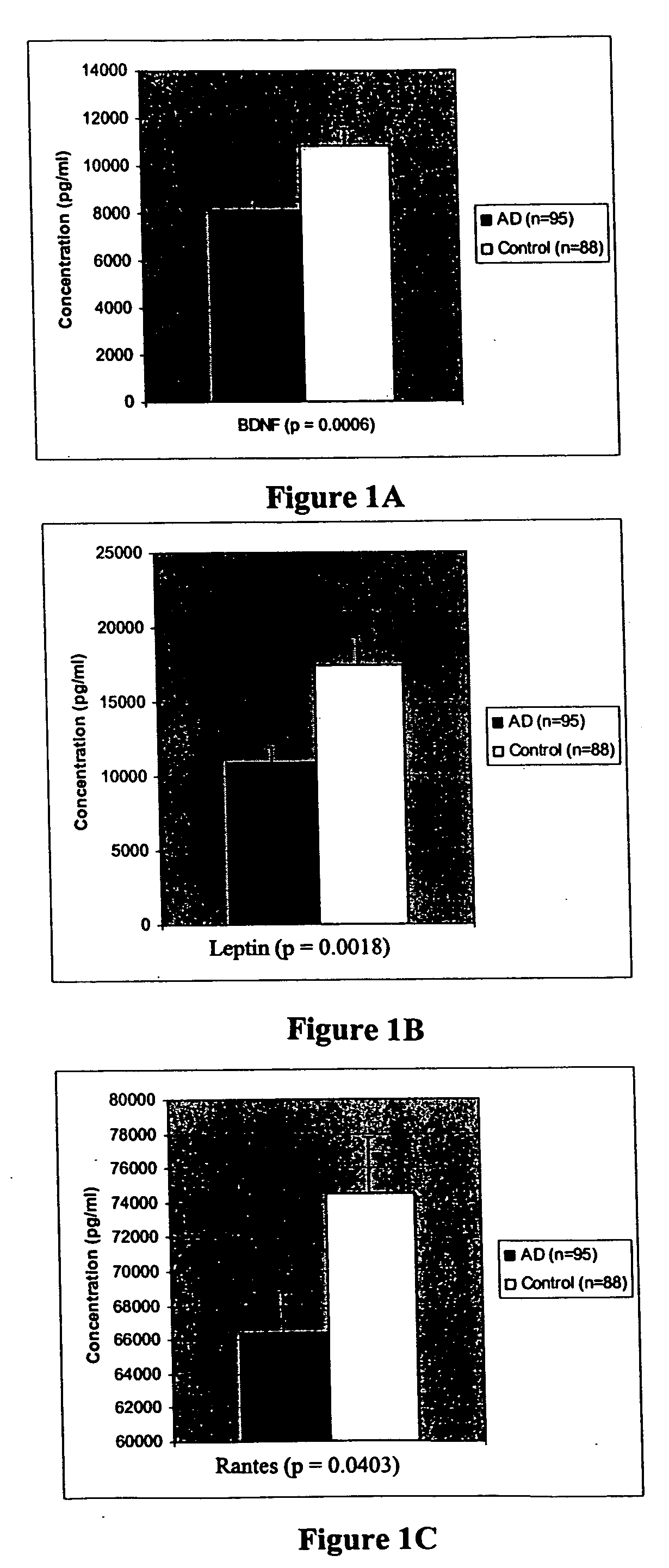
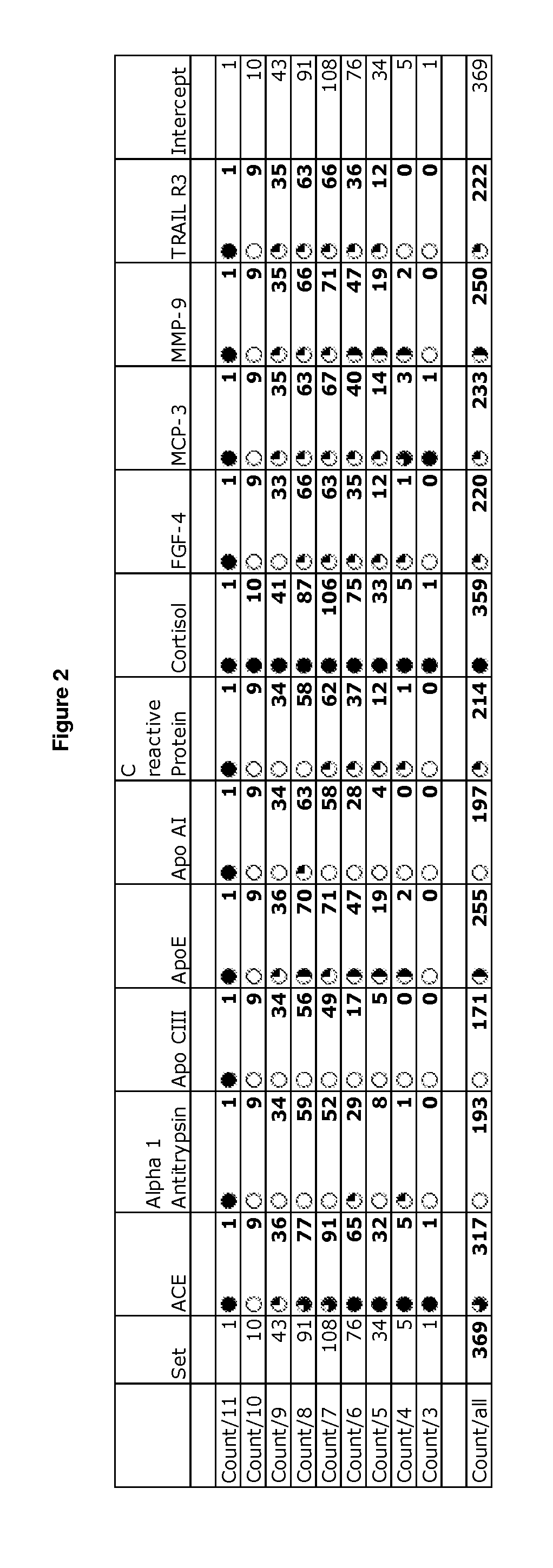
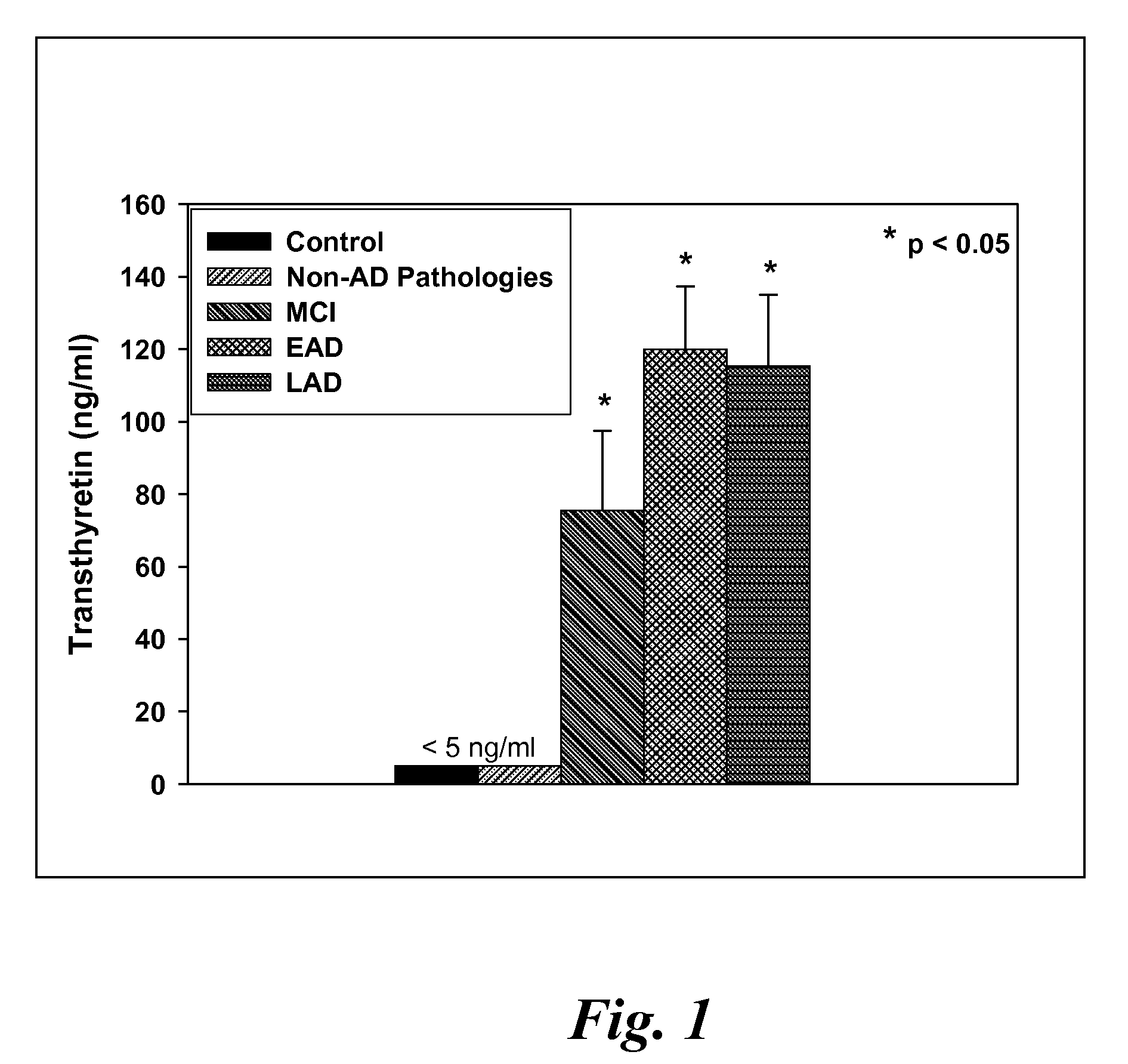
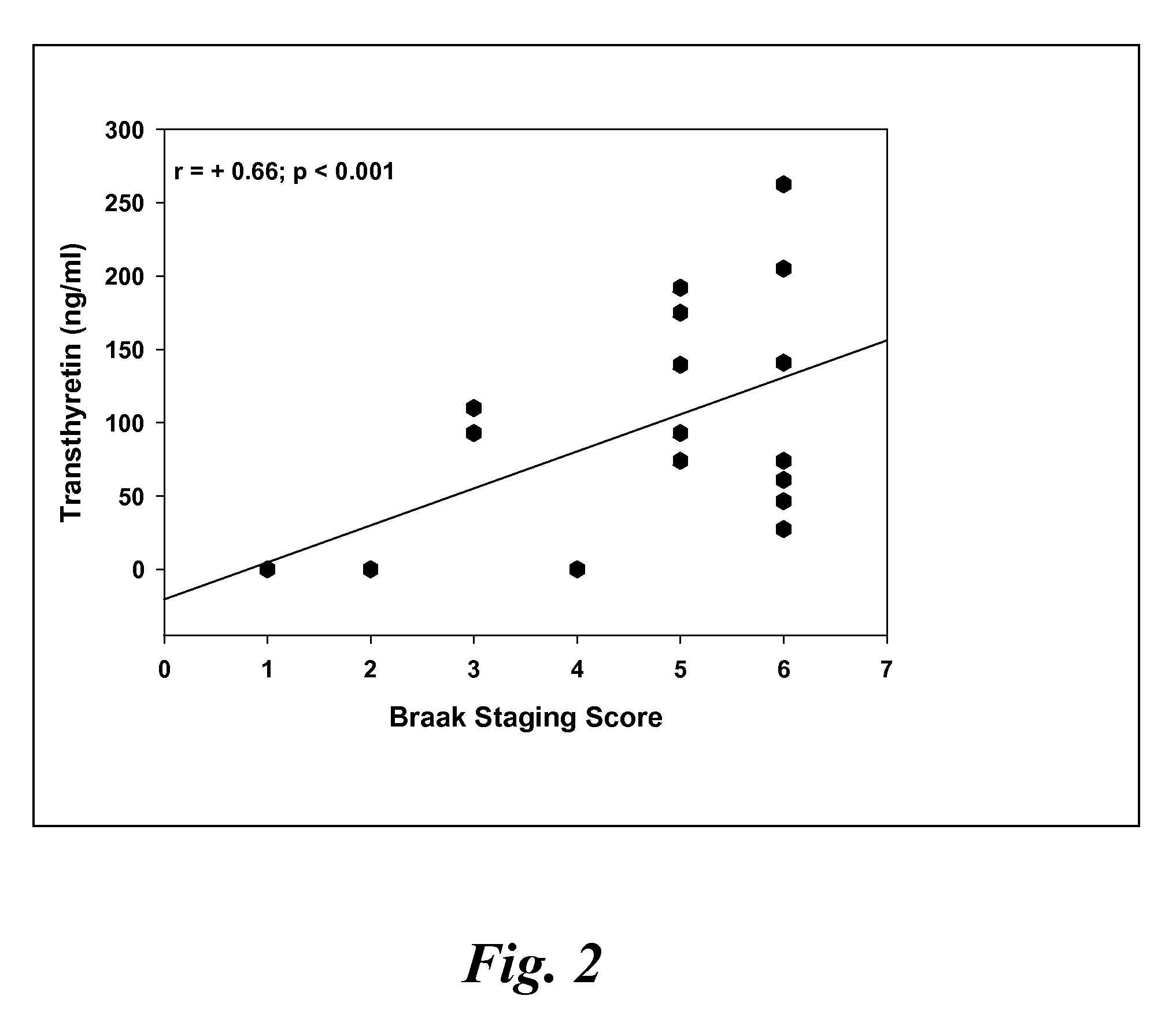
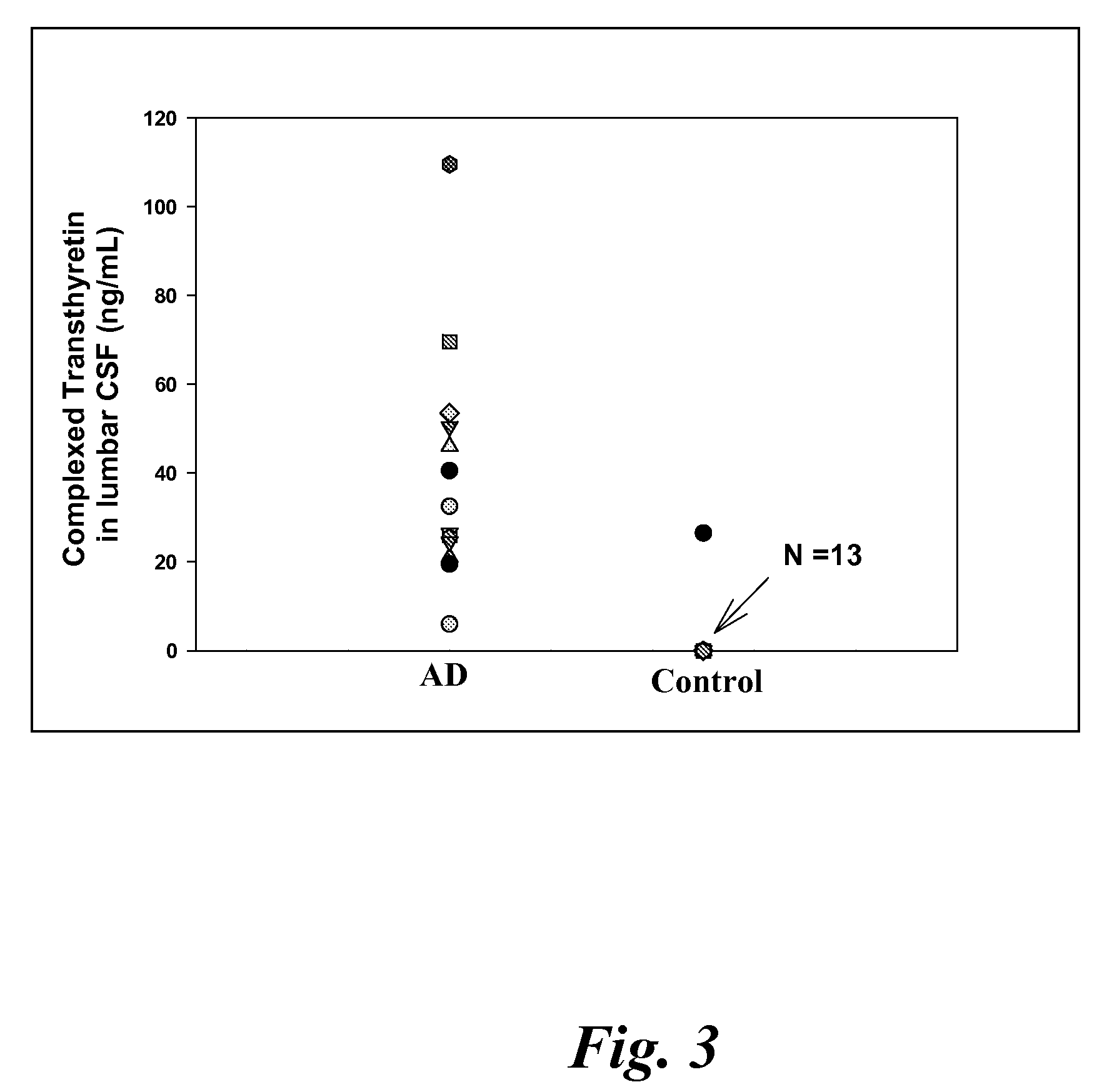
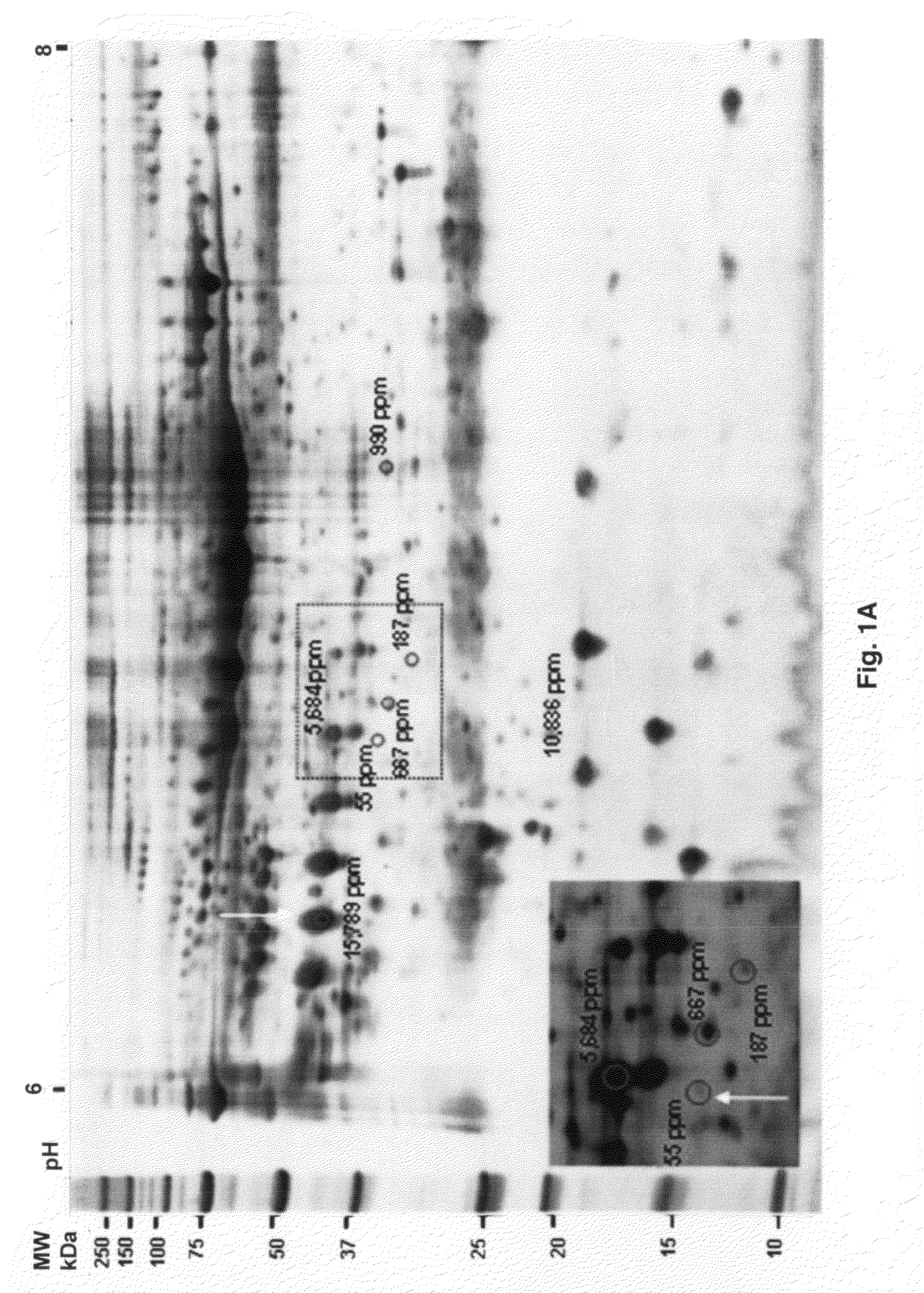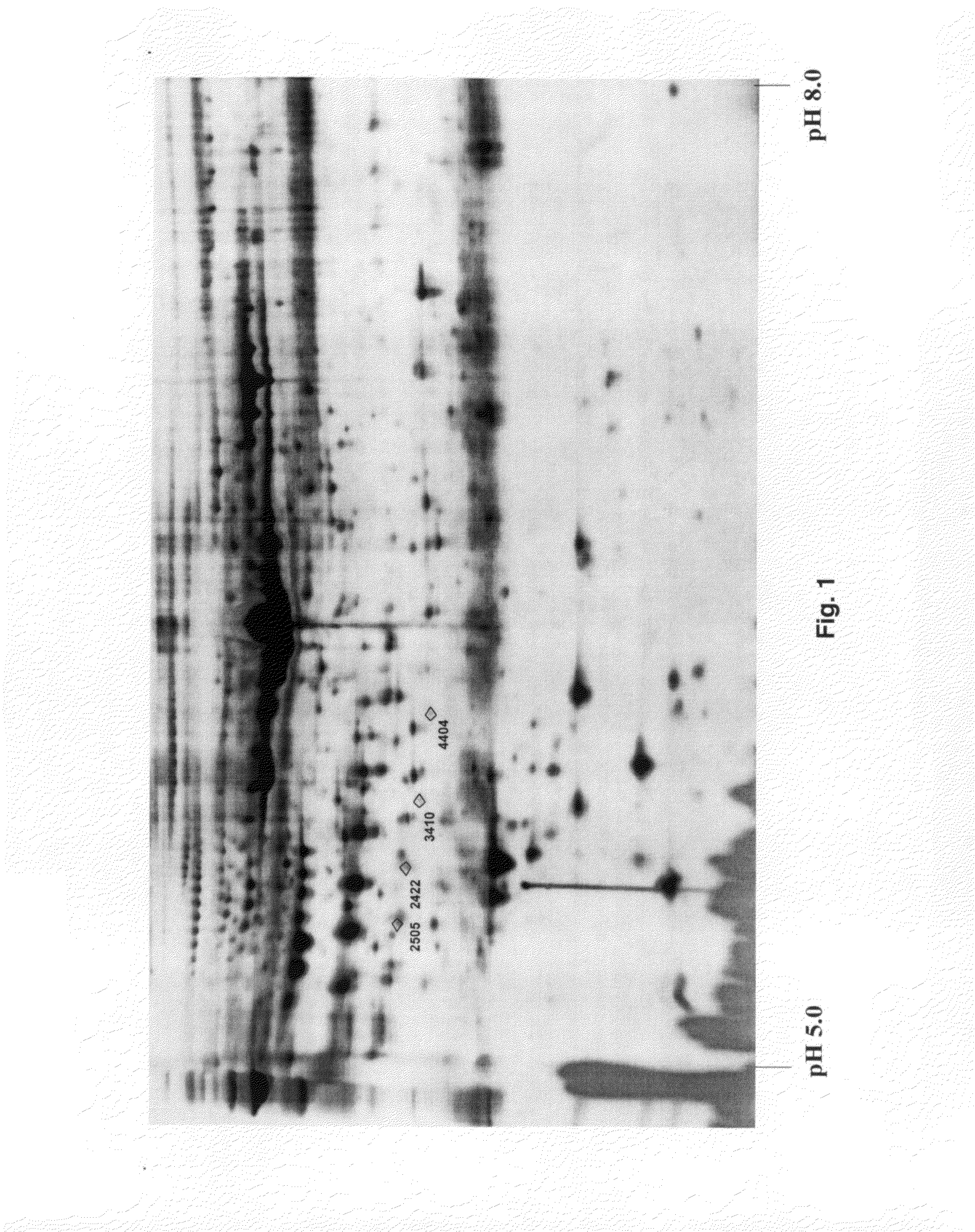Patents
Literature
184 results about "Protein biomarkers" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Protein biomarkers. Proteins are particularly useful molecules to use as biomarkers as they are often the effectors of diseases and the targets of therapeutic treatments. Using panels of protein biomarkers, healthcare experts can perform accurate disease diagnosis through convenient non-invasive testing.
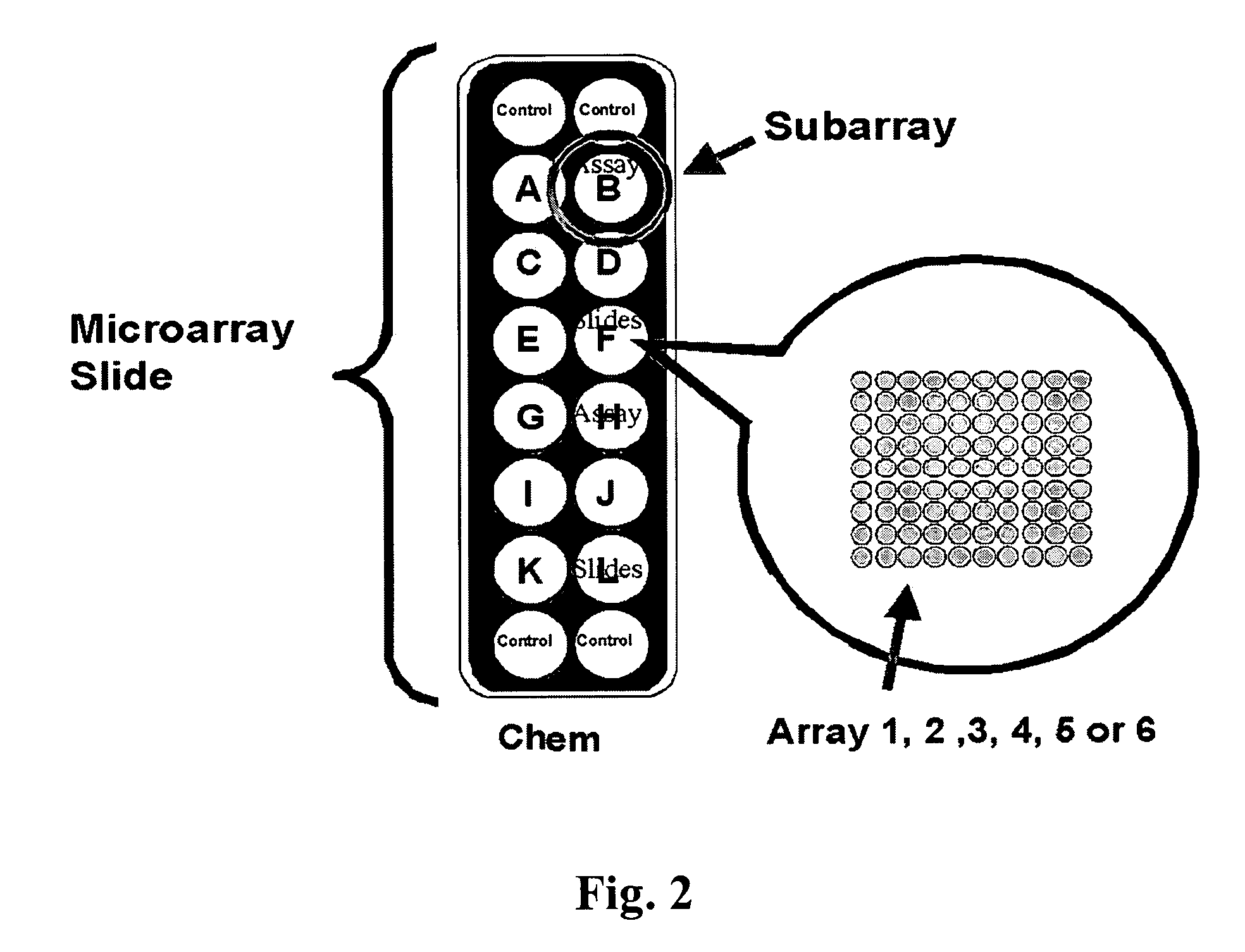
Assays based on liquid flow over arrays
InactiveUS20060275852A1Highly credible resultLow coefficient of variationBioreactor/fermenter combinationsBiological substance pretreatmentsAssayAnalyte
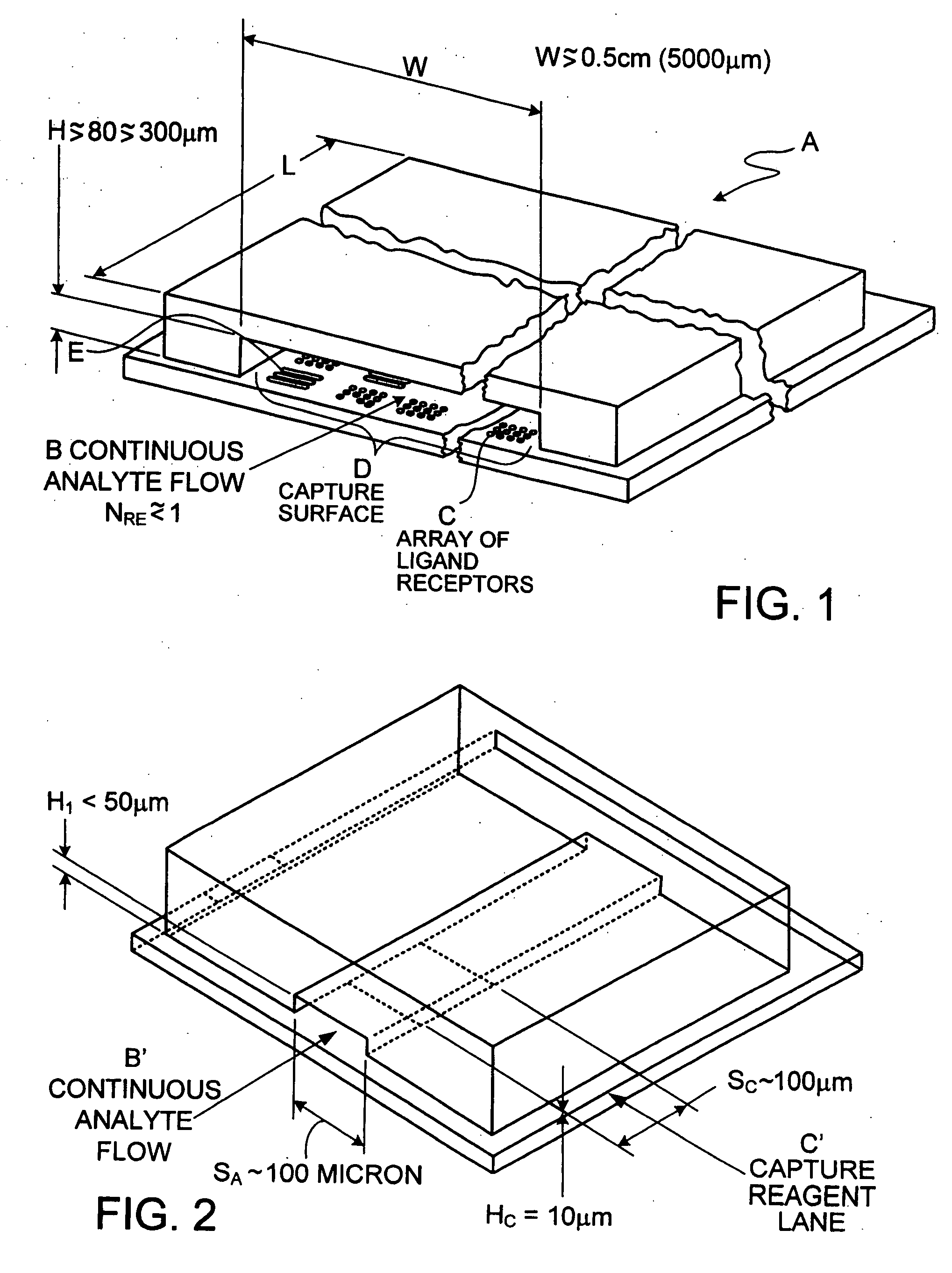
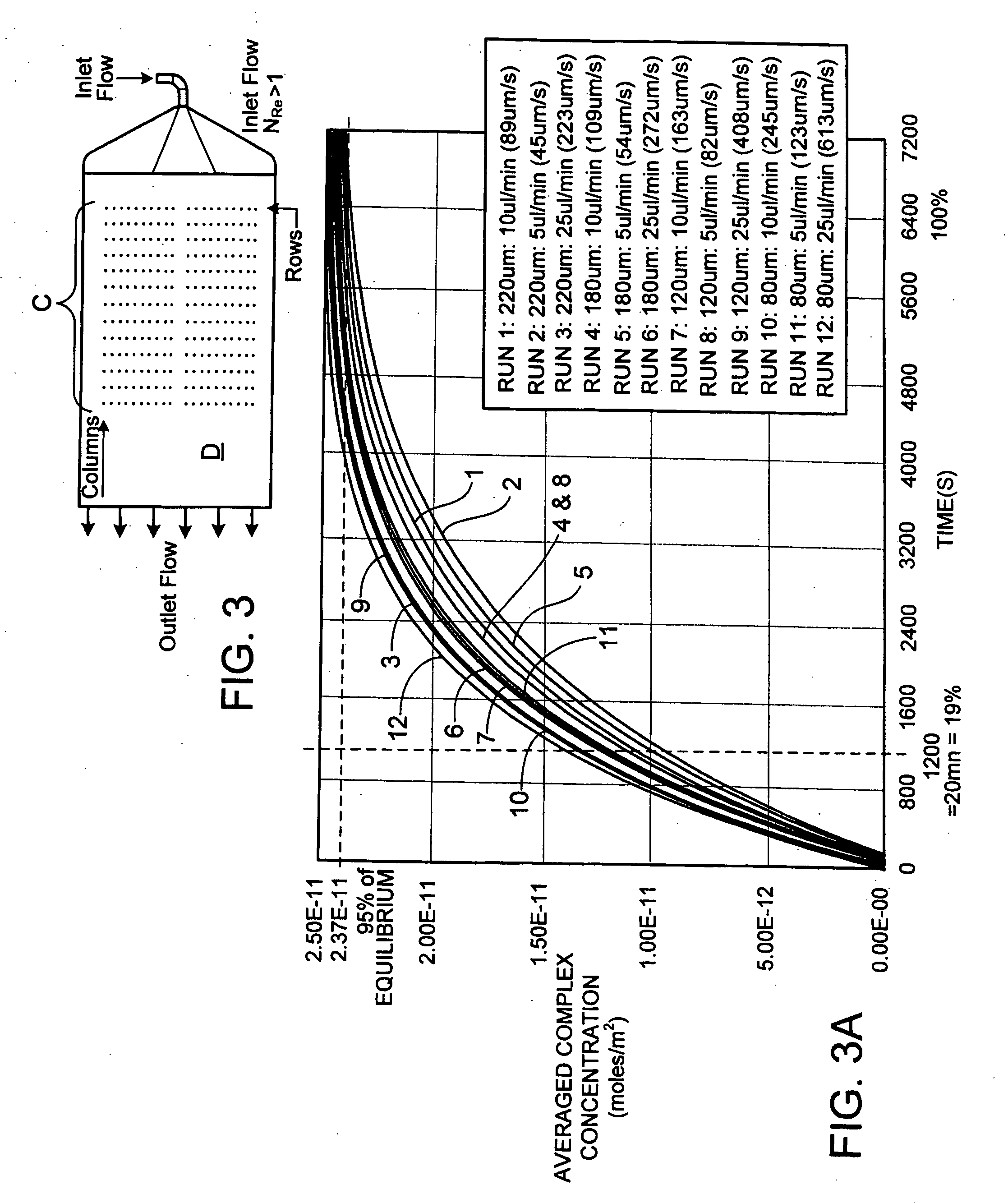
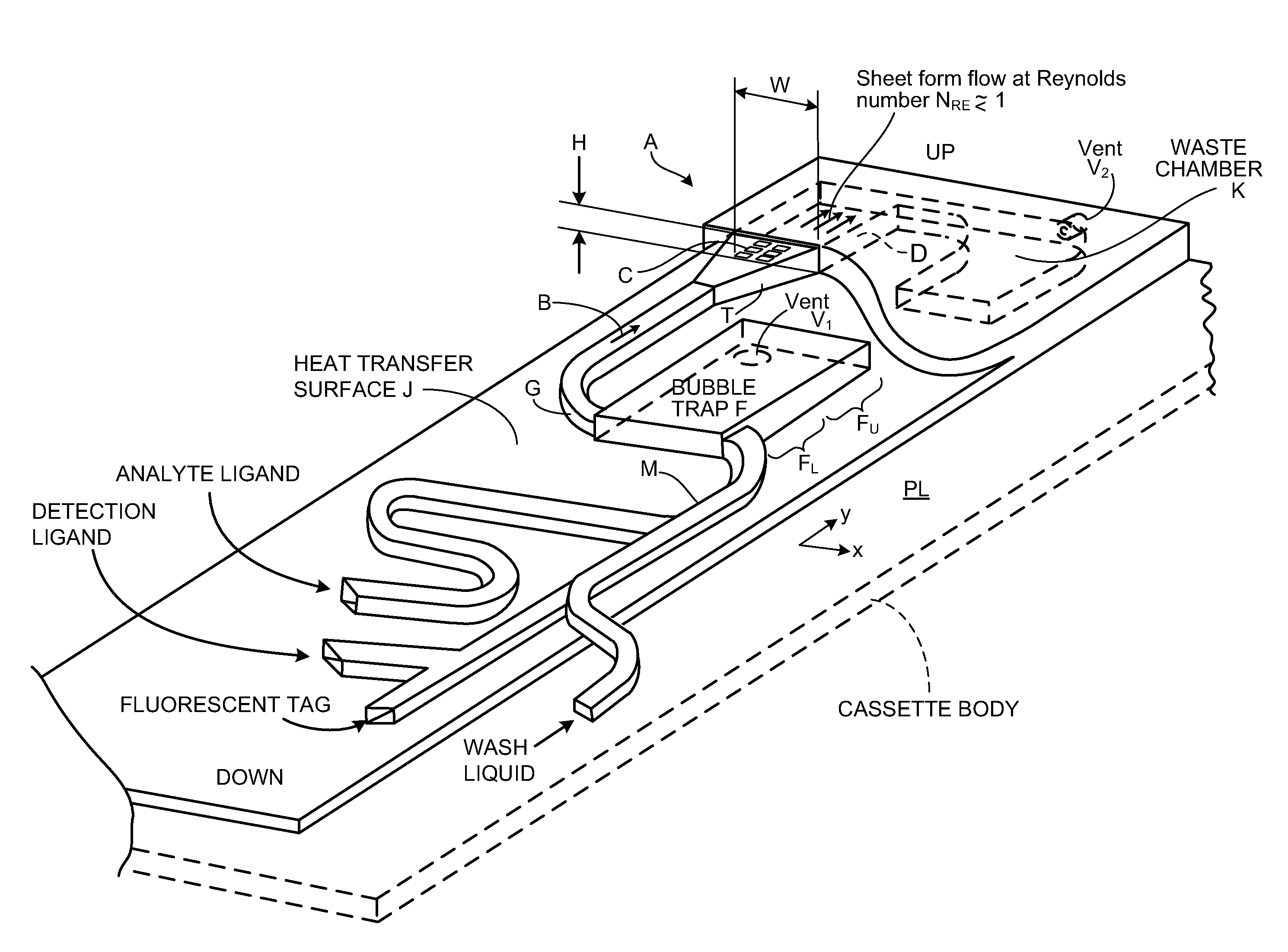
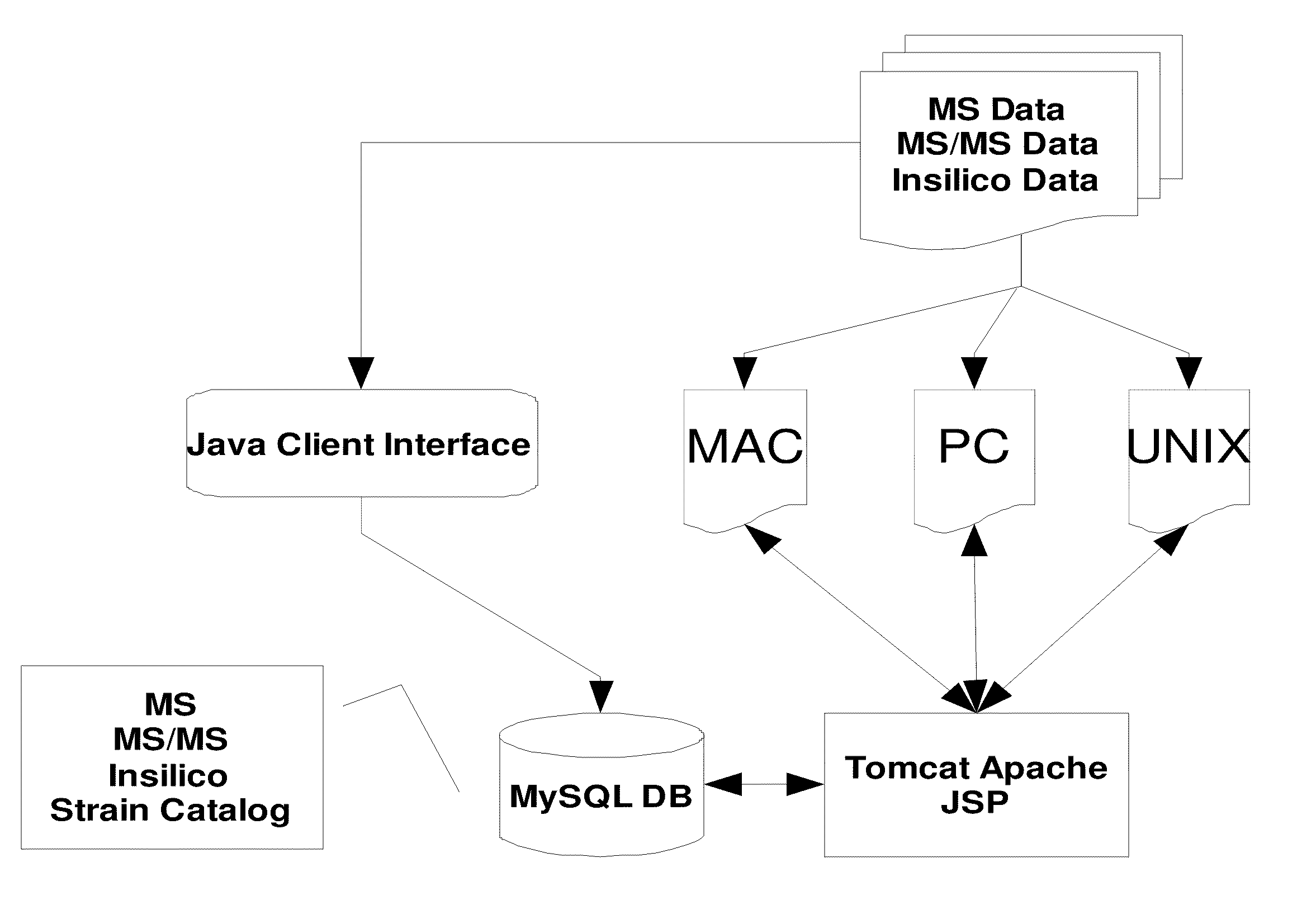
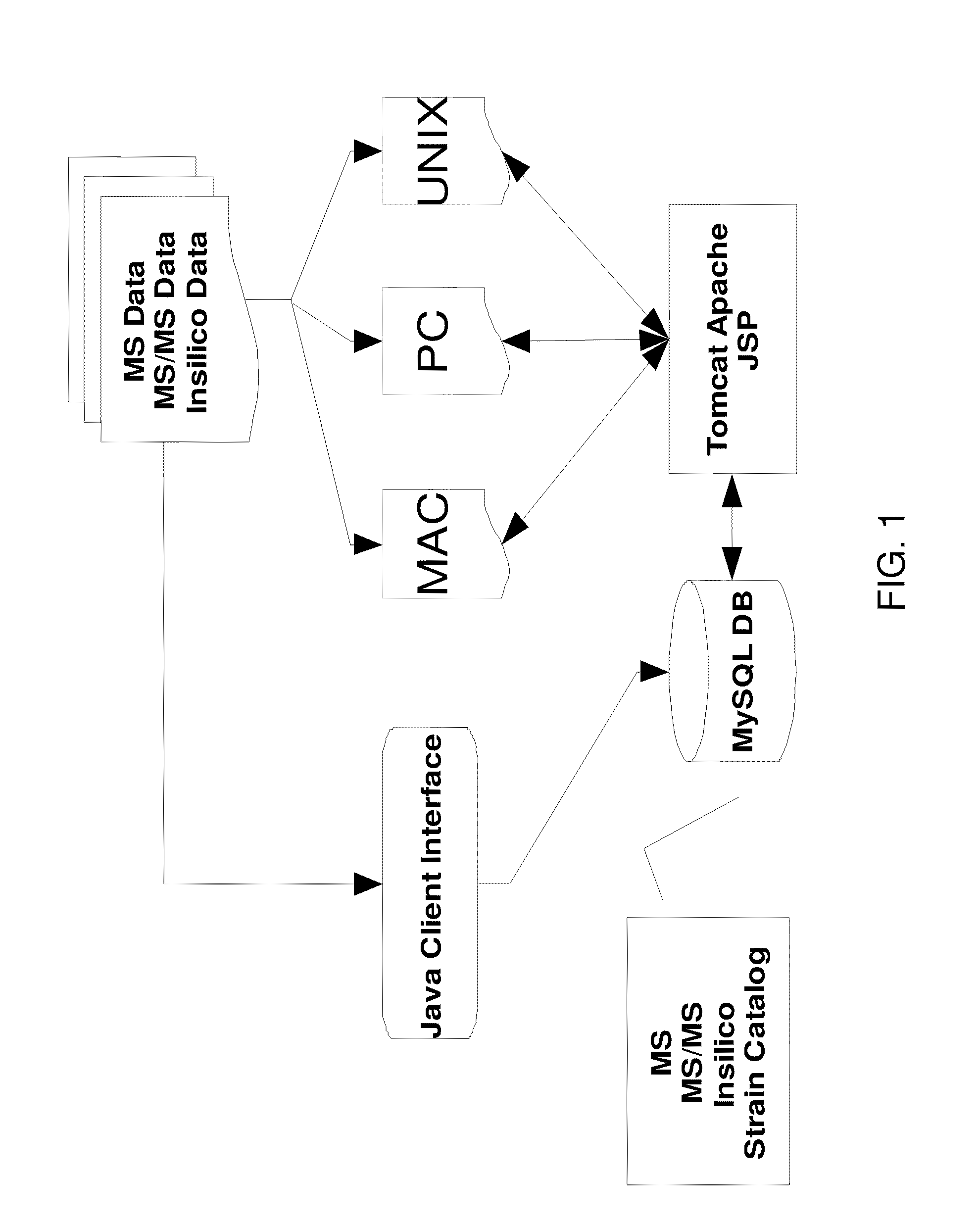
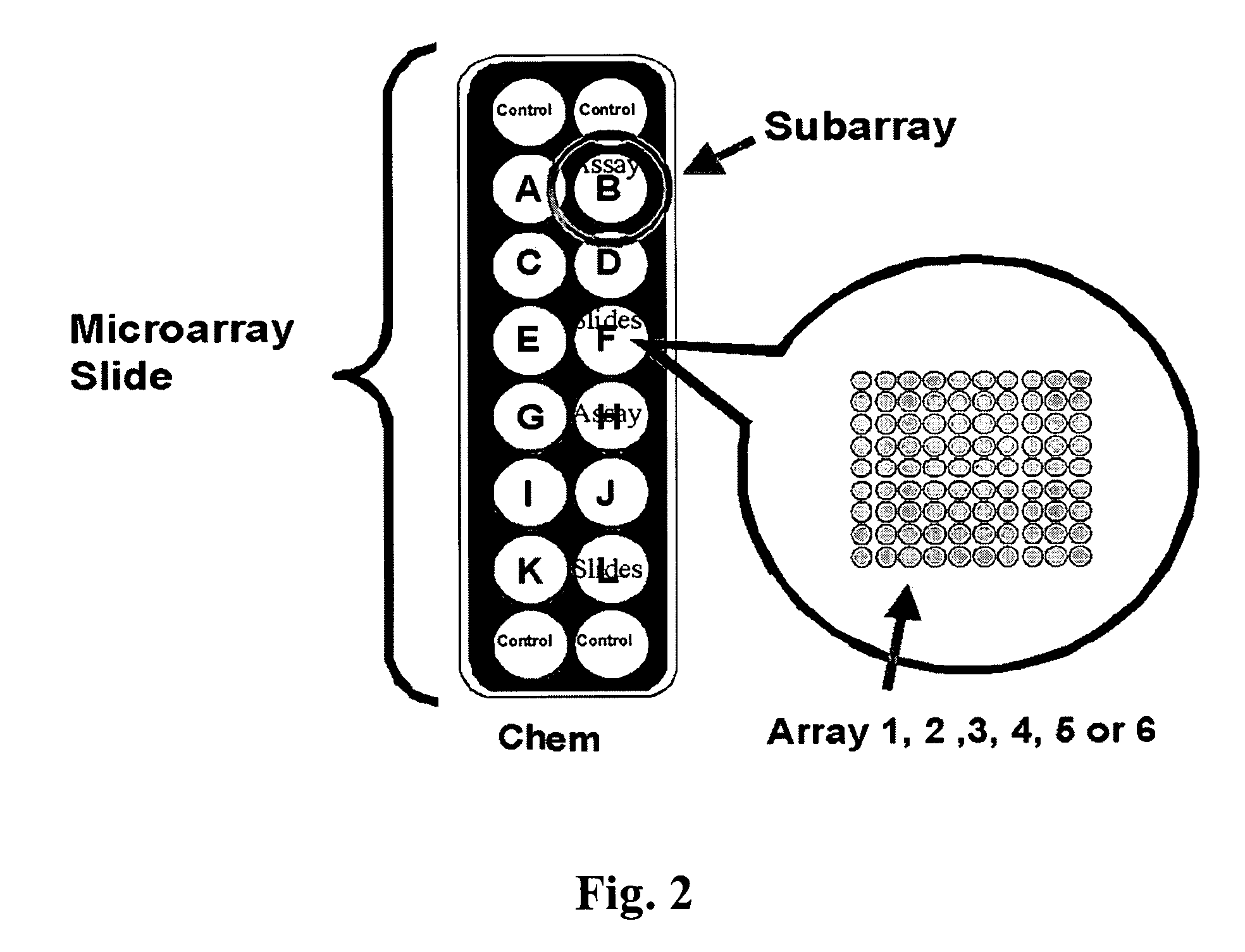
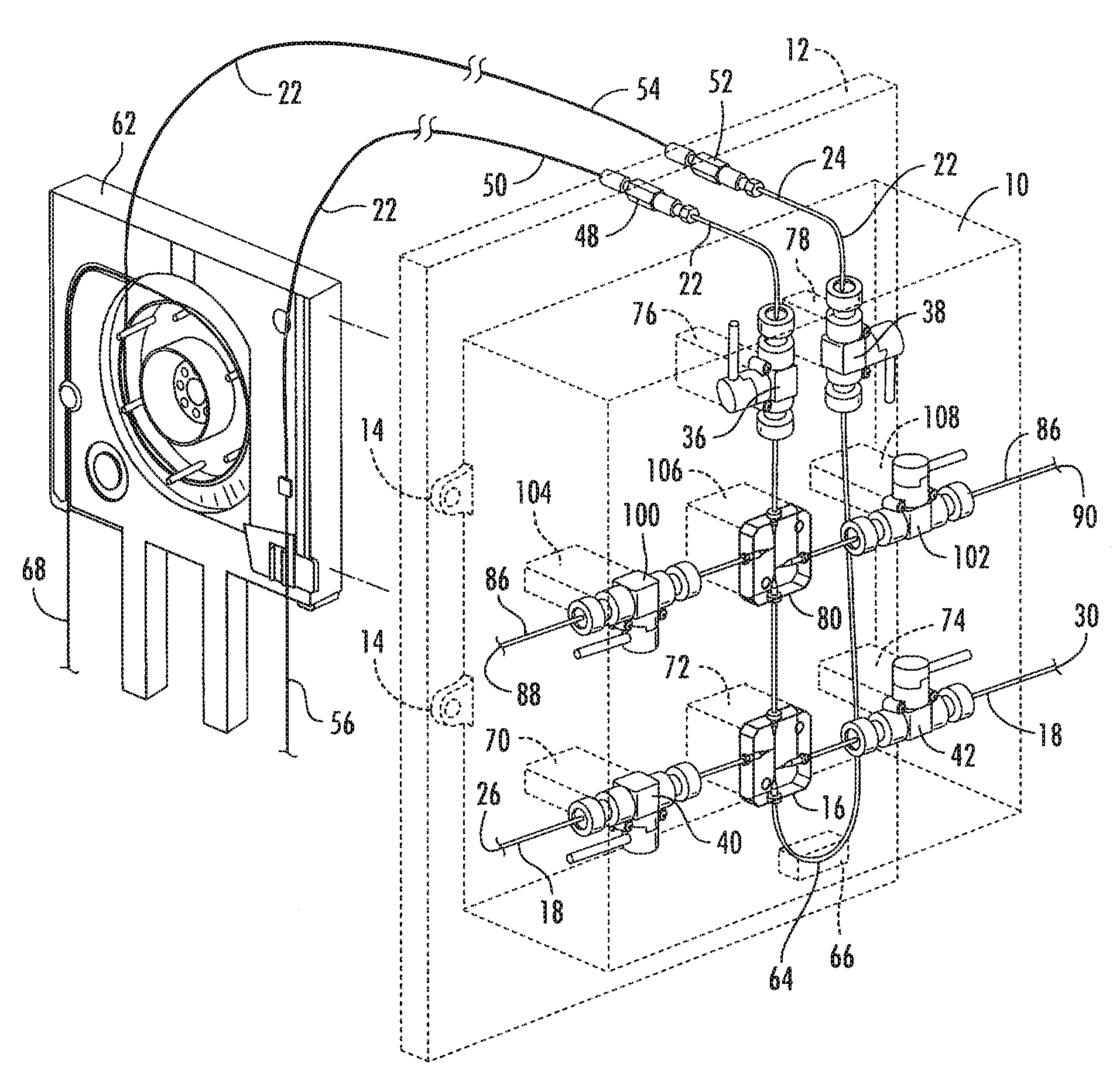
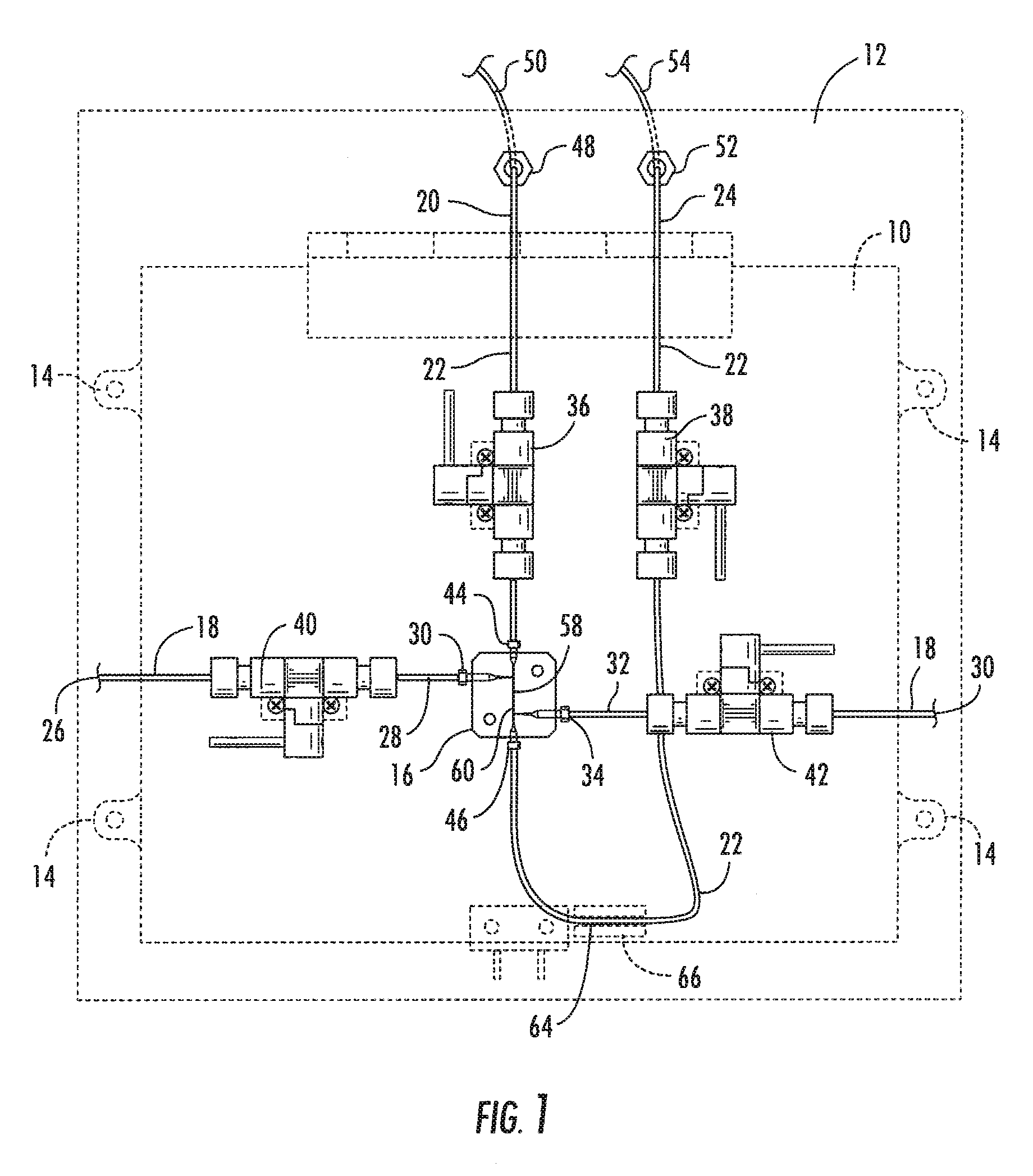
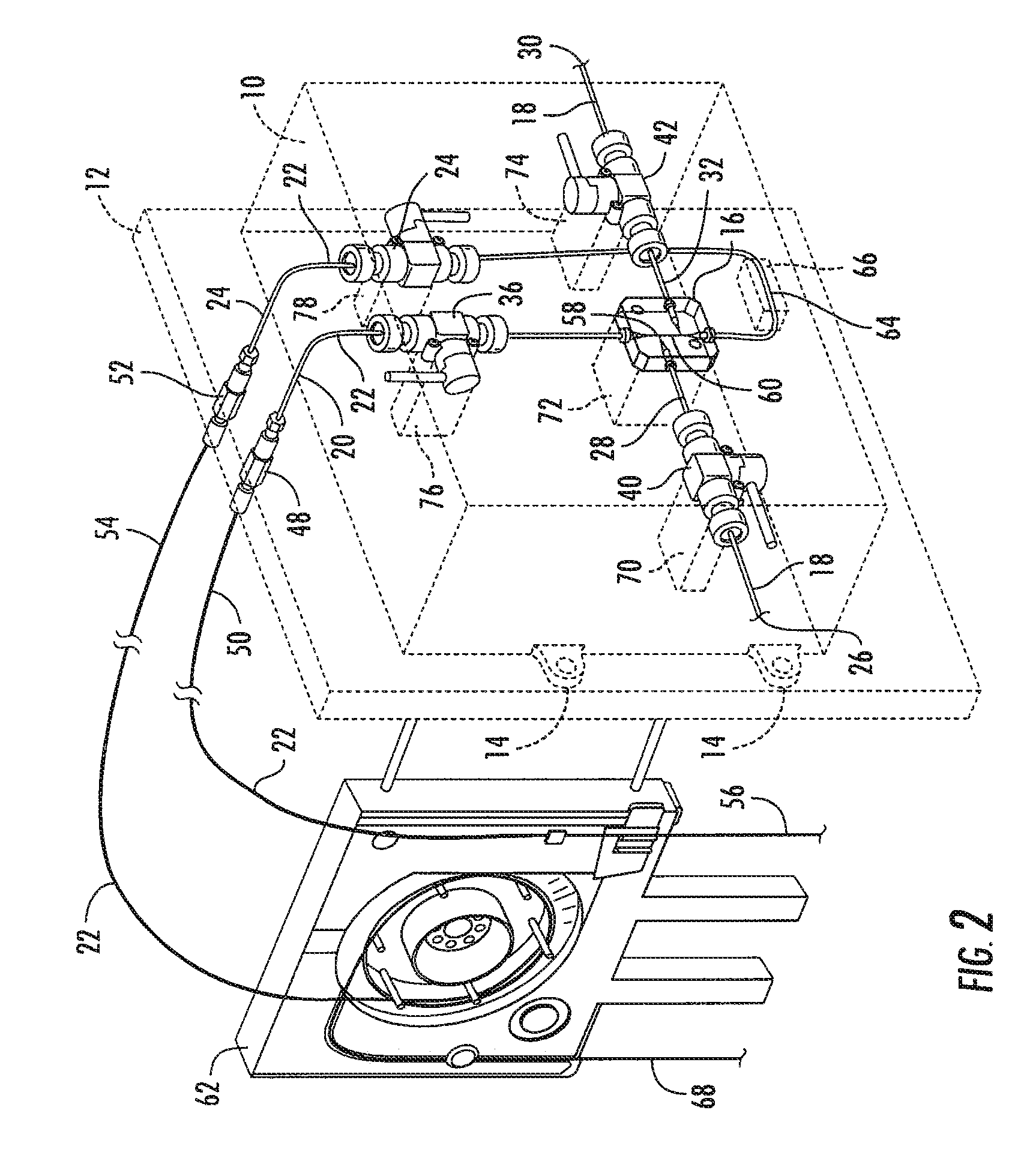
Cassette (50) performs assays, e.g. multiplexed protein biomarker assays. Wide, bubble-free, slow flows are produced from liquids stored on cassette (50), flowing over wide array (20) of ligand receptors on a capture surface. Flows of Reynolds Number less than about 1, preferably 1×10−1 to 5×10−3, are heated in region (34) preceding and including bubble removal system (128). Analyte is introduced through compressed septum (32). External actuations of displacement pumps (30, 37) and valves (137 A, B, and C) produce flows in response to flow-front optical sensors (150, 152). Elastic sheet provides pump and valve diaphragms and resilient expansion of mixing volume (131). Break-away cover portions are pistons. Heating is by conduction through cassette from external contact heater. Planar cassette body, when tilted from horizontal, enables upward flow from pumped storage (134, 135) to reaction (133) to waste (139), with buoyancy bubble removal before reaction. Reading of fluorescence is by external reader, employing calibration, control and reference features on capture surface. Extensive set of calibration features of differing intensities enables self-calibration.
Owner:AVANTRA BIOSCI CORP
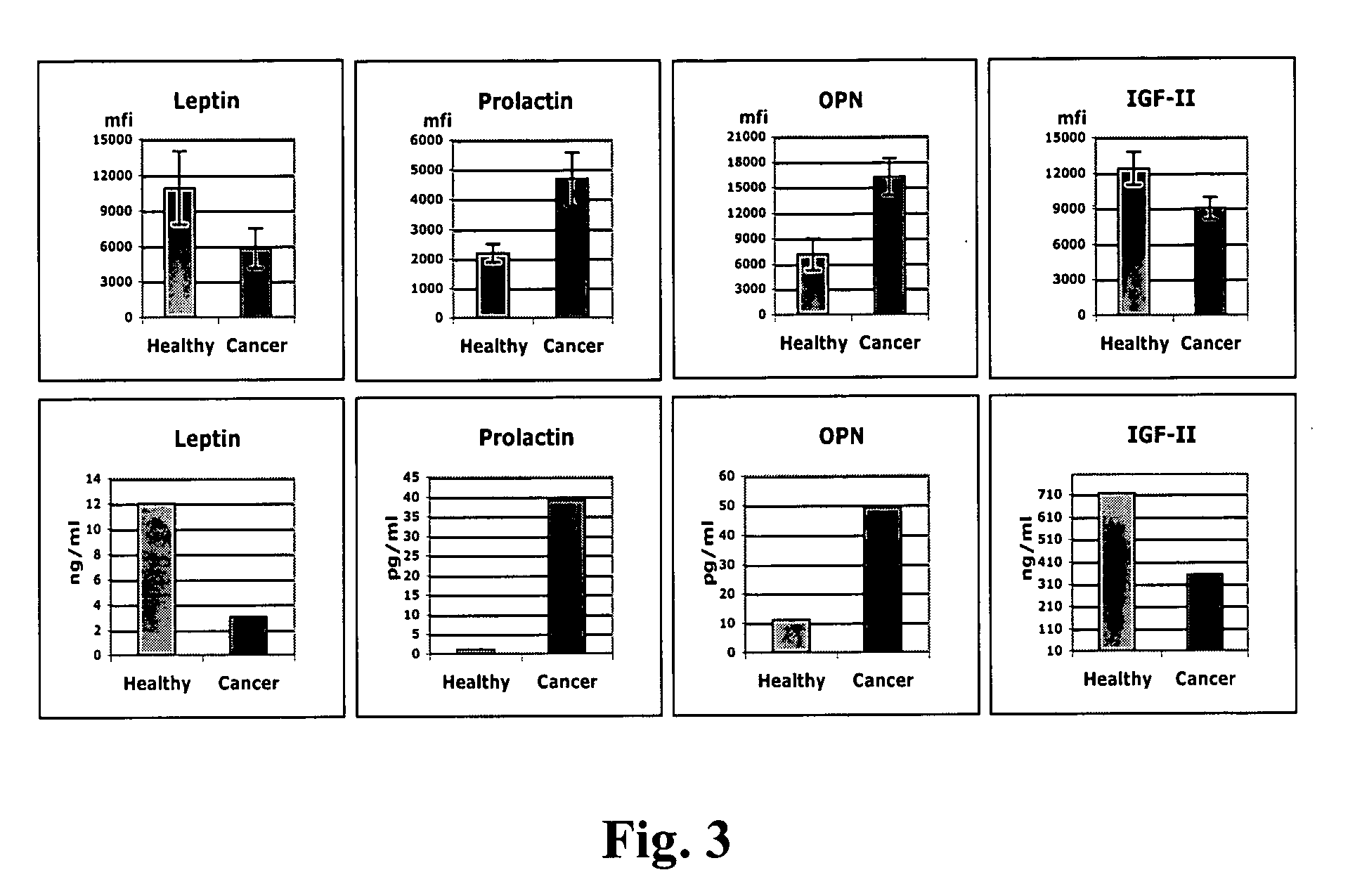
Identification of cancer protein biomarkers using proteomic techniques
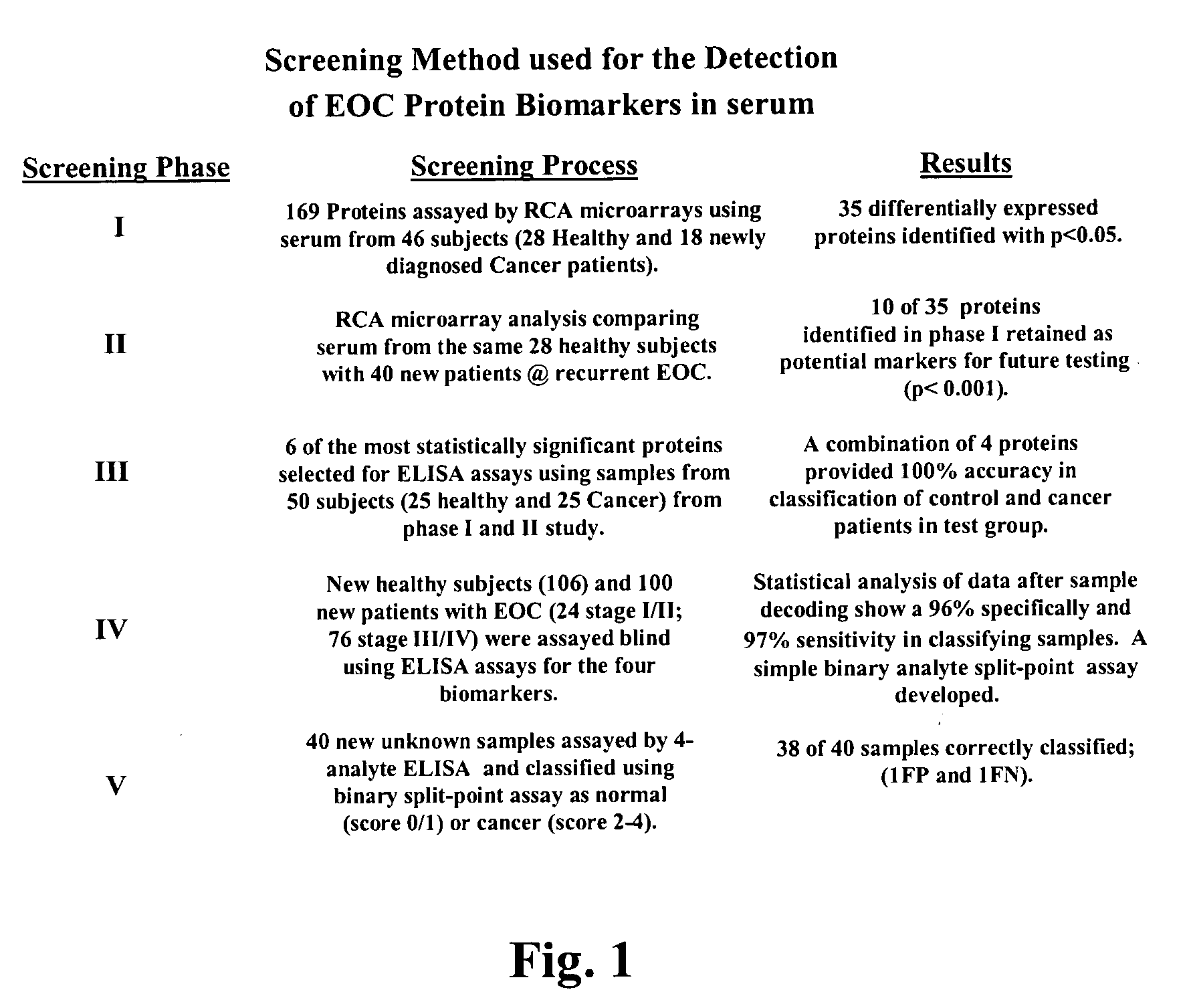
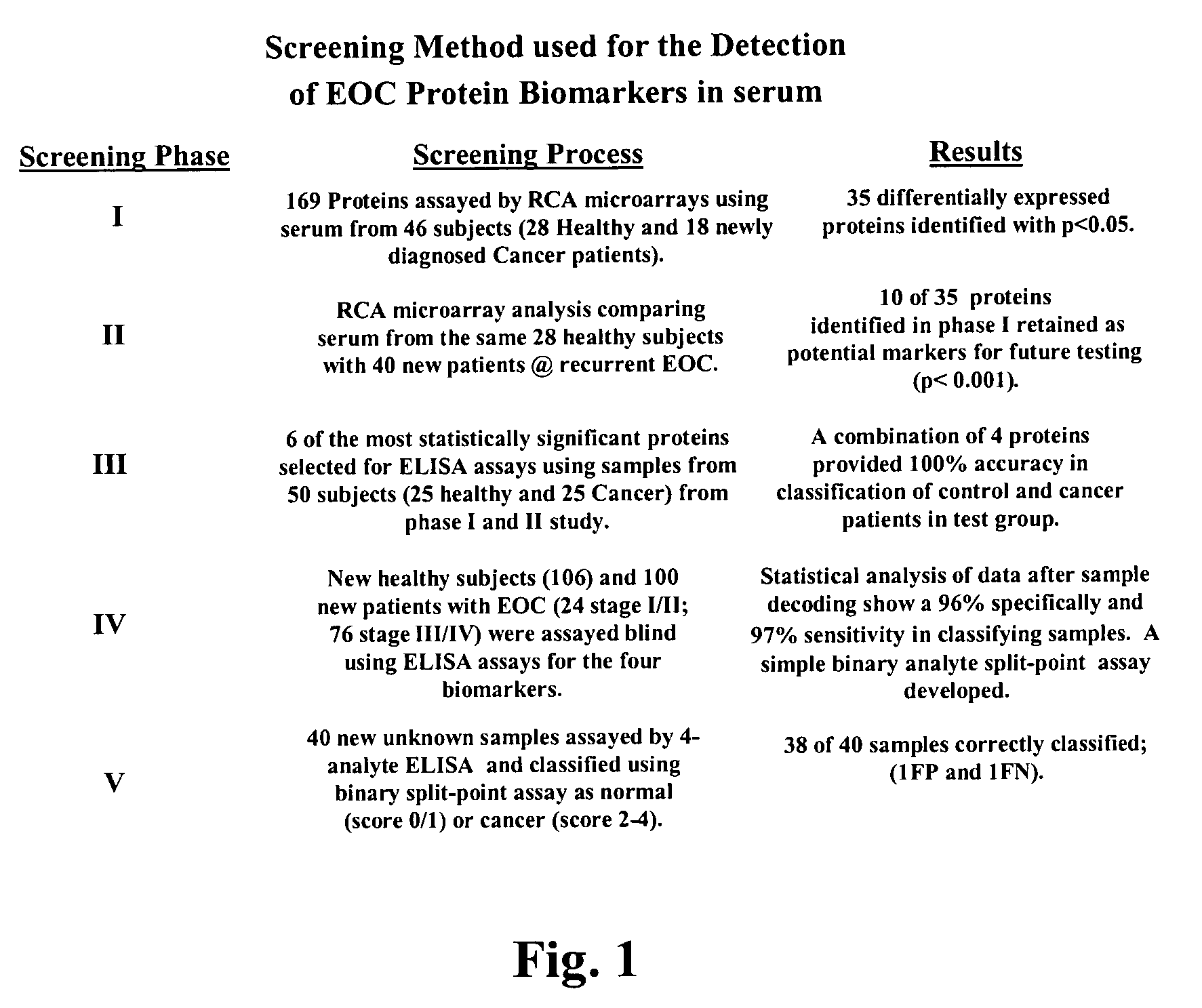
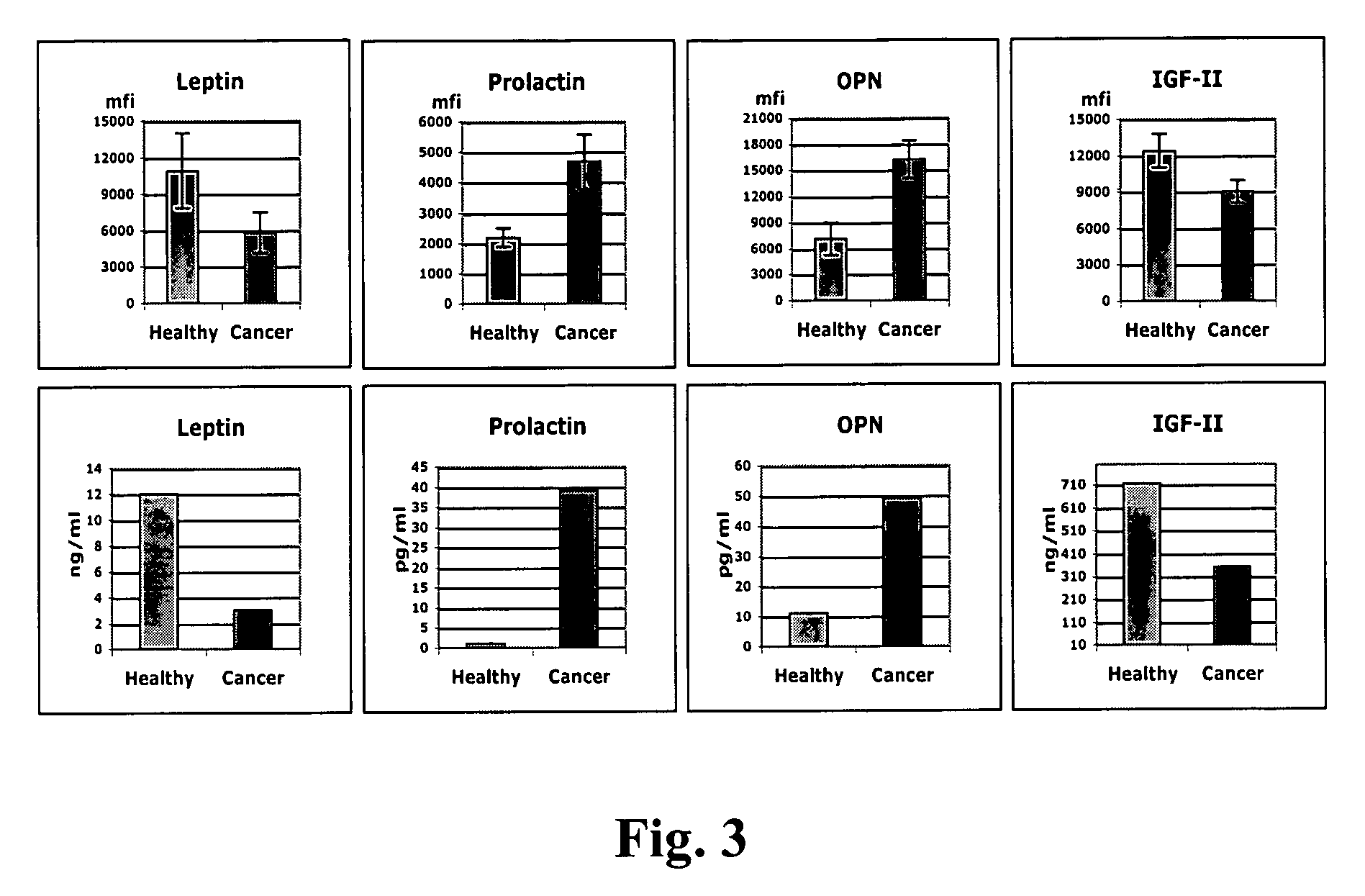
The claimed invention describes methods to diagnose or aid in the diagnosis of cancer. The claimed methods are based on the identification of biomarkers which are particularly well suited to discriminate between cancer subjects and healthy subjects. These biomarkers were identified using a unique and novel screening method described herein. The biomarkers identified herein can also be used in the prognosis and monitoring of cancer. The invention comprises the use of leptin, prolactin, OPN and IGF-II for diagnosing, prognosis and monitoring of ovarian cancer.
Owner:YALE UNIV
Biomarker assay for diagnosis and classification of cardiovascular disease
InactiveUS20110144914A1Reduce controlLow variabilityMicrobiological testing/measurementOmicsCardiovascular healthBiomarker (petroleum)
The disclosed methods, assays and kits identify biomarkers, particularly miRNA and / or protein biomarkers, for assessing the cardiovascular health of a human. In certain embodiments, methods, assays and kits, circulating miRNA and / or protein biomarkers are identified for assessing the cardiovascular health of a human.
Owner:AVIIR +1
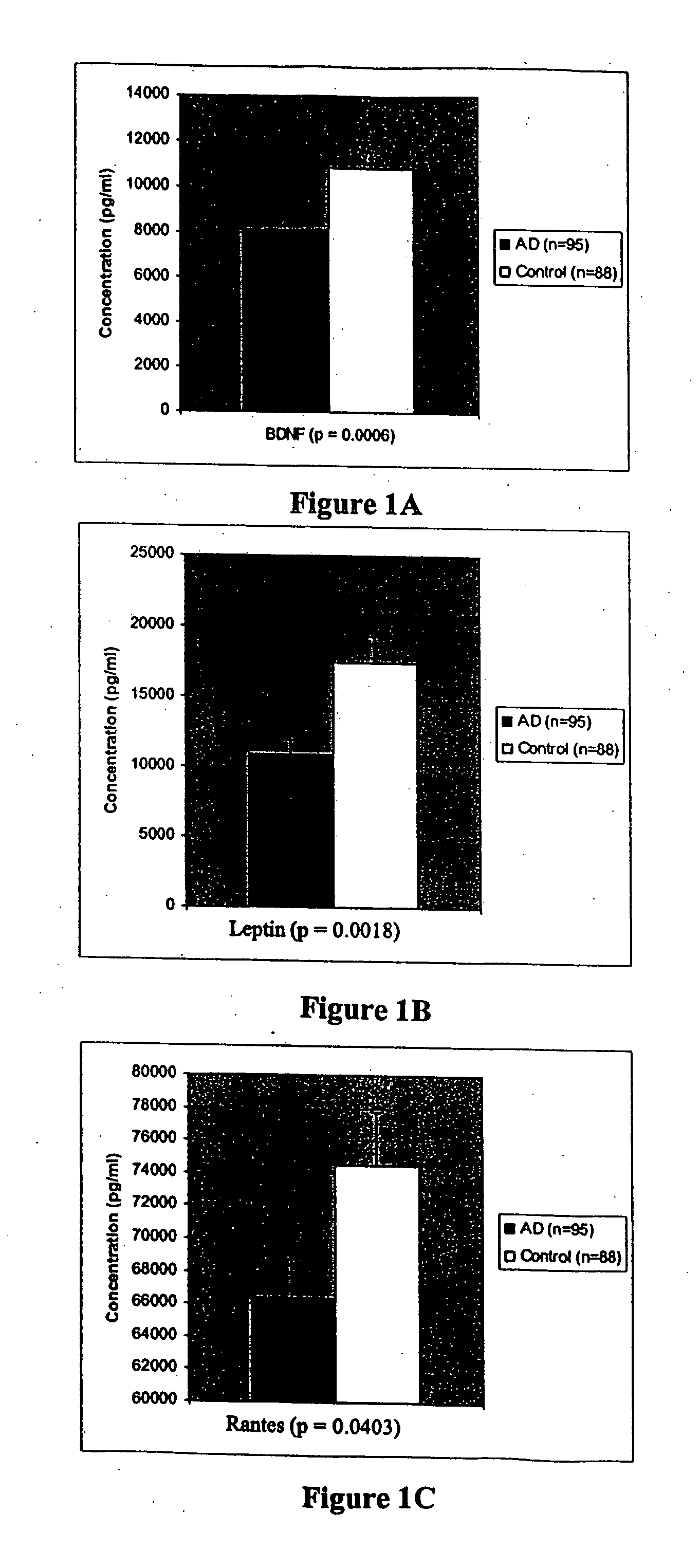
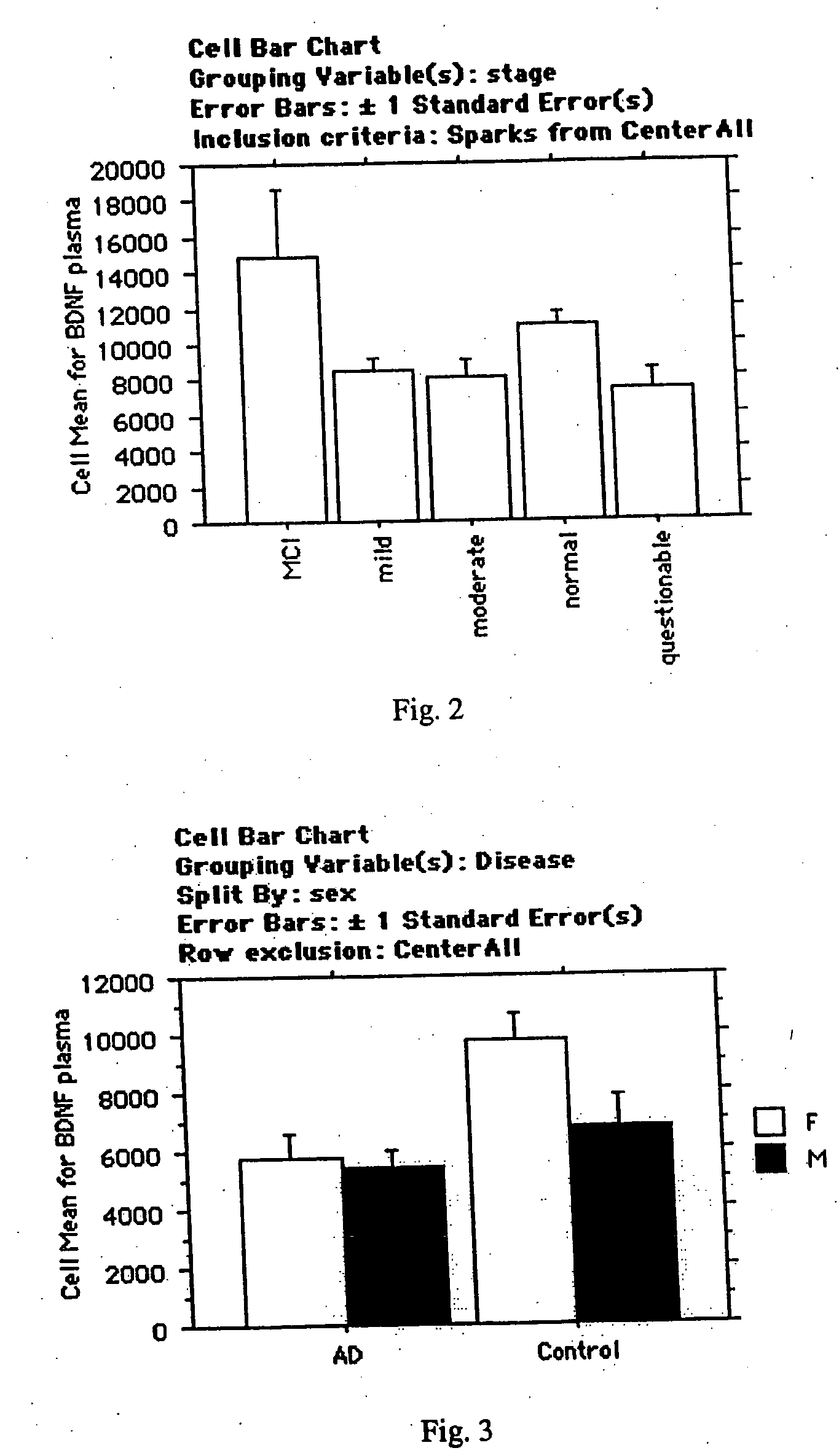
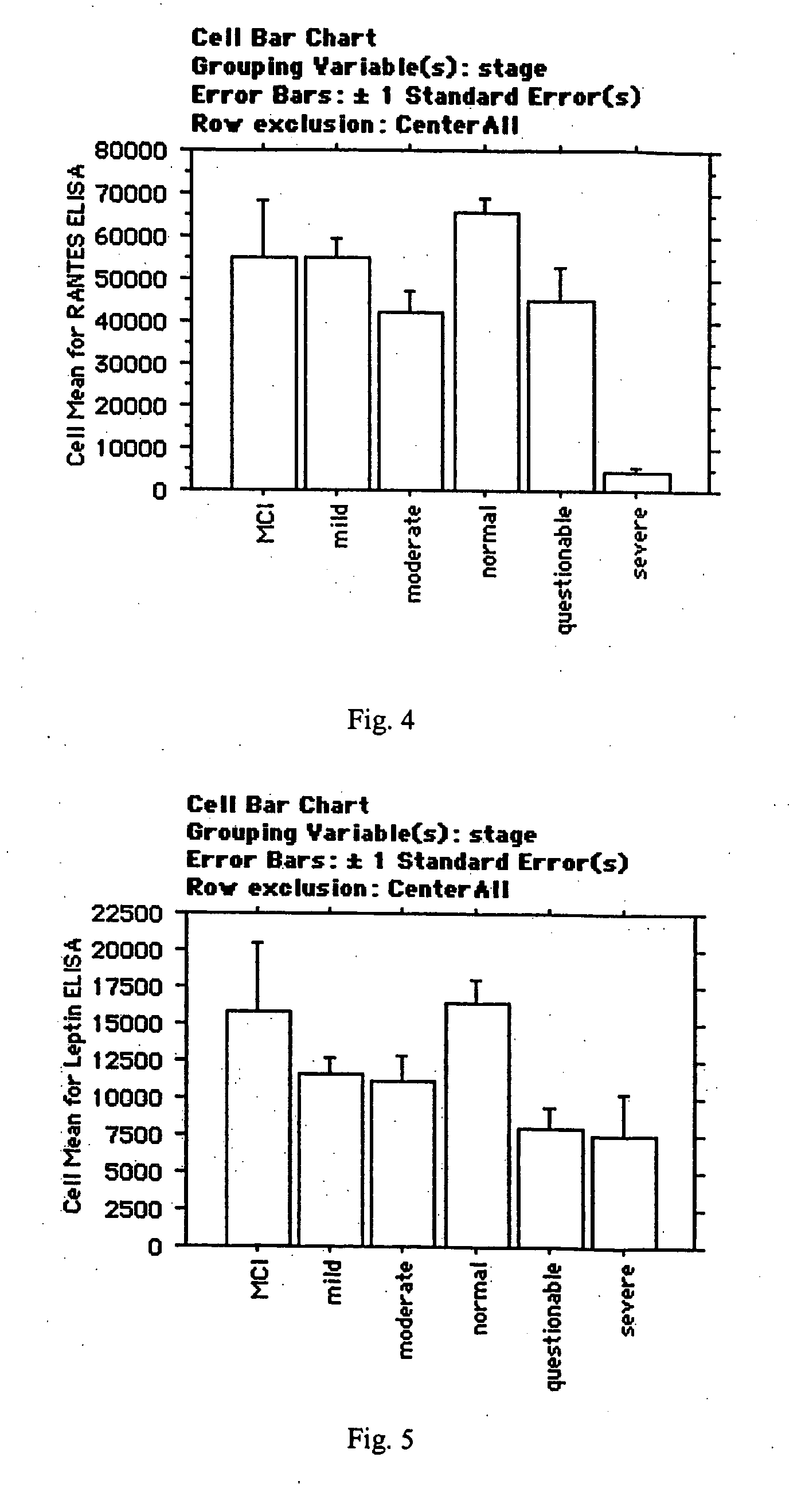
Collection of biomarkers for diagnosis and monitoring of alzheimer's disease in body fluids
InactiveUS20100124756A1Useful in detectionMonitor progressBioreactor/fermenter combinationsBiological substance pretreatmentsBody fluidBiomarker (petroleum)
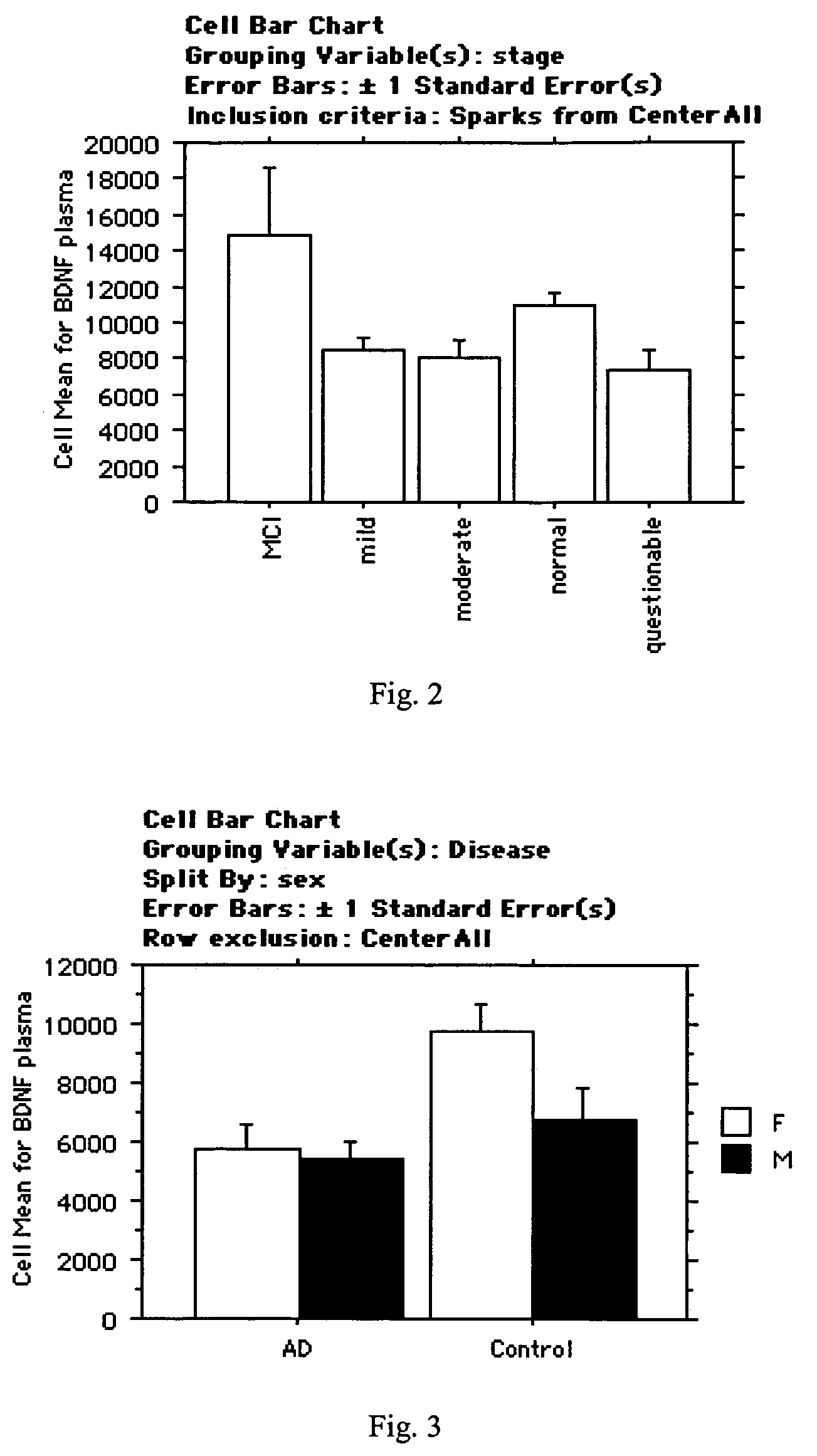
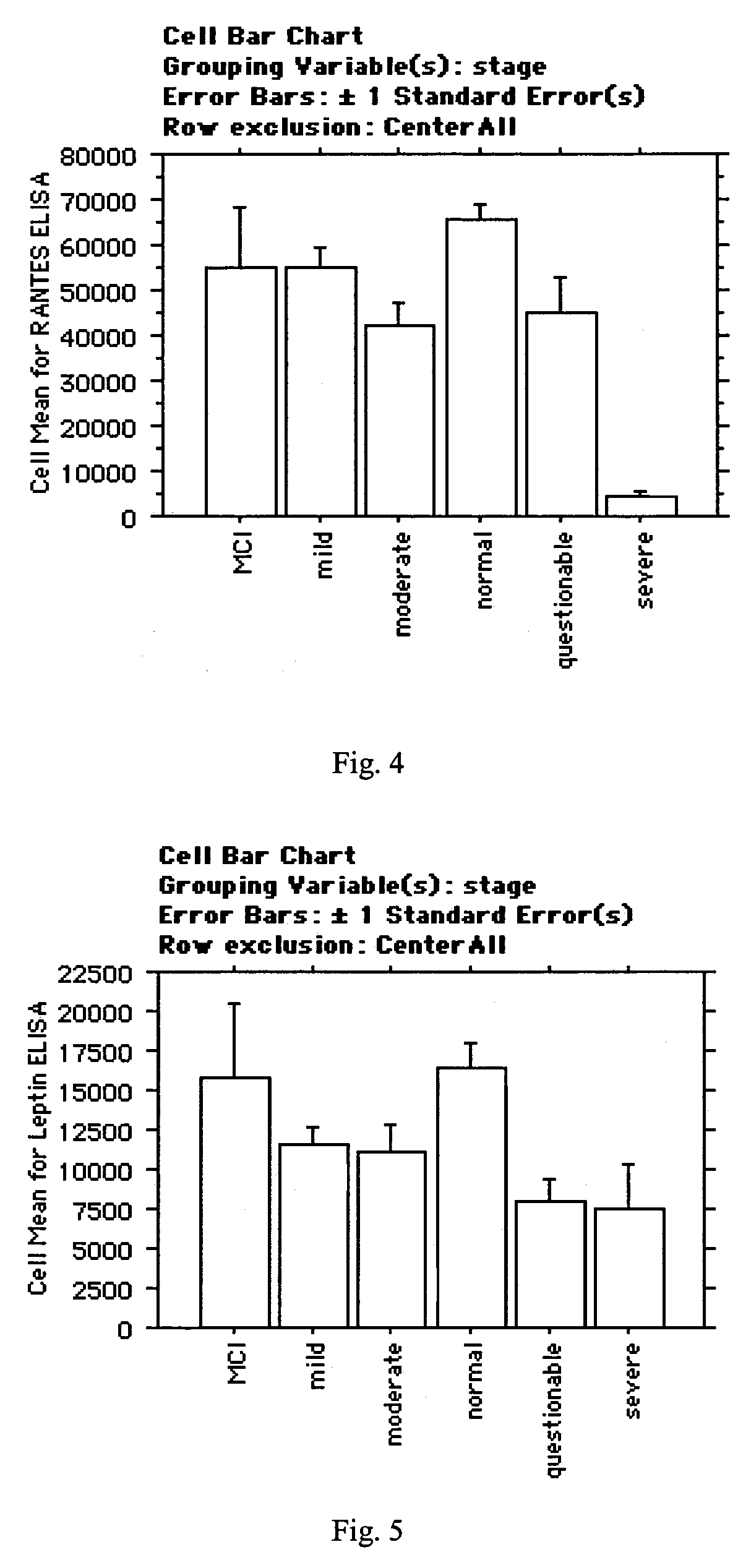
The inventors have discovered sets of proteinaceous biomarkers (“AD biomarkers”) which can be measured in peripheral biological fluid samples to aid in the diagnosis of neurodegenerative disorders, particularly Alzheimer's disease. The invention further provides methods of identifying candidate agents for the treatment of Alzheimer's disease by testing prospective agents for activity in modulating the levels of the AD biomarkers.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV +2
Classification of disease states using mass spectrometry data
InactiveUS20050048547A1Improve discriminationSamples introduction/extractionMicrobiological testing/measurementData setSpectroscopy
A method for identification of biological characteristics is achieved by collecting a data set relating to individuals having known biological characteristics and analyzing the data set to identify biomarkers potentially relating to selected biological state classes. A system for identification of biological characteristics is also provided. A methodology is also provided for utilizing mass spectroscopy data to identify peptide and protein biomarkers that can be used to optimally discriminate experimental from control samples—where the experimental samples may, for instance, be derived from patients with various diseases such as ovarian cancer.
Owner:ZHAO HONGYU +5
Methods and compositions for diagnosis, stratification, and monitoring of alzheimer's disease and other neurological disorders in body fluids
InactiveUS20060094064A1Improve the level ofLower Level RequirementsMicrobiological testing/measurementDisease diagnosisMild cognitive impairment (MCI)Body fluid
The inventors have discovered a collection of proteinaceous biomarkers (“AD biomarkers) which can be measured in peripheral biological fluid samples to aid in the diagnosis of neurodegenerative disorders, particularly Alzheimer's disease and mild cognitive impairment (MCI). The invention further provides methods of identifying candidate agents for the treatment of Alzheimer's disease by testing prospective agents for activity in modulating AD biomarker levels.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV +2
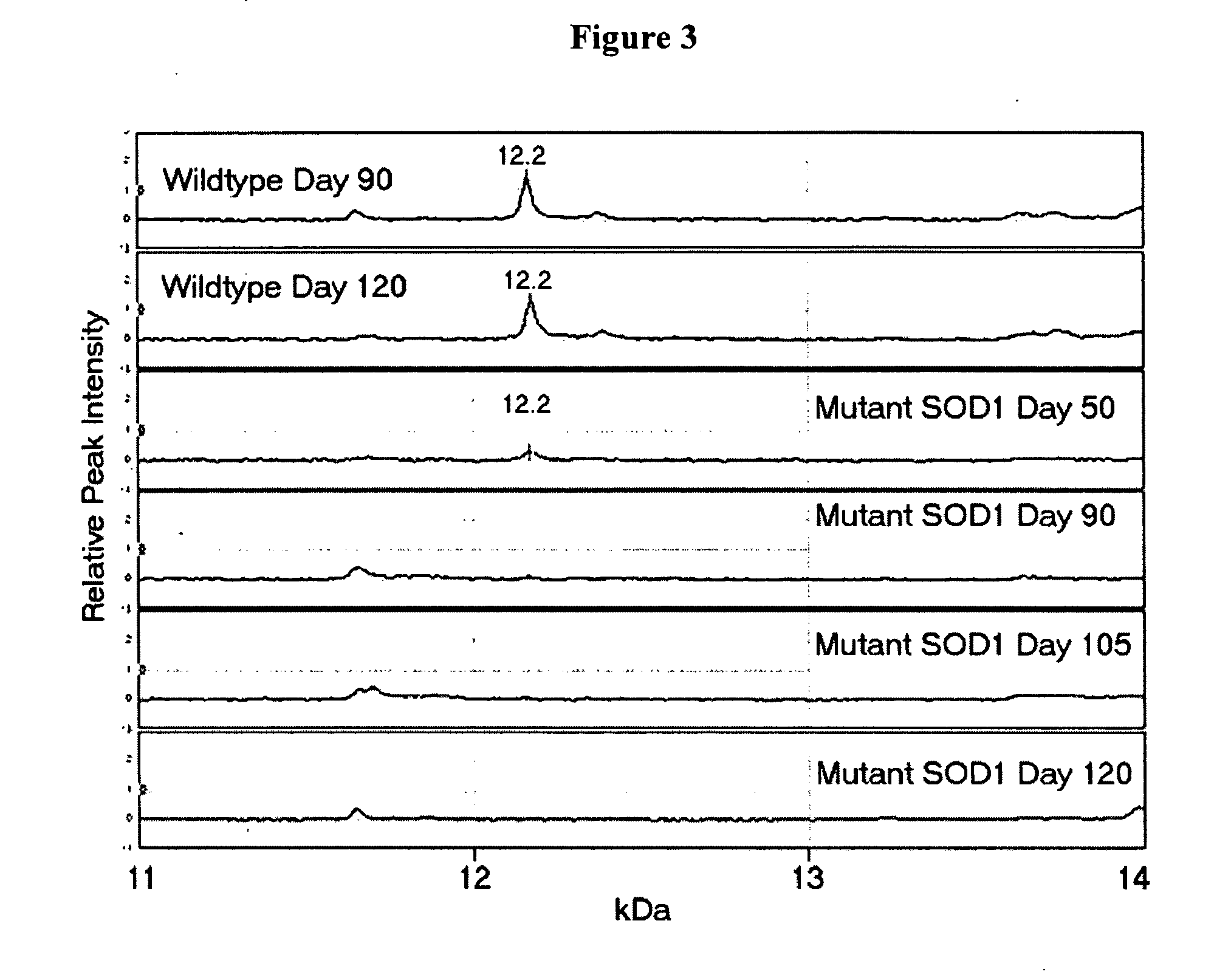
Protein biomarkers and therapeutic targets in an animal model for amyotrophic lateral sclerosis
The invention provides a method for determining the onset and / or progression of ALS in an animal. The method comprises (a) obtaining a sample from the animal, (b) analyzing the proteins in the sample by mass spectroscopy, and (c) determining a mass spectral profile for the sample. The invention also provides isolated protein biomarkers of ALS.
Owner:UNIVERSITY OF PITTSBURGH
Methods and compositions for diagnosis, stratification, and monitoring of Alzheimer's disease and other neurological disorders in body fluids
InactiveUS20070037200A1Improve the level ofLower Level RequirementsMicrobiological testing/measurementDisease diagnosisMild cognitive impairment (MCI)Body fluid
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV +2
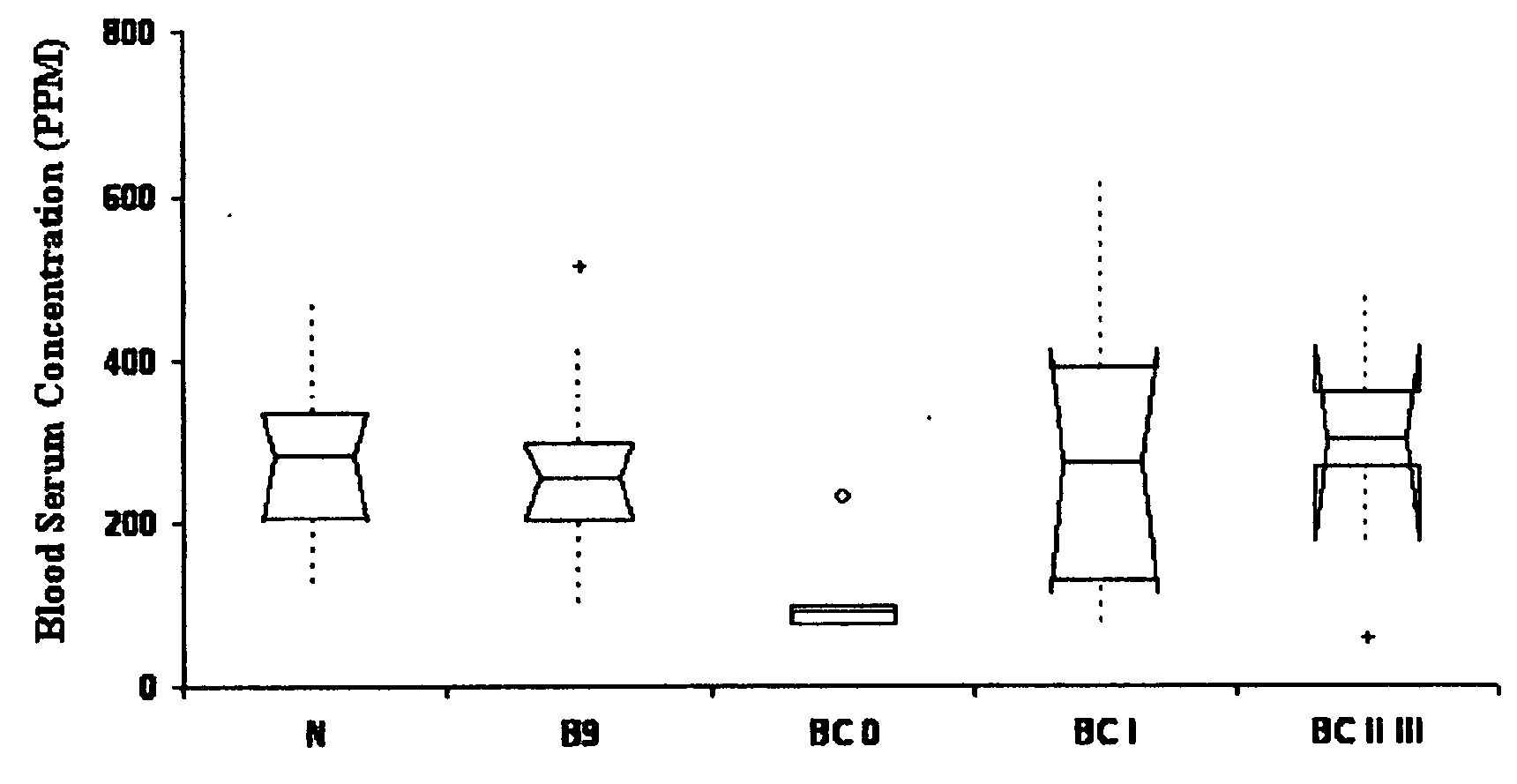
Multiple forms of Alzheimer's disease based on differences in concentrations of protein biomarkers in blood serum
The present invention relates to identification and uses of biomarkers for neurodegenerative disease, including Alzheimer's disease, and the related diseases. More specifically, the present invention relates to the identification of protein biomarkers useful for the screening, diagnosis, and differentiation of Alzheimer's disease from Parkinson's disease, other neurodegenerative diseases, and normal controls, and in the monitoring of Alzheimer's disease severity and disease mechanisms in patients.
Owner:NEOGENOMICS INC
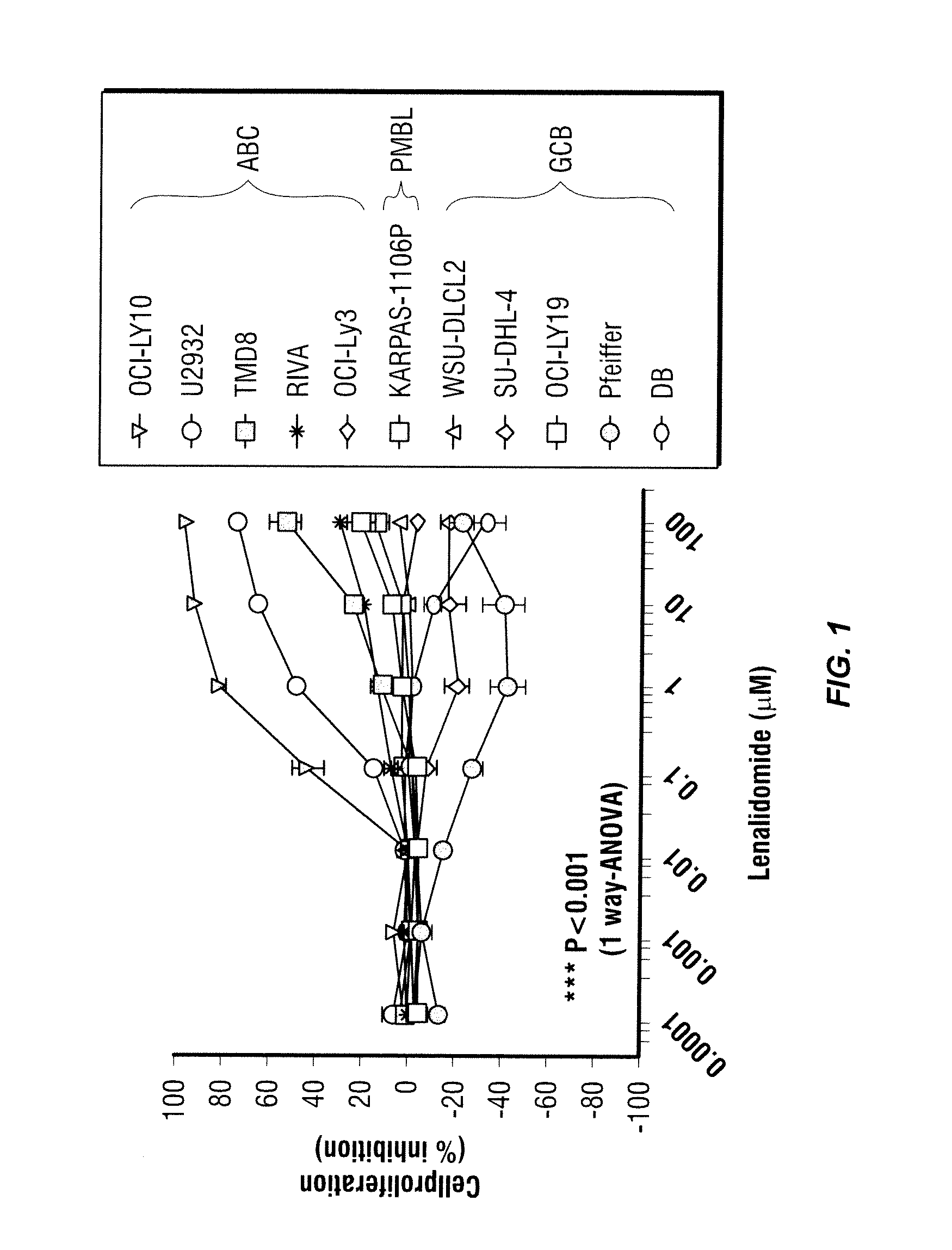
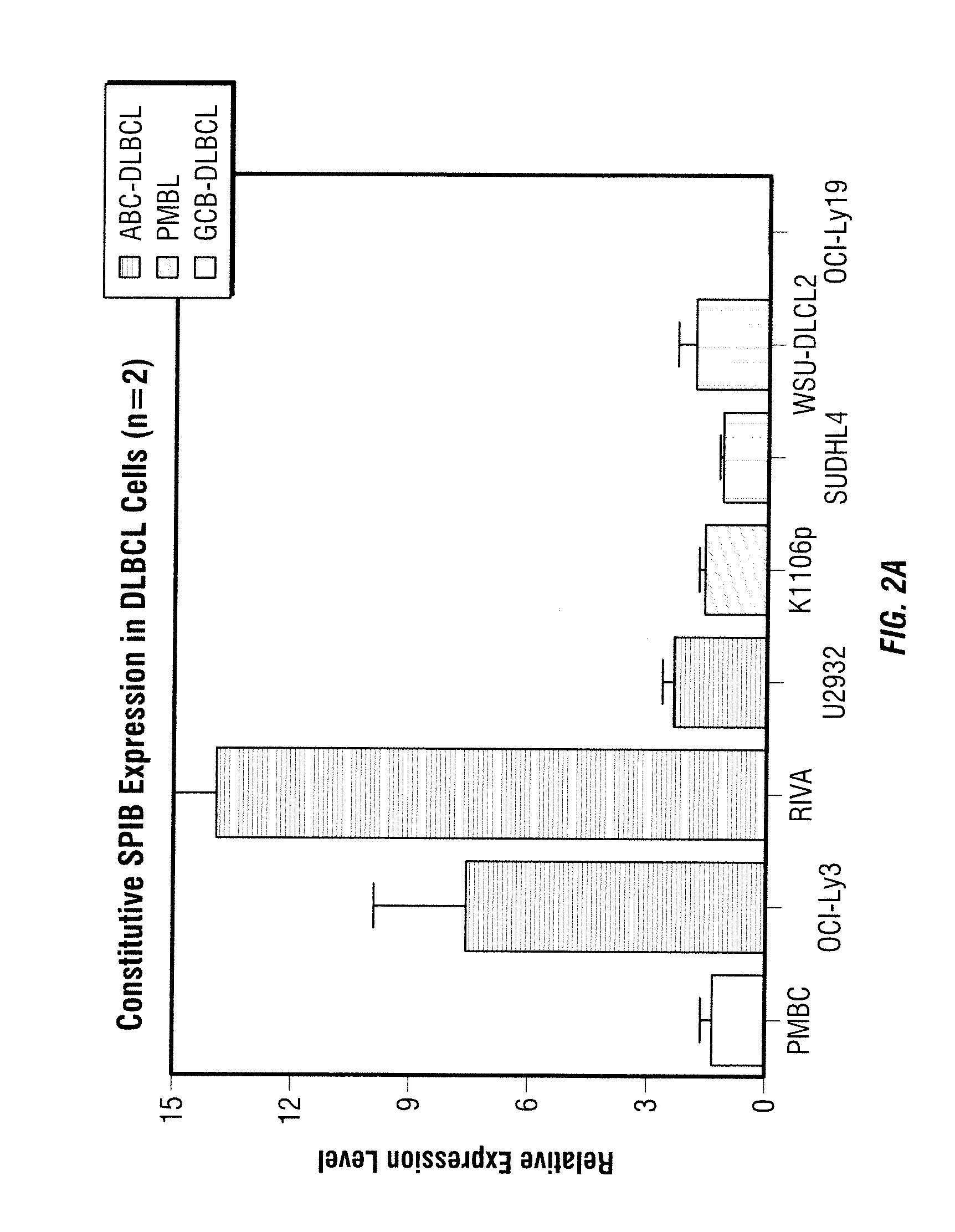
Methods for the treatment of non-hodgkin's lymphomas using lenalidomide, and gene and protein biomarkers as a predictor
InactiveUS20110223157A1Monitoring effectiveness of treatmentBiocideMicrobiological testing/measurementADAMTS ProteinsFhit gene
Methods of treating or managing specific cancers, including non-Hodgkin's lymphoma, by the administration of 3-(4-amino-1-oxo-1,3-dihydro-isoindol-2-yl)-piperidine-2,6-dione are disclosed. Methods of using gene and protein biomarkers as a predictor of non-Hodgkin's lymphoma response to treatment with 3-(4-amino-1-oxo-1,3-dihydro-isoindol-2-yl)-piperidine-2,6-dione are also disclosed.
Owner:SCHAFER PETER H +3
Prostate cancer biomarkers
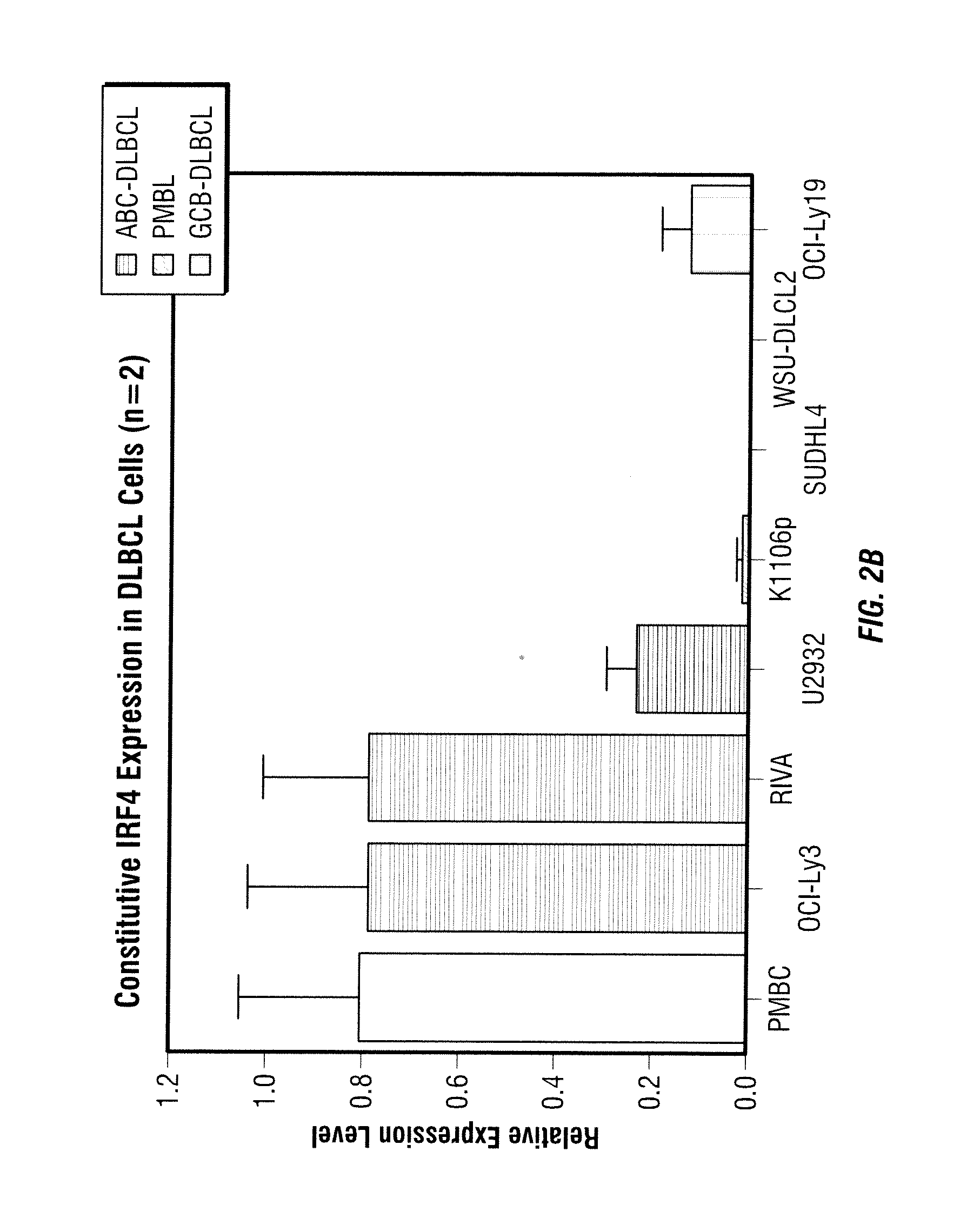
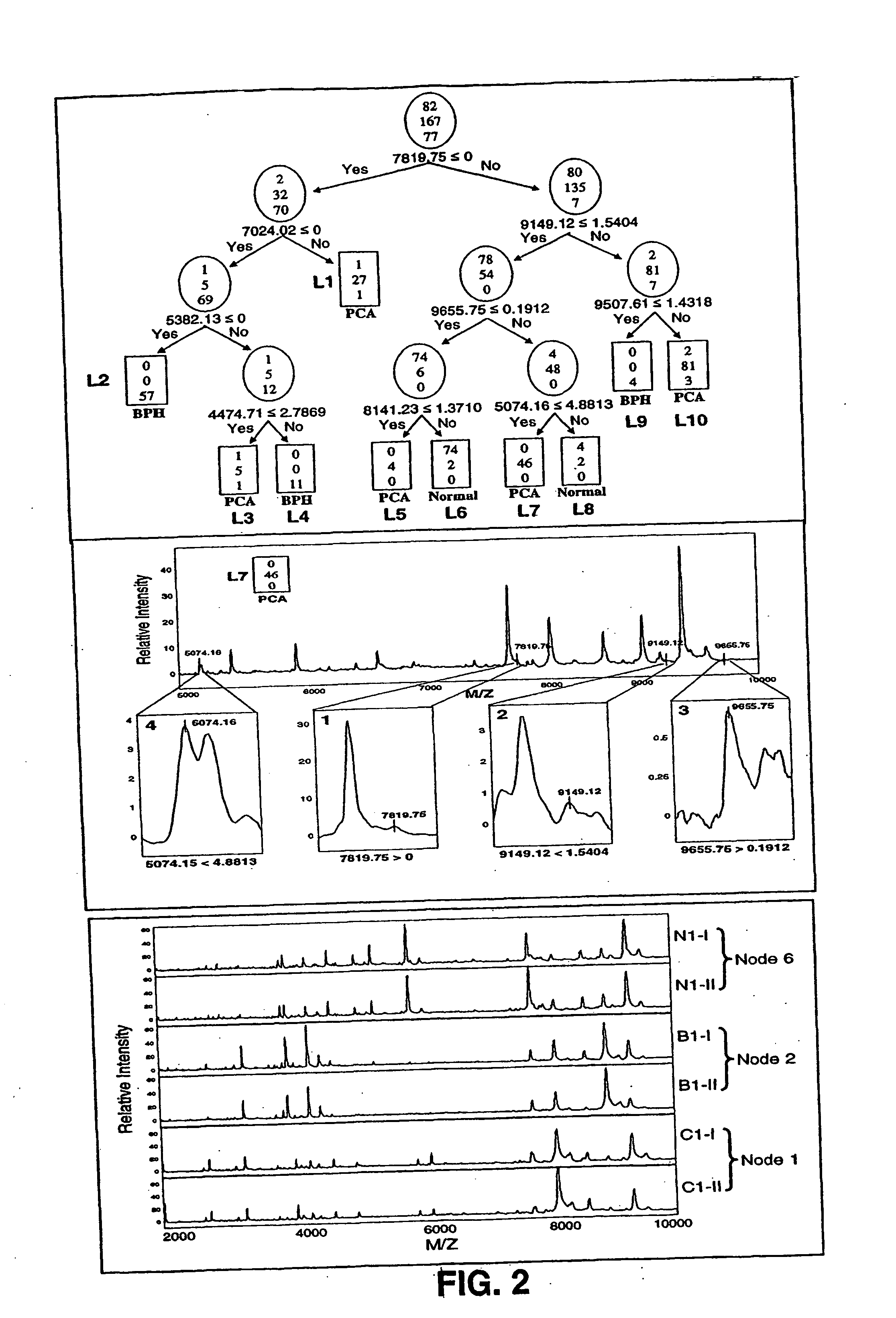
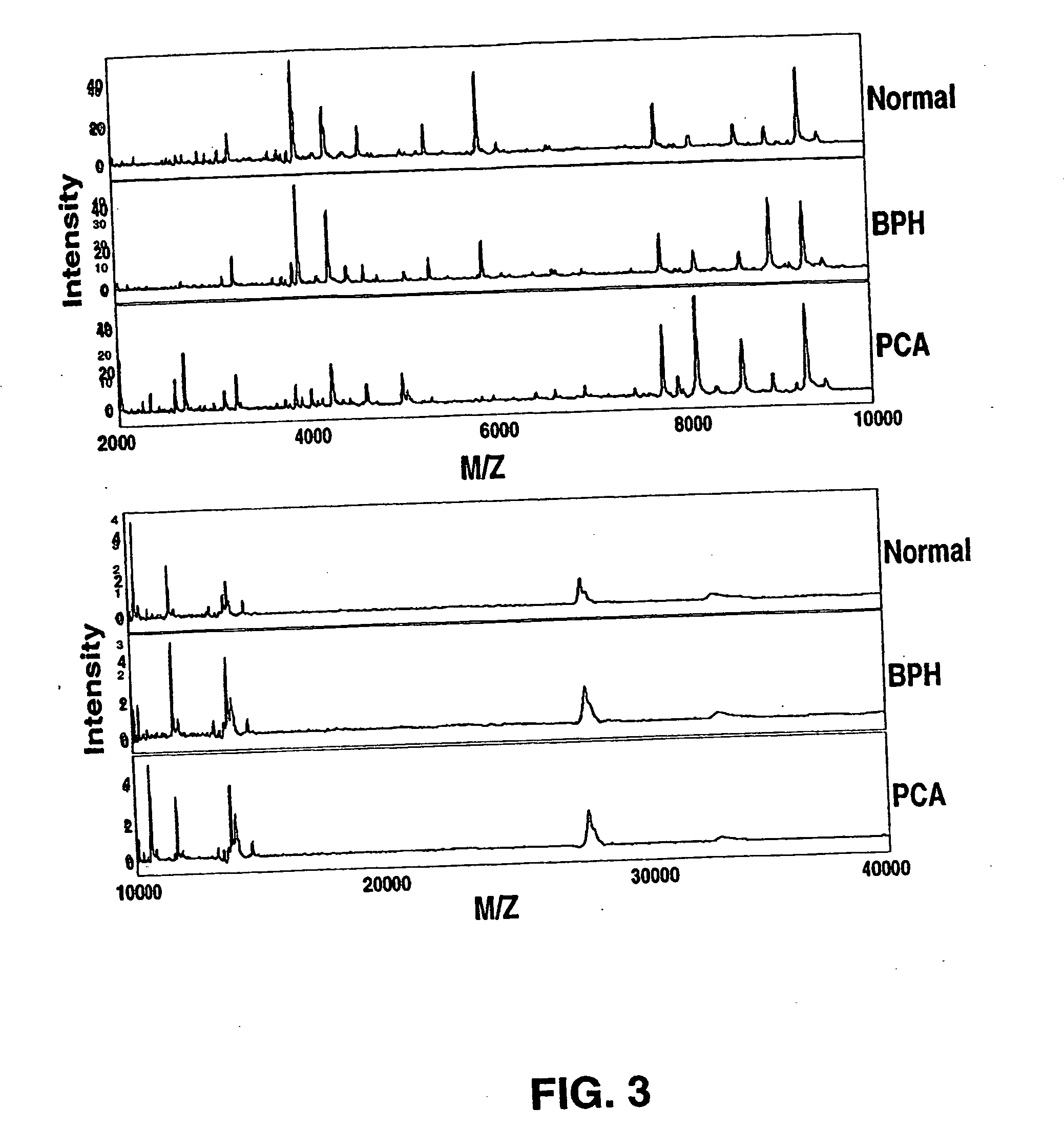
Protein biomarkers that may advantageously be utilized in diagnosing prostate cancer, benign prostate hyperplasia or to make a negative diagnosis are described. Accordingly, in one aspect of the invention, methods for aiding in, or otherwise making, a diagnosis of prostate cancer or benign prostate hyperplasia are provided. In one form of the invention, a method includes detecting various protein biomarkers of defined molecular weight and correlating the detection to a diagnosis of prostate cancer, benign prostate hyperplasia or to a negative diagnosis. In yet another aspect of the invention, kits are provided that may be utilized to detect the biomarkers described herein. In a further of the invention, methods of using a plurality of classifiers to make a probable diagnosis of prostate cancer of benign prostate hyperplasia are provided. In certain forms of the invention, the methods include use of a boosted decision tree analysis. Various computer readable media are also provided.
Owner:EASTERN VIRGINIA MEDICAL SCHOOL
System, method and computer program product for manipulating theranostic assays
InactiveUS20080243394A1Microbiological testing/measurementData visualisationProtein targetTissue sample
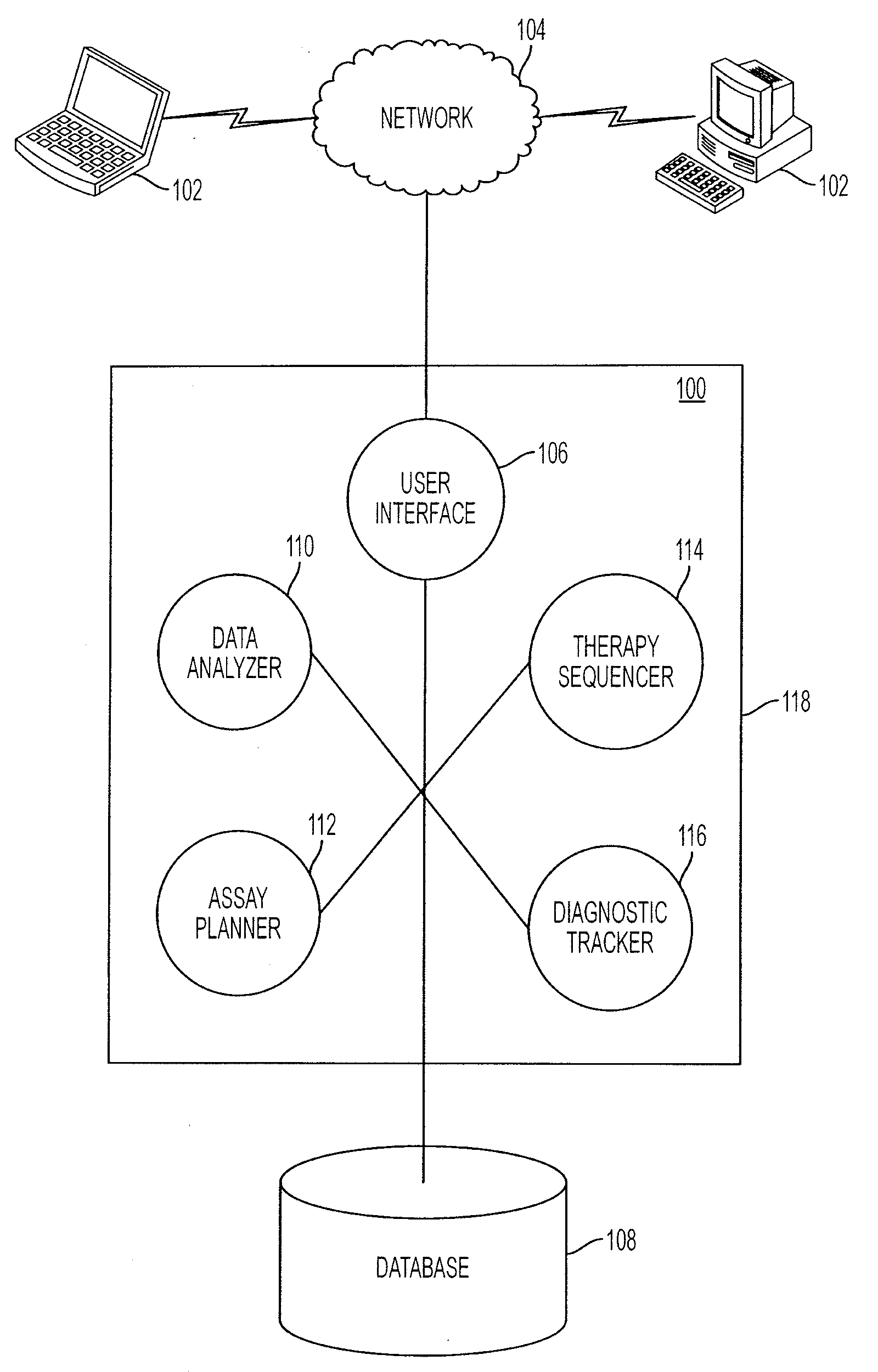
A theranostics technique for describing signaling pathway activity within a cellular or tissue sample may include analyzing a cellular sample to obtain sample quantitative values for a series of target protein modification levels reflected in a set of a plurality of protein biomarkers in the sample. The sample quantitative values may be compared to reference quantitative values for the same series of protein modification levels. The reference quantitative values may be statistically processed from a plurality of comparable samples. The sample quantitative values may be displayed in relation to the reference quantitative values in a way that may suggest a specific course of treatment.
Owner:THERANOSTICS HEALTH LLC
Assays Based on Liquid Flow Over Arrays
InactiveUS20110319279A1Reduce solubilityEasy to usePeptide librariesHeating or cooling apparatusAnalyteFluorescence
Cassette (50) performs assays, e.g. multiplexed protein biomarker assays. Wide, bubble-free, slow flows are produced from liquids stored on cassette (50), flowing over wide array (20) of ligand receptors on a capture surface. Flows of Reynolds Number less than about 1, preferably 1×10−1 to 5×10−3, are heated in region (34) preceding and including bubble removal system (128). Analyte is introduced through compressed septum (32). External actuations of displacement pumps (30, 37) and valves (137 A, B, and C) produce flows in response to flow-front optical sensors (150, 152). Elastic sheet provides pump and valve diaphragms and resilient expansion of mixing volume (131). Break-away cover portions are pistons. Heating is by conduction through cassette from external contact heater. Planar cassette body, when tilted from horizontal, enables upward flow from pumped storage (134, 135) to reaction (133) to waste (139), with buoyancy bubble removal before reaction. Reading of fluorescence is by external reader, employing calibration, control and reference features on capture surface. Extensive set of calibration features of differing intensities enables self-calibration.
Owner:COURTAGEN LIFE SCI
Protein Biomarkers and Methods for Diagnosing Kawasaki Disease
InactiveUS20110189698A1Improve diagnostic accuracyComponent separationDisease diagnosisKawasaki diseaseBiology
A method, kit and device for diagnosing Kawasaki Disease are provided. The invention provides detecting an expression level of at least two Kawasaki Disease diagnostic biomarkers in a biological sample from a patient with a capture agent and diagnosing the patient as having Kawasaki Disease when the expression levels of the at least two diagnostic biomarkers in the patient biological sample are higher than the normal expression levels of the same biomarkers in a biological sample from a control subject. The first Kawasaki Disease diagnostic biomarker disclosed in the present invention is a cardiomyocyte biomarker, and the second Kawasaki Disease diagnostic biomarker is an inflammatory biomarker. The invention further provides detecting an expression level of a third biomarker, interferon type-I biomarker, in the patient biological sample with a capture agent and diagnosing the patient as having Kawasaki Disease when the expression level of interferon type-I biomarker is lower than the expression level in a control subject.
Owner:RGT UNIV OF CALIFORNIA
Protein biomarkers and therapeutic targets for renal disorders
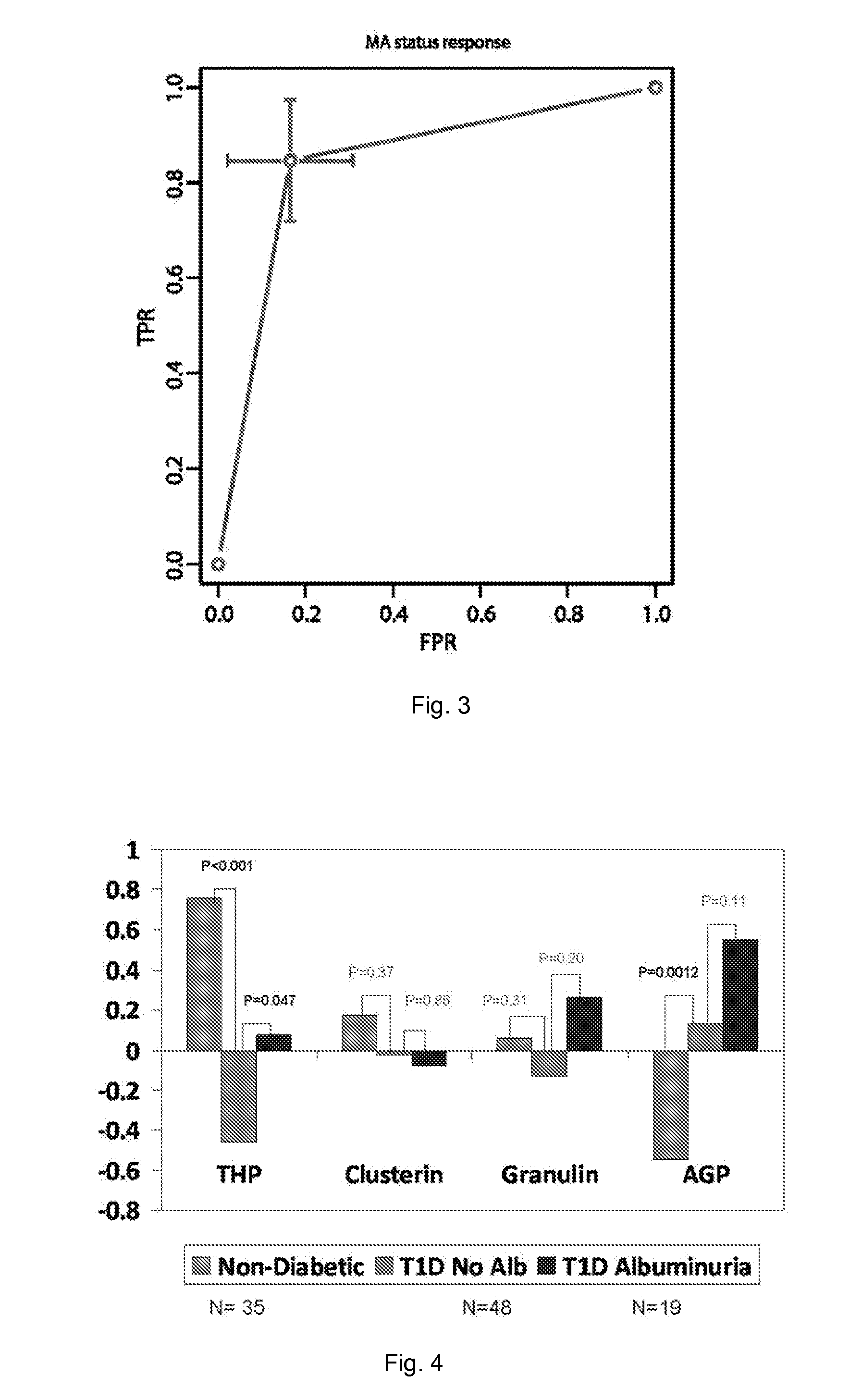
The present invention relates to a method of diagnosing a renal disorder. The method includes the steps of: (1) obtaining a biological sample from a subject; and (2) determining, in the biological sample, a level of one or more proteins whose abundance in urine change due to the renal disorder, wherein an increase or decrease in the level of one or more of the proteins compared to a control level is indicative of a renal disorder.
Owner:CHANCE MARK +1
Protein-based biomarkers for abdominal aortic aneurysm
InactiveUS20090093005A1Microbiological testing/measurementDisease diagnosisRadiologyProtein biomarkers
The present invention encompasses compositions and methods useful for diagnosing subjects with abdominal aortic aneurysms. The invention relates to the use of protein biomarkers whose levels are different in subjects with abdominal aortic aneurysms relative to normal subjects.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
Rapid identification of proteins and their corresponding source organisms by gas phase fragmentation and identification of protein biomarkers
InactiveUS20100057372A1Particle separator tubesBiological testingLaser desorption ionization mass spectrometryGas phase
Embodiments of the present invention relate to the identification of proteins using laser desorption ionization mass spectrometry, the identification of source organisms comprising the identified proteins and a computer readable storage medium storing instructions that, when executed by a computer cause the computer to perform a method for the identification of proteins using mass spectra generated through the application of laser desorption ionization mass spectrometry of the proteins.
Owner:UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC OF AGRI THE
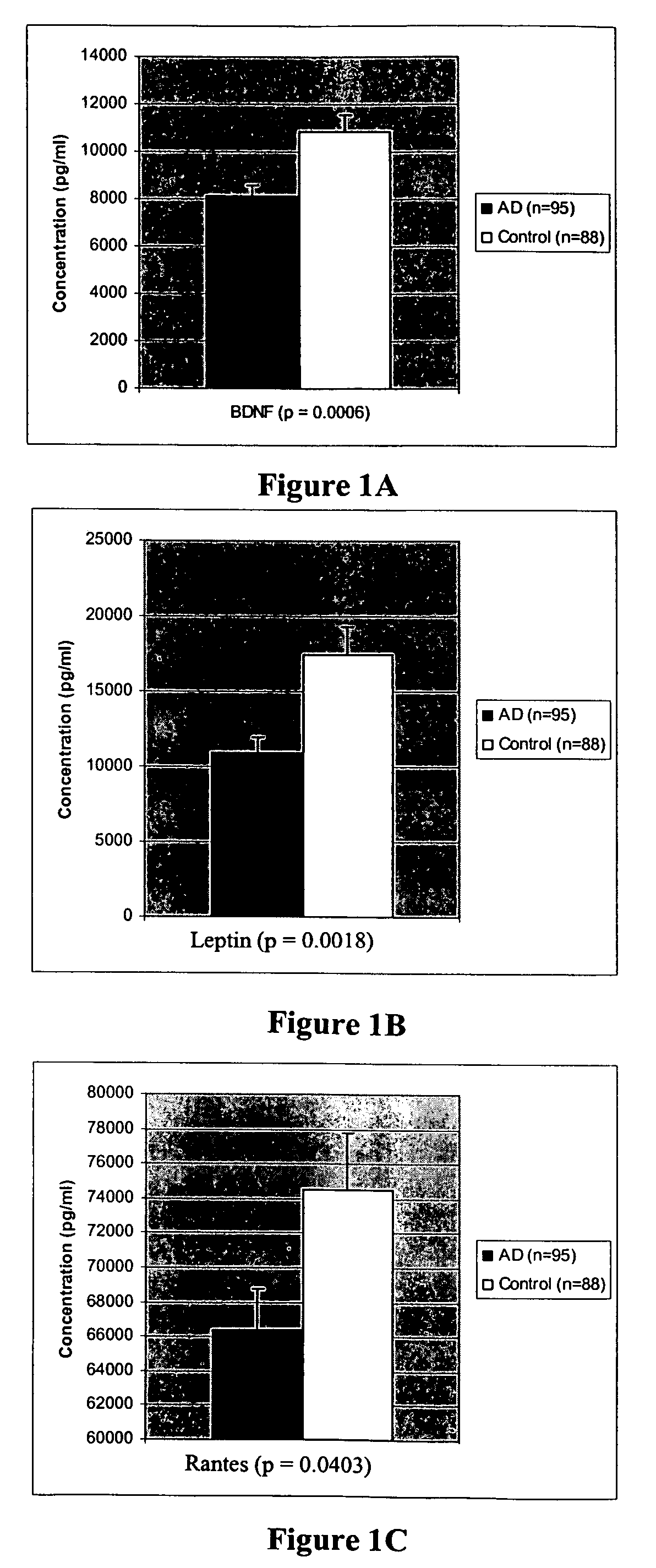
Novel groups of biomarkers for diagnosing alzheimer's disease
The inventors have discovered sets of proteinaceous biomarkers which can be measured in biological fluid samples to diagnosis or aid in the diagnosis of Alzheimer's disease and distinguish AD samples from non-demented samples.
Owner:RULES BASED MEDICINE
Biomarkers of mild cognitive impairment and alzheimer's disease
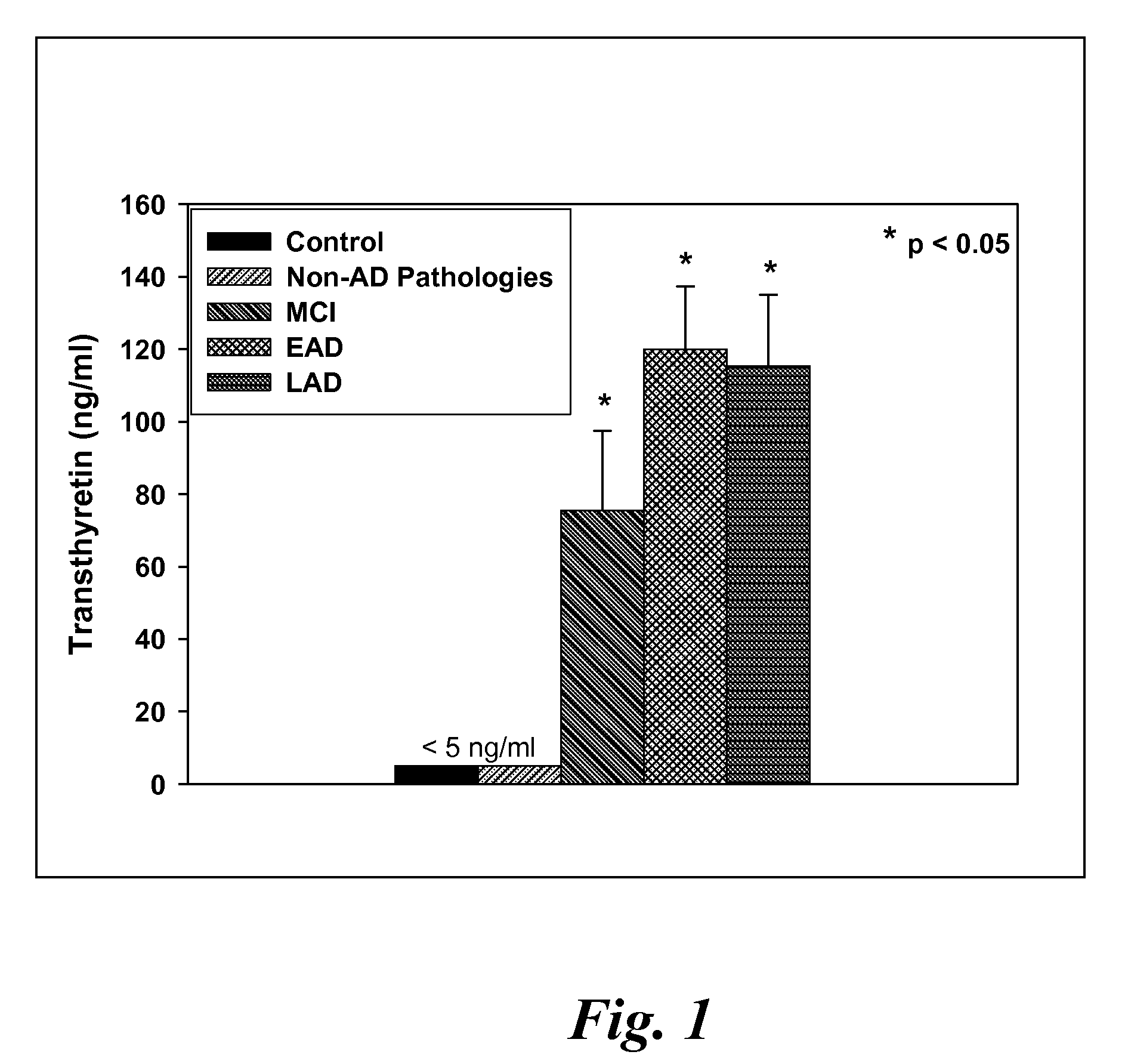
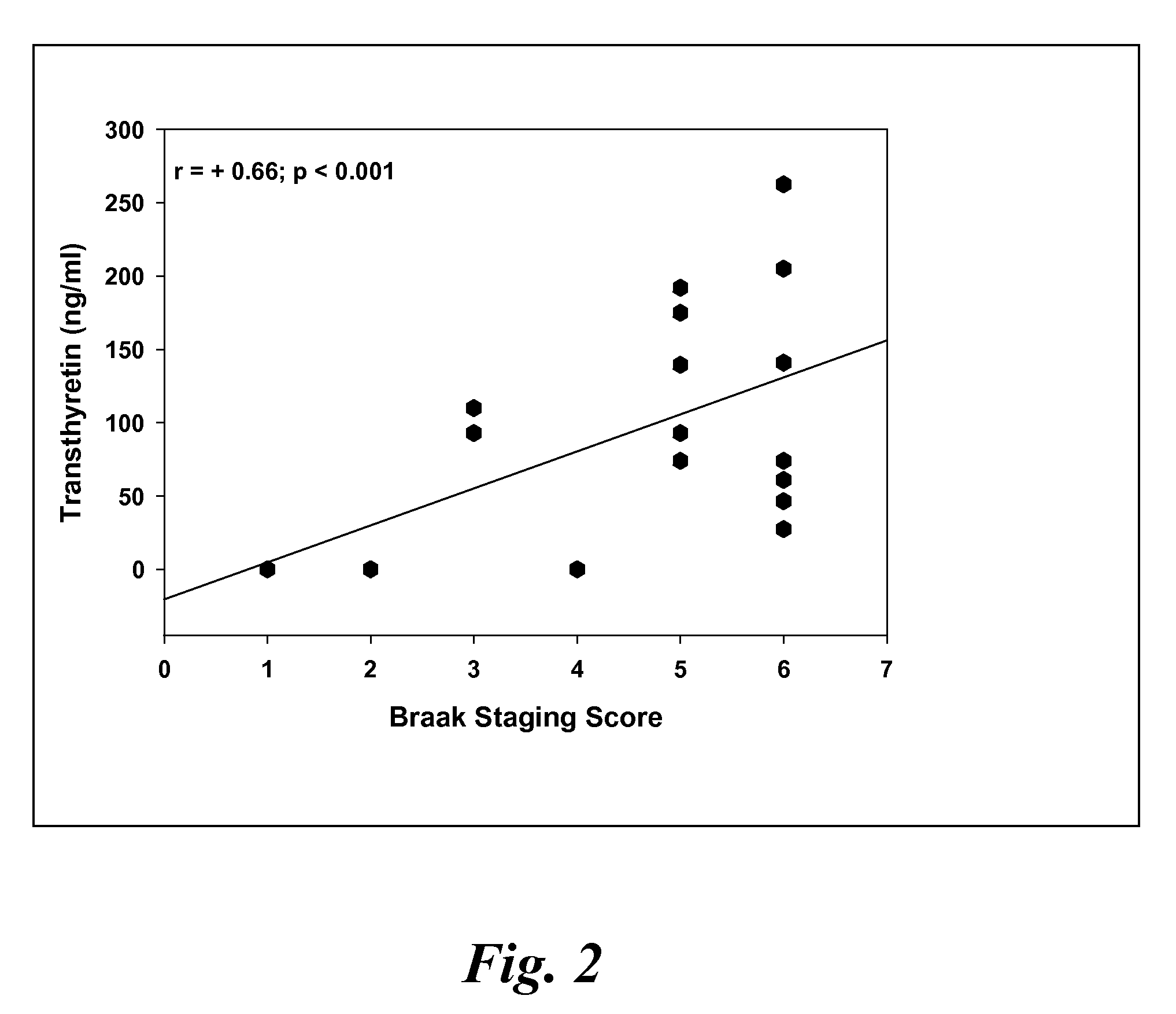
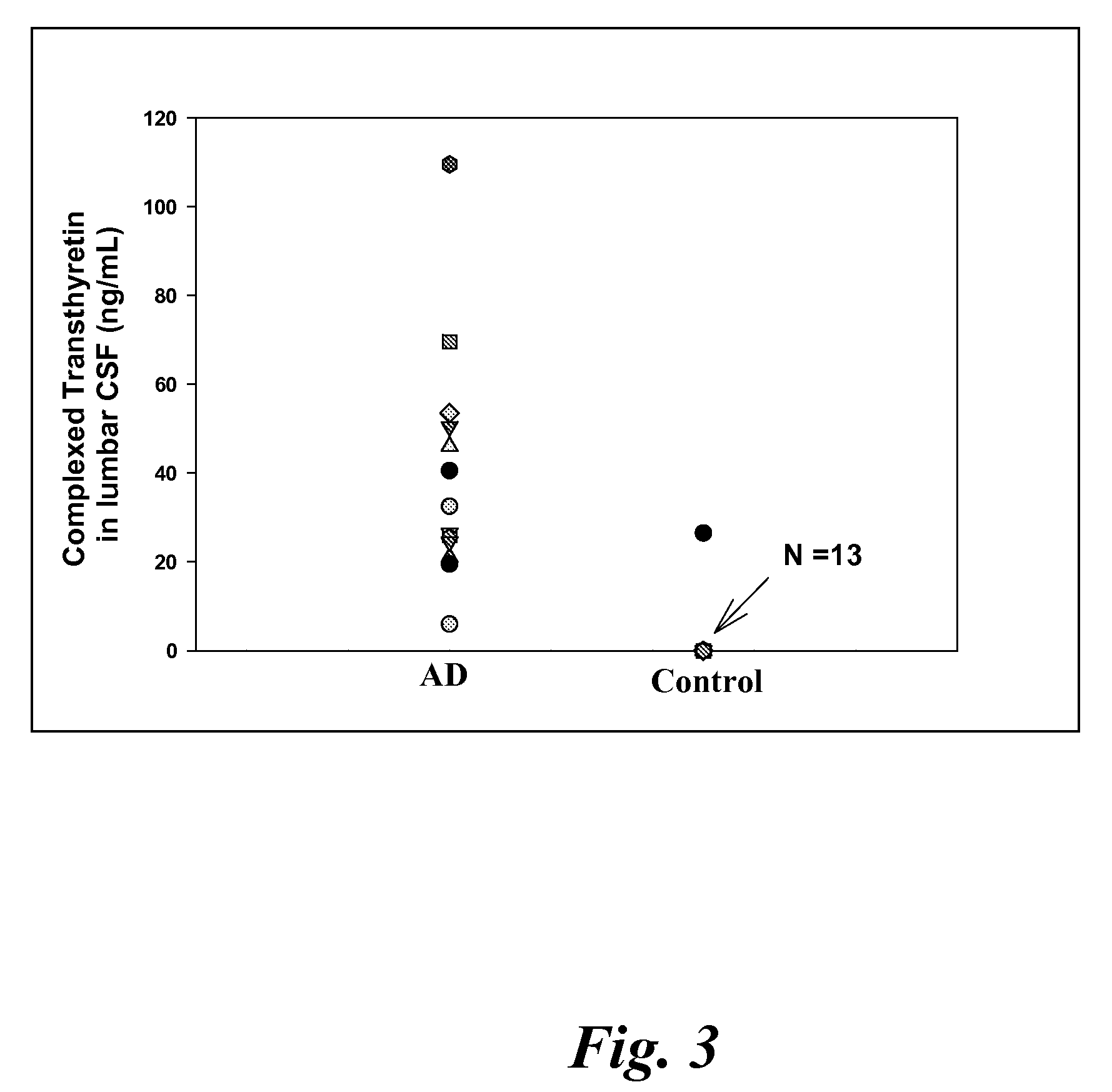
A method for quantifying a neurodegenerative disorder in a patient that includes obtaining a fluid sample from the subject; measuring a protein biomarker complex in said fluid sample and correlating the measurement with mild cognitive impairment or Alzheimer's disease status. The biomarkers include those that comprise at least one of a transthyretin protein and / or a prostaglandin-H2 D-isomerase protein, and at least one second, different protein selected from a transthyretin, prostaglandin-H2 D-isomerase, beta-2-microglobulin, cystatin C, superoxide dismutase [Cu—Zn], plasma retinol-binding protein, phosphatidylethanolamine-binding protein, carbonic anhydrase 2, prostaglandin-H2 D-isomerase, and / or serotransferrin protein.
Owner:UNIV OF KENTUCKY RES FOUND
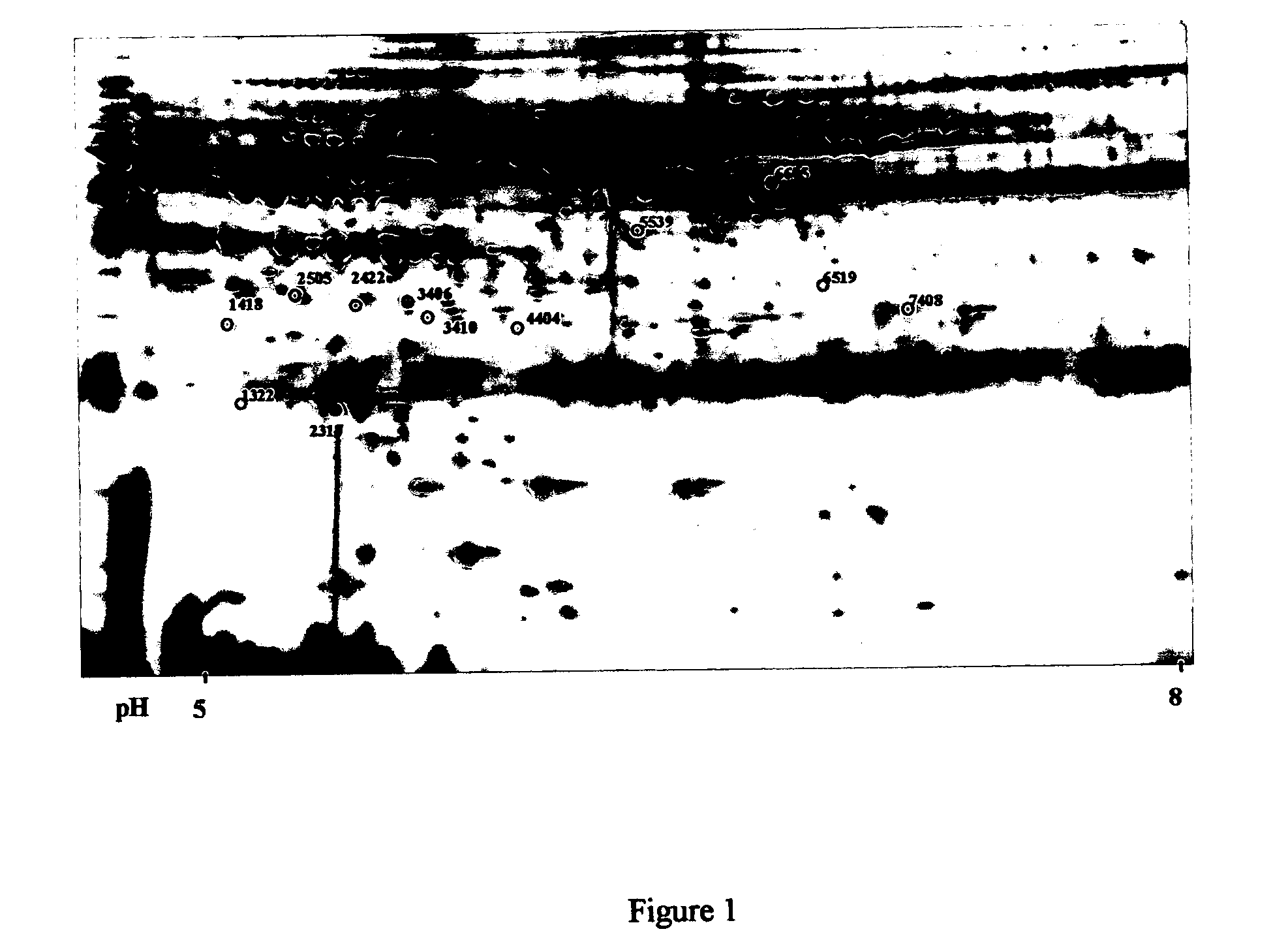
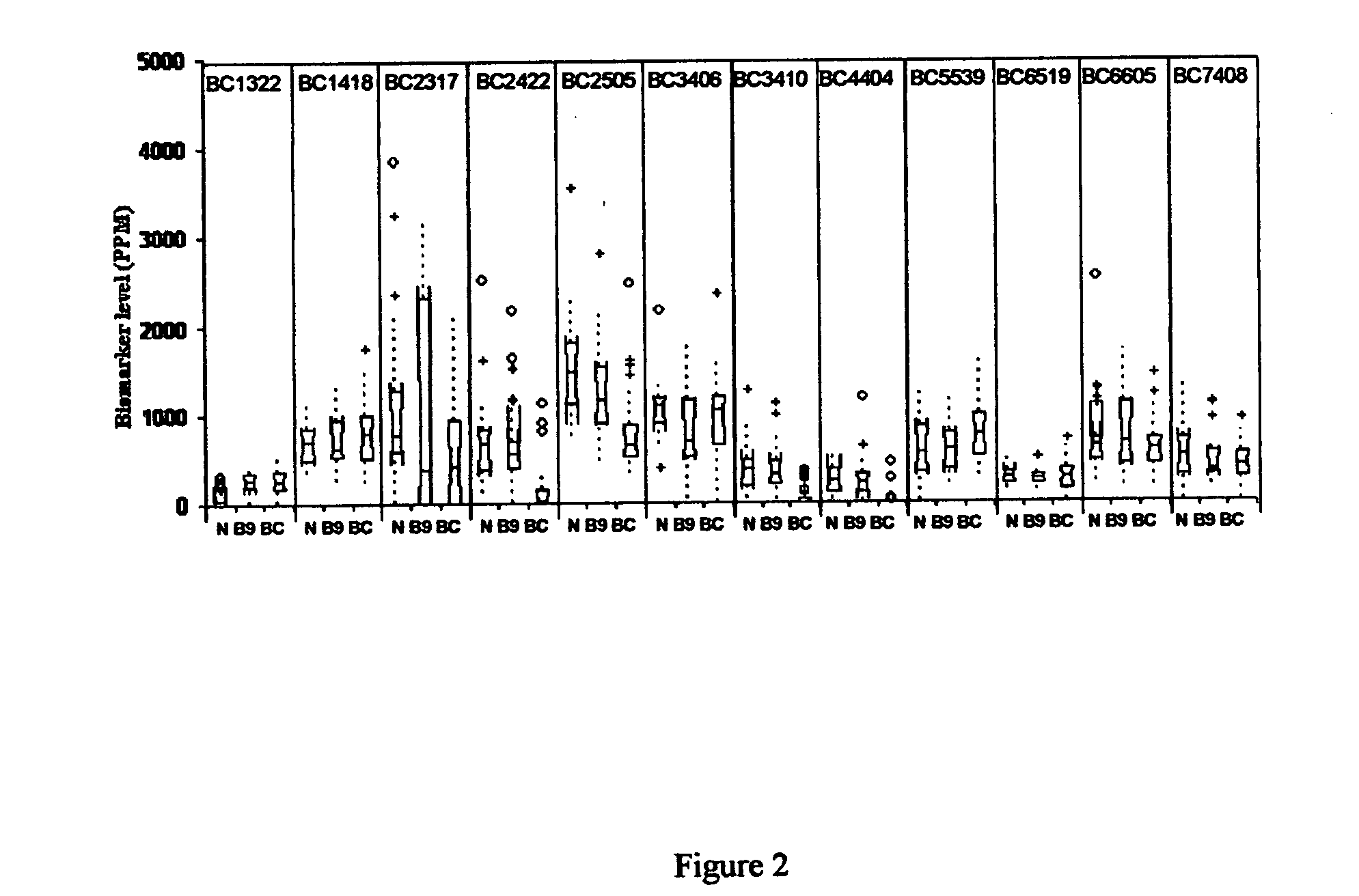
Twelve (12) protein biomarkers for diagnosis and early detection of breast cancer
InactiveUS20090035801A1More sensitiveMonitor responseMicrobiological testing/measurementBiological material analysisDisease severityGel electrophoresis
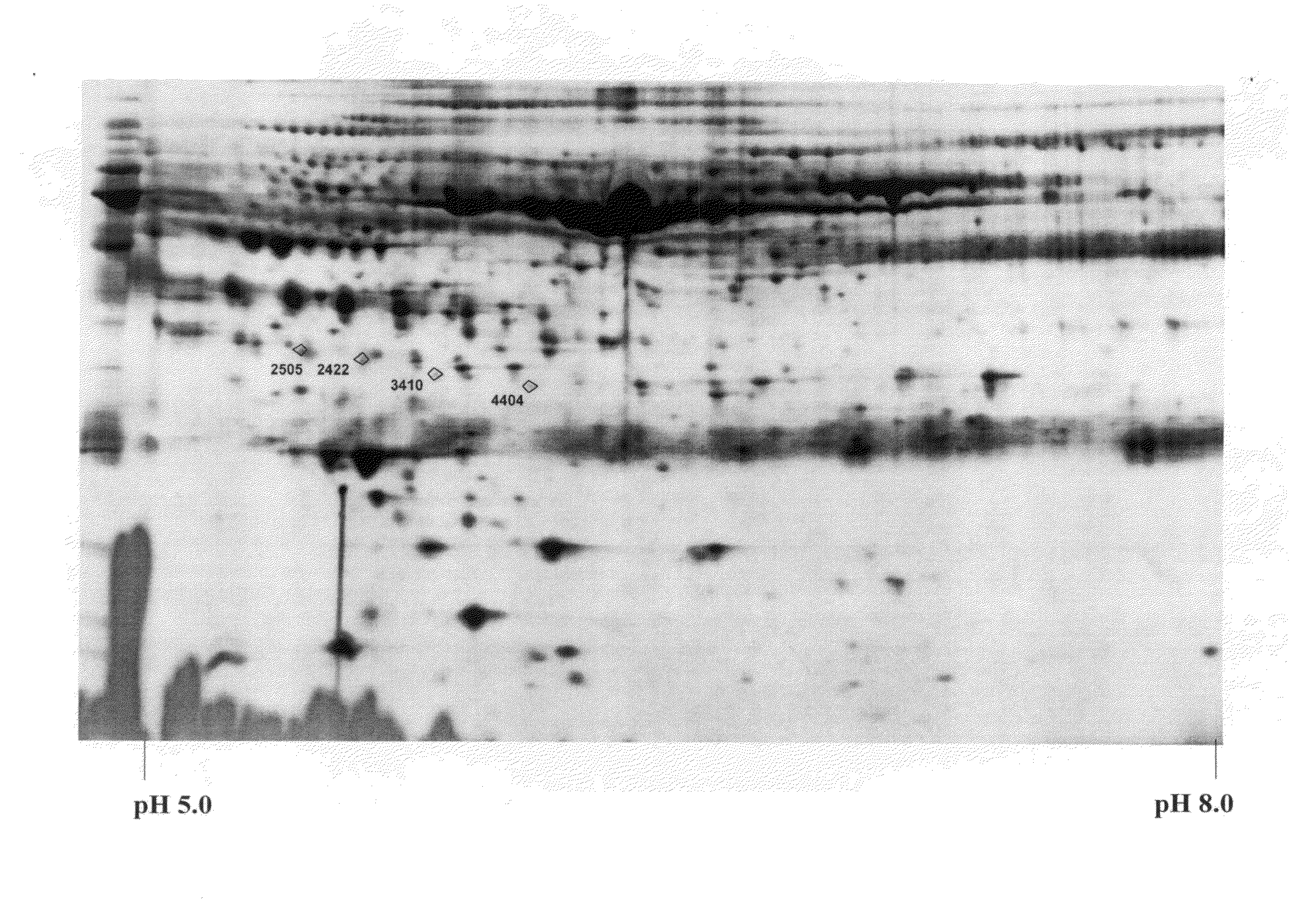
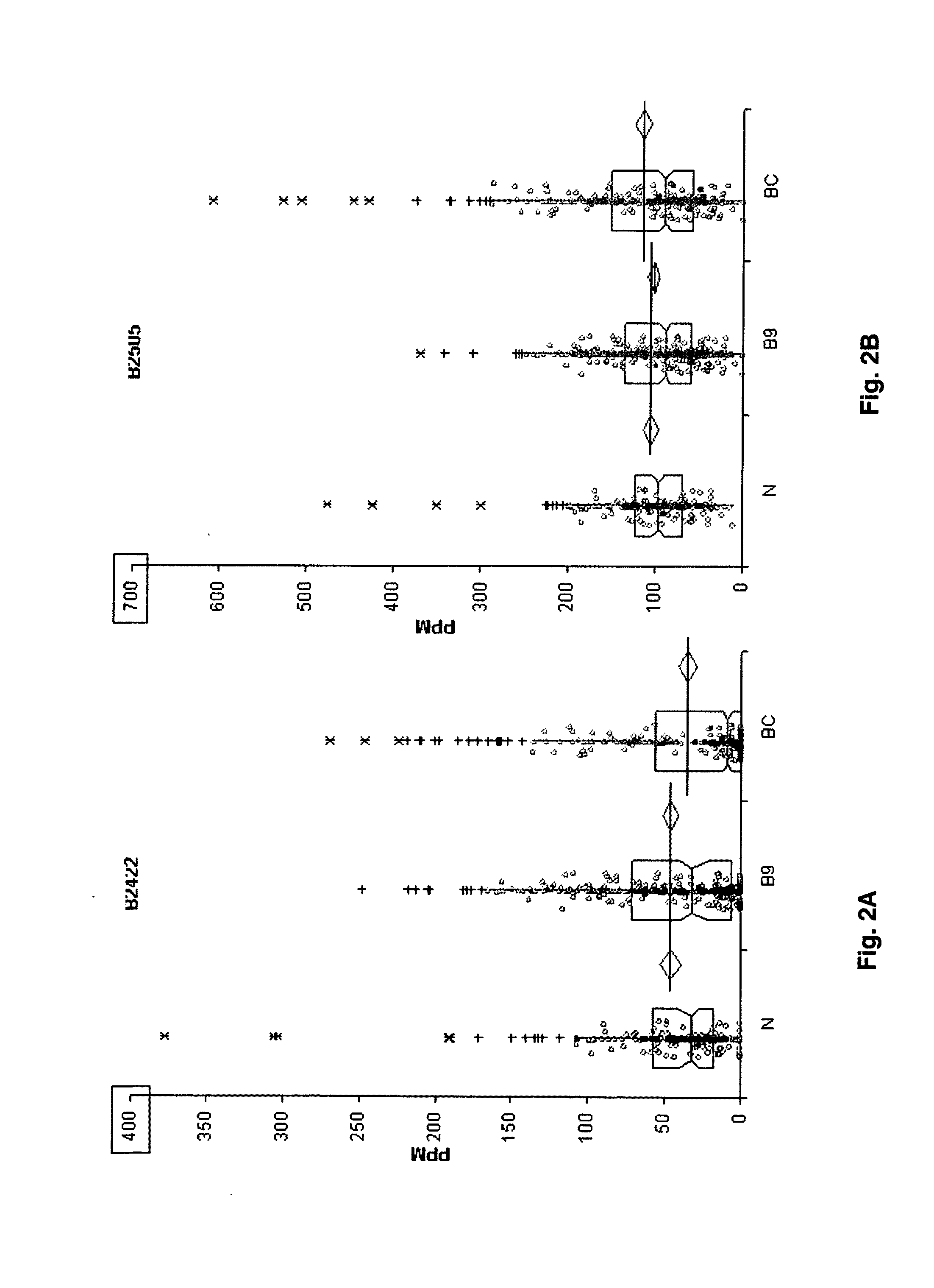
The invention relates to 12 identified protein biomarkers for diagnosis, determination of disease severity, and therapeutic response monitoring of patients with breast cancer. The method is based on the use of 2-dimensional (2D) gel electrophoresis to separate the complex mixture of proteins found in blood serum, the quantitation of up to 12 protein biomarkers, and statistical analysis of the concentration of the protein biomarkers.
Owner:NEOGENOMICS INC
Methods for diagnosis of Alzheimer's Disease in blood samples
InactiveUS7598049B2Improve the level ofLower Level RequirementsMicrobiological testing/measurementDisease diagnosisMild cognitive impairment (MCI)Neuro degeneration
The inventors have discovered a collection of proteinaceous biomarkers (“AD biomarkers) which can be measured in peripheral biological fluid samples to aid in the diagnosis of neurodegenerative disorders, particularly Alzheimer's disease and mild cognitive impairment (MCI). The invention further provides methods of identifying candidate agents for the treatment of Alzheimer's disease by testing prospective agents for activity in modulating AD biomarker levels.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV +2
Identification of cancer protein biomarkers using proteomic techniques
The claimed invention describes methods to diagnose or aid in the diagnosis of cancer. The claimed methods are based on the identification of biomarkers which are particularly well suited to discriminate between cancer subjects and healthy subjects. These biomarkers were identified using a unique and novel screening method described herein. The biomarkers identified herein can also be used in the prognosis and monitoring of cancer. The invention comprises the use of leptin, prolactin, OPN and IGF-II for diagnosing, prognosis and monitoring of ovarian cancer.
Owner:YALE UNIV
Isoform specificities of blood serum proteins and their use as differentially expressed protein biomarkers for diagnosis of breast cancer
InactiveUS20100129846A1More sensitiveMonitor responseMicrobiological testing/measurementBiological material analysisDiseaseMedicine
The present invention discloses twenty two 22 protein biomarkers of breast cancer. More specifically, the present invention discloses the identities, specificities, and uses of up to twenty two (22) protein biomarkers in blood serum for distinguishing between patients with earlier and later stages of breast cancer, patients with benign breast diseases or abnormalities, and normal individuals lacking breast abnormalities. More specifically, the present invention relates to specificities of isoforms of up to 22 protein biomarkers in blood serum for distinguishing between patients with earlier and later stages of breast cancer, patients with benign breast diseases or abnormalities, and normal individuals lacking breast abnormalities.
Owner:NEOGENOMICS INC
Biomarkers of mild cognitive impairment and alzheimer's disease
A method for quantifying a neurodegenerative disorder in a patient that includes obtaining a fluid sample from the subject; measuring a protein biomarker complex in said fluid sample and correlating the measurement with mild cognitive impairment or Alzheimer's disease status. The biomarkers include those that comprise at least one of a transthyretin protein and / or a prostaglandin-H2 D-isomerase protein, and at least one second, different protein selected from a transthyretin, prostaglandin-H2 D-isomerase, beta-2-microglobulin, cystatin C, superoxide dismutase [Cu—Zn], plasma retinol-binding protein, phosphatidylethanolamine-binding protein, carbonic anhydrase 2, prostaglandin-H2 D-isomerase, and / or serotransferrin protein;
Owner:UNIV OF KENTUCKY RES FOUND
In situ polypeptide identification
InactiveUS20070069122A1Material analysis by electric/magnetic meansIsotope separationProtein insertionFractionation
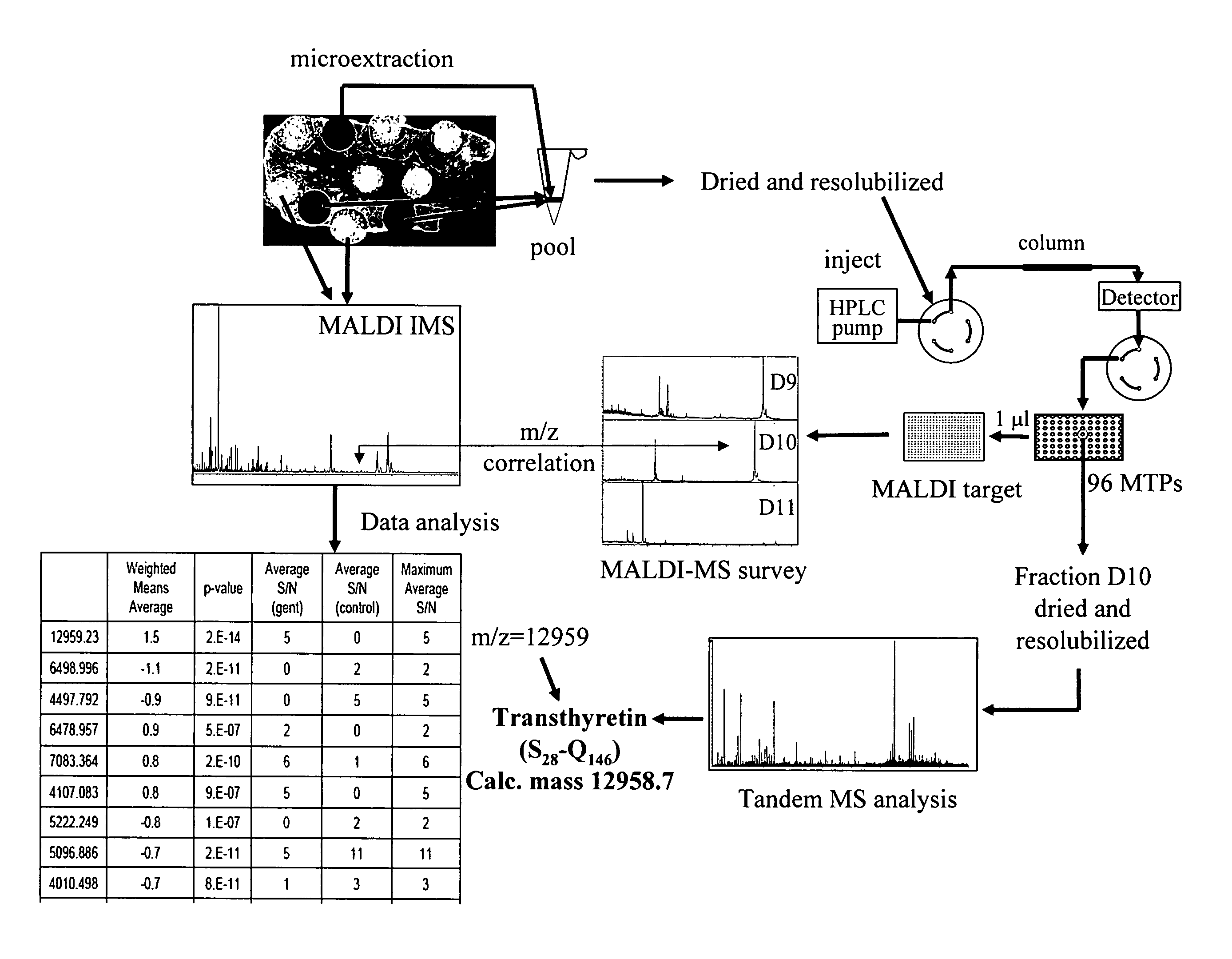
The invention relates to a method for identifying protein biomarkers directly in a specific region of interest of a tissue which comprises the identification of a mass of interest by analysis of MALDI imaging mass spectrometry results, followed by the identification of the protein represented by said mass of interest by direct elution from the specific region of interest of a tissue, fractionation and tandem mass spectrometry.
Owner:F HOFFMANN LA ROCHE INC
Markers for detection of complications resulting from in utero encounters
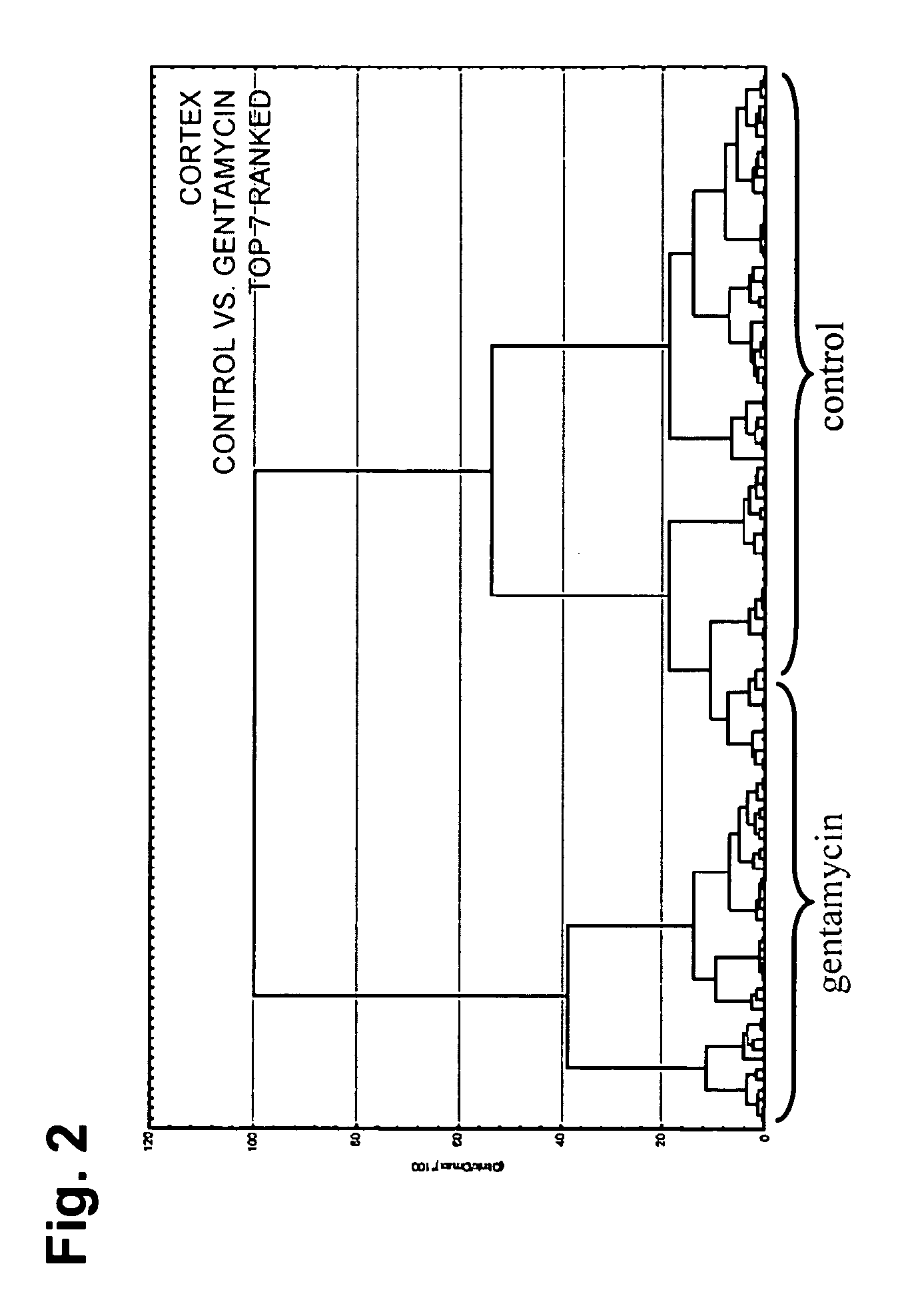
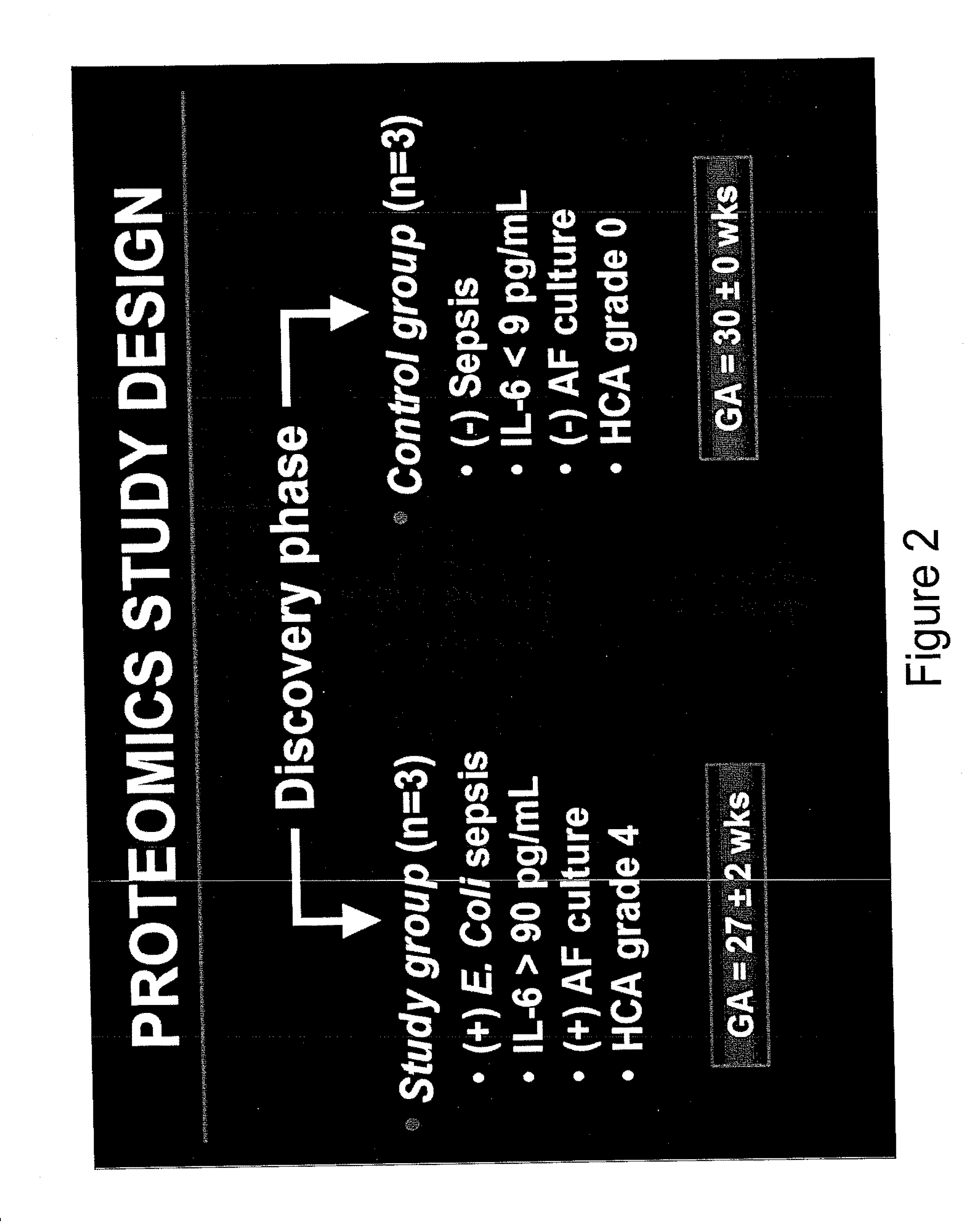
ActiveUS20120021442A1Unreliable resultMore targeted therapyDisease diagnosisNeonatal sepsisTraumatic intraventricular hemorrhage
Described herein are biomarkers, such as protein biomarkers, which are diagnostic of and predictive for complications that result from an in utero encounter, such as an infection by the fetus, that can lead to premature birth (PTB). The biomarkers can be used to identify fetuses and newborns at risk for complications of PTB, such as (Early Onset Neonatal Sepsis) EONS, intra-ventricular hemorrhage (IVH) and other poor outcomes.
Owner:YALE UNIV
Identities, specificities, and use of twenty two (22) differentially expressed protein biomarkers for blood based diagnosis of breast cancer
The present invention discloses twenty two 22 protein biomarkers of breast cancer. More specifically, the present invention discloses the identities, specificities, and uses of up to twenty two (22) protein biomarkers in blood serum for distinguishing between patients with earlier and later stages of breast cancer, patients with benign breast diseases or abnormalities, and normal individuals lacking breast abnormalities.
Owner:NEOGENOMICS INC
Method of predicting acute appendicitis
Embodiments of the invention provide method and devices for predicting the likelihood of acute appendicitis without invasive exploratory medical procedures. Several protein biomarkers: leucine-rich α-2-glycoprotein (LRG); S100-A8 (calgranulin); α-1-acid glycoprotein 1 (ORM); plasminogen (PLG); mannan-binding lectin serine protease 2 (MASP2); zinc-α-2-glycoprotein (AZGP1); Apolipoprotein D (ApoD); and α-1-antichymotrypsin (SERPINA3); are increased in the urine of patients with appendicitis. The method and devices comprise detecting the levels of these biomarkers and comparing with reference levels found in healthy individuals.
Owner:CHILDRENS MEDICAL CENT CORP
Method and apparatus for discovering patterns in binary or categorical data
The present invention relates to a computationally efficient method of finding patterns in any data that can be expressed in the form of arrays of binary features or arrays of categorical features. This includes data represented by continuous-valued attributes that can be transformed to a categorical representation, such as the discovery of patterns of genetic variability that may be causally related to diseases or traits, as well as the discovery of patterns of protein biomarkers that may be used for medical diagnostics, prognostics, and therapeutics. The invention further relates to a program storage device having instructions for controlling a computer system to perform the methods, and to a program storage device containing data structures used in the practice of the methods.
Owner:MOSER ALLAN ROBERT
Integrated modular unit including an analyte concentrator-microreactor device connected to a cartridge-cassette
ActiveUS8703061B2Easy interchangeBioreactor/fermenter combinationsBiological substance pretreatmentsMicroreactorCapillary electrophoresis
The present invention relates to an immunoaffinity device for capturing one or more analytes present at high or low concentrations in simple or complex matrices. The device is designed as an integrated modular unit and connected to capillary electrophoresis or liquid chromatography for the isolation, enrichment, separation and identification of polymeric macromolecules, primarily protein biomarkers. The integrated modular unit includes an analyte-concentrator-microreaction device connected to a modified cartridge-cassette.
Owner:PRINCETON BIOCHEM
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com