Process for preparing medicine grade recombined human urine trypase inhibitor
A technology of fusion protein and preparation process, which is applied in the direction of protease inhibitors, peptide preparation methods, botany equipment and methods, etc. It can solve the difficulties in determining the clinical value of rh-UTI, uneven N-terminal, and no large-scale production, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: the structure of recombinant human urine trypsin inhibitor (rh-UTI) engineering strain
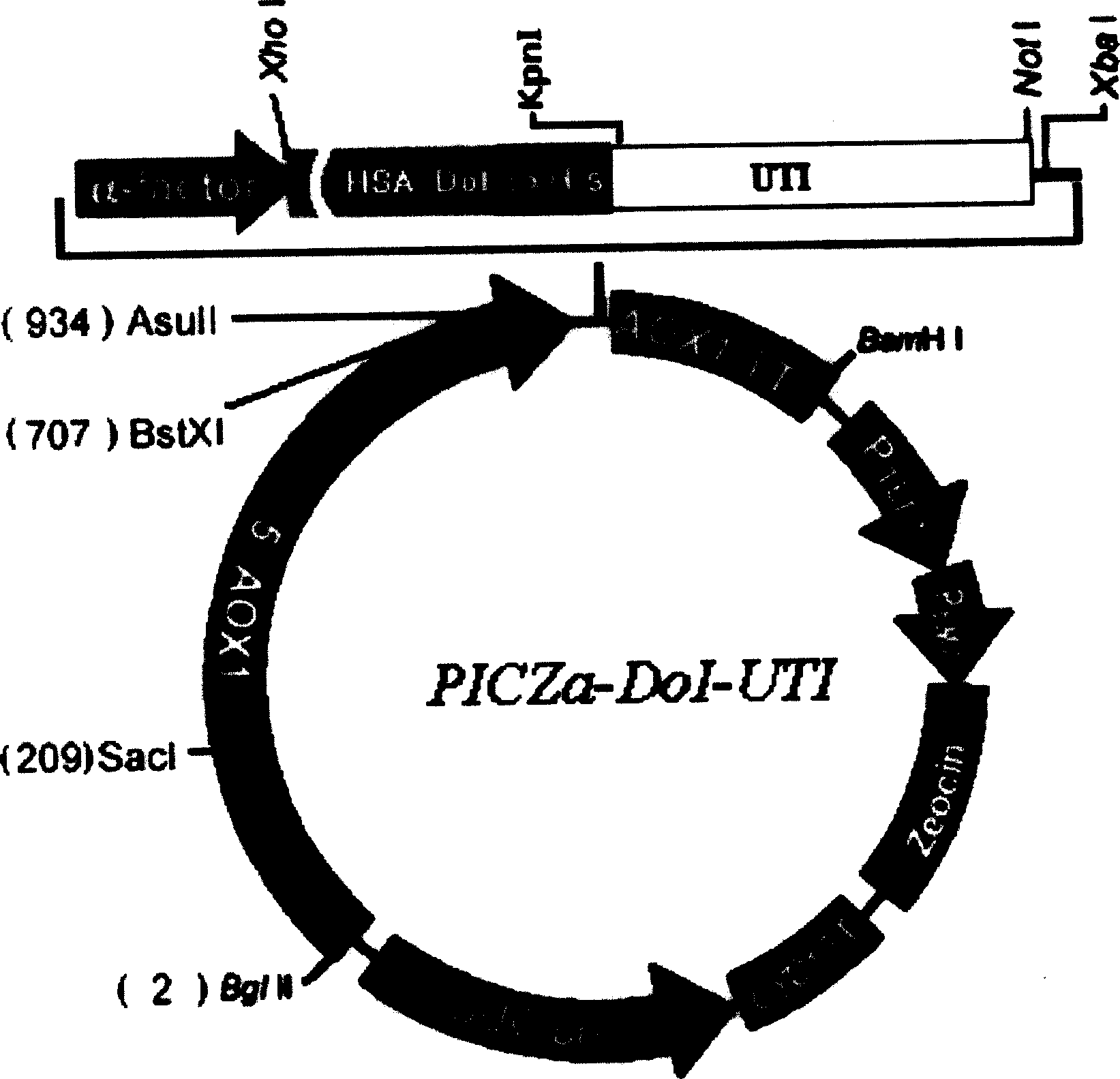
[0035] 1. The acquisition of rh-UTI gene and the construction of expression vector PICZa-DoI-UTI
[0036] Referring to the human Bikunin gene sequence in Genbank, two primers for hBIK5 and hBIK3 were synthesized:
[0037] hBIK5:5`-cat ggtacc gat gat gat gac aaa gct gtg cta ccc caa gaa gag-3`
[0038] hBIK3:5`-cat gcggccgc tta cag cag ctc ctc atc acc-3`
[0039] Using human normal fetal liver tissue cDNA (Lot: A604235, Biochain Institute, Inc) as a template, the human Bikunin gene fragment (see SEQ-1) was PCR-produced according to the following system and conditions.
[0040] 50ul PCR system:
[0041] cDNA (template) -------------------------- 2.0ul
[0042] hBIK5 (forward primer) ---------------------5.0ul
[0043] hBIK3 (reverse primer) ---------------------5.0ul
[0044] dNTP-------------------------------2.5ul
[0045] 10XPfu buffer --------------------...
Embodiment 2
[0056] Example 2: Fermentation and purification of recombinant human urinary trypsin inhibitor
[0057] The pilot fermentation and purification process selected GS115-UTI bacterial classification, and carried out in a 30L fermenter according to the description in the Invitrogen fermentation operation guide, the whole process of fermentation and purification (see Figure 4 with Figure 5 ) can be briefly described as:
[0058] 1. Preparation of Seed Solution
[0059]Streak the strain on the YPD plate, place it upside down in a 30°C incubator for 48 hours, pick a single clone and transfer it to a 250ml shaker flask filled with 25ml of YPD culture medium, and culture it at 30°C and 280rpm for 18 hours to OD 600 At 4 to 6 o'clock, after microscopic examination of the yeast with normal morphology and no bacterial contamination, transfer it to a 1L shaker flask containing 500ml of YPD culture medium at a ratio of 1:500, culture at 30°C and 280rpm for 18 hours, OD 600 After reachi...
Embodiment 3
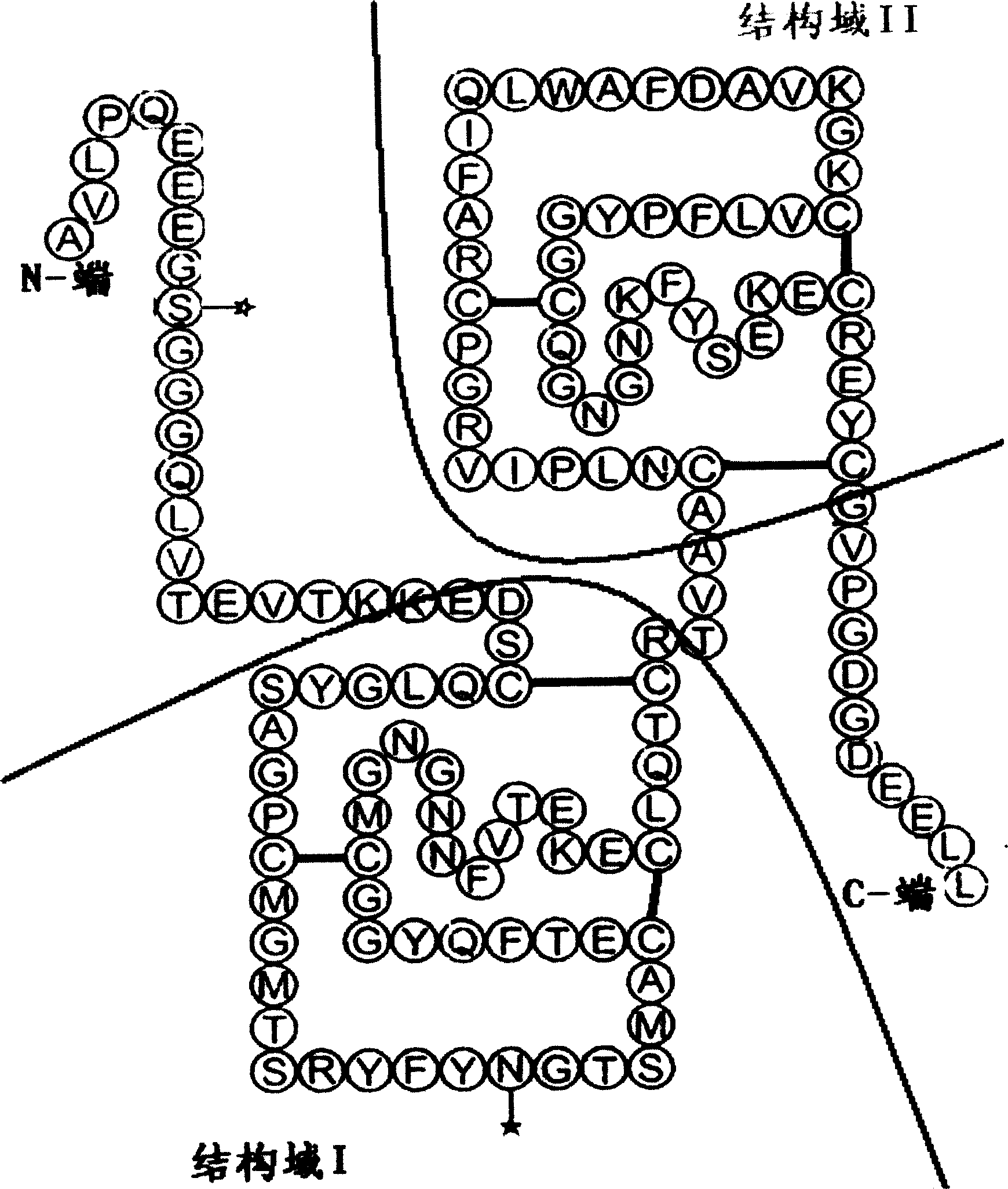
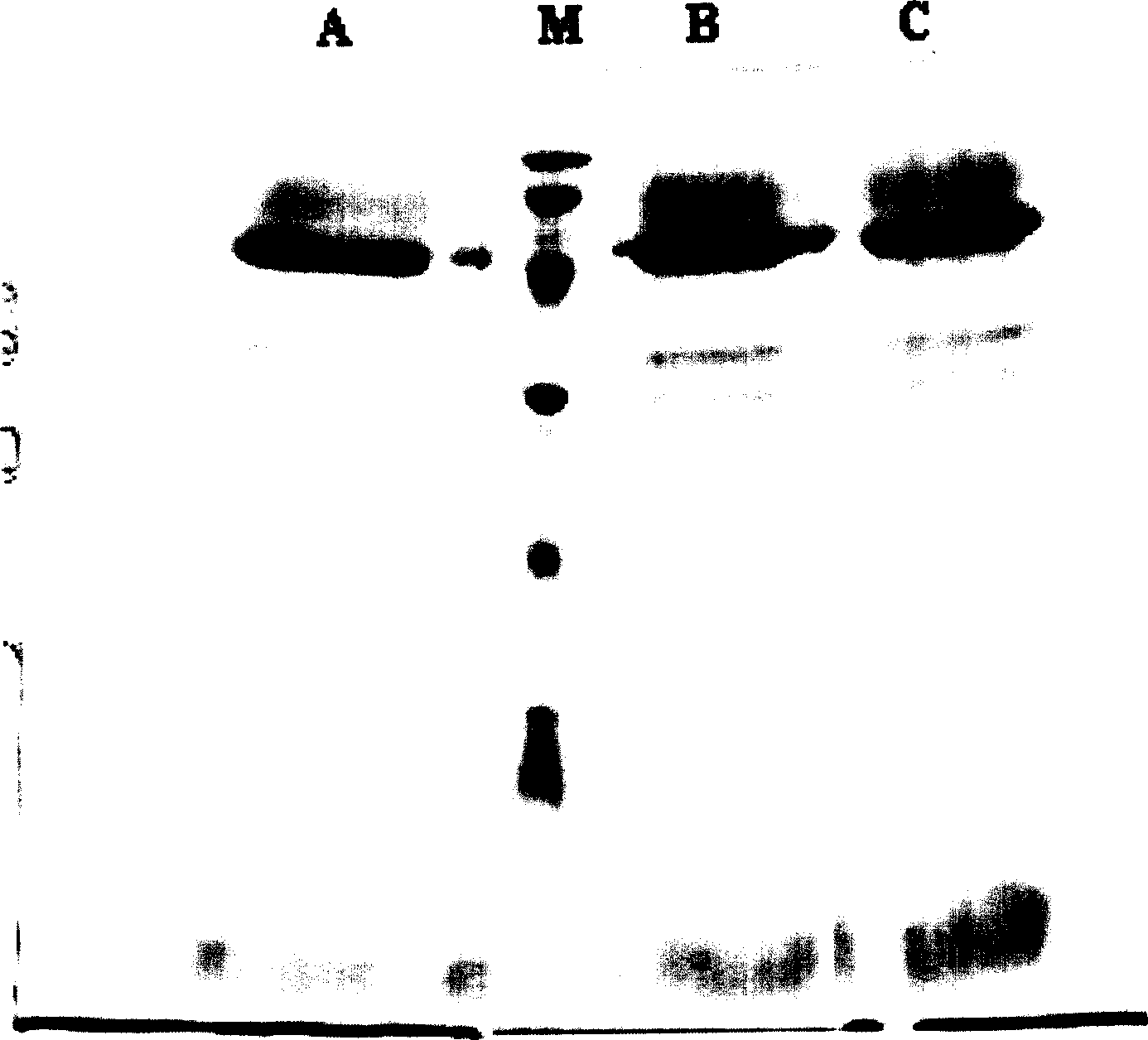
[0074] Example 3: Confirmation of the properties of recombinant human urinary trypsin inhibitor
[0075] The rh-UTI prepared according to the method in Example 2 has been studied in terms of protease inhibitory activity, specific activity, protein purity, molecular weight determination, isoelectric point, N-terminal-C-terminal sequencing, etc. according to the requirements of SFDA quality inspection standards. Tested to determine its basic properties.
[0076] 1. Protease inhibitory activity of h-UTI-specific activity determination
[0077] 1.1. Analysis of the inhibitory effect on trypsin
[0078] Use benzoyl-L-arg-p-NA as substrate, according to the method (Geige&Fritz, Methods of Enzymatic Analysis, Vol V, 3 rd ed., Bergmeyer (ed.), Verlag Chemie, Weinheim, p.121, 1984), and the released p-NA was detected with a spectrophotometer at a wavelength of 405 nm. Enzymes and inhibitors were pre-warmed for 15 minutes prior to adding substrate. The results showed that rh-UTI cou...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com