Method for biomarker and drug-target discovery for prostate cancer diagnosis and treatment as well as biomarker assays determined therewith
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
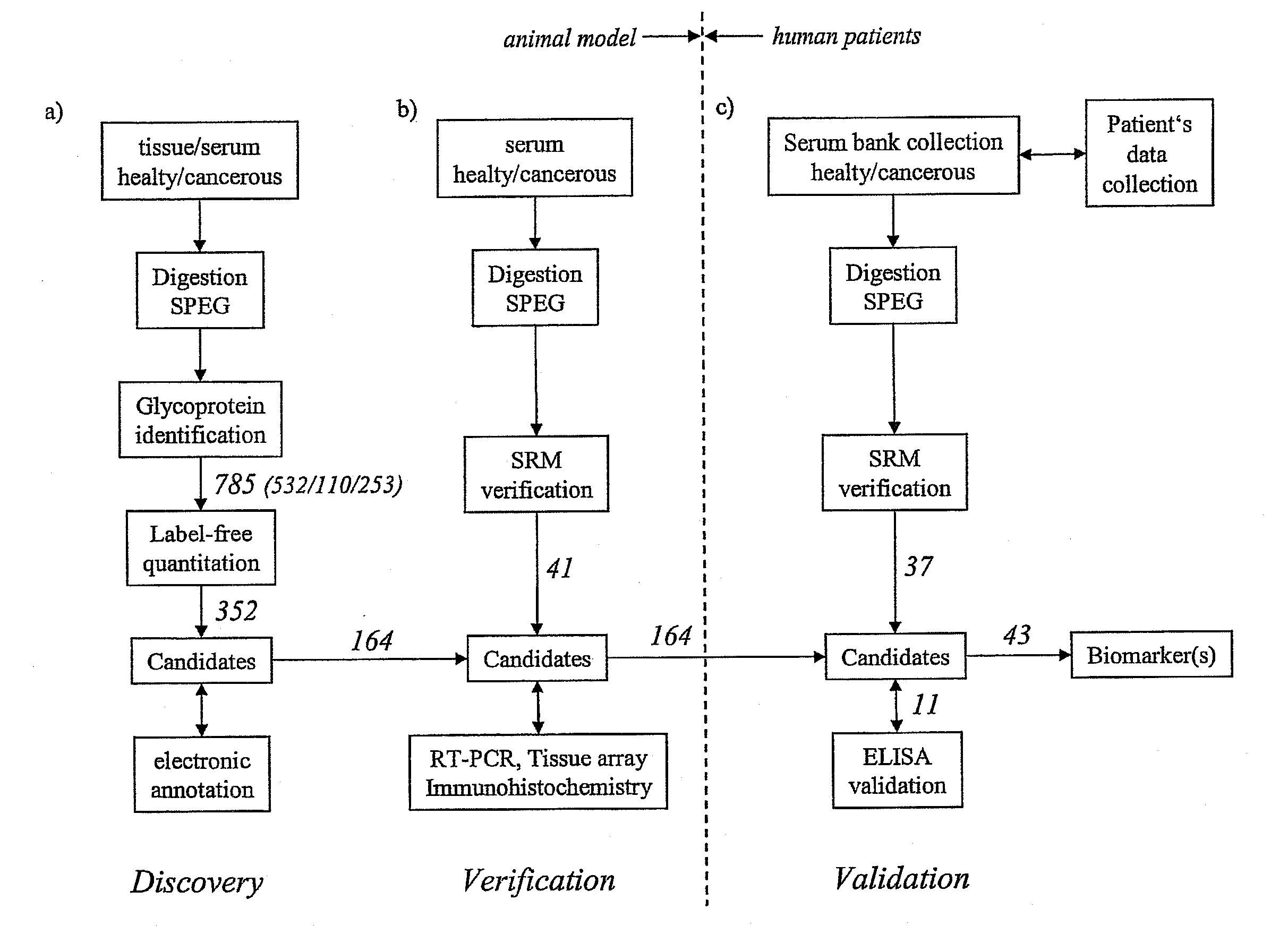
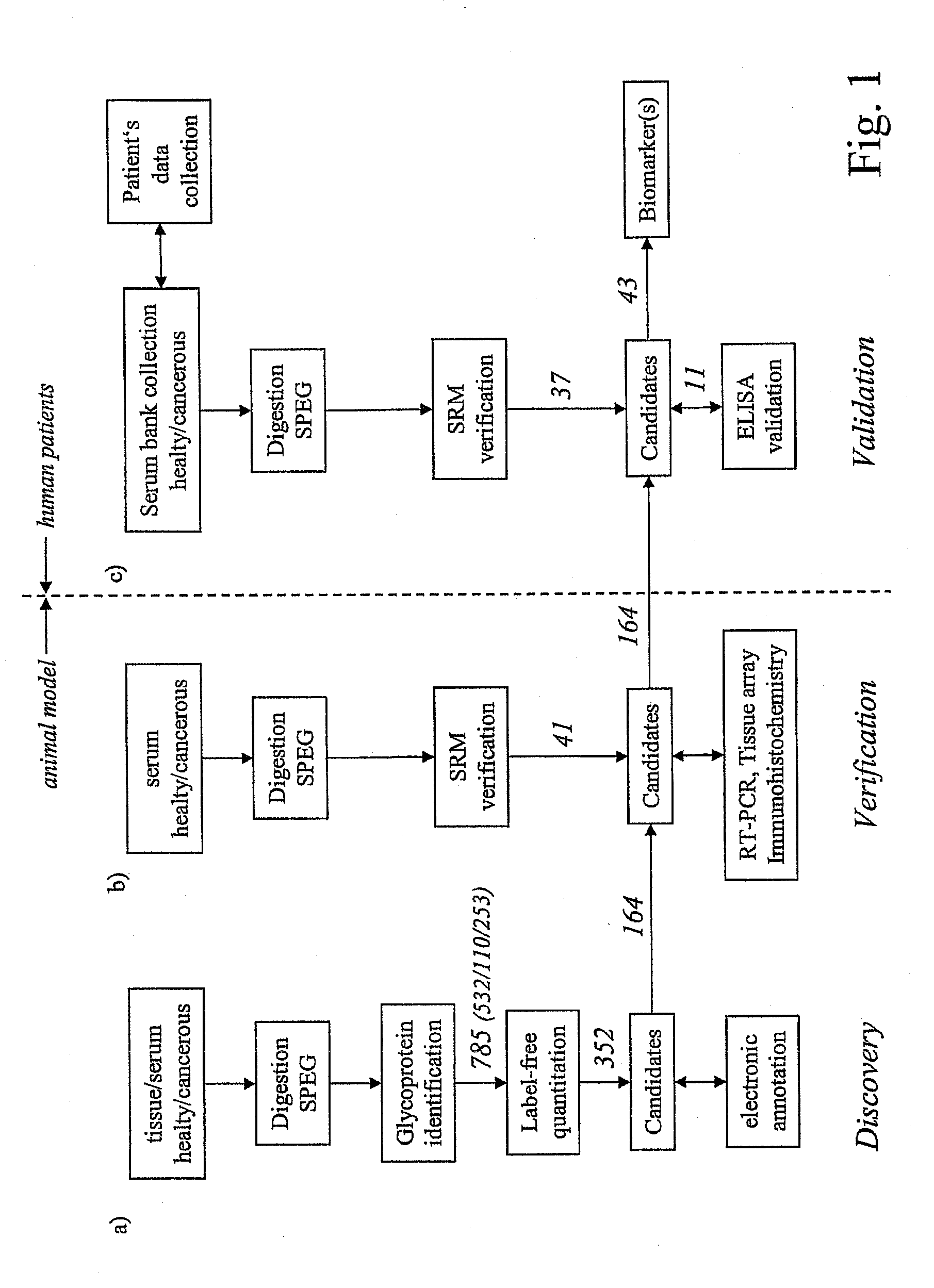
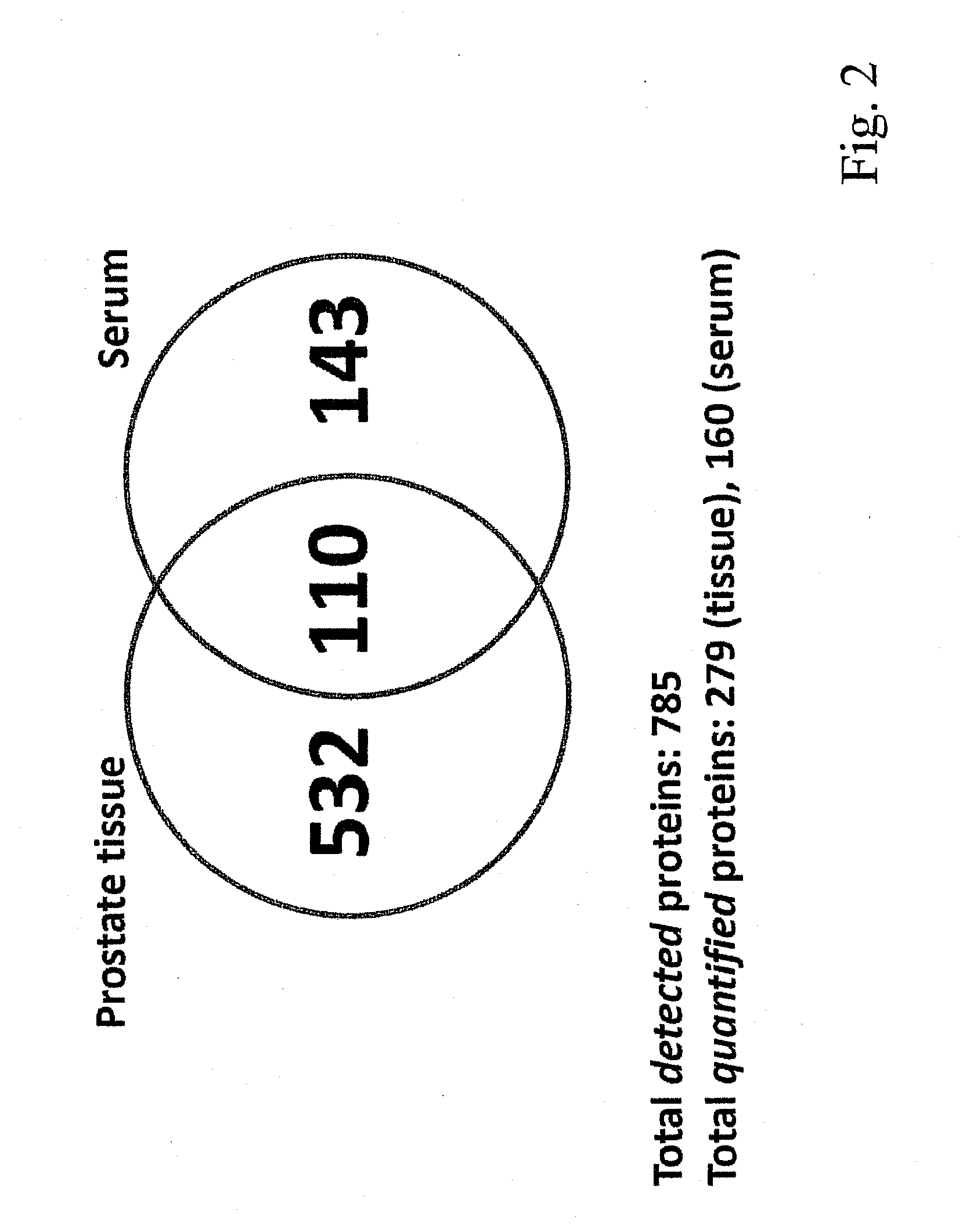
[0053]Referring to the drawings, which are for the purpose of illustrating the present preferred embodiments of the invention and not for the purpose of limiting the same, FIG. 1 shows an overview of the integrated proteomic approach for biomarker discovery, verification and validation. The scheme is divided in two main sections: First the discovery and verification phases (a) and (b) performed using an animal model (mice) and second the validation phase (c) with human patient samples.
[0054]In the following example the method is applied to the determination of biomarkers for prostate cancer. As outlined above, this shall however not be construed to the actual gist of the invention, as the method may equivalently be applied to other types of cancer such as breast cancer, lung cancer, ovarian cancer and the like and it may also equivalently be applied generally to other types of diseases or dysfunctions such as diabetes (mellitus and other types), neurodegenerative diseases: such as A...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com