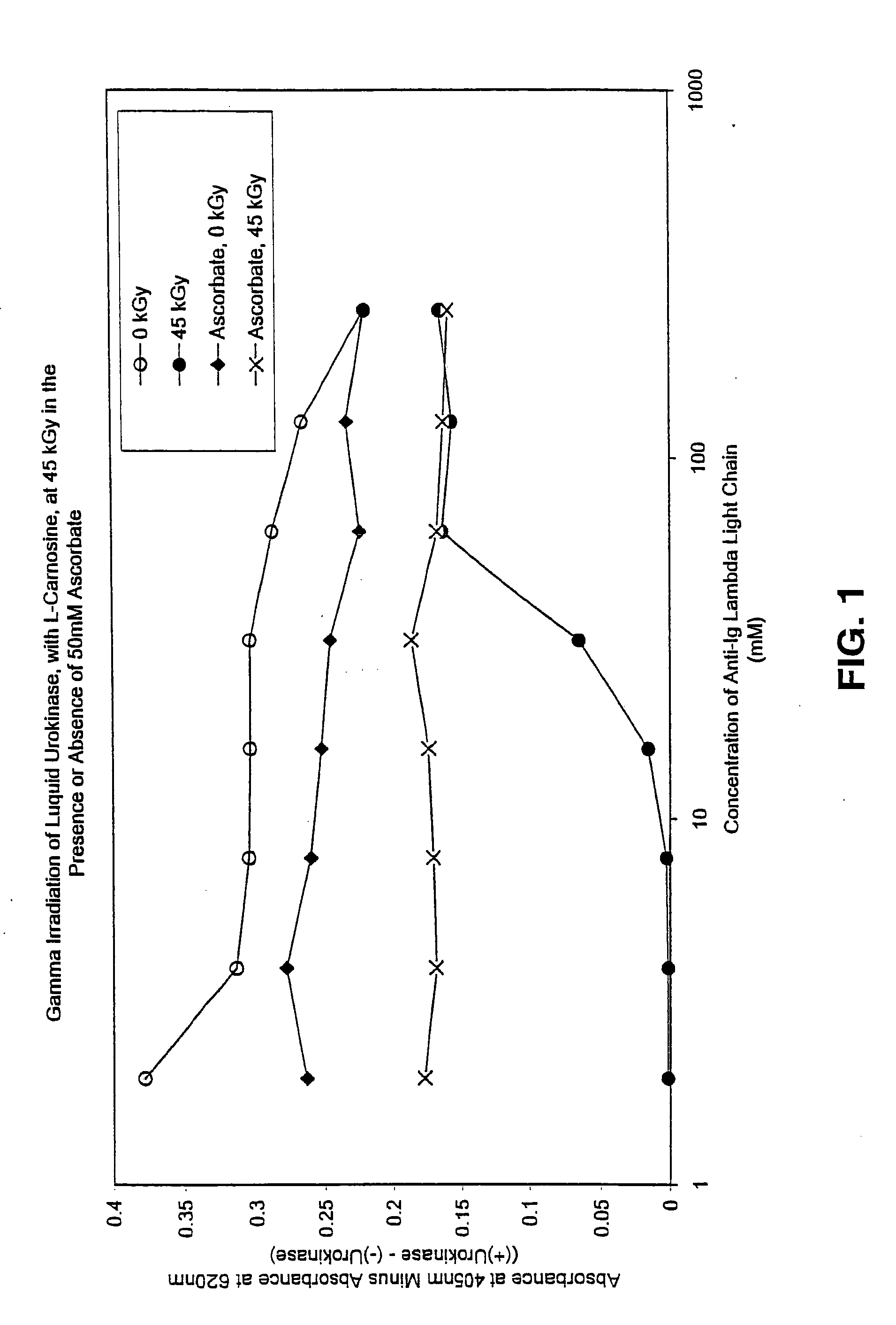
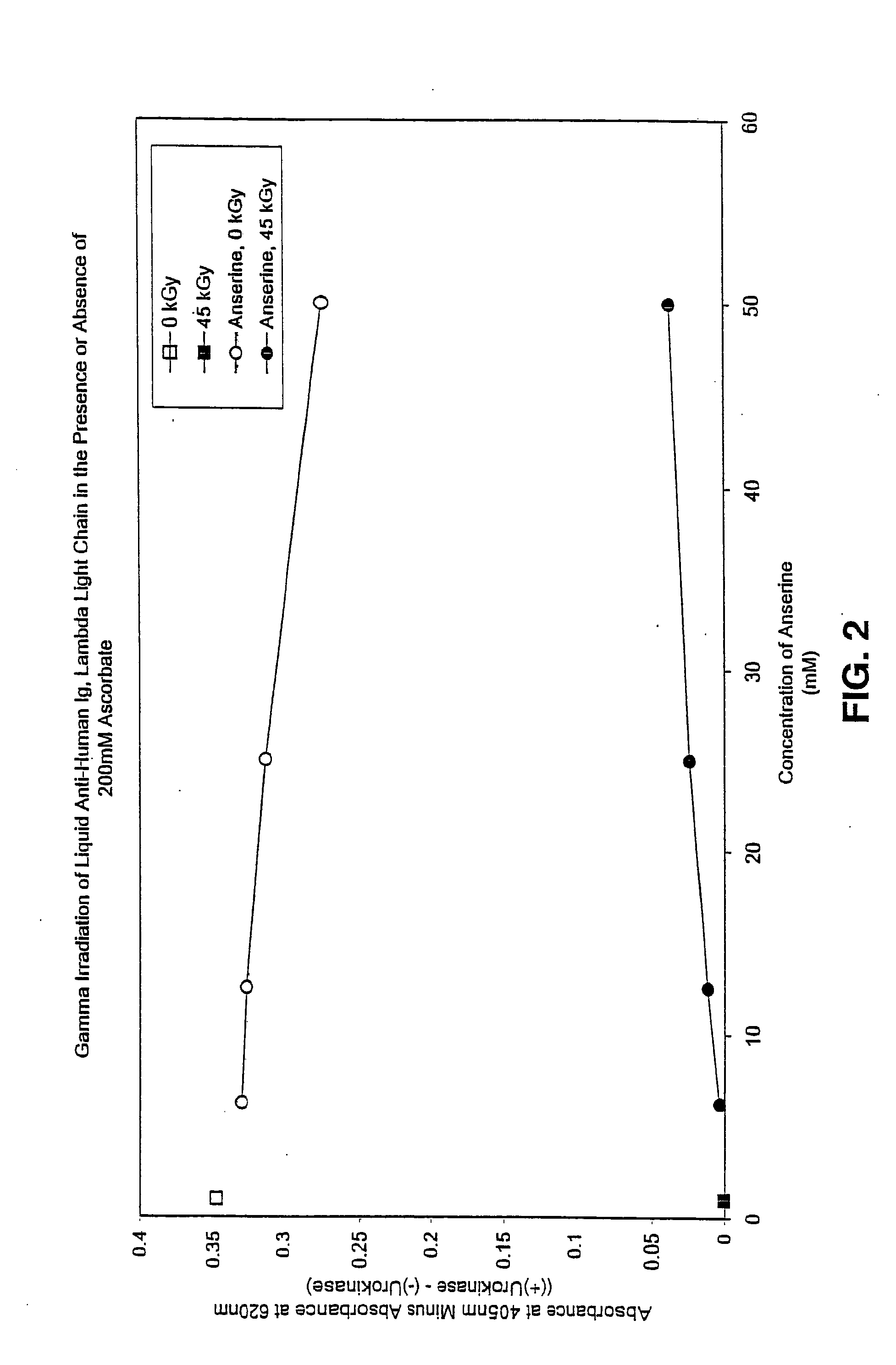
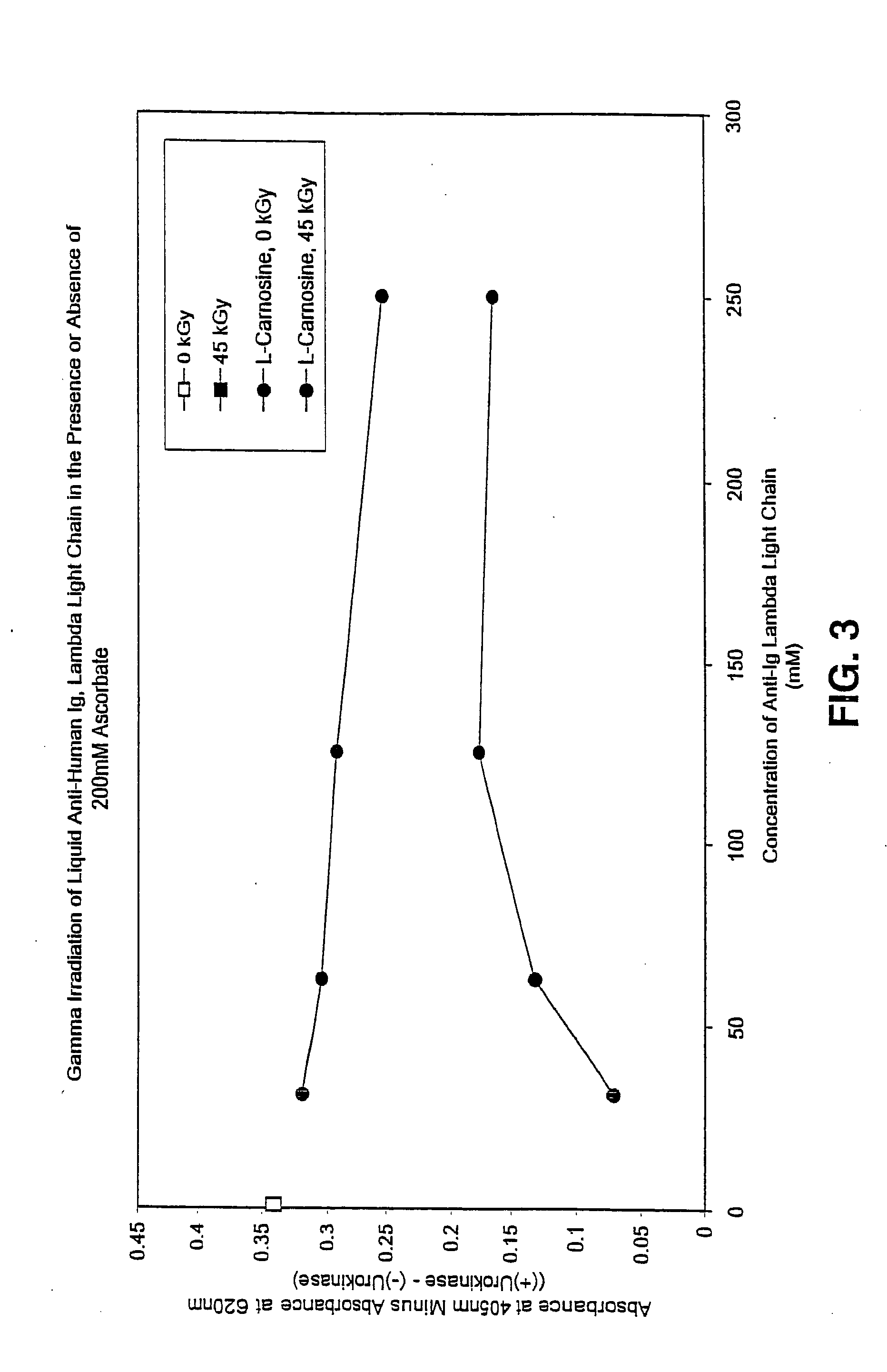
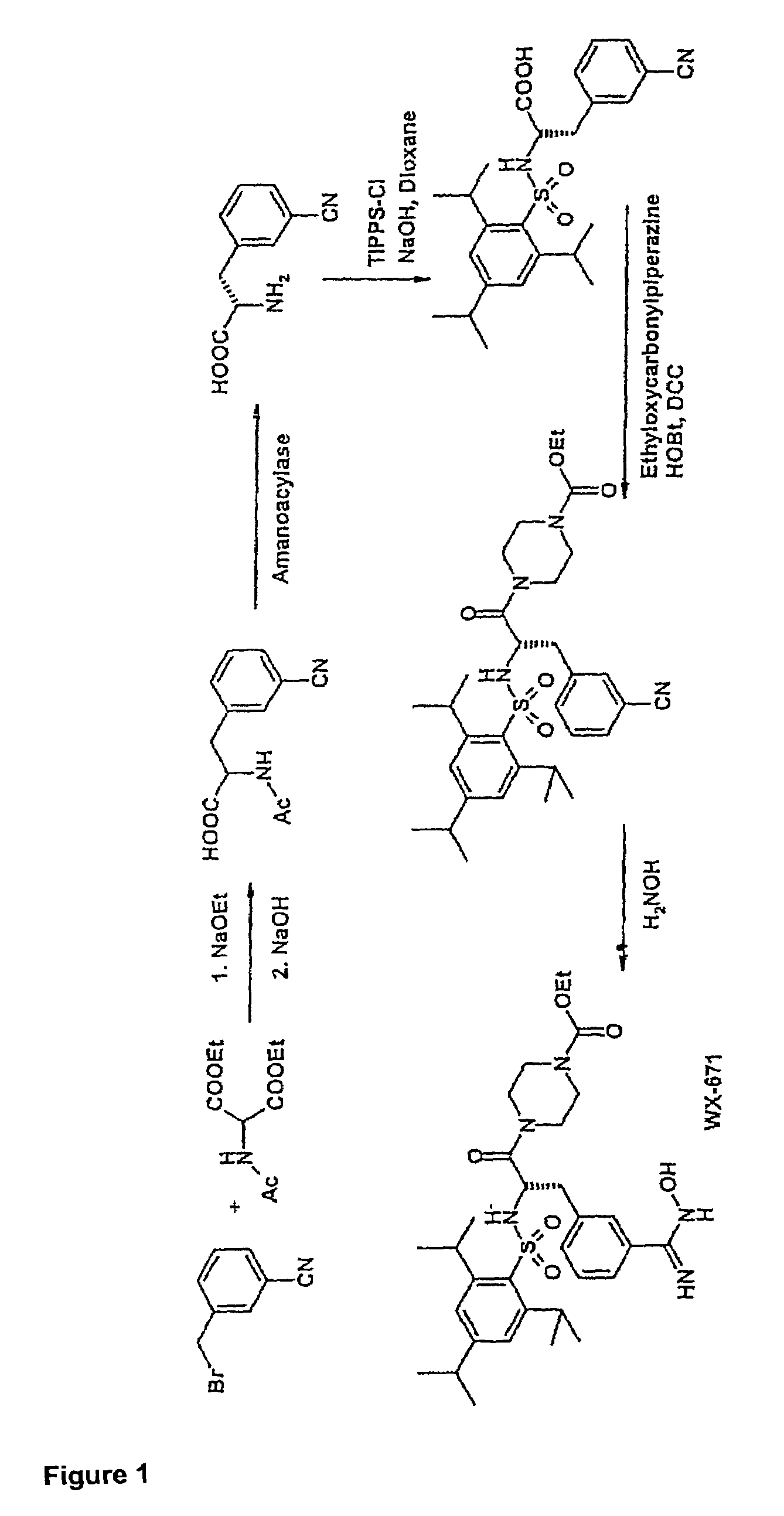
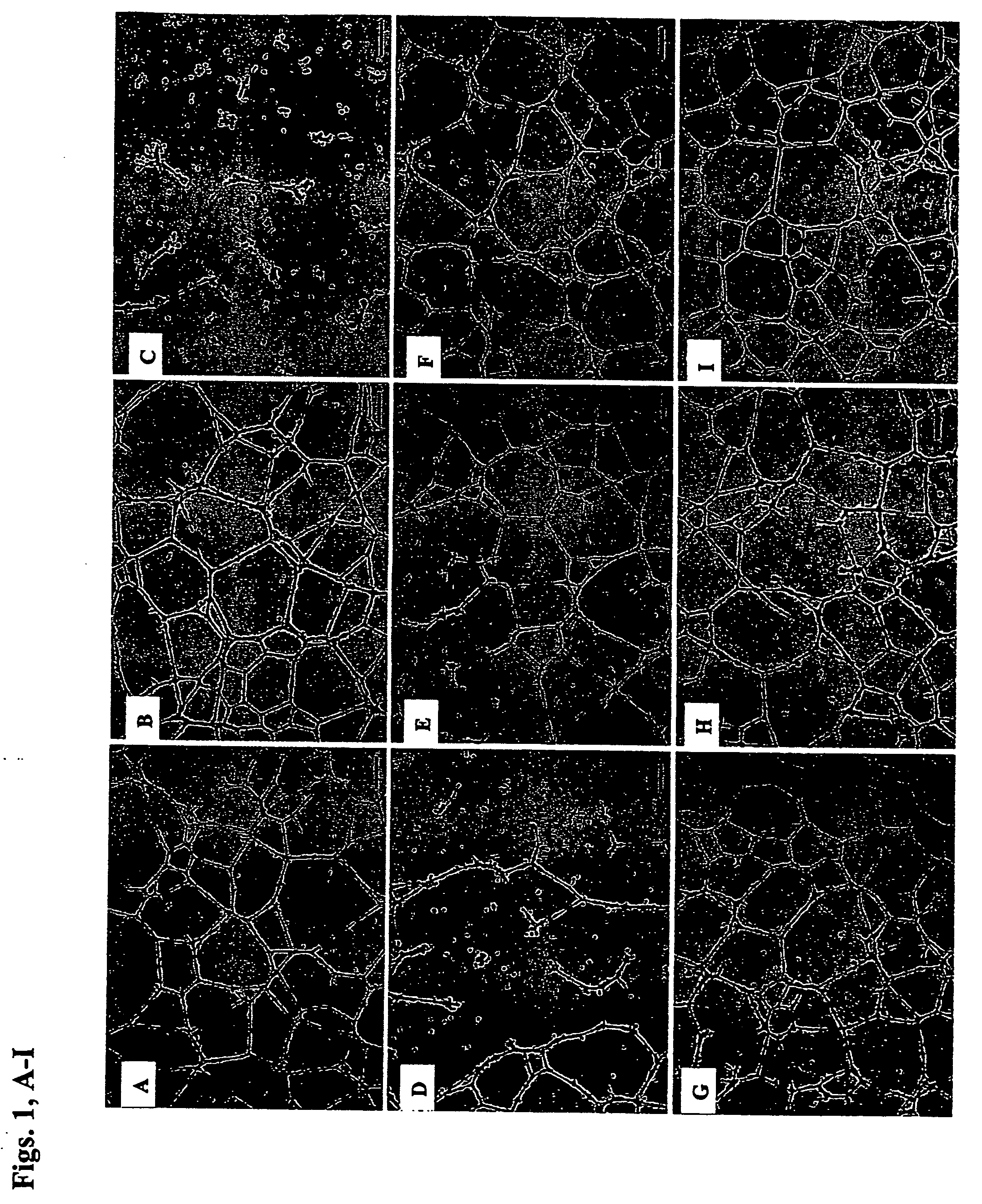
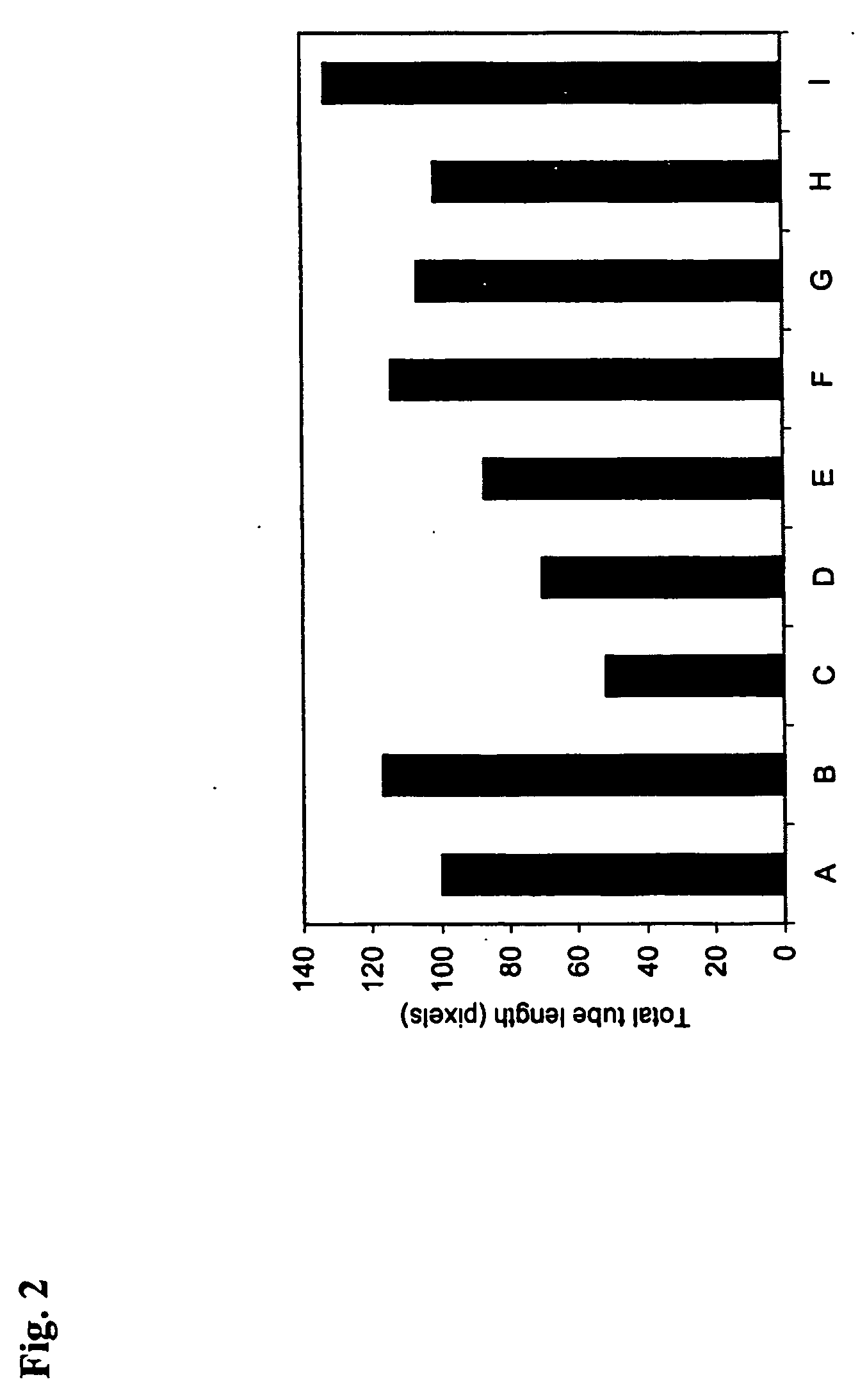
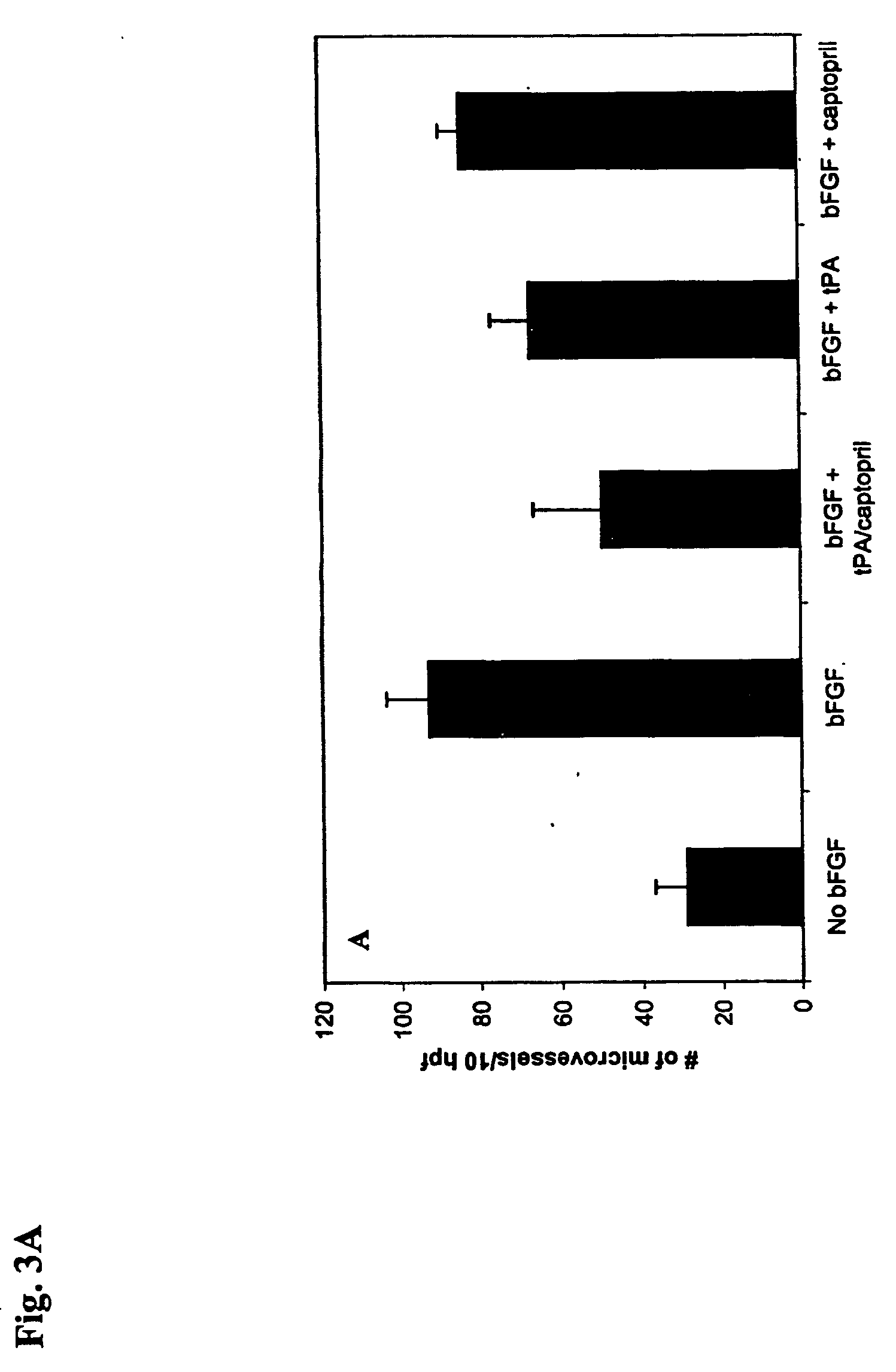
Patents
Literature
148 results about "Urokinase Plasminogen Activator" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Urokinase, also known as urokinase-type plasminogen activator (uPA), is a serine protease present in humans and other animals. The human urokinase protein was discovered, but not named, by McFarlane and Pilling in 1947.
Treating or preventing the early stages of degeneration of articular cartilage or subchondral bone in mammals using carprofen and derivatives
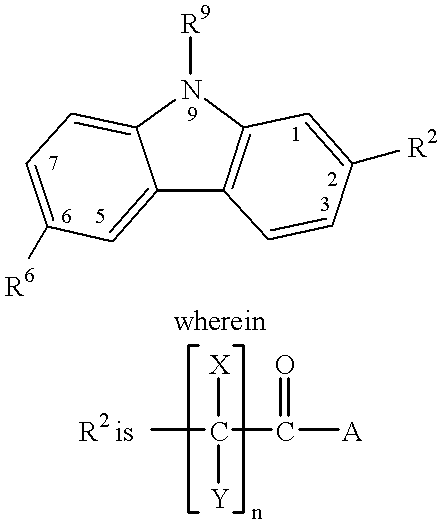
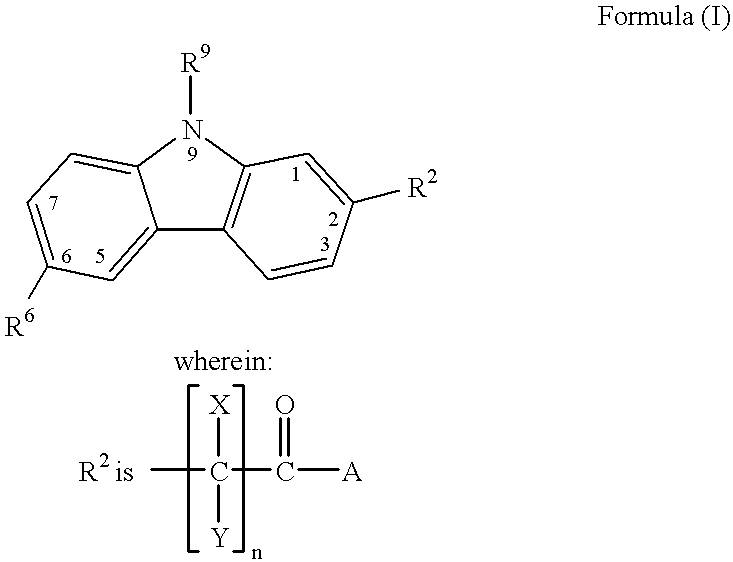
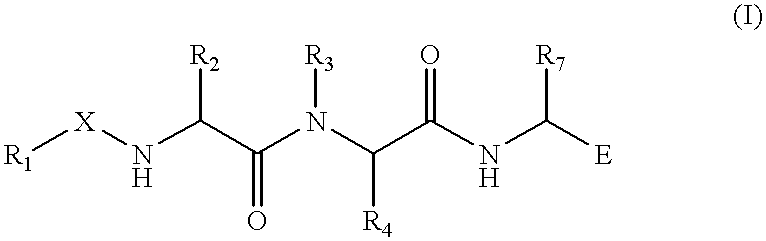
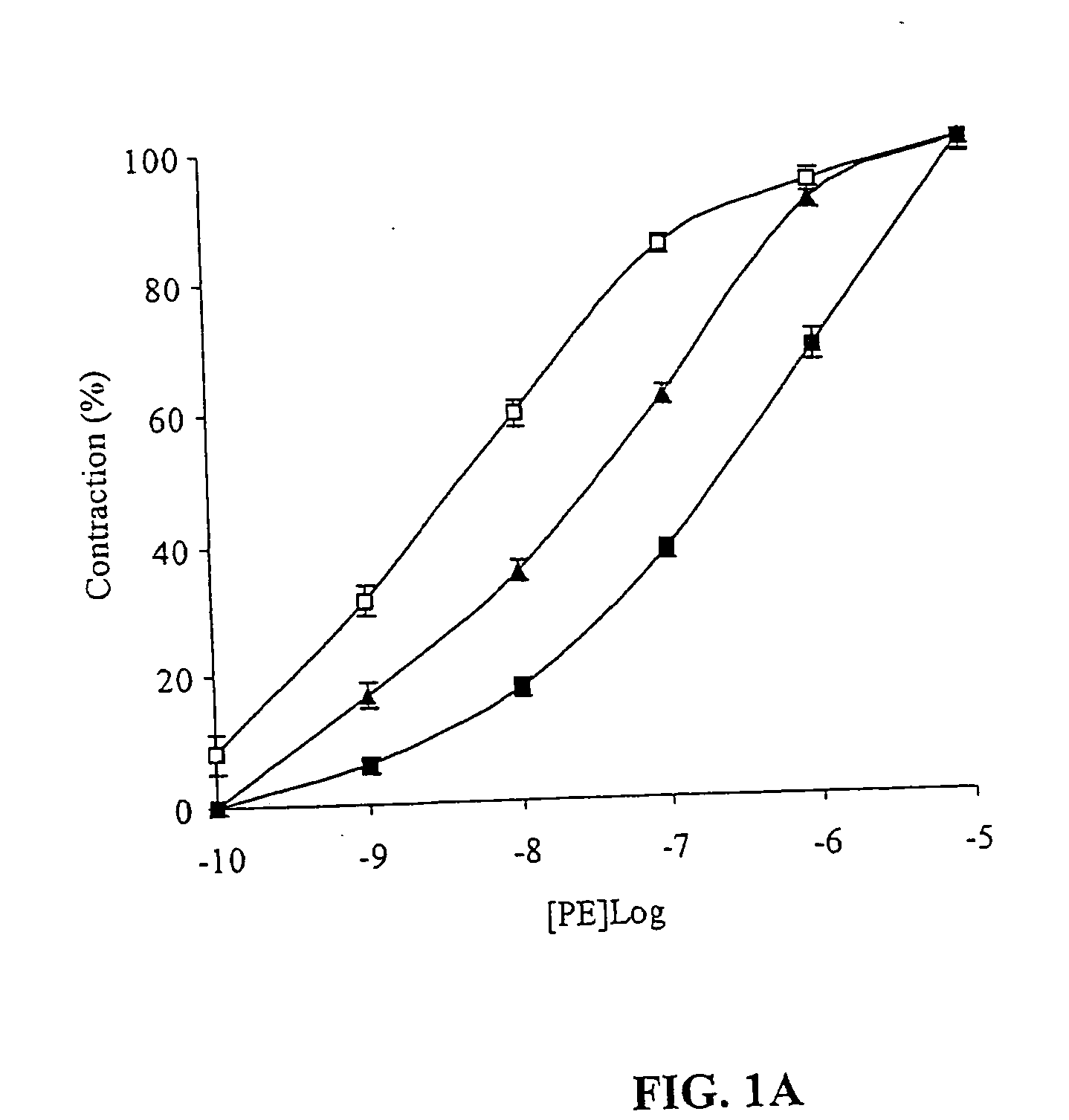
Treating or preventing the early stages of degeneration of articular cartilage or subchondral bone in the affected joint of a mammal is accomplished by administering a chondroprotective compound of Formula (I):where A is hydroxy, (C1-C4)alkoxy, amino, hydroxy-amino, mono-(C1-C2)alkylamino, di-(C1-C2)alkylamino; X and Y are independently H or (C1-C2)alkyl; and n is 1 or 2; R6 is halogen, (C1-C3)alkyl, trifluoromethyl, or nitro; R9 is H; (C1-C2)alkyl; phenyl or phenyl-(C1-C2)alkyl, where phenyl is optionally mono-substituted by fluoro or chloro; -C(=O)-R, where R is (C1-C2)alkyl or phenyl, optionally mono-substituted by fluoro or chloro; or -C(=O)-O-R', where R1 is (C1-C2)alkyl.This treatment ameliorates, diminishes, actively treats, reverses or prevents any injury, damage or loss of articular cartilage or subchondral bone subsequent to said early stage of said degeneration. Whether or not a mammal needs such treatment is determined by whether or not it exhibits a statistically significant deviation from normal standard values in synovial fluid or membrane from the affected joint, with respect to at least five of the following substances: increased interleukin-1 beta (IL-1beta); increased tumor necrosis factor alpha (TNFalpha); increased ratio of IL-1beta to IL-1 receptor antagonist protein (IRAP); increased expression of p55 TNF receptors (p55 TNF-R); increased interleukin-6 (IL-6); increased leukemia inhibitory factor (LIF); decreased insulin-like growth factor-1 (IGF-1); decreased transforming growth factor beta (TGFbeta); decreased platelet-derived growth factor (PDGF); decreased basic fibroblast growth factor (b-FGF); increased keratan sulfate; increased stromelysin; increased ratio of stromelysin to tissue inhibitor of metalloproteases (TIMP); increased osteocalcin; increased alkaline phosphatase; increased cAMP responsive to hormone challenge; increased urokinase plasminogen activator (uPA); increased cartilage oligomeric matrix protein; and increased collagenase.
Owner:PFIZER INC +1
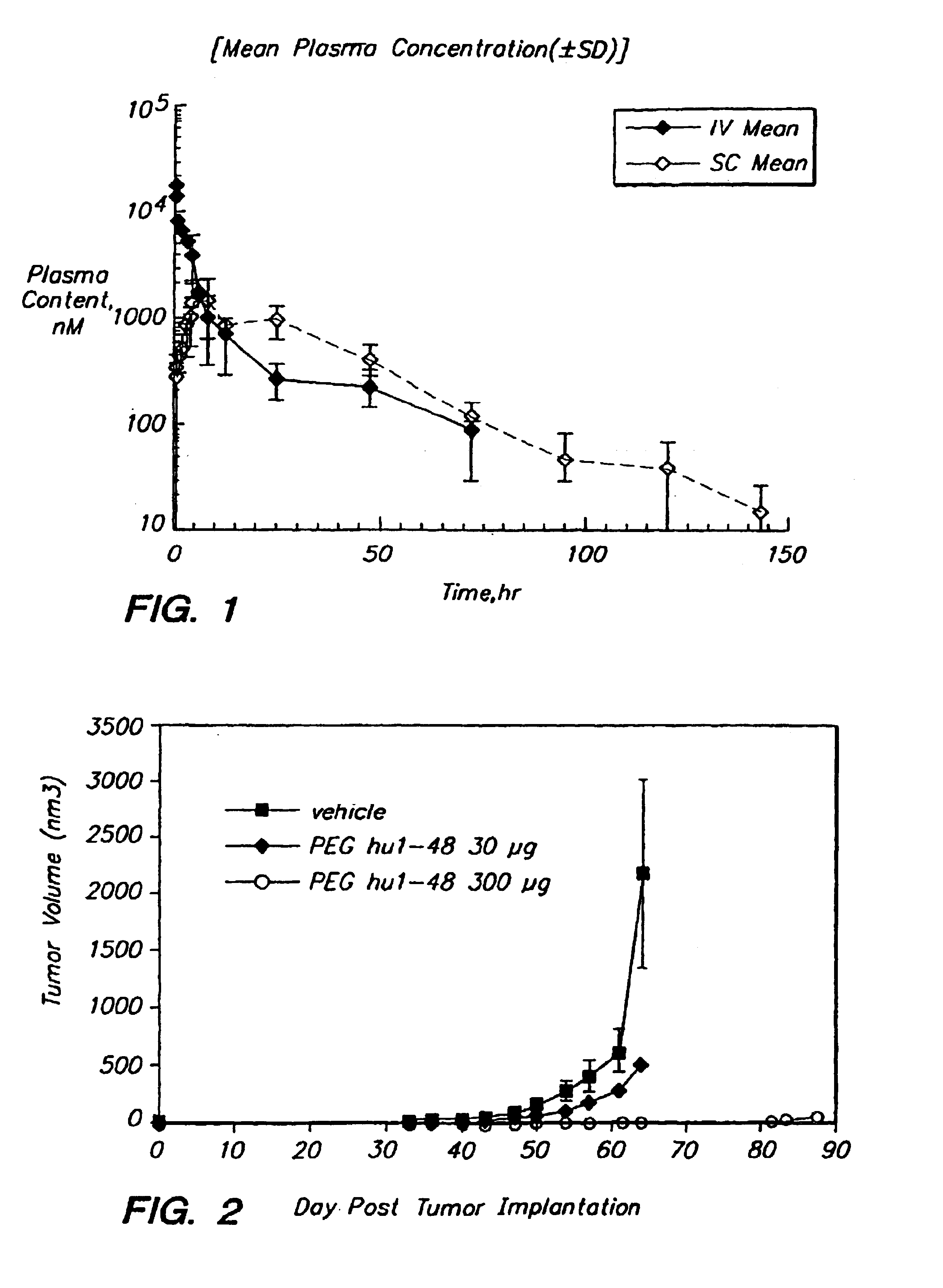
Method for increasing the serum half-life of a biologically active molecule
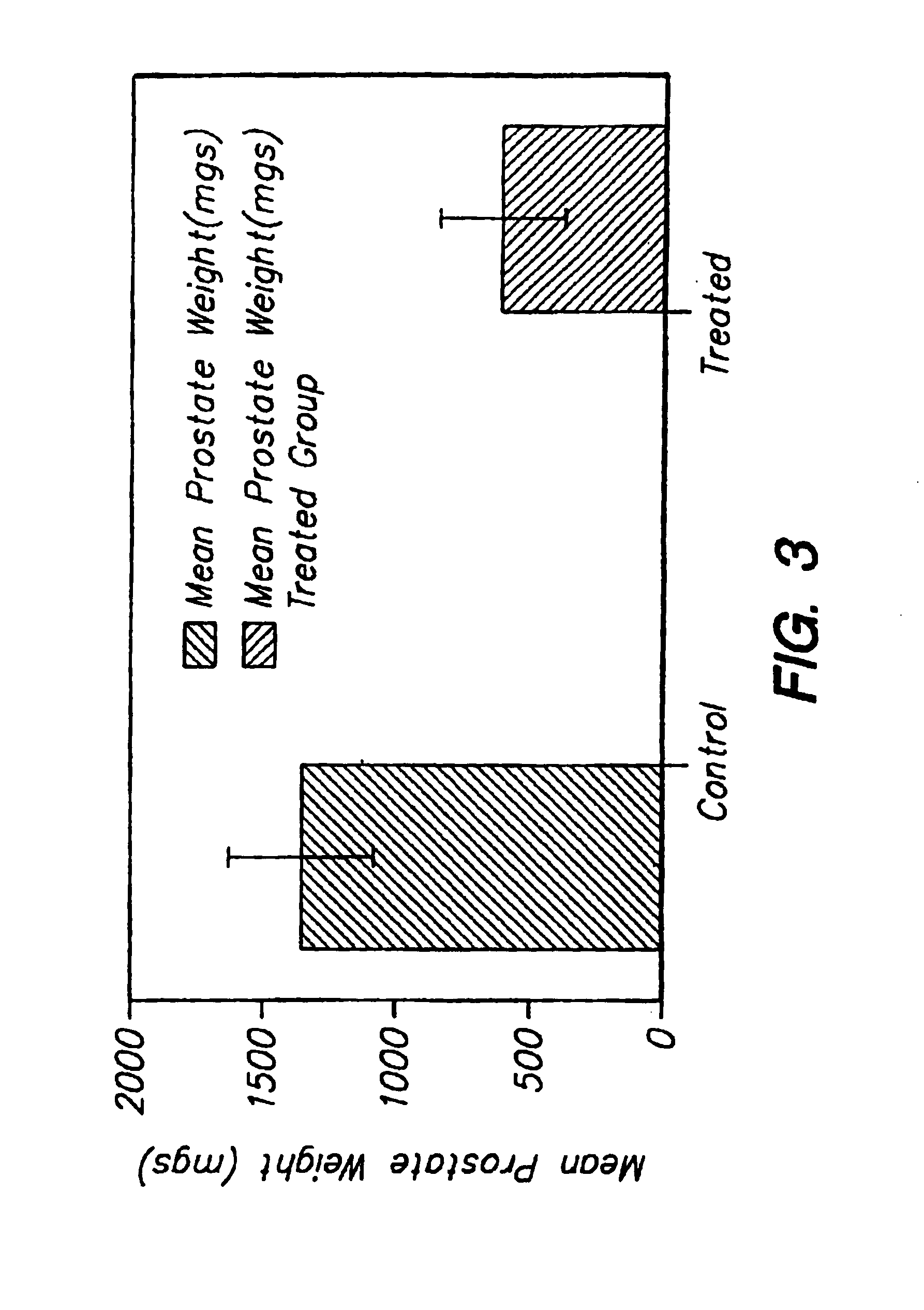
A method is provided for preparing a biologically active molecule having an increased serum half-life. The method involves conjugating a polymer such as polyethylene glycol to the biologically active molecule. Also provided are polypeptide drugs having an increased serum half-life, e.g., human urokinase plasminogen activator (human “uPA” or “hUPA”) or a fragment or derivative thereof. Pharmaceutical compositions containing such molecules and methods of using them to treat uPA-mediated and uPA receptor-mediated disorders are also provided.
Owner:CHIRON CORP
Method and tool for predicting cancer and other diseases
ActiveUS20100098705A1Monitor progressMicrobiological testing/measurementAntibody ingredientsUrokinase Plasminogen ActivatorReceptor
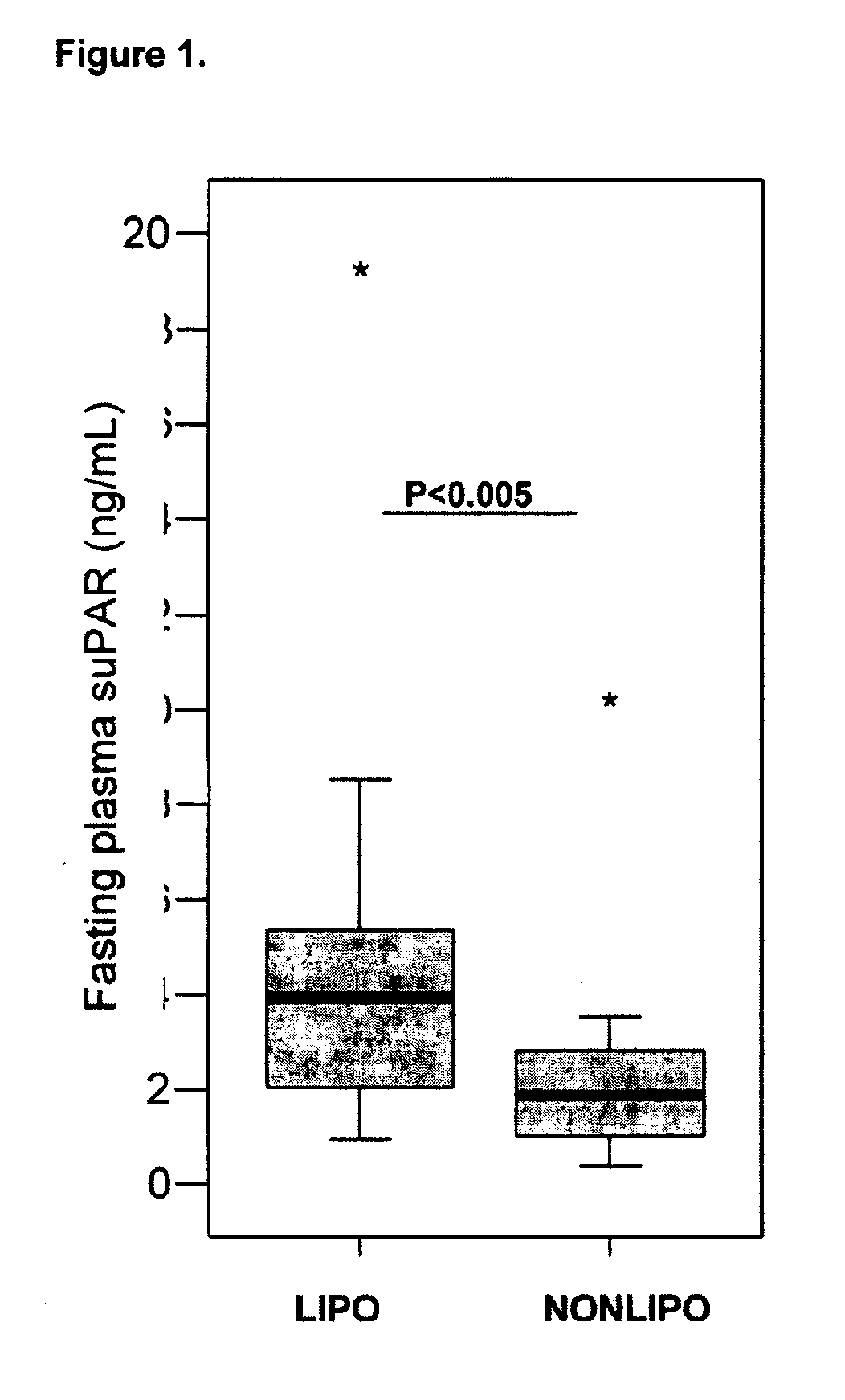
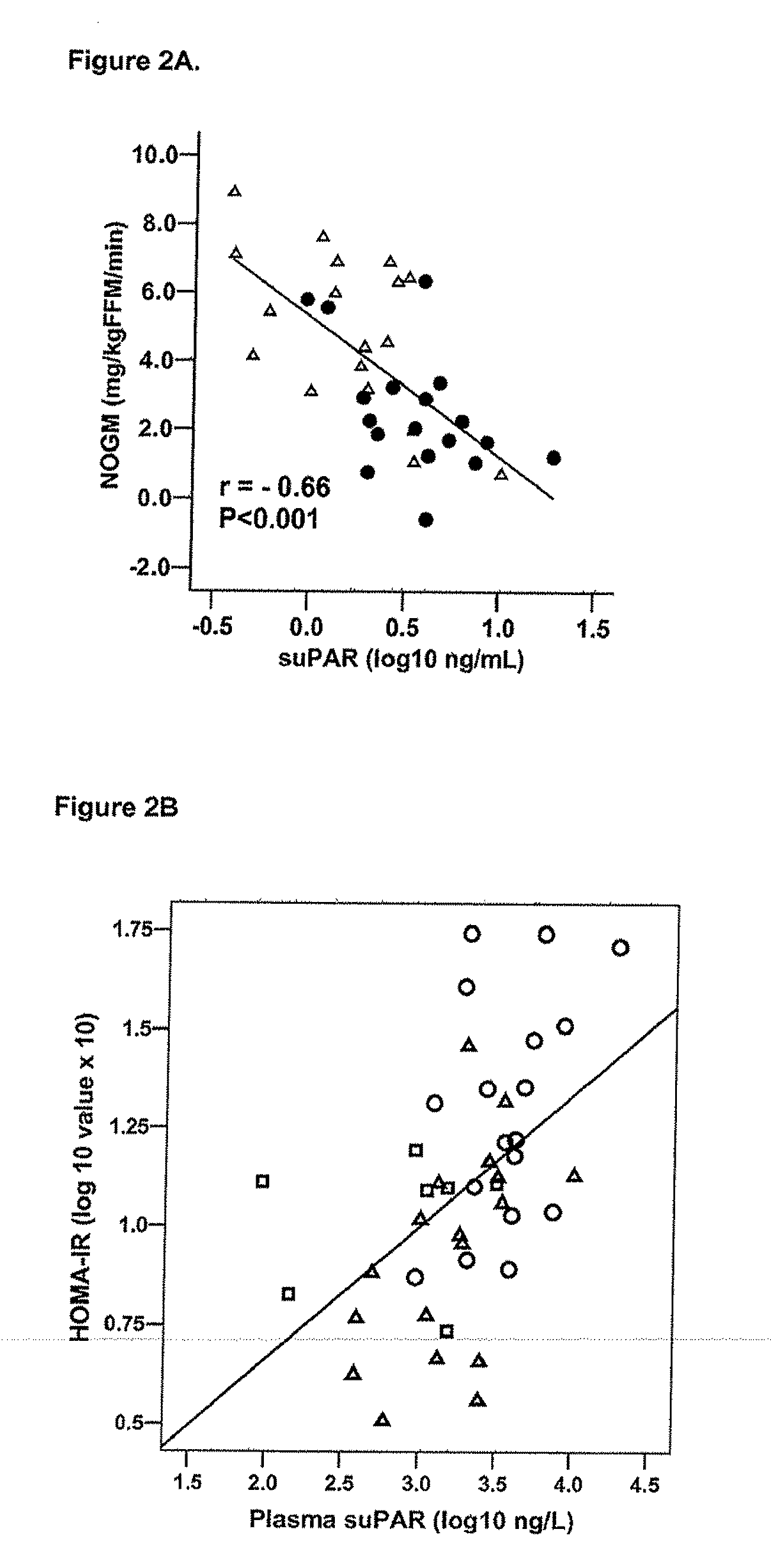
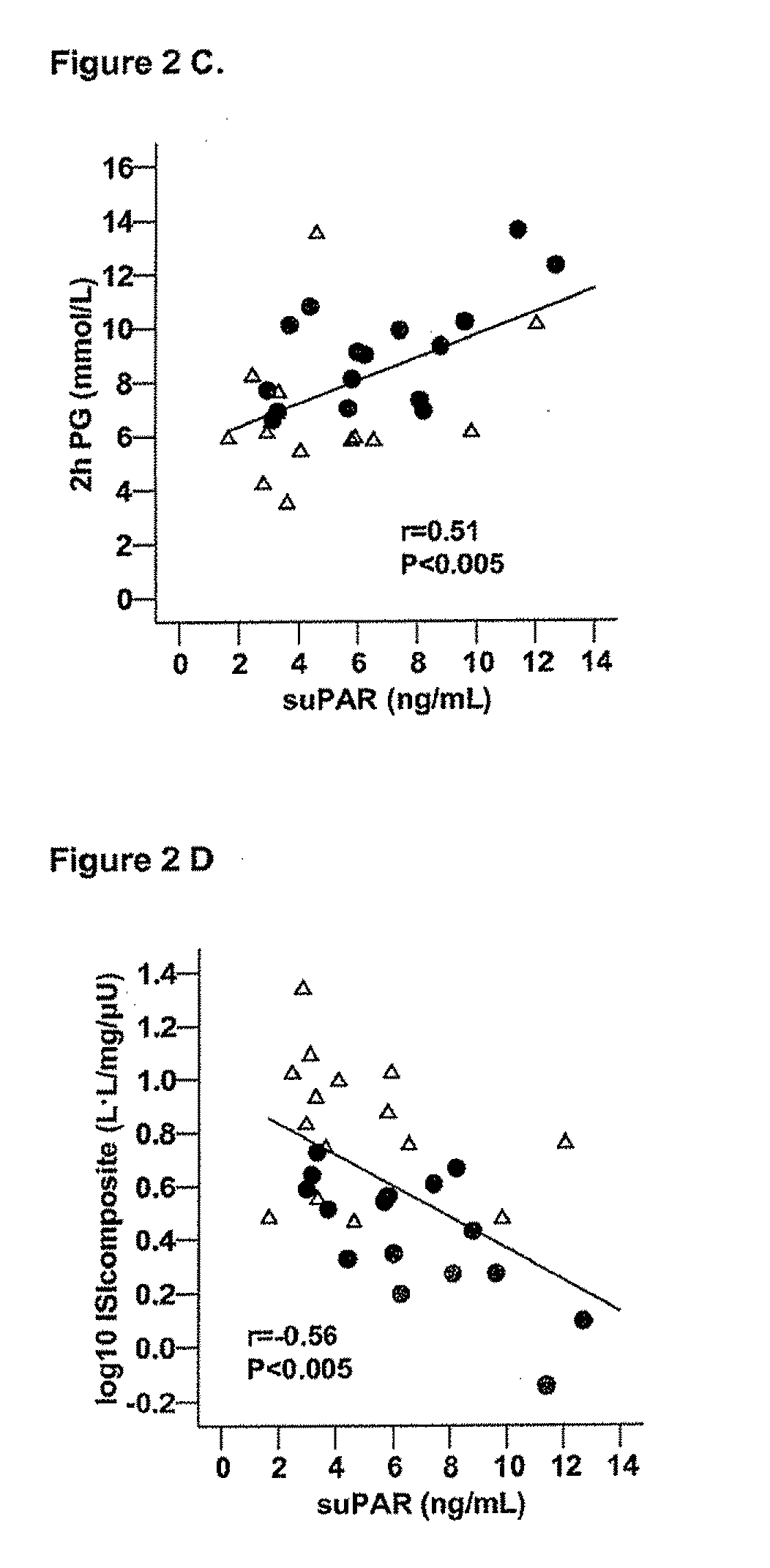
The invention concerns a marker for low-grade inflammation and metabolic syndrome (MS) and MS-related diseases and / or low-grade inflammation-related diseases such as cardiovasculardisease, ischemic heart disease and type 2 diabetes. More particularly it concerns the measurement of the concentration of soluble urokinase plasminogen activator receptor (suPAR) in human biological fluids (sputum, cystic fluid, ascites, serum, plasma, urine) as a tool of diagnosing and / or prognosticating low-grade inflammation and metabolic syndrome and the risk of development of the related diseases such as cancer, cardiovascular disease, ischemic heart disease and type 2 diabetes.
Owner:HVIDOVRE HOSPITAL
Antibodies and their use
InactiveUS6113897APeptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsLymphatic SpreadUrokinase Plasminogen Activator
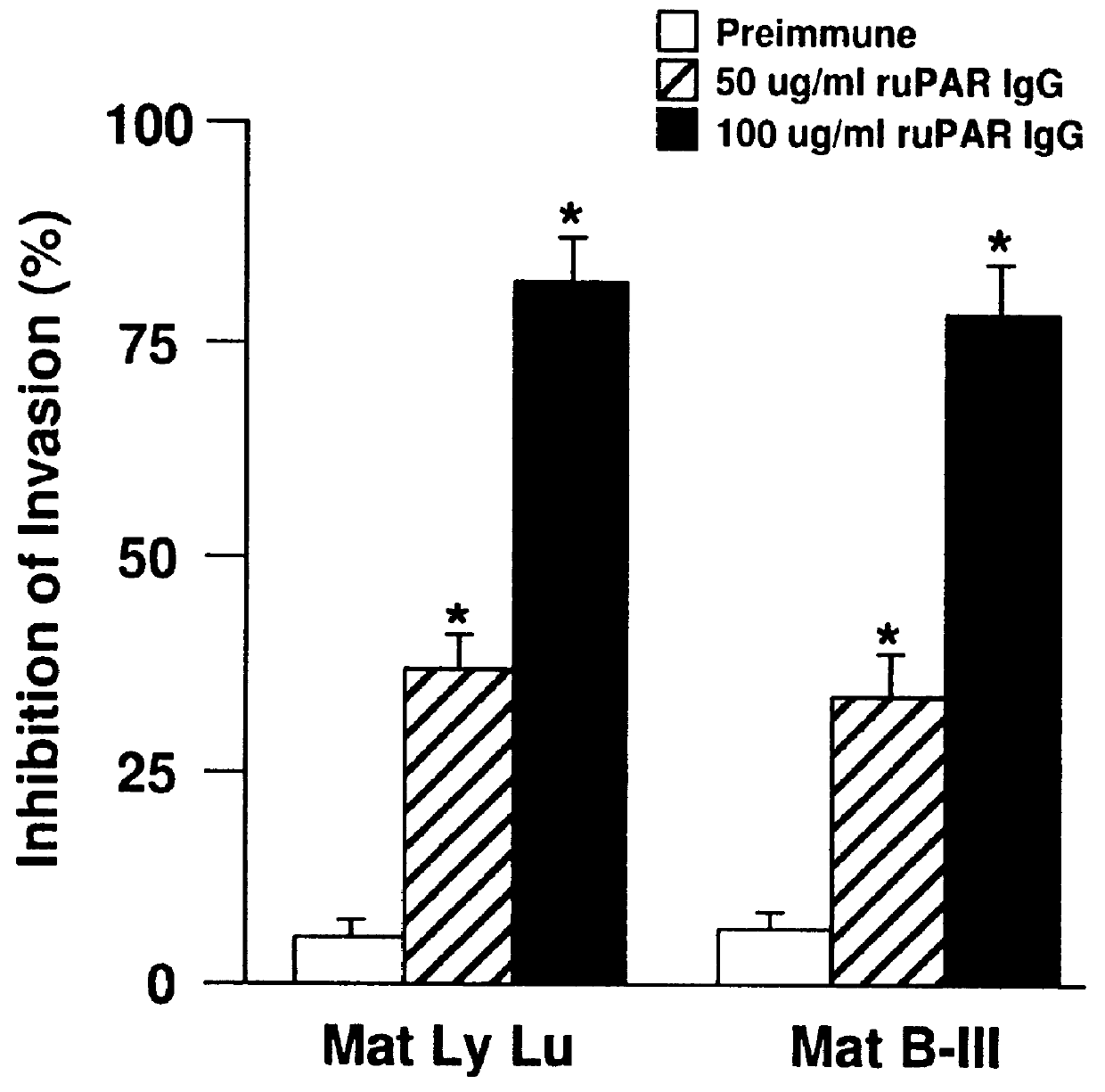
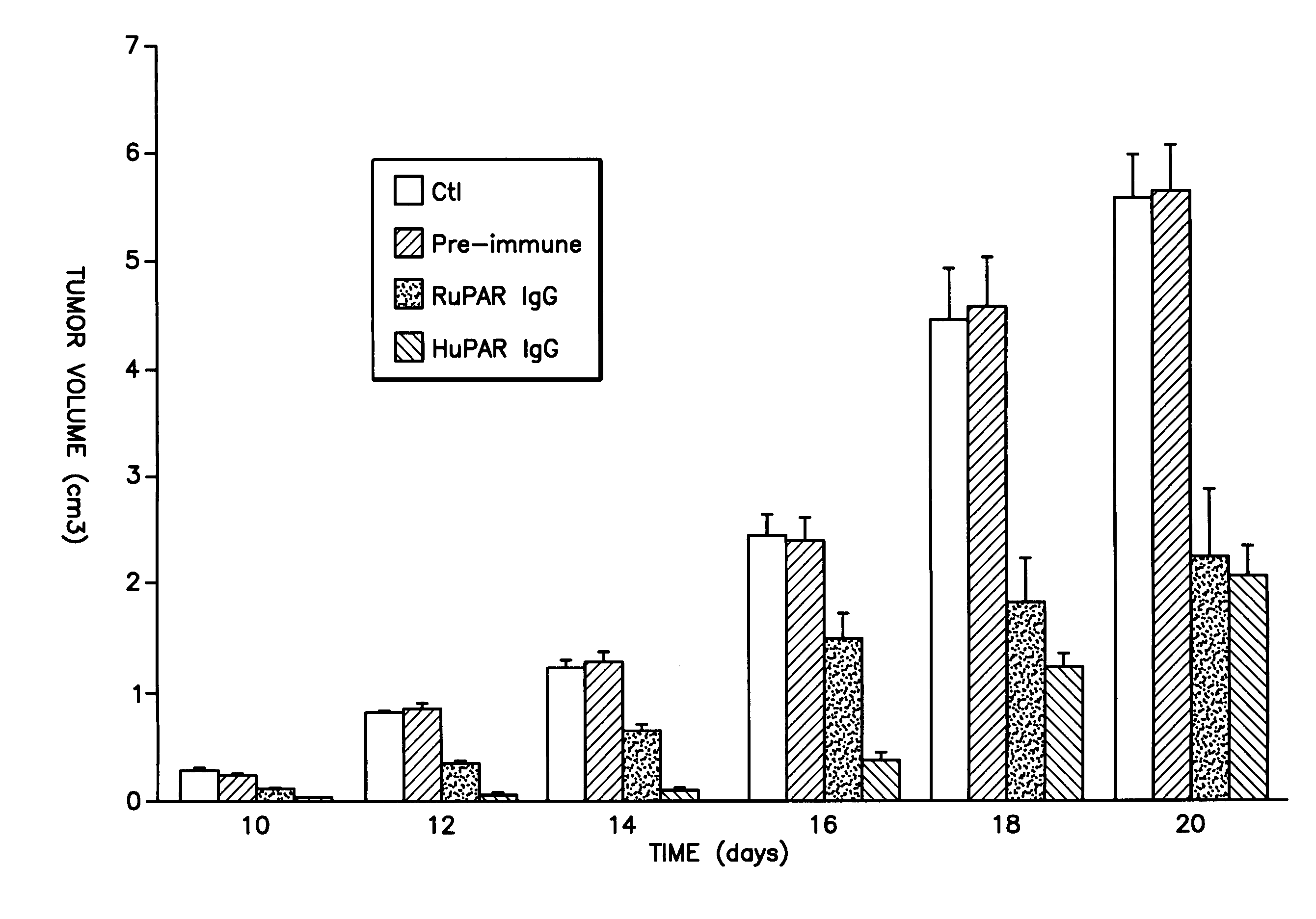
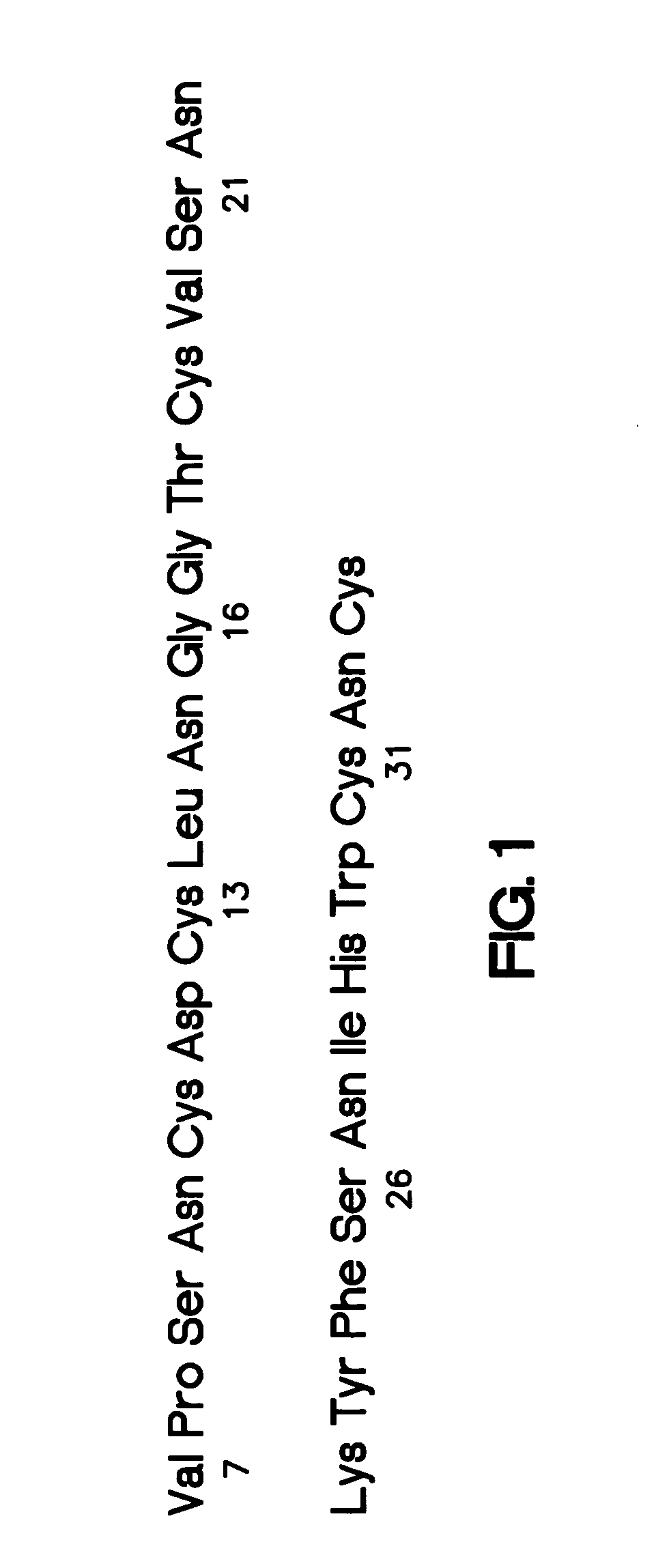
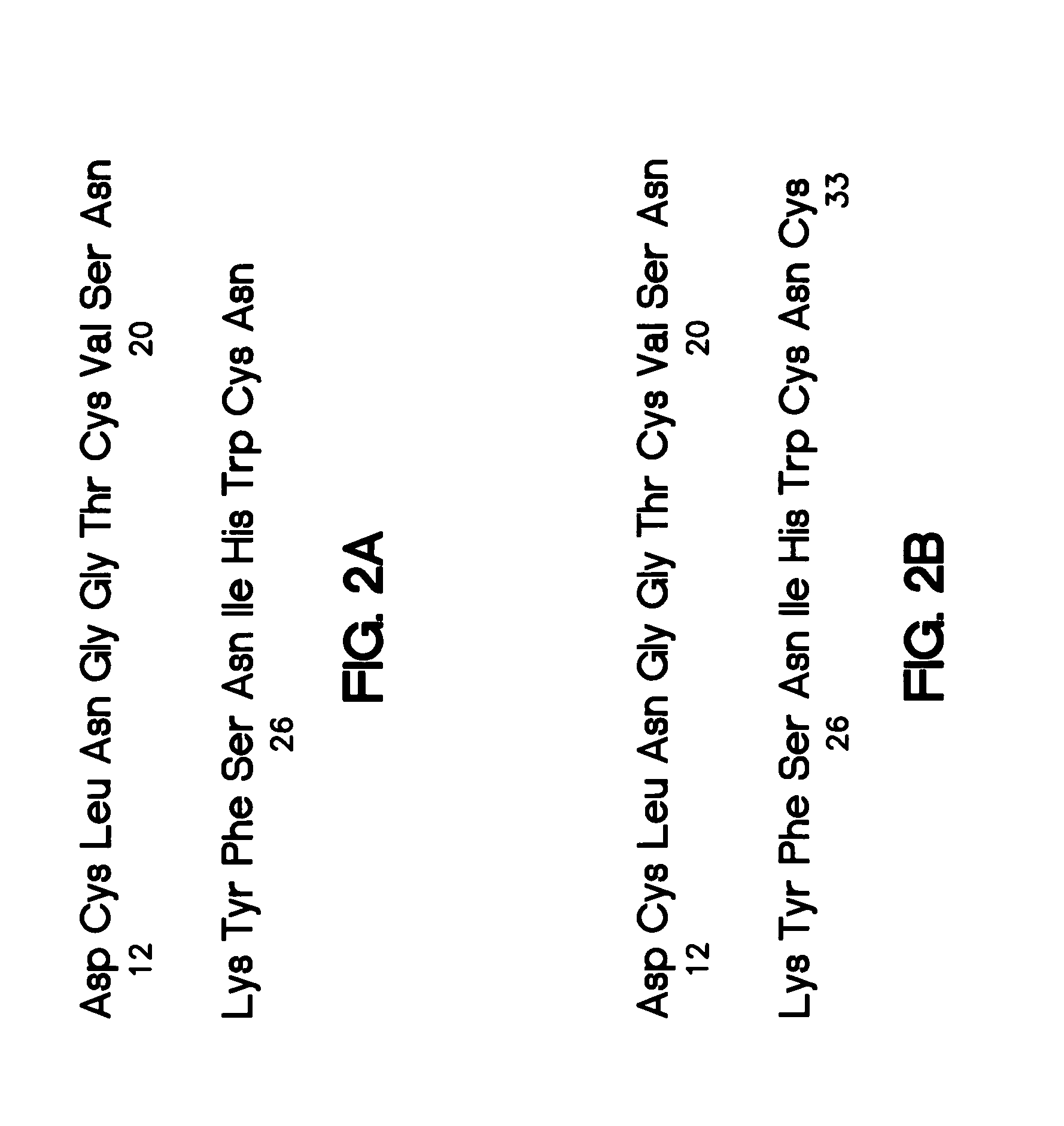
A monoclonal or polyclonal antibody directed against urokinase plasminogen activator receptor (u-PAR), or a subsequence, analogue or glycosylation variant thereof. Antibodies are disclosed which react with free u-PAR or with complexes between u-PA and u-PAR and which are capable of 1) catching u-PAR in ELISA, or 2) detecting u-PAR, e.g. in blotting, or 3) in radioimmunoprecipitation assay precipitate purified u-PAR in intact or fragment form, or 4) is useful for immunohistochemical detection of u-PAR, e.g. in immunostaining of cancer cells, such as in tissue sections at the invasive front, or 5) inhibits the binding of pro-u-PA and active u-PA and thereby inhibits cell surface plasminogen activation. Methods are disclosed 1) for detecting or quantifying u-PAR, 2) for targeting a diagnostic to a cell containing a u-PAR on the surface, 3) for preventing or counteracting proteolytic activity in a mammal. Methods for for selecting a substance suitable for inhibiting u-PA / u-PAR interaction, for preventing or counteracting localized proteolytical activity in a mammal, for inhibiting the invasion and / or metastasis comprise the use of the antibodies and of nude mice inoculated with human cancer cells which are known to invade and / or metastasize in mice and having a distinct color, f.x. obtained by means of an enzyme and a chromogenic substrate for the enzyme, the color being different from the cells of the mouse.
Owner:CANCERFORSKNINGSFONDEN AF 1989 FONDEN TIL FREMME
Non-covalent inhibitors of urokinase and blood vessel formation
InactiveUS20020037857A1Promote cell adhesionImprove cell adhesionDipeptide ingredientsDepsipeptidesUrokinase Plasminogen ActivatorIn vivo
Novel compounds having activity as non-covalent inhibitors of urokinase and having activity in reducing or inhibiting blood vessel formation are provided. These compounds have Pi a group having an amidino or guanidino moiety or derivative thereof. These compounds are useful in vitro for monitoring plasminogen activator levels and in vivo in treatment of conditions which are ameliorated by inhibition of or decreased activity of urokinase and in treating pathologic conditions wherein blood vessel formation is related to a pathologic condition.
Owner:KEVIN S HELMBACHER GENERAL COUNSEL +1
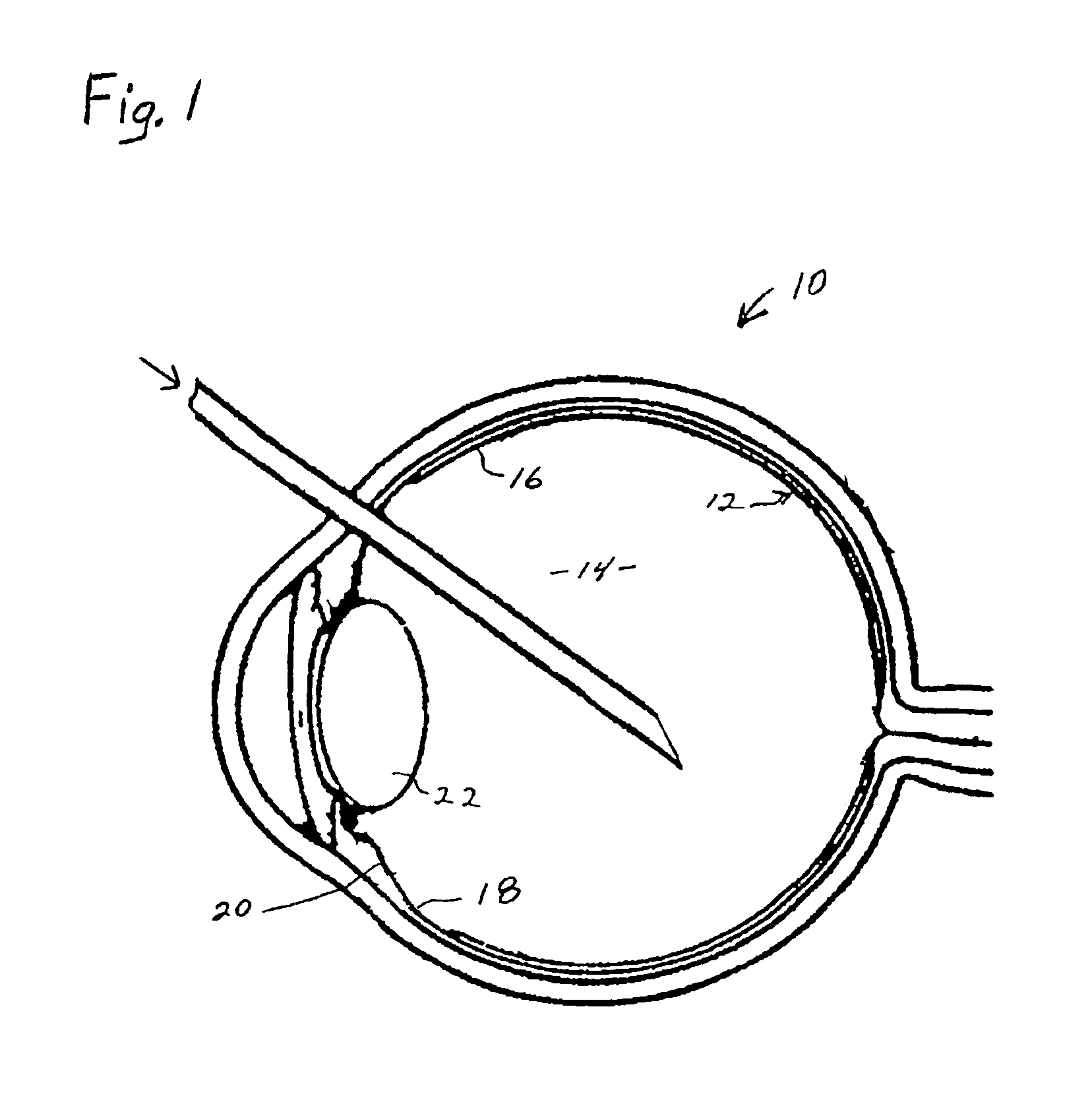
Process for generating plasmin in the vitreous of the eye and inducing separation of the posterior hyaloid from the retina
InactiveUS6899877B2Inhibiting retinal tearReducing and preventing effectSenses disorderEye implantsUrokinase Plasminogen ActivatorVascular proliferation
A process for inhibiting vascular proliferation separately introduces components into the eye to generate plasmin in the eye in amounts to induce complete posterior vitreous detachment where the vitreoretinal interface is devoid of cortical vitreous remnants. The process administers a combination of lysine-plasminogen, at least one recombinant plasminogen activator selected from the group consisting of urokinase, streptokinase, tissue plasminogen activator, chondroitinase, pro-urokinase, retavase, metaloproteinase, and thermolysin and a gaseous adjuvant to form a cavity in the vitreous. The composition is introduced into the vitreous in an amount effective to induce crosslinking of the vitreous and to induce substantially complete posterior vitreous detachment from the retina without causing inflammation of the retina. The gaseous adjuvant material is introduced into the vitreous simultaneously with or after the lysine-plasminogen and recombinant plasminogen activator such as recombinant urokinase to compress the vitreous against the retina while the composition induces the complete posterior vitreous detachment.
Owner:MINU
Methods for sterilizing preparations of urokinase
InactiveUS20050069453A1Effective sterilizationAvoid radiationLavatory sanitoryEnzymesUrokinase Plasminogen ActivatorFungal microorganisms
Methods are disclosed for sterilizing preparations of urokinase to reduce the level therein of one or more active biological contaminants or pathogens, such as viruses, bacteria (including inter- and intracellular bacteria, such as mycoplasmas, ureaplasmas, nanobacteria, chlamydia and rickettsias), yeasts, molds, fungi, single or multicellular parasites, and prions or similar agents responsible, alone or in combination, for TSEs. These methods involve sterilizing preparations of urokinase with irradiation.
Owner:CLEARANT
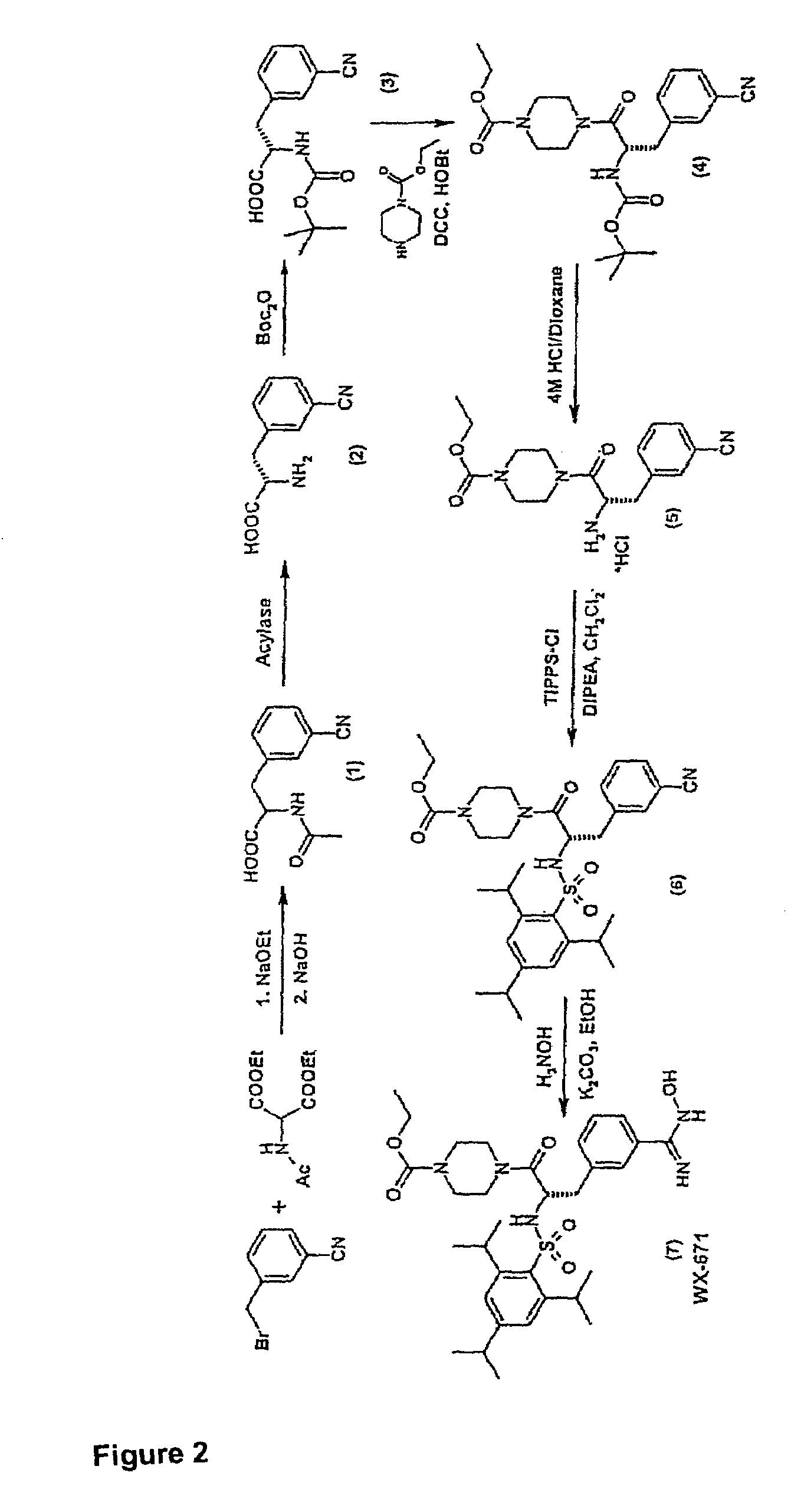
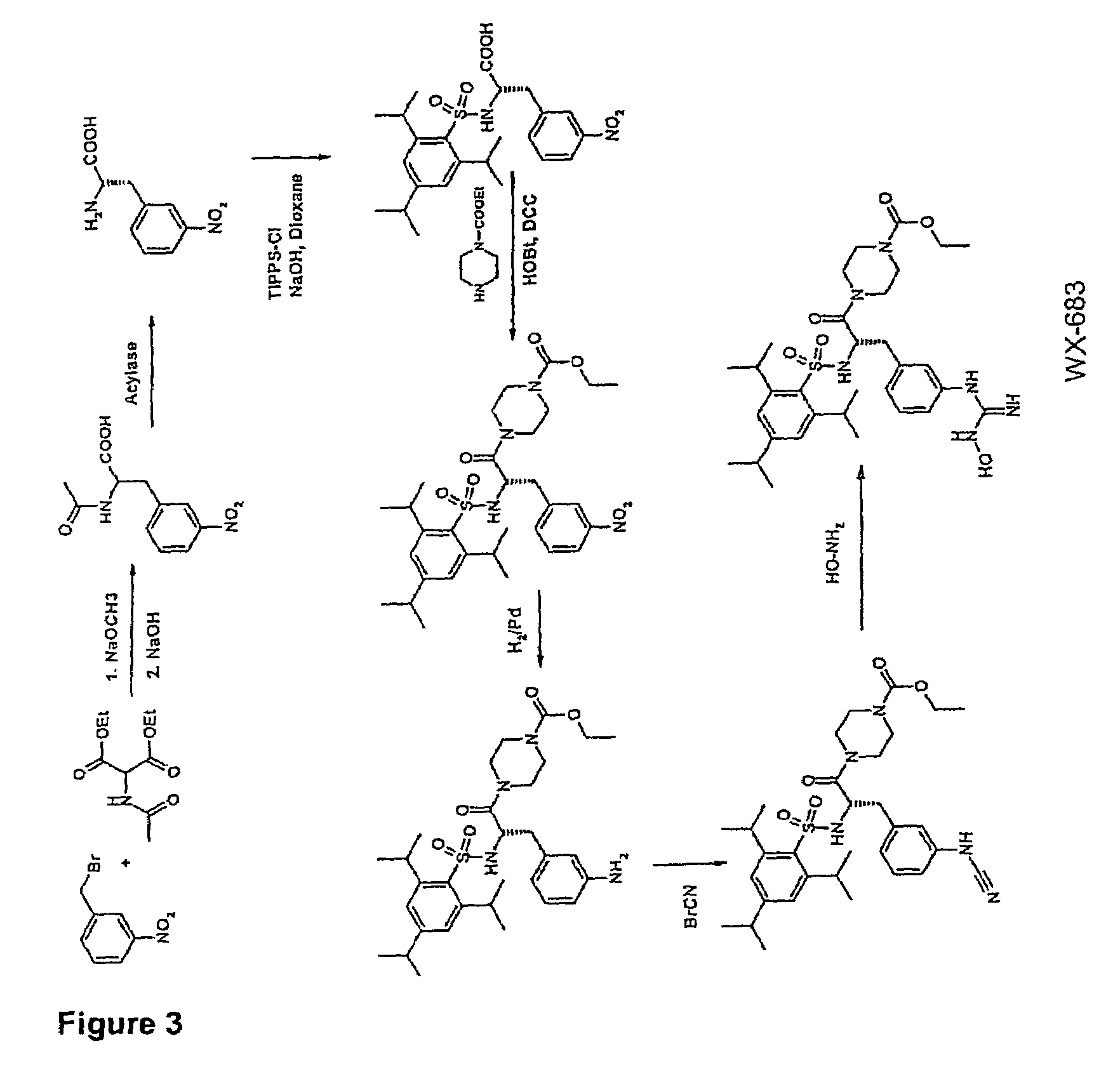
Hydroxyamidine and hydroxyguanidine compounds as urokinase inhibitors
ActiveUS7608623B2High activityImprove bioavailabilityOrganic active ingredientsDipeptidesUrokinase Plasminogen ActivatorUrokinase inhibitor
The present invention relates to novel compounds for inhibiting the urokinase plasminogen activator (uPA), which have high bioavailability and oral administerability, and also to the use thereof as therapeutic active compounds for the treatment of urokinase- or / and urokinase receptor-associated disorders such as, for example, tumors and metastasizing. The invention relates in particular to compounds containing hydroxyamidine or hydroxyguanidine groups.
Owner:WILEX AG
Urokinase plasminogen activator receptor as a target for diagnosis of metastases
InactiveUS6077508AUltrasonic/sonic/infrasonic diagnosticsPeptide/protein ingredientsZymogenUrokinase Plasminogen Activator
The present invention relates to the use of molecules capable of specifically binding a urokinase plasminogen activator receptor (uPAR) as diagnostic reagents for the detection of metastases in vivo. Such metastases can include, but are not limited to, micrometastases.
Owner:MCGILL UNIV +1
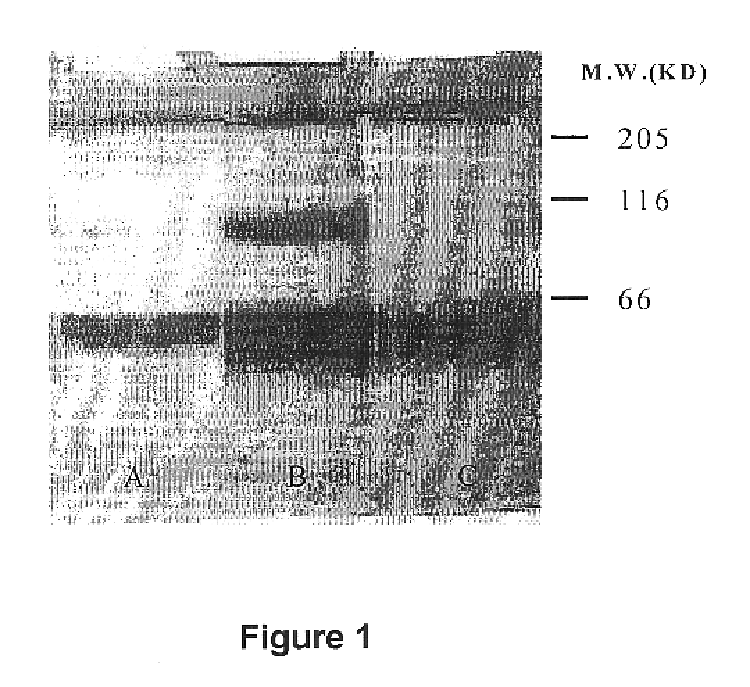
Purification of recommbined human urokinase zymogen
ActiveCN1680550AEasy to fillLarge amount of processingPeptide preparation methodsPeptidasesZymogenUrokinase Plasminogen Activator
The invention is about a method to purify the recombined human urokinase by the chromatography. The invention relates to recovery the gene engineering production from the mammal cell culture using the Streamline-SP, Sephacryl S-200, Sepharose Fast Flow and DEAE-Sepharose Fast Flow method. The recombined human urokinase can meet the SFDA standard and the percent recovery is above 70%, the purity is above 99% by using the method.
Owner:TASLY BIOPHARMACEUTICALS CO LTD
uPAR-binding molecule-drug conjugates and uses thereof
InactiveUS20070190068A1Effect be exertAntibody ingredientsPharmaceutical non-active ingredientsUrokinase Plasminogen ActivatorLymphatic Spread
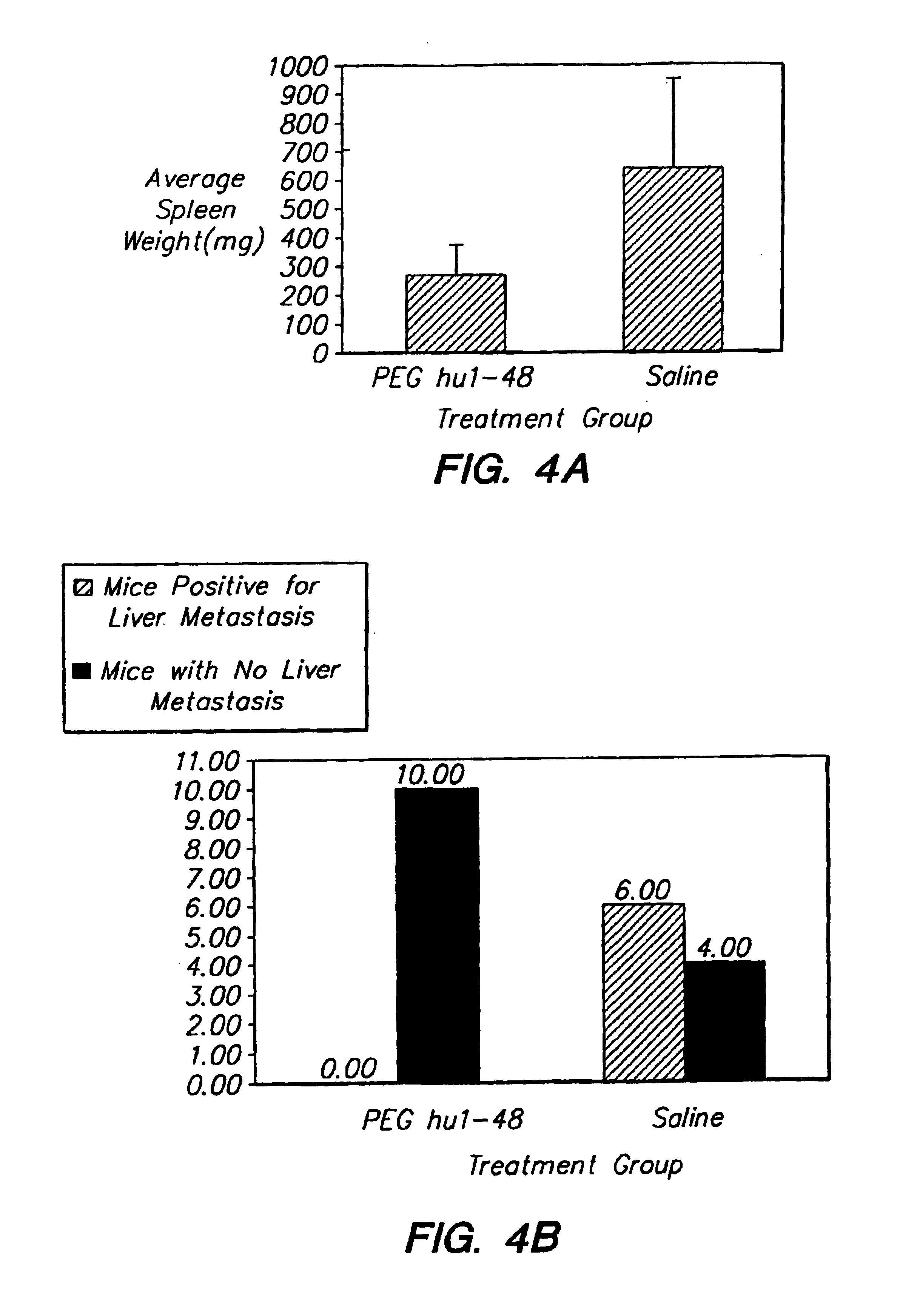
The present invention relates to the use of uPAR-binding molecule-drug conjugates capable of specifically binding a urokinase plasminogen activator receptor (uPAR) as therapeutic and diagnostic reagents for the treatment and monitoring of metastases. The present invention provides methods of treatment of metastases, comprising administering to a subject a uPAR-binding molecule-chemotherapeutic conjugate that is capable of binding to and internalizing into uPAR-expressing cells. The present invention further provides pharmaceutical compositions and kits comprising such conjugates. The present invention further provides methods and compositions relating to combination therapy for cancer involving or mediated by uPAR-expressing cells using uPAR-binding molecule-drug conjugates of the invention.
Owner:AMERICAN DIAGNOSTICA +1
Method for enriching urine protein directly
InactiveCN101863974AAvoid the collection stepLow costHydrolasesPeptide preparation methodsURINARY TRYPSIN INHIBITORUrokinase Plasminogen Activator
The invention relates to a urine protein enriching method. In the method, urine protein in urine is absorbed directly by an adsorbent in a urinal or a pee hopper, and the adsorbent for adsorbing the urine protein is conveyed to a working point for follow-up treatment; and based on the isoelectric point properties of specific urine protein, the urine protein such as urinary trypsin inhibitor, human urinary kininogenase, urokinase and the like is adsorbed directly and effectively by using the specific adsorbent such as ion exchange resin, and thus, the step of collecting the urine is avoided. The method does not influence sanitation of toilets obviously, reduces the cost of urine transportation substantially and solves a series of environmental problems.
Owner:GUANGDONG TECHPOOL BIO-PHARMA CO LTD +1
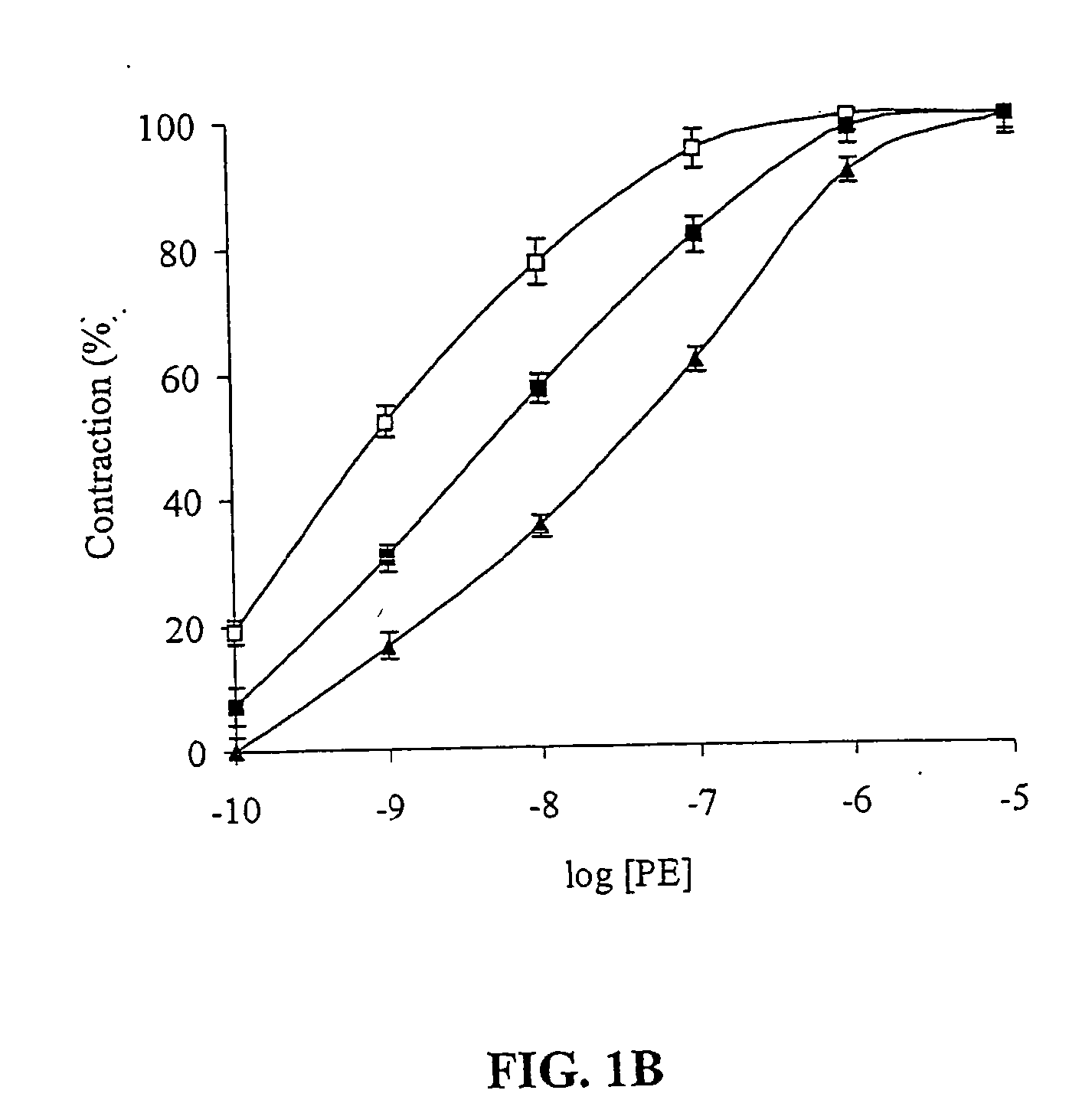
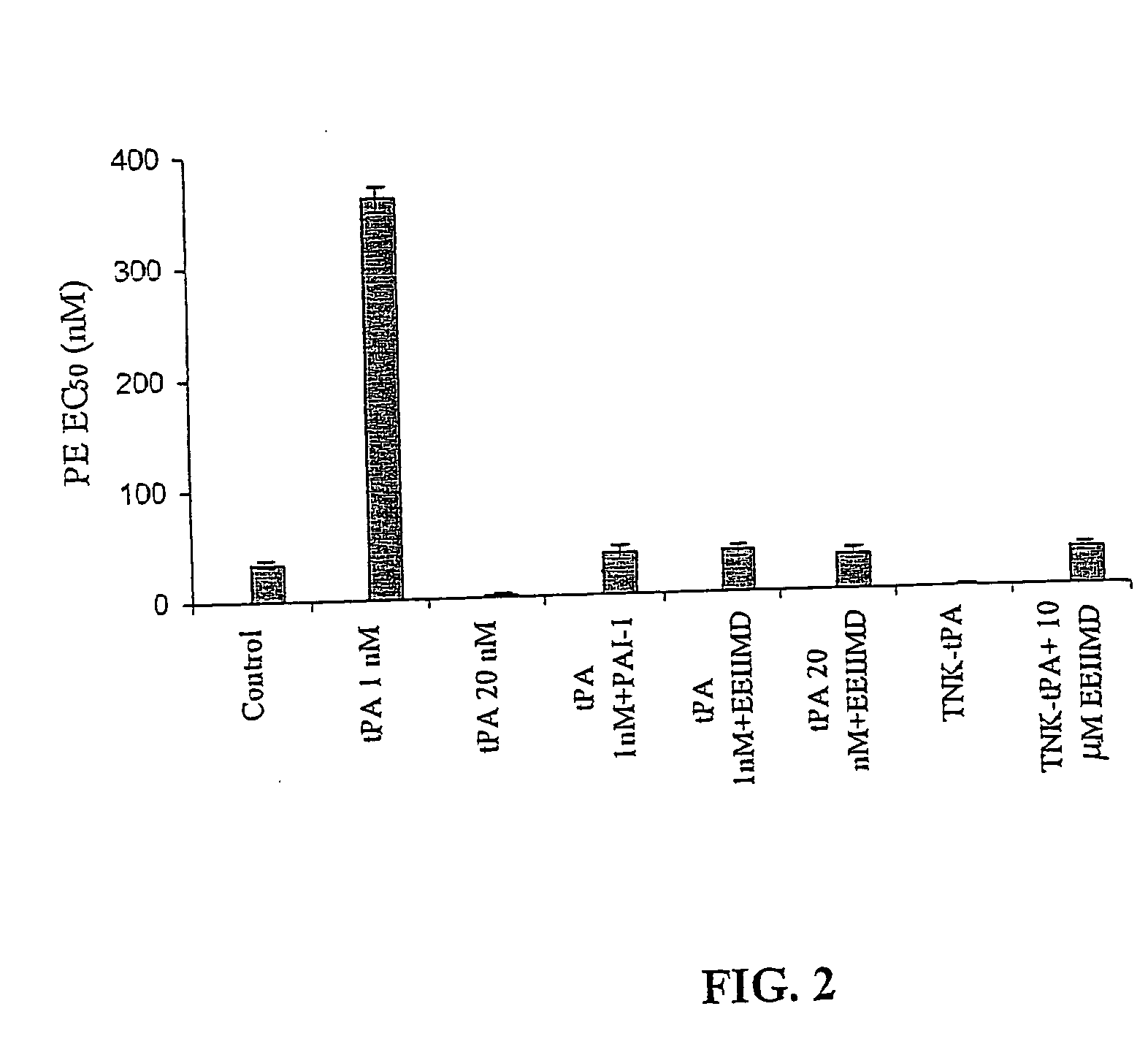
Peptide for regulation of urokinase plasminogen activator and method of optimizing therapeutic efficacy
InactiveUS20060069035A1Avoid side effectsHigh activityFibrinogenPeptide/protein ingredientsStreptokinaseLANOTEPLASE
The present invention relates to compositions of the polypeptide EEIIMID and one or more fibrinolytic agents selected from the group consisting of scuPA, tPA, uPA, tcuPA, streptokinase, rt-PA, alteplase, rt-PA derivatives, reteplase, lanoteplase, TNK-rt-PA, anisoylated plasminogen streptokinase complex, anistreplase, or a streptokinase derivative. The invention further relates to methods of enhancing the fibrinolytic activity, reducing the side effects due to vasoactivity caused by the fibrinolytic agents, or prolonging the half lives of the fibrinolytic agents by adding EEIIMD.
Owner:HIGAZI ABD AL ROOF
Expression, purification and crystallization of urokinase catalyst structure domain mutant
InactiveCN101024827ALow costImprove stabilityMicrobiological testing/measurementRecombinant DNA-technologySerine proteinasesPichia pastoris
The invneiton relates to expression, purification and crystallization of urokinase catalytic domain mutant, relating to the field of medicine, biotechnique, and structural biology. And the invention provides a techical method of expression, purification and crystallization of urokinase catalytic domain mutant in Pichia pastoris. And the cultured urokinase catalytic domain mutant has crystallographic space group R3, and unit cell parameters: a=b=120.48, c=42.45, alpha=beta=90.0deg, and gama=120.0 deg. And this protein has serine proteinase activity, appliable to high flux screening of urokinase inhibitor; and its high resolution crystal provides a research and development platform for design and development of urokinase inhibitor.
Owner:FUJIAN INST OF RES ON THE STRUCTURE OF MATTER CHINESE ACAD OF SCI
Specific purified high-molecular urokinase chromatography media as well as preparation method and application thereof
ActiveCN101693748AEasy to makeMild reaction conditionsEnzymesCross-linkUrokinase Plasminogen Activator
A specific purified high-molecular urokinase chromatography media is characterized by having a structure as follows: HOOC-(CH2)4(NH)C-O-R-O-C(NH)NH(CH2)4-CO-X,X=NHC6H5C(NH)NH2, wherein R represents cross-linked agarose substrate. The specific purified high-molecular urokinase chromatography media has the advantages of being simple in preparation process and mild for reaction conditions, being applicable to high-efficiency separation of high-molecular urokinase media and low-molecular urokinase media, and being capable of implementing industrial production of high-purification and high-molecular urokinase.
Owner:江西浩然生物制药有限公司
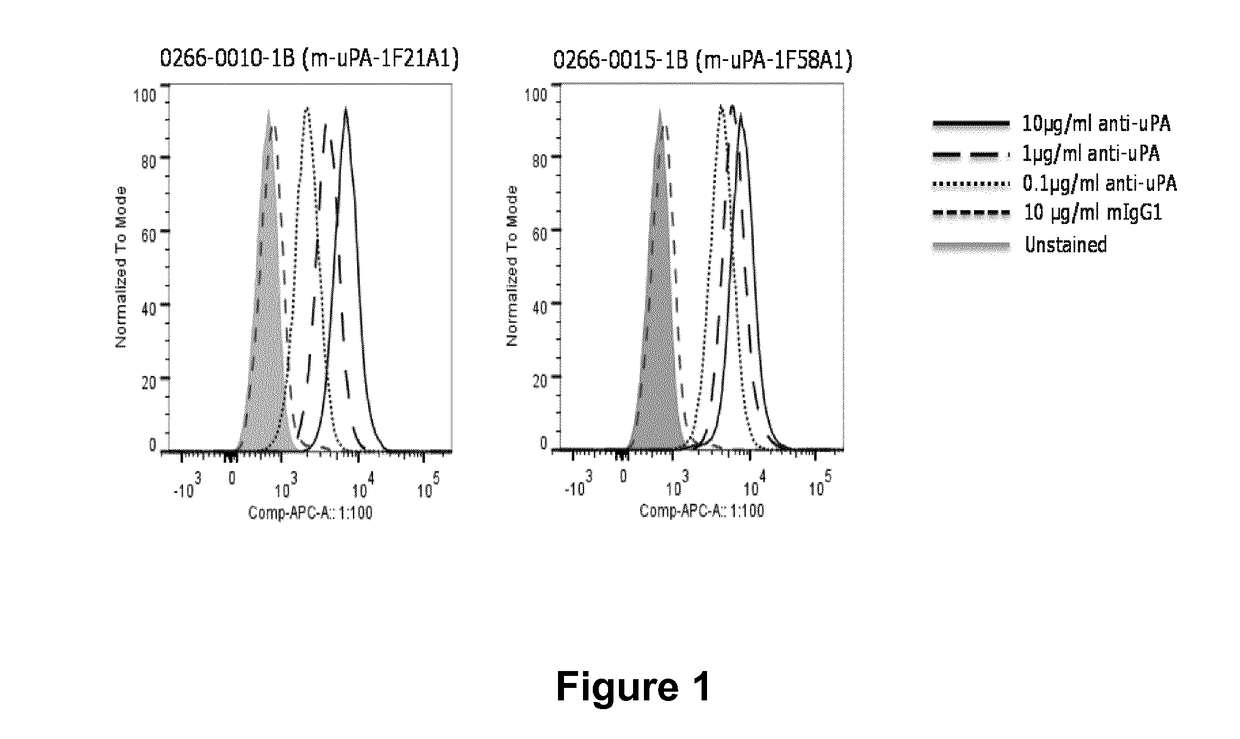
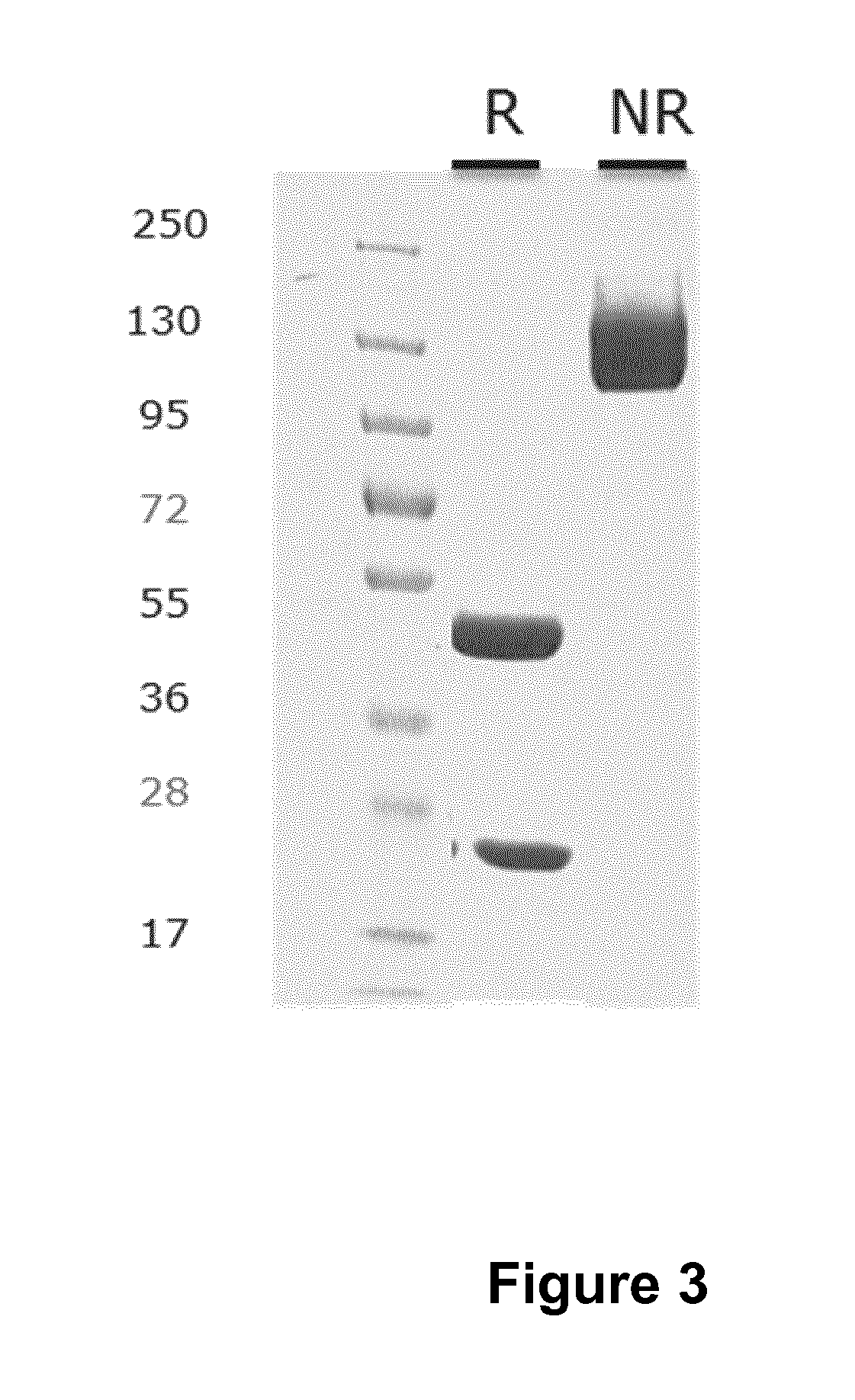
Antibodies that bind urokinase plasminogen activator
InactiveUS9908944B2Inhibitory activityCompound screeningApoptosis detectionUrokinase Plasminogen ActivatorAntibody
The present invention relates to a method of identifying urokinase plasminogen activator (uPA) antibodies. The invention also relates to antibodies that are capable of binding uPA and which are capable of reducing or inhibiting uPA activity. Furthermore, the invention relates to uses for such antibodies, such as therapeutic and pharmaceutical uses.
Owner:NOVO NORDISK AS
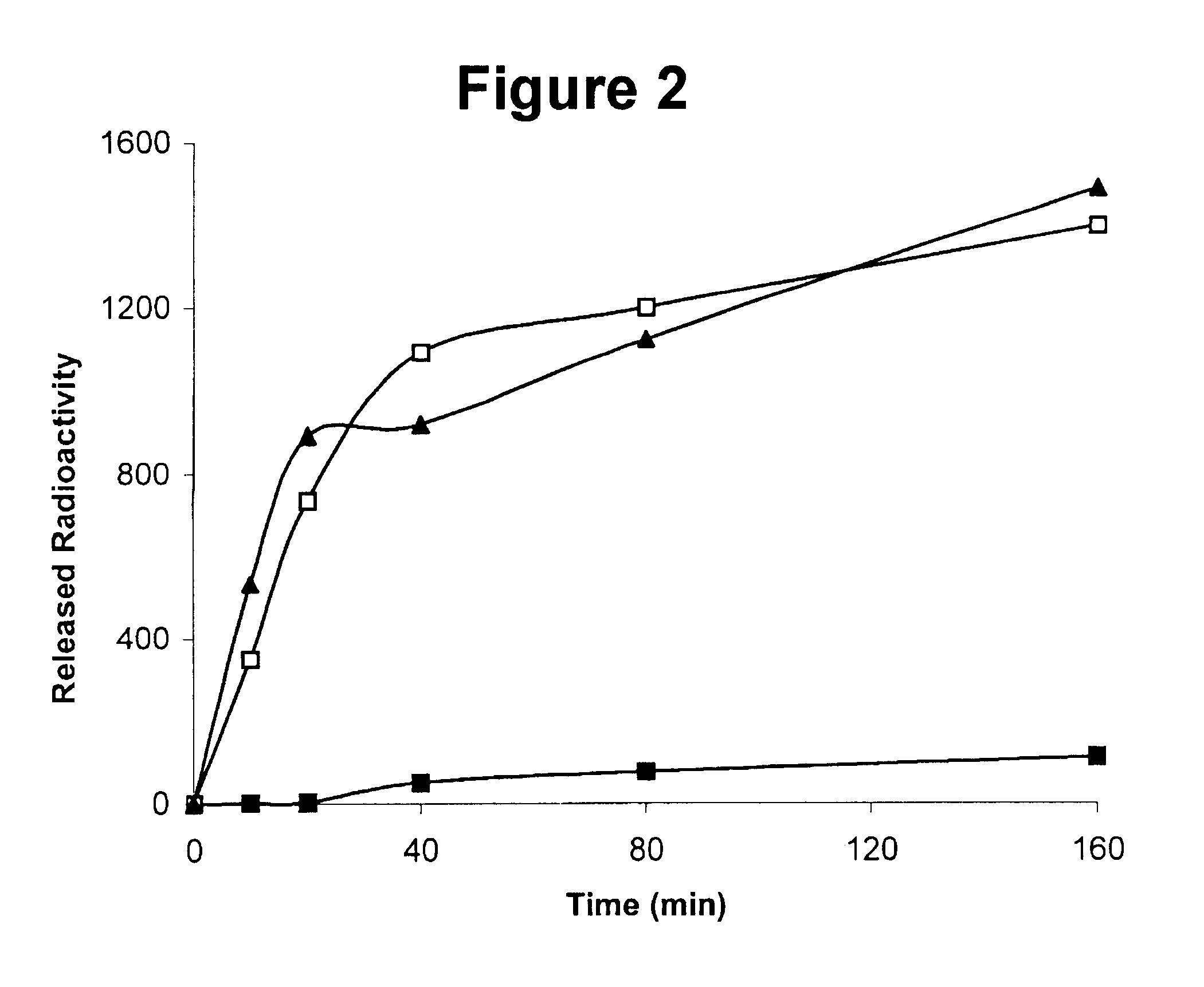
Use of cross-linked, covalently bound urokinase plasminogen activator (scuPA)-urokinase plasminogen activator receptor (suPAR) complex as a fibrinolytic agent
InactiveUS6759042B2Peptide/protein ingredientsMammal material medical ingredientsCross-linkUrokinase Plasminogen Activator
The present invention relates to the cross-linked scuPA / suPAR complex and / or tcuPA / suPAR cross-linked complex, the process of preparation of the covalently bound single compound having fibrinolytic activity and use of the cross-linked scuPA / suPAR or tcuPA / suPAR complex in the prevention and / or treatment of thrombolytic disorders. The invention further relates to combination compositions and / or therapy regimens, comprising the cross-linked scuPA / suPAR complex or tcuPA / suPAR and one or more currently used plasminogen activators to achieve improved therapeutic efficacy and / or reduce side effects.
Owner:THROMBOTECH LTD (IL)
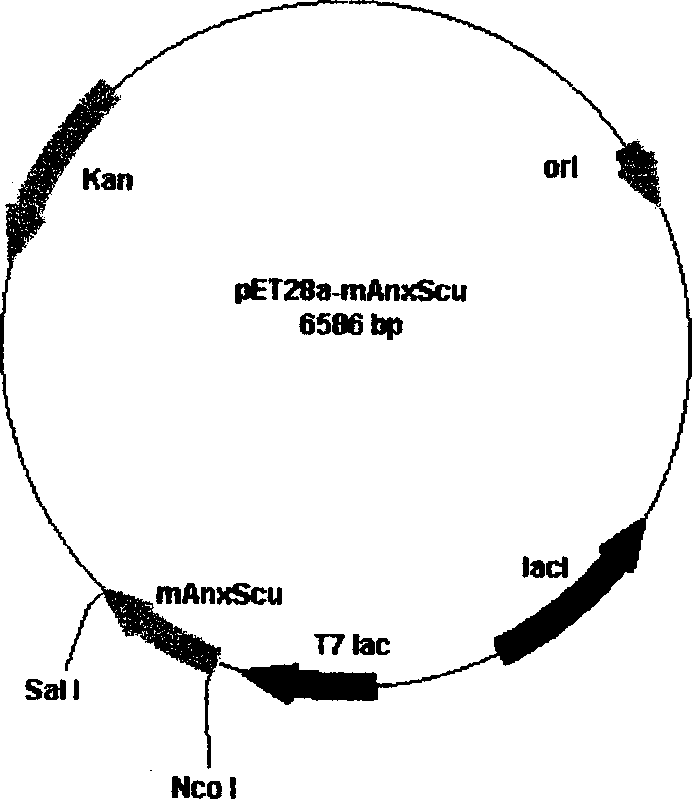
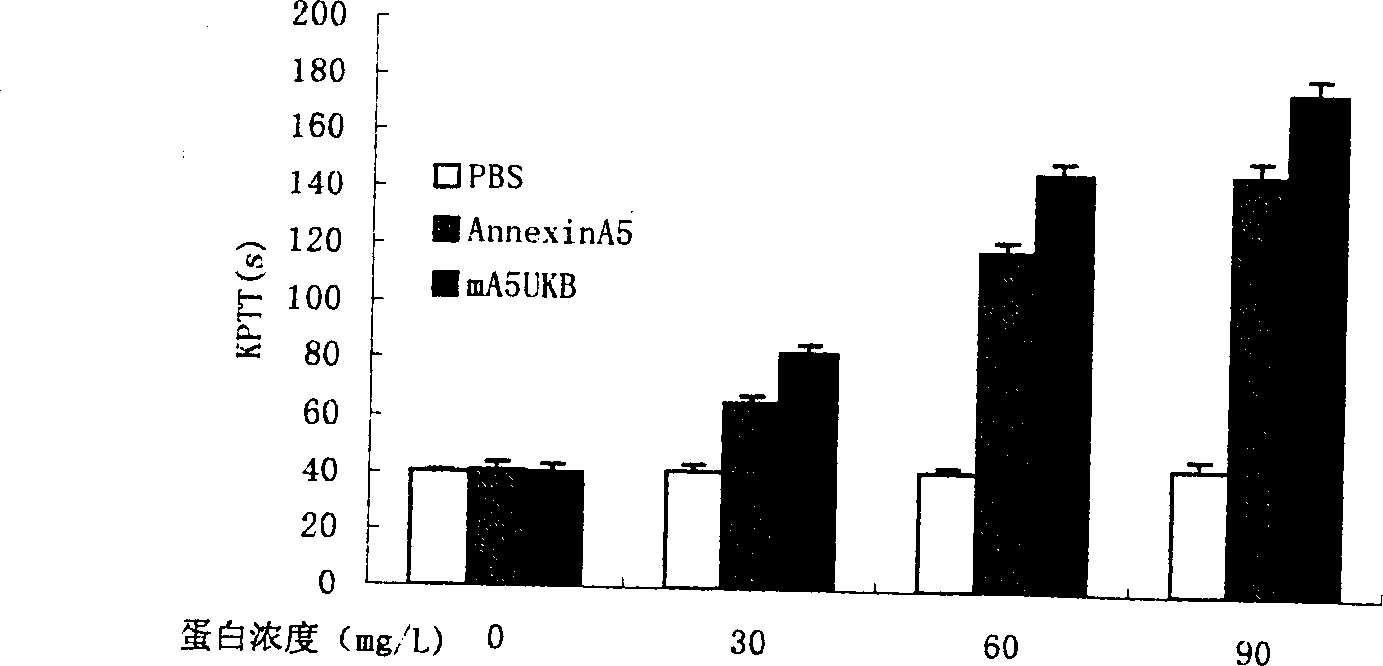
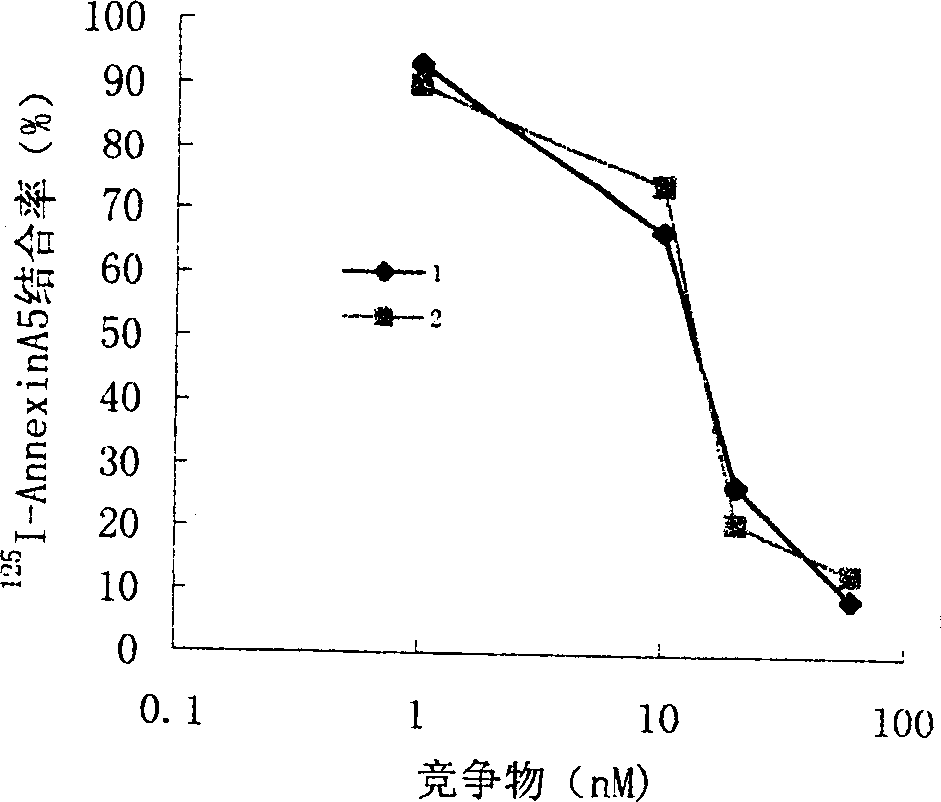
Anticoagulation and thrombolytic thrombus target fusion mA5UKB
InactiveCN1401662AHigh anticoagulant activityHigh affinityPeptide/protein ingredientsHybrid peptidesUrokinase Plasminogen ActivatorThrombus
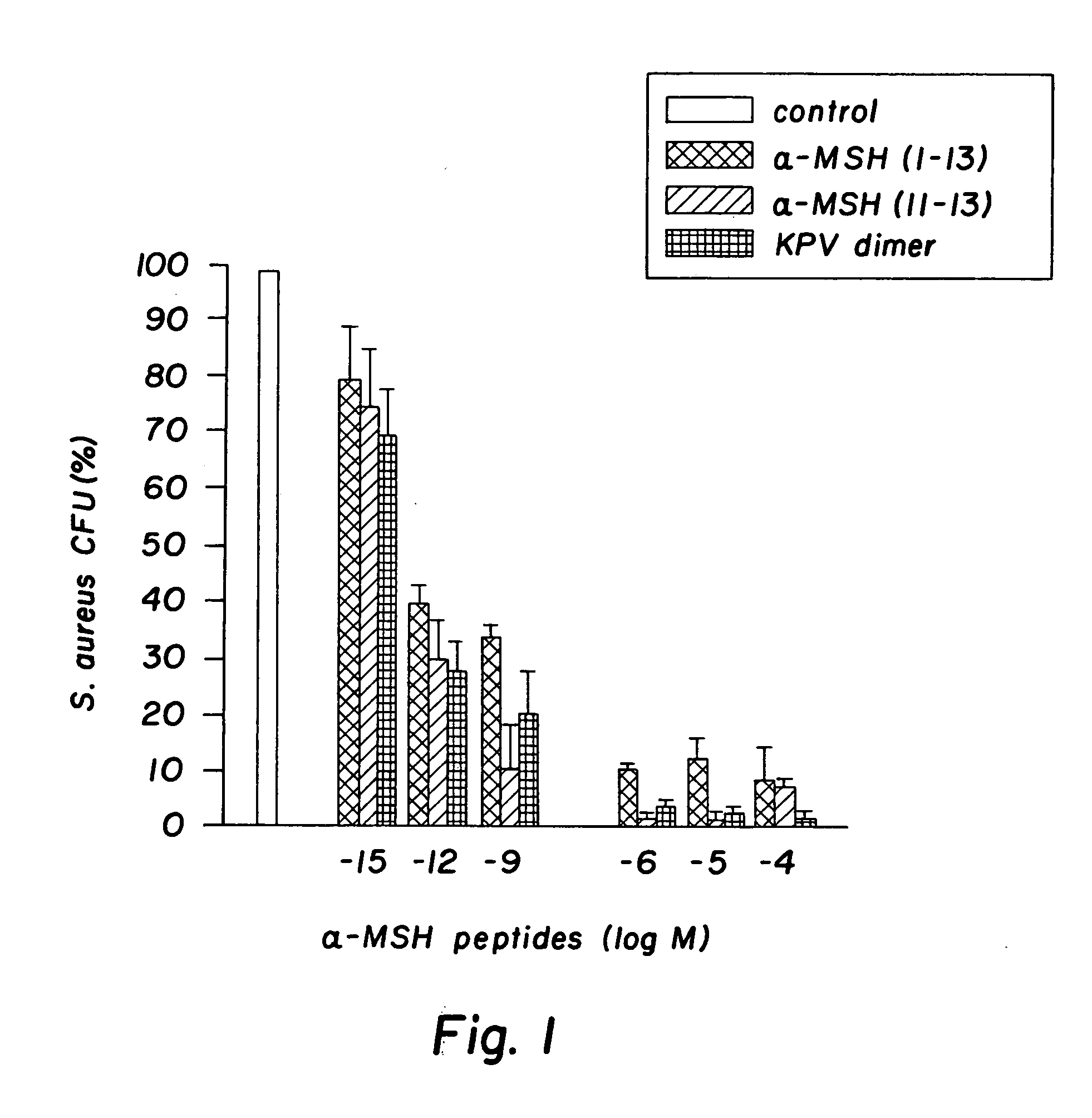
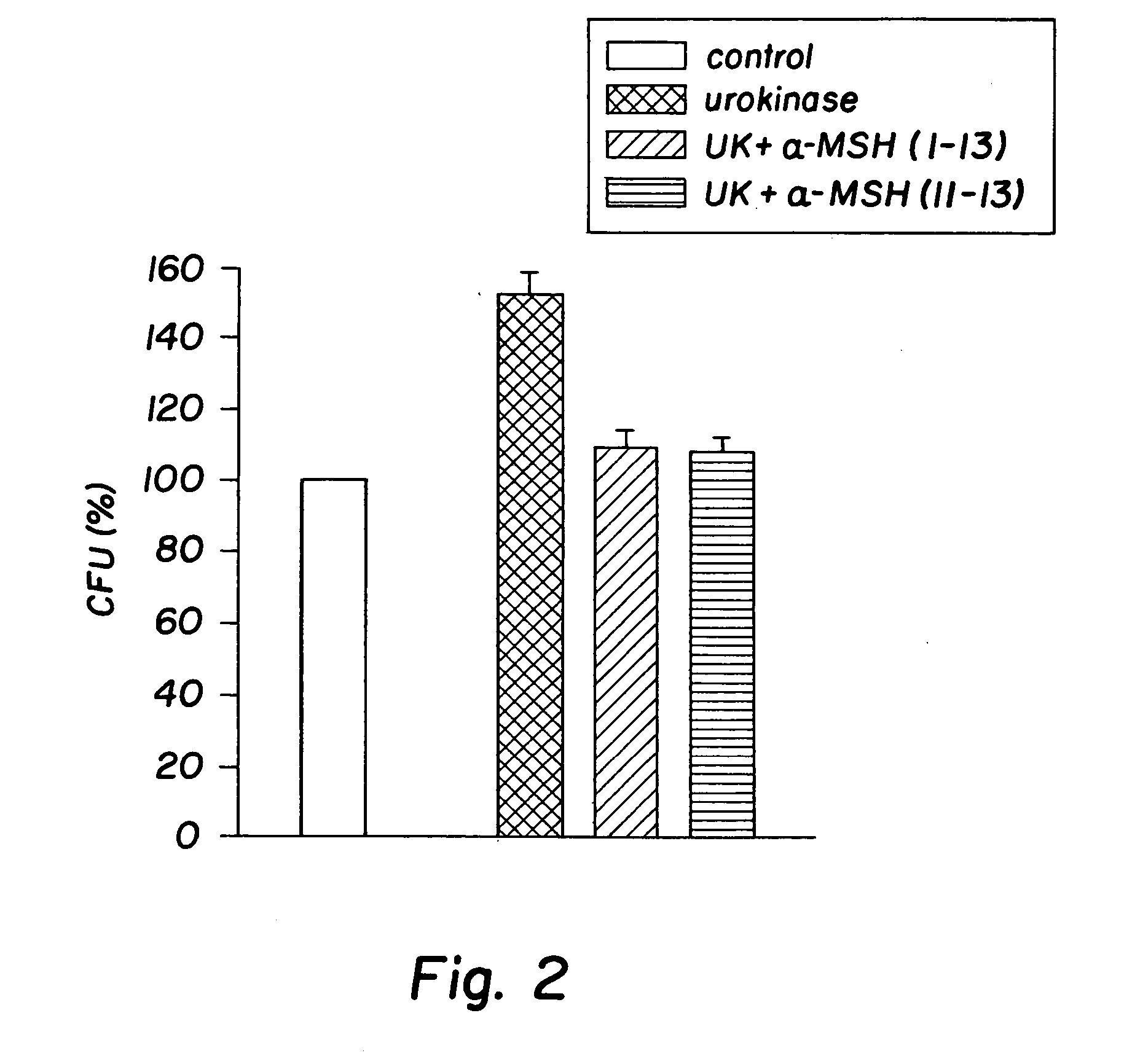
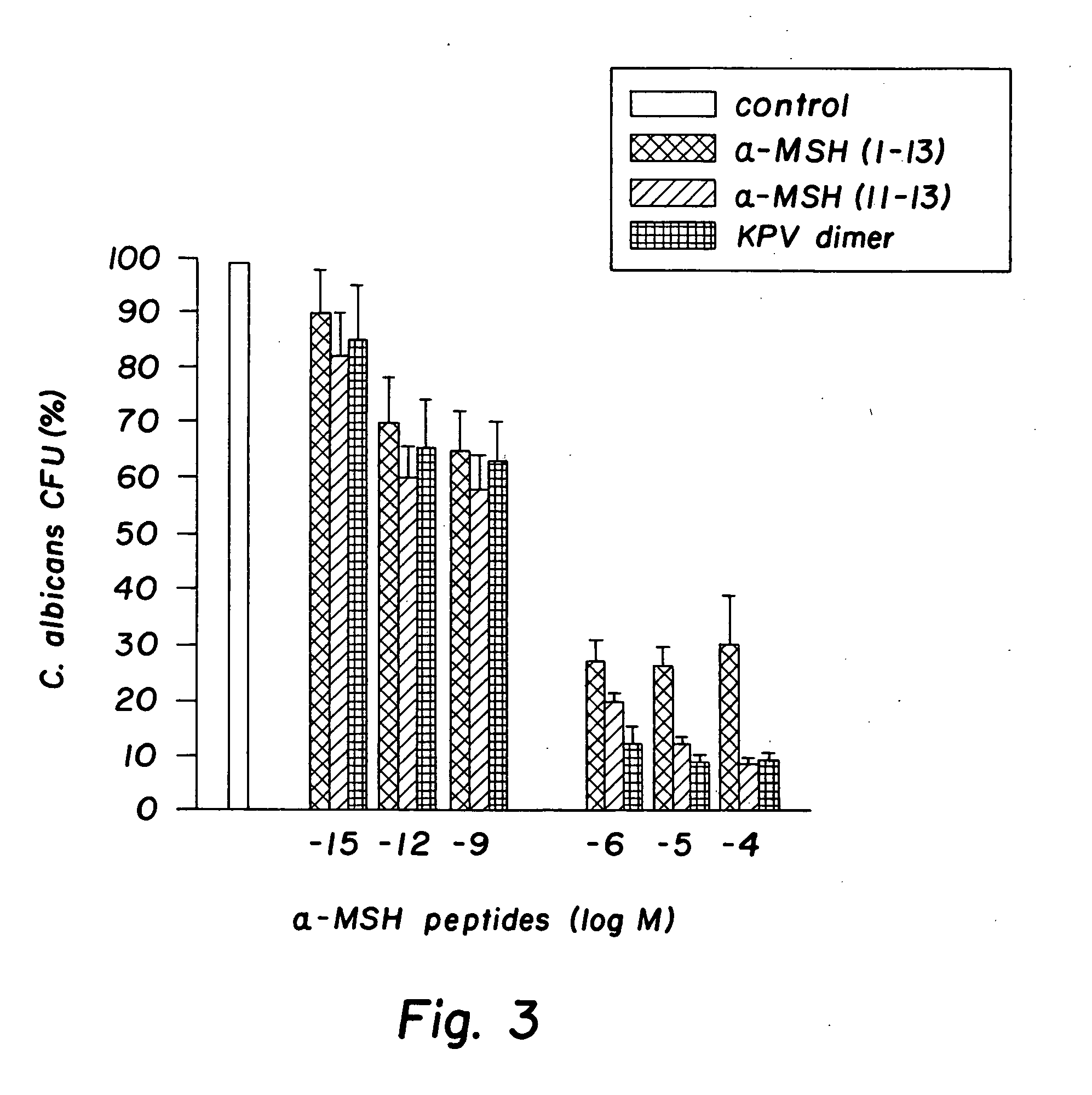
Antimicrobial amino acid sequences derived from alpha-melanocyte-stimulating hormone
InactiveUS20060111300A1Reduced viabilityAccelerate the accumulation processBiocideAntimycoticsDiseaseAmino acid
Owner:ZENGEN
Vector of uPA expression regulated by Tet-on system and application thereof
InactiveCN101948860AEasy constructionVector-based foreign material introductionAnimal husbandryUrokinase Plasminogen ActivatorNucleotide
The invention discloses an effect vector of uPA expression regulated by a Tet-on system. The nucleotide sequence of the effect vector comprises a bacteria tetracycline drug-resistant operon sequence, an immediate early promoter sequence of macrophages, a mice pro-urokinase activator expression cassette sequence and a sequence behind the last exon of the mice pro-urokinase activator expression cassette, the four sequences are serially connected in turn. The invention establishes a transgenic mice model of uPA expression regulated by the Tet-on system or tetracycline derivative inducible expression system, and lays solid foundation for further establishing a transgenic animal model of tissue specificity regulatable expression uPA for uPA function research in specific tissues.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
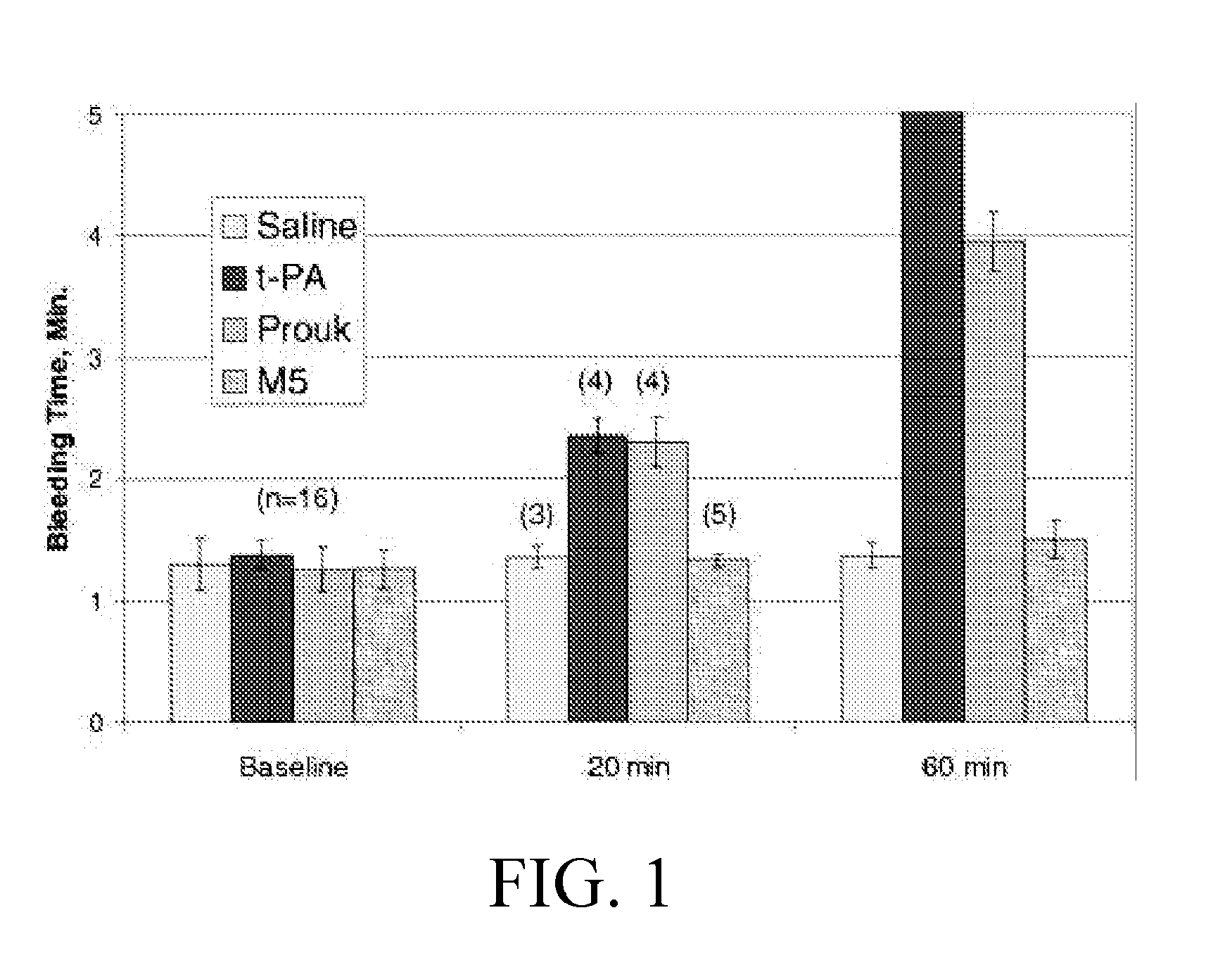
Use of Prourokinase and Variants Thereof in Facilitated Percutaneous Coronary Intervention in Patients with Acute Myocardial Infarction
ActiveUS20110229454A1High selectivityPrevent hemorrhagic complicationPeptide/protein ingredientsEnzymesUrokinase Plasminogen ActivatorCardiac muscle
In the field of biological medicines, a use of prourokinase (proUK) and variants thereof in facilitated percutaneous coronary intervention (PCI) in patients with acute myocardial infarction is provided. The use includes: within 6 hrs after a patient is afflicted with accurate myocardial infarction (AMI), firstly, performing thrombolytic therapy with proUK or variants thereof, and then, performing a PCI operation, to dredge the infarction related artery (IRA) as soon as possible, and re-establish an effective forward blood flow, such that an ischemic myocardium is reperfused. According to the present invention, the facilitated PCI for treatment of AMI with the proUK or variants thereof has an effect superior to that of direct PCI.
Owner:BOSTON PI CARDIO INC
Waterless self-circulating urine flushing equipment with function of collecting raw urokinase
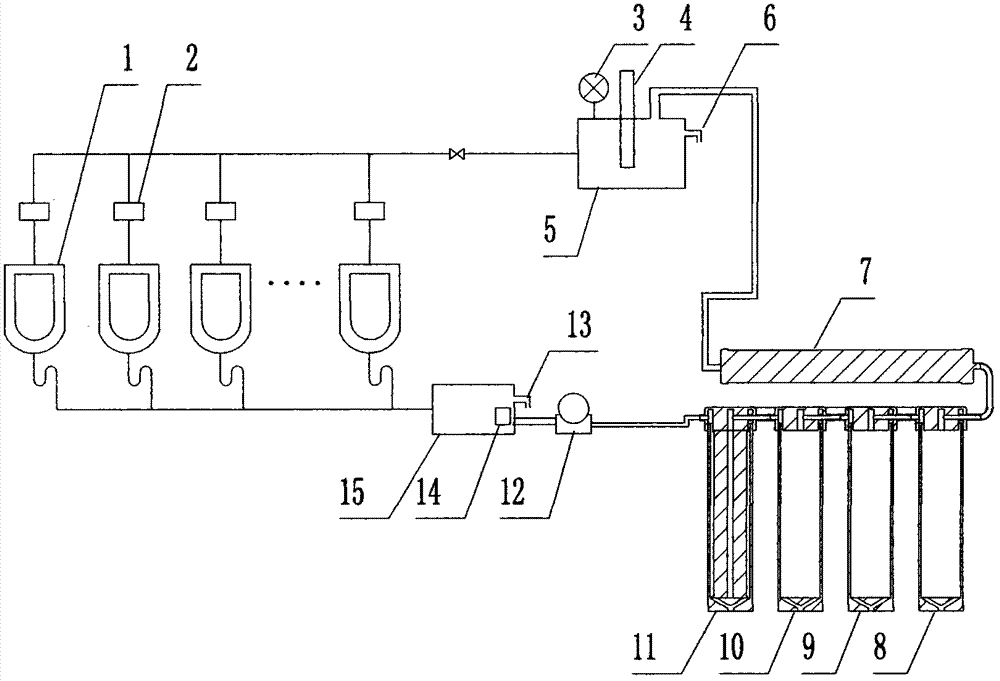
ActiveCN104278730AImprove recycling efficiencySimple structureUrinalsFlushing devicesUrokinase Plasminogen ActivatorLiquid storage tank
The invention relates to waterless self-circulating urine flushing equipment with a function of collecting raw urokinase. The equipment comprises a water incoming tank, urinals and a pipeline. The water incoming tank is an overhead purified water tank providing the urinals with water through the pipeline. A human body sensor is arranged over a water inlet of each urinal and is in control connection with an electromagnetic valve which controls the water incoming tank to supply water. A drain pipe under every urinal is communicated with a liquid storage tank. A water level sensor in control connection with a booster pump switch is arranged inside the liquid storage tank. A liquid outlet of a booster pump is communicated with a liquid filter system. The liquid filter system comprises a plurality of filter elements; each filter element is arranged inside a cylinder; the liquid discharged from the liquid storage tank by the booster pump passes the filter elements in order, and a purified water outlet is communicated with the overhead purified water tank. The waterless self-circulating urine flushing equipment is applicable to urinals, especially applicable to water-deficient areas, and has the advantages such that application and maintenance costs are lower, less water is consumed, the environment is protected, recycling efficiency of waste water and filtrate is high, and the equipment can be used normally in cold areas.
Owner:北京凯明阳热能技术有限公司
Method for producing antithrombotic medicine by using silk-worm
InactiveCN1404872AHigh clinical application valueHigh affinityPeptide/protein ingredientsBlood disorderAntithrombotic AgentUrokinase Plasminogen Activator
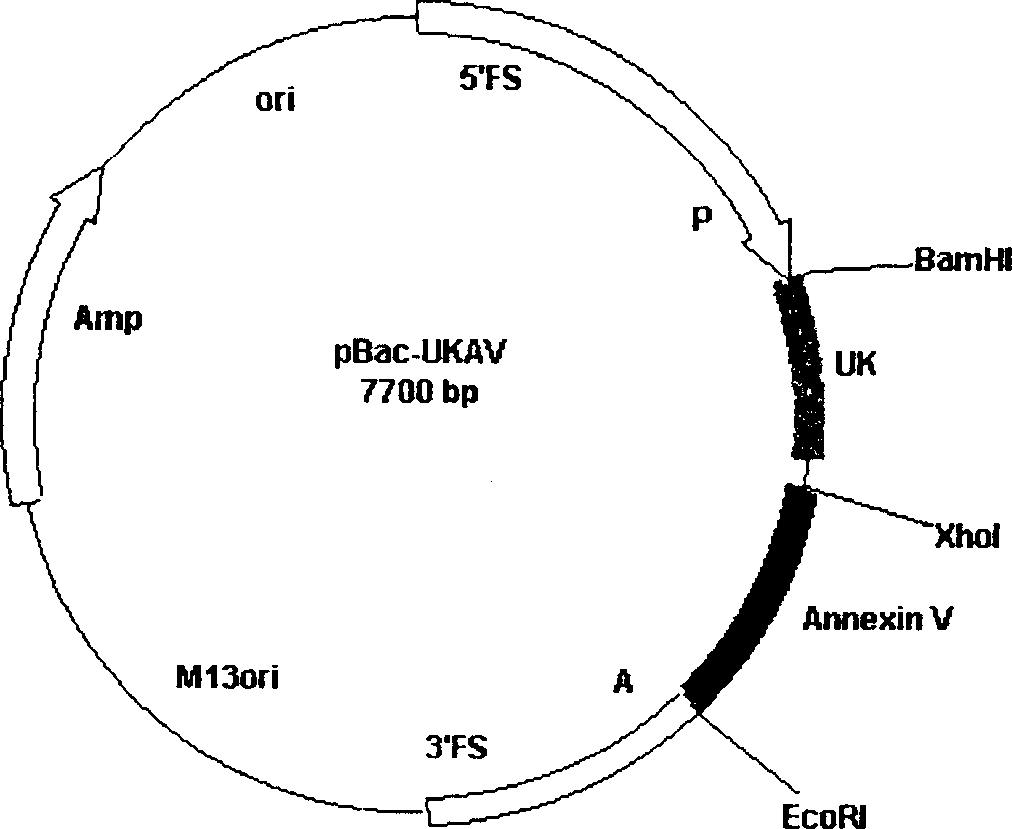
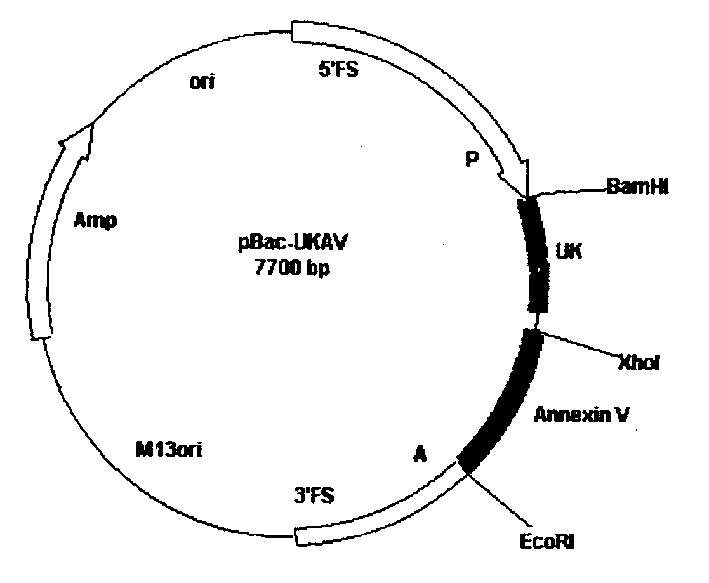
The method for producing antithrombotic medicine by using silkworm utilizes the Bombyx noriNuclear polyhedrosis virus expression system to recombine fusion protein gene with human Annexin V and low molecular weight urokinase to obtain recombinant virus. Said recombinant virus is inoculated in bombyx mori larva and chrysalis to make expression, through separation, purification and freeze-drying, the invented antithrombotic oral liquor and injection can be made up. The animal tests show that it possesses obvious antithrombotic action, and is a new type thrombolytic medicine.
Owner:浙江中奇生物药业股份有限公司
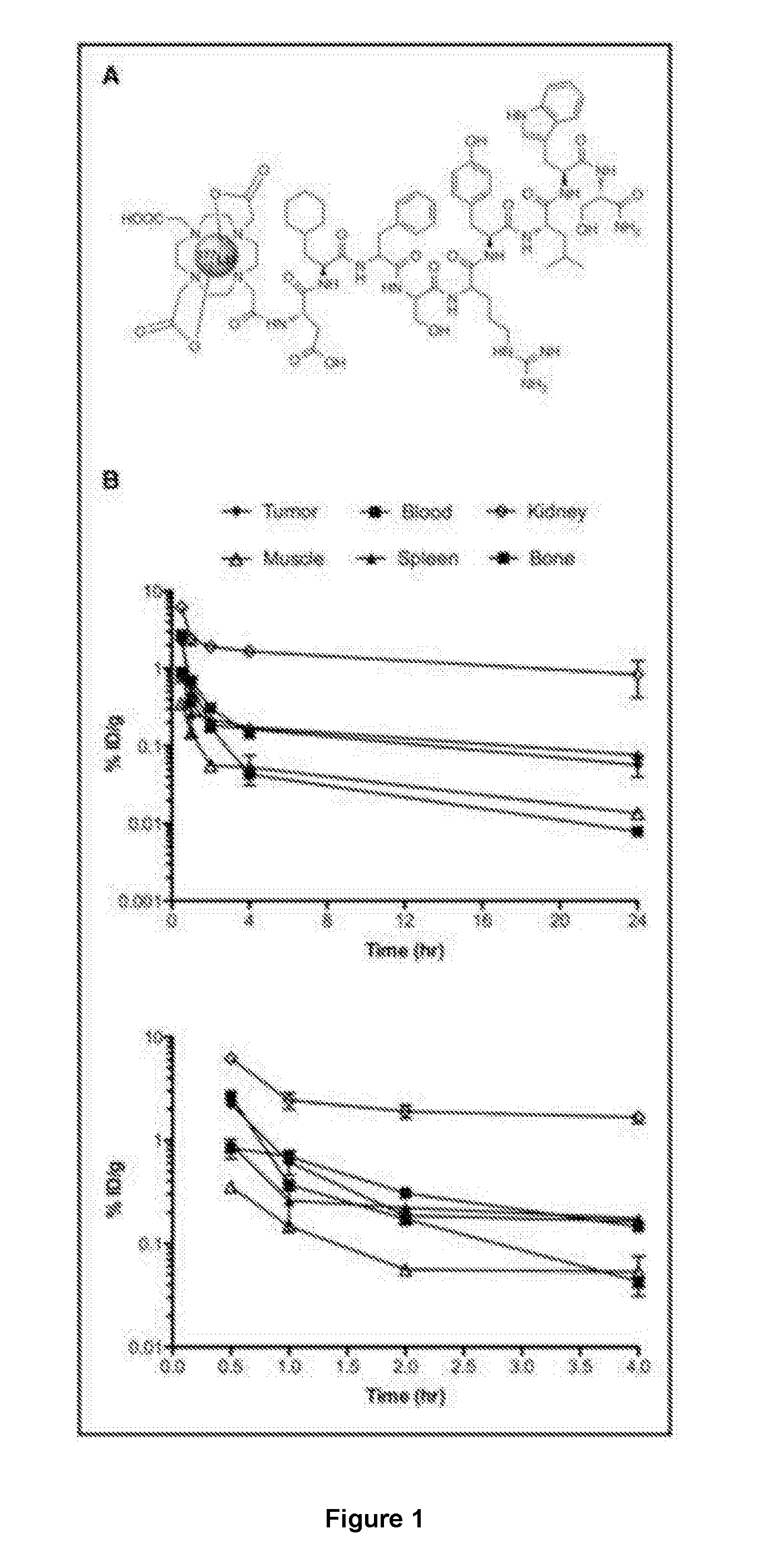
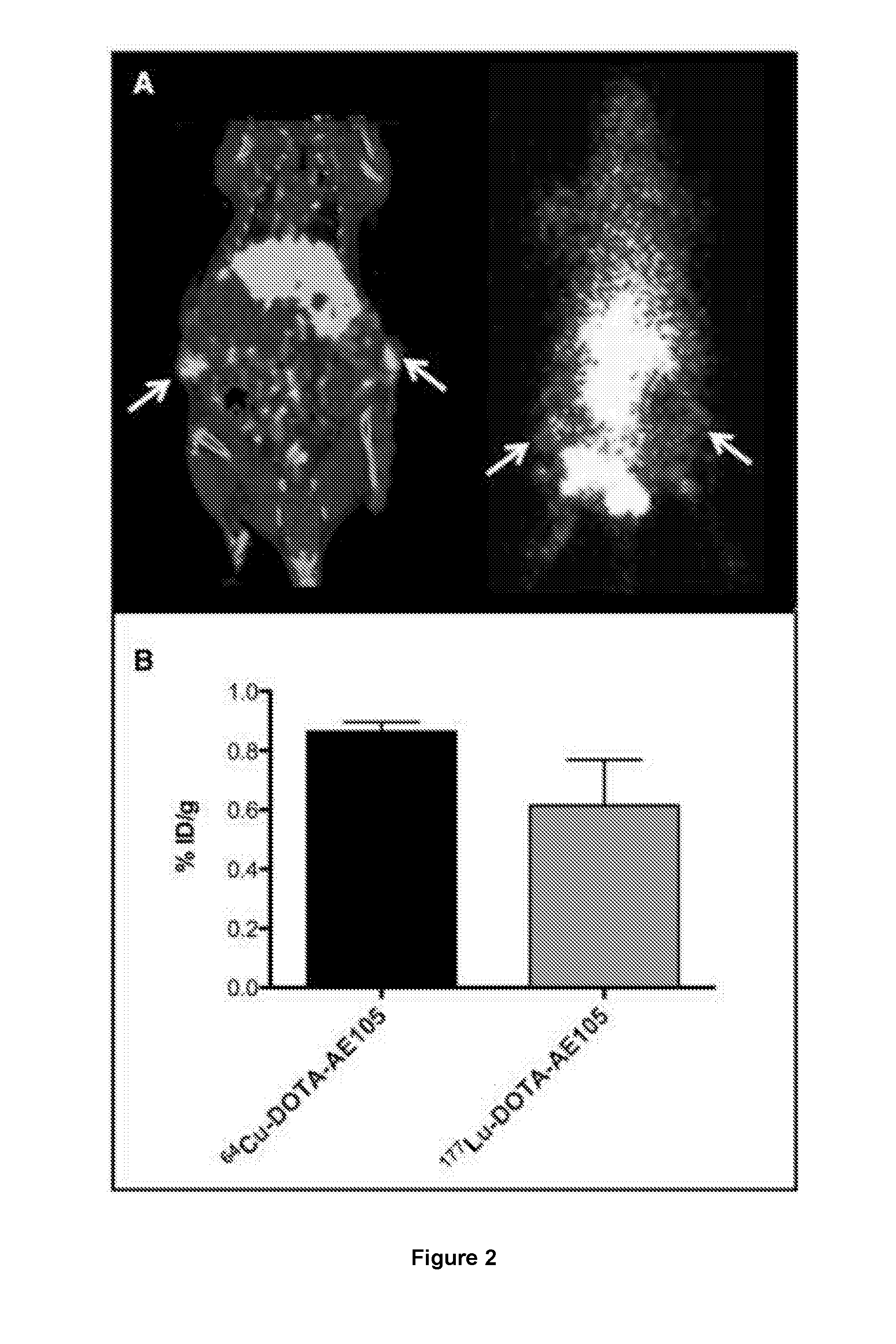
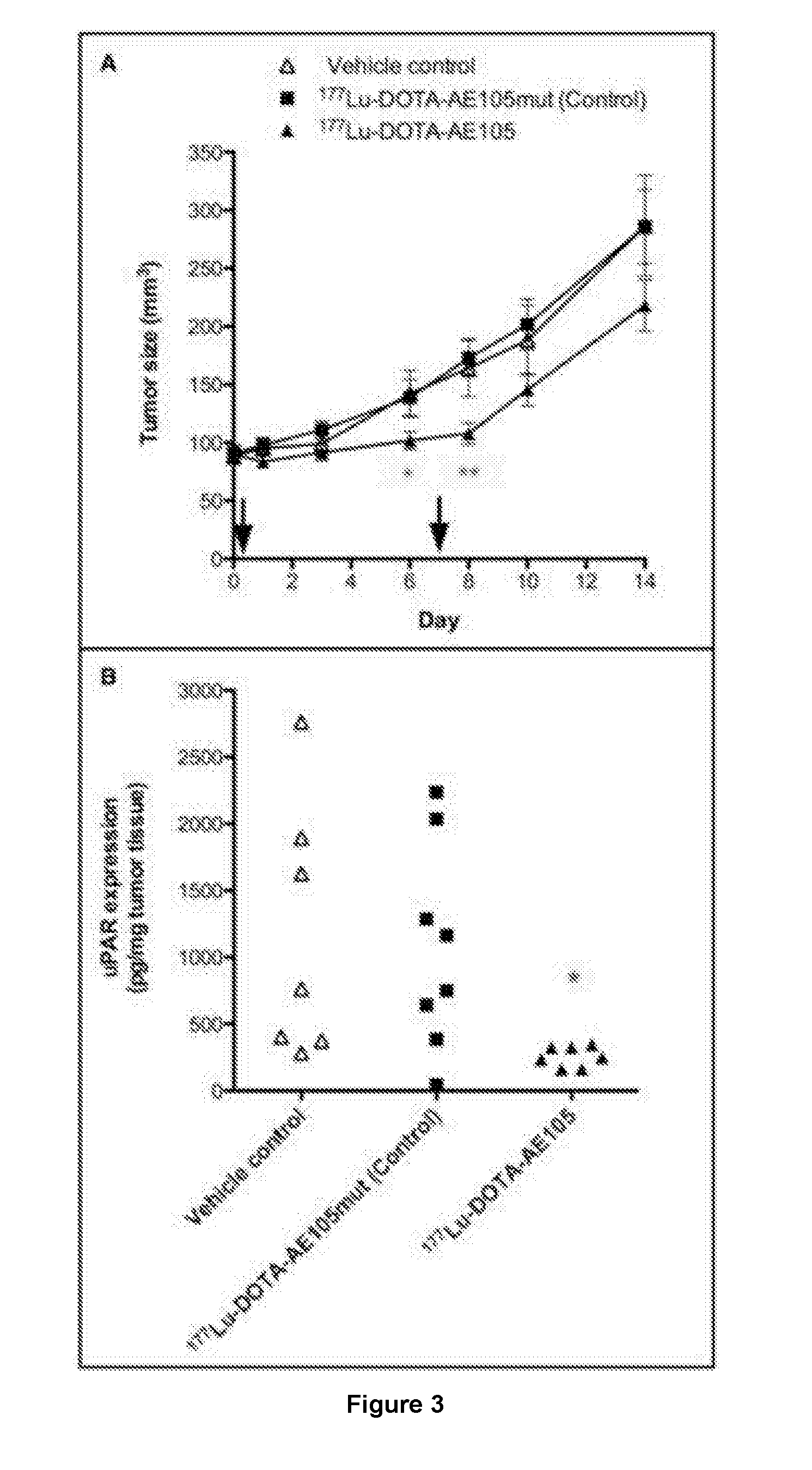
177-Lu LABELED PEPTIDE FOR SITE-SPECIFIC uPAR-TARGETING
ActiveUS20150132219A1Inducing effectImprove the level ofPeptide/protein ingredientsRadioactive preparation carriersDiseaseUrokinase Plasminogen Activator
There is provided a 177-Lu labelled peptide for site-specific targeting of the Urokinase Plasminogen Activator Receptor (uPAR) thereby enabling treatment of a cancer disease associated with high uPAR expression; e.g. treatment of colorectal cancer by administering to a patient an effective amount of the 177-Lu labelled peptide.
Owner:TRT INNOVATIONS APS
Extraction method of clotting genomic DNA (deoxyribonucleic acid)
InactiveCN102321613APromote decompositionFast extractionDNA preparationUrokinase Plasminogen ActivatorGenomic DNA
The invention provides an extraction method of clotting genomic DNA (deoxyribonucleic acid), which comprises the following steps that: nattokinase is added to a sample before extraction, and fibrin can be quickly decomposed by utilizing the thrombolytic force of the nattokinase several times more than that of a thrombolytic agent UK (urokinase), and further blood clots are separated, so that the blood clots become a homogeneous solution; and the obstacle of blood DNA extraction caused by sludged blood is removed, thereby the extraction speed of blood DNA is obviously improved, and simultaneously the purity and the yield of DNA product are greatly increased. The method provided by the invention is applicable to the extraction of DNA from sludged blood from a crime scene or dead bodies, the extraction of high-throughput blood gDNA and the extraction of blood gDNA in an antibody analysis experiment.
Owner:吴剑 +1
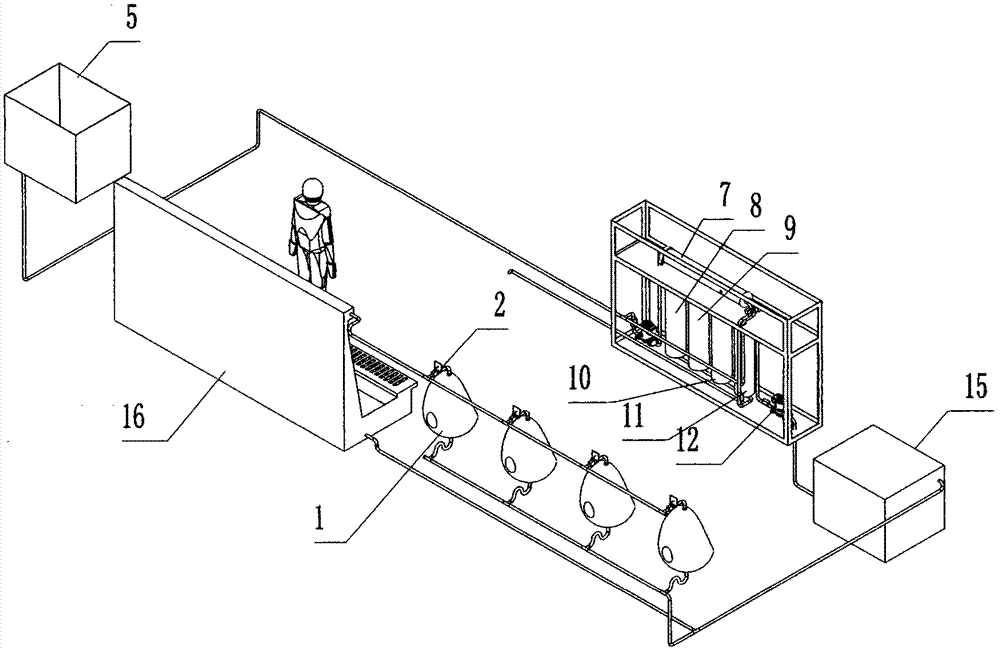
Rapid urokinase absorption bag
ActiveCN103263894AImprove adsorption capacityAdsorption instantOther chemical processesSolid sorbent liquid separationUrokinase Plasminogen ActivatorUrine collection
The present invention provides a rapid urokinase absorption bag, which comprises a mutton gauze layer, a modified cellulose layer, and a modified resin layer or a modified silica gel layer, wherein the mutton gauze layer, the modified cellulose layer, and the modified resin layer or the modified silica gel layer are sequentially arranged from the outside to the inside. The rapid urokinase absorption bag has the following characteristics that: a structure is simple; use is convenient; combination of the modified cellulose layer and the modified resin layer or the modified silica gel layer is adopted, such that the prepared absorption bag can timely and rapidly absorb urokinase in a toilet urinal so as to reduce steps of initial urine collection, transportation and the like in the traditional method, substantially simplify steps and workload of urokinase crude product extraction, provide characteristics of convenience and rapidness, and substantially save cost. In addition, large-scale industrial production of the rapid urokinase absorption bag is easily achieved.
Owner:NANJING NANDA PHARMA
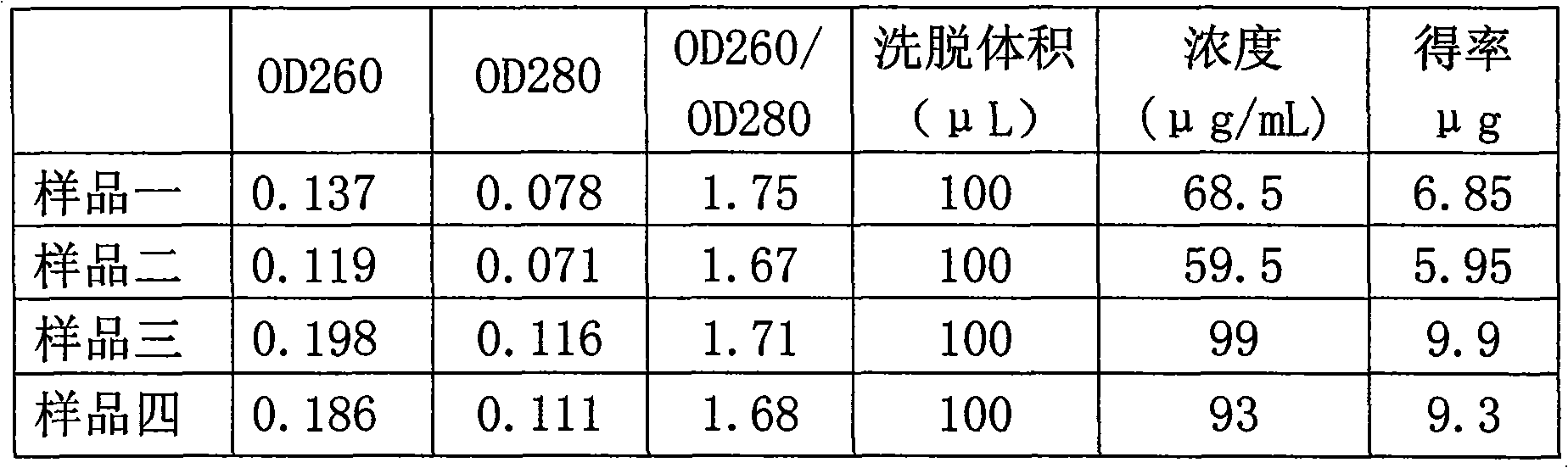
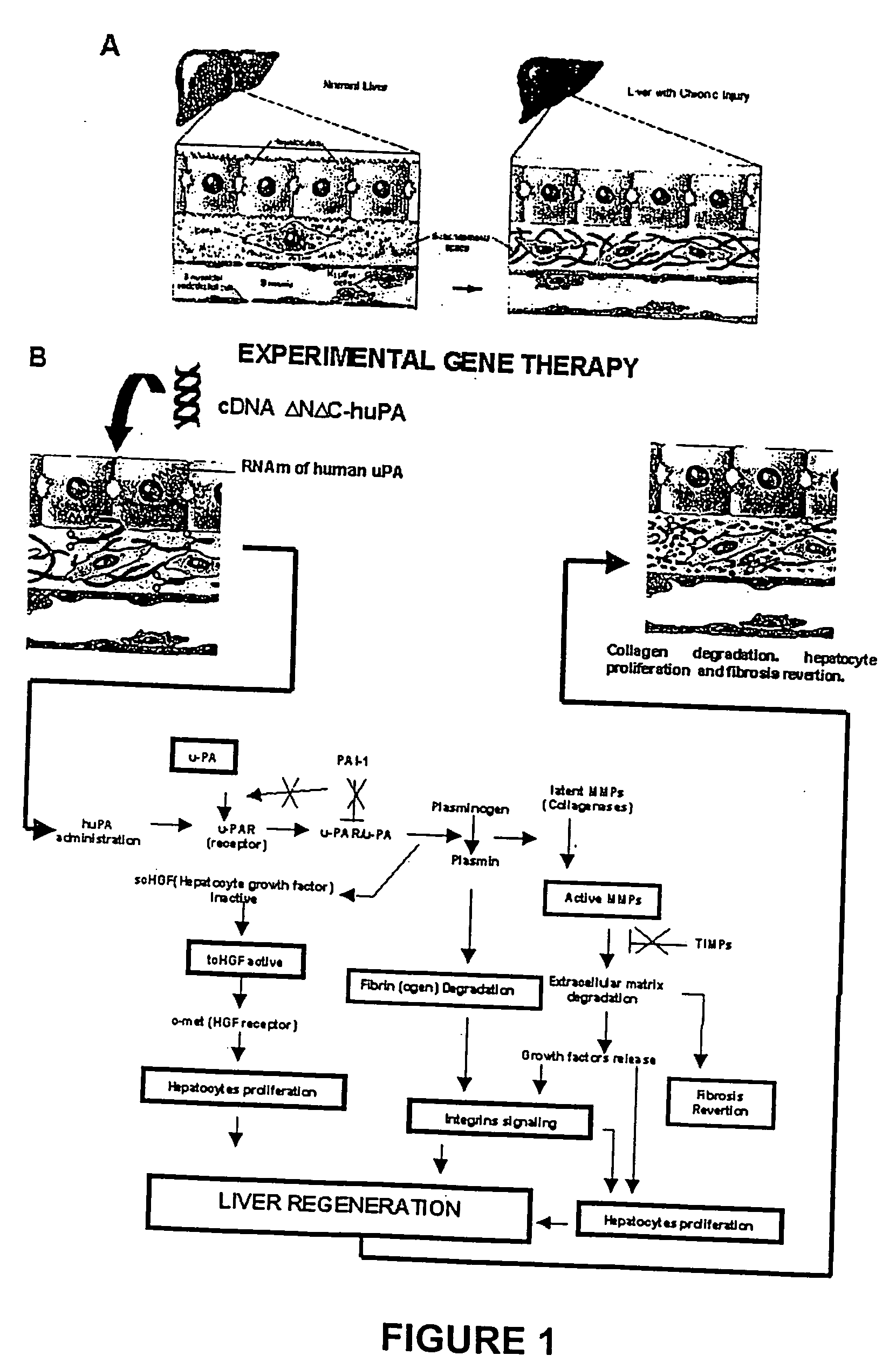
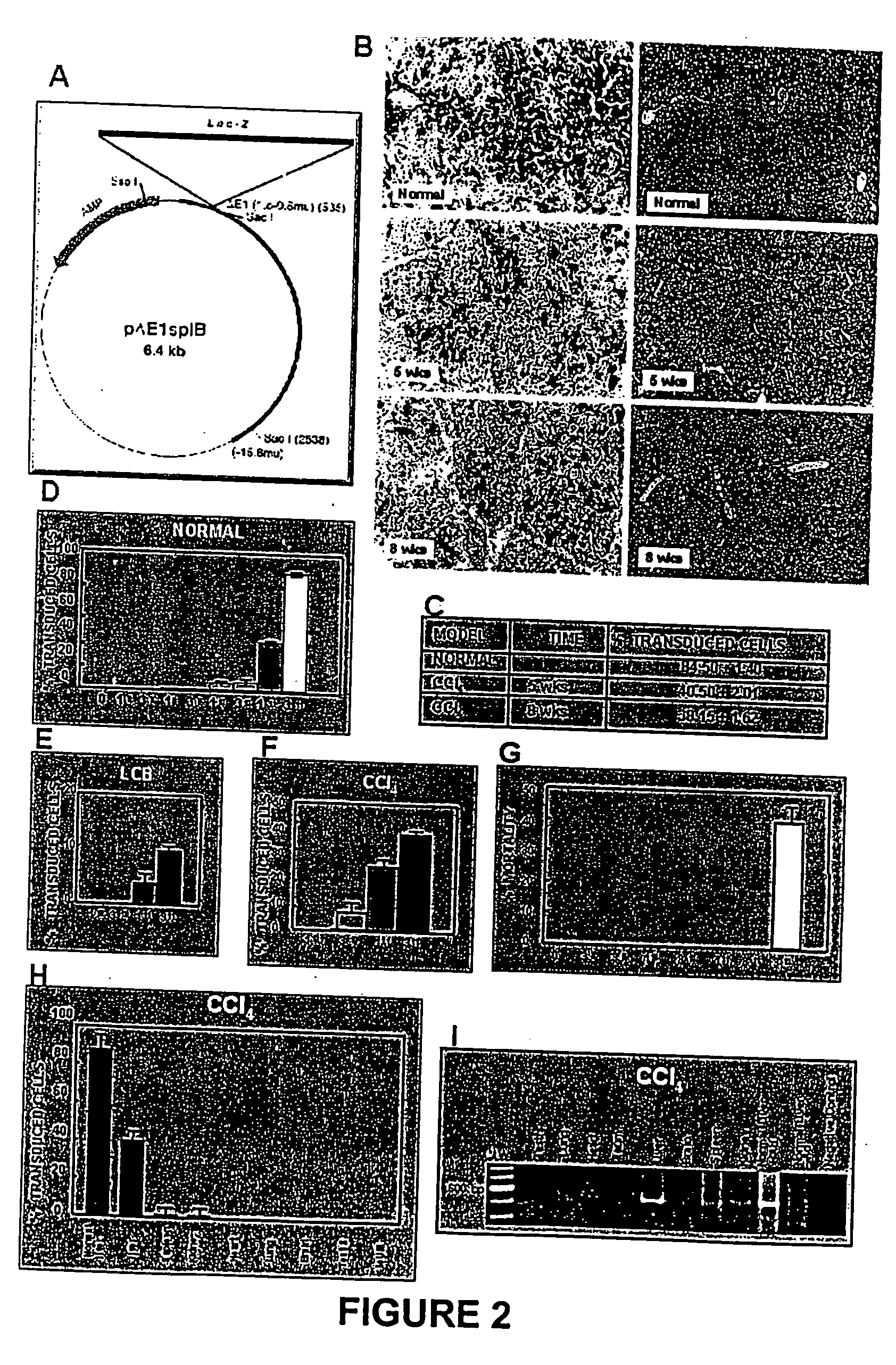
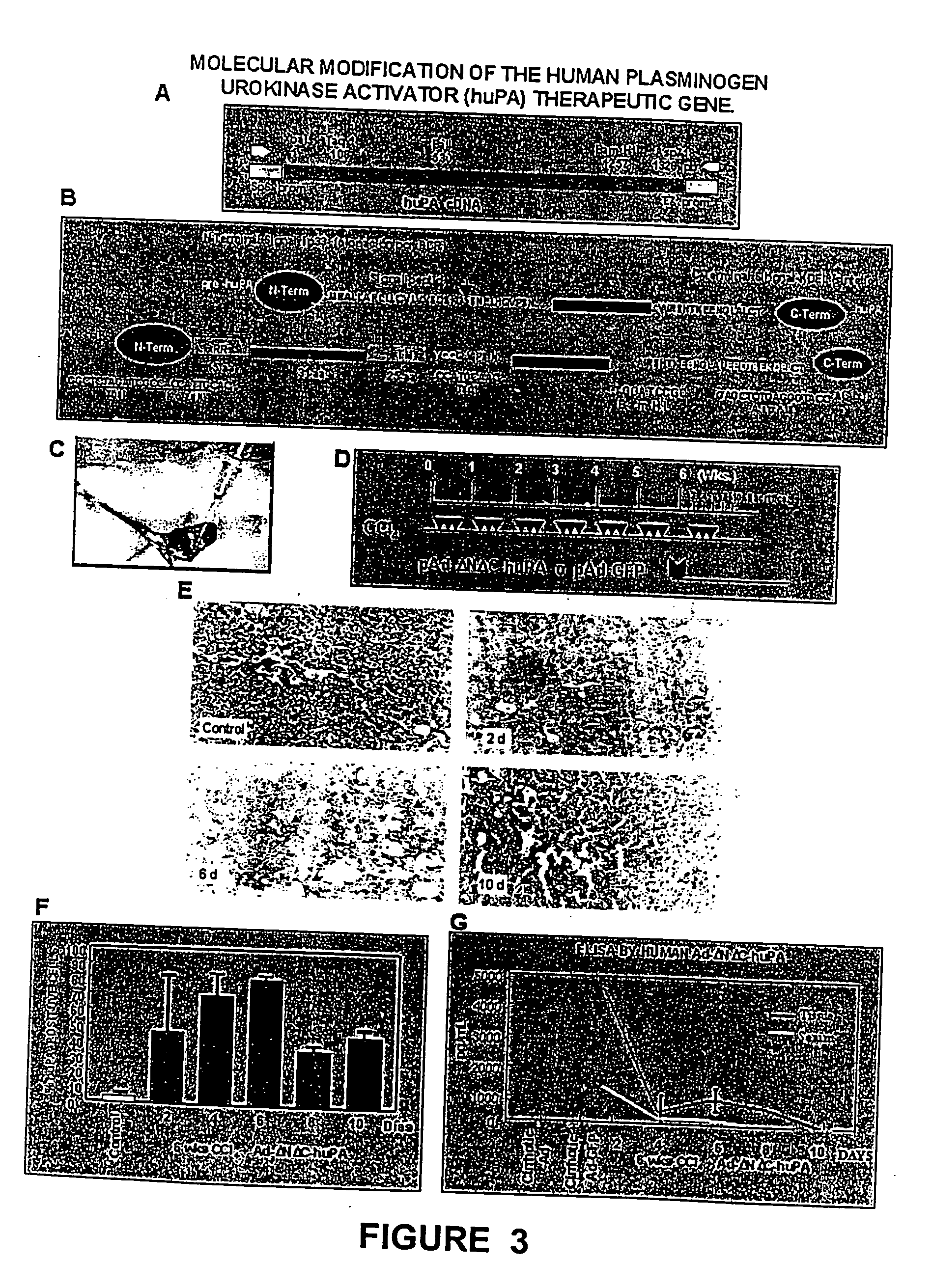
Recombinant viral and non-viral vectors containing the human urokinase plasminogen activator gene and its utilization in the treatment of various types of hepatic, pulmonary, pancreatic and cardiac fibrosis and hypertrophic scars
InactiveUS20040097455A1Reestablishment of liver functionFacilitated DiffusionPeptide/protein ingredientsGenetic material ingredientsCardiac fibrosisCause of death
Hepatic cirrhosis is considered a severe health problem in Mexico, since it is the third mortality cause in working-age people and there is no 100% effective treatment. Cirrhosis is characterized by an exacerbated increase of collagen in liver parenchyma, replacing the hepatocytes and thus provoking liver failure. This is one of the reasons why we have used a gene therapy through specific delivery to cirrhotic livers of the gene of human urokinase plasminogen activator (huPA), which activates mechanisms that induce the degradation of excess cellular matrix and stimulate hepatocyte proliferation, obtaining thus a fast re-establishment of the liver function. In the instant invention, the modified human uPA gene was inserted in the adenoviral vector (pAd-DeltahuPA), because it is not secreted and does not provoke hypercoagulation or spontaneous internal bleedings. Moreover, data from the bio-distribution essay with an adenoviral vector with reporter gene beta-gal have shown liver specificity as the target organ of the vector. Using ELISA, huPa protein was detected in liver homogenates (4500 pg / ml) in animals treated with pAd-DeltahuPA and was also intracellularly detected through immunochemistry in liver cuts (80% positive cells). huPa induced a dramatic fibrosis reduction (85%) on day 10 of vector administration, compared to control cirrhotic rats and 55% hepatocyte proliferation increase. Liver function tests (ALT, AST, alkaline phosphatase and bilirubin) dropped to nearly normal levels and hepatocyte proliferation was observed. Because of the two beneficial event cascades, gene therapy with modified huPA can be developed as a definite potential treatment for patients with liver cirrhosis.
Owner:TGT LAB DE C V
Nattokinase expression method and its special expression vector and engineering bacterium
The present invention discloses the expression method of natto kinase and its special expression vector and engineering bacterium. The expression vector is lactic acid bacteria constitutive expression vector containing constitutive expression promoter, natto kinase leading peptide and its mature peptide coding sequence; or lactic acid bacteria inducing expression vector containing inducing expression promoter, transmembrane signaling peptide coding sequence, natto kinase leading peptide and its mature peptide coding sequence. Experiment shows that the expressed product of the engineering bacterium has relatively high fibrinolysis activity up to 41.7 urokinase unit each milliliter of the fermented supernatant, eating safety, no toxic side effect on human body and high stability, is suitable for direct oral taking and may be added to various kinds of food. The present invention lays foundation for developing orally taken thrombolytic functional health food of natto kinase.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Manufacturing method of adjustable liver damage animal model and special DNA fragment thereof
The invention discloses a manufacturing method of an adjustable liver damage animal model and a special DNA fragment thereof; a DNA fragment I is formed by sequentially connecting liver super promoters and antisense tetracycline activator coding genes of a Tet-on gene expression system from upstream to downstream; an DNI fragment II is formed by sequentially connecting tetracycline reaction factors, promoters and prourokinase activator coding genes of the Tet-off / Tet-on gene expression system from upstream to downstream; a recombination expression vector carrying the DNI fragment II is used for converting mammalian cells, the cells are cultivated to obtain recombinant adenovirus. The DNA fragment I is led in the animal to obtain a transgenosis first-construction animal; the transgenosis first-construction animal and scid / bg animal are back-crossed to obtain the animal carrying the DNA fragment I with the scid / bg background; the recombinant adenovirus is used for injecting the animal carrying the DNA fragment I with the scid / bg background, so as to obtain the adjustable liver damage animal model; in the model, the prourokinase activator expression is activated by doxycycline, the expression intensity is changed along with the change of dosage, the specificity is very high.
Owner:PEKING UNIV
Protease activity of thrombin inhibits angiogenesis
InactiveUS20050232925A1Easy to produceOrganic active ingredientsSenses disorderUrokinase Plasminogen ActivatorProteinase activity
The present invention features pharmaceutical compositions and methods to inhibit angiogenesis, with implications to cancer therapy. These methods are based on the discovery that activated thrombin has antiangiogenic activity and that this antiangiogenic activity is at least in part, mediated through the activation of a class of thrombin receptors termed, Protease Activated Receptor (PAR). Pharmaceutical compositions and methods are also directed to a class of proteases which mediate this activation, particularly the urokinase plasminogen activator (uPA) polypeptide.
Owner:BETH ISRAEL DEACONESS MEDICAL CENT INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com