Patents
Literature
415 results about "Fungating tumour" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
High frequency thermal ablation of cancerous tumors and functional targets with image data assistance
InactiveUS6241725B1Ultrasonic/sonic/infrasonic diagnosticsSurgical needlesAbnormal tissue growthTumour volume
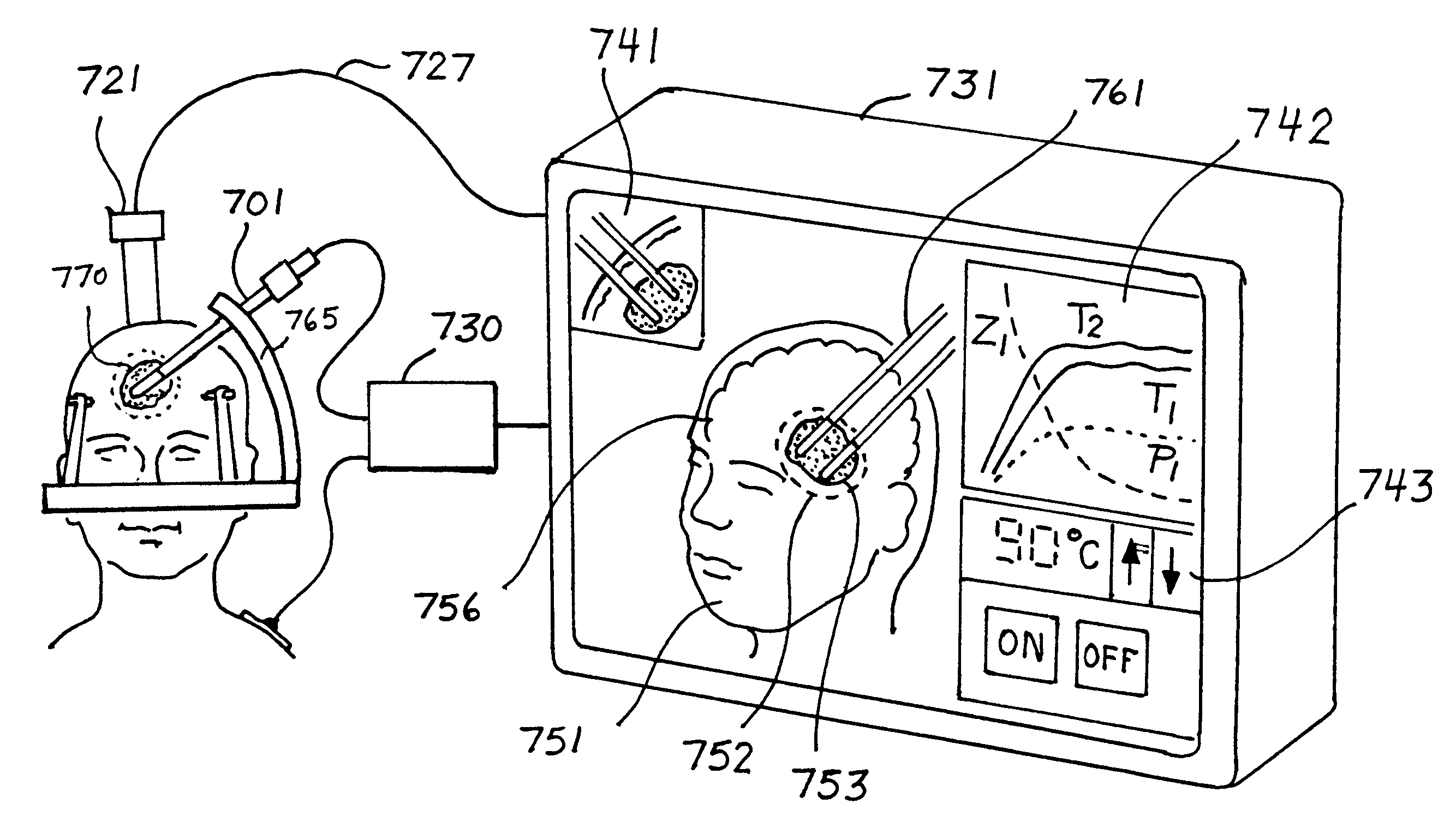
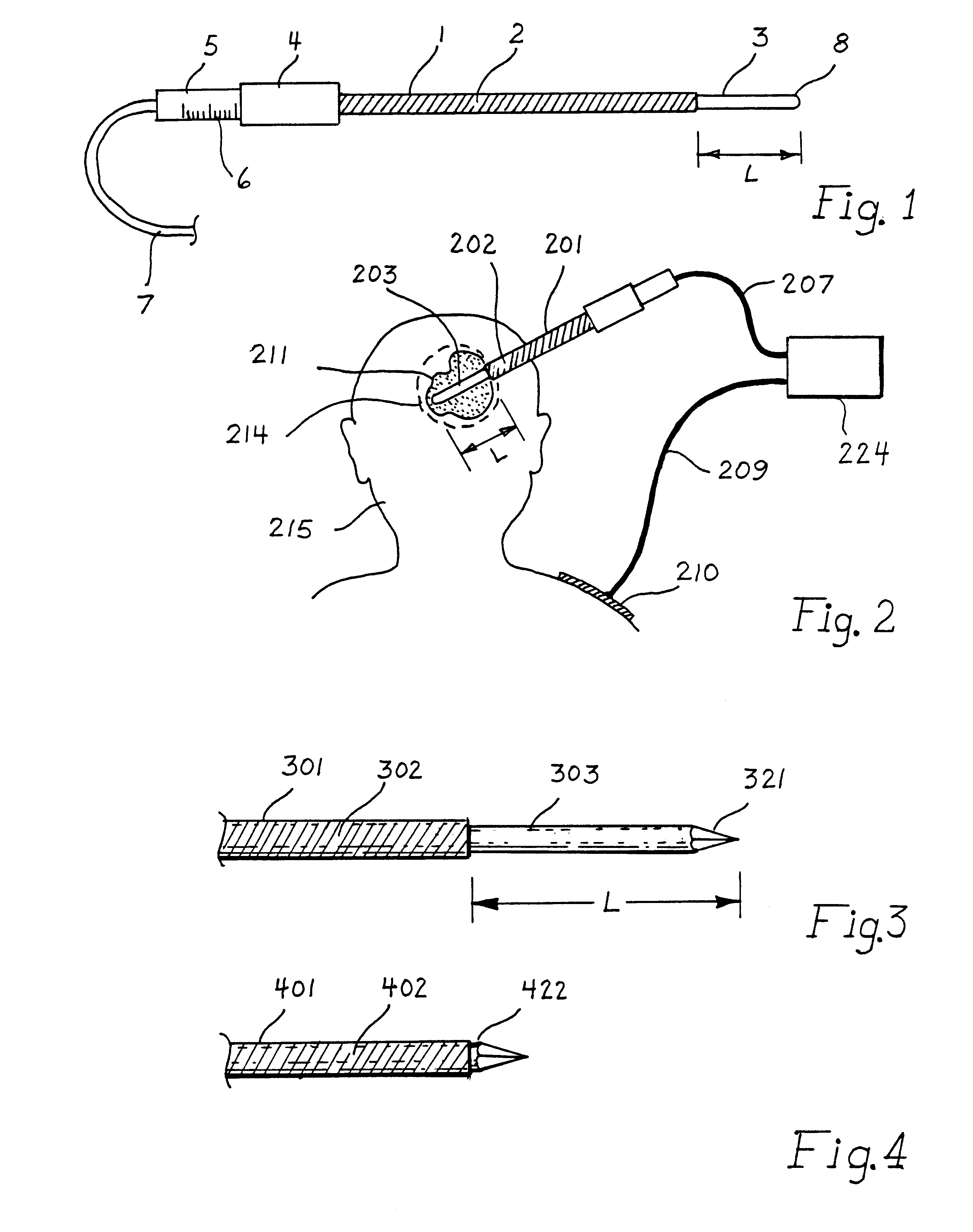
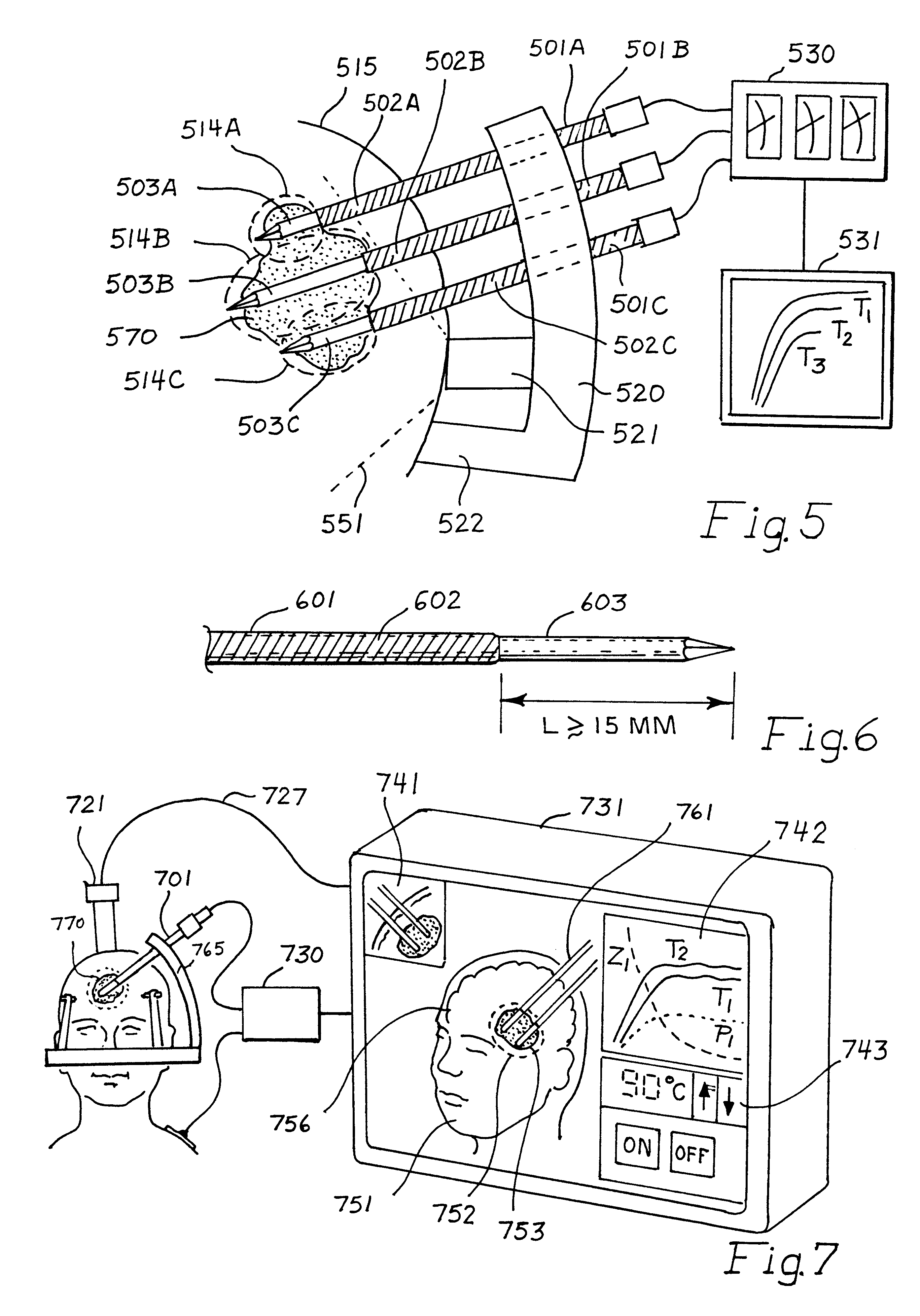
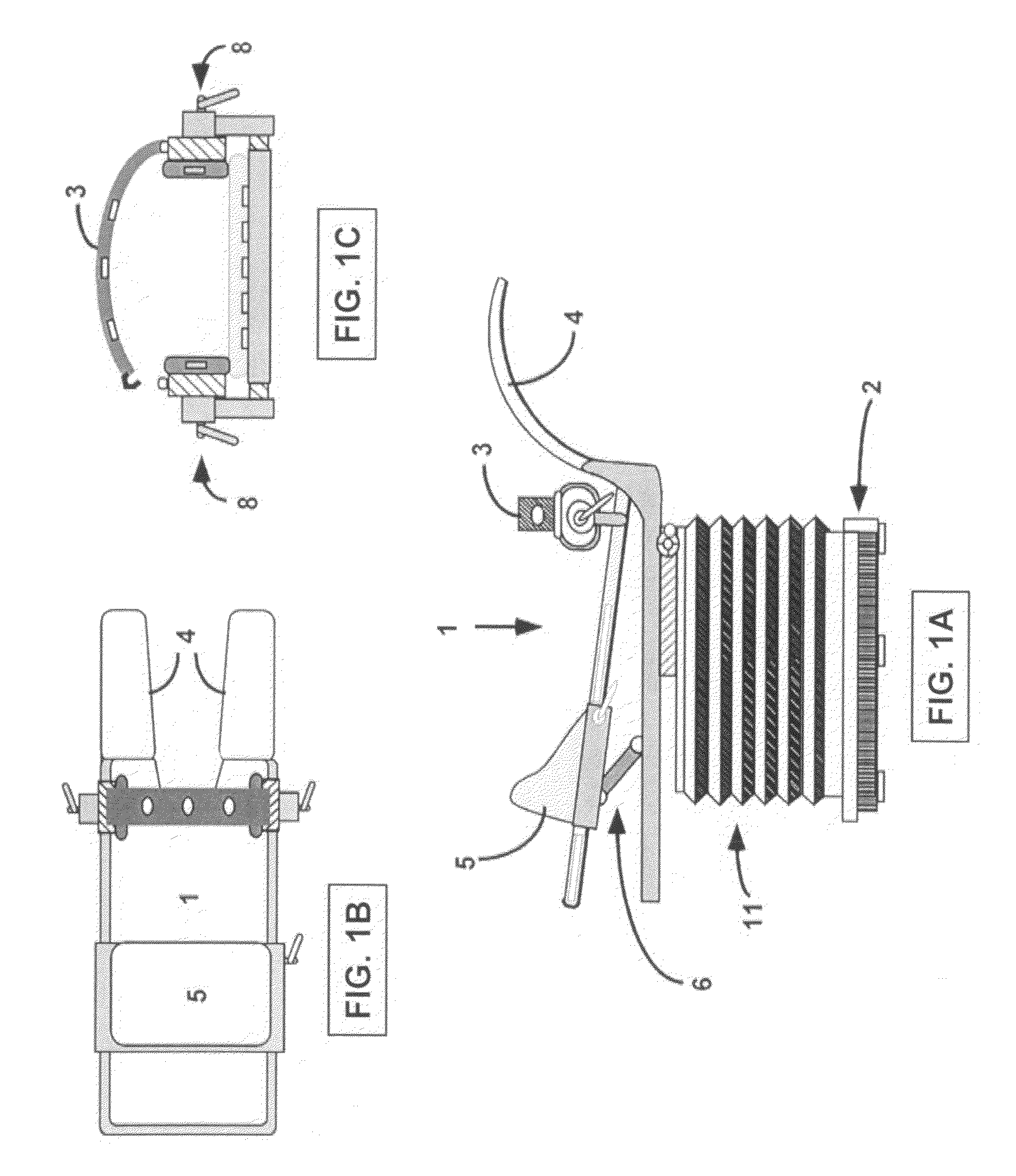
This invention relates to the destruction of pathological volumes or target structures such as cancerous tumors or aberrant functional target tissue volumes by direct thermal destruction. In the case of a tumor, the destruction is implemented in one embodiment of the invention by percutaneous insertion of one or more radiofrequency probes into the tumor and raising the temperature of the tumor volume by connection of these probes to a radiofrequency generator outside of the body so that the isotherm of tissue destruction enshrouds the tumor. The ablation isotherm may be predetermined and graded by proper choice of electrode geometry and radiofrequency (rf) power applied to the electrode with or without temperature monitoring of the ablation process. Preplanning of the rf electrode insertion can be done by imaging of the tumor by various imaging modalities and selecting the appropriate electrode tip size and temperature to satisfactorily destroy the tumor volume. Computation of the correct three-dimensional position of the electrode may be done as part of the method, and the planning and control of the process may be done using graphic displays of the imaging data and the rf ablation parameters. Specific electrode geometries with adjustable tip lengths are included in the invention to optimize the electrodes to the predetermined image tumor size.
Owner:COVIDIEN AG
Intraoperative, intravascular, and endoscopic tumor and lesion detection, biopsy and therapy
InactiveUS6096289ADiscriminationImprove discriminationUltrasonic/sonic/infrasonic diagnosticsSurgeryNon malignantAntibody fragments
Methods are provided for close-range intraoperative, endoscopic and intravascular deflection and treatment of lesions, including tumors and non-malignant lesions. The methods use antibody fragments or subfragments labeled with isotopic and non-isotopic agents. Also provided are methods for detection and treatment of lesions with photodynamic agents and methods of treating lesions with a protein conjugated to an agent capable of being activated to emit Auger electron or other ionizing radiation. Compositions and kits useful in the above methods are also provided.
Owner:IMMUNOMEDICS INC
Device for biopsy and treatment of breast tumors
A device for diagnosis and treatment of tumors and lesions within the body. A cannula adapted to apply suction through the lumen of the catheter to the tumor or lesion is described. The lumen has a self sealing valve through which a cryoprobe is inserted while the suction is being applied. The cryoprobe is then inserted into the lesion, and operated to ablate the lesion.
Owner:SCION MEDICAL
Device for biopsy tumors
A device and method of use for securing and coring of tumors within the body during a biopsy of the tumor, specifically breast tumors. An adhesion probe for securing the tumor is described. The probe secures the tumor by piercing the tumor and providing a coolant to the distal tip to cool the tip. The cooled tip adheres to the tumor. A coring instrument adapted for cutting a core sample of the tumor is described. The instrument is provided with a cannula that can cut a core sample of the tumor. The instrument is adapted for use with the probe with the probe fitting within the cannula. The instrument can be used in conjunction with the probe to secure and core a sample of the tumor for biopsy.
Owner:SCION MEDICAL
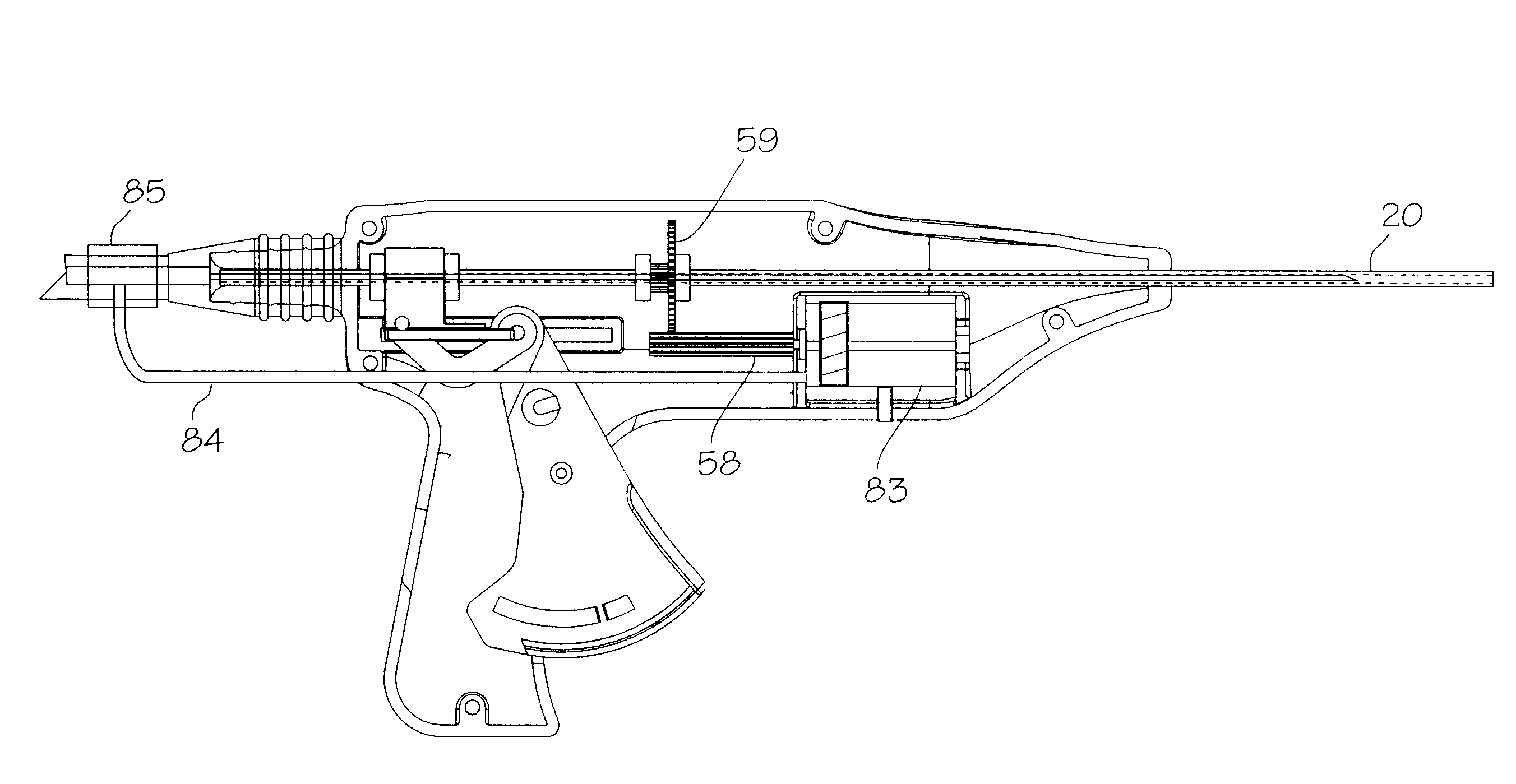
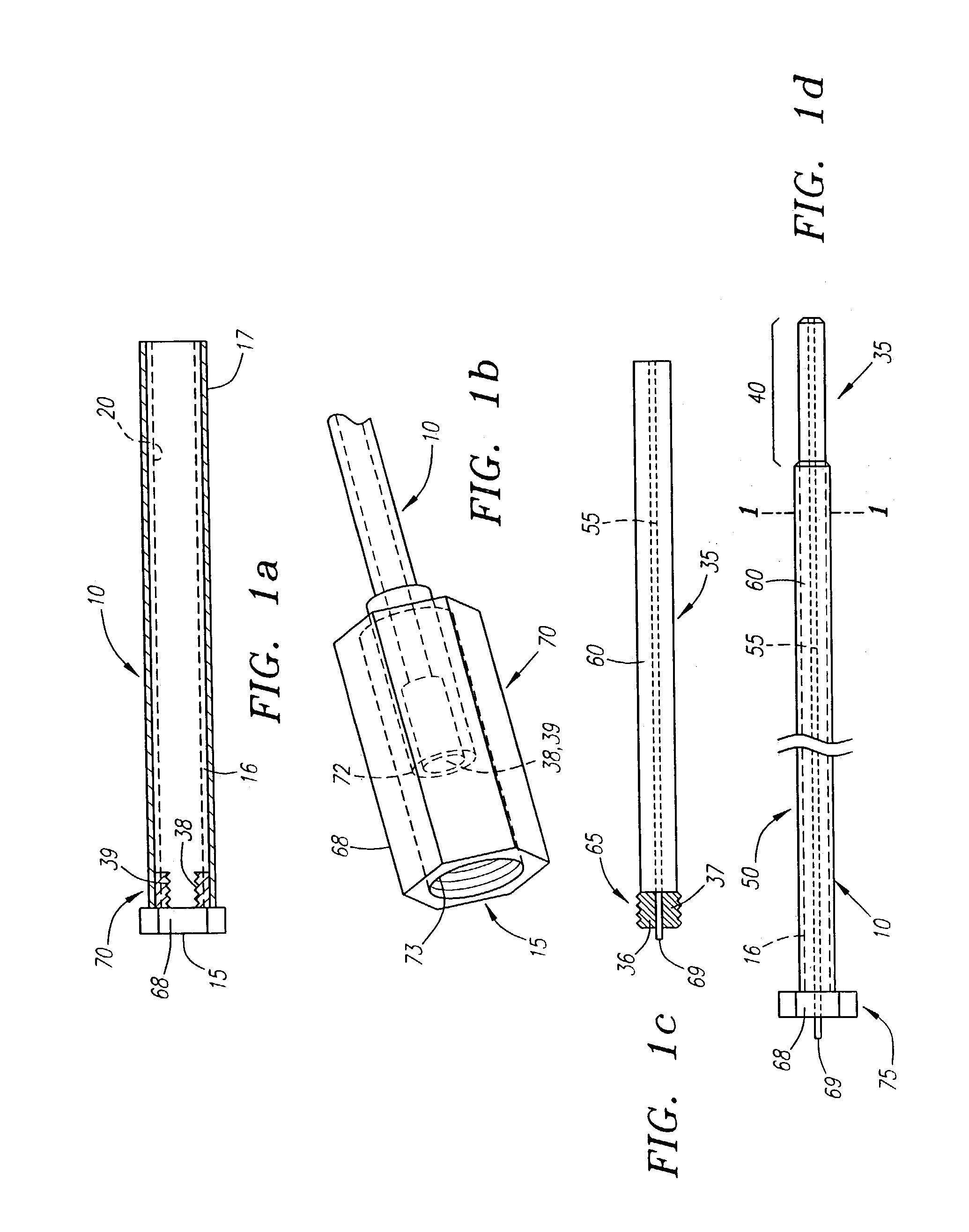
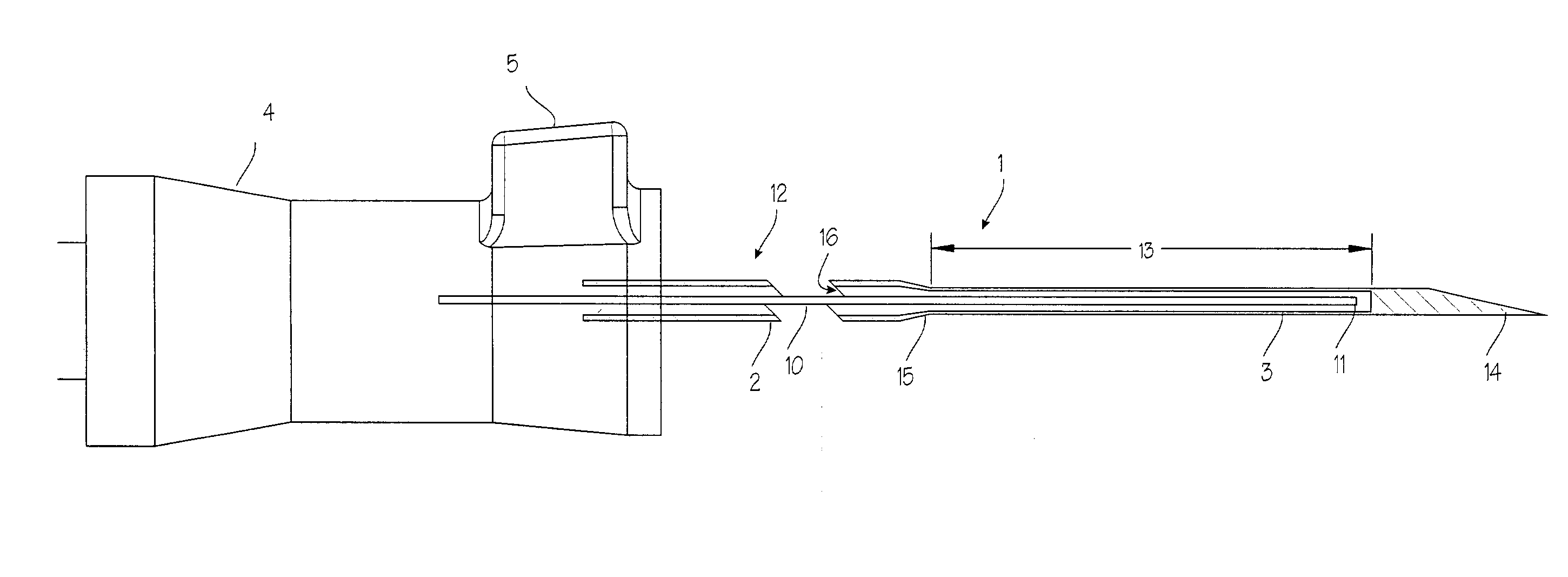
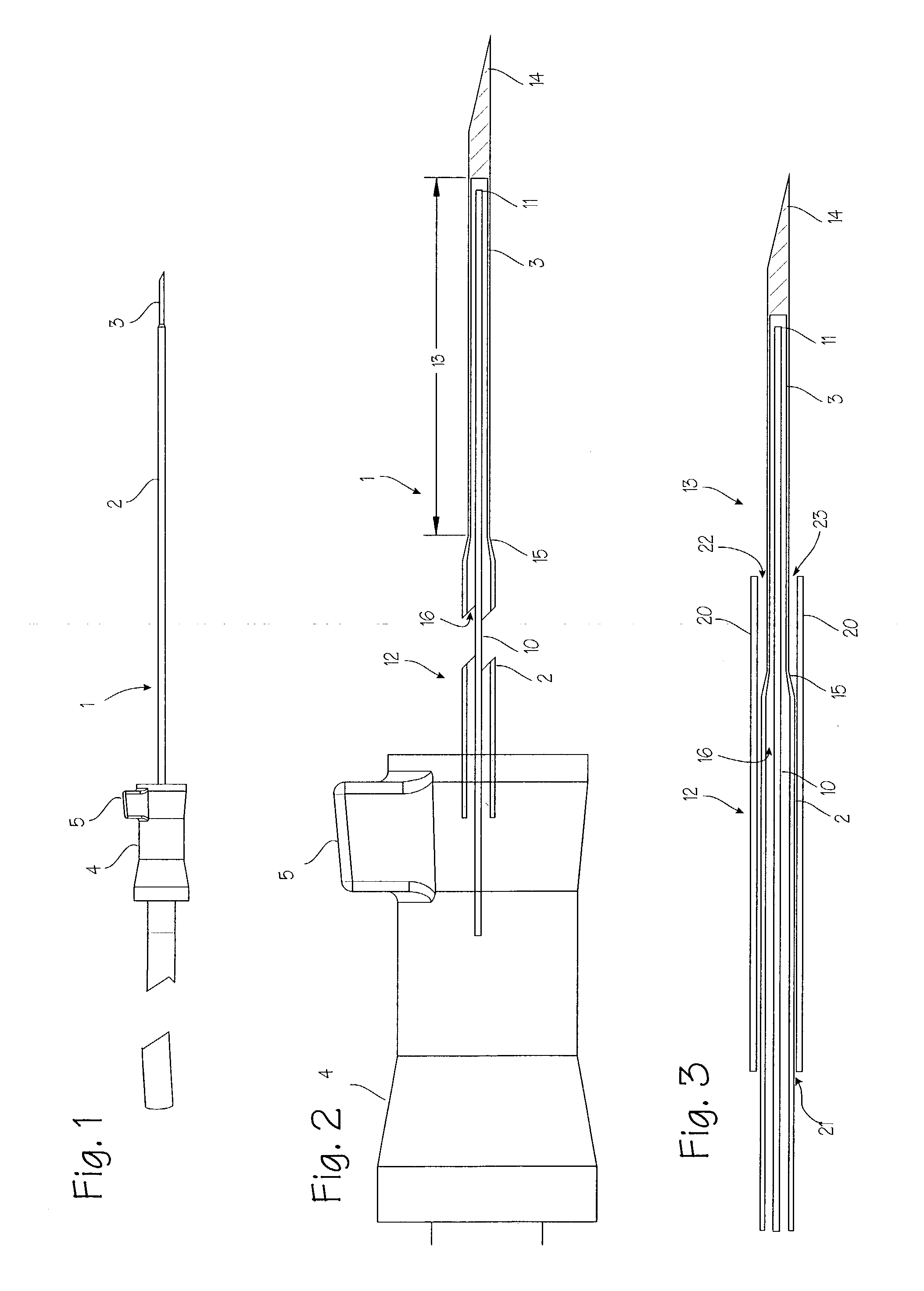
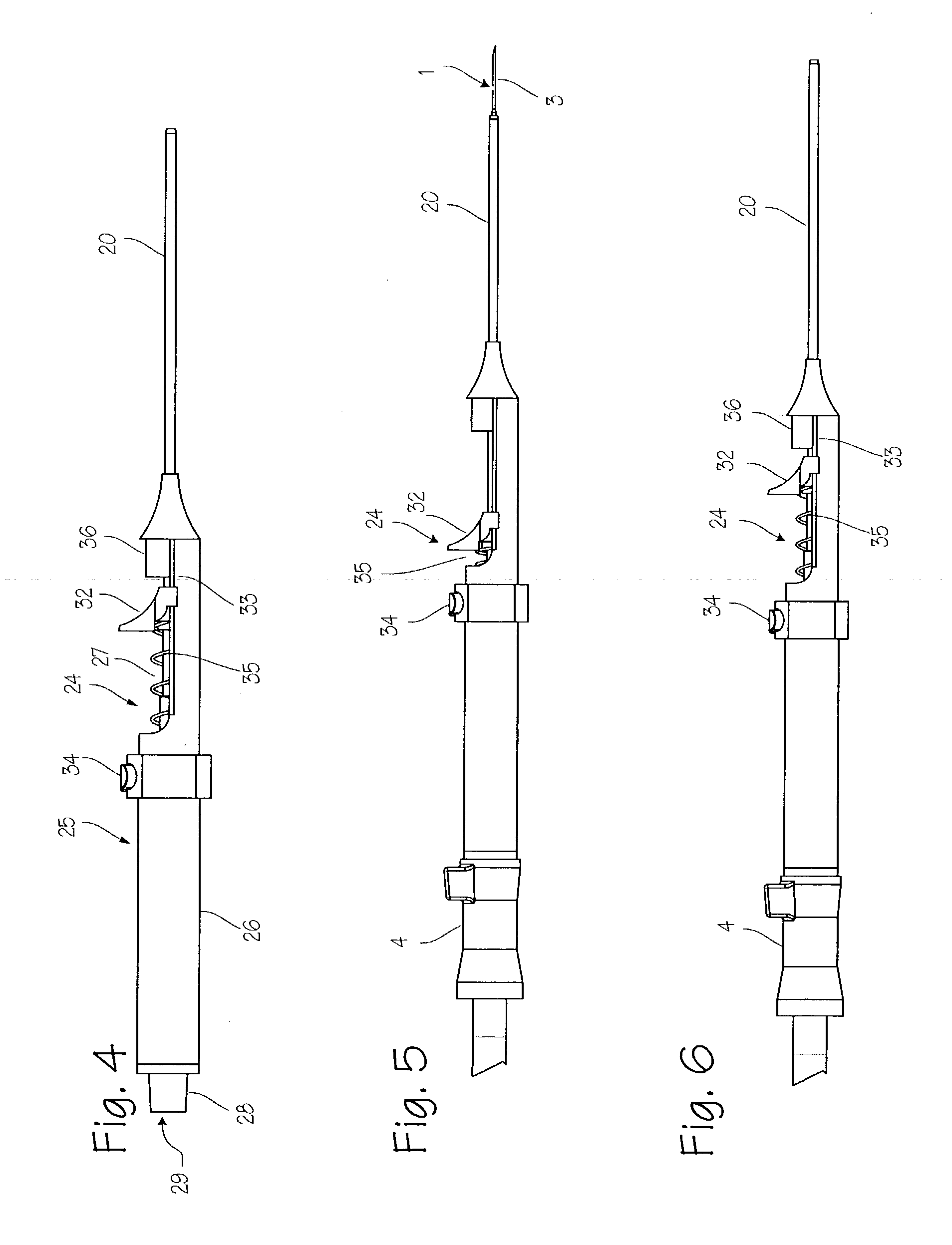
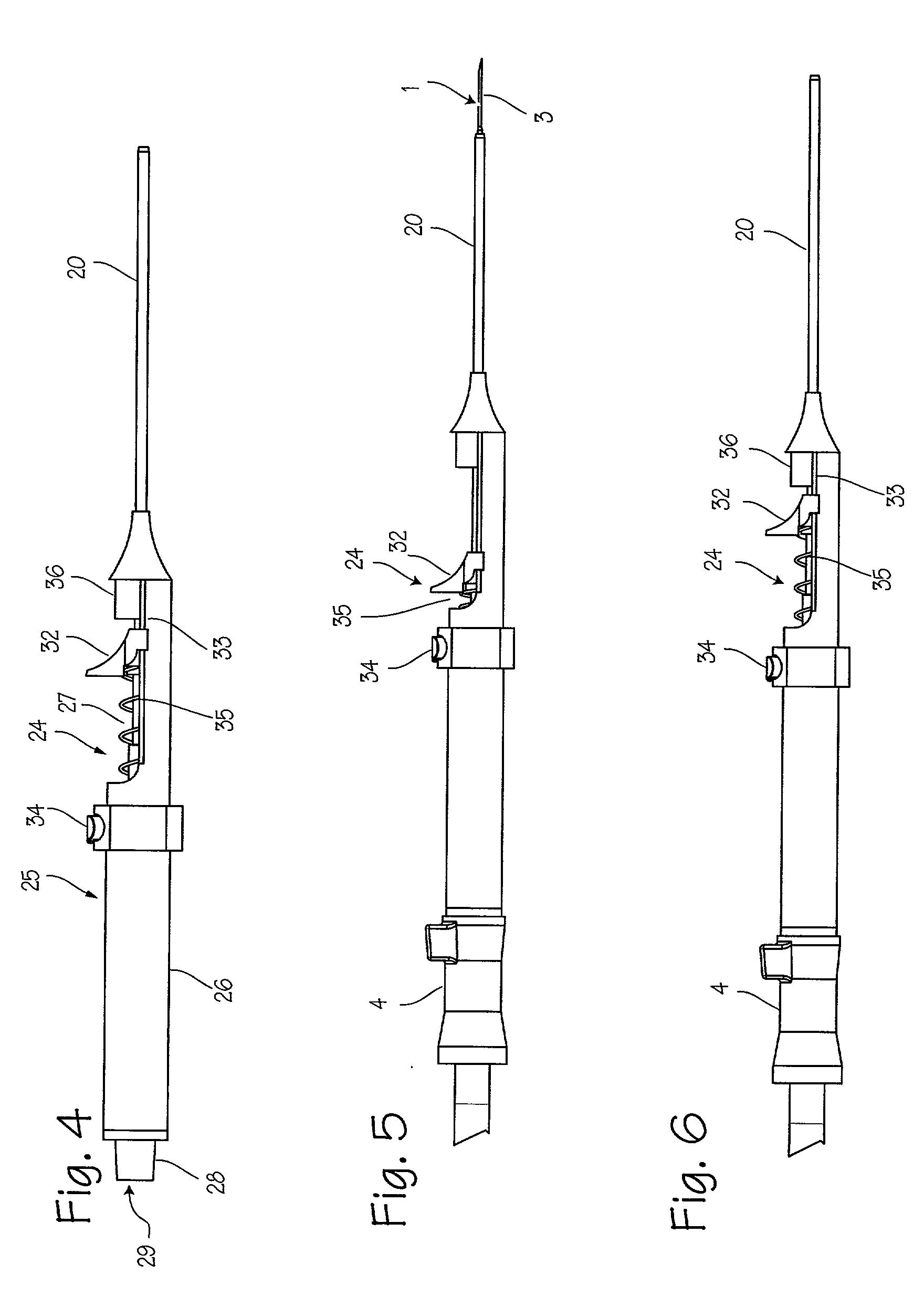
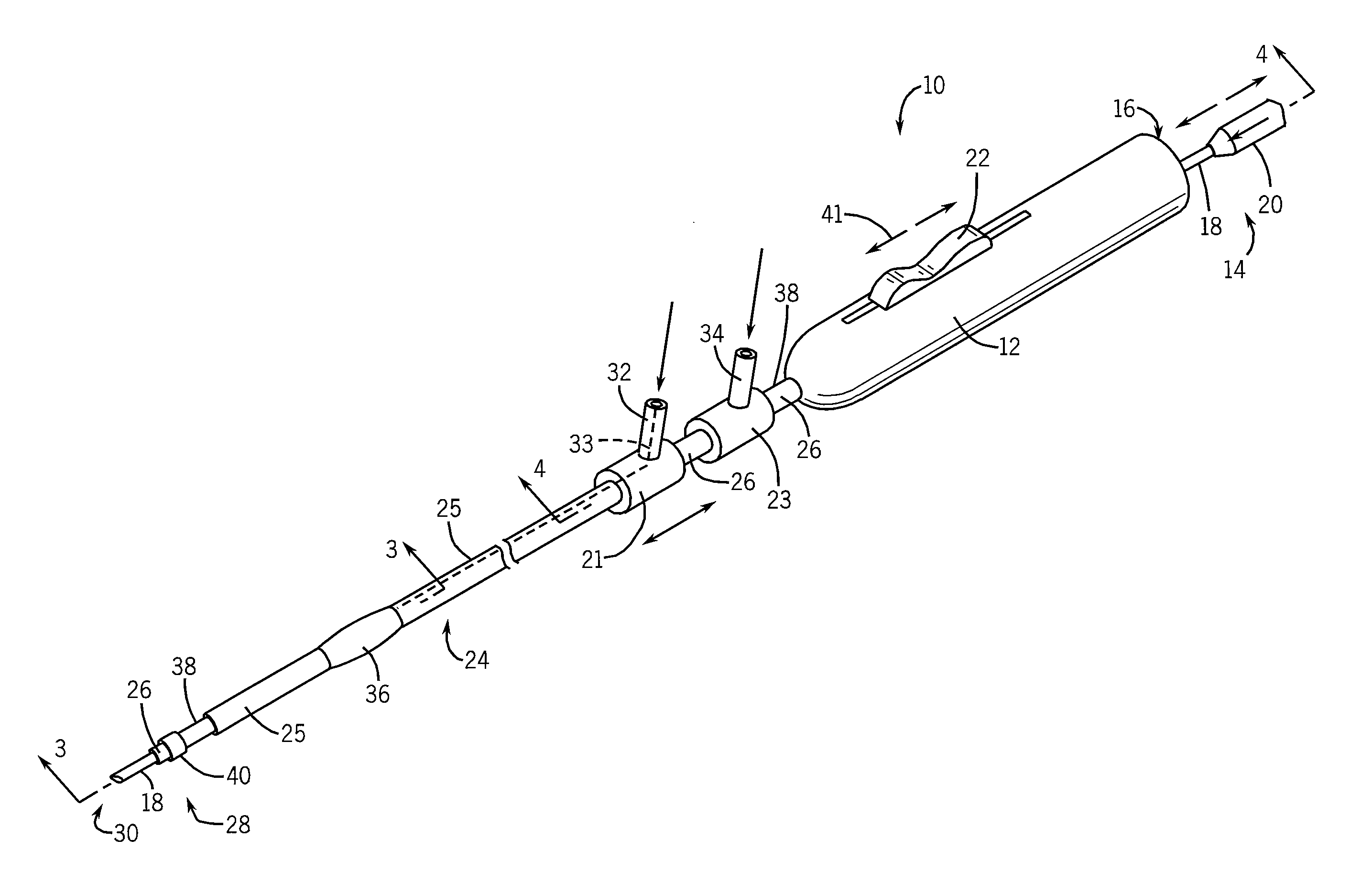
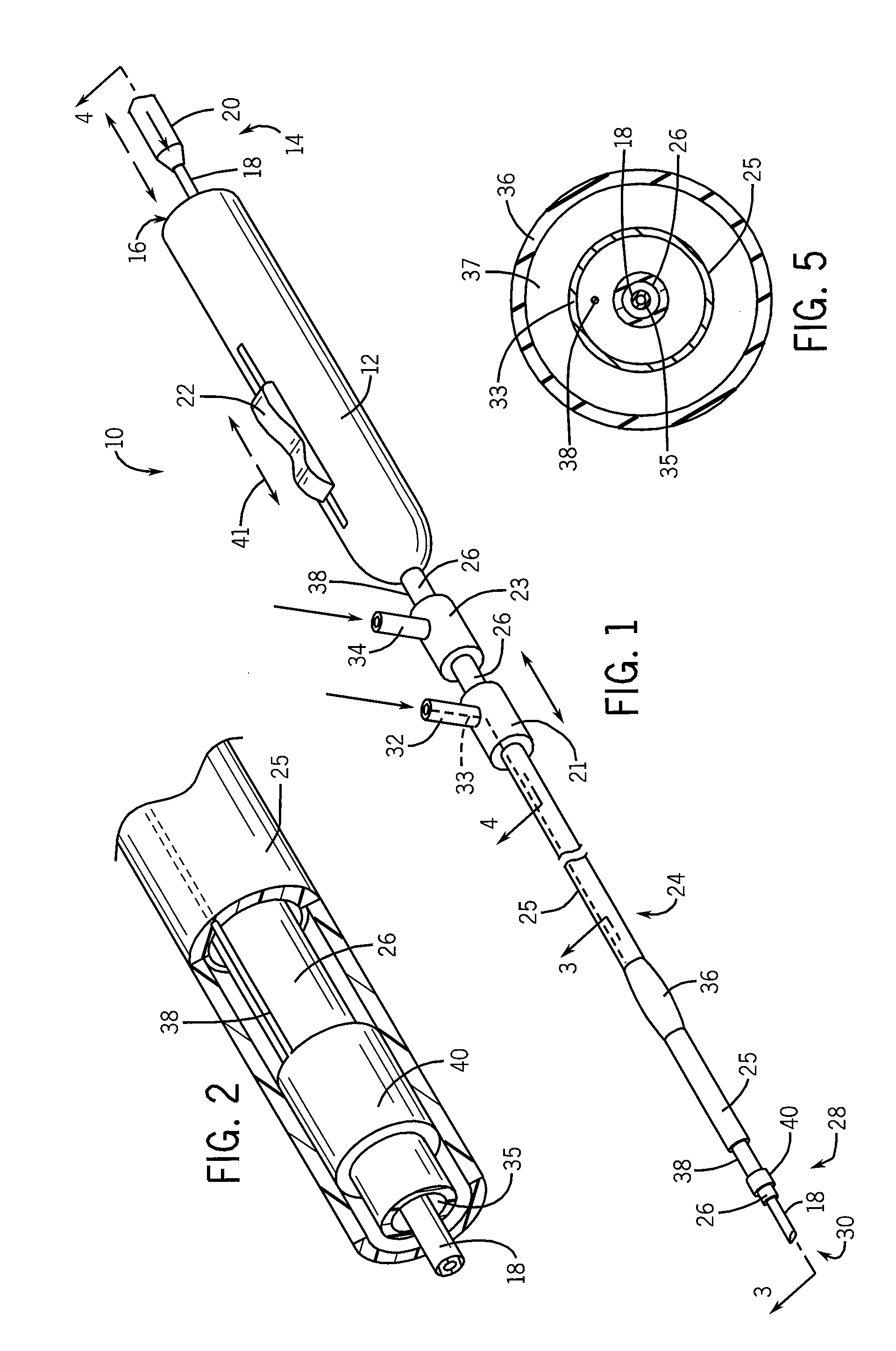
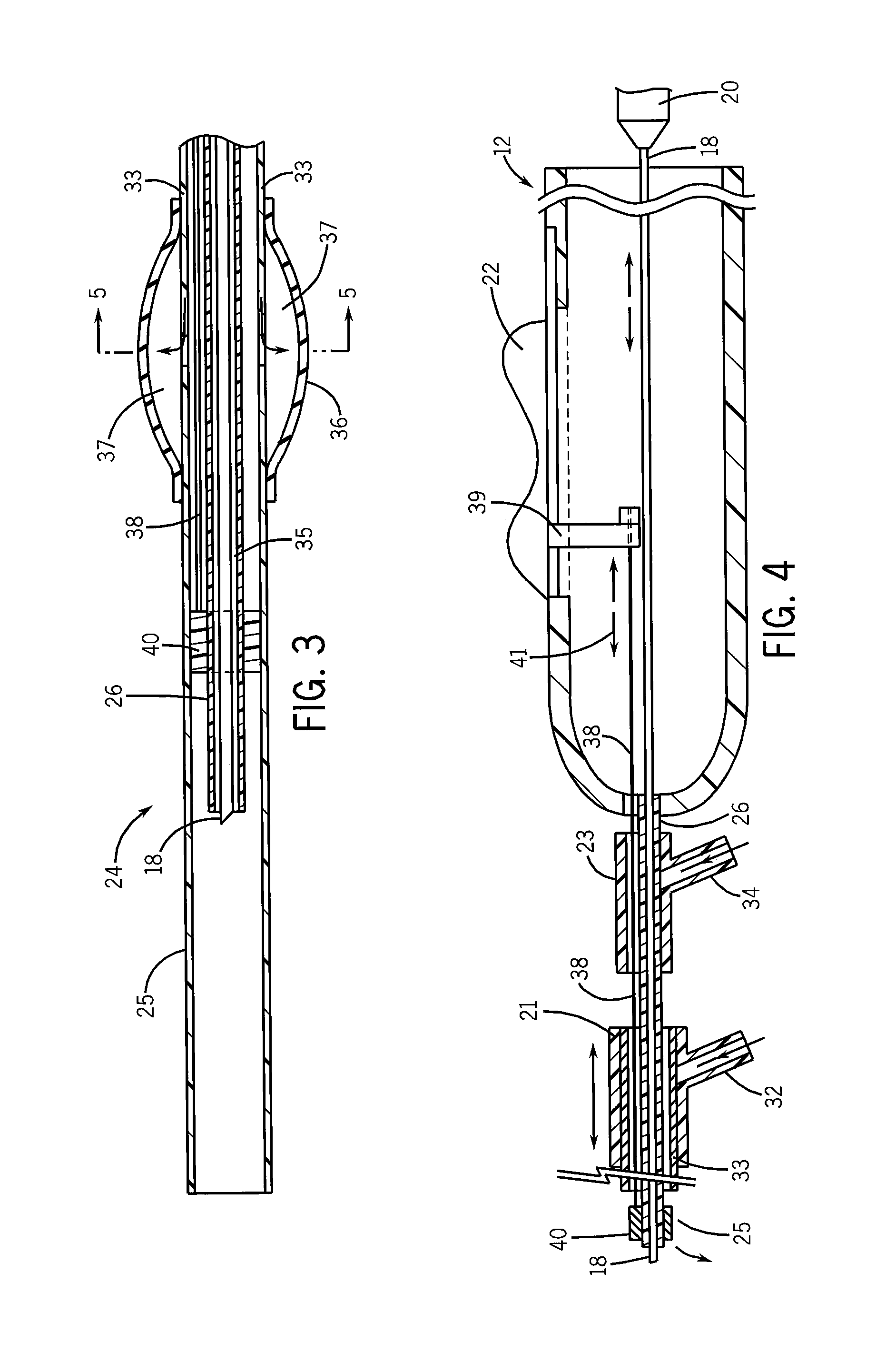
Needle kit and method for microwave ablation, track coagulation, and biopsy
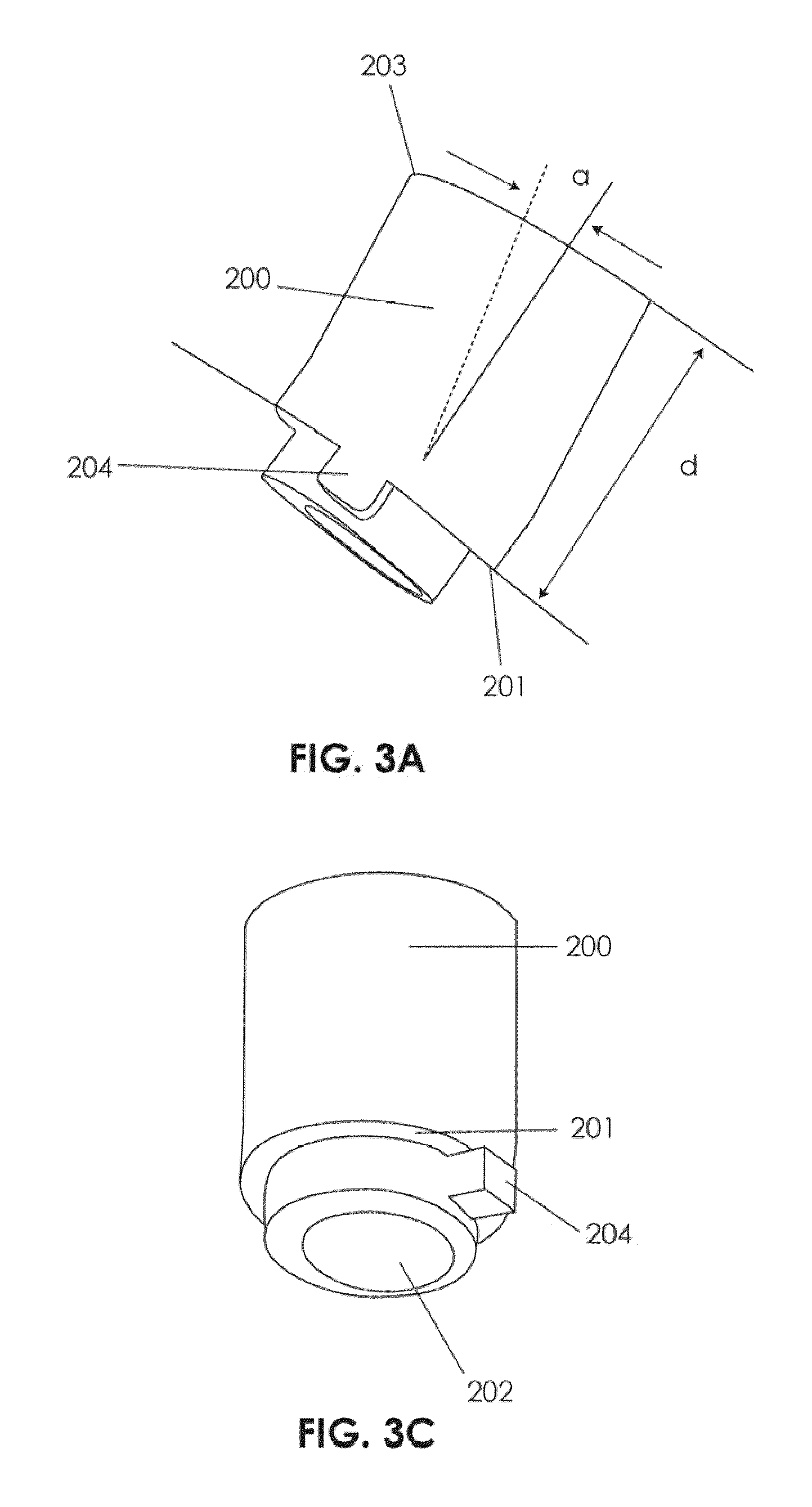
InactiveUS7160292B2Minimize damageMinimize bleedingSurgical needlesVaccination/ovulation diagnosticsFungating tumourMicrowave ablation
A modular biopsy, ablation and track coagulation needle apparatus is disclosed that allows the biopsy needle to be inserted into the delivery needle and removed when not needed, and that allows an inner ablation needle to be introduced and coaxially engaged with the delivery needle to more effectively biopsy a tumor, ablate it and coagulate the track through ablation while reducing blood loss and track seeding. The ablation needle and biopsy needle are adapted to in situ assembly with the delivery needle. In a preferred embodiment, the ablation needle, when engaged with the delivery needle forms a coaxial connector adapted to electrically couple to an ablating source. Methods for biopsying and ablating tumors using the device and coagulating the track upon device removal are also provided.
Owner:TYCO HEALTHCARE GRP LP
Transcorporeal spinal decompression and repair system and related method
A system and method are provided for making an access channel through a vertebral body to access a site of neural compression, decompressing it, and repairing the channel to restore vertebral integrity. System elements include an implantable vertebral plate, a guidance device for orienting bone cutting tools and controlling the path of a cutting tool, a bone cutting tool to make a channel in the vertebral body, a tool for opening or partially-resecting the posterior longitudinal ligament of the spine, a tool for retrieving a herniated disc, an implantable device with osteogenic material to fill the access channel, and a retention device that lockably-engages the bone plate to retain it in position after insertion. System elements may be included in a surgery to decompress an individual nerve root, the spinal cord, or the cauda equina when compressed, for example, by any of a herniated disc, an osteophyte, a thickened ligament arising from degenerative changes within the spine, a hematoma, or a tumor.
Owner:GLOBUS MEDICAL INC
Method for cryospray ablation
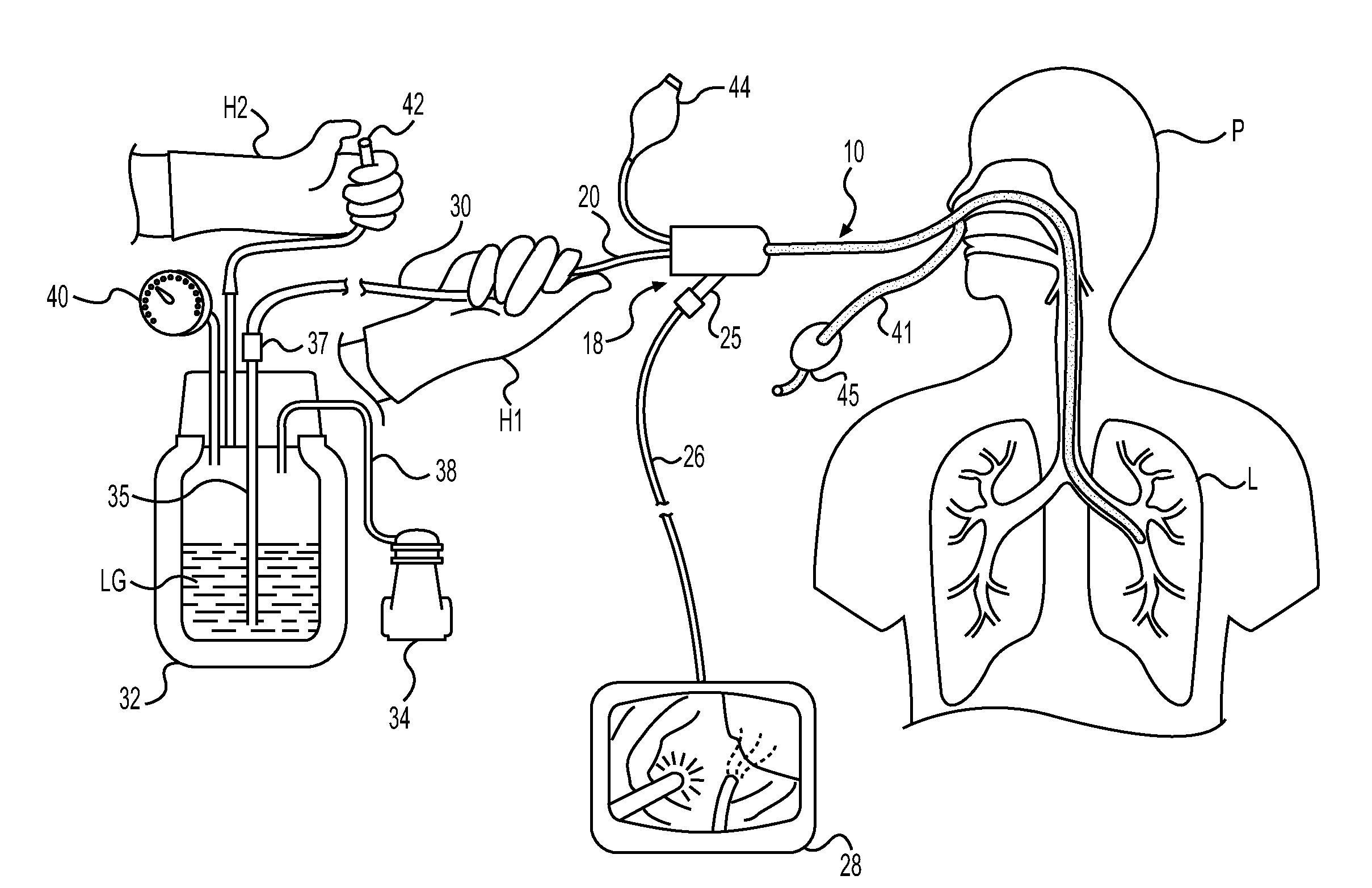
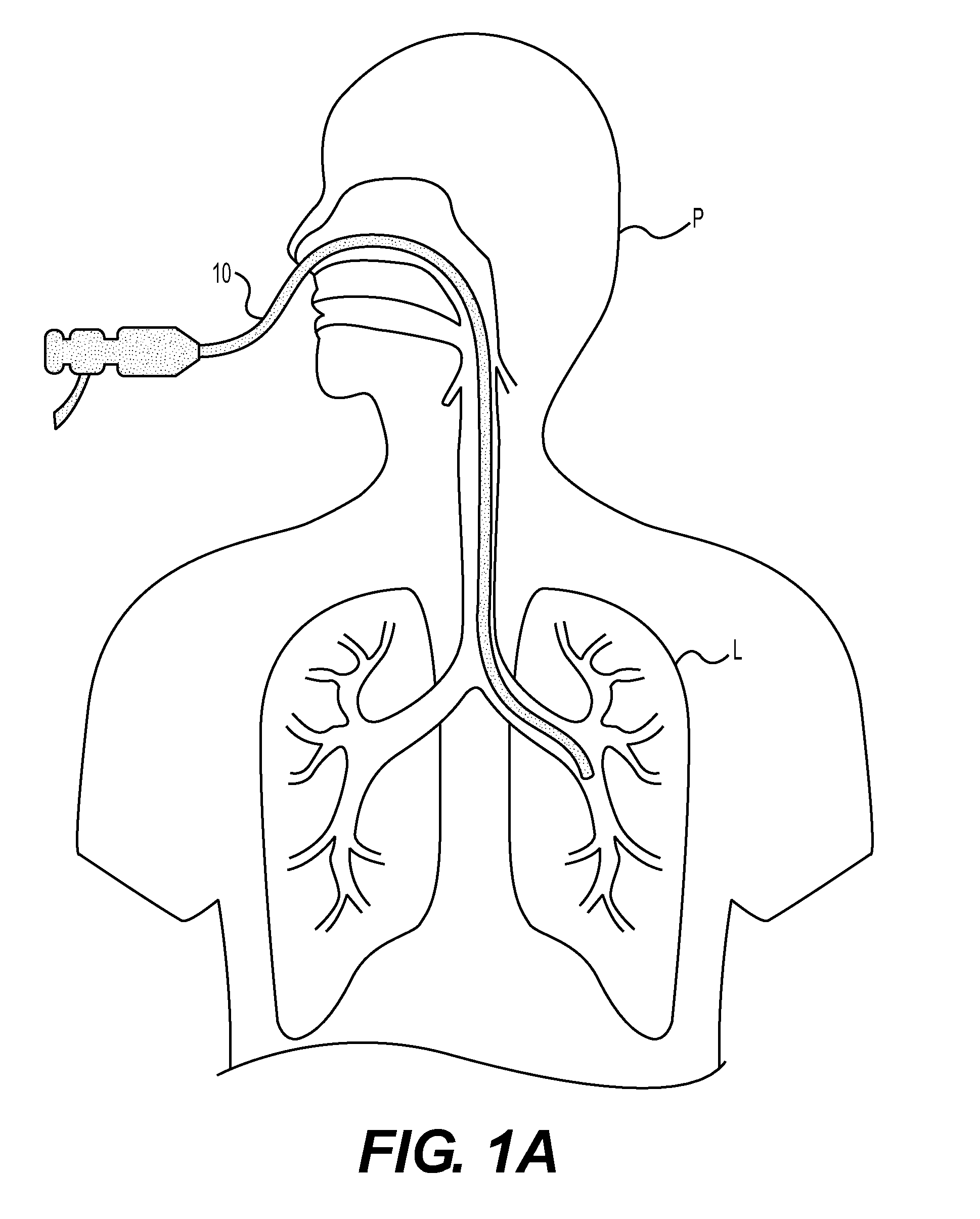
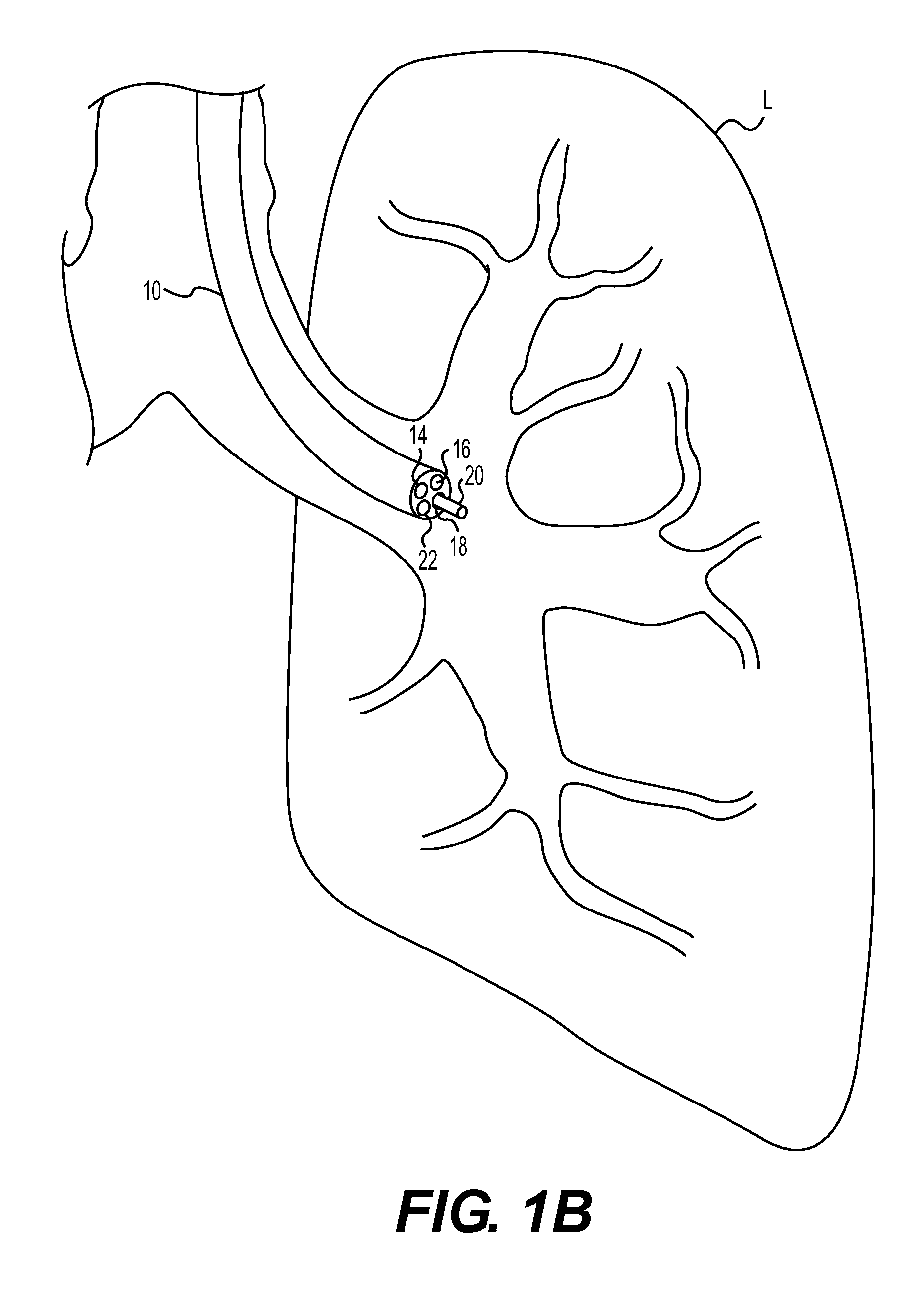
InactiveUS20090192505A1Increase load capacityAdequate doseUltrasonic/sonic/infrasonic diagnosticsEchographic/ultrasound-imaging preparationsThoracic structureDisease
The present invention relates to methods for treating tissue in the thoracic cavity of a subject by the application of a cryogen, or using the cryogen to create an isotherm in proximity to the tissue to be treated. A wide variety of conditions may be treated using the methods of the invention including asthma, neoplastic disease and a variety of conditions characterized by inflammation in lung and chest tissue.
Owner:RESET MEDICAL
Device for biopsy of tumors
InactiveUS20030195436A1Surgical needlesVaccination/ovulation diagnosticsAbnormal tissue growthCombined use
A device and method of use for securing and coring of tumors within the body during a biopsy of the tumor, specifically breast tumors. An adhesion probe for securing the tumor is described. The probe secures the tumor by piercing the tumor and providing a coolant to the distal tip to cool the tip. The cooled tip adheres to the tumor. A coring instrument adapted for cutting a core sample of the tumor is described. The instrument is provided with a cannula that can cut a core sample of the tumor. The instrument is adapted for use with the probe with the probe fitting within the cannula. The instrument can be used in conjunction with the probe to secure and core a sample of the tumor for biopsy.
Owner:SCION MEDICAL
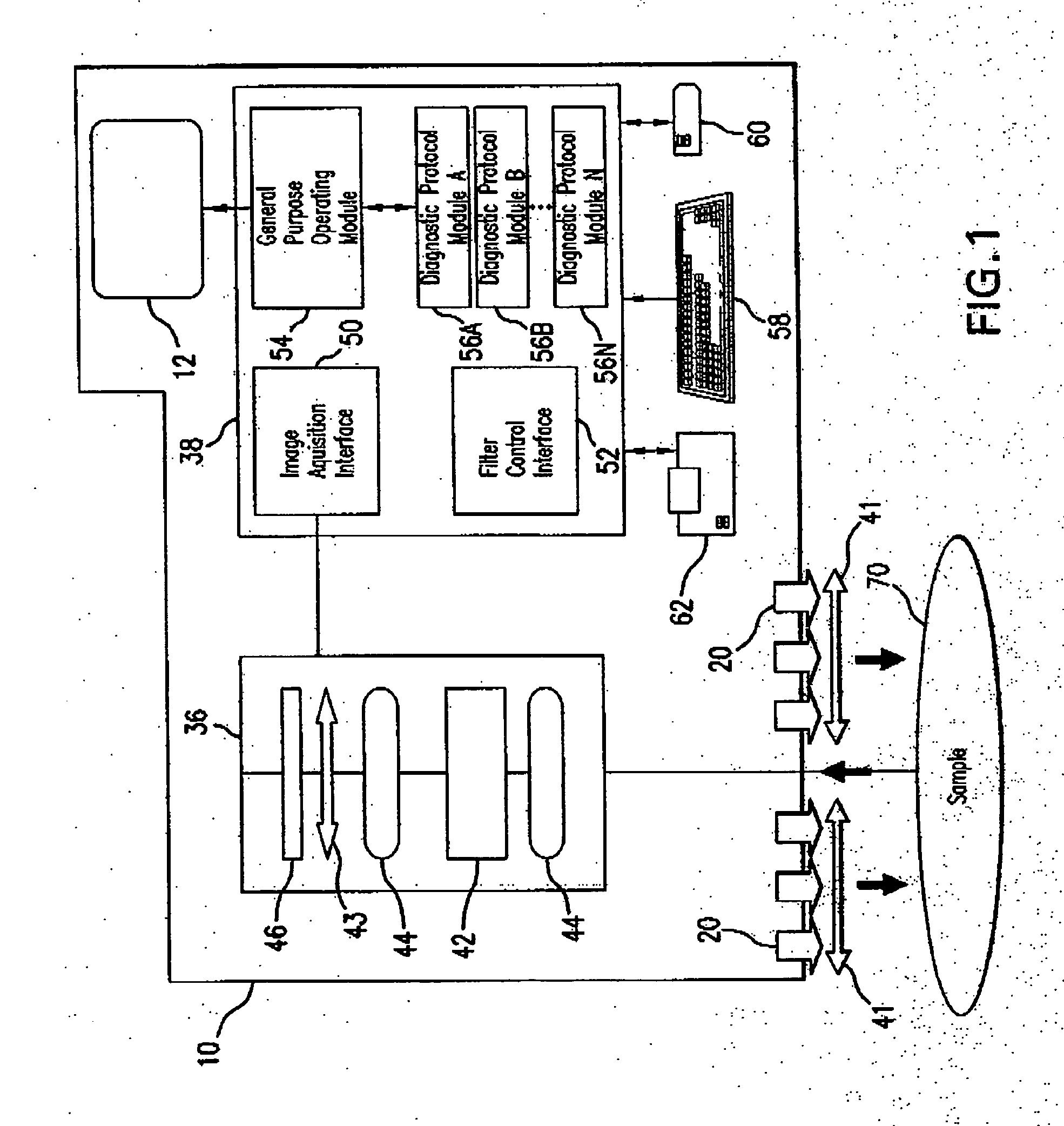
Medical hyperspectral imaging for evaluation of tissue and tumor
ActiveUS20060247514A1Diagnostics using lightPolarisation-affecting propertiesRadiologyFungating tumour
Apparatus and methods for hyperspectral imaging analysis that assists in real and near-real time assessment of biological tissue condition, viability, and type, and monitoring the above over time. Embodiments of the invention are particularly useful in surgery, clinical procedures, tissue assessment, diagnostic procedures, health monitoring, and medical evaluations, especially in the detection and treatment of cancer.
Owner:HYPERMED IMAGING
Device for biopsy of tumors
InactiveUS20020045842A1Surgical needlesVaccination/ovulation diagnosticsFungating tumourBreast tumor
A device and method of use for securing and coring of tumors within the body during a biopsy of the tumor, specifically breast tumors. An adhesion probe for securing the tumor is described. The probe secures the tumor by piercing the tumor and providing a coolant to the distal tip to cool the tip. The cooled tip adheres to the tumor. A coring instrument adapted for cutting a core sample of the tumor is described. The instrument is provided with a cannula that can cut a core sample of the tumor. The instrument is adapted for use with the probe with the probe fitting within the cannula. The instrument can be used in conjunction with the probe to secure and core a sample of the tumor for biopsy.
Owner:SCION MEDICAL
Isolation And Use Of Solid Tumor Stem Cells
InactiveUS20080178305A1Reduce spreadIncreased proliferationMaterial nanotechnologyMicrobiological testing/measurementAbnormal tissue growthMammary gland structure
A small percentage of cells within an established solid tumor have the properties of stem cells. These solid tumor stem cells give rise both to more tumor stem cells and to the majority of cells in the tumor that have lost the capacity for extensive proliferation and the ability to give rise to new tumors. Thus, solid tumor heterogeneity reflects the presence of tumor cell progeny arising from a solid tumor stem cell. We have developed a xenograft model in which we have been able to establish tumors from primary tumors via injection of tumor cells in the mammary gland of severely immunodeficient mice. These xenograft assay have allowed us to do biological and molecular assays to characterize clonogenic solid tumor stem cells. We have also developed evidence that strongly implicates the Notch pathway, especially Notch 4, as playing a central pathway in carcinogenesis.
Owner:ONCOMED PHARMA +1
Non-invasive location and tracking of tumors and other tissues for radiation therapy
InactiveUS20100198101A1Diagnostic recording/measuringSensorsForced expiratory vital capacityAbnormal tissue growth
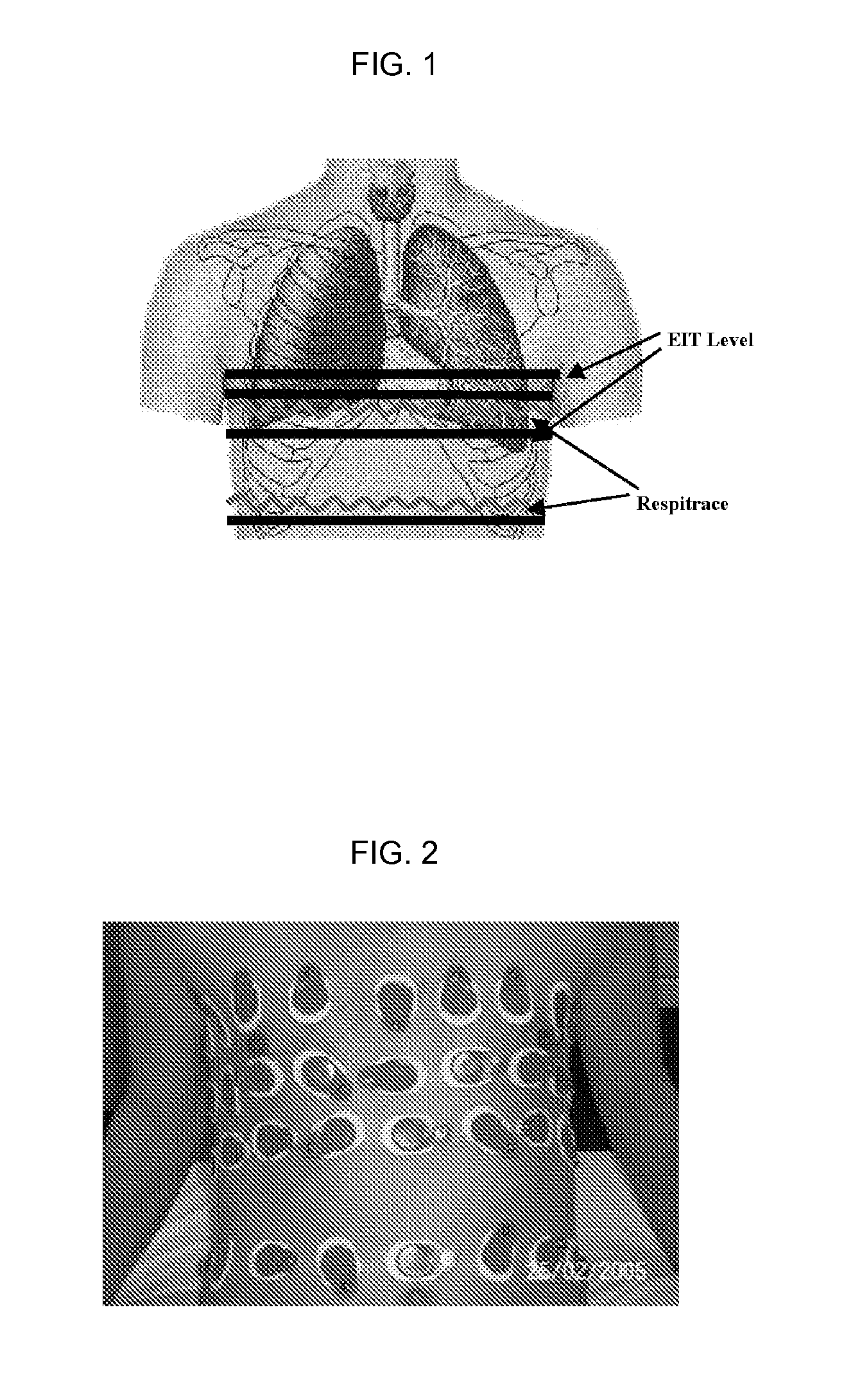
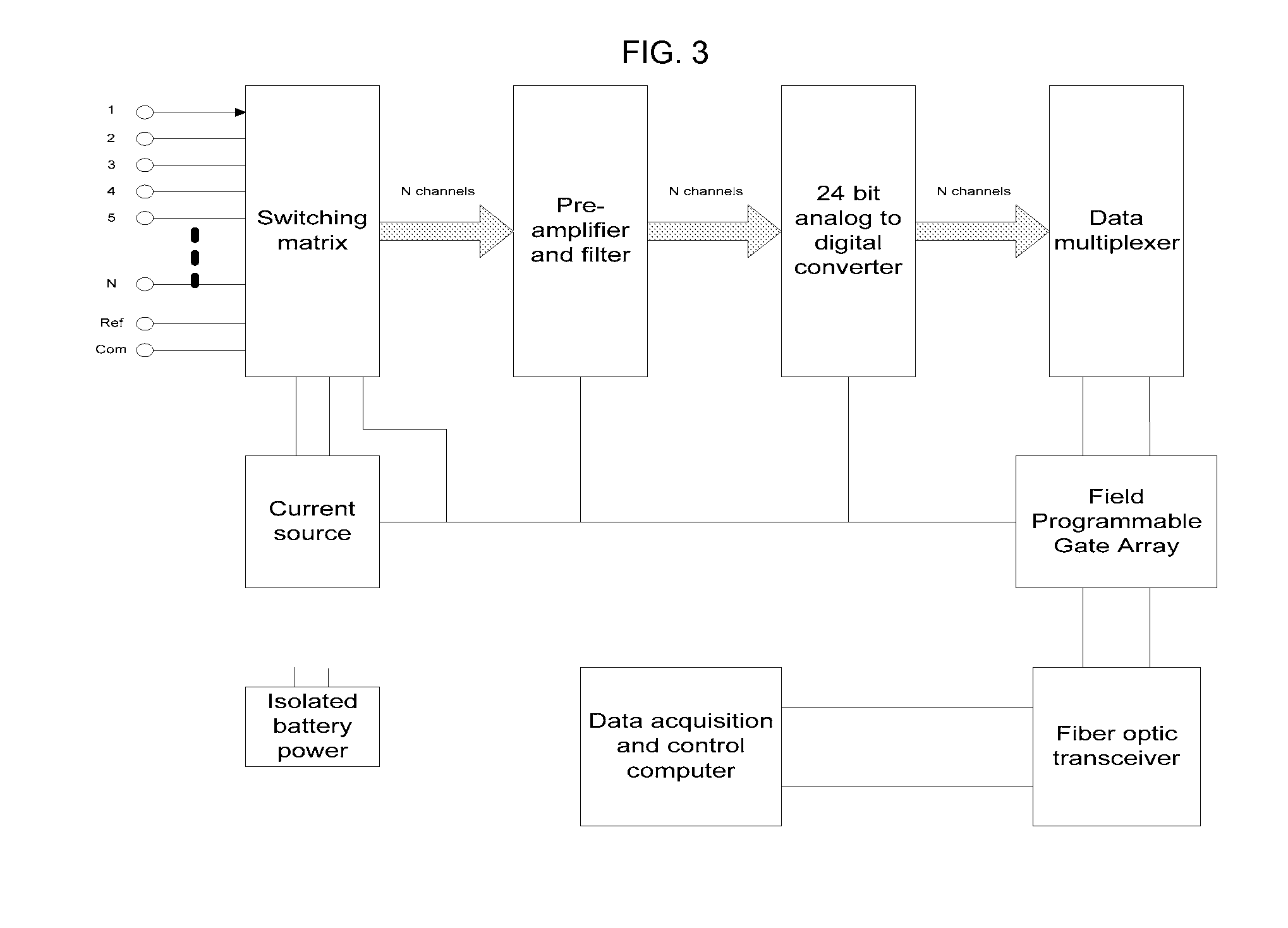
Embodiments herein provide a non-invasive tracking system that accurately predicts the location of tumors, such as lung tumors, in real time, while allowing patients to breathe naturally. This is accomplished by using Electrical Impedance Tomography (EIT), in conjunction with spirometry, strain gauge and infrared sensors, and by using sophisticated patient-specific mathematical models that incorporate the dynamics of tumor motion. With the direction and speed of lung tumor movement successfully tracked, radiation may be effectively delivered to the lung tumor and not to the surrounding healthy tissue, thus increased radiation dosage may be directed to improving local tumor control without compromising functional parenchyma.
Owner:OREGON HEALTH & SCI UNIV
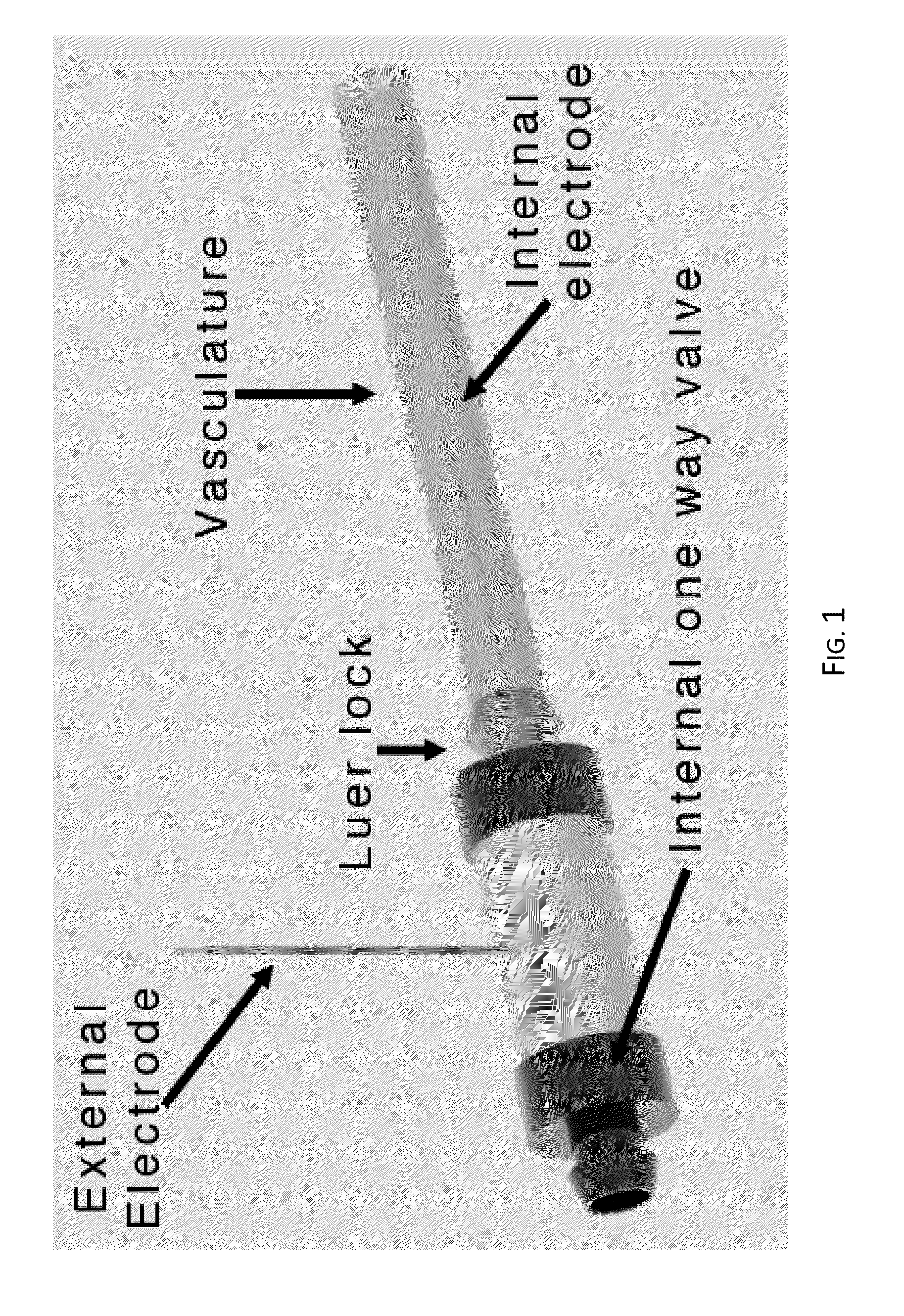
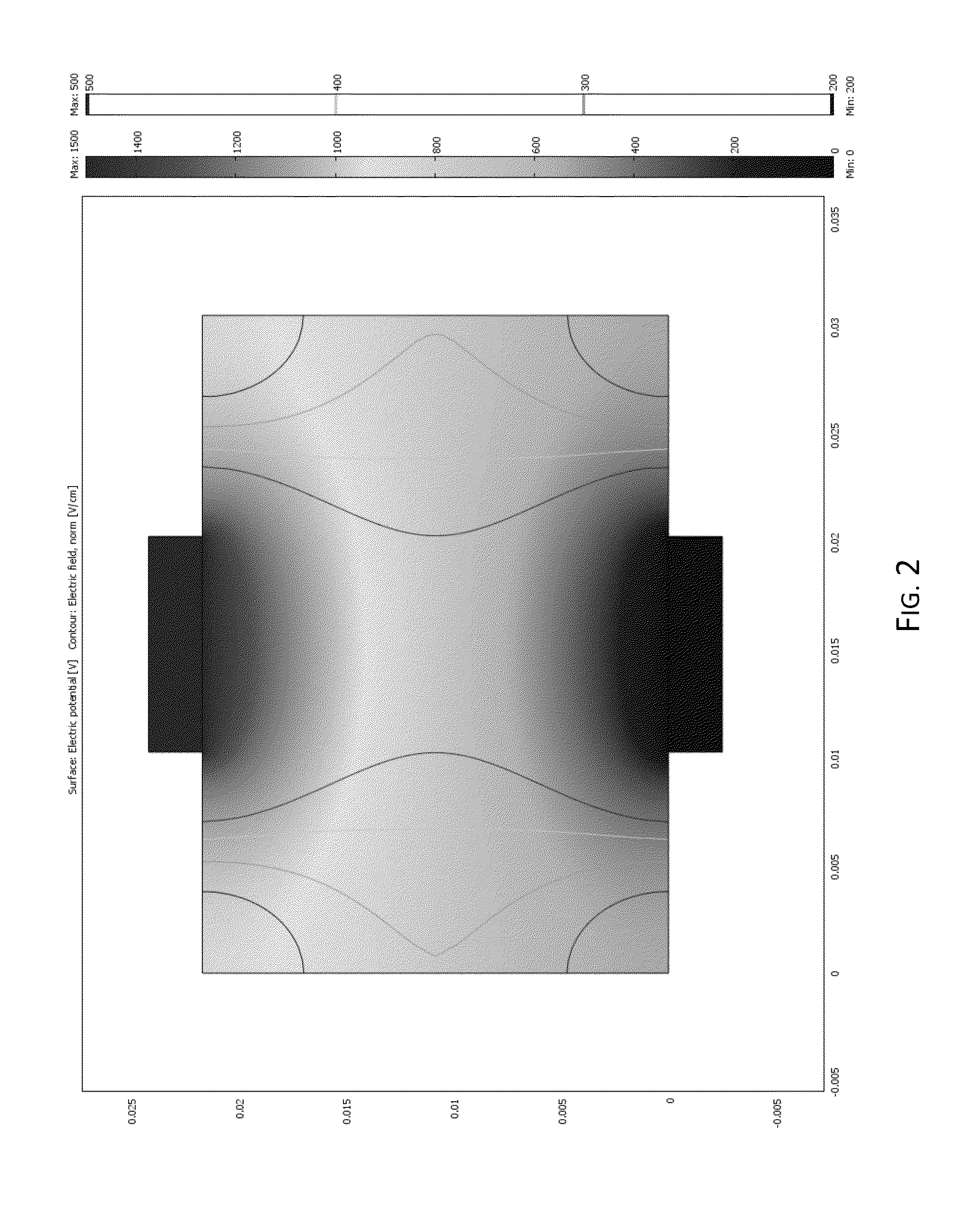
Irreversible electroporation using tissue vasculature to treat aberrant cell masses or create tissue scaffolds
ActiveUS20130253415A1Easy to storeImprove breathabilityElectrotherapyIntravenous devicesDiseaseNatural source
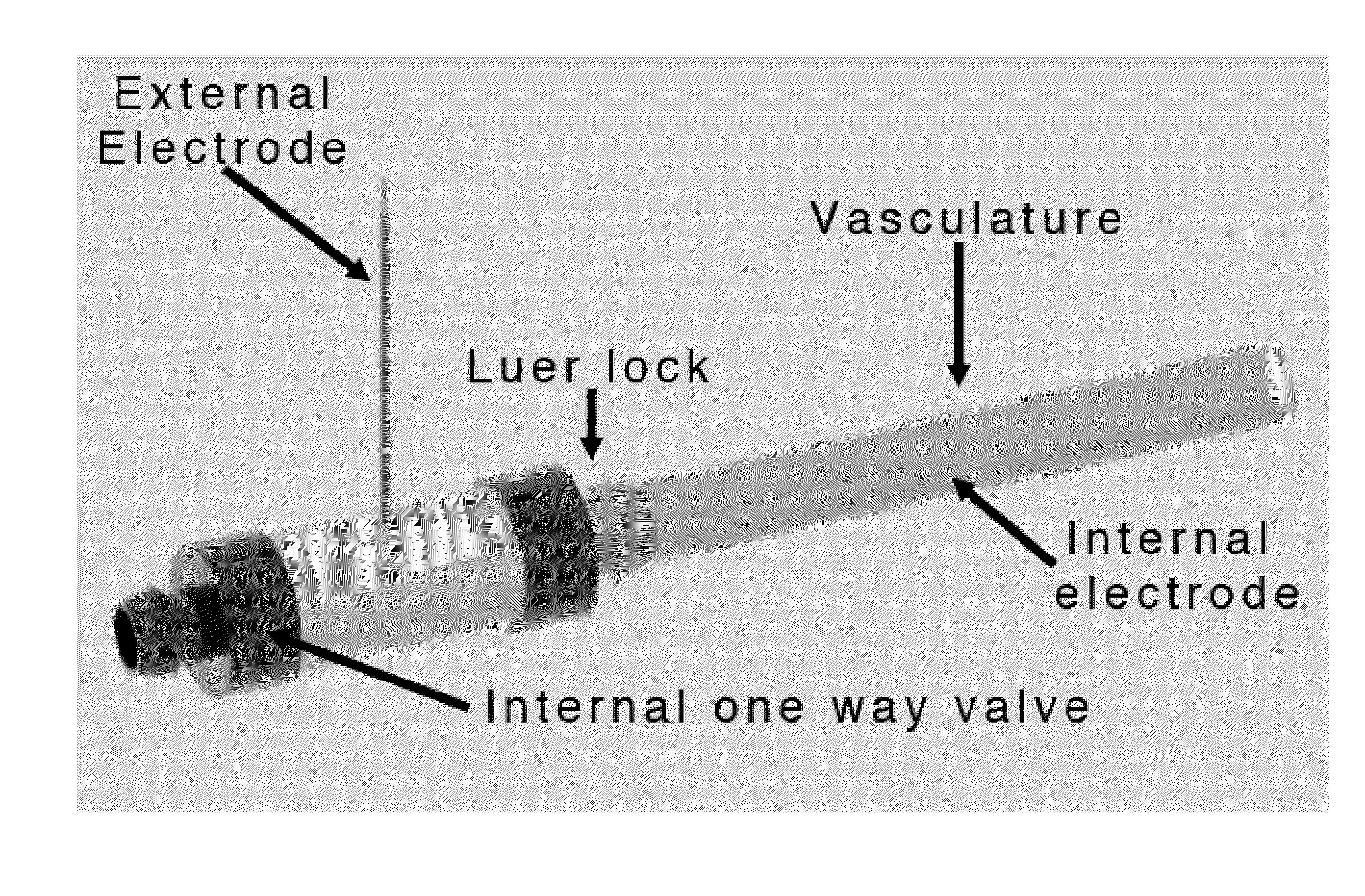
The present invention relates to the field of medical treatment of diseases and disorders, as well as the field of biomedical engineering. Embodiments of the invention relate to the delivery of Irreversible Electroporation (IRE) through the vasculature of organs to treat tumors embedded deep within the tissue or organ, or to decellularize organs to produce a scaffold from existing animal tissue with the existing vasculature intact. In particular, methods of administering non-thermal irreversible electroporation (IRE) in vivo are provided for the treatment of tumors located in vascularized tissues and organs. Embodiments of the invention further provide scaffolds and tissues from natural sources created using IRE ex vivo to remove cellular debris, maximize recellularization potential, and minimize foreign body immune response. The engineered tissues can be used in methods of treating subjects, such as those in need of tissue replacement or augmentation.
Owner:VIRGINIA TECH INTPROP INC
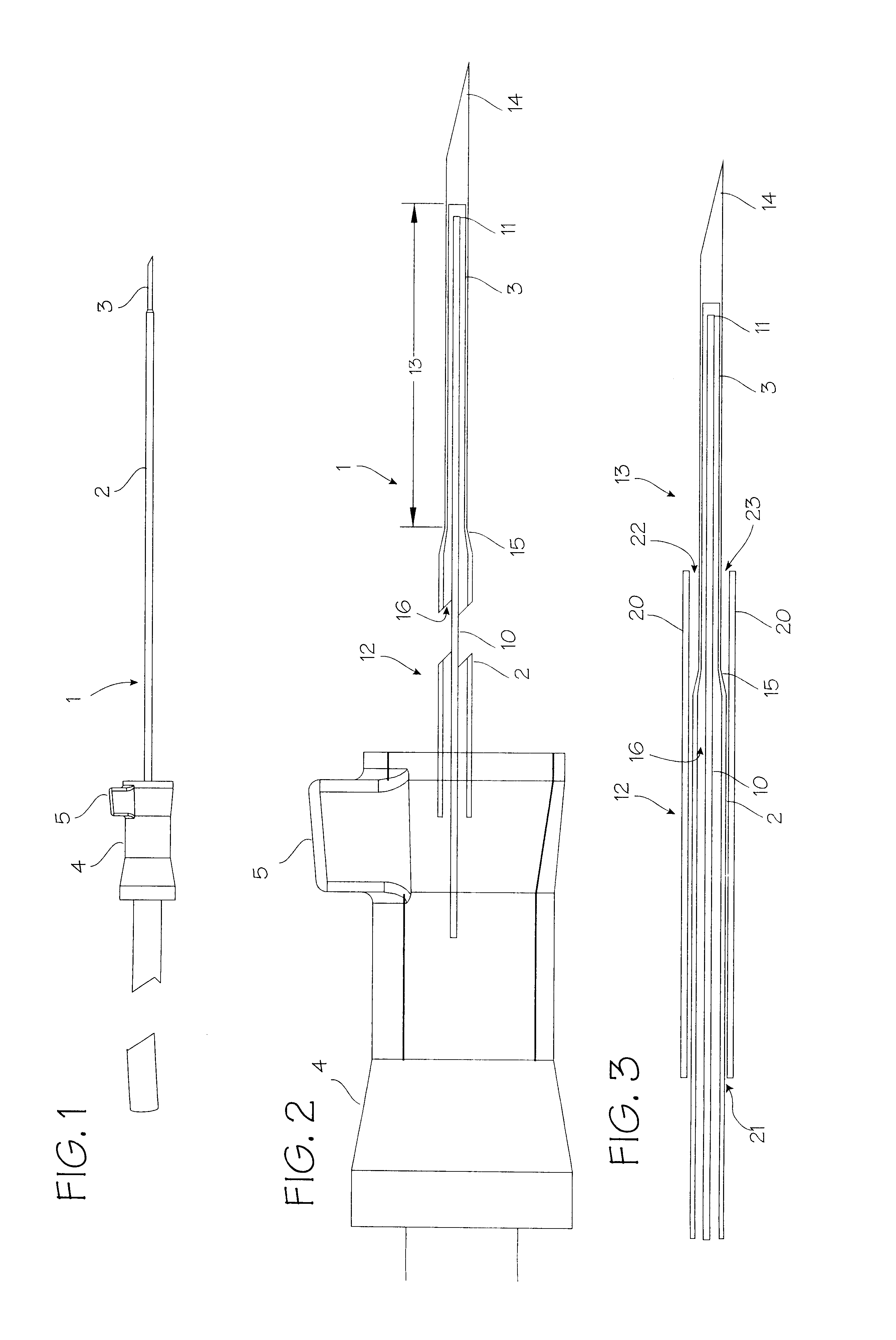
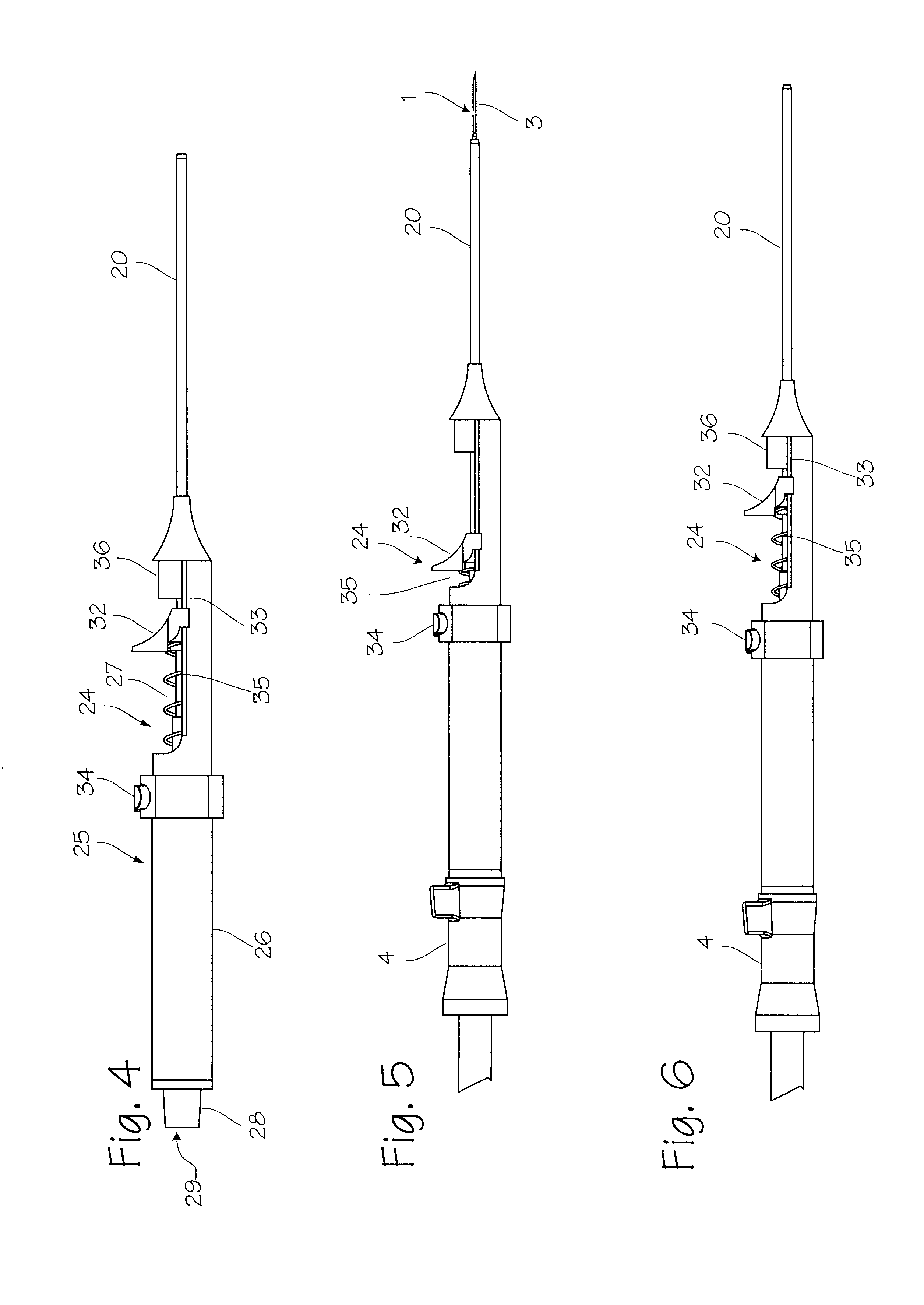
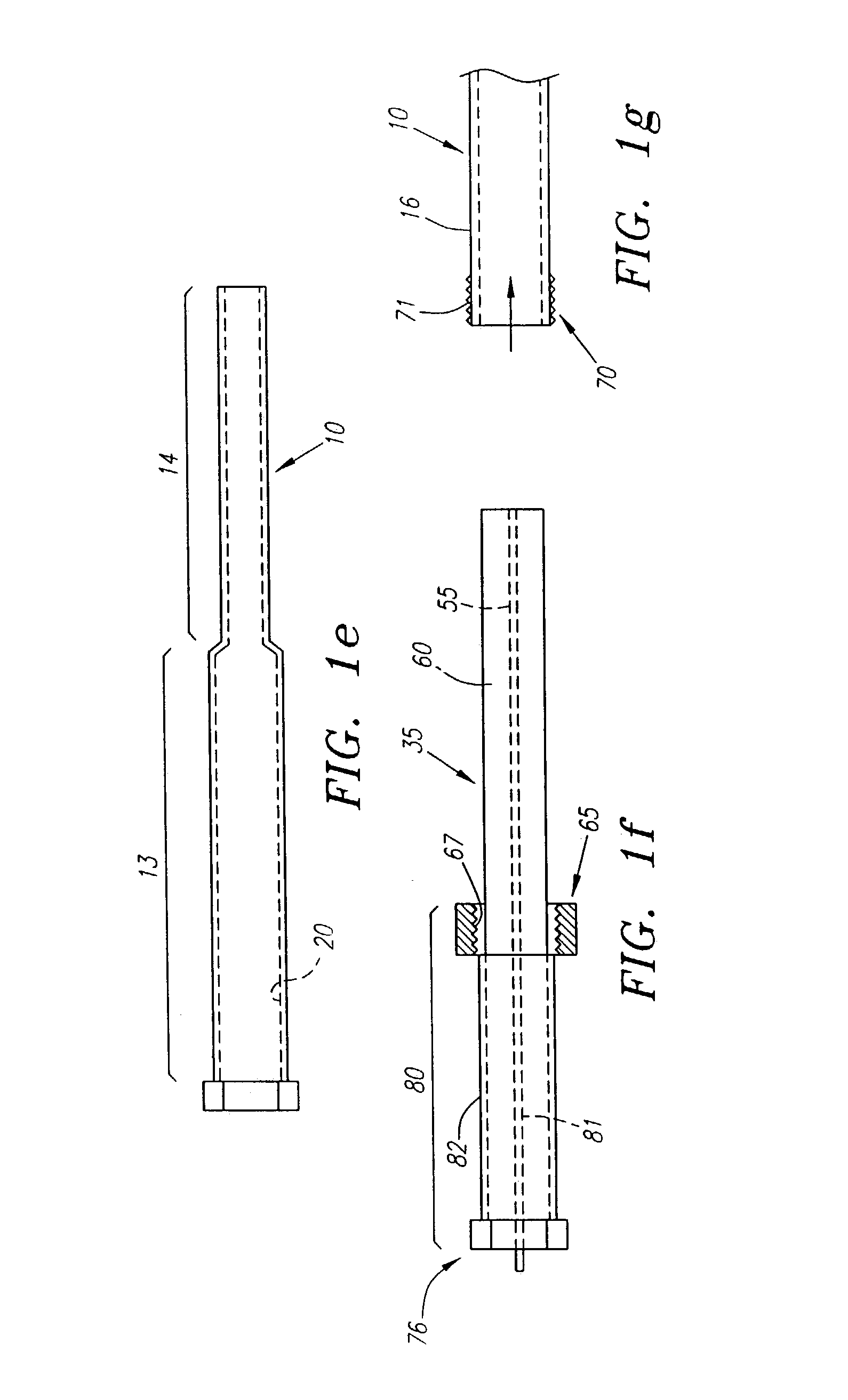
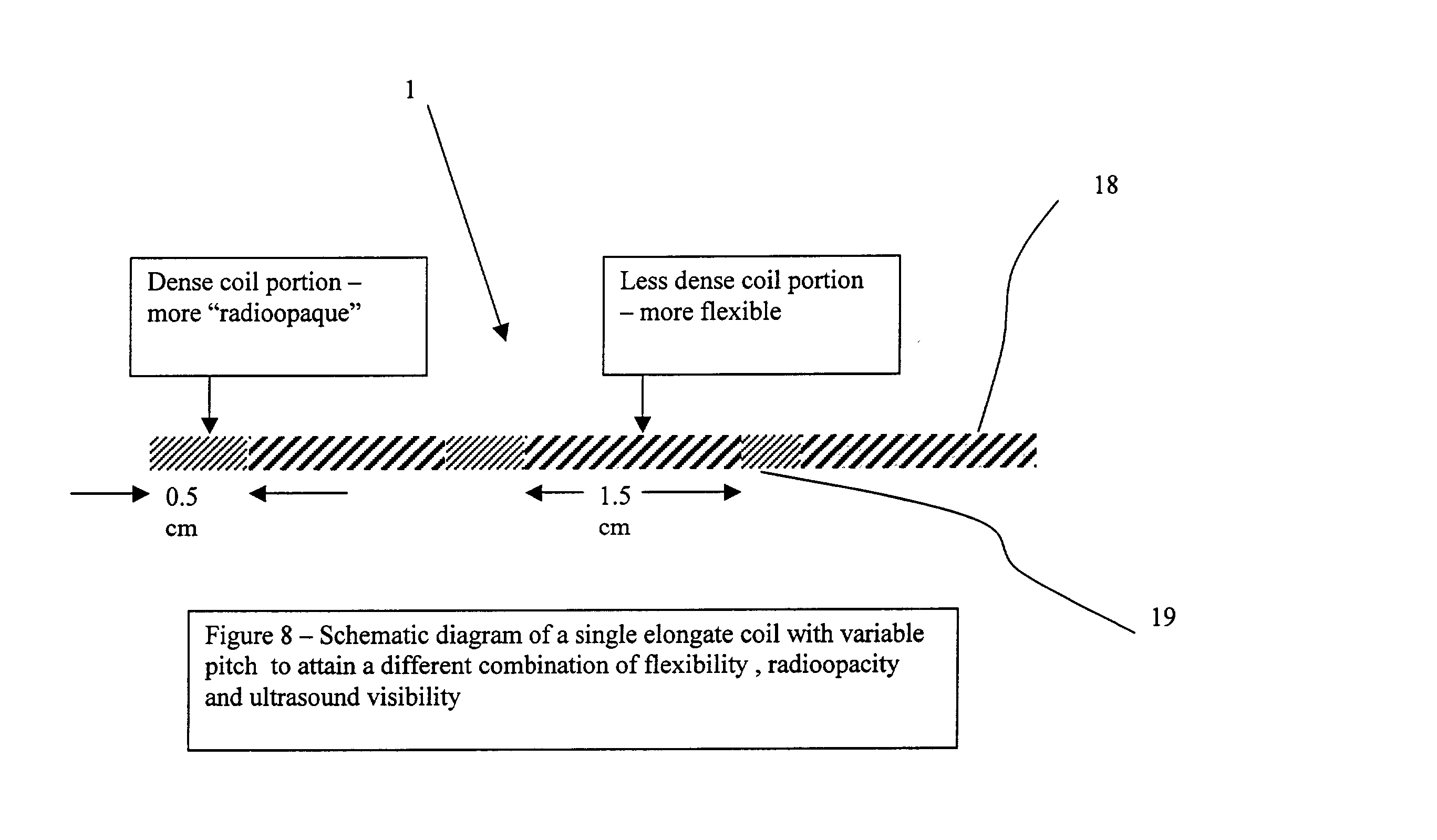
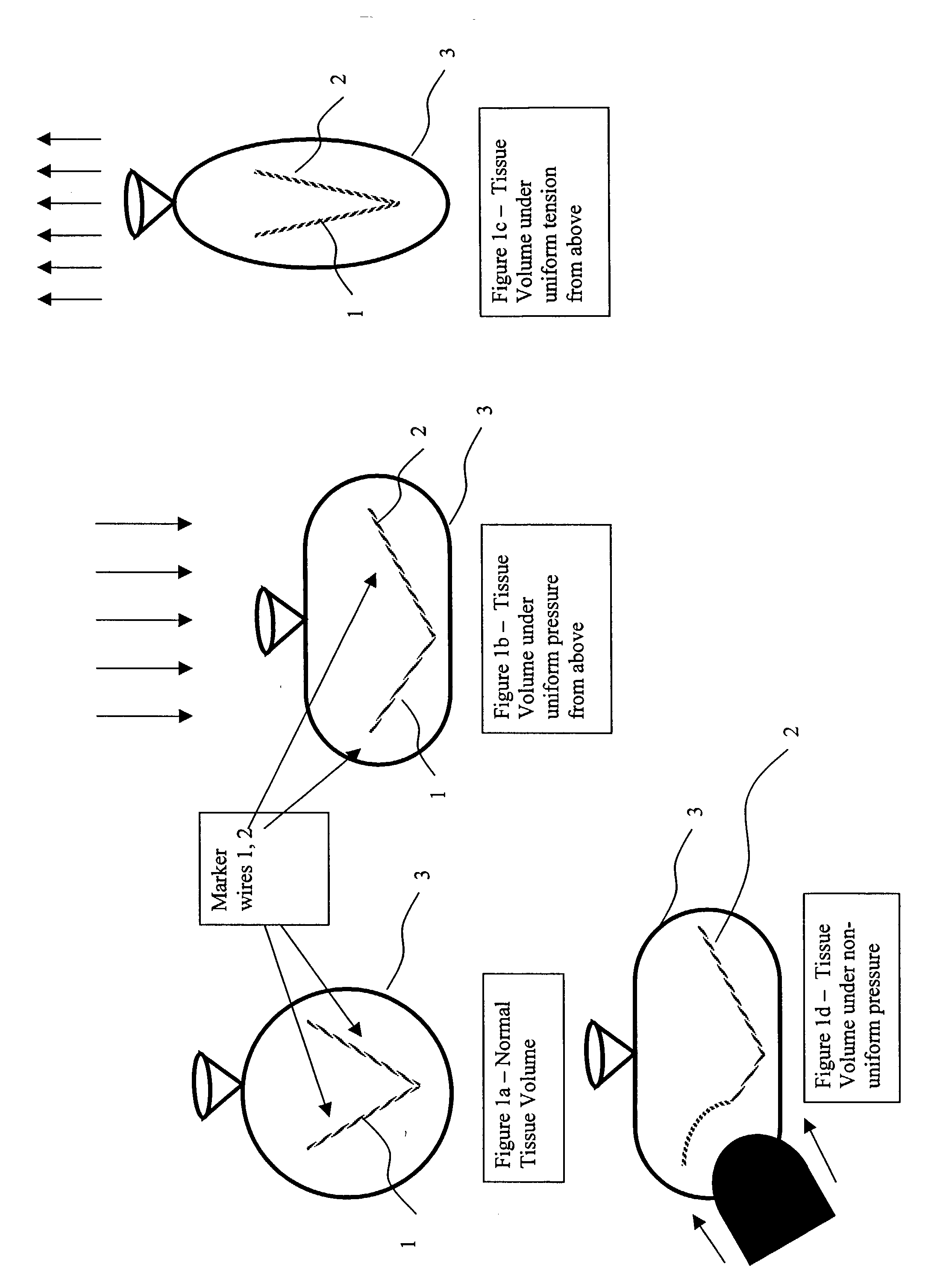
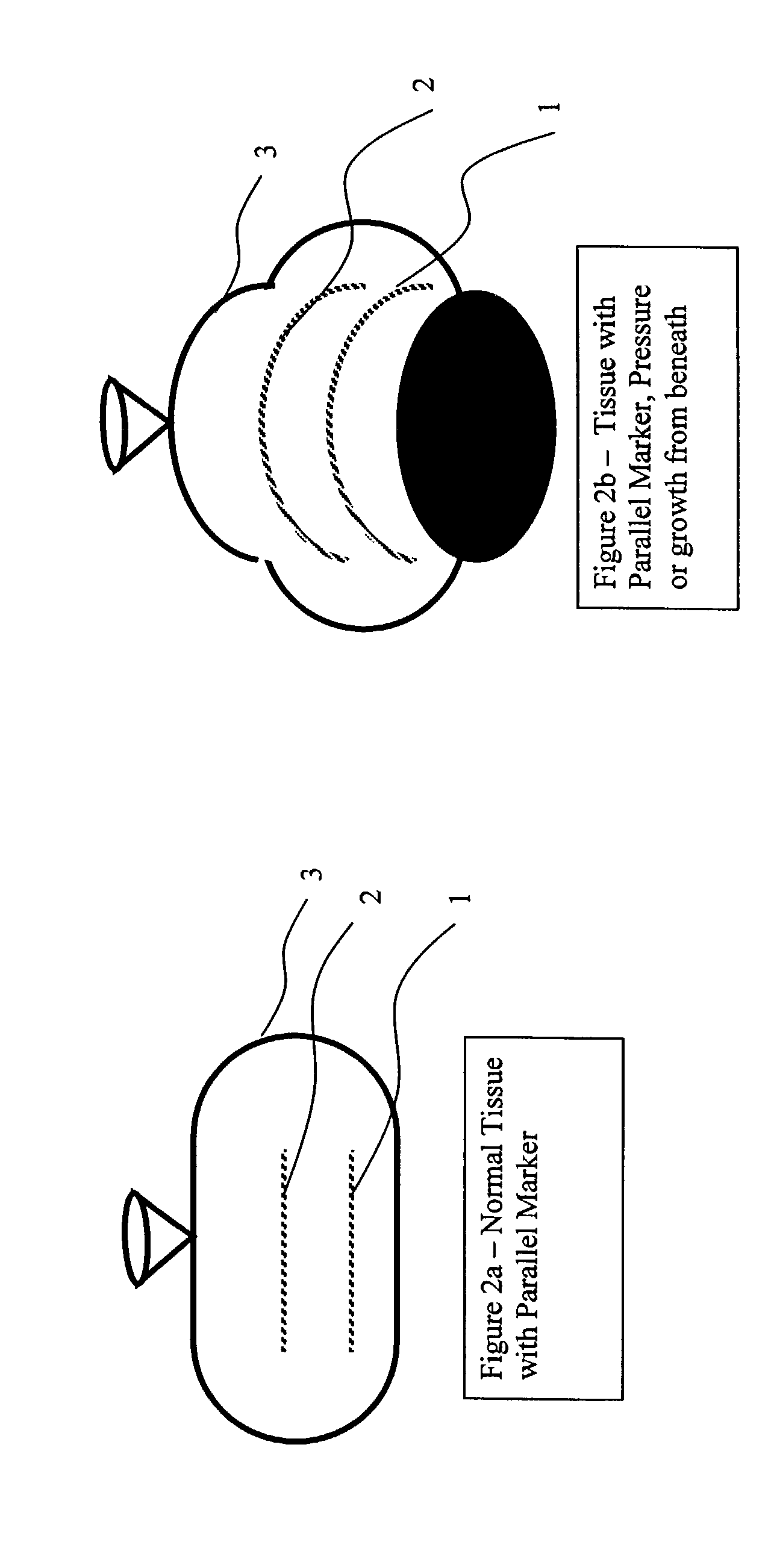
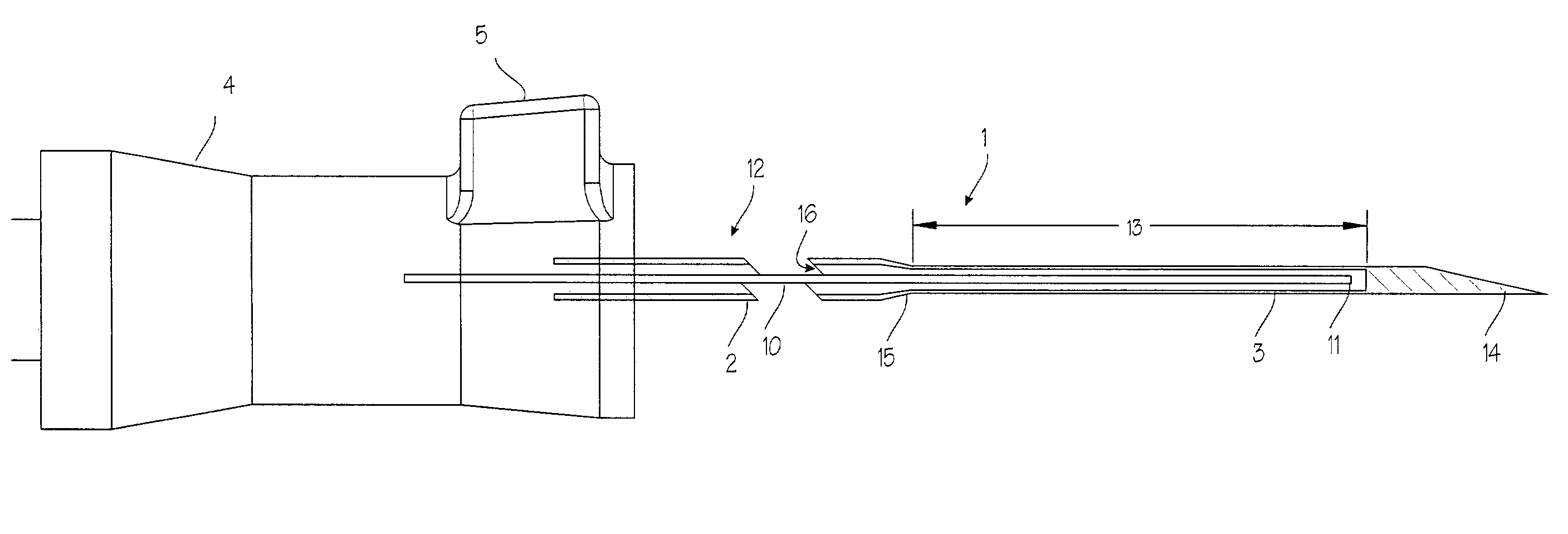
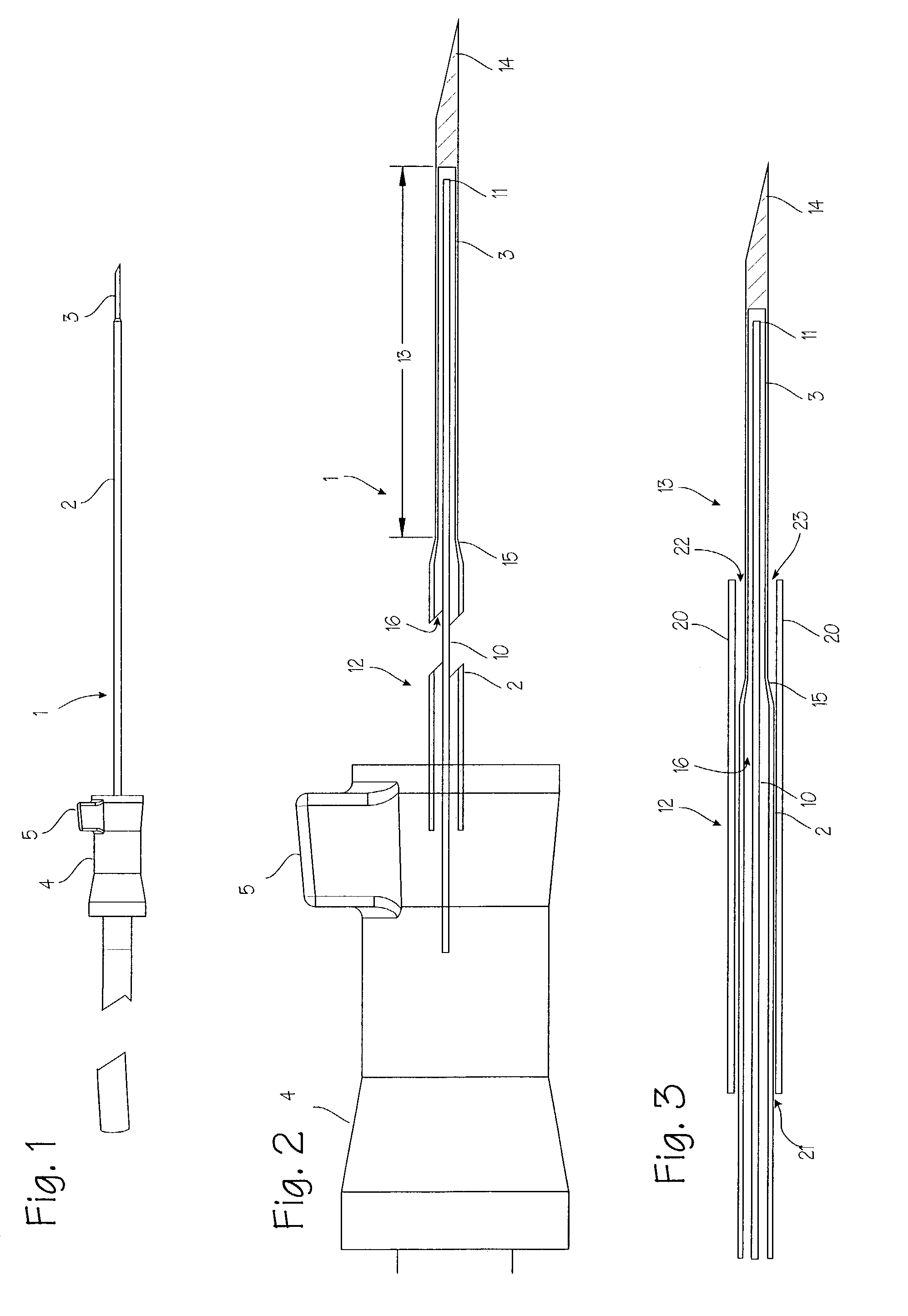
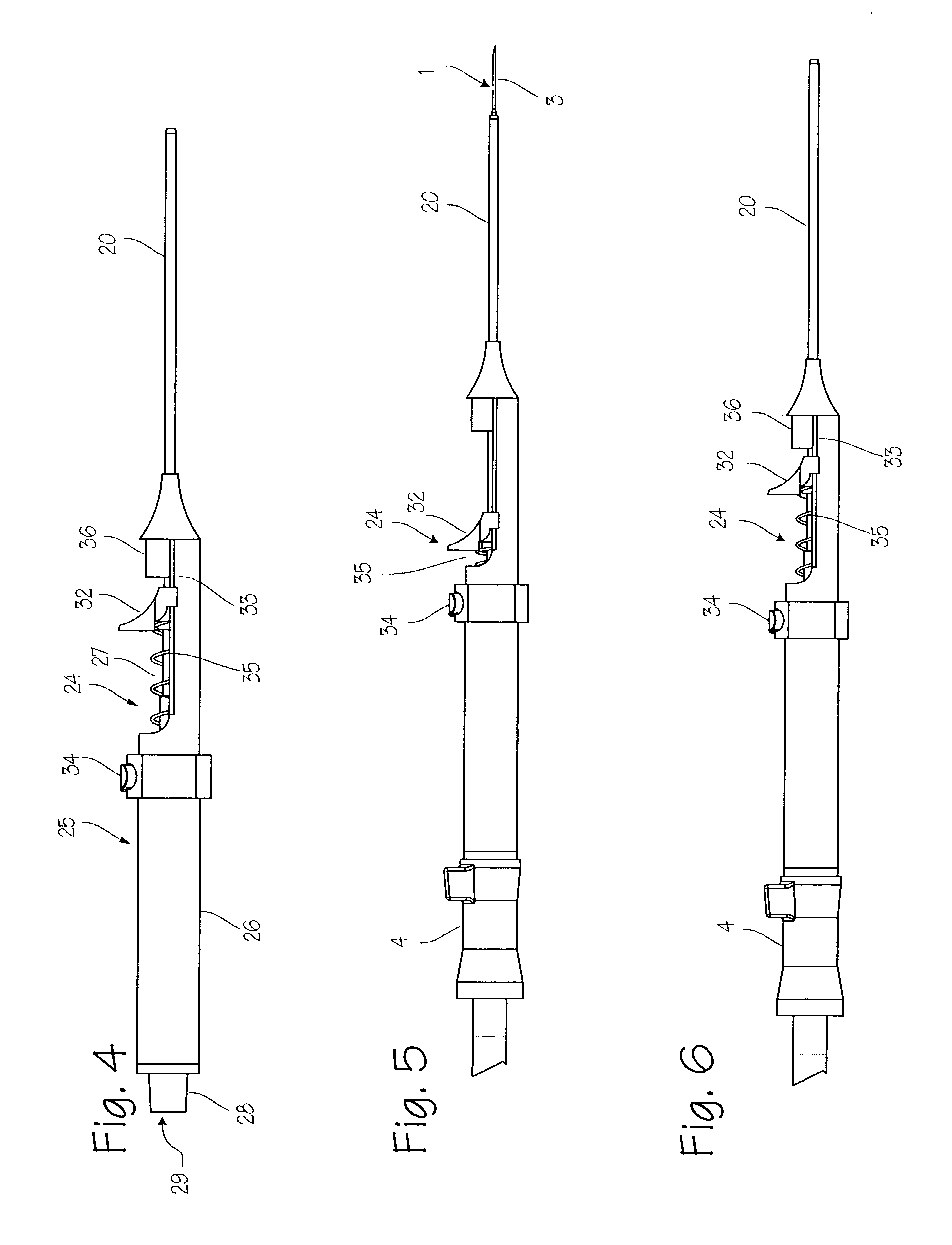
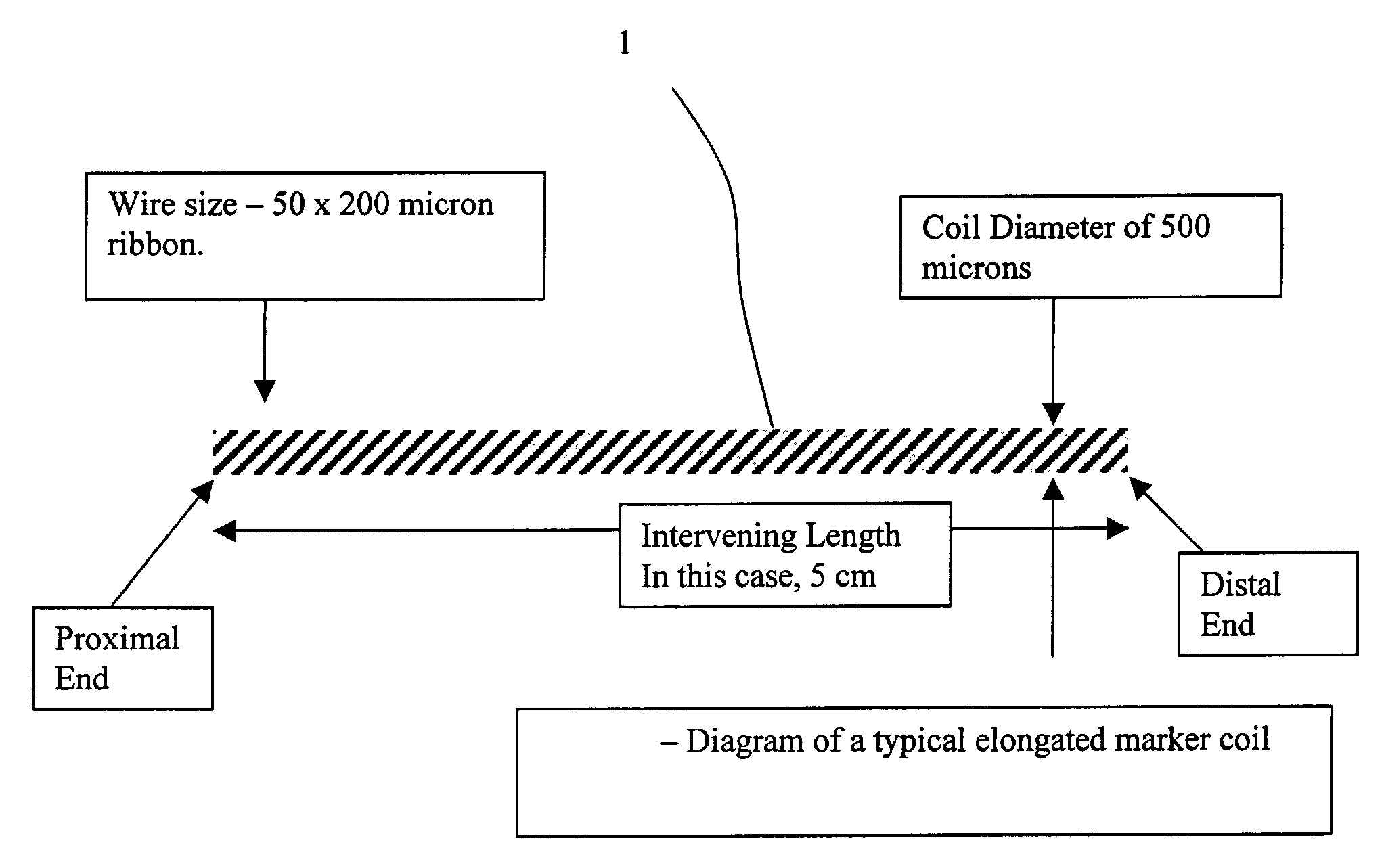
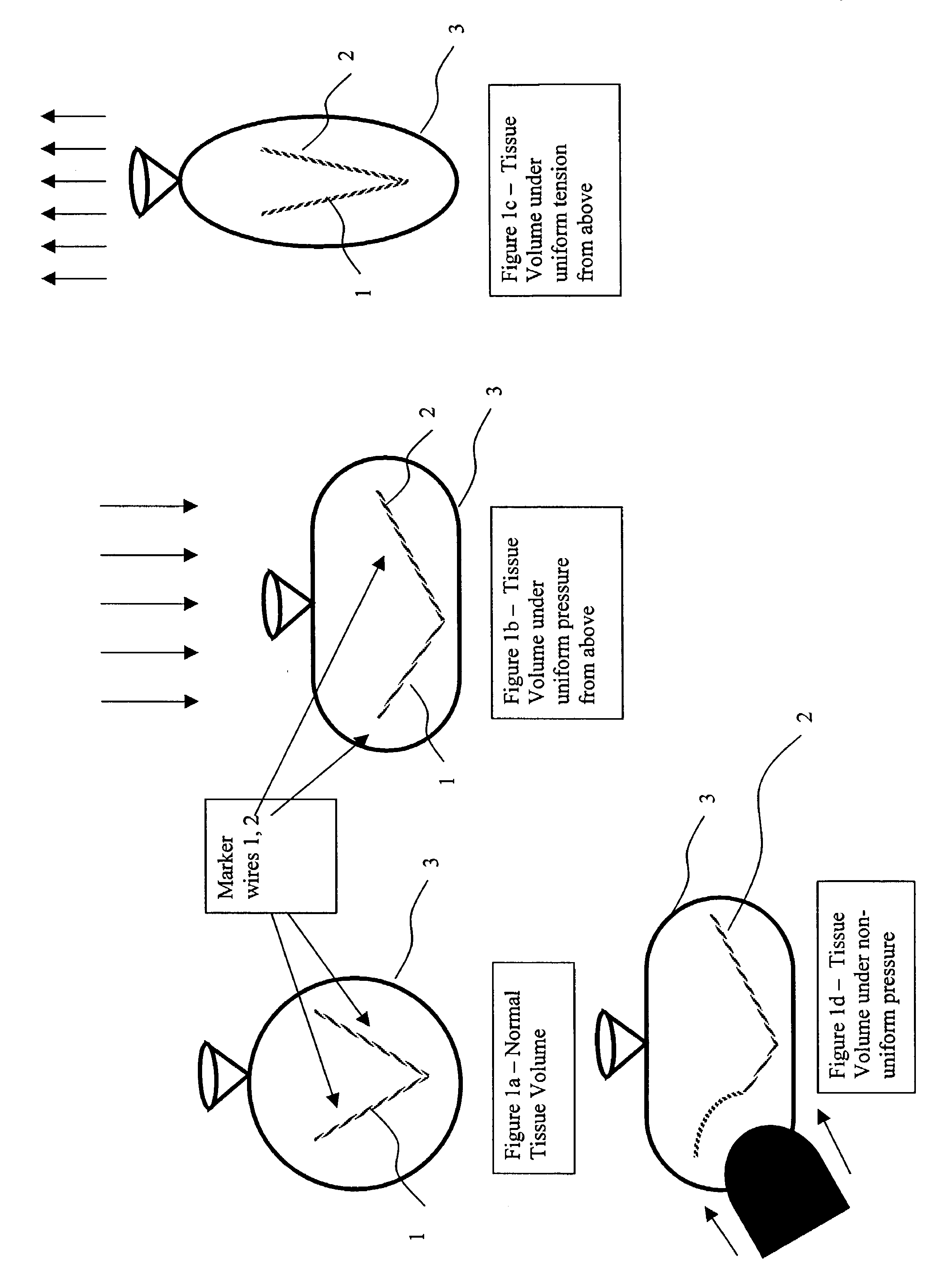
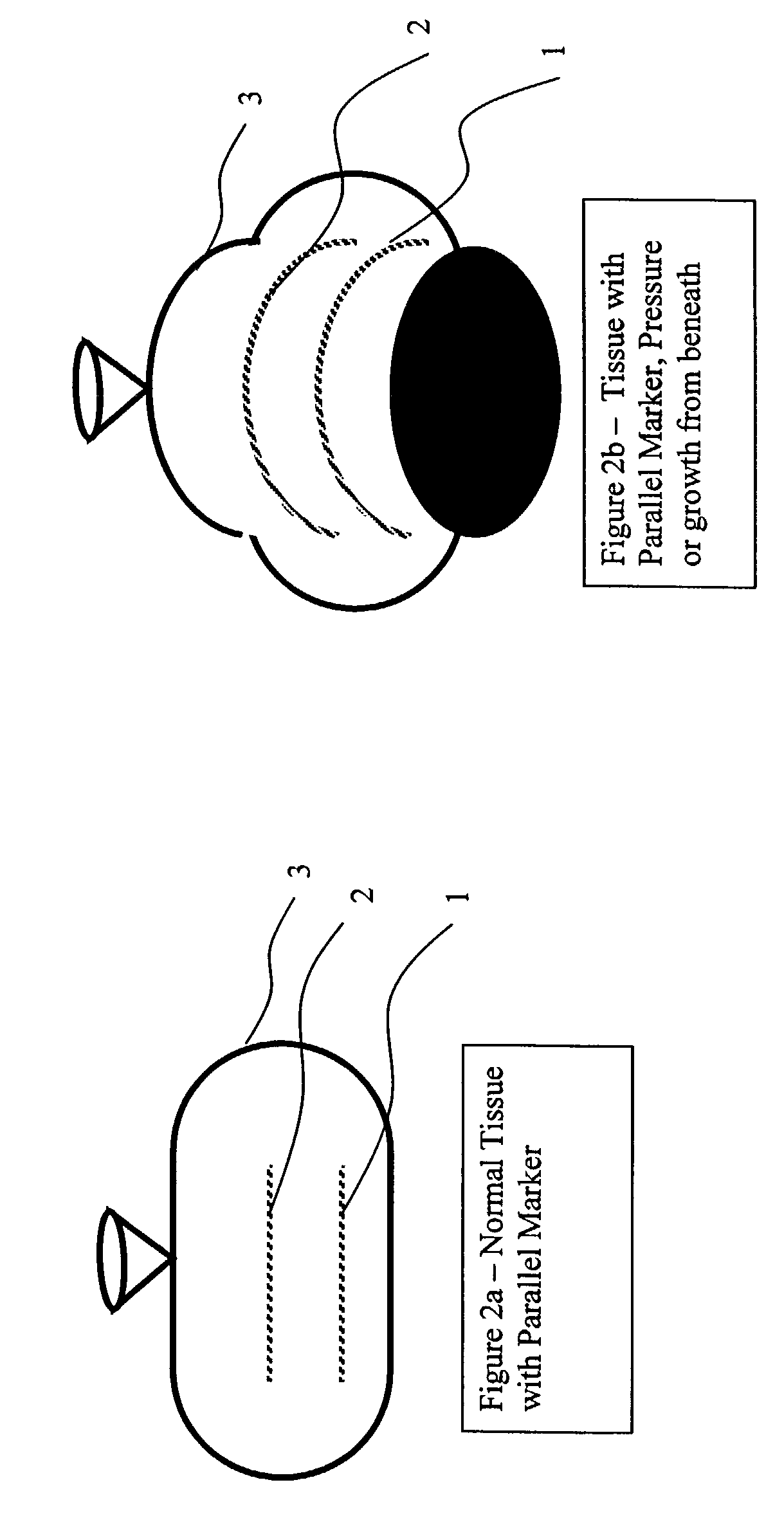
Elongated markers for soft tissue volume identification
ActiveUS20040073107A1Improve gripGood flexibilitySurgeryDiagnostic markersDiagnostic Radiology ModalityInterstitial marker
The present invention is related to an interstitial marker for localizing an organ, tumor or tumor bed within a mammalian body wherein said marker has a proximal end, a distal end, and a continuous intervening length, at least a portion of the intervening length of said marker being visible under at least one imaging modality and having a flexibility such that said marker follows movements and changes of shape of said organ, tumor or tumor bed.
Owner:RADIOMED
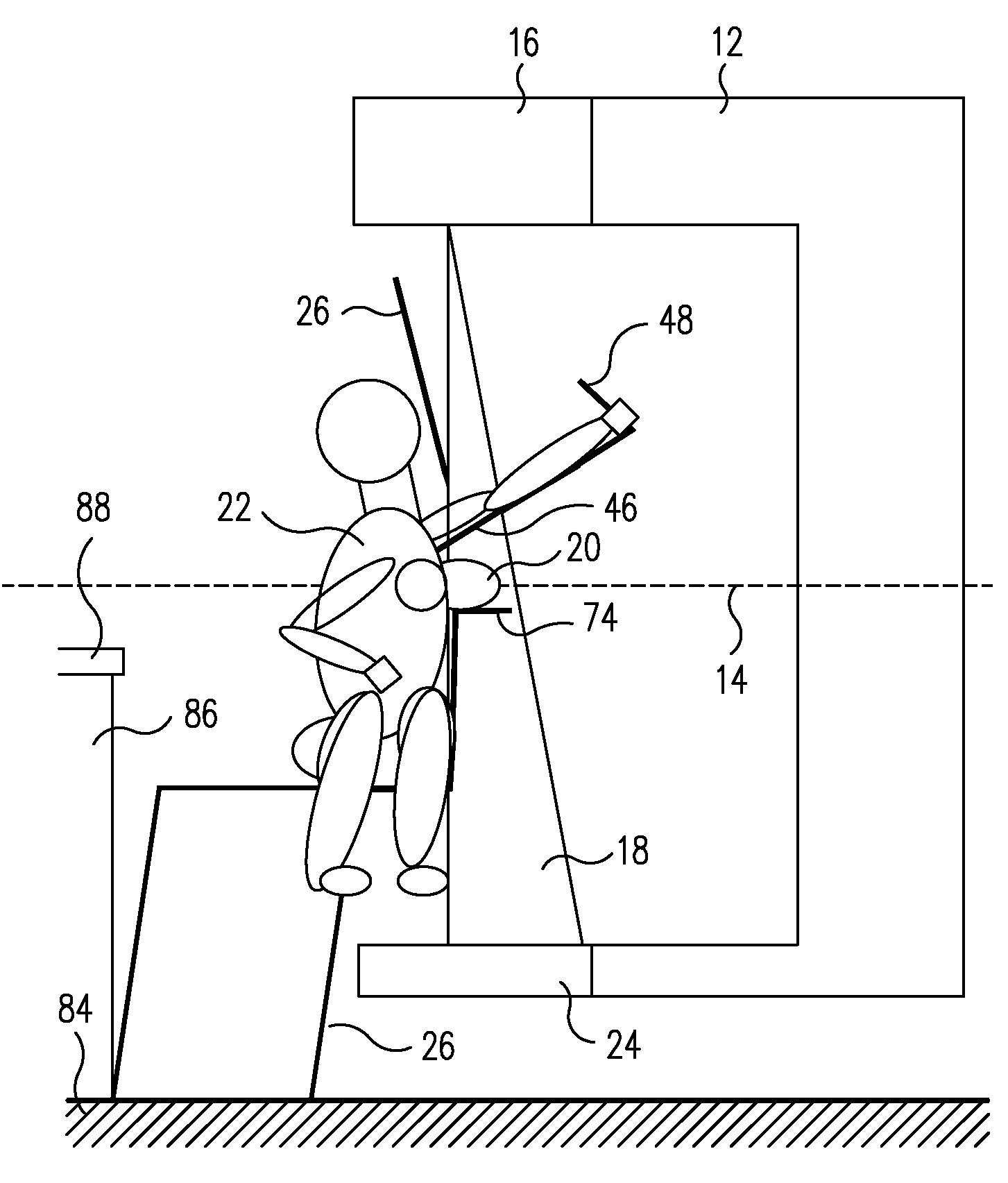
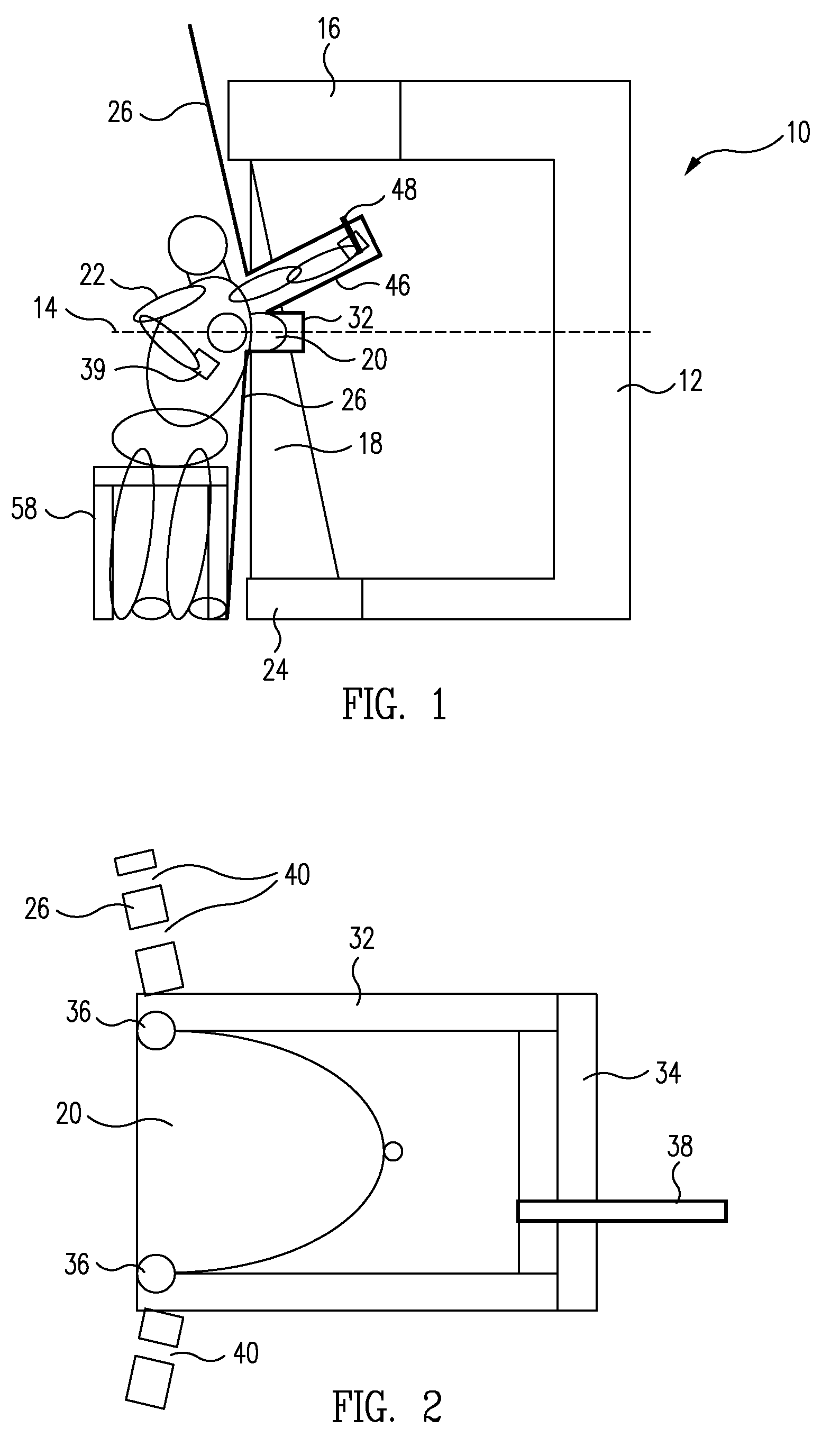
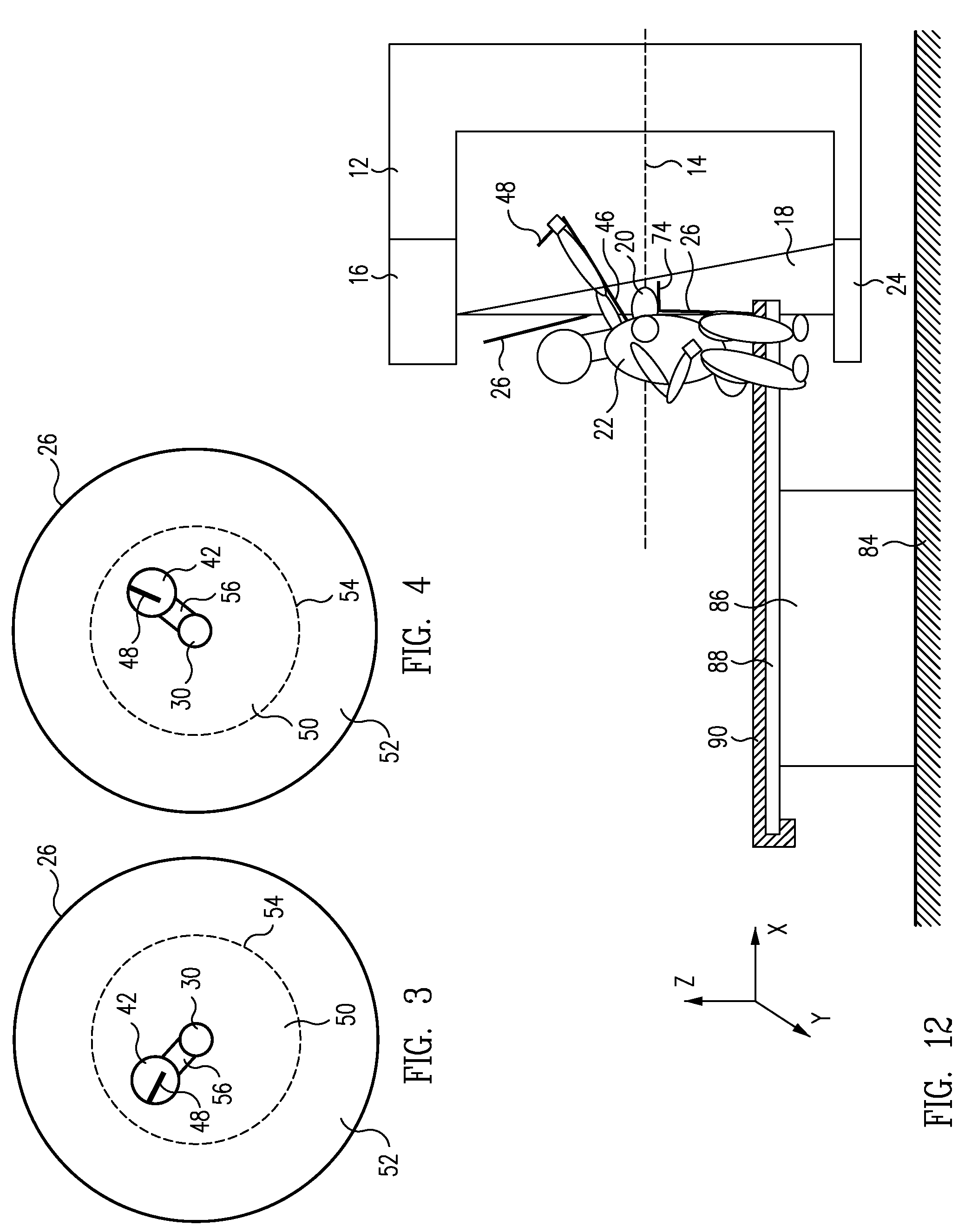
System and Method for Imaging and Treatment of Tumorous Tissue in Breasts Using Computed Tomography and Radiotherapy
InactiveUS20080317202A1Inhibition formationMinimize exposureMaterial analysis using wave/particle radiationRadiation/particle handlingTumour tissueMuscle tissue
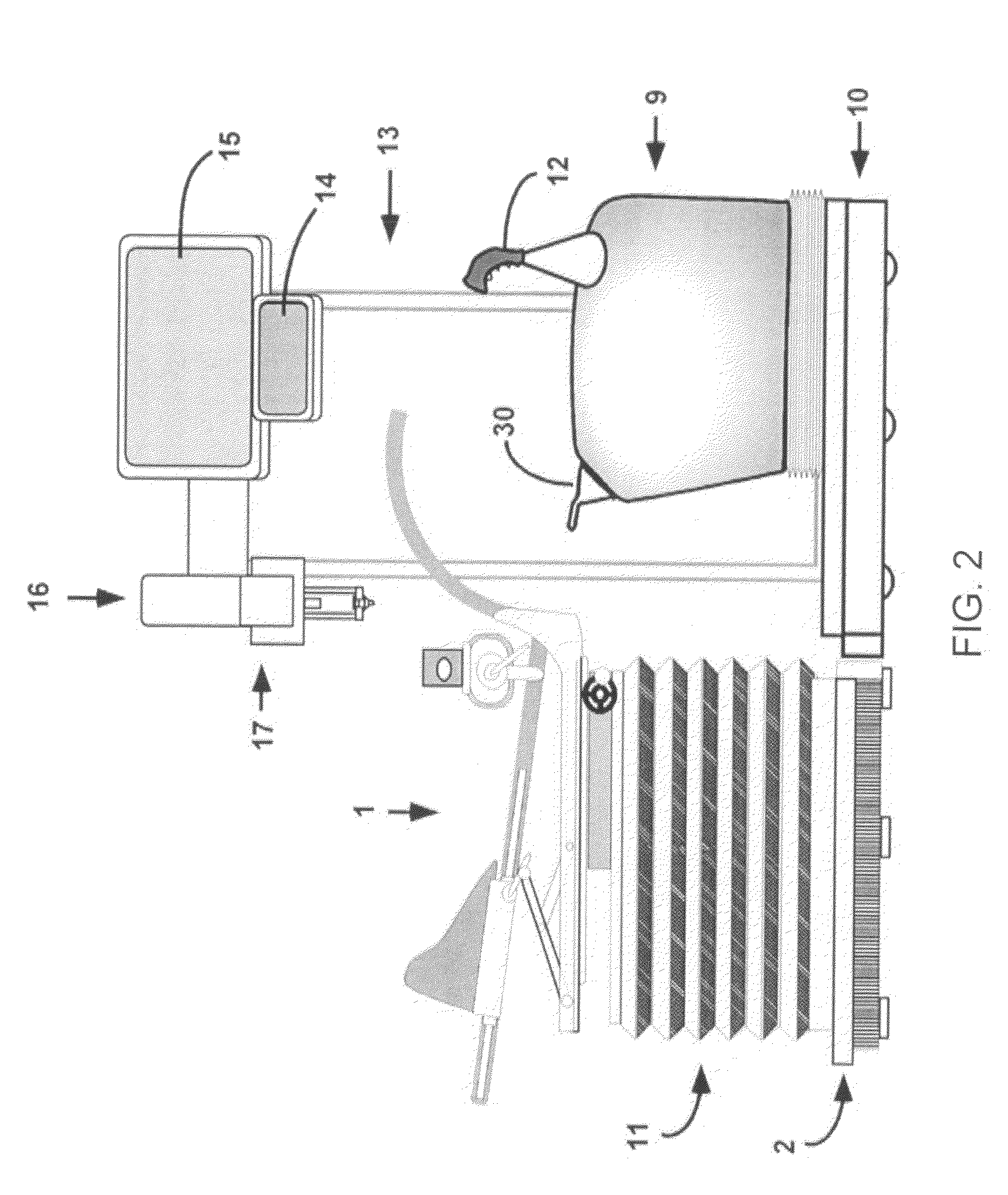
The present invention provides a system 10 for irradiating a breast 20 of a patient 22. The system 10 comprises a gantry 12 rotatable about a horizontal axis 14 and comprising a radiation source 16 for generating a radiation beam 18 and a detector 24 spaced from the radiation source 16, and a barrier 26 disposed between the patient 22 and the gantry 12. The barrier 26 is provided with an opening 30 adapted to allow a breast 20 passing therethrough to be exposed to the radiation beam 18. In some embodiments, the barrier 26 is provided with an opening 30 adapted to allow both the breast 20 and the tissue leading from the breast to axilla and the muscle tissue of the adjacent chest wall passing therethrough to be exposed to the radiation beam 18.
Owner:VARIAN MEDICAL SYSTEMS
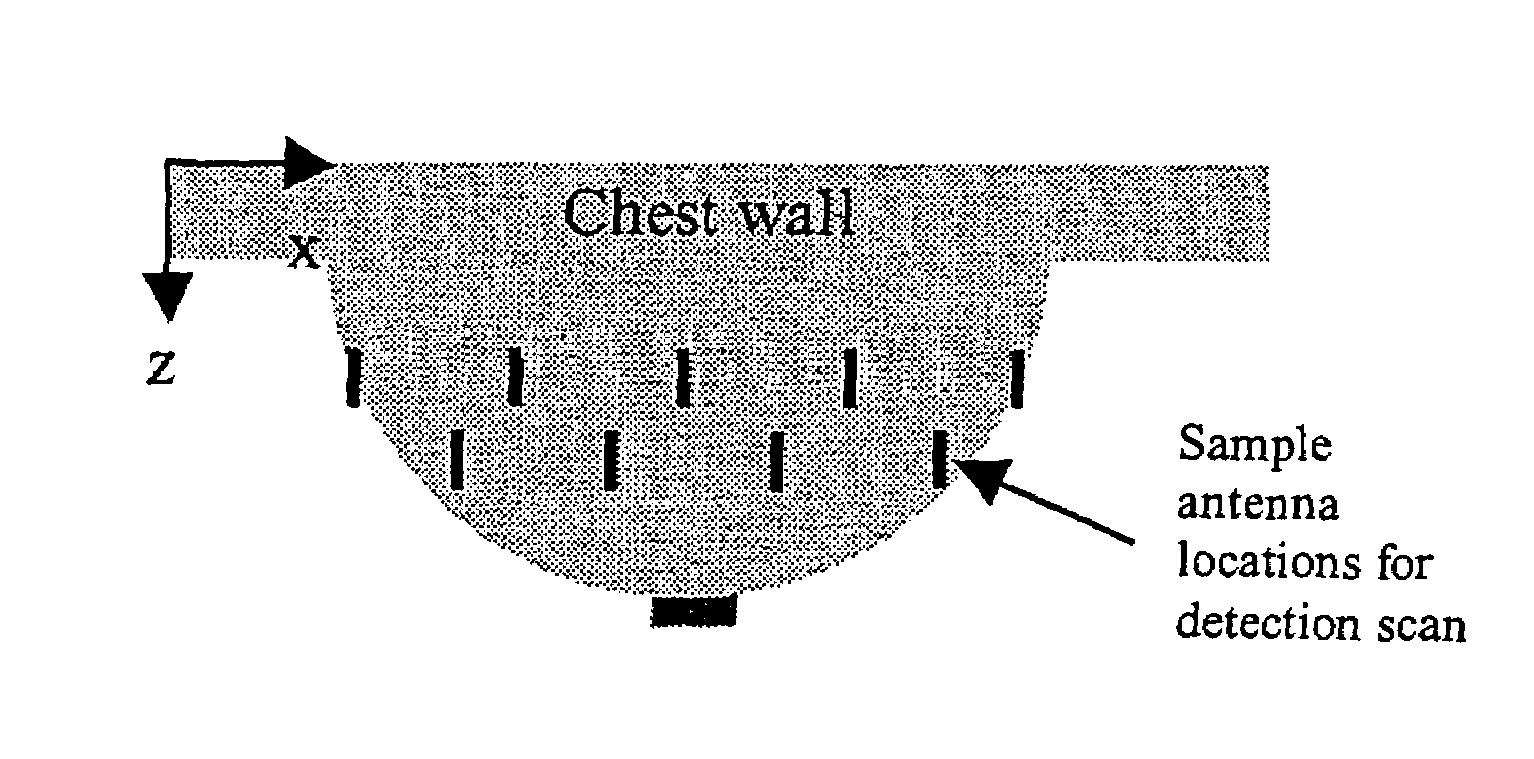
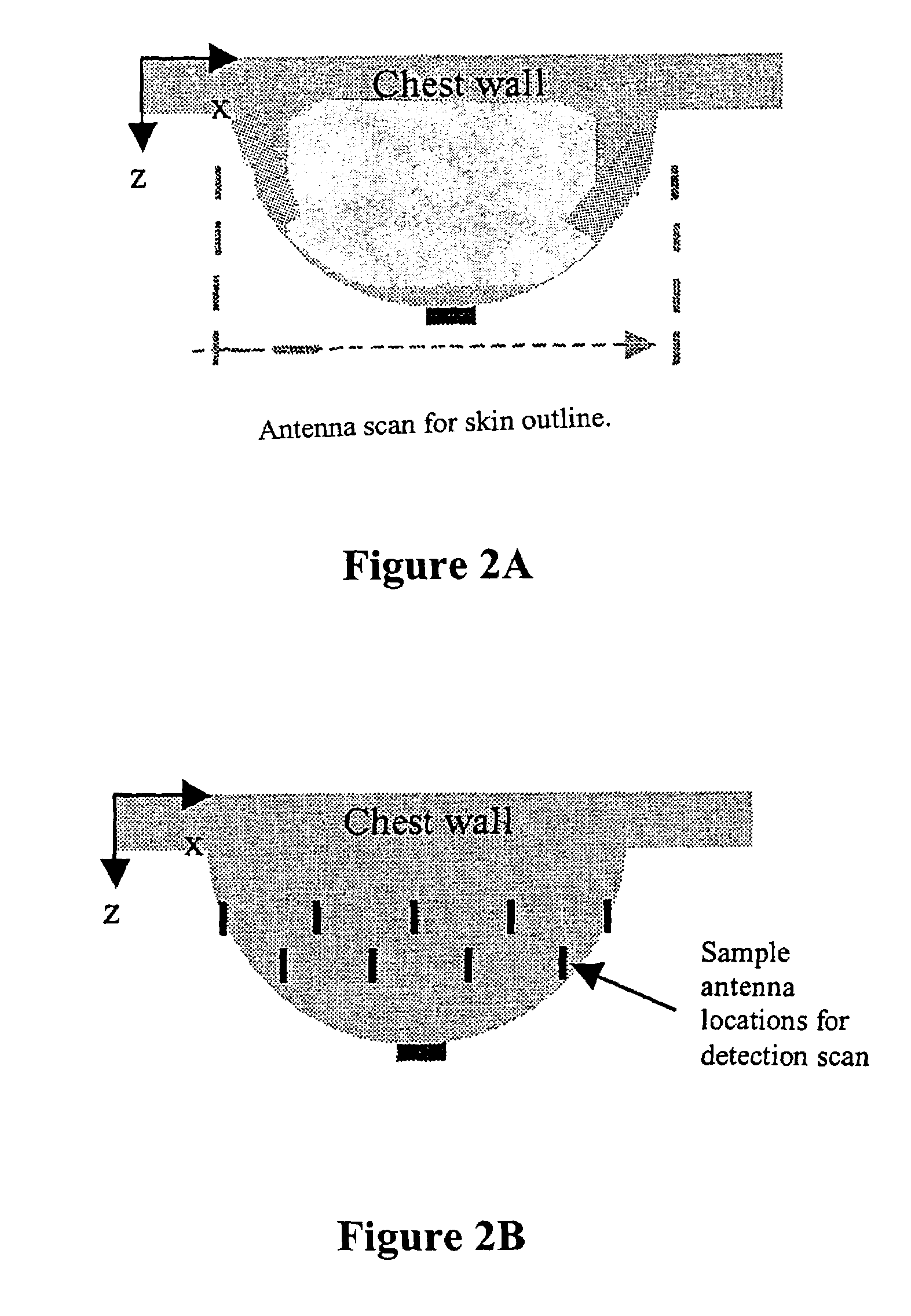
Tissue sensing adaptive radar imaging for breast tumor detection
InactiveUS7454242B2Big imageImage is often very smallElectrotherapyPolarisation/directional diversityMicrowaveRadar imaging
Owner:UTI LLP
Method for stimulating an immune response
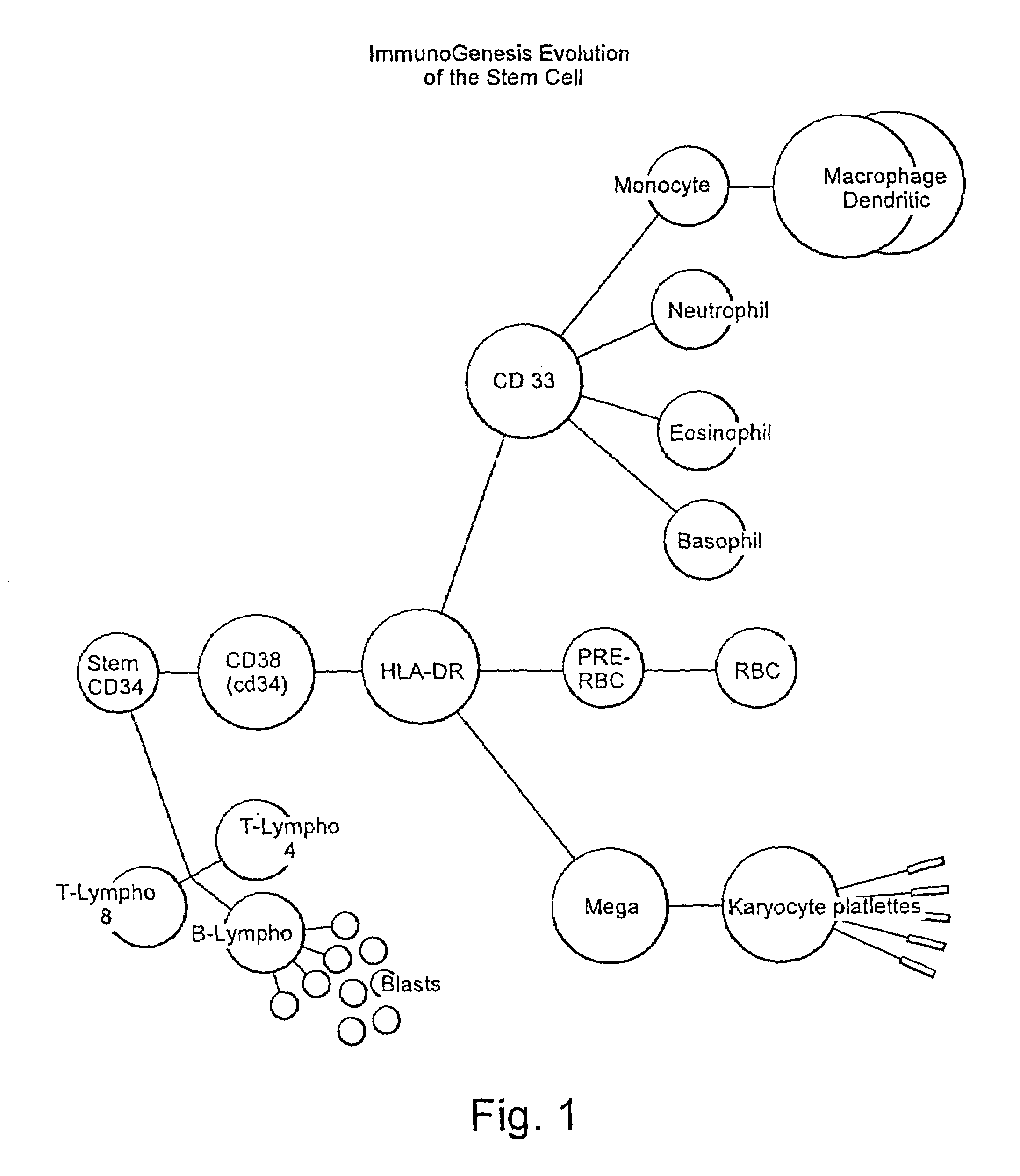
InactiveUS6977073B1Stimulating directed immune responseStimulate immune responseBiocideArtificial cell constructsAbnormal tissue growthHematopoietic cell
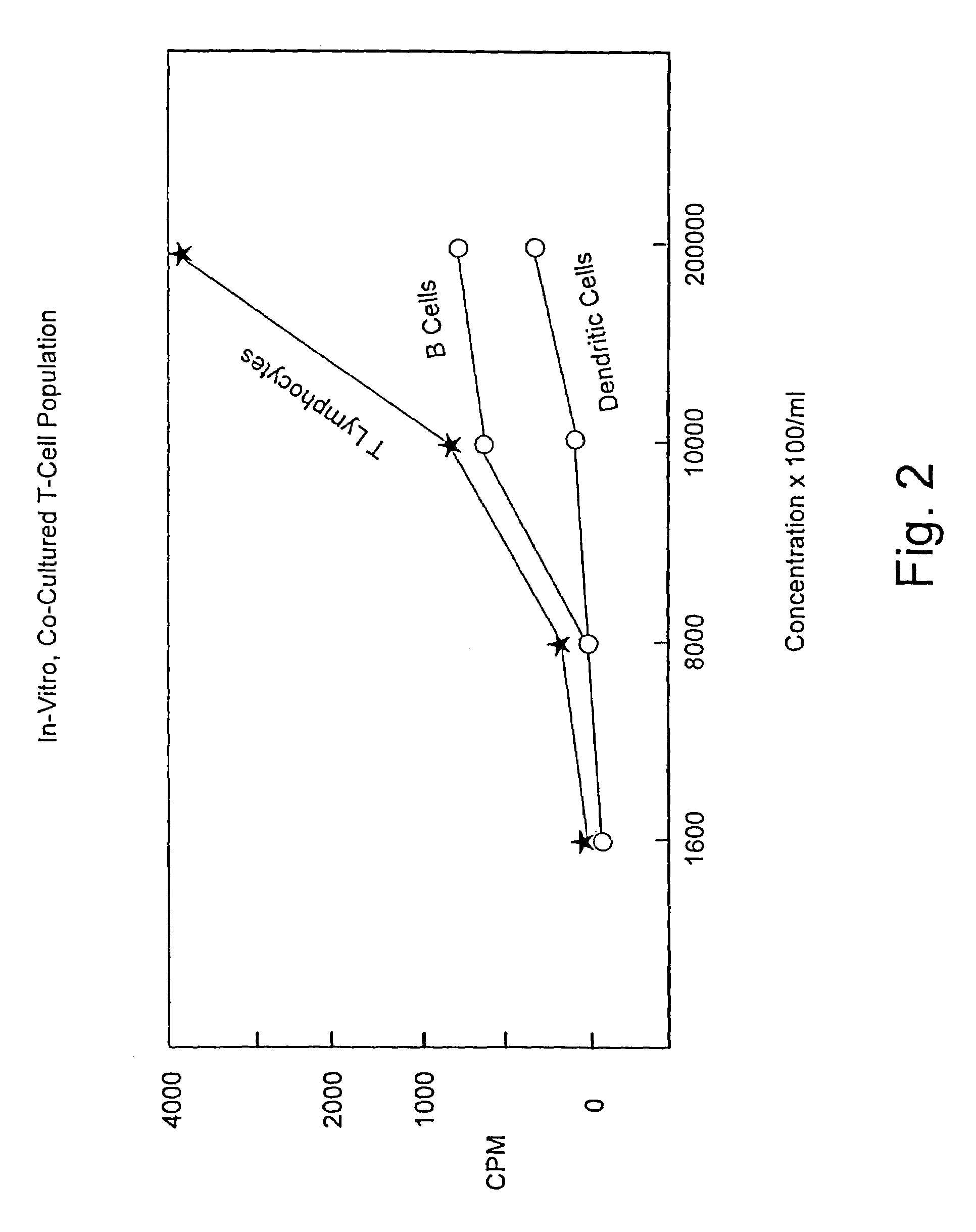
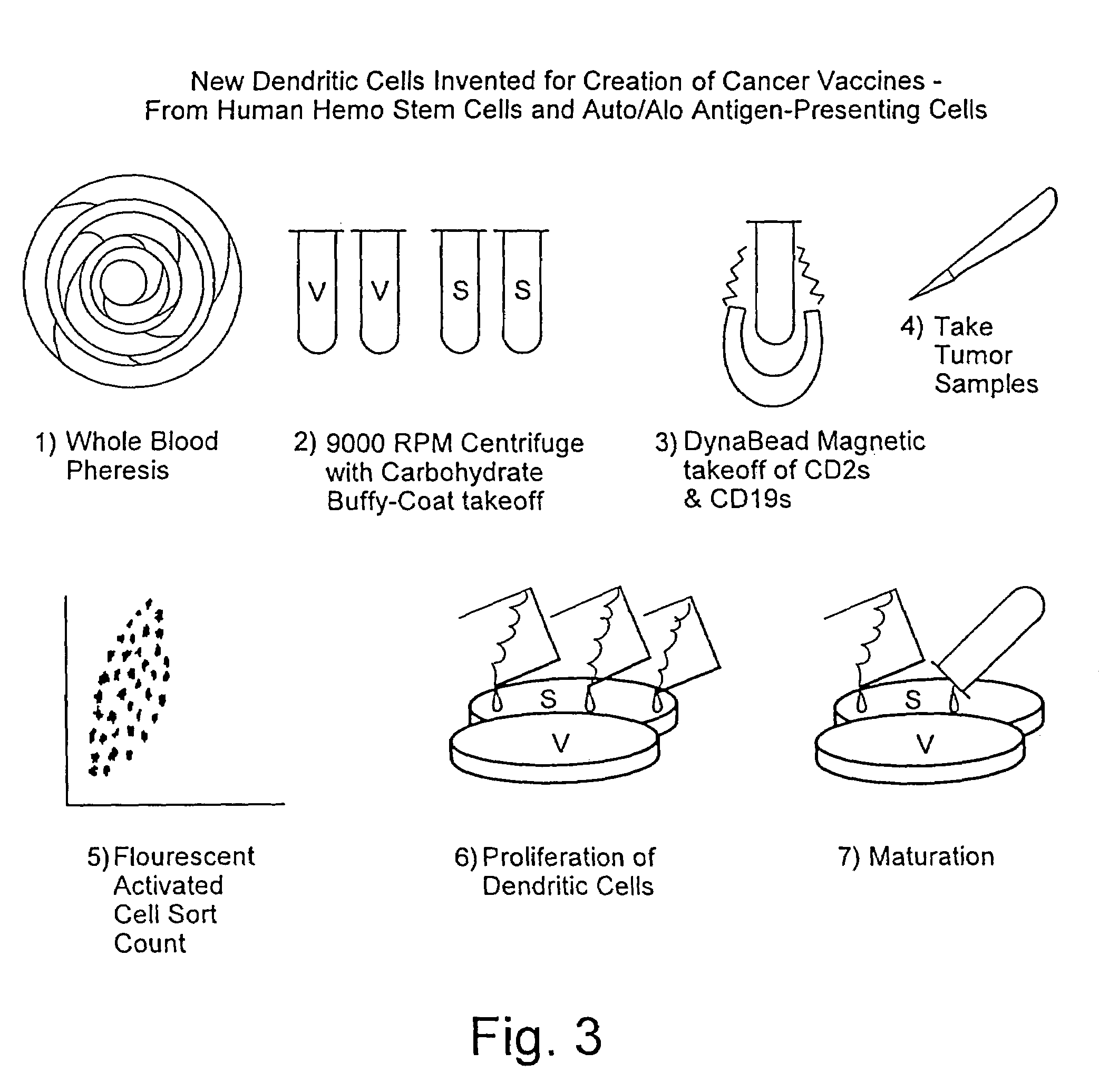
A method is described whereby dendritic cells derived from the CD34+ and CD 34−hematopoietic cell lineages are directed to become programmable antigen presenting cells. The programmed cells may be pulsed with tumor cell RNA or tumor cell RNA expression products. The protocol provides for directing the maturation of dendritic cells to become antigen presenting cells. The protocol further provides for isolating tumor cell RNA from biopsy material that has been prepared in paraffin block storage. The directed dendritic cell is provided with a plurality of tumor markers by using tumor RNA in toto, the poly A+RNA fraction or the expression product of such RNA. Once activated the dendritic cells are incubated with T4 and T8 lymphocytes to stimulate and sensitize the T lymphocytes which upon introduction either into a donor host or a nondonor recipient will provide immune response protection.
Owner:CEZAYIRLI CEM
Convection enhanced delivery catheter to treat brain and other tumors
InactiveUS6893429B2Less traumaticIncreased surgical riskPressure infusionCatheterBiological bodyAbnormal tissue growth
An apparatus and system is provided for delivering a therapeutic agent to selected sites within an organism. More particularly, the invention allows for the simultaneous delivery of therapeutics to multiple treatment locations from a single catheter using a single pumping source. The catheter utilizes a microporous membrane that allows for the distribution of therapeutic agents from multiple longitudinal positions.
Owner:MEDTRONIC INC
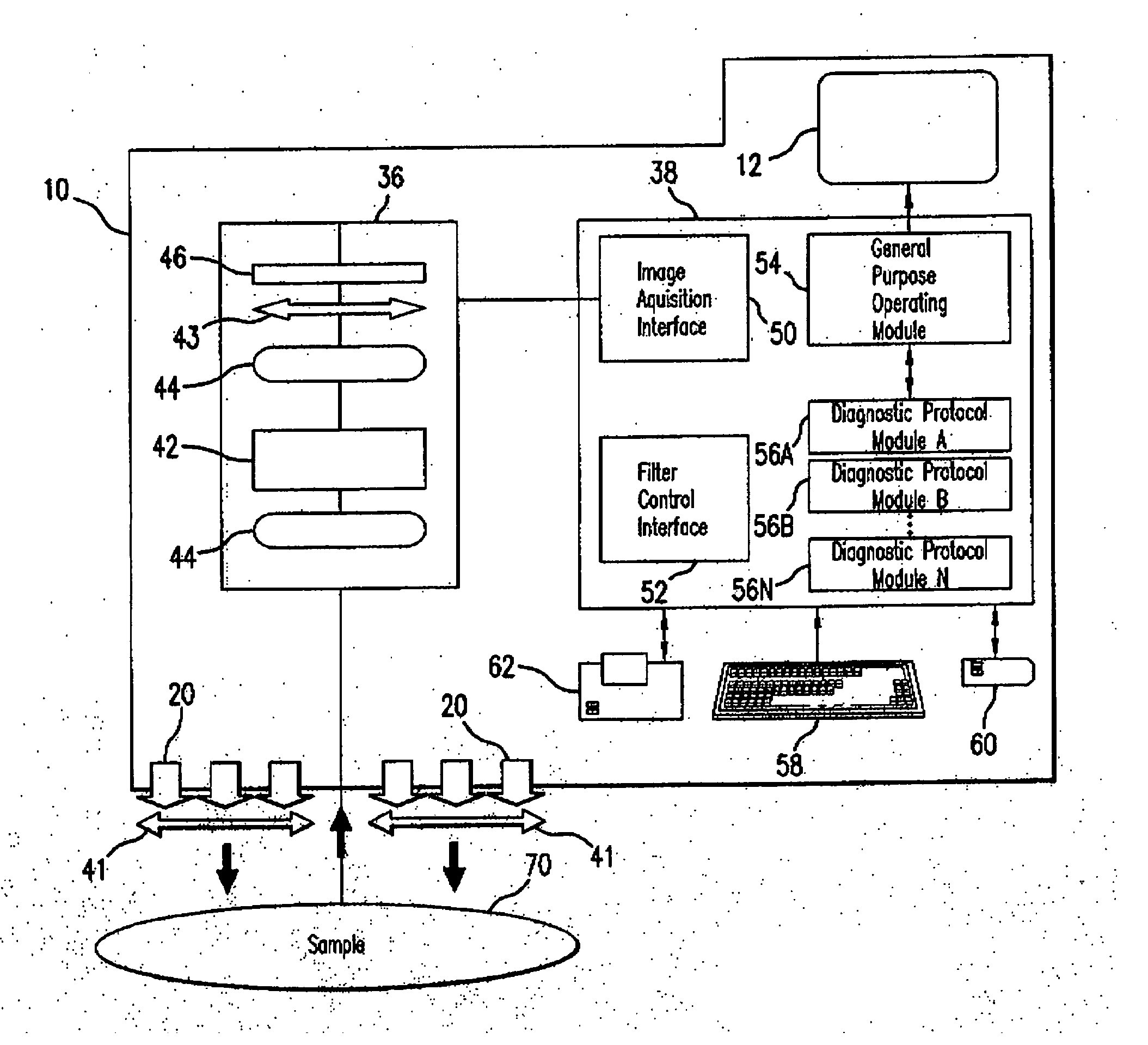
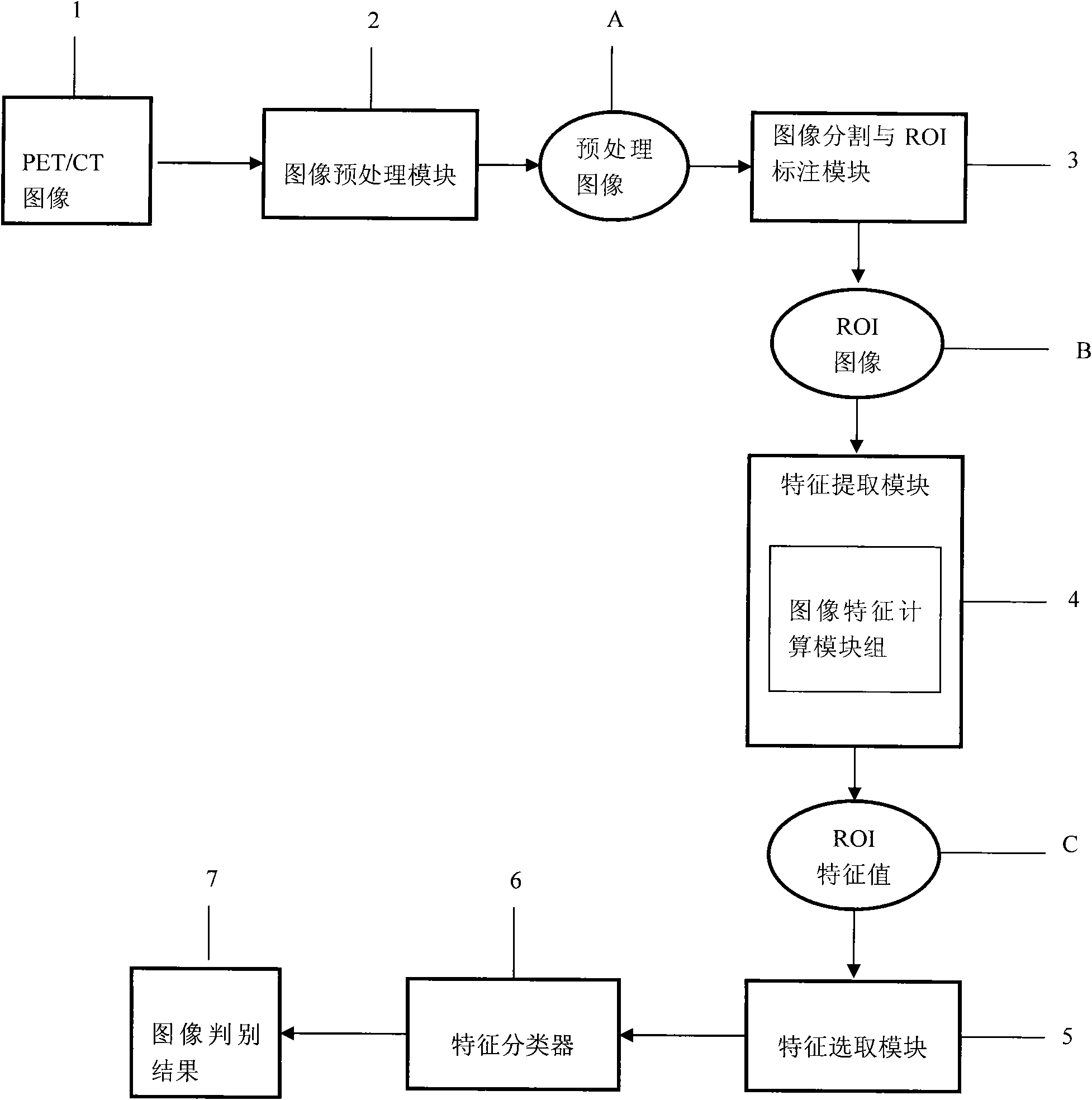
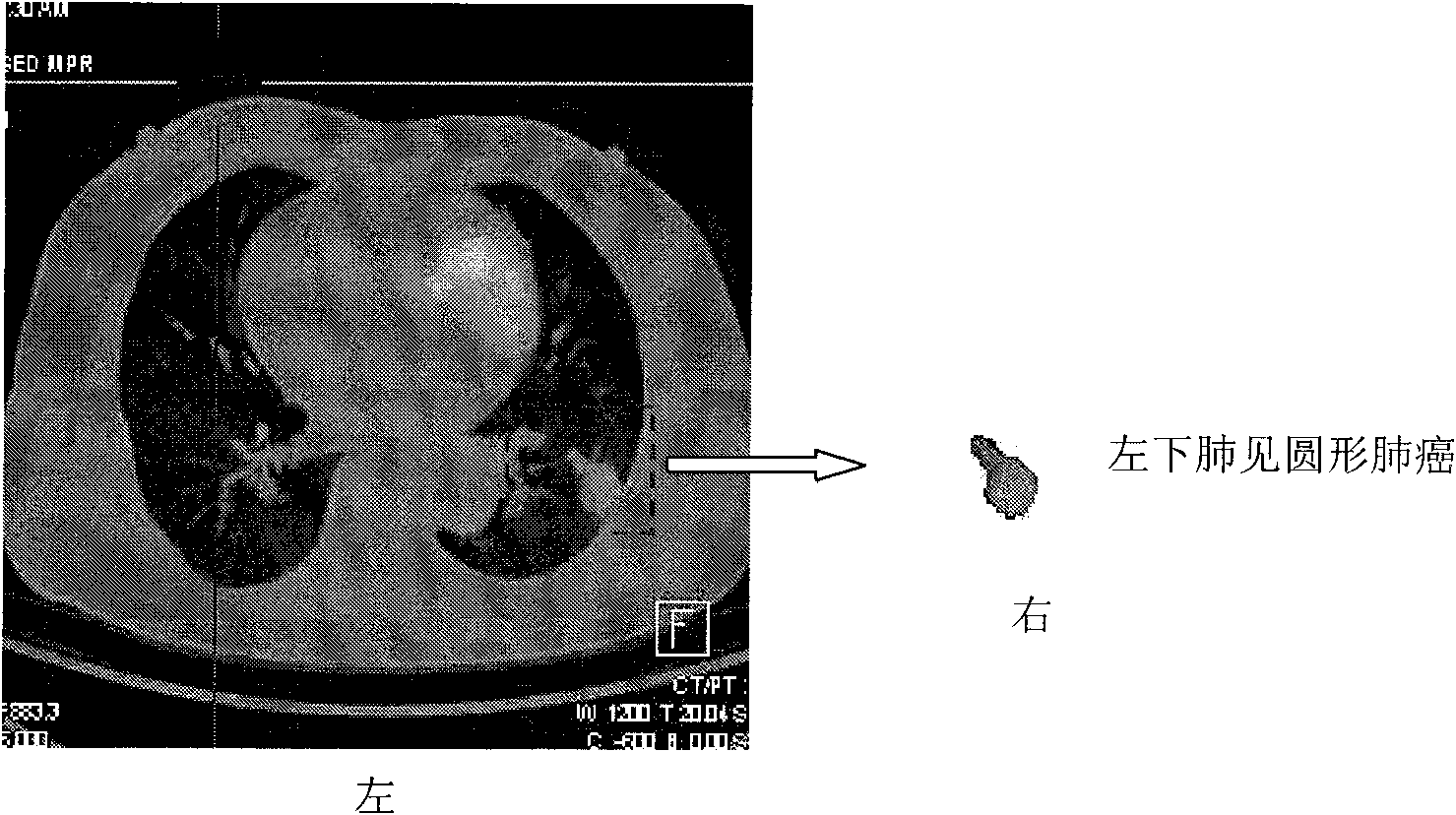
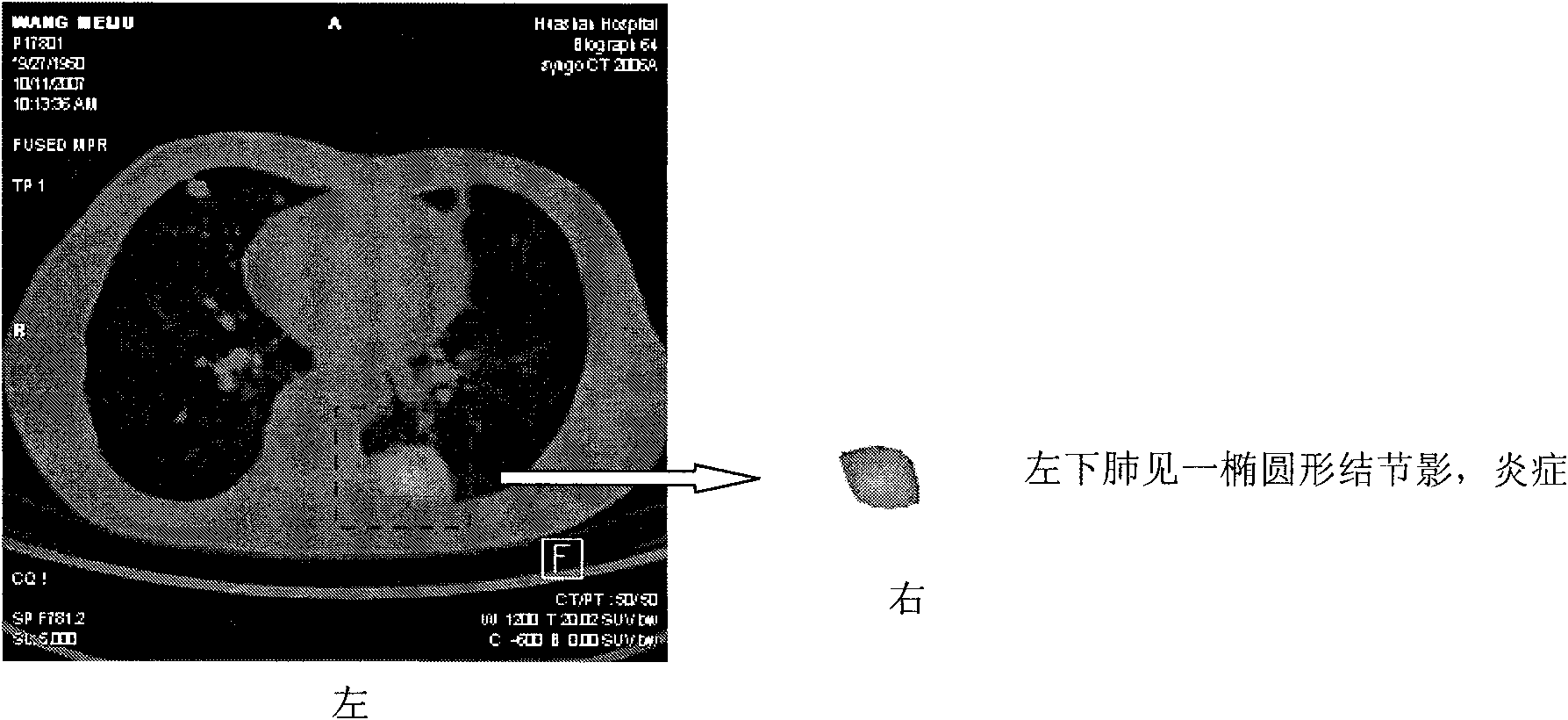
System for detecting pulmonary malignant tumour and benign protuberance based on PET/CT image texture characteristics
InactiveCN101669828AImprove processing efficiencyImprove user experienceImage analysisComputerised tomographsTumour tissueFungating tumour
The invention relates to a system for detecting a pulmonary malignant tumour and a benign protuberance based on PET / CT image texture characteristics, belonging to the technical field of medical digital image processing. The invention has the major function of searching useful texture characteristics from a PET / CT picture to better distinguish a pulmonary tumour tissue and a benign tissue. The system comprises the following steps: firstly, dividing a region of interest ROI from the PET / CT image and then extracting five texture characteristics of roughness, contrast, busyness, complexity and intensity of the ROI; then, carrying out classifying discrimination on the characteristics by distance calculation and a characteristic classifier and distinguishing the malignant tumour and the benign protuberance effectively by the combined data of various characteristics.
Owner:FUDAN UNIV
Device for biopsy of tumors
InactiveUS7311672B2Avoid destructionReduce dispersionSurgical needlesVaccination/ovulation diagnosticsAbnormal tissue growthCombined use
A device and method of use for securing and coring of tumors within the body during a biopsy of the tumor, specifically breast tumors. An adhesion probe for securing the tumor is described. The probe secures the tumor by piercing the tumor and providing a coolant to the distal tip to cool the tip. The cooled tip adheres to the tumor. A coring instrument adapted for cutting a core sample of the tumor is described. The instrument is provided with a cannula that can cut a core sample of the tumor. The instrument is adapted for use with the probe with the probe fitting within the cannula. The instrument can be used in conjunction with the probe to secure and core a sample of the tumor for biopsy.
Owner:SCION MEDICAL
Engineered CD20 targeting NKT cell and its preparation method and application
ActiveCN103820393AHigh transfection rateProlong survival timePolypeptide with localisation/targeting motifImmunoglobulin superfamilyCD20Tumor antigen
Owner:SHANGHAI CELLULAR BIOPHARMACEUTICAL GROUP LTD
Elongated markers for soft tissue volume identification
ActiveUS8027712B2Improve gripGood flexibilitySurgeryDiagnostic markersInterstitial markerImaging modalities
The present invention is related to an interstitial marker for localizing an organ, tumor or tumor bed within a mammalian body wherein said marker has a proximal end, a distal end, and a continuous intervening length, at least a portion of the intervening length of said marker being visible under at least one imaging modality and having a flexibility such that said marker follows movements and changes of shape of said organ, tumor or tumor bed.
Owner:RADIOMED
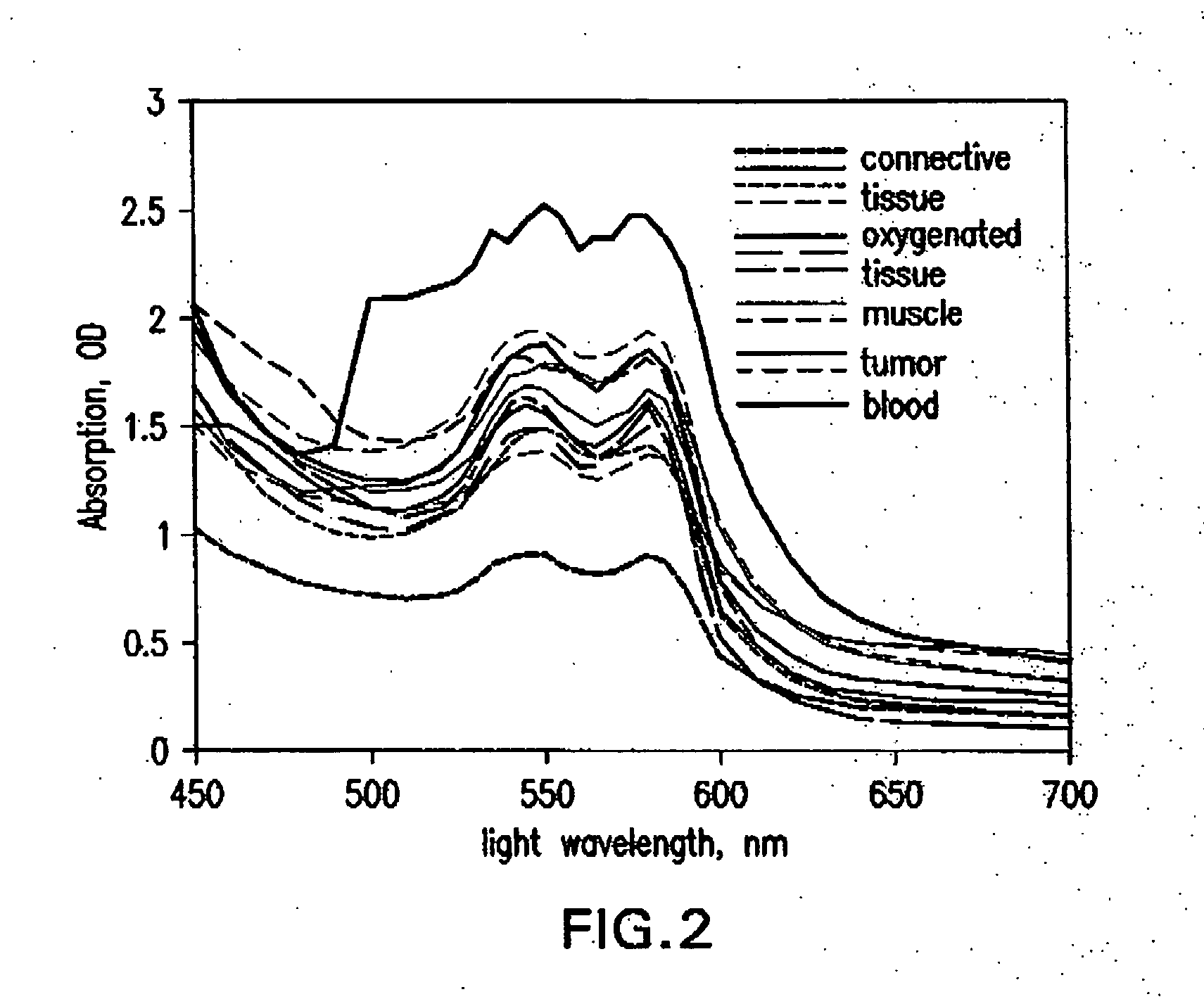
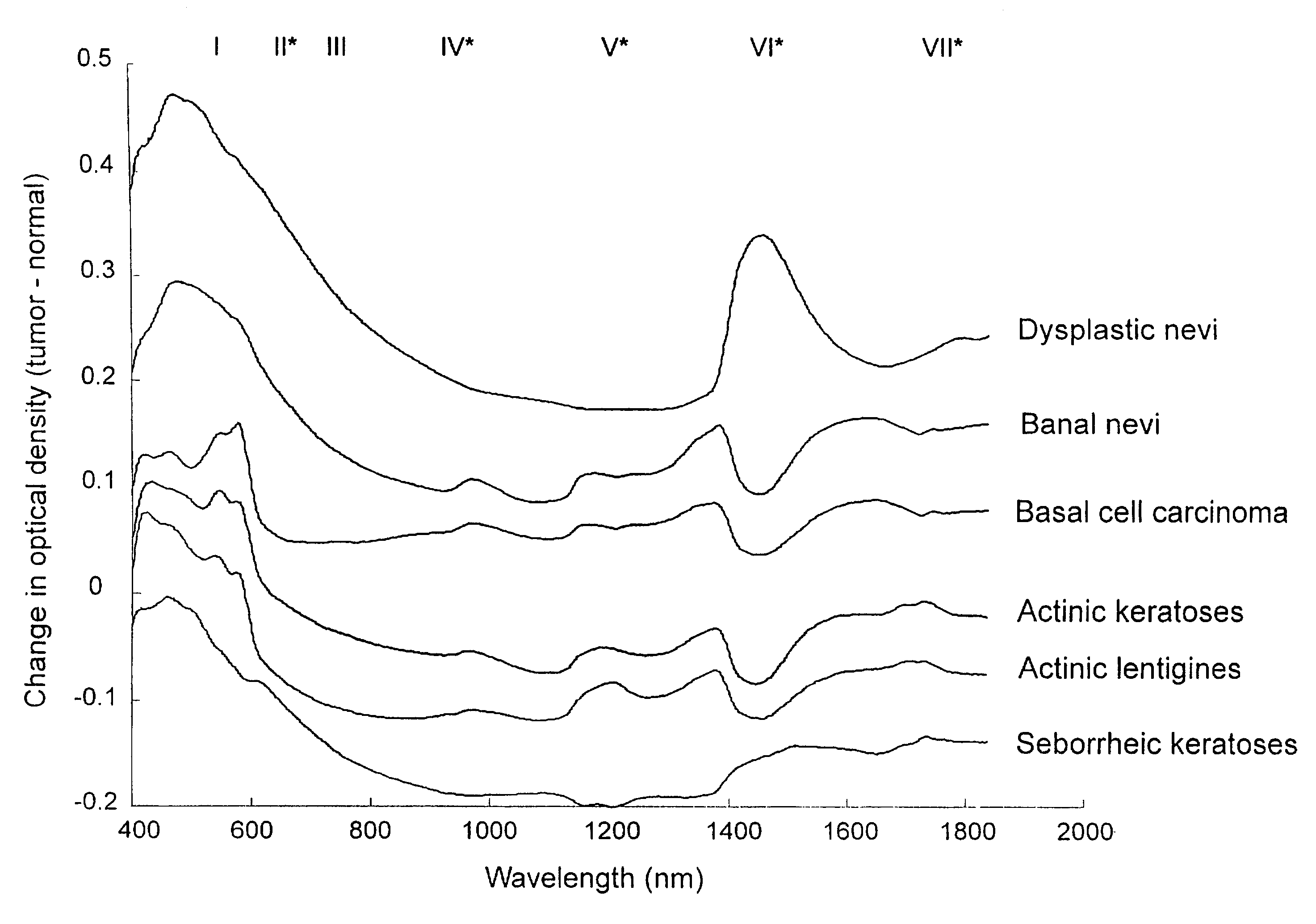
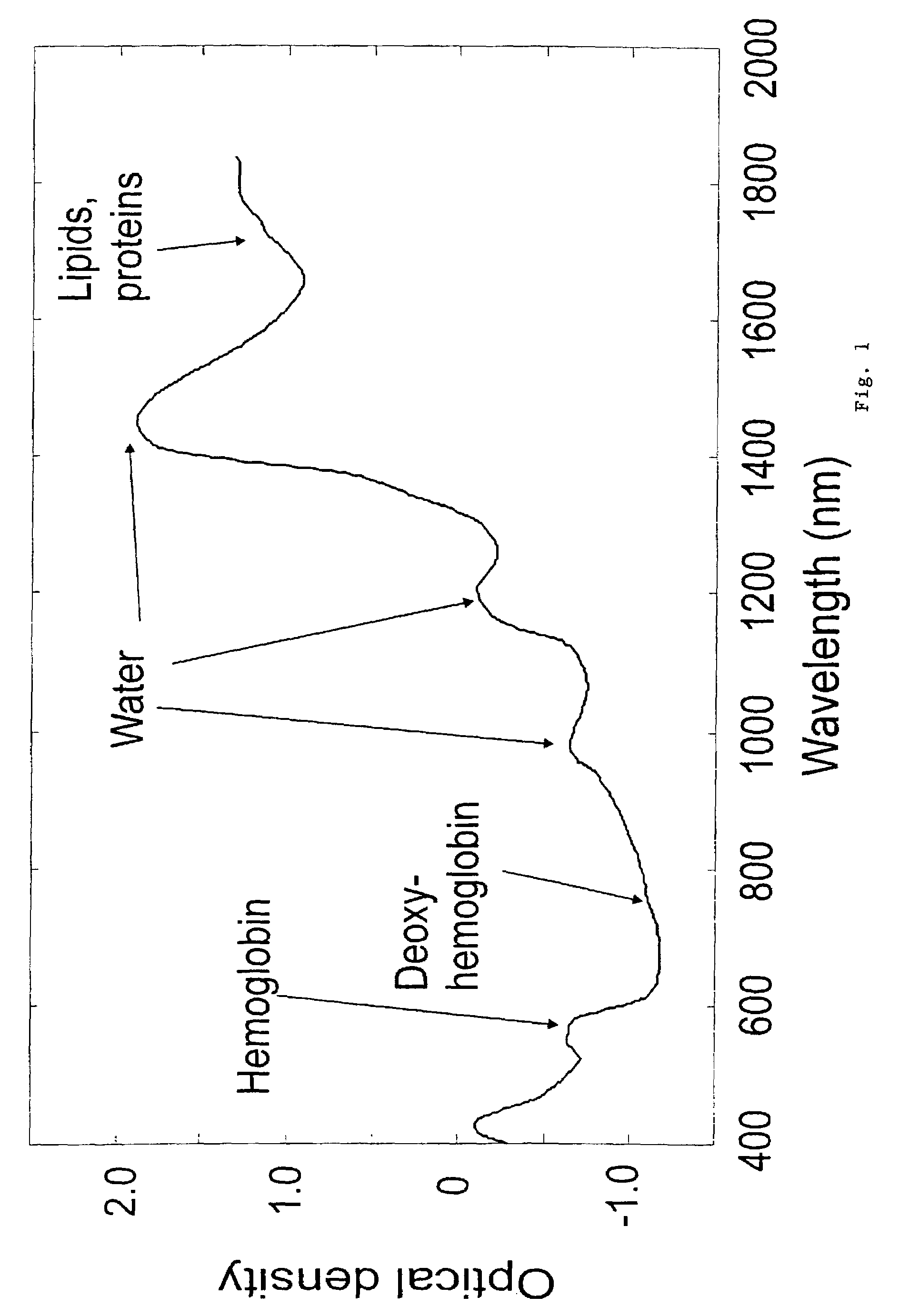
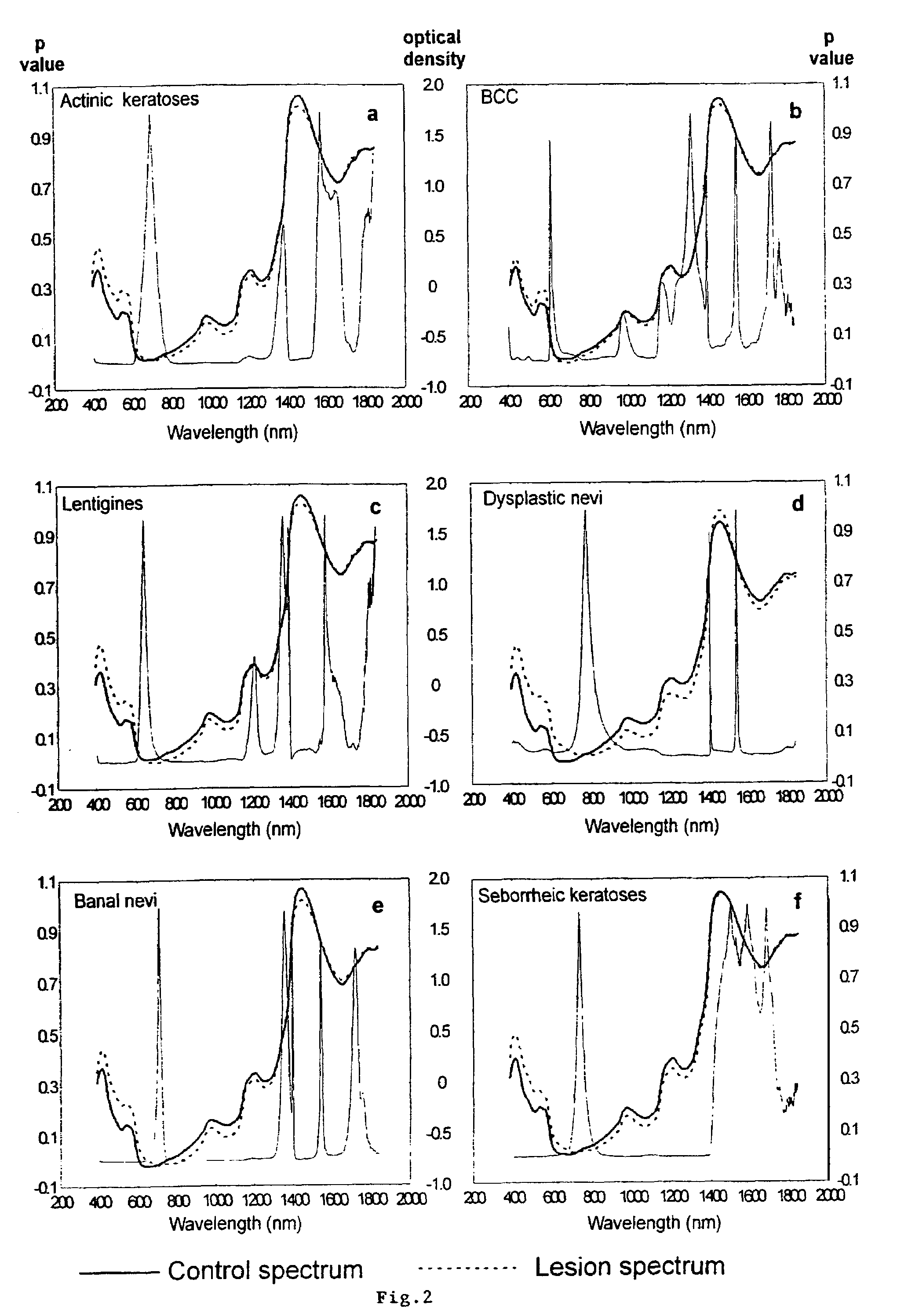
Non-invasive screening of skin diseases by visible/near-infrared spectroscopy
A non-invasive tool for skin disease diagnosis would be a useful clinical adjunct. The purpose of this study was to determine whether visible / near-infrared spectroscopy can be used to non-invasively characterize skin diseases. In-vivo visible- and near-infrared spectra (400-2500 nm) of skin neoplasms (actinic keratoses, basal cell carcinomata, banal common acquired melanocytic nevi, dysplastic melanocytic nevi, actinic lentigines and seborrheic keratoses) were collected by placing a fiber optic probe on the skin. Paired t-tests, repeated measures analysis of variance and linear discriminant analysis were used to determine whether significant spectral differences existed and whether spectra could be classified according to lesion type. Paired t-tests showed significant differences (p<0.05) between normal skin and skin lesions in several areas of the visible / near-infrared spectrum. In addition, significant differences were found between the lesion groups by analysis of variance. Linear discriminant analysis classified spectra from benign lesions compared to pre-malignant or malignant lesions with high accuracy. Visible / near-infrared spectroscopy is a promising non-invasive technique for the screening of skin diseases.
Owner:NAT RES COUNCIL OF CANADA
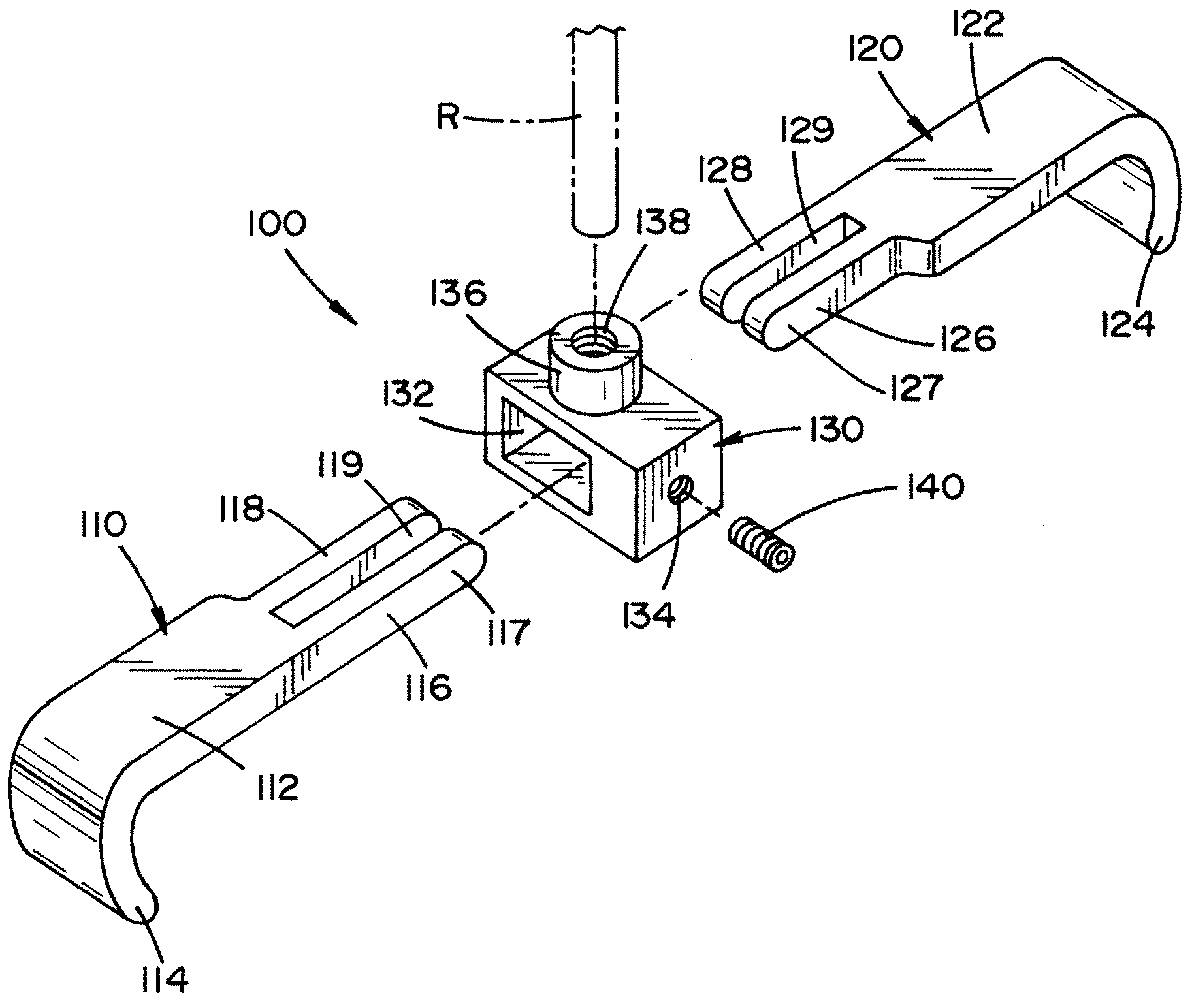
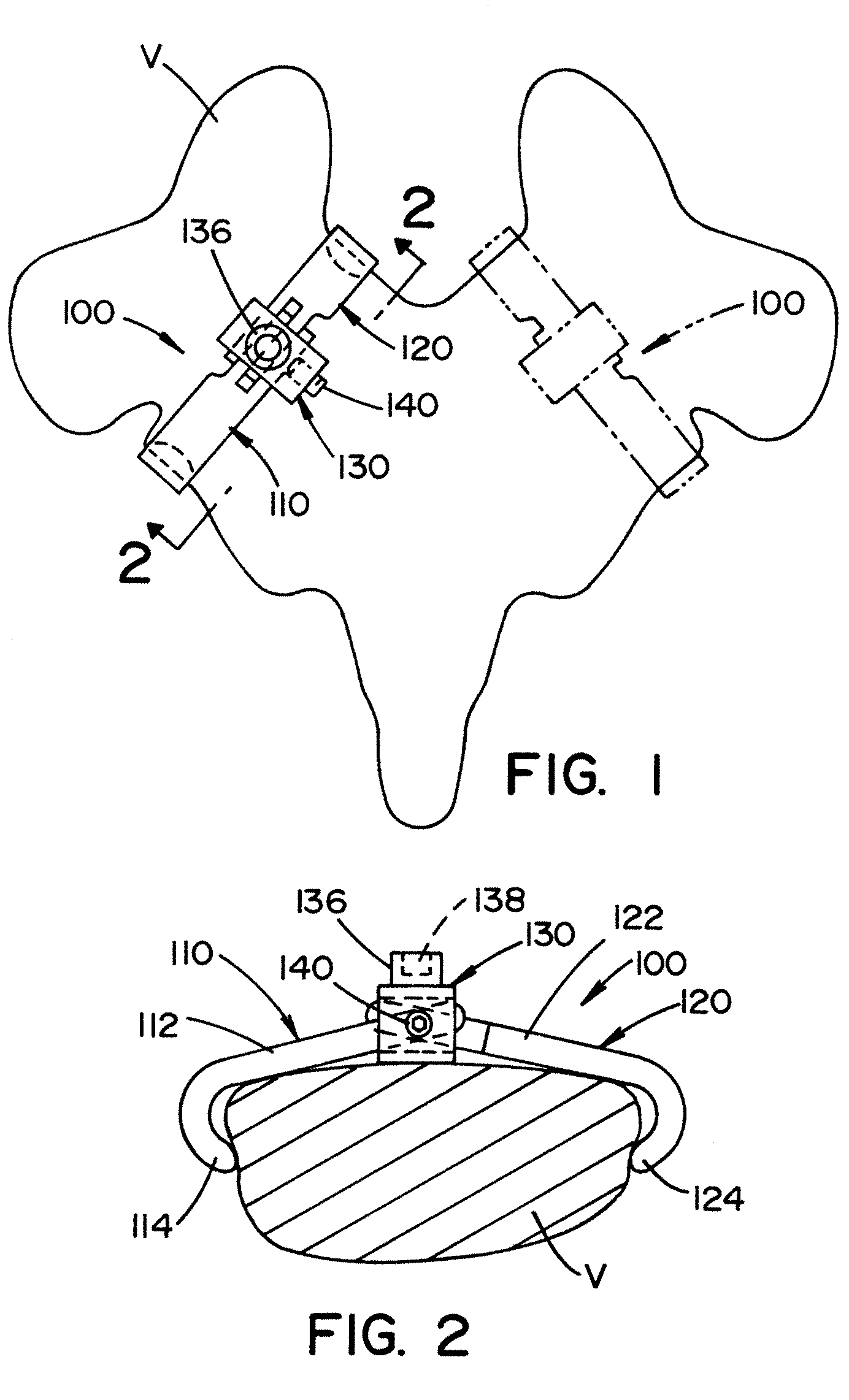
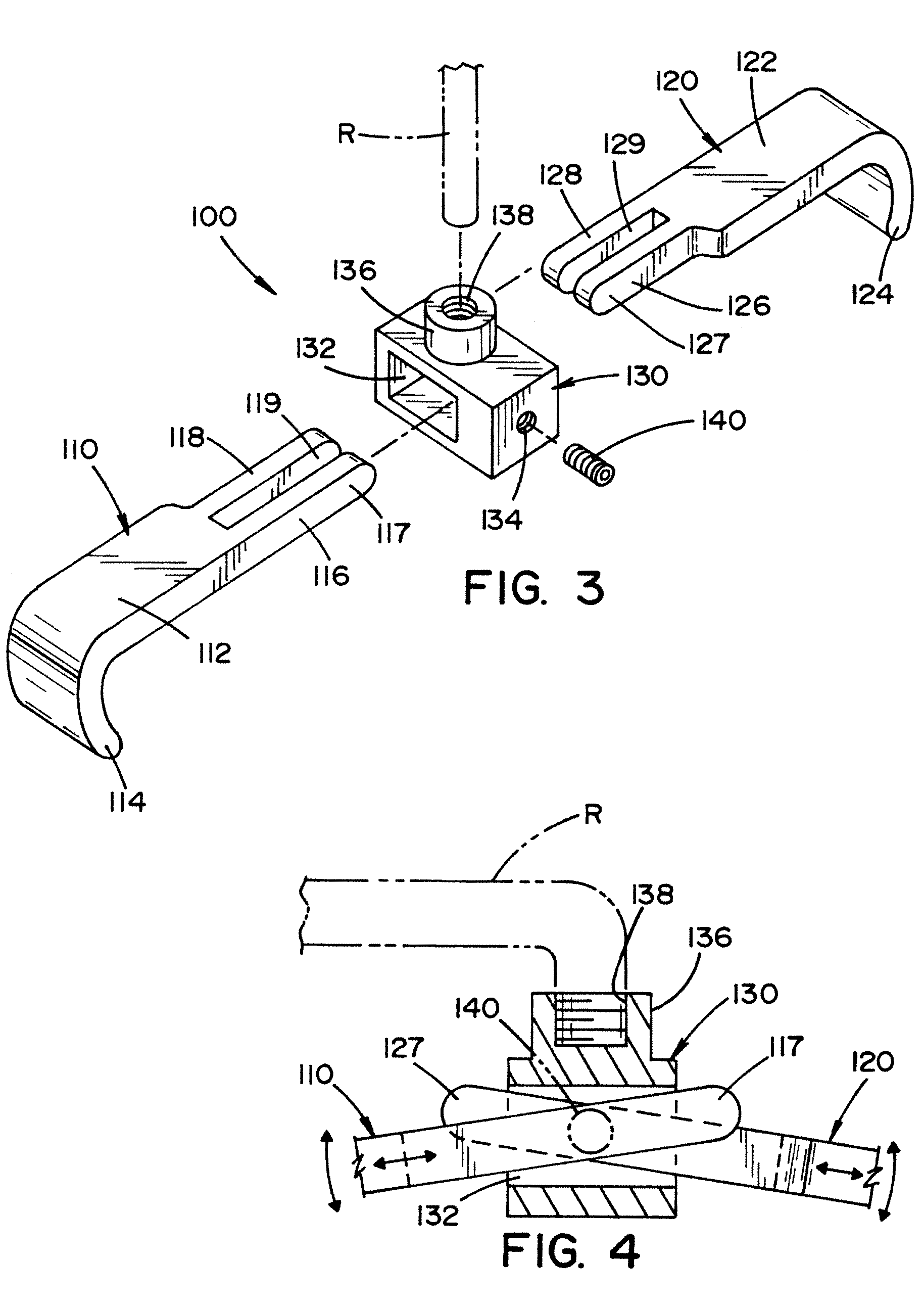
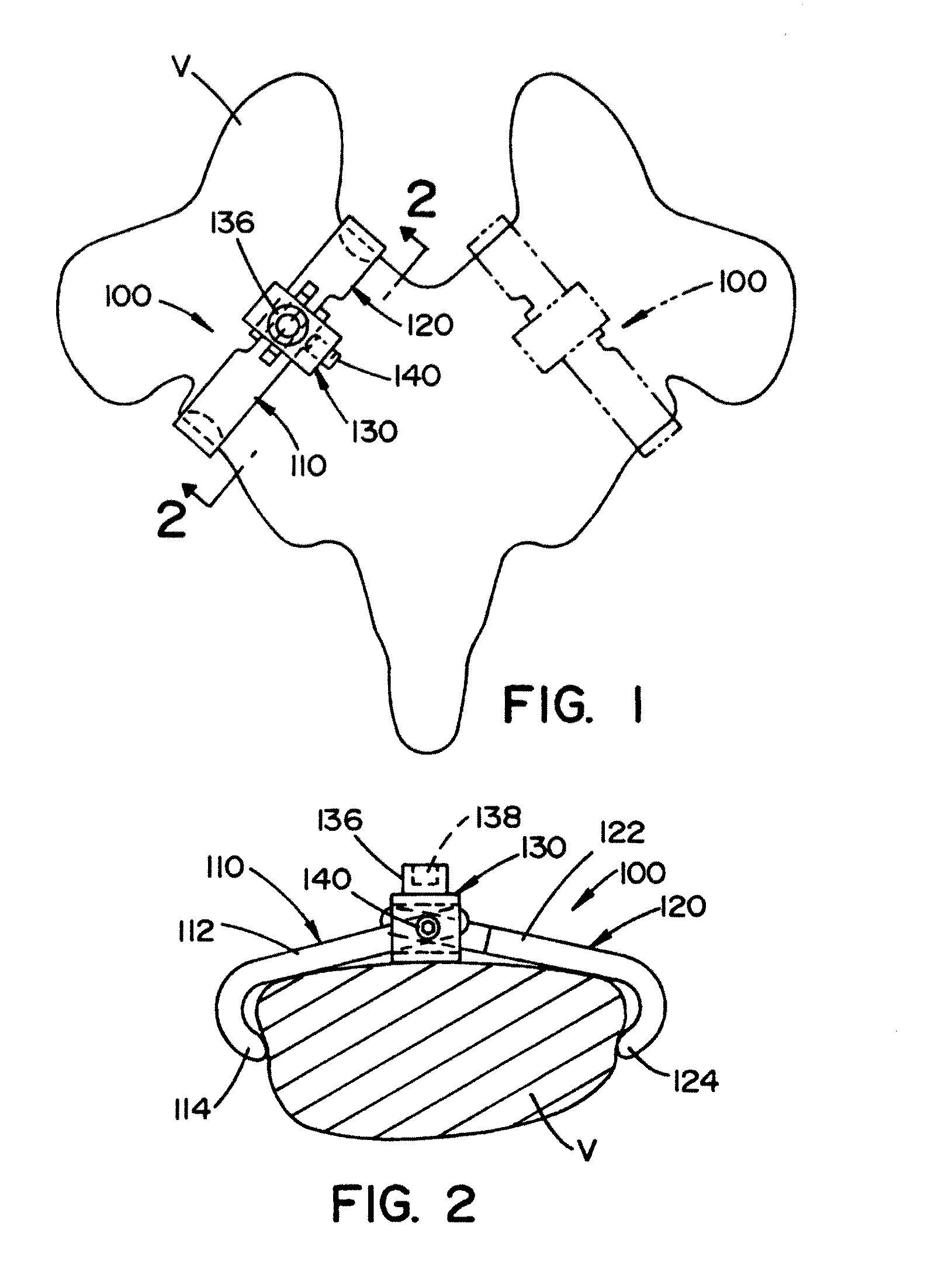
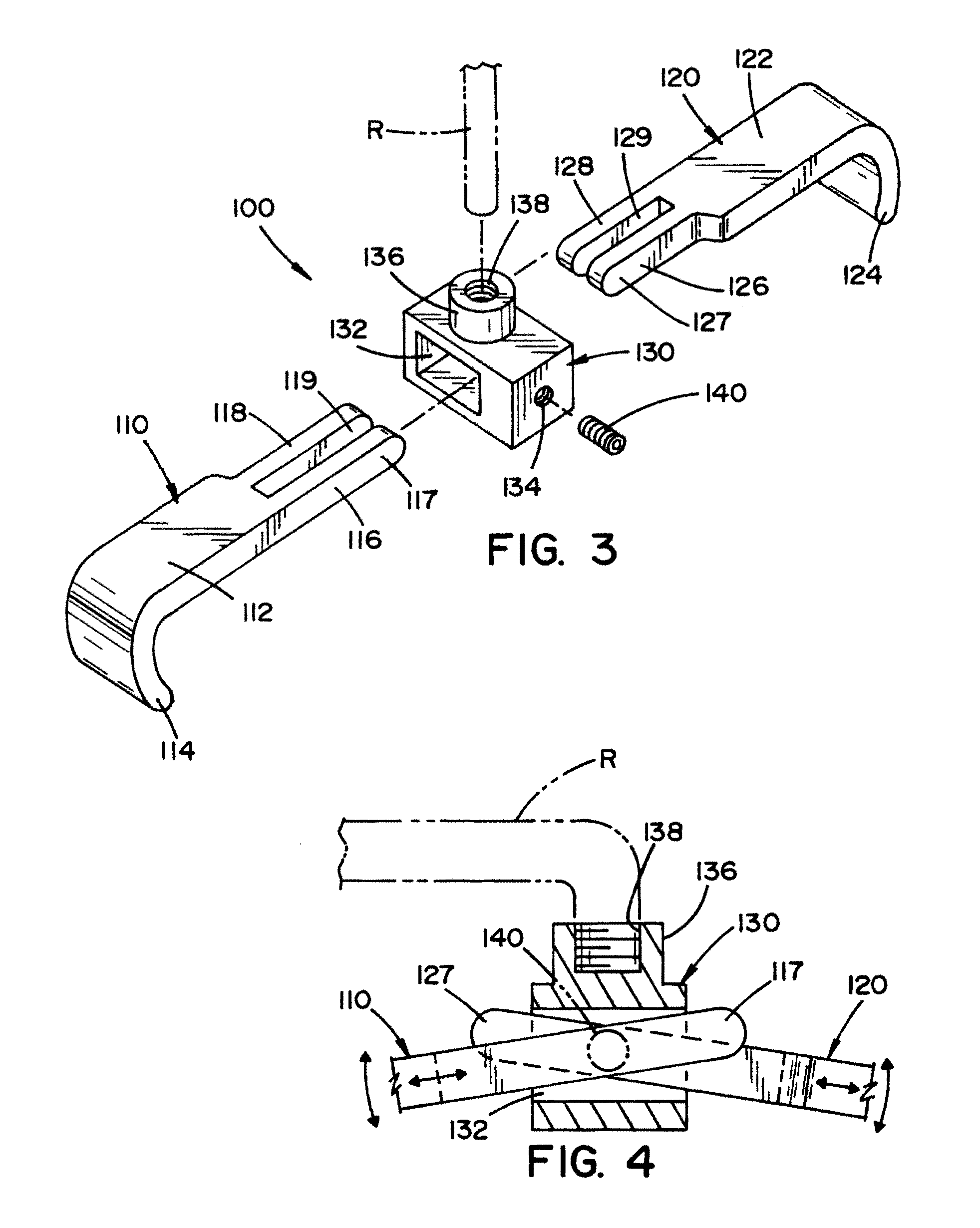
Vertebral pars interarticularis clamp a new spine fixation device, instrumentation, and methodology
InactiveUS7883532B2Improve the inconvenienceExtension of timeSuture equipmentsInternal osteosythesisTrauma surgerySpinal column
An improve spinal surgical implant used primarily in the posterior aspect of the spinal column for spinal reconstruction; revision surgery; deformity correction; and / or tumor surgery and / or trauma surgery of the cervical, thoracic and / or and lumbo-sacral spine.
Owner:SPINECO
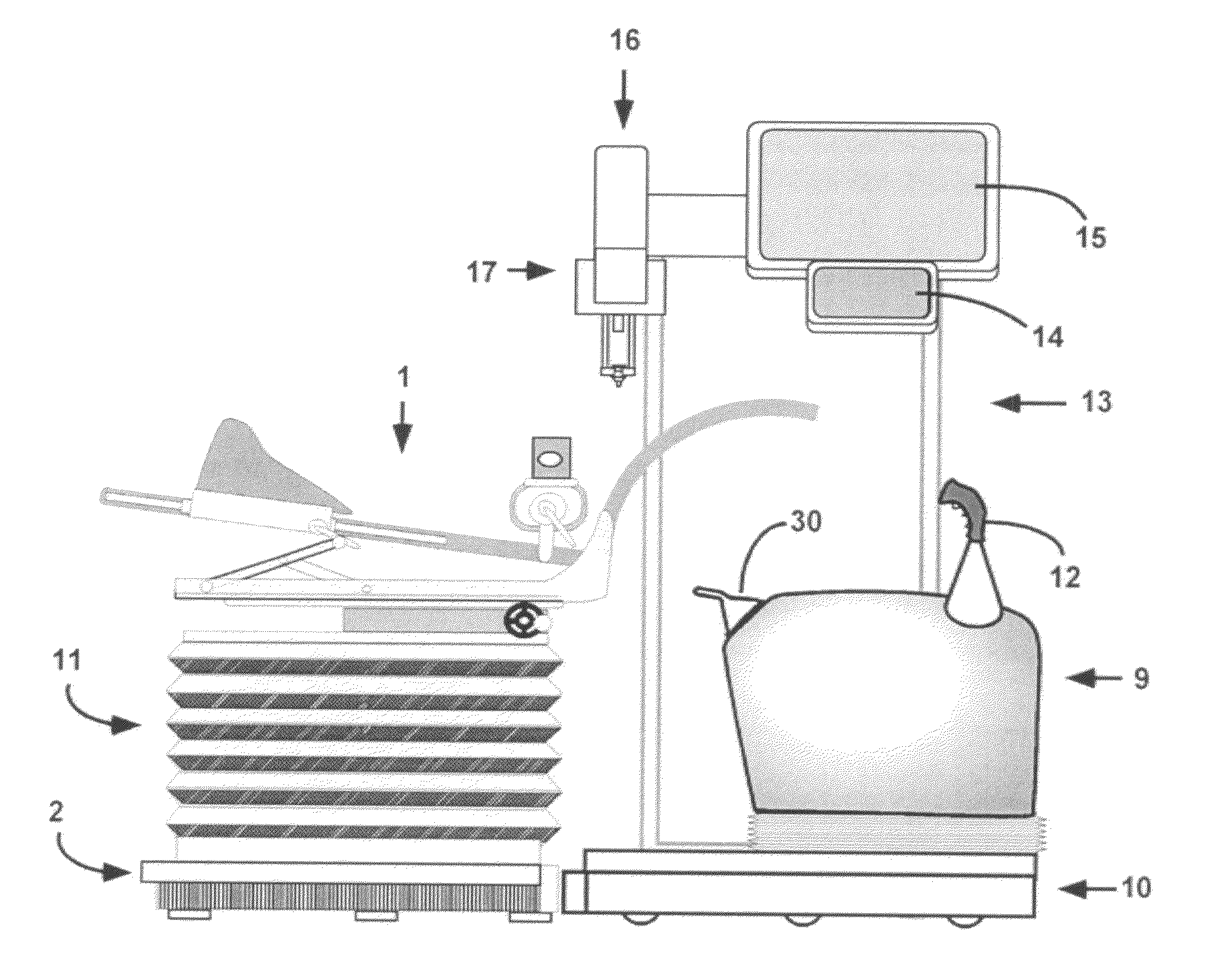
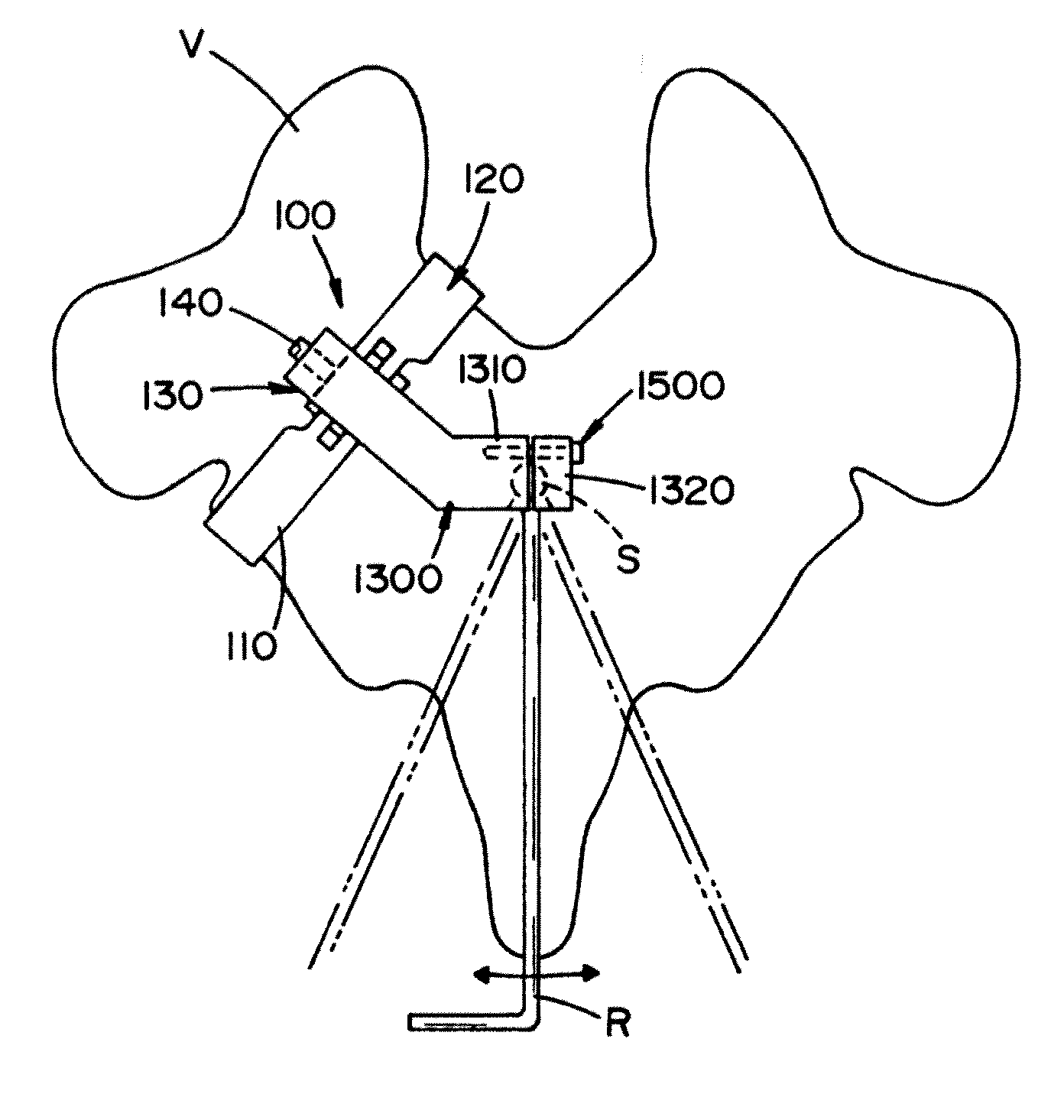
System for endosurgical removal of tumors by laser ablation with treatment verification - particularly tumors of the prostate
InactiveUS20100179522A1High safety factorImprove accuracyUltrasonic/sonic/infrasonic diagnosticsSurgical needlesAbnormal tissue growthHuman body
The disclosed invention is a unique, patient-friendly, laser-based tumor ablation system for the removal of malignant tumors of the prostate and, with modified delivery systems, may have application for other areas of the human body.The disclosed invention is an integrated, robotic treatment subsystem that takes advantage of the capabilities of the previously disclosed MedSci Detection, Mapping and Confirmation System, for the purpose of providing a patient friendly system and method for removing tumors detected by said diagnostic system. The invention is a laser-based endosurgical thermal treatment system that utilizes historical cancer mapping data together with real-time ultrasonic and other data to reliably target and control the eradication of cancer conditions. The system contains computer aided robotic control such that control of the boundary, size, position and orientation of the ablated volume of tissue has a tolerance of less than a millimeter. The disclosed system provides multimodal scanning methods for improved identification and localization of detected tumors, including multi-focal tumors. The disclosed system also provides multiple methods for monitoring the successful progress and conclusion of the treatment. The disclosed system provides the capability of closing the created cavity. The disclosed system resides in a subsystem module and when treatment is to be conducted, the treatment module is substituted in place of the previously disclosed ultrasonic diagnostic module of the MedSci system. The subject thermal treatment system meets the challenges confronting the advancement of thermal treatment systems in the search for a highly effective and patient-friendly cancer treatment.
Owner:MEDSCI TECH INC
Method and apparatus for inhibiting the growth of and shrinking cancerous tumors
InactiveUS20050245850A1Raise the possibilityPrecludes many persons from having their abnormalities corrected or physical attributes enhancedPenis support devicesPneumatic massageAbnormal tissue growthCancer cell
A dome for applying a vacuum to a patient's breast is comprised of a generally rigid dome capable of withstanding a pressure differential, with a rim and rim cushion underlying the rim of the dome for supporting a rim from the patient's skin surface. The rim may be generally wider than the dome in order to distribute the attendant forces across a greater surface and avoid tissue damage. A sticky sole underlies the rim cushion and seals the rim cushion to the patient's skin to thereby preserve the vacuum within the dome. The sticky sole may be comprised of any adhesive material or even be achieved through the use of an appropriate material for the rim cushion itself. A portable pump unit is connected to the domes and maintains the vacuum within the domes during a recommended protocol. By using the device in accordance with the prescribed protocol, the growth of cancer cells and tumors within the breasts are inhibited.
Owner:BRAVA
Treating or preventing the early stages of degeneration of articular cartilage or subchondral bone in mammals using carprofen and derivatives
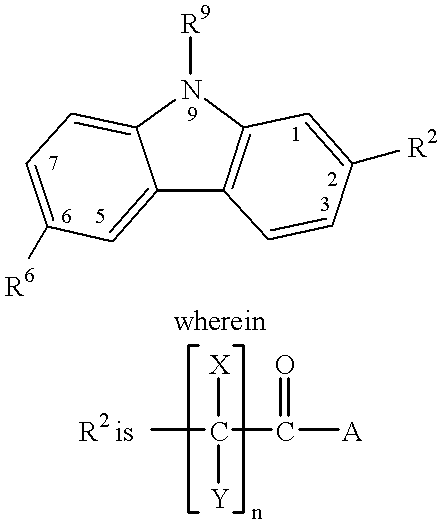
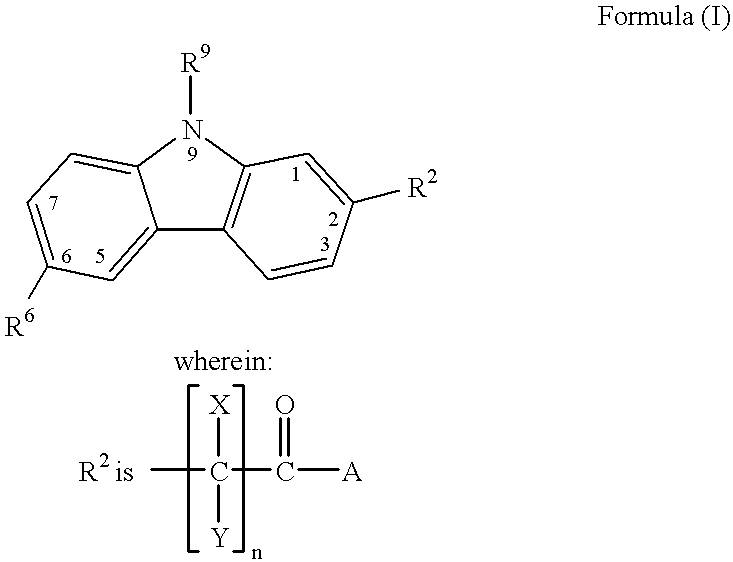
Treating or preventing the early stages of degeneration of articular cartilage or subchondral bone in the affected joint of a mammal is accomplished by administering a chondroprotective compound of Formula (I):where A is hydroxy, (C1-C4)alkoxy, amino, hydroxy-amino, mono-(C1-C2)alkylamino, di-(C1-C2)alkylamino; X and Y are independently H or (C1-C2)alkyl; and n is 1 or 2; R6 is halogen, (C1-C3)alkyl, trifluoromethyl, or nitro; R9 is H; (C1-C2)alkyl; phenyl or phenyl-(C1-C2)alkyl, where phenyl is optionally mono-substituted by fluoro or chloro; -C(=O)-R, where R is (C1-C2)alkyl or phenyl, optionally mono-substituted by fluoro or chloro; or -C(=O)-O-R', where R1 is (C1-C2)alkyl.This treatment ameliorates, diminishes, actively treats, reverses or prevents any injury, damage or loss of articular cartilage or subchondral bone subsequent to said early stage of said degeneration. Whether or not a mammal needs such treatment is determined by whether or not it exhibits a statistically significant deviation from normal standard values in synovial fluid or membrane from the affected joint, with respect to at least five of the following substances: increased interleukin-1 beta (IL-1beta); increased tumor necrosis factor alpha (TNFalpha); increased ratio of IL-1beta to IL-1 receptor antagonist protein (IRAP); increased expression of p55 TNF receptors (p55 TNF-R); increased interleukin-6 (IL-6); increased leukemia inhibitory factor (LIF); decreased insulin-like growth factor-1 (IGF-1); decreased transforming growth factor beta (TGFbeta); decreased platelet-derived growth factor (PDGF); decreased basic fibroblast growth factor (b-FGF); increased keratan sulfate; increased stromelysin; increased ratio of stromelysin to tissue inhibitor of metalloproteases (TIMP); increased osteocalcin; increased alkaline phosphatase; increased cAMP responsive to hormone challenge; increased urokinase plasminogen activator (uPA); increased cartilage oligomeric matrix protein; and increased collagenase.
Owner:PFIZER INC +1
Steerable sonohysterographic injection catheter for uterine cancer sentinel lymph node mapping
A steerable sonohysterographic injection catheter for identifying sentinel lymph nodes in uterine cancer patients includes a tubular shaft housing a needle extendible therefrom. The needle is preferably sized for intrauterine insertion and configured to deliver a fluid such as a contrast media to a tumor location. The catheter preferably includes a sealing device such as a selectively inflatable balloon disposed at a position along the outside of the catheter. The sealing device is configured for placement at an opening of a patient's cervix to prevent the leaking of the contrast media out of the uterus through the cervix. The distal end of the catheter is steerable so as to allow the tip of the needle to be positioned substantially near the tumor location.
Owner:WISCONSIN ALUMNI RES FOUND
Vertebral pars interarticularis clamp a new spine fixation device, instrumentation, and methodology
InactiveUS20110178552A1Improve the inconvenienceExtension of timeInternal osteosythesisJoint implantsSpinal columnTrauma surgery
An improved spinal surgical implant used primarily in the posterior aspect of the spinal column for spinal reconstruction; revision surgery; deformity correction; and / or tumor surgery and / or trauma surgery of the cervical, thoracic and / or lumbo-sacral spine.
Owner:SPINECO
Guidance and implantation of catheters
InactiveUS20100222668A1Ultrasonic/sonic/infrasonic diagnosticsSurgeryProstate cancerNavigation system
Owner:PEAK BIOSCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com