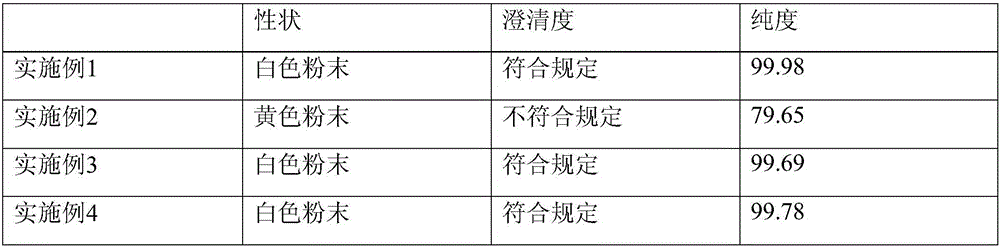
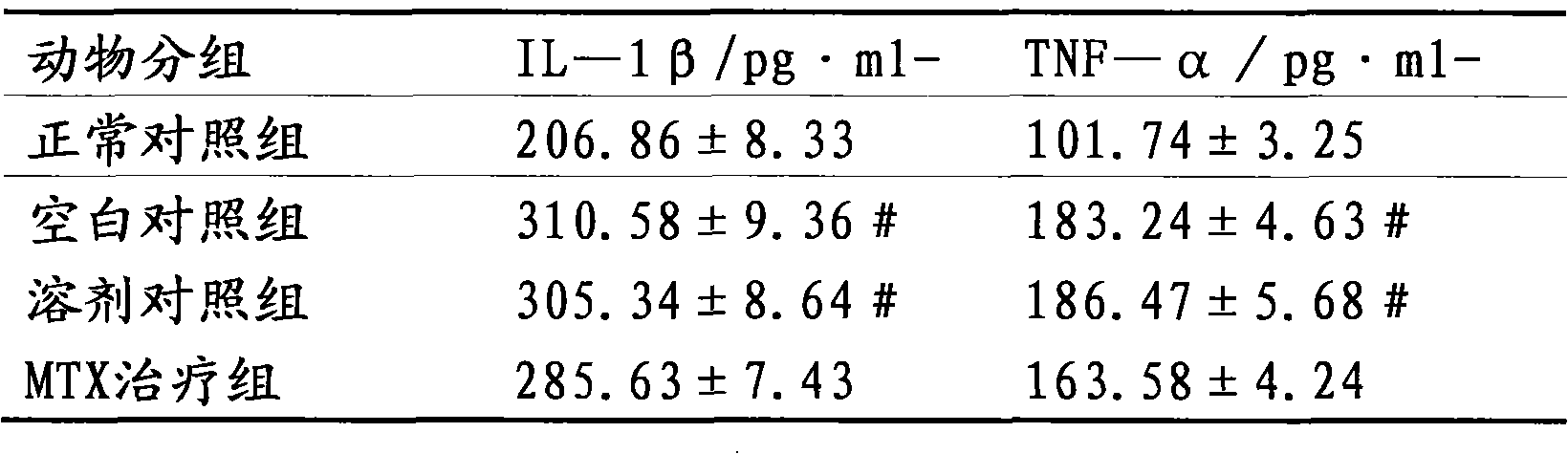Patents
Literature
88 results about "Ulinastatin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
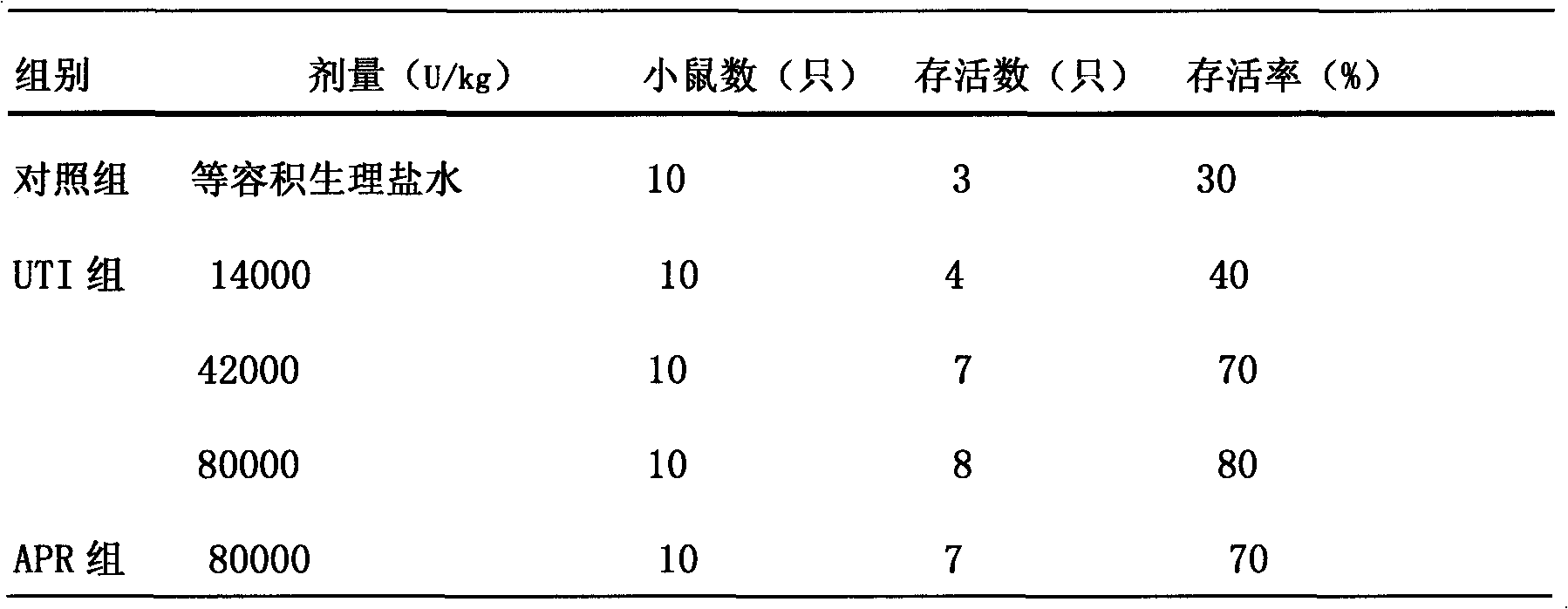
Ulinastatin, as an urinary trypsin inhibitor (UTI), is a glycoprotein that is isolated from healthy human urine or synthetically produced and has molecular weight of 25 - 25kDa. Highly purified ulinastatin has been clinically used for the treatment of acute pancreatitis, chronic pancreatitis, Stevens–Johnson syndrome, burns, septic shock, and toxic epidermal necrolysis (TEN).
Pyemia treating medicine composition
InactiveCN101020048AObvious therapeuticObvious/or preventive effectPowder deliveryPeptide/protein ingredientsFreeze-dryingThymulin
The present invention relates to the application of ulinastatin and / or alpha-1 thymulin in preparing medicine for preventing and treating pyemia and the medicine therewith. Ulinastatin and / or alpha-1 thymulin have obvious pyemia treating effect. The medicine is prepared into injection or freeze dried powder for injection.
Owner:GUANGDONG TECHPOOL BIO-PHARMA CO LTD
High purity ulinastatin and its prepn process and medicine composition
ActiveCN1931875AHigh purityImprove stabilityPeptide/protein ingredientsAntipyreticMetalloproteinPhosphate
The present invention relates to high purity ulinastatin and its medicine composition and their preparation process. Specially, the high purity ulinastatin in 50,000 U / ml concentration has optical absorption value at 405 nm not exceeding 0.05 and human urea kininogenase content not exceeding 0.0003 PNAU. The present invention purifies ulinastatin product through adsorption with hydrophobic column, purification in hydrophilic column, combination with metalloprotein in metal chelating column and elution with buffering phosphate solution.
Owner:GUANGDONG TECHPOOL BIO-PHARMA CO LTD
Medicament composition for improving stability of Ulinastatin
InactiveCN101439180AOvercome the problem of easy inactivation in clinical useImprove stabilityPeptide/protein ingredientsDigestive systemFreeze-dryingInjection powder
The invention discloses a stable ulinastatin sterile injection powder pharmaceutical composition which contains an effective dose of ulinastatin and a pharmaceutical excipient, and the excipient is one or the combination of a plurality of kinds of mannitol, hydrolyzed gelatin, dextran and sodium chloride. The composition is generally used in the form of the freeze-dried sterile injection powder, when in clinical application, the composition is dissolved in glucose or physiological saline and other large infusions, and the composition can significantly reduce the decrease of the activity of the ulinastatin in water solution.
Owner:GUANGDONG TECHPOOL BIO-PHARMA CO LTD
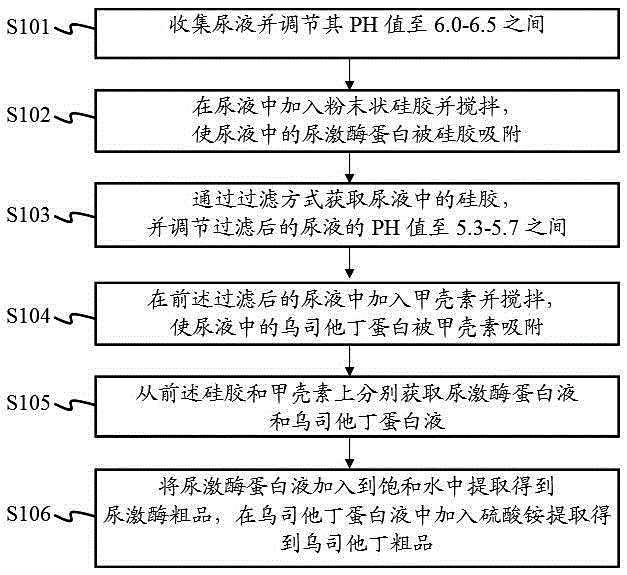
Method for extracting urokinase and ulinastatin from urine
InactiveCN105238771AHigh economic valueIncrease productionProtease inhibitorsAnimals/human peptidesMedicineSilica gel
The invention provides a method for extracting urokinase and ulinastatin from urine. The method comprises the following steps: collecting the urine, and adjusting the pH value of the urine; adding silica gel powder into the urine, and stirring so that urokinase protein in the urine is adsorbed by the silica gel; acquiring the silica gel through a filtering method, and adjusting the pH value of the filtered urine; adding chitin into the filtered urine so that ulinastatin protein in the urine is adsorbed by the chitin; acquiring urokinase protein fluid and ulinastatin protein fluid from the silica gel and the chitin, respectively; and adding the urokinase protein fluid into saturated water, and extracting to obtain urokinase, and adding ammonium sulfate into the ulinastatin protein fluid, and extracting to obtain ulinastatin. According to the method provided by the invention, the urokinase crude product and the ulinastatin crude product can be extracted from the urine, so that the economic value of the urine is greatly improved.
Owner:张昭
Stable water injection medicament composition containing Ulinastatin
ActiveCN101439181AConvenient treatmentPeptide/protein ingredientsDigestive systemSodium acetateFreeze-drying
The invention discloses a stable ulinastatin pharmaceutical composition which contains an effective dose of ulinastatin and a pharmaceutical excipient with a certain proportion. Wherein, the excipient can be one or more of mannitol, sodium chloride, sodium acetate and acetic acid, and the range of pH value is 4.2-6.0. The composition is generally used in the form of injection, when in clinical application, the composition can be directly used for intravenous infusion and the composition can significantly improve the problem that the activity of an ulinastatin freeze-dried sterile injection powder preparation is easy to decrease during the preparation.
Owner:GUANGDONG TECHPOOL BIO-PHARMA CO LTD
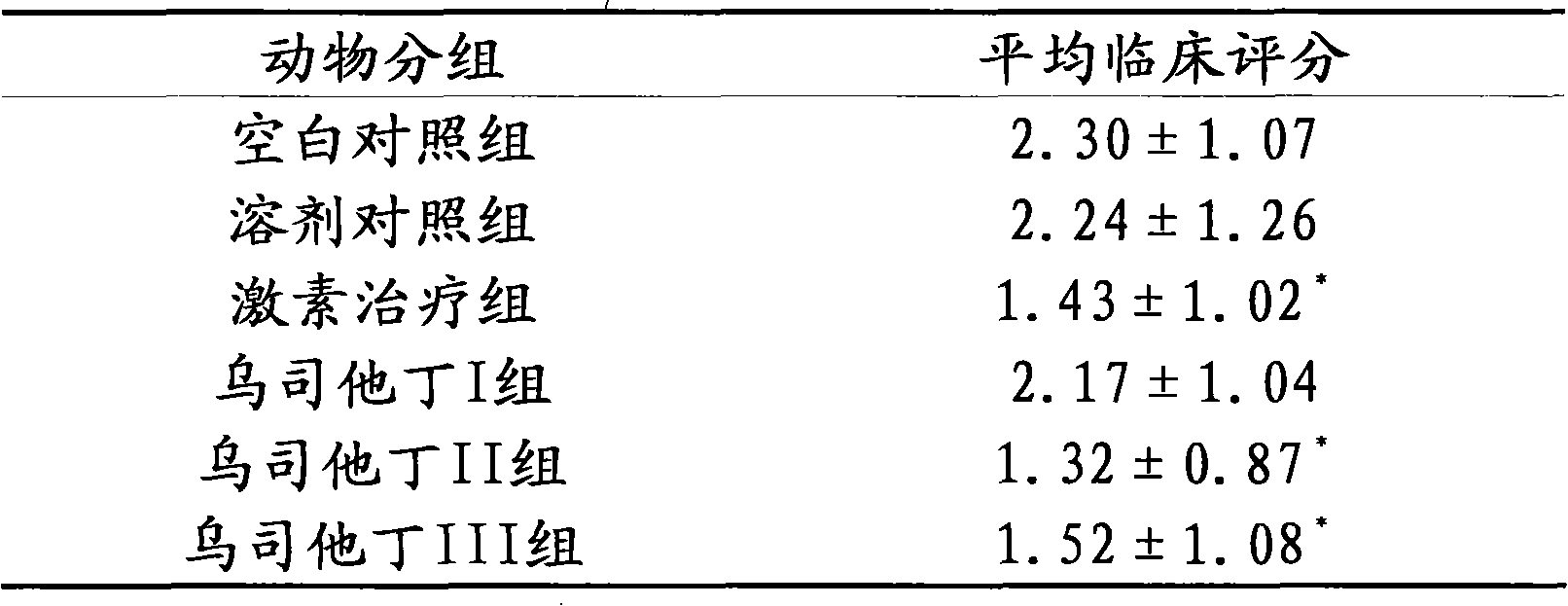
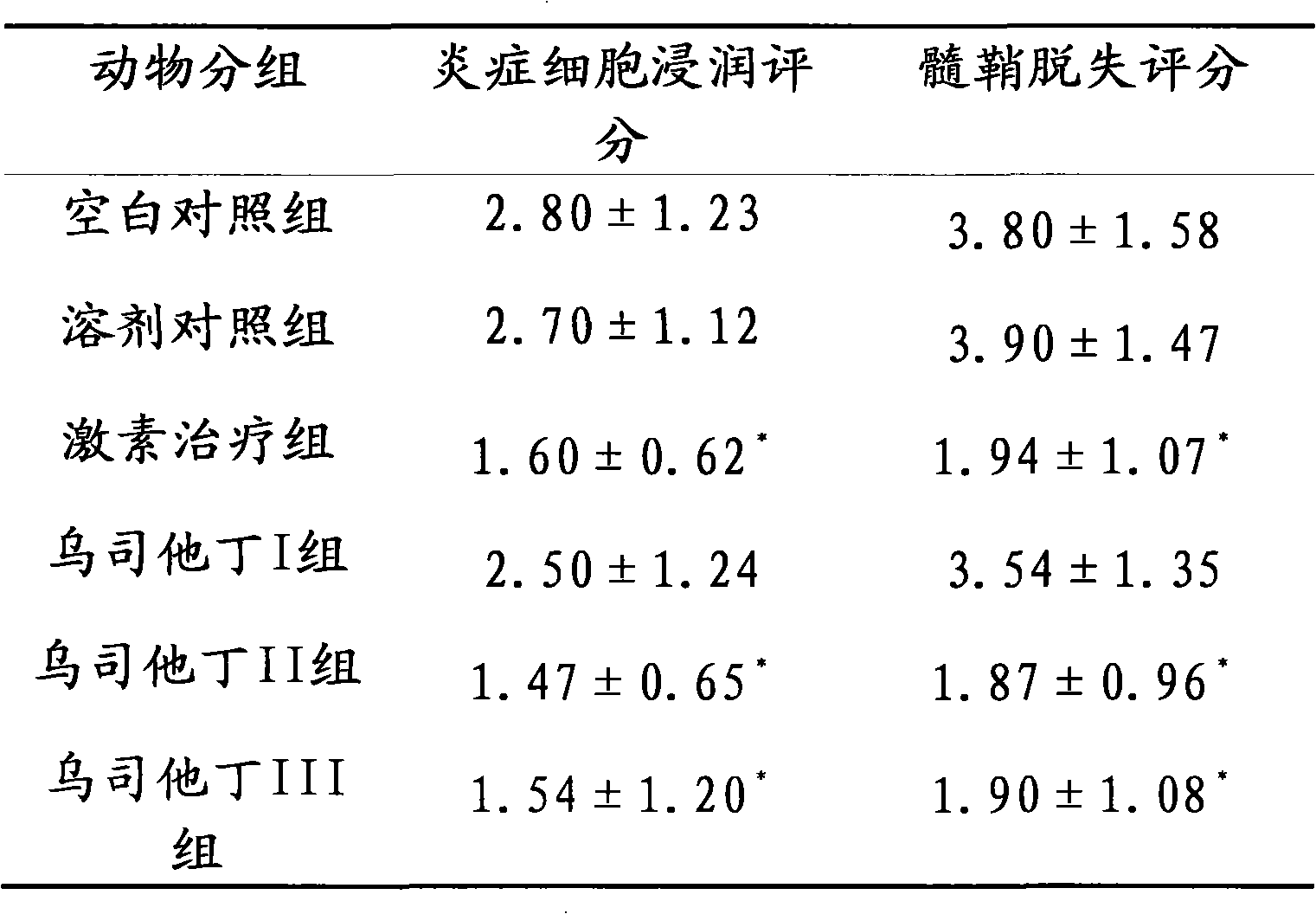
Application of Ulinastatin in preparing drug for curing autoimmune encephalomyelitis and pharmaceutical composition thereof
ActiveCN101972471AEffective treatmentTreatment safetyPowder deliveryNervous disorderSide effectLife quality
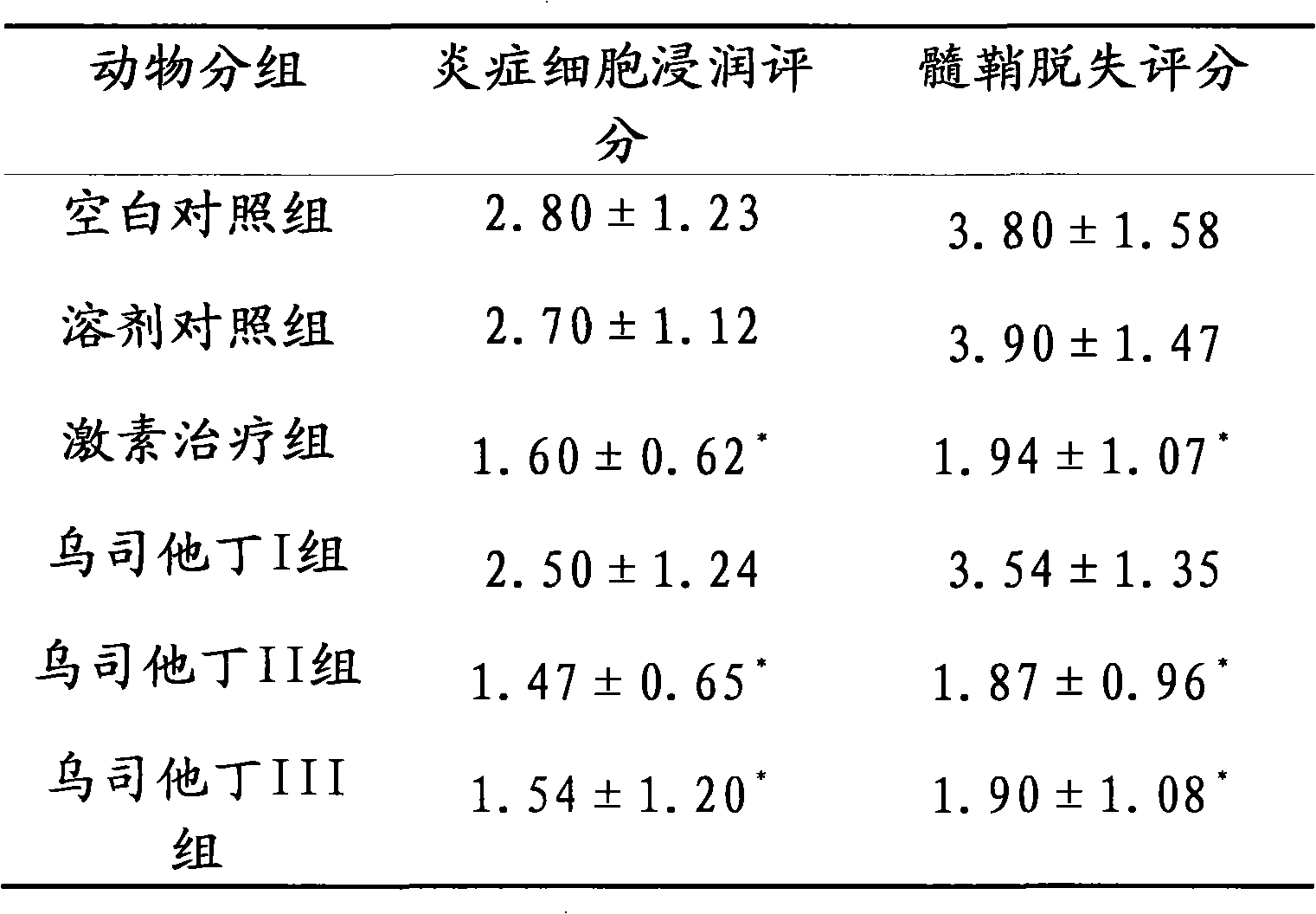
The invention relates to an application of Ulinastatin in preparing a drug for curing autoimmune encephalomyelitis, and the drug related by the invention is derived from natural protein drugs purified by human urine, has stable and reliable quality and less adverse effects, and overcomes the defects of more side effects, influenced life quality of patients even caused death and the like existing in the common drugs such as glucocorticoid and the like for curing autoimmune encephalomyelitis. Contrastive studies of curative effects on animal models of the autoimmune encephalomyelitis find that Ulinastatin has the same curative effect as hormone.
Owner:GUANGDONG TECHPOOL BIO-PHARMA CO LTD
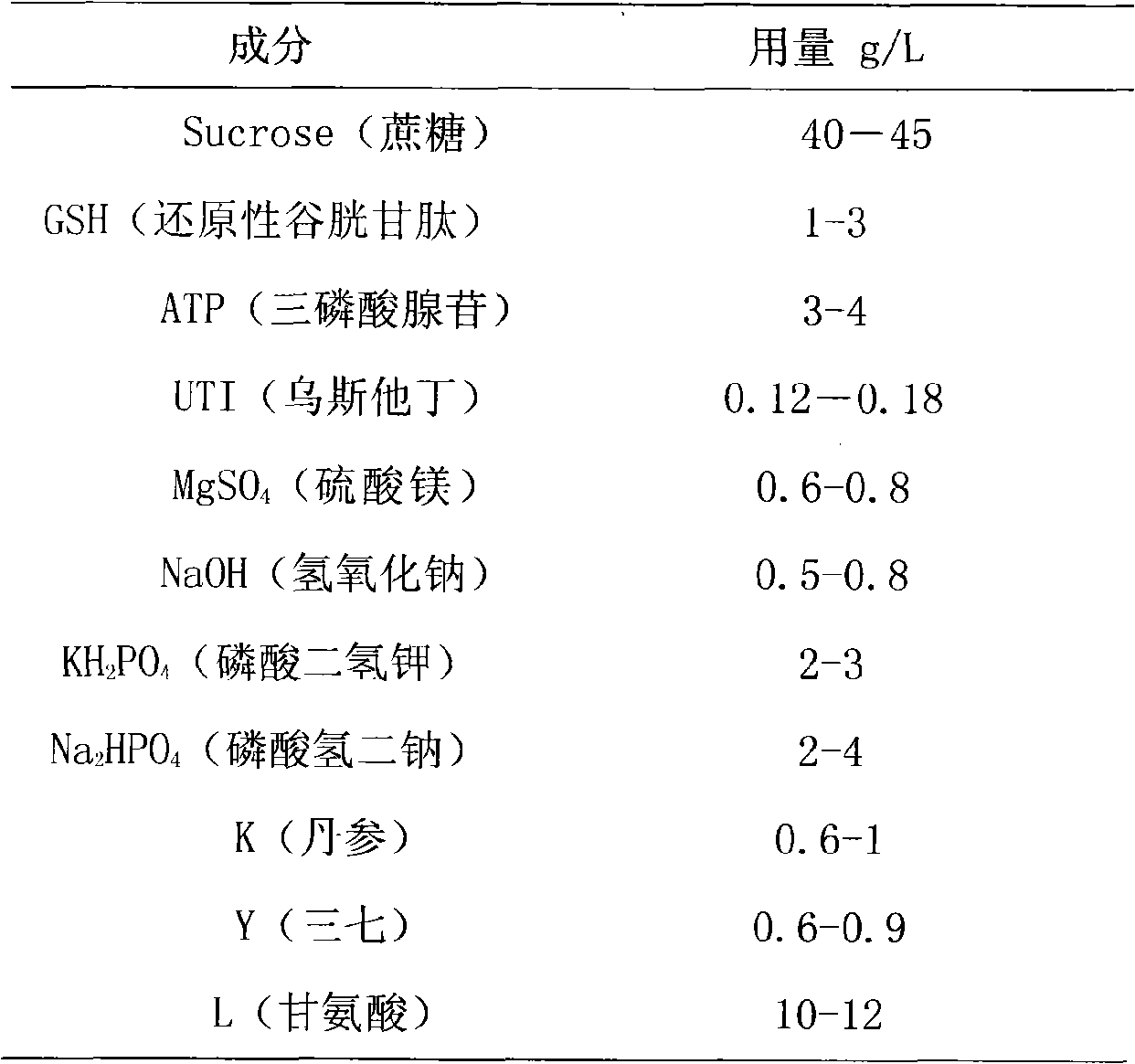
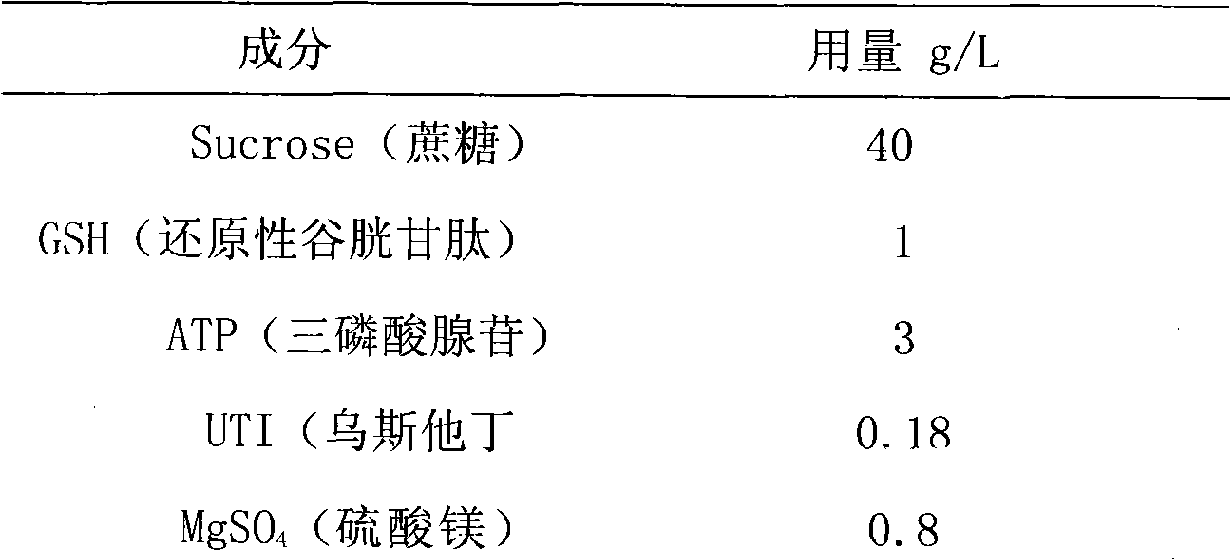
Organ preservation liquid
The invention relates to a (KYL) organ preservation solution. The organ preservation solution is prepared from the following raw materials by weight (dosage g / L): 40 to 45 grams of sucrose, 1 to 3 grams of reducibility glutathione, 3 to 4 grams of adenosine triphosphate, 0.12 to 0.18 gram of ulinastatin, 0.6 to 0.8 gram of magnesium sulfate, 0.5 to 0.8 gram of sodium hydroxide, 2 to 3 grams of potassium dihydrogen phosphate, 2 to 4 grams of disodium hydrogen phosphate, 0.6 to 1 gram of radix salviae miltiorrhizae, 0.6 to 0.9 gram of notoginseng, and 10 to 12 grams of glycine. The organ preservation solution has the advantages that: the organ preservation solution has simple preparation and an equivalent preservation effect compared with the UW solution; the organ preservation solution has a better effect than the UW solution regarding the protection for the hepatocyte functions, the hepar microcirculation and the sinus hepaticus endothelial cells; and the organ preservation solution has low price.
Owner:李立
Application of ulinastatin for preparing cervical cancer treatment medicine and pharmaceutical composition
ActiveCN104906558AOpen up the application directionSignificant synergistic anticancer effectOrganic active ingredientsPeptide/protein ingredientsSide effectCervical ca
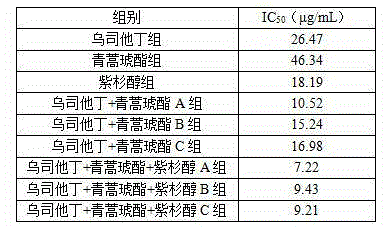
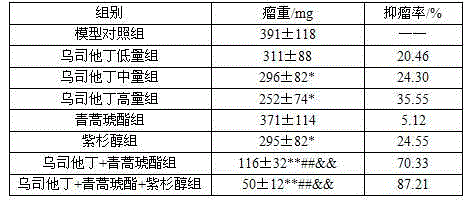
The invention belongs to the field of medical technology and discloses a new application of ulinastatin for preparing cervical cancer treatment medicine and a pharmaceutical composition for treating cervical cancer. The pharmaceutical composition includes ulinastatin and artesunate, and the pharmaceutical composition can include paclitaxel medicine specifically paclitaxel. The pharmaceutical composition has a synergistic effect for inhibiting cervical cancer and has advantages of low toxic and side effects, obvious cancer inhibition effect and the like.
Owner:GUANGDONG TECHPOOL BIO-PHARMA CO LTD
Pancreatic island freezing protective agent and using method thereof
InactiveCN102197802AInhibits the action of digestive enzymesAvoid degradationDead animal preservationHigh concentrationIslet cells
The invention provides a pancreatic island freezing protective agent and a using method thereof. The pancreatic island freezing protective agent comprises liquid A, liquid B, liquid C and liquid D, wherein the liquid A is a basic freezing culture medium and consists of 50 to 70 volume percent of M-199 culture medium, 30 to 50 volume percent of AB serum, 1 to 3 volume percent of 1MHepes and 100 to200 IU / ml of Ulinastatin; the liquid B is a 2MDMSO freezing culture medium and comprises 14.2 volume percent of DMSO and 85.8 volume percent of basic freezing culture medium; the liquid C is a 3MDMSOfreezing culture medium and comprises 1.3 volume percent of DMSO2 and 78.7 volume percent of basic freezing culture medium; and the liquid D is isopropanol. The pancreatic island is resuspended by a basic culture medium of high-concentration serum, and different concentrations of DMSO freezing liquid is added gradually incrementally, so that the DMSO can be permeated into pancreatic island cell masses. The isopropanol serves as a freezing medium and has the effect of reducing temperature slowly. The pancreatic island freezing protective agent is used for effectively preserving islet cells obtained through separation, so that the islet cells are prevented from being damaged by freezing and are provided for clinical application.
Owner:FUZHOU GENERAL HOSPITAL OF NANJING MILITARY COMMAND P L A
High purity ulinastatin and its prepn process and medicine composition
The present invention relates to high purity ulinastatin and its medicine composition and their preparation process. Specially, the high purity ulinastatin in 50,000 U / ml concentration has optical absorption value at 405 nm not exceeding 0.05 and human urea kininogenase content not exceeding 0.0003 PNAU. The present invention purifies ulinastatin product through adsorption with hydrophobic column, purification in hydrophilic column, combination with metalloprotein in metal chelating column and elution with buffering phosphate solution.
Owner:GUANGDONG TECHPOOL BIO-PHARMA CO LTD
Use of ulinastatin in preparation of medicament for treating systemic lupus erythematosus and medicinal composition of ulinastatin
ActiveCN101954071BLittle side effectsPeptide/protein ingredientsUrinary disorderSide effectSystemic lupus erythematosus
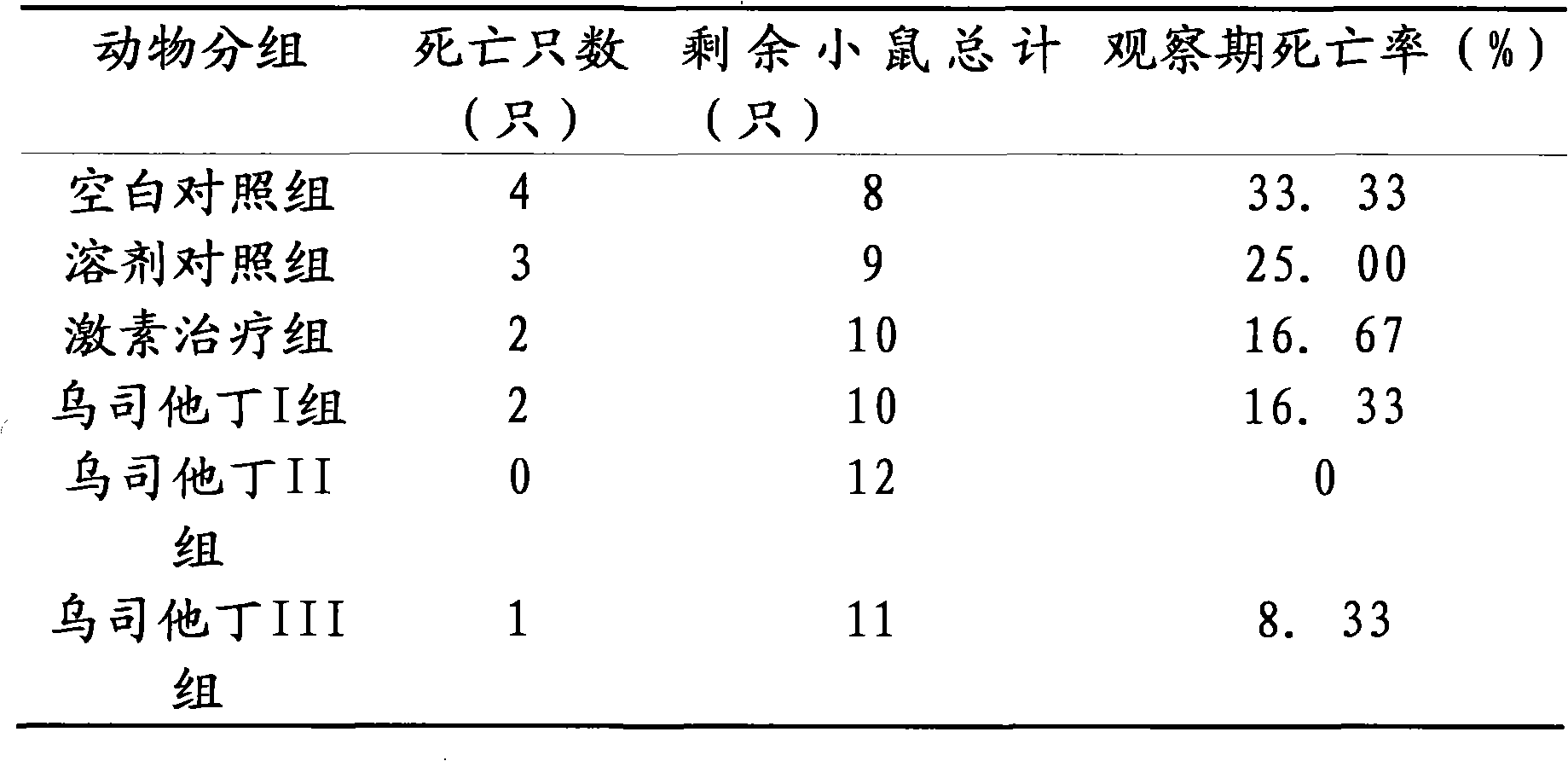
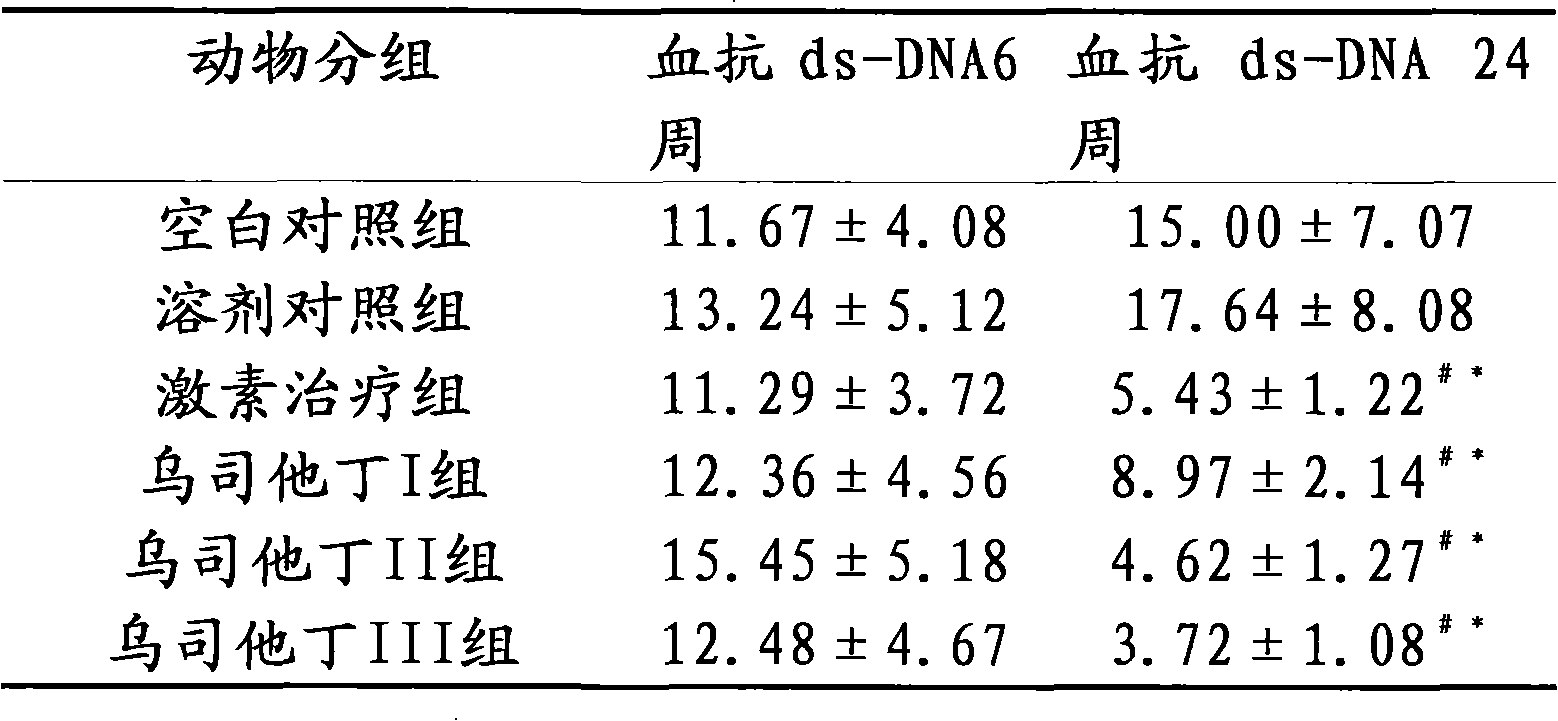
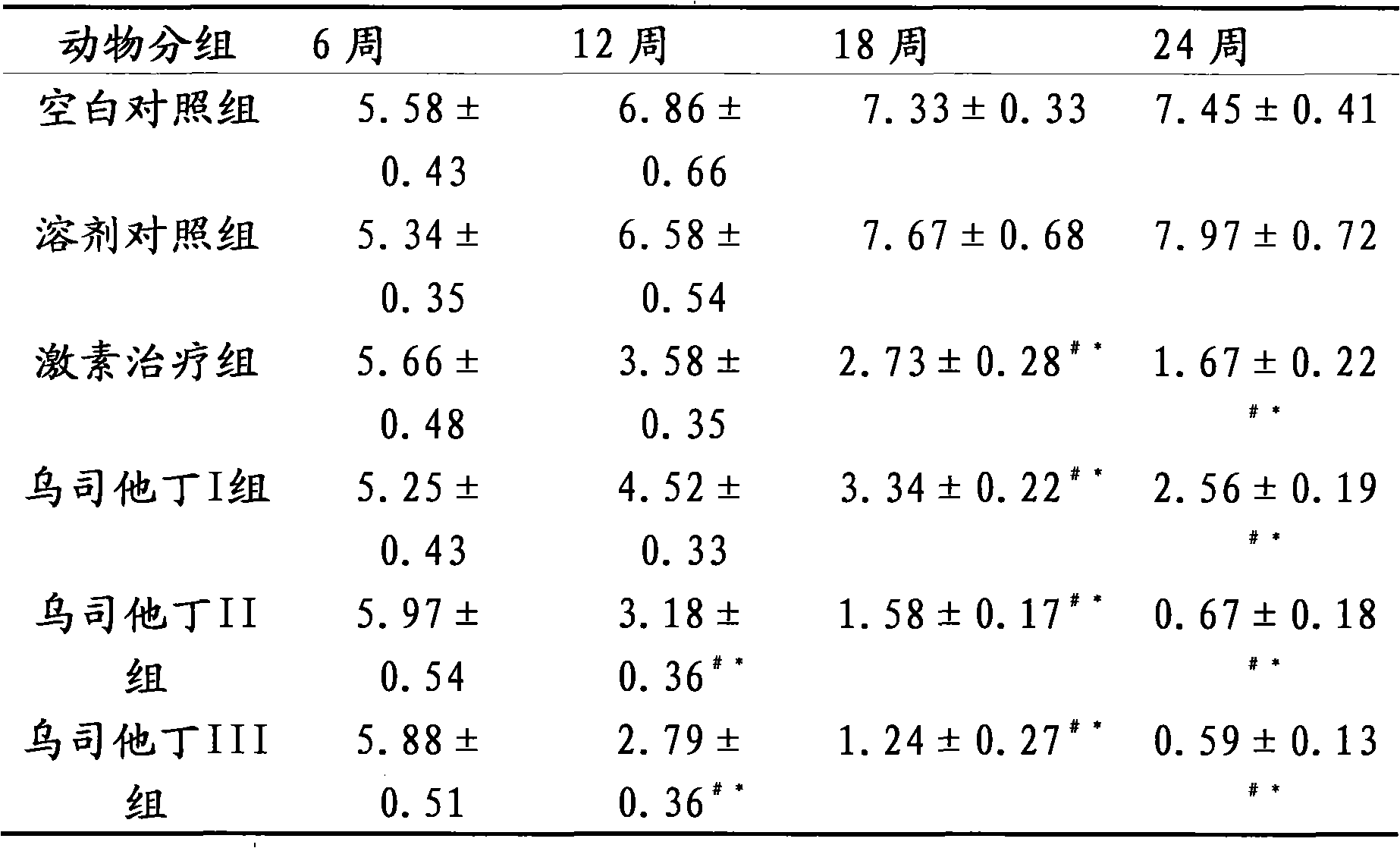
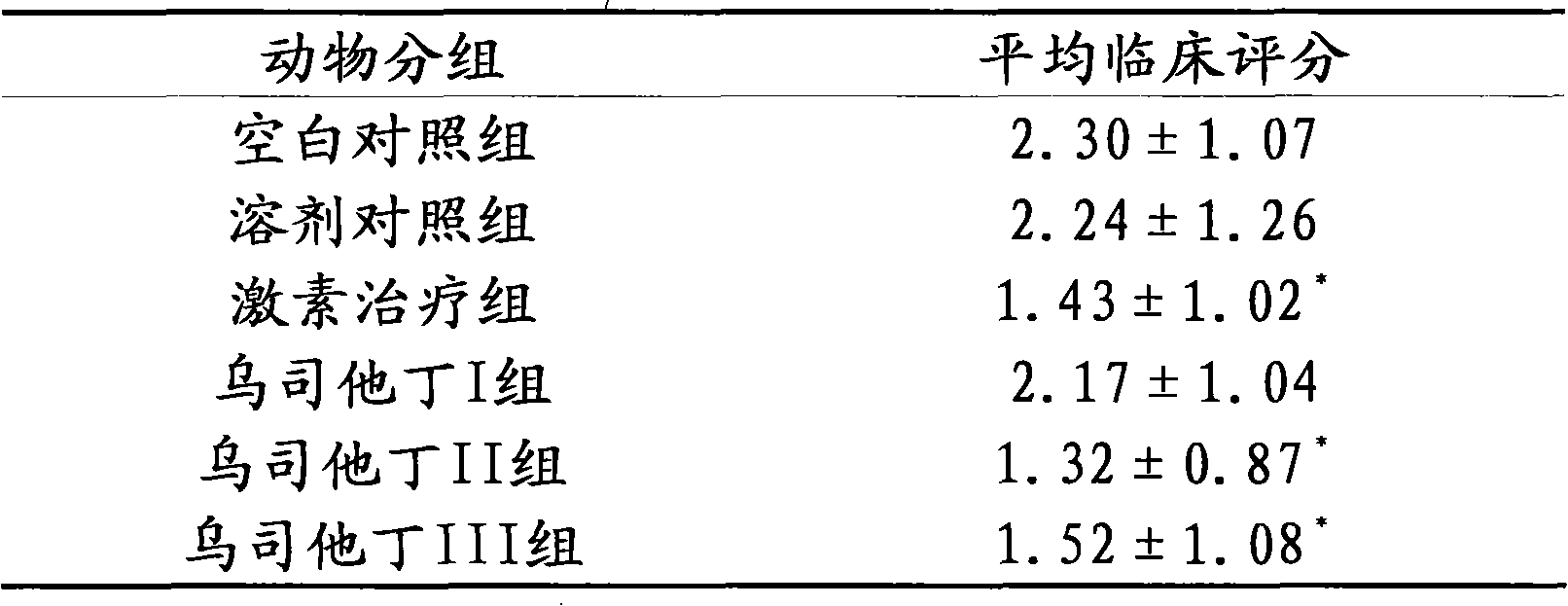
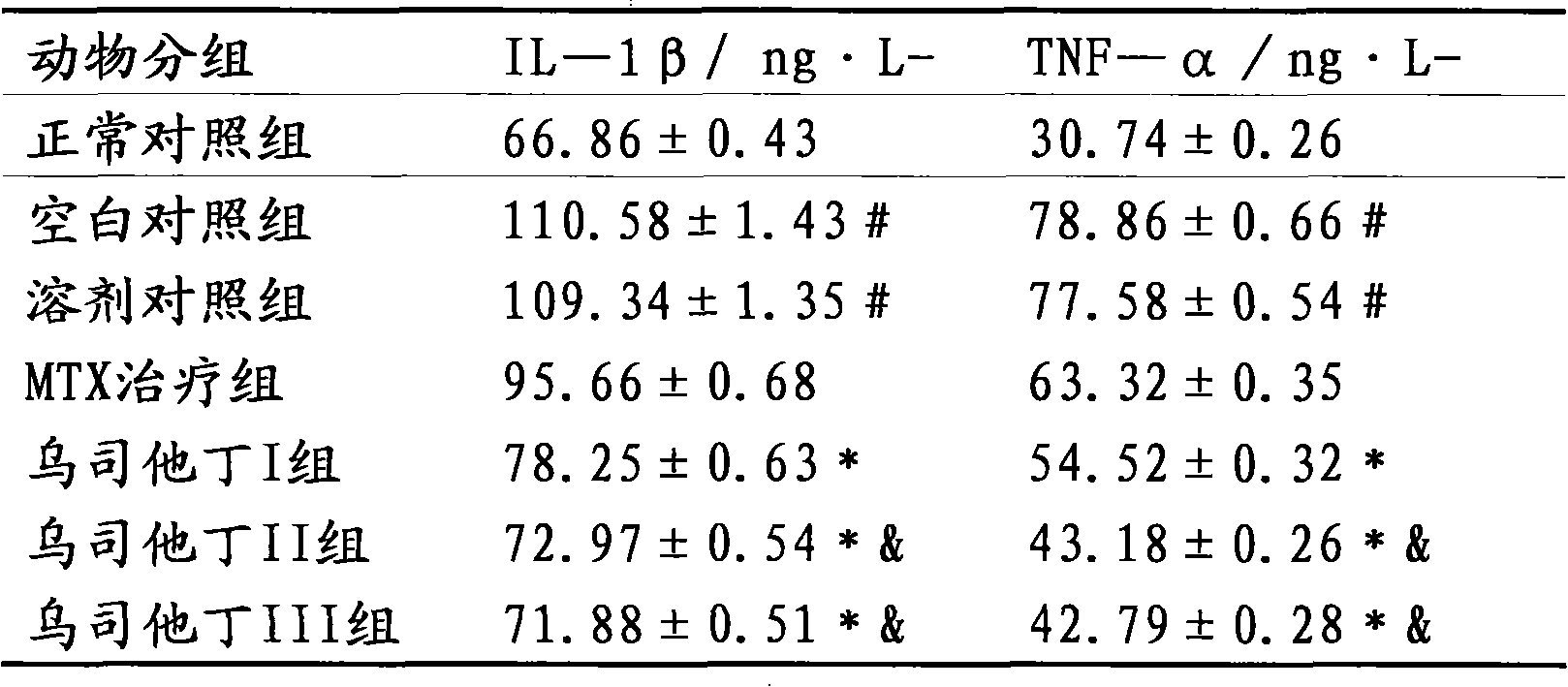
The invention relates to the use of ulinastatin in the preparation of a medicament for treating systemic lupus erythematosus. The medicament of the invention is derived from natural protein medicaments extracted from human urine, has a stable and reliable quality and fewer unwanted effects and overcomes the shortcomings of generating more side effects, influencing the living quality of a patient, even causing death of the patient and the like when the conventional medicaments such as glucocorticoid, yclophosphamide, azathioprine and cyclosporine A are used for treating the systemic lupus erythematosus. According to the comparative study on the treatment effect of lupous animal models, the treatment effect of the ulinastatin is the same as that of a hormone contrast group.
Owner:GUANGDONG TECHPOOL BIO-PHARMA CO LTD
Application of Ulinastatin in preparing drug for curing autoimmune encephalomyelitis and pharmaceutical composition thereof
ActiveCN101972471BEffective treatmentTreatment safetyPowder deliveryNervous disorderLife qualitySide effect
The invention relates to an application of Ulinastatin in preparing a drug for curing autoimmune encephalomyelitis, and the drug related by the invention is derived from natural protein drugs purified by human urine, has stable and reliable quality and less adverse effects, and overcomes the defects of more side effects, influenced life quality of patients even caused death and the like existing in the common drugs such as glucocorticoid and the like for curing autoimmune encephalomyelitis. Contrastive studies of curative effects on animal models of the autoimmune encephalomyelitis find that Ulinastatin has the same curative effect as hormone.
Owner:GUANGDONG TECHPOOL BIO-PHARMA CO LTD
Use of ulinastatin in preparation of drugs for treating rheumatoid arthritis and pharmaceutical composition thereof
InactiveCN101954072ALittle side effectsPeptide/protein ingredientsAntipyreticSide effectCurative effect
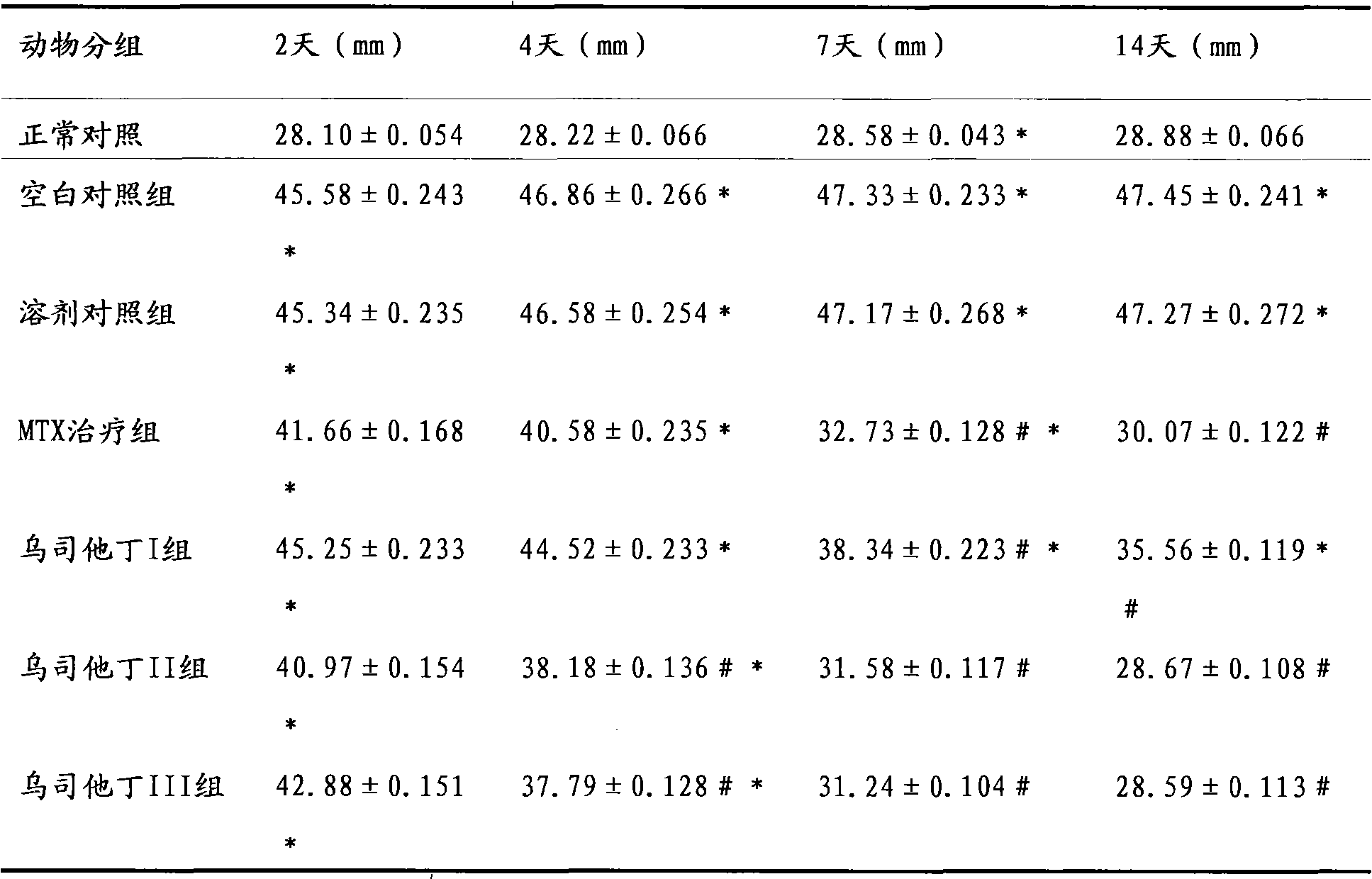
The invention relates to an application of ulinastatin in the preparation of drugs for treating rheumatoid arthritis. The related drugs are derived from natural proteins which are extracted from human urine, have stable and reliable quality and few adverse reactions and can overcome the shortcomings of more side effects and the like in the traditional drugs for treating the rheumatoid arthritis. The efficacy comparison studies of rheumatoid arthritis animal models prove that the efficacy of the ulinastatin is better than an amethopterin group, and the side effects are few.
Owner:GUANGDONG TECHPOOL BIO-PHARMA CO LTD
Use of ulinastatin in preparing medicine for treating and/or preventing spinal cord injury
InactiveCN101020049AObvious therapeuticObvious/or preventive effectPowder deliveryNervous disorderFreeze-dryingUlinastatin
The present invention relates to the application of ulinastatin in preparing medicine for preventing and treating spinal cord injury. Ulinastatin has obvious spinal cord injury preventing and treating effect. The medicine is prepared into injection or freeze dried powder for injection.
Owner:GUANGDONG TECHPOOL BIO-PHARMA CO LTD
Method for purifying ulinastatin based on cation exchange resin
InactiveCN105753976ARich purification methodsImprove recycling efficiencyPeptide preparation methodsProtease inhibitorsUltrafiltrationIon-exchange resin
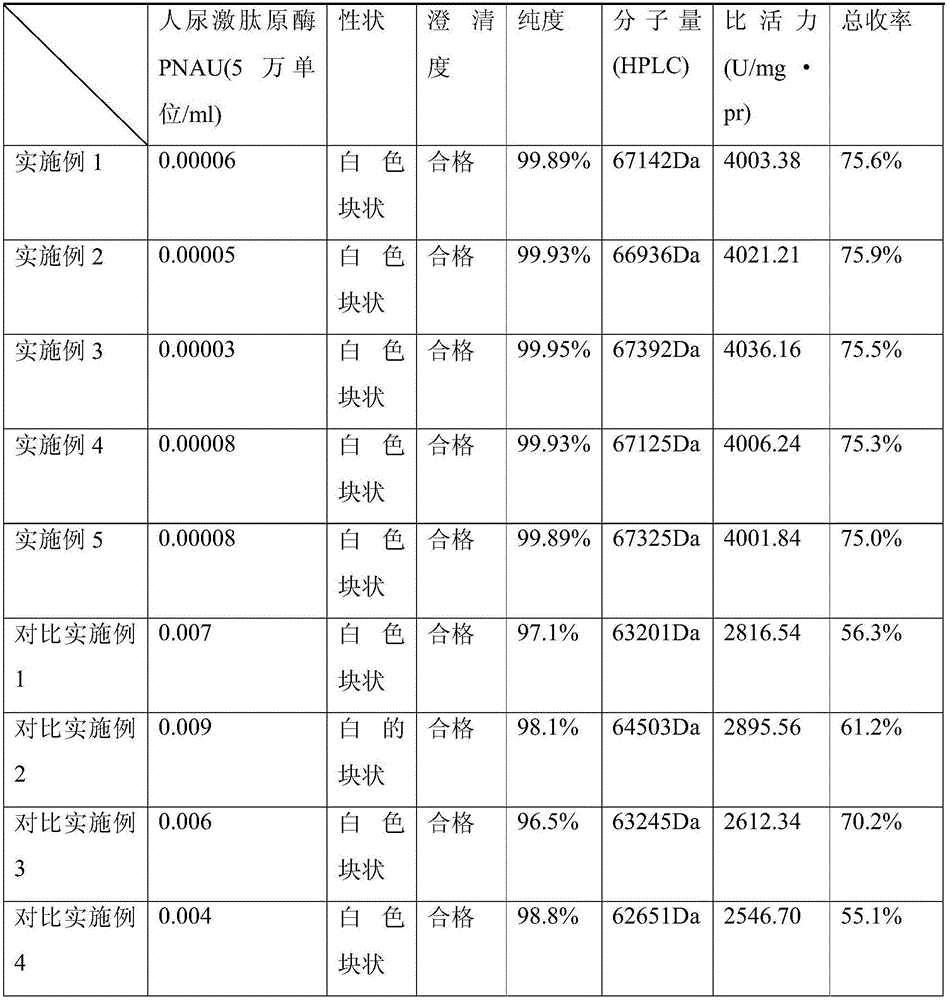
The invention belongs to the technical field of medicines, and in particular relates to a method for purifying ulinastatin based on cation exchange resin. The method comprises the following steps: mixing chitosan and alginate with primarily filtered urine, performing ultrafiltration, and purifying the trapped fluid obtained through ultrafiltration through cation exchange resin and ultrafiltration in sequence. By adoption of the method for purifying ulinastatin based on the cation exchange resin, the purification process is shortened, the yield and the purity of the ulinastatin are high, the yield of the ulinastatin is 75% or above, and the specific activity of the ulinastatin is up to 4750 IU / mg protein.
Owner:GUANGDONG TECHPOOL BIO-PHARMA CO LTD
Application of ulinastatin serving as rescue auxiliary medicament for toxic shock caused by acute abdomen
InactiveCN102205115APeptide/protein ingredientsDigestive systemURINARY TRYPSIN INHIBITORIntestinal loops
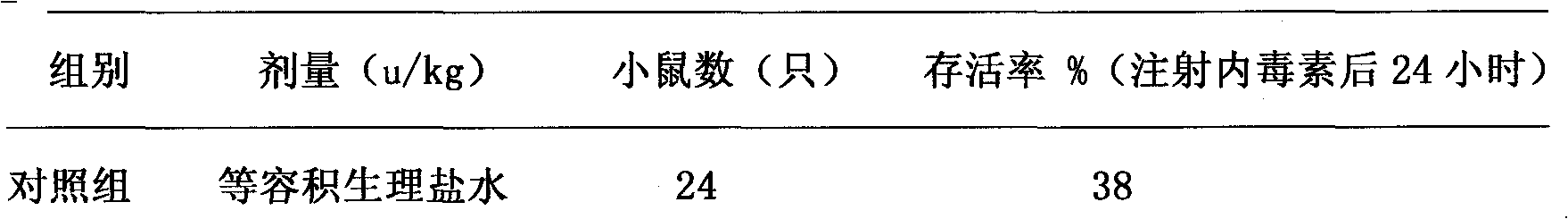
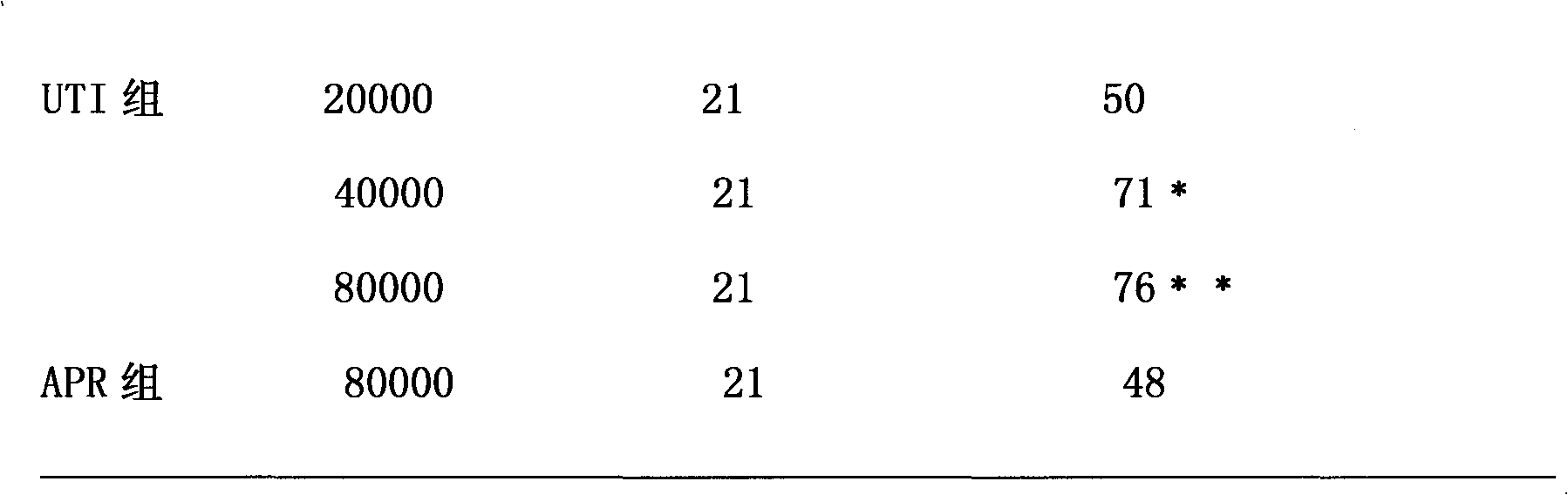
The invention relates to a rescue medicament in the field of medical treatment, in particular to a rescue medicament, namely ulinastatin or a urinary trypsin inhibitor (UTI) for toxic shock caused by acute abdomen (particularly mesenteric angiemphraxis and the like). The medicament is a preferred medicament for the toxic shock. In the pharmacodynamic study of the ulinastatin for resisting shock, the ulinastatin also can improve the average arterial blood pressure and the average arterial blood flow of superior mesenteric artery occlusion shock of rats, and has an extremely better effect on shock rescue.
Owner:徐宗昆 +3
Medicinal composition for treating arthritis
ActiveCN1751740AEasy to useUse progressPeptide/protein ingredientsSkeletal disorderGlycineURINARY TRYPSIN INHIBITOR
A composite medicine in the form of freeze-dried powder injection or liquid injection for treating osteoarthritis and rheumatoid arthritis is prepared from ulinastatin (a human Urinary trypsin inhibitor), sodium hyaluronate and optional mannitol, glycine and dextran.
Owner:GUANGDONG TECHPOOL BIO-PHARMA CO LTD
Purified ustading and its preparation method and medicinal composition containing said ustading
ActiveCN100425286CHigh purityImprove stabilityPeptide/protein ingredientsAntipyreticMetalloproteinPhosphate
A purified ulinastatin is prepared through adsorbing by hydrophobic column, separating by gel column, combining metalloprotein by metallic chelating column, and eluting with phosphate buffering liquid. A composite medicine containing the purified ulinastatin also disclosed.
Owner:GUANGDONG TECHPOOL BIO-PHARMA CO LTD
Ulinastatin purification method based on hydrophobic interaction column
InactiveCN105753975AEfficient removalHigh removal ratePeptide preparation methodsProtease inhibitorsPurification methodsFiltration
The invention belongs to the technical field of medicine, and particularly relates to a ulinastatin purification method based on a hydrophobic interaction column.The method specifically comprises the steps that pretreatment such as deposition, filtration and pH regulation is performed on crude ulinastatin, separation and purification are performed on the ulinastatin through the hydrophobic interaction column, impure protein and colored groups are removed, and the content of human urinary kallidinogenase in the ulinastatin is particularly and effectively decreased.According to the method, the total yield can be increased to 75% or above, and the product specific activity reaches 4000 U / mg.pr.
Owner:GUANGDONG TECHPOOL BIO-PHARMA CO LTD
Ulinastatin purification method based on hydrophobic column
ActiveCN107827976AAchieve reuseHigh purityPeptide preparation methodsProtease inhibitorsHydrogenPurification methods
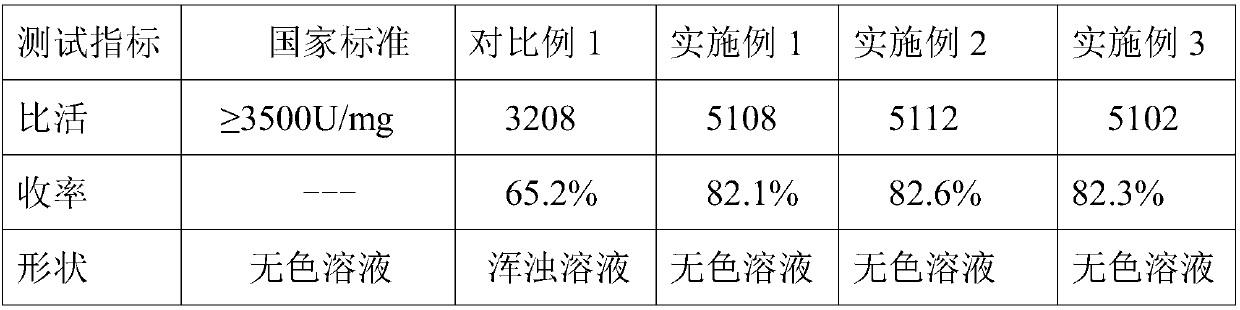
The invention discloses an ulinastatin purification method based on a hydrophobic column. The ulinastatin purification method comprises the following specific steps: S1: dissolving an ulinastatin crude product and pure water in a beaker together; then adding a proper amount of salt into the beaker; controlling the pH (Potential of Hydrogen) of a solution in the beaker to be 5 to 6; putting the beaker on a magnetic stirrer and uniformly stirring for 60min to 150min; then filtering by utilizing a filtering screen to obtain sediment for later use; S2: washing and drying the hydrophobic column andfixing the hydrophobic column on an operation platform; dissolving the sediment prepared by step S1 into 1 to 2 times of de-ionized water; after uniformly mixing, uniformly adding the mixture at theupper end of the hydrophobic column in sequence and then reacting for 1h to 2h; then adding a 1mol / L to 2mol / L salt type buffering solution for washing. According to the ulinastatin purification method based on the hydrophobic column, a test finds out that the average yield is 82 percent or above; the purity of purified ulinastatin reaches 99.9 percent or above and the specific activity is 5100U / mg protein at least; the shape of the ulinastatin is colorless liquid and standard requirements of China are met; a purification technology is simple and feasible and is worthy of being greatly popularized.
Owner:GUANGDONG TECHPOOL BIO-PHARMA CO LTD
Use of ulinastatin in preparation of medicament for treating systemic lupus erythematosus and medicinal composition of ulinastatin
ActiveCN101954071ALittle side effectsPeptide/protein ingredientsUrinary disorderSide effectTherapeutic effect
The invention relates to the use of ulinastatin in the preparation of a medicament for treating systemic lupus erythematosus. The medicament of the invention is derived from natural protein medicaments extracted from human urine, has a stable and reliable quality and fewer unwanted effects and overcomes the shortcomings of generating more side effects, influencing the living quality of a patient,even causing death of the patient and the like when the conventional medicaments such as glucocorticoid, yclophosphamide, azathioprine and cyclosporine A are used for treating the systemic lupus erythematosus. According to the comparative study on the treatment effect of lupous animal models, the treatment effect of the ulinastatin is the same as that of a hormone contrast group.
Owner:GUANGDONG TECHPOOL BIO-PHARMA CO LTD
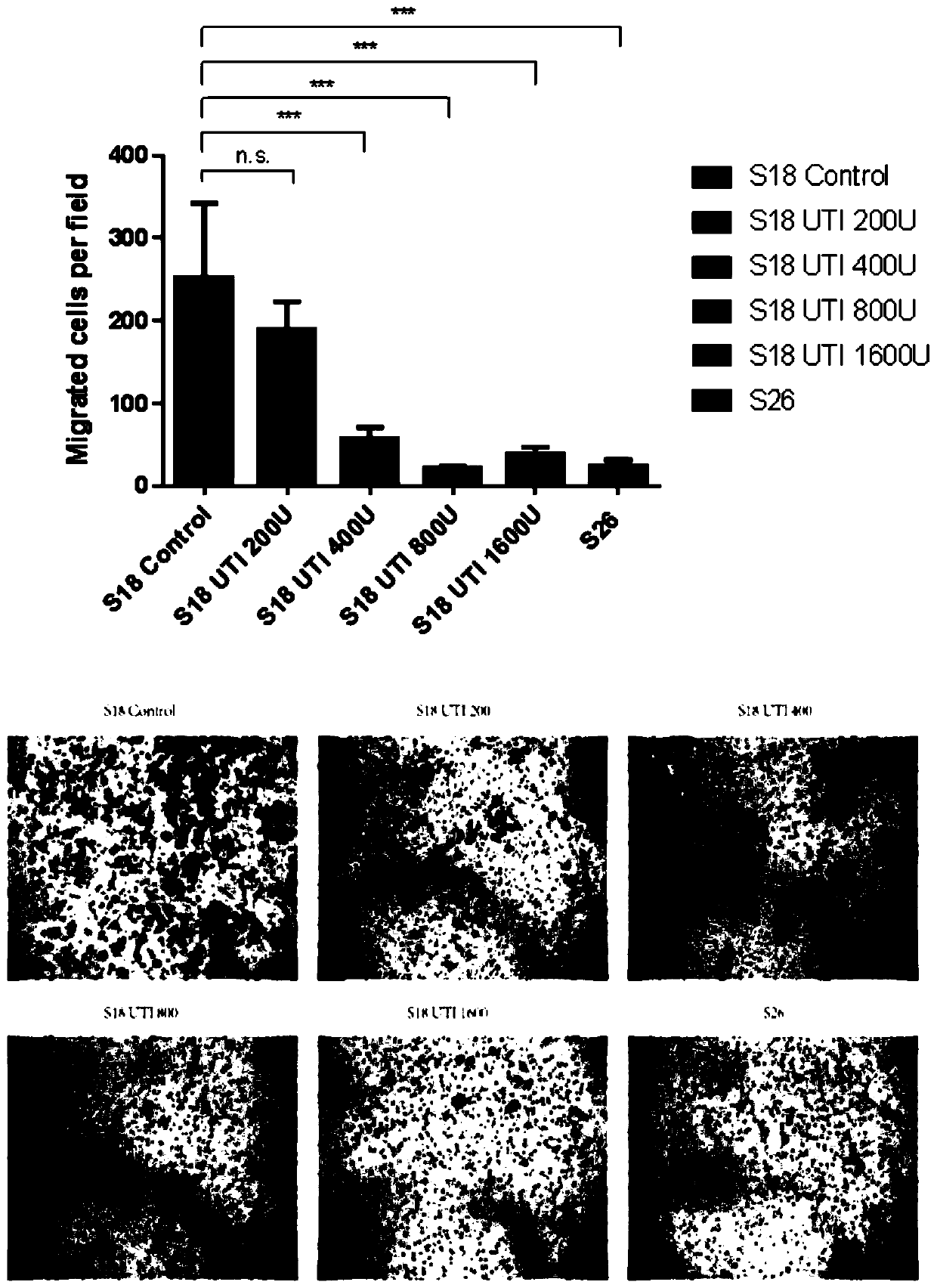
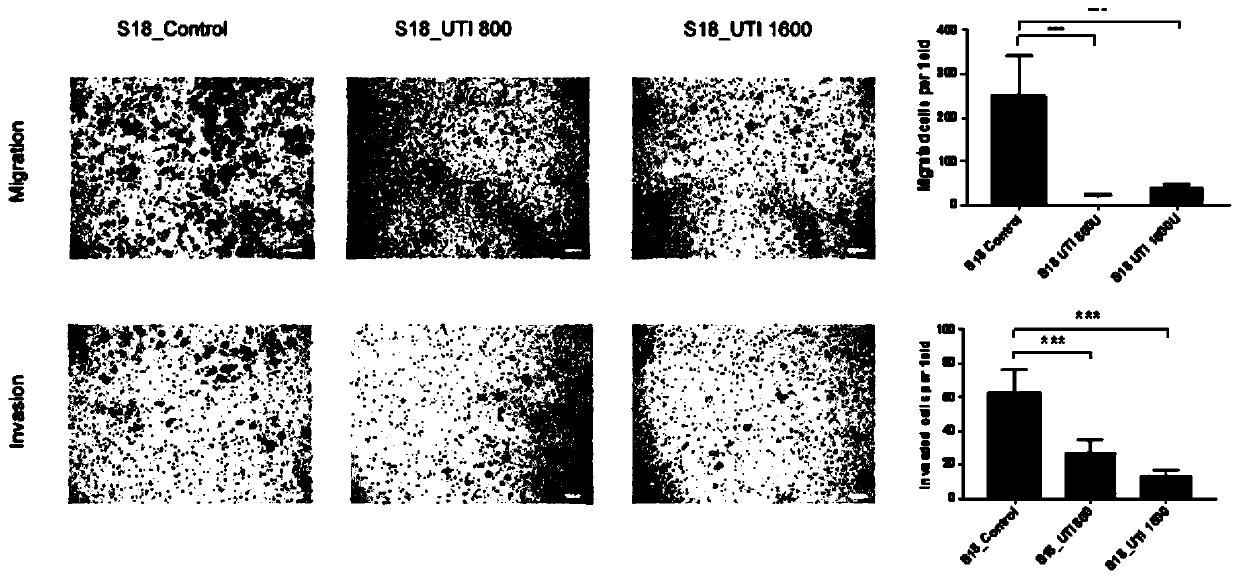
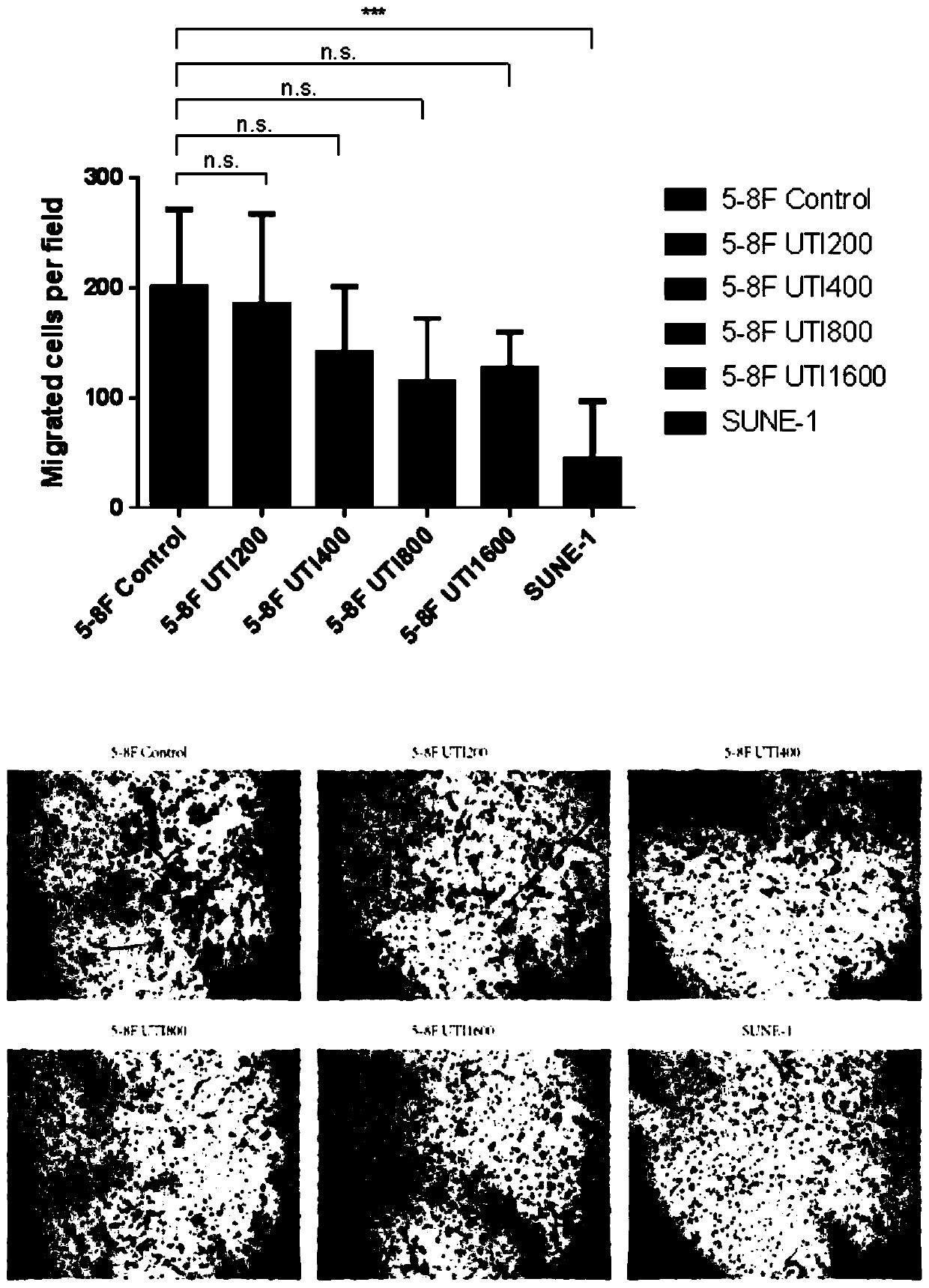
Application of ulinastatin in preparations of anti-nasopharyngeal carcinoma metastasis medicines
ActiveCN111529696AInhibit migrationPrevent invasionPowder deliveryPeptide/protein ingredientsPharmaceutical drugOncology
The present invention belongs to the technical field of medicines and specifically relates to an application of ulinastatin in preparations of anti-nasopharyngeal carcinoma metastasis medicines. Through cell in-vitro tests, the ulinastatin is found to be capable of inhibiting metastasis of nasopharyngeal carcinoma cells, does not inhibit growth of the nasopharyngeal carcinoma cells, and is less toxic and more conducive to rehabilitation of patients with nasopharyngeal carcinoma.
Owner:GUANGDONG TECHPOOL BIO-PHARMA CO LTD
Method for purifying ulinastatin based on anion exchange resin
InactiveCN105837685AIncrease molecular weight cut offHigh precisionPeptide preparation methodsProtease inhibitorsUltrafiltrationFreeze-drying
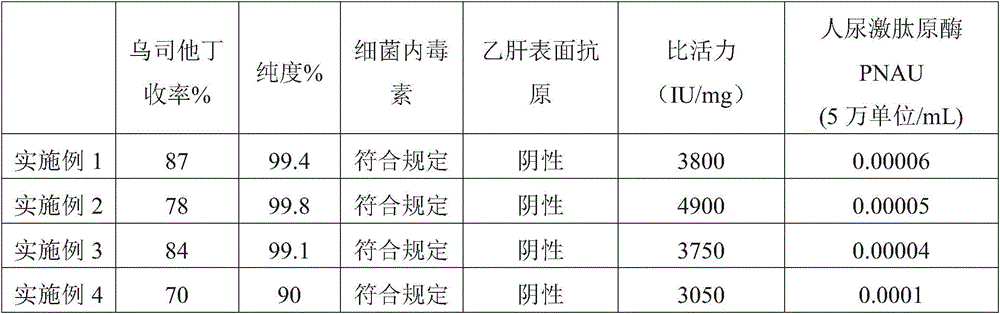
The invention belongs to the technical field of medicine and particularly relates to a method for purifying ulinastatin based on anion exchange resin. The method comprises the steps that firstly, a mixture of chitosan and chitin is used for adsorption separation, and a crude ulinastatin product is obtained; then, the crude product and alginate are mixed and then subjected to ultrafiltration, anion exchange resin purification and ultrafiltration in sequence; finally, interception liquid obtained after ultrafiltration is subjected to freezing drying, and a pure ulinastatin product is obtained. According to the method for purifying ulinastatin based on anion exchange resin, the purifying process is shortened, and the yield and purity of ulinastatin are high, wherein the yield of ulinastatin reaches 87%, and the specific activity reaches 4900 IU / mg protein.
Owner:GUANGDONG TECHPOOL BIO-PHARMA CO LTD
Application of ulinastatin in preparation of drug for treating gallbladder carcinomas
InactiveCN106110312AOpen up the application directionSignificant effectPeptide/protein ingredientsAntineoplastic agentsSide effectNude mouse
The invention belongs to the technical field of medicines, and particularly discloses application of ulinastatin in preparation of a drug for treating gallbladder carcinomas. It is proved through animal experiments that ulinastatin has an obvious inhibiting effect on human gallbladder carcinoma transplanted tumor in nude mice, is low in toxic and side effects and high in safety and can serve as a drug for treating the gallbladder carcinomas for use.
Owner:GUANGDONG TECHPOOL BIO-PHARMA CO LTD
Pharmaceutical composition for treating acute lung injury
InactiveCN106924740AEffective treatmentEffective protectionPeptide/protein ingredientsRespiratory disorderALI - Acute lung injuryPuerarin
The invention discloses a pharmaceutical composition for treating acute lung injury and belongs to the technical field of biopharmaceutical industry. The pharmaceutical composition comprises the following main components in parts by weight: 20-25 parts of puerarin, 0.2-0.4 part of verapamil, 1-2 parts of ulinastatin, 0.6-0.8 part of an NE inhibitor, 0.3-0.6 part of a salviae miltiorrhizae water extract and 0.4-0.8 part of glutamine. The invention provides the pharmaceutical composition for treating acute lung injury. By means of matched use of puerarin and verapamil, the pharmaceutical composition can effectively treat acute lung injury and inhibit rise of content of blood serum TNF-alpha, alleviate PMN infiltration, eliminate free radicals and alleviate the extent of lung tissue edema, and has an effective protecting action on acute lung injury; and moreover, the oxygenation index of arterial partial pressure of oxygen is obviously increased, and the respiratory function of a patient is improved. The pharmaceutical composition disclosed by the invention not only can treat acute lung injury effectively, but also plays a maintaining role on lungs, and is convenient to use and low in cost.
Owner:HENAN UNIV OF SCI & TECH
Ulinastatin purification method based on affinity chromatography column
InactiveCN105753974AEasy to operateIncrease productivityPeptide preparation methodsProtease inhibitorsPurification methodsFreeze-drying
The invention relates to a ulinastatin purification method based on an affinity chromatography column.The method comprises the following steps of urine processing, hydrophobic interaction column chromatography, affinity chromatography, gel filtration chromatography, washing and freeze-drying, and through a purification test on the finally obtained ulinastatin, it is found that the total yield of the ulinastatin is increased to 85% or above, and the total titer of the ulinastatin is 6500 iu / mg or above; the chromatography column is adopted for purification treatment, operation is easy, the production efficiency is improved through optimized packing and parameters such as the flow velocity, the production cost is greatly reduced, and further development and utilization values are achieved.
Owner:GUANGDONG TECHPOOL BIO-PHARMA CO LTD
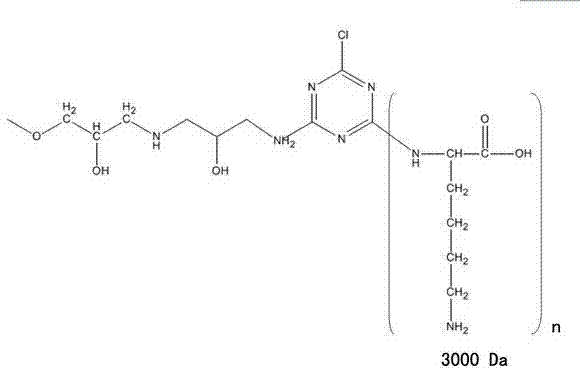
Method for preparing pure ulinastatin from ulinastatin affinity chromatography medium
ActiveCN103880951AMeet the requirementsShort time consumptionPeptide preparation methodsProtease inhibitorsSephadexPolylysine
The invention relates to a method for preparing pure ulinastatin from an ulinastatin affinity chromatography medium. The chromatography medium is formed by linking an ulinastatin affinity ligand and a common chromatography medium, wherein the affinity ligand is polylysine 3000; the common chromatography medium is sephadex; the affinity ligand and the chromatography medium are linked by a hydrophilic spacer arm, and then pure production is carried out on ulinastatin by utilizing the affinity chromatography medium. Ulinastatin with molecular weight of 37-43kDa is quickly separated from the raw materials and is purified via processes for adjusting the concentrations and pH values of various solutions and flow rates of washing and eluting and the like. One-step affinity chromatography is only needed in the whole process, and the yield and specific activity of pure ulinastatin obtained through purification are respectively more than 80% and more than 5000IU / mg.pr. The whole purifying step consumption time is short, and the activity recovery rate and the specific activity are relatively high, so that the method is suitable for large-scale popularization and application.
Owner:NANCHANG WANHUA BIOCHEM PHARMA
Application of compound in preparation of medicine for treating acute pancreatitis
ActiveCN111529526AMedication is simpleMedication convenienceOrganic active ingredientsDispersion deliverySerum amylasePharmaceutical drug
The invention discloses an application of a compound in preparation of a medicine for treating acute pancreatitis. The compound can remarkably reduce the content of serum amylase and has an obvious effect on treating acute pancreatitis, and the curative effect of the compound is superior to or not inferior to that of an existing treatment medicine, namely ulinastatin. The compound can be used fordeveloping a novel medicine for treating acute pancreatitis, and has an important medical prospect and economic value.
Owner:LINK HEALTH GRP
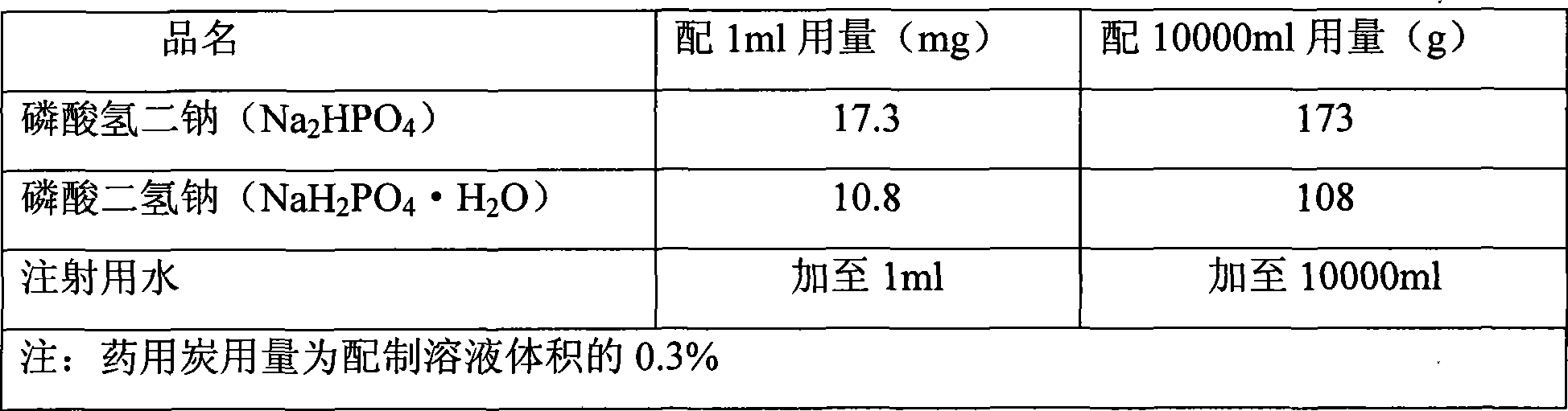
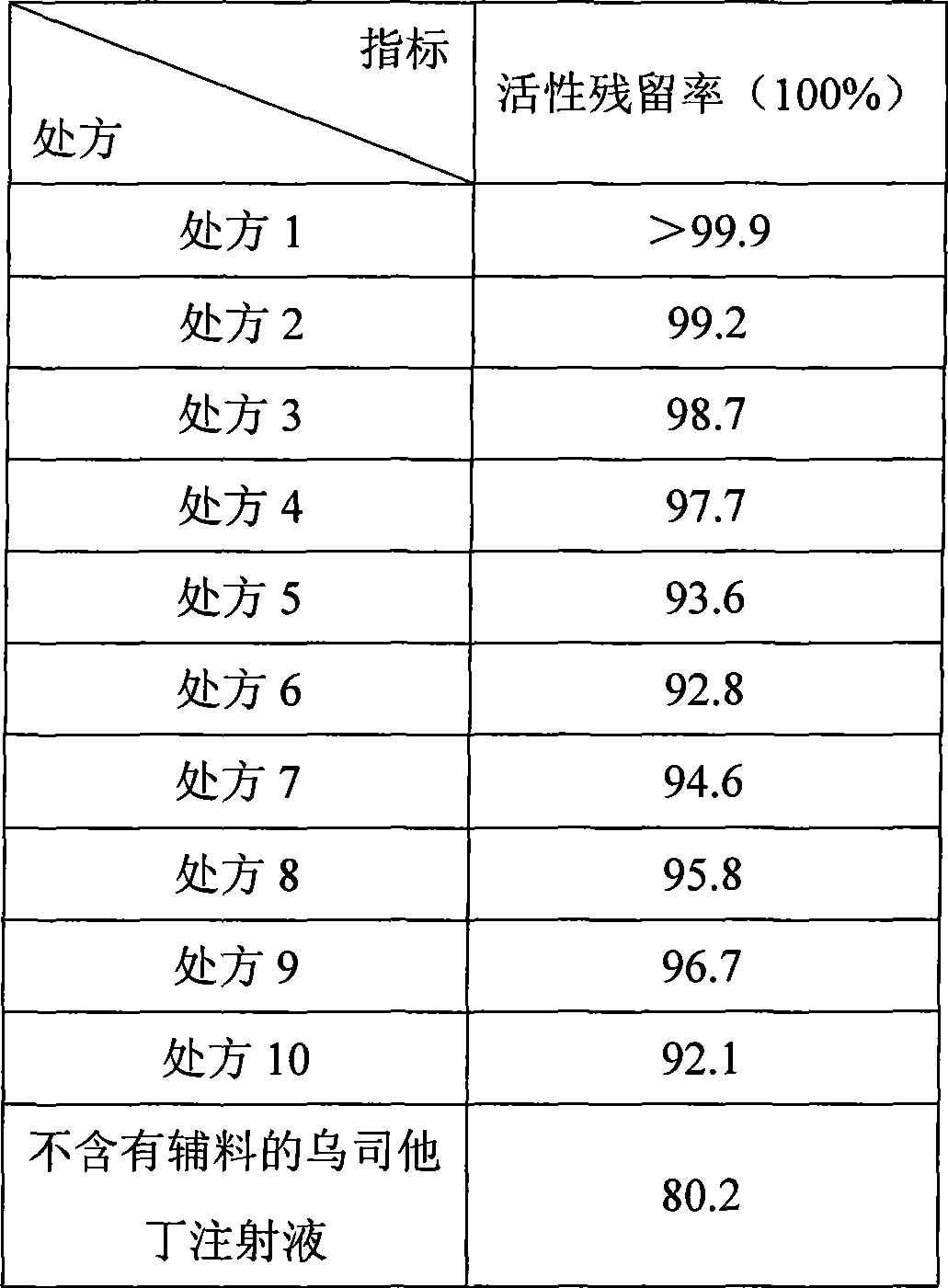
Preparation method for ulinastatin freeze-dried powder preparation
InactiveCN109010290APlay a protective effectSolve the problem of low temperature and difficult controlPowder deliveryNervous disorderFreeze-dryingBottle
The invention discloses a preparation method of an ulinastatin freeze-dried powder preparation, belonging to the field of pharmaceutical preparations. According a prescription for the ulinastatin freeze-dried powder preparation, every 1000 bottles of the ulinastatin freeze-dried powder preparation comprise 25,000,000 to 100,000,000 units of ulinastatin, 0-20 g of mannitol, 0-2 g of sodium chloride, 0-2 g of disodium hydrogen phosphate and 0-2 g of sodium dihydrogen phosphate. The preparation method for the ulinastatin freeze-dried powder preparation provided by the invention has the followingcharacteristics: preparation is implemented at room temperature, so the problem that industrial production temperature is low and difficult to control is overcome, and energy consumption is lowered; and a phosphate buffer solution is prepared at room temperature at first, and then the active ingredient ulinastatin is added, so the buffer solution has protective effect on the active ingredient ulinastatin. The ulinastatin freeze-dried powder preparation prepared by using the prescription and the preparation method provided by the invention is convenient for industrial production, reduces energyconsumption and can improve the stability of the active ingredient--an ulinastatin solution.
Owner:YANGZHOU AIDEA BIOTECH
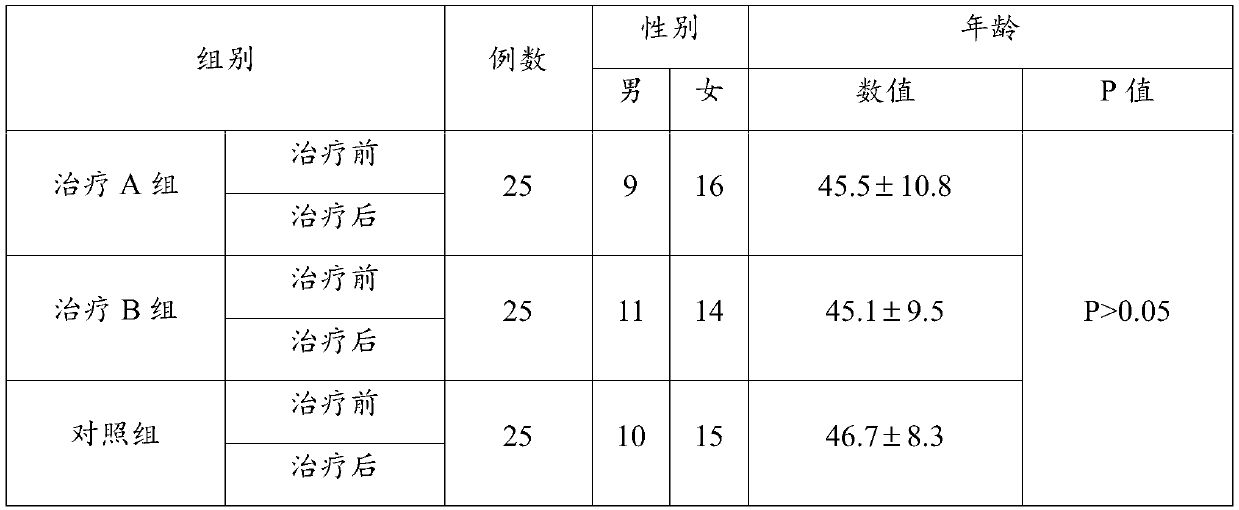
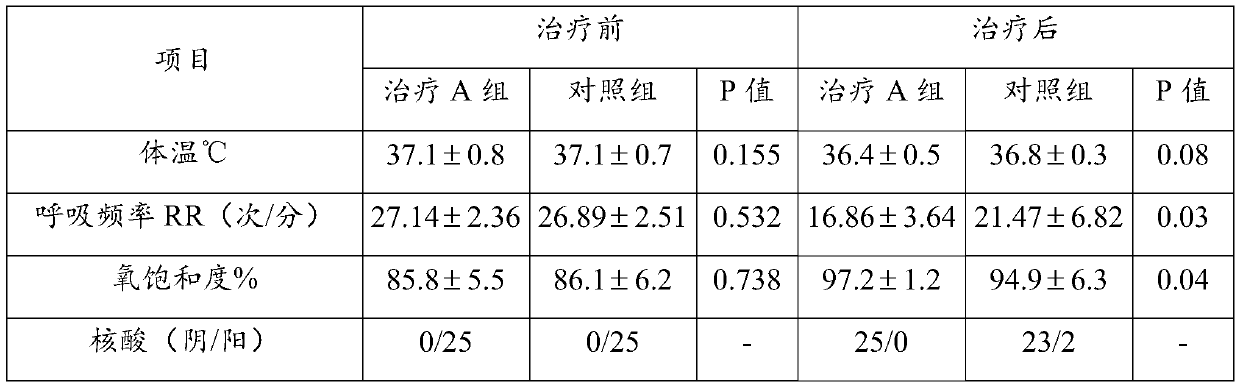
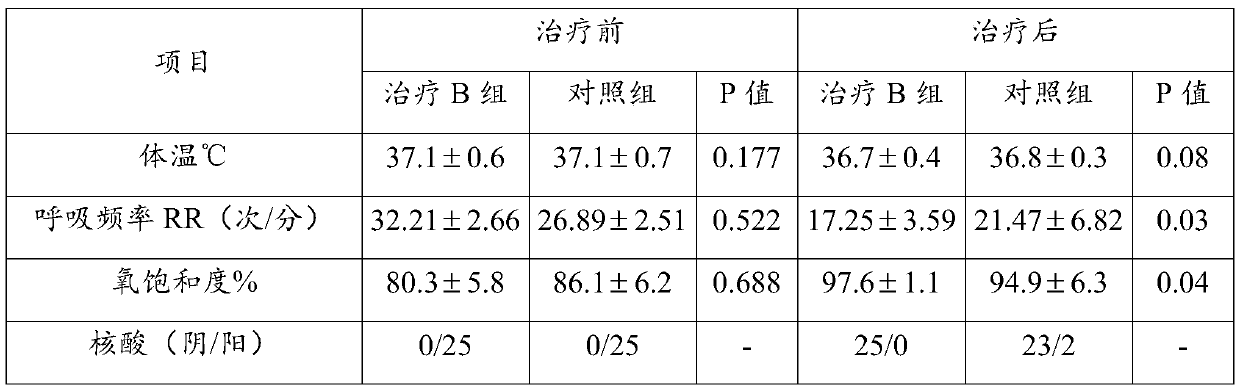
Use of ulinastatin in preparing medicines for treating new coronavirus pneumonia
InactiveCN111329999ASignificant clinical effectTargetedPeptide/protein ingredientsAntiviralsPharmaceutical drugClinical trial
The present invention belongs to the technical field of medicine and particularly relates to a use of ulinastatin in preparing medicines for treating new coronavirus pneumonia. The present invention provides the use of the ulinastatin in preparing the medicines for treating the new coronavirus pneumonia. Clinical trials find that the ulinastatin has a significant therapeutic effect on the new coronavirus pneumonia.
Owner:GUANGDONG TECHPOOL BIO-PHARMA CO LTD +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com