Expression, purification and crystallization of urokinase catalyst structure domain mutant
A technology of catalytic domains and mutants, applied in the fields of structural biology, biotechnology, and medicine, can solve the problems of unsuitable anti-tumor metastasis drugs, unclear interaction mechanism between urokinase and inhibitors, and achieve low cost, High stability and high expression effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0011] Example 1 Amplification of expression target gene
[0012] 1. The applicant designed and synthesized the following PCR primers by the primer synthesis company:
[0013] ①Starting primer (forward):
[0014] 5'-CCG CTCGAG AAAAGAATTATTGGGGGAGAATTCAC-3';
[0015] ②End primer (reverse): 5'-CCG CTCGAG TTATTCCTTGGTGTGACTGC-3';
[0016] ③ Mutation point (C279A) primer (forward): 5'-GACCATC GCC CTGCCCTC-3';
[0017] ④ Mutation point (N302Q) primer (reverse): 5'-GTCGGTAGA TTG CTCTTTTTCCAA-3';
[0018] Note: The underline in the ①② primer indicates the restriction endonuclease Xho I digestion site; ③④ The underline in the primer indicates the site of the mutated amino acid.
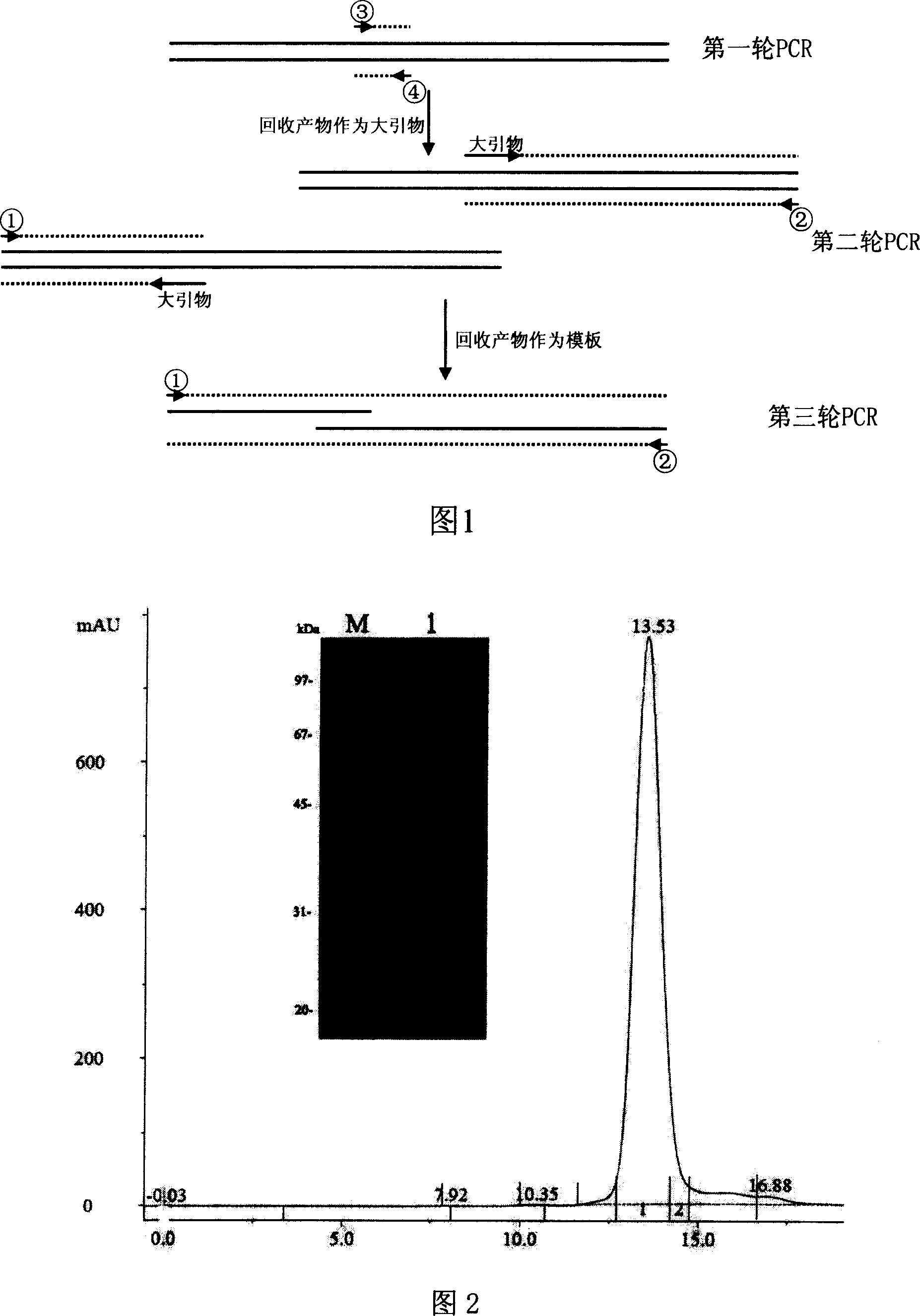
[0019] 2. Amplification and site-directed mutation of the catalytic domain of urokinase (C279A / N302Q) by overlap extension PCR (Fig.
[0020] 1): Using the full-length gene of urokinase as a template and using ③ and ④ as primers, perform the first round of PCR. The PCR conditions are: 94°C, 2 minut...
Embodiment 2
[0021] Example 2 Cloning of the target gene
[0022] Cloning of mutants in the catalytic domain of urokinase: the product was recovered by PCR, digested overnight with restriction endonuclease Xho I, and recovered by ethanol precipitation; similarly digested with restriction endonuclease Xho I to linearize Pichia pastoris expression vector pPICZαA, and remove Phosphorylated, gel recovery kit to recover linearized products. Mix the product recovered by PCR with the dephosphorylated plasmid pPICZαA, inactivate it with T4 DNA ligase, overnight at 16°C, and inactivate at 65°C for 5 minutes; take 1.5 μl of the linker and mix it with the pre-cooled competent cell TOP10F', discharge at 2.5kV, and electric shock , add 1 ml of SOC solution, incubate at 37°C for 1 hour, take 200 μl coated LB plate (25 μg / ml Zeocin), incubate at 37°C for 14 hours, select colonies, culture, small amount of recombinant plasmid extraction, enzyme digestion identification, enzyme Correctly identified clones...
Embodiment 3
[0023] Example 3 Expression and purification of mutant proteins
[0024] Expression and purification of recombinant plasmids in Pichia pastoris X-33: linearized recombinant plasmids were electrotransformed into yeast X-33 strains, coated with 100 μg / ml Zeocin YPDS plates, colonies were picked, genomes were extracted, and identified by PCR. The correct recombinant expression was induced by adding methanol at a final concentration of 0.5% every day, and the culture fluid was fermented for 4 days, and the supernatant was obtained by centrifugation, and the molecular weight was checked by 12% SDS-PAGE electrophoresis. Strains with high expression were expanded and cultured, induced by methanol for 4 days, and the supernatant was diluted to 1-fold volume and passed through a cation rapid exchange column SPFF pre-equilibrated with 20mM pH6.5 potassium phosphate buffer solution, 20mM pH6.5 potassium phosphate buffer solution (containing 0.5M chloride Sodium chloride) was eluted, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com