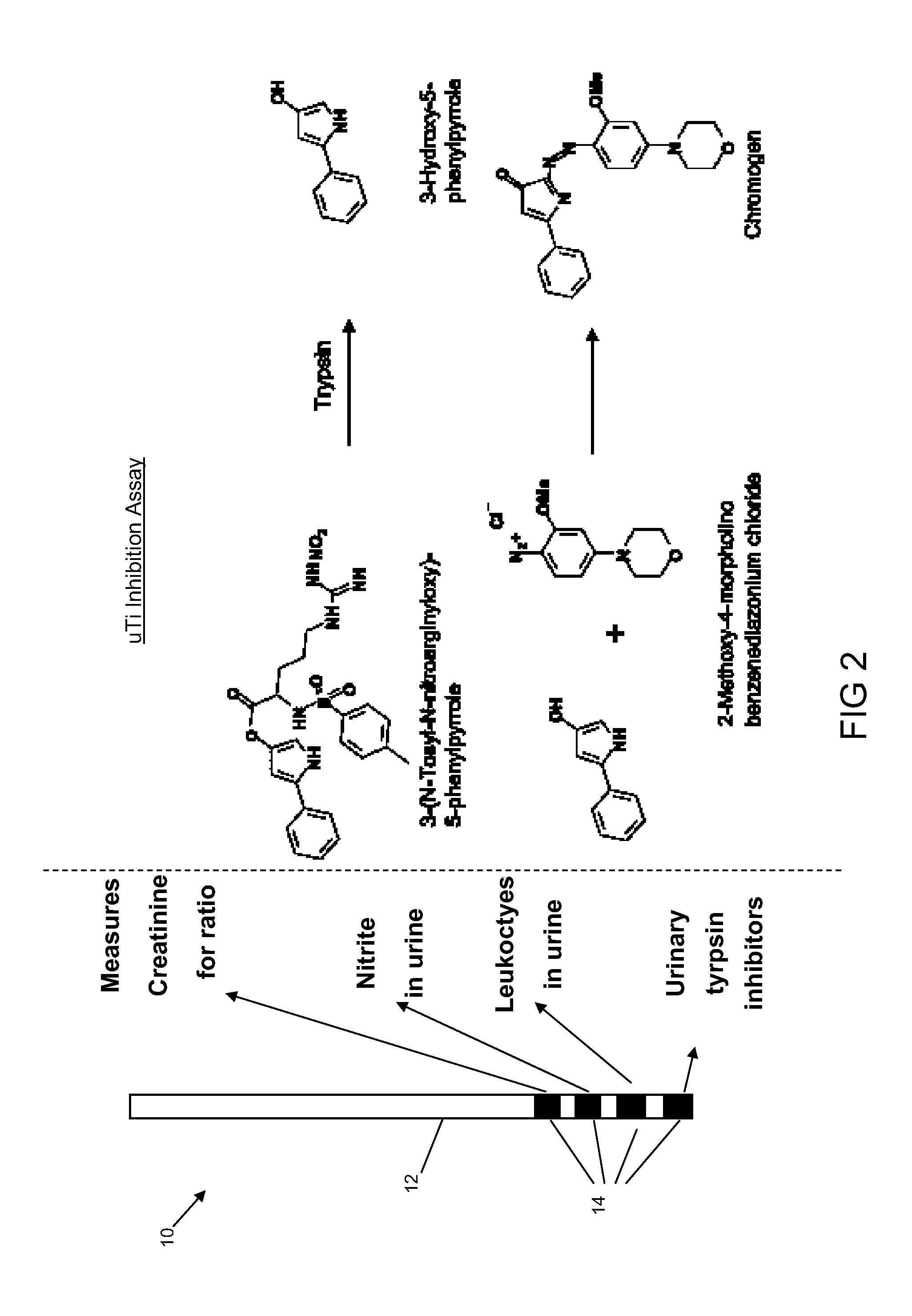
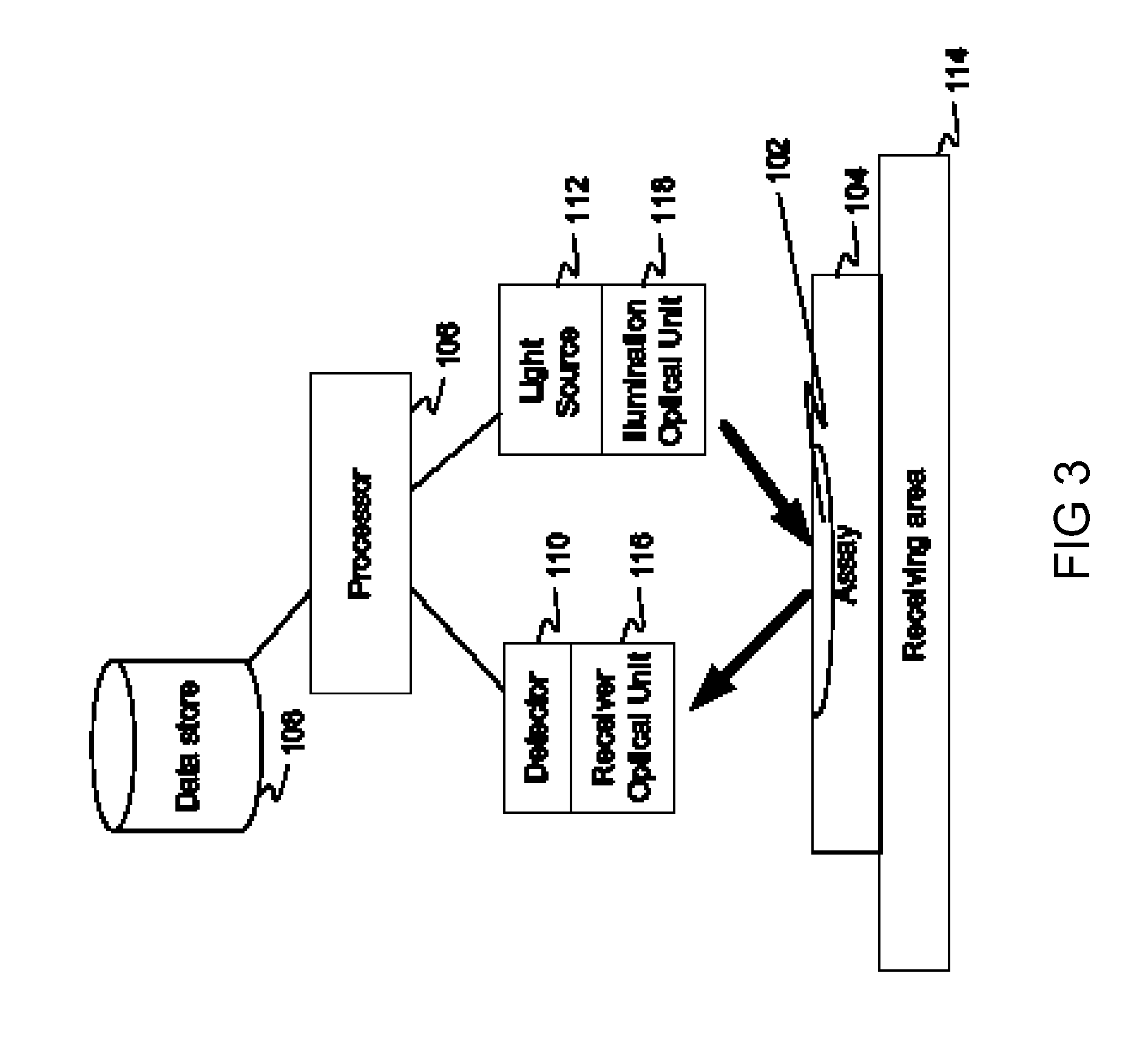
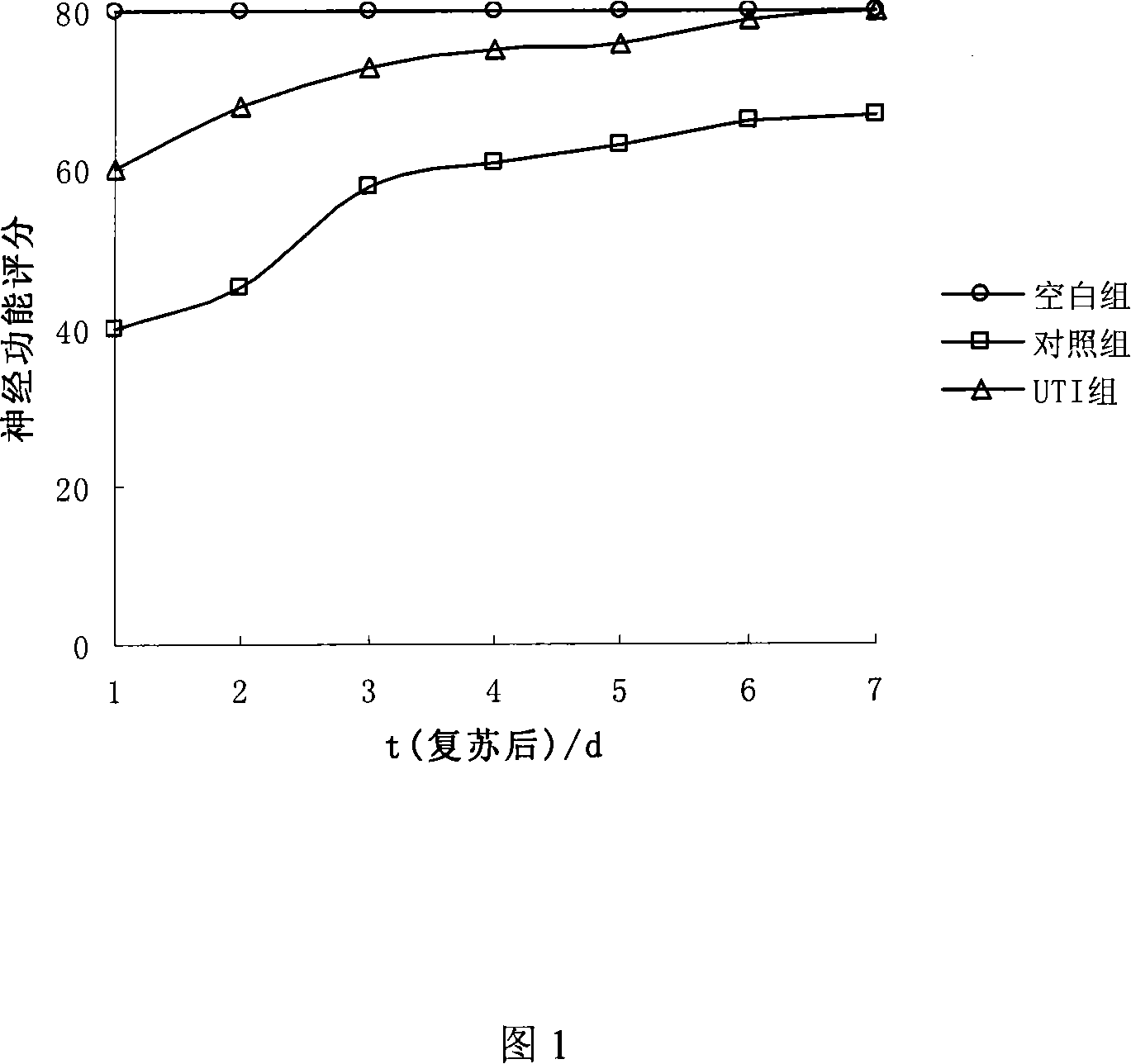
Patents
Literature
32 results about "URINARY TRYPSIN INHIBITOR" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
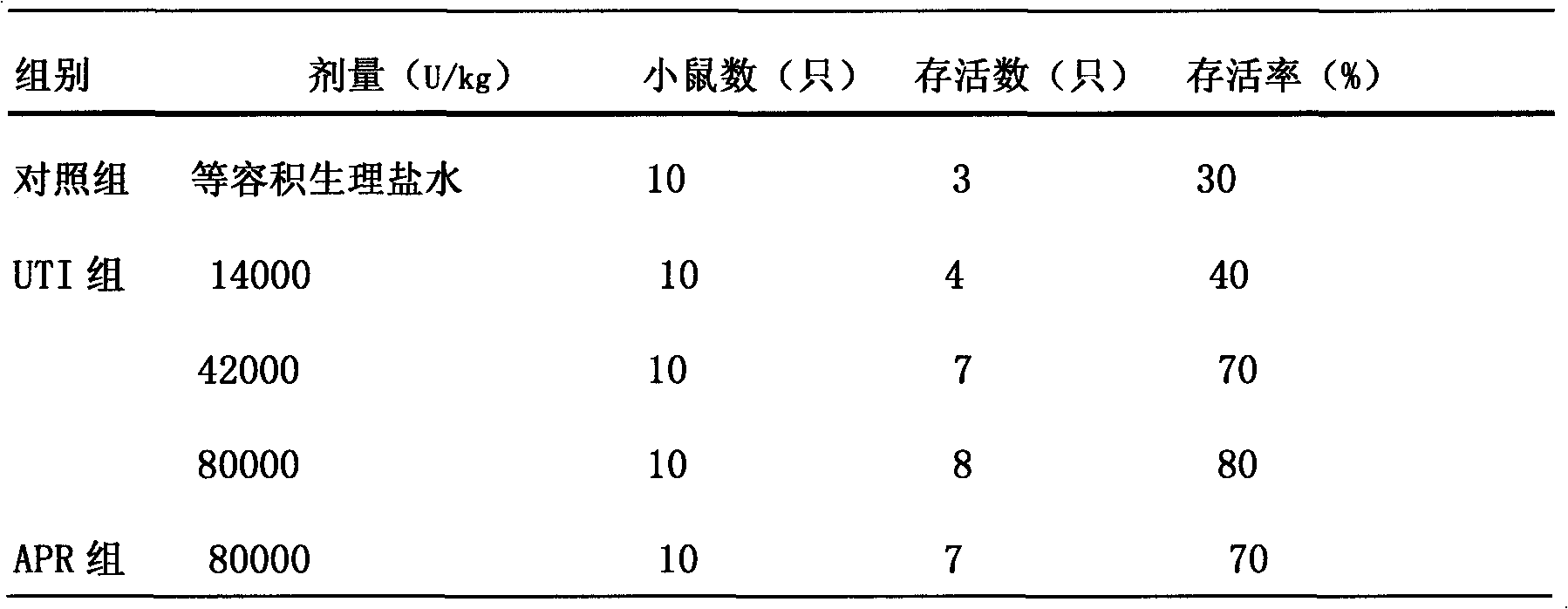
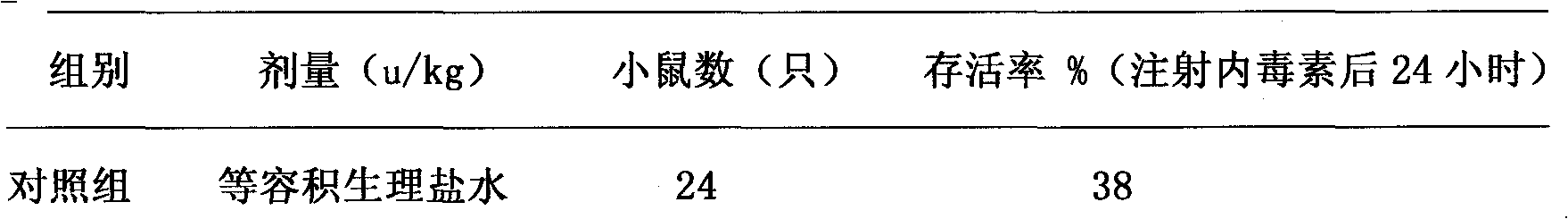
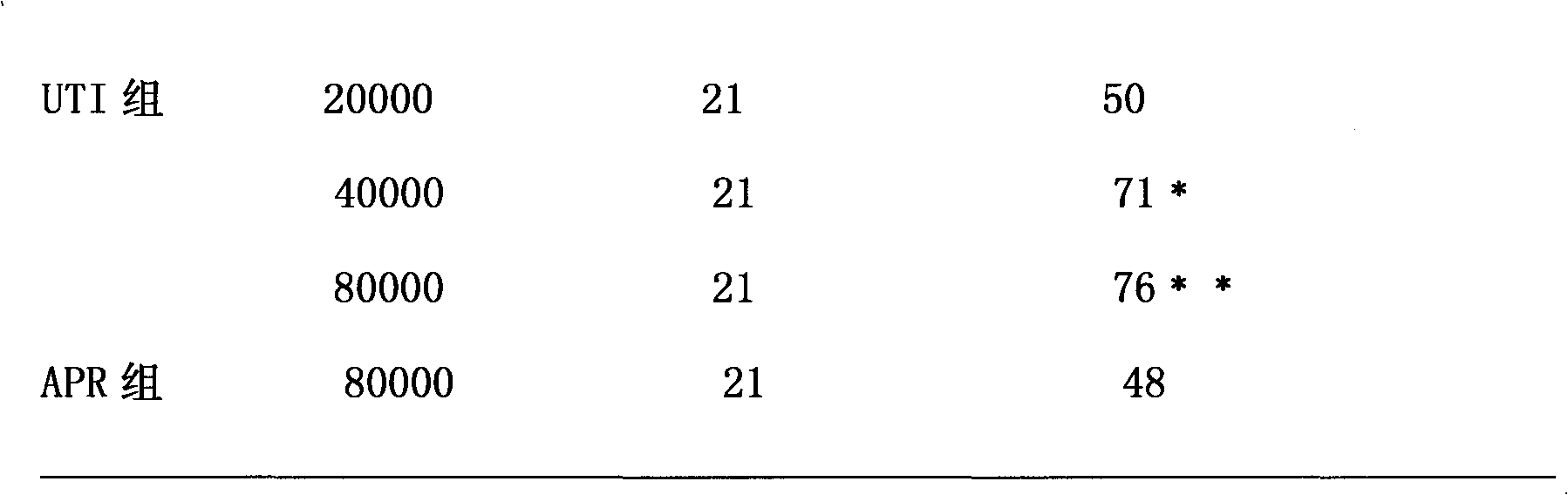
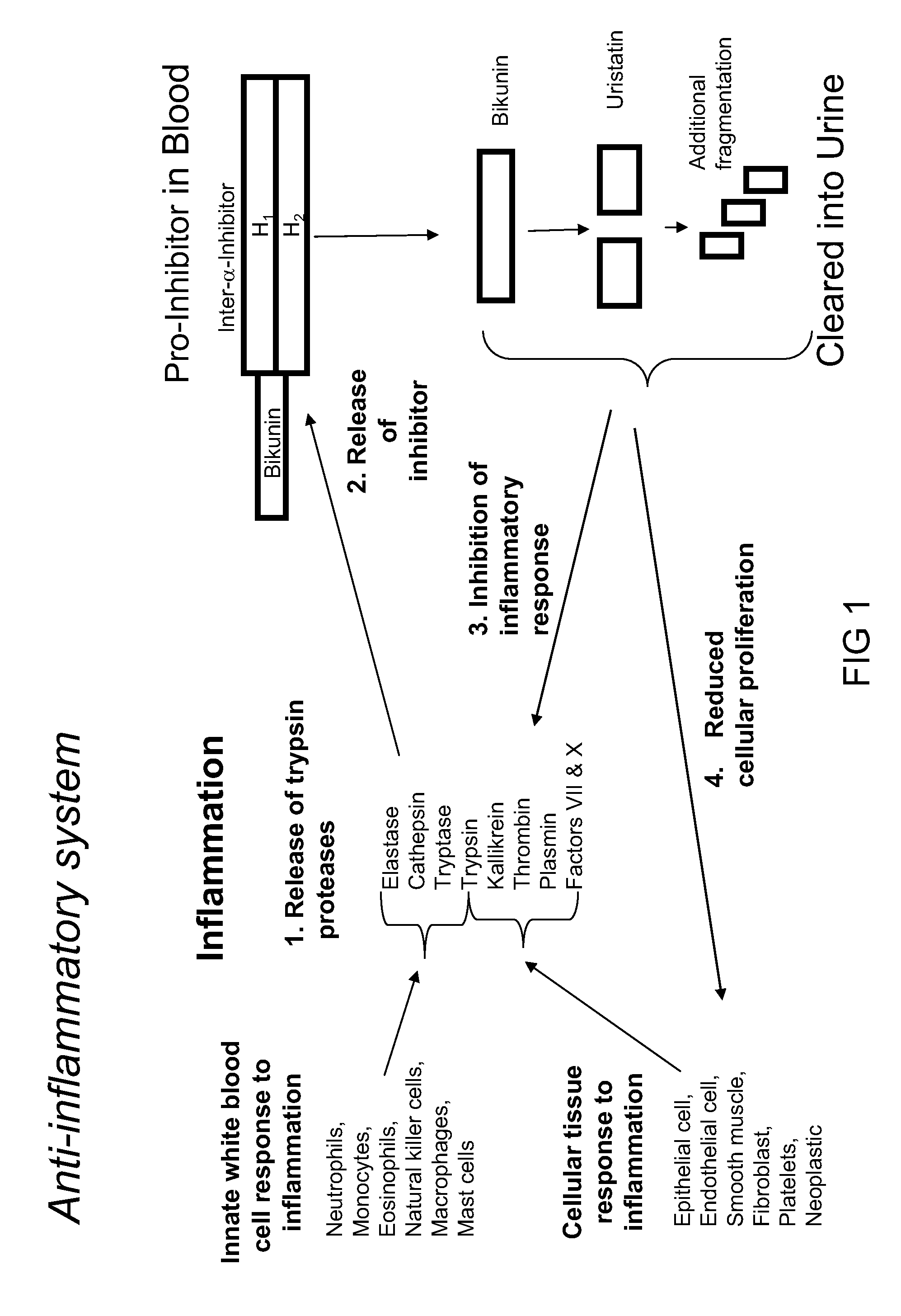
Ulinastatin, as an urinary trypsin inhibitor (UTI), is a glycoprotein that is isolated from healthy human urine or synthetically produced and has molecular weight of 25 - 25kDa.
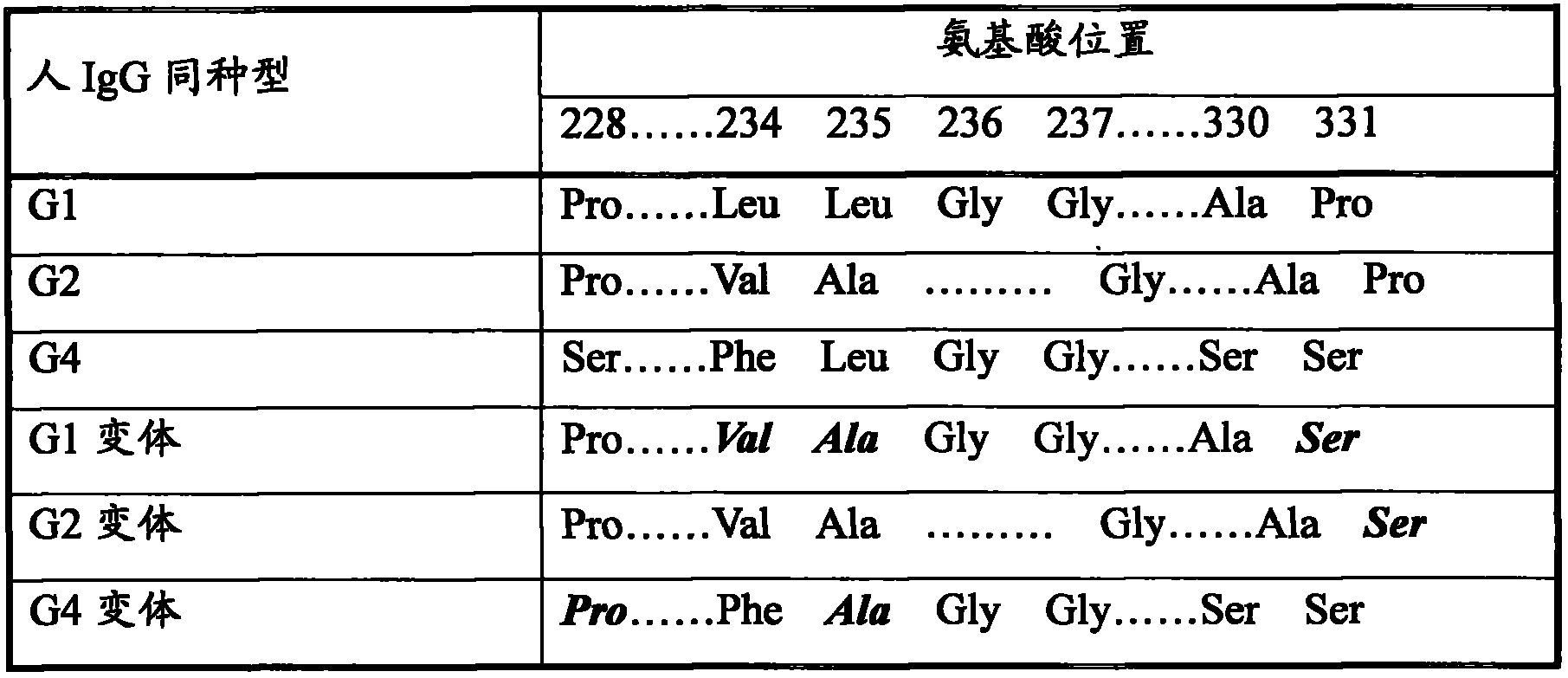
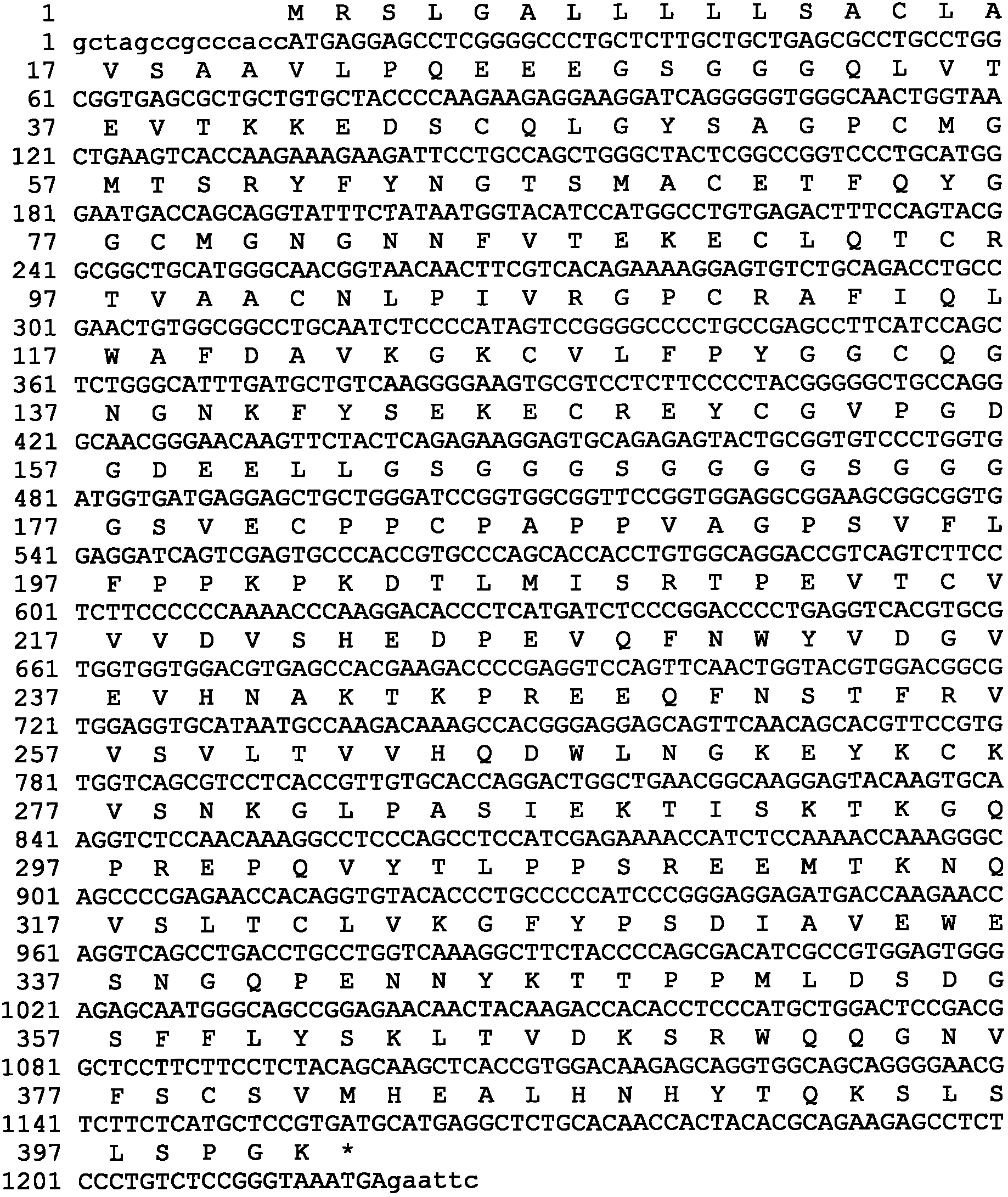
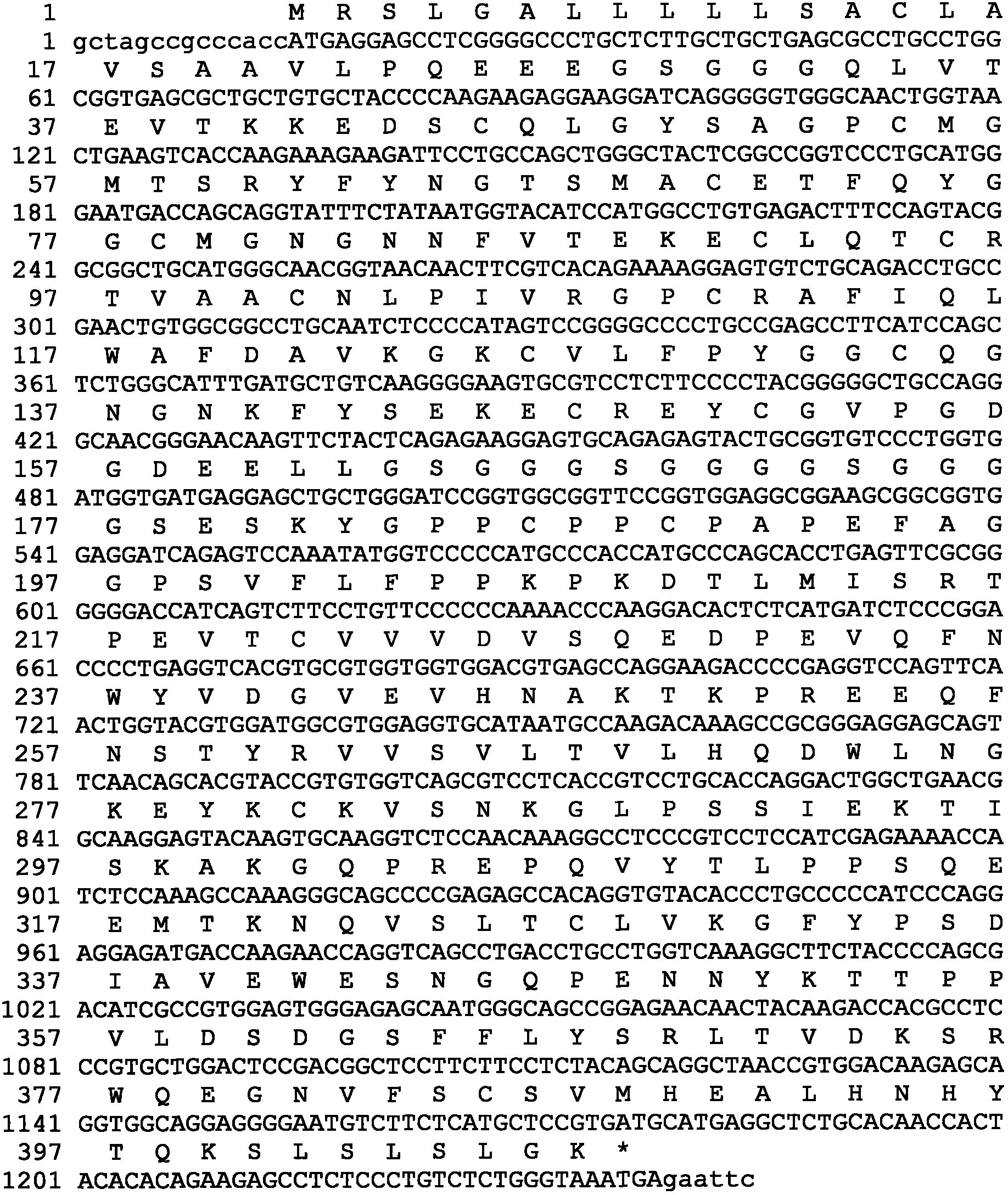
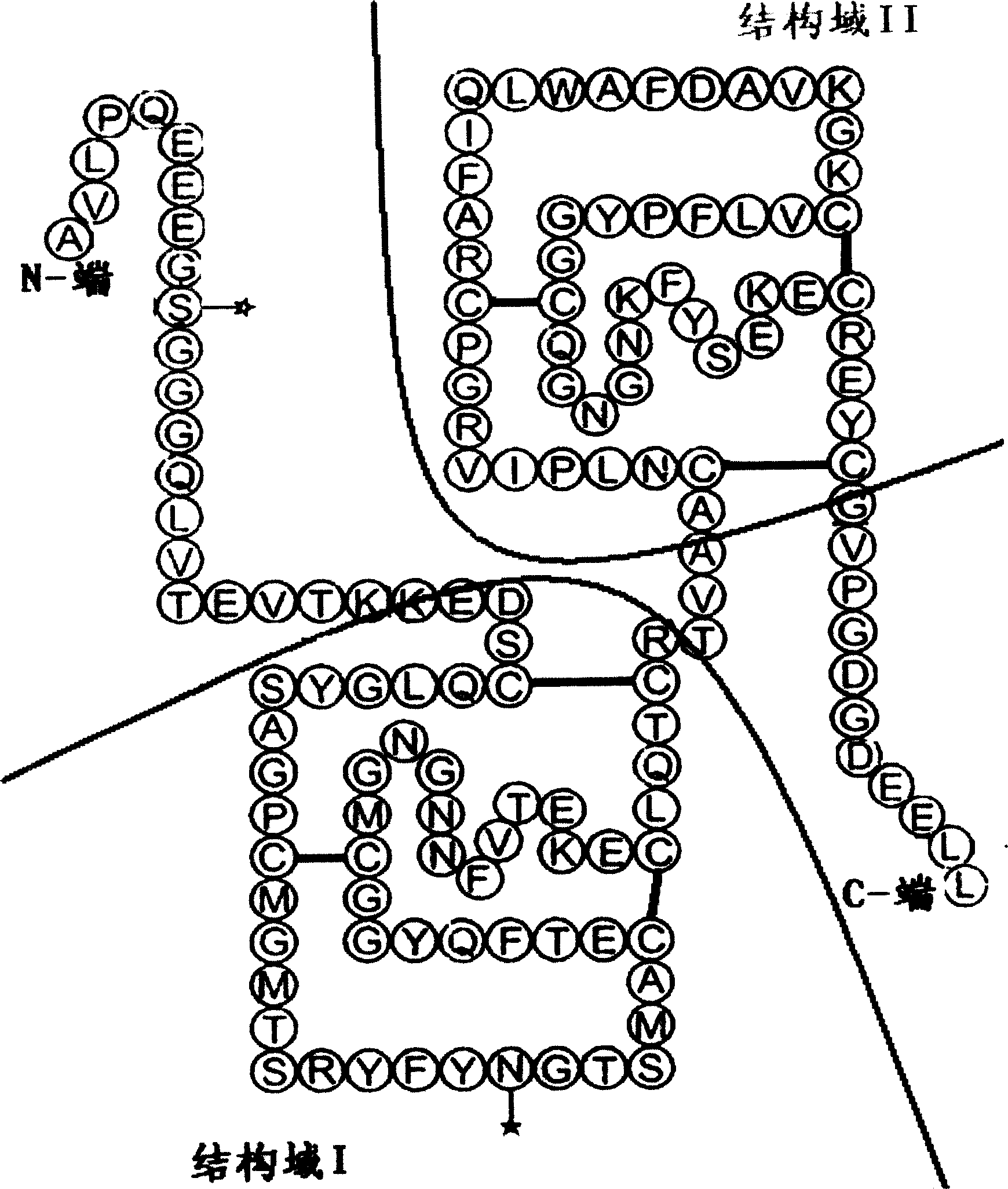
Human urinary trypsin inhibitor (hUTI) of reorganization-dimerization and preparation method and application thereof
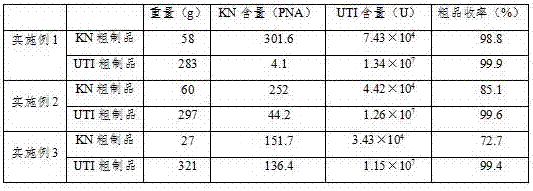
ActiveCN103044554AIncrease productionEfficient and convenient purificationPeptide/protein ingredientsAntipyreticURINARY TRYPSIN INHIBITORHalf-life
The invention discloses a human urinary trypsin inhibitor (hUTI) of reorganization-dimerization and preparation method and application thereof, wherein the hUTI protein of reorganization-dimerization successively comprises amino acid residue sequence of human UTI, a peptide linker and a human IgGFc variant from N-terminal to C-terminal. The hUTI protein of reorganization-dimerization has the in-vitro biological activity similar with that of the human urinary trypsin inhibitor, higher biological activity in vivo and an extended half-life period in vivo.
Owner:PHARMAB
Method for enriching urine protein directly
InactiveCN101863974AAvoid the collection stepLow costHydrolasesPeptide preparation methodsURINARY TRYPSIN INHIBITORUrokinase Plasminogen Activator
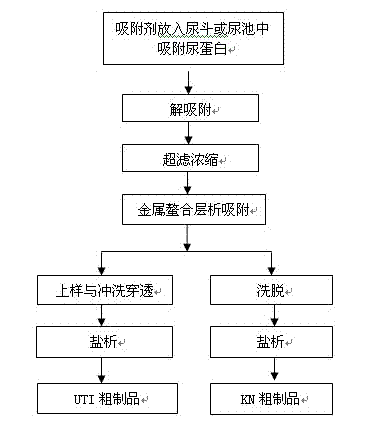
The invention relates to a urine protein enriching method. In the method, urine protein in urine is absorbed directly by an adsorbent in a urinal or a pee hopper, and the adsorbent for adsorbing the urine protein is conveyed to a working point for follow-up treatment; and based on the isoelectric point properties of specific urine protein, the urine protein such as urinary trypsin inhibitor, human urinary kininogenase, urokinase and the like is adsorbed directly and effectively by using the specific adsorbent such as ion exchange resin, and thus, the step of collecting the urine is avoided. The method does not influence sanitation of toilets obviously, reduces the cost of urine transportation substantially and solves a series of environmental problems.
Owner:GUANGDONG TECHPOOL BIO-PHARMA CO LTD +1
Process for preparing medicine grade recombined human urine trypase inhibitor
The extraction human urinary trypsase inhibitor (hUTI) from urea has been widely applied clinically. The present invention describes how to constitute fusion protein engineering strain capable of secreting and expressing recombinant human urinary trypsase inhibitor (rhUTI) in Pichia pastoris. Through fermentation, rough purification, restriction, fine purification and serial property comparison tests, engineering strain with yield, purity and pharmacological characteristic meeting the requirement of large scale production and clinical application and rhUTI producing process are finally determined.
Owner:上海万兴生物制药有限公司
Method for preparing urokinase
ActiveCN105087531AImprove adsorption capacitySimple structurePeptidasesURINARY TRYPSIN INHIBITORSilica gel
The invention relates to a urinary protein enriching method which comprises the steps of directly adsorbing urinary proteins in urine in urinals or urinating buckets with filter cloth bags loaded with modified silica gel, and transporting the urinary protein adsorbed filter cloth bags to working points for subsequent treatment. According to the method, the isoelectric point property of specific urinary proteins is utilized, and urinary proteins such as urinary trypsin inhibitors, human urinary kininogenase, urokinase and the like are effectively absorbed directly by using the modified silica gel, macroporous resin, chitin, ionic resin and the like, so that a urine collecting step is avoided. The method is free of obvious influence on sanitary conditions of toilets, and the cost for urine transportation and a series of environmental problems resulting from urine transportation are greatly reduced.
Owner:GUANGDONG TECHPOOL BIO-PHARMA CO LTD
Affinity medium for human urinary trypsin inhibitor as well as synthesis and application for same
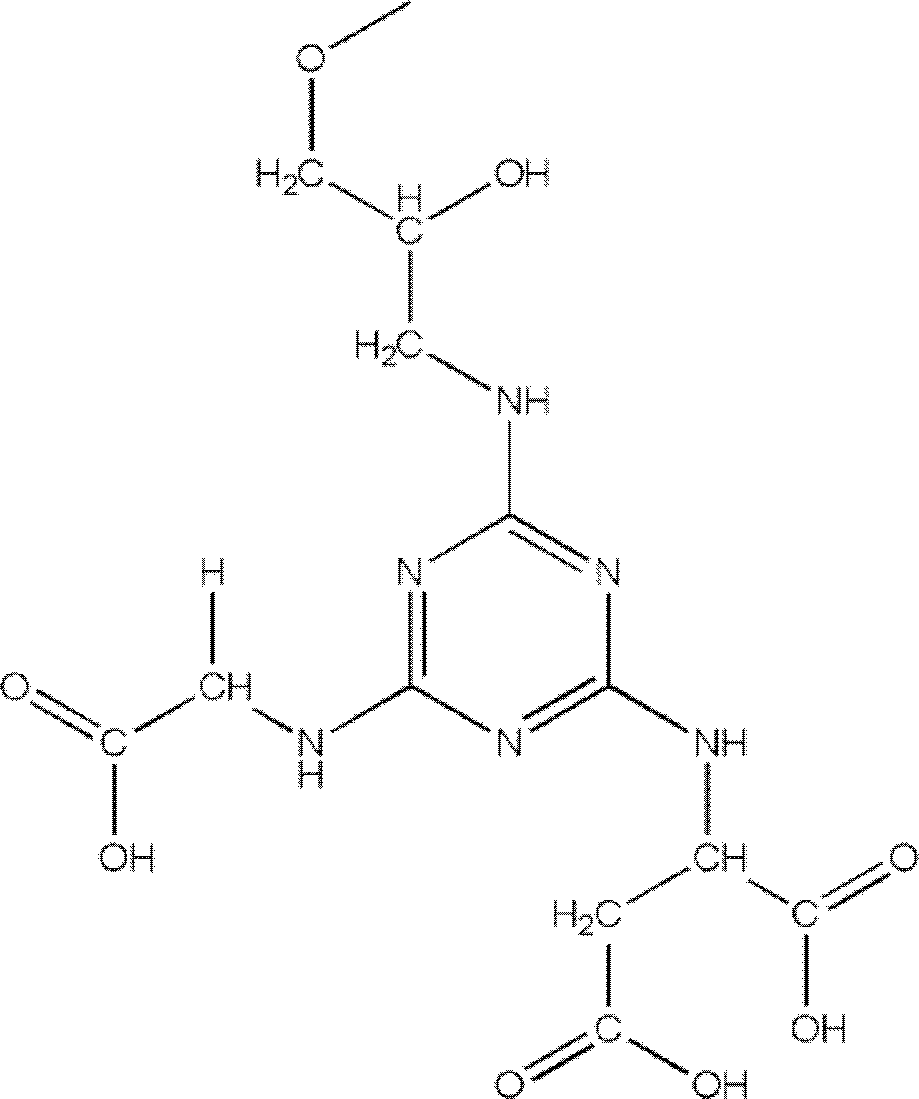
ActiveCN102516387AEfficient purificationImprove purification efficiencyPeptide preparation methodsProtease inhibitorsURINARY TRYPSIN INHIBITORSynthesis methods
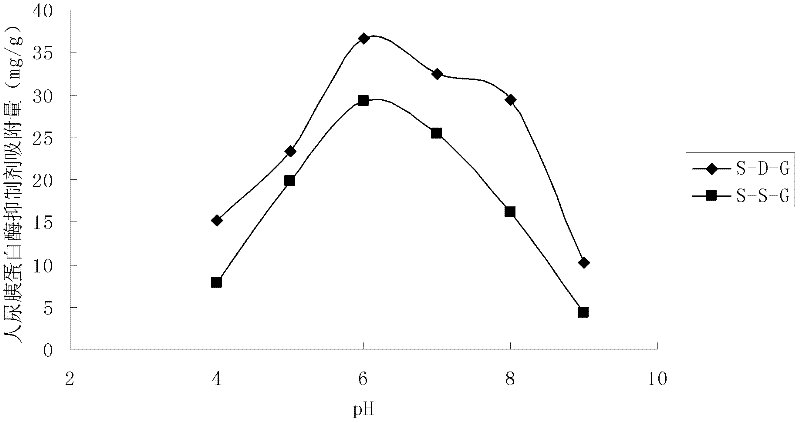
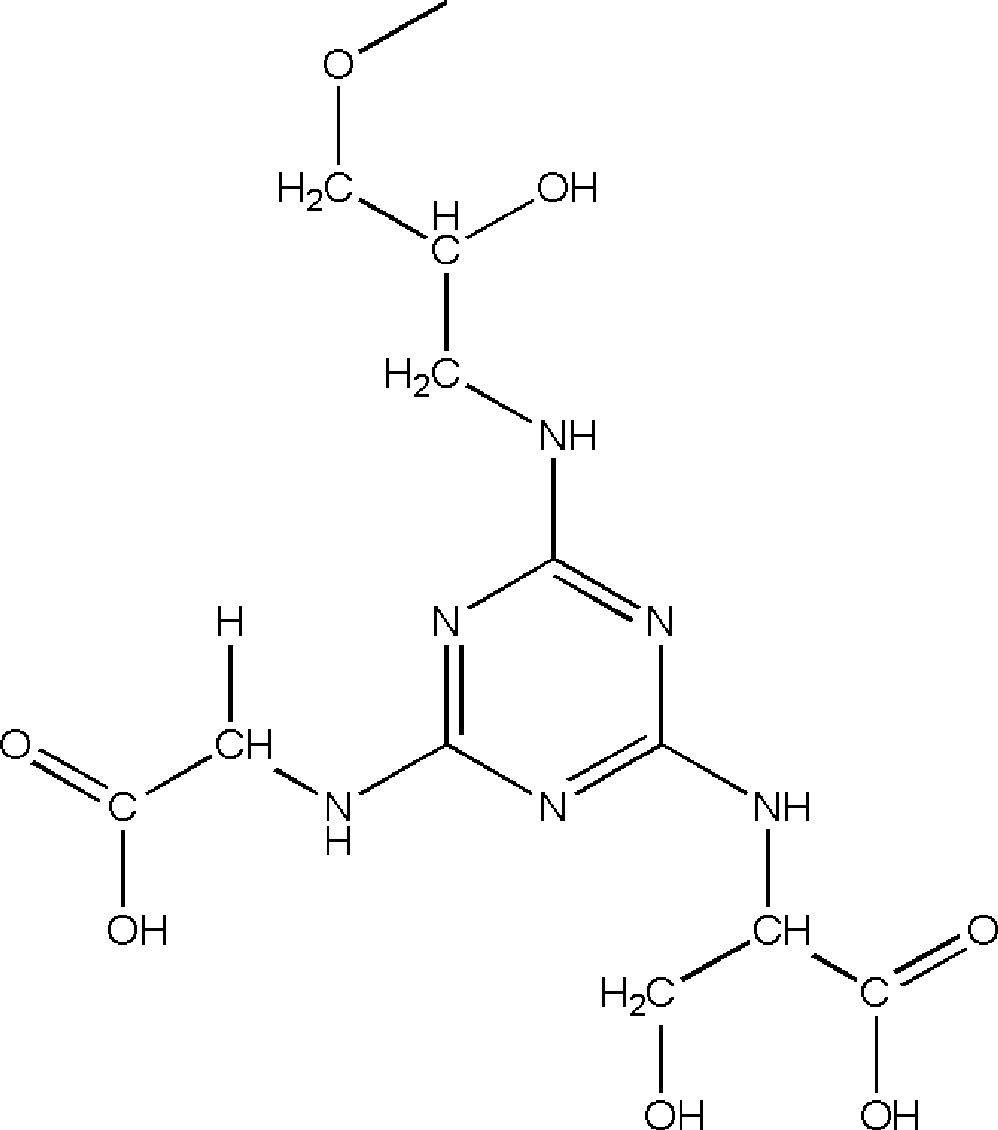
The invention relates to an affinity medium for human urinary trypsin inhibitor, formed by connecting an affinity ligand for human urinary trypsin inhibitor with a chromatography medium. Preferably, the affinity ligand for human urinary trypsin inhibitor is an analogue of the site S1 of serine protease, the chromatography medium is Sepharose, chitosan, silica gel and ceramic particles, and the affinity ligand for human urinary trypsin inhibitor is connected with the chromatography medium by using cyanuric chloride as a spacer arm. A synthesis method for the affinity medium for human urinary trypsin inhibitor, and a method for purifying human urinary trypsin inhibitor in a large scale by using the affinity medium for human urinary trypsin inhibitor are further provided. Human urinary trypsin inhibitor can be rapidly separated and purified in a large scale by using the affinity medium for human urinary trypsin inhibitor, and human urinary trypsin inhibitor pure product can be obtained in case of only one-step affinity chromatography; and the human urinary trypsin inhibitor pure product is short in the consumed time of whole purification step, high in the recovery rates of protein and activity, and suitable for large-scale popularization and application.
Owner:宁夏妙朗生物科技有限公司
Monoclonal antibodies for detection of urinary trypsin inhibitors
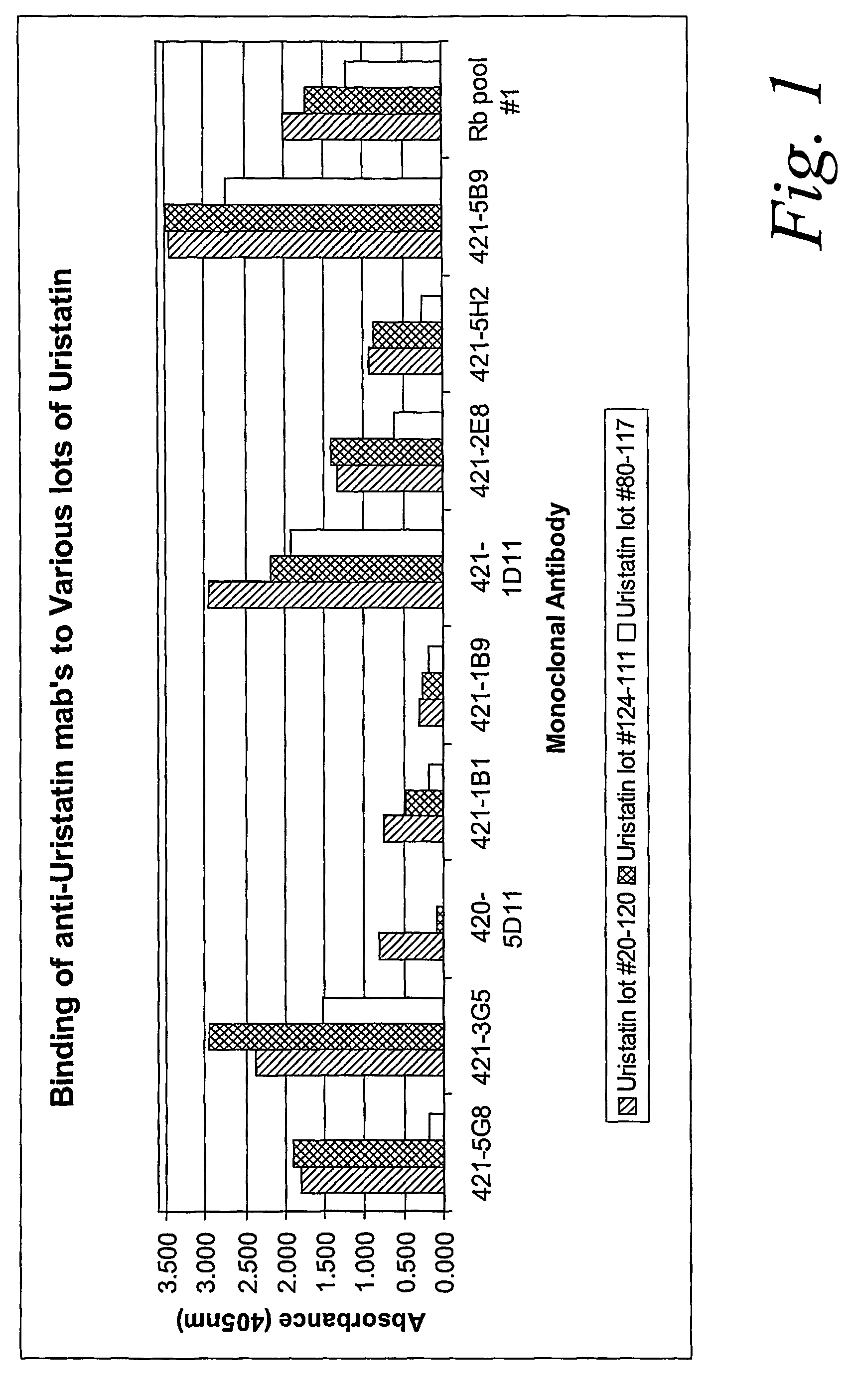
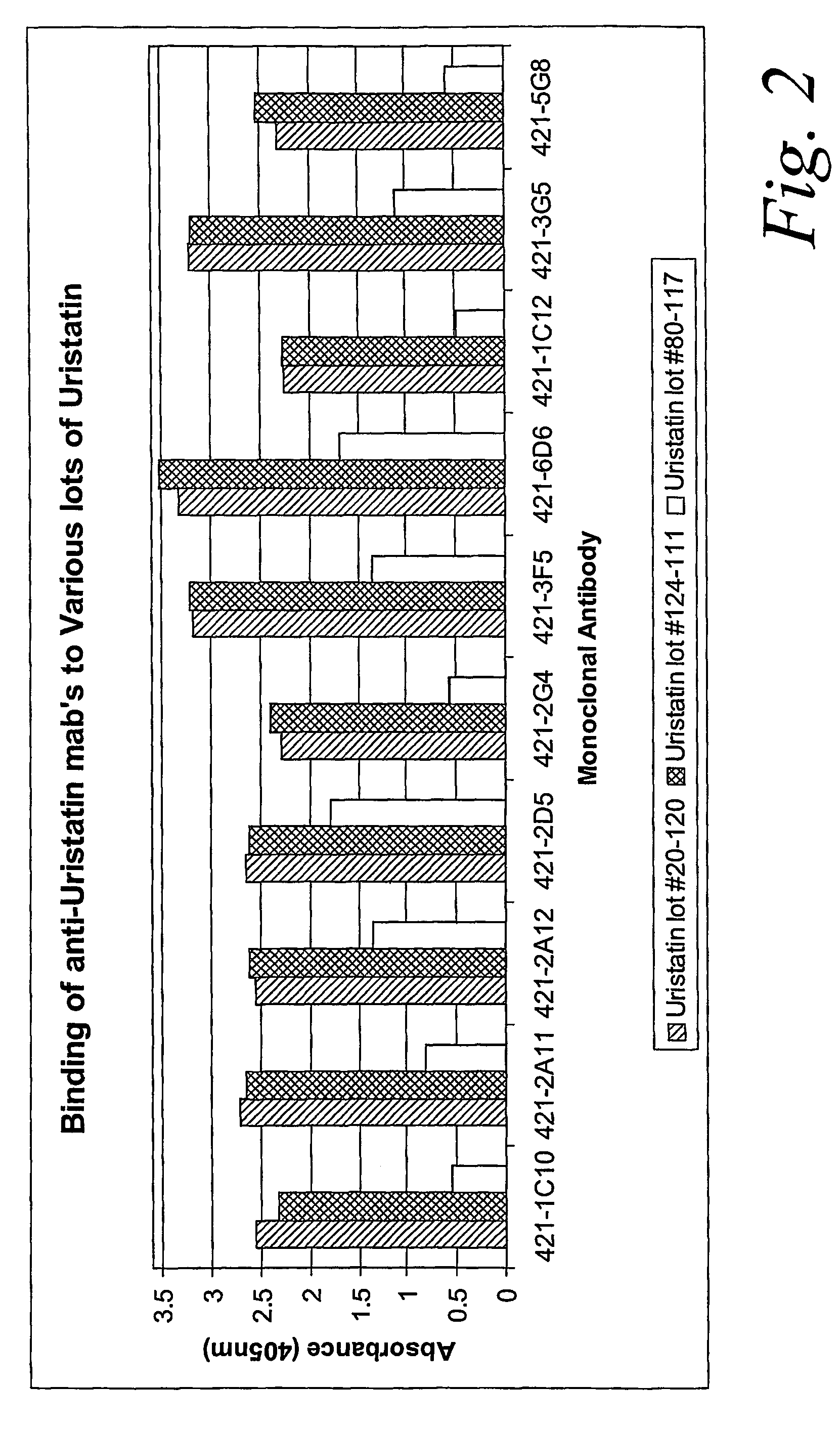
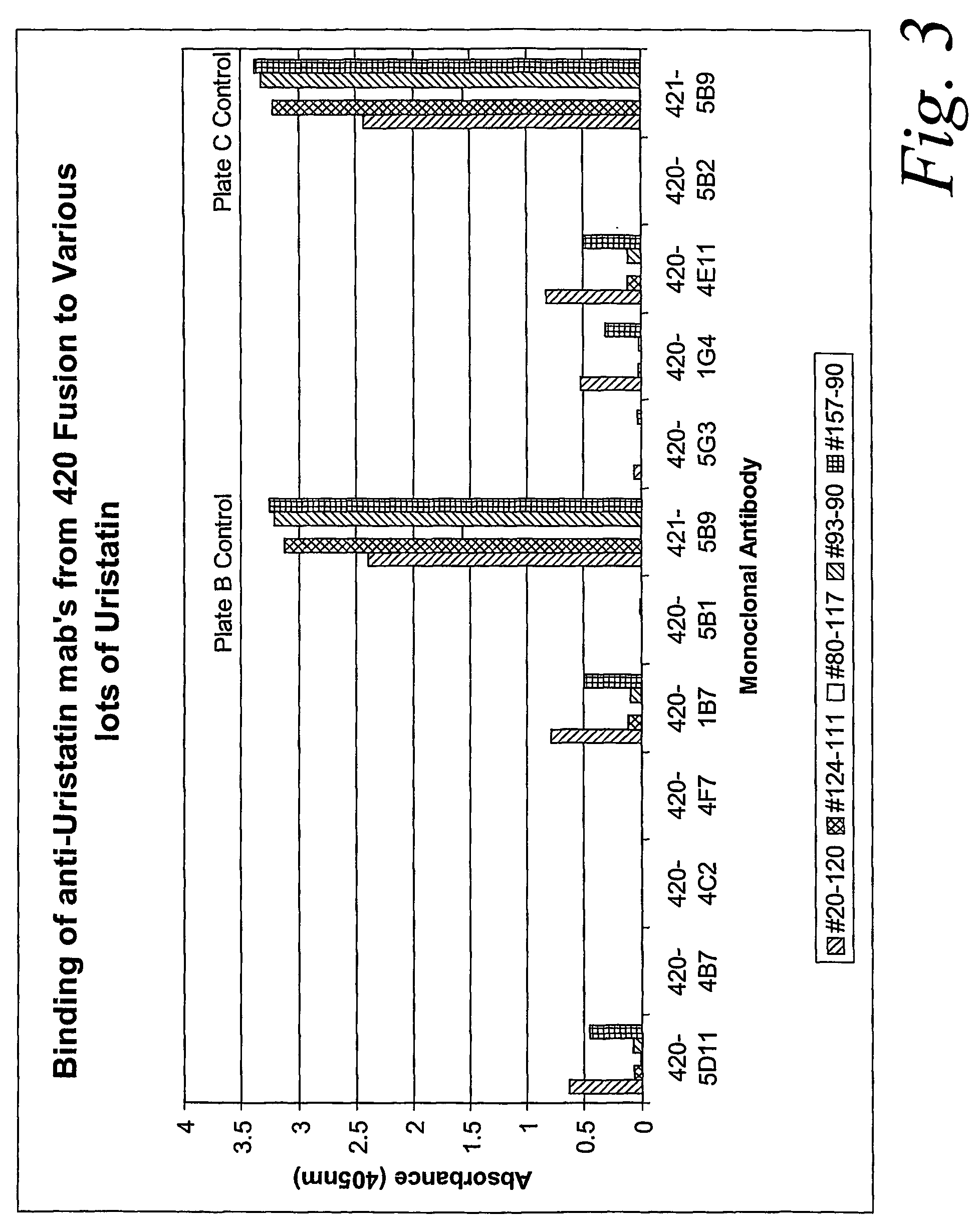
ActiveUS20070020683A1Immunoglobulins against animals/humansBiological material analysisURINARY TRYPSIN INHIBITORMonoclonal antibody
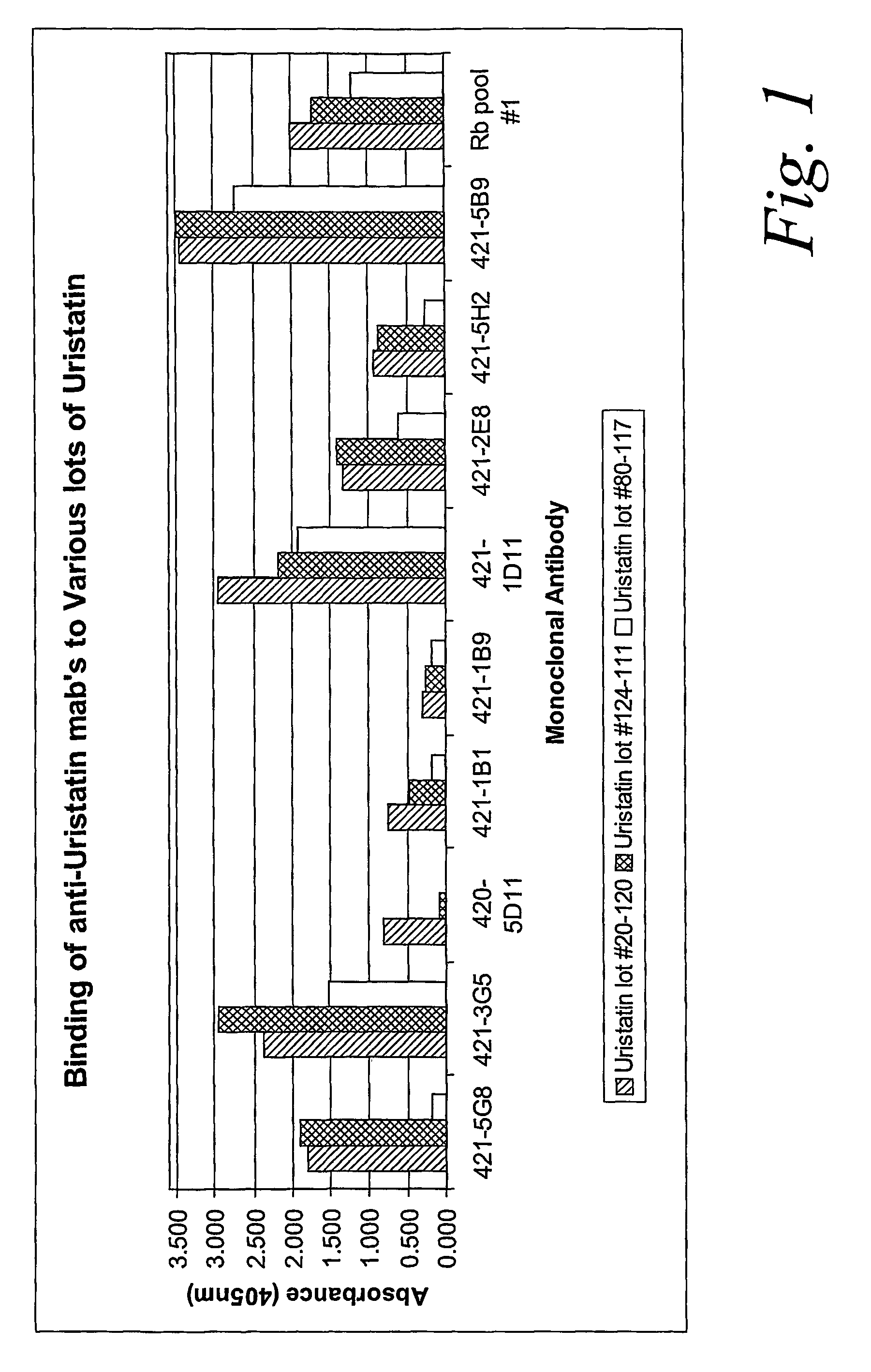
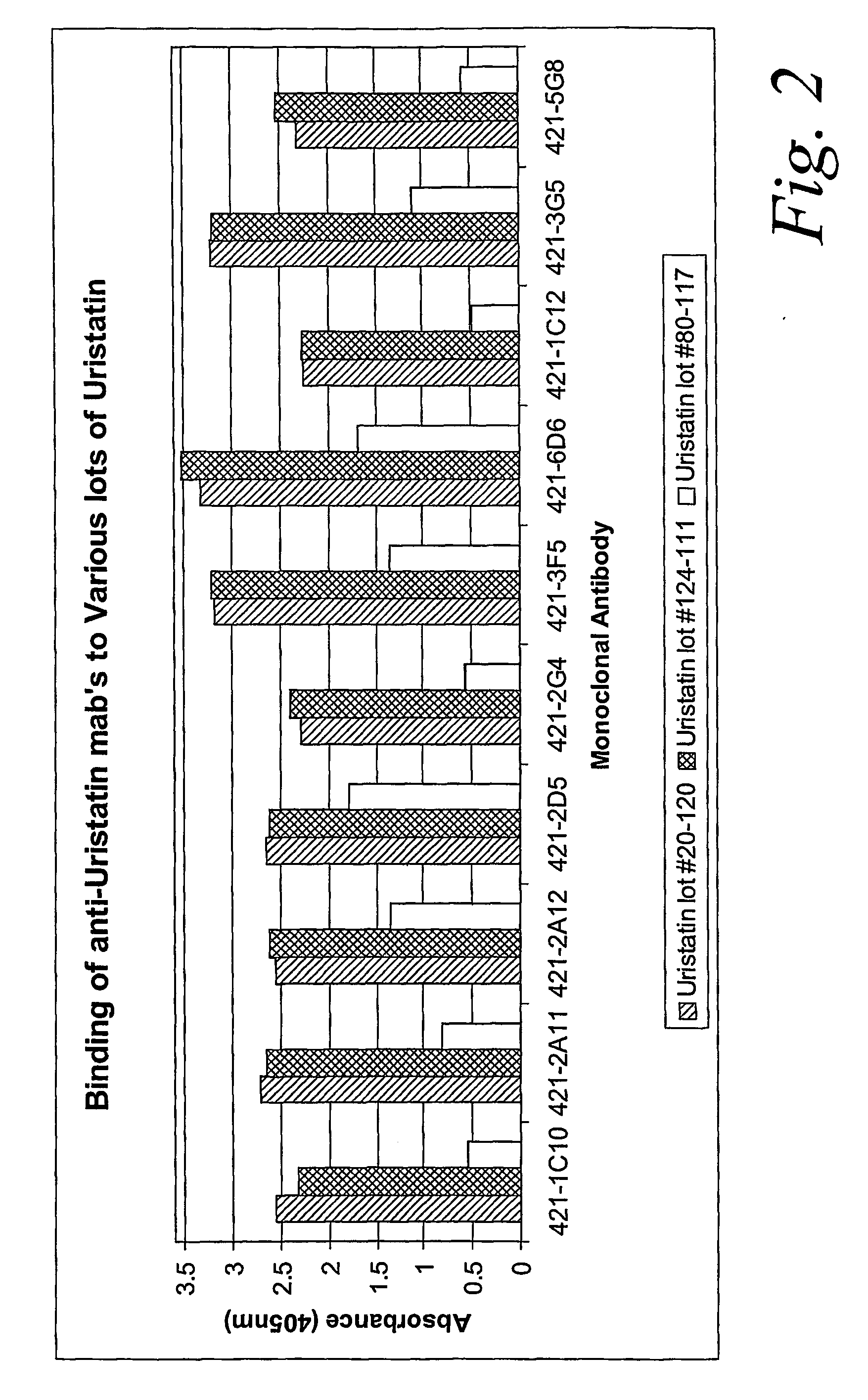
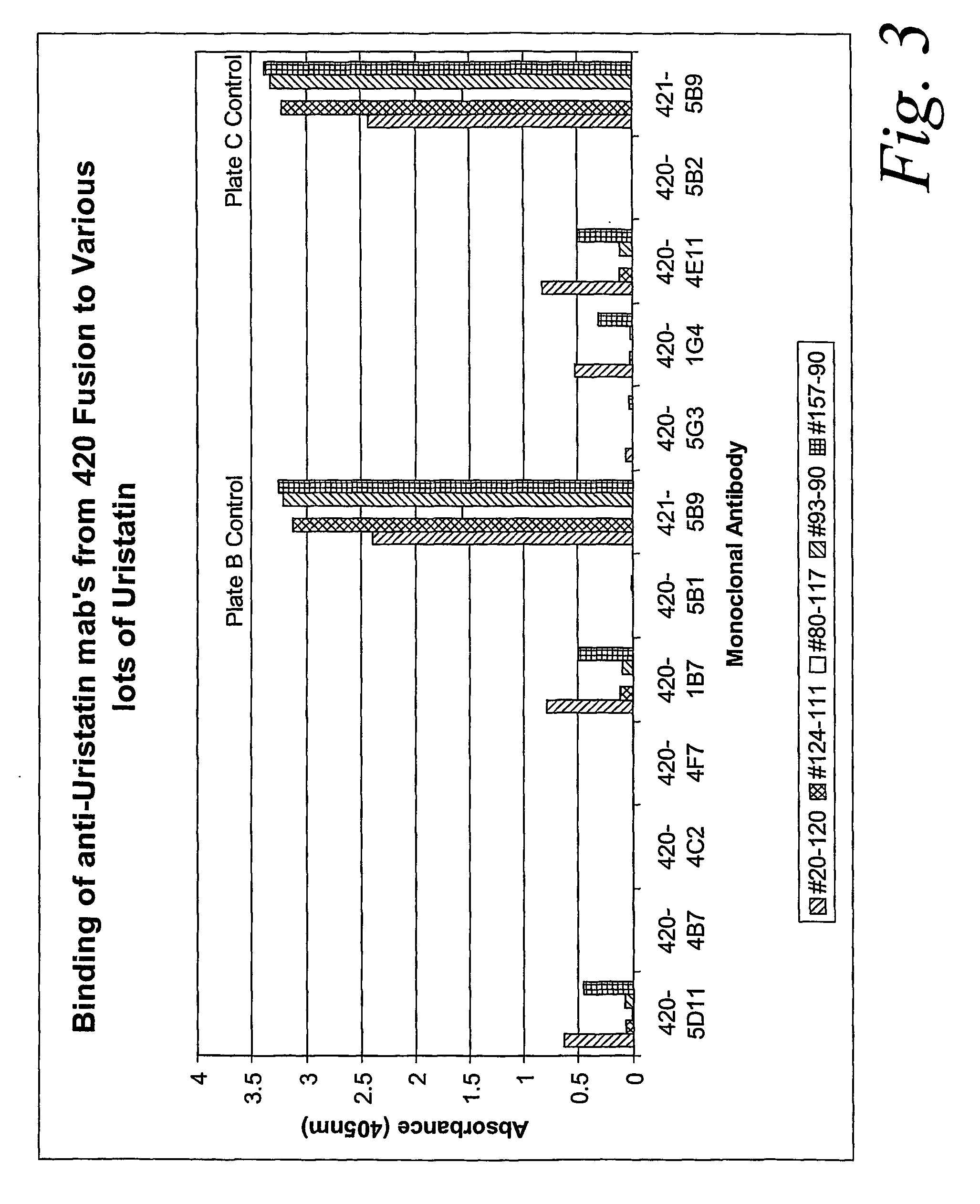
Certain monoclonal antibodies are able to detect urinary trypsin inhibitors (UTIs) that are characteristic of disease in humans. In particular, the UTIs include AMBK, Bikunin, Uristatin, Uristatin-1, Uristatin-2, as defined herein, also including the fragments and aggregates thereof.
Owner:SIEMENS HEALTHCARE DIAGNOSTICS INC
Application of ulinastatin serving as rescue auxiliary medicament for toxic shock caused by acute abdomen
InactiveCN102205115APeptide/protein ingredientsDigestive systemURINARY TRYPSIN INHIBITORIntestinal loops
The invention relates to a rescue medicament in the field of medical treatment, in particular to a rescue medicament, namely ulinastatin or a urinary trypsin inhibitor (UTI) for toxic shock caused by acute abdomen (particularly mesenteric angiemphraxis and the like). The medicament is a preferred medicament for the toxic shock. In the pharmacodynamic study of the ulinastatin for resisting shock, the ulinastatin also can improve the average arterial blood pressure and the average arterial blood flow of superior mesenteric artery occlusion shock of rats, and has an extremely better effect on shock rescue.
Owner:徐宗昆 +3
Medicinal composition for treating arthritis
ActiveCN1751740AEasy to useUse progressPeptide/protein ingredientsSkeletal disorderGlycineURINARY TRYPSIN INHIBITOR
A composite medicine in the form of freeze-dried powder injection or liquid injection for treating osteoarthritis and rheumatoid arthritis is prepared from ulinastatin (a human Urinary trypsin inhibitor), sodium hyaluronate and optional mannitol, glycine and dextran.
Owner:GUANGDONG TECHPOOL BIO-PHARMA CO LTD
Urinary trypsin inhibitors as diagnostic aid for interstitial cystitis
InactiveUS20120115174A1Bioreactor/fermenter combinationsBiological substance pretreatmentsURINARY TRYPSIN INHIBITORInterstitial cystitis
A method aiding the diagnosis of interstitial cystitis involving the combination of an infection marker and an inflammation marker. More specifically, the method includes correlating the presence of urinary trypsin inhibitors in urine with the absence of traditional infection markers in urine to aid in the diagnosis of interstitial cystitis. The method provides for a differential diagnosis between kidney disease, infection and chronic inflammation with a noninvasive urine test. Assay devices and kits, as well as analyzers and systems are also described that utilize the methodology.
Owner:SIEMENS HEALTHCARE DIAGNOSTICS INC
Use of ulinastain in the preparing of treating and/or preventing sudden arrest of heart beat tritocerebrum damnification medicine
InactiveCN101095948AAvoid damagePowder deliveryNervous disorderURINARY TRYPSIN INHIBITORFreeze-drying
The invention relates to the application of Urinary Trypsin Inhibitor (UTI)for preparing medicine that can prevent or treat brain damage after sudden arrest of heart beat. The effect is distinctive. The medicine is generally prepared into freeze dried or injection.
Owner:GUANGDONG TECHPOOL BIO-PHARMA CO LTD
Method for separating urokinase from human urinary trypsin inhibitor
ActiveCN103074319AHigh yieldEasy to separatePeptide preparation methodsProtease inhibitorsURINARY TRYPSIN INHIBITORBiochemistry
The invention discloses a method for separating urokinase from a human urinary trypsin inhibitor. The method comprises the following steps: regulating pH of a solution containing the urokinase and the human urinary trypsin inhibitor; slowly adding ammonium sulfate powder while stirring until the solution generates obvious protein precipitation; regulating the pH to 9.0 plus or minus 0.6; after standing, centrifuging or adding diatomite for filtering, collecting the precipitation rich in the urokinase, and obtaining the urokinase; continuing to adding ammonium sulfate into supernatant while stirring to dissolve the ammonium sulfate; and after standing, centrifuging or adding diatomite for filtering, collecting the precipitation 2 rich in the human urinary trypsin inhibitor, and obtaining the human urinary trypsin inhibitor. The method is simple, convenient and feasible and has a short period, the UK and UTI activity is effectively protected, the UK degradation is reduced, the UK quality is increased, special equipment is not needed, and the invested cost of the equipment is reduced.
Owner:YANGZHOU AIDEA BIOTECH
Production method of recombined human urinary trypsin inhibitor
InactiveCN103014057AMicroorganism based processesVector-based foreign material introductionURINARY TRYPSIN INHIBITORPhosphate
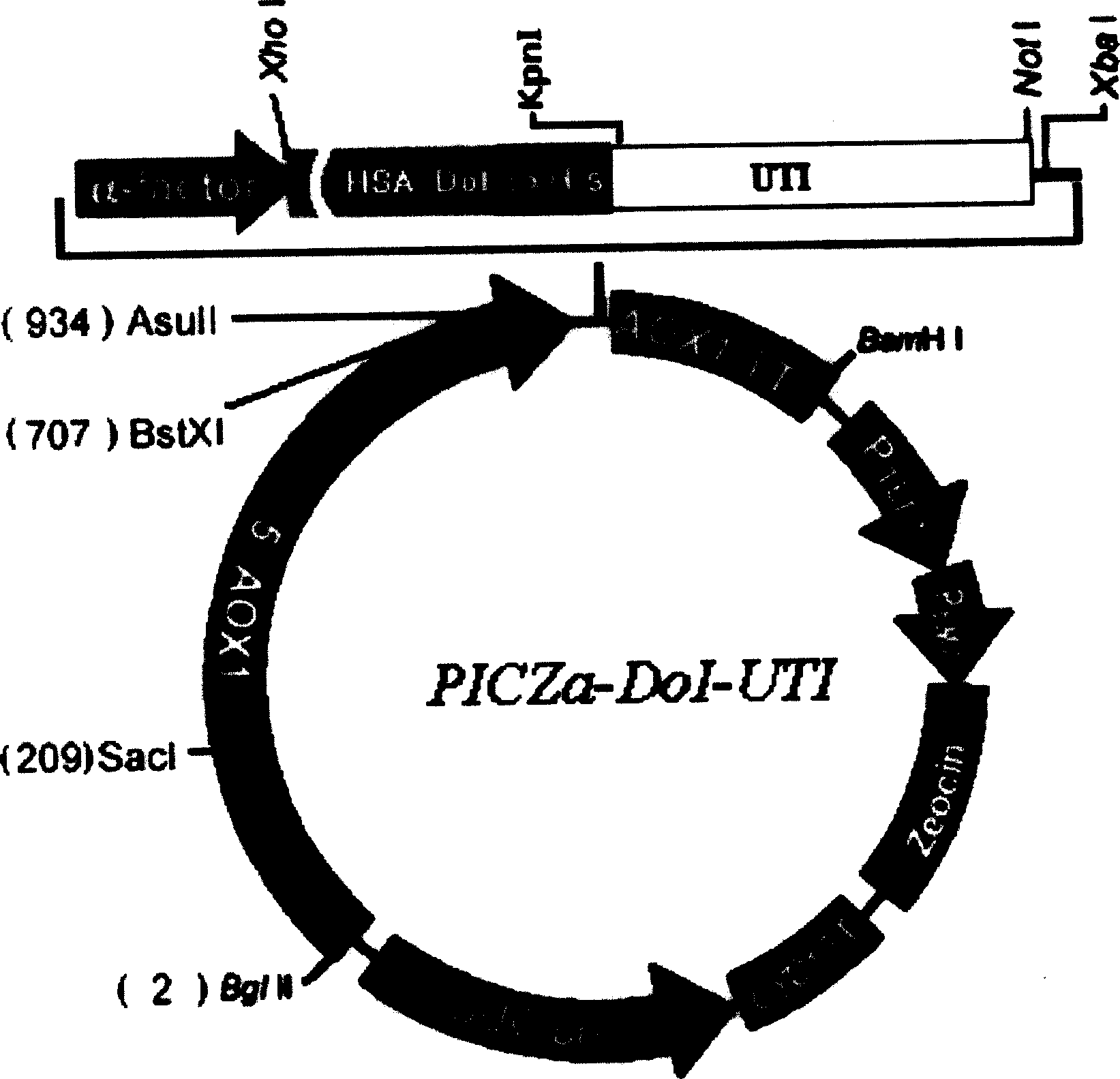
The invention discloses a method for producing a recombined human urinary trypsin inhibitor. The method comprises the steps of: fusing an h-UTI gene of a human body respectively with a first structural domain gene sequence in human serum albumin, inserting a fused body into a special expression carrier PICZa to be expressed, carrying out fermentation culture on a constructed engineering strain in a fermentation tank, and after the induction is completed, collecting fermenting supernatant in a centrifugal manner, adding NaC1, adjusting the pH of the fermenting supernatant to be at 7.4 by using NaOH, adding phosphate until reaching the final concentration of 50nM, and carrying out membrane filtration to obtain fermentation broth which can be used for Ni2 plus or minus chelating chromatography sampling. DoI-UTI fusion protein is eluted with 50mM imidazole, desalination is carried out and DoI-UTI is converted into a digestion buffer solution of enterokinase, and rh-UTI is obtained by a chelating chromatography medium and an ion exchange medium after digestion is completed. The method can solve the difficulty of nonuniformity of h-UTI expression in yeast, low expression output and the like.
Owner:CHENGDU UNIV
A method for preparing urokinase
ActiveCN105087531BImprove adsorption capacitySimple structurePeptidasesURINARY TRYPSIN INHIBITORUrokinase Plasminogen Activator
The invention relates to a urinary protein enriching method which comprises the steps of directly adsorbing urinary proteins in urine in urinals or urinating buckets with filter cloth bags loaded with modified silica gel, and transporting the urinary protein adsorbed filter cloth bags to working points for subsequent treatment. According to the method, the isoelectric point property of specific urinary proteins is utilized, and urinary proteins such as urinary trypsin inhibitors, human urinary kininogenase, urokinase and the like are effectively absorbed directly by using the modified silica gel, macroporous resin, chitin, ionic resin and the like, so that a urine collecting step is avoided. The method is free of obvious influence on sanitary conditions of toilets, and the cost for urine transportation and a series of environmental problems resulting from urine transportation are greatly reduced.
Owner:GUANGDONG TECHPOOL BIO-PHARMA CO LTD
Method for separating urokinase from human urinary trypsin inhibitor
ActiveCN103074319BHigh yieldEasy to separatePeptide preparation methodsProtease inhibitorsURINARY TRYPSIN INHIBITORUrokinase Plasminogen Activator
The invention discloses a method for separating urokinase from a human urinary trypsin inhibitor. The method comprises the following steps: regulating pH of a solution containing the urokinase and the human urinary trypsin inhibitor; slowly adding ammonium sulfate powder while stirring until the solution generates obvious protein precipitation; regulating the pH to 9.0 plus or minus 0.6; after standing, centrifuging or adding diatomite for filtering, collecting the precipitation rich in the urokinase, and obtaining the urokinase; continuing to adding ammonium sulfate into supernatant while stirring to dissolve the ammonium sulfate; and after standing, centrifuging or adding diatomite for filtering, collecting the precipitation 2 rich in the human urinary trypsin inhibitor, and obtaining the human urinary trypsin inhibitor. The method is simple, convenient and feasible and has a short period, the UK and UTI activity is effectively protected, the UK degradation is reduced, the UK quality is increased, special equipment is not needed, and the invested cost of the equipment is reduced.
Owner:YANGZHOU AIDEA BIOTECH
Method for preparing human urinary kallidinogenase crude product
ActiveCN102660525BEfficient separationReduce difficultyHydrolasesProtein solutionURINARY TRYPSIN INHIBITOR
The invention discloses a method for preparing a human urinary kallidinogenase crude product. The method for preparing the human urinary kallidinogenase crude product comprises the following steps of: after adsorbing urokinase protein in urine for a certain time by using an adsorbing agent, collecting the adsorbing agent in which the urokinase protein is adsorbed and centrally eluting; enabling the eluted urokinase protein solution to pass through a metal chelating affinity chromatography column, wherein a human urinary kallidinogenase inhibiting agent is not adsorbed and the human urinary kallidinogenase is combined onto the metal chelating affinity chromatography column, and the human urinary kallidinogenase inhibiting agent is separated from the human urinary kallidinogenase; and collecting an eluting peak to prepare the human urinary kallidinogenase crude product. Due to the adoption of the technical scheme, the human urinary kallidinogenase is efficiently separated from the humanurinary kallidinogenase inhibition agent in the urine, the subsequent purification treatment for the human urinary kallidinogenase and the human urinary kallidinogenase inhibiting agent is facilitated and the purification yield is increased; and the co-generation of two protein crude products is realized, and thus lower production cost is realized.
Owner:YANGZHOU AIDEA BIOTECH
Method for purifying human urinary trypsin inhibitor
ActiveCN101525598ASuitable for industrial productionHUTI higher than livePeptide/protein ingredientsDigestive systemURINARY TRYPSIN INHIBITORChemistry
The invention discloses a method for purifying a human urinary trypsin inhibitor (hUTI). The method comprises the following steps that: a, a solution containing the urinary trypsin inhibitor and cation exchange resin are contacted, so that the urinary trypsin inhibitor is adsorbed on the cation exchange resin; and b, a pH1-7 buffering liquid is used to elute the urinary trypsin inhibitor adsorbed on the cation exchange resin to obtain purified urinary trypsin inhibitor. The method is simple and high-efficient; and an obtained product has high purity.
Owner:SHANGHAI TECHWELL BIOPHARMACEUTICALS CO LTD
Method for preparing crude human urine kininogenase product
ActiveCN105754977AImprove adsorption capacityEasy to cleanPeptidasesURINARY TRYPSIN INHIBITORTrypsin inhibitor
The invention relates to a urine protein extraction method, in particular to a method for preparing a crude human urine kininogenase product.According to the method for preparing the crude human urine kininogenase product, anion exchange resin is adopted as an adsorbent to adsorb urine protein in urine, then the urine protein adsorbing resin is collected and eluted in a centralized mode, the eluant is used for adsorbing human urine kininogenase through cation exchange resin, and therefore human urine kininogenase and a human urine trypsin inhibitor are separated.Through the method for preparing the crude human urine kininogenase product, human urine kininogenase and the human urine trypsin inhibitor can be effectively separated, subsequent purification of human urine kininogenase and the human urine trypsin inhibitor is convenient, the purification rate is increased, co-production of two crude protein products is realized, and production cost can be greatly reduced.
Owner:GUANGDONG TECHPOOL BIO-PHARMA CO LTD
Monoclonal antibodies for detection of urinary trypsin inhibitors
ActiveUS7419665B2Immunoglobulins against animals/humansBiological material analysisURINARY TRYPSIN INHIBITORMonoclonal antibody
Certain monoclonal antibodies are able to detect urinary trypsin inhibitors (UTIs) that are characteristic of disease in humans. In particular, the UTIs include AMBK, Bikunin, Uristatin, Uristatin-1, Uristatin-2, as defined herein, also including the fragments and aggregates thereof.
Owner:SIEMENS HEALTHCARE DIAGNOSTICS INC
Method for inactivating virus contained in trypsin inhibitor extracted from human urine
ActiveCN103102409AInactivation achievedGood inactivation effectInactivation/attenuationPeptide preparation methodsURINARY TRYPSIN INHIBITORFiltration
The invention relates to a method for inactivating virus contained in a trypsin inhibitor extracted from human urine. The method comprises the following steps of dissolving a raw urinary trypsin inhibitor extracted from human urine in a buffer solution and heating at 60 DEG C; then eluting after being adsorbed on a DEAE (diethyl-aminoethanol) chromatographic column so as to obtain DEAE eluate; next sampling the DEAE eluate in ammonium sulfate solution and adsorbing on a hydrophobic chromatography column, then eluting with ammonium sulfate solution of 1M so as to obtain hydrophobic eluate; and after ultrafiltration of the hydrophobic eluate, incubating at low pH, and carrying out gel filtration chromatography to obtain the urinary trypsin inhibitor with virus inactivated. According to the method, a plurality of virus activation methods are utilized comprehensively, especially the two virus inactivation methods of thermally treating firstly and then incubating at low pH, and other separating medium are utilized for realizing inactivation of various virus, so that the inactivation effect of virus contained in the trypsin inhibitor extracted from human urine is further improved, and drug use safety is improved.
Owner:SHANGHAI FENGHUA PHARMA CO LTD
Method for large-scale production of high-purity ulinastatin (namely human urinary trypsin inhibitor)
ActiveCN105753977AEasy to operateImprove controllabilityPeptide preparation methodsProtease inhibitorsFractional PrecipitationURINARY TRYPSIN INHIBITOR
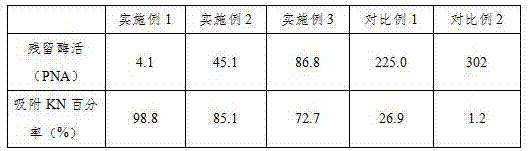
The invention belongs to the technical field of medicines, and particularly relates to a method for large-scale production of high-purity ulinastatin (namely human urinary trypsin inhibitor). The method comprises the following steps: mixing chitosan and alginate with urine after preliminary filtration so as to obtain a mixture, and then purifying the mixture in a manner combining ultrafiltration and ethanol fractional precipitation. The method provided by the invention is convenient to operate, high in controllability, simple in purification technology, and suitable for large-scale production; besides, the yield of the ulinastatin is as high as 72%, the molecular weight of protein obtained through purification is 66800D-67200D, and the activity of the ulinastatin for restraining trypsin is higher than 4780IU / mg protein.
Owner:GUANGDONG TECHPOOL BIO-PHARMA CO LTD
Method for purifying human urinary trypsin inhibitor by utilizing salt resistant mixed mode adsorbent
InactiveCN102167739BImprove purification efficiencyReduce process stepsPeptide preparation methodsProtease inhibitorsURINARY TRYPSIN INHIBITORPurification methods
Owner:江西浩然生物制药有限公司
Human urinary trypsin inhibitor (hUTI) of reorganization-dimerization and preparation method and application thereof
ActiveCN103044554BIncrease productionEfficient and convenient purificationPeptide/protein ingredientsAntipyreticURINARY TRYPSIN INHIBITORHalf-life
The invention discloses a human urinary trypsin inhibitor (hUTI) of reorganization-dimerization and preparation method and application thereof, wherein the hUTI protein of reorganization-dimerization successively comprises amino acid residue sequence of human UTI, a peptide linker and a human IgGFc variant from N-terminal to C-terminal. The hUTI protein of reorganization-dimerization has the in-vitro biological activity similar with that of the human urinary trypsin inhibitor, higher biological activity in vivo and an extended half-life period in vivo.
Owner:PHARMAB
Application of ulinastatin serving as rescue auxiliary medicament for toxic shock caused by acute abdomen
InactiveCN102205115BPeptide/protein ingredientsDigestive systemURINARY TRYPSIN INHIBITORIntestinal loops
The invention relates to a rescue medicament in the field of medical treatment, in particular to a rescue medicament, namely ulinastatin or a urinary trypsin inhibitor (UTI) for toxic shock caused by acute abdomen (particularly mesenteric angiemphraxis and the like). The medicament is a preferred medicament for the toxic shock. In the pharmacodynamic study of the ulinastatin for resisting shock, the ulinastatin also can improve the average arterial blood pressure and the average arterial blood flow of superior mesenteric artery occlusion shock of rats, and has an extremely better effect on shock rescue.
Owner:徐宗昆 +3
Method for purifying human urinary trypsin inhibitor
ActiveCN101525598BSuitable for industrial productionHUTI higher than livePeptide/protein ingredientsDigestive systemURINARY TRYPSIN INHIBITORChemistry
The invention discloses a method for purifying a human urinary trypsin inhibitor (hUTI). The method comprises the following steps that: a, a solution containing the urinary trypsin inhibitor and cation exchange resin are contacted, so that the urinary trypsin inhibitor is adsorbed on the cation exchange resin; and b, a pH1-7 buffering liquid is used to elute the urinary trypsin inhibitor adsorbedon the cation exchange resin to obtain purified urinary trypsin inhibitor. The method is simple and high-efficient; and an obtained product has high purity.
Owner:SHANGHAI TECHWELL BIOPHARMACEUTICALS CO LTD
A method for large-scale production of high-purity ulinastatin
ActiveCN105753977BEasy to operateImprove controllabilityPeptide preparation methodsProtease inhibitorsFractional PrecipitationURINARY TRYPSIN INHIBITOR
The invention belongs to the technical field of medicines, and particularly relates to a method for large-scale production of high-purity ulinastatin (namely human urinary trypsin inhibitor). The method comprises the following steps: mixing chitosan and alginate with urine after preliminary filtration so as to obtain a mixture, and then purifying the mixture in a manner combining ultrafiltration and ethanol fractional precipitation. The method provided by the invention is convenient to operate, high in controllability, simple in purification technology, and suitable for large-scale production; besides, the yield of the ulinastatin is as high as 72%, the molecular weight of protein obtained through purification is 66800D-67200D, and the activity of the ulinastatin for restraining trypsin is higher than 4780IU / mg protein.
Owner:GUANGDONG TECHPOOL BIO-PHARMA CO LTD
Novel application of recombinant human urinary trypsin inhibitor
ActiveCN106139136AEffective treatmentReduce expressionPeptide/protein ingredientsAntiinfectivesURINARY TRYPSIN INHIBITORCD80
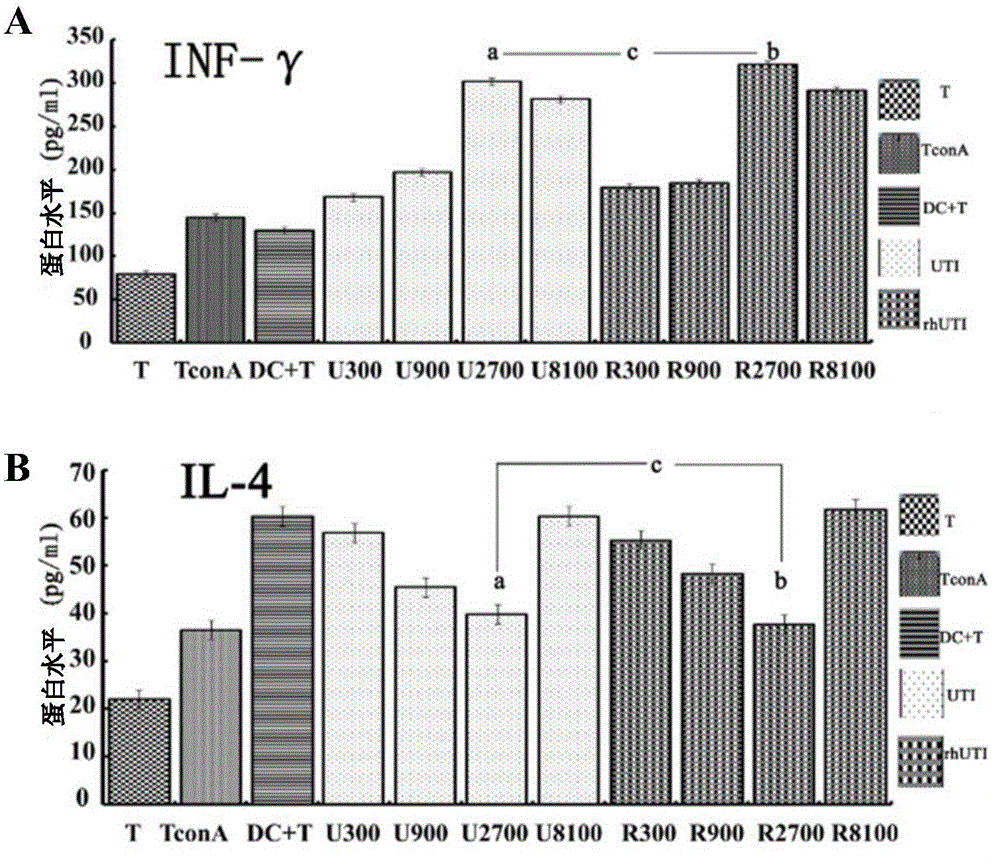
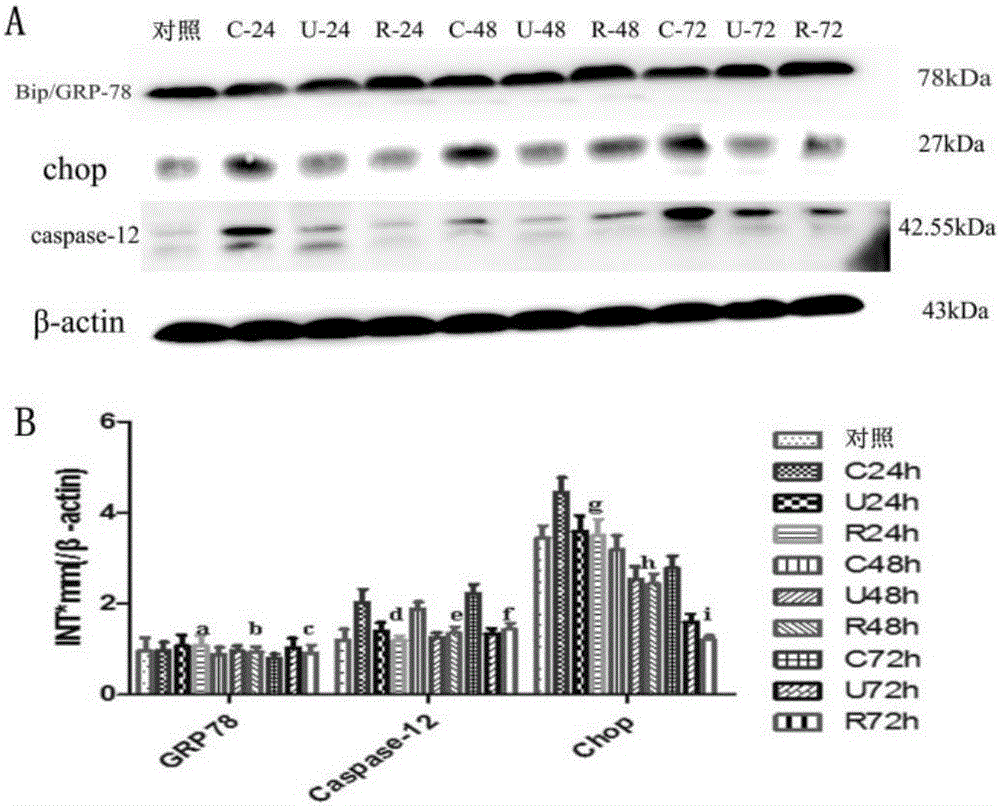
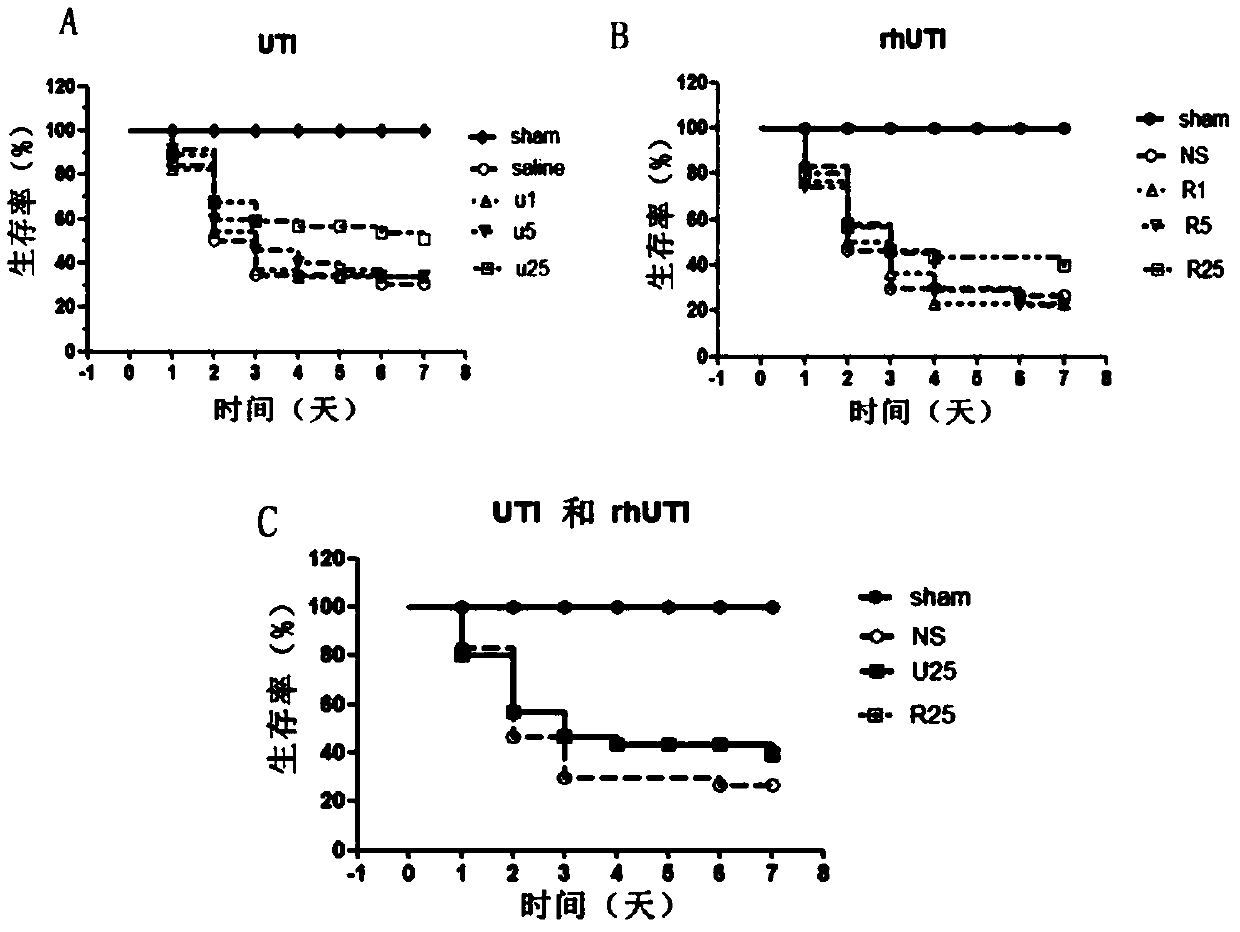
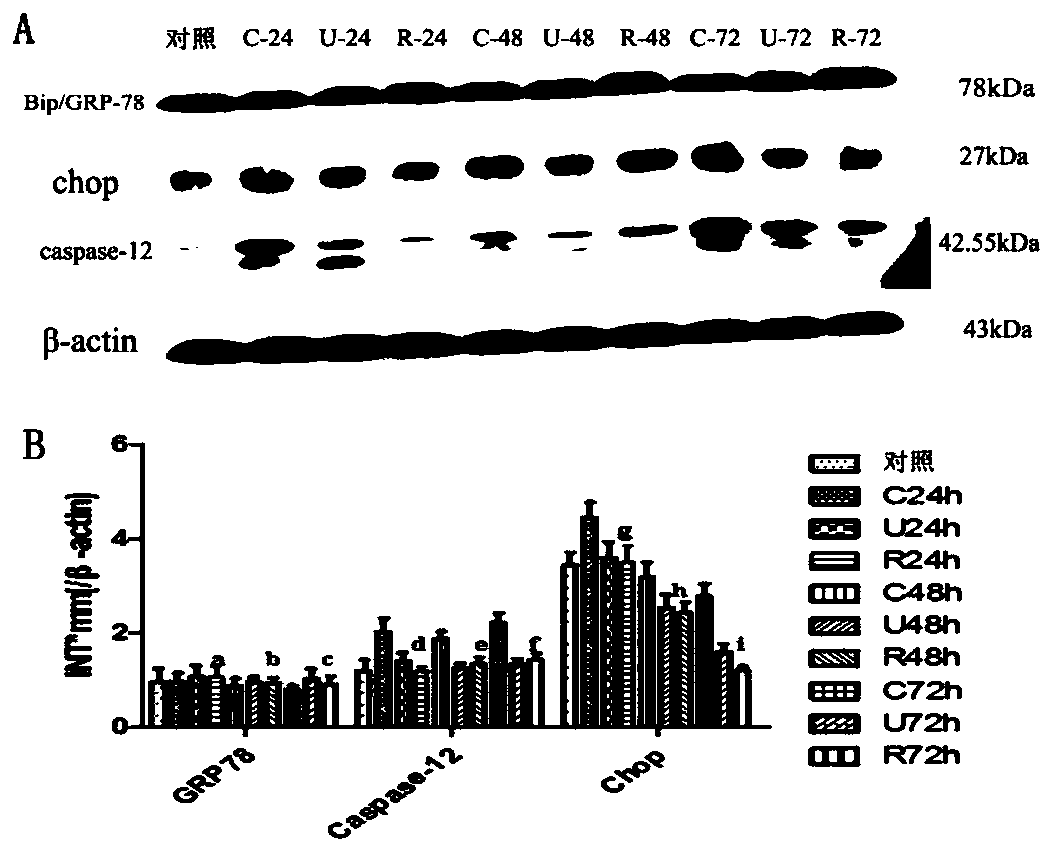
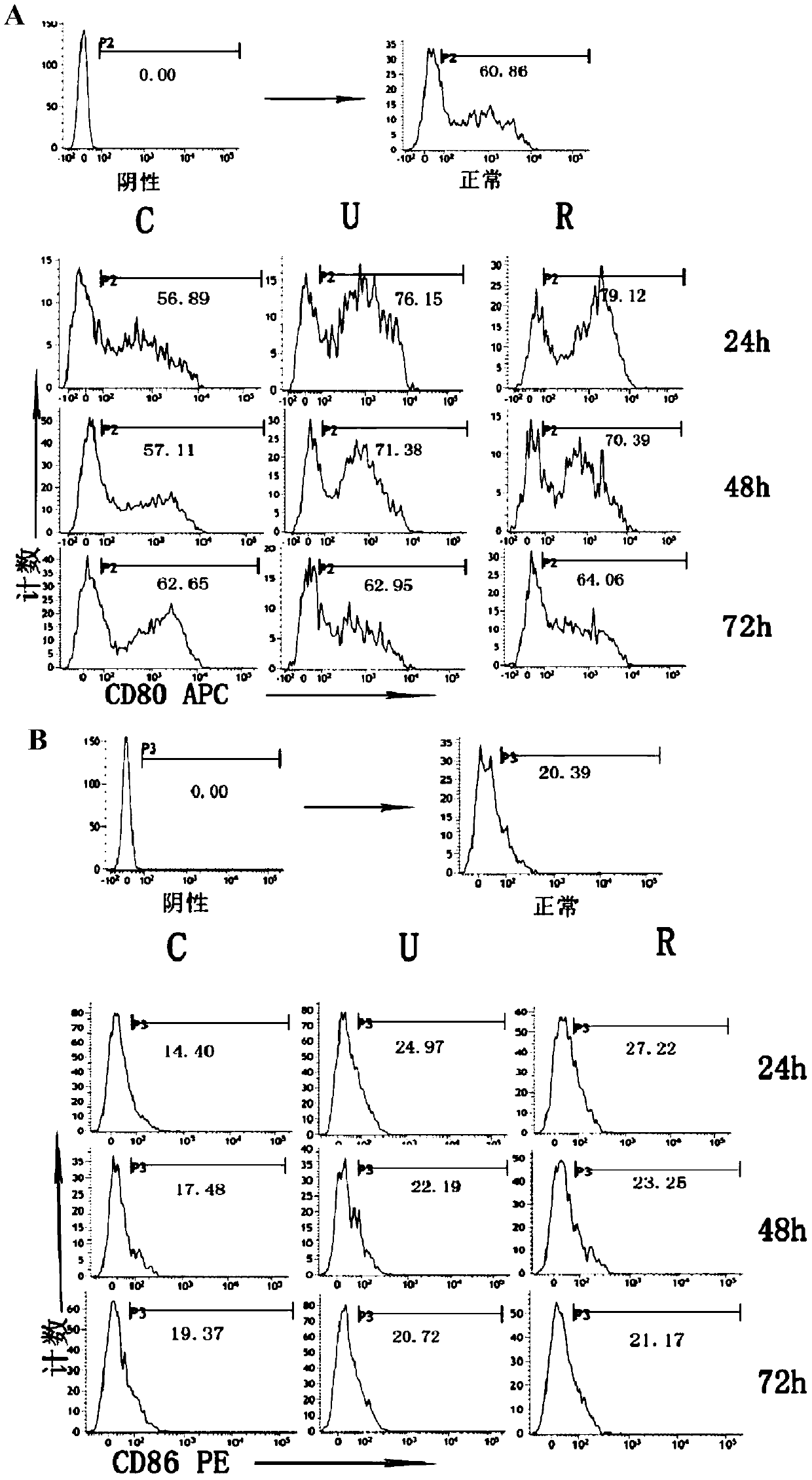
The invention discloses novel application of a recombinant human urinary trypsin inhibitor. According to the experimental research on animals, the recombinant human urinary trypsin inhibitor has the effects of regulating expression of DC surface molecules CD80, CD86 and MHC-II, influencing T cell proliferation and differentiation, improving maturation state of DC through the expression of surface molecules CD80, CD86 and MHC-II, and further influencing T cell proliferation and differentiation and relieving and regulating negative immunoregulation of T lymphocytes. The recombinant human urinary trypsin inhibitor can achieve the effects of relieving an immunosuppressive state caused by sepsis, particularly cellular immunosuppression, and improving the survival rate of mice suffering from sepsis and the symptoms of sepsis, and can be used for treating sepsis.
Owner:FIRST HOSPITAL AFFILIATED TO GENERAL HOSPITAL OF PLA
New application of recombinant human urinary trypsin inhibitor
ActiveCN106139136BEffective treatmentReduce expressionPeptide/protein ingredientsAntiinfectivesURINARY TRYPSIN INHIBITORCD80
The invention discloses novel application of a recombinant human urinary trypsin inhibitor. According to the experimental research on animals, the recombinant human urinary trypsin inhibitor has the effects of regulating expression of DC surface molecules CD80, CD86 and MHC-II, influencing T cell proliferation and differentiation, improving maturation state of DC through the expression of surface molecules CD80, CD86 and MHC-II, and further influencing T cell proliferation and differentiation and relieving and regulating negative immunoregulation of T lymphocytes. The recombinant human urinary trypsin inhibitor can achieve the effects of relieving an immunosuppressive state caused by sepsis, particularly cellular immunosuppression, and improving the survival rate of mice suffering from sepsis and the symptoms of sepsis, and can be used for treating sepsis.
Owner:FIRST HOSPITAL AFFILIATED TO GENERAL HOSPITAL OF PLA
A method for simultaneously preparing crude human urinary kininogenase and crude human urinary trypsin inhibitor
ActiveCN105754977BEfficient separationReduce difficultyPeptidasesURINARY TRYPSIN INHIBITORTrypsin inhibitor
The invention relates to a urine protein extraction method, in particular to a method for preparing a crude human urine kininogenase product.According to the method for preparing the crude human urine kininogenase product, anion exchange resin is adopted as an adsorbent to adsorb urine protein in urine, then the urine protein adsorbing resin is collected and eluted in a centralized mode, the eluant is used for adsorbing human urine kininogenase through cation exchange resin, and therefore human urine kininogenase and a human urine trypsin inhibitor are separated.Through the method for preparing the crude human urine kininogenase product, human urine kininogenase and the human urine trypsin inhibitor can be effectively separated, subsequent purification of human urine kininogenase and the human urine trypsin inhibitor is convenient, the purification rate is increased, co-production of two crude protein products is realized, and production cost can be greatly reduced.
Owner:GUANGDONG TECHPOOL BIO-PHARMA CO LTD
Medicinal composition for treating arthritis
ActiveCN1321692CEasy to useUse progressPeptide/protein ingredientsSkeletal disorderGlycineURINARY TRYPSIN INHIBITOR
A composite medicine in the form of freeze-dried powder injection or liquid injection for treating osteoarthritis and rheumatoid arthritis is prepared from ulinastatin (a human Urinary trypsin inhibitor), sodium hyaluronate and optional mannitol, glycine and dextran.
Owner:GUANGDONG TECHPOOL BIO-PHARMA CO LTD
Affinity medium for human urinary trypsin inhibitor as well as synthesis and application for same
ActiveCN102516387BEfficient purificationImprove purification efficiencyPeptide preparation methodsProtease inhibitorsURINARY TRYPSIN INHIBITORSynthesis methods
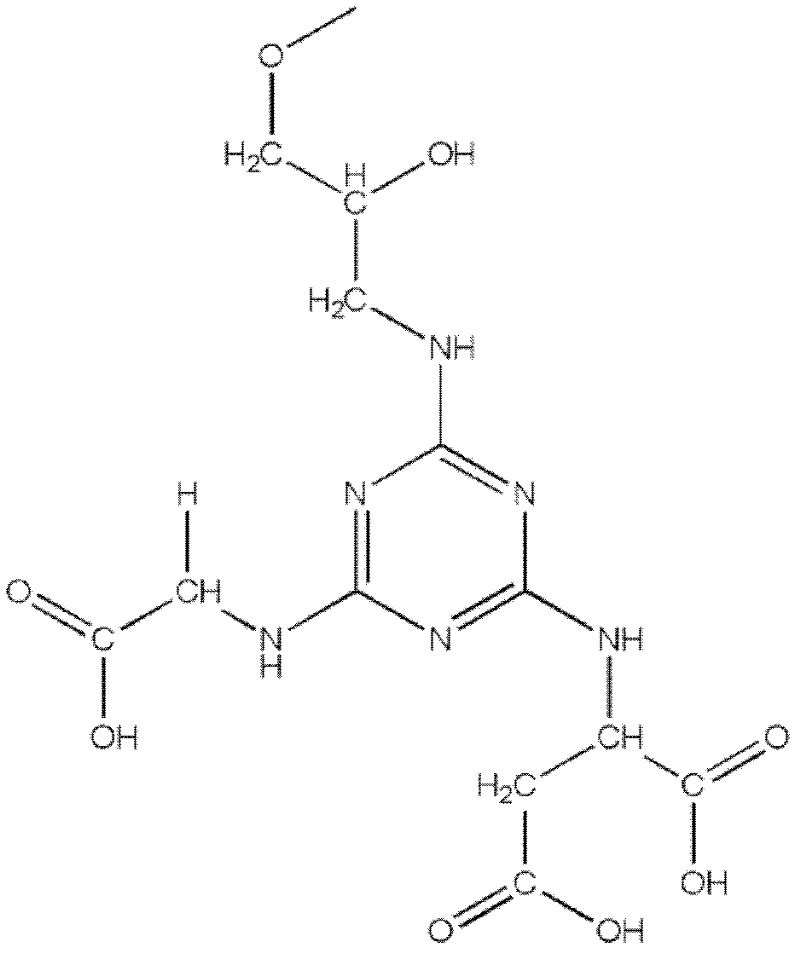
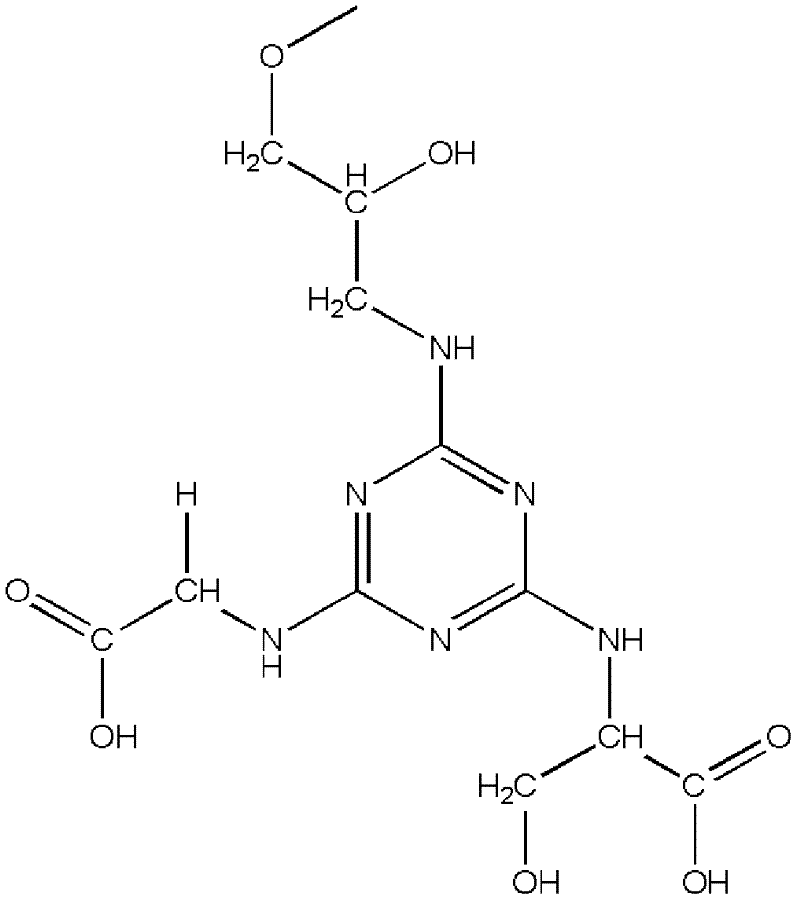
The invention relates to an affinity medium for human urinary trypsin inhibitor, formed by connecting an affinity ligand for human urinary trypsin inhibitor with a chromatography medium. Preferably, the affinity ligand for human urinary trypsin inhibitor is an analogue of the site S1 of serine protease, the chromatography medium is Sepharose, chitosan, silica gel and ceramic particles, and the affinity ligand for human urinary trypsin inhibitor is connected with the chromatography medium by using cyanuric chloride as a spacer arm. A synthesis method for the affinity medium for human urinary trypsin inhibitor, and a method for purifying human urinary trypsin inhibitor in a large scale by using the affinity medium for human urinary trypsin inhibitor are further provided. Human urinary trypsin inhibitor can be rapidly separated and purified in a large scale by using the affinity medium for human urinary trypsin inhibitor, and human urinary trypsin inhibitor pure product can be obtained in case of only one-step affinity chromatography; and the human urinary trypsin inhibitor pure product is short in the consumed time of whole purification step, high in the recovery rates of protein and activity, and suitable for large-scale popularization and application.
Owner:宁夏妙朗生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com