Serum/plasma miRNA marker related to assisted diagnosis of intrahepatic cholestasis of pregnancy and application of serum/plasma miRNA marker
A technique for cholestasis and auxiliary diagnosis, applied in the fields of genetic engineering and reproductive medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] The collection of embodiment 1 sample and the arrangement of sample data
[0063] The inventor collected a large number of peripheral blood samples of ICP patients and healthy control pregnant women from October 2016 to March 2017 from the Wuxi Maternal and Child Health Hospital affiliated to Nanjing Medical University (the samples used for research were collected during the same period, sampled, sub-packaged, and stored) The conditions are uniform), and by sorting out the sample data, the inventor selected 88 samples that meet the following criteria as the experimental samples for Agilent miRNA chip detection and a series of subsequent qRT-PCR verifications:
[0064] 1. The above research objects are second trimester Pregnant women who were confirmed to have ICP during ICP screening (refer to the guidelines for diagnosis and treatment of ICP patients (first edition)) were defined as cases.
[0065] 2. The above research objects are in second trimester No ICP occurr...
Embodiment 2
[0067] Agilent miRNA chip detection of miRNA in embodiment 2 serum / plasma
[0068] The above-mentioned 4 eligible ICP cases and 4 healthy controls were detected by Agilent miRNA chip to obtain relevant results. The specific steps are:
[0069] 1. Take 600ul of serum from the "intrahepatic cholestasis of pregnancy" group and the "healthy female control" group, and add 3 times the volume of Trizol reagent;
[0070] 2. Phase separation: place it at 37°C for 15 minutes after shaking for 1 minute, and add the internal reference gene hsa-mir-16 (primers are SEQ ID No.7 and SEQ ID No.8) to control the difference between samples before resting, so that The final concentration is 10 -4 pmol / μl. Add an equal volume of chloroform to the turbid solution, shake for 50 s, and keep at room temperature for 15 min. After resting, centrifuge at 14,000rpm at 4°C for 15min;
[0071] 3. RNA precipitation: transfer the water phase to a new 15ml centrifuge tube, add 1.5 times the volume of the ...
Embodiment 3
[0079] qRT-PCR experiment of miRNA in embodiment 3 serum / plasma
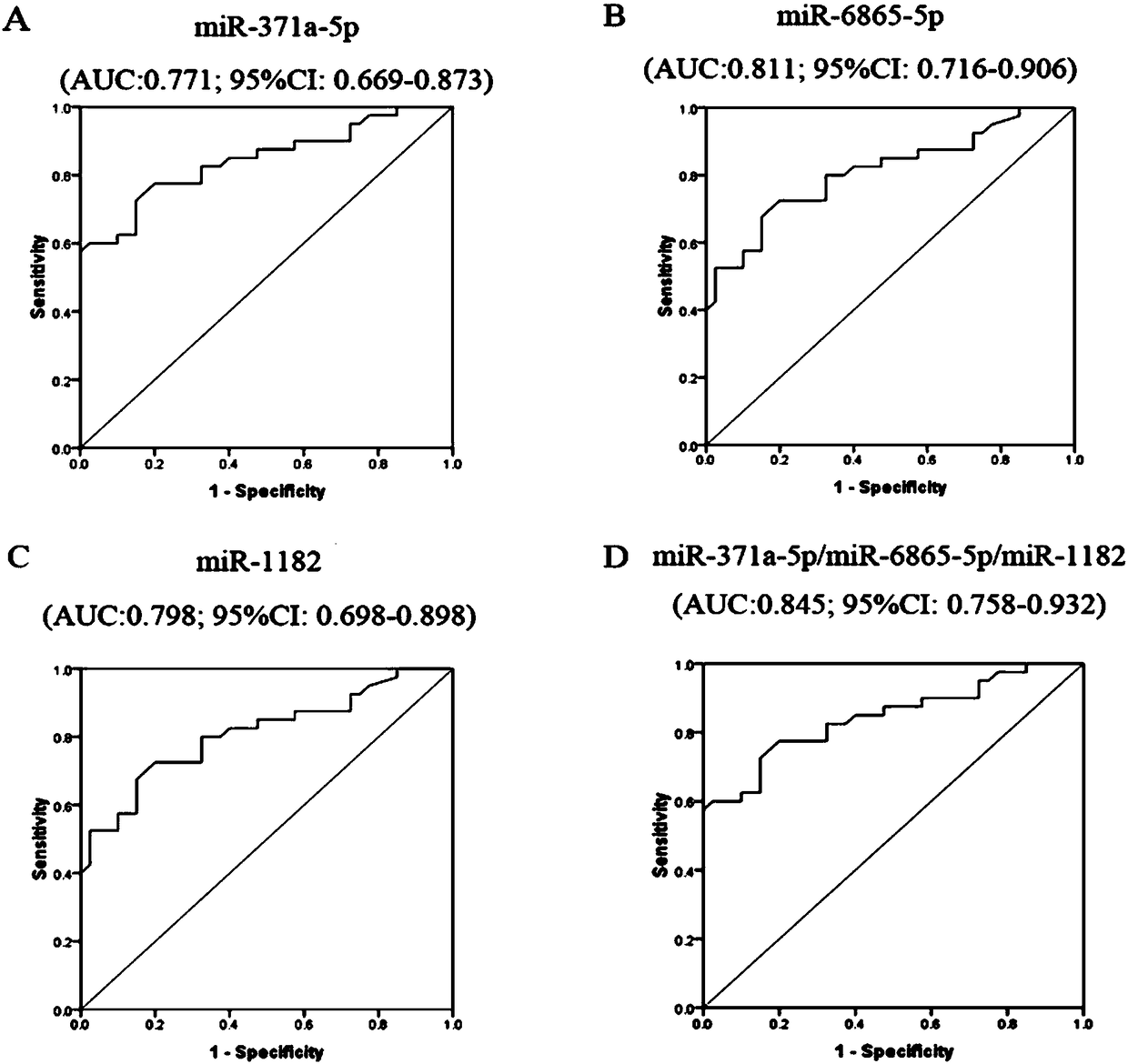
[0080] In the Agilent miRNA chip, the CT values of the two groups of research subjects were not greater than 35 and the miRNAs whose expression signals were relatively uniform among the samples of each group were selected for further verification by qRT-PCR method to improve the detection efficiency.
[0081] miRNAs meeting the above conditions include: miR-371a-5p, miR-6865-5p, miR-1182, miR-6803-5p.
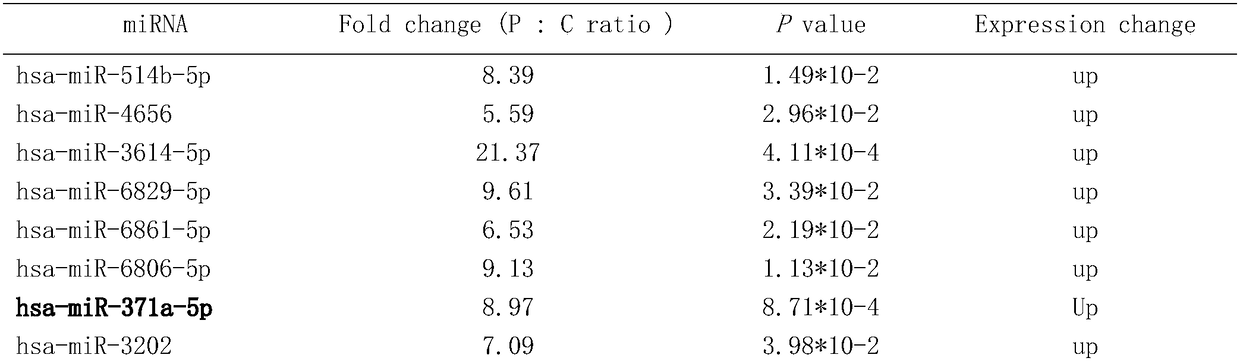
[0082] According to the above Agilent miRNA results, select miR-371a-5p, miR-6865-5p, miR-1182, miR-6803-5p to design primers for reverse transcription and qRT-PCR. The qRT-PCR detection of miRNA was performed on individual individuals in the serum of the "ICP case" group and the "healthy control" group, see Table 1.
[0083] Table 1: Differential expression of miRNA detected by miR array in ICP group and control group
[0084]
[0085]
[0086] More than 2.0-fold up- or down-regulated miRNAs in ICP (P)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com