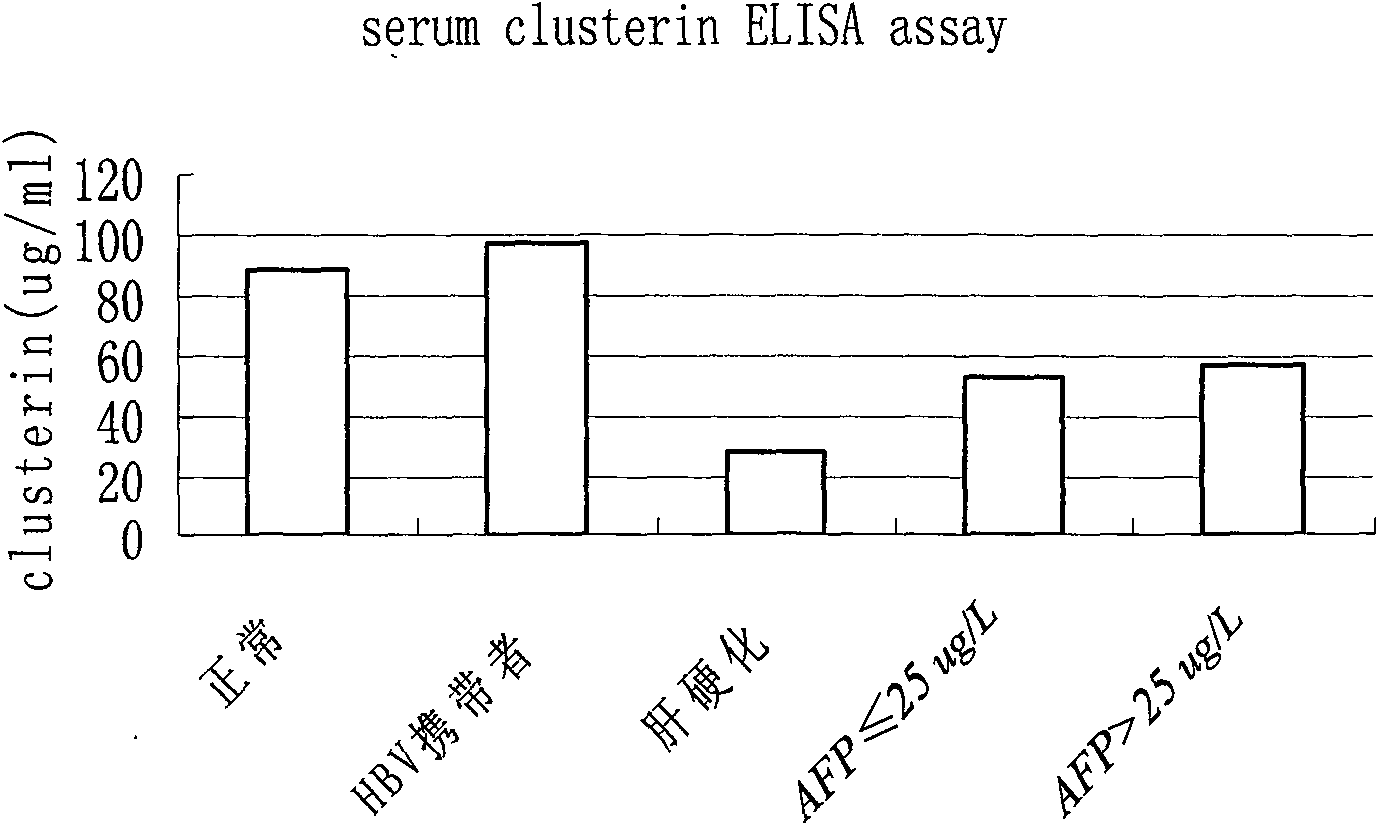
Kit for distinguishing and diagnosing hepatocirrhosis and early liver cancer and application of serum clusterin in preparing same
The invention discloses a kit for diagnosing primary hepatoma, preparation method and application thereof. The kit can quickly and accurately carry out the clusterin detection so as to carry out quickauxiliary diagnosis on the primary hepatoma of each period; and the kit especially has an important clinical application value for distinguishing hepatocirrhosis and early liver cancer. The kit comprises an enzyme-linked immunological diagnosis system established on the basis of a double-antibody sandwich principle. The kit has high sensitivity, strong specificity, simple operation, stable reagent, good repeatability, easy popularization and application and extensive market prospect.
Owner:SUN YAT SEN UNIV
Pancreatic ductal adenocarcinoma marker and screening method thereof
ActiveCN109239210AImprove diagnosis rateAccurately reflect differences in metabolic profilesComponent separationPancreas Ductal AdenocarcinomaMetabolite
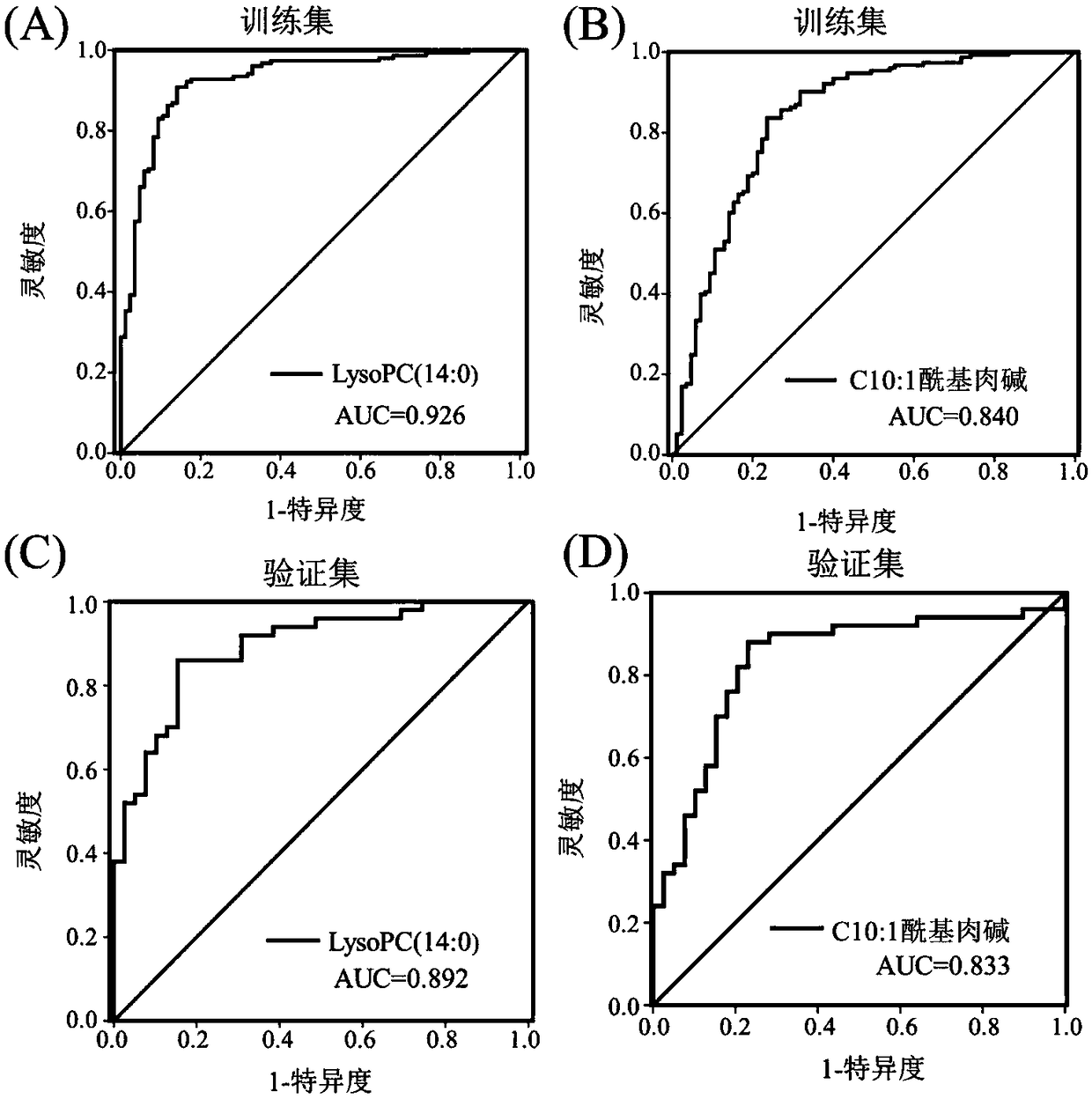
The invention discloses a pancreatic ductal adenocarcinoma marker and a screening method thereof, which belongs to the field of clinical test and diagnosis. In view of the problem that the detection sensitivity and the detection specificity of the existing pancreatic ductal adenocarcinoma diagnosis marker are poor, serum of an early patient of early stage pancreatic duct adenocarcinoma is subjected to a trace metabolomics analysis by the high-performance liquid chromatography-tandem mass spectrometry technology, and different metabolites between normal human and the early stage pancreatic ductal adenocarcinoma patients are found. The different metabolites between normal human and pancreatic ductal adenocarcinoma patients are further analyzed by this technique to find the specific differentmetabolites C10:1 acyl carnitine and lysophosphatidyl choline LysoPC (14:0) of pancreatic ductal adenocarcinoma patients caused by cancer, i.e., the diagnostic molecules of the pancreatic ductal adenocarcinoma. According to the pancreatic ductal adenocarcinoma marker and the screening method thereof, the method can assist CA19-9 in diagnosing pancreatic duct adenocarcinoma patients, and can improve the diagnosis rate for the CA19-9 negative patients by 85%. The method is suitable for the screening of tumor markers.
Owner:HARBIN INST OF TECH
Diabetic nephropathy and non-diabetic nephropathy differential diagnosis device
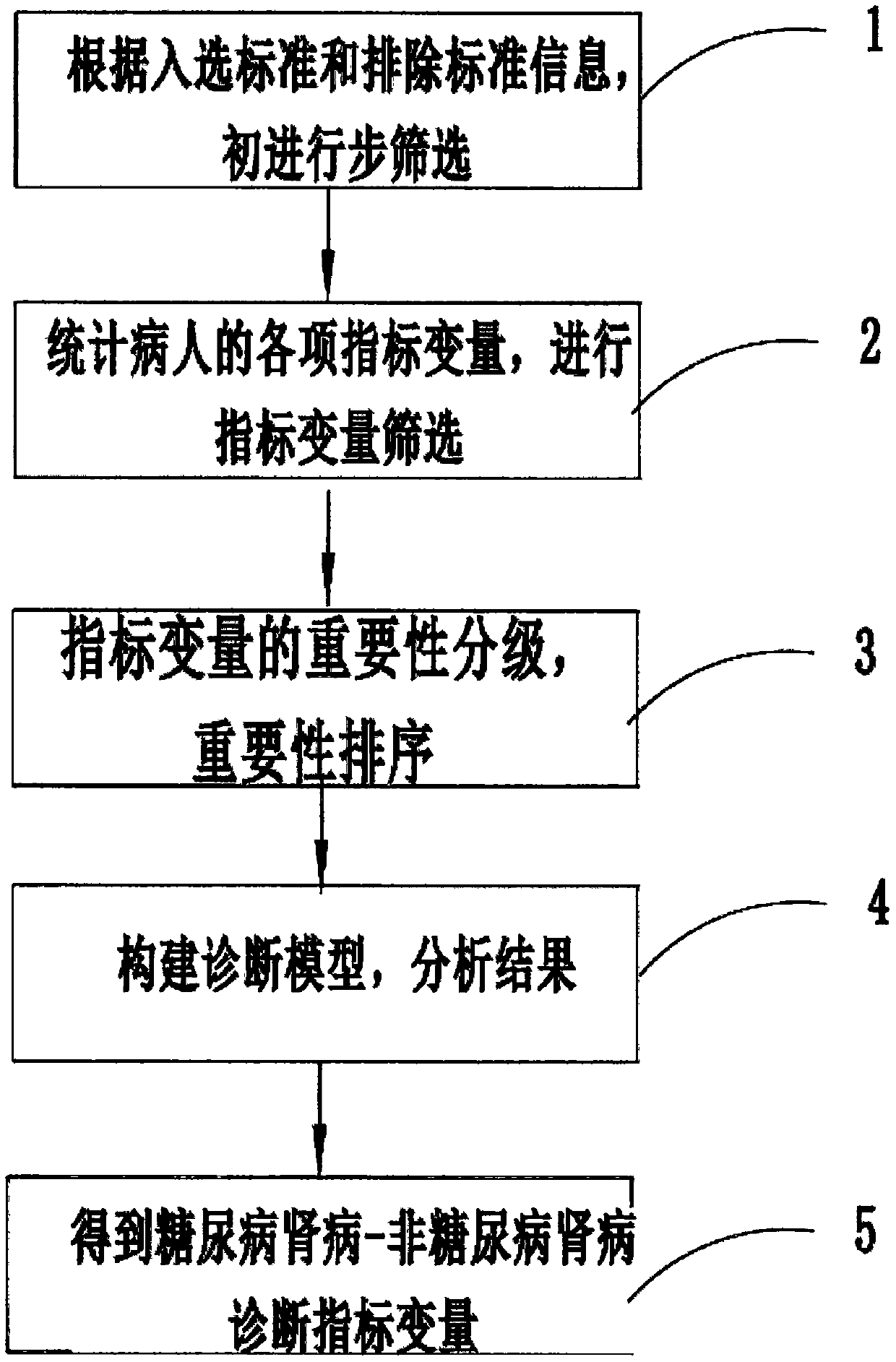
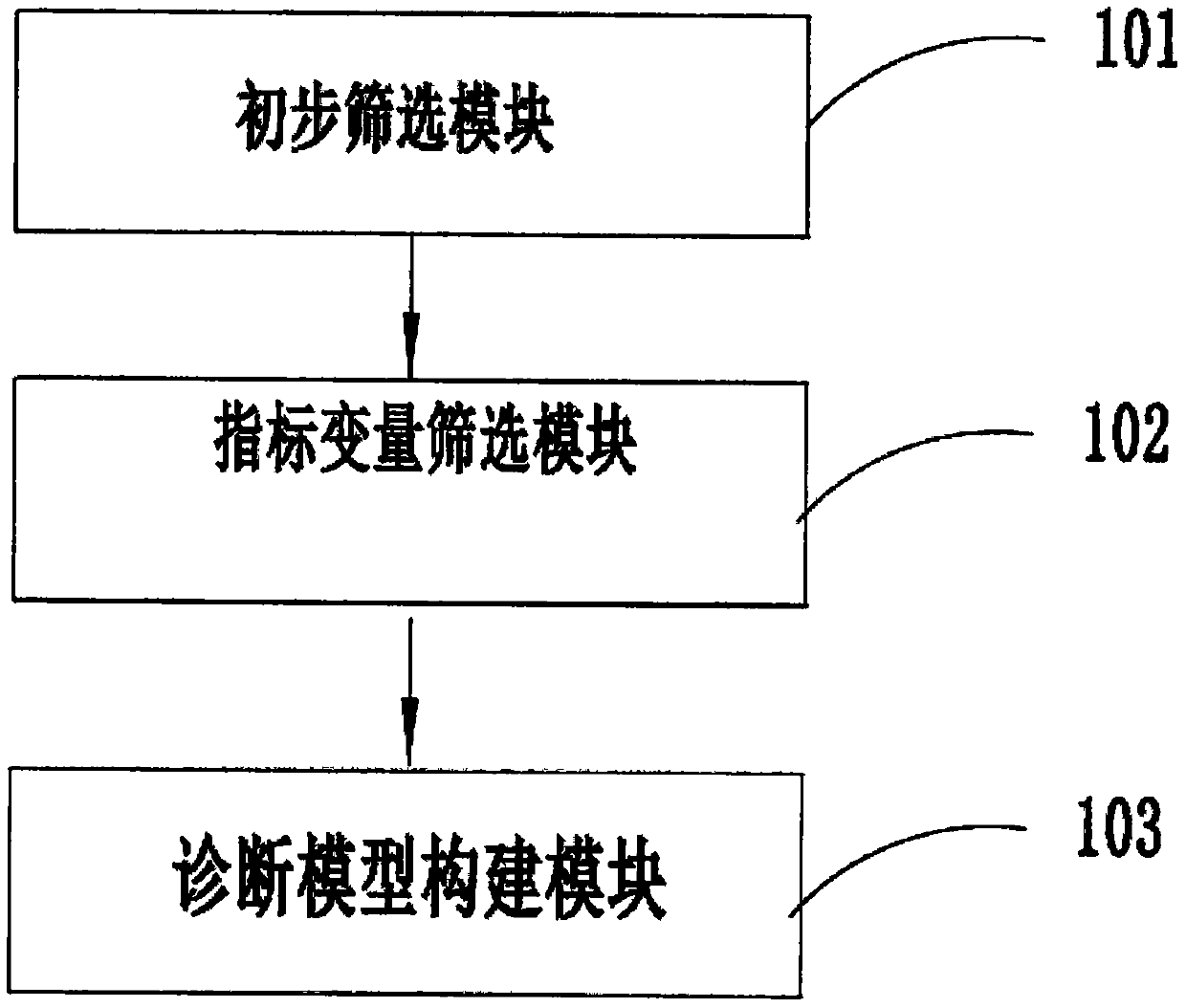
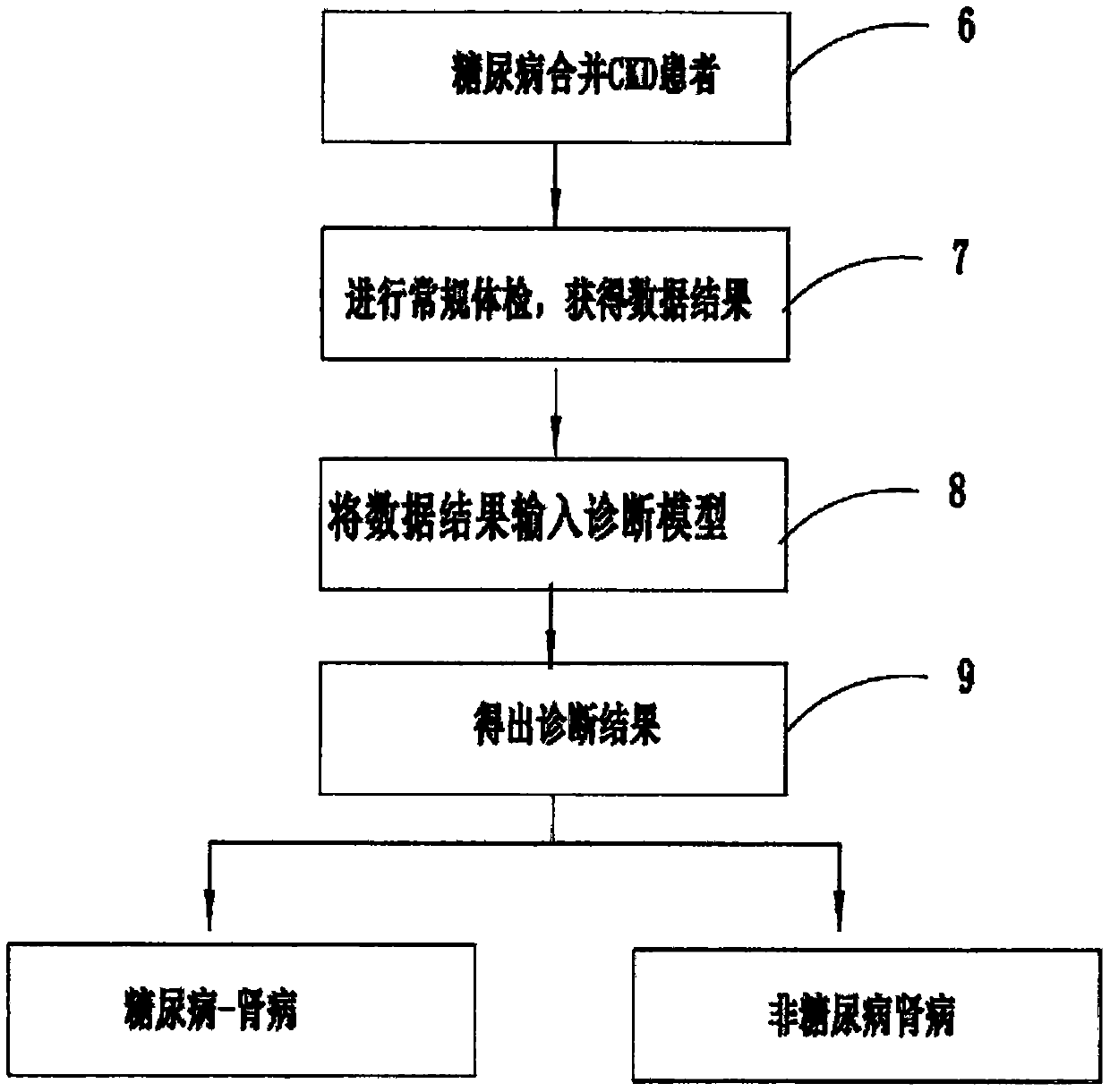
PendingCN110634563ARelieve painAccurate diagnosisMedical automated diagnosisDiagnosis standardsPuncture Biopsy
The invention provides a diabetic nephropathy-non-diabetic nephropathy differential diagnosis device and relates to the biological detection technology field. Defects that a standard in a traditionalchronic kidney disease clinical practice guide (KDOQI) is not objective and unified and is not suitable for serving as a diagnosis standard, and a traditional kidney puncture biopsy technology is large in trauma, can cause complications after operation and is high in technical difficulty. In a diabetic nephropathy-non-diabetic nephropathy differential diagnosis process provided in the invention, anew index is selected by adopting a machine learning method to construct a diabetic nephropathy and non-diabetic nephropathy differential diagnosis model, pain of a patient can be reduced, a diagnosis result is accurate, an application range is wide, and a good auxiliary diagnosis effect is provided for clinical practice.
Owner:GENERAL HOSPITAL OF PLA
Colloidal gold method detecting reagent for extrauterine pregnancy and number of pregnancy days, and preparation thereof
InactiveCN101294966AResolve detectionSolve time-consuming and costly problemsBiological testingCelluloseObstetrics
The invention discloses an ectopic pregnancy and pregnant days colloid gold detection agent and manufacturing method thereof used for the problem of fast detection for pregnancy, comprising a piece of detecting test paper and urine sample treatment solution; wherein, the detecting test paper consists of a base plate, a cellulose nitrate membrane, a gold label anti-Beta HCG monoclone antibody layer, an Alpha-HCG monoclone antibody line, a sheep anti-rat polyclonal antibody line, an absorbing pad and a sample pad; the matching composition of the urine sample treatment solution is that: 12.5 to 18.0 portions of disodium hydrogen phosphate dodecahydrate, 1.2 to 1.7 portions of sodium dihydrogen phosphate dihydrate, 40.0 to 48.0 portions of sodium chloride and 4600 to 5300 portions of distilled water; the ectopic pregnancy and pregnant days colloid gold detection agent and manufacturing method of the invention are characterized by fast and simple detection, the operation of blood-drawing detection for 2 to 6 hours can be replaced by 2 to 3 minutes with time-saving, manual-saving and cost-saving; besides, the ectopic pregnancy and pregnant days colloid gold detection agent and manufacturing method thereof have the advantages of detecting ectopic pregnancy and pregnant days in a semiquantitative way, etc., and can be used for external detection of the pregnant condition and artificial abortion and assisted diagnosis.
Owner:崔学礼
Serum/plasma miRNA marker related to assisted diagnosis of intrahepatic cholestasis of pregnancy and application of serum/plasma miRNA marker
ActiveCN108441552AConvenient for clinical operationEasy diagnosisMicrobiological testing/measurementDNA/RNA fragmentationAIDS diagnosisOncology
Owner:WUXI MATERNAL & CHILD HEALTH HOSPITAL
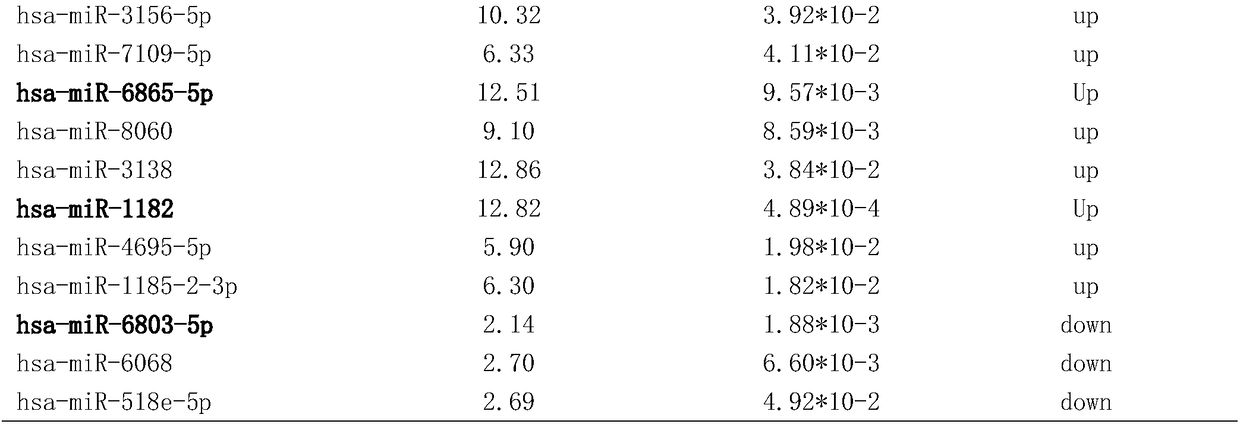
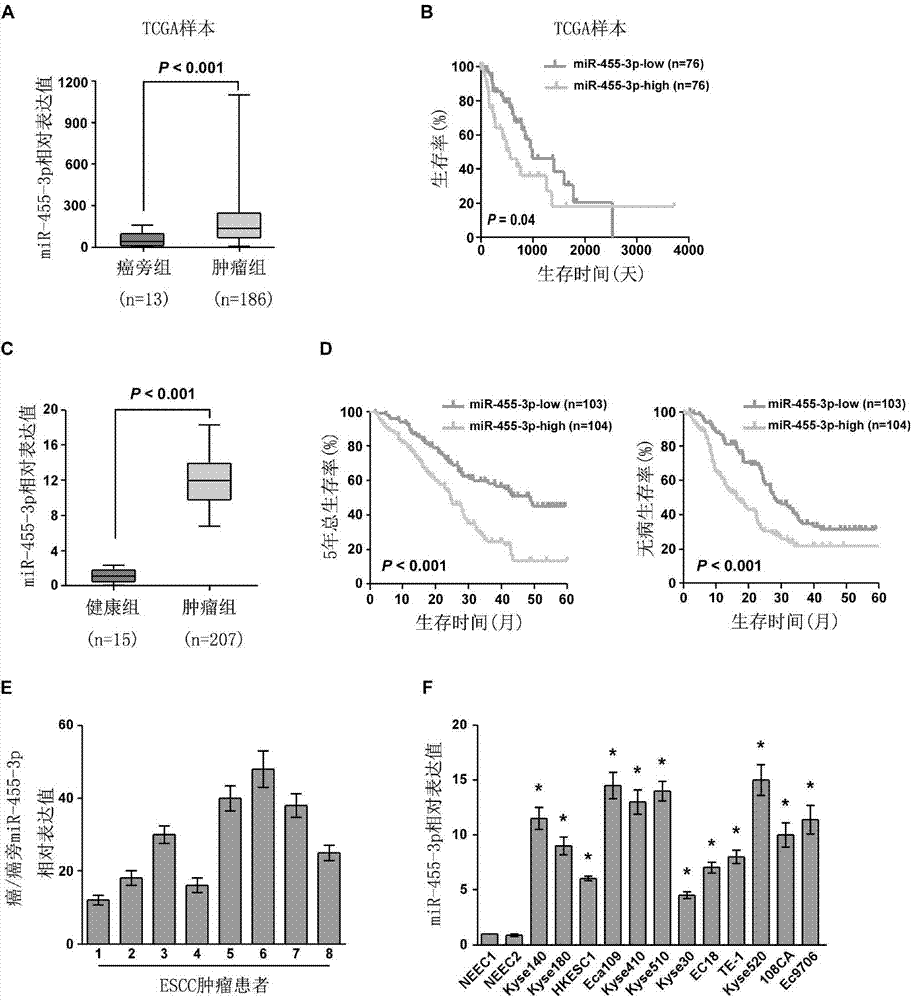
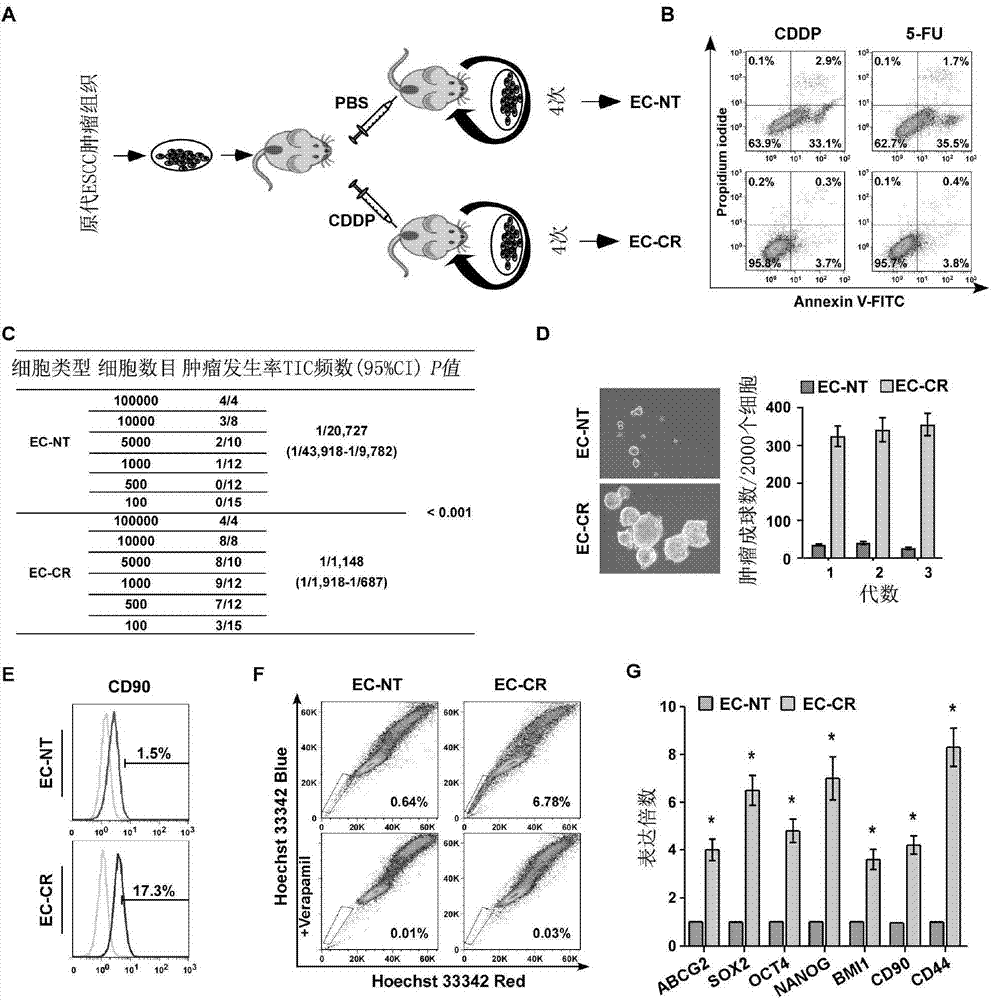
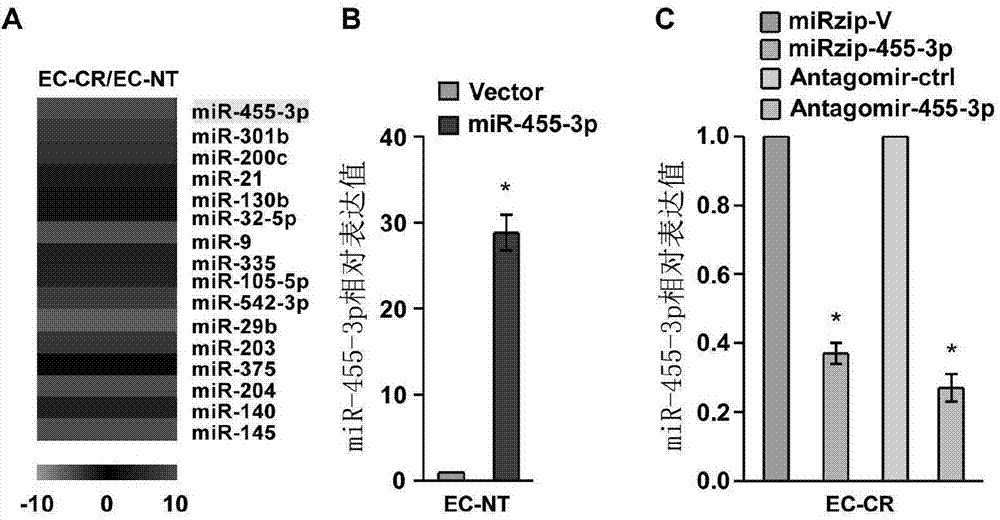
Applications of miR-455-3p in diagnosis, treatment and prognosis of esophageal squamous cancer
ActiveCN104774929APromote malignant developmentIncreased sensitivityOrganic active ingredientsMicrobiological testing/measurementDiseaseNovel treatment method
The invention discloses applications of miR-455-3p in the diagnosis, treatment, and prognosis of esophageal squamous cancer. The inventor finds that the obviously low survival rate of ESCC patients is related with the high expression of miR-455-3p in ESCC patients. The in-vitro and in-vivo experiment results show that miR-455-3p is capable of promoting the malignant progression of ESCC, by inhibiting the expression of miR-455-3p, the ESCC stem cell characteristics can be inhibited, and the sensitivity of ESCC stem cells to chemotherapy drugs is enhanced; so an expression inhibitor of miR-455-3p can inhibit the tumor stem cells, and is capable of inhibiting the tumor stem cell characteristics and enhancing the sensitivity of the cancer stem cells to chemotherapy drugs. The invention finds the expression of miR-455-3p in cancer patients for the first time, and the expression can be used as auxiliary diagnosis and / or prognosis of ESCC. The invention provides a novel diagnosis method, a novel treatment method, and a pharmaceutical drug screening platform for tumor diseases.
Owner:SUN YAT SEN UNIV CANCER CENT
Kit for comprehensively detecting four indexes of ovarian cancer through time-resolved fluorescence and application of kit
InactiveCN103575902AImprove linear rangeSimple detection operationDisease diagnosisBiological testingFluorescenceMedicine
The invention relates to the technical field of biological and medical detection, in particular relates to a time-resolved fluorescence immunodetection kit capable of simultaneously and comprehensively detecting four indexes of HE4, CA125, CEA and TPS in ovarian cancer tumor markers in a same reaction system, and also relates to application of the kit to comprehensive detection of the four indexes of HE4, CA125, CEA and TPS in the ovarian cancer tumor markers. The kit comprises a porous plate coated with a first monoclonal antibody mixture which resists the HE4, CA125, CEA and TPS respectively, an Sm<3+> labeled anti-HE4, Eu<3+> labeled anti-CA125, Tb<3+> labeled anti-CEA and Dy<3+> labeled anti-TPS second monoclonal antibody mixture, an analytical buffer solution, a common fluorescence enhancer, a cleaning solution and a quality control material. The kit provided according to the invention can be used for clinical auxiliary diagnosis, observation of curative effect and prognosis of ovarian cancer, has significance for treatment and prevention of the ovarian cancer tumor, and can be used for detecting the four tumor markers simultaneously, thus simplifying the detection steps and improving the specificity and sensitivity of comprehensive analysis of detected data.
Owner:河南生生医疗器械有限公司
Application method of machine learning classification model in adolescent autism auxiliary diagnosis
InactiveCN111009321AImprove forecast resultsImprove generalization abilityMedical automated diagnosisCharacter and pattern recognitionMedicineAIDS diagnosis
The invention discloses an application method of a machine learning classification model in adolescent autism auxiliary diagnosis. The method is characterized by being implemented according to the following mode. The method comprises the steps of 1, establishing a model training method; 2, constructing a model evaluation index; 3, performing characteristic engineering of the autism auxiliary diagnosis system; 4, performing data dimension reduction processing; 5, carrying out feature selection; and 6, carrying out model training and result analysis. According to the invention, a machine learning method is introduced into the field of autism research; the high efficiency and the reliability brought by the method are greatly helpful to the auxiliary diagnosis of autism. The application fieldof the invention can be embodied in: (1) disease diagnosis and treatment, (2) smoking addiction, network addiction and network game addiction, (3) cognition and other health fields, and the like.
Owner:UNIV OF ELECTRONICS SCI & TECH OF CHINA
Screening and application of feces gene marker
ActiveCN109658980AEnrich and gain insight into pathological mechanismsEquivalentBiostatisticsProteomicsGenomicsStool dna
The invention relates to the field of biomedicine, and particularly to screening and application of a feces gene marker based on high-flux metagenome sequencing analysis. According to the method, through extracting feces DNA for performing macro genome sequencing and ROC curve analysis by means of a shotgun method, the gene marker is screened, and furthermore the gene marker is verified through fluorescent real-time quantified PCR for obtaining consistency. According to the method, metagenome high-flux shotgun sequencing technical measurement is firstly performed in feces of Chinese PD patients, and a screening method for the feces gene marker is established; various different genes in the feces of the PD patient are found; 25 feces gene markers are screened as PD diagnosis markers for auxiliary diagnosis of the PD patient; and the method has important academic meaning and application prospect for enriching and further understanding the pathological mechanism of PD.
Owner:RUIJIN HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
SNP marker of mitochondria DNA related to asthenospermia with unknown clinical causes and application thereof
ActiveCN103773859AIncreased sensitivityImprove featuresMicrobiological testing/measurementDNA/RNA fragmentationMedicineAIDS diagnosis
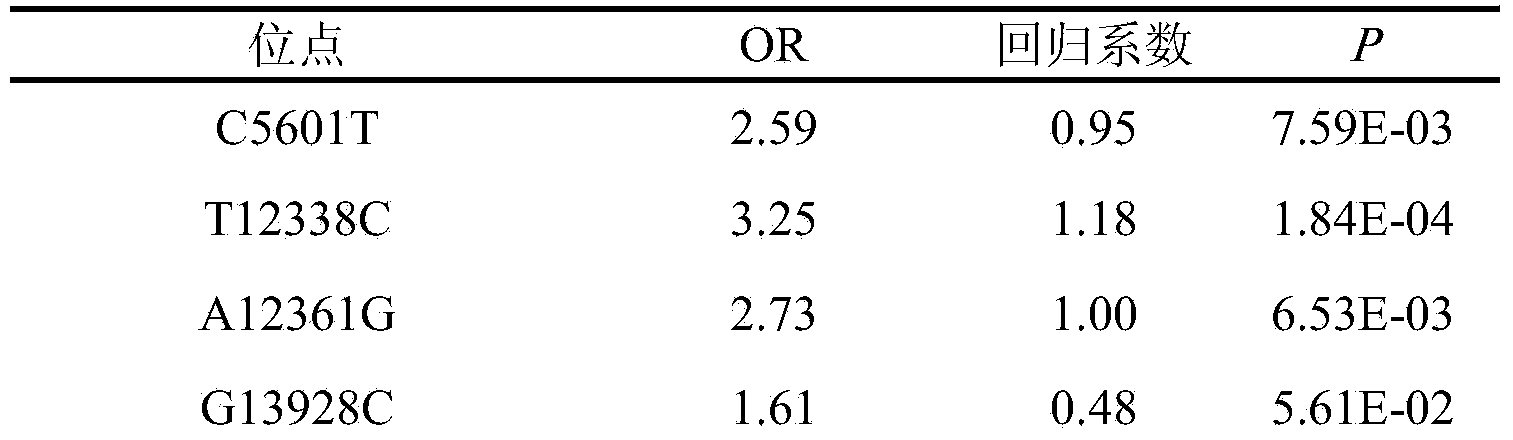
The invention belongs to the fields of genetic engineering and reproductive medicine and discloses an SNP marker of mitochondria DNA related to asthenospermia with unknown clinical causes and application thereof. The marker is a combination of C5601T, T12338C, Al2361G, G13928C, Al5851G, C16179T and G16291A, can be used for preparing an auxiliary diagnosis kit for asthenospermia with unknown clinical causes and has high specificity and sensitivity.
Owner:NANJING MEDICAL UNIV
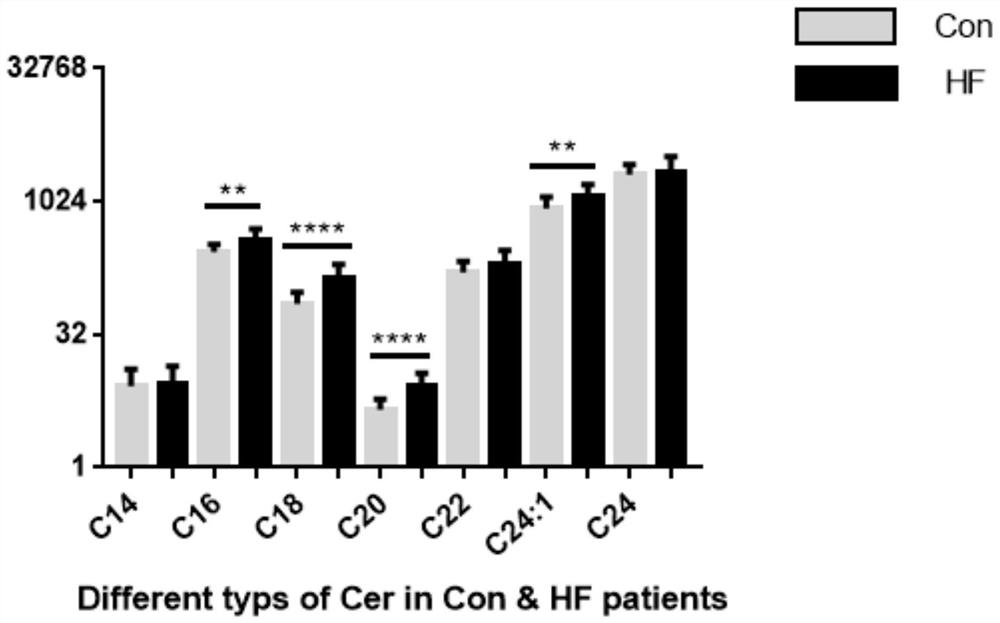
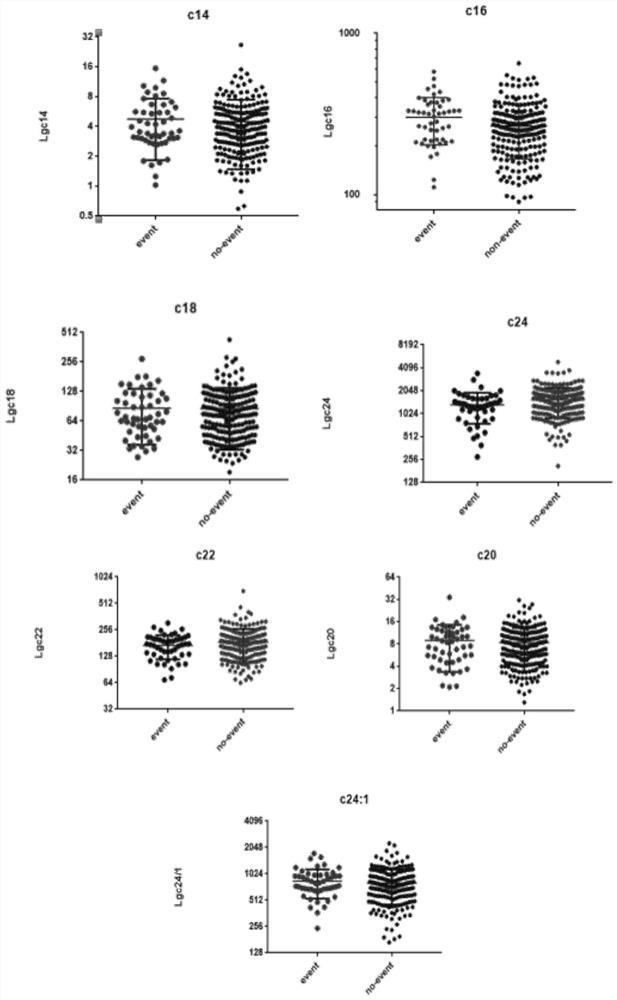
Application of ceramide in preparation of kit for evaluating adverse event risks of heart failure patients
The invention discloses application of a product for detecting ceramide in preparation of a kit. The application of the kit is at least one of the following (a1)-(a3): (a1) screening or assisting in diagnosing heart failure patients; (a2) evaluating or assisting in evaluating the prognosis risk of heart failure patients; and (a3) evaluating or assisting in evaluating the adverse event risk of heart failure patients. Research results show that the ratio of plasma ceramide cer (d18: 1 / 16: 0) to cer (d18: 1 / 24: 0) is an important predictive factor for the occurrence of adverse events of heart failure in patients with heart failure at the final stage, and is independent of currently used lipid markers, which facilitates the identification of high risk patients requiring more positive therapeutic interventions.
Owner:BEIJING INST OF HEART LUNG & BLOOD VESSEL DISEASES
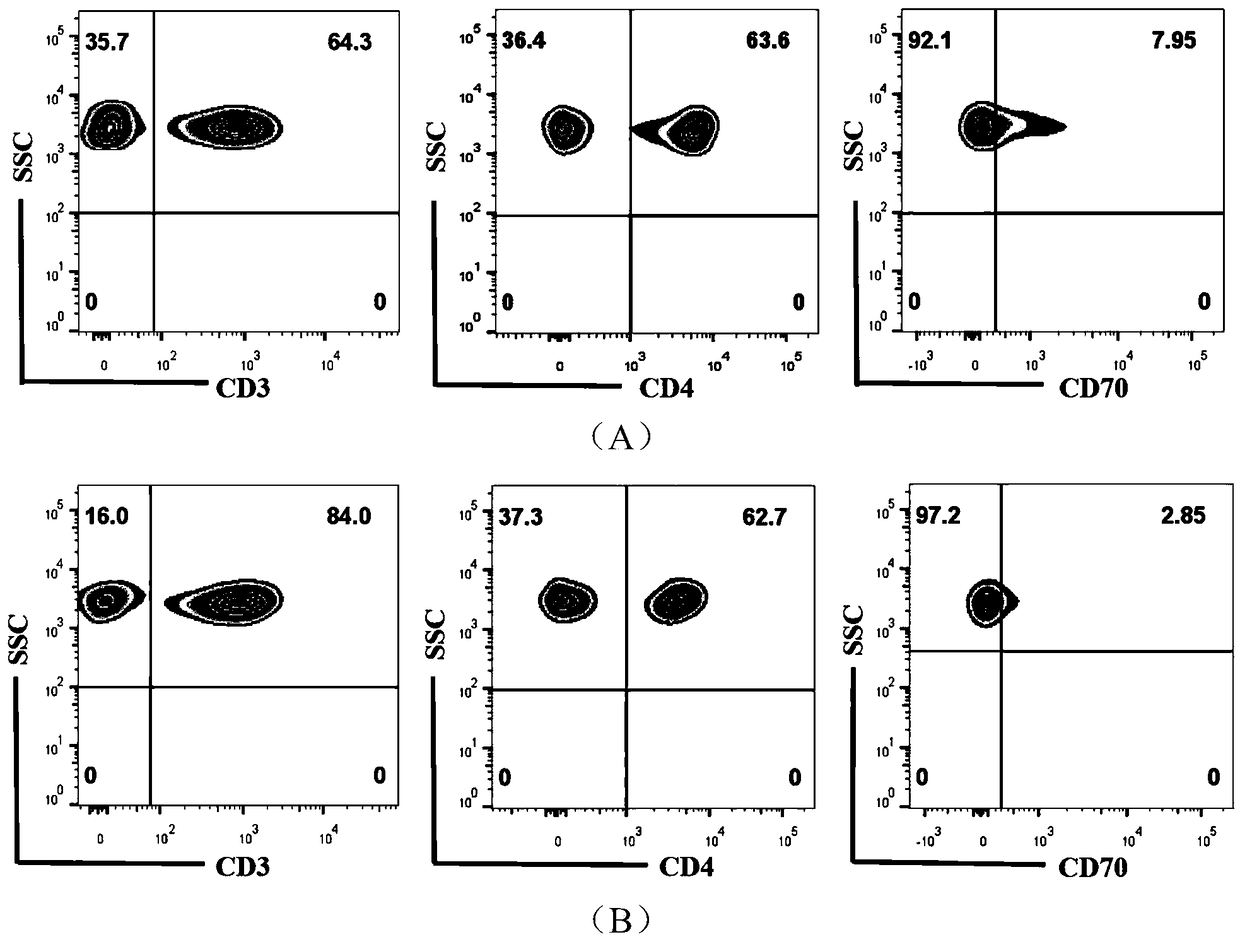
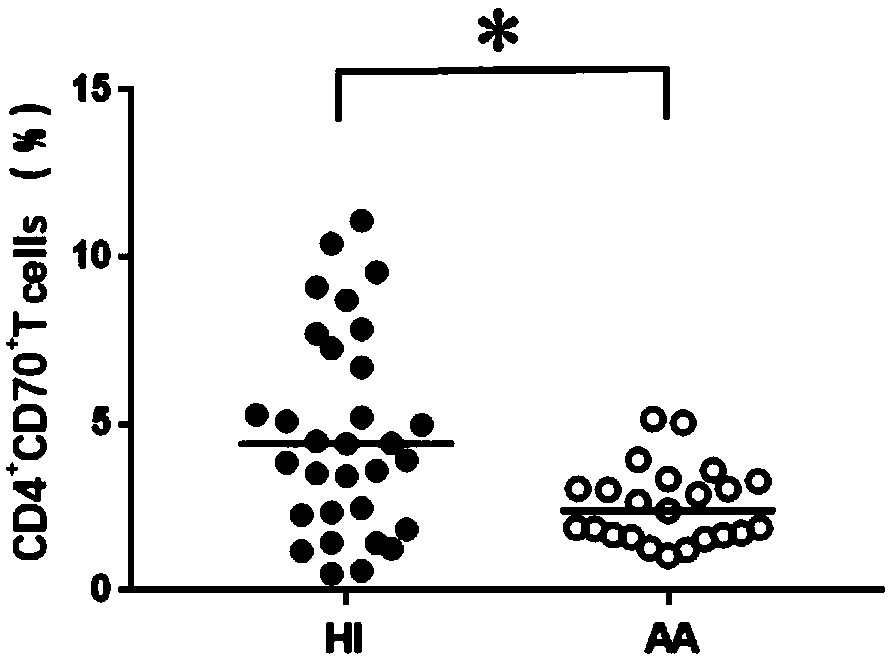
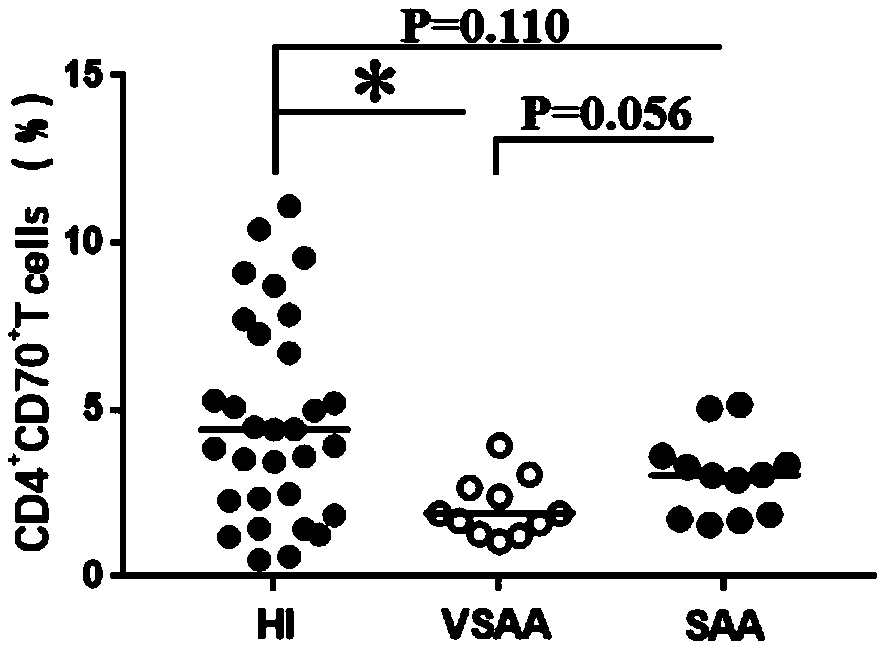
Application of CD4+CD70+T cell subset in preparing reagent for auxiliary diagnosis of very severe aplastic anemia
ActiveCN109187941AReduce the ratioDisease diagnosisIndividual particle analysisTreatment strategyLaboratory facility
The invention discloses an application of CD4+CD70+T cell subset in preparing reagent for auxiliary diagnosis of very severe aplastic anemia. According to the invention, the inventor finds for the first time that the proportion of CD4+CD70+T cell in the peripheral blood of AA patients is obviously reduced in A patients, especially, is most obvious in VSAA, which can be used as one of related testindices for auxiliary diagnosis of laboratory immunization of VSAA patients, and meanwhile, provides important reference data for selection of treatment strategy for VSAA patients clinically.
Owner:JINAN UNIVERSITY
Marker joint detection model for diagnosis of lung cancer
ActiveCN110376378AAccurate diagnosisImprove diagnostic accuracyMaterial analysisAlpha-enolaseCarcinoembryonic antigen
The invention discloses a marker joint detection model for diagnosis of the lung cancer. According to the invention, a carcinoembryonic antigen, a cancer antigen 125, an annexin A1 antibody and an alpha-enolase antibody are used as markers and are applied to preparation of a product for the diagnosis or assisted diagnosis of the lung cancer. According to the invention, the good optimization modelbeing optimal diagnosis combination of four kinds of biomarkers (the CEA, CA125, annexin A1 antibody and and alpha-enolase antibody) is established, wherein the e good optimization model has the better diagnosis capability superior to the single traditional marker diagnosis. The marker joint detection model as a novel method for lung cancer diagnosis has advantages of high sensitivity and specificity and has the important significance in accurate diagnosis of the lung cancer.
Owner:CANCER INST & HOSPITAL CHINESE ACADEMY OF MEDICAL SCI
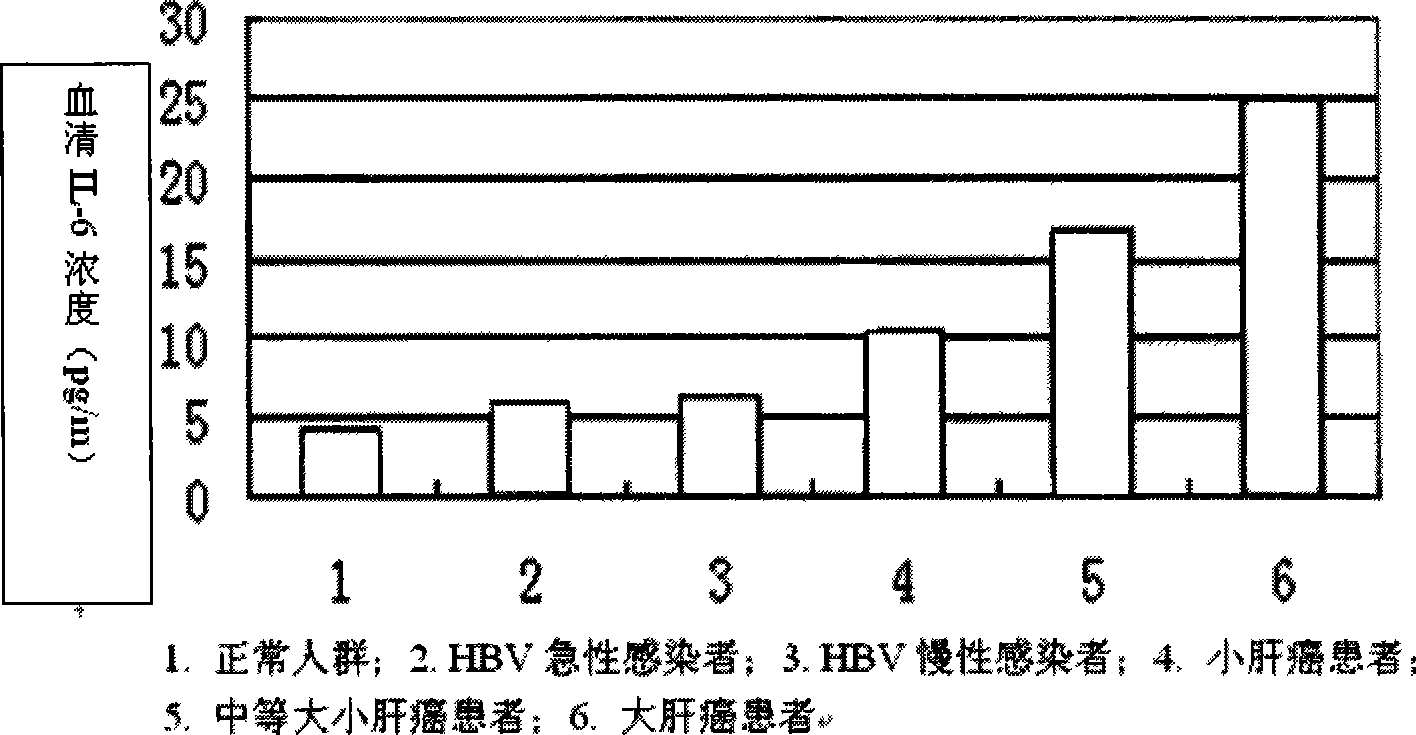
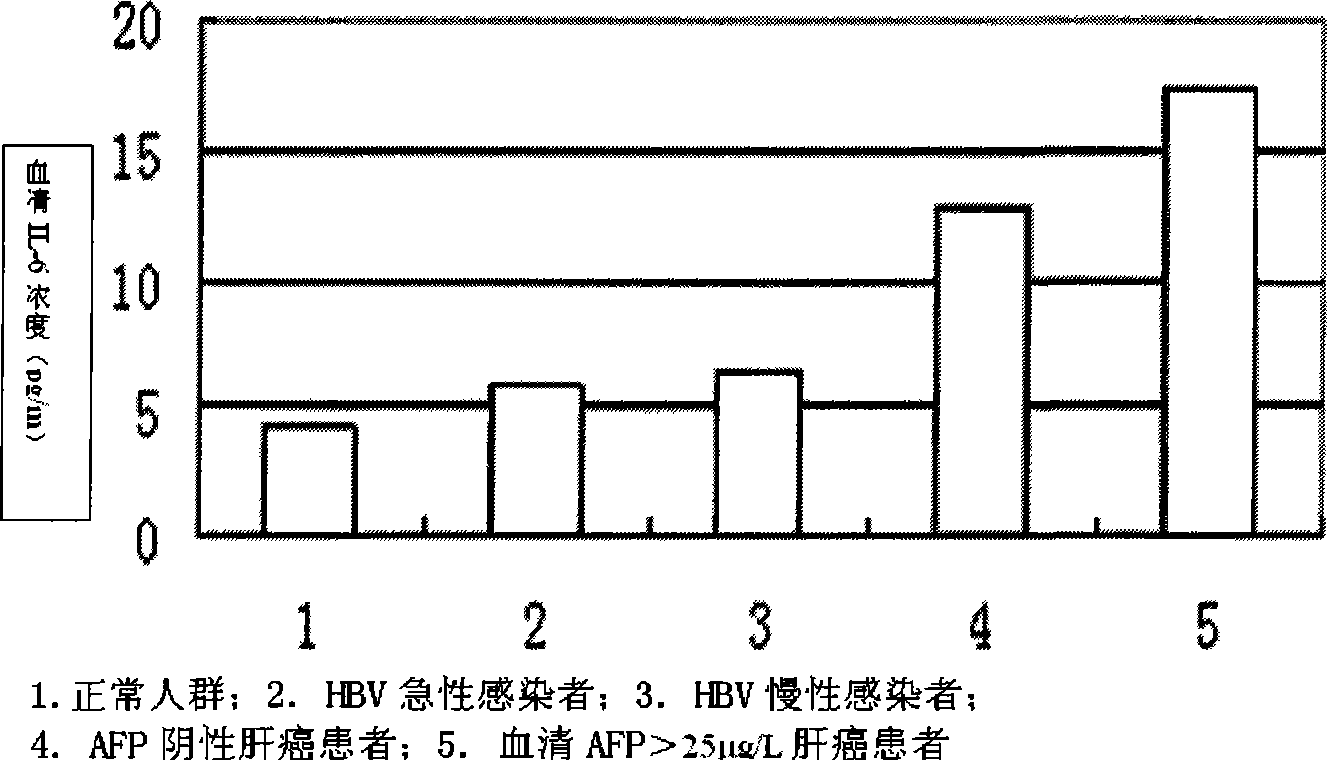
Applications of blood serum IL-6 for preparing diagnose kit for primary hepatocellular carcinoma
InactiveCN101393204AQuick checkAccurate detectionBiological testingHepatocellular carcinomaCancers diagnosis
The invention relates to a cancer diagnosis technology, in particular to application of serum IL-6 in the preparation of a primary hepatocellular carcinoma diagnostic reagent kit. The reagent kit can quickly and accurately detect the IL-6, thereby performing quick assistant diagnosis to primary hepatocellular carcinoma at each stage (particularly at early stage) and having important clinical application value. The invention comprises the establishment of an enzyme-linked immune diagnosis system based on a double antibody sandwich principle. The serum does not need to be diluted, so the sensitivity is greatly improved. The application has the advantages that the detection reagent kit has simple operation, steady reagent, good repetitiveness, strong specificity, and high sensitivity, is easy to promote and apply within large range, and has broad market prospect.
Owner:SUN YAT SEN UNIV
Reagent for auxiliary diagnosis of liver cancer, and application of reagent in preparation of kit
The invention belongs to the field of molecular biological detection, particularly belongs to the field of liver cancer detection, provides an application of a reagent in the preparation of a kit forauxiliary diagnosis of liver cancer, and also provides the reagent for auxiliary diagnosis of the liver cancer, and the kit containing the reagent. The reagent can be used for diagnosing liver cancerat least with the sensitivity of 82.35% and the specificity of 100%, and the area under a curve is 0.8720 or above.
Owner:SANSURE BIOTECH INC
Primer and probe composition for detecting HPV (Human Papillomavirus) high-risk type 16 by applying RPA (Recombinase Polymerase Amplification) technology
InactiveCN108330215AImprove accuracyHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationHuman papillomavirusFluorescence
The invention discloses a primer and probe composition for detecting HPV (Human Papillomavirus) high-risk type 16 by applying an RPA (Recombinase Polymerase Amplification) technology. A forward primersequence is shown as SEQ ID No. 1, a backward primer sequence is shown as SEQ ID No. 2 and a probe sequence is shown as SEQ ID No. 3. According to the method provided by the invention, a lot of RPA primers and probes are designed according to an HPV high-risk type 16 genome sequence, and one pair of the primer and probe composition capable of rapidly and effectively detecting HPV high-risk type 16 nucleic acid is screened. By applying a primer pair, a probe and a corresponding kit, provided by the invention, rapid constant-temperature fluorescence detection can be carried out on an HPV high-risk type 16 DNA (Deoxyribonucleic Acid) segment in unknown samples including woman cervical epithelial cells, genitalsecretion and the like. A detection result can be used for auxiliary diagnosis of HPV high-risk type 16 infection and early screening of cervical carcinoma, follow-up of cervical lesions and guidance of development of vaccines.
Owner:JIANGSU YINUOWAN CELL CLINIC CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com