Novel application of recombinant human urinary trypsin inhibitor
A trypsin inhibition and application technology, applied in the field of recombinant human urinary trypsin inhibitor, can solve the problems of endoplasmic reticulum-related cell apoptosis, tissue and organ damage, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
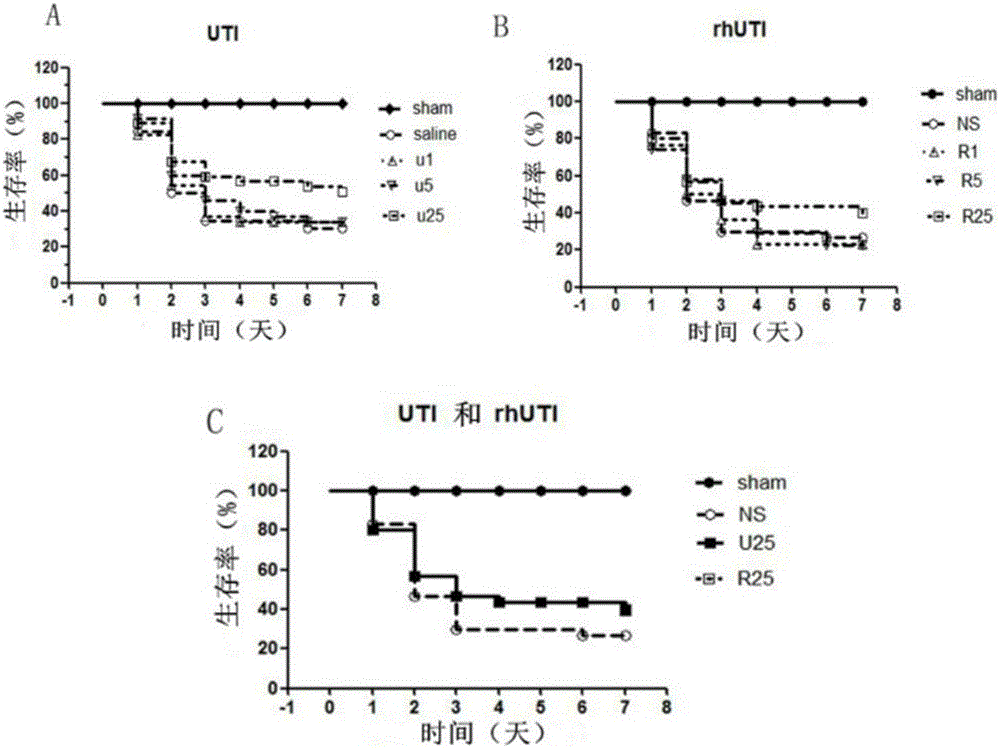
[0044] Example 1: Effect of rhUTI on Survival Rate of Mouse Sepsis Model
[0045] 1. Experimental animals and main reagents
[0046] Male BALB / c mice, 6-8w, 20±2g, were purchased from the Institute of Experimental Animals, Chinese Academy of Medical Sciences (animal certificate number: SCXK Beijing 2009-0007). Adaptive feeding for 1 week, free access to food and water, room temperature 25°C, 12h circadian rhythm.
[0047] Anesthetic drugs: Ketamine Hydrochloride Injection (Shanghai First Biochemical Pharmaceutical Company, China), Sumianxin II Injection (developed by Military Veterinary Research Institute, Academy of Military Medical Sciences, China) and 0.9% normal saline (Shandong Luxin Pharmaceutical Group, China) is used after mixing according to the volume ratio of 2:2.5:4.5.
[0048] rhUTI (Shanghai Wanxing Biopharmaceutical Co., Ltd., China): 100,000 U / bottle, ulinastatin for injection (Guangdong Tianpu Biochemical Pharmaceutical Co., Ltd., China): batch number 031211...
Embodiment 2
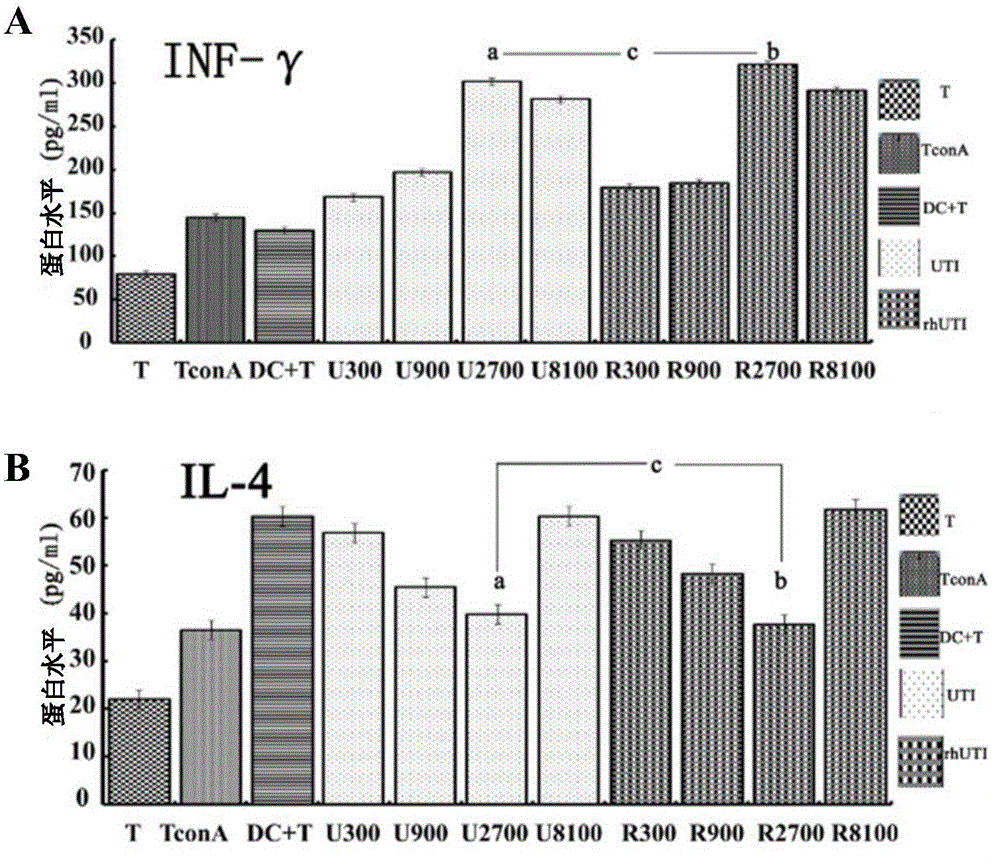
[0066] Example 2 Effect of rhUTI treatment on the functional status of spleen DC in septic mice
[0067] 1. Experimental animals and main reagents
[0068] The mice and rhUTI used in this example are the same as those in Example 1.
[0069] Mouse CD11c Isolation Kit, Mouse CD4 + T isolation kit: Miltenyi Biltec GmbH, Germany; mouse ELISpot (IL-12, TNF-α) mouse ELISPOT kit (IL-4, IFN-γ): Shanghai Yikesai Biological Products Co., Ltd., China; Anti-mouse CD-86PE, CD-80APC Mouse: Miltenyi Biltec GmbH, Germany; anti-mouse MHC-II, American eBioscience; rabbit anti-mouse GRP78 antibody, American Epitomics; rabbit anti-mouse CHOP antibody, rabbit anti Mouse Caspase-12 was purchased from Cell Signaling Technology, USA.
[0070] 2. Experimental steps:
[0071] (1) The construction of severe sepsis mouse model and the administration of rhUTI were the same as in Example 1.
[0072] (2) After the successful modeling of sepsis, the mice were sacrificed by neck dislocation after 1 day, ...
Embodiment 3
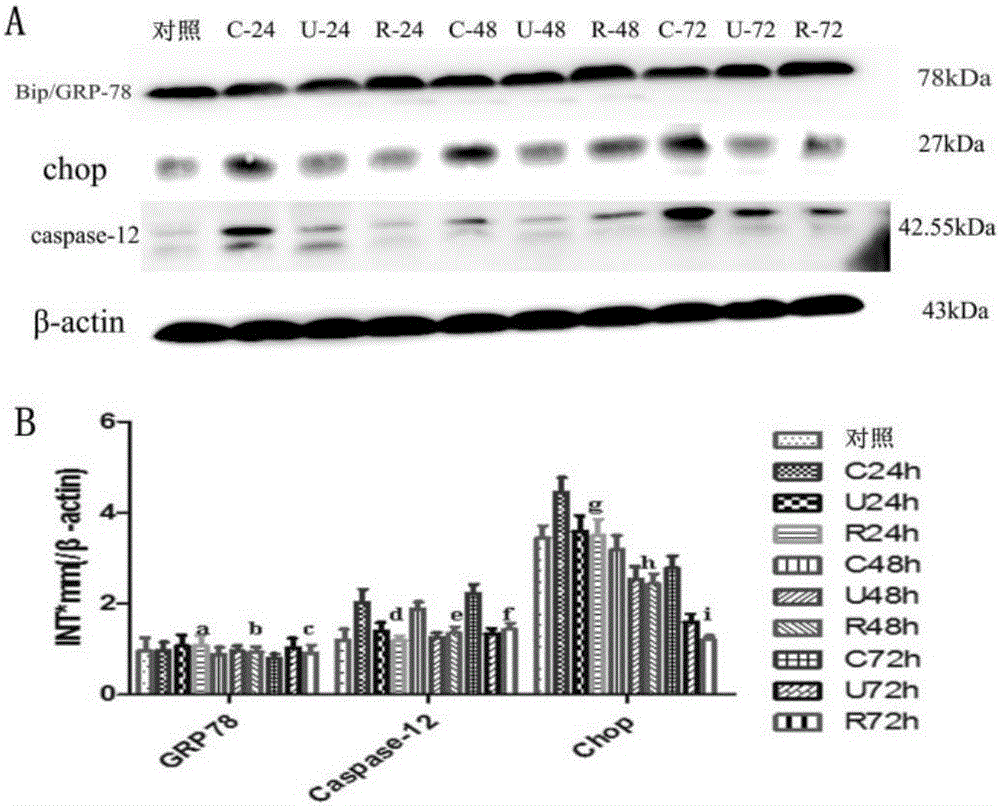
[0080] Example 3 Effect of rhUTI intervention on spleen DC apoptosis in septic mice
[0081] 1. Experimental animals and main reagents
[0082] The mice, rhUTI, and mouse CD11c isolation kits used in this example are the same as those in Example 3.
[0083] Annexin V-PE / 7-AAD Apoptosis Detection Kit (100test, containing 1ml Annexin-V-PE, 500μl 7-AAD and 80ml binding buffer) BD Company, USA.
[0084] 2. Experimental steps:
[0085] ① The construction of severe sepsis mouse model and rhUTI medication are the same as in Example 1.
[0086] ②One day after CLP modeling, they were killed by neck dislocation, and the spleen tissue was collected, minced and ground, and filtered through a 200-mesh steel sieve to separate mononuclear cells from the lymphocyte separation medium. DCs were separated by immunomagnetic beads, and the cell concentration was adjusted. V-PE and 7-AAD were co-incubated at room temperature for 15 min, and DC apoptosis was detected by flow cytometry.
[0087] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com