Patents
Literature
132 results about "Serum plasma" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
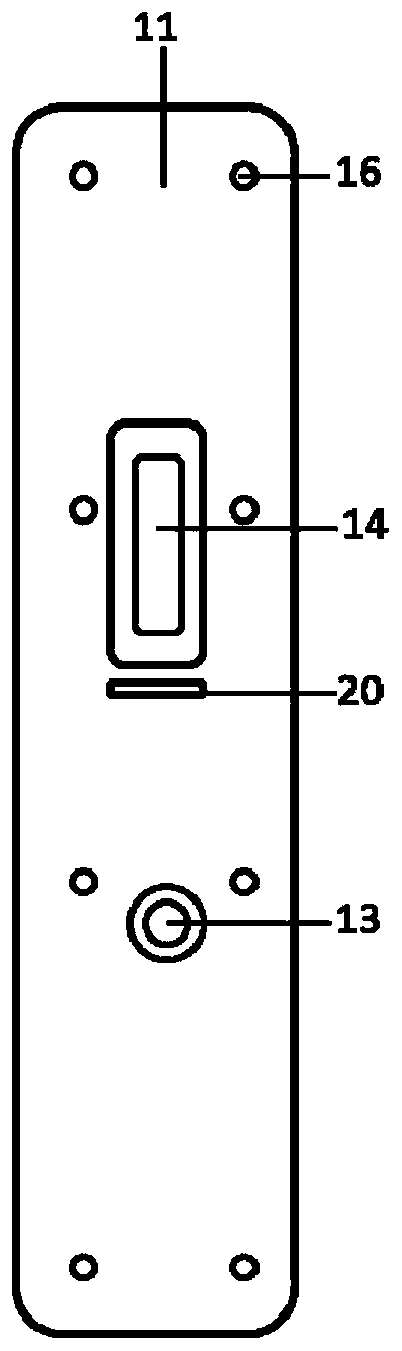
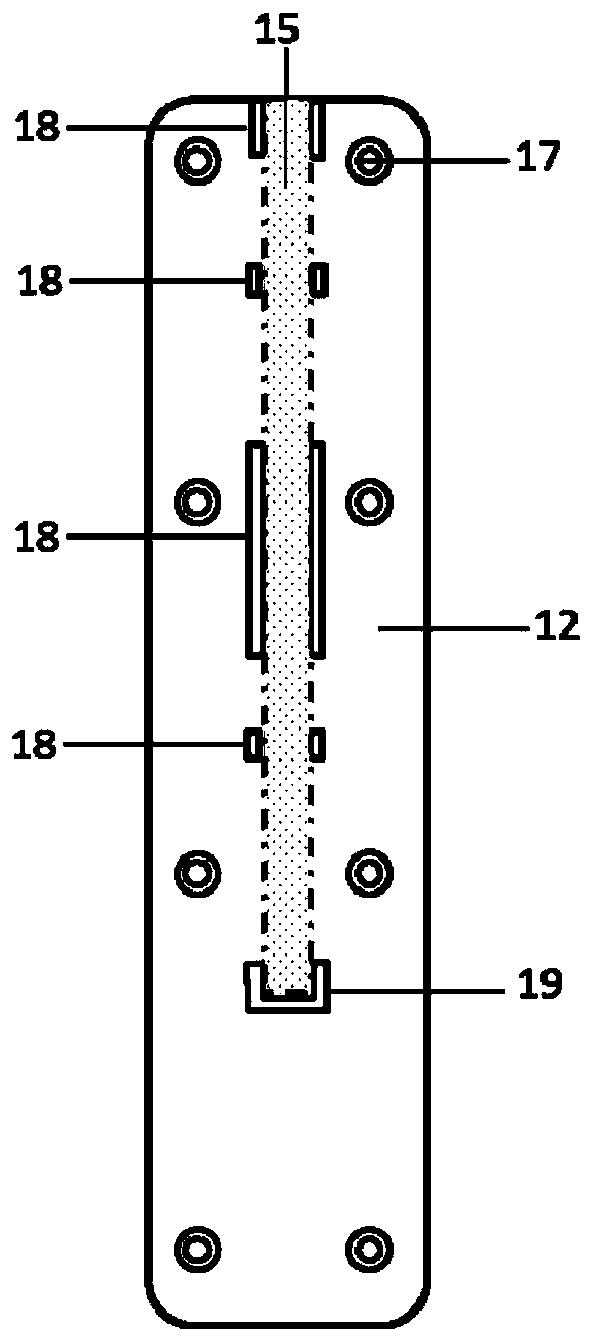
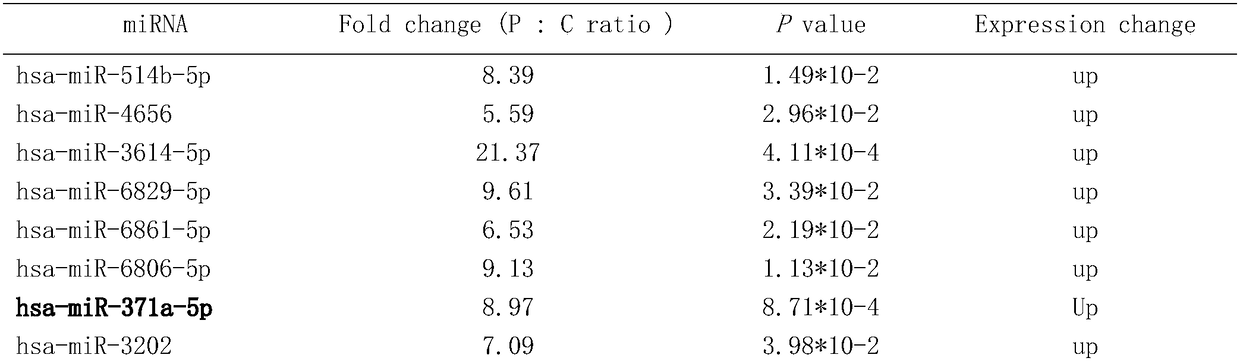
MiRNA with cell corpuscule as vector and preparation research approach thereof and application
InactiveCN101386848AEasy to storeWide detection rangeNervous disorderGenetic material ingredientsEfficacyDisease complication
The invention discloses micro ribonucleic acids (microRNA, miRNA) carried by cell microparticles (Microparticle, MP), a method for preparing the same, and application thereof in the technical field of biotechnological pharmacy. The invention provides a combination of the micro ribonucleic acids for evaluating the physiological and / or pathological states of a participant, and the combination contains all the micro ribonucleic acids which exist stably in serum / plasma particles of the participant and are detectable. At the same time, the invention provides an experimental method for preparing the cell microparticles containing specific micro ribonucleic acids and using the cell microparticles to perform gene-level regulation and control as well as modification on other cells and tissues. The combination and the method can be used for detecting and treating various diseases, including the aspects of the diagnosis and the differential diagnosis of various tumors, various acute and chronic infectious diseases and other acute and chronic diseases, the prediction and the curative effect evaluation of the occurrences of disease complications and the recurrences of malignant diseases, as well as the active ingredient screening, the efficacy evaluation and the judicial authentication of drugs, the detection of prohibited drugs and the like; besides, the combination and the method have the advantages of wide detection pedigree, high sensitivity, low detection cost, convenient available material, easy storage of samples and the like.
Owner:NANJING UNIV
Blood serum minuteness ribonucleic acid reagent kit and application in early diagnosis of hepatitis B
InactiveCN101368213AWide detection rangeHigh sensitivityMicrobiological testing/measurementBiotechnologyBlood plasma
invention discloses a kit for a serum micro-ribonucleic acid and the application in the early diagnosing of hepatitis B thereof which belong to the technical field of biotechnological pharmaceutics. The invention provides the combination of the micro-ribonucleic acid used for estimating the physiological and / or pathological states of a normal person, a HBVer and a patient with hepatitis B; the combination comprises all the micro-ribonucleic acids which have differential expression in the serum / plasm of the normal person, the HBVer and the patient with hepatitis B and prepares a diagnosing kit which can be used for the early diagnosing of hepatitis B. The combination and the method can be used for the early detection and nondirective therapy of hepatitis B; besides, the combination and the method have the advantages of high specificity, high efficiency, high sensitivity, low detection cost, convenient material obtaining, and the like. Besides, the samples are easily stored.
Owner:NANJING UNIV
Serum/plasma miRNA marker associated with breast cancer and application thereof
InactiveCN101921760AAids in diagnosisEasy to detectMicrobiological testing/measurementDNA/RNA fragmentationOncologyBlood plasma
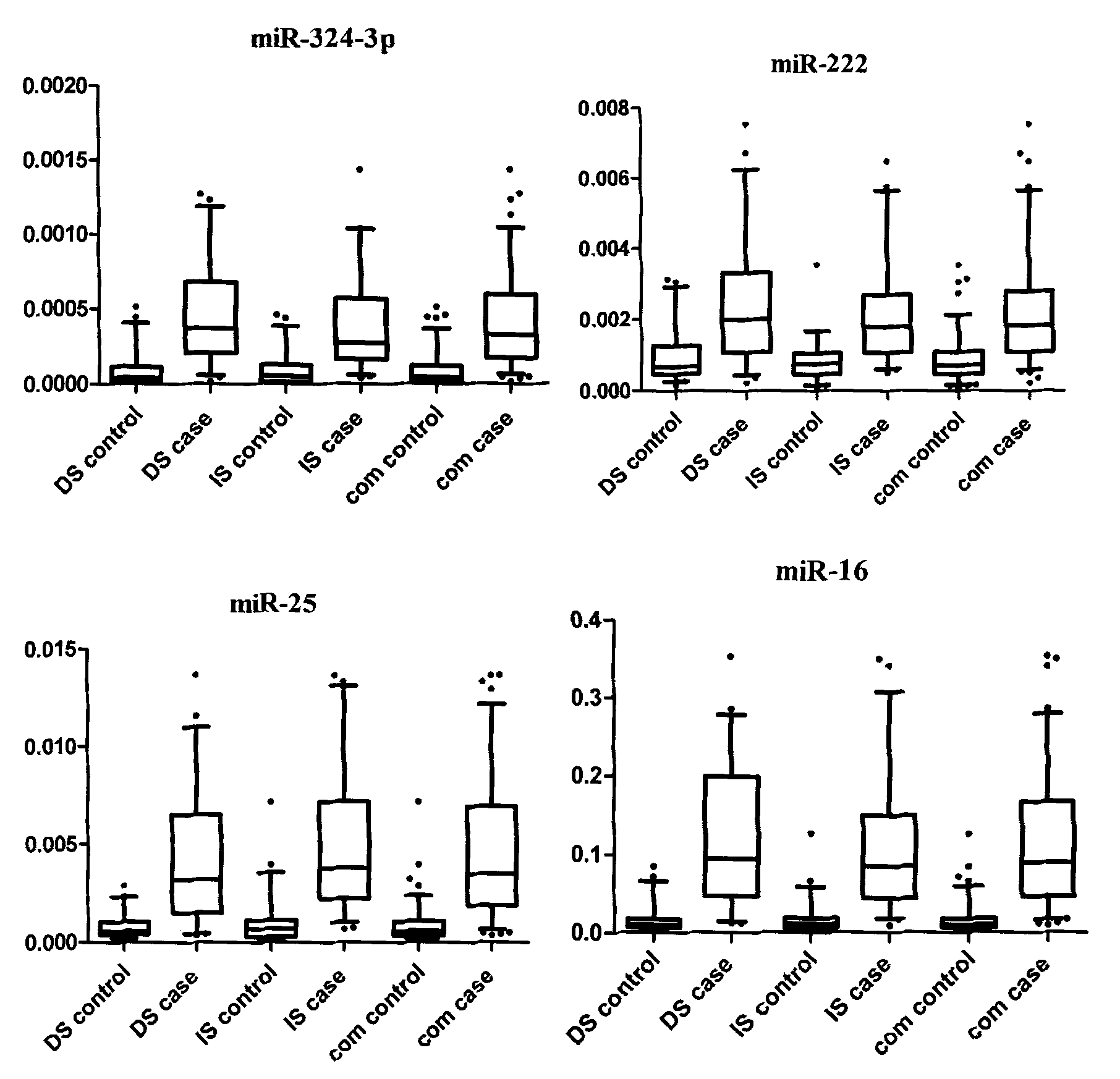
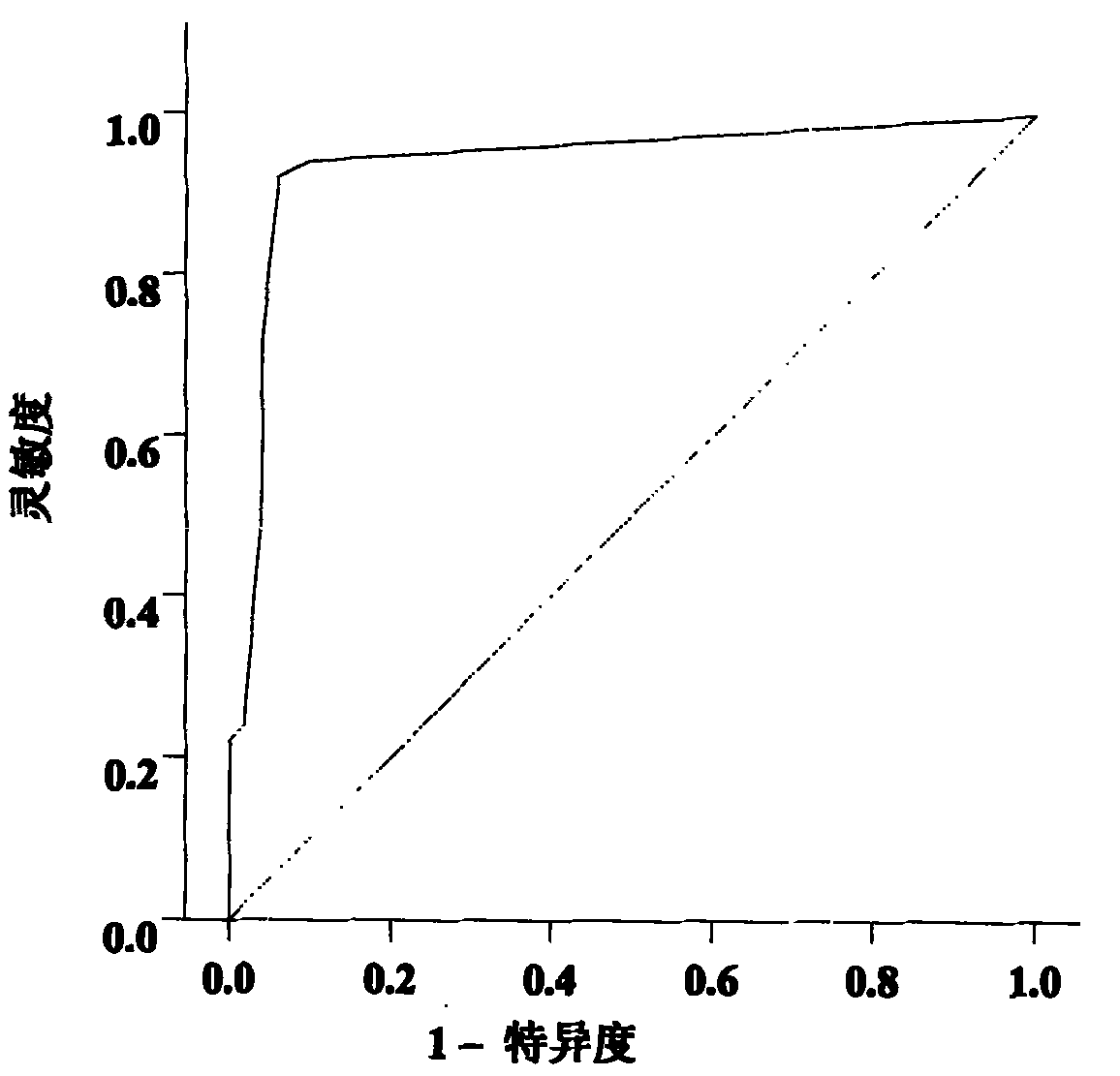
The invention belongs to the fields of gene engineering and oncology and discloses a serum / plasma miRNA marker associated with breast cancer and application thereof. The marker is a combination of miR-16, miR-25, miR-222 and miR-324-3p. The maker and primers thereof can be used for preparing a diagnosis kit and assisting early diagnosis of the breast cancer.
Owner:NANJING MEDICAL UNIV
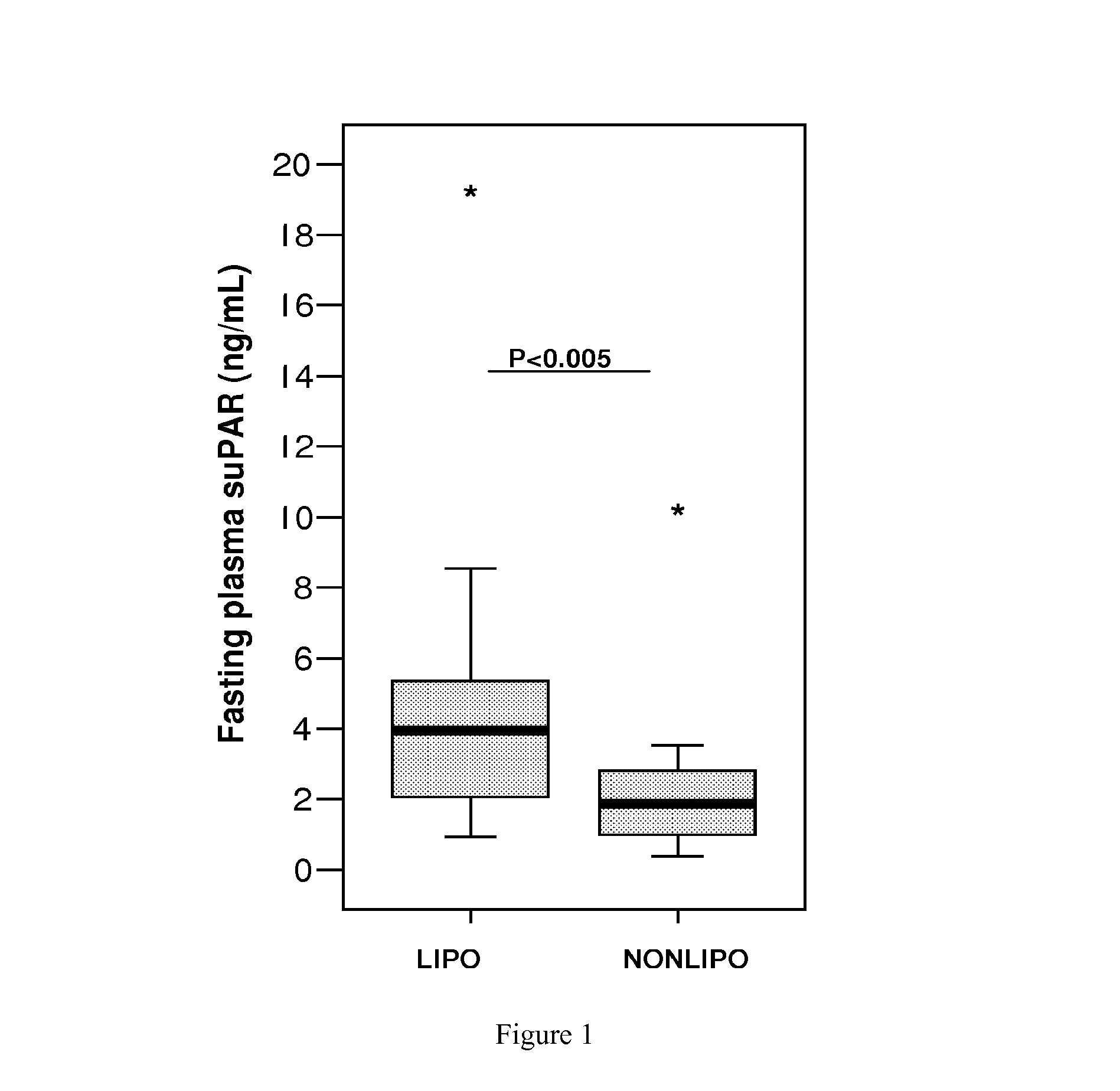
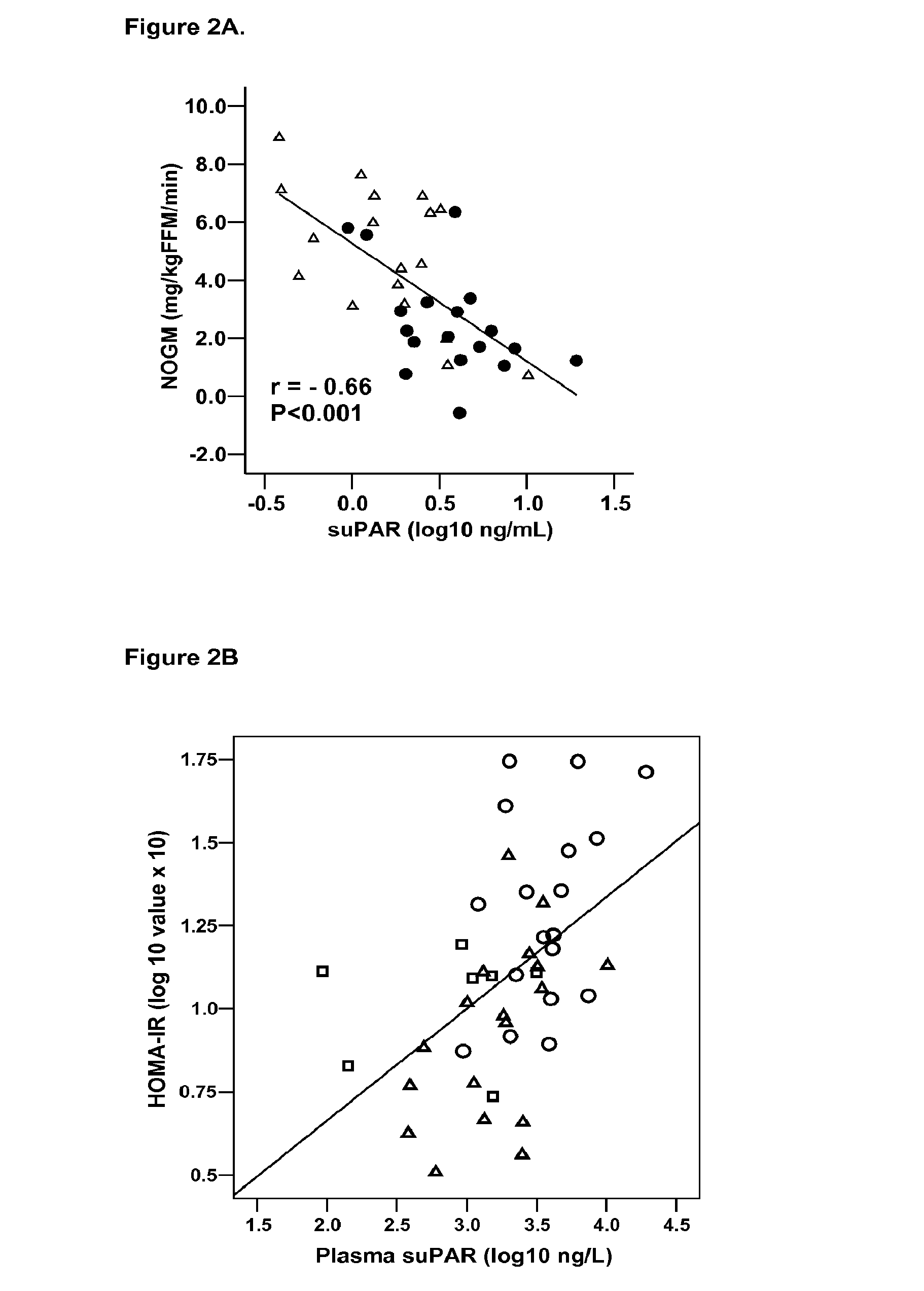
Method and tool for predicting cancer and other diseases
ActiveUS20100098705A1Monitor progressMicrobiological testing/measurementAntibody ingredientsUrokinase Plasminogen ActivatorReceptor
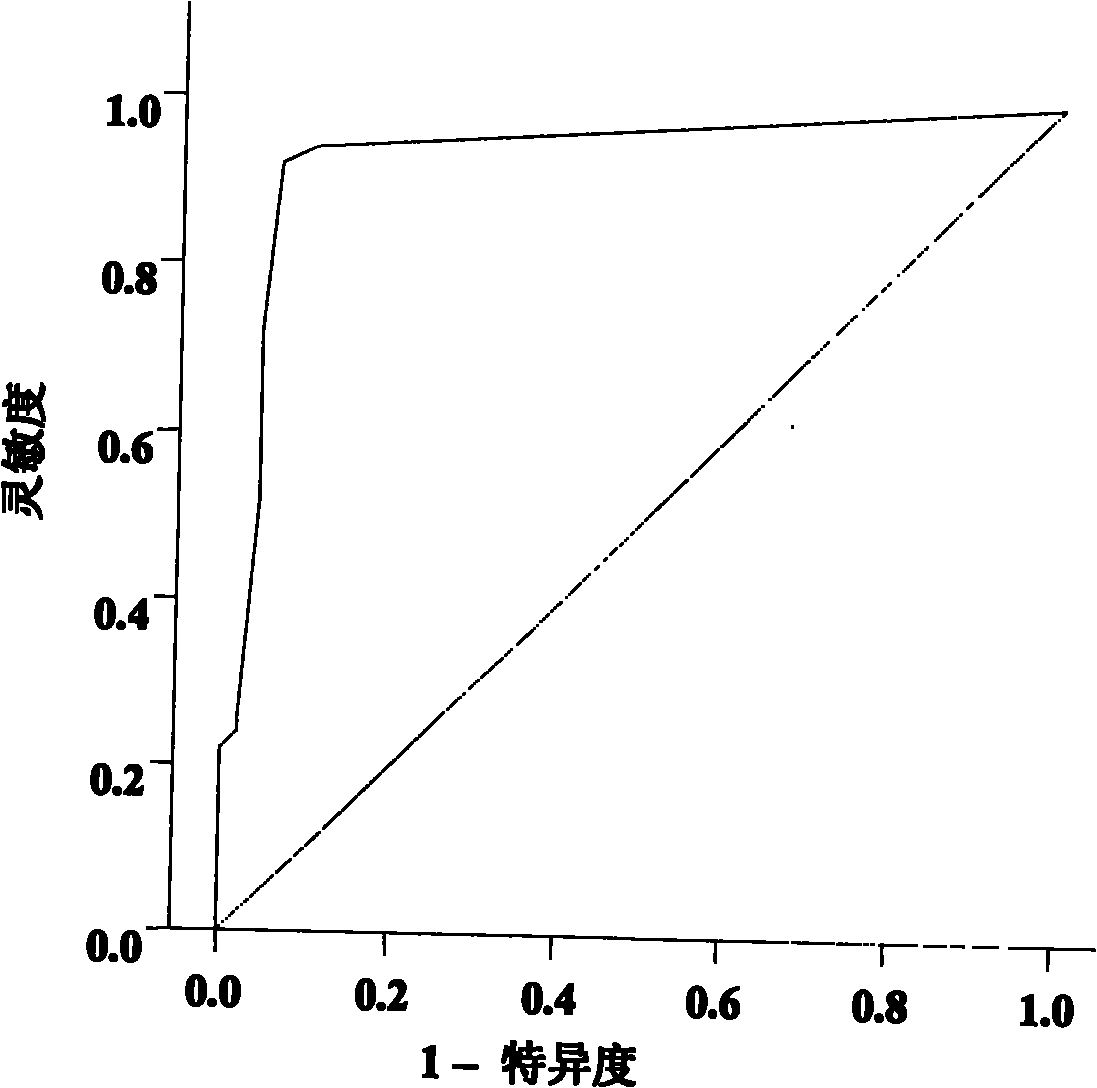
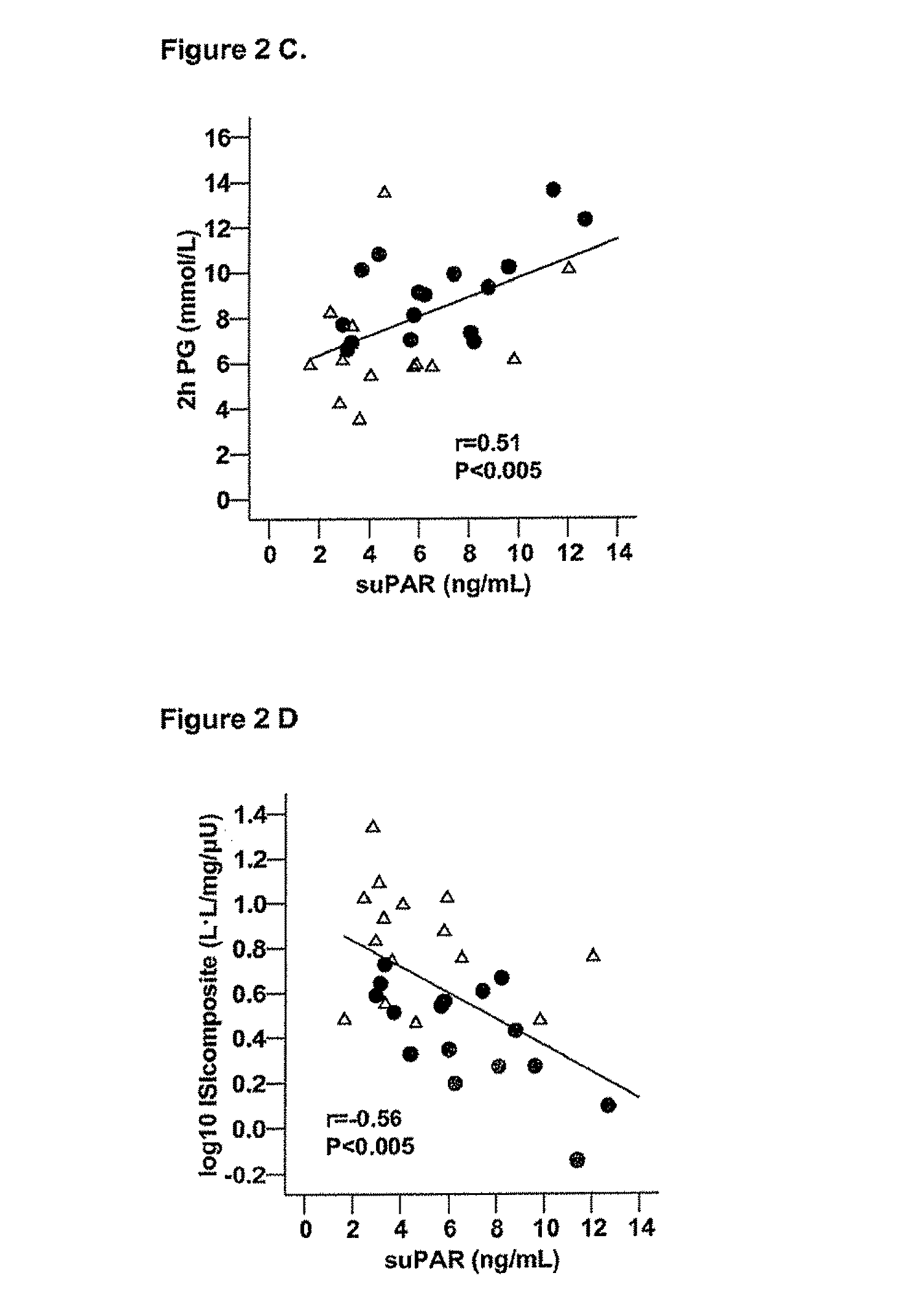
The invention concerns a marker for low-grade inflammation and metabolic syndrome (MS) and MS-related diseases and / or low-grade inflammation-related diseases such as cardiovasculardisease, ischemic heart disease and type 2 diabetes. More particularly it concerns the measurement of the concentration of soluble urokinase plasminogen activator receptor (suPAR) in human biological fluids (sputum, cystic fluid, ascites, serum, plasma, urine) as a tool of diagnosing and / or prognosticating low-grade inflammation and metabolic syndrome and the risk of development of the related diseases such as cancer, cardiovascular disease, ischemic heart disease and type 2 diabetes.
Owner:HVIDOVRE HOSPITAL
Polynucleotides and polypeptides of Anaplasma phagocytophilum and methods of using the same
InactiveUS20050142557A1Rapid diagnosisEnhance specificityBacteriaPeptide/protein ingredientsPhagocyteWhole blood units
We have successfully sequenced and cloned the gene expressing the Major Surface Protein 5 (MSP5) of A. phagocytophilum. The recombinant MSP5 (rMSP5) protein has been tested using sera from humans and dogs infected with A. phacytophilum. The polypeptide has been found to be immunogenic and useful as a diagnostic test antigen. The polypeptide antigen of the subject invention can provide the basis of a diagnostic assay that would allow the rapid, in-house, laboratory diagnosis of infection with A. phagocytophilum using a sample (e.g., serum, plasma, or whole blood) from an infected human or animal. Additionally, the subject invention provides methods of detecting the presence of A. phagocytophilum in biological or environmental samples utilizing antibodies provided by the subject invention. Furthermore, the use of the single antigen in the diagnosis of this important disease offers many advantages including enhanced test specificity, ease of testing and consistency of results using synthetically produced test antigens instead of cultured, whole organisms.
Owner:UNIV OF FLORIDA RES FOUNDATION INC +1
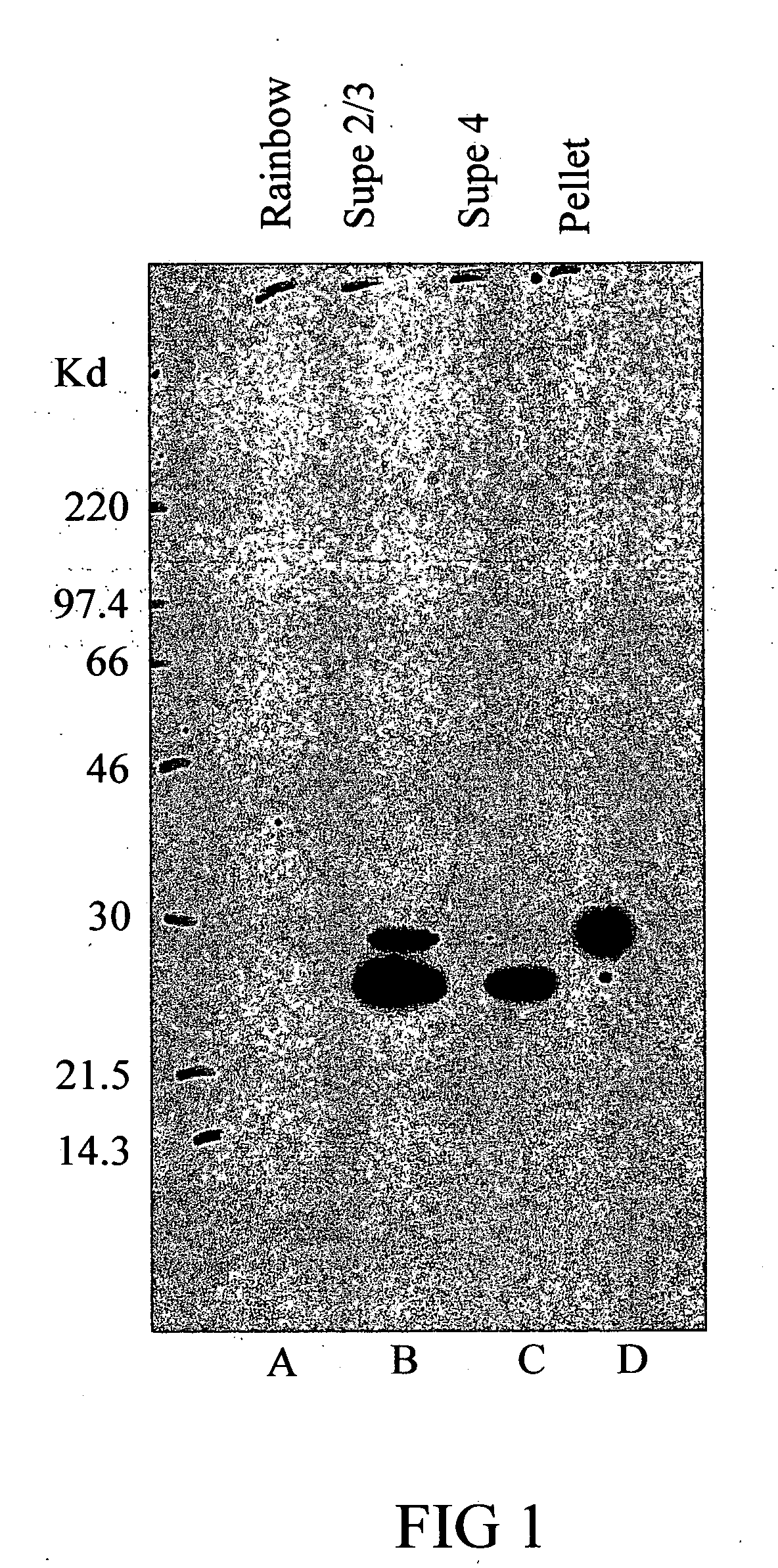
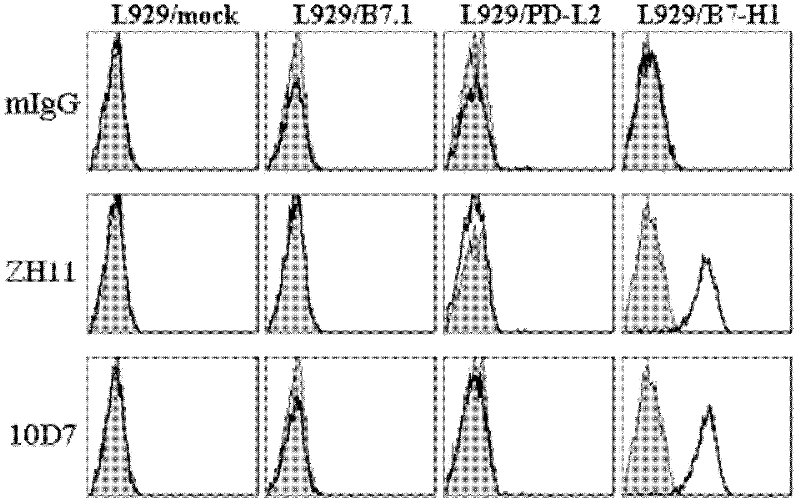
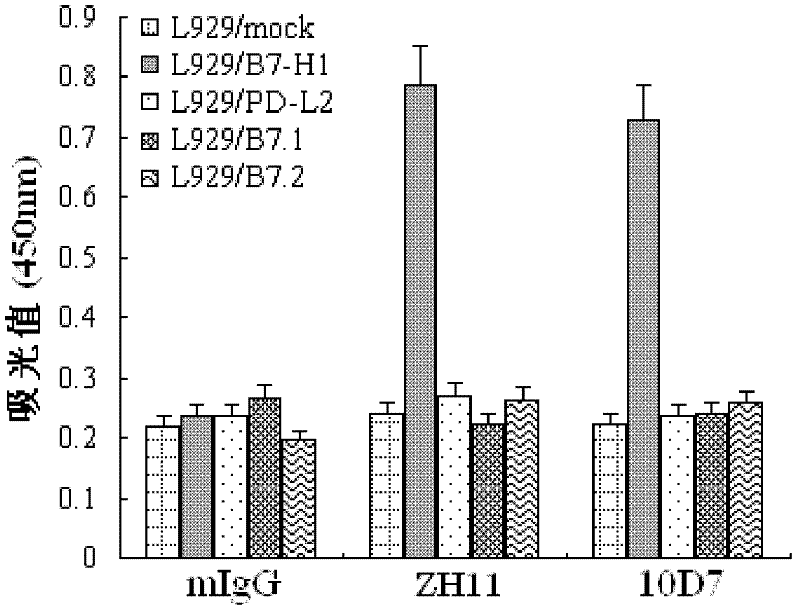
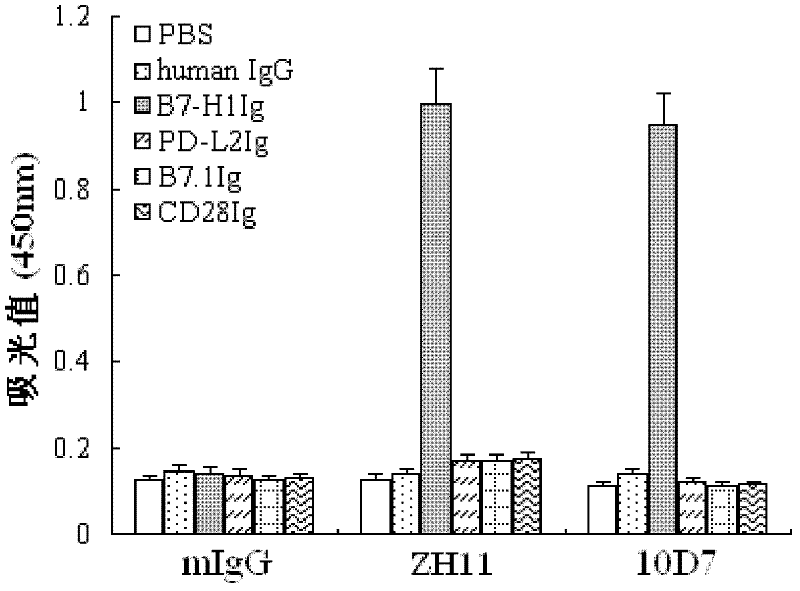
Soluble B7-H1 quantitative detection kit
InactiveCN102250911AQuantitative concentrationStrong specificityImmunoglobulins against animals/humansMicroorganism based processesAntiendomysial antibodiesNucleotide
The invention discloses a kit capable of quantitatively detecting soluble B7-H1, which comprises a horseradish peroxidase label, tetramethylbenzidine serving as a reaction substrate, bovine serum albumin, an elisa plate, washing liquor, stop solution, a coating antibody, a standard protein and a detection antibody, wherein the coating antibody is a mouse anti-human B7-H1 monoclonal antibody and the nucleotide sequence of the heavy chain of the coating antibody is represented by a nucleotide sequence SEQ ID No.3 in the sequence list, and the nucleotide sequence of the light chain of the coating antibody is represented by a nucleotide sequence SEQ ID No.4 in the sequence table. The kit capable of quantitatively detecting soluble B7-H1 has high specificity and can be used for accurately quantitative analysis on soluble B7-H1 protein factor concentration in liquid for human cell culture supernate, serum, plasma, hydrothorax and the like.
Owner:SUZHOU UNIV
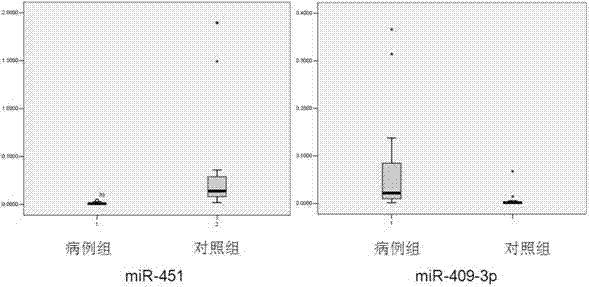
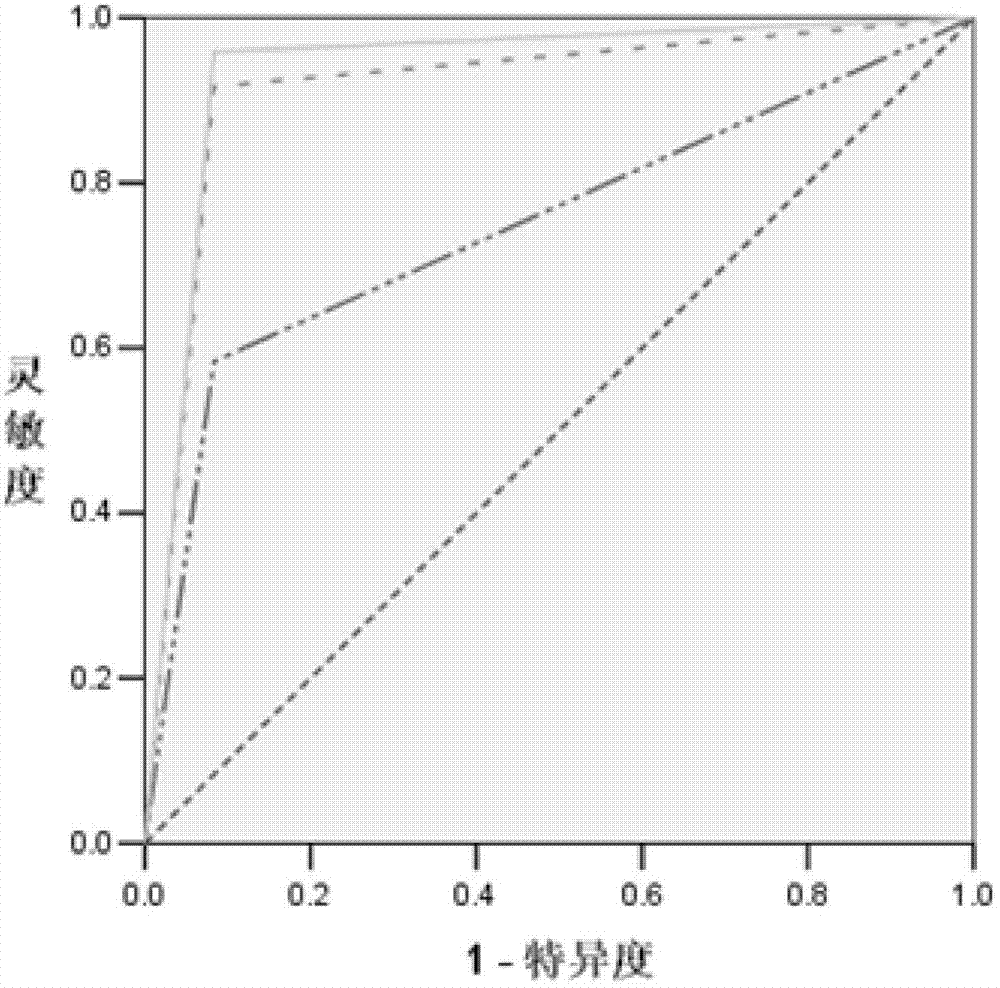
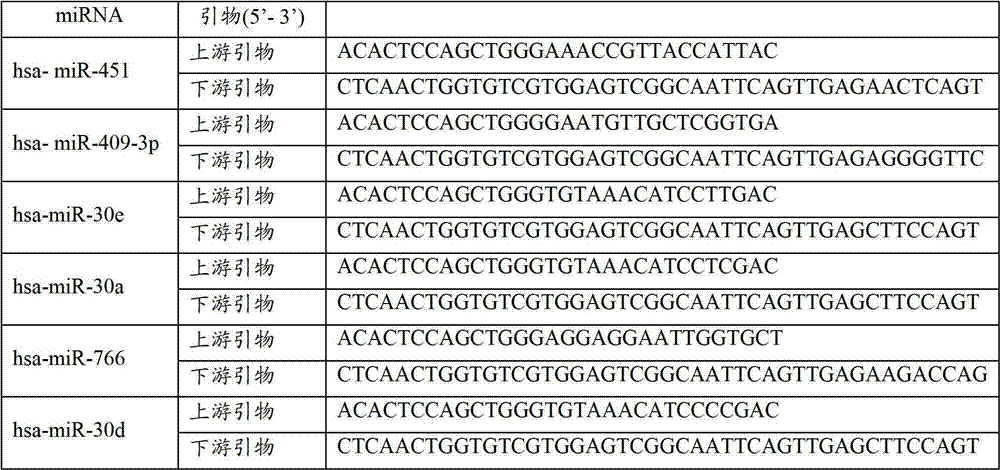
Blood serum/blood plasma micro ribonucleic acid (miRNA) marker relevant with pancreatic cancer and application thereof
ActiveCN102876676AAids in diagnosisEasy to detectMicrobiological testing/measurementDNA/RNA fragmentationBlood plasmaGenetic engineering
The invention belongs to the fields of genetic engineering and oncology, and discloses a blood serum / blood plasma micro ribonucleic acid (miRNA) marker relevant with pancreatic cancer and application thereof. The marker is formed by combining miR-451 and miR-490-3p. The marker and primers of the marker can be used for preparing a diagnostic kit and are used for the auxiliary diagnosis of pancreatic cancer.
Owner:NANJING MEDICAL UNIV
Blood serum/blood plasma miRNA marker related to non-small cell lung cancer (SCLC) prognosis and application thereof
InactiveCN101638656AIncreased sensitivityImprove featuresGenetic material ingredientsMicrobiological testing/measurementBlood plasmaGenetic engineering
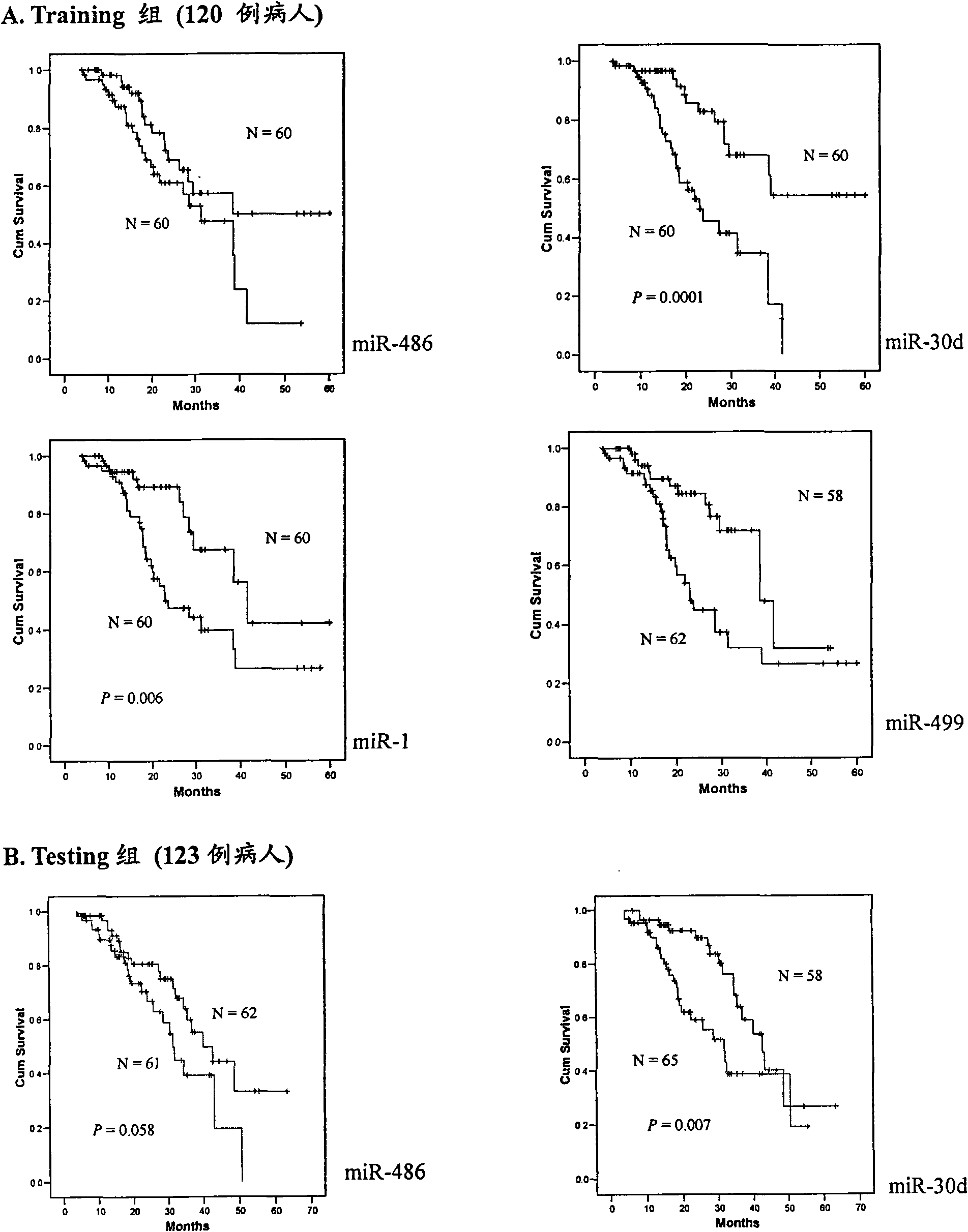
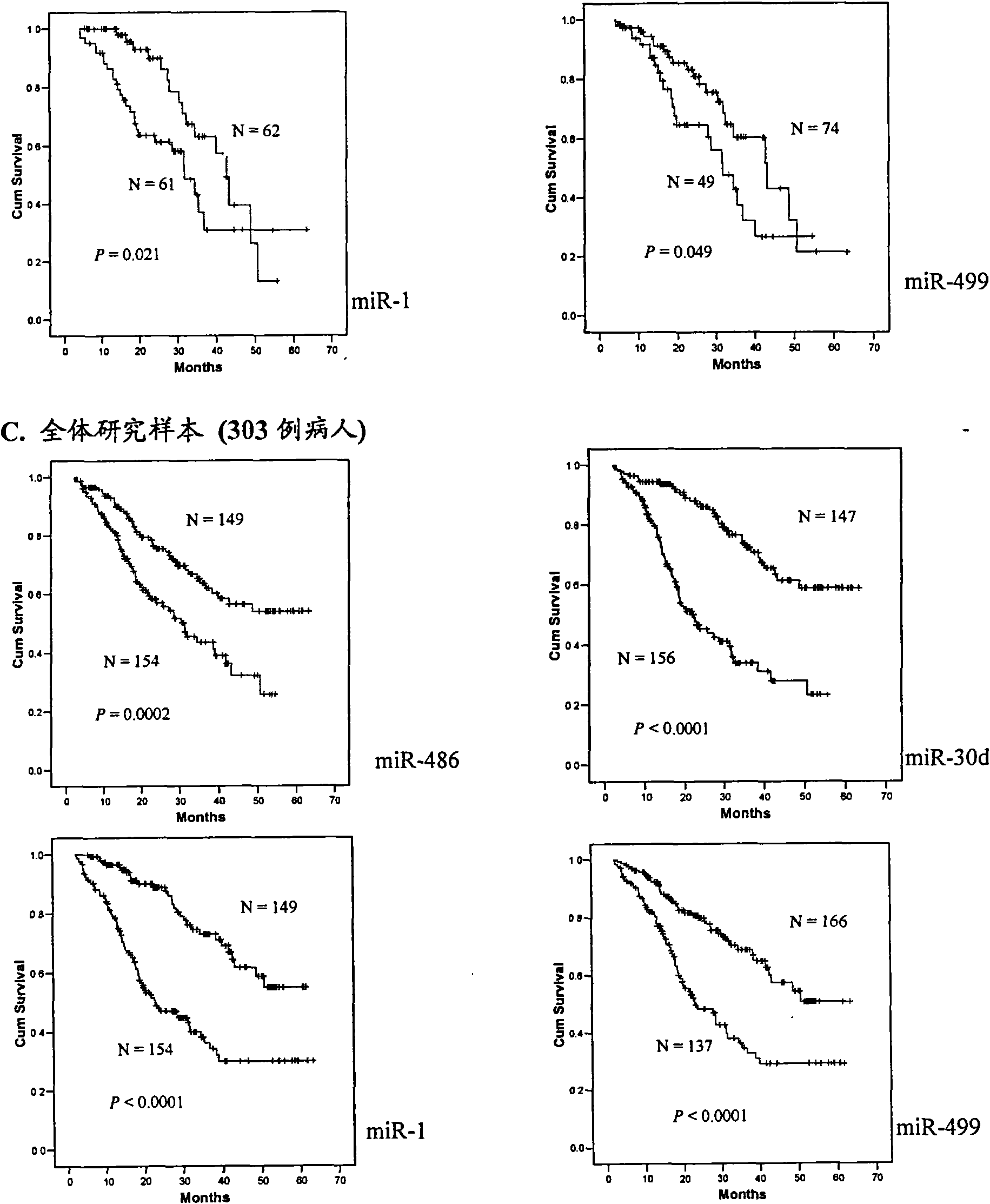
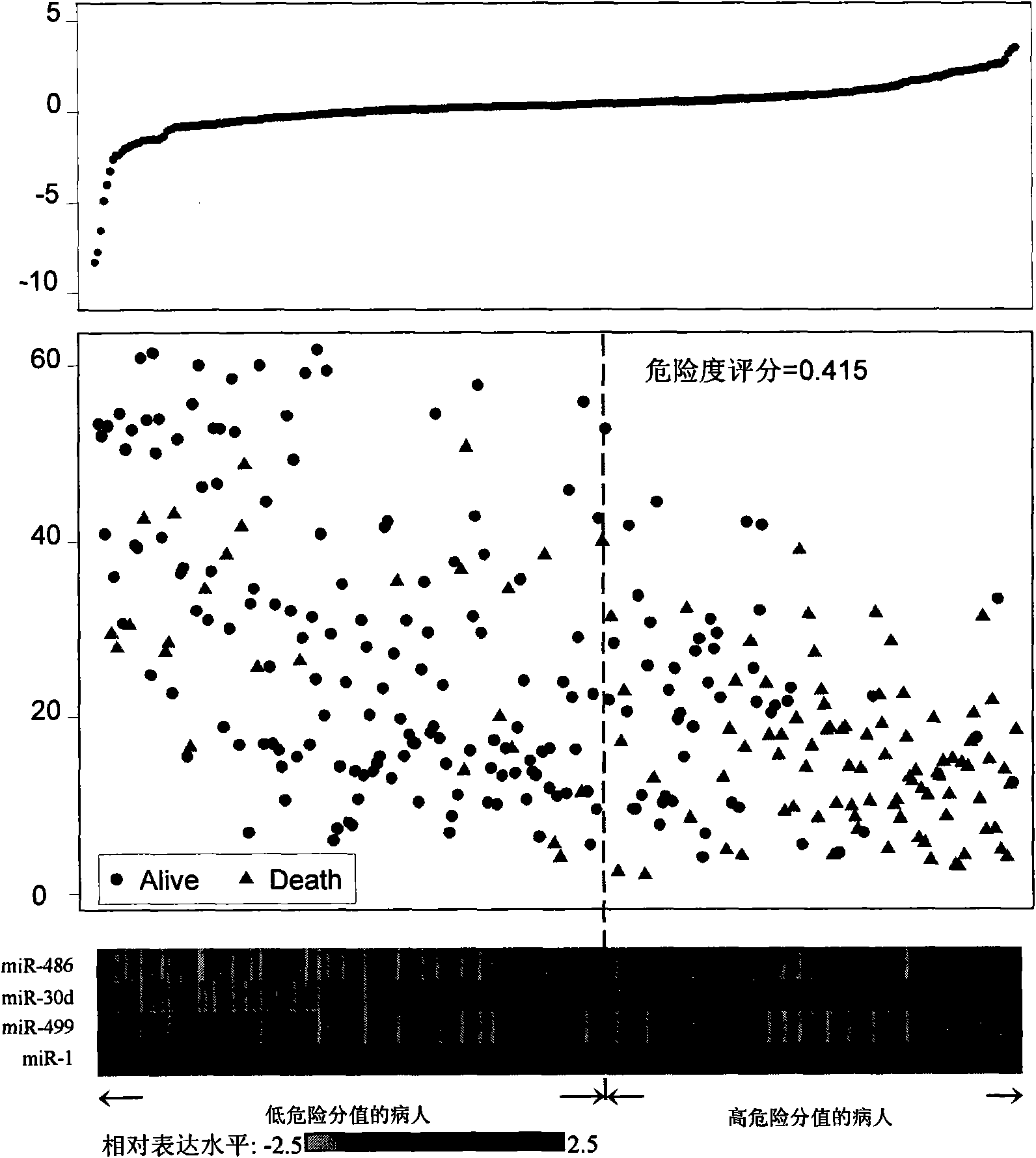
The invention belongs to the field of genetic engineering and phymatology, in particular to a blood serum / blood plasma miRNA marker related to non-small cell lung cancer (SCLC) prognosis and an application thereof. The marker is one or more of miR-486, miR30d, miR-1 or miR-499 and can be used for preparing an auxiliary diagnostic reagent kit for the non-SCLC prognosis or a medicine for treating the SCLC.
Owner:NANJING MEDICAL UNIV
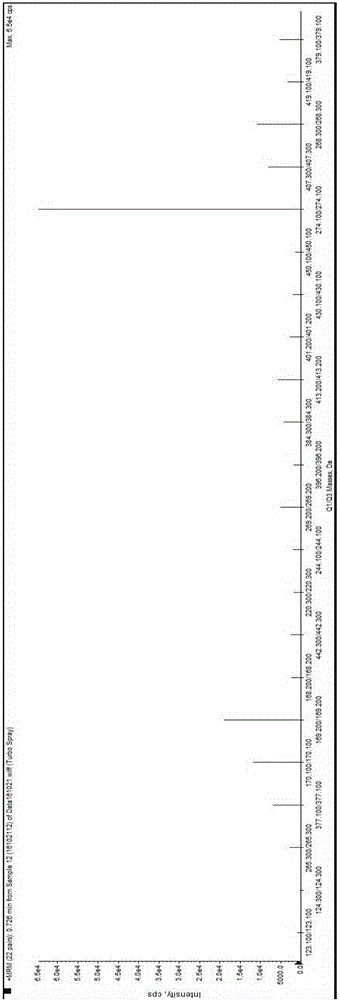
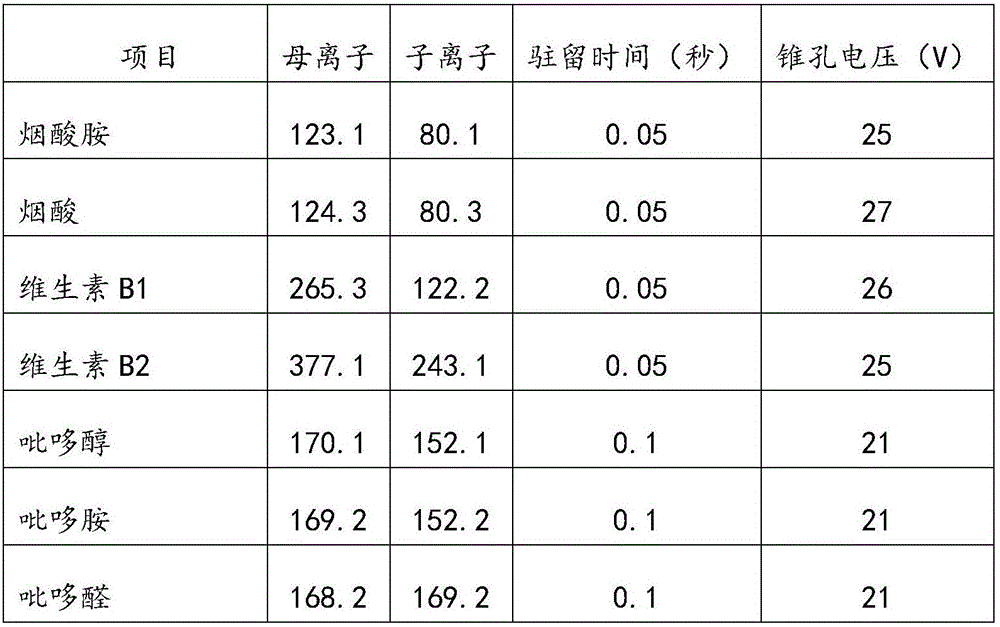
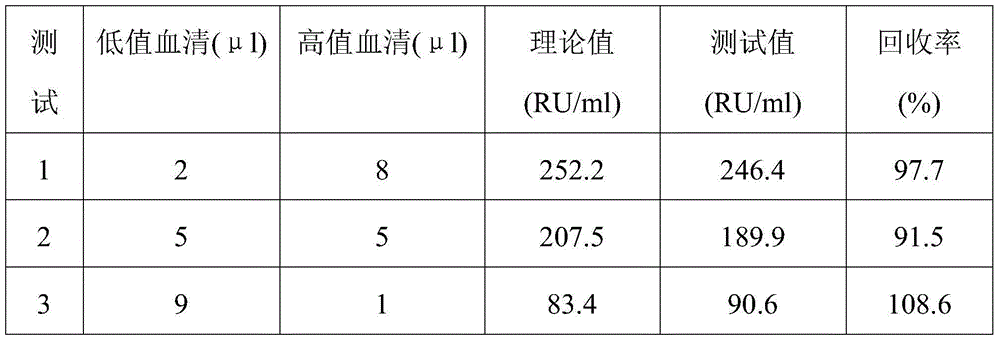
Method and kit for detecting various vitamins in serum/plasma at same time
The invention discloses a method for detecting various vitamins in serum / plasma at the same time. The method includes following steps: (1), well mixing to-be-detected serum / plasma with deproteinized liquid, centrifuging, and taking supernate; (2), well mixing the supernate obtained in the step (1) with internal standard mixed liquid, adopting tandem mass spectrometry for detection, and comparing ion strength of vitamins and internal standards of the vitamins to acquire content of the vitamins in the to-be-detected serum / plasma. In addition, the invention further discloses a kit for detecting various vitamins in serum / plasma at the same time. The kit comprises the internal standard mixed liquid, the deproteinized liquid, a mobile phase and probe washing liquid. By the method, various water-soluble and fat-soluble vitamins can be extracted from the serum / plasma, and a tandem mass spectrometer is utilized to quantify more than ten vitamins in one specimen within 2-3min, so that detection period is remarkably shortened, and vitamin detection results are higher in degree of precision and accuracy.
Owner:GUANGZHOU FENGHUA BIOENG
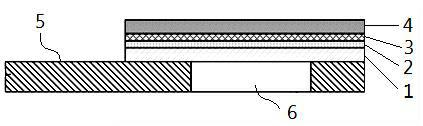
Multilayer film dry chemical reagent tablet for detecting alanine aminotransferase
InactiveCN102323399AExtend storage timeAccurate checkColor/spectral properties measurementsBiological testingChemistryBlood plasma
The invention discloses a multilayer film dry chemical reagent tablet for detecting alanine aminotransferase, which comprises a lower support layer; a test hole is disposed at one end of the lower support layer; the lower support layer is coated with a multilayer film; the multilayer film comprises an euphotic layer, a reagent layer, an auxiliary layer and a diffusion layer which are orderly superimposed from up to down; one side of the euphotic layer of the multilayer film is bonded and fixed at the test hole of the lower support layer. The multilayer film dry tablet for detecting alanine aminotransferase of the invention can rapidly and accurately detect alanine aminotransferase in serum and blood plasma, and can increase the preservation time of the dry tablet.
Owner:SUZHOU INST OF BIOMEDICAL ENG & TECH
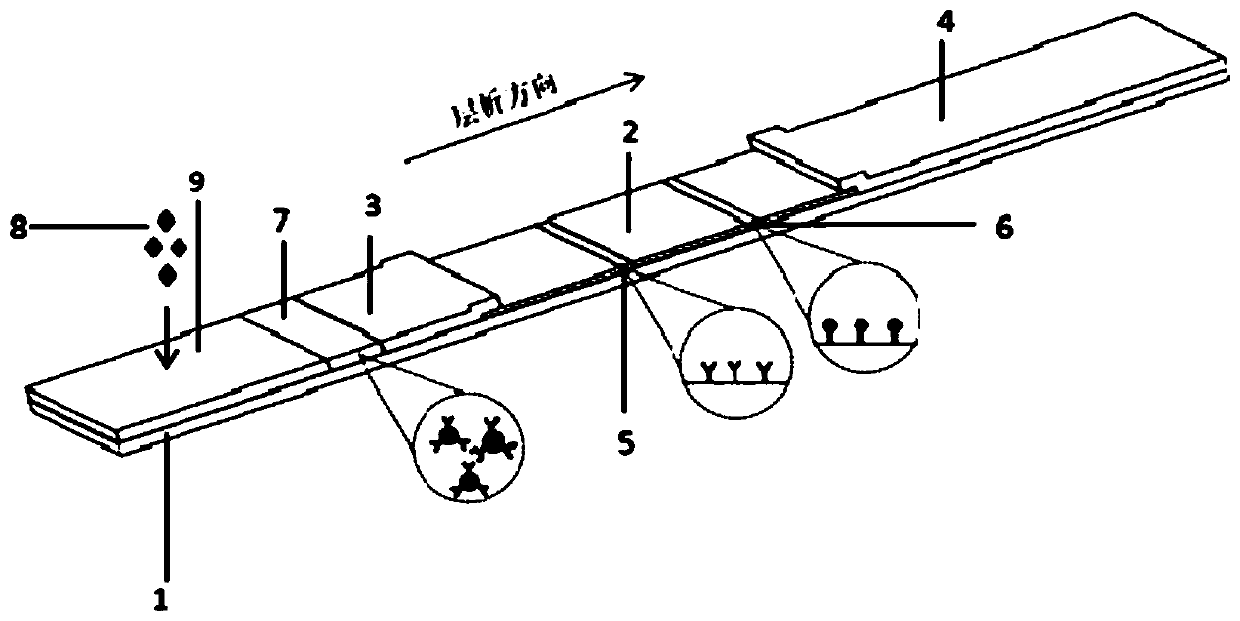
PLA2R (phospholipase A2 receptor) antibody detection strip and preparation method and detection method thereof
InactiveCN104880560AImprove stabilityQuantitative detection safetyDisease diagnosisSerum igeFluorescence
The invention relates to the biotechnology field and in particular relates to a PLA2R (phospholipase A2 receptor) antibody detection strip and a preparation method and a detection method of the PLA2R antibody detection strip. According to the PLA2R antibody detection strip, based on the double-antigen sandwiched antibody detection principle, PLA2R coats a nitrocellulose membrane, quantum dot coupled PLA2R is sprayed on a conjugate pad respectively, a sample pad, the conjugate pad, the nitrocellulose membrane and absorbent paper are sequentially lapped on a viscous PVC (polyvinyl chloride) bottom plate sequentially, the strip with certain width is cut out to form a detection card, the concentration value of the antibody is measured by a fluorescence analyzer, and the PLA2R antibody in the sample can be quantitatively detected; based on a quantum dot immunochromatographic method, the contents of PLA2R antibody in serum, plasma and whole blood can be quantitatively detected safely, accurately and rapidly in a noninvasive low-risk and low-cost manner, and the PLA2R antibody detection strip can provide assistance for preliminary screening of the idiopathic membranous nephropathy and illness monitoring.
Owner:SHENZHEN BLOT BIOTECH
Use method of immunochromatographic kit for rapidly detecting novel coronavirus N protein
InactiveCN111398588AThe detection process is fastEasy to operateImmunoassaysPolyclonal antibodiesBlood plasma
The invention relates to a use method of an immunochromatographic kit for rapidly detecting novel coronavirus N protein. The immunochromatographic kit comprises a test strip, the test strip comprisesa detection line and a quality control line, the detection line is coated with an anti-novel coronavirus N protein antibody, and the quality control line is coated with a goat anti-rabbit polyclonal antibody. The immunochromatographic kit for rapidly detecting the novel coronavirus N protein provided by the invention can be used for detecting the novel coronavirus N protein in serum, plasma and whole blood samples so as to be used for diagnosing novel coronavirus pneumonia.
Owner:BEIJING ELCOTEQ BIO TECH
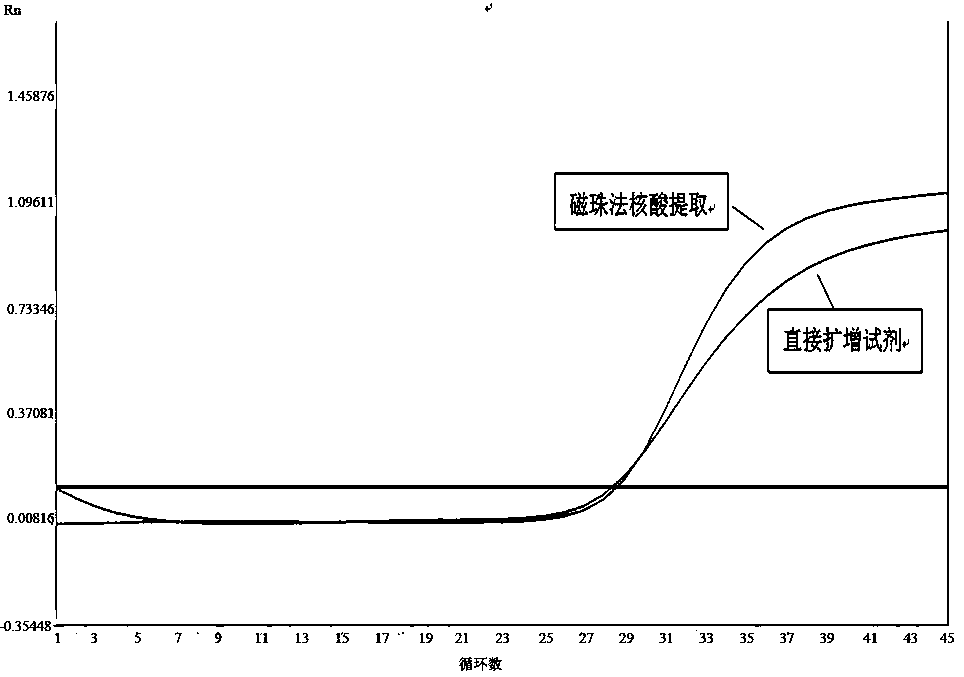
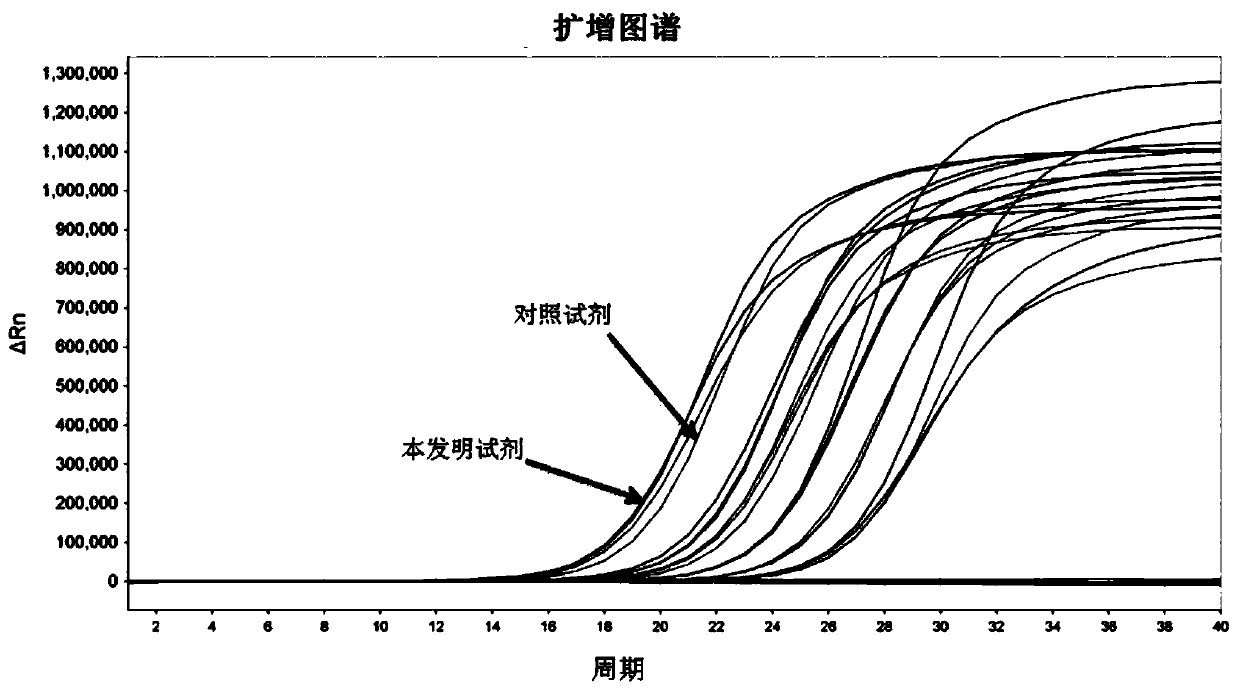
Extraction-free direct amplification reagent for real-time fluorescent quantitation PCR and application thereof
ActiveCN109402239AEasy to operateShort processMicrobiological testing/measurementHuman DNA sequencingFluorescence
The invention provides an extraction-free direct amplification reagent for real-time fluorescent quantitation PCR and application thereof. The direct amplification reagent comprises the following components: sodium hydroxide (NaOH), Surfactin, dimethyl sulfoxide (DMSO), sucrose and chelex-100. When the reagent is used, there is no need to extract and purify nucleic acid from a sample, only the sample and the reagent need to be mixed according to the equal volume ratio of 1:1. The mixture can be directly used for fluorescent PCR detection. The extraction-free direct amplification reagent is applicable to nucleic acid extraction of human genome, bacteria and viruses in mouth swabs, nasopharyngeal swabs, serum, plasma, genital tract secretion and other samples. The reagent is suitable for both the fluorescence PCR of a probe method and the PCR of a dying method, is suitable for a variety of clinical applications and has the advantages of low cost, simple operation, accurate results and the like, and detection can be simple and quickly completed.
Owner:贝南生物科技(厦门)有限公司
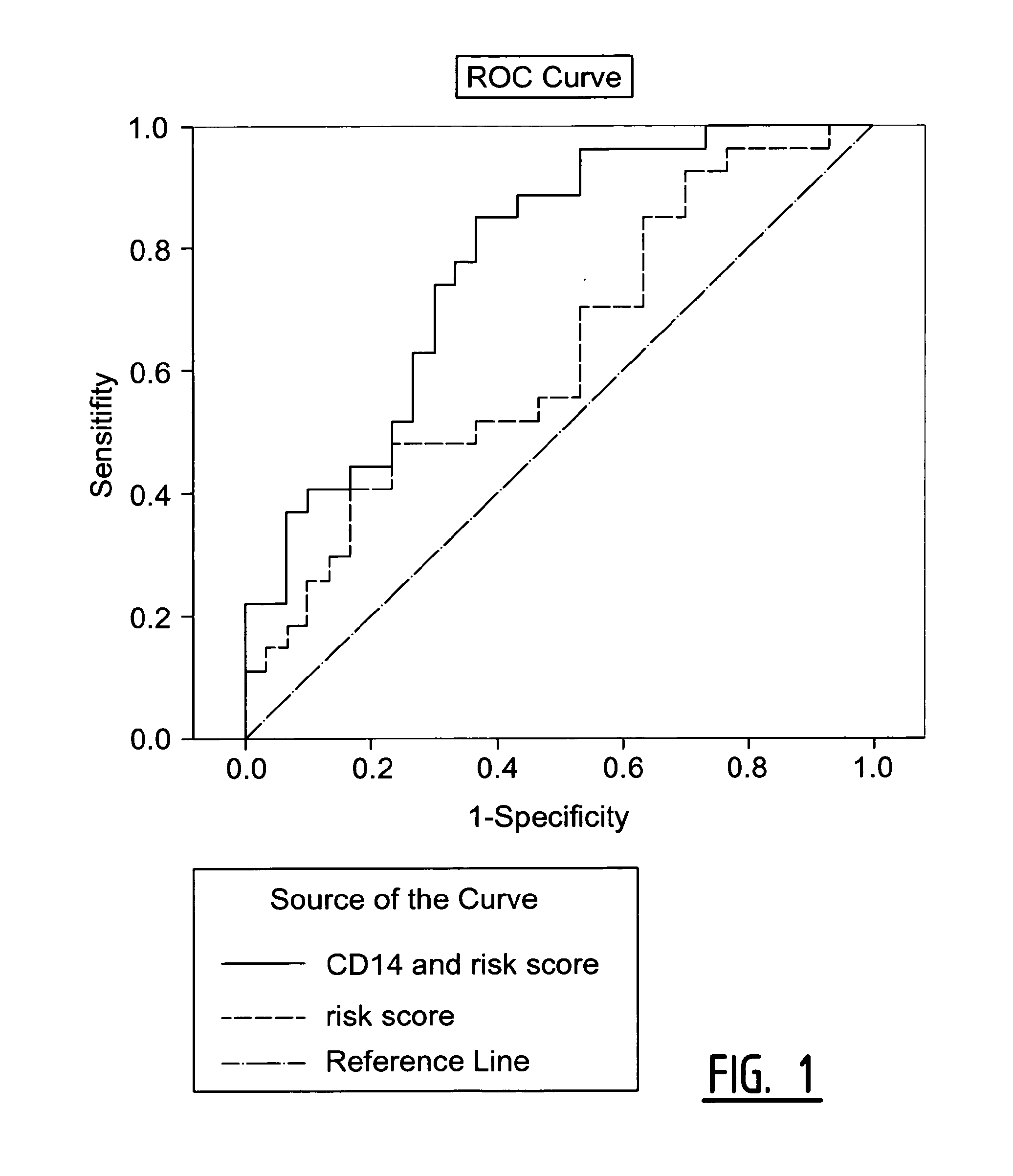
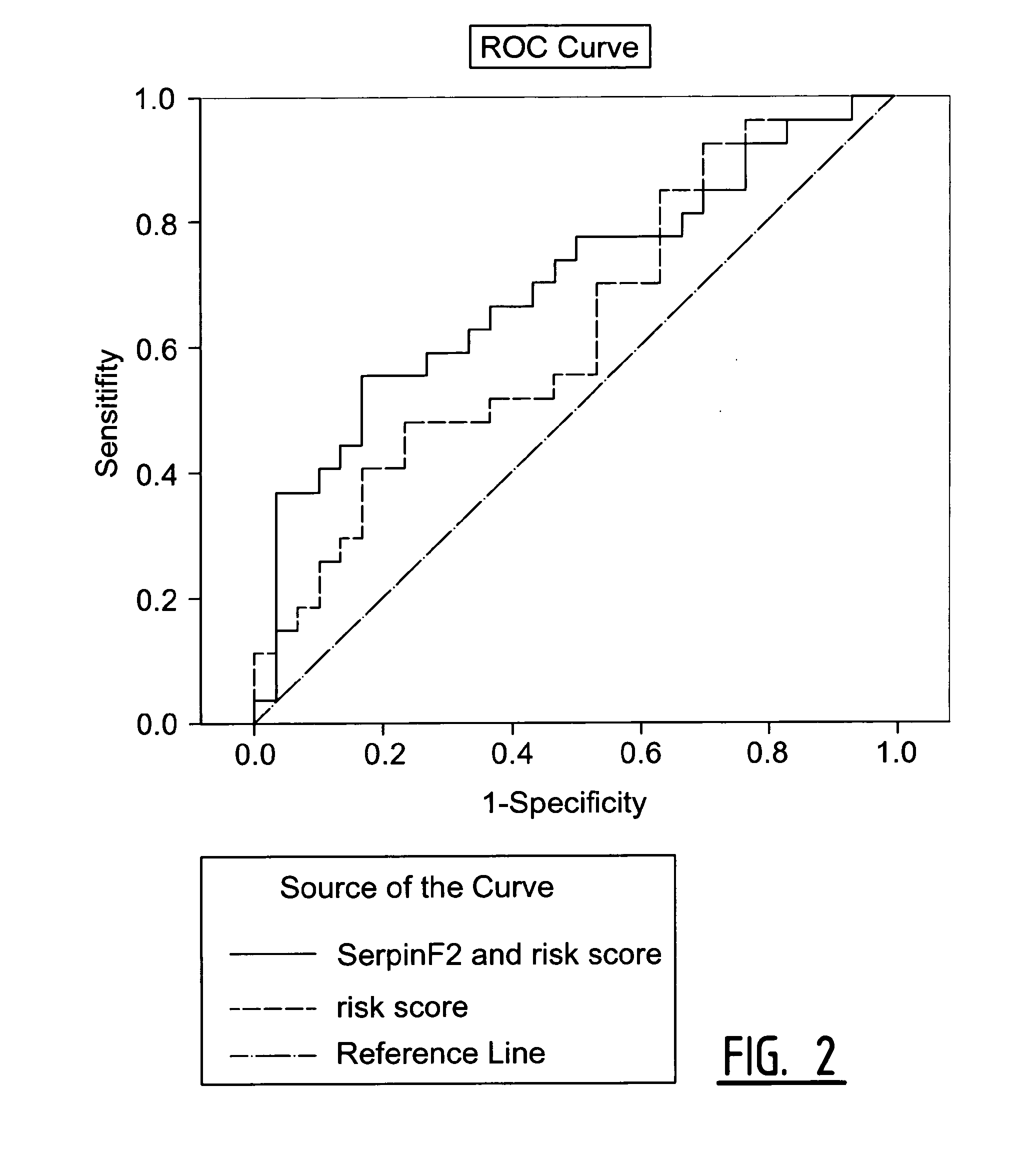
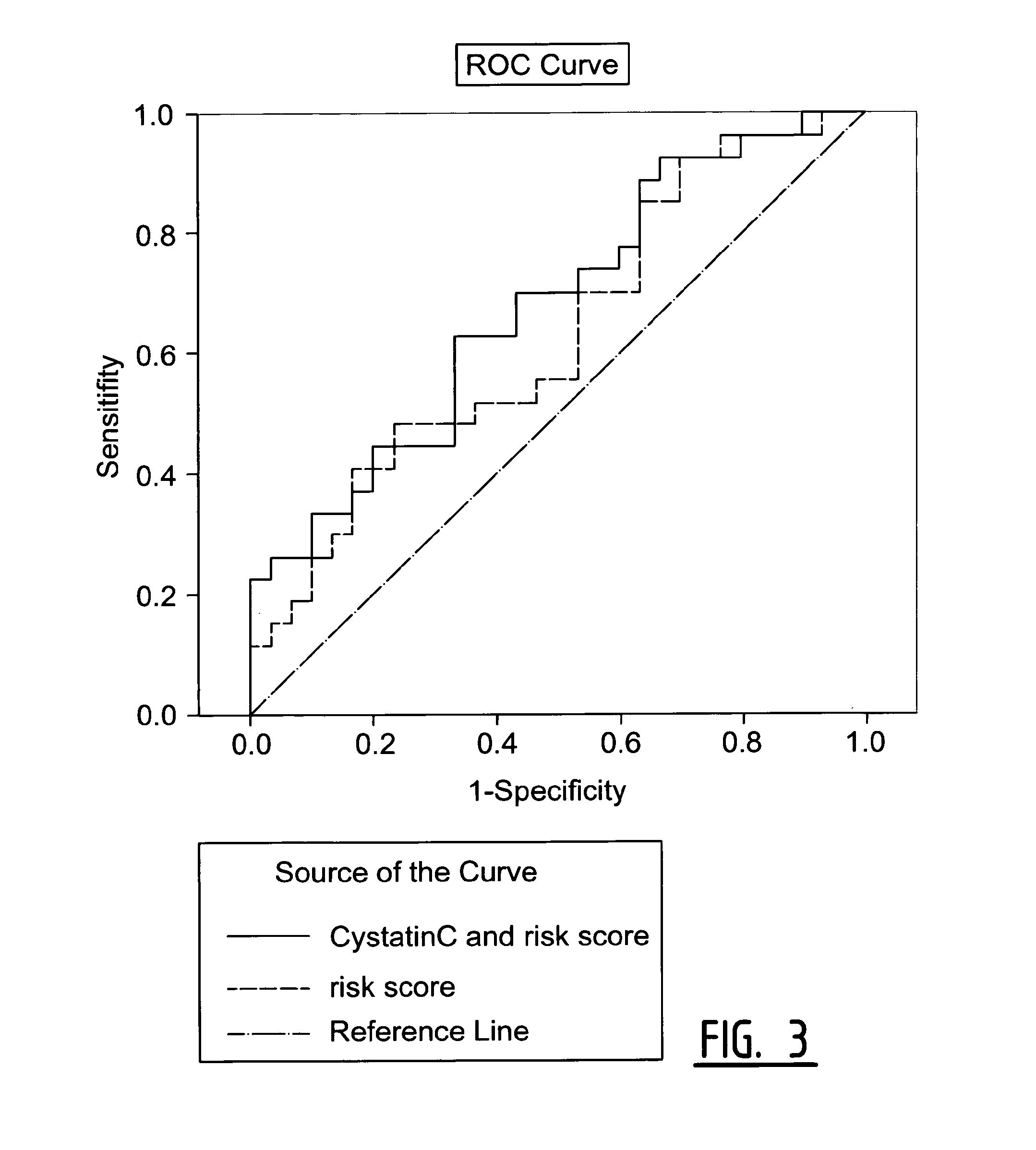
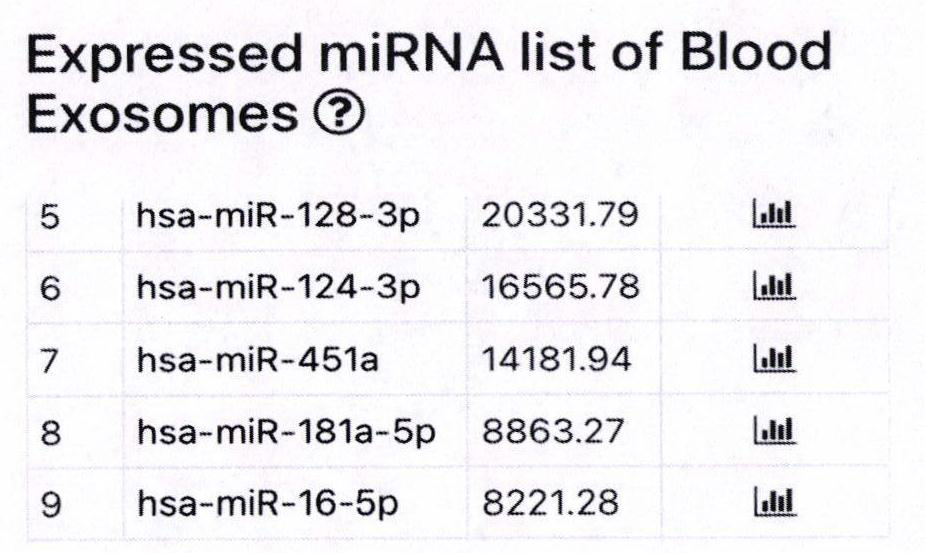
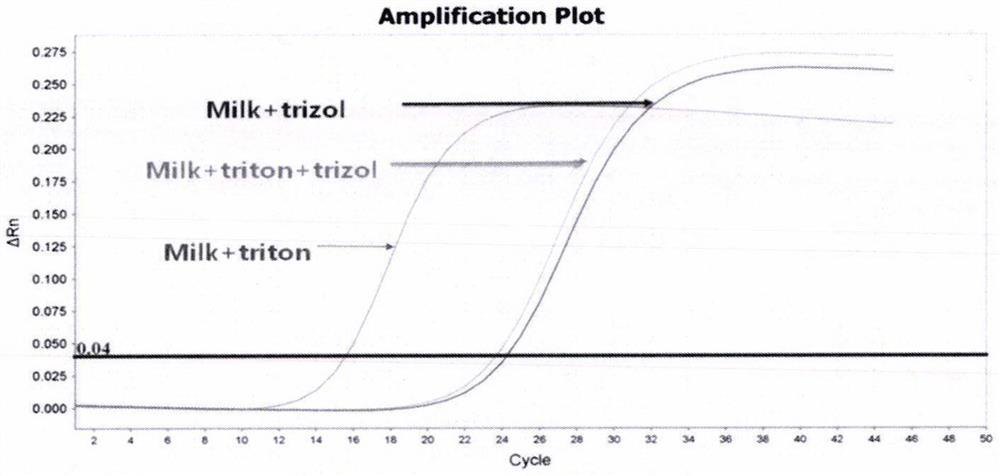
Determination of Exosomel Biomarkers for Predicting Cardiovascular Events
InactiveUS20120309041A1Reduce stepsComplicate to detectMicrobiological testing/measurementImmunoglobulins against animals/humansAmniotic fluidNGAL Protein
The present invention relates to a method of predicting the risk of a subject developing a cardiovascular event, comprising determining the presence of a biomarker that is indicative of the risk of developing a cardiovascular event in exosomes from the subject. The exosomes are suitably isolated from a body fluid selected from serum, plasma, blood, urine, amniotic fluid, malignant ascites, bronchoalveolar lavage fluid, synovial fluid, breast milk, saliva, in particular serum. The biomarker is selected from the proteins Vitronectin, Serpin F2, CD14, Cystatin C, Plasminogen, Nidogen 2 or any combination of two or more of these proteins.
Owner:ERASMUS UNIV MEDICAL CENT ROTTERDAM ERASMUS MC
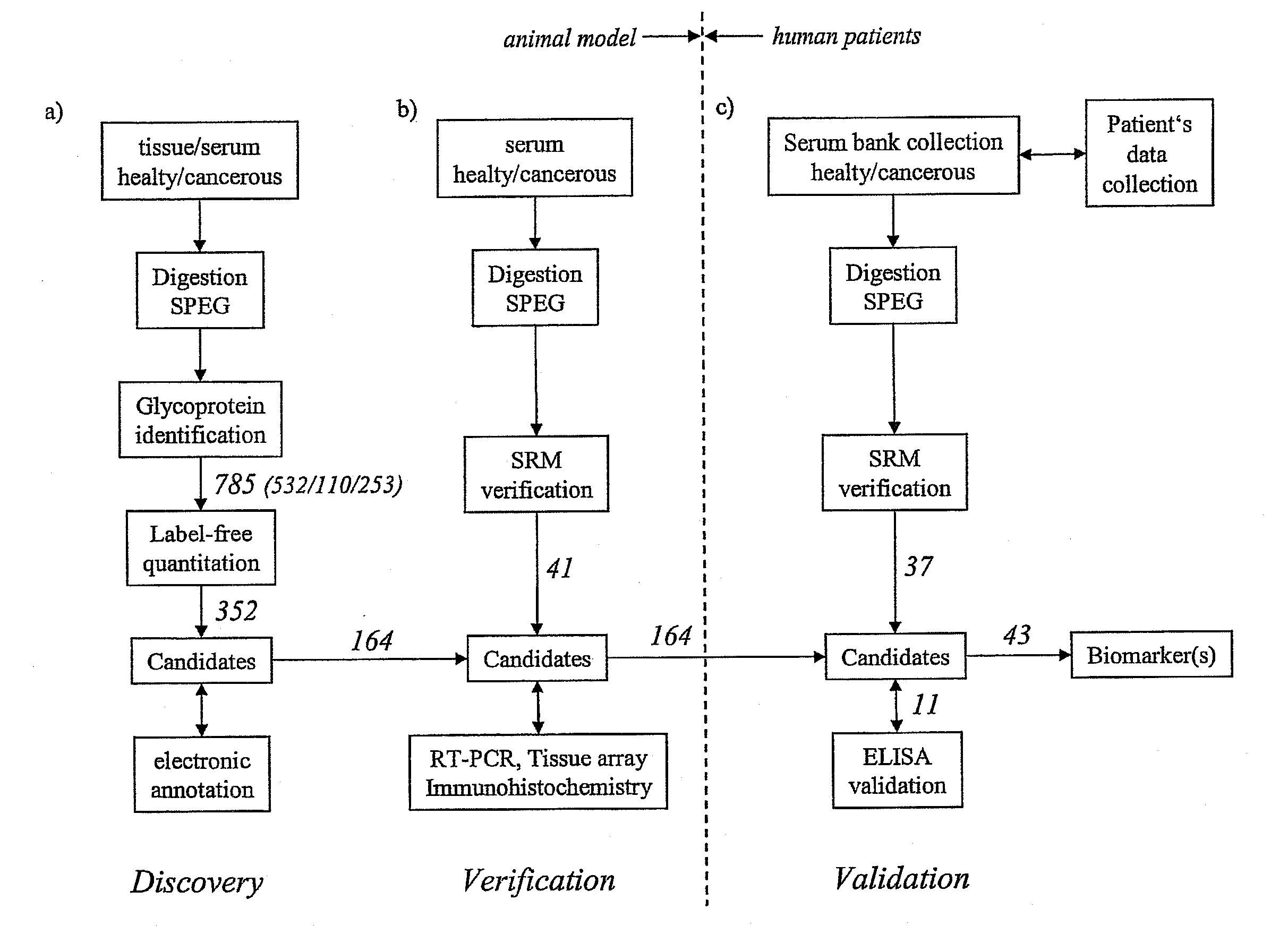
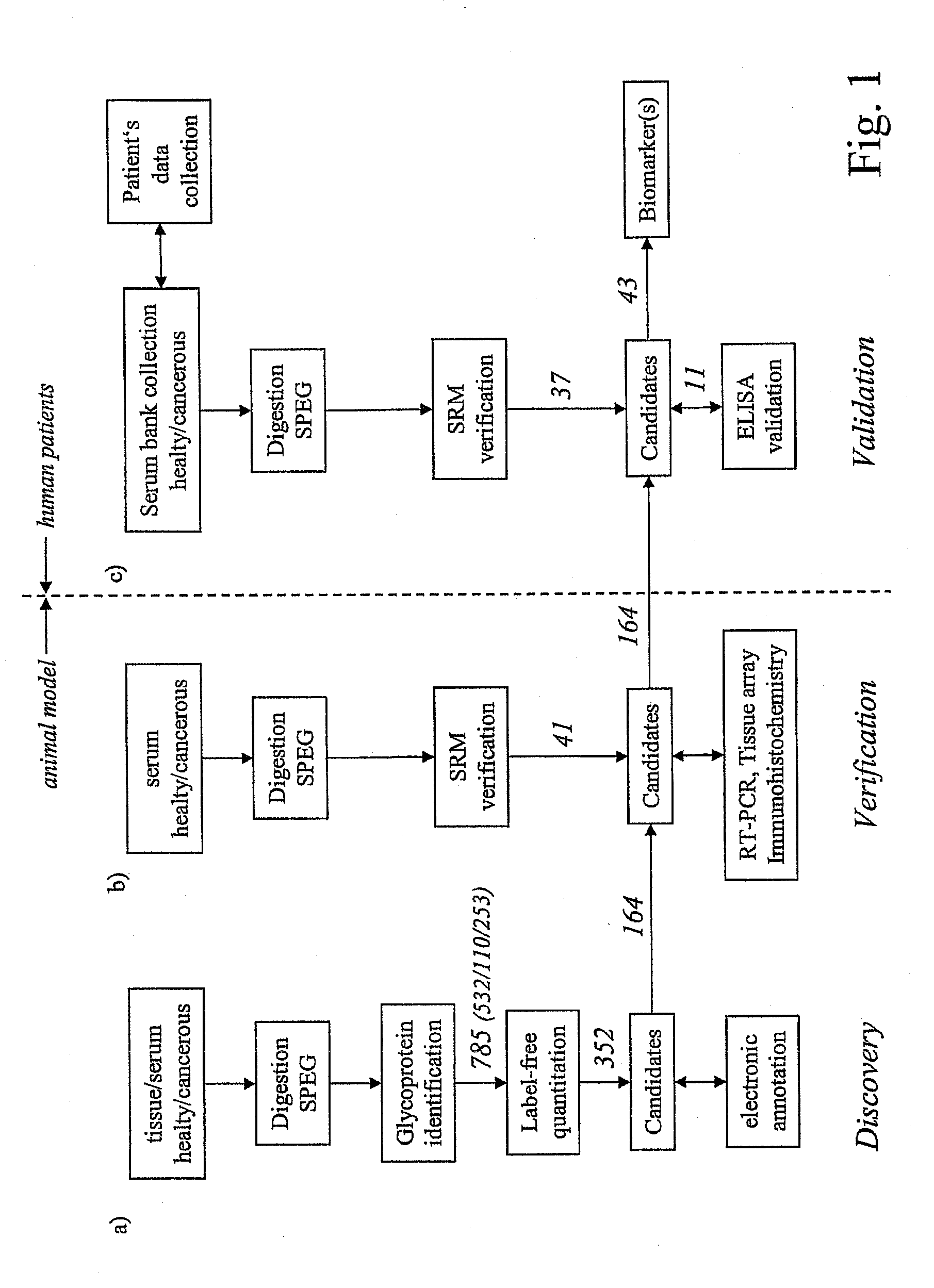
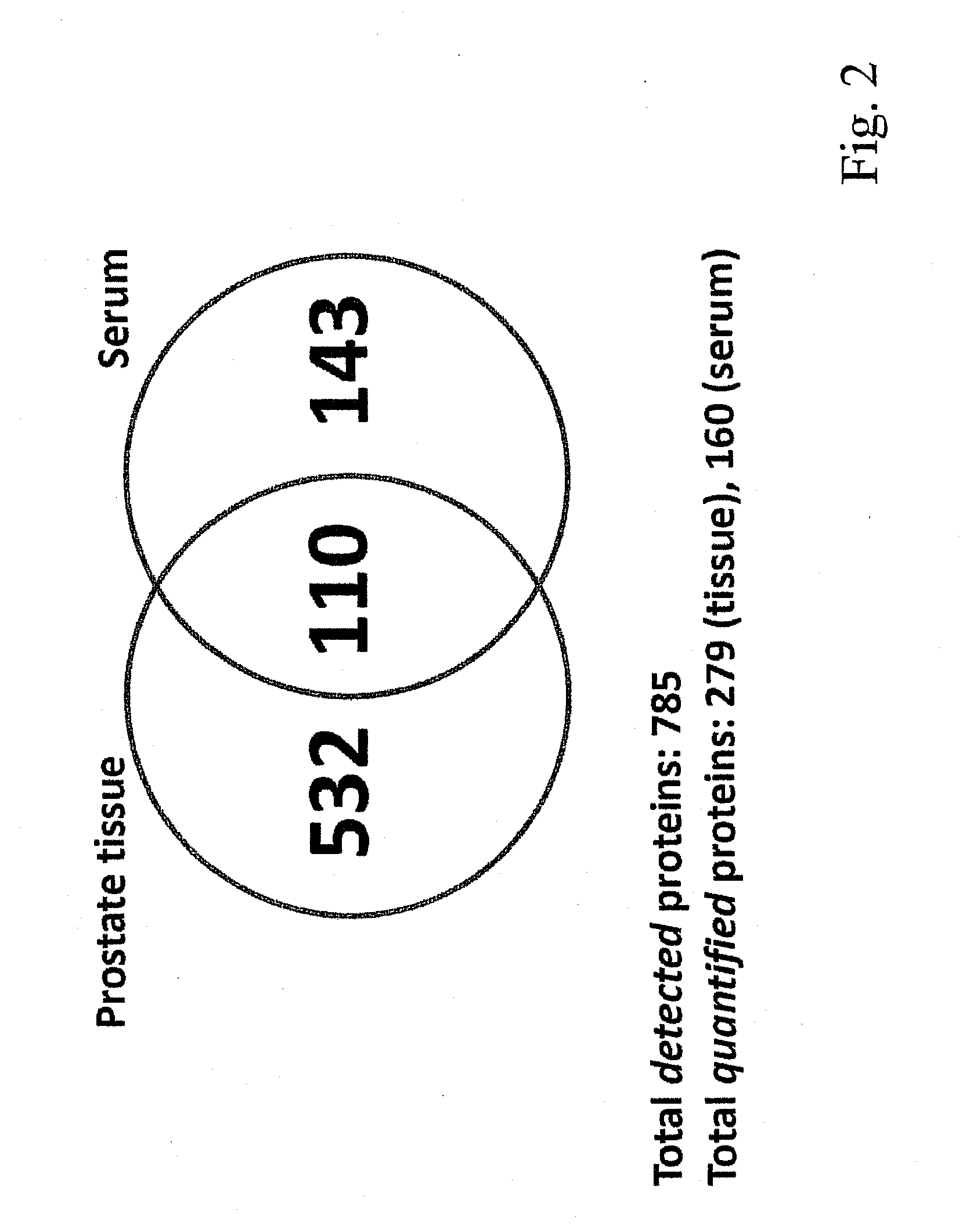
Method for biomarker and drug-target discovery for prostate cancer diagnosis and treatment as well as biomarker assays determined therewith
InactiveUS20110065605A1Improve accuracyImprove reliabilityMicrobiological testing/measurementLibrary screeningProtein proteinAntibody
The invention relates to a method for the determination of a cancer diagnostic / therapeutic biomarker assay and drug-targets including the following steps: (a) identification of potential candidate protein / peptide biomarkers and drug-targets based on the measurement of protein / peptide constituent concentrations in tissue sample proteomes as well as serum, plasma or any other derivatives of blood, or blood itself sample proteomes derived from healthy non-human mammalian individuals as well as from cancerous non-human mammalian individuals and qualitatively selecting as potential candidate protein / peptide biomarkers those which show a pronounced differential behaviour between healthy and cancerous sample proteomes; (b) optional verification of the potential candidate protein / peptide biomarkers as identified in step (a) by quantitative mass spectrometric measurement of the potential candidate protein biomarkers in serum, plasma or any other derivatives of blood, or blood itself sample proteomes derived from healthy non-human mammalian individuals as well as from cancerous non-human mammalian individuals and selecting as candidate protein / peptide biomarkers those which show a mass-spectrometrically measurable quantitative differential behaviour between healthy and cancerous sample proteomes; (c) validation of the candidate protein / peptide biomarkers as identified in step (a), or as optionally verified in step (b), by mass spectrometric measurement and / or antibody-based assays such as an Enzyme-Linked Immunosorbent Assay (ELISA) determination of the candidate protein biomarkers in serum, plasma or any other derivatives of blood, or blood itself sample proteomes derived from healthy human individuals as well as from cancerous human individuals and selecting as protein / peptide biomarkers those which show a mass-spectrometrically measurable and / or antibody-based assay detectable differential behaviour between healthy and cancerous sample proteomes; (d) application of statistical methods to uncover single or groups of protein / peptide biomarkers as validated in step (c) as signatures for the detection of patients with cancer. The invention furthermore relates to specific biomarker assays for the highly reliable diagnosis of cancer, specifically of localized or non-localized prostate cancer, using human serum, plasma or any other derivatives of blood, or blood itself.
Owner:ETH ZZURICH +1
Serum/plasma miRNA marker related to assisted diagnosis of intrahepatic cholestasis of pregnancy and application of serum/plasma miRNA marker
ActiveCN108441552AConvenient for clinical operationEasy diagnosisMicrobiological testing/measurementDNA/RNA fragmentationAIDS diagnosisOncology
Owner:WUXI MATERNAL & CHILD HEALTH HOSPITAL
Method for measuring the amount of constituent contained in a specific lipoprotein
InactiveUS7223546B2Microbiological testing/measurementEnzymologyBlood plasmaHigh-density lipoprotein
There is provided a method and kit for measuring the amount of an objective constituent contained in a specific lipoprotein in a biological sample such as serum and plasma, specifically for measuring the amount of cholesterol contained in high density lipoprotein, which can be applicable to clinical tests.
Owner:WAKO PURE CHEMICAL INDUSTRIES
Inducible heart attack animal model
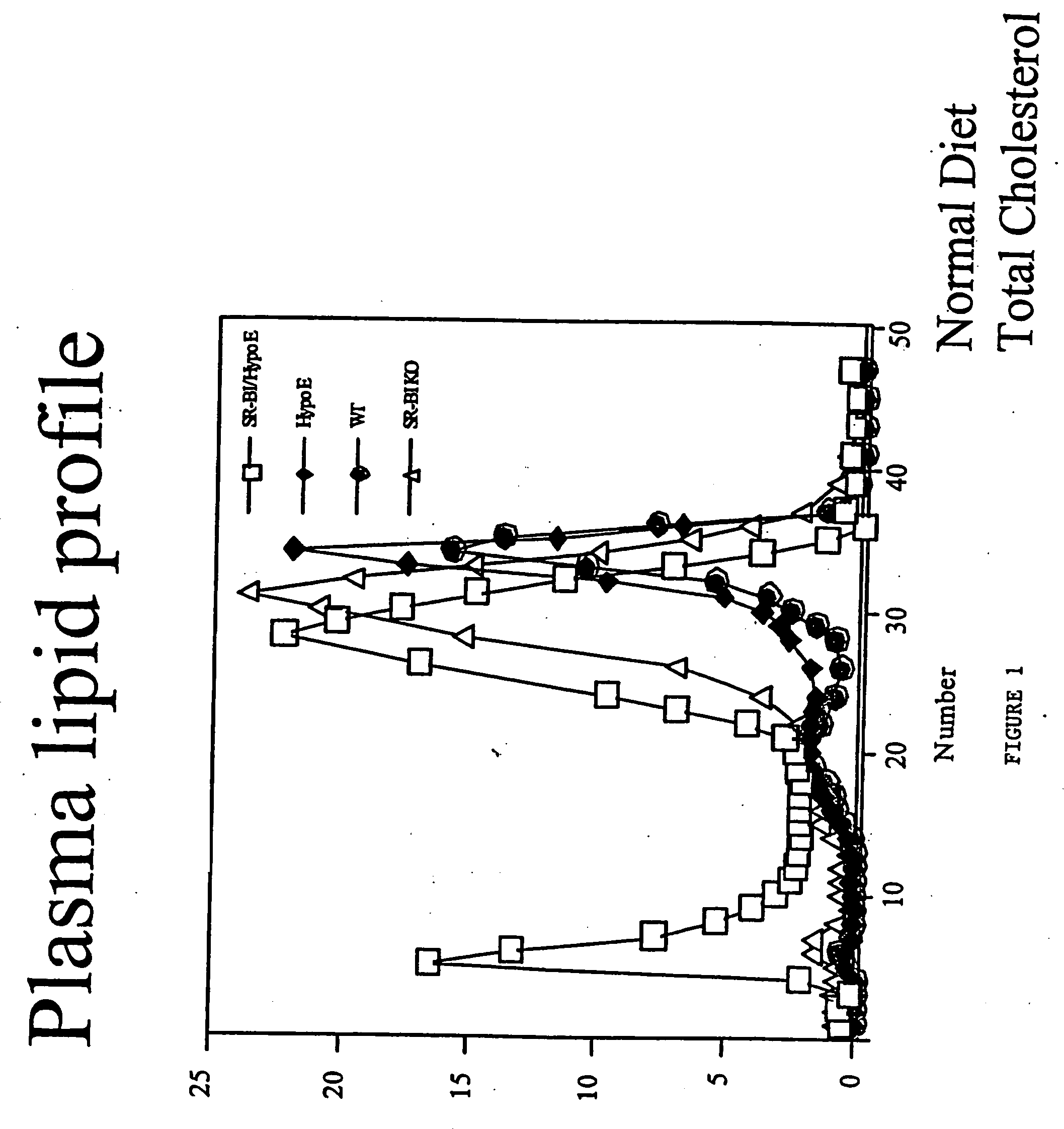
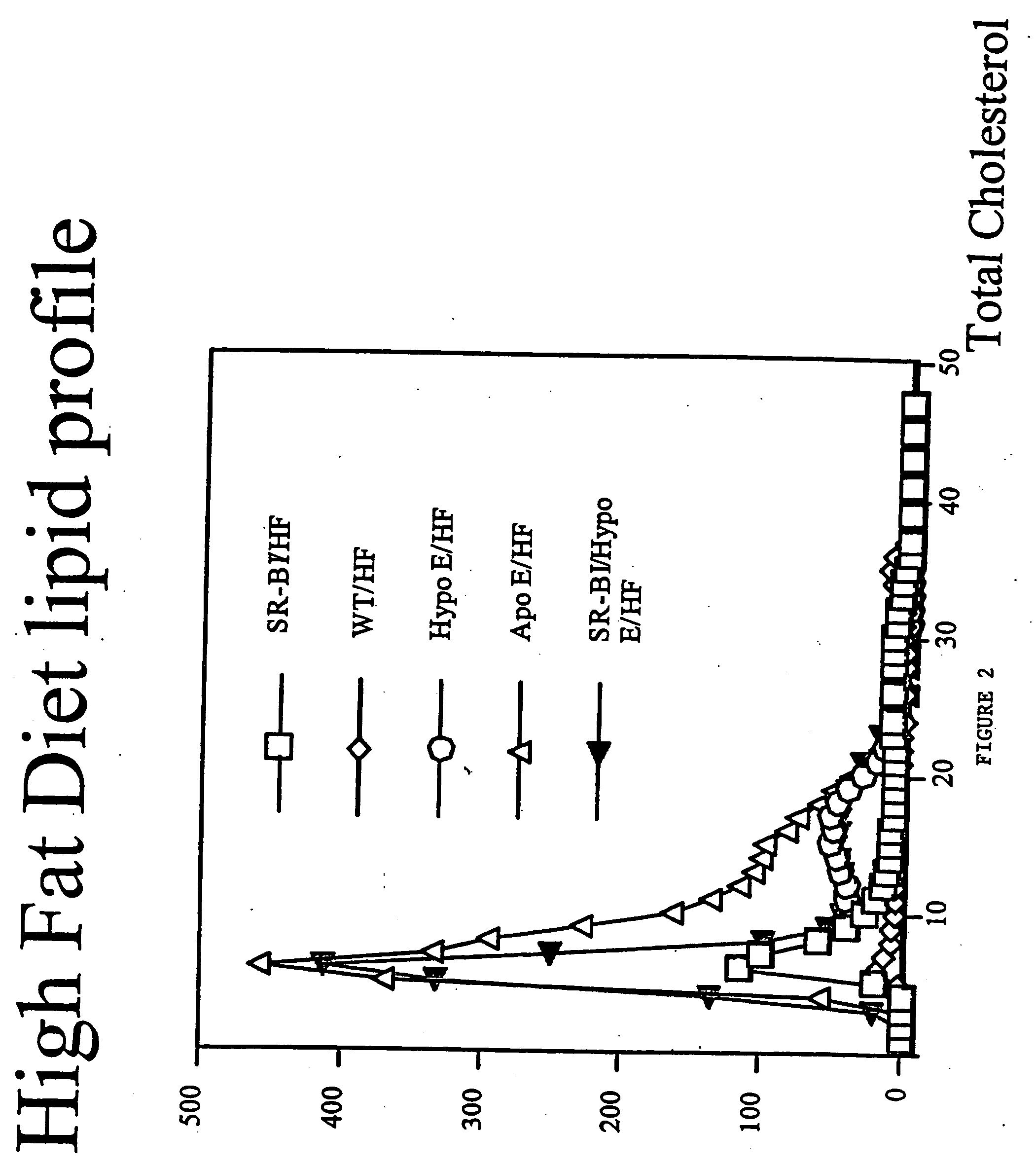
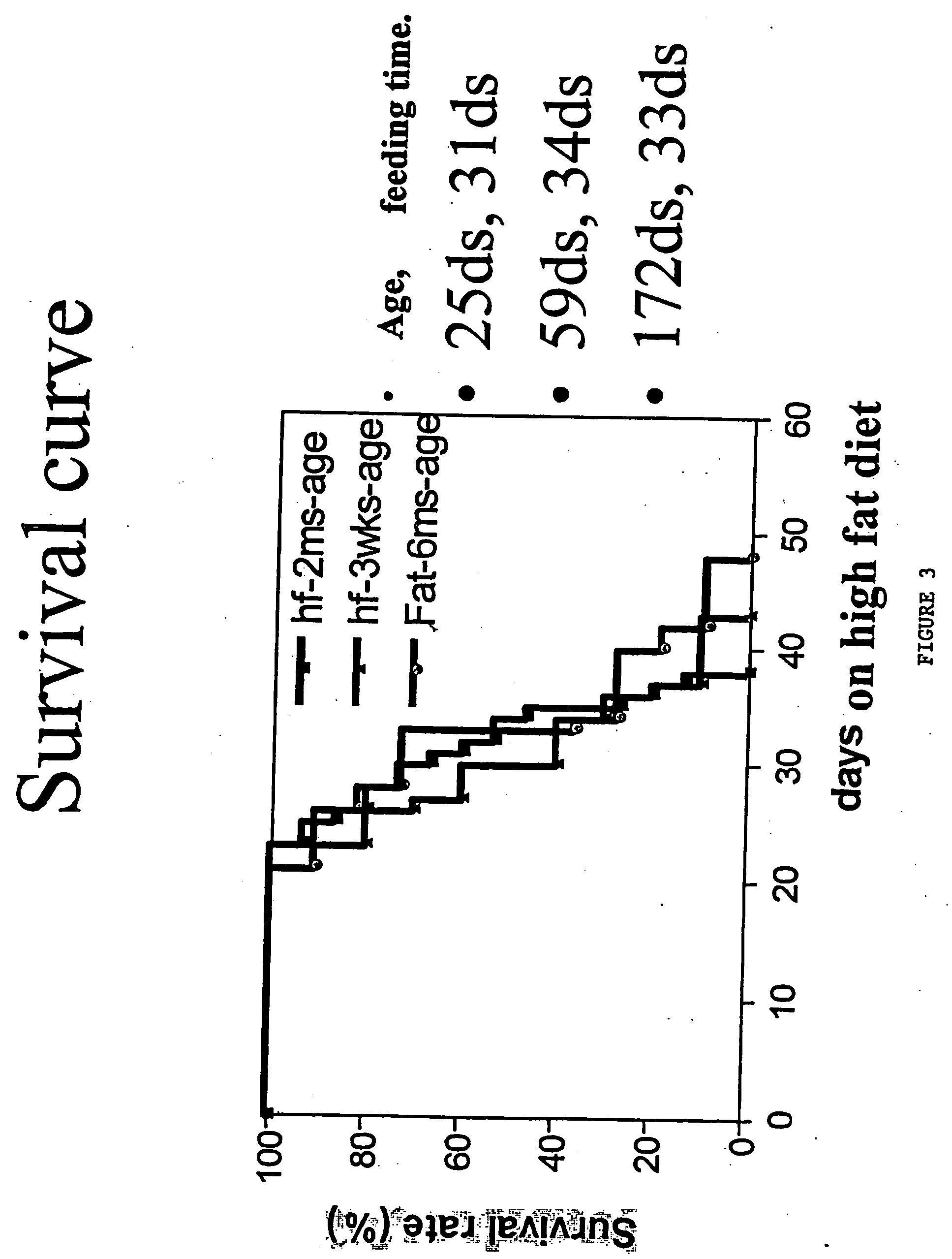
An animal model of coronary heart disease has been developed where myocardial infarct can be induced by altering the animal's diet. In all embodiments, this animal model is a result of reduced activity of scavenger receptor class BI (SR-BI) and apolipoprotein E (ApoE). In a preferred embodiment, the model is a result of crossbreeding two transgenic mouse lines: a knockout of SR-BI (SR-BI− / −) and an impaired ApoE expressor (hypoE). The impaired ApoE gene results in only 2-5% expression of ApoE and a reduction in cholesterol homeostasis. Resulting animals are predisposed to hypercholesterolemia but can live longer than a year on a normal low fat diet. Serum plasma levels can be significantly elevated by changing the animal's diet to one containing high levels of fat and cholesterol. Within a month on a high fat, high cholesterol diet, animals develop atherosclerosis and myocardial infarction occurs. Survival depends on the nature of the diet and the conditions of animal husbandry and can typically be around 20-30 days after administration of the modified diet depending on the specific conditions. Housing the animals alone or in groups significantly affects survival of these animals on a high fat diet. Analysis of B- and T-cell deficient SR-BI / ApoE / RAG2 triple knockout mice established that B- and T-lymphocytes do not play a key role in the pathophysiology of the SR-BI ApoE dKO model of human disease. These animal models can be used to study mechanisms and progression of CHD as a function of diet, treatment with drugs to be screened for efficacy or undesirable side effects, and social environmental effects.
Owner:MASSACHUSETTS INST OF TECH
Fast and quantitative PG (pepsinogen)II detection kit, production method thereof and PGII detection method
InactiveCN105467117AShort detection timeInexpensive Quantitative AssayDisease diagnosisMonoclonal antibodyQuantum dot
The invention relates to a fast and quantitative PG (pepsinogen)II detection kit, a production method thereof and a PGII detection method. The detection kit comprises a detection card and a sample buffer which are arranged in the kit, wherein a test strip in the detection card comprises a sample pad, a conjugate pad, a nitrocellulose membrane and absorbent paper which are in sequential lap joint; quantum dots coupled with one PGII monoclonal antibody are sprayed to the conjugate pad, and the nitrocellulose membrane is coated with another PGII monoclonal antibody as a detection line and is further coated with a goat-anti-mouse antibody or another anti-mouse antibody as a quality control line. According to the detection kit, the quantum dots are combined with immunochromatography, the detection kit has the characteristics of short detection time and high efficiency and can detect the stock of PGII very well. A quantum dot-immunochromatographic method is adopted, the content of the PGII in serum, plasma and whole blood is detected non-invasively, safely, accurately, fast and quantitatively with low risk and low cost, and an auxiliary function can be provided for gastric disease examination and monitoring.
Owner:SHENZHEN BLOT BIOTECH
Novel serum/plasma proteome analysis method
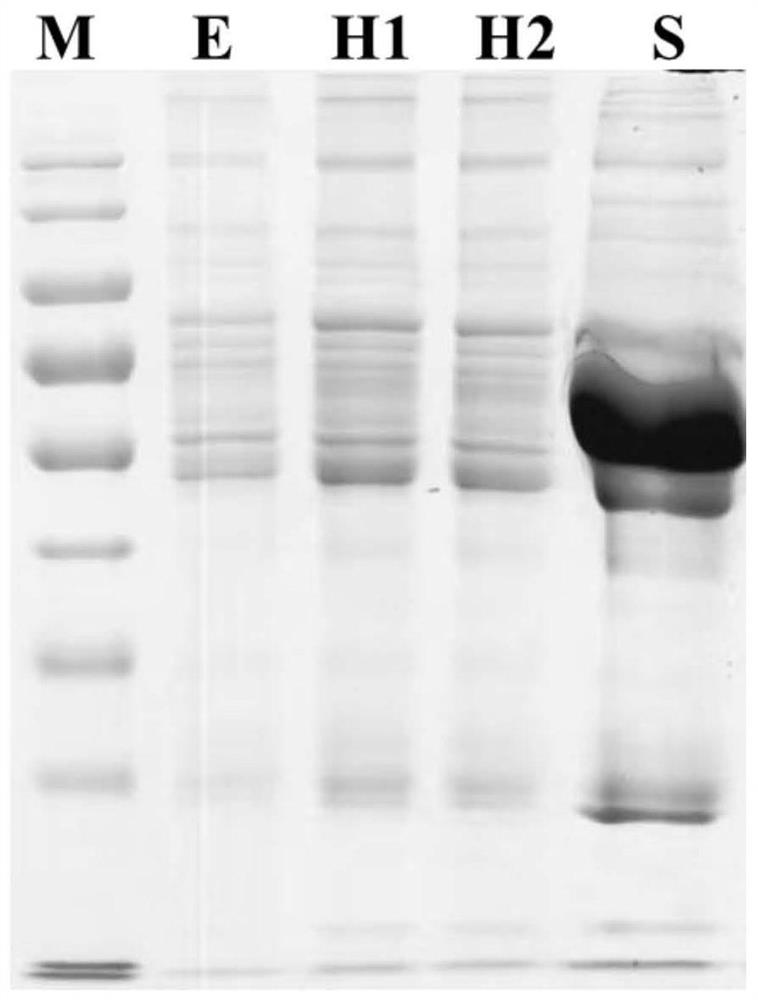
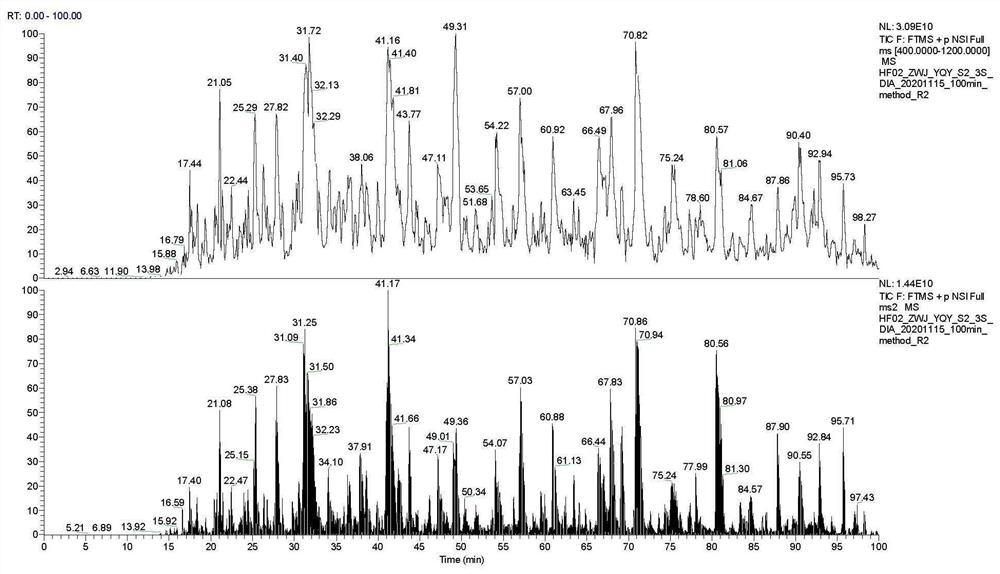
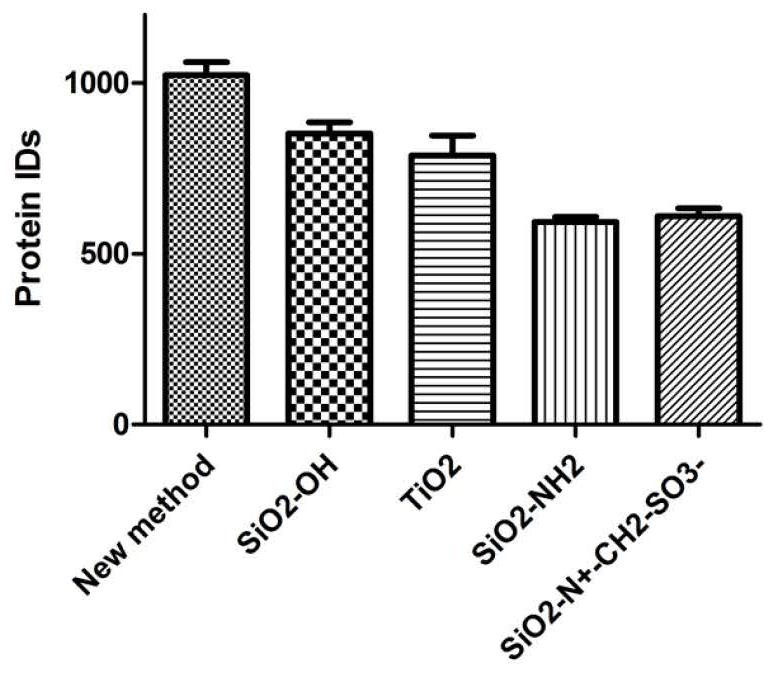
ActiveCN112710755AEasy to prepareVisible removal effectComponent separationAgainst vector-borne diseasesBlood plasmaMass spectrometric
The invention discloses a novel serum / plasma proteome analysis method. The mixed nanoparticles modified by different groups can effectively adsorb different types of serum / plasma proteins, selective enrichment of low-abundance proteins in serum / plasma is realized, and interference of high-abundance proteins in serum / plasma on low-abundance protein identification during mass spectrometry identification is effectively avoided. By combining reversed-phase C18 column online separation and a mass spectrometric data independent data acquisition mode, qualitative and quantitative analysis of more than 1500 serum / plasma proteins under 100 min chromatographic gradient can be realized. The method disclosed by the invention has the following advantages that an expensive commercialized serum / plasma high-abundance protein removal device is not needed, and selective enrichment of low-abundance proteins in serum / plasma can be completed only by using cheap and easily available nanoparticles; and according to the method, enrichment of low-abundance protein in serum / plasma can be completed only through four links of incubation, centrifugal cleaning and centrifugation, and errors caused by artificial complex operation are avoided.
Owner:ACADEMY OF MILITARY MEDICAL SCI
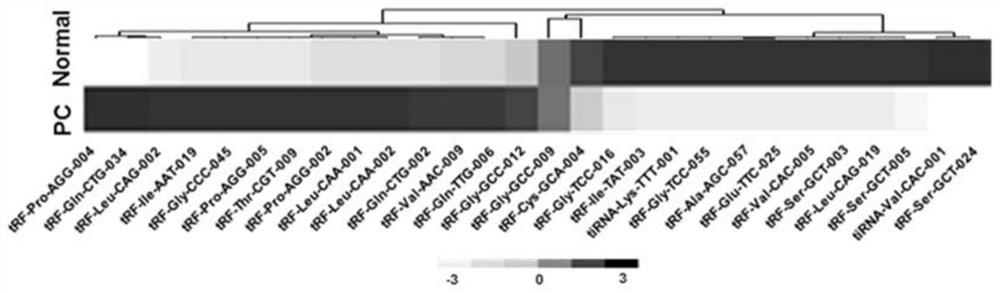
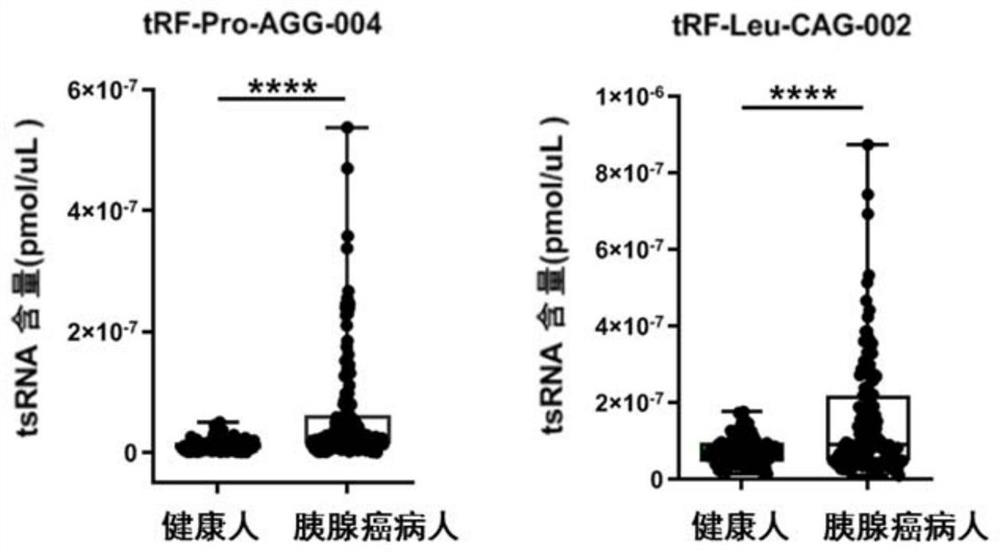
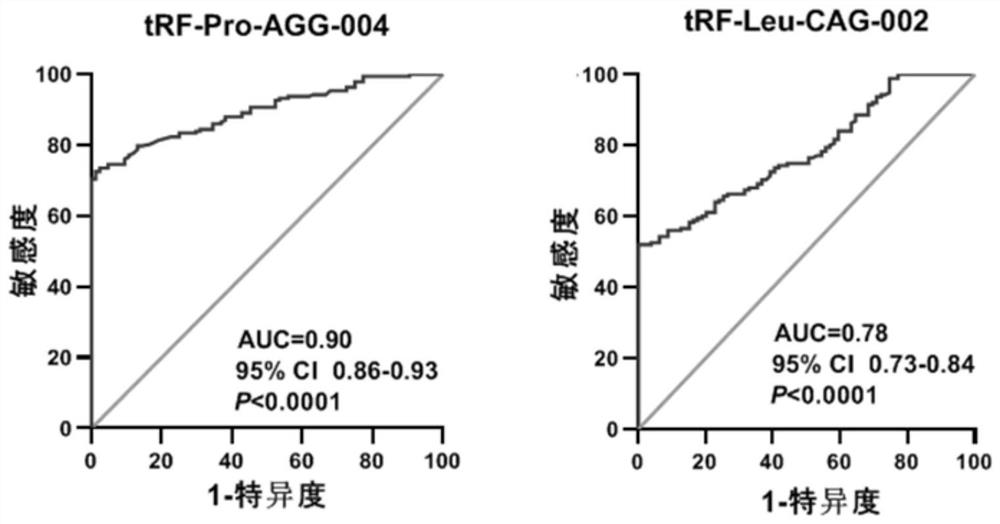
Pancreatic cancer-associated serum/plasma tsRNA marker and probe and application thereof
ActiveCN111826444AIncreased sensitivityImprove featuresMicrobiological testing/measurementDNA/RNA fragmentationProtein markersPancreas Cancers
The invention belongs to the field of molecular biology, and discloses a pancreatic cancer-associated serum / plasma tsRNA marker and application thereof. The marker is tRF-Pro-AGG-004 and / or tRF-Leu-CAG-002. The tsRNA screened by the invention has significantly higher specificity and sensitivity for detection of pancreatic cancer patients than protein markers currently used in clinical practice, and greatly improves the accuracy of diagnosis. The marker can be used to prepare a diagnostic kit for auxiliary early diagnosis of pancreatic cancer.
Owner:NANJING UNIV
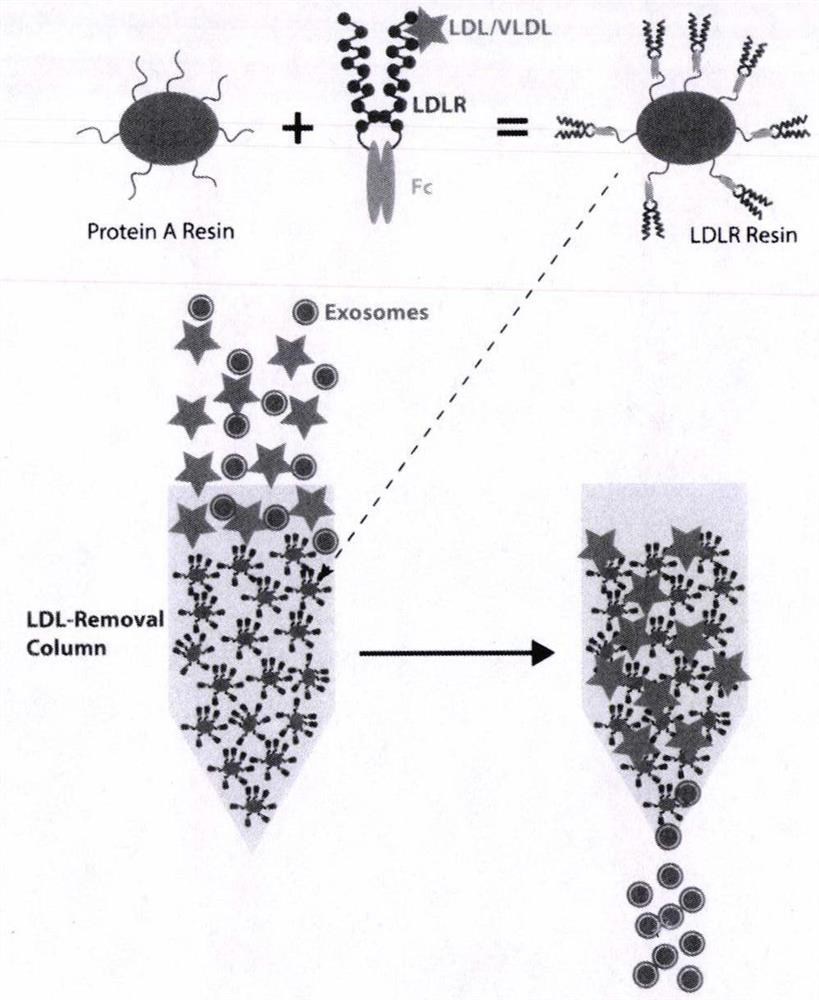
Extraction method and application of exosome
PendingCN113774008ACell dissociation methodsMicrobiological testing/measurementSurface markerA lipoprotein
The invention discloses an extraction method of exosomes. The method mainly comprises the operation steps: passing serum / plasma (a sodium citrate blood collection tube) through an LDLR affinity chromatography column, and enriching and concentrating the collected liquid with oxide. According to the invention, LDLR affinity chromatography is adopted to remove low-density lipoprotein in serum / plasma, and oxide is adopted to concentrate a collected buffer solution, so that a method for purifying exosome from serum is established. According to the invention, particle number is detected through nanoflow, exosome surface markers are detected through western blot, the transmission electron microscope detection results show that yield of the extracted exosome is high, the extracted exosome is further combined with a splitting method to release the internal substances of the exosome, and nucleic acid and protein can be subjected to the subsequent research. The method has advantages of simple operation steps, short extraction time, high purity of prepared exosomes, suitability for small samples and high cost performance, and is suitable for popularization and application in the field of in vitro diagnosis.
Owner:光武惠文生物科技(北京)有限公司 +1
PGI rapid quantitative detection kit and making method and detection method thereof
InactiveCN105548556AShort detection timeAccurate quantitative detectionDisease diagnosisBiological testingDisease monitoringNon traumatic
The invention relates to a PGI rapid quantitative detection kit and a making method and detection method thereof. The detection kit comprises a detection card and a sample buffer solution disposed in the kit, the test strip in the detection card comprises a sample pad, a conjugate pad, a nitrocellulose membrane and absorbent paper that are lapped in order. PGI monoclonal antibody coupled quantum dots are sprayed to the conjugate pad, the nitrocellulose membrane is coated with another strain of PGI monoclonal antibody as a detection line, and is also coated with goat-anti-mouse antibody or other anti-mouse antibodies as a quality control line. The kit provided by the invention combines quantum dot and immunochromatography technologies, has the characteristics of short detection time and high efficiency, and can well detect the storage of PGI. The kit and the detection method provided by the invention utilize quantum dot immune chromatography technology, which has the characteristics of short detection time and high efficiency, and can detect the stock of very well. The invention uses quantum dot immunochromatography, can realize non-traumatic, low risk, safe, cheap, accurate and rapid quantitative detection of PGI content in serum, plasma and whole blood, and can provide assistance for stomach disease inspection and disease monitoring.
Owner:SHENZHEN BLOT BIOTECH
Method for predicting cancer and other diseases
ActiveUS8815519B2Microbiological testing/measurementAntibody ingredientsUrokinase Plasminogen ActivatorReceptor
Owner:HVIDOVRE HOSPITAL
Kit for detecting immuno-gold synchronous scattering spectrum of human serum complement 3 and use method thereof
InactiveCN101718780ASimple and fast operationEasy to operateScattering properties measurementsBiological testingBlood plasmaComplement 3
The invention discloses a kit for detecting immuno-gold synchronous scattering spectrum of human serum complement 3 and a use method thereof. The kit comprises three reagents, wherein the reagent 1 is a calibrator containing the frozen dry plasma protein reference serum of the complement 3, the reagent 2 contains 111.5-164.5mM of Na2HPO4, 17.5-45.0mM of citric acid solution and 50-70g / ml of PEG6000, and the reagent 3 contains gold-labeled goat anti-human C3 antibody and 0.03-0.06 g / L of PEG20000. The use method comprises the following steps: firstly preparing a C3 standard series, adding the reagent 1, reagent 2 and reagent 3 according to a defined ratio, performing constant volume process, incubating for 15min in a ultrasonic reactor, scanning the synchronous scattering spectrum with a fluorescence spectrophotometer to detect the synchronous scattering value, and calculating the C3 content according to the standard curve. The kit of the invention can be used to accurately and qualitatively detect the complement C3, be suitable for the clinical and scientific research analysis of complement 3 in samples such as serum and plasma, and have the advantages of convenient, fast and sensitive operation, low detection limit, wide linear range, simple phase separation process and low sample consumption.
Owner:GUANGXI NORMAL UNIV
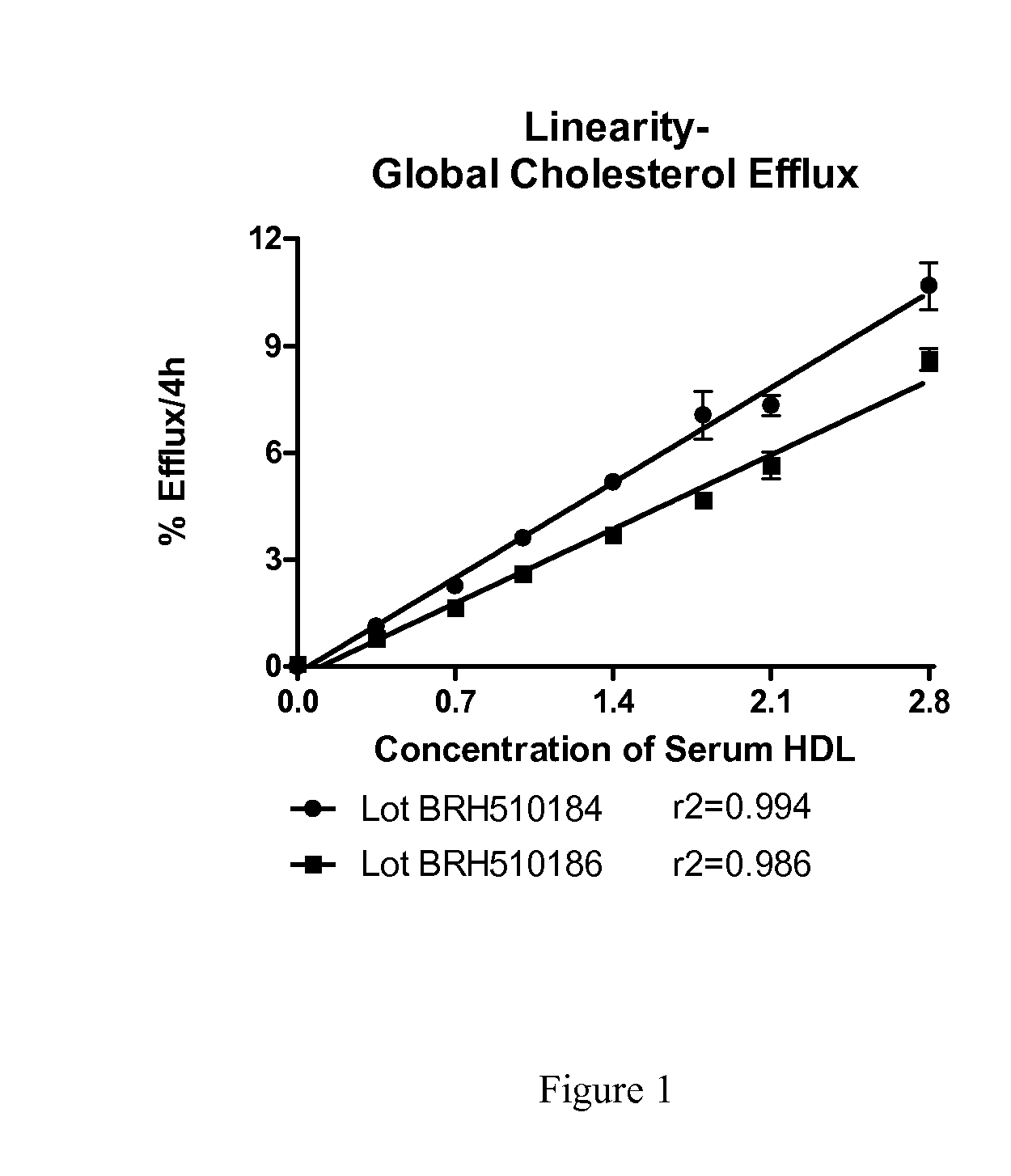
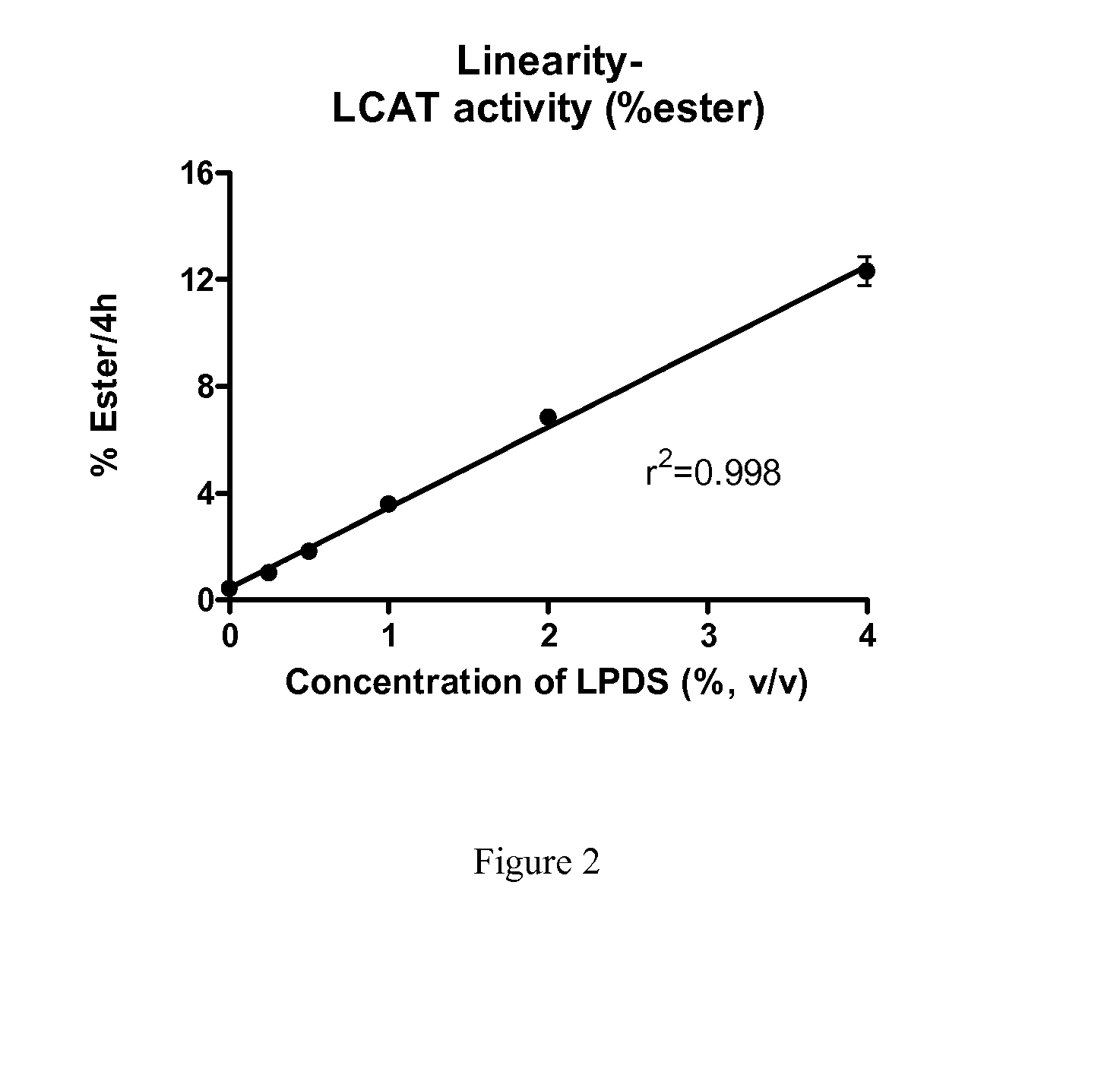
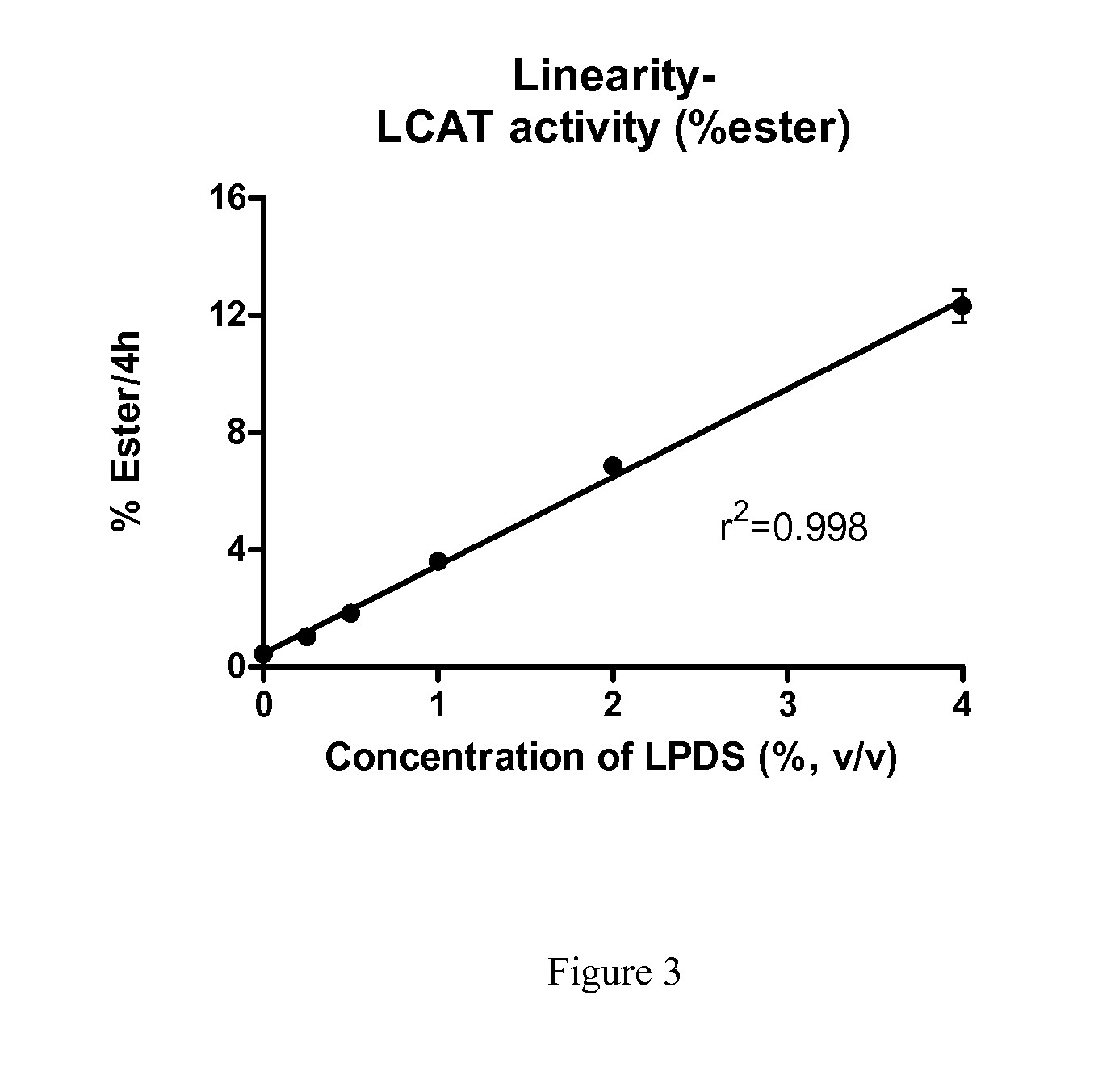
Method to Measure Endogenous Enzymatic Serum/Plasma Cholesterol Esterification by LCAT (Lecithin:Cholesterol Acyltransferase) Assay
InactiveUS20140322735A1Improvement to the biologic relevanceReduce usageMicrobiological testing/measurementBiological material analysisPhysiologyLCAT activity
The present invention provides a method for the assessment of cholesterol esterification through physiologically relevant pathways and incorporates the function of individual subject's endogenous HDL particles. This ex vivo approach avoids the use of an artificial substrate and provides for determination of LCAT activity that includes both the contribution of a subject's endogenous HDL function and endogenous LCAT protein, and is therefore a significant improvement to the biologic relevance.
Owner:VASCULARSTRATEGIES
Methods for inactivating pathogens using broad-spectrum pulsed light
InactiveCN1344170AImprove methodReliable methodPeptide/protein ingredientsInactivation/attenuationBovine Viral Diarrhea VirusesWhole blood product
A method of reducing pathogen content in a biologically derived composition by irradiating it with at least one high-intensity short-duration pulse of broad-spectrum incoherent polychromatic light. The biomolecule of interest retains biological activity in the resulting treated composition. Biologically derived compositions, such as serum, plasma or other blood products, including insulin, transferrin, heparin, collagen, coagulation factor VIII and / or coagulation factor IX, or containing monoclonal antibodies, or genetically engineered cell lines, etc. The content of pathogens, such as viruses, bacteria, pyrogens, fungi and / or prions present in the resulting protein composition is reduced by irradiation with broad spectrum pulsed light. A single pulse of broad spectrum light significantly reduces the amount of HIV-1, SV40, canine parvovirus or bovine viral diarrhea virus in a biologically derived composition.
Owner:PUREPULSE TECH
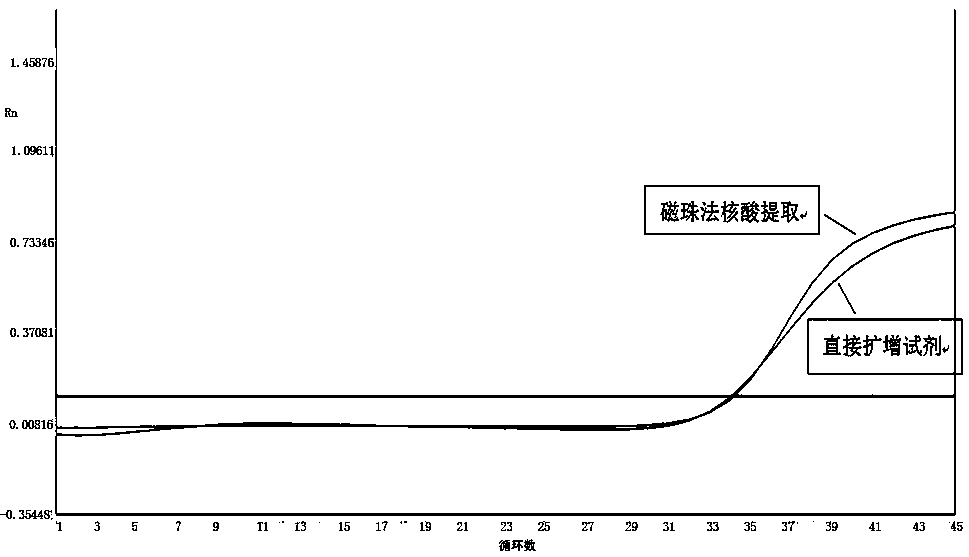
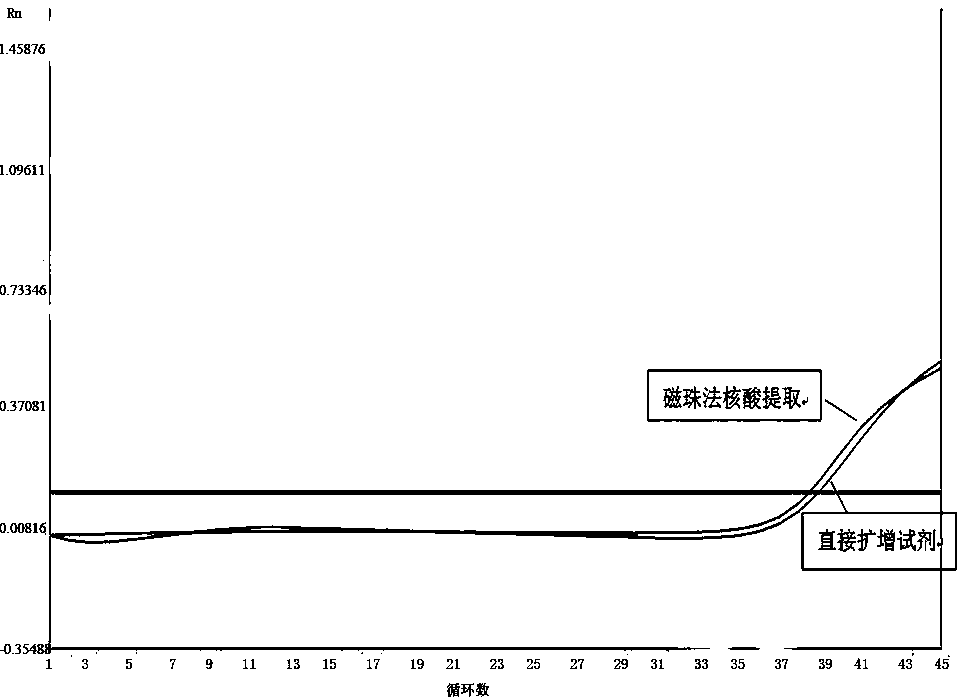
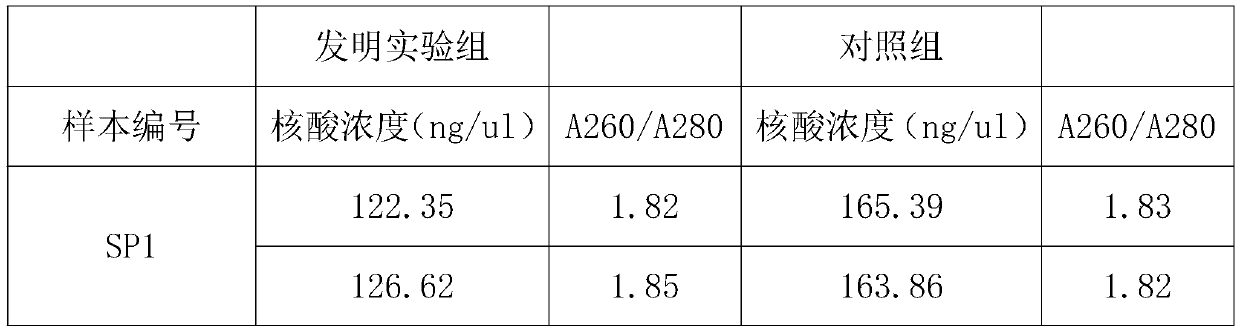
Nucleic acid extraction method based on nano magnetic beads and application of nucleic acid extraction method
InactiveCN110628762AExtract a wide range of samplesAutomatic extractionMicrobiological testing/measurementDNA preparationMagnetic beadTissue fluid
The invention discloses a nucleic acid extraction method based on nano magnetic beads. The nucleic acid extraction method comprises the following steps: step 1, adding a lysis buffer to a biological sample, and then forming a lysis solution containing nucleic acid after splitting and precipitating; step 2, adding nano magnetic beads to form magnetic beads-nucleic acid complex, applying an externalmagnetic field to aggregate the magnetic beads-nucleic acid complex; step 3, removing the lysis solution under the action of the external magnetic field, and adding a washing buffer to wash the magnetic beads-nucleic acid complex; step 4, removing the magnetic field, adding an elution buffer to elute the magnetic beads-nucleic acid complex, and then obtaining the required nucleic acid. The invention also discloses an application of the nucleic acid extraction method. The nucleic acid extraction method is used to extract the nucleic acid from faeces, whole blood, serum and plasma, tissue fluid, swab lotion, tissue homogenate, sputum, urine and saliva. The nucleic acid extraction method of the invention has advantages of realizing automatic, efficient, high-throughput extraction and purification of the nucleic acid, and convenient extraction operations.
Owner:中诺(杭州)基因科技有限责任公司
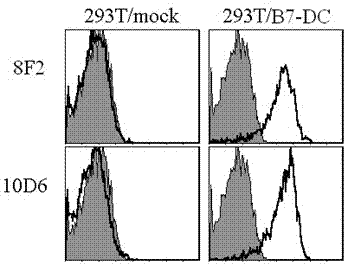
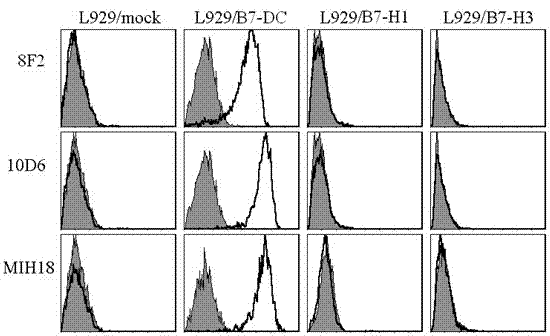
Human soluble B7-DC quantitative detection kit
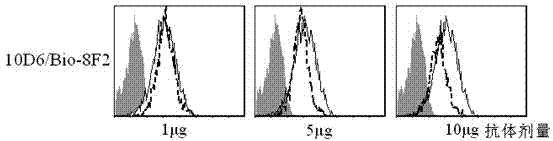
ActiveCN102250910AStrong specificityHigh sensitivityImmunoglobulins against animals/humansMicroorganism based processesAntiendomysial antibodiesTherapeutic effect
The invention discloses a kit capable of quantitatively detecting soluble B7-DC. The kit comprises a horse radish peroxidase marker, tetramethyl benzidine serving as a reaction substrate, bovine serum albumin, an elisa plate, a wash solution and a stop solution, and is characterized by further comprising a coated antibody, standard proteins and a detection antibody, wherein the coated antibody isa mouse anti-human B7-DC monoclonal antibody 8F2; and the detection antibody is a mouse anti-human B7-DC monoclonal antibody 10D6. The kit capable of quantitatively detecting soluble B7-DC has high specificity, can be used for accurately and quantitatively analyzing the concentrations of soluble B7-DC protein factors in liquids such as human cell culturing supernatant, blood serum, blood plasma, hydrothorax and the like, and can be applied to clinical differential diagnosis and treatment effects of asthma.
Owner:苏州市第五人民医院 +1
Antibody diluent for enzyme-linked immunoassay and preparation method thereof
PendingCN111077299ALong storage timeSimple systemMaterial analysisSodium lactateAntiendomysial antibodies
The invention provides an antibody diluent for enzyme-linked immunoassay and a preparation method of the antibody diluent, and relates to the technical field of enzyme-linked immunoassay. The antibodydiluent for enzyme-linked immunoassay comprises the following components, by weight, 80 to 90 parts of a 0.01 M PBS buffer solution, 0.5-1.5 parts of bovine serum albumin, 0.6-1.2 parts of cold fishskin gel, 0.1-0.2 parts of sodium trinitride, 0.3-0.5 parts of a Proclin 300 preservative, 0.2-0.4 parts of sodium lactate, 0.4-0.6 parts of gentamicin sulfate and 1-2 parts of an anticoagulant, wherein the 0.01 M PBS buffer solution is prepared from monopotassium phosphate, disodium hydrogen phosphate, sodium chloride, potassium chloride, tween-20 and deionized water. In the invention, preparation raw materials are reasonably used, and a system of the antibody diluent is optimized so that components of the antibody diluent of serum and plasma and peripheral blood are more reasonable, preservation time of the diluted antibody is effectively prolonged, and the antibody diluent is beneficial to medical use.
Owner:WUHAN KANGZHU BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com