Affinity medium for human urinary trypsin inhibitor as well as synthesis and application for same
A trypsin inhibition and urine trypsin technology is applied to the human urine trypsin inhibitor affinity medium and its synthesis and application fields, which can solve the problems of long purification time, low recovery rate of protein and activity, etc. The effect of large economic benefits and large industrial application potential
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
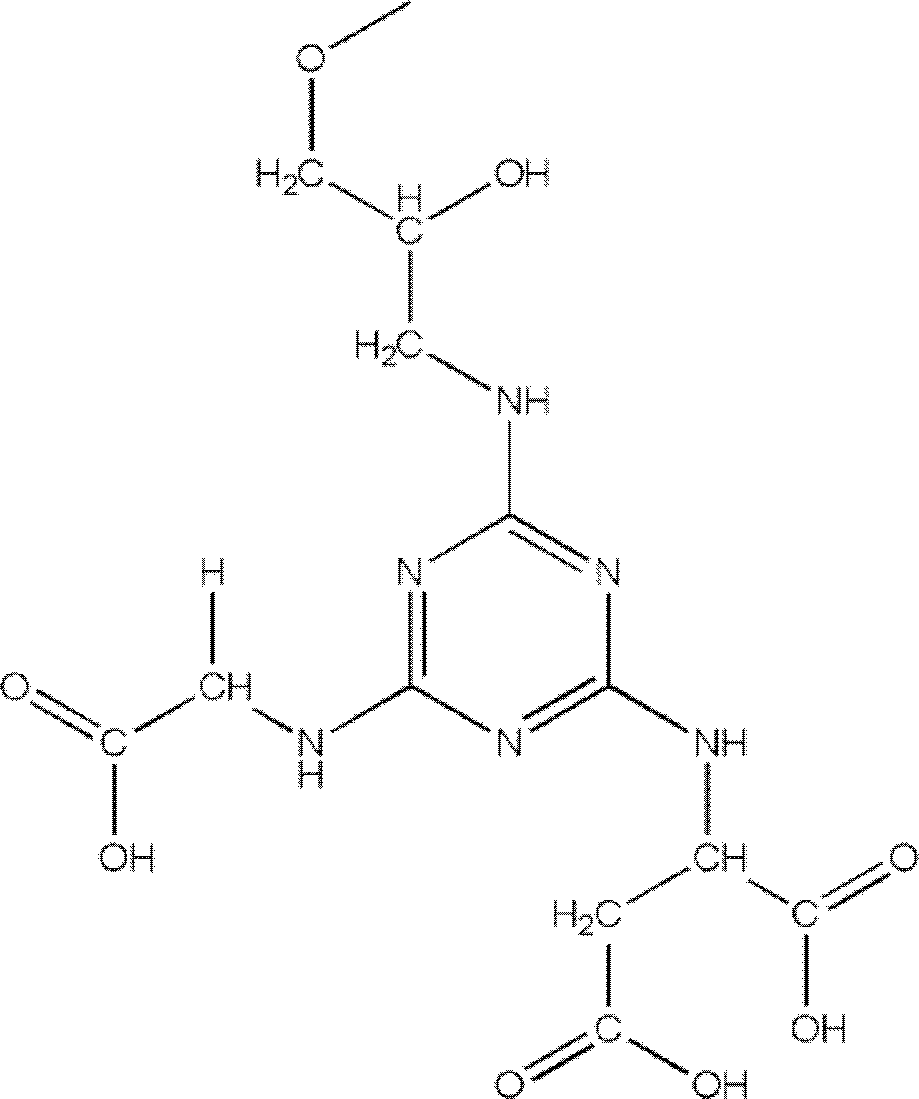
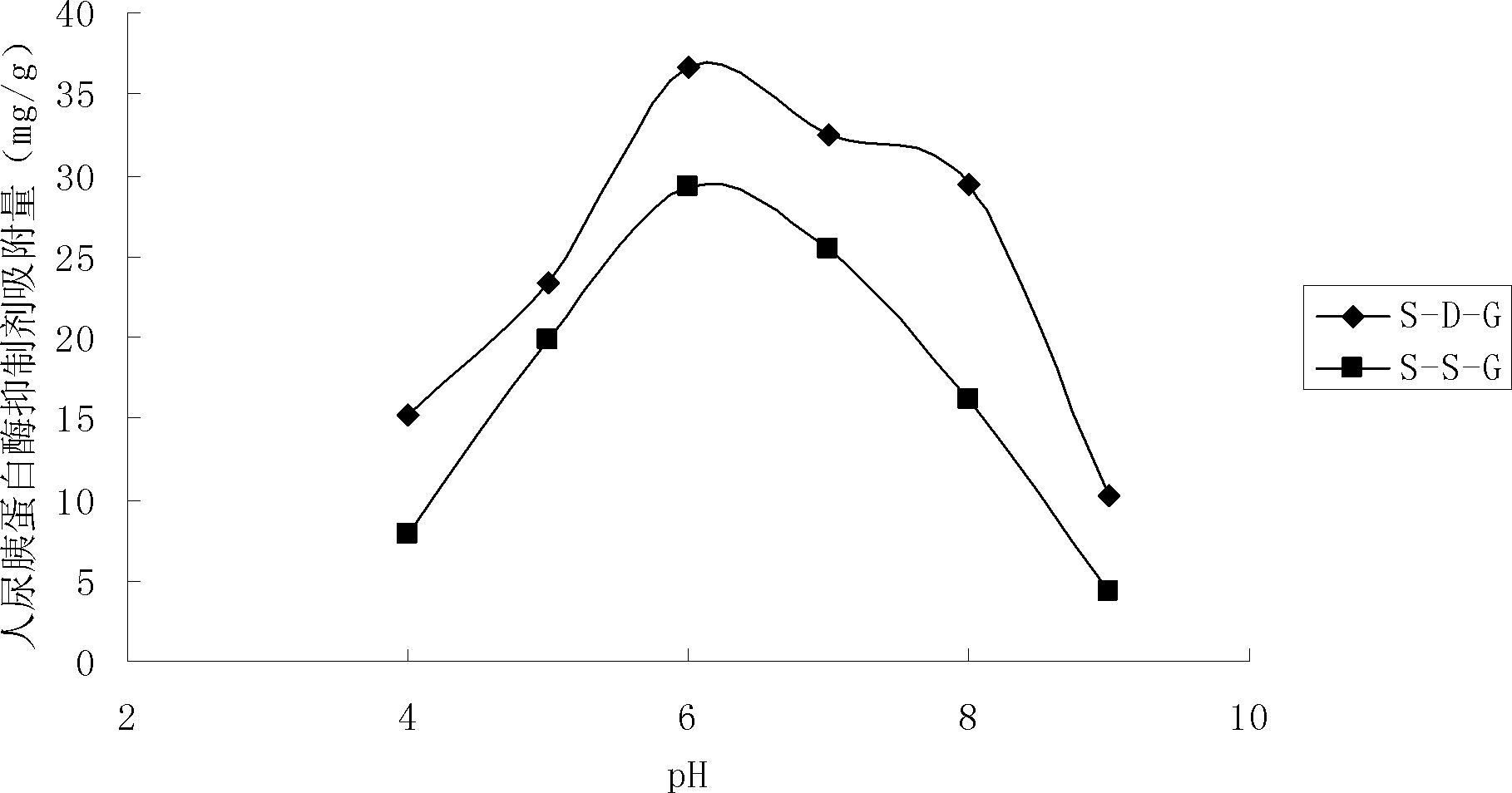
[0029] Embodiment 1: Synthesis and application of Sepharose-ASP-GLY
[0030] Sepharose CL 4B (100g) was washed with 10 times the volume of deionized water, and dried into a wet cake; suspended in 50ml activation buffer (0.8M NaOH, 20% dimethylsulfoxide, 10% epichlorohydrin, 0.5mg / ml sodium borohydride) shaken at 40°C for 2.5h, then poured into a glass frosted funnel, and washed with 10 times the volume of distilled water each time under suction filtration until the pH of the washing solution was neutral, and then drained to form a wet cake shape. The activated Sepharose CL 4B medium was suspended in 500ml of 0.1M NaOH solution, 20ml of ethylenediamine solution was added, and the gel was kept at 30°C for 12h under stirring (200rpm). Rinse with deionized water. NH 2 - Suspend Sepharose CL 4B in 350ml 50% (v / v) ice-bathed acetone solution, then dissolve 4g triazoxide in 80ml-20°C pre-cooled acetone, quickly add the medium suspension, check the pH value in real time, use satur...
Embodiment 2
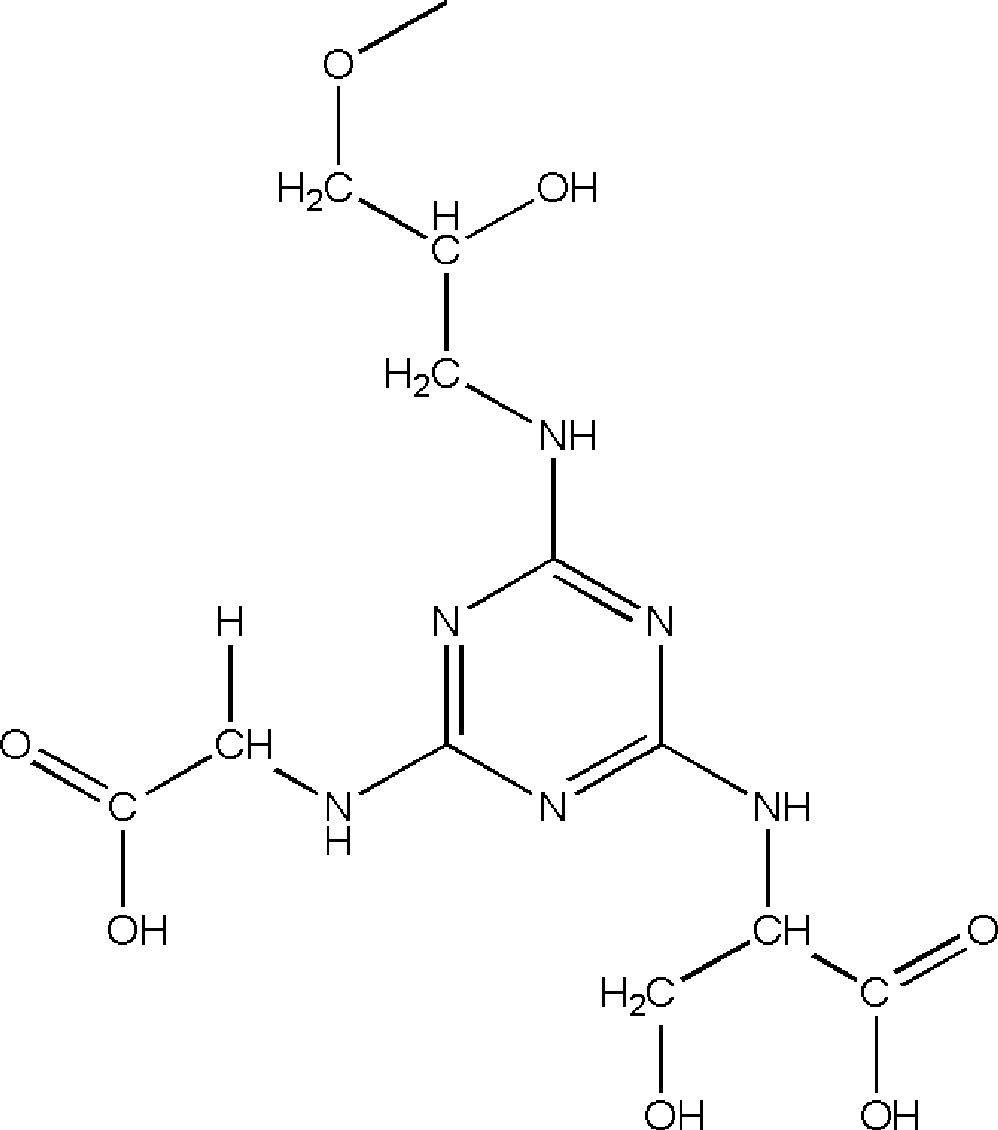
[0038] Embodiment 2: Synthesis and application of Sepharose-SER-GLY
[0039] Sepharose CL 4B (100g) was washed with 10 times the volume of deionized water, and dried into a wet cake; suspended in 50ml activation buffer (0.8M NaOH, 20% dimethylsulfoxide, 10% epichlorohydrin, 0.5mg / ml sodium borohydride) shaken at 40°C for 2.5h, then poured into a glass frosted funnel, and washed with 10 times the volume of distilled water each time under suction filtration until the pH of the washing solution was neutral, and then drained to form a wet cake shape. The activated Sepharose CL 4B medium was suspended in 500ml of 0.1M NaOH solution, 20ml of ethylenediamine solution was added, and the gel was kept at 30°C for 12h under stirring (200rpm). Rinse with deionized water. NH 2 - Suspend Sepharose CL 4B in 350ml 50% (v / v) ice-bathed acetone solution, then dissolve 4g triazoxide in 80ml-20°C pre-cooled acetone, quickly add the medium suspension, check the pH value in real time, use satur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com